Abstract
Human breath offers several benefits for diagnostic applications, including simple, noninvasive collection. Breath is a rich source of clinically-relevant biological information; this includes a volatile fraction, where greater than 1,000 volatile organic compounds (VOCs) have been described so far, and breath aerosols that carry nucleic acids, proteins, signaling molecules, and pathogens. Many of these factors, especially VOCs, are delivered to the lung by the systemic circulation, and diffusion of candidate biomarkers from blood into breath allows systematic profiling of organismal health. Biomarkers on breath offer the capability to advance early detection and precision medicine in areas of global clinical need. Breath tests are noninvasive and can be performed at home or in a primary care setting, which makes them well-suited for the kind of public screening program that could dramatically improve the early detection of conditions such as lung cancer. Since measurements of VOCs on breath largely report on metabolic changes, this too aids in the early detection of a broader range of illnesses and can be used to detect metabolic shifts that could be targeted through precision medicine. Furthermore, the ability to perform frequent sampling has envisioned applications in monitoring treatment responses. Breath has been investigated in respiratory, liver, gut, and neurological diseases and in contexts as diverse as infectious diseases and cancer. Preclinical research studies using breath have been ongoing for some time, yet only a few breath-based diagnostics tests are currently available and in widespread clinical use. Most recently, tests assessing the gut microbiome using hydrogen and methane on breath, in addition to tests using urea to detect Helicobacter pylori infections have been released, yet there are many more applications of breath tests still to be realized. Here, we discuss the strengths of breath as a clinical sampling matrix and the technical challenges to be addressed in developing it for clinical use. Historically, a lack of standardized methodologies has delayed the discovery and validation of biomarker candidates, resulting in a proliferation of early-stage pilot studies. We will explore how advancements in breath collection and analysis are in the process of driving renewed progress in the field, particularly in the context of gastrointestinal and chronic liver disease. Finally, we will provide a forward-looking outlook for developing the next generation of clinically relevant breath tests and how they may emerge into clinical practice.
Introduction
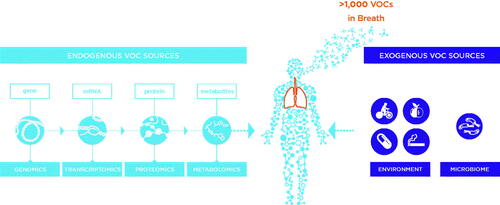
Human breath is a rich and diverse matrix that consists of much more than just atmospheric gases. Over the last 50 years, research has reported over 1000 different volatile organic compounds (VOCs) as well as an aqueous component – exhaled breath aerosols (EBAs) – that can include non-volatiles, proteins, nucleotides, and pathogens [Citation1–3]. An extensive range of techniques and technologies have been developed to enable the capture and analysis of breath components, each with their own benefits and drawbacks [Citation4,Citation5]. Notably, breath sampling can be completely noninvasive, making it well-suited for public screening programs and other early-stage investigations. Studies have also shown associations between breath and clinically-relevant processes. Increasingly, these studies have sought to associate breath biomarkers with clinically-relevant disease processes with a view to advance early detection and precision medicine (). However, to date, breath is rarely used in clinical tests and few breath biomarkers have been approved for use in diagnostics.
Figure 1. The opportunities for breath biomarkers to impact early detection and precision medicine in the clinical context.
Many clinical diagnostics are built around biological samples, most commonly tissues, biological fluids (blood, urine, or sputum), or feces. Tests in these matrices are an essential part of detecting, diagnosing, and treating almost all illnesses, yet these approaches have limitations in patient experience, accessibility, usability, and cost. These factors limit test utility and can make them less well suited for health screening applications. The development of breath as a novel diagnostic modality could overcome these limitations and help to address outstanding global healthcare challenges.
For many major illnesses, the chance of survival increases drastically, with a concomitant decrease in treatment costs, if patients are diagnosed early [Citation6,Citation7]. This is widely recognized in cancers, where the stage of diagnosis and morbidity both vary significantly by cancer type. However, there are other examples. Chronic liver diseases (CLDs) are estimated to affect over 1.5 billion people, with more than 50% of cases detected at late stages, when liver damage is typically irreversible [Citation8–10]. Given the self-regenerative ability of the liver, if disease could be detected earlier, progression might be halted or even reversed through targeted therapy or dietary and lifestyle alterations, reducing the burden on healthcare [Citation11]. Breath collection does not face the same hesitancy as more invasive samples for broad early-stage screening, where it could be deployed through regular screening programs and even be used for at-home testing in some contexts.
In certain settings, breath is already being used for precision medicine. Breath biomarkers associated with specific metabolic processes can offer direct readouts of disease activity and treatment response [Citation12]. For example, breath tests based on the detection of hydrogen and methane as biomarkers of bacterial fermentation are already in use for the diagnosis and treatment of gastrointestinal (GI) conditions, which can be difficult to classify based on symptoms [Citation13]. While GI illness is rarely fatal, it can be chronic, long lasting, and profoundly impacts quality of life. Patients often wait years for a clear diagnosis, and the consequent economic impact is significant [Citation14]. Over one billion people are affected and the annual cost to the US alone exceeds $20 billion [Citation15,Citation16].
Similarly, the detection of nitric oxide (NO) is widely assessed in diagnosis of respiratory diseases. A growing body of research evidence also supports the use of other breath biomarkers to guide precision treatment of chronic inflammatory airway diseases such as asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF), where inflammatory mechanisms have the potential to inform treatment choice [Citation17,Citation18]. Since COPD is the world’s third leading cause of death, this too represents a critical clinical need [Citation19].
In this review, we provide an overview of breath collection and analysis, with a focus on areas currently showing the greatest potential for clinical implementation. We will then discuss outlooks for breath tests in clinical diagnostics and the likely forms that such tests might take.
Biomarkers and breath
The origin of modern breath research is widely attributed to Linus Pauling and colleagues in the early 1970s [Citation20], although the history of breath and disease dates to the Ancient Greeks, who described fetor hepaticus – a distinct malodourous breath associated with liver disease [Citation21]. In Eastern medicine, the smell of breath has been used for disease diagnosis for over 3000 years [Citation22]. Similarly, high levels of acetone on breath can be the result of ketoacidosis, which may be an indicator of diabetes [Citation23]. Despite these examples, challenges related to reproducibility and standardization of sampling and analysis have limited clinical use of breath in many contexts.
Disease processes can modify underlying metabolic pathways, generating biomarkers that can report on aspects of disease development, progression, or treatment. Many VOCs on breath are products of endogenous biochemistry, making them relevant reporters of metabolic activity that may be altered by disease processes () [Citation24]. Metabolic biomarkers offer many advantages, including responsiveness to endogenous (e.g. genetic) and exogenous (e.g. Western diet) stimuli. As such, metabolic biomarkers provide a more direct readout of current disease state, while genetics, for example, is often only suitable for predicting the risk of disease. This distinction is particularly beneficial in treatment monitoring, where biomarkers may detect signs of response or changing severity before there are notable changes in symptoms.
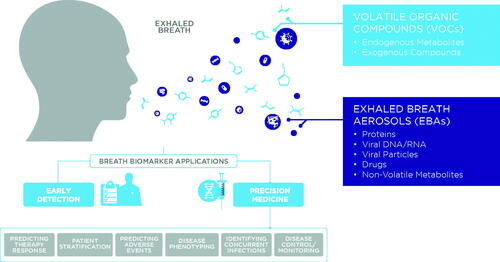
While breath has been most widely investigated in relation to respiratory diseases, the volatile nature of VOCs means that they readily exchange from the blood into the lung alveoli and, as such, they have the potential to report on diseases present in most parts of the body [Citation24,Citation25]. In contrast, EBA biomarkers typically arise in the lung airways as respiratory droplets. Biomarkers from EBAs can typically be analyzed using established approaches such as PCR or ELISA assays. In particular, EBAs have recently attracted attention for PCR-based SARS-CoV-2 detection [Citation26–28].
Since they currently present a wider range of prospective clinical applications and require more specialized analysis methods relative to EBAs, VOCs are the primary focus of this review. Research into VOC biomarkers from breath, and other biological samples, has demonstrated their relevance for the detection, treatment, and monitoring of a wide range of conditions including respiratory, hepatic, and GI illnesses, as well as cancer, infections, and inflammatory diseases. This potential is also highlighted by the successes of breath tests measuring urea for Helicobacter pylori infections, hydrogen and methane for GI conditions, and NO in respiratory medicine.
Breath sample collection and analysis tools
Breath has a great potential to be a valuable sample type for clinical tests. Breath sampling is noninvasive, painless, easy, and it can minimize stress for patients, particularly those with a fear of needles. Beath sampling can also be used with a wide range of patient groups, including children, the elderly, and even those with shortness of breath [Citation29]. The noninvasive nature of breath can facilitate collection in a wider range of contexts since it requires no clinical setting or specialist training, and it could enable patients to collect their own samples at home. Furthermore, a typical human produces around eight liters of breath per minute, making it an endlessly renewing source of information. This makes breath well-suited for regular or ongoing sampling to assess changes over time and means that it is easy to collect larger samples simply by collecting for a longer time.
