Abstract
Intense research has confirmed the formerly theoretical distribution of amyloids in nature, and studies on different systems have illustrated the role of these proteins in microbial adaptation and in interactions with the environment. Two lines of research are expanding our knowledge on functional amyloids: (i) structural studies providing insights into the molecular machineries responsible for the transition from monomer to fibers and (ii) studies showing the way in which these proteins might participate in the microbial fitness in natural settings. Much is known about how amyloids play a role in the social behavior of bacteria, or biofilm formation, and in the adhesion of bacteria to surfaces; however, we are still in the initial stages of understanding a complementary involvement of amyloids in bacteria–host interactions. This review will cover the following two topics: first, the key aspects of the microbial platforms dedicated to the assembly of the fibers, and second, the mechanisms by which bacteria utilize the morphological and biochemical variability of amyloids to modulate the immunological response of the host, plants and humans, contributing to (i) infection, in the case of pathogenic bacteria or (ii) promotion of the health of the host, in the case of beneficial bacteria.
Introduction
Functional amyloids are a very heterogeneous class of proteins that are widely distributed in nature from humans to bacteria and that have a variety of functions that are fundamentally different from the pathological features of their relatives “traditional” amyloids. In bacteria, some physiological processes known to be associated with functional amyloids are as follows: (i) bacterial cell attachment and biofilm formation (Chapman et al. Citation2002; Dueholm et al. Citation2010; Romero et al. Citation2010), (ii) reproduction (Claessen et al. Citation2003), (iii) control of plasmid replication (Molina-García et al. Citation2016), (iv) cytotoxicity (Hetz et al. Citation2002) and virulence (Oh et al. Citation2007). In eukaryotes, functional amyloids have been found to be dedicated to the following: (i) cell adhesion in yeasts (Ramsook et al. Citation2010), (ii) hyphae compatibility in filamentous fungi (Coustou-Linares et al. Citation2001), (iii) antimicrobial activity (Jang et al. Citation2011), iv) structural functions (Iconomidou et al. Citation2000; Kenney et al. Citation2002), and (v) detoxification in certain physiological processes (Fowler et al. Citation2005) and hormone storage (Maji et al. Citation2009). These examples are indicative of the versatility of the amyloid state in their contributions to multiple aspects of the biology of living organisms. In addition to differences in their biological significance, the idea that the polymerization process of functional amyloids is tightly controlled in comparison to their pathogenic siblings is widely supported; functional amyloids partner with themselves and with several other proteins in the system for localization and stabilization. Some functional amyloids are secreted to the outside of the cell, where they polymerize into long fibers that are used for attachment to surfaces or biofilm formation; others remain inside the cell, occupying optimal subcellular localizations and developing their functionality in normal cell physiological processes. Such is the case of Hfq, an RNA chaperone in Escherichia coli. The C-terminus of this protein displays typical amyloid features and the capacity to self-assemble into fibrils. This protein appears to be localized in patterns within the cell cytoplasm (Fortas et al. Citation2015).
Although there are no apparent similarities in their amino acid sequences, amyloids fold into fibers with a common quaternary structure characterized by a cross-β pattern, in which hydrogen-bonded β-strands run perpendicularly to the axis of the fibril (Knowles et al. Citation2014). In the native state, the folding pattern is determined by the intramolecular interactions that occur between the different amino acids, whereas in the amyloid state, intermolecular interactions are favored, resulting in the formation of the distinctive amyloid fiber (Fitzpatrick et al. Citation2015). Additionally, this β-sheet-enriched structure grants amyloids some characteristic tinctorial properties useful for their identification (LeVine 1993; Klunk et al. Citation1999). All these structural features provide amyloid fibers with great robustness and stability, which are the reasons behind the widespread presence of amyloids in nature. Thus, even though amyloids were first studied in the brain tissue deposits of patients suffering from Alzheimer’s disease, the pathogenic implications of some amyloids are apparently the exception; new possible and unpredictable functions are emerging for amyloid proteins traditionally associated with neurodegenerative disorders (Soscia et al. Citation2010).
1. The sophisticated machineries controlling polymerization of functional amyloids
1.1. The intrinsic abilities of proteins to transition to the amyloid state
Intrinsic disorder in protein structure also seems to be a common feature in amyloids. There are a large number of amyloid proteins that have regions within their amino acid sequences that lack any specific structure and are therefore classified as random coils. Both disease-related amyloids and highly functional proteins often have a high level of intrinsic disorder (Tompa Citation2009). For example, in the human proteome, several proteins involved in important functions, such as signaling or regulation, share the feature of having a high number of intrinsically disordered regions (for an extensive review of the relationship between amyloidogenesis and intrinsic disorder, see Tompa Citation2009), showing that a certain level of intrinsic disorder in proteins is required to maintain highly specialized and controlled functions, most likely functional amyloidogenesis. However, this means that proteins with a significant proportion of intrinsic disorder are also involved in a considerable number of diseases, even in neurodegenerative pathologies; a strong correlation exists between the presence of disordered regions in proteins and disease (Uversky et al. Citation2008). These regions, flexible in structure, allow the interactions of proteins with different molecules, including protein-protein interactions, and it is an interesting suggestion that the aggregation-prone behavior that is found in most amyloids can be somehow influenced by these intrinsically disordered regions. A recent study in fungal hydrophobins performed using molecular-dynamic simulations shows that, indeed, a small loop of 26 amino acids seems to be critical in regulating the aggregation of EAS from Neurospora crassa (Simone et al. Citation2012). The structural dynamics of the protein, which is highly soluble in solution but prone to aggregation at the air-water interface, show that conformational changes and the exposure of certain regions can drastically modify protein behavior, which, in fact, could be a good strategy under specific environmental circumstances. For these reasons, the prediction of intrinsic disorder within a protein sequence could be a good indicator of amyloidogenesis in addition to other evidence and could be used as a complementary tool. MetaDisorder (Kozlowski and Bujnicki Citation2012) is a disorder prediction web-based tool that combines several publicly available prediction methods to accurately find a consensus in the prediction of any given amino acid sequence.
1.2. The dedicated amyloid platforms
There are subtle differences in the fine structures of functional and traditionally pathological amyloids, and similar dynamics of polymerization presumably occur when they are both studied in vitro. A noticeable difference, however, appears to be related to the tight regulatory mechanisms that drive the polymerization of functional amyloids in contrast to those that drive the polymerization of pathological amyloids. Several strategies have been found in nature for the proper assembly of amyloid fibrils from monomers with the aim of reducing the accumulation of diffusible toxic oligomeric intermediates (Sengupta et al. Citation2016).
Chaperones are key players in the aggregation of several fungal prions. An example of this is the Sup35 prion protein in yeast, which is propagated in [PSI+] variants and was one of the first discovered hereditary elements not associated with genes, since the prion state is self-propagating and transmits itself. In this system, the interplay between several chaperones and the balance between aggregase/disaggregase activities in protein homeostasis seems to be critical in the regulation of prion formation (Serio and Lindquist Citation2001). When these processes are coordinated, Sup35 functions as a translation termination factor in protein synthesis. However, under certain conditions, Sup35 is misfolded, thereby generating the prion form, which is the amyloid state of Sup35 (Paushkin et al. Citation1996). In this process, the chaperone Hsp104 seems to be of great importance and functions by reducing the size of the Sup35 fibrils. As a result of this “trimming”, the fibrils turn more infective since these smaller seeds are also more diffusible (Grimminger-Marquardt and Lashuel, Citation2010). Therefore, Hsp104 is required for the maintenance and propagation of the [PSI+] variant, which shows the extent to which the process of amyloidogenesis is controllable or even reversible.
In some groups of bacteria, remarkable complex systems exist to manage the assembly of functional amyloids. In these systems, several proteins interact with the major protein component of the fiber to enable efficient polymerization. Here, we summarize some of the known functional amyloid systems (the main amyloid plus the additional proteins required for proper folding, assembly and localization of the fibrils) for gram-positive and gram-negative bacteria.
