Abstract
The quality of existing evidence about the impact of diet quality on colorectal cancer (CRC) risk has only rarely been assessed. In the current review, we searched PubMed, EMBASE, Web of Science, Cochrane, and the resulting references (up to January 2020) for studies that evaluated the role of high diet quality by extreme dietary index categorization and the risk of CRC. Two researchers independently performed the study selection, data extraction, and quality assessment. We then applied a random-effects meta-analysis to estimate the pooled odds ratios (ORs) and 95% confidence intervals (CIs) for CRC at the extremes of each dietary index, and we assessed the quality of the pooled results using the Grading of Recommendations Assessment, Development and Evaluation approach. A high diet quality was significantly associated with reduced CRC risk when patients had a low Diet Inflammatory Index score (OR, 0.66; 95%CI, 0.56–0.78), a high Mediterranean Diet Score (OR, 0.84; 95%CI, 0.78–0.90), high Dietary Approaches to Stop Hypertension adherence (OR, 0.83; 95%CI, 0.78–0.89), and a high Healthy Eating Index score (OR, 0.72; 95%CI, 0.64–0.80). The pooled results for all dietary indices were rated as being of low quality due to concerns over inconsistency or imprecision. We conclude that, despite a high diet quality appearing to have a preventive role in CRC, the evidence is currently of insufficient quality to develop dietary recommendations.
Introduction
Colorectal cancer (CRC) is a highly preventable cancer, yet it affects approximately 1.5 million people and leads to 800,000 cause specific deaths each year (Ferlay et al. Citation2019). In genetically susceptible individuals, diet is among the main environmental factors affecting CRC development (Herszenyi and Tulassay Citation2010). The significant manifest of dietary habits and food components on the risk of chronic complex diseases, including CRC (Song, Garrett, and Chan Citation2015), has led to the notion of diet modification as a tool for reducing the risk of disease. Thus, multiple diet indices have been created to quantify diet quality based on food components in preventing common chronic diseases.
Dietary indices vary in both their foundation and their dietary components. For instance, Diet Inflammatory Index (DII) is based on specific targeted biological mechanisms, including 45 evidence-based, inflammation-related dietary components (Shivappa et al. Citation2014), Mediterranean Diet Score (MDS) is based on epidemiologic findings on beneficial diet on cardiovascular health, composing of 11 components (Panagiotakos, Pitsavos, and Stefanadis Citation2006). Among other well-known indices, Dietary Approaches to Stop Hypertension (DASH), is built based on evidence for diet elements related to hypertension management, with eight food components (Appel et al. Citation1997) and the Healthy Eating Index (HEI-2010), quantified with 10 components, together with some regionally defined dietary indices are based on localized healthy eating guidelines (Kennedy et al. Citation1995) (Box 1). A low score on the DII and a high score on the DASH, HEI, and MDS are considered to indicate diets with fewer inflammatory properties, higher quality, and lower risk of CRC.
Assessments of the impact of diet quality, as quantified by diet indices, on CRC risk have yielded inconclusive results (Haslam et al. Citation2017; Jones et al. Citation2017; Schwingshackl and Hoffmann Citation2015a, Citation2015b). Some studies have demonstrated no association between CRC risk and the DII (Boden et al. Citation2019; Brouwer et al. Citation2017; Liu et al. Citation2017; Tabung et al. Citation2017), MDS (Fasanelli et al. Citation2017; Jafari Nasab et al. Citation2019; Lavalette et al. Citation2018; Petimar et al. Citation2018; Torres Stone et al. Citation2017), or HEI (Lavalette et al. Citation2018), whereas a large cohort study reported an approximate 30% risk reduction in CRC when people consumed a diet constituting a low DII, as an indicator of less inflammatory and high diet quality (CitationTabung et al. 2018). Similarly, consuming diet components that yield a high score of DASH, HEI (Erben et al. Citation2018) and MDS (Jones et al. Citation2017), are likely to reduce CRC risk. However, there were inconsistencies in the effects and in the directions of the effects between the diet indices and CRC risk arising from differences in the dietary components, applied dietary indices due to the course of time, study duration, study design, follow-up duration, study population, and tumor site (Galbete et al. Citation2018). Inevitably, the conclusions of any findings from related pooled analyses also carry these flaws, and this has been compounded by a failure to evaluate the overall quality of the findings of pooled analyses (Fan et al. Citation2017; Namazi, Larijani, and Azadbakht Citation2018; Schwingshackl and Hoffmann Citation2015b; Schwingshackl et al. Citation2017; Zhang, Wang, and Zhang Citation2018). To date, meta-analyses on the role of diet quality measured by dietary indices and CRC are also disputed due to the fact that pooled results were mostly driven from a single dietary index, different subtypes of a single dietary index (Schwingshackl and Hoffmann, 2015c), the inclusion of small numbers of studies (Bloomfield et al. Citation2016; Fowler and Akinyemiju Citation2017; Mohseni et al. Citation2019; Namazi, Larijani, and Azadbakht Citation2018; Schwingshackl and Hoffmann, Citation2015a, Citation2015b), findings were defined from Western dietary habits, thus not generalizable to other populations (Mohseni et al. Citation2019), and being reliant on a single pooled analysis of data from different dietary indices (Balter, Moller, and Fondell Citation2012). The observed inconsistency among individual studies, as well among previous pooled studies and the lack of qualified affirmative conclusion hinders the development of an effective and applicable dietary recommendation for the prevention of CRC.
Given the limitations in so far conducted meta-analyses, it is crucial to investigate the role of diet quality on CRC risk more comprehensively and to assess the quality of the overall findings. Accordingly, we conducted a systematic review and meta-analysis that included relevant observational studies. We evaluated the effect of diet quality measured by diet indices on CRC risk, measured the quality of current evidence, and assessed the strength of the findings. Ultimately, our goal was to conclude whether a dietary recommendation could be developed for the prevention of CRC based on this existing evidence.
