Abstract
Invasive Scedosporium spp. and Lomentospora prolificans infections are an emerging threat in immunocompromised and occasionally in healthy hosts. Scedosporium spp. is intrinsically resistant to most, L. prolificans to all the antifungal drugs currently approved, raising concerns about appropriate treatment decisions. High mortality rates of up to 90% underline the need for comprehensive diagnostic workup and even more for new, effective antifungal drugs to improve patient outcome. For a comprehensive analysis, we identified cases of severe Scedosporium spp. and L. prolificans infections from the literature diagnosed in 2000 or later and the FungiScope® registry. For 208 Scedosporium spp. infections solid organ transplantation (n = 58, 27.9%) and for 56 L. prolificans infection underlying malignancy (n = 28, 50.0%) were the most prevalent risk factors. L. prolificans infections frequently presented as fungemia (n = 26, 46.4% versus n = 12, 5.8% for Scedosporium spp.). Malignancy, fungemia, CNS and lung involvement predicted worse outcome for scedosporiosis and lomentosporiosis. Patients treated with voriconazole had a better overall outcome in both groups compared to treatment with amphotericin B formulations. This review discusses the epidemiology, prognostic factors, pathogen susceptibility to approved and investigational antifungals, and treatment strategies of severe infections caused by Scedosporium spp. and L. prolificans.
Introduction
Advances in medical care led to increasing numbers of patients with non-Aspergillus mold infections. Scedosporiosis is of particular concern due to intrinsic resistance to antifungal therapy (Douglas et al. Citation2016).
The most relevant pathogens causing invasive scedosporiosis and lomentosporiosis are Scedosporium apiospermum species complex [comprising amongst others S. apiospermum, S. boydii (traditionally and wrongly thought to be the teleomorph state of the anamorphic fungus S. apiospermum)], S. aurantiacum, and Lomentospora prolificans (formerly Scedosporium prolificans but renamed due to phylogenetic differences to Scedosporium spp.) (Lackner et al. Citation2014). In the absence of effective surveillance systems, data on the incidence of Scedosporium- and Lomentospora-related infections is scarce and differs by region (Tintelnot et al. Citation2009). In Spain, a population-based survey on clinically relevant fungi identified Scedosporium spp. as the second most relevant filamentous fungus after Aspergillus spp. (Alastruey-Izquierdo et al. Citation2013). In Italy, incidence of proven scedosporiosis was 0.08% among acute leukaemia patients (Caira et al. Citation2008). For Houston, Texas incidence increased from 0.82 to 1.33 cases per 100,000 inpatient days between 1993 and 2005 (Lamaris et al. Citation2006).
Patients with compromised immune status are at highest risk of developing invasive scedosporiosis or lomentosporiosis. In particular, those with prolonged neutropenia, solid organ transplant, and patients with inherited or acquired immunodeficiency. It has long been recognized that Scedosporium spp. can also cause severe infections in immunocompetent hosts, e.g. after near drowning in polluted water and after penetrating trauma (Panackal and Marr Citation2004; Katragkou et al. Citation2007). Clinical presentation depends on the route of infection and immune status and ranges from superficial and subcutaneous disease to deep tissue involvement and dissemination (Ishii et al. Citation2015; Daniele et al. Citation2017). Scedosporium spp. and L. prolificans infections can involve the central nervous system, in immunocompetent patients through contiguous spread from the sinuses, and in immunocompromised patients through haematogenous spread (Tortorano et al. Citation2014). L. prolificans is more prone to cause invasive disease than Scedosporium species, as it produces conidia in body fluid and tissue and disseminates through the bloodstream (Husain et al. Citation2005; Cooley et al. Citation2007; Tortorano et al. Citation2014).
Current treatment guidelines recommend combination of voriconazole or amphotericin B-based formulations with surgery (Rodríguez-Tudela et al. Citation2009; Tortorano et al. Citation2014; Lass-Flörl and Cuenca-Estrella Citation2017). Scedosporium spp. is often resistant to amphotericin B-based formulations but susceptible to posaconazole and voriconazole, whereas L. prolificans is usually pan-resistant (Cuenca-Estrella et al. Citation1999; Carrillo and Guarro Citation2001; Espinel-Ingroff Citation2001; Bouza and Muñoz Citation2004; Patterson et al. Citation2016). Mortality rates rise up to 90% owing to pathogenicity and limited treatment options (Blyth et al. Citation2014).
In this study, we comprehensively review scedosporiosis and lomentosporiosis cases selected from relevant literature and the FungiScope® registry, with an emphasis on assessing predictors of mortality.
Methods
FungiScope® is a registry study on rare invasive fungal diseases (IFD) currently active in 74 countries. Its methodology has been described elsewhere (Seidel et al. Citation2017). Data of scedosporiosis and lomentosporiosis cases were extracted and included for analysis.
In addition, we performed an electronic literature search for case reports in PubMed on August 18, 2017 using the search filter “(Scedospori* OR Pseudallescheri* OR Lomentospori*) AND ((invasive OR disseminated OR infection) AND (case OR patient OR report))”. Articles in English, French, German, Italian, and Spanish were chosen for further selection. Records presenting cases of invasive Scedosporium spp. or L. prolificans infection were selected on the basis of the title and abstract. Reference lists of the identified articles were checked for further studies. We chose the case reports with diagnosis made in 2000 or later, i.e. after marketing authorization of voriconazole for scedosporiosis and other IFD in 2002, and the compassionate use of voriconazole in the preceding years (Agatha et al. Citation2014). Each report was reviewed for demographics, fungal pathogens, underlying diseases and risk factors for IFD, site of infection, signs and symptoms at the time of diagnosis of IFD (imaging findings, fever, cough, dyspnoe, neurological signs), antifungal and surgical therapy from first sign of IFD, susceptibility assessed according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical & Laboratory Standards Institute (CLSI) reference method (Institute CaLaS Citation2008; The European Committee on Antimicrobial Susceptibility Testing Citation2017), clinical outcome, and autopsy findings. Observation time was defined from day of first sign of IFD to last patient contact.
Underlying conditions were selected as follows: treatment of hematological malignancy, allogeneic hematopoietic stem cell transplantation (HSCT), long-term immunosuppression (prolonged use of corticosteroids, treatment with other recognized T cell immunosuppressants, such as cyclosporine, TNF-α blockers, monoclonal antibodies, inherited severe immunodeficiency), HIV/AIDS, solid organ transplantation (SOT), cystic fibrosis (CF), diabetes mellitus (DM), near drowning, surgery, trauma, history of pulmonary tuberculosis (TB), and others. For each patient the main risk factor was identified. For example, in a car accident with traumatic injuries and near drowning, inhalation of polluted water was chosen as the dominating risk factor, if the primary site of infection was related to the near drowning event and not the injuries. In patients with lung transplant on a background of CF or TB, SOT would dominate.
Proven and probable IFD were included (De Pauw et al. Citation2008). Disseminated infection was defined as a positive blood culture or infection at ≥2 non-contiguous sites.
In vitro susceptibility was tested for approved antifungals and the novel orotomide antifungal olorofim (F901318) that inhibits the dihydroorotate dehydrogenase (Oliver et al. Citation2016; McCarthy et al. Citation2017) for eight S. apiospermum and seven L. prolificans isolates collected in FungiScope® using broth microdilution for filamentous fungi according to CLSI document M38-A2 (25).
For comparison analysis, cases were grouped according to the causative pathogen: (1) infection due to Scedosporium species including S. apiospermum, S. boydii and S. aurantiacum and (2) infection due to L. prolificans together with one case that had a mixed infection with S. apiospermum (Lackner et al. Citation2014).
Statistical analyses were performed using SPSS 25 (IBM Corp., USA). Characteristics of the patient population were compared by calculating the frequencies, means and medians. For the comparison analysis, Chi2, Fisher’s Exact Test or t-test were used as appropriate. Kaplan–Meier method was used to estimate survival and curves were compared statistically using the log rank test. The p value ≤0.05 was considered statistically significant. Mean survival of time to death was calculated using the mean and 95% confidence interval (CI 95%). Minimum inhibitory concentrations (MIC) were compared by calculating medians.
Results
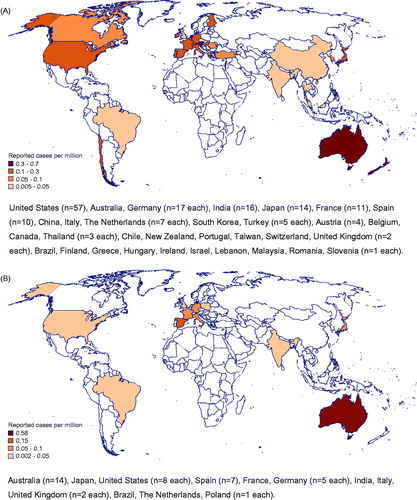
We identified 273 cases with invasive Scedosporium spp. and L. prolificans infection, 232 from the literature and 41 from the FungiScope® registry (D'Hondt et al. Citation2000; Muñoz et al. Citation2000; Bhatk and Naseeruddin Citation2001; Canet et al. Citation2001; Greig et al. Citation2001; Kiraz et al. Citation2001; Lavy et al. Citation2001; Luu et al. Citation2001; Nguyen Citation2001; Tirado-Miranda et al. Citation2001; Campagnaro et al. Citation2002; Farina et al. Citation2002; Levine et al. Citation2002; Mellinghoff et al. Citation2002; Miele et al. Citation2002; O'Bryan et al. Citation2002; Raj and Frost Citation2002; Safdar et al. Citation2002; Talbot et al. Citation2002; Taylor et al. Citation2002; Wu et al. Citation2002; Zaas Citation2002; Bosma et al. Citation2003; Chaveiro et al. Citation2003; Fernández-Mosteirín et al. Citation2003; Fietz et al. Citation2003; Gosbell et al. Citation2003; Horré et al. Citation2003; Howden et al. Citation2003; Leck et al. Citation2003; Nulens et al. Citation2003; Ochiai et al. Citation2003; Pennekamp et al. Citation2003; Posteraro et al. Citation2003; Saracli et al. Citation2003; Ahmed et al. Citation2004; Danaher and Walter Citation2004; CitationFigueroa et al. 2004; German et al. Citation2004; Kanafani et al. Citation2004; Kowacs et al. Citation2004; Perlroth and Miller Citation2004; Reimann et al. Citation2004; Riddell et al. Citation2004; Shand et al. Citation2004; Shu et al. Citation2004; Tan et al. Citation2004; Thiagalingam et al. Citation2004; Larocco and Barron Citation2005; O'Doherty et al. Citation2005; Schaenman et al. Citation2005; Singh and McCluskey Citation2005; Uenotsuchi et al. Citation2005; Vagefi et al. Citation2005; Verghese et al. Citation2005; Bates and Mims Citation2006; Buzina et al. Citation2006; Farina et al. Citation2006; Musk et al. Citation2006; Porte et al. Citation2006; Symoens et al. Citation2006; Tascini et al. Citation2006; Abgrall et al. Citation2007; Baumgartner et al. Citation2007; Bhat et al. Citation2007; Chen et al. Citation2007; Jain et al. Citation2007; Kooijman et al. Citation2007; Lainscak et al. Citation2007; Leechawengwongs et al. Citation2007; Oh et al. Citation2007; Pellón Dabén et al. Citation2007; Rogasi et al. Citation2007; Sahi et al. Citation2007; Sarvat and Sarria Citation2007; Shankar et al. Citation2007; Tong et al. Citation2007; Ananda-Rajah et al. Citation2008; Guignard et al. Citation2008; Li et al. Citation2008; Mesfin et al. Citation2008; Nochez et al. Citation2008; Satirapoj et al. Citation2008; Bibashi et al. Citation2009; Cardoso et al. Citation2009; Carod-Artal et al. Citation2009; Chanqueo et al. Citation2009; Elm et al. Citation2009; Ezzedine et al. Citation2009; Foo et al. Citation2009; García-Vidal et al. Citation2009; Grenouillet et al. Citation2009; Horré and Marklein Citation2009; Ikewaki et al. Citation2009; Matsumoto et al. Citation2009; Morales et al. Citation2009; Ngai et al. Citation2009; Sheu et al. Citation2009; Ahmad et al. Citation2010; Al-Jehani et al. Citation2010; Azofra et al. Citation2010; Baradkar et al. Citation2010; Beier et al. Citation2010; Gelabert-González et al. Citation2010; Kimura et al. Citation2010; Morio et al. Citation2010; O’Hearn et al. Citation2010; Ortmann et al. Citation2010; Sarva et al. Citation2010; Sireesha et al. Citation2010; Spanevello et al. Citation2010; Fernández Guerrero et al. Citation2011; Gottesman-Yekutieli et al. Citation2011; Lackner et al. Citation2011; Luijk et al. Citation2011; Makino et al. Citation2011; Nakamura et al. Citation2011; Nguyen and Raychaudhuri Citation2011; Ohashi et al. Citation2011; Ong et al. Citation2011; Rivier et al. Citation2011; Ruinemans et al. Citation2011; Solé Citation2011; Stur-Hofmann et al. Citation2011; Takeuchi et al. Citation2011; Tammer et al. Citation2011; Bose et al. Citation2012; Ceccarelli et al. Citation2012; Cetrulo et al. Citation2012; Harrison et al. Citation2012; Mays et al. Citation2012; Yoneda et al. Citation2012; Allen et al. Citation2013; Ergin et al. Citation2013; Fadzillah et al. Citation2013; Henao-Martínez et al. Citation2013; Holmes et al. Citation2013; Husain et al. Citation2013; Kubisiak-Rzepczyk et al. Citation2013; Larbcharoensub et al. Citation2013; Lin et al. Citation2013; Nakamura et al. Citation2013; Sayah et al. Citation2013; Slone et al. Citation2013; Wilson and Kennedy Citation2013; Yu et al. Citation2013; Agatha et al. Citation2014, Abela et al. Citation2018; Campa-Thompson et al. Citation2014; Kepez Yildiz et al. Citation2014; Moloney and Park Citation2014; Nishimori et al. Citation2014; Shimizu et al. Citation2014; Trubiano et al. Citation2014; Uno et al. Citation2014; Alpaydın et al. Citation2015; Boyce and Collins Citation2015; Clement et al. Citation2015; Cruz et al. Citation2015; He et al. Citation2015; Ishii et al. Citation2015; Kim et al. Citation2015; Ochi et al. Citation2015; Patel et al. Citation2015; Patel and Orlandi Citation2015; Sharma and Singh Citation2015; Smita et al. Citation2015; Strunk et al. Citation2015; Thomson et al. Citation2015; Balandin et al. Citation2016; Bui and Carvounis Citation2016; Chen et al. Citation2016; Denton et al. Citation2016; Goldman et al. Citation2016; Guber et al. Citation2016; Kelly et al. Citation2016; Kite and Heng Citation2016; Lahmer et al. Citation2016; Leek et al. Citation2016; Mohan and Gopakumar Citation2016; Ogawa et al. Citation2016; Roy et al. Citation2016; Tamaki et al. Citation2016; Tilakaratne et al. Citation2016; Wang et al. Citation2016; Williams et al. Citation2016; Ghosh et al. Citation2017; Hu and Chen Citation2017; Jain et al. Citation2017; Kim et al. Citation2017; Masukane et al. Citation2017; Mei et al. Citation2017; Signore et al. Citation2017; Stoneham et al. Citation2017; Tóth et al. Citation2017; Tsuji et al. Citation2017). Five cases were already included in the comprehensive review of 162 Scedosporium (Lomentospora) prolificans infections by Rodríguez-Tudela et al. (Citation2009) (Greig et al. Citation2001; Taylor et al. Citation2002; Gosbell et al. Citation2003; Howden et al. Citation2003; Singh and McCluskey Citation2005). Nine cases with unknown species were omitted from further analysis. Distribution and reported cases per million population by the country are given in .
