Abstract
Colorectal cancer (CRC) is the third most frequent cancer worldwide, accounts for about 10% of the total cancer cases, and ranks as the second cause of death by cancer. CRC is more prevalent in developed countries in close causal relation with occidental diets. Due to anatomy, the diet has a strong impact on CRC. High contents in meat are acknowledged risk factors whereas a diet rich in fruits and vegetables is an established CRC protective factor. Fruits and vegetables contain numerous Bioactive Food Components (BFCs), physiologically active food compounds, beneficial on health. Preventive and therapeutic benefits of BFCs in cancer have increasingly been reported over the past 20 years. BFCs show both chemopreventive and anti-tumor properties in CRC but more interestingly, abundant research describes BFCs as enhancers of conventional cancer treatments. Despite these promising results, their clinical transferability is slowed down by bioavailability interrogations and their poorly understood hormetic effect. In this review, we would like to reposition BFCs as well-fitted for applications in CRC. We provide a synthetic overview of trustworthy BFC applications in CRC, with a special highlight on combinatory approaches and conventional cancer treatment potentiation strategies.
Introduction
Colorectal cancer (CRC) is the third most frequent cancer worldwide, accounting for about 10% of the total cancer cases and ranks as the second cause of death by cancer. CRC is present especially in developed countries with about 60% of all CRC cases (Parkin et al. Citation2002). This high incidence was proven associated with occidental highly caloric diets (Ryan-Harshman and Aldoori Citation2007; Jin and Zhang Citation2020). Diet-linked CRC risk factors are multiple. Among them, important consumption of meat either red, processed, or cooked at high temperature (fried, broiled, or grilled) can increase the risk of developing CRC by up to 30 times (Imperial College London Citation2018). Conversely, regular physical activity and diets rich in fruits and vegetables are the main established CRC-specific protective factors (Imperial College London Citation2018).
Literature reports sufficient evidence to link obesity and higher risk of CRC, especially in men with low physical activity (Johnson and Lund Citation2007; Sawicki et al. Citation2021). The pathophysiological bases (Yang et al. Citation2022) include pro-oncogenic adipocyte metabolism (associated with lipid oxidation and energy storage) (Schwartz and Yehuda-Shnaidman Citation2014), low and chronic inflammation associated with pro-inflammatory cytokines secretion (Reilly and Saltiel Citation2017) and, stimulation of colorectal cell proliferation by adipokines and hormones (such as insulin) (Chen et al. Citation2011; Chen et al. Citation2018). In this context, unhealthy food consumption is closely related to obesity, ad increases the risk of CRC development.
The relationship between health and food regained intensive attention during the last 20 years to set the basis of numerous studies, first focusing on the cardiovascular impact and neurodegenerative diseases. The Mediterranean diet was early recognized as beneficial for health and nutrition (Altomare et al. Citation2013). Rich in fruits and vegetables, it prevents chronic cardiovascular diseases and several malignancies (reviewed in (Mentella et al. Citation2019; Widmer et al. Citation2015):). In parallel, the concept “French paradox” emerged, based on the observation that French people have a relatively low incidence of coronary heart diseases despite a rich diet, but including a moderate and regular consumption of red wine (Renaud and de Lorgeril Citation1992). Even if this theory was later refuted (Griswold et al. Citation2018), it triggered the identification of protective compounds in the wine and vine, and more broadly, in food.
The Bioactive Food Components (BFCs) are "physiologically active food compounds, derived from animal or plant sources, including compounds belonging to the food of a basic diet, for which a role has been shown to be healthy and the consumption of which is not harmful to health," according to the American Dietetic Association (Hasler et al. Citation2004). Noticeably, some BFCs have been long known in traditional medicines. For instance, Curcuma longa, with high content of curcumin, is widely used in India and China as a natural herbal remedy (reviewed in Hatcher et al. (Citation2008)). Interestingly, most BFCs exhibit hormesis, characterized by opposite effects at low and high doses, because of their anti/pro-oxidative properties.
Preventive and therapeutic benefits of BFCs in cancer have increasingly been reported over the past two decades in particular with regards to CRC (). Noticeably, BFCs showed both chemopreventive and anti-tumor properties in CRC (Yin et al. Citation2016; Ahmed et al. Citation2019) contributing to the general concept that a healthy way of life, including a balanced diet, is protective of CRC. Moreover, abundant literature describes BFCs as enhancers of conventional treatment efficiency in multiple cancers, suggesting a true biological relevance. Paradoxically, this profusion of information overflows important clues, hormesis adding confusion, which hinders their transfer to clinical applications. As our team gained expertise and improved comprehension of BFCs validity in cancer treatment, we would like to provide a synthetic overview of selected trustworthy applications in CRC deserving attention, with a special highlight on combinatory approaches.
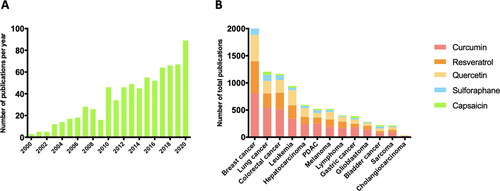
Figure 1. BFCs and cancers. (A) Number of reported publications per year in PubMed using the key words “cancer” and “bioactive food component” from January 2000 to December 2020. (B) Number of total reported publications in PubMed including key words “breast cancer” or “lung cancer” or “colorectal cancer” or “leukemia” or “hepatocarcinoma” or “melanoma” or “pancreatic ductal adenocarcinoma” (PDAC) or “lymphoma” or gastric cancer” or glioblastoma” or bladder cancer” or sarcoma” or “cholangiocarcinoma” and “curcumin” or “quercetin” or “resveratrol” or “capsaicin” or “sulforaphane” from January 2000 to December 2020.
Literature and clinical trial searching process
Systematic literature searches of articles published between 2000 and October 2021 were performed in the electronic PubMed database from the American National Institute of Health and the Google Scholar database. The searches were conducted using medical subject headings(MeSH: “colorectal cancer” or “colon cancer” or “rectal cancer” combined with “resveratrol” or “sulforaphane” or “quercetin” or curcumin” or “capsaicin”) and free-text words and limited to articles published in the English language in indexed and peer-reviewed journals.
A clinical trial search was performed using the clinicaltrials.gov website. To select international studies of interest, “colorectal cancer” was selected as the condition of interest, and either “resveratrol” or “sulforaphane” or “quercetin” or curcumin” or “capsaicin” keywords were used in the “other terms” section.
Main phytochemical BFC categories and compounds
As the “Bioactive Food Components” term brings together a wide range of molecules, originating from both plant and animal sources, it seems vain and counter-productive to provide an exhaustive list. We chose to focus on three major categories of BFCs carrying the most convincing level of evidence for utility in CRC prevention and treatment.
Polyphenols, the most studied bioactive food group in the field of cancerology, are present in most diets, at around 1 gram per day. They are mainly provided by fruits, vegetables, wine, tea, and chocolate (Manach et al. Citation2004). They contribute to the organoleptic properties of the leaves, fruits, and processed products such as wine or olive oil. Three main classes are studied in oncology: Phenolic acids, Flavonoids, and Stilbenes. Phenolic acids are phenolic compounds with one or more carboxylic acid groups, present for instance in coffee, red fruits, and onions. These include Curcumin, a derived BFC of the spice turmeric, with promising applications in oncology (Giordano and Tommonaro Citation2019). Flavonoids are found in onions and broccoli and are constituted by two benzene rings linked by a heterocycle. Among them, Quercetin showed recurrent beneficial anti-cancer effects (Rather and Bhagat Citation2020). Stilbenes are chemically close to flavonoids. They include resveratrol and its derived such as hydroxylated derivatives (piceatannol), dimerized derivatives (ε-viniferin and δ-viniferin), and other oligomers (vitisin B) for example. They are present in some fruits like blackberries, peanuts, and more particularly in grapes and their derivative products such as red wine. The properties of resveratrol have been widely studied in cancer demonstrating both chemopreventive and anti-cancer activity (Vervandier-Fasseur and Latruffe Citation2019; Berretta et al. Citation2020).
Heterosides are glycosylated derivatives of terpenes, phenols, and, more rarely, alkaloids. Glucosinolates, present in cruciferous vegetables like cabbage and broccoli, are hydrolyzed into bioactive isothiocyanates, including sulforaphane, with antioxidant properties potentially relevant for the prevention of neurodegenerative diseases (Tarozzi et al. Citation2013) and cancers (Grabacka, Gawin, and Pierzchalska Citation2014).
Alkaloids are basic nitrogen compounds extracted from plants. They provide medicine like morphine or the vincristine and taxol chemotherapies. They also count BFCs such as quinine and caffeine. Capsaicin, naturally occurring in chili peppers, represents the most current edible alkaloid of interest in cancerology applications.
BFCs in colorectal cancer prevention
Prevention of CRC has important public health implications and the influence of the diet has now been documented for many years. Several risk factors have been highlighted such as important consumption of meat or chronic alcohol consumption (Imperial College London Citation2018). The lower CRC frequency in Mediterranean countries positioned the Mediterranean diet as chemopreventive (Reviewed in Farinetti et al. (Citation2017)). Particularly rich in fruits and vegetables, this diet naturally contains many BFCs, especially polyphenols such as resveratrol and quercetin (Medina-Remón et al. Citation2017; Bagetta et al. Citation2020), conferring strong chemoprevention of CRC onset (Imperial College London Citation2018; Couto et al. Citation2011).