Online breath analysis
Online breath analysis methods offer real-time or near-real-time analysis of samples with immediate output of results. Although the readout of results is rapid, many online methods depend on recognition of breath fingerprints, or “breathprints,” rather than exact mass- or structure-based identification of analytes, which may limit their practicality for many clinical test applications. Online breath analysis encompasses eNoses, which, like the alcohol breathalyzer, use specific detection sensors to identify particular compounds in breath [Citation30]. Naturally, this limits the range of compounds that can be measured, and additional sensors are required for each new set of compounds that need to be detected. Examples of eNoses include the Aeonose (The Enose Company, NL) and Cyranose (Sensigent, US) devices, which have been tested in various breath contexts.
The strength of eNoses is in their portability and ease of use, as well as in their ability to give an immediate result. The resulting breathprint reflects the strength of signal detected by each of the sensors in the device. While breathprints differ between individuals and have been shown to be affected by various illnesses, the breathprint itself cannot be directly related to biological changes associated with disease onset or development. As such, several eNose studies have also used parallel offline approaches to identify biomarker changes between groups of test subjects [Citation31,Citation32].
The availability and affordability of relevant sensors can also be a limitation. While hydrogen sensors, for example, are cheap and readily available, sensors that respond consistently and sensitively to other compounds on breath are often prohibitively expensive, if they are available at all. Calibrating sensors to ensure consistency across all devices is also a recognized challenge with this approach.
In recent years, there have also been developments in tools for more in-depth online analysis. Approaches such as selected ion flow tube mass spectrometry (SIFT-MS) and proton transfer reaction mass spectrometry (PTR-MS) have both been applied to directly collect and analyze breath samples [Citation33,Citation34]. These offer broader detection capability and greater sensitivity than many other online methods, while still providing relative ease of use and a higher level of sensitivity.
While these approaches allow the contents of a breath sample to be more comprehensively examined and analyzed, the quality of the results is still not comparable to more established offline approaches. Compared to offline approaches, the analysis pipeline is much harder to standardize, and results are typically of lower quality as a result. In addition, the lack of a pre-separation stage prevents the unambiguous identification of individual VOCs within a sample. As with eNoses, this limitation poses an issue for clinical translation where biomarker identities are necessary for regulatory approval.
Offline breath analysis
Offline methods typically rely on gas chromatography (GC) coupled to a backend detector – often a mass spectrometer – and require breath sample collection and transport to a central laboratory for analysis. While collecting breath in bags, balloons, or tubes presents unique challenges in terms of sample storage and transportation for analysis, offline analysis has notable benefits in terms of the opportunity to detect and identify compounds with greater sensitivity. The practical opportunity to provide high-quality, high-capacity, centralized breath analysis services that are optimized for compound detection and reproducible biomarker discovery has been further improved by the development of next-generation collection technologies that capture samples in formats more suitable for storage.
As the gold standard for breath sample analysis, gas chromatography mass spectrometry (GC-MS) offers the opportunity to separate and identify a wide range of compounds from breath and to perform detailed analysis and quantifications of each of those compounds. GC has been the leading choice to separate compounds since the era of modern breath research was ushered in by Pauling and colleagues [Citation20]. Advances in mass spectrometry technologies have further increased our ability to distinguish and identify molecules on breath, first with two-dimensional chromatography methods such as GCxGC-MS and more recently with high resolution accurate mass (HRAM) GC-MS. GC-MS offers the ability to identify compounds by matching to reference standards or existing compound libraries, and the confidence in naming compounds allows potential biomarkers to be associated with specific biological pathways and disease processes. Demonstrating the metabolic origin for biomarkers helps demonstrate their disease relevance, establish their clinical validity, and inform further development of targeted sampling and analysis methods that are more suitable in clinical contexts. GC-MS approaches also show the highest sensitivity for low abundance biomarkers, as low as parts per trillion (ppt), and have a dynamic range of up to six orders of magnitude, which is necessary to reliably cover the various VOC abundances seen on breath.
Clinical breath analysis
The following sections consider breath analysis across various diseases and the future forms that clinical breath tests might take. While handheld devices have captured the popular expectation for breath analysis, the additional complexity required for reliable disease diagnostics would suggest that centralized, offline testing is likely to become the prevalent mode of breath testing, at least in the shorter term.
Breath tests in clinical gastroenterology
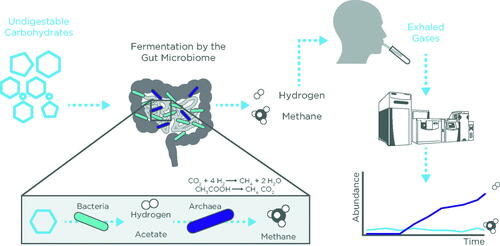
Breath testing to diagnose small intestinal bacterial overgrowth (SIBO), intestinal methanogen overgrowth (IMO), and carbohydrate malabsorption through detection of hydrogen and methane are established clinical applications of breath analysis. These GI conditions are characterized by excessive fermentation by the small intestinal and colonic microbiota, respectively, and can cause a range of GI symptoms such as bloating, abdominal pain, and diarrhea [Citation35]. Fermentation of carbohydrates by the gut microbiota leads to production of gases, short chain fatty acids (SCFAs), and other metabolites. Specifically, hydrogen and methane gases are produced exclusively by microorganisms and not by our own cells. Thus, hydrogen and methane in breath serve as distinctive biomarkers of bacterial fermentation.
Hydrogen and methane breath tests (HMBTs) are widely used for the diagnosis of SIBO. The development of SIBO is associated with motility disorders, after effects of surgery, structural disease, and stomach acid-suppression (i.e. achlorhydria or use of proton pump inhibitor drugs) [Citation36]. There is no established gold standard investigation for SIBO [Citation37]. Microbial culture of a jejunal aspirate has been used but is invasive, expensive, and not widely performed in clinical practice. In addition, aspirate cultures pose high risks of both false positives, through contamination by oral bacteria or saliva, and false negatives, through irregular distribution of bacteria through the bowel causing aspiration of a non-representative sample or of cultivation-resistant species [Citation38]. Consequently, the use of HMBT for the assessment of SIBO has been widely adopted in clinical practice as a safe and noninvasive alternative.
HMBT for SIBO involves administering an exogenous substrate, typically either glucose or lactulose, to stimulate bacterial fermentation (). The pattern of excreted breath hydrogen and methane is measured over time to reflect bacterial fermentation in response to oral carbohydrate load. Once collected using specialized bags or collection tubes, breath gases can be measured using portable handheld or desktop devices that measure gases using electrochemical sensors and lasers that can measure hydrogen and methane as low as 500 parts per million (ppm). These devices also assess O2 levels as a measure of sample validity with the O2 concentration of alveolar air estimated at 14% [Citation39]. The preferred approach for consistent clinical analysis is GC systems that enable a higher throughput of breath test analysis and also reduce the requirement for clinic appointments as breath samples can be provided using patient-friendly portable test-tube-based kits. GC systems equipped with a flame ionization detector and thermal conductor detector can reliably measure hydrogen and methane as well as CO2, which can serve as a correction factor to assess breath sample quality based on a CO2 concentration of an end-tidal breath as 5% [Citation39].
A positive result for SIBO is defined as a rise in hydrogen >20 ppm from the pretest baseline within 90 min after carbohydrate administration whereas methane levels of >10 ppm at any interval during the breath test are used to determine IMO (). IMO is a separate indication from SIBO since methane is produced by methanogens, which are archaea, not bacteria, that may overgrow in the small or large bowel [Citation40]. Similarly, an elevation in hydrogen gas >20 ppm from baseline can detect carbohydrate malabsorption. Lactose malabsorption affects over half of the global population, with prevalence much higher in the Middle East compared to Europe (70% versus 28%) [Citation41]. Lactose absorption depends on its hydrolysis by the enzyme lactase, which diminishes in most populations during childhood. Lactase deficiency may be primary (genetic) or secondary (acquired), such as through gastroenteritis, inflammatory bowel disease (IBD), celiac disease, and systemic sclerosis [Citation42]. Genetic tests can identify polymorphisms for primary lactase deficiency but are typically not recommended for clinical purposes [Citation42].
Figure 4. Potential additional mechanistic origins for VOC biomarkers relevant to gastrointestinal health. Adapted from [47].
