Abstract
Lupinus mutabilis has protein (32.0–52.6 g/100 g dry weight) and lipid (13.0–24.6 g/100 g dry weight) contents similar to soya bean (Glycine max). The Ω3, Ω6, and Ω9 contents are 1.9–3.0, 26.5–39.6, and 41.2–56.2 g/100 g lipid, respectively. Lupins can be used to fortify the protein content of pasta, bread, biscuits, salads, hamburgers, sausages, and can substitute milk and soya bean. Specific lupin protein concentrates or isolates display protein solubility (>90%), water-absorption capacity (4.5 g/g dry weight), oil-absorption capacity (3.98 g/g), emulsifying capacity (2000 mL of oil/g), emulsifying stability (100%, 60 hours), foaming capacity (2083%), foaming stability (78.8%, 36 hours), and least gelation concentration (6%), which are of industrial interest. Lupins contain bitter alkaloids. Preliminary studies on their toxicity suggest as lethal acute dose for infants and children 10 mg/kg bw and for adults 25 mg/kg bw. However, alkaloids can also have medical use for their hypocholesterolemic, antiarrhythmic, and immunosuppressive activity. Bitter lupins can be detoxified by biological, chemical, or aqueous processes. The shortest debittering process requires one hour. This review presents the nutritional composition of lupins, their uses (as food, medicine, and functional protein isolates), toxicology, and debittering process scenarios. It critically evaluates the data, infers conclusions, and makes suggestions for future research.
INTRODUCTION
Lupins (Lupinus spp.) are legumes (Haq, Citation1993) used principally as a protein source in human and animal nutrition (Güémes-Vera et al., Citation2008). According to FAO (Citation2012a) more than 934,426 metric tons of lupin were produced in 2010, in Germany, Poland, the Russian Federation, and Mediterranean countries as well as in Australia, South Africa, and South America. Four major species of lupins are cultivated, namely, Lupinus albus, Lupinus luteus, Lupinus angustifolius, and Lupinus mutabilis, of which the latter has the highest average content of protein (44% dry weight (dw)) and lipids (18% dw) (Pate et al., Citation1985). Lupins can be used as ingredients for many products such as cakes, snacks, hamburgers, biscuits, babyfoods, soups, salads, and substitutes for milk, meat, and soya bean (Cremer, Citation1983; Ruales et al., Citation1988; Villacrés et al., Citation2003; Güémes-Vera et al., Citation2008). Lupin protein isolates and concentrates display physical and functional properties comparable to those of soya bean (Doxastakis, Citation2000). Water and oil absorption; emulsifying capacity, activity, and stability; foaming capacity and stability; and gelation capacity are properties of lupin protein isolate that are valuable to the food and chemical industry (Sathe et al., Citation1982; Gueguen and Cerletti, Citation1994; Doxastakis, Citation2000; Moure et al., Citation2006). Alkaloids from lupins, apart from being toxic in human nutrition, could be useful in medical applications for their immunosuppressive, antiarrhytmic, and hypocholesterolemic capacity (Jiménez-Martínez et al., Citation2003a; Ciesiolka et al., Citation2005). In addition, lupins contain phenolic antioxidant compounds, and prebiotic oligosaccharides, which may favor the proliferation of bifidobacteria (Jiménez-Martínez et al., Citation2003c). However, despite these facts, little is known about the chemical structure, properties, and composition of the four main lupins species (Santos et al., Citation1997), when compared with soya bean (Gueguen and Cerletti, Citation1994). The factor limiting the use of lupins is the presence of quinolizidine alkaloids (QAs) (Jiménez-Martínez et al., Citation2003a), especially in bitter species or subspecies, which have to be removed before consumption (Australia New Zealand Food Authority, Citation2001).
To assess the potential of lupins, particularly of L. mutabilis, this review critically investigates published data on the composition, uses, toxicity, and processing scenarios for the detoxification and debittering of lupin species. Research needs are formulated on the basis of identified knowledge gaps. For each constituent, the published data were converted into the same units, and their average, minimum, and maximum values were calculated and reported.
Varieties of L. mutabilis cited in this study are, apart from a unspecified variety (Aguilera and Trier, Citation1978; Aguilera et al., Citation1983), “H-1” (Bleitgen et al., Citation1979), “Potosi” (Múzquiz et al., Citation1989; Santos et al., Citation1997; Carvalho et al., Citation2005), “Inti” (Gross et al., Citation1988; Santos et al., Citation1997), “2150-Inti” (Gross et al., Citation1988), “Multulopa” (Güémes-Vera et al., Citation2008), “CTC-177–1,” “Cumbre,” “Garz” (Múzquiz et al., Citation1989), “H-6” (Sathe et al., Citation1982), “Kayra” (Torres-Tello et al., Citation1980), and “Sweet Andino 450” (Villacrés et al., Citation2000).
Other lupins cited are L. albus “Multolupa” (Aguilera and Trier, Citation1978; King et al., Citation1985; Agosin et al., Citation1989; Múzquiz et al., Citation1989), “Astra” (Aguilera and Trier, Citation1978; Bleitgen et al., Citation1979), “Tifwhite” (Aguilera et al., Citation1983), “Ares” and “Typ Top” (D´Agostina et al., Citation2006), “SP,” “AL,” and “Kali” (Múzquiz et al., Citation1989); a L. angustifolius unspecified variety (Lqari et al., Citation2002), “Uniwhite” (Múzquiz et al., Citation1989), “Fest,” “Unicorp,” and “LCFM” (Múzquiz et al., Citation1989); a Luzula campestris unspecified variety (Jiménez-Martínez et al., Citation2003a); L. luteus “Aurea” (Aguilera and Trier, Citation1978), “Tremosilla,” “Gyulatanyai,” “SAH,” and “Afus” (Múzquiz et al., Citation1989); Lupinus termis (Rhama and Narasinga, Citation1984); and Lupinus tricolor SODIRO (Castillo, Citation1965).
NUTRIENT COMPOSITION OF LUPINS
Macronutrients
The average moisture content () of whole raw lupin (Lupinus spp.) seeds varies from 8.1–9.4 g/100 g fresh weight. The metabolic energy content varies slightly from 2032 kJ/100 g dw for L. angustifolius, to 2078 kJ/100 g dw for L. albus, and to 2164 kJ/100 g dw for L. luteus. These values are lower than those reported for L. mutabilis (2307 kJ/100 g dw) (Villacrés et al., Citation2000). This could be explained by the higher lipid content reported for L. mutabilis. The average crude protein content in lupins varies from 33.9–43.3 g/100 g dw. The lower value is for L. angustifolius and the higher for L. mutabilis. However, despite the fact that almost all publications agree that the protein content in L. mutabilis is highest amongst the major lupin species; this is based on averages only. When we consider data within the species, we observe, for example, for L. mutabilis, that crude protein ranges from 32.0–52.6 g/100 dw. This wide range in L. mutabilis is associated with genetic and agronomic factors. Indeed (Haq, Citation1993) mentioned that L. mutabilis has a wide genetic variability illustrating adaptation to microhabitats and natural selection. This variability has especially been noted in plant shape, vegetative growth, susceptibility to frost and diseases, protein, oil, and alkaloid content (Haq, Citation1993). Carvalho et al. (Citation2004) grew L. mutabilis “Potosi” in pots with a layer of gravel at the bottom and filled with sandy soil, watered every day, added no fertilizers and obtained seeds with just 11.2% of protein dw, 8.5% of oil dw, and 28.3% of crude fiber dw, showing that a limited availability of nutrients may affect the composition of lupin.
Table 1 Composition of lupin seeds
In addition, total protein content is often (but not always) estimated by multiplying the total nitrogen value by the factor 6.25 (Santos et al., Citation1997). However, according to several authors (Aguilera and Trier, Citation1978; Gueguen and Cerletti, Citation1994), this procedure overestimates the protein values because living tissues and legume seeds in particular, contain considerable amounts of nonprotein nitrogenous compounds and because of the high degree of amidation of these proteins (Doxastakis, Citation2000). Santos et al. (Citation1997) mentioned that a factor of 5.7 would be more suitable as a conversion factor for legume proteins, and for lupin seeds even a lower factor (5.4) was proposed (the difference resulting from the fact that in the case of lupin a portion of the nitrogen measured originates from alkaloids. Gueguen and Cerletti (Citation1994) and Aguilera and Trier (Citation1978) suggested 5.5 and 5.7 as conversion factors, respectively.
The reported lipid content in raw lupins () ranges from 5.5 g/100 g dw in L. luteus to 18.9 g/100 g dw in L. mutabilis. However, among the varieties of L. mutabilis, lipid content may range from 13.0–24.6 g/100 dw. This range in lipid content can be explained at least partially by genetic and agronomical factors (Haq, Citation1993; Carvalho et al., Citation2004). For example, Carvalho et al. (Citation2005) showed that the composition of lupin (and its lipid fraction particularly) can be affected by water stress, i.e., lipid content was reduced by half in conditions of water stress.
Francki et al. (Citation2002) mentioned that total-acid-glycerols (TAGs) are rapidly accumulated during mid-stages of seed development. However, the seeds of late-maturing varieties usually accumulate larger amounts of lipid than those of early-maturing varieties because the plants with a longer growing season have a longer time available to convert carbohydrates into lipids. This is only true, however, if late-maturing varieties get enough time in the field because the last stage of maturation is of critical importance for oil content (Bélteky and Kovács, Citation1984).
The average fiber content varies from 8.2 g/100 g dw in L. mutabilis to 16.0 g/100 g dw in L. angustifolius. We note that L. mutabilis has the lowest average fiber content of the lupin species reported in , and that the fiber content varies widely between lupin species. The reported average values for ash content vary from 3.0 g/100 g dw in L. angustifolius to 3.9 g/100 g dw in L. mutabilis. The variability in content of fiber and ash also can be explained partially by agronomic factors, i.e., crude fiber and ash decreased with about 10% as a result of water stress (95) (Carvalho et al., Citation2005).
The average carbohydrate content in lupin species was reported excluding the fiber content, and varied from 32.9 g/100 dw in L. mutabilis to 47.6 g/100 g dw in L. angustifolius. The differences in carbohydrate content probably can be explained by the same arguments that explain variations in other macronutrients; however, that it is not stated as such in the investigated studies since the carbohydrate content was generally determined by difference (Güémes-Vera et al., Citation2008).
Based on average values presented in , raw L. mutabilis has the highest protein and lipid, and the lowest fiber and carbohydrate content among the major lupin species. However, some minor species such as L. campestris (Jiménez-Martínez et al., Citation2003a) were reported to have similar amounts of protein (44.9 ± 2.0), lipid (13.1 ± 2.0), crude fiber (14.7 ± 1.1), ash (3.5 ± 0.1), and carbohydrate (24.7 ± 1.3 g/100 dw) as L. mutabilis.
Regarding the composition of whole debittered lupins, there is a paucity of published data, except for debittered L. mutabilis and L. campestris. The average composition of debittered L. mutabilis as presented in is higher in crude protein and carbohydrates than in L. campestris debittered by a wet process (Jiménez-Martínez et al., Citation2003a), which contained crude protein 50 ± 0.5, lipids 21.2 ± 0.5, fiber 10.2 ± 0.2, ash 3 ± 0.0, and carbohydrates 15.6 ± 0.2 g/100 dw. All data on the composition of whole debittered lupins were based on wet debittering processes, which obviously cause losses of soluble dry matter into the process water. This then can result in apparent increases of, e.g., the crude protein content due to preferential leaching-out of dissolved carbohydrates and minerals. Regarding the crude lipid content in debittered lupins, there is a different situation. In L. mutabilis the crude lipid content decreases and this might be because of a sort of micelle formation with lecithin present in this lupin (Rozan et al., Citation1997; FAO, Citation2012b). However, fat content in debittered L campestris increases compared to the raw material. We did not find an explanation in literature for this difference.
Regarding the fatty acid composition, the aqueous debittering process apparently does not significantly affect the profile of fatty acids of L. mutabilis (). This is important because lupin species contain approximately 80% of unsaturated fatty acids in the lipid fraction.
So far several reasons have been given for the wide variability in the macronutrient composition of raw and debittered lupins. However, that variability may also be influenced by the method of analysis. A wide array of methods for determining macronutrients was reported, such as gravimetry for estimating moisture, fiber, ash (Güémes-Vera et al., Citation2008); Kjeldahl (Ortiz and Mukherjee, Citation1982) and micro Kjeldahl (Gross et al., Citation1988; Güémes-Vera et al., Citation2008) for crude protein; solvent extractions (Torres-Tello et al., Citation1980; Güémes-Vera et al., Citation2008), and nuclear magnetic resonance (Gross et al., Citation1988) for lipids; and estimation of carbohydrates by difference (Güémes-Vera et al., Citation2008), whereas some authors even omitted to report the methodology used, which precludes an evaluation of exactitude. Moreover, some authors did not specify the variety of Lupinus analyzed, or did not present the standard deviation of the mean values or the range of variation, making it impossible to determine the precision of the results.
Minerals
The number of authors who investigated the mineral composition in lupins is rather low. Whole raw lupins present a mineral composition that shows a wide variability (); especially in the reported manganese content for L. albus (83.5 mg/100 g dw) when compared with other lupins (2.1–8.6 mg/100 g dw). These variations could be partly explained by agronomical aspects. Field studies on the accumulation of mineral elements provide evidence of significant differences between species grown at on the same site and, within a species, when grown on different soil types (Gladstones and Drover, Citation1962; Walton and Francis, Citation1975). The amount of mineral elements absorbed by a crop will obviously depend on its productivity of dry matter and on the availability of specific nutrients in the soil in which it is rooted (Pate et al., Citation1985). For example, a study conducted under greenhouse conditions showed that the lupin plant is sensitive to deficiencies of nitrogen, phosphorus, potassium, calcium, magnesium, sulfur, zinc, iron, and manganese (Rivadeneira et al., Citation2001). These deficiencies determine that the lupin plant can be dwarfed, weak, with necrosis, discoloration, and with lower content of minerals compared with a plant grown without mineral deficiencies (Rivadeneira et al., Citation2001).
In the case of whole debittered L. mutabilis, it can be noted that, with the exception of calcium, iron, and zinc, the other mineral contents are lower than in whole raw lupin. This reduction could be attributed to leaching. Remarkable is the decrease of potassium and magnesium in the debittered product. Perhaps these two minerals were present in a highly soluble chemical form. Increases in calcium, iron, and zinc contents could be due to their presence in the form of poorly soluble complexes with, e.g., phytic acid. Villacrés et al. (Citation2000) also suggests that increases in iron and zinc contents may be caused by contact of the product with debris present in water used for the debittering process.
Variations in mineral content may also be due to the use of different analytical methods. For example, Peñalosa et al. (Citation1991) determined calcium, potassium, magnesium, and phosphorus using an Auto Analyzer. Torres-Tello et al. (Citation1980) determined calcium by permanganometry, iron by orthophenanthroleine, magnesium by complexometry, and phosphorus by spectrophotometry. In addition, authors do not always mention analytical methods and variety of lupin analyzed, nor does they always present standard deviations or ranges. Therefore, we did not attempt to explain all observed differences based on limited information; rather, we gathered the scarce information to obtain an impression of the mineral contents in lupin, and to evaluate which knowledge is lacking.
Amino Acids
The essential amino acid profile of raw lupins shows little variation among species (). However, L. luteus has a higher cystine and leucine content, L. albus a higher tyrosine content, and L. mutabilis a higher lysine content.
Table 2 Amino acid composition of lupin seeds
The content of essential amino acids in debittered lupins was reported only once (Torres-Tello et al., Citation1980) for L. mutabilis (). In some cases, the reported data do not differ greatly from those of raw lupin (namely, for glutamic acid, glycine, isoleucine, and leucine); in others the contents are higher (for lysine, phenylalanine, proline, serine, and threonine), lower (for hystidine, tryptophan, tyrosine, and valine), or much lower (for cystine and methionine). It is suggested that the cystine and methionine contents diminish both when the seed is defatted and when the alkaloid extraction is done in an alkaline environment, as Torres-Tello et al. (Citation1980) did Gueguen and Cerletti (Citation1994). This is in agreement with other authors Cerletti et al. (Citation1978), Liener (Citation1994), Maga (Citation1984), Sgarbiere and Galeazzi (Citation1978) who have also suggested that alkaline processing can alter protein quality due to the possibility of disruption of the protein structure and degradation of some amino acids.
