Abstract
Lipopeptides (LPs) are a prominent class of molecules among the steadily growing spectrum of specialized metabolites retrieved from Pseudomonas, in particular soil-dwelling and plant-associated isolates. Among the multiple LP families, pioneering research focussed on phytotoxic and antimicrobial cyclic lipopeptides (CLPs) of the ubiquitous plant pathogen Pseudomonas syringae (syringomycin and syringopeptin). Their non-ribosomal peptide synthetases (NRPSs) are embedded in biosynthetic gene clusters (BGCs) that are tightly co-clustered on a pathogenicity island. Other members of the P. syringae group (Pseudomonas cichorii) and some species of the Pseudomonas asplenii group and Pseudomonas fluorescens complex have adopted these biosynthetic strategies to co-produce their own mycin and peptin variants, in some strains supplemented with an analogue of the P. syringae linear LP (LLP), syringafactin. This capacity is not confined to phytopathogens but also occurs in some biocontrol strains, which indicates that these LP families not solely function as general virulence factors. We address this issue by scrutinizing the structural diversity and bioactivities of LPs from the mycin, peptin, and factin families in a phylogenetic and evolutionary perspective. BGC functional organization (including associated regulatory and transport genes) and NRPS modular architectures in known and candidate LP producers were assessed by genome mining.
1. Introduction
Pseudomonas inhabit diverse environments and many species are thriving in the rhizosphere and phyllosphere of plants (Weller Citation2007; Silby et al. Citation2011). Most plant-associated Pseudomonas belong to the P. fluorescens lineage, which is composed of five phylogenetic groups or complexes: P. fluorescens, P. syringae, P. putida, P. asplenii, and P. lutea (Garrido-Sanz et al. Citation2016). Among those, certain species can be beneficial as biocontrol or growth-promoting agents, mostly within the P. fluorescens complex, whereas others exhibit phytopathogenicity, mainly belonging to the P. syringae complex or the P. asplenii group (Höfte and Vos Citation2007; Weller Citation2007). The P. syringae complex has been divided in more than 60 pathovars and 13 phylogroups, and many members produce diverse phytotoxins, acting as virulence factors in a wide range of hosts (Bender et al. Citation1999; Xin et al. Citation2018). These toxins include Lipopeptides (LPs), secondary metabolites composed of a fatty acid tail attached to a peptide (linear, partially, or fully cyclized), predominantly described for phylogroup-2 strains (Berge et al. Citation2014; Dillon et al. Citation2019). Typically, two types of cyclic LPs (CLPs), respectively from the “Mycin” and “Peptin” families, and a linear LP (LLP) from the “Factin” family, are produced (Ballio et al. Citation1988; Ballio et al. Citation1991; Lindeberg et al. Citation2008; Burch et al. Citation2014; and ). Subsequently, variants belonging to the different LPs families were characterized in diverse phytopathogens, such as P. cichorii (P. syringae complex, phylogroup-11), P. fuscovaginae (P. asplenii group), P. corrugata, and P. mediterranea (P. fluorescens complex) (Ballio et al. Citation1996; Flamand et al. Citation1996; Emanuele et al. Citation1998; Scaloni et al. Citation2004; Licciardello et al. Citation2012; Pauwelyn et al. Citation2013; Huang et al. Citation2015; Götze et al. Citation2019; and ). However, CLPs are well known to have anti-microbial properties against a wide range of soil-borne phytopathogens and several “mycin”/“peptin” producers within the P. fluorescens complex (P. corrugata and P. mandelii groups) have been proposed as biocontrol agents (Berry et al. Citation2014; Michelsen, Jensen, et al. Citation2015; Van Der Voort et al. Citation2015). In fact, the distinction between beneficial and phytopathogenic LP producers is not very strict and, even if CLPs have been described as virulence factors among well-known pathogens, the role of these molecules in other Pseudomonas species remains unclear.
Table 1. Pseudomonas LPs from the factin, mycin, and peptin families with known structure.
Table 2. Original LP profile of representative Pseudomonas strains.
LPs are assembled by non-ribosomal peptide synthetases (NRPSs) encoded by large biosynthetic gene clusters (BGCs) and many Pseudomonas strains possess multiple BGCs coding for the production of mycins, peptins, and/or factins (Gross and Loper Citation2009). However, for a particular producer, LPs are often chemically characterized one by one, with or without the stereochemistry, the corresponding BGCs and the biological activities, which makes the knowledge very disparate and the comparison arduous (Ballio et al. Citation1988; Ballio et al. Citation1991; Ballio et al. Citation1996; Emanuele et al. Citation1998). Intensive efforts were recently made in order to gather such information for a large number of LP families but the mycin/peptin/factin producers were not included or the diversity of their BGCs was not examined in detail (Geudens and Martins Citation2018; Götze and Stallforth Citation2020). In this review, we make the link between LP structures, BGC organizations and diversity, and biological activities for these families. This work has led us to complement the current knowledge with a non-exhaustive genome mining-based inventory to offer a comprehensive genus-wide overview on these LPs producers. Moreover, it allowed us to present a general comparison of BGC evolutionary relationships and to highlight gaps necessary to fill in order to clarify the boundary between beneficial and detrimental plant-Pseudomonas relationships.
2. Phytopathogenic Pseudomonas: LPs as virulence factors
2.1. Two different biosynthetic strategies for LP production in P. syringae
Several P. syringae strains co-produce LPs from the mycin, peptin, and factin families. In the late 1980s and early 1990s, the chlorinated CLPs syringomycin, syringostatin, syringotoxin, and pseudomycin were characterized (Ballio et al. Citation1988; Ballio et al. Citation1990; Ballio, Bossa, et al. Citation1994; Ballio, Collina, et al. Citation1994; Segre et al. Citation1989; Scaloni et al. Citation1994). These members of the mycin family are composed of a fatty acid tail ranging from 10 to 16 carbons attached to a fully cyclized peptide, made of nine amino acids (AAs), with the last being a chlorinated threonine (). The peptin family encompasses the largest known CLPs, and in P. syringae strains these compounds are made of a partially cyclized peptide (8-ringed) with either 22 AAs in syringopeptin SP22 (Ballio et al. Citation1991) and its variants SP22(SC) (Isogai et al. Citation1995), SP508 (Grgurina et al. Citation2005), and SP22(Phv) (Grgurina et al. Citation2002), or 25 AAs in syringopeptin SP25 (Ballio et al. Citation1991; Ballio et al. Citation1995) and it is variant SP25(Phe) (Scaloni et al. Citation1997). The factin family comprises LLPs with eight AAs and its first member, syringafactin, was identified in diverse P. syringae strains (Berti et al. Citation2007; Burch et al. Citation2014; , and S1).
The LPs of the peptin and factin families are synthesized co-linearly with the order of the modules present in their respective NRPS enzymes. The same biosynthesis scheme is used for the production of most Pseudomonas CLPs (reviewed by Götze and Stallforth Citation2020). A typical module is composed of three domains ordered as [C-A-T]: C (AA condensation), A (AA-specific adenylation), and T (AA thiolation for thioester binding). The fatty acid is attached to the first AA by a dedicated condensation domain with N-acylation activity (C1 or Cstarter mediating lipoinitiation). Subsequent additions of AAs are catalysed by either a regular condensation domain (LCL domain connecting two L-configured AAs) or, more frequently, by a condensation domain with built-in epimerization activity (C/E domain). The latter converts the configuration of the previously incorporated residue from L to D, avoiding the need for separate epimerization (E) domains, such as those present in most Pseudomonas pyoverdine synthetases (Visca et al. Citation2007). Release from the terminating enzyme, with concomitant cyclization in the case of CLPs, is catalysed by a thioesterase (TE) domain. Like most CLP systems in Pseudomonas, the peptin and factin terminal NRPSs carry a tandem of TE domains, while mycin synthetases only possess one TE domain ()). Elucidation of the syringomycin biosynthetic pathway has revealed a number of distinguishing features (Götze and Stallforth Citation2020; Jaremko et al. Citation2020). Syringomycin synthetase SyrE lacks an A-domain in the last module [C-T] which is provided by a separate enzyme with an [A-T] module (SyrB1; ; Myc-a-1). Threonine attached to SyrB1 is chlorinated by SyrB2 and then shuttled via SyrC to the last T-domain of SyrE for condensation of the co-linear octapeptide intermediate with L-4-chlorothreonine prior to cyclization. Yet another stand-alone enzyme (SyrP) participates in biosynthesis by hydroxylation of aspartate, serving as a substrate for the penultimate module of SyrE.
Figure 1. Module architecture of Pseudomonas NRPS enzymes involved in the biosynthesis of mycins (A and B), peptins (C), factins (D), brabantamide (E) and predicted LP-13 (F), and LP-8 (G). Representative domain configurations are shown, differing by the presence of a single (A and E) or tandem TE-domain (C, D, F, and G) and the number of regular [C-A-T] modules encoded by the respective genes (specified inside arrows). Deviations from the canonical module composition in mycin systems are highlighted: presence of an additional C-domain (+C); absence of an A-domain (−A). A variant of the regular SyrB1 module [A-T] (B:1) is expanded with an extra T-domain (B:2). The different enzyme configurations occurring in the mycin and peptin family are specified for the respective CLPs in Tables S2 and S3.
![Figure 1. Module architecture of Pseudomonas NRPS enzymes involved in the biosynthesis of mycins (A and B), peptins (C), factins (D), brabantamide (E) and predicted LP-13 (F), and LP-8 (G). Representative domain configurations are shown, differing by the presence of a single (A and E) or tandem TE-domain (C, D, F, and G) and the number of regular [C-A-T] modules encoded by the respective genes (specified inside arrows). Deviations from the canonical module composition in mycin systems are highlighted: presence of an additional C-domain (+C); absence of an A-domain (−A). A variant of the regular SyrB1 module [A-T] (B:1) is expanded with an extra T-domain (B:2). The different enzyme configurations occurring in the mycin and peptin family are specified for the respective CLPs in Tables S2 and S3.](/cms/asset/32fcb4e0-2c35-4177-8ad5-541547458254/imby_a_1794790_f0001_c.jpg)
2.2. P. syringae’s CLP island
In the genomes of the syringomycin producers P. syringae pv. syringae B301D (co-producing SP22) and B728a (co-producing SP22(Phv)), both BGCs are tightly clustered (). Similarly clustered BGCs are also present in the genomes of SP25-producing strains such as P. syringae pv. syringae HS191 and P. syringae pv. atrofaciens LMG 5095T. The SP22 and SP25 NRPS systems display similar organization with genes sypB (5 modules) and sypC (12 modules) but differ in sypA (five versus eight modules). SP25-type NRPS genes are also present in P. syringae pv. lapsa ATCC 10859, but the adjacent Mycin cluster appears to have a split equivalent, SyrE1 (five modules) and SyrE2 (four modules) (Myc-b-1, and ), of the common syringomycin synthetase SyrE (nine modules)(Myc-a-1, ). In P. syringae pv. syringae SM, compared to other SP25-type BGCs (HS191and LMG 5095T), the syrE gene is lost. Apparently, this loss is compensated by the acquisition of a different type of Mycin cluster (syrE1syrE2; similar to ATCC 10859; Myc-b-1, ). The latter NRPS genes are located about 300 kb upstream of the original syrE and on the opposite strand (). All these phylogroup-2 P. syringae strains carry a syringafactin BGC at about 34–38 kb downstream of their syringomycin NRPS gene(s). However, other P. syringae strains only harbour a factin BGC (lacking the mycin and peptin BGCs) such as the tomato pathogen P. syringae pv. tomato DC3000 (phylogroup-1) or Pseudomonas sp. SZ57 (phylogroup-2) (Berti et al. Citation2007; Mukherji et al. Citation2020).
