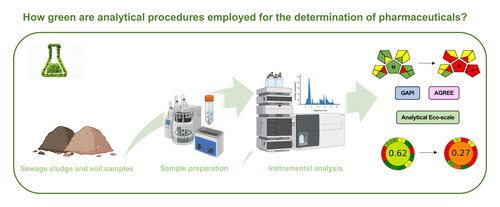
Abstract
The main difficulties when analyzing pharmaceutically active compounds (PhACs) in solid environmental samples is the complexity of the samples and the low concentration levels of such pollutants. Most efforts are focused in achieving good analytical performance parameters such as high recoveries or low detection limits without considering if the methods are environmentally friendly. In this work, the main tools proposed for assessing the greenness of analytical methodologies (Analytical Eco-scale, Green Analytical Procedure Index (GAPI), and Analytical GREEnness metric (AGREE)) have been applied to nine analytical procedures that include recent important analytical tendencies. The three metrics identified the paper spray ionization method as the greenest procedure since it used untreated samples for direct mass spectrometry analysis. Using Analytical Eco-scale, most of the evaluated procedures were rated as “acceptable green”. However, the use of internal standards resulted key in the environmental impact of the method which provided contradictory results versus other metrics. GAPI found greenness similarities between most of selected methods, hindering a greenness classification. AGREE allowed the weighting of each evaluation criterion providing a greenness ranking. The application of each metric detecting their weaknesses and strengths was discussed. The incorporation of validation analytical features in greenness metrics was a gap revealed.
Graphical Abstract
Introduction
The presence of pharmaceutically active compounds (PhACs) in the environment has caused a high concern due to their ubiquitous and inherent biological activity. PhACs have pernicious effects on all kinds of life forms because of their toxicological effects. For example, propranolol has been detected in effluent wastewater at 1900 ng/L.[Citation1] At a concentration of 1000 ng/L, this PhAC can cause changes in the reproduction of the crustacean Hyalella azteca.[Citation2] In the same way, 17β-estradiol, which can induce female characteristics in male fish at a concentration of 41.2 ng/L,[Citation3] was detected in surface water and effluent wastewater at concentrations up to 7901 ng/L[Citation4] and 506.4 ng/L,[Citation5] respectively. A suitable analytical method is the first prerequisite for further research to prioritize the substances and risk assessment.[Citation6,Citation7] Mejías et al.[Citation8] reported that, a total of 180 PhACs and 45 metabolites have been found in sewage sludge samples at concentrations from ng/g to µg/g dry matter. Some of the main difficulties to overcome when developing analytical methods for the determination of PhACs in solid environmental samples are the complexity of the matrix, the low concentration levels of PhACs and the presence of organic, inorganic, and biological materials that can cause interferences.[Citation8,Citation9]
Methods reported for the determination of PhACs in solid environmental samples commonly involves time-consuming and laborious protocols comprising extraction of PhACs from the matrix, removal of interferences and procedures to enhance sensitivity and selectivity. Soxhlet extraction,[Citation10] ultrasound-assisted extraction (UAE),[Citation11,Citation12] microwave-assisted extraction (MAE),[Citation13,Citation14] pressurized-liquid extraction (PLE),[Citation15,Citation16] matrix solid-phase dispersion (MSPD)[Citation17,Citation18] and Quick, Easy, Cheap, Effective, Rugged and Safe method (QuEChERS)[Citation19,Citation20] are the most commonly technique proposed for PhACs. After extraction, extract clean-up is usually applied to remove interfering compounds which could affect the analytical determination. The most used clean-up techniques are solid-phase extraction (SPE)[Citation21–23] and dispersive-SPE (d-SPE).[Citation24,Citation25] Analytical determination is carried out by liquid chromatography-tandem mass spectrometry (LC-MS/MS)[Citation26,Citation27] and, to a lesser extent, by gas chromatography-mass spectrometry (GC-MS).[Citation21,Citation28]
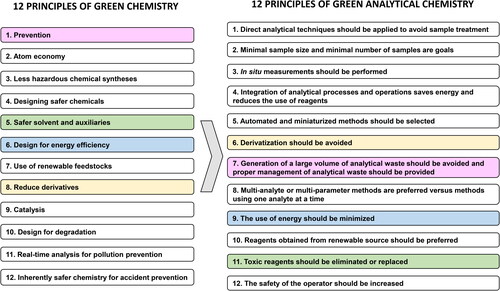
The development of analytical methods to determine PhACs in solid environmental sample has notably increased over the last 10 years.[Citation6,Citation7] In many of these papers, the authors claim that their proposed analytical methods are green[Citation29] however, this feature needs proper corroboration by the application of greenness metrics tools. Since the introduction of the concept of green analytical chemistry (GAC) in 2000, different metrics have been proposed for greenness assessment.[Citation30] Some of the widest scope metrics to assess the greenness of analytical methods are Analytical Eco-Scale,[Citation31] Green Analytical Procedure Index (GAPI)[Citation32] and Analytical GREEnness (AGREE) metric.[Citation33] Other metrics, such as Analytical Method Volume Intensity (AMVI) and High-Pressure Liquid Chromatography-Environmental Assessment Tool (HPLC-EAT), have been designed to be applied to LC determinations.[Citation34] These metrics consider a broad variety of GAC principles () such as the use of minimal sample sizes, low consumption of chemicals and energy, use of reusable and renewable materials and low waste generation.
Learning how to use these greenness analytical metrics to assess and limit the generation of hazardous environmental waste during analytical procedures becomes crucial. The aim of this work is to provide an overview of metrics available for a greenness assessment of analytical methods and to comparatively show their differences by their application to different procedures reported in recent literature for the determination of PhACs in solid environmental samples. Metrics selected for greenness assessment were Analytical Eco-Scale, GAPI, and AGREE. This work attempts to be useful and helpful for users working on analytical method development where GAC principles can be implemented to minimize environmental impact and it can inspire new strategies to reduce the weakest points in given analytical procedures.
Overview of widely use metrics for greenness assessment of analytical procedures
Analytical Eco-Scale, GAPI, and AGREE are relatively most widely used metrics because of their suitability to be applied to most of the analytical procedures.[Citation34] contains a summary with the protocol followed in each case as well as their advantages and disadvantages. The criteria followed in analytical Eco-Scale metric to assign penalty points (PPs), the pictogram color codes in GAPI or the AGREE scores are explained in Tables S1–S3, respectively.
Table 1. Advantages and disadvantages of greenness metrics.
Table 3. Application of analytical eco-scale, GAPI and AGREE metrics to selected methodologies for the determination of PhACs in sludge or soil.
