Abstract
The ever greater understanding of the composition and function of the gut microbiome has provided new opportunities with respect to understanding and treating human disease. However, the models employed for in vitro and in vivo animal studies do not always provide the required insights. As a result, one such alternative in vitro cell culture based system, organ-on-a-chip technology, has recently attracted attention as a means of obtaining data that is representative of responses in humans. Organ-on-a-chip systems are designed to mimic the interactions of different tissue elements that were missing from traditional two-dimensional tissue culture. While they do not traditionally include a microbiota component, organ-on-a-chip systems provide a potentially valuable means of characterising the interactions between the microbiome and human tissues with a view to providing even greater accuracy. From a dietary perspective, these microbiota-organ-on-a-chip combinations can help researchers to predict how the consumption of specific foods and ingredients can impact on human health and disease. We provide an overview of the relevance and interactions of the gut microbiota and the diet in human health, we summarise the components involved in the organ-on-a-chip systems, how these systems have been employed for microbiota based studies and their potential relevance to study the interplay between food-gut microbiota-host interactions.
1. Introduction
The role that the gut microbiota plays in human health has become ever more evident over the last decade. Increasing evidence suggests that bacteria, viruses, archaea and microbial eukaryotes impact directly, or through the metabolites they produce, on the human body. These microbes and metabolites, or depletion thereof have been associated with several suboptimal health conditions and diseases, both local and systemic, within the human body (Guinane and Cotter Citation2013; Belkaid and Hand Citation2014; Gibson et al. Citation2020). Some examples include the development of metabolic syndrome (Castaner et al. Citation2018), the severity of child and elderly malnutrition (Hermann and Foligne Citation2017; Salazar et al. Citation2017; Leng et al. Citation2019), irritable bowel syndrome (IBS) (Distrutti et al. Citation2016), inflammatory bowel disease (IBD) (Nishida et al. Citation2018), colorectal cancer (Lawrence et al. Citation2020), rheumatoid arthritis (Maeda and Takeda Citation2017), skin conditions (Codoñer et al. Citation2018) or conditions associated to the nervous system, such as multiple sclerosis (MS) (Wekerle and Hohlfeld Citation2016), autism (Garcia-Gutierrez et al. Citation2020), Parkinson’s disease (Miraglia and Colla Citation2019), Alzheimer’s disease (Kowalski and Mulak Citation2019) and depression (Bastiaanssen et al. Citation2019), among others. In addition to evidence of a role for the gut microbiota, it has been suggested that many of these conditions are linked to dietary habits in Western societies (Hills et al. Citation2019), including the increased consumption of processed foods. However, despite being linked with microbiota-related factors, the underlying mechanisms by which these health alterations take place are, in the majority of case, not well understood. In many instances, new experimental models are needed to establish causality, and identify the mechanisms involved (Lynch et al. Citation2019; Walter et al. Citation2020).
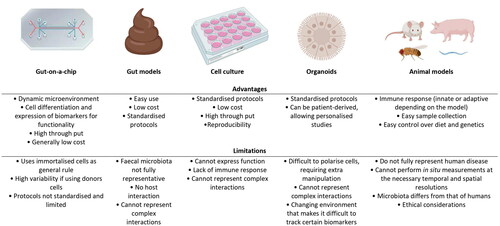
There are several in vitro and in vivo models that are currently used for studying the interactions between food components, the gut microbiota and the human host. However, as with any model, deficiencies exist (). In vitro models try to replicate certain characteristics of a living being artificially and are useful for screening purposes to identify elements of interest for further investigation in vivo. However, the biggest limitation of in vitro models is that the results do not always translate in vivo (Nogacka Citation2020). In vitro models include model gut systems, which become ex vivo models when faecal material is introduced. These models can be discontinuous or continuous models. The discontinuous models are more simple, comprising a closed bioreactor generally controlling temperature, oxygen and pH (O'Donnell et al. Citation2018). Continuous models more closely reflect in vivo conditions, and include a nutrient influx and a waste efflux. There are a number of available validated models, both continuous and discontinuous, that are frequently used for microbiota studies, such as EnteroMix, PolyFermS, CoMiniGut, TIM-1 and TIM-2, SHIME or SIMGI, that have been reviewed elsewhere (Williams et al. Citation2015; Mackie et al. Citation2020).
Figure 1. Advantages and limitations of the different models for the study of host-microbiota-food interactions. Created with BioRender.com.
Although these models, especially those inoculated with a faecal microbiota, can be very informative, it is well-established that faecal samples do not fully reflect the microbiota right throughout the gut, with, for example, the small intestinal and mucosal communities being particularly different from those found in faeces (Bajaj et al. Citation2012; Donaldson et al. Citation2016). Additionally, model gut systems generally do not incorporate the structural and immunological context of the human host, and consequently, the effect of both the supplemented food and the changes produced in and by the gut microbiota on certain aspects of host health cannot be determined (Nogacka Citation2020). Other in vitro models, such as those employing conventional static 2 D tissue culture, cannot support the co-culture of commensal microbiota in a balanced way for more than 24 h as bacteria rapidly overgrow and contaminate the human cell cultures. To overcome these limitations, traditional cell culture has incorporated elements, such as semipermeable membranes, to limit bacterial contamination of cell lines (Fritz et al. Citation2013), and have also evolved into 3 D cell culture-based systems, usually grown in spheroids or bioreactors, to try to mimic the growth of cells in their natural environment (Huh et al. Citation2011).
Another level of complexity can be achieved by incorporating different cell types in a culture, constituting an organoid, which mimics some of the functions that a given organ can display (Wang et al. Citation2018; Kim et al. Citation2020). Organoids can be constructed using pluripotent stem cells or by reprogramming adult stem cells (Bartfeld Citation2016). However, the organoid models are limited by the lack of supporting cell and tissue types found within the living intestine, such as endothelium-lined blood vessels and immune cells. Also, they do not experience fluid flows and cyclic mechanical deformations similar to those experienced in a host intestine that undergoes peristaltic movement, which contributes to intestinal health and function.
To increase complexity in a similar way to that which occurs in the human body, and, therefore, obtain relevant results, in vivo tests are also performed. There are a broad number of different in vivo models that are used for host-microorganism studies and to evaluate the response of the host to certain compounds, such as the moth Galleria mellonella, the nematode Caenorhabditis elegans, the zebrafish Danio rerio, and the fruit fly Drosophila melanogaster (Nogacka Citation2020). These models allow the study of a more complex level of interaction, due to the presence of an immune system. Rodent models such as rats and mice are also very commonly used for microbiota-host interactions in pathogenic and dietary models, as their mammalian guts share greater similarities with those of humans. However, the anatomy of the rodent gut and their gut microbiota are still quite different from that in a human and poorly reflects certain aspects of human physiology and conditions, such as inflammation (Kostic et al. Citation2013; Seok et al. Citation2013; Nguyen et al. Citation2015; Bein et al. Citation2018). The gut microbiota and physiology of other animals, such as that of pigs and monkeys, is more relatable to that of humans (Crespo-Piazuelo et al. Citation2019; Wang et al. Citation2019; Patil et al. Citation2020). However, higher costs and ethical considerations prevent their use on a large scale (Wilkinson Citation2019). One of the main pitfalls of animal models is the prediction of human responses in drug response studies, as drug toxicity is a feature that can vary considerably between species, producing potential loss of time and resources (Redfern et al. Citation2010; Bein et al. Citation2018). Ultimately, there is a need to identify and incorporate alternate models to study the impact of foods and food ingredients on gut microbiota-human cell interactions. Such models could help with the future design of strategies for the maintenance and restoration of health-associated microorganisms in the gut (Reid et al. Citation2011).
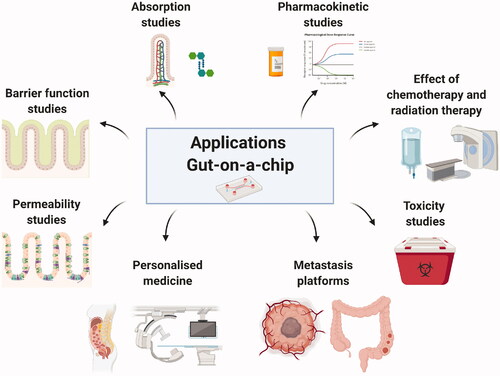
One such set of alternative models are experimental microfluidic organ-on-a-chip (OoC) systems. Such chip systems are generally not considered as replacements for culture models but rather as models that can be used as alternatives, or a means of complementing, animal models (Ingber Citation2018). OoC, and more specifically, gut-on-a-chip can allow the analysis of intestinal structure, function, physiology, and pathology of an integrated cell system representing the living human intestine by allowing analytical and imaging techniques that are not possible to use in other complex in vitro and ex vivo models, such as organoids, where the shape and structure changes on a constant basis, or in vivo models. Gut chip models can incorporate stimuli mimicking peristalsis, which has been proved to increase physiological, anatomical and metabolic representability when compared with in vivo parameters (Ingber Citation2018). Moreover, gut chip models can be connected with other OoC systems to study the interactions between these ‘organs’, with the potential of the eventual development of a body-on-a-chip system. Additionally, these models offer the possibility of using patient-derived stem cells obtained by biopsy (Spence et al. Citation2011; Kasendra et al. Citation2020). However, the most advantageous feature for the study of the interplay food-gut-microbiota is that gut chips enable stable co-culture of human cells with microbes over an extended period of time (Ingber Citation2018). Moreover, the microbiota can also be derived from specific consumers or patients, allowing studies to be performed that are of potential value with respect to personalised diagnostics and treatments (). More specifically, these models are providing key chemical, molecular, cellular and mechanical information about the response of the human intestine, both healthy and with induced enteropathic conditions, to different factors and have the potential to play a pivotal role in detailed understanding how foods/food ingredients and the microbiota influence the human host.
2. The gut-microbiota-food axis
2.1. The gut element
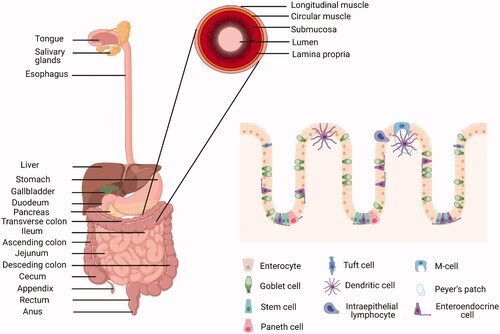
In order to fully digest food, the gut, which extends from the mouth to the anus, requires the contributions of accessory organs such as the salivary glands, the tongue, the liver, the pancreas and the gallbladder (). Anatomically, the gut is a cylindrical structure where food is digested at different points. Digestion starts in the mouth where, for examples, salivary amylase begins the digestion of starch, then progresses through the oesophagus to the stomach where gastric juices contribute to the digestion of proteins. The digestion process continues in the small intestine, where pancreatic enzymes and bile contribute to the breakdown of the food components and the digestion products begin to be absorbed in the form of monosaccharides, peptides, amino acids and fatty acids. The small intestine consists of the duodenum, jejunum and ileum. The duodenum is the closest to the stomach pylorus and receives chime, pancreatic juices from the pancreas, and bile from the liver. The duodenum is short, about 20 cm in length, and is also the location where the neutralisation of stomach acids takes place. Foods then proceed to the jejunum, about 2.5 m in length, the main function of which is the absorption of sugars, amino acids and fatty acids. Finally, the ileum is about 3 m in length and is responsible for the absorption of vitamin B12 and other nutrients, and the re-absorption of bile. The large intestine consists of the caecum, the appendix, ascending colon, transverse colon, descending colon, sigmoid colon, rectum and the anal canal. One of the main functions of the colon is the absorption, along with microbially-mediated breakdown of other food components.
Histologically, the gut comprises four concentric layers, i.e. the mucosa, the inner layer that is in contact with the digested food in the lumen, the submucosa, the muscular layer and the adventitia or serosa, which is the most outer layer. The mucosa contains the epithelium, where absorption and secretory processes take place; the lamina propria, connective tissue; and the muscularis mucosae, smooth muscle that produces agitation and peristalsis. The mucosae of each organ is adapted to the different conditions based on the function of the organ. The epithelium is the component that varies most extensively. In the intestine, the epithelium is formed by a single layer of cells that are renewed every 4–5 days through stem cells known as “proliferative cells,” located in crypts of the intestinal glands. The cells differentiate into seven different cell-types, migrating before starting apoptosis, and shedding off to be washed out. The apical surface of the cells is covered with microvilli, increasing the absorption surface. The main cell types are: the enterocytes, the most numerous and which play a key role in absorption; the goblet cells, that secrete the mucus layer; the enteroendocrine cells, that secrete enzymes; the Paneth cells, that produce antimicrobial peptides; the Microfold (M) cells, involved in immune functions and associated with Peyer’s patches in the small intestine; the Tuft cells, also with immune function; and the Cup cells, with unknown function. These cell types are connected by four types of cell junctions: gap junctions (a protein hexamer that leaves a channel in the middle to transport molecules and ions), desmosomes (strong cell-cell junction complexes), adherent junctions (multiprotein systems involved in the regulation of epithelial migration and cell polarity) and tight junctions (protein complexes involved in transport). Cell junctions are a key element for correct barrier function and, therefore, for maintaining gut health as, when disrupted, microbes can escape the gut in an uncontrolled manner and cause infections (Lee et al. Citation2018). The submucosa is formed by connective tissue, blood vessels, lymphatic and nerves. The submucosa comprises the submucosal plexus and the enteric nervous plexus. The muscular layer consists of two layers, i.e. an inner circular and an outer longitudinal layer, with the myenteric plexus in between, controlling peristalsis. Both muscular layers are coordinated to move the food forward, with the inner circular layer functioning to ensure that food continues in the correct direction, while the longitudinal layer shrinks the tract. The myenteric interstitial cells of Cajal are the ‘pacemaker’ cells responsible for the initiation of the peristaltic activity, but the rate can be modulated by the autonomic nervous system. The serosa membrane comprises several layers of connective tissue in the intraperitoneal part of the gut while the mesentery covers most of the stomach, duodenum, small intestine, caecum, appendix, transverse and sigmoid colon and rectum. In the retroperitoneal parts, the covering layer is referred to as the adventitia and surrounds the mouth, the oesophagus, pylorus, distal duodenum, ascending and descending colon and anal canal.
