Abstract
Disruptive innovation is an invention that disrupts an existing market and creates a new one by providing a different set of values, which ultimately overtakes the existing market. Typically, when disruptive innovations are introduced, their performance is initially less than existing standard technologies, but because of their ability to bring the cost down, and with gradual improvement, they end up replacing established service standards.
Disruptive technologies have their fingerprints in health care. Pathology and laboratory medicine are fertile soils for disruptive innovations because they are heavily reliant on technology. Disruptive innovations have resulted in a revolution of our diagnostic ability and will take laboratory medicine to the next level of patient care. There are several examples of disruptive innovations in the clinical laboratory. Digitizing pathology practice is an example of disruptive technology, with many advantages and an extended scope of applications. Next-generation sequencing can be disruptive in two ways. The first is by replacing an array of laboratory tests, which each requires expensive and specialized instruments and expertise, with a single cost-effective technology. The second is by disrupting the current paradigm of the clinical laboratory as a diagnostic service by taking it into a new era of preventive or primary care pathology. Other disruptive innovations include the use of dry chemistry reagents in chemistry analyzers and also point of care testing. The use of artificial intelligence is another promising disruptive innovation that can transform the future of pathology and laboratory medicine. Another emerging disruptive concept is the integration of two fields of medicine to create an interrelated discipline such as “histogenomics and radiohistomics.” Another recent disruptive innovation in laboratory medicine is the use of social media in clinical practice, education, and publication.
There are multiple reasons to encourage disruptive innovations in the clinical laboratory, including the escalating cost of health care, the need for better accessibility of diagnostic care, and the increased demand on the laboratory in the era of precision diagnostics. There are, however, a number of challenges that need to be overcome such as the significant resistance to disruptive innovations by current technology providers and governmental regulatory bodies. The hesitance from health care providers and insurance companies must also be addressed.
Adoption of disruptive innovations requires a multifaceted approach that involves orchestrated solutions to key aspects of the process, including creating successful business models, multidisciplinary collaborations, and innovative accreditation and regulatory oversight. It also must be coupled with successful commercialization plans and modernization of health care structure. Fostering a culture of disruptive innovation requires establishing unique collaborative models between academia and industry. It also requires uncovering new sources of unconventional funding that are open to high-risk high-reward projects. It should also be matched with innovative thinking, including new approaches for delivery of care and identifying novel cohorts of patients who can benefit from disruptive technology.
Introduction
We are at the beginning of a new era of revolutionized practice in the clinical laboratory. In “precision medicine,” we stratify patients into unique subgroups based on their distinct disease pathology, and in “personalized medicine,” we customize the treatment plan according to each patient’s unique pathogenesis and ability to respond to a particular treatment. There is currently a great shakeup in the history of pathology and laboratory medicine that will move pathologists and laboratory scientists into the center of patient care and redefine their role from being diagnostic specialists into active participants in patient care through prediction of disease risk, assessment of prognosis, and guiding treatment decision in addition to patient follow up after treatment [Citation1]. This revolution is triggered by two distinct classes of innovations: sustaining and disruptive innovations [Citation2]. Sustaining innovations can be simply defined as improvements in the performance of currently existing technologies and methodologies that eventually lead to an improved product (e.g. a laboratory test) or a better function (e.g. making an existing technology more efficient or enhancing the capability of a current platform) [Citation3]. There are plenty of examples of sustaining innovations in a laboratory setting. For instance, producing more efficient, more accurate, and higher throughput next-generation sequencing (NGS) instruments [Citation4] or faster chemistry analyzers that can assess a wider range of analytes in a shorter time with less cost [Citation5]. Disruptive innovation, on the other hand, is the introduction of a new concept/technology as explained below.
What is disruptive innovation?
A disruptive innovation can be defined as an innovation that disrupts (or in other words interrupts) an existing market and creates a new one by providing a different set of values, which ultimately, and unexpectedly, overtakes the existing market [Citation6–8]. In this process, an unconventional new product/service initially takes root as a simple application at the bottom of a market and then moves “upmarket,” eventually displacing established competitors [Citation6,Citation7]. As illustrated in (adopted from reference [Citation9]), when disruptive innovations are introduced, typically their performance usually targets the low-end segments of existing standard care technologies. For instance, they do not usually cover the entire spectrum of applications of a standard technology and can be less high-throughput or less sensitive (e.g. a strip pregnancy test is less accurate than a standard laboratory analysis for hCG in a clinical laboratory). What makes them appealing is that they can bring the cost down so that their adoption becomes inevitable. Other disruptive innovations address problems for which there is no other alternative (an unmet need). Eventually, their performance catches up (motivated by their value proposition and cost-effectiveness), leading to mass dissemination of the product/service.
Figure 1. Illustration of the concept of disruptive innovation. This figure depicts the difference between sustained and disruptive innovation. Sustained innovation is an incremental increase in an existing product/test aiming to enhance its performance. This usually leads to a more sophisticated test that is beyond the need of an ordinary customer. Disruptive innovation, on the other hand, is the creation of a product/test that is probably of less quality but has lower costs and suits the needs of regular customers/patients. Author/Copyright holder: Clayton Christensen. Copyright terms and license: All Rights Reserved. Reproduced with permission.
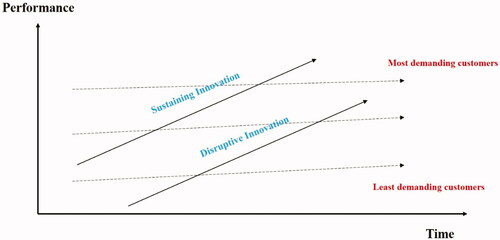
It is important to note that these innovations are not initially targeting the high demand, sophisticated users of the technology but rather directed toward the broader base of customers where cost-effectiveness dictates reasonable expectations about performance. Additionally, in some cases, an innovation that is disruptive allows a whole new population of consumers’ access to a product or service that is traditionally only accessible to wealthy customers who are able to afford it (e.g. automated/robotic chemistry autoanalyzers that can only be obtained by major hospitals or academic institutions) or highly-skilled customers who are able to utilize it (e.g. NGS, which requires multiple areas of expertise for sample preparation, processing, and results interpretation) [Citation10]. During its early stages, disruptive innovations (e.g. point-of-care testing in a laboratory setting) target a small and specific set of customers (or patients in a health care model) and use simple products with comparable results to a well-established, highly expensive existing standard of care approved solution. The main characteristics of a disruptive innovation are summarized in Box 1.
Box 1 The main characteristics of a disruptive innovation
Thinking outside the box: not just an incremental improvement of an existing technology/solution
Much cheaper than existing solutions → adoption
Creates a new market or opens opportunities to a new cohort of customers
Eventually replaces traditional industry/service
Initially grows slowly, but then expands exponentially to replace existing standards
Disruptive innovations: revolutionary changes in different industries
To obtain a better understanding of the impact of disruptive innovations, we can look at different industries [Citation11]. There are several success stories of how disruptive innovations were able to revolutionize markets by providing more convenient, less expensive solutions to well-established technologies, as outlined in recent reviews [Citation9,Citation10]. One clear example is the world of computer technology [Citation12]. In the early days of the nineteenth century, the computer world was dominated by large manufactures such as IBM, but computers were expensive, occupied a large space, and the data processors required special expertise. The revolution came with the introduction of mini-computers and laptops. In the beginning, the capacity and level of performance of these were far less than what you could obtain from a sophisticated computer facility. Years after when mini-computers and laptops gained market, they developed quickly to almost replace the need for highly sophisticated computers. They also opened a whole new market for nonprofessionals, allowing them to run their small computational solutions without the need for skilled experts.
Online brokerage is another example of a disruptive innovation that has enabled individuals from all classes to manage their own stock portfolios without the need for expensive brokerage experts from large firms. The introduction of android systems for mobile phones caused large companies like Nokia and Blackberry to be much less popular in the market with significantly reduced sales. Online shopping platforms (e.g. Amazon) are other examples of disruptive innovations. The car sharing models (e.g. Uber, Lyft, etc.) represent disruptive technologies that were able to challenge and to a certain degree outperform the classic Taxi models.
