Abstract
Coffee is one of the most consumed beverages in the world. Coffee provides to the consumer special sensorial characteristics, can help to prevent diseases, improves physical performance and increases focus. In contrast, coffee consumption supplies a significant source of substances with carcinogenic and genotoxic potential such as furan, hydroxymethylfurfural (HMF), furfural (F), and acrylamide (AA). The present review addresses the issues around the presence of such toxic substances formed in Maillard reaction (MR) during thermal treatments in food processing, from chemical and, toxicological perspectives, occurrences in coffee and other foods processed by heating. In addition, current strategies advantages and disadvantages are presented along with application of molecular imprinting technology (MIT) and poly (ionic liquid) s (PIL) as an alternative to reduce the furan, HMF, F and AA content in coffee and other foods.
Introduction
Coffee is one of the most appreciated and consumed beverages in the world (Folmer et al. Citation2017; Mussatto et al. Citation2011; Monsalve-Atencio et al. Citation2021), being Finland the largest consumer with an average of 12 kilograms of coffee per inhabitant per year, followed by Iceland, Norway, Sweden and Denmark, reaching an average of 9 kilograms of coffee per year per inhabitant (Bermudez Citation2020). Coffee is and will remain an inspiration to humanity with its trade and consumption (Vegro and de Almeida Citation2020); It has become relevant to consumers not only as an energy source but also as an important instrument for socialization and it is perceived as a symbol of contemporary life (R. Rodrigues, de Almeida, and Spers Citation2020).
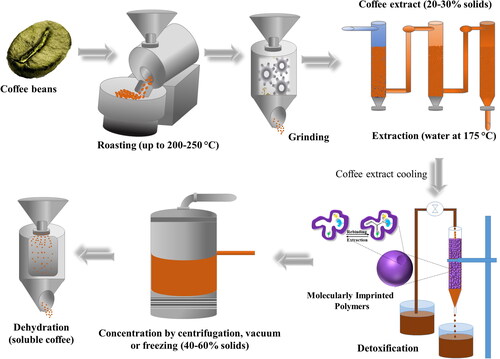
The coffee drink is usually obtained from the extraction of soluble solids from roasted and ground coffee beans. To obtain roasted coffee it is necessary to use green coffee as a starting material, with a typical humidity percentage of 10 − 12% which is subjected to the roasting process in which temperatures close to 200 − 250 °C are required for up to 20 min in order to generate the typical reactions of coffee roasting; this usually ends with a cooling process after losing between 12 − 20% of weight (Poisson et al. Citation2017). During this process physical changes closely related to flavor are generated in the grain such that, the color can vary from a blue-greenish gray to almost black.
Some important chemical reactions include MR involving amino acids and reducing carbohydrates (Henle, Walter, and Klostermeyer Citation1991), in which furanic compounds (FC) (Bedoya-Ramírez et al. Citation2017; Altaki, Santos, and Galceran Citation2011) and AA (Anese and Suman Citation2013) are generated, Streker degradation, pyrolysis, and caramelization also occur, as well as denaturation and degradation of proteins and acids (Schenker and Rothgeb Citation2017). This review describes some aspects related to the formation of FC and AA in coffee, including chemical, toxicological, and health factors; as well as some relevant strategies for the elimination of these substances from coffee and other processed foods, which can affect the sensory quality of coffee and have not shown good effectiveness and industrial applicability. For this reason, more efficient and industrially adapted methodologies are required so that sensory quality of coffee is not affected. For the first time, the applications of MIT and PIL used for the extraction of FC and AA from different matrices, especially in coffee, are analyzed in detail, and presented as a novel alternative, ecological and promising for the industrial detoxification of coffee. Also, aspects such as physicochemical interactions, biodegradability, toxicity and industrial application are considered important factors for an efficient environmental design.
Maillard reaction
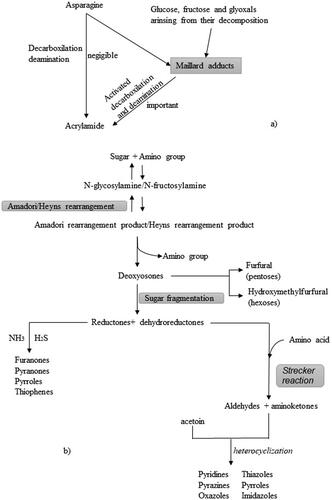
The MR corresponds to a network of various non-enzymatic reactions between reducing sugars and compounds with a free amino group (Maillard Citation1912), which can occur simultaneously and they can be influenced by each other as well as by milieu parameters (Wrodnigg and Eder Citation2001). In order to better understand the complexity of the reaction, MR is subdivided into three stages: early stage, intermediate stage, and final stage (Nursten Citation2005). During early stage,a condensation of amino groups and a reducing sugars occurs via Schiff’s base formation, leading to the formation of an N-glycosylamine in the case of an aldose sugar, followed by Amadori rearrangement (Nursten Citation2005) or Heyns product rearrangement if the reducing sugar is a ketose (Van Citation2006).The intermediate stage, starts from the product formed in the previous stage (Amadori or Henys) and includes the dehydration and fragmentation of sugar and the breakdown of amino acids (Strecker breakdown) releasing the amino group (Nursten Citation2005). There are three main different breakdown routes depending on the pH of the environment: (1) The 3-deoxyosone-pathway via the 1,2 enolisation route under acidic environments. (2) The 1-deoxyosone-pathway via the 2,3 enolisation route under neutral and alkaline environments. (3) The 4-deoxyosone-pathway under slightly alkaline conditions, and it is less common than the other two pathways (Van Boekel Citation1998). During the final stage the intermediate products previously generated react with each other via polymerization forming heterocyclic nitrogenous brown compounds (melanoidins) which have high molecular weights (up to about 100,000 g/mol) (Nursten Citation2005). Dehydration, fragmentation, cyclization, and polymerization reactions occur at this stage (Van Citation2006). During the roasting process, the MR is carried out in which compounds are generated with a direct impact on the organoleptic properties of the coffee (Belitz and Grosch Citation1999).
Formation of acrylamide and furanic compounds
Some of the products of the MR are AA (Anese and Suman Citation2013) and FC (furan, HMF, and F) (Altaki, Santos, and Galceran Citation2011) as shown in . One of the most important routes for furan formation is MR (Akıllıoğlu, Bahçeci, and Gökmen Citation2015) through thermal degradation and reorganization of sugars; however, there are other routes such as thermal oxidative degradation of ascorbic acid and polyunsaturated fatty acids (Rannou et al. Citation2016). HMF and F are formed in the second stage of MR from the degradation of Amadori products when hexoses or pentoses, respectively, are heated in the presence of amino acids or proteins, and can also be formed by caramelization (Gökmen and Morales Citation2014; Ho Citation1996) at high temperatures under acidic conditions (Gökmen and Morales Citation2014). As for AA, these can also be formed during the intermediate stage of MR by degrading asparagine in the presence of reducing sugars (Gökmen Citation2015; Stadler et al. Citation2004).
Figure 1. Maillard reaction pathways associated with the generation of (a) AA, (b) FC, and other compounds in coffee. Adapted from Ho (Citation1996) and Richarme et al. (Citation2016).
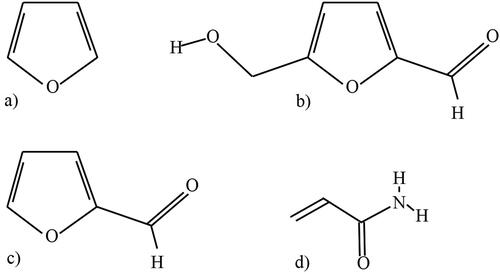
Occurrence and toxicological aspects of AA and FC of coffee
FC are characterized by the furan ring having one or more substitutes, as can be seen in , which shows the chemical structures of some important FC and AA. One of the FC corresponds to HMF, which is a multifunctional molecule highly soluble in water, ethanol, methanol, ethyl acetate, and petroleum ether (Gökmen and Morales Citation2014) that has several structural clues that present possible genotoxic and carcinogenic risks such as the furan ring, α, β-unsaturated carbonyl group, and an allyl hydroxyl group. Several studies have shown that HMF can induce genotoxic and mutagenic effects in bacterial and human cells and promote colon and liver cancer in rats and mice (Monien et al. Citation2012), and coffee is known to be the most important source of HMF in the diet (Arribas-Lorenzo and Morales Citation2010). As can be seen in , authors have evaluated the content of HMF in soluble and roasted ground coffee, the values of which ranged from 23.3 − 4112 mg/kg, and the content of HMF was significantly higher in soluble samples (p < 0.05) (Bedoya-Ramírez et al. Citation2017; Contreras-Calderón et al. Citation2016). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) defined a Tolerable Daily Intake (TDI) de 0.54 mg/person/day for HMF (JECFA Citation1996), while the Scientific Panel on food additives, flavorings, processing aids and materials in contact with foods (AFC) estimated a dietary HMF intake of 1.6 mg/person per day based on an mTAMDI (modified Theoretical Added Maximum Daily Intake)- approach (EFSA Citation2005). In contrast, Arribas-Lorenzo and Morales (2010), found an intake of 8.57 mg HMF/person per day due to high coffee consumption habits, which exceeds even up to more than 15 times the value established according to the TDI, representing a health risk, similar to that reported in the research of Rodrigues and Bragagnolo (2013), where the consumption of a cup (100 mL) of regular roasted ground coffee, decaffeinated roasted ground coffee, regular soluble coffee, and decaffeinated soluble coffee could supply 0.4, 0.6, 4.6, and 6.0 mg of HMF ().
Table 1. Occurrence and Tolerable Daily Intake (TDI) o suggested intake of AA and FC in coffee (Arribas-Lorenzo and Morales Citation2010; JECFA Citation1996; Rodrigues and Bragagnolo Citation2013; Chaichi et al. Citation2015; EFSA Citation2004; Rahn and Yeretzian Citation2019; Altaki, Santos, and Galceran Citation2011; Arisseto and Vicente Citation2015; Soares, Alves, and Oliveira 2015).
Furfural, also called 2-furaldehyde or furan-2-carbaldehyde, is a water-soluble aldehyde and consists of a furan ring in which the hydrogen in position 2 was replaced by a formyl group; it is known to be harmful to the eyes, mucous membranes, and skin (Abraham et al. Citation2011) and has genotoxic potential (Chávez-Servín, Castellote, and López-Sabater Citation2005). As shown in , F content has been found in soluble and roasted ground coffee with values from 18 − 135 mg/kg (Bedoya-Ramírez et al. Citation2017; Contreras-Calderón et al. Citation2016). Likewise, other authors found concentrations of up to 8.2 mg/L in brewed coffee (0.82 mg/100 mL cup) (Chaichi et al. Citation2015), so that the intake of one cup could exceed the TDI values established by the European Food Safety Authority (EFSA) corresponding to 0.5 mg/person per day (EFSA Citation2004).
On the other hand, furan was found to induce hepatocellular carcinomas and adenomas in rats and mice, and a high incidence of cholangiocarcinomas in rats at doses higher than 2 mg/kg per body weight (Moro et al. Citation2012). In 1995, the International Agency for Research on Cancer (IARC) classified furan as possibly carcinogenic to humans (group 2B) (IARC. Citation1996) and concentrations of furan in whole roasted grains have been found in the range of 2 − 7 mg/kg (Guenther et al. Citation2010). In the case of furan, the Panel on Contaminants in the Food Chain (CONTAM) decided that it was not appropriate to establish a TDI and used a margin of exposure (MOE) approach, which establishes the margin between a dose causing cancer in animal studies and the estimated human exposure to the substance, it has been considered that a MOE of 10,000 or higher would be of low concern from a public health point of view and might reasonably be considered as a low priority for risk management actions, however, for non‐neoplastic effects, the calculated MOEs for furan are below 100, which indicates a health concern (CONTAM et al. 2017). Rahn and Yeretzian (2019) found up to 99.05 µg/L of furan in brewed coffee. Other authors have found that daily intake of furan through coffee consumption corresponds to 2.1 − 26.6 µg/person per day (), considering a body weight of 70 Kg (Altaki, Santos, and Galceran Citation2011).