Anti-inflammatory properties
A wide range of BFCs is known to exert anti-inflammatory properties in many diseases and in particular cancer (Kim et al. Citation2009). Their activities take place at different levels of the inflammation process with for instance up-regulation of anti-inflammatory cytokines (Nguyen et al. Citation2006; Song et al. Citation2014) or downregulation of pro-inflammatory interleukin and enzyme synthesis, such as TNF-alpha and cyclooxygenase II (Manna, Mukhopadhyay, and Aggarwal Citation1950; Xagorari et al. Citation2001; Tong et al. Citation2005). Thus, BFCs can participate to maintain the balance between pro and anti-inflammatory mediators. They exhibit chemopreventive properties and could oppose CRC development by limiting chronic inflammation of intestine tissues (Arya, Kanthlal, and Linda Citation2020; Jantan et al. Citation2021). For instance, resveratrol and other dietary polyphenols downregulated inflammation-related enzymes and cytokines (Yin et al. Citation2016; Liang et al. Citation2001). The Association of curcumin and sulforaphane also exhibited a synergistic anti-inflammatory effect by induction of phase II antioxidant enzymes and down-regulation of pro-inflammatory cytokines like Tumor Necrosis Factor (TNF), nitric oxide (NO), or Prostaglandin E2 (PGE2) (Cheung, Khor, and Kong Citation2009). In another study, colorectal inflammation levels in mice under a classic diet were compared to that of a diet enriched in 4 BFCs including curcumin. The BFC-enriched diet group showed significantly reduced number and size of solid lesions, decreased histological inflammation scores, lowered pro-inflammatory cytokine mRNA expression, decreased number of low-grade dysplasia and high-grade dysplasia areas, as compared to the classic diet group (Girardi et al. Citation2018), suggesting a systemic effect of the BFC-enriched diet. In a colitis mouse model, oral administration of microencapsulated quercetin, preventing its absorption by the small intestine, led to decreased intestinal neutrophil recruitment, as well as diminution of histological alterations and macroscopical damages (Guazelli et al. Citation2013). Reduction of Interleukin 33 (IL-33) and IL-10 production also contributed to the anti-inflammatory effect. Interestingly, these results were not observed with free quercetin. Thus, quercetin absorption (and potent metabolization) by enterocytes seemed to impede its anti-inflammatory properties in the colon. Thus, BFCs seem to be able to reverse the inflammatory imbalance of digestive tissues and contribute to the prevention of CRC development.
Genetic stability
In non-cancer cells, resveratrol and other related polyphenols contributed to stabilizing histones H2AX and reduced replication stress-associated DNA double-strand breaks (DSB), resulting in increased genome stability of mouse embryonic fibroblasts and the prevention of gene mutations in the p53 pathway (Matsuno et al. Citation2020). In the same way, curcumin prevented telomere shortening and limited micronucleus formation in a transgenic model of Alzheimer’s disease (Thomas et al. Citation2009).
In CRC, mutant-APC mice fed with a curcumin-containing high-fat diet (HFD) displayed a significant reversion of HFD-induced polyps (Pettan-Brewer et al. Citation2011). Capsaicin was administrated to Wistar rats before and after treatment with 1,2-dimethylhydrazine (DMH), a DNA methylating agent known for its carcinogenesis properties (Caetano et al. Citation2018). The number and frequency of colorectal preneoplastic lesions were reduced in the capsaicin-treated group, suggesting that DMH genotoxicity was attenuated by capsaicin. In the same way, quercetin prevented DMH-induced colorectal inflammation and tumor development through the modulation of Wnt and nuclear factor erythroid-2-related factor 2 (NRF2) signaling pathways (Darband et al. Citation2020; Shree, Islam, and Sultana Citation2021). The chemopreventive role of a diet containing curcumin was also demonstrated in the healthy intestine. Mice receiving curcumin displayed fewer preneoplastic events, with reduced DNA damage in differentiated cells, possibly because DNA-damaged-Lgr5+ intestinal stem cells were more prone to apoptosis (Kim et al. Citation2019).
BFCs in colorectal cancer treatment
BFCs present direct antitumor properties on CRC, closely related to compound concentration and involving multiple cellular mechanisms. While low concentrations of BFCs seem to contribute to tumor prevention through their anti-oxidative properties, direct anti-tumor effects call for high compound concentrations and oxidative stress.
Upregulation of reactive oxygen species
Reactive oxygen species (ROS) are major determinants of the cell redox homeostasis and BFCs are potent modulators of ROS generation. ROS upregulation was commonly responsible for the colorectal antitumoral properties of BFCs. For instance, resveratrol and curcumin are pro-apoptotic for colorectal tumor cell lines in vitro in a ROS-dependent manner (Juan et al. Citation2008; Blanquer-Rosselló et al. Citation2017; Wang et al. Citation2017; Sritharan and Sivalingam Citation2021). Quercetin-induced ROS and derivatives positively modulated endoplasmic reticulum (ER) stress, especially by calcium transfer from the endoplasmic reticulum to mitochondria triggering intrinsic apoptosis (Khan et al. Citation2016).
Other cell death pathways may contribute, such as autophagy which was enhanced by ROS upregulation in human colon cancer cells treated with resveratrol (Miki et al. Citation2012). Oxidative stress induced by the flavonoid apigenin resulted in p53-independent senescence through p16, retinoblastoma protein (pRb), and p21 modulation (Banerjee and Mandal Citation2015).
BFCs contribute to ROS increase by direct regulation of enzymes involved in oxidative stress response. Resveratrol inhibited catalase and superoxide dismutase (SOD), both involved in ROS scavenging (Santandreu et al. Citation2011). By contrast, quercetin enhanced cyclooxygenase 2 (COX-2) activity, resulting in ROS generation upregulation and cytotoxicity increase on colorectal tumor cell lines (Raja et al. Citation2017).
DNA damages generation
One of the most deleterious effects of ROS is their ability to induce DNA damages when present at high concentrations in the nucleus. This observation set the basis of studies aiming at evaluating BFC-induced DNA damages. For instance, resveratrol was reported to increase the phosphorylation of histone gamma-H2AX, a chromatin modification mark at double-strand breaks (DSB), needed to initiate DSB DNA repair (San Hipólito-Luengo et al. Citation2017). In the same way, curcumin markedly increased DNA damage as measured by alkaline comet assay (Lu, Cai, and Ding Citation2011). When possible, DNA damages are resolved to preserve genomic integrity. BFCs hinder DNA repair, leading to synergistic toxicity by the direct increase of DNA damages and concomitant DNA repair inhibition. Sulforaphane analogs increase phosphorylation of histone gamma-H2AX, inhibit homologous recombination (HR), and non-homologous end joining (NHEJ) repair activities, notably by affecting the HAT/HDAC balance and histone acetylation status (Okonkwo et al. Citation2018). This cytotoxic synergy was also reported in other digestive cancers (Vendrely et al. Citation2019).
Cell cycle arrest
Because of unresolved DNA damage accumulation and inhibition of DNA repair pathways, BFCs also affect the cell cycle. The majority of studies found a typical accumulation of cells in the G2/M phase (Adams et al. Citation2005; Cheah et al. Citation2018; Wu, Yi, et al. Citation2019; Jozkowiak et al. Citation2020). The p53 pathway activation in stress conditions played a major role in cell cycle arrest. Resveratrol upregulated the Su(var)3-9, Enhancer-of-zeste and Trithorax (SET) domain-containing lysine methyltransferase 7/9 (SET7/9) in colorectal cell lines, which stabilized p53 by mono-methylation at lysine 372 (Liu et al. Citation2019). A resveratrol analog up-regulated p53 and p21 with subsequent cell cycle arrest and apoptosis (Cheah et al. Citation2018), as did capsaicin (Jin et al. Citation2014). Interestingly, looking deeper into the TP53/ATM/ATR DNA damage response pathway, curcumin-induced G2/M cell cycle arrest was not reversed by caffeine, an inhibitor of the ATM/ATR proteins, suggesting that additional pathways may be modulated (Lu, Cai, and Ding Citation2011).
Invasion and metastasis inhibition
Inhibition of tumor cell dissemination takes a large part of the BFC literature. For instance, resveratrol strongly impacted the epithelial to mesenchymal transition (EMT) by interfering with AKT, Wnt, TNF-β, or TGF-β signaling pathways (Ji et al. Citation2013; Ji et al. Citation2015; Yuan et al. Citation2019; Buhrmann et al. Citation2019). Metastasis inhibition by resveratrol involved focal adhesion protein suppression (Buhrmann et al. Citation2017) and overexpression of the translation inhibitor RNA binding protein tristetraprolin (TTP) (Lee et al. Citation2018). Oxyresveratrol, a natural hydroxyl derivate of resveratrol, modulated EMT-related miRNAs, resulting in EMT inhibition and cell migration reduction (Lin et al. Citation2021). More recently, up-regulation of miR-200c and associated target EPM5, a DNA binding histone-like protein, was the effector of curcumin-related EMT downregulation (Wang, Cai, et al. Citation2020). Curcumin downregulated the transcription of oncogenic microRNA miR-21 to inhibit invasion and metastasis of HCT116 cells in a chicken-embryo-metastasis assay (Mudduluru et al. Citation2011). Likewise, the inhibition of the TGF-β pathway by quercetin compromised EMT resulting in invasion/metastasis inhibition (Feng et al. Citation2018). The antimetastatic effect of curcumin, quercetin and their derivatives were described in several studies (Kee et al. Citation2016; Li et al. Citation2018; Calibasi-Kocal et al. Citation2019).
The interest of combination for BFCs
Unlike the majority of the studies, only a few reported effects of molecules used in combination. This topic was reviewed recently for chemoprevention (Rizeq et al. Citation2020). BFCs share common antitumor effectors leading to ROS induction or apoptosis but the upstream sequences of events seemed compound-dependent. Thus, combining different BFCs may carry additive or synergistic activities. In this way, combining curcumin with different BFCs enhanced global antitumor efficiency in different CRC models (Ravindranathan et al. Citation2018; Wu, Koh, et al. Citation2019). With resveratrol curcumin induced a greater impairment of epithelial growth factor receptor (EGFR) constitutive activation (Majumdar et al. Citation2009) and upregulation of several genes involved in apoptosis (Gavrilas et al. Citation2019). Similarly, four BFCs (Lycopene, Sulforaphane, Quercetin, and Curcumin) displayed additive proliferation inhibition while no toxic effect was observed for normal colon epithelial cells (Langner, Lemieszek, and Rzeski Citation2019). In a clinical setting, the combination treatment of quercetin and curcumin significantly decreased polyp number and size after 6 months of treatment of adenomatous polyposis coli (APC) patients (Cruz-Correa et al. Citation2006), with minimal adverse side effects and no routine biological parameters abnormalities.
BFCs as adjuvant agents for potentiation of conventional CRC treatments
BFCs may enhance the efficiency of conventional therapies, in numerous cancers (Lin et al. Citation2020). Combinations of BFCs were tested in CRC with chemotherapy, radiotherapy, or targeted therapies.
