Abstract
Human milk is the gold standard for newborn infants. Breast milk not only provides nutrients, it also contains bioactive components that guide the development of the infant’s intestinal immune system, which can have a lifelong effect. The bioactive molecules in breast milk regulate microbiota development, immune maturation and gut barrier function. Human milk oligosaccharides (hMOs) are the most abundant bioactive molecules in human milk and have multiple beneficial functions such as support of growth of beneficial bacteria, anti-pathogenic effects, immune modulating effects, and stimulation of intestine barrier functions. Here we critically review the current insight into the benefits of bioactive molecules in mother milk that contribute to neonatal development and focus on current knowledge of hMO-functions on microbiota and the gastrointestinal immune barrier. hMOs produced via genetically engineered microorganisms are now applied in infant formulas to mimic the nutritional composition of breast milk as closely as possible, and their prospects and scientific challenges are discussed in depth.
Introduction
It is widely accepted that breastfeeding is the gold standard for infant nutrition. It offers complete nutrition for the newborn as well as many bioactive components that contribute to healthy development of the newborn (Doare et al. Citation2018). These bioactive molecules can shape microbiota composition, modulate gastrointestinal physiology, promote proper development of the immune system, and enhance intestinal barrier function (Gila-Diaz et al. Citation2019). Many studies have focused on understanding the composition of mother milk and on identifying which factors contribute to healthy development of the child. This knowledge is essential for the development of effective infant formula that until several years ago was associated which higher frequencies of atopic allergies, a different microbiota composition, higher risks for infectious diseases, higher rates of obesity, and even higher frequency of diabetes when compared to children solely fed on mother milk (Binns, Lee, and Low Citation2016; Doare et al. Citation2018; Lodge et al. Citation2015). Effective formulations will have a profound impact on child health, as over 70% of all infants receive infant formula, and thereby depend on cow milk-based infant formulas for daily supply of nutrients and bioactive components (Victora et al. Citation2016). These cow milk-derived infant formulas do not have the same bioactive molecules as human milk (Aly et al. Citation2018).
The intestinal tract is the first organ that is in contact with bioactive molecules in mother milk. In the infant’s intestine the bioactive milk components impact the immature intestinal mucosa, which contains more than 80% of the body’s immune cells. The cells are matured by the bioactive factors facilitating the monitoring of luminal food components across the intestinal barrier and supporting regulation of defense-responses against undesired intruders. At the same time the maturation of immune cells supports regulatory responses to e.g. commensal bacteria that are needed for host survival (Lewis and Blutt Citation2019). The mucosal immunity is integrated into a physical barrier which is composed of a mucus layer and closely connected epithelial cells that protect the host from the harsh luminal content of the gut (Okumura and Takeda Citation2017). In newborn infants this barrier is more diffuse, and the maturation to an intact barrier is needed to prevent pathogens from entering the host (Torow et al. Citation2017). It is assumed that also closing the barrier is supported by bioactive components in mother milk (Cacho and Lawrence Citation2017). In addition, the bioactive molecules in mother milk support colonization of the more than 100 trillion microbiota in the small and large intestine that digest foods and produce fermentation products that are needed to support our metabolism, immunity and brain health (Figueroa-Lozano and de Vos Citation2019; McLeod et al. Citation2019).
There is increasing evidence that bioactive molecules in human milk have short- and long-term benefits in the developing intestinal system with impact on the development of the neonate as a whole (Lönnerdal Citation2014). These bioactive molecules involve a large group of compounds, such as growth factors (Vass et al. Citation2019), carbohydrates (Coppa et al. Citation1993; Nijman et al. Citation2018), cells (Cregan et al. Citation2007; Patki et al. Citation2010), cytokines (Meki et al. Citation2003) and immunoglobulins (Savel'ev et al. Citation2001). In order to gain insight into the function of these molecules in maintaining health or stimulating specific maturation processes, many studies have focused on comparing the composition of preterm and term delivered infants. Preterm infants are more prone to inflammatory diseases such as necrotizing enterocolitis, and by comparing preterm milk compositions with term milk compositions researchers try to identify essential elements in milk composition that might lead to novel formulations to prevent inflammatory events in preterm infants (Boyce et al. Citation2016; Cappelletti et al. Citation2016). More than that, over the course of lactation variations in the composition of mother milk has been reported, which is considered to be a response of the mother to the changing needs of the infant.
Not only identifying essential molecules for promoting health has been a major focus in infant formula research, finding cost effective means to include the molecules in infant formula has been a major effort as well, as most molecules are too complex to produce or are subject to many regulatory issues (Cicero, Fogacci, and Colletti Citation2017; Hill and Newburg Citation2015). Recently, major advances have been made with the inclusion of human milk oligosaccharides (hMOs) in infant formula (Vandenplas et al. Citation2018). hMOs are the most abundant bioactive molecules in human milk (Neu et al. Citation2019). Human milk contains more than 200 hMOs which are virtually absent in cow-milk based formulations (Bode Citation2012). Some hMOs that are abundantly present in mother milk can be produced via genetically engineered microorganisms and are now applied in infant formulas (Vandenplas et al. Citation2018). There is some evidence that this might lead to specific health benefits compared to traditional infant formulas that are supplemented with non-digestible carbohydrates (Puccio et al. Citation2017). Some hMOs have multiple functions which include support of growth of beneficial bacteria, anti-pathogenic effects, immune modulating effects and stimulating intestine barrier functions (Cheng et al. Citation2019; Kong et al. Citation2019; Triantis, Bode, and van Neerven Citation2018). Whether these effects can be achieved with the addition of single hMO instead of a mixture of hMOs is currently subject of study (Cheng et al. Citation2019; Kong et al. Citation2019; Salli et al. Citation2019).
In this review, we first briefly discuss the intestinal immunity in the small and large intestine, as most of the barrier immune effects of bioactive molecules start in the intestinal tract. Next, we critically review the current insight into the benefits of bioactive molecules in mother milk, including growth factors, antimicrobial proteins, cytokines, immunoglobulins, and cells in mother milk, that are all considered to contribute to neonatal development. The focus of the review will be on current knowledge of specific structure-activity relations of hMOs, as the first infant formulas with synthetic hMOs such as 2′-FL have reached the market. We discuss the impact they may have on microbiota and the gastrointestinal immune barrier. Finally, we discuss the currently applied hMOs in infant formula as well as their prospects and scientific challenges in the field of infant nutrition research.
The intestinal immune system
The intestinal mucosa of a healthy adult contains more than 80% of the body’s immune cells, which makes the gastrointestinal tract the largest immunologically active organ of the human body (Lewis and Blutt Citation2019). Its primary function is to protect the human body from undesired intruders from the lumen of the intestine while at the same time inducing tolerance to the 100 trillion bacteria that are needed for digestive processes and for production of beneficial fermentation products such as short chain fatty acids (SCFAs) (Figueroa-Lozano and de Vos Citation2019; McLeod et al. Citation2019). The mucosal immune system is integrated into a physical barrier which is composed of a mucus layer and closely connected epithelial cells that protect the host from the harsh luminal content of the gut (Okumura and Takeda Citation2017). In the small intestine, the mucosal immune system is highly organized, with fingerlike projections called villi, whose primary function is absorption of nutrients. It contains unique cells with specialized functions. At the base of the villi, in the crypts, the intestinal stem cells (ISCs) are located (Allaire et al. Citation2018), as well as the so-called Paneth cells which produce antimicrobial peptides and are responsible for maintaining a healthy balance in microbiota populations (Jansen Citation2019). Goblet cells are scattered in between the epithelial cells located at the villus and secrete gel-forming mucins (Knoop and Newberry Citation2018). Mucus is the principal barrier between the lumen and the underlying epithelial cells (Boedeker and Allen Citation2018).
The immune components of the gut-associated lymphoid tissue (GALT) are localized in microenvironments such as the Peyer’s patches (PPs), mesenteric lymph nodes (MLNs), and lamina propria (LP) (). The GALT is a secondary lymphoid organ that can be divided into an inductive site and an effector site (Brandtzaeg Citation2016). The induction site is compartmentalized into the small intestinal PPs and the MLNs, while the effector site is spread over the entire region of the intestinal LP (Brandtzaeg Citation2016). The small intestinal PPs are lymphoid tissues containing immune cells. The PPs are partly covered with specialized epithelial cells called microfold cells (M cells) that sample antigens from the lumen (Ahmad et al. Citation2017). The key function of the PPs is presenting antigens that are sampled by M cells or dendritic cells which protrude their dendrites into the lumen and regulate immune responses (Tezuka and Ohteki Citation2019). Due to this sampling and presentation function, the PPs are considered to be important for coordination of immune responses (Mowat and Agace Citation2014). Moreover, the PPs contain follicles where B cells are located. Those B cells secrete IgA which binds and neutralizes pathogens and undesired food antigens (Reboldi and Cyster Citation2016). The antigen presenting cells (APC) present in the PPs, such as CD103 positive dendritic cells, can migrate to the MLNs after uptake of antigens and activate naive lymphocytes (Tezuka and Ohteki Citation2019). Some of these cell populations are regulatory T cells (Treg), which induce tolerance to food antigens and microbiota, while more undesired antigens, such as those of pathogens, can induce formation of T helper (Th) cells (Vojdani Citation2015).
Figure 1. The intestinal immune barrier. (A) The small intestinal immune barrier. The mucus and the underlying closely connected epithelial cells form a barrier between the immune system and luminal content, which contains food and microbiota. Different cells contribute to the maintenance of barrier function and to balancing immune responses. These include intestinal epithelial cells, M cells, Paneth cells, stem cells and goblet cells. The immune components of the gut-associated lymphoid tissue are localized in Peyer’s patches, mesenteric lymph nodes, and lamina propria. (B) The large intestine. Colonocytes and goblet cells are the major cell types of the colonic crypt. Goblet cells produce mucus to form the outer and inner mucus layer which serves as protective barrier. The outer mucus layer harbors the commensal flora.
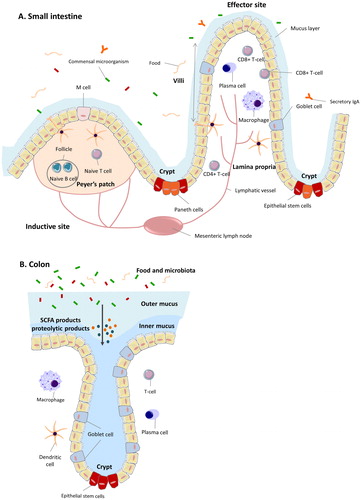
The large intestine is considered to be a less immunologically active site as compared to the small intestine. In the large intestine there is less direct contact between the luminal antigens and molecules and the mucosal immune system due to a firm mucus layer on top of the epithelial cells (). The large intestine has no villi, only crypts. The goblet cell is one of the major cell types of the colonic crypt, and is responsible for the secretion of gel forming mucins that provide protection against pathogenic intruders and supports growth of commensal bacteria (Allaire et al. Citation2018). Unlike the thinner mucus layer in the small intestine, that promotes nutrient absorption and antigen sampling, the large intestine has a two-layered mucus structure (Martens, Neumann, and Desai Citation2018). The colonic mucus is organized in an inner and an outer layer. The inner layer is firmly adherent to the epithelial cells while the thicker but looser outer layer on top of the inner layer harbors the commensal microflora (Johansson and Hansson Citation2016). Only at the top of the crypts, there is some contact between the mucosal immune cells from the colonic lamina propria and the luminal content (Allaire et al. Citation2018). It is still unclear whether this part of the colon is of significant importance for immune signaling and tolerance. However the colonic microbiota are producing large quantities of fermentation products, such as vitamins, SCFAs, and secondary bile acids, that can modulate the host’s metabolism and are essential for human health (Hillman et al. Citation2017). For example, butyrate, a major intestinal bacterial metabolite, is closely linked to promoting the generation of Treg cells that induce tolerance for food antigens in infants (Newton, Priyadharshini, and Turka Citation2016).