The curli amyloid fibers of Escherichia coli and Salmonella spp. are composed mainly of the major protein component CsgA (and its corresponding ortholog in Salmonella spp.) (Chapman et al. Citation2002). Nonetheless, the protein CsgB, encoded in the same operon csgABC, acts as a nucleator of curli fiber assembly. CsgB facilitates polymerization of CsgA into fibers upon interaction and seems to be responsible for the attachment of the fibers to the cell surface (Hammer et al. Citation2007; Shu et al. Citation2012). In addition to CsgB, there are 5 other proteins, CsgC-G, which are similarly important in fiber assembly even though they are not directly involved as components of the fiber. CsgD acts as a positive transcriptional regulator of the system (Hammar et al. Citation1995), whereas the other proteins have a more complex role. CsgG is responsible for the formation of a channel structure in the outer membrane that allows for the secretion of CsgA, CsgB, and CsgF from the periplasm to the outside of the cell (Robinson et al. Citation2006). CsgE interacts with CsgA by a signal-peptide dependent mechanism, conferring substrate specificity to the CsgG-mediated secretion (Nenninger et al. Citation2011). In addition, it has been shown that CsgE blocks the polymerization of CsgA in vitro, and it is therefore thought to be a molecular chaperone that restricts curli fiber formation to the extracellular space, thus preventing the production of toxic amyloid oligomers inside the cell (Nenninger et al. Citation2011). In a similar way, it has been shown that CsgC functions as a very selective and efficient amyloid inhibitor that is able to impede polymerization of CsgA and other amyloid proteins that share a common motif within their sequence (Evans et al. Citation2015). Finally, CsgF works as a chaperone that facilitates CsgB localization and function (Nenninger et al. Citation2009).
Accessory proteins that assist bacterial amyloid polymerization are also found in other gram-negative bacteria. The fapABCDEF operon is present in several members of the Beta-, Delta-, and Gammaproteobacteria (Dueholm et al. Citation2013a) and has been well characterized in Pseudomonas spp. This operon is responsible for encoding the Fap amyloid fibrils found in Pseudomonas spp. biofilms and plays a role in their hydrophobicity and robustness (Zeng et al. Citation2015). The protein FapC is the major protein component of the Fap fibers (Dueholm et al. Citation2010). However, the other Fap proteins play an important role in the proper assembly and function of the FapC fibril (Dueholm et al. Citation2013b). In addition to FapC, there are two other proteins present in a lower proportion in the fiber: FapB and FapE. FapB seems to function as a nucleator protein in a similar way as CsgB in curli, whereas the role of FapE is still uncertain. Similar to curli, all the Fap proteins are secreted via Sec across the inner membrane, and once located in the periplasm, they are sorted according to their final destinations. FapA and D remain in the periplasm, while FapB, C and E are potentially secreted to the outside of the cell by FapF, which has been identified by bioinformatic methods as a probable membrane-associated pore with a β-barrel conformation. This has been recently confirmed by the determination of the complete 3 D structure of FapF, which is defined as a trimer composed of three beta barrels plugged by α-helices in its closed state, resembling the classical structure of the Type V auto-transporter family (Rouse et al. Citation2017). In this protein, the N-terminal coiled-coil region aids the complex in forming a stable trimer and is required for the secretion of FapC outside the cell via a mechanism that probably involves a conformational change in the plugs, which have been shown to be additionally required for FapF-dependent FapC secretion. Evidence suggests that in the periplasm, FapA acts as a molecular chaperone, possibly facilitating the incorporation of FapC and B monomers to the growing fibers, whereas FapD might be a dedicated protease for the processing of other Fap proteins, which in collaboration with FapF might direct their transport to the extracellular milieu. Indeed, FapD has been characterized as a cysteine-peptidase active in the periplasm and required for FapC secretion, which most likely targets FapE since it has been demonstrated that the N-terminal region of FapE is cleaved during the secretion process (Rouse et al. Citation2017). It is also interesting to note that the C-terminal region of FapE contains amino acid repeats similar to the amyloid regions found in FapC and B suggesting a FapD-dependent processing of FapE prior to the formation of the amyloid fibril, which would facilitate FapC and B secretion through FapF. However, despite these efforts, there are still aspects of the Fap functional amyloid system that remain to be understood, and further experimental evidence is necessary to elucidate some of the proposed roles for the Fap auxiliary proteins.
Another interesting example of functional amyloids that can be found in gram-negative plant-pathogenic bacteria are the harpins. Harpins are a family of glycine-rich proteins present in the bacterial species Erwinia amylovora (Barny, Citation1995), several members of the Pseudomonas syringae group (He et al. Citation1993; Charkowski et al. Citation1998; Lee et al. Citation2001), Xanthomonas spp (Kim et al. Citation2003), and Ralstonia solanacearum (Li et al. Citation2010). One of the most distinctive features of this family of proteins is their ability to trigger the hypersensitive response (HR) in plants in the absence of bacterial cells () (Wei et al. Citation1992). It has been shown that some harpins are able to polymerize into fibrils with amyloid properties due to intrinsic properties of their own sequences. This feature has been extensively studied in a harpin called HpaG from Xanthomonas spp. in which the rate of fibril formation seems to be influenced by a domain located at the C-terminal half of the protein, whereas the α-helical domain at the amino-terminal half of the protein is essential for the induction of HR in plants (Oh et al. Citation2007).
Posttranslational modifications also play a role in the formation and function of bacterial amyloids. Microcin E492 (MccE492), a bacteriocin produced by Klebsiella pneumoniae, is a low molecular weight antimicrobial compound secreted by this bacterium (de Lorenzo & Pugsley Citation1985), which has the ability to form amyloid fibrils, both in vivo and in vitro, which seem to modulate the antibacterial activity of this protein since fibril formation is associated with the loss of antibacterial activity and fibrils can also act as reservoirs of toxic oligomeric intermediates (Bieler et al. Citation2005). In this antimicrobial amyloid, modification of the C-terminal end by addition of a small molecule seems to be important to confer antibacterial properties to this peptide. This modified form of the microcin accumulates during the stationary phase of bacterial growth and is less efficient in the formation of amyloid fibers. Contrarily, amyloids seeds of the unmodified version alone polymerize into fibrils with amyloid properties more rapidly (Marcoleta et al. Citation2013). Interestingly, it has been shown that this amyloid behaves as a prion-like protein in which the β-rich aggregated form (presumably, posttranslationally unmodified) induces the conversion of the soluble and active form into the latter, altering the regular function of the protein and acting as a regulator of the antimicrobial activity (Shahnawaz et al. Citation2017).
In the gram-positive bacterium Bacillus subtilis (and other related Bacilli), TasA is another example of a functional amyloid that requires accessory proteins for proper formation and function. TasA was initially discovered in the endospore coat and, in addition, showed a broad antibiotic spectrum; these two features led to the protein being called the Translocation-dependent antimicrobial spore component (Stöver and Driks Citation1999). However, it was later found that this protein is also essential in biofilm formation and is able to polymerize to form amyloid-like fibrils both in vivo and in vitro with the distinctive features of a functional amyloid (Romero et al. Citation2010; Diehl et al. Citation2018). Indeed, factors affecting the polymerization of TasA have been studied in vitro in a pre-aggregated conformation purified from the B. subtilis extracellular matrix, showing that environmental conditions are determinants in the triggering of the process of amyloidogenesis. Acidic pH and hydrophobic surfaces seem to be factors that promote complete fibrillation of these pre-formed aggregates (Chai et al. Citation2013). This behavior is not unprecedented; indeed, it has been demonstrated how local environmental conditions can determine the molecular switch of these proteins. Studies performed on other amyloids, such as the islet amyloid polypeptide (IAPP), an amyloid peptide produced by the β-cells that has been found to be a determinant in the development of type 2 diabetes mellitus, or the amyloid Aβ peptide have demonstrated that surface properties are critical to modulate amyloid polymerization and determine the abundance of the different amyloid species (Kowalewski and Holtzman Citation1999; Keller et al. Citation2011; Moores et al. Citation2011). Acidity or basicity are also chemical conditions that determine the protonation and charge states of radical chains of amino acids, therefore affecting the H bonds that form the β-sheets. Studies on aggregation of the recombinant amyloidogenic light chain variable domain showed that fibrils were obtained only at pH <7, demonstrating that pH conditions affect oligomer interactions (Zhu et al. Citation2002).
