Abstract
Since the first years of history, microbial fermentation products such as bread, wine, yogurt and vinegar have always been noteworthy regarding their nutritional and health effects. Similarly, mushrooms have been a valuable food product in point of both nutrition and medicine due to their rich chemical components. Alternatively, filamentous fungi, which can be easier to produce, play an active role in the synthesis of some bioactive compounds, which are also important for health, as well as being rich in protein content. Therefore, this review presents some important bioactive compounds (bioactive peptides, chitin/chitosan, β-glucan, gamma-aminobutyric acid, L-carnitine, ergosterol and fructooligosaccharides) synthesized by fungal strains and their health benefits. In addition, potential probiotic- and prebiotic fungi were researched to determine their effects on gut microbiota. The current uses of fungal based bioactive compounds for cancer treatment were also discussed. The use of fungal strains in the food industry, especially to develop innovative food production, has been seen as promising microorganisms in obtaining healthy and nutritious food.
Highlights
Fungal-based bioactive compounds have various health benefits.
Prebiotic fungi play an active role in the regulation of gut microbiota.
Anti-tumor effective fungal components will contribute to alternative medicine.
Beta-glucan and chitin are the most promising fungal metabolites for cancer treatment.
Introduction
Mushrooms are one of the foods that have been widely consumed as food since the first periods of history, used for health, and even considered sacred by some cultures (Bernart, Chang and, and Miles Citation2005). Even in the oldest civilizations of history, mushrooms have a special place; The Greeks believed that it gave strength to the soldiers, and in wars, the soldiers were fed mushrooms. The Romans, on the other hand, sanctified the mushroom even more and saw it as the ‘food of the gods’ and therefore consumed it on special days and holidays. In Chinese culture, mushrooms have been seen as a healthy food for centuries and have even been called the ‘elixir of life’ (Ogidi, Oyetayo, and Akinyele Citation2020). Mushrooms which have taken their place in health studies for centuries, have recently; it is preferred more in scientific studies for reasons such as easier production and increased efficiency and storage conditions (Bernart, Chang and, and Miles Citation2005).
Filamentous fungi such as mushrooms have been used traditionally for many years to obtain high-protein food products by the fermentation of agricultural products such as rice and soybeans, especially in Indonesia, Japan, China and Korea. Zygomycete Rhizopus oryzae, R. delemar, R. oligosporus species have been used in the preparation of fermented foods such as tempeh. Ascomycetes Aspergillus oryzae (it’s fermented products are shoyu, miso, and hamanatto), Neurospora intermedia (it’s fermented product is oncom) and Monascus purpureus (it’s fermented product is red rice) are also fungal species used to produce traditional fermented products (Denny, Aisbitt, and Lunn Citation2008; Filho, Andersson, et al. Citation2019; Moore and Chiu Citation2001; Sar, Ferreira, et al. Citation2020). Alternatively, the biomass produced by Fusarium venenatum currently provides an alternative protein source as QuornTM containing 50% protein in the market (Gmoser et al. Citation2020; Wiebe Citation2002). Single-cell protein obtained from these fungal species (mycoprotein) is known as generally recognized as safe (GRAS) (Sar, Larsson, et al. Citation2022).
Filamentous fungi can be grown not only in cereal products but also in various food by-products () since they can secrete many different enzymes (amylase, invertase, cellulase, xylanase, lipase, and protease) (Awasthi, Harirchi, et al. Citation2022; Ferreira et al. Citation2016). It is possible to obtain biomass abundantly after a short incubation period using these wastes from laboratory-scale shake-flasks to industrial-scale fermenters (Rousta, Hellwig, et al. Citation2021; Sar, Larsson, et al. Citation2022). Moreover, different cultivation strategies such as submerged or solid-state fermentation can be utilized for fungal biomass production (Awasthi et al. Citation2023). In addition, the obtained fungal biomass can be evaluated as a food and/or animal feed source according to its composition (protein, fatty acid, mineral, etc.) and the type of substrate used (Sar, Larsson, et al. Citation2022; Strong et al. Citation2022; Uwineza et al. Citation2021). Moreover, the biomass obtained from oat flour and bread left over from the market has become an alternative food product such as hamburger (Hellwig et al. Citation2020; Rousta, Hellwig, et al. Citation2021; Rousta et al. Citation2022).
Table 1. Potential food industry based substrates for fungal biomass production.
Fungi are very rich in easily digestible protein content, polyunsaturated fatty acids antioxidants, dietary fiber, vitamins, and minerals (Alberti, Foster, and Bailey Citation2017; Grangeia et al. Citation2011). The bioactive compounds produced by fungi can be divided into two groups: high molecular weight compounds (e.g., polysaccharides and enzymes) and low molecular compounds (e.g., phenolic compounds). As the common feature of these two groups that we have separated; thanks to the immunomodulator ability of these components show some biological activities such as antiallergic, antibacterial, antiviral, antioxidant, anticancer, neuroprotective, anti-inflammatory or hypoglycemic effects (Gargano et al. Citation2017; Madhu et al. Citation2022; Varsha et al. Citation2022).
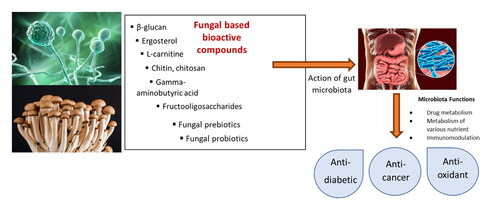
This study aimed to investigate the production and potential benefits of bioactive compounds (bioactive peptides, chitin/chitosan, β-glucan, gamma-aminobutyric acid, L-carnitine, ergosterol and fructooligosaccharides) synthesized by fungal species. In addition, potential probiotic and prebiotic fungi on gut microbiota were also mentioned. Moreover, the effects of bioactive compounds derived from fungi on health benefits were discussed in detail.
Bioactive compounds in fungi and their health benefits
Mushrooms and filamentous fungi are rich in protein, fibers, lipids, minerals and vitamins which are essential for human diet (Awasthi et al. Citation2023; Gmoser et al. Citation2020; Heleno et al. Citation2013; Sar, Larsson, et al. Citation2022). In addition, filamentous fungi can synthesize some important metabolites and components such as bioactive peptides, chitin/chitosan, β-glucan, gamma-aminobutyric acid, L-carnitine, ergosterol and fructooligosaccharides. These bioactive compounds synthesized by fungal strains are important in human nutrition and can play a critical role in the prevention of different diseases ( and ).
Table 2. Fungal based bioactive peptides and their potential health effects.
Table 3. Fungal based bioactive compounds and their potential health effects.
Bioactive peptides
Proteins are one of the fundamental macronutrients in the diet and their consumption is essential throughout one’s life. They have an important role in the organism’s growth and maintenance, and they act as enzymes, membrane transporters, metabolic process regulators, and oxygen transporters. Furthermore, proteins have also been shown to have health benefits whether intact or hydrolyzed (World Health Organization Citation2007). Hydrolyzed protein fragment, also known as bioactive peptides (Bp), have a positive impact on physiological functions and may eventually influence health in addition to their nutritional significance (Daliri, Oh, and Lee Citation2017; Korhonen and Pihlanto Citation2006; Sánchez and Vázquez Citation2017). Bioactive peptides have low molecular weight (<6000 D) and usually contain between 2 and 20 amino acids residues, and they may have an inherent resistance to digestion in the gastrointestinal tract. The peptides are inactive, when they are encrypted in the structure of proteins, once released due to proteolytic activity they may operate as physiological modulators with hormone-like activity (Lafarga and Hayes Citation2014; López-Barrios, Gutiérrez-Uribe, and Serna-Saldívar Citation2014; Montoya-Rodríguez et al. Citation2015; Mora and Hayes Citation2015; Sánchez and Vázquez Citation2017). The most studied bioactive peptides are present in meat, fish, dairy products, and legumes fermented using lactic acid bacteria, yeasts and Bacillus species (Cipolari, de Oliveira Neto, and Conceição Citation2020; Venegas-Ortega et al. Citation2019; Xing et al. Citation2019). Moreover, for filamentous fungi, legumes, particularly soybean, and some cereals such as quinoa, oat and lentil, are the most studied substrates for bioactive peptides (Magro et al. Citation2019; Nascimento et al. Citation2020; Tamam et al. Citation2019).
Producing bioactive peptide by fungal protease activity attract great attention from biotechnologists due to the ability of fungi to produce variety of protease which are active over a wide pH range as well as growing on low-cost substrate (Soukup, Keller, and Wiemann Citation2016). In addition using filamentous fungi as an starter culture for producing fermented food and beverages eliminates the need for extra enzymatic recovery processes, as well as the inconvenient extraction and exogenous treatment of enzymes (Chávez et al. Citation2015). Filamentous fungi are able to produce Bp through both solid-state fermentation (SSF) and submerged fermentation (SmF). The fungal based Bp composition may vary depending on the specificity of the fungal protease and the protein composition of the substrate. In addition to microbial species participating in fermentation, some physical characteristics such as temperature, pH, moisture content, salt extent, and cultivation time have a significant impact on the degree of proteolysis and synthesis of Bp (Martínez-Medina et al. Citation2019; CitationVerardo, Gómez-Caravaca, and Tabanelli 2020). Bioactive peptides, depending on the length and sequence of their amino acids, hydrophobicity and charge, exhibit different functional properties such as antioxidant (Lorenzo et al. Citation2018; D. P. Mohanty, Jena, et al. Citation2016), antimicrobial (Agyei and Danquah Citation2012; D. Mohanty, Jena, et al. Citation2016; Tan, Fu, and Ma Citation2021; Tan et al. Citation2022), immunomodulatory (Agyei and Danquah Citation2012; Santiago-López et al. Citation2016), antithrombic (Rojas-Ronquillo et al. Citation2012; Rutherfurd-Markwick and Moughan Citation2005), hypocholesterolemic (González-Ortega et al. Citation2015; Karami and Akbari-Adergani Citation2019; Peighambardoust et al. Citation2021) and antihypertensive activities (Aluko Citation2015; Kim, Ngo, and Vo Citation2012; Li et al. Citation2022).
Dipeptidyl peptidase (DPP) IV inhibitor peptides, Lys-Leu and Leu-Arg, derived from soybean fermented by A. oryzae, had previously been identified as antidiabetic agent (Sato et al. Citation2018). DPP-IV inactivates two hormones which promote insulin production. As a result DPP-IV inhibition is a molecular target for treating diabetes (Yan et al. Citation2019). Different studies showed the potent DPP-IV inhibitory peptides, composed of a branched chain amino acid such as leucine, isoleucine, or valine at their N-terminal position, or an aromatic residue with a polar group in the side chain mainly tryptophan (Nongonierma and FitzGerald Citation2019; Tulipano et al. Citation2015). Bioactive peptides also act as an antidiabetic agent by inhibitory effect on α-amylase and amyloglucosidase (glucoamylase). The inhibition of α-amylase up to 75% has been reported after fermentation of lentil flour by A. oryzae (Magro et al. Citation2019).
The antihypertensive impact of different peptides has been linked to the inhibition of the angiotensin-converting enzyme (ACE) resulted in lower of blood pressure. Aromatic amino acids as well as leucine, valine, lysine, arginine, and isoleucine have also been demonstrated to have a major impact on ACE inhibition (García-Mora et al. Citation2017; Pan et al. Citation2012). In addition, Gly-Tyr, Ala-Phe, Val-Pro, Ala-Ile, and Val-Gly are antihypertensive peptides generated by A. sojae after fermentation of soy sauce. The antihypertensive peptides Val-Pro-Pro and Ile-Pro-Pro were generated by fermenting miso with A. oryzae (Tamam et al. Citation2019).
The bioactive peptides with hydrophobic amino acids (proline, histidine, tyrosine or tryptophan), phenolics (tyrosine, tryptophan or phenylalanine) and reductive amino acids (methionine or cysteine) are well known with their antioxidant activities (Elias, Kellerby, and Decker Citation2008; He et al. Citation2012; Roblet et al. Citation2012). Antioxidative peptides were defined after fermentation of okara (known as soy pulp) by A. oryzae, R. oligosporus, and Actinomucor elegans. Moreover, antioxidant activities increased due to the significant accumulation of protein hydrolysis end products (peptides and amino acids) after fermentation of colored quinoa fermentation using by R. oligosporus, A. oryzae and N. intermedia (Starzyńska-Janiszewska et al. Citation2019). The tripeptide of Gln-Tyr-Pro in fermented meat sauce (FMS) by Aspergillus sp. showed extremely high antioxidant activity against OH radical (Ohata et al. Citation2016).
Anticancer peptides (ACPs) have a cationic charge (Chen et al. Citation2016). They are amphipathic and usually abundant in arginine and lysine. Anticancer activity of bioactive peptide has been reported after enzymatic hydrolyze of shellfish by A. oryzae. The sequence was partially purified as His-Phe-Asn-Ile-Gly-Asn-Arg-Cys-Leu-Cys at the N-terminus. This peptide effectively induced the death of prostate, breast, and lung cancer cells but not the normal liver cells (Cheong et al. Citation2013; Yang et al. Citation2012).
The antithrombotic bioactivity of filamentous fungi is referred to their ability to secret fibrinolytic protease. This enzyme specifically degrade fibrin, which is the main component of thrombus (CitationVerardo, Gómez-Caravaca, and Tabanelli 2020). This bioactivity has significant role on preventing and managing cardiovascular disease with removing blood clots. Many studies have been shown the anti-thrombotic activities of different types of filamentous fungi such as Neurospora sitophila, N. crassa (Duan et al. Citation2022), Mucor subtilissimus (Nascimento et al. Citation2017), A. oryzae (Shirasaka et al. Citation2012), Rhizopus sp. (Polanowska et al. Citation2020), R. chinensis (Xiao-Lan et al. Citation2005) and Fusarium sp. (Wu and Xu Citation2012). The bioactivities of Bp produced by filamentous fungi are shown in .
Chitin-chitosan
Chitin and chitosan are the major structural biopolymer in exoskeletons of invertebrates, insects, and crustaceans as well as in fungi (Hamed, Özogul, and Regenstein Citation2016). The molecule is classified as chitin or chitosan, based on the frequency of the latter monosaccharides and chitin can be converted to chitosan by partial deacetylation of the monomer N-acetyl-D-glucosamine to D-glucosamine (Stevens Citation2019). Chitin and chitosan provides important structural stability to fungal cell walls and their biosynthesis are catalyzed by chitin synthases (CHSs), a family of integral membrane proteins, by transferring N-acetylglucosamine from uridine diphosphate (UDP)-N-acetylglucosamine to a growing chitin chain (M. Li, Jiang, et al. Citation2016).
Among the fungal phyla, the cell wall of the Ascomycetes, Zygomycetes, Basidiomycetes, and Deuteromycetes have mostly chitin and chitosan (George et al. Citation2011). However, the chitin and chitosan contents of fungal cell walls vary between different fungal species (e.g., 2% in dry yeast cells and 60% in Mucor rouxii) (Abo Elsoud and El Kady Citation2019; Knezevic-Jugovic, Petronijevic, and Smelcerovic Citation2011). It appears that the chitin, chitosan content of the cell wall is higher in Zygomycetes, notably M. rouxii, M. mucedo, M. circinelloides, Rhizomucor miehei, Rhizopus oryzae, Phycomyces blakesleeanus, and Cunninghamella elegans (Knezevic-Jugovic, Petronijevic, and Smelcerovic Citation2011; Zamani et al. Citation2007). In addition to fungal strain, the age of the producing microbial cells, cultivation medium, and fermentation type influence the chitinous composition of the fungal cell wall. Li et al. (Citation2021) found that reducing the pH and supplementing extra nitrogen sources increased the chitinous content of A.oryzae.
Chitin and chitosan are well-studied bioactive compounds with numerous biological activities on human health. Chitin is an insoluble dietary fiber and its structure is analogous to indigestible cellulose in plants (Kostag and El Seoud Citation2021). It prevents and treats constipation as well as reduce the risk of colorectal condition such as hemorrhoids and diverticulitis (Debnath et al. Citation2019).
Several studies have found that chitin and chitosan are excellent cholesterol-lowering and fat-blocking substances (Chiu et al. Citation2019; Z. Wang, Ren, et al. Citation2019). In a randomized, double-blind, and placebo-controlled clinical study, oral chitosan ingestion significantly reduces low-density lipoprotein (LDL), cholesterol, and plasma cholesterol while improving the high-density lipoprotein (HDL) cholesterol/total cholesterol ratio. These investigations suggested that it was caused by increased bile acid excretion and/or impaired cholesterol absorption (Moraru et al. Citation2018). Today, chitosan is sold in the market as a dietary supplement for reducing cholesterol and body weight.
The antidiabetic effects of chitosan has been reported in animal models of type 1 and type 2 diabetes. Hayashi and Ito (Citation2002) designed to clarify the effects of low molecular weight (LMW) chitosan on hyperglycemia, and hyperinsulinemia, in genetically obese diabetic male KK-Ay mice. The result showed that LMW chitosan lowered the serum glucose levels and improved overdrinking and polyuria observed in these diabetic mice. The study by Jean et al. (Citation2012) revealed that chitosan nanoparticles can be applied for the gene therapy of diabetes in living organisms since they successfully transfected the human insulin gene in vivo and in vitro. Furthermore, several studies have pointed out and reviewed the other biological activities of chitosan and its derivatives such as antimicrobial, immune modulator, antitumor, anti-inflammatory, and anti-hypertensive agent (Naveed et al. Citation2019; Zhai et al. Citation2021). It has also been proven that the chitin and chitosan monomer units are essential to repair and maintain healthy cartilage and joint function (Jung and Park Citation2014). Chitin and chitosan have a wide range of applications in food, health, and pharmaceutical regarding their high biological activities.
However, due to their higher solubility and bioavailability, LMW and chitooligosaccharides (COS) have received the most attention (Gonçalves, Ferreira, and Lourenço Citation2021).
β-glucans
The non-starch polysaccharide β-glucans is a component of cell walls in yeast, filamentous fungi, and mushrooms. It is also found in the cell wall of some bacteria, and diversity of grains such as rye, barley, and oat (Barsanti et al. Citation2011). The synthesis of β-glucan in fungi is made up of β-1,3 chains with a variable degree of β-1,6 branching (Papaspyridi, Zerva, and Topakas Citation2018). This process is catalyzed by glucosyltransferase which utilizes urine diphosphate glucose (UDPG) as a sugar donor (Papaspyridi, Zerva, and Topakas Citation2018). β-glucan helps to strengthen fungal cell structure and serves as a food reserve (Yoshimi, Miyazawa, and Abe Citation2017). Properties of β-glucan syntheses are extremely variable depending on the fungal species, condition of growth media (especially pH and glucose content), and their developmental stage. Several studies have shown that the addition of various types and concentrations of carbon sources to the fermentation media significantly influenced the increase in β-glucan contents of S. cerevisiae, R. oligosporus and A. oryzae cells (Kusmiati et al. Citation2007; Rizal et al. Citation2020; Utama et al. Citation2021).
The molecular weight, concentration and conformation of β-glucan structure can define their biological activities (Liu et al. Citation2016; Özcan and Ertan Citation2018). The high molecular weight (MW) and high viscosity of β-glucan showed better hypocholesterolemic and hypoglycemic properties (Du et al. Citation2019). However, low molecular weight of β-glucan showed better immunostimulatory and anti-allergic activity (Jippo et al. Citation2015; Suárez et al. Citation2006).
The hypocholestromic and hypoglycemic effect of β-glucan have been well proven and documented in different in vitro and in vivo studies in animals and humans (Afiati et al. Citation2019; Kim et al. Citation2005; Pino, Mujica, and Arredondo Citation2021; Sima, Vannucci, and Vetvicka Citation2018). In a systematic review by Andrade et al. (Citation2015) concluded that intake of β-glucan is effective in decreasing glucose levels in diabetic patients, with higher or smaller doses for longer periods presenting better results. In addition, ingestion of β-glucan by diabetic rats showed anti-hyperglycemic, anti-hypertriglyceridemia, and anti-hypercholesterolemic activities (Afiati et al. Citation2019; Kim et al. Citation2005). Several mechanisms could account for hypocholesterolemic and hypoglycemic effect of β-glucan.