Chemotherapy
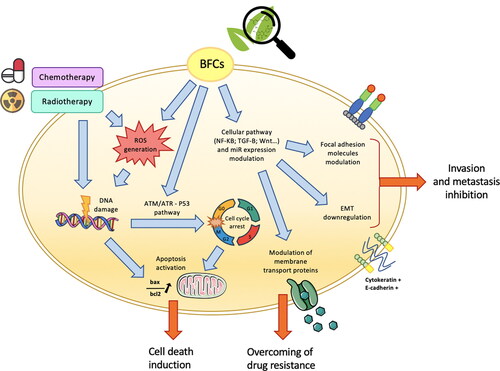
Colorectal cancer treatment includes surgery and chemotherapy (Recio-Boiles and Cancer Citation2020). Indications depend on the location, stage, histology, and patient’s general condition. The common chemotherapies include 5FU, irinotecan, oxaliplatin, doxorubicin, and capecitabin, often combined for better efficiency. BFCs and chemotherapies target common tumor cell key functions (). Improvement of chemotherapy antitumor activity by BFCs would greatly benefit patient management, especially if they do not carry systemic toxicity. This is particularly true for fragile patients for whom this strategy may allow to maintain disease control while reducing chemotherapy doses.
Figure 2. Bioactive food components (BFCs) as adjuvant agents for potentiation of conventional colorectal cancer (CRC) treatments. BFC and standard CRC treatments (chemotherapy/radiotherapy) target common tumor cell key functions. In particular, BFCs, as well as chemo and radiotherapy, induce oxidative stress associated with reactive oxygen species generation and DNA damages. BFCs are also able to modulate various signaling pathways resulting in, for instance, cellular cycle arrest, apoptosis induction and epithelial to mesenchymal transition (EMT) downregulation. Moreover, modulation of membrane transport proteins by BFCs can lead to reduce tumor drug resistance to standard chemotherapy treatments.
Several signaling pathways were regulated by BFCs with 5FU (Du et al. Citation2006; Marjaneh et al. Citation2018; Buhrmann et al. Citation2018; Chung et al. Citation2018; Zheng et al. Citation2021). For example, quercetin enhanced 5FU-induced apoptosis by overexpression of p53 in a CRC microsatellite instability (MSI) positive cell line. This was further supported by the fact the apoptosis enhancement disappeared in p53 deficient/knockdown CRC cell lines (Xavier et al. Citation2011). Up-regulation of cell-cell junction protein expression by resveratrol together with down-regulation of the NF-κB pathway led to inhibition of EMT and chemosensitization to 5FU (Buhrmann et al. Citation2015). Ginkgetin, a flavone BFC mainly isolated from Ginkgo biloba, and resveratrol suppressed VEGF-induced endothelial cell proliferation, migration, and invasion (Hu et al. Citation2019). They exerted a synergistic anti-tumor activity with 5FU by decreasing microvessel density inside the tumors. Concomitantly, combination treatment synergistically relieved the 5FU-induced inflammatory response by suppressing the expression of COX-2 and inflammatory cytokines. Quercetin and ellagic acid decreased human CRC cell proliferation, involving DNA damage and cell cycle modulation, to increase 5FU cell sensitivity. Moreover, tumors harboring BRAF mutations may be more responsive to vine pruning residues (VPE) than KRAS mutated tumors, suggesting that BFCs’ properties depend on CRC major oncogenic pathways (Jesus et al. Citation2020).
CRC oxaliplatin chemosensitization was reported mostly for curcumin and its derivates. Recently, oxaliplatin and curcumin co-treatment not only increased chemotherapy efficiency but also abolished oxaliplatin resistance (Patel et al. Citation2008; Chen et al. Citation2017; Han et al. Citation2020). Sulforaphane sensitized colon cancer cells to oxaliplatin-induced cell growth inhibition by both intrinsic and extrinsic apoptosis and programmed necrosis (Kaminski et al. Citation2011). WZ26, a curcumin synthetic analog, combined with cisplatin significantly inhibited tumor growth while attenuating body weight loss observed with cisplatin treatment (Zhang et al. Citation2019).
As oxaliplatin, doxorubicin is known to reach limited efficacy in CRC due to multi-drug resistance. Resveratrol inhibited the P-glycoprotein efflux pump activity, a transmembrane protein responsible for the multiple drug resistance, leading to accumulation of doxorubicin in tumor cells. More interestingly, MDR1 gene (coding for P-glycoprotein) expression remained stable under resveratrol treatment, suggesting direct ATPase inhibition (Khaleel et al. Citation2016). Quercetin enhanced the doxorubicin antitumor effect by downregulating the expression of the P-glycoprotein (Zhou et al. Citation2020). In a C26 colon carcinoma murine model, a PEGylated liposomal curcumin and doxorubicin combination demonstrated enhanced antitumor activity through suppressive effects of angiogenesis, inflammation, invasion, and resistance to apoptosis (Sesarman et al. Citation2019). Moreover, dysregulation of the Th1/Th2 cell axis in the tumor microenvironment favored the antineoplastic phenotype.
In CRC and other cancer types, cancer stem cells (CSCs) are classically responsible for treatment resistance. Therefore, the impact of BFCs on CSCs was investigated. Quercetin targeted C133+ CSCs of the HT-29 CRC cell line, especially by promoting cell cycle arrest and apoptosis (Atashpour et al. Citation2015). Similarly, curcumin significantly attenuated chemoresistance in an irinotecan-resistant LoVo CRC cell line (Su et al. Citation2018). A significant reduction of the expression levels of CSC-specific markers, associated with more apoptosis, occurred only in curcumin-treated tumor cells.
As mentioned before, CRC chemotherapy classically combines several molecules for more efficiency. Rather than testing combinations of BFCs and individual chemotherapies, the impact of curcumin was assessed in combination with FOLFOX, the association of 5FU, oxaliplatin, and folinic acid, commonly used for the treatment of CRC. A sequence treatment of FOLFOX followed by curcumin greatly reduced survival of HCT116 and HT-29 CRC cells by particular targeting of FOLFOX-resistant cells through inhibition of Epidermal Growth Factor Receptor (EGFR) and Insulin Growth Factor 1 receptor (IGF-1R) (Patel, Gupta, et al. Citation2010). At the clinical level, conventional chemotherapeutic regimens associated with curcumin emerges as a potential strategy to prevent chemoresistance of colon cancer cells, as exemplified by the randomized phase IIa clinical study (Howells et al. Citation2019), with the primary outcome assessing the safety of curcumin for patients receiving either FOLFOX or CUFOX (curcumin + FOLFOX). Similar adverse event profiles were observed in both arms, confirming curcumin safety. Importantly, overall survival (OS) was improved in the CUFOX arm (502 versus 200 days). However, these results deserve careful consideration and external validation because of the small cohort size (28 patients).
Radiotherapy
Although radiotherapy has limited indications for colon cancer, it is almost systematically used in the management of rectal cancer. Neoadjuvant treatments classically associate radiotherapy and chemotherapy to reduce tumor volume before surgical procedure. In addition to chemosensitization, some BFCs exhibit CRC radiosensitization, notably by oxidative stress-associated DNA damage generation (). Modulation of CRC radiosensitivity by curcumin was evidenced in 2 mouse models (Kunnumakkara et al. Citation2008; Yang et al. Citation2018). Both studies reported enhancement of radiotherapy efficacy when combined with curcumin through NF-kB pathway downregulation, apoptosis, angiogenesis, and DNA repair inhibition. Interestingly, curcumin also suppressed post-irradiation NF-kB activation and associated pro-survival response in human CRC cell lines (Sandur et al. Citation2009). Radiosensitization by BFCs like quercetin and resveratrol were recently confirmed in several tumor types (Vendrely et al. Citation2019; Lin et al. Citation2012; Amini et al. Citation2020).
Precision medicine
With the late development of precision medicine, the management of CRC becomes tailor-made, according to the tumor and patient specificities. The role of precision medicine in the management of digestive cancers was detailed in a recent publication (Matsuoka and Yashiro Citation2020). Regorafenib, a tyrosine kinase inhibitor approved by Food and Drug Administration (FDA) for CRC treatment, the antitumoral effect was enhanced by curcumin in the KRAS mutant HCT116 cell line, through autophagy and apoptosis. Curcumin acted as a MEK inhibitor. Interestingly, the KRAS wild-type HT-29 CRC cell was not responsive, suggesting that KRAS status influenced the curcumin potentiating effect (Wu, Wu, et al. Citation2019). Combination treatment of curcumin and dasatinib reduced cell growth, invasion, and colonosphere formation accompanied by chemosensitization of the cancer stem cell subpopulation (Nautiyal et al. Citation2011). Similarly, resveratrol sensitized CRC tumor cells to the anti-EGFR cetuximab (Wang, Wang, et al. Citation2020).
The immunomodulatory properties of BFCs seemed beneficial in combination with checkpoint inhibitors (Deng et al. Citation2020). For example, combination treatment of curcumin and sildenafil increased anti-PD1 antibody efficiency in a CRC mouse model (Dent et al. Citation2020). Moreover, BFCs combination reduced tumor expression of PD‐L1 and increased that of Class I human major histocompatibility complex (HMC). Resveratrol enhanced natural killer (NK) lymphocyte antitumor activity (Lee and Kim Citation2020; Lee, Shin, and Kim Citation2021), boosting the efficiency of checkpoint inhibitors (Chen and Musa Citation2021). These results also highlight the potentiality of BFCs as modulators of the tumor microenvironment (Nagaraju Citation2020).
Clinical trials
Few studies explored BFCs’ benefits in clinical setup for CRC. Results are not available for most of the trials, yet (see ). Briefly, 12 studies tested curcumin (6 Phase I, 5 Phase II, and one Phase III), 4 Phase I studies tested resveratrol and one study tested quercetin. Among all of these 17 studies, 13 included CRC patients, 2 included APC patients, 1 included patients with a moderate risk of CRC, and 1 study included healthy patients.
Table 1. Clinical trials interested in BFCs for chemoprevention and treatment of colorectal cancer.
