ABSTRACT
Objective: The aim of this study was to assess the mean of histamine concentration in food poisoning.
Design: Systematic review and meta-analysis of reports published between 1959 and 2013.
Study selection: Main criteria for inclusion of studies were: all report types that present outbreaks of “histamine poisoning' or “scombroid syndrome” from food, including histamine content and type of food. Health status of people involved must be nonpathological.
Results: Fifty-five (55) reports were included, these studies reported 103 incidents. All pooled analyses were based on random effect model; histamine mean concentration in poisoning samples was 1107.21 mg/kg with confidence interval for the meta-mean of 422.62–2900.78 mg/kg; heterogeneity index (I2) was 100% (P < 0.0001); prediction interval was 24.12–50822.78 mg/kg. Fish involved in histamine poisoning was mainly tuna or Istiophoridae species. No clues of association between concomitant conditions (female sex, alcohol consumption, previous medication, and consumption of histamine releasing food) and histamine poisoning, were highlighted.
Conclusions: This is the first systematic review and meta-analysis that analyzes all the available data on histamine poisoning outbreaks evaluating the histamine concentration in food involved. Histamine mean concentration in poisoning samples was fairly high. Our study suffers from some limitations, which are intrinsic of the studies included, for instance the lack of a complete anamnesis of each poisoning episode.
Protocol registration: Methods were specified in advance and have been published as a protocol in PROSPERO database (18/07/2012 -CRD42012002566).
Introduction
Scombroid syndrome/histamine poisoning occurs worldwide and it is considered one of—if not—the most common form of toxicity caused by fish consumption (Dalgaard, Emborg, et al., Citation2008). The number of cases is increasing, in spite of the improved knowledge on seafood safety; this is due to a change in the way in which seafood, and mainly tuna, is eaten, that is, as steaks or hamburger (Becker, Southwick, et al., Citation2001), or as canned tuna recipes (sandwiches, salads, pizza) (Cattaneo, Stella, Citation2001; Mclauchlin, Little, et al., Citation2006). Less is known about foods other than seafood and it is of utmost importance to assess the impact of all food types on this syndrome to implement specific prevention measures.
Periodically reviews on this item have been published (Lehane, Olley, Citation2000; Hungerford, Citation2010), although containing a lot of data they are not systematic reviews. Systematic review has not yet been performed on histamine poisoning. To assess histamine level of food associated with histamine poisonings, in the light of objective criteria, could lead to reliable information useful to control this hazard.
The general aim of this review is to perform the first systematic review about histamine food poisoning and meta-analysis of histamine content in food involved in these outbreaks.
Methods
According to the Cochrane Collaboration (www.cochrane.org) guidelines, the methods of the analysis and inclusion criteria were specified in advance and documented in a protocol that has been published in the International prospective register of systematic reviews (PROSPERO WEB site: http://www.crd.york.ac.uk/PROSPERO), on 18/07/2012 with registration number CRD42012002566.
Criteria for considering studies for this review
Types of studies
All report types of histamine food poisoning from food were considered for inclusion in the review. Reports of histamine poisonings from non-food sources (such as experimental studies with histamine administration) were not considered for inclusion. Only reports with histamine concentrations determined by chemical and ELISA methods were included. If the report was an experimental comparative one (e.g., experimental group versus control group) only data of group where occurred foodborne histamine intoxication were considered.
Eligible studies included any histamine poisoning outbreaks or single episodes that reported a measure of the histamine content and the type of the food involved in histamine poisoning.
The spatial interval for considering studies was set as worldwide. The time interval was set from 1959 through 2013, because in 1959 there was the first application of a specific and accurate quantitative method, the fluorimetric assay of histamine in tissues (Shore, Burkhalter, et al., Citation1959). Reports (abstract and full text) written in English, Italian, French, German, Portuguese, and Spanish were considered; considering a full text in other languages was decided case-by case by the potential relevance for this review of its English abstract.
Population
Only clinically healthy subjects were included; food allergic patients and other very sensitive people (due to serious illness or anomalous physical or psychic conditions), preschooler (< 6 years old) and very old (> 80 years old) people were excluded. If in a study nothing was reported about health status of people involved in histamine poisoning the health status was recorded as “unknown.”
Types of outcome measures
Primary outcomes
Number of histamine poisoning samples and histamine concentration in poisoning sample.
Secondary outcomes
Concomitant conditions relevant to histamine poisoning were considered as listed in Maintz and Novak (Citation2007): female sex, previous medication, food description (fish species, food recipe), consumption of alcohol during the meal; consumption of food recipe with suggested histamine-releasing capacities.
Search methods for identification of studies
Search strategies were optimized to detect all reports of histamine poisonings from foods that met inclusion criteria. A main form of search strategy was designed and modified to meet settings of databases consulted. We systematically identified all potentially relevant reports through the main electronic databases (); additional search was conducted by analyzing references of the selected articles.
Characteristics of consulted databases, specific search strategy and number of reports obtained, searched database are shown in . Unpublished and ongoing studies were also considered and detected if existing. The main search strategy is presented in , search terms included the following key word: “histamine,” “scombroid syndrome,” “histamine poisoning,” “food,” “seafood,” “meat products,” “fish”, “cheese”, “beer”, “wine”, and “biogenic amines.” To improve the effectiveness of keywords in the search strategy, a preliminary thesaurus study was performed. When multiple reports for a single study were present, it was used the most complete and updated version.