Nonetheless, there are two other proteins necessary for TasA fiber assembly: SipW and TapA. These three proteins are part of the tapA-sipW-tasA operon, which is transcriptionally regulated by SinI-SinR in response to several factors that also modulate biofilm formation (Vlamakis et al. Citation2013). SipW is a bifunctional signal peptidase in charge of processing and translocation of both TasA and TapA to the exterior of the cell, where the formation of the amyloid fibrils occurs. In addition, and independent of its role as a peptidase, SipW seems to act as a regulatory element of the expression of the tapA and eps operons in the formation of solid-surface-adhered biofilms by a mechanism yet unknown (Terra et al. Citation2012). TapA is required for biofilm and TasA fiber formation, and its absence leads to the following: i) defects in pellicle formation and colony architecture and ii) a dramatic decrease in the levels of TasA. The remaining TasA produced by this mutant is not able to polymerize efficiently and is observed to not have any apparent attachment to the cell surfaces. Thus, TapA has been proposed as being necessary for anchoring of the fibers to the cell by a yet unknown mechanism (Romero et al. Citation2011). In vitro, it has been shown that TapA enhances the speed of polymerization of TasA (Romero et al. Citation2014) approximately six-fold; therefore, it could act as a nucleator protein, similar to CsgB in the curli system. However, and in contrast to its possible analog in E. coli, TapA has not been proven to exhibit amyloid properties. A genomic region that contains orthologs of tasA and sipW but not tapA is also found in the closely related bacterium B. cereus, in which these two proteins are also involved in the formation of biofilms (Caro-Astorga et al. Citation2014). Interestingly, this region includes an additional gene called calY, an ortholog of tasA from B. subtilis. The two orthologous, B. cereus tasA and calY, are functional when they are expressed in a mutant of the tapA operon in B. subtilis, indicating that this region encodes the minimum requirement for the proper assembly of the fibers that are part of the extracellular matrix of bacilli biofilms. Although not yet demonstrated, CalY possesses properties that suggest that this protein could behave as a putative amyloid. However, TasA shows a much higher tendency towards aggregation when compared to CalY (Caro-Astorga et al. Citation2014). Therefore, whether these two proteins act in a coordinated manner during the assembly of the fibers required for biofilm formation is a subject that is being currently explored and remains to be understood.
The chaplins, a family of hydrophobic proteins found on the surface of hyphae of the filamentous gram-positive bacterium Streptomyces coelicolor, have the ability of self-assembly into fibrils with amyloid properties (Claessen et al. Citation2003). The chaplin proteins are divided in two subgroups: the long chaplins (ChpA-C) and the short chaplins (ChpD-H). The long chaplins contain two of the so-called “chaplin” hydrophobic domains, whereas the small chaplins only contain one. The fibrils from ChpE and ChpH, which are genes that become transcriptionally active when the mycelium is still submerged, are responsible for the formation of a thin film that reduces the tension at the air-water interface, allowing the hyphae to emerge into the air. Once in the exterior, the hyphae are then surrounded by a rodlet layer, a surface layer present in both the hyphae and the spores, which is formed by the action of the rodlins, RdlA and RdlB, along with the chaplins (Claessen et al. Citation2004). Interestingly, RdlB has also recently been shown to build fibrils or to bind specific dyes, features characteristic of amyloid proteins (Yang et al. Citation2017). Under this layer of rodlins, we find a mosaic of fibrils composed of different chaplins. It is hypothesized that diverse regions in the long chaplins cooperatively lead to the correct localization of the fibers outside the cells; the exclusive hydrophilicity permits the chaplins to cross the cell wall, and an LAXTG motif facilitates their covalent attachment to the peptidoglycan. These long fibers may serve as a stable structure for further co-assembly with the short chaplins and for their immobilization at the cell surface (Claessen et al. Citation2003). The intricate layers of chaplins possess chemical features exploitable for several functions: i) the hydrophobicities of the layers confer protection to the surface of the spore, ii) their association with cellulose in the biofilm improves bacterial adhesion to surfaces (De Jong et al. Citation2009), and iii) the external layer composed of rodlins ensures the development of spores in tight association with the colony, thereby avoiding their release. (Yang et al. Citation2017).
An interesting example of convergence in amyloid functions can be found in Solibacillus silvestris AM1, a recently isolated sporulating gram-positive bacteria from an estuarine ecosystem that is able to produce a bioemulsifier (surface-active molecules that are amphiphilic in nature and can form stable emulsions with several organic solvents) that exhibits amyloid properties. This functional amyloid, designated BE-AM1, is an extracellular glycoprotein that forms complexes of large molecular weight built from subunits that are 30 KDa in size. In addition, its ability to change surfaces properties, in a manner like the Chaplins of Streptomyces coelicolor (see above paragraph), might serve as an essential tool for colonization and persistence in several niches. In addition its properties as a bioemulsifier, this protein plays a role in cell adhesion to surfaces and in biofilm formation, as do many other functional amyloids that have been described throughout this review (Markande and Nerurkar Citation2016).
An intriguing model of bacterial amyloids are the phenol-soluble modulins (PSMs) of the human pathogen Staphylococcus sp. The phenol-soluble modulins (PSMs) are a group of small peptides with surfactant properties, which are encoded by different operons: the psmα and psmβ operons, responsible for the production of PSMα and PSMβ peptides, respectively, and the δ-toxin encoded by the RNAIII, all of which are transcriptionally regulated by the activity of the agr quorum sensing system (Vuong et al. Citation2004). These peptides show a tendency to aggregate in the form of fibrils with amyloid properties (Schwartz et al. Citation2012) and participate in a variety of functions. The PSMs are essential components of the extracellular matrix and contribute to the stabilization, maturation and disassembly of the biofilm structure (Periasamy et al. Citation2012; Schwartz et al. Citation2012). Moreover, the small sizes of these peptides along with their tendency to form aggregates make these small molecules perfect candidates for antimicrobial peptides (AMPs). The cytotoxicity of the amyloid state is a feature of these proteins that has been extensively studied, and therefore, it is not surprising to find functional amyloids in nature that are designed to work as biological weapons (Marshall et al. Citation2014). The toxicity of amyloids is given by the ability of these proteins to form small diffusible oligomers that form channels through biological membranes () (Kourie and Shorthouse, Citation2000). Indeed, PSMs exhibit antimicrobial activity against several microorganisms, such as Micrococcus luteus or Legionella pneumophila (Joo et al. Citation2011; Marchand et al. Citation2011). Furthermore, PSMs secreted by Staphylococcus epidermidis show a strong tendency to interact with synthetic membranes, causing leakage and permeabilization which might be reasoned to be the cause of their antimicrobial activity against potential bacterial pathogens occupying the same niche (Cogen et al. Citation2010b).
Figure 1. Schematic representation of amyloid aggregation from monomers to fibrils. (A) In the fibrillation process, the initial monomeric protein goes through a conformational change that favors the formation of the characteristic beta-sheet enriched structure of the robust amyloid fibers. In this process, different oligomeric intermediates are formed. These oligomers have been described as the most toxic species, since they are shaped as pore-like structure that interfere with biological membranes and are more diffusible than the large macromolecular amyloid fibrils. (B) Bacterial amyloids are secreted to the medium, where they coexist in different forms depending on the environmental conditions. Eventually, the oligomeric species may interact with the surface of the cells inducing the formation of channels in the membrane that may lead to cellular leakage and eventually cell death.
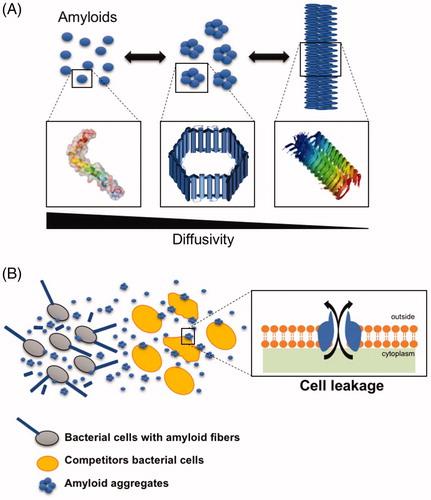
Additionally, apart from the different environmental conditions that modulate polymerization of PSMs and other amyloids, other factors are required to ensure and control the fibrillation process. In this case, extracellular DNA (eDNA) seems to contribute to the polymerization of PSMs by directly interacting with PSMα1 and promoting its polymerization into amyloid fibrils (Schwartz et al. Citation2016). However, not all the PSMs behave in a similar manner, and it has been demonstrated that a certain degree of difference in terms of polymerization and fibril formation exists within the same group of PSMs. Indeed, despite their high similarity in sequence, the four PSMα of Staphylococcus aureus have different physicochemical properties determined by the subtle differences in their sequences, which impacts their propensities to aggregate, and only PSMα1 and PSMα4 are the main constituents of the PSMα amyloid fibrils (Marinelli et al. Citation2016).