Functionally, the gut performs many different roles. In addition to food digestion and absorption, it has a barrier function to selectively filter and transport compounds and microorganisms through the system, protecting the body from toxic compounds and pathogens. The gut also has an immune system function via the intestinal mucosal barrier and the gut-associated lymphoid tissue (GALT). Pathogens are also controlled by the low pH of the stomach (Guinane and Cotter Citation2013; Belkaid and Hand Citation2014; Castaner et al. Citation2018; Gibson et al. Citation2020), along with the mucus containing IgA antibodies, and the enzymatic activity of saliva and bile. Beneficial bacteria also helps maintaining the immune homeostasis in the gut by, for example, producing antimicrobials (Garcia-Gutierrez et al. Citation2019). Food intake also regulates immune system in the gut, e.g. the intake of fibre can increase the induction of T-regulatory cells via the production of SCFA, reducing inflammation responses and allergies. It is also important to note that the gut also plays a key sensory role, communicating with the brain via the gut-brain axis in response to digestive by-products of microbial origins, such as short chain fatty acids (SCFA) or gamma aminobutyric acid GABA (Cryan et al. Citation2019).
The gut is in close contact with external elements that can impact health, such as pathogens and potentially harmful substances like xenobiotics, that can induce several disorders (Lee et al. Citation2018). Gastroenteritis is the most common condition of the gut. Bacteria and viruses are the primary cause of foodborne illnesses, producing inflammation in the stomach and the small intestine. If the inflammation is produced in the appendix, it can produce appendicitis, requiring surgical intervention. Cancer can developed along the gut, e.g. in mouth, tongue, oesophageal, stomach and colorectal. Diverticulosis is the condition when pouches form on the intestinal wall, and diverticulitis happens when these pouches get inflamed. Other conditions include coeliac disease, a form of autoimmune response that leads to malabsorption, triggered by the gluten proteins found in wheat, barley and rye. The autoimmune response causes villous atrophy in the small intestine and the only treatment is the lifelong dietary avoidance of the potentially harmful foodstuffs. IBD affects the bowel walls and includes subtypes Crohn’s disease, when affects the entire gut and is considered an autoimmune disease, and ulcerative colitis, when limited to the large intestine. The organs in close contact with the gut, such as the liver and pancreas, can also be bidirectionally affected by the alterations in the gut as they are connected by the enterohepatic and the portal circulation (Storck et al. Citation2019; Albillos et al. Citation2020).
2.2. The microbiome factor
The trillions of microorganisms that the human body harbours and their human hosts have developed a close relationship, co-evolving to the point that humans are considered super-organisms. The gut contains the greatest number of microorganisms, and it is estimated that the colon alone contains around 70% of all microbes in the human body (Jandhyala et al. Citation2015). Indeed, it has been reported that the gut contains a greater number of bacterial cells than there are host cells in the human body (Sender et al. Citation2016). With respect to genetic content, the gut microbiome is estimated to encode around eight million genes, which contrasts with the ∼23,000 unique genes in the human genome (Ceppa et al. Citation2019). Other important microbial communities are located in the mouth, skin, urogenitalia, lungs, breast milk and eyes. The initial colonisation of the gut by microbes occurs in infants during delivery and changes over a period of approximately two to three years, at which time a more settled adult-like community is established (Rodríguez et al. Citation2015; Milani et al. Citation2017). This mature community has greater resilience and is able to recover from exposure to acute stressors, such as the use of antibiotics. More sustained changes to the microbiota can be brought about by factors such as diet or lifestyle over a longer period (Ceppa et al. Citation2019). Later in life, the gut microbiota undergoes further changes, some of which have been linked with conditions such as cognitive impairment or elderly malnutrition (Xu et al. Citation2019). While high levels of gut microbiome richness and complexity, in terms of both genes and species, has generally been associated with health, it is important to note that there is considerable compositional variability in the gut microbiota among individuals that makes very difficult to define what constitutes a healthy microbiota (Shanahan et al. Citation2021). However, an unhealthy microbiota can, in some cases, be easier to identify. It has also been suggested that, given the level of functional redundancy that can exist, the functions encoded rather than taxonomic composition are of key importance with respect to how microbes influence our well-being (Johnson Citation2020).
The gut ecosystem includes bacteria, archaea, viruses and eukaryotes (Pasolli et al. Citation2017). The main bacterial phyla in the gut are Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia. Representative of these groups are not distributed evenly through the gut, reflecting the different micro-environments that exist. The factors that shape the distribution of specific taxa in the gut are the pH, water activity, gas composition, including the presence of oxygen, and the nutrient availability, among others (Lozupone et al. Citation2012; Donaldson et al. Citation2016). As a result, the small intestine is colonised by representatives of groups of facultative anaerobes from the phylum Firmicutes and Proteobacteria that tolerate the presence of bile salts and oxygen. The colon is more densely colonised, mainly by fermentative organisms that degrade complex compounds such as dietary fibre. These include the Bacteroidaceae, Prevotellaceae, Rikenellaceae (Bacteroidetes), Lachnospiraceae and Ruminococcaceae families. The communities also differ transversally in the gut, as the bacteria found in the lumen more closely resemble those found in faecal samples, while those found in the mucosal layer differ.
Despite the increasing knowledge of the gut microbiota, there are aspects that are poorly characterised, including uncultured microorganisms and other microorganisms that can be cultured but whose role is not known (Shanahan et al. Citation2021). Although bacteria are the most studied microorganisms in the gut, the relevance of other groups such as archaea, viruses and eukaryotes will be of considerable interest in the future to further elucidate the interactions that occur within the gut ecosystem. The human mycobiome refers to the diverse range of fungi found in or on the human body. Saccharomyces sp., Malassezia sp. and Candida sp. are among the most abundant fungi species in healthy human stool (Nash et al. Citation2017). Gut fungi influence, and are influenced by, bacteria. Notably there are a number of conditions where bacteria dysbiosis is evident in the gut and are associated with alterations in gut fungi, e.g. enrichment of Candida spp. has been described in patients suffering from primary sclerosing cholangitis, autism and cystic fibrosis (Strati et al. Citation2017; Liwinski et al. Citation2020; Tiew et al. Citation2020) Archaea can also be found in the human gut. 20 species have been identified, including eight methanogens and two halophiles isolated from stool samples (Nkamga et al. Citation2017). The main archaeal groups found in the gut are Euryarcheota and Crenarchaeota, with Methanobrevibacter smithii being estimated to be present in 50% of the adult population. It has been suggested that Archaea, and more specifically, methanogens, have a pivotal role in the removal of the hydrogen excess produced during the carbohydrate fermentation into methane, which is involved in intestinal transit (Nkamga et al. Citation2017). Archaea have also been associated with physiologic disorders, e.g. the abundance of M. smithii has been found to be lower in the gut of obese, in comparison with healthy, individuals (Million et al. Citation2013).
The gut virome is the name given to the community of viruses found within the human hosts. Bacteriophage are especially important, as they can impact the gut bacteriome by predation and horizontal gene transfer, and have also been studied as potential microbiome modulator tools (i.e. phage therapy) (Sutton and Hill Citation2019). Recently, high-throughput sequencing and analysis has allowed the identification of 142,000 new viral genomes, expanding the biodiversity of this previously under-studied group that can play an important role in the gut ecosystem balance (Camarillo-Guerrero et al. Citation2021). The human gut virome is regarded as being quite stable but is highly individual (Shkoporov et al. Citation2019). CrAss-like phage, Microviridae, Siphoviridae, Myoviridae and Podoviridae are regarded as the most abundant viral families in the gut (Shkoporov et al. Citation2019). Although information relating to human gut mycobiome, virome and archaeome has increased in recent years, there are other microorganisms, such as protozoans, that are often overlooked. When they are studied, protozoans are usually the focus of investigations relating to their parasitic effect on the human gut (e.g. Giardia sp. (Barash et al. Citation2017)), however, others such as Blastocystis sp. may also have a role as ecosystem engineers (Laforest-Lapointe and Arrieta Citation2018).
The liver, the gallbladder and the pancreas are among the major organs that have also been reported to have an associated microbiota, albeit at extremely low levels relative to the gut. It is also notable that although it was long believed that bile was sterile, recent culturing- and metagenomics-based studies have indicated the presence of a microbiota in bile, not only in patients with liver inflammation and cholestasis, where it was previously reported, but also in healthy patients (Warburton Citation2017; Molinero, Ruiz, Milani et al. Citation2019; Molinero, Ruiz, Sánchez et al. Citation2019). It has been hypothesised that the presence of microbiota in bile could be due to an impairment of the intestinal barrier and the consequent translocation of microbes and their products via enterohepatic circulation (Isaacs-Ten et al. Citation2020). In the other direction, it has been reported that alterations in liver and bile homeostasis can induce changes in the intestinal microbiota (Cabrera-Rubio et al. Citation2019). The existence of a pancreas-associated microbiota has been less controversial and it is widely accepted, as well as its connections with the gut microbiota (de Goffau et al. Citation2018; Thomas and Jobin Citation2020). Moreover, it has been proposed that a bidirectional crosstalk exists between the gut microbiota and the pancreas that could also be involved in the regulation of antimicrobial peptide (AMP) secretion by pancreatic cells, via SCFA produced by the gut microbiota (Sun et al. Citation2015). Alterations in this crosstalk might be involved in the development of diseases such as diabetes mellitus, pancreatitis and pancreatic cancer (Tilg and Moschen Citation2014; Riquelme et al. Citation2019; Thomas and Jobin Citation2020).
In addition to the SCFAs mentioned previously, the gut can be the source of a wide spectrum of other metabolites that interact with the human host, including lipids and glycolipids, oligosaccharides, terpenoids, polyketides, amino acids, non-ribosomal peptides and ribosomally synthesised post-translationally modified peptides (RiPPs), among many others (Donia and Fischbach Citation2015; Mousa et al. Citation2017; Morton et al. Citation2019; Villarreal-Soto et al. Citation2020). These metabolites can display a variety of functions in the human body, including immunomodulatory, cytotoxic, antioxidant and antimicrobial activities, although many metabolites and their activities are yet to be characterised. Unsurprisingly, changes in the gut microbiome can lead to different patterns of metabolite production that ultimately might alter the appropriate functioning of the body, and therefore, it has been proposed that dietary interventions that impact on the microbiota and/or the metabolites produced represent a logical approach to positively impact health (Devkota and Chang Citation2015; Rowan et al. Citation2017; Storck et al. Citation2019).
2.3. The food modulator
As noted, lifestyle and diet are major influencers of the gut microbiota. While some foods, such as particular processed foods, are associated with negative impacts on the microbiota and, in turn, health, other foods, such as fermented foods, are more frequently associated with positive effects. Therefore, it is rational to investigate the use of diet to modulate the microbiota to address certain conditions. Indeed, it has been suggested that the aforementioned cognitive impairment in the elderly might be linked with a loss of dietary diversity that ultimately leads to inflammation and frailty (Claesson et al. Citation2012; Shanahan et al. Citation2021). In addition, clinical studies involving dietary interventions, such as consumption of a Mediterranean diet, have shown changes in the microbiome, increasing abundance of taxa positively associated with biomarkers of lower frailty and negatively associated with inflammatory markers, and also increases in SCFA production and lower production of secondary bile acids, p-cresols, ethanol and carbon dioxide, independently of age and body mass index (Ghosh et al. Citation2020). Mediterranean diet and associated gut microbiota changes have also been linked to cholesterol reduction (Meslier et al. Citation2020), improved cognitive abilities and memory, immunity and bone strength (Ghosh et al. Citation2020; Merra et al. Citation2021). Mediterranean diet is characterised by a high consumption of fibre and polyphenols sourced from a mostly plant-based diet based on vegetables, fruit, beans and grains, complemented with dairy, eggs, fish and poultry and low levels of red meat (Azzini and Maiani Citation2015), and contrasts with Western diets, characterised by high consumption of processed foods, which have been associated with shifts in the gut microbiome of taxa associated with inflammation patterns and correlated with obesity and metabolic diseases (Broussard and Devkota Citation2016; Zinöcker and Lindseth Citation2018; Shi Citation2019).
Other studies have focussed on the effect of specific dietary components rather than general diet, e.g. polyphenols (Filosa et al. Citation2018; Koudoufio et al. Citation2020) or fibre (So et al. Citation2018; Myhrstad et al. Citation2020). Some of these dietary components can be considered as prebiotics, “a substrate that is selectively utilised by host microorganisms conferring a health benefit” (Gibson et al. Citation2017). Prebiotics have been typically considered carbohydrate-based, but other substances such as polyphenols and polyunsaturated fatty acids are now being considered within the scope of the definition. Thus, consumption of polyphenols has been linked to improvements in cognitive decline and brain function (Angeloni et al. Citation2020; Pontifex et al. Citation2021) whereas fibre consumption is associated with the production of SCFA in the gut that ultimately increase satiety responses and improves gut barrier integrity, reducing inflammation biomarkers (Holscher Citation2017; Makki et al. Citation2018). There have been extensive studies in this area, including a recent investigation that showed that fibre-associated Lachnospiraceae could reduce colorectal cancer tumorigenesis by modulating the tumor-immune microenvironment (Almeida et al. Citation2021).
