Abstract
Transposable elements (TE), small mobile genetic elements unable to exist independently of the host genome, were initially believed to be exclusively deleterious genomic parasites. However, it is now clear that they play an important role as bacterial mutagenic agents, enabling the host to adapt to new environmental challenges and to colonize new niches. This review focuses on the impact of insertion sequences (IS), arguably the smallest TE, on bacterial genome plasticity and concomitant adaptability of phenotypic traits, including resistance to antibacterial agents, virulence, pathogenicity and catabolism. The direct consequence of IS transposition is the insertion of one DNA sequence into another. This event can result in gene inactivation as well as in modulation of neighbouring gene expression. The latter is usually mediated by de-repression or by the introduction of a complete or partial promoter located within the element. Furthermore, transcription and transposition of IS are affected by host factors and in some cases by environmental signals offering the host an adaptive strategy and promoting genetic variability to withstand the environmental challenges.
Introduction
Insertion sequences (IS) are among the simplest mobile genetic elements (MGEs) and widespread in all domains of life. They can occur in a wide range of copy numbers in a particular genome and can move within a genome (between different DNA molecules or within an individual DNA molecule) or horizontally between genomes as part of other MGE vectors such as phages and plasmids. More than 4500 IS belonging to 29 families have been identified to date (Siguier et al. Citation2006, Citation2014). These small and compact elements (typically less than 3 kb), capable of independent transposition, are generally flanked by short terminal inverted repeats (IR) and carry one or two open reading frames encoding gene products essential for their mobility (Mahillon & Chandler Citation1998; Siguier et al. Citation2014). DNA cleavage (at the transposon tips) and strand transfer (of the transposon DNA into the target) necessary for transposition are catalyzed by a transposon-specific enzyme, the transposase. The IR are necessary for transposase-binding, donor DNA cleavage and strand transfer. Often, short directly repeated sequences of the target DNA, from 2 to 14 bp long and whose length is specific for a given element, are generated at the point of insertion (for reviews see Mahillon & Chandler Citation1998; Siguier et al. Citation2014). IS vary in the degree of sequence specificity in choice of their DNA target-site. Depending on the element, this can vary from highly sequence-specific to near random insertion (Craig Citation1997). For some elements, the potential DNA structure of the target site is also important (Bender & Kleckner Citation1992; Hallet et al. Citation1994; Hu & Derbyshire Citation1998).
Although IS classification is based on a variety of characteristics, the amino acid sequence similarity of their transposases is the principal parameter used for classification (Mahillon & Chandler Citation1998; Siguier et al. Citation2006, Citation2014). Transposase types include DDE, DEDD, HUH (Y1), Tyrosine (Y) and Serine (S). These names reflect amino acids located at their catalytic sites and are related to their transposition chemistry. IS carrying the DDE transposases represent the majority of presently identified IS elements (Siguier et al. Citation2015).
IS elements have been reviewed thoroughly with a focus on their classification, transposition mechanism and structure (Mahillon & Chandler Citation1998; Chandler & Mahillon Citation2002; Curcio & Derbyshire Citation2003; Bennett Citation2004; Nagy & Chandler Citation2004; Siguier et al. Citation2006, Citation2014, Citation2015). In this review, we inventory the diverse impact of IS transposition on phenotypic traits in bacteria and the putative modulation of IS transposition by environmental signals and stressors.
IS-mediated gene inactivation affecting virulence, resistance and metabolism
Perhaps the most common effect of IS transposition is gene inactivation (). Many cases have been described illustrating the modulation of resistance, virulence and metabolic activities by IS-mediated gene inactivation.
Figure 1. Possible effects of IS transposition in the function and expression of a target gene. The IS is shown as a blue rectangle delimited by short terminal inverted repeats (IR; blue triangles) and flanked by directed repeats (DR; yellow rectangles) and the target gene as a green rectangle. mRNA transcripts are represented by straight arrows, the target gene promoter sequence is represented by a curved arrow and detailed by −35 and −10 components, and transcriptional start site (+1). For IS elements carrying a complete outward-directed promoter (Pout), the target gene transcription start is altered and denoted as < +1>.
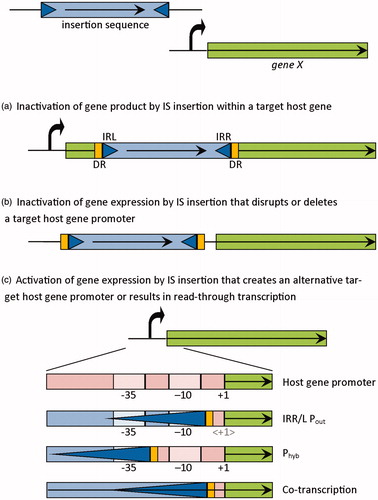
IS involvement in changing antimicrobial/xenobiotic resistance
A variety of cases where IS elements affect antibiotic/xenobiotic resistance by directly inactivating uptake determinants have been described. Examples, which are detailed in , include increased carbapenem resistance because of IS transposition into the porin-coding oprD gene of numerous Pseudomonas aeruginosa isolates (Wolter et al. Citation2004; Evans & Segal Citation2007; Ruiz-Martinez et al. Citation2011; Diene et al. Citation2013; Al Bayssari et al. Citation2014; Fowler & Hanson Citation2014; Rojo-Bezares et al. Citation2014; Al-Bayssari et al. Citation2015) and Pseudomonas putida (Kalantar-Neyestanaki et al. Citation2015), the porin-coding carO gene of Acinetobacter baumannii (Mussi et al. Citation2005); the porin-coding ompE36 gene of Enterobacter aerogenes (Chen et al. Citation2008b), and the porin-coding ompK36 gene of Klebsiella pneumoniae (Lee et al. Citation2007). Furthermore, IS-mediated inactivation of ompK36 also resulted in increased cefoxitin resistance (Hernandez-Alles et al. Citation1999). In contrast, inactivating efflux can result in hypersensitivity, e.g. to solvents due to insertional inactivation of acrB, part of the AcrAB-TolC efflux pump, by IS30 in Escherichia coli (Kobayashi et al. Citation2001).
Table 1. IS-mediated gene inactivation affecting virulence, resistance, and metabolism.
In addition to IS influence on transport, antibiotic/xenobotic processing or target sites can also be affected by IS transposition (). For instance, IS-mediated inactivation (ISAba11, IS701 family) of lipid A biosynthesis genes in Acinetobacter baumannii results in high-level colistin resistance because of complete loss of lipopolysaccharide production, the initial binding target of colistin (Moffatt et al. Citation2011). Insertional inactivation of the thymidylate synthase-encoding thyA gene in Deinococcus radiodurans, the 16S rRNA methyltransferase-encoding rsmG gene in Thermus thermophilus HB8 and the lytH gene involved in the biogenesis of the bacterial cell and the membrane protein-coding tcaA gene in Staphylococcus aureus results in increased trimethoprim (Mennecier et al. Citation2006), streptomycin (Gregory & Dahlberg Citation2008), methicillin (Fujimura & Murakami Citation2008) and glycopeptide resistance (Maki et al. Citation2004), respectively.
Next to a direct effect, IS elements can also affect regulatory pathways, thereby modulating expression of determinants leading to resistance (). IS-mediated derepression of efflux pumps has been observed in P. aeruginosa, by IS21 (IS21 family) insertion into the mexR repressor resulting in increased transcription of the mexAB-oprM efflux pump and increased β-lactam resistance (Boutoille et al. Citation2004); in P. putida, by ISS12 (IS21 family) insertion into the srpS repressor resulting in increased expression of the srpAC efflux pump and constitutive tolerance to toxic organic solvents (Wery et al. Citation2001; Sun & Dennis Citation2009); and in Cupriavidus metallidurans AE126, by inactivation of cnrY or cnrX, coding, respectively, for a membrane-bound anti-sigma factor (CnrY) and a sensor protein (CnrX), resulting in release of the extracytoplasmic function family (ECF) sigma factor CnrH that directs transcription of the cnrCBAT efflux pump and increased (non-specific) Zn2+ efflux (Collard et al. Citation1993; Grass et al. Citation2000; Tibazarwa et al. Citation2000; Vandecraen et al. Citation2016). Other examples are the insertional inactivation of the regulatory mgrB gene in K. pneumoniae and K. oxytoca resulting in colistin resistance (Cannatelli et al. Citation2014; Jayol et al. Citation2015; Poirel et al. Citation2015), of the ompR regulator in E. coli resulting in decreased porin expression and increased silver resistance (Graves et al. Citation2015; Randall et al. Citation2015) and of the amidase-coding ampD gene resulting in high-level β-lactam resistance (Bagge et al. Citation2002).
IS involvement in modulating virulence
The impact of IS transposition on virulence via modulation of biofilm formation and the production of extracellular polymeric substances has been described for different bacteria (). In Staphylococcus epidermidis and S. aureus, reversible IS256 transposition into the icaADBC locus, encoding the polysaccharide intercellular adhesin (a poly-β(1–6)-N-acetylglucosamine), results in phenotypic biofilm variation (Ziebuhr et al. Citation1999; Arciola et al. Citation2004; Kiem et al. Citation2004). Importantly, in S. epidermidis, the presence of the ica locus together with phenotypic evidence of biofilm production is used as a virulence marker (Arciola et al. Citation2015). IS256 has also been found to indirectly affect ica expression by inactivating rsbU (a positive regulator of the global stress response sigma factor σB) or sarA (encoding the staphylococcal accessory regulator) (Conlon et al. Citation2004). In Enterococcus faecalis, IS256 transposition mediated inactivation of gelatinase activity, which is essential in the initial steps of biofilm formation (Perez et al. Citation2015). Loss of extracellular polysaccharide production was also observed in Xanthomonas oryzaei via IS-mediated inactivation of the gumM gene, which is part of the gum cluster involved in the biosynthesis of extracellular polysaccharides (Rajeshwari & Sonti Citation2000). In Neisseria meningitidis, capsule expression and endogenous lipooligosaccharide sialylation, as well as an important antigen are affected by IS1301 (IS481 family) inactivation of essential biosynthesis genes such as siaA (sialic acid biosynthesis) and oatWY (O-acetyltransferase), and porA (porin), respectively (Hammerschmidt et al. Citation1996; Newcombe et al. Citation1998; Claus et al. Citation2003). In Rickettsia peacockii, ISRpe1 (IS481 family) transposition mediated the disruption of two genes involved in actin-tail polymerization and cell adhesion (Simser et al. Citation2005). In Ralstonia solanacearum, ISRso4 (IS5 family) transposition mediated the inactivation of the global regulatory gene phcA, which in turn modulated expression of extracellular polysaccharides (Jeong & Timmis Citation2000).
Next to biofilm formation and the production of extracellular polymeric substances, IS transposition also affected other virulence factors, including the above described phcA inactivation as the global regulator PhcA also modulates other virulence factors such as β-1,4-endoglucanase, pectin methylesterase, endopolygalacturonase A and motility (Clough et al. Citation1997; Jeong & Timmis Citation2000). Other examples include IS195 (IS982 family)-mediated inactivation of the protease-coding prtP gene in Porphyromonas gingivalis (Lewis & Macrina Citation1998), the IS1515 (IS5 family)-mediated inactivation of the ply gene coding for the virulence factor pneumolysin in a clinical isolate of Streptococcus pneumoniae (Garnier et al. Citation2007) and IS256-mediated modulation of S. aureus virulence by insertion into the rot (repressor of toxins) promoter, which drives expression of Rot, resulting in the derepression of cytotoxin expression and increased virulence (Benson et al. Citation2014).
IS involvement in modulating metabolic activities
Other cases were described that illustrate the modulation of metabolic activities such as carbon source utilization, electron donor usage, and production of primary and secondary metabolites by IS-mediated gene inactivation (). For instance, the ability to grow on different aromatic compounds can be modulated by insertion and precise excision of ISL3-family ISs in the o-, m- and p-xylene, and naphthalene catabolic pathway of Pseudomonas stutzeri OX1 (ISPst2) (Bolognese et al. Citation1999) and AN10 (ISPst9) (Christie-Oleza et al. Citation2008), respectively. Other examples are inactivation of protocatechuate catabolism by IS1236 (IS3 family) in Acinetobacter calcoaceticus (Gerischer et al. Citation1996) and constitutive expression of the lac genes in Lactococcus delbrueckii (Lapierre et al. Citation2002). In E. coli, IS1 insertion within rpoS, the master regulator of gene expression under stress conditions, confers higher fitness in glucose- and phosphate-limited chemostats thanks to loss of RpoS function mutation alleviating competition with other sigma factors, such as RpoD, presumably improving nutrient scavenging by increasing RpoD-dependent gene expression (Ferenci Citation2003; Gaffe et al. Citation2011). The ability of Thiobacillus ferrooxidans to oxidize Fe2+ (electron donor) can be affected by ISTfe1 (ISL3 family) insertion into the resB gene (Cabrejos et al. Citation1999).