At early stages in life, the intestinal immune system is immature and it develops rapidly in the early postnatal period under the influence of bioactive components in mother milk, and by encountering new dietary components and microbiota (Macpherson, de Agüero, and Ganal-Vonarburg Citation2017; Toscano et al. Citation2017). Breast milk components such as growth factors, carbohydrates, cells, and cytokines facilitate and expedite the maturation process (Gila-Diaz et al. Citation2019; Parigi et al. Citation2015). The bioactive molecules in breast milk regulate the diversity of the microbiota that impact immune maturation (Castanys-Muñoz, Martin, and Vazquez Citation2016; Conlon and Bird Citation2014; Gensollen et al. Citation2016) but they may also directly support development by interacting with the immune system (Doare et al. Citation2018). In the next sections, the different bioactive molecules in mother milk that contribute to neonatal development are reviewed in view of its possible impact on the infant’s gastrointestinal immune system or gut barrier.
Bioactive components in human milk
It has become recognized in the past decade that human milk is more than a source of nutrients. It is a source of bioactive components as well, such as complex proteins, lipids, and carbohydrates, which impact the infant’s metabolism and immune system (Andreas, Kampmann, and Mehring Le-Doare Citation2015). Bioactive components are defined as “elements that show effects on biological processes or substrates and thereby have an influence on body function or condition and ultimately, health” (Schrezenmeir et al. Citation2000). Human milk contains several essential bioactive components that regulate these processes. Some bioactive molecules are produced and secreted by the mammary epithelium, some are produced by the cells in breast milk, while others are from maternal serum and transferred across the mammary epithelium by receptor-mediated transport (Vass et al. Citation2019). In response to the needs of the infant, the presence and absence as well as quantities of these bioactive components may vary in preterm and term delivery, or over the course of lactation.
Growth factors
Human milk contains a large number of growth factors which impact infant development and gastrointestinal maturation. Epidermal growth factor (EGF) is one of these factors. It is important for intestinal and mucosal maturation and for development of a well-established gut barrier function. EGF has been found in breast milk but also in amniotic fluid (Wagner, Taylor, and Johnson Citation2008). The concentration of EGF in breast milk decreases during lactation, from approximately 100 ng/mL in colostrum to 50 ng/mL in mature milk (Dvorak Citation2010). The concentration of EGF in milk of mothers with extremely preterm neonates (less than 28 weeks) is 50–80% higher compared to that in milk of mothers with full-term infants, which is directly correlated with an improved mucosal maturation in preterm infants (Dvorak Citation2010; Dvorak et al. Citation2003). Insulin-like growth factor-I (IGF-I), IGF-II, and IGF binding proteins (IGFBP) are found in human milk too, which may play a role in preventing oxidative stress-induced intestinal damage (Baregamian et al. Citation2006; Blum and Baumrucker Citation2002). The levels of IGF are highest in colostrum and decline over time during lactation, which ranges from 20–50 ng/mL in colostrum and decrease to around 4 ng/mL in mature milk (Hoeflich and Meyer Citation2017). In addition, high concentrations of vascular endothelial growth factor (VEGF), which is important for lymphatic vascular development (Secker and Harvey Citation2015), are also present in breast milk. The concentration of VEGF is highest in colostrum, reaching a concentration of approximately 50 ng/mL, and lower in mature milk at a concentration of 20 ng/mL (Loui et al. Citation2012; Siafakas et al. Citation1999). Erythropoietin (Epo) is present in human milk as well (Kling et al. Citation1998). The concentration of Epo in human milk increases in time during lactation. It is in the range of 4 to 5 mU/mL in the first 1 to 2 months and increases to 100 to 150 mU/mL by lactation (Semba and Juul Citation2002). It enhances intestinal tight junctions and thereby contributes to the development of barrier function and gastrointestinal development and function, additionally, it is associated with prevention of necrotizing enterocolitis (Shiou et al. Citation2011).
Cells
A variety of cells are found in human milk too, including leukocytes and stem cells, which are involved in immunological development and modulation (Witkowska-Zimny and Kaminska-El-Hassan Citation2017). In colostrum, more than 106 cells/mL are found, but the numbers drop to approximately 103 cells/mL in the weeks after birth (Molès et al. Citation2018). The predominant leukocytes in human colostrum are macrophages (40–50%), followed by polymorphonuclear neutrophils (40–50%), and lymphocytes (5–10%) (Hassiotou and Geddes Citation2015). T cells constitute the majority of lymphocytes (more than 80%) over B cells (Vieira Borba, Sharif, and Shoenfeld Citation2018). The immune cells in human milk protect against pathogens in the lumen during the development of the immune system of the newborn (Cacho and Lawrence Citation2017). In addition, stem cells have been found in mother milk (Cregan et al. Citation2007). Hosseini et al. confirmed the presence of stem cells in human milk and demonstrated that they differentiate into neural cell lineages and thereby contribute to gastrointestinal function and development (Hosseini et al. Citation2014). Recently, Briere et al. compared breast milk samples from mothers of preterm and term infants, and found no significant differences in the numbers of stem-like cells in these two groups, while the authors did find differences in gene expression. Cell markers SOX2, Nanog, CD90, and CD105 are up-regulated in the preterm milk samples, and EpCAM and TJP1 were down-regulated compared to full-term milk samples (Briere et al. Citation2017). This might indicate different functions of stem cells in preterm and term milk. However, overall it is still largely unknown to which processes stem cells in breast milk contribute (Ninkina et al. Citation2019).
Besides human cells, breast milk is also a source of commensal and beneficial bacteria, which shape the neonatal gut microbiome and contribute to enhanced immune defense and reduction of the incidence of infections (Jost et al. Citation2015; Martín et al. Citation2005). Some studies demonstrate that breast milk contains 103 to 104 CFU/mL bacteria. This is considerable when taking into account that infants by taking mother milk consume approximately 105 to 107 bacteria every day (Fernández et al. Citation2013; Jeurink et al. Citation2013). Up to now, more than 200 bacterial species have been isolated from human milk. Most of these bacteria belong to Staphylococcus, Streptococcus, Corynebacterium, Propionibacterium, and Lactobacillus and Bifidobacterium spp. (Collado et al. Citation2019; McGuire and McGuire Citation2017). Some studies have investigated the potential relationship between time postpartum and the composition of the human milk microbiome which resulted in different conclusions. Cabrera-Rubio et al. (Citation2012) found that in colostrum, the predominant bacteria are Weisella, Leuconostoc, Staphylococcus, Streptococcus, and Lactococcus, whereas in 1- and 6-months postpartum milk samples, the typical bacteria of the oral cavity, i.e. Veillonella, Leptotrichia, and Prevotella, are significantly increased. Khodayar-Pardo et al. (Citation2014) reported that Bifidobacterium spp. and Enterococcus spp. counts increased during lactation as well. However, Urbaniak et al. (Urbaniak et al. Citation2016) showed that time postpartum has no effects on milk microbial composition. In another study Boix-Amorós, Collado, and Mira (Citation2016) demonstrated that colostrum (5 days), transition (6–15 days), and mature (after 15 days) milk show different bacterial diversity, but no statistical significant differences were observed between time points for any bacterial genus. Therefore, based on current knowledge, it is hard if not impossible to draw conclusions on changes in microbiota composition in human milk during lactation.
Cytokines and chemokines
Cytokines and chemokines found in breast milk can pass the intestinal barrier, protect and contribute to immune development in the systemic circulation of the infant (Dawod and Marshall Citation2019). Cytokines in human milk have been shown to expedite immune maturation, to defend against infections, and to prevent inflammation (Brenmoehl et al. Citation2018). The most abundant cytokine in breast milk is Transforming Growth Factor-β (TGF-β) (Sitarik et al. Citation2017). TGF-β attenuates too strong immune responses and induces tolerogenic signals, which may be instrumental in preventing allergic diseases (Brenmoehl et al. Citation2018). However, the key anti-inflammatory and immunoregulatory cytokine interleukin-10 (IL-10), is found in very high concentrations during the first 80 hours of lactation (Garofalo et al. Citation1995). It has been suggested that IL-10 inhibits Th1 responses, contributes to the survival and expansion of B cells, and downregulates major histocompatibility complex-II (MHC-II) expression on monocytes (Dawod and Marshall Citation2019). Another regulatory cytokine found in breast milk is IL-7. This cytokine is involved in thymic development and supports T cell longevity, and thereby contributes to immunological development and adaptive immunity (Hossny et al. Citation2020). Also, proinflammatory cytokines are abundantly present in mother milk. TNF-α, IL-6, IL-8, and interferon-γ (IFN-γ) have all been found in human milk, and they generally decrease in concentration over the course of lactation (Meki et al. Citation2003). The function of the proinflammatory cytokines in human milk is still a subject of debate, but it probably contributes to intestinal development and maturation of immune cells in the infants gastrointestinal tract (Brenmoehl et al. Citation2018).
Immunoglobulins
Immunoglobulins (Ig) are present in relatively high concentrations during early lactation and belong to the most studied immune mediators in human milk (Cacho and Lawrence Citation2017; Field Citation2005; Turfkruyer and Verhasselt Citation2015). Infants are born with an immature adaptive immune system, and they need to rely on antibodies from mother milk for defense against infectious organisms (Andreas, Kampmann, and Mehring Le-Doare Citation2015). The most predominant form of immunoglobulin found in breast milk is secretory IgA (SIgA) (Lönnerdal Citation2016). SIgA is the primary protective agent in human milk. The SIgA antibodies in breast milk are specific for enteric and respiratory pathogens such as Vibrio cholerae, Escherichia coli, Giardia lamblia (Le Doare and Kampmann Citation2014). SIgA shows a high concentration of 12 mg/mL in colostrum and decreases to approximately 1 mg/mL in mature milk (Goldman et al. Citation1982; Lawrence and Lawrence Citation2004). While SIgA is the most abundant antibody in breast milk, it contains IgG and IgM too, which both contribute to defenses and immunoregulation and becomes more abundant in later lactation (Gao et al. Citation2012; Rodríguez-Camejo et al. Citation2018). IgG can facilitate antigen uptake and is involved in the prevention of allergic and autoimmune diseases (Palmeira and Carneiro-Sampaio Citation2016). Besides, pentameric IgM has been shown to neutralize HIV-1 intracellularly and prevents transcytosis (Van De Perre Citation2003).
Antimicrobial proteins
A multifunctional iron-binding glycoprotein, i.e. lactoferrin (LF), is present in mother milk and affects microbiota activity by protecting against infection through degradation of gram-negative cell walls of pathogenic bacteria (Telang Citation2018). Lactoferrin is considered to be a so-called defensin (Adamkin Citation2012). Defensins are a family of proteins that are also produced by Paneth cells and reduce infection by deleting pathogens without affecting commensal bacteria (Chairatana and Nolan Citation2017). The concentration of LF is typically highest in colostrum and reaches concentrations between 5 and 6 mg/mL. It decreases over time during lactation when the child is developing its immune system (Lönnerdal Citation2016). Lactadherin, another glycoprotein found in human milk, known as milk fat globule-epidermal growth factor 8 (MFG-E8) as well, has been shown to have specific anti-viral functions and prevents rotaviral infection in the newborn (Sabha et al. Citation2018). More than that, it prevents intestinal inflammation by enhancing the phagocytosis of apoptotic cells (Aziz et al. Citation2017). In addition, a recent study showed that lactadherin can prevent necrotizing enterocolitis (NEC) by enhancing intestinal barrier integrity (Sabha et al. Citation2018). Milk fat globules (MFGs) are milk lipids present in the form of dispersed droplets (Martini, Salari, and Altomonte Citation2016). Several bioactive glycoproteins have been characterized in MFGs, which contain mucin 1 (MUC1) and mucin 4 (MUC4) (Hamosh et al. Citation1999). Both MUC1 and MUC4 inhibit the invasion of Salmonella enterica serovar typhimurium in intestinal epithelial cells (Liu et al. Citation2012).
Human milk oligosaccharides
One of the most important bioactive components of mother milk is the human milk oligosaccharides (hMOs). The concentration of hMOs ranges from 20 to 25 g/L in colostrum and 5 to 20 g/L in mature milk, which makes them the third-largest solid component in human milk (Bode Citation2012). hMOs are unique to humans and are not found in the same variety and composition in other mammals (Bode Citation2012). Several studies show that hMOs provide numerous health-promoting effects (Cheng et al. Citation2019; Doare et al. Citation2018; Kong et al. Citation2019). Before critically reviewing possible beneficial effects for gastrointestinal development and health, we review the structure and composition of hMOs.
