Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen responsible for the coronavirus disease 2019 (COVID-19) outbreaks that resulted in a catastrophic threat to global health, with more than 500 million cases detected and 5.5 million deaths worldwide. Patients with a COVID-19 infection presented with clinical manifestations ranging from asymptomatic to severe symptoms, resulting in acute lung injury, acute respiratory distress syndrome, and even death. Immune dysregulation through delayed innate immune response or impairment of the adaptive immune response is the key contributor to the pathophysiology of COVID-19 and SARS-CoV-2-induced cytokine storm. Symptomatic and supportive therapy is the fundamental strategy in treating COVID-19 infection, including antivirals, steroid-based therapies, and cell-based immunotherapies. Various studies reported substantial effects of immune-based therapies for patients with COVID-19 to modulate the over-activated immune system while simultaneously refining the body’s ability to destroy the virus. However, challenges may arise from the complexity of the disease through the genetic variance of the virus itself and patient heterogeneity, causing increased transmissibility and heightened immune system evasion that rapidly change the intervention and prevention measures for SARS-CoV-2. Cell-based therapy, utilizing stem cells, dendritic cells, natural killer cells, and T cells, among others, are being extensively explored as other potential immunological approaches for preventing and treating SARS-CoV-2-affected patients the similar process was effectively proven in SARS-CoV-1 and MERS-CoV infections. This review provides detailed insights into the innate and adaptive immune response-mediated cell-based immunotherapies in COVID-19 patients. The immune response linking towards engineered autologous or allogenic immune cells for either treatment or preventive therapies is subsequently highlighted in an individual study or in combination with several existing treatments. Up-to-date data on completed and ongoing clinical trials of cell-based agents for preventing or treating COVID-19 are also outlined to provide a guide that can help in treatment decisions and future trials.
1. Introduction
Coronavirus disease 2019 (COVID-19) is the official name for the coronavirus pneumonia 2019 caused by severe acute respiratory syndrome coronavirus (SARS-CoV)-2, as designated by the World Health Organization. Since the first COVID-19 patient was detected in Wuhan, China, in December 2019, more than 630 million confirmed cases and more than 6.5 million deaths had been reported worldwide [Citation1]. Coronaviruses are a large family of viruses categorized into α-genera, β-genera, γ-genera, and δ-genera CoVs. β-genera CoVs are enveloped, positive-sense, single-stranded RNA viruses surrounded by an envelope studded with spike-like glycoproteins, affording the virus the appearance of a crown, thus the name, coronavirus. The viral genomes comprise several open reading frames (ORF) encoding the primary viral proteins, including spike (S) protein, membrane (M) protein, nucleocapsid (N) protein, and envelope (E) protein [Citation2]. Severe acute respiratory syndrome-mediated virus (SARS-CoV), SARS-CoV-2, and Middle East respiratory syndrome-related coronavirus (MERS-CoV) are three human β-genera coronaviruses affecting the lower respiratory tract cells [Citation3]. Compared to the 2002–2003 SARS-CoV outbreak and MERS-CoV, SARS-CoV-2 has a longer incubation time and is more contagious, but the MERS-CoV-associated mortality rate is higher than SARS-CoV and SARS-CoV-2 [Citation4]. The SARS-CoV-2 genome is more homologous to the SARS-CoV genome than the MERS-CoV genome, with approximately 80% and 50% sequence identity similarity, respectively [Citation5]. The close genetic proximity saved scientists a lot of effort and time in deciphering the molecular structure of SARS-CoV-2 and interpreting the pathogenesis of COVID-19 infection.
The amassing knowledge on the sequential identity of the SARS-CoV-2 viral genome, the viral protein components, SARS-CoV-2-mediated host cells infectivity, and the mode of replication have paved the way for scientists to develop therapies and vaccines that target SARS-CoV-2 at unprecedented speed. Eleven vaccines targeting SARS-CoV-2 have been approved, and more than 12 billion COVID-19 vaccines have been distributed worldwide, with more than 60% of the world population being fully vaccinated [Citation6]. After more than two years of the COVID-19 pandemic, extensive human-to-human transmission resulted in various genomic mutations and new SARS-CoV-2 variants. The mutated SARS-CoV-2 strains show relative diversity in their transmissibility, infectivity, and ability to escape the host’s acquired immunity. The most recent emergent SARS-CoV-2 variant, Omicron, resulted in the fourth wave of the global pandemic due to the high level of alteration in its genetics, which is responsible for its high transmission and infectivity rate among the human population [Citation7]. With the number of infected patients and deaths drastically mounting, researchers rush to examine the functions and responses of neutralizing antibodies in previously infected COVID-19 patients or fully vaccinated individuals, especially since breakthrough infections following vaccination are being reported worldwide. High infectivity and fatality associated with SARS-CoV-2, lengthy research and development cycle, and high production and approval costs for novel therapeutic agents have shifted the research pathways toward the “repurposing of drugs” approach in drug discovery. This strategy is being implemented to clinically investigate FDA-approved interventional therapies based on their comprehensive scientific data, safety, and outcome in treating MERS-CoV and SARS-CoV [Citation8].
Cell-based therapy has pioneered a new era in regenerative medicine, in which natural physiological processes are employed to treat the patient. Currently, several types of cell-based therapies are undergoing clinical trials for the treatment of COVID-19 infection, such as stem cells (including mesenchymal stem cells (MSCs)), dendritic cells (DCs), natural killer (NK) cells, and T lymphocytes (i.e. T cells) [Citation9]. More than 100 clinical trials are in progress, exploring novel approaches and potential interventional therapies to challenge the viral pandemic. This review provides insights into the innate and adaptive immune responses that mediate various immune cell therapies in SARS-CoV-2-infected patients with mild to moderate or severe and critically ill cases. The development of autologous or allogeneic immune cells for preventative or therapeutic modalities is also highlighted. Updates on the enrollment of these treatment modalities in various clinical trial phases for COVID-19 treatment or as preventive vaccines are also outlined.
2. COVID-19 pathogenesis
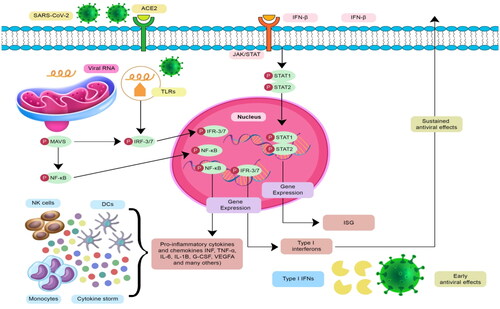
An infection of the respiratory system by SARS-CoV-2 is initiated by the association of the viral S glycoproteins with the angiotensin-converting enzyme 2 (ACE-2) receptors on the surface of the alveolar epithelial cells [Citation3]. These spike projections comprise two subunits, namely S1 and S2. The S1 subunit, with its outer receptor-binding domain (RBD), attaches to the ACE-2 receptors. To allow for cellular viral entrance, transmembrane serine protease 2 (TMPRSS2) is required for proteolytic cleavage of the S1 protein [Citation10,Citation11]. Once the ACE-2 and S1 proteins have been cleaved by TMPRSS2, the S2 domain fuses with the epithelia targeting SARS-CoV-2 to facilitate viral entry via endocytosis. The binding affinity of the S1 protein expressed by SARS-CoV-2 toward the ACE-2 receptors is 10 to 20 times higher than SARS-CoV, which explains the extreme contagiousness of COVID-19 [Citation12]. Human protein atlas and single-cell RNA sequencing data revealed that ACE-2 and TMPRSS2 are extensively co-expressed in alveolar type II cells in the lungs and the nasal, bronchial, and intestinal epithelial cells, providing a reasonable explanation for the predominant lung susceptibility to SARS-CoV-2 infection. It was reported that interferons (IFNs) play a regulatory role in ACE-2 gene expression [Citation13]. However, the role of IFN families (IFN-I and IFN-III members, IFN-λ1, INF-λ2, and IFN-λ3) in SARS-CoV-2 infection is still questioned. It has been noticed that SARS-CoV-2-infected patients presenting with high levels of IFNs (mainly IFN-λ1 and to some extent IFN-λ3, but not IFN-λ2 and IFN-I) in the upper airways are characterized by mild infection. IFN-λ1 production induces a significant level of interferon-stimulated genes (ISGs) that protect against the progression of COVID-19 and its complications. On the contrary, excessive production of IFN-λ2 and IFN- λ3 by DCs in the lungs compromises lung epithelial cell function, induces lung damage, and predisposes patients with severe COVID-19 to disastrous secondary bacterial infection. It was demonstrated that INF-λ2 and IFN-λ3 induce genes associated with lung epithelial cell apoptosis and decreased proliferation [Citation14,Citation15]. Additionally, several pro-inflammatory cytokines and chemokines are overexpressed in SARS-CoV-2 infection, including interleukin (IL-6, IL-1β, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), chemokine C-X-C motif ligand (CXCL)-1, CXCL-5, and CXCL-10/IFN-γ inducible protein (IP)-10, which contribute to the recruitment of adaptive immune cells into the lungs [Citation13].
