Abstract
Digestible maltodextrins are low-sweet saccharide polymers consisting of D-glucose units linked primarily linearly with alpha-1,4 bonds, but can also have a branched structure through alpha-1,6 bonds. Often, maltodextrins are classified by the amount of reducing sugars present relative to the total carbohydrate content; between 3 and 20 percent in the case of digestible maltodextrins. These relatively small polymers are used as food ingredients derived by hydrolysis from crops naturally rich in starch. Through advances in production technology, the application possibilities in food products have improved during the last 20 years. However, since glucose from digested maltodextrins is rapidly absorbed in the small intestine, the increased use has raised questions about potential effects on metabolism and health. Therefore, up-to-date knowledge concerning production, digestion, absorption, and metabolism of maltodextrins, including potential effects on health, were reviewed. Exchanging unprocessed starch with maltodextrins may lead to an increased glycemic load and therefore post meal glycaemia, which are viewed as less desirable for health. Apart from beneficial food technological properties, its use should accordingly also be viewed in light of this. Finally, this review reflects on regulatory aspects, which differ significantly in Europe and the United States, and, therefore, have implications for communication and marketing.
INTRODUCTION
Maltodextrins (MDs) are a class of carbohydrates (CHOs) extracted from a range of botanical sources. They are industrially produced by enzymatic or acid hydrolysis of the starch, followed by purification and spray drying (Takeiti et al., Citation2010). The resulting commercially available, mostly white, powders are of high purity and microbiological safety and are used in a wide range of food and beverage products, including baked goods and sports drinks (Chronakis, Citation1998; BeMiller and Whistler, Citation2009). Although confusing to the average consumer, both digestible and resistant-to-digestion type of MDs are commercially exploited as food ingredients under the same denominator (Whelan, Citation2008). While it is the case that MDs can be rendered indigestible to be used as a dietary fiber or prebiotic (Brouns et al., Citation2007), we will only highlight aspects of digestible MDs in the present paper.
With an energy value of approximately 16 kJ/g (4 kcal/g), as underlined by international food law standards (Regulations, Citation2009; EC, Citation2011), digestible MDs used in foods and beverages have long been considered to be a good source of energy (Altschul, Citation1989). Most MDs are fully soluble in water and exert other important functionalities, such as gelling or freeze control (Stephen et al., Citation2006). As such, MDs have found numerous applications in the food, beverage, dietetic, and medical food products, as well as in the pharmaceutical industry in tablet and powder applications (BeMiller and Whistler, Citation2009).
Sixteen years ago, relevant literature and data on the molecular characteristics, compositional properties, and structural-functional mechanisms of MDs were thoroughly reviewed (Chronakis, Citation1998). In recent years, concerns have been raised about the increased use of refined CHOs, including isolated starches and MDs, in food and beverage and its relation to increased obesity rates (Ogden et al., Citation2012). One of the main reasons for concern is that, depending on the quantity consumed, refined CHO sources can have a strong impact on the post-ingestion blood glucose, insulin, and lipid levels. Discernible changes in these markers have been linked to potential health risks, especially in vulnerable individuals such as diabetic patients (Johnson et al., Citation2009; Parker et al., Citation2010; Welsh et al., Citation2010).
In the present paper, we review available literature from food technology, food chemistry, behavioral nutrition, and biological sciences to provide an up-to-date reflection on the current use of digestible MDs. In doing so, we review in brief the production process of different MDs and the physicochemical, technological, and functional properties. Moreover, by incorporating literature streams from nutritional and medical sciences, the current knowledge on nutrition and health implications is reflected in the light of observed correlations between increased refined CHOs intake and increase in obesity rates and related health disorders (Astrup, Citation1999). In the case that the consumption of rapidly digested starch, as well as products derived thereof plays a significant role in the etiology of obesity, steps should be taken by regulatory authorities to inform the public appropriately. We discuss this conundrum with the aim of supplying a solid basis for our conclusions, which include health, commercial and regulatory aspects of our findings.
PRODUCTION AND COMPOSITION OF MALTODEXTRINS
As introduced, MDs are produced by hydrolysis of starch from different botanical sources. During the production process, native starch is heated in the presence of water, causing the crystalline structure of starch granules to swell and be broken irreversibly. This gelatinization process makes starch available for enzymatic or acidic degradation, or a combination of both (BeMiller and Whistler, Citation2009). After degradation, chains of D-glucose units are left with varying length and appearance. Digestible MDs ((C6H10O5)nH2O) have a relatively short chain length and can be defined as saccharide polymers obtained from edible starch having a so-called dextrose equivalency (DE) of less than 20 (Takeiti et al., Citation2010), as will be discussed later.
Before doing so, it is important to note that starch granules mainly contain varying amounts of two types of glucose polymers: amylose and amylopectin, which differ in molecular structure. In amylose, glucose units are linked in a linear structure by α1,4 glycosidic links while some glucose units in amylopectin are linked by α1,6 bonds, resulting in branched structures (Buléon et al., Citation1998; Tester et al., Citation2004). Most starches contain approximately 70–80% amylopectin and roughly 20–30% amylose. The latter is known to be less rapidly digested by pancreatic α-amylase (Topping et al., Citation1997; Englyst and Englyst, Citation2005). Thus, depending on the amylose content of the native starch (some specific selected crops have a high amylose starch content of up to 70%) differences in blood glucose response will occur. Accordingly, high amylose rice has a lower glycemic index (GI = 38) than low amylose rice (GI = 57) (Atkinson et al., Citation2008).
The amylose/amylopectin ratio in different native starches can also influence the properties and therefore technological applications of the MDs derived from these starches as well as their digestive properties (Coultate, Citation2009). When discussing the production process of MDs, differences in composition are often expressed by the dextrose equivalent (DE). This crude yet relatively simple measurement is often used to express the degree of hydrolysis of starch; the higher the degree of hydrolysis, the higher the DE (Fetzer et al., Citation1953). The DE corresponds to the amount of reducing sugars (in g) expressed as dextrose on 100 g dry matter in the product as shown in Equation 1.
