ABSTRACT
Inflammation is a major biological process regulating the interaction between organisms and the environment, including the diet. Because of the increase in chronic inflammatory diseases, and in light of the immune-regulatory properties of breastfeeding, the ability of dairy products to modulate inflammatory processes in humans is an important but unresolved issue. Here, we report a systematic review of 52 clinical trials investigating inflammatory markers in relation to the consumption of dairy products. An inflammatory score (IS) was defined to quantitatively evaluate this interaction. The IS was significantly positive for the entire data set, indicating an anti-inflammatory activity in humans. When the subjects were stratified according to their health status, the IS was strongly indicative of an anti-inflammatory activity in subjects with metabolic disorders and of a pro-inflammatory activity in subjects allergic to bovine milk. Stratifying the data by product categories associated both low-fat and high-fat products, as well as fermented products, with an anti-inflammatory activity. Remarkably, the literature is characterized by a large gap in knowledge on bioavailability of bioactive nutrients. Future research should thus better combine food and nutritional sciences to adequately follow the fate of these nutrients along the gastrointestinal and metabolic axes.
Introduction
Immunity is a major process among the biological phenomena regulating the interaction of higher organisms with the environment, in particular as it provides a mechanism by which external agents are either rejected (e.g., phagocytosis of pathogens) or internalized (e.g., oral tolerance to ingested food) by the organism. One main expression of the immune system is its ability to mount an inflammatory reaction to these stimuli. If sustained, the inflammatory response may, however, turn against the host's own tissues, leading to a range of chronic inflammatory diseases that have now supplanted infectious diseases worldwide (Hunter and Reddy, Citation2013). The Global Business Intelligence Research estimated the global inflammatory therapeutics market to reach $85.9 billion in 2017 (Global Business Intelligence Research, Citation2011).
Most chronic inflammatory diseases (e.g., obesity, diabetes) as well as allergic diseases are strongly influenced by nutrition, the metabolism of food being intimately associated with inflammatory processes (Hotamisligil, Citation2006). In addition, postprandial inflammation is part of the normal stress reaction of the cell in response to the ingestion of food (Hernandez-Aguilera et al., Citation2013). Nutrients thus appear to be able to modulate the inflammatory status of humans and inflammation has consequently emerged as an important research topic in food and nutrition sciences (Calder et al., Citation2011; Calder et al., Citation2013; Klop et al., Citation2012).
Dairy products represent a particularly interesting food type to study in the context of inflammation. From an evolutionary point of view, ancestors of mammalians may have possessed primitive apocrine-like glands in the skin, approximately 310 million years ago, that incorporated elements of the innate immune system in providing protection to the skin and to eggs that were moistened (Oftedal, Citation2012). Because of its ability to support the development of the immune system of the infant, to inhibit bacterial growth (e.g., lactoferrin) and to deliver anti-oxidative protection (e.g., vitamins or glutathione), the potential of maternal milk to inhibit inflammation in the offspring has consequently raised interest (Lepage and Van de Perre, Citation2012). Part of these properties may be maintained when boundaries across species and life cycles are crossed, i.e. in the context of the consumption of dairy products by human adults (Labonte et al., Citation2013). In addition, the importance of food in modulating the gut microbiota, a key regulator of immunity, has become more evident during the last decade (Kau et al., Citation2011). Milk is a natural and culturally accepted vector to deliver supplements to the human organism (Ceapa et al., Citation2013), in particular prebiotic and probiotics that both modulate the microflora and thus influence immune and inflammatory processes. Besides, milk is amenable to a wide range of technological transformations, including its fermentation by lactic acid bacteria to produce fermented dairy products such as yoghurt or cheese whose metabolites may further modulate the ability of milk to influence immune processes in humans (Augustin and Udabage, Citation2007). Milk and dairy products are major food products in human nutrition, amounting to 14% of the caloric intake in developed countries (FAO, Citation2013b). The Food and Agriculture Organization (FAO) forecasted a world milk production of 784 million tons in 2013 (FAO, Citation2013a), which amounts to an average of circa 100 L milk per year per human being. An evaluation of the ability of dairy products to modulate inflammatory processes in humans is, thus justified.
Studies addressing the impact of dairy products on inflammatory processes present a contradictory landscape. Indeed, dairy products were reported to be beneficial, inactive, as well as detrimental. For illustration, the ATTICA study reported an inverse relationship between the consumption of dairy products and markers of the metabolic syndrome, including the inflammatory markers associated with this syndrome (Panagiotakos et al., Citation2010). On the other hand, the relatively high concentrations of saturated fat and dietary antigens in cow milk have raised concern and some scientists claimed that dairy products are a major cause in the development of chronic inflammatory disorders and autoimmune diseases (Melnik, Citation2009). These opposite statements reflect the wide spectrum of information available in the scientific literature on the relationship between the consumption of dairy products and inflammation. Indeed, many articles have been published on this relationship, but systematic reviews are scarce (Labonte et al., Citation2013) and incomplete. The association between the consumption of dairy products and inflammation in humans, thus merits clarification for the following reasons: (i) milk and dairy products play qualitatively and quantitatively an important role in human nutrition (Haug et al., Citation2007); (ii) inflammation, in particular low-grade systemic inflammation, has a significant impact on human health and longevity (Candore et al., Citation2010); (iii) nutrient metabolism and inflammation are mechanistically closely interconnected (Hotamisligil, Citation2006; Calder et al., Citation2011; Klop et al., Citation2012; Calder et al., Citation2013; Hernandez-Aguilera et al., Citation2013).
The property of the foods investigated in human nutritional trials are often poorly documented what renders an objective evaluation of the clinical outcome very difficult. This review aimed to narrow the gap between food science and nutritional science. The information usually provided by reviews on medical topics (Moher et al., Citation2009) was thus complemented with product-related information that is usually requested by regulatory authorities to document the functional properties of the food products and nutrients of interest (EFSA Panel on Dietetic Products Nutrition and Allergies, Citation2011; FDA Office of Nutrition Labeling and Dietary Supplements, Citation2009).
The specific goals of this review are to:
Present a structured overview of published original human studies investigating the impact of the consumption of dairy products on inflammatory processes;
Develop a method to quantitatively evaluate the results extracted from these studies;
Use this method, in order to evaluate whether pro- or anti-inflammatory properties of dairy products can be concluded from these studies;
Identify research gaps that should be filled to allow a better evaluation of the anti- or pro-inflammatory properties of specific dairy products in specific human populations.
Methods
Literature search strategy
A review was conducted using Medline and Scopus search that includes all original research articles written in English, published since January 1990, on the relationship between inflammatory markers and the consumption of dairy products in humans.
A first Medline search was conducted on February 13, 2013. A search of the Scopus database was also conducted on June 18, 2013 and the entries not identified in Medline were included into the evaluation. Medline and Scopus were searched again on December 10, 2013 to identify and include additional articles published until November 30, 2013. The search strategies were as follows:
Medline search strategy. (milk OR cheese OR yog* OR dair*) AND inflam* NOT (“breast milk” NOT “human milk”) NOT review*. Filters: Case Reports; Clinical Trial; Clinical Trial, Phase I; Clinical Trial, Phase II; Comparative Study; Controlled Clinical Trial; Multicenter Study; Randomized Controlled Trial; Evaluation Studies; Meta-Analysis; Systematic Reviews; Humans; English;
Scopus search strategy. (((TITLE-ABS-KEY(milk OR cheese OR yog* OR dair*) AND TITLE-ABS-KEY(inflam*) AND NOT TITLE-ABS-KEY(“breast milk” not “human milk”)) AND DOCTYPE(ar)) AND (humans)) AND (inflammation) AND (LIMIT-TO(LANGUAGE,”English”)).
Data collection process
shows the flow diagram with the five phases leading to the quantitative analysis of the 52 clinical studies. Seventy-eight study results were extracted from these clinical studies to measure the impact of dairy products on inflammation in humans.
Figure 1. Flow diagram of the five phases conducted to establish an IS for the 78 study results extracted from the 52 human studies in which the impact of dairy products on inflammation was investigated.
