Abstract
While evolution proceeds through the generation of random variant alleles, the application of selective pressures can select for subsets of mutations that confer fitness-improving physiological benefits. This, in essence, defines the process of adaptive evolution. The rapid replication rate of bacteria has allowed for the design of experiments to study these processes over a reasonable timeframe within a laboratory setting. This has been greatly assisted by advances in tractability of diverse microorganisms, next generation sequencing technologies and bioinformatic analysis pipelines. Examining the processes by which organisms adapt their genetic code to cope with sub-optimal growth conditions has yielded a wealth of molecular insight into diverse biological processes. Here we discuss how the study of adaptive evolutionary trajectories in bacteria has allowed for improved understanding of stress responses, revealed important insight into microbial physiology, allowed for the production of highly optimised strains for use in biotechnology and increased our knowledge of the role of genomic plasticity in chronic infections.
Introduction
Natural selection has shaped all living organisms that inhabit our planet. The plasticity of microbial genomes has led to their unparalleled success in environments that are completely inhospitable to larger organisms. Creative laboratory evolution experiments allow us to closely analyse the alterations in bacterial genomes that facilitate increased fitness under a given selective pressure (i.e. adaptive mutations). With the development of cost-effective high throughput genomic sequencing, we are now capable of rapidly assigning causal mutations, thereby grounding evolutionary theories with the mechanistic molecular basis of evolution (Conrad et al. Citation2011).
There are many important considerations for the design of an effective laboratory evolution experiment. For example, convergent growth phenotypes are often observed from parallel evolved cultures but with distinct underlying transcriptional (Fong et al. Citation2005) and mutational states. This highlights an inherent need for replicate sampling if a complete appreciation of the routes to improved fitness is of interest. Evolution proceeds through the random generation of variant alleles, some of which may not be truly adaptive in a given condition. These neutral mutations are often carried together with adaptive mutations, in which case they are known as hitch hiker mutations. As such, in order to infer causality, one must experimentally confirm the role of individual mutations by phenotype interrogation with isogenic mutant strains (Long and Antoniewicz Citation2018). Oftentimes, metabolic rewiring is implied as a causal route to improved fitness in which case, direct assessment of metabolic flux through flux balance analysis should be considered (Long and Antoniewicz Citation2018).
Recent years have seen an increase in carefully designed, elegant studies within this theme. Perhaps none more so than the long-term evolution experiment (LTEE) performed by the Lenski group. This consists of twelve independent Escherichia coli populations adapted in minimal medium for what currently stands in excess of 73,000 generations and has yielded extremely interesting insights into several evolutionary themes including historical contingency (Blount et al. Citation2018), the emergence of hypermutator states (Sniegowski et al. Citation1997) and ecological specialisation (Leiby and Marx Citation2014) that are discussed later in this review. Within the era of synthetic biology, where extremes of genetic engineering and minimum requirements for bacterial life are being explored, adaptive evolution experiments can also play a vital role in repairing the scars of excessive genetic manipulations (Tenaillon Citation2018; Wannier et al. Citation2018; Choe et al. Citation2019).
In this review, we highlight some of the important advancements made in our understanding of bacterial processes through the analysis of adaptive evolution. We place particular emphasis on in vitro approaches, but also highlight studies involving evolution at the host-pathogen interface. We aim to illuminate important aspects of experimental set up, the evolutionary trajectories (mutations) observed and the physiological outcomes of adaptation to bottlenecks in growth. The research presented has widespread importance in the fields of biotechnology, genetics, microbial physiology and pathogenicity.
Evolutionary perspectives on bacterial physiology
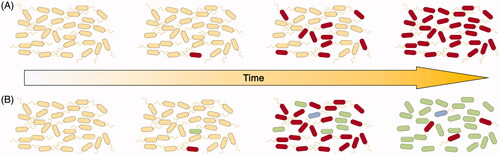
The LTEE performed on E. coli in glucose limited (25 μg ml−1) minimal medium represents an excellent example of how the study of bacterial populations under adaptive conditions can inform not only about bacterial physiology but also yield fascinating insight into evolution in a greater context. The level of glucose limitation in this experiment provided a strong nutritional selective pressure resulting in approximately 6.67 generations per day (Lenski et al. Citation1991). Samples were taken at 500 generation intervals throughout the experiment providing a fossil record which could be studied in detail using whole genome sequencing at a later date (Lenski et al. Citation1991; Good et al. Citation2017). The examination of the spread of mutations throughout the twelve independent populations during adaptation has revealed tremendous diversity in evolutionary trajectories and corresponding heterogeneity in fitness improvement. Adaptive mutations were often found to arise alongside neutral or deleterious hitch-hiker mutations with sub-clades of competing adaptive populations often co-existing for periods in excess of 10,000 generations (Good et al. Citation2017). Beneficial traits were often found to be subject to a period of refinement where further adaptive mutations allowed for increased fitness before fixation (Good et al. Citation2017). A simple model of adaptive evolution consists of a single clone bearing an adaptive mutation progressively outgrowing the progenitor and members of the population with neutral or deleterious mutations by purifying selection (). The findings of the LTEE challenge this notion and demonstrate further complexity in evolutionary trajectories with stable co-existence of multiple adaptive lineages and phenotypic traits often requiring refinements in order for maximal adaptive potential to be reached (). While fitness continued to increase over 60,000 generations, the rate at of fitness improvement reduced over time (Wiser et al. Citation2013; Lenski et al. Citation2015). Fitness gains were more consistent with a power-law model where decelerating fitness increases without an upper limit are observed rather than a hyperbolic model that is predictive of a hard upper limit (Lenski et al. Citation2015). The mutation rate was found to increase dramatically in half of the populations due to the development of a mutator phenotype, potentiated by mutation in genes with functions in DNA mismatch repair such as mutS, mutL and uvrD (Sniegowski et al. Citation1997; Good et al. Citation2017). These clones developed mutation rates between one and two orders of magnitude higher than their ancestors (Sniegowski et al. Citation1997) and were associated with increased rates of fitness improvement (Lenski et al. Citation2015).
Figure 1. Models of population diversification through adaptive evolutionary sweeps under fixed selective pressure. (A) Simplistic model of purifying selection whereby an adaptive mutation (red cell) confers a fitness advantage over the progenitor (yellow), leading to fixation of the mutation in the population. (B) More realistic model with stable co-existence of multiple lineages (yellow, red, green and blue), where some adaptive traits (green non-motile cells) require refinement before sweeping through the population. Further complexity in population dynamics are imparted by spatial organisation and fluctuations in selective pressure.
An interesting example of ecological diversification was observed in one of these LTEE mutator populations where aerobic citrate utilisation (Cit+) evolved after 31,000 generations (Blount et al. Citation2008; Citation2012). The phenotype was produced by a tandem duplication event leading to the insertion of the aerobically active rnk promoter upstream of the citrate transporter encoding gene citT (Blount et al. Citation2012). Importantly, attempts to “replay the tape” by evolving this metabolic capacity with 270 clones from various stages throughout the LTEE determined that 13 clones all from 20,000 generations onwards became Cit+ (Blount et al. Citation2008). This indicated the importance of historical contingency with potentiating mutations being required for diversification (Blount et al. Citation2018). Full citrate utilisation was facilitated by a mutation that led to increased expression of the dicarboxylate transporter gene dctA (Quandt et al. Citation2014). It was later observed that an early mutation in citrate synthase encoded by gltA facilitated the emergence of Cit+ with further gltA mutations allowing for refinement of the Cit+ phenotype by altering carbon flux through the TCA cycle (Quandt et al. Citation2015). This explains why Cit− and Cit+ clones co-existed for approximately 10,000 generations before fixation of the Cit+ lineage. Aerobic citrate utilisation is extremely rare in E. coli. Sodium citrate was originally intended to function as a chelating agent in the minimal medium used for the LTEE. The authors speculate that the development of such extremely novel traits by complex evolutionary trajectories may be akin to those occurring during speciation. Indeed, the Cit+ trait has recently been shown to result in death of large proportions of the bacterial population when cultured on citrate as a sole carbon source, even after 2,500 generations of adaptive evolution, highlighting an inherent incompatibility between aerobic citrate utilisation an stable cellular physiology in E. coli (Blount et al. Citation2020).
Exploring adaptative evolutionary routes to surmounting stress
The adaptive nature of the bacterial genome has allowed for success in environments of extreme physical and chemical stress. Such stresses include temperature, hydrostatic and osmotic pressures, fluid shear, antibiotics, solvents and detergents. In many habitats such pressures operate in dynamic spatial gradients, therefore rapid adaptation through positive selection is often a prerequisite for survival. Examining the nature of these adaptations in the laboratory can be informative not only from the perspective of stress tolerance but also in determining the mode of action of a given stressful agent.