Figure 2. Overview of the NRPS-based systems for synthesis of LPs of the mycin, peptin, and factin families in Pseudomonas. The phylogenetic tree is based on the concatenated analysis of 16S rRNA, gyrB, rpoB, and rpoD genes (Mulet et al. Citation2010). The distance matrix was calculated by the Jukes-Cantor method and the dendrogram was generated by neighbour joining (NJ, bootstrap 1000). Phylogenetic groups/complexes are delineated (dashed lines). Three strains of the P. putida group were included as an outgroup. Reference strains are indicated in orange and correspond to those used by Hesse et al. (Citation2018). Strains harbouring the LPQ island organization are highlighted in blue and an asterisk indicates those without the additional LP-8 BGC. The LPQ genomic sequence of thanamycin producer P. fluorescens DSM 11579 (not shown) is nearly identical (99%) to the one of Pseudomonas sp. 7SR1. The arrows represent NRPS genes and are coloured according to the type of CLP synthesized. The number of modules for incorporation of consecutive amino acids by the respective encoded NRPS enzymes is indicated. The number “1S” refers to a module lacking an A-domain (see ). Chemically characterized LPs are indicated in the figure (names aligned horizontally and vertically with corresponding producer and BGC, respectively) and listed, together with the peptide sequence of predicted ones, in . The mupirocin PKS BGC is drawn without individual genes and not to scale (broken arrow). Two types of uncharacterized hybrid NRPS-PKS systems, Hybrid NRPS-PKS-1 (three genes) and Hybrid NRPS-PKS-2 (four genes), are shown in light grey and their genes encoding a NRPS with carboxyterminal PKS domains are marked with an asterisk. Solid connectors between genes correspond to a distance <10 kb, dotted connectors 10–50 kb and blank space >50 kb. A detailed version of the BGCs, where described NRPS genes are labelled, is available in Figure S1.
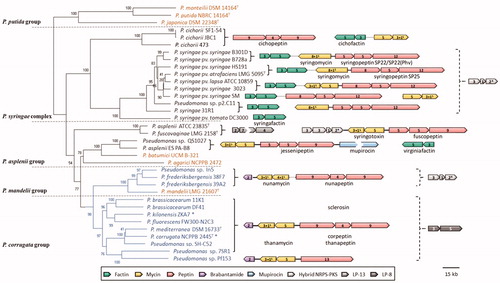
Figure 3. Genetic organization of the regions flanking representative Pseudomonas BGCs for LPs of the mycin, peptin, and factin families. The grey bullets represent the NRPS enzymes specified in (not to scale): B, brabantamide; F, factin; L, LP-13, M, mycin; P, peptin; A similar representation is used for the Hybrid NRPS-PKS-2 system (H2) and the mupirocin PKS system (A). The distances between non-adjacent genes (separated by a dotted line) are specified. The colour legend specifies the respective gene products, with the same colour used for homologs present in different strains. Genes that are discussed in the text are labelled. Pseudogenes or gene remnants are crossed. The distance between the thanamycin and thanapeptin clusters differs between strains SH-C52 (24.6 kb) and DSM 11579 (2.8 kb, not shown). The BGC organization of P. syringae strains ATCC 10859 and 3023 is identical to those of strains B728a and LMG 5095T, except for two or three additional genes in the region between the M and P clusters (only the latter part is shown).
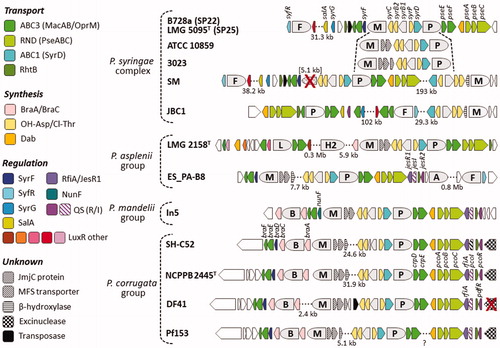
In addition, a cryptic BGC (Hybrid NRPS-PKS-1, ) comprising two NRPS genes and a hybrid NRPS-PKS gene can be present. Its second and third enzyme are homologous to RmoH and RmoI (at 44 and 48% AA identity, respectively) encoded by a syntenic gene pair required for synthesis of rimosamide by Streptomycetes (McClure et al. Citation2016).
2.3. LP archipelago of P. cichorii
Distinct members of the peptin and mycin families have been identified in the lipopeptidome of P. cichorii, another phytopathogen belonging to P. syringae complex. P. cichorii SF1-54 produces cichopeptin, also made of 22 AAs but differing from SP22 by the size of its macrocycle (five versus eight; ) and its AA composition (Huang et al. Citation2015), and cichofactin which differs from syringafactin by a single AA (, Pauwelyn et al. Citation2013; Götze et al. Citation2019). Molecular mass data suggest that a third mycin BGC yields pseudomycin (Ballio, Bossa, et al. Citation1994; Pauwelyn Citation2012). The genome of the closely related cichofactin producer P. cichorii JBC1 reveals that the tightly organized mycin-peptin BGCs, typical for most P. syringae strains, are not observed here (Götze et al. Citation2019). The three BGCs occur separately in the genome, hence the suggestion of an “archipelago” (). Interestingly, the Mycin BGC shows striking synteny with the one of P. syringae pv. syringae SM. The cichopeptin analogue produced by P. cichorii MAFF 730229 diverges at four different position and it will be of interest to genome sequence this strain for comparative analysis (Komatsu et al. Citation2019; ).
2.4. P. fuscovaginae: standing out from the phytopathogenic crowd
A more distant relative of P. syringae that produces CLPs from the Mycin and Peptin families is the rice pathogen P. fuscovaginae. Fuscopeptin produced by P. fuscovaginae UPB 264 resembles cichopeptin by its cyclization pattern (5-ringed) but differs by its shorter and different peptide sequence of 19 AAs (Ballio et al. Citation1996; ). This strain produces syringotoxin (Flamand et al. Citation1996; ), which is also reported in some P. syringae strains (Ballio et al. Citation1990; Fukuchi et al. Citation1992; ). In P. fuscovaginae LMG 2158T, the corresponding BGCs occur in a single cluster. This strain lacks a BGC for factin production but also carries a cryptic rimosamide-related BGC, however, equipped with an extra triple-modular NRPS gene (Hybrid NRPS-PKS-2, ). Another cryptic BGC consists of a tripartite NRPS operon that would generate a novel lipotridecapeptidic product (LP-13) ( and , Table S1). The structure and function of this molecule will be published elsewhere. Nearly identical syntenic BGCs are present in the genome of P. asplenii ATCC 23835T, suggesting that these closely related species, have a very similar capacity to produce several (lipo)peptidic secondary metabolites (Hu et al. Citation1998). It should be noted that Tohya et al. (Citation2020) recently suggested that P. fuscovaginae should be classified as a later heterotypic synonym of P. asplenii since the average nucleotide identity between the two type strains representing these species is 98.4%.
A fuscopeptin-related compound, jessenipeptin, was recovered from Pseudomonas sp. QS1027, another member of the P. asplenii group, isolated from Dictyostelium discoideum fruiting bodies (Arp et al. Citation2018; ). A distinguishing feature of this peptin producer is the presence, immediately downstream of the jessenipeptin cluster, of a large BGC (73.7 kb with 34 ORFs) for the known polyketide antibiotic mupirocin. To reconstruct the probable organization of the fragmented jessenipeptin BGC and its missing upstream region, we mapped several contigs of the QS1027 draft genome on a large contiguous genomic sequence of the closely related strain P. asplenii ES_PA-B8, also carrying near-identical mupirocin and jessenipeptin BGCs (Figure S2). This assembly also suggests that a Mycin BGC is located upstream of the jessenipeptin cluster (Myc-b-2, and ). The unlinked factin-type BGC of QS1027 yields virginiafactin (Götze et al. Citation2019; ). Neither strain appears to accommodate the rimosamide-related BGC of the P. asplenii and P. fuscovaginae type strains.
Interestingly, the MLSA phylogeny reveals a split P. asplenii group with P. asplenii ATCC 23835T and P. fuscovaginae LMG 2158T being separated from P. asplenii ES_PA-B8 and Pseudomonas sp. QS1027, in line with changes in BGCs composition and organization that have occurred during their divergent evolutionary history (). Strain ES_PA-B8 has only 84% average nucleotide identity with P. asplenii ATCC 23835T and needs to be reclassified (Tohya et al. Citation2020).
3. P. fluorescens complex
3.1. LPQ: an island not merely linked to phytopathogenicity
Within the P. fluorescens complex, CLPs of the Peptin family were characterized in strains affiliated with the P. corrugata and P. mandelii groups, e.g. corpeptin (Emanuele et al. Citation1998), nunapeptin (Michelsen, Watrous, et al. Citation2015), thanapeptin (Van Der Voort et al. Citation2015), and the LLP sclerosin (Berry et al. Citation2012; and ). Their structures, all containing a 22-AA peptide, bear more similarity to cichopeptin than to the shorter fuscopeptin/jessenipeptin and this is reflected in similar NRPS organizations: 9 − 4 − 9 modules for cichopeptin and cor/nuna/thanapeptin versus 5 − 5 − 9 for fusco/jessenipeptin (). On the other hand, Pseudomonas sp. Pf153 harbours a single NRPS gene coding for 13 modules for the synthesis of a putative short, possibly linear peptin (peptin-13 in ; Fuchs Citation2000).
Consistently, these strains co-produce a second CLP from the Mycin family, e.g. cormycin (Scaloni et al. Citation2004), nunamycin (Michelsen, Watrous, et al. Citation2015), or thanamycin (Watrous et al. Citation2012; Johnston et al. Citation2015) (). The corresponding mycin clusters encode two NRPSs whose module architecture can be differentiated by the phylogenetic affiliation of the producer ( and ), e.g. P. corrugata group (Myc-b-1) and P. mandelii group (Myc-c-1). Their respective Mycin and Peptin BGCs are tightly packed in P. fluorescens In5 and P. brassicacearum DF41, or with some non-conserved intervening region, ranging from 2.8 kb in thanamycin producer P. fluorescens DSM 11579 to about 32 kb in P. corrugata NCPPB 2445T (). In addition, members of the P. mandelii group, similarly to P. syringae strains, possess an unlinked rimosamide-related BGC (Hybrid NRPS-PKS-1) and most of the strains belonging to the P. corrugata group harbour a cryptic BGC consisting of a bipartite NRPS operon that would generate a novel lipo-octapeptidic product (LP-8, and , Table S1).
The closely co-integrated tandem BGCs organization is also present in Pseudomonas sp. FW300-N2C3. Although the corresponding metabolites have not yet been characterized, Melnyk et al. (Citation2019) showed that this genomic region is required for phytopathogenic effects in a gnotobiotic assay, also dependent on the activity of the associated quorum sensing (QS) genes. Hence, it was considered to represent a pathogenicity island, designated LPQ (lipopeptide/QS) island, conserved among members of the P. corrugata and P. mandelii groups ( and ). Interestingly, it was suggested that the gain or loss of the LPQ island might occur by homologous recombination. A putative excinuclease gene (uvrA2 homolog) is flanking the peptin BGC in the P. corrugata-type LPQ, but it is not known whether this may be functionally linked to the mobility of this island (Tark et al. Citation2008). Additionally to the peptin/mycin BGC, the LPQ island harbours QS genes (luxR/luxI) downstream of the peptin cluster and a brabantamide BGC downstream of the Mycin cluster ( and ).
