Abstract
With the important role of the gut microbiome in health and disease, it is crucial to understand key factors that establish the microbial community, including gut colonization during infancy. It has been suggested that the first bacterial exposure is via a placental microbiome. However, despite many publications, the robustness of the evidence for the placental microbiome and transfer of bacteria from the placenta to the infant gut is unclear and hence the concept disputed. Therefore, we conducted a systematic review of the evidence for the role of the placental, amniotic fluid and cord blood microbiome in healthy mothers in the colonization of the infant gut. Most of the papers which were fully assessed considered placental tissue, but some studied amniotic fluid or cord blood. Great variability in methodology was observed especially regarding sample storage conditions, DNA/RNA extraction, and microbiome characterization. No study clearly considered transfer of the normal placental microbiome to the infant gut. Moreover, some studies in the review and others published subsequently reported little evidence for a placental microbiome in comparison to negative controls. In conclusion, current data are limited and provide no conclusive evidence that there is a normal placental microbiome which has any role in colonization of infant gut.
Keywords:
Introduction
The conventional paradigm that the placenta is a sterile organ has been challenged by a number of findings indicating that it harbours a unique microbiome (Aagaard et al. Citation2012; Stout et al. Citation2013; Aagaard et al. Citation2014; Vinturache et al. Citation2016). Recent studies have suggested that these bacteria could influence reproductive function and may affect pregnancy outcomes (Lauder et al. Citation2016). Indeed, bacterial infection is a frequent cause of neonatal sepsis and preterm birth (Goldenberg et al. Citation2000).
Some published studies have presented evidence for the presence of microbes in normal healthy placenta, amniotic fluid, cord blood and meconium (Jimenez et al. Citation2005; Collado et al. Citation2016; Parnell et al. Citation2017). Several hypotheses have been suggested for potential transfer of the bacteria from mother to infant in utero (Doyle et al. Citation2017). Ascension from the vaginal tract might be the most evident route of entry of the placental bacteria. However, alternative routes have also been suggested, including bacteria derived from the maternal intestine and oral cavity by translocation through the choriodecidual barriers as well as inclusion of a uterine commensal microbiome into the developing placenta. In utero colonization of the fetus/infant could be hypothesized to affect immune function and metabolic control after birth and potentially throughout life (Neu Citation2016). Another intriguing issue is whether commensal microbes are necessary for healthy placental development and function, and if so, what the underlying mechanisms are for these effects (Nuriel-Ohayon et al. Citation2016). Consequently, placental bacteria may influence infant colonization and possibly long-term health outcomes. It is already known that pathogenic microorganisms colonizing the placenta can induce a pro-inflammatory state, which may lead to abortion, preterm birth or potentially an increased risk of chronic disease later in life. In a recent review of the mechanisms for the increased risk of adverse pregnancy outcomes in those with oral periodontal disease, oral–utero transmission was considered a strong possible mechanism (Figuero et al. Citation2020).
In the early 1980s, Kovalovszki et al. (Citation1982) were the first to describe the presence of aerobic bacteria in 16% of tested placentas using a culture-dependent approach without any histological evidence of chorioamnionitis. Since then several additional reports described the presence of bacteria in the human placenta (Nuriel-Ohayon et al. Citation2016). Two studies using culture-independent whole-genome shotgun sequencing of genomic DNA isolated from human placentas support the existence of a placental microbiome in healthy women. First, Aagaard et al. (Citation2012) identified a low abundance, but potentially metabolically rich microbiome, including a number of commensal species in both term and preterm placentas. Then Stout et al. (Citation2013) provided morphologic evidence that both gram-positive and gram-negative bacteria of diverse morphologies were present in a third of all placentas from preterm and term pregnancies and Doyle et al. (Citation2014) using a 16S rDNA pyrosequencing approach identified a distinct microbial community in placental membranes from that reported by Aagaard et al. (Citation2012). However more recent studies have cast doubt on the existence of a normal placental microbiome suggesting that contamination around delivery, or during sample processing and analysis may be a key factor in the detection of bacteria in placental samples (Lauder et al. Citation2016; de Goffau et al. Citation2019).
There are limited reports providing clear evidence on where microbes might reside in the placenta. Stout et al. (Citation2013) reported that placental microbes were intracellular, within the extravillous trophoblast (EVT) cells in the maternal decidua, suggesting that the endometrial epithelium of the non-pregnant uterus might harbour occult microbes which could become incorporated into the basal plate at the time of placental implantation. However, the impact of bacterial presence in the placenta and amniotic fluid needs to be determined, good or bad. For example, does the type of bacteria, the immune response they elicit or the ability of bacteria to reach critical numbers create the difference between normal and abnormal pregnancy outcomes? Overgrowth of one or a few strains may be harmful, while a diverse community of low numbers of cells may not interfere with pregnancy (Stout et al. Citation2013). Another important unanswered question is whether there is sufficient exposure of the fetus in utero to affect the colonization of the infant gut. This could be by direct colonization of the gut mucosa or by influencing later interaction with mucosal cells or immune cells.
The aim of the present study was therefore to systematically review and evaluate published studies related to the placental, amniotic fluid and/or cord blood microbiome in healthy women. We particularly focussed on finding evidence for factors influencing the colonization of the infant gut through placental transfer. This is the first systematic review to look at this. We assessed the quality of studies by considering potential contamination during sample collection and processing along with other key methodological considerations for microbiota characterization and clear detail on mode of delivery. Finally, we assessed how the “placental microbiome” may be important in normal full-term pregnancies in contrast to preterm infants and infants with low birthweight, or suffering from infection, neonatal sepsis and other complications.
