Abstract
Resuscitation promoting factors (Rpf) are peptidoglycan-hydrolyzing enzymes that are pivotal in the resuscitation of quiescent actinobacteria including Mycobacterium tuberculosis. From the published data, it is clear that Rpf are required for the resuscitation of non-replicating bacilli and pathogenesis in murine infection model of tuberculosis, although their direct influence on human Mycobacterium tuberculosis infection is ill-defined. In this review, we describe the progress in the understanding of the roles that Rpf play in human tuberculosis pathogenesis and importance of bacilli dependent upon Rpf for growth for the outcome of human tuberculosis. We outline how this research is opening up important opportunities for the diagnosis, treatment and prevention of human disease, progress in which is essential to attain the ultimate goal of tuberculosis eradication.
Introduction
Tuberculosis is a major global public health concern with an estimated 10.4 million incident cases and 1.4 million deaths in 2015 (WHO, Citation2016). Recent mathematical modeling predicted that as of 2014, 1.7 billion people were latently infected with Mycobacterium tuberculosis (Mtb) (Houben & Dodd, Citation2016). Mtb is transmitted primarily by respiratory droplet inhalation (Turner & Bothamley, Citation2015) and upon reaching the alveoli is phagocytosed by macrophages, neutrophils and dendritic cells (DCs) (Dorhoi & Kaufmann, Citation2014; Eum et al., Citation2010). Despite internalization, Mtb can continue to replicate intracellularly (Ahmad, Citation2011).
Protective immunity to Mtb requires a fine balance of innate and adaptive responses (Nunes-Alves et al., Citation2014). After initial infection, myeloid cells sense Mtb through cell surface receptors and facilitate T-helper1 (TH1) and T-helper17 (TH17) differentiation (Dorhoi & Kaufmann, Citation2014). TH1 cells promote inflammatory cell recruitment and enhance Mtb killing by macrophages (Nunes-Alves et al., Citation2014). TH17 cells are also important for protective immunity to Mtb, particularly in the early stages of infection (Gopal et al., Citation2014; Prezzemolo et al., Citation2014; van de Veerdonk et al., Citation2010). Macrophages and T-cells are key to granuloma formation, which ultimately controls primary infection but in 90% of cases fails to kill Mtb and provides a site for bacilli to latently infect the host (Ahmad, Citation2010; Dorhoi & Kaufmann, Citation2014; Ramakrishnan, Citation2012). At present, the physiological status of these bacteria is unknown, however it is hypothesized that conditions within granulomas may vary leading to heterogeneous populations consisting of replicating and non-replicating cells (Gideon & Flynn, Citation2011; Zhang et al., Citation2010). Moreover, differential killing of Mtb at the onset of adaptive immunity may lead to the formation of heterogeneous lesions, some of which may be sterilized and others may progress to a maximum Mtb burden (Lin et al., Citation2014). The precise fate of each lesion is probably influenced by multiple factors including the local environment, the physiological state of Mtb and the interplay of host immune factors.
In clinical medicine, latent tuberculosis infection (LTBI) is seen as a homogenous entity, characterized by evidence of immune sensitization to mycobacterial antigens with the absence of clinical and radiological signs of disease (Barry et al., Citation2009). The prevailing view, however, is that LTBI is best viewed as a range of infection states extending from infection eradicated by the host immune response to subclinical active infection, at particularly high risk of reactivation (Esmail et al., Citation2014). It is thought that bacillary replication is curtailed by the host immune response, however, if this balance is perturbed by factors such as immunosuppression due to human immunodeficiency virus (HIV) infection or anti-tumor necrosis factor therapy, bacillary replication resumes leading to reactivation and clinical disease (Paige & Bishai, Citation2010). The mechanisms by which bacterial growth restarts are not yet resolved, although it presumed to involve cell wall alterations mediated by enzymes termed resuscitation promoting factors (Rpf) (Kana & Mizrahi, Citation2010).
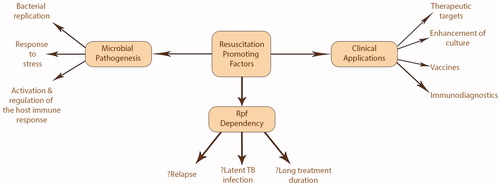
Rpf are a family of bacterial proteins, able to resuscitate non-replicating bacteria, was discovered in Micrococcus luteus (Mukamolova et al., Citation1998, Citation2002). Mtb possesses five rpf-like genes (rpf A–E) whose products exhibit similar biological activity to the Rpf from M. luteus (Mukamolova et al., Citation2002). The mechanism through which Rpf resuscitate “quiescent” Mtb is poorly understood although their enzymatic activity is essential for biological effects (Cohen-Gonsaud et al., Citation2005; Mukamolova et al., Citation2006) suggesting direct cell wall remodeling or muropeptide-mediated signaling (Kana & Mizrahi, Citation2010; Kaprelyants et al., Citation2012). The importance of Rpf in Mtb infection and the reactivation of chronic TB has been shown in mouse models and there is a growing body of evidence that Rpf play a central part in human tuberculosis (see ). In this review, we will discuss the current understanding of the role Rpf play in Mtb infection and how this knowledge may be applied to diagnose, treat, and prevent Mtb infection.
Rpf are critical for Mtb replication and reactivation in vivo
Rpf can be collectively deleted without significantly impairing Mtb growth in standard 7H9 medium (Kana et al., Citation2008), although this does not preclude a significant role in bacillary replication. Two studies characterizing the in vitro phenotype of rpf-deletion mutants in mononuclear phagocyte infection models produced discordant results. Russell-Goldman et al. demonstrated a ΔrpfAB (rpfA and rpfB deletion) mutant was attenuated for growth in murine macrophages (Russell-Goldman et al., Citation2008) whereas Kana et al. showed Mtb quadruple rpf deletion mutants were unimpaired for growth within human monocytes (Kana et al., Citation2008). Differences in the experimental set up between the studies may account for the discordant results. When the in vivo phenotype of rpf-deletion mutants are investigated in the mouse infection model, it is clear Rpf are important in the pathogenesis of infection (Kana & Mizrahi, Citation2010).
