Abstract
Natural carotenoids are secondary metabolites that exhibit antioxidant, anti-inflammatory, and anti-cancer properties. These types of compounds are highly demanded by pharmaceutical, cosmetic, nutraceutical, and food industries, leading to the search for new natural sources of carotenoids. In recent years, the production of carotenoids from bacteria has become of great interest for industrial applications. In addition to carotenoids with C40-skeletons, some bacteria have the ability to synthesize characteristic carotenoids with C30-skeletons. In this regard, a great variety of methodologies for the extraction and identification of bacterial carotenoids has been reported and this is the first review that condenses most of this information. To understand the diversity of carotenoids from bacteria, we present their biosynthetic origin in order to focus on the methodologies employed in their extraction and characterization. Special emphasis has been made on high-performance liquid chromatography-mass spectrometry (HPLC-MS) for the analysis and identification of bacterial carotenoids. We end up this review showing their potential commercial use. This review is proposed as a guide for the identification of these metabolites, which are frequently reported in new bacteria strains.
Introduction
Naturally occurring carotenoids are produced by eukaryotic cells, such as plants, algae, fungi, and prokaryotic cells like bacteria. In addition, some animals, such as flamingos accumulate carotenoids in their plumage from their diet (mollusks and crustaceans). These compounds are characterized by a striking range of colorations from red to yellow, through a spectrum of orange tones. Color is generated by at least six conjugated double bonds in a polyene chain.[Citation1,Citation2] The human eye is able to recognize colors within the range of 360 − 750 nm (yellow–blue). The ophthalmic color discrimination threshold approaches approximately 2 nm, and the Vis-detector limit is at best 0.1 nm.[Citation3–5] These natural pigments are derived from isoprenoids and are classified into two main groups: carotenes (hydrocarbon carotenoids) and oxygenated carotenoids (also known as xanthophylls). Carotenes are made of carbon and hydrogen atoms exclusively (e.g., phytoene, lycopene, and β-carotene). Xanthophyll possesses oxygen functional groups, including carotenols (e.g., zeaxanthin), carotenals (e.g., β-apo-8′-carotenal), carotenones (e.g., cantaxanthin), and carotenoid acids (e.g., 4,4′-diaponeurosporenoic acid).[Citation6–8] To date, more than 1200 carotenoids and carotenoid precursors from 722 organisms have been reported in the Carotenoid Database (http://carotenoiddb.jp/index.html), where 324 are from bacterial sources, 251 carotenoids being exclusive to these microorganisms.[Citation9] There are variations in the numbers and types of carbon units, for example, C30,[Citation10–12] C40,[Citation13] C45,[Citation14] and C50.[Citation15–18] Chains shorter than C30 occur in some bacteria via an alternate pathway. In general, thirty-seven molecules containing C30 carotenoids have been reported.[Citation9] The double bonds in these polyenes allow for the incorporation of environmentally free radicals, and delocalization of charges (positive or negative) along the chains.[Citation19] The antioxidant characteristics of carotenoids cause them to be very sensitive to light, heat, oxygen, isomerization (cis/trans), acidic conditions, and/or basic conditions.[Citation20]
Carotenoids exhibit a broad variety of applications and have been reported as beneficial treating ocular diseases due to their antioxidant and anti-inflammatory properties.[Citation19,Citation21–24] Lutein, zeaxanthin, meso-zeaxanthin are accumulated in the human eye where they act by filtering harmful UV (blue) light, thus preventing ocular oxidative stress.[Citation25] Reviews of carotenoids’ applications in nutraceutical products have demonstrated their potential.[Citation23,Citation26] Natural carotenoids, for instance astaxanthin (approved by the European Commission as food dye, E161j) and lycopene (E160d) are used as food colorants. In addition, astaxanthin is used as a dietary supplement, formulated as oils and tablets. Thus, the use of carotenoids obtained from microorganisms, such as Haematococcus pluvialis (microalgae), Xanthophyllomyces dendrorhous (yeast), and Blakeslea trispora (fungi), is an alternative to synthetic carotenoids in the food industry.[Citation27–29]
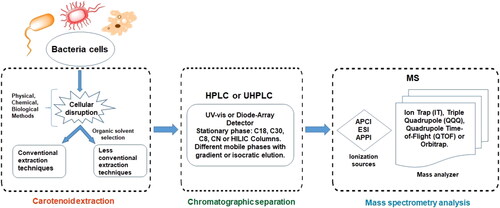
The main drawbacks of these microorganisms are their long cultivation times, and their dependence on climatic conditions. For example, astaxanthin production by H. pluvialis requires up to 288 h of culture and 156 h in X. dendrorhous. In addition, microalgae depend on light for the production of carotenoids.[Citation30,Citation31] Therefore, research on bacterial carotenoids has become an area of interests due to the short culture times compared to other microorganisms.[Citation32] In this sense, astaxanthin can be also obtained from the bacteria Halobacillus trueperi MXM-16 and Exiguobacterium Sps after 48 and 72 h, respectively.[Citation33,Citation34] Although bacteria are typically associated with human pathologies, carotenoids from bacteria are just as safe as those obtained from conventional sources like plants or by chemical synthesis. Thus, bacterial carotenoids could also be used in pharmaceuticals, nutraceuticals, cosmetics, and foods.[Citation35] Previous reviews mainly deal with carotenoids produced by yeasts, algae, and fungi over bacteria,[Citation36–40] or are mainly focused on the applications of these molecules.[Citation41–46] As far as we know, this is the first review about bacterial carotenoids including biosynthesis, protocols for their extraction, separation, and analysis (see ).
Biosynthesis
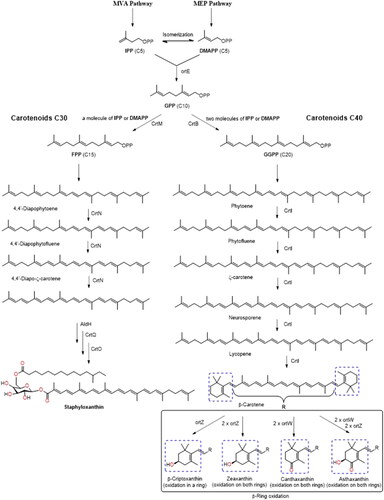
Carotenoids are secondary metabolites of plants and microorganisms; however, they are not involved in fundamental survival processes such as growth, development, and reproduction. Carotenoids are associated to the capacity of regulating light absorption – preventing the photodamage – and the oxidative stress, due to their antioxidant properties.[Citation26,Citation47,Citation48] Besides C40 carotenoids, some bacteria can synthesize C30 carotenoids and, to a lesser extent, other C45, C50, and C60 chains lengths. To understand the main types of carotenoids synthesized by bacteria; namely C30 and C40, a general description of the similarities and differences between both biosynthetic pathways is presented below, before tackling the extraction and identification methods for bacterial carotenoids.
The biosynthesis of carotenoids derived from bacteria starts from the C5 isoprenoid known as isopentenyl pyrophosphate (IPP), which isomerizes into dimethylallyl pyrophosphate (DMAPP) in the mevalonate (MVA) pathway (). Initially, a condensation reaction occurs between one IPP molecule and one DMAPP molecule, producing C10-geranyl diphosphate (GPP). However, depending on the bacteria, additional units of C5 may be added from the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway.[Citation1,Citation6,Citation36] Both MAV and MEP are synthesized during bacterial glycolysis. The MAV is generated from acetyl CoA, and the MEP from pyruvate. Chain elongation occurs during successive cyclical reactions, and is catalyzed by prenyl transferase (CrtE). This occurs between one or two IPP molecules with GPP, which produces C15-farnesyl pyrophosphate (FPP) and C20-geranylgeranyl diphosphate (GGPP), respectively. As a result, carotenoids derived from C30 and C40 chains take separate pathways. The synthesis of C30 occurs through the addition of two FPP units, while C40 synthesis is due to the addition of two GGDP units.[Citation41,Citation49,Citation50] In C30 carotenoid group, a colorless carotenoid precursor known as 4,4′-diapophytoene is produced, and in C40 group, 15-cis-phytoene is the first synthesized product. Both products are colorless and reactions are catalyzed by CrtM and CrtB enzymes, respectively. In both pathways, successive desaturation processes are carried out by the enzyme desaturase CrtN (C30) and CrtI (C40). shows the similarity in the initial steps of C30 and C40 carotenoids’ biosynthetic pathways. While carotenoids corresponding to C30 group are functionalized early by the oxidation processes (producing aldehyde, ketone, and/or carboxyl groups), carotenoids from the C40 group undergo dehydrogenation and cyclization processes prior to these oxidation reactions.[Citation46] Subsequently, addition, elimination, substitution, or rearrangement reactions produce a variety of molecules, including stereoisomers (cis/trans) and optical isomers (R/S).[Citation50–53]
Lycopene is a colored carotene C40 generated from four successive desaturations of the phytoene molecule. Through cyclic reactions, lycopene produces α-carotene and β-carotene, while a hydroxylation reaction in one of the β-carotene rings produces a β-cryptoxanthin molecule ().[Citation54] These three pro-vitamin A carotenoids are necessary for retinol synthesis in animals. One molecule of β-carotene generates two molecules of Vitamin A, a retinoid. This vitamin is necessary for vision, cellular communication, immune function, and human reproduction.[Citation21,Citation55,Citation56] Due to the poor absorption–conversion capacity of vitamin A by human body, it is necessary to consume foods that contain this retinoid. Lutein is a xanthophyll generated by the hydroxylation of α-carotene, that has been identified in the macula lutea region.[Citation25] In addition, it has been proposed that, due to its antioxidant and their light absorption characteristic, lutein may be involved in filtering light harmful to the eye.[Citation21,Citation22,Citation24,Citation25,Citation55]
Extraction methods
There is not a single accepted method for extracting carotenoids from bacteria, since the yield will depend on different factors (e.g., polarity of carotenoids, moisture content, and kind of cell wall[Citation36,Citation57]). Carotene’s extraction is favored by employing non-polar solvents (hexane), while xanthophylls are commonly extracted with polar solvents (acetone or ethanol [EtOH]). In addition, the moisture of the bacterial cells will determine whether the extraction is performed by a liquid–liquid or solid-liquid process. Bacteria’s cell wall is another important factor to consider; for instance, Gram-negative bacteria have inner and outer membranes separated by a thin peptidoglycan layer and a lipopolysaccharide (LPS) in the outer membrane, making them generally more permeable to organic solvents. On the other hand, Gram-positive bacteria have an inner membrane covered by a robust cell wall composed primarily of a peptidoglycan network, making these structures more resistant to organic solvents.[Citation36] In both cases, cell disruption of bacteria is simpler compared to microalgae and yeast, which are composed of more complex and rigid cell walls. shows a representation of zeaxanthin in the bacterial membrane of Pantoea sp. YR343;[Citation58] cell disruption is required for its extraction from intact bacterial cells (). Zeaxanthin is also found in lipid extracts used in the preparation of vesicles (). As a result, the extraction of these metabolites follows a similar process, which starts with cellular disruption or cell-membrane permeabilization, followed by carotenoid solubilization with organic solvent, and further removal of unwanted compounds.[Citation2] In addition, saponification processes are also carried out during the extraction, carotenoids are often bound to fatty acids of the bacterial membranes, which in many cases interferes in the carotenoids identification process. Extractions can be performed by using conventional or modern techniques as discussed in “Conventional extraction techniques” and “Advanced extraction techniques” subsections.
Figure 3. Representation of zeaxanthin in the bacterial membrane of Pantoea sp. YR343 (A). Intact bacterial cells (B) and vesicles prepared from bacterial lipids (C), both observed with confocal microscopy. Reprint from Kumar et al. [Citation58]
![Figure 3. Representation of zeaxanthin in the bacterial membrane of Pantoea sp. YR343 (A). Intact bacterial cells (B) and vesicles prepared from bacterial lipids (C), both observed with confocal microscopy. Reprint from Kumar et al. [Citation58]](/cms/asset/7827896a-2cde-4414-b2a0-92c11b20ec3d/batc_a_2016366_f0003_c.jpg)
Cellular disruption
Biomolecules, such as proteins, sugars, and fatty acids can lead to multiple problems during carotenoids’ extraction. Therefore, even if cell disruption is a crucial step previous to the extraction process, care must be taken to avoid carotenoids’ degradation by an increase of temperature during the treatment.[Citation59] After pelleting, cell disruption is carried out by physical, chemical, and/or biological methods.[Citation16] Use of mortar and pestle, vortex mixing with or without glass beads, orbital shaking, or incubation (may include temperature rise) are examples of physical methods. These approaches facilitate the entry of organic solvent into bacteria, leading to solubilization of carotenoids.[Citation29,Citation36] Whereas chemical methods use acids, bases, or surfactants, biological methods use enzymes, such as lysozyme, pectinase, or cellulase for cell disruption. Extraction techniques include conventional and non-conventional processes that may occur simultaneously during cellular disruption. Atmospheric pressure liquid extraction by maceration or Soxhlet is typical examples of conventional techniques. However, due to the heat-sensitive characteristic of carotenoids, it is not recommended because it would cause the degradation of these compounds. Nevertheless, advanced conventional techniques, such as microwaves (microwave-assisted extraction [MAE]), ultrasounds (ultrasound-assisted extraction [UAE]), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), gas expanded liquids (GXLs), and enzyme-assisted extraction (EAE) may also be employed to simultaneously disrupt and extract carotenoids.
Comparative analysis of three cell disruption methods of Arthrospira, including microwave, autoclave, and bead milling was conducted.[Citation60] In microwave, 0.5 g of cells were used at a frequency of 2450 MHz, with 1400 W of power for 120 s. In the autoclave process, 5 g of sample were treated for 30 min at 121 °C of temperature and 200 kPa of pressure. In the bead mill process, 150 g of A. spirulina cells were ground for 2 h at 60 rpm. Water was removed from the autoclaved samples with a vacuum dryer. In this study, microwave and bead milling provided the best yields. In a different study, cell lysis by sonication was evaluated on Formosa sp. KMW bacterium.[Citation61] When an ultrasonic bath was used, yields of 139.67 ± 7.00 µg/mg biomass were obtained, while employing a probe sonicator, the yield was 148.9 ± 19.5 µg/mg. However, the vortex presented the highest yield, with 1.83 ± 0.27 and 0.96 ± 0.9 mg/L, respectively. Recently, Park et al. performed cell lysis on freeze-dried cells of the cyanobacterium Arthrospira platensis,[Citation62] using a pre-cooled mortar and acetone containing 0.01% butylated hydroxytoluene as antioxidant.
