Abstract
Critical illness leads to millions of deaths worldwide each year, with a significant surge due to the COVID-19 pandemic. Patients with critical illness are frequently associated with systemic metabolic disorders and malnutrition. The idea of intervention for critically ill patients through enteral and parenteral nutrition has been paid more and more attention gradually. However, current nutritional therapies focus on evidence-based practice, and there have been lacking holistic approaches for nutritional support assessment. Metabolomics is a well-established omics technique in system biology that enables comprehensive profiling of metabolites in a biological system and thus provides the underlying information expressed and modulated by all other omics layers. In recent years, with the development of high-resolution and accurate mass spectrometry, metabolomics entered a new “generation”, promoting its broader applications in critical care nutrition. In this review, we first described the technological development and milestones of next-generation metabolomics in the past 20 years. We then discussed the emerging roles of next-generation metabolomics in advancing our understanding of critical care nutrition, such as nutritional deficiency risk evaluation, metabolic mechanisms of nutritional therapies, and novel nutrition target identification.
Introduction
Critical illness is a life-threatening and severe condition that usually requires specialized medical and nursing care in an intensive care unit (ICU) such as severe burn, heart attack, kidney failure, sepsis, and severe COVID-19. It has become a significant public health issue, and the annual ICU admissions from emergency departments are increasing as the population ages (Adhikari et al. Citation2010; Vincent and Singer Citation2010). Moreover, there was a dramatic surge of critically ill patients worldwide due to the COVID-19 pandemic (Tyrrell et al. Citation2021). The presence of malnutrition in ICU patients is common because these patients are often in a state of excessive catabolism due to the inflammatory stress responses, sympathoexcitation, and endocrine system disturbances (Cattani et al. Citation2020). It was estimated that over 30% of critically ill patients suffer from malnutrition (Lew et al. Citation2017). Even the patients who do not have malnutrition upon admission are very prone to develop malnutrition later during their stay in ICU, which may eventually lead to adverse clinical outcomes (Bistrian et al. Citation1976; Braunschweig, Gomez, and Sheean Citation2000; Lew et al. Citation2017). Both biological and observational studies support the association between nutrition status and adverse outcomes in the critically ill patients (Mogensen et al. Citation2015). In addition, nutrient deficiencies contribute to some of the clinical symptoms of critical illness, such as encephalopathy, muscle weakness, peripheral neuropathy, skin and mucosal lesions (Casaer and Bellomo Citation2019). Therefore, adequate nutritional support is particularly needed to ensure the basic functional operation of vital organs (Kondrup Citation2014), reduce the incidence of nutrition-related complications, and improve the prognosis (Jolliet et al. Citation1999). Although the significance of nutritional therapy is noted, the current nutritional risk assessment tools for ICU patients have apparent limitations and have not been broadly validated in clinical studies yet (Zhang et al. Citation2021). More work is still needed to develop reliable tools for assessing nutritional status in critical care, evaluating the effects of nutritional therapy, and optimizing the personalized and precision intervention approaches.
Metabolomics is defined as the comprehensive characterization of the small molecules (<1500 Da) or metabolome in a biological system. Compared to the transcriptome and proteome, the metabolome is the “snapshot” of the current status of an organism and the direct readout of the cellular machinery in response to endogenous and exogenous factors, including nutrients and drugs (Nicholson and Lindon Citation2008; Zheng et al. Citation2022). Classic metabolomic approaches with single quadrupole gas chromatography coupled with mass spectrometry (1 D GC-MS) and nuclear magnetic resonance (NMR) have disadvantages such as limited metabolome coverage and co-eluting interferences (Fiehn Citation2016). In the last decade, with the development of high-resolution and accurate mass spectrometry (HRAM), metabolomics came to the next generation, promoting its broader applications in nutrition-related fields.
In this review, we presented the technological development and milestones of metabolomics in the past 20 years and performed a scientometric analysis of the literature regarding the use of next-generation metabolomics in critical care nutrition. We then described the common malnutrition and nutritional therapy for critically ill patients. Finally, we discussed the emerging roles of next-generation metabolomics in screening ICU patients with nutritional deficiency risks, elucidating the molecular mechanisms of nutritional therapies, identifying novel nutrition targets, and highlighting the metabolic differences between different types of nutritional support. Overall, our review provides the implications of next-generation metabolomics toward precision and personalized nutritional support in critical care.
Next-generation metabolomics
As an emerging and evolving omics technology, metabolomics has been practiced for over 20 years and has undergone three primary stages (). The term metabolomics was coined in the late ‘90s, and the traditional metabolomics was mainly performed using single quadrupole gas chromatography coupled with mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) (Fiehn Citation2016; Nicholson, Lindon, and Holmes Citation1999; Alseekh and Fernie Citation2018; Li, Pidatala, et al. Citation2014). In the last decade, metabolomics entered into a next-generation represented by the widely used high-resolution accurate-mass instruments (HRAM) in the field, such as time of flight mass spectrometry (TOF MS), Fourier-transform ion cyclotron resonance MS (FT-ICR MS), and Orbitrap (Orbital ion-trap) MS (Dettmer, Aronov, and Hammock Citation2007; Li et al. Citation2021; Han et al. Citation2018; Makarov Citation2019). The metabolome coverage and mass identification accuracy are dramatically expanded in the next generation metabolomic platforms. Additionally, linear ion-trap (LIT) is often added in combination with other mass analyzers such as TOF and FT-ICR in metabolomics to further improve the sensitivity of HRAM (Fu et al. Citation2019). At the same time, to overcome the challenges of compound identification with HRAM, our team and a few other groups have developed several broad-spectrum targeted metabolomics platforms using triple quadrupole MS (QQQ), which also become important toolkits in next-generation metabolomics. The advanced QQQ MS with high scan speed, ultra-sensitivity and fast polarity switch enables the simultaneous quantification of hundreds of metabolites in a single run (Li et al. Citation2020; Li et al. Citation2017; Li, Wang, et al. Citation2014; Yuan et al. Citation2012).
Figure 1. The main development stages of metabolomics technologies.
Abbreviations: GC-MS: Gas chromatography coupled with mass spectrometry; NMR: Nuclear magnetic resonance; TOF: Time-of-flight; QTOF: Quadrupole Time-of-flight; FT-ICR: Fourier-transform ion cyclotron resonance; LC: liquid chromatography, CE: Capillary electrophoresis; MALDI: Matrix-assisted laser desorption/ionization; DESI: Desorption electrospray ionization; LAESI: Laser ablation electrospray ionization; GCIB-SIMS: gas cluster ion beam secondary ion mass spectrometry.
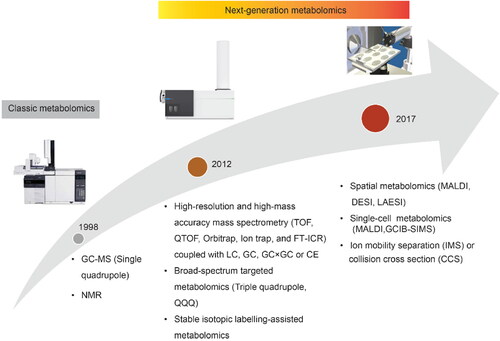
Chromatography-based metabolomics methods using GC, liquid chromatography (LC), and capillary electrophoresis (CE) separate the analytes based on their physical properties and fail to preserve the spatial distribution of metabolites. Recently, spatially resolved metabolomics approaches have been developed to quantify hundreds of metabolites while simultaneously providing their spatial information, which is valuable for understanding complex biological systems and physiological processes, particularly in heterogeneous tissues (Wang et al. Citation2022). Common MS imaging (MSI) tools used to explore the spatial information include matrix-assisted laser desorption ionization (MALDI) (Neumann et al. Citation2020), desorption electrospray ionization (DESI) (Huo et al. Citation2021), and laser ablation electrospray ionization (LAESI) mass spectrometry (Taylor, Lukowski, et al. Citation2021). In addition, to characterize the chemical heterogeneity between cells, an array of new techniques named single-cell metabolomics was developed using gas cluster ion beam secondary-ion mass spectrometry (GCIB-SIMS) (Taylor, Lukowski, et al. Citation2021), nanospray ionization mass spectrometry, and MALDI (Rappez et al. Citation2021; Seydel Citation2021; Castro et al. Citation2021). Single-cell metabolomics allows researchers to analyze the chemical contents of a single cell or even a single organelle (Castro et al. Citation2021).