The literature search was conducted by two investigators (EC, FC) by aid of an information expert and by consulting with CB and PC. Two authors (CB and FC) independently selected potentially eligible studies for inclusion. The decision to include articles was made on the basis of the study title, then of the study abstract and finally of the full text; disagreements between reviewers were resolved by consensus; if no agreement was reached, a third author (PC) decided.
A data extraction sheet was developed and pilot-tested on a randomly-selected subgroup of included studies, data sheet was refined accordingly. One author (CB) extracted data from extraction sheet; data extracted were checked by a second author (FC). Disagreements were resolved by discussion between the two review authors; if no agreement was reached, a third author decided (PC).
A unique identifier of report was included in the characteristics recorded.
All quantitative measures of histamine content and measures of their variability; method of analysis used to determine food histamine content (if no method was mentioned the value was set to “unknown”); foods involved in histamine poisoning; primary and secondary outcome values; country or other identifier of geographic locations; people health category, i.e., if participants belonged to an excluded category and which was this category (if participants did not belong to above categories the status of “normal” was recorded); the presence of “heterogeneous food” (referring to more food types being associated with a single histamine mean value); other report characteristics useful to improve the quality of information.
Assessment of risk of bias in individual studies
Two reviewers (FC and CB) assessed the quality independently and any disagreements were resolved by discussion between the two review authors; if no agreement was reached, a third author decided (PC). Quality of included studies was considered a surrogate of risk of bias, so a quality score, of reports included in review based on additional relevant details other than inclusion criteria, was calculated. For each of the following seven items, a score of 1 was given if a value was present, 0 for absent value. The scores were then summed to give the final quality score (Murphy, Pfeiffer, et al., Citation2009). Variability estimate of histamine concentration, source of medical diagnosis (e.g., hospital m.d., family m.d.) or reasons given to present data as “histamine poisoning /scombroid syndrome,” age, sex, health status, source of food involved in poisoning (restaurant, supermarket…), declaration of histamine content measurement method, number of patients involved in histamine poisoning; otherwise any element that could arise suspect of bias was recorded.
Summary measures
Concomitant conditions (“risk factors”) relevant to histamine poisoning” outcome were summarized as a contingency table of the declared risk factors versus the number of their occurrences. The “number of histamine poisonings” outcome was summarized as the overall sum of histamine poisoning samples.
The summary measure of histamine concentration in sample was set to “log- mean”; this term is defined as the value of the estimate of the mean of the logarithms of the raw data. If this log-mean value was not be given in reports it was calculated with documented methods to yield a log-mean and its standard error (Quan, Zhang, Citation2003; Higgins, White, et al., Citation2008).
Unit of the analysis
The unit of the analysis was the “histamine poisoning sample.” This unit is defined as one “histamine poisoning” that occurred to one group of people (for “group” is meant one or more people) that ate one sample of food (for “sample of food” is meant one or more foods that were involved in one poisoning.
Histamine poisoning sample concept
One “histamine poisoning sample” (as defined above) led to one observation for each of the three outcomes considered; the observation formats were: a count of one (1) case in “assessment of valid histamine poisoning cases outcome,” one histamine concentration log- mean in “histamine content” outcome and one list of values (i.e., the names of relevant concomitant factors) in “relevant concomitant factors” outcome. The number of patients involved in histamine poisoning sample was recorded. It was decided that all unexpected situations related to unit of analysis were assessed and managed and the management method recorded.
Methods to deal with missing data
Missing variability data in poisoning samples (when a mean is given for more than one food specimens being involved in a single poisoning sample) was derived with documented statistical method that were recorded.
If a single poisoning sample (unit of analysis) was associated with more than one food type (“heterogeneous food category”) and histamine values of single foods were given but not the mean, it was planned that histamine content value had to be recorded as the log- mean of the values and variability estimate had to be calculated, the single values being recorded. If any of single values were missing, it was planned that the mean and variability estimated had to be calculated and the presence of missing values recorded. Again, it was planned that if all, but one, values were missing histamine content had to be considered as a single value, this situation being recorded; moreover, all unexpected situations related to missing data had to be assessed and managed, possibly with documented methods that had to be recorded.
Synthesis of results methods
Punctual estimates and their 95% confidence intervals were calculated across all selected studies on statistical units according to the methods described above. Calculations were performed using the “metagen” procedure of “meta” package of R software (Schwarzer, Citation2010). As this meta-analysis was expected to yield a high degree of variability, the random effect model, described by DerSimonian and Laird (Citation1986), was selected over the fixed effect model, because it incorporates within and between study variability. The chosen level of significance for statistical tests was P < 0.05. Heterogeneity, i.e., variability among records, was assessed by the I-squared (I2) statistic (Higgins et al., Citation2003). Ninety-five percent (95%) prediction intervals were calculated by means of “metafor” R package (Viechtbauer, Citation2010).
Assessment of risk of bias across the studies
In general, due to the nature of this systematic review, no selective reporting bias was assessed; it was planned that, if there were clues of selective reporting, authors of reports had to be contacted asking them about other results or outcomes not reported and that, if this issue was not resolved, to decide, with reasons, to exclude such reports. Decision had to be kept independently by CB and FC; if disagreement occurred PC had to keep final decision. Whatever the decision, the bias clues detected had to be recorded.