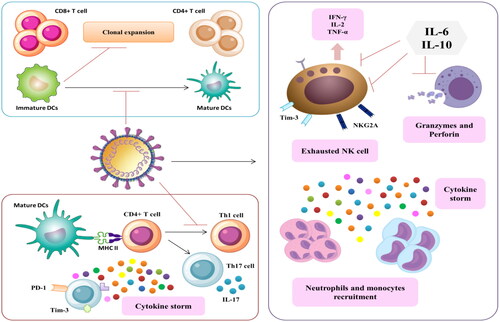
SARS-CoV-2-infected epithelial cells instigate an instantaneous immune response where viral proteins are recognized by the pattern recognition receptors (PRRs) expressed by epithelial cells and macrophages [Citation16]. When the PRRs are attached to viral pathogen-associated molecular patterns (PAMPs), they promote the activation of downstream transcription factor expression, including interferon regulatory factor (IRF), nuclear factor-kappa factor (NF-κB), and activator protein (AP)-1, followed by an overproduction of the type-I and type-III antiviral INF, IL-6, TNF-α, and other chemokines [Citation17]. The chemokines will recruit other immune response cells such as monocytes (Mo), NK cells, and DCs that produce chemokines like macrophage inflammatory protein (MIP)1A, MIP-1B, and MCP-1 to attract more lymphocytes to the infection site () [Citation18]. The ability of SARS-CoV-2 to evade the host immune response and delay SARS-CoV-2-induced innate response induces hypersecretion of the pro-inflammatory cytokines and chemokines that results in lung inflammation, a phenomenon known as cytokine storm. A substantial inflammatory response and compromised adaptive immune response trigger lung damage and induce macrophage recruitment and subsequent adaptive T cell and B lymphocyte (i.e. B cell) priming [Citation19].
Figure 1. The underlying immuno-regulatory mechanisms accompanied by T cell-dependent protective response or chemokine-mediated inflammatory response.
The clinical manifestations of COVID-19 patients range from asymptomatic to severe respiratory symptoms requiring mechanical ventilation. Generally, most COVID-19 patients experience coughing, high fever, chest tightness, and shortness of breath. Elderly patients and those with comorbidities may develop more severe symptoms that require hospitalization or admission to the intensive care unit [Citation4,Citation8]. To this date, antivirals such as remdesivir (Veklury), molnupiravir (Lagevrio), and nirmatrelvir with ritonavir (Paxlovid) are the only FDA-authorized treatment for COVID-19, alongside monoclonal antibodies such as bebtelovimab. Other therapeutic options, including anti-SARS-CoV-2 monoclonal antibodies and immunomodulators, are available under Food and Drug Administration (FDA)-issued Emergency Use Authorization to manage COVID-19 [Citation20].
A respiratory compromise mediated by SARS-CoV-2 is primarily caused by cytokine storm rather than the virus itself. Therefore, immunological therapies such as bamlanivimab/etesevimab or caseirivimab/imdevimab that attenuate cytokine storm are the treatment of choice for treating patients with severe COVID-19 and acute respiratory distress syndrome (ARDS). Conventional immunomodulating agents such as corticosteroids, IL-6 inhibitors, and Janus kinase (JAK) inhibitors, targeting one or two molecules, did not produce a robust immune response against SARS-CoV-2 [Citation21]. Recombinant INF-III is another promising interventional therapy for treating COVID-19; however, disease severity as well as the timing and duration of IFN-III administration should be carefully considered to avoid compromised lung epithelial barrier [Citation22,Citation23]. As major COVID-19 complications are ARDS, coagulation disorders, septic shock, metabolic acidosis, and multiple organ damage, symptomatic treatment of the SARS-CoV-2-established disease and its accompanied secondary infections is essential for preventing and treating the severe COVID-19 complications [Citation8].
3. Immune cell response in SARS-CoV-2 infection
3.1. Dendritic cell response
DCs are the most potent antigen-presenting cells (APCs) responsible for various pathogen-induced innate responses and adaptive immunity stimulation. DCs act as both respiratory sentinels and targets for SARS-CoV-2. ACE-2 receptors and CD147 demonstrate SARS-CoV-2-induced interstitial lung DC infection on their surfaces [Citation24]. DCs are the only immune components that cause antigen-specific naive CD8+ T cell activation. Type I and type II conventional DCs (cDCs), CD141+ and CD1c+, and plasmacytoid DC (pDCs) are the principal DC subtypes in the lungs [Citation25]. CD141+ are distributed in the mucosa and the vascular walls, whereas CD1c + are present in the lamina propria. pDCs are distributed in the lung’s parenchyma, airways, and alveolar septa, and they are the primary source of type I IFN involved in the viral response [Citation24,Citation25]. pDCs initiate an innate immune response and stimulate CD8+ T cell response to activate the production of type I and type III IFNs [Citation26]. IL-12 producing CD141+ cells are responsible for CD8+ T cell activation and subsequent stimulation of type I T helper (Th) cell response [Citation27]. Other factors include dendritic cell-specific intracellular adhesion molecule-grabbing nonintegrin (DC-SIGN) and its related protein, liver/lymph node-specific intracellular adhesion molecule-grabbing nonintegrin (L-SIGN), that signifies the infection in ACE-2 expressing DCs [Citation28]. Overexpression of furin enzyme and DC-SIGN displayed by SARS-CoV-2 infected monocyte-derived DCs (Mo-DCs) is another host factor contributing to SARS-CoV-2 acquisition and replication [Citation29].
The antiviral effect of DC is mediated by its action as APC and the secretion of IL-6 and IFN. Once the TLRs and C-type lectin receptors (CLRs) at the DC surface are activated, its downstream pathways, myeloid differentiation primary response 88, and TIR-domain-containing adapter-inducing interferon-β, are activated, leading to heightened expression of IFN regulatory factors (IRF-3, IRF-5, and IRF-7) and NF-κB, producing type I interferons (IFN-α and IFN-β) and pro-inflammatory cytokines such as IL-12 and TNF-α [Citation30]. The delayed IFN production or inability of DCs to produce IFN will result in uncontrolled viral replication and subsequent parenchyma and epithelial cell death, leading to hyper-innate inflammation in the lungs and respiratory compromise seen in severe COVID-19 infection [Citation31].
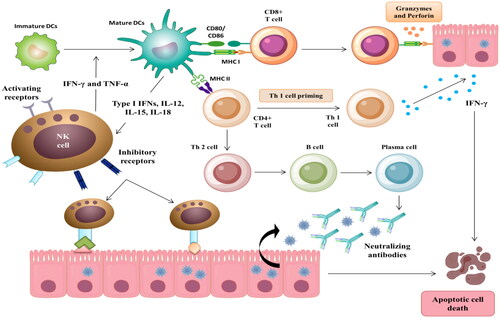
Interestingly, virus engulfment by DCs will not infect the DC itself [Citation31]. Instead, immature DCs internalize the viruses and process the newly endogenous synthesized peptides. The exogenous antigens from virus lysis will interact with major histocompatibility complex (MHC) class I and MHC class II molecules. The resultant complexes are transported to the DC surface for presentation [Citation32]. Mature DCs migrate to the lymph nodes, where DC-mediated pro-inflammatory cytokines, IL-12, IL-1β, IFN-α, IFN-β, IFN-γ, IL-4, IL-10, and TNF-α, are released. Also, costimulatory molecules (CD80 and CD86), T cell adhesive molecules (CD48 and CD58), and chemokine receptor (CCR7) are overexpressed, and the intracellular MHC II is relocated to the plasma membrane presenting DC phenotyping and maturation [Citation25]. In the lymph nodes, the CD8+ T and CD4+ T cells recognize MHC class I- and MHC class II-antigen complex causing clonal expansion of CD8+ T cells. The initial activation of CD8+ T cells and CD4+ T cells is followed by antibody production by B cells () [Citation33].
Figure 2. The crosstalk between NK cells, T cells, and DCs induces the innate and adaptive response in SARS-CoV-2-infected patients with mild to moderate symptoms.
In a normal situation, mature DCs and subsequent T cell activation are characterized by DC surface presentation of MHC, co-stimulatory and co-inhibitory molecules, and high cytokine production. Downregulation of MHC-I, MHC-II, and CD80/86 expression in COVID-19 infection reduces circulating mature cDC and pDC count and functionality. A high ratio of cDC/pDC indicates a significant decline in pDC levels and a subsequent decrease in IFN production, explaining the delayed SARS-CoV-2-induced innate responses [Citation34]. The defective DCs cannot efficiently activate allogenic and adaptative T-cell responses and induce NK-cell-mediated DC apoptosis () [Citation35]. Moreover, some virulent proteins expressed on the surface of SARS-CoV-2 could antagonize the effect of the DC-producing IFN and cytokines by suppressing the signal transducer and activator of transcription (STAT) and its downstream IFN signaling pathway activation, which in turn impairs the progression of the innate response to the adaptive response [Citation34]. These clinical manifestations are also responsible for the delayed deterioration in COVID-19 patients.
3.2. NK cell response
Natural killer (NK) cells, also known as large granulocyte lymphocytes, are the frontline cellular component modulating the innate immune response following a viral infection without needing any pre-stimulation with antibodies or MHC. Notably, NK cells constitute 10% of the total body lymphocyte reservoir. Unlike T cells and B cells, NK cells are uncharacterized cytotoxic lymphocytes that lack antigen specificity [Citation36].