Like other omics techniques, next-generation metabolomics produces information-rich and complex big data. Metabolomics data analysis is co-evolving to match the pace of the rapid development of analytical instrumentation. The metabolomic data analysis tools, databases, and resources developed before 2020 were reviewed (Misra Citation2021). From then on (2020 − 2022), several new tools have been reported primarily for multi-omics integration, network analysis and the application of machine learning and deep learning techniques. For example, MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) was recently built for LC-HRMS spectra processing and multi-omics integration (Pang et al. Citation2022). In addition, MetaboAnalyst 5.0 is also able to analyze the metabolomics data using the machine learning tools such as random forest, and support vector machines (SVM). A cloud-based knowledge database named foodMASST was recently created to support precision nutrition in many fields including critical care (West et al. Citation2022). Other useful databases for critical care nutrition include Human Metabolome Database (https://hmdb.ca/), foodDB (https://foodb.ca/), and PhytoHub (https://phytohub.eu/about).
Overview of the literature for the use of next-generation metabolomics in critical care nutrition
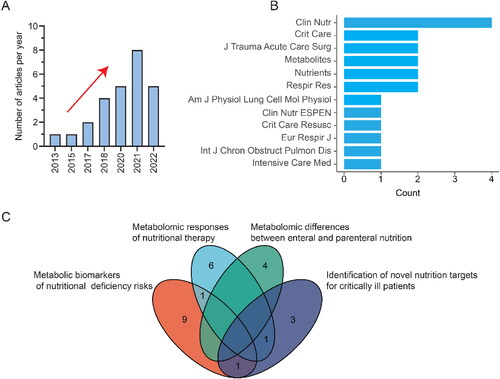
There is a rising trend toward the use of next-generation metabolomics in assessing nutrition support in critical care (). By April 1, 2022, 26 research articles were published and found in PubMed, and more than half of them were reported in the last 2 years. The top 12 journals are displayed in , and Clinical Nutrition is the most relevant journal. Keywords analysis showed that next-generation metabolomics plays an important role in personalized and precision nutrition support for critical illness. These include but are not limited to evaluating nutritional deficiency risks, elucidating the metabolic mechanisms of nutritional therapies, comparing enteral and parenteral nutrition, and identifying new nutrition targets ().
Malnutrition caused by metabolic dysregulation in critically ill patients and common nutritional support
Previous metabolomic studies revealed the presence of persistent metabolic abnormalities and inflammatory catabolic syndrome (PICS) in intensive care patients resulted in mild to severe malnutrition such as protein hydrolysis, electrolyte and minerals deficiency, vitamin deficiency, and fatty acids accumulation () (Wang et al. Citation2020; Langley et al. Citation2013; Chen et al. Citation2022). Adequate nutritional support is thus essential for these patients to meet their energy requirements during and after the ICU stay, protect against severe catabolism and prevent significant deconditioning (Singer Citation2019). The common nutritional therapies for ICU patients with malnutrition are summarized in .
Figure 3. Malnutrition caused by metabolic dysregulation in critically ill patients. Downward arrows in the text boxes and “-” sign indicate the decrease in concentration or activity. Upward arrows in the text boxes and “+” indicate the increase in concentration or activity.
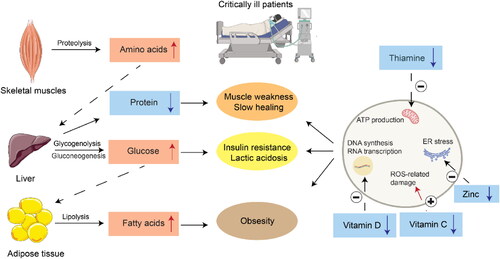
Table 1. Common nutritional therapy, metabolic pathways, and their clinical functions for critically ill patients.
Protein hydrolysis
Protein, the most important substance for human growth and development, has become the main energy source during critical illness (Sharma, Mogensen, and Robinson Citation2019). Since all proteins in the human body have specific functions, there is no reserve protein in the body to cope with the high metabolic state. Amino acids can only be transferred from peripheral tissues such as skeletal muscle to vital organs for gluconeogenesis and immune material synthesis, resulting in protein hydrolysis and related muscle loss (). The increased degradation of endogenous proteins is common in critically ill patients (Sharma, Mogensen, and Robinson Citation2019; Thiessen, Gunst, and Van den Berghe Citation2018; Hsu et al. Citation2021), and it was estimated that more than 40% of ICU patients had low plasma prealbumin levels in the acute stage of critical illness (Hsu et al. Citation2021; Bouharras El Idrissi et al. Citation2015). Therefore, protein supplementation has become an important part of nutritional intervention in critical care, and adequate protein supplementation might improve the clinical outcomes of critically ill patients (Hsu et al. Citation2021). Recent clinical trials reported that a high dose of protein (0.7 g/per kg body weight/day) could significantly reduce the 60-day mortality and the time course of ventilator utilization for critically ill patients with sepsis and pneumonia (Elke et al. Citation2014; Nicolo et al. Citation2016). The European Society of Parenteral and Enteral Nutrition (ESPEN) recommends 1.5 − 2.0 gram/kg body weight/day of protein for critically ill patients, including severe patients with COVID-19 (Singer et al. Citation2019). Overall, the amount of protein given to the patients should be dynamically adjusted based on the severity of the diseases.
Electrolytes and trace minerals disturbances
Electrolytes and trace elements play crucial roles in biochemical and physiological processes in the human body. Disturbances in electrolytes and trace minerals are among the most common clinical problems in the setting of critical care, such as hypomagnesemia and hypozincaemia (Limaye et al. Citation2011; Casaer and Van den Berghe Citation2014). Hypomagnesemia in ICU patients caused by insufficient intake and excessive loss of magnesium may lead to potentially fatal complications such as sepsis, respiratory failure, cardiac failure, lactic acidosis, and arrhythmia (Hansen and Bruserud Citation2018). Zinc, a cofactor for a wide range of enzymes, can enhance the catalytic activity of various enzymes and proteins (Jurowski et al. Citation2014), which in turn promote the normal function of mitochondria and oxidative phosphorylation, carbohydrate metabolism, as well as fatty acid metabolism (Yang et al. Citation2017). Zinc is also crucial for innate immunity by affecting the proliferation, differentiation, and maturation of leukocytes and lymphocytes (Hasan, Rink, and Haase Citation2013; Skalny et al. Citation2020). A previous study reported that plasma zinc concentration was below normal in critically ill patients and further reduced in the septic cohort (Besecker et al. Citation2011). In addition, lower zinc concentration in the plasma and serum of ICU patients was correlated with increased severity of illness, including cardiovascular dysfunction (Cander et al. Citation2011). As a result, the administration of zinc may be beneficial for critically ill patients (Casaer and Van den Berghe Citation2014; Al Sulaiman et al. Citation2021). However, electrolyte supplementation has been largely ignored in nutritional practice in the ICU (Hambidge Citation2000), and so far, there is inadequate evidence from clinical trials to recommend the routine administration of high-dose electrolytes in the critically ill (Heyland et al. Citation2008; Pieracci et al. Citation2014; Collie et al. Citation2017).
Vitamin depletion
Observational and clinical trial data indicate that vitamin depletion is common among critically ill patients, among which vitamin B1 (Thiamin), vitamin C, and vitamin D deficiencies are the most extensively studied (Casaer and Bellomo Citation2019; National Heart, Lung, Petal Clinical Trials Network Blood Institute et al. Citation2019).