HMO structures and compositions
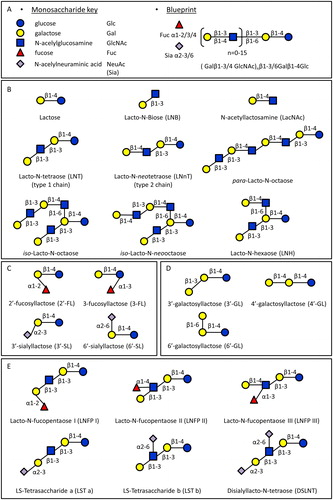
HMOs are composed of five monomers: d-glucose (Glc), d-galactose (Gal), N-acetylglucosamine (GlcNAc), l-fucose (Fuc), and sialic acid (NeuAc) (). All hMOs are synthesized from lactose (Galβ1-4Glc), which can be extended by the addition of β1-3 or β1-6 linked lacto-N-biose (Galβ1-3GlcNAc-, LNB, type 1 chain) or N-acetyllactosamine (Galβ1-4GlcNAc-, LacNAc, type 2 chain). The addition of LNB terminates the chain, while type 2 chain LacNAc can be further extended. The β1-6 linkage creates branched structures, which are designated as iso-hMO; the linear structures without branches are called para-hMO (). The hMO backbone can be either sialylated or fucosylated, and in the case of the shortest hMOs this forms trisaccharides such as 2′-fucosyllactose (2′-FL), 3- fucosyllactose (3-FL), 3′-sialyllactose (3′-SL) and 6′-sialyllactose (6′-SL) (). The lactose core can also be extended with β1-3, β1-4 and β1-6 galactosyl residues and form galactosyllactoses (GL) (), which are typically present in human colostrum rather than in mature milk (Kulinich and Liu Citation2016). Elongated hMOs can be fucosylated in α1-2, α1-3 or α1-4 linkages and/or sialylated in α2-3 or α2-6 linkage to form a variety of structural isomers as well (). hMOs are synthesized in the mammary gland. To date, approximately 200 different hMOs have been discovered and characterized (Thurl et al. Citation2017). The molecular structures and ratios vary among individuals and is depending on the expression of the α1-2- fucosyltransferase (FUT2) and α1-3/4-fucosyltransferase (FUT3) in lactocytes (Bode Citation2019). The hMOs profile is determined by Secretor (Se) and Lewis (Le) blood group genes (Elwakiel et al. Citation2018). The enzymes FUT2 and FUT3 are encoded by Se gene and Le gene, respectively. Individuals with an active Se locus are called secretors, and individuals with an active Le locus are classified as Lewis positive. Based on the expression of Se and Le genes, human milk can be divided into four groups: Le-positive Secretors (FUT 2 active, FUT3 active, Se+Le+), Le-negative Secretors (FUT 2 active, FUT3 inactive, Se+Le−), Le-positive non-Secretors (FUT2 inactive, FUT3 active, Se−Le+), and Le-negative non-Secretors (FUT2 inactive, FUT3 inactive, Se−Le−). FUT2 is responsible for connecting Fuc to terminal Gal through α1–2 linkages, and 2′-FL is the most abundant hMO in secretor women (Egge, Dell, and Von Nicolai Citation1983; Thurl et al. Citation2010). In contrast, the milk of non-secretor women does not contain α1-2-fucosylated hMOs, i.e. 2′-FL. FUT3 is responsible for connecting Fuc to GlcNAc in type 1 chains through an α1-4 linkage on type 1 chains, and as a result Le-negative woman do not have these specific α1-4-fucosylated hMOs, such as lacto-N-fucopentaose II (LNFP II) (Johnson and Watkins Citation1992).
Figure 2. Structures of hMOs. (A) Building blocks and structural blueprint of hMOs. (B) Lactose can be elongated by addition of either lacto-N-biose (type I) or N-acetyllactosamine (type II) disaccharides. The addition of a β1-6 linkage produces branched structures (iso-hMO); the β1-3 linkage leads to linear structures (para-hMO). (C) Lactose is fucosylated or sialylated through different linkages to generate trisaccharides. (D) Galactosyl residues are linked to lactose through β1-3, β1-4- and β1-6 linkages to form galactosyllactoses. (E) Elongated type I or II chains can be fucosylated or sialylated in different linkages to form a variety of structural isomers.
Beneficial effects of human milk oligosaccharides
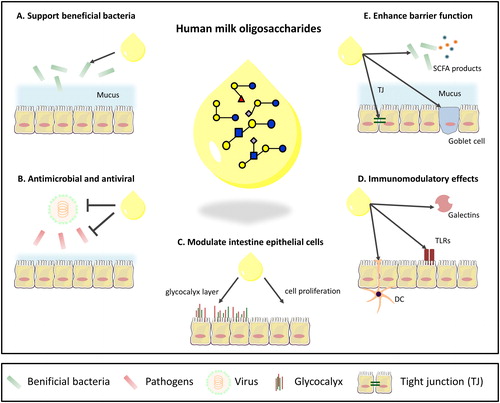
In this section, we focus on current knowledge of hMO functions. The evidence for these functions will be critically reviewed. illustrates some of the discussed effects. We critically review the evidence for the effects of hMOs on gut microbiota composition, immune defense, intestinal epithelial cells, and the gastrointestinal barrier. We also discuss the addition of hMOs to infant formula as well as the challenges for further studies to investigate the effects of hMOs and creation of optimal formulations for adding to infant formula.
Figure 3. Overview of known functions of hMOs in the intestine. (A) Support the growth and colonization of beneficial bacteria. (B) Antimicrobial and antiviral effects by serving as decoy for pathogens and/or inhibit the growth of pathogens. (C) Exert a direct influence on intestinal epithelial cells by enhancing barrier function. (D) Immunomodulatory effects. (E) Effects on intestinal barrier function.
hMOs and microbiota
hMOs are considered to have a strong impact on colonization of the intestine by bacteria that are essential for health (Jost et al. Citation2015). In early life, the intestine is colonized by 1014 bacterial cells (Hillman et al. Citation2017). The first year of life is critical for intestinal microbiome establishment, and infant diet is one of the most important factors for gut microbiome development (Tamburini et al. Citation2016). As many hMOs resist the gastric acidity and the host enzyme’s hydrolysis processes in the small intestine, high concentrations of hMO can reach both the small and large intestine, and modulate the activity and composition of the resident microbiota (Kirmiz et al. Citation2018; van den Elsen et al. Citation2019).
hMOs support beneficial bacteria
hMOs are specifically known to support the growth of beneficial microorganisms (), especially Bifidobacterium species, which is a dominant species in breast-fed infants (Kirmiz et al. Citation2018). One of the Bifidobacterium species, Bifidobacterium longum supsp. infantis (B. longum supsp. infantis) grows well when cultured with pooled hMOs isolated from human milk as the sole carbohydrate source. In those cultures, all of the hMOs are consumed entirely by this Bifidobacterium strain. The concentrations of 2′-FL, 3-FL, LDFT, LNT, LNnT, LNFP I, LNFP II, LNFP III, LNDFH I, and LNDFH II all rapidly decreased when the cells entered the logarithmic phase (Sadaki Asakuma et al. Citation2011). In the same in vitro study, it was found that Bifidobacterium longum subsp. longum (B. longum subsp. longum) , and Bifidobacterium breve (B.breve) are only able to consume lacto-N-tetraose (LNT) (Asakuma et al. Citation2011). This study demonstrates that multiple bifidobacteria species can consume hMOs but that not all Bifidobacterium species can use hMOs as only carbohydrate sources (Thomson, Medina, and Garrido Citation2018).
Bacteria can degrade hMOs using both intracellular and extracellular glycoside hydrolases (Garrido et al. Citation2015; Kitaoka Citation2012). Intracellular degradation, which can be done by B. infantis, B. breve, and B. longum subsp. longum, is based on the uptake of intact hMOs inside the bacteria. The uptake is accomplished by several Solute Binding Proteins (SBPs). After being transported into the bacteria, the intracellular glycosyl hydrolases (GHs) degrade the hMOs and release monosaccharides in the cytoplasm instead of outside the bacteria (Garrido et al. Citation2011, Citation2015). The extracellular degradation is different and found in, for example, Bifidobacterium bifidum (B. bifidum). In contrast to B. infantis, B. bifidum relies on the membrane-bounded extracellular GHs, which show similar enzymatic affinities for hMOs as in B. infantis (Kitaoka Citation2012). Gotoh et al. showed that the extracellular hMO degradation of B. bifidum results in the production of sugars and thereby stimulate the growth of other species that can utilize these carbohydrates as source of energy (Gotoh et al. Citation2018).hMOs do not only support the growth of bacteria, they can enhance binding of commensal bacteria to epithelial cells, and thereby support microbiota colonization in infants. For example, Kavanaugh et al. demonstrated that a mixture of 3′-SL and 6′-SL increase B. longum subsp. infantis ATCC 15697 adhesion to human HT-29 intestinal cells in vitro (Kavanaugh et al. Citation2013). Wickramasinghe et al. found that an hMO mixture enhanced the anti-inflammatory effects of bifidobacteria on intestinal cells in vitro (Wickramasinghe et al. Citation2015). Besides the direct influence of hMOs on bifidobacteria, it can modulate the growth and fermentation activity of other bacterial species (Salli et al. Citation2019; Schwab et al. Citation2017). Salli et al. found that 2′-FL promoted the growth of bifidobacteria, and significantly increased production of SCFAs as well as of lactic acid in a human study (Salli et al. Citation2019). Schwab et al. investigated the interaction between bifidobacteria and Eubacterium hallii (E. hallii), one of the first butyrate producers in the infant’s gut in a human study. They demonstrate that E. hallii consumes acetate, lactate and 1,2-propanediol, which are the hMOs fermentation products of bifidobacteria, and subsequently produces the SCFAs butyrate and propionate, thereby supporting gut barrier function and the immune system (Schwab et al. Citation2017).
hMOs prevent pathogen infection
hMOs can reduce pathogen infection in infants in several ways (). They can do so by serving as soluble decoy receptors or by inhibiting the growth of pathogens (Townsend Citation2019). Many viruses and pathogens need to attach to the intestinal epithelial glycocalyx to colonize or invade the host (Kong et al. Citation2019). By structural resemblance to the glycocalyx layer, hMOs can bind to pathogens and serve as antiadhesive antimicrobials, and prevent microbial infections (Patel and Kim Citation2018). For example, Campylobacter jejuni (C. jejuni), which is one of the most common causes of bacterial diarrhea and infant mortality, can bind to α1-2-fucosylated hMOs in vitro, such as 2′-FL, and reduce its binding and infection of intestinal cells (Ruiz-Palacios et al. Citation2003). In an in vitro study, Jantscher-Krenn et al. found that LNT significantly reduced the attachment and cytotoxicity of the protozoan parasite Entamoeba histolytica (E. histolytica) to intestinal epithelial cells (Jantscher-Krenn, Lauwaet, et al. Citation2012). Interestingly, although 2′-FL can reduce host-adhesion and infection of C. jejuni, it does not affect E. histolytica attachment and cytotoxicity, which indicates that the decoy effect of hMOs is species dependent.
Recent studies have shown that hMOs also exhibit antimicrobial and antibiofilm effects against Group B Streptococcus (GBS), which cause invasive infections in newborns (Ackerman et al. Citation2017; Lin et al. Citation2017). In these studies, hMOs isolated from human milk significantly inhibited the growth of GBS up to 89% and inhibited biofilm formation up to 90%. Ackerman et al. found that hMOs isolated from human milk possessed significant antimicrobial activity against Acinetobacter baumannii (A. baumannii) and could inhibit adhesion up to 11%. Moreover, it could inhibit biofilm formation of Staphylococcus aureus (S. aureus) up to 60% (Ackerman et al. Citation2018).
hMOs show direct modulatory effects on intestinal epithelial cells
hMOs do not only have a strong impact on microbes, they can directly influence intestinal epithelial cells (). An in vitro study by Kuntz et al. showed that both acidic and neutral hMOs isolated from human milk had inhibitory effects on intestinal epithelial cells proliferation under homeostatic conditions (Kuntz, Rudloff, and Kunz Citation2008). However, Wang et al. showed in an animal model that pooled hMOs could increase the proliferation of crypt cells under inflammatory conditions which might protect against necrotizing enterocolitis (Wang et al. Citation2019). These studies indicate that hMOs might have different functions depending on the nature of the inflammatory conditions. Holscher et al. found that in vitro, 6′-SL, 2′-FL, and LNnT all could inhibit proliferation of HT-29 and Caco-2Bbe cells, while they enhanced differentiation of HT-29 and Caco-2Bbe cells (Holscher, Bode, and Tappenden Citation2017). These results suggest that hMOs may have a specific role in the maturation of the gastrointestinal tract. The maturation of intestinal epithelium requires a shift from sialylation to fucosylation (Lenoir et al. Citation1995), and hMOs can modulate intestinal epithelial cells through modification of the intestinal glycome (Kavanaugh et al. Citation2015). There are additional studies showing that hMOs can alter the structure of the intestinal epithelial cell glycocalyx layer (Angeloni et al. Citation2004; Kong et al. Citation2019). Angeloni et al. found that in vitro, 3′-SL lowered gene expression of the sialyltransferases ST3Gal1, ST3Gal2, and ST3Gal4, which resulted in reduction in α2-3-, α2-6-sialylation on cell surface glycans of Caco2 cells, and as a consequence reduced the enteropathogenic Escherichia coli (EPEC) adherence to about 50% (Angeloni et al. Citation2004). Kong et al. showed 2′-FL as well as 3-FL significantly increased the thickness of the glycocalyx layer in vitro (Kong et al. Citation2019). These observations suggest that hMOs stimulate glycocalyx development in a structure-dependent manner.