Vitamins
Little information is available on the vitamin content in lupin species. Only one study (Castillo, Citation1965) reported on vitamins in a mixture of raw L. tricolor ‘Sodiro’ and L. mutabilis ‘Sweet’ (). Vitamins in debittered seeds were published by Castillo (Citation1965), Torres-Tello et al. (Citation1980). The carotene content was reported by Castillo (Citation1965) for the debittered mix as 0.6 mg/100 g dw. It is worth noting that the amount of carotene in the whole debittered mix was six times higher than the value reported by the same author in the raw material (0.1 mg/100 g dw). We did not find an explanation for this difference in the bibliography. Perhaps, it is because the author did not analyze the same samples before and after debittering. The author appeared to have taken random samples of raw and debittered lupin from markets. The reported thiamine content varied from 0.01 to 0.6 mg/100 g dw. The riboflavin content varied from 0.02 to 0.5 mg/100 g dw. The niacin content varied greatly from 0.0 to 4.1 mg/100 g dw. The lowest values were reported by Castillo (Citation1965), while the highest were reported by Torres-Tello et al. (Citation1980). Variation in vitamin content in whole debittered lupin could be related to the fact that Torres-Tello et al. (Citation1980) analyzed L. mutabilis “Sweet” and Castillo (Citation1965) analyzed a mix of L. mutabilis “Sweet” and L. tricolor “Sodiro.” In addition, variations might be caused by the debittering process applied. Torres-Tello et al. (Citation1980) boiled L. mutabilis for three times 5 minutes at 100°C, soaked the seeds in alkaline water, and then washed them in running water for 8–12 hours. Castillo (Citation1965) reported that mixed samples were cooked for 12 hours and washed in running water for seven to eight days. Castillo (Citation1965) analyzed mixed samples that stayed roughly 11 hours longer in boiling water and six days more in contact with running water, which might explain losses by leaching.
Table 3 Vitamins in lupin seeds
ALKALOIDS
Diversity and Their Occurrence
Almost 70 different QA have been reported to occur in Lupinus species (Australia New Zealand Food Authority, Citation2001), of which about 28 are free bases (soluble in organic solvents), and the remaining alkaloids are salts (Ortiz and Mukherjee, Citation1982). The most common alkaloids are shown in . QA are bitter and toxic compounds that can be structurally very similar to sweet-tasting molecules. It is agreed that 25 human G protein-coupled receptors mediate bitter taste perception. However, it is also possible to find alternative mechanisms that mediate bitter taste. For example, lipophylic bitter compounds and bitter salts may activate intracellular signals (Rodgers et al., Citation2005).
Table 4 Alkaloids in Lupinus spp.
In lupins, the reported total alkaloid content varies considerably between authors, and species (). On average, the lower total alkaloid content is reported for L. albus (0.186 g/100 g dw) and the higher for L. mutabilis with 2.8 g/100 g dw. However, also in L. mutabilis, low total alkaloid contents have been reported. For example, Haq (Citation1993) reported 0.007 g/100 g dw in L. mutabilis (unspecified variety), and Gross et al. (Citation1988) reported 0.08 g/100 g dw in L. mutabilis “Inti” and 0.018 g/100 g dw in L. mutabilis “2150.”
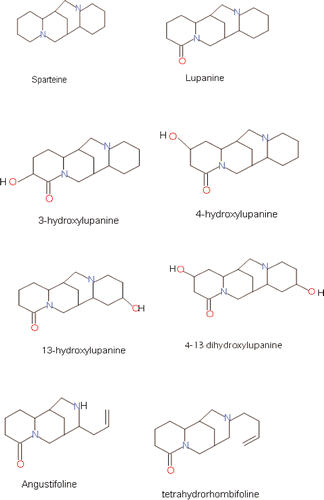
Regarding the diversity of alkaloids in the major lupin species, the principal alkaloid reported is lupanine (C15H24N2O) (). Next, 13-hydroxylupanine (C15H24N2O2) is reported in L. albus, L. angustifolius, and L. mutabilis. Sparteine (C15H26N2) is an important component of L. luteus and L. mutabilis. In L. mutabilis 4-hydroxylupanine, D-lupanine, sparteine, 3-hydroxylupanine, and minor components such as anagyrine, 11–12 dehydroasparteine, dehydrolupanine, and 17-oxolupanine, were also reported. The chemical structures of the most frequently occurring alkaloids are presented in .
The total alkaloid content was determined by titrimetry (INEN Instituto Ecuatoriano de Normalización, Citation2005), gas chromatography (GC) (Nossack et al., Citation2000), high-performance liquid chromatography (HPLC) (Jiménez-Martínez et al., Citation2003a), and capillary gas liquid chromatography (Gross et al., Citation1988), whereas the identity of alkaloids has been elucidated by gas-liquid chromatography (GLC), capillary GLC-mass spectrometry (Hatzold et al., Citation1983), GC and mass spectrometry (Jiménez-Martínez et al., Citation2007), and GC and thin layer chromatography (Múzquiz et al., Citation1989).
Apart from differences due to the analytical techniques used, variation in the reported alkaloid contents and their diversity can be explained, by (i) the fact that the studied species and variety were not always the same; (ii) the presence of a wide genetic variability illustrating adaptation to microhabitats and natural selection such as reported for L. mutabilis (Haq, Citation1993); (iii) environmental and agronomical conditions: favorable moisture conditions would reduce alkaloid content, whereas maritime conditions could be associated with higher levels (Bélteky and Kovács, Citation1984), the amount of nitrogen as well as the intensity of sunlight and temperature might affect the amount of alkaloids directly (Jambrina-Alonso, Citation1983; Wink and Witte, Citation1984) even in maturing seeds (Wink and Witte, Citation1984), and in the shade, the alkaloid content would increase (Bélteky and Kovács, Citation1984); and (iv) the “turnover” effect can affect alkaloid contents within the same plant, depending on the weather, day, and hour of the day (Wink and Witte, Citation1984). The turnover effect is manifested when alkaloids produced in leaves (from carbon and nitrogen) are then transported in the phloem like amino acids and rapidly degraded in the target tissues, which probably use the nitrogen and carbon for the synthesis of storage protein (Wink and Witte, Citation1984). Diurnal variation of QA formation, transport, and turnover was studied in fruiting lupins. In phloem sap of seeds of L. albus, alkaloid contents changed from about 4 mg/g at 17h00 to about 3 at 11h00 and 21h00 (Wink and Witte, Citation1984), showing that apparently QA are not waste or end products, but that they are metabolically dynamic compounds.
Toxicity in Humans
summarizes published data on toxic effects of alkaloids in man. Most of the information comes from reports of accidents. Alkaloids are associated with liver diseases and neuromuscular blockage (Camacho et al., Citation1991) caused by inhibiting the ganglionic transmission impulse of the sympathic nervous system (Jiménez-Martínez et al., Citation2003a). Intoxications with alkaloids can be acute or chronic. Regarding acute intoxication with orally administrated sparteine, one report mentioned that it was mortal in a dose >30 mg/kg body weight (bw), whereas mixed alkaloids orally administered to five people in a dose between 11 and 46 mg/kg bw were lethal for three, and caused a serious intoxication to two (Australia New Zealand Food Authority, Citation2001). Cremer (Citation1983) reported that alkaloid doses between 10 and 25, and 25–45 mg/kg bw were toxic for small children, and adults, respectively. Aguilera and Trier (Citation1978) mention similar toxic levels; however, in this report, the intake by adults was reported as nonfatal poisoning, and the intake by children was reported as fatal. In another study, a single dose of 10 mg of lupanine or 13-hydroxylupanine was administered orally to 11 volunteers. In all subjects, more than 90% of both alkaloids was excreted unchanged via the urine with a half-life of —six to seven hours (Australia New Zealand Food Authority, Citation2001). These results suggest that the minimum lethal acute dose is 10 mg total alkaloids/kg bw for infants and children, and 25 mg total alkaloids/kg bw for adults, respectively.
Table 5 Studies of alkaloids toxicity on humans
On the other hand, when chronic toxicity of lupins is studied in human beings, it should be considered that the use of debittered lupins in Europe and South America over thousands of years would provide indicative evidence of safety (Cremer, Citation1983; Petterson, Citation1998). In fact, nowadays Lupinus spp. are still consumed in the Andean region (Cremer, Citation1983) and around the world FAO (Citation2012b). Information about studies of chronic toxicity of lupin alkaloids in human beings is very scarce. Once a tolerance test was carried out with 20 military cadets in Perú. They received an average daily ration of 60 g of L. albus flour containing <0.02% alkaloids (equivalent with a daily dose of 12 mg alkaloids). That dose was served as 49 different dishes, which were administered during four weeks. Results showed good digestibility of lupin dishes and no significant changes in main blood indicators (Aguilera and Trier, Citation1978).
The maximum allowed total alkaloid content in debittered lupin seed was established as 700 mg/kg seed by the Ecuadorean Institute of Standards (INEN Instituto Ecuatoriano de Normalización, Citation2005). This is higher than the level of 400 or 500 mg/kg seed proposed by Múzquiz et al. (Citation1994) for food and feed use. Jiménez-Martínez et al. (Citation2003a) suggested even a lower maximum level of 300 mg/kg in feed, as higher levels would result “in a decrease in nutrient ingestion and consequently a decrease in animal growth.” In Europe, a daily dose of 0.35 mg/kg bw was reported to be tolerated in adults without adverse effects (Australia New Zealand Food Authority, Citation2001); however, this value was not considered safe for all individuals in the population. Therefore, a factor of 10 was applied to account for the uncertainties in the data and human variations. As a result the provisional tolerable daily intake for humans was suggested as 0.035 mg/kg bw/bw/d (Australia New Zealand Food Authority, Citation2001), which is very different from the 500 mg/d proposed as a safe dose by Aguilera and Trier (Citation1978). This huge difference has important implications for the amount of lupin that somebody would be allowed to eat. For example, considering 0.035 mg/kg/d as the maximum tolerated daily intake as proposed by the Australia New Zealand Food Authority (Citation2001) and the maximum alkaloid content in debittered lupin seeds according to the Ecuadorean Institute of Standards INEN (Citation2005) (0.07%), an adult weighing 70 kg would be allowed to eat a maximum of 3.5 g of (debittered) lupin per day. However, in the Andean region the portion size of debittered L. mutabilis is often much bigger than this amount, i.e., 5–10 times more (personal observation). On the other hand, considering 500 mg/d as the safe dose as proposed by Aguilera and Trier (Citation1978) and again the maximum alkaloid content according to the Ecuadorean Institute of Standards INEN (Citation2005) for debittered lupin seeds (700 mg/kg seed), a 70-kg adult could safely consume 714 g of debittered lupin per day. This shows the uncertainty about a safe daily amount of debittered lupin for human beings, and at the same time points out the necessity to determine this more accurately, especially where lupin is consumed not as just ingredient but also as snack or main dish.
Another toxicity risk associated with lupin consumption is the sometimes lethal effect of phomopsins, mycotoxins that can be formed by the fungus Diaporthe toxica, which occasionally infests lupins. The infested seeds are smaller, discolored, and less dense than noninfested seeds. The phomopsins are concentrated initially in the seed coats, and are not found in the cotyledons until there has been a heavy fungal invasion (Petterson, Citation1998). Clinical effects of phomopsins are functional failure of liver and fatty infiltrations. According to Petterson (Citation1998), the National Food Authority in Australia and the Department of Health in the United Kingdom mention the value of 5 μg phomopsin/kg seed as the maximum amount allowed for human consumption. Since discolored seeds are easily recognized and removed, both by manual grading and machine color-sorting, the only possible risk of phomopsin ingestion would seem to come from the consumption of very lightly discolored seed coats or from lupin flour made with infested seeds (Petterson, Citation1998). A moisture content of lupin seed below 10% () does not favor fungal activity (Petterson, Citation1998); however, the presence of phomopsin is a risk that needs to be considered at all times.
Allergenicity and Antinutritional Factors
A minority of people are lupin sensitive (Petterson, Citation1998). In a skin-shot test on 200 Chilean children using extracts from lupins and other foods, it was shown that sensitivity for lupin (3%) was similar to sensitivity for eggs (3%), wheat (2%), but much less than for cow's milk (8%) or soya beans (22%) (Petterson, Citation1998).
Antinutritional factors such as phytic acid, saponins, and tannins are present in lupins (). The amount of phytate in lupins is too low to be of concern (Petterson, Citation1998). The amount of phytic acid reported for lupins varies from 1.42 to 2.74 g/100 g dw (Múzquiz et al., Citation1989). The small amounts of sapogenins in seeds of lupins are also considered very low and of little concern (Pate et al., Citation1985). The saponin content in lupins of up 1.7 g/100 g dw was reported by Múzquiz et al. (Citation1989) as similar or lower than in soya bean. Concerning the toxicity of tannins, a possible relationship between the presence of condensed tannins and esophageal cancer was suggested (Jiménez-Martínez et al., Citation2003a). Although no-effect levels for tannins on growth have not yet been established, Jiménez-Martínez et al. (Citation2003a) reported that a 0.1% of concentration of tannic acid (a hydrolysable form) in diets given to chickens did not cause any harmful effect. The tannic acid content in L. mutabilis was reported as 58 mg/100 g by Jiménez Martínez et al. (Citation2007). In lupins small amounts of cyanogenic compounds, hemagglutinins and trypsin inhibitor activity were detected but considered not to be of antinutritional significance (Pate et al., Citation1985). Indeed, “several authors have reported the absence of hemagglutin activity in the test based on red blood cells of sheep, chickens, rabbits, and humans type O” (Múzquiz et al., Citation1989). In L. mutabilis the trypsin activity was reported as 1.16 trypsin inhibitor units (T.I.U.), which is considerably lower than in soya bean (30.1 T.I.U.) (Haq, Citation1993). Absence of vicine and convicine, based on a quantitative ultraviolet spectrophotometry test (the vicine and/or convicine detection limit of the test was 0.3 g/kg) (Olsen and Andersen, Citation1978), was reported for the main lupin species (Múzquiz et al., Citation1989).
Table 6 Allergenicity and anti-nutritional factors
Oligosaccharides may be considered antinutritional factors when occurring in large quantities, because they cannot be metabolized by monogastric animals and pass through to the colon, where bacterial digestion may produce carbon dioxide, methane, and hydrogen. The final result is discomfort and flatulence (Petterson, Citation1998), and the enhanced bowel movement may reduce nutrient uptake. The oligosaccharides in lupin species belong to the raffinose family (Petterson, Citation1998). The oligosaccharides found in lupins are stachyose, and raffinose (Múzquiz et al., Citation1989) (). Other reported oligosaccharides in L. mutabilis are verbascose, ranging from 0.8 g/100 g dw (Gross et al., Citation1988) to 4.5 g/100 g dw (Harpal and Gladstones, Citation1986), and ajugose 0.2 g/100 g dw (Andersen et al., Citation2005).
On the other hand, oligosaccharides are also reported to have health benefits because of their role as osmotic regulators in the gastrointestinal tract (Petterson, Citation1998). It will be of interest to investigate which oligosaccharides cause such beneficial effect and at what levels and conditions.
DEBITTERING PROCESSES
Biological Processes
Biological methods to debitter lupin are mainly based on fungal or bacterial fermentation as summarized in . A study on bacterial fermentation performed on L. albus “Multolupa” (Camacho et al., Citation1991) investigated the effects of Lactobacillus acidophilus, L. buchneri, L. cellobiosus, and L. fermentum, which revealed that the alkaloid content could be reduced to 41.1% of the initial value. This reduction was obtained at pH ≤ 4.5 with the strain Lactobacillus acidophilus B-1910. An additional reported benefit of L. acidophilus B-1910 was the reduction of the oligosaccharide content. Moreover, the riboflavin content was increased. Unfortunately, no control experiment was included in this study to assess the loss of alkaloids by leaching (diffusion) only. Santana and Empis (Citation2001) reported the reduction of the alkaloid content in L. albus flour by bacterial fermentation with two unnamed strains (IST20B and IST40D) (not identified but genetically closely related to Acidovorax) (Santana et al., Citation1996). Those strains were isolated from soil that had recently been used to produce L. luteus seed. The maximum QA reduction (50%) was obtained by using 5 g lupin flour in 20 mL of a suspension (IST20B) at pH 7 with a bacteria concentration of ≈ 0.85 g of dry biomass per liter, incubated during 120 hours at 31.2°C. Lactobacillus plantarum species were also reported to reduce the alkaloid content in L. albus (Szakács and Stankovics, Citation1983). In this study, soaked and dehulled seeds were put in contact with different lactic acid bacteria. The best results were reported at 37°C. The alkaloid content was reduced from 1.1% initially to 0.1% after soaking, dehulling, fermenting, and washing the seeds. In this study, the effect of the separate steps on alkaloid reduction was not presented. These results show that the use of different bacteria can reduce the alkaloid content by about 50% when suspensions or slurries of lupin are made. Apparently such acid cultures with a high alpha-galactosidase activity cause significant reductions in the amount of alkaloids (Camacho et al., Citation1991).