Thiamin is a cofactor for multiple key enzymes, including pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and transketolase, which act at key junctures for the TCA cycle, pentose-phosphate shuttle, and NAPDH production (Frank, Leeper, and Luisi Citation2007; Donnino et al. Citation2016). Thus, severe thiamin deficiency may lead to impaired oxidative metabolic function in energy-dependent organs such as the heart or the brain, resulting in increased lactate and death (Manzanares and Hardy Citation2011; Campbell Citation1984). New data indicate that thiamin deficiency occurs in up to 35% of septic shock patients. The use of diuretics, continuous renal replacement therapy (CRRT), and insufficient intake are the potential risk factors for thiamin deficiency in these patients (Donnino et al. Citation2016). Vitamin C is a water-soluble vitamin that plays important roles as an electron donor, antioxidant, and enzyme cofactor. Serum vitamin C level is shown to be significantly lower than the normal range during critical illness, leading to free radical excess and, consequently, severe clinical symptoms such as organ failure (Gundogan et al. Citation2022; McNamara et al. Citation2018). The efficacy of thiamin and vitamin C supplementation in critical care has been studied in multiple clinical trials, and two recent meta-analyses showed that the use of thiamin alone or in combination with vitamin C had a significant reduction in the sequential organ failure assessment (SOFA) score and the length of ICU stays in critically ill patients (Shokri-Mashhadi et al. Citation2022; Shrestha et al. Citation2021).
Vitamin D deficiency is also a common risk factor associated with greater morbidity and mortality among critically ill patients (Rech, Hunsaker, and Rodriguez Citation2014). Given the essential roles of vitamin D, vitamin D supplementation was proposed for ICU patients. So far, several randomized controlled trials (RCTs) have been conducted, and a recent meta-analysis of previous trials suggested that the administration of vitamin D did not provide additional advantages over the placebo for critically ill patients (Lan et al. Citation2020). Further studies are needed to investigate the potential benefits of vitamin D supplementation in acute critical illness.
Lipids and fatty acids
Fatty acids can influence the inflammatory and immune processes via regulating the structure and function of cell membranes, modulating the inflammatory mediator profiles, and altering gene expression (Singer et al. Citation2009; Patkova et al. Citation2017). Specifically, ω-3 polyunsaturated fatty acids (PUFAs) displace arachidonic acid from cell membranes, antagonize pro-inflammatory eicosanoids (e.g., Prostaglandin E2 and leukotriene B4), promote the production of less inflammatory eicosanoids (e.g., thromboxane and prostaglandin E3), and inhibit inflammatory mediators (e.g., inducible NO synthase) (Todd et al. Citation2008). A previous meta-analysis reported a 60% reduction in 60-day mortality through the continuous administration of omega-3 fatty acids and full enteral nutrition to ICU patients (Preiser et al. Citation2015). Lorenzo et al. (Pradelli, Mayer, et al. Citation2020) performed a meta-analysis of 49 RCTs and found that ω‐3 fatty‐acid enriched parenteral nutrition (PN) significantly reduces the risk of infections and length of both ICU and hospital stays compared with standard PN (Pradelli, Klek, et al. Citation2020). Proper use of fish fat mixture can also reduce the occurrence of oxidative stress, acute respiratory distress syndrome (ARDS), and sepsis (Stachowska et al. Citation2020).
Roles of next-generation nutritional metabolomics in critical illness
Next-generation metabolomics provides a comprehensive approach in assessing the nutritional status of critically ill patients
The nutritional status of critically ill patients should be screened regularly due to their high risk for malnutrition, which impairs patients quality of life. Several screening tools have been developed, such as the Malnutrition Universal Screening Tool (MUST), Short Nutritional Assessment Questionnaire (SNAQ), and the Nutritional Risk Screening-2002 (NRS-2002) for hospitalized patients (Zhou et al. Citation2022). However, these screening approaches are not designed to be used in ICU and have apparent limitations for critically ill patients who are often unable to report their food intake history. Recently, a modified nutrition risk in the critically ill (mNUTRIC) score was developed but has not been fully validated (Wang et al. Citation2021).
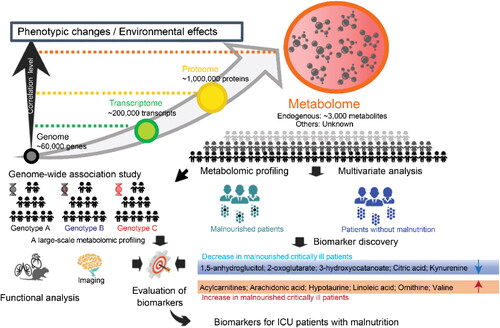
Next-generation metabolomics provides an objective and comprehensive strategy for evaluating the nutrition status of critically ill patients. Metabolomic analysis revealed significant differences in the plasma or serum metabolic patterns between critically ill patients with and without malnutrition (Mogensen et al. Citation2017; Lasky-Su et al. Citation2017; McMillan et al. Citation2017). For example, amino acids and the associated metabolites such as arginine, glutamine, kynurenine, phosphoserine, tryptophan, betaine, tyrosine, and valine were dramatically decreased in the plasma or serum of malnourished ICU patients () (Wen et al. Citation2022). The plasma or serum levels of metabolites from the tricarboxylic acid (TCA) cycle, such as citric acid and 2-oxoglutarate, were also observed to be decreased in patients with malnutrition (Alvarez et al. Citation2017). In contrast, acylcarnitines, n-6 polyunsaturated fatty acids, and arachidonic acid were increased in the plasma or serum of critically ill patients with malnutrition (). Additionally, serum vitamin D status was found to be correlated with plasma glutathione and glucuronidation-related metabolites (Lasky-Su et al. Citation2017).
Figure 4. Identification of metabolic biomarkers for nutritional deficiency risks in critically ill patients using the next-generation metabolomic technologies. Blue color indicates the trend of decrease in malnourished critically ill patients, and dark orange color indicates that the metabolites increase in the biological fluids of malnourished critically ill patients.
Next-generation metabolomics elucidates the metabolic mechanisms of nutritional therapies
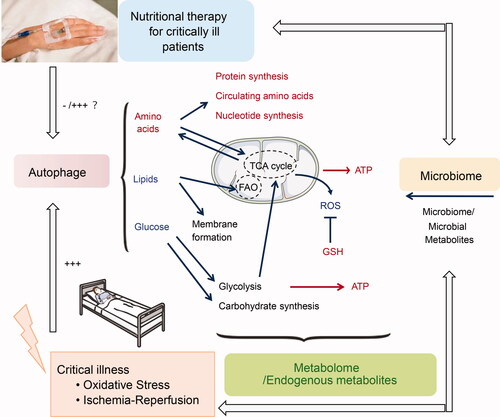
Conventional assessment of nutritional therapy is primarily based on the clinical outcomes for patients during the critical illness, such as the mortality, and length of ICU stay. However, it remains largely unclear why patients benefit from nutritional support. Next-generation metabolomics is now used to elucidate the metabolic changes in response to nutritional therapy, and global metabolomic profiling can reflect the desired treatment effects and mechanisms to restore homeostasis (). For example, metabolomic analysis of plasma samples from critically ill trauma patients revealed that peripheral amino acid infusions modulate many beneficial aspects of amino acid metabolism and may both decrease the inflammatory stimulus and lower the stress responses (Stolarski et al. Citation2022). An increase in 25-hydroxyvitamin D following high-dose vitamin D3 intervention resulted in favorable metabolomic changes involved in endothelial protection, enhanced innate immunity, and improved mitochondrial function (Amrein et al. Citation2021). In addition, the next-generation metabolomic analysis revealed sex-specific metabolic differences following vitamin D3 intervention, which represents an important move toward the understanding of personalized medicine (Chary et al. Citation2022). β-hydroxy-β-methylbutyrate (HMB), a metabolite of the essential amino acid leucine, is known to prevent or slow damage to muscle cells that occurs in critical illness. A recent RCT showed that daily HMB supplementation is associated with improved amino acid metabolism and reduced net protein breakdown (Viana et al. Citation2021).
Figure 5. The metabolomic changes in response to nutritional therapy in critically ill patients. +++ and red color indicate the increase after nutritional therapy, and – and blue color indicate decrease after nutritional therapy. Abbreviations: ROS: Reactive oxygen species; GSH: Reduced glutathione. TCA cycle: Tricarboxylic acid cycle; FAO: Fatty acid oxidation.