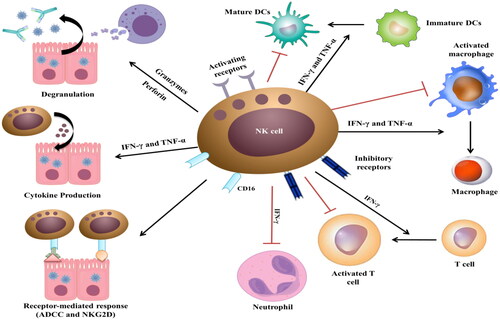
NK cells play a pivotal immuno-regulatory role against viral infections and cancer by bridging the innate immune response and the downstream adaptive immune response [Citation36]. Healthy cell-expressed MHC I serves as a ligand for the inhibitory receptors, killer Ig-like receptors (KIRs) as well as CD94/natural killer group 2D (NKG2D) on the NK cell surface, protecting these cells from the NK cell-induced cell death. Cancer and viral infections are associated with MHC I downregulation and/or KIR impairment, resulting in the upregulation of NK activating receptors, and subsequently, NK cell activation [Citation37]. Activated NK cells induce an antiviral response through a direct cytolytic effect against virus-infected cells and their ability to produce pro-inflammatory chemokines [Citation38]. NK cells attack the virus-infected cells and release perforin and granzyme B, causing the target cells to degranulate and thus exposing the viruses to adaptive antibodies. The receptor-mediated killing effect is induced by the association of CD16-expressing NK cells with the surface antibody-bound infected cells, leading to antibody-dependent cell cytotoxicity (ADCC) [Citation39]. Besides their essential role in evading viral infections, type I IFNs are the most important cytokines involved in NK cell-mediated immuno-regulatory responses. IFN-γ-producing NK cells stimulate immature Th cells (i.e. Th0) to differentiate into Th1 inflammatory cells and enhance DC maturation, followed by the activation of CD8+ T cell response [Citation40]. NK cells induce the immunomodulating effect of the adaptive response through their cytotoxic killing effect against the activated T cells. As the expression of the activating receptor NKG2D is upregulated and the manifestation of MHC I ligands is downregulated by the activated T cells, NK cells can easily recognize and lyse the activated T cells () [Citation37,Citation40].
One of the characteristics of SARS-CoV-2-mediated severe respiratory complications is the marked decrease in CD8+ lymphocytes and NK cells. It is reported that NK cell count decreases in SARS-CoV-2-infected patients and returns to normal after recovery [Citation41]. In some cases, functionally exhausted NK cells are produced in patients with COVID-19, causing mononuclear cell recruitment and the hypersecretion of cytokines and chemokines, leading to cytokine storm and ARDS [Citation41–44]. In severe cases, the systemic CD8+ and CD4+ T cells and NK cells reduced, while neutrophil level increased, resulting in lymphopenia, neutrophilia, and higher neutrophil lymphocyte ratio (NLR). NK cells induce cytokine-mediated lung injury either by producing chemokines, MCP-1 and IP-10, that recruits NK cells to the inflamed site, or through the abnormal effector functions of the inflammatory NK cell phenotypes that worsen lung inflammation [Citation45]. Finally, IL-10 and IL-6 decrease the cytotoxicity of NK cells by decreasing the perforin and granzyme B expression as well as reducing IFN-γ and IL-2 expression (). IFN-γ and TNF-α generated by the NK cells exert a cytolytic effect, and this effect is negatively correlated with low NK cells as well as IFN-γ and TNF-α production [Citation46]. The impacts of dampened immun0-regulatory and cytotoxicity would result in hyper-inflammation, as seen in the COVID-19 infected patients.
3.3. T cell response
T lymphocytes (i.e. T cells) are the arsenals of the innate and adaptive immune response. Viral dissemination and elimination are tightly controlled by synchronizing the natural and adaptive immune response based on adequate T cell count, satisfactory activation, and proper functionality [Citation47]. The antiviral activity of T cells involves the elimination of infected cells mediated by cytotoxic T lymphocytes (CTLs) and the activation of B cells induced by Th cells. Early CD8+ and CD4+ mediate protective effects in COVID-19 patients with mild symptoms [Citation48]. Generally, SARS-CoV-2 can evade the innate immune response and hinder T cells from producing type I and III IFN. Unsatisfactory or delayed innate immune response leads to exacerbation of the inflammatory response. Indeed, evidence has revealed that the T cell response in COVID-19 patients could be suboptimal, highly activated, or functionally exhausted, resulting in the debilitating immune response against SARS-CoV-2 infection [Citation41,Citation42]. Persistent SARS-CoV-2 stimulation contributes to the overexpression of T cell-mediated inhibitory checkpoints as well as the downregulation of cytokines and cytolytic molecule-encoded gene expression, resulting in the loss and exhaustion of T cell functionality [Citation49]. Upon activation, T cells express several inhibitory immune checkpoints, including programmed cell death protein 1 (PD-1), T-cell immunoglobulin and mucin domain containing (TIM)-3, cytotoxic T lymphocyte-associated protein (CTLA)-4, and T cell immunoreceptor with Ig and ITIM domains (TIGIT). Zheng et al. demonstrated that SARS-CoV-2-infected patients with severe symptoms displayed higher CD4+ T cells expressing low levels of IFN-γ, IL-2, and TNF-α, as well as increased CD8+ cells expressing PD-1, CTLA-4, TIGIT, granzyme A, and perforin [Citation50].
Highly activated and fully functional CD4+ and CD8+ T cells are usually pronounced within two weeks of the onset of symptoms in SARS-CoV-2-infected patients. One study revealed that COVID-19 patients exhibited main memory SARS-CoV-2-targeted CD4+ phenotypes, producing Th1 cytokines, and SARS-CoV-2-specific CD8+, expressing high levels of perforin and demonstrating effector effects [Citation51]. In another study, CD38- and human leukocyte antigen (HLA)-DR-expressing memory CD4+ and CD8+ T cells, CD4+ PD-1+ memory T cells, highly proliferative Ki76+, and non-naïve CD4+ and CD8+ T cells were found to be more pronounced in patients with severe infection [Citation52]. Excessive T cell activation results in either compromised or exaggerated T cell response. Compared to healthy individuals, dysregulation of T cell subsets was also apparent in SARS-CoV-2-infected patients. COVID-19 patients exhibited lower levels of naïve and central memory CD8+ T cells, comparable levels of the naïve, central memory, and effector CD4+ T cells, and higher levels of the terminally differentiated effector CD4+ and CD8+ T cells [Citation47]. The regulatory T cell (Treg) phenotypes are more pronounced in mild COVID-19 cases.
Lymphopenia is a hallmark biomarker that can provide insights into the severity and complications of COVID-19 infection. In non-surviving SARS-CoV-2-infected patients, lung autopsy revealed excessive Mo and T cell infiltration of the pulmonary interstitial, while macrophages and neutrophils infiltrated the alveolar lamina [Citation53]. Imbalances in immune cell sequestration and infiltration in the lungs elucidate the systemic lymphopenia and the increase in the neutrophil-to-lymphocyte ratio in non-surviving patients or surviving SARS-CoV-2-infected patients with severe symptoms [Citation54]. Another study reported that the influx of macrophages and neutrophils into the bronchoalveolar lavage fluid (BALF) was excessively higher than CD8+ T cells and DCs in patients with severe COVID-19. BALF-infiltrated CD8+ T cells in patients with severe symptoms exhibited a highly proliferative but less clonally expanded phenotype, suggesting that the SARS-CoV-2-induced T cell response was compromised [Citation47].
Additionally, the interplay between epithelial cells and infiltrated immune cells in a pro-inflammatory milieu is potentially the driving force of epithelial cell and alveolar damage in severe SARS-CoV-2 infection. Evidence also hints that the bronchial cells of COVID-19 patients exhibit higher expression of ACE-2 receptors, which is positively correlated to INF-γ expression and upregulation of C-C motif chemokine ligand (CCL)2, CCL3, CCL20, CXCL10, IL-8, and IL-1B gene expression, resulting in T cell recruitment [Citation54]. Collectively, the massive influx of immune cells into the lungs, the activation of CD8+, Th1, and NK cells, as well as the signature cytokine storm are among the factors that lead to the exacerbation of the inflammatory response, ARDS, and other disastrous consequences [Citation52].
3.4. Stem cell response
Mesenchymal stem cells (MSCs) are multipotent cells with the regenerative potential to repair damaged tissues. The characteristics of their immunomodulatory effects arise from their ability to secrete a wide variety of cytokines and growth factors [Citation55]. Reduced expression of MHC I and MHC II indicates that MSCs are non-immunogenic cells. The MSC-mediated inhibition of T cells, including NK cells and CD8+ T cells, and MSC-producing fibrinolytic factors are responsible for repairing damaged alveolar cells and preventing pulmonary fibrosis [Citation56]. The immunomodulatory properties of MSCs are temporary, as they disappear within six months to one year. However, the immunomodulatory effects and tissue regenerative activities have made MSCs an ideal cell-based therapy agent for treating incurable medical conditions, including SARS-CoV-2. Since MSCs are non-ACE-2- and/or TMPRSS2-expressing cells, they cannot be infected with SARS-CoV-2 [Citation57]. They also do not produce IFN and are refractory to extrinsic IFN. Further, persistent transcription of ISGs in an IFN-independent manner indicates that MSCs evade SARS-CoV-2 through ISG-expressed proteins [Citation58].