It is well known that HMF can generate AA from asparagine more efficiently than glucose in a model system (Gökmen et al. Citation2012). AA also known as 2-propenamide has a toxic potential in tissues, including in the reproductive and urinary systems. It is defined as group 2 A carcinogen by the IARC and studies in rodents show that it produces tumors in the liver, ovary, breast, thyroid, and is a neurotoxic compound (Çebi Citation2016). Currently, the European Union (EU) laid down a regulation establishing mitigation measures and reference levels to reduce the presence of AA in roasted and soluble coffee, which are 400 and 850 μg/kg, respectively (European Commission Citation2017). According to a report by the EFSA, the average content of AA in instant coffee was 1123 μg/kg (EFSA. Citation2012), value higher than the allowed limit according to EU regulations, and it has also been shown that the contribution of coffee to exposure to AA is up to 40% in countries that consume a lot of coffee (EFSA. Citation2011). As a genotoxic carcinogen, acrylamide is considered to have no threshold limit of exposure, i.e. a single exposure to one molecule of carcinogen can trigger the biological process leading to cancer (Felsot Citation2002), therefore, these compounds have been considered not to have a threshold in the dose-response relationship; i.e., there is not a dose or intake below which the risk is null (O’Brien et al. Citation2006). However, Arisseto and Vicente (Citation2015) recommend that when considering morphological changes in nerves, intakes greater than 1.4 μg/person per day (considering a body weight of 70 Kg) could suggest a higher concern; while in terms of reproductive, developmental, and other non-neoplastic effects, intakes higher than 14 μg/person per day () (considering a body weight of 70 Kg) should not be exceeded, which would result in MOEs lower than 10,000, showing high concern. For acrylamide a MOE of 300 for average consumers and of 75 for high consumers has been estimated, which indicates a health concern (WHO. Citation2005). Considering the consumption of roasted coffee beverages and a body weight of 70 Kg, the exposure to acrylamide varied from 0.21 − 11.97 and 4.13 to 31.92 μg/person per day (), for average and high consumers, depending on the country, respectively (Arisseto and Vicente Citation2015), exceeding up to 22 times the previously suggested intake. Likewise, other authors found that the acrylamide levels for different coffee beverages prepared from different brewing techniques can range from 6.0 − 75 μg/L de beverage (Soares, Alves, and Oliveira 2015), so the consumption of a cup of coffee could suggest health concern.
When considering the concentrations of furan, HMF, F, and AA in coffee beverages, as well as MOE values and intake of these toxic compounds from coffee consumption, it can be observed that exceed the TDI limits and MOE defined for each of these, which evidences that the consumption of coffee could represent a high concern and risk to health as mentioned above, however, in addition to the toxic substances that can be found in coffee, there are also bioactive compounds such as chlorogenic acids, trigonelline, melanoidins, hydroxycinnamic acids, and caffeine, of which there are evidence that they can reduce the risk of various diseases (Chronic-degenerative diseases such as Parkinson’s and Alzheimer’s, cirrhosis, asthma, type 2 diabetes and cardiovascular diseases.) and improve performance mental and physical (Folmer et al. Citation2017). Considering this, it is imperative to obtain coffees from which, consumers, can enjoy its antioxidant properties and health benefits without be exposed to the risk of ingesting toxic substances from it.
Mitigation strategies for acrylamide and furanic compounds in coffee
Due to the previously commented, different strategies have been applied to reduce or eliminate the contents of these coffee contaminants (Anese Citation2016; Rannou et al. Citation2016; Anese Citation2015; Anese and Suman Citation2013; Anese et al. Citation2013; Capuano and Fogliano Citation2011). One of the mitigation strategies in coffee consists of applying a medium or light degree of roasting (100 − 110 lange reflectance units (LRUs) for 240 s, where lower LRU values indicate darker roast colors) (Pavesi et al. Citation2011; Guenther et al. Citation2010), which compared to treatment with dark roasting (40 − 50 LRUs for 240 s), can reduce up to approximately 41% of the content of furan; however, this method has the disadvantage, since it increases the AA levels (Guenther et al. Citation2010). In the same way, an attempt has been made to substitute fine grinding for coarse grinding since it favors the reduction of the furan content (Crews et al. Citation2009); however, it has the disadvantage that there is low extraction (Castaño, Quintero, and León Citation2000). Evaporation has also been used for the removal of FC content using coffee with fine particle sizes and prolonged exposure to air at a relatively high temperature for 4 days achieving reductions of up to 20% (Guenther et al. Citation2010). However, although the elimination percentage is not high, there is a disadvantage related to the loss of processing time. In another study, vacuum was applied at 2.7 kPa and 60 °C for 10 min with a previous hydration step up to an aw of 0.7, achieving HMF and F eliminations of 20% and 100%, respectively. However, it is noteworthy that the vacuum treatments caused a significant decrease in the headspace of the total volatiles of coffee, which in turn was responsible for a lower odor intensity of the samples (Quarta and Anese Citation2012). On the other hand, the mitigation of HMF and AA in coffee by fermentative action of Saccharomyces cerevisiae has been investigated, finding that after 24 h the concentration of HMF was reduced by 61.2%, 75.7%, 93.6% and 99.2% in the fermentation media containing 0, 1, 5 and 10% of sucrose, respectively; after 48 h the AA concentration was reduced by approximately 70% (Akıllıoglu and Gökmen Citation2014). Nonetheless, this operation involves considerable extensions of process time and it is noted that no sensory analysis has been performed as in the work reported by Bedade, Sutar, and Singhal (Citation2019) in which an system of columns packed with chitosan-coated calcium alginate beads and functionalized with citric acid was developed, in which acrylamidase was covalently immobilized. The system allowed the elimination of AA in soluble coffee samples due to its degradation in acrylic acid and ammonia by the enzymatic action of acrylamidase. However, in this study, FC are not considered and the production, extraction, and purification stages of acrylamidase by fermentation are also required. In addition, the reuse of the system could be limited by the decrease in enzyme activity due to the accumulation of products in the beads during the enzyme reaction. As well as the latter, in several works they do not report sensory evaluations of the drink after being subjected to these treatments and that is so more studies are required to reveal the process conditions capable of minimizing the loss of sensory properties.
Alternative methods for the extraction of acrylamide and furanic compounds from coffee and other foods
Poly (ionic liquid)s
Overview
A possible innovative alternative for the decontamination of coffee concerning the previously commented procedures could refer to the use of polymeric/polymerized ionic liquids or poly (ionic liquid) s (PIL), which refer to a special type of polyelectrolytes generally based on the polymerization of ionic liquid monomers (ILM), that is, from ionic liquids (IL) that have polymerizable functional group. Thus, the cationic or anionic centers are constrained to the repeating units in the polymer chain, so, PIL have the advantage of combining their mechanical and thermal properties with some of the IL valuable properties (Patinhaa, Silvestre, and Marrucho 2019; Yuan and Antonietti Citation2011; Mei, Huang, and Chen Citation2019). For its part, IL corresponds to a group of salts that melt at temperatures below 100 °C or at room temperature, called Newton liquids (Khan et al. Citation2020). They are also defined as supramolecular nanostructures of type [(X)n+z (A)n)]z+ o [(X)n (A)n+z)]z-, where X represents the organic cation and A represents the anion (Gozzo et al. Citation2004). Among its properties of interest are self-assembly capacity, low vapor pressure, non-flammability, thermal stability, and a wide range of liquid phase (Das and Roy Citation2013). They are known as green media due to their nonvolatile nature and high chemical and thermal stability (Dong et al. Citation2015), which is why they have low levels of atmospheric pollution (Alviz and Alvarez Citation2017).
Conventionally they consist of large organic cations (for example, imidazolium, pyridinium, pyrrolidinium or quaternary ammonium) and small organic or inorganic anions (for example, trifluoromethylsulfonate, trifluoroethanoate or Cl-, Br-, PF6-) (Berthod, Ruiz-Ángel, and Carda-Broch Citation2018) that generally have certain levels of toxicity (Hartmann and Pereira Citation2016) and not all of them are biodegradable (Hou et al. Citation2013). However, the toxicity of food products contaminated with residual IL has not been confirmed (Martins, Braga, and de Rosso Citation2017) and furthermore, it has been reported that low doses of some IL in animal and in vitro tests did not cause cell damage (Martins, Braga, and de Rosso Citation2017). On the other hand, a new generation of biodegradable and reduced toxicity IL stands out (Hou et al. Citation2013) with cations based on amino acids (Tao et al. Citation2005) and choline (Hou et al. Citation2013), of which some may be edible (Vraneš et al. Citation2019; Schubert Citation2017); in the same way, anions of amino acid-based IL (Kirchhecker and Esposito Citation2016; Hou et al. Citation2013) and sugar-based IL have been developed (Chiappe, Marra, and Mele Citation2010).
According to life cycle studies, few IL are toxic and non-biodegradable (Hospido and Rodríguez Citation2019), which are important aspects for the care of the environment. Generally has been reported that as the length of the anion or the number of atoms in the alicyclic ring increases, the alkoxy or alkyl chain of the cation also increases the level of toxicity of IL, which could be related to the permeabilization of the biological membrane given the level of lipophilicity that increases with the length of the chain or number of atoms, contrary to what happens when symmetric chains are present in the cation since it has a steric effect on the cell surface, which leads to a decrease in the level of toxicity (Hartmann and Pereira Citation2016), which may also be favored by the inclusion of groups hydrophilic poles such as hydroxyls, carbonyls, amino and ether that also increase biodegradability, which in amino acids and alkanoates decreases with the presence of branched chains (Hou et al. Citation2013).
Conventional IL can be synthesized by different methods that generally include neutralization, quaternization, and ion exchange reaction. For its part, protic IL are synthesized by the reaction of an acid and a Brønsted base that involves the transfer of a proton. Aprotic IL can be synthesized including quaternization and metathesis reactions. They can also be functionalized by including functional groups such as − OH, −OR, −SH, NH2, among others (Ozokwelu et al. Citation2017a). There are also unconventional preparations that include microwave radiation and ultrasound-assisted reactions (Singh and Savoy Citation2020). In the particular case of IL-based on amino acids, they can be manufactured by protonation (if the amino acid is used as a cation) through potentiometric titration, alkylation of the amino group of amino acids and amino acid as nitrogen donors; or by neutralization (if the amino acid is used as an anion) through anion exchange or potentiometric titration and subsequent neutralization with an equimolar amount of amino acid (Kirchhecker and Esposito Citation2016). Opposite charges bind the cation and anion of IL. The lattice energy of crystalline compounds is proportional to the inverse of the distance between their lattice points, which is relatively short in ionic salts, so they have high reticular energy and consequently easy crystallization, unlike the IL whose complex shapes (larger multi-atomic cations and anions) is responsible for the largest distance between their lattice points, so that the asymmetry of IL ions reduces the lattice energy of the crystalline structure resulting in a low-melting-point salt, and it does not allow ions to easily form crystalline, stable, and ordered structures (Ozokwelu et al. Citation2017b; Doble and Kruthiventi Citation2007; Mehrkesh and Karunanithi Citation2016).
Different polymerization techniques are used in the synthesis of PIL, such as conventional and controlled radical polymerizations, step-growth polymerization, ring opening metathesis polymerization, among others. Similarly, two main strategies highlight, which consist of direct polymerization of ILM; and chemical modification of existing polymers. Typical ILM polymerization consists of conventional free radical polymerization using (meth)acryloyl, N-vinylimidazolium, or styrenic-based ILM, which can be homo- or copolymerized, or used as crosslinkers. Similarly, in recent years PIL have been developed via controlled/"living" radical polymerizations, focusing on atom transfer radical polymerization (ATRP) and reversible addition fragmentation chain transfer (RAFT), which have the advantage of precisely designing and controlling the macromolecular architecture of IL species on a meso-/nanoscale within a polymer matrix (Yuan and Antonietti Citation2011).