Figure 1. Geographical distribution of (A) 208 scedosporiosis cases and (B) 56 lomentosporiosis cases identified in the literature and FungiScope® presented as number of cases per million population. United States (n = 57), Australia, Germany (n = 17 each), India (n = 16), Japan (n = 14), France (n = 11), Spain (n = 10), China, Italy, The Netherlands (n = 7 each), South Korea, Turkey (n = 5 each), Austria (n = 4), Belgium, Canada, Thailand (n = 3 each), Chile, New Zealand, Portugal, Taiwan, Switzerland, United Kingdom (n = 2 each), Brazil, Finland, Greece, Hungary, Ireland, Israel, Lebanon, Malaysia, Romania, Slovenia (n = 1 each). Australia (n = 14), Japan, United States (n = 8 each), Spain (n = 7), France, Germany (n = 5 each), India, Italy, United Kingdom (n = 2 each), Brazil, The Netherlands, Poland (n = 1 each).
Scedosporium spp.
IFD due to Scedosporium spp. was reported in 208 patients. Five patients had mixed infection, four with Aspergillus spp., one with Candida spp. The majority were proven IFD (n = 183, 88%). Median age at diagnosis was 56 years (IQR 39–65 years), 128 (61.5%) patients were males.
Predisposing factors
The most common risk factors in immunocompromised patients (n = 118, 56.7%) were SOT (n = 58, 49.2%) and malignancy (n = 29, 24.6%) (). Concerning solid organ transplantation, most transplants were kidney or lung transplants (n = 22 each, 37.9%). IFD occurred in median after 365 days (IQR 98–1460 days) in kidney and after 82 days (IQR 26–461 days) in lung transplant patients. Eleven of 13 CF patients received a lung transplant. Regarding patients with underlying malignancy, most had leukaemia or lymphoma (n = 21, 72.4%). In total, eight (27.6%) patients received allogeneic hematopoietic stem cell transplantation for haematological malignancy. In 10 (34.5%) patients with underlying malignancy, neutropenia was reported. Nineteen of 33 (57.6%) patients with DM were immunocompromised due to SOT, malignancy or other long-term immunosuppression.
Table 1. Site of infection by dominant risk factor in 208 Scedosporium spp. and 56 L. prolificans infections.
In immunocompetent patients (n = 90, 43.3%) surgery or trauma were the most prevalent risk factors (n = 17, 18.9% each). The most frequent traumatic events were eye lacerations and penetrating injuries. DM was the sole known risk factor in five patients.
Clinical presentation
Fever at onset of the Scedosporium spp. infection was reported for 53 (25.5%) patients, similar for immunocompromised (30/118, 25.4%) and immunocompetent (23/90, 25.6%) patients. Fever was most frequent in HSCT recipients, other patients treated for malignancy and near drowning victims (62.5%, 42.9% and 50% respectively) but rarely reported for trauma patients (5.9%).
Scedosporiosis affected skin, lung, CNS, and eye in the majority of patients (n = 58, 27.9%; n = 51, 24.5%; n = 50, 24%; n = 47, 22.6%, respectively) (). Lung was frequently involved in patients with underlying malignancy, primarily in HSCT recipients (6/8, 75%) and near drowning victims (6/12, 50%) (). Pulmonary infection was identified in 13 of 24 (54.2%) patients receiving a lung transplant, but in none of the patients receiving a kidney or liver transplant. Four of the 51 patients with lung infection presented with cough, five with dyspnoea, and six with both. Chest pain was reported for only three immunocompromised patients. Abnormal computed tomography (CT) findings were presented for 17 (33.3%) patients with lung infection, mostly infiltrates, cavities with surrounding infiltrates and nodular lesions. No difference in imaging findings of the lung between immunocompetent (n = 5) and immunocompromised (n = 12) patients could be identified.
The CNS was frequently affected in transplant recipients and near drowning victims. The majority of the patients with CNS infection showed neurological symptoms (27/50, 54%), mostly decreased consciousness, confusion but also hemiparesis in few cases, and complained about headache (17/50, 34%). Occurrence of neurological symptoms were similar in immunocompetent and immunocompromised patients, but pain was reported more frequently in the immunocompetent group (50% versus 21.4% in immunocompromised patients). Lesions suggestive of IFD were detected by magnetic resonance imaging (MRI) in 23 (46%) patients with CNS infection and by CT in additional six (12%) patients.
Eye infections were localized in 28 (59.6%) patients and were involved in disseminated disease in 13 (27.7%). In additional six (12.7%) patients, infection affected adjacent sinuses or CNS. For 14 (29.8%) patients with eye infections, impaired or complete loss of vision was reported; for 21 (44.7%) pain in the eye was noted. Whereas impaired vision was reported slightly more frequently in immunocompromised patients (35% versus 25.9% in immunocompetent patients), pain was more frequently addressed in immunocompetent patients (48% versus 40% in immunocompromised patients).
Disseminated Scedosporium spp. infection was present in 31 (26.3%) of the immunocompromised, mainly in transplant recipients, and 15 (16.7%) of immunocompetent patients. Lung, CNS, skin, and eye were the most frequently involved organs in disseminated disease overall (n = 25, 54.3%; n = 22, 47.8%; n = 19, 41.3%; n = 13, 28.3%, respectively) (Supplementary Table 1(A)). In 12 (5.8%) patients with confirmed blood stream infection, the pathogen was isolated from at least one other organ (mostly CNS, heart or lung). If infection affected contiguous organs these were eyes, sinuses and/or CNS in all but two cases with deep soft tissue infection with involvement of the bone.
Treatment
All but four patients received antifungal drugs for treatment of scedosporiosis, in median for 90 days (IQR 34 – 217 days) (). Surgical resection, debridement or drainage of the infected site was performed in 62 (52.5%) immunocompromised patients and in 57 (63.3%) immunocompetent patients. Most patients with eye infections underwent vitrectomy, keratoplasty, enucleation or surgical drainage of abscesses (32/47, 68%). Pulmonary infections were mainly treated with systemic antifungals (49/51, 96%), eight (15.7%) infections were managed surgically in addition, of which six had localized disease. Brain lesions were resected or surgically drained in 15 of 50 (30%) patients, of which eight were immunocompromised and seven immunocompetent.
Table 2. Treatment administered to 208 patients with Scedosporium spp. and 56 L. prolificans infection.
The majority of the patients received systemic voriconazole, amphotericin B-based formulation (amphotericin B) or itraconazole (65.9%, 30.3%, and 27.4%, respectively) for treatment of scedosporiosis (). The most frequent monotherapies were voriconazole and itraconazole (Supplementary Table 2). In 31 of 46 (67.4%) patients treated with other than these two first line antifungals, treatment was switched to voriconazole. Switch of any antifungal drug to amphotericin B was rare. Most patients treated with any combination therapy, received more than one regimen. In the majority of the cases, voriconazole was co-administered, mostly with terbinafine or amphotericin B. Posaconazole was rarely used, never as first line monotherapy, but rather as salvage or in combination.
Outcome and presumed prognostic factors
All-cause mortality for each risk group and for disseminated Scedosporium spp. infection is shown in . Day 42 and overall mortality were higher in immunocompromised (22.2% and 43.6% respectively) compared to immunocompetent patients (14% and 30.7%, respectively). Mortality in patients with malignancy (55.2% overall), particularly in HSCT recipients (75%), was higher than in SOT recipients (39.7%) and other immunocompromised patients (40%) (Supplementary Figure 1 (A), ). Disseminated disease was associated with higher mortality in all risk groups, except near drowning, where four patients with CNS and lung or eye infection survived. All 12 patients with fungemia died and death was mostly attributed to IFD. Overall, attributable mortality was 76%; results for each risk group are presented in Supplementary Table 3.
Table 3. All-cause mortality overall and at day 42 in patients with Scedosporium spp. (n = 205) and L. prolificans (n = 55) infection.
Mean survival time varied depending on the risk group and dissemination of the infection (Supplementary Figure 1, Figure 2). HSCT recipients had a shorter mean survival time of 93 days (CI 95% 30–157) compared to patients with underlying malignancy without HSCT (236 days, CI 95% 41–156), SOT (271 days, CI 95% 230–312), and other conditions requiring long-term immunosuppression (220 days, CI 95% 156–283). Overall, for immunocompromised patients with disseminated Scedosporium spp. infection mean survival time was 156 days (CI 95% 98–214); in the seven patients with blood stream infection 42 days (CI 95% 0–107). If infection was localized, mean survival time was 270 days (CI 95% 236–305). In immunocompetent patients with disseminated disease, mean survival time was 175 days (CI 95% 87 – 262); in the five patients with blood stream infection 12 days (CI 95% 5–19). If infection was localized, mean survival time was 293 days (CI 95% 261–325) accordingly.
Bivariate analysis revealed that in SOT recipients, infection of the CNS and disseminated infection were associated with higher mortality (Supplementary Table 4(A)). In patients with underlying malignancy, infection of the lung predicted worse outcome. This holds true if HSCT recipients were excluded (Log Rank Test p = 0.011, data not shown). In immunocompromised patients who developed CNS complications, the 42-day mortality was higher compared to those with other infected sites, both in patients with localized (33.3% versus 15.9%) and disseminated disease (60% versus 18.8%). In immunocompetent patients with localized infection, CNS involvement was associated with higher 42-day mortality compared to other affected organs (23.1% versus 6.5%, Fisher’s Exact Test p = 0.095). No difference in 42-day mortality was identified in immunocompetent patients with disseminated disease with versus without CNS involvement (33.3% versus 33.3%).