3.2. Brabantamide: an immigrant BGC settled on the LPQ island
The braABC operon is conserved among strains harbouring a LPQ island organization and was previously considered to be part of the “syringomycin synthetase operon” (Melnyk et al. Citation2019; ). This BGC was independently shown to drive the synthesis of the cyclocarbamate antibiotic brabantamide in Pseudomonas sp. SH-C52 (Schmidt et al. Citation2014) and P. fluorescens DSM 11579 (equivalent operon lpiABC; Johnston et al. Citation2013). The lipodipeptide core of the molecule is assembled by a dual-module NRPS (BraB, ) and further modified by the monooxygenase BraC and the rhamnosyl transferase BraA. Two LPQ variants have evolved in the P. corrugata and P. mandelii groups by integrating the brabantamide BGC at a slightly shifted position, respectively, in front or behind a conserved triplet of genes involved in regulation and transport (see Sections 5 and 6). Compared to the SH-C52 enzymes, the BraBC proteins in P. corrugata and P. mandelii groups show strong sequence conservation (>95 and >80% identity, respectively).
Within the P. asplenii group, P. fuscovaginae LMG 2158T and Pseudomonas sp. QS1027, harbour differently organized brabantamide-like genes. Indeed, in P. fuscovaginae LMG 2158T only braA is found downstream the mycin cluster (), and two sets of braBC homologous are present: one upstream the LP-13 coding for a bi-modular braB and one far downstream the mycin-peptin BGC coding for a tri-modular braB. In Pseudomonas sp. QS1027 no braA was retrieved but a braBC set coding for a bi-modular BraB is present. The divergent NRPS sequences present in these strains (<45% identity to SH-C52 BraB) suggest that the structure of the corresponding compounds is likely different from brabantamide and needs further characterization.
4. Prominent differences between mycin and peptin/factin families
4.1. BGC organization and NRPS architecture
The peptin and factin NRPS systems are quite similar to those of most other CLP families in Pseudomonas, only differing by the overall number of modules and their distribution across one to three NRPS gene(s) ( and ; Götze and Stallforth Citation2020). A very similar modular design is apparent for the unlinked NRPS systems predicted to assemble a lipotridecapeptide (LP-13) or a lipo-octapeptide (LP-8).
The well-characterized syringomycin NRPS system is atypical for most Pseudomonas CLPs as it comprises one large mega-enzyme (SyrE) complemented with an external [A-T] enzyme (SyrB1) (Myc-a-1; ). However, in several known and predicted mycin producers architectural variations are noted. In most strains, including some P. syringae SP25 producers, the mycin BGC encodes two synthetases, further denoted as SyrE1 and SyrE2 (). Such SyrE2 retains the “split” architecture of the last module but features a tandem C-domain [C-C-A-T] in the first module (Myc-b; ). A further deviation from the syringomycin model, the lack of an A-domain in the last module of the first synthetase (Myc-c; ), was first noted for nunamycin and module shuffling (a module functioning twice) was put forward as a possible explanation (Michelsen, Watrous, et al. Citation2015). This domain architecture is also present in P. cichorii 473 but not in other inspected P. cichorii strains. For representative strains, the Myc subtypes and occurrence of an atypical [A-T-T] SyrB1 () are documented in Table S2.
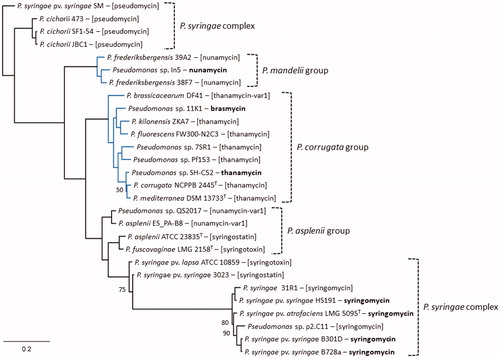
The conserved module composition of SyrE and concatenated SyrE1-E2 enables a global comparison of mycin NRPSs by phylogenetic analysis (). Among strains belonging to the P. syringae complex the mycin synthetases are distributed across distant clades and branches. The SyrE1-SyrE2 pair of P. syringae pv. syringae SM clusters with the P. cichorii enzymes (about 80% AA identity), in line with the striking synteny of their uncoupled mycin BGCs predicted to generate pseudomycin. The SyrE enzymes mediating syringomycin biosynthesis constitute a well-resolved clade, separated from two branches with concatenated SyrE1-SyrE2 enzymes from the potential producers of syringostatin, and syringotoxin. With the latter P. syringae enzymes, the syringomycin synthetases share only about 60% AA identity and similarity is even lower (48% AA identity) to strain SM pseudomycin synthetases. The SyrE1-SyrE2 sequences from the LPQ strains separate into two distinct clades (on average 66% AA identity) that reflect their differentiated CLP synthesis (nunamycin or thanamycin/thanamycin variant) and phylogenetic affiliation (P. mandelii or P. corrugata group). Similarly to the MLSA phylogeny of the P. asplenii group (), the NRPS concatenate phylogeny reveals two branches with (i) strains QS2017 and ES_PA-B8 potentially producing a new nunamycin variant and (ii) strains ATCC 23835T and LMG 2158T, respectively, predicted to produce syringostatin and syringotoxin. Interestingly, the enzymes of the last two strains are predicted to assemble the same peptide moiety as those of the P. syringae strains 3023 and ATCC 10859, but their biosynthetic systems show host group-associated divergence (about 84% AA identity).
Figure 4. Phylogenetic analysis of mycin synthetases. Maximum-likelihood phylogenetic tree (PhyML, JTT substitution model) inferred from multiple AA sequence alignment of single SyrE and concatenated SyrE1-SyrE2 homologous of representative Pseudomonas strains belonging to different phylogenetic groups/complexes (delineated by labelled right brackets; see ). The separate SyrB1 sequences are not included in the comparison. The LPQ cluster enzymes are indicated in blue. The DSM 11579 enzymes (not shown) share 99.7% AA identity with the 7SR1 orthologous. Characterized CLPs produced by these NRPS systems are shown in bold next to the corresponding producer strain. Other CLPs tentatively assigned based on the same or a similar predicted peptide sequence are shown in double brackets. The predicted AA sequence of P. corrugata NCPPB 2445T matches the one of thanamycin (Asp-3), whereas cormycin of original producer IPVCT 10.3 incorporates Asn-3. Only bootstrap values (percentages of 100 replicates) below 100 are shown. The scale bar represents 0.2 substitutions per site.
4.2. Peptide stereochemistry
The overall peptide stereochemistry in CLPs from the mycin/factin families shows a higher proportion of L-configured residues, setting them apart from those of the peptin family (and most other Pseudomonas CLPs) with predominantly D-AAs. In the mycin family, this is reflected in the balance between LCL and C/E domains in their NRPS enzymes (Tables S2 and S3). It should be noted that phylogeny-based classification of C-domains for stereo-chemical prediction has its limitations, as some of the experimentally determined AA-configurations conflict with the predicted ones (Balibar et al. Citation2005, “Balibar exceptions”, Tables S2 and S3). The starter C1-domain attaches the first AA to the fatty acid, most frequently a 3-hydroxy derivative but chain length can range from C8 to C16. Dihydroxy and mono-unsaturated fatty acids are less frequent (). A similar starter C1-domain is active in brabantamide biosynthesis. A C1-domain cladogram shows that sequences group consistently according to family affiliation of the NRPS and not according to chemical nature of the selected fatty acid moiety (Figure S3).
4.3. Conserved accessory and unknown genes
The non-proteinogenic residue Dab (2,4-diaminobutyric acid) is incorporated in all characterized peptins and most mycins (Tables S2 and S3) and the dab gene is present in the BGCs of all known (and predicted) peptin-mycin co-producers (Walsh et al. Citation2013; ). In P. syringae pv. syringae B301D, the dab gene is co-transcribed with biosynthesis and transport genes and is required for CLP production (Lu et al. Citation2002). In P. fuscovaginae it appears uncoupled from the fuscopeptin genes and integrated in the regulatory region of the cryptic LP-13 BGC (). It has not yet been elucidated which enzymes generate the three other non-proteinogenic AAs, Dhb (2,3-dehydroaminobutyric acid), Dhp (dehydro-2-aminopropanoic acid), and Dha (dehydroalanine) (Grgurina and Mariotti Citation1999; Götze and Stallforth Citation2020).
Synteny analysis of BGCs for mycins and peptins production revealed a set of 2 or 3 convergent genes of unknown function that are found, in many producers, right upstream of the mycin cluster. The two first genes encode for, respectively, a putative membrane-anchored protein with a periplasmic JmjC-family hydroxylase domain (Gao et al. Citation2018) and an unknown MFS efflux protein (DHA3 family; transporter classification TC 2.A.1.21). The third gene, absent from some clusters (e.g. nunamycin) or present individually (P. syringae SM and P. cichorii strains), is encoding a member of the β-hydroxylase family (Pfam PF05118) for which no function has yet been assigned (Reitz et al. Citation2019; ).
5. Multiple complementary systems for CLP export
Multiple transport-related genes are integrated in the mycin/peptin BGCs and they are co-expressed with the biosynthetic genes (Lu et al. Citation2005; Licciardello et al. Citation2018). The first is a tripartite export system required for CLP secretion, made of a cytoplasmic membrane protein (MacB), a periplasmic adaptor (MacA), and an outer-membrane protein (OMP) (Lu et al. Citation2005; Strano et al. Citation2015; Greene et al. Citation2018; Licciardello et al. Citation2018). The macAB gene pair (also pseEF or crpDE) is consistently situated downstream of the third Peptin gene (sypC and crpC) and the gene likely encoding the required OMP (nunGX in the P. mandelii group) is located downstream of the Mycin synthetase (syrE gene(s); ). The embracement of the NRPS BGCs by transporter genes is reminiscent of the typical gene organization in Pseudomonas strains, irrespective of the CLP family. Such “transporter hug”, consisting of well-conserved transporter genes flanking a BGC or BGC cluster at the distal NRPS genes, is even maintained if the NRPS genes are present at two distant genomic locations (). Similarly, within the P. corrugata group, the OMP genes are located downstream of the brabantamide operon (braEF), thus including the brabantamide BGC into this transporter hug. On the other hand, when the factin cluster is the only LP BGC, a macAB pair (syfCD) is present right downstream the biosynthetic genes (syfAB) and mediate the transport (Berti et al. Citation2007). This may explain why a second macAB module is still part of the separate cichofactin and LP-13 BGCs (respectively, in P. cichorii and P. fuscovaginae), whereas in others (e.g. P. syringae and P. asplenii) the export system seems to have evolved towards dual use, accommodating the export of factins and peptins.
A second secretory route is specified by homologs of the P. syringae genes pseABC, located in the downstream region of the macAB genes only separated by the dab gene (). This second tripartite export system, made of a pmf-dependent RND-superfamily transporter (PseC; TC 2.A.6.3.8), a periplasmic adaptor (PseB), and an OMP (PseA), appears necessary for syringomycin and syringopeptin secretion (to a greater extent for syringopeptin; Kang and Gross Citation2005), and the corresponding genes are co-transcribed with the respective biosynthetic genes (Lu et al. Citation2005; Licciardello et al. Citation2018). However, the similarities between the different components of these two first efflux systems are quite low (<25–30% identical AAs).