Particularly interesting is the case of fermented foods, which have been consumed by human cultures over millennia. The fermentation process involves the transformation of food complex constituents of the raw materials into simpler ones, improving the availability of elements such as trace minerals, vitamins and antioxidants, through the activity of fermenting microbes, typically lactic acid bacteria (LAB), but also other microbial species, that contribute to milk, meat, fish, vegetables, cereals and legumes fermentations, on an industrial or artisanal basis (Bell et al. Citation2018; Macori and Cotter Citation2018). Recently, the International Scientific Association for Probiotics and Prebiotics (ISAPP) has defined fermented foods and beverages as “foods made through desired microbial growth and enzymatic conversions of food components” (Marco et al. Citation2021). Some strains of LAB can also be considered potential probiotics (Diaz et al. Citation2019; Pasolli et al. Citation2020). The term probiotic is defined by the WHO as “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host” (Hill et al. Citation2014). There are several observational studies that support the link between the consumption of LAB-containing fermented foods with health benefits such as weight management, cardiovascular disease and high blood pressure, type 2 diabetes and impaired glucose metabolism, or mood and brain activity (McGrane et al. Citation2011; Mozaffarian et al. Citation2011; Chen et al. Citation2014; Arneth Citation2018; Firth et al. Citation2020). Moreover, the consumption of fermented foods has been correlated with lower mortality rates and lower cancer risk development (Zhang et al. Citation2019). Some of the metabolites produced by microorganisms in fermented foods have been linked to beneficial health effects (). However, fermented foods and probiotics not necessarily produce the same effect in every consumer and, in fact, some of their metabolites, such as biogenic amines, can produce detrimental effects, such as migraines or abdominal pain (Diaz et al. Citation2016; Simon Sarkadi Citation2017). It would be important for dietary interventions to elucidate which are, and to what extent, the foods and individual characteristics, including gut microbiota, that are associated with different outcomes.
Table 1. Some examples of microbial metabolites in fermented foods.
The microbial communities present in fermented foods can be considered as a transient microbiota (De Filippis et al. Citation2020). At the moment, it is unknown which fraction of this transient microbiota associated with foods is transferred to the gut microbiota. However, a recent study analysed 9445 metagenomes from human samples and found that closely related LAB strains occur in both food and gut environments, providing evidence that fermented foods could be considered a source of LAB for the gut microbiota (Pasolli et al. Citation2020). The bacterial ability to colonise the gut has been associated with certain traits, such as exopolysaccharide production, that can protect bacteria from the acid and bile activities in the gut, the ability to adhere to the intestinal epithelial cells and the colonic mucin, or the production of antimicrobial compounds, that might enhance their ability to compete against other bacteria (Nueno-Palop and Narbad Citation2011; Garcia-Gutierrez et al. Citation2019).
The effect that certain probiotic, prebiotic or specific diet produces in the gut microbiota may be impacted by the initial composition of that microbiota (Zmora et al. Citation2018). In practical terms, this means that individual studies might be required, e.g. in the case of supplementation with a probiotic, in addition to the importance of the specific strain, its dose might also need to be adjusted to each person to obtain the intended effect. Another consideration is that the formulation might require prebiotic supplementation for optimal effect. The combination of prebiotic and probiotic elements is defined as synbiotic. More specifically, the ISAPP has defined a synbiotic as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (Swanson et al. Citation2020). Indeed, recently it has been found that a synbiotic combination of the probiotic Lactobacillus rhamnosus GG (LGG) and the benzoic acid derivative salicylic acid increased the co-aggregation effect of LGG with Escherichia coli, increased L. rhamnosus anti-oxidant properties and also induced the cytotoxic effects of LGG against human colon cancer cells (Celebioglu Citation2021). Another example of a synbiotic application is the improvement in perception scores and C-reactive protein in IBD patients after the use of Bifidobacterium longum in combination with psyllium (Parian et al. Citation2018). It has been observed that some metabolites can exert their beneficial health action even if the microbes are not alive or present, by the consumption of the fermented matrices where bacteria have been inactivated after releasing these compounds. Such metabolites can be defined as “postbiotics” (Collado et al. Citation2019). In one such case, lipoteichoic acid (LTA) produced by Bifidobacterium animalis subsp. lactis BPL1 (CECT8145 ameliorated obesity biomarkers in a pre‐clinical model of C. elegans, exerting its function through the IGF‐1 pathway, as did the BPL1 strain, with a potential therapeutical application for metabolic syndrome and diabetes-associated disorders (Balaguer et al. Citation2021).
Overall, the evidence suggests that important health benefits can be conferred from dietary interventions but we need to identify the underlying mechanisms of action of the dietary components in order to exploit and maximise them. However, due to the current limitations, it is often difficult to separate association from causation. Therefore, we need methods that can help us assess how this interaction takes place.
3. Organs-on-a-chip
Organs-on-a-chip (OoC), at the interface between engineering and cell biology, have been developed as microfluidic options to overcome some of the limitations of other models. Originally they were fabricated by adapting the processes used for computer microchip production, and they retained the name (Ingber Citation2018). Microfluidics-based OoC have been used mainly for biomedical and pharmaceutical research, in toxicological and pharmacokinetics studies and, more recently, in pathophysiology and tissue development studies (Ingber Citation2018; Wang et al. Citation2018; Zhang et al. Citation2018; Jang et al. Citation2019). However, as the technology expands, other applications are being developed.
A successful OoC design requires a deep understanding and accurate reproduction of the functional structures of the organ, e.g. in the case of the gut-on-a-chip, the key functions would be the absorption and transport of nutrients, and the presence of an immune and endocrine systems along with an associated microbiota, with the villi being the location where many of these elements converge (Kasendra et al. Citation2020). In the case of a liver-on-a-chip, the smallest functional unit is the liver lobule, a hexagonal structure that has a central vein, and in the pancreas-on-a-chip, the pancreatic islet (Deng et al. Citation2019; Rogal et al. Citation2019).
The mimicking of certain organ conditions is another factor that increases the representativeness of the OoC model. Indeed, the fluid flow and shear stress within the human gut has been reported to be 0.002–0.08 dyne cm−2 approximately and, therefore, the culture medium pumped into the microchannels has been established around these values, where a volume of 10–100 µl h−1 provides 0.006–0.06 dyne cm−2 approximately (Kim et al. Citation2012). The modification of flow rate also allows the optimisation of important parameters involved in gut function, such as residence time as physiologically established for the colon (Degen and Phillips Citation1996; de Haan et al. Citation2021). Other parameters, such as the Reynolds number, important to calculate the rate of digestion and/or absorption of nutrients in the intestine, depend on the viscosity of the medium and the flow parameters. The Reynolds number obtained in experimental data suggested that there is a laminar flow in the human colon, in the range 0.11–0.34, which has been replicated in the gut-on-a-chip systems, with lower values, around 0.007 (Takahashi Citation2011; Kulthong et al. Citation2020). Overall, OoC allow a precise control of the parameters and conditions that ultimately can improve modelling.
As microfluidic devices, the OoC structures have in common the presence of channels, sensors and electrodes embedded in a transparent body of a polymeric gas-permeable material that allows the diffusion of oxygen and carbon dioxide. Depending on the organ model, there might be additional structures, like a porous membrane, in the case of the gut-on-a-chip, separating the channel of epithelial cells and the channel of vascular endothelial cells, lined in a confluent monolayer that mimics a blood vessel and recreates the barrier properties (Yeste et al. Citation2018; Kasendra et al. Citation2020). However, this feature is not always present, as some designs have not included this component, to make the model more similar to real tissue conditions (Trietsch et al. Citation2017). Other OoC might include membranes to mimic the blood-brain-barrier (van der Helm et al. Citation2016). As a general rule, the cells are seeded in static conditions and, once they are adhered, stimuli are introduced to mimic the in vivo microenvironment. Of particular importance is the mechanical strain that, in a representative manner for each organ, e.g. peristalsis-like in gut-on-a-chip or the breathing movements of the alveolar barrier in the lung-on-a-chip, increases the biological relevance of the system (Guenat and Berthiaume Citation2018; Ingber Citation2018).
There is a range of common analytical methods used to obtain accurate data from the different OoC models. Techniques such as surface plasmon resonance, multi-omics, high-resolution real time fluorescence imaging, electrochemistry or mass spectroscopy have been adapted to obtain quantitative real time information of the biological data obtained during the experimental process (Ingber Citation2018; Kilic et al. Citation2018). Optical methods are used to assess cell development, morphology and viability and, therefore, the suitability of the cell organisation and their structures. They involve light, fluorescent and confocal microscopy, but also colorimetric strategies to detect presence or absence of metabolic products of interest or assess cell and tissue integrity (McDonald and Whitesides Citation2002; Kilic et al. Citation2018). Generally, optical and electrochemical strategies are preferred, as they are non-invasive and can be used to quickly identify potential problems (Wang et al. Citation2018; Jalili-Firoozinezhad et al. Citation2019). The development and integration of biosensors in the chips allow in situ monitoring of cell behaviour (cell adhesion, detachment, death, response to osmotic stress, sepsis), mechanical and electrical stimulation, chemical gradients (hydrogen peroxide, glucose, lactate or cytokines detection and quantification), well beyond the traditional environmental parameters of temperature, pH, humidity and oxygen levels (Kilic et al. Citation2018).
Given the nature of the OoC, some techniques might be more relevant than others. Thus, for the gut-on-a-chip model, one of the most important parameters to assess is the integrity of the monolayers in order to ensure appropriate barrier function prior to any study. The protocols most commonly used for this validation rely on transepithelial electrical resistance (TEER) or the dyes Lucifer Yellow and Cascade Blue. The TEER protocol measures the electrical resistance as an indicator of the permeability and integrity of the cell layer. The TEER values depend on temperature, medium formulation, passage number of cells and the technique itself. It has been reported that late passage Caco-2 cells show higher TEER values, indicating the growth of multilayers (Lea et al. Citation2015; Srinivasan et al. Citation2015). TEER measurements are typically used in cell culture, but it was reported that values are typically higher in the OoC in comparison with the traditional 2 D culture (Odijk et al. Citation2015). Other methods to assess the integrity of the barrier function rely on dyes. Lucifer Yellow is a low molecular mass compound with innate fluorescence that has historically used for this purpose. However, Cascade Blue has a narrower emission spectrum, which is useful to avoid interferences later. Large molecules, e.g. polyethyleneglycol (PEG) or dextran-conjugated fluorophores, have been also used, but their size can result in a lower sensitivity of the assay (Novak et al. Citation2018). The study of the tight junction proteins can also be informative to establish the integrity of the monolayer, using staining and confocal immunofluorescence microscopy to obtain optical information (Kim et al. Citation2012).
Another important element is the monitoring of the cellular microenvironment. Thus, oxygen, pH and temperature are factors that will have a dramatic impact on the biochemical parameters of the OoC. The channel’s effluents can be designed to analyse pH and gas and metabolite concentrations. More recently, other developments have been coupled with OoC approaches in order to increase their analytical and application potential. In this regard, high-throughput analysis of biomarkers and metabolites can be performed using ELISA, PCR and the incorporation of mass-spectrometry (MS) platforms (Oedit et al. Citation2015; Wu et al. Citation2016; Junaid et al. Citation2017; Li and Tian Citation2018; Lin et al. Citation2020). Advances have been made also in remote on-line monitoring, with applications developed for hardware control from tablets, smartphones or smart-watches connected to the sensors placed in the OoC devices, that allow data visualisation and remote control of the parameters (Kilic et al. Citation2018).
3. 1. The materials
Although some materials, such as polymethyl methacrylate (PMMA) (Zhang et al. Citation2017; Sriram et al. Citation2018) and glass (Kulthong et al. Citation2018), are used on occasion for the microfluidic devices employed for OoC, the material most typically used for the support structure is polydimethylsiloxane (PDMS). PDMS is an inexpensive biologically inert, gas permeable, non-toxic polymer that displays low adhesion, allowing more cell-cell interactions than cell-substrate interactions (Mata et al. Citation2005; van Poll et al. Citation2007). Because of its transparency, it is suitable for the microscopy requirements that accompany the OoC platform. PDMS also has disadvantages, such as its hydrophobicity, which can induce the adsorption of lipophilic compounds to the material and the gas permeability, including oxygen and water vapour, which can make it difficult to maintain the appropriate gas levels for culture growth (Ochs et al. Citation2014). However, both problems can be mitigated by introducing surface modifications (Gokaltun et al. Citation2017; Wolf et al. Citation2018). Importantly, PDMS can be applied as a viscous liquid in 3 D printed scaffolds, so the chips can be easily manufactured, in contrast with the PMMA and glass alternatives (Dahlberg et al. Citation2018; Novak et al. Citation2018; Mikkonen et al. Citation2020). Currently, the chips can be purchased from different manufacturers, but there are also highly detailed published protocols that allow in-house constructions of chips and customisation upon requirements (Kim et al. Citation2012; Dahlberg et al. Citation2018; Novak et al. Citation2018).
Channels are separated by a membrane of a porous material, typically polycarbonate, PDMS, polyester and collagen (PET) (Ashammakhi et al. Citation2020). The choice of the material depends on the pore size required as this will control the transfer of molecules between chambers. Other structures, like the OrganoPlate, use a collagen type I-based gel instead of a membrane for the separation of the two channels (Virumbrales-Muñoz et al. Citation2020). Both the chambers and membranes can be coated with materials that will facilitate cell attachment, migration and differentiation, with the most commonly used coatings consisting of collagen, matrigel, gelatine, gelatine methacryloyl, fibrin and fibronectin (Ashammakhi et al. Citation2020). There can be a modification of the surface to introduce scaffolds to facilitate villus formation. Particularly in the case of the gut-on-a-chip, these modifications seem to have an impact on Caco-2 cell metabolism, increasing mitochondrial activity and lowering phosphatase activity to physiological levels that are more representative of than is the case for cells grown over flat surfaces (Ashammakhi et al. Citation2020).