Methods
Study protocol and search strategy
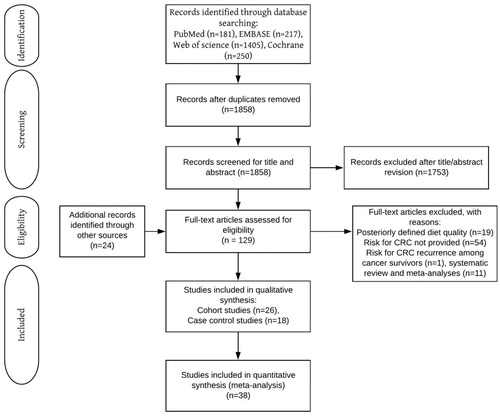
We formulated search strategies for the PubMed, Embase, Web of Science, and Cochrane online databases under the supervision of a medical librarian. We systematically searched these databases for studies evaluating the role of diet quality on CRC risk, published until January 2020, with no language restriction (Supplementary Table S1). To find further related studies, we manually searched the reference lists of included studies. Two investigators (SM and KJWS) conducted the entire procedures independently and resolved disagreement through discussion with a third investigator (BZA). The review process was based on the PRISMA guidelines (Moher et al. 2015).
Table 1. The overall quality of evidence on diet quality quantified by dietary indices on the risk of CRC in pooled findings from eligible observational studiesTable Footnotea.
Eligibility criteria and data extraction
Trials, cohort studies, and case-control studies were eligible if they quantified the association between diet quality and the risk of CRC as an outcome. Diet quality was required .i) to be quantified by either one or several of the existing dietary indices (e.g., DII, MDS, DASH, HEI, or regionally defined dietary indices) and calculated based on dietary guidelines; ii) CRC could be identified by self-reported questionnaire or pathology records reviewed by a trained physician, linkage to a cancer registry system, or linkage with a mortality records system. Studies were excluded if they met the following criteria: (i) CRC was not an outcome; (ii) determinants were other than diet quality measured by diet indiceses (iii) no summary estimate was provided in the form of an odds ratio (OR), relative risk (RR), or hazard ratio (HR), and there was no standard error or 95% confidence interval (CI).
SM and KJWS extracted the following information: study design, first author, publication year, study country, applied diet index (plus the number of food components included), gender, age (mean or range) of study population, number of total study population and incidental CRC cases in cohort studies, or number of cases and controls in cases control studies, and number of adjusted variables. They also recorded the most adjusted risk estimates with corresponding standard errors (SE) or 95% CIs for the highest compared to the lowest category of the diet index used.
Quality assessment
We evaluated the quality of eligible cohort and case-control studies using the Newcastle–Ottawa quality assessment scale (Stang Citation2010). Study quality was ranked as the following: low, ≤3 stars; moderate, 4–6 stars; and high, ≥7 stars. We awarded maximums of 9 starts to cohort and 8 stars to case-control studies. Disagreements were resolved by reaching consensus.
For cohort studies, the following items categorized in three levels were evaluated; i) selection, indicating the representativeness of exposed and unexposed study populations and the adequacy of demonstrating the outcome; ii) comparability, representing control for age/sex and as well as controlling for at least three additional risk factors such as body mass index (BMI), ethnicity, family history of gastrointestinal cancer, smoking, alcohol, physical activity, and dietary supplement intake; and iii) exposure/outcome, representing the methods applied for outcome assessment, the adequacy of follow-up for the outcome to occur (i.e., >10 years for CRC), and whether loss to follow-up was acceptable as being ≤5%.
For case-control studies, we modified the selection and exposure/outcome sections as follows: i) selection, adequacy and representativeness of case definition, the source population for selection of controls as community-based controls, etc., confirmation on no history of GI cancers among controls, and confirmation on no history of gastrointestinal cancer among controls; and ii) exposure, included the methods applied for exposure assessment (e.g., the use of secured records, validated questionnaires, or self-report assisted by a healthcare practitioner) and the comparability of methods applied for cases and controls.
Data analysis
Risk estimations for CRC by high diet quality were quantified differently using the DII (highest quality associated with the lowest category) and the other dietary indices (highest quality associated with the highest categories). We reported the most adjusted risk estimates for CRC by highest compared to the lowest quality. To aid comparison, we converted HRs and RRs to ORs as follows: HR or using the following formula; H/RR = OR/[(1 − P0) + (P0 × OR)], where P0 is the mean incidence of CRC in the general population within years of study conducted (Deeks, Higgins, Altman, 2017). Next, we calculated the corresponding SEs of ORs, using the following formula: SElog(RR) = {[SElog (OR) × log(RR)]/log(OR)} (Deeks, Higgins, Altman, 2017). The natural logarithms of the ORs, together with their SEs and 95% CIs, were calculated. For each diet index, the pooled results were estimated using the inverse variance method.
We assessed publication bias by visual evaluation of funnel plots, and Egger`s tests for funnel plot asymmetry. Trim and fill methods proposed by Duval and Tweedie were applied to handle publication bias (Duval and Tweedie Citation2000). Homogeneity was tested using the I2 Index, the Cochran’s Q statistic (χ2), and the associated P-value for heterogeneity (Phet). We consider I2≤25%, as an indication for low heterogeneity and applied fixed-effects model when I2≤25%, otherwise we used random-effects model to adjust for observed heterogeneity between studies. We conducted sensitivity analysis to assess consistency in the pooled results by excluding one study at a time and re-estimating the pooled OR. To check the impact of strata specific effect and to reveal potential sources of heterogeneity, we conducted stratified analyses for the following potential confounders: study design (strata set as cohort and case-control studies), geographical region (strata set as Asia, Europe, and North America), gender (strata set as men and women), and tumor site (strata set as colon and rectum). The meta-analysis was conducted with Review Manager (RevMan), Version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) and Comprehensive Meta-Analysis software, version 2.2 (Biostat, Englewood, New Jersey).
The quality of the pooled findings for the risk of CRC by each dietary index was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt et al. Citation2011). In this approach, the quality of the pooled results was downgraded if the following shortcomings were present: risk of bias, inconsistency, indirectness, imprecision, or other considerations (e.g. evidence restricted to published findings). These shortcomings were extracted by extensive review of the GRADE guidelines (Guyatt et al. Citation2011), and each was scored as follows: 0 = not serious (all components met the GRADE criteria), −1 = serious (1–2 components did not meet the GRADE criteria), and −2 = very serious (>2 components did not meet the GRADE criteria). The quality of the overall findings was upgraded in the presence of a large effect size, as follows: 0, when not present; +1, when the pooled effect size showed a 2-fold decrease in the risk of CRC; and +2, when the pooled effect size demonstrated a > 2 times decrease in the risk of CRC. Finally for the presence of dose-response in analyses, we scored no dose response as 0 and a presence of dose response as 1.