Adaptation to antibiotic stress
The evolution of antibiotic resistance remains one of the most prominent threats to human health worldwide. The ease at which antimicrobial resistance arises was well recognised by Alexander Fleming who noted in a 1945 interview that “there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant”. Resistance is simply classified as the inherited ability to grow at high concentrations of antibiotic, whereas tolerance occurs when an organism overcomes the killing effect of an agent by entering a transient quiescent state while concentrations of that compound remain high (Brauner et al. Citation2016). Tolerance and resistance have an adaptive mutational basis, distinct from persistence, which involves the production of non-growing antibiotic tolerant phenotypic variants with a genotype indistinguishable from that of the wild type (Balaban et al. Citation2004; Brauner et al. Citation2016). The mutations giving rise to tolerance are distinct from those associated with full resistance. Laboratory evolution experiments and mathematical modelling have shown that mutations conferring tolerance to ampicillin increase the probability of acquiring full resistance mutations (Levin-Reisman et al. Citation2017). Development of resistance is often costly under non-selective conditions and can require compensatory mutations to alleviate this burden (Schrag et al. Citation1997; Andersson and Hughes Citation2010). Accordingly, it has been shown that during 50,000 generations of growth in the absence of any selective pressure that populations with low level intrinsic resistance tend to become more susceptible to the majority of antibiotics as increased fitness evolves and that the causal mutations arise predominantly within the first 2,000 generations (Lamrabet et al. Citation2019).
The study of resistant populations in laboratory experiments has yielded interesting insight into the strategies bacteria employ to avoid these deleterious outcomes. Exposure of Salmonella enterica to cephalosporins has been shown to rapidly induce duplication of the blaTEM1 gene, with secondary mutations affecting expression or function of the porin proteins OmpC, OmpD, and OmpF (Sun et al. Citation2009). A similar duplication-dependent mechanism of multiple antibiotic resistance has been described in tetracycline adapted E. coli. Mechanistically, this involved an IS186 insertion in the promoter element of the Lon protease encoding gene (lon3) prior to duplication of a region encoding components (acrAB) of the AcrAB-TolC efflux pump (Nicoloff et al. Citation2006). These studies demonstrate that gene duplication can serve as a rapid and reversible means of overcoming transient antibiotic toxicity, while avoiding the often-detrimental effects of gene disruption.
Adaptation involves, in addition to stable mutation, the generation of bet-hedging phenotypic variants that carry transient beneficial traits. For example, it was observed that during exposure to kanamycin S. enterica cultures developed heterogeneity in the expression of the outer membrane porin OmpC, with reduced expression allowing adaptive resistance that was lost upon culture in the absence of selection (Sánchez-Romero and Casadesús Citation2014). It has also been shown that sub-MIC concentrations of aminoglycosides can result in high level resistance in S. enterica but via distinct adaptive strategies to those associated with lethal selection. Lethal selection was associated with rpsL (encoding ribosomal protein S12) mutation, while sub-lethal concentrations resulted in modification of the ribosomal RNA target (gidB mutation), a reduction in aminoglycoside uptake (cyoB, nuoG, and trkH mutation), and induction of an aminoglycoside-modifying enzyme AadA (znuA mutation) (Wistrand-Yuen et al. Citation2018). More recently, it has been shown that while lethal concentrations of tigecycline induce mutation predominantly in genes with well characterised roles in resistance (such as nfxB, mexC, mexD, parRS, rpsJ, orfN or pmrB), half of the mutations arising in sub-lethal selections were in genes without a previously ascribed function in resistance (including fleQ, fleR, flgE, bacA and mraW) (Sanz-García et al. Citation2020). The differentiation in resistance mechanisms arising from these mutations was further substantiated by the observation that sub-lethal selections produced mutants with lower MIC to tigecycline but with more cross-protection against structurally diverse antibiotics. These studies demonstrate that distinct evolutionary trajectories are employed in response to differing degrees of selective pressure.
The target for activity of many antimicrobials is often intracellular and as such, disruption of intracellular transport represents a rapid adaptive route to resistance. Identification of causal resistance mutations can therefore yield valuable insight into translocation mechanisms. This approach has proved particularly useful for the characterisation of uptake mechanisms of highly specific, bacterially produced protein antibiotics, known as bacteriocins. For example, early studies identified that four distinct mutations in the E. coli major outer membrane porin OmpF could confer resistance to colicin N, with two of these providing cross protection against colicin A (Fourel et al. Citation1993). While three of the mutations were found to be localised to surface exposed loops, presumably directly affecting binding of the bacteriocin, the fourth (G119D) was localised to an internal loop and resulted in protrusion of the peptide side chain into the lumen, subdividing it into two (3–4 Å diameter) channels (Jeanteur et al. Citation1994). These studies indicated the utility of adaptive mutants in not only identifying proteins of importance for translocation but characterising resistance mechanisms at an atomic level. More recently, resistant Shigella soneii and E. coli DH5 mutants have implicated the vitamin B12 transporter BtuB in the transport of colicins E2, E3 and E8 (Calcuttawala et al. Citation2015). Indeed, colicins such as colicin E9 are known to form a translocon complex consisting of an OmpF trimer and BtuB monomer that facilitates capture and threading of the bacteriocin through the outer membrane (Housden et al. Citation2013). Adaptive mutants of Pseudomonas aeruginosa strain E2 were found to acquire resistance to pyocin L1 through disruption of wbpz, a gene encoding a common polysaccharide antigen glycosyltransferase, thereby implicating the interaction between pyocin L1 and D-rhamnose rich LPS moieties in bactericidal activity (McCaughey et al. Citation2014). Similarly, in the Gram-positive organism Lactococcus lactis, the MalEFG maltose transporter, the zinc dependent metallopeptidase YvjB and undecaprenyl pyrophosphate phosphatase UppP have been reported as playing a role in the activities of garvicin ML, bacteriocin LsbB and lactococcin G through the isolation and sequence analysis of resistant mutants (Gabrielsen et al. Citation2012; Uzelac et al. Citation2013; Kjos et al. Citation2014). Developing a complete understanding of the evolutionary routes to bacteriocin resistance in will play a vital role in ensuring optimal therapeutic efficacy.
It should be noted that well shaken flask cultures that are typical of laboratory evolution experiments do not reflect the spatial organisation and concentration gradients characteristic of selection in natural environments. Baym et al. developed the microbial evolution and growth arena (MEGA)–plate (a simple yet fascinating experiment consisting of a four by two-foot agar substrate with increasing antibiotic concentrations) and observed that many of the previously modelled patterns of fixation and fitness of adaptive mutants do not hold true. In fact, multiple co-existing lineages were formed with variants of high resistance requiring compensatory mutations to gain full fitness and mutants with full fitness often becoming spatially trapped by less fit counterparts (Baym et al. Citation2016). This raises interesting questions about mutational diversity and spatial organisation of populations under selective pressure in natural environments. We will later discuss this in the context of bacterial biofilms.
Adaptation to chemical stress
Chemical selective pressure extends beyond antibiotic agents, with solvents, detergents, inorganic ions and even unfavourable nutritional compounds exerting growth inhibition on some microorganisms. Pathogenic E. coli are specialised towards causing infections at distinct sites within the body, including the intestine, the bladder and the brain (Kaper et al. Citation2004). In the case of the intestinal pathogen enterohaemorrhagic E. coli (EHEC), niche specificity is largely determined by the sensing of environmental cues and appropriate modulation of expression of its primary colonisation apparatus – a type 3 secretion system (T3SS) encoded on a pathogenicity island known as the locus of enterocyte effacement (LEE) (Bansal et al. Citation2007; Pacheco et al. Citation2012; Connolly et al. Citation2015; Kumar and Sperandio Citation2019). We observed the extremely rare occurrence of carriage of a complete, functional D-serine catabolism locus in strains harbouring the LEE (Connolly et al. Citation2015). Conversely, uropathogenic E. coli that cause infections in the bladder, where D-serine concentrations are up to one thousand-fold higher, are predominantly capable of metabolising D-serine (Anfora et al. Citation2007; Connolly et al. Citation2015). Importantly, while D-serine supports the growth of UPEC, it inhibits growth, induces an SOS-like response and causes transcriptional repression of the LEE in EHEC (Connolly et al. Citation2015; Connolly and Roe Citation2016). This indicates that E. coli occupying distinct niches have evolved contrasting responses to this metabolite. We expanded on this recently by showing that through adaptive evolution, EHEC could rapidly (within 48 h) overcome the inhibition of growth that it normally encounters upon exposure to D-serine (O’Boyle et al. Citation2020). Whole genome sequencing and transcriptomics revealed that resistance to this toxic metabolite could be achieved through two distinct mechanisms. Nonsense mutations in the D-serine transporter gene cycA and transcriptional repression of a second D-serine transporter encoded by sstT cooperatively contributed to D-serine resistance. Resistance was also conferred through genomic rearrangements that led to the constitutive activation of the previously silent D-serine deaminase gene dsdA. Mutants possessing both adaptive mechanisms were isolated from the same population indicating heterogeneity in evolutionary trajectories and stable co-existence of multiple adaptive lineages, as observed in the LTEE and summarised in . Adaptation resulted in enhanced ability to colonise epithelial cells in the presence of D-serine as mutants with defective D-serine transport no longer exhibited transcriptional repression of the LEE, while those constitutively expressing dsdA displayed enhanced growth in the presence of D-serine, thereby negating any detrimental effect of T3SS repression (O’Boyle et al. Citation2020). These findings illustrate that D-serine-dependent toxicity and colonisation repression can be overcome by multiple adaptive mechanisms and demonstrate a means by which this pathogen could expand to a niche where D-serine is present at normally intolerable concentrations.