MSC treatment controls the cytokine storm and thus modulates immune response without affecting the patient’s ability to fight infection. It was reported that exosome- and cytokine-enriched miR-455-3p contribute to the MSC-mediated inhibitory effect of Mo differentiation, inhibiting IL-6 production, thus relieving the cytokine storm [Citation59]. Low MHC I expression at the surface of MSCs and their ability to produce anti-inflammatory cytokines mediates the MSCs’ immunomodulatory effect. COVID-19-associated hyperimmune responses activate and induce MSCs to release anti-inflammatory-related factors, including IL-10, transforming growth factor (TGF)-β1, prostaglandin E2 (PGE2), hepatocyte growth factor (HGF), indoleamine-pyrrole 2,3-dioxygenase (IDO), and nitric oxide (NO) [Citation45]. Besides anti-inflammatory capabilities, MSCs also antagonize the secretion of pro-inflammatory cytokines. Evidence revealed that elevated IL-10 and reduced TNF-α activity increase the ratio of regulatory to inflammatory cytokines following MSC treatment in a rodents model of acute lung injury [Citation60]. Additionally, MSCs produce numerous inflammatory cytokines, mainly IL-6, that act as anti- and pro-inflammatory mediators depending on the activated signaling pathway of the targeted cells. The activation of the IL-6-mediated classical signaling pathway results in an anti-inflammatory response and promotes regeneration of alveolar cells, while the IL-6-induced trans-signaling pathway is associated with a pro-inflammatory response [Citation61].
MSC-induced immuno-regulatory effects balance the innate and adaptive immune response. MSC treatment modulates the proliferation and functionality of immune cells (T cells, B cells, macrophages, and DCs) (). Specifically, MSCs affect T cell function either through inflammatory stimulation or cytokine release. MSC-induced cytokine-related growth factors such as TGF-β and HGF exert an inhibitory effect on proliferation of activated T cells and modulate T cell phenotyping [Citation62]. Additionally, MSCs regulate B cell proliferation, differentiation, and antibody-producing abilities. The ability of these cells to block G0/G1 phase transition is associated with decreasing B cell proliferation. MSC-secreted Epstein-Barr virus-induced gene 3 (EBI3) increases the frequency of the regulatory B cells expressing IL-10 [Citation63]. One study revealed that MSC treatment inhibits the differentiation of Mo into DCs. However, MSC-secreted HGF induces DC maturation into the jagged-2 dependent regulatory DCs [Citation64]. The resultant regulatory DCs are beneficial immune cells due to their immunosuppressive effect and role in restoring immune homeostasis [Citation65]. It has been reported that MSC-treated COVID-19 patients exhibited markedly increased regulatory DCs [Citation57].
Figure 5. The idiosyncratic immune-modulatory effect is associated with the paracrine factors produced by MSC-mediated therapy.
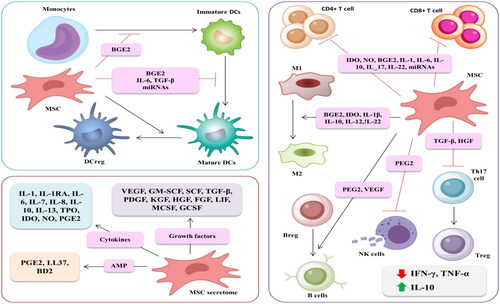
The distinguishing characteristic of MSC treatment is its ability and role in tissue repair. MSCs secrete regenerative mediators, including epidermal growth factor (EGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (FGF), HGF, and vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and keratinocyte growth factor (KGF). MSC-secreted KGF increases the expression of the α1 subunit of ACE-2 and promotes the sodium-potassium ATP enzyme in alveolar cells, thus enhancing alveolar fluid clearance to resolve pulmonary edema, protect the alveolar epithelial cells, and prevent pulmonary fibrosis and associated acute injuries [Citation64]. Yang et al. reported that alveolar regeneration mediated by MSC therapy is VEGF-dependent. Therefore, inhibition of VEGF expression abrogates MSCs’ constructive effects [Citation66]. Moreover, it was reported that the protective effect of MSCs against virus-induced lung fibrosis and epithelial cell apoptosis is HGF-dependent [Citation67].
MSCs exert either a direct or indirect antiviral effect against SARS-CoV-2. The mechanism for MSC-induced direct anti-SARS-CoV-2 activity is IFN-independent. MSCs produce numerous factors, including antimicrobial peptides (AMPs), IDO, IL-17, and many others, leading to the upregulation of many antiviral genes, such as interferon-induced transmembrane proteins (IFITM), an ISG component, and subsequently increasing the expression of viral protein structures associated with the invading-ability of the viruses [Citation31]. The antibacterial peptides LL-37 (Cathelicidin LL-37), human beta-defensin 2 (BD2), hepcidin, and lipocalin-2 are examples of MSC-secreted AMPs. It has been revealed that these AMPs could kill the SARS-CoV-2-infected cells directly or through interference with the synthesis of DNA, RNA, and other viral proteins in the infected cells. The indirect antiviral effect is based on the ability of MSCs to enhance phagocytic activities and modulate the pro-inflammatory- and anti-inflammatory-producing immune cells [Citation68]. Despite mounting evidence documenting the antimicrobial effect of MSCs, Schwartz et al. revealed a contradictory response, whereby naive MSCs administration was found to enhance mycobacterial growth in Bacille Calmette-Guerin (BCG)-infected mice compared to conditioned MSCs. Therefore, MSCs are effective antibacterial agents only if they are appropriately pre-conditioned [Citation69].
Recently, a critical macromolecule, leukemia inhibitory factor (LIF), was demonstrated to lessen the lung cytokine storm. LIF is a stem cell growth factor that targets the IL-6 pathway, thus regulating the inflammatory response and repairing damaged alveoli and blood capillaries [Citation70]. LIF was successfully used as an interventional therapy for treating multiple sclerosis and neurodegenerative diseases [Citation71]. By controlling the COVID-19-induced cytokine storm, MSC-derived LIF can be used not only for treating COVID-19 infection but possibly to prevent its progression.
4. Cell-based therapies and vaccine development in patients with COVID-19
Single-cell RNA sequencing showed that both ACE-2 and TMPRSS2 are not expressed in immune cells such as T cells, B cells, and macrophages, so these cells are unlikely to be infected with SARS-CoV-2. These results indicated that patients infected with SARS-CoV-2 could be treated with immunoglobulins and immune cell transplantation. Clinical trials that employ several immune cells for treating COVID-19 patients are underway (). Moreover, identifying the SARS-CoV-2 genome sequence and proteome empowered scientists to engineer vaccines targeting SARS-CoV-2 using viral RNA or viral protein epitopes as antigens. Immunization using immune cells, mainly DCs, is still under investigation. The ongoing clinical trials may take longer to confirm the safety and efficacy of such vaccines.
Table 1. Examples of ongoing interventional clinical trials registered at ClinicalTrials.gov employ cellular-based therapy to treat and prevent COVID-19 infection.
4.1. Sources of immune cells
4.1.1. Sources of DC subsets
Autologous or allogeneic DCs could be used in DC-based immunotherapy. CD14+ Mo isolated from the patient’s own peripheral blood mononuclear cells (PBMCs) is the primary source of autologous Mo-DCs. Despite being easy to obtain, isolate, and differentiate, the quality of PBMC-derived Mo in patients infected with SARS-CoV-2 should be carefully considered. Inflammatory cytokine hypersecretion mediated by SARS-CoV-2 decreases Mo count, with impaired phenotypes and functionality [Citation72]. According to several case reports, classical and non-classical Mo were significantly depleted in severe COVID-19 patients and patients with ARDS [Citation73].
Moreover, IFN signaling and ISG-mediated Mo activation were downregulated in patients with severe COVID-19 [Citation43]. SARS-CoV-2-infected Mo induces immune microenvironment remodeling and promotes the differentiation of infected Mo into macrophages instead of DCs [Citation74]. These drawbacks restricted the applicability of autologous Mo-derived DCs in vaccine development to prevent or treat COVID-19. Bone marrow (BM)-derived MSCs (BM-MSCs) are the ideal source of different cell-based therapies in inflammatory conditions due to their immunosuppressive effect. Autologous BM-MSC extraction is time-consuming, and the prompt administration of autologous BM-MSCs in acute infectious diseases such as COVID-19 is complex. As a result, the readily available and immediately administered allogeneic BM-MSCs are widely used instead [Citation75]. Human embryonic stem cell (hESC)-derived DCs were used by Lineage Cell Therapeutics Inc. to develop an allogenic DC-based vaccine (https://lineagecell.com/) [Citation76]. The rare hematopoietic stem cell (HSC)-derived CD34+ in the blood and BM act as DC precursors [Citation77]. Umbilical cord blood (UCB) is another preferred source of HSC-derived DCs due to its availability and mild graft versus host disease (GvHD) [Citation78]. UCB-derived HSC-differentiated DCs induce allogeneic T cell response [Citation79]. Finally, naturally occurring DCs unexposed to cytokines may also be used to develop DC-based vaccines. The systemic DC populations may be significantly increased by Flt3L administration before the naturally occurring DCs are isolated [Citation80].
