Abstract
Four tar-contaminated sites in Germany (Lünen, Castrop Rauxel, Stuttgart and Wülknitz) were investigated and 42 polycyclic aromatic compounds (PAC) were detected in significant concentration ranges including polycyclic aromatic hydrocarbons (PAH), substituted PAC, heterocyclic PAC containing nitrogen, sulphur and oxygen as well as their metabolites. A similar composition and pattern of PAH heterocyclic PAC and metabolites were found in corresponding groundwater samples. Identification and quantification were performed using liquid-liquid extraction and analyses with gas chromatography – electron impact – mass spectrometry. Detailed investigation (in Castrop Rauxel, Germany) showed that the distribution of non-polar compounds can only be detected close to the source of contamination, whereas the distribution of more polar compounds and degradation products is more widespread downstream of an aquifer.
INTRODUCTION
The formation of polycyclic aromatic compounds (PAC) is not limited to processing fossil fuels and their combustion, coal gasification sites, tar-oil distillation plants and wood-preserving facilities (Citation1). They are also assimilated by smoking (benz[a]pyrene) (Citation2, Citation3), insecticides (dibenzofuran) or by foods (Citation4, Citation5). Furthermore, in several European countries tar was used, for example, as corrosion protection for water pipes (Citation6, Citation7). Original tar contains a higher percentage of non-polar PAH compared to PAC-metabolites, substituted PAC and heterocyclic PAC (PASH, PANH, PAOH) as degradation and transformation products (Citation8, Citation9, Citation10, Citation11, Citation12, Citation13, Citation14, Citation15). Due to higher water solubility and corresponding lower KOW-values, polar PAH derivatives show an increasing tendency for distribution in groundwater flow direction and bioavailability. Owing to the increasing number of known tar-contaminated sites in Germany (Citation16, Citation17), research in the field of PAC-metabolites, heterocyclic PAC and PAC substituted by polar groups has been attracting heightened attention in recent years (Citation18). In particular the formation and distribution of polar PAC and their metabolites by intrinsic natural retention and degradation processes in the subsoil and aquifers of contaminated sites is the object of current research (Citation13, Citation19, Citation20, Citation21, Citation22, Citation23). Our knowledge about formation, identification, distribution and degradation of these usually more polar, and in some cases toxic, compounds compared to their parent non-polar and poorly water-soluble PAH (Citation24, Citation25, Citation26) is limited, nevertheless.
Consequently, the investigation of natural attenuation processes involving polar PAC was one of the principal tasks of the German National Research Project KORA (Retention and Degradation Processes to Reduce Contaminants in Groundwater and Soil). Natural Attenuation (NA) refers to the observed reduction in contaminant concentrations and/or contaminant mass flow rates as contaminants migrate from the source into environmental media. Under favorable conditions (depending mainly on the specific properties of the contaminants and the hydro-geological settings), Monitored Natural Attenuation (MNA) may be used as a land management option for tar-contaminated sites. Within this project, we investigated the identification, quantification and distribution of 68 assigned PAC and PAC derivatives in groundwater samples of known tar-contaminated sites at Stuttgart, Castrop Rauxel, Wülknitz and Lünen (Germany). These sites are included in the research projects KORA and RUBIN (German Permeable Reactive Barrier Network). The mixture of non-polar and polar PAC and corresponding metabolites required the development of appropriate analytical tools, including chromatographic and extraction techniques.
In this paper, we will report details about analytical designs, concentrations as well as the pattern of PAC found. In addition, results concerning the distribution of specially selected mother-daughter pairs of non-polar and polar PAC on tar-contaminated sites will be discussed.