A third type of transporter protein, SyrD, also designated cyclic peptide transporter, encoded by the syrD gene, located in the conserved intergenic region between the syringomycin and syringopeptin BGCs, was shown to be required for syringomycin production (Quigley et al. Citation1993; ). SyrD is similar to the ABC transporter PvdE, involved in the export of the pyoverdine precursor pseudobactin to the periplasm (Cornelis Citation2010). By analogy with PvdE, SyrD might serve to support the transport of (a precursor of) syringomycin to the periplasm, where it may be further translocated via the RND transport system PseABC. In strains with less tightly organized mycin/peptin BGCs, the syrD gene is nevertheless located in the 5′-region of the peptin operon. P. cichorii again shows a deviation from this common organization with the syrPD-syrB1B2C cluster being associated with the unlinked mycin BGC. Remarkably, in P. syringae pv. syringae SM a syntenic mycin BGC is present, apparently acquired to replace the original “regular” syrE that has been largely deleted without the loss of the associated syrPD-syrB1B2C (). As a result, this strain accommodates two clusters, an “indigenous” one associated with the peptin BGC and an “exogenous” one more similar to the P. cichorii mycin system. SyrD (Peptide-3 Exporter Family; TC 3.A.1.113) and Macrolide Exporter Family (MacB)) belong to different superfamilies of ATP-dependent transporters, respectively, ABC1 and ABC3, reflecting their different evolutionary origin (Wang et al. Citation2009).
6. A plethora of LP-associated LuxR regulators
All the regulators associated with the mycin, peptin, and factin BGCs belong to the LuxR superfamily and dispose of its characteristic carboxyterminal DNA-binding domain. Within LPQ strains but also in some belonging to the P. asplenii group, a subset of these LuxR regulators (JesR2, PcoR, and PdfR) are acyl homoserine lactone (AHL) receptors (harbouring the characteristic AHL-binding domain). Their cognate AHL synthase gene luxI (i.e. jesI, pcoI, or pdfI), a second regular luxR regulator gene, rfiA, and a divergent rhtB homolog, are also part of this well-conserved “QS cluster” (). The following hierarchical model has been described for corpeptin and jessenipeptin production: expression of rfiA, together with the AHL synthase gene, is under the control of LuxR; in turn, RfiA activates the expression of CLP biosynthetic and transport genes, resulting in a cell density-dependent CLP production (Licciardello et al. Citation2007, Citation2009; Licciardello et al. Citation2018; Arp et al. Citation2018). In Pseudomonas sp. DF41, a slightly different system occurs, where RfiA but not AHLs are essential for sclerosin production (Berry et al. Citation2014; Nandi et al. Citation2016). Even though the corresponding peptin has not yet been characterized, the inactivation of the equivalent luxR-luxI gene pair in Pseudomonas sp. FW300-N2C3 abolished its pathogenic phenotype (Melnyk et al. Citation2019). Interestingly, a luxR-solo gene (possessing an AHL-binding domain but without a cognate luxI) is present downstream of the cichopeptin genes, along with non-adjacent homologs of salA and syrG regulatory genes (Subramoni and Venturi Citation2009; ). RhtB has been previously characterized as a threonine and homoserine transporter possibly involved in AHL export (Zakataeva et al. Citation1999; Zakataeva et al. Citation2006). However, a rhtB gene is also present in P. fuscovaginae/P. cichorii and P. syringae strains, respectively, linked or unlinked to the RND transporter genes, lacking such QS pair and producing CLPs independently of QS (Quiñones et al. Citation2005; Mattiuzzo et al. Citation2011). P. syringae rhtB, located downstream of syrE, is co-expressed with the syp/syr genes (Lu et al. Citation2005). Such prominent conservation within all these BGCs suggests an as yet unexplored functional role.
In P. syringae, CLP production is subjected to the control of multiple LuxR regulators. It is not controlled by QS but depends on the GacS/GacA global regulatory system (Quiñones et al. Citation2005). A complex regulatory cascade composed of, in the order of hierarchy, SalA, SyrG, and SyrF, activates expression of the syp/syr genes (Wang, Lu, Records, et al. Citation2006; Wang, Lu, Yang, et al. Citation2006; Vaughn and Gross Citation2016). Likewise, the syrF homolog, nunF, is required for production of both nunamycin and nunapeptin (Hennessy et al. Citation2017). An homologous regulator, braD, is also conserved in the P. corrugata group but at a slightly shifted position, downstream the brabantamide operon. The syringafactin BGC is flanked by two additional luxR genes (), of which only the upstream gene (syfR) is strictly required for syringafactin production, at least in P. syringae pv. tomato DC3000 (Berti et al. Citation2007). Homologs of both regulators are also associated with the factin BGC of P. cichorii, whereas only a syfR homolog is retained in Pseudomonas sp. QS1027 and P. asplenii ES_PA-B8 ().
Remarkably, the syringotoxin/fuscopeptin BGC of P. fuscovaginae LMG 2158T lacks regulatory genes (). This species contains two QS systems required for virulence but neither regulates CLP production (Mattiuzzo et al. Citation2011). However, its unlinked BGC for the unknown LP-13 is equipped with four unrelated luxR genes, one downstream and three upstream of the NRPS genes.
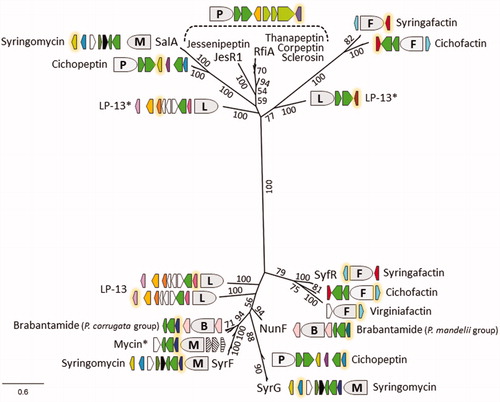
Phylogenetic analysis of this large set of non-QS LuxR regulators reveals two large clades (). The first mainly populated by the peptin-associated RfiA homologs (including JesR1) also accommodates the SalA homologs. The other broad clade encompasses the mycin/brabantamide-associated SyrF homologs, as well as a SyrG branch. The factin-associated LuxR regulators located, respectively, upstream (SyfR) and downstream of the NRPS genes, constitute separate branches in the SyrF-SyrG clade and in the RfiA-SalA clade. Remarkably, the quartette of LP-13 associated LuxR regulators in P. fuscovaginae, are also part of these clades but forming separate branches, and it is tempting to speculate that they may play a role in the coordination of CLP production. The occurrence of two LPQ groups is also reflected in the clustering of their LuxRs, in particular for the SyrF/BraD homologs.
Figure 5. Phylogeny of non-QS LuxR-family proteins associated with mycin, peptin, and factin BGCs. Maximum likelihood phylogenetic tree (PhyML, JTT substitution model) inferred from multiple AA sequence alignment of characterized regulators (JesR1, NunF, RfiA, SalA, SyfR, SyrF, and SyrG) and homologs linked to related BGCs of representative Pseudomonas strains. Asterisks indicate LuxRs that are associated with uncharacterized LPs and Mycin* refers to the predicted nunamycin-var1 producers Pseudomonas sp. QS1027 and P. asplenii ES_PA-B8. The respective regulator genes are shown with a yellow glow and their relative positioning towards the respective NRPS system is indicated (see ). The syringomycin label includes the sequences retrieved from P. syringae pv. syringae SM adjacent to its syrE remnant. Bootstrap values (percentages of 100 replicates) higher than 50 are shown. The scale bar represents 0.6 substitutions per site.
7. Biological properties of LPs
7.1. LPs as virulence factors
The CLPs produced by P. syringae, P. fuscovaginae, P. corrugata, and P. cichorii, contribute to virulence via their phytotoxic activities (Bender et al. Citation1999; Coraiola et al. Citation2008; Huang et al. Citation2015; Strano et al. Citation2015; Weeraratne et al. Citation2019). Generally, the amphipathic structure of mycins and peptins allows their insertion into the lipid bilayers of membranes to form pores which induces electrolyte leakage and subsequent death of plant cells (i.e. necrotic symptoms; Hutchison and Gross Citation1997; Coraiola et al. Citation2008). However, comparison of the outcome of diverse biological tests reported in the literature shows a trend that CLPs from the mycin family appear more efficient at causing haemolysis (Scaloni et al. Citation2004; Fiore et al. Citation2008) while CLPs from the peptin family show stronger phytotoxicity (Ballio et al. Citation1996; Emanuele et al. Citation1998; Grgurina et al. Citation2002; Scaloni et al. Citation2004). Additionally, their biosurfactant properties seem to have an important role in promoting the spread of the bacteria across the plant (Bender et al. Citation1999) and syringomycin was shown to contribute (to a greater extent than syringopeptin) to the fitness of P. syringae during apoplast colonization (Helmann et al. Citation2019). Mycins and peptins can work in synergy and altering the production of one or the other induces a strong reduction of virulence (Batoko et al. Citation1998; Bender et al. Citation1999; Strano et al. Citation2015), while in other cases no effect on virulence was observed (Kitten et al. Citation1998). It was hypothesized that toxin production may contribute differently to virulence depending on the host colonized by P. syringae (Bender et al. Citation1999). Supporting this idea, it was demonstrated that an important factor influencing pore formation is the composition of plant cell membranes, especially the type and abundance of sterols and sphingolipids (Julmanop et al. Citation1993; Feigin et al. Citation1997; Bender et al. Citation1999).
While syringomycin and syringopeptin have a direct phytotoxic activity on plants, syringafactin/cichofactin seems to have a more insidious effect on virulence. Syringafactin represents the major surfactant produced by P. syringae pv. syringae B728a and it contributes to fitness on leaves under fluctuating humidity (Burch et al. Citation2014). The activator of flagellar synthesis, FleQ, exerts a negative control on syringafactin production, apparently suppressing its biosynthesis under conditions favouring swimming motility (Nogales et al. Citation2015). Inactivation of cichofactin biosynthesis reduces pathogenicity, results in swarming deficiency but enhances biofilm formation (Pauwelyn et al. Citation2013). Moreover, it was demonstrated that syringafactin facilitates the multiplication of P. syringae by interacting with the waxy cuticle of leaves which increases cuticle permeability and allows the diffusion of nutrients out of the tissue (Burch et al. Citation2014). Perhaps disturbing the plant tissue barrier also facilitates the action of syringomycin and syringopeptin to cause necrosis.
7.2. Anti-microbial activities of LPs
Besides phytotoxicity, CLPs also exhibit a wide antimicrobial activity. This activity probably evolved to ward off microbial competitors that colonize the same niche as CLP producer. Considering the different types of biological tests (with mutants, crude extracts, or pure compounds) on a wide range of microorganisms and in a quite broad range of concentrations, it is clear that, comparison is difficult but it still provides a general view on the different CLP families. Exploring possible biological effects exerted in soil and plant environments, we focus on the biological tests involving microorganisms that are prominent inhabitants of the rhizosphere and/or phyllosphere of plants, particularly excluding human pathogens. Antifungal activity is a common feature but it appears that mycins and peptins show differential activity depending on the type of fungi tested for susceptibility. While it seems that among fungi from the Ascomycota branch mycins and 8-ringed peptins act in a species-specific manner, in the Basidiomycota branch mycins show a greater antifungal activity then 8-ringed peptins. Conversely, 5-ringed peptins have no or low activity on fungi (Ascomycota and Basidiomycota branches) but show activity against oomycetes (). Furthermore, all CLPs exhibit anti-bacterial activity against B. megaterium but the MIC values are completely different and dependent on the study and/or the strain of B. megaterium used (Ballio et al. Citation1996; Lavermicocca et al. Citation1997; Scaloni et al. Citation2004; Grgurina et al. Citation2005). Some studies are also reporting anti-bacterial activity against other Gram-positive bacteria, such as Rhodococcus fascians, Micrococcus luteus, Bacillus subtilis, or Mycobacterium smegmatis and peptins show higher inhibition then mycins (Lavermicocca et al. Citation1997; Grgurina et al. Citation2005; Arp et al. Citation2018). Jessenipeptin, produced by a grazing-resistant Pseudomonas, has amoebicidal activity against the bacterivorous amoeba Dictyostelium discoideum and antimicrobial activity against Gram-positive bacteria (Arp et al. Citation2018).
