Abstract
Fish and algae are the major sources of n-3 polyunsaturated fatty acids (n-3 PUFAs). Globally, there is a rapid increase in demand for n-3 PUFA-rich oils. Conventional oil production processes use high temperature and chemicals, compromising the oil quality and the environment. Hence, alternative green technologies have been investigated for producing oils from aquatic sources. While most of the studies have focused on the oil extraction and enrichment of n-3 PUFAs, less effort has been directed toward green refining of oils from fish and algae. Enzymatic processing and ultrasound-assisted extraction with environment-friendly solvents are the most promising green technologies for extracting fish oil, whereas pressurized extractions are suitable for extracting microalgae oil. Lipase-catalysed ethanolysis of fish and algae oil is a promising green technology for enriching n-3 PUFAs. Green refining technologies such as phospholipase- and membrane-assisted degumming deserve investigation for application in fish and algal oils. In the current review, we critically examined the currently existing research on technologies applied at each of the steps involved in the production of oils rich in n-3 PUFAs from fish and algae species. Special attention was placed on assessment of green technologies in comparison with conventional processing methods.
Introduction
Fish oils are an important source of long-chain polyunsaturated fatty acids (PUFAs), among which eicosapentaenoic acid (EPA; 20:5, n-3) and docosahexaenoic acid (DHA; 22:6, n-3) are of special relevance due to their importance as structural components in synaptic membranes in the brain and the retina (Dyall and Michael-Titus Citation2008) and their role in supporting the health of the heart and the cardiovascular system (Swanson, Block, and Mousa Citation2012). DHA occurs in large amounts in the gray brain matter, and therefore an adequate intake of this n-3 PUFA is especially important during pregnancy and breastfeeding in the neurogenesis and synaptogenesis of the developing child. A sufficient amount of DHA in the diet has shown benefits in the cognitive and visual functions in newborns, and increased school performance in children. Furthermore, DHA and DHA-derived neuroprotectin D-1 are linked to neuroprotective effects against neurodegenerative diseases and brain aging (Echeverría et al. Citation2017). EPA and DHA also reduce lipogenesis and downregulate inflammatory pathways, having shown potential in the management of nonalcoholic fatty liver disease (Valenzuela et al. Citation2020). One of the specific ways of PUFAs targeting these metabolic pathways are through binding to peroxisome proliferator-activated receptors, which triggers the transcription of genes involved in fatty acid (FA) β-oxidation and adipogenesis (Echeverría et al. Citation2016).
Although the health benefits of n-3 PUFAs are generally known, the intake from the diet is low, especially in the West. Pregnant and lactating women are also not getting enough of these FAs, (Barrera et al. Citation2018). The recommended daily intake of PUFAs is continuously being revised and updated by governments and health organizations (FAO/WHO, American Dietetic Association or American Heart Association). The current recommendations for total n-3 PUFAs range from 1.4 to 25 gꞏd−1, and for EPA + DHA from 140 to 600 mgꞏd−1. The amount can be fulfilled with a minimum of two servings of fish per week, of which one is from an oily fish, such as salmon, tuna or sardine (Molendi-Coste, Legry, and Leclercq Citation2011). Hence, to fulfill nutritional requirements n-3 PUFAs are of increasing demand as food ingredients and dietary supplements, as well as pharmaceutical products.
The FA composition of fishes varies among the species and is affected by factors such as the environment and feed. Lower contents of lipids have been reported in fishes from tropical climate compared to fishes from the Arctic region. Marine fish species have a higher content of n-3 PUFAs due to their feed on plankton, while freshwater fish has a higher content of monounsaturated FAs (MUFAs) reflecting the FA composition of the vegetation and plant materials as the major feed in fresh water (Sahena et al. Citation2009).
According to FAO (Citation2018), 170.9 million tons of fish products were produced in 2016, of which a major part was produced in developing countries (84% of total production). Fish industry generates a high amount of by-products, of which heads, viscera, skin, and scales are the main components (Olsen, Toppe, and Karunasagar Citation2014). In some cases, the yield of side streams may be as high as 70% of the whole fish. Fish meal and fish oil are currently the two main products produced from the valorization of the by-products fish processing. The production of fishmeal and fish oil have been stimulated by the significant increase in the price since the beginning of the 21st century rising from 800 to 1600 USD per ton for fishmeal and from 800 to 2400 USD per ton for fish oil by 2017 (FAO Citation2018). Hence, the production of fish oil from low value fish mass and side streams not suitable for direct consumption presents a unique solution to provide valuable n-3 PUFAs for human consumption. In addition, the production of fish oil from fish side-streams fulfills the principles of circular economy stating that the wastes or by-products of one industry become the raw materials for another one (European Commission Citation2015).
In addition to fish, various algae species have recently gained popularity as a source of several bioactive compounds for human consumption due to their high growth rates and high biomass production. The content of bioactive lipids in microalgae can reach up to 85% of the dry weight, being especially rich in PUFAs. They can also be grown in bioreactors under controlled conditions to maximize their performance (Gallego et al. Citation2018). Moreover, research has shown potential of using microalgae (e.g., Nannochloropsis sp.) cultivation to recover nutrients released as wastes from industrial processing, presenting a sustainable way of producing biomass rich in EPA and DHA (Polishchuk et al. Citation2015). A recent study showed a lipid extraction yield of 42 wt% from Isochrysis biomass using pressurized liquid extraction (PLE), also known as subcritical fluid extraction, with 90% aqueous ethanol (He et al. Citation2019). Recently, there has also been an increasing interest in macroalgae species as a source of nutrients and bioactive components for food and feed. Indeed, some species present high contents of PUFAs (Rodrigues et al. Citation2015); however, due to the generally low content of oil, currently oil extraction is not part of the common processing pipelines of macroalgae. Instead they are mainly devoted to direct use as food (mostly as dried algae) as well as to production of hydrocolloids and food supplements.
Currently, several conventional techniques are applied at industrial-scale to convert aquatic sources into high-value oil. Commonly, fish oil production involves cooking at high temperature, pressing and centrifugation to separate raw oil from water and solid materials, followed by several steps of refining. Typically, refining includes degumming to eliminate the phospholipidic (PL) fraction, deacidification by neutralization with NaOH followed by washing with water to eliminate the free FAs (FFAs), bleaching with an appropriate ratio of adsorbent/oil to remove the colorants and pollutants, and deodorization with steam distillation under vacuum conditions. Furthermore, to increase the content of n-3 PUFAs, fish oils are subjected to enrichment process yielding end products with the content of DHA and EPA together up to 85% of total FAs, either as ethyl esters (EEs) or as triacylglycerols (TAGs), the latter being established to provide a higher bioavailability for the n-3 PUFAs (Olsen, Toppe, and Karunasagar Citation2014; Neubronner et al. Citation2011). Moreover, acylglycerols with n-3 PUFAs bound to the sn-2 position have been proven to have higher oxidation stability compared to those with n-3 PUFAs bound to the sn-1,3 positions (Wijesundera et al. Citation2008). Additionally, the oxidation stability of the obtained n-3 PUFA-enriched oils is a matter of interest for researchers due to the high reactivity of the multiple double bounds, resulting into lipid oxidation products that cause unwanted off-flavors and reduce the value of the lipid-containing products. Further, free radicals formed during oxidation may participate in the development of atherosclerosis. Currently, the main approaches to enhance the oxidation stability and improve the shelf life of fish oil products include addition of antioxidants, encapsulation and modified atmosphere packaging (Arab-Tehrany et al. Citation2012).
The traditional oil production methods cause degradation of the labile PUFAs due to the use of harsh conditions such as high temperatures (Fournier et al. Citation2006). In addition, chemicals including toxic solvents are used, leaving harmful residues in the final product and causing environmental pollutions. With the aim to increase the efficiency of the whole process and to improve the quality of the final products, as well as to minimize the environmental impact (solvents and energy), it is essential to develop greener strategies for producing food and natural products (Chemat, Vian, and Cravotto Citation2012). The production of oil rich in n-3 PUFAs for human consumption is not an exception; there have been an increasing number of researches published on novel green technologies applied at different processing steps of fish oil production. A number of green extraction methods, including supercritical fluid extraction (SFE), PLE, enzyme-assisted processing, and fermentation, have been shown to improve the oil quality and stability by retaining the natural antioxidants and reducing oxidation (Gallego et al. Citation2018; Ozogul et al. Citation2018; Yang et al. Citation2011). Following the structure presented in Scheme 1, this review aims to critically investigate the current research on technologies applied at each of the main processing steps of the extraction and refining of oils rich in n-3 PUFAs from aquatic resources including fish and microalgae. Special attention is paid to the assessment of green technologies in comparison with conventional processing methods in terms of the yield, composition and quality of the oil as well as the potential impact on the environment. The review also aims to identify the gaps in the current research and knowledge, which deserves further investigation on the development of green technologies.
Extraction of crude oils
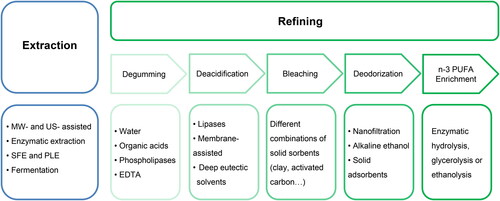
The first step in oil production is the extraction of crude oil from raw materials. Currently, available techniques can be classified into “conventional” and “non-conventional” techniques. Scheme 2 shows the methods and technologies investigated in oil for each processing step together with a brief description of them.
Scheme 2. Methods and technologies investigated for each processing step in oil from aquatic sources. (C) Conventional method of the step. MW, microwave; US, ultrasound; LAB, lactic acid bacteria; PLs, phospholipids; FFAs, free fatty acids; MASE membrane-assisted solvent extraction; SPD, short-paths distillation; PUFAs, polyunsaturated fatty acids; POPs, persistent organic pollutants; MUFAs, monounsaturated fatty acids.
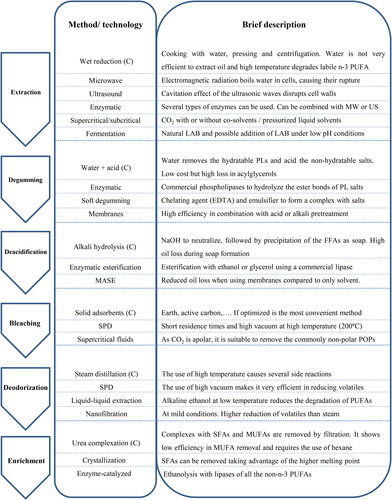
Conventional techniques refer to the ones traditionally used and globally accepted for fish oil extraction, which have been applied for many years in industrial extraction of fish oil, such as wet reduction (also known as rendering). Wet reduction starts with cooking the fish in water for a short time (ca. 30 min) at a temperature around 90 °C followed by pressing and centrifuging, resulting in a 3-phase system (from the bottom to top: solid matter, water and oil), from which the top layer (oil) is separated by decantation. Although the use of water is cheap, safe, and easy to operate in industrial systems, wet reduction is not highly efficient to extract oil. Moreover, the high temperature employed in the process leads to degradation of labile n-3 PUFAs. For this reason, in recent years non-conventional green techniques have emerged. Among the green alternatives with the highest potential for industrial application and thus, investigated here in more details, are physical pretreatment with microwave (MW) or ultrasounds (USs), enzymatic extraction, SFE and fermentation. In the following sections, we focus on the comparison between conventional oil extraction methods to green technologies in terms of yield and quality of the resulting oil. Typically, the quality markers for crude oil are FA composition and physicochemical parameters, including peroxide value (PV), p-anisidine value (AV), iodine value (IV) and acid value. These parameters are taken into account when giving an overview of each methodology as summarized in .