IL and PIL have an industrial appeal (Martins, Braga, and de Rosso Citation2017) explained by their costs and recyclability (de Melo et al. Citation2017), non-corrosive, green and ecological nature (Singh and Savoy Citation2020), with less toxicity and biodegradability (Toledo-Hijo et al. Citation2016; Hou et al. Citation2013), which is why they are considered ideal extraction mediums (Mei, Huang, and Chen Citation2019), friendly to the environment and allow extraction of various metabolites of the food industry, demonstrating that they can improve selectivity, efficiency and present a promising outlook for extraction on an industrial scale (Toledo-Hijo et al. Citation2016). Few applications of IL have been presented in the food industry compared to other academic or industrial fields and the literature on food science and technology involving IL is still scarce (Toledo-Hijo et al. Citation2016). The use of IL and PIL has been integrated with some techniques such as solid phase microextraction (SPME) and dispersive liquid-liquid microextraction (DLLME) (Kissoudi and Samanidou Citation2018; Nawała et al. Citation2018). Different substances of interest for the food industry have been one of the main objectives in analysis and extraction processes, among them are phenolic compounds, esters, heterocyclic aromatics, pesticides, estrogens, organic acids (Mei, Huang, and Chen Citation2019), essential oils, piperine, caffeine, fatty acids, and other food additives (Toledo-Hijo et al. Citation2016).
Application of ionic liquids and poly (ionic liquid) s in the extraction of acrylamide and furanic compounds
Regarding the application of IL and PIL in the extraction of the toxic substances treated in the present work such as AA and FC, mention can be made of some few studies such as the recently reported green method, where liquid-based vortex-forced matrix solid phase dispersion (IL-VFMSPD) was used to extract HMF from the dried ripe sarcocarp of the Cornus Officinalis plant, using ultra-high performance liquid chromatography; IL was used as a green elution reagent in a VFMSPD process with recoveries in the range of 95.2 − 103% (RSD < 5.0%); the results showed that the method was applied efficiently to extract and determine the HMF from the study matrix (Du et al. Citation2018). In another work, the concentration of a furfuryl in red wine samples was successfully determined with the help of stationary phase coatings for solid phase microextraction (SPME) based on PIL, so that when compared to a commercial reference of polydimethylsiloxane of similar film thickness, higher recovery percentages were achieved (Zhao, Meng, and Anderson Citation2008).
On the other hand, few cases of use of PIL for the extraction and determination of some toxic substances have been reported in coffee; however, to the author’s knowledge, until now only analytical studies have been reported and not as extraction processes or industrial detoxifications. shows some structural characteristics of the IL and PIL used for the extraction of FC and AA in coffee, which have been reported in different studies such as that reported by Cagliero, Nan, et al. (2016) in which a PIL-based sorbent coating for SPME was designed and successfully applied for the determination of the trace level of AA in brewed and powdered coffee using gas chromatography/mass spectrometry (GC-MS), where all PIL fibers showed excellent analytical precision and linearity, demonstrating matrix compatibility of PIL-based fibers with real complex samples. Cagliero, Ho, et al. (Citation2016) developed a PIL coating method on the SPME support, which demonstrated superior sensitivity in AA extraction compared to all commercially available SPME coatings and achieved results comparable to the international organization for standardization (ISO) method in the analysis of coffee powder samples. Similarly, Zhang et al. (Citation2017) used IL to extract AA from coffee samples prepared by DLLME that was coupled to gas chromatography (GC) and the effect of different structural characteristics of IL on extraction efficiency was evaluated, which exhibited good analytical precision, linearity and detection limits. Regarding the analysis of F and other FC in coffee, stands out the work carried out by López-Darias et al. (2011), in which coatings were used as sorbents for SPME based on PIL, which demonstrated an exceptional selectivity and extraction efficiency. Similarly, Toledo et al. (Citation2014) used PIL-based sorbent coatings for SPME of coffee aromatics among which some furans were identified.
Table 2. Structural characteristics of some imidazolium-based ILs used in the extraction of AA and FC, mainly from coffee.
Nondestructive extraction techniques based on poly (ionic liquids) s
To detoxify coffee in terms of AA and FC content, methods that provide the possibility of industrial application are required, which forces them not to be destructive with the matrix and to have appropriate scalability characteristics. Regarding PIL, there has been increasing interest in their integration with solid phase extraction (SPE) techniques (Marcinkowska et al. Citation2019; Patinhaa, Silvestre, and Marrucho 2019; Mei, Huang, and Chen Citation2019; Kissoudi and Samanidou Citation2018); however, few nondestructive techniques stand out with the sample and those techniques such as stir bar sorptive extraction (SBSE), stir cake sorptive extraction (SCSE), stir bar dispersive liquid microextraction (SBLME), hollow fiber-based liquid phase microextraction (HF-LPME), and headspace solid-phase microextraction (HS-SPME) that in some cases do not require substantially modifying the nature of the sample, usually present only analytical approaches and not industrial applications. However, some may be scalable, as is the case of the work carried out by Fiscal-Ladino et al. (2017), in which rotating disk sorption extraction (RDSE) was used for the extraction of low polarity compounds in wastewater samples, for which a new ecological material based on montmorillonite clays modified with ionic liquids was successfully used. Another similar and interesting application that turned out to be simple and profitable, refers to a new extraction device based on an SCSE approach for the extraction of a cocaine metabolite for which massive in situ polymerization of a molecularly imprinted polymer (MIP) was proposed in the surface of a polytetrafluoroethylene (PTFE) membrane placed between two magnetic rings (Sorribes-Soriano et al. Citation2019). Although they did not use PIL in this study, they used MIT, which is a technique that, integrated with IL, is promising given its applicability with industrial approaches and high specificity, as discussed below.
Molecular imprinting technology
Overview
Over time, traditional liquid-liquid extraction processes have been replaced by SPE that uses sorbents which are not usually selective and retains other compounds different than the analyte (Turiel and Martín- Esteban Citation2020). MIT presents a solution to this problem, through which MIP can be obtained, which are smart materials that are designed with the purpose of improving selectivity in the extraction of organic and inorganic analytes from many complex matrices, such as food, blood, urine and wastewater (Madikizela et al. Citation2018); since corresponding to synthetic analogs of the natural antigen-antibody biological systems and operate through a "key-lock" mechanism to selectively bind the molecule with which they were molded during production in preference to other closely related compounds (Turiel and Martín- Esteban Citation2020; BelBruno Citation2019), for which functional monomers (FM) and crosslinkers are polymerized around a template molecule (usually corresponds to the target molecule), generally in a porogenic solvent medium and initiator (Pichon, Delaunay, and Combès 2020), thus having a highly crosslinked three-dimensional polymer network, which is assembled through various interactions, such as the van der Waals forces, hydrogen bonding, π-π stacking, electrostatic and hydrophobic interaction (Ding et al. Citation2020). Subsequently, the template molecule is extracted, leaving binding sites with the shape, size, and functionalities within the complementary polymer network to the target compound, which allows rebinding of the analyte (Turiel and Martín- Esteban Citation2020).
There are different approaches to MIP synthesis, including covalent, semi-covalent, and non-covalent that relate to the type of bond formation between the template and monomers before polymerization, with the non-covalent approach being most used for the synthesis of MIP (Turiel and Martín- Esteban Citation2020) since it facilitates the elution of the template molecule at the end of the synthesis (Pichon, Delaunay, and Combès 2020). Typically there are different MIP preparation methods such as bulk manufacturing that involves grinding and is one of the most widely used due to its simplicity; precipitation polymerization that facilitates control over shape and size; suspension polymerization allowing to obtain spherical beads in a wide size range; iniferter and emulsion polymerization that increase monodispersibility helping to stabilize the particles to a single size; in molecular surface imprinting, emulsion polymerization is typically used to obtain MIP coatings on preformed particles consisting of solid cores such as silica spheres, polystyrene beads, and Fe3O4 that impart properties such as shape, fluorescence, porosity, and magnetism that facilitate the separation of the nanoparticles with the application of a magnetic field using a magnet, and direct synthesis in situ of a monolith by mass polymerization, which avoids the grinding step (Wackerlig and Schirhagl Citation2016; Kosheleva, Mitropoulos, and Kyzas Citation2019; Pichon, Delaunay, and Combès Citation2020).
The generation of MIP involves the creation of cavities containing functional sites within a highly cross-linked polymer matrix complementary to the template molecule. There is an increase in the number of MIP that have been developed with multi-templates where more than one target compounds are imprinted simultaneously (Chen et al. Citation2020). Likewise, the use of a single dummy template that is structurally related to two or more target compounds is being explored, which allows optimizing the use of MIP by minimizing the extraction phase needed to extract multiple structurally related compounds (Ncube et al. Citation2017). There has been a steady growth in the number of ionic liquids that have investigated as functional monomers for the synthesis of MIP due to their properties that are in line with green chemistry principles (Madikizela et al. Citation2018). However, some of the monomers typically used comprise functional groups such as 2 or 4-vinylpyridine (VPy), methacrylic acid (MAA), methacrylamide (MAAM), and N-Vinylpyrrolidone (NVP) which are selected when considering acidic or basic properties of the template molecule and the FM; for example, amides have been observed to have high selectivity with monomers containing primary amide (Turiel and Martín- Esteban Citation2020). On the other hand, some conventional crosslinking agents involve, for example, divinylbenzene (DVB), trimethylolpropane trimethacrylate (TRIM), and ethylene glycol dimethacrylate (EGDMA), the latter being one of the most widely used (Turiel and Martín- Esteban Citation2020). As a porogen, a non-polar or moderately polar aprotic solvent is usually used, such as acetonitrile, dichloromethane or toluene. However, there are also reports of the use of polar and protic media, such as methanol, ethanol and water; the use of the latter is considered a green approach for the production of MIP, as occurs in the case of the use of IL as porogens and FM (Pichon, Delaunay, and Combès Citation2020).
Complete desorption of the template molecule is very important because if it is not done correctly, it implies a decrease of cavities available for re-binding, which decreases the extraction efficiency. For re-usability, MIP are easily regenerated by simple washing with organic solvents such as acetic acid and methanol, and they show high chemical stability in those chemicals (Madikizela et al. Citation2018). Generally, MIP are often used in various extraction techniques such as SPE, dispersive solid phase extraction (d-SPE), magnetic solid phase extraction (SCSE), SPME, and SBSE (Speltini et al. Citation2017).
Molecularly imprinted polymers based on ionic liquids and their industrial application
With selectivity characteristics, desired molecular binding, physical robustness, re-usability, chemical and mechanical stability, easy preparation, and low cost (Turiel and Martín- Esteban Citation2020; Gunasekara and Zhao Citation2017; Madikizela et al. Citation2018), MIP can be used to carry out extractions or purifications at an industrial level and can be produced cost-effectively in large amounts and provide for long-time storage (Wackerlig and Schirhagl Citation2016), shows some examples of research with analytical application and industrial approach for the use of molecularly imprinted polymers based on ionic liquids monomers (IL-MIP). Some aspects that must be considered for the cost estimation for the industrial application of MIP correspond to the preparation cost of the MIP, removal capacity from the target, volume of fluid treated per unit mass of MIP, and reusability or number of cycles, which is variable and depends on factors such as method of production, FM, porogens, templates, solvents or surface modifiers, or crosslinkers, commercial availability, among others. However, different authors have concluded that the application of MIP presents excellent prospects due to its low cost in removal processes with an industrial approach, of substances such as pesticides in tea (Chen et al. Citation2020), cholesterol in milk (Kartal and Denizli Citation2020), lysozyme in egg white (Wei et al. Citation2019), quinoline in octane (Yang et al. Citation2015), wastewater treatment (Huang et al. Citation2015; Shen, Xu, and Ye Citation2013; Qu et al. Citation2020), heavy hetal ions and toxic dyes in water contaminated (Sharma and Kandasubramanian Citation2020), of which authors have calculated and demonstrated the cost-effectiveness of the material compared to commercial activated carbon (Krupadam, Khan, and Wate Citation2010). Most commercial applications of IPMs involve separation materials, which are led by companies such as MIP Technologies, MIP Diagnostics, Semorex, Ltd, and Sigma-Aldrich (BelBruno Citation2019).