Results
We retrieved 129 studies for full-text evaluation of which 44 met the selection criteria (). Among these, diet quality was quantified either by the DII in 15 studies, MDS in 17 studies, the DASH in 9 studies, the HEI in 9 studies, and on regional dietary guidelines about cancer prevention in 7 studies (Supplementary Table S2). We rated 25 cohort studies as high quality (≥7 stars) and 1 as moderate quality (6 stars), whereas we rated eight out of 18 case-control studies (44.4%) as high quality (≥7 stars), and 10 as moderate quality (5–6 stars) studies (Supplementary File 1).
DII and CRC risk
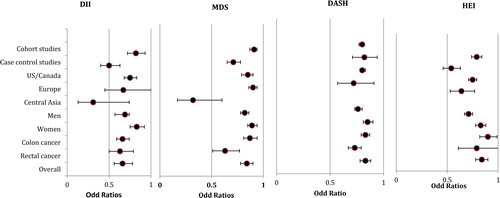
Eleven studies were included in final analysis, counting up to 1,027,206 subjects; Six cohort studies had a mean follow-up of 18.85 years) and five case-control studies with 16,896 included subjects, despite a visually detected asymmetry in funnel plot, Egger test demonstrated no significant publication bias (z = 1.04, p = 0.26) (Supplementary Figure S1A). Three studies showed extreme findings on the forest plots (Cho et al. Citation2016; Obon-Santacana et al. Citation2019; Rafiee et al. Citation2019) (Supplementary Figure S2); removing these improved the symmetry in the funnel plot. Significant heterogeneity was present (I2, 76%; Q statistics χ2 104.80; Phet <0.00001) and remained consistent after excluding the three studies with extreme findings (Cho et al. Citation2016; Obon-Santacana et al. Citation2019; Rafiee et al. Citation2019). Overall, comparing the lowest categories (as an indicator of diets with high anti-inflammatory quality) and the highest DII categories revealed a significant association with reduced CRC risk, having a pooled OR of 0.66 (95% CI: 0.56–0.78; p < 0.00001). Sensitivity analysis did not change the pooled estimates significantly. The preventive effects were consistent for three strata (i.e., geographic region, gender, and tumor site; ), and heterogeneity was significant by each of the four strata (Supplementary Figure S3A). Finally, the quality of evidence for the pooled results achieved a −2 rating (very low; ) due to inconsistency (scoring −1) and considerations (scoring −1; evidence restricted to published findings).
Figure 2. Summary risk estimates for CRC comparing the highest to lowest diet quality.
The data are stratified by study design (i.e., cohort studies/case-control studies), geographic region (i.e., US/Canada, Europe, Central Asia) gender (i.e., men, women), tumor site (i.e., colon and rectal cancers), and overall estimate. The summary risk estimates are pooled ORs for CRC comparing the effect of diet quality by the DII, MDS, DASH, and HEI. For the DII, diet quality is highest with the lowest categories and lowest with the highest categories. For the MDS, DASH, and HEI, diet quality is highest for the highest categories and lowest for the lowest categories. Abbreviations: CRC, colorectal cancer; DASH, Dietary Approach to Stop Hypertension; DII, Diet Inflammatory Index; HEI, Healthy Eating Index; MDS, Mediterranean Diet Score; OR, odds ratio; US, United State
MDS and CRC risk
Sixteen studies were included, consisting of 1,915,498 subjects; of which nine were cohort studies with a mean follow-up of 13.99 years and seven case-control studies including 74,924 subjects. A significant publication bias was detected by Egger test (z=-2.88, p = 0.0001; Supplementary Figure S1B), and heterogeneity was significant (I2, 76%; Q statistics χ2 74.19; Phet < 0.00001). Three studies with extreme findings were detected by visual forest plot assessment (Agnoli et al. Citation2013; Jafari Nasab et al. Citation2019; Rosato et al. Citation2016) (Supplementary Figure S2), and the detected heterogeneity was non-significant after excluding these studies (I2, 29%; χ2, 21.12; Phet = 0.13). A significant decrease was detected in the risk of CRC when comparing the highest and lowest MDS categories, giving a pooled OR of 0.84 (95% CI, 0.78–0.90; p < 0.00001). Unbiased pooled effect size, computed by trim and fill methods, showed similar findings (OR: 0.81, 95% CI: 0.74–0.88). The sensitivity analysis did not change the pooled estimates significantly, and the preventive effects remained significant (with comparable pooled ORs in the same direction) for analyses stratified by study design, geographic region, gender, and tumor site (excluding colon cancer; ). The heterogeneity became non-significant in the stratified analysis for cohort studies, geographic regions, women, and rectal cancer, but it remained significant for case-control studies, men, and colon cancers (Supplementary Figure S3B). Finally, the quality of evidence for the pooled effect of MDS on CRC risk was rated as −2 (very low; ) because of inconsistency (scoring −1), publication bias and restriction to published findings (scoring −1).
DASH and CRC risk
Eight studies were included being five cohort studies (1,238,750 subjects) with a mean follow-up of 15.13 years, and thee case-control studies consisting of 53,433 subjects. There was no apparent publication bias (Egger test intercept; Z = 0.12, P = 0.87) or significant heterogeneity (I2, 0%; Q statistics χ2 3.39; Phet = 0.64) (Supplementary Figure S1C and S2). Comparison between the highest and lowest categories of the DASH revealed a significant 1.20-fold (i.e., 1/0.83), decrease in the risk of CRC (95% CI, 1.37–4.34 P < 0.00001). Sensitivity analysis showed no significant change in the overall pooled effect sizes, and they remained significant in all strata, with no evidence of significant heterogeneity in any strata ( and Supplementary S3C). Finally, the pooled findings achieved a quality score of −2 (very low) due to indirectness (limited number of included studies and findings being restricted to specific geographic regions; scored as −1), and restriction to published findings (scored as −1, ).
