Abstract
HarvestPlus, part of the Consultative Group on Internation Agriculture research (CGIAR) Program on Agriculture for Nutrition and Health (A4NH) uses conventional plant breeding techniques to develop staple food crops that are rich in micronutrients, a food-based approach to reduce micronutrient malnutrition known as biofortification. The nutritional breeding targets are established based on the food intake of target populations, nutrient losses during storage and processing and bioavailability. This review collates the evidence on the retention of provitamin A carotenoid (pVAC) after processing, cooking, and storing of the staple crops targeted for pVAC biofortification: cassava, maize, and sweet potato. Sun drying was more detrimental to the pVAC levels (27–56% retention) in cassava than shade (59%) or oven (55–91%) drying, while the pVAC retention levels (66–96%) in sweet potato were not significantly different among the various drying methods. Overall, boiling and steaming had higher pVAC retention (80–98%) compared to baking (30–70%) and frying (18–54%). Gari, the most frequently consumed form of cassava in West Africa had the lowest pVAC retention (10–30%). The pVAC retention of maize grain and cassava and sweet potato flour reached levels as low as 20% after 1–4 months of storage and was highly dependent on genotype. Therefore, we recommend that an evaluation of the pVAC degradation rate among different genotypes be performed before a high pVAC crop is promoted.
INTRODUCTION
Hidden hunger, caused by a lack of micronutrients in the diet, afflicts billions of people. It is caused by a lack of micronutrients in the diet. Fruits, vegetables, and animal products are rich in micronutrients, but these foods are often not available to the poor. Their daily diet consists mostly of a few inexpensive staple foods, such as cassava, maize and sweet potato, which are mostly white in color and devoid of carotenoids. HarvestPlus, part of the Consultative Group on Internation Agriculture research (CGIAR) Program on Agriculture for Nutrition and Health (A4NH) uses conventional plant breeding techniques to develop varieties of staple food crops that are rich in micronutrients, a food-based approach to reduce micronutrient malnutrition known as biofortification. Our program is enhancing the levels of iron, zinc, and provitamin A carotenoids (pVAC), precursors of vitamin A, in staple foods without sacrificing essential agronomic qualities, such as high yield and resistance to disease and environmental stress. The contents of pVAC, in particular, are being increased in cassava, maize, and sweet potato to levels that will produce a measurable impact on the nutritional and health status in target African countries where the prevalence of vitamin A deficiency (VAD) is of a public health significance (WHO, Citation2009).
Vitamin A (retinol) is an essential micronutrient responsible for gene expression, growth, development, visual adaptation to darkness, and certain aspects of the immune system (Rando, Citation1990; West et al., Citation1991). An estimated 250 million preschool children and a substantial proportion of pregnant women in developing countries suffer from VAD, which can cause night blindness and increase the risk of child and maternal mortality (WHO, Citation2010). Over two decades ago, vitamin A supplementation was introduced globally as a short-term intervention to reduce VAD for children under five and postpartum women. Complementary food-based approaches have also been explored as a more sustainable and cost-effective strategies (Bouis et al., Citation2011).
The current target concentration of pVAC for each crop will provide half of the estimated average requirement (EAR) for the target population at average staple food consumption levels. The target levels of pVAC are calculated based on average staple food intake (grams/day, FW), the losses of pVAC with processing, storage, and cooking, and the bioavailability and the retinol equivalency of the ingested pVAC (Hotz & McClafferty, Citation2007). Studies undertaken to-date by HarvestPlus collaborators confirmed the high consumption rates of staple crops in rural areas of target African countries with a range of 150–250 g/day among children and 300–400 g/day among women for sweet potato and maize (Hotz et al., Citation2012a, Hotz et al., Citation2012b) and 700–900 g/day among women for cassava (unpublished data). Bioconversion rates of β-carotene to retinol were also high, with reported values of 7:1 (Li et al., Citation2010) and 3:1 (Muzhingi et al., Citation2011) for biofortified maize as compared to the current 12:1 conversion ratio recommended by the Institute of Medicine (IOM, Citation2001). However, the highly unsaturated structure of carotenoids makes them susceptible to post harvest degradation by heat, oxygen and light, and evaluation of pVAC retention in cassava, maize, and sweet potato by multiple laboratories has understandably produced varying results, depending on the post-harvest processing and cooking methods and the conditions and duration of storage.
This review critically evaluates the literature on pVAC-rich cassava, maize, and sweet potato, and assesses the findings on pVAC retention during processing, cooking, and storage. The percentage of pVAC retention as total carotenoids and/or β-carotene is presented and, when possible, the summarization of trans- to cis-isomer configuration is also reported.
BACKGROUND
Provitamin A Carotenoids
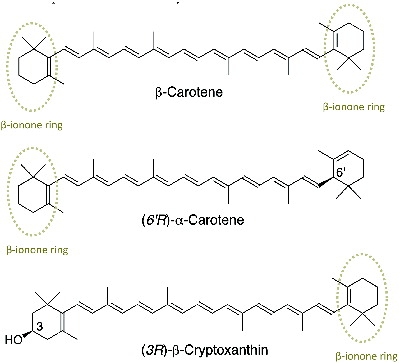
Carotenoids are usually C40 tetraterpenoids built from eight C5 isoprenoid units in an extensive conjugated double-bond system, which serves as the light-absorbing chromophore responsible for the yellow, orange, or red color in fruits and vegetables. The main condition for provitamin A activity is that the carotenoid must possess at least one β-ionone ring residue (). β-carotene has two β-ionone rings in its structure and is the most widespread of all carotenoids in food. Structurally, β-carotene can generate two molecules of vitamin A (retinol) when centrally cleaved, while α-carotene and β-cryptoxanthin can generate only one molecule. These three carotenoids are the most commonly found precursors of vitamin A in plant food consumed by humans.
Mechanisms of Carotenoid Degradation
The mechanisms of carotenoid degradation may involve: the reaction of carotenoids with atmospheric oxygen (autooxidation), light (photodegradation), and heat (thermal degradation), as well as degradation by the interactions of carotenoids with singlet oxygen, acid, metals, and free radicals. These mechanisms have been, for the most part, theorized based on a combination of studies in model systems, mostly in organic solutions; however, in food systems, the degradation mechanisms are more complex (Boon et al., 2010). In the intact fruit, vegetable, root/tuber or grain, the carotenoid molecules are less susceptible to degradation because they are protected within tissues through molecular interactions and association into supramolecular proteolipid complexes or crystal formation and the antioxidant regime within living cells. Differences in sequestration and intracellular localization of carotenoids in the tissue may be crucial factors in the susceptibility of these pigments to trans-cis isomerization and oxidation (Borsarelli & Mercadante 2009). Additionally, when the crop tissues are disrupted by cutting, chopping, shredding, cooking, or natural aging these physical barriers are affected thus rendering the carotenoids open to exposure to oxygen and oxidizing enzymes (Britton and Khachik, Citation2009). Given all these possible factors and their interactions, there are still many questions about the exact chain of reactions at the physiological level.
Isomerization
All-trans-carotenoids, the most common form of carotenoids present in foods, can be isomerized to the cis-isomer by exposure to direct sunlight. The twisting of the molecule during the isomerization process may lead to an unpaired spin state, which can react easily with oxygen to form carbon-peroxyl triplet diradicals unavailable for conversion to retinol. The isomerization can occur first, followed by the formation of a diradical, or they may both (isomerization and formation of diradical) occur simultaneously and reversibly (Mordi et al., 1993).
Photodegradation
The photochemical process may produce isomers and/or degraded products from oxidation or volatile compounds, such as β-ionone. The degraded products are lighter colored because of the resulting shorter chromophore, while in the isomerization process the cis-isomer retains most of the properties of the parent carotenoids. Furthermore, photooxidation produces species thought to be carotenoid radical (Mortensen and Skibsted, 1996). The photosensitized transformation of carotenoids has been studied using several sensitizer molecules, such as chlorophylls, iodine, rose Bengal (RB), and methylene blue (MB), and, in general terms, isomerization is the major pathway of reaction (Borsarelli & Mercadante, 2009).
Autooxidation
Formations of oxidation products with molecular weight lower than β-carotene have been extensively reported. Eccentric cleavage products such as retinal and β-apocarotenals, as well as epoxy carotenoids, were produced by autooxidation of β-carotene (Mordi et al 1993). Bechoff and colleagues investigating the β-carotene degradation in dried sweet potato chips at various temperature, water activity, and oxygen levels, observed that in all cases that, β-carotene submitted to oxidation was degraded into epoxides, apocarotenals, and apocarotenones, which were further oxidized into lighter and volatile compounds, e.g., β-cyclocitral, β-ionone, 5,6-epoxy-β-ionone, and dihydroactinidiolide (DHA) (Bechoff et al., Citation2010b). The authors suggested that the greater β-carotene degradation rate at lower water was due in particular to autooxidation.
Singlet Oxidation
Carotenoids can also interact with singlet oxygen present in the food matrix (which is more reactive than the triplet oxygen, present in the air) to yield triplet oxygen and an excited triplet carotenoid. The highly reactive molecules can interact with other free radicals, such as peroxyl or hydroxyl radicals, to generate further oxidative products (Britton G, 1995). Similarly to autooxidation, β-apocarotenals can also be originated from the oxidation of β-carotene with a radical generating reagent and singlet oxygen (McClure and Lieber, 1995 and Stratton et al., 1993).
Thermal Degradation
The isomerization and degradation of pure β-carotene were evaluated in an oven heated at temperatures between 50°C and 150°C for up to 30 minutes, as well as by reflux heating at 70°C for 140 minutes, using first-order kinetic decay (Chen and Huang 1998). The major isomers formed during heating were 13-cis-β-carotene, both under oven and reflux heating, while the 13,15-di-cis-β-carotene was only found at temperatures higher than 120°C. Similar results were obtained by Henry et al., 1998, heating β-carotene at several temperatures formed 13-cis-β-carotene in higher amounts, followed by 9-cis-β-carotene. In summary, isomerization is the main reaction that occurs during heating at atmospheric pressure and at temperatures lower than 100°C; the 13-cis-isomer is formed at higher rates than 9-cis-isomer; formation of oxidation products from β-carotene, such as epoxides and apo-carotenals, as well as di-cis isomers occur under higher temperature, longer time, and higher pressure (Mercadante, 2008).
Retention Analysis
Conditions necessary for isomerization and oxidation of carotenoids exist during preparation, processing, and storage of food, but are also artificially generated during laboratory analysis. Therefore, the inter-laboratorial discrepancies encountered in the literature are usually associated with the analytical practices during the extraction and measurements of the carotenoids and methodology used for calculating retention (true versus apparent retention). In general, errors associated with chromatography are minor, while those from extraction procedures are potentially significant (Howe & Tanumihardjo, Citation2006).