Phase 1. For phase 1, all studies identified by the search strategy were randomly split into six groups. Each group of studies was distributed to reviewers of one partner institution. Based on title and abstract, only studies that were clearly associated with inflammatory mediators and with the ingestion of dairy products (i.e., milk, cheese, yoghurt, fermented milk, whey products, and other dairy foods) by humans, were kept for phase 2 of the review process. Studies investigating human milk and/or breastfeeding, were excluded. Studies in which dairy products were used as a vector to deliver ingredients such as probiotics, prebiotics or bioactive nutrients such as vitamins or peptides, were excluded. However, studies were included if non-supplemented dairy products were used as control products and if information was available on the impact of these control products on inflammatory markers compared to the baseline values (e.g., comparison before and after treatment). Studies investigating isolated dairy proteins or lipids, were excluded. The information derived from the abstracts and the titles was summarized in tabulated form (see section “Tabulated summary” below) and used for selecting the studies to be evaluated in phase 2 of the review.
Phase 2. The studies retained, based on their abstracts, were again randomly split into six groups and each group of studies was distributed to reviewers of one partner institution. The tabulated summary was completed, based on the content of the articles. A workshop took place in Lisbon on June 4–6, 2013 during which the reviewers presented an overview of their evaluation of the studies. Based on these presentations the content and form of the tabulated summary were refined.
Phase 3. The study results were grouped into five subject categories (see section “Tabulated summary” below) and each group of studies was accordingly redistributed to the reviewers of one partner institution. The studies were re-evaluated to finalize the content of the tabulated summary. Finally, a non-systematic search of the literature was conducted by the reviewers, for each of the five subject categories, to identify human studies that may not have been identified by the previous searches. The form of the complementary search strategy was left to the discretion of the reviewing authors and no additional studies were identified.
Phase 4. The tabulated summary of all studies was finally revised by two reviewers from one institution, in order to harmonize its content. In particular, the status of each column in the tabulated summary was changed from the description of one clinical study per column to the description of one study result per column. This adaptation was motivated by the fact that several studies reported results for more than one dairy product or more than one subject category, each of these study results needing a separate evaluation.
Phase 5. A quantitative estimation of the ability of dairy products to modulate inflammation was conducted, for each study result, based on the content of the tabulated summary and on the establishment of the inflammatory score (IS) (see the next two sections).
Tabulated summary
The tabulated summary was not only defined in broad compliance with the reporting of systematic reviews according to the PRISMA checklist (Moher et al., Citation2009), but also integrated elements requested by regulatory authorities for the preparation of applications on health claims (EFSA Panel on Dietetic Products Nutrition and Allergies, Citation2011; FDA Office of Nutrition Labeling and Dietary Supplements, Citation2009). The tabulated summary contains the following descriptors:
Reference—Presents the bibliographic reference of the clinical trial from which each study result was extracted. Studies for which more than one study result was extracted are indicated and the study results are numbered.
Subject category—The articles are grouped into five categories based on the clinical status of the subjects enrolled in the selected studies:
HEALTH, for studies investigating healthy subjects;
MET, for studies on subjects with metabolic and cardiovascular disorders, including obesity and overweight;
GIT, for studies enrolling subjects with non-allergic gastrointestinal disorders;
HYPER, for studies with subjects suffering from food hypersensitivity, in particular allergy to dairy products, but not from lactose intolerance;
OTHERS, for studies describing subjects with all other disorders, in particular lung disease, joint disease, and infection.
Articles discussing both gastrointestinal disorders and food hypersensitivity are included in the category HYPER.
Target indication—Potential health benefit, clinical indication, or safety issue investigated in the study.
Target population—Population targeted by the target indication.
Fat content—The dairy product investigated is categorized as ‘high-fat’, ‘low-fat’, or, otherwise, “not available (n.a.)”. The classification between high-fat and low-fat dairy products was made based on the information given in the corresponding paper. When the authors did not mention the fat content of the investigated product or when they did not use special terminology such as “fat-reduced, skimmed, semi-skimmed, high-fat, normal-fat,” the study product was classified as “n.a.”.
Fermentation—The dairy product investigated is categorized as “fermented,” “non-fermented,” or, otherwise, “n.a.”.
Test and control products—Details on the foods used as test or control products (dairy or non-dairy) are reported. Only studies using dairy food products as the test or the control product are considered. For studies with more than one dairy product investigated, each dairy product is reported as a separate study result (one column for each product).
Test and control subjects—For each group enrolled in the study as test or control subjects, the number of subjects in the group, their gender (if available), age (including range) and health or disease status is provided (if appropriate). For studies with more than one group of subject investigated, each group is reported as a separate study result (one column for each group).
Diet—The composition of the dairy products investigated, its quantity, and the duration of the dairy products consumption during the study period is reported.
Controlled dairy test—Studies that are controlled and in which a dairy product is the test product are labeled as “yes,” otherwise as “no”.
Randomization—Studies that are randomized are labeled as “randomized,” otherwise either “non-randomized” or “n.a.”.
Time factor—The studies are categorized as either “longitudinal” or “cross-sectional”.
Study results—The study results are generally expressed by presenting the food products investigated, the inflammatory markers measured, and the direction of the effect. Depending on the study design, seven different types of outcome are presented:
Outcome 1 [Dairy vs Control], when dairy products are the test products and compared against control products;
Outcome 2 [Dairy (end time vs baseline)], when dairy products at baseline are compared under fasting conditions over several days (dn vs d0), weeks (wn vs w0), or months (mn vs m0);
Outcome 3 [Dairy (xh vs 0h)], when dairy products at baseline are compared over several hours in challenge postprandial studies (nh vs 0h);
Outcome 4 [Dairy (test subjects vs control subjects)], for studies in which the effects of dairy products are compared in two populations of subjects;
Outcome 5 [Dairy : Correlation], for studies in which the consumption of dairy products is quantitatively correlated to inflammatory markers. If available, adjustments for confounders are indicated;
Outcome 6 [Dietary pattern 1 vs Dietary pattern 2], for studies in which the relative impact on inflammation of different dietary patterns containing dairy products is evaluated;
Outcome 7 [Dietary patterns : Correlation], for studies in which dietary patterns containing dairy products are correlated with inflammatory markers. If available, adjustments for confounders are indicated.
The type of outcome (1–7) is indicated for each study result.
The strength of the effects was expressed by the direction of the statistically significant change in the inflammatory signal (→: no statistically significant effect; ↑: statistically significant increase; ↓: statistically significant decrease) or of the correlations (corr→: no statistically significant correlation; corr↑: statistically significant positive correlation; corr↓: statistically significant negative correlation). The criteria for statistical significance are indicated as reported in each study but are not documented in this review. To avoid bias, care was taken to document all results obtained with the inflammatory markers, including results in which no statistically significant changes were observed. Inflammatory markers are shown in italics in the table if their increase are associated with an anti-inflammatory effect.
Net change in inflammatory markers—The inflammatory markers shown in were considered for inclusion in this review. This list was extracted from recently published work that compiles a comprehensive list of inflammatory markers reported in nutritional studies (Calder et al., Citation2013). It offered clear harmonizing criteria for inclusion or exclusion of the IS that were evaluated by each reviewer. The net change in inflammatory markers was calculated for each study result by summing up the changes in all inflammatory results measured. A value of −1 was attributed for each change in inflammatory parameters contributing to a pro-inflammatory status (e.g., an increase in a pro-inflammatory parameter or a decrease in an anti-inflammatory parameter). A value of +1 was attributed for each change in inflammatory parameters contributing to an anti-inflammatory status (e.g., a decrease in a pro-inflammatory parameter or an increase in an anti-inflammatory parameter). A value of 0 was attributed for study results in which the inflammatory markers did not change. None of the 78 study results for which the net change in inflammatory markers was measured provided results in which both anti- and pro-inflammatory changes were observed together.
Table 1. List of inflammatory mediators selected for the evaluation of the articles1Footnote.
Sustainability of effect over time—This line reports whether sustainability of the inflammatory effect over time was “investigated,” “discussed,” or “not discussed”. A study result investigating and reporting a maintenance of the inflammatory effect after a washout phase of at least one week is labeled “yes”.
Dose-response—This line reports whether a dose-response relationship was investigated (yes) or not (no). If yes, a short description is presented.
Bioavailability data—Label as “yes” if information is provided on bioavailability of dairy product components, otherwise label as “no”. In cases where bioavailability data was obtained in the study (yes), a short presentation of the information is presented in the table.
Biological plausibility—This line presents whether the mechanism of action by which the dairy constituents exert their anti- or pro-inflammatory effects was discussed or investigated. The mechanism of action is shortly presented.
Bioactive components—If discussed or investigated, the components of the dairy products considered as responsible for the anti- or pro-inflammatory effect are shortly presented.
