Abstract
The cause of Alzheimer’s disease (AD), and the pathophysiological mechanisms involved, remain major unanswered questions in medical science. Oral bacteria, especially those species associated with chronic periodontitis and particularly Porphyromonas gingivalis, are being linked causally to AD pathophysiology in a subpopulation of susceptible individuals. P. gingivalis produces large amounts of proteolytic enzymes, haem and iron capture proteins, adhesins and internalins that are secreted and attached to the cell surface and concentrated onto outer membrane vesicles (OMVs). These enzymes and adhesive proteins have been shown to cause host tissue damage and stimulate inflammatory responses. The ecological and pathophysiological roles of P. gingivalis OMVs, their ability to disperse widely throughout the host and deliver functional proteins lead to the proposal that they may be the link between a P. gingivalis focal infection in the subgingivae during periodontitis and neurodegeneration in AD. P. gingivalis OMVs can cross the blood brain barrier and may accelerate AD-specific neuropathology by increasing neuroinflammation, plaque/tangle formation and dysregulation of iron homeostasis, thereby inducing ferroptosis leading to neuronal death and neurodegeneration.
Overview of Alzheimer’s disease and association with periodontitis
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, neuropathologically characterised by intracerebral, extracellular amyloid-β (Aβ) plaques and intraneuronal neurofibrillary tangles (NFTs). The cause of sporadic AD, and the pathophysiological mechanisms involved, remain major unanswered questions in medical science. While the greatest risk factor for sporadic AD is age, this complex syndrome depends on many genetic and environmental risk factors (Armstrong Citation2019).
AD and periodontitis are both inflammation-associated multifactorial diseases and a genetic susceptibility profile to inflammation is involved in the aetiology of both diseases. In particular, recent investigations suggest that polymorphisms in genes encoding several pro-inflammatory cytokines such as TNF-α, IL-1α, IL-1β and IL-6 are risk factors for both periodontitis and AD, which presents a commonality of susceptibility traits and a nexus between the two diseases (Singhrao et al. Citation2014). A wide range of non-genetic factors involving environmental, demographic, medical, psychiatric and lifestyle aspects are reported to affect both periodontitis and AD (Genco and Borgnakke Citation2013; Armstrong Citation2019). Periodontitis and AD thus share several risk factors.
As it is well acknowledged that Aβ plaque starts to build up in the brain up to 30 years before symptomatic onset of dementia (usually around 65 years of age) (Villemagne et al. Citation2013), the genesis of AD likely begins in mid-life, a time when periodontitis is becoming prevalent. Therefore, analogous to other important modifiable risk factors for AD, including midlife hypertension which also presents decades before the clinical symptoms of AD (Lennon et al. Citation2019), periodontitis might be conceived as a risk factor for future AD development, at least in a susceptible subpopulation. As periodontitis is an inflammatory disease mediated by bacteria, the emerging association between this disease and AD has prompted a deeper investigation of the roles periodontal bacteria may play in the pathogenesis of AD.
Microbiology of periodontitis
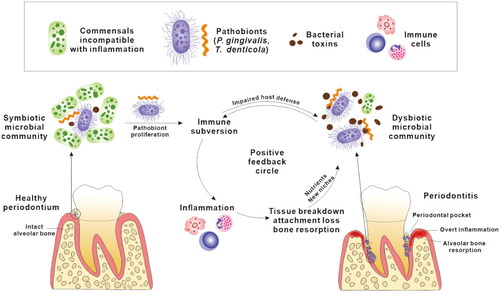
Periodontitis is a chronic inflammatory infectious disease affecting the periodontium, the structures surrounding and supporting the tooth, including gingiva, cementum, periodontal ligament and alveolar bone. It is induced by bacterial pathobionts that emerge in a polymicrobial community growing as a biofilm, accreted onto the surface of a tooth root in the subgingival crevice or periodontal pocket (Ng et al. Citation2016). In a suitable host environment (e.g. poor oral hygiene and/or host susceptibility), pathobionts like Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia can subvert innate immune responses to reinforce their fitness through several mechanisms including dysregulating complement function, inactivating antimicrobial peptides (AMPs) and manipulating adaptive immune responses (Moutsopoulos et al. Citation2012; Jauregui et al. Citation2013; Maekawa et al. Citation2014). Subversion of host immunity leads to pathobionts evading suppression by immune responses, and the normally symbiotic periodontal microbiota transforming and expanding into a dysbiotic, pathogenic community. This dysbiotic community is characterised by the production and secretion of proteolytic enzymes on outer membrane vesicles that hydrolyse host proteins causing tissue damage and further dysregulating the host response (). Meanwhile, these inflammophilic bacterial pathobionts procure nutrients from the inflammation-mediated tissue breakdown enabling them to outcompete commensal bacteria, reducing microbial diversity in deep subgingival pockets associated with progressing disease (Van Dyke et al. Citation2020). These bacterial pathobionts each have unique attributes which converge to synergism promoting their proliferation and virulence. In this way, dysbiosis and inflammation create a chronic and positive feedback cycle which fuels the progress of periodontitis ().
Figure 1. A positive feedback cycle in the pathogenesis of periodontitis. Pathobionts colonise subgingival niches, subvert host immune responses and proliferate, which leads to dysbiosis in the microbial community. The dysbiotic microbial community, characterised by overgrowth of a small number of pathobionts and overproduction of bacterial toxins, persistently stimulates local inflammatory responses. Inflammatory tissue breakdown in turn produces nutrients, including peptides, haem and ferrous iron, that further facilitate the proliferation of pathobionts. This also results in periodontal pockets deepening providing more suitable habitat for pathobionts. Collectively, the dysbiotic microbiota and inflammation share a mutually supportive relationship which is presented as a positive feedback cycle in the development of periodontitis.
