Abstract
Consumers’ demand for foods with high nutritional value and health benefits has fueled the development of prebiotic foods. In coffee industry, cherries transformation into roasted beans generates a large amount of waste/by-products (pulp/husks, mucilage, parchment, defective beans, silverskin and spent coffee grounds) that usually end up in landfills. The possibility to use coffee by-products as relevant sources of prebiotic ingredients is herein ascertained. As a prelude to this discussion, an overview of pertinent literature on prebiotic action was conducted, including on biotransformation of prebiotics, gut microbiota, and metabolites. Existing research indicates that coffee by-products contain significant levels of dietary fiber and other components that can improve gut health by stimulating beneficial bacteria in the colon, making them excellent candidates for prebiotic ingredients. Oligosaccharides from coffee by-products have lower digestibility than inulin and can be fermented by gut microbiota into functional metabolites, such as short-chain fatty acids. Depending on the concentration, melanoidins and chlorogenic acids may also have prebiotic action. Nevertheless, there is still a lack of in vivo studies to validate such findings in vitro. This review shows how coffee by-products can be interesting for the development of functional foods, contributing to sustainability, circular economy, food security, and health.
HIGHLIGHTS
Coffee by-product oligosaccharides increase short-chain fatty acid levels.
Melanoidins and chlorogenic acids promote the growth of lactobacillus and bifidobacteria.
Coffee by-products show prebiotic potential, but further in vivo studies are required.
Introduction
Health promotion by gut microbiota modulation through consumption of functional foods has been receiving more and more attention, and the use of prebiotics is being recognized as an effective way of dealing with different health issues (Jana et al. Citation2021). Prebiotics are substrates that beneficial microorganisms use selectively, resulting in benefits not only for the gastrointestinal system, but also with proven benefits for metabolic, bone, and mental health (Alves-Santos et al. Citation2020). For that, they must satisfy three requirements: (i) to be resistant to gastric juice, enzyme hydrolysis and gastrointestinal absorption; (ii) to show ability to be metabolized by the intestinal microbiota and promote the growth and/or activity of the bacteria with beneficial health effects; and (iii) do not cause negative effects to the host, such as pathogenic microorganisms’ growth or abdominal discomfort (Rezende, Lima, and Naves Citation2021). Besides, to a particular prebiotic be effective, the target bacteria must be present in a certain amount. If levels fall below a specific number, due to factors like aging or antibiotic use, the prebiotic may be ineffective in increasing the number of those beneficial bacteria to the desired level (Lordan et al. Citation2020).
The benefits of prebiotics are not only direct, that is, at the level of the gut or microbiota (Lordan et al. Citation2020). In fact, there is rising evidence that a prebiotic-rich diet can also benefit host immunity, as well as the microbiota-gut-brain axis (Yang et al. Citation2018). The bidirectional communication between the gut microbiota and central nervous system (CNS) is mediated through the enteric nervous system, autonomic nervous system, immune system, and endocrine system. Bacterial metabolites, such as short-chain fatty acids (SCFA), which are produced in the colon by bacterial fermentation of prebiotics, are also involved in this communication (Oroojzadeh, Bostanabad, and Lotfi Citation2022; Sandhu et al. Citation2017), Indeed, the gut microbiota and its metabolites can play a major role in the pathogenesis of neuropsychiatric and neurological diseases, such as depression, schizophrenia, autism spectrum disorder, Parkinson’s disease, epilepsy, and migraine (Socała et al. Citation2021).
Some dietary fibers, that is, carbohydrate polymers that are neither digested nor absorbed in the small intestine, are considered prebiotics. Dietary fibers are divided into subgroups: resistant oligosaccharides (fructooligosaccharides [FOS], mannanoligosaccharides [MOS], xylooligosaccharides [XOS], galactooligosaccharides [GOS], transgalactooligosaccharides [TOS], galactosides [raffinose, stachyose, verbascose]), non-starch polysaccharides (cellulose, hemicellulose, inulin, pectins, mannans, gums, mucilages, polydextrose and resistant dextrins), non-carbohydrate associated substances (lignin, waxes and chitins) and resistant starch (Rezende, Lima, and Naves Citation2021). Dietary fibers widely documented as prebiotics include FOS, GOS and inulin (Rezende, Lima, and Naves Citation2021). Others, such as MOS, XOS, galactosides, pectins, hemicellulose oligomers, resistant dextrin and polydextrose, are considered candidates to prebiotics, because they have been associated to beneficial changes in the gut microbiota, as increased SCFA production (Rezende, Lima, and Naves Citation2021). Lastly, dietary fibers not recognized as prebiotics include cellulose, lignin, and chitins (Rezende, Lima, and Naves Citation2021). Generally, soluble fibers (oligosaccharides and non-cellulosic polysaccharides) are fermented faster and to a higher extent than insoluble fibers (water-insoluble hemicellulose) (Rezende, Lima, and Naves Citation2021).
Recent evidence also suggests that certain non-carbohydrate substances, such as phenolic compounds, carotenoids, polyunsaturated fatty acids, and vitamins, may also have prebiotic properties by selectively stimulating beneficial bacteria and lowering illness incidence (Rodriguez-Daza et al. Citation2021; Rosa et al. Citation2021). Phenolic compounds are secondary metabolites found in plants that comprise one phenol ring (referred to as phenolic acids) or multiple phenol rings (referred to as polyphenols) (Capuano Citation2017). Due to their chemical structure, phenolic compounds are weakly absorbed and thus reach the colon, where they impact the local microbiota (Rodriguez-Daza et al. Citation2021). It is estimated that 5–10% of total dietary polyphenolic intake is absorbed in the small intestine, with the remaining 90–95% accumulate in the large intestinal lumen, where it is then exposed to the enzymatic activity of the colonic microbiota (Cardona et al. Citation2013; Frolinger et al. Citation2019).
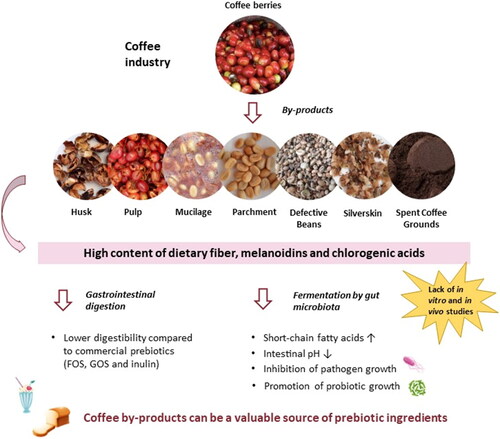
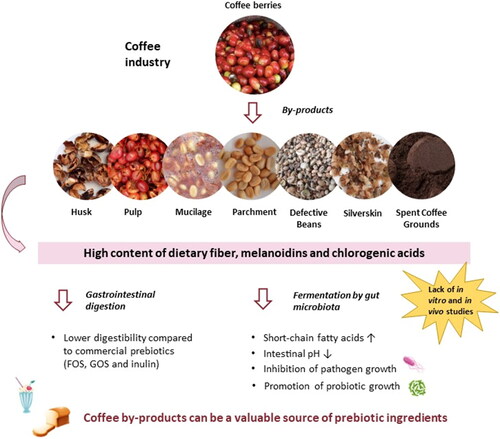
The use of agrifood wastes as a source of potential prebiotics has attracted increasing attention. For example, the coffee industry produces a large number of by-products (usually discarded), rich in polysaccharides and phenolic compounds, that can be recovered and used in this context (Desai et al. Citation2020; Iriondo-DeHond, García, Fernandez-Gomez, et al. Citation2019).
From the inside to the outside, the coffee cherry is composed of two beans, silverskin, parchment, mucilage, pulp, and outer skin. The only part used in beverage preparation is the bean (Santos et al. Citation2021). There are two main processing methods for acquiring green coffee beans: wet and dry. The dry method is recognized for being the simplest and most ecologically responsible, with the major by-product being coffee husk (), which includes the outer skin, pulp, mucilage, parchment, and portions of the silverskin (Hoseini et al. Citation2021; Klingel et al. Citation2020). Coffee husks account for 12 to 18% of the dried coffee cherry (Hoseini et al. Citation2021). The wet method consumes high amounts of water, involves more steps, and consequently produces a greater variety of by-products. During the pulping, fermentation, drying, and dehulling steps, the wet method generates mainly pulp, mucilage, and parchment () as by-products (Hoseini et al. Citation2021; Klingel et al. Citation2020).
The fermentation process involves the solubilization of mucilage by yeast, bacteria, and fungi, as well as the production of different enzymes, alcohols, and organic acids (Haile and Kang Citation2019; Siridevi et al. Citation2019). The wet method, despite being more complex, produces higher quality coffee beans (Klingel et al. Citation2020). After either method is completed, the green coffee beans are roasted, resulting in the release of a thin tegument known as silverskin (G. Oliveira et al. Citation2021). Once the coffee has been ground, it can be prepared in a variety of ways, including decoction (boiled coffee), steeping (French press), and brewing (filter coffee) (Soares et al. Citation2010). At the end of extraction, other by-product (spent coffee grounds, SCG) is obtained (Janissen and Huynh Citation2018). During the coffee industrial process, immature and defective beans are also separated, which must be removed to ensure the quality of the final product (Hoseini et al. Citation2021). These beans account for 15–20% of total coffee production (Esquivel and Jiménez Citation2012).
Some coffee by-products have been found to satisfy prebiotic requirements (Bhandarkar et al. Citation2021; Jiménez-Zamora, Pastoriza, and Rufián-Henares Citation2015; Puengsawada et al. Citation2021). According to a recent study, oligosaccharides derived from green coffee spent are resistant to artificial human gastric juice and can promote the growth of beneficial bacteria (Desai et al. Citation2020). However, prebiotic studies on by-products such as coffee pulp, parchment, and silverskin are still limited.
The present work aims to present a comprehensive review on the prebiotic potential of the different coffee by-products. In the first part of this review, and to contextualize, an overview of pertinent literature on prebiotic activity is conducted, which includes information on the biotransformation of prebiotics, the gut microbiota, and their metabolites. Next, the chemical composition of the coffee by-products is described, emphasizing those components with proven prebiotic activity. Finally, the prebiotic properties of coffee by-products as a whole are discussed, and the need for future work and new trends of functional foods are also brought into view. In sum, this review explores the potential to use coffee by-products in the development of functional foods or ingredients. This will contribute to their valorization, taking advantage of their potential health benefits and, simultaneously, address the current needs of sustainability and circular economy in the coffee chain.
Methodology
Bibliographic research was performed using the Scopus and Pubmed platforms as scientific databases. For each part of the work, the search strategy differed slightly, regarding its main objective.
Regarding the chapters that include information on the human gut microbiota and prebiotics and their biotransformation, a search was performed including the following keywords individually or in conjunction: “prebiotic,” “polyphenols,” “dietary fiber,” “coffee,” “gut microbiota,” “ileal microbiota,” “colonic microbiota,” “fermentation,” “degradation,” “metabolism,” “short-chain fatty acids,” “metabolites,” “probiotic,” “health,” among others. Given the large number of results, the most pertinent articles were selected to develop the abovementioned chapters. A total of 21 references were used.