Table 1. A summary of different methods for oil extraction from different aquatic sources.
MW and US-assisted extractions
MW-assisted extraction (MAE) is based on the capacity of a system to absorb the electromagnetic radiation (requires solvents with high dipole moment) and to transform it into thermal energy, resulting in a temperature rise. Due to this temperature increase, the water in cells evaporates producing massive cell wall disruption leading to an increase in the porosity, which facilitates the mass transfer to the solvent. US-assisted extraction (UAE), on the other hand, is based on the cavitation effect of the ultrasonic waves that facilitate the extraction and mass transport by disrupting cell walls. In UAE any solvent can be used (Chemat, Zill-e-Huma, and Khan Citation2011). Both techniques, especially the UAE, have been broadly applied for the extraction of a wide variety of compounds in food and natural products and scaling up is already ongoing (Chemat et al. Citation2017). Nevertheless, UAE has not been applied at industrial scale in the extraction of fish oil. Previous studies have compared the performance of MAE and UAE against extraction with Soxhlet and Bligh and Dyer methods at laboratory scale. UAE of fish oil was carried out from six fish species using ethanol as a solvent at room temperature for 90 min while MAE was applied at 600 W and 70 °C with an extraction time of 10 min. UAE resulted in higher oil yield and higher proportions of n-3 PUFAs in the oil compared to MAE. On the other hand, when MAE was employed after optimization by central composite rotatable experimental design, no significant differences in the yield and composition of the extract were found compared to the solvent-based Folch extraction, whereas the PV of the oil was 8-fold lower for the MAE method (Costa and Bragagnolo Citation2017).
Pretreatments rupturing the cell walls of microalgae are essential for extraction of oil from microalgae. A thorough review has been previously published on the efficiency of different technologies as pretreatment for improving the extraction lipid yield from different microalgae species (Lee et al. Citation2017) While many technologies are still in the early stage of investigation, high-pressure homogenization, enzymatic treatment, ultrasonication and MW treatment have proven to be effective techniques to break the cell walls increasing the oil extraction yield from microalgae (Lee et al. Citation2017; Xue et al. Citation2018). In the extraction of Crypthecodinium cohnii, a microalgae, UAE reduced the extraction time by 10-fold and increased the lipid yield by 5-fold compared to Soxhlet extraction, whereas MAE offered an increase in yield of less than 3-fold (Cravotto et al. Citation2008). The microalgae cells are difficult to disrupt due to the polymer network within the cell walls. Conventional extraction involves use of toxic solvents, such as chloroform, hexane and methanol, and they can be time consuming and harmful to the environment. MAE and UAE have shown to significantly improve the oil yield, reduce the extraction time and the environmental impact (Kapoore et al. Citation2018). MAE and UAE using environment-friendly solvents represent potential green solutions for extracting n-3 rich oils from fish and algae.
A new generation of non-conventional solvents called natural deep eutectic solvents (NADES) has emerged in recent years. The most commonly studied NADES are based on choline chloride (ChCl), carboxylic acids, and other hydrogen-bond donors, e.g., urea, citric acid, succinic acid and glycerol. NADES have similar characteristics to ionic liquids but they are cheaper to produce (lower cost of raw materials), less toxic, and often biodegradable. For extracting oil from Phaeodactylum tricornutum (a diatom), the combination of MW heating as a pretreatment and extraction with deep eutectic solvents resulted in total FA yields and profiles (EPA, other PUFAs, other FA and other lipids) comparable to the traditional Bligh and Dyer solvent extraction. The best results were achieved by using a NADES formed with ChCl and oxalic acid combined with MW, followed by extraction with dimethyl carbonate as an environmentally friendly solvent (Tommasi et al. Citation2017).
Based on the existing reports, UAE is a more advantageous technique than MAE, both in terms of quality of the crude oil obtained and the investment costs in instrumentation. In addition, UAE has already proven potential for scaling-up in the extraction of natural products using reactors up to 1000 L coupled with pump systems in order to fill the ultrasonic bath, to stir the mixture, and to empty the system at the end of the procedure (Chemat et al. Citation2017).
Enzymatic extraction
Enzyme-aided extraction of fish oil is carried out under mild temperature conditions by employing proteases together with an appropriate water/fish ratio to maximize the extraction efficiency. Senphan and Benjakul (Citation2015) compared organic solvent extraction to wet reduction, commercial Alcalase, and powdered crude protease extract (CPE) from hepatopancrease of Pacific white shrimp yielding very similar results in terms of oil yield and FA composition. Hence, the efficiency of the enzymatic extraction was comparable to the solvent extraction resulting in an oil recovery of 95% of the total lipids. Enzyme-assisted extraction using Alcalase has also been reported to give better results for oil extraction from tuna by-products when compared to solvent and wet reduction methods. Thecrude had a higher percentage of PUFAs as well as lower degree of oxidation compared to the conventional methods of organic solvent extraction and wet reduction (de Oliveira et al. Citation2017). Wet reduction using low temperature (15 °C instead of 95 °C) did not differ from the enzymatic treatment in terms of n-3 PUFA content, PV and AV; whereas conventional wet process with high-temperature cooking resulted in higher extent of oxidation of PUFAs.
Heating, MW, and US have also been studied as pretreatment steps before the enzymatic extraction on Labeo rohita head, concluding that MW- and US-pretreatments improved the oil yield from 55.9 to 69.8 and 68.1%, respectively (Bruno, Kudre, and Bhaskar Citation2019). Moreover, US enhanced the content of PUFAs, whereas MW reduced the stability of the oil by significantly increasing PV and AV. The same authors studied the structure of the fish mass after MW, US, and heat pretreatment by scanning electron microscopy, observing that the MW- and US-pretreated samples had more destroyed cells compared to the control samples. This caused an increase in the porosity of the matrix, thus facilitating the hydrolysis of proteins and consequent release of the intracellular lipids. Moreover, they observed that US was more efficient than MW due to the thermal unfolding and further aggregation of proteins resulting from the heat released in the MW-assisted procedure, which disturbs the protein hydrolysis.
For microalgae, a combination of different types of enzymes (cellulase, proteinase, lysozyme, pectinase), acting on different components of cell walls, have shown to be most efficient pretreatments improving the extraction yield of oils (Xue et al. Citation2018). US in combination with enzymatic treatment has also been applied in extraction of oil from microalgae. For Chlorella vulgaris, five different enzymes were investigated resulting in lipid recoveries ranging from 10% using Neutrase and Protease to more than 35% using Snailase and Trypsin. The highest lipid yield was achieved with a combined sonication-enzyme treatment at pH 4, which recovered 50% of the lipids present in the microalgae. Due to the diversity of algal types, selection of the most proper enzyme together with optimization of the processing conditions are of special importance in order to maximize the yield with optimal quality (Liang, Zhang, and Cong Citation2012). In a similar work, Zuorro et al. (Citation2016) optimized the lipid recovery from Nannochloropsis resulting in oil yields around 35% using Feedlyve ALPHAGAL and Feedlyve GMA, as endo-galactanase and endo-mannase, respectively.
Enzymatic treatment is a promising valorization method for a simultaneous extraction of oil, protein hydrolysate and bioactives from fish, fish by-products and algae (Araujo et al. Citation2020). The utilization of fish discards is currently of special interest due to the circular economy approach and the landing obligation by The European Common Fisheries Policy, which requires proper management of fish bycatch. However, the industrial processing may not be as efficient as at lab scale in the separation of the valuable oils. In their study, Vázquez et al. (Citation2020) showed that the oil recovery in the industrial pilot plant was significantly lower than at lab scale due to the oils forming an emulsion with the hydrolyzed proteins. The tricanter which was used instead of a centrifuge did not separate the oil phase well due to lower speed. The optimization of the process is essential to obtain the optimal yield and quality of the n-3 PUFA rich oils from specific types of raw materials, such as different fish and algae species and by-products. In a study reported by (Carvajal et al. Citation2015), an enzyme-assisted processing resulted in increased lipid oxidation compared to thermal treatment, demonstrating the importance of optimization of the process. The additions of antioxidants prior and during the processing, or running the process under anoxic conditions are important measures to decrease the level of oxidation. Despite higher costs, enzyme treatments are increasingly used in industrial scale to separate fish oil and muscle. The challenge remains in the structural changes and protein quality after the enzymatic treatment, which needs to be resolved by future innovations in order to obtain value added products from both oil and protein fractions.
Supercritical and subcritical fluid extractions
In a supercritical state, with the pressure and temperature above the critical point, solvent becomes supercritical fluid achieving density similar to a liquid and viscosity similar to a gas. These properties give advantages such as better transporting properties, efficient diffusion and faster extraction. Moreover, the properties of the fluid can be modified to optimize its performance, and the solvents used are compounds such as CO2 which are generally recognized as safe (Herrero, Cifuentes, and Ibañez Citation2006). SFE is used in industrial scale to produce high value berry seed oils and natural plant extracts with high contents of PUFAs and natural antioxidants (Tarvainen et al. Citation2015; Yang et al. Citation2011). SFE using CO2 alone or with co-solvents has been studied by several research groups to extract oil from fish products. SFE-CO2 extraction is especially suitable for the extraction of nonpolar lipids such as TAGs, but the method is restricted to dry biomass. Thus, the raw material, such as fish, is commonly freeze-dried prior the extraction, which consumes time and energy. PLE is a promising alternative for the SFE, and it can also be used for wet biomass, such as fish and algae. The process utilizes the subcritical state of the solvent, which remains liquid even at temperatures above the boiling point. The method can be easily modified by choosing different solvents according to their polarities; the commonly used solvents include ethanol, acetone and ethyl acetate (Derwenskus et al. Citation2019).
Sahena et al. (Citation2010) compared the performance of a Soxhlet system with various processes of SFE-CO2, such as continuous, ethanol as co-solvent, soaking, and pressure swing techniques. The Soxhlet and SFE-CO2 extractions resulted in a similar oil yield and composition of PUFAs with the only exception of the continuous SFE-CO2, which resulted in the lowest values. In addition, the amount of CO2 used for each extraction was the lowest for the pressure swing method, reducing it to half compared to the continuous system. The reason for the low consumption was long holding times, where the sample was incubated with CO2, and thus, no CO2 was consumed in these steps. In another study, the Soxhlet and SFE methods were applied for oil extraction from different parts of trout (head, spines and viscera). There were little variation in yield and FA compositions of the oil between the extraction methods but a higher variability between the different parts of the fish as raw material; the oil yield (calculated as oil/fish dry weight) was around 40% for the head and spine, and 70%–79% for the viscera (Fiori et al. Citation2012). Similarly, FA profiles found in fillets and viscera of carp fish extracted by the SFE and Soxhlet methods were more dependent on the fish material rather than the extraction technique, reinforcing that SFE is highly efficient in extracting fish oil from fish side streams (Kuvendziev et al. Citation2018). Furthermore, Hao et al. (Citation2015) compared SFE, wet reduction and enzymatic extraction of sturgeon oil in terms of oil composition and storage stability. Higher extraction yields (considering 100% for organic solvent extraction) were reported for protease and SFE (84% and 97%, respectively) compared to wet reduction (53%). Moreover, the quality of the crude oil with obtained SFE was better with higher PUFA content and lower PV and AV.