The fermentation of ethanol from diverse sugar substrates is one of the most economically important microbial bioprocesses. Fermentation is often limited by the accumulation of alcohol to levels that become toxic to the producing organism. Early studies suggested compromised membrane permeability (Sikkema et al. Citation1995), disruption in peptidoglycan cross-linking (Ingram and Vreeland Citation1980), and a broad modulation of global transcription (Gonzalez et al. Citation2003) in the mechanism of toxicity. In a more recent study, further insight into the mechanism of toxicity was revealed through the analysis of ethanol tolerant adaptive mutants. Mutants were isolated by exposure of duplicate populations of E. coli to increasing concentrations of ethanol (50, 55, 60, 65 g l−1) over 24 serial passages (Haft et al. Citation2014). In addition to genes with roles in cell envelope synthesis, disruption of genes involved in transcription and translation was noted in both populations. Mutation of the methionine biosynthesis repressor MetJ (ΔE91), the transcription termination protein Rho (L270M) and ribosomal protein S17 RpsQ (H31P) were confirmed as causal through the generation of an isogenic triple mutant. The H31P RpsQ mutation was found to decrease ethanol induced translational misreading. Ethanol stressed cells were found to exhibit increased transcriptional stalling at internal AUG codons which was reversible by deletion of MetJ, replacement with MetJΔE91 or supplementation with methionine, indicating that depletion of the methionine pool was responsible for transcriptional stalling. Ethanol was also found to cause premature termination of transcripts with low ribosome density, with adaptive mutation of Rho (L270M) resulting in enhanced readthrough and increased transcript length. This study elegantly indicates that ethanol induces toxic transcription and translation perturbation and that adaptive mutation of metJ, rpsQ and rho can help to alleviate these effects.
Biotechnological applications of experimentally adapted microbes
In addition to informing about basic microbial physiology, adaptive evolution can have practical applications. Slow growth represents perhaps the most obstructive bottleneck in microbial bioprocess. The Palsson group have been instrumental in discovering the many mechanisms by which E. coli can adapt to grow more rapidly, thereby allowing more efficient consumption of diverse substrates and production of desirable metabolites. The production of specific metabolites often requires genetically engineered strains with altered metabolism and consequently reduced fitness. Long-term evolutionary adaptation can often overcome these issues.
E. coli K-12 str. MG1655 displays an inherently poor growth rate in minimal medium with glycerol as a sole carbon source, despite possessing a complete glycerol catabolism pathway (Ibarra et al. Citation2002). Parallel adaptation of five independent populations to this condition for 44 days (700 generations) resulted in the identification of 13 mutations conferring an enhanced growth rate (Herring et al. Citation2006). Notably, the glycerol kinase GlpK was mutated in all five populations, with mutant variants exhibiting improved reaction kinetics or reduced inhibition by fructose 1,6 bisphosphate (FBP), demonstrating that weak GlpK enzymatic activity and inappropriately high allosteric inhibition by FBP contribute to glycerol intolerance in K-12. The authors also observed mutations in RNA polymerase subunits β (rpoB) and β’ (rpoC), suggesting that a reprogramming of global transcription may be a means of overcoming poor growth in glycerol. One such mutation – a 27 bp deletion in the SI3 domain of RpoC – was later found to result in a 60% increase in growth rate in glycerol but with a concurrent 30% decrease in rich medium (Conrad et al. Citation2010). Mutated RpoC displayed decreased open-complex longevity, decreased transcriptional pausing and increased transcript elongation rate at a rRNA promoter (i.e. decreased binding but compensatory increased transcription rate) (Conrad et al. Citation2010). Comparison of wild type and RpoCΔ27bp by chromatin immunoprecipitation of RNA polymerase β followed by hybridisation to a whole genome array (ChIP-chip) revealed a 15% reduction in signal at the rRNA transcriptional unit (TU), while microarray-based transcriptomics demonstrated strong downregulation of motility, chemotaxis, cell adhesion (fimbriae and curli), and acid resistance genes (Conrad et al. Citation2010). This indicated a redistribution from binding at rRNA TUs for the purpose of repressing potentially energetically costly TUs that lacked significant benefit for growth in glycerol.
A similar approach was employed with 11 replicate populations of E. coli K-12 evolving for 60 days in minimal medium with lactate as a sole carbon source. Between two and eight mutations were observed per endpoint, primarily in genes with roles in metabolism (such as the phosphoenolpyruvate synthase (ppsA) and glycine cleavage complex gene gcvT) and regulation (such as the cyclic AMP receptor protein (Crp) and stringent response regulator gene relA) (Conrad et al. Citation2009). An 82 bp deletion in the rph-pyrE operon appeared in seven populations and was hypothesised to reduce the efficacy of the pyrE 5′ transcriptional attenuator through removal of the native rph stop codon. This mutation resulted in a 15% increase in growth rate in wild type K-12 and a 30% increase in a strain with 2 further adaptive mutations. The authors hypothesise that this results in recovery from the previously reported pyrimidine synthesis defect possessed by K-12 (Jensen Citation1993). These results demonstrate that variable and sometimes synergistic adaptations can converge on the same growth phenotype, highlighting the importance of adapting multiple replicate populations to gain a complete understanding of adaptation under a given condition.
Adaptation to growth on glucose produces similar genetic outcomes to growth on glycerol and lactate with repair of the pyrimidine biosynthesis defect and mutation of genes (or regulatory regions adjacent to genes) with global regulatory functions (such as rpoB and hns) being observed (LaCroix et al. Citation2015; Sandberg et al. Citation2016). While the mutation of key global regulators was expected to cause large scale metabolic rewiring and a corresponding redirection of carbon flux through alternative pathways, 13 C-metabolic flux balance analysis indicated that such flux rewiring did not occur during glucose adaptation (Long et al. Citation2017). Instead, transport and all intracellular rates of central carbon metabolism were increased. This indicates that metabolic adaptive evolution responses are a matter of proteomic allocation rather than redirection of cellular processes and that reprogramming of global transcription may represent a means of redistributing cellular resources to overcome rate limiting steps in central metabolism.
The observation that E. coli K-12 can adapt to utilise a non-native carbon source represents another excellent example of adaptive mechanisms allowing for the surmounting of metabolic bottlenecks. While K-12 encodes a 1,2 propanediol (PD) oxidoreductase fucO, it lacks the ability to grow on PD as a sole carbon source due to the fact that transcription of fucO is not responsive to PD but rather fuculose 1 phosphate, a product of L-fucose catabolism (Cocks et al. Citation1974). The enzyme is also intrinsically inhibited by metal catalysed oxidation (MCO) (Cabiscol et al. Citation1994). Three populations of K-12 were cultured in exponentially decreasing glycerol concentrations over 11 days, with compensatory increases in PD, followed by 49 additional days of culture with PD as a sole carbon source, resulting in a specific growth rate of 0.35 h−1 after the 60 days of adaptation (Lee and Palsson Citation2010). A combination of a Leu8Met substitution that inhibited MCO, and an IS5 insertion upstream of fucO, that led to constitutive transcription, were fixed in all 3 populations and contributed most to increased growth rate. Accessory mutations in ilvG, ylbE1, and rrlD also contributed to improved growth, indicating further complexity in adaptation to PD catabolism.
