ABSTRACT
The contemporary pathophysiological model of non-alcoholic fatty liver disease (NAFLD) comprises multiple parallel pathways with a dynamic cross talk that cumulate in steatosis and inflammation, and ultimately fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma. So far, no pharmacological treatment has been approved. A major impediment of drugs, in general, is that they are intended to act on one single target in the pathology of a disease. However, the multitude of pathways involved in the pathogenesis of NAFLD underpins the need for treatments that address these various pathways. Interestingly, flavonoids have been found to have positive effects on lipid metabolism, insulin resistance, inflammation, and oxidative stress, the most important pathophysiological pathways in NAFLD. This puts flavonoids in the spotlight for the treatment of NAFLD and prompted us to review the existing evidence for the use of these food-derived compounds in the treatment of NAFLD.
Introduction
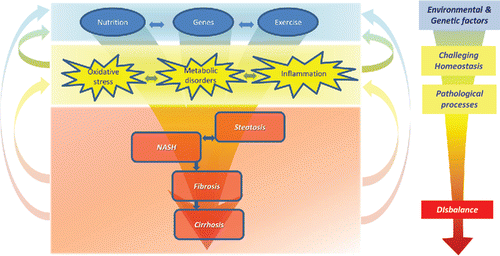
Non-alcoholic fatty liver disease (NAFLD) includes a spectrum of liver disorders, ranging from steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and hepatocellular carcinoma. The development of NAFLD is associated with metabolic syndrome. Around 20% of the general population in Western countries has NAFLD (Bedogni et al., Citation2005) and 2 to 3% develop NASH (Neuschwander-Tetri and Caldwell, Citation2003). The pathophysiological model that has evolved for the development of NAFLD includes multiple hits that do not follow a strict sequence () (Tilg and Moschen, Citation2010). In this model, metabolic disorders, oxidative stress, and local and systemic inflammation are the major processes involved in the progression of NAFLD. These processes appear to enforce each other, and a dynamic cross talk exists between different processes (). Some authors suggest that steatosis and steatohepatitis might be two distinct disease entities (Tilg and Moschen, Citation2010; Yilmaz Citation2012). However, progression from steatosis to NASH has been documented in patients (Pais et al., Citation2011).
Figure 1. Contemporary pathophysiological model of NAFLD. Environmental and genetic factors challenge homeostasis and lead to several pathological processes: metabolic disorders, oxidative stress, and inflammation. These processes also fortify each other and cause a disbalance, leading to the development of steatosis and NASH. NASH can eventually progress to fibrosis and cirrhosis.
To date, no evidence-based pharmacological treatment exists for NAFLD. Patients are given life style advice comprising dietary recommendations as well as encouragement to increase physical exercise to lose weight. That a multitude of pathways has been implicated in the etiology of NAFLD makes the treatment challenging; ideally, the treatment should address all the multiple pathways. The involvement of multiple pathways explains why no effective drug has been found for the treatment of NAFLD. Traditionally, drugs are designed as “silver bullets” that, according to the classical medicinal chemical approach, have a well-defined, specific biological target such as a receptor (). Since such a well-defined, specific biological target is missing in NAFLD, this traditional approach is deemed to fail.
Figure 2. “Traditional” concept of action of drugs versus the contemporary concept of action of bioactives such as flavonoids. While traditional drugs are developed to act on one target, leading to absence of disease, flavonoids act on multiple targets, affecting diverse pathological processes, leading to increased ability to adapt. This fits seamlessly in the pathophysiologic model of NAFLD, since diverse pathological processes are involved ().
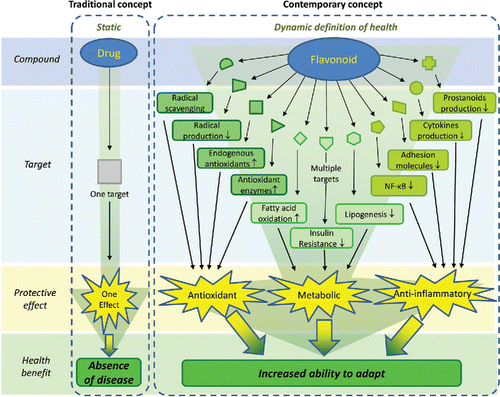
Natural compounds, such as flavonoids, have frequently been studied in models of NAFLD and seem to display beneficial effects. Flavonoids comprise a diverse group of compounds abundantly found in our diet. The intake of flavonoids has been associated with several health benefits. Initially, their health benefits were attributed to their potent antioxidant activity. However, further research revealed that other activities, such as anti-inflammatory and metabolic effects, also contribute. Apparently, flavonoids display a multitude of activities, and therefore these compounds were upgraded from antioxidant to bioactive compounds (Bast and Haenen, Citation2013). A bioactive produces a biological response via an array of subtle effects via different targets (). This multifarious mode of action of flavonoids seems to suit seamlessly in the treatment of NAFLD, in which various pathways are involved. This concept will be reviewed in the present paper.
First, an inventory of various pathways identified in the etiology of NAFLD will be made. Second, several biological activities of flavonoids will be presented. These activities will be linked to the pathways involved in NAFLD explaining the rational for the use of flavonoids in the treatment of NAFLD. Finally, the clinical studies on the efficacy of flavonoids in NAFLD will be evaluated, with the focus on the effect of flavonoids on different pathways.
To this end, a literature search was conducted on PubMed in November 2013 using the following search terms: flavonoids, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis. To increase the number of studies found, a search using the name of specific flavonoids was also included.
Pathogenesis of NAFLD
The pathogenesis of NAFLD is complex and multifactorial, comprising multiple hits that lead to steatosis and NASH (Tilg and Moschen, Citation2010; Polyzos et al., Citation2012). Although various pathways have been identified, the list is not complete as the etiology is still enigmatic and our knowledge of the disease is progressing.
A hallmark in the development of NAFLD is the accumulation of fat in the liver. Factors that are known to contribute to this accumulation include: (1) high free fatty acids (FFA) supply due to increased lipolysis from visceral and subcutaneous adipose tissue and dietary intake, (2) low FFA oxidation in relation to the supply of FFAs, (3) high hepatic lipogenesis, and (4) low hepatic excretion of very low-density lipoprotein (VLDL) (; Tilg and Moschen, Citation2010). Another hallmark is chronic inflammation causing fibrosis. Underlying processes include oxidative stress and lipid peroxidation, mitochondrial dysfunction, adipocytokine/cytokine imbalance, gut-derived bacterial endotoxins, hepatic stellate cell activation, and genetic factors (Polyzos et al., Citation2012). In addition, activation of Kupffer cells by cholesterol crystals is suggested to be a trigger for hepatic inflammation (Bieghs et al., Citation2013). Insulin resistance plays an important role in the development of both steatosis and inflammation (Polyzos et al., Citation2012). Because of insulin resistance, lipolysis is not inhibited by insulin. The release of FFAs cause inflammation, promote ectopic fat deposition, and further enhance insulin resistance, creating a self-propelling feed-forward process (; Polyzos et al., Citation2012). Furthermore, insulin resistance stimulates gluconeogenesis in hepatocytes and reduces glycogen formation. Increased glucose and insulin levels stimulate de novo lipogenesis via hepatic transcription factors such as sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate response element-binding protein (ChREBP). This causes stimulation of lipogenic enzymes such as glucokinase (gk), fatty acid synthase (FAS), and acetyl-coenzyme A carboxylase (ACC) (; Polyzos et al., Citation2012).
Figure 3. Schematic view of the pathophysiological pathways involved in NAFLD. Overview of the processes in the liver, adipose, and other tissues that are responsible for metabolic disturbances, oxidative stress, and inflammation in NAFLD. FFA = free fatty acids, SREBP-1c = sterol regulatory element-binding protein 1c, ChREBP = carbohydrate-responsive element-binding protein, FAS = fatty acid synthase, ACC = acetyl-CoA carboxylase, gk = glucokinase, VLDL = very low-density lipoprotein, ER = endoplasmic reticulum.
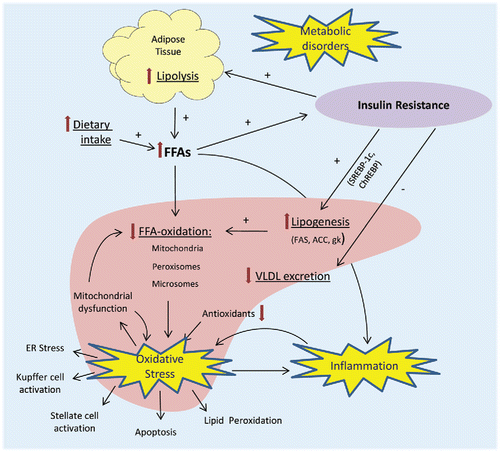
In the multifactorial etiology of NASH, oxidative stress represents a crucial process (Koek et al., Citation2011; Rolo et al., Citation2012; Ucar et al., Citation2013): The production of reactive oxygen species (ROS) is not balanced by the protection against ROS by antioxidants. Various potential sources of oxidative stress have been reported in NAFLD. ROS are produced during the mitochondrial and peroxisomal beta oxidation of FFAs and during the metabolism of FFAs by cytochrome P450 2E1 and 4A. ROS cause endoplasmic reticulum (ER) stress, which further promotes the accumulation of ROS within the cell (Ucar et al., Citation2013). In addition, reduction in antioxidant defenses will contribute to oxidative stress. Reduced glutathione (GSH) levels and decreased superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, and glutathione transferase activities are found in NASH and appear to be correlated to disease severity (Rolo et al., Citation2012). ROS react with biological compounds, including fatty acids, proteins, and DNA, causing lipid peroxidation, mitochondrial dysfunction, stellate cell activation, inflammation (via NF-κB activation), and apoptosis (; Koek et al., Citation2011; Ucar et al., Citation2013). Mitochondrial dysfunction and inflammation will lead to the formation of more ROS, further fueling the self-propelling feed-forward process.
Iron has also been implemented in the pathogenesis of NAFLD. In patients with NAFLD, elevated hepatic iron levels have been found (Valenti et al., Citation2010; Nelson et al., Citation2011). The precise role of iron in the pathogenesis of NAFLD has not yet been established, but it is well documented that iron increases oxidative stress, e.g., by its ability to generate hydroxyl radicals via the Fenton (Citation1894) reaction. Citrate, an intermediate product of lipid metabolism found to be elevated in NAFLD patients, has the ability to further increase this iron-induced oxidative stress by the stimulation of Fenton reaction (van de Wier et al., Citation2013).
Flavonoids
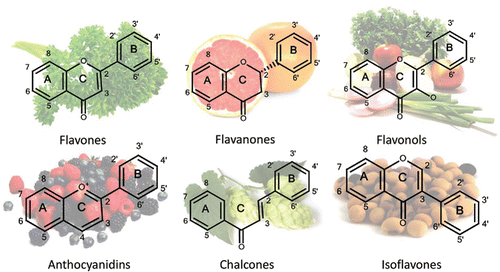
Flavonoids are polyphenolic compounds that are ubiquitously found in nature. They are secondary metabolites in plants and are frequently bound to sugars (glycosides) (Ross and Kasum, Citation2002). Flavonoids also occur as aglycones (without a sugar group) (Ross and Kasum, Citation2002). Most flavonoids () comprise three rings: two aromatic rings (A and B) and one heterocyclic ring (C). Flavonoids are categorized into subclasses based on variations in the C ring. The major subclasses are flavones, isoflavones, flavanols, flavanones, anthocyanidins, and chalcones (; Ross and Kasum, Citation2002).