Assessing the green profile of analytical procedures: Case studies
Green assessment of 9 analytical procedures for the determination of PhACs in sludge and soils
A total of nine analytical methods () using different techniques and different extraction approaches in complex sludge and soil samples have been selected for evaluation of their greenness. More information of each method can be found in . The analytical Eco-scale, GAPI and AGREE metrics have been applied to the selected procedures and the comparative results are shown in . The three metrics identified the PS-MS/MS method as the greenest procedure since it used untreated samples for direct mass spectrometry analysis.
Figure 2. Selected analytical procedures involving the determination of PhACs in sludge and soil samples.
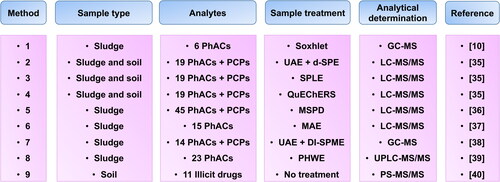
Table 2. Description of selected methodologies for the determination of PhACs in environmental solid samples.
The analytical Eco-Scale classified the greenness of selected methods as follows: PS-MS/MS (73) > MSPD; LC-MS/MS (71) = UAE + d-SPE; LC-MS/MS (71) > selective pressurized-liquid extraction (SPLE); LC-MS/MS (70) = QuEChERS; LC-MS/MS (70) > MAE; LC-MS/MS (69) > UAE + online direct inmersion-solid-phase microextraction (DI-SPME) on-fiber-derivatization; GC-MS (58) > Soxhlet; GC-MS (56) > pressurized hot water extraction (PHWE); SPE + ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) (39). All the procedures, except PHWE + SPE; UPLC-MS/MS, obtained scores in the range from 50-75 which are consider by analytical Eco-scale metric as “acceptable green analysis”. Detailed information of how analytical Eco-scale scores for each analytical procedure were obtained can be seen in Table S4. The main green drawback of PS-MS/MS procedure is the large number of internal standards (n = 7) used and their toxicity. Nevertheless, as internal standards are used at low volumes (10 µL), if analytical Eco-scale score is recalculated without considering the use of internal standards the score of PS-MS/MS procedure increases from 73 to 93, which is very close to the “ideal green analysis” (score: 100). The poor score obtained for PHWE + SPE; UPLC-MS/MS procedure (score: 39), despite the use of water as extraction solvent, is due to the use of hazardous reagents such as methanol (MeOH), silicon carbide and formic acid for extraction and clean-up steps and acetonitrile (ACN) as mobile phase solvent. Other procedures with a low score in analytical Eco-scale are those based on Soxhlet extraction; GC-MS (score: 56) and UAE-DI-SPME; GC-MS (score: 58). The main PPs in Soxhlet; GC-MS procedure are due to the high solvent volume. In UAE-DI-SPME; GC-MS the main PPs are due to the use of several internal standards and derivatization reagents. The strong influence on the final score of the use of internal standards, in turn conditioned by the number of analytes and therapeutic groups to analyze, makes difficult the comparison between the selected procedures using this metric. For instance, multi-residue methods are strongly penalized by the higher number of required internal standards.
The application of GAPI tool revealed PS-MS/MS procedure as the greenest one (8 categories in green, 5 categories in yellow and 2 categories in red) while the rest of the methodologies obtained the same profile: one category was colored in green, 5 categories in yellow and 9 categories in red, hindering a green classification of them. Detailed information of how GAPI green profiles were obtained can be seen in Table S5.
The AGREE approach classified the greenness of selected methods as follows: PS-MS/MS (0.62) > PHWE + SPE; UPLC-MS/MS (0.4) > MSPD; LC-MS/MS (0.34) > UAE-DI-SPME; GC-MS (0.33) = MAE; LC-MS/MS (0.33) > SPLE; LC-MS/MS (0.29) > Soxhlet; GC-MS (0.28) > UAE + d-SPE; LC-MS/MS (0.27) = QuEChERS; LC-MS/MS (0.27). Detailed information of how AGREE scores were obtained can be seen in Table S6. The greenest score was obtained for PS-MS/MS what it is in concordance with results from the other greenness metrics. Except PS-MS/MS and PHWE + SPE; UPLC-MS/MS methods, 7 out of 9 methodologies scored similar punctuation. The characteristics that significantly influenced in the lowest punctuations were due to the fact that the procedure was performed ex situ and consumed high volumes of hazardous solvents. For example, UAE + d-SPE; LC-MS/MS and QuEChERS; LC-MS/MS procedures, both with a score of 0.27, involved a high consumption of MeOH (up to 46.7 mL), an amount relatively higher than those regularly use with these techniques.
Green assessment of critical steps of analytical procedures for the determination of PhACs in sludge and soils
In this section, the green profile of the main parameters of the above-mentioned analytical procedures is comparatively evaluated by means of analytical Eco-scale, GAPI and AGREE metrics.
Sampling and pretreatment
Main problems of sampling of solid environmental samples are related with the lack of uniformity in its composition and the low representativeness of grab sampling. At-line system is not an option in this type of analysis. All the selected methodologies involved off-line sampling protocols and obtained the same punctuation in this step with AGREE and GAPI metrics. The analytical Eco-Scale metric does not consider the sampling step. Problems related to transportation and storage are also concerned and incompatible with GAC due to energy use. The use of passive sampling has been recently installed for water matrices to avoid periodic transportation to sampling site. However, it is a practice uncommon in sludge and soil samples. To our knowledge, some passive samplers have been developed to be placed in sediments, but they monitor contaminants in the sediment porewater, not in the sediment itself.
Once in the lab, sludge and soil samples require of a homogenization pretreatment. Although these operations are not polluting they require time and personal implication. Except the PS-MS/MS procedure, the selected methods required a homogenization pretreatment workflow consisting of drying, grinding, and sieving. Nevertheless, it must be point out that PS-MS/MS procedure was developed for soil samples whereas the others were developed for sewage sludge, which is a more heterogeneous matrix.
Extraction and clean-up steps
Procedures for the determination of PhACs in sludge and soil samples commonly require several extraction and/or clean-up steps to remove interferences and to enhance sensitivity and selectivity of the method. In AGREE metric, the number of steps to be performed is critical, with a relative penalization of 0.2 over 1 for each step. In GAPI metric, the number of steps of the methodology affects the score of two of the sixteen parameters evaluated: the type of method parameter (5th parameter) and additional treatments parameter (8th parameter). The analytical Eco-Scale metric does not consider the number of steps required by the methodologies.
Extraction procedure offers many options, and considerable efforts and progress is being made to make this step greener. A comparative table including advantages and disadvantages from a GAC perspective of main extraction techniques applied to determine PhACs in solid environmental samples is presented in .
Table 4. Comparative overview of sample treatment protocols reported for the determination of PhACs in solid environmental samples.