Table 3. General description of the composition and application approaches of the MIP, IL, PIL, and IL-MIP reported in the extraction processes of AA, FC, and some other substances of industrial interest.
Typically, applications are aimed at contaminants removal processes, among which mention can be made of the work carried out by Chen et al. (Citation2020) in which a MIP supported on silica gel was successfully synthesized by precipitation polymerization with double template imprinting for selective removal of two pesticides from tea polyphenols, obtaining elimination rates of 95.8 and 94.8% with an absorption capacity of up to 33.01 mg/g, so its application is promising to improve the quality and safety of tea since the enrichment with these pesticides during the process of obtaining the tea extract is unavoidable. In other research, Kartal and Denizli (2020) surprisingly managed to remove the cholesterol content of milk to reduce their daily intake and thus avoid some health problems; the developed MIP consisted of cryogel microspheres which allowed an absorption capacity of up to 288.72 mg/g, also showing excellent selectivity and reuse. On the other hand, Zeng et al. (2020) propose a MIP to improve the efficiency of the purification method in the industrial productivity of tylosin which is extracted from the Streptomyces fradiae fermentation broth with a broad antibacterial spectrum, allowing a successful extraction and purification, showing an adsorption capacity of 106.5 mg/g, which is applicable on a large scale for the purification of macrolide antibiotics. Considering the importance of wastewater treatments, Qu et al. (2020) worked to establish a fast and nondestructive method for the separation of phenol contaminants from water, for which they used a superficial MIT synthesizing a highly selective MIP in glucose-derived microporous carbon nanospheres, obtaining good adsorption yields and specific recognition with equilibrium adsorption capacity of up to 85.72 mg/g and relative selection factors close to 8.38, 7.96 and 6.67. Given the low efficiencies as well as the high costs and process times for the purification of bromelain for use in the pharmaceutical, cosmetic and food industries, Xu et al. (Citation2018) worked on developing an easy and effective ecological method by applying magnetic imprinted mesoporous polymers using carbon derived from Pericarp granati as a vehicle, with an adsorption capacity of 135.96 mg/g obtaining high recognition, binding capacity, selectivity, and reuse. Acid amides exhibit sensory numbing sensations typical of pepper flavor, pleasant in addition to different biological activities, which are usually obtained from Zanthoxylum bungeaum which is known as Chinese pepper, however, it is difficult to extract them due to the complex procedures required for their isolation and purification, so that Chen et al. (Citation2018) developed a method using MIP on an SPE column for the selective adsorption and extraction of acid amides achieving absorption capacities of up to 45.04 mg/g and an increase in purity of up to 92.40% compared to a pepper oil resin original (23.34%), showing great potential for industrial application. In the same way, other works carried out by different authors can be reviewed, which have applied the MIT for the extraction of compounds of industrial interest from sectors such as the chemical and pharmaceutical industries, as well as for environmental safety and the elimination of contaminants (Y. Zhang et al. Citation2014; Yang et al. Citation2015; Huang et al. Citation2015; Shen, Xu, and Ye Citation2013).
Despite the wide use of MIP, the type of FM and crosslinkers available for manufacturing is still limited (Ding et al. Citation2020). Therefore, IL have been integrated into MIT, highlighting advantages over conventional elements when used as FM, porogens, templates, solvents or surface modifiers improving aspects such as permeoselectivity and regeneration of MIP by functionalizing the matrix surface with oriented and multiple interaction sites, also helping to increase the efficiency of imprinting, mass transfer and also decreasing the nonspecific adsorption and swelling rate. However, their use as crosslinkers could be detracting given the excessive interactions that in turn lead to nonspecific adsorption, decreasing the efficiency of interaction extraction (Ding et al. Citation2020). Another important advantage of integrating ILM with MIP versus conventional monomers is a more environmentally friendly approach (Pichon, Delaunay, and Combès Citation2020).
There are also papers relating to the integration of ILM with MIP (IL-MIP) highlighting an approach directed toward industrial application as can be seen in , which are mostly polymerized by the conventional free radical technique. That is how Luo et al. (Citation2011) used IL as FM in the efficient development of an ionic liquid-based magnetic molecularly imprinted polymer (IL-MMIP) by inverse emulsion–suspension polymerization for the removal of acid dyes in factory wastewater, the IL-MMIP obtained had favorable stability and greater removal efficiency compared to conventional monomers, proving to be a reliable, effective and convenient method to remove and recycle water-soluble acid dyes in aqueous media. Tian, Bi, and Row (Citation2011) proposed an IL-MIP in which imidazolium-based IL with variations in functional groups such as carboxyl, amino and methyl were used to improve the extraction processes of the active compounds cryptotanshinone, tanshinone I and tanshinone II from the herb Sage miltiorrhiza bunge due to its importance for the treatment of diseases. Synephrine is an alkaloid obtained from the immature dried fruits of Citrus aurantium L, which due to its adverse effects, its extraction from citrus extracts is important, therefore Fan et al. (Citation2013) applied an IL-MIP for its extraction obtaining recoveries between 80 and 90%; for the manufacture of the MIP, IL was used as FM and precipitation polymerization. Similarly, He et al. (2015) developed an IL-MIP with industrial application in which the phase inversion technique was used to manufacture an imprinted membrane for the selective removal of salicylic acid from wastewater, in this case IL was used as a porogen. Recently Li et al. (Citation2019) prepared an IL-MIP as packaging material for the SPE of fucoidan and laminarin from marine algae due to its bioactive properties, obtaining extraction efficiencies of 95.5 and 87.6%, and absorption capacities of 95.6 and 63.6 mg/g respectively.
Application of molecularly imprinted polymers in the extraction of acrylamide and furanic compounds
The MIP have been applied in the extraction of AA and FC by different methods as can be seen in . For AA extraction, Fe3O4-supported MMIP as well as packed SPE systems have usually been developed, with adsorption or loading capacities ranging from 3.68 to 109.8 mg AA/g MIP; these are usually synthesized from the dummy template molecule corresponding to propanamide (PA), and FM such as 2- acrylamido-2-methylpropane sulfonic acid (AMPS), 3-aminopropyltrimethoxysilane (APTMS), acrylic acid and MAA. MAA is one of the most widely used FM for the extraction of FC such as F and furan, where DVB and EGDMA crosslinkers stand out, with sorption capacities of 69.64 and 101.8 mg/g MIP of furan and F, respectively. For both AA and FC, most MIP synthesis is carried out by conventional free radical polymerization using AIBN as initiator, and most approaches have been analytical, however, authors such as Peng et al. (Citation2010) proposed the use of MIP with industrial application approaches for coffee detoxification.
Regarding AA extraction, the MSPE and SPE in which packed SPE cartridges are used stand out. For its part, the MSPE has generated a lot of interest because its application allows rapid extraction processes, easy collection by a magnetic field, and high selectivity, which is potentiated with the specificity of the MIP. There are different reports where the MSPE has been applied as recently reported by Zhang et al. (Citation2020), who synthesized a MMIP in which propionamide was used as a dummy template molecule to avoid bleeding problems and Fe3O4 nanoparticles modified with carboxymethyl dextran as support, achieving adsorption capacities of 19.28 mg/g and percentages of recovery of up to 83.9 − 96.8%, showing excellent AA recognition, selectivity, and enrichment in potato chip samples. Similarly, Bagheria et al. (2019) synthesized MMIP using a green synthesis strategy for the extraction of AA in biscuit samples, obtaining recoveries between 86.0 − 98.3%. The use of MMIP for the extraction of AA in samples of potato chips and cookies has also been studied by Ning et al. (2017) and Arabi et al. (2016), who synthesized imprinted magnetic nanoparticles using Fe3O4 as a component or magnetic support functionalized with amine or graphene oxide, obtaining rapid magnetic separation and high capacity for AA adsorption, enrichment and elimination from food samples. On the other hand, the matrix solid-phase dispersion (MSPD) has also been studied for AA extraction, where molecularly imprinted functionalized silica nanoparticles packed in SPE cartridges were used, which were applied in a simple, easy, selective, highly efficient and fast method for the AA extraction from biscuits and breads samples (Arabi, Ghaedi, et al. Citation2016). Similarly, Xu et al. (Citation2012) successfully applied an AA extraction method in blank potato, twisted cruller, and potato chips samples using MIP in a packed in SPE cartridges system.
On the other hand, MIP have also been involved in the detection of FC, successfully using furan as a template molecule in the preparation of a MIP that presented specific adsorption for furan in soluble coffee, demonstrating its potential for furan SPE in food and drinks (Peng et al. Citation2010). For their part, Hashemi-Moghaddam and Ahmadifard (2016) developed a monolithic molecular imprinted polymeric fiber in which, due to the volatility of furan, the pyrrole molecule was used as a dummy template, the manufactured fiber was successfully applied for the extraction of the furan in HS-SPME in water and canned tuna samples. MIP have been used for the manufacture of different chemical sensors based on electrochemical transduction and surface plasmon resonance for the detection of HMF (Pesavento, Marchetti, et al. Citation2019) and F in aqueous samples and non-food matrices (transformer oil), which allowed the determination in a wide concentration range (Pesavento, Marchetti, et al. Citation2019; Pesavento, Cennamo et al. Citation2019; Cennamo et al. Citation2019; Citation2018; Citation2015; Citation2014).
In an investigation that includes an industrial application approach carried out by Kobayashi et al. (2002) molecularly imprinted membranes were developed for the extraction of dibenzofuran which showed permanent selective recognition and were useful for continuous operation in the treatment of large amounts of the solution with the solute, making them convenient for concentrating present environmental contaminating compounds in wastewater.
As mentioned above, MIT and PIL have great potential for removal of FC and AA from coffee, and an application presented here as a potential proposal could consist of the use of IL-MIP, packed in columns for solid-liquid extraction as shown in the detoxification stage (). Once the coffee extract is obtained, it is usually subjected to a concentration process before dehydration, which is usually done by spray drying or freeze-drying, thus obtaining soluble coffee powder. Coffee extract concentration is usually carried out at low temperatures using strategies such as concentration by centrifugation, vacuum and freezing, so the application of IL-MIP packed in columns for the removal of FC and AA is proposed, in a new stage (detoxification) of the soluble coffee manufacturing process, making the coffee extract pass through its bed once cold and just before carrying out the concentration process. Considering only an adsorption capacity of the MIP (amount of analyte/MIP) detailed in with values of 101.8, 69.64, y 109.8 mg/g for F, furan y AA, respectively, and concentrations of 8200, 99.05 y 75 µg/L in brewed coffee for F, furan y AA, respectively (as mentioned earlier), then it could be suggested to detoxify a liter of coffee brewed in approximately 100%, it would only be necessary to have 80.55, 1.42, y 0.68 mg of MIP.