Figure 4. Major subclasses of flavonoids found in significant amounts in the pictured fruits and vegetables.
Over 5000 different flavonoids have been identified. Since the group of flavonoids is a very heterogenic group of compounds that act on multiple biological targets, various, sometimes even paradoxical, effects have been described for different classes of flavonoids. In addition, the wide variation in models used to study the effects of flavonoids contributes to the variation in the effects found. This review addresses the potential of flavonoids and focuses on the reported positive effects of these bioactives in the treatment of NAFLD. These effects are differentiated in metabolic, antioxidant, and anti-inflammatory effects.
Metabolic effects
Peroxisome proliferator-activated receptors (PPARs)
The PPARs are promising targets in the treatment of NAFLD. PPARs are nuclear receptors that play a role in the regulation of lipid and glucose metabolism as well as inflammation (Tailleux et al., Citation2012). PPARα is highly expressed in the liver and regulates FFA transport and stimulates enzymes involved in β-oxidation (Kallwitz et al., Citation2008). Furthermore, it limits inflammation by inhibition of NF-κB and reduction of C-reactive protein expression (Tailleux et al., Citation2012). Studies have found evidences for a role of PPARα in NAFLD and its treatment (Kallwitz et al., Citation2008; Macdonald and Prins, Citation2004). PPARα−/− mice have increased susceptibility for the development of NAFLD when fed a high fat diet compared with wild-type mice (Kallwitz et al., Citation2008). In addition, PPARα agonists, such as fibrates, reduce steatosis, inflammation, and fibrosis in NASH models (Shiri-Sverdlov et al., Citation2006; Tailleux et al., Citation2012). Stimulation of PPARα is expected to decrease steatosis by stimulation of β-oxidation and to mitigate inflammation by inhibition of NF-κB. Several studies have demonstrated that flavonoids stimulate PPARα (Medjakovic et al., Citation2010; Chang et al., Citation2011; Cho et al., Citation2011; Goto et al., Citation2012; Lee et al., Citation2012; Jia et al., Citation2013; Malek et al., Citation2013). For this stimulation, various mechanisms of action have been proposed. Some studies claim that flavonoids are ligands and (partial) agonists of PPARα (; Medjakovic et al., Citation2010; Jia et al., Citation2013; Malek et al., Citation2013). Other studies conclude that flavonoids upregulate PPARα gene and/or protein expression (Chang et al., Citation2011; Cho et al., Citation2011; Goto et al., Citation2012; Lee et al., Citation2012), possibly involving adiponectin, a stimulator of PPARα (Goto et al., Citation2012). As stated above, the variation in reported mechanisms might originate from the variation in chemical structure between flavonoids and the multitude of targets on which the flavonoids act.
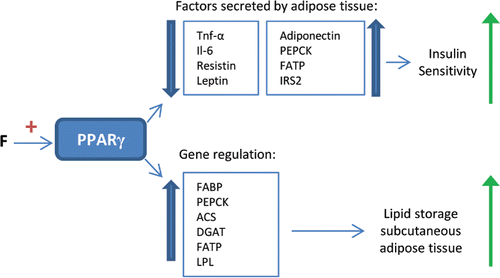
Peroxisome proliferator-activated receptor gamma (PPARγ) is mainly expressed in adipose tissue and its activation results in adipocyte differentiation and insulin sensitization (Medjakovic et al., Citation2010). Secretion of insulin resistance promoting factors by adipose tissue is reduced and secretion of insulin sensitivity promoting factors is increased (). By upregulation of adiponectin, PPARγ also activates PPARα. Furthermore, by activation of the involved genes (FABP, PEPCK, Acyl-CoA synthase, DGAT, FATP, and LPL), adipogenesis and lipid storage in subcutaneous adipose tissue are stimulated (). Consequently, fat from harmful visceral adipose tissue is redistributed to subcutaneous fat depots (Medjakovic et al., Citation2010). Moreover, FFA delivery to the liver is reduced (Medjakovic et al., Citation2010; Tailleux et al., Citation2012). PPARγ has also been reported to increase energy expenditure by induction of uncoupling protein-2 (UCP-2) (Kallwitz et al., Citation2008). Mutations in the PPARγ gene increase the risk of developing metabolic syndrome and NAFLD (Savage et al., Citation2003). In addition, glitazones, PPARγ agonists, improve insulin resistance and decrease aminotransferase levels and liver fat in NASH patients, whereas positive effects on histological markers of NASH are not always noted (Tailleux et al., Citation2012). These findings substantiate the use of PPARγ agonists in the treatment of NASH. Several studies report that flavonoids stimulate PPARγ (Xia et al., Citation2005; Chen et al., Citation2009; Medjakovic et al., Citation2010; Puhl et al., Citation2012; Lee et al., Citation2013; Sharma et al., Citation2013). Similar as for PPARα stimulation, various mechanisms of action are described. Flavonoids have been found to upregulate PPARγ gene expression (Xia et al., Citation2005; Chen et al., Citation2009; Sharma et al., Citation2011; Lee et al., Citation2013;). In addition, some flavonoids were observed to be agonists of PPARγ (Medjakovic et al., Citation2010; Puhl et al., Citation2012). An advantage of flavonoids over other drugs, such as glitazones, could be that the bioactives only partially activate PPARγ. This reduces the risk of serious side effects seen with the use of full agonists. For example, weight gain, an important side effect of glitazones, is not associated with the intake of isoflavones that also activate PPARγ. In several studies, intake of isoflavones even leads to a slight weight reduction (Medjakovic et al., Citation2010).
Figure 5. Effects of PPARγ stimulation by flavonoids. PPARγ stimulation by flavonoids (F) leads to changes in the factors secreted by adipose tissue, stimulating insulin sensitivity. Furthermore, it leads to the upregulation of specific genes, stimulating lipid storage in subcutaneous adipose tissue instead of the liver. TNF-α = tumor necrosis factor α, Il-6 = interleukin 6, PEPCK = phospoenolpyruvate carboxykinase, FATP = fatty acid transport protein, IRS2 = insulin receptor substrate 2, FABP = fatty acid-binding protein, ACS = acetyl-CoA synthase, DGAT = diglyceride acyltransferase, LPL = lipoprotein lipase.
Sterol regulatory element-binding protein-1c
Another target identified in the treatment of NAFLD is SREBP-1c. SREBP-1c is a transcription factor that controls de novo lipogenesis via induction of lipogenic enzymes (FAS, ACC, and gk; Ferre and Foufelle, Citation2010), which stimulate steatosis. In liver biopsies of NAFLD patients, the expression of SREBP-1c and liver X-receptor α (LXRα), which controls SREBP-1c transcription, and the expression of ACC and FAS are found to be significantly higher than in control biopsies (Higuchi et al., Citation2008). Several flavonoids inhibit SREBP-1c (Shin et al., Citation2007; Hwang et al., Citation2011; Liu et al., Citation2011; Sharma et al., Citation2011; Wu et al., Citation2011; Ahn et al., Citation2013). Multiple mechanisms of SREBP-1c inhibition have been implicated. Various studies have found that flavonoids downregulate SREBP-1c protein and gene expression (Hwang et al., Citation2011; Liu et al., Citation2011; Sharma et al., Citation2011; Wu et al., Citation2011; Ahn et al., Citation2013). The isoflavone genistein reduces the expression of site-1 proteases, which are necessary for SREBP-1c to act as a transcription factor (Shin et al., Citation2007). Furthermore, SREBP-1c is inhibited by the activation of 5′ adenosine monophosphate-activated protein kinase (AMPK; Hwang et al., Citation2011; Liu et al., Citation2011; Wu et al., Citation2011). Activation of AMPK inhibits LXRα, which controls SREBP-1c transcription (Yap et al., Citation2011). Flavonoids stimulate activation of AMPK (Hwang et al., Citation2011; Liu et al., Citation2011; Wu et al., Citation2011; Lee et al., Citation2012). In addition, flavonoids inhibit LXRα (Goldwasser et al., Citation2010; Sharma et al., Citation2011; Ahn et al., Citation2013). Since SREBP-1c transcription is stimulated by hyperinsulinemia, flavonoids might also reduce SREBP-1c expression by improving insulin sensitivity and normalizing insulin levels. Recently, activation of SREBP-1c was suggested to be one of the consequences of endoplasmic reticulum stress in steatotic liver (Ferre and Foufelle, Citation2010). Inhibition of endoplasmic reticulum stress is another way in which flavonoids might inhibit SREBP-1c.
Antioxidant effects
As a first line of defense, flavonoids reduce the production of radicals and other reactive species. Flavonoids inhibit pro-oxidant enzymes, such as xanthine oxidase (Garcia-Lafuente et al., Citation2009). In addition, inhibition of lipoxygenases and cyclooxygenases (COX; see Anti-Inflammatory Effects), enzymes that are capable to co-oxidize molecules other than their usual substrates, reduces the production of reactive species (Garcia-Lafuente et al., Citation2009).
Flavonoids have been found to be very effective scavengers. This is a pivotal biochemical mode of action of bioactives, although the importance of radical scavenging is exaggerated as well as undervalued (Bast and Haenen, Citation2013). Among antioxidants, flavonoids are at the top of the pecking order, meaning that they are the first in line to scavenge radicals. They scavenge a wide array of reactive species, including superoxide, hydroxyl, peroxyl, and peroxynitrite radicals (Bors et al., Citation1997; Haenen et al., Citation1997; Duthie and Crozier, Citation2000). During this scavenging, flavonoids are oxidized by the radical, resulting in a more stable, less reactive radical (Garcia-Lafuente et al., Citation2009). Among the various subclasses of flavonoids, the flavonols that comprise quercetin and related flavonoids display superior scavenging activity. This is due to a large conjugated π–system that delocalizes electrons over the entire molecule. Structure–activity relationship studies reveal that two pharmacophores are present in the flavonols: (1) a catechol moiety in ring B, and (2) a hydroxyl (OH) group at the 3 position at a 2,3-double bound, which is activated by the hydroxyl groups at the 5 and 7 positions (Heijnen et al., Citation2002). In NAFLD, radicals produced during peroxisomal and mitochondrial β-oxidation and the metabolism of FFA by Cytochrome P450 2E1 and 4A can be scavenged by flavonoids, which will result in a reduction of oxidative stress.
In addition to the direct radical scavenging effect, several flavonoids have the ability to chelate iron and other transition metals that contribute to the formation of radicals (Pietta Citation2000; Ross and Kasum, Citation2002). The quercetin-derived semi-synthetic flavonoid monoHER can scavenge OH-radicals at an extremely high apparent rate – ks = 980 × 108 M−1 s−1 – which is even quicker than the diffusion rate (˜100 × 108 M−1 s−1; Lemmens et al., in press). This can be explained by “site-specific scavenging.” Essential for this activity is that monoHER can chelate iron. The result of this chelation is that monoHER is present at the site of radical formation, i.e., the iron ion. This enables monoHER to immediately scavenge the newly formed radical. By this site-specific scavenging, monoHER is able to prevent damage to critical biomolecules such as lipids, proteins, or DNA, in spite of the high reactivity of radical (Haenen et al., Citation1993; Lemmens et al., in press). Since iron-mediated hydroxyl radical formation has been implied in NAFLD (O'Brien and Powell, Citation2011; van de Wier et al., Citation2013), this action of flavonoids is expected to be meaningful for the treatment of NAFLD.