The use of untreated samples for direct MS analysis began in earnest with the development of ambient desorption/ionization techniques.[Citation41] It requires minimal sample amount, minimal reagents and solvent consumption and minimal waste generation in addition to no sample preparation steps and a lower occupational risk. Despite being mainly used for liquid samples, PS-MS/MS has been proposed to the analysis of solid samples that can be easily placed or coated on a paper tip surface. However, matrix effects are unavoidable which results in selectivity and sensitivity problems, especially in complex samples such as sludge matrices. Previous studies have utilized this technique in clinical diagnostics, forensics, and in less proportion in environmental monitoring.[Citation42,Citation43] To our knowledge, only an application based on PS-MS/MS has been found in the literature although it was focus on the analysis of PhACs in soil samples and compounds ionized in positive mode.[Citation40]
Clean-up as an additional step uses extra reagents and resources. SPE and d-SPE are the most successfully applied due to the possibility of automatization and the vast diversity of commercial sorbents available (such as OASIS HLB, Strata X, or Sep-Pak C18 plus) which are generally applicable to a wide spectrum of organic pollutants. However, it is possible to have a significant negative impact in some cases due to poor recovery after purification. Recently, a green option considered to improve the sustainability of analysis is the propose of more selective adsorbent reducing the possibility of coelution of similar analytes and consequently the need for high-quality and expensive equipment to separate PhACs from interferences. Gilant et al.[Citation44] synthesized two novel polymers with strong cation-exchange character to be applied as selective sorbents for the SPE of PhACs and illicit drugs from wastewater samples. On the other hand, the use of commercial cartridges in large environmental screenings significantly increases the cost of the analytical process. As consequence, the use of natural and regenerated adsorbents is another important area of research and may also provide a solution to enhance the greenness. Cellulose-based sorbent was used for the determination of nonsteroidal anti-inflammatory drugs in environmental water samples.[Citation45]
While the use of miniaturized clean-up techniques to determine PhACs in water matrices is widely established, their used in solid environmental matrices is scarce and comparatively lower. Dowling et al.[Citation40] compared PS-MS/MS (without the need for off-line extraction) with salting-out assisted liquid-liquid extraction (SALLE) prior to PS-MS/MS. The addition of the SALLE step allowed six-fold lower limits of detection and an improvement of the precision and linearity of the method. The implementation of online coupling of treatment techniques with analytical instruments is a hot topic in the GAC since it allows to process samples in short times, to improve health and safety of the analyst, or to reduce solvent consumption.[Citation46] The application of online SPE allows for the efficient and automated preparation of small sample volumes with improved reliability and performance. Ferhi et al.[Citation47] developed an analytical method for the determination of 14 PhACs in soil and sewage sludge using online-SPE as clean-up step after an extraction procedure based on QuEChERS method. Besides the reduction of solvent consumption this strategy allowed to reduce plastic waste generation and analysis cost as cartridges are reusable for various samples.
Reagents and solvent consumption and waste generation
The amounts of toxic reagents or solvents used is a critical issue in the three metrics. Regarding the solvent choice, not significant differences were found between techniques. Mixtures MeOH:H2O are the most used for PhACs extraction. Mixtures of MeOH or ACN with other solvents, salts and acids have also been proposed. However, significant differences can be found in the amount of solvent and time consumed. It should be also underlined that despite using the same analytical technique differences exist in the literature reviewed in terms of the quantity of sample treated and the amount of solvent.[Citation7] For example, UAE technique was selected using from 1 g of digested sludge and 9 mL of MeOH:acetic acid (1:1; v/v),[Citation48] 2 g of secondary sludge and 30 mL MeOH:H2O (1:1; v/v)[Citation49] or to 0.5 g of primary sludge and 60 mL of MeOH.[Citation50]
The highest the sample mass is, the highest the solvent volumes required are. The sample mass used varied from 25 mg in direct analysis (PS-MS/MS) (no solvent) to 200 g used in Soxhlet extraction (300 mL solvent). The analytical Eco-Scale and GAPI establish PPs or assign colors based on the amounts of solvent/reagent used. In both metrics, the same ranges are proposed: < 10 mL (g); 10-100 mL (g) and > 100 mL (g). PS-MS/MS method was the only one classified as a micro-extraction method due to the low solvent volume used (165 µL). However, these ranges do not allow to positively differentiate microextraction procedures requiring micro- or nano- volumes. For example, a method employing 50 µL of an ethylenediaminetetraacetic acid solution[Citation39] would have the same PPs that a method using 6 mL of MeOH as extraction solvent.[Citation36] The same problem occurs when considering waste generation. In both analytical Eco-Scale and GAPI the same ranges are proposed for green evaluation of waste amount: < 1 mL (g) (green); 1-10 mL (g) (yellow) and > 10 mL (g) (red). Therefore, the same penalization was assigned for both procedures Soxhlet; GC-MS and UAE + DI-SPME; GC-MS (8 PPs with Analytical Eco-scale and red label using GAPI) even though the waste amount was twenty times higher with the former (301 mL of Soxhlet; GC-MS versus 13 mL of UAE + DI-SPME; GC-MS procedure). AGREE metric allows to introduce the specific amount of toxic reagents or solvents used and waste generation as the score is calculated from an equation where the specific volume or mass of reagent or waste is introduced.
Internal standards and derivatization agents
Methods using internal standards are strongly penalized by analytical Eco-Scale metric as this metric considers internal standards as any other type of reagents. Internal standards are used at very low volumes (just a few µL) but this fact does not allow to minimize their impact in the greenness assessment of the analytical procedure. For instance, the usage of 9 mL of an organic solvent for extraction would be assigned the same number of PPs as a few µL of internal standard. Only one of the selected methods, the one based on UAE + online-DI-SPME-on-fiber derivatization; GC-MS/MS[Citation38] required a derivatization step prior GC-MS determination. AGREE metric allows to introduce the CAS number of the derivatization agent to evaluate its toxicity whereas GAPI metric considers derivatization in one point of the whole evaluation (point 8, preparation pentagram, Figure S1). The analytical Eco-Scale does not specifically consider the derivatization step.
Analytical determination: LC and GC comparison
LC exhibits so far, a superior applicability due to PhACs polar nature. Nevertheless, methodologies involving LC determination could result less green than GC. For instance, they do not require derivatization agents but produce a large amount of organic solvents and reagents used as components of the mobile phase. For example, using LC with a 50:50 ratio of organic phase and buffer at 1 mL/min flow rate generates 720 mL of organic waste and 720 mL of aqueous buffer waste containing toxic chemicals in a single working day. Organic solvents like MeOH and ACN have a highly adverse environmental impact.