A phase II clinical trial, including 44 patients with 8 or more aberrant crypt foci (ACF) identified by colonoscopy, evaluated the preventive action of curcumin on the occurrence of colorectal neoplastic lesions. Patients were given an oral daily 2 or 4 g treatment of curcumin for 1 month. A significant 40% reduction in ACF number was observed with the 4 g dose (p < 0.005) but not with 2 g (Carroll et al. Citation2011). Importantly, both doses were well tolerated. Curcumin impact on intestinal adenomas was tested in a cohort of patients with familial adenomatous polyposis (FAP) (Cruz-Correa et al. Citation2018). There was no difference in the mean number or size of lower intestinal tract adenomas between curcumin (3 g/day for 12 weeks) and placebo. By contrast, significant FAP number and size decreased after 6 months with BFCs supplementation including quercetin (20 mg 3 times/day) and curcumin (480 mg 3 times/day) (Cruz-Correa et al. Citation2006). These contradictory results may be explained by the beneficial combinatory action of two BFCs and/or by a long-term effect with longer treatment.
Curcumin bioavailability and tolerability were studied in phase I trials (Sharma et al. Citation2001; Sharma et al. Citation2004; Garcea et al. Citation2005), finding no toxicity while biological effects were observed. Curcumin concentrations were measured in tumor and healthy tissues. As curcumin presents low bioavailability after oral administration, a liposomal form was recently developed and tested in a phase I study including metastatic CRC patients (Greil et al. Citation2018). The administration was carried out intravenously daily for 8 weeks. The authors considered the dose of 300 mg/m2 over 6 h as the maximum tolerated dose. Over this dose, some patients presented hemolysis or hemoglobin drops. Levels of resveratrol and its Phase II metabolites were higher in the right-side colon compared to the left side, in direct relation with the anatomy portion after the ileum, suggesting that resveratrol is not fully absorbed in the intestine or that a portion is released back into the colon (Patel, Brown, et al. Citation2010). Moreover, daily oral doses of resveratrol at 0.5 g or 1 g during 10 to 21 days were sufficient to obtain resveratrol and associated metabolites concentrations in the gastrointestinal tract to elicit anti-carcinogenic effects. Alternative administration routes have been developed and tested for enhanced resveratrol systemic availability. In a randomized phase, I trial, assessment of safety, pharmacokinetics, and pharmacodynamics of micronized resveratrol was performed in CRC patients with hepatic metastases eligible for hepatectomy (Howells et al. Citation2011). Treatment was well tolerated and plasmatic levels of resveratrol were increased as compared to non-micronized formulations. Additionally, authors reported a 39% significant increase of the apoptosis biomarker caspase 3, in malignant hepatic tissues with resveratrol treatment compared with tissues from the placebo group.
The tolerability of chemotherapy in association with BFCs was also evaluated. In a randomized clinical study, 72 patients with metastatic CRC cancer received leucovorin, 5-fluorouracil, and oxaliplatin in combination with either MB-6, a botanical preparation containing grape seeds and curcumin extracts, or a placebo for 16 weeks (Chen et al. Citation2014). Patients’ follow-up (70 weeks after treatment) revealed no significant difference in best overall response rate and OS between groups. However, patients in the MB-6 group exhibited a significant lower disease progression rate (0.0% vs 15.8%, p = 0.026). Moreover, no higher incidence of adverse events was observed in the MB-6 group compared to the placebo group. Curcumin was also tested in combination with FOLFOX in a phase I dose-escalation study (James et al. Citation2015). Authors reported curcumin to be a safe and tolerable adjunct to FOLFOX at doses up to 2 g daily. Concordant results were recently combining FOLFOX with oral curcumin (2 g daily) or placebo (Howells et al. Citation2019). Similar adverse event profiles were reported for both arms. More randomized trials considering OS and progression-free survival (PFS) as primary endpoints are needed to assess the antitumor benefit of BFCs in CRC.
Limits and future directions
Overall BFCs research is a worthy research domain because it holds promising chemopreventive or antitumor actions. However, attention should be given to their hormetic properties that could defeat their benefits. Indeed, enhancement of ROS production leading to antitumor effect was typically reported for high doses or concentrations of BFCs but low doses were cytoprotective. For instance, low concentrations of resveratrol (1 to 10 μM) enhanced the proliferation of the HT-29 CRC cell line, while higher concentrations were cytotoxic (San Hipólito-Luengo et al. Citation2017). Similarly, low doses of capsaicin promoted metastasis of CRC cell lines in vitro and in a murine preclinical model by triggering ROS generation and activation of the Akt-mTOR pathway (Yang et al. Citation2013). Similarly, in combination with 5-FU, a high concentration of resveratrol (200 μM) increased chemotherapy-triggered apoptosis of the HCT116 cell line (Chan et al. Citation2008). By contrast, lower doses (25 or 50 μM) inhibited the pro-apoptotic action of 5-FU, especially in p53 proficient cells. In the same way, sulforaphane annihilated resveratrol and capsaicin combined antitumor action in vivo (Vendrely et al. Citation2017). Sulforaphane inhibited T cell-mediated immune response, which may, in turn, interfere with checkpoint inhibitors’ efficiency (Liang et al. Citation2019). Therefore, BFCs association with conventional cancer therapies requires a deep understanding of the positive or negative synergy of combinations (Roell, Reif, and Motsinger-Reif Citation2017; Fernando, Rupasinghe, and Hoskin Citation2019).
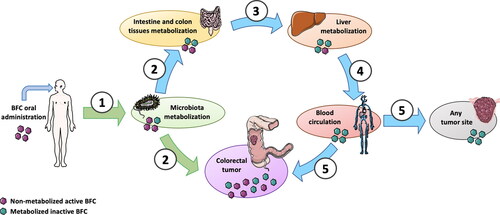
The recurrent concern about using BFCs in routine clinical practice is their challenged bioavailability. A large majority of compounds present low absorption rates or are actively transformed into less potent metabolites by digestive tract tissues and liver upon oral administration (). CRC may actually avoid this pitfall since non-metabolized oral-given BFCs can reach the tumors. More work, comparing the toxic effects of BFCs according to administration routes is however required.
Figure 3. Colorectal tumor anatomy allows direct intake of unmetabolized active BFCs after oral administration with entero-hepatic metabolization cycle shunt. 1. After oral administration, a part of the BFCs is metabolized by microbiota; 2. BFCs and metabolites can be directly uptake by normal intestine and colon tissues but also by colorectal tumors; 3. Enterocytes metabolize a part of the BFCs and release BFCs and their metabolites into portal circulation to the liver; 4. After hepatic metabolization, BFC metabolites are released into the general blood circulation; 5. BFCs metabolites can reach either colorectal tumor or any other tumor site through blood circulation. Unlike distant tumor sites receiving only metabolized non-active BFCs (blue path), colorectal tumors are directly exposed to active non-metabolized BFCs (green path). This figure was made using Servier Medical Art support (https://smart.servier.com).
To overcome bioavailability limits, researchers developed various galenic formulations of BFCs. Encapsulation into nanoparticles, either by physical, chemical, or physicochemical methods were frequently tested, as recently reviewed for polyphenols (Witika et al. Citation2021; Teng et al. Citation2021). The antitumor effect of curcumin on CRC cell lines was enhanced when encapsulated as Gemini surfactant nanoparticles (Ebrahimi et al. Citation2021) and nano encapsulated chrysin-curcumin combination showed a synergistic antitumor effect on the SW480 CRC cell line (Bagheri, Sanaat, and Zarghami Citation2018). This synergy was not observed with the free forms. Similarly, synthetic quercetin-caffeic-acid phenethyl ester co-loaded nanoparticles exhibited stronger anti-proliferative, anti-migration, and apoptotic properties than the free form of the combination (Colpan and Erdemir Citation2021). Recently, nanoparticles were used to combine curcumin with a silencer of the long non-coding (lnc) RNA colon cancer-associated transcript-1 (CCAT1) involved in CRC oncogenesis (Jia et al. Citation2021). The authors reported the synergistic anti-proliferative effect of the encapsulated combination better than drugs encapsulated individually. Thus, encapsulation can both enhance antitumor activity (by increasing bioavailability) as well as synergism of BFCs.
Excluding curcumin, for which several clinical studies have been carried out, BFC inclusion in clinical trials remains limited, and this is despite intense academic research. We believe that considering the high cost of clinical trials, the unpatented availability of BFCs is not attractive for pharmaceutical companies or private promoters. Patents may become possible with the development of new galenic forms or combination treatments. The time gap between basic research and clinical transfer is to deplore, especially for the patients, for whom the benefit may be important.
The toxicity of radiotherapy and chemotherapy is a major concern for oncologists and patients. Unlike standard treatments, BFCs do not induce major systemic toxicity, as shown by numerous phase I and II clinical trials. Surprisingly, very few studies explored this absence of toxicity on healthy cells compared to their tumor cell counterparts. Curcumin exerted a greater pro-apoptotic impact on hepatocarcinoma cell lines compared to healthy hepatocytes (Syng-Ai, Kumari, and Khar Citation2004), in close relation with intrinsic levels of the radical scavenger Glutathione. Curcumin also differentially modulated morphologic and elastic properties of mammalian cancerous and normal epithelial cells with a selective negative impact on tumor cells (Saab et al. Citation2013). In the same way, resveratrol was pro-oxidative in human astrocytoma cells, but not in primary healthy astrocytes (Gran et al. Citation2021). Thus, some BFCs exert tumor-specific cytotoxicity while sparing surrounding healthy tissue. This interesting property reinforces the cause of using BFCs in cancer, as reviewed for resveratrol (Mortezaee et al. Citation2020). Moreover, in combination with conventional treatments, BFCs could help maintain tumor control for fragile or comorbid patients necessitating treatment de-escalation. For instance, the combination of resveratrol and capsaicin associated with a gemcitabine dose reduced by 1/3 rescued the full dose anti-tumor effect (Vendrely et al. Citation2017).
In conclusion, the mechanisms ruling BFCs chemoprotective and antitumor activities in CRC are still unclear, especially accounting for their systemic metabolism and tumor bioavailability. Another important aspect of their use will no doubtedly explore pharmaceutical and galenic developments to optimize their oral bioavailability. Despite this, the current state of knowledge positions BFCs as important players for preventing and treating CRC, particularly because they are part of most diets, and because they do not carry systemic toxicity. However, clinical studies are still needed to fully reveal their health potential.
Declaration of interest statement
The authors have no conflicts of interest to declare.
Funding
This work was supported by SIRICBRIO (Bordeaux Recherche Intégrée en Oncologie).
References
- Adams, B. K., J. Cai, J. Armstrong, M. Herold, Y. J. Lu, A. Sun, J. P. Snyder, D. C. Liotta, D. P. Jones, and M. Shoji. 2005. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer. Drugs 16:263–75.