Another study evaluated three cell lysis methodologies in Rhodobacter sphaeroides including: acidic hydrolysis with HCl, grinding, and UAE,[Citation63] with total carotenoids extraction yields of 4650 µg/g, 1615 µg/g, and 645 µg/g, respectively. Optimized conditions for HCl-assisted disruption of cell walls (30 °C, solvent-solid ratio of 30:1, for 40 min) was the best option. Zeaxanthin, caloxanthin, nostoxanthin, adonixanthin, canthaxanthin, and erythroxanthin sulfate were extracted from Erythrobacter citreus LAMA 915 using methanol (MeOH) and acetone (1:1) with cell lysis by sonication for 5 min.[Citation64] In addition, a study on the extraction of carotenoids from cyanobacteria Synechococcus sp. using supercritical CO2 (SFE) was performed,[Citation65] and the best yield was achieved at 500 bar and 60 °C. However, when 15% EtOH was added as cosolvent in SFE, milder conditions were needed to complete the extraction: 300 bar and 50 °C. These conditions show a higher relative extraction yield compared to the conventional dimethylformamide (DMF) method. Moreover, higher selectivity was observed in the extraction of carotenoids using SFE compared to DMF, which also extracts high amounts of chlorophylls from cyanobacteria. Comparison of cell disruption by sonication or surfactant treatment did not show a significant increase in carotenoid extraction. Therefore, the most efficient cell disruption method for Synechococcus sp. is achieved through the combination of pressure, temperature, and EtOH.[Citation65] Similarly, cell disruption in Rhodothermus marinus was achieved by PLE, obtaining hydroxy- and free salinixanthin;[Citation66] as mentioned, since R. marinus is a Gram-negative bacterium, no need for a pretreatment previous to extraction. However, recent results obtained in our lab, using ethyl acetate (AcOEt), demonstrate that macerating the cell-pellet with mortar and pestle is necessary prior to the extraction of Staphyloccocus aureus’ (Gram-positive bacterium) carotenoids by PLE. Regarding enzymatic degradation of cell wall, lysozyme has been used as a biological method in the cell lysis of Formosa sp. KMW and Vitellibacter sp. NMW.[Citation67] Initially, the cells were suspended in a saline solution followed by the addition of the enzyme to proceed with the lysis (20 mg/mL). Finally, the addition of acetone allowed the solubilization of the carotenoids. Thus, cell disruption is a key step for improving extraction performance.
Organic solvent selection
Different solvents, such as hexane, acetone, dimethyl sulfoxide (DMSO), dichloromethane (DCM), AcOEt, MeOH, or EtOH have been used (either individually or combined) for the extraction of bacterial carotenoids, simultaneously or after cell disruption (see ).[Citation2,Citation16] The selection of the most appropriate organic solvent for each extraction will depend on the type of carotenoids present in the bacteria, as indicated above (xanthophylls or carotenes). Some bacteria only produce carotenes: β-carotene, lycopene, etc., and therefore, the choice of the extraction solvent may be easier. However, bacteria simultaneously producing carotenes and xanthophylls, including their esters, make the solvent selection step more complex. In addition, care must be taken on the high oxidation sensitivity of carotenoids; to avoid this reaction, ascorbic acid, butyl-hydroxytoluene (BHT), butyl-hydroquinone (TBHQ), and butyl-hydroxyanisol (BHA) have been suggested as antioxidants to stabilize carotenoids during the extraction process.[Citation2,Citation16,Citation107] Very often, the analysis of bacterial carotenoids is complex because of their tendency to form ester. Therefore, the hydrolysis of the carotenoid esters before the analysis is frequently required.
Table 1. Carotenoids obtained from different bacteria, extraction conditions, and analytical methodologies.
Saponification
Bacterial carotenoid extracts contain carotenes, carotenols, carotenoid esters, carotenoid glycosides, and carotenoids.[Citation7,Citation113] Carotenoid esters are more stable compared to carotenoids because of the increased hydrophobicity of the molecules, which prevents degradation by oxidation or isomerization due to high-temperature conditions and light exposure. Furthermore, with the increase in the degree of esterification of xanthophyll, higher stability of these compounds is observed. For example, lutein is less stable than its monoester, which is less stable than the diester.[Citation113] Carotenoid esters are more stable than free carotenoids; however, the identification of the esterified form can be challenging. Thus, saponification is an example of a chemical method employed to extract of carotenoids, which allows the proper identification of these metabolites.
Saponification of Bacillus spp. cells was performed with a freeze-dried sample suspended in a solution of NaOH (10% w/v) sonicated for 15–20 min at room temperature.[Citation12] Subsequently, NaOH was removed by centrifugation and carotenoids of the saponified extract were obtained with MeOH:CHCl3 (1:2) and a Tris-buffer saline. After saponification, the identification of carotenoids was easier, showing both lycopenoate and apo-8′-lycopenoate bonded only to glucoside. In the same way, confirmation of an intermediate species in the biosynthetic pathway of S. aureus carotenoids was accomplished by performing a saponification step of the lyophilized cells with a solution of methanolic KOH (6%) at 4 °C for 14 h,[Citation70] followed by centrifugation at 4000 rpm and 4 °C and drying by centrifugal evaporator. Afterwards, the extract was resuspended in AcOEt and NaCl solution (5.0 N) for removal of unwanted compounds. The by-product (4,4′-Diaponeurosporenoic acid) was found both in S. aureus and Escherichia coli recombinant bacteria. Since wild-type E. coli does not produce carotenoids, 4,4′-diaponeurosporenoic acid was obtained after insertion of genes from S. aureus into E. coli bacteria.
A comparative study of different temperatures and KOH concentrations was carried out to evaluate the appropriate combination for carotenoids’ saponification in microalgae.[Citation114] Thus, the solvent mixture EtOH:hexane:water (77:17:6) with 0–60% KOH ((g KOH/g dry biomass)×100) at temperatures between 25 and 80 °C were tested. Although results did not allow establishing a universally applicable method for the extraction of carotenoids from the eight microalgae studied, ideal conditions were found for each strain. Researchers found that P. reticulatum required a temperature of 40 °C and 10% KOH, while the best yields for T. suecica and H. pluvialis were achieved at 25 °C and 10% or 40% KOH, respectively. In I. galbana the ideal temperature was 60 °C and <10% KOH, while Chlorella sp. and S. almeriensis required a temperature of 80 °C and 40% KOH. Finally, for N. gaditana and K. veneficum, the best results were achieved at 60 °C without saponification. Therefore, it is not possible to establish standardized saponification conditions, since they depend on the target strain; this conclusion can be extended to other microorganisms, such as yeasts and bacteria.
Conventional extraction techniques
Conventional techniques for carotenoids’ extraction have been reported in different studies. For example, neurosporene, α-carotene, echinenone, canthaxanthin, and astaxanthin were obtained from Microbacterium sp. LEMMJ01 by maceration with MeOH, carotenoids with a significant photoprotective effect according to the results obtained from the biological tests carried out.[Citation115] Whereas liquid–liquid extraction was applied to S. aureus at 40 or 4 °C for 20–30 min, using EtOH, MeOH, or acetone.[Citation68,Citation70] A subsequent liquid–liquid extraction with a AcOEt:NaCl aqueous solution allowed the removing of the more polar compounds from the extract due to a salting-out effect. In both cases, several related intermediaries of the staphyloxanthin (STX) biosynthetic pathway or new carotenoid species in mutant strains were determined.[Citation70] STX is a saccharolipid derivate from a 4,4′-diaponeurosporenoic acid, which is associated with increased membrane stiffness and resistance to antimicrobial peptide activity.[Citation116] These studies helped deciphering the carotenoid biosynthesis in this human pathogen, allowing the identification of a potential target for anti-virulence therapy.[Citation10] Novel deinoxanthin derivatives were extracted from Deinococcus sp. AJ005 by maceration using MeOH.[Citation117] Similarly, Rezaeeyan et al.[Citation109] indicated that the extraction of neurosporene (a carotene with antioxidant activity and UV-B radiation protection) from Kocuria sp. QWT-12 can be achieved by incubating with MeOH at 60° for 15 min. Whereas Brevundimonas scallop has high carotenoids production (1304 ± 61 μg/g dry), being astaxanthin and 2-hydroxy astaxanthin the most important products, which were extracted by maceration with acetone and rotary shaker (100 rpm).[Citation95] Recently, Hartz et al.[Citation71] studied the carotenoids from Bacillus megaterium (4,4′-diapophytoene, 4,4′-diapophytofluene, 4,4′-diaponeurosporene, 4,4′-diapolycopene, 4,4′-diaponeurosporenic acid) using a cold extraction method with MeOH (−20 °C) combined with vortex and glass beads. This method prevents thermal degradation of carotenoids, which can occur during the incubation process. In addition, 4,4′-diaponeurosporene is a carotene which has the potential to treat inflammatory diseases. This carotene was selectively extracted from E. coli.[Citation118] A similar disruption of the cells using EtOH was carried out in Methylomonas sp. 16a.[Citation97] This disruption allowed the analysis of astaxanthin and canthaxanthin, that are not naturally produced by this bacterium, but obtained after genetic engineering. These carotenoids have been reported to have photoprotective, antioxidant, and anti-inflammatory effects. Also, isolation and characterization of astaxanthin, adonixanthin, hydroxy-astaxanthin, and dihydroxy-astaxanthin from marine Gram-negative bacteria Brevundimonas sp.[Citation28] and Erythrobacter[Citation13] was possible by extraction followed by shaking at 50 °C. Astaxanthin is characterized by its high antioxidant potential, greater than β-carotene (10 times) and vitamin E (500 times), and its health benefit are often associated to dermatological treatments.[Citation23] Adonixanthin has been reported to be even stronger than astaxanthin in protecting the brain from internal hemorrhages.[Citation119]
Different expeditions have found numerous bacterial strains in Antarctica. A comprehensive study on the carotenoids produced from 30 pigmented bacterial strains isolated from Fildes Peninsula, King George Island, was reported by Vila et al.[Citation120] Results suggested the important role of carotenoids in these bacteria, associated to their antioxidant activity, which increase the resistance to extreme temperature conditions, drastic light conditions, and high doses of UV-B. Carotenoids such as zeaxanthin, β-cryptoxanthin, and β-carotene were the main compounds extracted with MeOH. The toxicity of the selected solvent must always be considered, in particular, when scaling up the process to the industrial level. Therefore, green extraction techniques arise, which involve the use of more environmentally friendly solvents, such as EtOH, AcOEt, or water. Nevertheless, due to the physicochemical characteristics of carotenoids, water is not a viable option in the extraction of these pigments.
Advanced extraction techniques
An important aspect that may hinder the industrial application of carotenoids is the need of organic solvents during the extraction procedures, which may have adverse effects on the environment and human health. Hence, the search for environmentally friendly approaches based on green chemistry principles is of paramount importance. Moreover, non-conventional techniques may offer higher yields of carotenoids in shorter periods of time when compared to traditional techniques.[Citation57] For example, when PLE is used for carotenoids’ extraction, the high yield obtained is due to the change of the physico-chemical properties of the (non-toxic) solvents employed at high pressures and/or temperatures that favor the penetrability of the solvent in the matrix while decreasing their viscosity and increasing the solubility of the target compounds compared to other conventional extraction techniques. As far as we know, only few examples have been reported using PLE for the extraction of carotenoids from bacteria. Ron et al. employed PLE with EtOH, at 1600 psi and 100 °C for three extraction cycles of 2 min each, for the extraction of carotenoids from R. marinus strains DSM 4252 T, DSM 4253, and PRI 493.[Citation66] Four main xanthophylls were obtained: two carotenoid acyl glycoside (-hydroxyl and free) and salinixanthin (-hydroxyl and free).
Among non-conventional processes, UAE seems to be the least effective approach, even though it is the most employed for the extraction of carotenoids from bacteria. The lower efficiency could be due to the low extraction temperatures used to avoid their degradation. Astaxanthin has been extracted with UAE from H. trueperi MXM-16 bacterium employed a MeOH:acetone (1:1) mixture.[Citation33] Recently, Buddhi et al.[Citation88] reported the spirilloxanthin series analysis of Afifella aestuarii sp. nov. using UAE with an acetone:MeOH (9:0.5) mixture. Also, Giuffrida et al.[Citation18] used UAE with MeOH, analyzed C50 carotenoids, such as all-E-decaprenoxanthin, all-E-sarcinaxanthin, 9-Z-decaprenoxanthin, 15-Z-decaprenoxanthin produced by strains of the cheese-ripening bacterium Arthrobacter arilaitensis. The cyanobacteria Aphanotece microscopica Nägeli and Phormidum autumnale Gomont used a combination of ultrasound bath and vortex to extract their carotenoids, the main compounds being β-carotene and cis-echinenone with approximately 68% and 10% each one.[Citation121] Extraction of zeaxanthin from Formosa sp. KMW and Vitellibacter sp. NMW was achieved by EAE employing lysozyme with acetone as disruption and extraction media.[Citation67] Similarly, cis-β-carotene, all-trans-β-carotene, chlorobactene, and isorenieratene were obtained from Rhodococcus sp. B7740 with the same enzyme.[Citation77] Surprisingly, despite the claimed advantages of MAE, no reports on its use for carotenoids’ extraction from bacteria were found in the literature; most probably due to the time-consuming process of optimization, including solvent–sample ratio, solid–liquid ratio, and microwave power[Citation16] or to the high temperatures commonly employed.
In summary, the wide variety of carotenoids produced by bacteria requires continuous development and optimization of new extraction methods. Once the carotenoids fraction is obtained, chemical characterization is usually carried out by high-performance liquid chromatography-mass spectrometry (HPLC-MS) (see ) as described below. presents detailed information on the methods used for the extraction of bacterial carotenoids, as well as the conditions employed in their identification by HPLC-MS. We hope that the information condensed in will serve as a guide for the selection of extraction and characterization methods according to the type of bacteria (phylum) with which we are working.