In mice, infection of the respiratory tract is characterized by an initial exponential growth phase lasting several weeks. The subsequent transition to chronic persistent infection is marked by the development of cell-mediated immunity which stabilizes bacillary replication (Hu et al., Citation2015; Munoz-Elias et al., Citation2005). Throughout this period, the expression of rpfA–rpfE can be detected (Kesavan et al., Citation2009; Tufariello et al., Citation2006). Although the precise role of Rpf is unclear, the study of rpf deletion mutants underscores the importance of Rpf in the initial stages of infection. When single rpf genes are deleted, there is no impact on the course of acute infection or the immunopathology witnessed, perhaps indicating physiological redundancy (Gupta & Srivastava, Citation2012). However, further deletion of rpf genes reduces Mtb virulence which manifests as reduced growth and dissemination from the site of primary infection (Biketov et al., Citation2007; Kana et al., Citation2008; Kondratieva et al., Citation2011; Russell-Goldman et al., Citation2008). Ultimately, the mortality of mice infected with attenuated rpf deletion mutants is lower (Kondratieva et al., Citation2011; Russell-Goldman et al., Citation2008).
Interestingly, when individual rpf gene(s) are deleted, the degree of attenuation varies implying Rpf are functionally distinct. In one study of mice infected with rpf double deletion mutants via the respiratory tract, only the ΔrpfAB mutant showed lower lung and spleen colony forming units (CFUs) versus the wild type Mtb strain (Russell-Goldman et al., Citation2008). This contrasts with an intraperitoneal model of infection where ΔrpfAC was more attenuated for growth than ΔrpfAB although differences in host, strain or experimental conditions may account for this (Biketov et al., Citation2007). With triple rpf deletion mutants, the loss of rpfB from a ΔrpfAC mutant was more attenuating for growth in the mouse lung than rpfD loss (Downing et al., Citation2005).
In chronic persistent infection, the ability of Mtb to maintain low-level replication also varies by rpf deletion. Single rpf deletion mutants do not exhibit persistence defects, however with the progressive deletion of rpf genes, replication is impaired. These mutants require more time to establish chronic infection and replicate less compared to the wild type background strain (Biketov et al., Citation2007; Kana et al., Citation2008; Russell-Goldman et al., Citation2008; Tufariello et al., Citation2006). Notably, the effect of individual rpf deletions varies with the loss of rpfD from the mutant ΔrpfACB less impactful than rpfE (Kana et al., Citation2008).
The diminished replication exhibited by rpf mutants during acute and chronic persistent infection has been attributed to cell wall changes (Russell-Goldman et al., Citation2008). In vitro studies demonstrate mutants are less resilient to stress as shown by hypersensitivity to the surfactant sodium dodecylsulfate and increased susceptibility to antibiotics (Heidrich et al., Citation2002; Kana et al., Citation2008; Wivagg & Hung, Citation2012). Putatively, deficiency of Rpf may alter cell wall structure, so increasing sensitivity to stresses such as those imposed by the host immune response. It may be that Rpf enable Mtb to respond to adverse conditions in vivo although the precise mechanisms and roles remain unclear.
The reactivation of LTBI can be modeled by the administration of aminoguanidine, an inducible nitric oxide synthase inhibitor, to mice with chronic persistent Mtb infection (Flynn et al., Citation1998). During the recrudescence of infection, the expression of rpfA–rpfE is detectable (Tufariello et al., Citation2004). Asides from rpfC, it has been shown that rpf expression mirrors the level of bacillary replication (Tufariello et al., Citation2006). When mice are treated with Rpf inhibitors, recrudescence is suppressed but active replication is unaffected suggesting Rpf are important for the initiation of Mtb replication (Kaprelyants et al., Citation2012). This hypothesis is supported by the impact of rpf deletion upon reactivation growth kinetics. When single rpf genes are deleted, only rpfB deficiency delays recrudescence (Tufariello et al., Citation2006). Progressive rpf deletion reduces the rise in CFU counts with immunosuppression with triple rpf deletion mutants exhibiting no rise at all (Biketov et al., Citation2007; Russell-Goldman et al., Citation2008).
When the available evidence is summarized, it suggests Rpf are significant virulence factors with roles in bacillary replication during acute disease and reactivation of chronic persistent infection. At this moment, the exact function and regulation of individual Rpf during infection is unknown and awaits further investigation.
Rpf generate protective immunity
As Rpf are secreted or cell wall attached proteins, they are potential targets for the host immune system (Commandeur et al., Citation2013; Kana & Mizrahi, Citation2010; Yeremeev et al., Citation2003). As previously mentioned, Rpf are key virulence factors for Mtb (Kana et al., Citation2008), thus responses directed to Rpf may be protective. These have been assessed both in cellular assays and mouse models.
In vitro studies have concentrated on the immune responses to RpfB and RpfE proteins. Both induce the maturation of DCs via the Toll-like receptor 4 (Choi et al., Citation2015; Kim et al., Citation2013). Subsequently, activated DCs produce IL-12p70, which polarizes T-cell proliferation toward TH1 phenotype (Choi et al., Citation2015; Kim et al., Citation2013). RpfE also induces DC IL-23p19 secretion leading to TH17 development, which in addition to TH1 is required for optimal protection against Mtb (Gopal et al., Citation2014). The induction of IL-23p17 secretion by RpfB was not tested. Of note, RpfE does not induce antigen reactive T regulatory cells, which can suppress T-cell proliferation in response to Mtb antigens by IL-10 production (Choi et al., Citation2015). These findings show RpfB and RpfE induce what are considered to be protective immune responses in vitro and support their role as potential vaccine candidates (Choi et al., Citation2015; Kim et al., Citation2013).