Surgical treatment of eye infections was not associated with better overall survival in this case series. Day 42 mortality was 6.3% (2/32) and 13.3% (2/15) in patients with and without surgically treated eye infection, respectively (Fisher’s Exact Test p = 0.602). In patients with and without surgically treated brain infection, day 42 mortality was 20% (3/15) and 37.1% (13/35), respectively (Fisher’s Exact Test p = 0.328).
Overall, patients who received voriconazole for the treatment of scedosporiosis had a longer mean survival time compared to the patients treated with amphotericin B (276 days, CI 95% 248–304 versus 144 days, CI 95% 78–209) (Supplementary Figure 3(A)). For voriconazole similar mean survival times were seen in immunocompromised and immunocompetent patients (274 days, CI 95% 235–312 versus 280 days, CI 95% 238–321; Log Rank Test p = 0.651), whereas for amphotericin B survival time was longer in immunocompetent than in immunocompromised patients (206 days, CI 95% 104–309 versus 95 days, CI 9% 19–170; Log Rank Test p = 0.065).
Day 42 mortality was lower in patients treated with voriconazole compared to those treated with any formulation of amphotericin B in immunocompromised (11.3% versus 58.8%, Fisher’s Exact Test p < 0.001) and immunocompetent (14% versus 23.1%, Fisher’s Exact Test p = 0.416) patients. Treatment with voriconazole was associated with lower day 42 and overall mortality in localized and disseminated disease compared to treatment with amphotericin B.
Antifungal susceptibility
In vitro susceptibility to antifungals was available for clinical isolates of Scedosporium spp. from 34 patients (). Median MIC values were lowest for olorofim (0.0039 mg/L for all isolates), voriconazole (0.5 mg/L, IQR 0.25–1 mg/L) and posaconazole (1.5 mg/L, IQR 0.25–1 mg/L), and high for amphotericin B (12 mg/L, IQR 2.5–16 mg/L) and terbinafine (16 mg/L, IQR 3 – 32 mg/L). Highest MICs were determined for fluconazole (24 mg/L, IQR 16 – 56 mg/L), and flucytosine (128 mg/L for all isolates). In rare cases low MICs (≤0.5 mg/L) were determined for amphotericin B, itraconazole as well as for echinocandins but not for terbinafine, fluconazole and flucytosine for which MICs were >2 mg/L for all isolates.
Table 4. Median minimum inhibitory concentrations in Scedosporium spp. and L. prolificans clinical isolates assessed using the EUCAST and CLSI procedures.
Lomentospora prolificans
Fifty-six patients with L. prolificans infection were identified. Three had a mixed IFD with additional identification of Aspergillus spp., Exserohilum spp. or S. apiospermum. The majority were proven IFD (n = 52, 92.9%). Median age at diagnosis was 58 years (IQR 42–67 years), 32 (57.1%) patients were male.
Predisposing factors
Of 56 patients with L. prolificans infection, the majority were immunocompromised (n = 39, 69.6%), most related to an underlying malignancy (n = 28, 71.8%) or a SOT (n = 7, 17.9%) (). Leukaemia and lymphoma were the most frequent haematological diseases (n = 19, 67.9% and n = 4, 14.3%). Nine (32.1%) patients received HSCT and overall, for 13 (46.4%) patients neutropenia during treatment of the underlying malignancy was reported. Transplant patients had received a lung (n = 4), kidney (n = 2) or heart (n = 1). Lomentosporiosis was reported in 17 immunocompetent patients, mainly in surgical and trauma patients (n = 7, 41.2% and n = 5, 29.4%, respectively).
Clinical presentation
Fever was present before diagnosis of L. prolificans infection in 41% (16/39) of immunocompromised patients, mostly those with malignancy (15/28, 53.7%), and in 29.4% (5/17) of immunocompetent patients, mostly surgical patients (4/7, 57.1%). In none of the patients with traumatic injuries, long-term immunosuppression and in only one kidney transplanted patient fever was reported before diagnosis of IFD.
L. prolificans infection most frequently affected lung, eye, and heart (n = 22, 39.3%; n = 12, 21.4%; n = 11, 19.6%, respectively). Ten of 11 patients with infection of the heart had disseminated disease, seven with positive blood culture. Disseminated infection was more frequent in immunocompromised patients (n = 29, 74.4% versus n = 4, 23.5% of immunocompetent patients) and was mostly associated with fungemia in both groups (, Supplementary Table 1). Overall, in 8 of 26 (30.8%) patients with fungemia, no other infected organ was reported. In the other 18 fungemia cases as well as in cases with disseminated infection without reported blood stream infection (n = 7), on average a total of three affected organs was identified. Here, lung and/or heart were most frequently reported (Supplementary Table 1(B)). In 10 (45.5%) patients with lung infections, radiological signs were seen. CT scans showed mostly pulmonary areas of nodular consolidation without cavitation and less frequently infiltrates; halo or air-crescent signs were not reported. For seven (31.8%) patients with lung infection, dyspnoea or cough was reported.
Eye infections were localized in four (33.3%) patients. Pain in the eye was reported for four patients, for one of them and additional three patients, blurred vision was reported. Infection of the CNS was diagnosed in immunocompromised patients only (n = 6, 10.7%), in five the infection was disseminated, four with blood stream infection. Lesions on head MRI and CT scans were presented for four of these cases.
Overall, L. prolificans infection was localized in 10 (25.6%) immunocompromised patients and 13 (76.5%) immunocompetent patients; lung (n = 8), bone (n = 6) or eye (n = 4) were most frequently affected. For four of eight (50%) patients with localized lung infection, dyspnoea or cough before diagnosis was reported.
Treatment
All but one patient with L. prolificans infection received antifungals for treatment (). One patient with disseminated infection received intravitreal injection only and died after a few days. Nine (23.1%) immunocompromised and 15 (88.2%) immunocompetent patients underwent surgery. Two of 22 lung infections were treated with lobectomy, both patients were immunocompetent. Seven of 12 patients with eye infection underwent vitrectomy or enucleation. None of the brain infections were operated on.
Antifungals most frequently used for treatment of lomentosporiosis were voriconazole, amphotericin B, terbinafine, and echinocandins (67.9%, 48.2%, 39.3%, and 30.4%, respectively) (). Median treatment duration was 21 days (IQR 5–150 days). Sixteen patients received monotherapy, mostly amphotericin B, voriconazole or itraconazole for a median of 11 days (IQR 4–35 days). Two patients with fungemia and treated with echinocandin monotherapy died within 2 weeks. Half of the patients received combination therapy, mostly with voriconazole plus terbinafine or amphotericin B (Supplementary Table 2) for a median of 35 days (IQR 15–330 days). In 13 (26%) patients treated with combination therapy, more than one regime was administered.
Outcome
Overall mortality, as shown in for each risk group, was higher in immunocompromised than in immunocompetent patients with L. prolificans infection (74.4% versus 25%, Fisher’s Exact Test p = 0.001), being highest in patients with malignancy (85.7%), similar for patients with and without HSCT, and SOT (57.1%) (Supplementary Figure 1(B), ). Of the 14 patients with available information on attributable death, 10 (71.4%) died due to infection (Supplementary Table 3), all of which had disseminated disease, eight with confirmed blood stream infection.
Day 42 mortality was 59% in immunocompromised patients and 17.6% in immunocompetent patients (Fisher’s Exact Test p = 0.004). In patients with underlying malignancy, day 42 mortality was 71.4%, 90% of those had confirmed fungemia. Overall mortality and day 42 mortality in patients with disseminated disease was higher in all risk groups compared to the localized infection ().
Mean survival time in immunocompromised patients was 114 days (CI 95% 66–163) (Supplementary Figure 2(B)), in patients with blood stream infection 22 days (CI 95% 9–37). Mean survival time of patients with underlying malignancy and SOT was 63 days (CI 95% 21–105) and 242 days (CI 95% 127–357), respectively. Immunocompetent patients had a mean survival time of 302 days (CI 95% 239–367).
In patients with malignancy, disseminated disease was associated with a worse outcome and surgical intervention as antifungal treatment was associated with improved outcome overall (Supplementary Table 4(B)). Of five patients with eye infection who were not surgically treated, all died within 49 days, 4 had fungemia. Of seven patients with surgically treated eye infection, one patient with fungemia died after three weeks and six patients were alive with median follow up time of 180 days.
Overall mortality was lower in patients who received voriconazole for treatment compared to other antifungals (52.6% versus 68.8%, Fisher’s Exact Test p = 0.37) (Supplementary Figure 3(B)). Treatment with voriconazole together with terbinafine was not associated with improved day 42 and overall survival in patients with lomentosporiosis compared to treatment with voriconazole without terbinafine overall and in any of the tested subgroups (e.g. blood stream infection and localized disease, HSCT and no HSCT, all immunocompromised and all immunocompetent patients). Overall mortality was similar in patients treated with voriconazole and patients treated with voriconazole and terbinafine (50% and 55.3%, Chi2 p = 0.757).
Antifungal susceptibility
In vitro susceptibility to antifungals were available for 18 clinical isolates from different patients (). Median MIC were low for olorofim (0.0039 mg/L for all isolates). For all other antifungals tested, median MIC was 8 mg/L or higher. Occasionally isolates show MIC of 1 mg/L or lower for echinocandins, amphotericin B or voriconazole. Other than these, MICs were at least 4 mg/L for all antifungals in all isolates tested.
Discussion
Scedosporium spp. and L. prolificans infections are rare diseases as reflected by their reported frequency. Through literature search and in FungiScope® we identified 264 individual cases of severe IFD caused by Scedosporium spp. or L. prolificans that were diagnosed between 2000 and 2017. This translates to an average of only 16 reported cases per year. This may be due to a diagnostic and a reporting bias, and thus not reflect true epidemiology. Overall, Scedosporium spp. and L. prolificans infections account for less than 1% of all mold infections (Caira et al. Citation2008), and affect less than 1 in 60 000 in-patients in general (Mügge and Schömig Citation2017). Regional differences in incidences, such as seen with the relatively high incidence of these infections in Australia compared to other countries, may be due to climatic and environmental conditions that favour growth of the fungi. Soil pH has been suggested to play a role (Kaltseis et al. Citation2009). Similar pH ranges of the soil as in Australia are found in Mid East USA and Canada, Eastern South-America, Southern Europe, North and South Africa, and Mid and Central Asia (Global Soil Data Task Citation2014). Reported cases were predominately from those regions. Most Scedosporium spp. infections were reported in patients after organ transplantation, whereas L. prolificans infections were mostly diagnosed in patients with an underlying malignancy. Effective prophylaxis is particularly difficult in the case of L. prolificans, due to pan-resistance to virtually all systemically active antifungal agents currently available. Thus, incidence of break-through infections in haematological patients receiving posaconazole or voriconazole as first line antifungal prophylaxis is likely to be higher for L. prolificans than for Scedosporium spp.
Clinical manifestation differed between scedosporiosis and lomentosporiosis as well as between patient populations. Infection with Scedosporium spp. frequently disseminates to distant organs, often to the CNS even in immunocompetent patients, without primary bloodstream infection. L. prolificans infection often presents as fungemia, while brain infections are reported less commonly (Horré et al. Citation2000; Mellinghoff et al. Citation2002). Tropism for blood vessels and haematogenous spread is considerable in both diseases (Kowacs et al. Citation2004). Disseminated disease is independently associated with worse outcome, fungemia being the key determinant. In our dataset, fungemia proved fatal in all immunocompromised patients and in all but one immunocompetent patients.
Infection of the CNS was found to be associated with worse outcome in SOT patients with Scedosporium spp. infection. The true impact of brain infection on survival, also in other risk groups, is difficult to assess. Several aspects need to be considered. Despite CNS being reported as the sole site of infection for several patients, in the absence of previous brain injury or surgery, it is highly unlikely to be the primary site (O'Bryan Citation2005). If not all potentially infected sites are examined thoroughly, the additional impact of brain infection may be underestimated. On the contrary, CNS infections may not be identified before death. Reasons are manifold, involvement of the CNS is not suspected, patient’s condition does not allow transport to radiology or invasive diagnostic procedures, imaging findings may be inconclusive, or results from microbiological workup may remain negative. With fewer autopsies being performed nowadays, brain infections may not be reported altogether (Tietz et al. Citation2005). Due to low susceptibility of the fungus and low permeability of the blood-brain barrier for many antifungal drugs, it is undisputed that CNS infection is particularly difficult to treat, and mortality is high (Kantarcioglu et al. Citation2008).