Table 7 Debittering processes of lupin and impact on several variables
Fungal fermentation () is mostly carried out as a solid-state fermentation to produce tempeh or other mycelium-penetrated masses by dehulling, soaking, cooking, inoculating with fungal spores, and incubation of the legume seeds. These operations allow proliferation of, e.g., Rhizopus mycelium on and throughout the seed (Jiménez-Martínez et al., Citation2007). It is stated that solid-state fermentation can result in small increases in crude protein, riboflavine, and niacin contents. In addition, the process would decrease oligosaccharides and the QA content (Jiménez-Martínez et al., Citation2007). Peñaloza et al. (Citation1991) produced tempeh on L. mutabilis Sweet inoculated with Rhizopus oligosporus UCW-FF8001 at about 3 × 105 c.f.u./g of cooked beans. The appearance of the tempeh cake was reported as very good and comparable to that of tempeh from soya bean. Unfortunately, the rate of alkaloid reduction was not stated in the data. Jiménez-Martínez et al. (Citation2007) also made tempeh with L. mutabilis. In this study, 50 g of washed lupin seeds were soaked for 10–18 hours in a watery solution of lactic acid (1 g/L), washed again, dehulled, and autoclaved at 121°C and a pressure of 1 kg cm−2 during five minutes in a fresh solution of lactic acid of an equal concentration. Next, the seeds were washed and inoculated with R. oligosporus NRRL-2710 and incubated at 30°C for 48 hours. After soaking and cooking the alkaloid content was reduced to 65% of the initial value, and after fermentation to 9% of the initial value (7.9 g/kg). Agosin et al. (Citation1989) reported a study carried out on bitter (8.0 g/kg dw QA) and sweet (0.3 g/kg dw QA) L. albus “Multolupa.” The seeds were dehulled, 5 mm-ground, and inoculated with Rhizopus oligosporus NRRL 2710 spores. The complete process took two-hour energy (100°C) for cooking the seeds, and 45 hours (30°C) for the fermentation. In sweet lupin, even though the alkaloid content was reduced to about 43% of the initial value, the effect of fermentation (a reduction of 3.6%) was small compared to that of extraction during soaking and cooking. In addition, 50% of the lipid fraction was metabolized; linolenic and erucic acids were degraded, whereas no significant improvement of protein digestibility was observed. Sensory evaluation (on a 5-point hedonic scale by 28 untrained panelists) showed promising results for deep-fried lupin tempeh. In bitter lupin, the initial alkaloid content was reduced to 50% after soaking and cooking. The fermentation process did not cause any detectable reduction of alkaloids, indicating that R. oligosporus NRL 2710 could not degrade alkaloids of lupin. Cakes made from bitter lupin were reported less compact than those from sweet lupin. Alkaloids were determined by titration. The latter two studies showed different extents of reduction of alkaloid content in seeds treated by fermentation with R. oligosporus NRL 2710. We did not find an explanation for this difference in literature. It may be caused by the use of different lupin species, or by the use of an acid environment during soaking, which—as reported by Jiménez-Martínez et al. (Citation2007)—might facilitate the actions of R. oligosporus NRRL-2710 on alkaloid reduction in the fermentation process. Camacho et al. (Citation1991) reported a reduction of alkaloid content at pH 4.5. Another cause could be a deficiency in nutrients during fermentation as Peñaloza et al. (Citation1991) suggested.
Germination is another approach to reduce the alkaloid content. A study by Dagnia et al. (Citation1992) in L. angustifolius “Gungurru” showed that germination reduced the alkaloid content from 0.72 to 0.16 g/kg, which is equivalent to a 78% decrease after six days (with seven days total processing time). In this study, the phytate concentration also decreased, namely, from 4.7 to 1.6 g/kg.
The information about biological approaches to debitter lupins is scarce. Nevertheless, based on the studies presented above, some suggestions can be made. First, biological processes do not produce significant chemical residues; however, they require water for washing and sometimes lactic acid solutions. Second, biological processes require preparatory operations such as dehulling, optional grinding, soaking, and cooking. These physical treatments obviously contribute to the reduction of alkaloids in the seed. In order to distinguish the separate effects of biological and physical treatments, it is essential to perform biological studies that include appropriate controls. Third, all reported studies started with seeds that had initial alkaloid contents up to 11 g/kg. We did not find studies on the debittering of seeds with higher alkaloid contents such as 30 or 35 g/kg, which would facilitate an assessment of the applicability of biological methods to debitter lupin seeds such as L. mutabilis. Four, in general the biological methods might reduce antinutritional components of lupin seeds (Szakács and Stankovics, Citation1983; Beirao da Costa, Citation1989), and also in some cases fat, protein content and protein efficiency ratio (PER) values. Five, all reported treatments were carried out within 48 and 168 hours and required an amount of water between 8- and 40-fold the seed weight. Temperatures used varied between 30 and 37°C, except for the germination that was at 20–25°C. So the evidence to date shows that the debittering process cannot be achieved in less than two days and always uses substantial quantities of water and energy.
Chemical Extraction
In plant materials, alkaloids are known to occur partly as free bases and partly as salts that are insoluble in most organic solvents. A common practice to isolate alkaloids from plant sources, prior to their characterization, consists of a treatment with a base that converts such salts into free alkaloids, which, as they are soluble in organic solvents, can be easily recovered by extraction (Ortiz and Mukherjee, Citation1982). Chemical approaches to extract alkaloids can be distinguished as (i) extraction with hexane and basic solutions, (ii) basic extractions, and (iii) mixed alcohol extractions ().
Extractions with hexane and basic solutions were performed by Ortiz and Mukherjee (Citation1982) and Torres-Tello et al. (Citation1980). In these studies, L. mutabilis was crushed, flaked or dehulled, and split. Initially, the seeds were brought in contact with hexane, followed by a basic solution. These procedures extracted between 80% and 96.9% of original alkaloids and required between 3 and 24 hours of processing time.
Basic extractions have been tested with L. campestri (Jiménez-Martínez et al., Citation2003a) and L. mutabilis (Torres-Tello et al., Citation1980; Aguilera et al., Citation1983). These studies showed that this type of extraction can reduce alkaloid contents up to 99.9%. This required less than one day for whole seeds and less than one hour for lupin flour (90% passed 100 mesh screens). This might be because a reduction in particle size of lupin (especially when lupin is processed into flour) increases the contact with water, thus facilitating the diffusion of alkaloids, especially at raised temperatures. These processes also use energy up to six hours (i.e., about 50 MJ/kg) and cause material losses, mainly of carbohydrates. This could be explained by their solubility in an aqueous environment. The process carried out by Aguilera et al. (Citation1983) also extracted oil and protein from lupin. These authors did not report material loss and the explanation could be due the fact that they used centrifugation as a separation-extraction procedure. This principle might be an important asset for recovering material in other approaches as well.
Ethanol mixed with hexane or with CO2 can also be used to extract alkaloids (Torres-Tello et al., Citation1980; Nossack et al., Citation2000). In the first case, the seed was dehulled and split, and the alkaloid reduction (97.9%) was achieved in about 20 hours. In the latter case, the seed was powered to 70–100 mesh. The process was carried out in 0.33 hours and achieved a reduction of 39.8 mg of alkaloids/g of seed ().
All treatments discussed so far were carried out at laboratory scale. However, Chajuss (Citation1989) proposed a larger scale commercial procedure for extracting alkaloids and fat, and for producing protein concentrates and intermediate products from L. mutabilis and L. albus. This process includes dehulling, flaking, and treatment with hexane to extract lipids. The lipid-free fraction is treated with warm aqueous alcohol and then washed to separate protein concentrate and soluble extract (molasses). In this study, 2000 kg of raw lupin yielded 1000 kg of protein isolate (720 g protein/kg, 7 g oil/kg), 280 kg of food-grade, degummed, refined and bleached oil, 600 kg of lupin alkaloid-sugar extract (molasses 100 g protein/kg, 14 g oil/kg, 300 g moisture/kg, oligosaccharides, minor components), and 240 kg of hulls (80 g protein/kg, 20 g oil/kg). Molasses could be used as soil fertilizer, plant growth promoter, and insect repellent, whereas hulls could be used possibly as a “green manure” and soil conditioner, or as energy source.
Regarding the chemical alkaloid extraction scenarios, it is important to note that chemical extractions can be performed on lupin seeds with high alkaloid contents (between 19.4 and 42 g/kg). Basic debittering would decrease the methionine availability in lupin (Gueguen and Cerletti, Citation1994). This is an important nutritional issue to be considered. All chemical treatments require additional equipment and facilities for safe operation and disposal of waste. Chemical treatments might add residues, which could pose health risks and could affect the taste of the product. They require considerable amounts of water (24- to 60-fold the weight of lupin seed, or even more).
Aqueous Processing
Cold and warm aqueous processing of lupin seeds reduces the alkaloid content (). In a study on L. mutabilis “Kayra” (Torres-Tello et al., Citation1980), 95.4% of initial alkaloids were removed. The seeds were dehulled, split, and then cooked at 90°C for 0.5 hours followed by extraction with cold water for 72 hours. Villacrés et al. (Citation2000) reported a traditional process applied at commercial scale to whole seeds of L. mutabilis. The seeds were soaked for 14–20 hours at room temperature (≈15°C), then cooked for 0.5–2 hours and washed for 96–120 hours at room temperature again. The process took between 120 and 144 hours in total and removed 97.4% of the initial alkaloid content. In another study, Caicedo et al. (Citation2001) used warm water (40°C) to debitter whole seeds of L. mutabilis. This process was also carried out at commercial scale. It took 90 hours and used water in the ratio 63:1 (w: w) water: seed. The process removed 93.3% of alkaloids.
Aqueous alkaloid extraction has the following characteristics: (i) the alkaloid content is reduced, but it takes about three days when the seed is dehulled and split or —four to five days for whole seeds. (ii) The debittering process uses large volumes of water that can be treated and reused. (iii) At present, the cold aqueous extraction is the only food-grade method known and applied at a commercial scale. When destined as flour in formulated foods, lupin flour could be extracted more rapidly than whole seeds used for direct consumption as a snack or salad ingredient. (iv) It does not pose the risk of chemical residues nor requires the recovery of chemical reagents.
Uses
Food Uses
Lupin seeds are utilized both as food for human beings, and as feed for pigs, sheep, poultry, and ruminants (Cremer, Citation1983; Villacrés et al., Citation2000). For human consumption, debittered Lupinus can be eaten directly as a snack (Villacrés et al., Citation2003), and can be used as ingredient in many different products such as fresh salads, soups, cakes, snacks, hamburgers, biscuits, bread, foods for babies, substitutes of milk, and in main dishes (Cremer, Citation1983; Ruales et al., Citation1988; Villacrés et al., Citation2003; Güémes-Vera et al., Citation2008).
Nutritional Value of Lupin and Its Products
The PER of L. mutabilis was reported to be between 0.83 and 1 (Ortiz et al., Citation1975; Chango et al., Citation1993a; Petterson, Citation1998) and could be increased by adding methionine (Ortiz et al., Citation1975; Haq, Citation1993). Studies on rats show that whole seed supplemented with about 0.2% DL-methionine increased the PER value to about that of casein, i.e., 2.5 (Petterson, Citation1998).
In vitro protein digestibility of L. mutabilis flour and its protein concentrate were reported as 71.1 and 77.6%, respectively. Those values increased to 75.1 and 80.1%, respectively, when the samples were cooked for 30 minutes in moist heat (Sathe et al., Citation1982), while the apparent digestibility of L. mutabilis was reported as 81.8% compared with 87.1% for casein when fed to children (Petterson, Citation1998). These values are similar to those reported by Gueguen and Cerletti (Citation1994), who found an apparent digestibility of 84% for both raw and processed seeds, and oil cake. These authors also reported the true digestibility of L. mutabilis protein isolate as 92%, which is comparable with that of casein. Protein digestibility—corrected amino acid scores (PD-CAAS) of lupins were around 0.7, compared with 1.0 for casein and 0.7 for field peas (Pisum sativum) (Petterson, Citation1998).
L. mutabilis seeds, debittered with alcohol and/or water, and enriched with DL-methionine (20 g/kg of the protein) or fortified with complementary protein carriers rich in sulfur-containing amino acids, e.g., cereal proteins, were proposed as promising sources of nutrition for humans and animals (Gueguen and Cerletti, Citation1994).
Lupin seed and its derivates (flour, protein concentrates, and isolates) have also been used to improve the nutritional properties, specially the protein level, of lupin-enriched foods (Güémes-Vera et al., Citation2008). For example, the PER of bread with 10% of L. mutabilis flour rose from 28% (in bread without lupin) to 56% (control = casein 100%) (Gueguen and Cerletti, Citation1994), or according to Gross et al. (Citation1983) from 28 to 76%. In another study, Jiménez-Martínez et al. (Citation2003b) prepared milk from wild Lupinus campestri. In order to compare it with cow's milk and soya bean milk, these products were chemically analyzed. Results showed that the protein and fat content were the highest in L. campestri milk (protein 58.0% dw, fat 29.4%) compared with commercial soya bean milk (protein 39.1% dw, fat 7.0%) and cow's (semi skimmed) milk (protein 26.2% dw, fat 13.4%). In a similar study rice, a blend with Lupinus mutabilis (rice: lupin 80:20 w/w) and a blend with soya bean (Glycine max “Iniap-Jupiter”) (rice: soya bean 80:20 w/w) were used by Ruales et al. (Citation1988) to make extruded products. The chemical composition showed that the addition of dehulled soya bean grits and L. mutabilis flour increased the nutritional value of the product. However, the nutritional value of the product containing lupin was the highest (15.3% protein, 6.0% fat, 3.5% fiber, and 1.3% ashes) compared with the products made with soya bean grits (12.6% protein, 5.5% fat, 1.4% fiber, and 1.0% ashes) and with rice grits only (6.7% protein, 0.4% fat, 1.4% fiber, and 0.5% ashes). The mineral analysis showed that the rice-lupin product had the following composition (mg/kg dw): Zn 42.1, Fe 56.8, Ca 129, Mg 948, and Cu 9.2. The rice-soya bean product had (mg/kg dw): Zn 27.4, Fe 17.2, Ca 275, Mg 719, and Cu 10.9. Finally, the rice product had (mg/kg dw): Zn 14.8, Fe 13.7, Ca 114, Mg 399, and Cu 3.0. Note that the product made with the rice-lupin blend had the highest mineral content compared with two other products. Only the calcium content in the rice-lupin product was significantly lower than in the rice-soya bean product.
These results suggest that L. mutabilis can be used to improve the nutritional composition of different products because lupins increase the nutrient content (Jiménez-Martínez et al., Citation2003b). Lupins can also improve the biological quality of proteins when they are used in combination with cereals (Ruales et al., Citation1988; Jiménez-Martínez and Dávila-Ortiz, Citation2006).