Methods
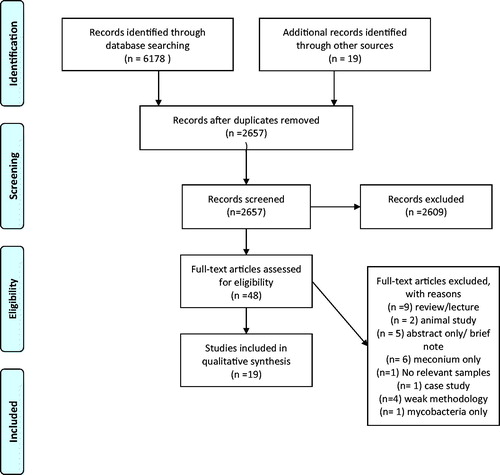
We conducted a systematic review to evaluate the evidence for bacterial transfer between mothers and infants via the placental route and if found, to assess the relationship between early colonization of the infant gut (prenatal and postnatal) and health outcomes (brain development, metabolic disease, immune system). Details of the literature review are available in PROSPERO register CRD42017039875 and a flow chart of the different steps is available in .
The following criteria were applied for inclusion, exclusion and critique of manuscripts:
Detection of bacteria in mother needed to be clearly described (amniotic fluid or placental tissues) and the timing of the sampling mentioned.
Detection of bacteria in cord blood needed to adhere to the same standards as for amniotic fluid and placenta samples. In studies where it was measured, infant faeces and sample collection timing including the frequency of sampling needed to be clearly described (close to birth, e.g. first stool, meconium or first sample after).
Number of study mothers and infants should be outlined.
DNA extraction kit should be named, sample storage conditions mentioned and, ideally, time between storage and DNA extraction outlined.
Information of the sequencing or PCR method including the PCR kit, targeted genes, primers sequences and sequencing details should be provided where relevant.
Data on quality criteria and collection of samples needed to be available: including methods used to avoid contamination.
Data around the “health-state” of the mother should be provided (abnormal/normal health and/or births, previous infections such as periodontitis and gut diseases, pre-eclampsia, vaginal infections, metabolic syndrome/GDM/obesity/weight data).
Mode of delivery (caesarean or vaginal) must be stated.
Antibiotic use and other relevant medications such as hypertension related medications should be declared.
Confirmation of maternal samples: e.g. placental, vaginal, amniotic fluid, stool.
Observational studies and controlled trials were included, reviews were not.
Searches were carried out using PUBMED, OVID, LILACS and PROQUEST. Further details regarding search strings as well as inclusion and exclusion criteria are available in PROSPERO register CRD42017039875. Searches were carried out from the beginning of records until June 1 2018. Reference lists of relevant papers, including reviews, were searched for additional studies for consideration.
Titles and abstracts of the studies identified by the search were screened by four independent assessors (CAE, AG, RR, CvLB) and discrepancies between decisions were presented and discussed with the whole group for final agreement. Full text reports of all included studies were assessed, data on sample collection (method, timing, contamination risk assessment, frequency, number of samples), long-term persistence of bacteria in infant tissues and faecal samples, sample handling after collection (DNA extraction kit name, time between storage and DNA extractions, storage conditions), study sample size (number of samples and study participants), sample analysis (main identification methods and statistics), maternal confounders (mode of delivery, birth conditions, maternal health profile) and other confounders (antibiotic use, other medications, mode of delivery) were collected.
Based on the information collected a quasi-risk of bias analysis was conducted as the traditional risk of bias analysis was not feasible for a systematic review of this kind. Paper quality was scored as high, medium, or low with all low graded papers rejected. The experts (CAE, AG, RR, CvLB) then discussed a final list of papers for inclusion into the systematic review results (Supplementary Table S1).
Results
Of 2657 non-duplicate papers found in the search on June 1 2018, 48 were assessed for eligibility at the full text screening stage (). Finally, 19 papers were included in the qualitative synthesis of which eight concentrated on normal term pregnancies (). The other eleven papers compared premature delivery, low birth weight or pre-eclampsia to normal term birth ().
Table 1. Summary of studies looking at normal pregnancies.
Table 2. Summary of studies looking at abnormal pregnancy.
The synthesized publications included data on placental, amniotic and/or cord blood microbiomes with between two and 168 samples each. The approaches used to avoid contamination of the placenta during sample collection varied in the details presented which were sometimes vague, especially in studies that included vaginal births where risk of contamination is high ( and ). Some studies considered only elective caesarean delivery (Collado et al. Citation2016) and/or only the placental structures that were not exposed to external contamination in utero (Lauder et al. Citation2016). A range of methods were used in the studies to identify bacteria including various staining approaches, PCR and metagenomics tools ( and ). Storage conditions of samples varied from −20 to −80 °C and the storage time of the DNA sample was usually not reported. There is differential deterioration of DNA between species of bacteria over time, so the storage time before DNA extraction is important and should be the same for all samples in a study. Methods used to extract DNA/RNA varied and were not always reported in detail. Some studies used a bead beating step (Lim et al. Citation2018), others used lysozyme incubation, and different extraction kits were used ( and ). The use of different extraction kits will likely lead to different results in terms of amount and quality of DNA isolation (Hart et al. Citation2015). In addition, different variable (V) regions of the 16S rRNA gene were targeted, for example either V4 or V6. Only one study looked at V1–V9 (Parnell et al. Citation2017), but then concentrated on V4. This makes direct comparisons of populations of bacteria in the microbiome between studies difficult. Negative controls were used in a few recent studies (Lauder et al. Citation2016; Parnell et al. Citation2017; Rehbinder et al. Citation2018) but not in earlier studies.