![Figure 4. Potential additional mechanistic origins for VOC biomarkers relevant to gastrointestinal health. Adapted from [47].](/cms/asset/a7244d02-d627-41d5-b452-535f8bbeb568/ilab_a_2038075_f0004_c.jpg)
More importantly, genetic tests do not detect secondary lactase deficiency or assess symptoms. As a provocation test, tracking patient symptoms concurrently with hydrogen breath tests provides a measure of true intolerance (i.e. symptomatic response to lactose ingestion). However, a rise in hydrogen may also be consequent to SIBO, which means that it is important to establish whether a patient has SIBO before testing for malabsorption. When lactose is administered to a patient with SIBO, the substrate could be fermented by microbes in the small intestine leading to a false positive result for lactose malabsorption [Citation13,Citation43]. Alternatively, a false-negative result for lactose malabsorption can be obtained in people with a non-hydrogen producing-microbiota capable of metabolizing lactose. Methanogens, such as Methanobrevibacter smithii, can convert hydrogen into methane. The extent of breath methane production is also associated with the severity of constipation as a symptom [Citation44]. HMBT is, therefore, favorable to traditional hydrogen breath testing to identify a cohort of patients with excessive methane production.
The sensitivity and the specificity of HMBT, including glucose and lactulose breath tests, have been reported to be widely variable [Citation45]. This can largely be attributed to methodological issues, differences in substrate and dose used, restrictions applied before and during the test, and interpretation of the results. The North American Consensus on HMBT in GI disorders was published in 2017 to develop standardized guidelines, including indications for testing, preparation, performance, interpretation of results, and identifying remaining gaps in knowledge to be targeted with further study in order to minimize the impact of these variables [Citation13].
Confounding factors in establishing clinical breath tests
Despite attempts to standardize HMBT, there is still some debate around their clinical utility relating to variable transit times and, specifically, the potential for false positives due to early arrival in the cecum, for example, caused by altered GI anatomy (i.e. following small bowel or bariatric surgery) [Citation46]. In addition, digestibility of substrate can affect results in SIBO since glucose is absorbed specifically in the proximal small bowel whereas lactulose is an indigestible sugar that traverses the entire small bowel.
Pre-study preparation is also a paramount consideration for standardization, including a controlled diet, to reduce baseline fermentation levels, and stopping medications that can influence transit time i.e. laxatives and prokinetics. Careful patient selection in addition to the consideration of individual patient factors by trained clinical staff is vital for reliable translation of raw HMBT data to an accurate diagnosis and beneficial clinical outcome for patients. All of these factors ought to be considered prior to undertaking breath VOC analysis for GI conditions.
Investigating breath biomarkers for other GI conditions
While HMBT has become a valuable tool for specific applications, there are other needs in GI medicine, especially in differentiating between irritable bowel syndrome (IBS), IBD, celiac disease, and gastric cancer, which all present with similar symptoms but have vastly different treatments and consequences. To this end, a number of studies have been undertaken to investigate VOC biomarkers on breath, and in other samples, that could be developed to help detect, diagnose, and treat digestive disorders [Citation47].
IBS is one of the most prevalent GI disorders affecting around 5–10% of the global population [Citation48]. The current understanding of IBS is multifactorial and related to diet, malabsorption, visceral hypersensitivity, immune response, the gut microbiome, and psychological factors. IBS is considered a disorder of gut–brain interaction and a positive diagnosis of IBS is based on a strict set of criteria [Citation49], which can be difficult to assess due to the heterogeneity of disease and the unreliability of biomarkers. Thus, diagnosis and targeted treatment of the various IBS subtypes is a significant clinical challenge, where new breath biomarkers could make a notable contribution to establishing personalized therapies [Citation50].
Given its prevalence, it is unsurprising that some of the larger studies in the area have focused on IBS, identifying a range of VOCs and correlating VOC abundances to symptoms [Citation51,Citation52]. The largest of these to date was by Baranska et al., who identified 16 VOCs that differentiated subjects with IBS from healthy controls and correlated with symptoms (). Other studies have also demonstrated the potential of VOCs using other sampling matrices, which could be translated into breath [Citation53,Citation54,Citation55].
Table 1. A summary of relevant studies using breath and other sources of VOCs to identify differences between gastrointestinal conditions, mainly IBD and IBS.
IBD is another focus for research, with the particular goal of differentiating IBD from IBS. Both conditions manifest with similar GI symptoms and may even overlap, but IBD is associated with increased risk of complications, such as malnutrition and colorectal cancer [Citation56,Citation57], and the underlying biology differs significantly. Despite this, there are currently no easy diagnostic methods for differentiating IBS from the two forms of IBD; Crohn’s disease (IBD-CD) and ulcerative colitis (IBD-UC). The current gold standard investigations for IBD are highly invasive and require biopsies taken from the intestinal mucosa. Less invasive laboratory biomarkers, such as fecal calprotectin and C-reactive protein, have been explored and are shown to be sensitive to active IBD but do not detect the disease in its quiescent state and struggle to differentiate IBD from other causes of inflammation, such as infections, neoplasia, and stress [Citation58].
Van Malderen et al. [Citation59] provided the most up-to-date review of studies looking at volatomics in IBD and IBS. Ten compounds unique to IBD were described in multiple studies and several breath markers have been proposed to distinguish IBD inactivity, such as a decrease of breath pentane in Crohn’s disease and ulcerative colitis patients in remission compared to active disease, which has been seen in three separate studies. A decrease in breath and fecal propan-1-ol following treatment for IBD was also described in four studies. Furthermore, propan-1-ol has been proposed to discriminate Crohn’s disease from irritable bowel syndrome with diarrhea (IBS-D).
Growing associations between VOCs and microbiota in GI conditions
As HMBT demonstrates, the gut microbiome is a uniquely valuable source of distinctive compounds relevant to GI health, with metabolic processes that are exclusive to bacteria-producing VOCs not found elsewhere in the human body. Smolinska et al.’s [Citation55] examination of microbiome-related VOCs demonstrates the potential of this resource, finding VOCs associated with several bacterial taxa, including SCFAs such as acetic, pentanoic, and propionic acid, which were lower in active Crohn’s disease states. Bifidobacteria, Blautia, and members of the Firmicutes phylum all decreased during active disease and correlated with the reduction in SCFAs. SCFAs are known to influence host physiology and detecting them on breath is of great interest for future diagnostics. The microbiome is also a key focus for GI health intervention, and VOC biomarkers linked to bacteria could help to identify suitable interventions for each patient.
Though not associated with disease per se, diet, nutrition, and prebiotics/probiotics have a nascent body of literature associated with breath testing. While our aim is not to fully review this field, a few brief examples are offered below.
Ajibola et al. stated in their 2013 review that “relatively little work on the dietary factors that may influence [volatile breath metabolites]” has been done and outlined the key metabolic pathways leading to nutrient-derived VOCs () [Citation60]. In many respects, the understanding of the connections between nutrition and VOCs has not changed much but there has been astronomical growth in interest and knowledge of the gut microbiome. As the authors note, host metabolism contributes to NO, ammonia, pentane, ethane, ethylene, acetone, and isoprene, while various members of the gut microbiota produce methane, hydrogen, SCFAs, branched chain fatty acids (BCFAs), phenolics, and hydrogen sulfide. While VOCs are often produced by many bacteria, the prevalence of individual VOCs varies by species and some, such as trimethylamine, are produced exclusively by bacteria without any contribution by the host metabolism [Citation61]. Gut microbiota transform dietary nutrients into compounds that are used by members of the microbiome and absorbed by the host. Some of the volatile compounds generated by microbial metabolism, such as SCFAs [Citation62], are signaling molecules and can be used for host energy metabolism, whereas others, such as trimethylamine derived from metabolism of dietary choline and carnitine, can be further metabolized to metabolites that have long-term toxic effects on the host [Citation63]. Breath VOC analysis applied to volatile metabolism in pediatric epileptic populations placed on ketogenic diets (KDs) for seizure control versus similar patients on non-KDs showed that acetaldehyde, acetone, 2-methylfuran, methyl-vinyl-ketone, and 2-pentanone were significantly elevated in the breath of children consuming the KD relative to the control diet group [Citation64]. The production of volatile ketones by increased beta-oxidation in the KD group is an expected outcome of consuming a modified Atkins diet high in triacylglycerols and low in carbohydrates, and the increased level of ketones on breath shows the potential of using breath VOC analysis to monitor nutrition-based therapies.
Table 2. Nutrient classes and examples of their derivative VOCs.
As with KDs, prebiotics are being used to treat or modify disease and have been similarly shown to alter breath VOCs. De Preter et al. [Citation65] assessed breath VOCs in a randomized, placebo-controlled study looking at prebiotics in patients with IBD. They found that 10 g/day of oligofructose-enriched inulin increased the abundance of Bifidobacterium longis in Crohn’s disease patients, which was positively correlated with a reduction in disease activity (R = 0.894; p = 0.02). Specifically, they identified a significant increase in the VOCs acetaldehyde and butyrate in the oligofructose-enriched inulin group. Clearly, VOCs could help to predict response to treatment, but current studies are highly limited and their role in ground-breaking therapies, such as fecal microbiota transplant, has yet to be explored [Citation66].
The most researched application for VOCs as biomarkers of GI illness is in cancer detection. Xiang et al. performed a systematic review and meta-analysis of 14 studies that utilized breath VOCs for GI cancer diagnosis [Citation67], including gastric, gastroesophageal, and colon cancers. The review noted an elevation of phenol compounds linked to cancer, which was proposed to be the result of amino acid degradation in cancer cells.