Carotenoid Extraction
Good laboratory practices include protection from light during analysis, which can be accomplished by the replacement of the laboratory lights to yellow lights. On the other hand, freeze-drying the samples prior to analysis is innocuous for the pVAC contents of fresh produce. No significant difference in the all-trans-β-carotene content was observed on seven OFSP samples after freeze-drying (Bengtsson et al., Citation2008). A major problem in retention studies is the greater efficiency of carotenoid extraction in processed foods compared to unprocessed foods. Processed food crops reporting retention values above 100% can be a result of increased carotenoid extraction efficiency in processed crops compared to raw food, rather than true increases in the carotenoid content, as the enzymes for biosynthesis of carotenoids have already been inactivated during processing (Rodriguez-Amaya, Citation1997).
Sampling
One source of variation is the sampling method used, because the same sample cannot be quantified for carotenoids in unprocessed and processed tissue. It is suggested that in the case of cassava and sweet potato, the root be quartered longitudinally and two opposite sections be taken for raw sample analysis, and the two remaining opposite sections be submitted for processing analysis (Rodriguez-Amaya, Citation1997; Ceballos et al., Citation2012).
The use of paired samples in retention studies is highly recommended to avoid any differences in the content of carotenoids in the raw versus the processed sample. Van Jaarsveld et al., Citation2006 reported a difference in the β-carotene content from 132 to 194 μg/g (FW) among sweet potatoes of the same size from the same harvest batch.
Calculation of Retention
Nutrient retention in cooked foods may be calculated as either apparent or true retention. True retention measures the proportion of a nutrient remaining in cooked food compared to the amount of that nutrient originally present in a given weight of the food before cooking.
True retention is the recommended method for calculating nutrient retention (Murphy et al., Citation1975; Rodriguez-Amaya, Citation1997). The formulas for calculating apparent and true retention are presented in Box 1.
Box 1. Calculation of Nutrient Retention in Cooked Foods
True retention
Apparent retention
Source: Murphy et al., Citation1975-Comparisons of Methods for Calculating Retentions of Nutrients in Cooked Foods
Apparent retention is defined by calculations based on the nutrient contents of the moisture-free raw and cooked foods. The apparent retention calculation assumes that solids are not lost to a significant extent, for example, by leaching into the steep water, which is later discarded. In cases where solid loss is negligible, some researchers have found that comparable retention results can be obtained by calculating retention on a dry weight basis instead of using the true retention formula (Li et al., Citation2007; Bengttson et al., Citation2008).
METHODOLOGY
A review of available retention literature was performed in English using Pubmed, Google Scholar, and Agricola (USDA, National Agricultural Library). Search terms included: [Provitamin A or Carotenoid], [Retention or Loss], and [Boiling or Drying or Flour or Roasting or Steaming or Frying or Microwaving]. It also included [Sweet potato] for sweet potato; [Maize or Corn] for maize and [Cassava or Manioc or Yucca] for cassava. Additionally, a number of experts and authors of recent retention publications were contacted for cross-checking the results of the literature review, locating specific articles, and providing unpublished data. A total of 30 studies (maize: 6, cassava: 8, and sweet potato: 16) were included in this review. Information on country of cultivation, cultivar, cooking process, the initial content of β-carotene or total carotenoid, and percentage of retention was extracted from the reports and is presented in table format in the present review. For organizational purposes, this review is presented in sections separated by crop and processing method.
CASSAVA
Cassava (Manihot esculenta Crantz), also known as manioc, mandioca, tapioca, or yuca, is a staple food in West Africa. Total cassava consumption has doubled in Africa from 24 million tons per year to 58 million tons per year, over the last 30 years (FAO, 2012). Nigeria is the largest producer of cassava in the world (FAO, 2008) with about 45 million metric tons and its cassava transformation is the most advanced in Africa (Egesi et al., Citation2006). According to the data from the Nigeria Food Consumption and Nutrition Survey 2001–2003, cassava consumption is approximately 200–250 g/day among children 4–6 years and 350–400 g/day among women in the southern region of Nigeria (Maziya-Dixon et al., Citation2007). Although cassava is a good source of carbohydrates and energy, it is a poor source of micronutrients. Given the high prevalence of VAD in the country—29.5% among children less than 5 years old and 13% among women of child-bearing age (Maziya-Dixon et al., Citation2007)—this root crop is an excellent vehicle for delivery of vitamin A through biofortified varieties with increased levels of pVAC.
Cassava roots can be boiled or steamed for consumption, but are usually processed into flour by oven drying or more laborious traditional West African methods. The processing of cassava can involve: chipping, crushing, milling, slicing or grating the roots, dehydration through pressing and decanting or drying in the sun, which can be followed by fermenting by soaking in water, sieving, and cooking under heat or roasting. In this paper, we examine how much pVAC is retained in cassava after drying, storage, and cooking by traditional West African methods.
Drying is used for production of flour and to extend shelf-life of stored cassava. Three drying methods have been investigated for cassava: direct sun-drying, shade-drying, and oven-drying (). Chavez et al., Citation2007 evaluated the effect of different drying methods on the true retention of β-carotene in light-yellow cassava roots. β-carotene was the main carotenoid in the three cassava genotypes evaluated, CM 2772–3, MBRA1324, and MCOL2401, with initial levels of 10.4, 7.2, and 13.5 μg/g DW, respectively, representing 93%, 79%, and 62% of total carotenoid (TC). The study was carefully designed; coarsely chopped chip samples (length 55 mm, width 10 mm, thickness 2 mm) from the same root were used in all three drying methods. To reach a final moisture content of 12% in the cassava chips, the period of drying varied according to the humidity and temperature conditions of each setting (sun: 2–3 days, shade: 6–7 days, oven: 24 hours at 60°C). The highest β-carotene retention was obtained by oven-drying (72%), followed by shade-drying (59%), and sun-drying (38%).
Table 1 Retention of carotenoids in cassava-effect of drying and storage
Similar results were reported by Iglesias et al., Citation1997 when studying the stability of carotene in response to different processing methods. Twenty-eight yellow cassava genotypes with an average initial TC content of 18.90 (range: 7.7 to 46.9) μg/g DW were evaluated. The average total TC retention was 27% (range: 10–55%) in sun-dried flour and 55% (range: 17–99%) in oven-dried flour. No information on the size of the cassava slices or temperature and period of drying was provided.
In an attempt to evaluate the changes in TC content at each stage of processing cassava, three improved yellow cassava varieties (TMS 94/0006, TMS 01/1235, and TMS 01/1371) were selected and made into flour and chips, either dried in the sun or in the oven (Maziya-Dixon et al., Citation2008). In this study, the authors collected samples for carotenoid analysis from peeled raw roots (initial carotenoid content) and at each stage of processing of flour (grated cassava mash, pressed grated mash, pulverized sieved mash/cake, and the final product) and chips (grated wet chips, grated wet pressed chips, and the final product). The wet material from flour and chip processing was equally divided to be sun-dried or oven-dried at 45°C for 72 hours. The samples were not weighed before and after each step, so true retention could not be calculated. The unadjusted calculations for the loss of moisture resulted in a false increase in total carotenoid retention, particularly for the chip processing. The original publication presented only the TC content at each stage. Therefore, we calculated the apparent retention presented in by applying the following formula:
Another observation was the significant variation in β-carotene retention among different cassava genotypes (Iglesias et al., Citation1997; Chavez et al., Citation2007). Iglesias et al., Citation1997, studying the stability of carotene in response to different processing methods, concluded that genotype is an important determinant of pVAC retention. Chavez et al., Citation2007 confirmed this finding through an analysis of variance conducted for all processing methods, including drying, storage of cassava as flour and chips, and two cooking processes.
In summary, sun drying produces the lowest rate of pVAC retention in cassava among the methods studied. Unfortunately, it is the most common method for drying cassava in African countries other than Nigeria where most cassava is processed directly into gari. The large drastic reduction in β-carotene retention in sun-drying compared to oven or shade-drying suggests a significant detrimental effect of light on the stability of this pigment (Chavez et al., 2007).
Blanching. Postharvest boiling or steaming for a short period of time prior to drying has been shown to improve carotenoid retention in dried foods by inactivating enzymes that can degrade carotenoids (Koca et al., Citation2005; Ndawula et al., Citation2004). The β-carotene content of slices of yellow cassava roots (4–5 mm) has been compared after they were blanched only by immersion into boiling water for 1.5 minutes, dried only at 70°C, or were subject to both processes (Nascimento et al., Citation2007). The β-carotene retention was 84% after blanching only, 91% after drying only, and 79% after blanching and drying. Therefore, blanching did not improve the retention of the carotenoids in cassava. However, after 20 days of storage, the slices that underwent blanching and oven drying retained 83% of the initial amount of β-carotene, compared to only trace amounts found in the samples that were not blanched prior to the drying process, suggesting that blanching may improve carotenoid stability over time. With only one study available, however, more data are needed to determine the effects of blanching on the shelf life of dried cassava, particularly in real-world settings.
Storage. Chavez et al., Citation2007 measured the β-carotene retention during a four-week storage period with yellow cassava flour and chips that had been either oven-dried or sun-dried. Oven-dried cassava initially retained 72% of β-carotene, which decreased to 40% and 32% after two and four weeks of storage at room temperature, respectively. Sun-dried cassava had a lower initial β-carotene retention (38%), which was further decreased to 24% and 18% after two and four weeks of storage, respectively. The β-carotene retention values for cassava chips were very similar to those of cassava flour (). Although data were not presented, the authors state that β-carotene retention did not vary significantly among the three yellow cassava cultivars during storage.
Even greater losses in TC content has been reported for cassava flour, from five yellow-cassava cultivars, stored at room temperature in the absence of light (Oliveira et al., Citation2010). The TC retention in cassava flour for all cultivars decreased with storage time, ranging from 24–40% and 11–30% after 5 and 12 days of storage, respectively. At 19 days of storage, only one cultivar had detectable levels of total carotenoids, and none by the 26th day of storage. This study showed that the cassava variety with the highest TC content as raw cassava (1751 Bonita: 18.92 μg/g) had lower retention (1.20 μg/g) when stored as flour for 12 days compared to another cultivar with lower initial TC levels (1752 Flor do Brasil: 11.63 μg/g) but higher retention (1.80 μg/g) after storage.