Not all samples from normal term pregnancies contained bacteria (Lauder et al. Citation2016; Amarasekara et al. Citation2015), whereas a randomized controlled trial with probiotics by Rautava et al. (Citation2012) reported DNA from lactobacilli and bifidobacteria in all placentas studied. In other studies, the bacteria isolated in the placenta were identified to be of an oral type (Aagaard et al. Citation2014). Very few studies considered possible bacteria transfer to the infant (to meconium or infant faeces) and overall, there was no substantial evidence for or against the role of a placental microbiome in the colonization of the infant gut in meconium or infant faeces.
Normal pregnancies
Placenta and amniotic fluid
Satokari et al. (Citation2009; ) analysed placenta samples from vaginal and caesarean deliveries, all full term, for the presence of Bifidobacterium and Lactobacillus species. Bacterial DNA was extracted directly from placenta samples and resected placenta homogenized tissue samples were cultured under anaerobic conditions using a non-selective medium. Selected colonies were subjected to 16S rDNA sequencing for species identification. DNA from bifidobacteria and/or L rhamnosus were found in most but not all placenta () but bifidobacteria or lactobacilli were not found by cultivation and any bacterial species which were cultured from placenta samples were suspected to be due to contamination during delivery.
Collado et al. (Citation2016; ) collected samples from several maternal sites (placenta, amniotic fluid, colostrum, and faeces). They compared these with paired infant meconium and faeces. These mothers were part of a probiotic intervention study. Deliveries were full term and by elective caesarean and careful and sterile processing procedures were used to avoid contamination of the samples (). However, negative controls for DNA extraction were not reported. Mothers with conditions that might affect placental and fetal physiology (e.g. pre-eclampsia, intrauterine growth retardation, fetal anomalies, onset of labour, asphyxia) or contaminate the placenta (rupture of membranes, vaginal delivery, infection) were excluded from the study. Bacteria were cultured and the microbiome assessed by 16S rRNA gene pyrosequencing, quantitative PCR, and denaturing gradient gel electrophoresis. The authors reported a distinct microbiota in placenta and amniotic fluid samples that was of low diversity and richness dominated by Proteobacteria especially Enterobacteriaceae. Enterobacter and Escherichia/Shigella. Lower abundances of these were also found in colostrum, meconium and infant faeces. Propionibacterium was also detected in placenta and amniotic fluid as well as in meconium and infant faeces. Over half the family level bacterial phylotypes found in meconium were identified in amniotic fluid and placenta or just in amniotic fluid.
Parnell et al. (Citation2017; ) suggested a niche-specificity to the placental microbiota, and that placental microbiome studies should consider regional differences in colonization which may affect maternal, fetal, and/or neonatal health and physiology. This study included at term pregnancies from 57 women with both singleton and twin births. Genomic DNA sequencing of multiple variable regions of the bacterial 16S ribosomal gene explored the microbial profiles from the basal plate (BP), which is in direct contact with maternal uterine, endothelial, and immune cells; placental villi (PV), which are bathed in maternal blood; and fetal membranes (FM), which encapsulate the amniotic cavity. Sterile techniques were used in sampling the placenta to avoid contamination. Human DNA was removed and negative controls were run for DNA at all stages with real time PCR was used to confirm results. The authors looked at all V1–V9 variable regions, but finally concentrated on V4. They identified most common OTUs and then species. The abundance and diversity of microbes differed by sampling location in the placenta. There were higher copy numbers in the BP and PV than the FM which also had lower diversity. BP was dominated by Proteobacteria, FM by Firmicutes and PV had no consistent pattern. Ralstonia insidiosa from the Burkholderiales order, (which has also been found in breastmilk) was found in 76% of validated BP samples. Finally, microbial profiles were not altered by mode of delivery (Parnell et al. Citation2017).
The control group from a study (Tuominen et al. Citation2018; ), designed to investigate the impact of existing human papillomavirus (HPV) infection on the placental microbiota was considered in this review. The microbiome was characterized by 16S rRNA gene sequencing. A total of 19/39 placenta samples (49%) were included in the analysis after exclusion of specimens with a low number of reads followed by filtering the OTUs present in the negative controls. For HPV negative samples (control group), the most abundant phylum in the placenta was Firmicutes (58.3%) followed by Proteobacteria (21.1%), Actinobacteria (13.8%) and Bacteroidetes (6.5%). At family level, Staphylococcaceae (22.5%), Enterococacceae (15.6%), Veillonellaceae (8.8%), Corynebacteriaceae (6.3%) and Moraxellaceae (6.1%) were the predominant groups. At genus level, Staphylococcus (22.9%); unclassified Enterococcaceae genus (15.6%), Corynebacterium (6.3%), and Acinetobacter (6.0%) were the most abundant.
Even though the previous studies suggested the presence of a commensal microbiota in the placenta and amniotic fluid, other studies have questioned this hypothesis. Rehbinder et al. (Citation2018; ) investigated the presence of a microbiota in sterilely collected amniotic fluid in uncomplicated pregnancies at term in the Preventing Atopic Dermatitis and Allergies in children (PreventADALL) study cohort. Amniotic fluid was randomly sampled at caesarean delivery from 65 pregnancies at term. Ten elective (planned, without on-going labour) caesarean deliveries with intact amniotic membranes were selected, while 14 with prior rupture of membranes were included as controls. Amniotic fluid was analysed by culture-independent and culture-dependent techniques. The median concentration of prokaryotic DNA was low for the group with intact membranes, while the group with rupture of amniotic membranes had >10-fold higher levels. Also, bacteria were detected in 50% of the ruptured amniotic membranes samples by anaerobic culturing, while none of the intact membranes samples showed bacterial growth. Sanger sequencing of the ruptured amniotic membrane samples identified bacterial species that are commonly part of the vaginal microbiota and/or associated with intrauterine infections. The authors concluded that fetal development in uncomplicated pregnancies occurs in the absence of an amniotic fluid microbiota and that the offspring microbial colonization starts after uterine contractions and rupture of amniotic membrane.