First, β-glucan, as a kind of viscose-soluble dietary fiber, both reduces the absorption rate of the digested nutrients such as glucose, monoglycerides, free fatty acids, and cholesterol. Second, β-glucan increases the fecal excretion through an increase in the viscosity of the contents of the stomach and small intestine. Third, β-glucan undergo fermentation in the intestinal microbiota generates short-chain fatty acids (SCFAs). These compounds, can inhibit the rate-limiting enzyme of cholesterol β-hydroxy-β-methylglutaryl coenzyme A (HMG-CoA) reductase to inhibit cholesterol synthesis and also, stimulate the release of the peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and ghrelin in entero-endocrine L cells, which boosts insulin secretion and satiety perception (Pino, Mujica, and Arredondo Citation2021; Vitaglione et al. Citation2009; Zaremba et al. Citation2018). Regarding mentioned health properties of β-glucan, the US Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) have authorized health claims for β-glucan (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Citation2011; Nishantha et al. Citation2018).
β-glucan possess other health promotion effects on human health including anti-tumor (Richter et al. Citation2016), anti-infection (Vetvicka and Vetvickova Citation2020), anti-inflammatory (Bacha et al. Citation2017) and antioxidant (Song and Moon Citation2006) properties. Furthermore, Du, Bian, and Xu (Citation2014) found that β-glucan plays an important function in skin health promotion as an active ingredient in anti-wrinkle activity, wound healing, antioxidant activity, and moisturizing effect. β-glucan is widely applied in the food, medical, and cosmetic industries regarding its positive health impacts.
Gamma-aminobutyric acid
Gamma-aminobutyric acid (GABA), a four carbon non-protein amino acid with molecular formula of C4H9NO2, is naturally found in plants, animals and microorganisms (Diana, Quílez, and Rafecas Citation2014). GABA is found in very low concentrations in biological tissues, making it difficult to adequately extract from wild species. Different attempts have been made to synthesize GABA chemically (Nikmaram et al. Citation2017). However, due to the various disadvantages of corrosive reactants required for chemical synthesis, biological GABA extraction has gained popularity (Rashmi et al. Citation2018a). Microbial fermentation is recognized as one of the most efficient methods of bioprocessing because of simple reaction procedure and high catalytic efficiency (Sarasa et al. Citation2020). The most studied GABA producer microorganisms are from genera of bacteria specifically lactic acid bacteria (Cui et al. Citation2020; Sang, Uyen, and Hung Citation2018). In addition to bacteria, filamentous fungi have also been found to produce GABA due to the presence of glutamic acid decarboxylase (GAD) in their cell (Cai et al. Citation2014; Kumar et al. Citation2000; Masuo et al. Citation2010). Producing and accumulating of GABA by filamentous fungi improve the tolerance to environmental acid stress (Krulwich, Sachs, and Padan Citation2011). The main pathway of GABA production by the conversion of alpha-ketoglutarate to succinate in TCA cycle, GABA and succinic semialdehyde via glutamate (Rowley et al. Citation2012).
Various studies have reported that the GABA content of the fungal product has been increased after cultivation of cereals by different types of filamentous fungi. Cai et al. (Citation2014) suggested that oats fermented by filamentous fungi (Aspergillus oryzae, A. oryzae var. effuses and Rhizopus oryzae) contained higher GABA (125-435 µg/g oat) compared to native oat (57 μg/g), and the resulting fungal product were functional foods. Similarly, GABA content of Thai rice grains is improved through Monascus purpureus cultivations (Jannoey et al. Citation2010). Under anaerobic conditions, high yield of GABA was achieved through fermentation of soybean by Rhizopus microsporus var. oligosporus to 15-17.4 g/kg (H. Aoki, Furuya, et al. Citation2003). Different parameters such as pH, temperature, cultivation time and media additives affect the GABA production rate of filamentous fungi (Dhakal, Bajpai, and Baek Citation2012). For example, the addition of sodium nitrate to the media increased GABA production by M. purpureus (Su et al. Citation2003).
GABA exerts a great influence on a wide range of health issues. One of the most known effects of GABA is blood pressure modulation. Many studies have shown the effect of GABA on reducing elevated blood pressure in humans and animals. Different studies showed that adding GABA-enriched fermented soybean to the diet of hypertensive rat significantly decreased blood pressure (Hideyuki Aoki, Furuya, et al. Citation2003; Dupont and Légat Citation2020; Shizuka et al. Citation2004). In addition, GABA effectively prevented diabetes by acting as a potent secretagogue of insulin from the pancreas (Q. Wang, Ren, et al. Citation2019). There is some evidence that GABA has anticancer properties and could slow or inhibit the invasion and metastasis of cancer cells, including mammary gland, colon, hepatic and lung cancer cells (H. Lee, Lin, et al. Citation2015; Minuk Citation2000; Oh and Oh Citation2004; Opolski et al. Citation2000).
GABA is involved in inhibitory neurotransmission in various pathways of the central nervous system as well as peripheral tissues (Watanabe et al. Citation2002). According to different researches, GABAergic neuron changes have been linked to Huntington’s disease, Parkinson’s disease, senile dementia, seizures, Alzheimer’s disease, stiff person syndrome, and schizophrenia (Iwakura and Nawa Citation2013; Sesack and Carr Citation2002; Shetty and Bates Citation2016).
GABA increases the levels of growth hormone and the rate of protein synthesis in the brain (Rashmi et al. Citation2018b). GABA ingestion can regulate pain and anxiety sensations, as well as improving memory and learning capacities. GABA has been used to treat a variety of physiological functions including relaxation, insomnia (Singh and Zhao Citation2017), and depression. Möhler (Citation2012) reported that normal GABA levels are beneficial in antidepressant medications and mood-stabilizing treatment.
L-carnitine
L-carnitine (β-hydroxy-γ-N-trimethylaminobutyric acid) is a quaternary ammonium compound that plays a crucial role in energy metabolism (Longo, Frigeni, and Pasquali Citation2016). The chemical and biotechnological processes are used for L-carnitine production. However, biological production has become more prominent due to less organic waste, less waste to incineration and less wastewater than to chemical process (Naidu et al. Citation2000). Biotechnologically, production of L-carnitine is achieved through the action of microorganisms (fungi and bacteria) on racemic mixture of D,L carnitine and achiral precursor such as crotonobetaine, γ-butyrobetaine, and dehydrocarnitine as substrates (Bernal et al. Citation2007; Evans and Fornasini Citation2003). Different ascomycetes and zygomycetes fungi such as Mucor, Rhizopus, Aspergillus, Neurospora, and Fusarium are used in the conversion DL-carnitine derivatives to L-carnitine through stereospecifically hydrolyzing (Naidu et al. Citation2000). Rhizomucor, Rhizopus and Neurospora are able to convert crotonobetaine to L-carnitine by hydrating step (Tian, Wang, and Zhang Citation2009). Some fungal strain such as Saccharomyces cerevisiae, Penicillium, Actinomucor, Mucor, Neurospora, Rhizopus and Aspergillus produce L-carnitine from γ-butyrobetaine via hydroxylation (Leung et al. Citation2010). The biotransformation of achiral compound to L-carnitine are much more productive method rather than racemic mixture (Meyer and Robins Citation2005). Lonza, commercially produce L-carnitine from γ-butyrobetaine as a starting material (Wutzke and Lorenz Citation2004).
Fermentative production of L-carnitine is the other biotechnological method using conventional medium with neither achiral nor racemic precursor. Aspergillus, Rhizopus, Neurospora, Mucor and Penicillium are the most fungi used for L-carnitine production through fermentation process (Naidu et al. Citation2000). These fungi are able to convert trimethyllysine which is synthesized from two essential amino acids, Lysine and methionine or comes from degradation of proteins to L-carnitine, through four enzymatic steps (Rousta, Ferreira, et al. Citation2021). Different studies have been reported L-carnitine production through solid state and submerged fermentation process by filamentous fungi. Rhizopus oligosporus strain has been used for L-carnitine production on range of substrates such as buckwheat (680.9 μg/kg, (Park et al. Citation2017)), quinoa (3.14 mg/kg (Hur et al. Citation2018)), wild ginseng (630 mg/kg (Lee et al. Citation2020)) via solid state fermentation. Lee et al. (Citation2018) reported that L-carnitine enriched (201.2 mg/kg) oyster mushroom (Pleurotus ostreatus) was produced using solid state fermentation of buckwheat by Rhizopus oligosporus. Rousta, Ferreira, et al. (Citation2021) reported the production of L-carnitine from filamentous fungi (A. oryzae 3 mg/g dried biomass; R. oligosporus 1.3 mg/g dried biomass, R. oryzae 1 mg/g dried biomass, and N. intermedia 0.4 mg/g dried biomass) grown in semi-synthetic medium, and this study showed much higher L-carnitine production rather than solid-state fermentation mentioned above.
L-carnitine has been postulated to have a variety of biological activities, but its principal purpose is to enhance the transfer of fatty acids, making them accessible for mitochondrial β-oxidation (Virmani and Cirulli Citation2022). Mitochondrial fatty acid oxidation is an important source of cellular energy, especially in cardiac and skeletal muscle (Shimada et al. Citation2004). L-carnitine is also thought to be necessary for serving as an acyl group acceptor in order to maintain enough levels of free coenzyme A (CoA) in the cell, and it may operate as an osmoprotectant in sensitive organs such as the kidney (Longo, Frigeni, and Pasquali Citation2016). Based on its functionality, L-carnitine has different effects on human health. Different studies have been shown the effect of L-carnitine on weight loss and promoting physical performance during intensive exercise (Fielding et al. Citation2018; Pooyandjoo et al. Citation2016; Talenezhad et al. Citation2020). L-carnitine enhances bone microstructural properties by decreasing bone turnover (Cao et al. Citation2011; Hooshmand et al. Citation2008; Manoli et al. Citation2004). It acts as an antioxidant and protect three mainly enzymes contribute to antioxidant defense system in the body from further peroxidative damage and it is effective in regulating age-related changes (Cao et al. Citation2011; Li et al. Citation2012; Ribas, Vargas, and Wajner Citation2014). Xu et al. (Citation2017) reported that the role of L-carnitine infusion and oral supplementation in diabetic patients to improve insulin sensitivity as well as increase non-oxidative glucose excretion and glycogen storage in skeletal muscle. In humans, L-carnitine deficiency in human causes skeletal myopathy, hyperlipoproteinemia, ketonemia, cardiac infarction and chronic kidney insufficiency (Magoulas and El-Hattab Citation2012). As a result of the biofunctionality of carnitine, there has also been an increasing demand for the potential uses of L-carnitine as a therapeutic agent and as a nutritional supplement.
Ergosterol
Ergosterol, a 5,7-diene oxysterol, the primary sterol in fungal membrane cells (yeast, filamentous fungi and mushroom) plays a variety of biological roles including permeability, fluidity, regulation, and cell cycle control (Nowak et al. Citation2022). Since ergosterol is essential for cell membrane integrity, its production pathway is critical for fungal growth and is a key target for many of the currently available antifungal drugs used to treat serious human fungal infections (Jamzivar et al. Citation2019). Furthermore, it is a good indicator for measuring fungal growth in solid substrate because of the significant relationship between ergosterol level and hyphae length, as well as the existence of ergosterol exclusively in fungi (Gessner Citation2020). Ergosterol synthesis is a complex and energy-intensive process that takes place in the endoplasmic reticulum through the sequential activity of 25 distinct enzymes (Jordá and Puig Citation2020). Squalene epoxidase and lanosterol synthase are two significant and necessary enzymes in the ergosterol production pathway (Hu et al. Citation2017). The concentration of ergosterol in fungal biomass is related to fungal species, culture age, developmental stage (growth phase, hyphae formation, and sporulation), and environmental conditions (pH and temperature). The average ergosterol content of fungal species ranges from 2.6 to 42 µg/ml of dry mass. Ergosterol concentrations in Aspergillus, Penicillium, Fusarium, and Rhizopus have ranged between 0.4 and 14.3 g/mg dry mass. (Pasanen et al. Citation1999).
Ergosterol possesses a wide spectrum of biological properties with promoting health effect, such as antioxidant (Corrêa et al. Citation2018), anti-inflammatory (Al-Rabia et al. Citation2021; Kobori et al. Citation2007; Nowak et al. Citation2022) anticancer (Chen et al. Citation2017; He et al. Citation2018; Kang et al. Citation2015; X. Li, Jiang, et al. Citation2016; Li et al. Citation2015; Yongxia et al. Citation2020) and anti-hypercholesteremic activities which reduced the incidence of cardiovascular disease. Hu et al. (Citation2006) found that two ergosterol-containing extracts of Pleurotus citrinopileatus significantly reduced the serum level of total cholesterol (TC) and triglycerides (TG) in rats. Likewise, the in vitro digestion study conducted by Gil-Ramírez et al. (Citation2014) demonstrated that ergosterol-enriched extracts from Agaricus bisporus could displace the cholesterol by 67%. The addition of 1-1.5% ergosterol to the high cholesterol diet in rats significantly decreased blood levels of TC and LDL (He et al. Citation2019). Some studies demonstrated that anti-hypercholesteremic effect of ergosterol by suppressing intestine cholesterol absorption and promoting fecal cholesterol excretion (He et al. Citation2020; He et al. Citation2019). Anti-hypercholestrolemic health claim for ergosterol has been fully approved by European Food Safety Authority (EFSA). In addition to the above-mentioned health effects of ergosterol, Jeong and Park (Citation2020) revealed the anti-obesity effect of ergosterol peroxide derived from an ethanolic extract of Ganoderma lucidum by inhibition the synthesis of triglycerides.
The other important role of ergosterol on health is related to synthesis of vitamin D2 (ergocalciferol) (Nowak et al. Citation2022). Ergosterol is known as a precursor of vitamin D2 that can be converted to vitamin D2 by exposure to natural or artificial UV irradiation (wavelengths in the range of 280-320 nm) (Jiang, Zhang, and Mujumdar Citation2020; Nölle et al. Citation2017). In the blood circulation, ergocalciferol convert to the biologically active form of vitamin D (calcitriol) after enzymatic processes in the liver and kidney (Cardwell et al. Citation2018; Charoenngam, Shirvani, and Holick Citation2019). A low plasma vitamin D level has been linked to the advancement of numerous metabolic diseases, including diabetes, hypertension, autoimmune and inflammatory diseases, cancer, immunological disorders, and infectious diseases (H. Wang, Chen, et al. Citation2017). Recently, some studies have been reported the correlation between vitamin D deficiency and mortality rate in COVID-19 (Annweiler, Cao, and Sabatier Citation2020; Giménez et al. Citation2020; Razdan, Singh, and Singh Citation2020). Since the rich diet source of vitamin D are animal products, the fungi as the only vegetarian source of vitamin D are highly considered.
Fructooligosaccharides
Fructooligosaccharides (FOSs) are non-digestible carbohydrates which represent a main classes of bifidogenic oligosaccharides (Gropper and Smith Citation2012; Monsan and Ouarné Citation2009). FOSs are a common name only for fructose oligomers that are mainly composed of 1-kestose (GF2), nystose (GF3), and lF-fructofuranosyl nystose (GF4) in which fructosyl units (F) are bound at the B-2,1 position of sucrose (GF) respectively (Zhang et al. Citation2017). They are present in trace amount in higher plants such as Chicory root, bananas, onions, agave, garlic, asparagus, jicama, and leeks (Nobre et al. Citation2015). However, they are produced commercially through enzymatic process from sucrose by microbial enzyme (Roupar et al. Citation2022). Two enzymes, namely fructosyltransferases, and β-fructofuranosidases are responsible for the production of fructooligosaccharides (Muñiz-Márquez et al. Citation2016) that can be found in wide variety of fungi including Aureobasidium sp. (Dominguez et al. Citation2012; Jung et al. Citation2011; Zhang et al. Citation2019), Aspergillus sp. (Choukade and Kango Citation2022; Nascimento et al. Citation2019; Ojwach et al. Citation2020) Fusarium sp. (Ganaie, Rawat, et al. Citation2014; Maiorano et al. Citation2020; Michel et al. Citation2016) Penicillium sp. (Rawat, Ganaie, and Kango Citation2015; Sánchez Martínez, Soto Jover, and López Gómez Citation2018) Scopulariopsis brevicaulis (Antosova and Polakovic Citation2002; Belorkar Citation2020; Yadav, Singh, and Shukla Citation2016), Rhizopus stolonifera (Flores-Maltos et al. Citation2016; Lateef and Gueguim-Kana Citation2012) and yeasts (Roupar et al. Citation2022; Yoshikawa et al. Citation2022). However, Aspergillus niger, Aspergillus japonicus, and Aureobasidium pullulans have a great potential for industrial production of FOSs. In addition to fungal species, optimization the nutritional and culture parameters are the main approaches to increase enzyme yield and productivity (Zhang et al. Citation2017). For example, improved media conditions containing 24% sucrose and 2.75% yeast extract boosted enzyme and, as a result, FOSs synthesis by Aspergillus japonicus by 180% (Mussatto and Teixeira Citation2010)
There has been a lot of research on the use of submerged fermentation for the synthesis of FOSs by various fungi (Ademakinwa, Ayinla, and Agboola Citation2017; Cunha et al. Citation2019; Flores-Maltos et al. Citation2016; Hayashi et al. Citation2000; L’Hocine et al. Citation2000; Lateef, Oloke, and Prapulla Citation2007; Muñiz-Márquez et al. Citation2019; Nascimento et al. Citation2019; Ottoni et al. Citation2012; Shin et al. Citation2004; Vandáková et al. Citation2004; Yang et al. Citation2016). Most of these studies used chemically formulated media for microbial fermentation. Fewer studies of the use of SSF in the production of FOSs have been published to that of submerged fermentation (Mussatto et al. Citation2013; Mussatto and Teixeira Citation2010; Sangeetha, Ramesh, and Prapulla Citation2004a, Citation2004b). However, more research has focused on SSF as a cost-effective alternative technique for FOSs production, using agricultural residual waste or by-product (Batista et al. Citation2020; de la Rosa et al. Citation2019; Ganaie et al. Citation2017; Mussatto et al. Citation2015). In a study by Mussatto et al. (Citation2015) investigated the economic and environmental effects of different fermentation processes for FOSs production. SSF has become more attractive due to its ability to produce FOSs with higher annual productivity and purity than the other process.
FOSs have various effects on human health due to its prebiotic properties. Short chain fatty acids (SCFAs) produced through the fermentation of FOSs can suppress inflammation and cancer, promote local immune response, and increase ammonia excretion (Liu et al. Citation2021; Vinolo et al. Citation2011). Several studies have shown that FOSs are therapeutic agent against ovarian (Oliveira-Ferrer et al. Citation2014), breast (Luo et al. Citation2003), prostate (Zhang et al. Citation2007) and colorectal cancer (Ding et al. Citation2020). Yu et al. (Citation2014) reported that the anti-tumor and immunostimulatory functions of FOSs produced by fermentation of wheat bran through Aureobasidium pullulans. In addition, FOS has been shown to lower serum lipid levels, have a hypotriglyceridemic and hypocholestrolemic effect therefore, reducing the risk of diabetes, obesity and atherosclerosis (Giacco et al. Citation2004; Ooi and Liong Citation2010; Rodríguez-Berdini and Caputto Citation2019). Different mechanisms have been proposed to explain these effects. One mechanism involves adjusting the concentrations of glucose or insulin. FOSs, as indigestible soluble prebiotic, decrease blood glucose peaks that occur after eating, and as a result, the production of glucose and insulin-induced lipidic enzymes is reduced significantly (Sabater-Molina et al. Citation2009). The other is related to SCFAs production in the colon. Propionate inhibits lipogenesis and cholesterogenesis pathways while acetate stimulates them, so the ratio of acetate to propionate reaching the liver may be one of the potential lipid-lowering properties of FOSs (Thandapilly et al. Citation2018). Moreover, FOS positively improves the absorption of minerals. It has been demonstrated that high SCFAs concentration resulting from the fermentation of FOS increases the water content and decreases the pH of the colon, which are two important factors for solubility/availability of minerals particularly, Ca and Mg, in several animal experimental studies (Scholz-Ahrens et al. Citation2007; Scholz-Ahrens and Schrezenmeir Citation2007).,
Another health benefits of FOSs relate to its anti-diabetic effect, which is achieved through several mechanisms, including increased glucose absorption in peripheral tissues, decreased gluconeogenesis, improved insulin tolerance, and increased insulin secretion. (Bharti et al. Citation2013; Erejuwa, Sulaiman, and Wahab Citation2011; Paul and Kumar Citation2020). Bharti et al. (Citation2013) reported that antidiabetic activity of FOSs produced by Aureobasidium pullulans using poloxamer-407 induced type 2 diabetes mellitus in rat. Since FOSs are not hydrolyzed by digestive enzymes, they cannot be used as a source of energy (calorie free) and are safe for diabetics and people on slimming diet.
