Abstract
Over the past several decades, the use of genetically engineered microorganisms (GEMs, often referred to as Genetically Modified Microorganisms or GMMs) has become widespread in the production of food processing aids and other food ingredients. GEMs are advancing food production by increasing efficiency, reducing waste and resource requirements, and ultimately enabling beneficial innovations such as the cost-effective fortification of food with essential nutrients, vitamins, and amino acids, and delivery of tailored enzymes to achieve unique food processing capabilities. Regulatory agencies, including those in the European Union, United States, and Canada review the safety of GEMs when evaluating food substances produced using GEMs to ensure that both the microorganism and the resulting food substance are safe. This paper provides a summary of historical and current use of GEMs in food manufacture, an overview of frameworks that regulate their use, and a description of the safety assessment of both GEMs and food substances produced with GEMs. The paper encourages regulatory agencies around the globe to take a more aligned approach to the safety evaluation and regulatory oversight of GEM-produced food ingredients and enzymes, a category of food substances that enables more sustainable consumer food choices.
Introduction
Microorganisms have been used throughout human history to aid in the production of food, even before humans knew that these organisms were responsible for the fermentation processes involved (Jay, Loessner, and Golden Citation2005; Zhang, Sun, and Ma Citation2017). Microorganisms, typically through production of their endogenous enzymes, have been critical to food production for thousands of years for foods like beer, yoghurt, kefir, cheese, soy sauce, wine, vinegar, and many more. Following the discovery of the microorganisms responsible in the 1830s (Barnett Citation2003), food producers began examining ways of harnessing these microorganisms to make them more efficient. While it took thousands of years for humans to discover the microorganisms responsible for the existence of many foods, once discovered it only took approximately one hundred years (and the discovery of DNA) for humans to begin using techniques to optimize the use of microorganisms, including the modification of their genetic makeup to make food production using microorganisms more efficient.
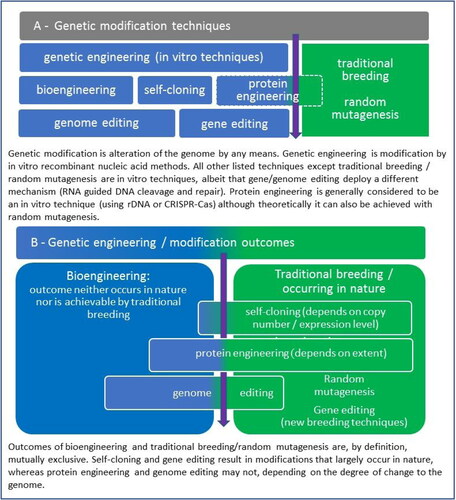
Modifications are made to the genetic makeup of microorganisms to either produce a new protein or other food ingredient, to improve/enhance the production of an existing protein/ingredient, or to tailor the characteristics of an existing protein to a new application. Multiple techniques are employed to make genetic changes in a microorganism, and the term Genetically Engineered Microorganisms (GEMs) specifically refers to microorganisms (i.e., bacteria or fungi, including yeasts) that humans have modified using in vitro molecular biology techniques (aka Modern Biotechnology) to perform a specific function. Many other techniques exist for modifying the genetic makeup of microorganisms, but not all of these techniques fall under regulatory definitions of genetically engineered or genetically modified. For example, chemical mutagenesis and interspecies crossing can be used to modify the genetic makeup of a microorganism (National Research Council of the United States Citation2004), yet these techniques do not typically fall under the regulatory definitions of genetically engineered (or genetically modified in the European Union). Later in this paper, some of the more common techniques used today for the modification of the genetic makeup of microorganisms will be compared.
The extraordinary achievements in biochemistry and molecular biology over the past several decades have led to a widespread use of GEMs in the production of medical and food substances, especially as these processes are increasingly recognized as environmentally-friendly, animal-friendly, and cost-effective means of production. For example, insulin is now produced in microbes rather than sacrificing pigs to harvest their pancreas, the original source of insulin. Similarly, microbially-produced trypsin and chymosin are available as alternatives to harvesting trypsin or rennet from animal sources such as pigs and cows. The benefits of GEM production are not limited to replacing animal-based production methods. For example, in comparison with traditional agricultural production of plant-based substances, such as stevia extracts and vanilla, GEM-based production of steviol glycosides (Philippe et al. Citation2014) and vanillin (Brochado et al. Citation2010) has many sustainability benefits including a reduction in land usage, production of less waste, and provision of a more stable and affordable supply to meet the rising consumer demand.
An excellent example of the adoption of GEMs as a production method for food ingredients is the production of riboflavin beginning in the 1990s through today, where nearly 100% of the commercially-produced riboflavin is manufactured using a GEM (Schwechheimer et al. Citation2016). Other examples of food ingredients produced today by GEMs include vitamins, amino acids, functional proteins (e.g., texturants), nutritional proteins, oligosaccharides, flavors, and sweeteners (Adrio and Demain Citation2010).
Another area where GEMs have found wide application is in the production of food enzymes (Olempska-Beer et al. Citation2006). Food enzymes are commonly used in food production to perform a number of different technical effects such as: reducing lactose content of foods (lactase), dough strengthening or starch modification in baking (amylases), vegetable oil refining (phospholipase), coffee production (mannanase), fruit- and vegetable processing (pectin esterase), conversion of starches into sugars and specialty products (carbohydrases such as amylase, glucoamylase, and transglucosidase), and hydrolysis of proteins (protease). Last but not least, cheese can be made with chymosin (the active milk protein coagulating enzyme in rennet) produced with GEMs, instead of harvesting the enzyme from calf stomachs.
Enzymes are proteins that have catalytic functions that can be leveraged in food processing. The microorganism produces the enzyme from a sequence of amino acid building blocks that define its properties including its catalytic function. Various methods of genetic modification of microorganisms are used to enable enzyme production, increase the yield of enzyme production, and to tailor enzyme functionality and stability via protein engineering if the application conditions (e.g., pH, temperature) differ from the natural conditions under which the source organism evolved.
Under many regulatory frameworks, GEMs used to produce food substances (ingredients or enzymes) are classified as processing aids as long as the organism itself is not detectable in the food substance, which is the case in examples above. While GEMs could also be incorporated as intact, live organisms into foods such as yogurt, kefir, or kombucha, this use falls outside of the scope of this paper.