EXPERIMENTAL
Standards and Reagents
Acetonitrile (Merck, Darmstadt, Germany) in extra pure quality and methyl pentanoate (Merck, Darmstadt, Germany) in analytical purity were used. A 16 EPA-PAH standard-solution mix with a purity of ≥99% was received from Ehrenstorfer (Augsburg, Germany) containing 10 μg/ml each in acetonitrile. The remaining PAC derivatives were supplied by Merck (Darmstadt, Germany), Aldrich and Fluka (Munich, Germany) as well as Promochem (Wesel, Germany) at best available purity (). Anthracene d10 (Ehrenstorfer, Augsburg, Germany) was used as internal standard with a concentration of 10 μg/ml (stock solution).
TABLE 1 Supplier and purity for all analysed heterocyclic PAC, PAC-metabolites sorted by functional groups. The 16 EPA-PAH purchased as standard-solution mix with a purity of ≥ 99% (Ehrenstorfer) are not shown in this table. a Additional analyzed non-PAC
Each PAC was dissolved in acetonitrile with a concentration of 1mg/ml and mixed and diluted to a concentration of 10 μg/ml for each compound. 500 μl of the 10 μg/ml PAC standard and 500 μl of the 10 μg/ml 16 EPA-PAH standard were mixed to a final concentration of 5 μg/ml. The PAH-standard solution mix was used as a reference standard containing 68 PAC.
Sample Collection
Groundwater samples from tar-oil contaminated sites within the KORA and RUBIN project were analysed in this study. Tar-contaminated sites were located in Stuttgart (South Germany, former gas plant), Wülknitz (East Germany, former impregnating plant), Castrop Rauxel and Lünen (West Germany, former coking plants). The samples were collected by the respective project members using representative groundwater sampling procedures in the years 2004 till 2007 (Citation27). The samples were fixed with hydrochloric acid (pH 2) and cooled (4°C) to avoid microbial activity.
Liquid-Liquid Extraction
Groundwater samples (100 ml) were extracted by liquid-liquid extraction with methyl pentanoate as extracting solvent at different pH values. In a first step the corresponding sample was saturated with 1 ml of the ester. After saturation each sample was extracted once with 2 ml of methyl pentanoate at pH 2, pH 7 and pH 13 using an overhead shaker for 20 min starting the extraction at pH 2. Before extraction, solutions were adjusted in consecutive steps from pH 2 to pH 7 and pH 13 by addition of sodium hydroxide. The volume of the resulting dried extract (using sodium sulphate) was 6 ml per sample. After addition of the internal standard, 1 ml of the extract was finally concentrated to 100 μ l, then the sample was analyzed by GC – electron impact ionization (EI)-MS. Satisfactory extraction recoveries were obtained for all PAC under consideration using this extracting procedure ().
TABLE 2 Liquid-liquid extraction recoveries of the different groups of PAC. a Ni represents the number of PAC included in each group.
Gas Chromatography/Mass Spectrometry
GC – Electron Impact (EI) – MS analyses were performed on a Perkin Elmer gas chromatograph Autosystem XL connected to a Perkin Elmer Turbomass mass spectrometer. An Optima - DB 5 - MS (30 m × 0.25 mm i.d. × 0.25 μ m film thickness) containing 5% phenyl-methylsiloxane capillary column was used for the identification and determination of the PAH, PAC-metabolites and heterocyclic PAC with the following temperature program: 70°C (held for 5 min) to 300°C (held for 5 min) at 5°C/min. For identification the full scan mode from 50 to 300 amu was used. The SIM (Selected Ion Monitoring)-mode was performed for quantification using two representative ions of each PAC. Detection limits were in the range of 0.2–30 ng/l. Accuracy criteria for the detection and quantification included the retention time for all m/z monitored for a given analyte of better than ±1 s, with a given signal-to-noise ratio of ≥3 for each compound. Furthermore, the ratio between the two monitored ions was always within 15% of the theoretical value. Quantification was carried out using anthracene d10 as internal standard.
This specially developed method enabled us to perform a routine analysis of all PAH, PAC-metabolites and heterocyclic PAC with a three-step liquid extraction and quantitative analyses by GC-(EI)-MS.