Due to their wide variety of physiological functions and health effects, FOSs have attracted great interest in the market and many biotechnological and pharmacological companies have been developed for their production (Ganaie, Lateef, et al. Citation2014).
Fungal probiotic and prebiotic properties and their effects on gut microbiota
The role of gut microbiota in health and disease is one of the most debated topics in human nutrition. This is especially true of interactions between resident microbiota and dietary ingredients that support their operations. It has been approved that the gut microbiome contains pathogenic and beneficial components, and the diet affects the gut microbiome composition (Steer et al. Citation2000). The use of probiotic (use of live microorganisms in food) and prebiotic (carbohydrates that are selectively metabolized by desirable flora moieties) improves the beneficial microbiota in the gut with a wide range of health effects (Kechagia et al. Citation2013). In this section, the fungi’s probiotic and prebiotic potentials have been discussed.
Probiotic fungi
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungal kingdom. Saccharomyces, a member of the Ascomycota, is the most well-known yeast in foods as baker’s yeast or brewer’s yeast (Levetin et al. Citation2016). Saccharomyces cerevisiae and Saccharomyces boulardii are used as human probiotics exerting many beneficial properties on human health (reviewed in Szajewska, Konarska, and Kołodziej (Citation2016), Wang et al. (Citation2015), and Tadesse et al. (Citation2021)). The strain S. cerevisiae IFST 062013 was found to have probiotic properties like tolerance to a wide pH and temperature ranges, gastric fluids, production of organic acids, assimilation of cholesterol, production of vitamins, siderophore, biofilm formation and enzymes. This strain was also showed antimicrobial and antioxidant activities (Fakruddin, Hossain, and Ahmed Citation2017). S. boulardii isolated form fruits in early 20th century is the only probiotic yeast investigated clinically and extensively studied for its beneficial effects on gastrointestinal system (Czerucka and Rampal Citation2002; Hossain et al. Citation2020; Pothoulakis Citation2009). Many studies have been performed using this strain in the treatment of diarrhea, Crohn’s disease, irritable bowel syndrome (ibs), inactivation of bacterial toxins, inhibition of toxin binding to receptors (Anoop et al. Citation2015; Offei et al. Citation2019). Using S. boulardii as a prophylactic supplement in the healthy intestine induced a mucosal immune response (Hudson et al. Citation2016). Moreover, the strains of S. boulardii isolated from different supplements showed antibacterial and antifungal activities on Salmonella Typhimurium and Aspergillus niger strains (Goktas, Dertli, and Sagdic Citation2021).
Many of other yeast strains are used in food fermentations or found in gastrointestinal system. Some yeast species other than Saccharomyces sp. were isolated from fermented dairy products such as Candida sp., Debaryomyces sp., Yarrowia sp., Kluyveromyces marxianus, Pichia fermentans, Candida guilliermondii with probiotic properties including specific enzyme productions, antioxidant, antimicrobial and antitumor activities and resistance to adverse environmental conditions like low pH or osmotic stress (Fredlund et al. Citation2002; Kumura et al. Citation2004; Mahasneh and Abbas Citation2010; Moslehi-Jenabian, Lindegaard, and Jespersen Citation2010; Qvirist et al. Citation2016; Tadesse et al. Citation2021; Utama et al. Citation2019). Candida tropicalis, Pichia sp., Aureobasidium pullulans have also beneficial properties like antimicrobial activities on pathogenic bacteria or enzymatic activities that can be used as promising probiotics in food and feed supplements (Syal and Vohra Citation2013; Wu et al. Citation2022).
Foods enriched with yeast probiotics as new functional products are of interest in recent years (Amorim, Piccoli, and Duarte Citation2018; Di Cagno et al. Citation2020). Senkarcinova et al. (Citation2019) showed the inclusion of strain S. boulardii for production of alcohol-free probiotic beer additional health benefits. Karaolis et al. (Citation2013) showed that S. boulardii stimulated the growth of lactic acid bacteria in yogurt without affecting organoleptic quality. Swieca et al. (Citation2019) used to lentil and bean sprouts as a carriers for S. cerevisiae var. boulardii and improved microbiological quality of the final sprouts without affecting the their nutritional properties.
It has been suggested that many filamentous fungi (Aspergillus awamori, A. niger, Rhizopus oligosporus, Acremonium charticola, Chrysonilia crassa, Scytalidium acidophilum) can be used as a probiotic (reviewed in Sugiharto (Citation2019)). Studies with the potential probiotic effects of filamentous fungi relate to broiler chicks, poultry and fish production (Poirier et al. Citation2022). In poultry, fungal-based probiotics could improve growth performance, muscle vitamin E content, skeletal muscle fatty acid profile, and reduce lipid peroxidation in the muscles (Saleh et al. Citation2011). In fish, some fungal probiotics can stimulate the antioxidant response and immune system, and also stimulate the production of various digestive enzymes (amylases, cellulases, β-glucanases, xylanases, proteases and lipases) (Melo-Bolívar et al. Citation2020).
Future research should focus on exploring new fungal probiotic strains that can be used in industrial, medical and agricultural applications. More in-depth research into genetic engineering on fungal probiotic strains will also help enhance their probiotic properties.
Prebiotic fungi
Innovative new trend foods have become popular today due to their various benefits on health. The most important functional group for consumers to prefer these foods is prebiotics (Belorkar Citation2020). Fungal polysaccharides (chitin, chitosan, and β-glucan) act as prebiotics as an alternative to prebiotics, which are usually provided by bacteria. In addition, some fungal species are widely used in the production of oligosaccharides with prebiotic effects (Awasthi, Tarafdar, et al. Citation2022). Fructooligosacharides (FOS) as explained above and xylooligosaccharides (XOS) are the other prebiotic compounds produced by fungi (Manisha and Yadav Citation2017). As fungal cultures, Trichoderma viride, Aspergillus foetidus, A. fumigatus, A. brasiliensis and recombinant A. nidulans are potential XOS producers (Carvalho et al. Citation2015; Chapla, Pandit, and Shah Citation2012; da Silva Menezes, Rossi, and Ayub Citation2017; da Silva Menezes et al. Citation2018; Manisha and Yadav Citation2017). In addition, Jiang et al. (Citation2021) reported that glycosylceramides, one of the glycosphingolipids abundantly found in Aspergillus, contribute to a prebiotic effects with prevention intestinal deterioration.
Prebiotics are considered as regulators of gut microbiota. These fungal based prebiotics can improve gut health and improve resistance to microbial infection (Strong et al. Citation2022). They are selectively fermented ingredients that particularly promote the growth of probiotics (Bifidobacteria and Lactobacillus sp.), especially increasing the microflora in the large intestine, as well as decreasing the population of pathogenic bacteria (Clostridium sp.) and reducing gas production (Flores-Maltos et al. Citation2016; Mussatto et al. Citation2015). Fermentation of prebiotics by bacteria in large intestine is led to liberation of some metabolites including short chain fatty acids (acetate, propionate, butyrate, lactate, etc.) with specific local physiological functions (Pascale et al. Citation2018).
Current use potentials of fungi on cancer treatment
While cells divide more rapidly in the first years of life, this rate of division slows down in adulthood. Nevertheless, these capacities of cells are limited, they cannot divide indefinitely. Each cell has the ability to divide a certain number of times during its lifetime (Shammas Citation2011). A healthy cell knows how much to divide, when to divide and how to die when necessary. The process of conscious death of the cell is called apoptosis or programmed death of the cell. Normally, the body needs to grow, divide, and produce more cells to function properly. But sometimes this process goes wrong, and cells continue to divide excessively and prematurely without the need for new cells. These unconsciously dividing cells are called cancer cells. Unconscious cancer cells begin to divide and multiply uncontrollably (Wong Citation2011). These excess clumps of cells form a huge size or tumor. Cancer cells that involve this irregular cell growth can be a subversive disease that can extended to more distant parts of the body via the lymphatic system or bloodstream, with some types of which overgrow in the area where they are found and form cancerous tissues (Cooper and Hausman Citation2000). Research on finding drugs and treatments for cancer, which is the second most common cause of death worldwide after heart diseases, is very important for scientists (Patel and Goyal Citation2012). For this reason, various ways have been tried for treatment and drug production for years, and different ways are still being tried. For example, mushrooms that were considered sacred in the first years of history and used in health research; since it is thought to be effective against cancer, alternative medicine and traditional medicine have also been used. As an example, various studies from Asian countries show that edible and medicinal mushrooms play an important role in the prevention and treatment of cancer. It is also known that the fruiting bodies of Inonotus obliquus are used as a medicine in the therapy of cancer in Eastern Europe due to the ergosterol peroxide and triterpenes they contain (Kang et al. Citation2015; Lindequist, Niedermeyer, and Jülich Citation2005; Molitoris Citation1994). Nevertheless, while plants and bacteria are given priority in drug preparation and research in modern medicine, these studies have focused on fungi in the light of recent studies (Ogidi, Oyetayo, and Akinyele Citation2020). Because there are problems with the use of natural products of plant origin although successful drug discoveries in general are from plant sources. Chief among these problems is the generally low production of bioactive compounds obtained using plant sources. Re-isolation of a compound to be produced is problematic because the metabolite composition is also affected by the environment. In addition, drug production time and research cost are also problematic (Newman and Cragg Citation2012). Considering these problems, other organisms have been sought for the production of bioactive compounds and have tried different microorganisms such as fungi as a resource to fight cancer. As a result of these studies, it was seen that; the use of mushrooms in the fight against cancer has many advantages. Fungi that respond well to routine cultures are relatively easier to amplify productivity than other organisms. Fungi can be produced in large quantities using large reactors, and this overproduction can be conveniently stored regardless of time, providing long-term availability of the resource organism and easier supply of the desired bioactive compounds. Being able to easily supply, for example, antibacterial penicillin, antifungal echinocandin B, immunosuppressive cyclosporine A, cholesterol-lowering lovastatin has been a good tool to demonstrate the importance of searching for fungal sources for new drugs (Cole, Jarvis, and Schweikert Citation2003; Dewick Citation1997; Turner and Aldridge Citation1983). Fungi produce metabolites belonging to a wide variety of structural classes. Examples of these are aromatic compounds, antacenones, butanolides, amino acids, butenolides, cytochalasans, macrolides, naphthalenes, pyrons, terpenes.
In recent studies, surprisingly, although there is no approved anti-cancer drug for fungi yet, but the active compounds of certain fungi with proven anti-cancer properties are of great interest as an area of research. Many clinical studies have been and continue to be conducted to evaluate the benefits of medicinal mushroom extracts used in cancer treatment. As a result of these studies, additional potential uses of mushrooms in the treatment of cancer have emerged individually. For example, mushrooms are known to supplement chemotherapy and radiation therapy by resisting the side effects of cancer such as bone marrow suppression, nausea, anemia and low resistance. As a result of these studies, a number of bioactive molecules, including anti-tumor agents of fungi, which have been known to have in traditional medicine effects for a long time, have been identified () (Bains et al. Citation2021; Lindequist, Niedermeyer, and Jülich Citation2005; Patel and Goyal Citation2012).
Table 4. The use of different fungal metabolites for cancer treatment.
Fungal gut microbiota dysbiosis and its role in carcinogenesis
The gut microbiota is a variety community consisting of bacteria, fungi and archaea. This complex ecosystem plays a veritably important part in the balance of body and health. These resident microorganisms in our gut can produce abundant bioproducts and metabolites that maintain homeostasis of the host and gut. The quantitative and qualitative changes in the composition of the gut microbiota are also defined as gut dysbiosis. Although this gut dysbiosis is known to play a role in the development of certain types of cancer, some bacterial and fungal components may also exert anti-tumor effects on the contrary (Kaźmierczak-Siedlecka et al. Citation2020). For example, as a result of a study about relationship cancer and gut microbiota by taking samples from patients with oral cancer and people with non-oral cancer, it is seen that Candida, which produces high ethanol derivative Acetaldehyde (ACH), isolated from patients with oral cancer, is more common than patients with non-oral cancer (Alnuaimi et al. Citation2016). In addition, Candida isolated from patients with oral cancer indicates hydrolytic enzyme activity along with huge biofilm mass and biofilm metabolic activity. As a result of this research, Candida promotes the development of oral cancers due to its ability to produce hydrolytic enzymes and metabolize alcohol to the carcinogenic ACH (Alnuaimi et al. Citation2016; Kaźmierczak-Siedlecka et al. Citation2020).
Fungal β-glucans used as adjuvants to treat cancer patients
Some components (lectin, krestin, lentinan, hispolon, calcaelin, illudin S, psilocybin, ganoderic acid, schizophilan, laccase; ) found in mushrooms/fungi may be promising in cancer treatment. Polysaccharides are best known as immunomodulatory and anti-tumor agents. β-glucan, which is polysaccharide, is the most versatile metabolite owing to its broad-spectrum biological activity. These β-glucans consist of a backbone of glucose residues linked by β (1-3)-glycosidic bonds, usually side chain glucose residues joined by β (1-6) bonds (Chen and Seviour Citation2007). Their mechanism of effect includes their recognition as non-self-molecules, so the immune system is induced by their entity. In fact, β-glucans purified from the cell walls of S. cerevisiae, as it is known, have been introduced as a healthy supplement that helps cancer treatment and prevention, weight loss, lowers cholesterol and increases vulnerable responses to infection (Volman, Ramakers, and Plat Citation2008). For some time, injectable soluble β-glucans, which are claimed in some studies, have been investigated as immune-enhancing agents with antibodies or chemotherapy in the treatment of cancer, and the results support this claim (Chen and Seviour Citation2007). What we see from the results of these studies seems to work primarily through the complementary intake of the tumor and the activation/renewal of neutrophils (Chen and Seviour Citation2007). After oral administration of particulate glucans, which mimics some of the benefits of injectable soluble β-glucans in animal models, orally administered particulate glucans are taken up by intestinal phagocytes, trafficked to the bone marrow and lymphoid organs, resulting in circulating soluble β-glucans, which can kill neutrophils and activate tumor cells (Hong et al. Citation2004). It is uncertain whether endogenous commensal fungi are converted to circulating β-glucan fractions, which are also undefended in "normal" situations or during fungal eruptions. Clinically, β-glucan blood assays are often used to identify invasive fungal complaint (Chen and Seviour Citation2007), and fungal colonization of the gut (without invasive complaint) may conduce to the changeable delivery status of circulating β-glucans detected. In addition, hispolon, that is a vigorous polyphenol component, additionally contained in mushrooms; it’s celebrated to own doubtless anti-neoplastic properties and doubtless the toxicity of chemotherapeutical agents. research to support the claims has accelerated in recent years (Arun et al. Citation2022). Although the findings show that some fungi add concert with commercial antitumor medicine as a good tool to treat drug-resistant cancers, there is not enough provability as it is still a new area of research. This shows us that mushrooms/fungi will take part in much more cancer research in the coming years (Patel and Goyal Citation2012).
Conclusion and future perspectives
In recent years, researches on both the production of valuable industrial metabolites (enzymes, organic acids, protein, pigment, etc.) and waste treatment through filamentous fungi have progressed exponentially, but their effects on the food industry need to be determined. In fact, the important bioactive compounds of mushrooms and their prevention on diseases (such as diabetes, hypertension, cancer, hypercholesterolemia and cardiovascular diseases) have been extensively studied and suggested to be used as a therapeutic supplement. Similarly, filamentous fungi with their high protein contents are important bioactive compounds producers. Studies on the antioxidant, antimicrobial and anticancer effects of these fungi-based bioactive compounds and secondary metabolites are promising in human health. Pharmacological investigation of existing bioactive compounds and secondary metabolites of fungi, which are easier to produce compared to mushrooms, and determination of their in vitro and in vivo health effects will make new generation food production more important.
Author contributions
Conceptualization: Taner Sar, Mohammad J. Taherzadeh; Visualization: Neda Rousta, Melissa Aslan, Taner Sar; Writing - original draft: Neda Rousta, Melissa Aslan, Meltem Yesilcimen Akbas, Taner Sar; Writing - review & editing: Neda Rousta, Meltem Yesilcimen Akbas, Ferruh Ozcan, Taner Sar, Mohammad J. Taherzadeh; Project administration: Mohammad J. Taherzadeh.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abo Elsoud, M. M., and E. M. El Kady. 2019. Current trends in fungal biosynthesis of chitin and chitosan. Bulletin of the National Research Centre 43 (1):59. doi: 10.1186/s42269-019-0105-y.
- Ademakinwa, A. N., Z. A. Ayinla, and F. K. Agboola. 2017. Strain improvement and statistical optimization as a combined strategy for improving fructosyltransferase production by Aureobasidium pullulans NAC8. Journal, Genetic Engineering & Biotechnology 15 (2):345–58. doi: 10.1016/j.jgeb.2017.06.012.
- Adnan, M., M. Patel, M. N. Reddy, and E. Alshammari. 2018. Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Scientific Reports 8 (1):1740. doi: 10.1038/s41598-018-20237-z.
- Afiati, F., Firza, S. F. Kusmiati, and Aliya, L. S. 2019. The effectiveness β-glucan of shiitake mushrooms and saccharomyces cerevisiae as antidiabetic and antioxidant in mice Sprague Dawley induced alloxan. AIP Conference Proceedings.
- Agyei, D., and M. K. Danquah. 2012. Rethinking food-derived bioactive peptides for antimicrobial and immunomodulatory activities. Trends in Food Science & Technology 23 (2):62–9. doi: 10.1016/j.tifs.2011.08.010.
- Akihisa, T., H. Tokuda, M. Ukiya, A. Kiyota, K. Yasukawa, N. Sakamoto, Y. Kimura, T. Suzuki, J. Takayasu, and H. Nishino. 2005. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of monascus pilosus fermented rice (red-mold rice). Chemistry & Biodiversity 2 (10):1305–9. doi: 10.1002/cbdv.200590101.
- Al-Rabia, M. W., G. A. Mohamed, S. R. M. Ibrahim, and H. Z. Asfour. 2021. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Natural Product Research 35 (23):5011–20. doi: 10.1080/14786419.2020.1762185.
- Alberti, F., G. D. Foster, and A. M. Bailey. 2017. Natural products from filamentous fungi and production by heterologous expression. Applied Microbiology and Biotechnology 101 (2):493–500. doi: 10.1007/s00253-016-8034-2.
- Alnuaimi, A. D., A. N. Ramdzan, D. Wiesenfeld, N. M. O’Brien-Simpson, S. D. Kolev, E. C. Reynolds, and M. J. McCullough. 2016. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Diseases 22 (8):805–14. doi: 10.1111/odi.12565.
- Aluko, R. E. 2015. Antihypertensive peptides from food proteins. Annual Review of Food Science and Technology 6 (1):235–62. doi: 10.1146/annurev-food-022814-015520.
- Amorim, J. C., R. H. Piccoli, and W. F. Duarte. 2018. Probiotic potential of yeasts isolated from pineapple and their use in the elaboration of potentially functional fermented beverages. Food Research International (Ottawa, ON) 107:518–27. doi: 10.1016/j.foodres.2018.02.054.
- Andrade, E. F., R. V. Lobato, T. V. de Araújo, M. G. Zangerônimo, R. V. de Sousa, and L. J. Pereira. 2015. Effect of beta-glucans in the control of blood glucose levels of diabetic patients: A systematic review. Nutricion Hospitalaria 31 (1):170–7.
- Annweiler, C., Z. Cao, and J.-M. Sabatier. 2020. Point of view: Should COVID-19 patients be supplemented with vitamin D? Maturitas 140:24–6. doi: 10.1016/j.maturitas.2020.06.003.
- Anoop, V., S. Rotaru, P. S. Shwed, A. F. Tayabali, and G. Arvanitakis. 2015. Review of current methods for characterizing virulence and pathogenicity potential of industrial Saccharomyces cerevisiae strains towards humans. FEMS Yeast Research 15 (6):fov057. doi: 10.1093/femsyr/fov057.