The third mode of action is that flavonoids can protect or enhance the endogenous antioxidant defense (Ross and Kasum, Citation2002; Stevenson and Hurst, Citation2007). Several flavonoids induce glutathione S-transferase, heme-oxygenase 1 (HO-1), and other antioxidants (Ross and Kasum, Citation2002; Yang et al., Citation2011; Sun et al., Citation2012; Zhang et al., Citation2012; Huang et al., Citation2013). An important pathway in this response is stimulation of nuclear factor erythroid derived 2 (nrf2), a transcription factor that binds to antioxidant response elements (AREs) in the promoter region of genes encoding various antioxidants and phase II detoxifying enzymes. This leads to the transcription of those enzymes, e.g., HO-1 and NAD(P)H–quinone oxidoreductase (Mann et al., Citation2009). Stimulation of nrf2 by flavonoids is reported in several studies (Yang et al., Citation2011; Sun et al., Citation2012; Zhang et al., Citation2012; Huang et al., Citation2013). Flavonoids increase nrf2 nuclear translocation to the nucleus and the binding of nrf2 to AREs (Yang et al., Citation2011; Huang et al., Citation2013). Since depletion of antioxidant defenses is seen in NASH patients, upregulation of antioxidants could be beneficial in the treatment of NASH (Rolo et al., Citation2012).
Anti-inflammatory effects
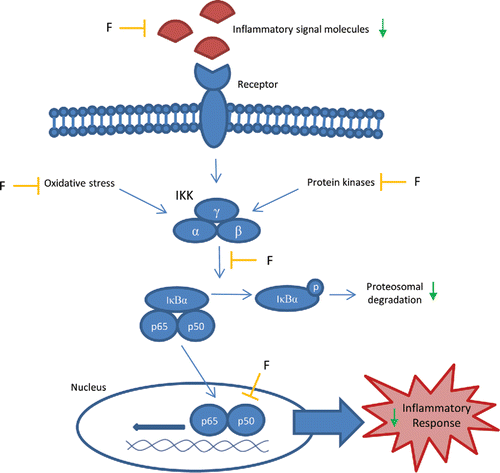
Anti-inflammatory effects of flavonoids have been mainly subscribed to the inhibition of NF-κB pathway (Gonzalez et al., Citation2011). The NF-κB pathway comprises canonical and non-canonical pathways. The canonical pathway is involved in inflammatory responses, while the non-canonical pathway regulates immunce cell differentiation and maturation and lymphoid organogenesis (Shih et al., Citation2011). Since the canonical pathway is most important for the initiation of inflammation, and effects of flavonoids focus on this part of the NF-κB pathway, our review is restricted to the canonical pathway. The canonical pathway is activated by pro-inflammatory signals, such as cytokines and oxidative stress (van den Berg et al., Citation2001). This causes the IκB kinase (IKK) complex to phosphorylate and designate NFκB inhibitor proteins (IκBs; α, β, or ϵ) for degradation. Degradation of IκBs releases NF-κB into the nucleus. Consequently, transcriptional activity occurs, resulting in an inflammatory response (Shih et al., Citation2011).
NF-κB activation occurs in various inflammatory diseases. Activation of the NF-κB pathway is observed in animal models of NASH as well as NASH patients (Marra Citation2008), illustrating a role of NF-κB activation in NASH. Flavonoids interfere with the NF-κB pathway in several ways (; Gonzalez et al., Citation2011). Flavonoids inhibit IKK complex and IκB-phosphorylation, thereby preventing NF-κB translocation to the nucleus and the transcription of genes involved in the inflammatory response. Flavonoids also inhibit protein kinases that control the activity of NF-κB (Kim et al., Citation2005). In addition, flavonoids inhibit protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), extracellular signal-related kinase (ERK), and Jun N-terminal kinase (JNK) (Kim et al., Citation2005). A mechanism proposed for inhibition of protein kinases is competitive binding to nucleotide-binding sites (Manthey Citation2000). Since oxidative stress also results in NF-κB activation (van den Berg et al., Citation2001), the antioxidant potency of flavonoids is also implicated in the inhibition of NF-κB pathway (Boots et al., Citation2008; Gloire et al., Citation2009; Salamone et al., Citation2012a).
Figure 6. Effects of flavonoids on the NFκB pathway. Inhibitory effects (—|) of flavonoids (F) on different processes in the canonical NF-κB pathway, leading to a decreased inflammatory response. IKK = IκB kinase, IκBα = NF-κB inhibitor-α, p65 = NF-κB complex subunit p65, p50 = NF-κB complex subunit p50.
Besides inhibitory effects on the NF-κB pathway, flavonoids also inhibit the activity of regulatory enzymes involved in the induction of inflammatory response, such as protein tyrosine kinases, PKC, phosphodiesterase, phospholipase A2, lipoxygenases, and COX (Manthey Citation2000; Kim et al., Citation2005). These enzymes are responsible for the activation of specialized cells involved in inflammation, e.g., by prostanoid biosynthesis via arachidonic acid metabolism (Manthey Citation2000). In addition, the production of various cytokines is inhibited by flavonoids, possibly involving inhibition of phosphodiesterase (Manthey Citation2000; Gonzalez-Gallego et al., Citation2010).
Furthermore, several flavonoids inhibit inducible nitric oxide (NO) synthase (iNOS) expression and the production of NO (Kim et al., Citation2005; Gonzalez-Gallego et al., Citation2010). NO serves as an inflammatory mediator, and leads to the formation of highly damaging peroxynitrite in conditions of oxidative stress (Garcia-Lafuente et al., Citation2009). In addition to the inhibition of NO production, flavonoids can scavenge NO (van Acker et al., Citation1995; Haenen and Bast, Citation1999) and peroxynitrite (Haenen et al., Citation1997). Inhibition of iNOS and COX-2 expression is also found to be related to the inhibition of NF-κB and activation of PPARγ (Gonzalez-Gallego et al., Citation2010).
Recently, it has been reported that flavonoids are able to prevent deterioration of anti-inflammatory effect of glucocorticoid cortisol in the presence of oxidative stress (Ruijters et al., Citation2014). Oxidative stress extinguishes the anti-inflammatory effect of cortisol, leading to cortisol resistance. Flavonoids reduce intracellular oxidative stress as well as the development of cortisol resistance. This further deciphers the enigmatic mechanism of flavonoids by which these bioactives exert their biological effect, and moreover, shows that their anti-inflammatory and antioxidant actions are intertwined.
Flavonoids in the treatment of NAFLD
Several flavonoids have been studied for the treatment of NAFLD. One of the most investigated flavonoids is silybin, one of the flavonoids in the flavonoid mixture silymarin. Green tea flavonoids and soy isoflavones are also extensively investigated. Together with quercetin and rutin, these are the most studied flavonoids for the treatment of NAFLD. These groups of flavonoids and their effects on NAFLD are reviewed separately.
Animal models
The majority of in vivo studies investigating the use of flavonoids in NAFLD are animal studies. Since NAFLD is a multifactorial disease and the patient population with NAFLD is very heterogenic, it is difficult to imitate all the facets of the disease in one animal model. Furthermore, NAFLD is seen as a hepatic manifestation of metabolic syndrome: patients do not only show liver abnormalities but also have obesity, dyslipidemia, and insulin resistance. Although many models may succeed to mirror the liver pathology correctly, this does not always reflect the right metabolic context (Larter et al., Citation2008).
Already many reviews have been devoted to the animal models of NAFLD (Nanji, Citation2004; Nagarajan et al., Citation2012; Takahashi et al., Citation2012). Therefore, this section will only evaluate the animal models used in the studies investigating the use of flavonoids in NAFLD. Most studies are mice or rat studies. In addition, one study investigated the development of NAFLD in gerbils. The animals used in different studies had various genetic backgrounds and some transgenic animal models were used. Furthermore, different diets were used to induce NAFLD, such as high fat and high fructose diets and methionine- and choline-deficient diets (MCD diet).
In order to validate the different models used, it has to be examined which model pictures the disease process most completely. Therefore, the models were compared regarding the development of liver damage (steatosis, inflammation, and fibrosis), the presence of the most important pathogenic pathways (metabolic abnormalities, oxidative stress, and inflammation), and the presence of other signs of metabolic syndrome (dyslipidemia, obesity, and insulin resistance).
First, the different dietary models are compared. The MCD diet causes lipid deposition in the liver by interfering with β-oxidation and VLDL secretion. The diet lacks methionine and choline, which are essential for hepatic β-oxidation and the production of VLDL. In addition to liver steatosis and inflammation, oxidative stress and changes in the liver cytokines and adipocytokines are found (Takahashi et al., Citation2012). The advantage of this model is that liver steatosis and inflammation are found within 10 days of the diet. Fibrosis is found after —eight to ten weeks (Takahashi et al., Citation2012). The MCD-model causes maximum inflammation, oxidative stress, and liver damage compared with other dietary models. However, the extent of damage is dependent on species, gender, and strain of the animals used (Kirsch et al., Citation2003). C57Bl/6 mice are found to develop maximum inflammation and necrosis, best approximating the histological features of NASH. Male gender and the strain Wistar rats are associated with the highest degree of steatosis (Kirsch et al., Citation2003). The disadvantage of the MCD-model is that it lacks the metabolic context of human NAFLD/NASH. Animals on the MCD-diet are found to lose weight, to have no insulin resistance, and unchanged or increased serum adiponectin levels (Kirsch et al., Citation2003). In some studies, this is resolved by using the MCD diet in genetically obese animals, such as ob/ob mice.
Various high fat diets have been used in the animal models of NAFLD. Sprague–Dawley rats on a high-fat diet are found to develop steatosis, inflammation, and oxidative damage in the liver. In addition, insulin resistance is found after three weeks of a high fat diet (Takahashi et al., Citation2012). However, development of steatohepatitis is dependent on the rodent species and the strain used. For example, while Sprague–Dawley rats do develop steatohepatitis, Wistar rats are found to be less susceptible to develop steatohepatitis on a high fat diet (Romestaing et al., Citation2007). C57Bl/6 mice are found to develop steatosis after 10 weeks of high fat diet. In addition, insulin resistance, increased plasma cholesterol levels, and obesity are found. However, slight inflammatory changes are only found after 35 weeks of high fat diet (Ito et al., Citation2007). The development of steatohepatitis in animals on a high fat diet is not only dependent on the rodent species and strain but also on the fat content in the diet, the composition of dietary fat, and the duration of treatment (Takahashi et al., Citation2012). Although the use of a high fat diet seems to reproduce the metabolic context of human NASH better, liver damage is less severe than with the use of MCD-diet. Among the various high fat diet models, the pathological changes in the intragastric-overfeeding model are found to resemble human NASH best (Ito et al., Citation2007).