Table 3. Antimicrobial activities of LPs.
8. Discussion
In this review, we scrutinized BGC organizations for the production of LPs from the factin (one type), the peptin (five types), and the mycin (four types) families, within a total of 35 Pseudomonas genomes ( and ; Table S1). Their MLSA tree is largely congruent with the different types of organization, indicating that the different BGCs have evolved in accordance to the evolutionary history of the Pseudomonas species in which they occur. Most of the strains harbour a tight organization of the mycin-peptin genes, forming a chimeric BGC, which indicates a co-evolution of these gene sets (). Auxiliary genes for transport and regulation are located in the genomic region between BGCs or positioned tightly downstream of the mycin and peptin NRPS clusters. The transporter genes located at each end are well conserved, even in looser organizations, forming a transporter hug seemly packing up all the required genes (). Interestingly, in LPQ strains a brabantamide operon has been integrated within this transporter hug which underlies an important function somehow supporting the action of CLPs.
Three types of peptin BGCs coexist within the P. syringae group, designated here as Pep-a and Pep-b configurations, respectively, coding for SP22 and SP25 (and their variants; Table S3) and the Pep-c organization of cichopeptin (). The Pep-a type possesses three extra modules compared to its shorter counterpart (Pep-b type), probably resulting from module duplication, while their multiple variants seem to originate mainly from mutations altering A-domain selectivity but keeping the hydrophobic nature of the side chain of AAs (e.g. Val ↔ Leu, Leu ↔ Dhb or Phe ↔ Tyr; Table S3). P. cichorii strains and most of the LPQ strains, on the other hand, harbour a completely different peptin-BGC organization (Pep-c type; ), which suggests that these BGCs have evolved from a common ancestral source, different from the P. syringae strains. In Pseudomonas sp. Pf153, a shortened version (Pep-e type) is found, apparently obtained by the deletion of nine modules (6–14 from the Pep-c type) and inter-genic rearrangement ( and Table S3). Conceivably, the peptin-BGC of strains from the P. asplenii group derive from the SP22-type BGC, with the two first NRPS genes being similar but having a shortened version of the third probably obtained by the deletion of three modules (i.e. modules 16–18 in SP22; Pep-d type , Table S3).
Basically, only two main types of mycin BGCs occur: the Myc-a type, with a single multi-domain SyrE gene, corresponding to the original syringomycin cluster; and the Myc-b/c types which accommodate two NRPS genes, supporting the synthesis of diverse mycins (, Table S2). This diversity of mycins most probably has arisen by gene rearrangement together with mutations altering A-domain selectivity and module shuffling (Table S2). Interestingly, mycin NRPS systems from LPQ strains, P. asplenii ATCC 23835T, P. fuscovaginae LMG 2158T, P. cichorii JBC1 and 473, seem to originate from a common ancestor but have further evolved compared to their counterparts in the P. syringae group (Myc-b-1 and -2 and Myc-c-1 types; and , Table S2). The Mycin BGCs of LPQ strains are well conserved within each group and bear less diversity. All the mycin producers lack an A-domain in the last module which is complemented by an extra gene (syrB1 in P. syringae with an [A-T] module). However, in some cases, a different version of SyrB1 is observed with an extra T-domain [A-T-T] module (, Table S2) and such tandem T-domains can increase biosynthetic flux (Zhang et al. Citation2020). The syrB1 gene, together with the other syr genes, is strictly conserved among all mycin producers which is not surprising considering the fact that this set of genes is responsible for the addition of their characteristic final chlorinated threonine (see Section 2.1).
The factin cluster, on the other hand, when present, is always found apart from this “Peptin-Mycin island” and possesses is own regulatory system (SyfR), which suggests that these clusters were acquired independently. However, in most of the cases it seems that these physically separated clusters have co-evolved long enough for the peptin-type transporter to accommodate the export of LPs from both families (peptin and factin). However, similarly to mono-factin producers of the P. syringae complex (Table S1), the cichofactin cluster still harbours cognate transporter genes, indicating that this cluster has been acquired more recently by P. cichorii.
P. syringae pv. syringae SM highlights an interesting case in which the factin/peptin/mycin BGCs seem to originate from vertical gene transfer (common ancestor with most P. syringae strains), but was followed by the acquisition, probably by horizontal gene transfer, of a new mycin BGC (pseudomycin) concomitant with the deletion of the initial mycin BGC (probably syringomycin). Supporting this hypothesis is the reminiscence of the set of conserved genes flanking the initial mycin BGC ().
Analysis of LP-BGCs illuminates how mutation and gene rearrangement, closely related to the modular nature of NRPSs, lead to evolutionary diversification. However, to counteract the high selective pressure bearing on such large BGCs, the corresponding metabolites must be essential for the fitness of these bacteria. As elaborated in Section 7, CLPs possess two main functions (phytotoxicity and antimicrobial activity), however, the boundary between phytopathogenic and beneficial (from the human point of view) Pseudomonas strains is difficult to delineate.
Within the P. syringae complex, most of the analysed strains that are plant pathogenic possess the factin, peptin, and mycin BGCs. However, predicted cichofactin producer strains belonging to the P. viridiflava (phylogroup-9) and P. asturiensis (phylogroup-7) species lack mycin/peptin BGCs, whereas in phytopathogens within the P. asplenii group and P. fluorescens complex (P. corrugata and P. mediterranea) the factin BGC is absent. It was demonstrated that syringafactin is crucial for the interaction with the upper part of plants and its producers appear to mainly cause symptoms above-ground, while most of strains belonging to the P. fluorescens complex seem to have more impact on roots or seeds (Höfte and Vos Citation2007; Melnyk et al. Citation2019). This might explain why most of the strains within the P. fluorescens complex do not feature a factin BGC. Pathogens of the P. asplenii group, which cause symptoms on leaves and leaf sheets, harbour a BGC encoding LP-13. This LP may play a role similar to factin-type LPs. However, within the P. corrugata group, where the overall BGC organization is well conserved and where CLPs are identical or very similar, some strains behave as phytopathogens (FW300-N2C3, DSM 16733T, and NCPPB 2445T) while others are qualified as commensal or biocontrol agent (DF41 and Pf153) (Fuchs Citation2000; Catara Citation2007; Berry et al. Citation2012; Michelsen, Watrous, et al. Citation2015; Melnyk et al. Citation2019; Gislason and de Kievit Citation2020; Yang et al. Citation2020). In the endophytic metagenome of sugarbeet (Carrión et al. Citation2019), we identified peptin, mycin, and brabantamide BGC sequences closely matching those of the LPQ island of P. mediterranea DSM 16733T (99.3% nucleotide sequence identity). Peptins seem to show stronger phytotoxicity and one intriguing similarity between some of these potential biocontrol agents is that they may possess a linear version of their peptins (i.e. sclerosin; predicted LP of Pf153). However, phytotoxicity assays are needed to confirm this hypothesis. It also appears that among LPQ strains, CLP production is mostly under the control of QS and thus may occur in a density-dependent manner (Licciardello et al. Citation2009; Licciardello et al. Citation2012; Berry et al. Citation2014; Arp et al. Citation2018). The active switch between low and high bacterial density could then explain how they can behave as commensal bacteria or as endophytes and shift to a pathogenic lifestyle when reaching a sufficient number of cells.
Plant signals were demonstrated to modulate CLP production in P. syringae (Mo and Gross Citation1991; Quigley and Gross Citation1994; Grgurina et al. Citation1996). In LPQ strains multiple uncharacterized LuxR-type regulators are present ( and ) and it seems possible that some of them also respond to specific secondary metabolites from plants (Subramoni et al. Citation2011). It also appears that the phytotoxic and antimicrobial activities of CLPs are linked to their ability to interact with membranes, where the nature of the sphingolipids and/or the sterols is of prime importance to determine whether or not a target is sensitive (Balleza et al. Citation2019). Membrane composition greatly varies according to the stage of development and the different part of the plant (roots, leaves, fruits….), and it is clear that the pathogenicity of LP-producing Pseudomonas is partly driven by the nature of the host but also dependent on the site of interaction. Thus, it is possible that a given Pseudomonas strain is pathogenic on above-ground plant parts but neutral or beneficial with biocontrol capacities on plant roots. In the simplest case where a non-sensitive host or part of the host is colonized, CLPs can act as antimicrobial compounds that inhibit or kill competing micro-organisms. If these microbial competitors are pathogenic on economically important plants, the producing Pseudomonas strains is considered as a biocontrol agent. It is then not surprising that some Pseudomonas species appear ambivalent, behaving on the one hand as a plant pathogen and on the other hand as biocontrol agent (Catara Citation2007; Melnyk et al. Citation2019; Gislason and de Kievit Citation2020). LP phytotoxicity is often assessed with a crude extract or purified compound on A. thaliana, tobacco or rice, but one needs to consider that (i) challenging different plant species or tissue (rhizosphere versus phyllosphere) may affect outcomes and (ii) the outcome of such assays cannot simply be extrapolated unless in situ production of (sufficient amount) LP by the bacteria was previously demonstrated. Finally, LP production is a fine-tuned mechanism under the control of different paths of regulation, and interactions with plants and other micro-organisms allow Pseudomonas species to decide whether or not the production of virulence factors, such as LPs, is appropriate to the situation they are in.
9. Perspectives
First, to better understand the fine-tuning of LP production, the function of multiple unknown LuxR-family regulators has to be characterized, particularly with respect to the chemical nature and origin of effector molecules that activate them (plant, microbial, or other environmental source). A subset of these regulators are part of a QS system controlling LP production and AHLs secreted by other bacteria occupying a common niche, pseudomonads as well as other AHL-producing bacteria, may influence LP production. Such QS cross-talk has been inferred from phenotypic complementation of LPQ-associated phytopathogenicity in a gnotobiotic Arabidopsis system upon co-inoculation with a non-virulent QS mutant and a different non-pathogenic LPQ strain (Melnyk et al. Citation2019). Cooperative phytopathogenicity driven by commensal AHL sharing has been observed for the olive tree pathogen P. savastanoi (Hosni et al. Citation2011; Caballo-Ponce et al. Citation2018). Conceivably, such signal sharing may also support cooperative microbial antagonism contributing to biocontrol (Besset-Manzoni et al. Citation2018). On the other hand, many plant-associated micro-organisms are also able to interfere with such interactions between community members by Quorum Quenching (QQ) mechanisms (Chan et al. Citation2011; Jafra et al. Citation2006). It will be of interest to determine to which extent interactions with other plant-associated micro-organisms can modulate LP production and, consequently, pathogenicity, and/or antimicrobial activity of the producing Pseudomonas strain.
Second, the inspection of Pseudomonas genomes allowed us to predict the peptide sequence of multiple new variants and to identify potential producers of known CLPs (e.g. syringotoxin, syringostatin, or syringopeptin SP508). P. cichorii is predicted to synthesize pseudomycin, in accordance with the molecular mass of a metabolite co-produced with cichofactin and cichopeptin (Pauwelyn Citation2012). Additional predictions awaiting validation comprise a novel variant of SP22 and cichopeptin, three non-described analogues of syringopeptin SP25 and additional LPQ island-derived peptins (Tables S2 and S3). Furthermore, new mycin variants are also awaiting characterization: strain DF41 strain likely produces a thanamycin peptide variant; although our peptide sequence prediction for brasmycin (strain 11K1) matches the one for thanamycin, the molecular masses are different (Michelsen, Jensen, et al. Citation2015; Zhao et al. Citation2019); and strain QS1027 is a candidate nunamycin analogue producer not affiliated with the P. mandelii LPQ-group.
