Abstract
The effectiveness of antibiotics has been challenged by the increasing frequency of antimicrobial resistance (AR), which has emerged as a major threat to global health. Despite the negative impact of AR on health, there are few effective strategies for reducing AR in food-producing animals. Of the antimicrobial resistant microorganisms (ARMs), extended-spectrum β-lactamases (ESBLs)-producing Enterobacteriaceae are an emerging global threat due to their increasing prevalence in livestock, even in animals raised without antibiotics. Many reviews are available for the positive selection of AR associated with antibiotic use in livestock, but less attention has been given to how other factors including soil, water, manure, wildlife, and farm workers, are associated with the emergence of ESBL-producing bacteria. Understanding of antibiotic resistance genes and bacteria transfer at the interfaces of livestock and other potential reservoirs will provide insights for the development of mitigation strategies for AR.
Introduction
Antimicrobial resistance (AR) is defined as “the resistance of a microorganism to an antimicrobial drug to which it was previously sensitive so that the standard treatments become ineffective and infections persist and may spread to others” (Demerec Citation1948; Alanis Citation2005). AR is one of the fundamental challenges affecting public health, claiming 23,000 estimated deaths annually and an approximate $55 billion/year in overall societal costs in the United States (US) alone (CDC Citation2013; Demirjian et al. Citation2015). The World Health Organisation (WHO) published a list of the most critical antimicrobial resistant microorganisms (ARMs) against which new antibiotics need to be developed urgently (WHO Citation2017). Among the ‘Highest Priority’ pathogens, extended-spectrum β-lactamases (ESBLs)-producing Enterobacteriaceae were identified as an emerging global threat due to their increasing prevalence in livestock in recent years after being mainly identified in human medicine in the past (Enoch et al. Citation2012; Reuland et al. Citation2013).
ESBLs can hydrolyse expanded-spectrum cephalosporins, including cefotaxime, ceftriaxone, ceftazidime, or cefepime and monobactams. More than 1,000 ESBL variants are known, including SHV, TEM, OXA, and CTX-M types, with more expected to be identified in the future (Allen et al. Citation2010; Jia et al. Citation2017). During the 1990s, the TEM- and SHV-β-lactamase families carried by Klebsiella pneumoniae and Escherichia coli were the main members of ESBLs (Coque et al. Citation2008). In recent years, the geographical distribution of ESBL-producing bacteria has increased dramatically, and ESBLs have been identified in other bacteria including K. pneumonia, E. coli, Acinetobacter calcoaceticus, Agrobacterium tumefaciens, Ochrobactrum spp., and Pseudomonas plecoglossicida, encoding blaSHV, blaCMY, blaVEB, blaOXA-2, blaTEM, and blaCTX-M genes (Zhou et al. Citation2015; Mir et al. Citation2016). In particular, the incidence of CTX-M type ESBLs has increased significantly during the past decade. A prototype of CTX-M type ESBLs has been found to originate from environmental bacteria Kluyvera species (Bevan et al. Citation2017). Most ESBL-producing bacteria encode the ESBL genes on plasmids facilitating the rapid spread through horizontal gene transfer (HGT) between bacteria, but recent findings indicate that the blaCTX-M genes are also encoded in chromosomal DNA (Teng et al. Citation2019).
The emergence of ESBL-producing bacteria in animals
The prevalence of ESBL-producing bacteria in swine farms has been reported to range from approximately 10% to 45%, and E. coli was the major ESBL producer (Geser et al. Citation2011; Randall et al. Citation2014; Li et al. Citation2015; Sabia et al. Citation2017; Liu et al. Citation2018). The most prevalent ESBL gene type at swine farms was blaCTX-M, while other β-lactamase genes such as blaCMY-2 blaTEM, blaSHV, blaOXA, blaKPC, and blaDHA were also identified (Dahms et al. Citation2015; Shin et al. Citation2017; Chah et al. Citation2018; Liu et al. Citation2018). Recently, the mcr-1 gene, which was first identified in China (Liu et al. Citation2016), was identified along with ESBL genes in the plasmid DNA in swine farms (Shafiq et al. Citation2019). E. coli isolates from cloacal and nasal swabs of swine in China were investigated, and 39.59% (78/197) of the E. coli isolates carried both mcr-1 and ESBL genes (blaCTX-M, blaSHV, and blaTEM) (Shafiq et al. Citation2019). Similarly, nine isolates out of 24 ESBL-producing E. coli from the rectal swabs of swine on farms and slaughterhouse were positive with mcr-1 gene (Malhotra-Kumar et al. Citation2016). Ji et al. reported 14% (4/28) prevalence of ESBL-producing E. coli encoding mcr-1 gene as well (Ji et al. Citation2019).
In the case of poultry, there are many studies investigating the occurrence of ESBL-producing bacteria, particularly in the entire broiler production pyramid (Nilsson et al. Citation2014; Zurfluh et al. Citation2014; Apostolakos et al. Citation2019; Dame-Korevaar et al. Citation2019; Oikarainen et al. Citation2019). The prevalence of ESBL-producing bacteria is different depending on the levels of broiler production pyramid. For instance, a high prevalence of ESBL and plasmid mediated AmpC-type cephalosporinase-producing E. coli was found in broiler parent flocks (92.5%, 95%CI 72.1–98.3%), which decreased to 20% (95%CI 12.9–29.6%) during the laying period (Apostolakos et al. Citation2019). The prevalence increased again to 69.2% (95%CI 53.6–81.3%) at the start of the production cycle in the fattening broilers, then dropped to 54.2% (95%CI 38.9–68.6%) in the last sampling right before slaughter (Apostolakos et al. Citation2019). Similarly, in Denmark, broiler parent flocks showed higher prevalence of ESBL-producing E. coli than broiler flocks (93.0 and 27.0%, respectively) (Agerso et al. Citation2014). In addition, broiler production practices (i.e., conventional or organic farms) can affect the prevalence of multi-drug resistant E. coli on eggshells (95% in conventional barns and 30% in organic farms, p < 0.05) (Dame-Korevaar et al. Citation2019) and chicken meats (100% on conventional and 84% on organic samples, p < 0.001) (Cohen Stuart et al. Citation2012). The majority of ESBL-producing bacteria in poultry farms was E. coli and Salmonella spp., and CTX-M was the most predominant ESBLs, while SHV and TEM were also reported (Saliu et al. Citation2017).
Cattle also appear to be an important reservoir of ESBL-producing bacteria among food-producing animals, with the increasing detection of these bacteria in cattle (Mir et al. Citation2018; Tymensen et al. Citation2018). ESBL-producing bacteria have been isolated from cattle in the US, Germany, France, and Asian countries including China, Japan, and Korea (Duan et al. Citation2006; Moon et al. Citation2007; Hiroi et al. Citation2012; Zheng et al. Citation2012; Schmid et al. Citation2013; Haenni et al. Citation2014; Mir et al. Citation2018). For example, in Switzerland, two independent studies showed 17.1% and 8.4% prevalence of ESBL-producing bacteria in the gastrointestinal tract of healthy cattle at abattoirs, primarily E. coli (Geser et al. Citation2011). In Taiwan, 42.2% of E. coli isolates from beef carcases produced ESBLs, with blaCTX-M-1 and blaCTX-M-9 as the most commonly identified ESBL genotypes (Chen et al. Citation2017).