Another recent study (Lim et al. Citation2018; ) concluded that amniotic fluid from healthy term pregnancies did not harbour a detectable microbial community. In this study, the virome and bacterial microbiota of amniotic fluid from 24 uncomplicated term pregnancies were characterized using next-generation sequencing methods. They did not identify a population of bacterial microbiota in the amniotic fluid from healthy term pregnancies that meaningfully differed in concentration or content from the sequences amplified from negative controls. Similarly, they found only limited evidence for viral presence using metagenomic sequencing of material that was subjected to preparation techniques optimized to recover DNA as well as RNA viruses, including DNA and RNA bacteriophages.
Lauder et al. (Citation2016; ) compared placental samples from 6 healthy deliveries to a matched set of contamination controls, as well as to oral and vaginal samples from the same women and quantified total 16S rRNA gene copies by quantitative PCR; equal volumes of the purified DNA of all samples were used in the assay. DNA was purified with two different kits. All placental and negative control samples were at the extreme low end of the range detectable by qPCR (a highly sensitive methodology). For both purification methods, the authors concluded that placental and control samples showed low and indistinguishable numbers of 16S rRNA gene copies. Thus, due to the low amounts of bacteria detected in placenta, some or all the bacterial DNA may come from contamination by dust or commercial reagents. These findings highlight the importance of negative controls which were generally not included in most other studies.
One study (Jimenez et al. Citation2015; ) isolated commensal bacteria from umbilical cord blood of 20 healthy neonates born by caesarean section. The blood samples were submitted to an enrichment step and then inoculated onto agar plates. Bacterial isolates were identified using PCR and 16S rDNA sequencing; they belonged to the genus Enterococcus, Streptococcus, Staphylococcus, or Propionibacterium.
Complicated pregnancies
To understand the potential existence and role of the placenta microbiome in normal pregnancy, it is useful to compare the data with that from abnormal pregnancies. A subset of the studies specifically addressed comparison between abnormal and a normal healthy control group.
Pre-eclampsia
Amarasekara et al. (Citation2015; ) confirmed the presence of bacteria (including Bacillus cereus, Listeria, Salmonella, Escherichia; Klebsiella pneumonia, Anoxybacillus, Variovorax, Prevotella, Porphyromonas, and Dialister). in 12.7% of placental tissues collected during caesarean delivery of 55 women with pre-eclampsia but not those of 55 matched controls using next generation sequencing (Illumina MiSeq). A small piece of placental tissue was immediately dissected from a cotyledon close to the insertion of the umbilical cord. Stringent aseptic measures were followed throughout the collection and analysis of the sample to prevent contamination.
Gestational diabetes
Bassols et al. (Citation2016) investigated the placental microbiome in women with gestational diabetes (). The study population consisted of 22 Caucasian women (11 with gestational diabetes mellitus (GDM) and 11 control non-GDM women with normal pregnancies). All infants were born at term. The placentas were collected after childbirth in the delivery room or operating room to ensure sterility and samples were collected from the inner surface of the placenta to avoid the placenta being contaminated by the vagina. The samples were then stored within 1 h at −80 °C until DNA and RNA extraction.
They found a distinct pattern of placental microbiota in GDM compared with controls. The diversity of placental microbiota was mainly related to four phyla: Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria in GDM. The proportion of Firmicutes was 7% higher and the proportion of Proteobacteria and Bacteroides were respectively reduced at 5 and 2% lower in GDM compared to levels in control women. A lower relative abundance of Acinetobacter was associated with increased glucose plasma levels, lower blood eosinophil count in the second and third trimester of pregnancy and lower placental expression of the anti-inflammatory cytokines IL10 and TIMP3. Furthermore, a lower relative abundance of Pseudomonadales was associated with lower blood neutrophil and lymphocyte counts in the second and third trimester of pregnancy and with lower placental expression of IL10, ITGAX, and MRC1MR (Bassols et al. Citation2016).
Preterm birth
DiGiulio et al. (Citation2008; ) reported microbial DNA in the amniotic cavity from women in preterm labour taking part in a retrospective cohort study (53 infants delivered at term and 113 preterm). Microbial genomic DNA was extracted from the amniotic fluid sample collected during amniocentesis and analysed by real time PCR. Microbial invasion of the amniotic cavity, defined by either a positive end-point PCR or culture of amniotic fluid, was found in only 15% (25/166) of patients. Seventeen bacterial species (belonging to five phyla) and 1 fungal species were identified. Six of the 17 bacteria taxa were detected by culture and PCR and others only by culture (4 taxa) or PCR only (7 taxa) (). The prevalence of a positive amniotic fluid was inversely related to gestational age at delivery.