4.1.2. Sources of NK cells
NK cells can be derived from various sources for clinical use as allogenic or autologous products. PBMCs are the primary source of NK cells [Citation37]. Unfortunately, the number of PBMC-derived NK cells recovered from the donor (approximately 20%) is insufficient to eliminate the virus. To obtain a sufficient yield of PBMC-derived NK cells, several aliquots of peripheral blood must be withdrawn from the patients, which would be troublesome and difficult [Citation81]. Stem cell mobilization is hence used to overcome this obstacle, whereby CD34+ cells are stimulated from the BM into the blood and cultured with granulocyte-colony stimulating factor (G-CSF). The most widely used source of NK cells is the UCB [Citation82]. Although UCB is easily collected with minimal adverse events and GvHD, the amount of UCB-derived NK cells is limited, and growth cultures are required to enhance the yield [Citation83]. Preparation of PBMC- and UCB-derived NK cell populations is time-consuming, incorporating essential isolation, expansion, validation, and purification processes before the cells can be infused into patients. Because of the invasiveness of the BM-based extraction method, NK cells are rarely extracted from the BM [Citation84]. hESCs and induced pluripotent stem cells (iPSCs) may be used to overcome the limitations associated with other sources. Despite the complicated process of culturing and differentiating hESCs and iPSCs to generate NK cells, the optimized and differentiated NK cell yield was 11% higher than the yield from other sources [Citation85].
Furthermore, the differentiation phase could be easily modified to produce various NK cell phenotypes and functions. Owing to the pluripotent cell-mediated unlimited proliferative capacity, libraries of the differentiated cells with numerous genetic modifications can be made and stored as an off-the-shelf supply for other diseases [Citation86]. In vitro transformed NK cells, mainly NK-92, were previously used in clinical trials due to their high proliferative abilities and facile cost-effective manufacturing. However, the cell-induced genetic abnormalities, carcinogenicity, and lethal irradiation required before infusion restrict their clinical use [Citation87].
4.1.3. Sources of T cells
PBMCs are the primary source of T cells. Allogenic and autologous SARS-CoV-2-specific T cells are isolated from the blood of convalescent or SARS-CoV-2-infected patients. In the presence of SARS-CoV-2-derived peptides, IL-2, granulocyte macrophage-colony stimulating factor (GM-CSF), and other cytokines, SARS-CoV-2-targeted T cells could be stimulated and clonally expanded before being infused into patients with severe COVID-19 infection. Clinical administration of adoptive T cell therapy for COVID-19 patients is accompanied by several drawbacks, including genetic restrictions that only allow the use of HLA-matched T cells. Subsequently, excessive stimulation of T cells with cytokines may result in the generation of an exhausted T cell phenotype that impairs their functionality. The infused T cells may also exacerbate cytokine storm, leading to acute lung injury, ARDS, and multiple organ disorders [Citation88]. Lymphoid progenitor cells are another source of off-the-shelf T cells immunotherapy. Herein, T cell precursors are isolated, expanded, and transferred into the patient [Citation89]. The transferred T cell precursors will differentiate in the patient’s thymus, controlled by MHC to produce well-tolerated T cells. The T cells produced this way would not stimulate GvHD reactions, which is the primary hurdle of T cell transfer therapy [Citation88]. As a result, strict patient-donor histocompatibility is not mandatory in T cell precursor immunotherapy.
Additionally, pluripotent-induced stem cells provide scientists with an infinite source of superlative T cells. iPSC-derived T cells are antigen-specific, validated, differentiated, and histocompatible [Citation90]. The development of iPSC-derived T cells is still a challenge as evidence reporting the potential therapeutic effectiveness of iPSC-derived T cells in vivo is lacking despite successful in vitro observation of well-differentiated and functional T cell immunotherapy [Citation91].
4.1.4. Sources of MSCs
Natural MSCs are derived from two primary sources: embryonic stem cells (ESCs) and adult stem cells. However, ESCs are not used clinically due to the ethical consideration and the risk of carcinogenicity [Citation92]. Numerous adult MSCs sources are now in clinical application, including umbilical cord (UC)-MSCs, adipose MSCs, amniotic membrane MSCs, Wharton’s jelly (WJ), and dental pulp. UC-MSCs use a noninvasive method to generate a high content of MSCs; hence, it is clinically applicable for treating systemic disease associated with multiple systemic complications, including COVID-19 [Citation56,Citation57]. UC-MSCs can be activated and clonally expanded under laboratory circumstances within a short period. The gene expression profile of UC-MSCs is highly similar to the ESC-derived MSCs, besides having several advantages, including fast amplification, easy manipulation, non-carcinogenicity, and the imperviousness of UC-extracted MSCs to the immune response due to their low MHC I and MHC II expression [Citation93]. Therefore, UC-MSCs are the ideal cellular therapy to be clinically investigated for treating COVID-19 patients. Autologous BM is another source of MSCs that is being clinically used. Despite its reasonable efficacy-safety profile, the BM-MSC yield is generally low, limiting its therapeutic use to localized diseases rather than systemic ones [Citation94].
4.2. Clinical trials for cell-based therapies against SARS-CoV-2 and COVID-19
4.2.1. DC-mediated therapy
The activation and proliferation of human immunodeficiency virus (HIV)-1-specific T cell response induced by DC vaccination in patients infected by HIV-1 was the basis for developing DC vaccines against viral diseases. Numerous clinical trials demonstrated the effectiveness of DC-based immunotherapy, alone or in combination with antiretroviral therapy, to promote CD8+ and CD4+ T cell response in HIV-1-infected patients [Citation95]. Consequently, DC vaccination was comprehensively extended for other viral infectious diseases, including COVID-19 (; Preventive Trials: DCs) ().
Figure 6. Schematic representation of various types of cell-based therapies used for prevention or treatment of patients with severe COVID-19, including the strategies to produce these immune cells and their mechanism of actions against SARS-CoV-2 infection.
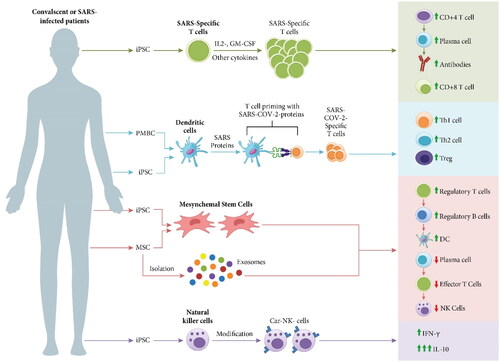
The distinctive features of DCs make them an ideal cellular component for developing vaccines against SARS-CoV-2. AV-COVID-19 is a personalized vaccine containing the participant’s immune cells loaded with SARS-CoV-2 antigen. Compared to other vaccines based on the production of viral proteins, it is postulated that this patient-specific platform stimulates a rapid adaptive immune response against SARS-CoV-2 without the common side effects associated with the intramuscular injection, including headache, fever, and arm pain [Citation96]. AV-COVID-19 entered an adaptive phase I trial with 27 confirmed negative COVID-19 participants with SARS-CoV-2 antibodies. Blood samples were collected from the participants to isolate peripheral blood Mo and differentiate them into DCs. DCs were incubated with spike protein that degraded into peptide sequences and incorporated at the dendrites of DCs. The autologous DCs loaded with ex vivo SARS-CoV-2 spike protein were mixed with saline with or without GM-CSF and injected subcutaneously within 5 h of preparation (ClinicalTrials.gov Identifier: NCT04690387). Phase I trial has been concluded, but the results have not been posted yet [Citation97]. In January 2022, an adaptive phase Ib-II trial of the previously mentioned DC-based vaccine will be initiated to prevent COVID-19 in adults (ClinicalTrials.gov Identifier: NCT04386252) [Citation98].
Another DC-based vaccine developed for treating COVID-19 patients is a synthetic minigene incorporated within a lentiviral vector (LV) system, known as LV-SMENP, expressing various COVID-19 proteins and immunomodulating genes. The LV-SMENP-DC vaccine internalizes, processes, and presents COVID-19-specific antigens, resulting in the activation of CTLs by the LV-SMENP-DC presenting the COVID-19 target antigen. LV-SMENP-DC vaccine and antigen-specific CTLs will be prepared within 7–21 days of administration. Some 5 × 106 cells of the LV-SMENP-DC vaccine will be injected subcutaneously, and 1 × 108 antigen-specific CTLs will be infused into healthy and COVID-19-infected patients. Phase I/II trial is now recruiting participants to investigate the safety and efficacy of the LV-DC and T cell vaccines (ClinicalTrials.gov Identifier: NCT04276896) [Citation99].