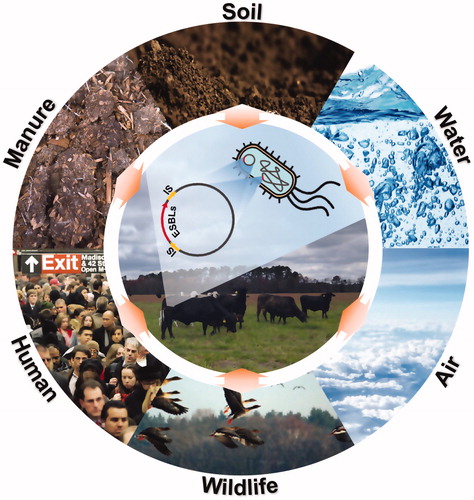
Recent findings propose another pillar of ESBL emergence. Beef cattle raised without antibiotics on pasture had a 15.8% prevalence of cefotaxime resistant bacteria (CRB) (Mir et al. Citation2016). Cefotaxime is frequently used to select ESBL-producing bacteria due to its strong antimicrobial activity against non-ESBL-producing bacteria (Eliopoulos and Bush Citation2001). All CRB isolates contained blaCTX-M genes as the predominant ESBL gene, and over 70% of the isolates carried more than two ESBL genes and 35% harboured more than three ESBL genes such as blaSHV (13%), blaOXA-2 (39%), and blaVEB (30%) (Mir et al. Citation2016). Mir et al. (Citation2018) also showed that the majority of the cattle (92%) during the first year of life had become colonised by CRB at least once (Mir et al. Citation2018). Cattle raised in cow/calf operation systems on pasture with limited use of antibiotics to occasional treatment carried CRB, although the prevalence was relatively low, 47.4%, compared to the prevalence of CRB in feedlot (83%) (Noyes et al. Citation2016; Markland et al. Citation2019). Furthermore, genetically similar ESBL-producing E. coli have been isolated in various hosts around the world (Teng et al. Citation2019). These observations suggest that AR in livestock can arise through various environmental pathways, even in the absence of anthropogenic antibiotic use (Li et al. Citation2015; Mir et al. Citation2016). There have been a number of reviews that have looked at the positive correlation between antibiotic use in livestock and the emergence of ARMs (Oliver et al. Citation2011; Landers et al. Citation2012; Andersson and Hughes Citation2014; Tang et al. Citation2017), while reviews on environmental factors adjacent to pastures and grazing area (e.g., the interface of wildlife and livestock, soil, and surface water) are limited. In this review, we focus on occurrence and transmission of ESBL-producing bacteria at the interface of food-producing animals, especially cattle, and the surrounding environment ().
Table 1. Reported occurrences of ESBL-producing bacteria in livestock-related environmental niches.
Potential sources of ESBL-producing bacteria in the environment
Soil
The soil has various natural antimicrobials, and the antibiotic residues in soil contaminated from animal manure, wastewater, and other sources, may serve as antimicrobial selective pressure to bacteria and develop antibiotic resistome (Riesenfeld et al. Citation2004; Torres-Cortés et al. Citation2011; Udikovic-Kolic et al. Citation2014; Jones-Dias et al. Citation2016; Tripathi and Cytryn Citation2017), such as CTX-M type ESBLs from Kluyvera spp. (Bevan et al. Citation2017). The prevalence of ESBL-producing bacteria as measured by screening for blaCTX-M genes using a real-time PCR method, was 18.3% (22/120) in soil samples obtained from Burgundy region in France, where beef cattle farms are densely located (Hartmann et al. Citation2012). In another study, soils in intensive agricultural practices (large inputs of pesticides) had the highest prevalence of ESBL-producing E. coli compared to soils in extensive (small inputs of pesticides) and organic (no inputs of pesticides) practices, showing that soil types can affect the prevalence of ESBL-producing bacteria (Jones-Dias et al. Citation2016). Although the anthropogenic impacts are critical to accelerate the occurrence of ARMs in soil, soil samples collected from undisturbed areas with no human activity and no antimicrobial selective pressure still contained ARMs, including AmpC β-lactamases (encoded on chromosome)- and ESBL-producing isolates (Upadhyay et al. Citation2016). In a similar study, novel and ancient β-lactam resistance determinants were found in the absence of selective pressure in areas of no anthropogenic activity in Alaska, suggesting that soil microbiota can contribute to the development of AR naturally (Allen et al. Citation2009). However, the occurrence of AR in remote areas and animal farm soils might be different, because AR in pristine areas is caused by naturally existed microorganisms without anthropogenic impacts, while most of the soil in farm environment is easily influenced by human activities. Furthermore, CRB prevalence in the gastrointestinal tract of cattle which were pasture-grazed was positively associated with the relative abundance of gamma-proteobacteria, a major antibiotic producer, in soil samples (Markland et al. Citation2019), suggesting that CRB may transmit at the interface of cattle and soil, and further studies will be necessary to understand the directionality of AR transmission between soil and cattle.
Water
ESBL-producing bacteria has been also found in water resources proximal to farm environment. A total of six E. coli isolates carrying blaTEM and blaCMY-2 genes (6/116, 5%) were obtained from 35 water samples used for drinking and washing dairy cattle in Thailand (Hinthong et al. Citation2017). In a similar study, water in beef cattle pens contained the ESBL and carbapenemase genes, and unique antimicrobial resistance genes (ARGs), suggesting that water in cattle farms could be another source of ESBL-producing bacteria (Noyes et al. Citation2016). Wastewater from sewage treatment plants and from hospitals have a higher predominance of ESBL-producing bacteria compared to surface water (rivers, canals, rivulets, lakes and the North Sea) in the Netherlands (Blaak et al. Citation2015) and similar results were obtained in another study in Tunisia, where wastewater had more ESBL-producing bacteria than surface water, 42.1% (24/57) vs. 1.7% (1/57) (Said et al. Citation2016). On the other hand, surface water in Switzerland (rivers and lakes) and in Bangladesh (lake) showed higher prevalence, 36.2% (21/58) and 75% (3/4), respectively (Zurfluh et al. Citation2013; Haque et al. Citation2014). There is also evidence showing the high prevalence of ESBL-producing K. pneumoniae at all stages of hospital sewage treatments (Prado et al. Citation2008). Substantial studies showed the presence of β-lactam resistance genes including blaTEM, blaIMP, and blaOXA-2 derivatives in sewage sludge in Europe (Tennstedt et al. Citation2005; Henriques et al. Citation2006; Mesa et al. Citation2006). Sewage sludge can provide ideal conditions for HGT because of high concentrations of bacteria (Gaze et al. Citation2011). Since the wastewater contributes to the presence of ARMs in surface water (Blaak et al. Citation2015) and soil through irrigation (Negreanu et al. Citation2012) and flooding (Devarajan et al. Citation2016), cattle grazing on pasture might be exposed to ESBL-producing bacteria by wastewater directly or indirectly.
Air
The aerosols in animal farms can harbour diverse microbes and microbes in farm animals can be associated with them (Yuan et al. Citation2010). The air collected from different points inside cattle farms and outside surroundings has been shown to contain TEM-1 producing E. coli isolates and the isolates harboured other resistance genes like sul1, sul2, tet(A), and tet(B) (Navajas-Benito et al. Citation2017). Airborne ESBL-producing isolates were reported from swine and broiler chicken farms. Gao et al. (Citation2015) reported that three out of four swine farms contained ESBL-producing bacteria in the air. ESBL-producing Enterobacteriaceae was detected in stable air samples from the pig farms (6/35) at the German-Dutch border region (Schmithausen et al. Citation2015). Similarly, 6% (2/36) ambient air samples in the vicinity of the pig barns and 9.5% (6/63) air samples inside the barn contained ESBL/AmpC-producing E. coli (von Salviati et al. Citation2015). In broiler chicken farms, air from inside and outside of the farms had a prevalence of ESBL-producing E. coli of 16% (10/63) and 7.5% (3/40), respectively (Laube et al. Citation2014). Huijbers et al. (Huijbers et al. Citation2016) isolated ESBL/AmpC-producing E. coli in air samples on an organic broiler farm as well. The presence of ESBL-producing bacteria in the air suggests that inhalation of contaminated air might provide another transmission route, contributing to the prevalence of ESBL-producing bacteria in farm animals (Dohmen et al. Citation2017a).