RESULTS AND DISCUSSION
Identified and Quantified PAC and PAC-Metabolites on Different Tar-Contaminated Sites in Germany
Out of 68 PAC, a total of 42 were detected above detection limits and quantified in 88 samples analyzed in total (). From the 42 detected PAC, 24 compounds were found at all four sites investigated.
TABLE 3 Minimum-maximum concentrations of non-polar PAH, PAC-metabolites, heterocyclic PAC and PAC substituted by polar functional groups in groundwater samples analysed of four tar-contaminated sites in Germany.
However, in at least one sample one of the compounds summarized in was below detection limit. Concentrations of above 1 mg/l were found for 1-methylnaphthalene, quinoline, 1-benzothiophene, 1-indanone, 1,8-naphthalic anhydride and 2-hydroxyquinoline. The highest concentration of 8071.2 μ g/l was found for naphthalene. Furthermore, it is noteworthy that the high-molecular PAH were never detected in any sample. PAH with high water solubility, especially naphthalene, were detected in groundwater samples collected far away from the source of contamination and found to have high concentrations. Without exception, naphthalene, acenaphthene, fluorene and phenanthrene were detected and found to have higher concentrations than all other PAC. It is assumed that, owing to the lower water solubility, the high-molecular PAH were still located near the tar-oil contaminated source and were therefore not detected in any groundwater sample.
In addition to the conventionally analyzed group of EPA-PAH, the heterocyclic PAC and PAC substituted by polar functional groups were detected in even higher concentrations compared to the non-polar PAH. The concentrations given in show that heterocyclic PAC, present in low concentrations in original tar in sum of max. 13% (Citation28, Citation29), were found in high concentrations in the groundwater samples analyzed. This finding is reasonable in view of the high water solubility and therefore mobility of polar PAC-derivatives. Quinoline, 2-methylquinoline, isoquinoline, carbazole, dibenzofuran, benzo[b]furan and 1-benzothiophene in particular were detected in notably high concentrations of up to 4000 μ g/l in groundwater samples even at different contaminated sites.
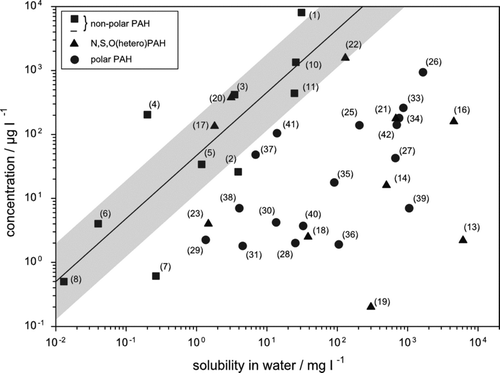
Concentrations found for all compounds from one of the most contaminated wells at the site, Lünen (West Germany, well 12Q), versus the corresponding solubility in water are shown in . Water solubilities were taken from (Citation30) and it must be emphasized that many solubilities are values estimated by using KOW, which are themselves highly uncertain, or even estimated. Hence, additional measurements of physico-chemical parameters such as solubilities are highly recommended and solubility data especially for metabolites of PAC should be used with care and for qualitative discussions only. Nevertheless, even with an uncertainty in solubility by an assumed factor of 10, some general conclusions can be drawn from . Assuming a sufficiently efficient source of all non-polar PAH and an already achieved equilibrium with respect to solubility, a correlation should be found for the concentrations in water and the corresponding solubilities (line shown in ). Within the uncertainties of concentrations and available solubility data (depicted qualitatively in as shaded area) this assumption is fulfilled. The correlation is therefore a theoretical borderline of a maximum possible concentration (but valid only for the conditions of the contaminated site) of a source with sufficiently high mass with respect to the corresponding solubility in water. In addition, three heterocyclic PAC in particular also fulfil this condition: carbazole, dibenzofuran and benzothiophene. Hence, at least these compounds are identified as important heterocyclic PAC of this site. Conversely, all polar PAC are found beyond the theoretical borderline. This emphasizes their dramatic increase in solubility in water by several decades (and their assumed increasing mobility in the aquifer) as well as their insufficient mass, not achieved equilibrium or further chemical transformation to reach the theoretical borderline. Examples of metabolites and mother-daughter pairs of non-polar PAH and heterocyclic PAC and their corresponding products are discussed below.