IS-mediated inactivation of primary and secondary metabolite production has been observed in Acinetobacter baumannii for lipid A biosynthesis (Moffatt et al. Citation2011) and in Lactobacillus sakei for lactocin S production (Skaugen & Nes Citation1994, Citation2000), and in Bacillus subtilis for poly-γ-glutamate production (Nagai et al. Citation2000) and in A. xylinum for cellulose production (Coucheron Citation1991), respectively. Noteworthy, the production of indole, often used as a biochemical characteristic for species differentiation, is inactivated in many Shigella strains via IS transposition into the tna operon (Rezwan et al. Citation2004).
IS-mediated modulation of expression of neighbouring genes
IS transposition into non-coding regions can lead to altered expression of adjacent genes (). IS elements can provide promoter sequences, which are either entirely included within the IS () or created by the correct placement of an outward-directed -35 promoter component (), often present within the IS ends, relative to a suitable -10 box in the flanking genomic region to form a hybrid promoter. These IS-provided promoter sequences can be oriented towards the right or the left IR (defined relative to the transcriptional orientation of the transposase gene).
Table 2. Modulation of expression of neighbouring genes by IS elements carrying a complete outward-directed promoter.
Table 3. Modulation of expression of neighbouring genes by IS elements carrying an outward-directed -35 promoter component.
IS elements carrying a complete outward-directed promoter
IS1380 family
ISEcp1-like elements are one of the most important genetic elements associated with increased expression of blaCTX-M genes that code for expanded-spectrum β-lactamases of the CTX-M type (Lartigue et al. Citation2004). They are often located at the 5′ ends of β-lactamase genes and may provide both −35 and −10 promoter sequences, located within the IS proximal to its right IR (IRR), for expression. Examples of genes which are activated in this way are: blaCTX-M-2, blaCTX-M-15, blaCTX-M-17 and blaCTX-M-19 carried by plasmids from Kluyvera ascorbata (Lartigue et al. Citation2006), enterobacterial isolates (Karim et al. Citation2001), K. pneumoniae BM4493 (Cao et al. Citation2002) and a clinical K. pneumoniae isolate (Poirel et al. Citation2003), respectively. In addition to driving β-lactamase gene expression, an ISEcp1-like element was also found to drive expression of rmtC, a 16S rRNA methyltransferase gene located on plasmid pARS68 from the aminoglycoside-resistant clinical Proteus mirabilis strain ARS68 (Wachino et al. Citation2006). RmtC methylates 16S rRNA, which decreases the affinity of aminoglycosides for their target, and thus prevent the antibiotic interfering with mRNA decoding. IS1380-family members in Bacteroides include multiple IS elements with putative internal promoter sequences, which are, however, oriented towards their IRL. These are presumed to promote expression of β-lactam and 5-nitroimidazole resistance by providing a promoter sequence for the silent carbapenemase gene cfiA (Podglajen et al. Citation2001; Kato et al. Citation2003; Soki et al. Citation2006) and nimC gene (Haggoud et al. Citation1994; Soki et al. Citation2006), respectively. Molecular data (e.g. transcription start mapping) evidencing a mobile promoter has been provided for ISEcp1, IS1170, IS1188 and IS942 (Trinh et al. Citation1995; Podglajen et al. Citation2001; Poirel et al. Citation2003; Kamruzzaman et al. Citation2015).
IS3 family
In E. coli, arginine biosynthesis and utilization of citrate and acetate were activated by an outward-directed promoter located on IS3 (Charlier et al. Citation1982; Treves et al. Citation1998; Blount et al. Citation2012). In Burkholderia cepacia isolates, lactose could be utilized thanks to IS407-promoted expression of the otherwise silent lac operon (Wood et al. Citation1991). In Mycobacterium tuberculosis, some evidence has been presented that IS6110 directed the expression of multiple genes (Safi et al. Citation2004). In ampicillin-resistant transformants of P. stutzeri, ISPst4 inserted in pUS23 and presumably promoted transcription of the ori region resulting in robust replication in P. stutzeri (Coleman et al. Citation2014). All outward-directed promoters from IS3-family members are oriented towards their IRR and molecular data (e.g. transcription start mapping, deletion and reporter strategies) evidencing a mobile promoter has been provided for IS3, IS6110 and ISPst4 (Charlier et al. Citation1982; Safi et al. Citation2004; Coleman et al. Citation2014).
IS4 family
Control of antibiotic resistance has been shown for a number of IS4-family members. In A. baumannii isolates, insertion of ISAba1 (blaampC and blaOXA-51-like genes) resulted in ceftazidime- and carbapenem-resistant isolates, respectively (Corvec et al. Citation2003; Segal et al. Citation2004; Poirel et al. Citation2005c; Heritier et al. Citation2006; Turton et al. Citation2006). ISPa12 induced expression of extended-spectrum β-lactamase (ESBL) PER-1 in a series of Gram-negative isolates, including Salmonella enterica, P. aeruginosa, Providencia stuartii and A. baumannii, resulting in resistance to penicillins, cefotaxime, ceftibuten, ceftazidime and aztreonam (Poirel et al. Citation2005a). IS1999-mediated constitutive expression of alternative ESBL VEB-1 and OXA-48 genes resulted in antibiotic-resistant clinical isolates of P. aeruginosa and K. pneumoniae, respectively (Aubert et al. Citation2003, Citation2006). IS4-family members in Bacteroides spp. such as B. fragilis promoted expression of β-lactam and 5-nitroimidazole resistance by providing a promoter sequence for the metallo-β-lactamase gene ccrA (Rasmussen & Kovacs Citation1991) and nimC genes (Trinh et al. Citation1995), respectively. In acrB-inactivated S. enterica serovar Typhimurium DT204 mutants, missing the functional AcrAB RND efflux pump, high-level resistance to fluoroquinolone can be restored by IS10-mediated increased expression (via pOUT) of the alternative multidrug efflux AcrEF transporter (Olliver et al. Citation2005). In addition to resistance, IS10 transposition has also been found to activate histidine biosynthesis (Wang & Roth Citation1988). All outward-directed promoters from IS4-family members are oriented towards their IRL and molecular data (e.g. transcription start mapping) evidencing a mobile promoter has been provided for ISAba1, ISPa12, IS1999, IS10 and IS942 (Simons et al. Citation1983; Podglajen et al. Citation2001; Aubert et al. Citation2003; Segal et al. Citation2004; ; Poirel et al. Citation2005a Kamruzzaman et al. Citation2015).
IS5 family
In Bacteroides spp., expression of carbapenem and 5-nitroimidazole resistance determinants (Haggoud et al. Citation1994; Podglajen et al. Citation1994, Citation1995) can be promoted via an outward-directed promoter oriented by IS1186 (Podglajen et al. Citation1994, Citation1995) and IS1168 (Haggoud et al. Citation1994; Soki et al. Citation2006), and IS1169 (Trinh et al. Citation1995) and ISBf6 (Soki et al. Citation2006), respectively. All outward-directed promoters from IS5-family members, except ISBf6, are oriented towards their IRR and transcription start mapping evidencing a mobile promoter has been provided for IS1168 and IS1186 (Podglajen et al. Citation1994, Citation2001).
Other families
IS elements from other families have also been found to carry complete outward-directed promoters. IS481 family members include IS481a in Bordetella pertussis, driving the expression of katA and bteA coding, respectively, for a catalase and a type III secretion system effector (IS481 family) (DeShazer et al. Citation1994; Han et al. Citation2011), and ISRme5 in C. metallidurans, driving the expression of the cnrCBAT efflux pump (Vandecraen et al. Citation2016). IS6 family members include Iso-ISS1 in L. lactis supsp. lactis, driving expression of a restriction modification system responsible for phage resistance (Cluzel et al. Citation1991), and IS257 in S. aureus, resulting in tetracycline resistance (Simpson et al. Citation2000). Singular examples of other IS families include IS1187 (IS982 family) in B. fragilis, driving the expression of carbapenem resistance, and IS1411 (ISL3 family) in P. putida, directing the expression of the phenol degradation genes pheBA (Kallastu et al. Citation1998).
All these outward-directed promoters are oriented towards their IRL, except ISRme5, and molecular data (e.g. transcription start mapping) evidencing a mobile promoter has been provided for all (Cluzel et al. Citation1991; DeShazer et al. Citation1994; Kallastu et al. Citation1998; Simpson et al. Citation2000; Podglajen et al. Citation2001; Han et al. Citation2011; Vandecraen et al. Citation2016).
Hybrid promoters
In addition to IS elements that provide entire promoter sequences, IS elements can also activate downstream genes via the formation of a hybrid promoter in which the -35 promoter sequence at the element's border is properly positioned close to the -10 promoter sequence of the flanking gene. It is noteworthy that some IS elements possess, in addition to a -35 hexamer at the right end, an inward directed -10 hexamer in their IRL. These elements all appear to transpose through a circular dsDNA intermediate and it has been demonstrated that they generate relatively strong promoters if two ends of such an element are juxtaposed, by formation of head-to-tail dimers or by circularization. This arrangement can lead to high transposase expression and consequent increased transposition activity (Mahillon & Chandler Citation1998; Prudhomme et al. Citation2002; Szeverenyi et al. Citation2003).
IS1 family
Insertion of IS1 into the blaT-1 promoter of plasmid pBR322 resulted in an active, although less efficient, hybrid promoter (Prentki et al. Citation1986), transposition in front of the emr gene resulted in enhanced erythromycin resistance (Barany et al. Citation1982) and fluoroquinolone resistance was acquired via activation of the acrEF efflux pump in S. typhimurium (Olliver et al. Citation2005). Similarly, in E. coli strain HB251, an IS1-like element upstream of blaT-6, coding for ESBL TEM-6, resulted in the creation of a hybrid promoter in which the -10 region was that of the TEM-6 promoter whereas the -35 canonical sequence TTGACA was provided by the right end of the IS1-like element (Goussard et al. Citation1991). In this case, the newly constructed promoter was 10 times more active and conferred higher levels of antibiotic resistance (Goussard et al. Citation1991).
IS256 family
In S. aureus and S. sciuri, IS256 transposition into an upstream region of, respectively, the llm and mecA gene resulted in a hybrid promoter that enhanced their transcription and elevated methicillin resistance (Maki & Murakami Citation1997; Couto et al. Citation2003). In Burkholderia cepacia isolates, the ability to utilize lactose and the herbicide 2,4,5-trichlorophenoxyacetic acid as a sole carbon source was promoted by IS406 and IS1490, respectively. IS406 promoted expression of the otherwise silent lac operon by formation of a hybrid promoter comprising a -35 promoter box at the right IR of IS406 and a duplicated -10 box from the native lac promoter (Wood et al. Citation1991). Whereas insertion of IS1490 110 bp upstream of tftA, coding for 2,4,5-trichlorophenoxyacetic acid oxygenase, created a fusion promoter responsible for high-level constitutive transcription (Hubner & Hendrickson Citation1997).
IS3 family
Insertion of IS2 in front of the emr gene resulted in enhanced erythromycin resistance (Barany et al. Citation1982) and fluoroquinolone resistance was acquired via activation of the acrEF efflux pump in E. coli. Likewise, insertion of IS2 into the E. coli ampC promoter resulted in a hybrid promoter with a 20-fold increased strength compared with the original ampC promoter (Jaurin & Normark Citation1983). Besides the formation of hybrid promoters involved in antibiotic resistance, IS2 was also found to modulate expression of the gal and arg operons (Saedler et al. Citation1974; Nevers & Saedler Citation1977; Glansdorff et al. Citation1981), and the xis and int genes of bacteriophage lambda (Mosharrafa et al. Citation1976; Pilacinski et al. Citation1977).
Another IS3-family member was described in L. lactis NZ9010, which displays a retarded growth rate under anaerobic conditions due to replacement of the las operon-encoded ldh genes with an erythromycin resistance gene cassette. Normal growth rate and the ability to produce lactate could be recovered by “unsilencing” ldhB with site-specific and directional IS981 insertions. Characterization of the upstream region of the ldhB gene in ten independently isolated lactate-producing derivatives of L. lactis NZ9010 indicated that IS981-mediated lactate production represents the predominant mechanism of the observed recovery to produce lactate (Bongers et al. Citation2003).