- Ahmed, K., S. F. Zaidi, Z.-G. Cui, D. Zhou, S. A. Saeed, and H. Inadera. 2019. Potential proapoptotic phytochemical agents for the treatment and prevention of colorectal cancer. Oncology Letters 18 (1):487–98.
- Altomare, R., F. Cacciabaudo, G. Damiano, V. D. Palumbo, M. C. Gioviale, M. Bellavia, G. Tomasello, and A. I. Lo Monte. 2013. The Mediterranean diet: A history of health. Iranian Journal of Public Health 42 (5):449–57.
- American Dietetic Association. ADA – comments on defining bioactive food components vdocuments .net.
- Amini, P., S. J. Nodooshan, M. Ashrafizadeh, S.-M. Eftekhari, T. Aryafar, L. Khalafi, A. E. Musa, S. R. Mahdavi, M. Najafi, and B. Farhood. 2020. Resveratrol induces apoptosis and attenuates proliferation of MCF-7 cells in combination with radiation and hyperthermia. Current Molecular Medicine 21:142–150.
- Arya, V. S., S. K. Kanthlal, and G. Linda. 2020. The role of dietary polyphenols in inflammatory bowel disease: A possible clue on the molecular mechanisms involved in the prevention of immune and inflammatory reactions. Journal of Food Biochemistry 44 (11):e13369. doi: 10.1111/jfbc.13369.
- Atashpour, S., S. Fouladdel, T. K. Movahhed, E. Barzegar, M. H. Ghahremani, S. N. Ostad, and E. Azizi. 2015. Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iranian Journal of Basic Medical Sciences 18 (7):635–43.
- Bagetta, D., A. Maruca, A. Lupia, F. Mesiti, R. Catalano, I. Romeo, F. Moraca, F. A. Ambrosio, G. Costa, A. Artese, et al. 2020. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. European Journal of Medicinal Chemistry 186:111903.
- Bagheri, R., Z. Sanaat, and N. Zarghami. 2018. Synergistic effect of free and nano-encapsulated chrysin-curcumin on inhibition of hTERT gene expression in SW480 colorectal cancer cell line. Drug Research 68 (6):335–43. doi: 10.1055/s-0043-121338.
- Banerjee, K., and M. Mandal. 2015. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biology 5:153–62. doi: 10.1016/j.redox.2015.04.009.
- Berretta, M., A. Bignucolo, R. D. Francia, F. Comello, G. Facchini, M. Ceccarelli, R. V. Iaffaioli, V. Quagliariello, and N. Maurea. 2020. Resveratrol in cancer patients: From bench to bedside. International Journal of Molecular Sciences 21:2945.
- Blanquer-Rosselló, M. D. M., R. Hernández-López, P. Roca, J. Oliver, and A. Valle. 2017. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochimica et Biophysica Acta. General Subjects 1861 (2):431–40. doi: 10.1016/j.bbagen.2016.10.009.
- Buhrmann, C., M. Yazdi, B. Popper, A. Kunnumakkara, B. Aggarwal, and M. Shakibaei. 2019. Induction of the epithelial-to-mesenchymal transition of human colorectal cancer by human TNF-β (lymphotoxin) and its reversal by resveratrol. Nutrients 11 (3):704. doi: 10.3390/nu11030704.
- Buhrmann, C., M. Yazdi, B. Popper, P. Shayan, A. Goel, B. Aggarwal, and M. Shakibaei. 2018. Resveratrol chemosensitizes TNF-β-induced survival of 5-FU-treated colorectal cancer cells. Nutrients 10 (7):888. doi: 10.3390/nu10070888.
- Buhrmann, C., P. Shayan, A. Goel, and M. Shakibaei. 2017. Resveratrol regulates colorectal cancer cell invasion by modulation of focal adhesion molecules. Nutrients 9 (10):1073. doi: 10.3390/nu9101073.
- Buhrmann, C., P. Shayan, P. Kraehe, B. Popper, A. Goel, and M. Shakibaei. 2015. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochemical Pharmacology 98 (1):51–68.
- Caetano, B. F. R., M. B. Tablas, N. E. F. Pereira, N. A. de Moura, R. F. Carvalho, M. A. M. Rodrigues, and L. F. Barbisan. 2018. Capsaicin reduces genotoxicity, colonic cell proliferation and preneoplastic lesions induced by 1,2-dimethylhydrazine in rats. Toxicology and Applied Pharmacology 338:93–102.
- Calibasi-Kocal, G., A. Pakdemirli, S. Bayrak, N. M. Ozupek, T. Sever, Y. Basbinar, H. Ellidokuz, and T. Yigitbasi. 2019. Curcumin effects on cell proliferation, angiogenesis and metastasis in colorectal cancer. Journal of BUON 24:1482–7.
- Carroll, R. E., R. V. Benya, D. K. Turgeon, S. Vareed, M. Neuman, L. Rodriguez, M. Kakarala, P. M. Carpenter, C. McLaren, F. L. Meyskens, et al. 2011. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prevention Research 4 (3):354–64. doi: 10.1158/1940-6207.CAPR-10-0098.
- Chan, J. Y., M. S. Phoo, M.-V. Clement, S. Pervaiz, and S. C. Lee. 2008. Resveratrol displays converse dose-related effects on 5-fluorouracil-evoked colon cancer cell apoptosis: The roles of caspase-6 and p53. Cancer Biology & Therapy 7 (8):1305–12. doi: 10.4161/cbt.7.8.6302.
- Cheah, F. K., K. H. Leong, N. F. Thomas, H. K. Chin, A. Ariffin, and K. Awang. 2018. Resveratrol analogue, (E)-N-(2-(4-methoxystyryl) phenyl) furan-2-carboxamide induces G2/M cell cycle arrest through the activation of p53-p21CIP1/WAF1 in human colorectal HCT116 cells. Apoptosis 23 (5-6):329–42. doi: 10.1007/s10495-018-1457-8.
- Chen, G.-P., Y. Zhang, Z.-Y. Xu, J.-F. Yu, and X. Wei. 2017. Curcumin combined with cis-platinum promote the apoptosis of human colorectal cancer HT29 cells and mechanism. International Journal of Clinical and Experimental Pathology 10 (12):11496–505.
- Chen, J., A. Katsifis, C. Hu, and X.-F. Huang. 2011. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Current Drug Discovery Technologies 8 (2):119–25.
- Chen, L., and A. E. Musa. 2021. Boosting immune system against cancer by resveratrol. Phytotherapy Research 35 (10):5514–5526.
- Chen, W. T.-L., T.-S. Yang, H.-C. Chen, H.-H. Chen, H.-C. Chiang, T.-C. Lin, C.-H. Yeh, T.-W. Ke, J.-S. Chen, K.-H. Hsiao, et al. 2014. Effectiveness of a novel herbal agent MB-6 as a potential adjunct to 5-fluoracil–based chemotherapy in colorectal cancer. Nutrition Research 34 (7):585–94.
- Chen, X., H. Liang, Q. Song, X. Xu, and D. Cao. 2018. Insulin promotes progression of colon cancer by upregulation of ACAT1. Lipids in Health and Disease 17 (1):122. doi: 10.1186/s12944-018-0773-x.
- Cheung, K. L., T. O. Khor, and A.-N. Kong. 2009. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharmaceutical Research 26 (1):224–31. doi: 10.1007/s11095-008-9734-9.
- Chung, S. S., P. Dutta, D. Austin, P. Wang, A. Awad, and J. V. Vadgama. 2018. Combination of resveratrol and 5-flurouracil enhanced anti-telomerase activity and apoptosis by inhibiting STAT3 and Akt signaling pathways in human colorectal cancer cells. Oncotarget 9 (68):32943–57. doi: 10.18632/oncotarget.25993.
- Colpan, R. D., and A. Erdemir. 2021. Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. Journal of Microencapsulation 38 (6):381–93. doi: 10.1080/02652048.2021.1948623.
- Couto, E., P. Boffetta, P. Lagiou, P. Ferrari, G. Buckland, K. Overvad, C. C. Dahm, A. Tjønneland, A. Olsen, F. Clavel-Chapelon, et al. 2011. Mediterranean dietary pattern and cancer risk in the EPIC cohort. British Journal of Cancer 104 (9):1493–9. doi: 10.1038/bjc.2011.106.
- Cruz-Correa, M., D. A. Shoskes, P. Sanchez, R. Zhao, L. M. Hylind, S. D. Wexner, and F. M. Giardiello. 2006. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clinical Gastroenterology and Hepatology 4:1035–8.
- Cruz-Correa, M., L. M. Hylind, J. H. Marrero, M. L. Zahurak, T. Murray-Stewart, R. A. Casero, E. A. Montgomery, C. Iacobuzio-Donahue, L. A. Brosens, G. J. Offerhaus, et al. 2018. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology 155 (3):668–73. doi: 10.1053/j.gastro.2018.05.031.
- Darband, S. G., S. Sadighparvar, B. Yousefi, M. Kaviani, F. Ghaderi-Pakdel, A. Mihanfar, Y. Rahimi, K. Mobaraki, and M. Majidinia. 2020. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sciences 253:117584. doi: 10.1016/j.lfs.2020.117584.
- Deng, L.-J., M. Qi, N. Li, Y.-H. Lei, D.-M. Zhang, and J.-X. Chen. 2020. Natural products and their derivatives: Promising modulators of tumor immunotherapy. Journal of Leukocyte Biology 108 (2):493–508.
- Dent, P., L. Booth, J. L. Roberts, A. Poklepovic, and J. F. Hancock. 2020. (Curcumin + sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. Journal of Cellular Physiology 235 (10):6862–74.
- Du, B., L. Jiang, Q. Xia, and L. Zhong. 2006. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 52 (1):23–8. doi: 10.1159/000090238.
- Ebrahimi, M., E. Babaei, F. Neri, and M. A. H. Feizi. 2021. Anti-proliferative and apoptotic effect of Gemini curcumin in p53-wild type and p53-mutant colorectal cancer cell lines. International Journal of Pharmaceutics 601:120592. doi: 10.1016/j.ijpharm.2021.120592.
- Farinetti, A., V. Zurlo, A. Manenti, F. Coppi, and A. V. Mattioli. 2017. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 43–44:83–8. doi: 10.1016/j.nut.2017.06.008.