Immunomodulatory effects of hMOs
Although the influence of hMOs on microbiota may affect the immune system indirectly, studies suggest that hMOs can modulate immune function in different ways as well (). An ex vivo study by He et al. shows that hMOs derived from pooled human colostrum can attenuate pathogen-associated molecular pattern (PAMP) induced production of inflammatory cytokines such as IL-8 in the immature intestinal mucosa (He et al. Citation2014). In addition, an elevation of levels of cytokines such as MIP-1-δ involved in tissue repair and homeostasis in immature human intestinal mucosa was observed. Such an hMO effect might help to promote the maturation of the intestinal mucosal immune system (He et al. Citation2014). He et al. also demonstrated in vitro that 2′-FL could attenuate type 1 pili pathogens derived lipopolysaccharide (LPS) induced IL-8 production in T84 and H4 cells by decreasing CD14 induction (He et al. Citation2016). The hMO disialyllacto-N-tetraose (DSLNT) was shown to suppress necrotizing enterocolitis-like inflammation in neonatal rats and is therefore identified as an immunomodulating and immune activation attenuating hMO (Jantscher-Krenn, Zherebtsov, et al. Citation2012). Besides that, hMOs have been shown to have immune-stimulating or maturation promoting properties. Kurakevich et al. (Citation2013) found that 3′-SL stimulates MLN CD11c + dendritic cells and increases TNF-α, TGF-β1, IL-12, and IFN-γ production in an animal model. Those cytokines typically induce increased Th1 and Th17 cell frequencies. Moreover, Thomas et al. (Citation2003) demonstrated in vivo that LNFP III induces strong Th2 responses and promotes dendritic cell 2 (DC2) maturation.
It is currently unknown which receptors and signaling pathways are involved in transducing hMO-mediated effects; however, some studies suggest that hMOs can interact with pattern recognition receptors (PRRs) and interfere with immune processes. It has been found that the immunomodulatory effects of hMOs can be induced via Toll-like receptors (TLRs), which are a family of PRRs. In an in vitro study, Asakuma et al. found that 3′-SL, 6′-SL, and 6′-GL enhanced both TLR2 and TLR4 expression, while LNFP I only increased the gene expression of TLR4 (Asakuma et al. Citation2010). 3-FL and LNT2 have been observed to activate TLRs. In an in vitro study by Cheng et al. 3-FL could activate TLR2, while LNT2 could activate all TLRs and induced both IL-10 and TNFα in THP1 macrophages. 6′-SL showed a synergistic effect on ssRNA40-induced TLR8 activation (Cheng et al. Citation2019). Beside activation effects, hMOs show inhibiting effects on TLRs signaling as well. He et al. (Citation2014) found ex vivo that 3′-GL, 4′-GL, and 6′-GL are able to attenuate the inflammatory response through TLR3. Cheng et al. demonstrated that 2′-FL, 6′-SL, and LNnT inhibit TLR5 and 7, while 3-FL inhibit TLR5, 7, and 8 in vitro (Cheng et al. Citation2019). This illustrates the complexity by which mixtures of hMOs modulate immune responses and how variations in composition can lead to different types of immune modulation opening novel venues to prevent diseases in infants on infant formula.
Galectins are β-galactoside binding proteins that are involved in many immune responses (Vasta Citation2012). Some hMOs contain β1-3- or β1-4-linked d-galactose at the non-reducing end, which can be the potential target for galectin-mediated interactions. The binding affinities of a library of 31 free hMOs with galectins Gal-1, Gal-3, and Gal-7 have been studied by catch-and-release electrospray ionization mass spectrometry (CaR-ESI-MS). It was shown that hMOs have high binding affinity to galectins (El-Hawiet et al. Citation2017). Whether hMOs are able to modulate galectin-mediated immune responses still needs further investigation, but the CaR-ESI-MS method provided another possible approach for rapid screening of the interaction between free hMOs and receptors to find the potential hMO receptors.
Since around 1% of the hMOs can reach the systemic circulation, it is plausible that hMOs not only impact the intestinal tract but also influence development of other organs (Plaza-Díaz, Fontana, and Gil Citation2018). An in vitro study showed that mixtures of hMOs isolated from pooled human milk significantly reduced uropathogenic Escherichia coli (UPEC) internalization in bladder epithelial cells. It attenuated the cytotoxic and proinflammatory effects induced by UPEC as well (Lin et al. Citation2014). Furthermore, Duska-McEwen et al. showed in vitro that hMOs can enhance innate immunity to airway infections (Duska-McEwen et al. Citation2014). 2′-FL could decrease respiratory syncytial virus (RSV) viral load while 6′-SL and LNnT were able to decrease influenza viral load in airway epithelial cells (Duska-McEwen et al. Citation2014). They also showed that 2’FL could attenuate RSV induced inflammatory cytokines IL-8 and TNF-α production in airway epithelial cells, while 6′-SL down-regulated TNF-α in RSV infected peripheral blood mononuclear cells (PBMCs) (Duska-McEwen et al. Citation2014). This again illustrates the specificity of different type of hMOs for health benefits.
Hmos enhance intestinal barrier function
The primary function of the gastrointestinal tract is to digest and absorb nutrients, while at the same time it has to act as a barrier and protect against toxic agents or potential pathogenic intruders (Figueroa-Lozano and de Vos Citation2019). However, the intestinal tract of neonates is functionally immature at birth (Lewis et al. Citation2017). hMOs support intestinal barrier function both through indirectly influencing microbiota composition and directly by modulating intestinal cells (). An animal study by Fukuda et al. shows that fermentation of hMOs by bifidobacteria leads to acetate formation, which can enhance gut barrier function and protect the host against lethal infection induced by enterohemorrhagic Escherichia coli O157:H7 (Fukuda et al. Citation2011). Fermentation products of hMOs secreted by the bifidobacterial strain B.infantis are reported to enhance epithelial cell barrier function. Guo et al. showed that B. infantis conditioned media (BCM) protects Caco2 cells against IL-1β stimulation through signaling through the NF-κB pathway in an in vitro study (Guo et al. Citation2017). BCM up-regulated protein expression of claudin-1 and occludin, which are responsible for the preservation of intestinal barrier integrity (Lewis et al. Citation2017). Therefore, hMOs can support intestinal barrier function by promoting the growth and supporting production of fermentation products by Bifidobacterium species. Chichlowski et al. found in vitro that B. infantis ATCC15697 grown on mixtures of hMOs isolated from mother milk had a significantly higher capacity to adhere to HT-29 cells, which induced higher expression of tight junction proteins such as occludin and junctional adhesion molecule (JAM-A) in gut epithelial cells (Chichlowski et al. Citation2012). Wu et al. demonstrated that hMOs isolated from pooled milk could increase mucin expression in intestinal cells both in vitro, ex vivo, and in vivo (Wu et al. Citation2018). They found that administration by oral gavage of hMOs to mouse pups increased MUC2 protein levels and decreased the permeability of the intestine to macromolecular dextran. Their study also showed that hMOs in human milk can induce MUC2 gene expression and decrease dextran permeability during an enterohemorrhagic Escherichia coli O157:H7 challenge in intestine epithelial cells (Wu et al. Citation2018).
hMOs in infant formula and regulatory considerations
Although the World Health Organization (WHO) recommends six months of exclusive breastfeeding after birth, this period is rarely fulfilled as breastfeeding is not always possible. For a variety of reasons, around 70% of the infants cannot be solely fed with breast milk (Martin, Ling, and Blackburn Citation2016). These infants receive cow milk-derived infant formulas, which attempts to mimic the nutritional composition of breast milk as closely as possible (Akkerman, Faas, and de Vos Citation2019). Despite many beneficial effects of hMOs, only 2′-FL and LNnT are currently used in infant formula, mainly because hMOs are complex molecules and because synthesis of hMOs is still challenging and expensive (Akkerman, Faas, and de Vos Citation2019; Vandenplas et al. Citation2018). Non-digestible fibers such as galacto-oligosaccharides (GOS) and inulin-type fructans are nowadays often added to commercially available infant formulas to substitute some of the hMO’s beneficial effects (Akkerman, Faas, and de Vos Citation2019). However, recent improvements in production processes make it possible to produce some smaller molecular weight hMO molecules in sufficient quantities to allow application in infant formulas (Vandenplas et al. Citation2018). The commercially available hMO analogs are not isolated from human milk but are the fermentation products of genetically engineered microorganisms, which include strains of E. coli and yeast (Sprenger, Baumgärtner, and Albermann Citation2017). Although hMOs are natural carbohydrates that are being recognized as safe for a long time, the use of chemical- or biotechnology-based processes to produce synthesized hMOs makes it necessary to evaluate the safety of the production procedure as well as the novel compounds through a series of registration processes (Bode et al. Citation2016).
Nowadays, the European Union (EU) considers 2′-FL and LNnT, two produced hMOs, as authorized novel foods that can be used in infant formulas (Commission Implemented Regulation (EU) 2017/2470). On 29 June 2015, the European Food Safety Authority (EFSA) concluded that a concentration up to 1.2 g/L of 2′-FL, alone or in combination with LNnT at a ratio of 2:1, is safe for infants when added to infant and follow-on formula (EFSA NDA Panel Citation2015a). In the same year, the Panel concluded that LNnT and 2′-FL are considered as safe as food supplements for 1–3 year old toddlers. Intake of these hMOs may not exceed 0.6 g of LNnT and 1.2 g of 2′-FL (alone or in combination) per day. For 4-18 years old children, 1.5 g of LNnT and 3 g of 2′-FL (alone or in combination) per day is recommended (EFSA NDA Panel Citation2015b). The USA’s Food and Drug Administration (FDA) considers three hMOs to be Generally Regarded as Safe (GRAS). Those hMOs are 2′-FL (GRAS notice no 546/571/650/735), LNnT (GRAS notice no 659), as well as 3′-SL (GRAS notice no 766). Through scientific procedures, the FDA considers 2′-FL to be GRAS at a maximum use level of 2.4 g/L in reconstituted formula; LNnT is GRAS at a maximum use level of 0.6 g/L in infant formula and 3 g/serving in follow-up formulas; and 3′-SL is GRAS at a level up to 0.23 g/L in infant formula and 3.1 g/serving in 12–24 months old toddler foods. As a consequence of these approvals, there are now hMOs in infant formulas on the market containing 2′-FL in the USA and 2′-FL and LNnT in Europe since 2016 (Austin et al. Citation2018; Vandenplas et al. Citation2018).
The safety assessments of more synthesized hMOs are currently ongoing. Lately, Pitt et al. conducted in vitro and in vivo safety assessment experiments of genetically modified E. coli K12 strain produced 3-FL and concluded that 3-FL is safe as a nutritional ingredient in foods (Pitt et al. Citation2019). More studies on the safety evaluation of hMOs are expected to follow soon, making it possible to consider more hMOs as novel foods in products in the near future.