Manufacturing food substances using microorganisms
Production of food substances using microorganisms is referred to as fermentation, which is the general process through which a microorganism converts an energy source into other substances. Fermentation is only the first step in the production of a refined food substance using a microorganism, regardless of whether that microorganism is genetically engineered. During fermentation, the microorganism is provided an energy source and other nutrients that are then converted into the food substance of interest. Unlike fermented foods (e.g. yoghurt, kefir, bread) where the microorganism is maintained intact, food substances are refined by several steps before being used as functional ingredients or processing aids in other foods. Depending on the food substance produced and the modifications made to the GEM, the substance may either be secreted by the microorganism into the fermentation media, or the GEM may be lysed in order to release the substance. Next, refinement begins with a recovery step where the food substance is separated by physical means such as centrifugation and/or filtration from the intact microorganism and/or fermentation medium. Recovery is then followed by additional purification steps, depending on the specific food substance, to remove other substances that could be present such as fermentation substrates, proteins, substances that could have been produced by the microorganism, intact GEM organisms, and recombinant DNA. A food substance manufactured in this manner is said to be ‘produced with’ a GEM (short for: “produced with the aid of” a GEM) rather than “produced from” a GEM.
Once the food substance has been appropriately refined or purified, it may undergo additional finishing steps. Finishing could include concentration of liquid food substances, addition of stabilizers or preservatives drying of food substances into a powder, or addition of other materials (such as carriers or granulation agents), depending on the need for the specific food substance. In this finished state, these food substances have been completely separated from any intact organism, as well as any of the genetic material (DNA) from the microorganism. In all cases, specifications that include both purity and impurity criteria are established to ensure that the food substance is safe.
While modern fermentation production facilities may appear futuristic, the production methods themselves are strikingly like food production processes that have used microorganisms for thousands of years (). For example, comparing modern fermentation to production of wine demonstrates that these processes are very similar. In wine production, microorganisms (yeast) are provided a substrate (the sugars present in grape juice) that the microorganism then converts into the desired substance (ethanol). Once wine fermentation is complete, a recovery step is used to separate the desired product from the microorganism (in wine, referred to as the lees), and additional purification steps may be employed such as filtration. Following recovery and purification, wine undergoes finishing steps such as aging in oak barrels before being bottled.
Table 1. Comparison of fermentation processes.
Common methods for modifying the genetic makeup of microorganisms used in food production
Numerous strains of microorganisms are used in the production of food substances using the process described above. The microorganisms used in this process generally must be modified unless they already express all the characteristics necessary to produce the desired food substance economically, at scale. The use of unmodified microorganisms is most common in traditional food processes such as those microorganisms used in the commercial production of bread, wine and beer, and yogurt, and is occasionally still used today in the production of some food enzymes. However, genetic modification of the microorganism is often needed to produce a desired substance altogether (e.g., if the source organism that naturally produces the substance of interest cannot be cultivated at scale), or genetic modification can be used to improve the efficiency and reduce cost of producing an endogenous enzyme or another food substance. The following section discusses four methods that can be used to genetically modify microorganisms to produce food substances.
Non-targeted mutagenesis
Until the advent of Modern Biotechnology, microorganisms were genetically modified for many decades by applying selection pressure or random mutagenesis induced by chemicals or UV irradiation. Modification approaches based on classical mutagenesis and selection are non-targeted as they introduce random DNA changes, followed by an extensive screening effort to select the microorganisms for increased production of a specific enzyme or for a desired phenotypic trait. The final genetic make-up of microbes produced with random mutagenesis cannot be predicted beforehand, and even today’s available high-throughput DNA sequencing does not always provide full clarity of all the DNA changes or their consequences.
Altogether, the utility of this approach today is limited to modifying traits for which the genetic basis is not well-understood and continued advances in molecular biology will likely lead to it being largely replaced with more precise methods of introducing genetic modifications via Modern Biotechnology techniques. However, random mutagenesis followed by repeated testing has been the basis for development of several robust, well characterized and safe microbial production platforms (so-called Safe Strain Lineages) for expression of new traits by genetic engineering (see below), such as Bacillus subtilis (US FDA Citation2018a; Ladics and Sewalt Citation2018), B. licheniformis (US FDA Citation2017; Sewalt, Reyes, and Bui Citation2018), and Trichoderma reesei (US FDA Citation2018b).
Genetic engineering/bioengineering/genome editing
Today, the primary mechanism for creating GEMs to produce food substances is through in vitro nucleic acid techniques, including the insertion of genes via recombinant DNA or related techniques into a selected, robust and safe microorganism that impart enhanced or new functionality to that organism. The resulting microorganism is said to be genetically engineered, a term differentiated from ‘genetic modification’ by the United States National Research Council (National Research Council of the United States Citation2004), and adopted in the vocabulary of various United States and Canadian regulatory guidance documents.
With genetic engineering, scientists begin by selecting or developing a robust, productive host microorganism that is known to be safe (not pathogenic and does not produce toxins). Once a suitable expression host has been selected/developed, molecular techniques are used to insert or delete one or more DNA sequence(s) into the genome of the microorganism that enhance existing or impart new functionality to that organism. Examples of the kinds of new or enhanced functionality include: production of an enzyme or other functional protein that is meant to be harvested from the microorganism for use in food production (e.g. α-amylase, lipase, protease, ice structure protein, leghemoglobin), production of enzymes that help the microorganism itself produce another food ingredient (e.g. riboflavin, steviol glycoside, or oligosaccharide), or additional modifications such as the deletion of endogenous genes or insertion of transporters aimed at making the microorganism a more effective production platform. Included with the DNA sequences that enable the expression of new or enhanced functionality are sequences that encode elements (e.g. promoter and terminator sequences) that help control the expression of functional genes in the microorganism. Although DNA sequences may be randomly inserted into the genome of the microorganism, often these sequences are intentionally inserted into specific points (called ‘loci’) of the microorganisms’ genome. Regardless, the insertion site can later be confirmed through sequencing of the entire genome.
