Abstract
Metabolomics research is rapidly gaining momentum in disease diagnosis, on top of other Omics technologies. Breathomics, as a branch of metabolomics is developing in various frontiers, for early and noninvasive monitoring of disease. This review starts with a brief introduction to metabolomics and breathomics. A number of important technical issues in exhaled breath collection and factors affecting the sampling procedures are presented. We review the recent progress in metabolomics approaches and a summary of their applications on the respiratory and non-respiratory diseases investigated by breath analysis. Recent reports on breathomics studies retrieved from Scopus and Pubmed were reviewed in this work. We conclude that analyzing breath metabolites (both volatile and nonvolatile) is valuable in disease diagnoses, and therefore believe that breathomics will turn into a promising noninvasive discipline in biomarker discovery and early disease detection in personalized medicine. The problem of wide variations in the reported metabolite concentrations from breathomics studies should be tackled by developing more accurate analytical methods and sophisticated numerical analytical alogorithms.
Introduction
A metabolome is defined as the global collection of small-molecule analytes (<1000–2000 Da) found within a biological sample, where the sample can be derived from a cellular organelles to cells, biofluids, tissues, organs, or even an entire organism. Metabolomics refers to studying metabolomes with regards to chemical processes involving metabolites, substrates, intermediates, and reaction products. Omics approaches connect more detailed molecular biology concepts to the general aspects of systems biology. Metablomics is an essential pillar of molecular medicine and the field is growing rapidly. According to the Human Metabolome Database (http://www.hmdb.ca) today, human body harbors at least 3396 “detected and quantified” metabolites. Given the large number of detected, expected or predicted metabolites (n = 114,265), we clearly lack information on the existence, let alone the function of these metabolites in human physiology. Regarding the most abundant or well-known metabolites, information exists mostly on their canonical function.
The workflow of metabolomics investigations include; sample collection, preparation, analysis, data handling, biomarker discovery and pathway discovery.[Citation1] Different biological samples including blood, plasma, feces, urine, cells, herbal extracts, exhaled breath (EB) and exhaled breath condensate (EBC) have been used in metabolomics studies. Compounds from endogenous or exogenous sources can penetrate from blood stream to the lung lining fluid, and depending on their solubility and volatility, appear in EB or EBC. On the other hand, the compounds from inhaled air can penetrate from lungs to the blood stream. Both parent compounds and their metabolites might go through penetration. EBC refers to the sample collected by trapping the exhaled aerosols originating from lung lining fluid and contains mainly nonvolatile analytes. It is obvious that some concentrations of volatile compounds could be found in EBC, especially those are soluble in aqueous solutions. EB refers to gaseous samples collected from exhaled breath and contains mainly volatile analytes and very small concentrations of nonvolatile analytes, especially those with lower boiling points. Metabolomic studies usually employ nuclear magnetic resonance (NMR), mass spectrometry (MS), and less commonly fluorescence spectroscopy (FS) to analyze the metabolic profiles in biological samples. Low sensitivity of NMR is a limiting factor, only allowing for detection of metabolites with high concentrations. However, the main advantage of NMR is that no sample pretreatment is required in this technique, while MS requires some pretreatments which may result in longer analysis time and loss of some metabolites in the pretreatment processes. In comparison though, MS is a more sensitive approach. FS is straightforward for analytes possessing native fluorescence but requires derivatization for other analytes. Near infrared (NIR) is another potential spectroscopic method that could be used in metabolomics investigations, and it has shown promising results in similar areas.[Citation2] Combining MS with different separation instruments such as gas chromatography (GC), high performance liquid chromatography (HPLC), capillary electrophoresis (CE), supercritical fluid chromatography (SFC), and ultra high performance liquid chromatography (UPLC) can provide complementary analytical data, since these techniques provide different and somewhat unique separation bases.
To provide a better overview of the findings, one may gather data from NMR, GC-MS, HPLC-MS, CE-MS, SFC-MS, UPLC-MS, and FS. Due to the high complexity and multidimensionality of data generated from untargeted metabolomics experiments, multivariate statistical tools such as principal component analysis (PCA), partial least squares analysis (PLS), and artificial neural networks (ANN) need to be applied in order to begin to understand the complex relationship between metabolic profiles and the disease or condition under study. Analysis of complex samples results in the complexity of obtained data. EB or EBC are interesting biological samples for detection of human diseases, especially in those of the respiratory system. On the other hand, the possibility of direct injection of EB or EBC samples to selected-ion flow-tube mass spectrometry (SIFT-MS),[Citation3] proton-transfer-reaction time of flight mass spectrometry (PTR-MS),[Citation4] atmospheric pressure ionization mass spectrometry (API-MS),[Citation5] LC-MS/MS,[Citation6–Citation8] or CE[Citation9] shows the feasibility of analyzing metabolites in breath analyses.
Breathomics in general refers to multidimensional analyses including identification and quantification of volatile compounds in EB (also called volatolomics) and volatile or nonvolatile compounds soluble in aqueous solutions in EBC. Breathomics is a growing research area in experimental and clinical investigations of respiratory and systemic diseases and provides useful information in personalized medicine, since its final clinical aim is to optimize treatments for patients by considering individual breath characteristics.
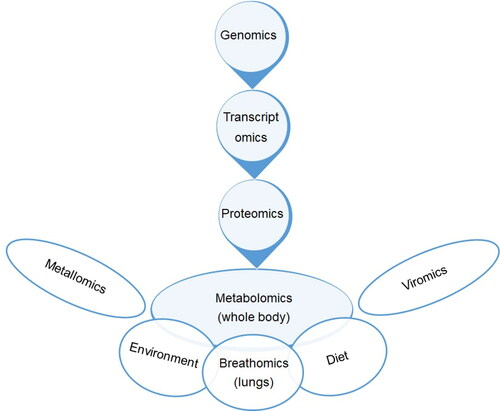
There are inter-connections between different omics approaches and also environment, diet etc. as schematically illustrated in .
Several articles have reviewed the increasing number of studies in breathomics. Lourenço and Turner[Citation10] reviewed methodological considerations for analysis of volatile organic compounds (VOCs) in breath samples. Kuo et al.[Citation11] introduced the human breathomics database (HBDB) by gathering available information on VOCs of healthy people and patients in a number of diseases. HBDB provides a useful resource for identifying potential biomarkers from breath of the patients in order to conduct complementary investigations. Metabolomics of chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis (CF) using different biological fluids was recently reviewed by Nobakht et al.[Citation12] Maniscalco et al.[Citation13] published a comprehensive review on metabolomics of EBC in chronic respiratory diseases. Neerincx et al.[Citation14] reviewed 12 reports on breathomics of VOCs in pediatric asthma covering the investigations published from 2010 to 2017. Finamore et al.[Citation15] reviewed 89 reports on the breathomics of VOCs in respiratory diseases, mesothelioma, pulmonary embolism, idiopathic pulmonary fibrosis, and gastroesophageal reflux disease (GERD). Azim et al.[Citation16] reviewed 66 reports on the assessment of VOCs in adult asthma and concluded that most of the studies are limited by a lack of standardization and external validations. Scheepers and Cocker[Citation17] provided a comprehensive review on the collection, analysis and interpretation of xenobiotics from occupational and environmental exposures along with their biomarkers in different biological fluids including EBC. Besides, Rahimpour et al.[Citation18] summarized the analytical techniques performed on EB/EBC samples with their main focus on drugs and biomarkers. Furthermore, Bruderer et al.[Citation19] reviewed the methodologies developed for on-line breath analysis with emphasis on the use of these technologies in diagnosing respiratory diseases, potential niche applications, and the promise of breath analysis for personalized medicine. However, there is still an obvious gap in reviewing the analytical techniques and sample preparation methods performed on EBC samples, which will be covered here. In this review, the latest articles published in breath analysis, focusing on breathomics are discussed.
EB and EBC collection
A breath sample is categorized into three fractions, including gaseous molecules, volatiles, and condensates. These fractions can be collected noninvasively through bags (for gaseous fraction), adsorbent traps (volatiles), and cold condensation chambers (condensates). The commonly used methods for sample collection for gaseous breath include (i) containers/bags: a simple and most commonly used sampling method where samples are collected in bags of varying volumes (typically 1–10 L) directly via a valve system to prevent rebreathing;[Citation20] (ii) reservoir/buffered sampling: These methods have been used to separate “upper” from “lower” respiratory tract air by retaining a portion of the breath from late expiration. Relatively simple solutions include the BioVOC and RTubeVOC which vent the initial breath and retain a fixed volume of end-tidal air (127 mL and 65 mL, respectively).[Citation21] A Breath Collection Apparatus (BCA) provides an integrated solution that also preferentially targets the breath from the end of expiration; and (iii) respiratory-cycle targeting: Another method to target late-expiratory air is to track respiration and sample breath based on the readouts from pressure or CO2 sensors.[Citation22,Citation23]
Such methods allow for a more personalized approach and accommodate very large differences in lung volume associated with age, gender, ethnicity and disease. Gaseous breath can be analyzed online or offline, with specific methods for capture and analysis for each method. Online measurement allows for rapid, near patient sample analysis and eliminates the need for sample storage. For offline measurement, the sample must be captured, stored and transported to the analytical instrument.[Citation24] Examples of analytical methods suitable for online analysis include forms of ion-mobility spectrometry (IMS), such as Selected Flow Tube-MS (SIFT-MS) and proton transfer reaction-MS (PTR-MS), which do not require sample pre-concentration and separation. Offline capturing of volatile compounds in gaseous phase includes their trapping onto a small, portable sorbent tube, solid phase micro extraction (SPME) fibers, or needle-trap devices.[Citation25,Citation26]
Although numerous gaseous breath tests have been validated for research purposes,[Citation27] few tests are currently approved for clinical use. Furthermore, some technical difficulties in chemical analysis of breath are: (i) the large numbers of VOCs (possibly 100 or more) found in breath, necessitating separation prior to the analysis (e.g., GC/MS), and (ii) the very low concentration of the compounds, which are below the limits of sensitivity of currently available instruments. The latter issue can be circumvented by the use of a breath collecting apparatus that concentrates the breath into a suitable sample for analysis.
EBC comprises another fraction of exhaled breath. Various commercial instruments have been developed for EBC collection. These devices work by rapidly cooling exhaled air with subsequent condensation of water vapor, as well as the sedimentation of aerosol particles onto a cold surface. The most common instruments according to the literature are: (i) the Ecoscreen (Erich Jaeger, Hoechberg, Germany) which is a stationary system, (ii) the RTubeTM (Respiratory Research, Charlottesville, VA, USA), which is a portable device, and (iii) Turbo-Deccs (Transportable Unit; ItalChill, Parma, Italy). In addition, various lab-made instruments have been described in the literature.[Citation28,Citation29] In pediatrics, exhalation maneuvers could be performed in children starting at the age of 6, and for younger children full facial mask and tidal breathing could be performed.[Citation30] With comparing sampling procedure for EBC and EB, sample collection for EB and its preservation for long periods are very difficult due to its gaseous nature. So, it is rather impossible to establish a biobank for these types of samples. Furthermore, the main pitfalls in sample collection for both EB/EBC are confounding factors on the concentration of the analytes such as diet, medications, smoking, physical activity and the ambient air of the sample collection area. The possible solutions for the latter factor include using purified high pressure air or subtracting inspired concentration of each analyte from expiratory concentration which requires further validations. The next critical point is to use a standard breath collection procedure, as this step has significant effect on the analysis results. Storage of the samples, especially for VOCs is another important point which should be considered in breathomics studies.[Citation14] In the case of patients under mechanical ventilation, the waste of the ventilator -which is the condensed form of lung lining fluid could also be collected and analyzed.[Citation31]
Factors affecting breath sampling
Nasal or oral inhalation
There are important differences between two breathing methods (i.e., nasal inhalation-oral exhalation and oral inhalation-oral exhalation) which may influence the concentration of analytes presented in EBC. During nasal inhalation, inhaled air is humidified in the upper airways and mediators formed in the nose and the sinuses enter the lower airways during nasal inhalation.[Citation32] This is an important issue to be noted, especially in patients with ongoing upper airway diseases. Measurement with oral inhalation-oral exhalation is preferred, so that only mouth-conditioned exhaled air enters the collection system.[Citation33]
Oral inhalation in EBC sampling may be performed with or without the use of a nose-clip. By using a nose-clip, subjects are forced to inhale through their mouths. In most studies, sample collection is performed by oral inhalation-oral exhalation.[Citation34]
Contaminations from salvia and nasal cavity
Many of the biomarkers in EBC are also found in significant concentrations in the salvia. For example, eicosanoids are present at high concentrations in saliva.[Citation35] Therefore, it is crucial to avoid EBC contamination with saliva. This can be achieved by measuring amylase concentrations[Citation32] or measurement of sample viscosity. Moreover, some inflammatory mediators formed in the nose and paranasal sinuses (e.g., leukotrienes and prostaglandins) can enter the oral expiratory air via the posterior nasopharynx. Therefore, it is crucial to exclude nasal contamination of EBC samples by measuring biomarker concentrations in three different experimental situations; (i) inspiring and expiring without a nose-clip, (ii) inhaling and exhaling with a nose-clip, and (iii) exhaling against a resistance to minimize nasal contamination.[Citation36] However, the potential for nasal/salivary contamination is different for each biomarker, and needs to be addressed in each individual case.
Temperature
Environmental and breath temperature may influence EBC results beyond the effects on volume yield. For example, there is a progressive increase in hydrogen peroxide (H2O2) and malondialdehyde concentrations and conductivity as the cooling temperature increases.[Citation37] Czebe et al. showed decreased pH with decreasing temperature[Citation38] and another study revealed that concentration of ammonia is lower in the ice form of condensate rather than the liquid form.[Citation34] So, the condensing temperature is important for those mediators/biomarkers that are thermally unstable, such as leukotrienes and purines. It is thus better for condensation temperature to be reported, to facilitate the comparison of data across laboratories.
Ambient air
EBC samples should not be left at room temperature or ambient air after collection. Ambient air contains molecules which may influence biomarker/compound concentrations in EBC through several possible mechanisms. For example, these molecules can (i) directly contribute to EBC, (ii) react and, therefore induce the degradation or formation of substances trapped in EBC, and (iii) promote inflammatory and biochemical changes in the airway that are subsequently reflected by changes in EBC composition. For instance, it has been shown that atmospheric NO reduces exhaled H2O2 levels.[Citation39]
Condenser system
Ahmadzai et al.[Citation40] listed a comparison of biomarkers measured in EBC that were collected using different devices. The design, inner coating materials used in the collecting device and temperature fluctuations over a condensate collection maneuver influence the concentration of biomarkers.[Citation41] Uneven condensing temperatures may not only be present between different EBC collection systems, but also in the case when the same condenser is used under different airflow or with different cooling temperatures. Moreover, variability in EBC pH between the various collection systems due to differences in the concentration of absorbed volatile salivary contaminants during collection (e.g., CO2, ammonia and acetic acid) can differentially influence the concentrations of biomarkers or nonvolatile compounds.[Citation28] Finally, to provide consistent data, the EBC collection system needs to be standardized with respect to collection temperature, coating material and flow design, as well as other parameters such as ventilatory patterns, tidal volumes, breathing frequencies, exhaled particles, etc.
Dilution
The airway lining fluid component of EBC is highly diluted by condensing vapor phase water.[Citation42] The concentration of EBC compounds depends on the efficiency of water vapor condensation, which is influenced by the temperature and surface area of collection. Without a reliable measure of a dilution factor, the precise concentration of compounds in the epithelial lining fluid (ELF) cannot be accurately determined. For this reason, a number of normalization factors have been proposed for nonvolatile substances in the literature. It would be preferable to use non-inflammatory reference indicators as internal standards that remain relatively unchanged in the respiratory fluid. Such indicators are ideally similar in concentration to those in the plasma and diffuse through the cell membranes, but are not produced in the alveoli or airways.[Citation43] Urea as a candidate molecule for this purpose is small, readily diffusible and equally distributed throughout the body. Furthermore, urea is not metabolized in the lungs. Thus, it would be possible to calculate an appropriate dilution factor for the nonvolatile compounds by measuring urea concentration in EBC, and estimating the interstitial urea.[Citation44] Measurement of urea concentration is based on degradation of urea to NH4+ by urease. However, urea concentration in EBC is relatively low, and serum values are more variable than those of the total electrolytes. Also, there may be a possible bacterial degradation of urea in infected lungs. Therefore, estimates of dilution with urea are consequently not accurate.[Citation43]
Other alternative ways are total nonvolatile cations, or conductivity measurement of EBC. Effros et al.[Citation43] have reported condensate electrolyte concentrations as a means to calculate the dilution of respiratory fluid droplets in the condensate. In one study in 2002,[Citation45] the effect of dilution was minimized by dividing condensate solute concentrations by the sum of the concentrations of the nonvolatile cations (Na+ + K+) in EBC. In a following work in 2003,[Citation43] the same group reported that conductivity can be used to estimate airway electrolyte concentrations and the dilution factor, under the condition that the ammonium ions are first removed by lyophilization. The observations in these studies suggest that the conductivity of lyophilized samples can be used as an inexpensive, simple, and reliable measure for estimating dilution of nonvolatile, hydrophilic biomarkers in EBC. Another reported correction for dilution is expressing concentrations of EBC components per time or per duration of EBC collection. However, this strategy has a significant drawback, because of the dependency of the collected volume of EBC on the volume of exhaled air in a given period of time. [Citation46] Although, dilution factor calculations are essential for interpretation of the obtained results from EBC studies, none of the above suggested strategies has proven to be a golden standard and the dilution factor calculations were omitted in most of the published articles on EBC.