Ultimately, the clinical use of breath VOCs has the capability to discriminate between GI diseases, as well as monitor disease activity and response to treatment. Breath VOCs could help to shift beyond the current limitations of HMBT owing to metabolic and microbial variation, such as providing information on the metabolite profile of bacteria in SIBO. Although the application of breath VOC analysis in clinical practice faces challenges due to the heterogeneity of patient populations as well as inconsistencies in analysis methods and result interpretation, combined with a lack of rigorous large-scale trials in GI disease. Future work should seek to reproducibly detect and validate consistent breath biomarkers in patient populations with specific GI disorders and validation cohorts.
Biomarkers of chronic liver diseases
The main factors leading to the development of CLDs worldwide are hepatitis B and C viruses, alcoholic liver disease, and nonalcoholic steatohepatitis (NASH), each showing different impact across countries and demographic groups, with NASH expected to overtake hepatitis B and C as the leading cause of CLD worldwide in the near future [Citation68]. The pathologic mechanisms underlying chronic liver injury for each of these indications are different but the end-stage outcome is the same: accumulation of fibrotic tissue referred to as cirrhosis [Citation8]. Independent of etiology, CLD progression is often slow and asymptomatic until liver fibrosis has become prominent. Therefore, ∼50% of the cases are detected at advanced stages in patients affected by episodes of liver decompensation [Citation8,Citation9,Citation69]. Inadequate early detection makes cirrhosis the 14th most common cause of death globally and 4th in central Europe [Citation8], despite the availability of therapeutic interventions, which if implemented at early stages prevent disease progression [Citation70].
The CLD diagnostic pathway frequently begins when the patient shows overt symptoms or alterations of blood biomarkers such as elevated bilirubin or decreased albumin. Noninvasive diagnosis through imaging modalities and indirect serum fibrosis tests offers excellent performance mainly at advanced stages [Citation8,Citation71]. Nevertheless, liver biopsy remains the gold standard diagnostic method despite its invasiveness and associated risk of complications [Citation72].
Accuracy of CLD diagnosis, etiology, and staging is crucial to establish treatment options, which include management of presented symptoms and correction of underlying factors, aiming to delay or stop disease progression [Citation70]. Almost all therapeutic interventions (i.e. viral suppression/eradication, alcohol abstinence, lifestyle corrections) administered at early stages have the potential to stop disease progression when liver function is still well preserved. However, efficient screening approaches, aiming at early detection, must be implemented to identify cases that remain undetected under current strategies [Citation73].
Metabolic alterations, associated with CLD, modify the profile of circulating metabolites [Citation74] as a result of changes in hepatic gene expression [Citation75–78], enzyme activity [Citation79–84], and liver extraction capacity due to portosystemic shunting and sinusoidal capillarization [Citation85,Citation86]. Routes of excretion for a subset of these metabolites include exhaled breath [Citation87]. Therefore, breath metabolomic analysis represents an attractive means for the identification of biomarkers associated with CLD that are suitable for a noninvasive test. The principle of breath testing was understood by Hippocrates (460–370 BC), who described fetor hepaticus, mentioned previously [Citation88]. More recently, GC techniques have been used to identify dimethyl sulfide as the main breath compound responsible for fetor hepaticus [Citation21,Citation89–91].
Perhaps due to the historical significance of liver diseases in breath research, a number of studies have been performed exploring VOCs on breath in association with CLDs, particularly cirrhosis (). The most consistent output of these studies, despite their variation in methodologies, has been the identification of elevated limonene on breath as a prospective biomarker associated with the development of cirrhosis [Citation92–96]. Unlike the classical approach of using endogenous biomarkers produced within the body, limonene on breath is thought to originate solely from exogenous dietary sources. Limonene is a terpene abundant in citrus fruit and is widely used in the food industry as flavoring agent. Dietary limonene is processed by CYP liver enzymes and, as such, it is plausible to expect that reduced liver function would slow or arrest the metabolic conversion of limonene [Citation97].
Table 3. A summary of relevant studies using breath sources of VOCs to identify chronic liver disease.
Limonene is valuable as a biomarker because its interaction with liver metabolism is well characterized and its exogenous origins mean it can be methodologically controlled and standardized, as recently reported [Citation98]. Perhaps the most striking association between limonene and cirrhosis comes from Fernandez del Rio et al. [Citation94], who not only demonstrated that it is elevated in cirrhosis patients compared to healthy controls but also showed that limonene levels decrease on breath following liver transplantation. A more recent study showed that limonene can help to distinguish pre-cirrhotic patients, and there is a lot of interest in examining whether limonene, either alone or in combination with other biomarkers, could help to detect early-stage liver diseases [Citation95]. Ferrandino et al. [Citation93] went further and showed that limonene could be positively correlated with measures of disease severity and blood biomarkers of liver function, including bilirubin and prothrombin time expressed as international normalized ratio, and negatively correlated with albumin. Morisco et al. [Citation99] also reported an unidentified monoterpene as the most effective single compound for classifying cirrhosis versus healthy control samples.
In addition to limonene, several studies have reported increased ketones on breath as markers of cirrhosis [Citation94,Citation99,Citation100], of which two reported significant elevations of 2-pentanone. The origins of this compound are less clear but may be linked to chronic inflammation.
In addition to discovery studies, hypothesis-based approaches have been tested to detect CLD through breath. The most striking example is represented by the methacetin breath test (MBT), which relies on the administration of 13C-labeled methacetin. In the liver, this drug is O-dealkylated by CYP1A2 to form paracetamol and 13C-formaldehyde, which is readily converted to 13CO2 and exhaled. The production of 13CO2 over baseline levels correlates with general liver function [Citation80,Citation101]. The latest evolution of this approach is the LiMAx test, which assesses maximum liver function capacity and consists of intravenous administration of 13C-methacetin followed by online sampling and analysis of the 13CO2/12CO2 ratio in the breath with a time resolution of 25 s and up to 40 min. This test has demonstrated an area under the curve (AUC) of 0.82 in a classification performance between patients with cirrhosis against healthy controls [Citation9] and is currently used to predict post-operative hepatic dysfunction in subjects undergoing hepatectomy [Citation102].
The examples of both limonene and methacetin serve to highlight the potential of administered compounds to stimulate metabolic responses associated with disease [Citation103], which has potential as a high-contrast approach to disease detection akin to the use of tracers or contrast agents in medical imaging.
Other applications for breath biomarkers
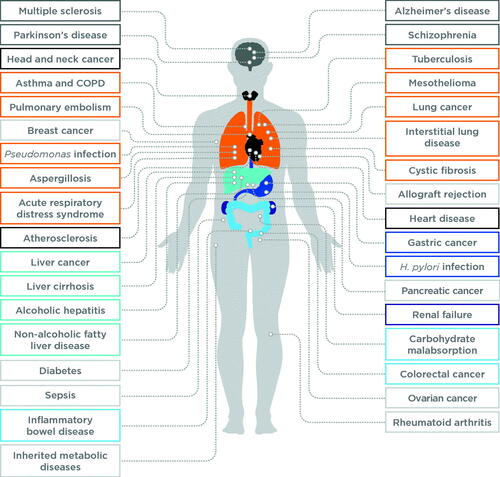
The examples covered in the previous sections illustrate just some selected examples of how breath biomarkers have been investigated to support early diagnosis and precision medicine. Other notable areas of investigation include respiratory diseases, inflammatory and infectious diseases, metabolic conditions, and of course, cancer ().
Figure 5. A summary of other conditions that have been investigated for detection using breath biomarkers.
Respiratory diseases
The most obvious applications, and the most extensively studied, are in respiratory diseases. Here, breath tests are already in use for detecting NO. Additionally, breath biomarkers could be used to report not only on systemic factors but also local biomarkers originating within the airways. Asthma and COPD are among the most common research topics, and IPF, interstitial lung disease (ILD), cystic fibrosis (CF), lung cancers, and mesothelioma have also been explored [Citation17,Citation104–111]. Breath tests for asthma and COPD have largely focused on improving differential diagnosis and treatment.
As inflammatory conditions, both asthma and COPD encompass various phenotypes that impact treatment [Citation112–115]. The ability to differentiate between cases dominated by eosinophils versus neutrophils could guide treatment decisions, improving symptom control for more patients sooner and dramatically reducing costs. Around half (46%) of asthma cases are eosinophilic, while neutrophilic inflammation is seen in 18% [Citation116]. While eosinophilic cases respond well to steroid treatments, other asthma phenotypes do not. A large study by Schleich et al. reported four VOCs capable of differentiating phenotypes [Citation18] and used VOCs to detect eosinophilic asthma (AUC = 0.72) with similar accuracy to FENO (AUC = 0.70) and blood eosinophil counts (AUC = 0.71). A systematic review of studies in adult asthma was performed by Azim et al. [Citation17].