The main goal in LC regarding GAC have been achieved by applying shorter and smaller diameter chromatographic columns, which implies high pressure system. The lower the diameter is, the lower flow-rate is required resulting in a lower mobile phase volumes required. From 4.6 mm to 2.1 mm internal diameter columns there is a 79% reduction in volume. Li et al.[Citation36] used a 4.6 mm of internal diameter column wasting a total mobile phase volume of 9 mL for sample (18 min of total run time and 0.5 mL/min of flow-rate) while Dorival García et al.[Citation37] employed a 2.1 mm of internal diameter column using a total mobile phase volume for each sample of 5.2 mL (13 min of total run time and 0.4 mL/min of flow-rate). UPLC and nano-LC has progressed based on HPLC. The average run time of UPLC is at least 20% less, due to the improved separation efficiency. The usage of short columns and fused core particle columns (with smaller sizes) are some proposals to make the elution faster and reduce waste. In relation to the column length, Dorival-García et al.[Citation37] can separate 15 PhACs in a Kinetex C18 column (100 mm x 2.1 mm i.d., 2.6 μm particle size) using up to 5.2 mL of mobile phase in 13 min of total run time while Svahn & Björklund (2019)[Citation39] get separate up to 23 PhACs employing a shorter column Acquity UPLC BEH C18 (50 mm x 2.1 mm i.d., 1.8 μm particle size) using 3.2 mL of mobile phase in 8 min of total run time.
Furthermore, it is logical to consider greener multiresidue methodologies since they can determine many analytes in the shortest time. However only AGREE considers this fact. The methodology developed by Gupta & Thakur[Citation10] based on Soxhlet + GC-MS determined the higher number of analytes by sample (up to 85 analytes from which 6 were PhACs), however, the analysis needed up to 1 sample per hour. With the method proposed by Dowling et al.[Citation40] (PS-MS/MS) up to 11 analytes were determined but up to 60 samples can be analyzed in 1 h. The time and the number of analytes have not been considered with analytical Eco-Scale and GAPI.
Energy
The three selected metrics classify the energy consumption into three ranges (≤0.1 kWh per sample, ≤1.5 kWh per sample and >1.5 kWh per sample).[Citation31–33] Using GAPI, all methodologies were classified as red. Energy consumption has quite low weight in the final analytical Eco-Scale score. Instruments that consume less than 0.1 kWh (i.e., vortex, centrifuge, or evaporator) are not penalized. If medium or high energy consumption instruments are used, the difference in penalty compared to not using any instrument is only one or two points respectively. AGREE considers a relative penalization of 0.5 over 1 for each range.
Selected methodologies involved MS determination, which implied in all cases an energy consumption of >1.5 kWh per sample. This fact makes irrelevant the energy consumption due to other equipment in the greenness evaluation of the complete procedure. It should be interesting to consider the energy consumption of sample treatment and analytical determination separately. Energy consumption is influenced by other variables such as the water content of the matrix, the amount of sample used or if the samples are treated in series or in parallel, as the energy requirements of the device should be divided by the number of samples processed simultaneously. For example, for the determination of PhACs in sludge samples the water content is usually removed. The greener and cheaper option of air-drying is uncommon due to contamination problems. Lyophilization is the preferred option to such end since it is a simple, time-effective method and PhACs are not degraded. Nonetheless it is an expensive apparatus with high consumption of energy[Citation51] (>1.5 kWh per sample). Centrifugation and decantation are an alternative to separate suspended solids from wastewater although the material is not completely dry.[Citation37] Heating in an oven at low temperature imply a greater period, may cause analyte degradation and it also consumes high energy content (> 0.1 kWh per sample).[Citation52]
Occupational risk
The analytical Eco-Scale and GAPI tool consider the existence of occupational risk during the development of a methodology. However, no differences were observed in this regard between selected methodologies, with the exception of PS-MS/MS method.[Citation40] The penalty or color assigned to occupational risk lacks specificity, only discriminating between “yes” if the process is hermitized or “no” if there is gas/vapors emission. AGREE tool considers the operator’s safety by selecting threats that are not avoided when performing the procedure, such as “bioacumulative”, “corrosive”, “persistent”, “highly flammable” or “explosive”, among others, with a relative penalization of 0.2 over 1 for each number of threats. However, other factors were missed in this section. For example, dried samples are routinely homogenized by grinding and sieving (<2 mm). These operations are not polluting but require time and personal implication.
Analytical features
The incorporation of analytical features (matrix effect, sensitivity, selectivity, etc.) in green metrics was a gap revealed in all cases. Although LC with diode array and fluorescence detectors may be a low-cost technology suited in some cases for PhACs in sewage sludge,[Citation11] the hyphenation with MS detection is increasingly the preferred technique for PhAC analysis because of its undeniably powerful qualitative and quantitative capabilities. Coupling LC with MS detection is key to increase sensitivity, selectivity, and structural confirmation in PhAC analysis. The main inconvenient is the powerful consumption and its elevated cost.
On the other hand, matrix effects negatively influence the quantification of PhACs in both LC-MS/MS and GC-MS analyses. To compensate matrix effects, besides the use of internal standard matrix-matched or isotope dilution are commonly employed. However, the use of these types of calibration entails the preparation of a different calibration curve per sample and contribute to enhanced reagent consumption, operator time and waste generation and require a significant economic investment, which is usually in disagreement with GAC principles. This fact was not considered in the evaluation of the greenness of analytical methodologies, but its contribution should not be underestimated.
Conclusions
The main procedures used to determine PhACs in solid environmental samples were examined from a green perspective using Analytical Eco-scale, GAPI, and AGREE metrics. Analytical Eco-Scale resulted an easy tool although the obtained score does not provide information about the stages of the analytical procedures responsible of the highest PPs values. GAPI allows at a glance clearly highlight the weakest greenness points of an analytical procedure. However, it was limited in its ability to evaluate the greenness of the methods for comparing purposes. AGREE resulted the most useful tool for comparing purposes and satisfies both requirements, it allows qualitative and quantitative analysis.
Although sludge and soil samples are complex matrices and sample preparation is tedious and time-consuming, significant efforts have been made to improve the greenness of analytical procedures. Developed procedures to determine PhACs in these matrices can be classified as acceptable green although still far of an excellent green analysis. PS-MS/MS was classified as the greenest methodology by the three metrics considered. It requires minimal sample preparation steps and chemicals. However, matrix effects are unavoidable which results in selectivity and sensitivity problems, especially in complex samples such as sludge matrices. Results of AGREE and GAPI also showed the green superiority of MSPD and PHWE over other extraction techniques such as Soxhlet, while discrepancy results were obtained using analytical Eco-Scale that sited PHWE as inadequate green analysis due to the use of internal standards. The incorporation of validation analytical features (accuracy, precision, or sensitivity) in green metrics was a gap revealed in all cases although all of them resulted suitable for its use in the assessment of the environmental impact. The implementation of GAC principles can affect the validation parameters; therefore, the compromise between these factors and GAC requirements must be achieved.