In the last decade, Reinhold and Knobloch[Citation47] and Rosias[Citation48] have recommended to standardize the concentration of any EBC component in relation to a defined volume (100 L) of exhaled air. Comparing different EBC components as exhaled per 100 L exhalate led to a decrease in the variability and to an increase in the repeatability and reproducibility of the obtained data.
In two cases, the dilution factor calculations are not necessary: (i) when multiple interactive or biologically related biomarkers are measured concurrently and their ratios are considered,[Citation49] and (ii) when a confident assay for a substance is needed, it serves as an on-off indicator of an abnormality. Examples include the presence of Mycobacterium tuberculosis DNA, gastric pepsin, rhinovirus RNA, and anthrax toxin.[Citation50]
It should be mentioned that the dilution factors are irrelevant for VOCs. The important factors affecting the VOC levels include water solubility, gas-liquid partition coefficients, temperature of the source fluid (airway lining fluid), temperature of the condenser, pH of the source fluid and EBC, and the opportunity to react within (and/or be captured by) the EBC matrix.[Citation50] Adsorption on the surface of the collection bag and/or containers is the next factor affecting the concentration of VOCs.
Biological influences
In addition to the above mentioned factors, the amount of biological variability that is present even in healthy subjects under physiological conditions needs to be evaluated before interpretation of data. This physiological variability includes intra-subject and inter-subject components. Inter-subject variability is determined by the homogeneity or non-homogeneity of a selected group. Intra-subject variability includes, for example, circadian influences, effect of diet, effect of respiration (tidal volumes, breathing frequency, airflow rates and the ratio between dead space volume and alveolar volume) or age-dependent effects.[Citation51]
Analytical advancements
Biological samples have complicated matrices and should be analyzed by sensitive techniques. Since the constituent substances in such samples have diverse physicochemical properties, several analytical techniques are sometimes needed to expand metabolite coverage. Different analytical instruments such as GC, HPLC, CE, SFC, NMR, and FT-IR have been used in metabolomics studies.
NMR
NMR is the most common method used in the analysis of different compounds in biological samples. In this method, nuclei are influenced by an external field and their transitions in magnetic energy levels are recorded. NMR spectroscopy allows for the visualization of single atoms and molecules in various media in solution as well as in solid state. NMR can be used for identification and quantification of different compounds with unbiased results. NMR results are repeatable, reproducible and accurate, and detection limits of micro to nano molar ranges can be obtained. Due to these advantages, NMR spectroscopy is used in metabolomics for studying different biological samples. In 2014, proton nuclear magnetic resonance spectroscopy (H-NMR) was used in profiling the EBC of patients with COPD.[Citation52] For this purpose, the collected EBC samples were mixed with sodium phosphate and sodium azide and the mixture was transferred for H-NMR analysis. To compare the results, EBC samples of healthy individuals were also analyzed. Using this method, acetone, lysine, valine, tyrosine, proline, serine, propionate, acetate, and lactate were quantified. The results showed that assessment of these compounds is informative for determining the pathological state of COPD. Metabolomics of EBC discriminated COPD patients from controls with an overall accuracy of 86%. Compared to controls, EBC from COPD featured significantly lower levels of acetone, valine and lysine, and significantly higher levels of lactate, acetate, propionate, serine, proline and tyrosine. H-NMR was applied to EBC samples obtained from the asthmatic patients.[Citation53] In this study, EBC samples were collected at different temperatures to follow the changes in the type and concentration of the substances in EBC. The experiments were done on EBC samples which were collected with two different devices collecting the EBCs at –23 and –4.8 °C. The results verified that the EBC collection device temperature affects the level and the type of substances detected in the samples. The samples were initially analyzed and assessed the within-day, between-day, and technical repeatabilities. The results showed that relative standard deviation (RSD) was ≤11.2%. In this method the samples were classified into three groups and analyzed together, disregarding the condensing temperature. The results showed that there are strong correlative models (95%) with high average-quality parameters for spectral profiling and targeted profiling.
Maniscalco et al.[Citation54] analyzed biological samples of asthmatic and COPD patients including saliva, serum, EBC, and urine with H-NMR and compared the compounds profiles of these samples. The results showed that H-NMR discriminates asthma and COPD patients with high sensitivity and specificity, and this may help the clinicians to decrease the number of erroneous diagnoses. This research group used H-NMR for surveying the differences between groups of subjects occupationally exposed to levels of airborne inhalable dust, formaldehyde, phenol, and VOCs below regulatory limits.[Citation55] The results demonstrated that NMR-based metabolomics of EBC is a promising strategy with potential to be successfully used in occupational health to discover subjects exposed to very low levels of airborne chemicals. In another work performed by D’Amato et al., the EBC profiling was performed in the EBC samples of bronchiectatic patients using NMR.[Citation56]
MS
MS is another analytical technique widely used in metabolomics. MS can analyze low concentrations of different substances with high resolution. In contrast to NMR, MS provides low linear range and its sensitivity is strongly related to the ionization efficiency of the compounds. However, coupling MS with separation systems such as LC, GC, and CE improves the separation and identification of new biomarkers. HPLC-MS technique was applied on EBC samples for metabolomics analysis.[Citation57] In this study, after removal of the proteins with precipitation, the samples were extracted with different sorbents during solid phase extraction (SPE). MS analysis led to detection of fatty acids, amino acids, fatty aldehydes, fatty amides, oxoanionic, imidazoles, sphingoid bases compounds, hydroxy acids and aliphatic acyclic acids.
Metabolomics profiles of saliva, EBC, and plasma was studied using HPLC-MS to detect air toxics.[Citation58] The samples were collected from individuals before and after a 2-hour road traffic exposure. The analysis yielded 7,110, 6,019, and 7,747 reproducible features in plasma, EBC, and saliva, respectively. Correlations were moderate-to-strong (R = 0.41 − 0.80) across all pairwise comparisons of feature intensity within profiles, with the strongest correlation being between EBC and saliva. EBC metabolomics analysis by UPLC-MS was used for early detection of CF and acute pulmonary exacerbations.[Citation59]
UPLC-MS was used to profile the EBC metabolites of clinically stable CF patients, CF patients who required hospitalization, 5 CF patients in recovery step, and 4 CF patients who were clinically stable at the time of EBC collection. The result showed that 3 discriminant metabolic features identified as 1) pyroglutamic acid, 2) short chain carboxylic acids and 3) hydroxyeicosatetraenoic acids differentiated the CF patients from healthy people. Pre-APE samples were distinguished from stable CF samples. In another study, EBC samples of patients suffering from CF were analyzed by ion mobility-MS,[Citation4] finding three metabolites discriminating patient EBCs from healthy subjects from both sources.
GC-time of flight (TOF)-MS can also be employed in metabolomics analysis of EBC samples. This method was applied for profiling volatile and nonvolatile compounds.[Citation60] The collected samples were extracted with Liquid–liquid extraction (LLE) and SPE methods. The results showed that LLE provided the widest information on the composition of EBC with tentative identification of 51 compounds. Among the identified compounds were fatty acids and other derivatives such as methyl esters and amide derivatives, which are not considered as volatile components. LLE provided an extraction recovery in the range of 77% in a single extraction. The method repeatability was examined on EBCs from 50 healthy individuals reporting a within-day variability below 7%. Other representative family of compounds found in EBC was that of prenol lipids such as terpenes, which are volatile. Castro and his group used EBCs to develop a screening tool for lung cancer discrimination between two groups with and without risk factor.[Citation61,Citation62]
The analyses of amino acid content in EBC samples has attracted more attention in identification of several diseases. For this purpose, Konieczna et al.[Citation63] developed a HPLC tandem mass spectrometry (MS/MS) method for simultaneous determination of 16 amino acids in EBC samples. Validation parameters such as precision (RSDs ≤ 11%), accuracy (RSDs ≤ 10%), recovery (52.2-108.2%) and linear range (0.25–500 ng/mL) were investigated and it was found that the developed method was robust and reliable for the determination of the selected amino acids.
Other methods
Gas sensing using tetrahertz radiation is a new opportunity to follow up VOCs. In this technique, heavy gas molecules display rotational resonance at microwave frequencies and light gas molecules in the mid-infrared frequencies.[Citation64] Electronic nose technology provides another opportunity for breathomics studies.[Citation65,Citation66] Sniffphone is a newly developed combination of nanoparticle based gas sensors with smart phones which provides a point of care tool for disease diagnosis.[Citation67] Nakhleh et al.[Citation68] reviewed the progresses made on using gold nanoparticles for breath analysis. Arrays of mono-layer-capped gold nanoparticle sensors provided fast, high-throughput and cost-effective methods for exhaled breath analysis. The produced results were used for numerical analyses in order to find out the pattern of variations of VOCs in different diseases.[Citation68] Tang et al.[Citation69] reviewed the applications of nanostructured metal sulfide materials for sensing of VOCs and inorganic gases such as CO2, NH3, H2S, SO2, CH4, H2 and nitric oxide. Recent advances in the role of eNoses in phenotyping of COPD were reviewed by Searlata et al.[Citation70] Mirzaei et al.[Citation71] review and compare devices used for Δ9-tetrahydrocannabinol detection in EB such as field asymmetric ion mobility spectrometry, semiconductor-enriched single-walled carbon nanotube chemiresistors, LC-MS/MS, microfluidic-based artificial olfaction, and optical-based gas sensing. They reported that amongst the examined platforms, from the portability and usability perspectives, optical-based, single-walled carbon nanotube chemiresistors, and artificial olfaction are the most promising, whereas field asymmetric ion mobility spectrometry and LC-MS/MS are best suited for highly sensitive and selective testing with toxicology lab confirmation.
FS is a simple and low cost method with minimum sample pretreatment, requiring a short analysis time for metabolomics studies. FS has been used for metabolomics investigations in plasma [Citation72] and urine;[Citation73] however, to the best of our knowledge, there is no report on the application of FS for EBC analysis.
Reactive oxygen species are chemically reactive species containing oxygen that are produced naturally during oxygen metabolism and are now known to possess important roles in cell signaling and homeostasis. Reactive oxygen species levels are elevated drastically under oxidative stress and can significantly damage cellular structures. These compounds have short half-lives and their determination in biological fluids is challenging. In this line, EBC can be a good candidate for analyzing oxidative products such as malondialdehyde. Different analytical methods can be used to quantify such oxidative products in EBC samples, as shown before for malondialdehyde.[Citation74–Citation76] Lačná et al. determined aldehyde metabolites in EBC samples with high sensitivity using CE with laser-induced fluorescence detection. In the project, one dialdehyde (malondialdehyde), eight saturated monoaldehydes, and an unsaturated aldehyde were investigated, as the main aldehydic metabolites in EBC.[Citation77] Under optimal conditions, target analytes could be well separated, and sensitive, repeatable, and reliable determination of the analytes was possible. The method was performed on EBC samples of smoker and nonsmoker men and women. The results showed that the concentrations of the compounds in EBC samples of male smokers were dramatically higher (∼eight folds) than those of male nonsmokers. However there was no significant difference between the smoker males and females.
Numerical and informatics methods in metabolomics research
Metabolomics (encompassing breathomics) analysis usually generates multi-dimensional data. Current metabolomics exploits classic analytical chemistry combined with cheminformatics and bioinformatics, to enable large-scale data analysis. Chemometrics is a branch of science dealing with data-driven means to extract information from chemical systems.[Citation78] In the majority of cases, the goal of metabolomics analysis in general and breathomics in particular is to separate metabolome snapshots from different samples or help identify biomarkers for various diseases. This feat is possible through classical statistics in parallel with multidimensional data analysis tools. The latter tools are essential in light of the large data that can be acquired using current technologies such as MS.[Citation79] In this regard, it is of utmost importance to recognize these tools and meticulously use them based on need and the area of application. Classic statistical tools such as t-test, Mann-Whitney test and one-way analysis of variance (ANOVA) have been extensively used and reviewed in the past. Therefore, in this section, we will focus on common multidimensional data analysis and multivariate statistical approaches used in metabolomics research. In this regard, robust data analysis pipelines are well established in transcriptomics and to a great extent in proteomics. Scientists in these areas have tried to deal with big data challenges over time. Such workflows have been also applied in metabolomics.
Usually a routine pipeline for biomarker discovery involves the following steps: automated detection of metabolites in the raw files,[Citation80] preprocessing (subtraction of background noise, peak detection and quantification) and cleaning of raw profiling data (removal of contaminants or missing values or imputation) and normalization. The data are then subjected to statistical analysis and selection of the most statistically significant and biologically relevant metabolites through feature selection, clustering, classification and pathway/network analyses. Finally, making the data sets publicly available is crucial, so that the community can reanalyze and reevaluate the quality of experimental outputs. In this line, Metabolomics Standards Initiative (MSI) [Citation81] is a community driven effort that sets global consensus for minimum reporting standards. Open-access online repositories such as MetaboLights[Citation82] and Metabolomics WorkBench allow for experimental data deposition, which in turn enables transparency and knowledge transfer. Furthermore, specific reporitories such as Human Breathomics Database can also be used for data/study deposition.[Citation11]
Multivariate data analysis
Once the data is cleaned, univariate analysis methods are applied to compare groups. Such methods include for example t-test, ANOVA, Kruskal–Wallis, etc. Different types of adjustments for multiple testing such as Bonferroni correction or Benjamini-Hochberg correction are also required to minimize the false discovery rates.[Citation83]
However, to comprehensively analyze a large data set, multivariate analysis methods are essential. Multivariate statistics encompasses methods that can be employed for simultaneous observation and analysis of data containing two or more variables. Through these methods, the dimensionality of data is reduced, improving their interpretation and visualization. Multivariate statistics can help to separate the signal from noise in high dimensional data. In addition, they can be useful in selection of variables such as biomarkers separating the samples or populations.[Citation84,Citation85]
Typical computational techniques used in metabolomics include but are not limited to PCA, clustering (e.g., hierarchical and k-means), and classification (e.g., Canonical Variate Analysis (CVA) and Support Vector Machine (SVM)). These analysis tools are mainly used for overall assessment of different biological systems or conditions, and/or quantifying the contribution of each metabolite to the differences between these systems and conditions. The top features separating the biosystems can thus be potential biomarkers.
Multivariate data analysis usually begins with unsupervised exploration of data. If unsupervised techniques do not yield satisfactory results, supervised methods such as classifiers can be used. After classification, internal cross validation strategies such as bootstrapping, split-half, or leave-one-out are used. Finally, external validation is performed by evaluating the performance of the model, using data that were not used in developing/training the model. The ultimate validation would be to test the model on a newly recruited population.[Citation86]
Exploratory data analysis by unsupervised tools
Exploratory data analysis tools are divided into projection methods and partitional clustering techniques. PCA is the most extensively used projection method in omics.[Citation66] PCA is an exploratory data analysis tool and a dimension reduction technique that is routinely used for data quality control, i.e., reproducibility between replicates or adjacency or relatedness of populations/samples. Furthermore, PCA is generally employed in omics studies including metabolomics for dimension reduction, to basically showcase separation and trends/patterns in the data.[Citation87] PCA output is a set of principal components. The first principal component has the largest possible variance and explains much of the variability in the data. By visualizing the samples in the PCA space, one can determine the variables most contributing to separation of samples within each component, most of the time even by naked eye. The other components explain less and less of the variation in the data in a step-wise manner. PCA loading can help differentiate the features (here metabolites) that have the most discriminatory power in separation of samples.