In an attempt to reduce β-carotene degradation during cassava flour storage, a vacuum was created using plastic bags to eliminate oxygen prior to storage (Chavez et al., Citation2007). Storage in a vacuum-packed plastic bag which is semipermeable to air did not seem to make a difference, since cassava flour in vacuum-packed bags displayed only slightly lower (statistical analysis was not performed) β-carotene retention (oven-dried: 32% and sun-dried: 17%) than the samples packed without a vacuum (oven-dried: 36% and sun-dried: 18%). Future research should be conducted with packaging material that is impermeable to air.
Cooking
Boiling. Simply boiling of cassava roots or rapid boiling of fermented cassava paste to prepare fufu is used in the traditional African cooking to reduce the total cyanogen content (Thakkar et al., Citation2009). Table 2 shows the results from five studies have evaluated the effect of boiling on cassava with the average pVAC retention among cultivars and studies ranging from 56 to 100% (Iglesias et al., 1997, Nascimento et al., Citation2006, Chavez et al., Citation2007, and Thakkar et al., Citation2009 and Ceballos et al., 2012). All studies followed the same protocol—boiling cassava for 30 minutes—with one study reporting to have boiled cassava in a covered pot (Chavez et al., Citation2007).
A strong genotype effect was observed among the three yellow cassava cultivars boiled for 30 minutes (CM 2772–3, MBRA 1324, and MCOL 2401) by Chavez and collaborators, with β-carotene retention reported as 16, 59, and 92%, respectively (Chavez et al., Citation2007). To further explore this effect, the study was expanded to include six different genotypes, in which the β-carotene retention ranged from 27 to 73%. Ten years earlier, a study of 28 genotypes using similar boiling conditions found that β-carotene retention ranged from 33 to 98% (Iglesias et al., 1997). In recent years, greater attention has been given to the differences in dry matter content among genotypes and the parts of the root used for the retention studies (Thakkar et al., Citation2009 and Ceballos et al., 2012).
Thakkar et al., Citation2009 evaluated the effect of boiling among the TMS 01/1371, TMS 01/1412, and TMS 01/1663 cassava cultivars which initially contained total β-carotene levels of 6.2–7.8 μg/g FW (25.9–40.9 μg/g DW). Due to the difference in the dry matter content before (18–25%) and after (19.1 ± 0.52%) boiling, the authors decided to express the results per unit of dry weight. However, the apparent retention of total β-carotene calculated on a dry weight basis exceeded 100%, proving that the formula of true retention should be used instead. Fresh roots containing lower dry matter (20–30%) tend to maintain their weight after boiling, but the carotenoids tend to lixiviate from the root samples into the water in higher proportion when the dry matter content is low (Ceballos et al., 2012).
The relationship between dry matter content and carotenoid levels in different sections (proximal, central, and distal) of the cassava root has been investigated by Ceballos et al., 2012. The results showed higher concentrations of total carotenoid in the proximal section of the root (proximal: 34, central: 32.9 and distal: 29.6, expressed as μg/g DW), similar to the pattern observed for the dry matter content (proximal: 36.3%, central: 34.0% and distal: 32.8%). Although the retention tended to be higher in the proximal and distal sections and lower in the central section, it was not significantly different. On average the TC retention among six cassava cultivars after boiling for 30 minutes was 87% (range: 76–97%). This was the highest reported cassava retention obtained among all studies included in this review. In conclusion, differences among genotypes were highly significant (p ≤ 0.01) whereas the variety by section of the root interaction was not significant (p ≥ 0.20).
Lastly, increases in the 13-cis (34%) and 15-cis (8%) isomers were observed in boiled cassava (Ceballos et al., 2012). The ratio of 13-cis to 9 cis- β-carotene (1.1–1.8) has also been reported to be almost twice in boiled cassava than that in their raw cassava counterpart (0.6–0.7) (Thakkar et al., Citation2009).
Frying is not a common method of preparing cassava for consumption in West Africa. One study evaluated the retention of β-carotene in fried cassava (Nascimento et al., Citation2006). Cassava roots purchased from the local market in Brazil were boiled for 20 minutes followed by 8-minutes' frying; the trans-β-carotene retention was 54%. The authors stated that higher levels of epoxy-β-carotene were found in the fried cassava, but no data were presented.
Gari is the most popular method of cassava processing in sub-Saharan Africa. Gari is a granular flour of varying texture made from cassava roots by peeling, washing, grating, fermenting for approximately four–five days, followed by pressing (to eliminate the water excess), and roasting the pressed cake into granules. Three yellow cassava varieties with an initial β-carotene content of 10.34 μg/g DW were made into gari and 34% (10–58%) of β-carotene was retained (Chavez et al., Citation2007). No information on the temperature and duration of roasting was provided. Gari made from high β-carotene transgenic cassava roots with average initial β-carotene content of 23.60 μg/g DW showed β-carotene retention of 23% (range: 16–30%) (Failla et al., Citation2012). In this study, the cassava was fermented for three days and roasted at high temperature (185°C) until granules reached 80°C and continued roasting at lower temperature (120°C) for 20 minutes. However, in another study, roasting cassava at 195°C for 20 minutes preserved approximately 10% of total β-carotene, whereas the retention was increased to 63% by roasting at 165°C for 10 minutes (Thakkar et al., Citation2009). It appears that the final β-carotene content in gari is a function of the intensity and duration of roasting.
Total carotenoid was measured in raw cassava and after each step of processing cassava into gari in an apparent retention study in Nigeria. The result showed an increase of 187% in the mean total carotenoid in gari compared to the raw cassava (Maziya-Dixon et al., Citation2008). The authors attributed the increase in TC concentration after processing to unaccounted moisture and soluble solid losses.
It is common practice in Southern Nigeria to add red palm oil during the roasting stage of gari production, which gives the finished gari a light yellow hue and particular taste characeristics. Red palm oil is a rich source of provitamin A containing average 600 μg/g of β-carotene and 230 μg/g of α-carotene (Trujilo-Quijano et al., Citation1990). No study in the present review had red palm oil added to gari. The yellow gari described in one of the publications was due to the use of yellow biofortified cassava varieties and not to the addition of red palm oil (Chavez et al., Citation2007).
Fufu is another traditional method of preparing cassava in sub-Saharan Africa (Montagnac et al., Citation2009). The entire process involves fermentation of the peeled and chopped roots followed by sieving and sedimentation for 24–72 hours or more, the elimination of excess water by means of a hydraulic press leads to an uncooked paste (uncooked fufu) which can be boiled or steamed and pounded into a cooked paste (cooked fufu). In some instances, the grated paste can be submitted to further fermentation (“double fermentation”) prior to boiling or steaming. The effect of fufu preparation on the pVAC retention was evaluated in three studies (Maziya-Dixon et al., Citation2008; Thakkar et al., Citation2009; Failla et al., Citation2012). Calculated apparent retention ranged from 44% in high β-carotene transgenic cassava (Failla et al., Citation2012) to 82% (Maziya-Dixon et al., 2008) and greater than 100% (Thakkar et al., Citation2009). Thakkar et al., Citation2009 found the ratio of 13-cis to 9-cis β-carotene to be similar in raw cassava and fufu, suggesting that the fermentation and the 10-minutes' boiling to prepare fufu were not sufficient to induce isomerization.
Flour. In Brazil, where 80% of the total cassava root production is used for cassava flour, the retention of total carotenoid was reported as 49% (29–67%) after cassava roots from five varieties with approximately 12 μg/g DW of total carotenoids were processed and oven dried at 120–150°C for 30 minutes (Oliveira et al., Citation2010). In a recent study with high β-carotene transgenic cassava roots (23.6 μg/g DW of β-carotene), the retention of β-carotene was higher in nonfermented flour 60% (35–78%) compared to the fermented flour 48% (39–56%). In both methods the cassava was dried in the oven at 37°C for 24 hours (Failla et al., Citation2012).
MAIZE
The majority of maize produced and consumed in Africa is white maize, which is essentially devoid of carotenoids. In Zambia, maize is by far the most important food crop, with production reaching a record high of 2.85 million tons in 2011/12 (FAOSTAT, 2014). It is mainly produced at the subsistence level for local household consumption, with only about 20% of rural households selling some of their maize after satisfying their household needs. The VAD problem persists in Zambia despite the biannual vitamin A supplementation program and the vitamin A sugar fortification which were introduced in 1992 and in 1998, respectively. The VAD prevalence among children aged less than five years reported in the past two National Surveys was 66% (NFNC, 1997) and 54% (NFNC, 2003). In our survey conducted in 2009 in two rural districts of Zambia, children showed a 48% prevalence of VAD with infection-adjusted plasma retinol (Hotz et al., Citation2012a).
This paper examines the retention of pVAC in maize after drying, storage, and traditional cooking methods, mainly porridge in Africa and nixtamalization, a cooking process commonly used in Mesoamerica.
Drying. The effect of drying on total carotenoid degradation was evaluated in three yellow dent inbred maize lines (A619, CG102, and CG60) and four high carotenoid lines (HiC-5, HiC-7, HiC-23, and HiC-26) from the breeding program at University of Guelph in Canada (Burt et al., Citation2010). The maize was dried in the oven either at 90°C for 8 hours or at 25°C (ambient temperature) for 72 hours, and the control samples frozen at –80°C for 48 hours and then lyophilized for another 48 hours. Surprisingly, none of the genotypes exhibited greater carotenoid loss at 90°C than at 25°C with an average of between 55 and 85% retention (Table 3). In an unpublished study the pVAC levels of two genotypes were compared after maize was dried under the sun as grain or cob, but no difference was found between the two genotypes (Natalia Palacios, CIMMYT Global Maize Program, oral communication). More studies are currently being carried out to better understand the effect of drying on the carotenoids retention in maize.
Storage. Maize can be stored from one harvest to the next, which is equivalent to 6–12 months, depending on the number of growing seasons per year in any given agro-ecological zone. After six months of storage, the average total carotenoid retention among four inbred lines of maize was reported to be 58% (Weber et al., 1987). The genotype with the lowest initial total carotenoids content (27.4 μg/g DW) showed the highest retention (67%). The degradation of total carotenoids in two genotypes (HiC-7 and HiC-26) was assessed in triplicate samples at 0, 3, 6, and 18 months of storage in the dark at 4°C and 35% relative humidity at the University of Guelph (Burt et al., Citation2010). Total carotenoids remained constant for the first three months but declined significantly by six months, remaining stable thereafter for an overall total carotenoid loss of 35–40%. In another experiment by the same authors, six genotypes were freeze-dried, fast oven-dried (90°C) or dried at ambient temperature (25°C) and stored for four months at 25°C in the dark. At four months, the total carotenoid retention ranged from 76% for HiC-23 at 90°C to 39% for HiC-5 at 25°C (Burt et al., Citation2010). In an unpublished study of two maize genotypes in which storage was not performed in dark conditions, 40–70% of the carotenoid content was degraded in the first four months. At four months, there was a significant genotype effect with retention ranging from 45 to 93% according to genotype; the genotype with lower initial β-carotene concentration had the higher retention at four months of storage. However, after 12 months of storage both genotypes displayed 90% degradation at either 22°C or 37°C (Natalia Palacios, CIMMyT, oral communication, unpublished data).