2. The role of bacterial amyloids during microbe–host interactions
Prompted by the wide presence of amyloids in so many different species, it is reasonable to think that the versatility of the amyloid state of proteins allows microorganisms to accomplish the fine tuning required for a variety of biological functions, which in the end provides them with adaptive advantages in the natural environment. In this section, we show some examples of how bacterial amyloids are relevant players in the interactions with other organisms.
2.1. Functional amyloids in bacteria-plant interactions
An example of the functionality of amyloids in plant-microbe interactions is the amyloid protein curli that facilitates the adhesion of bacterial cells of E. coli to plant surfaces (). The expression of the curli-dedicated operons in the K12 strain of E. coli, a natural non-producer of curli, restored the ability of the bacterium to form the curli amyloid fibrils and significantly increased the binding of the bacterium to alfalfa sprouts and seed coats (Jeter and Matthysse Citation2005). The adhesion of this bacterium to the plant material was comparable to that of several pathogenic strains of E. coli that naturally exhibit a slow binding that lasts several days. Interestingly, mutants defective in curli production of some of these diarrheagenic strains of E. coli are still able to bind the plant surfaces, which suggests that curli is an important element, although not essential, for plant-bacteria interaction in this species and that several and complementary strategies exist in E. coli that ensure their persistence in and colonization of plant tissues (Jeter and Matthysse Citation2005). Further transcriptomic analysis of two strains of E. coli, K12 and O157:H7, from lettuce or spinach leaves showed that, indeed, the expression of the curli genes csgA and csgB is only increased during the first stages of the interaction and decreases rapidly over the course of time (Fink et al. Citation2012). This finding, indicative of the relevance of curli fibers for the initial establishment of the bacteria on the plant surface, was confirmed by the lower attachment of csgA mutants of O157:H7 to the plant tissue compared to the WT strain during the initial stages of the interaction. Although the mutant in curli reaches adhesion levels similar to those of the wild-type strain, colonization appears to be impaired, which reflects the importance of the curli amyloid fiber in this stage of the interaction with the plant (Carter et al. Citation2016). A similar behavior has been found in other enterobacteria. In Salmonella enterica, the curli fimbriae also play a relevant role in initial attachment on plant surfaces (Barak et al. Citation2005). Interestingly, the curli gene product responsible for this interaction is the ortholog in Salmonella of the csgB gene in E. coli, which, as stated above, is required for the nucleation and polymerization of the fibrils, therefore suggesting a new biological role for this protein. Furthermore, curli does not only mediate interactions with biological surfaces. It has been demonstrated that the curli amyloid fibrils are also needed for the initial attachment and biofilm formation of E. coli O157:H7 on abiotic surfaces such as stainless steel or glass (Carter et al. Citation2016), materials that are commonly employed in the processing of fresh produce, highlighting the importance of precautionary measures and control during the handling of crops.
Figure 2. Bacterial functional amyloids mediate the interaction of bacterial cells with host plants. (A) Curli is a fundament structural element in the adhesion of E. coli cells to the plant leaves in initial stages of plant colonization. (B) Harpins of Xanthomonas are thought to polymerize in the form of fibers in the apoplast of plant tissues, targeting the membranes of plant cells and triggering the plant response known as HR (hypersensitive response). The picture in the inset is representative of a typical HR response characterized by necrotizing of the infiltrated zone. (C) Involvement of the amyloid-like protein TasA of B. subitlis in the colonization of plant rhizosphere. Plant rhizosphere secretions (malic acid and polysaccharides) induce the expression of the structural components of the extracellular matrix, TasA and EPS, necessaries for the assembly of biofilm. At the same time, it is suggested that TasA blocks the microbial triggered immunity (MTI) activated by foliar pathogens, an action that contributes to fully colonization of the rhizosphere and the beneficial contribution to the plant health.
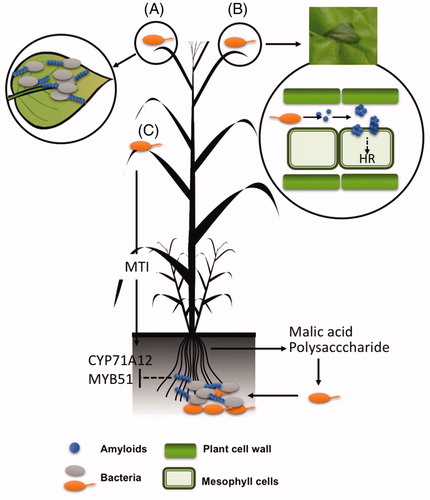
The pinnacle of the interaction of bacterial amyloids with plant hosts are, probably, the harpins. Harpins, as further described in Section 1.2 of this review, are well known for their ability to cause HR in plant hosts. They serve a variety of purposes during plant infection: (i) participating in the translocation of bacterial effectors into plant cells, (ii) as helpers in hrp (hypersensitive response and pathogenesis) type III protein secretion systems, and (iii), as toxins, forming pores in plant membranes which cause depolarization and further plant cell death (see Choi et al. Citation2013 for a comprehensive review concerning the different functions of harpins in plant pathogenesis). The amyloid fibrils and protofibrils from this protein induce the hypersensitive response (HR) in certain plant species, possibly because harpins, as demonstrated for other amyloid proteins, have the ability to interact with membranes through pore-like structures composed of several units (). This hypersensitive response occurs when harpins have already formed fibrils in apoplast-like conditions, which is evidence of the following: i) the connection between amyloid polymerization and the harpin-related HR activity and ii) the fact that acidic conditions, typically found in the plant apoplast (Grignon and Sentenac Citation1991), are conductive to fibrillation of distinct amyloid proteins (Chai et al. Citation2013; Chan et al. Citation2016) or peptides regardless of their amyloid nature (Su and Chang, Citation2001; Carrotta et al. Citation2005; Sasahara et al. Citation2008; Mawhinney et al. Citation2017).
This ability of harpins to induce HR in plants has proven very useful in agriculture. It has been shown that treatment of several cultivars of rice with a peptide corresponding to a fragment of 32 amino acids of the harpin HpaG from Xanthomonas oryzae pv. oryzicola, previously reported to retain HR-inducing properties with minimization of cell death, protects the crop against several diseases and increases the growth rate and yield of the plant (Chen et al. Citation2008). Bioactive products based on extracts from bacteria have been extensively used in safe crop production. Therefore, these studies show an interesting side of amyloids exploitable for the purpose of human applications or the development of amyloid-based products. In fact, a putative combination of these amyloid proteins with other biological control agents of plant diseases might be a promising management strategy in agriculture. Research has begun in this direction, showing that a derivative strain of B. subtilis 168 equipped to produce higher yields of surfactin is even more effective as a biocontrol agent when engineered to produce the harpin HpaG from Xanthomonas oryzae pv. oryzicola (Gao et al. Citation2013).