With voriconazole, known for its ability to cross the blood-brain barrier, an antifungal became available for successful treatment of brain lesions (Schwartz et al. Citation2005). Voriconazole showed high in vitro activity against S. apiospermum isolates (Meletiadis et al. Citation2002). Current guidelines recommend the use of voriconazole along with surgical resection (Tortorano et al. Citation2014). A comprehensive study on treatment efficacy in 107 scedosporiosis patients treated with voriconazole showed successful response in 57% of patients overall (Troke et al. Citation2008). In our analysis, day 42 and overall mortality was numerically lower in immunocompromised patients with scedosporiosis and lomentosporiosis treated with voriconazole compared to those treated with amphotericin B. Differences between voriconazole alone or in combination with amphotericin B were not seen. Combination therapies are widely used to exploit synergistic effects. Terbinafine for example, although demonstrating limited in vitro activity alone, showed synergy in combination with azoles in several in vitro studies (Ryder and Leitner Citation2001; Meletiadis et al. Citation2003). The additional value of terbinafine in combination treatment of patients with mold infections is controversial. Poor tissue penetration of terbinafine casts doubt on the clinical meaning of such synergy. Our analysis did not identify synergy of terbinafine. Olorofim is a new antifungal agent that shows promising antifungal activity in vitro. In our analysis, olorofim had low MICs against all Scedosporium spp. and L. prolificans strains, confirming recently published results (Wiederhold et al. Citation2017; Biswas et al. Citation2018). A clinical efficacy study on olorofim is upcoming. Similarly, APX001 (E1210) a novel antifungal agent that disrupts fungal cell wall assembly, showed good in vitro activity against Scedosporium spp. and L. prolificans and is currently tested in phase I clinical trial in patients with acute leukaemia (clinicaltrials.gov 2017; Castanheira et al. Citation2012).
Route of entry of Scedosporium spp. and L. prolificans is frequently through the respiratory tract, which explains the high incidence of lung lesions. In this case series, lung involvement predicted worse outcome of scedosporiosis in patients with malignancy in univariate analysis. Furthermore, disseminated infection was associated with worse outcome in patients with Scedosporium spp. infection post SOT and in patients with L. prolificans infection and malignancy. In our analysis, we did not identify additional potentially important predictors. Proper control of confounders is difficult due to heterogeneity of hosts and diverse clinical patterns of IFD, leaving us with small numbers not allowing multivariate analysis. Trends determined in univariate analysis need further confirmation in a larger dataset.
Infections caused by Scedosporium spp. and L. prolificans resemble other mold infections on cytological and histological examination, challenging timely diagnosis and targeted treatment (Guarro et al. Citation2006). High virulence of certain species, in particular L. prolificans, and less predictable susceptibility patterns urge for prompt pathogen identification. We detected low voriconazole MICs and slightly higher MICs for itraconazole and posaconazole in most Scedosporium spp. strains. In comparison, for L. prolificans MICs were higher for all antifungals and low voriconazole MICs were determined in individual cases only. Amphotericin B or echinocandin MICs were low in few Scedosporium spp. strains, which may offer a treatment option in respective patients. Supportive experimental in vivo data are scarce with varying results on efficacy of single and combination therapy with amphotericin B or echinocandins (Rodriguez et al. Citation2009; Lackner et al. Citation2014). The clinical predictive value of in vitro susceptibility of amphotericin B or echinocandins and thus, the additional therapeutic benefit for patients is still unknown.
Successful treatment of L. prolificans infections seems almost impossible. Currently available antifungals are ineffective against the vast majority of strains and surgical treatment is rarely an option due to the poor general condition of most patients and because disease presents as fungemia or complex disseminated disease in most cases. Thus, most patients are left with virtually no treatment option. This dilemma is clearly reflected by the high mortality rates in these patients and short survival time compared to Scedosporium spp. infections.
Despite being a feasible approach to comprehensively investigate the epidemiology and treatment patterns of these rare diseases, this study has obvious limitations. Due to recent changes in the taxonomy of Scedosporium and various standards in mycological diagnostics in different countries, reported species may or may not be correctly identified. Furthermore, one cannot be certain on the correct diagnosis of an invasive infection, due to lack of data in some cases. Predisposing host factors are sometimes difficult to assess, hampered by the frequently rapid deterioration of the fungal infection until death. Thus, impaired immune system may not have been recognized and patients may have been wrongly deemed immunocompetent. Nonetheless, Scedosporium spp. and L. prolificans infections have been reported in several immunocompetent patients with devastating outcome. Through direct wound inoculation potentially everyone is at risk for invasive fungal infections.
Infections caused by Scedosporium spp. and L. prolificans are extremely rare and patient populations at risk are diverse. CNS involvement, disseminated disease, and immunosuppression determine prognosis. During clinical management and eventually clinical trial design, these factors should be considered.
Collaborators
Björn Bachmannw, Kersten Borchertx, Alexander Burchardty, Arunaloke Chakrabartiz, Maximilian ChristopeitAA, Naima FasihAB, Khosro HekmatAC, Beléen Hernéandez RupéerezAD, Björn KemmerlingAE, Johanna KesselAF, Anupma Jyoti KindoAG, Nikolay KlimkoAH, Robert KrauseAI, Cornelia Lass-FlörlAD, Eric LevesqueAJ, Shawn LockhartAK, Jörg SteinmannAL, Alessandro MaritatiAM, Birgid MarkiefkaAN, Maria Teresa Martín GómezAO, Jacques MeisAP, Jarmo OksiAQ, Livio PaganoAR, Antonio Ramos Martinezs, Frederike ReischiesAS, Pere Soler PalacinAD and Edith VermeulenAT
Supplementary Figure 1.pdf
Download PDF (2.3 MB)Acknowledgements
We thank Sabine Wrackmeyer for her private donation to support the project. We thank Alexandra Laska and Maren Ziegler (Wisplinghoff Laboratories, Cologne, Germany), and Katharina Rosam (Medical University of Innsbruck, Austria) for their technical assistance, and Susann Bloßfeld (University Hospital Cologne, Germany) for her administrative support.
Disclosure statement
DS, JD, LDG, UA, GC, MPC, JC, RD, ILA, MM, AM, SM, MTM, DLP, EP, JSG, MSt, JT, HW having nothing to disclose.
AH reports personal fees from Astellas, personal fees from Gilead, personal fees from MSD, outside the submitted work.
RH reports personal fees from Astellas, personal fees from Basilea, personal fees from Gilead, personal fees from MSD, grants and personal fees from Pfizer, outside the submitted work.
PK eports personal fees from Cologne Cluster of Excellence - Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, non-financial support from Merck/MSD , non-financial support from MedImmune , other from Astellas, outside the submitted work.
ML reports grants and personal fees from Astellas Pharma , grants from MSD, personal fees from Forest Pharma , outside the submitted work.
MSl reports grants from Merck, personal fees from Gilead, personal fees from Merck, outside the submitted work.
JJV reports grants and personal fees from Merck / MSD, grants and personal fees from Astellas Pharma, grants and personal fees from Basilea, grants and personal fees from Deutsches Zentrum für Infektionsforschung, personal fees from Akademie für Infektionsmedizin, grants from Bundesministerium für Bildung und Forschung, personal fees from Uniklinik Freiburg / Kongress und Kommunikation, personal fees from Universität Manchester, personal fees from Deutsche Gesellschaft für Infektiologie, personal fees from Deutsche Gesellschaft für Innere Medizin, personal fees from Ärztekammer Nordrhein, personal fees from Uniklinik Aachen, personal fees from Back Bay Strategies, outside the submitted work.
MV reports personal fees from Merck/MSD, grants and personal fees from Astellas, grants and personal fees from Gilead Sciences, grants from 3M, personal fees from Berlin Chemie, grants and personal fees from DaVolterra, personal fees from Pfizer, personal fees from Organobalance, outside the submitted work.
OAC reports research grants from Actelion, Amplyx, Arsanis, Astellas, AstraZeneca, Basilea, Bayer, Cidara, , F2G, Gilead, GSK, Leeds University, Matinas, Medicines Company, MedPace, Melinta, Merck/MSD, Miltenyi, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres, is a consultant to Allecra Therapeutics, Amplyx, Actelion, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, IQVIA, Janssen, Matinas, Menarini, Merck/MSD, Paratek, PSI, Scynexis, Seres, Summit, Tetraphase, Vical, and received lecture honoraria from Astellas, Basilea, Gilead, Merck/MSD and Pfizer, outside the submitted work.
References
- Abela IA, Murer C, Schuurmans MM, Schmitt JW, Muller F, Imkamp F, Mueller NJ, Benden C. 2018. A cluster of scedosporiosis in lung transplant candidates and recipients: the Zurich experience and review of the literature. Transpl Infect Dis. 20:e12792.
- Abgrall S, Pizzocolo C, Michel CB, Martinod E, Martin A, Brauner M, Padoin C, Karoubi P, Lortholary O, Bouchaud O. 2007. Scedosporium apiospermum lung infection with fatal subsequent postoperative outcome in an immunocompetent host. Clin Infect Dis. 45:524–525.
- Agatha D, Krishnan KU, Dillirani VA, Selvi R. 2014. Invasive lung infection by Scedosporium apiospermum in an immunocompetent individual. Indian J Pathol Microbiol. 57:635–637.
- Ahmad S, Zia S, Sarwari AR. 2010. Scedosporium prolificans endocarditis: case report and review of literature. W V Med J. 106:24–26.
- Ahmed J, Ditmars DM, Sheppard T, del Busto R, Venkat KK, Parasuraman R. 2004. Recurrence of Scedosporium apiospermum infection following renal re-transplantation. Am J Transplant. 4:1720–1724.
- Alastruey-Izquierdo A, Mellado E, Peláez T, Pemán J, Zapico S, Álvarez M, Rodríguez-Tudela JL, Cuenca-Estrella M. 2013. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP Study). Antimicrob Agents Chemother. 57:4604.
- Al-Jehani H, Guiot MC, Torres C, Marcoux J. 2010. Scedosporium cerebral abscesses after extra-corporeal membrane oxygenation. Can J Neurol Sci. 37:671–676.
- Allen PB, Koka R, Kleinberg ME, Baer MR. 2013. Scedosporium apiospermum soft tissue infection as the initial presentation of acute myeloid leukemia: a case report. J Clin Oncol. 31:e98–100.
- Alpaydın S, Güler A, Çelebisoy N, Polat SH, Turhan T. 2015. Pseudallescheria boydii infection of the central nervous system: first reported case from Turkey. Acta Neurol Belg. 115:489–492.
- Ananda-Rajah MR, Grigg A, Slavin MA. 2008. Breakthrough disseminated Scedosporium prolificans infection in a patient with relapsed leukaemia on prolonged voriconazole followed by posaconazole prophylaxis. Mycopathologia. 166:83–86.
- Anonymous. on European Medicines Agency (EMA). http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000387/human_med_001135.jsp&mid=WC0b01ac058001d124.
- Azofra MM, Somovilla JLP, Porras MC, Carrillo LH, Pérez RD. 2010. Use of intralesional voriconazole for the treatment of cutaneous Scedosporium apiospermum infection. Clin Infect Dis. 51:255–257.
- Balandin B, Aguilar M, Sánchez I, Monzón A, Rivera I, Salas C, Valdivia M, Alcántara S, Pérez A, Ussetti P. 2016. Scedosporium apiospermum and S. prolificans mixed disseminated infection in a lung transplant recipient: an unusual case of long-term survival with combined systemic and local antifungal therapy in intensive care unit. Med Mycol Case Rep. 11:53–56.
- Baradkar VP, Mathur M, Kumar S. 2010. Invasive fungal sinusitis resulting in orbital apex syndrome in a HIV positive patient. Indian J Pathol Microbiol. 53:185–187.
- Bates DD, Mims JW. 2006. Invasive fungal sinusitis caused by Pseudallescheria boydii: case report and literature review. Ear Nose Throat J. 85:729–737.
- Baumgartner BJ, Rakita RM, Backous DD. 2007. Scedosporium apiospermum otomycosis. Am J Otolaryngol. 28:254–256.
- Beier F, Kittan N, Holzmann T, Schardt K, Andreesen R, Holler E, Hildebrandt GC. 2010. Successful treatment of Scedosporium apiospermum soft tissue abscess with caspofungin and voriconazole in a severely immunocompromised patient with acute myeloid leukemia. Transpl Infect Dis. 12:538–542.
- Bhat SV, Paterson DL, Rinaldi MG, Veldkamp PJ. 2007. Scedosporium prolificans brain abscess in a patient with chronic granulomatous disease: successful combination therapy with voriconazole and terbinafine. Scand J Infect Dis. 39:87–90.
- Bhatk V, Naseeruddin K. 2001. Invasive sino-nasal pseudallescheriasis in a non-immunocompromised patient. Indian J Otolaryngol Head Neck Surg. 53:148–150.
- Bibashi E, de Hoog GS, Kostopoulou E, Tsivitanidou M, Sevastidou J, Geleris P. 2009. Invasive infection caused by Pseudallescheria boydii in an immunocompetent patient. Hippokratia. 13:184–186.