Some prominent interactions between AA, FC and some functional monomers, important for the rational design of IL and MIP
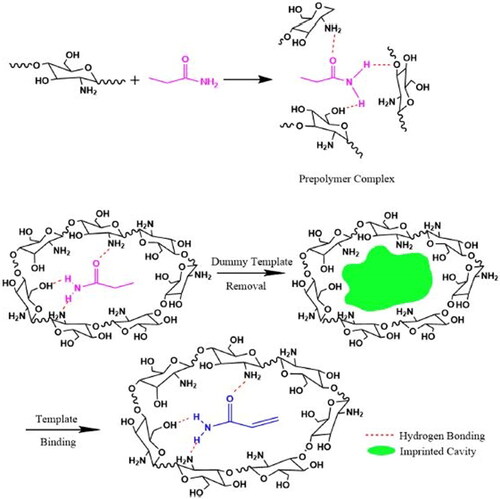
MIP are easily synthesized from various polymers and can be tailor-made for specific analytes and have attracted strong interest owing to the unique features of structure predictability (Jia et al. Citation2018), allowing rational design for which computational tools such as computational modeling can be used to demonstrate or investigate the strength of the bonds that result from the interaction between the functional monomer and the template molecules, allowing the selection of monomers and templates prior to synthesis, thus eradicating the trial-and-error method and thus avoiding the massive solid and liquid waste generation which end-up in contradiction with the green chemistry viewpoints (Madikizela et al. Citation2018). Therefore, one of the important aspects for a rational design consists in selecting a template molecule that contains functional groups capable of interacting with the monomer, in the case of AA, some aspects such as those mentioned by Zhang et al. (Citation2017), who evaluated the effect of IL aromatic and hydroxyl groups on the extraction efficiency of AA, IL [BC4IM][Br] was used as reference, in a comparative with hydroxyl groups [C2OHMIM][Cl], [BC2OHIM][Br] and benzyl [BC2OHIM][Br] were added. The authors found that with the Cl- anion, with respect to the Br- anion, superior extraction efficiencies were obtained, which was also substantially improved by the incorporation of hydroxyl and benzyl groups. AA is known to be a hydrophilic molecule that can interact with FM through interactions between the -NH2 groups present in both molecules or between the -OH groups of FM and -NH2 of AA, mainly through hydrogen bonds between atoms nitrogen, oxygen and hydrogen. Similarly, it is known that crosslinking agents such as EGDMA can establish hydrogen bonds between their groups (C = O) and (C-O) and -NH2 of AA. AA often acts as an FM, its double bond is crosslinked during the preparation of the MIP, which prevents the elimination of the eluents, due to this and its toxic nature, dummy template molecules are generally used that have the characteristic of being structural analogs such as propanamide (PA) whose main difference with AA lies in chain saturation () (C. Zhang et al. Citation2020; Bagheria et al. 2019; Lu et al. Citation2018; Ning et al. Citation2017; Arabi, Ghaedi, et al. Citation2016; Arabi, Ghaedi, et al. Citation2016; L. Xu et al. Citation2012).
Figure 4. Example of the process of preparing a MIP (polymerization, imprinting, desorption, and adsorption) for AA and illustration of some physicochemical interactions (Bagheri et al. Citation2019).
It is also known that π-π interactions can occur between HMF and IL such as [C2Mim][Br], [C4Mim][Br], and [C12Mim][HSO4]. The (C = O) and -OH groups of HMF can interact through hydrogen bonding and π-π interactions with the HSO4 anion and the imidazolium cations of IL, where it was also found that with respect to the Cl- anion, the anion Br− has more electrostatic affinity with HMF. Increased IL chain length leads to increased hydrophobic properties, affecting interactions between IL and HMF due to their hydrophilicity (Du et al. Citation2018). The volatile and semi-volatile compounds in coffee can experience hydrogen bonds and dipole-type interactions, among them are the FC, for its part, the use of furan as a template molecule is difficult due to volatility, so dummy templates molecules such as pyrrole are used (Hashemi-Moghaddam and Ahmadifard Citation2016). Taking PIL as [VC6Im][Cl], [VC16Im][NTf2] and [VBC16Im][NTf2] into account, it has been found that long chains such as those of ILM C16 usually favor dispersive nonspecific interactions and the benzyl π-π type interactions, dipole, and electron lone pair with FC (Toledo et al. Citation2014).
Future perspectives
MIP have many advantages including mechanical and chemical robustness, high selectivity for the target molecule, and low cost of preparation (Arabi, Ghaedi, et al. Citation2016). Regarding its integration with ILM, there are few reports on the application of IL as monomer and crosslinking agent in the preparation of MIP (Ma and Row Citation2018). Furthermore, it is highlighted that the continuous development of IL-MIP with specificity and adequate efficiencies is a subject of great current and future interest (Ding et al. Citation2020). In this regard, it should be noted that despite the different works published on the application of MIP or PIL in the extraction of AA and FC, to the best of our knowledge, there are no or very few reports on the use of IL-MIP for the extraction of these compounds in coffee and other food matrices. Therefore, the promotion of new IL-MIP-based systems for the extraction of these contaminants are interesting scientific developments with potential for analytical and industrial applications.
The importance of both the basic science and the industrial application of the MIP created by MIT in such important areas as the food industry and the development of new materials is evident. The area of production of different types of coffee is one of those fields that at a worldwide level moves many economic guilds and therefore implies the continuous improvement of its sensory and beneficial properties for health. However, contaminants must be removed to the maximum extent possible, and the various techniques described here for obtaining MIP that achieve the removal of such contaminants give us a broader perspective on the advancement of purification techniques in a much more specific and selective way.
Several authors show research related to the removal of AA and FC, with very promising results with the projection of being able to implement these methods at an industrial level. However, it is a technique that involves several stages of detailed chemistry and engineering, but with all these existing results, there are very good possibilities of implementation at the industry level. Very important is to understand the mechanism of interaction between analyte-MIP for a rational design, and therefore the correct interpretation of these interactions will allow the generation of new MIP for systems analogous to many of those already described in several papers in this review, which greatly enhances the application prospect in this area. But much more important is to incorporate IL in these systems, given the high versatility in their design and specificity, in addition to being able to involve systems with low environmental impact and minimize the toxicity effects of many components currently used for their implementation in the food industry.
Conclusion
To date, attempts have been made to apply different strategies to eliminate furan, HMF, F and AA from coffee, where although they have achieved significant reductions, the defects caused by the organoleptic properties still remain an issue. For the first time, a review is presented on the application of MIP and PIL as alternatives for the removal of these toxic substances from coffee. PIL show a promising outlook for industrial-scale extraction and have demonstrated the ability to improve selectivity and efficiency, but despite having been used for AA and FC extraction, their applications have been highlighted with analytical and non-industrial approaches. The MIP are characterized by presenting an industrial application in highly selective separation processes, mainly MSPE and SPE in which packed SPE cartridges, and they have also been combined with ILM, managing to improve their selectivity and efficiency. Similarly, MIP are known to have been used to selectively extract AA and some FC in some foods, but not in coffee and without the use of ILM, so the application of LI-based MIP could represent a potential alternative for extraction of AA and FC from coffee, where the functionalization of its structure with groups such as −NH2, −OH, −C = O, −C-O, HSO4−, and aromatic rings such as imidazole and benzene improve efficiency and specificity of extraction in soluble coffee, for which a highly selective method is required to avoid the extraction of aroma and flavor compounds and which is also industrial and nondestructive, which is why MIP and PIL turn out to be promising for its detoxification mediated by the extraction of AA and FC from the coffee extract using methods such as MSPE, SPE in packed cartridge, SPE or RDSE.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abraham, K., R. Gürtler, K. Berg, G. Heinemeyer, A. Lampen, and K. Appel. 2011. Toxicology and risk assessment of 5-hydroxymethylfurfural in food. Molecular Nutrition & Food Research 55 (5):667–78. doi: 10.1002/mnfr.201000564.
- Akıllıoğlu, H. G., K. S. Bahçeci, and V. Gökmen. 2015. Investigation and kinetic evaluation of furan formation in tomato paste and pulp during heating. Food Research International (Ottawa, Ont.) 78:224–30. doi: 10.1016/j.foodres.2015.10.005.
- Akıllıoglu, H. G., and V. Gökmen. 2014. Mitigation of acrylamide and hydroxymethyl furfural in instant coffee by yeast fermentation. Food Research International 61:252–6. doi: 10.1016/j.foodres.2013.07.057.
- Altaki, M. S., F. J. Santos, and M. T. Galceran. 2011. Occurrence of furan in coffee from Spanish market: contribution of brewing and roasting. Food Chemistry 126 (4):1527–32. doi: 10.1016/j.foodchem.2010.11.134.
- Alviz, P. L., and A. J. Alvarez. 2017. Comparative life cycle assessment of the use of an ionic liquid ([Bmim]Br) versus a volatile organic solvent in the production of acetylsalicylic acid. Journal of Cleaner Production 168:1614–24. doi: 10.1016/j.jclepro.2017.02.107.
- Anese, M. 2015. Furan and other furanic compounds in coffee: occurrence, mitigation strategies, and importance of processing. In Processing and impact on active components in food, ed. V. Preedy, 541–47. London: Academic Press. doi: 10.1016/B978-0-12-404699-3.00065-2.
- Anese, M. 2016. Acrylamide in coffee and coffee substitutes. In Acrylamide in food: analysis, content and potential health effects, ed. V. Gökmen, 181–95. London: Academic Press. doi: 10.1016/B978-0-12-802832-2.00009-7.
- Anese, M., L. Manzocco, S. Calligaris, and M. C. Nicoli. 2013. Industrially applicable strategies for mitigating acrylamide, furan, and 5-hydroxymethylfurfural in food. Journal of Agricultural and Food Chemistry 61 (43):10209–14. doi: 10.1021/jf305085r.
- Anese, M., and M. Suman. 2013. Mitigation strategies of furan and 5-hydroxymethylfurfural in food. Food Research International 51 (1):257–64. doi: 10.1016/j.foodres.2012.12.024.
- Arabi, M., M. Ghaedi, and A. Ostovan. 2016. Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chemistry 210:78–84. doi: 10.1016/j.foodchem.2016.04.080.
- Arabi, M., A. Ostovan, M. Ghaedi, and M. K. Purkait. 2016. Novel strategy for synthesis of magnetic dummy molecularly imprinted nanoparticles based on functionalized silica as an efficient sorbent for the determination of acrylamide in potato chips: optimization by experimental design methodology. Talanta 154:526–32. doi: 10.1016/j.talanta.2016.04.010.
- Arisseto, A. P., and E. Vicente. 2015. Chapter 65 – Estimate of acrylamide intake from coffee and health risk assessment. In Coffee in Health and Disease Prevention Preedy, ed. Victor R. B. T., 575–84. San Diego: Academic Press. doi: 10.1016/B978-0-12-409517-5.00065-6.
- Arribas-Lorenzo, G., and F. J. Morales. 2010. Estimation of dietary intake of 5-hydroxymethylfurfural and related substances from coffee to Spanish population. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 48 (2):644–9. doi: 10.1016/j.fct.2009.11.046.
- Bagheri, A. R., M. Arabi, M. Ghaedi, A. Ostovan, X. Wang, J. Li, and L. Chen. 2019. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 195:390–400. doi: 10.1016/j.talanta.2018.11.065.
- Bedade, D. K., Y. B. Sutar, and R. S. Singhal. 2019. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: process parameters and removal of acrylamide from coffee. Food Chemistry 275:95–104. doi: 10.1016/j.foodchem.2018.09.090.
- Bedoya-Ramírez, D., A. Cilla, J. Contreras-Calderón, and A. Alegría-Torán. 2017. Evaluation of the antioxidant capacity, furan compounds and cytoprotective/cytotoxic effects upon caco-2 cells of commercial colombian coffee. Food Chemistry 219:364–72. doi: 10.1016/j.foodchem.2016.09.159.
- BelBruno, J. J. 2019. Molecularly imprinted polymers. Chemical Reviews 119 (1):94–119. doi: 10.1021/acs.chemrev.8b00171.
- Belitz, H. D., Grosch, W. 1999. Coffee, tea and cocoa. In Food Chemistry, ed. H. D. Belitz and W. Grosch, 874–904. Berlin: Springer. doi: 10.1007/978-3-662-07281-3_22.
- Bermudez, J. 2020. ¿Cuáles Son Los Países Que Consumen Más Café En El Mundo? IAlimentos. Accessed October 15, 2021. https://www.revistaialimentos.com/cuales-son-los-paises-que-consumen-mas-cafe-en-el-mundo/.
- Berthod, A., M. J. Ruiz-Ángel, and S. Carda-Broch. 2018. Recent advances on ionic liquid uses in separation techniques. Journal of Chromatography. A 1559:2–16. doi: 10.1016/j.chroma.2017.09.044.
- Van Boekel, M. A. J. S. 1998. Effect of heating on maillard reactions in milk. Food Chemistry 62 (4):403. 14. doi: 10.1016/S0308-8146(98)00075-2.
- Cagliero, C., T. D. Ho, C. Zhang, C. Bicchi, and J. L. Anderson. 2016. Determination of acrylamide in brewed coffee and coffee powder using polymeric ionic liquid-based sorbent coatings in solid-phase microextraction coupled to gas chromatography-mass spectrometry. Journal of Chromatography. A 1449:2–7. doi: 10.1016/j.chroma.2016.04.034.
