Abstract
Over the past 30-years, the U.S. Dietary Guidelines for Americans have included recommendations around dairy consumption, largely based on meeting recommendations for calcium intake with the intended purpose of osteoporosis prevention. Although dairy products provide more bone-beneficial nutrients (e.g., calcium, magnesium, potassium, zinc, phosphorus, and protein) per unit of energy than any other food group, the relevance of dairy products for long-term bone health and fracture prevention has resurged as some observational studies have suggested consumption to be associated with a greater risk of fractures. Given this controversy, we sought to synthesize the evidence on dairy consumption and bone health across the lifespan. We searched the PubMed, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials databases for English-language publications through June 2, 2020. Case-controlled, cross-sectional, prospective cohort or nestled case-control (or case cohort), and clinical trials reporting the effect of dairy products on bone mineral density, bone mineral content, and/or fractures were included in the systematic review. Two reviewers independently performed data extractions. Data from 91 publications, including 30 RCTs, 28 prospective cohorts, 23 cross-sectional studies, and 10 case-control studies were included in the systematic review. We assigned a “D” grade or “insufficient evidence” for the effect of dairy in infants and toddlers (0- to <36-months), children (3- to <10-years), and young adults (19- to <50-years). A “C” grade or “limited evidence” was assigned for the effect of dairy in adolescents (10- to <19-years). A “B” grade or “moderate” evidence was assigned for the effect of dairy in middle aged to older adults (≥50-years). Research on bone mass in adults between the ages of 20- to 50-years and individuals from other ethnic groups apart from Chinese females and Caucasians is greatly needed. Daily intake of low or nonfat dairy products as part of a healthy habitual dietary pattern may be associated with improved BMD of the total body and at some sites and associated with fewer fractures in older adults.
Keywords:
Introduction
Dairy products represent one of the five core food groups embedded in most dietary guidelines worldwide. Over the past 30-years, the U.S. Dietary Guidelines for Americans have included recommendations around dairy consumption, largely based on meeting recommendations for calcium intake with the intended purpose of osteoporosis prevention. The 2015–2020 Dietary Guidelines for Americans currently recommend that adults consume 3 servings/day of fat-free or low-fat dairy (Dietary Guidelines Advisory Committee Citation2015). Although dairy products provide more bone-beneficial nutrients (e.g., calcium, magnesium, phosphorus, vitamin D, zinc, and protein) per unit of energy than any other food group (Heaney Citation2000, Citation2009) (), the relevance of dairy products for long-term bone health and prevention of fractures has recently been probed, as some observational studies have suggested consumption to be associated with a greater risk of fractures (Feskanich et al. Citation1997; Michaelsson et al. Citation2014), although longer follow-up and inclusion of dairy products other than milk may likely affect these results. The recently updated Canadian Food Guide now groups milk and milk alternatives with other proteins, instead of recommending several servings per day as it has since 1943 (Health Canada Citation2018).
Figure 1. Impact of dairy nutrients on bone strength. 1,25(OH)D = 1,25 dihydroxy vitamin D; FGF = fibroblast growth factor; IGF1 = insulin-like growth factor 1; IL7 = interlukin-7; PTH = parathyroid hormone; RANKL; TNFα = tumor necrosis factor alpha.
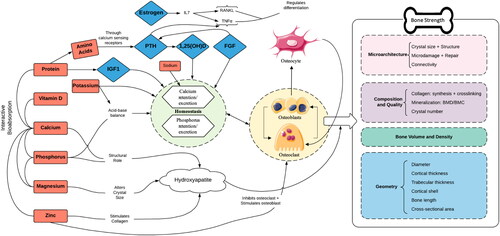
There is broad scientific consensus that high bone mineral density (BMD) is associated with a decreased risk of osteoporotic fractures later in life (Weaver et al. Citation2016). Maximizing bone during childhood and adolescence, and thus achieving the highest possible peak bone mass at the end of the skeletal maturation process, has been highlighted as a primary strategy for the prevention of osteoporotic fractures later in life (Weaver et al. Citation2016). Although >60% of the variance of peak bone mass is genetically determined, the remainder is influenced by modifiable lifestyle factors, including but not limited to adequate dietary intake of calcium, vitamin D, and dairy products as well as regular weight-bearing physical activity (Heaney et al. Citation2000; Rizzoli Citation2008; Rizzoli et al. Citation2010; Weaver et al. Citation2016). Just a 5–10% difference in accrual of peak bone mass has been suggested to be sufficient to account for a 25–50% difference in hip fracture rates later in life (Heaney et al. Citation2000; Weaver et al. Citation2016).
Using the newly proposed criteria for the clinical diagnosis of osteoporosis (Siris et al. Citation2014), the National Bone Health Alliance (NBHA) estimates that ∼16.0 and 29.9% of men and women age ≥50-years in the United States have osteoporosis, respectively (Wright et al. Citation2017). Standardized prevalence of osteoporosis is highest among those who are unemployed, individuals with a high poverty-to-income ratio, and those with a lower level of educational attainment, as well as among noncitizens in the United States (Tsai Citation2019). The National Osteoporosis Foundation (NOF) has published a “Clinician’s Guide to Prevention and Treatment of Osteoporosis,” which offers concise recommendations regarding prevention, risk assessment, diagnosis, and treatment of osteoporosis in postmenopausal women and men age ≥50-years (Cosman et al. Citation2014). The NOF supports the National Academy of Medicine recommendations that men age 50- to 70-years consume 1000 mg calcium/day and that women age ≥51-years and men age ≥71-years consume 1200 mg calcium/day (Ross et al. Citation2011), noting that primary dietary sources of both calcium and vitamin D are nonfat/low-fat dairy products and fortified foods (Cosman et al. Citation2014). However, dairy foods consist of a variety of nutrients within a complex matrix. The nature of this matrix can impact nutrient digestion and absorption, thereby modifying the overall nutritional properties of the food; thus, each food matrix may exhibit a different relationship with health and safety indicators (Thorning et al. Citation2017). For instance, the dairy matrix has been suggested to exert beneficial effects on muscle and bone health, greater than the sum of its nutrients, making assessment of whole foods vs. isolated nutrients in observational and intervention studies all the more important (Geiker et al. Citation2020). Likewise, recent research suggests that the assumed detrimental health effects of saturated fatty acids may be substantially modified by the food matrix in products like yogurt and cheese (Thorning et al. Citation2017; Astrup Citation2014).
Due to the recent disagreements regarding the efficacy of dairy intake for prevention of osteoporosis and related fractures, this review aimed to summarize current clinical and observational evidence regarding the role of dairy products and bone health across the lifespan, with a primary focus on fractures, BMD, and bone mineral content (BMC).
Methods
We followed the methods for conducting systematic reviews outlined in the National Academy of Science, Engineering, and Medicine’s Standards for Systematic Reviews (Eden et al. Citation2011) and report the study results according to the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al. Citation2009). Two reviewers (D.W. and T.C.W.) independently performed abstract and full-text screenings and data extraction. Disagreements between the reviewers were discussed until both parties were in agreement.
Data sources and searches
We searched the PubMed, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials databases through June 2, 2020 for (1) case-controlled, (2) cross-sectional, (3) prospective cohort or nested case-control (or case cohort), and (4) clinical trials assessing the effect of dairy products on BMD, BMC, and/or fractures. Dairy products of particular interest included total dairy, milk, yogurt, cheese, buttermilk, custard, curd, and kefir. Detailed search terms and search strategies used in each database are described in . Additionally, we searched the reference lists of four recent systematic reviews for articles not identified through our literature search (Bian et al. Citation2018; de Lamas et al. Citation2019; Fabiani, Naldini, and Chiavarini Citation2019; Shi et al. Citation2020; Weaver et al. Citation2016).
Table 1. Evidence grading system.
Study selection and data extraction
Study eligibility was restricted to peer-reviewed, English-language studies with no age restrictions. Prospective cohort studies and randomized controlled trials (RCTs) needed to have a minimum duration of 1-year and 6-months, respectively, to be included. Reference lists of relevant systematic reviews were cross-checked with our list of included studies to ensure that all relevant studies were assessed. We excluded commentaries, reviews, systematic reviews, letters to the editor, animal studies, in vitro studies, and non-human studies, as well as those articles not reporting values for the predefined markers/outcomes listed above. Also excluded were studies that compared fortified dairy to a dairy control (e.g., milk fortified with calcium vs. milk). A standardized data extraction form was used utilized to abstract data from each included study. Due to high heterogeneity within the studies, we did not conduct risk of bias or meta-analysis of the data.
Risk of bias
A modified version of the Jadad scale was employed to assess risk of bias (ROB) among clinical trials (Jadad et al. Citation1996; Boers et al. Citation2019). Standardized ROB tools for nutrition observational studies with varying designs are not available.
Grading of evidence
The results were graded using the evidence grading system provided in . This evidence grading system has been utilized widely in nutrition by prominent organizations such as the American Society for Nutrition (Cho et al. Citation2013), the American Diabetes Association (American Diabetes Association Citation2012), and the NOF (Wallace et al. Citation2016; Weaver et al. Citation2016) and is recommended by other experts (Woolf Citation2006). The assigned grade reflects the strength of available evidence and is based on consensus among the authors.
Results
Search results
Data from 91 studies, including 30 RCTs, 28 prospective cohorts, 23 cross-sectional studies, and 10 case-controlled study, were included in the present systematic review. shows PRISMA flow diagram depicting the flow of information through the various phases of systematic review. Included studies are organized by study design in subsequent subsections. The majority of studies predominantly reported BMC and/or BMC outcomes using dual-energy x-ray absorptiometry (DXA), with only a few of studies utilizing technologies such as peripheral quantitative computed tomography (pQCT), QCT, quantitative ultrasound (QUS), and single photon absorptiometry. Less than half of the published manuscripts (35 of 93) were funded, at least in part, by the industry. The scores on the Jadad scale were uniformly high, ranging from 7 to 10 out of 11 points (). In most cases, studies were described as randomized, while double-blinding was almost universally absent. Other common factors missing were justifications of the sample sizes and descriptions of the methods used to assess adverse effects.
Table 2. Studies assessing maternal dairy intake on offspring bone health.
All studies but of the included trials were described as randomized; however, only one study was described as double-blind due to the nature of the treatments.
Maternal dairy intake and bone health in offspring (any age)
Data from 1 prospective cohort study was identified in the literature search (). Ganpule et al. (Citation2006) found that maternal intake of dairy products during pregnancy to be associated with increases in total BMC, total BMD and spine BMD at 18-weeks post gestation. Intake was also associated with total BMD and spine BMD, but not total BMC, at 28-weeks post gestation. Total body BMD was greater in the children at age 6-years according to mother’s frequency of milk intake during pregnancy. Baseline dairy, calcium and protein intakes were very low among the 797 pregnant Indian women.
Evidence grading
We assigned a D-grade or “Insufficient” evidence based on the absence of data in this population.
Dairy intake and bone health in infants and toddlers (age 0- to 36-months)
Data from 1 RCT, 3 prospective cohort studies, and 1 cross sectional study were identified in the literature search (). Specker et al. (Citation1997) found no difference in total body BMC among infants given moderate or high mineral formula versus cow’s milk in a 6-month RCT of infants age 6-months at entry; however, baseline calcium and protein intakes were high among infants in the study. Volume and fat content of cow’s milk between ages 1- to 3-years did not seem to effect risk of fractures between 3- and 10-years of age in the TARGeT Kids! Study; however, baseline dairy intakes were relatively high on average (Allison et al. Citation2020). Another prospective cohort study (The Beginnings Study) showed formula fed infants to have different bone accretion trajectories than those breast-fed infants. Soy-based formula fed infants seemed to have lower bone mineralization in the first 3-months and greater accretion during the first year of life compared to those breast-fed or cow’s milk formula fed infants (Andres et al. Citation2013). A smaller prospective cohort (n = 31) found no differences in BMC among infants exposed to breast milk, cow’s milk-based formula or soy-based formula at 12-months (Hillman Citation1988; Hillman et al. Citation1988). A small (n = 35) cross-sectional investigation found no differences in breast milk versus cow’s milk-based formula on total body BMC in children age 2- to 5-months ( Park et al. Citation1998).
Table 3. Studies assessing dairy intake on infant and toddler bone health (age 0- to 36-months).
Evidence grading
We assigned a D-grade or “Insufficient” evidence based on the absence, heterogeneity, and inconsistency of data in this population.
Dairy intake and bone health in children (age 3- to <10-years)
Data from 5 publications, including 2 RCTs (Gibbons et al. Citation2004; Lau et al. Citation2004), 2 prospective cohort studies (Goulding et al. Citation2004; van den Hooven et al. Citation2015), and 1 cross-sectional study (Black et al. Citation2002), were identified in the literature search (). Gibbons et al. (Citation2004) found that calcium supplementation (i.e., high-calcium milk; 600 mg/day) with high habitual dietary calcium intake had no additional effects on bone mass in an RCT of white children (age 8- to 10-years) over a 30-month period compared with calcium-enriched water. Lau et al. (Citation2004) found that supplementing the diet with 80 g calcium-enriched milk powder (1300 mg calcium) was effective in enhancing bone accretion in an RCT of Chinese 9- to 10-year-old children over an 18-month duration. The group reported increases in the mean rate of change in hip BMD and BMC, and spine BMD. No effect was found on the mean rate of change for femoral neck, spine, and total body BMC or for femoral neck and total body BMD. Supplementing the diet with 40 mg calcium-enriched milk powder (650 mg calcium) increased mean rate of change in total body BMD but not for any sites measure. Goulding et al. (Citation2004) found that avoiding cow’s milk or calcium-rich food substitutes was associated with increased fracture frequency in a prospective cohort study with a 2-year follow-up period in children age 3- to 10-years. van den Hooven et al. (Citation2015) found dietary patterns characterized by high intakes of both dairy and whole grains to be associated with bone development in a prospective cohort study with a 6-year follow-up period in children with a mean age of 6 years. Significant effects were found on total body BMD and areal BMC but not BMC or bone area. Black et al. (Citation2002) found long-term avoidance of cow’s milk to be associated with poor bone health in a cross-sectional study of prepubertal children age 3- to 10-years. Avoidance of milk and subsequent lower calcium intakes resulted in lower total body BMC, bone area, and lower z scores at the femoral neck, hip, trochanter, lumbar spine, ultradistal radius, and 33% radius; however, total body areal bone mineral density (aBMD) was not significantly different compared to nonmilk avoidant peers.