- Biswas C, Law D, Birch M, Halliday C, Sorrell TC, Rex J, Slavin M, Chen SC. 2018. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol. 1–5. doi:10.1093/mmy/myx161. [Epub ahead of print].
- Blyth CC, Gilroy NM, Guy SD, Chambers ST, Cheong EY, Gottlieb T, McGuinness SL, Thursky KA. 2014. Consensus guidelines for the treatment of invasive mould infections in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern Med J. 44:1333–1349.
- Bose B, Sharma S, Derrington P, Divi D. 2012. Scedosporium apiospermum peritonitis in a patient undergoing peritoneal dialysis. Nephrology (Carlton).17:521–522.
- Bosma F, Voss A, van Hamersvelt HW, de Sévaux RG, Biert J, Kullberg BJ, Melchers WG, Verweij PE. 2003. Two cases of subcutaneous Scedosporium apiospermum infection treated with voriconazole. Clin Microbiol Infect. 9:750–753.
- Bouza E, Muñoz P. 2004. Invasive infections caused by Blastoschizomyces capitatus and Scedosporium spp. Clin Microbiol Infect. 10:76–85.
- Boyce Z, Collins N. 2015. Scedosporium apiospermum: an unreported cause of fungal sporotrichoid-like lymphocutaneous infection in Australia and review of the literature. Australas J Dermatol. 56:e39–e42.
- Bui DK, Carvounis PE. 2016. Favorable outcomes of filamentous fungal endophthalmitis following aggressive management. J Ocul Pharmacol Ther. 32:623–630.
- Buzina W, Feierl G, Haas D, Reinthaler FF, Holl A, Kleinert R, Reichenpfader B, Roll P, Marth E. 2006. Lethal brain abscess due to the fungus Scedosporium apiospermum (teleomorph Pseudallescheria boydii) after a near-drowning incident: case report and review of the literature. Med Mycol. 44:473–477.
- Caira M, Girmenia C, Valentini CG, Sanguinetti M, Bonini A, Rossi G, Fianchi L, Leone G, Pagano L. 2008. Scedosporiosis in patients with acute leukemia: a retrospective multicenter report. Haematologica. 93:104–110.
- Campagnaro EL, Woodside KJ, Early MG, Gugliuzza KK, Colomé-Grimmer MI, Lopez FA, Daller JA. 2002. Disseminated Pseudallescheria boydii (Scedosporium apiospermum) infection in a renal transplant patient. Transpl Infect Dis. 4:207–211.
- Campa-Thompson MM, West JA, Guileyardo JM, Spak CW, Sloan LM, Beal SG. 2014. Clinical and morphologic findings in disseminated Scedosporium apiospermum infections in immunocompromised patients. Proc (Bayl Univ Med Cent). 27:253–256.
- Canet JJ, Pagerols X, Sánchez C, Vives P, Garau J. 2001. Lymphocutaneous syndrome due to Scedosporium apiospermum. Clin Microbiol Infect. 7:648–650.
- Cardoso JC, Serra D, Cardoso R, Reis JP, Tellechea O, Figueiredo A. 2009. Cutaneous pseudallescheria boydii infection in a renal transplant patient: a case report. Dermatol Online J. 15:8.
- Carod-Artal FJ, Ferreira-Coral L, Mauro-Couto J, Gomes E, de Agassiz-Vasques M. 2009. Chronic spinal epidural abscess caused by Scedosporium prolificans in an immunocompetent patient. Spine (Phila Pa 1976). 34:E330–E332.
- Carrillo AJ, Guarro J. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob Agents Chemother. 45:2151–2153.
- Castanheira M, Duncanson FP, Diekema DJ, Guarro J, Jones RN, Pfaller MA. 2012. Activities of E1210 and comparator agents tested by CLSI and EUCAST broth microdilution methods against Fusarium and Scedosporium species identified using molecular methods. Antimicrob Agents Chemother. 56:352–357.
- Ceccarelli L, Calisti G, Delle Rose D, Ricciardi A, Maffongelli G, Sordillo P, Sarmati L, Andreoni M. 2012. Dapsone hypersensitivity syndrome complicated by Scedosporium apiospermum pneumonia in an immunocompetent patient. Infection. 40:459–462.
- Cetrulo CL, Jr., Leto Barone AA, Jordan K, Chang DS, Louie K, Buntic RF, Brooks D. 2012. A multi-disciplinary approach to the management of fungal osteomyelitis: current concepts in post-traumatic lower extremity reconstruction: a case report. Microsurgery. 32:144–147.
- Chanqueo L, Gutiérrez C, Tapia C, Silva V, Razeto L, Misad C. 2009. [Scedosporium apiospermum rhinosinusal infection in an immunocompetent host]. Rev Chil Infectol. 26:453–456.
- Chaveiro MA, Vieira R, Cardoso J, Afonso A. 2003. Cutaneous infection due to Scedosporium apiospermum in an immunosuppressed patient. J Eur Acad Dermatol Venerol. 17:47–49.
- Chen FK, Chen SD, Tay-Kearney ML. 2007. Intravitreal voriconazole for the treatment of endogenous endophthalmitis caused by Scedosporium apiospermum. Clin Experiment Ophthalmol. 35:382–385.
- Chen TC, Ho MW, Chien WC, Lin HH. 2016. Disseminated Scedosporium apiospermum infection in a near-drowning patient. J Formos Med Assoc.115:213–214.
- Clement ME, Maziarz EK, Schroder JN, Patel CB, Perfect JR. 2015. Scedosporium apiosermum infection of the "Native" valve: Fungal endocarditis in an orthotopic heart transplant recipient. Med Mycol Case Rep. 9:34–36.
- clinicaltrials.gov. 2017. Amplyx Pharmaceuticals. Safety and Pharmacokinetics of Intravenous and Oral APX001 in Patients With Acute Myeloid Leukemia (AML) and Neutropenia; NCT03333005. [accessed 2018 Apr 18]. https://clinicaltrials.gov/ct2/show/NCT03333005?term=APX001&rank =1.
- Cooley L, Spelman D, Thursky K, Slavin M. 2007. Infection with Scedosporium apiospermum and S. prolificans, Australia. Emerging Infect Dis. 13:1170–1177.
- Cruz R, Barros M, Reyes M. 2015. [Pulmonary non invasive infection by Scedosporium apiospermum]. Rev Chilena Infectol. 32:472–475.
- Cuenca-Estrella M, Ruiz-Díez B, Martínez-Suárez JV, Monzón A, Rodríguez-Tudela JL. 1999. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 43:149–151.
- Danaher PJ, Walter EA. 2004. Successful treatment of chronic meningitis caused by Scedosporium apiospermum with oral voriconazole. Mayo Clin Proc. 79:707–708.
- Daniele L, Le M, Parr AF, Brown LM. 2017. Scedosporium prolificans septic arthritis and osteomyelitis of the hip joints in an immunocompetent patient: a case report and literature review. Case Rep Orthop. 2017:1–3809735.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 46:1813–1821.
- Denton EJ, Smibert O, Gooi J, Morrissey CO, Snell G, McGiffin D, Paraskeva M. 2016. Invasive Scedosporium sternal osteomyelitis following lung transplant: Cured. Med Mycol Case Rep. 12:14–16.
- D'Hondt K, Parys-Van Ginderdeuren R, Foets B. 2000. Fungal keratitis caused by Pseudallescheria boydii (Scedosporium apiospermum). Bull Soc Belge Ophtalmol. 277:53–56.
- Douglas AP, Chen SC, Slavin MA. 2016. Emerging infections caused by non-Aspergillus filamentous fungi. Clin Microbiol Infect. 22:670–680.
- Elm MK, Ahmed A, Goksel D, Henning JS. 2009. Cutaneous and systemic infection with Scedosporium apiospermum. Cutis. 84:275–278.
- Ergin C, Kutlu M, Arıkan Akdağlı S, Sarıbaş Z, Aydeniz Ozansoy F, Sarı I, Dursunoğlu N. 2013. [Isolation of Scedosporium apiospermum (teleomorph: Pseudallescheria apiosperma) from an acute myeloid leukemia patient]. Mikrobiyol Bul. 47:351–355.
- Espinel-Ingroff A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J Clin Microbiol. 39:954–958.
- Ezzedine K, Wissing KM, Jacobs F, Rodríguez H, Malvy D, Simonart T. 2009. Recurrent Scedosporium apiospermum skin infection in a renal transplant recipient. J Eur Acad Dermatol Venereol. 23:95–96.
- Fadzillah MT, Ishak SR, Ibrahim M. 2013. Refractory Scedosporium apiospermum keratitis successfully treated with combination of Amphotericin B and Voriconazole. Case Rep Ophthalmol Med. 2013:413953.
- Farina C, Arosio M, Marchesi G, Amer M. 2002. Scedosporium apiospermum post-traumatic cranial infection. Brain Inj. 16:627–631.
- Farina C, Gotti E, Suter F, Goglio A. 2006. Scedosporium apiospermum soft-tissue infection: a case report and review of kidney transplant literature. Transplant Proc. 38:1333–1335.
- Fernández Guerrero ML, Askari E, Prieto E, Gadea I, Roman A. 2011. Emerging infectious endocarditis due to Scedosporium prolificans: a model of therapeutic complexity. Eur J Clin Microbiol Infect Dis. 30:1321–1324.
- Fernández-Mosteirín N, Salvador-Osuna C, Mayayo P, García-Zueco JC. 2003. [Scedosporium prolificans: disseminated infection in immunocompromised patient]. Med Clin (Barc). 120:317–318.
- Fietz T, Knauf W, Schwartz S, Thiel E. 2003. Intramedullary abscess in a patient with disseminated Scedosporium apiospermum infection. Br J Haematol. 120:724.
- Figueroa MS, Fortun J, Clement A, De Arévalo BF. 2004. Endogenous endophthalmitis caused by Scedosporium apiospermum treated with voriconazole. Retina. 24:319–320.
- Foo H, Ooi SY, Giles R, Jones P. 2009. Scedosporium apiospermum pacemaker endocarditis. Int J Cardiol. 131:e81–e82.
- García-Vidal C, Cabellos C, Ayats J, Font F, Ferran E, Fernández-Viladrich P. 2009. Fungal postoperative spondylodiscitis due to Scedosporium prolificans. Spine J. 9:e1–e7.
- Gelabert-González M, Llovo-Taboada J, Reyes-Santías R, Arcos-Algaba A, Serramito-García R, Peñalver-Barral MD, García-Allut A. 2010. [Scedosporium apiospermum brain abscess. Report of one case with literature review]. Neurocirugia (Astur). 21:125–131.
- German JW, Kellie SM, Pai MP, Turner PT. 2004. Treatment of a chronic Scedosporium apiospermum vertebral osteomyelitis. Case report. Neurosurg Focus. 17:E9.
- Ghosh R, Mishra P, Maiti PK, Debnandi A. 2017. Prompt diagnosis of Scedosporium apiospermum soft tissue infection: Life-saving in a renal transplant recipient. J Postgrad Med. 63:200–202.
- Goldman C, Akiyama MJ, Torres J, Louie E, Meehan SA. 2016. Scedosporium apiospermum infections and the role of combination antifungal therapy and GM-CSF: A case report and review of the literature. Med Mycol Case Rep. 11:40–43.
- Gosbell IB, Toumasatos V, Yong J, Kuo RS, Ellis DH, Perrie RC. 2003. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses. 46:233–236.
- Gottesman-Yekutieli T, Shwartz O, Edelman A, Hendel D, Dan M. 2011. Pseudallescheria boydii infection of a prosthetic hip joint – an uncommon infection in a rare location. Amer J Med Sci. 342:250–253.
- Greig JR, Khan MA, Hopkinson NS, Marshall BG, Wilson PO, Rahman SU. 2001. Pulmonary infection with Scedosporium prolificans in an immunocompetent individual. J Infect. 43:15–17.
- Grenouillet F, Botterel F, Crouzet J, Larosa F, Hicheri Y, Forel JM, Helias P, Ranque S, Delhaes L. 2009. Scedosporium prolificans: an emerging pathogen in France? Med Mycol. 47:343–350.
- Guarro J, Kantarcioglu AS, Horré R, Rodríguez-Tudela JL, Cuenca Estrella M, Berenguer J, de Hoog GS. 2006. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol. 44:295–327.
- Guber I, Bergin C, Majo F. 2016. Repeated intrastromal injections of voriconazole in combination with corneal debridement for recalcitrant Fungal Keratitis – a case series. Klin Monbl Augenheilkd. 233:369–372.
- Guignard S, Hubert D, Dupont B, Anract P, Alioua D, Guerini H, Paugam A, Dougados M. 2008. Multifocal Scedosporium apiospermum spondylitis in a cystic fibrosis patient. J Cyst Fibros. 7:89–91.