Mendes, Reis, and Palavra (Citation2006) compared SFE-CO2 and SFE-CO2 using ethanol as a co-solvent (CO2-ethanol) to Bligh and Dyer solvent extraction. The CO2-ethanol resulted in a 40% lipid recovery of Arthrospira maxima biomass while CO2 alone recovered only 32% of the lipids. Additionally, SFE-CO2 with ethanol increased the recovery of γ-linolenic acid compared to pure CO2, although both were significantly lower than that achieved with the Bligh and Dyer extraction. In another study, CO2 extraction recovered nearly 50% of the total lipids from Crypthecodinium cohnii biomass, of which 72% (wt/wt) of the total FAs were DHA (Couto et al. Citation2010). In contrast to other natural lipid sources, the extraction from microalgae requires higher pressures during the SFE process. The possible reason is that under low pressures, microalgae bind to the extracted lipids whereas at higher pressure the adsorption rate decreases (Sovová, Nobre, and Palavra Citation2016).
A recent study compared subcritical dimethyl ether extraction (SDEE) to SFE, enzymatic treatment and wet reduction in the extraction of oil from tuna liver. Dimethyl ether is a generally recognized as safe (GRAS) solvent for extraction purposes in the processing of foods. Furthermore, the low boiling point (-24.8 °C) allows it to evaporate freely from any food matrix, leaving practically no residues in the final product. The SDEE resulted in a very similar yield of PUFAs and oil to those obtained by SFE, however, SDEE consumes less energy and time because freeze-drying of raw materials is not required, and the pressures employed are lower (Fang et al. Citation2019).
PLE, on the other hand, has shown over 75% wt/wt lipid yields from microalgae, including Chlorella vulgaris and Phaeodactylum tricornutum using a pressure of 103.4 bar and a temperature of 150 °C. However, the adjustment of solvents is required for different species; medium-polar solvents such as ethyl acetate extracted lipids more efficiently from C. vulgaris biomass, whereas polar solvents like ethanol functioned better for P. tricornutum. These optimal solvents resulted in total FA yields of 85.9% for P. tricornutum (ethanol) and 76.5% wt/wt for C. vulgaris (ethyl acetate). C. vulgaris is rich in TAGs, and thus very polar or nonpolar solvents were inefficient for the extraction, the former due to poor solubility of TAGs, and the latter not being able to penetrate the water layer surrounding the cells (Derwenskus et al. Citation2019). A recent study from He et al. (Citation2019) reported that PLE with 90% aqueous ethanol resulted in a lipid extraction efficiency of 41.5% (wt/wt) from Isochrysis sp. biomass. Furthermore, over 90% (wt/wt) of the extract were FAs, proving that PLE is an efficient method to extract lipids from Isochrysis biomass. In addition to fish and microalgae, PLE was also used to extract lipids from a brown macroalgae Laminaria ochroleuca. Among the solvents tested, ethanol:water (1:1) was the most efficient in extracting oil from L. ochroleuca, while ethanol, ethyl acetate and hexane were less effective. The lipid recovery was 52% using ethanol:water and extraction temperature of 160 °C, while a lower temperature of 80 °C resulted in extraction yield of 37.5% (wt/wt). However, both ethanol and ethyl acetate enriched more unsaturated FAs in the oil compared to the two other solvents. The ratio of n-6 to n-3 FAs was also assessed concluding that ethanol and ethyl acetate resulted in the lowest values (Otero, López-Martínez, and García-Risco Citation2019).
Fermentation
Fermentation occurs naturally under anaerobic conditions due to microbial activity. Fish silage technology utilizes either acid treatment (organic acid, such as formic acid) or fermentation with lactic acid bacteria to break down the fish material. Low pH produces suitable conditions for the enzymes to break down fish proteins into smaller soluble units, and the acid helps to speed up their activity while preventing bacterial spoilage (Olsen, Toppe, and Karunasagar Citation2014). Rai et al. (Citation2010) studied the naturally present lactic acid bacteria (LAB) and added cultures (Ent. faecium HAB01 and Ped. acidilactici K7) for fish ensilaging followed by Bligh and Dyer extraction method to assess the FA composition of the resulting crude oil. However, no advantages were reported over the natural fermentation in terms of oil yield and FA composition. Another study compared the natural silage process to acid silage employing formic acid (3%, vol/wt), and fermentation with several LAB strains (Lb. plantarum, Pd. acidilactici, Ent. gallinarum, Lb. brevis, and Streptococcus spp.) supplemented with an antioxidant, a fungicide (potassium sorbate) and 15% molasses to aid in the fermentation process. The results showed lower PV and AV values in all of the added LAB fermented samples while the percentage of PUFAs did not significantly differ from the natural and acid fermentation processes (Özyurt et al. Citation2019). Additionally, some LAB produce natural antioxidants which prevent the oxidation of FAs, and the fermentation makes the proteins more digestible than those from acid silage (Vidotti, Carneiro, and Viegas Citation2002). In general, fish silage requires low investment, energy and labor costs which makes it a promising technique for industrial-scale fish oil processing.
Among the extraction techniques discussed above, enzymatic extraction and especially fermentation require lower investment and energy costs making them more attractive in an industrial context. On the other hand, MAE and SFE require costly and specific instrumentation but produce high-quality oil. Thus, MAE and SFE should be regarded as technique, which needs more development in the extraction plant to make them more economically feasible. Given their proven potential to extract high quality oil, they are probably more suitable for the extraction of microalgae in biorefineries as they can be produced in high yield and processed in situ.
A number of studies have investigated various green technologies for extraction of oil from different types of fish materials and algae. Overall, the characterization of the obtained crude has largely based on the extraction yield, oxidation parameters (PV, AV) and FA composition. The lipid class composition of the resulting crudes has seldomly been investigated. Distribution of lipid classes in the crude is important information guiding the optimization of oil extraction and purification processes since PLs have to be removed in the degumming step and FFAs in the deacidification step.
Technologies for refining crude oil
The crude oil obtained by any of the above detailed techniques does not yet fulfill the requirements for human consumption and further technological processing due to the presence of co-extractives, such as phospholipids, FFAs and pigments. Therefore, several steps are necessary to upgrade the quality of the crude oil. These steps commonly include: (1) degumming to eliminate the PLs, (2) deacidification to decrease the acidity of the oil by eliminating the FFAs, (3) bleaching to remove pigments and other contaminants, and (4) deodorization to remove volatile compounds. Changes in the FA composition and physicochemical parameters through the refining process have been studied for oils extracted from different fish species including tuna and anchovies (de Oliveira et al. Citation2016; Song, Dai, et al. Citation2018), nile tilapia and hybrid sorubim (Menegazzo, Petenuci, and Fonseca Citation2014), sardine (Soldo et al. Citation2019) as well as fish by-products such as carp viscera (Crexi et al. Citation2010). However, all the available reports rely on the conventional refining process, which includes degumming by using 1% of phosphoric acid, deacidification by neutralization with 1 M NaOH followed by centrifugation, washing with hot water and drying, and bleaching with a combination of adsorbents and deodorization by steam distillation under vacuum. In the following sections, we focus on reviewing research related to each of the above-mentioned refining steps, in which green alternatives are applied and compared with conventional methodologies.
Degumming
Degumming is the first step in the refining of the extracted crude oil. shows the different techniques applied to remove the PLs in fish oil and additional strategies that have been successfully assayed in vegetable oils. It is important to reduce the content of PLs in the oil because they tend to hydrolyze more easily than TAGs, generating FFAs and other reaction products that compromise the stability of the oil. Water degumming is the first stage of the refining process, which eliminates the hydratable fraction of PLs, i.e., PLs with polar moieties like hydroxyl or amino groups. In contrast, acid degumming is used for non-hydratable PLs, which consists primarily of phosphatidic acid having two free hydroxyl groups with high affinity for calcium and magnesium to form neutral, stable, and non-hydratable salts. Hence, the aim is to remove the phosphatidic acid yielding non-dissociated phosphatidic acid and the corresponding salts.
Table 2. Degumming processes applied for phospholipids (PLs) removal.
Although the acid degumming leads to significant loss in acylglycerols, it is still the methodology globally used and accepted due to the low cost of the chemicals used, profitable disposability of gums, and acceptable quality of oil. However, the effectiveness of the acid treatment is also dependent on the acid employed. Chakraborty and Joseph (Citation2015) determined that the use of phosphoric acid resulted in a better quality of the oil, i.e., lower PV and AV, but lower oil yield of 86% compared to use of acetic, oxalic, or citric acids, which resulted in yield ranging from 90 to 93%. Acid and water degumming treatments have also been employed on mixed algal oil from Chlorella species (Paisan, Chetpattananondh, and Chongkhong Citation2017). The most abundant PLs in mixed algal oil are non-hydratables, thus, acid degumming with phosphoric acid resulted in a greater PL reduction compared to the water degumming. The best removal up to 83% of total PLs was achieved with the following degumming conditions: 90 °C, 60 min and phosphoric acid 0.42 wt%. In contrast, the water degumming resulted in only removal of 19% of the PLs.
Although there are no reports comparing different degumming techniques for fish oil refining, studies comparing green alternatives have already emerged for the degumming of vegetable oils. Hence, they should be considered and assessed as possible alternatives to current conventional process applied in the fish oil degumming. Enzymatic degumming with commercial phospholipases has been examined for refining oils from corn, rapeseed and soybean. Phospholipases are a class of hydrolytic enzymes with the capacity to hydrolyze the ester bonds of PLs. Most commonly used phospholipases are phospholipase A1 (PLA1) and phospholipase A2 (PLA2) which catalyze the hydrolysis of FAs exclusively from the sn-1 and sn-2 positions, respectively. Instead, phospholipase C (PLC) is a phosphodiesterase catalyzing the cleavage of phosphatidylinositol, whereas phospholipase D (PLD) hydrolyzes the sn-3 phosphodiester bond of mostly phosphatidylcholine (PC) to generate a choline molecule and glycerophosphatidic acid (Richmond and Smith Citation2011). Turetkan et al. (Citation2018) compared acid hydrolysis with citric acid to two different enzyme-based approaches, namely Enzymax process, an industrial procedure by Lurgi and Röhm GmbH, as well as a direct enzymatic process with a commercial phospholipase PLA1 in degumming of crude corn oil. Both enzymatic methods resulted in a 10-fold enhanced performance in reducing the phosphorous content of the oil to levels of 5.7 and 6.2 ppm for the Enzymax and direct enzymatic degumming, respectively, in comparison with 54.9 ppm for the acid treatment. In another study, immobilized PLA1 showed a reduction in the phosphorus content of crude soybean oil from 63 ppm using the non-immobilized phospholipase to 10 and 7 ppm obtained with the bio-imprinted PLA1 (bi-PLA1) and the immobilized bio-imprinted PLA1 (im-bi-PLA1), respectively (Li et al. Citation2016). Phospholipases have also been applied after chemical degumming to enhance the efficiency of the process. In soybean oil, PLA1 treatment after chemical degumming enhanced the reduction of phosphorus content from 32 to 0.7 ppm in the oil (Sampaio et al. Citation2015). Similarly, the use of PLC reduced the phosphorus content of corn oil to less than half compared to water degumming (Sampaio et al. Citation2019). Ultrasonication prior to enzymatic hydrolysis of PLs by PLA was found to have a positive effect on the degumming of crude soybean oil, reducing the phosphorous content by additional 4% compared to the reduction of 94% achieved by the enzymatic hydrolysis alone. Moreover, the physicochemical parameters of oil were also improved by US-treatment resulting in PV, AV, and acid value of 0.3 mEq·kg−1, 0.7 mg·g−1, and 0.7%, respectively, in comparison to 0.3 mEq·kg−1, 0.8 mg·g−1, and 1.3% in the oil obtained with the enzymatic treatment alone (More and Gogate Citation2018). The optimization of the US parameters, such as temperature and power intensity together with pH and water addition for an optimal performance of the enzymes may further enhance the efficiency of the degumming process, not only in terms of the quality of the oil obtained, but also in terms of the reaction time.