Equation 1: Equation for calculation of the dextrose equivalent (DE) of a carbohydrate (CHO) (Coultate, Citation2009)
Given this equation, free D-glucose (dextrose) has a DE of 100. In glucose polymers, reducing sugars can be present in the ‘tail’ of the molecule, a so-called reducing end. As such, branched MDs are more likely to have high amounts of reducing sugars. With respect to the amylose and amylopectin content, the DE of a MD correlates to the ratio of amylose and amylopectin content in the starch used to produce it; a higher amylopectin content correlates to a higher DE of a MD (Coultate, Citation2009). Dried glucose syrups are, by definition, dried starch hydrolysis products with a DE greater than 20, whereas MDs are defined as dried starch hydrolysis products with a DE equal to or lower than 20, but higher than 3 (Chronakis, Citation1998).
Next to the DE, other factors are used to describe differences in MD properties (Kuntz, Citation1997; Hadnađev et al., Citation2011). Another metric used to describe the molecular composition of MDs is the degree of polymerization (DP), which stands for the average number of monosaccharide units per molecule. It is important to note that the DP of a MD decreases with increasing DE (BeMiller and Whistler, Citation2009). The relationship between DE and DP is depicted in Equation (2).
Equation 2: Equation depicting the relationship between the Dextrose Equivalent (DE) and degree of polymerization (DP) (BeMiller and Whistler, Citation2009)
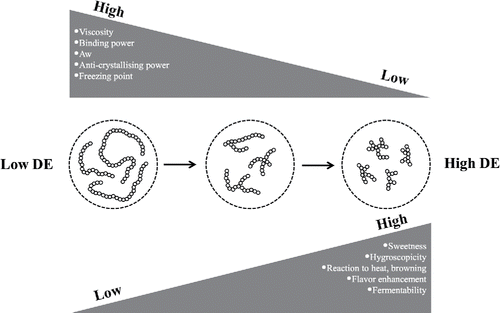
The applicability of MDs in food products is highly influenced by specific physicochemical and technological properties. Among these properties are viscosity, fermentability, solubility, hygroscopicity, freezing point depression, and osmolality. In turn, these properties of MDs strongly depend on their botanical source, production process, and therefore DE. An overview of the relationships between several important physicochemical properties and different DEs is depicted in .
MALTODEXTRINS IN THE HUMAN DIET
The digestive end product of MDs, glucose, is not considered to be an essential nutrient (Westman, Citation2002). Yet, glucose participates in many basic metabolic processes in the body. MDs are considered to be a good source of energy since glucose obtained from its digestion is readily absorbed in the small intestine and subsequently used in metabolism (Cabre et al., Citation1990). Although, large differences also occur here in the rate of branching of MDs (Lee et al., Citation2013). Since MDs are partially depolymerized starch granules, their digestion requires the same enzymes as required for the digestion of starch in vivo (Chronakis, Citation1998). Furthermore, starch, MDs and glucose all have a similar energy value of 4 kcal/g or 16 kJ/g (Livesey, Citation1991).
It is often suggested that there are differences in the rate of digestion and absorption of oral MDs compared to oral glucose. While glucose will be immediately available for absorption upon arrival in the small intestine (Man et al., Citation2006), MDs need to be digested by α-amylase and maltase first. The digestion of starch and MDs starts in the mouth by salivary α-amylase. This enzyme has the ability to breakdown MDs into maltose, a disaccharide consisting of two-linked D-glucose units. It appears that salivary amylase plays a rather small role in the MD breakdown due to the relatively short time that MDs reside in the mouth (Lebenthal, Citation1987). Subsequent to arrival in the stomach, the gastric contents need to be transferred into the small intestinal duodenum for digestion and further transit in the gut. The rate of gastric emptying is regulated by volume effects activating stretch receptors and small intestinal receptors, which sense the composition and quantity (load) of the macronutrients in the gastric effluent. As a result, the gastric emptying of CHO solution is regulated in such a way that an almost constant energy output from the stomach is realized (Brouns, Citation1998), explaining why diluted drinks (low macronutrient- energy content) empty more rapidly from the stomach than concentrated drinks (high macronutrient-energy content).
Pancreatic amylase, secreted in the small intestine, plays a final role in hydrolyzing the α, 1–4 linkages of MDs (Gray, Citation1975), a process that leads to the formation of maltose units. Maltose is either taken up by the gut epithelium directly or further broken down by brush border maltase, resulting in free glucose. The obtained free glucose is actively transported across the apical membrane of the enterocytes, and subsequently across their basement membrane into blood (Gray, Citation1975; Kellett and Helliwell, Citation2000; Ferraris, Citation2001). Due to the difference in digestion and absorption, when compared to glucose, it has often been suggested that low-DE MDs, as complex CHOs, will require more time for digestion and absorption, resulting in a lower glycemic response (Zhang and Hamaker, Citation2009).
This suggestion, however, is a misconception and is not supported by any research data. In contrast, the enzymic digestion of MDs appears to take place at a high rate leading to an absorption rate not being different from absorption after ingestion of pure glucose, as reflected also by comparable post-ingestive insulin responses at rest and during exercise, as well as oxidation rates during exercise (Hawley et al., Citation1992; Wagenmakers et al., Citation1993; Jeukendrup, Citation2004).
During absorption, a small fraction of glucose may be converted to lactic acid by the small intestinal cells or subsequently be taken up by liver, muscle, brain, and red blood cells, to serve as energy source. The nonmetabolized fraction of absorbed glucose will either be stored as liver and muscle glycogen (Nedergaard et al., Citation2003) under the influence of insulin (Guyton and Hall, Citation2000) or converted to lipid.
Health Aspects of Digestible Maltodextrins Consumption
The rise in consumption of refined CHO sources has been linked to an increased health risk (Johnson et al., Citation2009; Parker et al., Citation2010; Welsh et al., Citation2010). Although no causal relationship between the consumption of MDs and negative health effects has been reported, this does not mean that overconsumption of foods containing MDs will have no effect. The regular intake of calorie dense, low-fiber/protein foods or drinks with high levels of refined added CHOs, in particular soft drinks and sweet snacks, may easily induce a persistent positive energy balance resulting in weight gain, impaired insulin sensitivity as well as increased blood cholesterol and blood lipids (Gross et al., Citation2004; Johnson et al., Citation2009; Lustig et al., Citation2012; Lecoultre et al., Citation2013). Accordingly, consumers should consume in moderation and food and beverage producers should reduce the energy density of food and beverage while taking care for an appropriate nutrient, fiber, and protein level where possible.