4.2.2. NK cell-mediated therapy
NK cell immunotherapy has been successfully established in patients experiencing various types of cancer. Infusion of ex vivo expanded NK cells restored peripheral NK cell count, which improved patient immune response and gave them a higher chance of recovery. Owing to their low GvHD, NK cell transfer, including adoptive NK cells and chimeric antigen receptor (CAR)-NK cells, is a potentially effective and novel cellular therapy for treating patients infected with COVID-19. CAR-engineered NK cells exhibit improved specificity toward the targeted antigen [Citation100]. CAR-NK cells were derived by culturing the genetically modified NK-92 homogenous cell lines, producing an off-the-shelf allogeneic NK cell population in clinical trials [Citation101]. CAR-modified NK cells exert an antiviral response without secreting the inflammatory cytokine, IL-6; thus, these fabricated immune cells overwhelm the cytokine release storm associated with IL-6-producing CAR-T cell therapy [Citation100,Citation102]. Many clinical trials are currently investigating the activity of NK cells against COVID-19 infection. Others are interventional studies exploring the safety and efficacy of NK cell infusion in SARS-CoV-2-infected patients (; Treatment Trials: NK cells).
Diverse NK cell lines were employed for ex vivo expansion study, including IL15-NK cells, NKG2D CAR-NK cells, ACE-2 CAR-NK cells, and NKG2D-ACE-2 CAR-NK cells. The targeted CAR-NK cells were genetically engineered with ACE-2 and NKG2D receptors to enhance the binding of SARS-COV-2 particles and SARS-COV-2-infected cells with the infused cells, thus protecting the alveolar epithelial cells from being infected. The engineered CAR-NK cells were developed into IL-15 super agonists and GM-CSF neutralizing single chain fragment variable (scFv) secreting cells. IL-15 improves the survival time of the infused cells in the body and enhances their cytotoxicity, whereas GM-CSF neutralizing scFv protects against cytokine release syndrome. The expanded CAR-NK cells were infused into active SARS-CoV-2-infected patients experiencing mild to moderate symptoms or with severe symptoms and pneumonia. The patients were monitored for the safety and efficacy of NK cell treatment (ClinicalTrials.gov Identifier: NCT04324996) [Citation103]. Besides the innate NK cells, bioengineered NK cells may also be used as a targeted cell therapy for treating COVID-19 patients [Citation104]. Indeed, NK cells have been genetically modified to express CAR on their surface and preserve the innate activation and inhibitory receptors, which possess anticancer and antiviral activities.
The natural and bioengineered NK cell expansion should be used with extreme scrutiny in patients experiencing debilitating respiratory symptoms accompanied by cytokine storm and severe inflammatory conditions. Besides its cytolytic effect, replenishing NK cell infusion may overproduce cytokines and chemokines, aggravating lung inflammation and injury [Citation104]. Therefore, the expanded pro-inflammatory NK cells should be reprogrammed into the regulatory phenotypes that produce the anti-inflammatory cytokine, IL-10. The intrinsic epigenetic modification of the IL-10 gene locus promotes the production of the expanded regulatory NK cells, which secrete IL-10 upon stimulation with various cytokines such as IL-12 and IL-21 [Citation105]. Owing to the deleterious complications that may result from NK cell-mediated therapy, the timing of the cell-based interventions, symptom severity, potential complications, and disease prognosis should be considered before administration. NK cell-based therapy may be potentially useful as a targeted anti-viral therapy at the early stages of infection. The immune-competent milieu enhances viral elimination and circumvents disease progression into ARDS and lung injury.
4.2.3. T cell-mediated therapy
Allogenic or autologous SAR-CoV-2-targeted T cells represent a logical therapeutic approach for treating COVID-19 patients by reinvigorating an effective innate antiviral response. Despite the limitations of using adoptive T cells in clinical settings, several clinical trials investigating the safety and efficacy of novel adoptive T cells from recovered patients for treating patients with COVID-19 are underway (; Treatment Trials: T cells). SARS-CoV-2-infected patients are enrolled in Phase I-II to examine the safety and efficacy of virus-specific T cells. T cells will be derived from the convalescent donors and infused into patients with either mild and moderate COVID-19 or in patients with severe symptoms (ClinicalTrials.gov Identifier: NCT04457726) [Citation106]. Various T cells are being clinically investigated for treating COVID-19 patients. For example, gamma delta T cells were isolated from recovered donors, expanded, and administered to patients diagnosed with COVID-19 (ClinicalTrials.gov Identifier: NCT04834128) [Citation107]. Other clinical trials examined the efficacy of exosomes derived from SARS-CoV-2-specific T cells for treating patients with severe symptoms. Unlike the donor-derived SARS-CoV-2-specific T cells, these exosomes could be employed for any patient since they do not need HLA matching. The exosomes derived from SARS-CoV-2-specific T cells were examined in the phase I trial. Donor-derived virus-specific T cells were exposed to viral peptides in the presence of cytokines. The viral peptides would activate the T cells to secrete exosomes. Confirmed COVID-19 patients with early-stage pneumonia received these T cell derived-exosomes by inhalation to explore their efficacy and safety (ClinicalTrials.gov Identifier: NCT04389385) [Citation108]. Autologous exosomes could also be a potentially useful and effective approach for personalized treatment.
4.2.4. Stem cells-mediated therapy
MSCs have several qualities, namely their homing effect, ability to differentiate into alveolar cells without being infected, ability to repair damaged pulmonary tissues, and ability to prevent fibrosis, that make them a potential innovative cellular therapy in severe and critically ill SARS-CoV-2-infected patients with compromised lung function associated with acute lung injury and ARDS. Currently, growing bodies of clinical evidence employing MSC-based therapy, either alone or in combination with conventional treatments, were registered for clinical trials, most of them from China (; Treatment Trials: stem cells). The results of phase I and II trials are promising and some of them have already been published. A favorable safety profile of autologous or allogenic adult MSC therapy derived from various sources was documented in patients experiencing unresponsive medical conditions such as GvHD, ischemic heart disease, spinal cord injury, and ARDS [Citation102]. Currently, some published trials document the effectiveness and the safety of intravenous MSC transplantation in SARS-CoV-2-infected patients associated with pneumonia, acute lung injury, and ARDS [Citation109]. The body of evidence hints that the favorable MSC effects against SARS-CoV-2 infection may be attributed to the secretion of extracellular vesicles (EVs) containing paracrine factors, mainly exosomes. The presence of exosomes in chemokines, growth factors, and different nucleic acids and their anti-inflammatory, immune-modulatory, and regenerative effects are imperative to the healing effect of MSCs. These results provide insight into the use of exosomes rather than MSC itself as an interventional therapy for several reasons, including direct delivery of EVs into the lungs either intranasally or by inhalation instead of systemic administration. Besides, EVs are free from the complications associated with the immune cell, namely uncontrolled proliferation, apoptosis, and immune compatibility. The engineering of targeted pulsed EVs containing antivirals or inhibitors and conjugated with SARS-CoV-2 S protein is possible with facile production, scaling up, and storing large quantities as an off-the-shelf product [Citation110]. The safety and effectiveness of exosomes were investigated in several trials alone or in combination with MSC therapy (; Treatment Trials: MSC-derived EVs). Numerous preclinical and clinical studies demonstrated that exosomes influence the complications of cytokine storm by reducing alveolar inflammation and edema as well as enhancing tissue regeneration in patients with ARDS and acute lung injury. ExoFloTM is an allogenic BM-derived exosome intravenously administered to patients experiencing severe COVID-19 infection. ExoFloTM administration showed promising beneficial effects with minimal side effects [Citation111]. However, more clinical-based studies are needed to pave the way for the potential clinical application of EVs for treating SARS-CoV-2 infection.
MSCs showed the highest number of registered clinical trials in COVID-19 patients compared to other cell-based therapies. Owing to its intrinsic immunomodulating and differentiation properties, accessibility of the MSCs sources, satisfactory number of clinical-based studies, and the bankability of allogeneic MSCs, several MSC-based therapies are frequently used in the clinical setting. UC-MSCs are commonly utilized in most trials. Additionally, WJ-MSCs have attracted the attention of scientists due to their plasticity, strength, and non-carcinogenicity.
Autologous and allogeneic MSCs are administered via intravenous infusion in most trials. Other modes of MSC administration are not as frequently employed in trials. Besides the naturally derived MSCs, Desai and Shendi engineered a novel nanoparticle loaded with stem cells for treating SARS-CoV-2-infected patients. In vitro animal models and ex vivo studies showed that nasally administering this nano-conjugate resulted in a reduction in C-reactive protein and a release of cytokine. Additionally, it was reported that the fabricated stem cell-based nanotherapy induced angiopoietin-1 expression that stimulated fluid clearance and regeneration of alveolar epithelial cells [Citation112]. Advanced clinical studies recruiting a larger number of SARS-CoV-2-infected patients should be conducted to confirm the safety and effectiveness of MSC therapy in COVID-19 patients.