Characterization
The complex carotenoid extract from bacteria needs to be separated before identification. Hence, thin layer chromatography (TLC) and open column chromatography (OCC) have been traditionally employed for this separation step. These techniques involve off-line carotenoid separation frequently followed by mass spectrometric identification, as will be discussed in the next section. The major drawbacks of these techniques are the high sample concentration required and their low resolution, which curtails the ability to separate the wide variety of chemical structures reported for carotenoids and their intermediates (>1200 compounds). This complexity includes isomers (cis/trans), or modifications in the main chain that occur as a result of cyclization, addition of side chains, oxidation, hydrogenation, or dehydrogenation. Many of these structures are usually present in the bacterial carotenoid extract, making both their separation and identification very challenging. In addition, not all the carotenoid standards or their degradation products are available, which causes additional limitations to their identification.[Citation2]
Currently, the technique of choice to face all these analytical challenges is HPLC, usually coupled to a diode array detector (DAD) (or UV–visible detector) and MS. Therefore, the following paragraphs will discuss the HPLC separation methods reported on bacterial carotenoid extracts and analysis by DAD and MS.
Chromatographic separation
HPLC may use different types of columns available on the market: cyano (CN), octylsilane (C8), octadecylsilane (C18), or other more specific columns for separation of carotenoids (and some isomers), such as triacontyl (C30).[Citation52] Several reports have used C18 stationary phase columns in the analysis of bacterial carotenoids (see ). The drawback of C18 columns is the poor resolution in the separation of cis/trans isomers of carotenes with 40 or more carbon atoms. However, when xanthophylls are analyzed, similar profiles are observed using C18 and C30 columns.[Citation2,Citation122] Features such as better stability at a high pH, higher speed, and low cost make the C18 column useful and convenient for simple carotenoid analysis compared to the C30 column.[Citation123] C18 is also useful for other types of separation (vide infra). However, the higher retention capacity of both polar and non-polar compounds in C30 columns allows for a greater separation of carotenoids, thus favoring the resolution of geometric isomers, which is highly useful in complex carotenoid mixtures. Furthermore, C30 columns are stable at high pH and are more resistant to phase collapse than C18 columns. However, C30 columns generally require much longer analysis time than C18 columns.
Recently, Turcsi et al.[Citation124] compared the separation capacity of C18 and C30 stationary phases using a 100 carotenoids sample divided into six main groups: carotenes, hydroxycarotenoids, epoxycarotenoids, ketocarotenoids, and reduced κ carotenoids. The elution order of carotenoids in C18 columns was according to the polarity of each compound, with epoxy-carotenoids being the first compounds eluted, followed by hydroxy- and keto-carotenoids, ending with carotenes, which showed longer retention times. Similarly, the length of the molecule and the presence/number of polyenes are proportional to the retention times in C30 phases. C18 and C30 stationary phases, in combination with different elution modes, can be used to separate regioisomers of compounds such as zeaxanthin and lutein, including their epoxides carotenoid stereoisomers and epimers. Therefore, when analyzing an unknown extract, the initial analysis with C18 phase is recommended, and the characterization is complemented using a C30 phase.[Citation122]
Another example of the versatility of the C18 stationary phase in ultra-HPLC with a DAD (UHPLC-DAD) was reported for the separation and quantification of 13 carotenoids including three phytoene isomers, two ζ-carotene isomers, and two astaxanthin isomers.[Citation125] Analysis was performed in a BEH C18 column and a Cortecs C18 column (2.1 × 50 mm) at 35 °C with a flow rate of 400 µL/min, employing MeOH and ACN as mobile phase, with relatively short analysis times of 9 and 12 min. The method employed for both columns demonstrated a high level of reproducibility. However, the BEH C18 (1.7 µm) showed a slightly higher performance than Cortecs C18 (1.6 µm) in terms of recovery and less precision for the analyzed carotenoids. Selectivity of the chromatographic method was demonstrated by analyzing a carotenoid extract from H. pluvialis. Thus, this method could be applied not only to algae samples, but also to bacteria. Moreover, with the goal of producing a fast and efficient chemical identification method of these bacterial metabolites, Asker’s group and others have developed robust screening and profiling methods for the simultaneous analysis of diverse strains of bacteria producing carotenoids, which has emerged as an efficient alternative.[Citation13,Citation92] Meanwhile, an HPLC-MS/MS method for the analysis of microbial carotenoids and quinones produced by five bacteria (Brevibacterium linens, Micrococcus luteus, Panthoea agglomerans, Rhodococcus equi, Arthrobacter bergerei, and Arthrobacter protophormiae) used a C18 column. This method allowed the structural elucidation of acyclic carotenes, mono and bicyclo carotenes, mono and bicyclo xanthophylls, and acyclic xanthophylls.[Citation126]
Other reports have indicated the versatility of C8 columns in the separation of carotenoids. Thus, depending on the complexity of the carotenoid mixture, C8 columns have the advantage of shorter retention times compared to C18 and C30 columns; also, these columns show good resolving power when the carotenoids are in their ester form.[Citation123] Authors demonstrated the possibility of analyzing fucoxanthinol, fucoxanthin, mactraxanthin, violaxanthin, neoxanthin, and auroxanthin, being the latter one an isomer of violaxanthin at acidic pH.[Citation127]
In 2017, Abate-Pella et al.[Citation128] reported the use of a CN column in the efficient separation of more than 11 carotenoid standards in 9 or 18 min using MeOH as organic solvent. These carotenoids included carotene isomers (α-carotene, β-carotene, and lycopene) and xanthophyll isomers (canthaxanthin, α-cryptoxanthin, and β-cryptoxanthin). The applicability of this chromatographic method was demonstrated on three biological samples of strawberry leaf, chicken feed supplement, and the bacterium Chloroflexus aurantiacus. The resolution factor was 0.75 for the three carotenoids present in this bacterium. In addition, cis/trans isomerization of α-carotene was observed in the form of two peaks in the chromatogram.
In summary, HPLC is the (gold) standard technique for carotenoid separation. However, it is worth noting that carotenoids are influenced by the physical properties of the solvents used. Therefore, the selection of a suitable solvent in the mobile phase can lead to drastic changes in the chromatographic separation. For this reason, ACN, MeOH, THF, CHCl3, d-water, among other solvents, have been used (). In recent years, there has been an increase in the report of MeOH:MTBE:water mixtures as mobile phase with modifiers, such as ammonium acetate, ammonium formiate, acetic acid, or formic acid, among others,[Citation113,Citation122,Citation129–132] since their performances in the separation and identification of carotenoids has worked well ().
Figure 4. Chromatographic separation of mixed standards of carotenes and xanthophylls on a C30 column using the mixture MeOH:MTBE:water with ammonium acetate. Adapted from Hrvolová et al. [Citation129].
![Figure 4. Chromatographic separation of mixed standards of carotenes and xanthophylls on a C30 column using the mixture MeOH:MTBE:water with ammonium acetate. Adapted from Hrvolová et al. [Citation129].](/cms/asset/0f091ae4-ddbd-4af6-a589-6d324b00b4a7/batc_a_2016366_f0004_c.jpg)
However, many studies do not report the quantification of all carotenoids present in a sample because of the complexity of chromatograms due to the high variety of isomers and structurally related compounds. In many cases, carotenoid standards are not available or only one of the isomers is. Although carotenoid quantification requires the use of the specific standard; alternatively, a different carotenoid, typically β-carotene, is employed.[Citation133] Even if chromatographic separation is achieved, the lack of standards makes difficult to characterize this family of compounds. Mass spectrometry emerges as a useful technique for the identification and quantification of carotenoids.
Mass spectrometry analysis
MS provides structural information of the target compound though the molecular mass and fragmentation patterns that can be obtained in tandem mass spectrometry (MS/MS) mode.[Citation2,Citation57] MS selectivity allows the identification of compounds that cannot be separated by HPLC due to coelution. Therefore, HPLC-MS overcomes some of the problems related to the use of HPLC-DAD or HPLC-UV–vis for the identification of carotenoids since different families of these compounds have the same absorption in the UV–visible region.
In early studies, the identification of carotenoids was carried out using ionization methods such as electron ionization (EI), negative ion chemical ionization (CI), and fast atom bombardment (FAB).[Citation57,Citation134–136] The main drawback of EI and CI methods is the carotenoids thermolability. These ionization sources require solvent removal at relatively high temperatures prior to compounds ionization. Although FAB was also implemented for LC-MS analysis of carotenoids, this ionization technique presents low ionization capacity in negative mode.[Citation134,Citation136,Citation137] In addition, matrix-assisted laser desorption ionization (MALDI) has also been used in the identification of carotenoids;[Citation138–140] unfortunately, MALDI does not allow the hyphenation of HPLC and MS; thus, complex samples need to be previously fractionated off-line. For example, Yoshida et al. used TLC for the separation of carotenoids from Bacillus subtilis followed by MALDI-TOF MS.[Citation141] Similarly, off-line identification of carotenoids in Haloferax mediterranei was achieved using TLC-MALDI-TOF MS.[Citation142] In addition, Schöner et al.[Citation139] reported the characterization of bacterial carotenoids employed preparative HPLC separation and identification by MALDI-TOF MS.
Two main ionization sources are currently used for carotenoid analysis: electrospray ionization (ESI) and atmospheric pressure CI (APCI). While ESI is useful in the analysis of ionic and polar compounds such as xanthophylls,[Citation122,Citation143] APCI allows the characterization of both, carotenes and xanthophylls.[Citation122,Citation137] In recent years, a new ionization source, which is suitable for non-polar compounds, has been introduced. This source is known as atmospheric pressure photoionization (APPI).[Citation144] The ionization of metabolites in APPI is improved with the use of dopants; however, there is no universal solvent for this source, and the technique is still under development.[Citation122,Citation144]
In APCI, it is possible to use mobile phases without buffers and with high flow rates (greater than 800 µL/min), while ESI depends on the use of buffers or organic modifiers for efficient ionization and mobile phase flow rates lower or equal to 500 µL/min. In addition, NaOH solutions (0.5 mmol/L) have been used in ESI to increase the Na+ adducts of carotenoids, thus, improving the sensitivity of this ionization source.[Citation66] Another characteristic of APCI is the possibility of distinguishing some isomeric carotenoids by comparison of the fragmentation patterns between the positive and negative modes.[Citation137,Citation144] Thus, the best ionization source for the identification of carotenes and xanthophylls is still APCI. Examples of positive and negative ionization in APCI are presented in and , showing the different sensitivity and selectivity that these two ionization processes can provide in carotenoids analysis.
Figure 5. MS/MS spectra of xanthophylls in positive APCI: (A) astaxanthin; (B) β-cryptoxanthin; (C) lutein; and (D) zeaxanthin. Reprint from Van Breemen et al. [Citation137], with permission of Elsevier.
![Figure 5. MS/MS spectra of xanthophylls in positive APCI: (A) astaxanthin; (B) β-cryptoxanthin; (C) lutein; and (D) zeaxanthin. Reprint from Van Breemen et al. [Citation137], with permission of Elsevier.](/cms/asset/8e111dd7-acfc-457c-8531-975a54dfb4be/batc_a_2016366_f0005_b.jpg)
Figure 6. MS/MS spectra of carotenes in negative APCI: (A) β-carotene; (B) α-carotene; (C) lycopene; and (D) γ-carotene. Reprint from Van Breemen et al. [Citation137], with permission of Elsevier.
![Figure 6. MS/MS spectra of carotenes in negative APCI: (A) β-carotene; (B) α-carotene; (C) lycopene; and (D) γ-carotene. Reprint from Van Breemen et al. [Citation137], with permission of Elsevier.](/cms/asset/89bbb502-c989-41c6-b99a-708d1d151b14/batc_a_2016366_f0006_b.jpg)
A comparative study between the three sources of ionization at atmospheric pressure (ESI, APCI, and APPI) showed that the most powerful technique for carotenoids analysis is APCI.[Citation144] In this study, 11 xanthophylls and four carotenes were analyzed, and the effect of four dopants in APPI was evaluated. Carotenoids and carotenoid precursors observed with the strongest signal in APCI were: phytofluene, phytoene, echinenone, neoxanthin, antheraxanthin, astaxanthin, adonixanthin, zeaxanthin, β-apo-8′-carotenal, 3-hydroxyechinenone, β- and α-cryptoxanthin. ESI presented the best results for violaxanthin and lutein, whereas APPI exhibited the highest level of ionization for lycopene and β-carotene. In addition, the four dopants employed (acetone, toluene, anisole, and chlorobenzene) showed increases in the signal for all carotenoids analyzed in APPI. As a result, the highest sensitivity effect of dopants was observed for carotenes. Also, the addition of organic modifiers such as ammonium acetate in the mobile phase has been reported as a determining factor to increasing the ionization yield when using the APCI source.[Citation126]
Once these metabolites are separated and ionized, they move to the mass analyzer. Four types of analyzers are mainly used for the identification of carotenoids from bacteria (see ): ion trap (IT), triple quadrupole (QqQ), quadrupole time of flight (QTOF), and Orbitrap. IT allows the structural elucidation of unknown carotenoids by successive fragmentations in low-resolution tandem MS (MS2, MS3, MS4…). QqQ is mainly employed for targeted and quantitative analysis due to the high sensitivity obtained operating in multiple reaction monitoring (MRM) mode. QTOF and Orbitrap analyzers provide crucial structural information on the basis of the exact mass both molecular ions and fragments obtained by tandem-MS in high-resolution. summarizes some representative applications for these MS analyzers.