The data just presented seem to support the genotype-based classification proposed by Burt (Burt et al., Citation2010). The authors categorized the different genotypes into three groups: those with high losses due to drying and low loss due to storage (CG60, HiC-7); those with low losses due to drying, but high loss due to storage (CG102, HiC-5); and the intermediate genotypes with moderate losses due to both drying and storage (A619, HiC-23). The authors concluded that the understanding of the genotype effect has the potential to guide the development of high carotenoid maize inbred lines with good stability during both drying and storage.
Cooking
Porridge preparation from maize involves soaking, milling, and grinding of kernels. This staple food is often consumed as many as three times a day in many settings in Africa, Latin American, and Asia (Rooney & Serna-Saldivar, Citation2003). In certain African countries such as Benin, Ghana, Nigeria, and Togo, fermentation is also performed prior to boiling in a preparation known as Ogi (Jespersen et al., 2003). The effect of fermentation on pVAC degradation was evaluated in high β-carotene maize inbred lines (Li et al., Citation2007). Maize kernels were soaked in water at room temperature for 24 hours and wet milled into fine flour. The wet flour was fermented at room temperature in the dark for 48 hours (for fermented maize samples only). Fermented and unfermented slurries were heated at 93°C for 9 minutes in order to prepare the porridge. The apparent retention was measured at each stage of porridge preparation and found that the fermentation step prior to cooking reduced the β-carotene degradation during the cooking step. In summary, 7.3% of β-carotene was lost during soaking and milling. Spontaneous fermentation over 48 hours at 30°C in the dark (pH 4) caused an additional 10.2% β-carotene reduction, followed by 6.9% degradation during cooking. The unfermented slurry lost 18% of β-carotene during cooking. Therefore, the cumulative β-carotene loss of 24.5% for the fermented porridge (7.3% soaked/milled + 10.2% fermentation + 6.9% cooked) was almost identical to the 24.8% loss from the unfermented porridge (7.3% soaked/milled + 17.5% cooked). Fermentation does not adversely affect the retention of pVAC carotenoids in porridges prepared with high β-carotene maize.
Another study evaluated the effect of making thick and thin porridge from yellow maize on the level of degradation of individual carotenoids (Muzhingi et al., Citation2008). Unfortunately, the differences in moisture content and solid loss were not taken into consideration between the raw and cooked products and the concentration of all individual carotenoids were greater in the porridge than the raw uncooked maize flour. The results are shown in .
Table 2 Retention of carotenoids in cassava-effect of cooking
Table 3 Retention of carotenoids in maize-effect of drying and storage
Table 4 Retention of carotenoids in maize-effect of cooking
Nixtamalization. In Central America and Southern Mexico, maize is most commonly prepared as tortilla in a process involving nixtamalization (overnight steeping of maize in calcium hydroxide solution) followed by partial removal of pericarp and germ, grinding into a thick dough, which is molded by hand or machine into flat cakes that are roasted on an earthenware or metal flat plate (Rooney et al., Citation1987). Although losses in dry matter, thiamine, riboflavin, niacin, fat, and fiber are known to occur, alkali-cooking can, on the other hand, improve flavor, and the bioavailability of niacin and calcium (Bressani et al., Citation1990).
The pVAC retention levels observed by De la Parra et al., Citation2007 after the dough was deep-fried were much lower (18% for the high carotenoid variety, and not detected for the remaining maize varieties) than the 64% average reported by Lozano-Alejo et al., Citation2007. While time, temperature for the deep-frying, and a number of other variables were similar in both studies, differences that could explain the divergent results include: initial carotenoid levels in the maize, genotype, and time period the maize was lime-cooked prior to soaking. The highest pVAC maize (0.57 μg/g DW) evaluated by De la Parra et al., Citation2007 had lower carotenoid levels when compared with the lowest pVAC maize (0.70 μg/g DW) evaluated by Lozano-Alejo et al., Citation2007. The highest pVAC maize evaluated by Lozano-Alejo et al., Citation2007 had the highest retention (85%), but this was not a trend observed among the other 13 genotypes tested. Lastly, the boiling time of 25–50 minutes prior to steeping used by de la Parra et al., Citation2007 may have helped to increase the carotenoid degradation. For future analysis, we recommend the continued evaluation of various genotypes with their clear specifications reported, measurement of individual carotenoids at each step of the cooking process (steeping, baking, and frying), and recorded alterations of these processes by time and temperature to gauge their impact on pVAC concentrations—particularly when comparing commercial to household use.
SWEET POTATO
Sweet potato (Ipomoea batata (L.) Lam.) is a major staple crop in sub-Saharan Africa, with more than 100 million metric tons produced per year (FAOSTAT, 2012). The biofortified orange sweet potato varieties have β-carotene content ranging from 30 to 100 ppm, in contrast to the 2 ppm in local varieties of Mozambique and Uganda. The biofortified varieties could help to reduce the high rates of VAD prevalence among children 5–69 months estimated as 69% and 20% in Mozambique and Uganda, estimated as 69% and 20% in children aged 6–59 months, respectively. In fact, it has been estimated that consumption of biofortified orange sweet potato in Uganda could eliminate between 40–66% of the burden of VAD as measured by disability adjust life years (DALYs) (Meenakshi et al., 2010).
Retention studies were undertaken with orange sweet potato varieties that were considered for release in Mozambique (Resisto, MGCL-1, Jonathon, Gaba-Gaba) and Uganda (Ejumula, SPK004, SPK004/6, and SPK004/6/6). The main cooking method of orange sweet potato in Mozambique is boiling, while in Uganda involves boiling and steaming; however, four additional cooking methods of orange sweet potato (frying, roasting, microwaving, baking) were evaluated and are included in the review. In both countries, sweet potato is also dried as chips to make sweet potato flour for use in cooking after the harvest season; therefore, extensive studies of β-carotene retention following drying and throughout storage of the dried products were carried out and are presented here ( and ). The retention of sweet potato pVAC was reviewed recently (Boy & Miloff, Citation2009).
Table 5 Retention of carotenoids in sweet potato- effect of drying
Table 6 Retention of Carotenoids in Sweet Potato-Effect of Storage
Sun drying. The pVAC retention in sweet potato ranged from 66 to 95% after sun drying (Bengtsson et al., Citation2008, Bechoff et al., Citation2009, and Bechoff et al., Citation2010a, Bechoff et al., 2011a). A study conducted with American orange sweet potato reported retentions of 67 and 66% for total carotenoid and β-carotene, respectively, after sweet potato chips were dried for 8 hours under the sun (Bechoff et al., Citation2009). The fact that the weather was hot (29°C) and dry (39% humidity) allowed the sweet potato chips to dry in a shorter time, where traditionally in sub-Saharan African it would take 2–3 days, resulting in pVAC retention not significantly different from those obtained from solar drying.
In Uganda, seven improved sweet potato roots (variety Ejumula) had an average 84% β-carotene retention after sun-drying, which was not significantly different from the 88% retention from oven-dried samples or the 91% retention from solar-dried samples (Bengtsson et al., Citation2008). The time required for drying the samples was equivalent among the three methods, 6 to 10 hours for sun- and solar-drying, and 10 hours for oven-drying.
The environmental conditions for sun-drying had a significant effect on the pVAC retention (Bechoff et al., Citation2010a). Dry weather resulted in significantly higher (p < 0.05) carotenoid retention (90 and 95%) compared to wet weather (87 and 93%) in Ugandan sweet potato varieties Kakamega and Ejumula, respectively. This difference may have to do with the shorter sun exposure due to dry weather. During dry weather, the time required to dry the samples was almost half (5 hours) of that required during wet weather (9 hours).
In Mozambique, 95% of total carotenoid was retained after sun-drying for sweet potato variety MGCL01, independent of the thickness of the chips (Bechoff et al., 2011a). Sweet potato variety Resisto showed lower retention, 84% (thin chips) and 75% (thick chips), of total carotenoids after sun-drying. It is important to note that the Resisto variety required a greater time to dry than the MGCL01 variety, which had a higher retention rate.
Solar drying is achieved by direct sun radiation on the material covering the solar dryer, reaching internally higher temperatures, and similar or slightly lower humidity as the outside environment. The pVAC retention in sweet potato ranged from 77 to 96% after solar-drying (Mdziniso et al., Citation2006, Bengtsson et al., Citation2008, Bechoff et al., Citation2009, Bechoff et al., Citation2010a, and Bechoff et al., 2011a). In three studies, the pVAC retention from solar-drying was not significantly (p < 0.05) different from sun-drying (Bengtsson et al., Citation2008, Bechoff et al., Citation2009, Bechoff et al., Citation2010a). In one study, solar-drying showed lower total carotenoid retention in sweet potato variety MGCL01 (89%) compared to sun-drying (95%) (Bechoff et al., 2011a). Mdziniso et al., Citation2006 only measured the effect of solar-drying on carotenoid retention and, therefore, cannot be compared to sun-drying. The use of UV-resistant polythene material on the solar dryer did not increase carotenoid retention as compared to solar dryer with non-UV resistant materials or sun drying (Bechoff et al., Citation2010a). The authors concluded that direct sun exposure is as efficacious as solar drying in terms of pVAC retention in sweet potato.
Hot air drying was conducted with a wood device that has a heating system composed of a butane gas jet and centrifuge fan to circulate hot air 42°C (24–45°C) through the trays. In 2 hours the sweet potato chips reached 11% moisture and presented a higher retention of total carotenoids (87%) and β-carotene (84%) when compared to sun drying (67 and 66%), but was not significantly different from solar drying (79 and 77%) (Bechoff et al., Citation2009).
Shade drying is used to prevent direct sun exposure during drying with the purpose of reducing or preventing carotenoid degradation. Chips of sweet potato roots variety Zapallo dried outdoors in shade for 5 hours had 79% β-carotene retention, which reduced to 62% after the chips were made into flour (Kidmose et al., Citation2007). Although 13-cis-β-carotene was present in all three samples (raw, chips, and flour), 15-cis-β-carotene was present after the sweet potato was dried as chips and made into flour, and the 9-cis-β-carotene was present in the flour only.
