Farmed mussels have been collected on a monthly basis since 1999 from a remote site on the west coast of Scotland for polycyclic aromatic hydrocarbon (PAHs) analysis with the aim of establishing background concentrations as a benchmark against which to assess any environmental incident. Total PAH (2- to 6-ring parent and alkylated) concentrations ranged from 12.5 to 151.2 μg kg−1 wet weight. Seasonal trends were evident with concentrations being significantly higher for samples collected between November and March compared to those collected between April and October. By taking the median of medians for each of these time periods two background concentrations are suggested for the total PAH concentrations (2- to 6-ring PAHs parent and alkylated); for April to October: 31.2 μg kg−1 wet weight and for November to March: 62.9 μg kg−1 wet weight. Individual PAH concentrations were mainly below the OSPAR Background Assessment Concentrations (BACs), where they are specified, and were only exceeded for the heavier 4- and 5-ring PAHs (fluoranthene, pyrene, benz[a]anthracene and benzo[a]pyrene) in samples collected between November and March. Differences were also seen in the PAH profiles with season. Mussels collected between November and March had a higher proportion of the heavier PAHs compared to mussels collected in the summer and autumn.
INTRODUCTION
Blue mussels (Mytilus edulis) have been used extensively as sentinel indicator species for monitoring uptake and accumulation of hydrophobic contaminants in the marine environment, including polycyclic aromatic hydrocarbons (PAHs). PAHs can bioaccumulate in shellfish but are metabolized relatively effectively by fish (Citation1, Citation2, Citation3, Citation4, Citation5, Citation6, Citation7, Citation8, Citation9, Citation10). PAHs are widespread chemical pollutants that are introduced into the marine environment from a number of different sources. They are mainly produced by pyrolysis, but can also be of petrogenic origin from crude oils or refinery products. PAHs can enter the marine environment through atmospheric deposition, road run-off, and industrial discharges and as a result of oil spills. There is a continuing concern over the occurrence of PAHs in the marine environment due to their toxicity and persistence (Citation11). In general, the 2-ring compounds are acutely toxic and can cause tainting of fish and shellfish while some of the heavier 4- to 6-ring PAHs are mutagenic and carcinogenic (i.e., benz[a]anthracene, benzo[a]pyrene, dibenz[a,h]anthracene) (Citation11, Citation12). For these reasons they are listed on the Oslo and Paris Commission (OSPAR) List of Chemicals for Priority Action. In addition, PAHs are classed as Priority Hazardous Substances (PHS) by the Water Framework Directive (WFD) (Directive 2000/60/EC).
Mussels are filter-feeding bivalves and are exposed to both dissolved and particulate bound PAHs present in the water column. The more hydrophilic 2- and 3-ring PAHs, dissolved in the water column, will be readily available to mussels (Citation13, Citation14, Citation15). In addition, mussels will be exposed to the heavier (4- to 6-ring PAHs), more hydrophobic PAHs, associated with particulate matter (Citation13, Citation14, Citation15). The exposure of mussels to contaminated sediments or to particulate bound PAHs can result in bioaccumulation of PAHs and, on occasions, adverse biological effects.
Following any environmental incident, such as an oil spill, a monitoring program is put in place to assess the environmental impact of the incident. Following the Braer oil spill on Shetland in 1993, a Food and Environment Protection Act 1985 (FEPA) Exclusion Zone was put in place to prevent contaminated fish and shellfish reaching the market place (Citation16, Citation17). Up until the final lifting of the FEPA Exclusion Zone in 2000 (for mussels and Nephrops) a considerable amount of PAH monitoring was undertaken in fish, shellfish and sediment (Citation18, Citation19, Citation20, Citation21, Citation22). A long-term monitoring program of PAHs in mussels, collected both within and out with the Exclusion Zone (EZ) was undertaken (Citation19). As part of this monitoring exercise mussels from a reference site were transplanted to three sites within the EZ and suspended in mesh boxes from rafts. Samples were collected at regular intervals for PAH analysis. Seasonal trends were observed in the PAH concentrations; they were higher in winter and decreased in the spring. Total PAH concentrations in the EZ mussels at all three sites were consistently higher than concentrations found in the reference mussels. Despite there being no petrogenic taint, the EZ could not be lifted as PAH concentrations in mussels harvested from within the EZ were consistently higher than concentrations found in the reference mussels. Not until March 2000 could the EZ finally be lifted for mussels when further work demonstrated that the higher PAH concentrations were not due to contamination from Gullfaks crude oil. The PAH profiles were dominated by the heavier PAHs, and the lack of oil-related geochemical biomarkers confirmed that the higher PAH concentrations within the Zone were not due to Gullfaks crude oil.