Soft degumming by using a chelating agent, disodium ethylenediaminetetraacetate (EDTA) together with an emulsifying agent (sodium dodecyl sulfate, SDS) has been reported to improve the efficiency for reduction of phosphorous content of crude rapeseed oil. Optimization of the process by experimental design resulted in an additional reduction of phosphorous from 268 to 74 ppm (Szydłowska-Czerniak and Łaszewska Citation2017). Moreover, the technique is cheaper than enzymatic treatment and easy to scale-up. The main drawback of this process was the high stability of the emulsion formed during the process making the separation difficult. To solve this problem Crystallization and Degumming SPRL patented a procedure without SDS, obtaining similar elimination of PLs as in the original method but the separation of the oil phase from water became much easier, thus reducing oil loss. Moreover, this process was scaled up to process 500 t oil per day for soybean and rapeseed oil (Deffense Citation2009).
Lastly, membrane technology has also been widely investigated as a new approach to eliminate the PL fraction of oils using different membranes resulting in phosphorus reduction by 86%–93% in soybean oil (Subramanian et al. Citation1999). In combination with pretreatments of the oil with acid or alkali PL were eliminated almost completely from soya, sunflower and rapeseed crude oils (Hafidi, Pioch, and Ajana Citation2005). Membrane degumming is a green technology with high potential due to low consumption of energy and chemicals and low environmental impact (Chunduri et al. Citation2006).
Deacidification
Deacidification removes the FFAs which are present at concentrations of 5%–20% in the oils after the degumming step (Vaisali et al. Citation2015). shows the main alternatives for the reduction of the FFA content in fish oil. The conventional procedure for the deacidification includes the addition of NaOH to neutralize the acids, followed by precipitation of the FFAs as soap, which are then removed by centrifugation or washing. However, significant oil loss occurs during the soap formation due to alkali hydrolysis of TAGs, also known as saponification. Nontraditional neutralizing agents, such as Na2CO3 and NaHCO3, have also been tested in vegetable oils although NaOH was proven the most efficient yielding 0.4% FFAs versus 0.7 and 0.8% in oils processed with Na2CO3 and NaHCO3, respectively (De and Patel Citation2010).
Table 3. Deacidification processes for free fatty acids (FFAs) removal.
The main alternative to the conventional deacidification method used in fish oil processing is enzymatic esterification with ethanol or glycerol using a commercial lipase. Wang et al. (Citation2012) reported a reduction of p-AV and acid value from 44.4 and 10.2 to 26.4 mg·g−1 and 0.4 mg KOH·g−1 as well as an increase in PUFA content from 32 to 82% compared to the original crude oil. Thus, in addition to removing the FFAs, enrichment of PUFAs was achieved. The same type of enzymes were applied in several studies to enrich the PUFAs in the oil at the end of the refining process by performing a selective enzymatic glycerolysis followed by molecular distillation (Solaesa et al. Citation2016) or ethanolysis to achieve MAGs with a high content of PUFAs (He, Li, Kodali, Balle, et al. Citation2017). Although in most of the commercial formulations EPA and DHA are in the form of EEs, acylglycerols are the preferred forms because they confer better bioavailability and stability of EPA and DHA (Wang et al. Citation2012).
Charanyaa, Belur, and Regupathi (Citation2017) studied solvent extraction with methanol and membrane assisted pre-extraction combined with extraction with methanol for the deacidification of degummed fish oil. Initially, four short-chain alcohols methanol, ethanol, propanol and butanol were studied as solvents. The results showed that methanol was the most efficient by reducing the FFA content from 5.6 to 2.3%. However, the authors reported a high oil loss of 30% in the solvent extraction, whereas the membrane assisted solvent extraction (MASE) resulted in oil loss of 7%. In addition, the residual contents of methanol in the oil after extraction were 1% in the solvent extracted oil and 0.5% in the MASE oil. The high oil loss in the solvent extraction was probably due to the formation of stable oil-methanol micelles during the extraction. The membrane deacidification, on the other hand, occurs at 3 bars, resulting in a better separation of oil from the oil-methanol micelles. Further, the nonpolar PTEE membrane repels these micelles, leading to a reduced amount of methanol in the permeate. For green processing, the process should be optimized using ethanol to replace methanol. The use of NADES to replace traditional solvents in fish oil deacidification has not been reported yet; however, several betaine monohydrate-based NADES were studied to reduce the acidity of palm oil, obtaining a 49% acid reduction of palmitic acid while keeping the content of antioxidants stable (Zahrina et al. Citation2018).
Based on the research reported on various green alternatives for conventional deacidification, esterification of the FFAs with lipases shows the highest capabilities to replace the use of alkali neutralization; however, to our best knowledge, only FA composition and oxidation status of the oils has been studied to evaluate the impact on oil quality, but no research has been carried out to assess the oil loss. Hence, this alternative technology should be further investigated in order to fully assess its true potential.
Bleaching
Bleaching aims to remove several types of impurities, such as pigments, lipid oxidation products, and remains of PLs and soaps to further improve the quality and stability of the oil. Moreover, high contents of persistent organic pollutants (POPs) can be found in some fish oil products due to bioaccumulation in the fat tissue of fish in polluted marine areas and enrichment of these compounds during the oil extraction process (Rawn et al. Citation2009). Previously, bleaching studies () on fish oil have pointed mainly toward two different goals. The first one aims to improve the quality of fish oil by improving its physicochemical properties such as removal of colorants and lipid oxidation products. The second is focused on the reduction of POPs such as flame retardants, dioxins and polychlorinated biphenyls (PCBs) which are highly persistent and fat-soluble environmental pollutants that bioaccumulate in the food chain. Considerable levels of POPs have been detected in some of the most important fish species in the Baltic sea such as sprat (Sprattus sprattus) and herring (Clupea harengus) (Antelo et al. Citation2012) and in fish oil produced from Sprat caught in the North Sea (Oterhals et al. Citation2007). Effective measures are necessary to remove POPs from oil in order to make these oils safe for human consumption or use as ingredient of feed.
Table 4. Bleaching processes of fish oil using various combinations of bleaching agents.
Currently, the main methodology used for the bleaching of fish oil is the treatment with a solid adsorbent. Optimization of the process in terms of temperature, amount of adsorbent and contact time has resulted in effective reduction of oxidation products as shown in the reduction of PV and AV (García-Moreno et al. Citation2013). Monte et al. (Citation2015) carried out a similar study, where the processes using bleaching earth and activated carbon (AC) were optimized, yielding oil with a reduced content of lipid oxidation products and an improved color. Another study compared different solid adsorbents in sardine oil with remarkable results obtained when using a combination of AC and Fuller’s earth (FE). The refined oil had an enhanced PUFA content (from 25.6 to 26.6%) in addition to a reduced content of lipid oxidation products and an improved color (Chakraborty and Joseph Citation2015). This combination of adsorbents was especially efficient in reducing the color-related compounds, whereas the values of oxidation parameters were not noticeably better than the ones obtained with the other adsorbents.
Oterhals et al. (Citation2007) studied the effects of alkali bleaching (AB) and the combination of alkali and active charcoal (AC) for the elimination of polychlorinated dibenzo-p-dioxins, dibenzofurans (PCDD/F) and PCBs from fish oil. The procedure combining AB and AC bleaching proved to be very effective in the reduction of PCDDs and PCDFs, showing a reduction rate of 99%. However, it was less effective in reducing the PCB content, probably due to the planar molecular conformation of the AC, which inevitably limits its applicability. The non-ortho PCB was reduced by 87% and the mono-ortho PCB by 21%. In addition, the authors did not observe any negative effect on the oil quality after bleaching in terms of oxidation. Similarly, Ortiz et al. (Citation2011) studied 11 silicon- and 9 carbon-based adsorbents. The carbon-based adsorbents lead to reductions of 99, 70, and 27% of PCDDs, hexachlorobenzene (HCB), and PCBs, respectively, while treatment with the silicon-based adsorbents did not result in significant eliminations of POPs.
US-assisted bleaching (UAB) has been studied in canola oil (Icyer and Durak Citation2018), but not yet in fish oil. The study compared a conventional bleaching method with a process using acid-activated bleaching earth assisted with ultrasonication. The conventional bleaching relies on mixing bleaching earth with the oil while stirring and heating under partial vacuum (70 mmHg). No remarkable differences were reported between the two methods in the final composition of the oil, except a higher reduction of yellow color in the UAB treated oil. However, the US-treatment accelerated the process resulting in 50% reduction in the contact time. Also the same bleaching efficiency was obtained with a 25% reduction of processing temperature when compared to the non-UAB method using the same bleaching earth.
Short-path distillation (SPD) is a technique that employs short residence times and high vacuum levels (Antelo et al. Citation2012). The SPD technique was compared to alkali bleaching to refine sprat oil. The method resulted in lower PV, AV and total oxidation value (TOTOX); but the reduction of the initial POP content (76%) and the loss of vitamins (20%) were similar to what observed for alkali bleaching (Oterhals and Berntssen Citation2010). On the other hand, Oliveira and Miller (Citation2014) assessed SPD in regard to the oil quality since SPD bleaching requires higher temperatures (around 200 °C) compared to the adsorption bleaching using temperatures below 90 °C. High temperature might lead to the degradation of the beneficial PUFAs. The authors concluded that SPD was useful in the reduction of the oxidation products and FFAs, while keeping the FA profile unaltered. However, SPD involves high operating costs which have prevented the broad use of this technology by the industry to this date.
SFE with CO2 has also been studied as an alternative bleaching technique. As CO2 is a nontoxic and non-polar compound, it takes advantage of the rather non-polar molecular structures of many POPs to remove them efficiently from the oil. Kawashima et al. (Citation2009) examined the reduction of several classes of POPs in detail, obtaining promising results especially in the reduction of PCDF and PCB contents by 84% and 93%, respectively. However, an additional step with AC adsorption was required to enhance the efficiency of the whole procedure to reduce the PCDD content by 80% compared to 35% achieved with SFE-CO2 alone.
In conclusion, the conventional bleaching processes using solid adsorbents achieve good results and are cost-efficient for industrial applications. Additionally, the used bleaching material can be re-used as a bioorganic fertilizer (Loh et al. Citation2013). Hence, at this stage special attention should be paid to the optimization of the process in terms of oil/adsorbent ratio and bleaching temperature to maximize the removal of unwanted compounds while maintaining a high content of PUFAs.
Deodorization
Deodorization is the last step in the fish oil refining which aims to remove undesirable odorous compounds. Processes studied in fish oil deodorization are shown in . Currently, steam distillation is the most commonly used process in which undesirable odorous components, mostly aldehydes and ketones (lipid oxidation products), and residual FFAs are removed (Vaisali et al. Citation2015). However, the use of high temperatures (180–270 °C) can induce several chemical reactions, such as oxidation, isomerization and polymerization of lipid molecules. Additionally, high temperature and the simultaneous presence of a chloride ion source together with glycerol derivatives may favor the formation of 2-monochloropropane-1,3-diol (2-MCPD), 3-monochloropropane-1,2-diol (3-MCPD), and glycidyl esters (Zelinková et al. Citation2006). These compounds have been reported as possible human carcinogens (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Citation2013), hence, deodorization should be conducted at temperatures not higher than 180 °C (Fournier et al. Citation2006). Alternative deodorization strategies based on milder conditions have been a subject of research in the field of fish oil refining.