Concluding remarks and future considerations
The importance of hMOs in human milk and their beneficial effects on the infant are unequivocal, which have been previously studied in vitro (Cheng et al. Citation2019), ex vivo (He et al. Citation2014), in vivo (Wu et al. Citation2018), as well as in human studies (Salli et al. Citation2019). However, many questions about hMO synthesis, metabolism, and functions are still unanswered, and to date, there is little evidence available to support the beneficial function of individual hMOs. One of the major roadblocks remains the limited availability of synthesized hMOs to confirm their health in neonates. The limited resources and the high price of the synthesized hMOs make that scientists, and formula companies have to make decisions on which individual hMOs to study. At this moment, only some tri- and tetrasaccharides can be produced in kilogram quantities to study their functions, but the identification of benefits of more complex and/or the mixture of different hMOs still need more research efforts. The efforts are urgently needed as many studies have shown that health benefits of hMOs are very specific for specific hMO types and many more benefits are to be expected if mixtures with confirmed bioactivities can be applied in infant formula.
While the beneficial effects of mixtures of hMOs have been broadly proven, it is crucial in future efforts to identify the composition of the hMOs in the mixtures. As outlined in our review the composition varies considerably between breast milk from mothers with premature or mature infant and over the course of lactation. Also, non-secretor women have a different hMO composition than milk from secretor mothers. During recent years it has been shown by us and others that individual hMOs have different effects and that the final outcome of a specific health benefit dependents on the composition of the mixture and or quantity of individual hMOs (Cheng et al. Citation2019; Kong et al. Citation2019; Salli et al. Citation2019). Side-by-side comparison of hMO composition of the mixtures will contribute to a better understanding of sometimes contradictory results and may lead to a better understanding of the impact of specific hMOs for infant health.
Another change in experimental design which might lead to a better understanding of health benefits of hMOs is studying the impact of individual or well-defined mixtures of hMOs on human intestine cells. In particular, there is a need to understand whether different structures of hMOs modulate different types of cells such as epithelial cells, Paneth or goblet cells, in a structure-dependent manner. This knowledge is urgently needed to propose formulations of mixtures that support barrier function in e.g. premature babies with enhanced risk for development of NEC. It is also essential to understand which signaling pathways of different hMOs are involved in different cells to gain more insight in mechanisms involved and therewith to support acceptance of health benefits of hMOs. Besides, the impact on microbiota of infants requires more study. Interestingly it was recently shown that 2′-FL, which currently is approved for and applied in infant formula, is poorly fermentable by 2-12 weeks baby microbiota, and had lower production of acetate and lactate compared with lactose or GOS (Salli et al. Citation2019). What this implies for support of microbiota in infants requires further investigation but it seems that most effects of 2′-FL relate to direct effects on intestinal cells rather than on support of microbiota (Kong et al. Citation2019; Salli et al. Citation2019).
It has been shown that infant microbiota varies considerably in composition and diversity depending on the age of the infant (Chong, Bloomfield, and O’Sullivan Citation2018; Dominguez-Bello et al. Citation2010; Hill et al. Citation2017; Mueller et al. Citation2015). Especially in the first 8 weeks infants do not have the full enzymatic ability to ferment all hMOs (Mountzouris, McCartney, and Gibson Citation2002; Salli et al. Citation2019). Blood group-specific differences were found too (Ewald and Sumner Citation2018; Mäkivuokko et al. Citation2012). This is probably due to the fact that the mucus layer in the intestine has blood group-specific oligosaccharide epitopes (Tailford et al. Citation2015). As microbiota use mucus as substrate, this probably predisposes to blood group specific microbiota and utilization of hMOs. To gain more insight in efficacy of hMO on microbiota development and the colonization process it is needed to study the impact on microbiota of different age classes and blood groups. Also, there are indications that fermentation is different in healthy and diseased children (Ladirat Citation2014).
Understanding how and which hMOs are responsible for specific effects not only contributes to future design of hMO-containing infant formulas, in addition, it might provide new ways to explore the function of hMOs as therapeutics. For example, as members of the “ESKAPE” group of pathogens, the leading cause of multidrug-resistant (MDR) nosocomial infections throughout the world, A. baumannii and S. aureus both show methicillin-resistance (Santajit and Indrawattana Citation2016). The antimicrobial and antibiofilm properties of hMOs on A. baumannii and S. aureus could potentially be used as new therapeutics to treat or prevent infectious disease, which provides a new way to manage drug-resistant bacteria.
Disclosure statement
The authors have declared no conflict of interest.
Additional information
Funding
References
- Ackerman, D. L., K. M. Craft, R. S. Doster, J.-H. Weitkamp, D. M. Aronoff, J. A. Gaddy, and S. D. Townsend. 2018. Antimicrobial and antibiofilm activity of human milk oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus, and Acinetobacter baumannii. ACS Infectious Diseases 4 (3):315–24. doi: 10.1021/acsinfecdis.7b00183.
- Ackerman, D. L., R. S. Doster, J.-H. Weitkamp, D. M. Aronoff, J. A. Gaddy, and S. D. Townsend. 2017. Human milk oligosaccharides exhibit antimicrobial and antibiofilm properties against group B Streptococcus. ACS Infectious Diseases 3 (8):595–605. doi: 10.1021/acsinfecdis.7b00064.
- Adamkin, D. H. 2012. Mother’s milk, feeding strategies, and lactoferrin to prevent necrotizing enterocolitis. Journal of Parenteral and Enteral Nutrition 36 (1_suppl):25S–9S. doi: 10.1177/0148607111420158.
- Ahmad, T., M. Gogarty, E. G. Walsh, and D. J. Brayden. 2017. A comparison of three Peyer’s patch “M-like” cell culture models: Particle uptake, bacterial interaction, and epithelial histology. European Journal of Pharmaceutics and Biopharmaceutics 119:426–36. doi: 10.1016/j.ejpb.2017.07.013.
- Akkerman, R., M. M. Faas, and P. de Vos. 2019. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Critical Reviews in Food Science and Nutrition 59 (9):1486–12. doi: 10.1080/10408398.2017.1414030.
- Allaire, J. M., S. M. Crowley, H. T. Law, S. Y. Chang, H. J. Ko, and B. A. Vallance. 2018. The intestinal epithelium: Central coordinator of mucosal immunity. Trends in Immunology 39 (9):677–96. doi: 10.1016/j.it.2018.04.002.
- Aly, E., A. Ali Darwish, R. Lopez-Nicolas, C. Frontela-Saseta, and G. Ros-Berruezo. 2018. Bioactive components of human milk: Similarities and differences between human milk and infant formula. In Selected topics in breastfeeding. London: IntechOpen. doi: 10.5772/intechopen.73074.
- Andreas, N. J., B. Kampmann, and K. Mehring Le-Doare. 2015. Human breast milk: A review on its composition and bioactivity. Early Human Development 91 (11):629–35. doi: 10.1016/j.earlhumdev.2015.08.013.
- Angeloni, S., J. L. Ridet, N. Kusy, H. Gao, F. Crevoisier, S. Guinchard, S. Kochhar, H. Sigrist, and N. Sprenger. 2004. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 15 (1):31–41. doi: 10.1093/glycob/cwh143.
- Asakuma, S., E. Hatakeyama, T. Urashima, E. Yoshida, T. Katayama, K. Yamamoto, H. Kumagai, H. Ashida, J. Hirose, and M. Kitaoka. 2011. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. Journal of Biological Chemistry 286 (40):34583–92. doi: 10.1074/jbc.M111.248138.
- Asakuma, S., T. Yokoyama, K. Kimura, Y. Watanabe, T. Nakamura, K. Fukuda, and T. Urashima. 2010. Effect of Human milk oligosaccharides on messenger ribonucleic acid expression of toll-like receptor 2 and 4, and of MD2 in the intestinal cell line HT-29. Journal of Applied Glycoscience 57 (3):177–83. doi: 10.5458/jag.57.177.
- Austin, S., D. Cuany, J. Michaud, B. Diehl, and B. Casado. 2018. Determination of 2’-fucosyllactose and lacto-N-neotetraose in infant formula. Molecules 23 (10):2650. doi: 10.3390/molecules23102650.
- Aziz, M., L. W. Hansen, J. M. Prince, and P. Wang. 2017. Role of mfg-e8 in neonatal inflammation. In Dairy in human health and disease across the lifespan, 21–30. London: IntechOpen. doi:10.1016/B978-0-12-809868-4.00002-9.
- Baregamian, N., J. Song, M. G. Jeschke, B. M. Evers, and D. H. Chung. 2006. IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis. Journal of Surgical Research 136 (1):31–7. doi: 10.1016/j.jss.2006.04.028.
- Binns, C., M. Lee, and W. Y. Low. 2016. The long-term public health benefits of breastfeeding. Asia Pacific Journal of Public Health 28 (1):7–14. doi: 10.1177/1010539515624964.
- Blum, J. W., and C. R. Baumrucker. 2002. Colostral and milk insulin-like growth factors and related substances: Mammary gland and neonatal (intestinal and systemic) targets. Domestic Animal Endocrinology 23 (1-2):101–10. (02)00149-2 doi: 10.1016/S0739-7240(02)00149-2.
- Bode, L. 2012. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 22 (9):1147–62. doi: 10.1093/glycob/cws074.
- Bode, L. 2019. Human milk oligosaccharides: Next-generation functions and questions. Nestle Nutrition Institute Workshop Series 90:191–201. doi: 10.1159/000490306.
- Bode, L., N. Contractor, D. Barile, N. Pohl, A. R. Prudden, G. J. Boons, Y. S. Jin, and S. Jennewein. 2016. Overcoming the limited availability of human milk oligosaccharides: Challenges and opportunities for research and application. Nutrition Reviews 74 (10):635–44. doi: 10.1093/nutrit/nuw025.
- Boedeker, E. C., and A. Allen. 2018. The structure and function of gastrointestinal mucus. In Attachment of organisms to the gut mucosa, 3–12. London: IntechOpen. doi: 10.1201/9781351069977-2.
- Boix-Amorós, A., M. C. Collado, and A. Mira. 2016. Relationship between milk microbiota, bacterial load, macronutrients, and human cells during lactation. Frontiers in Microbiology 7 (APR):492. doi: 10.3389/fmicb.2016.00492.
- Boyce, C., M. Watson, G. Lazidis, S. Reeve, K. Dods, K. Simmer, and G. McLeod. 2016. Preterm human milk composition: A systematic literature review. British Journal of Nutrition 116 (6):1033–45. doi: 10.1017/S0007114516003007.
- Brandtzaeg, P. 2016. Immunity in the gut: Mechanisms and functions. In Viral gastroenteritis: molecular epidemiology and pathogenesis, 23–46. London: Academic Press. doi: 10.1016/B978-0-12-802241-2.00002-X.
- Brenmoehl, J., D. Ohde, E. Wirthgen, and A. Hoeflich. 2018. Cytokines in milk and the role of TGF-beta. Best Practice & Research Clinical Endocrinology & Metabolism 32 (1):47–56. doi: 10.1016/j.beem.2018.01.006.
- Briere, C. E., T. Jensen, J. M. McGrath, E. E. Young, and C. Finck. 2017. Stem-like cell characteristics from breast milk of mothers with preterm infants as compared to mothers with term infants. Breastfeeding Medicine 12 (3):174–9. doi: 10.1089/bfm.2017.0002.
- Cabrera-Rubio, R.,. M. C. Collado, K. Laitinen, S. Salminen, E. Isolauri, and A. Mira. 2012. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. The American Journal of Clinical Nutrition 96 (3):544–51. doi: 10.3945/ajcn.112.037382.
- Cacho, N. T., and R. M. Lawrence. 2017. Innate immunity and breast milk. Frontiers in Immunology 8 (May):584. doi: 10.3389/fimmu.2017.00584.
- Cappelletti, M., S. D. Bella, E. Ferrazzi, D. Mavilio, and S. Divanovic. 2016. Inflammation and preterm birth. Journal of Leukocyte Biology 99 (1):67–78. doi: 10.1189/jlb.3MR0615-272RR.
- Castanys-Muñoz, E., M. J. Martin, and E. Vazquez. 2016. Building a beneficial microbiome from birth. Advances in Nutrition 7 (2):323–30. doi: 10.3945/an.115.010694.
- Chairatana, P., and E. M. Nolan. 2017. Defensins, lectins, mucins, and secretory immunoglobulin A: Microbe-binding biomolecules that contribute to mucosal immunity in the human gut. Critical Reviews in Biochemistry and Molecular Biology 52 (1):45–56. doi: 10.1080/10409238.2016.1243654.