Table 4. Studies assessing dairy intake on child bone health (age 3–10 years).
Evidence grading
We assigned a D-grade or “Insufficient” evidence for 3- to 10-year-olds, based on scarce evidence from 2 RCTs, 2 prospective cohorts, and 1 cross-sectional study. One RCT showed no significant effects of a calcium enriched cocoa flavored dairy drink on total body or site-specific BMD (Gibbons et al. Citation2004). The other RCT found significant effects of milk powder supplementation at multiple bone sites (Lau et al. Citation2004) One high-quality prospective study with low direct relevance to dairy found dairy and whole grain intake in those without vitamin D supplements to have positive associations with total body BMD and aBMC (van den Hooven et al. Citation2015). Studies in this age group have major methodologic flaws, especially lack of specific relation to dairy that provides low confidence in the effect estimates.
Dairy intake and bone health in adolescents (10–<19 years)
Data from 18 publications, including 11 RCTs (Cadogan et al. Citation1997; Chan, Hoffman, and McMurry Citation1995; Cheng et al. Citation2005; Du et al. Citation2004; Lu et al. Citation2019; Malpeli et al. Citation2012; Merrilees et al. Citation2000; Vogel et al. Citation2017; Volek et al. Citation2003; Zhu et al. Citation2006, Citation2008), 2 prospective cohort studies (Matkovic et al. Citation2004; Moore et al. Citation2008), 3 cross-sectional studies (Budek et al. Citation2007; Du et al. Citation2002; Esterle et al. Citation2009), and 2 case-controlled studies (Konstantynowicz et al. Citation2007; Petridou et al. Citation1997), were identified in the literature search ().
Table 5. Studies assessing dairy intake on adolescent bone health (age 10–19 years).
RCTs
Cadogan et al. (Citation1997) found that 1 pint/day of whole or reduced-fat milk for 18-months significantly enhanced bone mineral acquisition in an RCT undertaken in 12-year-old adolescent white females. Significant effects were found on total body BMD, as well as total body, thoracic spine, pelvis, and leg BMC change, but not head, arm, rib, lumbar spine, or trunk BMC change. Chan, Hoffman, and McMurry (Citation1995) found that increased intake of dairy foods to the recommended dietary allowance of 1200 mg calcium/day increased total BMD at the lumbar spine and total body BMD in an RCT of 11-year-old adolescent white females over a 12-month duration. Dairy food intake did not increase overall total or saturated fat intake and was not associated with excessive weight gain or increased body fat. Cheng et al. (Citation2005) found that increasing calcium intake by consuming cheese appears to be more beneficial for cortical bone mass accrual than consumption of tablets containing similar amounts of calcium, calcium plus vitamin D, or placebo in an RCT of Tanner stage I–II 10- to 12-year-old adolescent (assumed white) females over a 2-year duration. Du et al. (Citation2004) found that consumption of 330 mL of calcium fortified milk per day for 2-years with (n = 260) or without (n = 238) added cholecalciferol, led to significant increases in size-adjusted total-body BMC and BMD, compared to the control group (n = 259) in an RCT undertaken in 10- to 12-year-old Chinese females. Those subjects receiving milk with added cholecalciferol showed significantly increased size-adjusted total body BMC and BMD, compared to those receiving milk alone (i.e., no added cholecalciferol). Lu et al. (Citation2019) found that consumption of milk powder fortified with 400 IU vitamin D and either 300, 600, or 900 mg of calcium for 1.5-years did not affect bone mineralization compared to the control in an RCT of 12- to 15-year-old Chinese adolescents (n = 207). Malpeli et al. (Citation2012) found that the effect of calcium was similar when given in the form of dairy products or supplements in regard to changes in BMD and BMC (no significant differences between the 2 forms of delivery) in an RCT of adolescent (assumed Hispanic) mothers aged ≤19-years postpartum. Changes in percent body weight and total calcium intake were predictors of total body BMD and BMC changes (Malpeli et al. Citation2012). Merrilees et al. (Citation2000) found that high calcium intake from dairy products increased trochanter BMC (but not total body, lumbar spine, and femoral neck BMC), as well as trochanter, spine, and femoral neck BMD (but total body BMD) in an RCT of 15- to 18-year-old white females over 2-years of supplementation with an additional year of follow-up. The benefits of the intervention were not sustained after an additional 1-year of follow-up (Merrilees et al. Citation2000). Vogel et al. (Citation2017) found no significant differences in the change of BMD, BMC, or bone area for total body, radius, lumbar spine, and total hip in an RCT of 8- to 15-year-old adolescents who consumed low amounts of dairy (<800 mg calcium/day) when supplemented with 3 servings of dairy (∼900 mg calcium/day) for a duration of 18-months. Volek et al. (Citation2003) found that increasing intake of milk versus juice in an RCT of physically active 13- to 17-year-old adolescent males enhanced total body BMD, but not site-specific BMD measures or total body and site-specific measures of BMC over a 12-week duration. Zhu et al. (Citation2006) reported that calcium and vitamin D–fortified milk improved percent change in total body BMC, bone area, BMC, and size-adjusted BMC compared to milk fortified with the control in an RCT of Chinese 10- to 12-year-old females over a duration of 2-years. Participants who consumed milk fortified with calcium and vitamin D also showed improvements in percent difference in total body BMD and size-adjusted BMC, but not percent difference in total body BMC and bone area, compared to milk fortified with calcium alone. After 3-years postintervention follow-up, no significant differences were detected in percent change since baseline in total body BMC, bone area, BMD or size-adjusted BMC (Zhu et al. Citation2006). Zhu et al. (Citation2008) further reported positive effects on bone mineral accretion when accounting for the change in skeletal size during growth in adolescent females (age 10–12 years), although the effects were mainly on the lower limbs.
Prospective cohort studies
Matkovic et al. (Citation2004) found beneficial effects of higher calcium intake from dairy products over a 7-year follow-up period in adolescents, mean age 10.8 years at baseline and ∼15- to ∼18-years during assessment. Dairy intake was associated with higher aBMD at various spine sites but not the femoral neck. Moore et al. (Citation2008) found beneficial effects of dairy consumption over a 12-year follow-up period in adolescents age 15- to17-years. Consumption of ≥2 servings of dairy/day was significantly associated with BMC at the arms, trunk, ribs, and pelvis but not spine compared to 2 servings of dairy/week. Higher intake was also significantly associated with bone area at the trunk and ribs, but not the arms, legs, pelvis, and spine (Moore et al. Citation2008).
Cross-sectional studies
Budek et al. (Citation2007) found a positive association between total and milk protein intake and size-adjusted total body and lumbar spine BMC even after correcting for energy, calcium, and physical activity in white females age 17-years. Du et al. (Citation2002) found both low and high milk intake to be associated with greater distal 33% radius and 10% distal radius BMD when compared with no reported milk consumption among adolescent Asian females age 12- to 14-years. Low milk intake was associated with greater distal 10% radius BMC compared to the no-milk group. Low, high, or total milk intake did not affect distal 33% radius BMC or bone width (BW); distal 33% ulna BMC, BMD, or BW; distal 10% radius BMD or BW; or distal 10% ulna BMC, BMD, or BW (Du et al. Citation2002). Esterle et al. (Citation2009) found that calcium from milk consumption, but not other dietary sources of calcium, was associated with higher lumbar spine BMC and BMD, but not L2–L4 area, in post-menarcheal (assumed white) females ages 12- to 22-years.
Case-controlled studies
Konstantynowicz et al. (Citation2007) found beneficial effects of a normal vs. a milk-free diet on fracture risk in girls but not boys in a study of children/adolescents, mean age 13-years. Petridou et al. (Citation1997) found no effect of calcium-rich dairy products on risk of fractures in a study of children/adolescents age 7- to14-years.
Evidence grading
We assigned a C-grade or “Limited” evidence for 10 to <19-year-olds based on equivocal evidence from 10 RCTs, 2 prospective cohort, 3 cross-sectional, and 2 case-controlled studies. We started with the B-grade or “Moderate” evidence assigned to the effect of dairy intake on development of peak bone mass from the 2016 NOF position paper (Weaver et al. Citation2016). Two large RCTs were not considered in the NOF position paper. Vogel et al. (Citation2017) found no effect of an 18-month dairy intervention in 240 adolescent boys and girls in the US. Zhu et al. (Citation2006) found positive effects in 501 Chinese adolescents with presumably lower calcium status than the participants in the Vogel et al. (Citation2017) study, but the intervention was with fortified milk and had inconsistent effects at different sites (i.e., milk fortified with calcium showed positive effects on arm BMD, while milk fortified with calcium and vitamin D showed positive effects on leg BMD).
Dairy intake and bone health in young adults (19–<50 years)
Data from 14 publications, including 3 RCTs (Labouesse et al. Citation2014; Liu et al. Citation2011; Rosado et al. Citation2011), 4 prospective cohorts (Feskanich et al. Citation1997; Feskanich, Willett, and Colditz Citation2003; Meyer et al. Citation1997; Nieves et al. Citation2010), and 8 cross-sectional studies (Bahtiri et al. Citation2014; Bierhals et al. Citation2019; Kalkwarf, Khoury, and Lanphear Citation2003; Movassagh et al. Citation2017; Opotowsky and Bilezikian Citation2003; Rulu et al. Citation2019; Torres-Costoso et al. Citation2019; Wadolowska et al. Citation2013), were identified in the literature search ().
Table 6. Studies assessing dairy intake on adult bone health.
RCTs
Labouesse et al. (Citation2014) found that following weight loss, adequate dairy intake resulted in significantly greater lumbar spine BMD, but not lumbar spine BMC, hip BMD, or hip BMC, compared to a low-dairy diet in a 15-week RCT of females age 19- to 45-years. Liu et al. (Citation2011) found that both milk and milk plus calcium supplementation was associated with greater arm, spine, and whole-body BMD (but not leg, femoral neck, intertrochanter, Ward’s, or total hip BMD) and suppressed bone resorption in an RCT of pregnant Chinese women (age 24- to 31-years) with habitual low dietary calcium intake at 6 weeks postpartum. Rosado et al. (Citation2011) found that when consumed 3 times/day, both low-fat milk on an energy-restricted diet (−500 kcal/day) and low-fat milk with added micronutrients on an energy-restricted diet (−500 kcal/day) suppressed total body BMC change compared to the control (i.e., energy-restricted diet [−500 kcal/day] alone) in a 16-week RCT of women (age 25- to 45-years).
Prospective cohort studies
Feskanich et al. (Citation1997) found that higher consumption of milk or other food sources of calcium did not protect against hip or forearm fractures in a prospective cohort study with a 12-year follow-up period in adult white women age 30- to 55-years. Dairy calcium but not total calcium was marginally associated (p = 0.05) with an increased relative risk of hip fractures, although the number of cases was low. Feskanich, Willett, and Colditz (Citation2003) also found that milk intake was not associated with a lower risk of postmenopausal osteoporotic fractures after menopause in a prospective cohort study with an 18-year follow-up period of white females age 30- to 55-years. Dietary vitamin D, but not total vitamin D, dietary calcium, or total calcium, was associated with a lower risk of postmenopausal osteoporotic fractures (data not extracted). Meyer et al. (Citation1997) found no significant effects of milk consumption on hip fractures in white men and women with a mean age 47-years over an average 11.2-year follow-up period. Nieves et al. (Citation2010) found higher intakes of dairy, skim milk, and total milk to be associated with a lower relative risk of stress fracture rates in a prospective cohort study with a 2-year follow-up period of females with a mean age of 21-years. Dairy, skim milk, and total milk intake was associated with a slower rate of annualized BMD loss in the total hip but not spine. Dairy intake, but not skim milk or total milk intake, was associated with a slower rate of annualized whole-body BMD loss. Skim milk and total milk, but not dairy intake, was associated with a slower rate of annualized whole-body BMC loss.
Cross-sectional studies
Bahtiri et al. (Citation2014) found that higher consumption of dairy products (i.e., milk, cheese, yogurt, pudding, and total dairy) was not related to higher BMD in a cross-sectional study of women age 22- to 65-years. Furthermore, calcium intake derived from dairy product consumption was not related to higher BMD. Dietary calcium intake from total dairy consumption was found to be significantly higher in the third tertile of BMD compared to the first and second tertiles of BMD (p < 0.05) (Bahtiri et al. Citation2014). Bierhals et al. (Citation2019) found males classified as “high” milk consumers to have a slightly lower BMD at the right femur site in a cross-sectional study of 3,109 adults aged 22-years. No significant associations were noted at this site in females. No associations were observed for milk consumption and whole body or lumbar spine BMD in males or females. Kalkwarf, Khoury, and Lanphear (Citation2003) found low retrospective reported milk intake during childhood and adolescence to be associated with lower BMD and BMC in adulthood and a greater risk of fracture in a cross-sectional study of adult women age ≥20-years. Significant effects were found on lifetime fractures with increased child and adolescent milk intake. Significant effects were also found on osteoporotic fractures with increased child but not adolescent milk intake (Kalkwarf, Khoury, and Lanphear Citation2003). Movassagh et al. (Citation2017) found that high versus low intake of milk and milk alternatives had a long-term beneficial effect on bone structure of the radius shaft in females but not males (mean age 29-years). No significant effects were observed for bone structure of the distal radius, distal tibia, and tibia shaft in either sex (Movassagh et al. Citation2017). Opotowsky and Bilezikian (Citation2003), after controlling for age and body mass index (BMI), reported that retrospective teenage milk consumption of >1 glass/day (versus <1 glass/week) was significantly associated with higher total hip, trochanter, intertrochanter, and femoral neck BMD in white, but not black women, age 20–39 years. After controlling for age and BMI, retrospective milk consumption of >1 glass/day (versus 1 glass/week) during childhood increased total hip and trochanter BMD, but not intertrochanter or femoral neck BMD, in white, but not black, women aged 20- to 39-years (Movassagh et al. Citation2017). Rulu et al. Citation2019 found milk intake to increase the risk of osteopenia or osteoporosis diagnosis by BMD; however, the study population age 20- to 70-years) did not separate findings by age as it did some other variables. Torres-Costoso et al. (Citation2019) found higher regular milk consumption to be associated with less total body BMD compared to those with lower regular milk consumption, even after controlling for different sets of confounders in a cross-sectional study of young adults 18 to 30-years-old (n = 239). The authors concluded that milk consumption, per se, does not have direct effects on bone development, because its association seems to be fully mediated by body composition variables (Torres-Costoso et al. Citation2019). Wadolowska et al. (Citation2013) found retrospective reported high consumption (third tertile) of dairy products during the preschool and school period to be associated with an increase in BMD among adult white women age 29- to 59-years. No relationship was found between current consumption of ≥28 servings of dairy/week, >400 mg calcium/day, or calcium-enriched food (Wadolowska et al. Citation2013).