Table 5. Deodorization processes employed in fish oil refining.
With the aim to reduce the extent of side reactions and degradation of PUFAs, Chung and Lee (Citation2009) studied six different types of zeolites, which resulted in a reduction of 20%–60% in the content of volatile compounds. In a recent study, Song, Zhang, et al. (Citation2018) compared the performance of conventional steam distillation with several alternative green processes, including short-path distillation (SPD), treatment with green tea polyphenol (GTP), which is a natural polymer consisting of six catechins with significant antioxidant and chelating properties (Heim, Tagliaferro, and Bobilya Citation2002), adsorbent-based processes such as activated clay, zeolites, and diatomite, as well as liquid-liquid (L-L) extraction with alkaline ethanol. Among the methods evaluated, the most efficient reduction of the volatiles was achieved with SPD, in which high vacuum was applied (around 10−4 mm Hg). SPD reduced the content of volatile compounds in the oil to 12% of the initial level, whereas L-L extraction resulted in a volatile content of 73%. The other deodorization methods resulted in volatile contents of 79%–98% of the level before the deodorization. Furthermore, the PV and FA compositions were not largely altered by any of the aforementioned processes. The PV ranged between 1.9 and 3.7 mEq O2·kg−1 and the percentage of PUFAs were 38% in the oils after the GTP treatment and L-L extraction in comparison with 34% in the crude oil. The a-based L-L extraction used low temperatures, which might have reduced peroxidation and degradation of the lipids, as well as the geometrical isomerization of the naturally present cis-double bond in the lipid molecules. Other advantages of the L-L extraction were high repeatability, low cost, and a simplified procedure.
Nanofiltration with membranes under mild operating conditions has also been studied as a low-temperature alternative to steam distillation in order to minimize the thermal degradation of lipids. Fang et al. (Citation2018) used membranes with a cutoff molecular weight of 360 Da, 20 bar, and room temperature in an experimental set-up to compare the performance of nanofiltration with that of steam distillation in refining of tuna and squid oil. Compared to the steam distillation, a 5- to 6-fold reduction in the volatile content was achieved by using nanofiltration. Additionally, the PUFA content increased from 2 to 4%, improving the FA profile of the oil.
To substitute high-temperature steam deodorization, the alternatives with a higher potential seem to be techniques operating at room temperature like alkaline liquid-liquid extraction with ethanol and nanofiltration, the former being less costly, while the latter giving better results in reducing the volatiles but requiring high investment costs for specific installations.
EPA and DHA enrichment
Although the refined oil is suitable for human consumption in terms of purity, color and odor, the fish oil TAGs still contain high proportions of SFAs and MUFAs. The total content of n-3 PUFAs is typically around 20%–30% of the total FAs in the refined oil, largely depending on the initial fish material (especially fish species), the extraction method, and the refining process applied. In order to achieve the health benefits, the daily intake of n-3 PUFAs, especially EPA and DHA should reach a sufficient level. Some clinical studies are using dosages of as much as 4 g·d of EPA, DHA or a combination of both, as recently reviewed (Watanabe and Tatsuno Citation2020). For this reason, enrichment of n-3 PUFAs, especially EPA and DHA, have also been an active field of research in order to obtain PUFA-concentrated products with higher added value.
Most commonly used methodologies for enriching PUFAs in fish oil are urea complexation, low temperature crystallization, and enzymatic purification. Other techniques, such as liquid and supercritical fluid chromatography and SFE, for concentrating n-3 PUFAs have been described as summarized in a review (Bonilla-Mendez and Hoyos-Concha Citation2018). In addition, studies using pressurized liquids have also emerged. Urea complexation relies on the ability of SFAs and MUFAs to form complexes with urea, while PUFAs remain in the non-urea-complexed fraction, which can easily be separated by filtration. This technique has been reported to be highly efficient in the removal of SFAs, but with limited efficiency in the reduction of MUFAs (C16:1, C18:1, and C20:1) (Zheng, Dai, and Shen Citation2018). Urea complexation has also shown promising results in enriching PUFAs of algal oil, where the percentage of DHA was increased from 47% in the original algal oil to 97% after enrichment. However, urea complexation does not fulfill the criteria of green extraction methods due to the use of hexane during the process (Senanayake and Shahidi Citation2000).
Molecular distillation, on the other hand, has been applied in a two-step process to increase the PUFA content of oil after the urea complexion, resulting in 2-fold increases in the contents of EPA and DHA (Magallanes et al. Citation2019). However, the use of urea for this purpose should be avoided due to the formation of ethyl carbamate, a human and animal carcinogen, during the process (Canas and Yurawecz Citation1999). In addition to lipid extraction, pressurized liquids can also be used for the fractionation of different lipids, such as n-3 acylglycerols and glycolipids. In a recent study, Castejón and Señoráns (Citation2019) extracted and enriched oil from algae species Nannochloropsis gaditana reaching an EPA concentration of up to 53% using PLE with hexane. In comparison, the PLE with ethanol resulted in EPA concentration of 36% of the total FAs. PLE with hexane resulted in an enriched fraction of TAGs whereas ethanol resulted in a fraction more concentrated with glycolipids and MAGs. The technique is based on different polarities of different lipid classes, which enable the fractionation of the oil into MAGs, diacylglycerols (DAGs), TAGs, FFAs and glycolipids. Although hexane resulted in concentration of EPA, further development is justified to replace hexane with a green solvent such as CO2.
SFAs can be crystallized from a solution in an organic solvent by low-temperature crystallization utilizing the high melting point of SFAs. The crystals can then be removed by filtration, and after that, the solvent is evaporated yielding FFA fractions with higher content of n-3 PUFAs (Morales-Medina et al. Citation2016). However, crystallization requires organic solvents like hexane to solubilize the mixture, thus it should be avoided when possible. Urea complexation and low-temperature crystallization have some limitations because they are methods only efficient for FFAs or FAEEs. Therefore, additional steps are required for the transformation of lipids to FFAs or FEEs before the process as well as re-esterification or trans-esterification with glycerol afterwards to yield the n-3 PUFA enriched TAGs. It has been demonstrated by several studies that acylglycerols provide better absorption and higher bioavailability of PUFAs than EEs, making acylglycerols the preferred form of PUFAs as ingredients of food and dietary supplements (Neubronner et al. Citation2011; Olsen, Toppe, and Karunasagar Citation2014). Hence, most of the state-of-the art research is being pointed toward the use of enzyme-catalyzed hydrolysis of TAGs to obtain PUFA-containing MAGs using refined fish oil as the substrate to reduce the number of steps of re-esterification and trans-esterification during the enrichment process.
Enzymatic production of PUFA-enriched MAGs has been carried out by three main approaches: hydrolysis, glycerolysis, and ethanolysis. Hydrolysis takes advantage of the ability of lipases to catalyze the fast removal of SFAs and MUFAs from the glycerol backbone in the presence of water. Unfortunately, this is a reversible reaction that yields low conversion rates and requires a second round of hydrolysis followed by a SPD (Kahveci and Xu Citation2011). Glycerolysis consists of a lipase, a hydrophobic oil phase and a hydrophilic glycerol phase. In addition, to overcome the poor miscibility between the oil and glycerol due to the difference in polarities, tertiary alcohols are needed. However, some of which are toxic and need to be removed at the end of the process, thus increasing the length and cost of the whole process. As alternatives, the use of food grade surfactants, such as lecithin, has been studied (Feltes et al. Citation2013). To further increase the concentration of PUFA-enriched MAGs, molecular distillation has been applied (Solaesa et al. Citation2016). Although hydrolysis and glycerolysis can be utilized for the enrichment of PUFAs in fish oil, their performance is not excellent. Hence, enzyme-catalyzed ethanolysis has recently attracted the greatest research attention as it enables an irreversible reaction by using ethanol both as a reactant and solvent (He et al. Citation2016; Rodrigues et al. Citation2015). Moreover, ethanol is a nontoxic, cheap, and environmentally friendly solvent.
The optimization of the process by selecting the most efficient lipase has been a matter of interest, and in this regard, lipase produced by fungi such as Candida antarctica and Thermomyces lanuginosus have been widely applied and genetically engineered to increase their performance. In addition, both immobilized and liquid forms of lipases have been studied. Immobilized lipases provide higher stability and reusability, but they are more expensive. Recently, a liquid lipase Candida Antarctica Lipase (CAL-A) and a lipase NS-40116 from a genetically modified C. antarctica demonstrated a superior, almost ideal, performance for the enrichment of n-3 PUFAs via ethanolysis using fish oil or microalgae oil as starting materials (He et al. Citation2016). According to the research, the most suitable lipase to obtain PUFA-concentrated glycerides via ethanolysis should fulfill the following criteria: (1) high FA selectivity to hydrolyze saturated and monounsaturated FAs without releasing PUFAs from the glycerol backbone; (2) non-regiospecificity in order to hydrolyze FAs in both sn-1/3 and sn-2 positions and (3) the ability to use ethanol in excess to favor the reaction. CAL-A is a near-ideal lipase with almost all required properties. He, Li, Kodali, Chen, et al. (Citation2017) elucidated the rationale behind the highly efficient concentration of n-3 PUFAs into MAGs; and they verified their conceptual hypothesis by revealing the catalytic mechanism through 13C-NMR analysis of starting materials and isolated products (Scheme 3).
Scheme 3. (a) The rationale to design the process protocol to obtain high purity n-3 enriched monoacylglycerols from low n-3 PUFA oil by using non-regiospecific, non-n-3 PUFA preferential lipase. (b) The practical results of representative reactions as shown by spectra of 13C-NMR analysis (Adapted from He, Li, Kodali, Chen, et al. (Citation2017)).
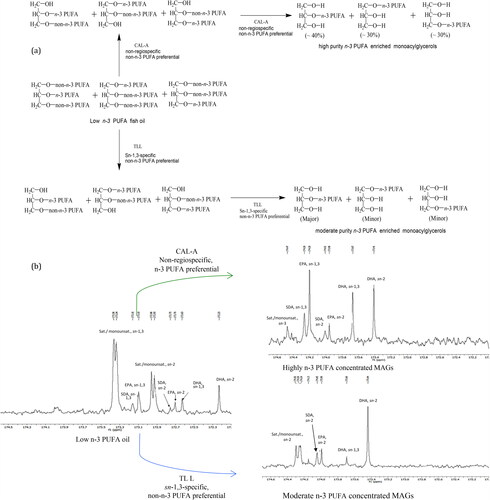
As previously mentioned, acylglycerols provide better bioavailability of PUFAs than EEs. However, n-3 PUFAs as PLs have shown a superior metabolic effects compared to TAGs in mice (Rossmeisl et al. 2012). A recent review (Ahmmed et al. Citation2020) stated that only the PL form of n-3 PUFAs can cross the blood-brain barrier, and EPA and DHA in PLs are also more resistant to oxidative degradation. Thus, the possibility of producing n-3 PUFA enriched PLs should be studied, although the processing can be challenging due to the emulsifying properties of PLs.