Disruptive innovation in health care
Disruptive technologies also have their fingerprints in health care. One example that stands out is the introduction of angioplasty. Years ago, the only solution to coronary artery disease was an open surgery with significant side effects. It was also expensive and required highly sophisticated surgical experience. The introduction of the noninvasive angioplasty with comparable results enabled better, quicker recovery as well as enabled a cohort of patients who were previously deemed unfit for an open surgery to be treated [Citation9,Citation10]. Another intriguing example is vision correction, which for many years has required a skilled professional optometrist to go through the time-consuming process of vision assessment. More recently, systems for customizing eyeglasses quickly and efficiently were introduced to the market. Another prominent example of disruptive innovation in health care is the introduction of “Nurse Practitioners” [Citation13] and “Physician Assistants” who can take over some of the simple tasks that are traditionally only done by a physician and thus provide quicker service at a much lower cost with the same or comparable effectiveness [Citation14,Citation15].
MinuteClinic (formerly known as QuickMedx) is another example of a successful disruptive innovation [Citation16]. With more than 1100 locations in 33 states, it offers fast and convenient testing for a flat payment of $35 USD per visit. The idea was to provide rapid testing, diagnosis, and prescriptions for 11 common illnesses, including influenza, ear infection, pink eye, and seasonal allergies. It also offers vaccination for Hepatitis B, tetanus, and the flu. Their stations are located in a famous grocery store chain and are staffed with nurse practitioners who provide care in a timely manner for these relatively simple illnesses [Citation10]. This model offers an affordable service that is quick and convenient compared to standard multi-step doctor visits with associated laboratory work.
Disruptive innovations were shown to be very helpful in providing non-conventional solutions for health disasters like the recent COVID-19 pandemic [Citation17]. Researchers at Johns Hopkins found that Twitter can be used effectively to keep care teams of pediatric intensive care units around the world informed and updated during the COVID-19 crisis [Citation17]. Robotic systems were able to remotely control ventilators in COVID-19 patient rooms. Also, a new app called “COVID Alert” has been developed in Canada to spot COVID-19 outbreaks [Citation18]. Artificial intelligence tools were able to predict heart problems in COVID-19 patients [Citation19].
There is a myriad of disruptive technologies and innovations that have revolutionized health care and continued to do so over many years [Citation7,Citation20,Citation21], which are out of the scope of this review that focuses on disruptive innovation in the field of laboratory medicine, as detailed below.
Disruptive innovation in laboratory medicine
Pathology and laboratory medicine are fertile soils for disruptive innovation because they are both heavily reliant on technology. Disruptive innovations have resulted in a revolution of our diagnostic ability and will take laboratory medicine into the new era of personalized health care where treatment is tailored according to every person’s specific needs. Below, we highlight in more details a number of established and evolving disruptive innovations in the clinical laboratory.
Digital pathology
The concept of digitizing pathology practice that got introduced in the late 1990s is a great example of disruptive technology in laboratory medicine. Digital pathology has many advantages and an extended scope of applications, as outlined in recent reviews [Citation22]. These include easy sharing of slides among institutions, which can have a great impact on improving the efficiency of consultations (through digital consults by pathologists with unique specializations worldwide). Taking into consideration that pathology is heading toward specialized practice is of special importance, but in many community hospitals, there is a lack of specialization compared to academic institutions where pathologists practice mainly one or a limited number of specialties like genitourinary or breast pathology. Some pathologists are even sub-specialized, such as “pediatric gastrointestinal pathology.” Thanks to the introduction of digital pathology, we can now obtain digital second opinions from expert pathologists across the globe in almost no time.
Also, with the introduction of telepathology, we are now able to provide better services to remote and underserved areas. Several recent studies have shown the value of telepathology in intraoperative consultation (frozen section diagnosis) by specialized pathologists for remote locations [Citation23,Citation24]. Pathology digitalization will also challenge the traditional need to retain glass slides for many years. By saving a digital copy instead of the glass slide, this reduces the need for space and decreases drawbacks and harms associated with storing glass. Simply stated, pathology digitalization will convert the world of pathology into a small village where diagnosis could be performed in a different location that is thousands of miles away from where the specimen was processed [Citation25–27].
Additionally, digital pathology is disruptive because it paves the way for computational pathology and artificial intelligence to transform pathology assessment from being qualitative (relying on subjective assessment by a pathologist) into quantitative assessment through image analysis [Citation28]. It is interesting to note that when high-resolution scanned pathology images were introduced it was a typical scenario of a disruptive innovation where the quality of the scanned image was much less and so was the digital diagnostic ability. With more investment and motivation by initial success, the diagnostic quality of digital whole slide imaging is now the same as microscopic diagnosis [Citation29].
Pathology digitization has its impact on education, through sharing online resources (scanned slides) nationally and internationally. The value of pathology digitization has also revolutionized the way examinations and resident evaluations are performed in many countries. For example, Canada fully digitalized the Royal College examination in pathology a couple of years ago [Citation30]. A study showed that resident’s performance was comparable between digitalized images and microscopes which led to a much-needed reduction in the cost of exam preparation and hassle of carrying a microscope to the examination center [Citation31].
The value of digital pathology exceeds the replacement of glass slides with digital images, with potential to revolutionize the workflow of pathology and redefine pathology practice [Citation32–34]. While other divisions of laboratory medicine (e.g. chemistry) have gone through significant automation, the practice of anatomical pathology has been almost the same for many decades, with the basic process of tissue processing and creating glass slides to be manually evaluated by pathologists. This workflow is manual, time-consuming, expensive, and is liable to multiple human errors throughout the process. Digitalization can help with the establishment of a faster and more efficient workflow. It can provide flexibility in the workspace and working hours for pathologists, in addition to the potential for performing image analysis thus enhancing the value of pathology diagnosis for patient care. It also allows proper integration into laboratory information systems, improving accessibility for the entire clinical team to pathology results.
A digital pathology workflow is especially useful in multicenter health care systems where multiple locations can be amalgamated into a single system. It also enables offering nationwide specialized pathology services (e.g. neuropathology) from one or a few locations. Another advantage is the ability to redirect cases between different sites and adjust workload according to volume changes and specific circumstances such as sick leaves, maternity leaves, etc. It can also be useful in special circumstances, like the recent COVID-19 pandemic. Another advantage is the time reduction for diagnosis (digital slides take less time because you do not need to confirm ID on the slide with patient ID) and reduction in error rate due to mixing up of cases.
Next-generation sequencing and molecular analysis
The promise of genomic pathology is that the cost of sequencing the human genome will become affordable so that it will inevitably find its way into fundamental aspects of health care – not only in disease diagnosis and management, but also prevention, risk mitigation, and health maintenance. The disruptive innovation that made this possible is NGS. Genomic data are becoming an integrated part of the pathology report of many cancers, including colon, breast, and lung cancers among many others [Citation35].
Sequencing of the human genome marked a new era of diagnostics and patient care. Along with the completion of the first Human Genome Project, multiple factors paved the road for genomics as a disruptive evolution of patient care [Citation36,Citation37]. These include the rapid significant improvement in NGS technology, the unprecedented advancement in bioinformatics and computer abilities, along with the significantly lower cost of sequencing every day. It is anticipated that in a few years, NGS technology will be adopted as a routine tool not only in major hospitals but in every non-hospital health care facility [Citation38,Citation39].
The evolution of NGS is very interesting, showing the power of disruptive innovation. Starting with two or three large manufacturers producing high-throughput instruments, many manufacturers are now moving to compact Personal Genome Machine (PGM) sequencers that are small in size, much cheaper, and have fast turnover rates but limited data throughput [Citation40–42]. New technologies, such as those developed by Oxford Nanopore Technologies (Oxford, United Kingdom), offer much smaller and cheaper sequencing instruments that are also quick and portable [Citation43].
As elegantly highlighted in a recent review [Citation35], genomic pathology represents disruption in two aspects: the first is by replacing an array of laboratory tests (biochemistry, microbiology, and molecular pathology) that each requires expensive and specialized instruments and a high level of expertise with a single cost-effective technology platform that is NGS. It will also disrupt the current paradigm of the clinical laboratory as a diagnostic service by taking it into a new era of preventive and primary care pathology.