- Harrison MK, Hiatt KH, Smoller BR, Cheung WL. 2012. A case of cutaneous Scedosporium infection in an immunocompromised patient. J Cutan Pathol. 39:458–460.
- He XH, Wu JY, Wu CJ, Halm-Lutterodt NV, Zhang J, Li CS. 2015. Scedosporium apiospermum Infection after Near-drowning. Chin Med J. 128:2119–2123.
- Henao-Martínez AF, Castillo-Mancilla JR, Barron MA, Nichol AC. 2013. Combination antifungal therapy in the treatment of scedosporium apiospermum central nervous system infections. Case Rep Infect Dis. 2013:589490.
- Holmes NE, Trevillyan JM, Kidd SE, Leong TY. 2013. Locally extensive angio-invasive Scedosporium prolificans infection following resection for squamous cell lung carcinoma. Med Mycol Case Rep. 2:98–102.
- Horré R, Feil E, Stangel AP, Zhou H, Gilges S, Wöhrmann A, de Hoog GS, Meis JF, Marklein G, Schaal KP. 2000. [Scedosporiosis of the brain with fatal outcome after traumatizatio of the foot. case report]. Mycoses. 43:33–36.
- Horré R, Jovanic B, Marklein G, Schumacher G, Friedrichs N, Neuhaus T, de Hoog GS, Becker WH, Choi SM, Schaal KP. 2003. Fatal pulmonary scedosporiosis. Mycoses. 46:418–421.
- Horré R, Marklein G. 2009. Isolation and clinical significance of Pseudallescheria and Scedosporium species. Med Mycol. 47:415–421.
- Howden BP, Slavin MA, Schwarer AP, Mijch AM. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur J Clin Microbiol Infect Dis. 22:111–113.
- Hu H, Chen J. 2017. Scedosporiosis presenting with subcutaneous nodules in an immunocompromised patient. Indian J Dermatol Venereol Leprol. 83:71–73.
- Husain N, Chen TC, Hou JK. 2013. An unusual cause of diarrhea in an immunocompromised patient. Scedosporium apiospermum Colitis and Brain Abscess. Gastroenterology.145(3):519, 697–698.
- Husain S, Muñoz P, Forrest G, Alexander BD, Somani J, Brennan K, Wagener MM, Singh N. 2005. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis. 40:89–99.
- Global Soil Data Task. 2014. Global Soil Data Products CD-ROM Contents (IGBP-DIS). Oak Ridge, Tennessee: ORNL DAAC. [accessed 2018 Apr 11]. https://doi.org/10.3334/ORNLDAAC/565.
- Ikewaki J, Imaizumi M, Nakamuro T, Motomura Y, Ohkusu K, Shinoda K, Nakatsuka K. 2009. Peribulbar fungal abscess and endophthalmitis following posterior subtenon injection of triamcinolone acetonide. Acta Ophthalmol. 87:102–104.
- Institute CaLaS. (2008). Clinical and Laboratory and Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. 2nd ed. Wayne, PA: Clinical and Laboratory and Standards Institute.
- Ishii S, Hiruma M, Hayakawa Y, Sugita T, Makimura K, Hiruma M, Yoshiike T. 2015. Cutaneous Pseudallescheria boydii/Scedosporium apiospermum Complex (Molecular type: Scedosporium apiospermum [Clade 4]) infection: a case report and literature review of cases from Japan. Med Mycol J. 56:E25–E30.
- Jain A, Egbert P, McCulley TJ, Blumenkranz MS, Moshfeghi DM. 2007. Endogenous Scedosporium apiospermum endophthalmitis. Arch Ophthalmol. 125:1286–1289.
- Jain P, Nagarajan P, Prayag P, Benton CB, Kadia T, Groisberg R, Kontoyiannis DP, Mulanovich VE, Pemmaraju N. 2017. Mixed angioinvasive exserohilum and scedosporium infection in a patient with AML. Am J Hematol. 92:119–120.
- Kaltseis J, Rainer J, De Hoog GS. 2009. Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Med Mycol. 47:398–405.
- Kanafani ZA, Comair Y, Kanj SS. 2004. Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur J Clin Microbiol Infect Dis. 23:836–840.
- Kantarcioglu AS, Guarro J, de Hoog GS. 2008. Central nervous system infections by members of the Pseudallescheria boydii species complex in healthy and immunocompromised hosts: epidemiology, clinical characteristics and outcome. Mycoses. 51:275–290.
- Katragkou A, Dotis J, Kotsiou M, Tamiolaki M, Roilides E. 2007. Scedosporium apiospermum infection after near-drowning. Mycoses. 50:412–421.
- Kelly M, Stevens R, Konecny P. 2016. Lomentospora prolificans endocarditis-case report and literature review. BMC Infect Dis. 16:36.
- Kepez Yildiz B, Hasanreisoglu M, Aktas Z, Aksu G, Kocak BC, Akata F. 2014. Fungal keratitis secondary to Scedosporium apiospermum infection and successful treatment with surgical and medical intervention. Int Ophthalmol. 34:305–308.
- Kim CM, Lim SC, Kim J, Jang HS, Chung JH, Yun NR, Kim DM, Jha P, Jha B, Kim SW, et al. 2017. Tenosynovitis caused by Scedosporium apiospermum infection misdiagnosed as an Alternaria species: a case report. BMC Infect Dis. 17:72.
- Kim SH, Ha YE, Youn JC, Park JS, Sung H, Kim MN, Choi HJ, Lee YJ, Kang SM, Ahn JY, et al. 2015. Fatal scedosporiosis in multiple solid organ allografts transmitted from a nearly-drowned donor. Am J Transplant. 15:833–840.
- Kimura M, Maenishi O, Ito H, Ohkusu K. 2010. Unique histological characteristics of Scedosporium that could aid in its identification. Pathol Int. 60:131–136.
- Kiraz N, Gülbas Z, Akgün Y, Uzun O. 2001. Lymphadenitis caused by Scedosporium apiospermum in an immunocompetent patient. Clin Infect Dis. 32:E59–E61.
- Kite BW, Heng T. 2016. An atypical foot infection. Aust Fam Physician. 45:819–820.
- Kooijman CM, Kampinga GA, de Hoog GS, Goudswaard WB, Reijnen MM. 2007. Successful treatment of Scedosporium aurantiacum osteomyelitis in an immunocompetent patient. Surg Infect (Larchmt). 8:605–610.
- Kowacs PA, Soares Silvado CE, Monteiro de Almeida S, Ramos M, Abrao K, Madaloso LE, Pinheiro RL, Werneck LC. 2004. Infection of the CNS by Scedosporium apiospermum after near drowning. Report of a fatal case and analysis of its confounding factors. J Clin Pathol. 57:205–207.
- Kubisiak-Rzepczyk H, Gil L, Zawirska A, Kubisiak-Michalska A, Mol A, Reich A, Komarnicki M, Adamski Z. 2013. Scedosporium prolificans fungaemia in a patient with acute lymphoblastic leukaemia. J Mycol Med. 23:261–264.
- Lackner M, de Hoog G, Yang L, Ferreira Moreno L, Ahmed S, Andreas F, Kaltseis J, Nagl M, Lass-Flörl C, Risslegger B, et al. 2014. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity. 67:1–10.
- Lackner M, De Man FH, Eygendaal D, Wintermans RGF, Kluytmans JA, Klaassen CH, Meis JF. 2011. Severe prosthetic joint infection in an immunocompetent male patient due to a therapy refractory Pseudallescheria apiosperma. Mycoses. 54:22–27.
- Lackner M, Fernandez-Silva F, Guarro J, Lass-Florl C. 2014. Assessing micafungin/triazole combinations for the treatment of invasive scedosporiosis due to Scedosporium apiospermum and Scedosporium boydii. J Antimicrob Chemother. 69:3027–3032.
- Lahmer T, Messer M, Ehmer U, Eser S, Beitz A, Fekecs L, Schmid RM, Huber W. 2016. Pseudallescheria boydii with Aspergillus fumigatus and Aspergillus terreus in a critically ill hematopoietic stem cell recipient with ARDS. Mycopathologia. 181:267–271.
- Lainscak M, Hocevar A, Logar D, Beović B, Matos T, Tomsic M. 2007. Subcutaneous infection with Pseudallescheria boydii in an immunocompromised patient. Clin Rheumatol. 26:1023–1024.
- Lamaris GA, Chamilos G, Lewis RE, Safdar A, Raad II, Kontoyiannis DP. 2006. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989–2006. Clin Infect Dis. 43:1580–1584.
- Larbcharoensub N, Chongtrakool P, Wirojtananugoon C, Watcharananan SP, Sumethkul V, Boongird A, Jirasiritham S. 2013. Treatment of a brain abscess caused by Scedosporium apiospermum and Phaeoacremonium parasiticum in a renal transplant recipient. Southeast Asian J Trop Med Public Health. 44:484–489.
- Larocco A, Jr, Barron JB. 2005. Endogenous scedosporium apiospermum endophthalmitis. Retina. 25:1090–1093.
- Lass-Flörl C, Cuenca-Estrella M. 2017. Changes in the epidemiological landscape of invasive mould infections and disease. J Antimicrob Chemother. 72:i5–i11.
- Lavy D, Morin O, Venet G, Maugars Y, Prost A, Berthelot JM. 2001. Pseudallescheria boydii knee arthritis in a young immunocompetent adult two years after a compound patellar fracture. Joint Bone Spine. 68:517–520.
- Leck A, Matheson M, Tuft S, Waheed K, Lagonowski H. 2003. Scedosporium apiospermum keratomycosis with secondary endophthalmitis. Eye (Lond). 17:841–843.
- Leechawengwongs M, Milindankura S, Liengudom A, Chanakul K, Viranuvatti K, Clongsusuek P. 2007. Multiple Scedosporium apiospermum brain abscesses after near-drowning successfully treated with surgery and long-term voriconazole: a case report. Mycoses. 50:512–516.
- Leek R, Aldag E, Nadeem I, Gunabushanam V, Sahajpal A, Kramer DJ, Walsh TJ. 2016. Scedosporiosis in a combined kidney and liver transplant recipient: a case report of possible transmission from a near-drowning donor. Case Rep Transplant. 2016:1.
- Levine NB, Kurokawa R, Fichtenbaum CJ, Howington JA, Kuntz C. 2002. An immunocompetent patient with primary Scedosporium apiospermum vertebral osteomyelitis. J Spinal Disord Tech. 15:425–430.
- Li JY, Yong TY, Grove DI, Coates PT. 2008. Successful control of Scedosporium prolificans septic arthritis and probable osteomyelitis without radical surgery in a long-term renal transplant recipient. Transplant Infect Dis. 10:63–65.
- Lin D, Kamili Q, Qurat-Ul-Ain K, Lai S, Musher DM, Hamill R. 2013. Cerebral Scedosporium apiospermum infection presenting with intestinal manifestations. Infection. 41:723–726.
- Luijk B, Ekkelenkamp MB, De Jong PA, Kwakkel-van Erp JM, Grutters JC, van Kessel DA, van de Graaf EA. 2011. Effective prolonged therapy with voriconazole in a lung transplant recipient with spondylodiscitis induced by scedosporium apiospermum. Case Rep Infect Dis. 2011:1.
- Luu KK, Scott IU, Miller D, Davis JL. 2001. Endogenous Pseudallescheria boydii endophthalmitis in a patient with ring-enhancing brain lesions. Ophthalmic Surg Lasers. 32:325–329.
- Makino K, Fukushima S, Maruo K, Egawa K, Nishimoto K, Ihn H. 2011. Cutaneous hyalohyphomycosis by Scedosporium apiospermum in an immunocompromised patient. Mycoses. 54:259–261.
- Masukane S, Kitahara Y, Okumoto J, Sasaki K, Nakano K. 2017. The effective treatment of lung infection due to Scedosporium prolificans with voriconazole and surgery. Intern Med. 56:973–977.
- Matsumoto Y, Oh IT, Nagai A, Ohyama F, Ooishi T, Tsuboi R. 2009. Case of cutaneous Scedosporium apiospermum infection successfully treated with voriconazole. J Dermatol. 36:98–102.
- Mays R, Gordon R, Wilson JM, LaPolla WJ, Sra KK, Madkan V, Tyring SK. 2012. Persistent erythematous plaque after minor trauma in an immunocompromised woman. Dermatol Online J. 18:2.
- McCarthy MW, Kontoyiannis DP, Cornely OA, Perfect JR, Walsh TJ. 2017. Novel agents and drug targets to meet the challenges of resistant fungi. J Infect Dis. 216:S474–S483.