Sensory Acceptance
In general, lupin products present a good sensory acceptance (Gross et al., Citation1976; Cremer, Citation1983; Jiménez-Martínez et al., Citation2003b), which can be higher than for soya bean products (Jiménez-Martínez et al., Citation2003b). Gross et al. (Citation1983) found that bread made with 90% wheat flour and 10% L. mutabilis flour had an acceptability (72.7/100) similar to bread made with 100% wheat flour (74.8/100). Bread made with 90% wheat flour + 10% L. albus flour scored slightly lower (71.6/100) than L. mutabilis bread, and bread made with 90% wheat flour + 10% soya bean flour had the lowest score (61.0/100). On the other hand, another study showed that the acceptance of lupin products can also be lower than that of traditional products (Alamanou et al., Citation1996; Güémes-Vera et al., Citation2008). For example, Lupinus albus “Graecus” protein isolate added at 1, 2, and 3% to frankfurter sausages had a lower acceptance than the control (0% addition) (Alamanou et al., Citation1996). For the sensory evaluation, panelists were instructed to evaluate the appearance, the texture, the flavor, and the juiciness of the products and express their overall acceptability on a 6-point hedonic scale (6 = extremely like, 1 extremely dislike). Results showed that sausages made with 1 or 2% protein isolate were liked (4.0/6). However, the scores were nearly 1 point lower than for sausages made without lupin isolate (4.8/6). Sausages made with 3% protein isolate had a very bad score of only 2 out of 6.
This suggests that there is room for improvement of the sensory attributes of lupin-based products (Linsberger-Martin et al., Citation2010). This improvement could be achieved by developing and adding flavors, colors, and additives but also by studying the effect of processing conditions on sensory attributes. For example, according to Gross et al. (Citation1983) roasting before milling considerably enhanced the organoleptic characteristics of the grain. By doing this, the lupin flour takes on a neutral flavor or a slightly nutty taste in accordance with the degree of roasting. Fermentation would also improve the taste and texture of some lupin products (Villacrés et al., Citation2006). We encourage researchers to study the sensory improvement of lupin-based products in order to increase their consumption.
Pharmaceutical Uses
QAs are known to have a high pharmacological activity (Jiménez-Martínez et al., Citation2003a). Many pharmaceutics and cosmetic uses for lupin seeds have been described since the sixteenth century (Aguilera and Trier, Citation1978). Ciesiolka et al. (Citation2005) suggested, based on in vitro studies, that the hypocholesterolemic activity was associated with stimulation of low-density lipoprotein receptors by a well-defined protein component of the lupin seeds. Extract from L. angustifolius (alkaloid content about 110 g/kg dw) showed pharmacological properties, such as a decrease of arterial blood pressure of rats (Ciesiolka et al., Citation2005). Sparteine is also used in cardiac medicine due to its antiarrhythmic capacity (Hatzold et al., Citation1983; Ciesiolka et al., Citation2005), and it is frequently used in obstetrics as it induces the contraction of the uterus and hastens partition (Hatzold et al., Citation1983).
COMPOSITION, STRUCTURE, PHYSICAL, AND FUNCTIONAL PROPERTIES OF LUPIN PROTEINS
The functional properties of proteins that are relevant to food production are related to their physicochemical and structural properties, such as size, shape, composition, hydrophobicity/hydrophilicity ratio, net charge, structural arrangements, and adaptability of domain structures of the whole molecule to changes in environmental conditions (Kinsella, Citation1976; Hettiarachchy and Ziegler, Citation1994). Lupin protein composition and structure are, therefore, presented and discussed to provide understanding of the functional properties of lupin flour and its derived products.
Composition and Structure of Lupin Proteins
The major protein classes in legume seeds are globulins and albumins (); prolamin and glutelin fractions are also present but in very low quantities (Doxastakis, Citation2000). Globulins are proteins extracted at high ionic strength, and represent 90% of the protein in soya bean and about 80% in L. albus) (Gueguen and Cerletti, Citation1994). L. mutabilis “Potosi” and “Inti” were reported to contain about 11 and 13% more globulin, respectively, than L. albus (Santos et al., Citation1997). Denaturing PAGE (Polyacrylamine Gel Electrophoresis) analysis showed that the globulins of L. mutabilis are composed of polypeptides with higher molecular masses than those of L. albus. Some of these polypeptides are linked by disulfide bonds (Santos et al., Citation1997). The complexity of the globulin fraction is due to the presence of different families of proteins (legumin-like and vicilin-like proteins, and lupin conglutins γ and δ), and the presence of oligomeric components (12S and m7S). These proteins and components have different types of associations, protomer sizes, and compositions (Doxastakis, Citation2000).
Table 8 Protein fractions in the different lupin species
Legumin-like proteins correspond to the fraction of globulin polypeptides with sedimentation coefficients of about 11–12S. In L. angustifolius this fraction shows molecular masses between 185 and 315 kDa (Doxastakis, Citation2000), similar to that of L. albus. However, legumin-like protein α-glutinin from L. mutabilis was reported to differ considerably in structure and composition from that of L. albus (Santos et al., Citation1997). In L. albus, the α-conglutin fraction represents about 33% of total protein (Duranti et al., Citation1981) and is composed of four main types of subunits, with molecular masses between 50 and 60 kDa (Santos et al., Citation1997). Upon reduction, each of the main subunits is split into an acid (heavier) polypeptide chain (38–50 kDa) and a basic (lighter) polypeptide chain (19 kDa) (Santos et al., Citation1997). In L. mutabilis “Potosi” α-conglutin is formed by four main types of subunits (50–65 kDa) and two minor types (40–42 kDa), which upon reduction produce a number of undetermined heavier polypeptide chains and two lighter ones (18 and 19 kDa). L. mutabilis “Inti” differs from “Potosi” as the first consists of five main types of subunits (namely of 32, 40, 45, 49, and 53 kDa), which upon reduction produce four main types of polypeptide chains (18, 19, 31, and 37 kDa) (Santos et al., Citation1997). In the case of soya bean the 11S fraction is glycinin, and represents 20–35% of total protein. In soya bean, 11S proteins are hexamers (αβ)6 of relative molecular weights of about 350–400 kDa. Each subunit of the hexamer consists of two components; the acid (α) of 40 kDa and the basic (β) of 20 kDa bound by disulphide bonds (Gueguen and Cerletti, Citation1994). For all these 11S-like proteins, the acid polypeptides have significantly lower hydrophobicity compared with the basic units and are mainly located on the exterior of the molecule (Doxastakis, Citation2000). Moreover, studies on L. albus have shown that assembly of the subunits in the oligomer is likely to be dictated by the distribution of polarity in the polypeptide (Duranti et al., Citation1988; Guerrieri and Cerletti, Citation1990). Consequently, the polarity of the 11S oligomer would be the result of its polypeptide composition (i.e., amount and type of polypeptides) and structure (spatial distribution of polypeptides in the oligomer). Because variations in the composition and structure are reported not only between species but also between different genotypes of soya bean (Gueguen and Cerletti, Citation1994) and L. mutabilis (Santos et al., Citation1997), we may expect different functional behavior of 11S fractions between and within species of lupins and soya bean.
Vicilin-like proteins are polypeptides with a sedimentation time of 7S. However, this group includes polypeptides 4S, 5S, 6S, and 7S for L. albus (Duranti et al., Citation1981) and polypeptides with 7.4S (Joubert, Citation1956) and β-conglutin (Aguilera and Garcia, Citation1989) for L. angustifolius and L. luteus (Doxastakis, Citation2000). For L. albus, the vicilin fraction represents about 44% of total protein (Duranti et al., Citation1981). Beta-conglutin from L. albus is composed of more than 20 polypeptide chains without disulfide bonds, with molecular masses ranging from 15 to 65 kDa (Santos et al., Citation1997). For L. mutabilis “Potosi” and “Inti,” β-conglutin is composed of seven major polypeptide chains (with molecular masses ranging from 50 to 67 kDa), two polypeptide chains with molecular masses in the range of 33–38 kDa, and a number of minor polypeptides. The presence of disulfide bonds was not detected (Santos et al., Citation1997). Blagrove and Gillespie (Citation1975) mention the presence of a 30 kDa subunit, a major component of β-conglutin, in L. angustifolius and other Old and New World lupin species and observed that it is absent in the American L. elegans and L. mutabilis. In soya bean, the 7S fraction (β and γ conglycinin) constitutes 30–35% of total protein (Peng et al., Citation1984). Beta-conglycinin, the major 7S fraction, has 6 components, from which three are principals (α, α′, and β) with relative molecular weights of 42–57 kDa. The γ conglycinin (i.e., the 7S minor fraction) has been less studied. The difference in the composition of the β-conglutin of L. mutabilis and L. albus (Santos et al., Citation1997) and the difference with the 7S fraction of soya bean (Gueguen and Cerletti, Citation1994) is evident. The vicilin protein shows surface hydrophobicity, which permits the self-association of proteins into micelle arrangements. At pH 6–6.8 this hydrophobicity is high and precipitated micelles show viscoelastic properties similar to wheat gluten (Gueguen and Cerletti, Citation1994). The variation in the composition of vicilin-like proteins from lupin species and soya bean are expected to influence the hydrophobic behavior of their proteins.
Oligomers 12S, 7S, and m7S, and System 12S↔7S
12S and 7S type proteins aggregate, forming a structure called 12S oligomer or 7S oligomer, respectively. The 12S oligomer is more compact and resistant to endogenous proteases, than the 7S oligomer (Duranti et al., Citation1988). This gives the 12S structure rigidity, not only due to the disulphide bonds but also because of hydrophobic interactions resulting from the high hydrophobicity of the basic subunits (Gueguen and Cerletti, Citation1994), which are present in the interior of the structure.
The 12S oligomer can dissociate to a smaller 7S species until an equilibrium is reached (system 12S↔7S). However, this equilibrium is dynamic. In L. albus, for instance, this equilibrium is reversibly shifted towards the high Mr form by increased ionic strength and protein concentration (Gueguen and Cerletti, Citation1994). On the other hand, when the equilibrium 12S↔7S is shifted toward the 7S form, the secondary structure and the net charge of the protein become more like those of m7S molecules (Duranti et al., Citation1988). This m7S molecule (158 kDa) is a modification of the 7S oligomer but without the capacity to produce 12S oligomers (Duranti et al., Citation1988). The system 12S↔7S has a structure that consists for 15% of α helix, 37% β strand and 48% coil, whereas this is 20%, 34 and 46%, respectively, in the m7S oligomer (Duranti et al., Citation1988). Thus the functional behavior of lupin protein will depend on the association state (i.e., the 12S↔7S equilibrium). This behavior depends especially on the composition of the subunits in the α-chains (Guerrieri and Cerletti, Citation1990).
Lupin conglutin γ is a globulin protein that in the case of L. albus and L. mutabilis “Potosi” and “Inti” is reported to consist of a single subunit (monomer) of 42–43 kDa composed of two polypeptide chains linked by disulfide bonds (18–30 kDa) (Santos et al., Citation1997). These monomers would be associated in various states of 92, 150, and 300 kDa representing 6% of total seed protein (Duranti et al., Citation1981). The small subunits precipitate at pH 5.6–5.9 and the large ones at pH 6.2–6.8 (Restani et al., Citation1981). In L. angustifolius the association has been reported to vary between 280 kDa (by sedimentation equilibrium) and 320 kDa (gel permeation). Dissociation generates monomers of 43–45 kDa and subunits of 28–30 kDa and 16.5 kDa (Blagrove et al., Citation1980). The small subunits precipitate at pH 6.9 and the larger ones at pH 7.8–8.0 (Blagrove et al., Citation1980). Of the globulins, conglutin γ from L. albus has the highest amount of bound sugar (Duranti et al., Citation1981). Pentoses and hexoses are present, with galactose as the major component (Duranti et al., Citation1981). In L. angustifolius also, a high (bound) carbohydrate content was reported (Gueguen and Cerletti, Citation1994).
Lupin conglutin δ is a sulfur-rich 2S globulin present in L. albus (Duranti et al., Citation1981; Cerletti, Citation1983), L. luteus (Gerritsen, Citation1956; Joubert, Citation1956), and L. angustifolius (Lilley, Citation1986a, Citation1986b). It was not reported in L. mutabilis. Lupin conglutin δ represents between 10 and 12% of total protein of L. albus (Duranti et al., Citation1981) and L. angustifolius (Lilley, Citation1986a). In L. angustifolius 80% of conglutin δ is conglutin δ2 of 14 kDa, composed of two subunits of 9.401 and 4.597 kDa with two intrachain and two interchain disulphide bonds and one free SH (Lilley and Iuglis, Citation1986). Conglutin δ2 can produce a dimer, conglutin δ1 (28 kDa, 2.8S), which at low ionic strength and neutral pH associates reversibly to an oligomer of 56 kDa and 4.1S (Gueguen and Cerletti, Citation1994). The presence of a disulphide crosslink (in the part of conglutin known as α helix) gives stability to the conglutins δ1 and δ2. However, addition of 1 M guanidine hydrochloride causes denaturation of the helix structure (Youle and Huang, Citation1981). Lupin conglutin δ is the most acidic globulin in lupin seed because of the high amounts of glutamic acid (Duranti et al., Citation1981). This acidic nature influences the behavior of the total protein (Gueguen and Cerletti, Citation1994) by increasing the hydrophilicity.
Albumins are defined as the water-soluble fraction of the protein from legume seeds, and represent 12.8, 15.4, between 5 and 10 and 10% of the total seed protein of L. albus, L. luteus, L. angustifolius, and soya bean, respectively (Hudson, Citation1994). L. albus is reported to have an albumin content that is about twice that of L. mutabilis “Potosi” and “Inti” (Santos et al., Citation1997). The albumin fraction includes molecules that belong to the functional proteins of the seed. Many are enzymes such as glycosidases and proteases. Others play an important role in plant defense, such as trypsin inhibitors and lectins. Albumin is characterized by a high lysine and sulfur amino acid content, especially methionine (Smith and Circle, Citation1978; Cerletti, Citation1983; Gueguen, Citation1991). However, Santos et al. (Citation1997) report that the presence of disulfide bonds is not apparent in L. albus and L. mutabilis “Inti” and “Potosi” after electrophoresis performed under nonreducing conditions, and that SDS electrophoresis on polyacrylamine gel (SDS-PAGE) showed about 20 polypeptides in L. albus (Cerletti et al., Citation1978) and L. angustifolius (Blagrove and Guillespie, Citation1978) and 13 in L. luteus (Konopoka-Waliszkiewicz, Citation1988) with apparent molecular masses from 117 to 6 kDa (Doxastakis, Citation2000). The polypeptide patterns of the two L. mutabilis analyzed by R (reducing)-SDS-PAGE are virtually identical but differ considerably from that of L. albus. Particularly evident is the presence of abundant 34 kDa albumin in L. mutabilis cotyledons (Santos et al., Citation1997), which are apparently not present in L. albus.
Physical and Functional Properties of Lupin Proteins (Lupin Flour, Protein Isolates, and Concentrates)
presents the physical and functional properties of the most important Lupinus spp. flours, their protein concentrates and isolates. In the following text, the term concentrate is used when the protein content is between 72.8 and 83.8%, and the term isolate when the protein content is 83.9–87.4%.
Table 9 Physical and functional properties of lupin flour, concentrates, and isolates
Isoelectric Point
The isoelectric point of the protein of L. mutabilis was reported to vary from pH 4.0 to 6.0 (Aguilera and Trier, Citation1978; Bleitgen et al., Citation1979; Aguilera et al., Citation1983). In L. albus “Multolupa,” it was between pH 4.2 to 6.4 (King et al., Citation1985) and in L. angustifolius between pH 4.3 (Lqari et al., Citation2002) and pH 4.5 (Sathe et al., Citation1982). These values confirm that lupin proteins consist of different subunits or groups, each with different properties. For example, the protein fraction from L. albus “Multolupa” that precipitates at pH 5.4, is reported to have a higher amino acid score than protein fractions obtained at a pH 4.2 or 6.4 (King et al., Citation1985).
Protein Solubility
Protein solubility is the percentage of soluble nitrogen/ total nitrogen. A higher solubility is attributed to an elevated charge and the electrostatic repulsion and ionic hydration occurring at a pH above and below the isoelectric pH (Doxastakis, Citation2000; Moure et al., Citation2006). For example, L. angustifolius slurry (20 mg flour one hour homogenized with 20 mL solution 0.1 M NaCl at pH 7) showed a protein solubility of 13.1% (Lqari et al., Citation2002). However, when—in the slurry—flour was replaced by L. angustifolius protein isolate, which first was solubilized at pH 10.5 or 12, and then precipitated at pH 4.3, the protein solubility increased to 19.2 and 33.8%, respectively (Lqari et al., Citation2002).