About management of reporting biases, being this concept difficult to apply due to the nature of this review, it was decided to discuss the publication bias issue according to data scenarios encountered during the review development.
Additional analyses
Subgroup analysis about country or other identifier of geographic locations of histamine poisoning samples.
Subgroup analysis about groups: (1) fresh seafood, (2) frozen seafood, (3) canned seafood, (4) fermented seafood, (5) seafood other than 1,2,3,4; (6) cheese and dairy; (7) other foods.
Sensitivity analysis conducted by quality score or quality categories of the reports
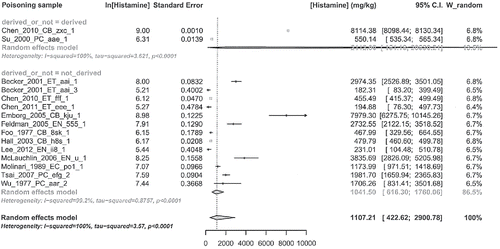
Sensitivity analysis on histamine concentration outcome conducting meta-analysis separately on two groups: one containing reports where variability was not derived (variability data value given in report) and one where variability was derived (variability data value not given in report, variability data inferred from other data).
Results
Study selection
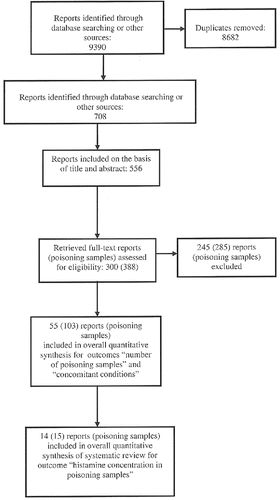
Searches yielded a total of 9390 references, after review and excluding duplicate reports 708 references were identified as potentially relevant. Of these, 556 records were included on the basis of title and abstract. We excluded 256 reports because they did not meet the adopted criteria and the full text of 300 reports was evaluated for report eligibility.
After excluding 248 full-text reports (corresponding to 285 poisoning samples), 52 reports (corresponding to 103 poisoning samples), listed in , were included in overall quantitative synthesis for outcomes “number of poisoning samples” and “concomitant conditions.” Fourteen reports among them, corresponding to 15 poisoning samples, were selected for quantitative synthesis of outcome “histamine concentration in poisoning samples.” The selection process is summarized in .
Table 1. Characteristics of studies included in meta-analysis. N for number of people involved in histamine outbreak, MD for missing data. Type of food (food category): (1) fresh, (2) frozen, (3) canned, (4) fermented, (5) other seafood, (6) cheese, and (7) other foods. Values of sample mean or sample standard deviation that were calculated have been rounded to two decimals.
Characteristics of included studies
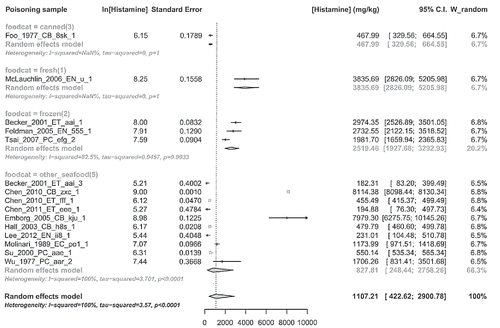
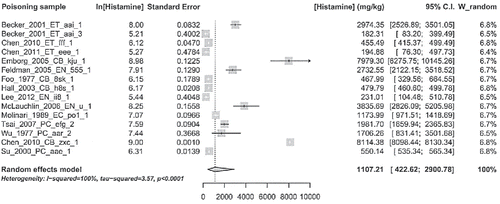
Below are summarized the characteristics of the 52 articles included; details are shown in . The overall analysis comprised a total number of 1171 people involved in 103 episodes of histamine intoxication, ranged from 1 to 347 (person/poisoning sample). In these outbreaks the sources of food were reported in 50 episodes (missing = 53). On the known 50 sources, 17 were related to institutional or company food services, 20 to restaurants, and only 9 (plus 4 unsure) linked to private home. Among the 103 poisoning samples, 101 were fish and seafood and only two were cheese. The raw data for each outbreaks of histamine intoxication are presented in . The meta-analysis of data from the 52 selected articles is summarized in Forest plot (); the mean histamine concentration in studied episodes is 1107.21 mg/kg with a confidence interval of 422.62–2900.78 mg/kg. Heterogeneity index (I2) was 100% (P < 0.0001), log-prediction interval was 3.18–10.84, equivalent to 24.12–50822.78 mg/kg. Secondary outcomes that are the concomitant conditions relevant to histamine poisoning were not evaluated, because in the most of included articles they are missing.
Figure 3. Meta-analysis of histamine concentration. The plot is centered on the meta-mean value. Lines are 95% confidence intervals for the means (c.i.) of the single poisoning samples. Lines with arrows indicate that plots of c.i. are truncated, due to the wide range of values. In the left size of the figure are shown values in the natural logarithm scale with their standard errors. In the right side are shown the above values transformed from log-mean and its standard error to the mean and confidence interval in the ordinary scale. At the bottom of the figure, from left to right, are shown the above items. Right: model used with main heterogeneity parameter estimates; center: scale of the values plus a polygon that plots confidence interval of the “meta-mean”; left: meta-mean and confidence interval actual values (ordinary scale).