All the examples presented above address the interactions of pathogenic bacteria and the above-ground parts of plants. B. subtilis, a soil dwelling bacterium, is known to live in association with plants to which it confers notable beneficial effects: i) defense against microbial diseases, either by direct interaction with the pathogen or indirectly inducing the defense mechanisms of plants, ii) promotion of plant growth and iii) resilience to drought (Chen et al. Citation2012; Beauregard et al. Citation2013). An outstanding inter-kingdom chemical communication mediates the establishment of this mutualistic interaction (): the plants produce and secrete organic acids (malic acid) and polysaccharides to the rhizosphere, which are sensed by B. subtilis cells, which respond by forming biofilms. Once established, B. subtilis cells trigger the immune system of the plant, a process called ISR (induced systemic resistance), which contributes to the repression of pathogenic infections aboveground. Microbe-associated molecular patterns is the name given to the bacterial factors that activate the plant innate immune system upon recognition (Lakshmanan et al. Citation2012). Some of them, such as flagellin, are indistinctly present on pathogenic and beneficial bacteria, while others, such as toxins, are specific to pathogenic bacteria; plants have evolved the ability to differentiate between them. The studies on the tripartite interaction of Pseudomonas syringae, a foliar pathogenic bacterium, with Arabidopsis plants and rhizospheric beneficial B. subtilis have furthered the understanding of the intriguing molecular aspects of this chemical signaling interplay (Lakshmanan et al. Citation2012). P. syringae applied to the phyllosphere apparently increases the rhizospheric secretions of malic acid, which, as mentioned earlier, mediates the recruitment of B. subtilis cells to the roots. However, at the same time, P. syringae triggers a long-distance defense mechanism in the roots, which could hypothetically antagonize the bacilli recruitment. Two lines of evidence led to the proposal of a hypothetical role for the amyloid-like protein TasA in the way by which B. subtilis cells manage these two antagonistic situations in a manner conducive to the efficient colonization of roots: first, by mediating the assembly of biofilms. TasA is a major component of the extracellular matrix and a TasA mutant is found to be arrested in biofilms not only in vitro but also on roots (Branda et al. Citation2006; Beauregard et al. Citation2013). Second, and most interestingly, this occurs by repressing the immune response that was initially triggered by the foliar pathogenic P. syringae in the roots (Lakshmanan et al. Citation2012). Indeed, wild type and mutants of the exopolysaccharide, another relevant component of the extracellular matrix, repressed the immune response but the tasA mutant did not. These findings expand the view of bacterial amyloids beyond the well-known functionality of adhesion to surfaces or development of multicellularity to mediate communication with the different hosts.
2.2. Functional amyloids in the interaction of bacteria with human hosts
The interaction between bacterial amyloids and human hosts has been poorly investigated, and curli is by far the most studied of the known bacterial functional amyloids in this scenario. Nevertheless, the findings highlight the multifaceted contribution of this amyloid protein to the pathogenesis of entero-gammaproteobacteria, suggesting a role for it in colonization, internalization, host defense evasion and as a weapon.
In a previous section, we discussed the implication of curli in the initial attachment of E. coli cells to plant surfaces. Similarly, curli fibers also mediate the attachment of E. coli cells to human cells and the extracellular matrices of different tissues. The extracellular matrix of mammal tissues is composed of a collection of diverse polymers and plays an important role in cell cohesion, mechanical behavior of tissues and degradation of toxic compounds (). Laminin is a heterotrimeric glycoprotein of the basal layer indispensable for the maintenance and survival of tissues. Fibronectin is a dimeric glycoprotein of the extracellular matrix that binds to integrins and plays a role in cell adhesion, growth, migration, and cell differentiation. Studies using lymphoma cell lines have proven the ability of curli to bind to these components of the extracellular matrix. The MHC-I (major histocompatibility complex class I) is a membrane glycoprotein present in all mammalian nucleated cell types, and curli was also demonstrated to adhere to this protein, showing a wide diversity in adhesion targets (Olsén et al. Citation1998, Citation1989). This effective adhesion of E. coli to host cells and tissues mediated by curli facilitates cell invasion and pathogenesis, revealing curli as an important bacterial factor in the first stages of human–bacterial interactions () (Gophna et al. Citation2002). However, this function is not exclusive to curli in E. coli given that the pili proteins (MPT) of Mycobacterium tuberculosis show similar amyloid properties and attachment behavior as curli in E. coli. The adhesiveness of pili is a feature exploited by a diversity of bacteria to colonize the host cells during infection. MPTs are small peptides which polymerize to form short pili which resemble the curli in E. coli, and lines of evidence have suggested their involvement in bacterial infection: First, MTPs are recognized by sera extracted from tuberculosis-affected patients, and second, purified MTPS are capable of binding laminin (Alteri et al. Citation2007). However, studies are expected to clarify this result. In the gram-negative pathogenic bacterium Chlamydia pneumoniae, the protein Pmp21 plays an important role in pathogenesis, mediating adhesion to host cells by binding to epidermal growth factor receptor EGFR and by working as an invasin. The Pmp21 protein also shows amyloid properties and a tendency to polymerize into fibrils with a secondary structure rich in β-sheets. This study goes into further detail and demonstrates that motifs FXXN (where X is any amino acid) of Pmp21 are partially responsible for adhesion, folding and polymerization (Luczak et al. Citation2016).
Figure 3. Bacterial functional amyloids mediate in microorganism–host interactions. (A) Curli in E. coli and MPT proteins in M. tuberculosis play an essential role in the first stages of colonization by adhering to laminin and fibronectin. Listeriolysin O (LLO) in L. monocytogenes mediates bacterial release from the endosome to the cytoplasm in a pH dependent manner, working as a functional protein inside the endosome disrupting the endosome membrane (pH ∼ 5.5) and polymerized as an amyloid and without membrane disruption properties in the cytoplasm (pH ∼ 7). (B) Amyloid proteins like PSM proteins from Staphylococcus haemolyticus and S. aureus induce neutrophil and erythrocyte lysis. Curli can also bind eDNA, forming DNA-amyloid complexes that interact with Toll-like Receptors (TLRs) leading to immune system wreck by inducing synthesis of auto-antibodies and caspases. DNA-amyloid complexes also activate neutrophils. Activated neutrophils and also other cells types produce the amyloid peptide LL-37 as antimicrobial peptide that block Curli polymerization at the same time that Curli inhibits the antimicrobial activity of LL-37.
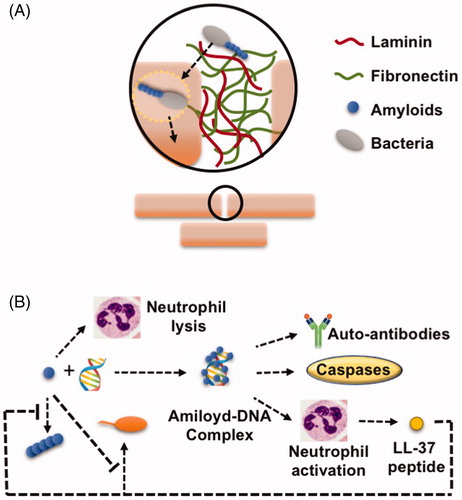
However, and as previously seen in bacterial-plant interactions, the functionality of curli in human-bacterial interactions is not restricted to adhesion. Recent studies have also shown that curli is recognized by the immune recognition Toll-like receptors (TLRs) and forms highly immunogenic complexes with eDNA from the extracellular matrix or DNA from the mammal host (). There are several TLRs; TLR2 resides on the cell surface and TLR9 on membranes of intracellular compartments. These specific TLRs recognize curli and trigger a cascade that leads to the activation of caspases, proinflammatory chemokines, and interleukins and induce the production of autoantibodies (Gallo et al. Citation2015; Tursi et al. Citation2017). This autoantibody production favors bacterial infection, causing perturbance of the host immune system and self-damage to the host. Within the immune response triggered by curli, CsgA specifically triggers the immune response in human epithelial cells and neutrophils, producing the antimicrobial peptide LL-37 from the cleavage of the holoenzyme cathelicidin (Gudmundsson et al. Citation1996). Functions associated with the human LL-37 are as follows: i) to be chemotactic for several immune cells, ii) to induce degranulation of mast cells and iii) to stimulate wound vascularization and re-epithelialization of healing skin (Zanetti Citation2004). LL-37 plays a critical role not only in the innate immune defense against invasive bacteria, fungi or viruses but also by affecting the host cells themselves (Xhindoli et al. Citation2016). In in vitro studies, curli has been shown to bind LL-37, clearing units of proteins and protecting bacterial cells against antimicrobial activity. The IC50 for LL-37 in the E. coli CsgA mutant is 50% lower than that in the wild type, which indicates the protection provided by CsgA to bacterial cells against LL-37. Interestingly, the peptide LL-37 also possesses amyloid properties, and upon binding to curli, it prevents the addition of more CsgA monomers to the growing fibers, thereby arresting curli fibrillation and biofilm formation of E. coli cells (Sood et al. Citation2008; Kai-Larsen et al. Citation2010). This is a fascinating finding that reflects the warfare between two amyloids with an output dependent on the strength and speed. In a manner similar to curli in E. coli, the PSM peptides of S. aureus may also play a role in virulence during infection of the human host. It has been shown that PSMs from Staphylococcus epidermidis are able to interact with AMPs from the host enhancing the antimicrobial effect exhibited by these peptides against Group A Streptococcus (GAS) and thus making PSMs part of the host innate immune response against potential bacterial pathogens occupying the same niche, which helps to the success of the microbial infection of the host (Cogen et al. Citation2010a). Moreover, studies done with methicillin-resistant strains of S. aureus have shown how these peptides act by recruiting and lysing neutrophils, which translates into the debilitation of the cellular immune system (Wang et al. Citation2007). Recently, similar behavior has been found in PSMs from Staphylococcus haemolyticus¸ the second causative agent of sepsis in nosocomial infections of S. aureus, in which these peptides are involved in hemolysis and destruction of neutrophils, contributing to the evasion of the immune response (Da et al. Citation2017).