Clinical evidence—If available, this line presents the results of clinical endpoints that, if changed, contribute to an upgrading of the overall effect. The list of clinical endpoints includes: non-systemic inflammatory markers (such as, cellular, organ inflammation, joint pain, flare), parameters formally recognized as being associated with the metabolic syndrome including changes in triglycerides, HDL cholesterol, blood pressure, plasma glucose, insulin tolerance, BMI, waist circumference, glucose tolerance, insulin resistance, waist:hip ratio, urinary albumin excretion, albumin:creatinine ratio, markers of oxidative stress known to promote inflammation and other clinical endpoints such as mortality or cardiovascular events.
Financing of research—This line mentions how the study was supported financially and is labeled as either “public”, “private”, “private and public”, or “not presented”.
Grading criteria—This line presents the grading criteria used to calculate the IS according to . The label “None” is attributed a value of 0, indicating a study result in which no net change in inflammatory markers was measured. The label “Anti” is attributed a value of +1, indicating a study result with a positive net change in inflammatory markers. The label “Pro” is attributed a value of −1, indicating a study result with a negative net change in inflammatory markers. For study results with a net change in inflammatory markers different from zero, the labels “Anti” and “Pro” are completed with the numbers 1–11 indicating which one of the quality criteria presented in were met. These criteria could be retrieved from the following descriptors in the tabulated summary: (1) “controlled dairy test”, (2) “randomization”, (3) “time factor”, (4) “test product” or “control product”, (5) “study results” and “net change in inflammatory marker”, (6–7) “study results”, (8) “sustainability of effect over time”, (9) “dose-response”, (10) “biological plausibility” or “bioactive components”, (11) “clinical evidence”.
Table 2. Criteria used to establish the IS to quantitatively evaluate the impact of dairy products on inflammatory processes in humans.
IS—The IS is the sum of the criteria reported above. Study results in which all criteria are fulfilled could thus theoretically reach an IS of −12 for results indicating a pro-inflammatory activity of dairy products and an IS of +12 for results indicating an anti-inflammatory activity of dairy products. Study results with an initial IS of 0 could not be modified by these criteria and the final IS thus remained 0, independently of the quality of the clinical study.
Table S1 provides an example of the calculation of the IS for one study result.
Determination of the IS for groups of study results
A median IS was calculated for the entire data set as well as for the following categories of study results:
Subjects category (HEALTH, MET, GIT, HYPER);
Fat content of dairy product (low-fat, high-fat);
Fermentation status of dairy product (non-fermented, fermented).
Non-parametric statistics were conducted to analyze the data (significance level: p<0.05). The two-sided Wilcoxon Signed-Rank test was conducted to identify whether the median IS of the selected categories were statistically different from zero (H0: median IS=0; Ha: median IS≠0). A mean IS>0 indicated an anti-inflammatory effect whereas a pro-inflammatory effect was indicated by a mean IS<0. The Kruskall-Wallis test was conducted to identify difference in the mean IS between different categories of study results.
Results
show the tabulated summary of the 78 study results extracted from the 52 human studies retained for this review. Each table contains 25 descriptors covering a wide range of study characteristics including, amongst others, a description of the enrolled subjects, the test and control products, the study designs, and the IS (documented in the last line). shows the data for study results with a positive IS, i.e., for results indicative of an anti-inflammatory effect of dairy products. shows the data for study results with a negative IS, i.e., for results indicative of a pro-inflammatory effect of dairy products. Finally, shows the data for study results with an IS=0, i.e., for results with no modulation of inflammatory processes by dairy products.
Table 3. Tabulated summary of the study results with evidence for an anti-inflammatory effect of dairy products.
Table 4. Tabulated summary of the study results with evidence for a pro-inflammatory effect of dairy products.
Table 5. Tabulated summary of the study results with no evidence for an inflammatory modulation of dairy products.
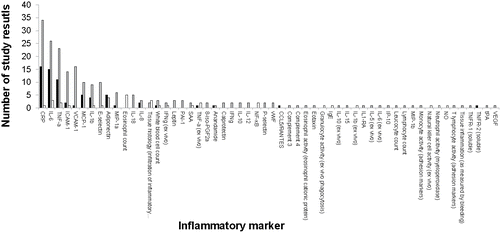
shows the overall distribution of the data obtained for each of the inflammatory markers listed in , that were measured at least once in the set of 78 study results reviewed. Out of the 98 inflammatory markers listed in , 57 markers were investigated at least once (58%). A total of 309 observations were reported with these inflammatory markers, 131 (42%) being accounted for by three cytokines, i.e., CRP (51 observations), IL-6 (44 observations), and TNF-α (36 observations). For each of these cytokines, the number of observations reporting no effect was the highest (CRP: 34 out of 51; IL-6: 26 out of 44; TNF-α: 23 out of 36) followed by the observations reporting an anti-inflammatory effect (CRP: 16 out of 51; IL-6: 15 out of 44; TNF-α: 11 out of 36). The number of these observations reporting a pro-inflammatory effect was the lowest for all three cytokines (CRP: 1 out of 51; IL-6: 3 out of 44; TNF-α: 2 out of 36). The only parameter systematically pointing to the pro-inflammatory state was ‘eosinophil count’ (5 out of 5), a parameter that was exclusively measured in studies investigating subjects with milk allergy and thus categorized in the subject category HYPER.
Figure 2. Distribution of the inflammatory markers measured in the 52 human studies. The x-axis presents the inflammatory markers. The y-axis presents the number of study results reporting a specific analytical result with the corresponding inflammatory marker. The color code indicates the direction of change of the inflammatory marker: significant anti-inflammatory change (black bars), no significant change (grey bar), significant pro-inflammatory change (white bars). The inflammatory markers are ranked in descending order with regard to their frequency of reporting in all 52 studies reviewed.
Taking into account the quality of all studies reviewed in the present article, we have developed a quantitative method that calculates an IS based on the range of eleven criteria listed in . presents the results of this analysis. Panel A first illustrates the number of study results identified with evidence for an anti-inflammatory activity (32 study results), a pro-inflammatory activity (19 results), or no change in inflammatory activity (27 study results). Panel B shows a distribution of the IS calculated for each of these study results, according to the criteria presented in . Although both panels in illustrate that the study results are well distributed among all three categories (anti-inflammatory, no effect, pro-inflammatory), the data indicating an anti-inflammatory activity appear to prevail over data pointing to a pro-inflammatory activity. This observation was confirmed by the positive mean IS for the set of 78 study results and the rejection of the null hypothesis for the median IS in the two-sided Wilcoxon Signed-Rank test, indicating an anti-inflammatory activity of dairy products ().
Figure 3. Distribution of the study results labeled as “anti-inflammatory”, “no effect”, and “pro-inflammatory” for the entire data set composed of 78 study results. (A) Number of study results labeled as “anti-inflammatory”, “no effect”, “pro-inflammatory” based on the initial grading defined in . (B) Distribution of the Inflammatory Score. The color code indicates the direction of change of the inflammatory marker, i.e., significant anti-inflammatory change (black bars), no significant change (grey bars), and significant pro-inflammatory change (white bars).
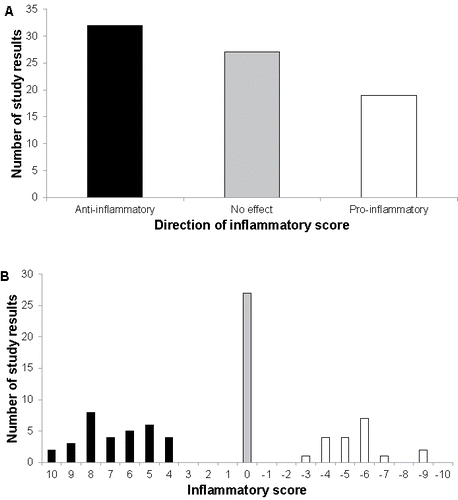
Table 6. Inflammatory Score for the impact of dairy products on humans.
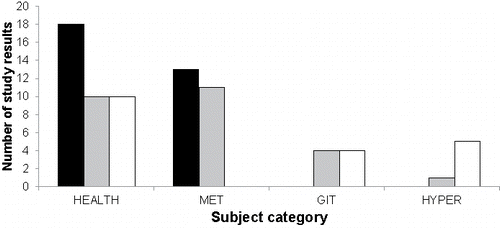
When the results were stratified according to subject categories, differences in the distribution of the study results appeared between these categories (). The group of 37 study results investigating healthy subjects, was characterized by study results covering each of the three possible effects (anti-inflammatory, no effect, pro-inflammatory). On the other hand, the group of 24 study results investigating subjects with metabolic disorders, including healthy obese subjects, was characterized by a lack of data pointing to a pro-inflammatory effect. The groups of study results investigating subjects with gastrointestinal disorders (8 study results) and of subjects with allergy to dairy products (6 study results) lacked study results indicative of an anti-inflammatory effect.