Bacterial strains often require genetic modification in order for their metabolism to be re-routed into production of commercially relevant metabolites. This can compromise growth under standard conditions. For example, lactate is produced by mixed-acid fermentation from glucose exclusively under anaerobic conditions which are challenging and costly to maintain (Portnoy et al. Citation2008). A cytochrome oxidase deficient mutant of E. coli K-12 (ΔcydAB, ΔcyoABCD, ΔcbdAB) displays 85% reduced oxygen uptake but loses the ability to grow on glucose as a sole carbon source (Portnoy et al. Citation2008). Adaptation for 17 days in exponentially decreasing concentrations of amino acid supplementation with compensatory increases in glucose, followed by 43 days of adaptation in glucose as a sole carbon source yielded a strain with a growth rate of 0.44 h−1, similar to that of K-12 when cultured on glucose in anaerobic conditions (Portnoy et al. Citation2008). All three replicate adapted populations produced lactate under aerobic conditions, with one strain displaying 80% conversion of glucose to lactate. Transcriptional analysis revealed overexpression of ygiN (quinol monooxygenase) and sodBC (superoxide dismutase) as being responsible for recovering O2 uptake in the adapted strains causing redirection of glycolytic flux. In an additional study, deletion of ygiN further replicated anaerobic physiology, reducing O2 uptake 98% and resulting in production of lactate as a sole fermentation product after adaptive evolution (Portnoy et al. Citation2010).
Interesting aspects of bacterial physiology can often be revealed during strain optimisation by adaptive evolution. For example, a novel function in xylose transport was revealed for the galactitol transporter GatC during optimisation of E. coli K-12 for growth and productivity during fermentation of D-lactate from xylose (Utrilla et al. Citation2012). D-lactate has commercial use as a precursor for polylactic acid polymers – a biodegradable alternative to petrochemical thermoplastics (Rasal et al. Citation2010). Deletion of components of genes in competing pathways (pflB, adhE and frdA) resulted in increased glycolytic flux to D-lactate but with a decrease in fitness due to adenosine triphosphate (ATP) imbalance (Utrilla et al. Citation2009). Deletion of the ATP driven xylose transporter encoded by xylFGH (JU01), followed by 15 successive batch fermentations with xylose as a sole carbon source (JU15) increased growth rate from 0.09 to 0.19 h−1 with production of D-lactate improving from 0.39 to 0.79 g l h−2 (Utrilla et al. Citation2012). Quantitative proteomics revealed elevated expression of glycolytic enzymes and comparative genomics identified a GatCS184L substitution as well as a 27.3 kb deletion in JU15. Recapitulation of the 27.3 kb deletion in the parent isolate had no effect. Deletion of gatC in JU01 or JU15 resulted in decreased growth rate, and expression of GatCS184L in JU01 improved growth rate indicating that GatC is a xylose transporter and the GatCS184L mutation improves growth and production of D-lactate from xylose (i.e. xylose transport is a limiting step in D-lactate fermentation).
Industrial bioprocess requires the accumulation of high concentrations of primary and secondary metabolites, which can often be toxic to the producing strain. By developing an understanding of adaptive mechanisms conferring tolerance to these metabolites, strains can be specifically engineered for enhanced productivity. Adaptation of a naturally ethanologenic E. coli isolate KC01 for 7 days (350 generation) in increasing concentrations of ethanol (from 10 to 40 g l−1) resulted in a two-fold increase in maximum ethanol tolerance (Wang et al. Citation2011). The derivation of ethanol from renewable cellulosic sugars is of great interest to the biotechnology industry (Zabed et al. Citation2017). Increased ethanol tolerance of adapted KC01 correlated with a 48% increase in rate of ethanol production and a 50% increase in final ethanol titre compared with the parental isolate following 72 h fermentation on 50 g l−1 xylose (Wang et al. Citation2011). In a separate study, six populations of E. coli W3110 were subjected to 1000 generations of growth in 50 g l−1 ethanol, resulting in a two-fold increase in growth rate (Horinouchi et al. Citation2010). While neither study employed comparative genomics to uncover the genetic basis of the ethanol adapted populations, microarray-based transcriptomics revealed increased expression of tryptophan and histidine biosynthesis genes in adapted W3110 (Horinouchi et al. Citation2010). The growth rate of the parental isolate could also be improved by supplementation with these amino acids, confirming their role in ethanol tolerance.
L-serine is an important building block for chemical synthesis in the cosmetic, medical and pharmaceutical industries. Mutation of genes responsible for serine deamination (sdaA, sdaB and tdcG) and serine conversion to glycine (glyA) allowed for the production of L-serine from glucose using E. coli K-12 (Mundhada et al. Citation2016). Accumulation of L-serine was highly toxic to the resulting strain, causing inhibition of growth and maximum yields of 8.3 g l−1. Five replicate populations of the serine producing strain were subjected to 45 days of automated adaptive evolution with passage at OD600 = 0.4 and increasing concentrations (3, 6, 12, 24, 50, 75, and 100 g l−1) of L-serine each time stable growth rate was recorded. The maximum yield of L-serine was found to increase from 8.3 to 37 g l−1 with growth rate in 50 g l−1 L-serine increasing 7.5-fold over wild type K-12. Genome sequencing of endpoints revealed that two populations had acquired mutator phenotypes through disruption of mismatch repair genes (mutS and mutT) and acquisition of over a hundred other mutations, thereby creating difficulty in inferring causality. The remaining three replicates contained independent mutations in thrA (homoserine dehydrogenase), while two of three replicates acquired mutations in rho, lrp and gcvB as well as mutations in rpoB, yojI, cycA and pykF in one replicate. ThrA plays a role in branched chain amino acid biosynthesis and has been shown to be inhibited by excess L-serine (Hama et al. Citation1990). Prior to adaptation, supplementation of medium with threonine was required to prevent inhibition of ThrA by L-serine and improve growth rate (Mundhada et al. Citation2016). The mutations observed in ThrA all conferred amino acid substitutions in the L-serine binding pocket and thus alleviated the requirement for threonine supplementation (Mundhada et al. Citation2017). In mixed glucose and glycine fermentation, adaptation of the serine producing strain led to a threefold increase in final L-serine titre and a four-fold increase in productivity over a 50-h fermentation.
Evolution in spatially ordered communities
In order to cope with varying nutritional status and stressors, bacteria in natural environments typically adopt a complex spatial organisation that is poorly reflected by planktonic culture in rich laboratory medium. Such spatially ordered structures are known as biofilms. These are often composed of multiple symbiotic/mutualistic species and can evolve enhanced resistance to antibiotic (Koch et al. Citation2014) and chemical (Mangalappalli-Illathu and Korber Citation2006) disruption. The study of evolution in biofilm conditions can inform about the regulatory processes governing biofilm formation, persistence and dispersal (Martin et al. Citation2016), in addition to offering insight into outcomes arising from social interactions. A study on E. coli in such spatially ordered communities alluded to the acquisition of mutations beneficial for this kind of lifestyle. Twenty two-day old biofilm cultures were shown to outgrow the parental isolate on a pre-established biofilm (Kraigsley and Finkel Citation2009), suggesting the acquisition of beneficial mutations. These mutations did not enhance competitiveness on sterile surfaces, indicating that such adaptations in E. coli likely affect biofilm processes downstream of initial attachment.
Due to the dynamic nature of biofilms, mutations rarely become fixed and instead give rise to diverse sub-populations with conditionally enhanced fitness. In the case of P. aeruginosa, distinct colony morphotypes arise from such mutations in a RecA (SOS response mediator)-dependent manner that Boles et al. have described as a form of evolutionary insurance, allowing protection during adverse events (Boles et al. Citation2004). A culture-impaired small colony variant of P. aeruginosa was found to emerge rapidly during adaptation to biofilm conditions, increasing in abundance from zero to 35% of the population within three days (Penterman et al. Citation2014). The culture-impaired phenotype was found to be due to mutation in lipopolysaccharide (LPS) O-antigen modification genes wbpA and wbpJ, that render the bacteria sensitive to self-produced pyocin R2 when grown in broth culture but confer an advantage when cultured in biofilm conditions. Modification of the O-antigen had previously been shown to shield P. aeruginosa from self-produced pyocin (Köhler et al. Citation2010), indicating a mechanism for culture-impairment in the wbpA/wbpJ mutants. Penterman et al. (Penterman et al. Citation2014) hypothesise that the corresponding increase in biofilm fitness may be due to enhanced energy conservation or aggregation. They describe this adaptation as an example of antagonistic pleiotropy with mutation conferring an advantage in one condition and a disadvantage in another.
Pseudomonads have long been known to form a distinctive pellicle at the air liquid interface of statically grown cultures. Adaptation of Pseudomonas fluorescens to this air-liquid interface biofilm condition, or biofilm formation on drip-fed glass columns, results in the emergence of a wrinkly spreader morphology, with mutation of the protein-glutamate methylesterase gene wspF being reported as a mediator of the phenotype in both conditions (Bantinaki et al. Citation2007; Udall et al. Citation2015). The Wsp surface sensing system becomes constitutively activated upon disruption of WspF due to a lack of demethylation of WspA by WspF, locking WspR in its active phosphorylated state (Francis et al. Citation2017). WspR is a GGDEF domain containing diguanylate cyclase, therefore wspF mutants exhibited increased intracellular accumulation of the second messenger cyclic-di-GMP and increased production of Psl polysaccharide, causing the wrinkled colony morphology. Adaptation of P. aeruginosa to oxidative stress with H2O2 for 120 generations also caused the emergence of rough small colony variants through mutation of wspF (Chua et al. Citation2016). While interesting from a physiological perspective, these adaptations have also been reported as clinically significant. Cystic fibrosis patients become chronically colonised with biofilm forming isolates of P. aeruginosa and other related strains, with the Wsp pathway being observed as a prominent target for adaptive mutations (Sousa and Pereira Citation2014; Blanka et al. Citation2015; Marvig et al. Citation2015).