APPLICATION OF MALTODEXTRINS IN FOOD PRODUCTS
Through advances in science and technology, the knowledge on the (functional) application possibilities of MDs in food and beverage products has improved significantly during the last 20 years. Due to their specific technological/functional properties and easy applicability, MDs can substitute sucrose (O'Brien-Nabors, Citation2011) or fat (Alexander, Citation1995; Hadnađev et al., Citation2011), and are being used in ice cream, dried instant food formulations, confectionary, cereals, snacks, and beverages (Takeiti et al., Citation2010). Below, we will elaborate on selected application areas with a focus on health-related aspects and regulatory environment.
Infant Nutrition
There is a strong nutritional reliance on lactose as a source of energy in early human development (Urashima et al., Citation2011), preferably as part of the mother's breast milk. However, lactase deficiency resulting in the inability to digest may lead to malabsorption-induced osmotic diarrhea in which approximately 40% of the energy provided may be lost (Siddiqui and Osayande, Citation2011). In such cases, MDs can be used as a substitute for lactose to provide energy (Maldonado et al., Citation1998). In this respect, it is suggested also that the use of MDs, instead of glucose is favorable since this helps reduce osmotic load and related intestinal distress (Gregorio et al., Citation2010). MDs are also used as a CHO source in nonallergic infant formulae containing nondairy proteins (soy) or hydrolyzed proteins (hypoallergenic formulas).
Clinical Nutrition
In clinical nutrition, MDs are applied in enteral and parenteral nutrition in which they can be combined with proteins for use of preoperative feeding and drinks (Cabre et al., Citation1990). Administering preoperative drinks containing MDs and protein, instead of using the conventional method of preoperative fasting, to patients undergoing major surgery for gastrointestinal malignancies seems to be a practical approach. For example, in one study, patients undergoing gastrointestinal surgery either received preoperative drinks containing 11% proteins, 70% MDs, and 19% sucrose (intervention group) or fasted prior to their surgery (control group). Results showed that the average postoperative hospital stay of patients in the intervention group was 50% lower compared to the controls. In addition, the patients in the intervention group had a lower postoperative inflammatory reaction than the patients who did not receive the preoperative drinks (Pexe-Machado et al., Citation2013). A different study investigated the effects of the administration of a preoperative drink containing MD and glutamine (GLN group) or only MD (CHO group) prior to laparoscopic cholecystectomy. Patients included in the control group fasted prior to their surgery. Results showed a reduced biological response to surgical trauma by improving insulin sensitivity in patients in the GLN group, but not the CHO group, compared to the control group (Dock-Nascimento et al., Citation2012).
Oral Rehydration Drinks
Early studies have indicated benefits of using MDs in oral rehydration solutions (ORS) for individuals suffering from diarrhea over the use of glucose. In this respect, an early paper of Sandhu et al. Citation(1982) concluded that solutions with lower sodium and glucose-polymer content, compared to higher sodium content and higher osmolality due to the use of glucose, might be of nutritional benefit in the oral rehydration of acute infantile diarrhea.
At the same dry-weight concentration, the osmolality increases with increasing DE of the saccharide (Marchal, Citation1999). Compared on a weight basis, the osmolality of MDs is significantly lower than that of disaccharide sugars (Shi et al., Citation1995; El-Mougi et al., Citation1996). El-Mougi et al Citation(1996) studied the use of MDs in ORS and observed that the osmolality of a solution containing 50 g/L MD with a DE of 11–14 still had a slightly lower osmolality than a solution containing only 20 g/L glucose (227 mmol/L vs. 311 mmol/L, respectively).
Sports Rehydration Drinks
A low beverage osmolality supports gastric emptying rate and helps reduce gastrointestinal stress (Vist and Maughan, Citation1995). Accordingly, aiming at a low beverage osmolality, MDs are being used to replace sucrose or glucose in sport drinks. This is relevant since hypertonicity and related postingestion gastrointestinal distress symptoms are significant performance-limiting factors during running events such as marathons and triathlons exercise (Brouns, Citation1991; Rehrer et al., Citation1994; Gregorio et al., Citation2010; Delzenne et al., Citation2010). Another effect of beverage hypertonicity is that it reduces water absorption rate. Vist and Maughan Citation(1995) evaluated the impact of CHO load and osmolality on gastric emptying rate. In this respect, they compared drinks with markedly different osmolalities and caloric contents. Two concentrated drinks containing either 18.8% glucose (1300 mOsmol/kg) or 18.8% MDs (237 msmol/kg) were consumed. The concentrated (188 g/L) MDs emptied much faster (t1/2 = 64 + 8 min) than the corresponding concentrated isoenergetic glucose solution (HG, 1300 mosmol.kg, t1/2 = 130 + 18 min). The strong hyperosmolality induced by an equivalent amount of glucose but avoided by the use of MDs appeared to have impacted significantly on gastric emptying rate. Recently, this area of research was reviewed by Shi and Passe Citation(2010). They concluded that water absorption in the human small intestine is influenced by osmolality, solute absorption, and the anatomical structures of gut segments (Shi and Passe, Citation2010).
Combining MDs with a fructose supplying CHO source may be beneficial when a high rate of CHO supply is warranted. Shi et al (1995) studied intestinal absorption of solutions containing either glucose or fructose combined with a glucose, either as free or directly transportable monosaccharides (glucose, fructose), bound as a disaccharide (sucrose), or as oligomers (maltodextrins). The authors showed that combining a glucose source with fructose resulted in better CHO-water absorption rates, while using MDs enabled osmolality to remain on the low side. The use of MDs can play a significant role in this respect especially at CHO concentration exceeding 40 g/l.
Sports Energy Drinks
Since there is a close relation between muscle fiber glycogen content and its ability to execute repeated high intensity contractions, either a reduced rate of glycogen breakdown or an increased glycogen content may help reduce fatigue and thus support performance capacity in field settings (Costill and Hargreaves, Citation1992; Hawley et al., Citation1992; Febbraio et al., Citation2000; Brouns, Citation2003). Examining the effects of MDs ingestion during exercise it was found that the ingestion of MD, like any other CHO, decreases net glycogen breakdown during long-duration exercise while maintaining a high whole-body CHO oxidation (Hawley et al., Citation1992; Wagenmakers et al., Citation1993; Wallis et al., Citation2005; Harger-Domitrovich et al., Citation2007). Such responses appear to be similar for men and women (Wallis et al., Citation2006).