One comparative analysis of shade-drying with two other methods (solar and sun), shade drying reported higher total carotenoid retention (92–100%) when compared to sun-drying (84–95%) and solar-drying (85–89%) between two sweet potato varieties in Mozambique (Bechoff et al., 2011a).
Oven drying of sweet potato roots resulted in average of 85% pVAC retention (range: 70–96%) (Hagenimana et al., Citation1999; Nascimento et al., Citation2007; Bengtsson et al., Citation2008). The β-carotene retention of sweet potato roots variety Ejumula oven-dried at 57°C for 10 hours was reported as 88% (Bengtsson et al., Citation2008). At higher temperature/time (65°C for 12 hours) of oven-drying the reported total carotenoid retention was 70% (Hagenimana et al., Citation1999). In addition to the differences in temperature and time, the study by Hagenimana and colleagues measured total carotenoids by spectrophotometer. Lastly, the true retention of β-carotene was 96% for sweet potato and 91% for cassava after these crops were oven-dried at 70°C (time was not reported) (Nascimento et al., Citation2007). One study was not included due to a pre-steaming step (Wu et al., Citation2008).
Storage
Twenty-five sweet potato cultivars from CIP (International Potato Center) were evaluated for the retention of total carotenoids after the sweet potato was cut into chips, dried, and stored at room temperature for 11 months (Hagenimana et al., Citation1999). The total carotenoid content was as low as 2 and as high as 632 μg/g DW that corresponded with a range white, yellow, orange, and purple flesh root colors. The average retention of total carotenoid in dried sweet potato chips was 70%, 64%, and 57% after drying for 3, 6, and 11 months of storage, respectively.
On the other hand, dried sweet potato chips packaged using different packaging materials presented variable total carotenoid retention, ranging from 23 to 36% over a 4-month period of storage at room temperature (Bechoff et al., Citation2010a) (). None, however, resulted in statistically different total carotenoid retention. There was also no significant genotypic effect on difference in retention among the six cultivars tested (Ejumula, Kakamega, SPK004/1, SPK004/1/1, SPK004/6 AND SPK004/6/6). The authors noted that none of the cultivars would provide a significant source of vitamin A to the diet after storage for four months even if 100g of finished product were consumed by children.
Table 7 Retention of carotenoids in sweet potato-effect of cooking
Another study performed in rural Mozambique examined sweet potato chips dried and stored using traditional methods, by hanging jute bags inside a house constructed from mud (Bechoff et al., 2011a). No effect of the chipping (thin, thick, or sliced by hand) was observed on the total carotenoid retention during storage with overall average retention of carotenoids after one, two, and four months of storage of 67.0%, 44.9%, and 16.3%, respectively. However, a significant effect of sweet potato variety was observed between MGCL01 and Resisto. The total carotenoid retention after 31, 62, and 125 days of storage was 61%, 37%, and 12%, respectively, for MGCL01, and 73%, 52%, and 21%, respectively, for Resisto. The variety Resisto also had a higher initial carotenoid content (366.2 μg/g) compared to MGCL01 variety (223.6 μg/g), which makes it a better variety for high carotenoid retention over a period of storage.
Degradation of carotenoids in dried foods has been demonstrated to follow a first-order kinetics. Bechoff et al., Citation2010b developed a model to predict the thermal degradation of β-carotene in sweet potato chips during storage at temperatures between 10–40°C taking into account oxygen and water activity. The kinetic model showed that the breakdown of trans-β-carotene followed this first-order kinetic's pattern. The authors tested how well the model predicted the pVAC degradation by storing dried Ejumula sweet potato chips in a laboratory inside of a jar for 88 days at room temperature. The difference between the experimental and the predicted value was 4.3% for total carotenoids and 3.5% for β-carotene. The total carotenoid and β-carotene retention measured after dried sweet potato chips were stored in a jar in the dark for 88 days was 41% and 38%, respectively. When the model was compared to data published in the literature (Bechoff et al., Citation2010a) where dried sweet potato was stored for 125 days under field conditions in Uganda, the difference between experimental and predicted values for total carotenoids was 9.3%.
Pretreatments to prevent carotenoid degradation in sweet potato have been evaluated (Nascimento et al., Citation2007 and Bechoff et al., 2011b). Blanching, a pretreatment to inactive enzymes, was carried out with sweet potato slices by immersion into boiling water for 1.5 minutes prior to drying and storage. Blanching sweet potato slices did not reduce the initial levels of β-carotene, as it did with cassava (16% loss), but it enhanced the stability of β-carotene of the dried sweet potato slices when stored at room temperature (Nascimento et al., Citation2007). After 20 days of storage, 45% of β-carotene was retained in unblanched samples as opposed to 80% retention in blanched samples. Blanched samples reached 50% β-carotene retention only at 70 days of storage. However, in a recent study, blanching of sweet potato for 8–11 minutes showed higher retention, but it was revealed that leaching of solids occurred during blanching and that was responsible for artificially increasing the retention levels (Bechoff et al., 2011b).
Pretreatment of dipping sweet potato chips with chemicals, such as sodium metabisulfite, acids (ascorbic acid, citric acid), and salt, singly or in combination, had little effect on carotenoid preservation after six-month storage (Bechoff et al., 2011b). The authors concluded that even though the pretreatment was effective, the soaking portion had a negative impact on the carotenoid content after drying.
Cooking
Boiling sweet potato for about 20 to 30 minutes had a minor effect on the pVAC degradation as the reported retention of total carotenoids/β-carotene ranged between 80–90% (Hagenimana et al., Citation1999; Nascimento et al., Citation2006; van Jaarsveld et al., Citation2006; Kidmose et al., Citation2007; Bengtsson et al., Citation2008; Wu et al., Citation2008). Boiling sweet potato fully immersed in water in a covered pot for 20 minutes had almost a 10% increase in the pVAC retention (92% versus 83%) compared to when only half of the pot was covered with water (van Jaarsveld et al., Citation2006). As expected, the period of boiling had a negative effect on the pVAC retention ranging from 99 to 68% if boiled for 10 versus 50 minutes (Wu et al., Citation2008). The authors demonstrated a strong linear reduction in pVAC content when plotted against boiling time (R2 = 0.97; p < 0.001; n = 5). The usual boiling time for sweet potato in Mozambique and Uganda is around 20–30 minutes, which is associated with 92–87% retention (Wu et al., Citation2008). Isomerization of 1% of trans-beta-carotene into 13-cis-β-carotene occurred during boiling raw sweet potato and increased to about 12% in mashed, boiled sweet potato (van Jaarsveld et al., Citation2006). The results of the studies are presented in Table 7.
Steaming sweet potato for 30 minutes resulted in pVAC retention in the range of 69–77% (Bengtsson et al., Citation2008, Wu et al., Citation2008, Tumuhimbise et al., 2009). Wu et al., Citation2008 reported similar pVAC retention for boiling and steaming until 30 minutes of cooking, at which point steamed sweet potato experienced a 17% greater reduction in pVAC content. Using a traditional steaming method of slicing sweet potato and wrapping in banana leaves, Bengtsson et al., Citation2008 documented that after 30 minutes of steaming the pVAC retention levels (77%) were roughly comparable to those in sweet potato boiled for 20 minutes (78% pVAC retention). Banana leaves seemed to have offered no protection against the high heat energy of steam in this time frame.
Frying over a short period of time (1 to 10 minutes) was found to be a relatively nondestructive process for pVAC content with an average of 70% retention (Range: 67–78%), Nascimento et al., (Citation2006), Bengtsson et al., (Citation2008) and Wu et al., (Citation2008). Although the pVAC retention did not decrease within a short frying period of 1 to 3 minutes, isomerization did increase significantly (p < 0.05) from 2.4 to 15% (Kidmose et al., 2006).
Roasting whole sweet potato on a grill was found to be less damaging to the pVAC content (85% retention; range: 49–100%), Kidmose et al., (Citation2007), than baking in the oven (69% retention) (Chandler and Schwartz, Citation1988). However, isomerization of β-carotene to the cis form was 12 and 11% when roasting or baking sweet potato, respectively. A moderate correlation was found between pVAC retention and cooking time (R2 = 0.36; p < 0.001).
THE EFFECT OF PROCESSING AND STORAGE ON CAROTENOID RETENTION
Drying Effect
The degradation of all-trans-β-carotene during drying are probably caused by oxidative degradation which is promoted by the presence of oxygen, high temperature, low water activity, and the presence of light, while isomerization appeared to have a minimum effect on degradation during the drying process (Bechoff et al., Citation2010b). No significant increase in the cis-isomers was observed when sweet potato was dried by three different methods: hot-air, solar, and sun-drying (Bechoff et al., Citation2009). In another study, cis-isomers corresponded to only 1% and 3% of total β-carotene content after sweet potato was dried as chips and flour, respectively, with the 13-cis- β-carotene as the predominant isomer, while only small amounts of 9-cis- β-carotene were detected in flour (Kidmose et al., Citation2007). Only when sweet potato had been heated prior to drying did the cis-isomers accounted for a large share (29%) of the total β-carotene content (Chandler & Schwartz, Citation1988).
The higher temperature-short time combination rather than low temperature-longer time resulted in higher pVAC retention in sweet potato (Bengtsson et al., Citation2008; Bechoff et al., Citation2009) and maize (Burt et al., Citation2010). Similar results have been reported for solar-dried mangoes, where the prolonged drying time at lower temperature reflected in a lower retention of all-trans-β-carotene and an increased isomerization rate (Pott et al., Citation2003). In this study, mango slices of cultivars Kent and Tommy Atkins dried at 75°C for 3–3.5 hours presented 93 and 69% retention of all-trans-β-carotene, respectively, compared to a lower retention, 66% and 58%, for cultivars Namdok Mai and Kaew, respectively, when dried for a longer period of time (7–8 hours) at a lower temperature (62°C). Furthermore, the 13-cis-β-carotene increased by 19% and the 9-cis-β-carotene was negligible when mango slices of Kent cultivar were dried in the dark; with sun exposure, the formation of 9-cis-β-carotene increased, representing 64% and 51% of total cis-isomers, respectively, for Kaew and Namdok Mai cultivars. Unfortunately, the genotype effect was a confounder in this study and could not be taken into account.