FIGURE 1 Concentrations found for all PAC at the Lünen site (West Germany, well 12Q) versus their corresponding solubilities in water. Numbers given correspond to the compounds summarized in . The line is the correlation of both concentrations for the non-polar PAH and the shaded area an assumed minimum uncertainty and variability in the concentrations used.
The identification of specific degradation products of non-polar PAH and original heterocyclic PAC is considered as one of the main results of the study. Hence, as an example, it was possible to quantify the well known metabolites 1-naphthol and 2-naphthol of naphthalene (Citation31, Citation32, Citation33). It has to be mentioned that PAC with phenolic groups as well as 1- and 2-naphthol can also be present in tar as original contaminants. However, the concentrations of phenolic PAC in tar are lower compared to concentrations found in the aquifer in contact with the corresponding organic phase (Citation9). Furthermore, additional mother-daughter pairs such as acenaphthene – acenaphthenole and fluorene – fluoren-9-one, 9-fluorenol were identified. Corresponding degradation products of the heterocyclic PAC acridine, dibenzothiophene and dibenzofuran were found to be 9(10)H-acridinone; dibenzothiophensulfone, 2-hydroxybiphenyl; 2-hydroxydibenzofuran and 2-hydroxycarbazole. As these metabolites were detected in significant concentrations (), it is therefore not sufficient to analyze only the EPA-PAH (Citation34). Hence, besides EPA-PAH, it is necessary to analyze the concentration and distribution of heterocyclic PAC and their degradation products in general to assess groundwater samples of tar-contaminated sites.
With regard to, and for comparison of the percentage composition of the groups of PAC detected in groundwater samples of tar-contaminated sites (), it was found that the original heterocyclic PAC, keto-PAC and OH-PAC as metabolites occur at up to 69% in the corresponding groundwater samples.
TABLE 4 Composition of PAC (%) in groundwater of four tar-contaminated sites in Germany. PAC were subdivided in non-polar PAH, heterocyclic PAC, OH-PAC, keto-PAC and other-PAC (including CN-PAC).
This demonstrates that heterocyclic PAC and polar PAC derivatives represent a high fraction of the total load of tar-contaminated groundwater. Variations between the sites investigated are caused in particular by differences in contamination with PAC-containing tar-oil, microbiological activity and therefore by the redox system in soil and groundwater.
Distribution of Naphthalene, Dibenzofuran and Selected PAC-Metabolites in the Groundwater Flow Direction of the Tar-Contaminated Site in Castrop Rauxel (West Germany)
The coking plant in Castrop Rauxel was used for mining and coal processing from 1905 until its closure in 1972 after which it was filled up with rubble. More than 740 tonnes of PAC and BTEX were transferred to the soil and contaminants are found at depth of up to at least 7 m of the aquifer. Contaminants are at least detec 250 m away from the source of contamination. Near the known source of contamination a test field of three rows of in sum 15 groundwater sampling sites separated by 7.4 m in the row and with a distance of 13.3 m between the rows was installed (). Every sampling site has been divided into three wells of 5, 6 and 7 m depth: in sum 45 wells. The analysis of depth profiles for selected PAC and PAC-metabolites as well as their distribution was thereby achieved.
shows the concentrations for naphthalene as the non-polar PAH with the highest concentration of all as well as dibenzofuran as a prototype for a heterocyclic PAC.
FIGURE 2 (A) Diagram of the 45 groundwater wells located on the test field of the former coal-mine Victor 3/4 in Castrop Rauxel (Germany). The 15 sampling sites were installed in three rows with a distance of 7.4 m and 13.3 m between the rows. Every row consists of five sampling sites with three wells in a depth of 5, 6 and 7 m each. (B) Corresponding concentrations and distribution profiles of naphthalene and dibenzofuran found for the 45 wells. A - row 1: sampling sites 15–11; B - row 2: sampling sites 10–6; C - row 3: sampling sites 5–1.