Rats and mice fed a fructose-rich diet have been found to be good models for metabolic syndrome (Takahashi et al., Citation2012). Liver damage is also found in these models. Wistar rats on a high fructose diet (70%) develop liver steatosis and inflammation (Kawasaki et al., Citation2009). However, the distribution pattern of steatosis in the liver is different from that in human NAFLD. While in human NAFLD steatosis is mostly present in zone 3, steatosis in Wistar rats on a high fructose diet is predominant in zone 1 (Takahashi et al., Citation2012). Interestingly, inflammation does follow the same pattern as in human NASH: predominantly lobular and not periportal (Kawasaki et al., Citation2009). Similar to the use of a high fat diet, the extent of liver damage developed on a high fructose diet is also dependent on the type and strain of animals used and the fructose content in the diet.
Transgenic animal models in studies investigating flavonoids in NAFLD include db/db mice, ob/ob mice, nSREBP-1c transgenic mice, obsese Zucker fa/fa rats, and obese diabetic Otsuka Long–Evans Tokushima Fatty (OLETF) rats.
In ob/ob mice, a spontaneous mutation in the leptin gene causes leptin deficiency, leading to hyperphagic, inactive, extremely obese, and diabetic mice that develop liver steatosis spontaneously (Nagarajan et al., Citation2012; Takahashi et al., Citation2012). However, ob/ob mice do not spontaneously develop steatohepatitis. Therefore, a second “hit” is needed, such as an MCD diet or high fat diet (Nagarajan et al., Citation2012; Takahashi et al., Citation2012).
Db/db mice carry a spontaneous mutation in the leptin-receptor gene. These mice have normal or increased levels of leptin but are resistant to its effects, which leads to obesity and insulin resistance (Nagarajan et al., Citation2012; Takahashi et al., Citation2012). Db/db mice also develop steatosis but require a second “hit” for progression to steatohepatitis, such as the MCD diet or high fat diet (Nagarajan et al., Citation2012; Takahashi et al., Citation2012). The advantage of both ob/ob and db/db mice models is that they develop NAFLD in conditions resembling the metabolic syndrome. A disadvantage is that they need a second hit for progression to steatohepatitis. Db/db mice fed with an MCD diet were found to have higher serum alanine transaminase (ALT) levels and more severe hepatic inflammation and fibrosis than ob/ob mice fed with the MCD diet (Takahashi et al., Citation2012).
Obese Zucker fa/fa rats also have a mutation in the leptin receptor gene leading to leptin resistance. Up to four weeks of age, these rats only display increased appetite (Nanji, Citation2004). At four to five weeks of age, the fat mass and the serum level of FFA increase and triglycerides accumulate in various organs, including the liver (Nanji, Citation2004). Hyperinsulinemia also develops, eventually leading to diabetes. Development of steatohepatitis in Zucker fa/fa rats is described after feeding a high fat, high cholesterol diet (Matsunami et al., Citation2010).
In SREBP-1c transgenic mice, SREBP-1c is overexpressed, causing congenital lipodystrophy and severe insulin resistance. At the age of one week, liver steatosis is found, which progresses to steatohepatitis within 20 weeks of age without the requirement of a second hit (Takahashi et al., Citation2012). A disadvantage of this model is that in contrast with human NAFLD/NASH, visceral fat is decreased in this animal model.
The last genetic model used to investigate flavonoids in the treatment of NAFLD is the OLETF rat. This is an established model of the metabolic syndrome, characterized by insulin resistance, abdominal obesity, hypertension, and dyslipidemia (Song et al., Citation2013). Because of a gentic deletion of the cholecystokinin 1 receptor, these rats lack the feeling of satiety. From eight weeks of age, the OLETF rats develop obesity and hyperinsulinemia. In addition, liver steatosis develops spontaneously in these rats at 18 weeks of age (Song et al., Citation2013). However, after 42 weeks of age steatosis declines and inflammation and fibrosis do not develop spontaneously (Song et al., Citation2013).
From all the studies evaluated, the models using rats/gerbils on a high fat diet (Haddad et al., Citation2011; Yao et al., Citation2011, Citation2013) (; Yalniz et al., Citation2007; Ronis et al., Citation2009; Ji et al., Citation2011) (; Kuzu et al., Citation2008; Xiao et al., Citation2013) (; Ying et al., Citation2013) (), or on a high fat diet combined with high fructose or carbohydrates (Panchal et al., Citation2011, Citation2012; ), seem to best approximate the human conditions of NAFLD. In addition, studies using genetic models of the metabolic syndrome combined with a high fat diet or MCD diet are useful (Kim et al., Citation2012; Salamone et al., Citation2012a, Citation2012b; ). Studies using the MCD diet only do not correctly mirror the circumstances of metabolic syndrome. Studies using mice on a high fat diet, Wistar rats on only a high fat diet, or genetic models of the metabolic syndrome without a special diet or second “hit,” do not seem to provoke sufficient liver damage compared with the human situation and can only be used to evaluate the development of steatosis.
Table 1. Effects of silybin/silymarin on the development of NAFLD in animal models
Table 2. Effects of Realsil in a randomized controlled clinical trial.
Table 3. Effects of soy isoflavones on the development of NAFLD in animal models.
Table 4. Effects of green tea extract/epigallocatechin-3-gallate on the development of NAFLD in animal models.
Table 5. Effects of quercetin on the development of NAFLD in animal models.
Silymarin and silybin
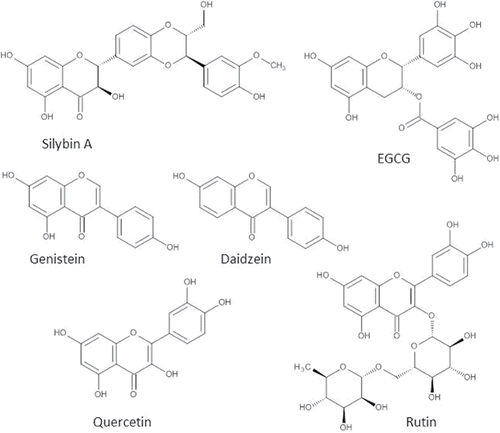
Silymarin is a flavonoid mix that originates from milk thistle extract. It was already used by doctors and herbalists to treat diverse liver and gallbladder disorders in ancient history (Abenavoli et al., Citation2012). Nowadays, 65% of the patients with liver diseases take herbal preparations, which are mainly derived from milk thistle (Loguercio et al., Citation2012). Silymarin contains at least eight different compounds: silybin (A and B), isosilybin (A and B), silichristin, silidianin, dehydrosilybin, taxifolin, and others. A small fraction of silymarin comprises polymerized polyphenolics that have not been identified yet (Skottova et al., Citation2003). About 50–70% of the silymarin extract comprise the flavonolignan silybin (), also known as silibinin, which is extensively studied and is regarded as the most active component of silymarin (Loguercio and Festi, Citation2011).
Figure 7. Most studied flavonoids in the treatment of NAFLD. Molecular structures of the most investigated flavonoids in the treatment of NAFLD: Silybin (mixture of two diateromers, of which one is pictured), genistein, daidzein, epigallocatechin-3-gallate (ECGC), quercetin, and rutin.
Silymarin flavanolignans have a limited bioavailability that was reported to be 0.45% in human volunteers (Calani et al., Citation2012). Similarly, the bioavailability of silybin in rats was calculated to be 0.95% (Wu et al., Citation2009). Extensive phase II metabolism, low permeability across intestinal epithelial cells, low solubility in water, and rapid excretion in bile and urine are the major causes of this limited bioavailability (Javed et al., Citation2011). To improve bioavailibility, derivatives of silybin have been synthesized, such as silybin–phosphatidylcholine (Javed et al., Citation2011; Loguercio and Festi, Citation2011). The silymarin that is not absorbed in the gastrointestinal tract is subjected to metabolism by bacteria in the colon. In the biological effect of polyphenolic compounds, the effect of colonic metabolites plays a key role (Mateo Anson et al., Citation2011). However, the contribution of colonic metabolites to the health effect of silymarin has not been examined.
In human studies, daily doses of up to 800-mg silymarin appeared to be safe. Only few and minor side effects of silymarin, primarily on the gastrointestinal tract, have been reported (Jacobs et al., Citation2002; Gazak et al., Citation2007).
The results of 10 identified in vivo studies investigating the use of silymarin/silybin in NAFLD models are summarized in (Serviddio et al., Citation2010; Shetty et al., Citation2010; Haddad et al., Citation2011; Yao et al., Citation2011; Kim et al., Citation2012; Qin et al., Citation2012; Salamone et al., Citation2012a, Citation2012b; Grattagliano et al., Citation2013; Yao et al., Citation2013). The molecular pathways implicated in the therapeutic effect of silymarin in NAFLD are elaborated in detail in , clustered in metabolic abnormalities, oxidative stress, and inflammation. An improvement in lipid metabolism is demonstrated by a decrease in serum and hepatic lipid values in numerous studies. This is caused by a stimulation of FFA oxidation and positive effects on coordinating factors of lipid metabolism, such as adiponectin and PPARα. In addition, insulin resistance is reduced in most studies. Antioxidant effects of silymarin represented by a decrease in lipid peroxidation and other oxidative stress markers might be caused by stimulation of endogenous antioxidants such as GSH and SOD. The anti-inflammatory effects of silymarin in NAFLD models include a decrease in the pro-inflammatory cytokine tumor necrosis factor α (TNF-α) and inhibition of NF-κB. Together, the metabolic, antioxidant, and anti-inflammatory effects of silymarin lead to a marked improvement of steatosis and liver inflammation in in vivo models of NAFLD ().
In a well-designed, randomized controlled trial (RCT), 138 patients with histologically proven NAFLD were treated with a silybin–phosphatidylcholine complex or placebo for 12 months (; Loguercio et al., Citation2012). Treatment showed positive effects on serum ALT, aspartate transaminase (AST), and gamma-glutamyl transpeptidase (γGT) levels, suggesting an improvement in hepatic damage. In addition, positive effects on insulin resistance and body mass index (BMI) were found. Patients of the treatment group that agreed to liver biopsy showed significant improvement in steatosis, lobular inflammation, ballooning, and fibrosis, while no improvement was seen in the biopsies of patients in the placebo group (Loguercio et al., Citation2012).
Taken together, the data indicate that silymarin and silybin show inhibitory effects on NAFLD progression. The influence of silymarin/silybin on NAFLD seems to be in line with the multifactorial mode of action of flavonoids; not one single mechanism of action but multiple mechanisms that reinforce each other emerge from the studies. The exact value of silymarin in the treatment of NAFLD still has to be established, but results so far are relatively consistent and encouraging.
Soy isoflavones
Unlike most flavonoids, isoflavones are not commonly found in a Western diet (Messina Citation2010). In fact, soybeans and products derived from soybeans are the only relevant sources of isoflavones (Messina Citation2010). Isoflavone intake in Western countries does not exceed 1 mg per day, whereas consumption of isoflavones in Japan and China can be as high as 40 mg/day (van Erp-Baart et al., Citation2003; Messina et al., Citation2006). The isoflavones in soy are genistein (), daidzein (), and glycitein. Generally, the content of genistein in soy is larger than the content of daidzein and glycetein (Larkin et al., Citation2008).
After intake of 5 to 7 h, genistein and daidzein plasma concentrations reach their maximum (Vitale et al., Citation2013). Plasma half-lives of genistein and daidzein aglycones are found to be 7 and 9 h respectively (Larkin et al., Citation2008). Because of extensive first-pass metabolism, isoflavone bioavailability is low (Larkin et al., Citation2008). Plasma genistein concentrations around 40 nM are measured in people consuming a Western diet, while concentrations of approximately 4 μM are measured in people consuming a traditional Japanese soybean-rich diet (Mann et al., Citation2009).