Canonical correlation analysis (CCA) is also gaining popularity in metabolomics. CCA is related to PCA, the difference is that while PCA works by defining coordinate systems that describe the variance in an individual data set, CCA defines the cross-variance between two data sets.[Citation88] CCA is an approach that is used to find the relationship between two multivariate sets of variables quantified for the same set of samples.[Citation89] Therefore, CCA is believed to be an extension of bivariate correlations. Among two vectors with random variables, CCA will discover linear combinations (called canonical variate) within the vectors that have maximum correlation with each other. The statistically significant canonical variates are then presented in a CCA score plot. Each point on such a plot corresponds to the combined information from the two vectors, whereas the shape of the cloud is indicative of the correlation between the two blocks of data. The relation between the two blocks of data is strong, only if the cloud of points forms a coherent scatter along a line and vice versa. CCA readout is the canonical coefficients, which show the contribution of the original parameters to the correlation. For example, Smolinska et al.[Citation90] used CCA to investigate the relationship between volatile metabolites in breath and gut microbiome in active and quiescent Crohn's disease (CD). In this study, CCA analysis identified 18 volatile metabolites significantly correlating with 19 fecal bacterial taxa (R = 0.91; p value = 3.5 × 10–4) in active disease, while in the quiescent disease 17 metabolites were correlating with 17 bacterial taxa (R = 0.96; p value = 2.8 × 10–4).
In this context, Factor Analysis also has special applications.[Citation91] Factor analysis is very similar to PCA, but these two methods are not identical. The aim of both methods is to reduce the dimensionality of the data. Unlike PCA, factor analysis is specifically tasked with the objective to determine a set of unobservable factors from the observed variables. This method helps to find a presumed small number of underlying variables by investigating a large number of observed variables.
Clustering methods comprise another type of exploratory data analysis tools. Basically, clustering is tasked with assigning group membership to samples in a data set and partitioning the data into clusters with homogenous members based only on quantitative data. The number of clusters are gradually reduced to reach a single cluster. In other words, the samples with similar features are assigned to the same cluster without a priori assumptions. This makes clustering an unbiased approach. Clusters are built in a way that samples within a cluster have the highest association with each other, while being only weakly associated with samples in other clusters.[Citation92] A proximity measure is used to partition the dataset into clusters in such methods. For example, in hierarchical clustering, distances are used to assess the similarity of samples and to organize them into an ordered grouping or a dendrogram. K-means uses a similar proximity measure, but the number of clusters is pre-defined by the user. Euclidean distance and Mahalanobis are the most commonly used distances. Clustering is routinely used in metabolomics for data exploration. [Citation91]
As an example, de Vries et al.[Citation66] applied a breathomics approach for phenotyping chronic airway disease. First, they used PCA to reduce the dimensionality of the data prior to clustering. Such dimension reduction would reduce the risk of overfitting. Subsequent unsupervised hierarchical clustering resulted in five significant combined asthma and COPD clusters that differed regarding ethnicity, systemic eosinophilia and neutrophilia, body mass index (BMI), exhaled nitric oxide fraction, atopy and exacerbation rate. The importance of these results is that these phenotypes lead to clusters that cannot be determined by diagnosis and are only reflected in clinical/inflammatory characteristics.
Supervised methods
The second major and fundamental part of the multivariate analysis is covered by classification methods, which aim at finding mathematical models that are capable of assigning membership of each population/sample to a proper class in the data set. In these analyses, each sample is assumed to belong to a relevant class. Such methods can be applied to any types of high dimensional data resulting from proteomics, spectroscopic, chromatographic and metabolomics analysis. In the case of metabolomics for example, the samples would be the quantified metabolomes and the data set would be the whole data obtained from the measurements. Supervised methods can also be used on relative abundance data as well as absolute quantification data.
Classification methods are divided into various types: linear and nonlinear, probabilistic and distance-based, pure classification methods and class-modeling methods. While the best linear boundary is used for class discrimination in linear classification methods, as the name suggests, the best curve or nonlinear boundary is used in non-linear methods. The classification in probabilistic methods are based on estimates of probability distributions, while distances between samples are used in distance-based methods. In pure classification methods, the hyperspace is separated into as many regions as the number of classes. However, in class-modeling methods, the focus is on modeling the analogies among the objects of a class.[Citation93]
CVAs and SVMs are two types of pure classifiers. In CVA, the samples are separated into classes by obtaining the minimal class variance and maximal between-class variance. Discriminant Analysis (LDA), Quadratic Discriminant Analysis (QDA) and Partial Least Squares-Discriminant Analysis (PLS-DA) are also similar or related to CVA. For example, Multilevel PLS was used for data reduction in a breathomics study to assess the effects of treatment and withdrawal with beclomethasone/formoterol inhalation in patients with COPD.[Citation94] PLS-DA as a supervised modeling approach is also gaining popularity in analysis of omics data, both in exploratory data overview and discovery of specific features in the data contributing to a condition under study.[Citation95] For example, in a recent tool called ProTargetMiner,[Citation96] PLS-DA modeling was used to contrast one proteome against others, to highlight the features that discriminate one treatment against others in the loading plot. Since this method can pull out the features that have the largest discriminatory power, such a paradigm can also be used in biomarker discovery in metabolomics and other omics studies. PLS-DA produces an R2 value representing the goodness of the model fit and a Q2 value, which corresponds to the model predictive power. The latter is calculated by an internal cross-validation, which is described in detail in the above article. For more detailed explanation, see Umetrics documentation.[Citation97]
SVM on the other hand, is tasked with classifying the data in space with a separator described by a hyperplane. Both linear and non-linear combinations of functions parametrized by support vectors can be used to express the hyperplane. This is why SVMs are suited and commonly used for non-linear separations. Other non-linear techniques include k-nearest neighbors (k-NN),[Citation94] Artificial Neural Networks and Random Forests.[Citation95] For example Daniel and Thangavel[Citation98] analyzed the breath of 49 gastric cancer and 30 gastric ulcer patients to distinguish the normal, suspected, and positive cases using back-propagation neural network. This analysis produced an accuracy of 93%, sensitivity of 94.38%, and specificity of 89.93%.
The class-modeling methods or so called one-class classifiers such as Soft-Independent Modeling of Class Analogy (SIMCA) can also have specific applications in metabolomics.[Citation99] In these methods, each class is modeled separately. These classifiers are usually employed when a reference class exists, for example in food authentication and toxicology.
Different classifiers can have various performances in various studies or applications. Therefore, they are sometimes tested simultaneously. For example, in a breathomics study, Arasaradnam et al.[Citation100] investigated exhaled VOC analysis to detect hepatic encephalopathy (HE). Breath samples from 22 HE patients and 20 control subjects were captured and analyzed using an uvFAIMS portable e-nose. Data were analyzed using 4 different classifiers including sparse logistic regression, Random Forests, SVM and Gaussian Process, showing the difference in the performance of these techniques.
In this section, we provided a brief introduction to the multivariate statistical tools in metabolomics and breathomics. This account is far from comprehensive. For a complete overview of the field, the reader is directed to other reviews.[Citation78,Citation79,Citation83–85,Citation93,Citation101–104] Saccenti et al.[Citation83] have interesting reflections on univariate and multivariate analysis of metabolomics data.
The great impact of databases on breathomics research
Breathomics research is accelerating especially by introduction of databases such as Human Breathomics Database (HBDB),[Citation11] METLIN[Citation105] and MassBank.[Citation106] HBDB for example contains a total of 913 VOCs in relation to human exhaled breath researches reported in 2,766 publications. The HBDB is the most comprehensive HBDB of VOCs in human breath to date. This along with the high number of publications in the field underlines the necessity of developing and advancing statistical tools in coping with this progress.[Citation79] Automation of metabolomics data analysis is also an emerging topic. For example, different clustering approaches have been developed such as DBSCAN and OPTICS and very recently VOCCluster.[Citation107]
Considerations and limitations
In the end, one should never forget the importance of method validation.[Citation108] Although many molecular fingerprints and biomarkers have been identified in the literature using the above computational techniques, only a fraction of the reported findings have been validated and proven to be useful. The large nature of data obtained in metabolomics requires good experimental design and identification of biological and technical issues that might give rise to bias, leading to false positive and negative discoveries. An efficient experimental design must consider different components such as formulation of hypothesis, proper and adequate definition of phenotype, power calculations, sample size calculations, optimization/standardization of protocols, quality control, multiple testing correction methods and plans for validations of findings.
A common problem in omics data analysis is the extremely large number of variables compared to the small number of samples. In ideal case, for accurate estimation of the covariance matrices, the number of samples must at least be equal to the number of variables. Otherwise, false discoveries arise due to overfitting of the diagnostic algorithm.[Citation101] Overfitting can be circumvented by adequate sample size or more often by proper correction for multiple comparisons.[Citation86,Citation109,Citation110] Other challenges in chemometrics include dealing with noisy data, data outliers, missing data, alignment of batch data and merging of multiomics datasets. These challenges must be prioritized as a research frontier in metabolomics in the coming years.
Reports on metabolomics analysis of EBC
Here we will review recent studies based on metabolomics analysis of EBC in lung diseases as well as non-respiratory disorders and animal studies.
Lung diseases
The complexity of lung disease is due to various mechanisms that are not present in all patients at a given time or in the same patient at different time points.[Citation111] Phenotyping of the patients using specific biomarkers is required in clinical practice for characterizing, prognosis and treatment of patients suffering from respiratory diseases. This is in line with the universal efforts toward personalized medicine. For such a complex disease, a single biomarker cannot be used and a panel of biomarkers is required for an accurate molecular level explanation of the disease.[Citation112] It is also important to identify the biomarkers that are normally present in EBC from those that appear later when one gets ill. In this line, Kazeminasab et al.[Citation113] discuss the significant role of exhaled breath biomarkers in COPD. They reported that reactive oxygen species production has the potential to damage lipids, proteins and nucleic acids as three classes of biomarkers in the EBC which act as indicators of biological or pathological processes in the lungs. Therefore, analysis of pulmonary biomarkers provides new insight into modern medicine that could be useful in aiding risk assessment, disease prevention, early and accurate diagnosis and monitoring treatment effectiveness in COPD.
Kononikhin et al.[Citation114] used a high resolution nano HPLC-MS method on EBC samples of newborns (20–40 days of life). This method was used to identify respiratory diseases by analyzing the EBC of 119 subjects and 164 metabolites were successfully characterized. To define respiratory disease-specific features, label-free semiquantitative proteomics and metabolomics data were obtained from 24 intubated neonates, through an easy, fast, and noninvasive method. The obtained differential biomarkers could be used in identification of respiratory deficiencies and diseases.
Similar to the above study, the composition of amino acids was investigated in EBC samples of children suffering from asthma.[Citation115] The results showed that amino acids levels especially glycine and methionine in children with a positive history of allergies can be an additional prognostic or diagnostic criterion for asthma. In another research amino acids and other asthma-related molecules were assessed in EBC and saliva samples using HPLC-quadruple-TOF (Q-TOF)-MS.[Citation116] According to this study, amino acids and several small molecules can be quantified in EBC samples, the concentration of which could be used for asthma diagnosis. On the other hand, 77 metabolites were identified in EBC samples obtained from lipids, spices, herbs, plants, food, herbicides, dipeptides, and polycyclic Aromatic Hydrocarbon (PAH) degradants. Interestingly, many of metabolites were undetectable in saliva samples and could only be found in EBC.
lists the published works on breathomics studies of lung diseases based on both EB and EBC samples.
Table 1. Metabolomics studies on respiratory diseases using EB or EBC (breathomics) samples.
Non-respiratory diseases
Multi-omics approaches including volatomics for biomarker discovery and target validation in different biological specimens for amylotrophic lateral sclerosis (ALS) were reviewed by Mitropoulos et al.[Citation167] Bannaga et al.[Citation168] reviewed VOCs of breath, urine and stool samples of patients with inflammatory bowel diseases and discussed their sensitivity and specificity.
Karyakin et al.[Citation169] used EBC and whole blood samples to determine glucose in diabetic patients. They used a biosensor based on glucose oxidase for blood and EBC samples. This procedure was performed on the samples collected in a 24-hour period. The results showed that EBC glucose levels correlate positively with blood glucose levels, therefore offering the prospect of a noninvasive approach to monitor diabetes. Furthermore, glucose metabolism to lactate was studied and glucose consumption in EBC was verified by observing the accumulation of lactate in living cells.[Citation170]
VOCs of children suffering from type 1 diabetes mellitus (N = 53) were investigated in comparison with a matched healthy control group (N = 60).[Citation26] As shown in , higher concentrations of isoprene, pentanol, dimethylsulfide, ethanol and isopropanol were observed in case group. In patient group, exhaled isopropanol was related to HbA1c (R = 0.57). In addition, exhaled isopropanol and pentanol levels were correlated with blood levels of cholesterol and LDL-cholesterol. Controversially, the research group did not observe the elevated exhaled acetone in diabetic patients whereas, previous investigations reported the elevated exhaled acetone (e.g., Ref. 170–172). Trefz et al.[Citation26] discussed this controversy by considering a reduction of acetone to isopropanol by nicotinamide adenine dinucleotide dependent redox reaction.
Figure 2. Box plots of exhaled concentrations of acetone, isoprene, pentanal and DMS (A) as well as limonene, ethanol and isopropanol (B). Black box plots: healthy controls; red box plots: T1DM patients; * and # indicate statistically significant differences with p < 0.001 and p = 0.002, respectively. ©Nature Publishing Group. Reproduced with permission Nature Publishing Group.[Citation26] Permission to reuse must be obtained from the rightsholder.
![Figure 2. Box plots of exhaled concentrations of acetone, isoprene, pentanal and DMS (A) as well as limonene, ethanol and isopropanol (B). Black box plots: healthy controls; red box plots: T1DM patients; * and # indicate statistically significant differences with p < 0.001 and p = 0.002, respectively. ©Nature Publishing Group. Reproduced with permission Nature Publishing Group.[Citation26] Permission to reuse must be obtained from the rightsholder.](/cms/asset/249a31cd-8aa1-439a-8707-38486626e12e/batc_a_1889961_f0002_c.jpg)
In another study, amino acid analysis were performed on EBC samples collected from children with leukemia. The differences in the levels of amino acids in patients were assessed, and alterations in the levels of amino acids were found to be specific to leukemia.[Citation63] The established procedure verifies the possibility of using amino acid profiles as leukemia biomarkers. In a review article, Robles and Priefer[Citation173] introduced hydrogen breath test as a relatively low cost, available, noninvasive and popular technology to aid in the diagnosis of many gastroenterological diseases with special focus on lactose intolerance. They reported that in various studies, the diagnosis of a positive breath test for lactose intolerance was either based on a rise in >20 ppm from baseline for hydrogen or >10 ppm for methane.
VOCs of exhaled breath of patients with mild- to- moderate kidney disease (N = 48) or patients with a functional renal transplant KTx (N = 8) were compared with those of a matched healthy control group (N = 60). As shown in , the levels of ammonia (a key molecule in the urea cycle removing nitrogen from protein metabolism and also a product of bacteria in saliva, respiratory and gastrointestinal tract), ethanol, isoprene (a by-product of cholesterol biosynthesis), pentanal and heptanal (markers of oxidative stress) in patient group were higher than those of healthy group and a reversed pattern was observed for methylamine (product of adrenaline deamination by monoamine oxidase).[Citation174] lists the published works on breathomics studies of non-respiratory diseases.
Figure 3. EBC levels of the biomarkers in healthy control (blue) and chronic kidney disease patients (red). ©Kluwer Academic. Reproduced by permission of Kluwer Academic.[Citation175] Permission to reuse must be obtained from the rightsholder.
![Figure 3. EBC levels of the biomarkers in healthy control (blue) and chronic kidney disease patients (red). ©Kluwer Academic. Reproduced by permission of Kluwer Academic.[Citation175] Permission to reuse must be obtained from the rightsholder.](/cms/asset/db1da8fe-5cc8-46dc-bdf2-7146d29c2335/batc_a_1889961_f0003_c.jpg)
Table 2. Metabolomics studies on non-respiratory diseases using EB or EBC (breathomics) samples.
Animal studies
The determination of breath constituents is of great importance in veterinary.[Citation46] The discovery of metabolic biomarkers in animals’ EBC may offer valuable insight to their metabolic origin and such results can be cautiously extrapolated or validated in humans for biomarker development. Recently, Carlos et al.[Citation212] reviewed progress on the detection of canine metabolites. They discussed the potential uses of metabolomics to discover biomarkers of diseases in dogs.