3.2. The cells
An OoC is typically formed by representative cells to form a functional unit of the organ. The choice of the cell types used to build the OoC are critical in determining how representative the model will be. As a general rule, OoC can be populated with commercial cell lines or with human induced pluripotent stem cells (hiPSCs) and primary stem cells, which allow differentiation into the cellular subtypes. Another option is the use of primary cells directly obtained from biopsies, which can be used to develop personalised medicine but, on the other hand, differences between donors can also make it difficult to obtain reproducible results.
In the case of the gut-on-a-chip models, it is important to recognise that, as previously noted, the intestinal epithelium comprises a variety of epithelial cells with different functions (enterocytes, goblet cells, M-cells, etc). To mimic this epithelial component, the cell line most widely used is the immortalised human colon carcinoma cell line Caco-2 (from Cancer Coli-2) (Lea et al. Citation2015). It was observed, during traditional 2 D cell culture, that Caco-2 were able to polarise with tight junctions and brush border at the apical side, and further differentiate by expressing different morphologies and functional properties, such as specific receptors and enzymes for the different epithelial cell types (Lea et al. Citation2015). There are other well-stablished cell lines, such as HT29, widely used to investigate interactions with microorganisms and nutrients (Nogacka et al. Citation2018). However, the Caco-2 cell layer, once differentiated, reports TEER values 4 times higher than the HT29 monolayers, which is more representative of an in vivo situation. Caco-2 cells express most receptors and enzymes, but no P-450 metabolising enzyme is reported in 2 D cell culture. However, P-450 activity is evident in Caco-2 cells differentiated in a gut-on-a-chip structure. Moreover, they differentiate into polarised epithelial cells and spontaneously form crypts and villus structures within five days (Sambuy et al. Citation2005; Kim and Ingber Citation2013), despite the fact that traditional protocols require 21 days for this to occur (Lea et al. Citation2015). The measurement of aminopeptidase activity is a strategy used to assess functionality in differentiated Caco-2 cells, since aminopeptidase enzyme is expressed in the brush border (Kim et al. Citation2012). The main setback is that Caco-2 cells are not able to produce a significant mucosal layer, which is especially important when considering that this layer plays a key influence on the permeability of drugs and molecules (Lea et al. Citation2015). To overcome this, primary cells have started to be used increasingly as epithelial cells in gut-on-a-chip devices. However, they are less robust, less accessible and require extra growth factors that need to be supplemented and the results can be less reproducible. Another strategy has been the construction of organoid-derived intestine chip using organoids derived from biopsies from endoscopic or surgical origins (Kasendra et al. Citation2020). This allows a better development of tissue architecture and function from patient cells, which ultimately brings more representative data for drug assessment. This was established by comparing the CYP450 cytochrome activity, particularly the expression and induction levels of CYP3A4, to the Caco-2 cell cultures (Kasendra et al. Citation2020).
In the case of the liver-on-a-chip, there are two types of functional epithelial cells, the parenchymal hepatocytes and the biliary epithelial cells. Other important cell types are the macrophages, i.e. Kupffer cells, and the fibroblasts, i.e. hepatic stellate cells. To construct the chips, three cell types have been used: primary hepatocytes, hepatic-derived cells lines (HepG2, HepaRG and C3A) and stem cells inducing hepatocytes. The primary hepatocytes reproduce the liver characteristics, but vary considerably between donors, are not suitable for long term use, can be difficult to isolate and be costly. In contrast, the cell lines can be used for long term, as they have unlimited lifespan, they are stable and can be easily manipulated. However, they quickly lose the ability to express differentiation biomarkers. When stem cells as a source of hepatocytes, they are also stable and can express differentiation biomarkers, but they are costly and difficult to manipulate (Deng et al. Citation2019).
Very recently, researchers have successfully built a device to mimic the pancreas-on-a-chip. Pancreatic ductal epithelial cells (PDECs), and pancreatic islets were investigated to mimic the functional unit of the pancreas (Shik Mun et al. Citation2019). Epithelial cell biomarkers for PDECs are cytokeratin 19 (KRT 19), E-cadherin, sodium transport channel (ENaC), and tight junction protein (ZO-1). To assess if PDECs are functional, the cystic fibrosis transmembrane conductance regulator (CFTR), which is key for maintaining fluid and pH within the pancreatic duct to deliver digestive enzymes, is measured using a cAMP-activating agonist forskolin assay. In addition, pancreatic islets are responsible for the production of insulin (β cells) and glucagon (α cells) to maintain an appropriate blood glucose level and functionality and viability is measured by immunostaining and by measuring the concentration of insulin and glucose in the media. The development of pancreas-on-a-chip has taken place quite recently and, so, it has not been used as widely as the gut-on-a-chip and liver-on-a-chip systems.
3.3. The stimuli
The OoC are systems that allow the introduction of physicochemical and mechanical stimuli. Whereas the physicochemical conditions can be achieved in other models, as cell culture requirements, and will not be further developed in this review, the mechanical stimuli is not as extended, being one of the distinctive characteristics of the OoC microfluidic systems. The mechanical stimuli has proved to modulate the proliferation, migration, phenotype, and/or differentiation of cells (Kaarj and Yoon Citation2019). Arguably, the fluid flow that provides shear stress to the cells is the most important mechanical strategy, since it has been reported that mucin-2 and actin expression were upregulated when compared with cells grown in a static Transwell model (Chi et al. Citation2015). Additionally, the stimulation provided by the flow induces Caco-2 polarisation, morphogenesis and differentiation into complex villi (Kim and Ingber Citation2013; Maurer et al. Citation2019). The differentiation of epithelial cells into different cell types can be confirmed by the expression of markers, such as α-defensin for Paneth cells, cytochrome P450 in enterocytes and MUC-2 in goblet cells. Peristalsis-like strain is another relevant mechanical stimulus for the gut-on-a-chip model. Peristalsis-like strain also induces morphogenesis in 3 D intestinal villi structures and the expression of tight junctions, presentation of a brush layer and the mucous production (Kim and Ingber Citation2013).
Mechanical forces are also important to study the relationship between the cell layer and the microbiota. Thus, the presence of flow, in contrast with a static model, reduced the number of adhered E. coli (Chi et al. Citation2015) whereas the cell differentiation induced by the mechanical stimuli was sufficient to show that Shigella infected the apical side of the enterocytes and used the crypt-like invaginations for early colonisation. When peristalsis was simulated, invasion increased, making the model a more realistic representative of in vivo activities.
There are different strategies to deliver these mechanical stimuli that have been reviewed elsewhere (Kaarj and Yoon Citation2019). Briefly, mechanical stimuli in OoC can be laminar or pulsatile, when it flows along the microfluidic channel. Laminar relates to one flow direction and pulsatile when the stress induced by the flow on the cell surfaces is bidirectional. Mechanical stress can also come from interstitial flow, through the extracellular matrix (ECM), but also from compressive forces on top of the cells. As a general rule, the stimuli can be delivered using syringe pumps or pressure regulators, but also with compression devices. In some instances this has been done passively, either by gravity or hydrostatically. The choice between strategies relies on the type of OoC, as different cell types require stimuli relatable to their biological microenvironment (Kaarj and Yoon Citation2019).
3.4. Connecting organs-on-a-chip
In order to study effects at a systemic level, different organs can be built together in a multi-organ platform, e.g. a three-component microfluidic device was designed to mimic the digestion process, including microenvironments for mouth, stomach and small intestine, with their specific pH, buffer and fluid composition, although without gut mucosa (Zhang et al. Citation2018; de Haan et al. Citation2019; Steinway et al. Citation2020). There are four groups of different strategies that can be used to connect organs in microfluidic devices that have been reviewed elsewhere (Wang et al. Citation2018). Briefly, the first of these are (a) static, e.g. Transwell platform; (b) single-pass microfluidic platforms that connect all organ modules in series in one fluid route or with additional routes if barrier tissues are involved; (c) pump-driven recirculating platforms that interconnect organ modules serially and/or in parallel in a closed-loop circuit. Separate fluidic pools or loops are needed for barrier tissues and (d) pumpless recirculating platforms utilise gravity-driven flow and a rocking motion to drive fluid through the organ module network, in which organ modules are connected in serial and/or parallel.
Multi-organ platforms can increase the biological relevance of the model. Gut-liver systems are very well explored, and have been particularly developed for toxicology and pharmacokinetic studies for preclinical drug screenings (Bricks et al. Citation2014; Tsamandouras et al. Citation2017; Chen et al. Citation2018). There are simple systems for studies where a gut-on-a-chip (using Caco-2 cells) and a liver-on-a-chip (using HepG2 cells) are recreated in a platform (Choe et al. Citation2017). The flow connecting these was in the gut to liver direction, mimicking the absorption and metabolism process, decreasing permeability of the Caco-2 barrier and both cell lines increased cytochrome P450 metabolic activities, indicating relevant biological behaviour. Another relevant process observed is that the transport of lipophilic molecules in a Caco-2 model can be determined by the presence of bile acids and phospholipids (Lea et al. Citation2015), a feature that can be optimised through the development of OoC in tandem for pharmacokinetic studies. The connection between the gut and liver was used for studies under inflammatory conditions, where it was reported that both gut and liver amplified responses and increased hepatic metabolism markers (Tsamandouras et al. Citation2017). A different gut-liver model was constructed using primary human intestinal cells, made immortal by transducing the cells with human telomerase reverse transcriptase (hTERT), and 3 D liver tissue, using HepG2 C3A cells. Both chambers were connected by media that flowed by gravity and the viability of the cells in the system was sustained up to 14 days (Chen et al. Citation2018). A system with four microfluidic components was constructed, where media flowed connecting the OoC, modelling a liver (with hepatocytes, Kupffer, and Stellate cells), a kidney (using primary human proximal tubular epithelial cells), a brain (iPSC-derived human neurons and astrocytes) and a gut in a Transwell model (Vernetti et al. Citation2017). Another system involving a gut-on-a-chip model, a liver organoid, a kidney organoid, and human skin biopsies was able to maintain viability over 28 days and was used for studying toxicity, including drug absorption, distribution, metabolism and excretion (Maschmeyer et al. Citation2015).
Another organ that is being studied in connection with the gut through a microfluidic approach is the brain. Recently, the “MINERVA” project commenced with the objective of mimicking the conditions for a complex gut-brain chip model that attempts to provide an environment for the study of the inflammation processes in the development of neurologic diseases such as Alzheimer’s and Parkinson’s, mimicking the immune system and the blood-brain-barrier (Bulutoglu et al. Citation2019; Donkers et al. Citation2021).
4. Organs-on-a-chip for the study of microbes and the microbiome
Co-culture protocols are well-established for microbial studies (e.g. adhesion, invasion, translocation, etc.), the main challenges for long-term co-culture being microbial overgrowth and the loss of epithelial viability, mainly due to the differences in growth requirements that ultimately lead to problems of control of microbial population density and metabolite production. OoC models can be used to overcome these problems and allow the study of the interactions between the microbiota and the human tissues. In fact, it has been noted that, currently, this is the only non-invasive approach available that can allow researchers to explore the influence of the human microbiota on the development of human tissues over time (Ingber Citation2018). The first successful study of gut-on-a-chip incorporating microbial elements was based in the co-culture of Caco-2 cells and L. rhamnosus GG (Kim et al. Citation2012) (). The chip was developed by adapting a previous design for a lung-on-a-chip that included vacuum chambers to simulate breathing deformation, which was converted to reflect peristaltic activity. Two microchannels separated by a 10 µm-porous PDMS membrane coated with ECM to facilitate cell adhesion. Caco-2 cells were seeded in the upper microchannel and, after observing optically their attachment to the porous membrane and the development of cell-cell adhesion, the flow rate helped to further establish the monolayer. The integrity of the cell monolayer was evaluated by staining occludin, a tight junction protein, and measuring TEER, and the cell functionality was assessed by measuring the aminopeptidase activity. Caco-2 cells were cultured until their barrier integrity reached a relevant TEER value, at which point the previously cultured L. rhamnosus GG was transferred to the apical surface of the Caco-2 cells. This first study was important because the gut-on-a-chip layout experiment was developed in parallel to other two combinations of conditions, one using the Transwell system (static chambers without fluid flow nor mechanical stimuli) and another using the gut-on-a-chip only with the flow but without the mechanical stimuli. The results showed that flow and shear stress accelerated the Caco-2 differentiation to 3 days in comparison with the static Transwell model, where cells remained flat after 3 weeks. TEER levels for intestinal barrier function were higher than cells in static growth and functionality marker was higher in the gut-on-a-chip model using flow and shear stress. Results showed that, in presence of L. rhamnosus GG, these differences were even higher and sustained in time, whereas in the Transwell model the monolayer died and detached in 48 h. Thus, those original challenges associated with microbial overgrowth and contamination with microbial metabolites, such as organic acids altering the medium, could be overcome by the effect of the flow, washing out unattached microbial cells and toxic metabolite products.
Figure 4. Examples of different complexity levels in gut-on-a-chip systems that use immortalised cells to study the interactions with gut microbiota. The example in the upper position represents the first study that co-cultured L. rhamnosus GG with Caco-2 cells (Kim et al. Citation2012). On the right hand side, another system incorporated Human Peripheral Blood Mononuclear Cells (PBMCs) to further study the gut-immune system interactions, like in the Humix system, that also allowed the growth of anaerobes such as Bacteroides caccae (Eain et al. Citation2017). On the right hand side, a representation of the system generated by Jalili-Firoozinezhad et al, including endothelial cells and a complex gut microbiota from humanised mice (Jalili-Firoozinezhad et al. Citation2019). Created with Biorender.com.