Listeriolysin O (LLO) toxin from Listeria monocytogenes is also of special interest for a number of reasons: first, the particular mechanism of action of this toxin, and second, because it represents an example of how the environmental pH modulates the amyloid properties and functionality during human-bacterial interaction. The internalization of L. monocytogenes occurs through phagolysosomes, which are organelles that are typically acidic (pH 5.5). When the bacteria reach the inside of the organelle, they produce LLO, which in these acidic conditions target the membranes and provoke the formation of pores that facilitate the escape of bacteria from the stressful and deleterious environment of the phagolysosome (Podobnik et al. Citation2015). Once in the cytoplasm, where the pH rises to pH 7, LLO toxic morphotypes are arrested into nontoxic amyloid aggregates, which eventually prevent the disruption of the cytosolic membrane and lead to a putative return of bacterial cells to the extracellular milieu. By doing so, the different stages of aggregation of the toxin allow L. monocytogenes to efficiently adapt to the predominant environmental conditions. In this way, amyloid properties play an important role in controlling the protein activity in a place-dependent manner (Bavdek et al. Citation2012).
As seen above, the contribution of B. subtilis to plant health implies, to some extent, the interference with the immune system which permits the efficient colonization of the roots. Studies on germfree mice showed how B. subtilis improves the immunological state of the host, promoting the development of gut-associated lymphoid tissues (GALT) and related outputs as mucosal immunity, and the development of the pre-immune antibody. Attempts to define the bacterial factors involved in this process disregarded the involvement of the two stress-related regulons SigB and SigD and the contribution of Spo0A, the master regulator that exclusively controls two cell fates: sporulation and biofilm formation (Rhee et al. Citation2004; Fujita et al. Citation2005). Apparently, TasA does not have a direct role in this beneficial contribution; a tasA mutant still promotes GALT development; however, the involvement of the TasA amyloid-like fibers in this host response should not be ruled out, given that TapA, the other component of the TasA amyloid-like fibers, can trigger this immunological state. However, this is a hypothetical contribution that needs further study.
Concluding remarks
Amyloids are among the more fascinating groups of proteins found in nature. Studies have shown many of their roles, especially in the multicellularity of bacteria, with special attention to bacterial cell-to-cell or cell-to-surface interactions. We know that amyloids transition from monomers to fibers and that certain environmental conditions are propitious to this molecular switch. Thus, it would be interesting to determine if the coexistence of these morphotypes provides the bacterial cells with the versatility necessary to respond and adapt to complex and variable environments such as human and plant tissues. The studies on evasion of immune system or interaction with plant host cells are going in this direction and giving a new and exciting perspective of amyloids beyond their well-known implications in adhesion and in structural roles. More studies on these aspects will provide us with insights on how functional amyloids are, indeed, proteins contributing to the wellness of the bacterial population in a multifaceted manner.
Disclosure statement
The authors report no conflicts of interest
Additional information
Funding
References
- Alteri CJ, Xicohténcatl-Cortes J, Hess S, Caballero-Olín G, Girón JA, Friedman RL. 2007. Mycobacterium tuberculosis produces pili during human infection. Proc Natl Acad Sci. 104:5145–5150.
- Barak JD, Gorski L, Naraghi-Arani P, Charkowski AO. 2005. Salmonella enterica virulence genes are required for bacterial attachment to plant tissue. Appl Environ Microbiol. 71:5685–5691.
- Barny M-A. 1995. Erwinia amylovora hrpN mutants, blocked in harpin synthesis, express a reduced virulence on host plants and elicit variable hypersensitive reactions on tobacco. Eur J Plant Pathol. 101:333–340.
- Bavdek A, Kostanjšek R, Antonini V, Lakey JH, Dalla Serra M, Gilbert RJC, Anderluh G. 2012. pH dependence of listeriolysin O aggregation and pore-forming ability. FEBS J. 279:126–141.
- Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. 2013. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA. 110:E1621–E1630.
- Bieler S, Estrada L, Lagos R, Baeza M, Castilla J, Soto C. 2005. Amyloid formation modulates the biological activity of a bacterial protein. J Biol Chem. 280:26880–26885.
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 59:1229–1238.
- Caro-Astorga J, Pérez-García A, de Vicente A, Romero D. 2014. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front Microbiol. 5:745.
- Carrotta R, Manno M, Bulone D, Martorana V, San Biagio PL. 2005. Protofibril formation of amyloid beta-protein at low pH via a non-cooperative elongation mechanism. J Biol Chem. 280:30001–30008.
- Carter MQ, Louie JW, Feng D, Zhong W, Brandl MT. 2016. Curli fimbriae are conditionally required in Escherichia coli O157:H7 for initial attachment and biofilm formation. Food Microbiol. 57:81–89.
- Chai L, Romero D, Kayatekin C, Akabayov B, Vlamakis H, Losick R, Kolter R. 2013. Isolation, characterization, and aggregation of a structured bacterial matrix precursor. J Biol Chem. 288:17559–17568.
- Chan SWS, Yau J, Ing C, Liu K, Farber P, Won A, Bhandari V, Kara-Yacoubian N, Seraphim TV, Chakrabarti N, et al. 2016. Mechanism of amyloidogenesis of a bacterial AAA + chaperone. Structure. 24:1095–1109.
- Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. 2002. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science (New York, NY). 295:851–855.
- Charkowski AO, Alfano JR, Preston G, Yuan J, He SY, Collmer A. 1998. The Pseudomonas syringae pv. tomato HrpW protein has domains similar to harpins and pectate lyases and can elicit the plant hypersensitive response and bind to pectate. J Bacteriol. 180:5211–5217.
- Chen L, Zhang S-J, Zhang S-S, Qu S, Ren X, Long J, Yin Q, Qian J, Sun F, Zhang C, et al. 2008. A fragment of the Xanthomonas oryzae pv. oryzicola harpin HpaG Xooc reduces disease and increases yield of rice in extensive grower plantings. Phytopathology 98:792–802.
- Chen Y, Cao S, Chai Y, Clardy J, Kolter R, Guo J-h, Losick R. 2012. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol. 85:418–430.
- Choi M-S, Kim W, Lee C, Oh C-S. 2013. Harpins, multifunctional proteins secreted by gram-negative plant-pathogenic bacteria. Mol Plant Microbe Interact. 26:1115–1122.
- Claessen D, Rink R, de JW, Siebring J, de VP, Boersma FGH, Dijkhuizen L, Wosten HAB. 2003. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 17:1714–1726.
- Claessen D, Stokroos I, Deelstra HJ, Penninga NA, Bormann C, Salas JA, Dijkhuizen L, Wösten HAB. 2004. The formation of the rodlet layer of streptomycetes is the result of the interplay between rodlins and chaplins. Mol Microbiol. 53:433–443.
- Cogen AL, Yamasaki K, Muto J, Sanchez KM, Crotty Alexander L, Tanios J, Lai Y, Kim JE, Nizet V, Gallo RL. 2010. Staphylococcus epidermidis antimicrobial delta-toxin (phenol-soluble modulin-gamma) cooperates with host antimicrobial peptides to kill group A Streptococcus. PLoS One. 5:e8557.
- Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, et al. 2010. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 130:192–200.
- Coustou-Linares V, Maddelein M-L, Bégueret J, Saupe SJ. 2001. In vivo aggregation of the HET-s prion protein of the fungus Podospora anserina. Mol Microbiol. 42:1325–1335.
- Da F, Joo H-S, Cheung GYC, Villaruz AE, Rohde H, Luo X, Otto M. 2017. Phenol-soluble modulin toxins of Staphylococcus haemolyticus. Front Cell Infect Microbiol. 7:206.