While NGS is a new technology, PCR is an example of an existing technology that has undergone revolutionary modification to cause disruptive innovation. A number of new protocols that use the basic PCR principle with significant modifications, such as quantitative real-time PCR analysis and digital droplet PCR, have been introduced in recent years [Citation2].
Disruptive innovation can occur through the introduction of a new technology or can simply be a revolutionary modification to an existing technology. PCR is a good example of this, with the introduction of a number of new protocols that use the basic PCR principle with significant modifications, such as quantitative real-time PCR analysis and then digital droplet PCR.
Point of care testing and disruptive innovations in clinical biochemistry
Examples of disruptive innovation include the use of dry chemistry reagents in chemistry analyzers in the core laboratory [Citation44,Citation45] and point of care testing (POCT) [Citation46,Citation47]. The latter is defined as an investigation done close to the patient at the time of the consultation. These are usually performed by a nurse without the need for a laboratory technologist, with instant availability of results to make immediate informed decisions about patient care. There is a growing list of POCT, including pregnancy tests, measuring blood sugar level by glucometers, cardiac biomarker tests, and the list continues to grow. POCT has the typical features of a disruptive innovation, including being cheaper, quicker, and easier to perform, but with less accuracy compared to a standard laboratory test. Handheld analyzers for testing whole blood are another example of a disruptive technology that can be used for POCT, even at home [Citation48].
The switch from manual labor-intensive laboratories into fully automated chemistry analyzers is another example of successful disruptive thinking. We are now incorporating even more advanced technology in the chemistry core laboratory, including mobile general-purpose dual arm robots that mark a new horizon for automations. These robots can perform certain repetitive tasks faster, cheaper, and more accurately than humans. They can be employed to perform sophisticated multiple-step tests like the enzyme-linked immunosorbent assay (ELISA) [Citation49].
Disruptive innovations include the use of matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry as a clinical tool for pathogen identification [Citation2]. Another emerging disruptive innovation, jointly developed by Google and Novartis, includes a glucose sensing electrode with telemetry so that glucose levels are monitored from the tears of the eye and transmitted to remote devices [Citation50]. The use of disposable electronics is an interesting example of a disruptive innovation in POCT. An example of this is the digital pregnancy test, “FirstResponse™ Pregnancy Pro,” which is a wireless technology-enabled pregnancy test that connects via Bluetooth to a smartphone [Citation51]. Another example is the “rHEALTH” sensor, which is a small portable device that is able to perform a large number of laboratory tests using one drop of blood [Citation2].
Disruptive innovations also extend into inventing robotic devices for automated blood drawing [Citation52]. These devices have the potential to replace standard blood drawing in clinical laboratories and thus save money, labor, and time. It also has the ability to improve the workflow in hospitals and private clinics, especially those associated with POCT equipment to provide quick results [Citation53].
Deep learning and artificial intelligence in laboratory medicine
The use of artificial intelligence (defined as using a computer system to perform tasks that normally require human intelligence) is another promising disruptive innovation that can transform the future of pathology and laboratory medicine, especially since medicine is transferring from being reactive to being proactive in the coming years [Citation54,Citation55]. Recent evidence shows that artificial intelligence represents another disruptive innovation that can replace (or better to say complement) the rule of molecular pathology. The potential utility of artificial intelligence in laboratory medicine has been recently reviewed [Citation55]. The concept of using artificial intelligence in the laboratory relies on the fact that computer systems have the ability to uncover and understand complex and non-linear associations. Because they are “thinking in a way different from humans” [Citation55] they will have better ability to provide diagnostic/prognostic models or algorithms and advance the analytical ability much further than what a human can do [Citation55].
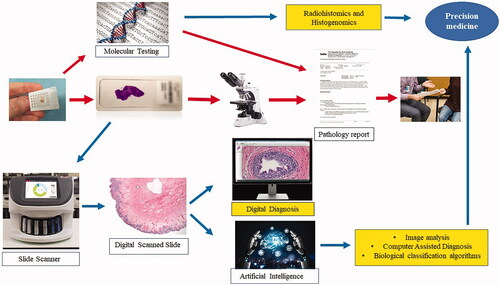
A diagrammatic representation of how artificial intelligence can lead to significant revolution of pathology practice is shown in . Scanned pathology images can be analyzed using deep learning, artificial intelligence, and machine learning technologies, and this will enable the development of pixel-pipeline-based workflow and diagnostic, prognostic, or predictive algorithms [Citation56]. Earlier studies have shown the ability of computer algorithms to assess a set of quantitative features from pathology slides and to create a predictive model for cancer severity [Citation57]. Recent studies have shown that deep learning can perform even better than the human eye for Gleason scoring of prostate cancer [Citation58]. Deep learning has greater sensitivity and specificity compared to specialized pathologists and can represent a new milestone toward computer-assisted diagnosis [Citation59]. A recent study showed a positive predictive value of 72% and a negative predictive value of 97% for breast cancer diagnosis [Citation60]. Deep learning algorithms have also been reported to accurately predict estrogen receptor status in breast cancer [Citation55]. Additionally, scientists have developed tools that can search for morphologically similar features in unannotated slides (features that are not marked manually by the pathologist) [Citation61]. This will allow exponential ability to navigate through millions of stored unannotated images [Citation59].
Figure 2. How disruptive innovation can revolutionize the workflow and outcome of pathology practice. The “classic” pathology workflow is indicated by the red arrows. The potential integration of image analysis and artificial intelligence is depicted by the blue arrows, and the potential added benefits of disruptive innovations are highlighted in yellow. In addition to creating a lenient workflow, image analysis can be an excellent tool for computer-assisted diagnosis by providing information beyond what the human eye can achieve. It can also create diagnostic algorithms to further classify disease into meaningful biological categories (prognostic and predictive). Eventually, the integration of molecular results and other diagnostic parameters (e.g. radiology and genomic data) will lead to enhanced precision diagnostics.
Artificial intelligence can also be utilized for big data analysis in chemistry, hematology, and other laboratory medicine specialties, as reviewed elsewhere [Citation55]. “Big data” is a term used to describe large datasets that have the four V’s: Volume, Variety, Velocity, and Value. The clinical laboratory typically produces millions of data points of test results every year. These data are connected to electronic medical records’ biometrics data. They are usually beyond simple statistical tools for analysis. In recent years, we have seen many success stories of artificial intelligence-based tools applied to big data that can be used to improve public health management and assist decision-making in health care [Citation55,Citation62,Citation63].
Another interesting upcoming utility for artificial intelligence is for predicting protein folding, which will most likely be a means to distinguish normal folding from the so-called “sick molecules,” as in amyloidosis.
Multidisciplinary disruptive innovations
Another emerging disruptive concept is the integration of two fields of medicine to create an interrelated discipline such as “histogenomics” [Citation64,Citation65] and “radiohistomics” [Citation66–68]. Over many years, there have been several efforts leading to incremental improvements in diagnostic accuracy in pathology through improving microscopy, better tissue processing, and adding special and immunostains. On the other hand, genomics has been improving by enhancing the accuracy of NGS and high-throughput production. The recently emerging concept of “histogenomics” represents the interface of histology and genomics. As detailed in our recent review, molecular/genomic analysis produces a very high-resolution report of the biology of the tumor but lacks spatial information. Understanding the morphological context of the molecular attributes, which is defined as molecular changes happening in tumor cells, the tumor microenvironment, or the normal cells around it, will allow a much better understanding of the clinical behavior of cancer. This will result in a four-dimensional analysis of cancer and other diseases through the incorporation of 3D images of digitally scanned slides and the molecular attributes of the lesion [Citation68].
Another interesting disruptive innovation is the field of “radiohistomics” or the interface between diagnostic imaging and anatomical pathology. This can even expand further to multi-dimensional analysis that includes clinical parameters, omics data, digitized morphology through pathology images, and 3D radiology, taking us into a completely new era of patient management [Citation68].