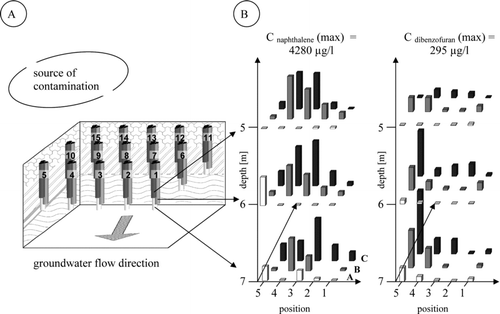
In assessing the distribution of contaminants, it must be considered that several factors have an influence on the distribution within the aquifer. These factors include attenuation or sorption, microbial degradation and limited degradation processes due to assumed bactericidal effect, especially as caused by benzene. Yet the interpretation of the results concerning distribution and degradation of PAC in view of these possible effects is still complicated. Nevertheless, as expected for these non-polar PAH the concentration profile of naphthalene shows a decrease in groundwater flow direction with increasing distance to the contamination source. Because of its high water solubility naphthalene was detected even downstream of the aquifer, far away from the source of contamination. A decrease in concentration from the source of contamination was also found for acenaphthene, acenaphthylen and fluorene. However, due to their lower water solubility and the corresponding correlation with KOW (compared to naphthalene) the distribution of these compounds in the aquifer is reduced. Hence, in comparison to naphthalene, concentrations of these high-molecular compounds were below detection limit downstream of the aquifer. Evidence of natural attenuation comes from degradation products of naphthalene as model PAH for natural degradation processes and a corresponding assessment is possible. Identified metabolites, which occur in the presence of naphthalene as mother PAH, were 1-naphthol and 2-naphthol (Citation31, Citation32, Citation33) in a concentration range of 0.8–351.7 μ g/l (). In addition to the distribution and degradation of naphthalene, selected heterocyclic PAC and PAC-metabolites were investigated in a related way. Dibenzofuran is chosen as a model compound for the distribution of heterocyclic PAC. Similarly to naphthalene the concentration of dibenzofuran in a range of < LOD – 294.6 μ g/l decreases in correlation with the distance to the source of contamination (). Dibenzofuran belongs to the slowly metabolizable heterocyclic PAC (Citation35). Hence, the assumed metabolite of dibenzofuran, 2-hydroxydibenzofuran, was found with relatively low maximum concentrations of up to 4.5 μ g/l, and therefore at a much lower concentration than the corresponding mother-heterocyclic PAC.
The chemical fate of quinoline is discussed using 2-hydroxyquinoline as degradation product ().
FIGURE 3 Distribution of the mother-daughter pair quinoline and 2-hydroxyquinoline on the test field of the former coal-mine Victor 3/4 (Castrop Rauxel, Germany) in groundwater flow direction.
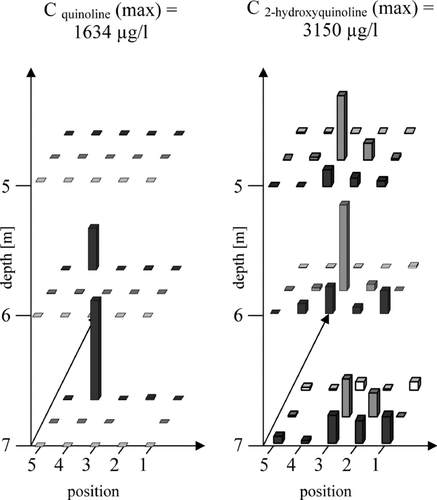
In contrast to quinoline (< LOD - 1634.2 μ g/l) the metabolite occurs in even higher concentrations of up to 3149.1 μ g/l along the groundwater flow direction as shown in . Not surprisingly, due to the assumed local contamination of quinoline this mother PAC was only locally detected in one of the 15 sampling sites near the original source of contamination. In contrast to quinoline, a widespread distribution of the degradation product 2-hydroxyquinoline was found in high concentrations downstream of the assumed source of quinoline. It should be emphasized that the metabolite was found also in high concentrations in groundwater samples where the parent quinoline was not detectable.