Jones et al. (Citation2009; ) investigated the prevalence and diversity of bacterial species in placental membranes of mothers suffering intrauterine infection compared with healthy mothers. They isolated bacterial DNA from fetal membranes and placenta using 16S rDNA and PCR cloning and sequencing and identified particular species, namely U. parvum, U. urealyticum, M. hominis, Fusobacterium spp. and S. agalactiae using qPCR. Of 74 women recruited, 26 (11 caesarean) exhibited prolonged rupture of membranes and delivered preterm infants <32 weeks gestational age; 19 (all vaginal birth) delivered preterm infants <32 weeks due to spontaneous labour; 8 with indicated preterm delivery <32 weeks; and 21 delivered term infants (11 caesarean). Twenty two (30%) women had bacterial DNA detected in fetal membranes and placental tissue. Thirty-two (43%) women were positive for bacterial DNA by species specific rtPCR, but only 14 of these women were positive using both methods. 50% (5/10) of term vaginal deliveries were positive for bacterial DNA. However, little spread was observed through tissues and species diversity was restricted. Only low levels of bacteria were detected in term elective section or indicated preterm deliveries. Bacterial prevalence was significantly increased in samples from spontaneous preterm labour with intact membranes (89% versus 50% in term vaginal delivery) and prolonged rupture of membranes with caesarean section (55% versus 0% in term elective caesarean section). Moreover, bacterial spread and diversity was greater in the preterm groups versus term vaginal deliveries. A positive PCR result was associated with histological chorioamnionitis in preterm deliveries.
Aagaard et al. (Citation2014; ) investigated a population-based cohort of placental specimens collected under sterile conditions from 48 subjects with extensive clinical data for comparative 16S ribosomal DNA–based and whole-genome shotgun (WGS) metagenomic studies. Placental specimens were passed immediately to trained personnel in a sterile clean container. Identified taxa and gene carriage patterns were compared to other human body site niches, including oral, skin, airway (nasal), vaginal, and gut microbiomes from non-pregnant controls. A unique placental microbiome niche was identified, composed of non-pathogenic commensal microbiota from the Firmicutes, Tenericutes, Proteobacteria, Bacteroidetes, and Fusobacteria phyla. In aggregate, the placental microbiome profiles were most akin (Bray-Curtis dissimilarity <0.3) to the human oral microbiome. 16S-based operational taxonomic unit analyses revealed associations of the placental microbiome with a remote history of antenatal infection.
In a short communication Doyle et al. (Citation2014; ) reported the analysis of bacteria DNA from placental membranes (chorion and amnion) of 14 mothers who gave birth preterm by spontaneous vaginal delivery (intake membranes at 8–32 weeks) all with chorioamnionitis and compared them with term births by caesarean (4 non-labouring mothers after 37 weeks with intact membranes) vaginal birth (6 mothers). Bacterial DNA was extracted and amplified by PCR, followed by pyrosequencing. There were many similarities between term deliveries but more lactobacilli in vaginal vs caesarean deliveries. There were more Fusobacterium, Streptococcus, Mycoplasma, Aerococcus, Gardnerella, Ureaplasma and Enterobacteriaceae in preterm placentas.
Antony et al. (Citation2015; ) showed that excess gestational weight gain (GWG) was associated with significant alterations in the composition of the placental microbiome and its bacterially encoded metabolic pathways, suggesting that the mechanism by which excess GWG results in preterm birth may be through aberrations in the placental microbiome. In this study, 320 placentas were collected from term and preterm pregnancies and sections taken from different areas of the placenta with very good procedures to reduce of contamination risk during sampling. The patterns of the RNA metagenomics were clustered by excess GWG and when the phyla level relative taxonomic abundance of mothers delivered preterm was compared by maternal weight gain, the authors reported increased abundance of Firmicutes, Actinobacteria, and Cyanobacteria and decreased relative abundance of Proteobacteria. This was also seen at family and genus level (Vibrio, Burkholderia, and Beijerinckia).
Prince et al. (Citation2016; ) found that the placental microbiome was altered among subjects with spontaneous preterm birth (delivered 32–36 weeks) with and without chorioamnionitis in a cross-sectional analysis with six nested spontaneous birth cohorts. Each cohort had 9 to 15 subjects (term gestations without chorioamnionitis, term with chorioamnionitis, preterm without chorioamnionitis, preterm with mild chorioamnionitis, preterm with severe chorioamnionitis, and preterm with chorioamnionitis and funisitis). Preterm subjects with chorioamnionitis had fewer bacterial constituents of their placental membrane microbiome and those with severe chorioamnionitis had high abundances of Ureaplasma parvum, Fusobacterium nucleatum, and Streptococcus agalactiae.
Doyle et al. (Citation2017; ) aimed to describe the microbiota found in placental tissue and fetal membranes (chorion and amnion) from a cohort of women in rural southern Malawi. They examined the microbial community structure and explored if this differed in placental tissue exhibiting chorioamnionitis. Participants (n = 1391) were enrolled prospectively into the main trial before 20 weeks gestation and followed throughout pregnancy, childbirth and beyond. Those with severe chorioamnionitis delivered on average −0.4 gestational weeks earlier and had a lower observed number of OTUs in the placenta and fetal membranes. A significantly higher mean bacterial load of Mycoplasmataceae, Leptotrichiaceae and Veillonaceae was observed in placental tissues with severe chorioamnionitis. Fusobacterium nucleatum, Ureaplasma sp. and Gemella asaccharolytica were associated with a shorter duration of pregnancy.
Low birth weight
Zheng et al. (Citation2015; ) demonstrated that placental microbiome also varied in association with low birth weight in a study of 12 low birth weight and 12 normal birth weight full-term neonates (gestational age 37w 0d–41w 6d). Placental samples were collected and immediately passed to trained personnel in a sterile clean container. The bacteria in placental tissues were characterized by 16S ribosomal DNA amplicon high-throughput sequencing. Low birthweight infants had lower diversity, less lactobacillus, lower relative abundance of Firmicutes but more Proteobacteria and Actinobacteria than normal birth weight infants. They also had a lower abundance of Enterococcus but more Lactococcus and Bacillus ().