The character of the immune response generated in vivo by Rpf antigens and their protective efficacy against challenge with virulent Mtb has been assessed in mouse models. The studies have taken different approaches to vaccination focusing predominantly upon RpfB and RpfE. To date immunization with RpfB has been performed using a plasmid DNA (pDNA) vector approach. When C57BL/6 mice were vaccinated intramuscularly, a significant polyfunctional T-cell response, particularly in CD8+ subsets was measured, suggesting the vaccine may be protective (Lee et al., Citation2014). Administration of a similar rpfB pDNA vaccine via the intramuscular route offered some protection against challenge with virulent Mtb, as shown by a moderate reduction in pulmonary and splenic burden compared to the control group. However, the vaccine was less efficacious than the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine (Romano et al., Citation2012). At 10 weeks post infection, the rpfB pDNA vaccine offered no protection possibly related to reduced rpfB expression hampering the protective action of the RpfB-specific T-cells (Romano et al., Citation2012).
Different vaccine approaches have been tried for RpfE with mixed results. The vaccination of C57BL/6 mice with a construct of RpfE fused to early secretory antigen 6 (ESAT-6) was highly immunogenic generating RpfE-specific IFNγ secreting T-cells (Xin et al., Citation2013). However, when challenged with virulent Mtb, no reduction in lung or spleen CFUs over those receiving the phosphate buffered saline control was seen (Xin et al., Citation2013). In another study, C57BL/6JCit mice were vaccinated subcutaneously with recombinant RpfE. Following exposure to virulent Mtb, they exhibited significantly prolonged survival times and lower lung and spleen CFUs compared to unvaccinated mice. The level of protection demonstrated by RpfE was comparable with ESAT-6 and Ag85B (Yeremeev et al., Citation2003).
The immune response to the other Rpf proteins has been less extensively studied. Immunization of C57BL/6 mice with an rpfD pDNA vaccine was disappointing eliciting only a minor IFNγ response (Romano et al., Citation2012). Pilot data described in a paper by Romano and colleagues suggest that rpfA and rpfC pDNA vaccines may induce significant humoral and cellular responses (Romano et al., Citation2012). Further study to determine the immunogenicity and protection against challenge with Mtb seems warranted to see if these Rpf are potential vaccine candidates.
In summary, RpfB and RpfE induce immune responses associated with resistance to Mtb and provide some protection in mouse models. The lack of standardization of vaccination approaches, use of different Mtb strains and mouse models complicates the interpretation of the data generated. Further human studies are required to determine whether the findings are replicated in humans and if Rpf should be considered for development as antigens in a subunit vaccine against Mtb.
Rpf expression in human infection
Akin to the findings from animal models, samples taken from patients with active tuberculosis demonstrate the transcription and expression of Rpf. Rachman and colleagues examined the transcriptome of Mtb isolated from human pulmonary tissue and found rpfA and rpfB transcripts (Rachman et al., Citation2006). The expression of Rpf in human infection was confirmed by Davies and colleagues by the application of immunohistochemistry performed on gut lymphoid tissue with anti-Rpf antibodies. The presence of Rpf in the vicinity of Mtb bacilli was established in areas of necrosis, in epithelioid/giant cells and in macrophages surrounding necrotic areas (Davies et al., Citation2008). However, the limitations of the method do not permit any conclusions to be drawn about the expression of specific Rpf during different phases of infection.
Studies have confirmed that Rpf are immunogenic in humans generating significant immune responses in both patients with active and latent disease (Chegou et al., Citation2012; Kassa et al., Citation2012; Riano et al., Citation2012). All Rpf proteins appear to be immunogenic with significant T-cell responses demonstrable in LTBI patients using interferon gamma release assays (IGRAs) (Commandeur et al., Citation2011; Geluk et al., Citation2014; Huang et al., Citation2013; Riano et al., Citation2012; Schuck et al., Citation2009). Whether immune responses to Rpf are protective or not is unclear. In a study of long term latently infected patients who did not develop tuberculosis, significant single and double cytokine producing CD4+ and CD8+ T-cells specific to RpfA were detected (Commandeur et al., Citation2011). Two studies showed that in patients with pulmonary tuberculosis, RpfA, B, D&E induced a significantly lower proportion of IFNγ producing CD4+ T-cells compared to those with LTBI (Riano et al., Citation2012; Schuck et al., Citation2009). Collectively this indirect evidence suggests that responses directed to Rpf antigens may strengthen immune surveillance in LTBI and prevent reactivation. This remains a hypothetical premise as a role of Rpf in Mtb reactivation in humans has not yet been verified.
In summary, this evidence suggests Rpf are produced during human Mtb infection and recognized by the host immune system, potentially leading to protective immune response. The data should, however, be interpreted with caution. The observed T-cell responses to Rpf antigens could simply represent ongoing exposure to environmental bacteria producing Rpf-like proteins (Commandeur et al., Citation2011). Further work to understand the role of Rpf in human infection may ultimately be therapeutically useful.
Rpf-dependency phenomenon
Rpf-dependent bacilli are those which cannot be cultured in standard media, requiring Rpf to initiate growth (Mukamolova et al., Citation2010; Shleeva et al., Citation2002). Exogenous Rpf can be provided in the form of recombinant Rpf proteins or Rpf-containing culture supernatant (Mukamolova et al., Citation2010). The populations of cells recovered in culture following supplementation by either sources of Rpf may be synonymous (Turapov et al., Citation2016), although recently it was shown that culture supernatant from a quintuple rpf deletion mutant exhibited resuscitation activity (Chengalroyen et al., Citation2016), possibly related to other factors such as cAMP (Shleeva et al., Citation2013), muropeptides (Nikitushkin et al., Citation2013), and lipids (Zhang et al., Citation2001) which are known to modulate the growth of non-replicating mycobacteria. It is unknown whether populations dependent upon Rpf-containing culture supernatant or recombinant Rpf exhibit phenotypic differences. For the purposes of this review, they will be considered interchangeable.