Evidence grading
We assigned a D-grade or “Insufficient” evidence for adults 19 to 50-years-old based on evidence from 3 RCTs, 3 prospective cohorts, and 8 cross-sectional studies. Limited conclusions can be made from the 3 RCT’s in adults because of small sample sizes (51 to 139 subjects in each study). Additionally, one of the RCT’s only obtained post-intervention bone measures (Liu et al. Citation2011). Two RCTs were weight loss studies where participants did not maintain energy-balance (Labouesse et al. Citation2014; Rosado et al. Citation2011). Maintenance of energy balance is important since the common practice of adjusting for BMI may lead to over-estimation of bone mineral mass, for instance, in patients with anorexia ( Achamrah et al. Citation2017 ). Data from three prospective cohort studies are available but two of these studies reported outcomes using the same study cohort (Nurses’ Health Study) (Feskanich et al. Citation1997; Feskanich, Willett, and Colditz Citation2003) and one study may have limited generalizability because it was undertaken in female competitive runners (Nieves et al. Citation2010). Dairy or calcium intake did not have a significant impact on risk of hip fractures based on analyses of the Nurses’ Health Study (∼77,000 women). Low fat milk and dairy product intake were associated with greater bone gains and lower stress fracture rates over a 2-year study interval in 125 female competitive runners. Beneficial effects on young adult fractures may be most pronounced when adequate dairy intakes accompany impact exercise. Other large well-designed prospective cohorts assessing fracture risk and those assessing BMD are needed. Seven cross-sectional studies were identified. Four of these studies were limited in sample size (Bahtiri et al. Citation2014; Movassagh et al. Citation2017; Torres-Costoso et al. Citation2019; Wadolowska et al. Citation2013) and one failed to control for BMI differences between groups (Bahtiri et al. Citation2014). The study by Beirhals showed no association between milk intake and BMD but has limitations due to retrospective methodology to assess food intake. Two of the cross-sectional studies carried out analyses using NHANES III data (Kalkwarf, Khoury, and Lanphear Citation2003; Opotowsky and Bilezikian Citation2003). Both of these relatively large, cross-sectional studies found a significant beneficial impact of early milk intake on bone mass and one found it to be beneficially associated with a subsequent risk of fracture (Kalkwarf, Khoury, and Lanphear Citation2003).
Dairy intake and bone health in Middle-aged to older adults (≥50-years)
Data from 50 studies, including 14 RCTs (Chee et al. Citation2003; Chen et al. Citation2015; Daly et al. Citation2005, Citation2008; Gui et al. Citation2012; Ilich et al. Citation2019; Lau et al. Citation2001, Citation2002; Manios et al. Citation2007; Moschonis et al. Citation2011; Prince et al. Citation2009; Storm et al. Citation1998; Ting et al. Citation2007; Tu et al. Citation2015), 17 prospective cohort studies (Aslam et al. Citation2019; Benetou et al. Citation2011; Biver et al. Citation2018; Cumming et al. Citation1997; Feart et al. Citation2013; Feskanich et al. Citation2014, Citation2018; Fujiwara et al. Citation1997; Holvik et al. Citation2019; Michaelsson et al. Citation2014, Citation2018; Nevitt et al. Citation2005; Owusu et al. Citation1997; Roy et al. Citation2003; Sahni et al. Citation2013, Citation2014, Citation2017), 10 cross-sectional studies (Chan et al. Citation2020; Eysteinsdottir et al. Citation2014; Lanyan et al. Citation2020; Lunt et al. Citation2001; Opotowsky and Bilezikian Citation2003; Mangano et al. Citation2019; McCabe et al. Citation2004; Murphy et al. Citation1994; Sato et al. Citation2015; Zhu et al. Citation2018), and 8 case-controlled studies (Cumming and Klineberg Citation1994; Jha et al. Citation2010; Jitapunkul, Yuktananandana, and Parkpian Citation2001; Johnell et al. Citation1995; Kanis et al. Citation1999; Lan et al. Citation2010; Nieves, Grisso, and Kelsey Citation1992; Tavani, Negri, and Vecchia Citation1995) were identified in the literature search ().
Table 7. Studies assessing dairy intake on middle-aged to older adult bone health (age ≥50 years).
RCTs
Chee et al. (Citation2003) found high-calcium skimmed milk powder (1200 mg calcium and 10 μg vitamin D taken as 2-glasses daily) versus the control to be effective in reducing BMD loss at the total body, lumbar spine, femoral neck, and total hip, after a 2-year RCT of postmenopausal Malaysian women age 55- to 65-years. Chen et al. (Citation2015) found consumption of high-calcium milk powder (450 mg calcium and 400 IU vitamin D) versus the control to be effective in reducing BMD loss at the lumbar spine, but not hip, after 2-years in an RCT of postmenopausal Chinese women age 50- to 65-years. Compliers were also found to have significantly reduced lumbar spine, but not hip, BMD loss after 2-years. Daly et al. (Citation2005) found that supplementing the diet with reduced-fat calcium and vitamin D3–enriched milk was effective to reduce age-related BMD loss at several skeletal sites including the femoral neck, total hip, ultradistal radius, and 33% radius, but not the lumbar spine, in an RCT of white men age >50-years over a 2-year duration. In a follow-up study, Daly et al. (Citation2008) found these BMD effects to be sustained, except at the 33% radius, in an 18-month follow-up study after discontinuation of the treatment. Gui et al. (Citation2012) found Chinese women aged 45- to 55-years consuming of 250 mg calcium through cow’s milk versus the control to have better BMD at the total hip and femoral neck, but not at the spine L1-L4, after an 18-month intervention. Ilich et al. (Citation2019) found that an energy-restricted weight loss study complemented with low-fat dairy foods (4–5 servings/day) did not lead to more favorable BMD outcomes in an RCT of postmenopausal women over a 6-month duration. Lau et al. (Citation2001) found that supplementing the diet with high-calcium milk powder prevented loss of total body, lumbar spine, femoral neck, and total hip BMD, but not intertrochanter BMD, over 2-years in an RCT of postmenopausal Chinese women age 55–59 years. In follow-up study, Lau et al. (Citation2002) found that supplementing the diet with high-calcium milk powder was effective in preventing bone loss over 3-years in an RCT of postmenopausal Chinese females age 55- to 59-years. Significant effects were found on lumbar spine bone area, total body and femoral neck BMC, total body, lumbar spine, total hip, and femoral neck BMD, but not total body, total hip, femoral neck, and intertrochanter bone area, lumbar spine, total hip, and intertrochanter BMC and intertrochanter BMD. After adjusting for the percent rate of change per year, the investigators found that the high-calcium milk intervention was effective in preventing total body, total hip, femoral neck, and intertrochanter BMD loss. Manios et al. Citation2007 found calcium and vitamin D fortified dairy products versus the control to have less BMD loss at the pelvis and total spine, but not the lumbar spine, arms, legs, and total body, in an RCT of postmenopausal white women, age 55- to 65-years. Moschonis et al. (Citation2011) reported that administration of 3 fortified dairy products (calcium plus vitamin D; calcium plus vitamin D plus vitamin K1; and calcium plus vitamin D plus vitamin K2) all increased total body BMD compared to the control in a 12-month RCT of white postmenopausal women age 55- to 65-years. The vitamin K1 and K2 fortified dairy groups had additional significant increases in L2–L4 lumbar spine BMD compared to the control. Prince et al. (Citation2009) found increased dietary calcium through milk powder intake along with exercise versus placebo to have less BMD loss at the trochanter, intertrochanter, and ultradistal ankle, but not the femoral neck, in an RCT of white women age 50- to 70-years-old, who were postmenopausal for at least 10-years. In a similar study, Storm et al. Citation1998 failed to find any effect of milk consumption on changes in BMD at the trochanter, femoral neck, and lumbar spine. Ting et al. (Citation2007) found beneficial effects of a high-calcium milk supplement on percent change in total body, spine L2–L4, femoral neck, and total hip BMD to still be evident in a 21-month RCT of postmenopausal Chinese females age 55- to 70-years (n = 139 of the original 173 subjects). The group had previously reported high-calcium milk to increase total body, spine L2–L4, femoral neck, and total hip BMD in this group over a 24-month duration (Chee et al. Citation2003). Tu et al. (Citation2015) found that kefir-fermented milk therapy was not associated with significant short-term changes in total hip, femoral neck, or spine BMD in an RCT of Taiwanese male and female osteoporotic patients (mean age 64- and 67-years, respectively) over a 6-month duration.
Prospective and retrospective cohort studies
Aslam et al. (Citation2019) no relationship between milk or total dairy consumption on fractures in a study of white women age ≥50-years after a 10-year follow-up period. Benetou et al. (Citation2011) found no relationship between dairy product intake and hip fracture incidence in a prospective cohort study of elderly Europeans after an 8-year follow-up period. Biver et al. (Citation2018) found that age-related cortical bone loss was attenuated at nonbearing bone sites in consumers of fermented dairy products in a prospective cohort study with a 3-year follow-up period in postmenopausal women with a mean age of 65-years. Fermented dairy product consumption was associated with attenuated loss of radius total volumetric BMD and of cortical volumetric BMD, area, and thickness. There was no difference in aBMD at the tibia. The associations were independent of total energy, calcium, or protein intakes. For other dairy product categories, only milk consumption was associated with a lower decrease of aBMD and of failure load at the radius. Cumming et al. (Citation1997) found milk intake to be associated with a decreased risk of ankle fractures, but not any nonvertebral, hip, proximal humerus, wrist or vertebral fractures, in a study of white women ≥65-years old after a 6.6-year average follow-up period. Feart et al. (Citation2013) found that low intake of dairy products (i.e., low dairy, yogurt, milk and cheese), in particular yogurt intake was associated with doubling of risk of wrist fracture but did not affect hip or vertebral fractures over 8-years in a prospective cohort study of older adults ≥67-years-old (n = 1,482). Feskanich et al. (Citation2014) found that reported teenage milk intake was not associated with hip fractures in a prospective cohort study of older adults >50-years-old enrolled in the Nurse’s Health Study or Health Professionals Follow-up Study (n = 96,927) after 22-years of follow-up. However, Feskanich et al. (Citation2018) found higher total dairy as well higher milk consumption to be associated with a lower risk of hip fractures in a prospective cohort study using the same two cohorts with a 32-year follow-up period (n = 123,906). Fujiwara et al. (Citation1997) found no effect of milk intake on hip fractures in Japanese men and women with a mean age of 58.5 years after a 14-year follow-up period. Holvik et al. (Citation2019) found no overall association between milk intake and hip fractures among older adults enrolled in two Norwegian cohorts (Norwegian Counties Study, n = 35,114; Five Counties Study, n = 23,259) over a ∼20-year follow-up. Michaelsson et al. (Citation2014) found high milk intake to be associated with a higher fracture incidence in white women age 39- to 74-years in a prospective cohort study with a 20-year follow-up period. However, fermented dairy intake resulted in a reduced incidence of fractures. There was no effect shown in a separate prospective cohort study of men age 45- to 79-years with an 11-year follow-up period. In a prospective cohort study with a 22-year follow-up period, Michaelsson et al. (Citation2018) found that the amount and type of dairy products as well as fruit and vegetable intake were differentially associated with hip fracture rates in white women age 39- to 74-years. The combination of fruits and vegetables (≥5 servings/day) with fermented milk (yogurt or soured milk; ≥2 servings/day) was associated with a lower rate of hip fracture in high consumers. Nevitt et al. (Citation2005) found retrospectively reported low milk intake during pregnancy (<1 glass per day) to be associated with a greater risk of incident vertebral fractures in a prospective cohort study of older women age 65 to 99-years-old (n = 7,238). Owusu et al. (Citation1997) found no association between milk intake and risk of forearm or hip fractures in mostly white men, age 40–75 years after an 8-year follow-up period. Roy et al. (Citation2003) found no association between milk intake and risk of incident vertebral fractures in a prospective cohort study of older European adults 50 to 75-years-old (n = 6,575). Sahni et al. (Citation2013) found milk and yogurt products to be associated with improved hip but not spine BMD in a prospective cohort study with a 12-year follow-up period in men and women primarily of European ancestry, age 26- to 85-years. Cream intake was suggested to adversely affect BMD. In another study, Sahni et al. (Citation2014) found greater intake of milk and milk plus yogurt to be associated with a lower risk of hip fractures in a prospective cohort study with a 12-year follow-up period in older men and women primarily of European ancestry, age 68- to 96-years. Sahni et al. (Citation2017) found higher intakes of milk, fluid dairy, and milk + yogurt + cheese to be associated with higher lumbar spine BMD, and a higher intake of milk + yogurt + cheese to be protective against trochanter BMD loss among vitamin D supplement users but not among nonusers, in a prospective cohort study of older adults aged 67- to 93-years-old with a follow-up period of 4-years (n = 628). No associations were found between dairy food intake and femoral neck BMD.