HEI and CRC risk
Eight studies were included consisting of five cohort studies of 1,263,312 subjects and with a mean follow-up of 14.52 years) and three case-control studies including 17,396 subjects. Moderate, but not significant, asymmetry was observed in the funnel plot (Egger test intercept; Z = −1.6, P = 0.17) (Supplementary Figure S1D) and heterogeneity was significant (I2, 71%; Q statistics χ2, 31.53; Phet = 0.0002; Supplementary Figure S2). Only one study had extreme findings in the forest plot analysis of the pooled results (Jafari Nasab et al. Citation2019) (Supplementary Figure S2), and excluding this study improved the asymmetry in the funnel plot and made the observed heterogeneity non-significant (I2, 36%; Q statistics χ2, 12.50; Phet = 0.13). We detected a significant protective effect for the HEI on CRC risk when comparing the highest and lowest categories, with a pooled OR estimate of 0.72 (95% CI, 0.64–0.80). The observed benefit persisted in the sensitivity analysis and in all stratified analyses (Supplementary Figure S3D). We detected no significant heterogeneity among the included studies when pooling the results for cohort studies, geographic region, men, and tumor site (Supplementary Figure S3D). Finally, this evidence gained the lowest quality rating (scoring as −3 overall; ) due to inconsistency (scored set as −1), indirectness (-1), and restriction to published findings (-1).
Discussion
In this systematic review and meta-analysis of 38 studies, covering more than 5.6 million people, we found that high diet quality quantified by the four main diet indices—the DII, the MDS, the DASH, and the HEI—was consistently and significantly associated with a lower risk of CRC. However, the estimated pooled findings of the protective effect on CRC risk of a high diet quality, as predicted by each index, were of low quality overall and were therefore unsuitable for use in developing recommendations.
DII and CRC
The preventive benefits of the lowest compared to the highest DII category in terms of CRC risk were in line with those seen in previous meta-analyses (Fan et al. Citation2017; Fowler and Akinyemiju Citation2017; Jayedi, Emadi, and Shab-Bidar Citation2018; Namazi, Larijani, and Azadbakht Citation2018; Shivappa et al. Citation2017; Zhang, Wang, and Zhang Citation2018). These included three (Fowler and Akinyemiju Citation2017), five (Namazi, Larijani, and Azadbakht Citation2018) eight (Fan et al. Citation2017), and nine (Shivappa et al. Citation2017a) of the 11 studies included in the present meta-analysis. For the included cohort and case-control studies, these reported 1.21- to 1.33-fold and 1.73- to 1.81-fold risk reductions in CRC risk with the lowest DII category, respectively.
All studies included in our meta-analysis used the latest version of the DII, comprising 18–45 food components validated based on their association with five inflammatory biomarkers of interleukins (IL)-1β, IL-4, IL-6, IL-10, plus tumor necrosis factor alpha (TNF-α) (Shivappa et al. Citation2014). In contrast to the overall pooled risk estimates, three studies in our meta-analysis reported a null effect of low DII scores on CRC risk (Boden et al. Citation2019; Brouwer et al. Citation2017; Liu et al. Citation2017). However, a closer look at the methods used in these studies revealed that the inconsistency probably resulted from a lack of data on food parameters with anti-inflammatory effects in the DII calculation, a failure to adjust for some confounders (e.g., history of NSAID use) (Boden et al. Citation2019), and reduced generalizability due to the study population (e.g. selected among health professionals (Liu et al. Citation2017) or people with Lynch syndrome (Brouwer et al. Citation2017). The heterogeneity observed in the pooled effect was also similar to that observed in previous meta-analyses (Fan et al. Citation2017b; Jayedi, Emadi, and Shab-Bidar Citation2018; Namazi, Larijani, and Azadbakht Citation2018; Shivappa et al. Citation2017; Zhang, Wang, and Zhang Citation2018). Given the consistency in benefit observed in all stratified analyses for study design, geographic region, gender, and tumor site, the protective effect of a low DII (i.e., a highly anti-inflammatory diet) is likely to be a true effect rather than the result of confounding. Nevertheless, sources of confounding and heterogeneity remain with the study design, sample size, differences in adjusted confounders, included dietary components in DII scoring system, chosen data analysis, and validation of the food frequency questionnaires. Additionally, the observed higher protective effect of healthy diet in overall findings compared to other diet quality indices was not consistent in stratified analysis by study design (the pooled effects from cohort studies demonstrated comparable pooled effect size with that of MDS, DASH and HEI). Therefore, further large population based investigations are warranted to confirm the superior risk predictive function of DII compared to other diet quality indices.
In summary, the beneficial effects of low DII scores on CRC risk lack the strength necessary to be used in developing dietary recommendations, primarily because of the high levels of inconsistency in the included studies. Given the low overall quality, the complex scoring system based on the inflammatory response to food components, and the wide range of food components (18–45), there is only a low level of transparency. This precludes moving toward making dietary recommendations for CRC prevention based on the DII scoring system.
MDS and CRC
The association of high MDS scores with a reduced risk of CRC was consistent with the findings from a recent meta-analysis that included 13 of the 16 studies considered eligible for our meta-analysis (Schwingshackl et al. Citation2017), reporting a 1.21-to-1.40 times risk reduction. The included studies took between 9 and 11 food components with a confirmed role in cardiovascular health. Notably, six of the included cohort studies reported non-significant effects of high MDS scores on CRC risk. The results may reflect high levels of heterogeneity due to differences in the study population (e.g., a specific socioeconomic class that was not representative of the general population) (Bamia et al. Citation2013; Fung et al. Citation2010; Lavalette et al. Citation2018; Petimar et al. Citation2018; Torres Stone et al. Citation2017) or the inclusion of a small number of incident cases (Lavalette et al. Citation2018). It is also worthy to notice that these studies were mainly conducted in North America and Europe (none Mediterranean regions), highlighting the role of variation in culture and food preference in predictive performance of a diet quality index, basically developed based on dietary habits in different geographic regions.