One of the questions that has been answered recently concerned maximizing CHO supply in periods of a high need, when absorption rate may be a limiting factor. It was shown that the synchronous intake of glucose + fructose favors a higher rate of CHO absorption than glucose sources only. Accordingly, recent research has been done on the favorable ratio of glucose to fructose. Wallis et al. Citation(2005) studied the oxidation of combined ingestion of MDs and fructose during exercise. They showed that with ingestion of substantial amounts of MD and fructose during cycling exercise, exogenous CHO oxidation can reach peak values of approximately 1.5 g·min, and this is markedly higher than oxidation rates from ingesting MD alone.
Sports Recovery Drinks
It has been shown that a combination of MDs with protein and/or amino acids can promote enhanced glycogen recovery and stimulate muscle protein synthesis following an intense exercise protocol (Costill and Hargreaves, Citation1992; Shi et al., Citation1995; Brouns, Citation2003; Kerksick et al., Citation2008). Some observations suggest that effects on postexercise glycogen recovery and also muscle protein synthesis can be enhanced when a combination of different CHOs and protein is used (Ivy et al., Citation2002; Nakhostin-Roohi and Khorshidi, Citation2013). This observation is often used by the sports nutrition industry to promote CHO–protein mixes for improving muscle strength, muscle power, and sports performance. However, results of studies into the effects of MDs + protein on postglycogen recovery are mixed, with some showing positive effects and some showing no effect (McCleave et al., Citation2011; Coletta et al., Citation2013).
Applications Related to Oral Health
Frequent exposure of sugars to teeth is known to cause dental caries (Anderson et al., Citation2009). This is a result of the fermentation of the sugars by microorganisms in dental plaque, leading to the formation of organic acids which in turn leads to the demineralization of enamel (Tahmassebi et al., Citation2006). Acid in drinks (as common in soft drinks and in juices) enhances this effect further (Cheng et al., Citation2009).
For the last 10–15 years, food industry has increasingly been adding ‘new’ CHOs, such as MDs and glucose syrups, to soft drinks instead of sucrose and fructose-glucose syrups (Moynihan, Citation2002; Tahmassebi et al., Citation2006). The effect of MDs on oral health has been addressed in a number of studies (Moynihan et al., Citation1996; Levine, Citation1998; Grenby and Mistry, Citation2000; Al-Khatib et al., Citation2001). The general outcome of these studies has been first that MDs are less potent in increasing acidity in the oral cavity compared to sucrose, and further that the rate of fermentation and acid formation increases with increasing DE. Al-Khatib et al Citation(2001) investigated the effect of three different MDs on the pH of dental plaque in vivo in 10 adult volunteers using the plaque harvesting method. The three MDs tested in this study were DE = 5.5, 14.0, and 18.5 (DE = dextrose equivalents), made up as 10% solutions. Three commercially available MD-containing children's drinks were also evaluated for their acidogenicity. 10% sucrose and 10% sorbitol solutions were used as positive and negative controls, respectively. The minimum pH achieved for DE = 5.5, 14.0, and 18.5 were 5.83 ± 0.30, 5.67 ± 0.24, and 5.71 ± 0.29, respectively, and were significantly higher compared with that for 10% sucrose (5.33 ± 0.17). The area under the curve was the least for DE = 5.5 (12.03 ± 4.64), followed by DE = 18.5 (13.13 ± 8.87) and DE = 14.0 (17.35 ± 6.43), but were all significantly smaller as compared with 10% sucrose (24.50 ± 8.64). It was concluded that, although a 10% MDs solution was significantly less acidogenic than a 10% sucrose solution, both solution were impacting on tooth enamel.
Applications as Fat Replacer
MDs can be used as fat replacers due to their ability to form smooth, fat-like gels and their relatively high viscosity (depending on DE/DP). Possible food categories for fat replacement via MDs are low-fat salad dressings, spreads, margarines and butters, mayonnaise, and dairy products (Sajilata and Singhal, Citation2005). Exchanging fat for MDs, on a w/w basis, will reduce the energy content of the food, as MDs contain less energy/g (resp. 16kJ vs. 38kJ). In this respect, it should be noted, however, that a replacement for fat will not necessarily lead to a reduction of food/energy intake (Stubbs et al., Citation2000).
One of the most important differences between MDs and fats is the hydrophilic behavior of MDs versus the lipophilic behavior of fats, properties that may affect the solubility of flavors and other compounds in a product. Recent findings suggest that the use of MDs in high-energetic food products may help reduce the fat content up to 50%, thus reducing energy density without altering important properties and characteristics of these products (Hadnađev et al., Citation2011). In practice, MDs do not mimic all sensory properties of fat, making its use as fat replacer complex (Hadnađev et al., Citation2011). Next to their fat-mimicking ability, research has shown that an additional benefit of the use of MDs is their inhibition of the release of volatile odor compounds, making them, for example, suitable as fat-replacers in low-fat meat products (Chevance et al., Citation2000; Junsi et al., Citation2012).
Applications Related to Appetite Control
There is a strong correlation between the macronutrient composition of foods and differences in the way and intensity that foods induce satiety. In order of satiating potential the macronutrients are generally classified as follows: protein > CHO > fat (Rolls et al., Citation1988; van der Klaauw et al., Citation2012).
MDs, as a source of glucose, are suggested to enhance feelings of satiety. To test this hypothesis, Yeomans et al. Citation(1998) investigated the effects of consuming a soup with added MDs on food intake, rated hunger, and fullness in 24 male volunteers. The soup was tested relative to a non-MD control soup, which was matched for sensory properties. Soup preloads were consumed 30 minutes before lunch and condition-order counterbalanced. Interestingly, the food intake at lunch was reduced significantly by 77 g (407 kJ) after the MD preload, and this reduced intake was associated with a significant reduction in eating rate but not in meal duration. Hunger ratings were significantly lower and fullness ratings significantly higher during the start of the meal after the MD preload when compared with the control (Yeomans et al., Citation1998). These results imply that the MD meal preloads can result in a reduced desire to eat, by mechanisms which remain to be clarified (Booth, Citation2009). However, several studies and a recent critical analysis of the literature revealed that the methodological set-up of satiety studies, using liquid meal pre-loads, has a significant influence on the outcomes of the studies (Rodin, Citation1991; Stewart et al., Citation1997; Delzenne et al., Citation2010; Allison, Citation2013). Accordingly, there is a lot of debate on the meaning of pre-load study results in relation to real life conditions.