In vitro Rpf-dependency can be induced by prolonged stationary growth phase culture, exposure to anti-tuberculous drugs, and gradual external acidification of culture media (Loraine et al., Citation2016; Mukamolova et al., Citation2002; Shleeva et al., Citation2011). Rpf-dependent mycobacteria are also generated in vivo, however the in vivo triggers for Rpf-dependency are as yet unresolved (Hu et al., Citation2015; Mukamolova et al., Citation2010; Turapov et al., Citation2014). Rpf-dependent mycobacteria have been grown from patients’ sputum (Chengalroyen et al., Citation2016; Mukamolova et al., Citation2010) and extrapulmonary samples (O'Connor et al., Citation2015). Moreover, Rpf-dependent bacilli frequently make up the vast majority of the sample population, however, some sputum samples do not contain detectable Rpf-dependent Mtb (Chengalroyen et al., Citation2016; Mukamolova et al., Citation2010).
Variation in the proportion of Rpf-dependency between individuals implicates host factors in its formation (Turapov et al., Citation2014). At present, the precise mechanism(s) by which Rpf-dependency is generated are unknown although recent studies have shed some light. When Rpf-dependent Mtb and M. bovis BCG were isolated from sputum and mouse lung homogenates, respectively, subsequent exposure of mycobacteria to these did not induce Rpf-dependency (Mukamolova et al., Citation2010; Turapov et al., Citation2014) so suggesting contact with the pulmonary environment itself is not the critical factor. More insight came from studying the dynamics of Rpf-dependency development, which indicates a potential role for the adaptive immune response (Turapov et al., Citation2014). When the lung CFU counts were measured at 24 h post infection of mice with M. bovis BCG Glaxo, no significant Rpf-dependent population was present, however, by 2 weeks Rpf-dependency had developed and persisted thereafter (Turapov et al., Citation2014). Notably, this correlates temporarily with the development of the adaptive immune response in mice (Vallerskog et al., Citation2010). When mycobacteria were incubated in mouse serum, which contains complement and immunoglobulins, Rpf-dependency was not generated, indicating the cellular arm of the adaptive immune response may be responsible (Hood et al., Citation2005; Turapov et al., Citation2014). The possible link of Rpf-dependency with the cellular immune response was illustrated also by the study of Mtb cells isolated from macrophages (Biketov et al., Citation2000) and the observation that HIV patients with CD4 T-cell counts >200 cells mm−3 or who were HIV negative had comparatively higher levels of cells dependent upon Rpf-containing culture supernatant for growth (Chengalroyen et al., Citation2016). At present, the mechanism by which the immune system may induce Rpf-dependency remains to be elucidated although immune-related stresses placed upon tubercule bacilli during infection may play a role. This area warrants further investigation as an understanding of how the immune system may induce Rpf-dependency could allow us to modulate the response so avoiding Rpf-dependent bacilli and their potential negative consequences for patients.
Rpf-dependent bacilli appear to represent a significant hurdle to the treatment of tuberculosis as they may underlie relapse and the need for prolonged chemotherapy for clinical cure. The putative role in relapse was illustrated in a study by Hu and colleagues where the eradication of Rpf-dependent Mtb bacilli in a Cornell mouse model with high dose rifampicin prevented relapse (Hu et al., Citation2015). Currently, there is no human data to confirm these findings. Of late, studies on sputum have demonstrated populations of Rpf-dependent Mtb bacilli that are tolerant to the action of rifampicin, isoniazid, and streptomycin and are low to clear from sputum (Mukamolova et al., Citation2010; Turapov et al., Citation2016). It may be that clearance is required for cure although further studies are needed to verify this. If this hypothesis is born out, quantification of Rpf-dependent bacilli during therapy may have potential use as a biomarker of response to treatment and better predict the efficacy of potential drug candidates (Turapov et al., Citation2016). Anti-tuberculous therapy (ATT) could be personalized so minimizing treatment duration without increasing relapse risk.
Application of Rpf to improve TB diagnosis, treatment and prevention
An understanding of the biology of Rpf and Rpf-dependency has opened up new potential avenues to improve upon mycobacterial culture, immune based diagnostics, vaccination and treatment approaches. These areas are expounded on below.
Immunodiagnostics
One of the most noteworthy developments in immunodiagnostic testing for Mtb infection was the development of IGRAs. IGRAs measure the cell mediated immune response by quantifying the IFNγ induced by microbial antigens (Darby et al., Citation2014). Current commercial IGRAs such as QuantiFERON® TB Gold In-Tube (Qiagen, Hilden, Germany) and T-SPOT® (Oxford Immunotec, Abingdon, UK) use culture filtrate protein 10 kDa (CFP-10) and ESAT-6 to detect exposure to Mtb (Pai et al., Citation2014). They are able to diagnose LTBI with a sensitivity of up to 90% and are unaffected by BCG vaccination status (Darby et al., Citation2014). These IGRAs are however limited to this narrow remit. They are unable to differentiate active from latent TB and cannot predict patients at high risk of progression to active disease who would most benefit from chemopreventative therapy (Pai et al., Citation2014).
By quantifying the production of IFNγ in response to stimulation by Rpf antigens, authors have shown they can differentiate between Mtb disease states potentially overcoming these limitations. In a non-endemic setting, Serra-Vidal and colleagues showed RpfD could distinguish uninfected, LTBI and active Mtb cases (Serra-Vidal et al., Citation2014). In a high burden endemic setting, where the usefulness of commercial IGRAs is limited by high LTBI rates, Chegou and colleagues showed RpfA–E performed well and could distinguish those with and without active tuberculosis (Chegou et al., Citation2012). Interestingly, Huang and colleagues showed RpfD was a highly specific antigen, even in an endemic setting, and was able to separate household contacts of active tuberculosis cases from community exposed persons (Huang et al., Citation2013). This is an important finding as household contacts are at high risk, particularly within the first year of developing active Mtb (Fox et al., Citation2013). Collectively these studies indicate that Rpf IGRAs may overcome the limitations of current commercial IGRAs although as the response to Rpf antigens can vary by location (Sutherland et al., Citation2013), these studies should be replicated in different settings. Furthermore, given the exposure of individuals to Rpf through, for example, BCG vaccination or nontuberculous mycobacterial disease, the diagnostic performance of Rpf IGRAs should be verified in these populations.