IS30 family
In E. coli, IS30 transposition upstream of cmr/mdfA in the cmlA1 strain creates a hybrid promoter region increasing Cmr/MdfA efflux pump expression leading to exclusion of the synthethic, non-metabolizable β-galactoside isopropyl-beta-d-thiogalactopyranoside (IPTG) and protects the cell against effects of plasmid-encoded IPTG-induced lethal genes.
Although overexpression of the efflux pump caused spectinomycin hypersensitivity, little is known concerning spectinomycin uptake. Possibly, CmlA1 overproduction may contribute to the increase of the intracellular concentration of spectinomycin (Bohn & Bouloc Citation1998). The latter exemplifies that, instead of inducing antibiotic resistance, IS-mediated expression of multidrug efflux pumps can also promote hypersensitivity to other antibiotics through an increased uptake.
The potential -35 transcriptional promoter at the right end of IS30, which after transposition may create a putative σ70 promoter, was already detected upstream of galK (Dalrymple & Arber Citation1985; Dalrymple Citation1987). In Acinetobacter, aminoglycoside resistance could be promoted by IS18-mediated expression of the otherwise silent aac(6’)-lj gene (Rudant et al. Citation1998).
IS6 family
IS140 mediated the expression of gentamycin resistance in R-plasmids encoding genes for aminoglycoside acetyltransferase enzymes (Brau et al. Citation1984a). The latter is another example of the involvement of IS elements in plasmids conferring antibiotic resistance to their host. Expression of ESBL SHV-2a in P. aeruginosa and of both aphA7, mediating resistance to aminoglycosides, and ESBL SHV-2a in K. pneumoniae clinical isolates was directed by a hybrid promoter made from the IRL of IS26 and the -10 region from the native promoter (Lee et al. Citation1990; Naas et al. Citation1999; Jones et al. Citation2005). In S. aureus, IS257 upstream the thyE-dfrA genes encoded on plasmids pSK1 and pSK818, and the tetA(K) gene encoded by a cointegrated copy of a pT181-like plasmid resulted in a hybrid promoter that induced high-level trimethoprim and tetracycline resistance, respectively (Leelaporn et al. Citation1994; Simpson et al. Citation2000). Interestingly, trimethoprim resistance could be abolished due to deletions just next to the IS257 copy as seen in other plasmids that induced only a low-level trimethoprim resistance, indicating that an optimal spacing between the outward-directed -35 promoter hexamer and the resident -10 hexamer is necessary for high-level resistance to trimethoprim (Leelaporn et al. Citation1994). Increased carbapenem resistance was described in A. baumannii via the formation of a hybrid promoter upstream the blaOXA-58 gene. Similarly, the -35 sequence was provided by the IRR of IS1008. However, the -10 sequence was not provided by the promoter of the blaOXA-58 gene but by an upstream ISAba3-like element in which IS1008 was transposed (Chen et al. Citation2008a).
Other families
IS elements from other families have also been found to form hybrid promoters. IS21 family members include ISBf1 in Bacteriodes fragilis, promoting high-level β-lactam resistance (Rogers et al. Citation1994), and ISKpn7 in K. pneumoniae, contributing to extended-spectrum β-lactamase expression (Naas et al. Citation2012). Singular examples of other IS families include IS982 (IS982 family) on the lactococcal plasmid pCIT264 from L. lactis biovar diacetylactis, increasing the expression of the citrate utilization citQRP cluster (Lopez de Felipe et al. Citation1996), and ISVa1 (IS5 family) on the virulence plasmid pJM1from the fish pathogen Vibrio anguilarum, increasing the expression of the fatDCBAangRT operon, thereby causing septicemic disease in salmonids and other fishes (Tolmasky & Crosa Citation1995; Di Lorenzo et al. Citation2003).
Co-transcription
Modulation of neighbouring genes is also possible via read-through transcription from the transposase promoter (). For instance, in the cyanobacterium Tolypothrix sp. PCC 7601, the gltX gene, coding for a glutamyl-tRNA synthetase, is located downstream of and co-transcribed with ISTosp1 (IS200/IS605 family) (Luque et al. Citation2006). For Francisella tularensis, the causative agent of tularemia, spermine-induced expression of the ISFtu1 (IS630 family) and ISFtu2 (IS5 family) transposase genes also resulted in increased expression of their adjacent genes in broth culture and in macrophages (Carlson et al. Citation2009). In the plasmid-encoded LlaKR21 restriction-modification (R-M) system of L. lactis supsp. lactis biovar diacetylactis KR2, an IS982 element (IS982 family) inserted in the intergenic region between the endonuclease llaKR2IR gene and the methyltransferase llaKR2IM gene. The latter was co-directionally transcribed with the IS982 transposase gene. The R-M system may originally have been more efficient at restricting phage proliferation when the IS element was absent. However, it is credible that if methylation activity was weakened in the presence of an active endonuclease also autodigestion of the hosts genomic DNA will occur. This stress imposed on the cell by the aberrant restriction-modification activity may have provoked the insertion of IS982, permitting enhancement of modification activity (Twomey et al. Citation1998).
Other cases
Topology
Insertion of a specific IS element can result in topological changes in DNA and transcriptional activation of adjacent genes. In E. coli, the normally cryptic, silent β-glucoside (bgl) catabolic operon is activated by the insertion of either IS5 or IS1 upstream or downstream of the promoter, probably disrupting the flanking silencer element sequences (Reynolds et al. Citation1981; Schnetz & Rak Citation1992). Moreover, it was shown for IS5 that activation was abolished by internal deletions but again restored by providing an IS5-encoded gene product in trans, necessary for transposition, indicating that interaction of IS5 with its ends in some way changes the topology of the bgl promoter region (Schnetz & Rak Citation1992). A second example is the enhanced motility of poorly motile E. coli after extended incubation in motility agar via IS1 or IS5 insertion at various locations upstream of the flhDC promoter, without altering the transcriptional start site. It was postulated that IS element insertion might activate transcription of the flhDC operon by reducing transcriptional repression (Barker et al. Citation2004). The role of regional topology in the regulation of flhDC expression was further confirmed by the fact that insertion of IS elements or a kanamycin gene far upstream the promoter or repressor binding sites also stimulated motility (Fahrner & Berg Citation2015). Another example is the mutation of E. coli crp (cAMP receptor protein) deletion strains, which carry a silent glpFK operon, to utilize glycerol. Interestingly, all mutants contained an IS5 element upstream of the glpFK promoter, always in the same position and orientation (Zhang & Saier Citation2009a). Insertion of IS5 activated the otherwise cryptic expression of the glpFK operon. The activation was caused by a short region at the 3′ end of IS5 that harbours a permanent bend and an overlapping nucleoid protein binding site for the integration host factor (IHF), both of which are required for maximal gene expression. Expression was still activated when a 10 bp fragment was inserted between IS5 and the glpFK promoter, but was completely abolished by insertion of a 5 bp fragment. Thus, positioning of the upstream IS5 at the correct phase angle relative to the promoter sequence of glpFK is essential for full promoter activity, emphasizing the important role of DNA topology (Zhang & Saier Citation2009b).
Furthermore, the lacZYA operon was also subject to IS5-mediated activation in the absence of CRP (Zhang & Saier Citation2009b). Finally, IS5 has also been shown to activate the cryptic ade gene encoding an adenine deaminase catalyzing deamination of adenine to hypoxanthine (Petersen et al. Citation2002). The activation was proposed to be caused by a relief of HNS-mediated normal repression by IS5 insertion (Petersen et al. Citation2002; Barker et al. Citation2004).
Attenuation
Several IS elements carry mobile attenuators at their 3′ end providing transcription terminators that may be adapted as 5′ regulators leading to repression of downstream genes (Naville & Gautheret Citation2010). Previously, in E. coli, rho-dependent termination sites were detected in IS1 and IS2, respectively, which block expression of downstream genes (Hinton & Musso Citation1983; Hubner et al. Citation1987).
Not determined
Several other cases of IS-mediated modulation of expression of neighbouring genes have been described for which the actual mechanism has not been determined (Supplementary Table 1). For instance, insertion of ISAac2 upstream of the orfA-ltxCABD operon increased its transcription and concomitant leukotoxin expression in Aggregatibacter actinomycetemcomitans (He et al. Citation1999). Although ISAac2 carries an outwardly directed -35 sequence at its IRR, which is positioned upstream from a potential -10 sequence, this putative promoter does not influence transcription of the ltx genes (Mitchell et al. Citation2003). ISAac2 carries in addition a -10-like element at its IRL that is apparently required for increased transcription of the ltx promoter (Schaeffer et al. Citation2008). However, no transcript spanning the ISAac2 transposase and orfA was detected (Mitchell et al. Citation2003; Schaeffer et al. Citation2008). Schaeffer et al. (Citation2008) concluded that either this -10 sequence has an indirect effect on the expression of orfA, which codes for an unknown protein that is required for the increased ltxCABD expression and interacts with the ltx promoter, or the transcript undergoes rapid post-transcriptional processing.
IS-mediated mobilization of neighbouring genes
It has been known for many years that IS can, under certain circumstances, mobilize neighbouring genes. In early studies, so-called compound (or composite) transposons were identified in which two flanking IS in inverted or directly repeated orientations could together mobilize one or more interstitial genes. Initial examples were limited to antibiotic resistance genes since this type of phenotypic trait was relatively easy to identify. Initial examples included the classical models Tn5 (flanking IS50), Tn10 (flanking IS10) as well as Tn9 (flanking IS1) (Alton & Vapnek Citation1979; Bennett Citation2008; Haniford & Ellis Citation2015). However, many other examples are now known with interstitial genes other than those coding for antibacterial traits (Siguier et al. Citation2014). In some instances, mutations have arisen in one or both flanking IS, which render them less autonomous and facilitate their recruitment in the transposon.
A number of TE, which are very closely related to known ISs, carry passenger genes involved in a variety of processes not linked to transposition, e.g. transcriptional regulation, methylation and antibiotic resistance (Siguier et al. Citation2014). Siguier et al. (Citation2009) coined the term transporter IS (tIS), since these elements are simpler than known transposons. Although the mechanisms involved in the acquisition of passenger genes are unclear, tISs do not appear to carry programmed recombination systems (as for instance some members of the Tn3 family seem to do). One route for the formation of tIS is from compound transposons in which one of the IS has undergone deletion. Several structures have been identified which could represent intermediates. These include an entire IS copy together with a surrogate IR located at some distance. Examples are IS elements such as IS911 (IS3 family) (Prere et al. Citation1990; Polard et al. Citation1994), IS102 (IS5 family) (Machida et al. Citation1982) and Ecp1-like elements (Poirel et al. Citation2003, Citation2005b). The latter (also referred to above) are often located upstream of several β-lactamase genes and their promiscuous transposases can recognize a variety of related DNA sequences as a functional IR. This enables the transposition and mobilization of the adjacent blaCTX-M genes (Poirel et al. Citation2005b). Other potential tISs of this type, containing a single IS and a short flanking sequence resembling an additional IR, harbour passenger genes involved in the resistance to bromoacetate (IS1380-family member IS1247 in Xanthobacter autotrophicus; van der Ploeg et al. Citation1995), triclosan (IS1182-family member IS1272 in Staphylococcus haemolyticus; Furi et al. Citation2016) and possibly colistin (IS30-family member ISApl1; Snesrud et al. Citation2016). tIS could be derived from such structures by mutation (deletion) of the internal end of the complete IS rendering the entire structure transposable. tIS might then undergo further deletion to generate minimal insertion cassettes (MICs) in which the IS transposase is removed leaving the transposable gene flanked by correctly oriented IR copies. At present, little is known concerning the evolutionary relationship between these structures and, indeed, between these structures and miniature inverted repeat transposable elements (MITES) in which two IR flank a short section of non-coding DNA (Siguier et al. Citation2014).
IS elements recognized by the cell's recombination machinery
As mentioned above, transposition of IS elements can locally destroy or alter gene function or expression. However, by amplification of their copy number within a genome, IS elements can also cause more extensive/general loss of genetic information by recombination events between identical individual copies (Cole et al. Citation2001; Preston et al. Citation2004). In an evolution experiment involving 12 populations of E. coli B, which have been propagated for more than 20,000 generations by daily serial transfers in minimal medium supplemented with glucose, multiple insertion, deletion and inversion events were detected (Papadopoulos et al. Citation1999; Schneider et al. Citation2000; Cooper et al. Citation2001). One deletion event was found in all 12 independent lines providing a fitness advantage in glucose medium and an example of parallel phenotypic and genomic evolution. This deletion was generated by a first transposition of IS150 (IS3 family) close to the rbs operon, encoding genes required for ribose utilization, followed by a recombination event with a second IS150 element, leading to deletion of (part of) the rbs operon (Cooper et al. Citation2001).