Due to the special anatomy of the respiratory system of cetaceans, EBC samples provide valuable information on body physiology. Aksenov et al.[Citation213] designed a portable device to trap EBC samples from cetaceans. The schematic representation of the device and the sampling process are presented in . The metabolomes of the collected EBC samples from trained and wild dolphins were analyzed by GC-MS and LC-MS. To analyze the metabolites with GC-MS, samples were pre-concentrated by a headspace equipped solid phase micro extraction (SPME) device. However, only a lyophilization step was carried out prior to the LC. After the determination step, results were analyzed by PLS-DA.
Figure 4. Schematic representation of the device for EBC sample collection from cetaceans. ©American Chemical Society. Reproduced by permission of American Chemical Society (https://pubs.acs.org/doi/abs/10.1021/ac5024217).[Citation213] Permission to reuse must be obtained from the rightsholder.
![Figure 4. Schematic representation of the device for EBC sample collection from cetaceans. ©American Chemical Society. Reproduced by permission of American Chemical Society (https://pubs.acs.org/doi/abs/10.1021/ac5024217).[Citation213] Permission to reuse must be obtained from the rightsholder.](/cms/asset/15aeffdd-7a99-4986-969c-e32403ee24c9/batc_a_1889961_f0004_c.jpg)
Results revealed that dolphin EBC contains a mixture of various volatile and nonvolatile compounds in trace concentrations. There was a similarity between the compounds detected from human and dolphins especially regarding the volatile compounds. However, some small amines (1-pentanamine, 5-(hydroxymethyl)-2-pyrrolidinone and bis (2-hydroxypropyl) amine) were reported in dolphins for the first time. Later, Zamuruyev et al.[Citation214] used the modified version of the device to collect EBC samples from small cetaceans. The fabricated device was used for reproducible EBC collection from bottlenose dolphins (Tursiops truncatus) as small cetaceans. After sampling, the metabolite contents were analyzed by GC-MS and LC-MS for the detection of volatile and nonvolatile compounds, respectively. Before analysis, the metabolites were extracted with polyacrylate based SPME approaches. In comparison with the previously developed device, the improved version could help quantify more than 230 and 2,400 metabolites with GC-MS and LC-MS, respectively.
Pasamontes et al.[Citation215] determined the metabolite content of wild cetaceans EBCs to investigate a possible relationship between EBC metabolites and pulmonary abnormalities using LC-MS/MS. Results showed meaningful correlations among EBC metabolites and health measures, suggesting possible use of EBC for evaluating health condition of wild cetaceans. Also, Borras et al.[Citation216] analyzed the EBC samples of bottlenose dolphin (Tursiops truncatus) to monitor their health conditions. The hydrophilic interaction and reverse-phased LC were used to separate and record metabolite peaks to observe any health alterations. Results suggested that various biomarkers and metabolites are related to specific diseases in dolphins.
Cumeras et al.[Citation217] collected EBCs whales (Eschrichtius robustus) to analyze metabolites important in health condition monitoring of whales. SPME approach was used to extract metabolites from EBC samples, which were then analyzed with GC-MS. About 70 compounds were separated and identified in the whale EBC samples, out of which about 44% were found in healthy human EBCs. In another work, whale hormones were assessed in EBC samples. Testosterone, progesterone, and cortisol concentrations were quantified by enzyme immunoassay kits (EIA) using urea as a normalizing agent. Results reveled that testosterone and cortisol levels were different in individual whales, and could be regarded as potential markers for investigation of adrenal activity of various whales.[Citation218] Also, Hogg et al.[Citation219] and Dunstan et al.[Citation220] evaluated LC-MS technique for determination of progesterone, testosterone and cortisol levels in EBC samples. Compared to LC-MS technique, EIA immunoassay showed better sensitivity with a Limit of Quantitation (LOQ) of 500 pg/mL for all studied hormones.
Asthma is a long-term chronic inflammatory disease of the lung airways which will affect about 400 million people around the world by the year 2025. Annually, about 250 thousand people pass away due to asthma. Hence, it is of great importance reduce asthma incidence, and also to detect this disease at the early stages. New biomarkers emerging from metabolomics studies can result in a decrease in the mortality rate of asthma. Fulcher et al.[Citation221] utilized NMR to distinguish the differences in the EBC samples of cats before and after asthma induction. Acquired spectra were analyzed with various chemometrics software to find possible biomarkers. Results suggested that acetone levels were increased in EBC samples and about 74% of cats with early asthma contained acetone in their EBCs. Also, the concentration of hydroxyphenyl-containing aromatic compounds was increased, whereas phthalate concentrations were decreased in about 60% of the cats.
Bos et al.[Citation222] pursued changes in EBC metabolites in two rat groups in which lung injury was induced by intratracheal (IT) and intravenous (IV) lipopolysaccharide (LPS). The mixture of EBC samples were determined by GC-MS. Metabolomic studies showed a decrease in hexanal, 6,10-dimethyl-5,9-undecadien-2-one and pentadecane concentrations for both IV- and IT-induced LPS groups. Based on metabolomic studies, some biomarkers were identified for lung injury and also nonanoic acid was proposed for alveolar neutrophil influx.
Fischer et al.[Citation223] conducted a study in which the effect of food intake on VOC composition and concentrations of nonvolatile in EBC samples collected from caprine was investigated. GC-MS was used as analytical data acquisition instrument. They showed that the concentration of hydrocarbons and alcohols were different before and after food intake. Also, Küntzel et al.[Citation224] designed a device for metabolite analysis in bovine EBC samples. They developed a proton transfer reaction TOF-MS(PTR-TOF-MS) device for real-time and direct EBC analysis. Bazzano et al.[Citation225] determined the metabolite composition of EBC and tracheal wash samples of healthy and asthma-affected horses. After collection, the samples were analyzed with H-NMR, approving eight metabolites of ethanol, methanol, formate, trimethylamine, acetone, acetate, lactate and butanone in EBC samples. Interestingly, the composition of the horse EBC is similar to humans. Neuhaus et al.[Citation226] compared the metabolites composition of EBC and bronchoalveolar lavage fluid collected from asthma-affected mice. To determine the concentration of metabolites, multi-capillary column ion mobility MS (MCC/IMS) was utilized for noninvasive detection. Histamine, nitric oxide, and arachidonic acid were detected by MCC/IMS.
Conclusions
As conclusion of this subsection, metabolomic studies of animals revealed that there is a significant overlap between human metabolites and those of other animals. The present and future needs of healthcare systems are to employ low cost, easy to use, noninvasive and reliable techniques for disease screening and diagnosis, as well as determining the disease stage. Such techniques would assist clinicians in early detection, accurate diagnosis, successful treatment and follow-up of patients. Metabolomics in general and breathomics in particular provide an opportunity to develop such analytical methods. In this regard breathomics is emerging as a noninvasive and patient-friendly diagnostic tool. In this work, an update on sample collection, metabolomics analysis, advances in analytical techniques, common numerical analyses used in data analysis and some clinical applications were reviewed.
Author contributions
MK and AJ conceived the concept of the review and finalized the manuscript, MA conducted literature search and drafting chemical analysis section, ER conducted literature search and drafting EBC collection section, AAS conducted literature search and drafting numerical analysis section, JS conducted literature search and drafting animal studies section. All authors contributed in final manuscript preparation.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y. V.; Eeckhaut, A. V. Analytical Techniques for Metabolomic Studies: A Review. Bioanalysis 2019, 11, 2297–2318. DOI: 10.4155/bio-2019-0014.
- Parastar, H.; van Kollenburg, G.; Weesepoel, Y.; van den Doel, A.; Buydens, L.; Jansen, J. Integration of Handheld NIR and Machine Learning to “Measure & Monitor” Chicken Meat Authenticity. Food Control 2020, 112, 107149. DOI: 10.1016/j.foodcont.2020.107149.
- Španěl, P.; Smith, D. Quantification of Volatile Metabolites in Exhaled Breath by Selected Ion Flow Tube Mass Spectrometry, SIFT-MS. Clin. Mass Spectrom. 2020, 16, 18–24. DOI: 10.1016/j.clinms.2020.02.001.
- Zang, X.; Pérez, J. J.; Jones, C. M.; Monge, M. E.; McCarty, N. A.; Stecenko, A. A.; Fernández, F. M. Comparison of Ambient and Atmospheric Pressure Ion Sources for Cystic Fibrosis Exhaled Breath Condensate Ion Mobility-Mass Spectrometry Metabolomics. J Am Soc Mass Spectrom. 2017, 28, 1489–1496. DOI: 10.1007/s13361-017-1660-9.
- Martínez-Lozano, P.; Fernández de la Mora, J. Direct Analysis of Fatty Acid Vapors in Breath by Electrospray Ionization and Atmospheric Pressure Ionization-Mass Spectrometry. Anal. Chem. 2008, 80, 8210–8215. DOI: 10.1021/ac801185e.
- Hamidi, S.; Amini, M.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V.; Sate, H.; Jouyban, A. LC-MS/MS Estimation of Propranolol Level in Exhaled Breath Condensate. Pharm. Sci. 2017, 24, 264–270. DOI: 10.15171/PS.2017.39.
- Montuschi, P.; Martello, S.; Felli, M.; Mondino, C.; Chiarotti, M. Ion Trap Liquid Chromatography/Tandem Mass Spectrometry Analysis of Leukotriene B4 in Exhaled Breath Condensate. Rapid Commun Mass Spectrom. 2004, 18, 2723–2729. DOI: 10.1002/rcm.1682.
- Montuschi, P.; Martello, S.; Felli, M.; Mondino, C.; Barnes, P. J.; Chiarotti, M. Liquid Chromatography/Mass Spectrometry Analysis of Exhaled Leukotriene B4 in Asthmatic Children. Respir. Res. 2005, 6, 119. DOI: 10.1186/1465-9921-6-119.
- Hamidi, S.; Khoubnasabjafari, M.; Ansarin, K.; Jouyban-Gharamaleki, V.; Jouyban, A. Direct Analysis of Methadone in Exhaled Breath Condensate by Capillary Zone Electrophoresis. CPA. 2016, 12, 137–145. DOI: 10.2174/1573412911666150911202647.
- Lourenço, C.; Turner, C. Breath Analysis in Disease Diagnosis: Methodological Considerations and Applications. Metabolites 2014, 4, 465–498. DOI: 10.3390/metabo4020465.
- Kuo, T. C.; Tan, C. E.; Wang, S. Y.; Lin, O. A.; Su, B. H.; Hsu, M. T.; Lin, J.; Cheng, Y. Y.; Chen, C. S.; Yang, Y. C.; et al. Human Breathomics Database. Database (Oxford) 2020, 2020, baz139. DOI: 10.1093/database/baz139.
- Nobakht M Gh, B. F.; Aliannejad, R.; Rezaei-Tavirani, M.; Taheri, S.; Oskouie, A. A. The Metabolomics of Airway Diseases, Including COPD, Asthma and Cystic Fibrosis. Biomarkers 2015, 20, 5–16. DOI: 10.3109/1354750X.2014.983167.
- Maniscalco, M.; Fuschillo, S.; Paris, D.; Cutignano, A.; Sanduzzi, A.; Motta, A. Clinical Metabolomics of Exhaled Breath Condensate in Chronic Respiratory Diseases. Adv. Clin. Chem. 2019, 121–149. pp DOI: 10.1016/bs.acc.2018.10.002.
- Neerincx, A. H.; Vijverberg, S. J. H.; Bos, L. D. J.; Brinkman, P.; van der Schee, M. P.; de Vries, R.; Sterk, P. J.; Maitland-van der Zee, A.-H. Breathomics from Exhaled Volatile Organic Compounds in Pediatric Asthma. Pediatr. Pulmonol. 2017, 52, 1616–1627. DOI: 10.1002/ppul.23785.
- Finamore, P.; Scarlata, S.; Incalzi, R. A. Breath Analysis in Respiratory Diseases: State-of-the-Art and Future Perspectives. Expert Rev. Mol. Diagn. 2019, 19, 47–61. DOI: 10.1080/14737159.2019.1559052.
- Azim, A.; Barber, C.; Dennison, P.; Riley, J.; Howarth, P. Exhaled Volatile Organic Compounds in Adult Asthma: A Systematic Review. Eur. Respir. J. 2019, 2019, 1900056. DOI: 10.1183/13993003.00056-2019.
- Scheepers, P. T. J.; Cocker, J. Human Biomonitoring with or without Limits? Progress in the Analysis of Biomarkers of Xenobiotics and Some Opportunities for Improved Interpretation. Trends Anal. Chem. 2019, 113, 116–123. DOI: 10.1016/j.trac.2019.02.001.
- Rahimpour, E.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V.; Jouyban, A. Non-Volatile Compounds in Exhaled Breath Condensate: Review of Methodological Aspects. Anal. Bioanal. Chem. 2018, 410, 6411–6440. DOI: 10.1007/s00216-018-1259-4.
- Bruderer, T.; Gaisl, T.; Gaugg, M. T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath Focus Review. Chem. Rev. 2019, 119, 10803–10828. DOI: 10.1021/acs.chemrev.9b00005.
- Preti, G.; Labows, J. N.; Kostelc, J. G.; Aldinger, S.; Daniele, R. Analysis of Lung Air from Patients with Bronchogenic Carcinoma and Controls Using Gas Chromatography-Mass Spectrometry. J. Chromatogr. B 1988, 432, 1–11. DOI: 10.1016/S0378-4347(00)80627-1.
- Poli, D.; Goldoni, M.; Caglieri, A.; Ceresa, G.; Acampa, O.; Carbognani, P.; Rusca, M.; Corradi, M. Breath Analysis in Non Small Cell Lung Cancer Patients after Surgical Tumour Resection. Acta Biomed. 2008, 79, 64–72. DOI: PMID: 18924311.
- Basanta, M.; Koimtzis, T.; Singh, D.; Wilson, I.; Thomas, C. L. P. An Adaptive Breath Sampler for Use with Human Subjects with an Impaired Respiratory Function. Analyst 2007, 132, 153–163. DOI: 10.1039/B608608J.
- Cope, K. A.; Watson, M. T.; Foster, W. M.; Sehnert, S. S.; Risby, T. H. Effects of Ventilation on the Collection of Exhaled Breath in Humans. J. Appl. Physiol. 2004, 96, 1371–1379. DOI: 10.1152/japplphysiol.01034.2003.
- Lawal, O.; Ahmed, W. M.; Nijsen, T. M. E.; Goodacre, R.; Fowler, S. J. Exhaled Breath Analysis: A Review of 'breath-taking' methods for off-line analysis . Metabolomics 2017, 13, 110 DOI: 10.1007/s11306-017-1241-8.
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of Volatile Organic Compounds as Biomarkers of Lung Cancer by SPME-GC-TOF/MS and Chemometrics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3360–3366. DOI: 10.1016/j.jchromb.2011.09.001.
- Trefz, P.; Obermeier, J.; Lehbrink, R.; Schubert, J. K.; Miekisch, W.; Fischer, D.-C. Exhaled Volatile Substances in Children Suffering from Type 1 Diabetes Mellitus: Results from a Cross-Sectional Study. Sci. Rep. 2019, 9, 15707. DOI: 10.1038/s41598-019-52165-x.
- Davis, M. D.; Fowler, S. J.; Montpetit, A. J. Exhaled Breath Testing - A tool for the clinician and researcher. Paediatr. Respir. Rev. 2019, 29, 37–41. DOI: 10.1016/j.prrv.2018.05.002.
- Frey, U.; Merkus, P. J. F. M., Eds. Paediatric Lung Function; European Respiratory Society Journals Ltd, 2010. DOI: 10.1183/1025448x.erm4710.
- Jouyban, A.; Khoubnasabjafari, M.; Ansarin, K.; Jouyban-Gharamaleki, V. Breath Sampling Setup 2013, Iranian patent 81363.
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C. S.; Pandya, H. C.; Thomas, C. P. Metabolomics Pilot Study to Identify Volatile Organic Compound Markers of Childhood Asthma in Exhaled Breath. Bioanalysis 2013, 5, 2239–2247. DOI: 10.4155/bio.13.184.
- Khoubnasabjafari, M.; Kezeminasab, S.; Emamalizadeh, B.; Jouyban, A. Exhaled Breath Condensate: A Non-Invasive Source for Tracking of Genetic and Epigenetic Alterations in Lung Diseases. Pharm. Sci. DOI: 10.34172/PS.2020.46.