- Cagliero, C., H. Nan, C. Bicchi, and J. L. Anderson. 2016. Matrix-compatible sorbent coatings based on structurally-tuned polymeric ionic liquids for the determination of acrylamide in brewed coffee and coffee powder using solid-phase microextraction. Journal of Chromatography. A 1459:17–23. doi: 10.1016/j.chroma.2016.06.075.
- Capuano, E., and V. Fogliano. 2011. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. Lwt - Food Science and Technology 44 (4):793–810. doi: 10.1016/j.lwt.2010.11.002.
- Castaño, J., G. Quintero, and V. León. 2000. Caracterización de Rendimiento de Extracción y El Contenido de Sólidos Solubles En La Bebida de Café. Cenicafe 51 (3):185–95. doi: 10.1016/j.chroma.2016.06.075 https://biblioteca.cenicafe.org/handle/10778/1001.
- Çebi, A. 2016. Acrylamide intake, its effects on tissues and cancer. In Acrylamide in Food, ed. V. Gökmen, 63–91. Singapore: World Scientific Publishing. doi: 10.1016/B978-0-12-802832-2.00004-8.
- Cennamo, N., L. De Maria, G. D’Agostino, M. Pesavento, and L. Zeni. 2014. Combined molecularly imprinted polymer and surface plasmon resonance transduction in plastic optical fiber for monitoring oil-filled power transformers. Procedia Engineering 87:532–5. doi: 10.1016/j.proeng.2014.11.541.
- Cennamo, N., L. De Maria, G. D’Agostino, L. Zeni, and M. Pesavento. 2015. Monitoring of low levels of furfural in power transformer oil with a sensor system based on a POF-MIP platform. Sensors (Basel, Switzerland) 15 (4):8499–511. doi: 10.3390/s150408499.
- Cennamo, N., L. Zeni, B. Andò, S. Baglio, S. Graziani, V. Marletta, A. Pistorio, M. Pesavento, and S. Marchetti. 2018. A novel chemical optical sensor based on molecularly imprinted polymer, optical fibers and inkjet printing technology. In 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), 1–15. Texas: IEEE. doi: 10.1109/I2MTC.2018.8409619.
- Cennamo, N., L. Zeni, M. Pesavento, S. Marchetti, V. Marletta, S. Baglio, S. Graziani, A. Pistorio, and B. Andò. 2019. A novel sensing methodology to detect furfural in water, exploiting MIPs, and inkjet-printed optical waveguides. IEEE Transactions on Instrumentation and Measurement 68 (5):1582–9. doi: 10.1109/TIM.2018.2879170.
- Chaichi, M., V. Ghasemzadeh-Mohammadi, M. Hashemi, and A. Mohammadi. 2015. Furanic compounds and furfural in different coffee products by headspace liquid-phase micro-extraction followed by gas chromatography-mass spectrometry: survey and effect of brewing procedures. Food Additives & Contaminants. Part B, Surveillance 8 (1):73–80. doi: 10.1080/19393210.2014.981601.
- Chávez-Servín, J. L., A. I. Castellote, and M. C. López-Sabater. 2005. Analysis of potential and free furfural compounds in milk-based formulae by high-performance liquid chromatography: evolution during storage. Journal of Chromatography A 1076 (1–2):133–40. doi: 10.1016/j.chroma.2005.04.046.
- Chen, J., X. Huang, L. Wang, C. Ma, S. Wu, and H. Wang. 2020. The synthesis of a dual-template surface molecularly imprinted polymer based on silica gel and its application in the removal of pesticides from tea polyphenols. Analytical Methods 12 (7):996–1004. doi: 10.1039/C9AY02708D.
- Chen, X., X. Jin, Y. Li, G. Chen, K. Chen, and J. Kan. 2018. Preparation and characterization of molecularly-imprinted polymers for extraction of sanshool acid amide compounds followed by their separation from pepper oil resin derived from Chinese prickly ash (Zanthoxylum bungeanum). Journal of Separation Science 41 (2):590–601. doi: 10.1002/jssc.201701014.
- Chiappe, C., A. Marra, and A. Mele. 2010. Synthesis and applications of ionic liquids derived from natural sugars. In Carbohydrates in sustainable development II, ed. A. P. Rauter, P. Vogel, and Y. Queneau, 177–95. Berlin, Heidelberg: Springer. doi: 10.1007/128_2010_47.
- Contreras-Calderón, J., D. Mejía-Díaz, M. Martínez-Castaño, D. Bedoya-Ramírez, N. López-Rojas, F. Gómez-Narváez, Y. Medina-Pinea, and O. Vega-Castro. 2016. Evaluation of antioxidant capacity in coffees marketed in colombia: relationship with the extent of non-enzymatic browning. Food Chemistry 209:162–70. doi: 10.1016/j.foodchem.2016.04.038.
- Crews, C., D. Roberts, S. Lauryssen, and G. Kramer. 2009. Survey of furan in foods and coffees from five European Union countries. Food Additives and Contaminants: Part B 2 (2):95–8. doi: 10.1080/02652030903095408.
- Das, R. N., and K. Roy. 2013. Advances in QSPR/QSTR models of ionic liquids for the design of greener solvents of the future. Molecular Diversity 17 (1):151–96. doi: 10.1007/s11030-012-9413-y.
- Ding, S., Z. Lyu, X. Niu, Y. Zhou, D. Liu, M. Falahati, D. Dan, and Y. Lin. 2020. Integrating ionic liquids with molecular imprinting technology for biorecognition and biosensing: a review. Biosensors & Bioelectronics 149:111830. doi: 10.1016/j.bios.2019.111830.
- Doble, M., and A. K. Kruthiventi. 2007. Chapter 5 - Alternate solvents. In Green chemistry and engineering, ed. M. Doble and A. K. Kruthiventi, 93–104. Burlington: Academic Press. doi: 10.1016/B978-012372532-5/50006-7.
- Dong, S. J., B. X. Zhang, Y. F. Gao, and X. M. Hu. 2015. An efficient process for pretreatment of lignocelluloses in functional ionic liquids. International Journal of Polymer Science 2015:1–6. doi: 10.1155/2015/978983.
- Du, K., J. Li, Y. Bai, M. An, X. M. Gao, and Y. X. Chang. 2018. A green ionic liquid-based vortex-forced MSPD method for the simultaneous determination of 5-HMF and iridoid glycosides from fructus corni by ultra-high performance liquid chromatography. Food Chemistry 244:190–6. doi: 10.1016/j.foodchem.2017.10.057.
- EFSA. 2004. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to furfural and furfural diethylacetal. The EFSA Journal 67:1–27.
- EFSA. 2005. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to flavouring group evaluation 13 (FGE. 13); furfuryl and furan derivatives with and without additional side‐chain substituent. EFSA Journal 3 (7):215.
- EFSA. 2011. Results on acrylamide levels in food from monitoring years 2007–2009. EFSA Journal 4 (9):2133. doi: 10.2903/j.efsa.2011.2133.
- EFSA. 2012. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA Journal 10 (10):2938. doi: 10.2903/j.efsa.2012.2938.
- European Commission. 2017. Commission regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. Official Journal of the European Union L304:24–44. http://data.europa.eu/eli/reg/2017/2158/oj.
- Fan, J. P., Z. Y. Tian, S. Tong, X. H. Zhang, Y. L. Xie, R. Xu, L. Qin, L. Li, J. H. Zhu, and X. K. Ouyang. 2013. A novel molecularly imprinted polymer of the specific ionic liquid monomer for selective separation of synephrine from methanol-water media. Food Chem 141 (4):3578–85. doi: 10.1016/j.foodchem.2013.06.040.
- Felsot, A. S. 2002. Acrylamide Angst: Another Annoying Distraction about Food Safety Agrichemical and Environmental News: A Monthly Report on Environmental and Pesticide Related Issues. Washington, DC: Washington State University.
- Fiscal-Ladino, J. A., M. Obando-Ceballos, D. F. Rosero-Moreano, M. Montaño, W. Cardona, L. F. Giraldo, and P. Richter. 2017. Ionic liquids intercalated in montmorillonite as the sorptive phase for the extraction of low-polarity organic compounds from water by rotating-disk sorptive extraction. Analytica Chimica Acta 953:23–31. doi: 10.1016/j.aca.2016.11.067.
- Folmer, B., A. Farah, L. Jones, and V. Fogliano. 2017. Human wellbeing—sociability, performance, and health. In The craft and science of coffee, ed. B. Former, 493–520. London: Academic Press. doi: 10.1016/B978-0-12-803520-7.00020-7.
- Gökmen, V. 2015. Acrylamide in food: analysis, content and potential health effects. New York: Academic Press. doi: 10.1016/C2014-0-02160-0.
- Gökmen, V., T. Kocadağlı, N. Göncüoğlu, and B. A. Mogol. 2012. Model studies on the role of 5-hydroxymethyl-2-furfural in acrylamide formation from asparagine. Food Chemistry 132 (1):168–74. doi: 10.1016/j.foodchem.2011.10.048.
- Gökmen, V., and F. J. Morales. 2014. Processing contaminants: hydroxymethylfurfural. Encyclopedia of Food Safety 2:404–8. doi: 10.1016/B978-0-12-378612-8.00209-2.
- Gozzo, F. C., L. S. Santos, R. Augusti, C. S. Consorti, J. Dupont, and M. N. Eberlin. 2004. Gaseous supramolecules of imidazolium ionic liquids: “magic” numbers and intrinsic strengths of hydrogen bonds . Chemistry (Weinheim an Der Bergstrasse, Germany) 10 (23):6187–93. doi: 10.1002/chem.200305742.
- Guenther, H., K. Hoenicke, S. Biesterveld, E. Gerhard-Rieben, and I. Lantz. 2010. Furan in coffee: pilot studies on formation during roasting and losses during production steps and consumer handling. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 27 (3):283–90. doi: 10.1080/19440040903317505.
- Gunasekara, R. W., and Y. Zhao. 2017. A general method for selective recognition of monosaccharides and oligosaccharides in water. Journal of the American Chemical Society 139 (2):829–35. doi: 10.1021/jacs.6b10773.
- Hartmann, D. O., and C. S. Pereira. 2016. Toxicity of ionic liquids: past, present, and future. In Ionic liquids in lipid processing and analysis, ed. X. Xu, Z. Guo, and L. Z. Cheong, 403–21. London: AOCS Press. doi: 10.1016/B978-1-63067-047-4.00013-1.
- Hashemi-Moghaddam, H., and M. Ahmadifard. 2016. Novel molecularly-imprinted solid-phase microextraction fiber coupled with gas chromatography for analysis of furan. Talanta 150:148–54. doi: 10.1016/j.talanta.2015.08.044.
- He, Z., M. Meng, L. Yan, W. Zhu, F. Sun, Y. Yan, Y. Liu, and S. Liu. 2015. Fabrication of new cellulose acetate blend imprinted membrane assisted with ionic liquid ([BMIM] Cl) for selective adsorption of salicylic acid from industrial wastewater. Separation and Purification Technology 145:63–74. doi: 10.1016/j.seppur.2015.03.005.
- Henle, T., H. Walter, and H. Klostermeyer. 1991. Evaluation of the extent of the early Maillard-reaction in milk products by direct measurement of the Amadori-product lactuloselysine. Zeitschrift Fur Lebensmittel-Untersuchung und -Forschung 193 (2):119–22. doi: 10.1007/BF01193359.
- Ho, C. 1996. Thermal Generation of maillard aromas. In The Maillard reaction consequences for the chemical and life sciences, ed. R. Irank, 27–53. Chichester, United Kingdom: John Wiley & Sons.
- Hospido, A., and H. Rodríguez. 2019. Life cycle assessment (LCA) of ionic liquids. In Encyclopedia of ionic liquids, ed. S. Zhang, 1–9. Singapore: Springer. doi: 10.1007/978-981-10-6739-6_54-1.