The source of the DNA that is expressed by the genetically engineered microorganism can be endogenous, from the same organism (e.g., to produce more of an existing enzyme), or exogenous, from a different organism. When the DNA is sourced exogenously from a closely related microorganism capable of natural DNA exchange (often within the same genus). this process is also referred to as ‘self-cloning’ by some regulatory frameworks. However, the source of the exogenous DNA is frequently another, more distant microorganism that cannot be grown efficiently under industrial conditions. It is through the creation of the new, bioengineered organism where the sum of the inserted sequences reaches its full potential to become a microorganism capable of producing the desired food substances at a commercial scale. As such, the term ‘bioengineering’ is a more narrowly defined term to indicate genetic engineering or genome editing steps that result in a modified organism that does not exist in nature or could not have been produced by traditional breeding and selection. When applied to food, the term ‘bioengineered’ is a regulatory term that determines consumer labeling requirements.
Whereas initially DNA was isolated from one microorganism, then amplified and transferred into another microorganism, today, synthetic DNA sequences created through other molecular biology techniques can be inserted into microorganisms to impart functionality. The use of synthetic DNA (rather than DNA physically isolated from another microorganism) allows for very fast development of genetically engineered microbes producing many variant enzyme proteins that can be tested in the target application. Moreover, the use of synthetic DNA completely avoids the inadvertent introduction of unintentional sequences such as DNA cloning remnants including even pathogens. The use of synthetic DNA allows molecular biologists to limit the transfer to just the beneficial gene sequence of interest, without ever being in contact with the pathogen and avoiding the transfer of sequences that could encode for pathogenicity. The safety considerations of one such example of a sequence originating from a potential pathogen are detailed by Sewalt, Reyes, and Bui (Citation2018) for an α-amylase sequence from Cytophaga sp., safely expressed in Bacillus licheniformis, and notified to FDA for use in carbohydrate processing (US FDA Citation2017).
Protein engineering
Protein engineering is often applied to optimize functionality of enzymes (or other proteins). This may target increased catalytic activity, but more often is employed to tailor the enzyme to function more effectively under the application conditions that may involve temperature, pH, or salt concentrations well outside the optimum range for the enzyme. For example, baking amylases can be engineered to withstand the high oven temperature longer, such that the same number of catalytic reactions can be achieved with a lower initial enzyme concentration before the enzyme is inactivated in the baking process.
Starting from an endogenous, wild-type enzyme, effective protein engineering often involves the generation of multiple variants by genetic engineering of the production organism (especially when expression levels need to increase as well) or, alternatively, gene editing (to merely test the impact of specific amino acid changes of an endogenous protein). These multiple variants are then tested in the application to produce multiple ‘hits’ with improved characteristics, sometimes followed by combining those hits in successive generations to maximize the improvement. A final selected variant enzyme protein may differ from the wild-type sequence in one amino acid or multiple amino acids.
Gene and genome editing
Today, CRISPR is the most common technique used for gene editing. CRISPR is a revolutionary technology that facilitates precise cutting and pasting of DNA by specialized proteins—inspired by nature, engineered by researchers. Gene editing proteins come in three varieties: zinc fingers, TALENs, and CRISPR-Cas. CRISPR-Cas has an elegant design and simple cell delivery and is now being used to treat genetic diseases, grow climate-resilient crops, and develop designer materials, foods, and drugs. CRISPR stands for Clustered Regularly Interspaced Palindromic Repeats, chunks of regularly recurring bits of DNA that arose in certain bacteria as an ancient defense system against viral invasions (Barrangou et al. Citation2007). CRISPR-Cas is a complex of enzymes (Cas proteins) and genetic guides (CRISPR sequences) that together finds and edits DNA.
Scientists have harnessed this powerful CRISPR-Cas system by designing guide RNA sequences that can recognize specific DNA code in any living cell, representing a genetic defect or an undesirable trait, and excise one or more base pairs from that DNA code. Replacement nucleic acids can be inserted into the exact spot where a target sequence was interrupted to repair the DNA, modifying the sequence or introducing a new beneficial gene, instead of merely knocking out the endogenous gene function. The biotechnology application in microbes was first described in 2012 by researchers at the Howard Hughes Medical Institute (Jinek et al. Citation2012). This manner of gene function deletion, restoration, or modification is called gene editing. The introduction of new DNA sequences into the genome by CRISPR-Cas is called genome editing. For an outline of different techniques and a comparison of their outcomes, see .
Labeling of GEM-produced food substances as “GMO”Footnote1
In addition to establishing criteria by which GEM-produced food substances are evaluated for safety, which will be described later in this paper, regulatory agencies also establish the regulations that determine whether food substances, or food products that contain those food substances, require disclosure as “GMO” (or in the United States, Bioengineered or BE). Interpretation of the regulatory requirements for labeling of GEM-produced food substances can appear daunting, especially if individuals are only familiar with how the regulations are applied to agricultural products or products derived from agricultural commodities. However, under most regulatory frameworks GEM-produced food substances are not considered “GMO” for the purpose of GMO labeling, through one or more of the following conditions:
Food substances or food “produced with” a GEM do not need to be labeled “GMO”
Food substances or food with no detectable DNA do not need to be labeled “GMO”
Food substances produced with certain genetic modification techniques do not need to be labeled “GMO” if the genetic modification occurs in nature or could otherwise be obtained through traditional breeding
Foods produced with GEM-derived processing aids/incidental additives do not need to be labeled “GMO”. In some jurisdictions, this may additionally require no detectable rDNA in the final food.
Under these regulatory frameworks, if the GEM-produced food substances meet one of these conditions, they (and finished foods that contain these substances) are not considered “GMO”. Below these conditions are discussed in more detail, along with an overview of whether several regulatory frameworks apply these conditions (). The situation in the EU is particularly complicated given a multitude of food regulations and GM regulations ().
Figure 2. EU Food and GM Regulations (December 2019 status). It is of note that the FIAP and Novel Foods regulations are mutually exclusive from the GM Food and Feed Regulation, even though GMMs may be used to produce enzymes or novel foods. Any approved food enzyme or novel food produced with a GMM is not a GM food. Conversely, any GM food cannot be a novel food and should be evaluated under the GM Food & Feed regulation.