In the study of Doyle et al. (Citation2017), carried out in a cohort of 1391 women in rural Malawi (see Preterm Birth section above), higher placental and vagina loads of Sneathia sanguinegens, Prevotella copri, Lachnospiraceae sp. and Phascolarctobacterium succinatutens were significantly associated with lower length for age z score in newborns.
Neonatal sepsis
Amniotic fluid and cord blood () were evaluated in a study of preterm births with intra amniotic infection (IAI) and/or early-onset neonatal sepsis (AEONS), Wang et al. (Citation2013; ). PTB cases were grouped as 1) Group 1– neonatal blood culture-positive AEONS (n = 6); 2) Group 2– neonatal blood culture-negative presumed AEONS with positive IAI (n = 16); 3) Group 3– neonatal blood culture-negative presumed AEONS with no IAI (n = 7); or 4) Group 4– no AEONS or IAI (n = 7). Samples from healthy term deliveries (n = 8) served as technical controls. Eighteen species (7 non redundant) were identified by 16SrRNA sequencing in cord blood in comparison to 31 (15 non redundant) in amniotic fluid. Few of these species were cultured. Many of the species found in cord blood were also found in the amniotic fluid of the same mother/infants. E. coli and F. nucleatum were the most common.
Intervention studies – transfer of bacteria from mother to infant
There were no studies that directly considered transfer of normal placental microbiome to the infant.
Discussion
In this systematic review, the key aim was to determine if there was evidence for a placental, amniotic fluid and/or cord blood microbiome in normal full-term births and whether this was transferred to the infant, influencing colonization of the infant gut. A further aim was then to establish if such microbiomes were present in and/or modulated in complicated pregnancies. Our findings reveal no evidence for any transfer of bacteria from the placenta to the infant in healthy pregnancies and conflicting evidence for the presence and role of the placental microbiome due to lack of consistency in methodology and in particular to weaknesses in prevention of contamination during sample collection, processing, storage and analysis.
Early histological studies visualized bacteria on the placenta. Stout et al. (Citation2013) identified intracellular bacteria in the basal plate for 195 placentas collected ≤12 h from delivery in term and preterm gestations using histochemical analyses. Gram-positive and Gram-negative intracellular bacteria of diverse morphologies were documented in the basal plates of 27% of all placentas. However, no attempt was made to use bacterial isolation and identification by molecular techniques.
Two major observational studies of healthy pregnancies included in our review supported the presence of commensal bacteria in the placenta (Aagaard et al. Citation2014,Collado et al. Citation2016). In addition to the studies in the systematic review, a probiotic intervention study by Rautava et al. (Citation2012) evaluated whether microbes in placenta or amniotic fluid can affect fetal innate immune gene expression during late pregnancy and whether innate immune gene expression profiles in the placenta and fetal gut can be modulated by dietary supplementation with probiotics. They did not look at infant faecal bacteria. Forty three pregnant women were randomized to receive Bifidobacterium lactis, B. lactis in combination with Lactobacillus rhamnosus GG (LGG) or placebo for 14 days before elective caesarean section at full term in a double-blind clinical trial. Only 29 mother-infant pairs completed the study. Bacteria in placenta were detected by quantitative (q)PCR and bacterial DNA for lactobacilli was detected in all placenta samples with DNA from bifidobacterium, bacteroides and Clostridia leptum groups in many but not all samples. Lactobacilli and bifidobacterium group DNA were also found in some, but not all, amniotic fluid samples. This was associated with changes in fetal intestinal innate immune function related gene expression profile.
These studies in humans contradicted the dogma that the fetus resides in a sterile environment, proposing the presence of microbes in the fetal maternal unit, which includes the placenta, amniotic fluid, cord blood and meconium. The concept of a maternal-fetal-microbial triad comprising a “holobiont,” that is highly interdependent has been proposed (Neu Citation2016). When the commensal microbiota is perturbed, an inflammatory response to altered intrauterine microbial communities has been linked to preterm delivery, as well as to increased risk for several brain, lung, and eye diseases in these infants (Neu Citation2016). In contrast, more recent studies contradict this in utero colonization hypothesis concluding that placenta and amniotic fluid from normal healthy pregnancies are free of bacteria (de Goffau et al. Citation2019; Lim et al. Citation2018; Rehbinder et al. Citation2018; Leiby et al. Citation2018; Perez-Munoz et al. Citation2017).
In challenged births (including preterm), there is more evidence for a placental microbiome and differences between normal and compromised births. This may be due to the higher levels of pathogenic bacteria which are more easily detected than a potential commensal microbiota ( and ). However, there were still many issues related to possible contamination of samples during collection, processing and DNA extraction that make interpretation of the data difficult. Adding to this are variations in both the reporting of and the actual DNA extraction methodology including the different regions of the rRNA gene studied which could influence the results. These confounding factors make it very difficult to compare studies, confirm observations and draw overall conclusions. The scarce results obtained with the placental microbiota and microbiome from women with GDM show that they differ from control women with lower levels of Pseudomonadales order and Acinetobacter genus, the lower abundance of placental Acinetobacter being associated to a more adverse metabolic and inflammatory phenotype (Bassols et al. Citation2016). Inflammation is a key part of the parturition process and external factors including infection or chronic low grade inflammation can increase the risk of premature labour (Behnia et al. Citation2016; Menon et al. Citation2019). A commensal placental microbiota in a non-healthy pregnancy could theoretically have a modulatory effect on the immune system, leading to a local anti-inflammatory environment, contributing to systemic protection from the GDM, but evidence for this is lacking.