Manure, farm environment and waste
In the US, about 14 million kilograms of antibiotics, approved for use in food-producing animals, were sold in 2016 (FDA Citation2017) and approximately 6.8 million tons of animal manure (FAO Citation2018) were spread onto pasture for forage and silage production as well as agricultural fields as a fertiliser to provide nutrients to crops and to improve soil quality. Therefore, continuous selective pressure by antibiotic residues in the treated soil may facilitate the acquisition of novel ARGs by the microorganisms. Soil treated with cattle manure has a higher abundance of β-lactam resistant bacteria than untreated soil, showing manure-amended soil could include more ARMs (Udikovic-Kolic et al. Citation2014). The resistome of cow manure including β-lactam, phenicol, aminoglycoside, and tetracycline resistance genes, showed a low identity of protein sequences against known reference genes, suggesting manure may carry various novel ARGs (Wichmann et al. Citation2014). Because of the residues of antimicrobials in cattle manure, animal manures may have a relatively higher prevalence of ARMs compared to other reported reservoirs in farm environments (Hou et al. Citation2015). In addition to manure, barn dust collected from cattle farms carried ESBL-producing E. coli (15.4% prevalence) (Schmid et al. Citation2013). Also, feed mixer, animal feed, and bedding in dairy farms were also shown to contain ESBL-producing E. coli (Braun et al. Citation2016), suggesting these bacteria are ubiquitous. A total of 56 environmental samples including water trough, feed, and bedding showed a prevalence of ESBL-producing E. coli as high as 28.6% (Braun et al. Citation2016). Isolates from the feed mixer and animal feed carried blaCTX-M-15 and blaTEM genes, and isolates from bedding had blaCTX-M-15, blaTEM, and blaOXA-1 genes (Braun et al. Citation2016).
Wildlife as potential reservoirs of ESBL-producing bacteria
Wildlife carry ARMs in a wide range of habitats, and they can affect the transmission dynamics of ARMs at the livestock-wildlife interface (Greig et al. Citation2015; Vittecoq et al. Citation2016). Although wildlife is not treated with antibiotics intentionally, they can acquire ARMs from a contaminated environment or natural resistome (Carroll et al. Citation2015; Swift et al. Citation2019), transferring the ARMs to livestock or vice versa via direct and indirect contacts through the use of shared resources such as pasture, water, or soil (Wiethoelter et al. Citation2015). Previous studies have highlighted the importance of wildlife, especially migratory birds as important agents of the ARM prevalence and spread (Greig et al. Citation2015). Birds can cover long distance during the migration season and they could potentially spread ARMs globally (Alcalá et al. Citation2016). One study reported 3% and 5.1% prevalence of bacteria encoding the blaCMY or blaCTX-M genes in wild songbirds on Ohio dairies, respectively (Mathys et al. Citation2017). Wild coastline birds (seagulls and pelicans) in Miami Beach, Florida had 14% prevalence of ESBL-producing bacteria (Poirel et al. Citation2012). Hasan et al. (Citation2016) reported that the prevalence of ESBL-producing E. coli and K. pneumoniae was 8% in wild birds. The isolates from different sources were closely related to each other according to the ERIC-PCR data (Hasan et al. Citation2016). Interestingly, Hasan et al. (Citation2016) reported that among five different bird species evaluated, cattle egrets, which forage at the feet of grazing cattle, showed the highest prevalence of ESBL-producing bacteria, suggesting a high probability of transmission of ARMs between birds and cattle in the environment where they co-inhabit (Hasan et al. Citation2016). Other wildlife including wolves, seabreams, lynxes, wild boars, foxes, deers, bats, and rodents can also be potential reservoirs of ESBL-producing bacteria (Nhung et al. Citation2015; Cristóvão et al. Citation2017; Schaufler et al. Citation2018; Wasyl et al. Citation2018; Garcês et al. Citation2019). In addition, flies near livestock have been shown to carry ESBL-producing bacteria (dos Santos Alves et al. Citation2018). Out of 1346 single flies obtained from an urban and rural area in Germany, 123 flies had ESBL producers (9.1% prevalence) (Schaumburg et al. Citation2016). Flies at poultry farms had a 10.5% prevalence of ESBL-producing E. coli (2/19) and houseflies and barn flies obtained from a cattle barn showed 10% prevalence (Usui et al. Citation2013; Blaak et al. Citation2014).
ESBL-producing bacteria in livestock farm workers
In the Netherlands, 2432 adults, who have lived in a livestock-dense area, were investigated to understand the risk of neighbouring residents regarding the carriage of ESBL-producing bacteria (Wielders et al. Citation2017). 4.5% (109/2432) of the adults carried ESBL- or AmpC-producing bacteria. Interestingly, keeping cows recreationally was one of the identified risk factors for colonisation with ESBL-producing bacteria (Wielders et al. Citation2017). Among the general population, farmers closely exposed to livestock and contacted animals directly had increased chances of transmission of ARMs (Klous et al. Citation2016). In Germany, faeces from farmers (5/73) and pig (15/17), cattle (6/11), and poultry (3/6) carried ESBL-producing E. coli. Five farmers (3 from cattle farms and 2 from pig farms) harboured ESBL-producing E. coli, showing 6.8% prevalence and one human isolate had the same multi-locus sequencing typing (MLST) (ST3891) and blaCTX-M allele as did cattle isolates from the same farm (Dahms et al. Citation2015). ESBL-producing E. coli have also been isolated from farm workers in Korea (Tamang et al. Citation2013) and Netherland (Dohmen et al. Citation2015; Dohmen et al. Citation2017a). The prevalence of ESBL-producing E. coli in human was significantly associated with job title (e.g., stable work, stabbing, dehairing, removal of organs, refrigeration, packaging, and expedition) in the abattoir, indicating the frequency of exposure to livestock enhance the transmission of ESBL-producing bacteria between human and livestock (Dohmen et al. Citation2017b). Therefore, understanding the role of animal carriage might be one of the key factors in understanding the prevalence of ESBL-producing bacteria in humans.
The movement of resistant bacteria and ARGs
Resistance genes can be transferred to other bacteria, even within a distantly related genus, through HGT with mobile elements such as bacteriophages, plasmids, and transposons (Andersson and Hughes Citation2010). However, due to the magnitude and complexity of the transmission and natural occurrence of ARMs at the interfaces, the process by which resistance is transferred between cattle and the environment within the food-animal production systems is poorly understood (Horigan et al. Citation2016). In this section, we summarise several studies which reported HGT and clonal transmission of ARMs between livestock and the environment to build a basic understanding of the transmission dynamics of ESBL-producing bacteria to postulate possible routes for colonisation of these bacteria in the gastrointestinal tract of cattle raised without antibiotic use.
Horizontal gene transfer among bacteria at the environmental interface
The presence of antimicrobial residues or high-density and high-complexity environments accelerates HGT among bacteria resulting in the spread and sharing ARGs at the interfaces of livestock, human, and the environment (Andersson and Hughes Citation2014; Fletcher Citation2015; von Wintersdorff et al. Citation2016; Sommer et al. Citation2017). Among HGT mechanisms, conjugation is the most common mechanism to spread ARGs among bacteria. Most ARGs are located on mobile genetic elements (MGEs), which help ARGs transfer among bacteria (Karkman et al. Citation2018). HGT has been reported in a diverse environment (von Wintersdorff et al. Citation2016), including soil bacteria and human pathogens that share ARGs and flanking regions of the ARGs (Forsberg et al. Citation2012). The identified ARGs in soil were relevant to β-lactams, aminoglycosides, amphenicols, sulphonamides, and tetracyclines, and those ARGs had a high identity with those found in clinical pathogens (Forsberg et al. Citation2012). Moreover, Nesme et al. (Citation2014) found ARGs from different environments (soil, ocean, and human faeces), and showed soil and human faeces shared ARGs (24/94), suggesting that genes flow between these environments (Nesme et al. Citation2014). Also, several MGEs such as conjugative plasmids including incompatibility groups (Inc) F, A/C, N, HI2, I1, and K with specific insertion sequences (IS) such as ISEcp1 and ISCR1 elements are frequently reported with ESBLs (Ali et al. Citation2016; Irrgang et al. Citation2017, Citation2018), indicating these IS elements are strongly associated with independent acquisition of the ESBL genes. IncN plasmids encoding CTX-M-1 have been found in bacteria isolated from pigs, farmers, and farm environments, such as manure and air, indicating the spread of conjugative IncN plasmids with blaCTX-M genes among pigs, farmers, and surroundings (Moodley and Guardabassi Citation2009). Similarly, distinct plasmids with the ESBL genes were shared between farm animals (pig and poultry) and humans (de Been et al. Citation2014). Lifshitz et al. (Lifshitz et al. Citation2018) reported that cattle and community derived isolates were related to each other in sharing CTX-M-15 and its surrounding MGEs (Tn3 or IS1380 families) (Lifshitz et al. Citation2018). Another study showed that ESBL-producing E. coli isolates from cows were carrying CTX-M-15 flanked with ISCR1 elements (Ali et al. Citation2016). ISEcp1–CTX-M-1–Δorf477, was identified regardless of plasmid origins such as human, cattle, and swine, demonstrating MGEs are critical units to spread ARGs among different environments (Jakobsen et al. Citation2015). ISEcp1 elements are also known to increase expression levels of ESBLs by providing a promoter at the upstream of the ESBL genes (Vandecraen et al. Citation2017).