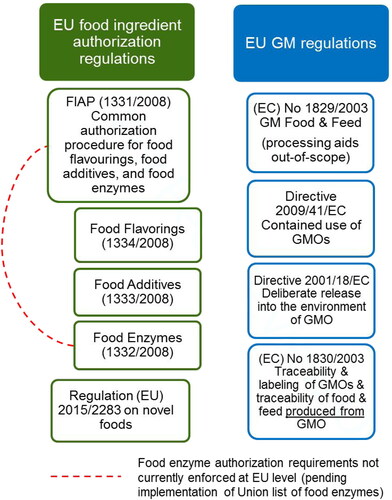
Table 2. Conditions that determine GMO labeling status for GEM-produced food substances.
Food substances or food “produced with” a GEM do not need to be labeled GMO
Many regulations make a distinction between food substances that are “derived from” or “produced from” a genetically engineered source, such as corn sugar derived from genetically engineered corn kernels, and food substances “produced with” a GMO, which includes food substances produced with (short for: produced with the help of) a GEM. Under these regulations, such as those in the European Union (Regulation (EC) No 1830/2003), the United States (7 CFR 66), and Canada (CAN/CGSB-32.315-2004), food substances “produced with” a GEM (the GEM is essentially a processing aid or incidental additive) do not require to be labeled as GMOFootnote2, while food substances “derived from/produced from” a GEM (the GEM and/or its genetic material remain present in the food substance) or other genetically engineered source must be labeled as GMO () unless they qualify for another exemption (such as processing aid status for enzymes in most jurisdictions or the exemption for highly refined ingredients in the United States National Bioengineered Food Disclosure regulation).
Food substances with no detectable DNA do not need to be labeled GMO
Several countries have established that food substances labeled GMO must be clearly differentiated from those not labeled GMO through the detection of DNA from the GMO source. Thus, if appropriate analytical methods cannot detect any DNA from the GEM in the food substance, it would not need to be labeled as GMO. As described in the previous section, GEM-produced food substances undergo significant refinement/purification steps following fermentation with the GEM. In most cases, these steps remove all traces of DNA from the GEM in the finished food substance. Therefore, under these regulatory frameworks, such as the recently finalized United States National Bioengineered Food Disclosure Standard (7 CFR 66) as well as under Regulation (EC) No 1830/2003 in the European Union, GEM-produced food substances would not meet this requirement to be labeled as GMO.
Food substances produced with certain genetic modification techniques do not need to be labeled GMO
The previous section discussed several techniques that can be used to modify the genetic makeup of a microorganism that is used to produce a food substance. Regulations often define which techniques are considered genetic engineering, and thus would require labeling as GMO. However, under some regulatory frameworks, not all the methods described above would be required to be labeled as GMO. Inconsistencies in these definitions between regulatory frameworks creates a complicated global marketplace where food substances that are required to be labeled GMO in one jurisdiction may be exempt from GMO labeling in another jurisdiction.
For example, while the use of CRISPR for gene editing is regarded to be outside the scope of definitions for Genetic Engineering or GMO labeling in the United States and Japan (as long as the modification occurs in nature or could otherwise be achieved by traditional breeding), a 2018 decision of the Court of Justice of the European Union ruled that any organism made with in vitro mutagenesis (such as CRISPR-Cas) was within the definition of GMO (Callaway Citation2018). There are efforts by the scientific community to drive a reconsideration of the decision in the European Union, which are seen as outdated in light of the nature of gene editing techniques (Urnov, Ronald, and Carroll Citation2018).
As another example, self-cloning refers to a process where in vitro techniques are used to over-express an endogenous gene or to express a gene from one microbial species into a strain from a related species with which it naturally exchanges genetic material. Closely related microbial species are known to exchange genetic material in a similar manner, usually within the same genus but sometimes even between species outside the genus. GEMs that are produced using in vitro techniques that qualify as “self-cloning” are not considered GMO for the purpose of consumer disclosure in several countries including the United States, Japan, and Australia.
Protein engineering is also subject to different regulatory interpretations regarding whether resulting food substances such as enzymes would be considered GMO. With this technique, some countries have established criteria to determine whether the resulting food substance is considered GMO based on the extent of protein engineering. For example, Australia considers the alteration of an endogenous protein by one amino acid to result in a novel protein, which triggers GMO labeling of the resulting food substance. Other jurisdictions may still consider a protein-engineered enzyme to be outside of the definition of GMO if the new sequence does not resemble that of another species more than the original, source organism.
As more techniques are developed, and if regulations are not updated on a timely basis and in a consistent manner to address these new methods, there is a risk that there will be additional inconsistencies between the GMO-labeling regulations, globally.
Food substances used as processing aids to produce foods do not need to be labeled GMO and neither does the final consumer food
This general principle applies to most major jurisdictions including the EU (), consistent with general food labeling requirements under which processing aids are not listed in the ingredient statement on consumer food labels. Hence, most of the other considerations regarding triggers for GMO labeling apply only to food substances not used as processing aids.
Other GMO labeling considerations
It is critical to highlight that under these regulatory frameworks, a food substance produced with a GEM typically does not require GMO labeling if it meets just one of the conditions described above. In practice, most GEM-produced food substances meet more than one of these conditions and therefore have multiple reasons why they do not require GMO labeling. Interestingly, foods produced via non-targeted mutagenesis techniques do not get labeled as GMO under any of these regulations, although they are genetically modified, and often resulting in a large phenotypic change. This applies to both conventional foods modified using non-targeted mutagenesis, such as ruby red grapefruit (Da Graca, Louzada, & Sauls, Citation2004), and foods produced with microorganisms modified through non-targeted mutagenesis, such as docosahexaenoic acid (DHA) algal oil (Fu et al. Citation2016).
It is important to note that most regulatory frameworks (Canada being a notable exception) define criteria by which a food must be labeled as containing GMOs, but not which foods would be considered non-GMO. While there is good regulatory standing for assuming that foods that do not require labeling as “contains GMOs” would necessarily be considered as “non-GMO”, this lack of explicit guidance has led, in several markets, to the creation of voluntary non-regulatory GMO labeling frameworks. These independent frameworks are often established by third-party certifying bodies, which then use their own criteria to certify food products as “non-GMO”. Third-party certifying bodies also apply restrictions to the use of genetic engineering when they qualify foods as ‘organic’. Hence, food substances either derived from or produced with GEMs are not currently compatible with organic food production, as genetic engineering is considered an excluded method as a synthetic process.