- Feng, J., D. Song, S. Jiang, XHui Yang, T. Ding, H. Zhang, J. Luo, J. Liao, and Q. Yin. 2018. Quercetin restrains TGF-β1-induced epithelial–mesenchymal transition by inhibiting Twist1 and regulating E-cadherin expression. Biochemical and Biophysical Research Communications 498 (1):132–8.
- Fernando, W., H. P. V. Rupasinghe, and D. W. Hoskin. 2019. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Letters 452:168–77. doi: 10.1016/j.canlet.2019.03.022.
- Garcea, G., D. P. Berry, D. J. L. Jones, R. Singh, A. R. Dennison, P. B. Farmer, R. A. Sharma, W. P. Steward, and A. J. Gescher. 2005. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiology, Biomarkers & Prevention 14:120–5.
- Gavrilas, L. I., D. Cruceriu, C. Ionescu, D. Miere, and O. Balacescu. 2019. Pro-apoptotic genes as new targets for single and combinatorial treatments with resveratrol and curcumin in colorectal cancer. Food & Function 10 (6):3717–26. doi: 10.1039/C9FO01014A.
- Giordano, A., and G. Tommonaro. 2019. Curcumin and cancer. Nutrients 11:2376. doi: 10.3390/nu11102376.
- Girardi, B., M. Principi, M. Pricci, F. Giorgio, A. Iannone, G. Losurdo, E. Ierardi, A. Di Leo, and M. Barone. 2018. Chemoprevention of inflammation-related colorectal cancer by silymarin-, acetyl-11-keto-beta-boswellic acid-, curcumin- and maltodextrin-enriched dietetic formulation in animal model. Carcinogenesis 39 (10):1274–82. doi: 10.1093/carcin/bgy104.
- Grabacka, M. M., M. Gawin, and M. Pierzchalska. 2014. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 7:913–42.
- Gran, E. R., V. Lotocki, Q. Zhang, J. Antel, A. Kakkar, and D. Maysinger. 2021. Human astrocytes and astrocytoma respond differently to resveratrol. Nanomedicine 37:102441. doi: 10.1016/j.nano.2021.102441.
- Greil, R., S. Greil-Ressler, L. Weiss, C. Schönlieb, T. Magnes, B. Radl, G. T. Bolger, B. Vcelar, and P. P. Sordillo. 2018. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemotherapy and Pharmacology 82 (4):695–706. doi: 10.1007/s00280-018-3654-0.
- Griswold, M. G., N. Fullman, C. Hawley, N. Arian, S. R. M. Zimsen, H. D. Tymeson, V. Venkateswaran, A. D. Tapp, M. H. Forouzanfar, J. S. Salama, et al. 2018. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet 392 (10152):1015–35. doi: 10.1016/S0140-6736(18)31310-2.
- Guazelli, C. F. S., V. Fattori, B. B. Colombo, S. R. Georgetti, F. T. M. C. Vicentini, R. Casagrande, M. M. Baracat, and W. A. Verri. 2013. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. Journal of Natural Products 76 (2):200–8.
- Han, W., H. Yin, H. Ma, Y. Wang, D. Kong, and Z. Fan. 2020. Curcumin regulates ERCC1 expression and enhances oxaliplatin sensitivity in resistant colorectal cancer cells through its effects on miR-409-3p. Evidence-Based Complementary and Alternative Medicine 2020:8394574.
- Hatcher, H., R. Planalp, J. Cho, F. M. Torti, and S. V. Torti. 2008. Curcumin: From ancient medicine to current clinical trials. Cellular and Molecular Life Sciences 65 (11):1631–52. doi: 10.1007/s00018-008-7452-4.
- Hasler, C. M., and A. C. Brown. 2009. Position of the American Dietetic Association: functional foods. Journal of the American Dietetic Association 109 (4):735–46. doi:10.1016/j.jada.2009.02.023. PMID:19338113
- Howells, L. M., C. O. O. Iwuji, G. R. B. Irving, S. Barber, H. Walter, Z. Sidat, N. Griffin-Teall, R. Singh, N. Foreman, S. R. Patel, et al. 2019. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. The Journal of Nutrition 149 (7):1133–9. doi: 10.1093/jn/nxz029.
- Howells, L. M., D. P. Berry, P. J. Elliott, E. W. Jacobson, E. Hoffmann, B. Hegarty, K. Brown, W. P. Steward, and A. J. Gescher. 2011. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases–safety, pharmacokinetics, and pharmacodynamics. Cancer Prevention Research 4 (9):1419–25. doi: 10.1158/1940-6207.CAPR-11-0148.
- Hu, W.-H., G. K.-L. Chan, R. Duan, H.-Y. Wang, X.-P. Kong, T. T.-X. Dong, K. W.-K. Tsim. 2019. Synergy of ginkgetin and resveratrol in suppressing VEGF-induced angiogenesis: A therapy in treating colorectal cancer. Cancers 11:1828. doi: 10.3390/cancers11121828.
- Imperial College London. 2018. WCRF/AICR systematic literature review Continuous Update Project report: The associations between food nutrition and physical activity and the risk of colorectal cancer -Revised 2018.
- James, M. I., C. Iwuji, G. Irving, A. Karmokar, J. A. Higgins, N. Griffin-Teal, A. Thomas, P. Greaves, H. Cai, S. R. Patel, et al. 2015. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Letters 364 (2):135–41. doi: 10.1016/j.canlet.2015.05.005.
- Jantan, I., M. A. Haque, L. Arshad, H. Harikrishnan, A. W. Septama, and Z.-A. Mohamed-Hussein. 2021. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. The Journal of Nutritional Biochemistry 93:108634.
- Jesus, M. S., A. C. Carvalho, J. A. Teixeira, L. Domingues, and C. Pereira-Wilson. 2020. Ohmic heating extract of vine pruning residue has anti-colorectal cancer activity and increases sensitivity to the chemotherapeutic drug 5-FU. Foods Basel Foods 9 (8):1102. doi: 10.3390/foods9081102.
- Ji, Q., X. Liu, X. Fu, L. Zhang, H. Sui, L. Zhou, J. Sun, J. Cai, J. Qin, J. Ren, et al. 2013. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One 8 (11):e78700. doi: 10.1371/journal.pone.0078700.
- Ji, Q., X. Liu, Z. Han, L. Zhou, H. Sui, L. Yan, H. Jiang, J. Ren, J. Cai, Q. Li, et al. 2015. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer 15:97. doi: 10.1186/s12885-015-1119-y.
- Jia, F., Y. Li, X. Deng, X. Wang, X. Cui, J. Lu, Z. Pan, and Y. Wu. 2021. Self-assembled fluorescent hybrid nanoparticles-mediated collaborative lncRNA CCAT1 silencing and curcumin delivery for synchronous colorectal cancer theranostics. Journal of Nanobiotechnology 19:238.
- Jin, H., and C. Zhang. 2020. High fat high calories diet (HFD) increase gut susceptibility to carcinogens by altering the gut microbial community. Journal of Cancer 11 (14):4091–8. doi: 10.7150/jca.43561.
- Jin, J., G. Lin, H. Huang, D. Xu, H. Yu, X. Ma, L. Zhu, D. Ma, and H. Jiang. 2014. Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. International Journal of Biological Sciences 10(3):285– 95.
- Johnson, I. T., and E. K. Lund. 2007. Review article: Nutrition, obesity and colorectal cancer. Alimentary Pharmacology & Therapeutics 26 (2):161–81.
- Jozkowiak, M., P. Skupin-Mrugalska, A. Nowicki, S. Borys-Wojcik, M. Wierzchowski, M. Kaczmarek, P. Ramlau, J. Jodynis-Liebert, and H. Piotrowska-Kempisty. 2020. The effect of 4′-hydroxy-3,4,5-trimetoxystilbene, the metabolite of resveratrol analogue DMU-212, on growth, cell cycle and apoptosis in DLD-1 and LOVO colon cancer cell lines. Nutrients 12 (5):1327. doi: 10.3390/nu12051327.
- Juan, M. E., U. Wenzel, H. Daniel, and J. M. Planas. 2008. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. Journal of Agricultural and Food Chemistry 56 (12):4813–8. doi: 10.1021/jf800175a.
- Kaminski, B. M., A. Weigert, B. Brüne, M. Schumacher, U. Wenzel, D. Steinhilber, J. Stein, and S. Ulrich. 2011. Sulforaphane potentiates oxaliplatin-induced cell growth inhibition in colorectal cancer cells via induction of different modes of cell death. Cancer Chemotherapy and Pharmacology 67 (5):1167–78. doi: 10.1007/s00280-010-1413-y.
- Kee, J.-Y., Y.-H. Han, D.-S. Kim, J.-G. Mun, J. Park, M.-Y. Jeong, J.-Y. Um, and S.-H. Hong. 2016. Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine 23 (13):1680–90. doi: 10.1016/j.phymed.2016.09.011.
- Khaleel, S. A., A. M. Al-Abd, A. A. Ali, and A. B. Abdel-Naim. 2016. Didox and resveratrol sensitize colorectal cancer cells to doxorubicin via activating apoptosis and ameliorating P-glycoprotein activity. Scientific Reports 6:36855.
- Khan, I., S. Paul, R. Jakhar, M. Bhardwaj, J. Han, and S. C. Kang. 2016. Novel quercetin derivative TEF induces ER stress and mitochondria-mediated apoptosis in human colon cancer HCT-116 cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 84:789–99. doi: 10.1016/j.biopha.2016.09.094.
- Kim, E., G. A. Wright, R. S. Zoh, B. S. Patil, G. K. Jayaprakasha, E. S. Callaway, I. Ivanov, N. D. Turner, and R. S. Chapkin. 2019. Establishment of a multicomponent dietary bioactive human equivalent dose to delete damaged Lgr5+ stem cells using a mouse colon tumor initiation model. European Journal of Cancer Prevention 28 (5):383–9. doi: 10.1097/CEJ.0000000000000465.
- Kim, Y. S., M. R. Young, G. Bobe, N. H. Colburn, and J. A. Milner. 2009. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prevention Research 2 (3):200–8. doi: 10.1158/1940-6207.CAPR-08-0141.
- Kunnumakkara, A. B., P. Diagaradjane, S. Guha, A. Deorukhkar, S. Shentu, B. B. Aggarwal, and S. Krishnan. 2008. Curcumin sensitizes human colorectal cancer xenografts in nude mice to γ-radiation by targeting nuclear factor-κB–regulated gene products. Clinical Cancer Research 14 (7):2128–36. doi: 10.1158/1078-0432.CCR-07-4722.