In another evolution experiment, where E. coli was adapted to extended starvation in liquid medium (Zambrano et al. Citation1993), mutants with increased fitness during stationary phase were isolated and an IS5-mediated gene loss involving the exchange of regulatory sequences was detected. First, transposition of an IS5 element occurred between the CRP-binding site and promoter of cstA, encoding for an oligonucleotide permease. Second, an inversion event occurred between the transposed IS5 copy and an IS5 copy located at 60 kb distance and upstream of the ybeJ-gltJKL-ybeK operon, involved in aspartate-glutamate transport. This inversion led to the inactivation of the cstA gene and CRP-dependent induction of the latter operon, conferring a new ability to grow on aspartate as a sole carbon source and grow faster with glutamate, asparagine and proline as carbon sources (Zinser et al. Citation2003). IS5-mediated deletion was also detected in E. coli populations evolved in glucose- and phosphate-limited chemostats (Gaffe et al. Citation2011). Here, a chromosomal deletion including mutY, involved in DNA repair, providing a mutator phenotype was identified in independent chemostats. As in the rbs deletion, a first transposition event was necessary before the subsequent recombination and deletion.
IS can also be involved in plasmid rearrangements. For instance, the large pAsa5 plasmid from the fish pathogen Aeromonas salmonicida encodes a type three secretion system, which is an important factor for virulence, but also harbours 11 IS elements, including three copies of ISAs11 (IS256 family). Stressful conditions such as growth at 25 °C caused ISAs11-mediated recombination events that resulted in pAsa5 rearrangements and loss of the type three secretion system and virulence (Tanaka et al. Citation2012).
As described above, IS elements can play an important role in the (in)activation of genes through transposition and recombination events. Furthermore, IS elements have been shown to specifically contribute to niche adaptation by promoting a variety of genetic rearrangements (detailed in Siguier et al. Citation2014). This is especially the case for pathogens which are dependent on their host for survival, since the host provides nutrition and protection. For example, comparative genetic analysis of Yersinia pestis, the causative agent of plague, has revealed that this organism has lost approximately 13% of its genome through first successive horizontal gene transfer of virulence factors and IS enrichment followed by IS-mediated massive genome decay (Chain et al. Citation2004). Yersinia pestis is not a unique example. Although this phenomenon would be detrimental in the free environment, it might be required for further niche adaptation among bacteria living in nutritionally protected environments such as S. enterica serovar Typhi (Parkhill et al. Citation2003), Mycobacterium leprae (Cole et al. Citation2001) and Mycobacterium ulcerans (Stinear et al. Citation2007).
IS transposition affected by host factors
Transposition rates per generation differ from element to element and host to host, depend on the type of donor molecule, and vary widely from 10−3 to 10−7 (Shen et al. Citation1987; Craig et al. Citation2002). Recently, Sousa et al. (Citation2013), using mutation accumulation and the vectorette PCR method, showed that the spontaneous transposition rate of multiple IS elements from one locus to another locus in the chromosome of E. coli K12 MG1655 srl::Tn10 mutS, without selective pressure during the analysis, ranged from 10−5 to 10−6. Transpositional activity of IS elements is strongly controlled, presumably to limit their detrimental effects to the host cell. IS transposition is regulated by the element itself, including transcriptional repressors (Zerbib et al. Citation1990; Hu et al. Citation1994), translational inhibitors (Kleckner et al. Citation1996), ribosome frame shifting (Escoubas et al. Citation1991), impinging of transcription by secondary mRNA structures (Beuzon et al. Citation1999), methylation sites (Roberts et al. Citation1985), transposase instability (Derbyshire et al. Citation1990) and target site preference (Olasz et al. Citation1998; Kiss et al. Citation2007). Furthermore, IS transposition can also be modulated by host factors, including, but not limited to, the DNA chaperones IHF, HU, HNS and FIS, the replication initiator DnaA, the protein chaperone/protease ClpX/P/A, the SOS control protein LexA, the DNA methylase Dam and GTP levels (Chandler & Mahillon Citation2002; Nagy & Chandler Citation2004; Coros et al. Citation2005; Twiss et al. Citation2005). In general, these effects are specific for each element (Mahillon & Chandler Citation1998). Recently, bacterial transposition reactions have also been shown to be regulated by Hfq, a well-studied RNA chaperone that binds small regulatory RNA (sRNA) and plays a central role in complex post-transcriptional regulatory networks in many bacteria (Vogel & Luisi Citation2011; Sobrero & Valverde Citation2012). For instance, IS10/Tn10 transposase translation is repressed by Hfq. When a single IS10 copy is present, Hfq directly binds to the translation initiation region of the transposase mRNA (RNA-IN) and blocks ribosome binding, when more IS10 copies are present, translation of RNA-IN is more efficiently inhibited by antisense pairing between RNA-IN and the cis-encoded sRNA, RNA-OUT (Ross et al. Citation2010; Ellis et al. Citation2015b). In addition, increased expression of the ChiX sRNA, for instance in stationary phase (Vogel et al. Citation2003), titrated Hfq away from RNA-IN, thereby derepressing RNA-IN translation (Ellis et al. Citation2015b). Another example is the post-transcriptional regulation of the expression of the IS200 transposase, an IS ubiquitous in Enterobacteriaceae (Beuzon et al. Citation2004). All three independent mechanisms, which include the tnpA mRNA secondary structure, Hfq binding to the tnpA mRNA and base-pairing of the cis-encoded sRNA, art200, with the tnpA mRNA, prevent ribosome binding and repress translation (Ellis et al. Citation2015a).
IS transposition affected by environmental stimuli
IS elements can be induced by environmental signals resulting in the creation of new genetic variability that can be useful to withstand stressful conditions. Multiple examples are described below; however, it is important to note that increased transposase transcription does not necessarily result in an increase in transposase.
Host environment
In F. tularensis, a highly virulent bacterial pathogen, environmental signals such as the presence of host cell-produced polyamines, including spermine and spermidine, dramatically alter cytokine induction in their host cells establishing a successful infection. Carlson et al. (Citation2009) showed that two classes of Francisella IS elements, ISFtu1 and ISFtu2, which are the most frequent in overall occurrence of six defined classes in F. tularensis (Rohmer et al. Citation2007), harboured spermine-responsive promoters leading to increased expression of their transposase genes and of adjacent genes via co-transcription. This increased expression was both observed in macrophages and in broth culture, where some of the cues encountering a mammalian host could be mimicked, including the presence of polyamines (Carlson et al. Citation2009).
A more general approach in M. tuberculosis indicated that IS6110 directed the expression of multiple genes via an outward-directed promoter in a 110 bp fragment, located near its IRR (Safi et al. Citation2004). Interestingly, this promoter is responsive to certain stress conditions, including the macrophage environment and the late phases of growth in broth, and thus has the ability to activate potential virulence genes during infection (Safi et al. Citation2004). In addition, IS6110 expression is also increased under microaerobic conditions (Ghanekar et al. Citation1999). Survival and growth in human monocytes and macrophages are major factors in the success of M. tuberculosis as a pathogen. Therefore, a mobile promoter, responsive to the macrophage environment, provides a mechanism for generating variants with potentially advantageous phenotypes.
Radiation and oxidative stress
Using a mutagenic assay to detect any ThyA inactivation events in D. radiodurans, five IS elements (ISDra2, ISDra3, ISDra4, ISDra5 and IS2621), belonging to different families, were found to be transpositionally active. In the presence of an active mismatch repair system, 75% of the mutations to trimethoprim resistance (TmpR) resulted from an IS insertion into the thyA coding region. ISDra2, which was rarely found among the spontaneous TmpR mutants, became the predominant IS among damage-induced mutants. Transposition of ISDra2 was induced by γ-irradiation (approximately 100-fold) and by UV-irradiation (approximately 50-fold) (Mennecier et al. Citation2006). It was later postulated that transposition of at least ISDra2 required single-strand DNA substrates and that its transposition thus could be triggered by the increase in its single-strand DNA substrate during irradiation (Pasternak et al. Citation2010). Induced transposition of ISDra2 by DNA damage is consistent with previous research where expression of the ISDra2 transposase was induced following a heat shock or treatment with H2O2 (Lipton et al. Citation2002; Schmid et al. Citation2005).
In E. coli, a mutagenesis assay system based on the phage 434 cI gene carried on a low-copy number plasmid seemed to have identified intermolecular IS10 transposition from the chromosome to the target plasmid inactivating cI. Moreover, this transposition appeared to be induced by UV-light in a SOS-induced manner. However, most of the UV-induced transposition of IS10 was accompanied by the integration of the target plasmid into the chromosome and by the production of extra IS10 copies (Eichenbaum & Livneh Citation1998).
Fingerprinting profiles of clinical isolates of B. cenocepacia showed substantial band variability that resulted from genomic rearrangements mediated by insertion sequences. Furthermore, transposition activity of an ISBcen20 homolog appeared to be induced when the ST-32 isolate CZ1238 was exposed to oxidative stress (Drevinek et al. Citation2010).
Metals and antibiotics
Subinhibitory zinc and cadmium concentrations induced the promoter activity of ISRme5 (IS481 family), IS1087B (IS3 family) and IS1088 (IS30 family) in C. metallidurans AE126 (Vandecraen et al. Citation2016). All three were involved in gene inactivation resulting in increased zinc resistance (see above and Vandecraen et al. Citation2016).
In addition, subinhibitory concentrations of antibiotics can also promote transposition of IS elements. ISEcp1-like elements are one of the most important genetic elements associated with blaCTX-M genes, which code for expanded-spectrum β-lactamases of the CTX-M type (Lartigue et al. Citation2004). Subinhibitory concentrations of several β-lactams, including cefotaxime, ceftazidime and piperacillin induced ISEcp1B-mediated transposition (Lartigue et al. Citation2006). However, amoxicillin/clavulanic acid, cefquinome and cefuroxime did not enhance ISEcp1B-mediated transposition (Nordmann et al. Citation2008). Transposition of ISEcp1B was also not promoted by nalidixic acid, a quinolone antibiotic, agreeing with previous work of Cattoir et al. (Citation2008) who found that ISEcp1-like elements mediated mobilization but not expression of qnrB genes coding for quinolone resistance. In addition, Lartigue et al. (Citation2006) showed that transposition might be temperature dependent as an enhanced transposition was detected at 40 °C compared with 37 °C.
Temperature
In B. subtilis, using an intermolecular transposition assay it seemed that the transposition frequency of IS4Bsu1 was increased under high temperature (4.4-fold, 49 °C compared with 37 °C), as well as under competence-inducing conditions (15.7-fold) (Takahashi et al. Citation2007).
Increased transposition of IS elements at high temperature (42 °C) was also observed in B. multivorans ATCC 17616 by using an IS trap with a positive sacB-based selection system (Ohtsubo et al. Citation2005). From the 13 active IS elements in B. multivorans ATCC 17616 (IS401 to IS408, IS411, IS415, ISBmu1, ISBmu2 and ISBmu3) (Lessie et al. Citation1990; Ohtsubo et al. Citation2005), eight were detected with this particular IS trap and transposition of IS401, IS402, IS406 and ISBmu3 was only detected under a high-temperature condition (Ohtsubo et al. Citation2005). In addition to high temperature, oxidative stress and starvation conditions were also evaluated but did not affect transposition activity in B. multivorans ATCC 17616 (Ohtsubo et al. Citation2005). Since, multiple IS elements (belonging to four different IS families) increased their transposition at high temperature, Ohtsubo et al. (Citation2005) suggested that this induction was not elicited by intrinsic transposase properties but by common host factor(s). In contrast, in E. coli, the transposition activities of several mobile elements (e.g. Tn3, IS1, IS30 and IS911) exhibit decreased transposition at 42 °C (Nagy & Chandler Citation2004) and the sensitivity of transposition to temperature has been considered to be an intrinsic feature of each transposase (Haren et al. Citation1997). For IS911, this decreased transposition resulted from an increased production of truncated transposase, devoid of the catalytic domain (Gueguen et al. Citation2006). Thus, environmental signals can also further repress the expression of transposases. For instance, in the cyanobacterium Tolypothrix sp. PCC 7601, expression of the ISTosp1 transposase is subjected to metabolic repression by ammonium ions via the presence of binding sites for the global regulator NtcA in the tnpA promoter (Luque et al. Citation2006).