- Antosova, M., and M. Polakovic. 2002. Fructosyltransferases: The enzymes catalyzing production of fructooligosaccharides. Chemical Papers-Slovak Academy of Sciences 55 (6):350–8.
- Aoki, H., Y. Furuya, Y. Endo, and K. Fujimoto. 2003. Effect of γ-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-tempeh) on the blood pressure of spontaneously hypertensive rats. Bioscience, Biotechnology, and Biochemistry 67 (8):1806–8.
- Aoki, H., I. Uda, K. Tagami, Y. Furuya, Y. Endo, and K. Fujimoto. 2003. The production of a new tempeh-like fermented soybean containing a high level of gamma-aminobutyric acid by anaerobic incubation with Rhizopus. Bioscience, Biotechnology, and Biochemistry 67 (5):1018–23. doi: 10.1271/bbb.67.1018.
- Arora, D., A. Kumar, P. Gupta, G. Chashoo, and S. Jaglan. 2017. Preparation, characterization and cytotoxic evaluation of bovine serum albumin nanoparticles encapsulating 5-methylmellein: A secondary metabolite isolated from Xylaria psidii. Bioorganic & Medicinal Chemistry Letters 27 (23):5126–30. doi: 10.1016/j.bmcl.2017.10.064.
- Arora, D., N. Sharma, V. Singamaneni, V. Sharma, M. Kushwaha, V. Abrol, S. Guru, S. Sharma, A. P. Gupta, S. Bhushan, et al. 2016. Isolation and characterization of bioactive metabolites from Xylaria psidii, an endophytic fungus of the medicinal plant Aegle marmelos and their role in mitochondrial dependent apoptosis against pancreatic cancer cells. Phytomedicine : international Journal of Phytotherapy and Phytopharmacology 23 (12):1312–20. doi: 10.1016/j.phymed.2016.07.004.
- Arun, K. B., R. Sindhu, D. Alex, P. Binod, A. Pughazhendi, T. C. Joseph, A. Pandey, M. Kuddus, S. Pillai, S. Emmanual, et al. 2022. Bacterial bioactive metabolites as therapeutic agents: From production to action. Sustainable Chemistry and Pharmacy 27:100650. doi: 10.1016/j.scp.2022.100650.
- Awasthi, M. K., S. Harirchi, T. Sar, V. Vs, K. Rajendran, R. Gómez-García, C. Hellwig, P. Binod, R. Sindhu, A. Madhavan, et al. 2022. Myco-biorefinery approaches for food waste valorization: Present status and future prospects. Bioresource Technology 360:127592. doi: 10.1016/j.biortech.2022.127592.
- Awasthi, M. K., V. Kumar, C. Hellwig, R. Wikandari, S. Harirchi, T. Sar, S. Wainaina, R. Sindhu, P. Binod, Z. Zhang, et al. 2023. Filamentous fungi for sustainable vegan food production systems within a circular economy: Present status and future prospects. Food Research International (Ottawa, ON) 164:112318. doi: 10.1016/j.foodres.2022.112318.
- Awasthi, M. K., A. Tarafdar, V. K. Gaur, K. Amulya, V. Narisetty, D. K. Yadav, R. Sindhu, P. Binod, T. Negi, A. Pandey, et al. 2022. Emerging trends of microbial technology for the production of oligosaccharides from biowaste and their potential application as prebiotic. International Journal of Food Microbiology 368:109610. doi: 10.1016/j.ijfoodmicro.2022.109610.
- Bacha, U., M. Nasir, S. Iqbal, and A. A. Anjum. 2017. Nutraceutical, anti-inflammatory, and immune modulatory effects of β-glucan isolated from yeast. BioMed Research International 2017:1–14. doi: 10.1155/2017/8972678.
- Bains, A., P. Chawla, S. Kaur, A. Najda, M. Fogarasi, and S. Fogarasi. 2021. Bioactives from mushroom: Health attributes and food industry applications. Materials 14 (24):7640. doi: 10.3390/ma14247640.
- Barsanti, L., V. Passarelli, V. Evangelista, A. M. Frassanito, and P. Gualtieri. 2011. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Natural Product Reports 28 (3):457–66. doi: 10.1039/c0np00018c.
- Batista, J. M. S., R. M. P. Brandão-Costa, M. N. Carneiro da Cunha, H. O. S. Rodrigues, and A. L. F. Porto. 2020. Purification and biochemical characterization of an extracellular fructosyltransferase-rich extract produced by Aspergillus tamarii Kita UCP1279. Biocatalysis and Agricultural Biotechnology 26:101647. doi: 10.1016/j.bcab.2020.101647.
- Belorkar, S. 2020. Fungal production of prebiotics. In Fungal biotechnology and bioengineering, 239–54. Switzerland AG: Springer Nature.
- Bernal, V., A. Sevilla, M. Cánovas, and J. L. Iborra. 2007. Production of L-carnitine by secondary metabolism of bacteria. Microbial Cell Factories 6 (1):31. doi: 10.1186/1475-2859-6-31.
- Bernart, M. W., S.-T. Chang and, and P. G. Miles. 2005. Mushrooms. Cultivation, nutritional value, medicinal effect, and environmental impact, 2nd Edition By (Chinese University of Hong Kong and State University of New York, respectively). CRC Press, Boca Raton. 2004. xx–+ 451. pp. 18.5 × 26 cm. $159.95. ISBN 0-8493-1043-1. Journal of Natural Products 68 (4):629–630. doi: 10.1021/np058221b.
- Bharti, S. K., S. Krishnan, A. Kumar, K. K. Rajak, K. Murari, B. K. Bharti, and A. K. Gupta. 2013. Antidiabetic activity and molecular docking of fructooligosaccharides produced by Aureobasidium pullulans in poloxamer-407-induced T2DM rats. Food Chemistry 136 (2):813–21. doi: 10.1016/j.foodchem.2012.08.083.
- Bryk, G., M. Z. Coronel, G. Pellegrini, P. Mandalunis, M. E. Rio, M. L. P. M. de Portela, and S. N. Zeni. 2015. Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. European Journal of Nutrition 54 (6):913–23. doi: 10.1007/s00394-014-0768-y.
- Cai, S., F. Gao, X. Zhang, O. Wang, W. Wu, S. Zhu, D. Zhang, F. Zhou, and B. Ji. 2014. Evaluation of γ- aminobutyric acid, phytate and antioxidant activity of tempeh-like fermented oats (Avena sativa L.) prepared with different filamentous fungi. Journal of Food Science and Technology 51 (10):2544–51. doi: 10.1007/s13197-012-0748-2.
- Cao, Y., H.-j. Qu, P. Li, C.-b. Wang, L.-x. Wang, and Z.-w. Han. 2011. Single dose administration of L-carnitine improves antioxidant activities in healthy subjects. The Tohoku Journal of Experimental Medicine 224 (3):209–13.
- Cardwell, G., J. F. Bornman, A. P. James, and L. J. Black. 2018. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 10 (10):1498. doi: 10.3390/nu10101498.
- Carvalho, A. F. A., P. de Oliva Neto, P. Z. De Almeida, J. B. Da Silva, B. Escaramboni, and G. M. Pastore. 2015. Screening of xylanolytic Aspergillus fumigatus for prebiotic xylooligosaccharide production using bagasse. Food Technology and Biotechnology 53 (4):428–35.
- Chapla, D., P. Pandit, and A. Shah. 2012. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresource Technology 115:215–21. doi: 10.1016/j.biortech.2011.10.083.
- Charoenngam, N., A. Shirvani, and M. F. Holick. 2019. Vitamin D for skeletal and non-skeletal health: What we should know. Journal of Clinical Orthopaedics and Trauma 10 (6):1082–93. doi: 10.1016/j.jcot.2019.07.004.
- Chávez, R., F. Fierro, R. O. García-Rico, and I. Vaca. 2015. Filamentous fungi from extreme environments as a promising source of novel bioactive secondary metabolites [Perspective]. Frontiers in Microbiology 6:903. doi: 10.3389/fmicb.2015.00903.
- Chen, J., and R. Seviour. 2007. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycological Research 111 (6):635–52. doi: 10.1016/j.mycres.2007.02.011.
- Chen, S., T. Yong, Y. Zhang, J. Su, C. Jiao, and Y. Xie. 2017. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Frontiers in Chemistry 5:85.
- Chen, W., H. Ding, P. Feng, H. Lin, and K.-C. Chou. 2016. iACP: A sequence-based tool for identifying anticancer peptides. Oncotarget 7 (13):16895–909. doi: 10.18632/oncotarget.7815.
- Cheong, S. H., E.-K. Kim, J.-W. Hwang, Y.-S. Kim, J.-S. Lee, S.-H. Moon, B.-T. Jeon, and P.-J. Park. 2013. Purification of a novel peptide derived from a shellfish, Crassostrea gigas, and evaluation of its anticancer property. Journal of Agricultural and Food Chemistry 61 (47):11442–6.
- Chiu, C. Y., T. E. Yen, S. H. Liu, and M. T. Chiang. 2019. Comparative effects and mechanisms of chitosan and its derivatives on hypercholesterolemia in high-fat diet-fed rats. International Journal of Molecular Sciences 21 (1):92. doi: 10.3390/ijms21010092.
- Choukade, R., and N. Kango. 2022. Purification of Aspergillus tamarii mycelial fructosyltransferase (m‐FTase), optimized FOS production, and evaluation of its anticancer potential. Journal of Food Science 87 (7):3294–306. doi: 10.1111/1750-3841.16173.
- Cipolari, O. C., X. A. de Oliveira Neto, and K. Conceição. 2020. Fish bioactive peptides: A systematic review focused on sting and skin. Aquaculture 515:734598. doi: 10.1016/j.aquaculture.2019.734598.
- Clementino, E. L., A. Sales, M. Cunha, A. Porto, and T. Porto. 2019. Produção e purificação integrada de protease fibrinolítica de Mucor subtilissimus UCP 1262. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 71 (2):553–62. doi: 10.1590/1678-4162-9495.
- Cole, J. R., B. B. Jarvis, and M. A. Schweikert. 2003. Secondary fungal metabolites. Amsterdam: Academic Press.
- Cooper, G. M., and R. E. Hausman. 2000. The cell: a molecular approach. Sunderland, MA: Sinauer Associates.[Google Scholar].
- Corrêa, R. C., L. Barros, Â. Fernandes, M. Sokovic, A. Bracht, R. M. Peralta, and I. C. Ferreira. 2018. A natural food ingredient based on ergosterol: Optimization of the extraction from Agaricus blazei, evaluation of bioactive properties and incorporation in yogurts. Food & Function 9 (3):1465–74. doi: 10.1039/c7fo02007d.
- Cui, Y., K. Miao, S. Niyaphorn, and X. Qu. 2020. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. International Journal of Molecular Sciences 21 (3):995. doi: 10.3390/ijms21030995.
- Cunha, J. S., C. A. Ottoni, S. A. Morales, E. S. Silva, A. E. Maiorano, and R. F. Perna. 2019. Synthesis and characterization of fructosyltransferase from Aspergillus oryzae IPT-301 for high fructooligosaccharides production. Brazilian Journal of Chemical Engineering 36 (2):657–68. doi: 10.1590/0104-6632.20190362s20180572.
- Czerucka, D., and P. Rampal. 2002. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes and Infection 4 (7):733–9. doi: 10.1016/S1286-4579(02)01592-7.
- da Silva Menezes, B., D. M. Rossi, and M. A. Z. Ayub. 2017. Screening of filamentous fungi to produce xylanase and xylooligosaccharides in submerged and solid-state cultivations on rice husk, soybean hull, and spent malt as substrates. World Journal of Microbiology & Biotechnology 33 (3):58. doi: 10.1007/s11274-017-2226-5.
- da Silva Menezes, B., D. M. Rossi, F. Squina, and M. A. Z. Ayub. 2018. Xylooligosaccharides production by fungi cultivations in rice husk and their application as substrate for lactic acid bacteria growth. Bioresource Technology Reports 2:100–6. doi: 10.1016/j.biteb.2018.05.004.
- Daliri, E. B., D. H. Oh, and B. H. Lee. 2017. Bioactive peptides. Foods 6 (5):32. doi: 10.3390/foods6050032.
- de la Rosa, O., A. C. Flores-Gallegos, D. Muñíz-Marquez, C. Nobre, J. C. Contreras-Esquivel, and C. N. Aguilar. 2019. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends in Food Science & Technology 91:139–46. doi: 10.1016/j.tifs.2019.06.013.
- de la Rosa, O., D. B. M. Márquez, J. E. W. Paz, R. Rodríguez, R. M. Rodríguez, J. C. Contreras, and C. Aguilar. 2017. Current trends in the biotechnical production fructooligosaccharides. In Applied chemistry and chemical engineering, 251–72. New York: Apple Academic Press.
- de Morais, E. C., A. G. Cruz, and H. M. A. Bolini. 2013. Gluten‐free bread: Multiple time–intensity analysis, physical characterisation and acceptance test. International Journal of Food Science & Technology 48 (10):2176–84.
- Debnath, S., S. Jawahar, H. Muntaj, V. Purushotham, G. Sharmila, K. Sireesha, and M. N. Babu. 2019. A review on dietary fiber and its application. Research Journal of Pharmacognosy and Phytochemistry 11 (3):109–13. doi: 10.5958/0975-4385.2019.00019.0.
- Denny, A., B. Aisbitt, and J. Lunn. 2008. Mycoprotein and health. Nutrition Bulletin 33 (4):298–310. doi: 10.1111/j.1467-3010.2008.00730.x.
- Dewick, P. M. 1997. Medicinal natural products. Chichester: John Wiley and Sons.
- Dhakal, R., V. K. Bajpai, and K.-H. Baek. 2012. Production of gaba (γ - Aminobutyric acid) by microorganisms: A review. Brazilian Journal of Microbiology: [Publication of the Brazilian Society for Microbiology] 43 (4):1230–41. doi: 10.1590/S1517-83822012000400001.
- Di Cagno, R., P. Filannino, V. Cantatore, A. Polo, G. Celano, A. Martinovic, I. Cavoski, and M. Gobbetti. 2020. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. International Journal of Food Microbiology 323:108591. doi: 10.1016/j.ijfoodmicro.2020.108591.
- Diana, M., J. Quílez, and M. Rafecas. 2014. Gamma-aminobutyric acid as a bioactive compound in foods: A review. Journal of Functional Foods 10:407–20. doi: 10.1016/j.jff.2014.07.004.
- Ding, Y., K. Hao, Z. Li, R. Ma, Y. Zhou, Z. Zhou, M. Wei, Y. Liao, Y. Dai, Y. Yang, et al. 2020. c‐Fos separation from Lamin A/C by GDF15 promotes colon cancer invasion and metastasis in inflammatory microenvironment. Journal of Cellular Physiology 235 (5):4407–21.
- Dominguez, A., C. Nobre, L. R. Rodrigues, A. M. Peres, D. Torres, I. Rocha, N. Lima, and J. Teixeira. 2012. New improved method for fructooligosaccharides production by Aureobasidium pullulans. Carbohydrate Polymers 89 (4):1174–9.
- Du, B., Z. Bian, and B. Xu. 2014. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytotherapy Research: PTR 28 (2):159–66. doi: 10.1002/ptr.4963.
- Du, B., M. Meenu, H. Liu, and B. Xu. 2019. A concise review on the molecular structure and function relationship of β-glucan. International Journal of Molecular Sciences 20 (16):4032. doi: 10.3390/ijms20164032.
- Duan, Y., P. Katrolia, A. Zhong, and N. K. Kopparapu. 2022. Production, purification and characterization of a novel antithrombotic and anticoagulant serine protease from food grade microorganism Neurospora crassa. Preparative Biochemistry & Biotechnology 52 (9):1008–1018. doi: 10.1080/10826068.2021.2023824.
- Dupont, A. G., and L. Légat. 2020. GABA is a mediator of brain AT1 and AT2 receptor-mediated blood pressure responses. Hypertension Research 43 (10):995–1005. doi: 10.1038/s41440-020-0470-9.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). 2011. Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781),“anti‐inflammatory properties”(ID 1882),“contributes to the upper respiratory tract health”(ID 3468),“can help to maintain a normal function of gastrointestinal tract”(3779), and “contributes to body defences against external agents”(ID 3467) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA Journal 9 (4):2033.
- Elias, R. J., S. S. Kellerby, and E. A. Decker. 2008. Antioxidant activity of proteins and peptides. Critical Reviews in Food Science and Nutrition 48 (5):430–41.
- Erejuwa, O. O., S. A. Sulaiman, and M. S. A. Wahab. 2011. Oligosaccharides might contribute to the antidiabetic effect of honey: A review of the literature. Molecules (Basel, Switzerland) 17 (1):248–66. doi: 10.3390/molecules17010248.
- Evans, A. M., and G. Fornasini. 2003. Pharmacokinetics of L-Carnitine. Clinical Pharmacokinetics 42 (11):941–67. doi: 10.2165/00003088-200342110-00002.
- Fakruddin, M., M. N. Hossain, and M. M. Ahmed. 2017. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complementary and Alternative Medicine 17 (1):64. doi: 10.1186/s12906-017-1591-9.
- Ferreira, J. A., P. R. Lennartsson, and M. J. Taherzadeh. 2014. Production of ethanol and biomass from thin stillage using food-grade zygomycetes and ascomycetes filamentous fungi. Energies 7 (6):3872–85. doi: 10.3390/en7063872.
- Ferreira, J. A., P. R. Lennartsson, and M. J. Taherzadeh. 2015. Production of ethanol and biomass from thin stillage by Neurospora intermedia: A pilot study for process diversification. Engineering in Life Sciences 15 (8):751–9. doi: 10.1002/elsc.201400213.
- Ferreira, J. A., A. Mahboubi, P. R. Lennartsson, and M. J. Taherzadeh. 2016. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresource Technology 215:334–45. doi: 10.1016/j.biortech.2016.03.018.
- Fielding, R., L. Riede, J. P. Lugo, and A. Bellamine. 2018. L-carnitine supplementation in recovery after exercise. Nutrients 10 (3):349. doi: 10.3390/nu10030349.
- Filho, P. F. S., D. Andersson, J. A. Ferreira, and M. J. Taherzadeh. 2019. Mycoprotein: Environmental impact and health aspects. World Journal of Microbiology & Biotechnology 35 (10):147. doi: 10.1007/s11274-019-2723-9.
- Filho, P. F. S., A. Zamani, and M. J. Taherzadeh. 2017. Production of edible fungi from potato protein liquor (PPL) in airlift bioreactor. Fermentation 3 (1):12. doi: 10.3390/fermentation3010012.
- Filho, P. F. S., A. Zamani, and M. J. Taherzadeh. 2019. Edible protein production by filamentous fungi using starch plant wastewater. Waste and Biomass Valorization 10 (9):2487–96. doi: 10.1007/s12649-018-0265-2.
- Flores-Maltos, D. A., S. I. Mussatto, J. C. Contreras-Esquivel, R. Rodríguez-Herrera, J. A. Teixeira, and C. N. Aguilar. 2016. Biotechnological production and application of fructooligosaccharides. Critical Reviews in Biotechnology 36 (2):259–67. doi: 10.3109/07388551.2014.953443.
- Fredlund, E., U. Druvefors, M. E. Boysen, K.-J. Lingsten, and J. Schnürer. 2002. Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Research 2 (3):395–402. doi: 10.1016/S1567-1356(02)00098-3.
- Ganaie, M. A., A. Lateef, and U. S. Gupta. 2014. Enzymatic trends of fructooligosaccharides production by microorganisms. Applied Biochemistry and Biotechnology 172 (4):2143–59. doi: 10.1007/s12010-013-0661-9.
- Ganaie, M. A., H. K. Rawat, O. A. Wani, U. S. Gupta, and N. Kango. 2014. Immobilization of fructosyltransferase by chitosan and alginate for efficient production of fructooligosaccharides. Process Biochemistry 49 (5):840–4. doi: 10.1016/j.procbio.2014.01.026.
- Ganaie, M. A., H. Soni, G. A. Naikoo, L. T. S. Oliveira, H. K. Rawat, P. K. Mehta, and N. Narain. 2017. Screening of low cost agricultural wastes to maximize the fructosyltransferase production and its applicability in generation of fructooligosaccharides by solid state fermentation. International Biodeterioration & Biodegradation 118:19–26. doi: 10.1016/j.ibiod.2017.01.006.