- Langner, E., M. K. Lemieszek, and W. Rzeski. 2019. Lycopene, sulforaphane, quercetin, and curcumin applied together show improved antiproliferative potential in colon cancer cells in vitro. Journal of Food Biochemistry 43 (4):e12802. doi: 10.1111/jfbc.12802.
- Lee, S.-R., H. Jin, W.-T. Kim, W.-J. Kim, S. Z. Kim, S.-H. Leem, and S. M. Kim. 2018. Tristetraprolin activation by resveratrol inhibits the proliferation and metastasis of colorectal cancer cells. International Journal of Oncology 53 (3):1269–78.
- Lee, Y., H. Shin, and J. Kim. 2021. In vivo anti-cancer effects of resveratrol mediated by NK cell activation. Journal of Innate Immunity 13 (2):94–106. doi: 10.1159/000510315.
- Lee, Y.-J., and J. Kim. 2020. Resveratrol activates natural killer cells through Akt- and mTORC2-mediated c-Myb upregulation. International Journal of Molecular Sciences 21 (24):9575. doi: 10.3390/ijms21249575.
- Li, M., G. G.-L. Yue, S. K.-W. Tsui, K.-P. Fung, and C. B.-S. Lau. 2018. Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo. Phytomedicine 46:131–41.
- Liang, J., G. M. Hänsch, K. Hübner, and Y. Samstag. 2019. Sulforaphane as anticancer agent: A double-edged sword? Tricky balance between effects on tumor cells and immune cells. Advances in Biological Regulation 71:79–87.
- Liang, Y. C., S. H. Tsai, D. C. Tsai, S. Y. Lin-Shiau, and J. K. Lin. 2001. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Letters 496 (1):12–8. doi: 10.1016/S0014-5793(01)02393-6.
- Lin, C., Y. Yu, H.-g. Zhao, A. Yang, H. Yan, and Y. Cui. 2012. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiotherapy and Oncology 104 (3):395–400. doi: 10.1016/j.radonc.2011.10.023.
- Lin, S.-R., C.-H. Chang, C.-F. Hsu, M.-J. Tsai, H. Cheng, M. K. Leong, P.-J. Sung, J.-C. Chen, and C.-F. Weng. 2020. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. British Journal of Pharmacology 177 (6):1409–23.
- Lin, T.-A., W.-S. Lin, Y.-C. Chou, K. Nagabhushanam, C.-T. Ho, and M.-H. Pan. 2021. Oxyresveratrol inhibits human colon cancer cell migration through regulating epithelial-mesenchymal transition and microRNA. Food & Function 12 (20):9658–68. doi: 10.1039/d1fo01920a.
- Liu, Z., X. Wu, J. Lv, H. Sun, and F. Zhou. 2019. Resveratrol induces p53 in colorectal cancer through SET7/9. Oncology Letters 17 (4):3783–9.
- Lu, J.-J., Y.-J. Cai, and J. Ding. 2011. Curcumin induces DNA damage and caffeine-insensitive cell cycle arrest in colorectal carcinoma HCT116 cells. Molecular and Cellular Biochemistry 354 (1-2):247–52.
- Majumdar, A. P. N., S. Banerjee, J. Nautiyal, B. B. Patel, V. Patel, J. Du, Y. Yu, A. A. Elliott, E. Levi, F. H. Sarkar, et al. 2009. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutrition and Cancer 61 (4):544–53.
- Manach, C., A. Scalbert, C. Morand, C. Rémésy, and L. Jiménez. 2004. Polyphenols: Food sources and bioavailability. The American Journal of Clinical Nutrition 79 (5):727–47.
- Manna, S. K., A. Mukhopadhyay, and B. B. Aggarwal. 1950. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. Journal of Immunology 164:6509–19.
- Marjaneh, R. M., F. Rahmani, S. M. Hassanian, N. Rezaei, M. Hashemzehi, A. Bahrami, F. Ariakia, H. Fiuji, A. Sahebkar, A. Avan, et al. 2018. Phytosomal curcumin inhibits tumor growth in colitis-associated colorectal cancer. Journal of Cellular Physiology 233 (10):6785–98.
- Matsuno, Y., Y. Atsumi, M. Alauddin, M. M. Rana, H. Fujimori, M. Hyodo, A. Shimizu, T. Ikuta, H. Tani, and H. Torigoe. 2020. Resveratrol and its related polyphenols contribute to the maintenance of genome stability. Scientific Reports 10:5388.
- Matsuoka, T., and M. Yashiro. 2020. Precision medicine for gastrointestinal cancer: Recent progress and future perspective. World Journal of Gastrointestinal Oncology 12 (1):1–20.
- Medina-Remón, A., R. Casas, A. Tressserra-Rimbau, E. Ros, M. A. Martínez-González, M. Fitó, D. Corella, J. Salas-Salvadó, R. M. Lamuela-Raventos, R. Estruch, et al. 2017. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. British Journal of Clinical Pharmacology 83 (1):114–28. doi: 10.1111/bcp.12986.
- Mentella, M. C., F. Scaldaferri, C. Ricci, A. Gasbarrini, and G. A. D. Miggiano. 2019. Cancer and Mediterranean diet: A review. Nutrients 11:2059. doi: 10.3390/nu11092059.
- Miki, H., N. Uehara, A. Kimura, T. Sasaki, T. Yuri, K. Yoshizawa, and A. Tsubura. 2012. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. International Journal of Oncology 40 (4):1020–8.
- Mortezaee, K., M. Najafi, B. Farhood, A. Ahmadi, D. Shabeeb, and A. E. Musa. 2020. Resveratrol as an adjuvant for normal tissues protection and tumor sensitization. Current Cancer Drug Targets 20 (2):130–45. doi: 10.2174/1568009619666191019143539.
- Mudduluru, G., J. N. George-William, S. Muppala, I. A. Asangani, R. Kumarswamy, L. D. Nelson, and H. Allgayer. 2011. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Bioscience Reports 31 (3):185–97. [Database] doi: 10.1042/BSR20100065.
- Nagaraju, G. P., ed. 2020. Phytochemicals targeting tumor microenvironment in gastrointestinal cancers. Springer International Publishing.
- Nautiyal, J., S. S. Kanwar, Y. Yu, and A. P. Majumdar. 2011. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. Journal of Molecular Signaling 6 (7):7.
- Nguyen, K. A., Y. Cao, J. R. Chen, C. M. Townsend, and T. C. Ko. 2006. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Annals of Surgery 243 (5):619–27.
- Okonkwo, A., J. Mitra, G. S. Johnson, L. Li, W. M. Dashwood, M. L. Hegde, C. Yue, R. H. Dashwood, and P. Rajendran. 2018. Heterocyclic analogs of sulforaphane trigger DNA damage and impede DNA repair in colon cancer cells: Interplay of HATs and HDACs. Molecular Nutrition & Food Research 62 (18):e1800228. doi: 10.1002/mnfr.201800228.
- Parkin, D., S. Whelan, J. Ferlay, L. Teppo, and D. B. Thomas. 2022. Cancer incidence in five continents, Vol. VIII. IARC Scientific Publication No. 155.
- Patel, B. B., D. Gupta, A. A. Elliott, V. Sengupta, Y. Yu, and A. P. N. Majumdar. 2010. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Research 30 (2):319–25.
- Patel, B. B., R. Sengupta, S. Qazi, H. Vachhani, Y. Yu, A. K. Rishi, and A. P. N. Majumdar. 2008. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. International Journal of Cancer 122 (2):267–73. doi: 10.1002/ijc.23097.
- Patel, K. R., V. A. Brown, D. J. L. Jones, R. G. Britton, D. Hemingway, A. S. Miller, K. P. West, T. D. Booth, M. Perloff, J. A. Crowell, et al. 2010. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Research 70 (19):7392–9. doi: 10.1158/0008-5472.CAN-10-2027.
- Pettan-Brewer, C., J. Morton, R. Mangalindan, and W. Ladiges. 2011. Curcumin suppresses intestinal polyps in APC Min mice fed a high fat diet. Pathobiology of Aging & Age Related Diseases 1:7281.
- Raja, S. B., V. Rajendiran, N. K. Kasinathan, A. P, S. Venkatabalasubramanian, M. R. Murali, H. Devaraj, and S. N. Devaraj. 2017. Differential cytotoxic activity of Quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food and Chemical Toxicology 106:92–106. doi: 10.1016/j.fct.2017.05.006.
- Rather, R. A., and M. Bhagat. 2020. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Medicine 9 (24):9181–92. doi: 10.1002/cam4.1411.
- Ravindranathan, P., D. Pasham, U. Balaji, J. Cardenas, J. Gu, S. Toden, and A. Goel. 2018. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Scientific Reports 8 (1):13869.
- Recio-Boiles, A., and C. B. Cancer. 2020. Colon. StatPearls. Treasure Island, FL: StatPearls Publishing.
- Reilly, S. M., and A. R. Saltiel. 2017. Adapting to obesity with adipose tissue inflammation. Nature Reviews. Endocrinology 13 (11):633–43.
- Renaud, S., and M. de Lorgeril. 1992. Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet 339 (8808):1523–6. doi: 10.1016/0140-6736(92)91277-F.
- Rizeq, B., I. Gupta, J. Ilesanmi, M. AlSafran, M. M. Rahman, and A. Ouhtit. 2020. The power of phytochemicals combination in cancer chemoprevention. Journal of Cancer 11 (15):4521–33. doi: 10.7150/jca.34374.
- Roell, K. R., D. M. Reif, and A. A. Motsinger-Reif. 2017. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Frontiers in Pharmacology 8:158.
- Ryan-Harshman, M., and W. Aldoori. 2007. Diet, and colorectal cancer. Canadian Family Physician Medecin de Famille Canadien 53 (11):1913–20.
- Saab, M.-B., N. Bec, M. Martin, E. Estephan, F. Cuisinier, C. Larroque, and C. Gergely. 2013. Differential effect of curcumin on the nanomechanics of normal and cancerous Mammalian epithelial cells. Cell Biochemistry and Biophysics 65 (3):399–411.