Next-generation metabolomics highlights metabolic differences between patients with enteral and parenteral nutrition
Enteral nutrition (EN) and parenteral nutrition (PN) are two common types of artificial nutritional therapy in ICU. Next-generation metabolomic profiling across various feeding approaches and biological fluids mechanistically presents a strategy to link the therapy actions to global metabolome changes. Metabolomic patterns differ between patients who received EN and PN, and the amount of energy supplied by EN or PN modulates metabolism (Gonzalez-Granda et al. Citation2021). In detail, the metabolic responses to EN were associated with the increase of amino acids, urea cycle upregulation, restoration of antioxidants, and the increase of RNA synthetic metabolites (Parent et al. Citation2017). In contrast, PN is less effective than those given enterally. It was reported that PN appears to only upregulates amino acid metabolism without influencing protein metabolism and antioxidant rebalance due to the bypass of the hepatic “first-pass” effect as EN (Parent et al. Citation2017). An energy deficit with the downregulation of the TCA cycle was reported in preterm newborns fed PN (Esturau-Escofet et al. Citation2022). The combination of EN and PN was shown to adequately cover the energy requirements for critically ill patients (Singer et al. Citation2021).
Next-generation metabolomics provides novel nutrition targets in critically injured patients
Critical illness induces metabolic changes that alter macro and micro-nutrient metabolism, while simultaneously, nutrient intake alters our physiologic responses to critical illness. MS-based next-generation metabolomics uniquely contributes to the direct analysis of small molecules as the potential new nutrition targets. For example, a recent metabolomic analysis revealed impaired nucleotide synthesis in critically ill trauma patients for the first 7 days after trauma compared to the healthy controls (Parent et al. Citation2016). Therefore, nucleotide supplementation is likely essential for these patients since the de novo nucleotide synthesis may not be sufficient in these critical periods. In addition, sulfur-containing amino acids (SAA), especially taurine and cysteine, were reported to decrease significantly with the progress of sepsis, which suggested that SAA supplementation may improve the prognosis of septic patients (Su et al. Citation2015). Previous metabolomic studies have highlighted the links between lysophosphatidylcholines (LPC) and their roles in sepsis pathology (Wang et al. Citation2020; Amunugama, Pike, and Ford Citation2021). They found that the major LPC molecular species (16:0 LPC, 18:0 LPC, 18:1 LPC, and 18:2 LPC) are significantly lower in serum and plasma of patients with sepsis (Park et al. Citation2019). Exogenous administration of LPC inhibits the production of the pro-inflammatory cytokines (IL-1β and TNF-α), reduces organ damage, and improves survival rates in septic mice and rats (Parra Millan et al. 2016; Yan et al. Citation2004).
Conclusions and future prospective
Critical care nutrition is still a young science, and the best practices are evolving. Proper timing, doses, nutrition routes, and duration of the nutritional support remain largely unknown (McKeever et al. Citation2021). In addition, current nutritional therapy has treated all patients as a homogenous group throughout the course of their ICU stay and has not accounted for heterogeneity in treatment effects (Stoppe et al. Citation2020). This array of clinical nutritional decisions will likely require further development in precision nutrition technologies, including next-generation metabolomics (O’Sullivan et al. Citation2018).
Despite the many applications described in this review, the impact of metabolomic profiling on critical care nutrition is still limited by access to MS technology owing to cost and the required expertise. We believe that this situation is changing as MS-based next-generation metabolomics is increasingly recognized as a powerful and versatile tool to elucidate mechanisms underlying nutritional interventions and monitor malnutrition. Nutritional metabolomics has also been hindered by the challenges of data integration with other -omics and clinical phenotypes and the lack of diet-related metabolite information in the databases, which limit our ability to extract meaningful conclusions from clinical trials. Therefore, there is a pressing need for multidisciplinary collaboration and standardized tools for comprehensive metabolomic measurements and big data analysis in critical care nutrition.
Anticipated further advancements in next-generation metabolomics, including MS-based instrumentation, software, and workflows, will enable the development of more personalized and predictive nutritional interventions for critically ill patients and eventually improve patient care in ICU.
Authors’ contributions
K. Li, H.HY. Tong, Y. Chen and J. Wang wrote and revised the paper. K. Li and J. Wang drew the figures used in the paper. Y. Sun offered insightful suggestions for the writing and revision of the paper. All authors read and approved the final manuscript.
| Abbreviations | ||
| EN | = | Enteral nutrition |
| PN | = | Parenteral nutrition |
| TCA | = | Tricarboxylic acid |
| FAO | = | Fatty acid oxidation |
| ROS | = | Reactive oxygen species |
| GSH | = | Reduced glutathione |
| ICU | = | Intensive care unit |
| MS | = | Mass spectrometry |
| GC-MS | = | Gas chromatography coupled with mass spectrometry |
| NMR | = | Nuclear magnetic resonance |
| HRAM | = | High-resolution accurate-mass instruments |
| TOF | = | Time of flight |
| QTOF | = | Quadrupole time of flight |
| FT-ICR | = | Fourier transform ion cyclotron resonance |
| QQQ | = | Triple quadrupole |
| LC | = | Liquid chromatography |
| CE | = | Capillary electrophoresis |
| MSI | = | MS imaging |
| MALDI | = | Matrix-assisted laser desorption ionization |
| DESI | = | Desorption electrospray ionization |
| LAESI | = | Laser ablation electrospray ionization |
| GCIB-SIMS | = | Gas cluster ion beam secondary-ion mass spectrometry |
| CCS | = | Collision cross section |
| IMS | = | Ion mobility separation |
| PICS | = | Persistent metabolic abnormalities and inflammatory catabolic syndrome |
| ESPEN | = | European Society of Parenteral and Enteral Nutrition |
| CRRT | = | Continuous renal replacement therapy |
| SOFA | = | Sequential organ failure assessment |
| PUFAs | = | Polyunsaturated fatty acids |
| ARDS | = | Acute respiratory distress syndrome |
| MUST | = | Malnutrition Universal Screening Tool |
| SNAQ | = | Short Nutritional Assessment Questionnaire |
| NRS-2002 | = | Nutritional Risk Screening-2002 |
| mNUTRIC | = | Modified nutrition risk in the critically ill |
| HMB | = | β-hydroxy-β-methylbutyrate |
| SAA | = | Sulfur-containing amino acids |
| LPC | = | lysophosphatidylcholines |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adhikari, N. K., R. A. Fowler, S. Bhagwanjee, and G. D. Rubenfeld. 2010. Critical care and the global burden of critical illness in adults. Lancet (London, England) 376 (9749):1339–46. doi: 10.1016/S0140-6736(10)60446-1.
- Al Sulaiman, K., O. Aljuhani, A. I. Al Shaya, A. Kharbosh, R. Kensara, A. Al Guwairy, A. Alharbi, R. Algarni, S. Al Harbi, R. Vishwakarma, et al. 2021. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: A two center propensity-score matched study. Critical Care (London, England) 25 (1):363. doi: 10.1186/s13054-021-03785-1.
- Alseekh, S., and A. R. Fernie. 2018. Metabolomics 20 years on: What have we learned and what hurdles remain? The Plant Journal: For Cell and Molecular Biology 94 (6):933–42. doi: 10.1111/tpj.13950.
- Alvarez, J. A., E. Y. Chong, D. I. Walker, J. D. Chandler, E. S. Michalski, R. E. Grossmann, K. Uppal, S. Li, J. K. Frediani, R. Tirouvanziam, et al. 2017. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: A pilot randomized study of high-dose vitamin D3 administration. Metabolism 70:31–41. doi: 10.1016/j.metabol.2017.02.006.
- Amrein, K., H. M. Oudemans-van Straaten, and M. M. Berger. 2018. Vitamin therapy in critically ill patients: Focus on thiamine, vitamin C, and vitamin D. Intensive Care Medicine 44 (11):1940–4. doi: 10.1007/s00134-018-5107-y.
- Amrein, K., J. A. Lasky-Su, H. Dobnig, and K. B. Christopher. 2021. Metabolomic basis for response to high dose vitamin D in critical illness. Clinical Nutrition (Edinburgh, Scotland) 40 (4):2053–60. doi: 10.1016/j.clnu.2020.09.028.
- Amunugama, K., D. P. Pike, and D. A. Ford. 2021. The lipid biology of sepsis. Journal of Lipid Research 62:100090. doi: 10.1016/j.jlr.2021.100090.