- García-Mora, P., M. Martín-Martínez, M. A. Bonache, R. González-Múniz, E. Peñas, J. Frias, and C. Martinez-Villaluenga. 2017. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chemistry 221:464–72.
- Gargano, M. L., L. J. L. D. van Griensven, O. S. Isikhuemhen, U. Lindequist, G. Venturella, S. P. Wasser, and G. I. Zervakis. 2017. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosystems - An International Journal Dealing with All Aspects of Plant Biology 151 (3):548–65. doi: 10.1080/11263504.2017.1301590.
- George, T. S., K. S. Guru, N. S. Vasanthi, and K. P. Kannan. 2011. Extraction, purification and characterization of chitosan from endophytic fungi isolated from medicinal plants. World Journal of Science and Technology 1 (4):43–8.
- Gessner, M. O. 2020. Ergosterol as a measure of fungal biomass. In Methods to study litter decomposition: A practical guide, edited by F. Bärlocher, M. O. Gessner, and M. A. S. Graça, 247–55. Switzerland AG: Springer International Publishing. doi: 10.1007/978-3-030-30515-4_27.
- Giacco, R., G. Clemente, D. Luongo, G. Lasorella, I. Fiume, F. Brouns, F. Bornet, L. Patti, P. Cipriano, A. A. Rivellese, et al. 2004. Effects of short-chain fructo-oligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clinical Nutrition (Edinburgh, Scotland) 23 (3):331–40. doi: 10.1016/j.clnu.2003.07.010.
- Gibbs, B. F., A. Zougman, R. Masse, and C. Mulligan. 2004. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Research International 37 (2):123–31. doi: 10.1016/j.foodres.2003.09.010.
- Gil-Ramírez, A., A. Ruiz-Rodríguez, F. R. Marín, G. Reglero, and C. Soler-Rivas. 2014. Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. Journal of Functional Foods 11:589–97. doi: 10.1016/j.jff.2014.08.025.
- Gil, Y.-G., S. Kang, A. Chae, Y.-K. Kim, D.-H. Min, and H. Jang. 2018. Synthesis of porous Pd nanoparticles by therapeutic chaga extract for highly efficient tri-modal cancer treatment [10..1039/C8NR07172A]. Nanoscale 10 (42):19810–7. doi: 10.1039/C8NR07172A.
- Giménez, V. M. M., F. Inserra, C. D. Tajer, J. Mariani, L. Ferder, R. J. Reiter, and W. Manucha. 2020. Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sciences 254:117808. doi: 10.1016/j.lfs.2020.117808.
- Gmoser, R., R. Fristedt, K. Larsson, I. Undeland, M. J. Taherzadeh, and P. R. Lennartsson. 2020. From stale bread and brewers spent grain to a new food source using edible filamentous fungi. Bioengineered 11 (1):582–98. doi: 10.1080/21655979.2020.1768694.
- Goktas, H., E. Dertli, and O. Sagdic. 2021. Comparison of functional characteristics of distinct Saccharomyces boulardii strains isolated from commercial food supplements. LWT 136:110340. doi: 10.1016/j.lwt.2020.110340.
- Gonçalves, C., N. Ferreira, and L. Lourenço. 2021. Production of low molecular weight chitosan and chitooligosaccharides (COS): A review. Polymers 13 (15):2466. doi: 10.3390/polym13152466.
- González-Ortega, O., A. R. López-Limón, J. F. Morales-Domínguez, and R. E. Soria-Guerra. 2015. Production and purification of recombinant hypocholesterolemic peptides. Biotechnology Letters 37 (1):41–54. doi: 10.1007/s10529-014-1657-4.
- Grangeia, C., S. A. Heleno, L. Barros, A. Martins, and I. C. F. R. Ferreira. 2011. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Research International 44 (4):1029–35. doi: 10.1016/j.foodres.2011.03.006.
- Gropper, S. S., and J. L. Smith. 2012. Advanced nutrition and human metabolism. Belmont, CA: Cengage Learning.
- Guarnieri, A., M. Triunfo, C. Scieuzo, D. Ianniciello, E. Tafi, T. Hahn, S. Zibek, R. Salvia, A. De Bonis, and P. Falabella. 2022. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Scientific Reports 12 (1):8084. doi: 10.1038/s41598-022-12150-3.
- Hamed, I., F. Özogul, and J. M. Regenstein. 2016. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends in Food Science & Technology 48:40–50. doi: 10.1016/j.tifs.2015.11.007.
- Hayashi, K., and M. Ito. 2002. Antidiabetic action of low molecular weight chitosan in genetically obese diabetic KK-Ay mice. Biological & Pharmaceutical Bulletin 25 (2):188–92. doi: 10.1248/bpb.25.188.
- Hayashi, S., T. Yoshiyama, N. Fujii, and S. Shinohara. 2000. Production of a novel syrup containing neofructo-oligosaccharides by the cells of Penicillium citrinum. Biotechnology Letters 22 (18):1465–9. doi: 10.1023/A:1005627828876.
- He, L., W. Shi, X. Liu, X. Zhao, and Z. Zhang. 2018. Anticancer action and mechanism of ergosterol peroxide from Paecilomyces cicadae fermentation broth. International Journal of Molecular Sciences 19 (12):3935. doi: 10.3390/ijms19123935.
- He, R., X. Ju, J. Yuan, L. Wang, A. T. Girgih, and R. E. Aluko. 2012. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Research International 49 (1):432–8. doi: 10.1016/j.foodres.2012.08.023.
- He, W.-S., D. Cui, L. Li, J. Rui, and L.-T. Tong. 2020. Plasma triacylglycerol-reducing activity of ergosterol linolenate is associated with inhibition of intestinal lipid absorption. Journal of Functional Foods 64:103686. doi: 10.1016/j.jff.2019.103686.
- He, W.-S., D. Cui, L. Li, L.-T. Tong, J. Rui, H. Li, H. Zhang, and X. Liu. 2019. Cholesterol-reducing effect of ergosterol is modulated via inhibition of cholesterol absorption and promotion of cholesterol excretion. Journal of Functional Foods 57:488–96. doi: 10.1016/j.jff.2019.04.042.
- Heleno, S. A., D. Stojković, L. Barros, J. Glamočlija, M. Soković, A. Martins, M. J. R. P. Queiroz, and I. C. F. R. Ferreira. 2013. A comparative study of chemical composition, antioxidant and antimicrobial properties of Morchella esculenta (L.) Pers. from Portugal and Serbia. Food Research International 51 (1):236–43. doi: 10.1016/j.foodres.2012.12.020.
- Hellwig, C., R. Gmoser, M. Lundin, M. J. Taherzadeh, and K. Rousta. 2020. Fungi burger from stale bread? A case study on perceptions of a novel protein-rich food product made from an edible fungus. Foods 9 (8):1112. doi: 10.3390/foods9081112.
- Hellwig, C., N. Rousta, R. Wikandari, M. J. Taherzadeh, G. Häggblom-Kronlöf, K. Bolton, and K. Rousta. 2022. Household fermentation of leftover bread to nutritious food. Waste Management (New York, NY) 150:39–47. doi: 10.1016/j.wasman.2022.06.038.
- Hong, F., J. Yan, J. T. Baran, D. J. Allendorf, R. D. Hansen, G. R. Ostroff, P. X. Xing, N.-K V. Cheung, and G. D. Ross. 2004. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. Journal of Immunology (Baltimore, MD: 1950) 173 (2):797–806. doi: 10.4049/jimmunol.173.2.797.
- Hooshmand, S., A. Balakrishnan, R. M. Clark, K. Q. Owen, S. I. Koo, and B. H. Arjmandi. 2008. Dietary l-carnitine supplementation improves bone mineral density by suppressing bone turnover in aged ovariectomized rats. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 15 (8):595–601. doi: 10.1016/j.phymed.2008.02.026.
- Hossain, M. N., S. Afrin, S. Humayun, M. M. Ahmed, and B. K. Saha. 2020. Identification and growth characterization of a novel strain of saccharomyces boulardii isolated from soya paste [original research]. Frontiers in Nutrition 7:27. doi: 10.3389/fnut.2020.00027.
- Hu, S.-H., J.-C. Wang, J.-L. Lien, E.-T. Liaw, and M.-Y. Lee. 2006. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Applied Microbiology and Biotechnology 70 (1):107–13. doi: 10.1007/s00253-005-0043-5.
- Hu, Z., B. He, L. Ma, Y. Sun, Y. Niu, and B. Zeng. 2017. Recent advances in ergosterol biosynthesis and regulation mechanisms in Saccharomyces cerevisiae. Indian Journal of Microbiology 57 (3):270–7. doi: 10.1007/s12088-017-0657-1.
- Huang, J., C. Wu, S. Tang, P. Zhou, J. Deng, Z. Zhang, Y. Wang, and Z. Wang. 2020. Chiral active β-glucan nanoparticles for synergistic delivery of doxorubicin and immune potentiation. International Journal of Nanomedicine 15:5083–95. doi: 10.2147/IJN.S258145.
- Hudson, L. E., C. D. McDermott, T. P. Stewart, W. H. Hudson, D. Rios, M. B. Fasken, A. H. Corbett, and T. J. Lamb. 2016. Characterization of the probiotic yeast Saccharomyces boulardii in the healthy mucosal immune system. PloS One 11 (4):e0153351. doi: 10.1371/journal.pone.0153351.
- Hur, J., T. T. H. Nguyen, N. Park, J. Kim, and D. Kim. 2018. Characterization of quinoa (Chenopodium quinoa) fermented by Rhizopus oligosporus and its bioactive properties. AMB Express 8 (1):143– doi: 10.1186/s13568-018-0675-3.
- Inoue, K., T. Gotou, H. Kitajima, S. Mizuno, T. Nakazawa, and N. Yamamoto. 2009. Release of antihypertensive peptides in miso paste during its fermentation, by the addition of casein. Journal of Bioscience and Bioengineering 108 (2):111–5. doi: 10.1016/j.jbiosc.2009.03.007.
- Iwakura, Y., and H. Nawa. 2013. ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: Pathological implications in schizophrenia and Parkinson’s disease [Review. Frontiers in Cellular Neuroscience 7:4. doi: 10.3389/fncel.2013.00004.
- Jamzivar, F., M. Shams-Ghahfarokhi, M. Khoramizadeh, N. Yousefi, M. Gholami-Shabani, and M. Razzaghi-Abyaneh. 2019. Unraveling the importance of molecules of natural origin in antifungal drug development through targeting ergosterol biosynthesis pathway. Iranian Journal of Microbiology 11 (6):448–59.
- Jannoey, P., H. Niamsup, S. Lumyong, T. Suzuki, T. Katayama, and G. Chairote. 2010. Comparison of gamma-aminobutyric acid production in Thai rice grains. World Journal of Microbiology and Biotechnology 26 (2):257–63. doi: 10.1007/s11274-009-0168-2.
- Jean, M., M. Alameh, D. De Jesus, M. Thibault, M. Lavertu, V. Darras, M. Nelea, M. D. Buschmann, and A. Merzouki. 2012. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences 45 (1–2):138–49. doi: 10.1016/j.ejps.2011.10.029.
- Jeong, Y. U., and Y. J. Park. 2020. Ergosterol Peroxide from the medicinal mushroom ganoderma lucidum inhibits differentiation and lipid accumulation of 3T3-L1 adipocytes. International Journal of Molecular Sciences 21 (2):460. doi: 10.3390/ijms21020460.
- Jiang, C., J. Ge, B. He, and B. Zeng. 2021. Glycosphingolipids in filamentous fungi: Biological roles and potential applications in cosmetics and health foods [Review]. Frontiers in Microbiology 12:690211. doi: 10.3389/fmicb.2021.690211.
- Jiang, Q., M. Zhang, and A. S. Mujumdar. 2020. UV induced conversion during drying of ergosterol to vitamin D in various mushrooms: Effect of different drying conditions. Trends in Food Science & Technology 105:200–10. doi: 10.1016/j.tifs.2020.09.011.
- Jippo, T., T. Suzuki, H. Sato, Y. Kobayashi, and M. Shigekawa. 2015. Water-soluble low-molecular-weight b-(1, 3–1, 6) D-Glucan inhibit cedar pollinosis. Functional Foods in Health and Disease 5 (2):80–8. doi: 10.31989/ffhd.v5i2.173.
- Jordá, T., and S. Puig. 2020. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 11 (7):795. doi: 10.3390/genes11070795.
- Jung, K., S. Bang, T. K. Oh, and H. Park. 2011. Industrial production of fructooligosaccharides by immobilized cells of Aureobasidium pullulans in a packed bed reactor. Biotechnology Letters 33 (8):1621–4. doi: 10.1007/s10529-011-0606-8.
- Jung, W.-J., and R.-D. Park. 2014. Bioproduction of chitooligosaccharides: Present and perspectives. Marine Drugs 12 (11):5328–56. doi: 10.3390/md12115328.
- Kang, J.-H., J.-E. Jang, S. K. Mishra, H.-J. Lee, C. W. Nho, D. Shin, M. Jin, M. K. Kim, C. Choi, and S. H. Oh. 2015. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. Journal of Ethnopharmacology 173:303–12. doi: 10.1016/j.jep.2015.07.030.
- Karami, Z., and B. Akbari-Adergani. 2019. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. Journal of Food Science and Technology 56 (2):535–47. doi: 10.1007/s13197-018-3549-4.
- Karaolis, C., G. Botsaris, I. Pantelides, and D. Tsaltas. 2013. Potential application of Saccharomyces boulardii as a probiotic in goat’s yoghurt: Survival and organoleptic effects. International Journal of Food Science & Technology 48 (7):1445–52. doi: 10.1111/ijfs.12111.
- Karimi, S., N. Mahboobi Soofiani, T. Lundh, A. Mahboubi, A. Kiessling, and M. J. Taherzadeh. 2019. Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: Protein, lipid, and mineral composition. Fermentation 5 (4):99. doi: 10.3390/fermentation5040099.
- Kaźmierczak-Siedlecka, K., A. Dvořák, M. Folwarski, A. Daca, K. Przewłócka, and W. Makarewicz. 2020. Fungal gut microbiota dysbiosis and its role in colorectal, oral, and pancreatic carcinogenesis. Cancers 12 (5):1326. doi: 10.3390/cancers12051326.
- Kechagia, M., D. Basoulis, S. Konstantopoulou, D. Dimitriadi, K. Gyftopoulou, N. Skarmoutsou, and E. M. Fakiri. 2013. Health benefits of probiotics: A review. ISRN Nutrition 2013:481651. doi: 10.5402/2013/481651.
- Kim, S.-K., D.-H. Ngo, and T.-S. Vo. 2012. Chapter 16 - Marine fish-derived bioactive peptides as potential antihypertensive agents. In Advances in food and nutrition research, ed. S.-K. Kim, 65, 249–60. Waltham, MA: Academic Press. doi: 10.1016/B978-0-12-416003-3.00016-0.
- Kim, Y.-W., K.-H. Kim, H.-J. Choi, and D.-S. Lee. 2005. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnology Letters 27 (7):483–7.
- Knezevic-Jugovic, Z., Z. Petronijevic, and A. Smelcerovic. 2011. Chitin and chitosan from microorganisms. In Chitin, chitosan, oligosaccharides and their derivatives, 25–36. New York: CRC Press.
- Kobori, M., M. Yoshida, M. Ohnishi‐Kameyama, and H. Shinmoto. 2007. Ergosterol peroxide from an edible mushroom suppresses inflammatory responses in RAW264. 7 macrophages and growth of HT29 colon adenocarcinoma cells. British Journal of Pharmacology 150 (2):209–19. doi: 10.1038/sj.bjp.0706972.
- Korhonen, H., and A. Pihlanto. 2006. Bioactive peptides: Production and functionality. International Dairy Journal 16 (9):945–60. doi: 10.1016/j.idairyj.2005.10.012.
- Kostag, M., and O. A. El Seoud. 2021. Sustainable biomaterials based on cellulose, chitin and chitosan composites-A review. Carbohydrate Polymer Technologies and Applications 2:100079. doi: 10.1016/j.carpta.2021.100079.
- Krulwich, T. A., G. Sachs, and E. Padan. 2011. Molecular aspects of bacterial pH sensing and homeostasis. Nature Reviews. Microbiology 9 (5):330–43. doi: 10.1038/nrmicro2549.
- Kumar, S., N. S. Punekar, V. SatyaNarayan, and K. V. Venkatesh. 2000. Metabolic fate of glutamate and evaluation of flux through the 4-aminobutyrate (GABA) shunt in Aspergillus niger. Biotechnology and Bioengineering 67 (5):575–84. doi: 10.1002/(SICI)1097-0290(20000305)67:5<575::AID-BIT8>3.0.CO;2-L.
- Kumura, H., Y. Tanoue, M. Tsukahara, T. Tanaka, and K. Shimazaki. 2004. Screening of dairy yeast strains for probiotic applications. Journal of Dairy Science 87 (12):4050–6. doi: 10.3168/jds.S0022-0302(04)73546-8.
- Kusmiati, K., S. R. Tamat, E. Jusuf, and R. I. A. Istiningsih. 2007. Beta glucan production from two strains of agrobacterium sp in medium containing of molases and uracil combine. Biodiversitas Journal of Biological Diversity 8 (2):123–129. doi: 10.13057/biodiv/d080210.
- L’Hocine, L., Z. Wang, B. Jiang, and S. Xu. 2000. Purification and partial characterization of fructosyltransferase and invertase from Aspergillus niger AS0023. Journal of Biotechnology 81 (1):73–84.
- Lafarga, T., and M. Hayes. 2014. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Science 98 (2):227–39. doi: 10.1016/j.meatsci.2014.05.036.
- Lan Liu, X., N. Kumar Kopparapu, H. Chen Zheng, P. Katrolia, Y. Ping Deng, and X. Qun Zheng. 2016. Purification and characterization of a fibrinolytic enzyme from the food-grade fungus, Neurospora sitophila. Journal of Molecular Catalysis B: Enzymatic 134:98–104.
- Lateef, A., and E. Gueguim-Kana. 2012. Utilization of cassava wastes in the production of fructosyltransferase by Rhizopus stolonifer LAU 07. Romanian Biotechnological Letters 17 (3):7309–16.
- Lateef, A., J. Oloke, and S. Prapulla. 2007. The effect of ultrasonication on the release of fructosyltransferase from Aureobasidium pullulans CFR 77. Enzyme and Microbial Technology 40 (5):1067–70. doi: 10.1016/j.enzmictec.2006.08.008.
- Lee, B.-J., J.-S. Lin, Y.-C. Lin, and P.-T. Lin. 2015. Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition (Burbank, Los Angeles County, CA) 31 (3):475–9.
- Lee, G., T. T. H. Nguyen, T. Y. Lim, J. Lim, B. Park, S. Lee, I.-K. Mok, K. Pal, S. Lim, and D. Kim. 2020. Fermented wild ginseng by rhizopus oligosporus improved l-carnitine and ginsenoside contents. Molecules (Basel, Switzerland) 25 (9):2111. https://www.mdpi.com/1420-3049/25/9/2111. doi: 10.3390/molecules25092111.
- Lee, H., J. I. Juncosa, and R. B. Silverman. 2015. Ornithine aminotransferase versus GABA aminotransferase: Implications for the design of new anticancer drugs. Medicinal Research Reviews 35 (2):286–305. doi: 10.1002/med.21328.
- Lee, T-k., T. T. H. Nguyen, N. Park, S.-H. Kwak, J. Kim, S. Jin, G.-M. Son, J. Hur, J.-I. Choi, and D. Kim. 2018. The use of fermented buckwheat to produce l-carnitine enriched oyster mushroom. AMB Express 8 (1):138. doi: 10.1186/s13568-018-0664-6.
- Leung, I. K. H., T. J. Krojer, G. T. Kochan, L. Henry, F. von Delft, T. D. W. Claridge, U. Oppermann, M. A. McDonough, and C. J. Schofield. 2010. Structural and mechanistic studies on γ-butyrobetaine hydroxylase. Chemistry & Biology 17 (12):1316–24. doi: 10.1016/j.chembiol.2010.09.016.
- Levetin, W. E., J. A. Horner, C. Scott, S. Barnes, G. L. Baxi, C. Chew, K. Grimes, D. Kennedy, J. D. Larenas-Linnemann, and E. Miller, Environmental Allergens Workgroup. 2016. Taxonomy of allergenic fungi. The Journal of Allergy and Clinical Immunology. In Practice 4 (3):375–85.e1.