Carotenoid extracts from bacteria very often contain high concentrations of lipids (mainly triglycerides, TAGs) that are part of the cell membranes. The two main disadvantages of TAGs are high background noise in mass spectrometry with APCI(+) source and similar fragmentation patterns to carotenoid esters.[Citation7,Citation113] Thus, a clean-up step of the extracts is often required, that may involve biological methods using enzymes or physical methods employing low temperatures. Although extraction and cleaning processes may seem time-consuming and cumbersome, especially the latter, they are suitable for subsequent MS identification.[Citation7,Citation113,Citation122]
Applications
Commercial carotenoids are mainly produced by synthesis. However, consumers’ interest in products with carotenoids of natural origin has increased in recent years.[Citation35] Carotenoids are used commercially for pharmaceutical, cosmetic, nutraceutical, food (human and animal), among others purposes because of their low toxicity, antioxidant activity, stability in acid, and neutral pH, high coloring capacity, and the possibility of mixing with other colors.[Citation145–147] The relevance of bacterial carotenoids is due to their usable characteristics during industrial production, such as large number of colors, no dependence on climatic conditions (seasons), short cultivation times, the low complexity of extraction processes, and no toxicity, being accepted in different applications.[Citation41]
Pharmaceutical
Carotenoids of bacterial origin such as esterified astaxanthin showed higher antioxidant capacity than those of synthetic origin (free astaxanthin). Also, free sarcinaxanthin, mono- and diglucosilated (C50 skeletons), saproxanthin, isorenieratene, and 3,3′-dihydroxyisorenieratene (C40 skeletons) () have antioxidant capacities up to 500 times greater than β-carotene.[Citation148,Citation149] Astaxanthin a showed a neuroprotective effect on HT22 cells against glutamate-induced neurotoxicity in a model of Alzheimer’s disease by reducing reactive oxygen species (ROS), decreasing the caspase activation, and modulating the Akt/GSK-3b pathway.[Citation150] Astaxanthin administration significantly reduces neuronal apoptosis by modulating anti-apoptotic signaling and the brain Akt/Bad survival pathway.[Citation151] Adonixanthin is another xanthophyll produced by the bacteria Paracoccus sp., Erythrobacter, and Agrobacterium sp. which protects the brain from hemorrhagic brain damage due to its antioxidant effect, which is greater than that of astaxanthin.[Citation119] On their part, a study on serum lutein in people over 80 years of age showed high relationships between this xanthophyll and cognitive characteristics associated to language, memory, and learning.[Citation152] In the industrial preparation of pharmaceutical tablets, canthaxanthin is used because of its bright red color.[Citation153] In a study in mice, fucoxanthin combined with fish oil was reported to markedly decrease weight gain, with results indicating that xanthophyll reduces blood glucose and plasma insulin.[Citation154] These features make that this group of carotenoids interesting for pharmaceutical applications.
Cosmetic
The skin is the largest organ of the human body and most exposed to weather conditions such as solar radiation, hence the need for skin protection through the constant use of skin photoprotective agents. High concentrations of carotenoids in human skin occur mainly on the forehead and palms of the hands.[Citation145] UV radiation on the skin is associated with premature aging, wrinkles, pigmentation, and dryness. Studies have shown the best antioxidant capacity of lycopene in the quenching of ROS and β-carotene showed interesting results in the protection of ultraviolet and infrared light photo-radiation.[Citation155] In addition, the carotenoid precursors phytoene and phytofluene have light absorption capacity of UV-B (abourt 5% of the UV radiation in the earth) and UV-A (about 95% of the UV radiation in the earth) respectively.[Citation26,Citation145,Citation155] Additionally, topical formulations containing phytoene and phytofluene (5 mg per day) led to skin lightening and additional positive anti-wrinkle and anti-aging effects, observing skin lightening results of up to 82%.[Citation26] Interesting results because these carotenoid precursors could replace the use of hydroquinone as a skin depigmenting agent, which is associated with the side effects of developing malignancies and irritation.[Citation156] The versatility of bacteria in synthesizing novel carotenoids (), as well as traditionally known carotenoids, which may have equal or greater photoprotective, antioxidant and anticarcinogenic activities is of interest to the dermo-cosmetic industry.[Citation148,Citation149] Therefore, the carotenoid potential for use in topical components such as sunscreens remains a challenge to overcome, due to the problem is the low stability. Although, its low polarity allows efficient solubilization in skin emulsions.
Nutraceutical
Consumption of foods or foods fortified with high concentrations of carotenoids has been associated with human health benefits, such as cancer prevention, cardiovascular disease, and ocular health. The benefit of daily consumption juice fortified with of β-cryptoxanthin has been reported with bone formation and inhibition of bone resorption, preventing osteoporosis in women.[Citation41] Therefore, bacteria such as Pseudomonas sp.,[Citation112] Zobellia laminarie 465, or B. linens,[Citation82] could be used in the industrial production of this carotenoid, complementing the nutritional values of other foods and have an impact on human health. On the other hand, ROS are produced mainly in the retina of the eye due to the interaction of oxygen with photons of light, necessary during the human vision process. For this reason, macular carotenoids (lutein, zeaxanthin, and meso-zeaxanthin) protect the eye from oxidative damage, both by absorption of harmful light and by quenching (chemical and physical) of ROS.[Citation25] The United Nations and the World Health Organization established an adequate daily intake between 0 and 2 mg of zeaxanthin per kilogram of body weight.[Citation41] Thus, zeaxanthin can be selectively obtained from the bacteria: Mesoflavibacter zeaxanthinifaciens, Formosa sp. KMW, Vitellibacter sp. NMW, Synechococcus elongatus PCC 7942, and Paracoccus zeaxanthinifaciens ATCC 21588 (see ). In relation to cancer prevention, preclinical, epidemiological, and toxicological studies suggest that carotenoids may decrease human carcinogenesis.[Citation54] Consumption of lycopene, β-carotene, β-cryptoxanthin, lutein, and zeaxanthin decreases the risk of developing prostate cancer by 15%.[Citation157] A meta-analysis on the effect of carotenoids found that lycopene and β-carotene are associated with a decrease of about 30% in the development of lung cancer.[Citation158]
Food
Food industry has focused on manufacturing products that have striking colors of natural origin. Bacterial carotenoids applications in the food industry include the use of B. linens in the fermentation of Limburger and Port-du-Salut cheeses, producing the characteristic color in these dairy products.[Citation82] Likewise, a carotenoid that has shown wide versatility is astaxanthin. When this carotenoid is obtained from Paracoccus carotinifaciens, is used in the pigmentation of salmon and rainbow trout;[Citation159,Citation160] whereas astaxanthin from Mycobacterium lacticola is employed in fish feed for its antioxidant and photo-protective nature.[Citation161] Additionally, canthaxanthin is profiteers in the pigmentation of processed foods such as margarine, butter, and confectionery. For example, canthaxanthin from Haloferax alexandrines increases the pigmentation of salmon flesh.[Citation162] Also, zeaxanthin from Flavobacterium sp. is being used as an additive in poultry feed to change the color of egg yolks and chicken skin to a darker yellow.[Citation163,Citation164] The industrial production of these carotenoids from bacteria such as Dietzia sp., Bradyrhizobium sp., or Gordonia alkanivorans could also potentially be employed in the food industry.[Citation100,Citation102,Citation165]
Opportunities and challenges
Several patents have been granted for bacteria producing different carotenoids: Flavobacterium sp. - ATCC 21588 (US Patent 5,827,652), S. multivorum (US Patent 5,308,759), and P, zeaxanthinifaciens (US Patent 8,853,460). The latest patent was developed by JX Nippon Oil and Energy for the coloring of animal feed.[Citation166] As well as the patent on the administration of organisms that synthesize carotenoids in situ (US Patent US 9,113,653 B2) has been approved in 2015.[Citation167] Now, industrial application of bacterial carotenoids must consider the optimization of pH conditions, carbon and nitrogen sources, temperature, and cultivation times, as reported by different researchers.[Citation82,Citation168,Citation169] The same should be done for the extraction methods used in order to achieve the highest possible yield.[Citation63] In addition, as bacteria have fast-growing times, easy growing conditions, and do not depend on light for their cultivation, they show an advantage in industrial scale-up compared to microalgae and yeasts. However, their low carotenoid production yields compared to microalgae remain a challenge.
The advantage of microorganisms (especially bacteria) over plants as a source of natural carotenoids is their easy modification by genetic engineering techniques. This branch of engineering is, therefore, highly relevant to industrial processes, allowing work with mutant bacteria, where certain enzymes could be inhibited or overexpressed, avoiding the formation of unwanted by-products. For example, the bacterium Brevundimonas spp. (wild type) synthesize 440–1060 μg/g astaxanthin while its mutant Brevundimonas sp. M7 produces 1.3 mg/g ().[Citation174] Other genetic engineering approaches would allow the construction of mutants that favor the biosynthesis of phytoene and phytofluene, carotenoid precursors that present interesting dermo-cosmetic properties. Then, lists a number of bacteria that are being used in industrial processes, e.g., Haloferax volcanii produces bacterioruberin, a carotenoid with an antioxidant effect three times greater than traditional β-carotene. Another example where the mutant strain increases the production of this carotenoid by almost 16 times more than the wild-type strain (). Whereas P.zeaxanthinifaciensc, Dietzia natronolimnaea HS-1, and Paracoccus carotinfaciens (E-396) produce considerable yields of zeaxanthin, canthaxanthin, and astaxanthin, respectively, making them attractive for industrial-scale production processes. Furthermore, promising bacterial species tentatively identified as Chryseobacterium proteolyticum 9670, Flavobacterium granuli Kw05, Arthrobacter flavus JCM 11,496, Novosphingobium rhizosphaerae JM-1, and Pseudomonas parafulva AJ 2129 could be used in different industrial applications, due to its selective capacity in the biosynthesis of carotenoids: zeaxanthin, lutein, canthaxanthin, and nostoxanthin (see yield ).
Table 2. Bacteria reported for high-production of carotenoids of industrial interest.
The positive characteristics of carotenoids listed here, the marketing of vitamin supplements containing β-carotene and other carotenoids have increased in recent years.[Citation27] Therefore, bacteria are biofactory for carotenoid production, which can be efficiently exploited for industrial interests. Although the human health effects of carotenoid consumption are favorable, the next hurdle to overcome is the absorption process, which is low compared to the concentrations consumed.
Conclusions and perspectives
The great interest in the consumption of carotenoids of natural origin has encouraged the search of new sources for these compounds. Bacteria stand out as an excellent alternative due to the large variety of carotenoids that can be produced by different species. Moreover, bacteria can be considered a renewable source of carotenoids. In this article, different methodologies frequently used for the extraction of bacterial carotenoids have been reviewed. Many of these methods involve multiple steps for extraction, concentration, and resuspension of extracts, using large amounts of organic solvents such as hexane, MeOH, and CHCl3, among others. Therefore, green extraction methodologies become environmentally friendly alternatives that should be considered in future research. Their use has been shown to be efficient in other microorganisms: algae and fungi.[Citation131,Citation132,Citation176] Moreover, bacterial carotenoids, very often found at low concentrations levels, make the identification a challenging task. Therefore, the use of sensitive and high-throughput analytical methodologies such as HPLC-MS for qualitative and quantitative analysis of bacterial carotenoids becomes an essential requirement. The chromatographic separations have been developed with different stationary phases, although C18 and C30 columns are the most commonly used. The APCI source shows the best performance in the ionization of carotenoids, regardless of the mass analyzer used. Thus, HPLC-DAD-APCI-MS/MS is the technique of choice for identifying carotenoids produced by new bacterial strains, that has been reported over the last few years.[Citation177–186] We predict a continuous development in the near future toward more sensitive and higher resolution methodologies that will allow to widen the coverage of carotenoids identified in bacteria through the improvement of chromatographic columns, comprehensive methodologies in two-dimensional Liquid Chromatography (LC × LC), ionization processes and MS analyzers. Finally, the search of new (or modified) bacteria as natural sources for carotenoids will remain a hot area of research for years to come.
Acknowledgments
The authors wish to thank to Colombian Ministerio de Ciencia, Tecnología e Innovación (MINCIENCIAS) for PhD Fellowship to Gerson-Dirceu López (No. 785, Call 2017), as well as the support to No. 80740-532-2019 project and the Grant No. 120480763040.
Disclosure statement
The authors declare no conflicts of interest.
Gerson-Dirceu López: conceptualization, methodology, validation, investigation, writing – original draft preparation, and writing – review and editing. Gerardo Álvarez-Rivera: methodology, validation, and writing – review and editing. Chiara Carazzone: conceptualization, supervision, and writing – review and editing. Elena Ibañez: methodology, validation, supervision, and writing – review and editing. Chad Leidy: conceptualization, supervision, and writing – review and editing. Alejandro Cifuentes: methodology, validation, supervision, and writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Additional information
Funding
References
- Liang, M. H.; Zhu, J.; Jiang, J. G. Carotenoids Biosynthesis and Cleavage Related Genes from Bacteria to Plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333. DOI: 10.1080/10408398.2017.1322552.
- Amorim-Carrilho, K. T.; Cepeda, A.; Fente, C.; Regal, P. Review of Methods for Analysis of Carotenoids. TrAC – Trend. Anal. Chem. 2014, 56, 49–73. DOI: 10.1016/j.trac.2013.12.011.
- Schaefer, B. Chapter 7 - Vitamins. In Natural Products in the Chemical Industry; Springer Berlin Heidelberg: Berlin, Heidelberg, Germany, 2014; pp 589–640. DOI: 10.1007/978-3-642-54461-3.
- Valduga, E.; Oliveira Tatsch, P.; Tiggemann, L.; Treichel, H.; Toniazzo, G.; Zeni, J.; Luccio, M.; Di; Fúrigo Júnior, A. Produção de Carotenoides: Microrganismos Como Fonte de Pigmentos Naturais. Quim. Nova 2009, 32, 2429–2436. DOI: 10.1590/S0100-40422009000900036
- Martínez, A.; Zeeshan, M.; Zaidi, A.; Sliwka, H.-R. R.; Naqvi, K.; Partali, V. On Infinitenes – Reliable Calculation of Λ∞ and Molecular Modeling of Lemniscate Structured Carotenoids. Comput. Theor. Chem. 2018, 1125, 133–141. DOI: 10.1016/j.comptc.2017.12.006.
- Das, A.; Yoon, S. H.; Lee, S. H.; Kim, J. Y.; Oh, D. K.; Kim, S. W. An Update on Microbial Carotenoid Production: Application of Recent Metabolic Engineering Tools. Appl. Microbiol. Biotechnol. 2007, 77, 505–512. DOI: 10.1007/s00253-007-1206-3.
- Mariutti, L. R. B.; Mercadante, A. Z. Carotenoid Esters Analysis and Occurrence: What Do We Know so Far? Arch. Biochem. Biophys. 2018, 648, 36–43. DOI: 10.1016/j.abb.2018.04.005.
- Sliwka, H. R.; Partali, V. Key to Xenobiotic Carotenoids. Molecules 2012, 17, 2877–2928. DOI: 10.3390/molecules17032877.
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. DOI: 10.1093/database/bax004.