Mycobacterial culture
The successful culture of Mtb from patient samples is an important component of clinical management (Tsara et al., Citation2009). It enables drug susceptibility testing, typing for outbreak investigations and advanced “omic” studies (Asmar & Drancourt, Citation2015; Hayer et al., Citation2013; Ryu, Citation2015). Despite advances in the molecular detection of Mtb, culture remains the gold standard for diagnosis (Asmar & Drancourt, Citation2015). It is more sensitive and is unencumbered by dead bacilli (Asmar & Drancourt, Citation2015). Nevertheless, culture sensitivity can be low, particularly for extrapulmonary samples (Sandgren et al., Citation2013), so improving culture performance would have undoubted clinical benefits.
The addition of Rpf to standard culture either as recombinant proteins or in culture supernatant is one potential approach. Mukamolova and colleagues showed Rpf could increase the yield of sputum culture (Mukamolova et al., Citation2010). In some samples, Rpf enabled the recovery of Mtb that would otherwise be negative by standard culture (Chengalroyen et al., Citation2016; Mukamolova et al., Citation2010). This was also demonstrated in one extrapulmonary tuberculosis case (O'Connor et al., Citation2015). Although highly preliminary, these findings suggest Rpf may improve culture performance.
One major drawback of tuberculosis culture is the slow growth of Mtb (Ghodbane et al., Citation2014). While recent advances have made the culture of Mtb in less than 48 h a possibility (Ghodbane et al., Citation2015), currently these techniques are technologically sophisticated and limited to reference laboratory/research settings. In comparison, Rpf may offer a simpler method of speeding up tuberculosis culture. This was illustrated by Wu and colleagues who demonstrated that recombinant RpfB added to mycobacterial growth indicator tubes (MGITs) improved the time-to-positivity of low inocula of quiescent Mtb H37Rv (Wu et al., Citation2008). Huang and colleagues showed the effect was replicable in clinical samples with both RpfB and RpfE reducing the time-to-positivity of sputum cultures that were positive after more than 20 days by standard culture methods (Huang et al., Citation2014).
These preliminary studies suggest Rpf supplementation of standard culture could improve time to detection and sensitivity. The veracity of these initial findings should be tested in a prospective clinical diagnostic study. As Rpf are unstable and difficult to produce (Mukamolova et al., Citation2010), work to produce an easy-to-use, durable form should be pursued.
Vaccination
The availability of an effective vaccine would greatly enhance progress toward the END TB goal of less than 10 cases per 100 000 per year by 2035 (Fletcher & Schrager, Citation2016). Modeling indicates that mass immunization of those with LTBI, particular adolescents and young adults who are the main sources of TB transmission, would have the most impact on new infections (Evans et al., Citation2013; Fletcher & Schrager, Citation2016). At present, the only licensed vaccine is BCG which was introduced in 1921 (Fletcher & Schrager, Citation2016; Kaufmann, Citation2014). While it prevents severe disease in younger children, in older children and adults its protection is limited and further boosting with BCG is unable to extend this (da Costa et al., Citation2015; Doherty et al., Citation2007; Roy et al., Citation2014). One promising alternative approach is a subunit vaccine consisting of key Mtb antigens (Xin et al., Citation2013). Rpf are strong contenders as they are appropriately immunogenic and may generate durable responses enabling long term control of LTBI (Choi et al., Citation2015; Geluk et al., Citation2014; Kim et al., Citation2013). Combined with latency antigens, they could induce host immune responses that eliminate quiescent Mtb as well as inhibiting Mtb reactivation (Geluk et al., Citation2014; Huang et al., Citation2013), so reducing the reservoir of infection as well as transition to clinical disease. It should be noted that in theory the vaccination of LTBI patients with Rpf could induce reactivation (Zhao et al., Citation2015). This potential risk should be investigated before widespread use is considered.
Treatment
In the era of increasing antibiotic resistance, targeting of bacterial virulence factors is being seen as a possible treatment strategy (Lynch & Wiener-Kronish, Citation2008). Agents antagonizing virulence factors may be useful as adjuncts to antibiotics or as therapies in their own right (Hauser et al., Citation2016). In Mtb, Rpf represent inviting targets for intervention. They are important virulence factors, they lack human homologs ensuring a high degree of drug specificity and their extracellular nature means small molecule inhibitors do not need to enter the cell for biological activity (Kana et al., Citation2008; Machowski et al., Citation2014). Inhibitors of Rpf, such as 2-nitrophenylthiocyanates, could be given to patients with LTBI who need treatment with anti-TNFα inhibitors to prevent the reactivation of tuberculosis (Demina et al., Citation2009; Kaprelyants et al., Citation2012). Contrarily, Rpf could be administered to patients with active infection, reactivating and sensitizing quiescent bacilli which are thought to be responsible for the requirement for long treatment courses so shortening the duration of treatment (Davies et al., Citation2008; Zhang et al., Citation2010). It is also possible that by interfering with the host immune response to minimize pathological host responses or to boost innate immunity (Nathan, Citation2012), the development of Rpf-dependency may be prevented. While these approaches are at the conceptual stage, the ability to control entry into and exit from non-replicating states could be therapeutically powerful and merit further research to determine their feasibility.