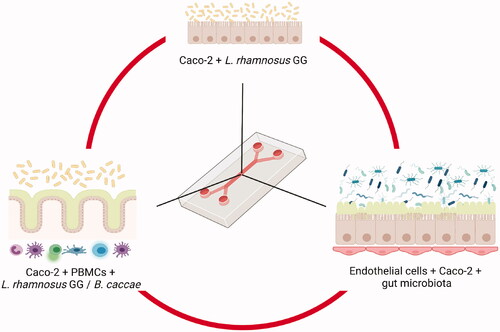
Many studies have adapted this technology since then, increasing the complexity and purposes of the models. Other systems were designed to incorporate another chamber to separate the microorganisms from the host cells (Shah et al. Citation2016). The three-chamber device, named HuMiX, contained oxygen sensors and TEER and fluorescent microscopy were used to assess barrier function, using L. rhamnosus GG, grown alone in aerobic or anaerobic conditions, or grown in combination with an obligate anaerobe, Bacteroides caccae, under anaerobic conditions. The system could be applied for the study of host-microbe interaction, drug screening and/or nutritional studies.
An important milestone was achieved with the design of a system that allows co-culturing aerobic and anaerobic microorganisms over at least five days, allowing an investigation of host-microorganism crosstalk (Jalili-Firoozinezhad et al. Citation2019). This device was able to overcome a major setback of gut-on-a-chip systems, optimising the conditions for the aerobic human cells that could be damaged in the presence of low oxygen concentrations, and the anaerobic microbiota, that could be damaged in the presence of higher oxygen concentrations. The chip was constructed using two channels, a lower one containing endothelial cells, and an upper one growing epithelial cells in contact with the microbiota. The oxygen gradient was built, mimicking the endothelium–epithelium–lumen axis, by flowing the oxygenated medium into the lower channel whereas the chip is in the anaerobic chamber. The oxygen was detected by fluorescent oxygen sensors. An initial simpler system was built using human intestinal microvascular endothelial cells, Caco-2 as epithelial cells and Bacteroides fragilis as anaerobe. The system was successful because 1) endothelial and epithelial cells remained viable; 2) Epithelial cells polarised and formed villi and tight junctions and produced mucus and 3) B. fragilis number of colony forming units increased, meaning that oxygen concentration was low enough. A further level of complexity was added by incorporating gut microbiota from humanised mice that was previously tested to be sure that it was within the range of composition that the Human Microbiome Project established (). This model was also successful because the epithelial barrier function was increased after the co-culture with the microbiota. A more complex system was built, as a proof of concept, using epithelial cells from an ileal biopsy of a patient, building a chip in a similar way, after co-culturing the cells with fresh faecal microbiota (Jalili-Firoozinezhad et al. Citation2019).
Some microbiota-gut-on-a-chip devices also study the immune element, which can be a valuable tool to elucidate the inflammatory process in the gut (Beaurivage et al. Citation2020). An initial inflammation gut-on-a-chip model was developed to study IBD at the interface of gut microbiota, intestinal mucosa and immune components. The device for studying the gut-immune interactions was built using Caco-2 cells and Human Peripheral Blood Mononuclear Cells (PBMCs) (Kim et al. Citation2016). The system had induced cyclic mechanical deformation and flowing medium and these conditions induced the production of a mucus layer. For this study, several microbial combinations were used, e.g. commensal microbes from the therapeutic formulation of probiotic bacteria (VSL#3), six of the eight bacterial strains within which were originally isolated from the human gut, three lactobacilli (L. acidophilus DSM 24735, L. plantarum DSM 24730, L. paracasei DSM 24734) and three bifidobacteria (B. breve DSM 24732, B. longum DSM 24736, and B. infantis DSM 24737) (De Simone Citation2018). Additionally, other non-commensal bacteria were also added to the system: a green fluorescence protein (GFP)-labelled E. coli, a pathogenic enteroinvasive (EIEC) E. coli (serotype O124:NM), and lipopolysaccharide (LPS) endotoxin from pathogenic E. coli (serotype O111:B4). Novel insights were gained from this approach that would not be possible using animal models. Notably, it was reported that high levels of IL-8 were necessary for proinflammatory cytokines IL-6, IL-1β, and TNF-α to exert their villus injury effects and the disruption of the intestinal barrier. The effect of the LPS and the non-commensal bacteria were also assessed, as the protective effect of the VSL#3 formulation against the overgrowth of the EIEC strain and its gut damage effect. The model allowed the characterisation of the immune response via gene expression profile, e.g. up-regulation of receptors that mediate immune cell recruitment, for histamine and leukotriene B4, commonly found in IBD patients, proving to be a potential informative method to characterise the molecular mechanisms underlying IBD.
Another study introduced human PBMCs containing a mixed population of innate (monocytes and granulocytes) and adaptive (lymphocytes) immune cells to examine their crosstalk with epithelial cells in the presence of different stimuli, like dextran sodium sulphate (DDS), LPS endotoxin, probiotic VSL#3, and non-pathogenic E. coli (Shin and Kim Citation2018). This model allowed the isolation of each component of the signalling cascade the measurements of proinflammatory cytokines and helped to identify compromised intestinal barrier function (Shin and Kim Citation2018).
Similarly, other microorganisms have been used in gut-on-a-chip devices to investigate their effects on the intestinal barrier. Such studies have involved the use of Shigella, Candida, coxsackievirus B1 (CBV1) and probiotic microorganisms other than those listed above (Villenave et al. Citation2017; Grassart et al. Citation2019; Maurer et al. Citation2019). These studies provided evidence that the tissue structure might be relevant for the infective processes of CBV1, that some lactobacilli have protective effects against opportunistic invasion of the epithelium by Candida and that the peristalsis-like stimuli induced the invasion capabilities of Shigella in the colonic epithelium. Moreover, the mimicking conditions of the gut-on-a-chip host have been suggested to provide some of the host-microbiota cross-talk culture requirements to be used to enrich previously uncultured gut microorganisms listed in the Human Microbiome Project’s Most Wanted taxa (Ma et al. Citation2014).
Ultimately, OoC can, provide valuable insights that can help construction of a more complete picture of host-microbiota interactions, when combined with other models.
5. Microfluidics platforms for the study of nutritional components: the NutriChip project
Given the influence that food can have in the gut, there have been attempts to determine to what extent this can be due to their immunomodulatory potential, initially using Transwell systems and more recently using microfluidics (Ramadan et al. Citation2013). However, the use of gut-on-a-chip devices for this purpose has not been extended, mainly due to the structural nature of the food, requiring complex sample processing and methods that are still yet to be developed (Xiang et al. Citation2020). Currently, the most advanced option has been the NutriChip, which was the focus of efforts to be validate through a human nutrition trial (Vergères et al. Citation2012). The objectives of the NutriChip project were to develop a structure that mimicked the digestion process, intestinal absorption, and the modulation of immune cells by the bioavailable nutrients, connected to a gut-on-a-chip structure for cell co-culture and imaging technologies and, ultimately, providing a platform for nutrient screening and validation in human nutrition. The ultimate success of this approach has yet to be revealed.
6. Gut-associated disease models based on the organ-on-a-chip technology and their clinical significance
As noted above, the presence of an immune element in the OoC could favour the study of certain inflammatory conditions associated with alterations in the gut microbiota, including a variety of chronic diseases (Beaurivage et al. Citation2020; Moreno-Gonzalez and Beraza Citation2021). However, the clinical applications of OoC have mainly focussed on understanding the physiological mechanisms underpinning these conditions, as well as studies relating to preclinical drug discovery and efficacy, including compound discovery, target validation, drug permeability, pharmacokinetics and pharmacodynamics (Esch et al. Citation2015; Beaurivage et al. Citation2019; Low et al. Citation2021; Ma et al. Citation2021).
A gut-on-a-chip model for the study of IBD has been developed to increase the biological and physiological relevance of the pre-existing in vitro and in vivo models (Beaurivage et al. Citation2020). The main elements employed to reproduce the key IBD characteristics were intestinal epithelial cells from human intestinal organoids and monocyte-derived macrophages, which reproduced the expression profile of human colon and mimicked the dysregulated pathways in IBD patients. This work allowed the study of gene expression when exposed to inflammatory triggers (Donkers et al. Citation2021). However, not all gut conditions are being studied using OoC, as gut-on-a-chip models for IBS and colon cancer are still under development (Chong et al. Citation2019).
A liver-on-a-chip technology has been used to investigate the development of fatty liver disease (FLD), a chronic liver disorder that affects up to 30% in Western countries and which most important risk factors are obesity and alcohol consumption (Hassan et al. Citation2020; Nawroth et al. Citation2020). More recently, a model was created to use alcohol as an inductor to study biomarkers such as oxidative stress and lipid accumulation and to generate a novel in vitro quantitative readout of alcoholic liver toxicity by measuring structural changes of the bile canaliculi network (Nawroth et al. Citation2020). There are also models for the study of NAFLD, which comprises different stages, from steatosis to steatohepatitis, cirrhosis and hepatocellular carcinoma (Gori et al. Citation2016; Bulutoglu et al. Citation2019; Cho et al. Citation2021; Freag et al. Citation2021).
Pancreas-on-a-chip are the OoC models that have been developed most recently. They are being used to investigate type-1 diabetes (Rogal et al. Citation2019), cystic fibrosis-related diabetes (Shik Mun et al. Citation2019), function restoration of the islets (Sokolowska et al. Citation2020) and the possibility of islet transplantation (Abadpour et al. Citation2020). A particularly interesting study focussed on the generation of a pancreas-liver tandem to assess the regulation of the glucose circulation by the pancreatic islets in the system (Bauer et al. Citation2017). Additionally, as the pancreas is involved in other body processes, multi-organ structures have been investigated to study absorption rates and mechanisms of action of anti-cancer therapies or immunosuppressive medications (Abadpour et al. Citation2020) .
Overall, OoC constitute a good strategy to personalise treatments in a context of high variation levels in the population. OoC can be developed using patient’s cells, which allows not only a personalised and precision medicine, but also a platform to investigate rare diseases (Ronaldson-Bouchard and Vunjak-Novakovic Citation2018; van den Berg et al. Citation2019). However, OoC protocols are not standardised, which could difficult the potential scale-up of these treatments. In the meantime, the relevance of the data obtained allows higher success in the selection of drug candidates, despite their lower high-throughput screening capacity, if compared with other in vitro methods.
7. Conclusions: organs-on-a-chip to assess food effects on host-gut microbiota interaction? Challenges, future perspectives and concluding remarks
The advances in microfluidics design and fabrication have allowed the development of OoC technology that can help to provide deeper insights into host-microbiome investigations. These include details relating to the interaction between microbes and the host at the mucosal surface of the intestine, the degree to which different metabolites produced by the microorganisms are absorbed by the host or the manner in which organs other than the gut influence, and are influenced, by the microbiota, allowing us to further investigate dietary and pharmaceutical interventions and their impact on host-microbiota dynamics. OoC can offer a landscape to help us to address target areas, such as the study of the spatial structure of the microbiota, the distribution of the communities through the oxygen gradient, the differences in communities between the mucosa and the lumen, and between the villi and the crypts, and their recovery to study metabolism, RNA expression and genetic and phenotypic composition. OoC offer in situ analyses and control over all the elements in the system where other research models were limited, providing a means of distinguishing between cause and effect for some microbiota studies and opening the range of scientific questions that can be addressed.
Despite all of these advantages, OoC systems have challenges (). They constitute a new system that requires further development towards standardising and balancing complexity, relevance and costs. An aspect that requires further development is the optimisation of the system by using relevant cells, given that immortalised cells are not fully representative of human physiology. The use of human derived cells have partially addressed this question, although their limited life span add another layer of difficulty. However, it is not only a matter of cell survival, but also a matter of expression of the differential functional biomarkers. The choice of biomaterials might be relevant in this question and therefore, the development of biosensors and materials that can be responsive to the stimuli would be a major parameter to study, with preference to biocompatible, inert, non-adsorbent, and non-leaching materials. Developing non-invasive sensors and imaging techniques in situ might back up the information obtained and help the research of physiology, pathology and nutrition of the gut. Moreover, another area that will benefit from further development is the analytical techniques to investigate by the integration of mass spectrometric analysis for the quantification of genetic, proteomic and metabolomics.
It is also worth noting that the use of host-microbiota models to address nutritional and dietary studies would benefit from the further development of protocols to address the pre-processing of food samples prior to their incorporation to the model. This is an important consideration as food structure is key factor with respect to some of the characteristics associated with each food type. Despite this, the questions can be narrowed and these platforms could be used for the study of the food effects on the gut microbiota-human gut tissue tandem, particularly in the immunomodulation effects that can help understand inflammation processes linked to food consumption and the developmental of systemic conditions in the human body.
Overall, the wide-scale application of OoC studies will require the validation of the systems and a standardisation of the designs and protocols as well as integration with standard laboratory tools and protocols.
Author contributions
EG-G and PDC conceived and designed the manuscript. EG-G wrote the manuscript. PDC critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Abadpour S, Aizenshtadt A, Olsen PA, Shoji K, Wilson SR, Krauss S, Scholz H. 2020. Pancreas-on-a-Chip Technology for Transplantation Applications. Curr Diab Rep. 820(12):72–72.
- Albillos A, de Gottardi A, Rescigno M. 2020. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 72(3):558–577.
- Almeida AS, Tran TTT, Ghosh TS, Ribiere C, Foley C, Alves LA, et al. 2021. Fiber-associated Lachnospiraceae reduce colon tumorigenesis by modulation of the tumor-immune microenvironment. bioRxiv.
- And HC, Hoover DG. 2003. Bacteriocins and their Food Applications. Compr Rev Food Sci Food Saf. 2(3):82–100.
- Angeloni C, Businaro R, Vauzour D. 2020. The role of diet in preventing and reducing cognitive decline. Curr Opin Psychiatry. 33(4):432–438.
- Annunziata G, Arnone A, Ciampaglia R, Tenore GC, Novellino E. 2020. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. focus on short-chain fatty acids. foods. Basel Switz. 9(8):999.
- Arneth BM. 2018. Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgrad Med J. 94(1114):446–452.