Conjugation
Finally, conjugative interaction, possibly even without transfer, can also induce transposition of IS elements as this was demonstrated for the transposition of ISPst9. The genomic dose and/or distribution of ISPst9 copies was changed when P. stutzeri AN10 cells were put in contact with a (plasmidless) conjugative strain compared with a non-conjugative E. coli strain (Christie-Oleza et al. Citation2009). The role of conjugational transfer in adaptive mutability was first shown by the recombination-mediated high frequency of complete Tn10 loss among Lac− and Lac+ bacteria that have experienced conjugational transfer of the F’128 episome harbouring a Tn10 linked to the mutated lacI33 allele (Godoy & Fox Citation2000).
Evolutionary dynamics of ISs
The many cases detailed above illustrate the impact of ISs on the bacterial genome. Depending on the target gene, IS movement can have positive, neutral or negative effects on cell fitness. Under selective conditions, e.g. antibiotic pressure, IS-mediated beneficial mutations are favored and fixation of the IS at that particular target site depends on its exerted effect (e.g. gene inactivation or modulation of expression).
On an evolutionary timescale, an interesting question is how ISs are maintained in a genome. This issue can be approached from different angles. One approach focuses more on the parasitic nature of these elements and their ability to self-replicate (Doolittle & Sapienza Citation1980), a second takes into account the fact that ISs can also provide selective benefits to the host, for example, by generating occasional beneficial mutations in promoting genetic variation (Blot Citation1994; Schneider & Lenski Citation2004; De Palmenaer et al. Citation2008; Bichsel et al. Citation2013). A third hypothesis was postulated by Iranzo et al. (Citation2014), who concluded via a large-scale genomic analysis that IS abundance and distribution are selectively neutral. However, these hypotheses are not necessarily mutually exclusive. Recently, Wu et al. (Citation2015) showed that transposition bursts accelerate the extinction of IS elements and that this process could be decelerated by mechanisms suppressing transposition activity, which ISs as well as their hosts have developed (see above). The latter corresponds with the findings of Iranzo et al. (Citation2014) that ISs and their hosts tend to coexist in a dynamic equilibrium. In addition, this still allows generating some genetic variability and occasional IS-induced mutations are beneficial to overcome environmental challenges or to adapt to new environmental niches.
Conclusions
The examples described here, highlighting the effect and regulation of IS transposition in bacteria, clearly show that IS elements can have an important impact on the evolution of their hosts. IS transposition can have different outcomes, from simple gene inactivation to constitutive expression or repression of adjacently located genes by delivering IS-specified (partial) promoter or terminator sequences, respectively. Furthermore, multiple copies of an identical IS element dispersed over a genome promote various genomic rearrangements such as inversion, deletion, duplication and fusion of two replicons. Additionally, sequences flanked by two identical IS elements, properly orientated, can become mobilized and dispersed across different strains, even to unrelated bacteria. For example, the dissemination of antibiotic resistance genes has become a major health problem. Moreover, the activity of, at least some, IS elements can be modulated by environmental signals to promote genome plasticity and genetic variability offering occasional beneficial mutations to overcome environmental challenges or to adapt to new environmental niches. Thus, whether or not IS elements are selfish and parasitic elements, their impact on the architecture of microbial genomes is undeniable.
IMBY_A_1303661_Supplementary_Information.docx
Download MS Word (28.7 KB)Disclosure statement
The authors report no declarations of interest.
References
- Al-Bayssari C, Valentini C, Gomez C, Reynaud-Gaubert M, Rolain JM. 2015. First detection of insertion sequence element ISPa1328 in the oprD porin gene of an imipenem-resistant Pseudomonas aeruginosa isolate from an idiopathic pulmonary fibrosis patient in Marseille, France. New Microbes New Infect. 7:26–27.
- Al Bayssari C, Diene SM, Loucif L, Gupta SK, Dabboussi F, Mallat H, Hamze M, Rolain JM. 2014. Emergence of VIM-2 and IMP-15 carbapenemases and inactivation of oprD gene in carbapenem-resistant Pseudomonas aeruginosa clinical isolates from Lebanon. Antimicrob Agents Chemother. 58:4966–4970.
- Alton NK, Vapnek D. 1979. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature, 282:864–869.
- Arciola CR, Campoccia D, Gamberini S, Rizzi S, Donati ME, Baldassarri L, Montanaro L. 2004. Search for the insertion element IS256 within the ica locus of Staphylococcus epidermidis clinical isolates collected from biomaterial-associated infections. Biomaterials. 25:4117–25.
- Arciola CR, Campoccia D, Ravaioli S, Montanaro L. 2015. Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects. Front Cell Infect Microbiol. 5:7.
- Aubert D, Naas T, Heritier C, Poirel L, Nordmann P. 2006. Functional characterization of IS1999, an IS4 family element involved in mobilization and expression of beta-lactam resistance genes. J Bacteriol. 188:6506–6514.
- Aubert D, Naas T, Nordmann P. 2003. IS1999 increases expression of the extended-spectrum beta-lactamase VEB-1 in Pseudomonas aeruginosa. J Bacteriol. 185:5314–5319.
- Bagge N, Ciofu O, Hentzer M, Campbell JIA, Givskov M, Hoiby N. 2002. Constitutive high expression of chromosomal -lactamase in Pseudomonas aeruginosa caused by a new insertion sequence (IS1669) located in ampD. Antimicrob Agents Chemother. 46:3406–3411.
- Barany F, Boeke JD, Tomasz A. 1982. Staphylococcal plasmids that replicate and express erythromycin resistance in both Streptococcus pneumoniae and Escherichia coli. Proc Natl Acad Sci. USA, 79:2991–2995.
- Barker CS, Pruss BM, Matsumura P. 2004. Increased motility of Escherichia coli by insertion sequence element integration into the regulatory region of the flhD operon. J Bacteriol. 186:7529–7537.
- Bender J, Kleckner N. 1992. Tn10 insertion specificity is strongly dependent upon sequences immediately adjacent to the target-site consensus sequence. Proc Natl Acad Sci USA. 89:7996–8000.
- Bennett PM. 2004. Genome plasticity. In: Genomics, proteomics, and clinical bacteriology. Totowa (NJ): Humana Press; p. 71–113.
- Bennett PM. 2008. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol. 153 Suppl 1:S347–S357.
- Benson MA, Ohneck EA, Ryan C, Alonzo F, 3rd, Smith H, Narechania A, Kolokotronis SO, Satola SW, Uhlemann AC, Sebra R, et al. 2014. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol Microbiol. 93:664–681.
- Beuzon CR, Chessa D, Casadesus J. 2004. IS200: an old and still bacterial transposon. Int Microbiol. 7:3–12.
- Beuzon CR, Marques S, Casadesus J. 1999. Repression of IS200 transposase synthesis by RNA secondary structures. Nucleic Acids Res. 27:3690–3695.
- Bichsel M, Barbour AD, Wagner A. 2013. Estimating the fitness effect of an insertion sequence. J Math Biol. 66:95–114.
- Blot M. 1994. Transposable elements and adaptation of host bacteria. Genetica 93:5–12.
- Blount ZD, Barrick JE, Davidson CJ, Lenski RE. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature. 489:513–518.
- Bohn C, Bouloc P. 1998. The Escherichia coli cmlA gene encodes the multidrug efflux pump Cmr/MdfA and is responsible for isopropyl-beta-d-thiogalactopyranoside exclusion and spectinomycin sensitivity. J Bacteriol. 180:6072–6075.
- Bolognese F, Di Lecce C, Galli E, Barbieri P. 1999. Activation and inactivation of Pseudomonas stutzeri methylbenzene catabolism pathways mediated by a transposable element. Appl Environ Microbiol. 65:1876–1882.
- Bongers RS, Hoefnagel MH, Starrenburg MJ, Siemerink MA, Arends JG, Hugenholtz J, Kleerebezem M. 2003. IS981-mediated adaptive evolution recovers lactate production by ldhB transcription activation in a lactate dehydrogenase-deficient strain of Lactococcus lactis. J Bacteriol. 185:4499–4507.
- Boutoille D, Corvec S, Caroff N, Giraudeau C, Espaze E, Caillon J, Plésiat P, Reynaud A. 2004. Detection of an IS21 insertion sequence in the mexR gene of Pseudomonas aeruginosa increasing β-lactam resistance. FEMS Microbiol Lett. 230:143–6.
- Brau B, Pilz U, Piepersberg W. 1984a. Genes for gentamicin-(3)-N-acetyltransferases III and IV: I. Nucleotide sequence of the AAC(3)-IV gene and possible involvement of an IS140 element in its expression. MGG Mol. General Genet. 193:179–187.
- Brau B, Pilz U, Piepersberg W. 1984b. Genes for gentamicin-(3)-N-acetyltransferases III and IV: I. Nucleotide sequence of the AAC(3)-IV gene and possible involvement of an IS140 element in its expression. Mol Gen Genet. 193:179–187.
- Cabrejos ME, Zhao HL, Guacucano M, Bueno S, Levican G, Garcia E, Jedlicki E, Holmes DS. 1999. IST1 insertional inactivation of the resB gene: implications for phenotypic switching in Thiobacillus ferrooxidans. FEMS Microbiol Lett. 175:223–229.
- Cannatelli A, Giani T, D'Andrea MM, Di Pilato V, Arena F, Conte V, Tryfinopoulou K, Vatopoulos A, Rossolini GM, Group CS. 2014. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother. 58:5696–5703.
- Cao V, Lambert T, Courvalin P. 2002. ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum beta-lactamase CTX-M-17. Antimicrob Agents Chemother. 46:1212–1217.
- Carlson PE, Jr., Horzempa J, O'Dee DM, Robinson CM, Neophytou P, Labrinidis A, Nau GJ. 2009. Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J Bacteriol. 191:6855–6864.
- Cattoir V, Nordmann P, Silva-Sanchez J, Espinal P, Poirel L. 2008. ISEcp1-mediated transposition of qnrB-like gene in Escherichia coli. Antimicrob Agents Chemother. 52:2929–2932.
- Chain PS, Carniel E, Larimer FW, Lamerdin J, Stoutland PO, Regala WM, Georgescu AM, Vergez LM, Land ML, Motin VL, et al. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci USA. 101:13826–13831.
- Chandler M, Mahillon J. 2002. Insertion Sequences Revisited. In: Mobile DNA II. Washington (DC): ASM Press.
- Charlier D, Piette J, Glansdorff N. 1982. IS3 can function as a mobile promoter in E. coli. Nucleic Acids Res. 10:5935–5948.
- Chen TL, Wu RC, Shaio MF, Fung CP, Cho WL. 2008a. Acquisition of a plasmid-borne blaOXA-58 gene with an upstream IS1008 insertion conferring a high level of carbapenem resistance to Acinetobacter baumannii. Antimicrob Agents Chemother. 52:2573–2580.
- Chen YG, Zhang Y, Yu YS, Qu TT, Wei ZQ, Shen P, Li LJ. 2008b. In vivo development of carbapenem resistance in clinical isolates of Enterobacter aerogenes producing multiple beta-lactamases. Int J Antimicrob Agents 32:302–307.
- Christie-Oleza JA, Lanfranconi MP, Nogales B, Lalucat J, Bosch R. 2009. Conjugative interaction induces transposition of ISPst9 in Pseudomonas stutzeri AN10. J Bacteriol. 191:1239–1247.
- Christie-Oleza JA, Nogales B, Martin-Cardona C, Lanfranconi MP, Alberti S, Lalucat J, Bosch R. 2008. ISPst9, an ISL3-like insertion sequence from Pseudomonas stutzeri AN10 involved in catabolic gene inactivation. Int Microbiol. 11:101–110.
- Claus H, Borrow R, Achtman M, Morelli G, Kantelberg C, Longworth E, Frosch M, Vogel U. 2003. Genetics of capsule O-acetylation in serogroup C, W-135 and Y meningococci. Mol Microbiol. 51:227–239.
- Clough SJ, Flavier AB, Schell MA, Denny TP. 1997. Differential Expression of Virulence Genes and Motility in Ralstonia (Pseudomonas) solanacearum during Exponential Growth. Appl Environ Microbiol. 63:844–850.