- De Jong W, Wösten HAB, Dijkhuizen L, Claessen D. 2009. Attachment of Streptomyces coelicolor is mediated by amyloidal fimbriae that are anchored to the cell surface via cellulose. Mol Microbiol.73:1128–1140.
- de Lorenzo V, Pugsley AP. 1985. Microcin E492, a low-molecular-weight peptide antibiotic which causes depolarization of the Escherichia coli cytoplasmic membrane. Antimicrob Agents Chemother. 27:666–669.
- Diehl A, Roske Y, Ball L, Chowdhury A, Hiller M, Moliere N, Kramer R, Stoppler D, Worth CL, Schlegel B, et al. 2018. Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc Natl Acad Sci USA. 115:3237–3242.
- Dueholm MS, Otzen D, Nielsen PH. 2013. Evolutionary insight into the functional amyloids of the pseudomonads. PLoS One. 8:e76630.
- Dueholm MS, Petersen SV, Sønderkaer M, Larsen P, Christiansen G, Hein KL, Enghild JJ, Nielsen JL, Nielsen KL, Nielsen PH, et al. 2010. Functional amyloid in pseudomonas. Mol Microbiol. 77:1009–1020.
- Dueholm MS, Søndergaard MT, Nilsson M, Christiansen G, Stensballe A, Overgaard MT, Givskov M, Tolker-Nielsen T, Otzen DE, Nielsen PH. 2013. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiol Op. 2:365–382.
- Evans ML, Chorell E, Taylor JD, Åden J, Götheson A, Li F, Koch M, Sefer L, Matthews SJ, Wittung-Stafshede P, et al. 2015. The bacterial curli system possesses a potent and selective inhibitor of amyloid formation. Mol Cell. 57:445–455.
- Fink RC, Black EP, Hou Z, Sugawara M, Sadowsky MJ, Diez-Gonzalez F. 2012. Transcriptional responses of Escherichia coli K-12 and O157:H7 associated with lettuce leaves. Appl Environ Microbiol. 78:1752–1764.
- Fitzpatrick AWP, Vanacore GM, Zewail AH. 2015. Nanomechanics and intermolecular forces of amyloid revealed by four-dimensional electron microscopy. Proc Natl Acad Sci USA. 112:3380–3385.
- Fortas E, Piccirilli F, Malabirade A, Militello V, Trépout S, Marco S, Taghbalout A, Arluison V. 2015. New insight into the structure and function of Hfq C-terminus. Biosci Rep 35. doi:10.1042/BSR20140128.
- Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. 2005. Functional amyloid formation within mammalian tissue. PLoS Biol. 4:e6.
- Fujita M, González-Pastor JE, Losick R. 2005. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 187:1357–1368.
- Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, Goulian M, Buttaro B, Caricchio R, Gallucci S, Tükel Ç. 2015. Amyloid-DNA composites of bacterial biofilms stimulate autoimmunity. Immunity 42:1171–1184.
- Gao S, Wu H, Wang W, Yang Y, Xie S, Xie Y, Gao X. 2013. Efficient colonization and harpins mediated enhancement in growth and biocontrol of wilt disease in tomato by Bacillus subtilis. Lett Appl Microbiol. 57:526–533.
- Gophna U, Oelschlaeger TA, Hacker J, Ron EZ. 2002. Role of fibronectin in curli-mediated internalization. FEMS Microbiol Lett. 212:55–58.
- Grignon C, Sentenac aH. 1991. pH and ionic conditions in the apoplast. Annu Rev Plant Physiol Plant Mol Biol. 42:103–128.
- Grimminger-Marquardt V, Lashuel HA. 2010. Structure and function of the molecular chaperone Hsp104 from yeast. Biopolymers 93:252–276.
- Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. 1996. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 238:325–332.
- Hammar M, Arnqvist A, Bian Z, Olsén A, Normark S. 1995. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol. 18:661–670.
- Hammer ND, Schmidt JC, Chapman MR. 2007. The curli nucleator protein, CsgB, contains an amyloidogenic domain that directs CsgA polymerization. Proc Natl Acad Sci USA. 104:12494–12499.
- He SY, Huang HC, Collmer A. 1993. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell 73:1255–1266.
- Hetz C, Bono MR, Barros LF, Lagos R. 2002. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci USA. 99:2696–2701.
- Iconomidou VA, Vriend G, Hamodrakas SJ. 2000. Amyloids protect the silkmoth oocyte and embryo. FEBS Lett. 479:141–145.
- Jang H, Arce FT, Mustata M, Ramachandran S, Capone R, Nussinov R, Lal R. 2011. Antimicrobial protegrin-1 forms amyloid-like fibrils with rapid kinetics suggesting a functional link. Biophys J. 100:1775–1783.
- Jeter C, Matthysse AG. 2005. Characterization of the binding of diarrheagenic strains of E. coli to plant surfaces and the role of curli in the interaction of the bacteria with alfalfa sprouts. Mol Plant Microbe Interact.: MPMI 18:1235–1242.
- Joo HS, Cheung GY, Otto M. 2011. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J Biol Chem. 286:8933–8940.
- Kai-Larsen Y, Lüthje P, Chromek M, Peters V, Wang X, Holm A, Kádas L, Hedlund K-O, Johansson J, Chapman MR, et al. 2010. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog. 6:e1001010.
- Keller A, Fritzsche M, Yu Y-P, Liu Q, Li Y-M, Dong M, Besenbacher F. 2011. Influence of hydrophobicity on the surface-catalyzed assembly of the islet amyloid polypeptide. ACS Nano. 5:2770–2778.
- Kenney JM, Knight D, Wise MJ, Vollrath F. 2002. Amyloidogenic nature of spider silk. Eur J Biochem. 269:4159–4163.
- Kim J-G, Park BK, Yoo C-H, Jeon E, Oh J, Hwang I. 2003. Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J Bacteriol. 185:3155–3166.
- Klunk WE, Jacob RF, Mason RP. 1999. Quantifying amyloid beta-peptide (Abeta) aggregation using the Congo red-Abeta (CR-abeta) spectrophotometric assay. Anal Biochem. 266:66–76.
- Knowles TPJ, Vendruscolo M, Dobson CM. 2014. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 15:496–396.
- Kourie JI, Shorthouse AA. 2000. Properties of cytotoxic peptide-formed ion channels. Am J Physiol Cell Physiol. 278:C1063–C1087.
- Kowalewski T, Holtzman DM. 1999. In situ atomic force microscopy study of Alzheimer’s -amyloid peptide on different substrates: new insights into mechanism of -sheet formation. Proc Natl Acad Sci. 96:3688–3693.
- Kozlowski LP, Bujnicki JM. 2012. MetaDisorder: a meta-server for the prediction of intrinsic disorder in proteins. BMC Bioinformatics. 13:111.
- Lakshmanan V, Kitto SL, Caplan JL, Hsueh Y-H, Kearns DB, Wu Y-S, Bais HP. 2012. Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 160:1642–1661.
- Lee J, Klusener B, Tsiamis G, Stevens C, Neyt C, Tampakaki AP, Panopoulos NJ, Nöller J, Weiler EW, Cornelis GR, et al. 2001. HrpZ(Psph) from the plant pathogen Pseudomonas syringae pv. phaseolicola binds to lipid bilayers and forms an ion-conducting pore in vitro. Proc Natl Acad Sci USA. 98:289–294.
- LeVine H, 3rd. 1993. Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Prot Sci. 2:404–410.
- Li J-G, Liu H-X, Cao J, Chen L-F, Gu C, Allen C, Guo J-H. 2010. PopW of Ralstonia solanacearum, a new two-domain harpin targeting the plant cell wall. Mol Plant Pathol. 11:371–381.
- Luczak SET, Smits SHJ, Decker C, Nagel-Steger L, Schmitt L, Hegemann JH. 2016. The Chlamydia pneumoniae adhesin Pmp21 forms oligomers with adhesive properties. J Biol Chem. 291:22806–22818.
- Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KPR, Simon R, Schubert D, et al. 2009. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 325:328–332.
- Marchand A, Verdon J, Lacombe C, Crapart S, Hechard Y, Berjeaud JM. 2011. Anti-legionella activity of staphylococcal hemolytic peptides. Peptides 32:845–851.