High total PAH concentrations (> 4,000 μg kg−1 wet weight) have also been found in farmed mussels from Loch Leven in the western Highland region of Scotland as a result of discharges from an aluminium smelter situated at the head of the loch (Citation23). The smelter finally closed in June 2000. Monitoring demonstrated that concentrations were considerably higher than had previously been reported in mussels sampled from elsewhere around the Scottish mainland. A monitoring program was put in place with samples being collected from two mussel farms in Loch Leven and a reference site at Loch Etive, to the south of Loch Leven. Since monitoring began in 1999 total PAH concentrations in Loch Leven mussels have decreased although concentrations have been consistently higher than in mussels from Loch Etive. The total PAH (2- to 6-ring PAH parent and branched) concentration in a mussel sample collected in December 2004 was 187.8 μg kg−1 wet weight, however, concentrations were still higher than found in farmed mussels, collected at the same time, from Loch Etive.
Environmental incidents, such as the Braer oil spill and the Loch Leven problem have highlighted the need to establish background concentrations for contaminants in a range of matrices so as to have a benchmark against which sites of potential contamination can be assessed. Therefore, sampling has continued at Loch Etive to provide data on background PAH concentrations in mussels. This paper presents the PAH data for these reference mussels and looks at the seasonal differences in the PAH concentrations and composition.
METHODS
Sample Collection
Since June 1999 mussel (Mytilus edulis) samples have been collected at monthly intervals, when possible, from a mussel farm in Loch Etive, situated in the western highland region of Scotland, UK (). Loch Etive is approximately 30 km long with a coastline of approximately 65 km. Farmed mussels from Loch Etive were delivered to FRS Marine Laboratory, Aberdeen, Scotland in insulated cool boxes. Each sample consisted of between 20 and 30 animals with lengths of 4–6 cm. The soft tissue was removed from each mussel and homogenised. Samples were stored in solvent washed aluminium cans at −18°C to −20°C until required for analysis.
Isolation of Hydrocarbons from Mussels (Citation19)
To a homogenized sample of mussel (∼10 g) was added deuterated aromatic standards (d8-naphthalene, d10-biphenyl, d8-dibenzothiophene, d10-anthracene, d10-pyrene and d12-benzo[a]pyrene). This was mixed with sodium hydroxide (10%, m/v) in methanol-water (9/1, v/v; 40 ml). The mixture was refluxed for 3 h 45 min before the addition of water (10 ml) and then refluxing continued for a further 15 min. The resulting hot solution was extracted with iso-hexane (2 × 80 ml). The combined extracts were washed with water (3 × 40 ml) before drying over Na2SO4. The dried extract was concentrated to approximately 300 μl then fractionated by isocratic, normal phase high performance liquid chromatography (HPLC), to separate the aliphatic and aromatic components. The second fraction, containing the PAHs, was collected between approximately 3 and 20 minutes and was concentrated to approximately 20 μl prior to analysis by gas chromatography-mass spectroscopy (GC-MS).
Determination of Polycyclic Aromatic Hydrocarbons (PAHs) by GC-MS (Citation19)
The concentration and composition of the PAHs (2- to 6-ring parent and alkylated) were determined by gas chromatography-mass spectroscopy (GC-MS) using an HP6890 Series gas chromatograph interfaced with an HP5973 MS and fitted with a cool on-column injector and a HP5 MS column, or equivalent (30 m × 0.25 mm id, 0.25 μm film thickness, Agilent Technologies, Stockport, England). Helium was used as the carrier gas in constant flow mode (0.7 ml min−1). Injections were made at 50°C and the oven temperature held constant for 3 minutes. Thereafter the temperature was raised at 20°C min−1 up to 100°C. This was followed by a slower ramp of 4°C min−1 up to a final temperature of 270°C. The MS was set for selective ion monitoring (SIM) with a dwell time of 50 ms. A total of 29 ions plus the 6 internal standard ions were measured over the period of the analysis. Thus the analysis incorporated 2- to 6-ring, parent and alkylated PAHs. Calibration standards, covering the concentration range 0.01 to 5.0 ng μl−1 were analyzed, in triplicate, and the average response used to compute the calibration curve. Correlation coefficients of at least 0.99 were achieved for all PAHs. The detection limits were between 0.05 to 0.20 μg kg−1 wet weight for all individual PAHs.