- Hou, X. D., Q. P. Liu, T. J. Smith, N. Li, and M. H. Zong. 2013. Evaluation of toxicity and biodegradability of cholinium amino acids ionic liquids. PloS One 8 (3):e59145. doi: 10.1371/journal.pone.0059145.
- Huang, D. L., R. Z. Wang, Y. G. Liu, G. M. Zeng, C. Lai, P. Xu, B. A. Lu, J. J. Xu, C. Wang, and C. Huang. 2015. Application of molecularly imprinted polymers in wastewater treatment: a review. Environmental Science and Pollution Research International 22 (2):963–77. doi: 10.1007/s11356-014-3599-8.
- IARC. 1996. Dry cleaning, some chlorinated solvents and other industrial chemicals IARC monographs on the evaluation of carcinogenic risks to humans. Cancer Causes Control 63:289–91. https://aplicacionesbiblioteca.udea.edu.co:2399/10.1007/BF00051307.
- JECFA. 1996. Toxicological evaluation of certain food additives. The forty‐fourth meeting of the joint fao/who expert committee on food additives and contaminants. In WHO Food Additives Series. Geneva: IFCS WHO.
- Jia, M., Z. Zhang, J. Li, X. Ma, L. Chen, and X. Yang. 2018. Molecular imprinting technology for microorganism analysis. TrAC Trends in Analytical Chemistry 106:190–201. doi: 10.1016/j.trac.2018.07.011.
- Kartal, F., and A. Denizli. 2020. Molecularly imprinted cryogel beads for cholesterol removal from milk samples. Colloids and Surfaces. B, Biointerfaces 190:110860. doi: 10.1016/j.colsurfb.2020.110860.
- Khan, A. S., Z. Man, A. Nasrullah, Z. Ullah, N. Muhammad, A. Rahim, A. Bustam, A. Idris, and M. Uroos. 2020. Conversion of biomass to chemicals using ionic liquids. In Green sustainable process for chemical and environmental engineering and science, ed. A. M. A. Asiri and S Kanchi, 1–30. India: Elsevier. doi: 10.1016/B978-0-12-817386-2.00001-9.
- Kirchhecker, S., and D. Esposito. 2016. Amino acid based ionic liquids: a green and sustainable perspective. Current Opinion in Green and Sustainable Chemistry 2:28–33. doi: 10.1016/j.cogsc.2016.09.001.
- Kissoudi, M., and V. Samanidou. 2018. Recent advances in applications of ionic liquids in miniaturized microextraction techniques. Molecules 23 (6):1437. doi: 10.3390/molecules23061437.
- Knutsen, H. K., J. Alexander, L. Barregård, M. Bignami, B. Brüschweiler, S. Ceccatelli, B. Cottrill, M. Dinovi, L. Edler, B. Grasl-Kraupp, et al. 2017. Risks for public health related to the presence of furan and methylfurans in food. EFSA Journal. European Food Safety Authority 15 (10):e05005. doi: 10.2903/j.efsa.2017.5005.
- Kobayashi, T., P. S. Reddy, M. Ohta, M. Abe, and N. Fujii. 2002. Molecularly imprinted polysulfone membranes having acceptor sites for donor dibenzofuran as novel membrane adsorbents: charge transfer interaction as recognition origin. Chemistry of Materials 14 (6):2499–505. doi: 10.1021/cm0109692.
- Kosheleva, R. I., A. C. Mitropoulos, and G. Z. Kyzas. 2019. New trends in molecular imprinting techniques. In Advanced low-cost separation techniques in interface science, ed. G. Z. Kyzas and A. C. Mitropoulos, 151–72. London: Academic Press. doi: 10.1016/B978-0-12-814178-6.00007-8.
- Krupadam, R. J., M. S. Khan, and S. R. Wate. 2010. Removal of probable human carcinogenic polycyclic aromatic hydrocarbons from contaminated water using molecularly imprinted polymer. Water Research 44 (3):681–8. doi: 10.1016/j.watres.2009.09.044.
- Li, G., Y. Dai, X. Wang, and K. Row. 2019. Molecularly imprinted polymers modified by deep eutectic solvents and ionic liquids with two templates for the simultaneous solid-phase extraction of fucoidan and laminarin from marine kelp. Analytical Letters 52 (3):511–25. doi: 10.1080/00032719.2018.1471697.
- López-Darias, J., J. L. Anderson, V. Pino, and A. M. Afonso. 2011. Developing qualitative extraction profiles of coffee aromas utilizing polymeric ionic liquid sorbent coatings in headspace solid-phase microextraction gas chromatography-mass spectrometry. Analytical and Bioanalytical Chemistry 401 (9):2965–76. doi: 10.1007/s00216-011-5394-4.
- Lu, X., Y. Yang, Y. Zeng, L. Li, and X. Wu. 2018. Rapid and reliable determination of P-nitroaniline in wastewater by molecularly imprinted fluorescent polymeric ionic liquid microspheres. Biosensors & Bioelectronics 99:47–55. doi: 10.1016/j.bios.2017.07.041.
- Luo, X., R. Dong, S. Luo, Y. Zhan, X. Tu, and L. Yang. 2013. Preparation of water‐compatible molecularly imprinted polymers for caffeine with a novel ionic liquid as a functional monomer. Journal of Applied Polymer Science 127 (4):2884–90. doi: 10.1002/app.36792.
- Luo, X., Y. Zhan, Y. Huang, L. Yang, X. Tu, and S. Luo. 2011. Removal of water-soluble acid dyes from water environment using a novel magnetic molecularly imprinted polymer. Journal of Hazardous Materials 187 (1–3):274–82. doi: 10.1016/j.jhazmat.2011.01.009.
- Ma, W., and K. H. Row. 2018. Solid-phase extraction of chlorophenols in seawater using a magnetic ionic liquid molecularly imprinted polymer with incorporated silicon dioxide as a sorbent. Journal of Chromatography. A 1559:78–85. doi: 10.1016/j.chroma.2018.01.013.
- Madikizela, L. M., N. T. Tavengwa, H. Tutu, and L. Chimuka. 2018. Green aspects in molecular imprinting technology: from design to environmental application. Trends in Environmental Analytical Chemistry 17:14–22. doi: 10.1016/j.teac.2018.01.001.
- Maillard, L. C. 1912. Action Des Acides Aminés Sur Les Sucres: Formation Des Mélanoidines Par Voie Méthodique. Comptes Rendus de l’ Academie Des Sciences Serie IIa. Sciences de. La Terre et Des Planets 154:66–8.
- Marcinkowska, R., K. Konieczna, Ł. Marcinkowski, J. Namieśnik, and A. Kloskowski. 2019. Application of ionic liquids in microextraction techniques: current trends and future perspectives. Trac Trends in Analytical Chemistry 119:115614. doi: 10.1016/j.trac.2019.07.025.
- Martins, P. L. G., A. R. Braga, and V. V. de Rosso. 2017. Can ionic liquid solvents be applied in the food industry? Trends in Food Science & Technology 66:117–24. doi: 10.1016/j.tifs.2017.06.002.
- Mehrkesh, A, and A. T. Karunanithi. 2016. New quantum chemistry-based descriptors for better prediction of melting point and viscosity of ionic liquids. Fluid Phase Equilibria 427:498–503. doi:10.1016/j.fluid.2016.07.006.
- Mei, M., X. Huang, and L. Chen. 2019. Recent development and applications of poly (ionic liquid)s in microextraction techniques. Trac Trends in Analytical Chemistry 112:123–34. doi: 10.1016/j.trac.2019.01.003.
- de Melo, M. M., A. J. Silvestre, I. Portugal, and C. M. Silva. 2017. Emerging technologies for the recovery of valuable compounds from coffee processing by-products. In Handbook of coffee processing by-products, 141–69. London: Academic Press. doi: 10.1016/B978-0-12-811290-8.00005-0.
- Monien, B. H., W. Engst, G. Barknowitz, A. Seidel, and H. Glatt. 2012. Mutagenicity of 5-hydroxymethylfurfural in V79 cells expressing human SULT1A1: identification and mass spectrometric quantification of DNA adducts formed. Chemical Research in Toxicology 25 (7):1484–92. doi: 10.1021/tx300150n.
- Monsalve-Atencio, R., K. Sanchez, J. Camaño, S. Lopera-Cardona, and B. Ortiz-Reyes. 2021. Determination of ochratoxin a in coffee by ELISA method and its relationship with the physical, physicochemical and microbiological properties. Vitae (2 SE-Foods: Science, Engineering and Technology) 28: 1–12. doi: 10.17533/udea.vitae.v28n2a343838.
- Moro, S., J. K. Chipman, J. W. Wegener, C. Hamberger, W. Dekant, and A. Mally. 2012. Furan in heat-treated foods: formation, exposure, toxicity, and aspects of risk assessment. Molecular Nutrition & Food Research 56 (8):1197–211. doi: 10.1002/mnfr.201200093.
- Mussatto, S. I., E. M. Machado, S. Martins, and J. A. Teixeira. 2011. Production, composition, and application of coffee and its industrial residues. Food and Bioprocess Technology 4 (5):661–72. doi: 10.1007/s11947-011-0565-z.
- Nawała, J., B. Dawidziuk, D. Dziedzic, D. Gordon, and S. Popiel. 2018. Applications of ionic liquids in analytical chemistry with a particular emphasis on their use in solid-phase microextraction. TrAC Trends in Analytical Chemistry 105:18–36. doi: 10.1016/j.trac.2018.04.010.
- Ncube, S., P. Kunene, N. T. Tavengwa, H. Tutu, H. Richards, E. Cukrowska, and L. Chimuka. 2017. Synthesis and characterization of a molecularly imprinted polymer for the isolation of the 16 US-EPA priority polycyclic aromatic hydrocarbons (PAHs) in solution. Journal of Environmental Management 199:192–200. doi: 10.1016/j.jenvman.2017.05.041.
- Ning, F., T. Qiu, Q. Wang, H. Peng, Y. Li, X. Wu, Z. Zhang, L. Chen, and H. Xiong. 2017. Dummy-surface molecularly imprinted polymers on magnetic graphene oxide for rapid and selective quantification of acrylamide in heat-processed (including fried) foods. Food Chemistry 221:1797–804. doi: 10.1016/j.foodchem.2016.10.101.
- Nursten, H. 2005. The maillard reaction – chemistry, biochemistry and implications. Cambridge: The Royal Society of Chemistry.
- O’Brien, J., A. G. Renwick, A. Constable, E. Dybing, D. J. G. Müller, J. Schlatter, W. Slob, W. Tueting, J. van Benthem, G. M. Williams, A, et al. 2006. Approaches to the risk assessment of genotoxic carcinogens in food: a critical appraisal. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 44 (10):1613–35. doi: 10.1016/j.fct.2006.07.004.
- Ozokwelu, D., S. Zhang, O. Okafor, W. Cheng, and N. Litombe. 2017a. Preparation and characterization of ionic liquids. In Novel catalytic and separation processes based on ionic liquids, ed. D. Ozokwelu, S. Zhang, O. Okafor, W. Cheng, and N. Litombe, 13–44. India: Elsevier. doi: 10.1016/B978-0-12-802027-2.00002-9.
- Ozokwelu, D., S. Zhang, O. Okafor, W. Cheng, and N. Litombe. 2017b. Properties of ionic liquids. In Novel catalytic and separation processes based on ionic liquids, 45–110. Elsevier. doi: 10.1016/B978-0-12-802027-2.00003-0.
- Patinha, D. J. S., A. J. D. Silvestre, and I. M. Marrucho. 2019. Poly(ionic liquids) in solid phase microextraction: recent advances and perspectives. Progress in Polymer Science 98:101148. doi: 10.1016/j.progpolymsci.2019.101148.
- Pavesi, A., E. Vicente, M. Soares-Ueno, S. A. Verdiani-Tfouni, and M. C. De Figueiredo-Toledo. 2011. Furan levels in coffee as influenced by species, roast degree, and brewing procedures. Journal of Agricultural and Food Chemistry 59 (7):3118–24. doi: 10.1021/jf104868g.