Conclusions
Substantial progress has been made understanding the biology of Rpf, illuminating the crucial role they play in tuberculosis pathogenesis (see ). Their immunogenicity and lack of human homologs make Rpf ideal targets for therapeutic and preventative interventions. The use of Rpf inhibitors as well as booster vaccines incorporating Rpf could significantly impact upon LTBI reactivation, reducing clinical cases and transmission of infection. Rpf-dependent populations appear to underlie the difficulty we have in improving TB treatment, yet their presence appears to be driven by both treatment and host factors. A greater understanding of the mechanisms underlying their genesis may yield novel approaches to improve treatment. Furthermore, quantification of the dynamics of the Rpf-dependent population during infection and treatment could predict individual patient outcomes as well as assess the efficacy of new drug candidates. Currently, this is technically demanding and would be greatly enhanced by the development of proxy biomarker. Future studies are required to test whether these scientific findings translate into successful diagnostic and therapeutic modalities to tackle our ancient scourge.
Disclosure statement
The authors report no declarations of interest.
Additional information
Funding
References
- Ahmad S. (2010). New approaches in the diagnosis and treatment of latent tuberculosis infection. Respir Res 11:169.
- Ahmad S. (2011). Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol 2011:814943.
- Asmar S, Drancourt M. (2015). Rapid culture-based diagnosis of pulmonary tuberculosis in developed and developing countries. Front Microbiol 6:1184.
- Barry CE, 3rd, Boshoff HI, Dartois V, et al. (2009). The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7:845–55.
- Biketov S, Mukamolova GV, Potapov V, et al. (2000). Culturability of Mycobacterium tuberculosis cells isolated from murine macrophages: a bacterial growth factor promotes recovery. FEMS Immunol Med Microbiol 29:233–40.
- Biketov S, Potapov V, Ganina E, et al. (2007). The role of resuscitation promoting factors in pathogenesis and reactivation of Mycobacterium tuberculosis during intra-peritoneal infection in mice. BMC Infect Dis 7:146.
- Chegou NN, Black GF, Loxton AG, et al. (2012). Potential of novel Mycobacterium tuberculosis infection phase-dependent antigens in the diagnosis of TB disease in a high burden setting. BMC Infect Dis 12:10.
- Chengalroyen MD, Beukes GM, Gordhan BG, et al. (2016). Detection and quantification of differentially culturable tubercle bacteria in sputum from tuberculosis patients. Am J Respir Crit Care Med 194:1532–40.
- Choi HG, Kim WS, Back YW, et al. (2015). Mycobacterium tuberculosis RpfE promotes simultaneous Th1- and Th17-type T-cell immunity via TLR4-dependent maturation of dendritic cells. Eur J Immunol 45:1957–71.
- Cohen-Gonsaud M, Barthe P, Bagneris C, et al. (2005). The structure of a resuscitation-promoting factor domain from Mycobacterium tuberculosis shows homology to lysozymes. Nat Struct Mol Biol 12:270–3.
- Commandeur S, van Meijgaarden KE, Lin MY, et al. (2011). Identification of human T-cell responses to Mycobacterium tuberculosis resuscitation-promoting factors in long-term latently infected individuals. Clin Vac Immunol: CVI 18:676–83.
- Commandeur S, van Meijgaarden KE, Prins C, et al. (2013). An unbiased genome-wide Mycobacterium tuberculosis gene expression approach to discover antigens targeted by human T cells expressed during pulmonary infection. J Immunol (Baltimore, MD: 1950) 190:1659–71.
- da Costa C, Walker B, Bonavia A. (2015). Tuberculosis vaccines – state of the art, and novel approaches to vaccine development. Int J Infect Dis: IJID 32:5–12.
- Darby J, Black J, Buising K. (2014). Interferon-gamma release assays and the diagnosis of tuberculosis: have they found their place? Intern Med J 44:624–32.
- Davies AP, Dhillon AP, Young M, et al. (2008). Resuscitation-promoting factors are expressed in Mycobacterium tuberculosis-infected human tissue. Tuberculosis (Edinburgh, Scotland) 88:462–8.
- Demina GR, Makarov VA, Nikitushkin VD, et al. (2009). Finding of the low molecular weight inhibitors of resuscitation promoting factor enzymatic and resuscitation activity. PLoS One 4:e8174.
- Doherty TM, Dietrich J, Billeskov R. (2007). Tuberculosis subunit vaccines: from basic science to clinical testing. Expert Opin Biol Ther 7:1539–49.
- Dorhoi A, Kaufmann SH. (2014). Perspectives on host adaptation in response to Mycobacterium tuberculosis: modulation of inflammation. Semin Immunol 26:533–42.
- Downing KJ, Mischenko VV, Shleeva MO, et al. (2005). Mutants of Mycobacterium tuberculosis lacking three of the five rpf-like genes are defective for growth in vivo and for resuscitation in vitro. Infect Immun 73:3038–43.
- Esmail H, Barry CE, 3rd, Young DB, Wilkinson RJ. (2014). The ongoing challenge of latent tuberculosis. Phil Trans R Soc Lond B Biol Sci 369:20130437.
- Eum SY, Kong JH, Hong MS, et al. (2010). Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137:122–8.
- Evans TG, Brennan MJ, Barker L, Thole J. (2013). Preventive vaccines for tuberculosis. Vaccine 31:B223–6.
- Fletcher HA, Schrager L. (2016). TB vaccine development and the end TB strategy: importance and current status. Trans R Soc Trop Med Hyg 110:212–18.
- Flynn JL, Scanga CA, Tanaka KE, Chan J. (1998). Effects of aminoguanidine on latent murine tuberculosis. J Immunol 160:1796–803.
- Fox GJ, Barry SE, Britton WJ, Marks GB. (2013). Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 41:140–56.
- Geluk A, van Meijgaarden KE, Joosten SA, et al. (2014). Innovative strategies to identify M. tuberculosis antigens and epitopes using genome-wide analyses. Front Immunol 5:256.