Quality Control
The PAHs analyzed were: naphthalenes (parent and C1-C4); acenaphthene; acenaphthylene; fluorene; phenanthrene and anthracene (parent and C1-C3); dibenzothiophenes (parent and C1-C3); fluoranthene and pyrene (parent and C1-C3); benz[a]anthracene, benz[b]anthracene, benzo[c]phenanthrene and chrysene (parent and C1-C2); benzofluoranthene, benzo[a]pyrene, benzo[e]pyrene and perylene (parent and C1-C2); indenopyrene, benzoperylene (parent and C1-C2); dibenz[a,h] anthracene. The total PAH concentration was the sum of these target PAHs.
The analytical method is accredited to ISO 17025 by the United Kingdom Accreditation Service (UKAS). This method was validated by the replicate analysis of standards and samples, and through spiking experiments. The replicate analysis (n = 7) of low and high standards gave CV% of less than 3% for individual PAHs. The replicate analysis (n = 7) of a mussel sample gave CV% < 8% for individual PAHs. Recoveries of greater than 80% were achieved for mussels spiked with 1 μg kg−1 wet weight of individual PAHs.
Internal quality control procedures include the analysis of a laboratory reference material (LRM) in each batch of samples. Procedural blanks were performed with each batch of samples, and the final concentration adjusted accordingly. The data obtained from the LRM was transferred onto NWA Quality Analyst and Shewhart charts were produced with warning and action limits being drawn at ±2× and ±3× the standard deviation of the mean. Quality assurance was further demonstrated through successful participation in QUASIMEME (Quality Assurance of Information for Marine Environmental Monitoring in Europe) Laboratory Performance Studies.
Statistical Analysis
Analysis of variance (ANOVA) at the 95% confidence level, using Minitab 14, was used to assess significant differences between the mean PAH concentrations of samples collected from the two time periods. Principal component analysis (PCA) was used to investigate any seasonal differences in the PAH composition of the Loch Etive mussels. PCA forms a lower number of variables from linear combinations of the original data. Clustering only occurs if samples have similar properties. In this case, PCA was applied to the parent and alkylated concentrations normalised to the total PAH concentration; that is, so the data are in the form of proportions of the total concentration. Minitab 14 was used for the PCA. The results of the analysis were viewed by plotting the principal components which had the greatest variance against one another.
Results and Discussion
Total PAH concentrations (2- to 6-ring parent and branched, including the 16 US Environmental Protection Agency (EPA) PAHs) in Loch Etive mussels collected over the six year period ranged from 12.5 μg kg−1 wet weight in a sample collected in August 2004 to 151.2 μg kg−1 wet weight in a sample collected in March 2000 (). Seasonal trends in PAH concentrations have been well documented (Citation19, Citation24, Citation25) and are clearly seen at Loch Etive (). PAH concentrations in the Loch Etive mussels increased over the winter and started to decrease in spring. Total PAH concentrations were significantly lower (p < 0.001, ANOVA) in mussels collected between April and October compared to those collected between November and March with time averaged means of 35.4 μg kg−1 wet weight (SD = 18.6 μg kg−1 wet weight, n = 30) and 80.5 μg kg−1 wet weight (SD = 33.3 μg kg−1 wet weight, n = 25), respectively. Mean concentrations for each year for April to October and November to March are shown in ; in all years concentrations were lower in samples collected between April and October. From autumn to spring, lipids are saved for gameteogenesis and the increase in lipid results in an increase in the uptake of hydrophobic contaminants such as PAHs. The monthly variation in the total PAH concentrations are shown in for each year, lowest concentrations were found in May and June. Spawning generally occurs in late spring/early summer, although the exact timing is dependent on external factors including water temperature. Spawning is associated with a decrease in PAH burden.