The development of more environmentally friendly analytical methods by the on-line coupling of some treatment steps with analytical instruments, the use of eco-friendly solvents, the application of environmentally friendly SPE sorbents, and the miniaturization (i.e., lower diameter and shorter columns, microfluidic analysis) and greater automatization of the techniques are some future tasks in this research area.
| Abbreviations | ||
| ACN | = | acetonitrile |
| AGREE | = | Analytical GREEnness |
| AMVI | = | Analytical Method Volume Intensity |
| DI-SPME | = | direct inmersion-solid-phase microextraction |
| d-SPE | = | dispersive solid-phase extraction |
| GAC | = | Green Analytical Chemistry |
| GC-MS | = | gas chromatography-mass spectrometry |
| GAPI | = | Green Analytical Procedure Index |
| HPLC-EAT | = | High-Pressure Liquid Chromatography-Environmental Assessment Tool |
| QuEChERS | = | Quick, Easy, Cheap, Effective, Rugged and Safe method |
| LC-MS/MS | = | liquid chromatography-tandem mass spectrometry |
| MAE | = | microwave-assisted extraction |
| MeOH | = | methanol |
| MSPD | = | matrix solid-phase dispersion |
| nano-LC | = | nano-liquid chromatography |
| PCPs | = | personal care products |
| PhACs | = | pharmaceutically active compounds |
| PHWE | = | pressurized-hot water extraction |
| PLE | = | pressurized-liquid extraction |
| PPs | = | penalty points |
| PS | = | paper spray ionization |
| PS-MS/MS | = | paper spray ionization-tandem mass spectrometry |
| SALLE | = | salting-out assisted liquid-liquid extraction |
| SPE | = | solid-phase extraction |
| SPLE | = | selective pressurized-liquid extraction |
| UAE | = | ultrasound-assisted extraction |
| UPLC-MS/MS | = | ultra-performance liquid chromatography-tandem mass spectrometry. |
Supplemental Material
Download PDF (407 KB)Additional information
Funding
References
- Mohapatra, S.; Huang, C.; Mukherji, S.; Padhye, L. P. Occurrence and Fate of Pharmaceuticals in WWTPs in India and Comparison with a Similar Study in the United States. Chemosphere 2016, 159, 526–535. DOI: 10.1016/j.chemosphere.2016.06.047.
- Hugget, D. B.; Brooks, B. W.; Peterson, B.; Foran, C. M.; Schlenk, D. Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. DOI: 10.1007/s00244-002-1182-7.
- Rose, J.; Holbech, H.; Lindholst, C.; Norum, U.; Povlsen, A.; Korsgaard, B.; Bjerregaard, P. Vitellogenin Induction by 17b-Estradiol and 17a-Ethinylestradiol in Male Zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 131, 531–539. DOI: 10.1016/S1532-0456(02)00035-2.
- Griffero, L.; Alcántara-Durán, J.; Alonso, C.; Rodríguez-Gallego, L.; Moreno-González, D.; García-Reyes, J. F.; Molina-Díaz, A.; Pérez-Parada, A. Basin-Scale Monitoring and Risk Assessment of Emerging Contaminants in South American Atlantic Coastal Lagoons. Sci. Total Environ. 2019, 697, 134058. DOI: 10.1016/j.scitotenv.2019.134058.
- Valdés, M. E.; Marino, D. J.; Wunderlin, D. A.; Somoza, G. M.; Ronco, A. E.; Carriquiriborde, P. Screening Concentration of E1, E2 and EE2 in Sewage Effluents and Surface Waters of the “Pampas” Region and the “Río de la Plata” Estuary (Argentina). Bull. Environ. Contam. Toxicol. 2015, 94, 29–33. DOI: 10.1007/s00128-014-1417-0.
- Martín-Pozo, L.; Alarcón-Gómez, B.; Rodríguez-Gómez, R.; García-Córcoles, M. T.; Çipa, M.; Zafra-Gómez, A. Analytical Methods for the Determination of Emerging Contaminants in Sewage Sludge Samples. A Review. Talanta 2019, 192, 508–533. DOI: 10.1016/j.talanta.2018.09.056.
- Pérez-Lemus, N.; López-Serna, R.; Pérez-Elvira, S. I.; Barrado, E. Analytical Methodologies for the Determination of Pharmaceuticals and Personal Care Products (PPCPs) in Sewage Sludge: A Critical Review. Anal. Chim. Acta 2019, 1083, 19–40. DOI: 10.1016/j.aca.2019.06.044.
- Mejías, C.; Martín, J.; Santos, J. L.; Aparicio, I.; Alonso, E. Occurrence of Pharmaceuticals and Their Metabolites in Sewage Sludge and Soil: A Review on Their Distribution and Environmental Risk Assessment. Trends Environ. Anal. Chem. 2021, 30, e00125. DOI: 10.1016/j.teac.2021.e00125.
- Santos, J. L.; Martín, J.; Mejías, C.; Aparicio, I.; Alonso, E. Pharmaceuticals and Their Metabolites in Sewage Sludge and Soils: Distribution and Environmental Risk Assessment. In Handbook of Environmental Chemistry; Nuñez-Delgado, A., Arias-Estévez, M., Eds.; Springer Science and Business Media: Deutschland GmbH, 2023, pp 19–36. DOI: 10.1007/698_2022_847.
- Gupta, A.; Thakur, I. S. Biodegradation of Wastewater Organic Contaminants Using Serratia sp. ISTVKR1 Isolated from Sewage Sludge. Biochem. Eng. J. 2015, 102, 115–124. DOI: 10.1016/j.bej.2015.02.007.
- Martín, J.; Santos, J. L.; Aparicio, I.; Alonso, E. Multi-Residue Method for the Analysis of Pharmaceutical Compounds in Sewage Sludge, Compost and Sediments by Sonication-Assisted Extraction and LC Determination. J. Sep. Sci. 2010, 33, 1760–1766. DOI: 10.1002/jssc.200900873.
- Huidobro-López, B.; López-Heras, I.; Alonso-Alonso, C.; Martínez-Hernández, V.; Nozal, L.; de Bustamante, I. Analytical Method to Monitor Contaminants of Emerging Concern in Water and Soil Samples from a Non-Conventional Wastewater Treatment System. J. Chromatogr. A 2022, 1671, 463006. DOI: 10.1016/j.chroma.2022.463006.
- Balakrishnan, V. K.; Exall, K. N.; Toito, J. M. The Development of a Microwave-Assisted Extraction Method for the Determination of Sulfonamide Antibiotics in Sediments and Soils. Can. J. Chem. 2014, 92, 369–377. DOI: 10.1139/cjc-2013-0457.