- Ghodbane R, Asmar S, Betzner M, et al. (2015). Rapid diagnosis of tuberculosis by real-time high-resolution imaging of Mycobacterium tuberculosis colonies. J Clin Microbiol 53:2693–6.
- Ghodbane R, Raoult D, Drancourt M. (2014). Dramatic reduction of culture time of Mycobacterium tuberculosis. Sci Rep 4:4236.
- Gideon HP, Flynn JL. (2011). Latent tuberculosis: what the host “sees?” Immunol Res 50:202–12.
- Gopal R, Monin L, Slight S, et al. (2014). Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathogens 10:e1004099.
- Gupta RK, Srivastava R. (2012). Resuscitation promoting factors: a family of microbial proteins in survival and resuscitation of dormant mycobacteria. Indian J Microbiol 52:114–21.
- Hauser AR, Mecsas J, Moir DT. (2016). Beyond antibiotics: new therapeutic approaches for bacterial infections. Clin Infect Dis 63:89–95.
- Hayer KS, Sitch AJ, Dedicoat M, Wood AL. (2013). Culture confirmation of tuberculosis cases in Birmingham, UK. Scand J Infect Dis 45:746–51.
- Heidrich C, Ursinus A, Berger J, et al. (2002). Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J Bacteriol 184:6093–9.
- Hood BL, Zhou M, Chan KC, et al. (2005). Investigation of the mouse serum proteome. J Proteome Res 4:1561–8.
- Houben RM, Dodd PJ. (2016). The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS One 13:e1002152.
- Hu Y, Liu A, Ortega-Muro F, et al. (2015). High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 6:641.
- Huang W, Qi Y, Diao Y, et al. (2014). Use of resuscitation-promoting factor proteins improves the sensitivity of culture-based tuberculosis testing in special samples. Am J Respir Crit Care Med 189:612–14.
- Huang W, Qi Y, Ren C, et al. (2013). Interferon-gamma responses to Mycobacterium tuberculosis Rpf proteins in contact investigation. Tuberculosis (Edinburgh, Scotland) 93:612–17.
- Kana BD, Gordhan BG, Downing KJ, et al. (2008). The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol Microbiol 67:672–84.
- Kana BD, Mizrahi V. (2010). Resuscitation-promoting factors as lytic enzymes for bacterial growth and signaling. FEMS Immunol Med Microbiol 58:39–50.
- Kaprelyants AS, Mukamolova GV, Ruggiero A, et al. (2012). Resuscitation-promoting factors (Rpf): in search of inhibitors. Protein Peptide Lett 19:1026–34.
- Kassa D, Ran L, Geberemeskel W, et al. (2012). Analysis of immune responses against a wide range of Mycobacterium tuberculosis antigens in patients with active pulmonary tuberculosis. Clin Vac Immunol: CVI 19:1907–15.
- Kaufmann SH. (2014). Tuberculosis vaccine development at a divide. Curr Opin Pulmon Med 20:294–300.
- Kesavan AK, Brooks M, Tufariello J, et al. (2009). Tuberculosis genes expressed during persistence and reactivation in the resistant rabbit model. Tuberculosis (Edinburgh, Scotland) 89:17–21.
- Kim JS, Kim WS, Choi HG, et al. (2013). Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J Leuk Biol 94:733–49.
- Kondratieva T, Rubakova E, Kana BD, et al. (2011). Mycobacterium tuberculosis attenuated by multiple deletions of rpf genes effectively protects mice against TB infection. Tuberculosis (Edinburgh, Scotland) 91:219–23.
- Lee J, Kim J, Shin SJ, Shin EC. (2014). DNA immunization of Mycobacterium tuberculosis resuscitation-promoting factor B elicits polyfunctional CD8(+) T cell responses. Clin Exp Vac Res 3:235–43.
- Lin PL, Ford CB, Coleman MT, et al. (2014). Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat Med 20:75–9.
- Loraine J, Pu F, Turapov O, Mukamolova GV. (2016). Development of an in vitro assay for detection of drug induced resuscitation-promoting factor dependent mycobacteria. Antimicrob Agents Chemother 60:6227–33.
- Lynch SV, Wiener-Kronish JP. (2008). Novel strategies to combat bacterial virulence. Curr Opin Crit Care 14:593–9.
- Machowski EE, Senzani S, Ealand C, Kana BD. (2014). Comparative genomics for mycobacterial peptidoglycan remodelling enzymes reveals extensive genetic multiplicity. BMC Microbiol 14:75.
- Mukamolova GV, Kaprelyants AS, Young DI, et al. (1998). A bacterial cytokine. Proc Natl Acad Sci USA 95:8916–21.
- Mukamolova GV, Murzin AG, Salina EG, et al. (2006). Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation. Mol Microbiol 59:84–98.
- Mukamolova GV, Turapov O, Malkin J, et al. (2010). Resuscitation-promoting factors reveal an occult population of tubercle Bacilli in Sputum. Am J Respir Crit Care Med 181:174–80.
- Mukamolova GV, Turapov OA, Young DI, et al. (2002). A family of autocrine growth factors in Mycobacterium tuberculosis. Mol Microbiol 46:623–35.
- Munoz-Elias EJ, Timm J, Botha T, et al. (2005). Replication dynamics of Mycobacterium tuberculosis in chronically infected mice. Infect Immun 73:546–51.
- Nathan C. (2012). Fresh approaches to anti-infective therapies. Sci Transl Med 4:140sr142.
- Nikitushkin VD, Demina GR, Shleeva MO, Kaprelyants AS. (2013). Peptidoglycan fragments stimulate resuscitation of “non-culturable” mycobacteria. Anton Van Leeuwen 103:37–46.
- Nunes-Alves C, Booty MG, Carpenter SM, et al. (2014). In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol 12:289–99.
- O'Connor BD, Woltmann G, Patel H, et al. 2015. Can resuscitation-promoting factors be used to improve culture rates of extra-pulmonary tuberculosis? Int J Tuberculosis Lung Dis 19:1556–1557.