FIGURE 2 Total PAH (2- to 6-ring parent and branched) concentrations in mussels collected from Loch Etive.
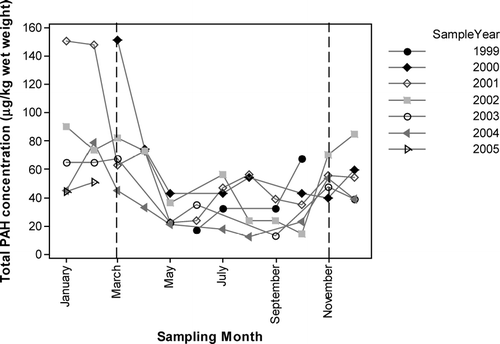
TABLE 1 Total PAH Concentrations (μg kg−1 wet weight) in Farmed Mussels Collected from Loch Etive between 1999 and 2005. The Samples were Grouped into Two Time Periods: April to October and November to March. At Least 4 Samples were Collected from All but Two of the Time Periods. SD = standard deviation
Oros and Ross reported PAH concentrations (25 PAHs, 2- to 6-ring parent and alkylated) in mussels from San Francisco estuary and from reference sites collected between 1993 and 2001 (Citation24). Total PAH concentrations in the reference site mussels ranged from 25–581 μg kg−1 dry weight. Multiplying by 0.2 (assuming 80% moisture) gave an approximate wet weight concentration range of 5–116.2 μg kg−1. Baumard and colleagues reported total PAH (14 PAHs, 3- to 6-ring parent and alkylated) concentrations of 280–480 μg kg−1 dry weight (56–96 μg kg−1 wet weight) in mussels collected in February 1995 from Arachon Bay, France, from the least contaminated sites (total PAH concentrations in sediment < 3000 μg kg−1 dry weight) and suggested a baseline concentration of 250 μg kg−1 dry weight (50 μg kg−1 wet weight) for the mussels from these sites (Citation14). Lower total PAH (14 PAHs, 3- to 6- ring parent and alkylated) concentrations were reported from the coast of Spain and France with a range of 25–82 μg kg−1 dry weight (5–16.4 μg kg−1 wet weight) in mussels collected in August 1996 (Citation13, Citation16). Azevedo et al. reported total PAH (PAHs not specified) concentrations of 41.5–375 μg kg−1 dry weight (8.3–75.0 μg kg−1 wet weight) in summer and 59.7–432 μg kg−1 dry weight (11.9–86.4 μg kg−1 wet weight) in winter in mussels from near the entrance of Guanabara Bay, Brazil (Citation25). The concentration ranges at Loch Etive were similar to these studies although higher concentrations than this were found in some of the Loch Etive spring and winter samples. However, the total comprised of a greater number of PAH compounds or groups of compounds (number = 40) compared to other studies.
PAH concentrations and sources were previously investigated in mussels from a range of coastal locations in Scotland. Mussel samples were collected during spring (March and April) 1998. The majority of the rope grown mussels from remote locations had low total PAH (36 PAHs, 2- to 6-ring PAHs) concentrations (< 50 μg kg−1 wet weight) whereas mussels from estuarine sites had higher total PAH concentrations (> 150 μg kg−1 wet weight) (Citation26). Only three Loch Etive samples gave total PAH concentrations close to 150 μg kg−1 wet weight. These were collected in March 2000 (151.2 μg kg−1 wet weight) and January and February 2001 (150.2 and 148.1 μg kg−1 wet weight respectively). Mean total PAH concentrations for April–October were mainly < 50 μg kg−1 wet weight, only in 2000 was this value just exceeded with a mean total PAH concentration for April–October of 51.7 μg kg−1 wet weight. The time-averaged mean total PAH concentration was < 50 μg kg−1 wet weight (35.4 μg kg−1 wet weight) for April to October and > 50 μg kg−1 wet weight for November to March (80.5 μg kg−1 wet weight).