For the sub-chapter about the coffee by-products and their chemical composition, 42 references were selected using the terms individually or in conjunction: “coffee,” “by-products,” “industry,” “chemical,” “nutritional,” “phenolic,” “polysaccharides,” “fiber,” “melanoidins,” “chlorogenic acids,” “sugars,” “peel,” “pulp,” “husk,” “silverskin,” “spent,” “defective,” “immature,” “beans,” “parchment” and “mucilage.”
Finally, to respond to the main objective of this review, the keywords used in the search were: “coffee” in conjunction with “prebiotic,” “prebiotics,” “gut microbiota,” “colonic microbiota,” “intestinal microbiota,” “probiotic,” “probiotics,” “peel,” “pulp,” “husk,” “silverskin,” “spent,” “beans,” “parchment” and “mucilage.” Only research articles published between 2004 and 2022, written exclusively in English, were considered. A total of 76 articles were collected and then screened regarding duplicity. Articles that did not coincide with the theme or did not test the impact of a coffee by-product on the intestinal microbiota and/or production of SCFA were excluded. A total of 22 articles were considered eligible for the sub-chapters: “compounds with prebiotic potential found in coffee by-products” and “prebiotic potential of coffee by-products in their original form.” Additional search on the prebiotic activity of coffee and their compounds was performed to complement the last two subchapters. In this search, the keywords used were: “coffee,” “oligosaccharides,” “melanoidins,” “polyphenol,” “chlorogenic acids,” “prebiotic,” “gut microbiota,” “probiotic,” among others. The most relevant articles were selected and used as reference.
In all searches, terms were examined in the title, abstract and keywords search fields. Some references were used in more than one chapter or sub-chapter.
Human gut microbiota
The human gut microbiota represents an ecological community of commensal, symbiotic, and potential pathogenic microorganisms that influence the host’s health and disease (Azad et al. Citation2018). The composition of the microbiota is dynamic and individualized, and it is highly influenced by factors such as age, diet, lifestyle, ethnicity, and host health (Linares, Ross, and Stanton Citation2016; Wieers et al. Citation2019). The main microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia, with the first two representing 90% of the gut microbiota (Rinninella et al. Citation2019). Bacteroides, Clostridium, Eubacterium, Fusobacterium, Peptococcus, Peptostreptococcus, Ruminococcus, and Bifidobacterium are the most common bacterial genera. Other genera, such as Escherichia and Lactobacillus, are found in considerably smaller amounts (Linares, Ross, and Stanton Citation2016). Some of these genera include probiotic species, that is, microorganisms that exert a positive effect on health. Probiotics are intended to promote host homeostasis, prevent disturbances, and reduce the risk of pathogen colonization. The substrates of probiotic bacteria are prebiotics, which increase their activity and production of crucial metabolites for human health (El Hage, Hernandez-Sanabria, and de Wiele Citation2017). Most probiotic bacterial strains belong to the species Lactobacillus (casei, paracasei, acidophilus, fermentum, brevis, gasseri, johnsonii, plantarum, reuteri, salivarius rhamnosus, and delbrueckii) and Bifidobacterium (bifidum, animalis, breve, adolescentis, and longum). Strains belonging to the genera Streptococcus (particularly thermophilus) and Propionibacterium are also considered as probiotic microorganisms (Linares, Ross, and Stanton Citation2016). Other bacterial species being investigated as emerging probiotics include members of Clostridium clusters IV, XIVa and XVIII, Akkermansia muciniphila, Faecalibacterium prausnitzii, Bacteroides fragilis, Bacteroides uniformis, and Eubacterium hallii (T. Chen et al., Citation2021; El Hage, Hernandez-Sanabria, and de Wiele Citation2017).
Prebiotics and their biotransformation
Although prebiotics are thought to be inert materials in the gastrointestinal tract, they are partially metabolized (Capuano Citation2017; Patel et al. Citation2021). Along the gastrointestinal tract, prebiotics undergo chemical and structural changes that impact their behavior during digestion. For example, the glycosidic linkages between the component monosaccharides and the ester linkages can be subjected to acid hydrolysis during the passage through stomach due to the very low pH (Capuano Citation2017).
In turn, phenolic compounds are extremely unstable in neutral or alkaline environments and are prone to break glycosidic bonds and/or ring fission of the parent molecule (J. Yang et al. Citation2021). However, as phenolic compounds are frequently linked to difficult-to-digest fibrous components, they can escape intestinal digestion and enter the colon. Stalmach et al. (Citation2010) analyzed 0–24 h post-ingestion ileal effluents and found that 30% of chlorogenic acids (CGA) intake from coffee is absorbed in stomach and/or small intestine, and 70% of the intake passes from the small intestine to the large intestine, where it is exposed to the activity of the colonic microbiota. 5-Caffeoylquinic acid (5-CQA), the major chlorogenic acid from coffee, is very little absorbed in stomach and/or small intestine because it is stable in gastric acid, duodenal fluid, and ileostomy effluent (Ludwig et al. Citation2013; Stalmach et al. Citation2010).
Before reaching the colon, prebiotics can be fermented by the microflora present in the ileum, which also depends on the carbohydrate fermentation as an energy source. The ileum fermentation is not very intense; however, a small significant part of dietary fibers can be metabolized in the ileum (Capuano Citation2017). Once in the colon, prebiotics undergo a very intense fermentation by the resident bacteria (Capuano Citation2017). These bacteria encode a variety of enzymes (CAZymes) that can hydrolyze complex carbohydrates that haven’t been digested and absorbed (Hills et al. Citation2019). The genome of some bacteria also encodes an array of phenolic compound associated enzymes (PAZymes), which are involved particularly in phenolics metabolization (Rodriguez-Daza et al. Citation2021). In general, the bacterial fermentation of prebiotics leads to the production of several metabolites, such as SCFA (acetate, propionate, and butyrate), gases (NO, CO2, H2, CH4, and H2S), organic acids (lactate, succinate, and pyruvate), and phenols (T. Ashaolu, J. Ashaolu, and Adeyeye Citation2021; Patel et al. Citation2021; Rodriguez-Daza et al. Citation2021).
The acidic metabolites (SCFA) lower the colonic pH, influencing the microbiota composition and the balance of microbial metabolites (Flint et al. Citation2012; Holscher Citation2017). Normal human colonic pH values range between 5.5 and 7.5 (Holscher Citation2017). Lower pH levels promote the development of beneficial bacteria such as Lactobacillus and Bifidobacterium while inhibiting the growth of potentially pathogenic species (Swennen, Courtin, and Delcour Citation2006). shows a correlation between gut-resident bacteria and the production of SCFA. Some gut bacterial genera capable of producing SCFA are Bifidobacterium, Eubacterium, Propionibacterium, Clostridium, Lactobacillus, Bacteroides, Roseburia, and Prevotella (Tran and Mohajeri Citation2021). Species of the probiotic genus Bifidobacterium, namely B. longum, B. animalis, and B. bifidum, are able to produce acetic, propionic, and butyric acids. SCFA production can be influenced by several factors, including the type and number of microorganisms present in the colon, the source and chemical structure of substrates, and the gut transit time (Usta-Gorgun and Yilmaz-Ersan Citation2020).
Table 1. Gut-residing bacteria found associated to the production of SCFA.
Overall, according to the most recent studies (T. Chen et al. Citation2021; Socała et al. Citation2021; Tran and Mohajeri Citation2021), the gut microbiota appears to have a considerably more important role in the host’s health than previously thought, and this microbiota can be selectively modulated by different groups of prebiotics. The oligosaccharides, melanoidins, and chlorogenic acids found in coffee by-products can provide health benefits comparable to or higher than established prebiotics. The following topic explores the prebiotic properties of various coffee by-products, considering their chemical composition.
Coffee by-products as potential prebiotics
Chemical composition of coffee by-products
The chemical composition of coffee by-products varies significantly depending on the method of processing, roasting, and beverage preparation. However, they are generally rich in dietary fiber, carbohydrates, phenolic compounds, and caffeine (Janissen and Huynh Citation2018). presents the chemical composition of the different by-products obtained along the coffee chain. The chemical composition of some by-products, such as defective coffee beans, mucilage, and parchment, is still largely unexplored.
Table 2. Chemical composition of the different by-products obtained along the coffee chain (values reported in dry weight).
Coffee husks are mainly composed of cellulose, hemicelluloses, and lignin (Collazo-Bigliardi, Ortega-Toro, and Boix Citation2018). Their sugar composition comprises approximately 49% xylose, 33% glucose, 13% arabinose, and 5% galactose, indicating that xylan is the predominant polysaccharide fraction in this by-product (Baeta et al. Citation2017). Chlorogenic, protocatechuic, and gallic acids are the most common phenolic compounds found in coffee husks (Rebollo-Hernanz et al. Citation2021).
Coffee mucilage is a highly hydrated viscous tissue made up of cellulose, pectic substances, and non-cellulosic polysaccharides (Avallone et al. Citation2000; Siridevi et al. Citation2019). About 60% of pectin is uronic acids, with a high degree of methyl esterification and a moderate degree of acetylation. In alcohol-insoluble residues, arabinose was the major neutral non-cellulosic monosaccharide followed by galactose, xylose, rhamnose, glucose (Avallone et al. Citation2000). The coffee mucilage comprises approximately 85.3% moisture, 7.2% protein, 0.7% fat, 1.1% ash, 1.5% crude fiber, 4.3% total sugars, and 1.05% caffeine. In terms of phenolic compounds, tannins and flavonoids have been found in mucilage (Kc et al. Citation2021).
Coffee parchment, which is high in dietary fiber and phenolic compounds, is one of the least explored coffee by-products. (Aguilera et al. Citation2019; Benitez et al. Citation2019). The dietary fiber of coffee parchment samples was solely composed by the insoluble fraction. Hemicelluloses (28%) are the most abundant polysaccharides in coffee parchment, followed by cellulose (6–12%) and pectic polysaccharides (4–7%). Hemicelluloses and celluloses are primarily composed of xylose and glucose, respectively. The peptide polysaccharides, on the other hand, are composed only of uronic acids. Neutral sugars such as mannose, arabinose, or galactose have not been found in coffee parchment. Thus, the dietary fiber composition of coffee parchment indicates an absence of galactomannans and arabinogalactans, in contrast to silverskin and SCG composition (Benitez et al. Citation2019).
Immature, black, and sour coffee green beans have a different chemical composition from non-defective coffee green beans. The mean percentage of CGA in green coffee beans is 4.43 for immature, 4.26 for black, 4.26 for sour, and 4.07 for non-defective (Habtamu and Belay Citation2020). Defective coffee green beans have a high fiber content, reaching 56% in dry weight (Prandi et al. Citation2021). Although the polysaccharide composition of defective coffee beans is unknown, it is thought to be comparable to that of non-defective coffee beans. The three main polysaccharides found in non-defective coffee beans are arabinogalactan, mannan, and cellulose. The building blocks of these polysaccharides are arabinose, galactose, mannose, and glucose. Other monosaccharides that are found in smaller amounts in non-defective coffee beans are rhamnose, xylose, galacturonic acid, and glucuronic acid. This shows that pectins, rhamnogalacturonan, and xylans are present (Redgwell et al., Citation2002).
Silverskin has a higher protein content compared to other coffee by-products, ranging between 10.9 and 20.6% dw (Bessada et al. Citation2018; Wen et al. Citation2021). The most prevalent components in this by-product are polysaccharides. Sugars are polymerized into hemicellulose and cellulose structures, which together make up 40.45% of the dry weight of silverskin. Xylose is the main sugar in silverskin hemicellulose (Ballesteros, Teixeira, and Mussatto Citation2014).