Potential sites of origin of the maternal-fetal microbiome include the vagina as most the probable location via ascension and translocation through the choriodecidual barriers. However, alternatives suggested include hematogenously derived sources such as maternal periodontitis and the maternal intestinal tract due to higher intercellular junctional permeability and/or dendritic cell transport (Neu Citation2016). Several papers have suggested a uterine commensal microbiome when not pregnant and some of these could be incorporated into the developing placenta (Baker et al. Citation2018; Benner et al. Citation2018).
It has been proposed by Wassenaar and Panigrahi (33) that there are three elements that determine whether the outcome of bacterial presence in the placenta and amniotic fluid is good or bad (Wassenaar and Panigrahi Citation2014). First, which bacteria are present and whether they can be eliminated or reduced by the immune system, Second, whether the resultant immune response itself becomes detrimental and lastly if the bacteria can survive and achieve pathological population levels.
Overgrowth of one or a few strains seems to be harmful, while a diverse community of low numbers of cells, do not seem to interfere with pregnancy. Consequently, the placenta may function as an active low biomass ecologic niche that harbours a unique microbiome, which could be hypothesized to protect against harmful microbial dominance. However, these low levels of colonization still need to be robustly confirmed, but if this happens, these observations challenge not only the assumed notions of “from when and where” our earliest microbes colonize (or are seeded), but also the concepts of inflammatory mediators, reproductive immunity, and whether microbes in such niches constitute more friend than foe (Prince et al. Citation2016). This process of colonization may influence health and disease not only early in life but in some cases throughout life. In fact, in utero colonization, if it occurs, could have major impact on the developing mammalian host in terms of development of immunity and metabolism that, with epigenetic modifications, may link to disease risk later in life (Neu Citation2016). As an example, gestational diabetes, a clinical situation that clearly influences pregnancy outcomes not only for the mother but also for the infant, has been recently associated with changes in placental microbiota, suggesting that placental microbiota may be a new target for therapy in for instance gestational diabetes (Bassols et al. Citation2016).
A challenge in working with specimens with low bacterial biomass, such as placental samples, is that some or all of the bacterial DNA may derive from contamination. In fact, Lauder et al. (Citation2016), quantified total 16S rRNA gene copies using quantitative PCR and two different DNA purification methods, and found that placental samples and negative controls contained low and indistinguishable copy numbers. Consequently, studies need to be designed and executed using rigorous quality standards and state-of-the-science metagenomics evaluating potential contamination. This is especially true with samples in which the placenta is collected after vaginal delivery and therefore prone to contamination from the birth canal (Nuriel-Ohayon et al. Citation2016).
A very recent study examined the potential microbiome in 537 pregnancies where infants with complicated and uncomplicated pregnancy were born by pre-labour Caesarean section (de Goffau et al. Citation2019). They used negative and positive controls and considered batch effects in the metagenomic and 16S rRNA sequencing data. They also controlled for mode of delivery and considered the possible origin of any bacteria detected to rule out vaginal contamination. The authors concluded that there was no evidence for a placental microbiome and most bacteria detected were contaminants during sample collection or from kits and reagents in both normal and complicated pregnancies. The only exception was the potential pathogen Streptoccocus agalactiae found in 5% of samples collected before labour started.
In conclusion, our study has shown that there is great variability in the detection of bacteria in the placenta of normal term births with little data available from the cord blood microbiome. Where a placenta microbiome is reported, there is a large variation in the type and diversity of bacteria found in different studies. This could indicate potential site specificity but also that different methodologies were used for extraction and analysis of the microbial DNA, and it is most likely that contamination had a major impact on the results. The pathological birth studies clearly differed compared to the normal birth studies, but even these may have detected potential contaminants rather than a true microbiome.
Our findings highlight the need for studies with improved quality and reproducibility of the methodologies and, in particular, avoidance of the risk of contamination. Overall, more studies carried out in a harmonized way are needed to ensure solid evidence for bacterial transfer from the mother to the infant. Specifically, future research approaches need to be more consistent with DNA extraction both in terms of timing and methods. Methods need to be harmonized to avoid contamination and agreement should be reached on which regions of the rRNA gene should be examined. Lastly, future experiments should focus on larger birth cohorts of normal birth mothers with a study set up that enables tracking of the bacteria to the gut of infants. Thus, the results from this systematic review support the notion that a placental microbiome may not be a normal phenomenon and may indeed be the result of contamination. There is no evidence that it plays a role in early gut microbial colonization of the infant.
Supp_Table_1_final.docx
Download MS Word (75.5 KB)Acknowledgements
The authors acknowledge the support of Matthieu Flourakis in the preparation of this manuscript.
This work was conducted by an expert group of the European branch of the International Life Sciences Institute, ILSI Europe. This publication was coordinated by the Early Nutrition and Long-Term Health Task Force. Industry members of this task force are listed on the ILSI Europe website at http://ilsi.eu/task-forces/nutrition/early-nutrition-and-long-term-health/. Experts are not paid for the time spent on this work; however, the non-industry members within the expert group were offered support for travel and accommodation costs from the Early Nutrition and Long-Term Task Forces to attend meetings to discuss the manuscript and a small compensatory sum (honoraria) with the option to decline. The expert group carried out the work, i.e. collecting/analysing data/information and writing the scientific paper separate to other activities of the task forces. The research reported is the result of a scientific evaluation in line with ILSI Europe’s framework to provide a precompetitive setting for public-private partnership (PPP). ILSI Europe facilitated scientific meetings and coordinated the overall project management and administrative tasks relating to the completion of this work. For further information about ILSI Europe, please email [email protected] or call +32 2 771 00 14. The opinions expressed herein and the conclusions of this publication are those of the authors and do not necessarily represent the views of ILSI Europe nor those of its member companies.