- Peng, N., F. C. Yan, L. Chen, and X. S. Huang. 2010. Preparation and binding characteristics of molecularly imprinted polymers for furan. Chinese Journal of Analytical Chemistry 4.
- Pesavento, M., N. Cennamo, G. Alberti, S. Marchetti, and L. Zeni. 2019. Sensing of furfural by molecularly imprinted polymers on plasmonic and electrochemical platforms. Proceedings 15 (1):48-50. doi: 10.3390/proceedings2019015048.
- Pesavento, M., S. Marchetti, L. De Maria, L. Zeni, and N. Cennamo. 2019. Sensing by molecularly imprinted polymer: evaluation of the binding properties with different techniques. Sensors 19 (6):1344. doi: 10.3390/s19061344.
- Pichon, V., N. Delaunay, and A. Combès. 2020. Sample preparation using molecularly imprinted polymers. Analytical Chemistry 92 (1):16–33. doi: 10.1021/acs.analchem.9b04816.
- Poisson, L., I. Blank, A. Dunkel, and T. Hofmann. 2017. The chemistry of roasting-decoding flavor formation. In The craft and science of coffee, ed. B. Folmer, 273–309. London: Academic Press. doi: 10.1016/B978-0-12-803520-7.00012-8.
- Qu, Y., L. Qin, X. Liu, and Y. Yang. 2020. Reasonable design and sifting of microporous carbon nanosphere-based surface molecularly imprinted polymer for selective removal of phenol from wastewater. Chemosphere 251:126376. doi: 10.1016/j.chemosphere.2020.126376.
- Quarta, B., and M. Anese. 2012. Furfurals removal from roasted coffee powder by vacuum treatment. Food Chemistry 130 (3):610–4. doi: 10.1016/j.foodchem.2011.07.083.
- Rahn, A., and C. Yeretzian. 2019. Impact of consumer behavior on furan and furan-derivative exposure during coffee consumption. A comparison between brewing methods and drinking preferences. Food Chemistry 272:514–22. doi: 10.1016/j.foodchem.2018.08.078.
- Rannou, C., D. Laroque, E. Renault, C. Prost, and T. Sérot. 2016. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Research International (Ottawa, Ont.) 90:154–76. doi: 10.1016/j.foodres.2016.10.037.
- Bagheri, A. R., M. Arabi, M. Ghaedi, A. Ostovan, X. Wang, J. Li, and L. Chen. 2019. Dummy Molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 195:390–400. doi: 10.1016/j.talanta.2018.11.065.
- Richarme, G., E. Marguet, P. Forterre, S. Ishino, and Y. Ishino. 2016. DJ-1 family maillard deglycases prevent acrylamide formation. Biochemical and Biophysical Research Communications 478 (3):1111–6. doi: 10.1016/j.bbrc.2016.08.077.
- Rodrigues, N. P., and N. Bragagnolo. 2013. Identification and quantification of bioactive compounds in coffee brews by HPLC–DAD–MSn. Journal of Food Composition and Analysis 32 (2):105–15. doi: 10.1016/j.jfca.2013.09.002.
- Rodrigues, R.,. L. de Almeida, and E. Spers. 2020. Coffee and health in the perspective of young consumers. In Coffee consumption and industry strategies in brazil, ed. L. F. de Almeida and E. E. Spers, 343–66. India: Woodhead Publishing. doi: 10.1016/B978-0-12-814721-4.00017-2.
- Schenker, S., and T. Rothgeb. 2017. The roast-creating the Beans’ signature. In The craft and science of coffee, ed. B. Folmer, 245–71. London: Academic Press. doi: 10.1016/B978-0-12-803520-7.00011-6.
- Schubert, T. 2017. Current and future. Ionic liquid markets. In Ionic liquids: current state and future directions, 35–65. Washington, DC: American Chemical Society.
- Sharma, G., and B. Kandasubramanian. 2020. Molecularly imprinted polymers for selective recognition and extraction of heavy metal ions and toxic dyes. Journal of Chemical & Engineering Data 65 (2):396–418. doi: 10.1021/acs.jced.9b00953.
- Shen, X., C. Xu, and L. Ye. 2013. Molecularly imprinted polymers for clean water: analysis and purification. Industrial & Engineering Chemistry Research 52 (39):13890–9. doi: 10.1021/ie302623s.
- Singh, S. K., and A. W. Savoy. 2020. Ionic liquids synthesis and applications: an overview. Journal of Molecular Liquids 297:112038. doi: 10.1016/j.molliq.2019.112038.
- Soares, C. M. D., R. C. Alves, M. Beatriz, and P. P. Oliveira. 2015. Chapter 24 - Factors affecting acrylamide levels in coffee beverages. In Coffee in health and disease prevention, ed. V. R. Preedy, 217–24. San Diego: Academic Press. doi: 10.1016/B978-0-12-409517-5.00024-3.
- Sorribes-Soriano, A., R. Arráez-González, F. A. Esteve-Turrillas, S. Armenta, and J. M. Herrero-Martínez. 2019. Development of a molecularly imprinted monolithic polymer disk for agitation-extraction of ecgonine methyl ester from environmental water. Talanta 199:388–95. doi: 10.1016/j.talanta.2019.02.077.
- Speltini, A., A. Scalabrini, F. Maraschi, M. Sturini, and A. Profumo. 2017. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: a review. Analytica Chimica Acta 974:1–26. doi: 10.1016/j.aca.2017.04.042.
- Stadler, R., F. Robert, S. Riediker, N. Varga, T. Davidek, S. Devaud, T. Goldmann, J. Hau, and I. Blank. 2004. In-depth mechanistic study on the formation of acrylamide and other vinylogous compounds by the Maillard reaction. Journal of Agricultural and Food Chemistry 52 (17):5550–8. doi: 10.1021/jf0495486.
- Tao, G. H., L. He, N. Sun, and Y. Kou. 2005. New generation ionic liquids: cations derived from amino acids. Chemical Communications 28 (28):3562–4. doi: 10.1039/b504256a.
- Tian, M., W. Bi, and K. H. Row. 2011. Molecular imprinting in ionic liquid-modified porous polymer for recognitive separation of three tanshinones from Salvia miltiorrhiza bunge. Analytical and Bioanalytical Chemistry 399 (7):2495–502. doi: 10.1007/s00216-010-4641-4.
- Toledo-Hijo, A. A., G. J. Maximo, M. C. Costa, E. A. Batista, and A. J. Meirelles. 2016. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustainable Chemistry & Engineering 4 (10):5347–69. doi: 10.1021/acssuschemeng.6b00560.
- Toledo, B. R., L. W. Hantao, T. D. Ho, F. Augusto, and J. L. Anderson. 2014. A chemometric approach toward the detection and quantification of coffee adulteration by solid-phase microextraction using polymeric ionic liquid sorbent coatings. Journal of Chromatography. A 1346:1–7. doi: 10.1016/j.chroma.2014.04.035.
- Turiel, E., and A. Martín- Esteban. 2020. Molecularly imprinted polymers. In Solid-phase extraction, ed. C. F. Poole, 215–33. Cambridge, USA: Elsevier. doi: 10.1016/B978-0-12-816906-3.00008-X.
- Van, M. 2006. Formation of flavour compounds in the Maillard reaction. Biotechnology Advances 24 (2):230–3. doi: 10.1016/j.biotechadv.2005.11.004.
- Vegro, C. L., and L. F. de Almeida. 2020. Global coffee market: socio-economic and cultural dynamics. In Coffee consumption and industry strategies in Brazil, ed. L. F. Almeida and E. E. Sper, 3–19. India: Woodhead Publishing. doi: 10.1016/B978-0-12-814721-4.00001-9.
- Vraneš, M., A. Tot, J. Panić, S. Papović, S. Gadžurić, and D. Četojević-Simin. 2019. Towards edible ionic liquids-cholinium taurate. Journal of the Serbian Chemical Society 84 (9):991–1004. doi: 10.2298/JSC190413047V.
- Wackerlig, J., and R. Schirhagl. 2016. Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: a review. Analytical Chemistry 88 (1):250–61. doi: 10.1021/acs.analchem.5b03804.
- Wei, X., Y. Wang, J. Chen, R. Ni, J. Meng, Z. Liu, F. Xu, and Y. Zhou. 2019. Ionic liquids skeleton typed magnetic core-shell molecularly imprinted polymers for the specific recognition of lysozyme. Analytica Chimica Acta 1081:81–92. doi: 10.1016/j.aca.2019.07.025.
- WHO. 2005. Summary report of the sixty-fourth meeting of the joint FAO/WHO expert committee on food additive (JECFA). Rome, Italy. Washington DC: The ILSI Press International Life Sciences Institute.
- Wrodnigg, T. M., and B. Eder. 2001. The Amadori and Heyns rearrangements: landmarks in the history of carbohydrate chemistry or unrecognized synthetic opportunities?. In Glycoscience: epimerisation, isomerisation and rearrangement reactions of carbohydrates, ed. A. E. Stütz, 115–52. Berlin, Heidelberg: Springer Berlin Heidelberg. doi: 10.1007/3-540-44422-X_6.
- Xu, L., X. Qiao, Y. Ma, X. Zhang, and Z. Xu. 2012. Preparation of a hydrophilic molecularly imprinted polymer and its application in solid-phase extraction to determine of trace acrylamide in foods coupled with high-performance liquid chromatography. Food Analytical Methods 6 (3):838–44. doi: 10.1007/s12161-012-9491-6.
- Xu, W., Q. Dai, Y. Wang, X. Hu, P. Xu, R. Ni, and J. Meng. 2018. Creating magnetic ionic liquid-molecularly imprinted polymers for selective extraction of lysozyme. RSC Advances 8 (39):21850–6. doi: 10.1039/C8RA03818J.
- Xu, X.,. R. Liu, P. Guo, Z. Luo, X. Cai, H. Shu, Y. Ge, C. Chang, and Q. Fu. 2018. Fabrication of a novel magnetic mesoporous molecularly imprinted polymer based on pericarpium granati-derived carrier for selective absorption of bromelain. Food Chemistry 256:91–7. doi: 10.1016/j.foodchem.2018.02.118.
- Yang, W., P. Ma, T. Fan, Z. Zhou, H. Liu, and W. Xu. 2015. Optimal design of an imprinted preassembled system by quantum chemical calculations and preparation of a surface‐imprinted material for the selective removal of quinoline. Journal of Applied Polymer Science 132 (15): 41730:1–41730:10. doi: 10.1002/app.41730.
- Yuan, J., and M. Antonietti. 2011. Poly(ionic liquid)s: polymers expanding classical property profiles. Polymer 52 (7):1469–82. doi: 10.1016/j.polymer.2011.01.043.
- Zeng, H., X. Yu, J. Wan, and X. Cao. 2020. Rational design and synthesis of molecularly imprinted polymers (MIP) for purifying tylosin by seeded precipitation polymerization. Process Biochemistry 94:329–39. doi: 10.1016/j.procbio.2020.03.025.
- Zhang, C., C. Cagliero, S. A. Pierson, and J. L. Anderson. 2017. Rapid and sensitive analysis of polychlorinated biphenyls and acrylamide in food samples using ionic liquid-based in situ dispersive liquid-liquid microextraction coupled to headspace gas chromatography. Journal of Chromatography. A 1481:1–11. doi: 10.1016/j.chroma.2016.12.013.
- Zhang, C., X. Shi, F. Yu, and Y. Quan. 2020. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chemistry 317:126431. doi: 10.1016/j.foodchem.2020.126431.
- Zhang, Y., X. Qu, J. Yu, L. Xu, Z. Zhang, H. Hong, and C. Liu. 2014. 13C NMR aided design of molecularly imprinted adsorbents for selectively preparative separation of erythromycin. J Mater Chem B 2 (10):1390–9. doi: 10.1039/c3tb21636e.
- Zhao, F., Y. Meng, and J. L. Anderson. 2008. Polymeric ionic liquids as selective coatings for the extraction of esters using solid-phase microextraction. Journal of Chromatography. A 1208 (1–2):1–9. doi: 10.1016/j.chroma.2008.08.071.