Spent coffee grounds are a waste rich in sugars polymerized into hemicellulose and cellulose structures, which account for over half of the material dry weight (45.3%) (Mussatto et al. Citation2011). These monosaccharide profiles consist of glucose (20%), arabinose (7%), galactose (27%), and mannose (46%) (Coelho et al. Citation2021; Simoes et al. Citation2013). The higher abundance of galactose and mannose leads to infer that galactomannans are the major polysaccharides in this biomass (Coelho et al. Citation2021; Simoes et al. Citation2013). In addition to polysaccharides, SCG also contain a significant protein content, ranging from 11.20 to 17.44% dw (Ballesteros, Teixeira, and Mussatto Citation2014; Martinez-Saez et al. Citation2017). Although the amount of melanoidins in this coffee residue is related to the roasting intensity, it appears to range between 9 and 16% dw (Cosío-Barrón, Hernández-Arriaga, and Campos-Vega Citation2020).
Considering the chemical composition, notably enriched in insoluble components, these by-products should have a significant effect on the gut microbiota (Jiménez-Zamora, Pastoriza, and Rufián-Henares Citation2015; Panzella et al. Citation2017).
Compounds with prebiotic potential found in coffee by-products
Non-digestible oligosaccharides
Non-digestible oligosaccharides, as opposed to non-digestible polysaccharides, have a lower polymerization degree, generally consisting of 2–20 monomers linked by a number of O-glycosidic linkages (Tian et al. Citation2017; Vera, Illanes, and Guerrero Citation2021). The prebiotic characteristics of oligosaccharides are highly influenced by structural conformation, including their degree of polymerization, monosaccharide composition, and glycosidic bond stereochemistry (Tian et al. Citation2017; Vera, Illanes, and Guerrero Citation2021). Oligosaccharides can be obtained from coffee by-products polysaccharides by means of chemical hydrolysis or enzymatic hydrolysis (Acosta-Fernández et al. Citation2020). It is important to note that the roasting process can also promote the release of oligosaccharides. Higher roasting intensity is related to greater structural diversity and oligosaccharides abundance (Tian et al. Citation2018). Several studies have isolated, identified, and characterized oligosaccharides from coffee by-products such as coffee husks, parchment, and SCG (Ávila, Martins, and Goldbeck Citation2021; Chiyanzu et al. Citation2014; Nguyen et al. Citation2019; Ratnadewi et al. Citation2020; Tian et al. Citation2017). These by-products are rich in mannans and xylans, making them possible sources of mannanoligosaccharides and xylooligosaccharides.
Acosta-Fernández et al. (Citation2020) describe the use of coffee parchment as a substrate for XOS production by applying an endo-1,4-β-xylanase produced by Aspergillus niger. Xylan was isolated from coffee parchment by autohydrolysis at 180 °C and then subjected to enzymatic hydrolysis in membrane bioreactors at 40 °C. After 30 minutes of reaction, the sugar fraction with a degree of polymerization between X1 and X20 increased rapidly to 89.6%. This range represents the most important sugar fraction due to its prebiotic properties. Ávila, Martins, and Goldbeck (Citation2021) and Ávila, Martins, Costa, et al. (Citation2020) also conducted research on the production of XOS with possible prebiotic effect from coffee husks. The results demonstrated that the enzymatic combination of endo-xylanase, feruloyl esterase, and α-l-arabinofuranosidase had a synergistic effect, producing a significant quantity of XOS, mainly xylobiose, xylotriose, and xylotetraose. XOS with less than 4 monomers are preferred in the production of functional foods, as they are more easily fermented by probiotic bacteria and have more prebiotic action (Ávila, Martins, Costa, et al. Citation2020; Marim and Gabardo Citation2021). In one of the studies involving coffee husks, it was reported that L. acidophilus, L. paracasei, and B. longum are capable of using XOS as carbon source. Moreover, a rapid decrease in the concentrations of xylobiose and xylotriose was observed after fermentation, confirming that these molecules are the preferred substrates. The compounds with a higher degree of polymerization, xylotetraose and xylopentaose, were also viable carbon sources, although their utilization kinetics were slower (Ávila, Martins, Costa, et al. Citation2020).
Wongsiridetchai et al. (Citation2018) found that MOS can be produced from SCG by alkali treatment and digestion with mannanase from Bacillus subtilis. More recently, Wongsiridetchai et al. (Citation2021) evaluated the prebiotic properties of MOS considering four criteria: gastrointestinal tolerance, growth of lactic acid bacteria (LAB), inhibition of pathogenic bacteria, and intestinal adhesion. The results revealed that MOS derived from SCG had prebiotic properties including the ability to promote LAB growth, resistance under gastrointestinal conditions, and pathogen growth suppression. Moreover, adding MOS to the LAB culture increased its hydrophobicity relative to the control. High hydrophobic interactions between the bacterial cell wall and intestinal cells are associated to a high adhesion ability (Wongsiridetchai et al. Citation2021). Perez-Burillo et al. (Citation2019) also observed that MOS produced from SCG increases microbial diversity by stimulating the growth of some beneficial genera such as Anaerostipes, Barnesiella, Bifidobacterium, Blautia, Butyricicoccus, Clostridium XVIb, Coprococcus, Intestinimonas and Ruminococcus. Furthermore, a higher MOS concentration translated into higher SCFA production, reinforcing the potential of MOS to act as a prebiotic (Perez-Burillo et al. Citation2019). Oligosaccharides from SCG showed lower digestibility compared to inulin, a well-known prebiotic. The non-digestibility of SCG oligosaccharides was estimated to be about 90% at pH 1. This indicates that SCG oligosaccharides can reach the colon without being degraded by human gastric acid, thus fulfilling the first requirement of prebiotics (Desai et al. Citation2020).
Chlorogenic acids
Green coffee beans are one of the most well-known natural sources of CGA, with the C. canephora species containing the highest levels (Farah and de Paula Lima Citation2019). CGA are a family of nonflavonoid compounds, formed by the esterification of quinic acid with one or more cinnamic acids, including caffeic, ferulic, and p-coumaric acids. The most common forms of CGA in coffee are caffeoylquinic acids (CQA), feruloylquinic acids (FQA), p-coumaroylquinic acids, dicaffeoylquinic acids, and caffeoylferuloylquinic acids (Ludwig et al. Citation2013). Isomerization, epimerization, and lactonization reactions can occur during roasting since chlorogenic acids are thermolabile compounds (Farah and de Paula Lima, Citation2019). As a result, significant levels of caffeoylquinic acid lactones and feruloylquinic acid lactones can be found in roasted coffee. Several isomers occur in coffee depending on the position of the hydroxycinnamic acid on the quinic acid moiety, with the 3-, 4-, and 5-CQA being the most prevalent (Ludwig et al. Citation2013).
According to previous research, the unabsorbed fraction of CGA in the human gastrointestinal tract acts as a substrate for beneficial intestinal bacteria, promoting their growth (Farah and de Paula Lima Citation2019). Mills et al. (Citation2015) used a stirred, anaerobic, pH-controlled, batch culture fermentation model to replicate the distal area of the human colon to study the impact of coffee and CGA on the growth of human fecal microbiota. The results showed that coffee with the highest levels of CGA (80.8 mg) induced a significant increase in the growth of Bifidobacterium spp. A similar quantity of pure 5-CQA (the most prevalent CGA in coffee) induced a significant increase in the growth of Bifidobacterium spp., suggesting that CGA are mediating compounds for prebiotic effects. A study of patients with type 2 diabetes and nonalcoholic fatty liver disease reported similar conclusions. The combination of chlorogenic acid and caffeine, the two main components of coffee, increased the amount of intestinal bifidobacteria (Mansour et al. Citation2020). Sales et al. (Citation2020) observed that coffee extracts with higher levels of CGA (medium roasted coffee) increased the growth of L. rhamnosus, L. acidophilus and B. animalis, in an in vitro study. CGA’s beneficial effect on the gut microbiota has been explored in other research that did not use coffee (Parkar, Trower, and Stevenson Citation2013; Raimondi et al. Citation2015; Shi et al. Citation2021; Song et al. Citation2019; Wang et al. Citation2019; P. Zhang et al. Citation2019). Thirty-two strains of Bifidobacterium from eight different species present in the human gut were evaluated for their ability to grow in the presence of different levels of CGA. The great majority of bifidobacteria, even those that do not participate in CGA degradation (B. bifidum, B. breve, B. catenulatum, and B. infantis), were resistant to 2 mmol/L CGA. This concentration is close to the maximum that may occur in the colon, considering CGA dietary uptake, 30% absorption in the small intestine, and 1 kg of colonic content. Bifidobacteria seem to be unaffected by physiological amounts of CGA. In turn, a considerable number of strains were unable to grow in the presence of 10 mmol/L of CGA (Raimondi et al. Citation2015). P. Zhang et al. (Citation2019) also found that CGA improves the relative abundance of organisms of the genus Lactobacillus and restores intestinal microbial diversity in a mouse model with induced colitis.
Microbial degradation of CGA in the colon involves several metabolic pathways. In the case of CQA and FQA, quinic acid is cleaved from the hydroxycinnamate part, resulting in the formation of caffeic acid and ferulic acid, respectively (Ludwig et al. Citation2013). This cleavage is most probably carried out by cinnamoyl esterase-producing bacteria such as E. coli, B. lactis, L. fermentum, L. acidophilus and L. reuteri (Ludwig et al. Citation2013; Raimondi et al. Citation2015; Sales et al. Citation2020; E. Santos, Schieber, and Weber Citation2018). Caffeic acid formation can affect the composition of the microbiota, increasing the abundance of Akkermansia, a potentially beneficial bacterium (Wan et al. Citation2021). Following that, the reductase enzyme reduces the double bond in the side chain, leading to the formation of dihydrocaffeic acid and dihydroferulic acid (Ludwig et al. Citation2013). The two dihydro derivatives are the primary end products of coffee CGA fecal fermentation (Ludwig et al. Citation2013; Mills et al. Citation2015). A recent experiment by Baeza et al. (Citation2017) demonstrates that dihydrocaffeic acid and dihydroferulic acid can significantly reduce the expression of P-selectin (a marker of platelet activation). Excessive platelet activation has been linked to physical blood vessel blockage and the development of chronic inflammation. The bacteria present in the human intestine are also able to demethoxylate, dehydroxylate and decarboxylate dihydrocaffeic acid and dihydroferulic acid into a series of compounds (Ludwig et al. Citation2013).
Melanoidins
Melanoidins, high-molecular weight brown polymers, are end products of Maillard reactions that contribute to food flavor, texture, and color (Alves et al. Citation2021; Bravo et al. Citation2012; H. Zhang et al. Citation2019). These molecules can be classified into two types: melanosaccharides (skeletons mostly formed of polysaccharides), which are found in beverages like coffee and beer; and melanoproteins (skeletons mostly formed of proteins), which are found in bakery products (Alves et al. Citation2021). In coffee, melanosaccharides are primarily generated during the roasting of green beans, although other heating-related processes, such as bean drying and decaffeination, may also contribute to their generation (Alves et al. Citation2020). Thus, melanosaccharides may be found in coffee by-products such as husks, silverskin, and SCG (Bravo et al. Citation2012; Iriondo-DeHond et al. Citation2020; Tores de la Cruz et al. Citation2019). Besides the polysaccharide core, coffee melanoidins can incorporate phenolic compounds (mainly hydroxycinnamic acids) and proteins (Iriondo-DeHond et al. Citation2021). Caffeic acid and ferulic acid are the main phenolic compounds incorporated into coffee melanosaccharides, representing on average 77% and 20% of the total bound phenolic compounds, respectively (Alves et al. Citation2020). These hydroxycinnamic acid derivatives detected in coffee melanosaccharides are the consequence of non-enzymatic hydrolysis of chlorogenic acids induced by the roasting process (Alves et al. Citation2020; Moreira et al. Citation2012).