Disclosure statement
Dr Ricardo Rueda is a full-time employee of Abbott Nutrition. Dr Schoemaker is currently a full-time employee of Friesland Campina. Prof. Stanton is a full-time employee of Teagasc. Prof. van der Beek is a part-time employee of Danone Nutricia Research. Dr van Loo Bouwman is a full-time employee of Yili Innovation Centre Europe. Dr. van Diepen is a full-time employee of Reckitt Benckiser/Mead Johnson.
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. 2014. The placenta harbors a unique microbiome. Sci Transl Med. 6:237–265.
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, et al. 2012. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 7:e36466.
- Amarasekara R, Jayasekara RW, Senanayake H, Dissanayake VH. 2015. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J Obstet Gynaecol Res. 41:662–669.
- Antony KM, et al. 2015. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 212:653.e1–653.e16.
- Baker JM, Chase DM, Herbst-Kralovetz MM. 2018. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 9:208.
- Bassols J, Serino M, Carreras-Badosa G, Burcelin R, Blasco-Baque V, Lopez-Bermejo A, Fernandez-Real JM. 2016. Gestational diabetes is associated with changes in placental microbiota and microbiome. Pediatr Res. 80:777–784.
- Behnia F, Sheller S, Menon R. 2016. Mechanistic differences leading to infectious and sterile inflammation. Am J Reprod Immunol. 75:505–518.
- Benner M, Ferwerda G, Joosten I, van der Molen RG. 2018. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum Reprod Update. 24:393–415.
- Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. 2016. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 6:23129.
- de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, Smith GCS. 2019. Human placenta has no microbiome but can contain potential pathogens. Nature. 572:329–334.
- DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. 2008. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 3:e3056.
- Doyle RM, Alber DG, Jones HE, Harris K, Fitzgerald F, Peebles D, Klein N. 2014. Term and preterm labour are associated with distinct microbial community structures in placental membranes which are independent of mode of delivery. Placenta. 35:1099–1101.
- Doyle RM, Harris K, Kamiza S, Harjunmaa U, Ashorn U, Nkhoma M, Dewey KG, Maleta K, Ashorn P, Klein N. 2017. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS One. 12:e0180167.
- Figuero E, Han YW, Furuichi Y. 2020. Periodontal diseases and adverse pregnancy outcomes: mechanisms. Periodontol 2000. 83:175–188.
- Goldenberg RL, Hauth JC, Andrews WW. 2000. Intrauterine infection and preterm delivery. N Engl J Med. 342:1500–1507.
- Hart ML, Meyer A, Johnson PJ, Ericsson AC. 2015. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PLoS One. 10:e0143334.
- Jimenez E, et al. 2015. Metagenomic analysis of milk of healthy and mastitis-suffering women. J Hum Lact. 31:406–415.
- Jimenez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodríguez JM, et al. 2005. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 51:270–274.
- Jones HE, Harris KA, Azizia M, Bank L, Carpenter B, Hartley JC, Klein N, Peebles D. 2009. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 4:e8205.
- Kovalovszki L, Villanyi Z, Pataki I, Veszelowvsky I, Nagy ZB. 1982. Isolation of aerobic bacteria from the placenta. Acta Paediatr Acad Sci Hung. 23:357–360.
- Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, Leite R, Elovitz MA, Parry S, Bushman FD. 2016. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 4:29.
- Leiby JS, McCormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, Hofstaedter CE, Roche AM, Mattei LM, Bittinger K, et al. 2018. Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome. 6:196.
- Lim ES, Rodriguez C, Holtz LR. 2018. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome. 6:87.
- Menon R, Richardson LS, Lappas M. 2019. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta. 79:40–45.
- Neu J. 2016. The microbiome during pregnancy and early postnatal life. Semin Fetal Neonatal Med. 21:373–379.
- Nuriel-Ohayon M, Neuman H, Koren O. 2016. Microbial changes during pregnancy, birth, and infancy. Front Microbiol. 7:1031.
- Parnell LA, Briggs CM, Cao B, Delannoy-Bruno O, Schrieffer AE, Mysorekar IU. 2017. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep. 7:11200.
- Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. 2017. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 5:48.
- Prince AL, et al. 2016. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. 214:627.e1–627.e16.
- Rautava S, Collado MC, Salminen S, Isolauri E. 2012. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 102:178–184.
- Rehbinder EM, et al. 2018. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? Am J Obstet Gynecol. 219:289.e1–289.e12.
- Satokari R, Gronroos T, Laitinen K, Salminen S, Isolauri E. 2009. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 48:8–12.
- Stout MJ, et al. 2013. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 208:226.e1–226.e7.
- Tuominen H, Rautava S, Syrjanen S, Collado MC, Rautava J. 2018. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci Rep. 8:9787.
- Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B; Preterm Birth International Collaborative (PREBIC). 2016. Maternal microbiome – a pathway to preterm birth. Semin Fetal Neonatal Med. 21:94–99.
- Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. 2013. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 8:e56131.
- Wassenaar TM, Panigrahi P. 2014. Is a fetus developing in a sterile environment? Lett Appl Microbiol. 59:572–579.
- Zheng J, Xiao X, Zhang Q, Mao L, Yu M, Xu J. 2015. The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients. 7:6924–6937.