Nevertheless, stratified analyses for cohort studies, as well as those conducted in North America, with women, and with rectal cancer, showed comparable results to the overall pooled estimates. Heterogeneity was high among case-control studies as well as those conducted in Europe, in men, and in colon cancer. Considering the inconsistencies with the overall findings, we could not generalize the association of a high MDS with a lower CRC risk. Consequently, the MDS lacked the required transparency to be used in the development of dietary recommendations, mainly due to the low quality of the overall findings, the positive scoring system for moderate alcohol use, and the downgrading of dairy products despite their evident role in preventing CRC.
DASH and CRC
The overall findings of benefit with the DASH were comparable to those reported by Mohseni et al. (Mohseni et al. Citation2019) who demonstrated risk reductions of 1.14-times and 1.09-times for CRC in the pooled findings of four cohort and two case-control studies, respectively. The included studies considered 7–10 dietary components associated with a decrease in the risk of hypertension. Moreover, the protective effect of a high DASH score on CRC risk remained consistent despite the individual studies applying different methods to calculate the scores, including the use of quintiles/servings as cutoff points or setting sex-specific standards (Erben et al. Citation2018; Miller et al. Citation2013; Park et al. Citation2017; Petimar et al. Citation2018; Torres Stone et al. Citation2017), applying the same standards for both sexes (Miller et al. Citation2013), and setting standards for sex, age, and physical activity (Miller et al. Citation2013). In contrast to our findings, Dixon et al. and Petimar et al. found that high DASH scores had no effect on CRC risk among men (Petimar et al. Citation2018; Torres Stone et al. Citation2017). However, these discrepancies could be accounted for by their inclusion of a specific study population with a high socioeconomic status and by limited reporting of confounders (e.g., family history of CRC). Despite the discrepancies with the overall findings and the variation in the scoring system, we detected minor heterogeneity in the findings of benefit. All but one study ((Erben et al. Citation2018)) were conducted in North America, precluding global generalization. The lack of geographic variation coupled with the small effect size implied indirectness in the findings of benefit, necessitating that we down rate the quality and transparency of the results for developing dietary recommendations. Nevertheless, the inclusion of food components with an evident role in reducing the risk of CRC and the use of gender, age, and physical activity cutoff standards meant that these data had higher transparency compared with either the DII or the MDS.
HEI and CRC
The observed preventive effect of a high HEI on CRC risk supported the findings of a recent meta-analysis of three cohort studies (Schwingshackl and Hoffmann Citation2015b) that reported a risk reduction of 1.29 for the highest compared to lowest HEI category. All studies in our analysis used either the HEI or HEI-2010, which was originally developed to quantify adherence to American dietary guidelines and comprises 10–12 dietary components with higher scores reflecting better diet quality (Guenther et al. Citation2013). Two studies also applied the alternate Healthy Eating Index (AHEI) (Park et al. Citation2017; Reedy et al. Citation2008), which comprises 9–11 dietary components and was developed to identify dietary patterns associated with a lower risk of chronic disease (Chiuve et al. Citation2012). Despite applying these different methods to HEI scoring, the findings were consistent with the pooled results in all but one eligible study (Lavalette et al. Citation2018). The findings in this might have been affected by including a small number of incident CRC cases and by selecting a study population from among people who were more aware of adverse health behaviors (Lavalette et al. Citation2018). The substantial consistency in the pooled results after all stratified analyses also hinders the effect of heterogeneity on the accuracy of the estimated effect size. Nonetheless, given the limited findings from Europe and Asia, further investigations from less represented areas will be necessary to confirm our findings. What’s more, the observed beneficiary role for HEI remained strong, and consistent in studies from North America, suggesting firstly testing HEI in other cultures is relevant, and secondly putting forward the hypothesis that development of a culturally/ethnically-relevant scoring method to qualify diet patterns will eventually yield a more efficient modification of CRC risk. The limitation of findings to North America and the presence of moderate publication bias despite a homogenous scoring system mean that the results lack sufficient transparency for developing dietary recommendations. Large prospective studies are now required from a range of geographic areas.
Factors impeding developing dietary recommendations using the existing diet indices:
The existing diet quality indices are majorly developed based on general dietary guidelines or aiming at prevention of chronic disease other than CRC. Thus, modifications are required in the scoring system of applied dietary indices for the food components with probable/evident role in prevention of CRC. For instance, micronutrients including vitamins A, C, E, D, B1, B2, B9, trace elements including iron, sodium, zinc, and carotenoids in addition to macronutrients such as various types of proteins and lipids with probable yet controversial role and calcium, fiber red and processed meet with evident role (Song, Garrett, and Chan Citation2015) should outweigh in diet quality scoring system. To achieve this goal, World Cancer Research Fund International has developed cancer specific, diet and life style factors quality index (Kerschbaum and Nüssler Citation2019). Yet, given the altered risk prediction performance of same diet quality indices in different populations, the recommendations should be tailored for ethnicities/genetically susceptibilities, cultural background, food preparation and preservation methods, excess calorie intake, adiposity, physical activity as well as the interaction between nutrients might lead to altered risk predictive performance of the same diet quality among different populations (Kuipers et al. Citation2015).
Study strengths and limitations
This systematic review and meta-analysis benefits from two main factors. First, we conducted a comprehensive assessment of the role of diet quality measured by commonly applied dietary indices on the risk for CRC. Second, we also rated the quality of the findings to generate an overview of the transparency of existing evidence for developing dietary recommendations about CRC prevention. However, the pooled findings were restricted to observational studies, leading to inherited limitations in findings. Nevertheless, to comprehensively address the existing evidence in qualitative and quantitative analyses, we did not restrict inclusion criteria to a specific study design. To handle the limitations with included studies e.g. case control studies, we assessed the quality of studies by the new Newcastle–Ottawa scale; all included case-control studies were of moderate to high quality (quality score ranging from 5–7 out of 8). Further, to address the possible bias in pooled effect sizes caused by case control studies, we conducted a stratified analysis for study design; the beneficiary effect for all four diet indices were consistent when pooling the effect sizes stratified for cohorts and case control studies. Our efforts to consider this issue when rating the quality of the overall findings may not have been sufficient. Additionally, the findings for the DII, MDS, and HEI had substantial heterogeneity, but we partly addressed this by showing consistency in the direction of overall benefit by stratified analysis for four major confounders (e.g., study design, geographic region, gender, and tumor site). Thus, the heterogeneity observed may have resulted from factors other than the main confounders, including sample size, number of variables, number of food components, dietary index categorization, and method of CRC case identification. Another limitation of the findings for the DASH and HEI were that the research was largely restricted to a specific geographic region, preventing generalization, and necessitating that we downgrade the quality of evidence for these indices. Finally, the dietary components for each included dietary index may affect the risk of CRC via different mechanisms (e.g., alterations in gut microbiota) that were not considered in the included studies.