Isoflavones are generally regarded as safe (Qin et al., Citation2013). Most clinical trials do not report any adverse effects. Side effects that have been reported include abdominal bloating, constipation, and hot flushes (Qin et al., Citation2013). Isoflavones have estrogen-like activity in women with low endogenous estrogen levels (Andres et al., Citation2011). Because of the estrogen-like activity of isoflavones, concerns were raised that isoflavones could stimulate development of breast cancer in postmenopausal women. This is an issue of major concern; however, the limited studies in humans that addressed this subject did not corroborate this serious side effect (Andres et al., Citation2011).
Thirteen in vivo studies investigating the use of soy isoflavones in the animal models of NAFLD were found (Ae Park et al., Citation2006; Gudbrandsen et al., Citation2006; Lee et al., Citation2006; Davis et al., Citation2007; Ustundag et al., Citation2007; Yalniz et al., Citation2007; Gudbrandsen et al., Citation2009; Mohamed Salih et al., Citation2009; Ronis et al., Citation2009; Kim et al., Citation2010; Crespillo et al., Citation2011; Ji et al., Citation2011; Kim et al., Citation2011). The various effects of soy isoflavones on metabolic abnormalities, oxidative stress, and inflammation are shown in detail in . Improvement of lipid metabolism, evidenced by a decrease in hepatic and serum lipid values, has been attributed to the stimulation of FFA oxidation, inhibition of lipogenesis, and interaction of soy isoflavones with coordinating factors of lipid metabolism, such as adiponectin, leptin, PPARα, PPARγ, and others (). Protection against oxidative stress is demonstrated by a decrease in lipid peroxidation and protein carbonyl levels. The mechanism proposed for this protection is stimulation of endogenous antioxidant levels. Inflammation is mitigated by inhibition of NF-κB activation and reduced production of pro-inflammatory cytokines (). All these subtle effects combined can lead to a decrease in steatosis. The few studies that have investigated histological signs of inflammation corroborate the anti-inflammatory effect of isoflavones.
Since isoflavones inhibit many of the pathogenic mechanisms, they are promising compounds for the treatment of NAFLD. Although several animal studies have found positive effects of the use of soy isoflavones in the treatment of NAFLD, no clinical trials have been conducted on the use of soy isoflavones in NAFLD patients.
Green tea flavonoids
Green tea, similar to oolong tea and black tea, is derived from the plant Camellia Sinensis (Masterjohn and Bruno, Citation2012). The main flavonoids in green tea are catechins (30–42% of solid weight). They comprise epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin-gallate (EGCG) (Paquay et al., Citation2000; Masterjohn and Bruno, Citation2012). The highest percentage of catechins in green tea comprise EGCG (50–75%) (). Other flavonoids that can be found in small amounts in green tea are quercetin and myricitin (Masterjohn and Bruno, Citation2012).
After ingestion of 1–3 h of green tea, maximal catechin plasma concentrations (0.1–4.4 μM) are reached (Masterjohn and Bruno, Citation2012). Plasma half-lives of green tea catechins range from 1.5 to 5.7 h (Masterjohn and Bruno, Citation2012). Similar values were obtained in studies investigating pure EGCG (Manach et al., Citation2005). Because of the rapid elimination of catechins from the body, intake of tea catechins might be beneficial only when they are consumed for several times a day (Masterjohn and Bruno, Citation2012).
In some studies, concerns were raised regarding the possible hepatoxicity of green tea extract (GTE; Sarma et al., Citation2008). Nevertheless, a review of several clinical trials showed that the use of GTE/EGCG up to doses of 800 mg/kg/day is safe and well tolerated in humans (Sarma et al., Citation2008). The only side effects that were reported were mild headache and fatigue. In a recent clinical trial, daily consumption of 714 mg GTE/day for three weeks did not lead to liver toxicity in healthy males (Frank et al., Citation2009).
Ten studies investigating the use of GTE or EGCG in the animal models of NAFLD were found (Bose et al., Citation2008; Bruno et al., Citation2008; Kuzu et al., Citation2008; Nakamoto et al., Citation2009; Ueno et al., Citation2009; Chen et al., Citation2011; Park et al., Citation2011; Chung et al., Citation2012; Park et al., Citation2012; Xiao et al., Citation2013). The effects of GTE/EGCG on metabolic abnormalities, oxidative stress, and inflammation are presented in detail in . Improvement in lipid metabolism, represented by a decrease in serum and hepatic lipid levels, might be caused by interaction of green tea catechins with coordinating factors of lipid metabolism or a decrease in lipogenesis (). Stimulation of antioxidants and inhibition of ROS production by green tea catechins leads to an attenuation of oxidative stress, demonstrated by a decrease in lipid peroxidation (). In addition, inflammation is reduced by green tea catechins by inhibition of NF-κB activity and a decrease in pro-inflammatory cytokines (). In the majority of studies, these effects led to a decrease in steatosis. Only one study did not find a reduction of steatosis (Nakamoto et al., Citation2009). Histological inflammation was only investigated in two studies (Chung et al., Citation2012; Xiao et al., Citation2013). Both studies found a diminution of inflammation.
Unfortunately, no studies investigating GTE/EGCG in NAFLD patients were found. In addition, most of the investigated animal studies focused on steatosis only. Although in general promising effects of GTE/EGCG on steatosis are reported, the effects of GTE/EGCG on inflammation in NAFLD models are not conclusive. Clinical trials are lacking to substantiate the therapeutic potential of green tea catechins in NAFLD. Furthermore, the rapid elimination from the body seriously questions the prospect of the use of green tea in NAFLD treatment.
Quercetin
Quercetin () is a flavonol that is one of the most abundant flavonoids in the human diet. It is found in various fruits and vegetables, such as onions, apples, and tomatoes. The average intake of quercetin is estimated to be 5–40 mg/day (Hertog et al., Citation1995). In diet, quercetin is often bound by sugars (quercetin glycosides).
Quercetin has a relatively high bioavailability compared with other flavonoids (Russo et al., Citation2012). The bioavailability depends on the type of quercetin glycoside. It has been reported that glucosides of quercetin (quercetin bound by glucose) are absorbed in the gastrointestinal tract better than aglycon (Hollman et al., Citation1995; Graefe et al., Citation2001). Fifty-two percent of quercetin glucosides from onions are absorbed in the gastrointestinal tract versus 24% of the quercetin aglycone (Hollman et al., Citation1995). Quercetin and its metabolites have long plasma half-lives, i.e., 11–28 h (Manach et al., Citation2005). Therefore, plasma concentrations can increase significantly upon frequent intake. Quercetin is normally found in human plasma in low nanomolar concentrations, but upon supplementation this can increase to high nanomolar or low micromolar concentrations (Hollman et al., Citation1996; Conquer et al., Citation1998).
No adverse effects of quercetin were reported in studies investigating oral intake of quercetin or quercetin glucosides in doses of 3–1000 mg/day for a period of up to 12 weeks (Harwood et al., Citation2007). In addition, for intravenous doses of ±10.8 mg/kg body weight, no adverse effects were reported. For higher intravenous doses of up to 51.3 mg/kg body weight, pronounced pain at the injection site, dyspnea, emesis, and transient nephrotoxicity were reported (Harwood et al., Citation2007). Clinical symptoms lasted for a period after each injection (Harwood et al., Citation2007).
Although in vitro toxicity studies have reported mutagenic effects of quercetin and two in vivo animal studies reported quercetin carcinogenicity, many other animal studies fail to show increased tumor incidence related to administration of quercetin (Harwood et al., Citation2007). Quercetin toxicity is assumed to be related to the formation of quercetin–quinone, which is produced when quercetin is oxidized by radicals (Boots et al., Citation2007; Boots et al., Citation2008). The quercetin–quinone binds to thiols and causes toxic effects such as increased membrane permeability and altered function of enzymes with a critical sulfhydryl group (Boots et al., Citation2007, Citation2008).
Six animal studies investigating the use of quercetin in the animal models of NAFLD were identified (Kobori et al., Citation2011; Marcolin et al., Citation2012; Panchal et al., Citation2012; Jung et al., Citation2013; Marcolin et al., Citation2013; Ying et al., Citation2013). Effects of quercetin on lipid metabolism, oxidative stress and inflammation are summarized in . Quercetin improves lipid metabolism by affecting coordinating factors of lipid metabolism, such as PPARα, PPARγ, and adiponectin. Inhibition of lipogenesis and stimulation of fatty acid oxidation by quercetin also contribute to the improvement of lipid metabolism (). Reduction of oxidative stress, demonstrated by a decrease in lipid peroxidation, is caused by a potent direct antioxidant effect, induction of endogenous antioxidant defences, and inhibition of iNOS expression in the liver (). In only two studies inflammatory markers, such as TNF-α and Il-6, were investigated and found to be decreased by quercetin (). In all studies, the quercetin administration led to a decrease in steatosis. In general, histological signs of liver inflammation were also reduced. Two studies that investigated fibrosis found a reduction in fibrosis as well.
In the in vivo studies, mainly positive effects of quercetin on NAFLD were demonstrated. A randomized clinical trial on the use of quercetin in NAFLD patients is lacking, whereas various clinical trials have investigated the use of quercetin in other diseases (Valensi et al., Citation2005; Edwards et al., Citation2007; Egert et al., Citation2009; Heinz et al., Citation2010; Boots et al., Citation2011). To substantiate the therapeutic effect of quercetin in NAFLD, clinical trials are mandatory. A concern for approval of such studies will be the potential carcinogenic effect of quercetin, although the relatively high intake of quercetin in the normal diet as well as the widely applied supplementation of quercetin have not pinpointed this as a problem. In fact, epidemiological studies reveal the opposite: Flavonoid intake, which for the substantial part is quercetin, has an inverse relationship with the incidence of cancer.
Rutin
Rutin is quercetin with the disaccharide rutinose covalently bound to the 3-OH group (quercetin-3-O-β-rutinoside; ). It is abundantly found in plants such as buckwheat (Fagopyrum esculentum) and citrus fruits such as oranges (Citrus sinensis) and grapefruits (Citrus paradisi) (Sharma et al., Citation2013). The uptake of rutin in the gastrointestinal tract is less than that of quercetin and quercetin mono-glucosides, and lower peak plasma concentrations of rutin are reached after intake of an equivalent dose compared with that of quercetin (Hollman et al., Citation1995; Graefe et al., Citation2001). The main reason for rutin's poor bioavailability is its poor solubility in aqueous media (Sharma et al., Citation2013) and the resulting low bioaccessibility. However, rutin also has advantages over other flavonoids. It is relatively stable and does not display prominent pro-oxidant activity. While some flavonoids are labeled as mutagenic and relatively cytotoxic, rutin is neither (Sharma et al., Citation2013).