- Kharitonov, S. A.; Barnes, P. J. Exhaled Markers of Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1693–1722. DOI: 10.1164/ajrccm.163.7.2009041.
- Horváth, I.; Hunt, J.; Barnes, P. J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W. J. C.; Corradi, M.; Dekhuijzen, R.; et al.; ATS/ERS Task Force on Exhaled Breath Condensate. Exhaled Breath Condensate: Methodological Recommendations and Unresolved Questions. Eur. Respir. J. 2005, 26, 523–548. DOI: 10.1183/09031936.05.00029705.
- Vass, G.; Huszár, E.; Barát, E.; Valyon, M.; Kiss, D.; Pénzes, I.; Augusztinovicz, M.; Horváth, I. Horváth, I. Comparison of Nasal and Oral Inhalation during Exhaled Breath Condensate Collection. Am. J. Respir. Crit. Care. Med. 2003, 167, 850–855. DOI: 10.1164/rccm.200207-716BC.
- Zakrzewski, J. T.; Barnes, N. C.; Costello, J. F.; Piper, P. J. Lipid Mediators in Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. Am. Rev. Respir. Dis. 1987, 136, 779–782. DOI: 10.1164/ajrccm/136.3.779.
- Montuschi, P.; Barnes, P. J. Analysis of Exhaled Breath Condensate for Monitoring Airway Inflammation. Trends Pharmacol. Sci. 2002, 23, 232–237. DOI: 10.1016/S0165-6147(02)02020-5.
- Goldoni, M.; Caglieri, A.; Andreoli, R.; Poli, D.; Manini, P.; Vettori, M. V.; Corradi, M.; Mutti, A. Influence of Condensation Temperature on Selected Exhaled Breath Parameters. BMC Pulm. Med. 2005, 5, 10 DOI: 10.1186/1471-2466-5-10.
- Czebe, K.; Barta, I.; Antus, B.; Valyon, M.; Horváth, I.; Kullmann, T. Influence of Condensing Equipment and Temperature on Exhaled Breath Condensate PH, Total Protein and Leukotriene Concentrations. Respir. Med. 2008, 102, 720–725. DOI: 10.1016/j.rmed.2007.12.013.
- Latzin, P.; Griese, M. Exhaled Hydrogen Peroxide, Nitrite and Nitric Oxide in Healthy Children: Decrease of Hydrogen Peroxide by Atmospheric Nitric Oxide. Eur. J. Med. Res. 2002, 7, 353–358.
- Ahmadzai, H.; Huang, S.; Hettiarachchi, R.; Lin, J.-L.; Thomas, P. S.; Zhang, Q. Exhaled Breath Condensate: A Comprehensive Update. Clin. Chem. Lab. Med. 2013, 51, 1343–1361. DOI: 10.1515/cclm-2012-0593.
- Tufvesson, E.; Bjermer, L. Methodological Improvements for Measuring Eicosanoids and Cytokines in Exhaled Breath Condensate. Respir. Med. 2006, 100, 34–38. DOI: 10.1016/j.rmed.2005.04.007.
- Corradi, M.; Goldoni, M.; Caglieri, A.; Folesani, G.; Poli, D.; Corti, M.; Mutti, A. Collecting Exhaled Breath Condensate (EBC) with Two Condensers in Series: A Promising Technique for Studying the Mechanisms of EBC Formation, and the Volatility of Selected Biomarkers. J. Aerosol Med. 2008, 21, 35–44. DOI: 10.1089/jam.2007.0644.
- Effros, R. M.; Biller, J.; Foss, B.; Hoagland, K.; Dunning, M. B.; Castillo, D.; Bosbous, M.; Sun, F.; Shaker, R. A Simple Method for Estimating Respiratory Solute Dilution in Exhaled Breath Condensates. Am. J. Respir. Crit. Care Med. 2003, 168, 1500–1505. DOI: 10.1164/rccm.200307-920OC.
- Dwyer, T. M. Sampling Airway Surface Liquid: non-volatiles in the exhaled breath condensate . Lung 2004, 182, 241–250. DOI: 10.1007/s00408-004-2506-3.
- Effros, R. M.; Hoagland, K. W.; Bosbous, M.; Castillo, D.; Foss, B.; Dunning, M.; Gare, M.; Lin, W.; Feng, S. Dilution of Respiratory Solutes in Exhaled Condensates. Am. J. Respir. Crit. Care. Med. 2002, 165, 663–669. DOI: 10.1164/ajrccm.165.5.2101018.
- Reinhold, P.; Knobloch, H. Exhaled Breath Condensate: Lessons Learned from Veterinary Medicine. J Breath Res . 2010, 4, 017001. DOI: 10.1088/1752-7155/4/1/017001.
- Reinhold, P.; Jaeger, J.; Schroeder, C. Evaluation of Methodological and Biological Influences on the Collection and Composition of Exhaled Breath Condensate. Biomarkers 2006, 11, 118–142. DOI: 10.1080/13547500600572764.
- Rosias, P. Methodological Aspects of Exhaled Breath Condensate Collection and Analysis. J. Breath Res. 2012, 6, 027102. DOI: 10.1088/1752-7155/6/2/027102.
- Scheuch, G.; Siekmeier, R. Novel Approaches to Enhance Pulmonary Delivery of Proteins and Peptides. J. Physiol. Pharmacol. 2007, 58, 615–625.
- Hunt, J. Exhaled Breath Condensate: An Overview. Immunol. Allergy Clin. North Am. 2007, 27, 587–596. DOI: 10.1016/j.iac.2007.09.001.
- Greenwald, R.; Fitzpatrick, A. M.; Gaston, B.; Marozkina, N. V.; Erzurum, S.; Teague, W. G. Breath Formate is a Marker of Airway S-Nitrosothiol Depletion in Severe Asthma. PLoS One 2010, 5, e11919. DOI: 10.1371/journal.pone.0011919.
- Bertini, I.; Luchinat, C.; Miniati, M.; Monti, S.; Tenori, L. Phenotyping COPD by 1H NMR Metabolomics of Exhaled Breath Condensate. Metabolomics 2014, 10, 302–311. DOI: 10.1007/s11306-013-0572-3.
- Motta, A.; Paris, D.; D'Amato, M.; Melck, D.; Calabrese, C.; Vitale, C.; Stanziola, A. A.; Corso, G.; Sofia, M.; Maniscalco, M. NMR Metabolomic Analysis of Exhaled Breath Condensate of Asthmatic Patients at Two Different Temperatures. J. Proteome Res. 2014, 13, 6107–6120. DOI: 10.1021/pr5010407.
- Maniscalco, M.; Paris, D.; Melck, D. J.; Molino, A.; Carone, M.; Ruggeri, P.; Caramori, G.; Motta, A. Differential Diagnosis between Newly Diagnosed Asthma and COPD Using Exhaled Breath Condensate Metabolomics: A Pilot Study. Eur. Respir. J. 2018, 51, 1701825. DOI: 10.1183/13993003.01825-2017.
- Maniscalco, M.; Paris, D.; Melck, D.; Chiariello, N.; Di Napoli, F.; Manno, M.; Iavicoli, I.; Motta, A. Biomonitoring of Workers Using Nuclear Magnetic Resonance-Based Metabolomics of Exhaled Breath Condensate: A Pilot Study. Toxicol. Lett. 2018, 298, 4–12. DOI: 10.1016/j.toxlet.2018.10.018.
- D’Amato, M.; Paris, D.; Molino, A.; Cuomo, P.; Fulgione, A.; Sorrentino, N.; Palomba, L.; Maniscalco, M.; Motta, A. The Immune-Modulator Pidotimod Affects the Metabolic Profile of Exhaled Breath Condensate in Bronchiectatic Patients: A Metabolomics Pilot Study. Front. Pharmacol. 2019, 10, 1–14. DOI: 10.3389/fphar.2019.01115.
- Fernández-Peralbo, M. A.; Calderón Santiago, M.; Priego-Capote, F.; Luque De Castro, M. D. Study of Exhaled Breath Condensate Sample Preparation for Metabolomics Analysis by LC-MS/MS in High Resolution Mode. Talanta 2015, 144, 1360–1369. DOI: 10.1016/j.talanta.2015.08.010.
- Ladva, C. N.; Golan, R.; Greenwald, R.; Yu, T.; Sarnat, S. E.; Flanders, W. D.; Uppal, K.; Walker, D. I.; Tran, V.; Liang, D.; et al. Metabolomic Profiles of Plasma, Exhaled Breath Condensate, and Saliva Are Correlated with Potential for Air Toxics Detection. J. Breath Res. 2017, 12, 016008. DOI: 10.1088/1752-7163/aa863c.
- Zang, X.; Monge, M. E.; McCarty, N. A.; Stecenko, A. A.; Fernández, F. M. Feasibility of Early Detection of Cystic Fibrosis Acute Pulmonary Exacerbations by Exhaled Breath Condensate Metabolomics: A Pilot Study. J. Proteome Res. 2017, 16, 550–558. DOI: 10.1021/acs.jproteome.6b00675.
- Peralbo-Molina, A.; Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque de Castro, M. D. Development of a Method for Metabolomic Analysis of Human Exhaled Breath Condensate by Gas Chromatography-Mass Spectrometry in High Resolution Mode. Anal. Chim. Acta 2015, 887, 118–126. DOI: 10.1016/j.aca.2015.07.008.
- Peralbo-Molina, A.; Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque De Castro, M. D. Metabolomics Analysis of Exhaled Breath Condensate for Discrimination between Lung Cancer Patients and Risk Factor Individuals. J. Breath Res. 2016, 10, 016011. DOI: 10.1088/1752-7155/10/1/016011.
- Peralbo-Molina, A.; Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque De Castro, M. D. Identification of Metabolomics Panels for Potential Lung Cancer Screening by Analysis of Exhaled Breath Condensate. J. Breath Res. 2016, 10, 026002. DOI: 10.1088/1752-7155/10/2/026002.
- Konieczna, L.; Pyszka, M.; Okońska, M.; Niedźwiecki, M.; Bączek, T. Bioanalysis of Underivatized Amino Acids in Non-Invasive Exhaled Breath Condensate Samples Using Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1542, 72–81. DOI: 10.1016/j.chroma.2018.02.019.
- Arshad, A. Z.; Munajat, Y.; Ghoshal, S. K.; Jamal, R.; Johdi, N. A.; Ibrahim, R. K. R.; Zulkhairi, H. Volatolomics Combined Terahertz Time Domain Spectral Analyses of Colon Cancer in Vitro. J. Teknol. 2019, 81, 105–112. DOI: 10.11113/jt.v81.13310.
- de Vries, R.; Muller, M.; van der Noort, V.; Theelen, W. S. M. E.; Schouten, R. D.; Hummelink, K.; Muller, S. H.; Wolf-Lansdorf, M.; Dagelet, J. W. F.; Monkhorst, K.; et al. Prediction of Response to Anti-PD-1 Therapy in Patients with Non-Small-Cell Lung Cancer by Electronic Nose Analysis of Exhaled Breath. Ann. Oncol. 2019, 30, 1660–1666. 10.1093/annonc/mdz279.
- De Vries, R.; Dagelet, Y. W. F.; Spoor, P.; Snoey, E.; Jak, P. M. C.; Brinkman, P.; Dijkers, E.; Bootsma, S. K.; Elskamp, F.; de Jongh, F. H. C.; et al. Clinical and Inflammatory Phenotyping by Breathomics in Chronic Airway Diseases Irrespective of the Diagnostic Label. Eur. Respir. J. 2018, 51, 1701817. DOI: 10.1183/13993003.01817-2017.
- Jaeschke, C.; Padilla, M.; Turppa, E.; Polaka, I.; Gonzalez, O.; Richardson, K.; Pajukanta, J.; Kortelainen, J. M.; Shani, G.; Shuster, G.; et al. Overview on SNIFFPHONE: A Portable Device for Disease Diagnosis. Presented at the ISOEN 2019 - 18th International Symposium on Olfaction and Electronic Nose, Fukuoka, Japan, 2019. DOI: 10.1109/ISOEN.2019.8823212.
- Nakhleh, M. K.; Broza, Y. Y.; Haick, H. Monolayer-Capped Gold Nanoparticles for Disease Detection from Breath. Nanomedicine (London) 2014, 9, 1991–2002. DOI: 10.2217/nnm.14.121.
- Tang, H.; Sacco, L. N.; Vollebregt, S.; Ye, H.; Fan, X.; Zhang, G. Recent Advances in 2D/Nanostructured Metal Sulfide-Based Gas Sensors: Mechanisms, Applications, and Perspectives. J. Mater. Chem. A 2020, 8, 24943–24976. DOI: 10.1039/D0TA08190F.
- Scarlata, S.; Finamore, P.; Meszaros, M.; Dragonieri, S.; Bikov, A. The Role of Electronic Noses in Phenotyping Patients with Chronic Obstructive Pulmonary Disease. Biosensors 2020, 10, 171. DOI: 10.3390/bios10110171.
- Mirzaei, H.; O'Brien, A.; Tasnim, N.; Ravishankara, A.; Tahmooressi, H.; Hoorfar, M. Topical Review on Monitoring Tetrahydrocannabinol in Breath. J. Breath Res. 2020, 14, 034002. DOI: 10.1088/1752-7163/ab6229.
- Lawaetz, A. J.; Bro, R.; Kamstrup-Nielsen, M.; Christensen, I. J.; Jørgensen, L. N.; Nielsen, H. J. Fluorescence Spectroscopy as a Potential Metabonomic Tool for Early Detection of Colorectal Cancer. Metabolomics 2012, 8, 111–121. DOI: 10.1007/s11306-011-0310-7.
- Rajasekaran, R.; Aruna, P. R.; Koteeswaran, D.; Padmanabhan, L.; Muthuvelu, K.; Rai, R. R.; Thamilkumar, P.; Murali Krishna, C.; Ganesan, S. Characterization and Diagnosis of Cancer by Native Fluorescence Spectroscopy of Human Urine. Photochem. Photobiol. 2013, 89, 483–491. DOI: 10.1111/j.1751-1097.2012.01239.x.
- Hasanzadeh, M.; Mokhtari, F.; Shadjou, N.; Eftekhari, A.; Mokhtarzadeh, A.; Jouyban-Gharamaleki, V.; Mahboob, S. Poly Arginine-Graphene Quantum Dots as a Biocompatible and Non-Toxic Nanocomposite: Layer-by-Layer Electrochemical Preparation, Characterization and Non-Invasive Malondialdehyde Sensory Application in Exhaled Breath Condensate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 247–258. DOI: 10.1016/j.msec.2017.02.025.
- Lačná, J.; Foret, F.; Kubáň, P. Sensitive Determination of Malondialdehyde in Exhaled Breath Condensate and Biological Fluids by Capillary Electrophoresis with Laser Induced Fluorescence Detection. Talanta 2017, 169, 85–90. DOI: 10.1016/j.talanta.2017.03.061.
- Wang, T.; Luo, D.; Chen, Z.; Qu, Y.; Ma, X.; Ye, J.; Chu, Q.; Huang, D. Sensitive Determination of Aldehyde Metabolites in Exhaled Breath Condensate Using Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Anal. Bioanal. Chem. 2018, 410, 7203–7210. DOI: 10.1007/s00216-018-1327-9.
- Lačná, J.; Ďurč, P.; Greguš, M.; Skřičková, J.; Doubková, M.; Pokojová, E.; Kindlová, D.; Dolina, J.; Konečný.; Foret, F. Capillary Electrophoretic Analysis of Ionic Content in Exhaled Breath Condensate and PH Monitoring as a Non-Invasive Method in Gastroesophageal Reflux Disease Diagnostics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1134–1135, 121857. DOI: 10.1016/j.jchromb.2019.121857.
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in Metabolomics-A Review in Human Disease Diagnosis. Anal. Chim. Acta 2010, 659, 23–33. DOI: 10.1016/j.aca.2009.11.042.
- Liu, X.; Locasale, J. W. Metabolomics: A Primer. Trends Biochem. Sci. 2017, 42, 274–284. DOI: 10.1016/j.tibs.2017.01.004.
- Meier, R.; Ruttkies, C.; Treutler, H.; Neumann, S. Bioinformatics Can Boost Metabolomics Research. J. Biotechnol. 2017, 261, 137–141. DOI: 10.1016/j.jbiotec.2017.05.018.
- Fiehn, O.; Robertson, D.; Griffin, J.; van der Werf, M.; Nikolau, B.; Morrison, N.; Sumner, L. W.; Goodacre, R.; Hardy, N. W.; Taylor, C.; et al. The Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 175–178. DOI: 10.1007/s11306-007-0070-6.