Innovation in publication and knowledge dissemination
An important recent disruptive innovation in laboratory medicine is the use of social media in clinical practice and education [Citation69]. Social media now represents a platform for communication, with e-mails becoming official documents of consultation and other forms of interactions among pathologists and clinical laboratorians. In addition, social media is gradually taking over the classic paper books for information dissemination [Citation70,Citation71]. There are a myriad of websites and blogs that pathologists can refer to for a quick image representation of a rare case, to identify the diagnostic criteria of an entity, or to inquire about the appropriate usage of immunostaining [Citation72–74]. Although useful and easily accessible, it should be noted that there is a risk associated with completely relying on social media-based information as it sometimes lacks accuracy and validation of the information provided.
Several evolving social networking platforms, such as “Doximity” [Citation75] and ” [Citation76], are directed specifically to educating and connecting the medical community. is an educational application in which medical professionals post and discuss interesting cases, often with an accompanying histology image. By offering a cost-effective and instantaneous method of communication and information sharing, social media allows pathologists in remote regions to collaborate, communicate, or share special cases with their colleagues across the country. The utility of social media in clinical biochemistry and its associated opportunities, challenges, and risks were recently reviewed [Citation77].
Social media represents another disruptive innovation in laboratory medicine education. It is very interesting to realize that social media is taking over the classic ways of communication for the new generation of learners [Citation69], and medicine is no exception [Citation78]. Academic institutions are increasingly using social media for reaching out to learners and sending out news about conferences, major achievements, and up to dates. The classic peer-reviewed publication model is also being challenged. Recently, new disruptive concepts are emerging, such as open access journals, non-peer-reviewed journals (e.g. bioRxiv) [Citation79], and post-publication review (e.g. F1000 research) [Citation80]. These disruptive models are still evolving with associated advantages, disadvantages, and risks that have to be carefully considered. Regardless, it seems like a wave of change is eventually coming and we will need to adapt to disruptive innovations in publications.
The case for disruptive innovation in laboratory medicine
An important question to ask is “why is there a need for disruptive innovation in the clinical laboratory?” There are multiple reasons for this, including the escalating cost of health care that is becoming unaffordable. It is predicted that soon, health care expenditure will reach 20% of the annual gross domestic product (GDP) in the USA [Citation2,Citation81]. This is of particular importance in the field of diagnostics where advanced technologies are almost always associated with a significant cost increase, especially for genome diagnostics. As recently suggested, instead of asking how we can afford the increasing cost of laboratory testing, we need to ask how we can make lab tests more affordable [Citation10].
A second motivation is accessibility of patient care. Highly sophisticated analyses are performed only in academic institutions but not in remote areas and community hospitals. Disruptive innovations can bridge this gap by providing innovative alternatives to make health care accessible to all. Disruptive technologies, if encouraged, can allow for better laboratory care in underserved areas and developing nations worldwide [Citation9].
Also, disruptive innovations can drive a whole new level of patient care. The current health system can be described as being suboptimal. To illustrate the need for innovation, the Dartmouth Institute for Health Policy & Clinical Practice estimated that 30–40% of all hospitalizations are avoidable and that, among regions, Medicare costs can vary 2–3-fold to treat similarly ill patients, without better outcomes [Citation82]. A key solution to improve health care is through improving the use of advanced laboratory testing that can accurately guide treatment decision and patient follow up after treatment. Innovative testing can also take us into an era of preventive health care that is proactive rather than reactive. The use of artificial intelligence, genomic signatures, image analysis, and others can take us into the era of P4 medicine (Predictive, Preventative, Personalized, and Participatory) where system approaches to biology and medicine will give patients and physicians the opportunity to have personalized information about the unique health experience of every individual in health and disease [Citation83]. This will enable more cost-effective personalized patient care that is customized to each person’s unique biology and lead to treatment of the causes rather than the symptoms of disease. Disruptive innovations are also needed to provide better solutions for difficult-to-treat diseases like amyloidosis, Alzheimer’s disease, and multiple sclerosis. There is a need to think outside the box for finding early diagnostic tools and better classification tests, in addition to tests that can guide and monitor treatment.
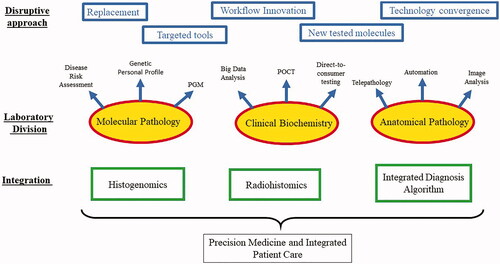
Lastly, innovative approaches through big data analysis can enable optimizing health utilization at the institutional level, thus reducing costs and increasing efficiency. The impact of disruptive innovations on laboratory medicine is multifaceted. As shown in , disruptive ideas can revolutionize all laboratory medicine disciplines. Multiple approaches can be perused, and interdisciplinary integration will ultimately lead to a new era of precision diagnostics.
Figure 3. Approaches and impact of disruptive innovation in laboratory medicine. Disruptive ideas can enhance performance in all laboratory medicine disciplines. The top panel (blue) shows the different disruptive innovation approaches that can be explored in the clinical laboratory. These approaches can be used interchangeably among laboratory medicine disciplines (the middle panel). The middle panel (red) shows some examples of significant disruptive innovations in each of laboratory medicine divisions. Intradisciplinary and interdisciplinary integration (lower panel, green) will ultimately lead to a new era of precision diagnostics.
Disruptive innovation approaches
As shown in , disruptive innovations represent a spectrum of approaches; a classic example is to replace an existing technology with another. Another approach is producing a simplified version of an existing technology for a specific task. An example of this is the newly emerging smaller capacity sequencers. Although these are not suitable for large-scale sequencing and have much less efficiency than larger comparable instruments, they can efficiently carry a specific limited task such as sequencing a small microbial genome or targeted gene panel testing.
Table 1. Different approaches for disruptive innovation.
Disruptive innovations can also be in the way we practice (workflow disruptive innovations), such as creating a task-specific assignment according to the specific needs of a particular patient cohort without sacrificing the quality of care. An interesting example that has been recently adopted is creating “pathologists’ assistant” positions to efficiently perform gross dissection of pathology specimens, thus saving time and money for the institution.
Innovations can also represent innovative specimen types. For generations, clinical laboratories functioned by testing enzymes, hormones, as well as chemicals in blood (chemistry laboratory) or by tissue assessment (anatomical pathology). The emergence of liquid biopsy [Citation85] expands the types of molecules that can be tested, including cell-free DNA, miRNAs [Citation86], long coding noncoding RNAs, peptides, and circulating tumor cells [Citation87]. A liquid biopsy can overcome the challenges and limitations of an invasive biopsy [Citation85].
An exciting dimension of disruptive innovation is through repurposing existing technologies. An example of this in the clinical realm is the use of smartphones for clinical purposes. There are a number of emerging innovations that will allow the use of smartphones supported by cloud computation and validated algorithms for medical care [Citation2]. In pathology, smartphones are being increasingly used for training assessment (residents’ and fellows’ evaluations, especially for competence by design programs). Also, the use of social media is increasing with the establishment of clinical specialty-oriented twitter groups or e-mail groups [Citation69].
3D printing technology also represents an upcoming disruptive innovation that will enable the on-demand manufacturing of specific small parts of laboratory equipment, resulting in a greater degree of autonomy for the clinical laboratory [Citation88]. The concept of technology convergence or the integration of two or more different technologies within a system or device is also evolving. Health testing is now being added as features that can be performed using a mobile phone. It started earlier with the ability of the cell phone to count walking steps or measure heart rate, but now there are glucose testing devices that can be plugged into a smartphone port (e.g. “iHealth Align” Sunnyvale, CA USA). This technology convergence might eventually replace the conventional glucose meters [Citation49].
Disruptive innovation can also be in the way that we deliver laboratory testing to patients. There are now a number of companies that provide the customer with a collection kit to measure different analytes (e.g. MammaPrint™ [Citation84] (Irvine, California, USA), HIV infection, pharmacogenomic testing, or drug exposure testing, etc.)) that are returned by courier to the laboratory for analysis.