FOOD REGULATORY ASPECTS RELEVANT TO MALTODEXTRINS
The potential to make statements concerning ingredient-related benefits or product composition depends on the food regulations enforced in the country concerned. In the following paragraphs, we aim to provide a short overview of current food regulatory aspects that apply to the use, marketing and communication of MDs.
European Regulations
Even though there is no legal definition for MDs in Europe, MDs are generally referred to as starch hydrolysates in ingredient labeling. It is admitted in the profession that MD in general has a DE < 20.
Eu Allergen Labeling
In Regulation (EU) n° 1169/2011 of the European parliament and the council of 25 October 2011 on the provision of food information to consumers (EC, Citation2011), wheat-based MDs are published on Annex II as products causing non-allergies or intolerances. The ‘wheat’ origin does not need to be labeled. In Europe, MDs are seen as gluten-free CHO sources and are used in gluten-free products.
Eu Nutritional Value
The conversion factors for the calculation of energy are also provided by the Regulation (EU) N° 1169/2011 in Annex XIV, which states that commercially available MDs can be considered 95% CHOs, of which 7% is glucose, and 5% is water. This infers that the inclusion of 1 gram of MDs is equivalent to 3.8 kcal (16.15 kJ).
Eu Health Claim: ‘Carbohydrates Contribute to the Maintenance of Normal Brain Function’
The European Food Safety Authority (EFSA) has recently approved CHO-related claims regarding brain and muscle function, which also apply for MDs. The Commission Regulation (EU) 1018/2013 (EC, Citation2013) added the following claim: ‘Carbohydrates contribute to the maintenance of normal brain function’ entering into force on 13th November 2013. In order to bear the claim, information shall be given to the consumer that the beneficial effect is obtained with a daily intake of 130 g of CHOs from all sources. The claim may be used for foods which contain at least 20 g CHOs that are metabolized by humans, excluding polyols, per quantified portion and complies with the nutrition claim ‘low sugars’ or ‘with no added sugars’, as listed in the Annex to Regulation (EC) n° 1924/2006. The claim should not be used for a food that is 100% sugars.
Eu Health Claim: ‘Carbohydrate-electrolyte Solutions Enhance the Absorption of Water during Physical Exercise’
Here, the panel considered that in order to bear the claim CHO-electrolyte solutions should contain 80–350 kcal/L from CHOs, and at least 75% of the energy should be derived from CHOs which induce a high glycemic response, such as glucose, glucose polymers, and sucrose. In addition, these beverages should contain between 20 mmol/L (460 mg/L) and 50 mmol/L (1,150 mg/L) of sodium, and have an osmolality between 200–330 mOsm/kg water. The target population is active individuals performing endurance exercise (EFSA Journal 2011;9(6):2211).
Eu Health Claim: ‘Carbohydrate-electrolyte Solutions Can Contribute to the Maintenance of Endurance Performance during Prolonged Endurance Exercise’
In order to make this claim, CHO-electrolyte solutions should contain 80–350 kcal/L from CHOs, and at least 75% of the energy should be derived from CHOs which induce a high glycemic response, such as glucose, glucose polymers, and sucrose. In addition, these beverages should contain between 20 mmol/L (460 mg/L) and 50 mmol/L (1,150 mg/L) of sodium, and have an osmolality between 200 and 330 mOsm/kg water. The target population is active individuals performing endurance exercise (EFSA Journal 2011;9(6):2211)
Eu Health Claim: ‘Glycemic Carbohydrates Contribute to Recovery of Normal Muscle Function (Contraction) after Strenuous Exercise’
The Panel considers that in order to achieve the claimed effect, glycemic CHOs should be consumed at doses of 4 g per kg of body weight in the first four to six hours following strenuous exercise. The target population is assumed to be active individuals performing exercise (EC, Citation2006)
Eu Infant Nutrition Regulation
The Regulation (EU) n° 609/2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control, was published on 29th June 2013 in the Official Journal of the European Union. It repealed the directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae, compositional and labeling requirements for infant formulae and follow-on formulae intended for use by infants in good health. The Regulation enters into force on 19 July 2013 and applies from 20 July 2016, with the exception of the Articles 11, 16, 18, and 19 empowering the Commission to adopt delegating acts, which apply from 19 July 2013. The delegating acts shall be adopted by 20 July 2015. Food intended for infants and young children are concerned by delegating acts. By waiting the delegating acts, the directive 2006/141/EC mentioned that MDs are one of the few CHOs that can be used in the development of infant and follow-on formulae. The nutritional content of the CHOs in the formulae should be within the range of 9–14 g per 100 kcal (2.2–3.4 g/100 kJ).
Regulations in the United States
In the United States, MDs are regulated under the Food and Drug Administration's (FDA's) Code of Federal Regulations (CFR) as a GRAS substance, meaning it is Generally Recognized As Safe. Food components can be affirmed GRAS by the FDA or can be self-determined GRAS which is done by the manufacturer. Under Title 21 of the CFR, Part 184, Section 1444 (21 CFR §184.1444) (Regulations, Citation2009), MDs are described as ‘a white powder or concentrated solution by partial hydrolysis of maize starch, potato starch, or rice starch with safe and suitable acids and enzymes.’ Starches other than maize, rice, or potato can be used to make MD as long as the company self-determines GRAS and the resulting MDs have the same chemical structure as the MDs made from maize, rice, or potato as described in the Food Chemicals Codex.
In contrast to the European Union, no health/function claims that apply to the use of MDs have been registered inn United States.
Allergen Labeling
According to the Food Labelling and Consumer Protection Act (FALCPA), if the MDs contain protein derived from wheat, the word ‘wheat’ must be included on the food label (e.g., MDs (wheat)). For USDA-regulated foods, which are meat products, poultry products, and egg products, only the common or usual name is required to be listed on the food label. Therefore, according to regulations, MDs may simply be labeled as ‘maltodextrin’ even if it contains protein derived from wheat. However, the Food Safety and Inspection Service (FSIS) does encourage the use of allergen statements that are consistent with FALCPA.