The modification of LPS has also been reported as a means of enhancing symbiotic growth of the soil dwelling organism Pseudomonas putida. When cultured in an environment with benzyl alcohol as the sole carbon source, P. putida relies on the presence of other bacteria, such as Acinetobacter, that can catabolise benzyl alcohol to benzoate. This in turn can be metabolised by P. putida to allow growth (Christensen et al. Citation2002). When cultured under flow, the spatial organisation of Acinetobacter/P. putida co-cultures changes from mutually exclusive, discrete microcolonies of Acinetobacter isolated from sparse aggregates of P. putida cells after 24 h, to a true commensalism after 5 days with P. putida growing in close association with Acinetobacter (Hansen et al. Citation2007). It was found that during this transition, rough colony morphotypes of P. putida evolve, with mutations in the LPS outer core assembly gene wapH being observed as causal for both the biofilm fitness and colony morphology. Fascinatingly, these mutations did not arise in pure culture biofilms or in mixed culture chemostats, indicating that both the multi-species interaction and the spatial organisation of the biofilm were necessary for this adaptation. These results demonstrate that evolution can stabilise and increase productivity of spatially organised mixed species communities.
The formation of a functionally diverse bacterial biofilm can be seen as a means of coping with nutritionally adverse conditions. It was recently reported that aging colonies of Bacillus subtilis develop actively proliferating microdomains/microcolonies after 25 days of culture, when the majority of the population has entered a quiescent state (Hashuel and Ben-Yehuda Citation2019). Sequencing of 17 mutants revealed a large degree of diversity in mechanisms leading to late-stage replication with 22 mutations being observed in total. Twelve of these were in sporulation related genes such as spo0A, kinA and spo0F, while ten were in genes with central functions such as replication, transcription, translation and metabolism. The majority of morphomutants displayed deficient sporulation, competence and entry into stationary phase (i.e. deficient differentiation) when grown in pure culture. This study confirmed that proliferation can be used as an alternative strategy to dormancy when coping with a challenging niche.
Examination of adaptive evolution in vivo
The examination of adaptive evolution in pure culture allows for simple manipulation of experimental conditions, validation of phenotypes by replicating experiments with defined mutants and ease of sequencing without contaminating nucleic acids of host/microbiome origin. While extremely beneficial for the development of technologically useful strains, these approaches may be somewhat limited by their failure to replicate the complexity of selective conditions present within host environments.
Longitudinal sampling from chronic bacterial infections such as cystic fibrosis offers an opportunity to study how pathogens evolve within a native and clinically relevant environment. Lieberman et al. sequenced the genomes of 112 epidemic Burkholderia dolosa isolates from 14 cystic fibrosis patients over 16 years and found that 17 bacterial genes acquired nonsynonymous mutations in multiple individuals, indicating parallel adaptive evolution (Lieberman et al. Citation2011). Adaptive mutations were acquired in genes involved in antibiotic resistance (gyrA), bacterial membrane composition/O-antigen presentation (restorative insertion of a SNP reactivated a glycosyltransferase encoded by wbaD), and oxygen-dependent regulation (fixLJ two component sensor). In particular, a role for FixLJ in pathogenicity had not previously been reported, indicating new potential targets for therapeutics. FixLJ was later shown to be activated in low oxygen environments (consistent with those observed in the respiratory mucus of the cystic fibrosis lung) and to regulate biofilm, intracellular invasion, and pathogenicity (Schaefers et al. Citation2017).
Sequencing of 474 P. aeruginosa isolates from 34 children over a one to ten-year period (mean = 4.8), starting from the first detection of P. aeruginosa colonisation, identified convergent evolutionary trajectories in many isolates (Marvig et al. Citation2015). Based on convergent evolution in distinct isolates, 52 pathoadaptive genes were designated. In addition to the previously discussed mutations acquired in the Wsp signal transduction pathway, pathoadaptive mutations were overrepresented in genes with functions in transcriptional regulation (mexZ, mucA, algU), antibiotic resistance (gyrA, mexA, nalD) and biofilm formation (bifA, lasR, morA). Longitudinal sampling allowed for the identification of interesting cases of evolutionary contingency. For example, mutations in the alginate activating sigma factor gene algU often occurred after mutation of its cognate anti-sigma factor mucA (20 of 25 cases where double mutation was observed). This resulted in a constitutive mucoid phenotype upon loss of MucA, that the authors hypothesise to confer a transient benefit requiring depletion of energy reserves, eventually offset by disruption of AlgU (Marvig et al. Citation2015). Convergent pathoadaptive mutations were also observed in genes with no previously described pathogenicity-related functions, thereby increasing the number of potential targets for therapeutic intervention.
As a consequence of the evolution of multidrug resistance, the treatment of urinary tract infections (UTI) and in particular recurrent UTI (rUTI) can become increasingly challenging. A recent study analysed the evolution of faecal and urinary isolates of E. coli over a five-year period from a single patient who had suffered with rUTI for almost 40 years (Forde et al. Citation2019). The clonal lineage P1A was associated with intestinal persistence and rUTI providing a compelling case for the intestinal reservoir as a source of bladder infection. Comparison of P1A isolates collected over time demonstrated high levels of diversity in carriage of IncF/IncI/IncN plasmids and mutations in chromosomal genes conferring antibiotic resistance including gyrA, parC and ampC. This provides important insight into adaptive evolution in the context of fluctuating antibiotic exposure and establishes a framework which may be employed to develop a precision therapeutic approach to combatting rUTI.
In addition to host physiology and antibiotic use, the diet can serve as a driver for the evolution of bacterial strains with enhanced fitness. The fruit fly Drosophila melanogaster has been used extensively for the study of symbiotic and pathogenic host-microbe relationships. For example, certain strains of Lactobacillus plantarum function as mutualists by increasing proteolytic activity in the gut, resulting in increased luminal free amino acids (Erkosar et al. Citation2015). Successive adaptive evolution of a strain of L. plantarum less capable of this growth inducing effect, over 20 Drosophila generations (2,000 bacterial generations) led to the rapid (2 Drosophila generations) emergence of a similar growth enhancement capability (Martino et al. Citation2018). This coincided with the acquisition of mutations in the acetate kinase gene ackA, that were fixed for the remainder of the experiment. Mutants displayed enhanced replication both in Drosophila larvae and larval growth medium and were found to produce high levels of N-acetyl amino acids. This included N-acetyl glutamine, which conferred a growth inducing effect to Drosophila even in the absence of L. plantarum. Interestingly, adaptive mutants with growth promoting ability also emerged upon culture in Drosophila growth medium in the absence of host larvae, indicating that adaptation to host diet rather than the host itself can foster the development of animal-microbe symbioses (Martino et al. Citation2018).
Inexpensive industrial scale production coupled with regulatory approval led to the global use of trehalose as a food additive with per individual consumption increasing from approximately 0.3 g per day (from natural sources) prior to its introduction to 34.4 g per day (Collins et al. Citation2019). Three epidemic lineages of Clostridium difficile (RT027, RT078 and RT017) were shown to have acquired the ability to metabolise low concentrations of trehalose (Collins et al. Citation2018, Citation2019). Ribotypes RT027 and RT017 were found to have up to 500-fold higher sensitivity to trehalose due to substitutions (L172I and C171S, respectively) in the effector binding pocket of the TreR repressor. The treR gene is encoded adjacent to the phosphotrehalase gene treA, allowing for treA transcription at concentrations of trehalose as low as 50 μM. RT078 isolates on the other hand were found to encode a horizontally acquired four-gene trehalose utilisation module including a transporter gene ptsT that was sufficient to confer enhanced trehalose metabolism to non RT078 isolates. Importantly, increased efficiency of trehalose metabolism in a murine infection model correlated with earlier mortality. Within three days, in vitro adaptive evolution experiments allowed for the isolation of similar mutants with enhanced trehalose metabolism all possessing mutations in treR (Collins et al. Citation2018). Taken together, these studies highlight the importance of understanding how dietary alterations can shape host-microbe relationships by modulating the selective environment.