The need for a business model (value proposition)
Successful adoption of disruptive technologies in laboratory medicine must be matched with a sustained business case. This includes novelty in the way we deliver care, clear value propositions, and identifying novel target populations of patients [Citation9]. There is also a need to foster business-model innovations, defined as the art of enhancing advantage and value creation by making simultaneous – and mutually supportive – changes both to an organization’s value proposition to customers and to its underlying operating model [Citation9]. A clear example of the importance of a value proposition can be seen in the printing industry when Canon was able to sweep away the once dominant Xerox by bringing smaller, lower throughput but also more affordable tabletop photocopiers to the market [Citation9]. Since the health care industry has different rules and regulations, an important question to ask then is if the same concept can be applied in health care, as discussed below.
As detailed in a recent review [Citation9], fostering disruptive innovations can be achieved through developing new business models, such as creating “autonomous business units” organized around the disruptive value proposition. In pathology and laboratory medicine practice, such a unit could be a digital pathology consultation group practice or a multidisciplinary preventive medicine unit that incorporates genome diagnostics through NGS for regular checkups of healthy individuals. These specialized units would create their own structure that would be profitable by being managed and marketed differently from a classic hospital-based or clinic-based system [Citation9]. One drawback of these units is the fragmentation of health care delivery. This will necessitate advancing the technology to enable sharing of electronic patient files among different institutions through an integrated health information system [Citation9].
Disruptive innovations can also create new markets. A promise of disruptive innovation in laboratory medicine is to be able to reach out to new cohorts of patients and healthy individuals by providing new applications like disease risk assessment and dose adjustment of expensive toxic therapies through pharmacogenetics, among others. We also need to change our approach to health care delivery by identifying distinctive subsets of objectives and searching for unique technologies that can be useful for these particular tasks. A classic example to illustrate this is the introduction of frozen section evaluation in intraoperative consultation. Frozen section is less accurate than a permanent histopathology section diagnosis, but the value proposition is that it is quick, cheap, affordable, and can provide a reasonable answer to the surgeon within minutes. Another classic disruptive innovation in pathology is pap smear testing for screening for cervical cancer. Although a pap smear is not a perfect diagnostic test, it is a great screening tool that is affordable, quick, easy, and readily accessible to a broad class of women [Citation9].
We also need to be innovative in the delivery of diagnostic care. Examples of this include integrated laboratory/clinical units like the “MinuteClinic [Citation16]” described above or the establishment of focused services like a “cardiology group” that incorporates diagnostic testing, imaging, and clinical management in one location using cost-efficient protocols [Citation9].
For digital pathology to be financially attractive, it must move beyond replacing a glass slide and a microscope with a digital slide and a computer screen to the development of a comprehensive integrated workflow solution, as detailed above [Citation89].
Challenges for the adoption of disruptive innovations
Although disruptive innovation has brought affordability and convenience to customers in a variety of industries, the health care system and the clinical laboratory are still behind in this regard, and as a result, health care remains expensive and inaccessible to many. A whole host of disruptive innovations, small and large, could end the crisis of health care service worldwide if we foster a culture of disruptive innovation, in order to be able to build a new system that is characterized by lower cost, higher quality, and greater convenience [Citation10]. There are, however, a number of challenges that need to be overcome if disruptive innovation is to be widespread in laboratory medicine, as outlined in Box 2.
Box 2. Challenges for adoption of disruptive innovations in laboratory medicine
Resistance from current technology and reagent providers
Licensing and quality standards regulations
Uncertainty and concerns of health care providers and patients
Lack of accessibility to the new technologies
The need to create a new business model to support disruptive innovation
Ethical and legal concerns
Availability of disruptive innovations as direct-to-consumer testing
Understandably, a significant resistance to disruptive innovations usually comes from current technology providers (large instrument manufacturers, reagent suppliers, etc.). Giant companies are investing in product improvement to maintain their monopoly, and disruptions can cause great firms to collapse. A clear example of this is digital cameras that significantly eliminated the traditional Kodak film industry. Added to this are the concerns of clinicians and insurance companies about the accuracy of new disruptive testing. Most of these stem from malpractice-related fears and the worry of providing substandard service to patients.
There is no easy solution to these challenges, but one idea is to make disruptive technologies accessible as an alternative option for the clinician and patient where standard services are not available. For instance, small portable chemistry analyzers can be an acceptable option for remote areas or patients who are immobile and cannot travel long distances to perform hospital-based tests.
Resistance comes also from governmental regulatory bodies, health authorities, and organizations responsible for quality standards. A bottleneck for each disruptive innovation in laboratory medicine is passing quality regulations. A new innovation must meet the criteria set by the regulatory bodies such as the Food and Drug Administration (FDA) or Clinical Laboratory Improvement Amendments (CLIA). The process of approval is usually expensive and lengthy such that small inventors might not be able to afford it. Regulatory controls significantly hinder the progress of disruptive innovations. Disruptive innovations typically offer less quality performance in the short term but provide users with other value-enhancing benefits. Looking at the big picture, on the other hand, one can realize that the high expense of the current technology can be cost prohibiting, thus limiting the ability of the health care system to provide quality service to all patients. There is a fine balance between providing a highly accurate vs. an affordable test. There is a need to start serious discussions on where to draw the line! We are fully supportive of the need for regulatory control to ensure a high standard of patient care, but we advocate that regulatory bodies should consider the big picture and accurately calculate risk vs. value.
One of the most popular challenges in the laboratory medicine profession for decades has been laboratory-developed tests (LDTs) vs. in vitro diagnostics (IVDs) [Citation90]. An LDT is defined as a diagnostic test that is developed and performed by an individual laboratory. An IVD test, on the other hand, refers to an FDA-cleared test sold as a complete kit that a laboratory purchases from a manufacturer, which comes with all of the procedures and controls to perform the test. Whether LDTs (many of them are also disruptive innovations) should be allowed remains a subject of a heated debate [Citation91–93]. The FDA recently published evidence suggesting there is a need for FDA oversight of LDTs [Citation94]. Regulatory barriers and certification requirements for the laboratory are becoming more and more sophisticated. This raises the question of whether a product with reasonable sensitivity and specificity could be adopted for “clinical trial” testing so long as clinicians and patients are aware of its limitations [Citation9].
Another challenge for the adoption of disruptive innovations is accessibility. New technologies need to be widely available to the public, and this requires infrastructure investment in many cases. Whether or not new innovations should be available directly to the public is another subject for debate [Citation95]. Direct-to-consumer (DTC) testing refers to testing (especially genetic testing) provided to the public over the counter without medical professional oversight. Some argue that new innovations should be directly accessible to individuals. Patient autonomy in decision-making necessitates providing consumers with the ability to know and make choices on assessing their genetic risks of disease. Whereas current clinical testing is tightly regulated, DTC testing is lightly regulated if at all. The regulation of DTC testing varies by country, but this is beyond the scope of this review. In the United States, some DTC tests are reviewed by the FDA while others are not. In general, DTC tests for non-medical, general wellness, or low-risk medical purposes are not reviewed by the FDA. For these tests, there are no mechanisms to ensure that results are precise, consistent, and clinically significant. Such tests also carry the risk of oversimplified interpretation or misinterpretation of the results. DTC tests are viewed by the public as advanced and accurate indicators of health, although they sometimes do not take into account numerous important factors that are not captured by sequencing, such as family history, lifestyle, age, and other environmental influences. Genetic testing can result in overdiagnosis and overtreatment. False positive tests can result in subsequent invasive procedures.
Some DTC tests, like 23andMe (Sunnyvale, CA), are now FDA approved [Citation96]. It should be noted that the FDA certifies clinical validity (accuracy, reliability, and reproducibility of a test) but not necessarily clinical utility (whether outcomes are improved for patients who received the test compared with those who did not), and an FDA approved test does not necessarily need to establish clinical utility [Citation97].
The roadmap to implementation of disruptive innovation
The adoption of disruptive innovation requires a multifaceted approach that involves orchestrated solutions to key aspects, as summarized in Box 3. In this review, we do not aim to provide answers to all questions, but we will attempt to highlight some key issues that need to be focused on and provide some potential solutions. Our purpose is to initiate the conversation and our suggestions should be followed by more extensive discussion.