Comparison to OSPAR Background Assessment Concentrations (BACs)
OSPAR Background Concentrations (BCs) are used to assess chemical monitoring data and identify areas of potential environmental concern (Citation27). Provisional BCs have been established for ten PAHs in mussels (). Observed concentrations are said to be ‘near background’ if the mean concentration is statistically significantly below the corresponding Background Assessment Concentration (BAC) (). Formally, BACs are designed for use with data collected under the Oslo and Paris Commission (OSPAR) annual trend monitoring programme. However, it is still instructive to compare the concentrations observed in the Loch Etive mussels to the BACs. The mean concentration and upper 95% confidence limits were estimated for each season. The upper confidence bounds for the 4- (fluoranthene, pyrene and benz[a]anthracene) and 5-ring (benzo[a]pyrene) PAHs were above the BACs in samples collected between November and March only.
TABLE 2 Mean Concentrations for Selected PAHs in the Loch Etive Mussels for April–October and November–March, Compared to the OSPAR Background Concentrations (BCs) and Provisional Background Assessment Concentrations (BACs). BACs were Only Exceeded for November to March for Fluoranthene, Pyrene, Benz[a]anthracene and Benzo[a]pyrene. The Figures in Brackets are the 95% Upper Confidence Bound, Values in Bold are Above the OSPAR BACs
From the Loch Etive data it can be seen that concentrations are dependent on the time of year sampling is undertaken. Therefore, this should be taken into consideration when comparing concentrations with any assessment criteria. From this data it can be seen that two background concentrations may be more appropriate, one covering the period April to October and one for November to March. By taking the median of the median total PAH concentration for each time period for April to October and November to March, background concentrations were calculated and found to be; for April to October: 31.2 μg kg−1 wet weight, and for November to March (omitting the 2000 concentration as only one sample available): 62.9 μg kg−1 wet weight. Further work may be required to establish assessment criteria from which to compare monitoring data against these background concentrations.
PAH Sources
Generally the dominant source of PAHs in the marine environment is pyrolytic and only following an oil spill is a strong petrogenic PAH profile observed. A high proportion of the heavier parent PAHs is indicative of a predominately pyrolytic input whereas a high proportion of alkylated 2- and 3-ring PAHs would suggest a petrogenic source. Study of PAH distributions and PAH concentration ratios can be used to distinguish PAHs of petrogenic and pyrolytic origin. Petrogenic sources yield mainly alkylated 2- and 3-ring compounds (naphthalene and phenanthrene) and thermodynamically favoured isomers whereas PAHs from pyrolytic sources are mainly the 4- to 6-ring parent compounds. However this approach should be treated with caution when looking at mussels as different PAHs will have quite different uptake and depuration rates which will alter their profiles. In addition, farmed rope grown mussels (in low turbidity water) would be expected to accumulate a higher proportion of the lower molecular weight, more water soluble PAHs through uptake from the water column (Citation13). Natural mussels collected from mussel beds, or rope grown mussels in more turbid water, may accumulate a higher proportion of the more hydrophobic, higher molecular weight PAHs, associated with particulate material. Baumard and colleagues reported a preferential accumulation of higher molecular weight PAHs in areas of high turbidity due to the resuspension of fine particles (Citation13, Citation14). Piccardo and colleagues estimated phenanthrene and pyrene to be absorbed through the gills at 88% and 74% respectively, while benzo[a]pyrene was thought to be totally absorbed through particle ingestion (Citation6).
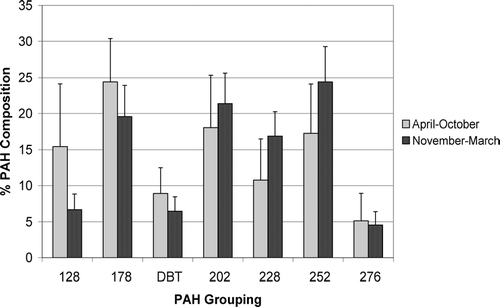
The mean seasonal PAH profile is shown in . The DBTs and 6-ring PAHs contributed the least to the total PAH concentrations, with the proportion of 3-ring (phenanthrenes), 4-rings and 5-rings having the largest contribution. Mussels collected between April and October had a higher proportion of 2- and 3-ring PAHs and a lower proportion of 4- and 5-ring PAHs compared to those collected between November and March. The proportion of parent PAHs was lower than normally found in sediments (< 40%). This is probably due to the preferential accumulation of the alkylated PAHs due to their higher octanol/water partition coefficient in comparison to the corresponding parent compounds and the higher biodegradation rate of the parent compound.