- Li, J.-L., Q.-Y. Wang, H.-Y. Luan, Z.-C. Kang, and C.-B. Wang. 2012. Effects of L-carnitine against oxidative stress in human hepatocytes: Involvement of peroxisome proliferator-activated receptor alpha. Journal of Biomedical Science 19 (1):32–9. doi: 10.1186/1423-0127-19-32.
- Li, J.-S., Y.-M. Chew, M.-C. Lin, Y.-Q. Lau, and C.-S. Chen. 2021. Enhanced glucosamine production from Aspergillus oryzae NCH-42 via acidic stress under submerged fermentation. CyTA - Journal of Food 19 (1):614–24. doi: 10.1080/19476337.2021.1946158.
- Li, M., C. Jiang, Q. Wang, Z. Zhao, Q. Jin, J.-R. Xu, and H. Liu. 2016. Evolution and functional insights of different ancestral orthologous clades of chitin synthase genes in the fungal tree of life. Frontiers in Plant Science 7:37.
- Li, T., X. Zhang, Y. Ren, Y. Zeng, Q. Huang, and C. Wang. 2022. Antihypertensive effect of soybean bioactive peptides: A review. Current Opinion in Pharmacology 62:74–81. doi: 10.1016/j.coph.2021.11.005.
- Li, X., Q. Wu, M. Bu, L. Hu, W. W. Du, C. Jiao, H. Pan, M. Sdiri, N. Wu, Y. Xie, et al. 2016. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget 7 (23):33948–59.
- Li, X., Q. Wu, Y. Xie, Y. Ding, W. W. Du, M. Sdiri, and B. B. Yang. 2015. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget 6 (19):17832–46.
- Lindequist, U., T. H. J. Niedermeyer, and W.-D. Jülich. 2005. The pharmacological potential of mushrooms. Evidence-Based Complementary and Alternative Medicine 2 (3):285–99. doi: 10.1093/ecam/neh107.
- Liu, L., Q. Li, Y. Yang, and A. Guo. 2021. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry [Review]. Frontiers in Veterinary Science 8:736739. doi: 10.3389/fvets.2021.736739.
- Liu, M., Y. Zhang, H. Zhang, B. Hu, L. Wang, H. Qian, and X. Qi. 2016. The anti-diabetic activity of oat β-d-glucan in streptozotocin–nicotinamide induced diabetic mice. International Journal of Biological Macromolecules 91:1170–6. doi: 10.1016/j.ijbiomac.2016.06.083.
- Liu, X., Y. Zhang, Z. Liu, and X. Xie. 2019. Anti-tumor effect of chitin oligosaccharide plus cisplatin in vitro and in vivo. OncoTargets and Therapy 12:7581–90. doi: 10.2147/ott.S220619.
- Longo, N., M. Frigeni, and M. Pasquali. 2016. Carnitine transport and fatty acid oxidation. Biochimica et Biophysica Acta 1863 (10):2422–35. doi: 10.1016/j.bbamcr.2016.01.023.
- López-Barrios, L., J. A. Gutiérrez-Uribe, and S. O. Serna-Saldívar. 2014. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. Journal of Food Science 79 (3):R273–R283. doi: 10.1111/1750-3841.12365.
- Lorenzo, J. M., P. E. S. Munekata, B. Gómez, F. J. Barba, L. Mora, C. Pérez-Santaescolástica, and F. Toldrá. 2018. Bioactive peptides as natural antioxidants in food products – A review. Trends in Food Science & Technology 79:136–47. doi: 10.1016/j.tifs.2018.07.003.
- Luo, Y., H. Zhou, M. Mizutani, N. Mizutani, R. A. Reisfeld, and R. Xiang. 2003. Transcription factor Fos-related antigen 1 is an effective target for a breast cancer vaccine. Proceedings of the National Academy of Sciences of the United States of America 100 (15):8850–5.
- Madhu, M., D. Kumar, R. Sirohi, A. Tarafdar, T. Dhewa, R. E. Aluko, P. C. Badgujar, and M. K. Awasthi. 2022. Bioactive peptides from meat: Current status on production, biological activity, safety, and regulatory framework. Chemosphere 307 (Pt 1):135650. doi: 10.1016/j.chemosphere.2022.135650.
- Magoulas, P. L., and A. W. El-Hattab. 2012. Systemic primary carnitine deficiency: An overview of clinical manifestations, diagnosis, and management. Orphanet Journal of Rare Diseases 7 (1):68. doi: 10.1186/1750-1172-7-68.
- Magro, A. E. A., L. C. Silva, G. B. Rasera, and R. J. S. de Castro. 2019. Solid-state fermentation as an efficient strategy for the biotransformation of lentils: Enhancing their antioxidant and antidiabetic potentials. Bioresources and Bioprocessing 6 (1):38. doi: 10.1186/s40643-019-0273-5.
- Mahasneh, A. M., and M. M. Abbas. 2010. Probiotics and traditional fermented foods: The eternal connection: Mini review. Jordan Journal of Biological Sciences 3 (4):133–40.
- Mahboubi, A., J. A. Ferreira, M. J. Taherzadeh, and P. R. Lennartsson. 2017a. Production of fungal biomass for feed, fatty acids, and glycerol by Aspergillus oryzae from fat-rich dairy substrates. Fermentation 3 (4):48. doi: 10.3390/fermentation3040048.
- Mahboubi, A., J. A. Ferreira, M. J. Taherzadeh, and P. R. Lennartsson. 2017b. Value-added products from dairy waste using edible fungi. Waste Management 59:518–25. doi: 10.1016/j.wasman.2016.11.017.
- Maiorano, A. E., E. S. da Silva, R. F. Perna, C. A. Ottoni, R. A. M. Piccoli, R. C. Fernandez, B. G. Maresma, and M. F. de Andrade Rodrigues. 2020. Effect of agitation speed and aeration rate on fructosyltransferase production of Aspergillus oryzae IPT-301 in stirred tank bioreactor. Biotechnology Letters 42 (12):2619–29. doi: 10.1007/s10529-020-03006-9.
- Manisha, and S. K. Yadav. 2017. Technological advances and applications of hydrolytic enzymes for valorization of lignocellulosic biomass. Bioresource Technology 245:1727–39. doi: 10.1016/j.biortech.2017.05.066.
- Manoli, I., M. U. De Martino, T. Kino, and S. Alesci. 2004. Modulatory effects of L‐carnitine on glucocorticoid receptor activity. Annals of the New York Academy of Sciences 1033 (1):147–57.
- Martínez-Medina, G. A., A. P. Barragán, H. A. Ruiz, A. Ilyina, J. L. Martínez Hernández, R. M. Rodríguez-Jasso, J. L. Hoyos-Concha, and C. N. Aguilar-González. 2019. Chapter 14 - Fungal proteases and production of bioactive peptides for the food industry. In Enzymes in food biotechnology, M. Kuddus, 221–46. London, United Kingdom: Academic Press. doi: 10.1016/B978-0-12-813280-7.00014-1.
- Masuo, S., Y. Terabayashi, M. Shimizu, T. Fujii, T. Kitazume, and N. Takaya. 2010. Global gene expression analysis of Aspergillus nidulans reveals metabolic shift and transcription suppression under hypoxia. Molecular Genetics and Genomics: MGG 284 (6):415–24. doi: 10.1007/s00438-010-0576-x.
- Melo-Bolívar, J. F., R. Y. Ruiz-Pardo, M. E. Hume, H. E. Sidjabat, and L. M. Villamil-Diaz. 2020. Probiotics for cultured freshwater fish. Microbiology Australia 41 (2):105–8. doi: 10.1071/MA20026.
- Meyer, H.-P., and K. T. Robins. 2005. Large scale bioprocess for the production of optically pure L-carnitine. Monatshefte Für Chemie - Chemical Monthly 136 (8):1269–77. doi: 10.1007/s00706-005-0330-y.
- Michel, M. R., R. M. Rodríguez-Jasso, C. N. Aguilar, S. M. Gonzalez-Herrera, A. C. Flores-Gallegos, and R. Rodríguez-Herrera. 2016. Fructosyltransferase sources, production, and applications for prebiotics production. In Probiotics and prebiotics in human nutrition and health, 169–90. London: InTech.
- Minuk, G. 2000. GABA and hepatocellular carcinoma. Molecular and Cellular Biochemistry 207 (1–2):105–8.
- Mohanty, D., R. Jena, P. K. Choudhury, R. Pattnaik, S. Mohapatra, and M. R. Saini. 2016. Milk derived antimicrobial bioactive peptides: A review. International Journal of Food Properties 19 (4):837–46. doi: 10.1080/10942912.2015.1048356.
- Mohanty, D. P., S. Mohapatra, S. Misra, and P. S. Sahu. 2016. Milk derived bioactive peptides and their impact on human health – A review. Saudi Journal of Biological Sciences 23 (5):577–83. doi: 10.1016/j.sjbs.2015.06.005.
- Moharib, S. A., M. Shehata, A. Salama, and M. Hegazi. 2014. Effect of fructooligosaccharides in Cynara scolymus and Allium cepa on carbohydrate and lipid metabolism in rats. Electronic Journal of Polish Agricultural Universities 17 (1).
- Molitoris, H. P. 1994. Mushrooms in medicine. Folia Microbiologica 39 (2):91–8. doi: 10.1007/BF02906801.
- Monsan, P. F., and F. Ouarné. 2009. Oligosaccharides derived from sucrose. In Prebiotics and probiotics science and technology, 293–336. New York: Springer.
- Montoya-Rodríguez, A., M. A. Gómez-Favela, C. Reyes-Moreno, J. Milán-Carrillo, and E. González de Mejía. 2015. Identification of bioactive peptide sequences from Amaranth (Amaranthus hypochondriacus) seed proteins and their potential role in the prevention of chronic diseases. Comprehensive Reviews in Food Science and Food Safety 14 (2):139–58. doi: 10.1111/1541-4337.12125.
- Moore, D., and S. W. Chiu. 2001. Fungal products as food. In Bio-exploitation of filamentous fungi, ed. S. B. Pointing and K. D. Hyde, 6, 223–51. Hong Kong: Fungal Diversity Press.
- Mora, L., and M. Hayes. 2015. Cardioprotective cryptides derived from fish and other food sources: Generation, application, and future markets. Journal of Agricultural and Food Chemistry 63 (5):1319–31. doi: 10.1021/jf505019z.
- Moraru, C., M. M. Mincea, M. Frandes, B. Timar, and V. Ostafe. 2018. A meta-analysis on randomised controlled clinical trials evaluating the effect of the dietary supplement chitosan on weight loss, lipid parameters and blood pressure. Medicina 54 (6):109. doi: 10.3390/medicina54060109.
- Moslehi-Jenabian, S., L. Lindegaard, and L. Jespersen. 2010. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2 (4):449–73. doi: 10.3390/nu2040449.
- Mudgal, J., P. P. Mudgal, M. Kinra, and R. Raval. 2019. Immunomodulatory role of chitosan-based nanoparticles and oligosaccharides in cyclophosphamide-treated mice. Scandinavian Journal of Immunology 89 (4):e12749. doi: 10.1111/sji.12749.
- Muñiz-Márquez, D. B., J. C. Contreras, R. Rodríguez, S. I. Mussatto, J. A. Teixeira, and C. N. Aguilar. 2016. Enhancement of fructosyltransferase and fructooligosaccharides production by A. oryzae DIA-MF in Solid-State Fermentation using aguamiel as culture medium. Bioresource Technology 213:276–82. doi: 10.1016/j.biortech.2016.03.022.
- Muñiz-Márquez, D. B., J. A. Teixeira, S. I. Mussatto, J. C. Contreras-Esquivel, R. Rodríguez-Herrera, and C. N. Aguilar. 2019. Fructo-oligosaccharides (FOS) production by fungal submerged culture using aguamiel as a low-cost by-product. LWT 102:75–9. doi: 10.1016/j.lwt.2018.12.020.
- Mussatto, S. I., L. M. Aguiar, M. I. Marinha, R. C. Jorge, and E. C. Ferreira. 2015. Economic analysis and environmental impact assessment of three different fermentation processes for fructooligosaccharides production. Bioresource Technology 198:673–81. doi: 10.1016/j.biortech.2015.09.060.
- Mussatto, S. I., L. F. Ballesteros, S. Martins, D. A. F. Maltos, C. N. Aguilar, and J. A. Teixeira. 2013. Maximization of Fructooligosaccharides and β-fructofuranosidase production by Aspergillus japonicus under solid-state fermentation conditions. Food and Bioprocess Technology 6 (8):2128–34. doi: 10.1007/s11947-012-0873-y.
- Mussatto, S. I., and J. A. Teixeira. 2010. Increase in the fructooligosaccharides yield and productivity by solid-state fermentation with Aspergillus japonicus using agro-industrial residues as support and nutrient source. Biochemical Engineering Journal 53 (1):154–7. doi: 10.1016/j.bej.2010.09.012.
- Möhler, H. 2012. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62 (1):42–53. doi: 10.1016/j.neuropharm.2011.08.040.
- Naidu, G. S. N., I. Y. Lee, E. G. Lee, G. H. Kang, and Y. H. Park. 2000. Microbial and enzymatic production of l-carnitine. Bioprocess Engineering 23 (6):627–35. doi: 10.1007/s004490000212.
- Nair, R. B., and M. J. Taherzadeh. 2016. Valorization of sugar-to-ethanol process waste vinasse: A novel biorefinery approach using edible Ascomycetes filamentous fungi. Bioresource Technology 221:469–76. doi: 10.1016/j.biortech.2016.09.074.
- Nakahara, T., A. Sano, H. Yamaguchi, K. Sugimoto, H. Chikata, E. Kinoshita, and R. Uchida. 2010. Antihypertensive effect of peptide-enriched soy sauce-like seasoning and identification of its angiotensin I-converting enzyme inhibitory substances. Journal of Agricultural and Food Chemistry 58 (2):821–7. doi: 10.1021/jf903261h.
- Nascimento, G. C. d., R. D. Batista, C. C. A. d A. Santos, E. M. d Silva, F. C. de Paula, D. B. Mendes, D. P. de Oliveira, and A. F. d Almeida. 2019. β-fructofuranosidase and β–D-fructosyltransferase from new Aspergillus carbonarius PC-4 strain isolated from canned peach syrup: Effect of carbon and nitrogen sources on enzyme production. The Scientific World Journal 2019:1–13. doi: 10.1155/2019/6956202.
- Nascimento, T. P., A. E. S. Conniff, J. A. S. Moura, J. Batista, S. Matias, R. Costa, P. Marcos, C. S. Porto, G. Takaki, and C. Maria. 2020. Protease from Mucor subtilissimus UCP 1262: Evaluation of several specific protease activities and purification of a fibrinolytic enzyme. Anais da Academia Brasileira de Ciências 92 (4):e20200882. doi: 10.1590/0001-3765202020200882.
- Nascimento, T. P., Sales, A. E. Porto, T. S. Costa, R. M. P. B. Breydo, L. Uversky, V. N. Porto, A. L. F, and Converti, A. 2017. Purification, biochemical, and structural characterization of a novel fibrinolytic enzyme from Mucor subtilissimus UCP 1262. Bioprocess and Biosystems Engineering 40 (8):1209–19. doi: 10.1007/s00449-017-1781-3.
- Naveed, M., L. Phil, M. Sohail, M. Hasnat, M. M. F. A. Baig, A. U. Ihsan, M. Shumzaid, M. U. Kakar, T. Mehmood Khan, M. D. Akabar, et al. 2019. Chitosan oligosaccharide (COS): An overview. International Journal of Biological Macromolecules 129:827–43.
- Newman, D. J., and G. M. Cragg. 2012. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Journal of Natural Products 75 (3):311–35. doi: 10.1021/np200906s.
- Nikmaram, N., B. N. Dar, S. Roohinejad, M. Koubaa, F. J. Barba, R. Greiner, and S. K. Johnson. 2017. Recent advances in γ-aminobutyric acid (GABA) properties in pulses: An overview. Journal of the Science of Food and Agriculture 97 (9):2681–9. doi: 10.1002/jsfa.8283.
- Nishantha, M., D. Jeewani, N. Xiaojun, and S. Weining. 2018. Beta-glucan: An overview of its properties, health benefits, genetic background and practical applications. Scholars Journal of Agriculture and Veterinary Sciences 5 (3):130–40.
- Nobre, C., M. Â. Cerqueira, L. R. Rodrigues, A. A. Vicente, and J. A. Teixeira. 2015. Chapter 19 - Production and extraction of polysaccharides and oligosaccharides and their use as new food additives. In Industrial biorefineries & white biotechnology, eds. A. Pandey, R. Höfer, M. Taherzadeh, K. M. Nampoothiri, and C. Larroche, 653–79. Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-444-63453-5.00021-5.
- Nongonierma, A. B., and R. J. FitzGerald. 2019. Features of dipeptidyl peptidase IV (DPP‐IV) inhibitory peptides from dietary proteins. Journal of Food Biochemistry 43 (1):e12451.
- Nowak, R., N. Nowacka-Jechalke, W. Pietrzak, and U. Gawlik-Dziki. 2022. A new look at edible and medicinal mushrooms as a source of ergosterol and ergosterol peroxide-UHPLC-MS/MS analysis. Food Chemistry 369:130927. doi: 10.1016/j.foodchem.2021.130927.
- Nuss, P. 2015. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatric Disease and Treatment 11:165–75. doi: 10.2147/ndt.S58841.
- Nölle, N., D. Argyropoulos, S. Ambacher, J. Müller, and H. K. Biesalski. 2017. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. LWT - Food Science and Technology 85:400–4. doi: 10.1016/j.lwt.2016.11.072.
- O’Keefe, S. J. D. 2016. Diet, microorganisms and their metabolites, and colon cancer. Nature Reviews. Gastroenterology & Hepatology 13 (12):691–706. doi: 10.1038/nrgastro.2016.165.
- Offei, B., P. Vandecruys, S. De Graeve, M. R. Foulquié-Moreno, and J. M. Thevelein. 2019. Unique genetic basis of the distinct antibiotic potency of high acetic acid production in the probiotic yeast Saccharomyces cerevisiae var. boulardii. Genome Research 29 (9):1478–94. doi: 10.1101/gr.243147.118.
- Ogidi, C. O., V. O. Oyetayo, and B. J. Akinyele. 2020. An introduction to mushroom. In Wild medicinal mushrooms: Potential applications in phytomedicine and functional foods. An Introduction to Mushroom, 118–126. London, United Kingdom: TechOpen.
- Oh, C.-H., and S.-H. Oh. 2004. Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. Journal of Medicinal Food 7 (1):19–23. doi: 10.1089/109662004322984653.
- Ohata, M., S. Uchida, L. Zhou, and K. Arihara. 2016. Antioxidant activity of fermented meat sauce and isolation of an associated antioxidant peptide. Food Chemistry 194:1034–9. doi: 10.1016/j.foodchem.2015.08.089.
- Ojwach, J., A. Kumar, S. Mukaratirwa, and T. Mutanda. 2020. Purification and biochemical characterization of an extracellular fructosyltransferase enzyme from Aspergillus niger sp. XOBP48: Implication in fructooligosaccharide production. 3 Biotech 10 (10):1–12. doi: 10.1007/s13205-020-02440-w.
- Oliveira-Ferrer, L., K. Rößler, V. Haustein, C. Schröder, D. Wicklein, D. Maltseva, N. Khaustova, T. Samatov, A. Tonevitsky, S. Mahner, et al. 2014. c-FOS suppresses ovarian cancer progression by changing adhesion. British Journal of Cancer 110 (3):753–63. doi: 10.1038/bjc.2013.774.
- Ooi, L.-G., and M.-T. Liong. 2010. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. International Journal of Molecular Sciences 11 (6):2499–522. https://www.mdpi.com/1422-0067/11/6/2499.
- Opolski, A., M. Mazurkiewicz, J. Wietrzyk, Z. Kleinrok, and C. Radzikowski. 2000. The role of GABA-ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. Journal of Experimental & Clinical Cancer Research: CR 19 (3):383–90.
- Ottoni, C., R. Cuervo-Fernández, R. Piccoli, R. Moreira, B. Guilarte-Maresma, E. Silva, M. Rodrigues, and A. Maiorano. 2012. Media optimization for β-fructofuranosidase production by Aspergillus oryzae. Brazilian Journal of Chemical Engineering 29 (1):49–59. doi: 10.1590/S0104-66322012000100006.