SUMMARIZING REMARKS
As discussed, the decrease in the consumption of ‘whole’ foods and dietary fiber, along with a rise in the consumption of rapidly digestible and absorbable CHO sources such as isolated starches, starch derivatives, and sugars, parallels an increase in the global prevalence of obesity, diabetes, and cardiovascular disease. Due to their characteristics and physicochemical, functional, technological, and nutritional properties, MDs have numerous applications in functional foods and beverages, as well as clinical nutrition, sports nutrition and infant nutrition. Accordingly, the rise in the overall consumption of MDs, amongst other refined CHO sources, can be attributed to a broader variety of foods containing them, thus potentially increased consumption. The use of MDs in specific circumstances, such as the use of concentrated energy drinks during endurance sports, may help reduce the risk of gastrointestinal distress compared to the use of glucose or sucrose, which would induce a high gastro-intestinal osmolality which may potentially induce gastrointestinal distress.
Next to their use as an energy source, applications of MDs include their uses in replacing fat, encapsulating vitamins, minerals and flavorants, enhancing shelf life, and increasing bulk of products, amongst other things. Furthermore, even though the use of MDs as CHO source is preferred to that of common sugars and their use as fat replacers leads to a reduction in the energy density (kJ/g) of food products containing them, a frequent consumption of these products should be judged in the light of the potential effects that this may have on health.
REFERENCES
- Al-Khatib, G. R., Duggal, M. S., and Toumba, K. J. (2001). An evaluation of the acidogenic potential of maltodextrins in vivo. J. Dent.. 29:409–414.
- Alexander, R. (1995). Fat replacers based on starch. Cereal Foods World. 40.
- Allison, D. B. (2013). Liquid calories, energy compensation and weight: what we know and what we still need to learn. Brit. J. Nutr. 111(3):384–386.
- Altschul, A. M. (1989). Low-calorie foods—a scientific status summary by the Institute of Food Technologists' expert panel of food safety and nutrition. Food Techno. 43:113.
- Anderson, C., Curzon, M., Van Loveren, C., Tatsi, C., and Duggal, M. (2009). Sucrose and dental caries: a review of the evidence. Obes. Rev. 10:41–54.
- Astrup, A. (1999). Macronutrient balances and obesity: the role of diet and physical activity. Public Health Nutr. 2:341–347.
- Atkinson, F. S., Foster-Powell, K., and Brand-Miller, J. C. (2008). International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 31: 2281–2283.
- BeMiller, J. N., and Whistler, R. L. (2009). Starch: Chemistry and Technology. London: Academic Press.
- Booth, D. A. (2009). Learnt reduction in the size of a meal. Measurement of the sensory-gastric inhibition from conditioned satiety. Appetite. 52:745–749.
- Brouns, F. (1991). Etiology of gastrointestinal disturbances during endurance events. Scand. J. Med. Sci. Sports. 1:66–77.
- Brouns, F. (1998). Gastric emptying as a regulatory factor in fluid uptake. Int. J. Sports Med. 19:S125–128.
- Brouns, F. (2003). Essentials of Sports Nutrition. West-Sussex, UK: Wiley.
- Brouns, F., Arrigoni, E., Langkilde, A. M., Verkooijen, I., Fassler, C., Andersson, H., Kettlitz, B., van Nieuwenhoven, M., Philipsson, H., and Amado, R. (2007). Physiological and metabolic properties of a digestion-resistant maltodextrin, classified as type 3 retrograded resistant starch. J. Agr. Food Chem. 55:1574–1581.
- Buléon, A., Colonna, P., Planchot, V., and Ball, S. (1998). Starch granules: structure and biosynthesis. Int. J. Biol. Macromol. 23:85–112.
- Cabre, E., Gonzalez-Huix, F., Abad-Lacruz, A., Esteve, M., Acero, D., Fernandez-Bañares, F., Xiol, X., and Gassull, M. A. (1990). Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology. 98: 715–720.
- Cheng, R., Yang, H., Shao, M., Hu, T., and Zhou, X. (2009). Dental erosion and severe tooth decay related to soft drinks: a case report and literature review. J. Zhejiang Univ. Sci. B. 10: 395–399.
- Chevance, F. F. V., Farmer, L. J., Desmond, E. M., Novelli, E., Troy, D. J., and Chizzolini, R. (2000). Effect of some fat replacers on the release of volatile aroma compounds from low-fat meat products. J. Agr. Food Chem. 48: 3476–3484.
- Chronakis, I. S. (1998). On the molecular characteristics, compositional properties, and structural-functional mechanisms of maltodextrins: a review. Crit. Rev. Food Sci. Nutr. 38:599–637.
- Coletta, A., Thompson, D. L., and Raynor, H. A. (2013). The influence of commercially-available carbohydrate and carbohydrate-protein supplements on endurance running performance in recreational athletes during a field trial. J. Int. Soc. Sports Nutr. 10:17.
- Costill, D. L., and Hargreaves, M. (1992). Carbohydrate nutrition and fatigue. Sports Med. 13:86–92.
- Coultate, T. (2009). Food: The Chemistry of its Components. Cambridge, UK: The Royal Society of Chemistry.
- Delzenne, N., Blundell, J., Brouns, F., Cunningham, K., De Graaf, K., Erkner, A., Lluch, A., Mars, M., Peters, H., and Westerterp-Plantenga, M. (2010). Gastrointestinal targets of appetite regulation in humans. Obes. Rev. 11: 234–250.
- Dock-Nascimento, D. B., de Aguilar-Nascimento, J. E., Faria, M. S. M., Caporossi, C., Slhessarenko, N., and Waitzberg, D. L. (2012). Evaluation of the effects of a preoperative 2-hour fast with maltodextrine and glutamine on insulin resistance, acute-phase response, nitrogen balance, and serum glutathione after laparoscopic cholecystectomy a controlled randomized trial. J. Parent. Enteral Nutr. 36:43–52.
- EC. (2006). Regulation (EC) No 1924/2006 of the European Parliament and of the council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union. oj l: 9–25.
- EC. (2011). Regulation (EC) No 1169/2011 of the European Parliament and of the council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union. oj l: 18–63.
- EC. (2013). Regulation (EC) No 1018/2013 of the European Parliament and of the council of 23 October 2013. Off. J. Eur. Union. oj l: 43–44.
- El-Mougi, M., Hendawi, A., Koura, H., Hegazi, E., Fontaine, O., and Pierce, N. (1996). Efficacy of standard glucose-based and reduced-osmolarity maltodextrin-based oral rehydration solutions: effect of sugar malabsorption. Bull. World Health Org. 74:471.