The ionic strength can also affect the solubility of lupin proteins. In a 1% slurry made from L. albus “Ares” and “Typ Top” isolates, the protein solubility was about 10% when the slurry was treated at pH 8.6 followed by precipitation of the protein at pH between 4.2 and 5.1 and then freeze dried (D´Agostina et al., Citation2006). In this case, the protein solubility was measured at pH 5 and at an ionic strength u = 0. This protein solubility, however, was increased to about 90% when all parameters remained the same, except for the ionic strength u, which was increased to 1.0 with sodium chloride. Other studies show the same behavior (Manrique et al., Citation1974; Sathe et al., Citation1982). The direct effect of ionic strength on protein solubility is clear. At pH 7, however, the effect of u on protein solubility is lower than at a pH closer to the isoelectric point. Temperature also affects protein solubility. On the one hand, heat treatments (80–100°C) have been reported to have greater adverse effects on solubility when the precipitate is kept at its isoelectric point (King et al., Citation1985). Apparently, the intermolecular attraction due to the pH at the isoelectric point is added to the effect of aggregation (coagulation) caused by the high temperatures. On the other hand, temperatures below 60°C are reported to be beneficial for protein solubility. King et al. (Citation1985) suggest that nitrogen solubility is increased up to 100% if isolates are taken to pH 6 before drying and subsequently heated at 60°C for 20 minutes. In this case, the heating could increase the protein dispersibility. All these results indicate that protein solubility depends on pH, temperature, and ionic strength. Isolates obtained by precipitation at the isoelectric pH, kept at that pH in an environment having an ionic strength of u = 0 and heated above 80°C will probably show very low solubility. On the other hand, isolates obtained by solubilization of protein at pH 8–10 in an environment with an ionic strength of u = 1, will show a higher solubility when they are precipitated at their isoelectric point and taken to pH 6–8 before drying and finally heated at temperatures below 60°C. In the latter case, the intermolecular repulsion would be greater, avoiding the formation of aggregates and thereby facilitating the solubility (King et al., Citation1985).
Water Absorption
The amount of water absorbed by flour, protein concentrate, or isolate is closely related to its amino acid profile, conformation, hydrophobicity, pH, thermal treatment, ionic strength, amount of protein, and presence of fat. The amount of water absorbed is also influenced by the technological process used to obtain the flour, concentrate or isolate, for example soaking, fermentation, or germination (Sathe et al., Citation1982; Moure et al., Citation2006). Moreover, even lupin protein derivates with the same protein content may show different functional properties because, for instance, the ratio of the different globulin fractions differs among lupin varieties (Cerletti et al., Citation1978).
The water absorption capacity of lupin flour reportedly varies from 2.4 g water/g flour dw in L. angustifolius (Lqari et al., Citation2002) and 2.3 g water/g flour dw in L. albus “Multolupa” (Agosin et al., Citation1989) to 1.2 g water/g flour dw in L. mutabilis (Sathe et al., Citation1982) (). This implies that the water absorption capacities of L. angustifolius and L. albus are similar to that of soya bean (2.0–2.4 water/g flour dw) (Sathe et al., Citation1982). The same authors hypothesized that the water absorption capacity of L. mutabilis flour is lower due to the presence of fat (17.9%). In addition, the low water absorption capacity can be related to a low availability of polar amino acids, which are the primary sites for water interaction of proteins (Sathe et al., Citation1982). This is corroborated by the probable absence of lupin conglutin δ and the lower amount of albumin in protein from L. mutabilis.
Water absorption for soya bean protein concentrate is reported to vary between 3.0 and 4.0 g water/ g of concentrate dw (Sathe et al., Citation1982) and soya bean protein isolates absorb up to 8 g water/g isolated dw. In protein concentrates and isolates of lupin the values of water absorption vary more widely, namely, between 0.5–6.0 g water/g of protein dw (Sathe et al., Citation1982; King et al., Citation1985; Lqari et al., Citation2002). This wide variation between and within species can at least be partially understood by the conditions in which those concentrates or isolates were obtained. For instance, Lqari et al. (Citation2002) found for L. angustifolius that isolates (83.9–87.4% protein) extracted at pH 12 or pH 10.5 with 0.25% Na2SO4 followed by precipitation at pH 4.3 showed a water absorption of 4.5 and 3.8 g/g protein, respectively. These data agree with King et al. (Citation1985), who found that a sample of L. albus “Multolupa” protein isolate could absorb water about six times its weight when that isolate was extracted at pH 8.6 followed by a precipitation at pH 4.2–5.1, then freeze-dried and later heated at 100°C for 20 minutes. However, the same lupin protein absorbed just 0.5 times its weight when the sample was neutralized before drying and the sample was not heated. Higher water absorption seems also to be related to the electric charge. Exposure to basic pH, especially 10–12, followed by acid precipitation could cause denaturation (unfolding) of proteins (Lqari et al., Citation2002), leading to an increase of the hydrophilicity thereby enhancing the water absorption. Isolates that in addition to exposure to a basic pH (8.6) and precipitation at an acidic pH (4.9) were heated (100 °C × 20 minutes) before drying showed higher water absorption than those treated at temperatures of 80°C or lower. Temperatures of 100°C might cause the unfolding of proteins too (King et al., Citation1985).
The protein content also would affect the water absorption capacity. Protein isolates of L. albus (95.7% protein) absorbed 6 g water/g protein dw (King et al., Citation1985). Water absorption apparently increased when the protein content in the isolate had more hydrophilic structures (acid polypeptides) in the periphery (11S-like proteins) (Gueguen and Cerletti, Citation1994; Moure et al., Citation2006) available to bind water molecules. Soaking also affects the water absorption capacity. L. mutabilis seeds increased their size by three times when soaked for 18 hours (Gross et al., Citation1983). Other factors such as germination, fermentation, toasting, and autoclaving reportedly increase the water absorption capacity of meals (Moure et al., Citation2006). This could be related to denaturation (unfolding) of proteins leading to increased hydrophilicity, which enhances higher water absorption. In addition certain processing steps, for instance, soaking combined with alkaline extraction of proteins, also improves the capacity of a protein isolate to absorb water because this process removes compounds such as lipids and polyphenols (Lqari et al., Citation2002).
Oil Absorption
Oil absorption amounts to 1.7 g oil/g seed dw for L. mutabilis, and 1.5 g oil/g dw flour for L. angustifolius (Lqari et al., Citation2002) (). Those values are higher than the 0.8 g oil/g flour reported for soya bean (Moure et al., Citation2006). Protein concentrates and isolates show higher oil absorption than lupin and soya bean flour. For L. mutabilis concentrate a value of 2.9 g oil/g full-fat concentrate was reported and 3.9 g oil/g defatted concentrate (Sathe et al., Citation1982), showing a inverse relation between fat content and oil absorption capacity, suggesting a lipophilic nature of lupin proteins (Sathe et al., Citation1982). Processing conditions also influence oil absorption capacity. L. angustifolius solubilized at pH 12 and then precipitated at pH 4.3 absorbed 2.0 g oil/g isolate. However, when the dissolution was done at pH 10.5 with 0.25% Na2SO3, followed by precipitation at pH 4.3, the absorption rose to 3.1 g oil/g isolate. On the other hand, oil absorption in L. albus is reported to be between 1.0–1.8 g/g isolate. In this case, the protein precipitation was at a pH between 7 and 4.5. This difference in the amount of oil absorbed would result from the effect of pH during processing. Alkaline extraction allows removal of undesirable compounds in the protein isolate (fiber, sugars, polyphenols, lipids, and alkaloids) (Lqari et al., Citation2002), thus increasing the functionality of protein isolates. However, alkaline processing can also alter protein quality due to the possibility of disruption of the protein structure and degradation of some amino acids (Cerletti et al., Citation1978; Sgarbiere and Galeazzi, Citation1978; Maga, Citation1984; Liener, Citation1994). To avoid degradation of amino acids, Lqari et al. (Citation2002) recommend alkaline processing at pH 10.5 but not at pH 12. In general, soya bean concentrates and isolates show oil absorption values between 0.9 to 2.9 g/g concentrate or isolate (Moure et al., Citation2006). These values are lower than reported for L. mutabilis, suggesting that the latter can be used to (partly) replace soya bean protein in foods to improve oil absorption capacity.
Emulsifying Capacity
Emulsifying capacity is defined as the quantity (in g) of emulsified oil per gram of flour, concentrate or isolate (Sathe et al., Citation1982). Some others report emulsifying capacity as mL of emulsified oil per gram of protein. In the case of L. mutabilis flour the emulsifying capacity is 55.1 g/g lupin flour and 89.9 g/g concentrate (Sathe et al., Citation1982). In both cases, the emulsifying capacity was measured on slurries with 2% flour or concentrate and 98% water. Apparently, the emulsifying capacity decreases with increasing amounts of concentrate in the slurry (). In the case of L. albus protein isolate, the reported emulsifying capacity varies between 370 and 570 mL/g isolate (D´Agostina et al., Citation2006), or 326–502 g/g isolate when the oil density is 0.88 kg/L (Sathe et al., Citation1982), in a slurry with a concentration of 1% (D, Agostina et al., Citation2006). In L. albus, the emulsifying capacity is reported to be 1000–2000 mL/g isolate in a slurry with a concentration of just 0.04% (King et al., Citation1985). According to King et al. (Citation1985), this concentration of 0.04% protein is the minimum value, which emulsified the maximum amount of oil. High protein concentrations did not emulsify more oil, possibly because it becomes more difficult to expose hydrophobic areas that can interact with the lipid phase at increasing concentrations (King et al., Citation1985). Emulsifying capacity is also pH dependent (Moure et al., Citation2006). In L. mutabilis, slurries with 2% protein concentrate had different emulsifying capacity depending on the pH. For example, the emulsifying capacity was reported as 315.5, 222.2, 80.0, 155.5, and 137.8 g/g concentrate at pH 2, 4, 6, 8, and 10, respectively (Sathe et al., Citation1982). In L. albus, the emulsifying capacity is reported as 1000 mL at pH 5 and 2000 mL at pH 8 (King et al., Citation1985). Note that the emulsifying capacity at pH 2 (in the case of L. mutabilis) or pH 8 (in the case of L. albus) is higher than at their isoelectric pH (4–5), probably due to an increased oil solubility in those conditions (King et al., Citation1985) by unfolding of proteins (12S oligomer), thereby facilitating the exposure of hydrophobic groups. Emulsifying properties show a good correlation with the presence of hydrophobic residues on the protein surface (Kato and Nakai, Citation1980). The emulsifying capacity of protein isolate also has an apparent inverse relation with its solubility in water. For example, King et al. (Citation1985) reported that soya bean concentrates and isolates with a high solubility in water showed an emulsifying capacity of 15 mL/g, but soya bean isolates and concentrates with a low solubility in water showed an emulsifying capacity of 66 mL/g. Apparently, highly water soluble proteins are poor emulsifiers because they can cause coalescence. The emulsifying capacity is also reported to depend on ionic strength (Kinsella, Citation1984). For L. albus and soya bean, the highest emulsifying capacity was recorded at an ionic strength of 0.5 (using sodium chloride) of the slurry (King et al., Citation1985). The values for the emulsifying capacity of lupin species are similar or higher than those reported for soya bean (between 15 and 191 mL/g) (King et al., Citation1985), which suggests that lupin isolates could well be used as emulsifiers.
Emulsifying Activity
The emulsifying activity is expressed as the volume of an emulsified layer at time 0 hours/total volume of all phases and multiplied by 100 (Lqari et al., Citation2002) to express the result as a percentage. The emulsifying activity of L. angustifolius in a slurry of 3.5% flour w/v (50 water: 50 oil) after homogenization for 2.5 minutes and centrifugation at 1100×g for five minutes was 74% (Lqari et al., Citation2002). When the flour was replaced by L. angustifolius isolate, the emulsifying activity ranged between 69.7 and 74.5% (Lqari et al., Citation2002). In both cases, the pH was 7. The composition of L. angustifolius flour (33.8% protein, 13.6% lipids, and 7.9% water) and its protein isolates (83.9–87.4% protein, ≈ 3.2% lipids, and ≈ 9.4% water) apparently does not affect the emulsifying activity. Slurries with 2% L. mutabilis concentrate showed an emulsifying activity of 100% at 21°C. These samples, however, were neither homogenized nor centrifuged (Sathe et al., Citation1982).
Emulsifying Stability
The emulsifying stability of protein is based on the ability to absorb the oil-water interfaces, unfold and stabilize oil droplets by forming cohesive and mechanically strong interfacial films which exhibit viscoelasticity (Chou and Morr, Citation1979; Graham and Phillips, Citation1980; Kiosseoglou et al., Citation1989; Velev et al., Citation1993). The emulsifying stability is expressed as the emulsifying activity after a specific period of time (Lqari et al., Citation2002; D´Agostina et al., Citation2006). Sathe et al. (Citation1982) reported that the emulsifying stability of a 2%-slurry made from L. mutabilis flour was 70.8% after 10 hours at 21°C. This value slightly decreased to 69% after 20 hours, and then remained the same up to 120 hours. The emulsifying stability of a 2%-slurry from L. mutabilis concentrate was 100% after 60 hours at 21°C and decreased to 91.4% after 120 hours (Sathe et al., Citation1982). This result indicates that the emulsifying stability of protein concentrate is better, in terms of time and amount of material stabilized, than of lupin flour. The ability of proteins to act as emulsifiers varies with their molecular properties (Kinsella, Citation1984). The emulsifying stability of a 3.5%-slurry from L. angustifolius flour measured after 0.25 hours at 85°C and 0.08 hours at 1100 × g was 69.4%, whereas a 3.5%-slurry from L. angustifolius isolate treated similarly as its flour had an emulsifying stability of 66.7–71.0% (Lqari et al., Citation2002). In this case, we do not see much difference between the emulsifying stability of the flour and its isolate, possibly because the measurements were taken after a very short period of time. It would be interesting to know the behavior of those samples after a couple of hours. The emulsifying stability of protein isolates is also influenced by the procedure used to obtain them. In L. albus, protein was isolated by two different approaches. In the first one, the protein was obtained by solubilization at pH 7 followed by precipitation at pH 4.5. In the second approach, protein was obtained by ultradiafiltration at pH 4.5, with a cut-off = 10 kDa. Next both protein isolates were emulsified (1 protein:10 oil:10 water) (w:v:v), homogenized at 11,000 rpm for five minutes, heated for 30 minutes at 80°C, stored for 12 hours at 5°C, and finally centrifuged for 10 minutes at 4500 × g and 20°C. The isolate obtained by the first treatment showed an emulsifying stability of 61–63%, against 74–93% for the second isolate (D´Agostina et al., Citation2006). The isolate obtained by ultra-/diafiltration had a higher emulsifying stability as it contains globulin, albumin and protein-polysaccharide complexes (Alamanou and Doxastakis, Citation1995), which enhance the emulsifying stability due to steric repulsion effects (Dickinson and Walstra, Citation1993). Isolate obtained by isoelectric precipitation does not contain albumin, and the amount of protein-polysaccharide complexes is lower. These finding agree with Kinsella (Citation1984), who reported that the emulsifying stability is influenced by conformation stability and charge.
Foaming Capacity
Like emulsions, foams are two-phase systems, with one phase dispersed in an aqueous continuous one. Foam formation is significantly affected by protein surface activity (Moure et al., Citation2006), processing procedure, and protein composition (Tolstogouzov, Citation1991). Different definitions exist for foaming capacity (Sathe et al., Citation1982; Lqari et al., Citation2002; D´Agostina et al., Citation2006); we use the definition that determines foaming capability as the relation, in percentage, between foam volume after whipping/initial volume of the protein solution × 100. In some cases, we recalculated the original data to express the foaming capacity similarly for all studies.