Risk of bias as quality score of individual reports
Quality items values and the overall quality score are presented in for each included report.
Table 2. Quality items values and overall quality score.
Risk of bias across reports
No elements pointing to selective reporting bias were detected. Publication bias was not assessed.
Additional analyses
Due to the nature of results about “concomitant conditions” outcome, this was not considered for additional analyses.
Subgroup analyses
Number of poisoning samples and histamine concentration outcomes by geographic locations were not analyzed because of too many different locations.
The number of poisoning samples and histamine concentration outcomes by food categories was analyzed (respectively and ).
Table 3. Sub-group analysis: Number of poisoning samples by food category.
Sensitivity analyses
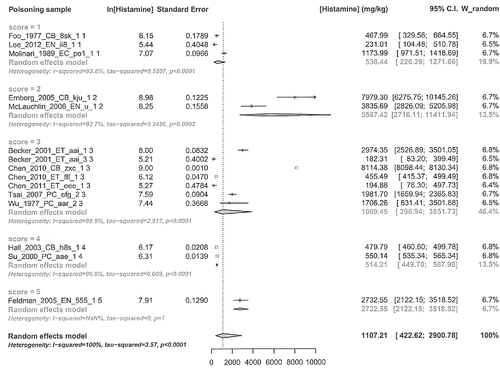
The following analyses were made: number of poisoning samples and histamine concentration outcomes by quality score categories; histamine concentration outcome by groups where variability was derived and where was not. Their results are respectively shown in and and .
Table 4. Sensitivity analysis: Number of poisoning samples by quality score.
Table A. Characteristics of searched database.
Discussion
About the fish species associated with poisoning samples, it is worth noting that, n. 59 (out of 101) belonged to species associated with a high amount of histidine, according to EU legislation, that establishes a legal limit of histamine for “Particularly fish species of the families: Scombridae, Clupeidae, Engraulidae, Coriphaenidae, Pomatomidae, Scomberosocidae.”(Communities, Citation2007), because these species are more likely to contain high histamine levels, as during spoilage some bacteria produce decarboxylase enzymes and convert histidine to histamine. Other 21 belonged to fish species without a legal limit in EU, and for a good 21 poisoning samples the species was unknown.
The data obtained by our review about canned tuna refute certain views that see this product as a main cause of poisoning. Among the 101 poisoning fish samples, only 22 consisted in canned products, mainly canned tuna () and all 22 poisoning samples were related to events happened before 1985, but two (Valentini, Levre, et al., Citation1991; Tsai, Kung, et al., Citation2005).
At present, canned tuna, and other canned fish belonging to species associated with the risk of histamine, have very low levels of histamine; this fact is likely due to the quality of canning process that is improving over the years due to widespread application of HACCP principles, from the caught fish on the vessel to the processed product (Cattaneo, Citation2011; Guillier, Thebault, et al., Citation2011).
Other three episodes regarded canned tuna as ingredient (tuna salad and tuna sandwiches) (Stell, Citation1997; Predy, Honish, et al., Citation2003; Jantschitsch, Kinaciyan, et al., Citation2011). In all three, tuna cans had been opened hours or even a week before the preparation or the consumption, with likely post-processing contamination and consequent histamine production.
Fresh or frozen fish, diversely prepared and cooked, and fish products differently processed (not canned) were cause of poisoning in 79 episodes. The species or the family mainly reported were (number, % of 79): tuna (26, 32.9%); scombridae other than tuna (7, 8.8%); mahi mahi (3, 3.8%); species of the family Istiophoridae (total 8, 10.1%) such as Makaira spp (5), Tetrapturus spp (2), sailfish (1); swordfish (2); others species (12, 15.2%).
Among the “others,” Seriola lalandi (n.3), Chanos chanos (n.1), Arripis trutta (n.4) were reported, fish species not considered in EU legislation, while having very high concentrations of histidine. Three other outbreaks (Eckstein, Serna, et al., Citation1999; Feldman, Werner, et al., Citation2005; Sinn, Citation2006) were attributed to Lepidocybium flavobrunneum, species whose meat has a very high content of wax ester that could cause gastrointestinal effects, but also has histidine levels as high as many Scombridae.
As to Istiophoridae and Xiphidae families, suborder Xiphiodei, in other countries they are associated with the risk of histamine because known to have very high free histidine levels or to be associated with SFP (Scombrotoxin Fish Poisoning) (F.A.O., Citation2014). Interestingly, the family Istiophoridae (Billfish) is placed in the Scombroidei suborder by Nelson (Citation2006). Both Billfish and scombrids have common characteristics that could explain the frequency of episodes of histamine intoxication caused by billfish. The complete list of fish species produced by our review can help to control imports and medical history of cases of suspected poisoning, as well as to cope with the problems arising from changes in international market trends of fishery products.
The source of poisoning (places where the poisoning samples were eaten) was not reported in 53 episodes (out of 103). The main reported sources were restaurants (20 cases, plus 3 unsure) and institutional foodservice, company or community canteens and cafeterias (17 cases), where the number of people involved is in terms of dozens or hundreds. The outbreaks occurred at home were 9 (plus 4 unsure); probably this kind of poisoning, involving a small number of persons for single episode, is little reported in the literature and could indicate a reporting bias (under-reporting).