Additionally, co-occurrence of a number of cryptic BGCs (NRPS and NRPS-PKS) was highlighted. The corresponding metabolites (LP-8, LP-13, rimosamide-related molecule) await chemical and functional characterization, including possible synergy with (other) LPs for phytopathogenic and/or antimicrobial activities. Rimosamide was shown to antagonize the antimicrobial activity of the Streptomyces griseochromogenes blasticidin against Bacillus cereus (McClure et al. Citation2016). On the other hand, Arp et al. (Citation2018) have demonstrated the synergistic activity of jessenipeptin and the antibiotic mupirocin towards S. aureus but the fact that each BGC possesses its own associated QS genes indicates that the production of each metabolite can (also) be regulated separately (Thomas et al. Citation2010). The brabantamide genes were shown to be co-transcribed with CLP BGCs and their clustering with CLP-associated transporter genes hints to potential co-secretion of brabantamide and CLPs by LPQ strains (Van Der Voort et al. Citation2015). Notably, in the study of Melnyk et al. (Citation2019), the brabantamide operon was co-deleted with CLP genes and, therefore, not individually accounted for the assessment of FW300-N2C3 mutant phenotypes regarding phytopathogenicity. Brabantamide is a glycosylated LP with phospholipase inhibitory activity and was shown to have a much broader antimicrobial activity than syringomycin, inhibiting a broad range of Gram-positive bacteria (Busby et al. Citation2000; Thirkettle et al. Citation2000; Reder-Christ et al. Citation2012). When considering the functions of these associated metabolites it is tempting to speculate that they can potentially contribute to antimicrobial or phytopathogenic behaviour.
Supplementary_material_Final_RDM.docx
Download MS Word (2.6 MB)Acknowledgment
This study was supported by the Excellence of Science grant EOS-30650620.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Arp J, Götze S, Mukherji R, Mattern DJ, García-Altares M, Klapper M, Brock DA, Brakhage AA, Strassmann JE, Queller DC, et al. 2018. Synergistic activity of cosecreted natural products from amoebae-associated bacteria. Proc Natl Acad Sci USA. 115(15):3758–3763.
- Balibar CJ, Vaillancourt FH, Walsh CT. 2005. Generation of D amino acid residues in assembly of arthrofactin by dual condensation/epimerization domains. Chem Biol. 12(11):1189–1200.
- Balleza D, Alessandrini A, Beltrán García MJ. 2019. Role of lipid composition, physicochemical interactions, and membrane mechanics in the molecular actions of microbial cyclic lipopeptides. J Membrane Biol. 252(2–3):131–157.
- Ballio A, Barra D, Bossa F, Collina A, Grgurina I, Marino G, Moneti G, Paci M, Pucci P, Segre A. 1991. Syringopeptins, new phytotoxic lipodepsipeptides of Pseudomonas syringae pv. syringae. FEBS Lett. 291(1):109–112.
- Ballio A, Barra D, Bossa F, DeVay JE, Grgurina I, Iacobellis NS, Marino G, Pucci P, Simmaco M, Surico G. 1988. Multiple forms of syringomycin. Physiol Mol Plant Pathol. 33(3):493–496.
- Ballio A, Bossa F, Camoni L, Di Giorgio D, Flamand MC, Maraite H, Nitti G, Pucci P, Scaloni A. 1996. Structure of fuscopeptins, phytotoxic metabolites of Pseudomonas fuscovaginae. FEBS Lett. 381(3):213–216.
- Ballio A, Bossa F, Collina A, Gallo M, Iacobellis NS, Paci M, Pucci P, Scaloni A, Segre A, Simmaco M. 1990. Structure of syringotoxin, a bioactive metabolite of Pseudomonas syringae pv. syringae. FEBS Lett. 269(2):377–380.
- Ballio A, Bossa F, Di Giorgio D, Ferranti P, Paci M, Pucci P, Scaloni A, Segre A, Strobel GA. 1994. Novel bioactive lipodepsipeptides from Pseudomonas syringae: the pseudomycins. FEBS Lett. 355(1):96–100.
- Ballio A, Bossa F, Giorgio D, Nola A, Manetti C, Paci M, Scaloni A, Segre AL. 1995. Solution conformation of the Pseudomonas syringae pv. syringae phytotoxic lipodepsipeptide syringopeptin 25-A. Two-dimensional NMR, distance geometry and molecular dynamics. Eur J Biochem. 234(3):747–758.
- Ballio A, Collina A, Nola AD, Manetti C, Pad M, Segre A. 1994. Determination of structure and conformation in solution of syringotoxin, a lipodepsipeptide from Pseudomonas syringae pv. syringae by 2D NMR and molecular dynamics. Struct Chem. 5(1):43–50.
- Batoko H, de Kerchove d’Exaerde A, Kinet JM, Bouharmont J, Gage RA, Maraite H, Boutry M. 1998. Modulation of plant plasma membrane H+-ATPase by phytotoxic lipodepsipeptides produced by the plant pathogen Pseudomonas fuscovaginae. Biochim Biophys Acta. 1372(2):216–226.
- Bender CL, Alarcón-Chaidez F, Gross DC. 1999. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev. 63(2):266–292.
- Berge O, Monteil CL, Bartoli C, Chandeysson C, Guilbaud C, Sands DC, Morris CE. 2014. A user’s guide to a data base of the diversity of Pseudomonas syringae and its application to classifying strains in this phylogenetic complex. PLoS One. 9(9):e105547.
- Berry CL, Brassinga AKC, Donald LJ, Fernando WGD, Loewen PC, de Kievit TR. 2012. Chemical and biological characterization of sclerosin, an antifungal lipopeptide. Can J Microbiol. 58(8):1027–1034.
- Berry CL, Nandi M, Manuel J, Brassinga AKC, Fernando WGD, Loewen PC, de Kievit TR. 2014. Characterization of the Pseudomonas sp. DF41 quorum sensing locus and its role in fungal antagonism. Biol Control. 69:82–89.
- Berti AD, Greve NJ, Christensen QH, Thomas MG. 2007. Identification of a biosynthetic gene cluster and the six associated lipopeptides involved in swarming motility of Pseudomonas syringae pv. tomato DC3000. J Bacteriol. 189(17):6312–6323.
- Besset-Manzoni Y, Rieusset L, Joly P, Comte G, Prigent-Combaret C. 2018. Exploiting rhizosphere microbial cooperation for developing sustainable agriculture strategies. Environ Sci Pollut Res Int. 25(30):29953–29970.
- Bultreys A, Gheysen I. 1999. Biological and molecular detection of toxic lipodepsipeptide-producing Pseudomonas syringae strains and PCR identification in plants. Appl Environ Microbiol. 65(5):1904–1909.
- Burch AY, Zeisler V, Yokota K, Schreiber L, Lindow SE. 2014. The hygroscopic biosurfactant syringafactin produced by Pseudomonas syringae enhances fitness on leaf surfaces during fluctuating humidity. Environ Microbiol. 16(7):2086–2098.
- Busby DJ, Copley RC, Hueso JA, Readshaw SA, Rivera A. 2000. SB-253514 and analogues: novel inhibitors of lipoprotein associated phospholipase A2 produced by Pseudomonas fluorescens DSM 11579. II. Physico-chemical properties and structure elucidation. J Antibiot. 53(7):670–676.
- Caballo-Ponce E, Meng X, Uzelac G, Halliday N, Cámara M, Licastro D, Passos da Silva D, Ramos C, Venturi V. 2018. Quorum sensing in Pseudomonas savastanoi pv. savastanoi and Erwinia toletana: role in virulence and interspecies interactions in the olive knot. Appl Environ Microbiol. 84:e00950–18.
- Carrión VJ, Perez-Jaramillo J, Cordovez V, Tracanna V, de Hollander M, Ruiz-Buck D, Mendes LW, van Ijcken WFJ, Gomez-Exposito R, Elsayed SS, et al. 2019. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 366(6465):606–612.
- Catara V. 2007. Pseudomonas corrugata: plant pathogen and/or biological resource? Mol Plant Pathol. 8(3):233–244.
- Chan KG, Atkinson S, Mathee K, Sam CK, Chhabra S, Cámara M, Koh CL, Williams P. 2011. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 11:51.
- Coraiola M, Paletti R, Fiore A, Fogliano V, Serra MD. 2008. Fuscopeptins, antimicrobial lipodepsipeptides from Pseudomonas fuscovaginae, are channel forming peptides active on biological and model membranes. J Pept Sci. 14(4):496–502.
- Cornelis P. 2010. Iron uptake and metabolism in pseudomonads. Appl Microbiol Biotechnol. 86(6):1637–1645.
- Dillon MM, Thakur S, Almeida RND, Wang PW, Weir BS, Guttman DS. 2019. Recombination of ecologically and evolutionarily significant loci maintains genetic cohesion in the Pseudomonas syringae species complex. Genome Biol. 20(1):3.
- Dudnik A, Dudler R. 2013. High-quality draft genome sequence of Pseudomonas syringae pv. syringae strain SM, isolated from wheat. Genome Announc. 1:e00610.
- Emanuele MC, Scaloni A, Lavermicocca P, Jacobellis NS, Camoni L, Di Giorgio D, Pucci P, Paci M, Segre A, Ballio A. 1998. Corceptins, new bioactive lipodepsipeptides from cultures of Pseudomonas corrugata. FEBS Lett. 433(3):317–320.
- Feigin AM, Schagina LV, Takemoto JY, Teeter JH, Brand JG. 1997. The effect of sterols on the sensitivity of membranes to the channel-forming antifungal antibiotic, syringomycin E. Biochim Biophys Acta. 1324(1):102–110.
- Fiore A, Mannina L, Sobolev AP, Salzano AM, Scaloni A, Grgurina I, Fullone MR, Gallo M, Swasey C, Fogliano V, et al. 2008. Bioactive lipopeptides of ice-nucleating snow bacterium Pseudomonas syringae strain 31R1. FEMS Microbiol Lett. 286(2):158–165.
- Flamand MC, Pelsser S, Ewbank E, Maraite H. 1996. Production of syringotoxin and other bioactive peptides by Pseudomonas fuscovaginae. Physiol Mol Plant Pathol. 48(4):217–231.
- Fuchs J. 2000. The laboratory medium used to grow biocontrol Pseudomonas sp. Pf153 influences its subsequent ability to protect cucumber from black root rot. Soil Biol Biochem. 32(3):421–424.
- Fukuchi N, Isogai A, Nakayama J, Takayama S, Yamashita S, Suyama K, Takemoto JY, Suzuki A. 1992. Structure and stereochemistry of three phytotoxins, syringomycin, syringotoxin and syringostatin, produced by Pseudomonas syringae pv. syringae. J Chem Soc Perkin Trans. 1(9):1149–1157.
- Gao SS, Naowarojna N, Cheng R, Liu X, Liu P. 2018. Recent examples of α-ketoglutarate-dependent mononuclear non-haem iron enzymes in natural product biosyntheses. Nat Prod Rep. 35(8):792–837.
- Garrido-Sanz D, Meier-Kolthoff JP, Göker M, Martín M, Rivilla R, Redondo-Nieto M. 2016. Genomic and genetic diversity within the Pseudomonas fluorescens Complex. PLoS One. 11(2):e0150183.
- Geudens N, Martins JC. 2018. Cyclic lipodepsipeptides from Pseudomonas spp. – biological swiss-army knives. Front Microbiol. 9:1867.
- Gislason AS, de Kievit TR. 2020. Friend or foe? Exploring the fine line between Pseudomonas brassicacearum and phytopathogens. J Med Microbiol. 69(3):347–360.