Unlike many existing methods, breath testing has shown to be suitable and even well tolerated for use with children. Since asthma is common in young children, this represents a critical benefit for early-age diagnosis. Smolinska et al. identified 17 VOCs, including ones linked to inflammation and oxidative stress, that correctly differentiated asthma from wheezers in 80% of cases [Citation117]. Neerincx et al. provide a thorough review of breath research for pediatric asthma [Citation118]. Exacerbations are a shared feature of respiratory inflammatory diseases. Since breath is well suited to regular sample collection, it can be effectively used for monitoring and exacerbation prediction. This too has been notably studied in childhood asthma, where studies in 2013 and 2017 found that VOCs have potential to predict exacerbations in a way that FENO and lung function tests do not [Citation119,Citation120].
Breath VOCs have also been more widely explored to detect respiratory infections in CF. Infections in CF can take hold quickly and have a severe impact on health. Infections that have been investigated include Staphylococcus, Pseudomonas, Stenotrophomonas, and Burkholderia species [Citation109,Citation121,Citation122]. Of course, various recent studies have also examined breath tests for COVID-19, although largely these have focused on EBAs and not VOCs [Citation123].
Cancer
The aim of creating a breath test for cancer has been widely promoted. The potential for cancer breath tests lies in early detection. During the early stages of cancer, while the mutagenic load on cancer cells is often less extensive, there may still be significant metabolic shifts. For example, cancer cells often have a much higher glycolytic flux due to the Warburg effect, and this is a property that is already used for cancer detection in positron emission tomography (PET) through the use of fluorodeoxyglucose (FDG) as a tracer agent. As such, metabolic biomarkers can be expected to be better suited to early detection of cancer than genetic or even proteomic markers. In addition to changes within cancer cells, VOCs may also detect immunological responses to cancer, as we have already seen in other contexts. The use of FDG in PET scans for cancer also serves to demonstrate how an exogenous VOC (EVOC) probe approach – detecting disease using an administered probe that can be monitored on breath – could be applied in cancer, with selected compounds being administered to measure metabolic flux through pathways affected by cancer.
The noninvasive and continuously renewing nature of VOCs on breath means that breath testing could become a valuable method for ongoing treatment monitoring and relapse detection. Taking a regular breath test could provide early warning when cancer starts to become resistant. A number of reviews and meta-analyses have already been published on breath VOCs for cancer [Citation110,Citation124–128]. Largely, these have concluded that the field needs to focus on progressing to larger, multi-center studies and standardization in collection, storage, analysis, and result reporting. These challenges were specifically examined by Hanna et al. in 2019 [Citation129].
Other diseases
The ability to reliably detect VOC biomarkers on breath, and use them to probe metabolism throughout the body, is still relatively novel and numerous opportunities remain to be explored. shows a selection of the conditions that have been examined using breath research.
We discussed previously how unique VOCs produced by the gut microbiome make breath tests an effective approach for monitoring gut dysbiosis. For similar reasons, breath research has also had success in detecting other infectious diseases [Citation61]. Tuberculosis [Citation130,Citation131], invasive aspergillosis [Citation132,Citation133] and Clostridium difficile infections are just some examples of this [Citation134]. Studies have also examined the use of breath to detect malaria parasites [Citation135–137].
VOCs on breath have also been investigated for arthritis [Citation138], diabetes [Citation139], Alzheimer’s [Citation140], Parkinson’s [Citation141], and multiple sclerosis [Citation142]. As things stand, breath tests appear to have vast potential to improve our ability to detect, diagnose, treat, and monitor many different illnesses. In particular, breath could become a common pre-selection approach, helping to reduce the number of patients requiring specialist consultation, invasive testing, or exploratory surgery.
Progressing breath research toward clinical application
In this review, we have presented an overview of the current progress in developing breath tests for clinical applications using VOC biomarkers. We have placed particular focus on fields where tests are already in use or have demonstrated promising progress toward clinical implementation. We have also highlighted some of the other disease areas where breath has been investigated and could be deployed for early detection, drug development, or precision medicine.
As a whole, the field is entering the analytical validation stage (). A large number of initial studies have been carried out across a wide range of disease areas and, through a diverse range of experimental approaches, have consistently demonstrated that breath tests have the potential to address critical clinical needs. In many cases, promising biomarkers have been proposed, some of which have been backed up by biological rationale or research studies using in vitro and animal models. Yet, to date, few breath biomarkers have been clinically validated.
Figure 6. An overview of the biomarker development pipeline and the potential for small-scale point of care tools to be deployed for clinical application on the basis of robust biomarker discovery and validation.
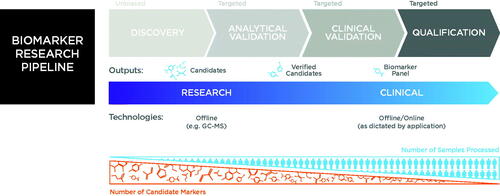
As we have seen, lack of consistency and standardization has become a key limitation for the field as numerous approaches to study design, breath collection, analysis, and reporting have been developed and tested. Recent progress has seen improvements in the quality of studies and technologies with an increasing focus on consistency, reproducibility, and validation. Notably, various tools and services for breath collection and analysis are now commercially available, which is driving significant advances in quality and consistency of results both within and between studies.
Over the coming years, we expect to see the publication of a growing number of validation studies seeking to reproduce and expand on the existing body of published research. From this, more validated breath biomarkers will likely emerge, which can then become the focus of targeted clinical test development. We also expect to see growth in the availability of biological evidence supporting the association of certain VOCs with disease processes. At present, this knowledge is only available for a relatively small number of VOCs, for example, the production of certain alkanes as a result of lipid peroxidation [Citation143] or the release of hydrogen and methane by the microbiome [Citation144]. Such insights will provide vital support to justify the use of biomarkers for medical testing and decision-making.
There is also a growing desire to standardize the reporting of results from breath studies to aid the sharing and reproduction of findings, this would be a significant collaborative undertaking that has the potential to drastically advance the field [Citation145]. Similarly, efforts such as the Peppermint consortium serve to highlight the desire for a deeper understanding of healthy variation within breath [Citation146]. Akin to the HapMap project in genomics [Citation147], this would give greater clarity over the extent of variation in breath VOCs across the human population, providing a vital resource for comparison to various research results and disease phenotypes. The need for this in breath research is even more dramatic than in genetics, since unlike genetics, VOCs on breath vary over time and are influenced by a wide range of external factors, making the frequency and extent of variation much greater and harder to predict.
The breath tests that have so far made the most progress toward clinical implementation are those based on administered substrates that lead to the production of well-characterized biomarkers. This use of a priori biological knowledge has enabled rapid test development by guiding both the method and approaches used. As with the carbohydrate substrates for gut health or limonene for liver disease, novel EVOC probes could be developed to assess the activity of other metabolic pathways through the production of biomarkers that can be measured on breath [Citation87]. A greater understanding of the biological origins of VOCs is essential to identifying more potential EVOC probes with relevance to other disease areas.
How breath could look in the clinic
One of the benefits of the diverse approaches that have been developed to collect and analyze breath samples is that it gives some insight into how breath tests may appear in the clinical context (). The key division is likely to be between online tests that provide immediate results and offline tests where sample collection and analysis are separated. In the long term, it seems likely that both approaches could be seen in clinical use and that the distinction between them will be guided by clinical utility.
As with current clinical tests, on blood or urine for example, offline solutions will most likely be sufficient for most applications across both early detection and precision medicine. Offline tests offer much greater scope for more wide-ranging and in-depth sample analysis, which simply is not possible for online point of care devices that need to be accessible and provide easy to interpret results. Similarly, offline testing methods could enable simultaneous testing of a greater number of disease markers associated with various relevant pathologies, expanding the potential value of each breath test.
In principle, offline breath tests could adapt or make use of existing technologies available within clinical laboratories, which may help to reduce the costs of implementing new tests and could take advantage of existing expertise. Alternatively, specialist services are becoming available to provide centralized, optimized support for breath testing, ensuring high consistency of results across sites.
Unlike other sampling matrices, the challenge for breath tests is in collecting and stabilizing a large enough sample of breath VOCs in a compact form that is suitable for easy storage, transportation, and analysis. Next-generation collection devices, such as ReCIVA® Breath Sampler [Citation148], are some of the first to provide this capability by collecting VOCs directly into cartridges of sorbent tubes, which trap and stabilize a wide range of VOCs without also collecting atmospheric gases. Even in the context of primary care testing or at-home breath tests, it seems likely that offline analysis will continue to have advantages for the foreseeable future.
Of course, online analysis avoids the need for sample storage but is much more challenging in terms of the requirements for creating a suitable testing device that provides accurate, consistent results. Building on the concept of alcohol breathalyzers, eNose devices [Citation149] and tools such as the FoodMarble Aire [Citation150] address the need for convenience but currently encounter issues with sensitivity and consistency. As discussed, the challenge with these sensor-based devices is that the patterns of responses they provide are not directly linked to specific molecules or disease processes. If we consider existing online tests in clinical use, they show clear connection between the purpose of the test (e.g. blood glucose, pregnancy, blood alcohol) and the biomarker being assayed. While eNoses have shown promise in early-stage clinical trials, more work is needed to show how the results they provide reflect underlying disease biology and whether these devices can provide robust results across many clinical sites.