Cross-sectional studies
Chan et al. Citation2020 found consumers versus non-consumers of dairy products had no effect on risk of fractures in Asian men and women with a mean age of 57.6-years. Eysteinsdottir et al. (Citation2014) found that regular milk consumption throughout life, from adolescence to old age, was associated with higher BMC and BMD in old age, but there were no differences in bone volume in a large cross-sectional study of white men and women age 66- to 96-years (n = 4,797). Lanyan et al. Citation2020 found postmenopausal women mean age 64.3-years with osteoporosis consume a high amount of vegetables but insufficient amounts of dairy products and calcium. Lunt et al. (Citation2001) found positive associations between consumption of dairy products and BMD at the spine, femoral neck, and trochanter in another large cross-sectional study of white men and women age 50–80 years (n = 4,000). Hard cheese had significant associations with spine, femoral neck, and trochanter BMD in men but not women. Soft cheese had a significant association with trochanter BMD in women but no association with spine and femoral neck in women or spine, femoral neck, or trochanter BMD in men. Yogurt had significant association with spine and femoral neck BMD in women but not trochanter BMD in women or spine, femoral neck, or trochanter BMD in men. Milk had a significant association with femoral neck and trochanter BMD in women but not spine BMD in women or spine, femoral neck, or trochanter BMD in men. Other milk products did not have any significant associations with spine, femoral neck, or trochanter BMD in men or women. Cumulative milk drinking was found to have a significant association with femoral neck and trochanter BMD in women but not spine BMD in women or spine, femoral neck, or trochanter BMD in men. Mangano et al. Citation2019 reported dairy food intakes (i.e., hard cheese, soft cheese, yogurt, milk and other milk products) to be associated with higher femoral neck, trochanter, and spine BMD; however results were not consistent across products in a cross-sectional study of older Puerto Rican adults aged 50- to 80-years-old from Boston (n = 904). McCabe et al. (Citation2004) found that higher dairy product consumption was associated with greater hip and femoral neck BMD in black and white men but not women age ≥60-years in a cross-sectional study. Murphy et al. (Citation1994) found frequent milk consumption before age 25-years to influence hip bone mass in a cross-sectional study of middle-aged and older white women age 44- to 74-years. Significant effects were found on total hip, femoral neck, and Ward’s triangle BMD, but not spine, trochanter, or intertrochanter BMD, when intake of milk before age 25-years was high. There was also a significant effect of milk intake from age 25- to 44-years on intertrochanter BMD. Milk intake did not affect spine, total hip, femoral neck, trochanter, or Ward’s triangle BMD when consumed between age 25- and 44-years or after age 44-years. Opotowsky and Bilezikian (Citation2003), after controlling for age and BMI, reported that retrospective childhood milk consumption of >1 glass/day (versus <1 glass/week) was significantly associated with higher trochanter BMD in white but not black postmenopausal females. No effects were found with retrospective milk consumption of >1 glass/day (versus 1 glass/week) during teenage years and BMD at any site in white or black postmenopausal females (results presented in ). Sato et al. (Citation2015) found that greater habitual milk intake was associated with higher total hip aBMD, but not lumbar spine or femoral neck aBMD or trabecular bone score, in a cross-sectional study of community-dwelling elderly Japanese men age ≥65-years. Greater habitual dietary calcium intake was associated with higher total hip and femoral neck aBMD and trabecular bone score but not lumbar spine aBMD. Zhu et al. (Citation2018) found ≥1 serving of milk per day to increase the risk of incident low-energy fractures in postmenopausal Asian women age ≤70-years.
Case-controlled studies
Cumming and Klineberg (Citation1994) found dairy product intake in younger years but not current age to increase the risk of hip fractures among white men and women age ≥65-years. Jha et al. (Citation2010) found consuming >1 glass of milk per day was associated with a decreased hip fracture risk in Indian men and women mean age 62.5-years. Jitapunkul, Yuktananandana, and Parkpian (Citation2001) found hip fracture risk to be increased when regular intake of milk was absent from the diet of postmenopausal Asian women age ≥50-years. Johnell et al. (Citation1995) also found milk intake to be associated with a reduced risk of hip fractures in postmenopausal white women age ≥50-years. Kanis et al. (Citation1999) found intake of cheese but not milk to decrease hip fracture risk in white men age ≥55-years. Lan et al. (Citation2010) found neither milk or cheese intake to have an effect on hip fracture risk in white women ≥45-years. Nieves, Grisso, and Kelsey (Citation1992) found that white women age 50- to 103-years who reported higher intakes of milk and recreational activity in their teenage years to have a reduced risk of hip fractures. Tavani, Negri, and Vecchia (Citation1995) found neither milk or cheese intake to be associated with hip fracture risk in white women age ≥45-years.
Evidence grading
We assigned a B-grade or “Moderate” evidence for older adults ≥50-years based on evidence from 14 RCTs, 17 prospective cohort studies, 10 cross-sectional studies, and 8 case-controlled studies. Most RCTs showed a benefit on BMC or BMD over 1- to 3-years with fortified dairy foods. The only RCTS with null associations for BMD were either short term (6-months) (Ilich et al. Citation2019; Tu et al. Citation2015), of which one RCT was a weight loss study (Ilich et al. Citation2019), or small in sample size with insufficient power (Storm et al. Citation1998). Data from 17 prospective cohort studies are available but two of these studies reported outcomes using the Nurses’ Health Study cohort (Feskanich et al. Citation2014, Citation2018) two using the Swedish Mammography cohort (Michaelsson et al. Citation2014, Citation2018), and two (one prospective and one retrospective) using the Study of Osteoporotic Fractures cohort (Cumming et al. Citation1997; Nevitt et al. Citation2005). The effect of dairy intake on fractures showed mixed results among cohort studies. Other large well-designed prospective cohorts assessing fracture risk are needed. Ten cross-sectional studies were identified. Four studies were relatively larger in size (Eysteinsdottir et al. Citation2014, Lunt et al. Citation2001 Opotowsky and Bilezikian 2003,; Zhu et al. Citation2018) and one of them carried out analyses using NHANES III data (Opotowsky and Bilezikian Citation2003). All of these relatively large, cross-sectional studies found a significant beneficial impact of milk intake on BMC and or BMD. However, it was unclear if milk intake consumed during childhood, young adulthood or cumulative intake over a lifetime was most beneficial later in life. All but one of the eight case-controlled studies found dairy intake to reduce hip fracture risk.
Discussion
Osteoporosis is considered the most common bone disorder in Western society and is associated with an imbalance in the rates of bone growth and remodeling, thereby resulting in a reduction in bone mass. Nutritional exposures across the lifespan have the potential to influence bone health; however, the risk of osteoporotic-related fractures in adults increases with age (Wright et al. Citation2014). Dairy products have a high frequency of consumption in both the United States and many countries across the globe and have traditionally been identified as having positive effects on the overall health of bone; thus, their intake could have large implications for public health.
Advances in nutrition science demonstrate that foods represent complex matrices of nutrients, minerals, bioactives, food structures and other factors with correspondingly complex effects on bone. The ability to properly absorb, store, and utilize minerals is greatly impacted in the body by the presence of other nutrients. Calcium and vitamin D, particularly 25-hydroxyvitamin D, are seen as corequisites to maintain bone health and calcium homeostasis (Haussler et al. Citation2013). Vitamin D plays a critical role in calcium metabolic processes. Dietary protein intake has recently been affirmed to be a critical component of the diet that influences long-term bone health (Rizzoli et al. 2018; Shams-White et al. Citation2017; Wallace Citation2019; Wallace and Frankenfeld Citation2017). Protein and calcium combined in dairy products have beneficial effects on calciotropic hormones, bone turnover markers, and BMD (Rizzoli et al. 2018). Protein has been shown to enhance both uptake and urinary excretion of calcium (Hunt, Johnson, and Fariba Roughead Citation2009; Kerstetter et al. Citation2005; Roughead et al. Citation2003). Vitamin C (ascorbic acid) is able to influence absorption of nonheme iron, alongside vitamin B12, vitamin A, folate, and riboflavin (Abbaspour, Hurrell, and Kelishadi Citation2014; Betancourt and Gaitan Citation2012).
Bone is a very active tissue that is sensitive to metabolic changes such as exercise and nutrition. It is likely that consumption of dairy has varying magnitudes of effects at different sites since the material properties of bone compartments differ. Over 80% of bone mass is in the cortical compartment. Trabecular bone has a lower calcium content but nearly 10 times the surface-to volume ratio as cortical bone, making its contribution to activation of bone metabolism greater, due to the increased number of osteoblasts and osteoclasts present (Ott Citation2018). It can therefore be assumed that the decrease in bone density caused by calcium inadequacy may occur in trabecular bone sooner than cortical bone. Both cortical and trabecular bone are important for bone strength and the relationships are complex. The spine is the classical trabecular bone site and vertebral compression fractures are a hallmark of osteoporosis; however, the thin cortical shell plays a substantial role. The hip is considered a cortical bone site but both cortical and trabecular bone contribute to femoral strength. Cortical bone supports bending in the distal region of the femoral neck and the trabecular bone supports the proximal load. Bone loss after menopause is more rapid in trabecular bone but since cortical bone accounts for ∼80% of the skeleton, the absolute amount of bone loss is similar from each compartment for the first 10-years. Later there is more loss from cortical bone (Seeman Citation2013). The above could influence outcomes of the studies included within the systematic review since most of the RCTs are only 6-months to 2-years in duration, and most cohorts do not assess vBMD while enrolling participants with a large range in age.
Because bone is a complex system and dairy is a complex food matrix, special attention should be given to the methods that researchers use to resolve remaining research gaps in the peer-reviewed literature. Several gaps in research exist in regard to the role of dairy products and bone health across the lifespan. First, our literature search failed to identify any RCTs that assessed the effects of dairy product intake on risk of fractures. Fractures represent the clinical outcome of utmost interest; however, changes in validated surrogate markers of bone health such as BMD and BMC provide valuable data in lieu of the large sample size and length of intervention needed for this primary outcome, given that osteoporosis is a long latency disease. To overcome limitations of DEXA, studies using pQCT are particularly needed to assess volumetric bone mineral density (vBMD) from each of the cortical and trabecular bones to provide a better prediction of fracture risk. The preponderance of studies report outcomes in adolescents and postmenopausal women, with some evidence in adults age <50-years and in men age >50-years. There is a lack of research in nonwhite or non-Asian (mostly Chinese) female populations; this is of significant concern, since genetic differences (e.g., lactose intolerance rates) can influence a population’s requisite for dairy alternatives and dietary supplements. There are a greater number of studies on calcium supplements than dairy likely due to logistical difficulties. RCTs with sufficient power have not directly compared dairy and calcium with vitamin D supplements to determine whether added benefits of dairy on bone exist. A rodent study undertaking such comparison found dairy to significantly increase bone size, density, and strength over nutritionally adequate diets with calcium salts (Weaver et al. Citation2009).
There is a great need for future research on the effects of dairy products during pregnancy and lactation. The single prospective cohort study showed maternal dairy intake during pregnancy to be associated with improvements in long-term offspring total body BMD at age 6-years; however, the study was relatively small in size (797 pregnant women and 698 children), baseline intakes of dairy, calcium, and protein were low, and the population limited to those of Indian decent (Ganpule et al. Citation2006) (). Our literature search only identified one RCT that reported the effects of dairy on maternal BMD (Liu et al. Citation2011). While the group supplemented with 45 g milk powder showed beneficial effects on BMD of the whole body, thoracic spine, and lateral spine over a 25-week period, the group supplemented with milk powder plus an additional 600 mg calcium showed more consistent effects on BMD across bone sites, likely due to low baseline calcium intake of the cohort (). A maternal dietary pattern that has the potential to influence bone health in both women and their offspring during pregnancy and lactation is an important topic that warrants future research.
Our study, similar to the recent NOF position statement found insufficient evidence to determine whether formula feeding versus breastfeeding had an effect on short- or long-term bone health in infants (Weaver et al. Citation2016). In the NOF position statement, formula-fed infants had better BMC and BMD in the first 6-months of life compared to breastfed infants in 2 observational studies (Butte et al. Citation2000; Kalkwarf, Khoury, and Lanphear Citation2003); however, breastfeeding was shown to be advantageous in 2 observational studies assessing later bone outcomes in 8-year-old children (Jones, Riley, and Dwyer Citation2000; Ma and Jones Citation2003) and 16-year-old adolescents (Jones, Hynes, and Dwyer Citation2013). These studies were excluded from this systematic review since it is not clear whether “infant formula” was comprised of cow’s milk. Although results from the single RCT, as well as the fairly large TARGeT Kids! prospective cohort study showed null effects assessed baseline calcium (in the RCT) and dairy (in the prospective cohort study) intakes were high per usual in North American studies (Specker et al. Citation1997; Allison et al. Citation2020). The Beginnings Study, a prospective cohort investigation found different trajectories of bone accretion among breast-fed, cow’s milk-based formula fed, and soy-based formula fed infants but was did not assess whether these relatively small differences had long-term impacts on bone (Andres et al. Citation2013). Two small studies, one prospective (n = 31) and another cross-sectional (n = 35) reported baseline serum 25OHD levels but not baseline intake of dairy, calcium or protein (Hillman Citation1988; Hillman et al. Citation1988; Park et al. Citation1998) (). Studies in toddlers (0- to 36-months) and complementary feeding in general are largely absent from the peer-reviewed literature as evidenced by a recent systematic review from the U.S. Department of Agriculture to support the 2020–2025 Dietary Guidelines Advisory Committee found insufficient evidence on the relationship of timing of introduction of complementary foods and beverages and types and/or amounts of complementary foods and beverages consumed and bone health (Obbagy et al. Citation2019).
In children, BMC is preferred over BMD as the measurement to evaluate changes in bone over time (Prentice, Parsons, and Cole Citation1994; Wren et al. Citation2005). Ten RCTs assessing effects of dairy products on BMD or BMC in children and adolescents were identified in the literature search; 8 of these studies showed statistically significant effects on at least one measured site, with none showing detrimental effects. Most studies were conducted in white female children and adolescents, and the larger studies were conducted in Chinese subjects with low baseline calcium intake. Huncharek, Muscat, and Kupelnick (Citation2008) previously highlighted in their meta-analysis that dairy products have a maximal benefit to improve total body BMC in children when calcium intake is <750 mg/day. Gains in a child’s bone mass increase with advancing age and are highly variable, even among children of the same age and sexual maturity. Linear growth is also highly variable. Calcium requirements to support growth and bone accretion therefore may be episodic and highly variable, especially during the ages when rapid growth and bone accretion take place (Lappe et al. Citation2015) ().