- Xue, L.; Chen, Y. Y.; Yan, Z.; Lu, W.; Wan, D.; Zhu, H. Staphyloxanthin: A Potential Target for Antivirulence Therapy. Infect. Drug Resist. 2019, 12, 2151–2160. DOI: 10.2147/IDR.S193649.
- Kim, J. W.; Choi, B. H.; Kim, J. H.; Kang, H. J.; Ryu, H.; Lee, P. C. Complete Genome Sequence of Planococcus Faecalis AJ003T, the Type Species of the Genus Planococcus and a Microbial C30 Carotenoid Producer. J. Biotechnol. 2018, 266, 72–76. DOI: 10.1016/j.jbiotec.2017.12.005.
- Perez-Fons, L.; Steiger, S.; Khaneja, R.; Bramley, P. M.; Cutting, S. M.; Sandmann, G.; Fraser, P. D. Identification and the Developmental Formation of Carotenoid Pigments in the Yellow/Orange Bacillus Spore-Formers. Biochim. Biophys. Acta. 2011, 1811, 177–185. DOI: 10.1016/j.bbalip.2010.12.009.
- Asker, D.; Awad, T. S.; Beppu, T.; Ueda, K. Screening and Profiling of Natural Ketocarotenoids from Environmental Aquatic Bacterial Isolates. Food Chem. 2018, 253, 247–254. DOI: 10.1016/j.foodchem.2018.01.066.
- Kallscheuer, N.; Moreira, C.; Airs, R.; Llewellyn, C. A.; Wiegand, S.; Jogler, C.; Lage, O. M. Pink- and Orange-Pigmented Planctomycetes Produce Saproxanthin-Type Carotenoids Including a Rare C45 Carotenoid. Environ. Microbiol. Rep. 2019, 11, 741–748. DOI: 10.1111/1758-2229.12796.
- Heider, S. A. E.; Peters-Wendisch, P.; Wendisch, V. F.; Beekwilder, J.; Brautaset, T. Metabolic Engineering for the Microbial Production of Carotenoids and Related Products with a Focus on the Rare C50 Carotenoids. Appl. Microbiol. Biotechnol. 2014, 98, 4355–4368. DOI: 10.1007/s00253-014-5693-8.
- Saini, R. K.; Keum, Y. S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. DOI: 10.1016/j.foodchem.2017.07.099.
- Silva, T. R.; Tavares, R. S. N.; Canela-Garayoa, R.; Eras, J.; Rodrigues, M. V. N.; Neri-Numa, I. A.; Pastore, G. M.; Rosa, L. H.; Schultz, J. A. A.; Debonsi, H. M.; et al. Chemical Characterization and Biotechnological Applicability of Pigments Isolated from Antarctic Bacteria. Mar. Biotechnol. 2019, 21, 416–429. DOI: 10.1007/s10126-019-09892-z.
- Giuffrida, D.; Sutthiwong, N.; Dugo, P.; Donato, P.; Cacciola, F.; Girard-Valenciennes, E.; Le Mao, Y.; Monnet, C.; Fouillaud, M.; Caro, Y.; et al. Characterisation of the C50 Carotenoids Produced by Strains of the Cheese-Ripening Bacterium Arthrobacter Arilaitensis. Int. Dairy J. 2016, 55, 10–16. DOI: 10.1016/j.idairyj.2015.11.005.
- Britton, G. Carotenoid Research: History and New Perspectives for Chemistry in Biological Systems. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158699. DOI: 10.1016/j.bbalip.2020.158699.
- Provesi, J. G.; Dias, C. O.; Amante, E. R. Changes in Carotenoids during Processing and Storage of Pumpkin Puree. Food Chem. 2011, 128, 195–202. DOI: 10.1016/j.foodchem.2011.03.027.
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. DOI: 10.3390/nu11102388.
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M. L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. DOI: 10.3390/nu10091321.
- Davinelli, S.; Nielsen, M. E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. DOI: 10.3390/nu10040522.
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Shahzad, T.; Malik, A.; Shariati, M. A.; Laishevtcev, A.; Plygun, S.; Heydari, M.; et al. Xanthophyll: Health Benefits and Therapeutic Insights. Life Sci. 2020, 240, 117104. DOI: 10.1016/j.lfs.2019.117104.
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P. S. The Macular Carotenoids: A Biochemical Overview. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2020, 1865, 158617. DOI: 10.1016/j.bbalip.2020.158617.
- Meléndez-Martínez, A. J.; Stinco, C. M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. DOI: 10.3390/nu11051093.
- Solymosi, K.; Latruffe, N.; Schoefs, B. Food Colour Additives of Natural Origin. In Colour Additives for Foods and Beverages; Elsevier Ltd.: Amsterdam, Netherlands, 2015; pp 3–34. DOI: 10.1016/B978-1-78242-011-8.00001-5.
- Asker, D. Isolation and Characterization of a Novel, Highly Selective Astaxanthin-Producing Marine Bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109. DOI: 10.1021/acs.jafc.7b03556.
- Ambati, R. R.; Gogisetty, D.; Aswathanarayana, R. G.; Ravi, S.; Bikkina, P. N.; Bo, L.; Yuepeng, S. Industrial Potential of Carotenoid Pigments from Microalgae: Current Trends and Future Prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. DOI: 10.1080/10408398.2018.1432561.
- Oslan, S. N. H.; Shoparwe, N. F.; Yusoff, A. H.; Rahim, A. A.; Chang, C. S.; Tan, J. S.; Oslan, S. N.; Arumugam, K.; Ariff, A.; Bin; Sulaiman, A. Z.; et al. A Review on Haematococcus Pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. DOI: 10.3390/biom11020256.
- Zheng, Y. G.; Hu, Z. C.; Wang, Z.; Shen, Y. C. Large-Scale Production of Astaxanthin by Xanthophyllomyces Dendrorhous. Food Bioprod. Process 2006, 84, 164–166. DOI: 10.1205/fbp.05030.
- Sajjad, W.; Din, G.; Rafiq, M.; Iqbal, A.; Khan, S.; Zada, S.; Ali, B.; Kang, S. Pigment Production by Cold-adapted Bacteria and Fungi: Colorful Tale of Cryosphere with Wide Range Applications. Extremophiles 2020, 24, 447–473. DOI: 10.1007/s00792-020-01180-2.
- Kharangate-Lad, A.; Bhosle, S. Studies on Siderophore and Pigment Produced by an Adhered Bacterial Strain Halobacillus Trueperi MXM-16 from the Mangrove Ecosystem of Goa, India. Indian J. Microbiol. 2016, 56, 461–466. DOI: 10.1007/s12088-016-0591-7.
- Sasidharan, P.; Raja, R.; Karthik, C.; Sharma, R. P, I. A. Isolation and Characterization of Yellow Pigment Producing Exiguobacterium Sps. J. Biochem. Technol. 2013, 4, 632–635.
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N. U.; Khan, A. L.; Shinwari, Z. K.; Al-Harrasi, A. Therapeutic Applications of Bacterial Pigments : A Review of Current Status and Future Opportunities. 3 Biotech. 2018, 8, 1–15. DOI: 10.1007/s13205-018-1227-x.
- Mussagy, C. U.; Winterburn, J.; Santos-Ebinuma, V. C.; Pereira, J. F. B. Production and Extraction of Carotenoids Produced by Microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. DOI: 10.1007/s00253-018-9557-5.
- Mendes-Silva, T. D. C. D.; Andrade, R. F. D. S.; Ootani, M. A.; Mendes, P. V. D.; Sá, R. A. D. Q. C. D.; Silva, M. R. F. D.; Souza, K. S.; Correia, M. T. D. S.; Silva, M. V. D.; Oliveira, M. B. M. D. Biotechnological Potential of Carotenoids Produced by Extremophilic Microorganisms and Application Prospects for the Cosmetics Industry. Adv. Microbiol. 2020, 10, 397–410. DOI: 10.4236/aim.2020.108029.
- Narsing Rao, M. P.; Xiao, M.; Li, W. J. Fungal and Bacterial Pigments: Secondary Metabolites with Wide Applications. Front. Microbiol. 2017, 8, 1113. DOI: 10.3389/fmicb.2017.01113.
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A. M. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites 2021, 11, 92. DOI: 10.3390/metabo11020092.
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G. K. The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 2021, 5, 603892. DOI: 10.3389/fsufs.2021.603892.
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S. R.; Mishra, S. Bacteria as an Alternate Biofactory for Carotenoid Production: A Review of Its Applications, Opportunities and Challenges. J. Funct. Foods 2020, 67, 103867. DOI: 10.1016/j.jff.2020.103867.
- Jinendiran, S.; Boopathi, S.; Sivakumar, N.; Selvakumar, G. Functional Characterization of Probiotic Potential of Novel Pigmented Bacterial Strains for Aquaculture Applications. Probiotics Antimicrob. Proteins 2019, 11, 186–197. DOI: 10.1007/s12602-017-9353-z.
- Venil, C. K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 1–17. DOI: 10.3389/fsufs.2020.00100.
- Velmurugan, P.; Venil, C. K.; Veera Ravi, A.; Dufossé, L. Marine Bacteria is the Cell Factory to Produce Bioactive Pigments: A Prospective Pigment Source in the Ocean. Front. Sustain. Food Syst. 2020, 4, 589655. DOI: 10.3389/fsufs.2020.589655.
- Pailliè-Jiménez, M. E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 1–8. DOI: 10.3389/fsufs.2020.590439.
- Mussagy, C. U.; Khan, S.; Kot, A. M. Current Developments on the Application of Microbial Carotenoids as an Alternative to Synthetic Pigments. Crit. Rev. Food Sci. Nutr. 2021. DOI: 10.1080/10408398.2021.1908222.
- Zhu, Y.; Graham, J. E.; Ludwig, M.; Xiong, W.; Alvey, R. M.; Shen, G.; Bryant, D. A. Roles of Xanthophyll Carotenoids in Protection against Photoinhibition and Oxidative Stress in the Cyanobacterium Synechococcus Sp. Strain PCC 7002. Arch. Biochem. Biophys. 2010, 504, 86–99. DOI: 10.1016/j.abb.2010.07.007.
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. DOI: 10.1007/s11418-019-01364-x.
- Frengova, G. I.; Beshkova, D. M. Carotenoids from Rhodotorula and Phaffia: Yeasts of Biotechnological Importance. J. Ind. Microbiol. Biotechnol. 2009, 36, 163–180. DOI: 10.1007/s10295-008-0492-9.
- Córdova, P.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Microbiological Synthesis of Carotenoids: Pathways and Regulation. In Progress in Carotenoid Research Pigments; Queiroz Zepka, L., Jacob-Lopes, E., Rosso, V. V., Eds.; IntechOpen: London, 2018; pp 63–83. DOI: 10.5772/intechopen.78343.
- Niu, F. X.; Lu, Q.; Bu, Y. F.; Liu, J. Z. Metabolic Engineering for the Microbial Production of Isoprenoids: Carotenoids and Isoprenoid-Based Biofuels. Synth. Syst. Biotechnol. 2017, 2, 167–175. DOI: 10.1016/j.synbio.2017.08.001.
- Wang, C.; Zhao, S.; Shao, X.; Park, J.; Bin; Jeong, S. H.; Park, H. J.; Kwak, W. J.; Wei, G.; Kim, S. W. Challenges and Tackles in Metabolic Engineering for Microbial Production of Carotenoids. Microb. Cell Fact. 2019, 18, 1–8. DOI: 10.1186/s12934-019-1105-1.
- Zhang, C. Biosynthesis of Carotenoids and Apocarotenoids by Microorganisms and Their Industrial Potential. In Progress in Carotenoid Research Pigments; Queiroz Zepka, L., Jacob-Lopes, E., Rosso, V. V., Eds.; BoD – Books on Demand: Norderstedt, Germany, 2018; pp 85–105. DOI: 10.5772/intechopen.79061.
- Rowles, J. L.; Erdman, J. W. Carotenoids and Their Role in Cancer Prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020, 1865, 158613. DOI: 10.1016/j.bbalip.2020.158613.
- Perera, C. O.; Yen, G. M. Functional Properties of Carotenoids in Human Health. Int. J. Food Prop. 2007, 10, 201–230. DOI: 10.1080/10942910601045271.
- Bohn, T.; Desmarchelier, C.; El, S. N.; Keijer, J.; Van Schothorst, E.; Rühl, R.; Borel, P. β-Carotene in the Human Body: Metabolic Bioactivation Pathways - From Digestion to Tissue Distribution and Excretion. Proc. Nutr. Soc. 2019, 78, 68–87. DOI: 10.1017/S0029665118002641.
- Pérez-Gálvez, A.; Roca, M. Recent Developments in the Analysis of Carotenoids by Mass Spectrometry. In Progress in Carotenoid Research; IntechOpen: London, 2018; pp 17–44. DOI: 10.5772/intechopen.79755.
- Kumar, S. V.; Taylor, G.; Hasim, S.; Collier, C. P.; Farmer, A. T.; Campagna, S. R.; Bible, A. N.; Doktycz, M. J.; Morrell-Falvey, J. Loss of Carotenoids from Membranes of Pantoea Sp. YR343 Results in Altered Lipid Composition and Changes in Membrane Biophysical Properties. Biochim. Biophys. Acta. Biomembr. 2019, 1861, 1338–1345. DOI: 10.1016/j.bbamem.2019.05.009.
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R. G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. DOI: 10.3390/fermentation7010036.
- Larrosa, A. P. Q.; Camara, Á. S.; Moura, J. M.; Pinto, L. A. A. Spirulina Sp. Biomass Dried/Disrupted by Different Methods and Their Application in Biofilms Production. Food Sci. Biotechnol. 2018, 27, 1659–1665. DOI: 10.1007/s10068-018-0397-y.
- Sowmya, R.; Sachindra, N. M. Carotenoid Production by Formosa Sp. KMW, a Marine Bacteria of Flavobacteriaceae Family: Influence of Culture Conditions and Nutrient Composition. Biocatal. Agric. Biotechnol. 2015, 4, 559–567. DOI: 10.1016/j.bcab.2015.08.018.
- Park, W. S.; Kim, H. J.; Li, M.; Lim, D. H.; Kim, J.; Kwak, S. S.; Kang, C. M.; Ferruzzi, M. G.; Ahn, M. J. Two Classes of Pigments, Carotenoids and c-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. DOI: 10.3390/molecules23082065.