At least in the early stages, it seems more likely that more analytical devices such as PTR-MS or SIFT-MS will be deployed where online testing is needed in the clinical setting. The limitations of the adoption of these devices are likely cost and clarity of results. If such tools are being used by clinical staff, it is likely that interface developments will be needed to make them easy for use by non-specialists.
The space where it seems most likely that we will see breath tests in a more portable online form is in the ongoing monitoring of rapidly changeable disease symptoms. As we have seen, breath has great potential to be used as a predictor for respiratory disease exacerbations and to assess changing symptom severity. This could become akin to glucose testing for diabetes, and of course a breath test for blood glucose could also be envisaged. Such approaches may also be relevant for treatment monitoring, particularly in cases where correct dosage is highly variable between patients or needs to be closely regulated over time.
| Abbreviations | ||
| AUC | = | area under the curve |
| BCFA | = | branched chain fatty acid |
| CF | = | cystic fibrosis |
| CLD | = | chronic liver disease |
| COPD | = | chronic obstructive pulmonary disease |
| CP | = | Child–Pugh |
| EBA | = | exhaled breath aerosol |
| EVOC | = | exogenous volatile organic compound |
| FDG | = | fluorodeoxyglucose |
| GC | = | gas chromatography |
| GC-MS | = | gas chromatography mass spectrometry |
| GI | = | gastrointestinal |
| HMBT | = | hydrogen and methane breath test |
| HRAM | = | high-resolution accurate mass |
| IBD | = | inflammatory bowel disease |
| IBD-CD | = | Crohn’s disease |
| IBD-UC | = | ulcerative colitis |
| IBS | = | irritable bowel syndrome |
| IBS-D | = | irritable bowel syndrome with diarrhea |
| ILD | = | interstitial lung disease |
| IMO | = | intestinal methanogen overgrowth |
| IPF | = | idiopathic pulmonary fibrosis |
| KD | = | ketogenic diet |
| MBT | = | methacetin breath test |
| NAFLD | = | nonalcoholic fatty liver disease |
| NASH | = | nonalcoholic steatohepatitis |
| NO | = | nitric oxide |
| PET | = | positron emission tomography |
| ppm | = | parts per million |
| ppt | = | parts per trillion |
| PTR-MS | = | proton transfer reaction mass spectrometry |
| SCFA | = | short chain fatty acid |
| SIBO | = | small intestinal bacterial overgrowth |
| SIFT-MS | = | selected ion flow tube mass spectrometry |
| VOC | = | volatile organic compound |
Acknowledgments
The authors thank Billy Boyle, Jason Kinchen, Olga Gandelman, and Marc van der Schee for their input and reviews in the planning and preparation of this manuscript. The authors also thank Edgard Delvin and the journal for inviting this review.
Disclosure statement
J. J. H., C. K. P., and A. R. H. are employees of Functional Gut Diagnostics Ltd. G. F., K. L. P., and J. L. D. L. are employed by Owlstone Medical Ltd. None of the authors declare any other interests.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Phillips M, Herrera J, Krishnan S, et al. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999;729(1–2):75–88.
- Fabian P, Brain J, Houseman EA, et al. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv. 2011;24(3):137–147.
- Bake B, Larsson P, Ljungkvist G, et al. Exhaled particles and small airways. Respir Res. 2019;20(1):8.
- Das S, Pal M. Review—Non-invasive monitoring of human health by exhaled breath analysis: a comprehensive review. J Electrochem Soc. 2020;167(3):037562.
- Khoubnasabjafari M, Mogaddam MRA, Rahimpour E, et al. Breathomics: review of sample collection and analysis, data modeling and clinical applications. Crit Rev Anal Chem. 2021. DOI:https://doi.org/10.1080/10408347.2021.1889961
- Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3(4):243–252.
- Organization WH. Early cancer diagnosis saves lives, cuts treatment costs 2017. [updated 3rd Feb 2017]. Available from: https://www.who.int/news/item/03-02-2017-early-cancer-diagnosis-saves-lives-cuts-treatment-costs
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761.
- Holzhütter H-G, Wuensch T, Gajowski R, et al. A novel variant of the 13C-methacetin liver function breath test that eliminates the confounding effect of individual differences in systemic CO2 kinetics. Arch Toxicol. 2020;94(2):401–415.
- Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666.
- Hossain N, Kanwar P, Mohanty SR. A comprehensive updated review of pharmaceutical and nonpharmaceutical treatment for NAFLD. Gastroenterol Res Pract. 2016;2016:7109270. 2016/02/23;2016:7109270.
- van der Schee M, Pinheiro H, Gaude E. Breath biopsy for early detection and precision medicine in cancer. Ecancermedicalscience. 2018;12:ed84.
- Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017;112(5):775–784.
- Henson SJ, Majowicz SE, Masakure O, et al. Estimation of the costs of acute gastrointestinal illness in British Columbia, Canada. Int J Food Microbiol. 2008;/127(1–2):43–52.
- Nirwan JS, Hasan SS, Babar Z-U-D, et al. Global prevalence and risk factors of gastro-oesophageal reflux disease (GORD): systematic review with meta-analysis. Sci Rep. 2020;10(1):5814.
- IBS IA. Facts about IBS – Statistics 2021. [cited 2021 13th Sept 2021]. Available from: https://aboutibs.org/what-is-ibs/facts-about-ibs/statistics/
- Azim A, Barber C, Dennison P, et al. Exhaled volatile organic compounds in adult asthma: a systematic review. Eur Respir J. 2019;54(3):1900056.
- Schleich FN, Zanella D, Stefanuto PH, et al. Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am J Respir Crit Care Med. 2019;200(4):444–453.
- Organization WH. The top 10 causes of death: World Health Organization; 2020. [updated 9th Dec 2020; cited 2021 13th Sept 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Pauling L, Robinson AB, Teranishi R, et al. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc Natl Acad Sci U S A. 1971;68(10):2374–2376.
- Tangerman A, Meuwese-Arends MT, Jansen JB. Cause and composition of foetor hepaticus. Lancet. 1994;343(8895):483.
- Wang Z, Wang C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J Breath Res. 2013;7(3):037109.
- Unger J. Measuring the sweet smell of success in diabetes management. Ann Transl Med. 2014;2(12):119–119.
- Phillips M, Greenberg J, Awad J. Metabolic and environmental origins of volatile organic compounds in breath. J Clin Pathol. 1994;47(11):1052–1053.
- Blanchet L, Smolinska A, Baranska A, et al. Factors that influence the volatile organic compound content in human breath. J Breath Res. 2017;11(1):016013.
- Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676–680.
- Li X, Li J, Ge Q, et al. Detecting SARS-CoV-2 in the breath of COVID-19 patients [Original research]. Front Med (Lausanne). 2021;8(210):604392.
- Smolinska A, Jessop DS, Pappan KL, et al. The SARS-CoV-2 viral load in COVID-19 patients is lower on face mask filters than on nasopharyngeal swabs. Sci Rep. 2021;11(1):13476.
- Holden KA, Ibrahim W, Salman D, et al. Use of the ReCIVA device in breath sampling of patients with acute breathlessness: a feasibility study. ERJ Open Res. 2020;6(4): 00119-2020.
- Wilson AD. Noninvasive early disease diagnosis by electronic-nose and related VOC-detection devices. Biosensors. 2020;10(7):73.
- Brinkman P, van de Pol MA, Gerritsen MG, et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin Exp Allergy. 2017;47(9):1159–1169.
- Saidi T, Zaim O, Moufid M, et al. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens Actuators, B. 2018;257:178–188.
- Španěl P, Smith D. Progress in SIFT-MS: breath analysis and other applications. Mass Spectrom Rev. 2011;30(2):236–267.
- Mayhew CAB, Herbig J, Beauchamp J. Proton transfer reaction mass spectrometry. In Beauchamp JD, Pleil J, editors. Breathborne biomarkers and the human volatilome. 2nd ed. New York, NY: Elsevier; 2020.
- Mohajeri MH, Brummer RJM, Rastall RA, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57(Suppl 1):1–14.
- Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3(2):112–122.
- Shah ED. Breath test or duodenal aspirate for small intestinal bacterial overgrowth: still no breath of fresh air. Dig Dis Sci. 2021;66(6):1770–1771.
- Erdogan A, Rao SS, Gulley D, et al. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;r27(4):481–489.
- Lee SM, Falconer IHE, Madden T, et al. Characteristics of oxygen concentration and the role of correction factor in real-time GI breath test. BMJ Open Gastroenterol. 2021;8(1):e000640.
- Pimentel M, Saad RJ, Long MD, et al. ACG clinical guideline: small intestinal bacterial overgrowth. Off J Am Coll Gastroenterol | ACG. 2020;115(2):165–178.
- Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):738–746.
- Misselwitz B, Butter M, Verbeke K, et al. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut. 2019;68(11):2080–2091.