Seventeen RCTs assessing effects of dairy products on BMD or BMC in adults age <50-years-old and >50-years-years-old (n = 3 and 14, respectively) were identified in the literature search ( and ); all but one small RCT with insufficient power and two short-duration (6-months) studies found beneficial effects at one or more sites, although not always consistent across studies particularly in younger adults < 50-years-old. Age-related changes in bone metabolism, baseline calcium and vitamin D status, and lack of compliance most likely explain the lack of consistent changes in BMD or bone biochemical measures in response to dairy products between individuals. It is also possible that there are critical timepoints across the lifespan during which nutrition may have a larger impact. Feskanich et al. (Citation1997) and Feskanich, Willett, and Colditz (Citation2003) failed to find a benefit of intake during younger adulthood on fractures later in life; however, Feskanich et al. (Citation2018) found benefits of consumption post-menopause on incidence of hip fractures with longer follow-up and larger sample size, which conferred greater power. Menopause is a timepoint where a significant amount of bone density is lost due to changes in hormonal status. A recent investigation (not included in this review) of the Study of Women’s Health Across the Nation (SWAN) found early commencement of calcium supplements in pre- versus peri-menopausal state to have protective effects on the annualized rate of BMD loss throughout the menopause transition and into older adulthood (Wallace et al. Citation2020). Although dairy consumption did not show similar effects on annualized rate of BMD loss, intake across the SWAN cohort was somewhat low (Bailey et al. Citation2020). This cohort study is also unique because it enrolled white, black, Chinese, and Japanese women prior to the menopause transition (Sowers et al. Citation2000). Interestingly, the follow-up study by Daly et al. (Citation2008) found that the treatment group tended to maintain a calcium intake closer to the EAR, compared to the control group, which may explain why most of the initial benefits on BMD were maintained (i.e., hence behavior change modification over the initial 2-year period in Daly et al. (Citation2005). Additional research should be conducted toward further investigating the effects of nutrition on bone during these proposed critical timepoints.
Comprehensive systematic reviews, such as the one presented here, are needed in nutrition science not only to help identify future research gaps but also to adequately inform policy and public health messaging, as limitations in both RCTs and observational studies exist. Although RCTs are considered to be the gold standard from a clinical research paradigm, there is a dearth of high-quality diet-related intervention trials with bone as the primary outcome, forcing the use of observational research to inform research and clinical practices (Bailey et al. Citation2019). There are a number of issues that make RCTs of dietary interventions challenging to conduct and interpret, including cost, the time commitment and difficulties with maintaining adherence to a given dietary protocol, health problems or medication changes, and ethical issues associated with assigning people to a nonintervention control comparison group (Blumberg et al. Citation2010; Crichton et al. Citation2012). Data synthesis from population-based, prospective cohort studies often allows for sufficient assessment of a dose-response relationship between dietary exposure and a long-term chronic disease outcome (Bailey et al. Citation2019), as RCTs are rarely designed to evaluate multiple doses. Synthesizing data from multiple well-designed prospective cohort studies should be undertaken to determine an effective dose(s) for RCTs, which are often initiated absent of these critical preclinical data.
On the other hand, while prospective cohort studies can be strong in study design, limitations in dietary assessment methods, risk of bias due to confounding and incomplete follow-ups, and heterogeneity in population characteristics and outcome definitions limit their sole use in developing policy and public health messaging. Synthesis of fracture data contained within prospective cohort studies can complement evidence synthesis of RCTs reporting BMC/BMC outcomes when crafting public health messaging. Bailey et al. (Citation2019) proposed best practices for conducting observational research with regard to nutrition and bone health. Adding to these considerations, a major limitation within several of the included prospective cohort studies in our review is the wide variation in participant age. As discussed by Bailey et al. (Citation2019), certain subpopulations such as perimenopausal women and elderly individuals are more prone to changes in bone and therefore should not be analyzed with other subpopulations that experience more minute changes in bone, such as younger adults and men.
Our study has several limitations. First, our literature search was narrowed to only assess the effect of dairy products on BMD, BMC, and fractures. Many other less accepted but emerging markers of bone health exist. The International Osteoporosis Foundation and the International Federation of Clinical Chemistry Bone Marker Standards Working Group identified C-terminal telopeptide of type I collagen (CTX-I) and N-terminal propeptide of type I procollagen (PINP) as reference markers of bone turnover for fracture risk prediction and monitoring of osteoporosis treatment (Vasikaran et al. Citation2011). The NBHA is currently working to better standardize CTX-I and PINP to increase their clinical and research utility (Szulc et al. Citation2017). Not included in this systematic review are small controlled trials assessing ultrasensitive changes in bone calcium balance using the rare, long-lived radiotracer 41Ca, measured by accelerator mass spectrometry. Retention of bone calcium after administration of dairy products may be explained by decreased bone resorption (Rogers et al. Citation2016). A recent study in postmenopausal women demonstrated that urinary 41Ca retention is increased with an increase in calcium and vitamin D intake, regardless of the source of calcium (Rogers et al. Citation2016). We chose not to assess risk of bias among the RCTs and prospective cohort studies included, as this methodology is typically employed alongside a meta-analysis or within systematic reviews that are narrower in scope. The studies presented in our systematic review are heterogenous in many aspects, including study design, participants, assessment of dietary intake (food frequency questionnaires, retrospective recall, etc.), measurement of markers such as BMD (pQCT, QUS, DXA, etc.), and statistical methods. Each individual study included provides unique data with both strengths and limitations. Furthermore, there is no protocol registration for observational studies, making reporting bias extremely difficult to assess. Because only published literature was included in the present systematic review, publication bias should be suspected.
Conclusion
Good nutrition is critical for bone health across the lifespan. It is difficult to fully appreciate the importance of good nutrition since the effects are subtle over long periods of time. Dairy products provide the raw materials for bone structure; however, other lifestyle choices also influence the growth and preservation of bone. Dairy intakes that provide adequate dietary calcium may enhance the effectiveness of physical activity on bone density and strength. Dairy intake does not seem to increase the risk of fractures. Daily intake of low or nonfat dairy products as part of a healthy habitual dietary pattern may be associated with improved BMD of the total body and at some sites and associated with fewer fractures in older adults.
| Abbreviations | ||
| aBMD | = | areal bone mineral density |
| BMC | = | bone mineral content |
| BMD | = | bone mineral density |
| BW | = | bone width |
| CTX-I | = | C-terminal telopeptide of type I collagen |
| DXA | = | dual-energy x-ray absorptiometry |
| NBHA | = | National Bone Health Alliance |
| NOF | = | National Osteoporosis Foundation |
| PINP | = | N-terminal propeptide of type I procollagen |
| pQCT | = | peripheral quantitative computed tomography |
| QUS | = | quantitative ultrasound |
| RCT | = | randomized controlled trial |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbaspour, N., R. Hurrell, and R. Kelishadi. 2014. Review on iron and its importance for human health. Journal of Research in Medical Sciences 19:164–74.
- Achamrah, N., M. Coëffier, P. Jésus, J. Charles, A. Rimbert, P. Déchelotte, and S. Grigioni. 2017. Bone mineral density after weight gain in 160 patients with anorexia nervosa. Frontiers in Nutrition (4). doi: https://doi.org/10.3389/fnut.2017.00046.
- Allison, R. M., C. S. Birken, G. Lebovic, A. W. Howard, M. R. L'Abbe, M.-E. Morency, and J. L. Maguire. 2020. Consumption of cow's milk in early childhood and fracture risk: A prospective cohort study. American Journal of Epidemiology 189 (2):146–55. doi: https://doi.org/10.1093/aje/kwz216.
- American Diabetes Association. 2012. Introduction: The American Diabetes Association's (ADA) evidence-based practice guidelines, standards, and related recommendations and documents for diabetes care. Diabetes Care 35 (Suppl 1):S1–S2.
- Andres, A., P. H. Casey, M. A. Cleves, and T. M. Badger. 2013. Body fat and bone mineral content of infants fed breast milk, cow's milk formula, or soy formula during the first year of life. The Journal of Pediatrics 163 (1):49–54. doi: https://doi.org/10.1016/j.jpeds.2012.12.067.
- Aslam, H., K. L. Holloway-Kew, M. Mohebbi, F. N. Jacka, and J. A. Pasco. 2019. Association between dairy intake and fracture in an Australian-based cohort of women: A prospective study. BMJ Open 9 (11):e031594. doi: https://doi.org/10.1136/bmjopen-2019-031594.
- Astrup, A. 2014. Yogurt and dairy product consumption to prevent cardiometabolic diseases: Epidemiologic and experimental studies. The American Journal of Clinical Nutrition 99 (5 Suppl):1235S–42S. doi: https://doi.org/10.3945/ajcn.113.073015.
- Bahtiri, E., H. Islami, R. Hoxha, H. QorrajBytyqi, T. MalokuGjergji, S. Rexhepi, S. Krasniqi, V. Zhjeqi, E. Halimi, S. Thaci, et al. 2014. Calcium and dairy products consumption and association with total hip bone mineral density in women from Kosovo. Medical Archives (Sarajevo, Bosnia and Herzegovina) 68 (4):259–62. doi: https://doi.org/10.5455/medarh.2014.68.259-262.
- Bailey, R. L., S. Sahni, P. Chocano-Bedoya, R. M. Daly, A. A. Welch, H. Bischoff-Ferrari, and C. M. Weaver. 2019. Best practices for conducting observational research to assess the relation between nutrition and bone: An international working group summary. Advances in Nutrition (Bethesda, Md.) 10 (3):391–409. doi: https://doi.org/10.1093/advances/nmy111.
- Bailey, R. L., P. Zou, T. C. Wallace, G. P. McCabe, B. A. Craig, S. Jun, J. A. Cauley, and C. M. Weaver. 2020. Calcium supplement use is associated with less bone mineral density loss but does not lessen the risk of bone fracture across the menopause transition: Data from the Study of Women's Health Across the Nation. JBMR Plus 4 (1):e10246. doi: https://doi.org/10.1002/jbm4.10246.
- Benetou, V., P. Orfanos, D. Zylis, S. Sieri, P. Contiero, R. Tumino, M. C. Giurdanella, P. H. M. Peeters, J. Linseisen, A. Nieters, et al. 2011. Diet and hip fractures among elderly Europeans in the EPIC cohort. European Journal of Clinical Nutrition 65 (1):132–9. doi: https://doi.org/10.1038/ejcn.2010.226.
- Betancourt, A. I., and H. F. Gaitan. 2012. Micronutrients: Sources, properties and health effects. Hauppauge, NY: Nova Science Publishers.
- Bian, S., J. Hu, K. Zhang, Y. Wang, M. Yu, and J. Ma. 2018. Dairy product consumption and risk of hip fracture: A systematic review and meta-analysis. BMC Public Health 18 (1):165. doi: https://doi.org/10.1186/s12889-018-5041-5.
- Bierhals, I. O., J. dos Santos Vaz, A. M. Baptista Menezes, F. C. Wehrmeister, L. Pozza, and F. M. C. Assuncao. 2019. Milk consumption, dietary calcium intake and nutrient patterns from adolescence to early adulthood and its effect on bone mass: The 1993 Pelotas (Brazil) birth cohort. Cadernos de Saude Publica 35 (8):e00192418.
- Biver, E., C. Durosier-Izart, F. Merminod, T. Chevalley, B. van Rietbergen, S. L. Ferrari, and R. Rizzoli. 2018. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporosis International 29 (8):1771–82. doi: https://doi.org/10.1007/s00198-018-4535-4.
- Black, R. E., S. M. Williams, I. E. Jones, and A. Goulding. 2002. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. The American Journal of Clinical Nutrition 76 (3):675–80. doi: https://doi.org/10.1093/ajcn/76.3.675.
- Blumberg, J., R. P. Heaney, M. Huncharek, T. Scholl, M. Stampfer, R. Vieth, C. M. Weaver, and S. H. Zeisel. 2010. Evidence-based criteria in the nutritional context. Nutrition Reviews 68 (8):478–84. doi: https://doi.org/10.1111/j.1753-4887.2010.00307.x.
- Boers, H. M., M. Alssema, D. J. Mela, H. P. F. Peters, R. J. Vonk, and M. G. Priebe. 2019. The rate of glucose appearance is related to postprandial glucose and insulin responses in adults: A systematic review and meta-analysis of stable isotope studies. The Journal of Nutrition 149 (11):1896–903. doi: https://doi.org/10.1093/jn/nxz150.
- Budek, A. Z., C. Hoppe, K. F. Michaelsen, and C. Molgaard. 2007. High intake of milk, but not meat, decreases bone turnover in prepubertal boys after 7 days. European Journal of Clinical Nutrition 61 (8):957–62. doi: https://doi.org/10.1038/sj.ejcn.1602605.
- Butte, N. F., W. W. Wong, J. M. Hopkinson, E. O. Smith, and K. J. Ellis. 2000. Infant feeding mode affects early growth and body composition. Pediatrics 106 (6):1355–66. doi: https://doi.org/10.1542/peds.106.6.1355.
- Cadogan, J., R. Eastell, N. Jones, and M. E. Barker. 1997. Milk intake and bone mineral acquisition in adolescent girls: Randomised, controlled intervention trial. BMJ (Clinical Research ed.) 315 (7118):1255–60. doi: https://doi.org/10.1136/bmj.315.7118.1255.
- Chan, C. Y., S. Subramaniam, N. Mohamed, S. Ima-Nirwana, N. Muhammad, A. Fairus, P. Y. Ng, N. A. Jamil, N. Abd Aziz, and K.-Y. Chin. 2020. Determinants of bone health status in a multi-ethnic population in Klang Valley. International Journal of Environmental Research and Public Health 17 (2):384. doi: https://doi.org/10.3390/ijerph17020384.
- Chan, G. M., K. Hoffman, and M. McMurry. 1995. Effects of dairy products on bone and body composition in pubertal girls. The Journal of Pediatrics 126 (4):551–6. doi: https://doi.org/10.1016/S0022-3476(95)70348-9.
- Chee, W. S., A. R. Suriah, S. P. Chan, Y. Zaitun, and Y. M. Chan. 2003. The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporosis International 14 (10):828–34. doi: https://doi.org/10.1007/s00198-003-1448-6.