Conclusions and future prospects
The demand for high quality oils from fish and microalgae is rapidly growing globally to meet the recommended intake of n-3 PUFAs. Green technologies for production of oils rich in n-3 PUFAs from aquatic sources is an increasingly popular field of research. While most research has focused on green strategies for extracting crude oil from raw materials, less effort has been directed toward the oil-refining processes. Enzyme-aided process and UAE are most promising extraction technologies offering higher yield and improved quality of oils from fish and microalgae. Pressurized extractions using supercritical and subcritical fluid of CO2 and other solvents have proven effective for extracting lipids from microalgae. Although all these processes are easy to up-scale and most of them have been applied in industrial scale for extraction of oils from different sources, the processes need to be carefully optimized in order to achieve the optimal performance on specific type of raw materials of fish and algae. SFE requires high investment in high pressure plant, which limits the industrial use. More attention should be placed on composition of lipid classes (PLs, FFAs and acylglycerols) in the oils when assessing green technologies for extraction and refining oils due to the importance of such composition for the quality, stability, as well as nutritional and technological properties of the oil. Lipase-catalysed ethanolysis is a promising green technology for enrichment of n-3 PUFAs from marine sources. Active research is being conducted to improve cost-efficiency by screening for effective lipases with low positional specificity (sn-1, 2 or 3) and high preference for non-n-3 FAs in TAGs.
In contrast to oil extraction and n-3 PUFA enrichment, the green technologies for refining oils extracted from fish and algae have not been investigated extensively. Green processes such as phospholipase- and membrane-assisted degumming should be tested for refining marine oils rich in n-3 PUFAs. Finally, at the deodorization step, alkaline ethanol liquid-liquid extraction should be further investigated as a possible gentle alternative to steam distillation and short path distillation. Nanofiltration represents a promising technique for removal of volatile compounds.
Green processes for oil production should be part of integrated processing strategies promoting sustainable utilization of fish and algae bioresources, as demonstrated in a review published on oil production from microalgae (Xue et al. Citation2018). Refined fish oil and algal oils and n-PUFA concentrates consist of mostly acylglycerols, whereas other lipid classes such as PLs, carotenoids, and sterols present in the crude extracts are removed during the oil refining processes. More effort should be directed to recovering and valorizing these fractions. Currently a dominating fraction of fish oil is refined from crude oils produced as side streams of fish meal production. There is an increasing interest in valorizing proteins of the so-called industrial fishes into high quality food products and protein concentrates. Pretreatment of raw materials and oil extraction methods have significant impact on structure, technological properties, as well as sensory and nutritional qualities of the protein fractions of fish and algae, which should be taken into consideration to enhance circular green strategies for processing these valuable bioresources.
CRediT author statement
Alexis Marsol-Vall: investigation, writing—original draft preparation, review and editing, and visualization. Ella Aitta: investigation, writing—original draft preparation, review and editing. Zheng Guo: visualization, writing—reviewing and editing. Baoru Yang: conceptualization, supervision, writing—reviewing and editing, project administration, and funding acquisition.
Disclosure statement
There are no relevant financial or non-financial competing interests to report.
Additional information
Funding
References
- Ahmmed, M. K., F. Ahmmed, H. (S.) Tian, A. Carne, and A. E.-D. Bekhit. 2020. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Comprehensive Reviews in Food Science and Food Safety 19 (1):64–123. doi: 10.1111/1541-4337.12510.
- Antelo, L. T., C. Lopes, A. Franco-Uría, and A. A. Alonso. 2012. Fish discards management: Pollution levels and best available removal techniques. Marine Pollution Bulletin 64 (7):1277–90. doi: 10.1016/j.marpolbul.2012.04.005.
- Arab-Tehrany, E., M. Jacquot, C. Gaiani, M. Imran, S. Desobry, and M. Linder. 2012. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends in Food Science & Technology 25 (1):24–33. doi: 10.1016/j.tifs.2011.12.002.
- Araujo, J., P. Sica, C. Costa, and M. C. Márquez. 2020. Enzymatic hydrolysis of fish waste as an alternative to produce high value-added products. Waste and Biomass Valorization: 1–9. doi: 10.1007/s12649-020-01029-x.
- Barrera, C., R. Valenzuela, R. Chamorro, K. Bascuñán, J. Sandoval, N. Sabag, F. Valenzuela, M.-P. Valencia, C. Puigrredon, and A. Valenzuela. 2018. The impact of maternal diet during pregnancy and lactation on the fatty acid composition of erythrocytes and breast milk of Chilean women. Nutrients 10 (7):839. doi: 10.3390/nu10070839.
- Bonilla-Mendez, J. R., and J. L. Hoyos-Concha. 2018. Methods of extraction, refining and concentration of fish oil as a source of omega-3 fatty acids. Corpoica Ciencia y Tecnologia Agropecuaria 19 (3):645–68. doi: 10.21930/rcta.vol19_num2_art:684.
- Bruno, S. F., T. G. Kudre, and N. Bhaskar. 2019. Impact of pretreatment‐assisted enzymatic extraction on recovery, physicochemical and rheological properties of oil from Labeo rohita head. Journal of Food Process Engineering 42 (3):e12990. doi: 10.1111/jfpe.12990.
- Canas, B. J., and M. P. Yurawecz. 1999. Ethyl carbamate formation during urea complexation for fractionation of fatty acids. Journal of the American Oil Chemists' Society 76 (4):537. doi: 10.1007/s11746-999-0038-y.
- Carvajal, A., R. Slizyte, I. Storrø, and M. Aursand. 2015. Production of High Quality Fish Oil by Thermal Treatment and Enzymatic Protein Hydrolysis from Fresh Norwegian Spring Spawning Herring By-Products. Journal of Aquatic Food Product Technology 24 (8):807–23. doi:10.1080/10498850.2013.814740.
- Castejón, N., and F. J. Señoráns. 2019. Simultaneous extraction and fractionation of omega-3 acylglycerols and glycolipids from wet microalgal biomass of Nannochloropsis gaditana using pressurized liquids. Algal Research 37:74–82. doi: 10.1016/j.algal.2018.11.003.
- Chaijan, M., S. Benjakul, W. Visessanguan, and C. Faustman. 2006. Changes of lipids in sardine (Sardinella gibbosa) muscle during iced storage. Food Chemistry 99 (1):83–91. doi:10.1016/j.foodchem.2005.07.022.
- Chakraborty, K., and D. Joseph. 2015. Production and characterization of refined oils obtained from Indian oil sardine (Sardinella longiceps). Journal of Agricultural and Food Chemistry 63 (3):998–1009. doi: 10.1021/jf505127e.
- Charanyaa, S., P. D. Belur, and I. Regupathi. 2017. A new strategy to refine crude Indian Sardine oil. Journal of Oleo Science 66 (5):425–34. doi: 10.5650/jos.ess16164.
- Chemat, F., N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier, and M. Abert-Vian. 2017. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry 34:540–60. doi: 10.1016/j.ultsonch.2016.06.035.
- Chemat, F., M. Vian, and G. Cravotto. 2012. Green extraction of natural products: Concept and principles. International Journal of Molecular Sciences 13 (7):8615–27. doi: 10.3390/ijms13078615.
- Chemat, F., Zill-e-Huma, and M. K. Khan. 2011. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonics Sonochemistry 18 (4):813–35. doi: 10.1016/j.ultsonch.2010.11.023.
- Chunduri, V., M. Rao, V. Balasubrahmanyam, and D. Bhowmick. 2006. Membrane degumming of crude rice bran oil: Pilot plant study. European Journal of Lipid Science and Technology 108 (9):746–52. doi: 10.1002/ejlt.200600086.
- Chung, K.-H., and K.-Y. Lee. 2009. Removal of trimethylamine by adsorption over zeolite catalysts and deodorization of fish oil. Journal of Hazardous Materials 172 (2-3):922–7. doi: 10.1016/j.jhazmat.2009.07.081.
- Costa, D. d. S. V., and N. Bragagnolo. 2017. Development and validation of a novel microwave assisted extraction method for fish lipids. European Journal of Lipid Science and Technology 119 (3):1600108. doi: 10.1002/ejlt.201600108.
- Couto, R. M., P. C. Simões, A. Reis, T. L. D. Silva, V. H. Martins, and Y. Sánchez‐Vicente. 2010. Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Engineering in Life Sciences 10 (2):158–64. doi: 10.1002/elsc.200900074.
- Cravotto, G., L. Boffa, S. Mantegna, P. Perego, M. Avogadro, and P. Cintas. 2008. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrasonics Sonochemistry 15 (5):898–902. doi: 10.1016/j.ultsonch.2007.10.009.
- Crexi, V. T., M. L. Monte, L. A. d S. Soares, and L. A. A. Pinto. 2010. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chemistry 119 (3):945–50. doi: 10.1016/j.foodchem.2009.07.050.
- De, B. K., and J. D. Patel. 2010. Effect of different degumming processes and some nontraditional neutralizing agent on refining of RBO. Journal of Oleo Science 59 (3):121–5. doi: 10.5650/jos.59.121.
- de Oliveira, D. A. S. B., S. Licodiedoff, A. Furigo, J. L. Ninow, J. A. Bork, R. Podestá, J. M. Block, and N. Waszczynskyj. 2017. Enzymatic extraction of oil from yellowfin tuna (Thunnus albacares) by-products: A comparison with other extraction methods. International Journal of Food Science & Technology 52 (3):699–705. doi: 10.1111/ijfs.13324.
- de Oliveira, D. A. S. B., M. G. Minozzo, S. Licodiedoff, and N. Waszczynskyj. 2016. Physicochemical and sensory characterization of refined and deodorized tuna (Thunnus albacares) by-product oil obtained by enzymatic hydrolysis. Food Chemistry 207:187–94. doi: 10.1016/j.foodchem.2016.03.069.
- Deffense, E. 2009. From organic chemistry to fat and oil chemistry. Oléagineux, Corps Gras, Lipides 16 (1):14–24. doi: 10.1051/ocl.2009.0238.
- Derwenskus, F., F. Metz, A. Gille, U. Schmid-Staiger, K. Briviba, U. Schließmann, and T. Hirth. 2019. Pressurized extraction of unsaturated fatty acids and carotenoids from wet Chlorella vulgaris and Phaeodactylum tricornutum biomass using subcritical liquids. GCB Bioenergy 11 (1):335–44. doi: 10.1111/gcbb.12563.
- Dyall, S. C., and A. T. Michael-Titus. 2008. Neurological benefits of omega-3 fatty acids. Neuromolecular Medicine 10 (4):219–35. doi: 10.1007/s12017-008-8036-z.
- Echeverría, F., M. Ortiz, R. Valenzuela, and L. A. Videla. 2016. Long-chain polyunsaturated fatty acids regulation of PPARs, signaling: Relationship to tissue development and aging. Prostaglandins, Leukotrienes, and Essential Fatty Acids 114:28–34. doi: 10.1016/j.plefa.2016.10.001.
- Echeverría, F., R. Valenzuela, M. Catalina Hernandez-Rodas, and A. Valenzuela. 2017. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins, Leukotrienes, and Essential Fatty Acids 124:1–10. doi: 10.1016/j.plefa.2017.08.001.
- European Commission. 2015. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Closing the loop—An EU action plan for the Circular Economy. Status of Data 2:2015.
- Fang, Y., S. Gu, J. Zhang, S. Liu, Y. Ding, and J. Liu. 2018. Deodorisation of fish oil by nanofiltration membrane process: Focus on volatile flavour compounds and fatty acids composition. International Journal of Food Science & Technology 53 (3):692–9. doi: 10.1111/ijfs.13644.