Coffee melanoidins have been described as "maillardized dietary fiber" since dietary fiber constitutes a significant portion of melanoidins (Silvan, Morales, and Saura-Calixto Citation2010). Melanoidins, in general, escape superior digestion and reach the colon intact, where they act as substrates for the intestinal microbiota (Castaldo, Izzo, et al. Citation2021; Iriondo-DeHond, Casas, and del Castillo Citation2021). Melanoidin-bound phenolics may be released during digestion as a result of stomach acidity and pancreatic activity in the intestinal fluid. It was found that the bioaccessibilities of melanoidin-bound phenolics from coffee after the gastric and intestinal stages were 12 and 23%, respectively. However, after 24 h of intestinal fermentation, the bioaccessibility of melanoidin-bound phenolics reached a maximum (50%) and thereafter decreased (38%), indicating that the phenolic compounds released by the gut microbiota were further metabolized (Alves et al. Citation2021).
Although the colonic fermentation of coffee melanoidins has not been well investigated, there is some evidence that they may have prebiotic properties. Jiménez-Zamora, Pastoriza, and Rufián-Henares (Citation2015) tested the prebiotic ability of melanoidins extracted from SCG at a concentration of 1 mg/ml and observed an increase in bifidobacteria and lactobacilli levels. Bacteroides and Clostridium numbers were reduced, although not significantly. However, when SCG melanoidins were tested at a concentration of 10 mg/ml, all bacteria tested, namely bifidobacteria, lactobacilli, clostridium, and bacteroides, showed a significant decrease. This is consistent with previous research indicating that coffee melanoidins can have antimicrobial activity depending on concentration (Jiménez-Zamora, Pastoriza, and Rufián-Henares Citation2015; Monente et al. Citation2015; Rufián-Henares and de la Cueva Citation2009). More recently, Perez-Burillo et al. (Citation2020) evaluated the impact of digested melanoidins from several food sources (coffee, SCG, beer, sweet wine, balsamic vinegar, chocolate, bread crust, cereals, and biscuits) on the microbial community and metabolite production. Coffee melanoidins promoted a significant expansion of members of the genus Bifidobacterium, which represented about ∼40% of all microbial cells. The SCG melanoidins, on the other hand, do not appear to have been fermented by the human fecal microbiota, since the community structure was very similar to the blank sample. Coffee melanoidins originated a substantially lower amount of SCFA compared to the bread crust, cereals, and beer melanoidins, where the genus Bifidobacterium represented an average of 92% of all microbial cells. Other investigations also involving melanosaccharide-type melanoidins have revealed prebiotic effects. Aljahdali et al. (Citation2020) assessed the impact of long-term melanoidin intake from barley malts at different concentrations on the mice gut microbiota. At days 14 and 21, the levels of butyrate, acetate, and propionate were higher in the mice that fed 100% melanoidin malts compared to 0% melanoidin malts, respectively. Moreover, Akkermansia spp. and Bifidobacterium spp. were significantly increased in mice consuming the highest concentrations of melanoidin, suggesting notable prebiotic potential.
In summary, the literature data suggests that melanoidins from coffee can be fermented and metabolized by human gut microbiota and appear to selectively stimulate the growth of bifidobacteria, suggesting that they may have potential prebiotic activity comparable to dietary fiber. However, broad conclusions concerning the prebiotic effects of melanoidins derived from coffee by-products are not achievable. Due to the high complexity and diversity of melanoidins (Moreira et al. Citation2012), their chemical characterization in each coffee by-product still needs to be better understood and the potential prebiotic effects should be further explored.
Prebiotic potential of coffee by-products
Following a brief discussion of the prebiotic properties of the compounds found in coffee by-products, it is important to consider the impact of the by-products in their natural form on the gut microbiota. Despite the lack of data on this topic, some by-products such as silverskin and spent coffee grounds have been investigated for their prebiotic potential. Borrelli et al. (Citation2004) investigated the ability of fecal bacteria to use silverskin as source of carbon and found an increase in bifidobacteria levels and a decrease in clostridia and bacteroides levels. Lactobacilli, on the other hand, showed a limited aptitude to use silverskin for their growth. Jiménez-Zamora, Pastoriza, and Rufián-Henares (Citation2015) found similar outcomes. After the digestion-fermentation step, there was a significant increase in the number of healthy bacteria, namely Bifidobacterium sp. and Lactobacillus sp. (Jiménez-Zamora, Pastoriza, and Rufián-Henares Citation2015). This suggests that silverskin could be a valuable ingredient in the development of novel foods with prebiotic action. As an example, in terms of acceptance, cookies enriched with silverskin received a positive score from the panelists in terms of color, texture, flavor-taste, and general acceptance (Gocmen et al. Citation2019). Considering the sensory evaluation of enriched cookies and the fact that consumers increasingly prefer natural foods with health benefits, the incorporation of coffee by-products, such as silverskin, could be a new market trend as well as a way to increase the economic value of the chain.
Jiménez-Zamora, Pastoriza, and Rufián-Henares (Citation2015) also evaluated the influence of SCG over the microbiological growth. The results showed that SCG in its natural form behaved similarly to the control. However, in the absence of melanoidins, SCG had a positive effect on beneficial bacteria, increasing the numbers of Lactobacillus spp. and Bifidobacteria spp. By this way, coffee melanoidins appeared to interfere with the prebiotic properties of SCG. In fact, when SCG were tested on beneficial bacteria, the melanoidins were at a concentration of 10 mg/ml, a value previously described as having an antimicrobial action. Lopez-Barrera et al. (Citation2016) also proved that SCG are fermented by the human gut microbiota. After submitting SCG to in vitro gastrointestinal digestion followed by colonic fermentation, they discovered an increase in SCFA production and a significant decrease in pH. It should be noted that such a decrease in pH can alter the microbiota composition, inhibiting the growth of some pathogenic bacteria such as Bacteroides spp., Salmonella spp., and Escherichia coli (Swann et al. Citation2020). According to Lopez-Barrera et al. (Citation2016), the most prevalent SCFA produced by colonic fermentation of SCG is acetic acid, followed by propionic and butyric acids. These metabolites exhibit potent anti-inflammatory activity by suppressing nitric oxide production and inhibiting inflammatory mediators (Lopez-Barrera et al. Citation2016). These results support the use of SCG in the food industry as a prebiotic source with health advantages. Remember that SCG, in contrast to silverskin, contain a high amount of moisture (about 60%), and therefore it is required to stabilize the by-product before utilizing it in food applications (Osorio-Arias et al. Citation2020). In this sense, the SCG generated during the industrial production of soluble coffee may be preferable to the SCG obtained during coffee beverage preparation (for example, in cafeterias). In microbiological terms, industrial SCG are more easily controllable, and the stabilization process could continue the production line. Implementing a SCG stabilization process in a cafeteria is more complex and incurs additional expenses that the owner may be unwilling to pay since it is waste. Collection may also not be fruitful. Due to the high moisture content, SCG easily degrades, making it unfit for human consumption.
A recent study explored whether the addition of 5% coffee pulp to the diet reverses obesity-induced microbiota changes in rats. The results showed that the coffee pulp intervention increased the abundance of Akkermansia muciniphila, a potential probiotic involved in the treatment of obesity (Nikhil S. Bhandarkar et al. Citation2021). Indeed, previous research has discovered a negative correlation between the abundance of Akkermansia muciniphila and overweight or obesity (Depommier et al. Citation2019). Nevertheless, other obesity-related genera were also increased as Clostridium saudiense, Cronobacter malonaticus and Cronobacter sakazakii. In addition to modulating the gut microbiota, coffee pulp reduced body weight, abdominal fat, and feed efficiency, normalized plasma concentrations of triglycerides, and improved glucose tolerance (Bhandarkar et al. Citation2021). Although the main objective of this study was not to investigate the prebiotic properties of coffee pulp, but rather to see if coffee pulp could improve metabolic syndrome in rats fed a diet high in fat and carbohydrates, significant growth of a possibly beneficial bacteria was observed after bacterial fermentation. The lack of research concerning coffee pulp and its prebiotic action must be tackled since this by-product is high in fiber and has the potential to influence the gut microbiota positively (Bhandarkar et al. Citation2021). Moreover, coffee pulp or husks has been approved as novel foods a pursuant to Regulation (EU) 2015/2283. Coffee pulp has already been exploited as a source of antioxidant dietary fiber to develop of "high in fiber" salty cookies. Consumers rated the pulp-enriched cookies as acceptable, with an acceptability value more than 6 (nine-point hedonic scale) (Moreno et al. Citation2019).
In comparison to by-products, studies involving coffee and its influence on the gut microbiota are more common. However, such studies can be useful to better understand the potential prebiotic effect of coffee by-products. Coffee beverages, like by-products, contain various compounds that have the potential to affect the microbiota, including fiber, polyphenols, and caffeine (Diamond et al. Citation2021). Coffee consumption, according to the literature, increased Lactobacillus and Bifidobacterium counts, suggesting that coffee acidifies the intestine (Jaquet et al. Citation2009; Mills et al. Citation2015; Nakayama and Oishi Citation2013; Sales et al. Citation2020). Furthermore, coffee may have antibacterial properties, decreasing E. coli and Clostridium spp. levels significantly (Nakayama and Oishi Citation2013). A study found that coffee consumption can also attenuate the increase in Firmicutes to Bacteroidetes ratio and Clostridium Cluster XI associated with high-fat diets. In terms of the metabolomic profile, coffee consumption increases SCFA production such as acetate, formate, and propionate (Cowan et al. Citation2014). Moreover, antibiotic use is frequently associated with an increase in potentially pro-inflammatory bacteria like Proteobacteria in the gut, resulting in dysbiosis. Diamond et al. (Citation2021) discovered that coffee intake can modulate amoxicillin-induced dysbiosis by decreasing Proteobacteria, specifically the family Burkholderiaceae. In general, previous studies suggest that coffee consumption may be advantageous to the host due to the potential of CGA and other coffee components to modify the colonic microbiota (Mills et al. Citation2015; Sales et al. Citation2020). It should be noted that the prebiotic effect of coffee can be affected by roasting and decaffeination (Sales et al. Citation2020). Conventional and decaffeinated coffee significantly increased levels of Akkermansia in sleep-deprived rats, but the effect of conventional coffee was the most obvious. Sleep deprivation is commonly associated with a decrease in Akkermansia (X. Gu et al. Citation2022).