- Cheng, L., M. B. G. Kiewiet, A. Groeneveld, A. Nauta, and P. de Vos. 2019. Human milk oligosaccharides and its acid hydrolysate LNT2 show immunomodulatory effects via TLRs in a dose and structure-dependent way. Journal of Functional Foods 59 (March):174–84. doi: 10.1016/j.jff.2019.05.023.
- Chichlowski, M., G. De Lartigue, J. Bruce German, H. E. Raybould, and D. A. Mills. 2012. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. Journal of Pediatric Gastroenterology and Nutrition 55 (3):321–7. doi: 10.1097/MPG.0b013e31824fb899.
- Chong, C. Y. L., F. H. Bloomfield, and J. M. O’Sullivan. 2018. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 10 (3):274. doi: 10.3390/nu10030274.
- Cicero, A. F. G., F. Fogacci, and A. Colletti. 2017. Potential role of bioactive peptides in prevention and treatment of chronic diseases: A narrative review. British Journal of Pharmacology 174 (11):1378–94. doi: 10.1111/bph.13608.
- Collado, M. C., M. Gueimonde, L. Ruiz, M. Aparicio, I. Castro, and J. M. Rodríguez. 2019. Baby’s first microbes: The microbiome of human milk. In How fermented foods feed a healthy gut microbiota, 3–33. Berlin: Springer. doi: 10.1007/978-3-030-28737-5_1.
- Conlon, M. A., and A. R. Bird. 2014. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7 (1):17–44. doi: 10.3390/nu7010017.
- Coppa, G. V., O. Gabrielli, P. Pierani, C. Catassi, A. Carlucci, and P. L. Giorgi. 1993. Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics 91 (3):637–41. www.aappublications.org/news.
- Cregan, M. D., Y. Fan, A. Appelbee, M. L. Brown, B. Klopcic, J. Koppen, L. R. Mitoulas, K. M. E. Piper, M. A. Choolani, Y.-S. Chong, et al. 2007. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell and Tissue Research 329 (1):129–36. doi: 10.1007/s00441-007-0390-x.
- Dawod, B., and J. S. Marshall. 2019. Cytokines and soluble receptors in breast milk as enhancers of oral tolerance development. Frontiers in Immunology 10 (JAN):16. doi: 10.3389/fimmu.2019.00016.
- Doare, K., Le, B. Holder, A. Bassett, and P. S. Pannaraj. 2018. Mother’s milk: A purposeful contribution to the development of the infant microbiota and immunity. Frontiers in Immunology 9 (Feb):361. doi: 10.3389/fimmu.2018.00361.
- Dominguez-Bello, M. G., E. K. Costello, M. Contreras, M. Magris, G. Hidalgo, N. Fierer, and R. Knight. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proceedings of the National Academy of Sciences of United States of America 107 (26):11971–5. doi: 10.1073/pnas.1002601107.
- Duska-McEwen, G., A. P. Senft, T. L. Ruetschilling, E. G. Barrett, and R. H. Buck. 2014. Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food and Nutrition Sciences 05 (14):1387–98. doi: 10.4236/fns.2014.514151.
- Dvorak, B. 2010. Milk epidermal growth factor and gut protection. The Journal of Pediatrics 156 (2):S31–S5. doi: 10.1016/j.jpeds.2009.11.018.
- Dvorak, B., C. C. Fituch, C. S. Williams, N. M. Hurst, and R. J. Schanler. 2003. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatric Research 54 (1):15–9. doi: 10.1203/01.PDR.0000065729.74325.71.
- EFSA NDA Panel. 2015a. Safety of 2′‐O‐fucosyllactose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA Journal 13 (7):4184. doi: 10.2903/j.efsa.2015.4184.
- EFSA NDA Panel. 2015b. Statement on the safety of lacto‐N‐neotetraose and 2′‐O‐fucosyllactose as novel food ingredients in food supplements for children. EFSA Panel on Dietetic Products. Nutrition and Allergies 13 (11):4299. doi: 10.2903/j.efsa.2015.4299.
- Egge, H., A. Dell, and H. Von Nicolai. 1983. Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Archives of Biochemistry and Biophysics 224 (1):235–53. (83)90207-2 doi: 10.1016/0003-9861(83)90207-2.
- El-Hawiet, A., Y. Chen, K. Shams-Ud-Doha, E. N. Kitova, Y. St-Pierre, and J. S. Klassen. 2017. High-throughput label-and immobilization-free screening of human milk oligosaccharides against lectins. Analytical Chemistry 89:28. doi: 10.1021/acs.analchem.7b00542.
- Elwakiel, M., J. A. Hageman, W. Wang, I. M. Szeto, J. B. Van Goudoever, K. A. Hettinga, and H. A. Schols. 2018. Human milk oligosaccharides in colostrum and mature milk of Chinese mothers: Lewis positive secretor subgroups. Journal of Agricultural and Food Chemistry 66 (27):7036–43. doi: 10.1021/acs.jafc.8b02021.
- Ewald, D. R., and S. C. J. Sumner. 2018. Human microbiota, blood group antigens, and disease. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 10 (3):e1413. doi: 10.1002/wsbm.1413.
- Fernández, L., S. Langa, V. Martín, A. Maldonado, E. Jiménez, R. Martín, and J. M. Rodríguez. 2013. The human milk microbiota: Origin and potential roles in health and disease. Pharmacological Research 69 (1):1–10. doi: 10.1016/j.phrs.2012.09.001.
- Field, C. J. 2005. The immunological components of human milk and their effect on immune development in infants. The Journal of Nutrition 135 (1):1–4. doi: 10.1093/jn/135.1.1.
- Figueroa-Lozano, S., and P. de Vos. 2019. Relationship between oligosaccharides and glycoconjugates content in human milk and the development of the gut barrier. Comprehensive Reviews in Food Science and Food Safety 18 (1):121–39. doi: 10.1111/1541-4337.12400.
- Fukuda, S., H. Toh, K. Hase, K. Oshima, Y. Nakanishi, K. Yoshimura, T. Tobe, J. M. Clarke, D. L. Topping, T. Suzuki, et al. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469 (7331):543–9. doi: 10.1038/nature09646.
- Gao, X., R. J. McMahon, J. G. Woo, B. S. Davidson, A. L. Morrow, and Q. Zhang. 2012. Temporal changes in milk proteomes reveal developing milk functions. Journal of Proteome Research 11 (7):3897–907. doi: 10.1021/pr3004002.
- Garofalo, R., S. Chheda, F. Mei, K. H. Palkowetz, H. E. Rudloff, F. C. Schmalstieg, D. K. Rassin, and A. S. Goldman. 1995. Interleukin-10 in human milk. Pediatric Research 37 (4):444–9. doi: 10.1203/00006450-199504000-00010.
- Garrido, D., J. H. Kim, J. B. German, H. E. Raybould, and D. A. Mills. 2011. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS One. 6 (3):e17315. doi: 10.1371/journal.pone.0017315.
- Garrido, D., S. Ruiz-Moyano, D. G. Lemay, D. A. Sela, J. B. German, and D. A. Mills. 2015. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Scientific Reports 5 (1):13517. doi: 10.1038/srep13517.
- Gensollen, T., S. S. Iyer, D. L. Kasper, and R. S. Blumberg. 2016. How colonization by microbiota in early life shapes the immune system. Science 352 (6285):539–44. doi: 10.1126/science.aad9378.
- Gila-Diaz, A., S. M. Arribas, A. Algara, M. A. Martín-Cabrejas, Á. L. López de Pablo, M. Sáenz de Pipaón, and D. Ramiro-Cortijo. 2019. A review of bioactive factors in human breastmilk: A focus on prematurity. Nutrients 11 (6):1307. doi: 10.3390/nu11061307.
- Goldman, A. S., C. Garza, B. L. Nichols, and R. M. Goldblum. 1982. Immunologic factors in human milk during the first year of lactation. The Journal of Pediatrics 100 (4):563–7. (82)80753-1 doi: 10.1016/S0022-3476(82)80753-1.
- Gotoh, A., T. Katoh, M. Sakanaka, Y. Ling, C. Yamada, S. Asakuma, T. Urashima, Y. Tomabechi, A. Katayama-Ikegami, S. Kurihara, et al. 2018. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Scientific Reports 8 (1):13958. doi: 10.1038/s41598-018-32080-3.
- Guo, S., T. Gillingham, Y. Guo, D. Meng, W. Zhu, W. A. Walker, and K. Ganguli. 2017. Secretions of Bifidobacterium infantis and Lactobacillus acidophilus protect intestinal epithelial barrier function. Journal of Pediatric Gastroenterology and Nutrition 64 (3):404–12. doi: 10.1097/MPG.0000000000001310.
- Hamosh, M., J. A. Peterson, T. R. Henderson, C. D. Scallan, R. Kiwan, R. L. Ceriani, M. Armand, N. R. Mehta, and P. Hamosh. 1999. Protective function of human milk: The milk fat globule. Seminars in Perinatology 23 (3):242–9. (99)80069-X doi: 10.1016/S0146-0005(99)80069-X.
- Hassiotou, F., and D. T. Geddes. 2015. Immune cell–mediated protection of the mammary gland and the infant during breastfeeding. Advances in Nutrition 6 (3):267–75. doi: 10.3945/an.114.007377.
- He, Y., S. Liu, S. Leone, and D. S. Newburg. 2014. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunology 7 (6):1326–39. doi: 10.1038/mi.2014.20.
- He, Y. Y., S. B. Liu, D. E. Kling, S. Leone, N. T. Lawlor, Y. Huang, S. B. Feinberg, D. R. Hill, and D. S. Newburg. 2016. The human milk oligosaccharide 2′-fucosyllactose modulates CD14 expression in human enterocytes, thereby attenuating LPS-induced inflammation. Gut 65 (1):33–46. doi: 10.1136/gutjnl-2014-307544.
- Hill, C. J., D. B. Lynch, K. Murphy, M. Ulaszewska, I. B. Jeffery, C. A. O’Shea, C. Watkins, E. Dempsey, F. Mattivi, K. Tuohy, et al. 2017. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 5 (1):4. doi: 10.1186/s40168-016-0213-y.
- Hill, D. R., and D. S. Newburg. 2015. Clinical applications of bioactive milk components. Nutrition Reviews 73 (7):463–76. doi: 10.1093/nutrit/nuv009.
- Hillman, E. T., H. Lu, T. Yao, and C. H. Nakatsu. 2017. Microbial ecology along the gastrointestinal tract. Microbes and Environments 32 (4):300–13. doi: 10.1264/jsme2.ME17017.
- Hoeflich, A., and Z. Meyer. 2017. Functional analysis of the IGF-system in milk. Best Practice & Research Clinical Endocrinology & Metabolism 31 (4):409–18. doi: 10.1016/j.beem.2017.10.002.
- Holscher, H. D., L. Bode, and K. A. Tappenden. 2017. Human Milk oligosaccharides influence intestinal epithelial cell maturation in vitro. Journal of Pediatric Gastroenterology and Nutrition 64 (2):296–301. doi: 10.1097/MPG.0000000000001274.
- Hosseini, S. M., T. Talaei-Khozani, M. Sani, and B. Owrangi. 2014. Differentiation of human breast-milk stem cells to neural stem cells and neurons. Neurology Research International 2014:1–8. doi: 10.1155/2014/807896.
- Hossny, E. M., D. H. El-Ghoneimy, R. H. El-Owaidy, M. G. Mansour, M. T. Hamza, and A. F. El-Said. 2020. Breast milk interleukin-7 and thymic gland development in infancy. European Journal of Nutrition 59 (1):111–8. doi: 10.1007/s00394-018-01891-5.
- Jansen, M. 2019. Marching out of the crypt. Science 365 (6454):642–3. doi: 10.1126/science.aay5861.
- Jantscher-Krenn, E., T. Lauwaet, L. A. Bliss, S. L. Reed, F. D. Gillin, and L. Bode. 2012. Human milk oligosaccharides reduce Entamoeba histolytica attachment and cytotoxicity in vitro. British Journal of Nutrition 108 (10):1839–46. doi: 10.1017/S0007114511007392.
- Jantscher-Krenn, E., M. Zherebtsov, C. Nissan, K. Goth, Y. S. Guner, N. Naidu, B. Choudhury, A. V. Grishin, H. R. Ford, and L. Bode. 2012. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 61 (10):1417–25. doi: 10.1136/gutjnl-2011-301404.