Conclusions
Given the clear evidence that a high diet quality offers benefit in the prevention of CRC, we advocate that general dietary advice can be offered in clinical settings regarding these benefits. However, at present, for an efficient prevention of CRC, further investigations are warranted on the role of recommendations tailored for food components and life style factors with evident role in CRC prevention, as well as for varying ethnicities and geographic regions.
bfsn_a_1786353_sm4165.zip
Download Zip (5 MB)Acknowledgements
We thank Karin Sijtsma (UMCG) for her assistance in formulating the search strategy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Box 1. The dietary indices used to quantify diet quality.
Additional information
Funding
Notes on contributors
Sara Moazzen
SM designed the study, conducted the study, ran the data analyses, and prepared the first draft of the manuscript. BZA and GHdB designed the study, conceived the study, and edited the manuscript. SM and KWJS independently ran the search strategy, extracted the data, and assessed the quality of the included studies and pooled findings. GHdB and BZA commented on the study design, data analyses, inference of the results, and critically edited the manuscript. All authors read and approved the final manuscript draft.
Kimberley W. J van der Sloot
SM designed the study, conducted the study, ran the data analyses, and prepared the first draft of the manuscript. BZA and GHdB designed the study, conceived the study, and edited the manuscript. SM and KWJS independently ran the search strategy, extracted the data, and assessed the quality of the included studies and pooled findings. GHdB and BZA commented on the study design, data analyses, inference of the results, and critically edited the manuscript. All authors read and approved the final manuscript draft.
Geertruida H. de Bock
SM designed the study, conducted the study, ran the data analyses, and prepared the first draft of the manuscript. BZA and GHdB designed the study, conceived the study, and edited the manuscript. SM and KWJS independently ran the search strategy, extracted the data, and assessed the quality of the included studies and pooled findings. GHdB and BZA commented on the study design, data analyses, inference of the results, and critically edited the manuscript. All authors read and approved the final manuscript draft.
Behrooz Z. Alizadeh
SM designed the study, conducted the study, ran the data analyses, and prepared the first draft of the manuscript. BZA and GHdB designed the study, conceived the study, and edited the manuscript. SM and KWJS independently ran the search strategy, extracted the data, and assessed the quality of the included studies and pooled findings. GHdB and BZA commented on the study design, data analyses, inference of the results, and critically edited the manuscript. All authors read and approved the final manuscript draft.
References
- Agnoli, C., S. Grioni, S. Sieri, D. Palli, G. Masala, C. Sacerdote, P. Vineis, R. Tumino, M. C. Giurdanella, V. Pala, et al. 2013. Italian Mediterranean Index and risk of colorectal cancer in the Italian section of the EPIC cohort. International Journal of Cancer 132 (6):1404–11. doi: 10.1002/ijc.27740.
- Appel, L. J., T. J. Moore, E. Obarzanek, W. M. Vollmer, L. P. Svetkey, F. M. Sacks, G. A. Bray, T. M. Vogt, J. A. Cutler, M. M. Windhauser, et al. 1997. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New England Journal of Medicine 336 (16):1117–24. doi: 10.1056/NEJM199704173361601.
- Balter, K., E. Moller, and E. Fondell. 2012. The effect of dietary guidelines on cancer risk and mortality. Current Opinion in Oncology 24 (1):90–102. doi: 10.1097/CCO.0b013e32834e0531.
- Bamia, C., P. Lagiou, G. Buckland, S. Grioni, C. Agnoli, A. J. Taylor, C. C. Dahm, K. Overvad, A. Olsen, A. Tjonneland, et al. 2013. Mediterranean diet and colorectal cancer risk: Results from a European cohort. European Journal of Epidemiology 28 (4):317–28. doi: 10.1007/s10654-013-9795-x.
- Bloomfield, H. E., E. Koeller, N. Greer, R. MacDonald, R. Kane, and T. J. Wilt. 2016. Effects on health outcomes of a mediterranean diet with no restriction on fat intake: A systematic review and meta-analysis. Annals of Internal Medicine 165 (7):491–500. doi: 10.7326/m16-0361.
- Boden, S., R. Myte, M. Wennberg, S. Harlid, I. Johansson, N. Shivappa, J. R. Hebert, B. Van Guelpen, and L. M. Nilsson. 2019. The inflammatory potential of diet in determining cancer risk; A prospective investigation of two dietary pattern scores. PLoS One 14 (4):e0214551. doi: 10.1371/journal.pone.0214551.
- Brouwer, J. G., M. Makama, G. J. van Woudenbergh, H. F. Vasen, F. M. Nagengast, J. H. Kleibeuker, E. Kampman, and F. J. van Duijnhoven. 2017. Inflammatory potential of the diet and colorectal tumor risk in persons with Lynch syndrome. American Journal of Clinical Nutrition 106 (5):1287–94. doi: 10.3945/ajcn.117.152900.
- Chiuve, S. E., T. T. Fung, E. B. Rimm, F. B. Hu, M. L. McCullough, M. Wang, M. J. Stampfer, and W. C. Willett. 2012. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of Nutrition 142 (6):1009–18. doi: 10.3945/jn.111.157222.
- Cho, Y. A., J. Lee, J. H. Oh, A. Shin, and J. Kim. 2016. Dietary inflammatory index and risk of colorectal cancer: A case-control study in Korea. Nutrients 8 (8):469. doi: 10.3390/nu8080.