While these independent frameworks can align closely with the regulatory frameworks, often the criteria developed by these agencies deviate in subtle ways from both the regulatory criteria as well as from each other. These deviations in the criteria for evaluating whether a food meets a definition of non-GMO leads to a complicated environment where a food product could be considered GMO under one framework and non-GMO under another. This is made even more complex by the fact that independent frameworks developed by private companies evolve much more rapidly than the legislative frameworks implemented by regulations.
How food ingredients or food enzymes produced using GEMs are evaluated is an area where discrepancies can be seen between these independent frameworks and established regulations. As more food production moves toward these sustainable, cost-effective methods for food production, it will be curious to see how these independent frameworks (as well as organic regulations) adapt to consider the benefits of GEM production (such as sustainability and food access), evolving definitions of what is GMO (e.g. gene editing and chemical mutagenesis), and more global alignment of regulatory definitions of GMO.
Safety evaluation and authorization of GEMs and GEM-produced food substances
Authorization of GEM-produced food substances
Food manufacturers must meet the regulatory requirements of each country prior to placing a new food substance on the market. The regulatory frameworks established by different countries in general have similar expectations for novel food ingredients, even if the specifics of regulations differ. For example, while the processes established by the United States and European Union have many contrasts, the definition of a novel food substance is very much aligned, as are the basic principles of safety evaluation.
The regulatory frameworks established in both the United States and European Union both consider many foods produced with GEMs as novel foods. This categorization applies to both when the food substance itself had not previously been on the market (e.g. a novel oligosaccharide that had not previously been included in a particular food type), and when the food substance itself has been on the market previously but for which production by a GEM represents a novel production process (e.g. a food substance produced by extraction from an agricultural source that is currently on market has an advancement in technology that allows it to be produced by a new, more efficient process).
In some cases, the introduction of a GEM into the food production process may not result in significant changes in the composition or structure of a food substance, and thus may not be interpreted as novel under these frameworks. However, food manufacturers may still choose to have their food substances evaluated through these regulatory processes as a way of gaining an independent verification of their determination that the use of a GEM is considered safe and meets all regulatory requirements.
Under the regulatory frameworks of the European Union and the United States, even in cases where a substance is considered “novel” because of a novel production process, the conclusion of the evaluation of the novel food substance is focused on the food substance itself, rather than the production process. However, the evaluation of any novel food substance does include an assessment of the entire manufacturing process, including the production organism, fermentation media, equipment, filters, processing aids, and any formulation ingredients. The conclusion of the evaluation of a novel food is published to the Novel Food Union List in the European Union (European Commission, Citation2017), and in the United States most commonly to the list of substances notified to FDA as Generally Recognized As Safe (US FDA Citation2019).
It is important to note that in both the European Union and the United States, both the food substance and the production organism are identified in the listed approval for the food substance, but not all processing aids, equipment and other materials that are evaluated as part of the process. Thus, just as there is no established list of filters that have been evaluated during review of novel food substances, there is also no established positive list of GEMs used in food production that have been reviewed in either the European Union or United States.
This is an efficient way to manage this information, since in most cases a unique GEM is used to produce a single unique food substance (1:1 alignment). Thus, creation of a list of GEMs would be a redundant effort, and likely one that would be a much larger effort to catalog all potential materials that could be used to produce foods (including other processing aids, filters, equipment, and starting materials). It is critical for these materials to be reviewed during the evaluation of novel food substances, however, the control of many of these materials (such as filters) are also covered by regulations that require that food manufacturers ensure the safety of products through Good Manufacturing Processes that enforce that only food grade or equivalent quality materials are used in production of food substances.
Several evaluations have been finalized in the European Union and United States for GEM-produced novel foods, including: steviol glycosides, oligosaccharides, and a structural protein, yet there are other food ingredients produced with genetically modified microbes (modified by either non-targeted mutagenesis or genetic engineering) that do not appear on these lists. This includes vitamins such as riboflavin (Schwechheimer et al. Citation2016) and other B vitamins (Acevedo-Rocha et al. Citation2019; Sych, Lacroix, and Stevens Citation2016), amino acids such as methionine (Willke Citation2014), and other food additives such as citric acid (Max et al. Citation2010). Most of these food substances have been part of the food supply for over a decade, and production by a GEM would only represent a novel production process for an otherwise identical molecule.
While food enzymes are often evaluated under the same regulatory frameworks as other food substances utilizing the same food risk assessment principles, there are several unique factors for food enzymes that provide an interesting contrast to other GEM-produced food substances (). Under a new European Union regulatory framework, the Food Improvement Agents Package or FIAP (European Commission Citation2012; European Commission Citation2011), food enzymes (including those produced with GEMs) must undergo an authorization that is separate from the novel food authorization process. However, while this separate regulatory process for food enzymes is being established, the safety evaluation of GEMs used to produce food enzymes relies on the same European Food Safety Authority (EFSA) risk assessment guidance. Until the European Union food enzyme list is established in the next several years, member country national legislations remain in force, which involves mandatory approval processes in France and Denmark.
Table 3. Comparison of food enzymes and food ingredients produced with GEMs.
In the United States, food enzymes (including those produced with GEMs) are typically reviewed through the same Generally Recognized As Safe (GRAS) process used to review novel food ingredients (Hanlon, Frestedt, and Magurany Citation2017). As in the European Union, the review of GEMs used to produce food enzymes uses the same principles to evaluate safety as those used to produce other food substances. It is of further note that the early safety evaluation guidelines for enzymes produced with modern biotechnology were developed in the late 1990s and formally updated to reflect industry practices (Pariza and Johnson Citation2001). The safety evaluation of GEM-produced food substances is described in more detail in the following section.
Safety evaluation of GEM-produced food substances
As discussed above, under most regulatory frameworks, the authorization of GEM-produced food substances is a single process that focuses on the final food substance. From the perspective of the safety assessment, the review is also focused on the final food substance while it considers all aspects of the ingredient including all parts of the manufacturing process. This includes an evaluation of the inherent safety of the GEM production organism, the degree to which the production organism is carried over into the finished ingredient, and any other potential impact the production organism could have on the finished ingredient.