5. Conclusion
The COVID-19 pandemic imposed a tremendous social, health, and economic burden with mounting numbers of infected patients as well as individuals at high risk of being infected worldwide. The concerted efforts of researchers and clinicians are directed toward engineering vaccines targeting SARS-CoV-2 and clinically investigating existing antivirals targeting SARS-CoV and MERS-CoV for the treatment of COVID-19. A growing number of studies demonstrated that COVID-19 is an immune-related viral infection, in which the delay or impairment in the spontaneous immune response against SARS-CoV-2 leads to a systemic hyperinflammatory response, lung damage, subsequent poor disease prognosis, and even death. Characterizing immune cell responses that include DCs, NK cells, T cells, and B cells in SARS-CoV-2 infection is crucial to better understand the disease prognosis. Currently, the widespread clinical use of different cell therapies for treating incurable diseases is based on their ability to differentiate into different cell lineages, their capacity to self-regenerate, and their immunomodulatory effect. Further studies are in dire need to predict their efficacy and safety profile in humans, besides considering the hurdles involved including immunogenicity, tumorigenicity, and large-scale production in clinical settings. Immune cell adoptive transfer therapy in combination with other conventional therapies and immune cell-based vaccines that boost antiviral immunity and suppress both immunopathology and SARS-CoV-2 evasion are promising clinical strategies to defeat the viral pandemic.
| Abbreviations | ||
| ACE-2 | = | angiotensin converting enzyme 2 |
| ADCC | = | antibody-dependent cell cytotoxicity |
| AMP | = | antimicrobial peptide |
| AP | = | activator protein |
| APC | = | antigen-presenting cell |
| ARDS | = | acute respiratory distress syndrome |
| BALF | = | bronchoalveolar lavage fluid |
| BCG | = | Bacille Calmette-Guerin |
| BM | = | bone marrow |
| CAR | = | chimeric antigen receptor |
| CCL | = | C-C motif chemokine ligand |
| cDC | = | conventional dendritic cell |
| CLR | = | C-type lectin receptor |
| COVID-19 | = | Coronavirus disease 2019 |
| CTL | = | cytotoxic T lymphocyte |
| CTLA | = | cytotoxic T lymphocyte-associated protein |
| CXCL | = | chemokine C-X-C motif ligand |
| DC | = | dendritic cell |
| DC-SIGN | = | dendritic cell-specific intracellular adhesion molecule-grabbing nonintegrin |
| EBI3 | = | Epstein-Barr virus-induced gene 3 |
| EGF | = | epidermal growth factor |
| ESC | = | embryonic stem cell |
| EV | = | extracellular vesicle |
| FDA | = | Food and Drug Administration |
| FGF | = | fibroblast growth factor |
| G-CSF | = | granulocyte-colony stimulating factor |
| GM-CSF | = | granulocyte macrophage-colony stimulating factor |
| GvHD | = | graft versus host disease |
| hESC | = | human embryonic stem cell |
| HGF | = | hepatocyte growth factor |
| HIV | = | human immunodeficiency virus |
| HLA | = | human leukocyte antigen |
| HSC | = | hematopoietic stem cell |
| IDO | = | indoleamine-pyrrole 2,3-dioxygenase |
| IFITM | = | interferon-induced transmembrane proteins |
| IFN | = | interferon |
| IGF | = | insulin-like growth factor |
| IL | = | interleukin |
| IP | = | inducible protein |
| iPSC | = | induced pluripotent stem cell |
| IRF | = | interferon regulatory factor |
| ISG | = | interferon-stimulated gene |
| JAK | = | Janus kinase |
| KIR | = | killer Ig-like receptor |
| KGF | = | keratinocyte growth factor |
| LIF | = | leukemia inhibitory factor |
| LV | = | lentiviral vector |
| PBMC | = | peripheral blood mononuclear cell |
| PD-1 | = | programmed cell death protein 1 |
| PDGF | = | platelet-derived growth factor |
| MCP | = | monocyte chemoattractant protein |
| MERS-CoV | = | Middle East respiratory syndrome coronavirus |
| MHC | = | major histocompatibility complex |
| MIP | = | macrophage inflammatory protein |
| Mo | = | monocyte |
| MSC | = | mesenchymal stem cell |
| NF-κB | = | nuclear factor-kappa B |
| NK | = | natural killer |
| NKG2D | = | natural killer group 2D |
| NLR | = | neutrophil lymphocyte ratio |
| NO | = | nitric oxide |
| ORF | = | open reading frames |
| PAMPs | = | pathogen-associated molecular pattern |
| pDC | = | plasmacytoid dendritic cell |
| PGE2 | = | Prostaglandin E2 |
| PRR | = | pattern recognition receptors |
| RBD | = | receptor-binding domain |
| RNA | = | ribonucleic acid |
| SARS-CoV | = | severe acute respiratory syndrome coronavirus |
| scFv | = | single chain fragment variable |
| STAT | = | signal transducer and activator of transcription |
| TGF | = | transforming growth factor |
| Th | = | T helper |
| TIM | = | T cell immunoglobulin and mucin containing domain |
| TIGIT | = | T cell immunoreceptor with Ig and ITIM domains |
| TMPRSS2 | = | transmembrane serine protease 2 |
| TNF-α | = | tumor necrosis factor-α |
| Treg | = | regulatory T cell |
| UC | = | umbilical cord |
| UCB | = | umbilical cord blood |
| VEGF | = | vascular endothelial growth factor |
| WJ | = | Wharton’s Jelly |
Acknowledgments
The authors thank the School of Pharmacy, Monash University Malaysia for the support in the preparation and completion of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Coronavirus Resource Center [Internet]. Baltimore (MD): Johns Hopkins University; 2020-2022 [cited 2022 Nov 2]. Available from: https://coronavirus.jhu.edu/map.html
- Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328.
- Huang Y, Yang C, Xu X-F, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–1149.
- Li H, Liu SM, Yu XH, et al. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55(5):105951.
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574.
- Coronavirus (COVID 19) vaccinations- [Internet]. Oxford: Our World in Data; 2020–2022 [cited 2022. Jan 1]. Available from: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
- Kannan SR, Spratt AN, Sharma K, et al. Omicron SARS-CoV-2 variant: unique features and their impact on pre-existing antibodies. J Autoimmun. 2022;126:102779.
- Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges, and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58.
- Cancio M, Ciccocioppo R, Rocco P, et al. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy. 2020;22(9):474–481.
- Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1–12.
- Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3(9):e202000786.
- Lukassen S, Chua RL, Trefzer T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10):e105114.
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9.
- Broggi A, Granucci F, Zanoni I. Type III interferons: balancing tissue tolerance and resistance to pathogen invasion. J Exp Med. 2020;217(1):e20190295.
- Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369(6504):706–712.
- Fung TS, Liu DX. Human coronavirus: host-pathogen interaction. Annu Rev Microbiol. 2019;73:529–557.
- Martín-Vicente M, Resino S, Martínez I. Early innate immune response triggered by the human respiratory syncytial virus and its regulation by ubiquitination/deubiquitination processes. J Biomed Sci. 2022;29(1):11.
- Tang Y, Liu J, Zhang D, et al. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708.
- Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374.
- Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet]. Windsor Mill (MD): US Department of Health and Human Services; [cited 2021 Nov 13]. Available from: https://www.covid19treatmentguidelines.nih.gov/
- van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med. 2022;28(1):39–50.
- Sposito B, Broggi A, Pandolfi L, et al. The interferon landscape along the respiratory tract impacts the severity of COVID-19. Cell. 2021;184(19):4953–4968.e16.
- Clementi N, Ghosh S, De Santis M, et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev. 2021;34(3):e00103–e00120.
- Galati D, Zanotta S, Capitelli L, et al. A bird’s eye view on the role of dendritic cells in SARS-CoV-2 infection: perspectives for immune-based vaccines. Allergy. 2022;77(1):100–110.
- Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20.
- Kotenko SV, Rivera A, Parker D, et al. Type III IFNs: beyond antiviral protection. Semin Immunol. 2019;43:101303.
- Harpur CM, Kato Y, Dewi ST, et al. Classical type 1 dendritic cells dominate priming of Th1 responses to herpes simplex virus type 1 skin infection. J Immunol. 2019;202(3):653–663.
- Marzi A, Gramberg T, Simmons G, et al. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78(21):12090–12095.
- Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV- Spike glycoprotein. Cell. 2020;181(2):281–292.e6.
- Carlin AF, Plummer EM, Vizcarra EA, et al. An IRF-3-, IRF-5-, and IRF-7-independent pathway of dengue viral resistance utilizes IRF-1 to stimulate type I and II interferon responses. Cell Rep. 2017;21(6):1600–1612.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422.
- Law HKW, Cheung CY, Ng HY, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374.
- Chu H, Zhou J, Wong BH, et al. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;:454–455:197–205.
- Han J, Sun J, Zhang G, et al. DCs-based therapies: potential strategies in severe SARS-CoV-2 infection. Int J Med Sci. 2021;18(2):406–418.
- Yang D, Chu H, Hou Y, et al. Attenuated interferon and proinflammatory response in SARS-CoV-2-Infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J Infect Dis. 2020;222(5):734–745.
- Zhou R, To KK, Wong YC, et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864.e5–877.e5.
- Abel AM, Yang C, Thakar MS, et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869.
- Belizário JE, Neyra JM, Setúbal Destro Rodrigues MF. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. Innate Immun. 2018;24(8):452–465.
- Schuster IS, Coudert JD, Andoniou CE, et al. “Natural regulators”: NK cells as modulators of T cell immunity. Front Immunol. 2016;14(7):235.