FIGURE 3 Mean PAH composition in samples collected between April and October and samples collected between November and March, including error bars (standard deviations), of mussel samples collected from Loch Etive between 1999 and 2004. 128, naphthalenes (parent and C1-C4); 178, phenanthrene/anthracene (parent and C1-C3); DBT, dibenzothiophenes (parent and C1-C3); 202, fluoranthene/pyrene (parent and C1-C3); 228, benzanthracene/benzophenanthrenes/chrysene/triphenylenes (parent and C1-C2); 252, benzofluoranthene/benzopyrene/perylene and 276, indenopyrene/benzoperylene (parent and C1-C2).
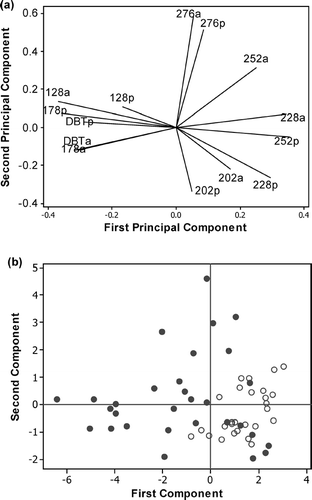
The PAH profiles of Loch Etive mussels were investigated further using PCA. The first two principal components accounted for 38.5 and 5.1% of the variance in the data respectively. The first component was a contrast between the heavier (4- to 6-ring PAHs) and lighter PAHs (2- and 3-ring PAHs) with the alkylated PAHs being positively correlated with the corresponding parent compounds (). The score plot showed a separation on the first component between samples collected between November and March and those collected between April and October (). The majority of samples (20 of the 30 samples) collected between November and March had a negative component 1 indicating these samples had a higher proportion of the heavier PAHs. This may be due to the increased turbidity of the water due to storms, resulting in sediment being re-suspended in the water column; any PAHs associated with this suspended particulate material (mainly the heavier 4- to 6-ring PAHs) would be more bioavailable to the mussels.
FIGURE 4 (a) Loading plot and (b) score plot for the PCA of the parent (p) and alkylated (a) concentrations in each PAH group, expressed as proportions of the total PAH concentration. Samples collected between November and March are shown as open circles and samples collected between April and October as filled circles.
CONCLUSIONS
Total PAH concentrations were measured in mussels collected on a monthly basis over a six year period from a remote location on the west cost of Scotland with the aim of establishing background PAH concentrations. Total PAH concentrations were mainly < 150 μg kg−1 wet weight. Mean total PAH concentrations in samples collected between April and October (< 50 μg kg−1 wet weight) were significantly lower than those collected between November and March (> 50 μg kg−1 wet weight). OSPAR BACs were only exceeded for the heavier 4- and 5-ring PAHs (fluoranthene, pyrene, benz[a]anthracene and benzo[a]pyrene) in samples collected between November and March. Mussel samples collected between November and March also showed a higher proportion of heavier PAHs, probably due to the re-suspension of sediments and, therefore, increased bioavailability of the more hydrophobic PAHs.
The authors would like to thank Rob Fryer for statistical advice.