- Fang, Y., S. Liu, W. Hu, J. Zhang, Y. Ding, and J. Liu. 2019. Extraction of oil from high-moisture tuna livers by subcritical dimethyl ether: A comparison with different extraction methods. European Journal of Lipid Science and Technology 121 (2):1800087. doi: 10.1002/ejlt.201800087.
- FAO. 2018. The state of world fisheries and aquaculture 2018: Meeting the sustainable development goals. Rome, Italy: FAO. http://www.fao.org/documents/card/en/c/I9540EN/.
- Feltes, M. M. C., D. de Oliveira, J. M. Block, and J. L. Ninow. 2013. The production, benefits, and applications of monoacylglycerols and diacylglycerols of nutritional interest. Food and Bioprocess Technology 6 (1):17–35. doi: 10.1007/s11947-012-0836-3.
- Fiori, L., M. Solana, P. Tosi, M. Manfrini, C. Strim, and G. Guella. 2012. Lipid profiles of oil from trout (Oncorhynchus mykiss) heads, spines and viscera: Trout by-products as a possible source of omega-3 lipids? Food Chemistry 134 (2):1088–95. doi: 10.1016/j.foodchem.2012.03.022.
- Fournier, V., F. Destaillats, P. Juanéda, F. Dionisi, P. Lambelet, J.-L. Sébédio, and O. Berdeaux. 2006. Thermal degradation of long-chain polyunsaturated fatty acids during deodorization of fish oil. European Journal of Lipid Science and Technology 108 (1):33–42. doi: 10.1002/ejlt.200500290.
- Gallego, R., L. Montero, A. Cifuentes, E. Ibáñez, and M. Herrero. 2018. Green extraction of bioactive compounds from microalgae. Journal of Analysis and Testing 2 (2):109–23. doi: 10.1007/s41664-018-0061-9.
- García-Moreno, P. J., A. Guadix, L. Gómez-Robledo, M. Melgosa, and E. M. Guadix. 2013. Optimization of bleaching conditions for sardine oil. Journal of Food Engineering 116 (2):606–12. doi: 10.1016/j.jfoodeng.2012.12.040.
- Głowacz-Różyńska, A., M. Tynek, E. Malinowska-Pańczyk, D. Martysiak-Żurowska, R. Pawłowicz, and I. Kołodziejska. 2016. Comparison of oil yield and quality obtained by different extraction procedures from salmon ( Salmo salar ) processing byproducts. European Journal of Lipid Science and Technology 118 (11):1759–67. doi:10.1002/ejlt.201500269.
- Hafidi, A., D. Pioch, and H. Ajana. 2005. Membrane-based simultaneous degumming and deacidification of vegetable oils. Innovative Food Science & Emerging Technologies 6 (2):203–12. doi: 10.1016/j.ifset.2004.12.001.
- Hao, S., Y. Wei, L. Li, X. Yang, J. Cen, H. Huang, W. Lin, and X. Yuan. 2015. The effects of different extraction methods on composition and storage stability of sturgeon oil. Food Chemistry 173:274–82. doi: 10.1016/j.foodchem.2014.09.154.
- He, Y., Z. Huang, C. Zhong, Z. Guo, and B. Chen. 2019. Pressurized liquid extraction with ethanol as a green and efficient technology to lipid extraction of Isochrysis biomass. Bioresource Technology 293:122049. doi: 10.1016/j.biortech.2019.122049.
- He, Y., J. Li, S. Kodali, T. Balle, B. Chen, and Z. Guo. 2017. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresource Technology 224:445–56. doi: 10.1016/j.biortech.2016.10.087.
- He, Y., J. Li, S. Kodali, B. Chen, and Z. Guo. 2016. The near-ideal catalytic property of Candida antarctica lipase A to highly concentrate n-3 polyunsaturated fatty acids in monoacylglycerols via one-step ethanolysis of triacylglycerols. Bioresource Technology 219:466–78. doi: 10.1016/j.biortech.2016.08.007.
- He, Y., J. Li, S. Kodali, B. Chen, and Z. Guo. 2017. Rationale behind the near-ideal catalysis of Candida antarctica lipase A (CAL-A) for highly concentrating ω-3 polyunsaturated fatty acids into monoacylglycerols. Food Chemistry 219:230–9. doi: 10.1016/j.foodchem.2016.09.149.
- Heim, K. E., A. R. Tagliaferro, and D. J. Bobilya. 2002. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. The Journal of Nutritional Biochemistry 13 (10):572–84. (02)00208-5 doi: 10.1016/S0955-2863.
- Herrero, M., A. Cifuentes, and E. Ibañez. 2006. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chemistry 98 (1):136–48. doi: 10.1016/j.foodchem.2005.05.058.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2013. Some chemicals present in industrial and consumer products, food and drinking-water. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 101. Lyon, France: International Agency for Research on Cancer.
- Icyer, N. C., and M. Z. Durak. 2018. Ultrasound-assisted bleaching of canola oil: Improve the bleaching process by central composite design. LWT - Food Science and Technology 97:640–7. doi: 10.1016/j.lwt.2018.07.030.
- Kahveci, D., and X. Xu. 2011. Repeated hydrolysis process is effective for enrichment of omega 3 polyunsaturated fatty acids in salmon oil by Candida rugosa lipase. Food Chemistry 129 (4):1552–8. doi: 10.1016/j.foodchem.2011.05.142.
- Kapoore, R. V., T. O. Butler, J. Pandhal, and S. Vaidyanathan. 2018. Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology 7 (1):18. doi: 10.3390/biology7010018.
- Kawashima, A., S. Watanabe, R. Iwakiri, and K. Honda. 2009. Removal of dioxins and dioxin-like PCBs from fish oil by countercurrent supercritical CO2 extraction and activated carbon treatment. Chemosphere 75 (6):788–94. doi: 10.1016/j.chemosphere.2008.12.057.
- Kuvendziev, S., K. Lisichkov, Z. Zeković, M. Marinkovski, and Z. H. Musliu. 2018. Supercritical fluid extraction of fish oil from common carp (Cyprinus carpio L.) tissues. The Journal of Supercritical Fluids 133:528–34. doi: 10.1016/j.supflu.2017.11.027.
- Lee, S. Y., J. M. Cho, Y. K. Chang, and Y.-K. Oh. 2017. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresource Technology 244 (Pt 2):1317–28. doi: 10.1016/j.biortech.2017.06.038.
- Li, Z., H. Liu, G. Zhao, P. Wang, L. Wang, H. Wu, X. Fang, X. Sun, X. Wu, and Z. Zheng. 2016. Enhancing the performance of a phospholipase A1 for oil degumming by bio-imprinting and immobilization. Journal of Molecular Catalysis B: Enzymatic 123:122–31. doi: 10.1016/j.molcatb.2015.11.018.
- Liang, K., Q. Zhang, and W. Cong. 2012. Enzyme-assisted aqueous extraction of lipid from microalgae. Journal of Agricultural and Food Chemistry 60 (47):11771–6. doi: 10.1021/jf302836v.
- Loh, S. K., S. James, M. Ngatiman, K. Y. Cheong, Y. M. Choo, and W. S. Lim. 2013. Enhancement of palm oil refinery waste–Spent bleaching earth (SBE) into bio organic fertilizer and their effects on crop biomass growth. Industrial Crops and Products 49:775–81. doi: 10.1016/j.indcrop.2013.06.016.
- Magallanes, L. M., L. V. Tarditto, N. R. Grosso, M. C. Pramparo, and M. F. Gayol. 2019. Highly concentrated omega-3 fatty acid ethyl esters by urea complexation and molecular distillation: Concentrated omega-3 FAEE by UC and MD. Journal of the Science of Food and Agriculture 99 (2):877–84. doi: 10.1002/jsfa.9258.
- Mendes, R. L., A. D. Reis, and A. F. Palavra. 2006. Supercritical CO2 extraction of γ-linolenic acid and other lipids from Arthrospira (Spirulina) maxima: Comparison with organic solvent extraction. Food Chemistry 99 (1):57–63. doi: 10.1016/j.foodchem.2005.07.019.
- Menegazzo, M. L., M. E. Petenuci, and G. G. Fonseca. 2014. Production and characterization of crude and refined oils obtained from the co-products of Nile tilapia and hybrid sorubim processing. Food Chemistry 157:100–4. doi: 10.1016/j.foodchem.2014.01.121.
- Molendi-Coste, O., V. Legry, and I. Leclercq. 2011. Why and how meet n-3 PUFA dietary recommendations? Gastroenterology Research and Practice 2011:364040. doi: 10.1155/2011/364040.
- Monte, M. L., M. L. Monte, R. S. Pohndorf, V. T. Crexi, and L. A. A. Pinto. 2015. Bleaching with blends of bleaching earth and activated carbon reduces color and oxidation products of carp oil: Carotenoids losses and oxidation in bleaching of carp oil. European Journal of Lipid Science and Technology 117 (6):829–36. doi: 10.1002/ejlt.201400223.
- Morales-Medina, R., G. De León, M. Munio, A. Guadix, and E. Guadix. 2016. Mass transfer modeling of sardine oil polyunsaturated fatty acid (PUFA) concentration by low temperature crystallization. Journal of Food Engineering 183:16–23. doi: 10.1016/j.jfoodeng.2016.03.009.
- More, N. S., and P. R. Gogate. 2018. Ultrasound assisted enzymatic degumming of crude soybean oil. Ultrasonics Sonochemistry 42:805–13. doi: 10.1016/j.ultsonch.2017.12.031.
- Neubronner, J., J. P. Schuchardt, G. Kressel, M. Merkel, C. von Schacky, and A. Hahn. 2011. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. European Journal of Clinical Nutrition 65 (2):247–54. doi: 10.1038/ejcn.2010.239.
- Oliveira, C. M. A., and R. M. Miller. 2014. Purification of Alaskan walleye pollock (Gadus chalcogrammus) and New Zealand Hoki (Macruronus novaezelandiae) liver oil using short path distillation. Nutrients 6 (5):2059–76. doi: 10.3390/nu6052059.
- Olsen, R. L., J. Toppe, and I. Karunasagar. 2014. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends in Food Science & Technology 36 (2):144–51. doi: 10.1016/j.tifs.2014.01.007.
- Ortiz, X., L. Carabellido, M. Martí, R. Martí, X. Tomás, and J. Díaz-Ferrero. 2011. Elimination of persistent organic pollutants from fish oil with solid adsorbents. Chemosphere 82 (9):1301–7. doi: 10.1016/j.chemosphere.2010.12.017.
- Oterhals, Å., and M. H. G. Berntssen. 2010. Effects of refining and removal of persistent organic pollutants by short-path distillation on nutritional quality and oxidative stability of fish oil. Journal of Agricultural and Food Chemistry 58 (23):12250–9. doi: 10.1021/jf102660v.
- Oterhals, Å., M. Solvang, R. Nortvedt, and M. H. G. Berntssen. 2007. Optimization of activated carbon-based decontamination of fish oil by response surface methodology. European Journal of Lipid Science and Technology 109 (7):691–705. doi: 10.1002/ejlt.200700083.
- Otero, P., M. I. López-Martínez, and M. R. García-Risco. 2019. Application of pressurized liquid extraction (PLE) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). Journal of Pharmaceutical and Biomedical Analysis 164:86–92. doi: 10.1016/j.jpba.2018.09.057.
- Ozogul, Y., Y. Ucar, F. Takadaş, M. Durmus, A. R. Köşker, and A. Polat. 2018. Comparision of green and conventional extraction methods on lipid yield and fatty acid profiles of fish species. European Journal of Lipid Science and Technology 120 (12):1800107. doi: 10.1002/ejlt.201800107.