- González AJ, Cleenwerck I, De Vos P, Fernández-Sanz AM. 2013. Pseudomonas asturiensis sp. nov., isolated from soybean and weeds. Syst Appl Microbiol. 36(5):320–324.
- Götze S, Arp J, Lackner G, Zhang S, Kries H, Klapper M, García-Altares M, Willing K, Günther M, Stallforth P. 2019. Structure elucidation of the syringafactin lipopeptides provides insight in the evolution of nonribosomal peptide synthetases. Chem Sci. 10(48):10979–10990.
- Götze S, Stallforth P. 2020. Structure, properties, and biological functions of nonribosomal lipopeptides from pseudomonads. Nat Prod Rep. 37(1):29–54.
- Greene NP, Kaplan E, Crow A, Koronakis V. 2018. Antibiotic resistance mediated by the MacB ABC transporter family: a structural and functional perspective. Front Microbiol. 9:2318.
- Grgurina I, Bensaci M, Pocsfalvi G, Mannina L, Cruciani O, Fiore A, Fogliano V, Sorensen KN, Takemoto JY. 2005. Novel cyclic lipodepsipeptide from Pseudomonas syringae pv. lachrymans strain 508 and syringopeptin antimicrobial activities. Antimicrob Agents Chemother. 49(12):5037–5045.
- Grgurina I, Gross DC, Iacobellis NS, Lavermicocca p, Takemoto JY, Benincasa M. 1996. Phytotoxin production by Pseudomonas syringae pv. syringae: syringopeptin production by syr mutants defective in biosynthesis or secretion of syringomycin. FEMS Microbiol Lett. 138(1):35–39.
- Grgurina I, Mariotti F. 1999. Biosynthetic origin of syringomycin and syringopeptin 22, toxic secondary metabolites of the phytopathogenic bacterium Pseudomonas syringae pv. syringae. FEBS Lett. 462(1–2):151–154.
- Grgurina I, Mariotti F, Fogliano V, Gallo M, Scaloni A, Iacobellis NS, Lo Cantore P, Mannina L, Axel Castelli V, van Greco ML, et al. 2002. A new syringopeptin produced by bean strains of Pseudomonas syringae pv. syringae. Biochim Biophys Acta. 1597(1):81–89.
- Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep. 26(11):1408–1446.
- Helmann TC, Deutschbauer AM, Lindow SE. 2019. Genome-wide identification of Pseudomonas syringae genes required for fitness during colonization of the leaf surface and apoplast. Proc Natl Acad Sci USA. 116(38):18900–18910.
- Hennessy RC, Phippen CBW, Nielsen KF, Olsson S, Stougaard P. 2017. Biosynthesis of the antimicrobial cyclic lipopeptides nunamycin and nunapeptin by Pseudomonas fluorescens strain In5 is regulated by the LuxR-type transcriptional regulator NunF. MicrobiologyOpen. 6(6):e00516.
- Hesse C, Schulz F, Bull CT, Shaffer BT, Yan Q, Shapiro N, Hassan KA, Varghese N, Elbourne LDH, Paulsen IT, et al. 2018. Genome-based evolutionary history of Pseudomonas spp. Environ Microbiol. 20(6):2142–2159.
- Höfte M, Vos DP. 2007. Plant pathogenic Pseudomonas species. In: Gnanamanickam SS, editor. Plant-associated bacteria. Dordrecht, Netherlands: Springer; p. 507–533.
- Hosni T, Moretti C, Devescovi G, Suarez-Moreno ZR, Fatmi MB, Guarnaccia C, Pongor S, Onofri A, Buonaurio R, Venturi V. 2011. Sharing of quorum-sensing signals and role of interspecies communities in a bacterial plant disease. ISME J. 5(12):1857–1870.
- Hu FP, Young JM, Fletcher MJ. 1998. Preliminary description of biocidal (syringomycin) activity in fluorescent plant pathogenic Pseudomonas species. J Appl Microbiol. 85(2):365–371.
- Huang CJ, Pauwelyn E, Ongena M, Debois D, Leclère V, Jacques P, Bleyaert P, Höfte M. 2015. Characterization of cichopeptins, new phytotoxic cyclic lipodepsipeptides produced by Pseudomonas cichorii SF1-54 and their role in bacterial midrib rot disease of lettuce. Mol Plant Microbe Interact. 28(9):1009–1022.
- Hutchison ML, Gross DC. 1997. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol Plant Microbe Interact. 10(3):347–354.
- Isogai A, Fukuchi N, Yamashita S, Suyama K, Suzuki A. 1990. Structures of syringostatins A and B, novel phytotoxins produced by pv. isolated from lilac blights. Tetrahedron Lett. 31(5):695–698.
- Isogai A, Iguchi H, Nakayama J, Kusai A, Takemoto JY, Suzuki A. 1995. Structural analysis of new syringopeptins by tandem mass spectrometry. Biosci Biotechnol Biochem. 59(7):1374–1376.
- Jafra S, Przysowa J, Czajkowski R, Michta A, Garbeva P, Van der Wolf JM. 2006. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can J Microbiol. 52(10):1006–1015.
- Jaremko MJ, Davis TD, Corpuz JC, Burkart MD. 2020. Type II non-ribosomal peptide synthetase proteins: structure, mechanism, and protein–protein interactions. Nat Prod Rep. 37(3):355–379.
- Johnston CW, Skinnider MA, Wyatt MA, Li X, Ranieri MRM, Yang L, Zechel DL, Ma B, Magarvey NA. 2015. An automated genomes-to-natural products platform (GNP) for the discovery of modular natural products. Nat Commun. 6:8421.
- Johnston CW, Zvanych R, Khyzha N, Magarvey NA. 2013. Nonribosomal assembly of natural lipocyclocarbamate lipoprotein-associated phospholipase inhibitors. Chembiochem. 14(4):431–435.
- Julmanop C, Takano Y, Takemoto JY, Miyakawa T. 1993. Protection by sterols against the cytotoxicity of syringomycin in the yeast Saccharomyces cerevisiae. J Gen Microbiol. 139(10):2323–2327.
- Kang H, Gross DC. 2005. Characterization of a resistance-nodulation-cell division transporter system associated with the syr-syp genomic island of Pseudomonas syringae pv. syringae. Appl Environ Microbiol. 71(9):5056–5065.
- Karasov TL, Almario J, Friedemann C, Ding W, Giolai M, Heavens D, Kersten S, Lundberg DS, Neumann M, Regalado J, et al. 2018. Arabidopsis thaliana and Pseudomonas pathogens exhibit stable associations over evolutionary timescales. Cell Host Microbe. 24(1):168–179.e4.
- Kitten T, Kinscherf TG, McEvoy JL, Willis DK. 1998. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol. 28(5):917–929.
- Komatsu H, Shirakawa T, Uchiyama T, Hoshino T. 2019. Chemical structure of cichorinotoxin, a cyclic lipodepsipeptide that is produced by Pseudomonas cichorii and causes varnish spots on lettuce. Beilstein J Org Chem. 15:299–309.
- Kong J, Jiang H, Li B, Zhao W, Li Z, Zhu S. 2016. Complete genome sequence of Pseudomonas syringae pv. lapsa strain ATCC 10859, isolated from infected wheat. Genome Announc. 4(2):e00024–16.
- Lam BS, Strobel GA, Harrison LA, Lam ST. 1987. Transposon mutagenesis and tagging of fluorescent Pseudomonas: antimycotic production is necessary for control of Dutch elm disease. Proc Natl Acad Sci USA. 84(18):6447–6451.
- Lavermicocca P, Iacobellis NS, Simmaco M, Graniti A. 1997. Biological properties and spectrum of activity of Pseudomonas syringae pv. syringae toxins. Physiol Mol Plant Pathol. 50(2):129–140.
- Le CN, Kruijt M, Raaijmakers JM. 2012. Involvement of phenazines and lipopeptides in interactions between Pseudomonas species and Sclerotium rolfsii, causal agent of stem rot disease on groundnut. J Appl Microbiol. 112(2):390–403.
- Licciardello G, Bertani I, Steindler L, Bella P, Venturi V, Catara V. 2007. Pseudomonas corrugata contains a conserved N-acyl homoserine lactone quorum sensing system; its role in tomato pathogenicity and tobacco hypersensitivity response. FEMS Microbiol Ecol. 61(2):222–234.
- Licciardello G, Bertani I, Steindler L, Bella P, Venturi V, Catara V. 2009. The transcriptional activator rfiA is quorum-sensing regulated by cotranscription with the luxI homolog pcoI and is essential for plant virulence in Pseudomonas corrugata. Mol Plant Microbe Interact. 22(12):1514–1522.
- Licciardello G, Caruso A, Bella P, Gheleri R, Strano CP, Anzalone A, Trantas EA, Sarris PF, Almeida NF, Catara V. 2018. The LuxR regulators PcoR and RfiA co-regulate antimicrobial peptide and alginate production in Pseudomonas corrugata. Front Microbiol. 9:521.
- Licciardello G, Strano CP, Bertani I, Bella P, Fiore A, Fogliano V, Venturi V, Catara V. 2012. N-acyl-homoserine-lactone quorum sensing in tomato phytopathogenic Pseudomonas spp. is involved in the regulation of lipodepsipeptide production. J Biotechnol. 159(4):274–282.
- Lindeberg M, Myers CR, Collmer A, Schneider DJ. 2008. Roadmap to new virulence determinants in Pseudomonas syringae: insights from comparative genomics and genome organization. Mol Plant Microbe Interact. 21(6):685–700.
- Lindow SE. 1987. Competitive exclusion of epiphytic bacteria by ice Pseudomonas syringae mutants. Appl Environ Microbiol. 53(10):2520–2527.
- Lu SE, Scholz-Schroeder BK, Gross DC. 2002. Characterization of the salA, syrF, and syrG regulatory genes located at the right border of the syringomycin gene cluster of Pseudomonas syringae pv. syringae. Mol Plant Microbe Interact. 15(1):43–53.
- Lu SE, Wang N, Wang J, Chen ZJ, Gross DC. 2005. Oligonucleotide microarray analysis of the SalA regulon controlling phytotoxin production by Pseudomonas syringae pv. syringae. Mol Plant Microbe Interact. 18(4):324–333.
- Mattiuzzo M, Bertani I, Ferluga S, Cabrio L, Bigirimana J, Guarnaccia C, Pongor S, Maraite H, Venturi V. 2011. The plant pathogen Pseudomonas fuscovaginae contains two conserved quorum sensing systems involved in virulence and negatively regulated by RsaL and the novel regulator RsaM. Environ Microbiol. 13(1):145–162.
- McClure RA, Goering AW, Ju KS, Baccile JA, Schroeder FC, Metcalf WW, Thomson RJ, Kelleher NL. 2016. Elucidating the rimosamide-detoxin natural product families and their biosynthesis using metabolite/gene cluster correlations. ACS Chem Biol. 11(12):3452–3460.
- Melnyk RA, Hossain SS, Haney CH. 2019. Convergent gain and loss of genomic islands drive lifestyle changes in plant-associated Pseudomonas. ISME J. 13(6):1575–1588.
- Michelsen CF, Jensen H, Venditto VJ, Hennessy RC, Stougaard P. 2015. Bioactivities by a crude extract from the greenlandic Pseudomonas sp. In5 involves the nonribosomal peptides, nunamycin and nunapeptin. PeerJ. 3:e1476.
- Michelsen CF, Watrous J, Glaring MA, Kersten R, Koyama N, Dorrestein PC, Stougaard P. 2015. Nonribosomal peptides, key biocontrol components for Pseudomonas fluorescens In5, isolated from a greenlandic suppressive soil. MBio. 6(2):e00079.