Four studies examining the use of rutin in the animal models of NAFLD were found (Hsu et al., Citation2009; Ziaee et al., Citation2009; Panchal et al., Citation2011; Gao et al., Citation2013). The effects of rutin on metabolism, oxidative stress, and inflammation are presented in . Hepatic and/or serum lipid values decreased in all studies, demonstrating an improvement in lipid metabolism. The mechanism of action was not investigated extensively in the studies. Inhibition of leptin and SREBP-1c was suggested to play a role (). Only two studies examined the effects of rutin on oxidative stress. Both studies concluded that administration of rutin reduced oxidative stress and implied as mechanism stimulation of endogenous antioxidant levels and inhibition of the production of ROS (). One study reported that administration of rutin reduced inflammatory markers such as TNF-α (; Gao et al., Citation2013). The combined effects of rutin on metabolism, oxidative stress, and inflammation can explain decrease in steatosis found in all studies. Liver inflammation was investigated in two studies and found to be reduced in both studies (Ziaee et al., Citation2009; Panchal et al., Citation2011). The only study that examined the effect of rutin on liver fibrosis did find a reduction in fibrosis (Panchal et al., Citation2011).
Table 6. Effects of rutin on the development of NAFLD in animal models.
Rutin is not extensively investigated in the animal models of NAFLD. In addition, no clinical trials have been performed. The promising results for the use of rutin against NAFLD summarized in show the potential of rutin and indicate that further studies are warranted. A disadvantage of rutin is its poor bioavailability. Semi-synthetic analogues of rutin with increased bioavailability might be more promising. In MonoHER, a hydroxyethyl group is attached to the oxygen to the 7-OH group, which increases water solubility. MonoHER is proven to be a very potent antioxidant (Haenen et al., Citation1993, Citation1997; van Acker et al., Citation1995; van Acker et al., Citation2000) with anti-inflammatory characteristics (Abou El Hassan et al., Citation2003).
Other flavonoids
A wide spectrum of flavonoids has been investigated in the animal models of NAFLD. However, the number of studies on many flavonoids is limited to one or two studies. These flavonoids are presented in .
Table 7. Other flavonoids investigated in animal models of NAFLD.
Naringenin, a flavanone found in grapefruit, completely reverses steatosis in LDLr−/− mice with fed a high fat diet. It also reduces dyslipidemia, hyperglycemia, hyperinsulinemia, and body weight (Mulvihill et al., Citation2009; Mulvihill et al., Citation2010).
Cyanidin 3-O-β-D-glucoside, an anthocyanin found in various plants and fruits that gives them a purple color, reduces hepatic steatosis, oxidative stress, and inflammation in diabetic db/db mice (Guo et al., Citation2012; Zhu et al., Citation2012). Furthermore, it reduces hyperglycemia and insulin resistance in db/db mice as well as C57Bl/6J mice fed with a high fat diet (Guo et al., Citation2012). Cyanidin 3-O-β-D-glucoside also decreases body weight and hepatic lipid content in C57Bl/6J mice (Tsuda et al., Citation2003).
Xanthohumol, a chalcone from the hop plant (Humulus lupulus), decreases steatosis, inflammation, and fibrosis in the murine models of NAFLD (Dorn et al., Citation2010; Doddapattar et al., Citation2013). Also, the other flavonoids noted in were found to have positive effects on NAFLD and other factors contributing to NAFLD, such as dyslipidemia, body weight, and insulin resistance (Guo et al., Citation2009; Zheng et al., Citation2009; Lee et al., Citation2012; Citation2013; Mei et al., Citation2012).
Since the number of different flavonoids known is huge, up to more than 5000 different chemical entities, it is mandatory to select to keep clinical research feasible from a practical point of view. Although the miscellaneous flavonoids mentioned in this paragraph might have merit in the treatment of NAFLD, their biochemical profile is not that different from the more extensively investigated flavonoids. Therefore, we focus on the latter group of more extensively studied flavonoids in this review.
Summary and perspective
The contemporary pathophysiological model of NAFLD comprises multiple parallel pathways with a dynamic cross talk that cumulate in steatosis and inflammation, and ultimately fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma. The multitude of pathways involved in the pathogenesis underpins the need for treatments that address these various pathways.
Flavonoids are compounds derived from plants with subtle effects on multiple targets that finally accumulate in a substantial health benefit. Interestingly, flavonoids have been found to have positive effects on lipid metabolism, insulin resistance, inflammation, and oxidative stress, the most important pathological processes in the etiology of NAFLD. This puts flavonoids in the spotlight for the treatment of NAFLD. In this review, the existing evidence for the use of flavonoids in the treatment of NAFLD is evaluated.
Flavonoids and flavonoid mixtures that have been widely investigated in the animal models of NAFLD include silymarin, silybin, soy isoflavones, green tea flavonoids, quercetin, and rutin. The protective biochemical profile of these flavonoids on lipid metabolism, insulin resistance, oxidative stress, and inflammation as well as their beneficial therapeutic effect on steatosis and liver inflammation in most of the studies form the scientific fundaments for the use of flavonoids in the treatment of NAFLD. However, further clinical studies are needed to examine the exact value of flavonoids in the treatment of NAFLD patients.
Further studies examining the use of flavonoids in NAFLD should include double-blinded randomized clinical trials. In designing and interpreting clinical studies, it is of importance to carefully consider the heterogeneity of the NAFLD patient group. A caveat is that high heterogeneity will negatively affect the power of the study. Moreover, NAFLD patients with a very different biochemical profile might benefit from flavonoids that display a biochemical profile that fits these different profiles the best. This means that it is unlikely that a uniform treatment for all types of NAFLD will be found. Personalized treatment with close monitoring of the therapeutic effect seems warranted to come to optimal treatment. To reach this stage, the most promising flavonoids should be tested first. The spectrum of flavonoids includes over 5000 different compounds with their own unique profile, illustrating that it is impossible to study all the flavonoids. Therefore, the compounds best suitable for further investigation have to be identified. In identifying the most promising compounds in a large series, structure–activity relationships are mandatory, although these relationships should always be critically evaluated (Haenen et al., Citation2006). Criteria that can be used to evaluate the therapeutic potential and to form a rational basis for selection of flavonoids for further investigation are their molecular mechanism of action and clinical evidence, bioavailability, and safety.
Regarding their molecular mechanism of action, none of the flavonoids seems to protrude from the animal studies. All flavonoids have been found to improve lipid metabolism, insulin resistance, oxidative stress, and inflammatory markers. However, in structure–activity relationship studies, quercetin, rutin, and its derivates are found to belong to the most potent antioxidants (Heijnen et al., Citation2002). Most clinical evidence is found for silymarin and silybin because these compounds are widely investigated in the animal models of NAFLD, and silybin was also examined in a randomized clinical trial.
Regarding bioavailability, quercetin has the best characteristics. Bioavailability of rutin is slightly lower but can be improved by the use of semi-synthetic derivates, such as MonoHER, which is more water-soluble. Bioavailability of silybin and silymarin is considerably lower and can be improved by conjugation to polar and hydrophilic moieties, e.g., as in silybin–phosphatidylcholine. Because of rapid elimination from the body and consequent necessity of frequent administration, green tea flavonoids are not likely to get a place in NAFLD treatment.
Although quercetin appears to possess superior antioxidant potential and bioavailability, quercetins safety is still debated due to its potential carcinogenicity. Rutin, which seems to be devoid of carcinogenic properties but displays similar antioxidant potential and only slightly lower bioavailability as quercetin, seems to be a better option. Rutin derivates, developed to increase bioavailability, seem to be even more appealing compounds for further investigation. For example, MonoHER, rutin with a hydroxyethyl group attached to the oxygen on the 7-position, has shown to be a very potent antioxidant (Haenen et al., Citation1993; Lemmens et al., in press).
In conclusion, with their multifaceted actions, flavonoids seem to suit perfectly in the pathophysiological model of NAFLD. The heterogeneity of the disease should be carefully considered in the design of clinical studies investigating a treatment for NAFLD. Already, multiple in vivo studies show encouraging results for the use of flavonoids in the treatment of NAFLD, which calls for additional research. Silybin has the advantage that it is the most studied flavonoid. Nevertheless, rutin and its derivatives, such as MonoHER, seem to be the most appealing flavonoids for further investigation due to their high antioxidant potential, bioavailability, and safety.
References
- Abenavoli, L., Milic, N., et al. (2012). Anti-oxidant therapy in non-alcoholic fatty liver disease: the role of silymarin. Endocrine. 42(3):754–755.
- Abou El Hassan, M. A., Verheul, H. M. et al. (2003). The new cardioprotector Monohydroxyethylrutoside protects against doxorubicin-induced inflammatory effects in vitro. Br. J. Cancer. 89(2):357–362.
- Ae Park, S., Choi, M. S. et al. (2006). Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sci. 79(12):1207–1213.
- Ahn, T. G., Yang, G. et al. (2013). Molecular mechanisms underlying the anti-obesity potential of prunetin, an O-methylated isoflavone. Biochem. Pharmacol. 85(10):1525–1533.
- Andres, S., Abraham, K. et al. (2011). Risks and benefits of dietary isoflavones for cancer. Crit. Rev. Toxicol. 41(6):463–506.
- Bast, A. and Haenen, G. R. (2013). Ten misconceptions about antioxidants. Trend. Pharmacol. Sci. 34(8):430–436.
- Bedogni, G., Miglioli, L. et al. (2005). Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology. 42(1):44–52.
- Bieghs, V., Walenbergh, S. M. et al. (2013). Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver Int. 33(7):1056–1061.
- Boots, A. W., Drent, M. et al. (2011). Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 30(4):506–512.
- Boots, A. W., Haenen, G. R. et al. (2008). Health effects of quercetin: From antioxidant to nutraceutical. Eur. J Pharmacol. 585(2–3):325–337.
- Boots, A. W., Li, H. et al. (2007). The quercetin paradox. Toxicol. Appl. Pharmacol. 222(1):89–96.
- Bors, W., Michel, C. et al. (1997). Antioxidant effects of flavonoids. Bio. Factors. 6(4):399–402.
- Bose, M., Lambert, J. D. et al. (2008). The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 138(9):1677–1683.
- Bruno, R. S., Dugan, C. E. et al. (2008). Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 138(2):323–331.
- Calani, L., Brighenti, F. et al. (2012). Absorption and metabolism of milk thistle flavanolignans in humans. Phytomedicine 20(1):40–46.
- Chang, C. J., Tzeng, T. F. et al. (2011). Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARalpha levels. Planta Medica. 77(17):1876–1882.
- Chen, N., Bezzina, R. et al. (2009). Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 29(11):784–793.
- Chen, Y. K., Cheung, C. et al. (2011). Effects of green tea polyphenol (-)-epigallocatechin-3-gallate on newly developed high-fat/western-style diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 59(21):11862–11871.
- Cho, K. W., Kim, Y. O. et al. (2011). Dietary naringenin increases hepatic peroxisome proliferators-activated receptor alpha protein expression and decreases plasma triglyceride and adiposity in rats. Eur. J. Nutr. 50(2):81–88.
- Chung, M. Y., Park, H. J. et al. (2012). Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J. Nutr. Biochem. 23(4):361–367.
- Conquer, J. A., Maiani, G. et al. (1998). Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 128(3):593–597.
- Crespillo, A., Alonso, M. et al. (2011). Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 164(7):1899–1915.
- Davis, J., Higginbotham, A. et al. (2007). Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metabol. 51(1):42–52.
- Doddapattar, P., Radovic, B. et al. (2013). Xanthohumol ameliorates atherosclerotic plaque formation, hypercholesterolemia, and hepatic steatosis in ApoE-deficient mice. Mol. Nutr. Food Res. 57(10):1718–1728.
- Dorn, C., Kraus, B. et al. (2010). Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol. Nutr. Food Res. 54(Suppl 2):S205–S213.