Figure 4. Distribution of the study results labeled as “anti-inflammatory”, “no effect”, and “pro-inflammatory” among the subject categories. Subject categories: HEALTH, healthy subjects; MET, subject with metabolic disorders including obesity; GIT, subjects with gastrointestinal disorders; HYPER, subjects with hypersensitivity, including allergy, to milk products. The color code indicates the direction of change of the inflammatory marker, i.e., significant anti-inflammatory change (black bars), no significant change (grey bars), and significant pro-inflammatory change (white bars).
These observations were statistically confirmed by comparing the distribution of the IS for the groups of study results investigating healthy subjects and subjects with metabolic disorders (). Both mean IS were positive and the null hypothesis for the median IS in the two-sided Wilcoxon Signed-Rank test was rejected, pointing to an anti-inflammatory activity of dairy products in these two subject categories. The mean IS of the MET subject category were higher than for the HEALTH subject category, but the Kruskal-Wallis test did not point to a statistically significant difference in the median IS between both subject categories. The mean IS for the GIT subject category was negative, but the Wilcoxon Signed-Rank test on the median IS did not point to a statistically significant effect. However, the mean IS for the HYPER subject category was negative and the null hypothesis for the median IS in the two-sided Wilcoxon Signed-Rank test was rejected, indicating a pro-inflammatory effect of dairy products in subjects allergic to dairy products. Finally, a group of studies in which the subjects could not be attributed to any of the above categories, had a median IS that was statistically not different from zero.
In order to investigate the impact of dairy product processing, in particular fat processing and fermentation on the IS, the study results were stratified according to the fat content and fermentation status of the dairy products investigated.
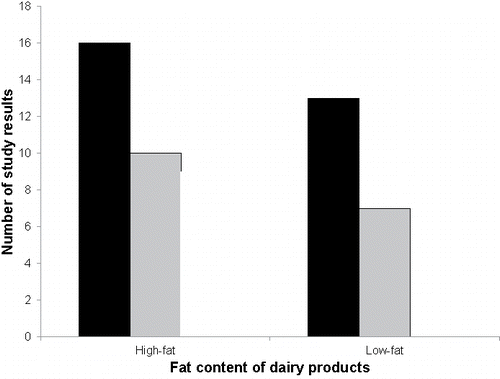
Thirty-five study results with high-fat dairy products and 20 study results with low-fat products were reported (). In contrast to the high-fat products, none of the study results with low-fat products indicated a pro-inflammatory activity. The mean IS of the low-fat product category was, indeed, lower than for the high-fat product category but the Kruskal-Wallis test on the median IS did not demonstrate this difference to reach statistical significance (p=0.083). However, the mean IS of each product category was positive and the null hypothesis for the median IS in the two-sided Wilcoxon Signed-Rank test was rejected, indicating an anti-inflammatory activity for both low-fat and high-fat dairy products ().
Figure 5. Distribution of the study results labeled as “anti-inflammatory”, “no effect”, and “pro-inflammatory” among the dairy product categories “high-fat” and “low-fat”. The color code indicates the direction of change of the inflammatory marker, i.e., significant anti-inflammatory change (black bars), no significant change (grey bars), and significant pro-inflammatory change (white bars).
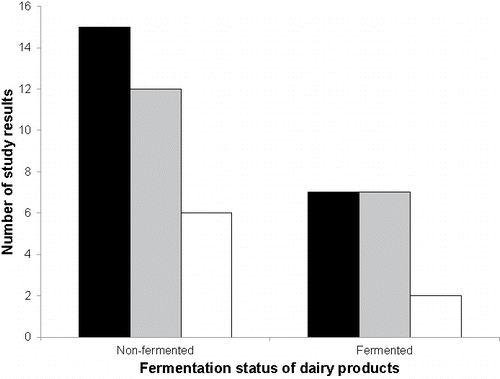
Thirty-three study results could be identified in which non-fermented dairy products were investigated, whereas 16 study results were reported with fermented products (). The mean IS of both the non-fermented and fermented product category were positive, but the two-sided Wilcoxon Signed-Rank test on the median IS only indicated a significant anti-inflammatory activity for the fermented product category ().
Figure 6. Distribution of the study results labeled as “anti-inflammatory”, “no effect”, and “pro-inflammatory” among the dairy product categories “fermented” and “non-fermented”. The color code indicates the direction of change of the inflammatory marker, i.e., significant anti-inflammatory change (black bars), no significant change (grey bars), and significant pro-inflammatory change (white bars).
In an attempt to identify the bioactive nutrients potentially modulating inflammation, and to complement the human data with preclinical data, we conducted a non-systematic and non-quantitative evaluation of the literature available on the inflammatory properties of dairy products in animal models (unpublished data). Most of these studies reported an anti-inflammatory effect; however, due to the different animal models and protocols used in the selected articles, it was not possible to compare results and to perform an analysis as we did for human studies. It was anyway clear that the importance of identifying the molecule(s) responsible for the effect, and its mechanism of action, is poorly considered in animal studies, too.
Discussion
Pro- and ant-inflammatory properties of dairy products
Overall, the IS of the entire data set composed of 78 study results, extracted from 52 human studies indicates that the consumption of dairy products is associated with anti-inflammatory properties in humans. We qualify this association as weak, although significant, because the IS has a low magnitude that is indicative of a low level of confidence in the effect estimate.
By stratifying the study results according to the health status of the enrolled subjects, we identified a pro-inflammatory activity of dairy products in subjects with milk allergy. This result is mechanistically expected, as hypersensitive reactions can obviously be linked to the pro-inflammatory state (Savilahti and Westerholm-Ormio, Citation2004). We therefore conclude that the IS is an adequate tool to evaluate the impact of food and dietary patterns on inflammation.
A systematic review recently assessed eight randomized controlled nutritional intervention studies, which have investigated the impact of dairy product consumption on biomarkers of inflammation in overweight and obese adults (Labonte et al., Citation2013). The authors concluded that the consumption of dairy products did not exert adverse effects on biomarkers of inflammation in these subjects, and that limitations among these studies did not allow for the differentiation between a beneficial or neutral impact of dairy products on inflammation. In our review, stratifying the data according to the health status of the subjects, allowed us to identify 24 study results in the MET subject category. The IS of this data set indicates an anti-inflammatory property of dairy products in subjects with metabolic disorders. Noteworthy, the significantly positive IS was also indicative of an anti-inflammatory effect of dairy products in the HEALTH group. We found, however, a trend towards a higher IS in the MET group, compared to the HEALTH group suggesting a stronger evidence for an anti-inflammatory activity of dairy products in the former subject category. This finding is illustrated by the identification of ten studies reporting a pro-inflammatory activity of dairy products in the HEALTH group, whereas the MET group is the only category in which none of the studies reported a pro-inflammatory activity of dairy products. The specific reactivity of the MET group may be linked mechanistically to the inflammatory nature of obesity. Obesity is associated with a low-grade systemic chronic inflammatory state, characterized by the abnormal production of inflammatory cytokines (Guri and Bassaganya-Riera, Citation2011;Schwander et al., Citation2014). As low-grade systemic inflammation links obesity to metabolic pathologies, including insulin resistance, cardiovascular diseases, or type-2 diabetes, targeting obesity-related inflammatory components may be a useful preventive strategy. Low-grade chronic inflammation is modulated by nutrients such as fatty acids, glucose, bioactive plant compounds, vitamins and minerals, which either enhance or alleviate the inflammatory state (Hirai et al., Citation2010). In this context, as obese subjects are characterized by low-grade systemic inflammation, the MET group may be more prone to the anti-inflammatory action of dairy products than metabolically healthy subjects.