- Haug, K.; Salek, R. M.; Conesa, P.; Hastings, J.; De Matos, P.; Rijnbeek, M.; Mahendraker, T.; Williams, M.; Neumann, S.; Rocca-Serra, P.; et al. MetaboLights - An Open-Access General-Purpose Repository for Metabolomics Studies and Associated Meta-Data. Nucleic Acids Res. 2013, 41, D781–786. DOI: 10.1093/nar/gks1004.
- Saccenti, E.; Hoefsloot, H. C. J.; Smilde, A. K.; Westerhuis, J. A.; Hendriks, M. M. W. B. Reflections on Univariate and Multivariate Analysis of Metabolomics Data. Metabolomics 2014, 10, 361–374. DOI: 10.1007/s11306-013-0598-6.
- Alonso, A.; Marsal, S.; Julià, A. Analytical Methods in Untargeted Metabolomics: State of the Art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. DOI: 10.3389/fbioe.2015.00023.
- Liland, K. H. Multivariate Methods in Metabolomics - From Pre-Processing to Dimension Reduction and Statistical Analysis. Trends in Analytical Chemistry 2011, 30, 827–841. DOI: 10.1016/j.trac.2011.02.007.
- Boots, A. W.; Bos, L. D.; van der Schee, M. P.; van Schooten, F. J.; Sterk, P. J. Exhaled Molecular Fingerprinting in Diagnosis and Monitoring: Validating Volatile Promises. Trends Mol. Med. 2015, 21, 633–644. DOI: 10.1016/j.molmed.2015.08.001.
- Bennett, L.; Ciaffoni, L.; Denzer, W.; Hancock, G.; Lunn, A. D.; Peverall, R.; Praun, S.; Ritchie, G. A. D. A Chemometric Study on Human Breath Mass Spectra for Biomarker Identification in Cystic Fibrosis. J. Breath Res. 2009, 3, 046002. DOI: 10.1088/1752-7155/3/4/046002.
- Härdle, W. K.; Hlávka, Z. Canonical correlation analysis. In Multivariate Statistics Springer: Berlin, 2015. DOI: 10.1007/978-3-642-36005-3_16.
- Wilks, D. S. Canonical Correlation Analysis (CCA); Elsevier, 2011. DOI: 10.1016/B978-0-12-385022-5.00013-0.
- Smolinska, A.; Tedjo, D. I.; Blanchet, L.; Bodelier, A.; Pierik, M. J.; Masclee, A. A. M.; Dallinga, J.; Savelkoul, P. H. M.; Jonkers, D. M. A. E.; Penders, J.; et al. Volatile Metabolites in Breath Strongly Correlate with Gut Microbiome in CD Patients. Anal. Chim. Acta 2018, 1025, 1–11. DOI: 10.1016/j.aca.2018.03.046.
- Fens, N.; Van Rossum, A. G. J.; Zanen, P.; Van Ginneken, B.; Van Klaveren, R. J.; Zwinderman, A. H.; Sterk, P. J. Subphenotypes of Mild-to-Moderate COPD by Factor and Cluster Analysis of Pulmonary Function, CT Imaging and Breathomics in a Population-Based Survey. COPD J. Chronic Obstr. Pulm. Dis. 2013. 10, 277–285. DOI: DOI: 10.3109/15412555.2012.744388.
- Weatherall, M.; Shirtcliffe, P.; Travers, J.; Beasley, R. Use of Cluster Analysis to Define COPD Phenotypes. Eur. Respir. J. 2010, 36, 472–474. DOI: 10.1183/09031900035210.
- Blekherman, G.; Laubenbacher, R.; Cortes, D. F.; Mendes, P.; Torti, F. M.; Akman, S.; Torti, S. V.; Shulaev, V. Bioinformatics Tools for Cancer Metabolomics. Metabolomics 2011, 7, 329–343. DOI: 10.1007/s11306-010-0270-3.
- Montuschi, P.; Santini, G.; Mores, N.; Vignoli, A.; Macagno, F.; Shoreh, R.; Tenori, L.; Zini, G.; Fuso, L.; Mondino, C.; et al. Breathomics for Assessing the Effects of Treatment and Withdrawal with Inhaled Beclomethasone/Formoterol in Patients with COPD. Front. Pharmacol. 2018, 9, 258. DOI: 10.3389/fphar.2018.00258.
- Smolinska, A.; Klaassen, E. M. M.; Dallinga, J. W.; Van De Kant, K. D. G.; Jobsis, Q.; Moonen, E. J. C.; Van Schayck, O. C. P.; Dompeling, E.; Van Schooten, F. J. Profiling of Volatile Organic Compounds in Exhaled Breath as a Strategy to Find Early Predictive Signatures of Asthma in Children. PLoS One 2014, 9, e95668. DOI: 10.1371/journal.pone.0095668.
- Saei, A. A.; Beusch, C. M.; Chernobrovkin, A.; Sabatier, P.; Zhang, B.; Tokat, Ü. G.; Stergiou, E.; Gaetani, M.; Végvári, Á.; Zubarev, R. A. ProTargetMiner as a Proteome Signature Library of Anticancer Molecules for Functional Discovery. Nat. Commun. 2019, 10, 5715. DOI: 10.1038/s41467-019-13582-8.
- Triba, M. N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D. N.; Savarin, P. PLS/OPLS Models in Metabolomics: The Impact of Permutation of Dataset Rows on the K-Fold Cross-Validation Quality Parameters. Mol. Biosyst. 2015, 11, 13–19. DOI: 10.1039/C4MB00414K.
- Daniel, D.; Thangavel, K. Breathomics for Gastric Cancer Classification Using Back-Propagation Neural Network. J. Med. Signals Sens. 2016, 6, 172–182. DOI: 10.4103/2228-7477.186879.
- Vanden Branden, K.; Hubert, M. Robust Classification in High Dimensions Based on the SIMCA Method. Chemom. Intell. Lab. Syst. 2005, 79, 10–21. DOI: 10.1016/j.chemolab.2005.03.002.
- Arasaradnam, R. P.; McFarlane, M.; Ling, K.; Wurie, S.; O'Connell, N.; Nwokolo, C. U.; Bardhan, K. D.; Skinner, J.; Savage, R. S.; Covington, J. A. Breathomics - Exhaled Volatile Organic Compound Analysis to Detect Hepatic Encephalopathy: A Pilot Study. J. Breath Res. 2016, 10, 016012. DOI: 10.1088/1752-7155/10/1/016012.
- Broadhurst, D. I.; Kell, D. B. Statistical Strategies for Avoiding False Discoveries in Metabolomics and Related Experiments. Metabolomics 2007, 2, 171–196. DOI: 10.1007/s11306-006-0037-z.
- Gardinassi, L. G.; Xia, J.; Safo, S. E.; Li, S. Bioinformatics Tools for the Interpretation of Metabolomics Data. Curr. Pharmacol. Rep. 2017, 3, 374–383. DOI: 10.1007/s40495-017-0107-0.
- Sugimoto, M.; Kawakami, M.; Robert, M.; Soga, T.; Tomita, M. Bioinformatics Tools for Mass Spectroscopy-Based Metabolomic Data Processing and Analysis. Curr. Bioinform. 2012, 7, 96–108. DOI: 10.2174/157489312799304431.
- Szymańska, E.; Davies, A. N.; Buydens, L. M. C. Chemometrics for Ion Mobility Spectrometry Data: Recent Advances and Future Prospects. Analyst 2016, 141, 5689–5708. DOI: 10.1039/C6AN01008C.
- Smith, C. A.; Maille, G. O. ?; Want, E. J.; Qin, C.; Trauger, S. A.; Brandon, T. R.; Custodio, D. E.; Abagyan, R.; Siuzdak, G. METLIN: A Metabolite Mass Spectral Database. Ther. Drug Monit. 2005, 27, 747–751. DOI: 10.1097/01.ftd.0000179845.53213.39.
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A Public Repository for Sharing Mass Spectral Data for Life Sciences. J. Mass Spectrom. 2010, 45, 703–714. DOI: 10.1002/jms.1777.
- Alkhalifah, Y.; Phillips, I.; Soltoggio, A.; Darnley, K.; Nailon, W. H.; McLaren, D.; Eddleston, M.; Thomas, C. L. P.; Salman, D. VOCCluster: Untargeted Metabolomics Feature Clustering Approach for Clinical Breath Gas Chromatography/Mass Spectrometry Data. Anal. Chem. 2020, 92, 2937–2945. DOI: 10.1021/acs.analchem.9b03084.
- Wheelock, C. E.; Goss, V. M.; Balgoma, D.; Nicholas, B.; Brandsma, J.; Skipp, P. J.; Snowden, S.; Burg, D.; D'Amico, A.; Horvath, I.; et al.; U-BIOPRED Study Group. Application of ’Omics Technologies to Biomarker Discovery in Inflammatory Lung Diseases. Eur. Respir. J. 2013, 42, 802–825. DOI: 10.1183/09031936.00078812.
- Leopold, J. H.; Bos, L. D. J.; Sterk, P. J.; Schultz, M. J.; Fens, N.; Horvath, I.; Bikov, A.; Montuschi, P.; Di Natale, C.; Yates, D. H.; et al. Comparison of Classification Methods in Breath Analysis by Electronic Nose. J. Breath Res. 2015, 9, 046002. DOI: 10.1088/1752-7155/9/4/046002.
- Miekisch, W.; Herbig, J.; Schubert, J. K. Data Interpretation in Breath Biomarker Research: Pitfalls and Directions. J. Breath Res. 2012, 6, 036007. DOI: 10.1088/1752-7155/6/3/036007.
- Agusti, A. The Path to Personalised Medicine in Copd. Thorax 2014, 69, 857–864. DOI: 10.1136/thoraxjnl-2014-205507.
- Maniscalco, M.; Motta, A. Metabolomics of Exhaled Breath Condensate: A Means for Phenotyping Respiratory Diseases? Biomark. Med. 2017, 11, 405–407. DOI: 10.2217/bmm-2017-0068.
- Kazeminasab, S.; Emamalizadeh, B.; Jouyban, A.; Shoja, M. M.; Khoubnasabjafari, M. Macromolecular Biomarkers of Chronic Obstructive Pulmonary Disease in Exhaled Breath Condensate. Biomark. Med. 2020, 14, 1047–1063. DOI: 10.2217/bmm-2020-0121.
- Kononikhin, A. S.; Starodubtseva, N. L.; Chagovets, V.; V; Ryndin, A. Y.; Burov, A. A.; Popov, I. A.; Bugrova, A. E.; Dautov, R. A.; Tokareva, A. O.; Podurovskaya, Y. L.; et al. Exhaled Breath Condensate Analysis from Intubated Newborns by Nano-HPLC Coupled to High Resolution MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1047, 97–105. DOI: 10.1016/j.jchromb.2016.12.036.
- Antypkin, Y. G.; Chumachenko, N. G. Amino Acid Composition of Blood Serum and Exhaled Breath Condensate in Children with Bronchial Asthma. Perinatol. I Pediatr. 2017, 4, 99–105. DOI: 10.15574/pp.2017.72.99.
- Cruickshank-Quinn, C.; Armstrong, M.; Powell, R.; Gomez, J.; Elie, M.; Reisdorph, N. Determining the Presence of Asthma-Related Molecules and Salivary Contamination in Exhaled Breath Condensate. Respir. Res. 2017, 18, 57. DOI: 10.1186/s12931-017-0538-5.
- Bos, L. D. J.; Weda, H.; Wang, Y.; Knobel, H. H.; Nijsen, T. M. E.; Vink, T. J.; Zwinderman, A. H.; Sterk, P. J.; Schultz, M. J. Exhaled Breath Metabolomics as a Noninvasive Diagnostic Tool for Acute Respiratory Distress Syndrome. Eur. Respir. J. 2014, 44, 188–197. DOI: 10.1183/09031936.00005614.
- Carraro, S.; Rezzi, S.; Reniero, F.; Héberger, K.; Giordano, G.; Zanconato, S.; Guillou, C.; Baraldi, E. Metabolomics Applied to Exhaled Breath Condensate in Childhood Asthma. Am. J. Respir. Crit. Care Med. 2007, 175, 986–990. DOI: 10.1164/rccm.200606-769OC.
- Čáp, P.; Chládek, J.; Pehal, F.; Malý, M.; Petrů, V.; Barnes, P. J.; Montuschi, P. Gas Chromatography/Mass Spectrometry Analysis of Exhaled Leukotrienes in Asthmatic Patients. Thorax 2004, 59, 465–470. DOI: 10.1136/thx.2003.011866.
- Dallinga, J. W.; Robroeks, C. M. H. H.; T.; Van Berkel, J. J. B. N.; Moonen, E. J. C.; Godschalk, R. W. L.; Jöbsis, Q.; Dompeling, E.; Wouters, E. F. M.; Van Schooten, F. J. Volatile Organic Compounds in Exhaled Breath as a Diagnostic Tool for Asthma in Children. Clin. Exp. Allergy 2010, 40, 68–76. DOI: 10.1111/j.1365-2222.2009.03343.x.
- De Laurentiis, G.; Paris, D.; Melck, D.; Montuschi, P.; Maniscalco, M.; Bianco, A.; Sofia, M.; Motta, A. Separating Smoking-Related Diseases Using NMR-Based Metabolomics of Exhaled Breath Condensate. J. Proteome Res. 2013, 12, 1502–1511. DOI: 10.1021/pr301171p.
- Zang, X.; Monge, M. E.; Gaul, D. A.; McCarty, N. A.; Stecenko, A.; Fernández, F. M. Early Detection of Cystic Fibrosis Acute Pulmonary Exacerbations by Exhaled Breath Condensate Metabolomics. J. Proteome Res. 2020, 19, 144–152. DOI: 10.1021/acs.jproteome.9b00443.
- Robroeks, C. M. H. H. T.; Van Berkel, J. J. B. N.; Dallinga, J. W.; Jöbsis, Q.; Zimmermann, L. J. I.; Hendriks, H. J. E.; Wouters, M. F. M.; Van Der Grinten, C. P. M.; Van De Kant, K. D. G.; Van Schooten, F. J.; et al. Metabolomics of Volatile Organic Compounds in Cystic Fibrosis Patients and Controls. Pediatr. Res. 2010, 68, 75–80. DOI: 10.1203/PDR.0b013e3181df4ea0.
- Montuschi, P.; Paris, D.; Melck, D.; Lucidi, V.; Ciabattoni, G.; Raia, V.; Calabrese, C.; Bush, A.; Barnes, P. J.; Motta, A. NMR Spectroscopy Metabolomic Profiling of Exhaled Breath Condensate in Patients with Stable and Unstable Cystic Fibrosis. Thorax 2012, 67, 222–228. DOI: 10.1136/thoraxjnl-2011-200072.
- Gordon, S. M.; Szidon, J. P.; Krotoszynski, B. K.; Gibbons, R. D.; O'Neill, H. J. Volatile Organic Compounds in Exhaled Air from Patients with Lung Cancer. Clin. Chem. 1985, 31, 1278–1282. DOI: 10.1093/clinchem/31.8.1278.
- O'Neill, H. J.; Gordon, S. M.; O'Neill, M. H.; Gibbons, R. D.; Szidon, J. P. A Computerized Classification Technique for Screening for the Presence of Breath Biomarkers in Lung Cancer. Clin. Chem. 1988, 34, 1613–1618. DOI: 10.1093/clinchem/34.8.1613.
- Phillips, M.; Gleeson, K.; Hughes, J. M. B.; Greenberg, J.; Cataneo, R. N.; Baker, L.; McVay, W. P. Volatile Organic Compounds in Breath as Markers of Lung Cancer: A Cross-Sectional Study. Lancet 1999, 353, 1930–1933. DOI: 10.1016/S0140-6736(98)07552-7.
- Barash, O.; Peled, N.; Hirsch, F. R.; Haick, H. Sniffing the Unique “Odor Print” of Non-Small-Cell Lung Cancer with Gold Nanoparticles. Small 2009, 5, 2618–2624. DOI: 10.1002/smll.200900937.
- Song, G.; Qin, T.; Liu, H.; Xu, G. B.; Pan, Y. Y.; Xiong, F. X.; Gu, K. S.; Sun, G. P.; Chen, Z. D. Quantitative Breath Analysis of Volatile Organic Compounds of Lung Cancer Patients. Lung Cancer 2010, 67, 227–231. DOI: 10.1016/j.lungcan.2009.03.029.