Clonal transmission of ESBL-producing bacteria at the interfaces
The intersected sequencing types (STs) across human and animal populations and the environment were identified in Tanzania. ESBL-producing E. coli ST38, ST131, and ST2852 were isolated across these three interfaces, showing dissemination of clonal isolates regardless of the original sources (Seni et al. Citation2018). In another study, molecular relatedness between ESBL-producing isolates from human and animal populations was substantially close (Dorado-García et al. Citation2018). Dorado-García et al. (Citation2018) conducted a meta-analysis of ESBL-producing isolates from 35 studies in the Netherlands to understand major ESBL gene types, plasmid replicons, and E. coli STs using proportional similarity index (PSIs) and principal component analysis (PCA). Isolates from humans who live near farms showed higher similarity to the isolates from their animals including broilers and pigs, whereas isolates from humans in the general population were similar with human clinical samples, surface, and sewage water, and wild birds (Dorado-García et al. Citation2018). Dahms et al. (Citation2015) reported that farm workers, who contacted livestock frequently, carried identical ST with cattle isolates, indicative of zoonotic transfer of ESBL-producing bacteria between humans and their animals. Similarly, uropathogenic ESBL-producing E. coli from diverse sources (livestock, human, surface water, and food) shared ST10 carrying CTX-M-1 (Müller et al. Citation2016). In poultry farms, the ESBL-producing E. coli isolates from all parasitic bird flies, and excreted manure carried identical ST with the blaTEM-52 gene, suggesting a clonal transfer between flies and birds happening at the poultry farms (Blaak et al. Citation2014). Bui et al. (Bui et al. Citation2018) found identical strains, based on genotyping using pulsed-field gel electrophoresis (PFGE), among chickens in the same farms. The isolates carried blaCTX-M-55 and blaCTX-M-65 in common, suggesting bird-to-bird transmission (Bui et al. Citation2018). Furthermore, ESBL-producing E. coli isolates from faeces, and farm air in broiler chicken farms were clonal variants, suggesting the ESBL-producing E. coli from broiler farms can spread to the surrounding environments and beyond (Laube et al. Citation2014). The indistinguishable ESBL genes, plasmids, and ST of ESBL-producing E. coli isolates were identified from retail chicken meat and humans, suggesting the potential transmission of ARMs in food production systems as well as in wildlife areas (Leverstein-van Hall et al. Citation2011).
Needs for high-resolution analysis of ESBL transmission
Many studies have shown transmission of ARMs between livestock and surroundings, but most studies used low-resolution techniques, such as PFGE, PCR-based genotyping technologies, and MLST, to evaluate genetic similarity to conclude gene or bacterial transmission at the interfaces. However, pan-genome based single nucleotide polymorphism (SNP) analysis has provided high-resolution tools to verify whether isolated strains at the interfaces are truly clonal variants or not (Bekal et al. Citation2016). Not surprisingly, isolates defined as the same strains by low-resolution techniques were found to be non-clonal variants (Knudsen et al. Citation2017; Guo et al. Citation2018). In our recent study (Teng et al. Citation2019), strains belonging to the same ST with an identical profile of virulence genes did not cluster together in a phylogenetic tree. To overcome the issues raised by low-resolution techniques, the GenomeTrakr network has been created by the Food and Drug Administration (FDA) to track pathogens that caused foodborne outbreaks, by comparing variant SNPs in the whole genomes of the isolated strains (Wang et al. Citation2016). However, there are still limited studies showing ESBL transmission by applying whole genome sequencing (WGS). Schaufler et al. showed the interspecies transmission of ESBL-producing E. coli ST410 through SNP analysis (Schaufler et al. Citation2016), showing a small number of SNPs (45 total SNPs, 8.6 SNPs/Mbp) between isolates from wild mute swan and humans. Furthermore, human isolates were closely related with avian and dog isolates with 24 and 29 SNPs, respectively (Schaufler et al. Citation2016). Similarly, Ma et al. identified identical plasmid groups, IncFIB and FII, and similar virulence factors in intrauterine pathogenic E. coli (IUPEC) strains from different dairy cows using WGS (Ma et al. Citation2018). However, the IUPEC strains were not clonal variants by phylogenetic tree analysis of whole genomes, revealing no animal to animal transmission of IUPEC (Ma et al. Citation2018). Therefore, we propose that accurate and correct understanding of gene and ARM transmission using high-resolution tools such as WGS is pivotal to identify transmission origin and route of ESBL-producing bacteria at the interfaces of livestock and the environment.
Mitigation for antibiotic resistance
Total elimination of AR is an impossible task, but effective strategies would slow down the development of new types of AR and its spread (Livermore et al. Citation2006). Antimicrobial stewardship for the selection, dosage, and duration of antimicrobial treatment is critical to reduce AR occurrence in the livestock industry (Ma et al. Citation2019). Efforts to control ARM transmission at the interfaces of livestock and environment would be extremely challenging due to the range of factors involved in the transmission of ARMs. To reduce the occurrence and acquisition of ARMs via multiple sources as has been discussed here, critical control points, where transmission of ARMs between livestock and environment is expected or occurring, should be identified and managed effectively (Berendonk et al. Citation2015). Good farming management such as improved farm hygiene and biosecurity can become one way to decrease the prevalence and concentration of ARMs in livestock (Markland et al. Citation2019). For example, the frequency of cleaning drinking water troughs was negatively associated with the prevalence of ARMs and the presence of quarantine programmes in farms, and burial/burning of deceased animals were related to the reduction of ARMs detection in cattle (Markland et al. Citation2019). A better understanding of interactions among microbiological, animal and environmental factors would provide insights into predicting the occurrence of ARMs and the creation of novel management strategies. In addition, the transmission rates of ARMs via different vehicles or sources need to be quantified, and modelling of transmission should be developed with such results to evaluate the risks of transmission at the interfaces and colonisation of ARMs in hosts. There are still data gaps to quantify the relative contribution of each factor responsible for ARMs transmission in the beef cattle industry (Horigan et al. Citation2016), indicating the urgency of collecting ecological and epidemiological data for mitigation of AR. Currently, there are available alternatives to antibiotics, such as prebiotics and probiotics, phage therapy, vaccines, antimicrobial peptides, antimicrobial polymers, and combination of synergistic antibiotics (Ma et al. Citation2019), although the effectiveness of these methods are not fully evaluated. Development and application of better AR mitigation strategies will be needed to reduce the risks of AR.
Conclusions
Multi-drug resistant pathogens are a major threat to global public health due to the increasing frequency of antimicrobial resistance and reducing efficacy of antibiotics. The lack of fully understanding of how ESBLs-producing Enterobacteriaceae are arising at the interfaces of livestock, wildlife, and the environment (), inhibits our ability to develop mitigation strategies. Quantitative mathematical modelling of risks of ARG/ARM transmission may help identify control points at the interfaces. High-resolution technologies may be required to accurately identify ARM transmission pathways, in order to facilitate tracking of the causative pathogens in outbreak settings. There is limited information available to determine the critical natural carrier/reservoir of ESBL-producing bacteria. Effective mitigation strategies such as farm management, biosecurity, and hygiene might facilitate the reduction of ESBL-producing bacteria in livestock. New strategies are needed to combine knowledge of the environmental, animal, and bacterial aspects to tackle this global issue.
Figure 1. Potential transmission routes of the ESBL genes and ESBL-producing bacteria at the interfaces of livestock and the environment. ESBL-producing bacteria in livestock can naturally occur and transmit through the environmental sources, such as manure, soil, water, air, wildlife, and human (especially farm workers) by horizontal gene transfer of the ESBL genes in chromosomal DNA and on plasmids medicated by IS elements or bacterial transmission.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Agerso Y, Jensen JD, Hasman H, Pedersen K. 2014. Spread of extended spectrum cephalosporinase-producing Escherichia coli clones and plasmids from parent animals to broilers and to broiler meat in a production without use of cephalosporins. Foodborne Pathog Dis. 11:740–746.