Lupin proteins have the lowest foaming capacity among the proteins from legumes and oilseed crops (Gueguen and Cerletti, Citation1994). A slurry with 2% of L. mutabilis flour had a foaming capacity of 132% after whipping for five minutes at 21°C. However, in the same conditions, this value increased to 180 and 186% when the flour content in the slurry was increased to 6 and 10%, respectively (Sathe et al., Citation1982). The foaming capacity for concentrates of L. mutabilis is slightly better than for the flour (Sathe et al., Citation1982) (). The addition of extra flour, concentrates or isolates to the slurry increases the amount of proteins in the system. Since proteins are surfactant materials (Moure et al., Citation2006), the interfacial tension in the slurry is reduced (Hettiarachchy and Ziegler, Citation1994) and the foam volume is increased. Defatting L. mutabilis concentrate also increased its foaming capacity, namely from 150 to 158%, probably because defatting reduces the possible competitive effect of lipids in the interface (Moure et al., Citation2006). However, defatting also could reduce the foaming capacity when the solvent used, for instance hexane, removes no polar lipids such as triglycerides and excludes polar lipids such as fatty acids and phospholipids (Doxastakis, Citation2000). This may cause a degree of denaturation in the protein molecule that affects foam formation (Alamanou and Doxastakis, Citation1997; Kiosseoglou and Perdikis, Citation1994). Other lupins have a higher foaming capacity than L. mutabilis. For instance, in the case of a 3%-slurry of L. angustifolius flour that was homogenized at 10,000 rpm and pH 7, the foaming capacity was 214% (Lqari et al., Citation2002). For a 5%-slurry of L. albus protein isolate the foaming capacity was between 1102 and 2083% (D´Agostina et al., Citation2006), which is about 5 to 10 times the foaming capacity of L. mutabilis. Such a difference can be understood, at least partially, by the fact that foaming capacity is not only related to the protein content of the isolate but also to its structure. Special attention should be given to the ratio vicilin/legumin proteins. In pea, the vicilin fraction has been shown to be more active at the air/water interface than legumin (Dagorn-Scaviner et al., Citation1987). In addition, vicilin had a higher diffusion coefficient and showed higher flexibility than legumin-like protein (Dagorn-Scaviner et al., Citation1987). Consequently, the equilibrium surface pressure in the interfaces is reached more quickly for the vicilin fraction (Dagorn-Scaviner et al., Citation1987). L. albus and L. mutabilis have a very different vicilin and legumin protein structure and composition, as stated earlier, and this difference is apparently in favor of the foaming capacity of protein from L. albus.
The addition of NaCl and carbohydrates may improve foaming capacity of lupin protein. Sathe et al. (Citation1982) reported an increase in the foaming capacity from 150 to 174% when 0.6% of salt (NaCl) was added to a slurry of 2% L. mutabilis concentrate, which may have been due to increased protein solubility (Sathe et al., Citation1982). The same authors reported that carbohydrates, such as potato starch, amylopectin, sucrose, and amylose, at a concentration of 0.25 g/g concentrate increased the foaming capacity. However, galactose, gum arabic, and pectin had the opposite effect. The increase in the foaming capacity is in some cases attributed to the formation of protein- polysaccharide complexes that generate stability due to steric repulsion effects (Dickinson and Walstra, Citation1993), which are absent with others carbohydrates.
Foam Stability
According to Doxastakis (Citation2000), proteins play an important role in accumulating at the bubble surface to produce a viscoelastic adsorbed layer that protects the fill against ruptures and prevents or retards Oswald ripening. Foaming stability is defined as the relation between the foam volume and time (D´Agostina et al., Citation2006). The foam stability of a 2% slurry of L. mutabilis flour at 21°C is reported as 93.9, 92.4, and 78.8% after 1, 2, and 36 hours, respectively (Sathe et al., Citation1982). For a 3% slurry of L. angustifolius flour the reported value is 82% after 1 hour and 79.2% after 2 hours (Lqari et al., Citation2002), and for a 2% slurry of L. mutabilis concentrate 94.5, 88 and 76% after 1, 2, and 36 hours, respectively (Sathe et al., Citation1982). For a 3% slurry of L. angustifolius isolate the foam stability was about 80% after 1 or 2 hours (Lqari et al., Citation2002). In the case of a 5%-slurry of L. albus isolate, the foam stability varied from 68 to 95% after 1 hours (D´Agostina et al., Citation2006). Variations in foam stability are attributed to protein surface activity, which is related to conformation and ability to unfold at interfaces, as determined by molecular factors (i.e., flexibility, conformational stability, and distribution of hydrophobic and hydrophilic residues in the primary structure) (Damodaran, Citation1997; van Vliet et al., Citation2002). pH and carbohydrates can also affect foaming stability. Sathe et al. (Citation1982) reported higher foaming stability for a 2% slurry of L. mutabilis concentrate at pH 2 after 2 hours (128%) as compared to foaming stability at pH 4 (114%), 6 (116%) or 8 (108%). The higher foaming stability at the acidic pH range may be due to the formation of stable molecular layers in the air-water interface, which impart texture, stability and elasticity to the foam (Sathe et al., Citation1982). The addition of 0.25 g carbohydrates (galactose, sucrose, amylose, amylopectin, potato starch, gum arabic, and pectin)/g protein decreased foaming stability after 36 hours to 106, 110, 108, 105, 102, 100, and 100%, respectively, compared with the slurry without carbohydrates (114%). The adverse effects of carbohydrates on foaming stability of lupin proteins may result from thinning of the films due to a random distribution of carbohydrates and also by an increased coalescence of gas bubbles dispersed in the liquid (Sathe et al., Citation1982).
Despite the lower efficiency of proteins as compared to low molecular weight surfactants in reducing surface tension, the foams formed with proteins are more stable because proteins give more flexibility and stability to the air-water system by decreasing the interfacial tension (Moure et al., Citation2006). In other words, lupin proteins have lower foaming capacity than low molecular weight surfactants, but higher foaming stability.
Least Gelation Concentration
Least gelation concentration is understood as the minimum amount of material that has the capacity of producing a stable gel. A gel is stable when a boiled and cooled sample does not fall down or slip from an inverted test tube (Sathe et al., Citation1982). This relation is expressed as a percentage of the weight of lupin flour, concentrate, or isolate per volume. Heating soya bean protein slurries above their denaturation temperature results in the formation of a high-viscosity progel (Gueguen and Cerletti, Citation1994). Upon cooling, the proteins—in their unfolded conformation—form the gel through disulfide, hydrogen, and hydrophobic interactions (Gueguen and Cerletti, Citation1994). For L. mutabilis, the least gelation concentration has been reported as 14% for its flour and as 8% for its isolate (Sathe et al., Citation1982). For L. angustifolius, this value was 6% for its flour and 10–12% for its isolate. Both determinations were at pH 7 (Lqari et al., Citation2002). The high variability in the least gelation concentration of proteins may be related to their composition and degree of unfolding. For instance, in soya bean the 7 S protein formed a gel at a lower concentration than the 11 S protein (Gueguen and Cerletti, Citation1994), and so differences in the degree of the denaturalization might explain why commercial isolates may have different gelling properties that depend on the preparation process (Gueguen and Cerletti, Citation1994). For instance, the pH used during the preparation of isolates and concentrates affects the last gelation concentration of proteins. For L. albus isolate, the reported values are 14% for isolate obtained at pH 4.9 and 16% after additional neutralization at pH 7 (King et al., Citation1985). The acid side of the isoelectric pH helps to form stable gels of globulins because in this environment carboxylic groups are less dissociated, and the interactions between protein molecules and the solvent increase (Gueguen and Cerletti, Citation1994). In addition, the variability in the least gelation concentration of proteins may be ascribed to the relative ratios of components other than proteins (Thompson and Casey, Citation1983), such us carbohydrates and lipids, suggesting interactions between those components (Sathe et al., Citation1982). In general, the least gelation properties of protein from lupin species are similar or in some cases better than those of soya bean, which was reported as 13% for protein isolate (King et al., Citation1985).
Improving the Functional Properties of Lupin Proteins
According to Feeney and Whitaker (Citation1985), the functional properties of lupin proteins can be improved by modifying protein structures and conformation at different levels, and by optimizing characteristics such as size, the hydrophobicity/hydrophilicity ratio (specially at the surface) and the molecular flexibility of proteins. Modification can be achieved by denaturation of proteins using various treatments, like physicochemical (pH variations), physical (heat), chemical (acylation and succinylation), and enzymatic treatments (Gueguen and Cerletti, Citation1994). The effect of pH and heat was discussed before under the heading Physical and functional properties of lupin proteins (lupin flour, protein isolates, and concentrates).
Acylation with succinic anhydride (acting on Lys and Tyr) improves the solubility, as well as the emulsifying, foaming, and gelling properties (Beuchat, Citation1977; Kinsella and Shetty, Citation1979) by affecting the charge distribution and net charge of protein molecules (Moure et al., Citation2006). These functional properties depend, however, on the degree of acylation (Gueguen and Cerletti, Citation1994). For instance, for faba bean (Vicia faba) proteins, 50–70% of acylation produced a good gel, whereas the emulsion stability and viscosity were significantly enhanced for 97% of modification (Muschiolik et al., Citation1987).
Succinylation also increases some functional properties of proteins (Moure et al., Citation2006). However, the degree of succinylation affects the physical properties of different materials in different ways. For instance, in faba bean the maximum foaming capacity was reached at >80% succinylation (Gueguen and Cerletti, Citation1994) and the maximum foaming stability for soya bean glycinin at 25% succinylation. Other chemical treatments that improve functional properties are acid hydrolysis, alkylation, oxidation, esterification, amidation, deamidation, and phosphorylation (Moure et al., Citation2006).
Enzymatic hydrolysis can increase protein solubility (Arias and Felacio, Citation1986; Were et al., Citation1997) by breaking up peptide bonds to produce peptides with desired size, charge and surface properties (Moure et al., Citation2006) to achieve an elevated charge and electrostatic repulsion. Trypsin treatment of protein products results in higher solubility and water hydration capacity than in the corresponding untreated product (Jones and Tung, Citation1983). Protein hydrolysis increases the foaming capacity and stability and gelation capacity of flour (Hrčkova et al., Citation2002; Taha and Ibrahim, Citation2002).
Physical treatments other than the use of heat, such us high-pressure, improve the functional properties by unfolding and exposing hydrophobic sites (Molina et al., Citation2002). Coprecipitation of proteins from different vegetable sources with whey proteins yield protein isolates with better functional properties than those of their individual isolates (Lawhon et al., Citation1980).
Finally, as each protein source may react in a different way to physical, physicochemical, chemical, and enzymatic modification treatments, it is necessary to determine and standardize the appropriate treatment for each specific protein application.
CONCLUSIONS
Variation in the Composition of Lupinus Mutabilis
This review shows that most of the reported values on the nutrient contents of raw and debittered lupins vary greatly. The causes of these variations are not precisely known, but several assumptions can be made. The variations may be due to the quality of the sample (obtained from a few or several plants, from markets or research institutes, storage conditions, and age of the sample), the analytical methods used (accuracy and precision), the variety of lupin analyzed, variations within each sample due to agronomical conditions (physical and chemical soil quality, and availability of water and sunlight), genetic aspects (which influence plant form, susceptibility to frost and diseases, growth cycle, protein, oil, and alkaloid contents) (Haq, Citation1993), and different factors used for converting free nitrogen into protein (namely, 5.4, 5.5, 5.7, and 6.25). In the case of debittered lupin, the methods used for debittering could also influence its nutrient content. However, in spite of the observed variations, L. mutabilis showed the highest protein and fat contents among the main lupin species. Most essential amino acids and a substantial amount of unsaturated fatty acids are present in raw lupin.
Alkaloid Content
The alkaloid content in L. mutabilis reportedly varies between 0.07 and 4.5 g/100 g dw. In general, the alkaloid content in L. mutabilis is higher than that of the other main lupin species. Observed variations in alkaloid content in lupins depend on different factors such as analytical procedures, subspecies or ecotype studied, genetic variability, agronomical factors, and environmental influences. The main alkaloids reported in L. mutabilis are lupanine, sparteine, 3-hydroxylupanine, 13-hydroxylupanine, and 4-hydroxylupanine. From those lupanine is also the main alkaloid reported in other lupins.
Toxicity
Most of the information on toxicity of alkaloids in humans comes from reports of accidental events and from a few studies. Therefore, the toxicity in humans (specially the chronic toxicity) is not well known, but several assumptions can be made. Infants and children are more susceptible to alkaloids than adults. The provisional minimum lethal acute dose of total alkaloids for infants and children is considered to be 10 mg/kg bw and for adults this is 25 mg/kg bw. As to chronic toxicity, there are no established safe daily doses. Values reported as safe vary from 0.035 mg/kg bw/d up to 500 mg/d. Based on the Andean region, the amount of alkaloids in the diet is clearly higher than 0.035 mg/kg/d, suggesting that this value is underestimated. This would imply one of the following options (i) the Andean population developed a certain resistance to alkaloids as compared to the European population, (ii) lupin consumers in the Andean population are suffering from chronic disease, or (iii) the value 0.035 mg/kg bw/d is wrong. On the other hand, the value of 500 mg/d seems to contradict even acute doses of 10 mg/kg bw in children, suggesting that this value is also wrong or at least overestimated. In addition, Jiménez-Martínez et al. (Citation2003a) suggested that alkaloid contents in the seed that are above 0.03% could result in a decrease in animal growth. This value is lower than the 0.07% that is the maximum value accepted by the Ecuadorean Institute of Standards (INEN Instituto Ecuatoriano de Normalización, Citation2005). In short, there is no established safe daily amount (dose) of alkaloids for human beings, pointing out the necessity to do research in this field.
Allergenicity and Anti-nutritional Factors
Taking into account the test of allergenicity and the antinutritional content of lupins we might consider them as safe for human consumption. However, because there is just one reported study of allergenicity, it is necessary to do more research in this field to supplement the initial findings.
Debittering Processes
Biological, chemical, and aqueous debittering processes can reduce the alkaloid content in lupin seeds with different outcomes depending on the conditions. First, bacterial or fungal fermentation reduced alkaloid contents, but from seeds with low alkaloid contents (lower than 1%). The applicability of the fermentation process as a means to reduce alkaloids in lupin seeds with higher alkaloid contents remains to be investigated. In addition, part of the alkaloid reduction by the fermentation approach is due to the initial processing steps, namely soaking and cooking. For tempeh fabrication, the simultaneous debittering-fermentation process can be accelerated by using lupin seeds that are first dehulled, crashed or flaked, soaked, and cooked, since these operations facilitate the contact between alkaloids and strains. In addition, hydration increases the water content in the seed and facilitates alkaloid extraction during the following steps. Cooking is essential to destroy the germinative capacity of seeds, inhibit enzymatic activity (by lipase, lipoxygenase), eliminate microorganisms adhered to the seed (which could produce toxins), reduce the loss of proteins through their coagulation and to facilitate the physical washing away of the alkaloids, oligosaccharides, or other antinutritional factors (because of increased cell wall permeability) (Gross et al., Citation1983; Jiménez-Martínez et al., Citation2003a). During fermentation the K+ content (and perhaps that of other nutrients) might have to be monitored because the efficiency of the process was reported as K+ dependent. Fermentation changes the taste and texture of lupin, which can be an advantage or disadvantage depending on the food type that consumers prefer. In addition, most fermentation processes need to use energy for several days, which is a disadvantage for economic and sustainability reasons.
Chemical treatments can reduce the alkaloid content in lupins, even in seeds with high amounts of alkaloids (up to 4.2%), and in some cases in a short time (i.e., less than one hour). Basic treatments diminish the methionine content and therefore reduce lupin PER. Chemical treatments also cause about 13% material losses. In addition, there is still uncertainty regarding the safety of these chemically treated products, customer acceptance, disposal of chemical compounds, and possibilities of water reutilization.
Aqueous treatments can also reduce the alkaloid content in lupin seeds, even in those with a high alkaloid content. These processes do not require the disposal of chemicals, nor a complicated infrastructure. Moreover, they do not change the availability of methionine. For debittering whole seeds to be used as food for humans, the aqueous treatment is the only process known to be applied on a commercial scale. The aqueous treatment reduces the alkaloid content in the whole seed without changing its natural flavor. This is especially important when the whole seed is eaten as a snack. This process uses significant amounts of water and time, and causes material loss. However, material lost can be recovered by centrifugation, decantation, or flocculation. The water used can be treated and reused several times and the speed of the process can possibly be improved by enhancing the diffusion of alkaloids during processing.
When the above methods are compared, we see that particle reduction is applied in biological and chemical processes to speed up the removal of alkaloids, but it is not used in aqueous debittering. Incorporating this pretreatment in the aqueous debittering process could also speed up the washing out of alkaloids and perhaps of some antinutritional factors. Particle size reduction could also diminish the use of water, energy, and labor and can be applied when debittered lupin will be used as flour, or a food ingredient.