- Gu, Z.; Deming, C.; Yongbin, H.; Zhigang, C.; Feirong, G. Optimization of Carotenoids Extraction from Rhodobacter Sphaeroides. LWT - Food Sci. Technol. 2008, 41, 1082–1088. DOI: 10.1016/j.lwt.2007.07.005.
- Niero, H.; da Silva, M. A. C.; de Felicio, R.; Trivella, D. B. B.; Lima, A. O. d S. Carotenoids Produced by the Deep-Sea Bacterium Erythrobacter Citreus LAMA 915: Detection and Proposal of Their Biosynthetic Pathway. Folia Microbiol. (Praha) 2021, 66, 441–456. DOI: 10.1007/s12223-021-00858-0.
- Montero, O.; Macìas-Sánchez, M. D.; Lama, C. M.; Lubián, L. M.; Mantell, C.; Rodríguez, M.; De La Ossa, E. M. Supercritical CO2 Extraction of β-Carotene from a Marine Strain of the Cyanobacterium Synechococcus Species. J. Agric. Food Chem. 2005, 53, 9701–9707. DOI: 10.1021/jf051283n.
- Ron, E. Y. C.; Plaza, M.; Kristjansdottir, T.; Sardari, R. R. R.; Bjornsdottir, S. H.; Gudmundsson, S.; Hreggvidsson, G. O.; Turner, C.; van Niel, E. W. J.; Nordberg-Karlsson, E. Characterization of Carotenoids in Rhodothermus Marinus. MicrobiologyOpen 2018, 7, 7:e536. DOI: 10.1002/mbo3.536.
- Sowmya, R.; Sachindra, N. M. Biochemical and Molecular Characterization of Carotenogenic Flavobacterial Isolates from Marine Waters. Pol. J. Microbiol. 2016, 65, 77–88. DOI: 10.5604/17331331.1197278.
- Pelz, A.; Wieland, K. P.; Putzbach, K.; Hentschel, P.; Albert, K.; Götz, F. Structure and Biosynthesis of Staphyloxanthin from Staphylococcus Aureus. J. Biol. Chem. 2005, 280, 32493–32498. DOI: 10.1074/jbc.M505070200.
- Mijts, B. N.; Lee, P. C.; Schmidt-Dannert, C. Identification of a Carotenoid Oxygenase Synthesizing Acyclic Xanthophylls: Combinatorial Biosynthesis and Directed Evolution. Chem. Biol. 2005, 12, 453–460. DOI: 10.1016/j.chembiol.2005.02.010.
- Kim, S. H.; Lee, P. C. Functional Expression and Extension of Staphylococcal Staphyloxanthin Biosynthetic Pathway in Escherichia Coli. J. Biol. Chem. 2012, 287, 21575–21583. DOI: 10.1074/jbc.M112.343020.
- Hartz, P.; Milhim, M.; Trenkamp, S.; Bernhardt, R.; Hannemann, F. Characterization and Engineering of a Carotenoid Biosynthesis Operon from Bacillus Megaterium. Metab. Eng. 2018, 49, 47–58. DOI: 10.1016/j.ymben.2018.07.017.
- Kim, J. H.; Kang, H. J.; Yu, B. J.; Kim, S. C.; Lee, P. C. Planococcus Faecalis Sp. Nov., a Carotenoid-producing Species Isolated from Stools of Antarctic Penguins. Int. J. Syst. Evol. Microbiol. 2015, 65, 3373–3378. DOI: 10.1099/ijsem.0.000423.
- Heo, J.; Kim, S. H.; Lee, P. C. New Insight into the Cleavage Reaction of Nostoc Sp. Strain PCC 7120 Carotenoid Cleavage Dioxygenase in Natural and Nonnatural Carotenoids. Appl. Environ. Microbiol. 2013, 79, 3336–3345. DOI: 10.1128/AEM.00071-13.
- Ganapathy, A.; Jayavel, S.; Natesan, S. Draft Genome Sequence of Carotenoid Producing Yellow Pigmented Planococcus Maritimus MKU009. J. Genomics. 2016, 4, 23–25. DOI: 10.7150/jgen.15533.
- Shindo, K.; Endo, M.; Miyake, Y.; Wakasugi, K.; Morritt, D.; Bramley, P. M.; Fraser, P. D.; Kasai, H.; Misawa, N. Methyl 5-glucosyl-5,6-dihydro-apo-4,4'-lycopenoate, a Novel Antioxidative glyco-C(30)-carotenoic Acid Produced by a Marine Bacterium Planococcus maritimus [Corrected]. J. Antibiot. (Tokyo) 2008, 61, 729–735. DOI: 10.1038/ja.2008.86.
- Widyastuti, Y.; Nugraheni, S. A.; Khoeri, M. M.; Kusmita, L.; Radjasa, O. K. Characterization of Carotenoid Pigments from Bacterial Symbionts of Seagrass Thalassia Hemprichii Knowledge Management on Low Emission Strategy into Coastal Management in NTB and NTB Province View Project DNA Barcoding View Project CHARACTERIZATION oF CAR. J. Coast. Dev. 2010, 14, 51–60. DOI: 10.13140/RG.2.1.3348.6562.
- Chen, Y.; Xie, B.; Yang, J.; Chen, J.; Sun, Z. Identification of Microbial Carotenoids and Isoprenoid Quinones from Rhodococcus Sp. B7740 and Its Stability in the Presence of Iron in Model Gastric Conditions. Food Chem. 2018, 240, 204–211. DOI: 10.1016/j.foodchem.2017.06.067.
- Tao, L.; Cheng, Q. Novel Beta-carotene Ketolases from Non-photosynthetic Bacteria for Canthaxanthin Synthesis. Mol. Genet. Genomics. 2004, 272, 530–537. DOI: 10.1007/s00438-004-1083-8.
- Tao, L.; Wagner, L. W.; Rouvière, P. E.; Cheng, Q. Metabolic Engineering for Synthesis of Aryl Carotenoids in Rhodococcus. Appl. Microbiol. Biotechnol. 2006, 70, 222–228. DOI: 10.1007/s00253-005-0064-0.
- Arpin, N.; Liaaen-Jensen, S.; Trouilloud, M. Bacterial Carotenoids. 38. C 50 -carotenoids. 9. Isolation of Decaprenoxanthin Mono- and Diglucoside from an Arthrobacter sp. Acta Chem. Scand. 1972, 26, 2524–2526. DOI: 10.3891/acta.chem.scand.26-2524.
- Takaichi, S.; Maoka, T.; Akimoto, N.; Carmona, M. L.; Yamaoka, Y. Carotenoids in a Corynebacterineae, Gordonia Terrae AIST-1: Carotenoid Glucosyl Mycoloyl Esters. Biosci. Biotechnol. Biochem. 2008, 72, 2615–2622. DOI: 10.1271/bbb.80299.
- Applique, L. D. M.; Alimentaires, I. Production of Carotenoids by Brevibacterium Linens: Variation among Strains, Kinetic Aspects and HPLC Profiles. J. Ind. Microbiol. Biotechnol. 2000, 24, 64–70. DOI: 10.1038/sj.jim.2900761.
- Hyeon, J. W.; Jeon, C. O. Roseomonas Aerofrigidensis Sp. Nov., Isolated from an Air Conditioner. Int. J. Syst. Evol. Microbiol. 2017, 67, 4039–4044. DOI: 10.1099/ijsem.0.002246.
- Ramana, V. V.; Sasikala, C.; Ramaprasad, E. V. V.; Ramana, C. V. Description of Ectothiorhodospira Salini Sp. Nov. J. Gen. Appl. Microbiol. 2010, 56, 313–319. DOI: 10.2323/jgam.56.313.
- Zhao, C.; Yue, H.; Cheng, Q.; Chen, S.; Yang, S. What Caused the Formation of the Absorption Maximum at 421 Nm in Vivo Spectra of Rhodopseudomonas Palustris. Photochem. Photobiol. 2014, 90, 1287–1292. DOI: 10.1111/php.12334.
- Ramaprasad, E. V. V.; Sasikala, C.; Ramana, C. V. Neurosporene is the Major Carotenoid Accumulated by Rhodobacter Viridis JA737. Biotechnol. Lett. 2013, 35, 1093–1097. DOI: 10.1007/s10529-013-1181-y.
- Rahul, K.; Azmatunnisa, M.; Sasikala, C. H.; Ramana, C. V. Hoeflea Olei Sp. Nov., a Diesel-Oil-Degrading, Anoxygenic, Phototrophic Bacterium Isolated from Backwaters and Emended Description of the Genus Hoeflea. Int. J. Syst. Evol. Microbiol. 2015, 65, 2403–2409. DOI: 10.1099/ijs.0.000277.
- Buddhi, S.; Suresh, G.; Gupta, D.; Sasikala, C.; Ramana, C. V. Afifella Aestuarii Sp. Nov., a Phototrophic Bacterium. Int. J. Syst. Evol. Microbiol. 2020, 70, 327–333. DOI: 10.1099/ijsem.0.003756.
- Jiménez, M. E. P.; Pinilla, C. M. B.; Rodrigues, E.; Brandelli, A. Extraction and Partial Characterisation of Antioxidant Pigment Produced by Chryseobacterium Sp. Kr6. Nat. Prod. Res. 2019, 33, 1541–1549. DOI: 10.1080/14786419.2017.1423304.
- Alvares, J. J.; Furtado, I. J. Characterization of Multicomponent Antioxidants from Haloferax alexandrinus GUSF-1 (KF796625). 3 Biotech. 2021, 11, 58–12. DOI: 10.1007/s13205-020-02584-9.
- Kulichevskaya, I. S.; Guzev, V. S.; Gorlenko, V. M.; Liesack, W.; Dedysh, S. N. Rhodoblastus Sphagnicola Sp. Nov., a Novel Acidophilic Purple Non-Sulfur Bacterium from Sphagnum Peat Bog. Int. J. Syst. Evol. Microbiol. 2006, 56, 1397–1402. DOI: 10.1099/ijs.0.63962-0.
- Asker, D. High Throughput Screening and Profiling of High-Value Carotenoids from a Wide Diversity of Bacteria in Surface Seawater. Food Chem. 2018, 261, 103–111. DOI: 10.1016/j.foodchem.2018.03.109.
- Liu, H.; Tan, K. S.; Zhang, X.; Zhang, H.; Cheng, D.; Ting, Y.; Li, S.; Ma, H.; Zheng, H. Comparison of Gut Microbiota between Golden and Brown Noble Scallop Chlamys Nobilis and Its Association with Carotenoids. Front. Microbiol. 2020, 11, 36. DOI: 10.3389/fmicb.2020.00036.
- Yokoyama, A.; Izumida, H.; Miki, W. Production of Astaxanthin and 4-Ketozeaxanthin by the Marine Bacterium. Agrobact. Aurantiac. Biosci. Biotechnol. Biochem. 1994, 58, 1842–1844. DOI: 10.1271/bbb.58.1842.
- Liu, H.; Zhang, C.; Zhang, X.; Tan, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys Nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629. DOI: 10.1016/j.foodchem.2020.126629.
- Asker, D.; Beppu, T.; Ueda, K. Sphingomonas Astaxanthinifaciens Sp. Nov., a Novel Astaxanthin-Producing Bacterium of the Family Sphingomonadaceae Isolated from Misasa, Tottori, Japan. FEMS Microbiol. Lett. 2007, 273, 140–148. DOI: 10.1111/j.1574-6968.2007.00760.x.
- Ye, R. W.; Yao, H.; Stead, K.; Wang, T.; Tao, L.; Cheng, Q.; Sharpe, P. L.; Suh, W.; Nagel, E.; Arcilla, D.; et al. Construction of the Astaxanthin Biosynthetic Pathway in a Methanotrophic Bacterium Methylomonas Sp. Strain 16a. J. Ind. Microbiol. Biotechnol. 2007, 34, 289–299. DOI: 10.1007/s10295-006-0197-x.
- Ye, R. W.; Stead, K. J.; Yao, H.; He, H. Mutational and Functional Analysis of the Beta-carotene Ketolase Involved in the Production of Ccanthaxanthin and Astaxanthin. Appl. Environ. Microbiol. 2006, 72, 5829–5837. DOI: 10.1128/AEM.00918-06.
- Mageswari, A.; Subramanian, P.; Srinivasan, R.; Karthikeyan, S.; Gothandam, K. M. Astaxanthin from Psychrotrophic Sphingomonas Faeni Exhibits Antagonism against Food-Spoilage Bacteria at Low Temperatures. Microbiol. Res. 2015, 179, 38–44. DOI: 10.1016/j.micres.2015.06.010.
- Silva, T. P.; Paixão, S. M.; Alves, L. Ability of: Gordonia Alkanivorans Strain 1B for High Added Value Carotenoids Production. RSC Adv. 2016, 6, 58055–58063. DOI: 10.1039/C6RA08126F.
- Lorquin, J.; Molouba, F.; Dreyfus, B. L. Identification of the Carotenoid Pigment Canthaxanthin from Photosynthetic Bradyrhizobium Strains. Appl. Environ. Microbiol. 1997, 63, 1151–1154. DOI: 10.1128/aem.63.3.1151-1154.1997.
- Venugopalan, V.; Tripathi, S. K.; Nahar, P.; Saradhi, P. P.; Das, R. H.; Gautam, H. K. Characterization of Canthaxanthin Isomers Isolated from a New Soil Dietzia Sp. and Their Antioxidant Activities. J. Microbiol. Biotechnol. 2013, 23, 237–245. DOI: 10.4014/jmb.1203.03032.
- Graham, J. E.; Lecomte, J. T. J.; Bryant, D. A. Synechoxanthin, an Aromatic C40 Xanthophyll That is a Major Carotenoid in the Cyanobacterium Synechococcus Sp. PCC 7002. J. Nat. Prod. 2008, 71, 1647–1650. DOI: 10.1021/np800310b.
- Asker, D.; Beppu, T.; Ueda, K. Mesoflavibacter Zeaxanthinifaciens Gen. Nov., Sp. Nov., a Novel Zeaxanthin-Producing Marine Bacterium of the Family Flavobacteriaceae. Syst. Appl. Microbiol. 2007, 30, 291–296. DOI: 10.1016/j.syapm.2006.12.003.