Colonisation and transmission are key aspects of the lifestyle of infectious bacteria and refinements in the genes required for each of these processes play a key role in determining pathogen success. Experimental evolution of Streptococcus pneumoniae in distinct murine models comprising nasal carriage and pneumonic disease revealed both parallel and niche specific adaptive mutations (Green et al. Citation2020). Nasopharyngeal adaptations resulted in improved colonisation efficiency while lineages adapted in the lung displayed a reduction in virulence. Of particular interest, multiple nasopharyngeal lineages acquired the same mutation in gpsA, encoding a glycerol-3-phosphate dehydrogenase. This protein has a role in lipid metabolism and importantly has also been shown to be subject to mutation during natural colonisation of infants with S. pneumoniae (Chaguza et al. Citation2020). This study illustrates how manipulation of experimental parameters of an in vivo experimental infection model can allow for dissection of adaptive processes specific to distinct features of the pathogenic lifestyle.
While in vivo experimental evolution often offers the most relevant environmental conditions for studying adaptation, the merits of in vitro experimentation should not be overlooked. Animal models can fail to recapitulate key features of the human host that manipulate the selective environment. The rapid growth rate of bacteria in vitro, even in nutrient limiting or stressful conditions allows for comparatively long evolutionary time frames to be studied, maximising the potential for adaptive refinement. Pure culture experimentation allows for ease of bacterial enumeration, enabling accurate assessment of generation times, something which is particularly challenging in vivo. Perhaps most importantly, in vitro experimentation allows precise modification of experimental parameters without the complexity of host interference. Adopting both in vitro and in vivo approaches to study bacterial evolution will maximise the potential for new insights into how bacteria find a way to thrive in the most challenging of environments.
Conclusion
The studies presented here allude to the importance of revealing the evolutionary basis of improved fitness in order to further develop our understanding of basic microbial biology. As sequencing technologies become more advanced and cost effective, one would hope that more complex experiments could be performed. For example, while difficult to conceive methodologically at present, the analysis of all of the adaptive mutations occurring within a complex community of interacting microorganisms would represent a major advancement (Lenski Citation2017). It should be noted however that sequencing power alone will not be sufficient to significantly improve our understanding of the complexities of bacterial evolution in the coming years. As mentioned, a combination of carefully designed in vivo and in vitro experimental models will be extremely valuable. Classical molecular biology approaches will continue to be of the utmost importance to validate the functionality of the adaptive mutations observed in such experiments. This will allow for insight into not only the physiological processes affected by adaptive mutations but also into protein functionality at a molecular level. Adaptation can lead to large scale alterations in the transcriptional (Fong et al. Citation2005; Horinouchi et al. Citation2010; O’Boyle et al. Citation2020) and proteomic (Utrilla et al. Citation2012) landscape, therefore combining transcriptomics or proteomics with whole genome sequencing will allow for a more complete understanding of the processes affected by adaptation. In silico modelling of bacterial evolution (reviewed elsewhere (Hindré et al. Citation2012)) offers many advantages over traditional wet lab experimentation and will certainly continue to play a vital complementary role in the future. Experiments are performed with “digital microorganisms” constructed from known physiological, metabolic and genetic characteristics in precisely defined theoretical environments and population dynamics can be recorded. This allows for exhaustive replicates over limitless evolutionary timescales and facilitates experimentation with unculturable bacteria. Given the significance of adaptive responses to diet, host, antimicrobial and nutritional factors presented here, it is clear that analysing evolutionary outcomes of adaptation should remain a key strategy for improving understanding of host-microbe relationships. This will allow for the development of microbes with enhanced fitness and suitability for use in the biotechnology industry, development of therapeutics which target genes that are of importance during niche specific adaptation of pathogens and most importantly improve our basic understanding of evolution within prokaryotes and beyond.
Disclosure statement
All authors declare no conflicting interests.
Additional information
Funding
References
- Andersson DI, Hughes D. 2010. Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Microbiol. 8(4):260–271.
- Anfora AT, Haugen BJ, Roesch P, Redford P, Welch RA. 2007. Roles of serine accumulation and catabolism in the colonization of the murine urinary tract by Escherichia coli CFT073. Infect Immun. 75(11):5298–5304.
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science (80-). 305(5690):1622–1625.
- Bansal T, Englert D, Lee J, Hegde M, Wood TK, Jayaraman A. 2007. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 75(9):4597–4607.
- Bantinaki E, Kassen R, Knight CG, Robinson Z, Spiers AJ, Rainey PB. 2007. Adaptive divergence in experimental populations of Pseudomonas fluorescens. III. Mutational origins of wrinkly spreader diversity. Genetics. 176(1):441–453.
- Baym M, Lieberman TD, Kelsic ED, Chait R, Gross R, Yelin I, Kishony R. 2016. Spatiotemporal microbial evolution on antibiotic landscapes. Science (80-). 353(6304):1147–1151.
- Blanka A, Düvel J, Dötsch A, Klinkert B, Abraham W-R, Kaever V, Ritter C, Narberhaus F, Häussler S. 2015. Constitutive production of c-di-GMP is associated with mutations in a variant of Pseudomonas aeruginosa with altered membrane composition. Sci Signal. 8(372):ra36.
- Blount ZD, Barrick JE, Davidson CJ, Lenski RE. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature. 489(7417):513–518.
- Blount ZD, Borland CZ, Lenski RE. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc Natl Acad Sci USA. 105(23):7899–7906.
- Blount ZD, Lenski RE, Losos JB. 2018. Contingency and determinism in evolution: replaying life’s tape. Science (80-). 362(6415):eaam5979.
- Blount ZD, Maddamsetti R, Grant NA, Ahmed ST, Jagdish T, Baxter JA, Sommerfeld BA, Tillman A, Moore J, Slonczewski JL, et al. 2020. Genomic and phenotypic evolution of Escherichia coli in a novel citrate-only resource environment. Elife. 9:e55414.
- Boles BR, Thoendel M, Singh PK. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc Natl Acad Sci USA. 101(47):16630–16635.
- Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 14(5):320–330.
- Cabiscol E, Aguilar J, Ros J. 1994. Metal-catalyzed oxidation of Fe2+ dehydrogenases. Consensus target sequence between propanediol oxidoreductase of Escherichia coli and alcohol dehydrogenase II of Zymomonas mobilis. J Biol Chem. 269(9):6592–6597.
- Calcuttawala F, Hariharan C, Pazhani GP, Ghosh S, Ramamurthy T. 2015. Activity spectrum of colicins produced by Shigella sonnei and genetic mechanism of colicin resistance in conspecific S. sonnei strains and Escherichia coli. Antimicrob Agents Chemother. 59(1):152–158.
- Chaguza C, Senghore M, Bojang E, Gladstone RA, Lo SW, Tientcheu P-E, Bancroft RE, Worwui A, Foster-Nyarko E, Ceesay F, et al. 2020. Within-host microevolution of Streptococcus pneumoniae is rapid and adaptive during natural colonisation. Nat Commun. 11(1):3442.
- Choe D, Lee JH, Yoo M, Hwang S, Sung BH, Cho S, Palsson B, Kim SC, Cho B-K. 2019. Adaptive laboratory evolution of a genome-reduced Escherichia coli. Nat Commun. 10(1):935.
- Christensen BB, Haagensen JAJ, Heydorn A, Molin S. 2002. Metabolic commensalism and competition in a two-species microbial consortium. Appl Environ Microbiol. 68(5):2495–2502.
- Chua SL, Ding Y, Liu Y, Cai Z, Zhou J, Swarup S, Drautz-Moses DI, Schuster SC, Kjelleberg S, Givskov M, et al. 2016. Reactive oxygen species drive evolution of pro-biofilm variants in pathogens by modulating cyclic-di-GMP levels. Open Biol. 6(11):160162.
- Cocks GT, Aguilar J, Lin ECC. 1974. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 118(1):83–88.
- Collins J, Danhof H, Britton RA. 2019. The role of trehalose in the global spread of epidemic Clostridium difficile. Gut Microbes. 10(2):204–209.
- Collins J, Robinson C, Danhof H, Knetsch CW, van Leeuwen HC, Lawley TD, Auchtung JM, Britton RA. 2018. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature. 553(7688):291–294.
- Connolly JPR, Goldstone RJ, Burgess K, Cogdell RJ, Beatson SA, Vollmer W, Smith DGE, Roe AJ. 2015. The host metabolite D-serine contributes to bacterial niche specificity through gene selection. Isme J. 9(4):1039–1051.
- Connolly JPR, Roe AJ. 2016. Intracellular D-serine accumulation promotes genetic diversity via modulated induction of RecA in enterohemorrhagic Escherichia coli. J Bacteriol. 198(24):3318–3328.
- Conrad TM, Frazier M, Joyce AR, Cho B-K, Knight EM, Lewis NE, Landick R, Palsson BØ. 2010. RNA polymerase mutants found through adaptive evolution reprogram Escherichia coli for optimal growth in minimal media. Proc Natl Acad Sci USA. 107(47):20500–20505.