- Duthie, G. and Crozier, A. (2000). Plant-derived phenolic antioxidants. Curr. Opin. Lipidol. 11(1):43–47.
- Edwards, R. L., Lyon, T. et al. (2007). Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 137(11):2405–2411.
- Egert, S., Bosy-Westphal, A. et al. (2009). Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 102(7):1065–1074.
- Fenton, H. J. H. (1894). Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 65:899–911.
- Ferre, P. and Foufelle, F. (2010). Hepatic steatosis: A role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 12(Suppl 2):83–92.
- Frank, J., George, T. W. et al. (2009). Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J. Nutr. 139(1):58–62.
- Gao, M., Ma, Y. et al. (2013). Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice. Pharm. Res. 30(11):2940–2950.
- Garcia-Lafuente, A., Guillamon, E. et al. (2009). Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflammat. Res. 58(9):537–552.
- Gazak, R., Walterova, D. et al. (2007). Silybin and silymarin – new and emerging applications in medicine. Curr. Med. Chem. 14(3):315–338.
- Gloire, G. and Piette, J. (2009). Redox regulation of nuclear post-translational modifications during NF-kappaB activation. Antioxid. Redox Signal. 11(9):2209–2222.
- Goldwasser, J., Cohen, P. Y. et al. (2010). Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PloS One. 5(8):e12399.
- Gonzalez, R., Ballester, I. et al. (2011). Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 51(4):331–362.
- Gonzalez-Gallego, J., Garcia-Mediavilla, M. V. et al. (2010). Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 104(Suppl. 3):S15–S27.
- Goto, T., Teraminami, A. et al. (2012). Tiliroside, a glycosidic flavonoid, ameliorates obesity-induced metabolic disorders via activation of adiponectin signaling followed by enhancement of fatty acid oxidation in liver and skeletal muscle in obese–diabetic mice. J. Nutr. Biochem. 23(7):768–776.
- Graefe, E. U., Wittig, J. et al. (2001). Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 41(5):492–499.
- Grattagliano, I., Diogo, C. V. et al. (2013). A silybin-phospholipids complex counteracts rat fatty liver degeneration and mitochondrial oxidative changes. World J. Gastroenterol. 19(20):3007–3017.
- Gudbrandsen, O. A., Wergedahl, H., et al. (2009). A casein diet added isoflavone-enriched soy protein favorably affects biomarkers of steatohepatitis in obese Zucker rats. Nutrition. 25(5):574–580.
- Gudbrandsen, O. A., Wergedahl, H., et al. (2006). Dietary soya protein concentrate enriched with isoflavones reduced fatty liver, increased hepatic fatty acid oxidation and decreased the hepatic mRNA level of VLDL receptor in obese Zucker rats. Brit. J. Nutr. 96(2):249–257.
- Guo, H. X., Liu, D. H. et al. (2009). Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol. Sinic. 30(11):1505–1512.
- Guo, H., Xia, M. et al. (2012). Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 23(4):349–360.
- Haddad, Y., Vallerand, D. et al. (2011). Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid. Based Compl. Alt. Med. 2011:nep164.
- Haenen, G. R., Arts, M. J. et al. (2006). Structure and activity in assessing antioxidant activity in vitro and in vivo A critical appraisal illustrated with the flavonoids. Environ. Toxicol. Pharmacol. 21(2):191–198.
- Haenen, G. R. and Bast, A. (1999). Nitric oxide radical scavenging of flavonoids. Methods Enzymol. 301:490–503.
- Haenen, G. R. M. M., Jansen, F. P. et al. (1993). The antioxidant properties of five O-(β-hydroxyethyl)-rutosides of the flavonoid mixture venoruton. Phlebology. 8(suppl 1):10–17.
- Haenen, G. R. M. M., Paquay, J. B. G. et al. (1997). Peroxynitrite scavenging by flavonoids. Biochem. Biophys. Res. Commun. 236(3):591–593.
- Harwood, M., Danielewska-Nikiel, B. et al. (2007). A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 45(11):2179–2205.
- Heijnen, C. G., Haenen, G. R. et al. (2002). Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic. Res. 36(5):575–581.
- Heinz, S. A., Henson, D. A. et al. (2010). Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacol. Res. 62(3):237–242.
- Hertog, M. G., Kromhout, D. et al. (1995). Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Internal Med. 155(4):381–386.
- Higuchi, N., Kato, M. et al. (2008). Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 38(11):1122–1129.
- Hollman, P. C., de Vries, J. H. et al. (1995). Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am.J. Clin. Nutr. 62(6):1276–1282.
- Hollman, P. C., vd Gaag, M. et al. (1996). Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 21(5):703–707.
- Hsu, C. L., Wu, C. H. et al. (2009). Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 57(2):425–431.
- Huang, C. S., Lii, C. K. et al. (2013). Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch. Toxicol. 87(1):167–178.
- Hwang, Y. P., Choi, J. H. et al. (2011). Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 31(12):896–906.
- Ito, M., Suzuki, J. et al. (2007). Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol. Res. 37(1):50–57.
- Jacobs, B. P., Dennehy, C. et al. (2002). Milk thistle for the treatment of liver disease: A systematic review and meta-analysis. Am. J. Med. 113(6):506–515.
- Javed, S., Kohli, K. et al. (2011). Reassessing bioavailability of silymarin. Altern. Med. Rev. 16(3):239–249.
- Ji, G., Yang, Q. et al. (2011). Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int. Immunopharmacol. 11(6):762–768.
- Jia, Y., Kim, J. Y. et al. (2013). Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochimica et Biophysica Acta. 1831(4):698–708.
- Jung, C. H., Cho, I. et al. (2013). Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Research PTR. 27(1):139–143.
- Kallwitz, E. R., McLachlan, A. et al. (2008). Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J. Gastroenterol. 14(1):22–28.
- Kawasaki, T., Igarashi, K. et al. (2009). Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J. Nutr. 139(11):2067–2071.
- Kim, M. H., Kang, K. S. et al. (2010). The inhibitory effect of genistein on hepatic steatosis is linked to visceral adipocyte metabolism in mice with diet-induced non-alcoholic fatty liver disease. Br. J. Nutr. 104(9):1333–1342.
- Kim, H., Kong, H. et al. (2005). Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm. Res. 22(9):1499–1509.
- Kim, K. D., Lee, H. J. et al. (2012). Silibinin regulates gene expression, production and secretion of mucin from cultured airway epithelial cells. Phytother. Res. PTR. 26(9):1301–1307.
- Kim, M. H., Park, J. S. et al. (2011). Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int. J. Obes. 35(8):1019–1030.
- Kim, M., Yang, S. G. et al. (2012). Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: Analysis of isolated hepatic stellate cells. Int. J. Mol. Med. 30(3):473–479.
- Kirsch, R., Clarkson, V. et al. (2003). Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J. Gastroenterol. Hepatol. 18(11):1272–1282.
- Kobori, M., Masumoto, S. et al. (2011). Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 55(4):530–540.
- Koek, G. H., Liedorp, P. R. et al. (2011). The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta. 412(15–16):1297–1305.
- Kuzu, N., Bahcecioglu, I. H. et al. (2008). Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 23(8, Pt 2):e465–e470.
- Larkin, T., Price, W. E. et al. (2008). The key importance of soy isoflavone bioavailability to understanding health benefits. Crit. Rev. Food Sci. Nutr. 48(6):538–552.
- Larter, C. Z. and Yeh, M. M. (2008). Animal models of NASH: Getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 23(11):1635–1648.
- Lee, Y.-S., Cha, B.-Y. et al. (2013). Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J. Nutr. Biochem. 24(1):156–162.
- Lee, J. W., Choe, S. S. et al. (2012). AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J. Lipid Res. 53(7):1277–1286.
- Lee, Y. M., Choi, J. S. et al. (2006). Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition. 22(9):956–964.
- Lemmens, K. J. A., Van de Wier, B. et al. (in press). The flavonoid 7-mono-O-(β-hydroxyethyl)-rutoside is able to protect endothelial cells by a direct antioxidant effect. Toxicol. in vitro. 28(4):538–543.
- Liu, J. F., Ma, Y. et al. (2011). Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother. Res. 25(4):588–596.
- Loguercio, C., Andreone, P. et al. (2012). Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free Radic. Biol. Med. 52(9):1658–1665.
- Loguercio, C. and Festi, D. (2011). Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 17(18):2288–2301.
- Macdonald, G. A. and Prins, J. B. (2004). Peroxisomal fatty acid metabolism, peroxisomal proliferator-activated receptors and non-alcoholic fatty liver disease. J.Gastroenterol.Hepatol. 19(12):1335–1337.
- Malek, M. A., Hoang, M. H. et al. (2013). Ombuin-3-O-beta-D-glucopyranoside from Gynostemma pentaphyllum is a dual agonistic ligand of peroxisome proliferator-activated receptors alpha and delta/beta. Biochem.Biophys. Res. Commun.. 430(4):1322–1328.
- Manach, C., Williamson, G. et al. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am.J.Clin. Nutr. 81(1 Suppl):230S–242S.
- Mann, G. E., Bonacasa, B. et al. (2009). Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 9(2):139–145.
- Manthey, J. A. (2000). Biological properties of flavonoids pertaining to inflammation. Microcirculation. 7(6, Pt 2):S29–S34.
- Marcolin, E., Forgiarini, L. F. et al. (2013). Quercetin decreases liver damage in mice with non-alcoholic steatohepatitis. Basic Clin. Pharmacol. Toxicol. 112(6):385–391.
- Marcolin, E., San-Miguel, B. et al. (2012). Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J. Nutr. 142(10):1821–1828.
- Marra, F. (2008). Nuclear factor-kappaB inhibition and non-alcoholic steatohepatitis: inflammation as a target for therapy. Gut. 57(5):570–572.
- Masterjohn, C. and Bruno, R. S. (2012). Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutr. Rev. 70(1):41–56.
- Mateo Anson, N., Aura, A. M. et al. (2011). Bioprocessing of wheat bran in whole wheat bread increases the bioavailability of phenolic acids in men and exerts antiinflammatory effects ex vivo. J. Nutr. 141(1):137–143.
- Matsunami, T., Sato, Y. et al. (2010). Regulation of oxidative stress and inflammation by hepatic adiponectin receptor 2 in an animal model of nonalcoholic steatohepatitis. Int. J. Clin. Exp. Pathol. 3(5):472–481.
- Medjakovic, S., Mueller, M. et al. (2010). Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutrients. 2(3):241–279.
- Mei, L., Mochizuki, M. et al. (2012). Hepatoprotective effects of pycnogenol in a rat model of non-alcoholic steatohepatitis. Phytother. Res. PTR. 26(10):1572–1574.
- Messina, M. (2010). A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 140(7):1350S–1354S.
- Messina, M., Nagata, C. et al. (2006). Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer. 55(1):1–12.
- Mohamed Salih, S., Nallasamy, P. et al. (2009). Genistein improves liver function and attenuates non-alcoholic fatty liver disease in a rat model of insulin resistance. J. Diabetes. 1(4):278–287.
- Mulvihill, E. E., Allister, E. M. et al. (2009). Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 58(10):2198–2210.
- Mulvihill, E. E., Assini, J. M. et al. (2010). Naringenin decreases progression of atherosclerosis by improving dyslipidemia in high-fat-fed low-density lipoprotein receptor-null mice. Arterioscler. Throm. Vascul. Biol. 30(4):742–748.