Stratifying the data according to categories of dairy products, revealed an anti-inflammatory activity for both low-fat and high-fat dairy products. The IS indicated an anti-inflammatory activity of high-fat dairy products despite the fact that nine studies were identified in which these products were associated with a pro-inflammatory activity. The pro-inflammatory activity identified with high-fat dairy products in these studies was mainly attributed to the presence of saturated fat. Fat consumption, in particular saturated fat (Steinberg, Citation2005) and trans-fatty acids (Micha and Mozaffarian, Citation2009), has been associated with inflammatory processes in humans. However, recent opinions in nutrition research advocate that the adverse health effects formerly associated with saturated fats, were most likely due to other factors (Lawrence, Citation2013). The positive IS, calculated for the high-fat products, is thus in line with this reevaluation of the impact of fat consumption on human health. Additionally, as both low-fat and high-fat products were associated with a positive IS, the molecules with a potential anti-inflammatory activity in milk may cover a broad range of nutrients, including polyunsaturated fatty acids (German and Dillard, Citation2006), proteins (Chatterton et al., Citation2013), and glycans (Newburg, Citation2013).
The IS of the product category “fermented dairy products” indicates a beneficial anti-inflammatory contribution, possibly resulting from the bacteria present in dairy products or their metabolic activity. The anti-inflammatory activity of strains of lactic acid bacteria and bifidobacteria has indeed been reported (Lomax & Calder, Citation2009;Tsai et al., Citation2012). The recent awareness of the role of the gut microbiota in the modulation of the immune system (Hakansson and Molin, Citation2011), further raises interest in the integration of bacteria with anti-inflammatory properties into dairy products (Dunne et al., Citation2001). Moreover, products deriving from the fermentation of milk with bacteria, in particular bioactive peptides (Ceapa et al., Citation2013) and glycans (Newburg, Citation2013), which both interact with gut microbes or immune cells, may contribute to an anti-inflammatory activity of dairy products.
Research gaps
Our review also aimed at identifying research gaps preventing a comprehensive understanding of inflammatory processes in food and nutrition sciences. In particular, we have identified the following gaps:
No consensus is available yet which clearly defines clinically relevant inflammatory markers. For illustration in Europe, the EFSA was required, following a consultation of stakeholders, to give guidance on potential markers of inflammation. In its response, the EFSA stated that “for function claims referring to reduction of inflammation, a change in markers of inflammation such as various interleukins does not indicate a beneficial physiological effect per se, but should be accompanied by a beneficial physiological or clinical outcome” (EFSA Panel on Dietetic Products, Citation2011). This position is an important challenge to the food and nutrition research community, given the difficulties associated with the identification of validated clinical markers of disease reduction by dietary interventions. In that context, the importance of validating sets of molecules present in the circulation as biomarkers of low-grade inflammation has been emphasized (Calder et al., Citation2013). At the same time, the predictive value tentatively attributed by the authors of this review to these sets of inflammatory markers, illustrates the gap with the position of regulatory authorities. The present review further highlights this gap: human studies complementing the inflammatory markers with convincingly addressing clinical outcomes, as described by the descriptor “Clinical evidence” in , are unsurprisingly scarce.
Validation issues are raised by new analytical technologies that now allow researchers to quantitate large sets of inflammatory markers in a single measurement (Liu et al., Citation2005; Breen et al., Citation2011; Thompson et al., Citation2012). Although these analytical issues were not discussed in the set of human trials reviewed, particular care should be taken in the future to better characterize the performance of these tests.
Regulatory authorities clearly highlight the importance of characterizing the food products investigated in human trials in their guidance for the authorization of health claims (EFSA Panel on Dietetic Products Nutrition and Allergies, Citation2011; FDA Office of Nutrition Labeling and Dietary Supplements, Citation2009). However, the studies reported in this review give little emphasis on the characterization of the dairy products investigated, as illustrated by a range of uncharacterized descriptors in (e.g., identification of bioactive nutrients, bioavailability data, dose-response effects, sustainability of the effect of the food product over time). In particular, integrating the variable ‘dose’ into study designs could allow researchers to draw a causal relationship between the food investigated and the physiological response measured in humans (Schwander et al., Citation2014). Also, although dozens of nutrients with immunomodulatory activity have been proposed in the literature (Ballard and Morrow, Citation2013), the bioactive nutrients potentially modulating inflammation in the reviewed studies, remain largely unknown even considering animal studies. The major reason for this gap is clearly inherent to the complex molecular composition of food. In light of the importance of the food matrix on the properties of bioactive nutrients, we endorse that food and nutrition research should shift its focus from the characterization of the nutritional and immunomodulatory properties of isolated nutrients to the characterization of foods, meals, and even dietary patterns.
The scientific basis for claims on bioactive food and nutrients established by national regulatory authorities is not harmonized, thereby hindering internationally harmonized market access (Aggett et al., 2012). To date, a very high number of requested health claims (more than 80%) have been rejected by the EFSA's NDA Panel, who underlined the need to identify the molecule(s) responsible of the claimed effect, and their mechanisms of action. The mechanisms of action of bioactives are usually studied in vitro, whereas in vivo studies are very often focused on demonstrating an effect on specific endpoints, without considering the underlying mechanisms. Evidence of the anti-inflammatory effectiveness of dairy components could be retrieved from in vitro studies, but they were not considered in this review for a specific reason, i.e., bioactive components are just one part of food, embedded in a very complex matrix. Cell supplementation in in vitro studies, as well as intervention studies administering bioactives as pure compounds assume that there are no confounding effects related to the food matrix. The food matrix, as well as food processing (Bordoni et al., Citation2011) can, indeed modify the digestibility and bioavailability of bioactive compounds, thus introducing a fundamental bias when translating in vitro data to humans. The ideal in vitro study should thus digest food in a static or dynamic model of digestion, have the digested nutrients transported through an intestinal cellular layer mimicking the gastrointestinal barrier, ideally with a model integrating the gut microbiota, and finally measure the ability of the absorbed nutrients to modulate inflammation. Such integrated in vitro models have not yet been successfully developed, although first steps in that direction have already been taken (Vergeres et al., Citation2012). Meanwhile, the COST action FA1005 ‘Improving health properties of food by sharing our knowledge on the digestive process’ (INFOGEST) has published an harmonized protocol of in vitro digestion (Minekus et al., Citation2014). To perform in vitro digestion prior to in vitro studies will help to bypass the enormous, and unscientific, gap in our knowledge related to the assumption, without any demonstration, that the in vivo effects of foods are related to the mechanisms of action observed in vitro supplementing cells with pure molecules. In vitro studies supplementing cells with digested food can mimic in a closer way the in vivo effects and underlying mechanism of actions of food bioactives, thus evidencing the cause-effect relationship as requested by the body authorities.
Strengths and limitations of the IS
The literature focusing on the impact of dairy products on inflammatory processes in humans revealed a very heterogeneous methodological landscape. The IS was therefore defined in order to take these limitations into account as follows:
Inflammation is a complex phenomenon that cannot be described by a single biomarker (Calder et al., Citation2013). Indeed, more than fifty inflammatory markers were reported in the pool of the 52 human studies reviewed. The data consisted of cellular markers of inflammation and measures of tissue infiltration, but the majority of studies concentrated on a few soluble circulatory cytokines. Furthermore, the number of markers measured in each study varied from one to more than ten. These points all raised the issue of the weighting of each study result in this heterogeneous environment. For the sake of simplicity, and to avoid over-interpreting the data, we decided to (i) rate each of the inflammatory markers listed in at the same level and (ii) to increase the IS by one unit in cases in which changes in the concentration of more than one inflammatory markers were pointing in the same direction (see point 5 in ). Note, however, that the IS was not upgraded by additional grades for studies in which more than two inflammatory markers were concordantly changed as this would have given too much weight to this criterion compared to the ten other criteria presented in .
As milk is amenable to a wide range of technological transformations and important in human diets, a large spectrum of dairy products was investigated in the 52 reviewed studies. As each of these products may differently modulate inflammation, we addressed this issue by defining a limited range of product categories in which the data could be stratified and analyzed (low-fat vs. high fat; fermented vs. non-fermented).
The health status of the subjects enrolled in the 52 studies was quite diverse, reflecting the generic importance of inflammatory processes in modulating human health and disease. The clinical indications targeted by these studies were consequently heterogeneous and we therefore classified the study results according to a limited, but clinically meaningful, set of subject categories (HEALTH, MET, GIT, HYPER).
Given the relative paucity of high-quality studies on the topic of dairy and inflammation, we chose an inclusive strategy which means that we considered all available publications on dairy and systemic inflammation, including randomized controlled trials, cross-over design trials and longitudinal cohort studies. This approach enabled us to analyze data from studies per se not considered in systemic reviews and we could thus provide a wide overview of studies dealing with dairy and inflammation. The downside of this strategy is that some studies of low quality, small sample size and short duration, were included in this review.