- Broza, Y. Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L. A.; Haick, H. A Nanomaterial-Based Breath Test for Short-Term Follow-up after Lung Tumor Resection. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 15–21. DOI: 10.1016/j.nano.2012.07.009.
- Bajtarevic, A.; Ager, C.; Pienz, M.; Klieber, M.; Schwarz, K.; Ligor, M.; Ligor, T.; Filipiak, W.; Denz, H.; Fiegl, M.; et al. ;. Noninvasive Detection of Lung Cancer by Analysis of Exhaled Breath. BMC Cancer 2009, 9, 348. DOI: 10.1186/1471-2407-9-348.
- Bousamra, M.; Schumer, E.; Li, M.; Knipp, R. J.; Nantz, M. H.; Van Berkel, V.; Fu, X. A. Quantitative Analysis of Exhaled Carbonyl Compounds Distinguishes Benign from Malignant Pulmonary Disease. J. Thorac. Cardiovasc. Surg. 2014, 148, 1074–1080. DOI: 10.1016/j.jtcvs.2014.06.006.
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of Volatile Lung Cancer Markers by Gas Chromatography-Mass Spectrometry: Comparison with Discrimination by Canines. Anal. Bioanal. Chem. 2012, 404, 141–146. DOI: 10.1007/s00216-012-6102-8.
- Chen, X.; Cao, M.; Li, Y.; Hu, W.; Wang, P.; Ying, K.; Pan, H. A Study of an Electronic Nose for Detection of Lung Cancer Based on a Virtual SAW Gas Sensors Array and Imaging Recognition Method. Meas. Sci. Technol. 2005, 16, 1535–1546. DOI: 10.1088/0957-0233/16/8/001.
- Corradi, M.; Poli, D.; Banda, I.; Bonini, S.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Andreoli, R.; Ampollini, L.; Casalini, A.; et al. Exhaled Breath Analysis in Suspected Cases of Non-Small-Cell Lung Cancer: A Cross-Sectional Study. J. Breath Res. 2015, 9, 027101. DOI: 10.1088/1752-7155/9/2/027101.
- Crohns, M.; Saarelainen, S.; Laitinen, J.; Peltonen, K.; Alho, H.; Kellokumpu-Lehtinen, P. Exhaled Pentane as a Possible Marker for Survival and Lipid Peroxidation during Radiotherapy for Lung Cancera Pilot Study. Free Radic. Res. 2009, 43, 965–974. DOI: 10.1080/10715760903159162.
- Deng, C.; Zhang, X.; Li, N. Investigation of Volatile Biomarkers in Lung Cancer Blood Using Solid-Phase Microextraction and Capillary Gas Chromatography-Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 808, 269–277. DOI: 10.1016/j.jchromb.2004.05.015.
- Feinberg, T.; Alkoby-Meshulam, L.; Herbig, J.; Cancilla, J. C.; Torrecilla, J. S.; Gai Mor, N.; Bar, J.; Ilouze, M.; Haick, H.; Peled, N. Peled, N. Cancerous Glucose Metabolism in Lung Cancer - Evidence from Exhaled Breath Analysis. J. Breath Res. 2016, 10, 026012. DOI: 10.1088/1752-7155/10/2/026012.
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative Analyses of Volatile Organic Compounds (VOCs) from Patients, Tumors and Transformed Cell Lines for the Validation of Lung Cancer-Derived Breath Markers. J. Breath Res. 2014, 8, 027111. DOI: 10.1088/1752-7155/8/2/027111.
- Fu, X.-A.; Li, M.; Knipp, R. J.; Nantz, M. H.; Bousamra, M. Noninvasive Detection of Lung Cancer Using Exhaled Breath. Cancer Med. 2014, 3, 174–181. DOI: 10.1002/cam4.162.
- Fuchs, P.; Loeseken, C.; Schubert, J. K.; Miekisch, W. Breath Gas Aldehydes as Biomarkers of Lung Cancer. Int. J. Cancer 2009, 126, 2663–2670. DOI: 10.1002/ijc.24970.
- Gaspar, E. M.; Lucena, A. F.; Duro da Costa, J. Chaves Das Neves, H. Organic Metabolites in Exhaled Human Breath-A Multivariate Approach for Identification of Biomarkers in Lung Disorders. J. Chromatogr. A 2009, 1216, 2749–2756. DOI: 10.1016/j.chroma.2008.10.125.
- Ha, H.; Usuba, A.; Maddula, S.; Baumbach, J. I.; Mineshita, M.; Miyazawa, T. Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry. PLoS One 2014, 9, e114555. DOI: 10.1371/journal.pone.0114555.
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E. M.; Trefz, P.; Amann, A.; Schubert, J. K. Breath Biomarkers for Lung Cancer Detection and Assessment of Smoking Related Effects - Confounding Variables, Influence of Normalization and Statistical Algorithms. Clin. Chim. Acta 2010, 411, 1637–1644. DOI: 10.1016/j.cca.2010.06.005.
- Li, M.; Yang, D.; Brock, G.; Knipp, R. J.; Bousamra, M.; Nantz, M. H.; Fu, X. A. Breath Carbonyl Compounds as Biomarkers of Lung Cancer. Lung Cancer 2015, 90, 92–97. DOI: 10.1016/j.lungcan.2015.07.005.
- Ligor, T.; Pater, Ł.; Buszewski, B. Application of an Artificial Neural Network Model for Selection of Potential Lung Cancer Biomarkers. J. Breath Res. 2015, 9, 027106. DOI: 10.1088/1752-7155/9/2/027106.
- Ma, H.; Li, X.; Chen, J.; Wang, H.; Cheng, T.; Chen, K.; Xu, S. Analysis of Human Breath Samples of Lung Cancer Patients and Healthy Controls with Solid-Phase Microextraction (SPME) and Flow-Modulated Comprehensive Two-Dimensional Gas Chromatography (GC × GC). Anal. Methods 2014, 6, 6841. DOI: 10.1039/C4AY01220H.
- Ma, W.; Gao, P.; Fan, J.; Hashi, Y.; Chen, Z. Determination of Breath Gas Composition of Lung Cancer Patients Using Gas Chromatography/Mass Spectrometry with Monolithic Material Sorptive Extraction. Biomed. Chromatogr. 2015, 29, 961–965. DOI: 10.1002/bmc.3385.
- Peled, N.; Hakim, M.; Bunn, P. A.; Miller, Y. E.; Kennedy, T. C.; Mattei, J.; Mitchell, J. D.; Hirsch, F. R.; Haick, H. Non-Invasive Breath Analysis of Pulmonary Nodules. J. Thorac. Oncol. 2012, 90, 92–97. DOI: 10.1097/JTO.0b013e3182637d5f.
- Peng, G.; Tisch, U.; Adams, O.; Hakim, M.; Shehada, N.; Broza, Y. Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosing Lung Cancer in Exhaled Breath Using Gold Nanoparticles. Nat. Nanotechnol. 2009, 4, 669–673. DOI: 10.1038/nnano.2009.235.
- Peng, G.; Hakim, M.; Broza, Y. Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. DOI: 10.1038/sj.bjc.6605810.
- Phillips, M.; Cataneo, R. N.; Cummin, A. R. C.; Gagliardi, A. J.; Gleeson, K.; Greenberg, J.; Maxfield, R. A.; Rom, W. N. Detection of Lung Cancer with Volatile Markers in the Breath. Chest 2003, 123, 2115–2123. DOI: 10.1378/chest.123.6.2115.
- Phillips, M.; Altorki, N.; Austin, J. H. M.; Cameron, R. B.; Cataneo, R. N.; Kloss, R.; Maxfield, R. A.; Munawar, M. I.; Pass, H. I.; Rashid, A.; et al. Detection of Lung Cancer Using Weighted Digital Analysis of Breath Biomarkers. Clin. Chim. Acta 2008, 393, 76–84. DOI: 10.1016/j.cca.2008.02.021.
- Poli, D.; Carbognani, P.; Corradi, M.; Goldoni, M.; Acampa, O.; Balbi, B.; Bianchi, L.; Rusca, M.; Mutti, A. Exhaled Volatile Organic Compounds in Patients with Non-Small Cell Lung Cancer: Cross Sectional and Nested Short-Term Follow-up Study. Respir. Res. 2005, 6, 71. DOI: 10.1186/1465-9921-6-71.
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of Aldehydes in Exhaled breath of patients with Lung Cancer by Means of on-Fiber-Derivatisation SPME-GC/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2643–2651. DOI: 10.1016/j.jchromb.2010.01.022.
- Rudnicka, J.; Kowalkowski, T.; Ligor, T.; Buszewski, B. Determination of Volatile Organic Compounds as Biomarkers of Lung Cancer by SPME-GC-TOF/MS and Chemometrics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 3360–3366. DOI: 10.1016/j.jchromb.2011.09.001.
- Sakumura, Y.; Koyama, Y.; Tokutake, H.; Hida, T.; Sato, K.; Itoh, T.; Akamatsu, T.; Shin, W. Diagnosis by Volatile Organic Compounds in Exhaled Breath from Lung Cancer Patients Using Support Vector Machine Algorithm. Sensors (Switzerland) 2017, 17, 287. DOI: 10.3390/s17020287.
- Schallschmidt, K.; Becker, R.; Jung, C.; Bremser, W.; Walles, T.; Neudecker, J.; Leschber, G.; Frese, S.; Nehls, I. Comparison of Volatile Organic Compounds from Lung Cancer Patients and Healthy Controls - Challenges and Limitations of an Observational Study. J. Breath Res. 2016, 10, 046007. DOI: 10.1088/1752-7155/10/4/046007.
- Schumer, E. M.; Trivedi, J. R.; van Berkel, V.; Black, M. C.; Li, M.; Fu, X.-A.; Bousamra, M. High Sensitivity for Lung Cancer Detection Using Analysis of Exhaled Carbonyl Compounds. J. Thorac. Cardiovasc. Surg. 2015, 150, 1517–1522. DOI: 10.1016/j.jtcvs.2015.08.092.
- Schumer, E. M.; Black, M. C.; Bousamra, M.; Trivedi, J. R.; Li, M.; Fu, X. A.; van Berkel, V. Normalization of Exhaled Carbonyl Compounds after Lung Cancer Resection. .Ann. Thorac. Surg. 2016, 102, 1095–1100. DOI: 10.1016/j.athoracsur.2016.04.068.
- Skeldon, K. D.; McMillan, L. C.; Wyse, C. A.; Monk, S. D.; Gibson, G.; Patterson, C.; France, T.; Longbottom, C.; Padgett, M. J. Application of Laser Spectroscopy for Measurement of Exhaled Ethane in Patients with Lung Cancer. Respir. Med. 2006, 100, 300–306. DOI: 10.1016/j.rmed.2005.05.006.
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The Application of Statistical Methods Using VOCs to Identify Patients with Lung Cancer. J. Breath Res. 2011, 5, 046008. DOI: 10.1088/1752-7155/5/4/046008.
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The Analysis of Volatile Organic Compounds Biomarkers for Lung Cancer in Exhaled Breath, Tissues and Cell Lines. CBM. 2012, 11, 129–137. DOI: 10.3233/CBM-2012-00270.
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S.; Ledochowski, M.; Grabmer, C.; Gastl, G. A.; Amann, A. Lung Cancer Detection by Proton Transfer Reaction Mass-Spectrometric Analysis of Human Breath Gas. Int. J. Mass Spectrom. 2007, 265, 49–59. DOI: 10.1016/j.ijms.2007.05.012.
- Van Oort, P. M. P.; De Bruin, S.; Hans, W.; Knobel, H. H.; Schultz, M. J.; Bos, L. D., On Behalf of the MARS Consortium. Exhaled Breath Metabolomics for the Diagnosis of Pneumonia in Intubated and Mechanically-Ventilated Intensive Care Unit (ICU)-Patients. IJMS. 2017, 18, 449. DOI: 10.3390/ijms18020449.
- Fowler, S. J.; Basanta-Sanchez, M.; Xu, Y.; Goodacre, R.; Dark, P. M. Surveillance for Lower Airway Pathogens in Mechanically Ventilated Patients by Metabolomic Analysis of Exhaled Breath: A Case-Control Study. Thorax 2015, 70, 320–325. DOI: 10.1136/thoraxjnl-2014-206273.
- Mitropoulos, K.; Katsila, T.; Patrinos, G. P.; Pampalakis, G. Multi-Omics for Biomarker Discovery and Target Validation in Biofluids for Amyotrophic Lateral Sclerosis Diagnosis. OMICS 2018, 22, 52–64. DOI: 10.1089/omi.2017.0183. [InsertedFromOnline[pubmedMismatch]]
- Bannaga, A. S.; Farrugia, A.; Arasaradnam, R. P. Diagnosing Inflammatory Bowel Disease Using Noninvasive Applications of Volatile Organic Compounds: A Systematic Review. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1113–1122. DOI: 10.1080/17474124.2019.1685873.
- Karyakin, A. A.; Nikulina, S. V.; Vokhmyanina, D. V.; Karyakina, E. E.; Anaev, E. K. H.; Chuchalin, A. G. Diabetes through Analysis of the Exhaled Breath Condensate (Aerosol). Electrochem. Commun. 2017, 83, 81–84. DOI: 10.1016/j.elecom.2017.09.005.
- Nelson, N.; Lagesson, V.; Nosratabadi, A. R.; Ludvigsson, J.; Tagesson, C. Exhaled Isoprene and Acetone in Newborn Infants and in Children with Diabetes Mellitus. Pediatr. Res. 1998, 44, 363–367. DOI: 10.1203/00006450-199809000-00016.
- Turner, C. Potential of Breath and Skin Analysis for Monitoring Blood Glucose Concentration in Diabetes. Expert Rev. Mol. Diagn. 2011, 11, 497–503. DOI: 10.1586/erm.11.31.
- Minh, T. D. C.; Blake, D. R.; Galassetti, P. R. The Clinical Potential of Exhaled Breath Analysis for Diabetes Mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. DOI: 10.1016/j.diabres.2012.02.006.
- Robles, L.; Priefer, R. Lactose Intolerance: What Your Breath Can Tell You. Diagnostics 2020, 10, 412. DOI: 10.3390/diagnostics10060412.
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J. K.; Staude, H.; Fischer, D.-C.; Miekisch, W. Exhaled Volatile Substances Mirror Clinical Conditions in Pediatric Chronic Kidney Disease. PLoS One 2017, 12, e0178745. DOI: 10.1371/journal.pone.0178745.
- Shuster, G.; Gallimidi, Z.; Reiss, A. H.; Dovgolevsky, E.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Engel, A.; Shiban, A.; Tisch, U.; et al. Classification of Breast Cancer Precursors through Exhaled Breath. Breast Cancer Res. Treat 2011, 126, 791–796. DOI: 10.1007/s10549-010-1317-x.
- Barash, O.; Zhang, W.; Halpern, J. M.; Hua, Q. L.; Pan, Y. Y.; Kayal, H.; Khoury, K.; Liu, H.; Davies, M. P. A.; Haick, H. Differentiation between Genetic Mutations of Breast Cancer by Breath Volatolomics. Oncotarget 2015, 6, 44864–44876. DOI: 10.18632/oncotarget.6269.
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of Potential Breath Biomarkers for the Early Diagnosis of Breast Cancer Using Gas Chromatography-Mass Spectrometry. Clin. Chim. Acta 2014, 436, 59–67. DOI: 10.1016/j.cca.2014.04.030.
- Phillips, M.; Cataneo, R. N.; Ditkoff, B. A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C. S.; Rahbari-Oskoui, F.; Wong, C. Volatile Markers of Breast Cancer in the Breath. Breast J. 2003, 9, 184–191. DOI: 10.1046/j.1524-4741.2003.09309.x.
- Wang, C.; Sun, B.; Guo, L.; Wang, X.; Ke, C.; Liu, S.; Zhao, W.; Luo, S.; Guo, Z.; Zhang, Y.; et al. Volatile Organic Metabolites Identify Patients with Breast Cancer, Cyclomastopathy, and Mammary Gland Fibroma. Sci. Rep. 2014, 4, 5383. DOI: 10.1038/srep05383.
- Bodelier, A. G. L.; Smolinska, A.; Baranska, A.; Dallinga, J. W.; Mujagic, Z.; Vanhees, K.; Van Den Heuvel, T.; Masclee, A. A. M.; Jonkers, D.; Pierik, M. J.; et al. Volatile Organic Compounds in Exhaled Air as Novel Marker for Disease Activity in Crohn’s Disease: A Metabolomic Approach. Inflamm. Bowel Dis. 2015, 21, 1776–1785. DOI: 10.1097/MIB.0000000000000436.