- Özyurt, G., A. S. Özkütük, Y. Uçar, M. Durmuş, and Y. Ozogul. 2019. Evaluation of the potential use of discard species for fish silage and assessment of its oils for human consumption. International Journal of Food Science & Technology 54 (4):1081–8. doi: 10.1111/ijfs.13954.
- Paisan, S., P. Chetpattananondh, and S. Chongkhong. 2017. Assessment of water degumming and acid degumming of mixed algal oil. Journal of Environmental Chemical Engineering 5 (5):5115–23. doi: 10.1016/j.jece.2017.09.045.
- Polishchuk, A., D. Valev, M. Tarvainen, S. Mishra, V. Kinnunen, T. Antal, B. Yang, J. Rintala, and E. Tyystjärvi. 2015. Cultivation of Nannochloropsis for eicosapentaenoic acid production in wastewaters of pulp and paper industry. Bioresource Technology 193:469–76. doi: 10.1016/j.biortech.2015.06.135.
- Rai, A. K., H. C. Swapna, N. Bhaskar, P. M. Halami, and N. M. Sachindra. 2010. Effect of fermentation ensilaging on recovery of oil from fresh water fish viscera. Enzyme and Microbial Technology 46 (1):9–13. doi: 10.1016/j.enzmictec.2009.09.007.
- Rawn, D. F. K., K. Breakell, V. Verigin, H. Nicolidakis, D. Sit, and M. Feeley. 2009. Persistent organic pollutants in fish oil supplements on the Canadian market: Polychlorinated biphenyls and organochlorine insecticides. Journal of Food Science 74 (1):T14–9. doi: 10.1111/j.1750-3841.2008.01020.x.
- Richmond, G. S., and T. K. Smith. 2011. Phospholipases A1. International Journal of Molecular Sciences 12 (1):588–612. doi: 10.3390/ijms12010588.
- Rodrigues, D., A. C. Freitas, L. Pereira, T. A. P. Rocha-Santos, M. W. Vasconcelos, M. Roriz, L. M. Rodríguez-Alcalá, A. M. P. Gomes, and A. C. Duarte. 2015. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chemistry 183:197–207. doi: 10.1016/j.foodchem.2015.03.057.
- Rossmeisl, M., Z. M. Jilkova, O. Kuda, T. Jelenik, D. Medrikova, B. Stankova, B. Kristinsson, G. G. Haraldsson, H. Svensen, I. Stoknes, et al. 2012. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS One 7 (6):e38834. doi: 10.1371/journal.pone.0038834.
- Sahena, F., I. S. M. Zaidul, S. Jinap, M. H. A. Jahurul, A. Khatib, and N. A. N. Norulaini. 2010. Extraction of fish oil from the skin of Indian mackerel using supercritical fluids. Journal of Food Engineering 99 (1):63–9. doi: 10.1016/j.jfoodeng.2010.01.038.
- Sahena, F., I. S. M. Zaidul, S. Jinap, N. Saari, H. A. Jahurul, K. A. Abbas, and N. A. Norulaini. 2009. PUFAs in fish: Extraction, fractionation, importance in health. Comprehensive Reviews in Food Science and Food Safety 8 (2):59–74. doi: 10.1111/j.1541-4337.2009.00069.x.
- Sampaio, K. A., N. Zyaykina, E. Uitterhaegen, W. De Greyt, R. Verhé, A. J. de Almeida Meirelles, and C. V. Stevens. 2019. Enzymatic degumming of corn oil using phospholipase C from a selected strain of Pichia pastoris. LWT - Food Science and Technology 107:145–50. doi: 10.1016/j.lwt.2019.03.003.
- Sampaio, K. A., N. Zyaykina, B. Wozniak, J. Tsukamoto, W. D. Greyt, and C. V. Stevens. 2015. Enzymatic degumming: Degumming efficiency versus yield increase: Enzymatic degumming efficiency of PLA1. European Journal of Lipid Science and Technology 117 (1):81–6. doi: 10.1002/ejlt.201400218.
- Senanayake, S. P. J. N., and F. Shahidi. 2000. Concentration of docosahexaenoic acid (DHA) from algal oil via urea complexation. Journal of Food Lipids 7 (1):51–61. doi: 10.1111/j.1745-4522.2000.tb00160.x.
- Senphan, T., and S. Benjakul. 2015. Impact of enzymatic method using crude protease from Pacific white shrimp hepatopancreas on the extraction efficiency and compositions of lipids. Food Chemistry 166:498–506. doi: 10.1016/j.foodchem.2014.06.054.
- Solaesa, Á. G., M. T. Sanz, M. Falkeborg, S. Beltrán, and Z. Guo. 2016. Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation. Food Chemistry 190:960–7. doi: 10.1016/j.foodchem.2015.06.061.
- Soldo, B., V. Šimat, J. Vlahović, D. Skroza, I. Ljubenkov, and I. Generalić Mekinić. 2019. High quality oil extracted from sardine by-products as an alternative to whole sardines: Production and refining. European Journal of Lipid Science and Technology 121 (7):1800513. doi: 10.1002/ejlt.201800513.
- Song, G., Z. Dai, Q. Shen, X. Peng, and M. Zhang. 2018. Analysis of the changes in volatile compound and fatty acid profiles of fish oil in chemical refining process. European Journal of Lipid Science and Technology 120 (2):1700219. doi: 10.1002/ejlt.201700219.
- Song, G., M. Zhang, X. Peng, X. Yu, Z. Dai, and Q. Shen. 2018. Effect of deodorization method on the chemical and nutritional properties of fish oil during refining. LWT - Food Science and Technology 96:560–7. doi: 10.1016/j.lwt.2018.06.004.
- Sovová, H., B. P. Nobre, and A. Palavra. 2016. Modeling of the kinetics of supercritical fluid extraction of lipids from microalgae with emphasis on extract desorption. Materials 9 (6):423. doi: 10.3390/ma9060423.
- Subramanian, R., M. Nakajima, A. Yasui, H. Nabetani, T. Kimura, and T. Maekawa. 1999. Evaluation of surfactant-aided degumming of vegetable oils by membrane technology. Journal of the American Oil Chemists' Society 76 (10):1247–53. doi: 10.1007/s11746-999-0101-8.
- Swanson, D., R. Block, and S. A. Mousa. 2012. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition (Bethesda, Md.) 3 (1):1–7. doi: 10.3945/an.111.000893.
- Szydłowska-Czerniak, A., and A. Łaszewska. 2017. Optimization of a soft degumming process of crude rapeseed oil—Changes in its antioxidant capacity. Food and Bioproducts Processing 105:26–35. doi: 10.1016/j.fbp.2017.05.012.
- Tarvainen, M., A. Nuora, K.-W. Quirin, H. Kallio, and B. Yang. 2015. Effects of CO2 plant extracts on triacylglycerol oxidation in Atlantic salmon during cooking and storage. Food Chemistry 173:1011–21. doi: 10.1016/j.foodchem.2014.10.125.
- Tommasi, E., G. Cravotto, P. Galletti, G. Grillo, M. Mazzotti, G. Sacchetti, C. Samorì, S. Tabasso, M. Tacchini, and E. Tagliavini. 2017. Enhanced and selective lipid extraction from the microalga P. tricornutum by dimethyl carbonate and supercritical CO2 using deep eutectic solvents and microwaves as pretreatment. ACS Sustainable Chemistry & Engineering 5 (9):8316–22. doi: 10.1021/acssuschemeng.7b02074.
- Turetkan, G., C. Tasdelen-Yucedag, G. Ustun, and M. Tuter. 2018. Enzymatic degumming process for crude corn oil with phospholipase A. International Journal Series in Engineering Science 4:14. doi: 10.1000/ijses.v0i0.153.
- Vaisali, C., S. Charanyaa, P. D. Belur, and I. Regupathi. 2015. Refining of edible oils: A critical appraisal of current and potential technologies. International Journal of Food Science & Technology 50 (1):13–23. doi: 10.1111/ijfs.12657.
- Valenzuela, R., M. Ortiz, M. C. Hernández-Rodas, F. Echeverría, and L. A. Videla. 2020. Targeting n-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease. Current Medicinal Chemistry 27 (31):5250–72. doi: 10.2174/0929867326666190410121716.
- Vázquez, J. A., J. Fraguas, J. Mirón, J. Valcárcel, R. I. Pérez-Martín, and L. T. Antelo. 2020. Valorisation of fish discards assisted by enzymatic hydrolysis and microbial bioconversion: Lab and pilot plant studies and preliminary sustainability evaluation. Journal of Cleaner Production 246:119027. doi: 10.1016/j.jclepro.2019.119027.
- Vidotti, R. M., D. J. Carneiro, and E. Viegas. 2002. Growth rate of pacu, Piaractus mesopotamicus, fingerlings fed diets containing co-dried fish silage as replacement of fish meal. Journal of Applied Aquaculture 12 (4):77–88. doi: 10.1300/J028v12n04_07.
- Wang, W., T. Li, Z. Ning, Y. Wang, B. Yang, Y. Ma, and X. Yang. 2012. A process for the synthesis of PUFA-enriched triglycerides from high-acid crude fish oil. Journal of Food Engineering 109 (3):366–71. doi: 10.1016/j.jfoodeng.2011.11.020.
- Watanabe, Y., and I. Tatsuno. 2020. Prevention of cardiovascular events with omega-3 polyunsaturated fatty acids and the mechanism involved. Journal of Atherosclerosis and Thrombosis 27 (3):183–98. doi: 10.5551/jat.50658.
- Wijesundera, C., C. Ceccato, P. Watkins, P. Fagan, B. Fraser, N. Thienthong, and P. Perlmutter. 2008. Docosahexaenoic acid is more stable to oxidation when located at the sn-2 position of triacylglycerol compared to sn-1(3). Journal of the American Oil Chemists' Society 85 (6):543–8. doi: 10.1007/s11746-008-1224-z.
- Xue, Z., F. Wan, W. Yu, J. Liu, Z. Zhang, and X. Kou. 2018. Edible oil production from microalgae: A review. European Journal of Lipid Science and Technology 120 (6):1700428. doi: 10.1002/ejlt.201700428.
- Yang, B., M. Ahotupa, P. Määttä, and H. Kallio. 2011. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Research International 44 (7):2009–17. doi: 10.1016/j.foodres.2011.02.025.
- Zahrina, I., M. Nasikin, E. Krisanti, and K. Mulia. 2018. Deacidification of palm oil using betaine monohydrate-based natural deep eutectic solvents. Food Chemistry 240:490–5. doi: 10.1016/j.foodchem.2017.07.132.
- Zelinková, Z., B. Svejkovská, J. Velíšek, and M. Doležal. 2006. Fatty acid esters of 3-chloropropane-1,2-diol in edible oils. Food Additives & Contaminants 23 (12):1290–8. doi: 10.1080/02652030600887628.
- Zheng, Z., Z. Dai, and Q. Shen. 2018. Enrichment of polyunsaturated fatty acids from seal oil through urea adduction and the fatty acids change rules during the process. Journal of Food Processing and Preservation 42 (5):e13593. doi: 10.1111/jfpp.13593.
- Zuorro, A., S. Miglietta, G. Familiari, and R. Lavecchia. 2016. Enhanced lipid recovery from Nannochloropsis microalgae by treatment with optimized cell wall degrading enzyme mixtures. Bioresource Technology 212:35–41. doi: 10.1016/j.biortech.2016.04.025.