The high variability in the pVAC retention after drying (55–85% for maize, 10–99% for cassava, and 66–96% for sweet potato), was associated not only with the drying method applied, temperature, and duration of exposure to heat but also with the differences in genotype (Clydesdale and Francis, Citation1976; Britton, Citation1992). The only physical attribute that has been associated with genotype in the literature has been the differences in the dry matter content. Significant correlations between initial dry matter content and carotenoid losses (Pearson coefficient R = –0.518; p < 0.05) and between initial total carotenoid content in fresh sweet potato chips and carotenoid losses (Pearson coefficient R = 0.589; p < 0.05) have been observed (Bechoff et al., Citation2009). The authors suggested that there is a linkage between both initial dry matter and initial carotenoid content and percentage loss of carotenoid during drying. Bengtsson et al., Citation2008 suggested that for an equivalent drying time, cultivars with higher moisture content and with higher initial carotenoid content tended to lose more carotenoids during the drying process. Mdziniso et al., Citation2006, also observed that sweet potato exhibited the least β-carotene loss (5–6% dry basis) whereas carrots exhibited the highest (49–68%), and their respective fresh moisture contents were 76 and 91%.
Overall, shade-drying was not as damaging to the carotenoid content in cassava as drying under the sun and it may be a better option for drying cassava in the rural environment where oven drying is not available (Chavez et al., Citation2007). On the contrary, drying sweet potato under the sun did not show a significant difference in the pVAC retention levels as compared to solar-drying (Bengtsson et al., Citation2008; Bechoff et al., Citation2009). Only one study (Burt et al., Citation2010) investigated the pVAC retention in maize after drying, showing that high heat (90°C) was not more detrimental than low heat (above 25C°) when maize was dried in the oven. A study with cowpea leaf and mango showed greater β-carotene retention when dried by solar-dryer than under the sun, furthermore, blanching cowpea leaves improved the β-carotene retention by 15% (Ndawula et al., Citation2004). In cassava, the blanching process reduced the initial levels of β-carotene (84% retention), but not in sweet potato (100% retention) (Nascimento et al., Citation2007). However, blanched samples showed higher β-carotene retention than unblanched samples during storage for both crops. In a most recent study, although blanched samples of sweet potato had the highest carotenoid content after drying compared to other pretreatments, e.g., salt, sodium metabisulfite, and ascorbic acid, the authors concluded that blanching artificially increased the level of retention by soluble solids that occurred during the process (Bechoff et al., 2011b). Blanching sweet potato for 2 and 10 minutes has been shown to reduce the solid content by 15 and 28%, respectively (Chandler and Schwartz, Citation1988).
In conclusion, in rural settings, sweet potato can be dried under the sun with little detriment to the pVAC levels as compared to other drying methods, while shade-drying is recommended for cassava. More research is needed to recommend the best drying method for maize. Therefore, the drying method of choice for better pVAC retention and most cost-effective will depend on the crop and the specific genotype. Future research should investigate the genotype-related elements that influence the higher or lower pVAC retention in those crops during the drying process so that the traits associated with protection against degradation may be included in the biofortification plant-breeding program.
Storage Effect
Storage conditions and duration had a greater negative impact on pVAC retention in cassava, maize, and sweet potato compared to drying and cooking. After one month of storage, cassava flour and chips presented 40 and 20% of the original pVAC levels when oven-dried and sun-dried, respectively (Chavez et al., Citation2007). Specific maize genotypes stored in the dark for four months showed 60–65% pVAC retention in one study (Burt et al., Citation2010); and other genotypes stored under subtropic conditions (20°C/20% RH) during a similar period displayed, pVAC retention between 30 and 60% (unpublished data). The case was less dramatic for sweet potato. The Resisto variety on average showed 10% higher pVAC retention (73%, 52%, and 21%) compared to the MGCL01 variety (61%, 37%, and 12%) after one, two, and four months of storage as chips, respectively (Bechoff et al., 2011a). Although at this point it is not clear what makes one variety more resistant to pVAC degradation than another, it is recommended that a retention study be conducted as part of the screening process to select the best biofortified variety for release and that the judgment not be solely based on the high pVAC content. Additionally, because of the intrinsic instability of carotenoids and retinoids, storage practices of pVAC crops should be optimized when they are deployed in target countries.
Autooxidation was the suggested main mechanism of carotenoid degradation during storage of dried sweet potato (Bechoff et al., Citation2010b). The authors reported that oxygen, in the range studied of 0–21%, had a greater effect on β-carotene degradation than water activity (0.13–0.76) and temperature (10–40°C). Furthermore, lower β-carotene degradation occurred at higher water activities in sweet potato demonstrating that enzymatic degradation is unlikely to occur due to low enzymatic activity in those conditions. It has also been hypothesized that because the oxidative degradation occurs through a free-radical process that water can have a protective effect on carotenoid stability through its interaction with free-radicals (Haralampu and Karel, 1983). Chen et al., Citation1996 studying stability of carotenoids in stored carrot juice showed that those stored in glass bottles under light had greater β-carotene degradation than those stored in aluminium foil bags (dark); and the formation of 9-cis-β-carotene was favored when the carrot juice was stored under light, while 13-cis-β-carotene was the main isomer when stored in the dark. In this study, higher temperature of storage (35°C vs. 4°C) was also detrimental to carotenoids.
In order to avoid pVAC degradation during storage of sweet potato, Bechoff et al., Citation2009 conducted experiments by using different types of plastic bags (dark vs. clear, or zipped vs. sealed) to store sweet potato chips. There was no difference among all forms of storage with an average of 30% pVAC retention after four months of storage at room temperature. Most recently, several pretreatments with antioxidants and alkaline solutions were applied prior to drying and although there was some improvement in the pVAC retention it was not maintained after four months of storage (Bechoff et al., 2011b). Chavez et al., Citation2007 applied vacuum to the bags of cassava flour prior to storage. The cassava flour packed in plastic bags under vacuum resulted in higher losses than the ones packed in plastic bags without vacuum, probably because the plastic bags were not impermeable to oxygen.
In summary, the pVAC losses during storage were considerable in all three crops. Selection based on the genotype through retention studies is the most practical method available, while more research on better understanding the genetic factors, such as the Or cauliflower gene (Li et al., Citation2012) that makes one variety less susceptible to pVAC degradation is needed as well as studies investigating pretreatments and optimal packaging conditions to decrease pVAC degradation during storage.
Cooking Effect
The average pVAC retention among different cooking methods can be ranked from highest to lowest as: boiling and steaming (80%) > baking (70% for cassava and sweet potato and 30% for maize) > frying (50% for sweet potato, 54% for cassava and 18% for maize) > gari and fufu (30%). Except for frying, which is not typical among the rural African traditional foods, gari and fufu presented the lowest pVAC retention among the cooking processes included in this review. Gari is the most popular form of cassava consumed in West Africa that involves fermentation and roasting (Ezedimna et al., 2008). According to Thakkar et al., Citation2009, the fermentation step had minimal effect on pVAC retention with 92% of total β-carotene remaining after cassava was fermented for three days; however, high levels of isomerization, mainly 13-cis-β-carotene, were evident following the roasting step. The retention was particularly dependent on the temperature/time combination. When cassava was roasted at higher temperature (195°C) for 20 minutes, only 10% of β-carotene was retained. The β-carotene degradation followed a similar pattern whether the biofortified cassava was conventionally bred or genetically modified (Failla et al., Citation2012). Further retention studies are currently underway with conventionally bred pVAC cassava in Nigeria to investigate the steps that are most critical for carotenoid degradation in the processing of gari and the best method to minimize degradation will be recommended.
As observed with gari, fermentation does not seem to have a dramatic effect on pVAC retention in other fermented products of maize and cassava. Thakkar et al., Citation2009 reported that fermentation and brief boiling of cassava during the preparation of fufu did not induce isomerization from all-trans- β-carotene to 13-cis-β-carotene. The same occurred with maize, despite losses during the fermentation phase, the final product of fermented and unfermented maize porridges did not differ significantly in their β-carotene retention (75%) (Li et al., Citation2007).
IMPLICATIONS FOR THE BIOFORTIFICATION INTERVENTION PROGRAM
The overall β-carotene retention in maize stored for four months after harvesting (50%), which may then be wet milled (93%), fermented (90%), and boiled (92%) is approximately 38%. In the biofortification program, the original assumption for retention was 50%, which would provide 50% of the vitamin A estimated average requirement (EAR) for children four –to six years of age and women of child bearing age considering the assumptions for maize consumption and β-carotene bioavailability and bioconversion remained constant. However, the biofortified maize has shown higher bioconversion of β-carotene to retinol: 7:1 (Li et al., Citation2010) and 3:1 (Muzhingi et al., Citation2011) than the 12:1 originally assumed (IOM, Citation2001). All things considered, 15 μg/g of pVAC for biofortified maize remains as the biofortification target which will contribute an additional 50% to the vitamin A EAR of women and children. Regarding extreme cooking effects, such as those practiced for production of gari, as little as 20% of the original pVAC will remain when the gari is finally consumed, which may still provide the 50% vitamin A EAR, given that average per capita consumption of cassava by adult women in rural Nigeria and the bioconversion/bioavailability of β-carotene in cassava are about 900g (FW) and 15%, respectively. Cassava, maize and OFSP biofortified with pVACs are expected to be good sources of β-carotene and biofortification a promising food-based intervention for prevention of vitamin A deficiency in women and children in developing countries.
The pVAC retention varied among maize, cassava, and sweet potato, indicating a difference in the protection or exposure of carotenoids to degradation according to the food matrix. Several studies also showed a strong genotype effect on pVAC retention within the same crop. More research is needed to understand the matrix and genotype effect on the protection of carotenoid degradation from those crops and provide guidance to plant breeders to seek genetic traits associated with these characteristics as well. Therefore, it is necessary to evaluate in more detail the differences among the genotypes to elucidate the reason of the different degradation pattern.
Furthermore, studies evaluating pVAC retention in processed and cooked maize, cassava, and sweet potato can be flawed if raw and cooked samples are not equivalent, extraction efficiency for the raw and cooked samples differ, enzymatic oxidation occurs, and calculation of retention is not corrected for weight changes during cooking. Recommendations for the best practices in retention studies have been compiled in this review.
More recent literature has reported the retention values as both, fresh weight and dry weight (Ceballos et al., 2012). This is a recommendation for future publications as there are different needs and practices within the various scientific research communities.
ACKNOWLEDGMENTS
The authors acknowledge the valuable discussion with Prof. Peter Beyer from Albert-Ludwigs Universität Freiburg and thank Benjamin Uchitelle-Pierce and Amy Saltzman from HarvestPlus for their careful proofreading of the manuscript.
REFERENCES
- Aman, R., Schierber, A. and Carle, R. (2005). Effects of heating and illumination on trans-cis isomerization and degradation of β-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 35:8159–2159.