- Ashammakhi N, Nasiri R, Barros N. R d, Tebon P, Thakor J, Goudie M, Shamloo A, Martin MG, Khademhosseini A. 2020. Gut-on-a-chip: Current progress and future opportunities. Biomaterials. 255:120196.
- Azzini E, Maiani G. 2015. Chapter 23 - mediterranean diet: antioxidant nutritional status. In: Preedy VR, Watson RR, editors. The mediterranean diet [Internet]. San Diego: Academic Press. p. 249–257. Available from: https://www.sciencedirect.com/science/article/pii/B9780124078499000233.
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. 2012. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 303(6):G675–85.
- Balaguer F, Enrique M, Llopis S, Barrena M, Navarro V, Álvarez B, Chenoll E, Ramón D, Tortajada M, Martorell P, et al. 2021. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: a novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb Biotechnol. 1–12.
- Barash NR, Maloney JG, Singer SM, Dawson SC. 2017. Giardia Alters Commensal Microbial Diversity throughout the Murine Gut. Appleton JA, editor. Infect Immun. 85(6):e00948–16.
- Barbieri F, Montanari C, Gardini F, Tabanelli G. 2019. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods Basel Switz. 8(1):17.
- Bartfeld S. 2016. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev Biol. 420(2):262–270.
- Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. 2019. Making sense of … the microbiome in psychiatry. Int J Neuropsychopharmacol. 22(1):37–52.
- Bauer S, Wennberg Huldt C, Kanebratt KP, Durieux I, Gunne D, Andersson S, Ewart L, Haynes WG, Maschmeyer I, Winter A, et al. 2017. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci Rep. 7(1):14620.
- Beaurivage C, Kanapeckaite A, Loomans C, Erdmann KS, Stallen J, Janssen RAJ. 2020. Development of a human primary gut-on-a-chip to model inflammatory processes. Sci Rep. 10(1):21475.
- Beaurivage C, Naumovska E, Chang YX, Elstak ED, Nicolas A, Wouters H, et al. 2019. Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int J Mol Sci. 20(22):5661.
- Bein A, Shin W, Jalili-Firoozinezhad S, Park MH, Sontheimer-Phelps A, Tovaglieri A, Chalkiadaki A, Kim HJ, Ingber DE. 2018. Microfluidic organ-on-a-chip models of human intestine. Cell Mol Gastroenterol Hepatol. 5(4):659–668.
- Belkaid Y, Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell. 157(1):121–141.
- Bell V, Ferrão J, Pimentel L, Pintado M, Fernandes T. 2018. One health, fermented foods, and gut microbiota. Foods Basel Switz. 7(12):195.
- Beulens JWJ, Booth SL, van den Heuvel EGHM, Stoecklin E, Baka A, Vermeer C. 2013. The role of menaquinones (vitamin K2) in human health. Br J Nutr. 110(8):1357–1368.
- Bricks T, Paullier P, Legendre A, Fleury M-J, Zeller P, Merlier F, Anton PM, Leclerc E. 2014. Development of a new microfluidic platform integrating co-cultures of intestinal and liver cell lines. Toxicol in Vitro. 28(5):885–895.
- Broussard JL, Devkota S. 2016. The changing microbial landscape of Western society: diet, dwellings and discordance. Mol Metab. 5(9):737–742.
- Bulutoglu B, Rey-Bedón C, Kang YB, Mert S, Yarmush ML, Usta OB. 2019. A microfluidic patterned model of non-alcoholic fatty liver disease: applications to disease progression and zonation. Lab Chip. 19(18):3022–3031.
- Cabrera-Rubio R, Patterson AM, Cotter PD, Beraza N. 2019. Cholestasis induced by bile duct ligation promotes changes in the intestinal microbiome in mice. Sci Rep. 9(1):12324.
- Camarillo-Guerrero LF, Almeida A, Rangel-Pineros G, Finn RD, Lawley TD. 2021. Massive expansion of human gut bacteriophage diversity. Cell. 184(4):1098–1109.e9.
- Castaner O, Goday A, Park Y-M, Lee S-H, Magkos F, Shiow S-ATE, Schröder H. 2018. The Gut Microbiome Profile in Obesity: A Systematic Review. Int J Endocrinol.2018:4095789–4095789.
- Celebioglu HU. 2021. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch Microbiol. 203(3):1221–1229. Available from:.
- Ceppa F, Mancini A, Tuohy K. 2019. Current evidence linking diet to gut microbiota and brain development and function. Int J Food Sci Nutr. 70(1):1–19.
- Chen HJ, Miller P, Shuler ML. 2018. A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip. 18(14):2036–2046.
- Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. 2014. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 12(1):215–215.
- Chi M, Yi B, Oh S, Park D-J, Sung JH, Park S. 2015. A microfluidic cell culture device (μFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed Microdevices. 17(3):9966.
- Cho H‐J, Kim H‐J, Lee KJu, Lasli S, Ung A, Hoffman T, Nasiri R, Bandaru P, Ahadian S, Dokmeci MR, et al. 2021. Liver-on-a-chip: bioengineered multicellular liver microtissues for modeling advanced hepatic fibrosis driven through non-alcoholic fatty liver disease (Small 14/2021). Small. 17(14):2170061.
- Choe A, Ha SK, Choi I, Choi N, Sung JH. 2017. Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed Microdev. 19(1):4.
- Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. 2019. The microbiome and irritable bowel syndrome - a review on the pathophysiology, current research and future therapy. Front Microbiol.10:1136–1136.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. 2012. Gut microbiota composition correlates with diet and health in the elderly. Nature. 488(7410):178–184.
- Codoñer FM, Ramírez-Bosca A, Climent E, Carrión-Gutierrez M, Guerrero M, Pérez-Orquín JM, Horga de la Parte J, Genovés S, Ramón D, Navarro-López V, et al. 2018. Gut microbial composition in patients with psoriasis. Sci Rep. 8(1):3812.
- Collado MC, Vinderola G, Salminen S. 2019. Postbiotics: facts and open questions. A position paper on the need for a consensus definition. Benef Microbes. 10(7):711–719.
- Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. (10):777–3:788.
- Crespo-Piazuelo D, Migura-Garcia L, Estellé J, Criado-Mesas L, Revilla M, Castelló A, Muñoz M, García-Casco JM, Fernández AI, Ballester M, et al. 2019. Association between the pig genome and its gut microbiota composition. Sci Rep. 9(1):8791.
- Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. 2019. The microbiota-gut-brain axis. Physiol Rev. 99(4):1877–2013.
- Dahlberg T, Stangner T, Zhang H, Wiklund K, Lundberg P, Edman L, Andersson M. 2018. 3D printed water-soluble scaffolds for rapid production of PDMS micro-fluidic flow chambers. Sci Rep. 8(1):3372.
- De Filippis F, Pasolli E, Ercolini D. 2020. The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol Rev. 44(4):454–489.
- de Goffau MC, Lager S, Salter SJ, Wagner J, Kronbichler A, Charnock-Jones DS, Peacock SJ, Smith GCS, Parkhill J. 2018. Recognizing the reagent microbiome. Nat Microbiol. 3(8):851–853.
- de Haan P, Ianovska MA, Mathwig K, van Lieshout GAA, Triantis V, Bouwmeester H, Verpoorte E. 2019. Digestion-on-a-chip: a continuous-flow modular microsystem recreating enzymatic digestion in the gastrointestinal tract. Lab Chip. 19(9):1599–1609.
- de Haan P, Santbergen MJC, van der Zande M, Bouwmeester H, Nielen MWF, Verpoorte E. 2021. A versatile, compartmentalised gut-on-a-chip system for pharmacological and toxicological analyses. Sci Rep. 11(1):4920.
- De Simone C. 2018. Letter: what gastroenterologists should know about VSL#3. Aliment Pharmacol Ther. 47(5):698–699.
- Degen LP, Phillips SF. 1996. Variability of gastrointestinal transit in healthy women and men. Gut. 39(2):299–305.
- Deng J, Wei W, Chen Z, Lin B, Zhao W, Luo Y. 2019. Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: a review. Micromachines. 10(10):676.
- Devkota S, Chang EB. 2015. Interactions between diet, bile acid metabolism, gut microbiota, and inflammatory bowel diseases. Dig Dis. 33(3):351–356.
- Diaz M, Kellingray L, Akinyemi N, Adefiranye OO, Olaonipekun AB, Bayili GR, Ibezim J, Du Plessis AS, Houngbédji M, Kamya D, et al. Comparison of the microbial composition of African fermented foods using amplicon sequencing. Sci Rep. 2019. 9(1):13863.
- Diaz M, Ladero V, Redruello B, Sanchez-Llana E, del Rio B, Fernandez M, Martin MC, Alvarez MA. 2016. A PCR-DGGE method for the identification of histamine-producing bacteria in cheese. Food Control. 63:216–223.
- Distrutti E, Monaldi L, Ricci P, Fiorucci S. 2016. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J Gastroenterol. 22(7):2219–2241.
- Donaldson GP, Lee SM, Mazmanian SK. 2016. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 14(1):20–32.
- Donia MS, Fischbach MA. 2015. Human Microbiota. Small molecules from the human microbiota. Science. 349(6246):1254766.
- Donkers JM, Eslami Amirabadi H, van de Steeg E. 2021. Intestine-on-a-chip: next level in vitro research model of the human intestine. Curr Opin Toxicol.25:6–14.
- Eain MMG, Baginska J, Greenhalgh K, Fritz JV, Zenhausern F, Wilmes P. 2017. Engineering solutions for representative models of the gastrointestinal human-microbe interface. Engineering. 3(1):60–65.
- Esch EW, Bahinski A, Huh D. 2015. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 14(4):248–260.
- Filosa S, Di Meo F, Crispi S. 2018. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res. 13(12):2055–2059.
- Firth J, Gangwisch JE, Borisini A, Wootton RE, Mayer EA. 2020. Food and mood: how do diet and nutrition affect mental wellbeing? BMJ.369:m2382.
- Freag MS, Namgung B, Reyna Fernandez ME, Gherardi E, Sengupta S, Jang HL. 2021. Human nonalcoholic steatohepatitis on a chip. Hepatol Commun. 5(2):217–233.
- Fritz JV, Desai MS, Shah P, Schneider JG, Wilmes P. 2013. From meta-omics to causality: experimental models for human microbiome research. Microbiome. 1(1):14.
- Garcia-Gutierrez E, Mayer MJ, Cotter PD, Narbad A. 2019. Gut microbiota as a source of novel antimicrobials. Gut Microbes. 10(1):1–21.
- Garcia-Gutierrez E, Narbad A, Rodríguez JM. 2020. Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front Neurosci.14:578666.
- Garcia-Gutierrez E. 2019. New antimicrobials to target gut and food pathogens. [Thesis]. University of East Anglia
- Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, et al. 2020. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 69(7):1218–1228.
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. 2017. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 14(8):491–502.
- Gibson PR, Halmos EP, Muir JG. 2020. Review article: FODMAPS, prebiotics and gut health-the FODMAP hypothesis revisited. Aliment Pharmacol Ther. 52(2):233–246.
- Gokaltun A, Yarmush ML, Asatekin A, Usta OB. 2017. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology. 5(1):1–12.
- Gori M, Simonelli MC, Giannitelli SM, Businaro L, Trombetta M, Rainer A. 2016. Investigating nonalcoholic fatty liver disease in a liver-on-a-chip microfluidic device. PLOS One. 11(7):e0159729.
- Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, Mercenier A, et al. 2005. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 102(29):10321–10326.
- Grassart A, Malardé V, Gobaa S, Sartori-Rupp A, Kerns J, Karalis K, Marteyn B, Sansonetti P, Sauvonnet N. 2019. Bioengineered human organ-on-chip reveals intestinal microenvironment and mechanical forces impacting shigella infection. Cell Host Microbe. 26(3):435–444.e4.
- Guenat OT, Berthiaume F. 2018. Incorporating mechanical strain in organs-on-a-chip: lung and skin. Biomicrofluidics. 12(4):042207–042207.
- Guinane CM, Cotter PD. 2013. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 6(4):295–308.
- Hassan S, Sebastian S, Maharjan S, Lesha A, Carpenter A-M, Liu X, Xie X, Livermore C, Zhang YS, Zarrinpar A, et al. 2020. Liver-on-a-chip models of fatty liver disease. Hepatology. 71(2):733–740.
- Hermann E, Foligne B. 2017. Healthy gut microbiota can resolve undernutrition. Hepatobiliary Surg Nutr. 6(2):141–143.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. 2014. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 11(8):506–514.
- Hills RD, Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. 2019. Gut microbiome: profound implications for diet and disease. Nutrients. 11(7):1613.
- Holscher HD. 2017. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 8(2):172–184.
- Huh D, Hamilton GA, Ingber DE. 2011. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21(12):745–754.
- Ingber DE. 2018. Developmentally inspired human ‘organs on chips. Dev Camb Engl. 145(16):dev156125.
- Isaacs-Ten A, Echeandia M, Moreno-Gonzalez M, Brion A, Goldson A, Philo M, et al. 2020. Intestinal microbiome-macrophage crosstalk contributes to cholestatic liver disease by promoting intestinal permeability. Hepatology. 72:2090–2108. Available from:.
- Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, Bein A, Swenor B, Nestor B, Cronce MJ, Tovaglieri A, et al. 2019. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 3(7):520–531.
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. 2015. Role of the normal gut microbiota. World J Gastroenterol. 21(29):8787–8803.
- Jang K-J, Otieno MA, Ronxhi J, Lim H-K, Ewart L, Kodella KR, Petropolis DB, Kulkarni G, Rubins JE, Conegliano D, et al. 2019. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med. 11(517):eaax5516.
- Johnson KVA. 2020. Gut microbiome composition and diversity are related to human personality traits. Hum Microb J. 15. DOI:https://doi.org/10.1016/j.humic.2019.100069
- Junaid A, Mashaghi A, Hankemeier T, Vulto P. 2017. An end-user perspective on Organ-on-a-Chip: assays and usability aspects. Curr Opin Biomed Eng. 1:15–22.