When lupin protein is used as functional food ingredient, it is important to consider that most of its functional properties will be modified if protein denaturation occurs during the isolation, for instance, due to physicochemical (pH variations), physical (heat), chemical (acylation), or enzymatic treatments. For example, faba bean protein precipitated at pH 2 has a decreased solubility in both alkaline and acid conditions, but its water adsorption capacity is increased about threefold (Gueguen and Cerletti, Citation1994).
Debittering of lupin seeds with high alkaloid contents requires further research, especially with respect to the efficiency, sensorial quality, and economic feasibility.
Uses of Lupins
After debittering, lupins can be used as a food: eaten directly as snack, or as an ingredient of many products and meals because of the nutritional value of the seed, especially for L. mutabilis, which is comparable to that of soya bean. The nutritional value of the seed can be affected by the debittering process applied. However, on the other hand, the nutritional value can be increased by fortifying with DL-methionine or by eating lupin in combination with a product rich in sulfur-containing amino acids, such us cereals. Doing so increases the PER value.
Alkaloids from lupins can be used in the medical field. Some studies suggest that certain QA have pharmacological activity. However, more research is needed to validate preliminary results, and to establish action mechanisms, doses, protocols, and contraindications.
The similarities and differences between lupin and soya proteins in terms of physical characteristics point at opportunities for increasing the use of lupin as an ingredient in the food industry, or even the replacement of soya as a food ingredient in countries where lupin is abundant and when lupin protein shows a better or similar physical behavior as soya. For example, the structural changes during protein gelation appear to be similar for L. albus and soya bean proteins (Kiosseoglou et al., Citation1999). Lupin applications can benefit from the extensive research in the area of soya bean based food (Doxastakis, Citation2000). The available research shows that lupins could be used as a functional ingredient for the following properties:
| – | L. mutabilis concentrate for its oil absorption capacity, emulsifying activity, emulsifying stability, protein solubility, foaming stability, and least gelation concentration. | ||||
| – | L. angustifolius isolate for its water absorption capacity and flour for its water absorption capacity and least gelation concentration. | ||||
| – | L. albus isolate for its emulsifying capacity, protein solubility, and foaming capacity. | ||||
However, there are so many variables and interactions, which are not yet fully understood, that affect the behavior of lupin isolates and concentrates, that the suggested applications should be regarded as preliminary and only valid for the conditions and varieties studied.
RECOMMENDATIONS
With respect to future research, we recommend:
To pay more attention to experimental procedures, such as sampling, storage conditions, specification of genetic make-up, and agronomical conditions of samples to obtain more precise information about the cause of biological variation. In addition more attention should be given to the reporting of the accuracy and precision of the experimental methods and obtained results.
To investigate lupin seeds as protein and fat sources (especially for L. mutabilis), their flours, protein isolates, and subproducts (alkaloids, oligosaccharides, and molasses).
To determine the maximum tolerable alkaloid content in the human diet and in the debittered seeds, as the safe doses for humans are still unclear.
To further investigate the debittering processes with a focus on the nutritional quality of the debittered seed, the effectiveness of the process expressed as extracted alkaloids, energy and time used, residues generated, solids lost, consumer acceptance, and the possibility to reutilize (or to reduce) processing water (and, if applicable, chemicals), and economic feasibility of the applied technique.
FUNDING
This research was conducted in the context of the TELFUN project, supported by the INREF fund (Wageningen University, The Netherlands) and Universidad San Francisco de Quito, Ecuador.
REFERENCES
- Agosin, E., Diaz, D., Aravena, R. and Yañez, E. (1989). Chemical and nutritional characterization of lupine tempeh. J. Food Sci. 54:102–104, 107.
- Aguilera, J. M. and Garcia, H. D. (1989). Protein extraction from lupin seeds: a mathematical model. Int. J. Food Sci. Technol. 24:17–27.
- Aguilera, J. M., Gerngross, M. F. and Lusas, E. W. (1983). Aqueous processing of lupin seed. J. Food Technol. 18:327–333.
- Aguilera, J. M. and Trier, A. (1978). The revival of the lupin. J. Food Technol. August:70–76.
- Alamanou, S., Bloukas, J. G., Paneras, E. D. and Doxastakis, G. (1996). Influence of protein isolate from lupin seeds (Lupinus albus ssp. Graecus) on processing and quality characteristics of frankfurters. Meat Sci. 42:79–93.
- Alamanou, S. and Doxastakis, G. (1995). Thermoreversible size selective swelling polymers as a means of purifications and concentration of lupin seed proteins (Lupinus albus ssp. Graecus). Food Hydrocolloids. 9:103–109.
- Alamanou, S. and Doxastakis, G. (1997). Effect of wet extraction methods on the emulsifying and foaming properties of lupin seed protein isolates (Lupinus albus ssp. Graecus). Food Hydrocolloids. 11:409–413.
- Andersen, K. E., Bjergegaard, C., Møller, P., Sørensen, J. C. and Sørensen, H. (2005). Compositional variations for α-galactosides in different species of leguminosae, brassicaceae, and barley: a chemotaxonomic study based on chemometrics and high-performance capillary electrophoresis. J. Agr. Food Chem. 53:5809–5817.
- Arias, G. M. and Felacio, C. H. (1986). Estudio exploratorio de la hidrólis enzimática de la proteína de ajonjolí. Tecnología. 28:21–36.
- Australia New Zealand Food Authority (2001). Lupin alkaloids in food. In: Technical Report Series No. 3, pp. 1–21. Australia New Zealand Food Authority, Australia.
- Beirao da Costa, M. L. D. M. (1989). Aspects of lupin composition as food. In: Lupin Production and Bioprocessing for Feed, pp. 94–105. Food and Other By-Products, Jerusalem, Israel, .
- Bélteky, B. and Kovács, I. (1984). In: Lupin the New Break, p. 140. Edwards, J. G., (Ed.). Bradford on Avon:Panagri.
- Beuchat, L. R. (1977). Functional and electrophoretic characteristics of succinylated peanut flour protein. J. Agr. Food Chem. 25:258–261.
- Blagrove, R. J. and Gillespie, J. M. (1975). Isolation, purification and characterization of the seed globulins of Lupinus angustifolius. Aust. J. Plant Physiol. 2:13–27.
- Blagrove, R. J., Gillespie, J. M., Lilley, G. C. and Woods, E. F. (1980). Physicochemical studies of conglutin gamma, a storage globulin from seeds of Lupinus angustifolius. Aust. J. Plant Physiol. 7:1–13.
- Blagrove, R. J. and Guillespie, J. M. (1978). Comparative studies on the proteins from seeds of Lupinus angustifolius L. Aust. J. Plant Physiol. 5:651–663.
- Bleitgen, R., Gross, R. and Gross, U. (1979). Die lupine-ein beitrag zur nahrungsversorgung in den Anden, 5. einige beobachtungen zur traditionellen entbitterung von lupinen im wasser. Zeitschrift für Ernährungswissenschaft. 18:104–111.
- Caicedo, C., Peralta, E., Villacrés, E. and Rivera, M. (2001). Poscosecha y mercado del chocho (Lupinus mutabilis Sweed) en Ecuador, Vol 105. INIAP-FUNDACIT, Quito, Ecuador. In: Chocho o Lupino Producción, Fitonutrición, Enfermedades y Plagas, Zonificación, Mercado y Postcosecha, Agroindustria, Guía del Cultivo de Chocho y Costos de Producción, Peralta, E., Ed. Published as CD by INIAP-FUNDACIT. Quito, Ecuador.
- Camacho, L., Sierra, C., Marcus, D., Guzmán, E., Campos, R., Von Bäer, D. and Trugo, L. (1991). Nutritional quality of lupine (Lupinus albus cv. Multolupa) as affected by lactic acid fermentation. Int. J. Food Microbiol. 14:277–286.
- Carvajal-Larenas, F. E., van Boekel, M. A. J. S., Koziol, M., Nout, M. J. R. and Linnemann, A. (2014) Effect of processing on the diffusion of alkaloid and quality of Lupinus mutabilis Sweet. J. Food Process. Preserv. 38(4):1461–1471.
- Carvalho, I. S., Chaves, M. and Ricardo, C. P. (2005). Influence of water stress on the chemical composition of seeds of two lupins (Lupinus albus and Lupinus mutabilis). J. Agron. Crop Sci. 191:95–98.
- Carvalho, I. S., Ricardo, C. P. and Chaves, M. (2004). Quality and distribution of assimilates within the whole plant of lupines (L. albus and L. mutabilis) influenced by water stress. J. Agron. Crop Sci. 190:205–210.
- Castillo, R. (1965). Estudio sobre Lupinus (chocho) en el Ecuador. Archivos Venez. de Nutr. 15:87–93.
- Cerletti, P. (1983). Lupin seed proteins. In: Developments in Food Proteins, pp. 133–171. Hudson, B. J. F., Ed., Applied Science Publishiers, Essex, U.K.
- Cerletti, P., Fumagalli, A. and Venturin, D. (1978). Protein composition of seeds of Lupinus albus. J. Food Sc. 43:1409–1414.
- Chajuss, D. (1989). Some technological aspects of lupin versus soybean processing. In: Lupin Production and Bioprocessing for Feed, Food and Other by-Products, Jerusalem, Israel, Commission of the European Communities. pp. 106–114.
- Chango, A., Bau, H. M., Villaume, C., Schwertz, A., Nicolas, J. P. and Mejean, L. (1993a). Effets des traitements (chauffage et fermentation par Rhizopus oligosporus sp-T3) de la graine de lupin blanc doux sur certains facteurs de son utilisation nutritionnelle. Reprod. Nutr. Dev. 33:89–98.
- Chango, A., Villaume, C., Bau, H. M., Nicolas, J. P. and Mejean, L. (1993b). Debittering of lupin (Lupinus luteus L) protein by calcium alginate and nutritional evaluation. J. Sci. Food Agr. 63:195–200.
- Chou, D. H. and Morr, C. V. (1979). Protein-water interactions and functional properties. J. Amer. Oil Chem. Soc. 56:53A–62A.
- Ciesiolka, D., Gulewicz, P., Martínez-Villaluenga, C., Pilarski, R., Bednarczyk, M. and Gulewicz, K. (2005). Products and biopreparations from alkaloid-rich lupin in animal nutrition and ecological agriculture. Folia Biologica (Kraków). 53:59–66.
- Cremer, H.-D. (1983). Current aspects of legumes as a food constituent in Latin America with special emphasis on lupines: Introduction. Qual. Plant., Plant Foods Human Nutr. 32:95–100.
- Dagnia, S. G., Petterson, D. S., Bell, R. R. and Flanagan, F. V. (1992). Germination alters the chemical-composition and protein quality of lupin seeds. J. Sci. Food Agr. 60:419–423.
- Dagorn-Scaviner, C., Gueguen, J. and Lefebvre, J. (1987). Emulsifying properties of pea globulins as related to their adsorption behaivors. J. Food Sc. 52:335–341.
- Damodaran, S. (1997). Food proteins: An overview. In: Food Proteins and Their Applications, pp. 1–21. Damodaran, S. and Paraf, A., Eds., Marcel Dekker, New York.
- Dickinson, E. and Walstra, P. (1993). Food Colloids and Polymers: Stability and Mechanical Properties. Royal Society of Chemistry, London.
- Doxastakis, G. (2000). Lupin seed proteins. In: Novel Macromolecules in Food Systems. Doxastakis, G. and Kiosseoglou, V., Eds., Elsevier Science B. V., Thessaloniki, Grece.
- Duranti, M., Guerrieri, N., Takahashi, T. and Cerletti, P. (1988). The legumin-like storage protein of Lupinus albus seeds. Phytochemestry. 27:15–23.
- Duranti, M., Restani, P., Poniatowska, M. and Cerletti, P. (1981). The seed globulins of Lupinus albus. Phytochemestry. 20:2071–2075.
- D´Agostina, A., Antonioni, C., Resta, D., Arnoldi, A., Bez, J., Knauf, U. and Wäsche, A. (2006). Optimization of a pilot-scale process for producing lupin protein isolates with valuable technological properties and minimum thermal damage. J. Agr. Food Chem. 54:92–98.
- FAO. (2012a). FAO Statistical Databases. FAO, United Nations. Available from http://faostat.fao.org., Rome.
- FAO. (2012b). Neglected Crops:1492 from a Different Perspective. FAO, United Nations. Available from http://fao.org/docrep/t0646e/T0646E0f.htm, Rome.
- Feeney, R. E. and Whitaker, J. R. (1985). Chemical and enzymatic modifications of plant proteins. In: New Protein Foods: Seed Storage Proteins, p. 471. Wilckle, H. L. and Attschul, A. M., Eds., Academic Press, New York.
- Francki, M. G., Whitaker, P., Smith, P. M. and Atkins, C. A. (2002). Differencial expression of a novel gene during seed triacyglycerol accumulation in lupin species (Lupinus angustifolius L. and L. mutabilis). Funct. Integral Genom. 2:292–300.
- Gerritsen, T. H. (1956). Lupin seed proteins: iv. aminoacid composition of the globulins from Lupinus angustifolius and Lupinus luteus. Biochimica et Biophysica Acta. 22:269–273.
- Gladstones, J. S. and Drover, D. P. (1962). The mineral composition of lupins. 1. A survey of the cupper, molybdenum and manganese contents of lupins in the south-west of Western Australia. Aust. J. Exper. Agr. Anim. Husb. 2:46–53.
- Graham, D. E. and Phillips, M. C. (1980). Proteins at liquid interfases. V. Shear properties. J. Colloid Interfase Sci. 76:240–250.
- Gross, U., Godomar-Galindo, R. and Schoeneberger, H. (1983). The development and acceptability of lupine (Lupinus mutabilis) products. Qual. Plant./Plant Foods Human Nutr. 32:155–164.
- Gross, U., Reyes, J., Gross, R. and von Baer, E. (1976). Die lupine, ein beitrag zur nahrungsversorgung in den Anden, 4. Die akzeptabilität des mehles von Lupinus albus. Zeitschrift für Ernährungswissenschaft. 15:395–402.
- Gross, R., von Baer, E., Koch, F., Marquard, R., Trugo, L. and Wink, M. (1988). Chemical composition of a new variety of the Andean lupin (Lupinus mutabilis cv. Inti) with low-alkaloid content. J. Food Composit. Anal. 1:353–361.
- Gueguen, J. (1991). Pea and fababean proteins. In: Developments in Food Proteins-7, pp. 35–78. Hudson, B. J. F., Ed., Elsevier Applied Science, Amsterdam.
- Gueguen, J. and Cerletti, P. (1994). Proteins of some legume seeds: soybean, pea, fababean and lupin. In: New and Developing Sources of Food Proteins, pp. 145–193. Hudson, B. J. F., Ed., Chapman & Hall, London.
- Guerrieri, N. and Cerletti, P. (1990). Association and folding in legumin oligomers of lupin seed. J. Protein Chem. 9:397–405.
- Güémes-Vera, N., Peña-Bautista, R. J., Jiménez-Martínez, C., Dávila-Ortiz, G. and Calderón-Domínguez, G. (2008). Effective detoxification and decoloration of Lupinus mutabilis seed derivatives, and effect of these derivatives on bread quality and acceptance. J. Sci. Food Agr. 88:1135–1143.
- Haq, N. (1993). Lupins (Lupinus species). In: Pulses and Vegetables, pp. 103–129. Williams, J. T., Ed., Chapman & Hall, London.
- Harpal, S. S. and Gladstones, J. S. (1986). Variability in the total and component galactosyl sucrose oligosaccharides of Lupinus species. Aust. J. Agr. Res. 37:157–166.
- Hatzold, T., Elmadfa, I., Gross, R., Wink, M., Hartmann, T. and Witte, L. (1983). Quinolizidine alkaloids in seeds of Lupinus mutabilis. J. Agr. Food Chem. 31:934–938.
- Hettiarachchy, N. S. and Ziegler, G. R. (1994). Protein Functionality in Food Systems. p. 519. Marcel Dekker, Inc, New York.
- Hrčkova, M., Rusňaková, M. and Zemanovič, J. (2002). Enzymatic hydrolisis of deffatted soy flour by three different proteases and their effect on the functional properties of resulting protein hydrolysates. Czech J. Food Sc. 20:7–14.