- Perets TT, Hamouda D, Layfer O, et al. Small intestinal bacterial overgrowth may increase the likelihood of lactose and sorbitol but not fructose intolerance false positive diagnosis. Ann Clin Lab Sci. 2017;47(4):447–451.
- Chatterjee S, Park S, Low K, et al. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102(4):837–841.
- Losurdo G, Leandro G, Ierardi E, et al. Breath tests for the non-invasive diagnosis of small intestinal bacterial overgrowth: a systematic review with meta-analysis. J Neurogastroenterol Motil. 2020;26(1):16–28.
- Jirapinyo P, Makuvire TT, Dong WY, et al. Impact of oral-cecal transit time on the interpretation of lactulose breath tests after RYGB: a personalized approach to the diagnosis of SIBO. Obes Surg. 2019;29(3):771–775.
- Kurada S, Alkhouri N, Fiocchi C, et al. Review article: breath analysis in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;41(4):329–341.
- Oka P, Parr H, Barberio B, et al. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;/5(10):908–917.
- Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):P1393–1407.E5.
- Camilleri M. Review article: biomarkers and personalised therapy in functional lower gastrointestinal disorders. Aliment Pharmacol Ther. 2015;42(7):818–828.
- Baranska A, Mujagic Z, Smolinska A, et al. Volatile organic compounds in breath as markers for irritable bowel syndrome: a metabolomic approach. Aliment Pharmacol Ther. 2016;44(1):45–56.
- Cauchi M, Fowler DP, Walton C, et al. Application of gas chromatography mass spectrometry (GC–MS) in conjunction with multivariate classification for the diagnosis of gastrointestinal diseases. Metabolomics. 2014;10(6):1113–1120.
- Ahmed I, Greenwood R, Costello Bde L, et al. An investigation of fecal volatile organic metabolites in irritable bowel syndrome. PLoS One. 2013;8(3):e58204.
- Arasaradnam RP, Westenbrink E, McFarlane MJ, et al. Differentiating coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis-a pilot study. PLoS One. 2014;9(10):e107312.
- Smolinska A, Tedjo DI, Blanchet L, et al. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal Chim Acta. 2018;1025:1–11.
- Hwang C, Ross V, Mahadevan U. Micronutrient deficiencies in inflammatory bowel disease: from a to zinc. Inflamm Bowel Dis. 2012;18(10):1961–1981.
- Wan Q, Zhao R, Xia L, et al. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol. 2021;147(4):1077–1087.
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454.
- Van Malderen K, De Winter BY, De Man JG, et al. Volatomics in inflammatory bowel disease and irritable bowel syndrome. EBioMedicine. 2020;54:102725.
- Ajibola OA, Smith D, Spaněl P, et al. Effects of dietary nutrients on volatile breath metabolites. J Nutr Sci. 2013;2:e34.
- Belizario JE, Faintuch J, Malpartida MG. Breath biopsy and discovery of exclusive volatile organic compounds for diagnosis of infectious diseases. Front Cell Infect Microbiol. 2020;10(564194):564194.
- Raninen KJ, Lappi JE, Mukkala ML, et al. Fiber content of diet affects exhaled breath volatiles in fasting and postprandial state in a pilot crossover study. Nutr Res. 2016;36(6):612–619.
- Hartiala J, Bennett BJ, Tang WH, et al. Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler Thromb Vasc Biol. CARDIoGRAM Consortium. 2014;34(6):1307–1313.
- Ruzsanyi V, Peter Kalapos M, Schmidl C, et al. Breath profiles of children on ketogenic therapy. J Breath Res. 2018;12(3):036021.
- De Preter V, Joossens M, Ballet V, et al. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn's disease patients: a double-blinded randomized controlled trial. Clin Transl Gastroenterol. 2013;4(1):e30.
- De Preter V, Hamer HM, Windey K, et al. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health? Mol Nutr Food Res. 2011;55(1):46–57.
- Xiang L, Wu S, Hua Q, et al. Volatile organic compounds in human exhaled breath to diagnose gastrointestinal cancer: a meta-analysis. Front Oncol. 2021;11: 606915–606915.
- GBD-Cirrhosis-Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266.
- Ratib S, Fleming KM, Crooks CJ, et al. 1 And 5 year survival estimates for people with cirrhosis of the liver in England, 1998–2009: a large population study. J Hepatol. 2014;60(2):282–289.
- Sharma A, Nagalli S. Chronic liver disease. Treasure Island, FL: StatPearls; 2021.
- Papastergiou V, Tsochatzis E, Burroughs AK. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25(3):218–231.
- Neuberger J, Patel J, Caldwell H, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69(8):1382–1403.
- Harman DJ, Ryder SD, James MW, et al. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross-sectional diagnostic study utilising transient elastography. BMJ Open. 2015;5(4):e007516.
- Khan V, Putluri N, Sreekumar A, et al. Current applications of metabolomics in cirrhosis. Metabolites. 2018;8(4):67.
- Hoang SA, Oseini A, Feaver RE, et al. Gene expression predicts histological severity and reveals distinct molecular profiles of nonalcoholic fatty liver disease. Sci Rep. 2019;9(1):12541.
- Gerhard GS, Legendre C, Still CD, et al. Transcriptomic profiling of obesity-related nonalcoholic steatohepatitis reveals a core set of fibrosis-specific genes. J Endocr Soc. 2018;2(7):710–726.
- Govaere O, Cockell S, Tiniakos D, et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci Transl Med. 2020;12(572):eaba4448.
- Suppli MP, Rigbolt KTG, Veidal SS, et al. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal-weight individuals. Am J Physiol Gastrointest Liver Physiol. 2019;316(4):G462–G472.
- Miele L, Grieco A, Armuzzi A, et al. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol. 2003;98(10):2335–2336.
- Braden B, Faust D, Sarrazin U, et al. 13C-methacetin breath test as liver function test in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2005;21(2):179–185.
- Park GJ, Katelaris PH, Jones DB, et al. The C-caffeine breath test distinguishes significant fibrosis in chronic hepatitis B and reflects response to lamivudine therapy. Aliment Pharmacol Ther. 2005;22(5):395–403.
- Li H, Toth E, Cherrington NJ. Alcohol metabolism in the progression of human nonalcoholic steatohepatitis. Toxicol Sci. 2018;164(2):428–438.
- Dietrich CG, Gotze O, Geier A. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World J Gastroenterol. 2016;22(1):72–88.
- Elbekai RH, Korashy HM, El-Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5(2):157–167.
- Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144(3):512–527.
- Straub AC, Clark KA, Ross MA, et al. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J Clin Invest. 2008;118(12):3980–3989.
- Gaude E, Nakhleh MK, Patassini S, et al. Targeted breath analysis: exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J Breath Res. 2019;13(3):032001.
- Dweik RA, Amann A. Exhaled breath analysis: the new frontier in medical testing. J Breath Res. 2008;2(3):030301.
- Kaji H, Hisamura M, Saito N, et al. Evaluation of volatile sulfur compounds in the expired alveolar gas in patients with liver cirrhosis. Clin Chim Acta. 1978;85(3):279–284.
- Dadamio J, Van den Velde S, Laleman W, et al. Breath biomarkers of liver cirrhosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;905:17–22.
- Pijls KE, Smolinska A, Jonkers DM, et al. A profile of volatile organic compounds in exhaled air as a potential non-invasive biomarker for liver cirrhosis. Sci Rep. 2016;6:19903.
- Friedman MI, Preti G, Deems RO, et al. Limonene in expired lung air of patients with liver disease. Dig Dis Sci. 1994;39(8):1672–1676.
- Ferrandino G, Orf I, Smith R, et al. Breath biopsy assessment of liver disease using an exogenous volatile organic compound-toward improved detection of liver impairment. Clin Transl Gastroenterol. 2020;11(9):e00239.
- Fernandez Del Rio R, O'Hara ME, Holt A, et al. Volatile biomarkers in breath associated with liver cirrhosis – comparisons of pre- and post-liver transplant breath samples. EBioMedicine. 2015;2(9):1243–1250.
- Sinha R, Lockman KA, Homer NZM, et al. Volatomic analysis identifies compounds that can stratify non-alcoholic fatty liver disease. JHEP Rep. 2020;2(5):100137.
- Thomas JN, Roopkumar J, Patel T. Machine learning analysis of volatolomic profiles in breath can identify non-invasive biomarkers of liver disease: a pilot study. PLoS One. 2021;16(11):e0260098.
- Miyazawa M, Shindo M, Shimada T. Metabolism of (+)- and (-)-limonenes to respective carveols and perillyl alcohols by CYP2C9 and CYP2C19 in human liver microsomes. Drug Metab Dispos. 2002;30(5):602–607.
- Murgia A, Ahmed Y, Sweeney K, et al. Breath-taking perspectives and preliminary data toward early detection of chronic liver diseases. Biomedicines. 2021;9(11):1563.
- Morisco F, Aprea E, Lembo V, et al. Rapid "breath-print" of liver cirrhosis by proton transfer reaction time-of-flight mass spectrometry. A pilot study. PLoS One. 2013;8(4):e59658.