The last issue that became evident during the reviewing process, is the usage of dairy products as controls in human studies actually aiming at investigating the ability of other food products to modulate inflammatory processes. This phenomenon was particularly the case for clinical studies using the milk matrix to supplement the test meals with bioactive components. Given the potential bioactivity of dairy products, we decided to also evaluate their properties even when used as control products, although this might pose the risk of misleading information when comparing data against baseline within randomized groups (Bland and Altman, Citation2011).
Conclusions
We have established the IS as a new tool to conduct a quantitative evaluation of human studies investigating the impact of dairy products on inflammation. Taken together, our review suggests that dairy products, in particular fermented products, have anti-inflammatory properties in humans not suffering from allergy to milk, in particular in subjects with metabolic disorders. As the clinical relevance of inflammatory markers is currently debated among researchers and regulatory authorities, the translation of these findings into dietary guidelines remains to be clarified.
Table S1.docx
Download MS Word (36.7 KB)Acknowledgments
We thank Ueli Bütikofer and Diklah Geva for support on the statistical analyses. We also thank Capucine Musard for a preliminary analysis of the literature on the topic of this review.
Funding
The authors of this review are members of the FA COST Action FA1005 “Improving health properties of food by sharing our knowledge on the digestive process” (INFOGEST) that financed the travel costs for the meetings of the MindTheGap project team. This work was, furthermore financed by the institutions employing the authors of this report. The work of PP and CNS was supported by Fundação para a Ciência e Tecnologia (PEst-OE/EQB/LA0004 /2011 and IF/01097/2013).
References
- Aggett, P. J., Hathcock, J., Jukes, D., Richardson, D. P., Calder, P. C., Bischoff-Ferrari, H., Nicklas, T., Muhlebach, S., Kwon, O., Lewis, J., Lugard, M. J. and Prock, P. (2012). Nutrition issues in Codex: health claims, nutrient reference values and WTO agreements: a conference report. Eur. J. Nutr. 51(Suppl 1):S1–S7.
- Arvola, T., Ruuska, T., Keranen, J., Hyoty, H., Salminen, S. and Isolauri, E. (2006). Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics 117:e760–e768.
- Asemi, Z., Samimi, M., Tabassi, Z., Sabihi, S. S. and Esmaillzadeh, A. (2013). A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition 29:619–624.
- Augustin, M. A. and Udabage, P. (2007). Influence of processing on functionality of milk and dairy proteins. Adv. Food Nutr. Res. 53:1–38.
- Ballard, O. and Morrow, A. L. (2013). Human milk composition: nutrients and bioactive factors. Pediatr. Clin. North Am. 60:49–74.
- Beavers, K. M., Serra, M. C., Beavers, D. P., Cooke, M. B. and Willoughby, D. S. (2009). Soymilk supplementation does not alter plasma markers of inflammation and oxidative stress in postmenopausal women. Nutr Res 29:616–622.
- Bland, J. M. and Altman, D. G. (2011). Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials 12:264.
- Bordoni, A., Picone, G., Babini, E., Vignali, M., Danesi, F., Valli, V., Di, N. M., Laghi, L. and Capozzi, F. (2011). NMR comparison of in vitro digestion of Parmigiano Reggiano cheese aged 15 and 30 months. Magn. Reson. Chem. 49(Suppl 1):S61–S70.
- Breen, E. C., Reynolds, S. M., Cox, C., Jacobson, L. P., Magpantay, L., Mulder, C. B., Dibben, O., Margolick, J. B., Bream, J. H., Sambrano, E., Martinez-Maza, O., Sinclair, E., Borrow, P., Landay, A. L., Rinaldo, C. R. and Norris, P. J. (2011). Multisite comparison of high-sensitivity multiplex cytokine assays. Clin. Vaccine Immunol. 18:1229–1242.
- Calder, P. C., Ahluwalia, N., Albers, R., Bosco, N., Bourdet-Sicard, R., Haller, D., Holgate, S. T., Jonsson, L. S., Latulippe, M. E., Marcos, A., Moreines, J., M'Rini, C., Muller, M., Pawelec, G., van Neerven, R. J., Watzl, B. and Zhao, J. (2013). A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 109(Suppl 1):S1–34.
- Calder, P. C., Ahluwalia, N., Brouns, F., Buetler, T., Clement, K., Cunningham, K., Esposito, K., Jonsson, L. S., Kolb, H., Lansink, M., Marcos, A., Margioris, A., Matusheski, N., Nordmann, H., O'Brien, J., Pugliese, G., Rizkalla, S., Schalkwijk, C., Tuomilehto, J., Warnberg, J., Watzl, B. and Winklhofer-Roob, B. M. (2011). Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 106(Suppl 3):S5–78.
- Candore, G., Caruso, C., Jirillo, E., Magrone, T. and Vasto, S. (2010). Low grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievement. Curr. Pharm. Des. 16:584–596.
- Ceapa, C., Wopereis, H., Rezaiki, L., Kleerebezem, M., Knol, J. and Oozeer, R. (2013). Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health. Best Pract. Res. Clin. Gastroenterol. 27:139–155.
- Chatterton, D. E., Nguyen, D. N., Bering, S. B. and Sangild, P. T. (2013). Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 45:1730–1747.
- Dalbeth, N., Ames, R., Gamble, G. D., Horne, A., Wong, S., Kuhn-Sherlock, B., MacGibbon, A., McQueen, F. M., Reid, I. R. and Palamano, K. (2012). Effects of skim milk powder enriched with glycomacropeptide and G600 milk fat extract on frequency of gout flares: a proof-of-concept randomised controlled trial. Ann Rheum.Dis 71:929–934.
- Dawczynski, C., Massey, K. A., Ness, C., Kiehntopf, M., Stepanow, S., Platzer, M., Grun, M., Nicolaou, A. and Jahreis, G. (2013). Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clin. Nutr. 32:686–696.
- Dawczynski, C., Schubert, R., Hein, G., Muller, A., Eidner, T., Vogelsang, H., Basu, S. and Jahreis, G. (2009). Long-term moderate intervention with n-3 long-chain PUFA-supplemented dairy products: effects on pathophysiological biomarkers in patients with rheumatoid arthritis. Br. J Nutr 101:1517–1526.
- De Aguilar-Nascimento, J. E., Prado Silveira, B. R. and Dock-Nascimento, D. B. (2011). Early enteral nutrition with whey protein or casein in elderly patients with acute ischemic stroke: a double-blind randomized trial. Nutrition 27:440–444.
- Deopurkar, R., Ghanim, H., Friedman, J., Abuaysheh, S., Sia, C. L., Mohanty, P., Viswanathan, P., Chaudhuri, A. and Dandona, P. (2010). Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 33:991–997.
- Dunne, C., O'Mahony, L., Murphy, L., Thornton, G., Morrissey, D., O'Halloran, S., Feeney, M., Flynn, S., Fitzgerald, G., Daly, C., Kiely, B., O'sullivan, G. C., Shanahan, F. and Collins, J. K. (2001). In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 73:386S–392S.
- EFSA Panel on Dietetic Products Nutrition and Allergies. (2011). Scientific and technical guidance for the preparation and presentation of an application for authorisation of a health claim (revision 1). 9 edn, p. 2170.
- EFSA Panel on Dietetic Products, N. a. A. (2011). Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 9:1984.
- Esmaillzadeh, A., Kimiagar, M., Mehrabi, Y., Azadbakht, L., Hu, F. B. and Willett, W. C. (2007). Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 137:992–998.
- FAO. (2013a). Food outlook - Biannual report on global food markets.
- FAO. (2013b). Milk and dairy products in human nutrition.
- FDA Office of Nutrition Labeling and Dietary Supplements. (2009). Guidance for Industry: Evidence-Based Review System for the Scientific Evaluation of Health Claims.
- German, J. B. and Dillard, C. J. (2006). Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit. Rev. Food Sci. Nutr. 46:57–92.
- Global Business Intelligence Research. (2011). Anti-Inflammatory Therapeutics Market to 2017 - Respiratory Diseases and Arthritis Continue to Dominate.
- Gonsalves, N., Yang, G. Y., Doerfler, B., Ritz, S., Ditto, A. M. and Hirano, I. (2012). Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 142:1451–1459.
- Guri, A. J. and Bassaganya-Riera, J. (2011). Systemic effects of white adipose tissue dysregulation and obesity-related inflammation. Obesity (Silver Spring) 19:689–700.