- San Hipólito-Luengo, Á., A. Alcaide, M. Ramos-González, E. Cercas, S. Vallejo, A. Romero, E. Talero, C. F. Sánchez-Ferrer, V. Motilva, C. Peiró, et al. 2017. Dual effects of resveratrol on cell death and proliferation of colon cancer cells. Nutrition and Cancer 69 (7):1019–27.
- Sandur, S. K., A. Deorukhkar, M. K. Pandey, A. M. Pabón, S. Shentu, S. Guha, B. B. Aggarwal, and S. Krishnan. 2009. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-κB activity. International Journal of Radiation Oncology, Biology, Physics 75 (2):534–42.
- Santandreu, F. M., A. Valle, J. Oliver, and P. Roca. 2011. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cellular Physiology and Biochemistry 28 (2):219–28. doi: 10.1159/000331733.
- Sawicki, T., M. Ruszkowska, A. Danielewicz, E. Niedźwiedzka, T. Arłukowicz, and K. E. Przybyłowicz. 2021. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 13 (9):2025. doi: 10.3390/cancers13092025.
- Schwartz, B., and E. Yehuda-Shnaidman. 2014. Putative role of adipose tissue in growth and metabolism of colon cancer cells. Frontiers in Oncology 4:164.
- Sesarman, A., L. Tefas, B. Sylvester, E. Licarete, V. Rauca, L. Luput, L. Patras, S. Porav, M. Banciu, A. Porfire, et al. 2019. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Delivery and Translational Research 9 (1):260–72.
- Sharma, R. A., H. R. McLelland, K. A. Hill, C. R. Ireson, S. A. Euden, M. M. Manson, M. Pirmohamed, L. J. Marnett, A. J. Gescher, W. P. Steward, et al. 2001. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clinical Cancer Research 7 (7):1894–900.
- Sharma, R. A., S. A. Euden, S. L. Platton, D. N. Cooke, A. Shafayat, H. R. Hewitt, T. H. Marczylo, B. Morgan, D. Hemingway, S. M. Plummer, et al. 2004. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clinical Cancer Research 10 (20):6847–54. [Database] doi: 10.1158/1078-0432.CCR-04-0744.
- Shree, A., J. Islam, and S. Sultana. 2021. Quercetin ameliorates reactive oxygen species generation, inflammation, mucus depletion, goblet disintegration, and tumor multiplicity in colon cancer: Probable role of adenomatous polyposis coli, β-catenin. Phytotherapy Research 35 (4):2171–84.
- Song, J., S. Y. Cheon, W. Jung, W. T. Lee, and J. E. Lee. 2014. Resveratrol induces the expression of interleukin-10 and brain-derived neurotrophic factor in BV2 microglia under hypoxia. International Journal of Molecular Sciences 15 (9):15512–29.
- Sritharan, S., and N. Sivalingam. 2021. Curcumin induced apoptosis is mediated through oxidative stress in mutated p53 and wild type p53 colon adenocarcinoma cell lines. Journal of Biochemical and Molecular Toxicology 35: e22616.
- Su, P., Y. Yang, G. Wang, X. Chen, and Y. Ju. 2018. Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells. International Journal of Oncology 53 (3):1343–53.
- Syng-Ai, C., A. L. Kumari, and A. Khar. 2004. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Molecular Cancer Therapeutics 3 (9):1101–8.
- Tarozzi, A., C. Angeloni, M. Malaguti, F. Morroni, S. Hrelia, and P. Hrelia. 2013. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxidative Medicine and Cellular Longevity 2013:415078.
- Teng, H., Y. Zheng, H. Cao, Q. Huang, J. Xiao, and L. Chen. 2021. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: A review. Critical Reviews in Food Science and Nutrition, 1–16. doi: 10.1080/10408398.2021.1947772.
- Thomas, P., Y.-J. Wang, J.-H. Zhong, S. Kosaraju, N. J. O’Callaghan, X.-F. Zhou, and M. Fenech. 2009. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutation Research 661 (1-2):25–34. doi: 10.1016/j.mrfmmm.2008.10.016.
- Tong, X., L. Yin, S. Joshi, D. W. Rosenberg, and C. Giardina. 2005. Cyclooxygenase-2 regulation in colon cancer cells: Modulation of RNA polymerase II elongation by histone deacetylase inhibitors. The Journal of Biological Chemistry 280 (16):15503–9.
- Vendrely, V., E. Peuchant, E. Buscail, I. Moranvillier, B. Rousseau, A. Bedel, A. Brillac, H. de Verneuil, F. Moreau-Gaudry, S. Dabernat, et al. 2017. Resveratrol and capsaicin used together as food complements reduce tumor growth and rescue full efficiency of low dose gemcitabine in a pancreatic cancer model. Cancer Letters 390:91–102. doi: 10.1016/j.canlet.2017.01.002.
- Vendrely, V., S. Amintas, C. Noel, I. Moranvillier, I. Lamrissi, B. Rousseau, S. Coulibaly, A. Bedel, F. Moreau-Gaudry, E. Buscail, et al. 2019. Combination treatment of resveratrol and capsaicin radiosensitizes pancreatic tumor cells by unbalancing DNA repair response to radiotherapy towards cell death. Cancer Letters 451:1–10. doi: 10.1016/j.canlet.2019.02.038.
- Vervandier-Fasseur, D., and N. Latruffe. 2019. The potential use of resveratrol for cancer prevention. Molecules 24:4506.
- Wang, H., X. Cai, and L. Ma. 2020. Curcumin modifies epithelial-mesenchymal transition in colorectal cancer through regulation of miR-200c/EPM5. Cancer Management and Research 12:9405–15.
- Wang, J., L. Qi, L. Mei, and Z. Wu. 2017. Curcumin inhibits the proliferation and induces apoptosis in HT-29 cell lines through a reactive oxygen species (ROS)-dependent mechanism. Pakistan Journal of Pharmaceutical Sciences 30 (5):1671–7.
- Wang, Y., W. Wang, X. Wu, C. Li, Y. Huang, H. Zhou, and Y. Cui. 2020. Resveratrol sensitizes colorectal cancer cells to cetuximab by connexin 43 upregulation-induced Akt inhibition. Frontiers in Oncology 10:383. doi: 10.3389/fonc.2020.00383.
- Widmer, R. J., A. J. Flammer, L. O. Lerman, and A. Lerman. 2015. The Mediterranean diet, its components, and cardiovascular disease. The American Journal of Medicine 128 (3):229–38. doi: 10.1016/j.amjmed.2014.10.014.
- Witika, B. A., P. A. Makoni, S. K. Matafwali, L. L. Mweetwa, G. C. Shandele, and R. B. Walker. 2021. Enhancement of biological and pharmacological properties of an encapsulated polyphenol: Curcumin. Molecules 26 (14):4244. doi: 10.3390/molecules26144244.
- Wu, C.-S., S.-Y. Wu, H.-C. Chen, C.-A. Chu, H.-H. Tang, H.-S. Liu, Y.-R. Hong, C.-Y F. Huang, G.-C. Huang, C.-L. Su, et al. 2019. Curcumin functions as a MEK inhibitor to induce a synthetic lethal effect on KRAS mutant colorectal cancer cells receiving targeted drug regorafenib. The Journal of Nutritional Biochemistry 74:108227.
- Wu, J., J. Yi, Y. Wu, X. Chen, J. Zeng, J. Wu, and W. Peng. 2019. 3, 3’-dimethylquercetin inhibits the proliferation of human colon cancer RKO cells through inducing G2/M cell cycle arrest and apoptosis. Anti-Cancer Agents in Medicinal Chemistry 19 (3):402–9. doi: 10.2174/1871520618666181106120718.
- Wu, X., G. Y. Koh, Y. Huang, J. W. Crott, R. T. Bronson, and J. B. Mason. 2019. The combination of curcumin and salsalate is superior to either agent alone in suppressing pro-cancerous molecular pathways and colorectal tumorigenesis in obese mice. Molecular Nutrition & Food Research 63 (8):e1801097. doi: 10.1002/mnfr.201801097.
- Xagorari, A., A. Papapetropoulos, A. Mauromatis, M. Economou, T. Fotsis, and C. Roussos. 2001. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. The Journal of Pharmacology and Experimental Therapeutics 296 (1):181–7.
- Xavier, C. P. R., C. F. Lima, M. Rohde, and C. Pereira-Wilson. 2011. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemotherapy and Pharmacology 68 (6):1449–57.
- Yang, G., J. Qiu, D. Wang, Y. Tao, Y. Song, H. Wang, J. Tang, X. Wang, Y. U. Sun, Z. Yang, et al. 2018. Traditional Chinese medicine curcumin sensitizes human colon cancer to radiation by altering the expression of DNA repair-related genes. Anticancer Research 38:131–6.
- Yang, H., G. G. L. Yue, P. C. Leung, C. K. Wong, and C. B. S. Lau. 2022. A review on the molecular mechanisms, the therapeutic treatment including the potential of herbs and natural products, and target prediction of obesity-associated colorectal cancer. Pharmacological Research 175:106031.
- Yang, J., T. Z. Li, G. H. Xu, B. B. Luo, Y. X. Chen, and T. Zhang. 2013. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 60 (4):364–72. doi: 10.4149/neo_2013_048.
- Yin, T.-F., M. Wang, Y. Qing, Y.-M. Lin, and D. Wu. 2016. Research progress on chemopreventive effects of phytochemicals on colorectal cancer and their mechanisms. World Journal of Gastroenterology 22 (31):7058–68.
- Yuan, L., M. Zhou, D. Huang, et al. 2019. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial- mesenchymal transition via the AKT/GSK-3β/Snail signaling pathway. Molecular Medicine Reports 20:2783–95.
- Zhang, T., P. Zheng, X. Shen, R. Shao, B. Wang, H. Shen, J. Zhang, Y. Xia, and P. Zou. 2019. Curcuminoid WZ26, a TrxR1 inhibitor, effectively inhibits colon cancer cell growth and enhances cisplatin-induced cell death through the induction of ROS. Free Radical Biology & Medicine 141:93–102.
- Zheng, X., X. Yang, J. Lin, F. Song, and Y. Shao. 2021. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine 85:153547. doi: 10.1016/j.phymed.2021.153547.
- Zhou, Y., J. Zhang, K. Wang, W. Han, X. Wang, M. Gao, Z. Wang, Y. Sun, H. Yan, H. Zhang, et al. 2020. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. European Journal of Pharmacology 881:173185.