- Conrad TM, Joyce AR, Applebee MK, Barrett CL, Xie B, Gao Y, Palsson BØ. 2009. Whole-genome resequencing of Escherichia coli K-12 MG1655 undergoing short-term laboratory evolution in lactate minimal media reveals flexible selection of adaptive mutations. Genome Biol. 10(10):R118.
- Conrad TM, Lewis NE, Palsson BØ. 2011. Microbial laboratory evolution in the era of genome-scale science. Mol Syst Biol. 7(1):509.
- Erkosar B, Storelli G, Mitchell M, Bozonnet L, Bozonnet N, Leulier F. 2015. Pathogen virulence impedes mutualist-mediated enhancement of host juvenile growth via inhibition of protein digestion. Cell Host Microbe. 18(4):445–455.
- Fong SS, Joyce AR, Palsson BØ. 2005. Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res. 15(10):1365–1372.
- Forde BM, Roberts LW, Phan M-D, Peters KM, Fleming BA, Russell CW, Lenherr SM, Myers JB, Barker AP, Fisher MA, et al. 2019. Population dynamics of an Escherichia coli ST131 lineage during recurrent urinary tract infection. Nat Commun. 10(1):3643.
- Fourel D, Mizushima S, Bernadac A, Pagès JM. 1993. Specific regions of Escherichia coli OmpF protein involved in antigenic and colicin receptor sites and in stable trimerization. J Bacteriol. 175(9):2754–2757.
- Francis VI, Stevenson EC, Porter SL. 2017. Two-component systems required for virulence in Pseudomonas aeruginosa. FEMS Microbiol Lett. 364(11):1–22.
- Gabrielsen C, Brede DA, Hernández PE, Nes IF, Diep DB. 2012. The maltose ABC transporter in Lactococcus lactis facilitates high-level sensitivity to the circular bacteriocin garvicin ML. Antimicrob Agents Chemother. 56(6):2908–2915.
- Gonzalez R, Tao H, Purvis JE, York SW, Shanmugam KT, Ingram LO. 2003. Gene array-based identification of changes that contribute to ethanol tolerance in ethanologenic Escherichia coli: comparison of KO11 (parent) to LY01 (resistant mutant). Biotechnol Prog. 19(2):612–623.
- Good BH, McDonald MJ, Barrick JE, Lenski RE, Desai MM. 2017. The dynamics of molecular evolution over 60,000 generations. Nature. 551(7678):45–50.
- Green AE, Howarth D, Chaguza C, Echlin H, Langendonk RF, Munro C, Barton TE, Hinton JCD, Bentley SD, Rosch JW, et al. 2020. Identification of colonisation and virulence determinants of Streptococcus pneumoniae via experimental evolution in mouse infection models. bioRxiv [Internet]. 2020 Jan 1;2020.09.08.287698. Available from: http://biorxiv.org/content/early/2020/09/08/2020.09.08.287698.abstract.
- Haft RJF, Keating DH, Schwaegler T, Schwalbach MS, Vinokur J, Tremaine M, Peters JM, Kotlajich MV, Pohlmann EL, Ong IM, et al. 2014. Correcting direct effects of ethanol on translation and transcription machinery confers ethanol tolerance in bacteria. Proc Natl Acad Sci USA. 111(25):E2576–E2585.
- Hama H, Sumita Y, Kakutani Y, Tsuda M, Tsuchiya T. 1990. Target of serine inhibition in Escherichia coli. Biochem Biophys Res Commun. 168(3):1211–1216.
- Hansen SK, Rainey PB, Haagensen JAJ, Molin S. 2007. Evolution of species interactions in a biofilm community. Nature. 445(7127):533–536.
- Hashuel R, Ben-Yehuda S. 2019. Aging of a bacterial colony enforces the evolvement of nondifferentiating mutants. MBio. 10(5):e01414–e01419.
- Herring CD, Raghunathan A, Honisch C, Patel T, Applebee MK, Joyce AR, Albert TJ, Blattner FR, van den Boom D, Cantor CR, et al. 2006. Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat Genet. 38(12):1406–1412.
- Hindré T, Knibbe C, Beslon G, Schneider D. 2012. New insights into bacterial adaptation through in vivo and in silico experimental evolution. Nat Rev Microbiol. 10(5):352–365.[Mismat
- Horinouchi T, Tamaoka K, Furusawa C, Ono N, Suzuki S, Hirasawa T, Yomo T, Shimizu H. 2010. Transcriptome analysis of parallel-evolved Escherichia coli strains under ethanol stress. BMC Genomics. 11(1):579.
- Housden NG, Hopper JTS, Lukoyanova N, Rodriguez-Larrea D, Wojdyla JA, Klein A, Kaminska R, Bayley H, Saibil HR, Robinson CV, et al. 2013. Intrinsically disordered protein threads through the bacterial outer-membrane porin OmpF. Science. 340(6140):1570–1574.
- Ibarra RU, Edwards JS, Palsson BO. 2002. Escherichia coli K-12 undergoes adaptive evolution to achieve in silico predicted optimal growth. Nature. 420(6912):186–189.
- Ingram LO, Vreeland NS. 1980. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J Bacteriol. 144(2):481–488.
- Jeanteur D, Schirmer T, Fourel D, Simonet V, Rummel G, Widmer C, Rosenbusch JP, Pattus F, Pagès JM. 1994. Structural and functional alterations of a colicin-resistant mutant of OmpF porin from Escherichia coli. Proc Natl Acad Sci USA. 91(22):10675–10679.
- Jensen KF. 1993. The Escherichia coli K-12 “wild types" W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels”. J Bacteriol. 175(11):3401–3407.
- Kaper JB, Nataro JP, Mobley HLT. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol. 2(2):123–140.
- Kjos M, Oppegård C, Diep DB, Nes IF, Veening J-W, Nissen-Meyer J, Kristensen T. 2014. Sensitivity to the two-peptide bacteriocin lactococcin G is dependent on UppP, an enzyme involved in cell-wall synthesis. Mol Microbiol. 92(6):1177–1187.
- Koch G, Yepes A, Förstner KU, Wermser C, Stengel ST, Modamio J, Ohlsen K, Foster KR, Lopez D. 2014. Evolution of resistance to a last-resort antibiotic in Staphylococcus aureus via bacterial competition. Cell. 158(5):1060–1071.
- Köhler T, Donner V, van Delden C. 2010. Lipopolysaccharide as shield and receptor for R-pyocin-mediated killing in Pseudomonas aeruginosa. J Bacteriol. 1;192(7):1921 LP–1928.
- Kraigsley AM, Finkel SE. 2009. Adaptive evolution in single species bacterial biofilms. FEMS Microbiol Lett. 293(1):135–140.
- Kumar A, Sperandio V. 2019. Indole signaling at the host-microbiota-pathogen interface. MBio. 10(3):e01031–19.
- LaCroix RA, Sandberg TE, O'Brien EJ, Utrilla J, Ebrahim A, Guzman GI, Szubin R, Palsson BO, Feist AM. 2015. Use of adaptive laboratory evolution to discover key mutations enabling rapid growth of Escherichia coli K-12 MG1655 on glucose minimal medium. Appl Environ Microbiol. 81(1):17–30.
- Lamrabet O, Martin M, Lenski RE, Schneider D. 2019. Changes in intrinsic antibiotic susceptibility during a long-term evolution experiment with Escherichia coli. MBio. 10(2):e00189–19.
- Lee D-H, Palsson BØ. 2010. Adaptive evolution of Escherichia coli K-12 MG1655 during growth on a nonnative carbon source, L-1,2-propanediol. Appl Environ Microbiol. 76(13):4158–4168.
- Leiby N, Marx CJ. 2014. Metabolic erosion primarily through mutation accumulation, and not tradeoffs, drives limited evolution of substrate specificity in Escherichia coli. PLoS Biol. 12(2):e1001789.
- Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat. 138(6):1315–1341.
- Lenski RE, Wiser MJ, Ribeck N, Blount ZD, Nahum JR, Morris JJ, Zaman L, Turner CB, Wade BD, Maddamsetti R, et al. 2015. Sustained fitness gains and variability in fitness trajectories in the long-term evolution experiment with Escherichia coli. Proc Biol Sci. 282(1821):20152292.
- Lenski RE. 2017. Experimental evolution and the dynamics of adaptation and genome evolution in microbial populations. Isme J. 11(10):2181–2194.
- Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. 2017. Antibiotic tolerance facilitates the evolution of resistance. Science. 355(6327):826–830.
- Lieberman TD, Michel J-B, Aingaran M, Potter-Bynoe G, Roux D, Davis MR, Skurnik D, Leiby N, LiPuma JJ, Goldberg JB, et al. 2011. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat Genet. 43(12):1275–1280.