- Bechoff, A., Dhuique-Mayer, C., Dornier, M., Tomlins, K., Boulanger, R., Dufour, D. and Westby, A. (2010b). Relationship between the kinetics of β-carotene degradation and formation of norisoprenoids in the storage of dried sweet potato chips. Food Chem. 121:348–357.
- Bechoff, A., Dufour, D. and Dhuique-Mayer, C. (2009). Effect of hot air, solar and sun drying treatments on provitamin A retention in orange-fleshed sweet potato. J. Food Eng. 92(2):164–171.
- Bechoff, A., Westby, A., Owori, C., Menya, G., Dhuique-Mayer, C., Dufour, D. and Tomlins, K. (2010a). Effect of drying and storage on the degradation of total carotenoids in orange-fleshed sweet potato cultivars. J. Sci. Food Agric. 90(4):622–629.
- Bechoff, A., Tomlins, K., Dhuique-Mayer, C., Dove, R. and Westby, A. (2011a). On-farm evaluation of the impact of drying and storage on the carotenoid content of orange-fleshed sweet potato (Ipomea batata Lam.) International Journal of Food Science & Technology 46:52–60.
- Bechoff, A., Westby, A., Menya, G. and Tomlins, K. (2011b) Effect of pre-treatments for retaining total carotenoids in dried and stored orange-fleshed-sweet potato chips. Journal of Food Quality 34:259–267.
- Bechoff, A., Poulaert, M., Tomlins, K., Westby, A., Menya, G., Young, S. and Dhuique-Mayer, C. (2011c). Retention and bioaccessibility of β-carotene in blended foods containing orange-fleshed sweet potato flour. Journal of Agricultural and Food Chemistry 59:10373–10380.
- Bengtsson, A., Namutebib, A. and Almingera, M. L. (2008). Effects of various traditional processing methods on the all-trans-β-carotene content of orange-fleshed sweet potato. J. Food Composition Anal. 21(2):134–143.
- Blandino, A., Macías, M. and Cantero, D. (2003). Calcium alginate gel as encapsulation matrix for coimmobilized enzyme systems. Appl. Biochem. Biotechnol. 110(1):53–60.
- Bouis, H. E., Hotz, C., McClafferty, B., Meenakshi, J. V. and Pfeiffer, W. H., (2011). Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 32(1 Suppl):S31–S40.
- Boon, C., McClement J. and Weiss J. (2010). Factors influencing the chemical stability of carotenoids in foods. Critical Reviews in Food Science and Nutrition. 50:515–532.
- Borsarelli C. and Mercadante A. (2010). Thermal and photochemical degradation of carotenoids. In: Landrum J. (ed.) Carotenoids: physical, chemical, and biological functions and properties. CRC Press, Boca Raton, USA, pp 229–253.
- Boy, E. and Miloff, A. (2009). Provitamin A carotenoid retention in orange sweet potato—A review of the literature. Sight and Life Mag. 3:27–33.
- Bressani, R., Benavides, V., Acevedo, E. and Ortiz, M. A. (1990). Changes in selected nutrient contents and in protein quality of common and quality-protein maize during rural tortilla preparation. Cereal Chem. 67:515–518.
- Britton, G. and Khachik, F. (2009). Carotenoids in food. Chapter 3. In: Carotenoids. Volume 5: Nutrition and Health, pp. 45–66. George Britton, Synnove iaaen-Jense, Hanspeter Pfander Eds., Virkhauser Verlag.
- Britton, G. (1992). Carotenoids. In: Natural Foods Colorants, pp. 141–148. Hendry, G. F. Ed., G. F. Blackie, New York.
- Burt, A. J., Grainger, C. M., Young, J. C., Shelp, B. J. and Lee, E. A. (2010). Impact of postharvest handling on carotenoid concentration and composition in high-carotenoid maize (Zea mays L.) kernels. J. Agric. Food Chem. 58(14):8286–8292.
- Ceballos, H., Luna, J., Escobar, A. F., Ortiz, D., Pérez, J. C., Sánchez, T., Pachón, H. and Dufour, D. (2012). Spatial distribution of dry matter in yellow fleshed cassava roots and its influence on carotenoid retention upon boiling. Food Res. Int. 45:52–59.
- Chandler, L. A. and Schwartz, S. J. (1988). Isomerization and losses of trans- β-carotene in sweet potatoes as affected by processing treatments. J. Agric. Food Chem. 36:129–133.
- Chavez, A. L., Sánchez, T., Ceballos, H., Rodriguez-Amaya, D. B., Nestel, P., Tohme, J. and Ishitani, M. (2007). Retention of carotenoids in cassava roots submitted to different processing methods. J. Sci. Food Agric. 87:388–393.
- Chen, B. and Huang, J. (1998). Degradation and isomerization of chlorophyll a and β-carotene as affected by various heating and illumination treatments. Food Chemistry. 62:299–307.
- Chen, H. E., Peng, H. Y. and Chen, B. H. (1996). Stability of carotenoids and vitamin A during storage of carrot juice. Food Chemistry. 57(4):497–503.
- Chuma, I. E. and Nkang M. N. (2008). The effect of quality on gari prices in Nigeria: A hedonic analysis. (2008). J. Food Agric. Environ. 6(1):18–23.
- Clydesdale, F. M. and Francis, F. J. (1976). Pigments. In: Principles of Food Science—Food Chemistry, pp. 417–430. Fennema, O. R. Ed., Marcel Dekker, New York.
- De la Parra, C., Saldivar, S. O. and Liu, R. H. (2007). Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J. Agric. Food Chem. 55(10):4177–4183.
- Egesi, C., Mbanaso, E., Ogbe, F., Okogbenin, E. and Fregene, M. (2006). Development of cassava varieties with high value root quality through induced mutations and marker-aided breeding. NRCRI. Umudike Annual Report 2006:2–6.
- Etejere, E. O. and Bhat, R. M. (1985). Traditional preparation and uses of cassava in Nigeria. Economic Botany. 39:157–164.
- Failla, M. L., Chitchumroonchokchai, C., Siritunga, D., De Moura, F. F., Fregene, M., Manary, M. J. and Sayre, R. T., (2012). Retention during processing and bioaccessibility of β-carotene in high β-carotene transgenic cassava root. J. Agric. Food Chem. 60(15):3861–3866.
- FAO and CIMMYT. (1997). White Maize: A Traditional Food Grain in Developing Countries. Available from http://www.fao.org/docrep/w2698e/ w2698e00.htm#Contents. Accessed: January 2010.
- FAO. (1994). Tropical root and tuber crops. Production, perspectives, and future prospects. FAO, Rome, Italy.
- FAO. (2009). Food Outlook-Global Market Analysis. December 9, 2009. FAO, Rome, Italy.
- FAOSTAT. (2012). Available from http://faostat.fao.org/site/339/default.aspx. Access February 22, 2012.
- FAOSTAT. (2014). http://faostat3.fao.org/faostat-gateway/go/to/browse/Q/QC/E Accessed on August 12, 2014.
- Gouado, I., Demasse, A. D., Ruphine, S. M. O., Some, T. S. and Félicité, T. M. (2008). Provitamin A carotenoid content of dried fermented cassava flour: The effect of palm oil addition during processing. Int. J. Food Eng. 4(11):1–10.
- Hagenimana, V., Carey, E., Gichuki, S. T., Oyunga, M. A. and Imungi, J. K. (1999). Carotenoid contents in fresh, dried and processed sweetpotato products. Ecol. Food Nutr. 37:455–473.
- Haralampu, S. and Karel, M. (1983). Kinetic models for moisture dependence of ascorbic-acid and β-carotene degradation in dehydrated sweet potato. Journal of Food Science. 48:1872–1873.
- Haralampu, S. G. and Karel, M. (1983). Kinetic models for moisture dependence of ascorbic acid and β-carotene degradation in dehydrated sweet potato. J. Food Sci., 48:1872–1873.
- Henry, L., Catignani G. and Schwartz S. (1998). Oxidative degradation kinetics of lycopene, lutein and 9-cis- and all-trans β-carotene. J. Am. Oil Chem. Soc. 75:823–829.
- Hidalgo, A. H. and Brandolini, A (2008). Kinetics of carotenoid degradation during the storage of einkorn (Triticum monococcum L. ssp. monococcum) and bread wheat (Triticum aestivum L. ssp. aestivum) flours. J. Agric. Food Chem. 56:11300–11305.
- Hotz, C., Chileshe, J., Siamusantu, W., Palaniappan, U. and Kafwembe, E. (2012a). Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr. 15(9):1688–1696. doi:10.1017/S1368980012000924.
- Hotz, C., Loechl, C., de Brauw, A., Eozenou, P., Gilligan, D., Moursi, M., Munhaua, B., van Jaarsveld, P., Carriquiry, A. and Meenakshi, J. V. (2012b). A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br. J. Nutr. 10:1–14.
- Hotz, C. and McClafferty, B. (2007). From harvest to health: challenges for developing biofortified staple foods and determining their impact on micronutrient status. Food Nutr. Bull. 28(2 Suppl):S271–S279.
- Howe, J. A. and Tanumihardjo, S. A. (2006). Evaluation of analytical methods for carotenoid extraction from biofortified maize (Zea mays sp.). J. Agric. Food Chem. 54:7992–7997.
- Iglesias, C., Mayer, J. and Chavez, L. and Calle, F. (1997). Genetic potential and stability of carotene content in cassava roots. Euphytica. 94:367–373.
- Institute of Medicine. (2001). Dietary Reference Intakes for Vitamin, A., Vitamin, K., Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine, NATIONAL ACADEMY PRESS, Washington, DC.
- Irvine, F. R. (1969). African Crops, 3rd edition. Oxford Univ. Press, London.
- Jaren-Galan, M., Carmona-Ramon, C. and Mınguez-Mosquera, M. I. (1999). Interaction between chloroplast pigments and lipoxygenase enzymatic extract of olives. J. Agric. Food Chem. 47:2671–2677.
- Kidmose, U., Christensen, L. P. and Agili, S. M. (2007). Effect of home preparation practices on the content of provitamin A carotenoids in coloured sweet potato varieties (Ipomoea batatas Lam.) from Kenya. Innovative Food Sci. & Emerg. Technol. 8(3):399–406.
- Kidmose, U., Yang, R., Thilsted, S., Christensen, L., and Brandt, K. (2006). Content of carotenoids in commonly consumed Asian vegetables and stability and extractability during frying. J Food Comp Anal. 19:562–571.