- Jeurink, P. V., J. van Bergenhenegouwen, E. Jiménez, L. M. J. Knippels, L. Fernández, J. Garssen, J. Knol, J. M. Rodríguez, and R. Martín. 2013. Human milk: A source of more life than we imagine. Beneficial Microbes 4 (1):17–30. doi: 10.3920/BM2012.0040.
- Johansson, M. E. V., and G. C. Hansson. 2016. Immunological aspects of intestinal mucus and mucins. Nature Reviews Immunology 16 (10):639–49. doi: 10.1038/nri.2016.88.
- Johnson, P. H., and W. M. Watkins. 1992. Purification of the Lewis blood-group gene associated α-3/4-fucosyltransferase from human milk: An enzyme transferring fucose primarily to Type 1 and lactose-based oligosaccharide chains. Glycoconjugate Journal 9 (5):241–9. doi: 10.1007/BF00731136.
- Jost, T., C. Lacroix, C. Braegger, and C. Chassard. 2015. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutrition Reviews 73 (7):426–37. doi: 10.1093/nutrit/nuu016.
- Kavanaugh, D., J. O'Callaghan, M. Kilcoyne, M. Kane, L. Joshi, and R. M. Hickey. 2015. The intestinal glycome and its modulation by diet and nutrition. Nutrition Reviews 73 (6):359–75. doi: 10.1093/nutrit/nuu019.
- Kavanaugh, D. W., J. O’Callaghan, L. F. Buttó, H. Slattery, J. Lane, M. Clyne, M. Kane, L. Joshi, and R. M. Hickey. 2013. Exposure of Bifidobacterium longum subsp. infantis to milk oligosaccharides increases adhesion to epithelial cells and induces a substantial transcriptional response. PLoS One 8 (6):e67224. doi: 10.1371/journal.pone.0067224.
- Khodayar-Pardo, P., L. Mira-Pascual, M. C. Collado, and C. Martínez-Costa. 2014. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. Journal of Perinatology 34 (8):599–605. doi: 10.1038/jp.2014.47.
- Kirmiz, N., R. C. Robinson, I. M. Shah, D. Barile, and D. A. Mills. 2018. Milk glycans and their interaction with the infant-gut microbiota. Annual Review of Food Science and Technology 9 (1):429–50. doi: 10.1146/annurev-food-030216-030207.
- Kitaoka, M. 2012. Bifidobacterial enzymes involved in the metabolism of human milk oligosaccharides. Advances in Nutrition 3 (3):422S–9S. doi: 10.3945/an.111.001420.
- Kling, P. J., T. M. Sullivan, R. A. Roberts, A. F. Philipps, and O. Koldovský. 1998. Human milk as a potential enteral source of erythropoietin. Pediatric Research 43 (2):216–21. doi: 10.1203/00006450-199802000-00010.
- Knoop, K. A., and R. D. Newberry. 2018. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunology 11 (6):1551–7. doi: 10.1038/s41385-018-0039-y.
- Kong, C., M. Elderman, L. Cheng, B. J. de Haan, A. Nauta, and P. de Vos. 2019. Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non-digestible carbohydrates. Molecular Nutrition & Food Research 63 (17):1900303. doi: 10.1002/mnfr.201900303.
- Kulinich, A., and L. Liu. 2016. Human milk oligosaccharides: The role in the fine-tuning of innate immune responses. Carbohydrate Research 432:62–70. doi: 10.1016/j.carres.2016.07.009.
- Kuntz, S., S. Rudloff, and C. Kunz. 2008. Oligosaccharides from human milk influence growth-related characteristics of intestinally transformed and non-transformed intestinal cells. British Journal of Nutrition 99 (3):462–71. doi: 10.1017/S0007114507824068.
- Kurakevich, E., T. Hennet, M. Hausmann, G. Rogler, and L. Borsig. 2013. Milk oligosaccharide sialyl(α2,3)lactose activates intestinal CD11c + cells through TLR4. Proceedings of the National Academy of Sciences of Sciences 110 (43):17444–9. America, doi: 10.1073/pnas.1306322110.
- Ladirat, S. E. 2014. Galacto-oligosasaccharides to counter the side effects of antibiotic treatments. https://library.wur.nl/WebQuery/wda/2050554
- Lawrence, R. M., and R. A. Lawrence. 2004. Breast milk and infection. Clinics in Perinatology 31 (3):501–28. doi: 10.1016/j.clp.2004.03.019.
- Le Doare, K., and B. Kampmann. 2014. Breast milk and group B streptococcal infection: Vector of transmission or vehicle for protection? Vaccine 32 (26):3128–32. doi: 10.1016/j.vaccine.2014.04.020.
- Lenoir, D., D. Ruggiero-Lopez, P. Louisot, and M. C. Biol. 1995. Developmental changes in intestinal glycosylation: Nutrition-dependent multi-factor regulation of the fucosylation pathway at weaning time. Biochimica et Biophysica Acta (Bba) - Biomembranes 1234 (1):29–36. doi: 10.1016/0005-2736(94)00254-M.
- Lewis, D. E., and S. E. Blutt. 2019. Organization of the immune system. In Clinical immunology. doi: 10.1016/B978-0-7020-6896-6.00002-8.
- Lewis, E. D., C. Richard, B. M. Larsen, and C. J. Field. 2017. The importance of human milk for immunity in preterm infants. Clinics in Perinatology 44 (1):23–47. doi: 10.1016/j.clp.2016.11.008.
- Lin, A. E., C. A. Autran, S. D. Espanola, L. Bode, and V. Nizet. 2014. Human milk oligosaccharides protect bladder epithelial cells against uropathogenic Escherichia coli invasion and cytotoxicity. Journal of Infectious Diseases 209 (3):389–98. doi: 10.1093/infdis/jit464.
- Lin, A. E., C. A. Autran, A. Szyszka, T. Escajadillo, M. Huang, K. Godula, A. R. Prudden, G.-J. Boons, A. L. Lewis, K. S. Doran, et al. 2017. Human milk oligosaccharides inhibit growth of group B Streptococcus. Journal of Biological Chemistry 292 (27):11243–9. doi: 10.1074/jbc.M117.789974.
- Liu, B., Z. Yu, C. Chen, D. E. Kling, and D. S. Newburg. 2012. Human milk mucin 1 and mucin 4 inhibit Salmonella enterica Serovar Typhimurium invasion of human intestinal epithelial cells in vitro. The Journal of Nutrition 142 (8):1504–9. doi: 10.3945/jn.111.155614.
- Lodge, C., D. Tan, M. Lau, X. Dai, R. Tham, A. Lowe, G. Bowatte, K. Allen, and S. Dharmage. 2015. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatrica 104:38–53. doi: 10.1111/apa.13132.
- Lönnerdal, B. 2014. Infant formula and infant nutrition: Bioactive proteins of human milk and implications for composition of infant formulas. American Journal of Clinical Nutrition 99 (3):712S–717S. doi: 10.3945/ajcn.113.071993.
- Lönnerdal, B. 2016. Human milk: Bioactive proteins/peptides and functional properties. Nestle Nutrition Institute Workshop Series 86:97–107. doi: 10.1159/000442729.
- Loui, A., E. Eilers, E. Strauss, A. Pohl-Schickinger, M. Obladen, and P. Koehne. 2012. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor 1 (sFlt-1) levels in early and mature human milk from mothers of preterm versus term infants. Journal of Human Lactation 28 (4):522–8. doi: 10.1177/0890334412447686.
- Macpherson, A. J., M. G. de Agüero, and S. C. Ganal-Vonarburg. 2017. How nutrition and the maternal microbiota shape the neonatal immune system. Nature Reviews Immunology 17 (8):508–17. doi: 10.1038/nri.2017.58.
- Mäkivuokko, H., S. J. Lahtinen, P. Wacklin, E. Tuovinen, H. Tenkanen, J. Nikkilä, M. Björklund, K. Aranko, A. C. Ouwehand, and J. Mättö. 2012. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiology 12 (1):94. doi: 10.1186/1471-2180-12-94.
- Martens, E. C., M. Neumann, and M. S. Desai. 2018. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nature Reviews Microbiology 16 (8):457–70. doi: 10.1038/s41579-018-0036-x.
- Martin, C. R., P. R. Ling, and G. L. Blackburn. 2016. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 8 (5):279. doi: 10.3390/nu8050279.
- Martín, R., M. Olivares, M. L. Marín, L. Fernández, J. Xaus, and J. M. Rodríguez. 2005. Probiotic potential of 3 lactobacilli strains isolated from breast milk. Journal of Human Lactation 21 (1):8–17. doi: 10.1177/0890334404272393.
- Martini, M.,. F. Salari, and I. Altomonte. 2016. The macrostructure of milk lipids: The fat globules. Critical Reviews in Food Science and Nutrition 56 (7):1209–21. doi: 10.1080/10408398.2012.758626.
- McGuire, M. K., and M. A. McGuire. 2017. Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Current Opinion in Biotechnology 44:63–8. doi: 10.1016/j.copbio.2016.11.013.
- McLeod, K. H., J. L. Richards, Y. A. Yap, and E. Mariño. 2019. Dietary short chain fatty acids: How the gut microbiota fight against autoimmune and inflammatory diseases. In. Bioactive food as dietary interventions for arthritis and related inflammatory diseases, 139–59. London: Academic Press. doi: 10.1016/b978-0-12-813820-5.00007-6.
- Meki, A. R. M. A., T. H. Saleem, M. H. Al-Ghazali, and A. A. Sayed. 2003. Interleukins -6, -8 and -10 and tumor necrosis factor-alpha and its soluble receptor I in human milk at different periods of lactation. Nutrition Research 23 (7):845–55. (03)00035-6 doi: 10.1016/S0271-5317(03)00035-6.
- Molès, J. P., E. Tuaillon, C. Kankasa, A. S. Bedin, N. Nagot, A. Marchant, J. M. McDermid, and P. Van de Perre. 2018. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatric Allergy and Immunology 29 (2):133–43. doi: 10.1111/pai.12841.
- Mountzouris, K. C., A. L. McCartney, and G. R. Gibson. 2002. Intestinal microflora of human infants and current trends for its nutritional modulation. British Journal of Nutrition 87 (5):405–20. doi: 10.1079/BJN2002563.
- Mowat, A. M., and W. W. Agace. 2014. Regional specialization within the intestinal immune system. Nature Reviews Immunology 14 (10):667–85. doi: 10.1038/nri3738.
- Mueller, N. T., E. Bakacs, J. Combellick, Z. Grigoryan, and M. G. Dominguez-Bello. 2015. The infant microbiome development: Mom matters. Trends in Molecular Medicine 21 (2):109–17. doi: 10.1016/j.molmed.2014.12.002.
- Neu, J., B. Poindexter, A. L. Morrow, and D. S. Newburg. 2019. Human milk oligosaccharide. In Gastroenterology and nutrition, 43–57. doi: 10.1016/B978-0-323-54502-0.00004-9.
- Newton, R., B. Priyadharshini, and L. A. Turka. 2016. Immunometabolism of regulatory T cells. Nature Immunology 17 (6):618–25. doi: 10.1038/ni.3466.
- Nijman, R. M., Y. Liu, A. Bunyatratchata, J. T. Smilowitz, B. Stahl, and D. Barile. 2018. Characterization and quantification of oligosaccharides in human milk and infant formula. Journal of Agricultural and Food Chemistry 66 (26):6851–9. doi: 10.1021/acs.jafc.8b01515.
- Ninkina, N.,. M. S. Kukharsky, M. V. Hewitt, E. A. Lysikova, L. N. Skuratovska, A. V. Deykin, and V. L. Buchman. 2019. Stem cells in human breast milk. Human Cell 32 (3):223–30. doi: 10.1007/s13577-019-00251-7.
- Okumura, R., and K. Takeda. 2017. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Experimental & Molecular Medicine 49 (5):e338–e338. doi: 10.1038/emm.2017.20.
- Palmeira, P., and M. Carneiro-Sampaio. 2016. Immunology of breast milk. Revista da Associação Médica Brasileira 62 (6):584–93. doi: 10.1590/1806-9282.62.06.584.
- Parigi, S. M., M. Eldh, P. Larssen, S. Gabrielsson, and E. J. Villablanca. 2015. Breast milk and solid food shaping intestinal immunity. Frontiers in Immunology 6 (Jul):415. doi: 10.3389/fimmu.2015.00415.