- Deeks J. J., J. P. T. Higgins, D. G. Altman, ed., on behalf ofthe Cochrane Statistical Methods Group. 2017. Chapter 9: Analysing data and undertaking metaanalyses. In Cochrane handbook for systematic reviews of interventions version 5.2.0 (updated June 2017), ed. J. P. T. Higgins, R. Churchill, J. Chandler, M. S. Cumpston. London, UK: Cochrane.
- Duval, S., and R. Tweedie. 2000. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 (2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x.
- Erben, V., P. R. Carr, B. Holleczek, C. Stegmaier, M. Hoffmeister, and H. Brenner. 2018. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Preventive Medicine 111:101–9. doi: 10.1016/j.ypmed.2018.02.025.
- Fan, Y., X. Jin, C. Man, Z. Gao, and X. Wang. 2017. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget 8 (35):59592–600. doi: 10.18632/oncotarget.19233.
- Fasanelli, F.,. D. Zugna, M. T. Giraudo, V. Krogh, S. Grioni, S. Panico, A. Mattiello, G. Masala, S. Caini, R. Tumino, et al. 2017. Abdominal adiposity is not a mediator of the protective effect of Mediterranean diet on colorectal cancer. International Journal of Cancer 140 (10):2265–71.,doi: 10.1002/ijc.30653.
- Ferlay, J., M. Colombet, I. Soerjomataram, C. Mathers, D. M. Parkin, M. Piñeros, A. Znaor, and F. Bray. 2019. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer 144 (8):1941–53. doi: 10.1002/ijc.31937.
- Fowler, M. E., and T. F. Akinyemiju. 2017. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. International Journal of Cancer 141 (11):2215–27. doi: 10.1002/ijc.30922.
- Fung, T. T., F. B. Hu, K. Wu, S. E. Chiuve, C. S. Fuchs, and E. Giovannucci. 2010. The mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. The American Journal of Clinical Nutrition 92 (6):1429–35. doi: 10.3945/ajcn.2010.29242.
- Galbete, C., L. Schwingshackl, C. Schwedhelm, H. Boeing, and M. B. Schulze. 2018. Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. European Journal of Epidemiology 33 (10):909–31. doi: 10.1007/s10654-018-0427-3.
- Guenther, P. M., K. O. Casavale, J. Reedy, S. I. Kirkpatrick, H. A. Hiza, K. J. Kuczynski, L. L. Kahle, and S. M. Krebs-Smith. 2013. Update of the healthy eating index: HEI-2010. Journal of the Academy of Nutrition and Dietetics 113 (4):569–80. doi: 10.1016/j.jand.2012.12.016.
- Guyatt, G. H., A. D. Oxman, H. J. Schünemann, P. Tugwell, and A. Knottnerus. 2011. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 64 (4):380–2. doi: 10.1016/j.jclinepi.2010.09.011.
- Haslam, A., S. Wagner Robb, J. R. Hebert, H. Huang, M. D. Wirth, N. Shivappa, and M. H. Ebell. 2017. The association between Dietary Inflammatory Index scores and the prevalence of colorectal adenoma. Public Health Nutrition 20 (9):1609–16. doi: 10.1017/s1368980017000453.
- Herszenyi, L., and Z. Tulassay. 2010. Epidemiology of gastrointestinal and liver tumors. European Review for Medical and Pharmacological Sciences. 14:249–58.
- Jafari Nasab, S., A. Bahrami, P. Rafiee, A. Hekmatdoust, M. Ghanavati, B. Rashidkhani, A. Sadeghi, H. Asadzadeh Aghdaei, F. Naja, and E. Hejazi. 2019. Healthy eating index-2010 and mediterranean-style dietary pattern score and the risk of colorectal cancer and adenoma: A case-control study. Nutrition and Cancer 5:1–10. doi: 10.1080/01635581.2019.1683212.
- Jayedi, A., A. Emadi, and S. Shab-Bidar. 2018. Dietary inflammatory index and site-specific cancer risk: a systematic review and dose-response meta-analysis. Advances in Nutrition (Bethesda, Md.) 9 (4):388–403. doi: 10.1093/advances/nmy015.
- Jones, P., J. E. Cade, C. E. L. Evans, N. Hancock, and D. C. Greenwood. 2017. The mediterranean diet and risk of colorectal cancer in the UK Women’s Cohort Study. International Journal of Epidemiology 46 (6):1786–96. doi: 10.1093/ije/dyx155.
- Kennedy, E. T., J. Ohls, S. Carlson, and K. Fleming. 1995. The healthy eating index: Design and applications. Journal of the American Dietetic Association 95 (10):1103–8. doi: 10.1016/S0002-8223(95)00300-2.
- Kerschbaum, E., and V. Nüssler. 2019. Cancer prevention with nutrition and lifestyle. Visceral Medicine 35 (4):204–9. doi: 10.1159/000501776.
- Kuipers, E. J., W. M. Grady, D. Lieberman, T. Seufferlein, J. J. Sung, P. G. Boelens, C. J. van de Velde, and T. Watanabe. 2015. Colorectal cancer. Nature Reviews. Disease Primers 1:15065. doi: 10.1038/nrdp.2015.65.
- Lavalette, C., M. Adjibade, B. Srour, L. Sellem, T. Fiolet, S. Hercberg, P. Latino-Martel, P. Fassier, M. Deschasaux, E. Kesse-Guyot, et al. 2018. Cancer-specific and general nutritional scores and cancer risk: Results from the prospective NutriNet-Sante Cohort. Cancer Research 78 (15):4427–35. doi: 10.1158/0008-5472.CAN-18-0155.
- Liu, L.,. R. Nishihara, Z. R. Qian, F. K. Tabung, D. Nevo, X. Zhang, M. Song, Y. Cao, K. Mima, Y. Masugi, et al. 2017. Association between inflammatory diet pattern and risk of colorectal carcinoma subtypes classified by immune responses to tumor. Gastroenterology 153 (6):1517–30. e1514. doi: 10.1053/j.gastro.2017.08.045.
- Miller, P. E., A. J. Cross, A. F. Subar, S. M. Krebs-Smith, Y. Park, T. Powell-Wiley, A. Hollenbeck, and J. Reedy. 2013. Comparison of 4 established DASH diet indexes: Examining associations of index scores and colorectal cancer. American Journal of Clinical Nutrition 98 (3):794–803. doi: 10.3945/ajcn.113.063602.