- Van den Velde S, Nevens F, Van Hee P, et al. GC-MS analysis of breath odor compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875(2):344–348.
- Gorowska-Kowolik K, Chobot A, Kwiecien J. 13C Methacetin Breath Test for assessment of microsomal liver function: methodology and clinical application . Gastroenterol Res Pract. 2017;2017:7397840.
- Stockmann M, Lock JF, Malinowski M, et al. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB. 2010;12(2):139–146.
- Stavropoulos G, van Munster K, Ferrandino G, et al. Liver impairment-the potential application of volatile organic compounds in hepatology. Metabolites. 2021;11(9):618.
- Rufo JC, Madureira J, Fernandes EO, et al. Volatile organic compounds in asthma diagnosis: a systematic review and meta-analysis. Allergy. 2016;71(2):175–188.
- Finamore P, Scarlata S, Incalzi RA. Breath analysis in respiratory diseases: state-of-the-art and future perspectives. Expert Rev Mol Diagn. 2019;19(1):47–61.
- Ratiu IA, Ligor T, Bocos-Bintintan V, et al. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma and COPD. J Clin Med. 2020;10(1):32.
- Yamada YI, Yamada G, Otsuka M, et al. Volatile organic compounds in exhaled breath of idiopathic pulmonary fibrosis for discrimination from healthy subjects. Lung. 2017;195(2):247–254.
- Weber R, Haas N, Baghdasaryan A, et al. Volatile organic compound breath signatures of children with cystic fibrosis by real-time SESI-HRMS. ERJ Open Res. 2020;6(1): 00171-2019.
- Neerincx AH, Geurts BP, van Loon J, et al. Detection of Staphylococcus aureus in cystic fibrosis patients using breath VOC profiles. J Breath Res. 2016;10(4):046014.
- Töreyin ZN, Ghosh M, Göksel Ö, et al. Exhaled breath analysis in diagnosis of malignant pleural mesothelioma: systematic review. Int J Environ Res Public Health. 2020;17(3):1110.
- Brinkman P, Zee AM, Wagener AH. Breathomics and treatable traits for chronic airway diseases. Curr Opin Pulm Med. 2019;25(1):94–100.
- Schleich F, Brusselle G, Louis R, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med. 2014;08(12):1723–1732.
- Barnes PJ. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249–1256.
- Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. 2019;54(2):1900651.
- Segal LN, Martinez FJ. Chronic obstructive pulmonary disease subpopulations and phenotyping. J Allergy Clin Immunol. 2018;141(6):1961–1971.
- Schleich FN, Manise M, Sele J, et al. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med. 2013;13:11.
- Smolinska A, Klaassen EMM, Dallinga JW, et al. Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children. PLoS One. 2014;9(4):e95668.
- Neerincx AH, Vijverberg SJH, Bos LDJ, et al. Breathomics from exhaled volatile organic compounds in pediatric asthma. Pediatr Pulmonol. 2017;52(12):1616–1627.
- van Vliet D, Smolinska A, Jöbsis Q, et al. Can exhaled volatile organic compounds predict asthma exacerbations in children? J Breath Res. 2017;11(1):016016.
- Robroeks CM, van Berkel JJ, Jöbsis Q, et al. Exhaled volatile organic compounds predict exacerbations of childhood asthma in a 1-year prospective study. Eur Respir J. 2013;42(1):98–106.
- Dryahina K, Sovová K, Nemec A, et al. Differentiation of pulmonary bacterial pathogens in cystic fibrosis by volatile metabolites emitted by their in vitro cultures: Pseudomonas aeruginosa, Staphylococcus aureus, Stenotrophomonas maltophilia and the Burkholderia cepacia complex. J Breath Res. 2016;10(3):037102.
- Kos R, Brinkman P, Neerincx AH, et al. Targeted exhaled breath analysis for detection of Pseudomonas aeruginosa in cystic fibrosis patients. J Cyst Fibros. 2021;21:e28–e34.
- Giovannini G, Haick H, Garoli D. Detecting COVID-19 from breath: a game changer for a big challenge. ACS Sens. 2021;6(4):1408–1417.
- Krilaviciute A, Heiss JA, Leja M, et al. Detection of cancer through exhaled breath: a systematic review. Oncotarget. 2015;6(36):38643–38657.
- Queralto N, Berliner AN, Goldsmith B, et al. Detecting cancer by breath volatile organic compound analysis: a review of array-based sensors. J Breath Res. 2014;8(2):027112.
- Sun X, Shao K, Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem. 2016;408(11):2759–2780.
- Jia Z, Patra A, Kutty VK, et al. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites. 2019;9(3):52.
- Di Lena M, Porcelli F, Altomare DF. Volatile organic compounds as new biomarkers for colorectal cancer: a review. Colorectal Dis. 2016;18(7):654–663.
- Hanna GB, Boshier PR, Markar SR, et al. Accuracy and methodologic challenges of volatile organic compound-based exhaled breath tests for cancer diagnosis: a systematic review and meta-analysis. JAMA Oncol. 2019;5(1):e182815.
- Sahota AS, Gowda R, Arasaradnam RP, et al. A simple breath test for tuberculosis using ion mobility: a pilot study. Tuberculosis . 2016;99:143–146.
- Phillips M, Basa-Dalay V, Blais J, et al. Point-of-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis. 2012;92(4):314–320.
- Koo S, Thomas HR, Daniels SD, et al. A breath fungal secondary metabolite signature to diagnose invasive Aspergillosis. Clin Infect Dis. 2014;59(12):1733–1740.
- Chambers ST, Bhandari S, Scott-Thomas A, et al. Novel diagnostics: progress toward a breath test for invasive Aspergillus fumigatus. Med Mycol. 2011;49(Suppl 1):S54–S61.
- Teny John LHK, Pappan O, Birch, et al. Volatile compound analysis of breath from patients with Clostridium difficile infection for biomarker discovery and biological insight. Poster. Breath Biopsy Conference. Breath Biopsy Community; 2020. Available from: https://www.owlstonemedical.com/media/uploads/files/Final_-_CC1_poster.pdf
- Berna AZ, McCarthy JS, Wang RX, et al. Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J Infect Dis. 2015;212(7):1120–1128.
- Berna AZ, McCarthy JS, Wang XR, et al. Diurnal variation in expired breath volatiles in malaria-infected and healthy volunteers. J Breath Res. 2018;12(4):046014.
- Schaber CL, Katta N, Bollinger LB, et al. Breathprinting reveals malaria-associated biomarkers and mosquito attractants. J Infect Dis. 2018;217(10):1553–1560.
- Brekelmans MP, Fens N, Brinkman P, et al. Smelling the diagnosis: the electronic nose as diagnostic tool in inflammatory arthritis. A case-reference study. PLoS One. 2016;11(3):e0151715.
- Das S, Pal S, Mitra M. Significance of exhaled breath test in clinical diagnosis: a special focus on the detection of diabetes mellitus. J Med Biol Eng. 2016;36(5):605–624.
- Tiele A, Wicaksono A, Daulton E, et al. Breath-based non-invasive diagnosis of Alzheimer's disease: a pilot study. J Breath Res. 2020;14(2):026003.
- Tisch U, Schlesinger I, Ionescu R, et al. Detection of Alzheimer's and Parkinson's disease from exhaled breath using nanomaterial-based sensors. Nanomedicine. 2013;8(1):43–56.
- Broza YY, Har-Shai L, Jeries R, et al. Exhaled breath markers for nonimaging and noninvasive measures for detection of multiple sclerosis. ACS Chem Neurosci. 2017;8(11):2402–2413.
- Ratcliffe N, Wieczorek T, Drabińska N, et al. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: an aid to understanding the origins of volatile organic compounds from the human body. J Breath Res. 2020;14(3):034001.
- McKay LF, Holbrook WP, Eastwood MA. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol Microbiol Immunol Scand B. 1982;90(3):257–260.
- Beauchamp JD, Pleil JD. Simply breath-taking? Developing a strategy for consistent breath sampling. J Breath Res. 2013;7(4):042001.
- Henderson B, Ruszkiewicz DM, Wilkinson M, et al. A benchmarking protocol for breath analysis: the peppermint experiment. J Breath Res. 2020;14(4):046008.
- Gibbs RA, Belmont JW, Hardenbol P, et al. The international HapMap project. Nature. 2003;426(6968):789–796.
- Harshman SW, Pitsch RL, Davidson CN, et al. Evaluation of a standardized collection device for exhaled breath sampling onto thermal desorption tubes. J Breath Res. 2020;14(3):036004.
- Farraia MV, Cavaleiro Rufo J, Paciência I, et al. The electronic nose technology in clinical diagnosis: a systematic review. Porto Biomed J. 2019;4(4):e42.
- Shrestha A, Prodhan UK, Mitchell SM, et al. Validity of a portable breath analyser (AIRE) for the assessment of lactose malabsorption. Nutrients. 2019;11(7):1636.