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J. K.; Staude, H.; Fischer, D.-C.; Miekisch, W. Exhaled Volatile Substances Mirror Clinical Conditions in Pediatric Chronic Kidney Disease. PLoS One 2017, 12, e0178745. DOI: 10.1371/journal.pone.0178745.
- Altomare, D. F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; De Gennaro, G. Exhaled Volatile Organic Compounds Identify Patients with Colorectal Cancer. Br. J. Surg. 2013, 100, 144–150. DOI: 10.1002/bjs.8942.
- Amal, H.; Leja, M.; Funka, K.; Lasina, I.; Skapars, R.; Sivins, A.; Ancans, G.; Kikuste, I.; Vanags, A.; Tolmanis, I.; et al. Breath Testing as Potential Colorectal Cancer Screening Tool. Int. J. Cancer 2016, 138, 229–236. DOI: 10.1002/ijc.29701.
- Wang, C.; Ke, C.; Wang, X.; Chi, C.; Guo, L.; Luo, S.; Guo, Z.; Xu, G.; Zhang, F.; Li, E. Noninvasive Detection of Colorectal Cancer by Analysis of Exhaled Breath. Anal. Bioanal. Chem. 2014. DOI: 10.1007/s00216-014-7865-x.
- Yan, Y.; Wang, Q.; Li, W.; Zhao, Z.; Yuan, X.; Huang, Y.; Duan, Y. Discovery of Potential Biomarkers in Exhaled Breath for Diagnosis of Type 2 Diabetes Mellitus Based on GC-MS with Metabolomics. RSC Adv. 2014, 4, 25430–25439. DOI: 10.1039/C4RA01422G.
- Abela, J. E.; Skeldon, K. D.; Stuart, R. C.; Padgett, M. J. Exhaled Ethane Concentration in Patients with Cancer of the Upper Gastrointestinal Tract - A Proof of Concept Study. Biosci. Trends 2009, 3, 110–114.
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Španěl, P.; Smith, D.; Hanna, G. B. Selected Ion Flow Tube Mass Spectrometry Analysis of Exhaled Breath for Volatile Organic Compound Profiling of Esophago-Gastric Cancer. Anal. Chem. 2013, 85, 6121–6128. DOI: 10.1021/ac4010309.
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; MacKenzie, H. A.; Veselkov, K. A.; Hoare, J. M.; Lovat, L. B.; Spanel, P.; Smith, D.; Hanna, G. B. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 981–990. DOI: 10.1097/SLA.0000000000001101.
- Amal, H.; Leja, M.; Broza, Y. Y.; Tisch, U.; Funka, K.; Liepniece-Karele, I.; Skapars, R.; Xu, Z. Q.; Liu, H.; Haick, H. Geographical Variation in the Exhaled Volatile Organic Compounds. J. Breath Res. 2013, 7, 047102. DOI: 10.1088/1752-7155/7/4/047102.
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of Precancerous Gastric Lesions and Gastric Cancer through Exhaled Breath. Gut 2016, 65, 400–407. DOI: 10.1136/gutjnl-2014-308536.
- Xu, Z. Q.; Broza, Y. Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y. Y.; Xiong, F. X.; Gu, K. S.; et al. A Nanomaterial-Based Breath Test for Distinguishing Gastric Cancer from Benign Gastric Conditions. Br. J. Cancer 2013, 108, 941–950. DOI: 10.1038/bjc.2013.44.
- Gruber, M.; Tisch, U.; Jeries, R.; Amal, H.; Hakim, M.; Ronen, O.; Marshak, T.; Zimmerman, D.; Israel, O.; Amiga, E.; et al. Analysis of Exhaled Breath for Diagnosing Head and Neck Squamous Cell Carcinoma: A Feasibility Study. Br. J. Cancer 2014, 111, 790–798. DOI: 10.1038/bjc.2014.361.
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of Head-and-Neck Cancer from Exhaled Breath. Br. J. Cancer 2011, 104, 1649–1655. DOI: 10.1038/bjc.2011.128.
- Patel, N.; Alkhouri, N.; Eng, K.; Cikach, F.; Mahajan, L.; Yan, C.; Grove, D.; Rome, E. S.; Lopez, R.; Dweik, R. A. Metabolomic Analysis of Breath Volatile Organic Compounds Reveals Unique Breathprints in Children with Inflammatory Bowel Disease: A Pilot Study. Aliment. Pharmacol. Ther. 2014, 40, 498–507. DOI: 10.1111/apt.12861.
- Tiele, A.; Wicaksono, A.; Kansara, J.; Arasaradnam, R. P.; Covington, J. A. Breath Analysis Using Enose and Ion Mobility Technology to Diagnose Inflammatory Bowel Disease — A Pilot Study. Biosensors 2019, 9, 55. DOI: 10.3390/bios9020055.
- Monasta, L.; Pierobon, C.; Princivalle, A.; Martelossi, S.; Marcuzzi, A.; Pasini, F.; Perbellini, L. Inflammatory Bowel Disease and Patterns of Volatile Organic Compounds in the Exhaled Breath of Children: A Case-Control Study Using Ion Molecule Reaction-Mass Spectrometry. PLoS One 2017, 12, e0184118. DOI: 10.1371/journal.pone.0184118.
- Dryahina, K.; Smith, D.; Bortlík, M.; Machková, N.; Lukáš, M.; Španěl, P. Pentane and Other Volatile Organic Compounds, Including Carboxylic Acids, in the Exhaled Breath of Patients with Crohn’s Disease and Ulcerative Colitis. J. Breath Res. 2017, 12, 016002. DOI: 10.1088/1752-7163/aa8468.
- Arasaradnam, R. P.; McFarlane, M.; Daulton, E.; Skinner, J.; O’Connell, N.; Wurie, S.; Chambers, S.; Nwokolo, C.; Bardhan, K.; Savage, R.; et al. Non-Invasive Exhaled Volatile Organic Biomarker Analysis to Detect Inflammatory Bowel Disease (IBD). Dig. Liver Dis. 2016, 48, 148–153. DOI: 10.1016/j.dld.2015.10.013.
- Hicks, L. C.; Huang, J.; Kumar, S.; Powles, S. T.; Orchard, T. R.; Hanna, G. B.; Williams, H. R. T. Analysis of Exhaled Breath Volatile Organic Compounds in Inflammatory Bowel Disease: A Pilot Study. J. Crohns Colitis 2015, 9, 731–737. DOI: 10.1093/ecco-jcc/jjv102.
- Smolinska, A.; Bodelier, A. G. L.; Dallinga, J. W.; Masclee, A. A. M.; Jonkers, D. M.; van Schooten, F. J.; Pierik, M. J. The Potential of Volatile Organic Compounds for the Detection of Active Disease in Patients with Ulcerative Colitis. Aliment. Pharmacol. Ther 2017, 45, 1244–1254. DOI: 10.1111/apt.14004.
- Rieder, F.; Kurada, S.; Grove, D.; Cikach, F.; Lopez, R.; Patel, N.; Singh, A.; Alkhouri, N.; Shen, B.; Brzezinski, A.; et al. A Distinct Colon-Derived Breath Metabolome is Associated with Inflammatory Bowel Disease, but Not Its Complications. Clin. Transl. Gastroenterol. 2016, 7, e201. DOI: 10.1038/ctg.2016.57.
- García, R. A.; Morales, V.; Martín, S.; Vilches, E.; Toledano, A. Volatile Organic Compounds Analysis in Breath Air in Healthy Volunteers and Patients Suffering Epidermoid Laryngeal Carcinomas. Chromatographia 2014, 77, 501–509. DOI: 10.1007/s10337-013-2611-7.
- Qin, T.; Liu, H.; Song, Q.; Song, G.; Wang, H. Z.; Pan, Y. Y.; Xiong, F. X.; Gu, K. S.; Sun, G. P.; Chen, Z. D. The Screening of Volatile Markers for Hepatocellular Carcinoma. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 2247–2253. DOI: 10.1158/1055-9965.EPI-10-0302.
- Ionescu, R.; Broza, Y.; Shaltieli, H.; Sadeh, D.; Zilberman, Y.; Feng, X.; Glass-Marmor, L.; Lejbkowicz, I.; Müllen, K.; Miller, A.; et al. Detection of Multiple Sclerosis from Exhaled Breath Using Bilayers of Polycyclic Aromatic Hydrocarbons and Single-Wall Carbon Nanotubes. ACS Chem. Neurosci. 2011, 2, 687–693. DOI: 10.1021/cn2000603.
- Sukul, P.; Schubert, J. K.; Trefz, P.; Miekisch, W. Natural Menstrual Rhythm and Oral Contraception Diversely Affect Exhaled Breath Compositions. Sci. Rep. 2018, 8, 10838. DOI: 10.1038/s41598-018-29221-z.
- Mendis, S.; Sobotka, P. A.; Euler, D. E. Pentane and Isoprene in Expired Air from Humans: Gas-Chromatographic Analysis of Single Breath. Clin. Chem. 1994, 40, 1485–1488. DOI: 10.1093/clinchem/40.8.1485.
- Phillips, M.; Greenberg, J.; Sabas, M. Alveolar Gradient of Pentane in Normal Human Breath. Free Radic. Res. 1994, 20, 333–337. DOI: 10.3109/10715769409145633.
- Bouza, M.; Gonzalez-Soto, J.; Pereiro, R.; De Vicente, J. C.; Sanz-Medel, A. Exhaled Breath and Oral Cavity VOCs as Potential Biomarkers in Oral Cancer Patients. J. Breath Res. 2017, 11, 016015. DOI: 10.1088/1752-7163/aa5e76.
- Szabó, A.; Tarnai, Z.; Berkovits, C.; Novák, P.; Mohácsi, Á.; Braunitzer, G.; Rakonczay, Z.; Turzó, K.; Nagy, K.; Szabó, G. Volatile Sulphur Compound Measurement with OralChromaTM: A methodological Improvement. J. Breath Res. 2015, 9, 016001. DOI: 10.1088/1752-7155/9/1/016001.
- Amal, H.; Shi, D. Y.; Ionescu, R.; Zhang, W.; Hua, Q. L.; Pan, Y. Y.; Tao, L.; Liu, H.; Haick, H. Assessment of Ovarian Cancer Conditions from Exhaled Breath. Int. J. Cancer 2015, 136, E614–E622. DOI: 10.1002/ijc.29166.
- Guo, L.; Wang, C.; Chi, C.; Wang, X.; Liu, S.; Zhao, W.; Ke, C.; Xu, G.; Li, E. Exhaled Breath Volatile Biomarker Analysis for Thyroid Cancer. Transl. Res. 2015, 166, 188–195. DOI: 10.1016/j.trsl.2015.01.005.
- Carlos, G.; dos Santos, F. P.; Fröehlich, P. E. Canine Metabolomics Advances. Metabolomics 2020, 16, 16–19. DOI: 10.1007/s11306-020-1638-7.
- Aksenov, A. A.; Yeates, L.; Pasamontes, A.; Siebe, C.; Zrodnikov, Y.; Simmons, J.; McCartney, M. M.; Deplanque, J.-P.; Wells, R. S.; Davis, C. E. Metabolite Content Profiling of Bottlenose Dolphin Exhaled Breath. Anal. Chem. 2014, 86, 10616–10624. DOI: 10.1021/ac5024217.
- Zamuruyev, K. O.; Aksenov, A. A.; Baird, M.; Pasamontes, A.; Parry, C.; Foutouhi, S.; Venn-Watson, S.; Weimer, B. C.; Delplanque, J.-P.; Davis, C. E. Enhanced Non-Invasive Respiratory Sampling from Bottlenose Dolphins for Breath Metabolomics Measurements. J. Breath Res. 2016, 10, 046005. DOI: 10.1088/1752-7155/10/4/046005.
- Pasamontes, A.; Aksenov, A. A.; Schivo, M.; Rowles, T.; Smith, C. R.; Schwacke, L. H.; Wells, R. S.; Yeates, L.; Venn-Watson, S.; Davis, C. E. Noninvasive Respiratory Metabolite Analysis Associated with Clinical Disease in Cetaceans: A Deepwater Horizon Oil Spill Study. Environ. Sci. Technol. 2017, 51, 5737–5746. DOI: 10.1021/acs.est.6b06482.
- Borras, E.; Aksenov, A. A.; Baird, M.; Novick, B.; Schivo, M.; Zamuruyev, K. O.; Pasamontes, A.; Parry, C.; Foutouhi, S.; Venn-Watson, S.; et al. Exhaled Breath Condensate Methods Adapted from Human Studies Using Longitudinal Metabolomics for Predicting Early Health Alterations in Dolphins. Anal. Bioanal. Chem. 2017, 409, 6523–6536. DOI: 10.1007/s00216-017-0581-6.
- Cumeras, R.; Cheung, W. H. K.; Gulland, F.; Goley, D.; Davis, C. E. Chemical Analysis of Whale Breath Volatiles: A Case Study for Non-Invasive Field Health Diagnostics of Marine Mammals. Metabolites 2014, 4, 790–806. DOI: 10.3390/metabo4030790.
- Burgess, E. A.; Hunt, K. E.; Kraus, S. D.; Rolland, R. M. Quantifying Hormones in Exhaled Breath for Physiological Assessment of Large Whales at Sea. Sci. Rep. 2018, 8, 10031. DOI: 10.1038/s41598-018-28200-8.
- Hogg, C. J.; Vickers, E. R.; Rogers, T. L. Determination of Testosterone in Saliva and Blow of Bottlenose Dolphins (Tursiops Truncatus) Using Liquid Chromatography–Mass Spectrometry. J. Chromatogr. B 2005, 814, 339–346. DOI: 10.1016/j.jchromb.2004.10.058.
- Dunstan, J.; Gledhill, A.; Hall, A.; Miller, P.; Ramp, C. Quantification of the Hormones Progesterone and Cortisol in Whale Breath Samples Using Novel, Non-Invasive Sampling and Analysis with Highly-Sensitive ACQUITY UPLC and Xevo TQ-S. Waters Appl. Note 2012, 1–8.
- Fulcher, Y. G.; Fotso, M.; Chang, C.-H.; Rindt, H.; Reinero, C. R.; Van Doren, S. R. Noninvasive Recognition and Biomarkers of Early Allergic Asthma in Cats Using Multivariate Statistical Analysis of NMR Spectra of Exhaled Breath Condensate. PLoS One 2016, 11, e0164394. DOI: 10.1371/journal.pone.0164394.
- Bos, L. D. J.; Van Walree, I. C.; Kolk, A. H. J.; Janssen, H.-G.; Sterk, P. J.; Schultz, M. J. Alterations in Exhaled Breath Metabolite-Mixtures in Two Rat Models of Lipopolysaccharide-Induced Lung Injury. J. Appl. Physiol. (1985) 2013, 115, 1487–1495. DOI: 10.1152/japplphysiol.00685.2013.
- Fischer, S.; Bergmann, A.; Steffens, M.; Trefz, P.; Ziller, M.; Miekisch, W.; Schubert, J. S.; Köhler, H.; Reinhold, P. Impact of Food Intake on in Vivo VOC Concentrations in Exhaled Breath Assessed in a Caprine Animal Model. J. Breath Res. 2015, 9, 047113. DOI: 10.1088/1752-7155/9/4/047113.
- Küntzel, A.; Oertel, P.; Trefz, P.; Miekisch, W.; Schubert, J. K.; Köhler, H.; Reinhold, P. Animal Science Meets Agricultural Practice: Preliminary Results of an Innovative Technical Approach for Exhaled Breath Analysis in Cattle under Field Conditions. Berl. Munch. Tierarztl. Wochenschr. 2018, 131, 444–452. DOI: 10.2376/0005-9366-17101.
- Bazzano, M.; Laghi, L.; Zhu, C.; Magi, G. E.; Serri, E.; Spaterna, A.; Tesei, B.; Laus, F. Metabolomics of Tracheal Wash Samples and Exhaled Breath Condensates in Healthy Horses and Horses Affected by Equine Asthma. J. Breath Res. 2018, 12, 046015. DOI: 10.1088/1752-7163/aade13.
- Neuhaus, S.; Seifert, L.; Vautz, W.; Nolte, J.; Bufe, A.; Peters, M. Comparison of Metabolites in Exhaled Breath and Bronchoalveolar Lavage Fluid Samples in a Mouse Model of Asthma. J. Appl. Physiol. (1985) 2011, 111, 1088–1095. DOI: 10.1152/japplphysiol.00476.2011.