- Pan, D., J. Cao, H. Guo, and B. Zhao. 2012. Studies on purification and the molecular mechanism of a novel ACE inhibitory peptide from whey protein hydrolysate. Food Chemistry 130 (1):121–6. doi: 10.1016/j.foodchem.2011.07.011.
- Papaspyridi, L.-M., A. Zerva, and E. Topakas. 2018. Biocatalytic synthesis of fungal β-glucans. Catalysts 8 (7):274. doi: 10.3390/catal8070274.
- Parchami, M., J. Ferreira, and M. Taherzadeh. 2021. Brewing process development by integration of edible filamentous fungi to upgrade the quality of Brewer’s spent grain (BSG). BioResources 16 (1):1686–701. doi: 10.15376/biores.16.1.1686-1701.
- Park, N., T.-K. Lee, T. T. H. Nguyen, E.-B. An, N. M. Kim, Y.-H. You, T.-S. Park, and D. Kim. 2017. The effect of fermented buckwheat on producing l-carnitine- and γ-aminobutyric acid (GABA)-enriched designer eggs. Journal of the Science of Food and Agriculture 97 (9):2891–7. doi: 10.1002/jsfa.8123.
- Pasanen, A.-L., K. Yli-Pietila, P. Pasanen, P. Kalliokoski, and J. Tarhanen. 1999. Ergosterol content in various fungal species and biocontaminated building materials. Applied and Environmental Microbiology 65 (1):138–42. doi: 10.1128/AEM.65.1.138-142.1999.
- Pascale, A., N. Marchesi, C. Marelli, A. Coppola, L. Luzi, S. Govoni, A. Giustina, and C. Gazzaruso. 2018. Microbiota and metabolic diseases. Endocrine 61 (3):357–71. doi: 10.1007/s12020-018-1605-5.
- Patel, S., and A. Goyal. 2012. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2 (1):1–15. doi: 10.1007/s13205-011-0036-2.
- Paul, I., and C. G. Kumar. 2020. Fungal biofactories as potential inulinase sources for production of fructooligosaccharides. In New and future developments in microbial biotechnology and bioengineering, 183–210. Amsterdam, Netherlands: Elsevier.
- Peighambardoust, S. H., Z. Karami, M. Pateiro, and J. M. Lorenzo. 2021. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 11 (5):631. doi: 10.3390/biom11050631.
- Pino, J. L., V. Mujica, and M. Arredondo. 2021. Effect of dietary supplementation with oat β-glucan for 3 months in subjects with type 2 diabetes: A randomized, double-blind, controlled clinical trial. Journal of Functional Foods 77:104311. doi: 10.1016/j.jff.2020.104311.
- Poirier, M., C. Hugot, M. Spatz, G. Da Costa, A. Lapiere, C. Michaudel, C. Danne, V. Martin, P. Langella, M.-L. Michel, et al. 2022. Effects of five filamentous fungi used in food processes on in vitro and in vivo gut inflammation. Journal of Fungi 8 (9):893. doi: 10.3390/jof8090893.
- Polanowska, K., A. Grygier, M. Kuligowski, M. Rudzińska, and J. Nowak. 2020. Effect of tempe fermentation by three different strains of Rhizopus oligosporus on nutritional characteristics of faba beans. LWT 122:109024. doi: 10.1016/j.lwt.2020.109024.
- Pooyandjoo, M., M. Nouhi, S. Shab‐Bidar, K. Djafarian, and A. Olyaeemanesh. 2016. The effect of (L‐) carnitine on weight loss in adults: A systematic review and meta‐analysis of randomized controlled trials. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 17 (10):970–6.
- Pothoulakis, C. 2009. Review article: Anti-inflammatory mechanisms of action of Saccharomyces boulardii. Alimentary Pharmacology & Therapeutics 30 (8):826–33. doi: 10.1111/j.1365-2036.2009.04102.x.
- Pyo, Y. H., and T. C. Lee. 2007. The potential antioxidant capacity and angiotensin I‐converting enzyme inhibitory activity of Monascus‐fermented soybean extracts: Evaluation of Monascus‐fermented soybean extracts as multifunctional food additives. Journal of Food Science 72 (3):S218–S223.
- Qiu, J., H. Zhang, Z. Wang, D. Liu, S. Liu, W. Han, J. M. Regenstein, and L. Geng. 2018. The antitumor effect of folic acid conjugated-Auricularia auricular polysaccharide-cisplatin complex on cervical carcinoma cells in nude mice. International Journal of Biological Macromolecules 107 (Pt B):2180–9. doi: 10.1016/j.ijbiomac.2017.10.087.
- Qvirist, L. A., C. De Filippo, F. Strati, I. Stefanini, M. Sordo, T. Andlid, G. E. Felis, P. Mattarelli, and D. Cavalieri. 2016. Isolation, identification and characterization of yeasts from fermented goat milk of the Yaghnob Valley in Tajikistan [Original Research]. Frontiers in Microbiology 7:1690. doi: 10.3389/fmicb.2016.01690.
- Rahmani, J., A. Miri, R. Černevičiūtė, J. Thompson, N. N. de Souza, R. Sultana, H. K. Varkaneh, S. M. Mousavi, and A. Hekmatdoost. 2019. Effects of cereal beta-glucan consumption on body weight, body mass index, waist circumference and total energy intake: A meta-analysis of randomized controlled trials. Complementary Therapies in Medicine 43:131–9.
- Rashmi, D., R. Zanan, S. John, K. Khandagale, and A. Nadaf. 2018a. Chapter 13 - γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and applications. In Studies in natural products chemistry, R. Atta ur, 57, 413–52. Amsterdam, Netherlands: Elsevier. doi: 10.1016/B978-0-444-64057-4.00013-2.
- Rashmi, D., R. Zanan, S. John, K. Khandagale, and A. Nadaf. 2018b. γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and applications. Studies in Natural Products Chemistry 57:413–52.
- Rawat, H. K., M. A. Ganaie, and N. Kango. 2015. Production of inulinase, fructosyltransferase and sucrase from fungi on low-value inulin-rich substrates and their use in generation of fructose and fructo-oligosaccharides. Antonie Van Leeuwenhoek 107 (3):799–811. doi: 10.1007/s10482-014-0373-3.
- Razdan, K., K. Singh, and D. Singh. 2020. Vitamin D levels and COVID-19 susceptibility: Is there any correlation? Medicine in Drug Discovery 7:100051. doi: 10.1016/j.medidd.2020.100051.
- Reece, K. M., E. D. Richardson, K. M. Cook, T. J. Campbell, S. T. Pisle, A. J. Holly, D. J. Venzon, D. J. Liewehr, C. H. Chau, D. K. Price, et al. 2014. Epidithiodiketopiperazines (ETPs) exhibit in vitro antiangiogenic and in vivo antitumor activity by disrupting the HIF-1α/p300 complex in a preclinical model of prostate cancer. Molecular Cancer 13 (1):91. doi: 10.1186/1476-4598-13-91.
- Ren, T., J. Gou, W. Sun, X. Tao, X. Tan, P. Wang, Y. Zhang, H. He, T. Yin, and X. Tang. 2018. Entrapping of nanoparticles in yeast cell wall microparticles for macrophage-targeted oral delivery of cabazitaxel. Molecular Pharmaceutics 15 (7):2870–82. doi: 10.1021/acs.molpharmaceut.8b00357.
- Ribas, G. S., C. R. Vargas, and M. Wajner. 2014. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533 (2):469–76. doi: 10.1016/j.gene.2013.10.017.
- Richter, J., V. Kral, J. Pohorska, L. R. Dobiasova, and V. Vetvicka. 2016. Effects of b-glucan on natural killer cells in patients recovering from cancer treatment: Clinical trial. International Journal of Clinical and Experimental Medical Sciences 2 (2):26–30. doi: 10.11648/j.ijcems.20160202.12.
- Rizal, S., M. Murhadi, M. E. Kustyawati, and U. Hasanudin. 2020. Growth optimization of Saccharomyces cerevisiae and Rhizopus oligosporus during fermentation to produce tempeh with high?-glucan content. Biodiversitas Journal of Biological Diversity 21 (6): 2667–2673. doi: 10.13057/biodiv/d210639.
- Roblet, C., J. Amiot, C. Lavigne, A. Marette, M. Lessard, J. Jean, C. Ramassamy, C. Moresoli, and L. Bazinet. 2012. Screening of in vitro bioactivities of a soy protein hydrolysate separated by hollow fiber and spiral-wound ultrafiltration membranes. Food Research International 46 (1):237–49. doi: 10.1016/j.foodres.2011.11.014.
- Rodríguez-Berdini, L., and B. L. Caputto. 2019. Lipid metabolism in neurons: A brief story of a novel c-Fos-dependent mechanism for the regulation of their synthesis. Frontiers in Cellular Neuroscience 13:198. doi: 10.3389/fncel.2019.00198.
- Rojas-Ronquillo, R., A. Cruz-Guerrero, A. Flores-Nájera, G. Rodríguez-Serrano, L. Gómez-Ruiz, J. P. Reyes-Grajeda, J. Jiménez-Guzmán, and M. García-Garibay. 2012. Antithrombotic and angiotensin-converting enzyme inhibitory properties of peptides released from bovine casein by Lactobacillus casei Shirota. International Dairy Journal 26 (2):147–54. doi: 10.1016/j.idairyj.2012.05.002.
- Roupar, D., M. C. Coelho, D. A. Gonçalves, S. P. Silva, E. Coelho, S. Silva, M. A. Coimbra, M. Pintado, J. A. Teixeira, and C. Nobre. 2022. Evaluation of microbial-fructo-oligosaccharides metabolism by human gut microbiota fermentation as compared to commercial inulin-derived oligosaccharides. Foods 11 (7):954. doi: 10.3390/foods11070954.
- Rousta, N., J. A. Ferreira, and M. J. Taherzadeh. 2021. Production of L-carnitine-enriched edible filamentous fungal biomass through submerged cultivation. Bioengineered 12 (1):358–68. doi: 10.1080/21655979.2020.1863618.
- Rousta, N., C. Hellwig, S. Wainaina, L. Lukitawesa, S. Agnihotri, K. Rousta, and M. J. Taherzadeh. 2021. Filamentous fungus aspergillus oryzae for food: From submerged cultivation to fungal burgers and their sensory evaluation—A pilot study. Foods 10 (11):2774. doi: 10.3390/foods10112774.
- Rousta, N., K. Larsson, R. Fristedt, I. Undeland, M. J. Taherzadeh, and S. Agnihotri. 2022. Production of fungal biomass from oat flour for the use as a nutritious food source. NFS Journal 29:8–15. doi: 10.1016/j.nfs.2022.09.001.
- Rowley, N. M., Madsen, K. K. Schousboe, A. S, and White, H. 2012. Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochemistry International 61 (4):546–58. doi: 10.1016/j.neuint.2012.02.013.
- Rutherfurd-Markwick, K. J., and P. J. Moughan. 2005. Bioactive peptides derived from food. Journal of AOAC International 88 (3):955–66. doi: 10.1093/jaoac/88.3.955.
- Sabater-Molina, M., E. Larqué, F. Torrella, and S. Zamora. 2009. Dietary fructooligosaccharides and potential benefits on health. Journal of Physiology and Biochemistry 65 (3):315–28. doi: 10.1007/BF03180584.
- Saleh, A. A., Y. Z. Eid, T. A. Ebeid, K. Amber, T. Kamizono, A. Ohtsuka, and K. Hayashi. 2011. Aspergillus awamori as probiotic in broiler chickens. Proceedings of the 9th Asia Pacific Poultry Conference, Taipei, Taiwan.
- Sánchez, A., and A. Vázquez. 2017. Bioactive peptides: A review. Food Quality and Safety 1 (1):29–46. doi: 10.1093/fqsafe/fyx006.
- Sánchez Martínez, M. J., S. Soto Jover, and A. López Gómez. 2018. Nuevos procesos biotecnológicos de fabricación de scFOS en la industria de azúcares líquidos, PhD Thesis, Universidad Politécnica de Cartagena (in Spanish).
- Sang, V. T., L. P. Uyen, and N. D. Hung. 2018. The increased gamma-aminobutyric acid content by optimizing fermentation conditions of bacteria from kimchi and investigation of its biological activities. Eurasian Journal of Biosciences 12:369–76.
- Sangeetha, P., M. Ramesh, and S. Prapulla. 2004a. Production of fructo-oligosaccharides by fructosyl transferase from Aspergillus oryzae CFR 202 and Aureobasidium pullulans CFR 77. Process Biochemistry 39 (6):755–60. doi: 10.1016/S0032-9592(03)00186-9.
- Sangeetha, P., M. Ramesh, and S. Prapulla. 2004b. Production of fructosyl transferase by Aspergillus oryzae CFR 202 in solid-state fermentation using agricultural by-products. Applied Microbiology and Biotechnology 65 (5):530–7.
- Santiago-López, L., A. Hernández-Mendoza, B. Vallejo-Cordoba, V. Mata-Haro, and A. F. González-Córdova. 2016. Food-derived immunomodulatory peptides. Journal of the Science of Food and Agriculture 96 (11):3631–41. doi: 10.1002/jsfa.7697.
- Sar, T., V. H. Arifa, M. R. Hilmy, J. A. Ferreira, R. Wikandari, R. Millati, and M. J. Taherzadeh. 2022. Organosolv pretreatment of oat husk using oxalic acid as an alternative organic acid and its potential applications in biorefinery. Biomass Conversion and Biorefinery 1–10. doiSS: 10.1007/s13399-022-02408-1.
- Sar, T., J. A. Ferreira, and M. J. Taherzadeh. 2020. Bioprocessing strategies to increase the protein fraction of Rhizopus oryzae biomass using fish industry sidestreams. Waste Management 113:261–9. doi: 10.1016/j.wasman.2020.06.005.
- Sar, T., J. A. Ferreira, and M. J. Taherzadeh. 2021. Conversion of fish processing wastewater into fish feed ingredients through submerged cultivation of Aspergillus oryzae. Systems Microbiology and Biomanufacturing 1 (1):100–10. doi: 10.1007/s43393-020-00009-5.
- Sar, T., K. Larsson, R. Fristedt, I. Undeland, and M. J. Taherzadeh. 2022. Demo-scale production of protein-rich fungal biomass from potato protein liquor for use as innovative food and feed products. Food Bioscience 47:101637. doi: 10.1016/j.fbio.2022.101637.
- Sar, T., M. Ozturk, M. J. Taherzadeh, and J. A. Ferreira. 2020. New insights on protein recovery from olive oil mill wastewater through bioconversion with edible filamentous fungi. Processes 8 (10):1210. doi: 10.3390/pr8101210.
- Sarasa, S. B., R. Mahendran, G. Muthusamy, B. Thankappan, D. R. F. Selta, and J. Angayarkanni. 2020. A brief review on the non-protein amino acid, gamma-amino butyric acid (GABA): Its production and role in microbes. Current Microbiology 77 (4):534–44. doi: 10.1007/s00284-019-01839-w.
- Saravanakumar, K., A. V. A. Mariadoss, A. Sathiyaseelan, K. Venkatachalam, X. Hu, and M.-H. Wang. 2021. pH-sensitive release of fungal metabolites from chitosan nanoparticles for effective cytotoxicity in prostate cancer (PC3) cells. Process Biochemistry 102:165–72. doi: 10.1016/j.procbio.2020.12.005.
- Sathiyaseelan, A., K. Saravanakumar, A. V. A. Mariadoss, and M.-H. Wang. 2020. Biocompatible fungal chitosan encapsulated phytogenic silver nanoparticles enhanced antidiabetic, antioxidant and antibacterial activity. International Journal of Biological Macromolecules 153:63–71. doi: 10.1016/j.ijbiomac.2020.02.291.
- Sato, K., S. Miyasaka, A. Tsuji, and H. Tachi. 2018. Isolation and characterization of peptides with dipeptidyl peptidase IV (DPPIV) inhibitory activity from natto using DPPIV from Aspergillus oryzae. Food Chemistry 261:51–6. doi: 10.1016/j.foodchem.2018.04.029.
- Scholz-Ahrens, K. E., P. Ade, B. Marten, P. Weber, W. Timm, Y. Açil, C.-C. Glüer, and J. Schrezenmeir. 2007. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. The Journal of Nutrition 137 (3 Suppl 2):838S–46S.
- Scholz-Ahrens, K. E., and J. r. Schrezenmeir. 2007. Inulin and oligofructose and mineral metabolism: The evidence from animal trials. The Journal of Nutrition 137 (11):2513S–23S. doi: 10.1093/jn/137.11.2513S.
- Seif, M., J. Hoppstädter, F. Breinig, and A. K. Kiemer. 2017. Yeast-mediated mRNA delivery polarizes immuno-suppressive macrophages towards an immuno-stimulatory phenotype. European Journal of Pharmaceutics and Biopharmaceutics: Official Journal of Arbeitsgemeinschaft Fur Pharmazeutische Verfahrenstechnik e.V 117:1–13. doi: 10.1016/j.ejpb.2017.03.008.
- Senkarcinova, B., I. A. Graça Dias, J. Nespor, and T. Branyik. 2019. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 100:362–7. doi: 10.1016/j.lwt.2018.10.082.
- Sesack, S. R., and D. B. Carr. 2002. Selective prefrontal cortex inputs to dopamine cells: Implications for schizophrenia. Physiology & Behavior 77 (4–5):513–7. doi: 10.1016/S0031-9384(02)00931-9.
- Shammas, M. A. 2011. Telomeres, lifestyle, cancer, and aging. Current Opinion in Clinical Nutrition and Metabolic Care 14 (1):28–34. doi: 10.1097/MCO.0b013e32834121b1.
- Shetty, A. K., and A. Bates. 2016. Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer’s and Parkinson’s diseases. Brain Research 1638 (Pt A):74–87. doi: 10.1016/j.brainres.2015.09.019.
- Shimada, K., Y. Sakuma, J. Wakamatsu, M. Fukushima, M. Sekikawa, K. Kuchida, and M. Mikami. 2004. Species and muscle differences in L-carnitine levels in skeletal muscles based on a new simple assay. Meat Science 68 (3):357–62. doi: 10.1016/j.meatsci.2004.04.003.
- Shin, H., S. Baig, S. Lee, D. Suh, S. Kwon, Y. Lim, and J. Lee. 2004. Production of fructo-oligosaccharides from molasses by Aureobasidium pullulans cells. Bioresource Technology 93 (1):59–62.
- Shirasaka, N., M. Naitou, K. Okamura, Y. Fukuta, T. Terashita, and M. Kusuda. 2012. Purification and characterization of a fibrinolytic protease from Aspergillus oryzae KSK-3. Mycoscience 53 (5):354–64. doi: 10.1007/S10267-011-0179-3.
- Shizuka, F., Y. Kido, T. Nakazawa, H. Kitajima, C. Aizawa, H. Kayamura, and N. Ichijo. 2004. Antihypertensive effect of γ-amino butyric acid enriched soy products in spontaneously hypertensive rats. BioFactors (Oxford, England) 22 (1–4):165–7.
- Sima, P., L. Vannucci, and V. Vetvicka. 2018. β-glucans and cholesterol. International Journal of Molecular Medicine 41 (4):1799–808. doi: 10.3892/ijmm.2018.3411.
- Singh, A., and K. Zhao. 2017. Treatment of insomnia with traditional Chinese herbal medicine. International Review of Neurobiology 135:97–115.
- Song, H.-S., and K.-Y. Moon. 2006. In vitro antioxidant activity profiles of ${\beta} $-glucans isolated from yeast Saccharomyces cerevisiae and mutant Saccharomyces cerevisiae IS2. Food Science and Biotechnology 15 (3):437–40.
- Soukup, A. A., N. P. Keller, and P. Wiemann. 2016. Enhancing nonribosomal peptide biosynthesis in filamentous fungi. In Nonribosomal peptide and polyketide biosynthesis: Methods and protocols, ed. B. S. Evans, 149–60. New York: Springer New York. doi: 10.1007/978-1-4939-3375-4_10.
- Souza Filho, P. F., R. B. Nair, D. Andersson, P. R. Lennartsson, and M. J. Taherzadeh. 2018. Vegan-mycoprotein concentrate from pea-processing industry byproduct using edible filamentous fungi. Fungal Biology and Biotechnology 5 (1):5. doi: 10.1186/s40694-018-0050-9.