- Chen, Y., Q. Zhang, Y. Wang, Y. Xiao, R. Fu, H. Bao, and M. Liu. 2015. Estimating the causal effect of milk powder supplementation on bone mineral density: A randomized controlled trial with both non-compliance and loss to follow-up. European Journal of Clinical Nutrition 69 (7):824–30. doi: https://doi.org/10.1038/ejcn.2015.3.
- Cheng, S., A. Lyytikainen, H. Kroger, C. Lamberg-Allardt, M. Alen, A. Koistinen, Q. J. Wang, M. Suuriniemi, H. Suominen, A. Mahonen, et al. 2005. Effects of calcium, dairy product, and vitamin D supplementation on bone mass accrual and body composition in 10-12-y-old girls: A 2-y randomized trial. The American Journal of Clinical Nutrition 82 (5):1115–26, quiz 47–8. doi: https://doi.org/10.1093/ajcn/82.5.1115.
- Cho, S. S., L. Qi, G. C. Fahey, Jr., and D. M. Klurfeld. 2013. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. The American Journal of Clinical Nutrition 98 (2):594–619. doi: https://doi.org/10.3945/ajcn.113.067629.
- Cole, Z. A., C. R. Gale, M. K. Javaid, S. M. Robinson, C. Law, B. J. Boucher, S. R. Crozier, K. M. Godfrey, E. M. Dennison, and C. Cooper. 2009. Maternal dietary patterns during pregnancy and childhood bone mass: A longitudinal study. Journal of Bone and Mineral Research 24 (4):663–8. doi: https://doi.org/10.1359/jbmr.081212.
- Cosman, F., S. J. de Beur, M. S. LeBoff, E. M. Lewiecki, B. Tanner, S. Randall, and R. Lindsay. 2014. Clinician's guide to prevention and treatment of osteoporosis. Osteoporosis International 25 (10):2359–81. doi: https://doi.org/10.1007/s00198-014-2794-2.
- Crichton, G. E., P. R. Howe, J. D. Buckley, A. M. Coates, K. J. Murphy, and J. Bryan. 2012. Long-term dietary intervention trials: Critical issues and challenges. Trials 13:111. doi: https://doi.org/10.1186/1745-6215-13-111.
- Cumming, R. G., S. R. Cummings, M. C. Nevitt, J. Scott, K. E. Ensrud, T. M. Vogt, and K. Fox. 1997. Calcium intake and fracture risk: Results from the study of osteoporotic fractures. American Journal of Epidemiology 145 (10):926–34. doi: https://doi.org/10.1093/oxfordjournals.aje.a009052.
- Cumming, R. G., and R. J. Klineberg. 1994. Case-control study of risk factors for hip fractures in the elderly. American Journal of Epidemiology 139 (5):493–503. doi: https://doi.org/10.1093/oxfordjournals.aje.a117032.
- Daly, R. M., M. Brown, S. Bass, S. Kukuljan, and C. Nowson. 2005. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: A 2-year randomized controlled trial. Journal of Bone and Mineral Research 21 (3):397–405. doi: https://doi.org/10.1359/JBMR.051206.
- Daly, R. M., N. Petrass, S. Bass, and C. A. Nowson. 2008. The skeletal benefits of calcium- and vitamin D3-fortified milk are sustained in older men after withdrawal of supplementation: An 18-mo follow-up study. The American Journal of Clinical Nutrition 87 (3):771–7. doi: https://doi.org/10.1093/ajcn/87.3.771.
- de Lamas, C., M. J. de Castro, M. Gil-Campos, Á. Gil, M. L. Couce, and R. Leis. 2019. Effects of dairy product consumption on height and bone mineral content in children: A systematic review of controlled trials. Advances in Nutrition (Bethesda, Md.) 10 (suppl_2):S88–S96. doi: https://doi.org/10.1093/advances/nmy096.
- Dietary Guidelines Advisory Committee. 2015. Dietary guidelines for Americans 2015–2020. Washington, DC: Government Printing Office.
- Du, X. Q., H. Greenfield, D. R. Fraser, K. Y. Ge, Z. H. Liu, and W. He. 2002. Milk consumption and bone mineral content in Chinese adolescent girls. Bone 30 (3):521–8. doi: https://doi.org/10.1016/S8756-3282(01)00698-6.
- Du, X., K. Zhu, A. Trube, Q. Zhang, G. Ma, X. Hu, D. R. Fraser, and H. Greenfield. 2004. School-milk intervention trial enhances growth and bone mineral accretion in Chinese girls aged 10-12 years in Beijing. The British Journal of Nutrition 92 (1):159–68. doi: https://doi.org/10.1079/BJN20041118.
- Durosier-Izart, C., E. Biver, F. Merminod, B. van Rietbergen, T. Chevalley, F. R. Herrmann, S. L. Ferrari, and R. Rizzoli. 2017. Peripheral skeleton bone strength is positively correlated with total and dairy protein intakes in healthy postmenopausal women. The American Journal of Clinical Nutrition 105 (2):513–25. doi: https://doi.org/10.3945/ajcn.116.134676.
- Eden, J., L. Levit, A. Berg, and S. Morton. 2011. Finding what works in health care: Standards for systematic reviews. Washington, DC: National Academies Press.
- Esterle, L., J. P. Sabatier, F. Guillon-Metz, O. Walrant-Debray, G. Guaydier-Souquieres, F. Jehan, and M. Garabedian. 2009. Milk, rather than other foods, is associated with vertebral bone mass and circulating IGF-1 in female adolescents. Osteoporosis International 20 (4):567–75. doi: https://doi.org/10.1007/s00198-008-0708-x.
- Eysteinsdottir, T., T. I. Halldorsson, I. Thorsdottir, G. Sigurdsson, S. Sigurðsson, T. Harris, L. J. Launer, V. Gudnason, I. Gunnarsdottir, and L. Steingrimsdottir. 2014. Milk consumption throughout life and bone mineral content and density in elderly men and women. Osteoporosis International 25 (2):663–72. doi: https://doi.org/10.1007/s00198-013-2476-5.
- Fabiani, R., G. Naldini, and M. Chiavarini. 2019. Dietary patterns in relation to low bone mineral density and fracture risk: A systematic review and meta-analysis. Advances in Nutrition 10 (2):219–36. doi: https://doi.org/10.1093/advances/nmy073.
- Feart, C., S. Lorrain, V. Ginder Coupez, C. Samieri, L. Letenneur, D. Paineau, and P. Barberger-Gateau. 2013. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporosis International 24 (12):3031–41. doi: https://doi.org/10.1007/s00198-013-2421-7.
- Feskanich, D., H. A. Bischoff-Ferrari, A. L. Frazier, and W. C. Willett. 2014. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatrics 168 (1):54–60. doi: https://doi.org/10.1001/jamapediatrics.2013.3821.
- Feskanich, D., H. E. Meyer, T. T. Fung, H. A. Bischoff-Ferrari, and W. C. Willett. 2018. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporosis International 29 (2):385–96. doi: https://doi.org/10.1007/s00198-017-4285-8.
- Feskanich, D., W. C. Willett, and G. A. Colditz. 2003. Calcium, vitamin D, milk consumption, and hip fractures: A prospective study among postmenopausal women. The American Journal of Clinical Nutrition 77 (2):504–11. doi: https://doi.org/10.1093/ajcn/77.2.504.
- Feskanich, D., W. C. Willett, M. J. Stampfer, and G. A. Colditz. 1997. Milk, dietary calcium, and bone fractures in women: A 12-year prospective study. American Journal of Public Health 87 (6):992–7. doi: https://doi.org/10.2105/ajph.87.6.992.
- Fujiwara, S., F. Kasagi, M. Yamada, and K. Kodama. 1997. Risk factors for hip fracture in a Japanese cohort. Journal of Bone and Mineral Research 12 (7):998–1004. doi: https://doi.org/10.1359/jbmr.1997.12.7.998.
- Ganpule, A., C. S. Yajnik, C. H D. Fall, S. Rao, D. J. Fisher, A. Kanade, C. Cooper, S. Naik, N. Joshi, H. Lubree, et al. 2006. Bone mass in Indian children - relationships to maternal nutritional status and diet during pregnancy: The Pune Maternal Nutrition Study. The Journal of Clinical Endocrinology & Metabolism 91 (8):2994–3001. doi: https://doi.org/10.1210/jc.2005-2431.
- Geiker, N. R. W., C. Mølgaard, S. Iuliano, R. Rizzoli, Y. Manios, L. J. C. van Loon, J.-M. Lecerf, G. Moschonis, J.-Y. Reginster, I. Givens, et al. 2020. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporosis International 31 (4):601–15. doi: https://doi.org/10.1007/s00198-019-05229-7.
- Gibbons, M. J., N. L. Gilchrist, C. Frampton, P. Maguire, P. H. Reilly, R. L. March, and C. R. Wall. 2004. The effects of a high calcium dairy food on bone health in pre-pubertal children in New Zealand. Asia Pacific Journal of Clinical Nutrition 13:341–7.
- Goulding, A., J. E. Rockell, R. E. Black, A. M. Grant, I. E. Jones, and S. M. Williams. 2004. Children who avoid drinking cow's milk are at increased risk for prepubertal bone fractures. Journal of the American Dietetic Association 104 (2):250–3. doi: https://doi.org/10.1016/j.jada.2003.11.008.
- Gui, J.-C., J. R. Brašić, X.-D. Liu, G.-Y. Gong, G.-M. Zhang, C.-J. Liu, and G.-Q. Gao. 2012. Bone mineral density in postmenopausal Chinese women treated with calcium fortification in soymilk and cow's milk. Osteoporosis International 23 (5):1563–70. doi: https://doi.org/10.1007/s00198-012-1895-z.
- Haussler, M. R., G. K. Whitfield, I. Kaneko, C. A. Haussler, D. Hsieh, J. C. Hsieh, and P. W. Jurutka. 2013. Molecular mechanisms of vitamin D action. Calcified Tissue International 92 (2):77–98. doi: https://doi.org/10.1007/s00223-012-9619-0.
- Health Canada. 2018. Welcome to Canada’s food guide. Accessed August 16, 2019. https://food-guide.canada.ca/en/.
- Heaney, R. P. 2000. Calcium, dairy products and osteoporosis. Journal of the American College of Nutrition 19 (2 Suppl):83S–99S. doi: https://doi.org/10.1080/07315724.2000.10718088.
- Heaney, R. P. 2009. Dairy and bone health. Journal of the American College of Nutrition 28 (sup1):82S–90S. doi: https://doi.org/10.1080/07315724.2009.10719808.
- Heaney, R. P., S. Abrams, B. Dawson-Hughes, A. Looker, R. Marcus, V. Matkovic, and C. Weaver. 2000. Peak bone mass. Osteoporosis International 11 (12):985–1009. doi: https://doi.org/10.1007/s001980070020.
- Hillman, L. S. 1988. Bone mineral content in term infants fed human milk, cow milk-based formula, or soy-based formula. The Journal of Pediatrics 113 (1 Pt 2):208–12. doi: https://doi.org/10.1016/s0022-3476(88)80613-9.
- Hillman, L. S., W. Chow, S. S. Salmons, E. Weaver, M. Erickson, and J. Hansen. 1988. Vitamin D metabolism, mineral homeostasis, and bone mineralization in term infants fed human milk, cow milk-based formula, or soy-based formula. The Journal of Pediatrics 112 (6):864–74. doi: https://doi.org/10.1016/s0022-3476(88)80206-3.
- Holvik, K., H. E. Meyer, I. Laake, D. Feskanich, T. K. Omsland, and A.-J. Sogaard. 2019. Milk drinking and risk of hip fracture: The Norwegian Epidemiologic Osteoporosis Studies (NOREPOS). British Journal of Nutrition 121 (6):709–18. doi: https://doi.org/10.1017/S0007114518003823.
- Huncharek, M., J. Muscat, and B. Kupelnick. 2008. Impact of dairy products and dietary calcium on bone-mineral content in children: Results of a meta-analysis. Bone 43 (2):312–21. doi: https://doi.org/10.1016/j.bone.2008.02.022.
- Hunt, J. R., L. K. Johnson, and Z. K. Fariba Roughead. 2009. Dietary protein and calcium interact to influence calcium retention: A controlled feeding study. The American Journal of Clinical Nutrition 89 (5):1357–65. doi: https://doi.org/10.3945/ajcn.2008.27238.
- Ilich, J. Z., O. J. Kelly, P.-Y. Liu, H. Shin, Y. Kim, Y. Chi, K. K. A. S. Wickrama, and I. Colic-Baric. 2019. Role of calcium and low-fat dairy foods in weight-loss outcomes revisited: Results from the randomized trial of effects on bone and body composition in overweight/obese postmenopausal women. Nutrients 11 (5):1157. doi: https://doi.org/10.3390/nu11051157.
- Jadad, A. R., R. A. Moore, D. Carroll, C. Jenkinson, D. J. M. Reynolds, D. J. Gavaghan, and H. J. McQuay. 1996. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials 17 (1):1–12. doi: https://doi.org/10.1016/0197-2456(95)00134-4.
- Jha, R. M., A. Mithal, N. Malhotra, and E. M. Brown. 2010. Pilot case-control investigation of risk factors for hip fractures in the urban Indian population. BMC Musculoskeletal Disorders 11:49. doi: https://doi.org/10.1186/1471-2474-11-49.
- Jitapunkul, S., P. Yuktananandana, and V. Parkpian. 2001. Risk factors of hip fracture among Thai female patients. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 84 (11):1576–81.
- Johnell, O., Gullberg, B. Kanis, J. Allander, A. E. Elffors, L. Dequeker, J. Dilsen, G. Gennari, C. Vaz, A. L. Lyritis, et al. 1995. Risk factors for hip fracture in European women: The MEDOS study. Journal of Bone and Mineral Research 10 (11):1802–15. doi: https://doi.org/10.1002/jbmr.5650101125.
- Jones, G., K. L. Hynes, and T. Dwyer. 2013. The association between breastfeeding, maternal smoking in utero, and birth weight with bone mass and fractures in adolescents: A 16-year longitudinal study. Osteoporosis International 24 (5):1605–11. doi: https://doi.org/10.1007/s00198-012-2207-3.