Regarding the result of meta-analysis, the meta-mean of histamine concentration that summarizes the 14 reports (Foo, Citation1977; Molinari, Montagnoli, et al., Citation1989; Wu, Yang, et al., Citation1997; Su, Chou, et al., Citation2000; Becker, Southwick, et al., Citation2001; Hall, Citation2003; Emborg, Laursen, et al., Citation2005; Feldman, Werner, et al., Citation2005; Mclauchlin, Little, et al., Citation2006; Tsai, Hsieh, et al., Citation2007; Chen, Huang, et al., Citation2010; Chen, Lee, et al., Citation2011; Lee, Huang, et al., Citation2012) used for the statistical calculation () is about 1000 ppm, a very high value if compared with what assumed by FDA (F.D.A., Citation2014) indicating, in most cases, histamine levels in illness-causing fish of about 200 or 500 ppm. On the other side, our result is in agreement with McLaughlin et al. (Mclauchlin, Little, et al., Citation2006) who wrote that ingestion of fish containing histamine at levels around 1000 ppm can result in illness. Shalaby (Citation1996) emphasized that poisoning does occur at histamine concentrations lower than 100 mg/100 g and levels of histamine in fish of 5–20 mg/100 g (50–200 ppm) are possibly toxic. This could be congruent with the lower limit of overall predictive interval of histamine concentration from meta-analysis (24.12 ppm) although, due to the highest heterogeneity amount estimated, this value is questionable. Either way, EU maximum limit (Communities, Citation2007) seems to be proper to protect the consumer, also respect to the meeting report of FAO/WHO (F.A.O., Citation2014), where an oral NOAEL (No Observed Adverse Effect Level) of 50 mg was identified, from which was derived a histamine limit of 200 mg/kg, considering a service size of 250 g.
Due to the highest (100%) level of heterogeneity estimated for the overall meta-analysis the limits both for the meta-mean confidence interval and the predictive interval are questionable. More reliable are the values for subgroups, where moderate amount of heterogeneity was estimated.
Subgroup analysis of histamine concentration outcome by food categories did not show significant difference between subgroups due to the overlapping of confidence interval. Moreover, the food category “fermented” (4) is missing, while categories “fresh” (1) and “canned” (3) consist of only one record and food category “other seafood” (5) is highly heterogeneous.
Sensitivity analysis of histamine concentration outcome by quality categories did not show separation of the values of quality categories (overlapping of confidence interval) but this cannot lead to declare absent the quality category effect, due to remarkable difference between the means of the categories, high degree of heterogeneity of each category and finally presence of single-record categories.
About sensitivity analysis of histamine concentration by derived or not variability, also this is poorly interpretable, due to high heterogeneity amount in each group and very unbalanced sample size of the two groups (2 vs. 13). Moreover, the overlapping of confidence intervals is scarcely meaningful because it is very large value in “variability derived” category.
Single-specimen poisoning samples were excluded from histamine concentration meta-analysis in order to not confound within—and between specimen variability.
Conclusions
The main goal of our systematic review was to remove noise as more as possible from information about values of histamine in foods involved into poisoning; this goal has been reached by producing objective estimates.
To attribute precisely the responsibility of the poisoning event, increasing knowledge, allowing the food business operators to improve their practice or processing, as well as guaranteeing the customer also legally, it is fundamental to approach this topic with pragmatism. We hope that these estimates could be a valid reference for operators and consumers.
The estimate of the mean was found to be fairly high, its precision was unfortunately impaired by a lot of variability (heterogeneity).
Too few suitable data are presently available to conduct a reliable analysis on homogeneous subsets of food.
It is recommended that histamine poisoning episodes are recorded and published including the values of all important variables pointed out in this review, moreover, the variability within poisoning sample should be stated analyzing at least twice the histamine content for each sample. About the conditions concomitant to the poisonings, the role of several health conditions, drugs and meal composition on the proceeding of an event of histamine (scombroid) poisoning has been underlined several times (Sattler, Hesterberg, et al., Citation1985; Taylor, Citation1986; Maintz, Novak, Citation2007; Hungerford, Citation2010). Alcoholic beverages can increase the seriousness of the episodes enhancing the absorption of histamine contained in the meal, but even if the importance of alcohol is reported in a previous review (Lehane, Olley, Citation2000) and other reports (Geiger, Citation1955; Zee, Simard, et al., Citation1981; Zimatkin, Anichtchik, Citation1999; Maintz, Novak, Citation2007), our results point out lack of this information, so it is recommended to physicians to include such items in the anamneses of the poisoning cases.
Contributions of authors
CB building protocol, study selection, data extraction and writing review; EC bibliographic search; FC building protocol, study selection, data extraction and analysis and writing review; PC: building protocol, supervising of all phases and writing review.
Declarations of interest
This systematic review is a phase of a research commissioned from a food industry.
Sources of support
Internal sources
Università degli Studi di Milano (Italy): EC work in this systematic review was rewarded by a salary.
External sources
Generale Conserve company, Italy: sponsored research about histamine poisoning in tunafish.
Acknowledgments
The authors thank Angela Moccia for her contributtion as information expert.
Funding
The authors thank Generale Conserve for funding this systematic review.
References
- Anonymous. (1985). Food poisoning and salmonella surveillance in England and Wales: 1983. British Medical Journal (Clinical Research ed.) 291:394–396.