As another example of a mother-daughter relationship, the heterocyclic PAC acridine and the corresponding degradation product 9(10)H-acridinone will be discussed. In contrast to acridine the metabolite is not biodegradable (Citation36, Citation37) and can be used as an indicator for groundwater contaminated with tar (Citation28). In contrast to quinoline a more diffused distribution of acridine was found ().
FIGURE 4 Distribution of the mother-daughter pair acridine and 9(10)H-acridinone on the test field of the former coal-mine Victor 3/4 (Castrop Rauxel, Germany) in groundwater flow direction.
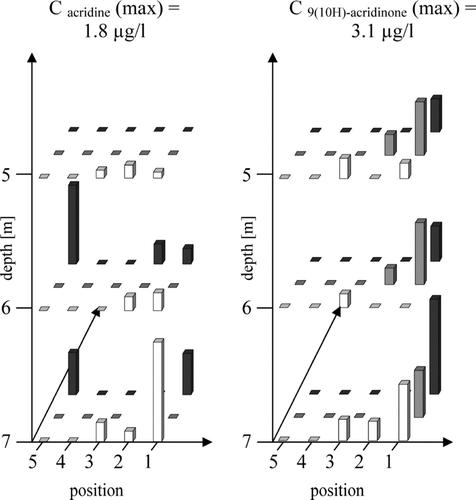
Low maximum concentrations for acridine (1.8 μ g/l) and 9(10H)-acridinone (3.1 μ g/l) were found compared to most of the PAC analyzed. Relatively high concentrations of the degradation product were found especially at the outer edge of the test field with small concentration differences in groundwater flow direction.
CONCLUSIONS
The analysis of 68 PACs including PAH, heterocyclic PAC and PAC-metabolites has shown that polar compounds play a major role in contaminated groundwater samples. Most polar PACs and their metabolites were found at a concentration range comparable to the non-polar PAHs. In contrast to non-polar PAH, polar PAC have a higher water solubility and therefore higher mobility and show a long-range distribution downstream of the aquifer. Most mother PACs discussed in this paper were detected in high concentrations only close to the source of contamination. This research shows that, beyond the analyzed conventional non-polar PAHs, sufficient consideration must be given to heterocyclic PACs and polar PAC-metabolites in the assessment of tar-contaminated sites.
We are grateful to the Federal Ministry of Education and Research in Germany (BMBF, project numbers 02WN0366 and 02WR0763) for their support. Furthermore, we thank all members involved in the KORA-project for support, discussion and especially providing groundwater samples.
Notes
a N = number of analyzed groundwater samples;
b 26 PAC found at all four sites investigated.
REFERENCES
- Ledesma , E. B. , Kalish , M. A. , Nelson , P. F. , Wornat , M. J. and Mackie , J. C. 2000 . Formation and fate of PAH during the pyrolysis and fuel-rich combustion of coal primary tar . Fuel , 79 : 1801 – 1814 .
- Hoffmann , D. and Hoffmann , I. 1997 . The changing cigarette, 1950-1995 . J. Toxicol. Env. Health , 50 : 307 – 364 .
- Baron , I. and Szustakowski , M. 1997 . Correlation between PAH, tar and ashes in tobacco smoke . Gefahrstoffe, Reinhaltung der Luft , 57 : 463 – 465 .
- Nieva-Cano , M. J. , Rubio-Barroso , S. and Santos-Delgado , M. J. 2001 . Determination of PAH in food samples by HPLC with fluorimetric detection following sonication extraction without sample clean-up . The Analyst , 126 : 1326 – 1331 .