- Jones, G., M. Riley, and T. Dwyer. 2000. Breastfeeding in early life and bone mass in prepubertal children: A longitudinal study. Osteoporosis International 11 (2):146–52. doi: https://doi.org/10.1007/PL00004176.
- Kalkwarf, H. J., J. C. Khoury, and B. P. Lanphear. 2003. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. The American Journal of Clinical Nutrition 77 (1):257–65. doi: https://doi.org/10.1093/ajcn/77.1.257.
- Kalkwarf, H. J., B. S. Zemel, K. Yolton, and J. E. Heubi. 2013. Bone mineral content and density of the lumbar spine of infants and toddlers: Influence of age, sex, race, growth, and human milk feeding. Journal of Bone and Mineral Research 28 (1):206–12. doi: https://doi.org/10.1002/jbmr.1730.
- Kanis, J., O. Johnell, B. Gullberg, E. Allander, L. Elffors, J. Ranstam, J. Dequeker, G. Dilsen, C. Gennari, A. Vaz, et al. 1999. Risk factors for hip fracture in men from southern Europe: The MEDOS study. Osteoporosis International 9 (1):45–54. doi: https://doi.org/10.1007/s001980050115.
- Kerstetter, J. E., K. O. O’Brien, D. M. Caseria, D. E. Wall, and K. L. Insogna. 2005. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. The Journal of Clinical Endocrinology & Metabolism 90 (1):26–31. doi: https://doi.org/10.1210/jc.2004-0179.
- Konstantynowicz, J., T. V. Nguyen, M. Kaczmarski, J. Jamiolkowski, J. Piotrowska-Jastrzebska, and E. Seeman. 2007. Fractures during growth: Potential role of a milk-free diet. Osteoporosis International 18 (12):1601–7. doi: https://doi.org/10.1007/s00198-007-0397-x.
- Labouesse, M. A., E. R. Gertz, B. D. Piccolo, E. C. Souza, G. U. Schuster, M. G. Witbracht, L. R. Woodhouse, S. H. Adams, N. L. Keim, and M. D. Van Loan. 2014. Associations among endocrine, inflammatory, and bone markers, body composition and weight loss induced bone loss. Bone 64:138–46. doi: https://doi.org/10.1016/j.bone.2014.03.047.
- Lan, T.-Y., S.-M. Hou, C.-Y. Chen, W.-C. Chang, J. Lin, C.-C. Lin, W.-J. Liu, T.-F. Shih, and T.-Y. Tai. 2010. Risk factors for hip fracture in older adults: A case-control study in Taiwan. Osteoporosis International 21 (5):773–84. doi: https://doi.org/10.1007/s00198-009-1013-z.
- Lanyan, A., P. Marques-Vidal, E. Gonzalez-Rodriguez, D. Hans, and O. Lamy. 2020. Postmenopausal women with osteoporosis consume high amounts of vegetables but insuffient dairy products and calcium to benefit their virtues: The CoLaus/OsteoLaus cohort. Osteoporosis International 31 (5):875–86. doi: https://doi.org/10.1007/s00198-019-05225-x.
- Lappe, J. M., P. Watson, V. Gilsanz, T. Hangartner, H. J. Kalkwarf, S. Oberfield, J. Shepherd, K. K. Winer, and B. Zemel. 2015. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. Journal of Bone and Mineral Research 30 (1):156–64. doi: https://doi.org/10.1002/jbmr.2319.
- Lau, E. M., H. Lynn, Y. H. Chan, W. Lau, and J. Woo. 2004. Benefits of milk powder supplementation on bone accretion in Chinese children. Osteoporosis International 15 (8):654–8. doi: https://doi.org/10.1007/s00198-004-1593-6.
- Lau, E. M., H. Lynn, Y. H. Chan, and J. Woo. 2002. Milk supplementation prevents bone loss in postmenopausal Chinese women over 3 years. Bone 31 (4):536–40. doi: https://doi.org/10.1016/S8756-3282(02)00853-0.
- Lau, E. M., J. Woo, V. Lam, and A. Hong. 2001. Milk supplementation of the diet of postmenopausal Chinese women on a low calcium intake retards bone loss. Journal of Bone and Mineral Research 16 (9):1704–9. doi: https://doi.org/10.1359/jbmr.2001.16.9.1704.
- Liu, Z., L. Qiu, Y. M. Chen, and Y. X. Su. 2011. Effect of milk and calcium supplementation on bone density and bone turnover in pregnant Chinese women: A randomized controlled trail. Archives of Gynecology and Obstetrics 283 (2):205–11. doi: https://doi.org/10.1007/s00404-009-1345-0.
- Lu, J. X., H. Pan, X. Q. Hu, Z. W. Huang, and Q. Zhang. 2019. Effects of milk powder intervention on bone mineral density and indicators related to bone metabolism in Chinese adolescents. Osteoporosis International 30 (11):2231–9. doi: https://doi.org/10.1007/s00198-019-05105-4.
- Lunt, M., P. Masaryk, C. Scheidt-Nave, J. Nijs, G. Poor, H. Pols, J. A. Falch, G. Hammermeister, D. M. Reid, L. Benevolenskaya, et al. 2001. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: The EVOS study. Osteoporosis International 12 (8):688–98. doi: https://doi.org/10.1007/s001980170069.
- Ma, D., and G. Jones. 2003. The association between bone mineral density, metacarpal morphometry, and upper limb fractures in children: A population-based case-control study. The Journal of Clinical Endocrinology & Metabolism 88 (4):1486–91. doi: https://doi.org/10.1210/jc.2002-021682.
- Malpeli, A., M. Apezteguia, J. L. Mansur, A. Armanini, M. Macias Couret, R. Villalobos, M. Kuzminczuk, and H. F. Gonzalez. 2012. Calcium supplementation, bone mineral density and bone mineral content. Predictors of bone mass changes in adolescent mothers during the 6-month postpartum period. Archivos Latinoamericanos de Nutrición 62:30–6.
- Mangano, K. M., S. E. Noel, S. Sahni, and K. L. Tucker. 2019. Higher dairy intakes are associated with higher bone mineral density among adults with sufficient vitamin D status: Results from the Boston Puerto Rican Osteoporosis Study. The Journal of Nutrition 149 (1):139–48. doi: https://doi.org/10.1093/jn/nxy234.
- Manios, Y., Moschonis, G. Trovas, G. and G. P. Lyritis. 2007. Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: The postmenopausal health study. The American Journal of Clinical Nutrition 86 (3):781–9. doi: https://doi.org/10.1093/ajcn/86.3.781.
- Matkovic, V., J. D. Landoll, N. E. Badenhop-Stevens, E.-Y. Ha, Z. Crncevic-Orlic, B. Li, and P. Goel. 2004. Nutrition influences skeletal development from childhood to adulthood: A study of hip, spine, and forearm in adolescent females. The Journal of Nutrition 134 (3):701S–5S. doi: https://doi.org/10.1093/jn/134.3.701S.
- McCabe, L. D., B. R. Martin, G. P. McCabe, C. C. Johnston, C. M. Weaver, and M. Peacock. 2004. Dairy intakes affect bone density in the elderly. The American Journal of Clinical Nutrition 80 (4):1066–74. doi: https://doi.org/10.1093/ajcn/80.4.1066.
- Merrilees, M. J., E. J. Smart, N. L. Gilchrist, C. Frampton, J. G. Turner, E. Hooke, R. L. March, and P. Maguire. 2000. Effects of diary food supplements on bone mineral density in teenage girls. European Journal of Nutrition 39 (6):256–62. doi: https://doi.org/10.1007/s003940070004.
- Meyer, H. E., J. I. Pedersen, E. B. Loken, and A. Tverdal. 1997. Dietary factors and the incidence of hip fracture in middle-aged Norwegians: A prospective study. American Journal of Epidemiology 145 (2):117–23. doi: https://doi.org/10.1093/oxfordjournals.aje.a009082.
- Michaelsson, K., A. Wolk, S. Langenskiold, S. Basu, E. Warensjo Lemming, H. Melhus, and L. Byberg. 2014. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ (Clinical Research ed.) 349:g6015. doi: https://doi.org/10.1136/bmj.g6015.
- Michaelsson, K., A. Wolk, E. W. Lemming, H. Melhus, and L. Byberg. 2018. Intake of milk or fermented milk combined with fruit and vegetable consumption in relation to hip fracture rates: A cohort study of Swedish women. Journal of Bone and Mineral Research 33 (3):449–57. doi: https://doi.org/10.1002/jbmr.3324.
- Moher, D., A. Liberati, J. Tetzlaff, and D. G. Altman. 2009. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine 6 (7):e1000097. doi: https://doi.org/10.1371/journal.pmed.1000097.
- Moore, L. L., M. L. Bradlee, D. Gao, and M. R. Singer. 2008. Effects of average childhood dairy intake on adolescent bone health. The Journal of Pediatrics 153 (5):667–73. doi: https://doi.org/10.1016/j.jpeds.2008.05.016.
- Moschonis, G., S. Kanellakis, N. Papaioannou, A. Schaafsma, and Y. Manios. 2011. Possible site-specific effect of an intervention combining nutrition and lifestyle counselling with consumption of fortified dairy products on bone mass: The Postmenopausal Health Study II. Journal of Bone and Mineral Metabolism 29 (4):501–6. doi: https://doi.org/10.1007/s00774-010-0256-2.
- Movassagh, E. Z., S. Kontulainen, A. D. G. Baxter-Jones, S. Whiting, M. Szafron, M. Papadimitropoulos, and H. Vatanparast. 2017. Are milk and alternatives and fruit and vegetable intakes during adolescence associated with cortical and trabecular bone structure, density, and strength in adulthood? Osteoporosis International 28 (2):609–19. doi: https://doi.org/10.1007/s00198-016-3775-4.
- Murphy, S., K. T. Khaw, H. May, and J. E. Compston. 1994. Milk consumption and bone mineral density in middle aged and elderly women. BMJ 308 (6934):939–41. doi: https://doi.org/10.1136/bmj.308.6934.939.
- Nevitt, M. C., S. R. Cummings, K. L. Stone, L. Palermo, D. M. Black, D. C. Bauer, H. K. Genant, M. C. Hochberg, K. E. Ensrud, T. A. Hillier, et al. 2005. Risk factors for a first-incident radiographic vertebral fracute in women ≥65 years of age: The Study of Osteoporotic Fractures. Journal of Bone and Mineral Research 20 (1):131–40. doi: https://doi.org/10.1359/jbmr.2005.20.1.131.
- Nieves, J. W., J. A. Grisso, and J. L. A. Kelsey. 1992. A case-control study of hip fracture: Evaluation of selected dietary variables and teenage physical activity. Osteoporosis International 2 (3):122–7. doi: https://doi.org/10.1007/BF01623818.
- Nieves, J. W., K. Melsop, M. Curtis, J. L. Kelsey, L. K. Bachrach, G. Greendale, M. F. Sowers, and K. L. Sainani. 2010. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM&R 2 (8):740–50, quiz 94. doi: https://doi.org/10.1016/j.pmrj.2010.04.020.
- Obbagy, J. E, L. K. English, Y. P. Wong, N. F. Butte, K. G. Dewey, M. K. Fox, F. R. Greer, N. F. Krebs, K. S. Scanlon, and E. E. Stoody. 2019. Complementary feeding and bone health: A systematic review. The American Journal of Clinical Nutrition 109 (Suppl_7):872S–8S. doi: https://doi.org/10.1093/ajcn/nqy227.
- Opotowsky, A. R., and J. P. Bilezikian. 2003. Racial differences in the effect of early milk consumption on peak and postmenopausal bone mineral density. Journal of Bone and Mineral Research 18 (11):1978–88. doi: https://doi.org/10.1359/jbmr.2003.18.11.1978.
- Ott, S. M. 2018. Cortical or trabecular bone: What's the difference? American Journal of Nephrology 47 (6):373–5. doi: https://doi.org/10.1159/000489672.
- Owusu, W., W. C. Willett, D. Feskanich, A. Ascherio, D. Spiegelman, and G. A. Colditz. 1997. Calcium intake and the incidence of forearm and hip fractures among men. The Journal of Nutrition 127 (9):1782–7. doi: https://doi.org/10.1093/jn/127.9.1782.
- Park, M. J., R. Namgung, D. H. Kim, and R. C. Tsang. 1998. Bone mineral content is not reduced despite low vitamin D status in breast milk-fed infants versus cow's milk based formula-fed infants. J. Pediatr 132 (4):641–5. doi:https://doi.org/10.1016/S0022-3476(98)70353-1.
- Petridou, E., T. Karpathios, N. Dessypris, E. Simou, and D. Trichopoulos. 1997. The role of dairy products and non alcoholic beverages in bone fractures among schoolage children. Scandinavian Journal of Social Medicine 25 (2):119–25. doi: https://doi.org/10.1177/140349489702500209.
- Prentice, A., T. J. Parsons, and T. J. Cole. 1994. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. The American Journal of Clinical Nutrition 60 (6):837–42. doi: https://doi.org/10.1093/ajcn/60.6.837.
- Prince, R., A. Devine, I. Dick, A. Criddle, D. Kerr, N. Kent, R. Price, and A. Randell. 2009. The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. Journal of Bone and Mineral Research 10 (7):1068–75. doi: https://doi.org/10.1002/jbmr.5650100711.
- Rizzoli, R. 2008. Nutrition: Its role in bone health. Best Practice & Research. Clinical Endocrinology & Metabolism 22 (5):813–29. doi: https://doi.org/10.1016/j.beem.2008.08.005.
- Rizzoli, R., M. L. Bianchi, M. Garabedian, H. A. McKay, and L. A. Moreno. 2010. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone 46 (2):294–305. doi: https://doi.org/10.1016/j.bone.2009.10.005.
- Rizzoli, R., E. Biver, J. P. Bonjour, V. Coxam, D. Goltzman, J. A. Kanis, J. Lappe, L. Rejnmark, S. Sahni, C. Weaver, et al. 2018. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporosis International 29 (9):1933–48. doi: https://doi.org/10.1007/s00198-018-4534-5.