- Patel, A. L., and J. H. Kim. 2018. Human milk and necrotizing enterocolitis. Seminars in Pediatric Surgery 27 (1):34–8. doi: 10.1053/j.sempedsurg.2017.11.007.
- Patki, S., S. Kadam, V. Chandra, and R. Bhonde. 2010. Human breast milk is a rich source of multipotent mesenchymal stem cells. Human Cell 23 (2):35–40. doi: 10.1111/j.1749-0774.2010.00083.x.
- Pitt, J., M. Chan, C. Gibson, O. Hasselwander, A. Lim, P. Mukerji, R. Mukherjea, A. Myhre, P. Sarela, P. Tenning, et al. 2019. Safety assessment of the biotechnologically produced human-identical milk oligosaccharide 3-Fucosyllactose (3-FL). Food and Chemical Toxicology 134:110818. doi: 10.1016/j.fct.2019.110818.
- Plaza-Díaz, J., L. Fontana, and A. Gil. 2018. Human milk oligosaccharides and immune system development. Nutrients 10 (8):1038. doi: 10.3390/nu10081038.
- Puccio, G., P. Alliet, C. Cajozzo, E. Janssens, G. Corsello, N. Sprenger, S. Wernimont, D. Egli, L. Gosoniu, and P. Steenhout. 2017. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. Journal of Pediatric Gastroenterology and Nutrition 64 (4):624–31. doi: 10.1097/MPG.0000000000001520.
- Reboldi, A., and J. G. Cyster. 2016. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunological Reviews 271 (1):230–45. doi: 10.1111/imr.12400.
- Rodríguez-Camejo, C.,. A. Puyol, L. Fazio, A. Rodríguez, E. Villamil, E. Andina, V. Cordobez, H. Díaz, M. Lemos, G. Siré, et al. 2018. Antibody profile of colostrum and the effect of processing in human milk banks: Implications in immunoregulatory properties. Journal of Human Lactation 34 (1):137–47. doi: 10.1177/0890334417706359.
- Ruiz-Palacios, G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguia, and D. S. Newburg. 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fucα1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. Journal of Biological Chemistry 278 (16):14112–20. doi: 10.1074/jbc.M207744200.
- Sabha, B. H., F. Alzahrani, H. A. Almehdar, V. N. Uversky, and E. M. Redwan. 2018. Disorder in milk proteins: Lactadherin multifunctionality and structure. Current Protein & Peptide Science 19 (10):983–97. doi: 10.2174/1389203719666180608091849.
- Salli, K., H. Anglenius, J. Hirvonen, A. A. Hibberd, I. Ahonen, M. T. Saarinen, K. Tiihonen, J. Maukonen, and A. C. Ouwehand. 2019. The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; A pilot study in comparison to GOS and lactose. Scientific Reports 9 (1):13232. doi: 10.1038/s41598-019-49497-z.
- Santajit, S., and N. Indrawattana. 2016. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Research International 2016:1–8. doi: 10.1155/2016/2475067.
- Savel'ev, A. N., T. G. Kanyshkova, A. A. Kulminskaya, V. N. Buneva, E. V. Eneyskaya, M. V. Filatov, G. A. Nevinsky, and K. N. Neustroev. 2001. Amylolytic activity of IgG and sIgA immunoglobulins from human milk. Clinica Chimica Acta 314 (1-2):141–52. (01)00691-X doi: 10.1016/S0009-8981(01)00691-X.
- Schrezenmeir, J., H. Korhonen, C. Williams, H. S. Gill, and N. Shah. 2000. Foreword. British Journal of Nutrition 84 (S1):1. doi: 10.1017/S0007114500002178.
- Schwab, C., H. J. Ruscheweyh, V. Bunesova, V. T. Pham, N. Beerenwinkel, and C. Lacroix. 2017. Trophic interactions of infant bifidobacteria and Eubacterium hallii during L-fucose and fucosyllactose degradation. Frontiers in Microbiology 8 (Jan):95. doi: 10.3389/fmicb.2017.00095.
- Secker, G. A., and N. L. Harvey. 2015. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Developmental Dynamics 244 (3):323–331. doi: 10.1002/dvdy.24227.
- Semba, R. D., and S. E. Juul. 2002. Erythropoietin in human milk: Physiology and role in infant health. Journal of Human Lactation 18 (3):252–261. doi: 10.1177/089033440201800307.
- Shiou, S. R., Y. Yu, S. Chen, M. J. Ciancio, E. O. Petrof, J. Sun, and E. C. Claud. 2011. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. Journal of Biological Chemistry 286 (14):12123–12132. doi: 10.1074/jbc.M110.154625.
- Siafakas, C. G., F. Anatolitou, R. D. Fusunyan, W. A. Walker, and I. R. Sanderson. 1999. Vascular endothelial growth factor (VEGF) is present in human breast milk and its receptor is present on intestinal epithelial cells. Pediatric Research 45 (5):652–657. doi: 10.1203/00006450-199905010-00007.
- Sitarik, A. R., K. R. Bobbitt, S. L. Havstad, K. E. Fujimura, A. M. Levin, E. M. Zoratti, H. Kim, K. J. Woodcroft, G. Wegienka, D. R. Ownby, et al. 2017. Breast milk transforming growth factor β is associated with neonatal gut microbial composition. Journal of Pediatric Gastroenterology and Nutrition 65 (3):e60–e67. doi: 10.1097/MPG.0000000000001585.
- Sprenger, G. A., F. Baumgärtner, and C. Albermann. 2017. Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations. Journal of Biotechnology 258:79–91. doi: 10.1016/j.jbiotec.2017.07.030.
- Tailford, L. E., E. H. Crost, D. Kavanaugh, and N. Juge. 2015. Mucin glycan foraging in the human gut microbiome. Frontiers in Genetics 6 (Feb):81. doi: 10.3389/fgene.2015.00081.
- Tamburini, S., N. Shen, H. C. Wu, and J. C. Clemente. 2016. The microbiome in early life: Implications for health outcomes. Nature Medicine 22 (7):713–722. doi: 10.1038/nm.4142.
- Telang, S. 2018. Lactoferrin: A critical player in neonatal host defense. Nutrients 10 (9):1228. doi: 10.3390/nu10091228.
- Tezuka, H., and T. Ohteki. 2019. Regulation of IgA production by intestinal dendritic cells and related cells. Frontiers in Immunology 10:1891. doi: 10.3389/fimmu.2019.01891.
- Thomas, P. G., M. R. Carter, O. Atochina, A. A. Da’Dara, D. Piskorska, E. McGuire, and D. A. Harn. 2003. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a toll-like receptor 4-dependent mechanism. The Journal of Immunology 171 (11):5837–5841. doi: 10.4049/jimmunol.171.11.5837.
- Thomson, P., D. A. Medina, and D. Garrido. 2018. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiology 75:37–46. doi: 10.1016/j.fm.2017.09.001.
- Thurl, S., M. Munzert, G. Boehm, C. Matthews, and B. Stahl. 2017. Systematic review of the concentrations of oligosaccharides in human milk. Nutrition Reviews 75 (11):920–933. doi: 10.1093/nutrit/nux044.
- Thurl, S., M. Munzert, J. Henker, G. Boehm, B. Müller-Werner, J. Jelinek, and B. Stahl. 2010. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. British Journal of Nutrition 104 (9):1261–1271. doi: 10.1017/S0007114510002072.
- Torow, N., B. J. Marsland, M. W. Hornef, and E. S. Gollwitzer. 2017. Neonatal mucosal immunology. Mucosal Immunology 10 (1):5–17. doi: 10.1038/mi.2016.81.
- Toscano, M., R. De Grandi, E. Grossi, and L. Drago. 2017. Role of the human breast milk-associated microbiota on the newborns’ immune system: A mini review. Frontiers in Microbiology 8 (Oct):2100. doi: 10.3389/fmicb.2017.02100.
- Townsend, S. D. 2019. Human milk oligosaccharides: Defense against pathogens. Breastfeeding Medicine 14 (S1):S-5–S6. doi: 10.1089/bfm.2019.0039.
- Triantis, V., L. Bode, and J. R. J. van Neerven. 2018. Immunological effects of human milk oligosaccharides. Frontiers in Pediatrics 6:190. doi: 10.3389/fped.2018.00190.
- Turfkruyer, M., and V. Verhasselt. 2015. Breast milk and its impact on maturation of the neonatal immune system. Current Opinion in Infectious Diseases 28 (3):199–206. doi: 10.1097/QCO.0000000000000165.
- Urbaniak, C., M. Angelini, G. B. Gloor, and G. Reid. 2016. Human milk microbiota profiles in relation to birthing method, gestation and infant gender. Microbiome 4 (1):1. doi: 10.1186/s40168-015-0145-y.
- Van De Perre, P. 2003. Transfer of antibody via mother’s milk. Vaccine 21 (24):3374–3376. (03)00336-0 doi: 10.1016/S0264-410X(03)00336-0.
- van den Elsen, L. W. J., J. Garssen, R. Burcelin, and V. Verhasselt. 2019. Shaping the gut microbiota by breastfeeding: The gateway to allergy prevention? Frontiers in Pediatrics 7 (FEB):47. doi: 10.3389/fped.2019.00047.
- Vandenplas, Y., B. Berger, V. Carnielli, J. Ksiazyk, H. Lagström, M. Sanchez Luna, N. Migacheva, J.-M. Mosselmans, J.-C. Picaud, M. Possner, et al. 2018. Human milk oligosaccharides: 2’-fucosyllactose (2’-FL) and lacto-n-neotetraose (LNnT) in infant formula. Nutrients 10 (9):1161. doi: 10.3390/nu10091161.
- Vass, R. A., A. Kemeny, T. Dergez, T. Ertl, D. Reglodi, A. Jungling, and A. Tamas. 2019. Distribution of bioactive factors in human milk samples. International Breastfeeding Journal 14 (1):9. doi: 10.1186/s13006-019-0203-3.
- Vasta, G. R. 2012. Galectins as pattern recognition receptors: Structure, function, and evolution. Advances in Experimental Medicine and Biology 946:21–36. doi: 10.1007/978-1-4614-0106-3_2.
- Victora, C. G., R. Bahl, A. J. D. Barros, G. V. A. França, S. Horton, J. Krasevec, S. Murch, M. J. Sankar, N. Walker, and N. C. Rollins. 2016. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. The Lancet 387 (10017):475–490. (15)01024-7 doi: 10.1016/S0140-6736(15)01024-7.
- Vieira Borba, V., K. Sharif, and Y. Shoenfeld. 2018. Breastfeeding and autoimmunity: Programing health from the beginning. American Journal of Reproductive Immunology 79 (1):e12778. doi: 10.1111/aji.12778.
- Vojdani, A. 2015. Oral tolerance and its relationship to food immunoreactivities. Alternative Therapies in Health and Medicine 21:23–32. http://www.ncbi.nlm.nih.gov/pubmed/25599183.
- Wagner, C. L., S. N. Taylor, and D. Johnson. 2008. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clinical Reviews in Allergy & Immunology 34 (2):191–204. doi: 10.1007/s12016-007-8032-3.
- Wang, C., M. Zhang, H. Guo, J. Yan, F. Liu, J. Chen, Y. Li, and F. Ren. 2019. Human milk oligosaccharides protect against necrotizing enterocolitis by inhibiting intestinal damage via increasing the proliferation of crypt cells. Molecular Nutrition & Food Research 63 (18):1900262. doi: 10.1002/mnfr.201900262.
- Wickramasinghe, S., A. R. Pacheco, D. G. Lemay, and D. A. Mills. 2015. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiology 15 (1):172. doi: 10.1186/s12866-015-0508-3.
- Witkowska-Zimny, M., and E. Kaminska-El-Hassan. 2017. Cells of human breast milk. Cellular & Molecular Biology Letters 22 (1):11. doi: 10.1186/s11658-017-0042-4.
- Wu, R. Y., B. Li, Y. Koike, P. Määttänen, H. Miyake, M. Cadete, K. C. Johnson-Henry, S. R. Botts, C. Lee, T. R. Abrahamsson, et al. 2018. Human milk oligosaccharides increase mucin expression in experimental necrotizing enterocolitis. Molecular Nutrition & Food Research 63 (3):1800658–11. doi: 10.1002/mnfr.201800658.