- Anonymous. (1988). Scombroid fish poisoning–New Mexico 1987. MMWR. Morbidity and Mortality Weekly Report. 37:451.
- Anonymous. (2000). Scombroid fish poisoning–Pennsylvania 1998. MMWR: Morbidity & Mortality Weekly Report. 49:398–400.
- Anonymous. (2007). Scombroid fish poisoning associated with tuna steaks–Louisiana and Tennessee, 2006. MMWR. Morbidity and Mortality Weekly Report, vol. 56, pp. 817–819, United States.
- Becker, K., Southwick, K., Reardon, J., Berg, R. and Maccormack, J. (2001). Histamine poisoning associated with eating tuna burgers. JAMA 285(10):1327–1330.
- Bedry, R., Gabinski, C. and Paty, M. (2000). Diagnosis of scombroid poisoning by measurement of plasma histamine. N. Engl. J. Med. 342:520–521.
- Bremer, P. J., Fletcher, G. C. and Osborne, C. (2003). Scombrotoxin in seafood. New Zealand Institute for Crop & Food Research Limited, Christchurch, NZ.
- Cattaneo, P. (2011). Sindrome sgombroide-intossicazione da istamina. Food. 2:5–55.
- Cattaneo, P. and Stella, S. (2001). Un episodio di intossicazione da istamina per consumo di pizza al tonno. Arch. Vet. Ital. 52(5/6):13.
- Chen, H. C., Huang, Y. R., Hsu, H. H., Lin, C. S., Chen, W. C., Lin, C. M. and Tsai, Y. H. (2010). Determination of histamine and biogenic amines in fish cubes (Tetrapturus angustirostris) implicated in a food-borne poisoning. Food Control. 21:13–18.
- Chen, H. C., Kung, H. F., Chen, W. C., Lin, W. F., Hwang, D. F., Lee, Y. C. and Tsai, Y. H. (2008). Determination of histamine and histamine-forming bacteria in tuna dumpling implicated in a food-borne poisoning. Food Chem. 106:612–618.
- Chen, H. C., Lee, Y. C., Hwang, D. F., Chiou, T. K. and Tsai, Y. H. (2011). Determination of histamine in mahi–mahi fillets (Coryphaena hippurus) implicated in a foodborne poisoning. J. Food Saf. 31:320–325.
- Chen, H. C., Lee, Y. C., Lin, C. M., Hwang, D. F. and Tsai, Y. H. (2010). Determination of histamine and bacterial isolation in marlin fillets (Makaira nigricans) implicated in a foodborne poisoning. J. Food Saf. 30:699–710.
- Chianea, D., Drouillard, I., Puyhardy, J. M., Corbe, H., Adam, F., Bietrix, P., Cariou, M., Helies, C. and Boutin, J. P. (1998). Collective histamine poisoning confirmed by a rapid high pressure liquid chromatography mass spectrometry technique. Ann. Biol. Clin. 56:578–579.
- Communities, C. O. T. E. (2007). Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union. L322:12, 13.
- D'aloia, A., Vizzardi, E., Pina, P. D., Bugatti, S., Magro, F. D., Raddino, R., Curnis, A. and Cas, L. D. (2011). A scombroid poisoning causing a life-threatening acute pulmonary edema and coronary syndrome in a young healthy patient. Cardiovasc. Toxicol. 11:280–283.
- Dalgaard, P., Emborg, J., Kjølby, A., Sørensen, N. D., Ballin, N. Z., Dalgaard, P., Emborg, J., Kjølby, A., Sørensen, N. D. and Ballin, N. Z. (2008). Histamine and biogenic amines: formation and importance in seafood. In Improving Seafood Products for the Consumer, pp. 292–324. Børresen, T., and Børresen, T., Eds., Cambridge: British Welding Research Association.
- Demoncheaux, J. P., Michel, R., Mazenot, C., Duflos, G., Iacini, C., De Laval, F., Saware, E. M. and Renard, J. C. (2012). A large outbreak of scombroid fish poisoning associated with eating yellowfin tuna (Thunnus albacares) at a military mass catering in Dakar. Epidemiol Infect, vol. 140, pp. 1008–1012.
- Dersimonian, R. and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials. 7:177–188.
- Doeglas, H. M., Huisman, J. and Nater, J. P. (1967). Histamine intoxication after cheese. Lancet 2:1361–1362.
- Eckstein, M., Serna, M., Delacruz, P. and Mallon, W. K. (1999). Out-of-hospital and emergency department management of epidemic scombroid poisoning. Acad. Emerg. Med. 6:916–920.
- Emborg, J. and Dalgaard, P. (2006). Formation of histamine and biogenic amines in cold-smoked tuna: An investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. J. Food Prot. 69:897–906.
- Emborg, J., Laursen, B. G. and Dalgaard, P. (2005). Significant histamine formation in tuna (Thunnus albacares) at 2(degrees)C–Effect of vacuum- and modified atmosphere-packaging on psychrotolerant bacteria. Int. J. Food Microbiol. 101:263–279.
- F.A.O. (2014). Joint FAO/WHO Expert Meeting on the Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. In).
- F.D.A. (2014). Scombrotoxin (Histamine) formation. In Fish and Fishery Products Hazards and Controls Guidance. 4th ed.