- Fismes , J. , Schwartz , C. , Perrin-Ganier , C. , Morel , J. , Charissou , A.-M. and Jourdain , M.-J. 2004 . Risk of contamination for edible vegetables growing on soil polluted by polycyclic aromatic hydrocarbons . Polycycl. Aromat. Comp. , 24 : 827 – 836 .
- Maier , M. , Maier , D. and Lloyd , B. J. 2000 . Factors influencing the mobilisation of polycyclic aromatic hydrocarbons (PAHs) from the coal-tar lining of water mains . Water Res. , 34 : 773 – 786 .
- Merkel , T. , Maier , M. , Sacher , F. and Maier , D. 1998 . Reactions of PAH with chlorine and chlorine dioxide in coal tar lined pipes . Acta Hydroch. Hydrob. , 26 : 279 – 287 .
- Kershaw , J. R. 1993 . The chemical composition of a coal-tar pitch . Polycycl. Aromat. Comp. , 3 : 185 – 197 .
- Zander , M. 1995 . Aspects of coal tar chemistry/a review . Polycycl. Aromat. Comp. , 7 : 209 – 221 .
- Islas , C. A. , Suelves , I. , Apicella , B. , Herod , A. A. , Kandiyoti , R. , Carter , F. F. and Li , W. 2003 . Structure and composition of coal tars: an attempt to correlate molecular structure with increasing molecular mass . Combust. Sci. Technol. , 175 : 775 – 791 .
- Ramaswami , A. and Luthy , R. G. 1997 . Mass transfer and bioavailability of PAH compounds in coal tar NAPL-slurry systems. 1. Model development . Environ. Sci. Technol. , 31 : 2260 – 2267 .
- Ramaswami , A. , Ghoshal , S. and Luthy , R. G. 1997 . Mass transfer and bioavailability of PAH compounds in coal tar NAPL-slurry systems. 2. Experimental evaluations . Environ. Sci. Technol. , 31 : 2268 – 2276 .
- Ghiorse , W. C. , Herrick , J. B. , Sandoli , R. L. and Madsen , E. L. 1995 . Natural selection of PAH-degrading bacterial guilds at coal-tar disposal sites . Environ. Health Persp. , 103 : 107 – 112 .
- Ramaswami , A. , Ghoshal , S. and Luthy , R. G. 1994 . Mass transfer and biodegradation of PAH compounds from coal tar . Water Sci. Technol. , 30 : 61 – 70 .
- Johnston , N. , Sadler , R. , Shaw , G. R. and Connell , D. W. 1993 . Environmental modification of PAH composition in coal tar containing samples . Chemosphere , 27 : 1151 – 1158 .
- Collin , G. and Zander , M. 1985 . Ullmans Enzyklopädie der Technischen Chemie , 411 – 446 . Weinheim : Verlag Chemie . (chapter 22)
- Mansfeldt , T. 2000 . Ehemalige gaswerk- und zechen-kokereistandorte in Nordrhein -Westfalen. Ein aktueller Überblick . Zeitschrift der Umweltchemie & Ökotoxikologie , 12 : 122 – 123 .
- Jacob , J. , Grimmer , G. and Hildebrandt , A. 1996 . Trends in environmental pollution by PAH in Germany during the period 1985–1995 . Polycycl. Aromat. Comp. , 9 : 143 – 150 .
- Cuypers , C. , Clemens , R. , Grotenhuis , T. and Rulkens , W. 2001 . Prediction of petroleum hydrocarbon bioavailability in contaminated soils and sediments . J. Soil Sediment Contam. , 10 : 459 – 482 .
- Thorsen , W. A. , Cope , W. G. and Shea , D. 2004 . Bioavailability of PAHs: Effects of soot carbon and PAH source . Environ. Sci. Technol. , 38 : 2029 – 2037 .
- Lors , Ch and Mossmann , J.-R. 2005 . Characterisation of PAHs intrinsic degradation in two coke factory soils . Polycycl. Aromat. Comp. , 25 : 67 – 85 .