- Koca, N.; Burdurlu, H. S. and Karadeniz, F. (2005). Kinetics of colour changes in dehydrated carrots. J. Food Eng. 78:449–455.
- Lancaster, P. A., Ingram, J. S., Lim, M. Y. and Coursey, D. G. (1982). Traditional cassava-based foods: Survey of processing techniques. Econ. Botany. 36:12–45.
- Lavelli, V., Zanoni, B. and Zaniboni, A. (2007). Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chem. 104:1705–1711.
- Li, L., Yang, Y., Xu, Q., Owsiany K., Welsch, R., Chitchumroonchokchai C., Lu, S., Van Eck, J., Deng, X., Failla, M. and Thannhauser, T. (2012) The Or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Mol Plant. 5:339–352.
- Li Li et al. (2012) The Or gene enhances carotenoid accumulation and stability during post-harvest storage of e. Molec. Plant. 5(2):339–352.
- Li, S., Nugroho, A., Rocheford, T. and White, W. S. (2010). Vitamin A equivalence of the ß-carotene in ß-carotene-biofortified maize porridge consumed by women. Am. J. Clin. Nutr. 92(5):1105–1112.
- Li, S., Tayie, F. A. K. and Young, M. F. (2007). Retention of provitamin A carotenoids in high carotene maize (Zea mays) during traditional African household processing. J. Agric. Food Chem. 55:10744–10750.
- Low, J. W., Arimond, M., Osman, N., Cunguara, B., Zano, F. and Tschirley, D. (2007). A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J. Nutr. 137(5):1320–1327.
- Lozano-Alejo, N., Carrillo, G. V. and Pixley, K. (2007). Physical properties and carotenoid content of maize kernels and its nixtamalized snacks. Innov. Food Sci. Emerg. 8:385–389.
- Maziya- Dixon, B., Akinyele, I. O., Oguntona, E. B., Nogkoe, S. and Harris, E. (2007). Nigeria Food Consumption and Nutrition Survey 2001 – 2003. Summary. IITA, Ibadan, Nigeria.
- Maziya-Dixon, B., Dixon, A. G. O. and Ssemakula, G. (2008). Changes in total carotenoid content at different stages of traditional processing of yellow-fleshed cassava genotypes. International J. Food Sci. & Technology. 44:2350–2357.
- Mdziniso, P., Hinds, M. J. and Bellmer, D. D. (2006). Physical quality and carotene content of solar-dried green leafy and yellow succulent vegetables. Plant Foods Hum. Nutr. 61(1):13–21.
- Mercadante A. (2008). Carotenoids in foods: Sources and stability during processing and storage. In: Socaciu C. (ed.) Food Colorants: Chemical and functional properties. CRC Press, Boca Raton, USA, pp 213–240.
- Meenakshi, J., Banerji, A., Manyong, V., Tomlins, K., Hamukwala, P., Zulu, R. and Mungoma, C. (2010). Consumer acceptance of provitamin A orange maize in rural Zambia. HarvestPlus Working Paper, number 4. International Food Policy Research Institute, Washington DC, USA, pp. 1–39.
- Montagnac, J. A., Davis, C. R. and Tanumihardjo, S. A. (2009). The nutritional value of cassava for use as a staple food and recent advances for improvement. CRFSFS. 8:181–194.
- Mortensen A. and Skibsted L. (1996). Kinetics of photobleaching of β-carotene in chloroformand formation of transient carotenoid species absorbing in the near infrared. Free Rad. Res. 25:355–368.
- Mulokozi, G. and Svanberg, U. (2003). Effect of traditional open sun-drying and solar cabinet drying on carotene content and vitamin A activity of green leafy vegetables. Plant Foods Hum. Nutr. 58:1–15.
- Murphy, E. W., Criner, P. E. and Gray, B. C. (1975). Comparisons of methods for calculating retentions of nutrients in cooked foods. J. Agric. Food Chem. 23(6):1153–1157.
- Muzhingi, T., Yeum, K. J., Russell, R. M., Johnson, E. J., Qin, J. and Tang, G. (2008) Determination of carotenoids in yellow maize, the effects of saponification and food preparations. Int. J. Vitam. Nutr. Res. 78:112–120.
- Muzhingi, T., Gadaga, T. H., Siwela, A. H.,, Grusak, M. A., Russell, R. M. and Tang, G. (2011). Yellow maize with high β-carotene is an effective source of vitamin A in healthy Zimbabwean men. Am J Clin Nutr. 94(2):510–519.
- Nascimento, P., Fernandes, N. S., Mauro, M. A. and Kimura, M. (2007). Beta-carotene stability during drying and storage of cassava and sweet potato. In: Book of Abstract of the 2nd International Symposium on the Health Benefits of Fruits and Vegetables, Houston, USA. Acta Horticulturae, Wellington, NZ.
- Nascimento, P., Kimura, M. and Fernandes, N. S. (2006). Beta-carotene retention of boiled and fried sweetpotato and cassava. In: Proceedings of 13th World Congress of Food Science and Technology - IUFOST, Nantes, France.
- National Food and Nutrition Commission (NFNC, Zambia). National survey on vitamin A deficiency in Zambia: A random cluster survey study for children (0–5 years) and mothers attending national immunization days in August 1997. Unpublished report, 1997.
- National Food and Nutrition Commission (NFNC, Zambia) and Centers for Disease Control and Prevention (CDC, USA). Report of the national survey to evaluate the impact of vitamin A interventions in Zambia in July and November 2003. Unpublished report, 2004.
- National Population Commission (NPC) [Nigeria] and ORC Macro. (2004). Nigeria Demographic and Health Survey 2003. Calverton, Maryland.
- Ndawula, J., Kabasa, J. D. and Byaruhanga, Y. B. (2004). Alterations in fruit and vegetable β-carotene and vitamin C content caused by open-sun drying, vesqueen-covered and polyethylene-covered solar dryers. Africa Health Sci. 4:125–130.
- Oliveria, R. G. A., Carvalho, M. J. L., Nutt, R. M., de Carvalho, L. V. J. and Gonçalves, F. W. (2010). Assessment and degradation study of total carotenoid and β-carotene in bitter yellow cassava (Manihot esculenta Crantz) varieties. Afr. J. Food Sci. 4(4):148–155.
- Olsen, A., Halm, M. and Jakobsen, M. (1995). The antimicrobial activity of lactic acid bacteria from fermented maize (kenkey) and their interactions during fermentation. J. Appl. Bacteriol. 79(5):506–512.
- Pott, I., Marx, M., Neidhart, S., Muhlbauer, W. and Carle, R. (2003). Quantitative determination of beta-carotene stereoisomers in fresh, dried, and solar-dried mangoes (Mangifera indica L.). J. Agric. Food Chem. 51:4527–4531.
- Rando, R. R. (1990). The chemistry of vitamin A and vision. Angew. Chem. Int. 29:461–480.
- Rodriguez-Amaya, D. B. (1997). Carotenoids and Food Preparation: The Retention of Provitamin A Carotenoids in Prepared, Processed, and Stored Foods.OMNI/USAID, Washington, DC.
- Rooney, L. W. and Serna-Saldivar, S. O. (1987). Food uses of whole corn and dry-milled fractions. Cereal Chemistry.
- Rooney, L. W. and Serna-Saldivar, S. O. (2003). Food use of whole corn and dry-milled fractions. In: White, P.J., and Johnson, L.A. (eds) Corn Chemistry and Technology 2nd ed. American Association of Cereal Chemists, St. Paul, MN, pp 495–535.
- Stratton S. and Liebler D. (1993). Isolation and identification of synglet oxygen oxidation products of β-carotene. Chem. Res. Toxicol. 6: 542–547.
- Teniola, O. S. A. (2001). The effects of processing methods on the levels of lysine, methionine and the general acceptability of ogi processed using starter cultures. Int. J. Food Microbiol. 63:1–9.
- Thakkar, S. K., Huo, T., Maziya-Dixon, B. and Failla, M. L. (2009). Impact of style of processing on retention and bioaccessibility of b-Carotene in cassava (Manihot esculanta, Crantz). J. Agric. Food Chem. 54:1344–1348.
- Theerakulkait, C. and Barret, D. M. (1995). Lipoxygenase in sweet corn germ: Isolation and physicochemical properties. J. Food. Sci. 60:1029–1033 & 1040.
- Trujillo-Quijano, J. A., Rodriguez-Amaya, D. B., Esteves, W. and Plonis, G. F. (1990). Carotenoid composition and vitamin A values of oils from palm fruits. Fat Sci. Technol. 6:222–226.
- Tumuhimbise, G., Namutebi, A. and Muyonga, J. (2009). Microstructure and in vitro β-carotene bioaccessibility of heat processed orange fleshed sweet potato. Plant Foods Hum Nutr. 64:312–318.
- Van Jaarsveld, P. J., Faber, M., Tanumihardjo, S. A., Nestel, P., Lombard, C. J. and Benadé, A. J. (2005). β-carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am. J. Clin. Nutr. 81(5):1080–1087.
- Van Jaarsveld, P. J., Marais, D. W. and Harmse, E. (2006). Retention of β-carotene in boiled, mashed orange-fleshed sweet potato. J. Food Composition Anal. 19(4):321–329.
- Walter, W. M. and Purcell, A. E. (1974). Lipid autoxidation in precooked dehydrated sweet potato flakes stored in air. J. Agric. Food Chem., 22:298–302
- Walton, J., Burton, G., Ingold, K., Lindsay, D. and Moffatt, D. (1993). Oxidative degradation of β-carotene and β-apo-8’-carotenal. Tetrahedron. 49:911–928.
- Weber E. (1987). Carotenoids and tocols of corn grain determined by HPLC. JAOCS 64:1129–1134.
- West, C. E., Rombout, J. M., Van der Ziypp, A. J. and Siytsma, S. R. (1991). Vitamin A and immune function. Proc. Nutr. Soc. 50:251–262.
- World Health Organization. (1996). Micronutrient Series-Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes. WHO, Geneva, Switzerland.
- World Health Organization. (2009). Global prevalence of vitamin A deficiency in populations at risk 1995–2005. In: WHO Global Database on Vitamin A Deficiency. WHO, Geneva, Switzerland.
- World Health Organization. (2010). Micronutrient Deficiencies. Available from http://www.who.int/nutrition/topics/vad/en./ Accessed September 5, 2010.
- Wu, X., Sun, C. and Zeng, L. Y. G. (2008). β-carotene content in sweet potato varieties from China and the effect of preparation on β-carotene retention in the Yanshu No. 5. Innovative Food Sci. Emerg. Technol. 9(4):581–586.