- Besecker, B. Y., M. C. Exline, J. Hollyfield, G. Phillips, R. A. Disilvestro, M. D. Wewers, and D. L. Knoell. 2011. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. The American Journal of Clinical Nutrition 93 (6):1356–64. doi: 10.3945/ajcn.110.008417.
- Bistrian, B. R., G. L. Blackburn, J. Vitale, D. Cochran, and J. Naylor. 1976. Prevalence of malnutrition in general medical patients. Jama 235 (15):1567–70. doi: 10.1001/jama.1976.03260410023017.
- Bouharras El Idrissi, H., J. Molina Lopez, I. Perez Moreno, D. I. Florea, G. Lobo Tamer, L. Herrera-Quintana, A. Perez De La Cruz, M. R. Elvira, and E. M. Planells Del Pozo. 2015. Imbalances in protein metabolism in critical care patient with systemic inflammatory response syndrome at admission in intensive care unit. Nutricion Hospitalaria 32 (6):2848–54. doi: 10.3305/nh.2015.32.6.9827.
- Braunschweig, C., S. Gomez, and P. M. Sheean. 2000. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. Journal of the American Dietetic Association 100 (11):1316–22. doi: 10.1016/S0002-8223(00)00373-4.
- Campbell, C. H. 1984. The severe lacticacidosis of thiamine deficiency: Acute pernicious or fulminating beriberi. Lancet (London, England) 2 (8400):446–9. doi: 10.1016/s0140-6736(84)92918-0.
- Cander, B., Z. D. Dundar, M. Gul, and S. Girisgin. 2011. Prognostic value of serum zinc levels in critically ill patients. Journal of Critical Care 26 (1):42–6. doi: 10.1016/j.jcrc.2010.06.002.
- Casaer, M. P., and G. Van den Berghe. 2014. Nutrition in the acute phase of critical illness. The New England Journal of Medicine 370 (25):2450–1. doi: 10.1056/NEJMc1404896.
- Casaer, M. P., and R. Bellomo. 2019. Micronutrient deficiency in critical illness: An invisible foe? Intensive Care Medicine 45 (8):1136–9. doi: 10.1007/s00134-019-05678-y.
- Castro, D. C., Y. R. Xie, S. S. Rubakhin, E. V. Romanova, and J. V. Sweedler. 2021. Image-guided MALDI mass spectrometry for high-throughput single-organelle characterization. Nature Methods 18 (10):1233–8. doi: 10.1038/s41592-021-01277-2.
- Cattani, A., I. C. Eckert, J. E. Brito, R. F. Tartari, and F. M. Silva. 2020. Nutritional risk in critically ill patients: How it is assessed, its prevalence and prognostic value: A systematic review. Nutrition Reviews 78 (12):1052–68. doi: 10.1093/nutrit/nuaa031.
- Chary, S., K. Amrein, S. H. Mahmoud, J. A. Lasky-Su, and K. B. Christopher. 2022. Sex-specific catabolic metabolism alterations in the critically ill following high dose vitamin D. Metabolites 12 (3):207. doi: 10.3390/metabo12030207.
- Chen, Q., X. Liang, T. Wu, J. Jiang, Y. Jiang, S. Zhang, Y. Ruan, H. Zhang, C. Zhang, P. Chen, et al. 2022. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis. Journal of Translational Medicine 20 (1):123. doi: 10.1186/s12967-022-03320-y.
- Collie, J. T. B., R. F. Greaves, O. A. H. Jones, Q. Lam, G. M. Eastwood, and R. Bellomo. 2017. Vitamin B1 in critically ill patients: Needs and challenges. Clinical Chemistry and Laboratory Medicine 55 (11):1652–68. doi: 10.1515/cclm-2017-0054.
- Dettmer, K., P. A. Aronov, and B. D. Hammock. 2007. Mass spectrometry-based metabolomics. Mass Spectrometry Reviews 26 (1):51–78. doi: 10.1002/mas.20108.
- Donnino, M. W., L. W. Andersen, M. Chase, K. M. Berg, M. Tidswell, T. Giberson, R. Wolfe, A. Moskowitz, H. Smithline, L. Ngo, Center for Resuscitation Science Research Group, et al. 2016. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: A pilot study. Critical Care Medicine 44 (2):360–7. doi: 10.1097/CCM.0000000000001572.
- Elke, G., M. Wang, N. Weiler, A. G. Day, and D. K. Heyland. 2014. Close to recommended caloric and protein intake by enteral nutrition is associated with better clinical outcome of critically ill septic patients: Secondary analysis of a large international nutrition database. Critical Care 18 (1):R29. doi: 10.1186/cc13720.
- Esturau-Escofet, N., E. Rodriguez de San Miguel, M. Vela-Amieva, M. E. Garcia-Aguilera, C. C. Hernandez-Espino, L. Macias-Kauffer, C. Lopez-Candiani, J. J. Naveja, and I. Ibarra-Gonzalez. 2022. A longitudinal (1)H NMR-based metabolic profile analysis of urine from hospitalized premature newborns receiving enteral and parenteral nutrition. Metabolites 12 (3):255. doi: 10.3390/metabo12030255.
- Fiehn, O. 2016. Metabolomics by gas chromatography-mass spectrometry: Combined targeted and untargeted profiling. Current Protocols in Molecular Biology 114 (1):30.4.1–30.4.32. doi: 10.1002/0471142727.mb3004s114.
- Frank, R. A., F. J. Leeper, and B. F. Luisi. 2007. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cellular and Molecular Life Sciences: CMLS 64 (7–8):892–905. doi: 10.1007/s00018-007-6423-5.
- Fu, C., Q. Wu, Z. Zhang, Z. Xia, H. Ji, H. Lu, and Y. Wang. 2019. UPLC-ESI-IT-TOF-MS metabolomic study of the therapeutic effect of Xuefu Zhuyu decoction on rats with traumatic brain injury. Journal of Ethnopharmacology 245:112149. doi: 10.1016/j.jep.2019.112149.
- Gonzalez-Granda, A., B. Seethaler, M. Haap, R. Riessen, and S. C. Bischoff. 2021. Effect of an intensified individual nutrition therapy on serum metabolites in critically ill patients - A targeted metabolomics analysis of the ONCA study. Clinical Nutrition ESPEN 43:267–75. doi: 10.1016/j.clnesp.2021.04.002.
- Gostyńska, A., M. Stawny, K. Dettlaff, and A. Jelińska. 2019. Clinical nutrition of critically ill patients in the context of the latest ESPEN guidelines. Medicina (Kaunas) 55 (12):770. doi: 10.3390/medicina55120770.
- Gundogan, K., F. S. Yucesoy, N. T. Ozer, S. Temel, S. Sahin, G. G. Sahin, M. Sungur, A. Esmaoglu, T. Talih, C. Yazici, et al. 2022. Serum micronutrient levels in critically ill patients receiving continuous renal replacement therapy: A prospective, observational study. JPEN. Journal of Parenteral and Enteral Nutrition 46 (5):1141–8. doi: 10.1002/jpen.2378.
- Hambidge, M. 2000. Human zinc deficiency. The Journal of Nutrition 130 (5S Suppl):1344S–9S. doi: 10.1093/jn/130.5.1344S.
- Han, J., Y. Xia, L. Lin, Z. Zhang, H. Tian, and K. Li. 2018. Next-generation metabolomics in the development of new antidepressants: Using albiflorin as an example. Current Pharmaceutical Design 24 (22):2530–40. doi: 10.2174/1381612824666180727114134.
- Hansen, B. A., and O. Bruserud. 2018. Hypomagnesemia in critically ill patients. Journal of Intensive Care 6:21. doi: 10.1186/s40560-018-0291-y.
- Hasan, R., L. Rink, and H. Haase. 2013. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immunity 19 (3):253–64. doi: 10.1177/1753425912458815.
- Heyland, D. K., N. Jones, N. Z. Cvijanovich, and H. Wong. 2008. Zinc supplementation in critically ill patients: A key pharmaconutrient? JPEN. Journal of Parenteral and Enteral Nutrition 32 (5):509–19. doi: 10.1177/0148607108322402.