- Preuss , S. , Wittneben , D. , Zink , G. and Lorber , K. E. 1993 . Microbial degradation of PAH and hetero-PAH in aqueous systems and oil-containing wastewater . Vom Wasser , 83 : 255 – 264 .
- Zink , G. and Lorber , K. E. 1995 . Mass spectral identification of metabolites formed by microbial degradation of PAH . Chemosphere , 31 : 4077 – 4084 .
- Bleeker , E. A. J. , Wiegmann , S. , de Vogt , P. , Kraak , M. , Leslie , H. A. , de Haas , E. and Admiraal , W. 2002 . Toxicity of azaarenes . Rev. Environ. Contam. T. , 173 : 39 – 83 .
- Bleeker , E. A. J. , Van Der Geest , H. G. , Klamer , H. J. C. , De Voogt , P. , Wind , E. and Kraak , M. H. S. 1999 . Toxic and genotoxic effects of azaarenes: Isomers and metabolites . Polycycl. Aromat. Comp. , 13 : 191 – 203 .
- Gissel-Nielsen , G. and Nielsen , T. 1996 . Phytotoxicity of acridine, an important representative of a group of tar and creosote contaminants, N-PAC compounds . Polycycl. Aromat. Comp. , 8 : 243 – 249 .
- Schlanges , I. , Meyer , D. , Palm , W.-U. and Ruck , W. 2007 . Identifizierung und Quantifizierung toxikologisch relevanter PAK-Metabolite und -Begleitstoffe, Hetero -PAK und substituierter PAK an Altlastenstandorten und ihr Verhalten im Aquifer (final report of the KORA project)
- Zamfirescu , D. and Grathwohl , P. 2001 . Occurrence and attenuation of specific organic compounds in the groundwater plume at a former gasworks site . J. Contam. Hydrol. , 53 : 407 – 427 .
- Johansen , S. S. , Hansen , A. B. , Mosbaek , H. and Arvin , E. 1996 . Method development for trace analysis of heteroaromatic compounds in contaminated groundwater . J. Chromatogr. A , 738 : 295 – 304 .
- 2007 . EPI-SUITE, Version 3.20 , U. S. Environmental Protection Agency . Available from the internet via: http://www.epa.gov/opptintr/exposure/pubs/episuitedl.htm
- Wilson , A. S. , Davis , C. D. , Williams , D. P. , Buckpitt , A. R. , Pirmohamed , M. and Park , B. K. 1997 . Characterisation of the toxic metabolite(s) of naphthalene: Quinones of l-naphthol rather than naphthalene-1,2 epoxide are the toxic metabolites . Toxicology , 114 : 233 – 242 .
- Cerniglia , C. E. 1992 . Biodegradation of polycyclic aromatic hydrocarbons . Biodegradation , 3 : 351 – 368 .
- Chen , C. F. and Hung , I. F. 1999 . Determination of 1-naphthol in urine by HPLC method . Polycycl. Aromat. Comp. , 17 : 171 – 177 .
- Andersson , J. T. , Hegazi , A. H. and Roberz , B. 2006 . Polycyclic aromatic sulphur heterocycles as information carriers in environmental studies . Anal. Bioanal. Chem. , 386 : 891 – 905 .
- Rockne , K. and Strand , S. E. 1998 . Biodegradation of bicyclic and polycyclic aromatic hydrocarbons in anaerobic enrichments . Environ. Sci. Technol. , 32 : 3962 – 3967 .
- Ondrus , M. G. and Steinheimer , T. R. 1990 . High-performance liquid chromatographic determination of azaarenes and their metabolites in groundwater affected by creosote wood preservatives . J. Chromatogr. Sci. , 28 : 324 – 330 .
- Knezovich , J. P. , Bishop , D. J. , Kulp , T. J. , Gribic-Galic , D. and Dewitt , J. 1990 . Anaerobic microbial degradation of acridine and the application of remote fiber spectroscopy to monitor the transformation process . Environ. Toxicol. Chem , 9 : 1235 – 1243 .