REFERENCES
- Richardson , D. M. , Davies , I. M. , Moffat , C. F. , Pollard , P. and Stagg , R. M. 2001 . Biliary PAH Metabolites and EROD Activity in Flounder (Platichthys flesus) from a Contaminated Estuarine Environment . Journal of Environmental Monitoring , 3 : 610 – 615 . [INFOTRIEVE] [CSA]
- Stagg , R. M. , McIntosh , A. D. and Mackie , P. 1995 . The Induction of Hepatic Mono-oxygenase Activity in Dab (Limanda limanda) in Relation to Environmental Contamination with Petroleum Hydrocarbons in the North Sea . Aquatic Toxicology , 33 : 254 – 264 . [CSA] [CROSSREF]
- Gowland , B. T. G. , McIntosh , A. D. , Davies , I. M. , Moffat , C. F. and Webster , L. 2002 . Implications from a Field Study Regarding the Relationship between Polycyclic Aromatic Hydrocarbons and Glutathione S-Transferase Activity in Mussels . Marine Environmental Research , 54 : 231 – 235 . [CSA] [CROSSREF]
- Meador , J. P. , Stein , J. E. , Reichert , W. L. and Varanasi , U. 1995 . Bioaccumulation of Polycyclic Aromatic Hydrocarbons by Marine Organisms . Reviews of Environmental Contamination and Toxicology , 143 : 79 – 165 . [INFOTRIEVE] [CSA]
- Baumard , P. , Budzinski , H. , Michon , Q. , Garrigues , P. , Burgeot , T. and Bellocq , J. 1998 . Origin and Bioavailability of PAHs in the Mediterranean Sea from Mussel and Sediment Records . Estuarine Coastal and Shelf Science , 47 : 77 – 90 . [CSA] [CROSSREF]
- Piccardo , M. T. , Coradeghini , R. and Valerio , F. 2001 . Polycyclic Aromatic Hydrocarbon Pollution in Native and Caged Mussels . Marine Pollution Bulletin , 42 : 951 – 956 . [INFOTRIEVE] [CSA] [CROSSREF]
- Hellou , J. and Law , R. J. 2003 . Stress on Stress Response of Wild Mussels, Mytilus edulis and Mytilus trossulus, as an Indicator of Ecosystem Health . Environmental Pollution , 126 : 407 – 416 . [INFOTRIEVE] [CSA] [CROSSREF]
- Hellou , J. , Stellar , S. , Leonard , J. , Langille , M. A. and Tremblay , D. 2005 . Partitioning of Polycyclic Aromatic Hydrocarbons between Water and Particles Compared to Bioaccumulation in Mussels: A Harbour Case . Marine Environmental Research , 59 : 101 – 117 . [INFOTRIEVE] [CSA] [CROSSREF]
- Law , R. J. , Kelly , C. , Baker , K. , Jones , J. , McIntosh , A. D. and Moffat , C. F. 2002 . Toxic Equivalency Factors for PAH and Their Applicability in Shellfish Pollution Monitoring Studies . Journal of Environmental Monitoring , 4 : 383 – 388 . [INFOTRIEVE] [CSA] [CROSSREF]
- Bosveld , A. T. C. , de Bie , P. A. F. , den Brink , N. W. , Jongepier , H. and Klomp , A. V. 2002 . In Vitro EROD Induction Equivalency Factors for the 10 PAHs Generally Monitored in Risk Assessment Studies in The Netherlands . Chemosphere , 49 : 75 – 83 . [INFOTRIEVE] [CSA] [CROSSREF]
- OSPAR Commission . 2002 . Polycyclic Aromatic Hydrocarbons , London : OSPAR Priority Substances, OSPAR Commission .
- Davies , H. K. , Moffat , C. F. and Shepherd , N. J. 2002 . Experimental Tainting of Marine Fish by Three Chemical Dispersed Petroleum Products, with Comparisons to the Braer Oil Spill . Spill Science and Technology Bulletin , 7 : 257 – 278 . [CSA] [CROSSREF]
- Baumard , P. , Budzinski , H. , Garrigues , P. , Narbonne , J. F. , Burgeot , T. , Michel , X. and Belloccq , J. 1999 . Polycyclic Aromatic Hydrocarbon Burden of Mussels (Mytilus sp.) in Different Marine Environments in Relation with Sediment and PAH Contamination and Bioavailability . Marine Environmental Research , 47 : 415 – 439 . [CSA] [CROSSREF]
- Baumard , P. , Budzinski , H. and Garrigues , P. 1998 . PAHs in Arachon Bay, France: Origin and Monitoring with Caged Organisms . Maine Pollution Bulletin , 36 : 577 – 586 . [CSA] [CROSSREF]
- Baumard , P. , Budzinski , H. , Garrigues , P. , Sorbe , J. C. , Burgeot , T. and Bellocq , J. 1998 . Concentrations of PAHs (Polycyclic Aromatic Hydrocarbons) in Various Marine Organisms in Relation to Those in Sediments and to Trophic Level . Marine Pollution Bulletin , 36 : 951 – 960 . [CSA] [CROSSREF]
- Whittle , K. J. , Anderson , A. D. , Mackie , P. R. , Moffat , C. F. , Shepherd , N. J. and McVicar , A. H. 1997 . “ The Impact of Braer Oil on Caged Salmon ” . In The Impact of an Oil Spill in Turbulent Waters: The Braer , Edited by: Davies , J. M. and Topping , G. 144 – 160 . Edinburgh : The Stationery Office . ch. 11
- Topping , G. , Davies , J. M. , Mackie , P. R. and Moffat , C. F. 1997 . “ The Impact of the Braer Spill on Commercial Fish and Shellfish ” . In The Impact of an Oil Spill in Turbulent Waters: The Braer , Edited by: Davies , J. M. and Topping , G. 121 – 143 . Edinburgh : The Stationery Office . ch. 10,
- Stagg , R. M. , McIntosh , A. M. , Moffat , C. F. , Robinson , C. , Smith , S. , Bruno , D. W. and Secombes , C. J. 1997 . “ The Sub-Lethal Effects of the Braer Oil Spill, Shetland Isles, Scotland, on Farmed Atlantic Salmon (Salmo salar) and the Common Dab (Limanda limanda) ” . In The Impact of an Oil Spill in Turbulent Waters: The Braer , Edited by: Davies , J. M. and Topping , G. 182 – 208 . Edinburgh : The Stationery Office .