- Hudson, B. J. F. (1994). New and Developing Sources of Food Proteins. p. 371. Chapman & Hall, London.
- INEN Instituto Ecuatoriano de Normalización (2005). Norma Técnica Ecuatoriana NTE INEN 2 390:2004. Leguminosas. Grano desamargado de chocho. Requisitos. pp. 1–7. Instituto Ecuatoriano de Normalización, Quito, Ecuador.
- Jambrina-Alonso, J. L. (1983). La génetica de los alkaloides en el género Lupinus. In: Comunicaciones I.N.I.A., pp. 1–12. Agrarias, I. N. d. I., Ed., Servicio de Publicaciones Agrarias Madrid.
- Jiménez-Martínez, C. and Dávila-Ortiz, G. (2006). Elaboración de un producto tipo botana a base de harina de trigo fortificada con aislado proteico de Lupinus mutabilis. In: Industria Alimentaria, pp. 12–20. Alfa Editores Técnicos, México, D.F. Miami: Chris Luke.
- Jiménez-Martínez, C., Hernández-Sánchez, H. and Dávila-Ortiz, G. (2003a). Lupines: An alternative for debittering and utilization in foods. In: Food Science and Food Biotechnology, pp. 233–252. Gutiérrez-López, G. and Barbosa-Cánovas, G., Eds., CRC Press, Boca Raton, FL, USA.
- Jiménez-Martínez, C., Hernández-Sánchez, H. and Dávila-Ortiz, G. (2003b). Production of a yogurt-like product from Lupinus campestris seeds. J. Sci. Food Agr. 83:515–522.
- Jiménez-Martínez, C., Hernández-Sánchez, H. and Dávila-Ortíz, G. (2007). Disminution of quinolizidine alkaloids, oligosaccharides and phenolic compounds from two species of Lupinus and soybean seeds by the effect of Rhizopus oligosporus. J. Sci. Food Agr. 87:1315–1322.
- Jiménez-Martínez, C., Loarca-Piña, G. and Dávila-Ortíz, G. (2003c). Antimutagenic activity of phenolic compounds, oligosaccharides and quinolizidinic alkaloids from Lupinus campestris seeds. Food Addit. Contam. 20:940–948.
- Jones, L. J. and Tung, M. A. (1983). Functional properties of modified oilseed protein concentrates and isolates. Can. Inst. Food Sci. Technol. J. 16:57–62.
- Joubert, F. J. (1956). Lupin seed proteins.III. A physico-chemical study of the proteins from white lupin seed (Lupinus albus). Biochim. et Biophys. Acta. 19:172–173.
- Kato, A. and Nakai, S. (1980). Hydrophobicity determined by a florescence probe method and its correlation with surface properties of proteins. Biochim. et Biophys. Acta. 624:13–20.
- King, J., Aguirre, C. and de Pablo, S. (1985). Functional properties of lupin protein isolates (Lupinus albus cv Multolupa). J. Food Sci. 50:82–87.
- Kinsella, J. E. (1976). Functional properties of proteins in foods: a survey. Crit. Rev. Food Sci. Nutr. 7:219–280.
- Kinsella, J. E. (1984). Milk proteins: physicochemical and functional properties. Crit. Rev. Food Sci. Nutr. 21:197–262.
- Kinsella, J. E. and Shetty, K. J. (1979). Chemical modifications for improving functional properties of plan and yeast proteins. In: Symposium Series, 92 (Functionality and Protein Structure), pp. 37–63. Pour-El, A., Ed., American Chemical Society, Washington D.C.
- Kiosseoglou, A., Doxastakis, G., Alevisopoulos, S. and Kasapis, S. (1999). Physical characterization of thermally induced networks of lupin protein isolates prepared by isoelectric precipitation and dialysis. Int. J. Food Sci. Technol. 34:253–263.
- Kiosseoglou, V. and Perdikis, A. (1994). Stability of bovine serum albumin-stabilized olive oil-in-water emulsions and the role of the oil minor surface-active lipids. Food Hydrocolloids. 8:27–32.
- Kiosseoglou, V., Theodorakis, K. and Doxastakis, G. (1989). The rheology of tomato seed protein isolate films at the corn oil-water interface. Colloid Polym. Sci. 267:834–838.
- Konopoka-Waliszkiewicz, L. (1988). Characterization of albumins from Lupinus luteus L. cotyledons. Biologia Plantarum (PRAHA). 30:461–464.
- Lawhon, J. T., Manak, L. J. and Lusas, E. W. (1980). An improved process for isolation of glandless cottonseed protein using industrial membrane systems. J. Food Sci. 45:197–199, 203.
- Liener, I. E. (1994). Implications of antinutritional components in soybean foods. Crit. Rev. Food Sci. Nutr. 34:31–67.
- Lilley, G. G. (1986a). The isolation of conglutin δ, a sulphur-rich protein from the seeds of Lupinus angustifolius. J. Sci. Food Agr. 37:20–30.
- Lilley, G. G. (1986b). The subunit structure and stability of a conglutin δ, a sulphur-rich protein from the seed of Lupinus angustifolius L. J. Sci. Food Agr. 37:895–907.
- Lilley, G. G. and Iuglis, A. S. (1986). Aminoacid sequense of conglutin δ a sulphur-rich seed protein of Lupinus angustifolius L. Sequence homology with the C.III α-amylase inhibitor from wheat. FEBS Lett. 195:235–241.
- Linsberger-Martin, G., Schöenlechner, R. and Berghofer, E. (2010). Physical, nutritional and sensory properties of cookies, pasta and expanded snack products made of the legumes common white beans, peas and lupines. In: 2010 AACC International Annual Meeting. Cereal Foods World Supplement, pp. 55, A58. Savannah Convention Center, Savannah, Georgia.
- Lqari, H., Vioque, J., Pedroche, J. and Millán, F. (2002). Lupinus angustifolius protein isolates: chemical composition, functional properties and protein characterization. Food Chem. 76:349–356.
- Maga, J. A. (1984). Lysinoalanine in foods. J. Agr. Food Chem. 32:955–964.
- Manrique, J., Coles, S. J. and Edwards, R. A. (1974). Cereal Chemistry. In: 24th Anual Conference Australian Chemistry Institute, Melbourne, Australia, p. 68.
- Molina, E., Papadopoulou, A., Defaye, A. and Ledward, D. A. (2002). Functional properties of soy proteins as influenced by high pressure: emulsifying activity. Prog. Biotechnol. (Trends in High Pressure Biosci. Biotechnol.). 19:557–562.
- Moure, A., Sineiro, J., Domínguez, H. and Parajó, J. C. (2006). Functionality of oilseed protein products: a review. Food Res. Int. 39:945–963.
- Muschiolik, G., Dickinson, E., Murray, B. and Stainsby, G. (1987). Interfacial and emulsifying behavior of acetylated field bean protein isolate. Food Hydrocoll. 1:191–196.
- Múzquiz, M., Burbano, C., Gorospe, M. J. and Ródenas, I. (1989). A chemical study of Lupinus hispanicus seed-toxic and antinutritional components. J. Sci. Food Agr. 47:205–214.
- Múzquiz, M., Cuadrado, C., Ayet, G., de la Cuadra, C., Burbano, C. and Osagie, A. (1994). Variation of alkaloids components of lupin seeds in 49 Genotypes of Lupinus albus L. From different countries and locationes. J. Agr. Food Chem. 42:1447–1450.
- Nossack, A. C., Vilegas, J. H. Y., von Baer, D. and Lanças, F. M. (2000). Supercritical fluid extraction and chromatographic analysis (HRGC-FID and HRGC-MS) of Lupinus spp. alkaloids. J. Brazilian Chem. Soc. 11:495–501.
- Olsen, H. S. and Andersen, J. H. (1978). The estimation of vicine and convicine in fababean protein. J. Sci. Food Agr. 29:323–331.
- Ortiz, C., Gross, R. and von Baer, E. (1975). Die lupine, ein beitrag zur nahrungsversorgung in den Anden. 2. Die proteinqualität von Lupinus mutabilis im vergleich zu Lupinus albus, Lupinus luteus und Soja max. Zeitschrift für Ernährungswissenschaft. 14:229–233.
- Ortiz, J. G. F. and Mukherjee, K. D. (1982). Extraction of alkaloids and oil from bitter lupin seed. JAOCS (J. Am. Oil Chem. Soc.). 59:241–244.
- Pate, J. S., Williams, W. and Farrington, P. (1985). Lupin (Lupinus spp.). In: Grain Legume Crops, pp. 699–746. Summerfield, R. J. and Roberts, E. H., Eds., Collins Professional and Technical Books, London.
- Peng, J. C., Quass, D. W., Dayton, W. R. and Allen, C. E. (1984). The physicochemical and functional properties of soybean 11S globulin-a review. Cereal Chem. 61:480–490.
- Petterson, D. S. (1998). Composition and food uses of lupins. In: Lupins as Crop Plants Biology, Production and Utilization, pp. 353–384. Gladstones, J. S., Atkins, C. A. and Hamblin, J., Eds., CAB INTERNATIONAL, Australia.
- Petterson, D. S. and Crosbie, G. B. (1990). Potential for lupins as food for humans. Food Aust. 42:266–268.
- Petterson, D. S. and Mackintosh, J. B. (1994). The Chemical Composition and Nutritive Value of Australian Grain Legumes. Grains Research and Development Corporation, Camberra.
- Peñaloza, W., Davey, C. L., Hedger, J. N. and Kell, D. B. (1991). Stimulation by potassium ions of the growth of Rhizopus oligosporus during liquid-and solid-substrate fermentations. World J. Microbiol. Biotechnol. 7:260–268.
- Restani, P., Duranti, M., Cerletti, P. and Simonetti, P. (1981). Subunit composition of the seed globulins of Lupinus albus. Phytochemistry. 20:2077–2083.
- Rhama, E. M. and Narasinga, R. M. S. (1984). Effect of debittering treatment on the composition and protein components of lupine seed (Lupinus termis) flour. J. Agr. Food Chem. 32:1026–1030.
- Rivadeneira, J., Córdova, J. and Peralta, E. (2001). Fitonutrición del cultivo del chocho. In: El Cultivo del Chocho Lupinus mutabilis Sweet: Fitonutrición, Enfermedades y Plagas, en el Ecuador, pp. 12–18. Caicedo, C. and Peralta, E., Eds., INIAP-FUNDACIT, Quito, Ecuador. In: Chocho o lupino producción, fitonutrición, enfermedades y plagas, zonificación, mercado y postcosecha, agroindustria, guía del cultivo de chocho y costos de producción, ed. Peralta, E., Published as CD by INIAP-FUNDACIT, Quito, Ecuador.
- Rodgers, S., Busch, J., Peters, H. and Christ-Hazelhof, E. (2005). Building a tree of knowledge: Analysis of bitter molecules. Chem. Senses. 30:547–557.
- Rozan, P., Lamghari R, Linder, M., Villaume, C., Fanni, J., Parmentier, M. and Méjean, L. (1997). In vivo and in vitro digestibility of soybean, lupine, and rapeseed meal proteins after various technological processes. J. Agr. Food Chem. 45:1762–1769.
- Ruales, J., Pólit, P. and Nair, B. M. (1988). Nutritional quality of blended foods of rice, soy and lupins, processed by extrusion. Food Chem. 29:309–321.
- Santana, F. C. and Empis, J. (2001). Bacterial removal of quinolizidine alkaloids from Lupinus albus flours. Eur. Food Res. Technol. 212:217–224.
- Santana, F. M. C., Fialho, A. M., Sá-Correia, I. and Empis, J. M. A. (1996). Isolation of bacterial strains capable of using lupanine, the predominant quinolizidine alkaloid in white lupin, as sole carbon and energy source. J. Ind. Microbiol. 17:110–115.
- Santos, C. N., Ferreira, R. B. and Teixeira, A. R. (1997). Seed proteins of Lupinus mutabilis. J. Agr. Food Chem. 45:3821–3825.
- Sathe, S. K., Deshpande, S. S. and Salunkhe, D. K. (1982). Functional properties of lupin seed (Lupinus mutabilis) proteins and protein concentrates. J. Food Sc. 47:491–497.
- Sgarbiere, V. C. and Galeazzi, M. A. M. (1978). Some physicochemical and nutritional properties of a sweet lupin (Lupinus albus var. Multolupa) protein. J. Agr. Food Chem. 26:1438–1442.
- Smith, A. k. and Circle, S. J. (1978). Soybeans: Chemistry and Technology, vol 1, 2 Ed., Avi Pub Co, Westport, Connecticut.
- Szakács, G. and Stankovics, L. (1983). Debittering of lupine seed by lactic acid fermentation. In: Symposium on Microbiology 1983, Budapest, pp. 273–274 (1984).
- Taha, F. S. and Ibrahim, M. A. (2002). Effect of degree of hydrolysis on the functional properties of some oilseed proteins. Grasas y Aceites. 53:273–281.
- Thompson, R. and Casey, R. (1983). Perspectives for peas and lupin crops. p. 380. M. Nijhoff, Hague.
- Tolstogouzov, V. B. (1991). Functional properties of food proteins and role of protein-polysaccharide interactions. Food Hydrocol. 4:429–468.
- Torres-Tello, F., Nagata, A. and Dreifuss-Spiegel, W. (1980). Métodos de eliminación de alcaloides en la semilla de Lupinus mutabilis, Sweet [Methods of eliminating alkaloids from the seeds of Lupinus mutabilis Sweet]. Arch. Latinoamericanos de Nutr. 30:200–209.
- van Vliet, T., Martin, A. H. and Bos, M. A. (2002). Gelation and interfacial behavior of vegetable proteins. Curr. Opin. Colloid Interface Sci. 7:462–468.
- Velev, D. O., Nikolov, D. A., Denkov, D. N., Doxastakis, G., Kiosseoglou, V. and Stalidis, G. (1993). Investigation of the mechanisms of stabilization of food emulsion by vegetable proteins. Food Hydrocol. 7:55–71.
- Villacrés, E., Caicedo, C. and Peralta, E. (2000). Diagnóstico del procesamiento artesanal, comercialización y consumo del chocho. In: Zonificación Potencial, Sistemas de Producción y Procesamiento Artesanal del Chocho (Lupinus mutabilis Sweed) en Ecuador, pp. 24–41. Caicedo, C. and Peralta, E. (Eds.), INIAP-FUNDACIT, Quito, Ecuador. In: Chocho o lupino producción, fitonutrición, enfermedades y plagas, zonificación, mercado y postcosecha, agroindustria, guía del cultivo de chocho y costos de producción, ed. Peralta, E., Published as CD by INIAP-FUNDACIT. Quito, Ecuador.
- Villacrés, E., Peralta, E. and Alvarez, M. (2003). Chochos en su punto, Vol 118. INIAP- FUNDACYT, Quito, Ecuador. In Chochos. Recetarios. Disfrute Cocinando con Chochos, ed. Peralta, E., Published as CD by INIAP-FUNDACYT. Quito, Ecuador.
- Villacrés, E., Rubio, A., Egas, L. and Segovia, G. (2006). Usos alternativos del chocho. In: Instituto Nacional Autónomo de Investigaciones Agropecuarias, pp. 1–19. I. N. I. A. P., Ed., Fundación para la Ciencia y la Tecnología, FUNDACYT, Quito, Ecuador.
- Walton, G. H. and Francis, C. M. (1975). Genetic influences on the split seed disorder in Lupinus angustifolius L. Aust. J. Agr. Res. 26:641–646.
- Were, L., Hettiarachchy, N. S. and Kalapathy, U. (1997). Modified soy protein with improved foaming and water hydration properties. J. Food Sc. 62:821–823, 850.
- Wink, M. and Witte, L. (1984). Turnover and transport of quinolizidine alkaloids. Diurnal fluctuations of lupanine in the pholem sap, leaves and fruits of Lupinus albus L. Planta. 161:519–524.
- Youle, R. J. and Huang, A. H. C. (1981). Occurrence of low molecular weight and high cysteine containing storage proteins in oilseeds of diverse species. Amer. J. Botany. 68:44–48.
- Zdunczyk, Z., Juskiewicz, J., Frejnagel, S., Flis, M. and Godycka, I. (1994). Chemical composition of the cotyledons and seed coat and nutritional value of whole and hulled seeds of yellow lupin. J. Anim. Feed Sci. 3:141–148.