- Alanis AJ. 2005. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 36(6):697–705.
- Alcalá L, Alonso CA, Simón C, González-Esteban C, Orós J, Rezusta A, Ortega C, Torres C. 2016. Wild birds, frequent carriers of extended-spectrum β-lactamase (ESBL) producing Escherichia coli of CTX-M and SHV-12 types. Microb Ecol. 72(4):861–869.
- Ali T, Ur Rahman S, Zhang L, Shahid M, Zhang S, Liu G, Gao J, Han B. 2016. ESBL-producing Escherichia coli from cows suffering mastitis in China contain clinical class 1 integrons with CTX-M linked to ISCR1. Front Microbiol. 7:1931
- Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. 2010. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 8(4):251–259.
- Allen HK, Moe LA, Rodbumrer J, Gaarder A, Handelsman J. 2009. Functional metagenomics reveals diverse β-lactamases in a remote Alaskan soil. ISME J. 3(2):243–251.
- Andersson DI, Hughes D. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 8(4):260–271.
- Andersson DI, Hughes D. 2014. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol. 12(7):465–478.
- Apostolakos I, Mughini-Gras L, Fasolato L, Piccirillo A. 2019. Assessing the occurrence and transfer dynamics of ESBL/pAmpC-producing Escherichia coli across the broiler production pyramid. PLoS One. 14(5):e0217174.
- Bekal S, Berry C, Reimer AR, Van Domselaar G, Beaudry G, Fournier E, Doualla-Bell F, Levac E, Gaulin C, Ramsay D, et al. 2016. Usefulness of high-quality core genome single-nucleotide variant analysis for subtyping the highly clonal and the most prevalent Salmonella enterica serovar Heidelberg clone in the context of outbreak investigations. J Clin Microbiol. 54(2):289–295.
- Berendonk TU, Manaia CM, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons M-N, et al. 2015. Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol. 13(5):310–317.
- Bevan ER, Jones AM, Hawkey PM. 2017. Global epidemiology of CTX-M beta-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 72(8):2145–2155.
- Blaak H, Hamidjaja RA, van Hoek AH, de Heer L, de Roda Husman AM, Schets FM. 2014. Detection of ESBL-producing Escherichia coli on flies at poultry farms. Appl Environ Microbiol. 80(1):239–246.
- Blaak H, Lynch G, Italiaander R, Hamidjaja RA, Schets FM, de Roda Husman AM. 2015. Multidrug-resistant and extended spectrum beta-lactamase-producing Escherichia coli in Dutch surface water and wastewater. PLoS One. 10(6):e0127752.
- Braun SD, Ahmed MF, El-Adawy H, Hotzel H, Engelmann I, Weiß D, Monecke S, Ehricht R. 2016. Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile Delta, Egypt. Front Microbiol. 7:1020
- Bui TKN, Bui TMH, Ueda S, Le DT, Yamamoto Y, Hirai I. 2018. Potential transmission opportunity of CTX-M-producing Escherichia coli on a large-scale chicken farm in Vietnam. J Glob Antimicrob Resist. 13:1–6.
- Carroll D, Wang J, Fanning S, McMahon BJ. 2015. Antimicrobial resistance in wildlife: implications for public health. Zoonoses Public Health. 62(7):534–542.
- CDC. 2013. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- Chah KF, Ugwu IC, Okpala A, Adamu KY, Alonso CA, Ceballos S, Nwanta JN, Torres C. 2018. Detection and molecular characterisation of extended-spectrum beta-lactamase-producing enteric bacteria from pigs and chickens in Nsukka, Nigeria. J Glob Antimicrob Resist. 15:36–40.
- Chen CM, Ke SC, Li CR, Wu YC, Chen TH, Lai CH, Wu XX, Wu LT. 2017. High diversity of antimicrobial resistance genes, class 1 Integrons, and genotypes of multidrug-resistant Escherichia coli in beef carcasses. Microb Drug Resist. 23(7):915–924.
- Cohen Stuart J, van den Munckhof T, Voets G, Scharringa J, Fluit A, Hall ML. 2012. Comparison of ESBL contamination in organic and conventional retail chicken meat. Int J Food Microbiol. 154(3):212–214.
- Coque T, Baquero F, Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 13:19044.
- Cristóvão F, Alonso CA, Igrejas G, Sousa M, Silva V, Pereira JE, Lozano C, Cortés-Cortés G, Torres C, Poeta P. 2017. Clonal diversity of extended-spectrum beta-lactamase producing Escherichia coli isolates in fecal samples of wild animals. FEMS Microbiol Lett. 364:fnx039.
- Dahms C, Hübner N-O, Kossow A, Mellmann A, Dittmann K, Kramer A. 2015. Occurrence of ESBL-producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS One. 10(11):e0143326.
- Dame-Korevaar A, Fischer EAJ, van der Goot J, Stegeman A, Mevius D. 2019. Transmission routes of ESBL/pAmpC producing bacteria in the broiler production pyramid, a literature review. Prev Vet Med. 162:136–150.
- de Been M, Lanza VF, de Toro M, Scharringa J, Dohmen W, Du Y, Hu J, Lei Y, Li N, Tooming-Klunderud A, et al. 2014. Dissemination of cephalosporin resistance genes between Escherichia coli strains from farm animals and humans by specific plasmid lineages. PLoS Genet. 10(12):e1004776.
- Demerec M. 1948. Origin of bacterial resistance to antibiotics. J Bacteriol. 56(1):63–74.
- Demirjian A, Sanchez GV, Finkelstein JA, Ling SM, Srinivasan A, Pollack LA, Hicks LA, Iskander JK. 2015. CDC Grand Rounds: getting smart about antibiotics. MMWR Morb Mortal Wkly Rep. 64(32):871–873.
- Devarajan N, Laffite A, Mulaji CK, Otamonga J-P, Mpiana PT, Mubedi JI, Prabakar K, Ibelings BW, Poté J. 2016. Occurrence of antibiotic resistance genes and bacterial markers in a tropical river receiving hospital and urban wastewaters. PLoS One. 11(2):e0149211.
- Dohmen W, Bonten MJ, Bos ME, van Marm S, Scharringa J, Wagenaar JA, Heederik DJ. 2015. Carriage of extended-spectrum β-lactamases in pig farmers is associated with occurrence in pigs. Clin Microbiol Infect. 21(10):917–923.
- Dohmen W, Schmitt H, Bonten M, Heederik D. 2017. Air exposure as a possible route for ESBL in pig farmers. Environ Res. 155:359–364.
- Dohmen W, Van Gompel L, Schmitt H, Liakopoulos A, Heres L, Urlings B, Mevius D, Bonten M, Heederik D. 2017. ESBL carriage in pig slaughterhouse workers is associated with occupational exposure. Epidemiol Infect. 145(10):2003–2010.
- Dorado-García A, Smid JH, van Pelt W, Bonten MJM, Fluit AC, van den Bunt G, Wagenaar JA, Hordijk J, Dierikx CM, Veldman KT, et al. 2018. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: a pooled analysis. J Antimicrob Chemother. 73(2):339–347.
- dos Santos Alves T, Lara GHB, Maluta RP, Ribeiro MG, da Silva Leite D. 2018. Carrier flies of multidrug-resistant Escherichia coli as potential dissemination agent in dairy farm environment. Sci Total Environ. 633:1345–1351.
- Duan R, Sit TH, Wong SS, Wong RC, Chow K, Mak GC, Yam W, Ng L, Yuen K, Ho P. 2006. Escherichia coli producing CTX-M β-lactamases in food animals in Hong Kong. Microb Drug Resist. 12(2):145–148.
- Eliopoulos GM, Bush K. 2001. New β-lactamases in Gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis. 32(7):1085–1089.
- Enoch DA, Brown F, Sismey AW, Mlangeni DA, Curran MD, Karas JA, Cone DB, Aliyu SH, Dhanji H, Doumith M, et al. 2012. Epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae in a UK district hospital; an observational study. J Hosp Infect. 81(4):270–277.
- FAO. 2018. FAOSTAT Statistics Database. http://www.fao.org/faostat/en/#data/EMN/visualize.
- FDA. 2017. Summary report on antimicrobials sold or distributed for use in food-producing animals. http://www.fda.gov/media/119332/download.