- Starzyńska-Janiszewska, A., B. Stodolak, A. M. Gómez- Caravaca, B. Mickowska, B. Martin-Garcia, and Ł. Byczyński. 2019. Mould starter selection for extended solid-state fermentation of quinoa. LWT 99:231–7. doi: 10.1016/j.lwt.2018.09.055.
- Steer, T., H. Carpenter, K. Tuohy, and G. R. Gibson. 2000. Perspectives on the role of the human gut microbiota and its modulation by pro-and prebiotics. Nutrition Research Reviews 13 (2):229–54. doi: 10.1079/095442200108729089.
- Stevens, C. V. 2019. Chitin and Chitosan: Properties and applications. Hoboken, NJ: Wiley series in renewable resources.
- Strong, P. J., R. Self, K. Allikian, E. Szewczyk, R. Speight, I. O’Hara, and M. D. Harrison. 2022. Filamentous fungi for future functional food and feed. Current Opinion in Biotechnology 76:102729. doi: 10.1016/j.copbio.2022.102729.
- Su, N.-W., Y.-L. Lin, M.-H. Lee, and C.-Y. Ho. 2005. Ankaflavin from monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on hep G2 cells. Journal of Agricultural and Food Chemistry 53 (6):1949–54. doi: 10.1021/jf048310e.
- Su, Y.-C., J.-J. Wang, T.-T. Lin, and T.-M. Pan. 2003. Production of the secondary metabolites γ-aminobutyric acid and monacolin K by Monascus. Journal of Industrial Microbiology & Biotechnology 30 (1):41–6. doi: 10.1007/s10295-002-0001-5.
- Suárez, E. R., R. Syvitski, J. A. Kralovec, M. D. Noseda, C. J. Barrow, H. S. Ewart, M. D. Lumsden, and T. B. Grindley. 2006. Immunostimulatory polysaccharides from chlorella pyrenoidosa. A new galactofuranan. Measurement of molecular weight and molecular weight dispersion by DOSY NMR. Biomacromolecules 7 (8):2368–76. doi: 10.1021/bm060365x.
- Sugiharto, S. 2019. A review of filamentous fungi in broiler production. Annals of Agricultural Sciences 64 (1):1–8. doi: 10.1016/j.aoas.2019.05.005.
- Sugimoto, S., T. Fujii, T. Morimiya, O. Johdo, and T. Nakamura. 2007. The fibrinolytic activity of a novel protease derived from a tempeh producing fungus, Fusarium sp. BLB. Bioscience, Biotechnology, and Biochemistry 71 (9):2184–9. doi: 10.1271/bbb.70153.
- Svensson, S. E., L. Bucuricova, J. A. Ferreira, P. F. Souza Filho, M. J. Taherzadeh, and A. Zamani. 2021. Valorization of bread waste to a fiber-and protein-rich fungal biomass. Fermentation 7 (2):91. doi: 10.3390/fermentation7020091.
- Swieca, M., M. Kordowska-Wiater, M. Pytka, U. Gawlik-Dziki, L. Seczyk, U. Złotek, and I. Kapusta. 2019. Nutritional and pro-health quality of lentil and adzuki bean sprouts enriched with probiotic yeast Saccharomyces cerevisiae var. boulardii. LWT 100:220–6. doi: 10.1016/j.lwt.2018.10.081.
- Syal, A., and P. Vohra. 2013. Probiotic potential of yeasts isolated from traditional Indian fermented foods. International Journal of Microbiology Research 5 (2):390–8. doi: 10.9735/0975-5276.5.2.390-398.
- Szajewska, H., Z. Konarska, and M. Kołodziej. 2016. Probiotic bacterial and fungal strains: Claims with evidence. Digestive Diseases 34 (3):251–9. doi: 10.1159/000443359.
- Tabassum, S., S. Ahmad, S. Madiha, S. Khaliq, S. Shahzad, Z. Batool, and S. Haider. 2017. Impact of oral supplementation of Glutamate and GABA on memory performance and neurochemical profile in hippocampus of rats. Pakistan Journal of Pharmaceutical Sciences 30: 1013–1021.
- Tadesse, T., D. Yimer, T. Tibebu, M. Workie, B. Kebede, S. Abera, A. Tilahun, M. Alemu, T. Daba, and A. Eshetu. 2021. Evaluating the probiotic potential of Yeasts isolated from Ethiopian traditionally fermented foods and dairy products. International Journal of Scientific Research Updates 1 (1):1–10.
- Talenezhad, N., M. Mohammadi, N. Ramezani-Jolfaie, H. Mozaffari-Khosravi, and A. Salehi-Abargouei. 2020. Effects of l-carnitine supplementation on weight loss and body composition: A systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clinical Nutrition ESPEN 37:9–23. doi: 10.1016/j.clnesp.2020.03.008.
- Tamam, B., D. Syah, M. T. Suhartono, W. A. Kusuma, S. Tachibana, and H. N. Lioe. 2019. Proteomic study of bioactive peptides from tempe. Journal of Bioscience and Bioengineering 128 (2):241–8. doi: 10.1016/j.jbiosc.2019.01.019.
- Tan, P., H. Fu, and X. Ma. 2021. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today 39:101229. doi: 10.1016/j.nantod.2021.101229.
- Tan, P., Q. Tang, S. Xu, Y. Zhang, H. Fu, and X. Ma. 2022. Designing self-assembling chimeric peptide nanoparticles with high stability for combating piglet bacterial infections. Advanced Science 9 (14):2105955. 10.1002/advs.202105955. doi: 10.1002/advs.202105955.
- Thandapilly, S. J., S. P. Ndou, Y. Wang, C. M. Nyachoti, and N. P. Ames. 2018. Barley β-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food & Function 9 (6):3092–6. doi: 10.1039/c8fo00157j.
- Thunuguntla, R., A. Mahboubi, J. A. Ferreira, and M. J. Taherzadeh. 2018. Integration of membrane bioreactors with edible filamentous fungi for valorization of expired milk. Sustainability 10 (6):1940. doi: 10.3390/su10061940.
- Tian, J., Q. Wang, and Z. Zhang. 2009. A novel strategy to improve the bioconversion of l-carnitine from crotonobetaine. European Food Research and Technology 229 (4):721–4. doi: 10.1007/s00217-009-1097-x.
- Tulipano, G., L. Faggi, A. Nardone, D. Cocchi, and A. M. Caroli. 2015. Characterisation of the potential of β-lactoglobulin and α-lactalbumin as sources of bioactive peptides affecting incretin function: In silico and in vitro comparative studies. International Dairy Journal 48:66–72. doi: 10.1016/j.idairyj.2015.01.008.
- Turner, W. B., and D. C. Aldridge. 1983. Fungal metabolites II. London: Academic Press.
- Utama, G. L., C. Dio, J. Sulistiyo, F. Yee Chye, E. Lembong, Y. Cahyana, D. Kumar Verma, M. Thakur, A. R. Patel, and S. Singh. 2021. Evaluating comparative β-glucan production aptitude of Saccharomyces cerevisiae, Aspergillus oryzae, Xanthomonas campestris, and Bacillus natto. Saudi Journal of Biological Sciences 28 (12):6765–73. doi: 10.1016/j.sjbs.2021.07.051.
- Utama, G. L., S. Meliana, M. Djali, T. Yuliana, and R. L. Balia. 2019. Probiotic candidates yeast isolated from Dangke - Indonesian traditional fermented buffalo milk. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 67 (1):179–87. doi: 10.11118/actaun201967010179.
- Uwineza, C., T. Sar, A. Mahboubi, and M. J. Taherzadeh. 2021. Evaluation of the cultivation of Aspergillus oryzae on organic waste-derived VFA effluents and its potential application as alternative sustainable nutrient source for animal feed. Sustainability 13 (22):12489. doi: 10.3390/su132212489.
- Vandáková, M., Z. Platková, M. Antosová, V. Báles, and M. Polakovic. 2004. Optimization of cultivation conditions for production of fructosyltransferase by Aureobasidium pullulans. Chemical Papers-Slovak Academy of Sciences 58 (1):15–22.
- Varsha, K. K., V. Narisetty, K. K. Brar, A. Madhavan, M. P. Alphy, R. Sindhu, M. K. Awasthi, S. Varjani, and P. Binod. 2022. Bioactive metabolites in functional and fermented foods and their role as immunity booster and anti-viral innate mechanisms. Journal of Food Science and Technology 1–10. doi: 10.1007/s13197-022-05528-8.
- Venegas-Ortega, M. G., A. C. Flores-Gallegos, J. L. Martínez-Hernández, C. N. Aguilar, and G. V. Nevárez-Moorillón. 2019. Production of bioactive peptides from lactic acid bacteria: A sustainable approach for healthier foods. Comprehensive Reviews in Food Science and Food Safety 18 (4):1039–51. doi: 10.1111/1541-4337.12455.
- Verardo, V., A. M. Gómez-Caravaca, and G. Tabanelli. 2020. Bioactive components in fermented foods and food by-products. Foods 9 (2):153. doi: 10.3390/foods9020153.
- Vetvicka, V., and J. Vetvickova. 2020. Anti-infectious and anti-tumor activities of β-glucans. Anticancer Research 40 (6):3139–45. doi: 10.21873/anticanres.14295.
- Vinolo, M. A., H. G. Rodrigues, R. T. Nachbar, and R. Curi. 2011. Regulation of inflammation by short chain fatty acids. Nutrients 3 (10):858–76. doi: 10.3390/nu3100858.
- Virmani, M. A., and M. Cirulli. 2022. The role of l-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. International Journal of Molecular Sciences 23 (5):2717. doi: 10.3390/ijms23052717.
- Vitaglione, P., R. B. Lumaga, A. Stanzione, L. Scalfi, and V. Fogliano. 2009. β-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite 53 (3):338–44. doi: 10.1016/j.appet.2009.07.013.
- Volman, J. J., J. D. Ramakers, and J. Plat. 2008. Dietary modulation of immune function by β-glucans. Physiology & Behavior 94 (2):276–84. doi: 10.1016/j.physbeh.2007.11.045.
- Wang, H., W. Chen, D. Li, X. Yin, X. Zhang, N. Olsen, and S. G. Zheng. 2017. Vitamin D and chronic diseases. Aging and Disease 8 (3):346–53. doi: 10.14336/AD.2016.1021.
- Wang, J., Z. Xing, W. Tang, Y. Zheng, and Y. Wang. 2015. Isolation, identification, and potential probiotic characterization of one Lactococcus from Kefir grain. Food Science and Biotechnology 24 (5):1775–80. doi: 10.1007/s10068-015-0231-8.
- Wang, Q., L. Ren, Y. Wan, and G. J. Prud’homme. 2019. GABAergic regulation of pancreatic islet cells: Physiology and antidiabetic effects. Journal of Cellular Physiology 234 (9):14432–44. doi: 10.1002/jcp.28214.
- Wang, R., R. Gmoser, M. J. Taherzadeh, and P. R. Lennartsson. 2021. Solid-state fermentation of stale bread by an edible fungus in a semi-continuous plug-flow bioreactor. Biochemical Engineering Journal 169:107959. doi: 10.1016/j.bej.2021.107959.
- Wang, Y., P. Li, F. Chen, L. Jia, Q. Xu, X. Gai, Y. Yu, Y. Di, Z. Zhu, Y. Liang, et al. 2017. A novel pH-sensitive carrier for the delivery of antitumor drugs: Histidine-modified auricularia auricular polysaccharide nano-micelles. Scientific Reports 7 (1):4751. doi: 10.1038/s41598-017-04428-8.
- Wang, Z., F. Zhang, Y. Yan, Z. Zhang, L. Wang, and C. Qin. 2019. Lipid-lowering activities of chitosan and its quaternary ammonium salt for the hyperlipidemia rats induced by high-fat diets. International Journal of Biological Macromolecules 132:922–8.
- Watanabe, M., K. Maemura, K. Kanbara, T. Tamayama, and H. Hayasaki. 2002. GABA and GABA receptors in the central nervous system and other organs. International Review of Cytology 213:1–47. doi: 10.1016/s0074-7696(02)13011-7.
- Wiebe, M. 2002. Myco-protein from Fusarium venenatum: A well-established product for human consumption. Applied Microbiology and Biotechnology 58 (4):421–7. doi: 10.1007/s00253-002-0931-x.
- Wong, R. S. Y. 2011. Apoptosis in cancer: From pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research 30 (1):87. doi: 10.1186/1756-9966-30-87.
- World Health Organization. 2007. Protein and amino acid requirements in human nutrition. Geneva, Switzerland: World Health Organization (Vol. 935).
- Wu, B., and J. Xu. 2012. Antithrombotic effect of a novel protein from Fusarium sp. CPCC 480097 in a rat model of artery-vein bypass thrombosis. Pharmaceutical Biology 50 (7):866–70. doi: 10.3109/13880209.2011.641023.
- Wu, J., Z. Lin, X. Wang, Y. Zhao, J. Zhao, H. Liu, L. J. Johnston, L. Lu, and X. Ma. 2022. Limosilactobacillus reuteri SLZX19-12 protects the colon from infection by enhancing stability of the gut microbiota and barrier integrity and reducing inflammation. Microbiology Spectrum 10 (3):e02124-02121. doi: 10.1128/spectrum.02124-21.
- Wutzke, K. D., and H. Lorenz. 2004. The effect of l-carnitine on fat oxidation, protein turnover, and body composition in slightly overweight subjects. Metabolism: Clinical and Experimental 53 (8):1002–6. doi: 10.1016/j.metabol.2004.03.007.
- Xiao-Lan, L., D. Lian-Xiang, L. Fu-Ping, Z. Xi-Qun, and X. Jing. 2005. Purification and characterization of a novel fibrinolytic enzyme from Rhizopus chinensis 12. Applied Microbiology and Biotechnology 67 (2):209–14. doi: 10.1007/s00253-004-1846-5.
- Xing, L., R. Liu, S. Cao, W. Zhang, and Z. Guanghong. 2019. Meat protein based bioactive peptides and their potential functional activity: A review. International Journal of Food Science & Technology 54 (6):1956–66. doi: 10.1111/ijfs.14132.
- Xu, Y., W. Jiang, G. Chen, W. Zhu, W. Ding, Z. Ge, Y. Tan, T. Ma, and G. Cui. 2017. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University 26 (2):333–8.
- Yadav, R., P. K. Singh, and P. Shukla. 2016. Production of fructooligosaccharides as ingredients of probiotic applications, future scope and trends. In: Microbial Biotechnology, 311–223, NewYork: CRC Press.
- Yan, J., J. Zhao, R. Yang, and W. Zhao. 2019. Bioactive peptides with antidiabetic properties: A review. International Journal of Food Science & Technology 54 (6):1909–19. doi: 10.1111/ijfs.14090.
- Yang, H., Y. Wang, L. Zhang, and W. Shen. 2016. Heterologous expression and enzymatic characterization of fructosyltransferase from Aspergillus niger in Pichia pastoris. New Biotechnology 33 (1):164–70.
- Yang, T., Z. Lu, L. Meng, S. Wei, K. Hong, W. Zhu, and C. Huang. 2012. The novel agent ophiobolin O induces apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signaling pathways. Bioorganic & Medicinal Chemistry Letters 22 (1):579–85. doi: 10.1016/j.bmcl.2011.10.079.
- Yi, C., M. Fu, X. Cao, S. Tong, Q. Zheng, C. K. Firempong, X. Jiang, X. Xu, and J. Yu. 2013. Enhanced oral bioavailability and tissue distribution of a new potential anticancer agent, Flammulina velutipes sterols, through liposomal encapsulation. Journal of Agricultural and Food Chemistry 61 (25):5961–71. doi: 10.1021/jf3055278.
- Yongxia, Z., X. Jian, H. Suyuan, N. Aixin, and Z. Lihong. 2020. Isolation and characterization of ergosterol from Monascus anka for anti-lipid peroxidation properties. Journal de Mycologie Medicale 30 (4):101038. doi: 10.1016/j.mycmed.2020.101038.
- Yoshikawa, J., Y. Honda, Y. Saito, D. Sato, K. Iwata, S. Amachi, Y. Kashiwagi, and K. Maehashi. 2022. Isolation and identification of Zalaria sp. Him3 as a novel fructooligosaccharides‐producing yeast. Journal of Applied Microbiology 132 (2):1104–11. doi: 10.1111/jam.15259.
- Yoshimi, A., K. Miyazawa, and K. Abe. 2017. Function and biosynthesis of cell wall α-1, 3-glucan in fungi. Journal of Fungi 3 (4):63. doi: 10.3390/jof3040063.
- Yu, X., R. Yang, Z. Gu, S. Lai, and H. Yang. 2014. Anti-tumor and immunostimulatory functions of two feruloyl oligosaccharides produced from wheat bran and fermented by Aureobasidium pullulans. BioResources 9 (4). doi: 10.15376/biores.9.4.6778-6790.
- Yuanita, L., P. R. Wikandari, and W. B. Sabtiawan. 2017. The role of yacon fructooligosaccharides on the bile acids binding and bile salt hydrolase activities. Advanced Science Letters 23 (12):11982–5. doi: 10.1166/asl.2017.10557.
- Zamani, A., L. Edebo, B. Sjöström, and M. J. Taherzadeh. 2007. Extraction and precipitation of chitosan from cell wall of zygomycetes fungi by dilute sulfuric acid. Biomacromolecules 8 (12):3786–90. doi: 10.1021/bm700701w.
- Zaremba, S. M. M., I. F. Gow, S. Drummond, J. T. McCluskey, and R. E. Steinert. 2018. Effects of oat β-glucan consumption at breakfast on ad libitum eating, appetite, glycemia, insulinemia and GLP-1 concentrations in healthy subjects. Appetite 128:197–204. doi: 10.1016/j.appet.2018.06.019.
- Zhai, X., C. Li, D. Ren, J. Wang, C. Ma, and A. Abd El-Aty. 2021. The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydrate Polymers 266:118132. doi: 10.1016/j.carbpol.2021.118132.
- Zhang, J.-H., E. Tatsumi, C.-H. Ding, and L.-T. Li. 2006. Angiotensin I-converting enzyme inhibitory peptides in douchi, a Chinese traditional fermented soybean product. Food Chemistry 98 (3):551–7. doi: 10.1016/j.foodchem.2005.06.024.
- Zhang, J., C. Liu, Y. Xie, N. Li, Z. Ning, N. Du, X. Huang, and Y. Zhong. 2017. Enhancing fructooligosaccharides production by genetic improvement of the industrial fungus Aspergillus niger ATCC 20611. Journal of Biotechnology 249:25–33. doi: 10.1016/j.jbiotec.2017.03.021.
- Zhang, S., H. Jiang, S. Xue, N. Ge, Y. Sun, Z. Chi, G. Liu, and Z. Chi. 2019. Efficient conversion of cane molasses into fructooligosaccharides by a glucose derepression mutant of Aureobasidium melanogenum with high β-fructofuranosidase activity. Journal of Agricultural and Food Chemistry 67 (49):13665–72.
- Zhang, Y., N. Wang, Z. Zhang, Y. Sun, X. Shi, H. Cao, and S. Wang. 2015. Purification and characterisation of a fibrinolytic enzyme from Rhizopus micro sporus var. tuberosus. Food Technology and Biotechnology 53 (2):243–8. doi: 10.17113/ftb.53.02.15.3874.
- Zhang, X., L. Zhang, H. Yang, X. Huang, H. Otu, T. A. Libermann, W. C. DeWolf, R. Khosravi-Far, and A. F. Olumi. 2007. c-Fos as a proapoptotic agent in TRAIL-induced apoptosis in prostate cancer cells. Cancer Research 67 (19):9425–34.
- Zhou, X., K. Ling, M. Liu, X. Zhang, J. Ding, Y. Dong, Z. Liang, J. Li, and J. Zhang. 2019. Targeted delivery of cisplatin-derived nanoprecursors via a biomimetic yeast microcapsule for tumor therapy by the oral route. Theranostics 9 (22):6568–86. doi: 10.7150/thno.35353.
- Özcan, Ö., and F. Ertan. 2018. Beta-glucan content, antioxidant and antimicrobial activities of some edible mushroom species. Food Science and Technology 6 (2):47–55. doi: 10.13189/fst.2018.060201.