- Kaarj K, Yoon J-Y. 2019. Methods of delivering mechanical stimuli to organ-on-a-chip. Micromachines. 10(10):700.
- Kasendra M, Luc R, Yin J, Manatakis DV, Kulkarni G, Lucchesi C, et al. 2020. Duodenum Intestine-Chip for preclinical drug assessment in a human relevant model. eLife.9:e50135.
- Kilic T, Navaee F, Stradolini F, Renaud P, Carrara S. 2018. Organs-on-chip monitoring: sensors and other strategies. Microphysiol Syst. 1:1–1. [cited 2018 Jan 1]; Available from: http://mps.amegroups.com/article/view/4689.
- Kim HJ, Huh D, Hamilton G, Ingber DE. 2012. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 12(12):2165–2174.
- Kim HJ, Ingber DE. 2013. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol. 5(9):1130–1140.
- Kim HJ, Li H, Collins JJ, Ingber DE. 2016. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci USA. 113(1):E7–15.
- Kim J, Koo B-K, Knoblich JA. 2020. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 21(10):571–584.
- Konstantinov SR, Smidt H, de Vos WM, Bruijns SCM, Singh SK, Valence F, et al. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci. 105(49):19474.
- Kostic AD, Howitt MR, Garrett WS. 2013. Exploring host-microbiota interactions in animal models and humans. Genes Dev. 27(7):701–718.
- Koudoufio M, Desjardins Y, Feldman F, Spahis S, Delvin E, Levy E. 2020. Insight into polyphenol and gut microbiota crosstalk: are their metabolites the key to understand protective effects against metabolic disorders? Antioxid Basel Switz. 9(10):982.
- Kowalski K, Mulak A. 2019. Brain-gut-microbiota axis in alzheimer's disease. J Neurogastroenterol Motil. 25(1):48–60.
- Kulthong K, Duivenvoorde L, Mizera BZ, Rijkers D, Dam G. Oegema G, Puzyn T, Bouwmeester H, van der Zande M. 2018.Implementation of a dynamic intestinal gut-on-a-chip barrier model for transport studies of lipophilic dioxin congeners. RSC Adv. 8(57):32440–32453.
- Kulthong K, Duivenvoorde L, Sun H, Confederat S, Wu J, Spenkelink B, de Haan L, Marin V, van der Zande M, Bouwmeester H, et al. 2020. Microfluidic chip for culturing intestinal epithelial cell layers: Characterization and comparison of drug transport between dynamic and static models. Toxicol in Vitro. 65:104815.
- Laforest-Lapointe I, Arrieta M-C. 2018. Microbial eukaryotes: a missing link in gut microbiome studies. mSystems. 3(2):e00201–17.
- Lawrence GW, Begley M, Cotter PD, Guinane CM. 2020. The more we learn, the less we know: deciphering the link between human gut fusobacteria and colorectal cancer. Dig Med Res. 3:21–21. [Internet].[cited 2020 Jan 1].
- Lea T. 2015. Caco-2 cell line. In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, et al. editors. The impact of food bioactives on health: in vitro and ex vivo models [Internet]. Cham: Springer International Publishing. p. 103–111. Available from:.
- Lebeer S, Claes I, Tytgat HLP, Verhoeven TLA, Marien E, von Ossowski I, Reunanen J, Palva A, Vos W. M d, Keersmaecker SCJD, et al. 2012. Functional analysis of lactobacillus rhamnosus gg pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol. 78(1):185–193.
- Lee B, Moon KM, Kim CY. 2018. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J Immunol Res.2018:2645465–2645465.
- Leng B, Sørensen MB, Kot W, Thymann T, Krych L, Nielsen DS. 2019. Severe gut microbiota dysbiosis caused by malnourishment can be partly restored during 3 weeks of refeeding with fortified corn-soy-blend in a piglet model of childhood malnutrition. BMC Microbiol. 19(1):277.
- Li C, Kwok L-Y, Mi Z, Bala J, Xue J, Yang J, Ma Y, Zhang H, ChenY 2017Characterization of the angiotensin-converting enzyme inhibitory activity of fermented milks produced with Lactobacillus casei. J Dairy Sci. . 100(12):9495–9507.
- Li X, Tian T. 2018. Recent advances in an organ-on-a-chip: biomarker analysis and applications. Anal Methods. 10(26):3122–3130.
- Lin A, Sved Skottvoll F, Rayner S, Pedersen-Bjergaard S, Sullivan G, Krauss S, Ray Wilson S, Harrison S. 2020. 3D cell culture models and organ-on-a-chip: Meet separation science and mass spectrometry. Electrophoresis.41(1-2):56–64.
- Linares DM, Gómez C, Renes E, Fresno JM, Tornadijo ME, Ross RP, Stanton C. 2017. Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front Microbiol. 8:846–846.
- Linares DM, O'Callaghan TF, O'Connor PM, Ross RP, Stanton C. 2016. Streptococcus thermophilus APC151 Strain Is Suitable for the Manufacture of Naturally GABA-Enriched Bioactive Yogurt. Front Microbiol.7:1876–1876.
- Liwinski T, Zenouzi R, John C, Ehlken H, Rühlemann MC, Bang C, Groth S, Lieb W, Kantowski M, Andersen N, et al. 2020. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 69(4):665–672.
- Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA. 2021. Organs-on-chips: into the next decade. Nat Rev Drug Discov. 20(5):345–361.
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature. 489(7415):220–230.
- Lynch KE, Parke EC, O’Malley MA. 2019. How causal are microbiomes? A comparison with the Helicobacter pylori explanation of ulcers. Biol Philos. 34(6):62.
- Ma C, Peng Y, Li H, Chen W. 2021. Organ-on-a-chip: a new paradigm for drug development. Trends Pharmacol Sci. 42(2):119–133.
- Ma L, Kim J, Hatzenpichler R, Karymov MA, Hubert N, Hanan IM, Chang EB, Ismagilov RF. 2014. Gene-targeted microfluidic cultivation validated by isolation of a gut bacterium listed in Human Microbiome Project's Most Wanted taxa. Proc Natl Acad Sci USA. 111(27):9768–9773.
- Mackie A, Mulet-Cabero A-I, Torcello-Gómez A. 2020. Simulating human digestion: developing our knowledge to create healthier and more sustainable foods. Food Funct. 11:9397–9431. DOI:https://doi.org/10.1039/D0FO01981J
- Macori G, Cotter PD. 2018. Novel insights into the microbiology of fermented dairy foods. Curr Opin Biotechnol.49:172–178.
- Maeda Y, Takeda K. 2017. Role of gut microbiota in rheumatoid arthritis. J Clin Med. 6(6):60.
- Mah J-H, Park YK, Jin YH, Lee J-H, Hwang H-J. 2019. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods Basel Switz. 8(2):85.
- Makki K, Deehan EC, Walter J, Bäckhed F. 2018. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 23(6):705–715.
- Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, Gänzle M, Kort R, Pasin G, Pihlanto A, et al. 2017. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol.44:94–102.
- Marco ML, Sanders ME, Gänzle M, Arrieta MC, Cotter PD, De Vuyst L, Hill C, Holzapfel W, Lebeer S, Merenstein D, et al. 2021.The international scientific association for probiotics and prebiotics (isapp) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. 18(3):196–208. Available from:.
- Maschmeyer I, Lorenz AK, Schimek K, Hasenberg T, Ramme AP, Hübner J, Lindner M, Drewell C, Bauer S, Thomas A, et al. 2015. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 15(12):2688–2699.
- Mata A, Fleischman AJ, Roy S. 2005. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed Microdevices. 7(4):281–293.
- Maurer M, Gresnigt MS, Last A, Wollny T, Berlinghof F, Pospich R, Cseresnyes Z, Medyukhina A, Graf K, Gröger M, et al. 2019. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials.220:119396.
- McDonald JC, Whitesides GM. 2002. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res. 35(7):491–499.
- McGrane MM, Essery E, Obbagy J, Lyon J, Macneil P, Spahn J, Van Horn L. 2011. Dairy consumption, blood pressure, and risk of hypertension: an evidence-based review of recent literature. Curr Cardiovasc Risk Rep. 5(4):287–298.
- Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, et al. 2021. Influence of mediterranean diet on human gut microbiota. Nutrients. 13(1):7.
- Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, Giacco R, Mennella I, Ferracane R, Pons N, et al. 2020. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 69(7):1258–1268.
- Mikkonen R, Puistola P, Jönkkäri I, Mäntysalo M. 2020. Inkjet printable polydimethylsiloxane for all-inkjet-printed multilayered soft electrical applications. ACS Appl Mater Interfaces. 12(10):11990–11997.
- Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. 2017. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 81(4):e00036–e00017.
- Million M, Angelakis E, Maraninchi M, Henry M, Giorgi R, Valero R, Vialettes B, Raoult D. 2013. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes. 37(11):1460–1466.
- Miraglia F, Colla E. 2019. Microbiome, parkinson’s disease and molecular mimicry. Cells. 8(3):222.
- Molinero N, Ruiz L, Milani C, Gutiérrez-Díaz I, Sánchez B, Mangifesta M, Segura J, Cambero I, Campelo AB, García-Bernardo CM, et al. 2019. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome. 7(1):100.
- Molinero N, Ruiz L, Sánchez B, Margolles A, Delgado S. 2019. Intestinal bacteria interplay with bile and cholesterol metabolism: implications on host physiology. Front Physiol.10:185–185.
- Moreno-Gonzalez M, Beraza N. 2021. The role of the microbiome in liver cancer. Cancers. 13(10). DOI:https://doi.org/10.3390/cancers13102330
- Morton JT, Aksenov AA, Nothias LF, Foulds JR, Quinn RA, Badri MH, Swenson TL, Van Goethem MW, Northen TR, Vazquez-Baeza Y, et al. 2019. Learning representations of microbe-metabolite interactions. Nat Methods. 16(12):1306–1314.
- Mousa WK, Athar B, Merwin NJ, Magarvey NA. 2017. Antibiotics and specialized metabolites from the human microbiota. Nat Prod Rep. 34(11):1302–1331.
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. 2011. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 364(25):2392–2404.
- Myhrstad MCW, Tunsjø H, Charnock C, Telle-Hansen VH. 2020. Dietary Fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients. 12(3):859.
- Nampoothiri KM, Beena DJ, Vasanthakumari DS, Ismail B. 2017. Chapter 3 - Health Benefits of Exopolysaccharides in Fermented Foods. In: Frias J, Martinez-Villaluenga C, Peñas E, editors. Fermented Foods in Health and Disease Prevention [Internet]. Boston: Academic Press; p. 49–62. Available from: http://www.sciencedirect.com/science/article/pii/B9780128023099000030.
- Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, et al. 2017. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 5(1):153.
- Nawroth JC, Petropolis DB, Manatakis DV, Maulana TI, Burchett G, Schlünder K, et al. 2020. Modeling alcoholic liver disease in a human Liver-Chip. bioRxiv.
- Nguyen TLA, Vieira-Silva S, Liston A, Raes J. 2015. How informative is the mouse for human gut microbiota research? Dis Model Mech. 8(1):1–16.
- Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. 2018. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 11(1):1–10.
- Nkamga VD, Henrissat B, Drancourt M. 2017. Archaea: essential inhabitants of the human digestive microbiota. Hum Microbiome J.3:1–8.
- Nogacka AM, Ruas-Madiedo P, Gómez E, Solís G, Fernández N, Suárez M, Suárez A, Salazar N, de Los Reyes-Gavilán CG, Gueimonde M, et al. 2018. Real-time monitoring of HT29 epithelial cells as an in vitro model for assessing functional differences among intestinal microbiotas from different human population groups. J Microbiol Methods.152:210–216.
- Nogacka AM. 2020. Modelos de interacción microbiota-hospedador en el entorno intestinal y su aplicación en el desarrollo de productos probióticos y prebióticos [Tesis Doctoral]. Oviedo: Universidad de Oviedo.
- Novak R, Didier M, Calamari E, Ng CF, Choe Y, Clauson SL, et al. 2018. Scalable fabrication of stretchable, dual channel, microfluidic organ chips. J Vis Exp JoVE.140:58151.
- Nueno-Palop C, Narbad A. 2011. Probiotic assessment of Enterococcus faecalis CP58 isolated from human gut. Int J Food Microbiol. 145(2-3):390–394.
- Ochs CJ, Kasuya J, Pavesi A, Kamm RD. 2014. Oxygen levels in thermoplastic microfluidic devices during cell culture. Lab Chip. 14(3):459–462.
- Odijk M, van der Meer AD, Levner D, Kim HJ, van der Helm MW, Segerink LI, Frimat J-P, Hamilton GA, Ingber DE, van den Berg A, et al. 2015. Measuring direct current trans-epithelial electrical resistance in organ-on-a-chip microsystems. Lab Chip. 15(3):745–752.
- O'Donnell MM, Rea MC, Shanahan F, Ross RP. 2018. The use of a mini-bioreactor fermentation system as a reproducible, high-throughput ex vivo batch model of the distal colon. Front Microbiol.9:1844.
- Oedit A, Vulto P, Ramautar R, Lindenburg PW, Hankemeier T. 2015. Lab-on-a-Chip hyphenation with mass spectrometry: strategies for bioanalytical applications. Curr Opin Biotechnol.31:79–85.
- Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M. 2019. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch.471(11-12):1441–1453.
- Parian AM, Mullin GE, Langhorst J, Brown AC. 2018. Chapter 50 - inflammatory bowel disease. In: Rakel D, editor. Integrative medicine 4th ed. [Internet]. Philadelphia (PA): Elsevier; p. 501–516.e8. Available from: https://www.sciencedirect.com/science/article/pii/B9780323358682000505.