- Feldman, K. A., Werner, S. B., Cronan, S., Hernandez, M., Horvath, A. R., Lea, C. S., Au, A. M. and Vugia, D. J. (2005). A large outbreak of scombroid fish poisoning associated with eating escolar fish (Lepidocybium flavobrunneum). Epidemiol. Infect. 133:29–33.
- Fernández, M. A., González, J. M. B. and Rozas, S. F. (2001). Escombrointoxicación por consumo de bonito. Emergencias. 13:132–135.
- Foo, L. Y. (1975a). The content of histamine and fish food poisoning. N. Z. Med. J. 82:381–383.
- Foo, L. Y. (1975b). Scombroid-type poisoning induced by the ingestion of smoked kahawai. N. Z. Med. J. 81:476–477.
- Foo, L. Y. (1977). Scombroid poisoning: Recapitulation on the role of histamine. N. Z. Med. J. 85:425–427.
- Geiger, E. (1955). Role of histamine in poisoning with spoiled fish. Science 121:865–866.
- Gellert, G. A., Ralls, J., Brown, C., Huston, J. and Merryman, R. (1992). Scombroid fish poisoning: Underreporting and prevention among noncommercial recreational fishers. West. J. Med. Brit. Med. J. 157:645–647.
- Guillier, L., Thebault, A., Gauchard, F., Pommepuy, M., Guignard, A. and Malle, P. (2011). A risk-based sampling plan for monitoring of histamine in fish products. J. Food Prot. 74:302–310.
- Guly, H. R. and Grant, I. C. (2006). Case of the month: Lesson of the week: Don't forget scombroid. Emerg. Med. J. 23:955–956.
- Hall, M. (2003). Something fishy: Six patients with an unusual cause of food poisoning!. Emerg. Med. 15:293–295.
- Higgins, J. P. T., Thompson, S. G., Deeks, J. J. and Altman, D. G. (2003). Measuring inconsistency in metaanalyses. Brit. Med. J. 327:557–560.
- Higgins, J. P. T., White, I. R. and Anzures-Cabrera, J. (2008). Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 27:6072–6092.
- Hobbs, G. (1983). Food poisoning and fish. J. R. Soc. Promot. Health. 103:144–149.
- Hungerford, J. M. (2010). Scombroid poisoning: A review. In Toxicon, vol. 56, pp. 231–243. England: Elsevier Ltd.
- Hwang, D. F., Chang, S. H., Shiua, C. Y. and Tuu-Jyi, C. (1997). High-performance liquid chromatographic determination of biogenic amines in fish implicated in food poisoning. J. Chrom. B. 693:23–29.
- Iguchi, S., Sugita, M., Nomura, T., Sekii, H., Ookubo, H. and Yamaguchi, N. (2008). A local outbreak of scombroid fish poisoning in Japan. Clin. Toxicol. 46:621–621.
- Jantschitsch, C., Kinaciyan, T., Manafi, M., Safer, M. and Tanew, A. (2011). Severe scombroid fish poisoning: An underrecognized dermatologic emergency. J. Am. Acad. Dermatol. 65:246–247.
- Jiang, D. D. S., Liu, C. M., Wang, S. C., Lin, C. W. and Chen, H. R. (2009). Histamine-induced allergic outbreak in among Junior/Senior High School Students: A Case–control study. Epidemiol. Bull. 25(5):316–331.
- Kanki, M., Yoda, T., Ishibashi, M. and Tsukamoto, T. (2004). Photobacteriumphosphoreum caused a histamine fish poisoning incident. Int. J. Food Microbiol. 92:79–87.
- Kelso, J. M. and Lin, F. L. (2009). Skin testing for scombroid poisoning. Ann. Allergy, Asthma Immunol. 103:447–447.
- Kim, R. (1979). Flushing syndrome due to mahimahi (scombroid fish) poisoning. Arch. Dermatol. 115:963–965.
- Kow-Tong, C. and Malison, M. D. (1987). Outbreak of scombroid fish poisoning, Taiwan. Am. J. Public Health. 77:1335–1336.
- Leask, A., Yankos, P. and Ferson, M. J. (2004). Fish, so foul! Foodborne illness caused by combined fish histamine and wax ester poisoning. Commun. Dis. Intell. 28:83–85.
- Lee, Y. C., Huang, T. C., Lin, C. S., Lin, C. M. and Tsai, Y. H. (2012). Determination of histamine and histamine-forming Bacteria in Striped Marlin Fillets (Tetrapturus audax) Implicated in a Food-borne Poisoning. Toxicon 60:161–162.
- Lehane, L. and Olley, J. (2000). Histamine fish poisoning revisited. Int. J. Food Microbiol. 58:1–37.
- Maintz, L. and Novak, N. (2007). Histamine and histamine intolerance. Am. J. Clin. Nutr. 85(5):1185–1196.
- Mclauchlin, J., Little, C. L., Grant, K. A. and Mithani, V. (2006). Scombrotoxic fish poisoning. J. Public Health. 28(1):61–62.
- Molinari, G., Montagnoli, G., Pellegrini, G. and Caroli, G. (1989). [Hygiene and health importance of histamine as an unhealthy factor in several food products]. Annali Di Igiene: Medicina Preventiva E Di Comunità 1:637–646.