- Mei Y, Chen X, Sun K, Lv J, Sun H, Zhang J. 2018. Scedosporium apiospermum infection: lethal complication after extracorporeal cardiopulmonary resuscitation. Perfusion. 33(1):71–73.
- Meletiadis J, Meis JF, Mouton JW, Rodriquez-Tudela JL, Donnelly JP, Verweij PE, Network E. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob Agents Chemother. 46:62–68.
- Meletiadis J, Mouton JW, Meis JF, Verweij PE. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob Agents Chemother. 47:106–117.
- Mellinghoff IK, Winston DJ, Mukwaya G, Schiller GJ. 2002. Treatment of Scedosporium apiospermum brain abscesses with posaconazole. Clin Infect Dis. 34:1648–1650.
- Mesfin FB, Tobin E, Adamo MA, DiRisio D. 2008. Fungal vertebral osteomyelitis due to Scedosporium apiospermum after near-drowning – Case report. J Neurosurg Spine. 9:58–61.
- Miele PS, Levy CS, Smith MA, Dugan EM, Cooke RH, Light JA, Lucey DR. 2002. Primary cutaneous fungal infections in solid organ transplantation: a case series. Am J Transplant. 2:678–683.
- Mohan R, Gopakumar TS. 2016. Clinico-radiological improvement in an immunocompetent patient presented with scedosporium apiospermum osteomyelitis. J Clin Orthop Trauma. 7:134–137.
- Moloney TP, Park J. 2014. Pseudallescheria endophthalmitis: four cases over 15 years in Queensland, Australia, and a review of the literature. Retina. 34:1683–1701.
- Morales P, Galán G, Sanmartín E, Monte E, Tarrazona V, Santos M. 2009. Intrabronchial instillation of amphotericin B lipid complex: a case report. Transplant Proc. 41:2223–2224.
- Morio F, Horeau-Langlard D, Gay-Andrieu F, Talarmin JP, Haloun A, Treilhaud M, Despins P, Jossic F, Nourry L, Danner-Boucher I, et al. 2010. Disseminated Scedosporium/Pseudallescheria infection after double-lung transplantation in patients with cystic fibrosis. J Clin Microbiol. 48:1978–1982.
- Mügge T, Schömig E. 2017. Jahresabschluss 2016, on Uniklinik Köln. [accessed 2017 Nov 01]. https://www.uk-koeln.de/uniklinik-koeln/die-uniklinik/jahres-qualitaetsberichte/.
- Muñoz P, Marín M, Tornero P, Martín Rabadán P, Rodríguez-Creixems M, Bouza E. 2000. Successful outcome of Scedosporium apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin Infect Dis. 31:1499–1501.
- Musk M, Chambers D, Chin W, Murray R, Gabbay E. 2006. Successful treatment of disseminated scedosporium infection in 2 lung transplant recipients: review of the literature and recommendations for management. J Heart Lung Transplant. 25:1268–1272.
- Nakamura Y, Suzuki N, Nakajima Y, Utsumi Y, Murata O, Nagashima H, Saito H, Sasaki N, Fujimura I, Ogino Y, et al. 2013. Scedosporium aurantiacum brain abscess after near-drowning in a survivor of a tsunami in Japan. Respir Investig. 51:207–211.
- Nakamura Y, Utsumi Y, Suzuki N, Nakajima Y, Murata O, Sasaki N, Nitanai H, Nagashima H, Miyamoto S, Yaegashi J, et al. 2011. Multiple Scedosporium apiospermum abscesses in a woman survivor of a tsunami in northeastern Japan: a case report. J Med Case Reports. 5:526.
- Ngai JC, Lam R, Ko FW, To KW, Hui DS. 2009. Pulmonary scedosporium infection as a complication of infliximab therapy for ankylosing spondylitis. Thorax. 64:184–184.
- Nguyen BD. 2001. Pseudallescheriasis of the lung and central nervous system: multimodality imaging. AJR Am J Roentgenol. 176:257–258.
- Nguyen CT, Raychaudhuri SP. 2011. Scedosporium infection in a patient with anti-tnfα therapy. Indian J Dermatol. 56:82–83.
- Nishimori M, Takahashi T, Suzuki E, Kodaka T, Hiramoto N, Itoh K, Tsunemine H, Yarita K, Kamei K, Takegawa H, et al. 2014. Fatal fungemia with Scedosporium prolificans in a patient with acute myeloid leukemia. Med Mycol J. 55:E63–E70.
- Nochez Y, Arsene S, Le Guellec C, Bastides F, Morange V, Chaumais MC, Pisella PJ. 2008. Unusual pharmacokinetics of intravitreal and systemic voriconazole in a patient with Scedosporium apiospermum endophthalmitis. J Ocul Pharmacol Ther. 24:87–90.
- Nulens E, Eggink C, Rijs AJ, Wesseling P, Verweij PE. 2003. Keratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazole. J Clin Microbiol. 41:2261–2264.
- O'Bryan TA, Browne FA, Schonder JF. 2002. Scedosporium apiospermum (Pseudallescheria boydii) endocarditis. J Infect. 44:189–192.
- O'Bryan TA. 2005. Pseudallescheriasis in the 21st century. Expert Rev anti Infect Ther. 3:765–773.
- Ochi Y, Hiramoto N, Takegawa H, Yonetani N, Doi A, Ichikawa C, Imai Y, Ishikawa T. 2015. Infective endocarditis caused by Scedosporium prolificans infection in a patient with acute myeloid leukemia undergoing induction chemotherapy. Int J Hematol. 101:620–625.
- Ochiai N, Shimazaki C, Uchida R, Fuchida S, Okano A, Ashihara E, Inaba T, Fujita N, Nakagawa M. 2003. Disseminated infection due to Scedosporium apiospermum in a patient with acute myelogenous leukemia. Leuk Lymphoma. 44:369–372.
- O'Doherty M, Hannan M, Fulcher T. 2005. Voriconazole in the treatment of fungal osteomyelitis of the orbit in the immunocompromised host. Orbit. 24:285–289.
- Ogawa Y, Sato M, Tashiro M, Miyazaki M, Nagata K, Takahashi N, Kasahara K, Izumikawa K, Yano H, Mikasa K. 2016. Rapid development of a mycotic aneurysm of the intracranial artery secondary to Scedosporium apiospermum sinusitis. Med Mycol Case Rep. 14:30–32.
- Oh IK, Baek S, Huh K, Oh J. 2007. Periocular abscess caused by Pseudallescheria boydii after a posterior subtenon injection of triamcinolone acetonide. Graefes Arch Clin Exp Ophthalmol. 245:164–166.
- Ohashi R, Kato M, Katsura Y, Takekawa H, Hoshika Y, Sugawara T, Yoshimi K, Togo S, Nagaoka T, Seyama K, et al. 2011. Breakthrough lung Scedosporium prolificans infection with multiple cavity lesions in a patient receiving voriconazole for probable invasive aspergillosis associated with monoclonal gammopathy of undetermined significance (MGUS). Med Mycol J. 52:33–38.
- O’Hearn TM, Geiseler PJ, Bhatti RA, Eliott D. 2010. Control of disseminated scedosporium prolificans infection and endophthalmitis. Retin Cases Brief Rep. 4:18–19.
- Oliver JD, Sibley GE, Beckmann N, Dobb KS, Slater MJ, McEntee L, du Pre S, Livermore J, Bromley MJ, Wiederhold NP, et al. 2016. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci USA. 113(45):12809–12814.
- Ong A, Blyth CC, Bency R, Vicaretti M, Harun A, Meyer W, Shingde M, Gilroy N, Chapman J, Chen SCA. 2011. Fatal mycotic aneurysms due to Scedosporium and Pseudallescheria infection. J Clin Microbiol. 49:2067–2071.
- Ortmann C, Wüllenweber J, Brinkmann B, Fracasso T. 2010. Fatal mycotic aneurysm caused by Pseudallescheria boydii after near drowning. Int J Legal Med. 124:243–247.
- Panackal AA, Marr KA. 2004. Scedosporium/Pseudallescheria infections. Semin Respir Crit Care Med. 25:171–181.
- Patel MS, Wright AJ, Kohn R, Markmann JF, Kotton CN, Vagefi PA. 2015. Successful long-term management of invasive cerebral fungal infection following liver transplantation. Mycoses. 58:181–186.
- Patel R, Orlandi RR. 2015. Fungal septal abscess complicating maxillary sinus fungus balls in an immunocompetent host. Allergy Rhinol (Providence). 6:184–187.
- Patterson TF, Thompson GR, 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, et al. 2016. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 63:433–e60.
- Pellón Dabén R, Marco de Lucas E, Martín Cuesta L, Piedra Velasco T, Arnaiz García J, Landeras R, López Duarte M, Bermúdez A. 2007. Imaging findings of pulmonary infection caused by Scedosporium prolificans in a deep immunocompromised patient. Emerg Radiol. 15:47–49.
- Pennekamp PH, Diedrich O, Zhou H, Kraft CN. 2003. [Foot injury as a rare cause of scendosporiosis with fetal outcome]. Unfallchirurg. 106:865–868.
- Perlroth MG, Miller J. 2004. Pseudoallescheria boydii pneumonia and empyema: a rare complication of heart transplantation cured with voriconazole. J Heart Lung Transplant. 23:647–649.
- Porte L, Khatibi S, Hajj LE, Cassaing S, Berry A, Massip P, Linas MD, Magnaval JF, Sans N, Marchou B. 2006. Scedosporium apiospermum mycetoma with bone involvement successfully treated with voriconazole. Trans R Soc Trop Med Hyg. 100:891–894.
- Posteraro P, Frances C, Didona B, Dorent R, Posteraro B, Fadda G. 2003. Persistent subcutaneous Scedosporium apiospermum infection. Eur J Dermatol. 13:603–605.
- Raj R, Frost AE. 2002. Scedosporium apiospermum fungemia in a lung transplant recipient. Chest. 121:1714–1716.
- Reimann D, Büssemaker E, Gross P. 2004. Successful treatment due to vacuum seal technique of a severe Scedosporium apiospermum skin infection in a renal transplant recipient. Nephrol Dial Transplant. 19:245–248.
- Riddell J, Chenoweth CE, Kauffman CA. 2004. Disseminated Scedosporium apiospermum infection in a previously healthy woman with HELLP syndrome. Mycoses. 47:442–446.
- Rivier A, Perny J, Debourgogne A, Thivillier C, Lévy B, Gérard A, Machouart M. 2011. Fatal disseminated infection due to Scedosporium prolificans in a patient with acute myeloid leukemia and posaconazole prophylaxis. Leukemia Lymphoma. 52:1607–1610.
- Rodriguez MM, Calvo E, Serena C, Marine M, Pastor FJ, Guarro J. 2009. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob Agents Chemother. 53:2153–2155.
- Rodríguez-Tudela JL, Berenguer J, Guarro J, Kantarcioglu AS, Horré R, de Hoog GS, Cuenca-Estrella M. 2009. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med Mycol. 47:359–370.
- Rogasi PG, Zanazzi M, Nocentini J, Fantoni E, Trotta M, Faggi E, Fontanelli A, Bertoni E, Salvadori M, Leoncini F. 2007. Disseminated Scedosporium apiospermum infection in renal transplant recipient: long-term successful treatment with voriconazole: a case report. Transplant Proc. 39:2033–2035.
- Roy R, Panigrahi PK, Pal SS, Mukherjee A, Bhargava M. 2016. Post-traumatic endophthalmitis secondary to keratomycosis caused by scedosporium apiospermum. Ocul Immunol Inflamm. 24:107–109.
- Ruinemans GM, Haagsma CJ, Hendrix R. 2011. Tenosynovitis caused by a pseudallescheria boydii infection and symptoms of reflex sympathetic dystrophy after a dog bite. J Clin Rheumatol. 17:363–364.
- Ryder NS, Leitner I. 2001. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species. Med Mycol. 39:91–95.
- Safdar A, Papadopoulos EB, Young JW. 2002. Breakthrough Scedosporium apiospermum (Pseudallescheria boydii) brain abscess during therapy for invasive pulmonary aspergillosis following high-risk allogeneic hematopoietic stem cell transplantation. Scedosporiasis and recent advances in antifungal therapy. Transpl Infect Dis. 4:212–217.
- Sahi H, Avery RK, Minai OA, Hall G, Mehta AC, Raina P, Budev M. 2007. Scedosporium apiospermum (Pseudoallescheria boydii) infection in lung transplant recipients. J Heart Lung Transplant. 26:350–356.
- Saracli MA, Erdem U, Gonlum A, Yildiran ST. 2003. Scedosporium apiospermum keratitis treated with itraconazole. Med Mycol. 41:111–114.
- Sarva ST, Manjunath SK, Baldwin HS, Robins DB, Freire AX. 2010. Lung scedosporiosis in human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Med Sci. 339:300–303.