A summary of the prebiotic properties of coffee and its by-products is presented in . In general, oligosaccharides from coffee by-products meet prebiotic requirements: low digestibility, sometimes lower than inulin; promote the growth of beneficial bacteria such as L. acidophilus, L. casei, L. fermentum, L. gasseri, L. paracasei, L. plantarum, L. reuteri, and B. longum; inhibit the growth of pathogenic bacteria such as E. coli, Bacillus cereus and Salmonella paratyphi; and increase the production of SCFA with potential health benefits in a dose-dependent manner. However, melanoidins from coffee by-products, more specifically from SCG, to meet prebiotic requirements must be ingested in regulated amounts in order to avoid a decrease in potentially beneficial bacteria. Although the CGA present in coffee by-products have not been explored for their prebiotic potential, one research found that CGA-rich coffee can be metabolized by the gut microbiota and stimulate the growth of the beneficial genus Bifidobacterium (Mills et al. Citation2015). The gut microbiota appears to be influenced positively by CGA and melanoidins from coffee by-products, although further research is needed.
Table 3. An overview of the prebiotic effect of coffee and their by-products.
Researchers observed that SCG phenolic extracts promoted the probiotic growth at low concentrations, including L. plantarum, L. casei, L. bulgaricus, L. acidophilus, L. brevis, B. bifidum, and B. breve, while inhibited pathogens including E. coli, B. cereus, Staphylococcus aureus and Salmonella Typhimurium (Klangpetch Citation2017). In fact, the majority of the bacteria tested do not participate in the degradation of CGA, one of the most prevalent phenols in the extract, but L. acidophilus is a cinnamoyl esterase-producing bacteria that can degrade CGA. Most probably, the presence of antibacterial compounds like caffeine and trigonelline in phenolic extracts can explains the low probiotic growth. In this way, it may be more advantageous to evaluate the prebiotic effect of coffee by-products after extraction of alkaloids or other compounds that, at a certain concentration, exhibit antimicrobial activity. One example is SCG, which, after removing melanoidins, stimulates the growth of beneficial bacteria. However, other coffee by-products, such as coffee pulp and silverskin, revealed interesting results in terms of prebiotic activity even without extraction.
Most studies involving by-products are performed in vitro and, in some cases, do not include pre-digestion, which could increase the validity of the work. Although the current data suggest that by-products can act as prebiotics in the human large intestine, further in vivo research is required to corroborate these findings (Mills et al. Citation2015). As far as we know, the effects of parchment, mucilage, and defective coffee beans on gut microbiota have not yet been explored.
Conclusions
In summary, coffee industry by-products can be taken as a source of unique functional ingredients owing to their relevant impact on the gut microbiota. The fermentation of oligosaccharides derived from coffee by-products produces short-chain fatty acids, which are important in a variety of physio-biological functions such as reducing intestinal pH and suppressing pathogens. Non-carbohydrate compounds found in coffee by-products, like as melanoidins and chlorogenic acids, have also been shown to promote probiotic growth. However, in the case of melanoidins, it is preferred to separate them from the by-product and provide them in a previously defined concentration to assure that there is no antimicrobial action.
Overall, all coffee industry by-products can be good sources of prebiotics, although additional in vitro and in vivo research is needed. Parchment, mucilage, and defective coffee beans have received minimal attention in terms of their chemical composition and ability to act as a substrate for probiotic bacteria. Adding value to coffee by-products can help to reduce their negative impact on the environment and contribute to the development of a new generation of functional foods. Simultaneously, that could answer to the increasing needs for food security and to the sustainability and circular economy requirements.
| Abbreviations | ||
| CNS | = | central nervous system |
| SCFA | = | short-chain fatty acids |
| FOS | = | fructooligosaccharides |
| MOS | = | mannanoligosaccharides |
| XOS | = | xylooligosaccharides |
| GOS | = | galactooligosaccharides |
| TOS | = | transgalactooligosaccharides |
| CGA | = | chlorogenic acids |
| CQA | = | caffeoylquinic acid |
| CAZymes | = | carbohydrate-active enzymes |
| PAZymes | = | polyphenol-associated enzymes |
| FQA | = | feruloylquinic acid |
| GPR | = | G-coupled protein receptors |
| SCG | = | spent coffee grounds |
| LAB | = | lactic acid bacteria |
| HMF | = | hydroxymethylfurfural |
| PBS | = | phosphate buffer saline |
| OS | = | oligosaccharides |
| BCAA | = | branched-chain amino acids |
Acknowledgments
F. Marlene Machado thanks to FCT/MCTES (Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) and ESF (European Social Fund) through NORTE 2020 (Programa Operacional Região Norte) for the PhD grant (ref. 2021.04907.BD). Rita C. Alves thanks to FCT/MCTES for funding through the Scientific Employment Stimulus - Individual Call (Ref. CEECIND/01120/2017).
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Acosta-Fernández, R., T. Poerio, D. Nabarlatz, L. Giorno, and R. Mazzei. 2020. Enzymatic hydrolysis of xylan from coffee parchment in membrane bioreactors. Industrial & Engineering Chemistry Research 59 (16):7346–54. doi: 10.1021/acs.iecr.9b06429.
- Aguilera, Y., M. Rebollo-Hernanz, S. Canas, D. Taladrid, and M. A. Martin-Cabrejas. 2019. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food & Function 10 (8):4739–50. doi: 10.1039/c9fo00544g.
- Aljahdali, N., P. Gadonna-Widehem, P. M. Anton, and F. Carbonero. 2020. Gut microbiota modulation by dietary barley malt melanoidins. Nutrients 12 (1):241. doi: 10.3390/nu12010241.
- Alves-Santos, A. M., C. S. A. Sugizaki, G. C. Lima, and M. M. V. Naves. 2020. Prebiotic effect of dietary polyphenols: A systematic review. Journal of Functional Foods 74:104169. doi: 10.1016/j.jff.2020.104169.
- Alves, G., L. A. Lobo, R. Domingues, M. Monteiro, and D. Perrone. 2021. Bioaccessibility and gut metabolism of free and melanoidin-bound phenolic compounds from coffee and bread. Frontiers in Nutrition 8:708928. doi: 10.3389/fnut.2021.708928.
- Alves, G., P. Xavier, R. Limoeiro, and D. Perrone. 2020. Contribution of melanoidins from heat-processed foods to the phenolic compound intake and antioxidant capacity of the Brazilian diet. Journal of Food Science and Technology 57 (8):3119–31. doi: 10.1007/s13197-020-04346-0.
- Ashaolu, T. J., J. O. Ashaolu, and S. A. O. Adeyeye. 2021. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. Journal of Applied Microbiology 130 (3):677–87. doi: 10.1111/jam.14843.
- Ateş, G., and Y. Elmacı. 2019. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. Journal of Food Measurement and Characterization 13 (1):755–63. doi: 10.1007/s11694-018-9988-9.
- Avallone, S., J. P. Guiraud, B. Guyot, E. Olguin, and J. M. Brillouet. 2000. Polysaccharide constituents of coffee-bean mucilage. Journal of Food Science 65 (8):1308–11. doi: 10.1111/j.1365-2621.2000.tb10602.x.
- Ávila, P. F., M. Martins, F. A. A. Costa, and R. Goldbeck. 2020. Xylooligosaccharides production by commercial enzyme mixture from agricultural wastes and their prebiotic and antioxidant potential. Bioactive Carbohydrates and Dietary Fibre 24:100234. doi: 10.1016/j.bcdf.2020.100234.
- Ávila, P. F., M. Martins, and R. Goldbeck. 2021. Enzymatic production of xylooligosaccharides from alkali-solubilized arabinoxylan from sugarcane straw and coffee husk. BioEnergy Research 14 (3):739–51. doi: 10.1007/s12155-020-10188-7.
- Azad, M. A. K., M. Sarker, T. Li, and J. Yin. 2018. Probiotic species in the modulation of gut microbiota: An overview. BioMed Research International 2018:9478630. doi: 10.1155/2018/9478630.
- Baeta, B. E. L., P. H. M. Cordeiro, F. Passos, L. V. A. Gurgel, S. F. de Aquino, and F. Fdz-Polanco. 2017. Steam explosion pretreatment improved the biomethanization of coffee husks. Bioresource Technology 245 (Pt A):66–72. doi: 10.1016/j.biortech.2017.08.110.
- Baeza, G., E. M. Bachmair, S. Wood, R. Mateos, L. Bravo, and B. de Roos. 2017. The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food & Function 8 (3):1333–42. doi: 10.1039/c6fo01404f.
- Ballesteros, L. F., J. A. Teixeira, and S. I. Mussatto. 2014. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food and Bioprocess Technology 7 (12):3493–503. doi: 10.1007/s11947-014-1349-z.
- Bekalo, S. A., and H.-W. Reinhardt. 2010. Fibers of coffee husk and hulls for the production of particleboard. Materials and Structures 43 (8):1049–60. doi: 10.1617/s11527-009-9565-0.
- Benitez, V., M. Rebollo-Hernanz, Y. Aguilera, S. Bejerano, S. Canas, and M. A. Martin-Cabrejas. 2021. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food & Function 12 (3):1097–110. doi: 10.1039/d0fo02295k.
- Benitez, V., M. Rebollo-Hernanz, S. Hernanz, S. Chantres, Y. Aguilera, and M. A. Martin-Cabrejas. 2019. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Research International (Ottawa, Ont.) 122:105–13. doi: 10.1016/j.foodres.2019.04.002.
- Bessada, S. M. F., R. C. Alves, A. S. G. Costa, M. A. Nunes, and M. Oliveira. 2018. Coffea canephora silverskin from different geographical origins: A comparative study. The Science of the Total Environment 645:1021–8. doi: 10.1016/j.scitotenv.2018.07.201.
- Bhandarkar, N. S., P. Mouatt, P. Goncalves, T. Thomas, L. Brown, and S. K. Panchal. 2020. Modulation of gut microbiota by spent coffee grounds attenuates diet-induced metabolic syndrome in rats. FASEB Journal 34 (3):4783–97. doi: 10.1096/fj.201902416RR.
- Bhandarkar, N. S., P. Mouatt, M. E. Majzoub, T. Thomas, L. Brown, and S. K. Panchal. 2021. Coffee pulp, a by-product of coffee production, modulates gut microbiota and improves metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Pathogens 10 (11):1369. doi: 10.3390/pathogens10111369.
- Borrelli, R. C., F. Esposito, A. Napolitano, A. Ritieni, and V. Fogliano. 2004. Characterization of a new potential functional ingredient: Coffee silverskin. Journal of Agricultural and Food Chemistry 52 (5):1338–43. doi: 10.1021/jf034974x.
- Brand, D., A. Pandey, S. Roussos, and C. R. Soccol. 2000. Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzyme and Microbial Technology 27 (1–2):127–33. doi: 10.1016/s0141-0229(00)00186-1.
- Bravo, J., I. Juaniz, C. Monente, B. Caemmerer, L. W. Kroh, M. P. De Pena, and C. Cid. 2012. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. Journal of Agricultural and Food Chemistry 60 (51):12565–73. doi: 10.1021/jf3040594.
- Capuano, E. 2017. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Critical Reviews in Food Science and Nutrition 57 (16):3543–64. doi: 10.1080/10408398.2016.1180501.
- Cardona, F., C. Andres-Lacueva, S. Tulipani, F. J. Tinahones, and M. I. Queipo-Ortuno. 2013. Benefits of polyphenols on gut microbiota and implications in human health. The Journal of Nutritional Biochemistry 24 (8):1415–22. doi: 10.1016/j.jnutbio.2013.05.001.