Box 3 Key factors that can facilitate the adoption of disruptive innovations
Creating a new, successful business model to support disruptive innovation
Multidisciplinary collaborations
Unconventional funding
Innovative accreditation and regulatory oversight
Successful commercialization plans
Modernization of health care structure and delivery
Enhancing knowledge translation
Fostering a culture of disruptive innovation
Multidisciplinary collaborations
Adoption of disruptive innovations require the assembly of multidisciplinary teams. Integrated efforts between different areas of expertise in multiple disciplines are needed to ensure technical accuracy, clinical efficiency, and an attractive value proposition. This necessitates coordinated efforts between research scientists, clinicians, bioinformaticians, marketing experts, and health authorities [Citation89]. Collaborations for knowledge transfer can take different forms, including joint research projects, contract research, consulting, and university–industry venture capital, as well as entrepreneurship. It is also important to realize that disruptive innovation works best in a non-conventional environment. Disruptive innovation hubs could be provided with some degree of independence, although they might still be linked to a university or a governmental organization.
Unconventional funding
Disruptive innovation requires coordinated efforts between governmental agencies, funding organizations, academic institutions, investors, and entrepreneurs. Traditionally, academia and industry have been walking in parallel with a lack of proper collaborative efforts. This is now slowly moving into a new era of partnerships [Citation98]. A “Triple Helix” that encompasses government, academia, and medical industry has been successful in connecting these previously detached entities [Citation10,Citation99].
Funding for disruptive innovation is different from regular funding by classic funding agencies, as these agencies bring people into the system and the strict grant judgment criteria do not give enough room for risk-taking and disruptive innovation. One way to push this forward is by providing smaller, high-risk high-reward grants like the National Institutes of Health (NIH)-Small Business Innovation Research (NIH-SBIR) and NIH-Small Business Technology Transfer (NIH-SBTR) [Citation100] that focus on demonstrating feasibility. Another example is the Canadian Cancer Society Research Institute innovation grants [Citation101] that require minimal to no preliminary evidence and accept high-risk highly innovative grants.
Accreditation and regulatory oversight
For disruptive innovations to pass the critical step of meeting clinical standards of performance, there is a need for a mindset change that stems from the realization that disruption is an evolutionary change that is necessary for the survival of clinical diagnostics. Clinical trial style validation projects are lengthy and expensive. An important challenge is to find the fine balance between such projects or instead taking the risk and in the meantime having an acceptable level of confidence in these innovations. Potential solutions include encouraging the use of LDTs that fall short of FDA approval but can be a step forward for building clinical validation data. Other ideas include temporary or emergency use authorization where the FDA provides flexibility to states and local authorities to authorize experimental use of LDT testing, as is the case for many tests created during the COVID-19 pandemic [Citation102].
Clarity about indications, limitations, and associated risks
Successful adoption relies on a clear understanding of testing and technology limitations. The legal and ethical framework for test use has to be carefully addressed. We also need to clearly outline the indications and clinical utility for each test. It has to be considered that innovation in health care creates risks that are unevenly distributed, as outlined in a recent publication [Citation21]. Disruptive innovation resembles early and late diversification experiments (diversification is modifying a compound at either an early or late stage during the process of the synthesis of this compound).
On one hand, early diversification (early adoption) of changes will carry higher risk, while late diversification (late adoption) will lead to lower risk. The former harbors the potential for disruption (with higher associated risk) and the latter offers the potential for growth rather than disruption (with lower risk).
Successful commercialization
There is a need for professional marketing and commercialization experts who can help move disruptive innovations forward [Citation55]. Also, the adoption of disruptive innovation is tightly linked to creating new markets. We need to identify new patient cohorts who would specifically benefit from a new disruptive innovation. Saving time and money is key for a viable business model. Successful examples of this include initial digital screening of blood smears in hematopathology to identify “suspicious” cells for manual evaluation, or automated initial screening of pap smears to identify abnormal cells for pathologist evaluation. A similar disruptive idea would be the use of artificial intelligence for initial screening to identify slides containing tumor tissue from prostate or breast cancer resection specimens. These slides would be forwarded to a pathologist for evaluation. This way we can save the pathologist’s time; instead of having to screen 50–60 slides, they can focus only on the diagnostic slides.
Modernization of health care structure and delivery
In order to enable disruptive innovations, we need to modify the health care system structure and workflow to match the level of skills to the level of medical difficulty and expertise needed, as suggested in recent articles [Citation9,Citation10]. Creative ideas such as home testing, virtual care, mobile laboratory testing units, and stepwise management protocols can be of great value. Implementation of disruptive innovations might also require changing clinical practice styles. An example of this is redefining the inclusion criteria for clinical trials from being disease-based (e.g. colon cancer patients) to molecular-based criteria using companion diagnostics (e.g. all patients with KRAS mutations regardless of the type of cancer they have) [Citation89].
Enhancing knowledge translation
The core mission of academic institutions has been teaching and research, but a third component known as knowledge translation (translating scientific discoveries into the clinic for improving health and socioeconomic progress) is recently being emphasized [Citation10]. This is a multidisciplinary effort that involves research innovation, entrepreneurship, and cutting-edge science in addition to clinical authorities. Successful knowledge translation units that serve as hubs for innovation are emerging, like the Cambridge Innovation Center [Citation103] and Toronto Innovation Acceleration Partners (TIAP) at the University of Toronto [Citation104].
Fostering a culture of innovation
The pathology and laboratory medicine community needs to promote and nurture a culture of disruptive innovation. This is a long-term objective that takes multiple steps. It starts by educating trainees at an early level on the importance of disruptive innovation and thinking outside the box. To foster a culture of innovation, we need to think unconventionally. In a typical well-structured system like medical research, we are used to following trends and evaluating them based on our standard ways of looking at research and the expected achievement (the benchmark or metric for evaluation). This will only result in sustaining innovations (incremental advances in current technology/test) but not disruptive innovations, which are high-risk high-reward projects. One way to stimulate an innovative attitude is to establish special granting opportunities by providing access to hospital lab datasets and computer facilities and challenge researchers through either monetary compensation or to be stakeholders in a future patent [Citation28]. There are several examples of this that now exist [Citation105–107].
In order to accelerate production of disruptive innovations in the clinical laboratory, we also need to share information [Citation80]. Publicly available databases are becoming a game-changer in modern research. A recent review suggested that data are more valuable than gold, and the more you have of it the more valuable it becomes [Citation28]. For artificial intelligence and image analysis in pathology, a large amount of highly curated data is the cornerstone for discovery and validation and are important for developing, testing, and refining new computer algorithms that can push digital pathology forward [Citation28]. A number of initiatives for digital image sharing are now emerging, including PathXchange, to allow the exchange of knowledge and images among pathologists worldwide [Citation108].
Big data availability is vital. Several successful examples of highly curated open access datasets include The Cancer Genome Atlas (TCGA), the International Cancer Genome Consortium (ICGC), Gene Expression Omnibus (GEO), and single nucleotide polymorphism (SNP) databases. Data-sharing can facilitate disruptive discoveries by providing access to high-quality data that can be used for initial testing of a research hypothesis or utilized as an independent validation set for experimental results. This is of special importance considering the high-risk nature of disruptive innovation. Open access also includes access to gene analysis platforms and sophisticated informatics tools that can be of great help to researchers.
Conclusions
Disruptive innovations offer solutions with different benchmarks or new value propositions. They represent a new paradigm in diagnostics. They hold a great promise of redefining the standard of care not only to improve and accelerate care but also make it available at a more reasonable cost. We are in a unique era, which is the end of the beginning of the integration of multidisciplinary disruptive approaches in pathology and laboratory medicine.