- Honeywell, S., R. Zelig, and D. R. Radler. 2019. Impact of Intravenous Lipid emulsions containing fish oil on clinical outcomes in critically ill surgical patients: A literature review. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition 34 (1):112–22. doi: 10.1002/ncp.10224.
- Hsu, C. C., C. Y. Sun, C. Y. Tsai, M. Y. Chen, S. Y. Wang, J. T. Hsu, C. N. Yeh, and T. S. Yeh. 2021. Metabolism of proteins and amino acids in critical illness: From physiological alterations to relevant clinical practice. Journal of Multidisciplinary Healthcare 14:1107–17. doi: 10.2147/JMDH.S306350.
- Huo, M., Z. Wang, W. Fu, L. Tian, W. Li, Z. Zhou, Y. Chen, J. Wei, and Z. Abliz. 2021. Spatially resolved metabolomics based on air-flow-assisted desorption electrospray ionization-mass spectrometry imaging reveals region-specific metabolic alterations in diabetic encephalopathy. Journal of Proteome Research 20 (7):3567–79. doi: 10.1021/acs.jproteome.1c00179.
- Jolliet, P., C. Pichard, G. Biolo, R. Chioléro, G. Grimble, X. Leverve, G. Nitenberg, I. Novak, M. Planas, J. C. Preiser, et al. 1999. Enteral nutrition in intensive care patients: A practical approach. Clinical Nutrition (Edinburgh, Scotland) 18 (1):47–56. doi: 10.1054/clnu.1998.0001.
- Jurowski, K., B. Szewczyk, G. Nowak, and W. Piekoszewski. 2014. Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. Journal of Biological Inorganic Chemistry : JBIC: A Publication of the Society of Biological Inorganic Chemistry 19 (7):1069–79. doi: 10.1007/s00775-014-1139-0.
- Kiabi, F., A. Alipour, H. Darvishi-Khezri, A. Aliasgharian, and A. Zeydi. 2017. Zinc supplementation in adult mechanically ventilated trauma patients is associated with decreased occurrence of ventilator-associated pneumonia: A secondary analysis of a prospective, observational study. Indian Journal of Critical Care Medicine 21 (1):34–9. doi: 10.4103/0972-5229.198324.
- Kondrup, J. 2014. Nutritional-risk scoring systems in the intensive care unit. Current Opinion in Clinical Nutrition and Metabolic Care 17 (2):177–82. doi: 10.1097/MCO.0000000000000041.
- Lan, S. H., C. C. Lai, S. P. Chang, L. C. Lu, S. H. Hung, and W. T. Lin. 2020. Vitamin D supplementation and the outcomes of critically ill adult patients: A systematic review and meta-analysis of randomized controlled trials. Scientific Reports 10 (1):14261. doi: 10.1038/s41598-020-71271-9.
- Langley, R. J., E. L. Tsalik, J. C. van Velkinburgh, S. W. Glickman, B. J. Rice, C. Wang, B. Chen, L. Carin, A. Suarez, R. P. Mohney, et al. 2013. An integrated clinico-metabolomic model improves prediction of death in sepsis. Science Translational Medicine 5 (195):195ra95. doi: 10.1126/scitranslmed.3005893.
- Lasky-Su, J., A. Dahlin, A. A. Litonjua, A. J. Rogers, M. J. McGeachie, R. M. Baron, L. Gazourian, D. Barragan-Bradford, L. E. Fredenburgh, A. M. K. Choi, et al. 2017. Metabolome alterations in severe critical illness and vitamin D status. Critical Care (London, England) 21 (1):193. doi: 10.1186/s13054-017-1794-y.
- Lew, C. C. H., R. Yandell, R. J. L. Fraser, A. P. Chua, M. F. F. Chong, and M. Miller. 2017. Association between malnutrition and clinical outcomes in the intensive care unit: A systematic review. JPEN. Journal of Parenteral and Enteral Nutrition 41 (5):744–58. doi: 10.1177/0148607115625638.
- Li, C., S. Chu, S. Tan, X. Yin, Y. Jiang, X. Dai, X. Gong, X. Fang, and D. Tian. 2021. Towards higher sensitivity of mass spectrometry: A perspective from the mass analyzers. Frontiers in Chemistry 9:813359. doi: 10.3389/fchem.2021.813359.
- Li, K., J. C. Naviaux, A. T. Bright, L. Wang, and R. K. Naviaux. 2017. A robust, single-injection method for targeted, broad-spectrum plasma metabolomics. Metabolomics: Official Journal of the Metabolomic Society 13 (10):122. doi: 10.1007/s11306-017-1264-1.
- Li, K., J. C. Naviaux, J. M. Monk, L. Wang, and R. K. Naviaux. 2020. Improved dried blood spot-based metabolomics: A targeted, broad-spectrum, single-injection method. Metabolites 10 (3):82. doi: 10.3390/metabo10030082.
- Li, K., V. R. Pidatala, R. Shaik, R. Datta, and W. Ramakrishna. 2014. Integrated metabolomic and proteomic approaches dissect the effect of metal-resistant bacteria on maize biomass and copper uptake. Environmental Science & Technology 48 (2):1184–93. doi: 10.1021/es4047395.
- Li, K., X. Wang, V. R. Pidatala, C. P. Chang, and X. Cao. 2014. Novel quantitative metabolomic approach for the study of stress responses of plant root metabolism. Journal of Proteome Research 13 (12):5879–87. doi: 10.1021/pr5007813.
- Limaye, C. S., V. A. Londhey, M. Y. Nadkart, and N. E. Borges. 2011. Hypomagnesemia in critically ill medical patients. The Journal of the Association of Physicians of India 59:19–22.
- Makarov, A. 2019. Orbitrap journey: Taming the ion rings. Nature Communications 10 (1):3743. doi: 10.1038/s41467-019-11748-y.
- Manzanares, W., and G. Hardy. 2011. Thiamine supplementation in the critically ill. Current Opinion in Clinical Nutrition and Metabolic Care 14 (6):610–7. doi: 10.1097/MCO.0b013e32834b8911.
- McKeever, L., S. J. Peterson, O. Lateef, and C. Braunschweig. 2021. The influence of timing in critical care nutrition. Annual Review of Nutrition 41:203–22. doi: 10.1146/annurev-nutr-111120-114108.
- McMillan, A., A. E. Orimadegun, M. W. Sumarah, J. Renaud, M. M. da Encarnacao, G. B. Gloor, O. O. Akinyinka, G. Reid, and S. J. Allen. 2017. Metabolic derangements identified through untargeted metabolomics in a cross-sectional study of Nigerian children with severe acute malnutrition. Metabolomics 13 (2):1–14. doi: 10.1007/s11306-016-1150-2.
- McNamara, R., A. M. Deane, J. Anstey, and R. Bellomo. 2018. Understanding the rationale for parenteral ascorbate (vitamin C) during an acute inflammatory reaction: A biochemical perspective. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine 20 (3):174–9.
- Misra, B. B. 2021. New software tools, databases, and resources in metabolomics: Updates from 2020. Metabolomics: Official Journal of the Metabolomic Society 17 (5):49. doi: 10.1007/s11306-021-01796-1.
- Mogensen, K. M., J. Lasky-Su, A. J. Rogers, R. M. Baron, L. E. Fredenburgh, J. Rawn, M. K. Robinson, A. Massarro, A. M. Choi, and K. B. Christopher. 2017. Metabolites associated with malnutrition in the intensive care unit are also associated with 28-Day mortality. Journal of Parenteral and Enteral Nutrition 41 (2):188–97. doi: 10.1177/0148607116656164.
- Mogensen, K. M., M. K. Robinson, J. D. Casey, N. S. Gunasekera, T. Moromizato, J. D. Rawn, and K. B. Christopher. 2015. Nutritional status and mortality in the critically ill. Critical Care Medicine 43 (12):2605–15. doi: 10.1097/CCM.0000000000001306.
- National Heart, Lung, Petal Clinical Trials Network Blood Institute, Ginde, A. A., R. G. Brower, J. M. Caterino, L. Finck, V. M. Banner-Goodspeed, C. K. Grissom, D. Hayden, C. L. Hough, et al. 2019. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. The New England Journal of Medicine 381 (26):2529–40. doi: 10.1056/NEJMoa1911124.