The processes established in the European Union and United States for the safety assessment of GEM-produced food substances are representative of the processes established in many other countries, including Canada, Australia, and New Zealand. In the United States, the safety assessment is performed by the company according to the GRAS requirements, followed by an optional review by the FDA. In the European Union, the safety assessment is completed by EFSA based on a dossier also assembled by the applicant. While the conclusion of safety is published by EFSA or the FDA on the finished ingredient, the review encompasses the feedback from multiple expert groups that focus on specific aspects of the safety evaluation, including the safety of the GEM.
In the United States, the expert group at the FDA tasked with novel food review and GMO review is the Division of Biotechnology and GRAS Notice Review in the Office of Food Additive Safety (OFAS), in the Center for Food Safety and Applied Nutrition. This group within OFAS maintains lists of both novel foods that have been reviewed (the GRAS Notification Inventory), and GMO plants that have been reviewed (Consultations on Food from GE Plant Varieties). It should be noted that while the FDA does not maintain a list of the safety evaluations that have been completed on GEMs used in the production of food ingredients or food enzymes, that the safety evaluation of each microbial GRAS substance includes an assessment of the safety of the GEMs as part of the overall safety assessment.
Similarly, in the European Union EFSA houses both expert groups that would be involved in the scientific evaluation of GEM-produced food substances. Within EFSA, distinct panels have the primary responsibility for the safety review of novel foods and enzymes, respectively, and for publishing the safety opinion. In addition, for food substances produced by GEMs, the EFSA Panel on Genetically Modified Organisms is responsible for providing input into the safety of the GEM itself.
While the underlying evaluation of a GEM used to produce a food ingredient or food enzyme is the same, the frameworks implemented in the European Union and United States differentiate the evaluation of finished GEM-produced food substances based on the extent that the GEM is carried over into the finished food substances. The primary difference between GEM-produced food enzymes and other GEM-produced food substances is the presence of organic solids other than the target molecule in the enzyme preparation. This is often referred to as Total Organic Solids (TOS), which includes the enzyme protein as well as fermentation solubles and other metabolites from the microorganism, such as amino acids, vitamins, organic acids, etc.
This distinction between GEM-produced enzymes and other food substances is what historically sorted these substances into different EFSA categories for evaluations. The EFSA guidance on risk assessment of GEMs (EFSA Citation2011b) classified enzymes into Category 2 “Complex products in which both GEMs and newly introduced genes are no longer present (e.g. cell extracts, most enzyme preparations)”, and other GEM-produced food substances into Category 1 “Chemically defined purified compounds and their mixtures in which both GEMs and newly introduced genes have been removed (e.g. amino acids, vitamins)”. Although these categories may still exist, a more holistic view across both genetically engineered and classical strains was recently taken by the EFSA panel on Food Contact Materials, Enzymes and Processing Aids (CEP) in its guidance for the characterization of microorganisms used for the production of food enzymes (EFSA Citation2019). While EFSA also defines two additional categories of food substances that use GEMs in production, those categories are outside of the scope of this paper (e.g. microorganisms that are carried over intact into the final food product, like yoghurt).
Under these EFSA guidance documents, manufacturers of GEM-produced food enzymes and other food substances are required to provide detailed information about the GEM itself including information about the parental organism, the inserted sequences, the method of genetic modification, along with the other information that is required to be provided with all applications for food enzymes and novel foods, such as information on the manufacturing process and specifications for the finished food substance.
Regardless of the categorization of the food substance, the key component in the evaluation of GEMs used to produce either food ingredients or food enzymes is the safety assessment of the production strain, and an evaluation of its pathogenic and toxigenic potential. ‘Pathogenic’ refers to the ability of a microorganism to produce an infection (e.g. pathogenic E. coli and Salmonella sp.), whereas ‘toxigenic’ refers to the ability of a microorganism to produce toxins that would harm someone consuming them (e.g. botulinum toxin and Staphylococcus aureus).
In the United States, similar guidance, that includes a decision tree, has been published for enzymes produced with GEMs (Pariza and Johnson Citation2001) and these guidelines have also been used to evaluate the safety of GEMs used to produce food ingredients. The number of unique food enzymes produced with GEMs far exceeds the number of unique food ingredients produced by GEMs, and therefore represents a robust dataset that demonstrates the effectiveness of this guidance on accurately determining the safety of GEM-produced food substances (Sewalt et al. Citation2016). This methodology, which has been broadly adopted by the enzyme industry, provides a framework for the FDA and other regulators in their assessment, and serves as an example for other food ingredient categories (Sewalt Citation2017; Sewalt Citation2018). This topic was recently discussed more extensively (e.g., Ladics and Sewalt Citation2018; Sewalt, Reyes, and Bui Citation2018a,Sewalt et al. Citation2018b; Ladics et al. Citation2020; JECFA Citation2019), including the concept of so-called ‘Safe Strain Lineages’ as a tool to streamline safety evaluation of microbial enzymes. The decision tree’s suitability for evaluating GEM-produced food ingredients other than enzymes stems from its adaptiveness to novel substances (i.e., substances without a history of safe use) and novel production strains (each of which might trigger the need for new toxicology data) and to use of ingredients at higher concentrations than those typical for enzymes.
Importantly, the scientific risk assessments required by European and United States agencies for GEM-produced food substances differ significantly from the risk assessments conducted for genetically engineered plants. In the European Union, EFSA has issued a completely separate guidance document for the risk assessment of food and feed from genetically modified plants (EFSA Citation2011a). The US FDA also provides guidance on the types of information that are required to evaluate the safety of foods derived from genetically modified plants (US FDA Citation1997). It is critical to differentiate the safety evaluation of GEM-derived food substances, which are produced within contained systems, as opposed to plants which have many additional considerations because of their potential interaction with the environment.
As GEMs become an even more integral part of sustainable food practices, along with the increasing globalization of the food supply, it is critical for global regulatory agencies to seek aligned approaches to the safety evaluation and categorization of these food substances and the production organisms used to produced them. In that light, initial efforts by ASTM International (formerly known as the American Society for Testing and Materials, a developer of international voluntary consensus standards) to classify industrial microbes in Standard 3214-19 (ASTM Citation2019) based on genotype, biosafety, use, and available sequence information, rather than the specific modification method, are noteworthy. The ASTM classification elaborates four genotype classes, ranging from (A) microbes without any intentional alteration, (B) microorganisms subjected to deliberate genetic alteration without introducing any non-native DNA (i.e., inclusive of both self-cloned and mutant organisms), and (C) microbial strains altered with addition of non-native DNA to (D) novel microbes that contain or produce chemical substances not previously observed in nature (e.g., non-natural amino acids or novel substances produced through engineered biochemical pathways). In addition to genotype class, three additional classification fields represent biosafety risk grouping, mode/intent of use (contained vs open release), and the extent of available genome sequence information. Initiatives such as the ASTM classification are intended to serve as a science-based inspiration to a broad swath of stakeholders toward a more refined, risk-focused regulatory classification of GEMs beyond a simplistic “GMO” designation.