- Mo YY, Gross DC. 1991. Plant signal molecules activate the syrB gene, which is required for syringomycin production by Pseudomonas syringae pv. syringae. J Bacteriol. 173(18):5784–5792.
- Mukherji R, Zhang S, Chowdhury S, Stallforth P. 2020. Chimeric LuxR transcription factors rewire natural product regulation. Angew Chem. 59:6192–6195.
- Mulet M, Lalucat J, García-Valdés E. 2010. DNA sequence-based analysis of the Pseudomonas species. Environ Microbiol. 12(6):1513–1530.
- Nandi M, Berry C, Brassinga AKC, Belmonte MF, Fernando WGD, Loewen PC, Kievit TR. 2016. Pseudomonas brassicacearum strain DF41 kills Caenorhabditis elegans through biofilm-dependent and biofilm-independent mechanisms. Appl Environ Microbiol. 82(23):6889–6898.
- Nogales J, Vargas P, Farias GA, Olmedilla A, Sanjuán J, Gallegos MT. 2015. FleQ coordinates flagellum-dependent and -independent motilities in Pseudomonas syringae pv. tomato DC3000. Appl Environ Microbiol. 81(21):7533–7545.
- Pauwelyn E. 2012. Epidemiology and pathogenicity mechanisms of Pseudomonas cichorii, the causal agent of midrib rot in greenhouse-grown butterhead lettuce (Lactuca sativa L.). Ghent, Belgium: Ghent University. Faculty of Bioscience Engineering.
- Pauwelyn E, Huang CJ, Ongena M, Leclère V, Jacques P, Bleyaert P, Budzikiewicz H, Schäfer M, Höfte M. 2013. New linear lipopeptides produced by Pseudomonas cichorii SF1-54 are involved in virulence, swarming motility, and biofilm formation. Mol Plant Microbe Interact. 26(5):585–598.
- Quigley NB, Gross DC. 1994. Syringomycin production among strains of Pseudomonas syringae pv. syringae: conservation of the syrB and syrD genes and activation of phytotoxin production by plant signal molecules. Mol Plant Microbe Interact. 7(1):78–90.
- Quigley NB, Mo YY, Gross DC. 1993. SyrD is required for syringomycin production by Pseudomonas syringae pathovar syringae and is related to a family of ATP-binding secretion proteins. Mol Microbiol. 9(4):787–801.
- Quiñones B, Dulla G, Lindow SE. 2005. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant Microbe Interact. 18(7):682–693.
- Ramkumar G, Lee SW, Weon HY, Kim BY, Lee YH. 2015. First report on the whole genome sequence of Pseudomonas cichorii strain JBC1 and comparison with other Pseudomonas species. Plant Pathol. 64(1):63–70.
- Ravindran A, Jalan N, Yuan JS, Wang N, Gross DC. 2015. Comparative genomics of Pseudomonas syringae pv. syringae strains B301D and HS191 and insights into intrapathovar traits associated with plant pathogenesis. MicrobiologyOpen. 4(4):553–573.
- Reder-Christ K, Schmidt Y, Dörr M, Sahl HG, Josten M, Raaijmakers JM, Gross H, Bendas G. 2012. Model membrane studies for characterization of different antibiotic activities of lipopeptides from Pseudomonas. Biochim Biophys Acta. 1818(3):566–573.
- Reitz ZL, Hardy CD, Suk J, Bouvet J, Butler A. 2019. Genomic analysis of siderophore β-hydroxylases reveals divergent stereocontrol and expands the condensation domain family. Proc Natl Acad Sci USA. 116(40):19805–19814.
- Ruinelli M, Blom J, Pothier JF. 2017. Complete genome sequence of Pseudomonas viridiflava CFBP 1590, isolated from diseased cherry in France. Genome Announc. 5(30):17.
- Scaloni A, Bachmann RC, Takemoto JY, Barra D, Simmaco M, Ballio A. 1994. Stereochemical structure of syringomycin, a phytotoxic metabolite of Pseudomonas syringae pv. syringae. Nat Prod Lett. 4(3):159–164.
- Scaloni A, Camoni L, Giorgio D, di Scortichini M, Cozzolino R, Ballio A. 1997. A new syringopeptin produced by a Pseudomonas syringae pv. syringae strain isolated from diseased twigs of laurel. Physiol Mol Plant Pathol. 51(4):259–264.
- Scaloni A, Dalla Serra M, Amodeo P, Mannina L, Vitale RM, Segre AL, Cruciani O, Lodovichetti F, Greco ML, Fiore A, et al. 2004. Structure, conformation and biological activity of a novel lipodepsipeptide from Pseudomonas corrugata: cormycin A. Biochem J. 384(Pt 1):25–36.
- Schmidt Y, Voort M, van der Crüsemann M, Piel J, Josten M, Sahl HG, Miess H, Raaijmakers JM, Gross H. 2014. Biosynthetic origin of the antibiotic cyclocarbamate brabantamide A (SB-253514) in plant-associated Pseudomonas. Chembiochem. 15(2):259–266.
- Segre A, Bachmann RC, Ballio A, Bossa F, Grgurina I, Iacobellis NS, Marino G, Pucci P, Simmaco M, Takemoto JY. 1989. The structure of syringomycins A1, E and G. FEBS Lett. 255(1):27–31.
- Silby MW, Winstanley C, Godfrey SAC, Levy SB, Jackson RW. 2011. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev. 35(4):652–680.
- Smith JA, Métraux J-P. 1991. Pseudomonas syringae pv. syringae induces systemic resistance to Pyricularia oryzae in rice. Physiol Mol Plant Pathol. 39(6):451–461.
- Strano CP, Bella P, Licciardello G, Fiore A, Lo Piero AR, Fogliano V, Venturi V, Catara V. 2015. Pseudomonas corrugata crpCDE is part of the cyclic lipopeptide corpeptin biosynthetic gene cluster and is involved in bacterial virulence in tomato and in hypersensitive response in Nicotiana benthamiana. Mol Plant Pathol. 16(5):495–506.
- Subramoni S, Gonzalez JF, Johnson A, Péchy-Tarr M, Rochat L, Paulsen I, Loper JE, Keel C, Venturi V. 2011. Bacterial subfamily of LuxR regulators that respond to plant compounds. Appl Environ Microbiol. 77(13):4579–4588.
- Subramoni S, Venturi V. 2009. PpoR is a conserved unpaired LuxR solo of Pseudomonas putida which binds N-acyl homoserine lactones. BMC Microbiol. 9:125.
- Tark M, Tover A, Koorits L, Tegova R, Kivisaar M. 2008. Dual role of NER in mutagenesis in Pseudomonas putida. DNA Repair (Amst). 7(1):20–30.
- Thirkettle J, Alvarez E, Boyd H, Brown M, Diez E, Hueso J, Elson S, Fulston M, Gershater C, Morata ML, et al. 2000. SB-253514 and analogues; novel inhibitors of lipoprotein-associated phospholipase A2 produced by Pseudomonas fluorescens DSM 11579. I. Fermentation of producing strain, isolation and biological activity. J Antibiot. 53(7):664–669.
- Thomas CM, Hothersall J, Willis CL, Simpson TJ. 2010. Resistance to and synthesis of the antibiotic mupirocin. Nat Rev Microbiol. 8(4):281–289.
- Tohya M, Watanabe S, Tada T, Tin HH, Kirikae T. 2020. Genome analysis-based reclassification of Pseudomonas fuscovaginae and Pseudomonas shirazica as later heterotypic synonyms of Pseudomonas asplenii and Pseudomonas asiatica, respectively. Int J Syst Evol Micr. 70:3547–3552..
- Van Der Voort M, Meijer HJG, Schmidt Y, Watrous J, Dekkers E, Mendes R, Dorrestein PC, Gross H, Raaijmakers JM. 2015. Genome mining and metabolic profiling of the rhizosphere bacterium Pseudomonas sp. SH-C52 for antimicrobial compounds. Front Microbiol. 6:693.
- Vassilev V, Lavermicocca P, Digiorgio D, Iacobellis N. 1997. Syringomycins and syringopeptins in the basal glume rot of wheat incited by Pseudomonas syringae pv. atrofaciens. In: Rudolph K, Burr TJ, Mansfield JW, Stead D, Vivian A, von Kietzell J, editors. Pseudomonas syringae pathovars and related pathogens. Dordrecht, Netherlands: Springer Netherlands; p. 210–214.
- Vaughn VL, Gross DC. 2016. Characterization of salA, syrF, and syrG genes and attendant regulatory networks involved in plant pathogenesis by Pseudomonas syringae pv. syringae B728a. PLoS One. 11(3):e0150234.
- Visca P, Imperi F, Lamont IL. 2007. Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol. 15(1):22–30.
- Walsh CT, O’Brien RV, Khosla C. 2013. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew Chem Int Ed Engl. 52(28):7098–7124.
- Wang B, Dukarevich M, Sun EI, Yen MR, Saier MH. 2009. Membrane porters of ATP-binding cassette transport systems are polyphyletic. J Membr Biol. 231(1):1–10.
- Wang N, Lu SE, Records AR, Gross DC. 2006. Characterization of the transcriptional activators SalA and SyrF, which are required for syringomycin and syringopeptin production by Pseudomonas syringae pv. syringae. J Bacteriol. 188(9):3290–3298.
- Wang N, Lu SE, Yang Q, Sze SH, Gross DC. 2006. Identification of the syr-syp box in the promoter regions of genes dedicated to syringomycin and syringopeptin production by Pseudomonas syringae pv. syringae B301D. J Bacteriol. 188(1):160–168.
- Watrous J, Roach P, Alexandrov T, Heath BS, Yang JY, Kersten RD, van der Voort M, Pogliano K, Gross H, Raaijmakers JM, et al. 2012. Mass spectral molecular networking of living microbial colonies. Proc Natl Acad Sci USA. 109(26):E1743–E1752.
- Weeraratne N, Stodart BJ, Venturi V, Höfte M, Hua GKH, Ongena M, Savocchia S, Steel C, Ash GJ. 2019. Syringopeptin contributes to the virulence of Pseudomonas fuscovaginae, based on sypA biosynthesis mutant analysis. Phytopathology. 110:780–789.
- Weller DM. 2007. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology. 97(2):250–256.
- Xin XF, Kvitko B, He SY. 2018. Pseudomonas syringae: what it takes to be a pathogen. Nat Rev Microbiol. 16(5):316–328.
- Yang M, Mavrodi DV, Mavrodi OV, Thomashow LS, Weller DM. 2020. Exploring the pathogenicity of Pseudomonas brassicacearum Q8r1-96 and other strains of the Pseudomonas fluorescens complex on tomato. Plant Dis. 104(4):1026–1031.
- Yu SM, Lee YH. 2012. First report of Pseudomonas cichorii associated with leaf spot on soybean in South Korea. Plant Dis. 96(1):142–142.
- Zakataeva NP, Aleshin VV, Tokmakova IL, Troshin PV, Livshits VA. 1999. The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett. 452(3):228–232.
- Zakataeva NP, Kutukova EA, Gronskiĭ SV, Troshin PV, Livshits VA, Aleshin VV. 2006. Export of metabolites by the proteins of the DMT and RhtB families and its possible role in intercellular communication. Mikrobiologiia. 75(4):509–520.
- Zhang JJ, Tang X, Huan T, Ross AC, Moore BS. 2020. Pass-back chain extension expands multimodular assembly line biosynthesis. Nat Chem Biol. 16(1):42–49.
- Zhao H, Liu YP, Zhang LQ. 2019. In silico and genetic analyses of cyclic lipopeptide synthetic gene clusters in Pseudomonas sp. 11K1. Front Microbiol. 10:544.