- Neumann, E. K., L. G. Migas, J. L. Allen, R. M. Caprioli, R. Van de Plas, and J. M. Spraggins. 2020. Spatial metabolomics of the human kidney using MALDI trapped ion mobility imaging mass spectrometry. Analytical Chemistry 92 (19):13084–91. doi: 10.1021/acs.analchem.0c02051.
- Nicholson, J. K., and J. C. Lindon. 2008. Systems biology: Metabonomics. Nature 455 (7216):1054–6. doi: 10.1038/4551054a.
- Nicholson, J. K., J. C. Lindon, and E. Holmes. 1999. Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica; the Fate of Foreign Compounds in Biological Systems 29 (11):1181–9. doi: 10.1080/004982599238047.
- Nicolo, M., D. K. Heyland, J. Chittams, T. Sammarco, and C. Compher. 2016. Clinical outcomes related to protein delivery in a critically ill population: A multicenter, multinational observation study. JPEN. Journal of Parenteral and Enteral Nutrition 40 (1):45–51. doi: 10.1177/0148607115583675.
- O’Sullivan, A., B. Henrick, B. Dixon, D. Barile, A. Zivkovic, J. Smilowitz, D. Lemay, W. Martin, J. B. German, and S. E. Schaefer. 2018. 21st century toolkit for optimizing population health through precision nutrition. Critical Reviews in Food Science and Nutrition 58 (17):3004–15. doi: 10.1080/10408398.2017.1348335.
- Oudemans-van Straaten, H. M., A. M. Spoelstra-de Man, and M. C. de Waard. 2014. Vitamin C revisited. Critical Care (London, England) 18 (4):460. doi: 10.1186/s13054-014-0460-x.
- Pang, Z., G. Zhou, J. Ewald, L. Chang, O. Hacariz, N. Basu, and J. Xia. 2022. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nature Protocols 17 (8):1735–61. doi: 10.1038/s41596-022-00710-w.
- Parent, B. A., M. Seaton, D. Djukovic, H. Gu, B. Wheelock, S. L. Navarro, D. Raftery, and G. E. O’Keefe. 2017. Parenteral and enteral nutrition in surgical critical care: Plasma metabolomics demonstrates divergent effects on nitrogen, fatty-acid, ribonucleotide, and oxidative metabolism. The Journal of Trauma and Acute Care Surgery 82 (4):704–13. doi: 10.1097/TA.0000000000001381.
- Parent, B. A., M. Seaton, R. F. Sood, H. Gu, D. Djukovic, D. Raftery, and G. E. O’Keefe. 2016. Use of metabolomics to trend recovery and therapy after injury in critically ill trauma patients. JAMA Surgery 151 (7):e160853. doi: 10.1001/jamasurg.2016.0853.
- Park, J. M., J. Y. Noh, M. J. Kim, T. G. Yun, S. G. Lee, K. S. Chung, E. H. Lee, M. H. Shin, N. S. Ku, S. Yoon, et al. 2019. MALDI-TOF mass spectrometry based on parylene-matrix chip for the analysis of lysophosphatidylcholine in sepsis patient sera. Analytical Chemistry 91 (22):14719–27. doi: 10.1021/acs.analchem.9b04019.
- Parra Millan, R., M. E. Jimenez Mejias, V. Sanchez Encinales, R. Ayerbe Algaba, A. Gutierrez Valencia, M. E. Pachon Ibanez, C. Diaz, J. Perez Del Palacio, L. F. Lopez Cortes, J. Pachon, et al. 2016. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrobial Agents and Chemotherapy 60 (8):4464–70. doi: 10.1128/AAC.02708-15.
- Patkova, A., V. Joskova, E. Havel, M. Kovarik, M. Kucharova, Z. Zadak, and M. Hronek. 2017. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: A systematic review. Advances in Nutrition 8 (4):624–34. doi: 10.3945/an.117.015172.
- Pieracci, F. M., R. T. Stovall, B. Jaouen, M. Rodil, A. Cappa, C. C. Burlew, D. N. Holena, R. Maier, S. Berry, J. Jurkovich, et al. 2014. A multicenter, randomized clinical trial of IV iron supplementation for anemia of traumatic critical illness. Critical Care Medicine 42 (9):2048–57. doi: 10.1097/CCM.0000000000000408.
- Pradelli, L., K. Mayer, S. Klek, A. J. Omar Alsaleh, R. A. C. Clark, M. D. Rosenthal, A. R. Heller, and M. Muscaritoli. 2020. Omega-3 fatty-acid enriched parenteral nutrition in hospitalized patients: Systematic review with meta-analysis and trial sequential analysis. Journal of Parenteral and Enteral Nutrition 44 (1):44–57. doi: 10.1002/jpen.1672.
- Pradelli, L., S. Klek, K. Mayer, A. J. Omar Alsaleh, M. D. Rosenthal, A. R. Heller, and M. Muscaritoli. 2020. Omega-3 fatty acid-containing parenteral nutrition in ICU patients: Systematic review with meta-analysis and cost-effectiveness analysis. Critical Care (London, England) 24 (1):634. doi: 10.1186/s13054-020-03356-w.
- Preiser, J. C., A. R. van Zanten, M. M. Berger, G. Biolo, M. P. Casaer, G. S. Doig, R. D. Griffiths, D. K. Heyland, M. Hiesmayr, G. Iapichino, et al. 2015. Metabolic and nutritional support of critically ill patients: Consensus and controversies. Critical Care (London, England) 19:35. doi: 10.1186/s13054-015-0737-8.
- Rappez, L., M. Stadler, S. Triana, R. M. Gathungu, K. Ovchinnikova, P. Phapale, M. Heikenwalder, and T. Alexandrov. 2021. SpaceM reveals metabolic states of single cells. Nature Methods 18 (7):799–805. doi: 10.1038/s41592-021-01198-0.
- Rech, M. A., T. Hunsaker, and J. Rodriguez. 2014. Deficiency in 25-hydroxyvitamin D and 30-day mortality in patients with severe sepsis and septic shock. American Journal of Critical Care: An Official Publication, American Association of Critical-Care Nurses 23 (5):e72-9–e79. doi: 10.4037/ajcc2014723.
- Seydel, C. 2021. Single-cell metabolomics hits its stride. Nature Methods 18 (12):1452–6. doi: 10.1038/s41592-021-01333-x.
- Sharma, K., K. M. Mogensen, and M. K. Robinson. 2019. Pathophysiology of critical illness and role of nutrition. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition 34 (1):12–22. doi: 10.1002/ncp.10232.
- Shokri-Mashhadi, N., A. Aliyari, Z. Hajhashemy, S. Saadat, and M. H. Rouhani. 2022. Is it time to reconsider the administration of thiamine alone or in combination with vitamin C in critically ill patients? A meta-analysis of clinical trial studies. Journal of Intensive Care 10 (1):8. doi: 10.1186/s40560-022-00594-8.
- Shrestha, D. B., P. Budhathoki, Y. R. Sedhai, S. K. Mandal, S. Shikhrakar, S. Karki, R. K. Baniya, M. G. Kashiouris, X. Qiao, and A. A. Fowler. 2021. Vitamin C in critically ill patients: An updated systematic review and meta-analysis. Nutrients 13 (10):3564. doi: 10.3390/nu13103564.
- Singer, P. 2019. Preserving the quality of life: Nutrition in the ICU. Critical Care (London, England) 23 (Suppl 1):139. doi: 10.1186/s13054-019-2415-8.
- Singer, P., A. R. Blaser, M. M. Berger, W. Alhazzani, P. C. Calder, M. P. Casaer, M. Hiesmayr, K. Mayer, J. C. Montejo, C. Pichard, et al. 2019. ESPEN guideline on clinical nutrition in the intensive care unit. Clinical Nutrition (Edinburgh, Scotland) 38 (1):48–79. doi: 10.1016/j.clnu.2018.08.037.
- Singer, P., I. Bendavid, R. Mesilati-Stahy, P. Green, M. Rigler, S. Lev, S. Schif-Zuck, A. Amiram, M. Theilla, and I. Kagan. 2021. Enteral and supplemental parenteral nutrition enriched with omega-3 polyunsaturated fatty acids in intensive care patients - A randomized, controlled, double-blind clinical trial. Clinical Nutrition 40 (5):2544–54. doi: 10.1016/j.clnu.2021.03.034.