- Muller, G. J., Lamprecht, J. H., Barnes, J. M., De Villiers, R. V. P., Honeth, B. R. and Hoffman, B. A. (1992). Scombroid poisoning. Case series of 10 incidents involving 22 patients. S Afr. Med. J. 81:427–430.
- Murphy, G., Pfeiffer, R., Camargo, M. C. and Rabkin, C. S. (2009). Meta-analysis shows that prevalence of Epstein–Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology 137(3):824–833.
- Nalinee Hongchumpon Ouppapong, T. P. J., et al. (2010). Scombrotoxin Food Poisoning Outbreak among Frozen Seafood Factory Workers Samut Prakan Province, Thailand, July 2007. Outbreak, Surveill. Invest. Rep. 2(1):5.
- Nelson, J. S. (2006). Fishes of the World (4th ed.). Hoboken, NJ: John Wiley and Sons.
- Ohnuma, S., Higa, M., Hamanaka, S., Matsushima, K. and Yamamuro, W. (2001). An outbreak of allergy-like food poisoning. Intern. Med. 40:833–835.
- Predy, G., Honish, L., Hohn, W. and Jones, S. (2003). Was it something she ate? Case report and discussion of scombroid poisoning. CMAJ: Can. Med. Assoc. J. 168:587–588.
- Quan, H. and Zhang, J. (2003). Estimate of standard deviation for a log-transformed variable using arithmetic means and standard deviations. Stat. Med. 22:2723–2736.
- Sanchez-Guerrero, I. M., Vidal, J. B. and Escudero, A. I. (1997). Scombroid fish poisoning: A potentially life-threatening allergic-like reaction. J. Allergy Clin. Immunol. 100:433–434.
- Sanders, W. E. (1987). Intoxications from the seas: Ciguatera, scombroid, and paralytic shellfish poisoning. Infect. Dis. Clin. North Am. 1:665–676.
- Sattler, J., Hesterberg, R., Lorenz, W., Schmidt, U., Crombach, M. and Stahlknecht, C. D. (1985). Inhibition of human and canine diamine oxidase by drugs used in an intensive care unit: relevance for clinical side effects?. Agents Actions 16:91–94.
- Schulze, K., Reusse, V. and Tillack, J. (1979). Histamine food poisoning after consumption of sardines in oil. Arch. Lebensmittelhygiene 30:56–59.
- Schwarzer G. (2010). Meta-Analysis with R. R package version 1.6-0.
- Shalaby, A. R. (1996). Significance of biogenic amines to food safety and human health. Food Res. Int. 29:675–690.
- Shore, P. A., Burkhalter, A. and Cohn, V. H. (1959). A method for the fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 127(3):182–186.
- Sinn, G. (2006). Histamine poisoning and gastroenteritis breakout after participation in a school lunch with fish burgers. Gesundheitswesen 68:216–217.
- Stell, I. M. (1997). Trouble with tuna: Two cases of scombrotoxin poisoning. J. Accid Emerg. Med. 14:110–111.
- Su, S. C., Chou, S. S., Chang, P. C. and Hwang, D. F. (2000). Determination of biogenic amines in fish implicated in food poisoning by micellar electrokinetic capillary chromatography. J. Chromatogr. B: Biomed. Sci. Appl. 749:163–169.
- Taylor, S. L. (1986). Histamine food poisoning: Toxicology and clinical aspects. Crit. Rev. Toxicol. 17:91–128.
- Taylor, S. L., Keefe, T. J., Windham, E. S. and Howell, J. F. (1982). Outbreak of histamine poisoning associated with consumption of Swiss cheese. J. Food Prot. 45:455–457.
- Tsai, Y. H., Hsieh, H. S., Chen, H. C., Cheng, S. H., Chai, T. J. and Hwang, D. F. (2007). Histamine level and species identification of billfish meats implicated in two food-borne poisonings. Food Chem. 104:1366–1371.
- Tsai, Y. H., Kung, H. F., Chen, H. C., Chang, S. C., Hsu, H. H. and Wei, C. I. (2007). Determination of histamine and histamine-forming bacteria in dried milkfish (Chanos chanos) implicated in a food-borne poisoning. Food Chem. 105:1289–1296.
- Tsai, Y. H., Kung, H. F., Lee, T. M., Chen, H. C., Chou, S. S., Wei, C. I. and Hwang, D. F. (2005). Determination of histamine in canned mackerel implicated in a food borne poisoning. Food Control 16:579–585.
- Valentini, P., Levre, E., Molinari, G., Brunetti, M., Domenici, D. and Caroli, G. (1991). Histamine and histamine-producing enterobacteria in canned tuna fish responsible for the scombrotoxic syndrome. Ig. Mod. 95:154–164.
- Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36(3):1–48.
- Wu, M. L., Yang, C. C., Yang, G. Y., Cer, J. and Deng, J. F. (1997). Scombroid fish poisoning: An overlooked marine food poisoning. Vet. Hum. Toxicol. 39:236–241.
- Zee, J. A., Simard, R. E., Vaillancourt, R. and Boudreau, A. (1981). Effect of Lactobacillus brevis, Saccharomyces uvarum and grist composition on amine formation in beers. Can. Inst. Food Sci. Technoogyl. 14(4):321–325.
- Zimatkin, S. M. and Anichtchik, O. V. (1999). Alcohol–histamine interactions. Alcohol Alcohol. 34:141–147.