- Castaldo, L., L. Izzo, A. Narvaez, Y. Rodriguez-Carrasco, M. Grosso, and A. Ritieni. 2021. Colon bioaccessibility under in vitro gastrointestinal digestion of different coffee brews chemically profiled through UHPLC-Q-Orbitrap HRMS. Foods 10 (1):179. doi: 10.3390/foods10010179.
- Castaldo, L., S. Lombardi, A. Gaspari, M. Rubino, L. Izzo, A. Narvaez, A. Ritieni, and M. Grosso. 2021. In vitro bioaccessibility and antioxidant activity of polyphenolic compounds from spent coffee grounds-enriched cookies. Foods 10 (8):1837. doi: 10.3390/foods10081837.
- Cavalcanti, M. H., J. P. S. Roseira, E. S. Leandro, and S. F. Arruda. 2022. Effect of a freeze-dried coffee solution in a high-fat diet-induced obesity model in rats: Impact on inflammatory response, lipid profile, and gut microbiota. PLoS One 17 (1):e0262270. doi: 10.1371/journal.pone.0262270.
- Chen, T., R. Wang, Z. Duan, X. Yuan, Y. Ding, Z. Feng, F. Bu, L. Liu, Q. Wang, J. Zhou, et al. 2021. Akkermansia muciniphila protects against psychological disorder-induced gut microbiota-mediated colonic mucosal barrier damage and aggravation of colitis. Frontiers in Cellular and Infection Microbiology 11:723856. doi: 10.3389/fcimb.2021.723856.
- Chiyanzu, I., M. Brienzo, M. P. Garcia-Aparicio, and J. F. Gorgens. 2014. Application of endo-beta-1,4,D-mannanase and cellulase for the release of mannooligosaccharides from steam-pretreated spent coffee ground. Applied Biochemistry and Biotechnology 172 (7):3538–57. doi: 10.1007/s12010-014-0770-0.
- Coelho, G. O., M. J. A. Batista, A. F. Ávila, A. S. Franca, and L. S. Oliveira. 2021. Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. Journal of Food Engineering 289:110083. doi: 10.1016/j.jfoodeng.2020.110083.
- Collazo-Bigliardi, S., R. Ortega-Toro, and A. C. Boix. 2018. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydrate Polymers 191:205–15. doi: 10.1016/j.carbpol.2018.03.022.
- Cosío-Barrón, A. C. G., A. M. Hernández-Arriaga, and R. Campos-Vega. 2020. Spent coffee (Coffea arabica L.) grounds positively modulate indicators of colonic microbial activity. Innovative Food Science & Emerging Technologies 60:102286. doi: 10.1016/j.ifset.2019.102286.
- Costa, A. S. G., R. C. Alves, A. F. Vinha, E. Costa, C. S. G. Costa, M. A. Nunes, A. A. Almeida, A. Santos-Silva, and M. Oliveira. 2018. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chemistry 267:28–35. doi: 10.1016/j.foodchem.2017.03.106.
- Cowan, T. E., M. S. Palmnas, J. Yang, M. R. Bomhof, K. L. Ardell, R. A. Reimer, H. J. Vogel, and J. Shearer. 2014. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. The Journal of Nutritional Biochemistry 25 (4):489–95. doi: 10.1016/j.jnutbio.2013.12.009.
- Cruz, R., M. M. Cardoso, L. Fernandes, M. Oliveira, E. Mendes, P. Baptista, S. Morais, and S. Casal. 2012. Espresso coffee residues: A valuable source of unextracted compounds. Journal of Agricultural and Food Chemistry 60 (32):7777–84. doi: 10.1021/jf3018854.
- da Silva, M. R., F. S. Bragagnolo, R. L. Carneiro, I. O. C. Pereira, J. A. A. Ribeiro, C. M. Rodrigues, R. E. Jelley, B. Fedrizzi, and C. S. Funari. 2022. Metabolite characterization of fifteen by-products of the coffee production chain: From farm to factory. Food Chemistry 369:130753. doi: 10.1016/j.foodchem.2021.130753.
- Depommier, C., A. Everard, C. Druart, H. Plovier, M. V. Hul, S. Vieira-Silva, G. Falony, J. Raes, D. Maiter, N. M. Delzenne, et al. 2019. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nature Medicine 25 (7):1096–103. doi: 10.1038/s41591-019-0495-2.
- Desai, N. M., G. S. Martha, N. V. Harohally, and P. S. Murthy. 2020. Non-digestible oligosaccharides of green coffee spent and their prebiotic efficiency. LWT 118:108784. doi: 10.1016/j.lwt.2019.108784.
- Diamond, E., K. Hewlett, S. Penumutchu, A. Belenky, and P. Belenky. 2021. Coffee consumption modulates amoxicillin-induced dysbiosis in the murine gut microbiome. Frontiers in Microbiology 12:637282. doi: 10.3389/fmicb.2021.637282.
- Dias, M., M. M. Melo, R. F. Schwan, and C. F. Silva. 2015. A new alternative use for coffee pulp from semi-dry process to beta-glucosidase production by Bacillus subtilis. Letters in Applied Microbiology 61 (6):588–95. doi: 10.1111/lam.12498.
- El Hage, R., E. Hernandez-Sanabria, and T. V. de Wiele. 2017. Emerging trends in "smart probiotics": Functional consideration for the development of novel health and industrial applications. Frontiers in Microbiology 8:1889. doi: 10.3389/fmicb.2017.01889.
- Esquivel, P., and V. M. Jiménez. 2012. Functional properties of coffee and coffee by-products. Food Research International 46 (2):488–95. doi: 10.1016/j.foodres.2011.05.028.
- Farah, A., and J. de Paula Lima. 2019. Consumption of chlorogenic acids through coffee and health implications. Beverages 5 (1):11. doi: 10.3390/beverages5010011.
- Flint, H. J., K. P. Scott, S. H. Duncan, P. Louis, and E. Forano. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3 (4):289–306. doi: 10.4161/gmic.19897.
- Frolinger, T., S. Sims, C. Smith, J. Wang, H. Cheng, J. Faith, L. Ho, K. Hao, and G. M. Pasinetti. 2019. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Scientific Reports 9 (1):3546. doi: 10.1038/s41598-019-39994-6.
- Gocmen, D., Y. Sahan, E. Yildiz, M. Coskun, and I. A. Aroufai. 2019. Use of coffee silverskin to improve the functional properties of cookies. Journal of Food Science and Technology 56 (6):2979–88. doi: 10.1007/s13197-019-03773-y.
- Gu, J., W. Pei, S. Tang, F. Yan, Z. Peng, C. Huang, J. Yang, and Q. Yong. 2020. Procuring biologically active galactomannans from spent coffee ground (SCG) by autohydrolysis and enzymatic hydrolysis. International Journal of Biological Macromolecules 149:572–80. doi: 10.1016/j.ijbiomac.2020.01.281.
- Gu, X., S. Zhang, W. Ma, Q. Wang, Y. Li, C. Xia, Y. Xu, T. Zhang, L. Yang, and M. Zhou. 2022. The impact of instant coffee and decaffeinated coffee on the gut microbiota and depression-like behaviors of sleep-deprived rats. Frontiers in Microbiology 13:778512. doi: 10.3389/fmicb.2022.778512.
- Habtamu, D., and A. Belay. 2020. First order derivative spectra to determine caffeine and chlorogenic acids in defective and nondefective coffee beans. Food Science & Nutrition 8 (9):4757–62. doi: 10.1002/fsn3.1723.
- Haile, M., and W. H. Kang. 2019. The role of microbes in coffee fermentation and their impact on coffee quality. Journal of Food Quality 2019 (11):1–6. doi: 10.1155/2019/4836709.
- Hijosa-Valsero, M., J. Garita-Cambronero, A. I. Paniagua-Garcia, and R. Diez-Antolinez. 2018. Biobutanol production from coffee silverskin. Microbial Cell Factories 17 (1):154. doi: 10.1186/s12934-018-1002-z.
- Hills, R. D., B. A. Pontefract, H. R. Mishcon, C. A. Black, S. C. Sutton, and C. R. Theberge. 2019. Gut microbiome: Profound implications for diet and disease. Nutrients 11 (7):1613. doi: 10.3390/nu11071613.
- Holscher, H. D. 2017. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8 (2):172–84. doi: 10.1080/19490976.2017.1290756.
- Hoseini, M., S. Cocco, C. Casucci, V. Cardelli, and G. Corti. 2021. Coffee by-products derived resources. A review. Biomass and Bioenergy 148:106009. doi: 10.1016/j.biombioe.2021.106009.
- Iriondo-DeHond, A., N. A. García, B. Fernandez-Gomez, E. Guisantes-Batan, F. V. Escobar, G. P. Blanch, M. I. S. Andres, S. Sanchez-Fortun, and M. D. del Castillo. 2019. Validation of coffee by-products as novel food ingredients. Innovative Food Science & Emerging Technologies 51:194–204. doi: 10.1016/j.ifset.2018.06.010.
- Iriondo-DeHond, A., A. S. Elizondo, M. Iriondo-DeHond, M. B. Rios, R. Mufari, J. A. Mendiola, E. Ibanez, and M. D. del Castillo. 2020. Assessment of healthy and harmful maillard reaction products in a novel coffee cascara beverage: Melanoidins and acrylamide. Foods 9 (5):620. doi: 10.3390/foods9050620.
- Iriondo-DeHond, A., M. B. Rios, T. Herrera, A. Rodriguez-Bertos, F. Nunez, M. I. S. Andres, S. Sanchez-Fortun, and M. D. del Castillo. 2019. Coffee silverskin extract: Nutritional value, safety and effect on key biological functions. Nutrients 11 (11):2693. doi: 10.3390/nu11112693.
- Iriondo-DeHond, A., A. R. Casas, and M. D. del Castillo. 2021. Interest of coffee melanoidins as sustainable healthier food ingredients. Frontiers in Nutrition 8:730343. doi: 10.3389/fnut.2021.730343.
- Jana, U. K., R. K. Suryawanshi, B. P. Prajapati, and N. Kango. 2021. Prebiotic mannooligosaccharides: Synthesis, characterization and bioactive properties. Food Chemistry 342:128328. doi: 10.1016/j.foodchem.2020.128328.
- Janissen, B., and T. Huynh. 2018. Chemical composition and value-adding applications of coffee industry by-products: A review. Resources, Conservation and Recycling 128:110–7. doi: 10.1016/j.resconrec.2017.10.001.
- Jaquet, M., I. Rochat, J. Moulin, C. Cavin, and R. Bibiloni. 2009. Impact of coffee consumption on the gut microbiota: A human volunteer study. International Journal of Food Microbiology 130 (2):117–21. doi: 10.1016/j.ijfoodmicro.2009.01.011.
- Jiménez-Zamora, A., S. Pastoriza, and J. A. Rufián-Henares. 2015. Revalorization of coffee by-products. Prebiotic, antimicrobial and antioxidant properties. LWT - Food Science and Technology 61 (1):12–8. doi: 10.1016/j.lwt.2014.11.031.
- Kc, Y., R. Subba, L. D. Shiwakoti, P. K. Dhungana, R. Bajagain, D. K. Chaudhary, B. R. Pant, T. R. Bajgai, J. Lamichhane, S. Timilsina, et al. 2021. Utilizing coffee pulp and mucilage for producing alcohol-based beverage. Fermentation 7 (2):53. doi: 10.3390/fermentation7020053.