- Nagarajan, P., Mahesh Kumar, M. J. et al. (2012). Genetically modified mouse models for the study of nonalcoholic fatty liver disease. World J. Gastroenterol. 18(11):1141–1153.
- Nakamoto, K., Takayama, F. et al. (2009). Beneficial effects of fermented green tea extract in a rat model of non-alcoholic steatohepatitis. J. Clin. Biochem. Nutr. 44(3):239–246.
- Nanji, A. A. (2004). Animal models of nonalcoholic fatty liver disease and steatohepatitis. Clin. Liver Dis. 8(3):559–574, ix.
- Nelson, J. E., Wilson, L. et al. (2011). Relationship between the pattern of hepatic iron deposition and histological severity in non alcoholic fatty liver disease. Hepatology. 53(2):448–457.
- Neuschwander-Tetri, B. A. and Caldwell, S. H. (2003). Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 37(5):1202–1219.
- O'Brien, J. and Powell, L. P. (2011). Non-alcoholic fatty liver disease: Is iron relevant? Hepatol. Int. 6(1):332–341.
- Pais, R., Pascale, A. et al. (2011). Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clin. Res. Hepatol. Gastroenterol. 35(1):23–28.
- Panchal, S. K., Poudyal, H. et al. (2011). Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 141(6):1062–1069.
- Panchal, S. K., Poudyal, H. et al. (2012). Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 142(6):1026–1032.
- Paquay, J. B., Haenen, G. R. et al. (2000). Protection against nitric oxide toxicity by tea. J. Agric. Food Chem. 48(11):5768–5772.
- Park, H. J., DiNatale, D. A. et al. (2011). Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. J. Nutr. Biochem. 22(4):393–400.
- Park, H. J., Lee, J. Y. et al. (2012). Green tea extract suppresses NFkappaB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. J. Nutr. 142(1):57–63.
- Pietta, P. G. (2000). Flavonoids as antioxidants. J. Nat. Prod. 63(7):1035–1042.
- Polyzos, S. A., Kountouras, J. et al. (2012). Nonalcoholic fatty liver disease: Multimodal treatment options for a pathogenetically multiple-hit disease. J. Clin. Gastroenterol. 46(4):272–284.
- Puhl, A. C., Bernardes, A. et al. (2012). Mode of peroxisome proliferator-activated receptor gamma activation by luteolin. Mol. Pharmacol. 81(6):788–799.
- Qin, Y., Niu, K. et al. (2013). Isoflavones for hypercholesterolaemia in adults. Cochrane Database System. Rev. 6:CD009518.
- Qin, R., Zhang, J. et al. (2012). Protective effects of gypenosides against fatty liver disease induced by high fat and cholesterol diet and alcohol in rats. Arch. Pharm. Res. 35(7):1241–1250.
- Rolo, A. P., Teodoro, J. S. et al. (2012). Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 52:59–69.
- Romestaing, C., Piquet, M. A. et al. (2007). Long-term highly saturated fat diet does not induce NASH in Wistar rats. Nutr. Metab. 4:4.
- Ronis, M. J., Chen, Y. et al. (2009). Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 139(8):1431–1438.
- Ross, J. A. and Kasum, C. M. (2002). Dietary flavonoids: bioavailability, metabolic effects, and safety. Ann. Rev. Nutr. 22:19–34.
- Ruijters, E. J. B., Haenen, G. R. M. M. et al. (2014). The cocoa flavanol (−)-epicatechin protects the cortisol response. Pharmacol. Res. 79(0):28–33.
- Russo, M., Spagnuolo, C. et al. (2012). The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem. Pharmacol. 83(1):6–15.
- Salamone, F., Galvano, F. et al. (2012a). Silibinin modulates lipid homeostasis and inhibits nuclear factor kappa B activation in experimental nonalcoholic steatohepatitis. Transl. Res J. Lab. Clin. Med. 159(6):477–486.
- Salamone, F., Galvano, F. et al. (2012b). Silibinin improves hepatic and myocardial injury in mice with nonalcoholic steatohepatitis. Dig. Liver Dis. 44(4):334–342.
- Sarma, D. N., Barrett, M. L. et al. (2008). Safety of green tea extracts: A systematic review by the US Pharmacopeia. Drug Saf. Int. J. Med. Toxicol. Drug Exp. 31(6):469–484.
- Savage, D. B., Tan, G. D. et al. (2003). Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 52(4):910–917.
- Serviddio, G., Bellanti, F. et al. (2010). A silybin-phospholipid complex prevents mitochondrial dysfunction in a rodent model of nonalcoholic steatohepatitis. J. Pharmacol. Exp. Ther. 332(3):922–932.
- Sharma, S., Ali, A. et al. (2013). Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Invest. Drugs. 22(8):1063–1079.
- Sharma, A. K., Bharti, S. et al. (2011). Up-regulation of PPARgamma, heat shock protein-27 and -72 by naringin attenuates insulin resistance, beta-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 106(11):1713–1723.
- Shetty, S. N., Mengi, S. et al. (2010). A study of standardized extracts of Picrorhiza kurroa Royle ex Benth in experimental nonalcoholic fatty liver disease. J. Ayur. Integr. Med. 1(3):203–210.
- Shih, V. F., Tsui, R. et al. (2011). A single NFkappaB system for both canonical and non-canonical signaling. Cell Res. 21(1):86–102.
- Shin, E. S., Lee, H. H. et al. (2007). Genistein downregulates SREBP-1 regulated gene expression by inhibiting site-1 protease expression in HepG2 cells. J. Nutr. 137(5):1127–1131.
- Shiri-Sverdlov, R., Wouters, K. et al. (2006). Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J. Hepatol. 44(4):732–741.
- Skottova, N., Vecera, R. et al. (2003). Effects of polyphenolic fraction of silymarin on lipoprotein profile in rats fed cholesterol-rich diets. Pharmacol. Res. 47(1):17–26.
- Song, Y. S., Fang, C. H. et al. (2013). Time course of the development of nonalcoholic Fatty liver disease in the Otsuka long-evans Tokushima fatty rat. Gastroenterol. Res. Pract. 2013:342648.
- Stevenson, D. E. and Hurst, R. D. (2007). Polyphenolic phytochemicals – just antioxidants or much more? Cell. Mol. Life Sci. 64(22):2900–2916.
- Sun, G. B., Sun, X. et al. (2012). Oxidative stress suppression by luteolin-induced heme oxygenase-1 expression. Toxicol. Appl. Pharmacol. 265(2):229–240.
- Tailleux, A., Wouters, K. et al. (2012). Roles of PPARs in NAFLD: potential therapeutic targets. Biochim. Biophys. Acta. 1821(5):809–818.
- Takahashi, Y., Soejima, Y. et al. (2012). Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 18(19):2300–2308.
- Tilg, H. and Moschen, A. R. (2010). Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology. 52(5):1836–1846.
- Tsuda, T., Horio, F. et al. (2003). Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 133(7):2125–2130.
- Ucar, F., Sezer, S. et al. (2013). The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. Comm. Free Radic. Res. 18(4):127–133.
- Ueno, T., Torimura, T. et al. (2009). Epigallocatechin-3-gallate improves nonalcoholic steatohepatitis model mice expressing nuclear sterol regulatory element binding protein-1c in adipose tissue. Int. J. Mol. Med. 24(1):17–22.
- Ustundag, B., Bahcecioglu, I. H. et al. (2007). Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig. Dis. Sci. 52(8):2006–2014.
- Valensi, P., Le Devehat, C. et al. (2005). A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy. A preliminary report. J. Diab. Complications. 19(5):247–253.
- Valenti, L., Fracanzani, A. L. et al. (2010). HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 138(3):905–912.
- van Acker, F. A., Schouten, O. et al. (2000). Flavonoids can replace alpha-tocopherol as an antioxidant. FEBS Lett. 473(2):145–148.
- van Acker, S. A., Tromp, M. N. et al. (1995). Flavonoids as scavengers of nitric oxide radical. Biochem. Biophys. Res. Communications. 214(3):755–759.
- van den Berg, R., Haenen, G. R. et al. (2001). Transcription factor NF-kappaB as a potential biomarker for oxidative stress. Br. J. Nutr. 86(Suppl 1):S121–S127.
- van de Wier, B., Balk, J. M. et al. (2013). Elevated citrate levels in non-alcoholic fatty liver disease: The potential of citrate to promote radical production. FEBS Lett. 587(15):2461–2466.
- van Erp-Baart, M. A., Brants, H. A. et al. (2003). Isoflavone intake in four different European countries: The VENUS approach. Br. J. Nutr. 89(Suppl 1):S25–S30.
- Vitale, D. C., Piazza, C. et al. (2013). Isoflavones: Estrogenic activity, biological effect, and bioavailability. Eur.J. Drug Metab. Pharmacokinet. 38(1):15–25.
- Wood, N. (2004). Hepatolipidemic effects of naringenin in high corn starch- versus high coconut oil-fed rats. J. Med. Food. 7(3):315–319.
- Wu, C. H., Lin, M. C. et al. (2011). Rutin inhibits oleic acid induced lipid accumulation via reducing lipogenesis and oxidative stress in hepatocarcinoma cells. J. Food Sci. 76(2):T65–T72.
- Wu, J. W., Lin, L. C. et al. (2009). Drug–drug interactions of silymarin on the perspective of pharmacokinetics. J. Ethnopharmacol. 121(2):185–193.
- Xia, M., Hou, M. et al. (2005). Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor {gamma}-liver X receptor {alpha}-ABCA1 pathway. J. Biol. Chem. 280(44):36792–36801.
- Xiao, J., Ho, C. T. et al. (2013). Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 53(1):187–199.
- Yalniz, M., Bahcecioglu, I. H. et al. (2007). Preventive role of genistein in an experimental non-alcoholic steatohepatitis model. J. Gastroenterol. Hepatol. 22(11):2009–2014.
- Yang, Y. C., Lii, C. K. et al. (2011). Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 51(11):2073–2081.
- Yao, J., Zhi, M. et al. (2013). Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz. J. Med. Biol. Res. 46(3):270–277.
- Yao, J., Zhi, M. et al. (2011). Effect of silybin on high-fat-induced fatty liver in rats. Braz.J. Med.Biol.Res. 44(7):652–659.
- Yap, F., Craddock, L. et al. (2011). Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int. J. Biol. Sci. 7(5):645–650.
- Yilmaz, Y. (2012). Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment. Pharmacol. Therapeut. 36(9):815–823.
- Ying, H. Z., Liu, Y. H. et al. (2013). Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 52:53–60.
- Zhang, Z., Cui, W. et al. (2012). Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCalpha and PI3K/AKT signaling pathways. J. Agric. Food Chem. 60(33):8171–8182.
- Zheng, P., Ji, G. et al. (2009). Therapeutic effect of puerarin on non-alcoholic rat fatty liver by improving leptin signal transduction through JAK2/STAT3 pathways. Am. J.. Chin. Med. 37(1):69–83.
- Zhu, W., Jia, Q. et al. (2012). The anthocyanin cyanidin-3-O-beta-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Radic. Biol. Med. 52(2):314–327.
- Ziaee, A., Zamansoltani, F. et al. (2009). Effects of rutin on lipid profile in hypercholesterolaemic rats. Basic Clin. Pharmacol. Toxicol. 104(3):253–258.