- Hakansson, A. and Molin, G. (2011). Gut microbiota and inflammation. Nutrients 3:637–682.
- Haug, A., Hostmark, A. T. and Harstad, O. M. (2007). Bovine milk in human nutrition—a review. Lipids Health Dis. 6:25.
- Henderson, C. J., Abonia, J. P., King, E. C., Putnam, P. E., Collins, M. H., Franciosi, J. P. and Rothenberg, M. E. (2012). Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol 129:1570–1578.
- Hernandez-Aguilera, A., Rull, A., Rodriguez-Gallego, E., Riera-Borrull, M., Luciano-Mateo, F., Camps, J., Menendez, J. A. and Joven, J. (2013). Mitochondrial dysfunction: a basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators Inflamm. 2013:135698.
- Hirai, S., Takahashi, N., Goto, T., Lin, S., Uemura, T., Yu, R. and Kawada, T. (2010). Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediators Inflamm. 2010:367838.
- Hlebowicz, J., Persson, M., Gullberg, B., Sonestedt, E., Wallstrom, P., Drake, I., Nilsson, J., Hedblad, B. and Wirfalt, E. (2011). Food patterns, inflammation markers and incidence of cardiovascular disease: the Malmo Diet and Cancer study. J Intern Med 270:365–376.
- Holmer-Jensen, J., Karhu, T., Mortensen, L. S., Pedersen, S. B., Herzig, K. H. and Hermansen, K. (2011). Differential effects of dietary protein sources on postprandial low-grade inflammation after a single high fat meal in obese non-diabetic subjects. Nutr J 10:115.
- Hotamisligil, G. S. (2006). Inflammation and metabolic disorders. Nature 444:860–867.
- Hunter, D. C., Brown, R., Green, T., Thomson, C., Skeaff, M., Williams, S., Todd, J. M., Lister, C. E., McGhie, T., Zhang, J., Martin, H., Rippon, P., Stanley, R. and Skinner, M. A. (2012). Changes in markers of inflammation, antioxidant capacity and oxidative stress in smokers following consumption of milk, and milk supplemented with fruit and vegetable extracts and vitamin C. Int J Food Sci Nutr 63:90–102.
- Hunter, D. J. and Reddy, K. S. (2013). Noncommunicable diseases. N. Engl. J. Med. 369:1336–1343.
- Iacono, G., Cavataio, F., Montalto, G., Florena, A., Tumminello, M., Soresi, M., Notarbartolo, A. and Carroccio, A. (1998). Intolerance of cow's milk and chronic constipation in children. N Engl J Med 339:1100–1104.
- Iwasa, M., Aoi, W., Mune, K., Yamauchi, H., Furuta, K., Sasaki, S., Takeda, K., Harada, K., Wada, S., Nakamura, Y., Sato, K. and Higashi, A. (2013). Fermented milk improves glucose metabolism in exercise-induced muscle damage in young healthy men. Nutr. J. 12:83.
- Jimenez-Flores, R., Heick, J., Davis, S. C., Hall, K. G. and Schaffner, A. (2012). A comparison of the effects of a high carbohydrate vs. a higher protein milk supplement following simulated mountain skirmishes. Mil. Med 177:723–731.
- Jones, K. W., Eller, L. K., Parnell, J. A., Doyle-Baker, P. K., Edwards, A. L. and Reimer, R. A. (2013). Effect of a dairy- and calcium-rich diet on weight loss and appetite during energy restriction in overweight and obese adults: a randomized trial. Eur. J. Clin. Nutr. 67:371–376.
- Jyonouchi, H., Sun, S. and Itokazu, N. (2002). Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology 46:76–84.
- Kagalwalla, A. F., Shah, A., Li, B. U., Sentongo, T. A., Ritz, S., Manuel-Rubio, M., Jacques, K., Wang, D., Melin-Aldana, H. and Nelson, S. P. (2011). Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr. Gastroenterol Nutr 53:145–149.
- Kagalwalla, A. F., Amsden, K., Shah, A., Ritz, S., Manuel-Rubio, M., Dunne, K., Nelson, S. P., Wershil, B. K. and Melin-Aldana, H. (2012). Cow's milk elimination: a novel dietary approach to treat eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 55:711–716.
- Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. and Gordon, J. I. (2011). Human nutrition, the gut microbiome and the immune system. Nature 474:327–336.
- Klop, B., Proctor, S. D., Mamo, J. C., Botham, K. M. and Castro, C. M. (2012). Understanding postprandial inflammation and its relationship to lifestyle behaviour and metabolic diseases. Int. J. Vasc. Med. 2012:947417.
- Kristjansson, G., Venge, P. and Hallgren, R. (2007). Mucosal reactivity to cow's milk protein in coeliac disease. Clin Exp. Immunol 147:449–455.
- Labonte, M. E., Couture, P., Richard, C., Desroches, S. and Lamarche, B. (2014). Impact of dairy products on biomarkers of inflammation: a systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am. J. Clin. Nutr. 97:706–717.
- Lawrence, G. D. (2013). Dietary fats and health: dietary recommendations in the context of scientific evidence. Adv. Nutr. 4:294–302.
- Lee, Y. M., Skurk, T., Hennig, M. and Hauner, H. (2007). Effect of a milk drink supplemented with whey peptides on blood pressure in patients with mild hypertension. Eur J Nutr 46:21–27.
- Lepage, P. and Van de Perre, P. (2012). The immune system of breast milk: antimicrobial and anti-inflammatory properties. Adv. Exp. Med. Biol. 743:121–137.
- Liu, M. Y., Xydakis, A. M., Hoogeveen, R. C., Jones, P. H., Smith, E. O., Nelson, K. W. and Ballantyne, C. M. (2005). Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin. Chem. 51:1102–1109.
- Lomax, A. R. and Calder, P. C. (2009). Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr. Pharm. Des. 15:1428–1518.
- Melnik, B. C. (2009). Milk–the promoter of chronic Western diseases. Med. Hypotheses. 72:631–639.
- Meyer, A. L., Elmadfa, I., Herbacek, I. and Micksche, M. (2007). Probiotic, as well as conventional yogurt, can enhance the stimulated production of proinflammatory cytokines. J Hum Nutr Diet 20:590–598.
- Meyer, J., Doring, A., Herder, C., Roden, M., Koenig, W. and Thorand, B. (2011). Dietary patterns, subclinical inflammation, incident coronary heart disease and mortality in middle-aged men from the MONICA/KORA Augsburg cohort study. Eur J Clin Nutr 65:800–807.
- Micha, R. and Mozaffarian, D. (2009). Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 5:335–344.
- Minekus, M., Alminger, M., Alvito, P., Ballance, S., Bohn, T., Bourlieu, C., Carrière, F., Boutrou, R., Corredig, M., Dupont, D., Dufour, C., Egger, L., Golding, M., Karakaya, S., Birkhus, B., Le Feunteun, S., Lesmes, U., Maczierzanka, A., MacKie, A., Marze, S., McClements, D. J., Ménard, O., Recio, I., Santos, C. N., Singh, R. P., Vegarud, G. E., Wickham, M. S. J., Weitschies, W. and Brodkorb, A. (2014). A standardized static in vitro digestion method suitable for food - an international consensus. Food. Funct. Jun;5(6):1113–1124.
- Moher, D., Liberati, A., Tetzlaff, J. and Altman, D. G. (2009). Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys. Ther. 89:873–880.
- Monagas, M., Khan, N., Andres-Lacueva, C., Casas, R., Urpi-Sarda, M., Llorach, R., Lamuela-Raventos, R. M. and Estruch, R. (2009). Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr 90:1144–1150.
- Nestel, P. J., Pally, S., MaCintosh, G. L., Greeve, M. A., Middleton, S., Jowett, J. and Meikle, P. J. (2012). Circulating inflammatory and atherogenic biomarkers are not increased following single meals of dairy foods. Eur J Clin Nutr 66:25–31.
- Nettleton, J. A., Steffen, L. M., Mayer-Davis, E. J., Jenny, N. S., Jiang, R., Herrington, D. M. and Jacobs, JR D. R. (2006). Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 83:1369–1379.
- Newburg, D. S. (2013). Glycobiology of human milk. Biochemistry (Mosc) 78:771–785.
- Oftedal, O. T. (2012). The evolution of milk secretion and its ancient origins. Animal 6:355–368.
- Pal, S. and Ellis, V. (2011). Acute effects of whey protein isolate on blood pressure, vascular function and inflammatory markers in overweight postmenopausal women. Br.J Nutr 105:1512–1519.