We are optimistic that disruptive innovations will have a greater impact on the clinical laboratory in the next decades compared to sustaining innovations. They will ultimately lead to lowered cost of clinical laboratory testing without compromising performance. An open dialogue and a multidisciplinary approach are needed to overcome the challenges that face the implementation of disruptive innovations in laboratory medicine.
| Abbreviations | ||
| CLIA | = | Clinical Laboratory Improvement Amendments |
| DTC | = | direct-to-consumer |
| ELISA | = | enzyme-linked immunosorbent assay; |
| FDA | = | Food and Drug Administration |
| GDP | = | gross domestic product |
| GEO | = | Gene Expression Omnibus |
| ICGC | = | International Cancer Genome Consortium |
| IVD | = | in vitro diagnostic |
| MALDI-TOF | = | matrix-assisted laser desorption/ionization-time of flight |
| NGS | = | next-generation sequencing |
| NIH | = | National Institutes of Health |
| PGM | = | Personal Genome Machine |
| POCT | = | point of care testing |
| SBIR | = | Small Business Innovation Research |
| SBTR | = | Small Business Technology Transfer |
| SNP | = | single nucleotide polymorphism |
| TCGA | = | The Cancer Genome Atlas |
| TIAP | = | Toronto Innovation Acceleration Partners. |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ibrahim R, Pasic M, Yousef GM. Omics for personalized medicine: defining the current we swim in. Expert Rev Mol Diagn. 2016;16(7):719–722.
- Rifai N, Topol E, Chan E, et al. Disruptive innovation in laboratory medicine. Clin Chem. 2015;61(9):1129–1132.
- Disruptive and sustaining innovation: Deloitte Israel; 2020. Available from: https://www2.deloitte.com/il/en/pages/innovation/article/disruptive_vs_sustaining.html#
- Koboldt DC, Steinberg KM, Larson DE, et al. The next-generation sequencing revolution and its impact on genomics. Cell. 2013;155(1):27–38.
- Murphy MJ. Automation in UK clinical biochemistry. Ann Clin Biochem. 2013;50(Pt 3):285–286.
- Jonsson B. Disruptive innovation and EU health policy. Eur J Health Econ. 2017;18(3):269–272.
- Galea S. Will disruptive innovation in health care improve the health of populations? Milbank Q. 2018;96(4):619–622.
- Bower JL, Christensen CM. Disruptive technologies: catching the wave. Harv Bus Rev. 1995;73(1):43–53.
- Hwang J, Christensen CM. Disruptive innovation in health care delivery: a framework for business-model innovation. Health Aff . 2008;27(5):1329–1335.
- Christensen CM, Bohmer R, Kenagy J. Will disruptive innovations cure health care? Harv Bus Rev. 2000;78(5):102–112.
- Christensen CM. The encyclopedia of human-computer interaction. 2nd ed. Aarhus, Denmark: Interaction Design Foundation.
- Team M. What is disruptive innovation: MJV technology & innovation; 2019. Available from: https://www.mjvinnovation.com/blog/disruptive-innovation/
- Wilson R, Godfrey CM, Sears K, et al. Exploring conceptual and theoretical frameworks for nurse practitioner education: a scoping review protocol. JBI Database System Rev Implement Rep. 2015;13(10):146–155.
- Hooker RS, Moloney-Johns AJ, McFarland MM. Patient satisfaction with physician assistant/associate care: an international scoping review. Hum Resour Health. 2019;17(1):104.
- Kleinpell RM, Grabenkort WR, Kapu AN, et al. Nurse practitioners and physician assistants in acute and critical care: a concise review of the literature and data 2008–2018. Crit Care Med. 2019;47(10):1442–1449.
- Polinski JM, Barker T, Gagliano N, et al. Patients’ satisfaction with and preference for telehealth visits. J Gen Intern Med. 2016;31(3):269–275.
- Medicine JH. Coronavirus (COVID-19) information and updates; 2020. Available from: https://www.hopkinsmedicine.org/coronavirus/index.html
- Alert C. Health Canada/Santé Canada; 2020. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/covid-alert-app.html
- Tantibanchachai C. Researchers use machine learning to predict heart damage in COVID-19 patients. Baltimore (MD): Johns Hopkins University; 2020.
- Bradley R, Harnett J, Cooley K, et al. Naturopathy as a model of prevention-oriented, patient-centered primary care: a disruptive innovation in health care. Medicina. 2019;55(9):603.
- Dodd GD, 3rd, Restauri NL, Kondo KL, et al. Driving innovation in radiology: a summary of the 2015 intersociety committee summer conference. J Am Coll Radiol. 2016;13(12 Pt A):1477–1482.
- Gabril MY, Yousef GM. Informatics for practicing anatomical pathologists: marking a new era in pathology practice. Mod Pathol. 2010;23(3):349–358.
- Evans AJ, Chetty R, Clarke BA, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: the University Health Network experience. Semin Diagn Pathol. 2009;26(4):165–176.
- Evans AJ, Chetty R, Clarke BA, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: the University Health Network experience. Hum Pathol. 2009;40(8):1070–1081.
- Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol. 2019;20(5):e253–e261.
- Griffin J, Treanor D. Digital pathology in clinical use: where are we now and what is holding us back? Histopathology. 2017;70(1):134–145.
- Williams BJ, Bottoms D, Treanor D. Future-proofing pathology: the case for clinical adoption of digital pathology. J Clin Pathol. 2017;70(12):1010–1018.
- Hart SN. Will digital pathology be as disruptive as genomics? J Pathol Inform. 2018;9:27.
- Vergani A, Regis B, Jocolle G, et al. Noninferiority diagnostic value, but also economic and turnaround time advantages from digital pathology. Am J Surg Pathol. 2018;42(6):841–842.
- Mirham L, Naugler C, Hayes M, et al. Performance of residents using digital images versus glass slides on certification examination in anatomical pathology: a mixed methods pilot study. CMAJ Open. 2016;4(1):E88–E94.
- Yousef GM. Navigation through a new age of digital pathology: promises and challenges. Can J Pathol. 2017;9(1):4–6.
- Fraggetta F, Garozzo S, Zannoni GF, et al. Routine digital pathology workflow: the Catania experience. J Pathol Inform. 2017;8:51.
- Retamero JA, Aneiros-Fernandez J, Del Moral RG. Complete digital pathology for routine histopathology diagnosis in a multicenter hospital network. Arch Pathol Lab Med. 2020;144(2):221–228.
- Stathonikos N, Nguyen TQ, Spoto CP, et al. Being fully digital: perspective of a Dutch academic pathology laboratory. Histopathology. 2019;75(5):621–635.
- Saffitz JE. Genomic pathology: a disruptive innovation. Per Med. 2012;9(3):237–239.
- Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature. 2015;526(7571):29–31.
- Collins FS, Morgan M, Patrinos A. The human genome project: lessons from large-scale biology. Science. 2003;300(5617):286–290.
- Diamandis M, White NM, Yousef GM. Personalized medicine: marking a new epoch in cancer patient management. Mol Cancer Res. 2010;8(9):1175–1187.
- Pasic MD, Samaan S, Yousef GM. Genomic medicine: new frontiers and new challenges. Clin Chem. 2013;59(1):158–167.
- Hwang SM, Lee KC, Lee MS, et al. Comparison of ion personal genome machine platforms for the detection of variants in BRCA1 and BRCA2. Cancer Res Treat. 2018;50(1):255–264.
- Zopf A, Raim R, Danzer M, et al. Introduction of the hybcell-based compact sequencing technology and comparison to state-of-the-art methodologies for KRAS mutation detection. Biotechniques. 2015;58(3):126–134.
- Kumar KR, Cowley MJ, Davis RL. Next-generation sequencing and emerging technologies. Semin Thromb Hemost. 2019;45(7):661–673.
- Technologies OO. Oxford nanopore technologies; 2020 November 25. Available from: https://nanoporetech.com/
- Lanevschi A, Kramer JW. Comparison of two dry chemistry analyzers and a wet chemistry analyzer using canine serum. Vet Clin Pathol. 1996;25(1):10–13.
- Flatland B, Breickner LC, Fry MM. Analytical performance of a dry chemistry analyzer designed for in-clinic use. Vet Clin Pathol. 2014;43(2):206–217.
- Goble JA, Rocafort PT. Point-of-care testing. J Pharm Pract. 2017;30(2):229–237.
- Ferreira CES, Guerra JCC, Slhessarenko N, et al. Point-of-care testing: general aspects. Clin Lab. 2018;64(1):1–9.
- Kost GJ, Shirey TL. New whole-blood testing for laboratory support of critical care at cardiac transplant centers and US hospitals. Arch Pathol Lab Med. 1990;114(8):865–868.
- Kricka LJ. Emerging and disruptive technologies. EJIFCC. 2016;27(3):253–258.