- Chan CJ, Smyth MJ, Martinet L. Molecular mechanisms of natural killer cell activation in response to cellular stress. Cell Death Differ. 2014;21(1):5–14.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992.e3–1000.e3.
- Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535.
- Wilk AJ, Rustagi A, Zhao NQ, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070–1076.
- Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92(10):1733–1734.
- Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, et al. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75.
- Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274.
- Chen Z, Wherry J. E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536.
- Chiappelli F, Khakshooy A, Greenberg G. CoViD-19 immunopathology and immunotherapy. Bioinformation. 2020;16(3):219–222.
- Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17(5):541–543.
- Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5(48):eabd2071.
- Tan M, Liu Y, Zhou R, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268.
- Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36.
- Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–979.
- Regmi S, Pathak S, Kim JO, et al. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98(5-8):151041.
- Muraca M, Pessina A, Pozzobon M, et al. Mesenchymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID-19 pneumonia? J Control Release. 2020;325:135–140.
- Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228.
- da Silva KN, Gobatto ALN, Costa-Ferro , et al. Is there a place for mesenchymal stromal cell-based therapies in the therapeutic armamentarium against COVID-19? Stem Cell Res Ther. 2021;12:425.
- Shao M, Xu Q, Wu Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):1–13.
- Xu YL, Liu YL, Wang Q, et al. Intravenous transplantation of mesenchymal stem cells attenuates oleic acid induced acute lung injury in rats. Chin Med J. 2012;125(11):2012–2018.
- Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–1247.
- Chen QH, Wu F, Liu L, et al. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther. 2020;11(1):91.
- Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372.
- Lu Z, Chang W, Meng S, et al. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 2019;10(1):372.
- Liu X, Qu X, Chen Y, et al. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189(3):1182–1192.
- Yang Y, Hu S, Xu X, et al. The vascular endothelial growth factors-expressing character of mesenchymal stem cells plays a positive role in treatment of acute lung injury in vivo. Mediators Inflamm. 2016;2016:2347938.
- Cahill EF, Kennelly H, Carty F, et al. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-Induced pulmonary fibrosis. Stem Cells Transl Med. 2016;5(10):1307–1318.
- Alcayaga-Miranda F, Cuenca J, Khoury M. Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol. 2017;8:339.
- Schwartz YS, Belogorodtsev SN, Filimonov PN, et al. BCG infection in mice is promoted by naïve mesenchymal stromal cells (MSC) and suppressed by poly(A: u)-conditioned MSC. Tuberculosis. 2016;101:130–136.
- Foronjy RF, Dabo AJ, Cummins N, et al. Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection. BMC Immunol. 2014;15:41.
- Janssens K, Van den Haute C, Baekelandt V, et al. Leukemia inhibitory factor tips the immune balance towards regulatory T cells in multiple sclerosis. Brain Behav Immun. 2015;45:180–188.
- Jafarzadeh A, Chauhan P, Saha B, et al. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102.
- Schulte-Schrepping J, Reusch N, Paclik D, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell. 2020;182(6):1419–1440.e23.
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362.
- Saxena M, Bhardwaj N. Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer. 2018;4(2):119–137.
- Lineage cell therapeutics [Internet]. Carlsbad (CA): lineage Cell Therapeutics, Inc; 2021 [cited 2022 Mar 11]. Available from: https://lineagecell.com/products-pipeline/vac2/
- Bernhard H, Disis ML, Heimfeld S, et al. Generation of immunostimulatory dendritic cells from human CD34+ hematopoietic progenitor cells of the bone marrow and peripheral blood. Cancer Res. 1995;55(5):1099–1104.
- Balan S, Kale VP, Limaye LS. A simple two-step culture system for the large-scale generation of mature and functional dendritic cells from umbilical cord blood CD34+ cells. Transfusion. 2009;49(10):2109–2121.
- Than UTT, Le HT, Hoang DH, et al. Induction of antitumor immunity by exosomes isolated from cryopreserved cord blood Monocyte-Derived dendritic cells. Int J Mol Sci. 2020;21(5):1834.
- Waskow C, Liu K, Darrasse-Jèze G, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9(6):676–683.
- Leong JW, Chase JM, Romee R, et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20(4):463–473.
- Sarvaria A, Jawdat D, Madrigal JA, et al. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329.
- Mu YX, Zhao YX, Li BY, et al. A simple method for in vitro preparation of natural killer cells from cord blood. BMC Biotechnol. 2019;19(1):80.
- Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2(4):274–283.
- Galat Y, Dambaeva S, Elcheva I, et al. Cytokine-free directed differentiation of human pluripotent stem cells efficiently produces hemogenic endothelium with lymphoid potential. Stem Cell Res Ther. 2017;8(1):67.
- Zeng J, Tang SY, Toh LL, et al. Generation of "off-the-Shelf" natural killer cells from peripheral blood cell-derived induced pluripotent stem cells. Stem Cell Rep. 2017;9(6):1796–1812.
- Suck G, Odendahl M, Nowakowska P, et al. NK-92: an 'off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65(4):485–492.
- Caccamo N, Sullivan LC, Brooks AG, et al. Harnessing HLA-E-restricted CD8 T lymphocytes for adoptive cell therapy of patients with severe COVID-19. Br J Haematol. 2020;190(4):e185–e187.
- Shah DK, Zúñiga-Pflücker JC. An overview of the intrathymic intricacies of T cell development. J Immunol. 2014;192(9):4017–4023.
- Inoue H, Nagata N, Kurokawa H, et al. iPS cells: a game changer for future medicine. EMBO J. 2014;33(5):409–417.
- Nishimura T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(1):114–126.
- Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113(9):2806–2812.
- Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014;6(2):195–202.
- Sanapati J, Manchikanti L, Atluri S, et al. Do regenerative medicine therapies provide long-term relief in chronic low back pain: a systematic review and metaanalysis. Pain Physician. 2018;21(6):515–540.
- Routy JP, Boulassel MR, Yassine-Diab B, et al. Immunologic activity and safety of autologous HIV RNA-electroporated dendritic cells in HIV-1 infected patients receiving antiretroviral therapy. Clin Immunol. 2010;134(2):140–147.
- SARS-CoV-2 vaccine [Internet]. Irvine (CA): AIVITA Biomedical; 2020 [cited 2022 May 2022]. Available from: https://aivitabiomedical.com/programs/sars-cov-2/
- Aivita Biomedical, Inc. Adaptive phase I clinical trial of preventive vaccine consisting of autologous dendritic cells previously incubated with S-protein from SARS-CoV-2, in subjects negative for COVID-19 infection and anti-SARS-CoV-2 antibodies. ClinicalTrials.gov [Internet]. 2021 Dec 16 [cited 2022 May 15]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04690387
- Aivita Biomedical, Inc. Adaptive phase I-II clinical trial of preventive vaccine consisting of autologous dendritic cells previously incubated with S-protein from severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), in subjects negative for COVID-19 infection and anti-SARS-CoV-2 antibodies. ClinicalTrials.gov [Internet]. 2021 Dec 16 [cited 2022 May 15]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04386252
- Shenzhen Geno-Immune Medical Institute. Phase I/II multicenter trial of lentiviral minigene vaccine (LV-SMENP) of covid-19 coronavirus. ClinicalTrials.gov [Internet]. 2020 Mar 19 [cited 2022 May 18]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04276896
- Rezvani K, Rouce R, Liu E, et al. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25(8):1769–1781.
- Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-Positive lymphoid tumors. N Engl J Med. 2020;382(6):545–553.
- Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Opin Immunol. 2018;51:146–153.
- Chongqing Public Health Medical Center. A phase I/II study of universal off-the-shelf NKG2D-ACE2 CAR-NK cells secreting IL15 superagonist and GM-CSF-neutralizing scFv for therapy of COVID-19. ClinicalTrials.gov [Internet]. 2020 Nov 17 [cited 2022 May 18]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04324996
- Tarrio ML, Lee SH, Fragoso MF, et al. Proliferation conditions promote intrinsic changes in NK cells for an IL-10 response. J Immunol. 2014;193(1):354–363.
- Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15(1):39.
- KK Women’s and Children’s Hospital. Part two of novel adoptive cellular therapy with SARS-CoV-2 specific T cells in patients with severe COVID-19. ClinicalTrials.gov [Internet]. 2020 July 7 [cited 2022 May 19]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04457726
- TC Biopharm. A phase II safety and tolerability, inter-patient pre-defined dose study of ex-vivo expanded allogeneic γδ T-lymphocytes (TCB008) in patients diagnosed with COVID-19. ClinicalTrials.gov [Internet]. 2022 Mar 24 [cited 2022 May 20]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04834128
- Mustafa Cetin, TC Erciyes University. Aerosol inhalation of the exosomes derived from allogenic COVID-19 T cell in the treatment of early stage novel coronavirus pneumonia. clinicalTrials.gov [Internet]. 2020 May 15 [cited 2022 May 20]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04389385
- Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31):e21429.
- Mahida RY, Matsumoto S, Matthay MA. Extracellular vesicles: a new frontier for research in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2020;63(1):15–24.
- Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754.
- Desai D, Shende P. Nanoconjugates-based stem cell therapy for the management of COVID-19. Stem Cell Rev Rep. 2021;17(1):231–240.