- Cluzel PJ, Chopin A, Ehrlich SD, Chopin MC. 1991. Phage abortive infection mechanism from Lactococcus Lactis Subsp lactis, expression of which is mediated by an Iso-ISS1 element. Appl Environ Microbiol. 57:3547–3551.
- Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, et al. 2001. Massive gene decay in the leprosy bacillus. Nature. 409:1007–1011.
- Coleman NV, Richardson-Harris J, Wilson NL, Holmes AJ. 2014. Insertion sequence ISPst4 activates pUC plasmid replication in Pseudomonas stutzeri. FEMS Microbiol Lett. 356:242–249.
- Collard JM, Provoost A, Taghavi S, Mergeay M. 1993. A new type of Alcaligenes eutrophus CH34 zinc resistance generated by mutations affecting regulation of the cnr cobalt-nickel resistance system. J Bacteriol. 175:779–784.
- Conlon KM, Humphreys H, O'Gara JP. 2004. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J Bacteriol. 186:6208–6219.
- Cooper VS, Schneider D, Blot M, Lenski RE. 2001. Mechanisms causing rapid and parallel losses of ribose catabolism in evolving populations of Escherichia coli B. J Bacteriol. 183:2834–2841.
- Coros AM, Twiss E, Tavakoli NP, Derbyshire KM. 2005. Genetic Evidence that GTP Is Required for Transposition of IS903 and Tn552 in Escherichia coli. J Bacteriol. 187:4598–4606.
- Corvec S, Caroff N, Espaze E, Giraudeau C, Drugeon H, Reynaud A. 2003. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J Antimicrob Chemother. 52:629–635.
- Coucheron DH. 1991. An Acetobacter xylinum insertion sequence element associated with inactivation of cellulose production. J Bacteriol. 173:5723–5731.
- Couto I, Wu SW, Tomasz A, de Lencastre H. 2003. Development of methicillin resistance in clinical isolates of Staphylococcus sciuri by transcriptional activation of the mecA homologue native to species. J Bacteriol. 185:645–653.
- Craig NL. 1997. Target site selection in transposition. Annu Rev Biochem. 66:437–474.
- Craig NL, Craigie R, Gellert M, Lambowitz AM. 2002. Mobile DNA II. Washington, DC: American Society of Microbiology.
- Curcio MJ, Derbyshire KM. 2003. The outs and ins of transposition: from mu to kangaroo. Nat Rev Mol Cell Biol. 4:865–877.
- Dalrymple B. 1987. Novel rearrangements of IS30 carrying plasmids leading to the reactivation of gene expression. Mol Gen Genet. 207:413–420.
- Dalrymple B, Arber W. 1985. Promotion of RNA transcription on the insertion element IS30 of E. coli K12. EMBO J. 4:2687–2693.
- De Palmenaer D, Siguier P, Mahillon J. 2008. IS4 family goes genomic. BMC Evol Biol. 8:18.
- Derbyshire KM, Kramer M, Grindley ND. 1990. Role of instability in the cis action of the insertion sequence IS903 transposase. Proc Natl Acad Sci USA. 87:4048–4052.
- DeShazer D, Wood GE, Friedman RL. 1994. Molecular characterization of catalase from Bordetella pertussis: identification of the katA promoter in an upstream insertion sequence. Mol Microbiol. 14:123–130.
- Di Lorenzo M, Stork M, Tolmasky ME, Actis LA, Farrell D, Welch TJ, Crosa LM, Wertheimer AM, Chen Q, Salinas P, et al. 2003. Complete sequence of virulence plasmid pJM1 from the marine fish pathogen Vibrio anguillarum strain 775. J Bacteriol. 185:5822–5830.
- Diene SM, L'Homme T, Bellulo S, Stremler N, Dubus JC, Mely L, Leroy S, Degand N, Rolain JM. 2013. ISPa46, a novel insertion sequence in the oprD porin gene of an imipenem-resistant Pseudomonas aeruginosa isolate from a cystic fibrosis patient in Marseille, France. Int J Antimicrob Agents. 42:268–271.
- Doolittle WF, Sapienza C. 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature. 284:601–603.
- Drevinek P, Baldwin A, Lindenburg L, Joshi LT, Marchbank A, Vosahlikova S, Dowson CG, Mahenthiralingam E. 2010. Oxidative stress of Burkholderia cenocepacia induces insertion sequence-mediated genomic rearrangements that interfere with macrorestriction-based genotyping. J Clin Microbiol. 48:34–40.
- Eichenbaum Z, Livneh Z. 1998. UV light induces IS10 transposition in Escherichia coli. Genetics. 149:1173–1181.
- Ellis MJ, Trussler RS, Haniford DB. 2015a. A cis-encoded sRNA, Hfq and mRNA secondary structure act independently to suppress IS200 transposition. Nucleic Acids Res. 43:6511–6527.
- Ellis MJ, Trussler RS, Haniford DB. 2015b. Hfq binds directly to the ribosome-binding site of IS10 transposase mRNA to inhibit translation. Mol Microbiol. 96:633–650.
- Escoubas JM, Prere MF, Fayet O, Salvignol I, Galas D, Zerbib D, Chandler M. 1991. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 10:705–712.
- Evans JC, Segal H. 2007. A novel insertion sequence, ISPa26, in oprD of Pseudomonas aeruginosa is associated with carbapenem resistance. Antimicrob Agents Chemother. 51:3776–3777.
- Fahrner KA, Berg HC. 2015. Mutations that stimulate flhDC expression in Escherichia coli. J Bacteriol. 197:3087–3096.
- Ferenci T. 2003. What is driving the acquisition of mutS and rpoS polymorphisms in Escherichia coli? Trends Microbiol. 11:457–461.
- Fowler RC, Hanson ND. 2014. Emergence of carbapenem resistance due to the novel insertion sequence ISPa8 in Pseudomonas aeruginosa. PLoS One. 9:e91299.
- Fujimura T, Murakami K. 2008. Staphylococcus aureus clinical isolate with high-level methicillin resistance with an lytH mutation caused by IS1182 insertion. Antimicrob Agents Chemother. 52:643–647.
- Furi L, Haigh R, Al Jabri ZJ, Morrissey I, Ou HY, Leon-Sampedro R, Martinez JL, Coque TM, Oggioni MR. 2016. Dissemination of novel antimicrobial resistance mechanisms through the insertion sequence mediated spread of metabolic genes. Front Microbiol. 7:1008.
- Gaffe J, McKenzie C, Maharjan RP, Coursange E, Ferenci T, Schneider D. 2011. Insertion sequence-driven evolution of Escherichia coli in chemostats. J Mol Evol. 72:398–412.
- Garnier F, Janapatla RP, Charpentier E, Masson G, Grelaud C, Stach JF, Denis F, Ploy MC. 2007. Insertion sequence 1515 in the ply gene of a type 1 clinical isolate of Streptococcus pneumoniae abolishes pneumolysin expression. J Clin Microbiol. 45:2296–2297.
- Gerischer U, D'Argenio DA, Ornston LN. 1996. IS1236, a newly discovered member of the IS3 family, exhibits varied patterns of insertion into the Acinetobacter calcoaceticus chromosome. Microbiology. 142:1825–1831.
- Ghanekar K, McBride A, Dellagostin O, Thorne S, Mooney R, McFadden J. 1999. Stimulation of transposition of the Mycobacterium tuberculosis insertion sequence IS6110 by exposure to a microaerobic environment. Mol Microbiol. 33:982–993.
- Glansdorff N, Charlier D, Zafarullah M. 1981. Activation of gene expression by IS2 and IS3. Cold Spring Harb Symp Quant Biol. 45 Pt 1:153–156.
- Godoy VG, Fox MS. 2000. Transposon stability and a role for conjugational transfer in adaptive mutability. Proc Natl Acad Sci USA. 97:7393–7398.
- Goussard S, Sougakoff W, Mabilat C, Bauernfeind A, Courvalin P. 1991. An IS1-like element is responsible for high-level synthesis of extended-spectrum beta-lactamase TEM-6 in Enterobacteriaceae. J Gen Microbiol. 137:2681–2687.
- Grass G, Grosse C, Nies DH. 2000. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. J Bacteriol. 182:1390–1398.
- Graves JL, Jr., Tajkarimi M, Cunningham Q, Campbell A, Nonga H, Harrison SH, Barrick JE. 2015. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front Genet. 6:42.
- Gregory ST, Dahlberg AE. 2008. Transposition of an insertion sequence, ISTth7, in the genome of the extreme thermophile Thermus thermophilus HB8. FEMS Microbiol Lett. 289:187–192.
- Gueguen E, Rousseau P, Duval-Valentin G, Chandler M. 2006. Truncated forms of IS911 transposase downregulate transposition. Mol Microbiol. 62:1102–1116.
- Haggoud A, Reysset G, Azeddoug H, Sebald M. 1994. Nucleotide sequence analysis of two 5-nitroimidazole resistance determinants from Bacteroides strains and of a new insertion sequence upstream of the two genes. Antimicrob Agents Chemother. 38:1047–1051.
- Hallet B, Rezsohazy R, Mahillon J, Delcour J. 1994. IS231A insertion specificity: consensus sequence and DNA bending at the target site. Mol Microbiol. 14:131–139.
- Hammerschmidt S, Hilse R, van Putten JP, Gerardy-Schahn R, Unkmeir A, Frosch M. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15:192–198.
- Han HJ, Kuwae A, Abe A, Arakawa Y, Kamachi K. 2011. Differential expression of type III effector BteA protein due to IS481 insertion in Bordetella pertussis. PLoS One. 6:e17797.
- Haniford DB, Ellis MJ. 2015. Transposons Tn10 and Tn5. Microbiol Spectr. 3:MDNA3.
- Haren L, Betermier M, Polard P, Chandler M. 1997. IS911-mediated intramolecular transposition is naturally temperature sensitive. Mol Microbiol. 25:531–540.
- He T, Nishihara T, Demuth DR, Ishikawa I. 1999. A novel insertion sequence increases the expression of leukotoxicity in Actinobacillus actinomycetemcomitans clinical isolates. J Periodontol. 70:1261–1268.
- Heritier C, Poirel L, Nordmann P. 2006. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin Microbiol Infect. 12:123–130.
- Hernandez-Alles S, Benedi VJ, Martinez-Martinez L, Pascual A, Aguilar A, Tomas JM, Alberti S. 1999. Development of resistance during antimicrobial therapy caused by insertion sequence interruption of porin genes. Antimicrob Agents Chemother. 43:937–939.
- Hinton DM, Musso RE. 1983. Specific in vitro transcription of the insertion sequence IS2. J Mol Biol. 169:53–81.
- Hu ST, Hwang JH, Lee LC, Lee CH, Li PL, Hsieh YC. 1994. Functional analysis of the 14 kDa protein of insertion sequence 2. J Mol Biol. 236:503–513.
- Hu WY, Derbyshire KM. 1998. Target choice and orientation preference of the insertion sequence IS903. J Bacteriol. 180:3039–3048.
- Hubner A, Hendrickson W. 1997. A fusion promoter created by a new insertion sequence, IS1490, activates transcription of 2,4,5-trichlorophenoxyacetic acid catabolic genes in Burkholderia cepacia AC1100. J Bacteriol. 179:2717–2723.
- Hubner P, Iida S, Arber W. 1987. A transcriptional terminator sequence in the prokaryotic transposable element IS1. Mol Gen Genet. 206:485–490.
- Iranzo J, Gomez MJ, Lopez de Saro FJ, Manrubia S. 2014. Large-scale genomic analysis suggests a neutral punctuated dynamics of transposable elements in bacterial genomes. PLoS Comput Biol. 10:e1003680.
- Jaurin B, Normark S. 1983. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell 32:809–816.
- Jayol A, Poirel L, Villegas MV, Nordmann P. 2015. Modulation of mgrB gene expression as a source of colistin resistance in Klebsiella oxytoca. Int J Antimicrob Agents. 46:108–110.
- Jellen-Ritter AS, Kern WV. 2001. Enhanced expression of the multidrug efflux pumps AcrAB and AcrEF associated with insertion element transposition in Escherichia coli mutants selected with a fluoroquinolone. Antimicrob Agents Chemother. 45:1467–1472.
- Jeong EL, Timmis JN. 2000. Novel insertion sequence elements associated with genetic heterogeneity and phenotype conversion in Ralstonia solanacearum. J Bacteriol. 182:4673–4676.
- Jones LA, McIver CJ, Kim MJ, Rawlinson WD, White PA. 2005. The aadB gene cassette is associated with blaSHV genes in Klebsiella species producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 49:794–797.