- Morales-Toledo, A.; Afonso-Olivares, C.; Montesdeoca-Esponda, S.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. Optimization and Development of SPE and MAE Combined with UHPLCFD for the Determination of Acetylsalicylic Acid, Naproxen, Ibuprofen and Gemfibrozil in Sewage and Sludge Samples. Curr. Anal. Chem. 2016, 12, 545–552. DOI: 10.2174/1573411012666160113235153.
- Pamreddy, A.; Hidalgo, M.; Havel, J.; Salvadó, V. Determination of Antibiotics (Tetracyclines and Sulfonamides) in Biosolids by Pressurized Liquid Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1298, 68–75. DOI: 10.1016/j.chroma.2013.05.014.
- Salvia, M. V.; Fieu, M.; Vulliet, E. Determination of Tetracycline and Fluoroquinolone Antibiotics at Trace Levels in Sludge and Soil. Appl. Environ. Soil Sci. 2015, 2015, 1–10. DOI: 10.1155/2015/435741.
- Cerqueira, M. B. R.; Soares, K. L.; Caldas, S. S.; Primel, E. G. Sample as Solid Support in MSPD: A New Possibility for Determination of Pharmaceuticals, Personal Care and Degradation Products in Sewage Sludge. Chemosphere 2018, 211, 875–883. DOI: 10.1016/j.chemosphere.2018.07.165.
- Castro, G.; Roca, M.; Rodríguez, I.; Ramil, M.; Cela, R. Identification and Determination of Chlorinated Azoles in Sludge Using Liquid Chromatography Quadrupole Time-of-Flight and Triple Quadrupole Mass Spectrometry Platforms. J. Chromatogr. A 2016, 1476, 69–76. DOI: 10.1016/j.chroma.2016.11.020.
- Benedetti, B.; Majone, M.; Cavaliere, C.; Montone, C. M.; Fatone, F.; Frison, N.; Laganà, A.; Capriotti, A. L. Determination of Multi-Class Emerging Contaminants in Sludge and Recovery Materials from Waste Water Treatment Plants: Development of a Modified QuEChERS Method Coupled to LC–MS/MS. Microchem. J. 2020, 155, 104732. DOI: 10.1016/j.microc.2020.104732.
- Rashid, A.; Mazhar, S. H.; Zeng, Q.; Kiki, C.; Yu, C. P.; Sun, Q. Simultaneous Analysis of Multiclass Antibiotic Residues in Complex Environmental Matrices by Liquid Chromatography with Tandem Quadrupole Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1145, 122103. DOI: 10.1016/j.jchromb.2020.122103.
- Ademoyegun, O. T.; Okoh, O. O.; Okoh, A. I. Method Validation and Investigation of the Levels of Pharmaceuticals and Personal Care Products in Sludge Ofwastewater Treatment Plants and Soils of Irrigated Golf Course. Molecules 2020, 25, 3114. DOI: 10.3390/molecules25143114.
- Verma, R.; Dhingra, G.; Malik, A. K. A Comprehensive Review on Metal Organic Framework Based Preconcentration Strategies for Chromatographic Analysis of Organic Pollutants. Crit. Rev. Anal. Chem. 2023, 53, 415–441. DOI: 10.1080/10408347.2021.1964344.
- Verma, R.; Dhingra, G.; Singh, G.; Singh, J.; Dureja, N.; Malik, A. K. Efficient Turn-On Zr Based Metal Organic Framework Fluorescent Sensor for Ultrafast Detection of Danofloxacin in Milk Samples. J. Fluoresc. 2023, DOI: 10.1007/s10895-023-03379-w.
- Shi, X.; Zhang, S.; Zhang, Y.; Geng, Y.; Wang, L.; Peng, Y.; He, Z. Novel and Simple Analytical Method for Simultaneous Determination of Sulfonamide, Quinolone, Tetracycline, Macrolide, and Chloramphenicol Antibiotics in Soil. Anal. Bioanal. Chem. 2022, 414, 6497–6506. DOI: 10.1007/s00216-022-04206-0.
- Dorival-García, N.; Labajo-Recio, C.; Zafra-Gómez, A.; Juárez-Jiménez, B.; Vílchez, J. L. Improved Sample Treatment for the Determination of 17 Strong Sorbed Quinolone Antibiotics from Compost by Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. Talanta 2015, 138, 247–257. DOI: 10.1016/j.talanta.2015.03.011.
- Muriuki, C.; Kairigo, P.; Home, P.; Ngumba, E.; Raude, J.; Gachanja, A.; Tuhkanen, T. Mass Loading, Distribution, and Removal of Antibiotics and Antiretroviral Drugs in Selected Wastewater Treatment Plants in Kenya. Sci. Total Environ. 2020, 743, 140655. DOI: 10.1016/j.scitotenv.2020.140655.
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qin, S.; Luo, Y. Occurrence and Distribution of Sulfonamides, Tetracyclines, Quinolones, Macrolides, and Nitrofurans in Livestock Manure and Amended Soils of Northern China. Environ. Sci. Pollut. Res. Int. 2015, 22, 4545–4554. DOI: 10.1007/s11356-014-3632-y.
- Bo, L.; Feng, L.; Fu, L.; Li, X.; Li, P.; Zhang, Y. The Fate of Typical Pharmaceuticals in Wastewater Treatment Plants of Xi’an City in China. J. Environ. Chem. Eng. 2015, 3, 2203–2211. DOI: 10.1016/j.jece.2015.08.001.
- Hussein, A. R.; Gburi, M. S.; Muslim, N. M.; Azooz, E. A. A Greenness Evaluation and Environmental Aspects of Solidified Floating Organic Drop Microextraction for Metals: A Review. Trends Environ. Anal. Chem. 2023, 37, e00194. DOI: 10.1016/j.teac.2022.e00194.
- Imam, M. S.; Abdelrahman, M. M. How Environmentally Friendly is the Analytical Process? A Paradigm Overview of Ten Greenness Assessment Metric Approaches for Analytical Methods. Trends Environ. Anal. Chem. 2023, 38, e00202. DOI: 10.1016/j.teac.2023.e00202.
- Gałuszka, A.; Migaszewski, Z. M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. Trends Anal. Chem. 2012, 37, 61–72. DOI: 10.1016/j.trac.2012.03.013.
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. DOI: 10.1016/j.talanta.2018.01.013.
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE - Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. DOI: 10.1021/acs.analchem.0c01887.
- Sajid, M.; Płotka-Wasylka, J. Green Analytical Chemistry Metrics: A Review. Talanta 2022, 238, 123046. DOI: 10.1016/j.talanta.2021.123046.
- Malvar, J. L.; Santos, J. L.; Martín, J.; Aparicio, I.; Alonso, E. Comparison of Ultrasound-Assisted Extraction, QuEChERS and Selective Pressurized Liquid Extraction for the Determination of Metabolites of Parabens and Pharmaceuticals in Sludge. Microchem. J. 2020, 157, 104987. DOI: 10.1016/j.microc.2020.104987.