- Fletcher S. 2015. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ Health Prev Med. 20(4):243–252.
- Forsberg KJ, Reyes A, Wang B, Selleck EM, Sommer MO, Dantas G. 2012. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 337(6098):1107–1111.
- Gao L, Tan Y, Zhang X, Hu J, Miao Z, Wei L, Chai T. 2015. Emissions of Escherichia coli carrying extended-spectrum β-lactamase resistance from pig farms to the surrounding environment. IJERPH. 12(4):4203–4213.
- Garcês A, Correia S, Amorim F, Pereira JE, Igrejas G, Poeta P. 2019. First report on extended-spectrum beta-lactamase (ESBL) producing Escherichia coli from European free-tailed bats (Tadarida teniotis) in Portugal: a one-health approach of a hidden contamination problem. J Hazard Mater. 370:219–224.
- Gaze WH, Zhang L, Abdouslam NA, Hawkey PM, Calvo-Bado L, Royle J, Brown H, Davis S, Kay P, Boxall ABA, et al. 2011. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J. 5(8):1253–1261.
- Geser N, Stephan R, Kuhnert P, Zbinden R, Kaeppeli U, Cernela N, Haechler H. 2011. Fecal carriage of extended-spectrum β-lactamase–producing Enterobacteriaceae in swine and cattle at slaughter in Switzerland. J Food Prot. 74(3):446–449.
- Greig J, Rajić A, Young I, Mascarenhas M, Waddell L, LeJeune J. 2015. A scoping review of the role of wildlife in the transmission of bacterial pathogens and antimicrobial resistance to the food chain. Zoonoses Public Health. 62(4):269–284.
- Guo Q, Yang J, Forsythe S, Jiang Y, Han W, He Y, Niu B. 2018. DNA sequence-based re-assessment of archived Cronobacter sakazakii strains isolated from dairy products imported into China between 2005 and 2006. BMC Genomics. 19(1):506.
- Haenni M, Châtre P, Métayer V, Bour M, Signol E, Madec JY, Gay E. 2014. Comparative prevalence and characterization of ESBL-producing Enterobacteriaceae in dominant versus subdominant enteric flora in veal calves at slaughterhouse, France. Vet Microbiol. 171(3–4):321–327.
- Haque A, Yoshizumi A, Saga T, Ishii Y, Tateda K. 2014. ESBL-producing Enterobacteriaceae in environmental water in Dhaka, Bangladesh. J Infect Chemother. 20(11):735–737.
- Hartmann A, Amoureux L, Locatelli A, Depret G, Jolivet C, Gueneau E, Neuwirth C. 2012. Occurrence of CTX-M producing Escherichia coli in soils, cattle, and farm environment in France (Burgundy region). Front Microbiol. 3:83
- Hasan B, Laurell K, Rakib MM, Ahlstedt E, Hernandez J, Caceres M, Järhult JD. 2016. Fecal carriage of extended-spectrum β-lactamases in healthy humans, poultry, and wild birds in León, Nicaragua—a shared pool of blaCTX-M genes and possible interspecies clonal spread of extended-spectrum β-lactamases-producing Escherichia coli. Microb Drug Resist. 22(8):682–687.
- Henriques I, Moura A, Alves A, Saavedra MJ, Correia A. 2006. Analysing diversity among β-lactamase encoding genes in aquatic environments. FEMS Microbiol Ecol. 56(3):418–429.
- Hinthong W, Pumipuntu N, Santajit S, Kulpeanprasit S, Buranasinsup S, Sookrung N, Chaicumpa W, Aiumurai P, Indrawattana N. 2017. Detection and drug resistance profile of Escherichia coli from subclinical mastitis cows and water supply in dairy farms in Saraburi Province, Thailand. PeerJ. 5:e3431.
- Hiroi M, Yamazaki F, Harada T, Takahashi N, Iida N, Noda Y, Yagi M, Nishio T, Kanda T, Kawamori F, et al. 2012. Prevalence of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in food-producing animals. J Vet Med Sci. 74(2):189–195.
- Horigan V, Kosmider R, Horton R, Randall L, Simons R. 2016. An assessment of evidence data gaps in the investigation of possible transmission routes of extended spectrum β-lactamase producing Escherichia coli from livestock to humans in the UK. Prev Vet Med. 124:1–8.
- Hou J, Wan W, Mao D, Wang C, Mu Q, Qin S, Luo Y. 2015. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ Sci Pollut Res Int. 22(6):4545–4554.
- Huijbers PM, Graat EA, van Hoek AH, Veenman C, de Jong MC, van Duijkeren E. 2016. Transmission dynamics of extended-spectrum β-lactamase and AmpC β-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev Vet Med. 131:12–19.
- Irrgang A, Falgenhauer L, Fischer J, Ghosh H, Guiral E, Guerra B, Schmoger S, Imirzalioglu C, Chakraborty T, Hammerl JA, et al. 2017. CTX-M-15-producing E. coli isolates from food products in Germany are mainly associated with an IncF-type plasmid and belong to two predominant clonal E. coli lineages. Front Microbiol. 8:2318
- Irrgang A, Hammerl JA, Falgenhauer L, Guiral E, Schmoger S, Imirzalioglu C, Fischer J, Guerra B, Chakraborty T, Käsbohrer A. 2018. Diversity of CTX-M-1-producing E. coli from German food samples and genetic diversity of the blaCTX-M-1 region on IncI1 ST3 plasmids. Vet Microbiol. 221:98–104.
- Jakobsen L, Bortolaia V, Bielak E, Moodley A, Olsen SS, Hansen DS, Frimodt-Møller N, Guardabassi L, Hasman H. 2015. Limited similarity between plasmids encoding CTX-M-1 β-lactamase in Escherichia coli from humans, pigs, cattle, organic poultry layers and horses in Denmark. J Glob Antimicrob Resist. 3(2):132–136.
- Ji X, Zheng B, Berglund B, Zou H, Sun Q, Chi X, Ottoson J, Li X, Lundborg CS, Nilsson LE. 2019. Dissemination of extended-spectrum beta-lactamase-producing Escherichia coli carrying mcr-1 among multiple environmental sources in rural China and associated risk to human health. Environ Pollut. 251:619–627.
- Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, et al. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 45(D1):D566–D573.
- Jones-Dias D, Manageiro V, Caniça M. 2016. Influence of agricultural practice on mobile bla genes: IncI1‐bearing CTX‐M. Environ Microbiol. 18:260–272.
- Karkman A, Do TT, Walsh F, Virta MP. 2018. Antibiotic-resistance genes in waste water. Trends Microbiol. 26(3):220–228.
- Klous G, Huss A, Heederik DJ, Coutinho RA. 2016. Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health. 2:65–76.
- Knudsen GM, Nielsen JB, Marvig RL, Ng Y, Worning P, Westh H, Gram L. 2017. Genome‐wide‐analyses of Listeria monocytogenes from food‐processing plants reveal clonal diversity and date the emergence of persisting sequence types. Environ Microbiol Rep. 9(4):428–440.
- Landers TF, Cohen B, Wittum TE, Larson EL. 2012. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 127(1):4–22.
- Laube H, Friese A, Von Salviati C, Guerra B, Rösler U. 2014. Transmission of ESBL/AmpC-producing Escherichia coli from broiler chicken farms to surrounding areas. Vet Microbiol. 172(3–4):519–527.
- Leverstein-van Hall M, Dierikx C, Cohen Stuart J, Voets G, Van Den Munckhof M, van Essen ‐Zandbergen A, Platteel T, Fluit A, Van de Sande ‐Bruinsma N, Scharinga J. 2011. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect. 17:873–880.
- Li B, Yang Y, Ma L, Ju F, Guo F, Tiedje JM, Zhang T. 2015. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. Isme J. 9(11):2490–2502.
- Li S, Song W, Zhou Y, Tang Y, Gao Y, Miao Z. 2015. Spread of extended-spectrum beta-lactamase-producing Escherichia coli from a swine farm to the receiving river. Environ Sci Pollut Res Int. 22(17):13033–13037.
- Lifshitz Z, Sturlesi N, Parizade M, Blum SE, Gordon M, Taran D, Adler A. 2018. Distinctiveness and similarities between extended-spectrum β-Lactamase-producing Escherichia coli isolated from cattle and the community in Israel. Microb Drug Resist. 24(6):868–875.