- Kalantar-Neyestanaki D, Emaneini M, Jabalameli F, Taherikalani M, Mirsalehian A. 2015. ISPpu22, a novel insertion sequence in the oprD porin gene of a carbapenem-resistant Pseudomonas aeruginosa isolate from a burn patient in Tehran, Iran. Iran J Microbiol. 7:247–250.
- Kallastu A, Horak R, Kivisaar M. 1998. Identification and characterization of IS1411, a new insertion sequence which causes transcriptional activation of the phenol degradation genes in Pseudomonas putida. J Bacteriol. 180:5306–5312.
- Kamruzzaman M, Patterson JD, Shoma S, Ginn AN, Partridge SR, Iredell JR. 2015. Relative strengths of promoters provided by common mobile genetic elements associated with resistance gene expression in Gram-negative bacteria. Antimicrob Agents Chemother. 59:5088–5091.
- Karim A, Poirel L, Nagarajan S, Nordmann P. 2001. Plasmid-mediated extended-spectrum beta-lactamase (CTX-M-3 like) from India and gene association with insertion sequence ISEcp1. FEMS Microbiol Lett. 201:237–241.
- Kato N, Yamazoe K, Han CG, Ohtsubo E. 2003. New insertion sequence elements in the upstream region of cfiA in imipenem-resistant Bacteroides fragilis strains. Antimicrob Agents Chemother. 47:979–985.
- Kiem S, Oh WS, Peck KR, Lee NY, Lee J-Y, Song J-H, Hwang ES, Kim E-C, Cha CY, Choe K-W. 2004. Phase variation of biofilm formation in Staphylococcus aureus by IS256 insertion and its impact on the capacity adhering to polyurethane surface. J Korean Med Sci. 19:779–782.
- Kiss J, Nagy Z, Toth G, Kiss GB, Jakab J, Chandler M, Olasz F. 2007. Transposition and target specificity of the typical IS30 family element IS1655 from Neisseria meningitidis. Mol Microbiol. 63:1731–1747.
- Kleckner N, Chalmers RM, Kwon D, Sakai J, Bolland S. 1996. Tn10 and IS10 transposition and chromosome rearrangements: Mechanism and regulation in vivo and in vitro. In: Transposable Elements. p. 49–82.
- Kobayashi K, Tsukagoshi N, Aono R. 2001. Suppression of hypersensitivity of Escherichia coli acrB mutant to organic solvents by integrational activation of the acrEF operon with the IS1 or IS2 element. J Bacteriol. 183:2646–2653.
- Lapierre L, Mollet B, Germond JE. 2002. Regulation and adaptive evolution of lactose operon expression in Lactobacillus delbrueckii. J Bacteriol. 184:928–935.
- Lartigue MF, Poirel L, Aubert D, Nordmann P. 2006. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring beta-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob Agents Chemother. 50:1282–1286.
- Lartigue MF, Poirel L, Nordmann P. 2004. Diversity of genetic environment of bla(CTX-M) genes. FEMS Microbiol Lett. 234:201–207.
- Lee CH, Chu C, Liu JW, Chen YS, Chiu CJ, Su LH. 2007. Collateral damage of flomoxef therapy: in vivo development of porin deficiency and acquisition of blaDHA-1 leading to ertapenem resistance in a clinical isolate of Klebsiella pneumoniae producing CTX-M-3 and SHV-5 beta-lactamases. J Antimicrob Chemother. 60:410–413.
- Lee KY, Hopkins JD, Syvanen M. 1990. Direct involvement of IS26 in an antibiotic resistance operon. J Bacteriol. 172:3229–3236.
- Leelaporn A, Firth N, Byrne ME, Roper E, Skurray RA. 1994. Possible role of insertion sequence IS257 in dissemination and expression of high- and low-level trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 38:2238–2244.
- Lessie TG, Wood MS, Byrne A, Ferrante A. 1990. Transposable gene-activating elements in Pseudomonas cepacia. In: Pseudomonas: biotransformations, pathogenesis, and evolving biotechnology. Washington (DC): American Society for Microbiology; p. 279–291.
- Lewis JP, Macrina FL. 1998. IS195, an insertion sequence-like element associated with protease genes in Porphyromonas gingivalis. Infect Immunun. 66:3035–3042.
- Lipton MS, Pasa-Tolic L, Anderson GA, Anderson DJ, Auberry DL, Battista JR, Daly MJ, Fredrickson J, Hixson KK, Kostandarithes H, et al. 2002. Global analysis of the Deinococcus radiodurans proteome by using accurate mass tags. Proc Natl Acad Sci USA. 99:11049–11054.
- Lopez de Felipe F, Magni C, de Mendoza D, Lopez P. 1996. Transcriptional activation of the citrate permease P gene of Lactococcus lactis biovar diacetylactis by an insertion sequence-like element present in plasmid pCIT264. Mol Gen Genet. 250:428–436.
- Luque I, Andujar A, Jia L, Zabulon G, de Marsac NT, Flores E, Houmard J. 2006. Regulated expression of glutamyl-tRNA synthetase is directed by a mobile genetic element in the cyanobacterium Tolypothrix sp. PCC 7601. Mol Microbiol. 60:1276–1288.
- Machida Y, Machida C, Ohtsubo E. 1982. A novel type of transposon generated by insertion element IS102 present in a pSC101 derivative. Cell. 30:29–36.
- Mahillon J, Chandler M. 1998. Insertion sequences. Microbiol Mol Biol Rev. 62:725–774.
- Maki H, McCallum N, Bischoff M, Wada A, Berger-Bachi B. 2004. tcaA inactivation increases glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 48:1953–1959.
- Maki H, Murakami K. 1997. Formation of potent hybrid promoters of the mutant llm gene by IS256 transposition in methicillin-resistant Staphylococcus aureus. J Bacteriol. 179:6944–6948.
- Mennecier S, Servant P, Coste G, Bailone A, Sommer S. 2006. Mutagenesis via IS transposition in Deinococcus radiodurans. Mol Microbiol. 59:317–325.
- Mitchell C, Gao L, Demuth DR. 2003. Positive and negative cis-acting regulatory sequences control expression of leukotoxin in Actinobacillus actinomycetemcomitans 652. Infect Immunun. 71:5640–5649.
- Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. 2011. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother. 55:3022–3024.
- Mosharrafa E, Pilacinski W, Zissler J, Fiandt M, Szybalski W. 1976. Insertion sequence IS2 near the gene for prophage lambda excision. Mol Gen Genet. 147:103–109.
- Mussi MA, Limansky AS, Viale AM. 2005. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob Agents Chemother. 49:1432–1440.
- Naas T, Cuzon G, Truong HV, Nordmann P. 2012. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob Agents Chemother. 56:4753–4759.
- Naas T, Philippon L, Poirel L, Ronco E, Nordmann P. 1999. An SHV-derived extended-spectrum beta-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 43:1281–1284.
- Nagai T, Tran LS, Inatsu Y, Itoh Y. 2000. A new IS4 family insertion sequence, IS4Bsu1, responsible for genetic instability of poly-gamma-glutamic acid production in Bacillus subtilis. J Bacteriol. 182:2387–2392.
- Nagy Z, Chandler M. 2004. Regulation of transposition in bacteria. Res Microbiol. 155:387–398.
- Naville M, Gautheret D. 2010. Premature terminator analysis sheds light on a hidden world of bacterial transcriptional attenuation. Genome Biol. 11:R97.
- Nevers P, Saedler H. 1977. Transposable genetic elements as agents of gene instability and chromosomal rearrangements. Nature. 268:109–115.
- Newcombe J, Cartwright K, Dyer S, McFadden J. 1998. Naturally occurring insertional inactivation of the porA gene of Neisseria meningitidis by integration of IS1301. Mol Microbiol. 30:453–454.
- Nordmann P, Lartigue MF, Poirel L. 2008. Beta-lactam induction of ISEcp1B-mediated mobilization of the naturally occurring bla(CTX-M) beta-lactamase gene of Kluyvera ascorbata. FEMS Microbiol Lett. 288:247–249.
- Ohtsubo Y, Genka H, Komatsu H, Nagata Y, Tsuda M. 2005. High-temperature-induced transposition of insertion elements in Burkholderia multivorans ATCC 17616. Appl Environ Microbiol. 71:1822–1828.
- Olasz F, Kiss J, Konig P, Buzas Z, Stalder R, Arber W. 1998. Target specificity of insertion element IS30. Mol Microbiol. 28:691–704.
- Olliver A, Valle M, Chaslus-Dancla E, Cloeckaert A. 2005. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar Typhimurium DT204 acrB mutants selected with fluoroquinolones. Antimicrob Agents Chemother. 49:289–301.
- Papadopoulos D, Schneider D, Meier-Eiss J, Arber W, Lenski RE, Blot M. 1999. Genomic evolution during a 10,000-generation experiment with bacteria. Proc Natl Acad Sci USA. 96:3807–3812.
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MT, Churcher CM, Bentley SD, Mungall KL, et al. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 35:32–40.
- Pasternak C, Ton-Hoang B, Coste G, Bailone A, Chandler M, Sommer S. 2010. Irradiation-induced Deinococcus radiodurans genome fragmentation triggers transposition of a single resident insertion sequence. PLoS Genet. 6:e1000799.
- Perez M, Calles-Enriquez M, del Rio B, Ladero V, Martin MC, Fernandez M, Alvarez MA. 2015. IS256 abolishes gelatinase activity and biofilm formation in a mutant of the nosocomial pathogen Enterococcus faecalis V583. Can J Microbiol. 61:517–519.
- Petersen C, Moller LB, Valentin-Hansen P. 2002. The cryptic adenine deaminase gene of Escherichia coli. Silencing by the nucleoid-associated DNA-binding protein, H-NS, and activation by insertion elements. J Biol Chem. 277:31373–31380.
- Pilacinski W, Mosharrafa E, Edmundson R, Zissler J, Fiandt M, Szybalski W. 1977. Insertion sequence IS2 associated with int-constitutive mutants of bacteriophage lambda. Gene. 2:61–74.
- Podglajen I, Breuil J, Casin I, Collatz E. 1995. Genotypic identification of two groups within the species Bacteroides fragilis by ribotyping and by analysis of PCR-generated fragment patterns and insertion sequence content. J Bacteriol. 177:5270–5275.
- Podglajen I, Breuil J, Collatz E. 1994. Insertion of a novel DNA sequence, 1S1186, upstream of the silent carbapenemase gene cfiA, promotes expression of carbapenem resistance in clinical isolates of Bacteroides fragilis. Mol Microbiol. 12:105–114.
- Podglajen I, Breuil J, Rohaut A, Monsempes C, Collatz E. 2001. Multiple mobile promoter regions for the rare carbapenem resistance gene of Bacteroides fragilis. J Bacteriol. 183:3531–3535.
- Poirel L, Cabanne L, Vahaboglu H, Nordmann P. 2005a. Genetic environment and expression of the extended-spectrum beta-lactamase blaPER-1 gene in Gram-negative bacteria. Antimicrob Agents Chemother. 49:1708–1713.
- Poirel L, Decousser JW, Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a bla(CTX-M) beta-lactamase gene. Antimicrob Agents Chemother. 47:2938–2945.
- Poirel L, Jayol A, Bontron S, Villegas MV, Ozdamar M, Turkoglu S, Nordmann P. 2015. The mgrB gene as a key target for acquired resistance to colistin in Klebsiella pneumoniae. J Antimicrob Chemother. 70:75–80.
- Poirel L, Lartigue MF, Decousser JW, Nordmann P. 2005b. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob Agents Chemother. 49:447–450.
- Poirel L, Marque S, Heritier C, Segonds C, Chabanon G, Nordmann P. 2005c. OXA-58, a novel class D {beta}-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 49:202–208.
- Polard P, Seroude L, Fayet O, Prere MF, Chandler M. 1994. One-ended insertion of IS911. J Bacteriol. 176:1192–1196.
- Prentki P, Teter B, Chandler M, Galas DJ. 1986. Functional promoters created by the insertion of transposable element IS1. J Mol Biol. 191:383–393.
- Prere MF, Chandler M, Fayet O. 1990. Transposition in Shigella dysenteriae: isolation and analysis of IS911, a new member of the IS3 group of insertion sequences. J Bacteriol. 172:4090–4099.
- Preston A, Parkhill J, Maskell DJ. 2004. The bordetellae: lessons from genomics. Nat Rev Microbiol. 2:379–390.