- Li, M.; Sun, Q.; Li, Y.; Lv, M.; Lin, L.; Wu, Y.; Ashfaq, M.; Yu, C.-P. Simultaneous Analysis of 45 Pharmaceuticals and Personal Care Products in Sludge by Matrix Solid-Phase Dispersion and Liquid Chromatography Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 4953–4964. DOI: 10.1007/s00216-016-9590-0.
- Dorival-García, N.; Zafra-Gómez, A.; Camino-Sánchez, F. J.; Navalón, A.; Vílchez, J. L. Analysis of Quinolone Antibiotic Derivatives in Sewage Sludge Samples by Liquid Chromatography-Tandem Mass Spectrometry: Comparison of the Efficiency of Three Extraction Techniques. Talanta 2013, 106, 104–118. DOI: 10.1016/j.talanta.2012.11.080.
- López-Serna, R.; Marín-de-Jesús, D.; Irusta-Mata, R.; García-Encina, P. A.; Lebrero, R.; Fdez-Polanco, M.; Muñoz, R. Multiresidue Analytical Method for Pharmaceuticals and Personal Care Products in Sewage and Sewage Sludge by Online Direct Immersion SPME on-Fiber Derivatization – GCMS. Talanta 2018, 186, 506–512. DOI: 10.1016/j.talanta.2018.04.099.
- Svahn, O.; Björklund, E. Extraction Efficiency of a Commercial Espresso Machine Compared to a Stainless-Steel Column Pressurized Hot Water Extraction (PHWE) System for the Determination of 23 Pharmaceuticals, Antibiotics and Hormones in Sewage Sludge. Appl. Sci. 2019, 9, 1509. DOI: 10.3390/app9071509.
- Dowling, S.; McBride, E. M.; McKenna, J.; Glaros, T.; Manicke, N. E. Direct Soil Analysis by Paper Spray Mass Spectrometry: Detection of Drugs and Chemical Warfare Agent Hydrolysis Products. Forensic Chem. 2020, 17, 100206. DOI: 10.1016/j.forc.2019.100206.
- Zacometti, C.; Tata, A.; Stella, R.; Leone, S.; Pallante, I.; Merenda, M.; Catania, S.; Pozzato, N.; Piro, R. DART-HRMS Allows the Detection of Toxic Alkaloids in Animal Autopsy Specimens and Guides the Selection of Confirmatory Methods in Accidental Plant Poisoning. Anal. Chim. Acta 2023, 1264, 341309. DOI: 10.1016/j.aca.2023.341309.
- Alves, M. V. S.; Maciel, L. I. L.; Passos, J. O. S.; Morais, C. L. M.; Dos Santos, M. C. D.; Lima, L. A. S.; Vaz, B. G.; Pegado, R.; Lima, K. M. G. Spectrochemical Approach Combined with Symptoms Data to Diagnose Fibromyalgia through Paper Spray Ionization Mass Spectrometry (PSI-MS) and Multivariate Classification. Sci. Rep. 2023, 13, 4658. DOI: 10.1038/s41598-023-31565-0.
- Bartella, L.; Mazzotti, F.; Talarico, I. R.; Santoro, I.; Di Donna, L. Paper Spray Tandem Mass Spectrometry for Assessing Oleic, Linoleic and Linolenic Acid Content in Edible Vegetable Oils. Separations 2023, 10, 26. DOI: 10.3390/separations10010026.
- Gilart, N.; Cormack, P. A. G.; Marcé, R. M.; Fontanals, N.; Borrull, F. Selective Determination of Pharmaceuticals and Illicit Drugs in Wastewaters Using a Novel Strong Cation-Exchange Solid-Phase Extraction Combined with Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1325, 137–146. DOI: 10.1016/j.chroma.2013.12.012.
- Abujaber, F.; Zougagh, M.; Jodeh, S.; Rios, A.; Guzman-Bernardo, F. J.; Rodriguez-Martin-Doimeadios, R. C. Magnetic Cellulose Nanoparticles Coated with Ionic Liquid as a New Material for the Simple and Fast Monitoring of Emerging Pollutants in Waters by Magnetic Solid Phase Extraction. Microchem. J. 2018, 137, 490–495. DOI: 10.1016/j.microc.2017.12.007.
- Mejias, C.; Santos, J. L.; Martin, J.; Aparicio, I.; Alonso, E. Automatised on-Line SPE-Chiral LC-MS/MS Method for the Enantiomeric Determination of Main Fluoroquinolones and Their Metabolites in Environmental Water Samples. Microchem. J. 2023, 185, 108217. DOI: 10.1016/j.microc.2022.108217.
- Ferhi, S.; Bourdat-Deschamps, M.; Daudin, J. J.; Houot, S.; Nélieu, S. Factors Influencing the Extraction of Pharmaceuticals from Sewage Sludge and Soil: An Experimental Design Approach. Anal. Bioanal. Chem. 2016, 408, 6153–6168. DOI: 10.1007/s00216-016-9725-3.
- Abril, C.; Santos, J. L.; Malvar, J. L.; Martín, J.; Aparicio, I.; Alonso, E. Determination of Perfluorinated Compounds, Bisphenol A, Anionic Surfactants and Personal Care Products in Digested Sludge, Compost and Soil by Liquid-Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1576, 34–41. DOI: 10.1016/j.chroma.2018.09.028.
- Shafrir, M.; Avisar, D. Development Method for Extracting and Analyzing Antibiotic and Hormone Residues from Treated Wastewater Sludge and Composted Biosolids. Water Air Soil Pollut. 2012, 223, 2571–2587. DOI: 10.1007/s11270-011-1049-5.
- Lonappan, L.; Pulicharla, R.; Rouissi, T.; Brar, S. K.; Verma, M.; Surampalli, R. Y.; Valero, J. R. Diclofenac in Municipal Wastewater Treatment Plant: Quantification Using Laser Diode Thermal Desorption-Atmospheric Pressure Chemical Ionization-Tandem Mass Spectrometry Approach in Comparison with an Established Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry Method. J. Chromatogr. A 2016, 1433, 106–113. DOI: 10.1016/j.chroma.2016.01.030.
- Kazarin, P.; Shivkumar, G.; Tharp, T.; Alexeenko, A. A.; Shang, S. Lyophilization Scale-Up to Industrial Manufacturing: A Modeling Framework Including Probabilistic Success Prediction. Chem. Eng. Res. Des. 2023, 192, 441–455. DOI: 10.1016/j.cherd.2023.02.044.
- Amorim, M. L.; Soares, J.; Coimbra, J. S. R.; Leite, M. O.; Albino, L. F. T.; Martins, M. A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. DOI: 10.1080/10408398.2020.1768046.