- Liu X, Liu H, Wang L, Peng Q, Li Y, Zhou H, Li Q. 2018. Molecular characterization of extended-spectrum β-Lactamase-Producing Multidrug Resistant Escherichia coli From Swine in Northwest China. Front Microbiol. 9:1756
- Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, et al. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 16(2):161–168.,
- Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G, Ayala J, Coque TM, Kern-Zdanowicz I, Luzzaro F, et al. 2006. CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother. 59(2):165–174.
- Ma Z, Ginn A, Kang M, Galvao K, Jeong KC. 2018. Genomic and virulence characterization of intrauterine pathogenic Escherichia coli with multi-drug resistance isolated from cow uteri with metritis. Front Microbiol. 9:3137.
- Ma Z, Lee S, Jeong KC. 2019. Mitigating antibiotic resistance at the livestock-environment interface: a review. J Microbiol Biotechnol. 29(11):1683–1692.
- Malhotra-Kumar S, Xavier BB, Das AJ, Lammens C, Hoang HT, Pham NT, Goossens H. 2016. Colistin-resistant Escherichia coli harbouring mcr-1 isolated from food animals in Hanoi, Vietnam. Lancet Infect Dis. 16(3):286–287.
- Markland S, Weppelmann TA, Ma Z, Lee S, Mir R, Teng L, Ginn A, Lee C, Ukhanova M, Galindo S, et al. 2019. High prevalence of cefotaxime resistant bacteria in grazing beef cattle: a cross sectional study. Front Microbiol. 10:176.
- Mathys DA, Mathys BA, Mollenkopf DF, Daniels JB, Wittum TE. 2017. Enterobacteriaceae harboring AmpC (blaCMY) and ESBL (blaCTX-M) in migratory and nonmigratory wild songbird populations on Ohio dairies. Vector Borne Zoonotic Dis. 17(4):254–259.
- Mesa RJ, Blanc V, Blanch AR, Cortés P, Gonzalez JJ, Lavilla S, Miro E, Muniesa M, Saco M, Tórtola MT. 2006. Extended-spectrum β-lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage). J Antimicrob Chemother. 58(1):211–215.
- Mir RA, Weppelmann TA, Johnson JA, Archer D, Morris JG, Jr, Jeong KC. 2016. Identification and characterization of cefotaxime resistant bacteria in beef cattle. PLoS One. 11(9):e0163279.
- Mir RA, Weppelmann TA, Teng L, Kirpich A, Elzo MA, Driver JD, Jeong KC. 2018. Colonization dynamics of cefotaxime resistant bacteria in beef cattle raised without cephalosporin antibiotics. Front Microbiol. 9:500.
- Moodley A, Guardabassi L. 2009. Transmission of IncN plasmids carrying blaCTX-M-1 between commensal Escherichia coli in pigs and farm workers. Antimicrob Agents Chemother. 53(4):1709–1711.
- Moon JS, Lee AR, Kang HM, Lee ES, Kim MN, Paik Y, Park YH, Joo YS, Koo H. 2007. Phenotypic and genetic antibiogram of methicillin-resistant staphylococci isolated from bovine mastitis in Korea. J Dairy Sci. 90(3):1176–1185.
- Müller A, Stephan R, Nüesch-Inderbinen M. 2016. Distribution of virulence factors in ESBL-producing Escherichia coli isolated from the environment, livestock, food and humans. Sci Total Environ. 541:667–672.
- Navajas-Benito EV, Alonso CA, Sanz S, Olarte C, Martínez-Olarte R, Hidalgo-Sanz S, Somalo S, Torres C. 2017. Molecular characterization of antibiotic resistance in Escherichia coli strains from a dairy cattle farm and its surroundings. J Sci Food Agric. 97:362–365.
- Negreanu Y, Pasternak Z, Jurkevitch E, Cytryn E. 2012. Impact of treated wastewater irrigation on antibiotic resistance in agricultural soils. Environ Sci Technol. 46(9):4800–4808.
- Nesme J, Cécillon S, Delmont TO, Monier J-M, Vogel TM, Simonet P. 2014. Large-scale metagenomic-based study of antibiotic resistance in the environment. Curr Biol. 24(10):1096–1100.
- Nhung NT, Cuong NV, Campbell J, Hoa NT, Bryant JE, Truc VNT, Kiet BT, Jombart T, Trung NV, Hien VB, et al. 2015. High levels of antimicrobial resistance among Escherichia coli isolates from livestock farms and synanthropic rats and shrews in the Mekong Delta of Vietnam. Appl Environ Microbiol. 81(3):812–820.
- Nilsson O, Borjesson S, Landen A, Bengtsson B. 2014. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother. 69(6):1497–1500.
- Noyes NR, Yang X, Linke LM, Magnuson RJ, Dettenwanger A, Cook S, Geornaras I, Woerner DE, Gow SP, McAllister TA, et al. 2016. Resistome diversity in cattle and the environment decreases during beef production. Elife. 5:e13195.
- Oikarainen PE, Pohjola LK, Pietola ES, Heikinheimo A. 2019. Direct vertical transmission of ESBL/pAmpC-producing Escherichia coli limited in poultry production pyramid. Vet Microbiol. 231:100–106.
- Oliver SP, Murinda SE, Jayarao BM. 2011. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog Dis. 8(3):337–355.
- Poirel L, Potron A, De La Cuesta C, Cleary T, Nordmann P, Munoz-Price LS. 2012. Wild coastline birds as reservoirs of broad-spectrum β-lactamase-producing Enterobacteriaceae, Miami Beach. Antimicrob Agents Chemother. 56(5):2756–2758.
- Prado T, Pereira WC, Silva D, Seki L, Carvalho ADA, Asensi M. 2008. Detection of extended‐spectrum β‐lactamase‐producing Klebsiella pneumoniae in effluents and sludge of a hospital sewage treatment plant. Lett Appl Microbiol. 46:136–141.
- Randall LP, Lemma F, Rogers JP, Cheney TE, Powell LF, Teale CJ. 2014. Prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli from pigs at slaughter in the UK in 2013. J Antimicrob Chemother. 69(11):2947–2950.
- Reuland EA, Overdevest IT, Al Naiemi N, Kalpoe JS, Rijnsburger MC, Raadsen SA, Ligtenberg-Burgman I, van der Zwaluw KW, Heck M, Savelkoul PH, et al. 2013. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin Microbiol Infect. 19(6):542–549.
- Riesenfeld CS, Goodman RM, Handelsman J. 2004. Uncultured soil bacteria are a reservoir of new antibiotic resistance genes. Environ Microbiol. 6(9):981–989.
- Sabia C, Stefani S, Messi P, de Niederhausern S, Bondi M, Condo C, Iseppi R, Anacarso I. 2017. Extended-spectrum β-lactamase and plasmid-mediated AmpC genes in swine and ground pork. J Food Safety. 37(1):e12282.
- Said LB, Jouini A, Alonso CA, Klibi N, Dziri R, Boudabous A, Slama KB, Torres C. 2016. Characteristics of extended-spectrum β-lactamase (ESBL)-and pAmpC beta-lactamase-producing Enterobacteriaceae of water samples in Tunisia. Sci Total Environ. 550:1103–1109.
- Saliu EM, Vahjen W, Zentek J. 2017. Types and prevalence of extended-spectrum beta-lactamase producing Enterobacteriaceae in poultry. Anim Health Res Rev. 18(1):46–57.
- Schaufler K, Nowak K, Düx A, Semmler T, Villa L, Kourouma L, Bangoura K, Wieler LH, Leendertz FH, Guenther S. 2018. Clinically relevant ESBL-producing K. pneumoniae ST307 and E. coli ST38 in an urban west African rat population. Front Microbiol. 9:150.
- Schaufler K, Semmler T, Wieler LH, Wöhrmann M, Baddam R, Ahmed N, Müller K, Kola A, Fruth A, Ewers C, et al. 2016. Clonal spread and interspecies transmission of clinically relevant ESBL-producing Escherichia coli of ST410—another successful pandemic clone? FEMS Microbiol Ecol. 92(1):fiv155.
- Schaumburg F, Onwugamba FC, Akulenko R, Peters G, Mellmann A, Köck R, Becker K. 2016. A geospatial analysis of flies and the spread of antimicrobial resistant bacteria. Int J Med Microbiol. 306(7):566–571.