- Singer, P., M. M. Berger, G. Van den Berghe, G. Biolo, P. Calder, A. Forbes, R. Griffiths, G. Kreyman, X. Leverve, and C. Pichard. 2009. ESPEN guidelines on parenteral nutrition: Intensive care. Clinical Nutrition (Edinburgh, Scotland) 28 (4):387–400. doi: 10.1016/j.clnu.2009.04.024.
- Skalny, A. V., L. Rink, O. P. Ajsuvakova, M. Aschner, V. A. Gritsenko, S. I. Alekseenko, A. A. Svistunov, D. Petrakis, D. A. Spandidos, J. Aaseth, et al. 2020. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). International Journal of Molecular Medicine 46 (1):17–26. doi: 10.3892/ijmm.2020.4575.
- Stachowska, E., M. Folwarski, D. Jamioł-Milc, D. Maciejewska, and K. Skonieczna-Żydecka. 2020. Nutritional support in Coronavirus 2019 Disease. Medicina (Kaunas) 56 (6):289. doi: 10.3390/medicina56060289.
- Stolarski, A. E., L. Young, J. Weinberg, J. Kim, E. Lusczek, D. G. Remick, B. Bistrian, and P. Burke. 2022. Early metabolic support for critically ill trauma patients: A prospective randomized controlled trial. Journal of Trauma and Acute Care Surgery 92 (2):255–65. doi: 10.1097/TA.0000000000003453.
- Stoppe, C., S. Wendt, N. M. Mehta, C. Compher, J. C. Preiser, D. K. Heyland, and A. S. Kristof. 2020. Biomarkers in critical care nutrition. Critical Care (London, England) 24 (1):499. doi: 10.1186/s13054-020-03208-7.
- Su, L., H. Li, A. Xie, D. Liu, W. Rao, L. Lan, X. Li, F. Li, K. Xiao, H. Wang, et al. 2015. Dynamic changes in amino acid concentration profiles in patients with sepsis. PloS One 10 (4):e0121933. doi: 10.1371/journal.pone.0121933.
- Taylor, M. J., J. K. Lukowski, and C. R. Anderton. 2021. Spatially resolved mass spectrometry at the single cell: Recent innovations in proteomics and metabolomics. Journal of the American Society for Mass Spectrometry 32 (4):872–94. doi: 10.1021/jasms.0c00439.
- Taylor, M. J., S. Mattson, A. Liyu, S. A. Stopka, Y. M. Ibrahim, A. Vertes, and C. R. Anderton. 2021. Optical microscopy-guided laser ablation electrospray ionization ion mobility mass spectrometry: Ambient single cell metabolomics with increased confidence in molecular identification. Metabolites 11 (4):200. doi: 10.3390/metabo11040200.
- Thiessen, S. E., J. Gunst, and G. Van den Berghe. 2018. Role of glucagon in protein catabolism. Current Opinion in Critical Care 24 (4):228–34. doi: 10.1097/MCC.0000000000000509.
- Todd, S. R., E. A. Gonzalez, K. Turner, and R. A. Kozar. 2008. Update on postinjury nutrition. Current Opinion in Critical Care 14 (6):690–5. doi: 10.1097/MCC.0b013e3283196562.
- Tyrrell, C. S. B., O. T. Mytton, S. V. Gentry, M. Thomas-Meyer, J. L. Y. Allen, A. A. Narula, B. McGrath, M. Lupton, J. Broadbent, A. Ahmed, et al. 2021. Managing intensive care admissions when there are not enough beds during the COVID-19 pandemic: A systematic review. Thorax 76 (3):302–12. doi: 10.1136/thoraxjnl-2020-215518.
- Viana, M. V., F. Becce, O. Pantet, S. Schmidt, G. Bagnoud, J. J. Thaden, G. A. M. Ten Have, M. Engelen, A. Voidey, N. E. P. Deutz, et al. 2021. Impact of beta-hydroxy-beta-methylbutyrate (HMB) on muscle loss and protein metabolism in critically ill patients: A RCT. Clinical Nutrition 40 (8):4878–87. doi: 10.1016/j.clnu.2021.07.018.
- Vincent, J. L., and M. Singer. 2010. Critical care: Advances and future perspectives. Lancet (London, England) 376 (9749):1354–61. doi: 10.1016/S0140-6736(10)60575-2.
- Wang, J., Y. Sun, S. Teng, and K. Li. 2020. Prediction of sepsis mortality using metabolite biomarkers in the blood: A meta-analysis of death-related pathways and prospective validation. BMC Medicine 18 (1):83. doi: 10.1186/s12916-020-01546-5.
- Wang, L., X. Xing, X. Zeng, S. R. Jackson, T. TeSlaa, O. Al-Dalahmah, L. Z. Samarah, K. Goodwin, L. Yang, M. R. McReynolds, et al. 2022. Spatially resolved isotope tracing reveals tissue metabolic activity. Nature Methods 19 (2):223–30. doi: 10.1038/s41592-021-01378-y.
- Wang, N., M. P. Wang, L. Jiang, B. Du, B. Zhu, and X. M. Xi. 2021. Association between the modified Nutrition Risk in Critically Ill (mNUTRIC) score and clinical outcomes in the intensive care unit: A secondary analysis of a large prospective observational study. BMC Anesthesiology 21 (1):220. doi: 10.1186/s12871-021-01439-x.
- Wen, B., J. M. Njunge, C. Bourdon, G. B. Gonzales, B. M. Gichuki, D. Lee, D. S. Wishart, M. Ngari, E. Chimwezi, J. Thitiri, et al. 2022. Systemic inflammation and metabolic disturbances underlie inpatient mortality among ill children with severe malnutrition. Science Advances 8 (7):eabj6779. doi: 10.1126/sciadv.abj6779.
- West, K. A., R. Schmid, J. M. Gauglitz, M. Wang, and P. C. Dorrestein. 2022. foodMASST a mass spectrometry search tool for foods and beverages. NPJ Science of Food 6 (1):22. doi: 10.1038/s41538-022-00137-3.
- Wijnands, K. A., T. M. Castermans, M. P. Hommen, D. M. Meesters, and M. Poeze. 2015. Arginine and citrulline and the immune response in sepsis. Nutrients 7 (3):1426–63. doi: 10.3390/nu7031426.
- Yan, J. J., J. S. Jung, J. E. Lee, J. Lee, S. O. Huh, H. S. Kim, K. C. Jung, J. Y. Cho, J. S. Nam, H. W. Suh, et al. 2004. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nature Medicine 10 (2):161–7. doi: 10.1038/nm989.
- Yang, X., H. Wang, C. Huang, X. He, W. Xu, Y. Luo, and K. Huang. 2017. Zinc enhances the cellular energy supply to improve cell motility and restore impaired energetic metabolism in a toxic environment induced by OTA. Scientific Reports 7 (1):14669. doi: 10.1038/s41598-017-14868-x.
- Yuan, M., S. B. Breitkopf, X. Yang, and J. M. Asara. 2012. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nature Protocols 7 (5):872–81. doi: 10.1038/nprot.2012.024.
- Zhang, P., Y. Bian, Z. Tang, and F. Wang. 2021. Use of nutrition risk in critically ill (NUTRIC) scoring system for nutrition risk assessment and prognosis prediction in critically ill neurological patients: A prospective observational study. JPEN. Journal of Parenteral and Enteral Nutrition 45 (5):1032–41. doi: 10.1002/jpen.1977.
- Zheng, J., K. Wang, Y. Wang, and K. Li. 2022. Precautions for study design and data interpretation of clinical metabolomics. Proceedings of the National Academy of Sciences 119 (5):e2118654119. doi: 10.1073/pnas.2118654119.
- Zhou, X., J. Liu, Q. Zhang, S. Rao, X. Wu, J. Zhang, and J. Li. 2022. Comparison of the suitability between NRS2002 and MUST as the first-step screening tool for GLIM Criteria in hospitalized patients with GIST. Frontiers in Nutrition 9:864024. doi: 10.3389/fnut.2022.864024.