- Pai M, Denkinger CM, Kik SV, et al. (2014). Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20.
- Paige C, Bishai WR. (2010). Penitentiary or penthouse condo: the tuberculous granuloma from the microbe's point of view. Cell Microbiol 12:301–9.
- Prezzemolo T, Guggino G, La Manna MP, et al. (2014). Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol 5:180.
- Rachman H, Strong M, Ulrichs T, et al. (2006). Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect Immun 74:1233–42.
- Ramakrishnan L. (2012). Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 12:352–66.
- Riano F, Arroyo L, Paris S, et al. (2012). T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia. Tuberculosis (Edinburgh, Scotland) 92:148–59.
- Romano M, Aryan E, Korf H, et al. (2012). Potential of Mycobacterium tuberculosis resuscitation-promoting factors as antigens in novel tuberculosis sub-unit vaccines. Microb Infect/Institut Pasteur 14:86–95.
- Roy A, Eisenhut M, Harris RJ, et al. (2014). Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 349:g4643.
- Russell-Goldman E, Xu J, Wang X, et al. (2008). A Mycobacterium tuberculosis Rpf double-knockout strain exhibits profound defects in reactivation from chronic tuberculosis and innate immunity phenotypes. Infect Immun 76:4269–81.
- Ryu YJ. (2015). Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberculosis Respir Dis 78:64–71.
- Sandgren A, Hollo V, van der Werf MJ. (2013). Extrapulmonary tuberculosis in the European Union and European Economic Area, 2002 to 2011. Euro Surveill: Bull Eur sur les maladies transmissibles = Eur Commun Dis Bull 18. Available from: http://www.eurosurveillance.org/images/dynamic/EE/V18N12/art20431.pdf
- Schuck SD, Mueller H, Kunitz F, et al. (2009). Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS One 4:e5590.
- Serra-Vidal MM, Latorre I, Franken KL, et al. (2014). Immunogenicity of 60 novel latency-related antigens of Mycobacterium tuberculosis. Front Microbiol 5:517.
- Shleeva M, Goncharenko A, Kudykina Y, et al. (2013). Cyclic AMP-dependent resuscitation of dormant Mycobacteria by exogenous free fatty acids. PLoS One 8:e82914.
- Shleeva MO, Bagramyan K, Telkov MV, et al. (2002). Formation and resuscitation of “non-culturable” cells of Rhodococcus rhodochrous and Mycobacterium tuberculosis in prolonged stationary phase. Microbiology (Reading, England) 148:1581–91.
- Shleeva MO, Kudykina YK, Vostroknutova GN, et al. (2011). Dormant ovoid cells of Mycobacterium tuberculosis are formed in response to gradual external acidification. Tuberculosis (Edinburgh, Scotland) 91:146–54.
- Sutherland JS, Lalor MK, Black GF, et al. (2013). Analysis of host responses to Mycobacterium tuberculosis antigens in a multi-site study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS One 8:e74080.
- Tsara V, Serasli E, Christaki P. (2009). Problems in diagnosis and treatment of tuberculosis infection. Hippokratia 13:20–2.
- Tufariello JM, Jacobs WR, Jr, Chan J. (2004). Individual Mycobacterium tuberculosis resuscitation-promoting factor homologues are dispensable for growth in vitro and in vivo. Infect Immun 72:515–26.
- Tufariello JM, Mi K, Xu J, et al. (2006). Deletion of the Mycobacterium tuberculosis resuscitation-promoting factor Rv1009 gene results in delayed reactivation from chronic tuberculosis. Infect Immun 74:2985–95.
- Turapov O, Glenn S, Kana B, et al. (2014). The in vivo environment accelerates generation of resuscitation-promoting factor-dependent mycobacteria. Am J Respir Crit Care Med 190:1455–7.
- Turapov O, O'Connor BD, Sarybaeva AA, et al. (2016). Phenotypically adapted Mycobacterium tuberculosis populations from sputum are tolerant to first-line drugs. Antimicrob Agents Chemother 60:2476–83.
- Turner RD, Bothamley GH. (2015). Cough and the transmission of tuberculosis. J Infect Dis 211:1367–72.
- Vallerskog T, Martens GW, Kornfeld H. (2010). Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol (Baltimore, MD: 1950) 184:6275–82.
- van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, et al. (2010). Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leuk Biol 88:227–32.
- WHO. (2016). Global Tuberculosis Report 2016.
- Wivagg CN, Hung DT. (2012). Resuscitation-promoting factors are required for beta-lactam tolerance and the permeability barrier in Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:1591–4.
- Wu X, Yang Y, Han Y, et al. (2008). Effect of recombinant Rv1009 protein on promoting the growth of Mycobacterium tuberculosis. J Appl Microbiol 105:1121–7.
- Xin Q, Niu H, Li Z, et al. (2013). Subunit vaccine consisting of multi-stage antigens has high protective efficacy against Mycobacterium tuberculosis infection in mice. PLoS One 8:e72745.
- Yeremeev VV, Kondratieva TK, Rubakova EI, et al. (2003). Proteins of the Rpf family: immune cell reactivity and vaccination efficacy against tuberculosis in mice. Infect Immun 71:4789–94.
- Zhang Y, Yang Y, Woods A, et al. (2001). Resuscitation of dormant Mycobacterium tuberculosis by phospholipids or specific peptides. Biochem Biophys Res Commun 284:542–7.
- Zhang Y, Yew WW, Barer MR. (2010). Targeting persisters for tuberculosis control. Antimicrob Agents Chemother 56:2223–30.
- Zhao S, Song X, Zhao Y, et al. (2015). Protective and therapeutic effects of the resuscitation-promoting factor domain and its mutants against Mycobacterium tuberculosis in mice. Pathog Dis 73:ftu025. Available from: https://academic.oup.com/femspd/article/73/3/ftu025/528906/Protective-and-therapeutic-effects-of-the?searchresult=1