- Chen, Y.; Cao, Q.; Deng, S.; Huang, J.; Wang, B.; Yu, G. Determination of Pharmaceuticals from Various Therapeutic Classes in Dewatered Sludge by Pressurized Liquid Extraction and High Performance Liquid Chromatography and Tandem Mass Spectrometry (HPLC-MS/MS). Int. J. Environ. Anal. Chem. 2013, 93, 1159–1173. DOI: 10.1080/03067319.2012.717271.
- Ekpeghere, K. I.; Lee, J. W.; Kim, H. Y.; Shin, S. K.; Oh, J. E. Determination and Characterization of Pharmaceuticals in Sludge from Municipal and Livestock Wastewater Treatment Plants. Chemosphere 2017, 168, 1211–1221. DOI: 10.1016/j.chemosphere.2016.10.077.
- Garcia-Rodríguez, A.; Sagristà, E.; Matamoros, V.; Fontàs, C.; Hidalgo, M.; Salvadó, V. Determination of Pharmaceutical Compounds in Sewage Sludge Using a Standard Addition Method Approach. Int. J. Environ. Anal. Chem. 2014, 94, 1199–1209. DOI: 10.1080/03067319.2014.921292.
- Hu, S.; Song, Y. H.; Bai, X. H.; Jiang, X.; Yang, X. L. Derivatization and Solidification of Floating Dispersive Liquid-Phase Microextraction for the Analysis of Kanamycin in Wastewater and Soil by HPLC with Fluorescence Detection. CLEAN Soil Air Water 2014, 42, 364–370. DOI: 10.1002/clen.201200374.
- Luque-Muñoz, A.; Vílchez, J. L.; Zafra-Gómez, A. Multiclass Method for the Determination of Pharmaceuticals and Personal Care Products in Compost from Sewage Sludge Using Ultrasound and Salt-Assisted Liquid–Liquid Extraction Followed by Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry Analysis. J. Chromatogr. A 2017, 1507, 72–83. DOI: 10.1016/j.chroma.2017.05.051.
- Tian, Y.; Li, L.; Li, X.; Li, J.; Meng, J. Sample Pretreatment and Analytical Methodology for the Determination of Antibiotics in Swine Wastewater and Activated Sludge. Environ. Sci. Pollut. Res. Int. 2022, 29, 83671–83685. DOI: 10.1007/s11356-022-21595-y.
- Vakondios, N.; Mazioti, A. A.; Koukouraki, E. E.; Diamadopoulos, E. An Analytical Method for Measuring Specific Endocrine Disruptors in Activated Sludge (Biosolids) Using Solid Phase Microextraction-Gas Chromatography. J. Environ. Chem. Eng. 2016, 4, 1910–1917. DOI: 10.1016/j.jece.2016.03.018.
- Lu, X.; Feng, Z.; Zhou, Y.; Zhang, J.; Ren, Y.; Peng, Y. Determination of Multi-Class Ntibiotic Residues in Compost by Microwave-Enhanced Accelerated Solvent Extraction and Ultra Performance Convergence Chromatography with Tandem Mass Spectrometry. J. Sep. Sci. 2019, 42, 1281–1288. DOI: 10.1002/jssc.201801032.
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of 90 Emerging Contaminants in Liquid and Solid Environmental Matrices by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1431, 64–78. DOI: 10.1016/j.chroma.2015.12.036.
- Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J. J. The Use of Microwave Assisted Extraction and on-Line Chromatography-Mass Spectrometry for Determining Endocrine-Disrupting Compounds in Sewage Sludges. Water Air Soil Pollut. 2013, 224, 1486. DOI: 10.1007/s11270-013-1486-4.
- Wang, H.; Ding, J.; Ding, J.; Ren, N. Analysis of Sulfonamides in Soil, Sediment, and Sludge Based on Dynamic Microwave-Assisted Micellar Extraction. Environ. Sci. Pollut. Res. Int. 2016, 23, 12954–12965. DOI: 10.1007/s11356-016-6383-0.
- Mastroianni, N.; Postigo, C.; De Alda, M. L.; Barcelo, D. Illicit and Abused Drugs in Sewage Sludge: Method Optimization and Occurrence. J. Chromatogr. A 2013, 1322, 29–37. DOI: 10.1016/j.chroma.2013.10.078.
- Sun, S.; Meng, F.; Qi, H. Simultaneous Determination of Fourteen Pharmaceuticals in Sewage Sludge Using Online Solid-Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry Combined with Accelerated Solvent Extraction. Environ. Sci. Pollut. Res. 2023, 30, 62522–62531. DOI: 10.1007/s11356-023-26072-8.
- Ezzariai, A.; Riboul, D.; Lacroix, M. Z.; Barret, M.; El Fels, L.; Merlina, G.; Bousquet-Melou, A.; Paturear, D.; Pinelli, E.; Hafidi, M. A Pressurized Liquid Extraction Approach Followed by Standard Addition Method and UPLC-MS/MS for a Fast Multiclass Determination of Antibiotics in a Complex Matrix. Chemosphere 2018, 211, 893–902. DOI: 10.1016/j.chemosphere.2018.08.021.
- Sun, H.; Qiao, F.; Liu, G.; Liang, S. Simultaneous Isolation of Six Fluoroquinolones in Serum Samples by Selective Molecularly Imprinted Matrix Solid-Phase Dispersion. Anal. Chim. Acta 2008, 625, 154–159. DOI: 10.1016/j.aca.2008.07.025.
- Triñanes, S.; Casais, M. C.; Mejuto, M. C.; Cela, R. Matrix Solid-Phase Dispersion Followed by Liquid Chromatography Tandem Mass Spectrometry for the Determination of Selective Ciclooxygenase-2 Inhibitors in Sewage Sludge Samples. J. Chromatogr. A 2016, 1462, 35–43. DOI: 10.1016/j.chroma.2016.07.044.
- Ferhi, S.; Bourdat-Deschamps, M.; Daudin, J. J.; Houot, S.; Nélieu, S. Factors Influencing the Extraction of Pharmaceuticals from Sewage Sludge and Soil: An Experimental Design Approach. Anal. Bioanal. Chem. 2016, 408, 6153–6168. DOI: 10.1007/s00216-016-9725-3.
- Rossini, D.; Ciofi, L.; Ancillotti, C.; Checchini, L.; Bruzzoniti, M. C.; Rivoira, L.; Fibbi, D.; Orlandini, S.; Del Bubba, M. Innovative Combination of QuEChERS Extraction with on-Line Solid-Phase Extract Purification and Pre-Concentration, Followed by Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Non-Steroidal anti-Inflammatory Drugs and Their Metabolites in Sewage Sludge. Anal. Chim. Acta 2016, 935, 269–281. DOI: 10.1016/j.aca.2016.06.023.