- Prudhomme M, Turlan C, Claverys JP, Chandler M. 2002. Diversity of Tn4001 transposition products: the flanking IS256 elements can form tandem dimers and IS circles. J Bacteriol. 184:433–443.
- Rajeshwari R, Sonti RV. 2000. Stationary-phase variation due to transposition of novel insertion elements in Xanthomonas oryzae pv. oryzae. J Bacteriol. 182:4797–4802.
- Randall CP, Gupta A, Jackson N, Busse D, O'Neill AJ. 2015. Silver resistance in Gram-negative bacteria: a dissection of endogenous and exogenous mechanisms. J Antimicrob Chemother. 70:1037–1046.
- Rasmussen BA, Kovacs E. 1991. Identification and DNA sequence of a new Bacteroides fragilis insertion sequence-like element. Plasmid. 25:141–144.
- Reynolds AE, Felton J, Wright A. 1981. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature. 293:625–629.
- Rezwan F, Lan R, Reeves PR. 2004. Molecular basis of the indole-negative reaction in Shigella strains: extensive damages to the tna operon by insertion sequences. J Bacteriol. 186:7460–7465.
- Roberts D, Hoopes BC, McClure WR, Kleckner N. 1985. IS10 transposition is regulated by DNA adenine methylation. Cell 43:117–130.
- Rogers MB, Bennett TK, Payne CM, Smith CJ. 1994. Insertional activation of cepA leads to high-level beta-lactamase expression in Bacteroides fragilis clinical isolates. J Bacteriol. 176:4376–4384.
- Rohmer L, Fong C, Abmayr S, Wasnick M, Larson Freeman TJ, Radey M, Guina T, Svensson K, Hayden HS, Jacobs M, et al. 2007. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 8:R102.
- Rojo-Bezares B, Estepa V, Cebollada R, de Toro M, Somalo S, Seral C, Castillo FJ, Torres C, Saenz Y. 2014. Carbapenem-resistant Pseudomonas aeruginosa strains from a Spanish hospital: characterization of metallo-beta-lactamases, porin OprD and integrons. Int J Med Microbiol. 304:405–414.
- Ross JA, Wardle SJ, Haniford DB. 2010. Tn10/IS10 transposition is downregulated at the level of transposase expression by the RNA-binding protein Hfq. Mol Microbiol. 78:607–621.
- Rudant E, Courvalin P, Lambert T. 1998. Characterization of IS18, an element capable of activating the silent aac(6')-Ij gene of Acinetobacter sp. 13 strain BM2716 by transposition. Antimicrob Agents Chemother. 42:2759–2761.
- Ruiz-Martinez L, Lopez-Jimenez L, d'Ostuni V, Fuste E, Vinuesa T, Vinas M. 2011. A mechanism of carbapenem resistance due to a new insertion element (ISPa133) in Pseudomonas aeruginosa. Int Microbiol. 14:51–58.
- Saedler H, Reif HJ, Hu S, Davidson N. 1974. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 132:265–289.
- Safi H, Barnes PF, Lakey DL, Shams H, Samten B, Vankayalapati R, Howard ST. 2004. IS6110 functions as a mobile, monocyte-activated promoter in Mycobacterium tuberculosis. Mol Microbiol. 52:999–1012.
- Schaeffer LM, Schmidt ML, Demuth DR. 2008. Induction of Aggregatibacter actinomycetemcomitans leukotoxin expression by IS1301 and orfA. Microbiology (Reading, Engl.). 154:528–538.
- Schmid AK, Lipton MS, Mottaz H, Monroe ME, Smith RD, Lidstrom ME. 2005. Global whole-cell FTICR mass spectrometric proteomics analysis of the heat shock response in the radioresistant bacterium Deinococcus radiodurans. J Proteome Res. 4:709–718.
- Schneider D, Duperchy E, Coursange E, Lenski RE, Blot M. 2000. Long-term experimental evolution in Escherichia coli. IX. Characterization of insertion sequence-mediated mutations and rearrangements. Genetics. 156:477–488.
- Schneider D, Lenski RE. 2004. Dynamics of insertion sequence elements during experimental evolution of bacteria. Res Microbiol. 155:319–327.
- Schnetz K, Rak B. 1992. IS5: a mobile enhancer of transcription in Escherichia coli. Proc Natl Acad Sci USA. 89:1244–1248.
- Segal H, Nelson EC, Elisha BG. 2004. Genetic environment and transcription of ampC in an Acinetobacter baumannii clinical isolate. Antimicrob Agents Chemother. 48:612–614.
- Shen MM, Raleigh EA, Kleckner N. 1987. Physical analysis of Tn10- and IS10-promoted transpositions and rearrangements. Genetics. 116:359–369.
- Siguier P, Filee J, Chandler M. 2006. Insertion sequences in prokaryotic genomes. Curr Opin Microbiol. 9:526–531.
- Siguier P, Gagnevin L, Chandler M. 2009. The new IS1595 family, its relation to IS1 and the frontier between insertion sequences and transposons. Res Microbiol. 160:232–241.
- Siguier P, Gourbeyre E, Chandler M. 2014. Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol Rev. FEMS Microbiol Rev. 38:865–891.
- Siguier P, Gourbeyre E, Varani A, Ton-Hoang B, Chandler M. 2015. Everyman’s guide to bacterial insertion sequences. In: Mobile DNA III. Washington (DC): American Society of Microbiology.
- Simons RW, Hoopes BC, McClure WR, Kleckner N. 1983. Three promoters near the termini of IS10: pIN, pOUT, and pIII. Cell 34:673–682.
- Simpson AE, Skurray RA, Firth N. 2000. An IS257-derived hybrid promoter directs transcription of a tetA(K) tetracycline resistance gene in the Staphylococcus aureus chromosomal mec region. J Bacteriol. 182:3345–3352.
- Simser JA, Rahman MS, Dreher-Lesnick SM, Azad AF. 2005. A novel and naturally occurring transposon, ISRpe1 in the Rickettsia peacockii genome disrupting the rickA gene involved in actin-based motility. Mol Microbiol. 58:71–79.
- Skaugen M, Nes IF. 1994. Transposition in Lactobacillus sake and its abolition of lactocin S production by insertion of IS1163, a new member of the IS3 family. Appl Environ Microbiol. 60:2818–2825.
- Skaugen M, Nes IF. 2000. Transposition in Lactobacillus sakei: inactivation of a second lactocin S operon by the insertion of IS1520, a new member of the IS3 family of insertion sequences. Microbiology. 146:1163–1169.
- Snesrud E, He S, Chandler M, Dekker JP, Hickman AB, McGann P, Dyda F. 2016. A model for transposition of the colistin resistance gene mcr-1 by ISApl1. Antimicrob Agents Chemother. 60:6973–6976.
- Sobrero P, Valverde C. 2012. The bacterial protein Hfq: much more than a mere RNA-binding factor. Crit Rev Microbiol. 38:276–299.
- Soki J, Gal M, Brazier JS, Rotimi VO, Urban E, Nagy E, Duerden BI. 2006. Molecular investigation of genetic elements contributing to metronidazole resistance in Bacteroides strains. J Antimicrob Chemother. 57:212–220.
- Sousa A, Bourgard C, Wahl LM, Gordo I. 2013. Rates of transposition in Escherichia coli. Biol Lett. 9:20130838.
- Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, Garnier T, Meurice G, Simon D, Bouchier C, Ma L, et al. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 17:192–200.
- Sun X, Dennis JJ. 2009. A novel insertion sequence derepresses efflux pump expression and preadapts Pseudomonas putida S12 for extreme solvent stress. J Bacteriol. 191:6773–6777.
- Szeverenyi I, Nagy Z, Farkas T, Olasz F, Kiss J. 2003. Detection and analysis of transpositionally active head-to-tail dimers in three additional Escherichia coli IS elements. Microbiology (Reading, Engl.). 149:1297–1310.
- Takahashi K, Sekine Y, Chibazakura T, Yoshikawa H. 2007. Development of an intermolecular transposition assay system in Bacillus subtilis 168 using IS4Bsu1 from Bacillus subtilis (natto). Microbiology (Reading, Engl.). 153:2553–2559.
- Tanaka KH, Dallaire-Dufresne S, Daher RK, Frenette M, Charette SJ. 2012. An insertion sequence-dependent plasmid rearrangement in Aeromonas salmonicida causes the loss of the type three secretion system. PLoS One. 7:e33725.
- Tibazarwa C, Wuertz S, Mergeay M, Wyns L, van der Lelie D. 2000. Regulation of the cnr cobalt and nickel resistance determinant of Ralstonia eutropha (Alcaligenes eutrophus) CH34. J Bacteriol. 182:1399–1409.
- Tolmasky ME, Crosa JH. 1995. Iron transport genes of the pJM1-mediated iron uptake system of Vibrio anguillarum are included in a transposonlike structure. Plasmid. 33:180–190.
- Treves DS, Manning S, Adams J. 1998. Repeated evolution of an acetate-crossfeeding polymorphism in long-term populations of Escherichia coli. Mol Biol Evol. 15:789–797.
- Trinh S, Haggoud A, Reysset G, Sebald M. 1995. Plasmids Pip419 and Pip421 from Bacteroides – 5-nitroimidazole resistance genes and their upstream insertion-sequence elements. Microbiology-UK. 141:927–935.
- Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 258:72–77.
- Twiss E, Coros AM, Tavakoli NP, Derbyshire KM. 2005. Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol Microbiol. 57:1593–1607.
- Twomey DP, McKAy LL, O'Sullivan DJ. 1998. Molecular characterization of the Lactococcus lactis LlaKR2I restriction-modification system and effect of an IS982 element positioned between the restriction and modification genes. J Bacteriol. 180:5844–5854.
- van der Ploeg J, Willemsen M, van Hall G, Janssen DB. 1995. Adaptation of Xanthobacter autotrophicus GJ10 to bromoacetate due to activation and mobilization of the haloacetate dehalogenase gene by insertion element IS1247. J Bacteriol. 177:1348–1356.
- Vandecraen J, Monsieurs P, Mergeay M, Leys N, Aertsen A, Van Houdt R. 2016. Zinc-induced transposition of insertion sequence elements contributes to increased adaptability of Cupriavidus metallidurans. Front Microbiol. 7:359.
- Vogel J, Bartels V, Tang TH, Churakov G, Slagter-Jager JG, Huttenhofer A, Wagner EG. 2003. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 31:6435–6443.
- Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat Rev Microbiol. 9:578–589.
- Wachino J, Yamane K, Kimura K, Shibata N, Suzuki S, Ike Y, Arakawa Y. 2006. Mode of transposition and expression of 16S rRNA methyltransferase gene rmtC accompanied by ISEcp1. Antimicrob Agents Chemother. 50:3212–3215.
- Wang A, Roth JR. 1988. Activation of silent genes by transposons Tn5 and Tn10. Genetics 120:875–885.
- Wery J, Hidayat B, Kieboom J, de Bont JA. 2001. An insertion sequence prepares Pseudomonas putida S12 for severe solvent stress. J Biol Chem. 276:5700–5706.
- Wolter DJ, Hanson ND, Lister PD. 2004. Insertional inactivation of oprD in clinical isolates of Pseudomonas aeruginosa leading to carbapenem resistance. FEMS Microbiol Lett. 236:137–143.
- Wood MS, Byrne A, Lessie TG. 1991. IS406 and IS407, two gene-activating insertion sequences for Pseudomonas cepacia. Gene. 105:101–105.
- Wu Y, Aandahl RZ, Tanaka MM. 2015. Dynamics of bacterial insertion sequences: can transposition bursts help the elements persist? BMC Evol Biol. 15:288.
- Zambrano MM, Siegele DA, Almiron M, Tormo A, Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760.
- Zerbib D, Polard P, Escoubas JM, Galas D, Chandler M. 1990. The regulatory role of the IS1-encoded InsA protein in transposition. Mol Microbiol. 4:471–477.
- Zhang Z, Saier MH. Jr. 2009a. A mechanism of transposon-mediated directed mutation. Mol Microbiol. 74:29–43.
- Zhang Z, Saier MH. Jr. 2009b. A novel mechanism of transposon-mediated gene activation. PLoS Genet. 5:e1000689.
- Ziebuhr W, Krimmer V, Rachid S, Lossner I, Gotz F, Hacker J. 1999. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 32:345–356.
- Zinser ER, Schneider D, Blot M, Kolter R. 2003. Bacterial evolution through the selective loss of beneficial genes. Trade-offs in expression involving two loci. Genetics. 164:1271–1277.