- Long CP, Antoniewicz MR. 2018. How adaptive evolution reshapes metabolism to improve fitness: recent advances and future outlook. Curr Opin Chem Eng. 22:209–215.
- Long CP, Gonzalez JE, Feist AM, Palsson BO, Antoniewicz MR. 2017. Fast growth phenotype of E. coli K-12 from adaptive laboratory evolution does not require intracellular flux rewiring. Metab Eng. 44:100–107.
- Mangalappalli-Illathu AK, Korber DR. 2006. Adaptive resistance and differential protein expression of Salmonella enterica serovar Enteritidis biofilms exposed to benzalkonium chloride. Antimicrob Agents Chemother. 50(11):3588–3596.
- Martin M, Hölscher T, Dragoš A, Cooper VS, Kovács ÁT. 2016. Laboratory evolution of microbial interactions in bacterial biofilms. J Bacteriol. 198(19):2564 LP–2571.
- Martino ME, Joncour P, Leenay R, Gervais H, Shah M, Hughes S, Gillet B, Beisel C, Leulier F. 2018. Bacterial adaptation to the host's diet is a key evolutionary force shaping drosophila-lactobacillus symbiosis. Cell Host Microbe. 24(1):109.e6–119.e6.
- Marvig RL, Sommer LM, Molin S, Johansen HK. 2015. Convergent evolution and adaptation of Pseudomonas aeruginosa within patients with cystic fibrosis. Nat Genet. 47(1):57–64.
- McCaughey LC, Grinter R, Josts I, Roszak AW, Waløen KI, Cogdell RJ, Milner J, Evans T, Kelly S, Tucker NP, et al. 2014. Lectin-like bacteriocins from Pseudomonas spp. utilise D-rhamnose containing lipopolysaccharide as a cellular receptor. PLoS Pathog. 10(2):e1003898–e1003898.
- Mundhada H, Schneider K, Christensen HB, Nielsen AT. 2016. Engineering of high yield production of L-serine in Escherichia coli. Biotechnol Bioeng. 113(4):807–816.
- Mundhada H, Seoane JM, Schneider K, Koza A, Christensen HB, Klein T, Phaneuf PV, Herrgard M, Feist AM, Nielsen AT, et al. 2017. Increased production of L-serine in Escherichia coli through adaptive laboratory evolution. Metab Eng. 39:141–150.
- Nicoloff H, Perreten V, McMurry LM, Levy SB. 2006. Role for tandem duplication and Lon protease in AcrAB-TolC- dependent multiple antibiotic resistance (Mar) in an Escherichia coli mutant without mutations in marRAB or acrRAB. J Bacteriol. 188(12):4413–4423.
- O’Boyle N, Connolly JPR, Tucker NP, Roe AJ. 2020. Genomic plasticity of pathogenic Escherichia coli mediates D-serine tolerance via multiple adaptive mechanisms. Proc Natl Acad Sci. 117(36):202004977. Available from: http://www.pnas.org/content/early/2020/08/25/2004977117.abstract.
- Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature. 492(7427):113–117.
- Penterman J, Nguyen D, Anderson E, Staudinger BJ, Greenberg EP, Lam JS, Singh PK. 2014. Rapid evolution of culture-impaired bacteria during adaptation to biofilm growth. Cell Rep. 6(2):293–300.
- Portnoy VA, Herrgård MJ, Palsson BØ. 2008. Aerobic fermentation of D-glucose by an evolved cytochrome oxidase-deficient Escherichia coli strain. Appl Environ Microbiol. 74(24):7561–7569.
- Portnoy VA, Scott DA, Lewis NE, Tarasova Y, Osterman AL, Palsson BØ. 2010. Deletion of genes encoding cytochrome oxidases and quinol monooxygenase blocks the aerobic-anaerobic shift in Escherichia coli K-12 MG1655. Appl Environ Microbiol. 76(19):6529–6540.
- Quandt EM, Deatherage DE, Ellington AD, Georgiou G, Barrick JE. 2014. Recursive genomewide recombination and sequencing reveals a key refinement step in the evolution of a metabolic innovation in Escherichia coli. Proc Natl Acad Sci USA. 111(6):2217–2222.
- Quandt EM, Gollihar J, Blount ZD, Ellington AD, Georgiou G, Barrick JE. 2015. Fine-tuning citrate synthase flux potentiates and refines metabolic innovation in the Lenski evolution experiment. Elife. 4:e09696.
- Rasal RM, Janorkar AV, Hirt DE. 2010. Poly(lactic acid) modifications. Prog Polym Sci. 35(3):338–356.
- Sánchez-Romero MA, Casadesús J. 2014. Contribution of phenotypic heterogeneity to adaptive antibiotic resistance. Proc Natl Acad Sci. 111(1):355 LP–360.
- Sandberg TE, Long CP, Gonzalez JE, Feist AM, Antoniewicz MR, Palsson BO. 2016. Evolution of E. coli on [U-13C]glucose reveals a negligible isotopic influence on metabolism and physiology. PLoS One. 11(3):e0151130.
- Sanz-García F, Sánchez MB, Hernando-Amado S, Martínez JL. 2020. Evolutionary landscapes of Pseudomonas aeruginosa towards ribosome-targeting antibiotic resistance depend on selection strength. Int J Antimicrob Agents. 55(6):105965.
- Schaefers MM, Liao TL, Boisvert NM, Roux D, Yoder-Himes D, Priebe GP. 2017. An oxygen-sensing two-component system in the Burkholderia cepacia complex regulates biofilm, intracellular invasion, and pathogenicity. PLoS Pathog. 13(1):e1006116.
- Schrag SJ, Perrot V, Levin BR. 1997. Adaptation to the fitness costs of antibiotic resistance in Escherichia coli. Proc Biol Sci. 264(1386):1287–1291.
- Sikkema J, de Bont JA, Poolman B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 59(2):201–222.
- Sniegowski PD, Gerrish PJ, Lenski RE. 1997. Evolution of high mutation rates in experimental populations of E. coli. Nature. 387(6634):703–705.
- Sousa AM, Pereira MO. 2014. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis Lungs-A Review. Pathogens. 3(3):680–703.
- Sun S, Berg OG, Roth JR, Andersson DI. 2009. Contribution of gene amplification to evolution of increased antibiotic resistance in Salmonella typhimurium. Genetics. 182(4):1183–1195.
- Tenaillon O. 2018. Experimental evolution heals the scars of genome-scale recoding. Proc Natl Acad Sci USA. 115(12):2853–2855.
- Udall YC, Deeni Y, Hapca SM, Raikes D, Spiers AJ. 2015. The evolution of biofilm-forming Wrinkly Spreaders in static microcosms and drip-fed columns selects for subtle differences in wrinkleality and fitness. FEMS Microbiol Ecol. 91(6):1–9.
- Utrilla J, Gosset G, Martinez A. 2009. ATP limitation in a pyruvate formate lyase mutant of Escherichia coli MG1655 increases glycolytic flux to D-lactate. J Ind Microbiol Biotechnol. 36(8):1057–1062.
- Utrilla J, Licona-Cassani C, Marcellin E, Gosset G, Nielsen LK, Martinez A. 2012. Engineering and adaptive evolution of Escherichia coli for D-lactate fermentation reveals GatC as a xylose transporter. Metab Eng. 14(5):469–476.
- Uzelac G, Kojic M, Lozo J, Aleksandrzak-Piekarczyk T, Gabrielsen C, Kristensen T, Nes IF, Diep DB, Topisirovic L. 2013. A Zn-dependent metallopeptidase is responsible for sensitivity to LsbB, a class II leaderless bacteriocin of Lactococcus lactis subsp. lactis BGMN1-5. J Bacteriol. 195(24):5614–5621.
- Wang Y, Manow R, Finan C, Wang J, Garza E, Zhou S. 2011. Adaptive evolution of nontransgenic Escherichia coli KC01 for improved ethanol tolerance and homoethanol fermentation from xylose. J Ind Microbiol Biotechnol. 38(9):1371–1377.
- Wannier TM, Kunjapur AM, Rice DP, McDonald MJ, Desai MM, Church GM. 2018. Adaptive evolution of genomically recoded Escherichia coli. Proc Natl Acad Sci USA. 115(12):3090–3095.
- Wiser MJ, Ribeck N, Lenski RE. 2013. Long-term dynamics of adaptation in asexual populations. Science (80-). 342(6164):1364–1367.
- Wistrand-Yuen E, Knopp M, Hjort K, Koskiniemi S, Berg OG, Andersson DI. 2018. Evolution of high-level resistance during low-level antibiotic exposure. Nat Commun. 9(1):1599.
- Zabed H, Sahu JN, Suely A, Boyce AN, Faruq G. 2017. Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sustain Energy Rev. 71:475–501.