- Pasolli E, De Filippis F, Mauriello IE, Cumbo F, Walsh AM, Leech J, Cotter PD, Segata N, Ercolini D. 2020. Large-scale genome-wide analysis links lactic acid bacteria from food with the gut microbiome. Nat Commun. 11(1):2610.
- Pasolli E, Schiffer L, Manghi P, Renson A, Obenchain V, Truong DT, Beghini F, Malik F, Ramos M, Dowd JB, et al. 2017. Accessible, curated metagenomic data through Experiment Hub. Nat Methods. 14(11):1023–1024.
- Patil Y, Gooneratne R, Ju X-H. 2020. Interactions between host and gut microbiota in domestic pigs: a review. Gut Microbes. 11(3):310–334.
- Pessione E, Cirrincione S. 2016. Bioactive molecules released in food by lactic acid bacteria: encrypted peptides and biogenic amines. Front Microbiol.7:876.
- Pontifex MG, Malik MMAH, Connell E, Müller M, Vauzour D. 2021. Citrus polyphenols in brain health and disease: current perspectives. Front Neurosci.15:115.
- Ramadan Q, Jafarpoorchekab H, Huang C, Silacci P, Carrara S, Koklü G, Ghaye J, Ramsden J, Ruffert C, Vergeres G, et al. 2013. NutriChip: nutrition analysis meets microfluidics. Lab Chip. 13(2):196–203.
- Redfern WS, Bialecki R, Ewart L, Hammond TG, Kinter L, Lindgren S, Pollard CE, Rolf M, Valentin J-P. 2010. Impact and prevalence of safety pharmacology-related toxicities throughout the pharmaceutical life cycle. Seventh Annu Focus Issue Methods Saf Pharmacol Part II. 62(2):e29.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. 2011. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 9(1):27–38.
- Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. 2019. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 178(4):795–806.e12.
- Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et al. 2015. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 26:26050–26050.
- Rogal J, Zbinden A, Schenke-Layland K, Loskill P. 2019. Stem-cell based organ-on-a-chip models for diabetes research. Adv Drug Deliv Rev.140:101–128.
- Ronaldson-Bouchard K, Vunjak-Novakovic G. 2018. Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell. 22(3):310–324.
- Rowan S, Jiang S, Korem T, Szymanski J, Chang M-L, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R, et al. 2017. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci USA. 114(22):E4472–E4481.
- Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG. 2017. Nutrition and the gut microbiome in the elderly. Gut Microbes. 8(2):82–97.
- Sambuy Y, De Angelis I, Ranaldi G, Scarino M, Stammati A, Zucco F. 2005. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 21(1):1–26.
- Sangwan V, Tomar SK, Ali B, Singh RRB, Singh AK. 2015. Production of β-galactosidase from streptococcus thermophilus for galactooligosaccharides synthesis. J Food Sci Technol. 52(7):4206–4215.
- Şanlier N, Gökcen BB, Sezgin AC. 2019. Health benefits of fermented foods. Crit Rev Food Sci Nutr. 59(3):506–527.
- Saqib S, Akram A, Halim SA, Tassaduq R. 2017. Sources of β-galactosidase and its applications in food industry. 3 Biotech. 7(1):79–79.
- Saubade F, Hemery YM, Guyot J-P, Humblot C. 2017. Lactic acid fermentation as a tool for increasing the folate content of foods. Crit Rev Food Sci Nutr. 257(18):3894–3910.
- Sender R, Fuchs S, Milo R. 2016. Are we really vastly outnumbered? revisiting the ratio of bacterial to host cells in humans. Cell. 164(3):337–340.
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 110(9):3507–3512.
- Shah P, Fritz JV, Glaab E, Desai MS, Greenhalgh K, Frachet A, Niegowska M, Estes M, Jäger C, Seguin-Devaux C, et al. 2016. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat Commun. 7(1):11535.
- Shakerian M, Razavi SH, Ziai SA, Khodaiyan F, Yarmand MS, Moayedi A. 2015. Proteolytic and ACE-inhibitory activities of probiotic yogurt containing non-viable bacteria as affected by different levels of fat, inulin and starter culture. J Food Sci Technol. 52(4):2428–2433.
- Shanahan F, Ghosh TS, O'Toole PW. 2021. The Healthy microbiome-what is the definition of a healthy gut microbiome?. Gastroenterology. 160(2):483–494.
- Sharma N, Angural S, Rana M, Puri N, Kondepudi KK, Gupta N. 2020. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci Technol. 96:1–12.
- Shi Z. 2019. Gut microbiota: an important link between Western Diet and chronic diseases. Nutrients. 11(10):2287.
- Shik Mun K, Arora K, Huang Y, Yang F, Yarlagadda S, Ramananda Y, Abu-El-Haija M, Palermo JJ, Appakalai BN, Nathan JD, et al. 2019. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun. 10(1):3124.
- Shimizu H, Masujima Y, Ushiroda C, Mizushima R, Taira S, Ohue-Kitano R, Kimura I. 2019. Dietary short-chain fatty acid intake improves the hepatic metabolic condition via FFAR3. Sci Rep. 9(1):16574.
- Shin W, Kim HJ. 2018. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc Natl Acad Sci USA. 115(45):E10539–E10547.
- Shkoporov AN, Clooney AG, Sutton TDS, Ryan FJ, Daly KM, Nolan JA, McDonnell SA, Khokhlova EV, Draper LA, Forde A, et al. 2019. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe. 26(4):527–541.e5.
- Silva CCG, Silva SPM, Ribeiro SC. 2018. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front Microbiol. 9:594–594.
- Simon Sarkadi L. 2017. Chapter 27 - Biogenic Amines in Fermented Foods and Health Implications. In: Frias J, Martinez-Villaluenga C, Peñas E, editors. Fermented foods in health and disease prevention. Boston: Academic Press. p. 625–651. Available from: https://www.sciencedirect.com/science/article/pii/B9780128023099000273.
- So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, Shanahan ER, Staudacher HM, Campbell KL. 2018. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am J Clin Nutr. 107(6):965–983.
- Sokolowska P, Janikiewicz J, Jastrzebska E, Brzozka Z, Dobrzyn A. 2020. Combinations of regenerative medicine and Lab-on-a-chip systems: New hope to restoring the proper function of pancreatic islets in diabetes. Biosens Bioelectron.167:112451.
- Sokovic Bajic S, Djokic J, Dinic M, Veljovic K, Golic N, Mihajlovic S, Tolinacki M. 2019. GABA-producing natural dairy isolate from artisanal zlatar cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro. Front Microbiol.10:527.
- Spano G, Russo P, Lonvaud-Funel A, Lucas P, Alexandre H, Grandvalet C, Coton E, Coton M, Barnavon L, Bach B, et al. 2010. Biogenic amines in fermented foods. Eur J Clin Nutr.64(Suppl 3):S95–S100.
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 470(7332):105–109.
- Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. 2015. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 20(2):107–126.
- Sriram G, Alberti M, Dancik Y, Wu B, Wu R, Feng Z, Ramasamy S, Bigliardi PL, Bigliardi-Qi M, Wang Z, et al. 2018. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater Today. 21(4):326–340.
- Steinway SN, Saleh J, Koo B-K, Delacour D, Kim D-H. 2020. Human microphysiological models of intestinal tissue and gut microbiome. Front Bioeng Biotechnol. 8:725–725.
- Storck LJ, Imoberdorf R, Ballmer PE. 2019. Nutrition in gastrointestinal disease: liver, pancreatic, and inflammatory bowel disease. J Clin Med. 8(8):1098.
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, et al. 2017. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 5(1):24–24.
- Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J, et al. 2015. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity. 43(2):304–317.
- Sutton TDS, Hill C. 2019. Gut bacteriophage: current understanding and challenges. Front Endocrinol.10:784.
- Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. 2020. The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 17(11):687–701.
- Takahashi T. 2011. Flow Behavior of Digesta and the Absorption of Nutrients in the Gastrointestine. J Nutr Sci Vitaminol. 57(4):265–273.
- Thomas RM, Jobin C. 2020. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 17(1):53–64.
- Tiew PY, Mac Aogain M, Ali NABM, Thng KX, Goh K, Lau KJX, Chotirmall SH. 2020. The mycobiome in health and disease: emerging concepts, methodologies and challenges. Mycopathologia. 185(2):207–231.
- Tilg H, Moschen AR. 2014. Microbiota and diabetes: an evolving relationship. Gut. 63(9):1513–1521.
- Trietsch SJ, Naumovska E, Kurek D, Setyawati MC, Vormann MK, Wilschut KJ, Lanz HL, Nicolas A, Ng CP, Joore J, et al. 2017. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat Commun. 8(1):262.
- Tsamandouras N, Chen WLK, Edington CD, Stokes CL, Griffith LG, Cirit M. 2017. Integrated gut and liver microphysiological systems for quantitative in vitro pharmacokinetic studies . 9(5):1499–1512.
- van den Berg A, Mummery CL, Passier R, van der Meer AD. 2019. Personalised organs-on-chips: functional testing for precision medicine. Lab Chip. 19(2):198–205.
- van der Helm MW, van der Meer AD, Eijkel JCT, van den Berg A, Segerink LI. 2016. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers. 4(1):e1142493.
- van Poll ML, Zhou F, Ramstedt M, Hu L, Huck WTS. A self-assembly approach to chemical micropatterning of Poly(dimethylsiloxane). Angew Chem Int Ed Engl. 2007. 46(35):6634–6637.
- Vergères G, Bogicevic B, Buri C, Carrara S, Chollet M, Corbino-Giunta L, Egger L, Gille D, Kopf-Bolanz K, Laederach K, et al. 2012. The NutriChip project-translating technology into nutritional knowledge. Br J Nutr. 108(5):762–768.
- Vernetti L, Gough A, Baetz N, Blutt S, Broughman JR, Brown JA, Foulke-Abel J, Hasan N, In J, Kelly E, et al. 2017. Corrigendum: functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal Muscle. Sci Rep. 7(1):44517.
- Villarreal-Soto SA, Bouajila J, Pace M, Leech J, Cotter PD, Souchard J-P, Taillandier P, Beaufort S. 2020. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int J Food Microbiol. 333:108778.
- Villenave R, Wales SQ, Hamkins-Indik T, Papafragkou E, Weaver JC, Ferrante TC, Bahinski A, Elkins CA, Kulka M, Ingber DE, et al. 2017. Human gut-on-a-chip supports polarized infection of coxsackie B1 virus in vitro. PLoS One.12(2):e0169412.
- Vinderola G, Reinheimer J, Salminen S. 2019. The enumeration of probiotic issues: from unavailable standardised culture media to a recommended procedure? Int Dairy J.96:58–65.
- Virumbrales-Muñoz M, Ayuso JM, Gong MM, Humayun M, Livingston MK, Lugo-Cintrón KM, McMinn P, Álvarez-García YR, Beebe DJ. 2020. Microfluidic lumen-based systems for advancing tubular organ modeling. Chem Soc Rev. 49(17):6402–6442.
- Walter J, Armet AM, Finlay BB, Shanahan F. 2020. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 180(2):221–232.
- Walther B, Karl JP, Booth SL, Boyaval P. 2013. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 4(4):463–473.
- Wang H, Yan Y, Yi X, Duan Y, Wang J, Li S, Luo L, Huang T, Inglis B, Li X, et al. 2019. Histopathological features and composition of gut microbiota in rhesus monkey of alcoholic liver disease. Front Microbiol.10:165.
- Wang YI, Carmona C, Hickman JJ, Shuler ML. 2018. Multiorgan microphysiological systems for drug development: strategies, advances, and challenges. Adv Healthc Mater. 7(2):10.
- Warburton R. 2017. A microbiological assessment of the human biliary tract in health and disease. [Thesis]. University of East Anglia.
- Wekerle H, Hohlfeld R. 2016. Chapter 9 - gut microbiota in multiple sclerosis: a bioreactor driving brain autoimmunity. In: Arnon R, Miller A, editors. Translational neuroimmunology in multiple sclerosis. San Diego: Academic Press; p. 113–125. Available from: http://www.sciencedirect.com/science/article/pii/B978012801914600009X.
- Wilkinson M. 2019. The potential of organ on chip technology for replacing animal testing. Leiden: Brill. p. 639–653. Available from: https://brill.com/view/book/edcoll/9789004391192/BP000033.xml.
- Williams CF, Walton GE, Jiang L, Plummer S, Garaiova I, Gibson GR. 2015. Comparative analysis of intestinal tract models. Annu Rev Food Sci Technol. 6(1):329–350.
- Wolf MP, Salieb-Beugelaar GB, Hunziker P. 2018. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog Polym Sci.83:97–134.
- Wu J, He Z, Chen Q, Lin J-M. 2016. Biochemical analysis on microfluidic chips. TrAC Trends Anal Chem. 80:213–231.
- Xiang Y, Wen H, Yu Y, Li M, Fu X, Huang S. 2020. Gut-on-chip: recreating human intestine in vitro. J Tissue Eng. 11:2041731420965318.
- Xu C, Zhu H, Qiu P. 2019. Aging progression of human gut microbiota. BMC Microbiol. 19(1):236.
- Yeste J, Illa X, Alvarez M, Villa R. 2018. Engineering and monitoring cellular barrier models. J Biol Eng.12:18–18.
- Zhang B, Korolj A, Lai BFL, Radisic M. 2018. Advances in organ-on-a-chip engineering. Nat Rev Mater. 3(8):257–278.
- Zhang K, Dai H, Liang W, Zhang L, Deng Z. 2019. Fermented dairy foods intake and risk of cancer. Int J Cancer. 144(9):2099–2108.
- Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, Riahi R, Chae S, Hu N, et al. 2017. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci USA. 114(12):E2293–E2302.
- Zinöcker MK, Lindseth IA. 2018. The Western Diet–microbiome-host interaction and its role in metabolic disease. Nutrients. 10(3):365.
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RB-Z, et al. 2018. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 174(6):1388–1405.e21.