- Panagiotakos, D. B., Pitsavos, C. H., Zampelas, A. D., Chrysohoou, C. A. and Stefanadis, C. I. (2010). Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: the ATTICA study. J Am Coll. Nutr 29:357–364.
- Panagiotakos, D. B., Pitsavos, C. H., Zampelas, A. D., Chrysohoou, C. A. and Stefanadis, C. I. (2010). Dairy products consumption is associated with decreased levels of inflammatory markers related to cardiovascular disease in apparently healthy adults: the ATTICA study. J. Am. Coll. Nutr. 29:357–364.
- Pintus, S., Murru, E., Carta, G., Cordeddu, L., Batetta, B., Accossu, S., Pistis, D., Uda, S., Elena, G. M., Mele, M., Secchiari, P., Almerighi, G., Pintus, P. and Banni, S. (2013). Sheep cheese naturally enriched in alpha-linolenic, conjugated linoleic and vaccenic acids improves the lipid profile and reduces anandamide in the plasma of hypercholesterolaemic subjects. Br. J. Nutr. 109:1453–1462.
- Raff, M., Tholstrup, T., Basu, S., Nonboe, P., Sorensen, M. T. and Straarup, E. M. (2008). A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory, or diabetic risk markers in healthy young men. J Nutr 138:509–514.
- Rebholz, C. M., Reynolds, K., Wofford, M. R., Chen, J., Kelly, T. N., Mei, H., Whelton, P. K. and He, J. (2013). Effect of soybean protein on novel cardiovascular disease risk factors: A randomized controlled trial. Eur. J. Clin. Nutr. 67:58–63.
- Romeo, J., Warnberg, J., Garcia-Marmol, E., Rodriguez-Rodriguez, M., Diaz, L. E., Gomez-Martinez, S., Cueto, B., Lopez-Huertaz, E., Cepero, M., Boza, J. J., Fonolla, J. and Marcos, A. (2011). Daily consumption of milk enriched with fish oil, oleic acid, minerals and vitamins reduces cell adhesion molecules in healthy children. Nutr Metab Cardiovasc. Dis 21:113–120.
- Rosti, L., Braga, M., Fulcieri, C., Sammarco, G., Manenti, B. and Costa, E. (2011). Formula milk feeding does not increase the release of the inflammatory marker calprotectin, compared to human milk. Pediatr.Med Chir 33:178–181.
- Savilahti, E. and Westerholm-Ormio, M. (2004). Gut inflammation and extraintestinal manifestation of food allergy. J. Pediatr. Gastroenterol. Nutr. 39(Suppl 3):S742–S743.
- Schwander, F., Kopf-Bolanz, K. A., Buri, C., Portmann, R., Egger, L., Chollet, M., mcTernan, P. G., Piya, M. K., Gijs, M. A. M., Vionnet, N., Pralong, F., Laederach, K. and Vergères, G. (2014). A dose-response strategy reveals differences between normal weight and obese men in their metabolic and inflammatory responses to a high-fat meal. J. Nutr. Oct;144(10):1517–1523.
- Sofi, F., Buccioni, A., Cesari, F., Gori, A. M., Minieri, S., Mannini, L., Casini, A., Gensini, G. F., Aabbate, R. and Anatongiovanni, M. (2010). Effects of a dairy product (pecorino cheese) naturally rich in cis-9, trans-11 conjugated linoleic acid on lipid, inflammatory and haemorheological variables: a dietary intervention study. Nutr Metab Cardiovasc. Dis 20:117–124.
- Spegel, J. M., Andrews, T., Brown-Whitehorn, T. F., Beausoleil, J. L. and Liacouras, C. A. (2005). Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol 95:336–343.
- Stancliffe, R. A., Thorpe, T. and Zemel, M. B. (2011). Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J Clin Nutr 94:422–430.
- Steinberg, D. (2005). Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II: the early evidence linking hypercholesterolemia to coronary disease in humans. J. Lipid. Res. 46:179–190.
- Strisciuglio, C., Giannetti, E., Martinelli, M., Sciorio, E., Staiano, A. and Miele, E. (2013). Does cow's milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr. 102:e273–e278.
- Sugawara, K., Takahashi, H., Kashiwagura, T., Yamada, K., Yanagida, S., Homma, M., Dairiki, K., Sasaki, H., Kawagoshi, A., Satake, M. and Shioya, T. (2012). Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir.Med 106:1526–1534.
- Thompson, D. K., Huffman, K. M., Kraus, W. E. and Kraus, V. B. (2012). Critical appraisal of four IL-6 immunoassays. PLoS One 7:e30659.
- Topuz, E., Derin, D., Can, G., Kurklu, E., Cinar, S., Aykan, F., Cevikbas, A., Disci, R., Durna, Z., Sakar, B., Saglam, S., Tanyeri, H., Deniz, G., Gurer, U., Tas, F., Guney, N. and Aydiner, A. (2008). Effect of oral administration of kefir on serum proinflammatory cytokines on 5-FU induced oral mucositis in patients with colorectal cancer. Invest New Drugs 26:567–572.
- Tsai, Y. T., Cheng, P. C. and Pan, T. M. (2012). The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl. Microbiol. Biotechnol. 96:853–862.
- Ulsemer, P., Toutounian, K., Kressel, G., Schmidt, J., Karesten, U., Hahn, A. and Goletz, S. (2012). Safety and tolerance of Bacteroides xylanisolvens DSM 23964 in healthy adults. Benef Microbes 3:99–111.
- Unknown. (1994). Dietary and other risk factors of ulcerative colitis. A case-control study in Japan. Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Clin Gastroenterol 19:166–171.
- Van Bussel, B. C., Henry, R. M., Schalkwijk, C. G., Ferreira, I., Feskens, E. J., Streppel, M. T., Smulders, Y. M., Twisk, J. W. and Stehouwer, C. D. (2011). Fish consumption in healthy adults is associated with decreased circulating biomarkers of endothelial dysfunction and inflammation during a 6-year follow-up. J Nutr 141:1719–1725.
- Van Meijl, L. E. and Mensink, R. P. (2010). Effects of low-fat dairy consumption on markers of low-grade systemic inflammation and endothelial function in overweight and obese subjects: an intervention study. Br.J Nutr 104:1523–1527.
- Vazquez-Agell, M., Urpi-Sarda, M., Sacanella, E., Camino-Lopez, S., Chiva-Blanch, G., Llorente-Cortes, V., Tobias, E., Roura, E., Andres-Lacueva, C., Lamuela-Raventos, R. M., Badimon, L. and Estruch, R. (2013). Cocoa consumption reduces NF-kappaB activation in peripheral blood mononuclear cells in humans. Nutr.Metab Cardiovasc.Dis. 23:257–263.
- Vergeres, G., Bogicevic, B., Buri, C., Carrara, S., Chollet, M., Corbino-Giunta, L., Egger, L., Gille, D., Kopf-Bolanz, K., Laederach, K., Portmann, R., Ramadan, Q., Ramsden, J., Schwander, F., Silacci, P., Walther, B. and Gijs, M. (2012). The NutriChip project–translating technology into nutritional knowledge. Br. J. Nutr. 108:762–768.
- Wang, H., Steffen, L. M., Vessby, B., Basu, S., Steinberger, J., Moran, A., Jacobs, D. R., JR., Hong, C. P. and Sinaiko, A. R. (2011). Obesity modifies the relations between serum markers of dairy fats and inflammation and oxidative stress among adolescents. Obesity (Silver Spring) 19:2404–2410.
- Wennersberg, M. H., Smedmann, A., Turpeinen, A. M., Retterstol, K., Tengblad, S., Lipre, E., Aro, A., Mutanen, P., Seljeflot, I., Basu, S., Pedersen, J. I., Mutanen, M. and Vessby, B. (2009). Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study. Am J Clin Nutr 90:960–968.
- Wojcik, J. R., Walber-Rankin, J., Smith, L. L. and Gwazdauskas, F. C. (2001). Comparison of carbohydrate and milk-based beverages on muscle damage and glycogen following exercise. Int J Sport Nutr Exerc. Metab 11:406–419.
- Zemel, M. B. and Sun, X. (2008). Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr 138:1047–1052.
- Zemel, M. B., Sun, X., Sobhani, T. and Wilson, B. (2010). Effects of dairy compared with soy on oxidative and inflammatory stress in overweight and obese subjects. Am J Clin Nutr 91:16–22.