- Rogers, T. S., M. G. Garrod, J. M. Peerson, D. J. Hillegonds, B. A. Buchholz, E. Demmer, C. Richardson, E. R. Gertz, and M. D. Van Loan. 2016. Is bone equally responsive to calcium and vitamin D intake from food vs. supplements? Use of (41)calcium tracer kinetic model. Bone Reports 5:117–23. doi: https://doi.org/10.1016/j.bonr.2016.05.001.
- Rosado, J. L., O. P. Garcia, D. Ronquillo, D. Hervert-Hernández, M. D. C. Caamaño, G. Martínez, J. Gutiérrez, and S. García. 2011. Intake of milk with added micronutrients increases the effectiveness of an energy-restricted diet to reduce body weight: A randomized controlled clinical trial in Mexican women. Journal of the American Dietetic Association 111 (10):1507–16. doi: https://doi.org/10.1016/j.jada.2011.07.011.
- Ross, A. C., C. L. Taylor, A. L. Yaktine, and H. B. Del Valle. 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press.
- Roughead, Z. K., L. K. Johnson, G. I. Lykken, and J. R. Hunt. 2003. Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. The Journal of Nutrition 133 (4):1020–6. doi: https://doi.org/10.1093/jn/133.4.1020.
- Roy, D. K., T. W. O'Neill, J. D. Finn, M. Lunt, A. J. Silman, D. Felsenberg, G. Armbrecht, D. Banzer, L. I. Benevolenskaya, A. Bhalla, et al. 2003. Determinants of incident vertebral fracture in men and women: Results from the European Prospective Osteoporosis Study (EPOS). Osteoporosis International 14 (1):19–26. doi: https://doi.org/10.1007/s00198-002-1317-8.
- Rulu, P., M. Dhall, R. Tyagi, K. S. Devi, N. Feroz, S. Kapoor, M. G. Tungdim, and S. Thakur. 2019. Factors influencing bone mineral density among adults of Delhi: A gender differential. Journal of Health Management 21 (2):199–209. doi: https://doi.org/10.1177/0972063419835096.
- Sahni, S., K. M. Mangano, D. P. Kiel, K. L. Tucker, and M. T. Hannan. 2017. Dairy intake is protective against bone loss in older vitamin D supplement users: The Framingham Study. Journal of Nutrition 147:646–52.
- Sahni, S., K. M. Mangano, K. L. Tucker, D. P. Kiel, V. A. Casey, and M. T. Hannan. 2014. Protective association of milk intake on the risk of hip fracture: Results from the Framingham Original Cohort. Journal of Bone and Mineral Research 29 (8):1756–62. doi: https://doi.org/10.1002/jbmr.2219.
- Sahni, S., K. L. Tucker, D. P. Kiel, L. Quach, V. A. Casey, and M. T. Hannan. 2013. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: The Framingham Offspring Study. Archives of Osteoporosis 8:119. doi: https://doi.org/10.1007/s11657-013-0119-2.
- Sato, Y., M. Iki, Y. Fujita, J. Tamaki, K. Kouda, A. Yura, J. S. Moon, R. Winzenrieth, H. Iwaki, R. Ishizuka, et al. 2015. Greater milk intake is associated with lower bone turnover, higher bone density, and higher bone microarchitecture index in a population of elderly Japanese men with relatively low dietary calcium intake: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Osteoporosis International 26:1585–94.
- Seeman, E. 2013. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 68 (10):1218–25. doi: https://doi.org/10.1093/gerona/glt071.
- Shams-White, M. M., M. Chung, M. Du, Z. Fu, K. L. Insogna, M. C. Karlsen, M. S. LeBoff, S. A. Shapses, J. Sackey, T. C. Wallace, et al. 2017. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. The American Journal of Clinical Nutrition 105 (6):1528–43. doi: https://doi.org/10.3945/ajcn.116.145110.
- Shi, Y., Y. Zhan, Y. Chen, and Y. Jiang. 2020. Effects of dairy products on bone mineral density in healthy postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Archives of Osteoporosis 15 (1):48. doi: https://doi.org/10.1007/s11657-020-0694-y.
- Siris, E. S., R. Adler, J. Bilezikian, M. Bolognese, B. Dawson-Hughes, M. J. Favus, S. T. Harris, S. M. Jan de Beur, S. Khosla, N. E. Lane, et al. 2014. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporosis International 25 (5):1439–43. doi: https://doi.org/10.1007/s00198-014-2655-z.
- Sowers, M. F. R., S. L. Crawford, B. Sternfeld, D. Morganstein, E. B. Gold, G. A. Greendale, D. A. Evans, R. Neer, K. A. Matthews, and S. Sherman. 2000. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In Menopause: Biology and pathobiology, eds. Rogerio A. Lobo, Robert Marcus and Jennifer Kelsey. 175–88. San Diego, CA: Academic Press.
- Specker, B. M., A. H. Beck, Kalkwarf, and M. Ho. 1997. Randomized trial of varying mineral intake on total body bone mineral accretion during the first year of life. Pediatrics 99 (6):E12. doi: https://doi.org/10.1542/peds.99.6.e12.
- Storm, D. E., R. E. S. Porter, K. Musgrave, D. Vereault, C. Patton, C. Kessenich, S. Mohan, T. Chen, M. F. Holick, and C. J. Rosen. 1998. Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: A randomized placebo controlled trial. The Journal of Clinical Endocrinology & Metabolism 83 (11):3817–25. doi: https://doi.org/10.1210/jcem.83.11.5289.
- Szulc, P., K. Naylor, N. R. Hoyle, R. Eastell, and E. T. Leary. 2017. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporosis International 28 (9):2541–56. doi: https://doi.org/10.1007/s00198-017-4082-4.
- Tavani, A., E. Negri, and L. C. Vecchia. 1995. Calcium, dairy products, and the risk of hip fracture in women in northern Italy. Epidemiology (Cambridge, Mass.) 6 (5):554–7. doi: https://doi.org/10.1097/00001648-199509000-00017.
- Teegarden, D., R. M. Lyle, G. P. McCabe, L. D. McCabe, W. R. Proulx, K. Michon, A. P. Knight, C. C. Johnston, and C. M. Weaver. 1998. Dietary calcium, protein, and phosphorus are related to bone mineral density and content in young women. The American Journal of Clinical Nutrition 68 (3):749–54. doi: https://doi.org/10.1093/ajcn/68.3.749.
- Thorning, T. K., H. C. Bertram, J.-P. Bonjour, L. de Groot, E. Feeney, R. Ipsen, J. M. Lecerf, A. Mackie, M. C. McKinley, M.-C. Michalski, et al. 2017. Whole dairy matrix or single nutrients in assessment of health effects: Current evidence and knowledge gaps. The American Journal of Clinical Nutrition 105 (5):1033–45. doi: https://doi.org/10.3945/ajcn.116.151548.
- Ting, G. P., S. Y. Tan, S. P. Chan, C. Karuthan, Y. Zaitun, A. R. Suriah, and W. S. Chee. 2007. A follow-up study on the effects of a milk supplement on bone mineral density of postmenopausal Chinese women in Malaysia. Journal of Nutrition, Health and Aging 11:69–73.
- Torres-Costoso, A., P. Lopez-Munoz, A. Ferri-Morales, E. Bravo-Morales, V. Martinez-Vizcaino, and M. Garrido-Miguel. 2019. Body mass index, lean mass, and body fat percentage as mediators of the relationship between milk consumption and bone health in young adults. Nutrients 11 (10):2500. doi: https://doi.org/10.3390/nu11102500.
- Tsai, A. J. 2019. Disparities in osteoporosis by race/ethnicity, education, work status, immigrant status, and economic status in the United States. European Journal of Internal Medicine 64:85–9. doi: https://doi.org/10.1016/j.ejim.2019.04.011.
- Tu, M. Y., H. L. Chen, Y. T. Tung, C. C. Kao, F. C. Hu, and C. M. Chen. 2015. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS One 10 (12):e0144231. doi: https://doi.org/10.1371/journal.pone.0144231.
- van den Heuvel, E., and J. Steijns. 2018. Dairy products and bone health: How strong is the scientific evidence? Nutrition Research Reviews 31 (2):164–78. doi: https://doi.org/10.1017/S095442241800001X.
- van den Hooven, E. H., D. H. Heppe, J. C. Kiefte-de Jong, C. Medina-Gomez, H. A. Moll, A. Hofman, V. W. Jaddoe, F. Rivadeneira, and O. H. Franco. 2015. Infant dietary patterns and bone mass in childhood: The Generation R Study. Osteoporosis International 26 (5):1595–604. doi: https://doi.org/10.1007/s00198-015-3033-1.
- Vasikaran, S., R. Eastell, O. Bruyere, A. J. Foldes, P. Garnero, A. Griesmacher, M. McClung, H. A. Morris, S. Silverman, T. Trenti, et al. 2011. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporosis International 22 (2):391–420. doi: https://doi.org/10.1007/s00198-010-1501-1.
- Vogel, K. A., B. R. Martin, L. D. McCabe, M. Peacock, S. J. Warden, G. P. McCabe, and C. M. Weaver. 2017. The effect of dairy intake on bone mass and body composition in early pubertal girls and boys: A randomized controlled trial. The American Journal of Clinical Nutrition 105 (5):1214–29. doi: https://doi.org/10.3945/ajcn.116.140418.
- Volek, J. S., A. L. Gomez, T. P. Scheett, M. J. Sharman, D. N. French, M. R. Rubin, N. A. Ratamess, M. M. McGuigan, and W. J. Kraemer. 2003. Increasing fluid milk favorably affects bone mineral density responses to resistance training in adolescent boys. Journal of the American Dietetic Association 103 (10):1353–6. doi: https://doi.org/10.1016/S0002-8223(03)01073-3.
- Wadolowska, L., K. Sobas, J. W. Szczepanska, M. A. Slowinska, M. Czlapka-Matyasik, and E. Niedzwiedzka. 2013. Dairy products, dietary calcium and bone health: Possibility of prevention of osteoporosis in women: The Polish experience. Nutrients 5 (7):2684–707. doi: https://doi.org/10.3390/nu5072684.
- Wallace, T. C. 2019. Optimizing dietary protein for lifelong bone health: A paradox unraveled. Nutrition Today 54 (3):107–15. doi: https://doi.org/10.1097/NT.0000000000000340.
- Wallace, T. C., D. C. Bauer, R. F. Gagel, S. L. Greenspan, J. M. Lappe, M. S. LeBoff, R. R. Recker, K. G. Saag, and A. J. Singer. 2016. The National Osteoporosis Foundation's methods and processes for developing position statements. Archives of Osteoporosis 11:22. doi: https://doi.org/10.1007/s11657-016-0276-1.
- Wallace, T. C., and C. L. Frankenfeld. 2017. Dietary protein intake above the current RDA and bone health: A systematic review and meta-analysis. Journal of the American College of Nutrition 36 (6):481–96. doi: https://doi.org/10.1080/07315724.2017.1322924.
- Wallace, T. C., S. Jun, P. Zou, G. P. McCabe, C. Bruce, J. A. Cauley, C. M. Weaver, and R. L. Bailey. 2020. Dairy intake is not associated with improvements in bone mineral density or risk of fractures across the menopause transition: Data from the Study of Women's Health Across the Nation. Menopause 27:879–86. doi: https://doi.org/10.1097/GME.0000000000001555.
- Weaver, C. M. 2017. Nutrition and bone health. Oral Diseases 23 (4):412–5. doi: https://doi.org/10.1111/odi.12515.
- Weaver, C. M., C. M. Gordon, K. F. Janz, H. J. Kalkwarf, J. M. Lappe, R. Lewis, M. O'Karma, T. C. Wallace, and B. S. Zemel. 2016. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporosis International 27 (4):1281–386. doi: https://doi.org/10.1007/s00198-015-3440-3.
- Weaver, C. M., E. Janle, B. Martin, S. Browne, H. Guiden, P. Lachcik, and W. H. Lee. 2009. Dairy versus calcium carbonate in promoting peak bone mass and bone maintenance during subsequent calcium deficiency. Journal of Bone and Mineral Research 24 (8):1411–9. doi: https://doi.org/10.1359/jbmr.090303.
- Woolf, S. H. 2006. Weighing the evidence to formulate dietary guidelines. Journal of the American College of Nutrition 25 (3 Suppl):277S–84S. doi: https://doi.org/10.1080/07315724.2006.10719578.
- Wren, T. A., X. Liu, P. Pitukcheewanont, and V. Gilsanz. 2005. Bone acquisition in healthy children and adolescents: Comparisons of dual-energy x-ray absorptiometry and computed tomography measures. The Journal of Clinical Endocrinology and Metabolism 90 (4):1925–8. doi: https://doi.org/10.1210/jc.2004-1351.
- Wright, N. C., A. C. Looker, K. G. Saag, J. R. Curtis, E. S. Delzell, S. Randall, and B. Dawson-Hughes. 2014. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of Bone and Mineral Research 29 (11):2520–6. doi: https://doi.org/10.1002/jbmr.2269.
- Wright, N. C., K. G. Saag, B. Dawson-Hughes, S. Khosla, and E. S. Siris. 2017. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporosis International 28 (4):1225–32. doi: https://doi.org/10.1007/s00198-016-3865-3.
- Zhu, K., H. Greenfield, X. Du, Q. Zhang, G. Ma, X. Hu, C. T. Cowell, and D. R. Fraser. 2008. Effects of two years' milk supplementation on size-corrected bone mineral density of Chinese girls. Asia Pacific Journal of Clinical Nutrition 17 (Suppl 1):147–50.
- Zhu, K., Q. Zhang, L. H. Foo, A. Trube, G. Ma, X. Hu, X. Du, C. T. Cowell, D. R. Fraser, and H. Greenfield. 2006. Growth, bone mass, and vitamin D status of Chinese adolescent girls 3 y after withdrawal of milk supplementation. The American Journal of Clinical Nutrition 83 (3):714–21. doi: https://doi.org/10.1093/ajcn.83.3.714.
- Zhu, Y., S. Liu, W. Chen, B. Liu, H. Lv, X. Zhang, and Y. Zhang. 2018. Epidemiology of low-energy fracture in Chinese postmenopausal women: Changing trend of incidence since menopause and associated risk factors, a national population-based survey. Menopause 26 (3):286–92. doi: https://doi.org/10.1097/GME.0000000000001211.