- Charles L, Nelson MKS, Jin RY, et al. Inventor; Abbott Diabetes Care Inc., assignee. Glucose measuring device for use in personal area network. Patent US9730584B2. 2017.
- Cole LA, Sutton-Riley JM, Khanlian SA, et al. Sensitivity of over-the-counter pregnancy tests: comparison of utility and marketing messages. J Am Pharm Assoc (2003). 2005;45(5):608–615.
- Balter ML, Leipheimer JM, Chen AI, et al. Automated end-to-end blood testing at the point-of-care: integration of robotic phlebotomy with downstream sample processing. Technology (Singap World Sci). 2018;6(2):59–66.
- Price CP, Smith I, Van den Bruel A. Improving the quality of point-of-care testing. Fam Pract. 2018;35(4):358–364.
- Hood L, Friend SH. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat Rev Clin Oncol. 2011;8(3):184–187.
- Naugler C, Church DL. Automation and artificial intelligence in the clinical laboratory. Crit Rev Clin Lab Sci. 2019;56(2):98–110.
- Yousef GM. Artificial intelligence: the best is yet to come. Can J Pathol. 2019;11(3):4.
- Beck AH, Sangoi AR, Leung S, et al. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci Transl Med. 2011;3(108):108ra113.
- Nagpal K, Foote D, Liu Y, et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer. NPJ Digit Med. 2019;2:48.
- Parwani AV. Next generation diagnostic pathology: use of digital pathology and artificial intelligence tools to augment a pathological diagnosis. Diagn Pathol. 2019;14(1):138.
- Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
- Hegde N, Hipp JD, Liu Y, et al. Similar image search for histopathology: SMILY. NPJ Digit Med. 2019;2:56.
- Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20(5):e262–e273.
- Olivera P, Danese S, Jay N, et al. Big data in IBD: a look into the future. Nat Rev Gastroenterol Hepatol. 2019;16(5):312–321.
- Perco P, Oberbauer R. Integrative analysis of -omics data and histologic scoring in renal disease and transplantation: renal histogenomics. Semin Nephrol. 2010;30(5):520–530.
- Perco P, Kainz A, Wilflingseder J, et al. Histogenomics: association of gene expression patterns with histological parameters in kidney biopsies. Transplantation. 2009;87(2):290–295.
- Chetty R. Pathology and radiology taking medical ‘hermeneutics’ to the next level? J Clin Pathol. 2017;70(7):553–554.
- Filice RW. Deep-learning language-modeling approach for automated, personalized, and iterative radiology-pathology correlation. J Am Coll Radiol. 2019;16(9 Pt B):1286–1291.
- Barsoum I, Tawedrous E, Faragalla H, et al. Histo-genomics: digital pathology at the forefront of precision medicine. Diagnosis. 2019;6(3):203–212.
- Yousef GM. Use of social media in pathology: threats and opportunities. Can J Pathol. 2019;11(4):4.
- Isom J, Walsh M, Gardner JM. Social media and pathology: where are we now and why does it matter? Adv Anat Pathol. 2017;24(5):294–303.
- Bellis M, Metias S, Naugler C, et al. Digital pathology: attitudes and practices in the Canadian pathology community. J Pathol Inform. 2013;4:3.
- Yousef AG, Shipilova I, Kelada B, et al. Social media and digital resources in pathology: current attitudes and future perspectives for implementation. Can. J Pathol. 2019;11(3):51–62.
- ImmunoQuery. Available from: https://www.immunoquery.com/
- Tissue pathology. Available from: https://tissuepathology.com/
- Doximity. Available from: https://www.doximity.com/
- Yousef GM. Navigating through a new age of digital pathology: promises and challenges. Can. J Pathol. 2019;9(1):4–6.
- Saenger AK, Berkwits M, Carley S, et al. The power of social media in medicine and medical education: opportunities, risks, and rewards. Clin Chem. 2018;64(9):1284–1290.
- Nix JS, Gardner JM, Costa F, et al. Neuropathology education using social media. J Neuropathol Exp Neurol. 2018;77(6):454–460.
- bioRxiv. Available from: https://www.biorxiv.org/
- Yousef GM. Publicly available data: a game changer in modern research? Can J Pathol. 2018;10(3):4.
- Lee R, Davies G. Technology: the cure for rising healthcare costs? MIT Technology Review. 2013.
- Enthoven AC. Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10):S284–S90.
- Flores M, Glusman G, Brogaard K, et al. P4 medicine: how systems medicine will transform the healthcare sector and society. Per Med. 2013;10(6):565–576.
- Brandao M, Ponde N, Piccart-Gebhart M. Mammaprint™: a comprehensive review. Future Oncol. 2019;15(2):207–224.
- Di Meo A, Bartlett J, Cheng Y, et al. Liquid biopsy: a step forward towards precision medicine in urologic malignancies. Mol Cancer. 2017;16(1):80.
- Di Meo A, Brown MD, Finelli A, et al. Prognostic urinary miRNAs for the assessment of small renal masses. Clin Biochem. 2020;75:15–22.
- Di Meo A, Batruch I, Brown MD, et al. Identification of prognostic biomarkers in the urinary peptidome of the small renal mass. Am J Pathol. 2019;189(12):2366–2376.
- Berry R. Will 3D printing make obsolescence obsolete? 2015. Available from: https://3dprint.com/60963/3d-printing-obsolete/
- Singh A. Digital imaging and anatomic pathology’s care delivery model: biomagene. Available from: https://www.executivewarcollege.com/wp-content/uploads/2012/03/Singh-1.pdf
- Mamuszka H. The neverending LDT vs IVD debate: the journal of precision medicine. Available from: https://www.thejournalofprecisionmedicine.com/wp-content/uploads/2019/06/jpm219-Mamuszka.pdf
- Genzen JR, Mohlman JS, Lynch JL, et al. Laboratory-developed tests: a legislative and regulatory review. Clin Chem. 2017;63(10):1575–1584.
- Lieberman JA, Pepper G, Naccache SN, et al. Comparison of commercially available and laboratory-developed assays for in vitro detection of SARS-CoV-2 in clinical laboratories. J Clin Microbiol. 2020;58(8):e00821-20.
- Genzen JR. Regulation of laboratory-developed tests. Am J Clin Pathol. 2019;152(2):122–131.
- (FDA) TFaDA. Discussion paper on laboratory developed tests (LDTs); 2017 January 13. Available from: https://www.fda.gov/media/102367/download.
- Yousef GM. Direct-to-consumer genetic testing: the fine line between helping and hurting. Can J Pathol. 2019;11(1):4.
- Nam SH. Why disruptive innovations matter in laboratory diagnostics. Clin Chem. 2015;61(7):935–937.
- Heitzer E, Haque IS, Roberts CES, et al. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20(2):71–88.
- Yousef GM. Academic industrial relationship: a symbiotic future. Can J Pathol. In Press.
- Etzkowitz H, Leydesdorff L. The dynamics of innovation: from National Systems and ‘‘Mode 2’’ to a Triple Helix of university–industry–government relations. NH Elsevier. 2000;29(2):109–123.
- SBIR. Available from: https://www.sbir.gov/about
- Canadian Cancer Society. Available from: https://www.cancer.ca/en/research/grants-and-awards/current-funding-opportunities/innovation-grants/
- COVID-19 update: FDA authorizes first diagnostic test where results can be read directly from testing card: food and arug administration (FDA). Available from: https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-authorizes-first-diagnostic-test-where-results-can-be-read-directly-testing-card#:∼:text=Silver%20Spring%2C%20MD%20%2D%2D%20Today,design%20to%20some%20pregnancy%20tests
- Cambridge Innovation Center. Available from: https://cic.com/
- Toronto Innovation Acceleration Partners. Available from: https://tiap.ca/
- Veta M, van Diest PJ, Willems SM, et al. Assessment of algorithms for mitosis detection in breast cancer histopathology images. Med Image Anal. 2015;20(1):237–248.
- Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199–2210.
- Precision FDA challenges – precision FDA. Available from: http://www.precision.fda.gov/challenges
- Roche tissue diagnostics. Available from: https://diagnostics.roche.com/global/en/about/roche-tissue-diagnostics.html