- Marcoleta A, Marin M, Mercado G, Valpuesta JM, Monasterio O, Lagos R. 2013. Microcin e492 amyloid formation is retarded by posttranslational modification. J Bacteriol. 195:3995–4004.
- Marinelli P, Pallares I, Navarro S, Ventura S. 2016. Dissecting the contribution of Staphylococcus aureus α-phenol-soluble modulins to biofilm amyloid structure. Sci Rep. 6:34552.
- Markande AR, Nerurkar AS. 2016. Bioemulsifier (BE-AM1) produced by Solibacillus silvestris AM1 is a functional amyloid that modulates bacterial cell-surface properties. Biofouling 32:1153–1162.
- Marshall KE, Marchante R, Xue W-F, Serpell LC. 2014. The relationship between amyloid structure and cytotoxicity. Prion 8(2).
- Mawhinney MT, Williams TL, Hart JL, Taheri ML, Urbanc B. 2017. Elucidation of insulin assembly at acidic and neutral pH: characterization of low molecular weight oligomers. Proteins 85:2096–2110.
- Molina-García L, Gasset-Rosa F, Álamo MM-d, Fernández-Tresguerres ME, Espina SM, Ddl Lurz R, Giraldo R. 2016. Functional amyloids as inhibitors of plasmid DNA replication. Sci Rep. 6:25425.
- Moores B, Drolle E, Attwood SJ, Simons J, Leonenko Z. 2011. Effect of Surfaces on amyloid fibril formation. Plos One. 6:e25954.
- Nenninger AA, Robinson LS, Hammer ND, Epstein EA, Badtke MP, Hultgren SJ, Chapman MR. 2011. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol Microbiol. 81:486–499.
- Nenninger AA, Robinson LS, Hultgren SJ. 2009. Localized and efficient curli nucleation requires the chaperone-like amyloid assembly protein CsgF. Proc Natl Acad Sci USA. 106:900–905.
- Oh J, Kim J-G, Jeon E, Yoo C-H, Moon JS, Rhee S, Hwang I. 2007. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. J Biol Chem. 282:13601–13609.
- Olsén A, Jonsson A, Normark S. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 338:652–655.
- Olsén A, Wick MJ, Mörgelin M, Björck L. 1998. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Inf Immun. 66:944–949.
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. 1996. Propagation of the yeast prion-like [psi+] determinant is mediated by oligomerization of the SUP35-encoded polypeptide chain release factor. Embo J. 15:3127–3134.
- Periasamy S, Joo H-S, Duong AC, Bach T-HL, Tan VY, Chatterjee SS, Cheung GYC, Otto M. 2012. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci USA. 109:1281–1286.
- Podobnik M, Marchioretto M, Zanetti M, Bavdek A, Kisovec M, Cajnko MM, Lunelli L, Serra MD, Anderluh G. 2015. Plasticity of listeriolysin O pores and its regulation by pH and unique histidine. Sci Rep. 5:srep09623.
- Ramsook CB, Tan C, Garcia MC, Fung R, Soybelman G, Henry R, Litewka A, O’Meally S, Otoo HN, Khalaf RA, et al. 2010. Yeast cell adhesion molecules have functional amyloid-forming sequences. Euk Cell. 9:393–404.
- Rhee K-J, Sethupathi P, Driks A, Lanning DK, Knight KL. 2004. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J Immunol. 172:1118–1124.
- Robinson LS, Ashman EM, Hultgren SJ, Chapman MR. 2006. Secretion of curli fibre subunits is mediated by the outer membrane-localized CsgG protein. Mol Microbiol. 59:870–881.
- Romero D, Aguilar C, Losick R, Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci USA. 107:2230–2234.
- Romero D, Vlamakis H, Losick R, Kolter R. 2011. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol Microbiol. 80:1155–1168.
- Romero D, Vlamakis H, Losick R, Kolter R. 2014. Functional analysis of the accessory protein TapA in Bacillus subtilis amyloid fiber assembly. J Bacteriol. 196:1505–1513.
- Rouse SL, Hawthorne WJ, Berry J-L, Chorev DS, Ionescu SA, Lambert S, Stylianou F, Ewert W, Mackie U, Morgan RML, et al. 2017. A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat Commun. 8:263.
- Sasahara K, Yagi H, Sakai M, Naiki H, Goto Y. 2008. Amyloid nucleation triggered by agitation of beta2-microglobulin under acidic and neutral pH conditions. Biochemistry 47:2650–2660.
- Schwartz K, Ganesan M, Payne DE, Solomon MJ, Boles BR. 2016. Extracellular DNA facilitates the formation of functional amyloids in Staphylococcus aureus biofilms. Mol Microbiol. 99:123–134.
- Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. 2012. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 8:e1002744.
- Sengupta U, Nilson AN, Kayed R. 2016. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine 6:42–49.
- Serio TR, Lindquist SL. 2001. [PSI+], SUP35, and chaperones. Adv Protein Chem. 57:335–366.
- Shahnawaz M, Park K-W, Mukherjee A, Diaz-Espinoza R, Soto C. 2017. Prion-like characteristics of the bacterial protein Microcin E492. Sci Rep. 7:45720.
- Shu Q, Crick SL, Pinkner JS, Ford B, Hultgren SJ, Frieden C. 2012. The E. coli CsgB nucleator of curli assembles to -sheet oligomers that alter the CsgA fibrillization mechanism. Proc Natl Acad Sci. 109:6502–6507.
- Simone AD, Kitchen C, Kwan AH, Sunde M, Dobson CM, Frenkel D. 2012. Intrinsic disorder modulates protein self-assembly and aggregation. Proc Natl Acad Sci. 109:6951–6956.
- Sood R, Domanov Y, Pietiäinen M, Kontinen VP, Kinnunen PKJ. 2008. Binding of LL-37 to model biomembranes: insight into target vs host cell recognition. Biochim Biophys Acta. 1778:983–996.
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, et al. 2010. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 5:e9505.
- Stöver AG, Driks A. 1999. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J Bacteriol. 181:1664–1672.
- Su Y, Chang PT. 2001. Acidic pH promotes the formation of toxic fibrils from beta-amyloid peptide. Brain Res. 893:287–291.
- Terra R, Stanley-Wall NR, Cao G, Lazazzera BA. 2012. Identification of Bacillus subtilis SipW as a bifunctional signal peptidase that controls surface-adhered biofilm formation. J Bacteriol. 194:2781–2790.
- Tompa P. 2009. Structural disorder in amyloid fibrils: its implication in dynamic interactions of proteins. Febs J. 276:5406–5415.
- Tursi SA, Lee EY, Medeiros NJ, Lee MH, Nicastro LK, Buttaro B, Gallucci S, Wilson RP, Wong GCL, Tükel Ç. 2017. Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLoS Pathog. 13:e1006315.
- Uversky VN, Oldfield CJ, Dunker AK. 2008. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 37:215–246.
- Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R. 2013. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 11:157–168.
- Vuong C, Dürr M, Carmody AB, Peschel A, Klebanoff SJ, Otto M. 2004. Regulated expression of pathogen-associated molecular pattern molecules in Staphylococcus epidermidis: quorum-sensing determines pro-inflammatory capacity and production of phenol-soluble modulins. Cell Microbiol. 6:753–759.
- Wang R, Braughton KR, Kretschmer D, Bach T-HL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, et al. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 13:1510–1514.
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. 1992. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 257:85–88.
- Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A. 2016. The human cathelicidin LL-37–A pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta. 1858:546–566.
- Yang W, Willemse J, Sawyer EB, Lou F, Gong W, Zhang H, Gras SL, Claessen D, Perrett S. 2017. The propensity of the bacterial rodlin protein RdlB to form amyloid fibrils determines its function in Streptomyces coelicolor. Sci Rep. 7:42867.
- Zanetti M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 75:39–48.
- Zeng G, Vad BS, Dueholm MS, Christiansen G, Nilsson M, Tolker-Nielsen T, Nielsen PH, Meyer RL, Otzen DE. 2015. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front Microbiol. 6:6.
- Zhu M, Souillac PO, Ionescu-Zanetti C, Carter SA, Fink AL. 2002. Surface-catalyzed amyloid fibril formation. J Biol Chem. 277:50914–50922.