- Khochapong, W., S. Ketnawa, Y. Ogawa, and N. Punbusayakul. 2021. Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract. Food Chemistry 348:129094. doi: 10.1016/j.foodchem.2021.129094.
- Klangpetch, W. 2017. Evaluation of antioxidant, anti-pathogenic and probiotic growth stimulatory activities of spent coffee ground polyphenol extracts. International Food Research Journal 24 (5):2246–52.
- Klingel, T., J. I. Kremer, V. Gottstein, T. R. de Rezende, S. Schwarz, and D. W. Lachenmeier. 2020. A review of coffee by-products including leaf, flower, cherry, husk, silverskin, and spent grounds as novel foods within the European Union. Foods 9 (5):665. doi: 10.3390/foods9050665.
- Li, S., Y. Chen, Y. Zhang, H. Lv, L. Luo, S. Wang, and X. Guan. 2022. Polyphenolic extracts of coffee cherry husks alleviated colitis-induced neural inflammation via NF-kB signaling regulation and gut microbiota modification. Journal of Agricultural and Food Chemistry 70 (21):6467–77. doi: 10.1021/acs.jafc.2c02079.
- Linares, D. M., P. Ross, and C. Stanton. 2016. Beneficial microbes: The pharmacy in the gut. Bioengineered 7 (1):11–20. doi: 10.1080/21655979.2015.1126015.
- Littardi, P., M. Rinaldi, M. Grimaldi, A. Cavazza, and E. Chiavaro. 2020. Effect of addition of green coffee parchment on structural, qualitative and chemical properties of gluten-free bread. Foods 10 (1):5. doi: 10.3390/foods10010005.
- Lopez-Barrera, D. M., K. Vazquez-Sanchez, M. G. Loarca-Pina, and R. Campos-Vega. 2016. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chemistry 212:282–90. doi: 10.1016/j.foodchem.2016.05.175.
- Lordan, C., D. Thapa, R. P. Ross, and P. D. Cotter. 2020. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 11 (1):1–20. doi: 10.1080/19490976.2019.1613124.
- Ludwig, I. A., M. P. de Pena, C. Concepcion, and C. Alan. 2013. Catabolism of coffee chlorogenic acids by human colonic microbiota. BioFactors (Oxford, England) 39 (6):623–32. doi: 10.1002/biof.1124.
- Mansour, A., M. R. Mohajeri-Tehrani, S. Karimi, M. Sanginabadi, H. Poustchi, S. Enayati, S. Asgarbeik, J. Nasrollahzadeh, and A. Hekmatdoost. 2020. Short term effects of coffee components consumption on gut microbiota in patients with non-alcoholic fatty liver and diabetes: A pilot randomized placebo-controlled, clinical trial. EXCLI Journal 19:241–50. doi: 10.17179/excli2019-2021.
- Marim, A. V. C., and S. Gabardo. 2021. Xylooligosaccharides: Prebiotic potential from agro-industrial residue, production strategies and prospects. Biocatalysis and Agricultural Biotechnology 37:102190. doi: 10.1016/j.bcab.2021.102190.
- Martinez-Saez, N., A. T. Garcia, I. D. Perez, M. Rebollo-Hernanz, M. Mesias, F. J. Morales, M. A. Martin-Cabrejas, and M. D. del Castillo. 2017. Use of spent coffee grounds as food ingredient in bakery products. Food Chemistry 216:114–22. doi: 10.1016/j.foodchem.2016.07.173.
- Mills, C. E., X. Tzounis, M. J. Oruna-Concha, D. S. Mottram, G. R. Gibson, and J. P. Spencer. 2015. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. The British Journal of Nutrition 113 (8):1220–7. doi: 10.1017/S0007114514003948.
- Mirón-Mérida, V. A., J. Yáñez-Fernández, B. Montañez-Barragán, and B. E. B. Huerta. 2019. Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT 101:167–74. doi: 10.1016/j.lwt.2018.11.013.
- Monente, C., J. Bravo, A. I. Vitas, L. Arbillaga, M. P. de Peña, and C. Cid. 2015. Coffee and spent coffee extracts protect against cell mutagens and inhibit growth of food-borne pathogen microorganisms. Journal of Functional Foods 12:365–74. doi: 10.1016/j.jff.2014.12.006.
- Moreira, A. S., F. M. Nunes, M. R. Domingues, and M. A. Coimbra. 2012. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food & Function 3 (9):903–15. doi: 10.1039/c2fo30048f.
- Moreno, J., S. Cozzano, A. M. Pérez, P. Arcia, and A. Curutchet. 2019. Coffee pulp waste as a functional ingredient: Effect on salty cookies quality. Journal of Food and Nutrition Research 7 (9):632–8. doi: 10.12691/jfnr-7-9-2.
- Murthy, P. S., and M. M. Naidu. 2012. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food and Bioprocess Technology 5 (3):897–903. doi: 10.1007/s11947-010-0363-z.
- Mussatto, S. I., L. M. Carneiro, J. P. A. Silva, I. C. Roberto, and J. A. Teixeira. 2011. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydrate Polymers 83 (2):368–74. doi: 10.1016/j.carbpol.2010.07.063.
- Nakayama, T., and K. Oishi. 2013. Influence of coffee (Coffea arabica) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiology Letters 343 (2):161–8. doi: 10.1111/1574-6968.12142.
- Nguyen, Q. A., E. J. Cho, D. S. Lee, and H. J. Bae. 2019. Development of an advanced integrative process to create valuable biosugars including manno-oligosaccharides and mannose from spent coffee grounds. Bioresource Technology 272:209–16. doi: 10.1016/j.biortech.2018.10.018.
- Niglio, S., A. Procentese, M. E. Russo, G. Sannia, and A. Marzocchella. 2019. Investigation of enzymatic hydrolysis of coffee silverskin aimed at the production of butanol and succinic acid by fermentative processes. BioEnergy Research 12 (2):312–24. doi: 10.1007/s12155-019-09969-6.
- Oliveira, G., C. P. Passos, P. Ferreira, M. A. Coimbra, and I. Goncalves. 2021. Coffee by-products and their suitability for developing active food packaging materials. Foods 10 (3):683. doi: 10.3390/foods10030683.
- Oliveira, L. S., A. S. Franca, J. C. F. Mendonça, and M. C. Barros-Júnior. 2006. Proximate composition and fatty acids profile of green and roasted defective coffee beans. LWT - Food Science and Technology 39 (3):235–9. doi: 10.1016/j.lwt.2005.01.011.
- Oroojzadeh, P., S. Y. Bostanabad, and H. Lotfi. 2022. Psychobiotics: The influence of gut microbiota on the gut-brain axis in neurological disorders. Journal of Molecular Neuroscience: MN 72 (9):1952–64. doi: 10.1007/s12031-022-02053-3.
- Osorio-Arias, J., S. Delgado-Arias, L. Cano, S. Zapata, M. Quintero, H. Nuñez, C. Ramírez, R. Simpson, and O. Vega-Castro. 2020. Sustainable management and valorization of spent coffee grounds through the optimization of thin layer hot air-drying process. Waste and Biomass Valorization 11 (9):5015–26. doi: 10.1007/s12649-019-00793-9.
- Panzella, L., S. Perez-Burillo, S. Pastoriza, M. A. Martin, P. Cerruti, L. Goya, S. Ramos, J. A. Rufian-Henares, A. Napolitano, and M. d’Ischia. 2017. High antioxidant action and prebiotic activity of hydrolyzed spent coffee grounds (HSCG) in a simulated digestion-fermentation model: Toward the development of a novel food supplement. Journal of Agricultural and Food Chemistry 65 (31):6452–9. doi: 10.1021/acs.jafc.7b02302.
- Parkar, S. G., T. M. Trower, and D. E. Stevenson. 2013. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 23:12–9. doi: 10.1016/j.anaerobe.2013.07.009.
- Patel, A. K., R. R. Singhania, M. K. Awasthi, S. Varjani, S. K. Bhatia, M. L. Tsai, S. L. Hsieh, C. W. Chen, and C. D. Dong. 2021. Emerging prospects of macro- and microalgae as prebiotic. Microbial Cell Factories 20 (1):112. doi: 10.1186/s12934-021-01601-7.
- Patil, S., V. Pimpley, K. Warudkar, and P. S. Murthy. 2022. Valorisation of coffee pulp for development of innovative probiotic beverage using kefir: Physicochemical, antioxidant, sensory analysis and shelf life studies. Waste and Biomass Valorization 13 (2):905–16. doi: 10.1007/s12649-021-01554-3.
- Perez-Burillo, S., S. Pastoriza, A. Fernandez-Arteaga, G. Luzon, N. Jimenez-Hernandez, G. D’Auria, M. P. Francino, and J. A. Rufian-Henares. 2019. Spent coffee grounds extract, rich in mannooligosaccharides, promotes a healthier gut microbial community in a dose-dependent manner. Journal of Agricultural and Food Chemistry 67 (9):2500–9. doi: 10.1021/acs.jafc.8b06604.
- Perez-Burillo, S., S. Rajakaruna, S. Pastoriza, O. Paliy, and J. A. Rufian-Henares. 2020. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chemistry 316:126309. doi: 10.1016/j.foodchem.2020.126309.
- Prandi, B., M. Ferri, S. Monari, C. Zurlini, I. Cigognini, S. Verstringe, D. Schaller, M. Walter, L. Navarini, A. Tassoni, et al. 2021. Extraction and chemical characterization of functional phenols and proteins from coffee (Coffea arabica) by-products. Biomolecules 11 (11):1571. doi: 10.3390/biom11111571.
- Puengsawada, P., C. Piyapittayanuna, T. Sawangwanb, and S. Chantorna. 2021. Characterization of Bacillus subtilis GA2(1) mannanase expressed in Escherichia coli Rosetta (DE3) for enzymatic production of manno-oligosaccharides from spent coffee grounds and in vitro assessment of their prebiotic properties. Agriculture and Natural Resources 55 (3):319–30. doi: 10.34044/j.anres.2021.55.3.01.
- Raimondi, S., A. Anighoro, A. Quartieri, A. Amaretti, F. A. Tomas-Barberan, G. Rastelli, and M. Rossi. 2015. Role of bifidobacteria in the hydrolysis of chlorogenic acid. MicrobiologyOpen 4 (1):41–52. doi: 10.1002/mbo3.219.
- Ratnadewi, A. A. I., M. H. Amaliyah Zain, A. A. N. Nara Kusuma, W. Handayani, A. S. Nugraha, and T. A. Siswoyo. 2020. Lactobacillus casei fermentation towards xylooligosaccharide (XOS) obtained from coffee peel enzymatic hydrolysate. Biocatalysis and Agricultural Biotechnology 23 (2):101446. doi: 10.1016/j.bcab.2019.101446.
- Rebollo-Hernanz, M., S. Canas, D. Taladrid, V. Benitez, B. Bartolome, Y. Aguilera, and M. A. Martin-Cabrejas. 2021. Revalorization of coffee husk: Modeling and optimizing the green sustainable extraction of phenolic compounds. Foods 10 (3):653. doi: 10.3390/foods10030653.
- Redgwell, R. J., V. Trovato, D. Curti, and M. Fischer. 2002. Effect of roasting on degradation and structural features of polysaccharides in Arabica coffee beans. Carbohydrate Research 337 (5):421–31. doi: 10.1016/S0008-6215(02)00010-1.