- Sarvat B, Sarria JC. 2007. Implantable cardioverter-defibrillator infection due to Scedosporium apiospermum. J Infect. 55:e109–e113.
- Satirapoj B, Ruangkanchanasetr P, Treewatchareekorn S, Supasyndh O, Luesutthiviboon L, Supaporn T. 2008. Pseudallescheria boydii brain abscess in a renal transplant recipient: first case report in Southeast Asia. Transplant Proc. 40:2425–2427.
- Sayah DM, Schwartz BS, Kukreja J, Singer JP, Golden JA, Leard LE. 2013. Scedosporium prolificans pericarditis and mycotic aortic aneurysm in a lung transplant recipient receiving voriconazole prophylaxis. Transpl Infect Dis. 15:E70–E74.
- Schaenman JM, DiGiulio DB, Mirels LF, McClenny NM, Berry GJ, Fothergill AW, Rinaldi MG, Montoya JG. 2005. Scedosporium apiospermum soft tissue infection successfully treated with voriconazole: potential pitfalls in the transition from intravenous to oral therapy. J Clin Microbiol. 43:973–977.
- Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 106:2641–2645.
- Seidel D, Durán Graeff L, Vehreschild MJGT, Wisplinghoff H, Ziegler M, Vehreschild JJ, Liss B, Hamprecht A, Köhler P, Racil Z, et al. 2017. FungiScope™ – Global Emerging Fungal Infection Registry. Mycoses. 60:508–516.
- Shand JM, Albrecht RM, Burnett HF, 3rd, Miyake A. 2004. Invasive fungal infection of the midfacial and orbital complex due to Scedosporium apiospermum and mucormycosis. J Oral Maxillofac Surg. 62:231–234.
- Shankar S, Biswas J, Gopal L, Bagyalakshmi R, Therese L, Borse NJ. 2007. Anterior chamber exudative mass due to Scedosporium apiospermum in an immunocompetent individual. Indian J Ophthalmol. 55:226–227.
- Sharma A, Singh D. 2015. Scedosporium apiospermum causing brain abscess in a renal allograft recipient. Saudi J Kidney Dis Transpl. 26:1253–1256.
- Sheu R, Bricker AO, Sahi H, Mohammed TL. 2009. Pseudallescheria boydii (Scedosporium species) in 3 lung transplant recipients: computed tomography findings and literature review. J Comput Assist Tomogr. 33:247–252.
- Shimizu J, Yoshimoto M, Takebayashi T, Ida K, Tanimoto K, Yamashita T. 2014. Atypical fungal vertebral osteomyelitis in a tsunami survivor of the Great East Japan Earthquake. Spine (Phila Pa 1976). 39:E739–E742.
- Shu T, Green JM, Orihuela E. 2004. Testicular involvement in disseminated fungal infection by Pseudallescheria boydii. Urology. 63:981–982.
- Signore SC, Dohm CP, Schütze G, Bähr M, Kermer P. 2017. Scedosporium apiospermum brain abscesses in a patient after near-drowning - a case report with 10-year follow-up and a review of the literature. Med Mycol Case Rep. 17:17–19.
- Singh RP, McCluskey P. 2005. Scedosporium prolificans sclerokeratitis 10 years after pterygium excision with adjunctive mitomycin C. Clin Experiment Ophthalmol. Ophthalmol. 33:433–434.
- Sireesha P, Kumar CHM, Setty CR. 2010. Thyroid abscess due to Scedosporium apiospermum. Indian J Med Microbiol. 28:409–411.
- Slone HW, Kontzialis M, Kiani B, Triola C, Oettel DJ, Bourekas EC. 2013. MRI with Magnetic Resonance Spectroscopy of multiple brain abscesses secondary to Scedosporium apiospermum in two immunocompromised patients. Clin Imaging. 37:361–366.
- Smita S, Sunil S, Amarjeet K, Anil B, Yatin M. 2015. Surviving a recurrent Scedosporium prolificans endocarditis: mention if consent was taken. Indian J Med Microbiol. 33:588–590.
- Solé A. 2011. Scedosporium apiospermum disseminated infection in a single lung transplant recipient. Rev Iberoam Micol. 28:139–142.
- Spanevello M, Morris KL, Kennedy GA. 2010. Pseudoaneurysm formation by Scedosporium prolificans infection in acute leukaemia. Intern Med J. 40:793.
- Stoneham AC, Stoneham SE, Wyllie SA, Pandya AN. 2017. Surgical resection of a rare cutaneous manifestation of Scedosporium apiospermum in a patient who underwent renal transplant. BMJ Case Rep. 2017:pii:bcr2016217923.
- Strunk T, Blume JH, Szeimies RM. 2015. [Deep skin infection with Scedosporium apiospermum-infection in a renal transplant patient]. Hautarzt. 66:195–198.
- Stur-Hofmann K, Stos S, Saxa-Enenkel M, Rappersberger K. 2011. Primary cutaneous infection with Scedosporium apiospermum successfully treated with voriconazole. Mycoses. 54:e201–e204.
- Symoens F, Knoop C, Schrooyen M, Denis O, Estenne M, Nolard N, Jacobs F. 2006. Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation. J Heart Lung Transplant. 25:603–607.
- Takeuchi M, Yoshida C, Ota Y, Fujiwara Y. 2011. Deep skin infection of Scedosporium apiospermum in a patient with refractory idiopathic thrombocytopenic purpura. Intern Med. 50:1339–1343.
- Talbot TR, Hatcher J, Davis SF, Pierson RN, 3rd, Barton R, Dummer S. 2002. Scedosporium apiospermum pneumonia and sternal wound infection in a heart transplant recipient. Transplantation. 74:1645–1647.
- Tamaki M, Nozaki K, Onishi M, Yamamoto K, Ujiie H, Sugahara H. 2016. Fungal meningitis caused by Lomentospora prolificans after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 18:601–605.
- Tammer I, Tintelnot K, Braun-Dullaeus RC, Mawrin C, Scherlach C, Schluter D, Konig W. 2011. Infections due to Pseudallescheria/Scedosporium species in patients with advanced HIV disease-a diagnostic and therapeutic challenge. Int J Infect Dis. 15:e422–e429.
- Tan TY, Liou CW, Kung LC. 2004. Meningitis caused by Pseudallescheria boydii. Chang Gung Med J. 27:228–232.
- Tascini C, Bongiorni MG, Leonildi A, Giannola G, Soldati E, Arena G, Doria R, Germenia C, Menichetti F. 2006. Pacemaker endocarditis with pulmonary cavitary lesion due to Scedosporium prolificans. J Chemother. 18:667–669.
- Taylor A, Wiffen SJ, Kennedy CJ. 2002. Post-traumatic Scedosporium inflatum endophthalmitis. Clin Experiment Ophthalmol. 30:47–48.
- The European Committee on Antimicrobial Susceptibility Testing. 2017. EUCAST method for susceptibility testing of moulds (version 9.3.1 valid from 15 January, 2017). [accessed 2017 Sep 01]. http://www.eucast.org/ast_of_fungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_moulds/.
- Thiagalingam S, Fernando GT, Tan K, O'Donnell BA, Weeks K, Branley M. 2004. Orbital apex syndrome secondary to Pseudallescheria boydii fungal sinusitis in an immunocompetent patient. Clin Exp Ophthalmol. 32:545–547.
- Thomson S, Alibhai K, Winkelaar G, Lien D, Halloran K, Kapasi A, Weinkauf J. 2015. Case report of vertebral osteomyelitis and mycotic abdominal aortic aneurysm caused by Scedosporium apiospermum in a lung transplant patient with cystic fibrosis. Transplant Proc. 47:204–209.
- Tietz H-J, Nennoff P, Ullmann AJ. 2005. Organmykosen auf einen Blick: Diagnostik und Therapie lebensbedrohlicher Pilzinfektionen. Thieme.
- Tilakaratne D, Ryan E, Pearce A. 2016. Concurrent pyoderma gangrenosum and infection with Scedosporium apiospermum. Australas J Dermatol. 57:e46–e48.
- Tintelnot K, Just-Nübling G, Horré R, Graf B, Sobottka I, Seibold M, Haas A, Kaben U, De Hoog GS. 2009. A review of German Scedosporium prolificans cases from 1993 to 2007. Med Mycol. 47:351–358.
- Tirado-Miranda R, Solera-Santos J, Brasero JC, Haro-Estarriol M, Cascales-Sánchez P, Igualada JB. 2001. Septic arthritis due to Scedosporium apiospermum: case report and review. J Infect. 43:210–212.
- Tong SY, Peleg AY, Yoong J, Handke R, Szer J, Slavin M. 2007. Breakthrough Scedosporium prolificans infection while receiving voriconazole prophylaxis in an allogeneic stem cell transplant recipient. Transplant Infect Dis. 9:241–243.
- Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Muñoz P, Johnson E, Meletiadis J, Pana ZD, Lackner M, et al. 2014. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 20:27–46.
- Tóth EJ, Nagy GR, Homa M, Ábrók M, Kiss IÉ, Nagy G, Bata-Csörgő Z, Kemény L, Urbán E, Vágvölgyi C, et al. 2017. Recurrent Scedosporium apiospermum mycetoma successfully treated by surgical excision and terbinafine treatment: a case report and review of the literature. Ann Clin Microbiol Antimicrob. 16:31.
- Troke P, Aguirrebengoa K, Arteaga C, Ellis D, Heath CH, Lutsar I, Rovira M, Nguyen Q, Slavin M, Chen SC. 2008. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 52:1743–1750.
- Trubiano JA, Paratz E, Wolf M, Teh BW, Todaro M, Thursky KA, Slavin MA. 2014. Disseminated Scedosporium prolificans infection in an ‘extensive metaboliser’: navigating the minefield of drug interactions and pharmacogenomics. Mycoses. 57:572–576.
- Tsuji G, Takei K, Takahara M, Matsuda T, Nakahara T, Furue M, Uchi H. 2017. Cutaneous Pseudallescheria boydii/Scedosporium apiospermum complex infection in immunocompromised patients: a report of two cases. J Dermatol. 44:1067–1068.
- Uenotsuchi T, Moroi Y, Urabe K, Tsuji G, Koga T, Matsuda T, Furue M. 2005. Cutaneous Scedosporium apiospermum infection in an immunocompromised patient and a review of the literature. Acta Derm Venereol. 85:156–159.
- Uno K, Kasahara K, Kutsuna S, Katanami Y, Yamamoto Y, Maeda K, Konishi M, Ogawa T, Yoneda T, Yoshida K, et al. 2014. Infective endocarditis and meningitis due to Scedosporium prolificans in a renal transplant recipient. J Infect Chemother. 20:131–133.
- Vagefi MR, Kim ET, Alvarado RG, Duncan JL, Howes EL, Crawford JB. 2005. Bilateral endogenous Scedosporium prolificans endophthalmitis after lung transplantation. Am J Ophthalmol. 139:370–373.
- Verghese S, Padmaja P, Chellamma MT, Leelavathy S, Nayar P. 2005. Prosthetic valve endocarditis caused by Scedosporium apiospermum. Indian J Med Microbiol. 23:264–266.
- Wang XY, Yu SL, Chen S, Zhang WH. 2016. CNS infection caused by Pseudallescheria boydii in a near-drowning traveller from a traffic accident. J Travel Med. 23:tav018.
- Wiederhold NP, Law D, Birch M. 2017. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. J Antimicrob Chemother. 72:1977–1980.
- Williams JR, Tenforde MW, Chan JD, Ko A, Graham SM. 2016. Safety and clinical response of intraventricular caspofungin for Scedosporium apiospermum complex central nervous system infection. Med Mycol Case Rep. 13:1–4.
- Wilson HL, Kennedy KJ. 2013. Scedosporium apiospermum brain abscesses in an immunocompetent man with silicosis. Med Mycol Case Rep. 2:75–78.
- Wu Z, Ying H, Yiu S, Irvine J, Smith R. 2002. Fungal keratitis caused by Scedosporium apiospermum: report of two cases and review of treatment. Cornea. 21:519–523.
- Yoneda K, Nakai K, Moriue T, Ishikawa E, Demitsu T, Ohkusu K, Kubota Y. 2012. Scedosporium apiospermum skin infection mimicking tuberous xanthoma. J Dermatol. 39:316–318.
- Yu Z, Hu L, Jiang M, Ning H, Xu C, Li B, Li Y, Lou X, Wang J, Hu J, et al. 2013. Dermatic Scedosporium apiospermum infection after autologous bone marrow transplantation. Intern Med. 52:689–693.
- Zaas D. 2002. Cases from the Osler medical service at Johns Hopkins University. Scedosporium apiospermum mycetoma of the lung. Am J Med. 113:760–762.