- Moher, D., L. Shamseer, M. Clarke, D. Ghersi, A. Liberati, M. Petticrew, P. Shekelle, and L. A. Stewart, PRISMA-P Group. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 4:1. doi: 10.1186/2046-4053-4-1.
- Mohseni, R., F. Mohseni, S. Alizadeh, and S. Abbasi. 2019. The association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: A meta-analysis of observational studies. Nutrition and Cancer 72 (5):778–790. doi: 10.1080/01635581.2019.1651880.
- Namazi, N.,. B. Larijani, and L. Azadbakht. 2018. Association between the dietary inflammatory index and the incidence of cancer: A systematic review and meta-analysis of prospective studies. Public Health 164:148–56. doi: 10.1016/j.puhe.2018.04.015.
- Obon-Santacana, M., D. Romaguera, E. Gracia-Lavedan, A. Molinuevo, E. Molina-Montes, N. Shivappa, J. R. Hebert, A. Tardon, G. Castano-Vinyals, and F. Moratalla. 2019. Dietary inflammatory index, dietary non-enzymatic antioxidant capacity, and colorectal and breast cancer risk (MCC-Spain Study). Nutrients 11 (6):1406–426.. doi: 10.3390/nu11061406.
- Panagiotakos, D. B., C. Pitsavos, and C. Stefanadis. 2006. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 16 (8):559–68. doi: 10.1016/j.numecd.2005.08.006.
- Park, S. Y., C. J. Boushey, L. R. Wilkens, C. A. Haiman, and L. Le Marchand. 2017. High-quality diets associate with reduced risk of colorectal cancer: Analyses of diet quality indexes in the multiethnic cohort. Gastroenterology 153 (2):386–94. e382. doi: 10.1053/j.gastro.2017.04.004.
- Petimar, J., S. A. Smith-Warner, T. T. Fung, B. Rosner, A. T. Chan, F. B. Hu, E. L. Giovannucci, and F. K. Tabung. 2018. Recommendation-based dietary indexes and risk of colorectal cancer in the nurses' health study and health professionals follow-up study. The American Journal of Clinical Nutrition 108 (5):1092–103. doi: 10.1093/ajcn/nqy171.
- Rafiee, P., N. Shivappa, J. R. Hebert, S. J. Nasab, A. Bahrami, A. Hekmatdoost, B. Rashidkhani, A. Sadeghi, M. Houshyari, and E. Hejazi. 2019. Dietary inflammatory index and odds of colorectal cancer and colorectal adenomatous polyps in a case-control study from Iran. Nutrients 11 (6):1213. doi: 10.3390/nu11061213.
- Reedy, J., P. N. Mitrou, S. M. Krebs-Smith, E. Wirfalt, A. Flood, V. Kipnis, M. Leitzmann, T. Mouw, A. Hollenbeck, A. Schatzkin, et al. 2008. Index-based dietary patterns and risk of colorectal cancer: The NIH-AARP diet and health study. American Journal of Epidemiology 168 (1):38–48. doi: 10.1093/aje/kwn097.
- Rosato, V., V. Guercio, C. Bosetti, E. Negri, D. Serraino, A. Giacosa, M. Montella, C. La Vecchia, and A. Tavani. 2016. Mediterranean diet and colorectal cancer risk: A pooled analysis of three Italian case-control studies. British Journal of Cancer 115 (7):862–5. doi: 10.1038/bjc.2016.245.
- Schwingshackl, L., and G. Hoffmann. 2015a. Adherence to mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Medicine 4 (12):1933–47. doi: 10.1002/cam4.539.
- Schwingshackl, L., and G. Hoffmann. 2015b. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. Journal of the Academy of Nutrition and Dietetics 115 (5):780–800.e785. doi: 10.1016/j.jand.2014.12.009.
- Schwingshackl, L., C. Schwedhelm, C. Galbete, and G. Hoffmann. 2017. Adherence to mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 9 (10):1063. doi: 10.3390/nu9101063.
- Shivappa, N., J. Godos, J. R. Hebert, M. D. Wirth, G. Piuri, A. F. Speciani, and G. Grosso. 2017. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients 9 (9):1043. doi: 10.3390/nu909.
- Shivappa, N., S. E. Steck, T. G. Hurley, J. R. Hussey, and J. R. Hébert. 2014. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutrition 17 (8):1689–96. doi: 10.1017/s1368980013002115.
- Song, M., W. S. Garrett, and A. T. Chan. 2015. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 148 (6):1244–60.e1216. doi: 10.1053/j.gastro.2014.12.035.
- Stang, A. 2010. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology 25 (9):603–5. doi: 10.1007/s10654-010-9491-z.
- Tabung, F. K., L. Liu, W. Wang, T. T. Fung, K. Wu, S. A. Smith-Warner, Y. Cao, F. B. Hu, S. Ogino, C. S. Fuchs, et al. 2018. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncology 4 (3):366–73. doi: 10.1001/jamaoncol.2017.4844.
- Tabung, F. K., S. E. Steck, Y. Ma, A. D. Liese, J. Zhang, D. S. Lane, G. Y. F. Ho, L. Hou, L. Snetselaar, J. K. Ockene, et al. 2017. Changes in the inflammatory potential of diet over time and risk of colorectal cancer in postmenopausal women. American Journal of Epidemiology 186 (5):514–23. doi: 10.1093/aje/kwx115.
- Torres Stone, R. A., M. E. Waring, S. L. Cutrona, C. I. Kiefe, J. Allison, and C. A. Doubeni. 2017. The association of dietary quality with colorectal cancer among normal weight, overweight and obese men and women: A prospective longitudinal study in the USA. BMJ Open 7 (6):e015619doi: 10.1136/bmjopen-2016-015619.
- Zhang, C., W. Wang, and D. Zhang. 2018. association between dietary inflammation index and the risk of colorectal cancer: A meta-analysis. Nutr Cancer 70 (1):14–22. doi: 10.1080/01635581.2017.1374418.