- Rezende, E. S. V., G. C. Lima, and M. M. V. Naves. 2021. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition (Burbank, Los Angeles County, Calif.) 89:111217. doi: 10.1016/j.nut.2021.111217.
- Rinninella, E., P. Raoul, M. Cintoni, F. Franceschi, G. A. D. Miggiano, A. Gasbarrini, and M. C. Mele. 2019. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7 (1):14. doi: 10.3390/microorganisms7010014.
- Rodriguez-Daza, M. C., E. C. Pulido-Mateos, J. Lupien-Meilleur, D. Guyonnet, Y. Desjardins, and D. Roy. 2021. Polyphenol-mediated gut microbiota modulation: Toward prebiotics and further. Frontiers in Nutrition 8:689456. doi: 10.3389/fnut.2021.689456.
- Rosa, M. C., M. R. S. Carmo, C. F. Balthazar, J. T. Guimarães, E. A. Esmerino, M. Q. Freitas, M. C. Silva, T. C. Pimentel, and A. G. Cruz. 2021. Dairy products with prebiotics: An overview of the health benefits, technological and sensory properties. International Dairy Journal 117:105009. doi: 10.1016/j.idairyj.2021.105009.
- Rufián-Henares, J. A., and S. P. de la Cueva. 2009. Antimicrobial activity of coffee melanoidins - A study of their metal-chelating properties. Journal of Agricultural and Food Chemistry 57 (2):432–8. doi: 10.1021/jf8027842.
- Sales, A. L., J. de Paula, C. M. Silva, A. Cruz, M. A. L. Miguel, and A. Farah. 2020. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food & Function 11 (2):1410–24. doi: 10.1039/c9fo02589h.
- Sandhu, K. V., E. Sherwin, H. Schellekens, C. Stanton, T. G. Dinan, and J. F. Cryan. 2017. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Translational Research: The Journal of Laboratory and Clinical Medicine 179:223–44. doi: 10.1016/j.trsl.2016.10.002.
- Santos, E. A. A., A. Schieber, and F. Weber. 2018. Site-specific hydrolysis of chlorogenic acids by selected Lactobacillus species. Food Research International (Ottawa, Ont.) 109:426–32. doi: 10.1016/j.foodres.2018.04.052.
- Santos, É. M. d., L. M. d. Macedo, L. L. Tundisi, J. A. Ataide, G. A. Camargo, R. C. Alves, M. B. P. P. Oliveira, and P. G. Mazzola. 2021. Coffee by-products in topical formulations: A review. Trends in Food Science & Technology 111:280–91. doi: 10.1016/j.tifs.2021.02.064.
- Sarghini, F., F. Marra, A. de Vivo, P. Vitaglione, G. Mauriello, D. Maresca, A. D. Troise, and E. Echeverria-Jaramillo. 2021. Acid hydrolysis of spent coffee grounds: Effects on possible prebiotic activity of oligosaccharides. Chemical and Biological Technologies in Agriculture 8 (1):67. doi: 10.1186/s40538-021-00262-3.
- Shi, A., T. Li, Y. Zheng, Y. Song, H. Wang, N. Wang, L. Dong, and H. Shi. 2021. Chlorogenic acid improves NAFLD by regulating gut microbiota and GLP-1. Frontiers in Pharmacology 12:693048. doi: 10.3389/fphar.2021.693048.
- Silvan, J. M., F. J. Morales, and F. Saura-Calixto. 2010. Conceptual study on maillardized dietary fiber in coffee. Journal of Agricultural and Food Chemistry 58 (23):12244–9. doi: 10.1021/jf102489u.
- Simoes, J., F. M. Nunes, M. R. Domingues, and M. A. Coimbra. 2013. Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments. Carbohydrate Polymers 97 (1):81–9. doi: 10.1016/j.carbpol.2013.04.067.
- Siridevi G. B., D. Havare, K. Basavaraj, and P. S. Murthy. 2019. Coffee starter microbiome and in-silico approach to improve Arabica coffee. LWT - Food Science and Technology 114:108382. doi: 10.1016/j.lwt.2019.108382.
- Soares, C. M., R. C. Alves, S. Casal, M. B. Oliveira, and J. O. Fernandes. 2010. Development and validation of a matrix solid-phase dispersion method to determine acrylamide in coffee and coffee substitutes. Journal of Food Science 75 (3):T57–T63. doi: 10.1111/j.1750-3841.2010.01545.x.
- Socała, K., U. Doboszewska, A. Szopa, A. Serefko, M. Włodarczyk, A. Zielińska, E. Poleszak, J. Fichna, and P. Wlaź. 2021. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacological Research 172:105840. doi: 10.1016/j.phrs.2021.105840.
- Song, J., N. Zhou, W. Ma, X. Gu, B. Chen, Y. Zeng, L. Yang, and M. Zhou. 2019. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food & Function 10 (5):2947–57. doi: 10.1039/c8fo02599a.
- Stalmach, A., H. Steiling, G. Williamson, and A. Crozier. 2010. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Archives of Biochemistry and Biophysics 501 (1):98–105. doi: 10.1016/j.abb.2010.03.005.
- Swann, O. G., M. Kilpatrick, M. Breslin, and W. H. Oddy. 2020. Dietary fiber and its associations with depression and inflammation. Nutrition Reviews 78 (5):394–411. doi: 10.1093/nutrit/nuz072.
- Swennen, K., C. M. Courtin, and J. A. Delcour. 2006. Non-digestible oligosaccharides with prebiotic properties. Critical Reviews in Food Science and Nutrition 46 (6):459–71. doi: 10.1080/10408390500215746.
- Tian, T., S. Freeman, M. Corey, J. B. German, and D. Barile. 2017. Chemical characterization of potentially prebiotic oligosaccharides in brewed coffee and spent coffee grounds. Journal of Agricultural and Food Chemistry 65 (13):2784–92. doi: 10.1021/acs.jafc.6b04716.
- Tian, T., S. Freeman, M. Corey, J. B. German, and D. Barile. 2018. Effect of roasting on oligosaccharide abundance in Arabica coffee beans. Journal of Agricultural and Food Chemistry 66 (38):10067–76. doi: 10.1021/acs.jafc.8b02641.
- Tores de la Cruz, S., A. Iriondo-DeHond, T. Herrera, Y. Lopez-Tofino, C. Galvez-Robleno, M. Prodanov, F. Velazquez-Escobar, R. Abalo, and M. D. del Castillo. 2019. An assessment of the bioactivity of coffee silverskin melanoidins. Foods 8 (2):68. doi: 10.3390/foods8020068.
- Tran, S. M., and M. H. Mohajeri. 2021. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients 13 (3):732. doi: 10.3390/nu13030732.
- Usta-Gorgun, B., and L. Yilmaz-Ersan. 2020. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electronic Journal of Biotechnology 47:29–35. doi: 10.1016/j.ejbt.2020.06.004.
- Vasconcelos, A. L. S., A. S. Franca, M. B. A. Glória, and J. C. F. Mendonça. 2007. A comparative study of chemical attributes and levels of amines in defective green and roasted coffee beans. Food Chemistry 101 (1):26–32. doi: 10.1016/j.foodchem.2005.12.049.
- Vazquez-Sanchez, K., N. Martinez-Saez, M. Rebollo-Hernanz, M. D. del Castillo, M. Gaytan-Martinez, and R. Campos-Vega. 2018. In vitro health promoting properties of antioxidant dietary fiber extracted from spent coffee (Coffee arabica L.) grounds. Food Chemistry 261:253–9. doi: 10.1016/j.foodchem.2018.04.064.
- Vera, C., A. Illanes, and C. Guerrero. 2021. Enzymatic production of prebiotic oligosaccharides. Current Opinion in Food Science 37:160–70. doi: 10.1016/j.cofs.2020.10.013.
- Wan, F., R. Zhong, M. Wang, Y. Zhou, Y. Chen, B. Yi, F. Hou, L. Liu, Y. Zhao, L. Chen, et al. 2021. Caffeic acid supplement alleviates colonic inflammation and oxidative stress potentially through improved gut microbiota community in mice. Frontiers in Microbiology 12:784211. doi: 10.3389/fmicb.2021.784211.
- Wang, Z., K. L. Lam, J. Hu, S. Ge, A. Zhou, B. Zheng, S. Zeng, and S. Lin. 2019. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Science & Nutrition 7 (2):579–88. doi: 10.1002/fsn3.868.
- Wen, L., C. Álvarez, Z. Zhang, M. M. Poojary, M. N. Lund, D.-W. Sun, and B. K. Tiwari. 2021. Optimisation and characterisation of protein extraction from coffee silverskin assisted by ultrasound or microwave techniques. Biomass Conversion and Biorefinery 11 (5):1575–85. doi: 10.1007/s13399-020-00712-2.
- Wieers, G., L. Belkhir, R. Enaud, S. Leclercq, J. M. P. de Foy, I. Dequenne, P. de Timary, and P. D. Cani. 2019. How probiotics affect the microbiota. Frontiers in Cellular and Infection Microbiology 9:454. doi: 10.3389/fcimb.2019.00454.
- Wongsiridetchai, C., W. Chiangkham, N. Khlaihiran, T. Sawangwan, P. Wongwathanarat, T. Charoenrat, and S. Chantorn. 2018. Alkaline pretreatment of spent coffee grounds for oligosaccharides production by mannanase from Bacillus sp. GA2(1). Agriculture and Natural Resources 52 (3):222–7. doi: 10.1016/j.anres.2018.09.012.
- Wongsiridetchai, C., V. Jonjaroen, T. Sawangwan, T. Charoenrat, and S. Chantorn. 2021. Evaluation of prebiotic mannooligosaccharides obtained from spent coffee grounds for nutraceutical application. LWT 148:111717. doi: 10.1016/j.lwt.2021.111717.
- Yang, J., Y. Hao, N. Li, C. Wang, and Y. Liu. 2021. Metabolic and microbial modulation of phenolic compounds from raspberry leaf extract under in vitro digestion and fermentation. International Journal of Food Science & Technology 56 (10):5168–77. doi: 10.1111/ijfs.15083.
- Yang, X. D., L. K. Wang, H. Y. Wu, and L. Jiao. 2018. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiology 18 (1):177. doi: 10.1186/s12871-018-0642-1.
- Ye, X., S. Shen, Z. Xu, Q. Zhuang, J. Xu, J. Wang, Z. Dong, and X. Wan. 2021. Sodium butyrate alleviates cholesterol gallstones by regulating bile acid metabolism. European Journal of Pharmacology 908:174341. doi: 10.1016/j.ejphar.2021.174341.
- Zhang, H., H. Zhang, A. D. Troise, and V. Fogliano. 2019. Melanoidins from coffee, cocoa, and bread are able to scavenge alpha-dicarbonyl compounds under simulated physiological conditions. Journal of Agricultural and Food Chemistry 67 (39):10921–9. doi: 10.1021/acs.jafc.9b03744.
- Zhang, P., H. Jiao, C. Wang, Y. Lin, and S. You. 2019. Chlorogenic acid ameliorates colitis and alters colonic microbiota in a mouse model of dextran sulfate sodium-induced colitis. Frontiers in Physiology 10:325. doi: 10.3389/fphys.2019.00325.