Conclusions
Food that includes substances produced with GEMs are becoming an integral part of the food supply. As a result, regulatory agencies are evolving to more efficiently assess these foods. While there is general alignment between the major regulatory agencies on how to assess safety, their stance on GMO labeling is less consistent, and confusing to consumers especially in the context of independent, non-regulatory bodies that have created their own definitions of GMO. Consumer knowledge on the technical topic of GMOs in general is limited and is likely to be even more limited in regard to GEMs. Therefore, the topic of GEM-produced food substances has the potential to amplify consumer confusion on GMO labels. There are many benefits of GEMs in the production of food from cost and resource perspectives, and as consumers integrate sustainability into their food choices this could also lead to inconsistencies between the objective of GMO labeling and the intent of consumers for making more sustainable food choices. We need to ensure safety, and we may need to modify how we look at the safety of common food products made via this novel manufacturing process, just like how we evaluate the complex processes used to manufacture other ingredients (filtering, grinding, extracting, purifying, concentrating, enriching). With the increasing globalization of the food supply, as GEMs become more important for sustainable food practices it is critical for regulatory agencies around the globe to have aligned approaches to the safety evaluation and categorization of these food substances to avoid unnecessary trade barriers that could arise through inconsistencies in global regulations.
Acknowledgments
Thanks are due to Azmy Azmy, Kees Broekhuizen, Greg Ladics, Paul Tenning, and Fred Wondergem for critically reading and providing suggestions to improve this review paper.
Disclosure statement
Paul Hanlon is a full-time employee of Abbott Nutrition. Vincent Sewalt is a full-time employee of DuPont Nutrition & Biosciences.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Notes
1 The abbreviation GMO (Genetically Modified Organism) will be used in this paper in relation to consumer transparency, acknowledging that consumers are often most familiar with the term GMO. For the purposes of brevity, when referring to consumer product labelling this paper will use only the term ‘GMO’ to represent all requirements, even in cases like the United States where a different term (Bioengineered or BE) has been established as the regulatory term.
2 Organisms altered with recombinant DNA are referred to as ‘genetically modified’ (GM) in Europe and as ‘genetically engineered’ (GE) in Canada and the US.
References
- Acevedo-Rocha, C. G., L. S. Gronenberg, M. Mack, F. M. Commichau, and H. J. Genee. 2019. Microbial cell factories for the sustainable manufacturing of B vitamins. Current Opinion in Biotechnology 56:18–29. doi: https://doi.org/10.1016/j.copbio.2018.07.006.
- Adrio, J. L., and A. L. Demain. 2010. Recombinant organisms for production of industrial products. Bioengineered Bugs 1 (2):116–31. doi: https://doi.org/10.4161/bbug.1.2.10484.
- ASTM. 2019. E3214-19, Standard classification for industrial microorganisms. West Conshohocken, PA: ASTM International.
- Barnett, J. A. 2003. Beginnings of microbiology and biochemistry: The contribution of yeast research. Microbiology 149 (3):557–67. doi: https://doi.org/10.1099/mic.0.26089-0.
- Barrangou, R., C. Fremaux, H. Deveau, M. Richards, P. Boyaval, S. Moineau, D. A. Romero, and P. Horvath. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315 (5819):1709–12. doi: https://doi.org/10.1126/science.1138140.
- Brochado, A. R., C. Matos, B. L. Møller, J. Hansen, U. H. Mortensen, and K. R. Patil. 2010. Improved vanillin production in baker’s yeast through in silico design. Microbial Cell Factories 9 (1):84. doi: https://doi.org/10.1186/1475-2859-9-84.
- Callaway, E. 2018. CRISPR plants now subject to tough GM laws in European Union. Nature 560 (7716):16. doi: https://doi.org/10.1038/d41586-018-05814-6.
- Da Graca, J., E. Louzada, and J. W. Sauls. 2004. The origins of red pigmented grapefruits and the development of new varieties. Proceedings of the International Society of Citriculture 1:369–74.
- European Commission. 2017. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union List of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Official Journal of the European Union.
- European Commission. 2012. Commission Implementing Regulation (EU) No 562/2012 of 27 June 2012 amending Commission Regulation (EU) No 234/2011 with regard to specific data required for risk assessment of food enzymes. Official Journal of the European Union.
- European Commission. 2011. Commission Regulation (EU) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council establishing a common authorisation procedure for food additives, food enzymes and food flavourings. Official Journal of the European Union.
- EFSA. 2011a. Guidance on the risk assessment of food and feed from genetically modified plants. EFSA Journal 9 (5):2150.
- EFSA. 2011b. Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA Journal 9 (6):2193.
- EFSA. 2019. Characterisation of microorganisms used for the production of food enzymes. EFSA Journal 17 (6):5741.
- Fu, W., A. Chaiboonchoe, B. Khraiwesh, D. R. Nelson, D. Al-Khairy, A. Mystikou, A. Alzahmi, and K. Salehi-Ashtiani. 2016. Algal cell factories: Approaches, applications, and potentials. Marine Drugs 14 (12):225.
- Hanlon, P. R., J. Frestedt, and K. Magurany. 2017. GRAS from the ground up: Review of the Interim Pilot Program for GRAS notification. Food and Chemical Toxicology 105:140–150. doi: https://doi.org/10.1016/j.fct.2017.03.064.
- Jay, J. M., M. J. Loessner, and D. A. Golden. 2005. Modern food microbiology (7th ed.). New York: Springer.