- Sarnaik, A.; Nambissan, V.; Pandit, R.; Lali, A. Recombinant Synechococcus Elongatus PCC 7942 for Improved Zeaxanthin Production under Natural Light Conditions. Algal Res. 2018, 36, 139–151. DOI: 10.1016/j.algal.2018.10.021.
- Berry, A.; Janssens, D.; Hümbelin, M.; Jore, J. P. M.; Hoste, B.; Cleenwerck, I.; Vancanneyt, M.; Bretzel, W.; Mayer, A. F.; Lopez-Ulibarri, R.; et al. Paracoccus Zeaxanthinifaciens Sp. Nov., a Zeaxanthin-Producing Bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 231–238. DOI: 10.1099/ijs.0.02368-0.
- Henke, N. A.; Frohwitter, J.; Peters-Wendisch, P.; Wendisch, V. F. Carotenoid Production by Recombinant Corynebacterium Glutamicum: Strain Construction, Cultivation, Extraction, and Quantification of Carotenoids and Terpenes. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, 2018; Vol. 1852. pp 127–141. DOI: 10.1007/978-1-4939-8742-9_8.
- Antolak, H.; Oracz, J.; Otlewska, A.; Żyżelewicz, D.; Kręgiel, D. Identification of Carotenoids and Isoprenoid Quinones from Asaia Lannensis and Asaia Bogorensis. Molecules 2017, 22, 1608. DOI: 10.3390/molecules22101608.
- Rezaeeyan, Z.; Safarpour, A.; Amoozegar, M. A.; Babavalian, H.; Tebyanian, H.; Shakeri, F. High Carotenoid Production by a Halotolerant Bacterium, Kocuria Sp. Strain QWT-12 and Anticancer Activity of Its Carotenoid. EXCLI J. 2017, 16, 840–851. DOI: 10.17179/excli2017-218.
- Jinendiran, S.; Dahms, H. U.; Dileep Kumar, B. S.; Kumar Ponnusamy, V.; Sivakumar, N. Diapolycopenedioic-Acid-Diglucosyl Ester and Keto-Myxocoxanthin Glucoside Ester: Novel Carotenoids Derived from Exiguobacterium Acetylicum S01 and Evaluation of Their Anticancer and anti-Inflammatory Activities. Bioorg. Chem. 2020, 103, 104149. DOI: 10.1016/j.bioorg.2020.104149.
- Jinendiran, S.; Dileep Kumar, B. S.; Dahms, H. U.; Arulanandam, C. D.; Sivakumar, N. Optimization of Submerged Fermentation Process for Improved Production of β-Carotene by Exiguobacterium Acetylicum S01. Heliyon 2019, 5, e01730. DOI: 10.1016/j.heliyon.2019.e01730.
- Fukaya, Y.; Takemura, M.; Koyanagi, T.; Maoka, T.; Shindo, K.; Misawa, N. Structural and Functional Analysis of the Carotenoid Biosynthesis Genes of a Pseudomonas Strain Isolated from the Excrement of Autumn Darter. Biosci. Biotechnol. Biochem. 2018, 82, 1043–1052. DOI: 10.1080/09168451.2017.1398069.
- Mercadante, A. Z.; Rodrigues, D. B.; Petry, F. C.; Mariutti, L. R. B. Carotenoid Esters in Foods - A Review and Practical Directions on Analysis and Occurrence. Food Res. Int. 2017, 99, 830–850. DOI: 10.1016/j.foodres.2016.12.018.
- Cerón-García, M. C.; González-López, C. V.; Camacho-Rodríguez, J.; López-Rosales, L.; García-Camacho, F.; Molina-Grima, E. Maximizing Carotenoid Extraction from Microalgae Used as Food Additives and Determined by Liquid Chromatography (HPLC). Food Chem. 2018, 257, 316–324. DOI: 10.1016/j.foodchem.2018.02.154.
- Reis-Mansur, M. C. P. P.; Cardoso-Rurr, J. S.; Silva, J. V. M. A.; de Souza, G. R.; Cardoso, V. D. S.; Mansoldo, F. R. P.; Pinheiro, Y.; Schultz, J.; Lopez Balottin, L. B.; da Silva, A. J. R.; et al. Carotenoids from UV-Resistant Antarctic Microbacterium Sp. LEMMJ01. Sci. Rep. 2019, 9, 9554. DOI: 10.1038/s41598-019-45840-6.
- Mishra, N. N.; Liu, G. Y.; Yeaman, M. R.; Nast, C. C.; Proctor, R. A.; McKinnell, J.; Bayer, A. S. Carotenoid-Related Alteration of Cell Membrane Fluidity Impacts Staphylococcus Aureus Susceptibility to Host Defense Peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. DOI: 10.1128/AAC.00680-10.
- Choi, J. Y.; Lee, K.; Lee, P. C. Characterization of Carotenoid Biosynthesis in Newly Isolated Deinococcus Sp. AJ005 and Investigation of the Effects of Environmental Conditions on Cell Growth and Carotenoid Biosynthesis. Mar. Drugs 2019, 17, 705. DOI: 10.3390/md17120705.
- Jing, Y.; Liu, H.; Xu, W.; Yang, Q. Amelioration of the DSS-Induced Colitis in Mice by Pretreatment with 4,4'-diaponeurosporene-producing Bacillus subtilis. Exp. Ther. Med. 2017, 14, 6069–6073. DOI: 10.3892/etm.2017.5282.
- Iwata, S.; Imai, T.; Shimazawa, M.; Ishibashi, T.; Hayashi, M.; Hara, H.; Nakamura, S. Protective Effects of the Astaxanthin Derivative, Adonixanthin, on Brain Hemorrhagic Injury. Brain Res. 2018, 1698, 130–138. DOI: 10.1016/j.brainres.2018.08.009.
- Vila, E.; Hornero-Méndez, D.; Azziz, G.; Lareo, C.; Saravia, V. Carotenoids from Heterotrophic Bacteria Isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnol. Rep. (Amst). 2019, 21, e00306. DOI: 10.1016/j.btre.2019.e00306.
- Manzoni Maroneze, M.; Jacob-Lopes, E.; Queiroz Zepka, L.; Roca, M.; Pérez-Gálvez, A. Esterified Carotenoids as New Food Components in Cyanobacteria. Food Chem. 2019, 287, 295–302. DOI: 10.1016/j.foodchem.2019.02.102.
- López, G.-D.; Suesca, E.; Álvarez-Rivera, G.; Rosato, A. E.; Ibáñez, E.; Cifuentes, A.; Leidy, C.; Carazzone, C. Carotenogenesis of Staphylococcus Aureus: New Insights and Impact on Membrane Biophysical Properties. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 2021, 1866, 158941. DOI: 10.1016/j.bbalip.2021.158941.
- Saha, S.; Walia, S.; Sharma, K.; Banerjee, K. Suitability of Stationary Phase for LC Analysis of Biomolecules. Crit. Rev. Food Sci. Nutr. 2020, 60, 2856–2873. DOI: 10.1080/10408398.2019.1665494.
- Turcsi, E.; Nagy, V.; Deli, J. Study on the Elution Order of Carotenoids on Endcapped C18 and C30 Reverse Silica Stationary Phases. A Review of the Database. J. Food Compos. Anal. 2016, 47, 101–112. DOI: 10.1016/j.jfca.2016.01.005.
- Jin, H.; Lao, Y. M.; Zhou, J.; Zhang, H. J.; Cai, Z. H. Simultaneous Determination of 13 Carotenoids by a Simple C18 Column-Based Ultra-High-Pressure Liquid Chromatography Method for Carotenoid Profiling in the Astaxanthin-Accumulating Haematococcus Pluvialis. J. Chromatogr. A. 2017, 1488, 93–103. DOI: 10.1016/j.chroma.2017.01.088.
- Kaiser, P.; Geyer, R.; Surmann, P.; Fuhrmann, H. LC-MS Method for Screening Unknown Microbial Carotenoids and Isoprenoid Quinones. J. Microbiol. Methods. 2012, 88, 28–34. DOI: 10.1016/j.mimet.2011.10.001.
- Bridoux, M. C.; Sobiechowska, M.; Pérez-Fuentetaja, A.; Alben, K. T. LC-PDA/APCIitMS Identification of Algal Carotenoid and Oxysterol Precursors to Fatty Acid Esters in Hydrolyzed Extracts of the Freshwater Mussel Dreissena Bugensis. Anal. Bioanal. Chem. 2017, 409, 6745–6760. DOI: 10.1007/s00216-017-0562-9.
- Abate-Pella, D.; Freund, D. M.; Slovin, J. P.; Hegeman, A. D.; Cohen, J. D. An Improved Method for Fast and Selective Separation of Carotenoids by LC-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1067, 34–37. DOI: 10.1016/j.jchromb.2017.09.039.
- Hrvolová, B.; Martínez-Huélamo, M.; Colmán-Martínez, M.; Hurtado-Barroso, S.; Lamuela-Raventós, R. M.; Kalina, J. Development of an Advanced HPLC–MS/MS Method for the Determination of Carotenoids and Fat-Soluble Vitamins in Human Plasma. Int. J. Mol. Sci. 2016, 17, 1719. DOI: 10.3390/ijms17101719.
- Schex, R.; Lieb, V. M.; Jiménez, V. M.; Esquivel, P.; Schweiggert, R. M.; Carle, R.; Steingass, C. B. HPLC-DAD-APCI/ESI-MSn Analysis of Carotenoids and α-Tocopherol in Costa Rican Acrocomia Aculeata Fruits of Varying Maturity Stages. Food Res. Int. 2018, 105, 645–653. DOI: 10.1016/j.foodres.2017.11.041.
- Gallego, R.; Martínez, M.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Development of a Green Downstream Process for the Valorization of Porphyridium Cruentum Biomass. Molecules 2019, 24, 1564. DOI: 10.3390/molecules24081564.
- Gallego, R.; Arena, K.; Dugo, P.; Mondello, L.; Ibáñez, E.; Herrero, M. Application of Compressed Fluid-based Extraction and Purification Procedures to Obtain Astaxanthin-enriched Extracts from Haematococcus pluvialis and Characterization by Comprehensive Two-dimensional Liquid Chromatography Coupled to Mass Spectrometry. Anal. Bioanal. Chem. 2020, 412, 589–599. DOI: 10.1007/s00216-019-02287-y.
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O. H. M.; Suarez-Álvarez, S.; Ferragut, J. A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. García-Cañas, V. Pressurized Liquid Extraction of Neochloris Oleoabundans for the Recovery of Bioactive Carotenoids with anti-Proliferative Activity against Human Colon Cancer Cells. Food Res. Int. 2017, 99, 1048–1055. DOI: 10.1016/j.foodres.2016.05.021.
- van Breemen, R. B. Mass, Spectrometry of Carotenoids. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, 2001; pp F2.4.1–F2.4.13. DOI: 10.3891/acta.chem.scand.28b-0385.
- Carnevale, J.; Cole, E. R.; Nelson, D.; Shannon, J. S. Chemical Ionization Mass Spectrometry of Carotenoids. Biol. Mass Spectrom. 1978, 5, 641–546. DOI: 10.1002/bms.1200051109.
- Breemen, R. B.; Van; Schmitz, H. H.; Schwartz, S. J. Fast Atom Bombardment Tandem Mass Spectrometry of Carotenoids. J. Agric. Food Chem. 1995, 43, 384–389. DOI: 10.1021/jf00050a024.
- Van Breemen, R. B.; Dong, L.; Pajkovic, N. D. Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry of Carotenoids. Int. J. Mass Spectrom. 2012, 312, 163–172. DOI: 10.1016/j.ijms.2011.07.030.
- Huang, S.; Xu, J.; Wu, J.; Hong, H.; Chen, G.; Jiang, R.; Zhu, F.; Liu, Y.; Ouyang, G. Rapid Detection of Five Anesthetics in Tilapias by in Vivo Solid Phase Microextraction Coupling with Gas Chromatography-Mass Spectrometry. Talanta 2017, 168, 263–268. DOI: 10.1016/j.talanta.2017.03.045.
- Schöner, T. A.; Gassel, S.; Osawa, A.; Tobias, N. J.; Okuno, Y.; Sakakibara, Y.; Shindo, K.; Sandmann, G.; Bode, H. B. Aryl Polyenes, a Highly Abundant Class of Bacterial Natural Products, Are Functionally Related to Antioxidative Carotenoids. Chembiochem 2016, 17, 247–253. DOI: 10.1002/cbic.201500474.
- Stafsnes, M. H.; Dybwad, M.; Brunsvik, A.; Bruheim, P. Large Scale MALDI-TOF MS Based Taxa Identification to Identify Novel Pigment Producers in a Marine Bacterial Culture Collection. Anton. Leeuwenhoek 2013, 103, 603–615. DOI: 10.1007/s10482-012-9844-6.
- Yoshida, K.; Ueda, S.; Maeda, I. Carotenoid Production in Bacillus Subtilis Achieved by Metabolic Engineering. Biotechnol. Lett. 2009, 31, 1789–1793. DOI: 10.1007/s10529-009-0082-6.
- Manikandan, M.; Hasan, N.; Wu, H. F. Rapid Detection of Haloarchaeal Carotenoids via Liquid-Liquid Microextraction Enabled Direct TLC MALDI-MS. Talanta 2013, 107, 167–175. DOI: 10.1016/j.talanta.2013.01.005.
- Neto, F. C.; Guaratini, T.; Costa-Lotufo, L.; Colepicolo, P.; Gates, P. J.; Lopes, N. P. Re-Investigation of the Fragmentation of Protonated Carotenoids by Electrospray Ionization and Nanospray Tandem Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2016, 30, 1540–1548. DOI: 10.1002/rcm.7589.
- Rivera, S.; Vilaró, F.; Canela, R. Determination of Carotenoids by Liquid Chromatography/Mass Spectrometry: Effect of Several Dopants. Anal. Bioanal. Chem. 2011, 400, 1339–1346. DOI: 10.1007/s00216-011-4825-6.
- Zerres, S.; Stahl, W. Carotenoids in Human Skin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158588. DOI: 10.1016/j.bbalip.2019.158588.
- Clugston, R. D. Carotenoids and Fatty Liver Disease: Current Knowledge and Research Gaps. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2020, 1865, 158597. DOI: 10.1016/j.bbalip.2019.158597.