- Englyst, K. N., and Englyst, H. N. (2005). Carbohydrate bioavailability. Brit. J. Nutr. 94:1–11.
- Febbraio, M. A., Keenan, J., Angus, D. J., Campbell, S. E., and Garnham, A. P. (2000). Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: effect of the glycemic index. J. Appl. Physiol. 89: 1845–1851.
- Ferraris, R. P. (2001). Dietary and developmental regulation of intestinal sugar transport. Biochem. J. 360:265–276.
- Fetzer, W., Crosby, E., Engel, C., and Kirst, L. (1953). Effect of acid and heat on dextrose and dextrose polymers. Ind. Eng. Chem. 45: 1075–1083.
- Gray, G. M. (1975). Carbohydrate digestion and absorption. New England J. Med. 292: 1225–1230.
- Gregorio, G. V., Gonzales, M. L. M., Dans, L. F., and Martinez, E. G. (2010). Cochrane review: Polymer-based oral rehydration solution for treating acute watery diarrhoea. Evid. Based Child Health: A Cochrane Rev. J. 5: 1612–1675.
- Grenby, T., and Mistry, M. (2000). Properties of maltodextrins and glucose syrups in experiments in vitro and in the diets of laboratory animals, relating to dental health. Brit. J. Nutr. 84: 565–574.
- Gross, L. S., Li, L., Ford, E. S., and Liu, S. (2004). Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Amer. J Clin. Nutr. 79: 774–779.
- Guyton, A. C., and Hall, J. E. (2000). Medical Physiology. Saunders, Philadelphia, PA pp, 1064.
- Hadnađev, M., Dokić, L., Hadnađev, T. D., Pajin, B., and Krstonošić, V. (2011). The impact of maltodextrin-based fat mimetics on rheological and textural characteristics of edible vegetable fat. J. Texture Stud. 42: 404–411.
- Harger-Domitrovich, S. G., McClaughry, A. E., Gaskill, S. E., and Ruby, B. C. (2007). Exogenous carbohydrate spares muscle glycogen in men and women during 10 h of exercise. Med. Sci. Sports Exer. 39: 2171.
- Hawley, J. A., Dennis, S. C., and Noakes, T. D. (1992). Oxidation of carbohydrate ingested during prolonged endurance exercise. Sports Med. 14: 27–42.
- Ivy, J. L., Goforth, H. W., Damon, B. M., McCauley, T. R., Parsons, E. C., and Price, T. B. (2002). Early postexercise muscle glycogen recovery is enhanced with a carbohydrate-protein supplement. J. Appl. Physiol. 93: 1337–1344.
- Jeukendrup, A. E. (2004). Carbohydrate intake during exercise and performance. Nutrition. 20:669–677.
- Johnson, R. K., Appel, L. J., Brands, M., Howard, B. V., Lefevre, M., Lustig, R. H., Sacks, F., Steffen, L. M., and Wylie-Rosett, J. (2009). Dietary sugars intake and cardiovascular health a scientific statement from the american heart association. Circulation. 120: 1011–1020.
- Junsi, M., Usawakesmanee, W., and Siripongvutikorn, S. (2012). Effect of using starch on off-odors retention in tuna dark meat. Int. Food Res. J. 19: 709–714.
- Kellett, G. L., and Helliwell, P. A. (2000). The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem. J. 350:155.
- Kerksick, C., Harvey, T., Stout, J., Campbell, B., Wilborn, C., Kreider, R., Kalman, D., Ziegenfuss, T., Lopez, H., and Landis10, J. (2008). International Society of Sports Nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 5:17.
- Klaauw, A. A., Keogh, J. M., Henning, E., Trowse, V. M., Dhillo, W. S., Ghatei, M. A., and Farooqi, I. S. (2013). High protein intake stimulates postprandial GLP1 and PYY release. Obesity 21(8):1602–1607.
- Kuntz, L. A. (1997). Making the most of maltodextrins. Food Products Design. 8:89–104.
- Lebenthal, E. (1987). Role of salivary amylase in gastric and intestinal digestion of starch. Dig. Dis. Sci. 32: 1155–1157.
- Lecoultre, V., Egli, L., Theytaz, F., Despland, C., Schneiter, P., and Tappy, L. (2013). Fructose-induced hyperuricemia is associated with a decreased renal uric acid excretion in humans. Diab. Care. 36: e149–e150.
- Lee, B.-H., Yan, L., Phillips, R. J., Reuhs, B. L., Jones, K., Rose, D. R., Nichols, B. L., Quezada-Calvillo, R., Yoo, S.-H., and Hamaker, B. R. (2013). Enzyme-synthesized highly branched maltodextrins have slow glucose generation at the mucosal α-glucosidase level and are slowly digestible in vivo. PloS One. 8: e59745.
- Levine, R. S. (1998). Briefing paper: maltodextrins and caries. Brit. Dental J. 185: 392.
- Livesey, G. (1991). The energy value of carbohydrate and “fibre” for man. In: Proc. Nutr. Soc. Aust, Norwich, UK: AFRC Institute of Food Research, pp. 79–87.
- Lustig, R. H., Schmidt, L. A., and Brindis, C. D. (2012). Public health: The toxic truth about sugar. Nature. 482: 27–29.
- Maldonado, J., Gil, A., Narbona, E., and Molina, J. A. (1998). Special formulas in infant nutrition: a review. Early Hum. Dev. 53(Supplement 1): S23–S32.
- Man, C., Camilleri, M., and Cobelli, C. (2006). A system model of oral glucose absorption: validation on gold standard data. Biomed. Eng. IEEE Trans. on. 53: 2472–2478.
- Marchal, L. M., Beeftink, H. H., and Tramper, J. (1999). Towards a rational design of commercial maltodextrins: a mechanistic approach. Trends in Food Science & Technology. 10(11): 345–355.
- McCleave, E. L., Ferguson-Stegall, L., Ding, Z., Doerner III, P. G., Wang, B., Kammer, L. M., and Ivy, J. L. (2011). A low carbohydrate–protein supplement improves endurance performance in female athletes. J. Strength Cond. Res. 25: 879–888.
- Moynihan, P. J. (2002). Dietary advice in dental practice. Brit. Dental J. 193: 563–568.