- Webster , L. , Angus , L. , Topping , G. , Dalgarno , E. J. and Moffat , C. F. 1997 . Long-Term Monitoring of Polycyclic Aromatic Hydrocarbons in Mussels (Mytilus edulis) Following the Braer Oil Spill . Analyst , 122 : 1491 – 1495 . [CSA] [CROSSREF]
- Webster , L. , McIntosh , A. D. , Moffat , C. F. , Dalgarno , E. J. , Brown , N. A. and Fryer , R. J. 2000 . Analysis of Sediments from Shetland Island Voes for Polycyclic Aromatic Hydrocarbons, Steranes and Triterpanes . Journal of Environmental Monitoring , 2 : 29 – 38 . [CSA] [CROSSREF]
- Webster , L. , Fryer , R. J. , Dalgarno , E. J. , Megginson , C. and Moffat , C. F. 2001 . The Polycyclic Aromatic Hydrocarbon Composition of Sediments from Voes and Coastal Areas in the Shetland and Orkney Islands . Journal of Environmental Monitoring , 3 : 591 – 601 . [CSA] [CROSSREF]
- Webster , L. , McIntosh , A. D. , Russell , M. , Walsham , P. , Packer , G. , Dalgarno , E. J. and Moffat , C. F. 2005 . “ Composition and Concentration of Hydrocarbons in Sediment Samples Collected during 2003 from West Burra and St. Magnus Bay ” . Shetland Islands, Fisheries Research Services Contract Report: No. 06/05
- McIntosh , A. D. , Moffat , C. F. , Packer , G. and Webster , L. 2003 . Polycyclic Aromatic Hydrocarbon (PAH) Concentration and Composition Determined in Farmed Blue Mussels (Mytilus edulis) in a Sea Loch Pre- and Post-Closure of an Aluminium Smelter . Journal of Environmental Monitoring , 6 : 209 – 218 . [CSA] [CROSSREF]
- Oros , D. R. and Ross , J. R. M. 2005 . Polycyclic Aromatic Hydrocarbons in Bivalves from the San Fransisco Estuary: Spatial Distribution, Temporal Trends and Sources (1993–2001) . Marine Environmental Research , 60 : 466 – 488 . [INFOTRIEVE] [CSA] [CROSSREF]
- Azevedo , L. A. , de Andrade Bruning , I. M. R. and Moreira , I. 2004 . Hydrocarbon Contamination in Mussels from Guanabara Bay . Marine Pollution Bulletin , 49 : 1120 – 1122 . [INFOTRIEVE] [CSA] [CROSSREF]
- Webster , L. , McIntosh , A. D. , Megginson , C. , Shepherd , N. J. and Moffat , C. F. 2002 . The Polycyclic Aromatic Hydrocarbon Composition of Mussels (Mytilus edulis) from Scottish Coastal Waters . Journal of Environmental Monitoring , 5 : 150 – 159 . [CSA] [CROSSREF]
- Moffat , C. F. , Pijnenburg , J. and Trass , T. , eds. . Report of the joint OSPAR/ICES Workshop . February 9–13 2004 , The Hague, Netherlands.