- Schmid A, Hörmansdorfer S, Messelhäusser U, Käsbohrer A, Sauter-Louis C, Mansfeld R. 2013. Prevalence of extended-spectrum β-lactamases producing Escherichia coli on Bavarian dairy and beef cattle farms. Appl Environ Microbiol. 79(9):3027–3032.
- Schmithausen RM, Schulze-Geisthoevel SV, Stemmer F, El-Jade M, Reif M, Hack S, Meilaender A, Montabauer G, Fimmers R, Parcina M, et al. 2015. Analysis of transmission of MRSA and ESBL-E among pigs and farm personnel. PLoS One. 10(9):e0138173.
- Seni J, Moremi N, Matee M, van der Meer F, DeVinney R, Mshana S, D Pitout J. 2018. Preliminary insights into the occurrence of similar clones of extended‐spectrum beta‐lactamase‐producing bacteria in humans, animals and the environment in Tanzania: a systematic review and meta‐analysis between 2005 and 2016. Zoonoses Public Health. 65(1):1–10.
- Shafiq M, Huang J, Ur Rahman S, Shah JM, Chen L, Gao Y, Wang M, Wang L. 2019. High incidence of multidrug-resistant Escherichia coli coharboring mcr-1 and blaCTX-M-15 recovered from pigs. IDR. 12:2135–2149.
- Shin SW, Jung M, Won HG, Belaynehe KM, Yoon IJ, Yoo HS. 2017. Characteristics of transmissible CTX-M- and CMY-type beta-lactamase-producing Escherichia coli isolates collected from pig and chicken farms in South Korea. J Microbiol Biotechnol. 27(9):1716–1723.
- Sommer MO, Munck C, Toft-Kehler RV, Andersson DI. 2017. Prediction of antibiotic resistance: time for a new preclinical paradigm? Nat Rev Microbiol. 15(11):689–696.
- Swift BMC, Bennett M, Waller K, Dodd C, Murray A, Gomes RL, Humphreys B, Hobman JL, Jones MA, Whitlock SE, et al. 2019. Anthropogenic environmental drivers of antimicrobial resistance in wildlife. Sci Total Environ. 649:12–20.
- Tamang MD, Nam HM, Gurung M, Jang GC, Kim SR, Jung SC, Park YH, Lim SK. 2013. Molecular characterization of CTX-M β-lactamase and associated addiction systems in Escherichia coli circulating among cattle, farm workers, and farm environment. Appl Environ Microbiol. 79(13):3898–3905.
- Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, Polachek AJ, Ganshorn H, Sharma N, Kellner JD, et al. 2017. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 1(8):e316–e327.
- Teng L, Lee S, Ginn A, Markland SM, Mir R, DiLorenzo N, Boucher C, Prosperi M, Johnson J, Morris G, et al. 2019. Genomic comparison reveals natural occurrence of clinically relevant multi-drug resistant extended-spectrum β-lactamase producing Escherichia coli. Appl Environ Microbiol. 85(13): e03030–18.
- Tennstedt T, Szczepanowski R, Krahn I, Puhler A, Schluter A. 2005. Sequence of the 68,869 bp IncP-1alpha plasmid pTB11 from a waste-water treatment plant reveals a highly conserved backbone, a Tn402-like integron and other transposable elements. Plasmid. 53(3):218–238.
- Torres-Cortés G, Millán V, Ramírez‐Saad HC, Nisa‐Martínez R, Toro N, Martínez‐Abarca F. 2011. Characterization of novel antibiotic resistance genes identified by functional metagenomics on soil samples. Environ Microbiol. 13:1101–1114.
- Tripathi V, Cytryn E. 2017. Impact of anthropogenic activities on the dissemination of antibiotic resistance across ecological boundaries. Essays Biochem. 61:11–21.
- Tymensen L, Zaheer R, Cook SR, Amoako KK, Goji N, Read R, Booker CW, Hannon SJ, Neumann N, McAllister TA. 2018. Clonal expansion of environmentally-adapted Escherichia coli contributes to propagation of antibiotic resistance genes in beef cattle feedlots. Sci Total Environ. 637:657–664.
- Udikovic-Kolic N, Wichmann F, Broderick NA, Handelsman J. 2014. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proc Natl Acad Sci Usa. 111(42):15202–15207.
- Upadhyay S, Mustafa M, Joshi S. 2016. Naturally evolving extended spectrum cephalosporin resistance in soil borne isolates of Enterobacteriaceae. Natl Acad Sci Lett. 39(3):181–184.
- Usui M, Iwasa T, Fukuda A, Sato T, Okubo T, Tamura Y. 2013. The role of flies in spreading the extended-spectrum β-lactamase gene from cattle. Microb Drug Resist. 19(5):415–420.
- Vandecraen J, Chandler M, Aertsen A, Van Houdt R. 2017. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit Rev Microbiol. 43(6):709–730.
- Vittecoq M, Godreuil S, Prugnolle F, Durand P, Brazier L, Renaud N, Arnal A, Aberkane S, Jean-Pierre H, Gauthier-Clerc M, et al. 2016. Antimicrobial resistance in wildlife. J Appl Ecol. 53(2):519–529.
- von Salviati C, Laube H, Guerra B, Roesler U, Friese A. 2015. Emission of ESBL/AmpC-producing Escherichia coli from pig fattening farms to surrounding areas. Vet Microbiol. 175(1):77–84.
- von Wintersdorff CJ, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, Savelkoul PH, Wolffs PF. 2016. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 7:173
- Wang S, Weller D, Falardeau J, Strawn LK, Mardones FO, Adell AD, Switt A. 2016. Food safety trends: from globalization of whole genome sequencing to application of new tools to prevent foodborne diseases. Trends Food Sci Technol. 57:188–198.
- Wasyl D, Zając M, Lalak A, Skarżyńska M, Samcik I, Kwit R, Jabłoński A, Bocian Ł, Woźniakowski G, Hoszowski A, et al. 2018. Antimicrobial resistance in Escherichia coli isolated from wild animals in Poland. Microb Drug Resist. 24(6):807–815.
- WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1.
- Wichmann F, Udikovic-Kolic N, Andrew S, Handelsman J. 2014. Diverse antibiotic resistance genes in dairy cow manure. MBio. 5(2):e01017–01013.
- Wielders CCH, van Hoek AHAM, Hengeveld PD, Veenman C, Dierikx CM, Zomer TP, Smit LAM, van der Hoek W, Heederik DJ, de Greeff SC, et al. 2017. Extended-spectrum β-lactamase-and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin Microbiol Infect. 23(2):120.e1–e8.
- Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM. 2015. Global trends in infectious diseases at the wildlife–livestock interface. Proc Natl Acad Sci USA. 112(31):9662–9667.
- Yuan W, Chai T, Miao Z. 2010. ERIC-PCR identification of the spread of airborne Escherichia coli in pig houses. Sci Total Environ. 408(6):1446–1450.
- Zheng H, Zeng Z, Chen S, Liu Y, Yao Q, Deng Y, Chen X, Lv L, Zhuo C, Chen Z, et al. 2012. Prevalence and characterisation of CTX-M β-lactamases amongst Escherichia coli isolates from healthy food animals in China. Int J Antimicrob Agents. 39(4):305–310.
- Zhou K, Lokate M, Deurenberg RH, Arends J, Foe LT, Grundmann H, Rossen JW, Friedrich AW. 2015. Characterization of a CTX-M-15 producing Klebsiella pneumoniae outbreak strain assigned to a novel sequence type (1427). Front Microbiol. 6:1250.
- Zurfluh K, Hächler H, Nüesch-Inderbinen M, Stephan R. 2013. Characteristics of extended-spectrum beta-lactamase (ESBL)-and carbapenemase-producing Enterobacteriaceae isolated from rivers and lakes in Switzerland. Appl Environ Microbiol. 79(9):3021–3026.
- Zurfluh K, Wang J, Klumpp J, Nuesch-Inderbinen M, Fanning S, Stephan R. 2014. Vertical transmission of highly similar blaCTX-M-1-harboring IncI1 plasmids in Escherichia coli with different MLST types in the poultry production pyramid. Front Microbiol. 5:519.