- Moynihan, P. J., Gould, M. E., Huntley, N., and Thorman, S. (1996). Effect of glucose polymers in water, milk and a milk substitute on plaque pH in vitro. Int. J. Paediat. Den. 6:19–24.
- Nakhostin-Roohi, B., and Khorshidi, M. (2013). The effect of glutamine and maltodextrin acute supplementation on anaerobic power. Asian J. Sports Med. 1:131–136.
- Nedergaard, M., Ransom, B., and Goldman, S. A. (2003). New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 26:523–530.
- O'Brien-Nabors, L. (2011). Alternative Sweeteners. Boca Raton, Florida, USA: CRC Press.
- Ogden, C. L., Carroll, M. D., Kit, B. K., and Flegal, K. M. (2012). Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Jama. 307:483–490.
- Parker, K., Salas, M., and Nwosu, V. C. (2010). High fructose corn syrup: Production, uses and public health concerns. Biotechnol. Mol. Biol. Rev. 5:71–78.
- Pexe-Machado, P. A., de Oliveira, B. D., Dock-Nascimento, D. B., and de Aguilar-Nascimento, J. E. (2013). Shrinking preoperative fast time with maltodextrin and protein hydrolysate in gastrointestinal resections due to cancer. Nutrition. 29: 1054–1059.
- Regulations, C. o. F. (2009). Code of Federal Regulations. Washington DC: ProStar Publications.
- Rehrer, N., Brouns, F., Beckers, E., and Saris, W. (1994). The influence of beverage composition and gastrointestinal function on fluid and nutrient availability during exercise. Scand. J. Med. Sci. Sports. 4:159–172.
- Rodin, J. (1991). Effects of pure sugar vs. mixed starch fructose loads on food intake. Appetite. 17: 213–219.
- Rolls, B. J., Hetherington, M., and Burley, V. J. (1988). The specificity of satiety: the influence of foods of different macronutrient content on the development of satiety. Physiol. Behavior. 43: 145–153.
- Sajilata, M., and Singhal, R. S. (2005). Specialty starches for snack foods. Carbohydr. Polym. 59: 131–151.
- Sandhu, B. K., Jones, B., Brook, C., and Silk, D. (1982). Oral rehydration in acute infantile diarrhoea with a glucose-polymer electrolyte solution. Arch. Disease Childhood. 57: 152–154.
- Shi, X., and Passe, D. H. (2010). Water and solute absorption from carbohydrate-electrolyte solutions in the human proximal small intestine: a review and statistical analysis. Int. J. Sport Nutr. Exer. Metabol. 20: 427–442.
- Shi, X., Summers, R. W., Schedl, H. P., Flanagan, S. W., Chang, R., and Gisolfi, C. V. (1995). Effects of carbohydrate type and concentration and solution osmolality on water absorption. Med. Sci. Sports Exer. 27:1607.
- Siddiqui, Z., and Osayande, A. S. (2011). Selected disorders of malabsorption. Primary Care. 38: 395–414; vii.
- Stephen, M. S., Philips, G. O., and Williams, P. A. (2006). Food Polysaccharides and Their Applications. CRC Press, Boca Raton.
- Stewart, S. L., Black, R. M., Wolever, T., and Anderson, G. H. (1997). The relationship between the glycaemic response to breakfast cereals and subjective appetite and food intake. Nutr. Res. 17: 1249–1260.
- Stubbs, J., Ferres, S., and Horgan, G. (2000). Energy density of foods: effects on energy intake. Crit. Rev. Food Sci. Nutr. 40: 481–515.
- Tahmassebi, J. F., Duggal, M. S., Malik-Kotru, G., and Curzon, M. E. (2006). Soft drinks and dental health: a review of the current literature. J. Dent. 34: 2–11.
- Takeiti, C., Kieckbusch, T., and Collares-Queiroz, F. (2010). Morphological and physicochemical characterization of commercial maltodextrins with different degrees of dextrose-equivalent. Int. J. Food Propert. 13: 411–425.
- Tester, R. F., Karkalas, J., and Qi, X. (2004). Starch—composition, fine structure and architecture. J. Cereal Sci. 39: 151–165.
- Topping, D. L., Gooden, J. M., Brown, I. L., Biebrick, D. A., McGrath, L., Trimble, R. P., Choct, M., and Illman, R. J. (1997). A high amylose (amylomaize) starch raises proximal large bowel starch and increases colon length in pigs. J. Nutr. 127: 615–622.
- Urashima, T., Fukuda, K., and Messer, M. (2011). Evolution of milk oligosaccharides and lactose: a hypothesis. Animal. 6:369.
- Vist, G. E., and Maughan, R. J. (1995). The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J. Physiol. 486: 523–531.
- Wagenmakers, A. J., Brouns, F., Saris, W. H., and Halliday, D. (1993). Oxidation rates of orally ingested carbohydrates during prolonged exercise in men. J. Appl. Physiol. 75: 2774–2780.
- Wallis, G. A., Dawson, R., Achten, J., Webber, J., and Jeukendrup, A. E. (2006). Metabolic response to carbohydrate ingestion during exercise in males and females. American J. Physiol.-Endocrinol. Metabol. 290: E708–E715.
- Wallis, G. A., Rowlands, D. S., Shaw, C., Jentjens, R., and Jeukendrup, A. E. (2005). Oxidation of combined ingestion of maltodextrins and fructose during exercise. Med. Sci. Sports Exerc. 37: 426–432.
- Welsh, J. A., Sharma, A., Abramson, J. L., Vaccarino, V., Gillespie, C., and Vos, M. B. (2010). Caloric sweetener consumption and dyslipidemia among US adults. JAMA:J Amer. Med. Assoc. 303: 1490–1497.
- Westman, E. C. (2002). Is dietary carbohydrate essential for human nutrition? Amer. J. Clin. Nutr. 75:951–953.
- Whelan, W. J. (2008). The wars of the carbohydrates, Part 6: What a name! IUBMB Life. 60: 555–556.
- Yeomans, M. R., Gray, R. W., and Conyers, T. H. B. (1998). Maltodextrin preloads reduce food intake without altering the appetiser effect. Physiol. Behav. 64: 501–506.
- Zhang, G., and Hamaker, B. R. (2009). Slowly digestible starch: concept, mechanism, and proposed extended glycemic index. Crit. Rev. Food Sci. Nutr. 49: 852–867.