- Silva, T. R. E.; Silva, L. C. F.; de Queiroz, A. C.; Alexandre Moreira, M. S.; de Carvalho Fraga, C. A.; de Menezes, G. C. A.; Rosa, L. H.; Bicas, J.; de Oliveira, V. M.; Duarte, A. W. F. Pigments from Antarctic Bacteria and Their Biotechnological Applications. Crit. Rev. Biotechnol. 2021, 41, 809–826. DOI: 10.1080/07388551.2021.1888068.
- Osawa, A.; Ishii, Y.; Sasamura, N.; Morita, M.; Kasai, H.; Maoka, T.; Shindo, K. Characterization and Antioxidative Activities of Rare C(50) Carotenoids-sarcinaxanthin, Sarcinaxanthin Monoglucoside, and Sarcinaxanthin Diglucoside-obtained from Micrococcus yunnanensis. J. Oleo Sci. 2010, 59, 653–659. DOI: 10.5650/jos.59.653.
- Wagener, S.; Völker, T.; De Spirt, S.; Ernst, H.; Stahl, W. 3. 3,3'-Dihydroxyisorenieratene and Isorenieratene Prevent UV-induced DNA Damage in Human Skin Fibroblasts. Free Radic. Biol. Med. 2012, 53, 457–463. DOI: 10.1016/j.freeradbiomed.2012.05.022.
- Wen, X.; Huang, A.; Hu, J.; Zhong, Z.; Liu, Y.; Li, Z.; Pan, X.; Liu, Z. Neuroprotective Effect of Astaxanthin against Glutamate-Induced Cytotoxicity in HT22 Cells: Involvement of the Akt/GSK-3β Pathway. Neuroscience 2015, 303, 558–568. DOI: 10.1016/j.neuroscience.2015.07.034.
- Zhang, X. S.; Zhang, X.; Wu, Q.; Li, W.; Zhang, Q. R.; Wang, C. X.; Zhou, X. M.; Li, H.; Shi, J. X.; Zhou, M. L. Astaxanthin Alleviates Early Brain Injury following Subarachnoid Hemorrhage in Rats: Possible Involvement of Akt/Bad Signaling. Mar. Drugs. 2014, 12, 4291–4310. DOI: 10.3390/md12084291.
- Johnson, E. J.; Vishwanathan, R.; Johnson, M. A.; Hausman, D. B.; Davey, A.; Scott, T. M.; Green, R. C.; Miller, L. S.; Gearing, M.; Woodard, J.; et al. Relationship between Serum and Brain Carotenoids, Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 1–13. DOI: 10.1155/2013/951786.
- Honda, M. Nutraceutical and Pharmaceutical Applications of Carotenoids. In Pigments from Microalgae Handbook; Springer International Publishing: Cham, Switzerland, 2020; pp 449–469. DOI: 10.1007/978-3-030-50971-2_18.
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary Combination of Fucoxanthin and Fish Oil Attenuates the Weight Gain of White Adipose Tissue and Decreases Blood Glucose in Obese/Diabetic KK-Ay Mice. J. Agric. Food Chem. 2007, 55, 7701–7706. DOI: 10.1021/jf071569n.
- Stahl, W. Sies, H. β-Carotene and Other Carotenoids in Protection from Sunlight. Am. J. Clin. Nutr. 2012, 96, 1179–1184. DOI: 10.3945/ajcn.112.034819.
- Lee, C. M. Fifty Years of Research and Development of Cosmeceuticals: A Contemporary Review. J. Cosmet. Dermatol. 2016, 15, 527–539. DOI: 10.1111/jocd.12261.
- Rowles, J. L.; Ranard, K. M.; Applegate, C. C.; Jeon, S.; An, R.; Erdman, J. W. Processed and Raw Tomato Consumption and Risk of Prostate Cancer: A Systematic Review and dose-response meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 319–336. DOI: 10.1038/s41391-017-0005-x.
- Abar, L.; Vieira, A. R.; Aune, D.; Stevens, C.; Vingeliene, S.; Navarro Rosenblatt, D. A.; Chan, D.; Greenwood, D. C.; Norat, T. Blood Concentrations of Carotenoids and Retinol and Lung Cancer Risk: An Update of the WCRF-AICR Systematic Review of Published Prospective Studies. Cancer Med. 2016, 5, 2069–2083. DOI: 10.1002/cam4.676.
- Grewe, C.; Menge, S.; Griehl, C. Enantioselective Separation of All-E-Astaxanthin and Its Determination in Microbial Sources. J. Chromatogr. A. 2007, 1166, 97–100. DOI: 10.1016/j.chroma.2007.08.002.
- Bories, G.; Brantom, P.; J. B. De, B.; Chesson, A.; Sandro, P.; Debski, B.; Dierick, N.; Franklin, A.; Gropp, J.; Halle, I. Opinion of the Scientific Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP) on Safety and Efficacy of Panaferd-AX (Red Carotenoid-Rich Bacterium Paracoccus Carotinifaciens) as Feed Additive for Salmon and Trout. EFSA J. 2007, 5, 1–30. DOI: 10.2903/j.efsa.2007.546.
- Kirti, K.; Amita, S.; Priti, S.; Mukesh Kumar, A.; Jyoti, S. Colorful World of Microbes: Carotenoids and Their Applications. Adv. Biol. 2014, 2014, 1–13. DOI: 10.1155/2014/837891.
- Manikandan, K.; Felix, N.; Prabu, E. A Review on the Application and Effect of Carotenoids with Respect to Canthaxanthin in the Culture of Fishes and Crustaceans. Int. J. Fish. Aquat. Stud. 2020, 8, 128–133. DOI: 10.22271/fish.2020.v8.i5b.2314.
- Pasarin, D.; Rovinaru, C. Sources of Carotenoids and Their Uses as Animal Feed Additives - a Review. Sci. Papers-Ser. D-Anim. Sci. 2018, 61, 74–85.
- Masetto, A.; Flores-Cotera, L. B.; Díaz, C.; Langley, E.; Sanchez, S. Application of a Complete Factorial Design for the Production of Zeaxanthin by Flavobacterium Sp. J. Biosci. Bioeng. 2001, 92, 55–58. DOI: 10.1263/jbb.92.55.
- Barreiro C.; Barredo, J. L. Carotenoids Production: A Healthy and Profitable Industry. In Microbial Carotenoids. Methods in Molecular Biology; Barreiro, C., Barredo, J. L., Eds.; Humana Press: New York, NY, 2018; Vol. 1852. DOI: 10.1007/978-1-4939-8742-9_2.
- Dufossé, L. Microbial Pigments from Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries; Elsevier Inc.: Amsterdam, Netherlands, 2018; Vol. 7. DOI: 10.1016/C2016-0-00380-7.
- Synthesize, O. T. Methods of Administering Probiotic Organisms That Synthesize Carotenoid Compounds in Sity to Enhance Human Helth and Nutrition. US Patent 113–653 B2, 2015.
- Sahli, K.; Gomri, M. A.; Esclapez, J.; Gómez-Villegas, P.; Ghennai, O.; Bonete, M. J.; León, R.; Kharroub, K. Bioprospecting and Characterization of Pigmented Halophilic Archaeal Strains from Algerian Hypersaline Environments with Analysis of Carotenoids Produced by Halorubrum Sp. BS2. J. Basic Microbiol. 2020, 60, 624–638. DOI: 10.1002/jobm.202000083.
- Thanapimmetha, A.; Suwaleerat, T.; Saisriyoot, M.; Chisti, Y.; Srinophakun, P. Production of Carotenoids and Lipids by Rhodococcus Opacus PD630 in Batch and Fed-Batch Culture. Bioprocess Biosyst. Eng. 2017, 40, 133–143. DOI: 10.1007/s00449-016-1681-y.
- Zalazar, L.; Pagola, P.; Miró, M. V.; Churio, M. S.; Cerletti, M.; Martínez, C.; Iniesta-Cuerda, M.; Soler, A. J.; Cesari, A.; De Castro, R. Bacterioruberin Extracts from a Genetically Modified Hyperpigmented Haloferax Volcanii Strain: Antioxidant Activity and Bioactive Properties on Sperm Cells. J. Appl. Microbiol. 2019, 126, 796–810. DOI: 10.1111/jam.14160.
- Joshi, C.; Singhal, R. S. Zeaxanthin Production by Paracoccus Zeaxanthinifaciens ATCC 21588 in a Lab-Scale Bubble Column Reactor: Artificial Intelligence Modelling for Determination of Optimal Operational Parameters and Energy Requirements. Korean J. Chem. Eng. 2018, 35, 195–203. DOI: 10.1007/s11814-017-0253-4.
- Gharibzahedi, S. M. T.; Razavi, S. H.; Mousavi, M. Feeding Strategies for the Improved Biosynthesis of Canthaxanthin from Enzymatic Hydrolyzed Molasses in the Fed-Batch Fermentation of Dietzia Natronolimnaea HS-1. Bioresour. Technol. 2014, 154, 51–58. DOI: 10.1016/j.biortech.2013.12.013.
- Hirasawa, K.; Tsubokura, A. Method for Separating Carotenoid. US Patent 8,853,460 B2, Oct. 7, 2014.
- Asker, D.; Awad, T. S.; Beppu, T.; Ueda, K. Purification and Identification of Astaxanthin and Its Novel Derivative Produced by Radio-Tolerant Sphingomonas Astaxanthinifaciens. Methods Mol. Biol. 2018, 1852, 171–192. DOI: 10.1007/978-1-4939-8742-9_10.
- Asker, D.; Awad, T. S.; Beppu, T.; Ueda, K. Screening, Isolation, and Identification of Zeaxanthin-Producing Bacteria. In Microbial Carotenoids: Methods and Protocols, Methods in Molecular Biology; Springer New York: New York, NY, 2018; Vol. 1852, pp 193–209. DOI: 10.1007/978-1-4939-8742-9_11.
- Gallego, R.; Alves, M. J.; Tardif, C.; Ibáñez, E.; Parreira, C.; Herrero, M.; Guerra, T. Simultaneous Extraction and Purification of Fucoxanthin from Tisochrysis Lutea Microalgae Using Compressed Fluids. J. Separat. Sci. 2020, 43, 1–11. DOI: 10.1002/jssc.202000021.
- Wang, N. N.; Li, C. M.; Li, Y. X.; Du, Z. J. Aquimarina Celericrescens Sp. Nov., Isolated from Seawater. Int. J. Syst. Evol. Microbiol. 2018, 68, 1683–1688. DOI: 10.1099/ijsem.0.002733.
- Wu, Y. H.; Zhou, P.; Jian, S. L.; Liu, Z. S.; Wang, C. S.; Oren, A.; Xu, X. W. Pontibacter Amylolyticus Sp. Nov., Isolated from a Deep-Sea Sediment Hydrothermal Vent Field. Int. J. Syst. Evol. Microbiol. 2016, 66, 1760–1767. DOI: 10.1099/ijsem.0.000944.
- Xamxidin, M.; Wu, Y. H.; Jian, S. L.; Zhou, Y. D.; Wang, C. S.; Tohty, D.; Xu, X. W. Aquaticitalea Lipolytica Gen. Nov., Sp. Nov., Isolated from Antarctic Seawater. Int. J. Syst. Evol. Microbiol. 2016, 66, 2657–2663. DOI: 10.1099/ijsem.0.001101.
- Neelam, D. K.; Agrawal, A.; Tomer, A. K.; Bandyopadhayaya, S.; Sharma, A.; Jagannadham, M. V.; Mandal, C. C.; Dadheech, P. K. A Piscibacillus Sp. Isolated from a Soda Lake Exhibits Anticancer Activity against Breast Cancer Mda-Mb-231 Cells. Microorganisms 2019, 7, 34. DOI: 10.3390/microorganisms7020034.
- Zhou, L. Y.; Yu, Z. L.; Xu, W.; Mu, D. S.; Du, Z. J. Maribellus Luteus Gen. Nov., Sp. Nov., a Marine Bacterium in the Family Prolixibacteraceae Isolated from Coastal Seawater. Int. J. Syst. Evol. Microbiol. 2019, 69, 2388–2394. DOI: 10.1099/ijsem.0.003495.
- Geng, Y.; Zhang, Y.; Qin, K.; Liu, J.; Tian, J.; Huang, Y.; Wei, Z.; Zhang, F.; Peng, F. Sphingomonas Paeninsulae Sp. Nov., Isolated from Soil Sampled at Fildes Peninsula, Antarctica. Int. J. Syst. Evol. Microbiol. 2019, 69, 3702–3709. DOI: 10.1099/ijsem.0.003504.
- Liu, Y. H.; Fang, B. Z.; Dong, Z. Y.; Li, L.; Mohamad, O. A. A.; Zhang, Y. G.; Egamberdieva, D.; Xiao, M.; Li, W. J. Croceibacterium Gen. Nov., with Description of Croceibacterium Ferulae Sp. Nov., an Endophytic Bacterium Isolated from Ferula Sinkiangensis k. m. Shen and Reclassification of Porphyrobacter Mercurialis as Croceibacterium Mercuriale Comb. Nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 2547–2554. DOI: 10.1099/ijsem.0.003540.
- Cai, H.; Cui, H.; Zeng, Y.; An, M.; Jiang, H. Sandarakinorhabdus Cyanobacteriorum Sp. Nov., a Novel Bacterium Isolated from Cyanobacterial Aggregates in a Eutrophic Lake. Int. J. Syst. Evol. Microbiol. 2018, 68, 730–735. DOI: 10.1099/ijsem.0.002571.
- Chun, B. H.; Lee, Y.; Jin, H. M.; Jeon, C. O. Cloacibacterium Caeni Sp. Nov., Isolated from Activated Sludge. Int. J. Syst. Evol. Microbiol. 2017, 67, 1688–1692. DOI: 10.1099/ijsem.0.001841.
- Jia, L.; Feng, X.; Zheng, Z.; Han, L.; Hou, X.; Lu, Z.; Lv, J. Polymorphobacter Fuscus Sp. Nov., Isolated from Permafrost Soil, and Emended Description of the Genus Polymorphobacter. Int. J. Syst. Evol. Microbiol. 2015, 65, 3920–3925. DOI: 10.1099/ijsem.0.000514.