- JECFA. 2006. General specifications and considerations for enzyme preparations used in food processing. Accessed November 29, 2019. http://www.fao.org/3/a0691e/A0691E03.htm
- JECFA. 2019. Expert meeting of Working Group established to consider the evaluation of enzyme preparations used in the manufacture of foods – Draft report. Accessed December 9, 2019. https://www.who.int/docs/default-source/food-safety/final-enzyme-report-1-3-f-draft-nov-2019e4cf41981c82443bb2c5496a9adda8c4.pdf?sfvrsn=8651b8a5_4
- Jinek, M., K. Chylinski, I. Fonfara, M. Hauer, J. A. Doudna, and E. Charpentier. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 (6096):816–821. doi: https://doi.org/10.1126/science.1225829.
- Ladics, G., L. Fan, V. J. Sewalt, and A. Spok. 2020. Safety regulations of food enzymes. In Enzymes, ed. M. Eskin. New York, NY: Elsevier. (In press).
- Ladics, G. S., and V. Sewalt. 2018. Industrial microbial enzyme safety: What does the weight-of-evidence indicate? Regulatory Toxicology and Pharmacology 98:151–154. doi: https://doi.org/10.1016/j.yrtph.2018.07.016.
- Max, B., J. M. Salgado, N. Rodriguez, S. Cortes, A. Converti, and J. M. Dominguez. 2010. Biotechnological production of citric acid. Brazilian Journal of Microbiology 41 (4):862–875. doi: https://doi.org/10.1590/S1517-83822010000400005.
- National Research Council of the United States. 2004. Safety of genetically engineered foods: Approaches to assessing unintended health effects. Washington, DC: The National Academies Press.
- Olempska-Beer, Z. S., R. I. Merker, M. D. Ditto, and M. J. DiNovi. 2006. Food-processing enzymes from recombinant microorganisms–A review. Regulatory Toxicology and Pharmacology 45 (2):144–158. doi: https://doi.org/10.1016/j.yrtph.2006.05.001.
- Pariza, M. W., and E. A. Johnson. 2001. Evaluating the safety of microbial enzyme preparations used in food processing: Update for a new century. Regulatory Toxicology and Pharmacology 33 (2):173–186. doi: https://doi.org/10.1006/rtph.2001.1466.
- Philippe, R. N., M. De Mey, J. Anderson, and P. K. Ajikumar. 2014. Biotechnological production of natural zero-calorie sweeteners. Current Opinion in Biotechnology 26:155–161. doi: https://doi.org/10.1016/j.copbio.2014.01.004.
- Schwechheimer, S. K., E. Y. Park, J. L. Revuelta, J. Becker, and C. Wittmann. 2016. Biotechnology of riboflavin. Applied Microbiology and Biotechnology 100 (5):2107–2119. doi: https://doi.org/10.1007/s00253-015-7256-z.
- Sewalt, V., D. Shanahan, L. Gregg, J. La Marta, and R. Carrillo. 2016. The Generally Recognized as Safe (GRAS) process for industrial microbial enzymes. Industrial Biotechnology 12 (5):295–302. doi: https://doi.org/10.1089/ind.2016.0011.
- Sewalt, V. J. 2017. Performance enzymes for food ingredients at the BIO world congress-enabling biotechnologies and supporting capabilities join in a model for successful commercialization of food ingredients. Industrial Biotechnology, 13 (5):219–220. doi: https://doi.org/10.1089/ind.2017.29098.vjs.
- Sewalt, V. J. 2018. Use of standardized enzyme safety evaluation methodology in the GRAS process – a model for other food ingredients and jurisdictions. 8th International Conference on Food Safety and Regulatory Measures. June 11-12, 2018, Barcelona, Spain. Journal of Food Microbiology, Safety & Hygiene (3):45. doi: https://doi.org/10.4172/2476-2059-C1-008.
- Sewalt, V. J., T. F. Reyes, and Q. Bui. 2018. Safety evaluation of two alpha-amylase enzyme preparations derived from Bacillus licheniformis expressing an alpha-amylase gene from Cytophaga species. Regulatory Toxicology and Pharmacology 98:140–150. doi: https://doi.org/10.1016/j.yrtph.2018.07.015.
- Sewalt, V., D. Shanahan, J. LaMarta, and L. Viebrock. 2018. GRAS conclusions: An uncompromising approach to facilitate market access for safe food ingredients from industrial biotechnology. Discussion Panel Organized by the Enzyme Technical Association (ETA) in Collaboration with the US Food and Drug Administration at the 2018 BIO World Congress, 19 July 16-Philadelphia.
- Sych, J., C. Lacroix, and M. Stevens. 2016. Vitamin B 12 - Physiology, production and application. In Industrial biotechnology of vitamins, biopigments, and antioxidants, 129–160. Hoboken, NJ: John Wiley & Sons, Inc. doi: https://doi.org/10.1002/9783527681754.ch6.
- Urnov, F. D., P. C. Ronald, and D. Carroll. 2018. A call for science-based review of the European court’s decision on gene-edited crops. Nature Biotechnology 36 (9):800–802. doi: https://doi.org/10.1038/nbt.4252.
- US FDA. 1997. Consultation procedures under FDA’s 1992 statement of policy for foods derived from new plant varieties. Accessed November 29, 2019. https://www.fda.gov/food/ingredients-additives-gras-packaging-guidance-documents-regulatory-information/consultation-procedures-under-fdas-1992-statement-policy-foods-derived-new-plant-varieties
- US FDA. 2017. GRN No. 664 Alpha-amylase from Cytophaga sp. expressed in Bacillus licheniformis. Accessed 29 November 2019. https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=664.
- US FDA. 2018a. GRN No. 714 Subtilisin from Bacillus amyloliquefaciens produced in Bacillus subtilis. Accessed November 29, 2019. https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=714
- US FDA. 2018b. GRN No. 727 Trehalase from Trichoderma reesei produced in Trichoderma reesei. Accessed November 29, 2019. https://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=727
- US FDA. 2019. GRAS notices inventory. Accessed November 29, 2019. http://www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices.
- Willke, T. 2014. Methionine production–A critical review. Applied Microbiology and Biotechnology 98 (24):9893–9914. doi: https://doi.org/10.1007/s00253-014-6156-y.
- Zhang, Y. P., J. Sun, and Y. Ma. 2017. Biomanufacturing: History and perspective. Journal of Industrial Microbiology & Biotechnology 44 (4-5):773–784. doi: https://doi.org/10.1007/s10295-016-1863-2.