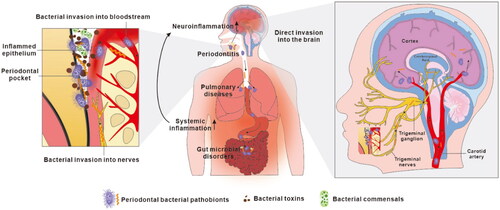
Increasing evidence strongly implicates oral bacteria, especially those related to periodontitis and P. gingivalis in particular, in extra-oral inflammation and systemic diseases including AD (Han and Wang Citation2013). The current proposed mechanisms of action of these candidate pathobionts underlying AD fall into two broad categories, direct infection of the central nervous system (CNS) by the bacteria, or indirect impact through the induction of chronic systemic inflammation, which spreads to the CNS and amplifies neuroinflammation (). Building on knowledge gained over the last decade on the physiology and ecology of P. gingivalis, and particularly the characterisation of its outer membrane vesicles (OMVs) and their roles in disease we propose a mechanism linking a focal infection by this periodontal pathobiont in the oral cavity with the pathogenesis of AD. In this proposal OMVs mediate the causal link between P. gingivalis and AD, and their proteinases (gingipains), haem and iron capture systems provide the molecular pathogenic mechanisms including plaque/tangle formation and ferroptosis in the brain.
Figure 2. Current proposals on how oral bacteria may promote the progression of Alzheimer’s disease. Oral bacteria have been proposed to promote AD pathology by two mechanisms. Firstly, oral bacteria may impact the central nervous system indirectly by causing periodontitis, by aggravating pulmonary diseases after aspiration to the respiratory system and by provoking microbiota-gut-brain axis disorders in the alimentary tract (Wu et al. Citation2017; Xue et al. Citation2020). This induces chronic systemic inflammation which subsequently extends to the brain adding to the load of neuroinflammation. Secondly, discontinuous sites within inflamed periodontal pocket epithelia allow periodontal bacteria to invade adjacent primary afferent nerves and blood vessels, along which they escape the oral cavity and directly access the brain, leading to brain infection.
P. gingivalis in AD
Although a number of oral bacteria have been associated with the neuropathology of AD we here focus on P. gingivalis as this bacterium is by far the best studied and a major bacterial pathobiont that emerges as part of a subgingival pathogenic polymicrobial community during periodontitis () (Ng et al. Citation2016). The presence of a variety of P. gingivalis biomolecules including nucleic acids, lipopolysaccharide (LPS) and surface located proteinases, known as gingipains, has been determined in multiple regions of human AD brains, including cortical gray matter, the basal forebrain, and hypothalamic regions (Poole et al. Citation2013; Bennett et al. Citation2019; Dominy et al. Citation2019; Patel et al. Citation2021). These biomolecules have been detected within neurons and associated with NFTs, in the brain of AD patients. Cerebral loads of gingipains have been significantly correlated with AD diagnosis, tauopathy and ubiquitin pathology (Dominy et al. Citation2019). Ubiquitin is a highly conserved 76 amino acid peptide that acts as a marker for intracellular protein degradation by proteasomes and has been observed to accumulate in neuritic plaques and neurofibrillary tangles of patients with AD. Higher serum anti-P. gingivalis immunoglobulin G (IgG) titres have been reported as a risk factor for AD development (Stein et al. Citation2012), increased AD incidence (Beydoun et al. Citation2020) and impaired delayed recall and calculation (Noble et al. Citation2009). A recent study of 20 AD patients and 20 patients with dementia of other forms detected elevated levels of anti-P. gingivalis IgG antibodies in cerebrospinal fluid for both groups (Laugisch et al. Citation2018). Other studies have failed to find similar associations (Noble et al. Citation2014; Panzarella et al. Citation2020), although the definition of a control population is problematic in these studies. However, the tendency towards a higher detection of P. gingivalis-related biomolecules in brains and sera of AD patients is clear, which suggests the intracerebral and systemic contributions of this pathobiont in the pathogenesis of AD. In the last five years, a number of studies have also demonstrated that administration of P. gingivalis or its virulence factors generates AD-like phenotypes including neuroinflammation, Aβ accumulation, tau hyperphosphorylation, neurodegeneration and cognitive impairments in small animal models (Ishida et al. Citation2017; Wu et al. Citation2017; Ding et al. Citation2018; Ilievski et al. Citation2018; Zhang et al. Citation2018; Díaz-Zúñiga et al. Citation2020; Gu et al. Citation2020; Hu et al. Citation2020; Liu et al. Citation2020; Bahar et al. Citation2021; Tang et al. Citation2021). It is important to note that to date no viable oral bacteria have been recovered from brain tissue of humans or from animal models of disease. Imaging of brain tissue in these studies has also failed to detect the presence of clusters of oral bacterial cells as would be expected if whole bacteria were invading the brain and growing. This raises the distinct possibility that P. gingivalis in the oral cavity is acting as a focal infection with regard to AD and that its products are disseminating to the brain causing collateral damage. Interestingly, P. gingivalis components that have been frequently detected in the brain, i.e. nucleic acids, LPS and gingipains are all carried by P. gingivalis OMVs. This invites us to consider if OMVs, as a transport vehicle of a range of bacterial biomolecules, are the mechanistic link between subgingival P. gingivalis and brain pathology.
P. gingivalis OMVs
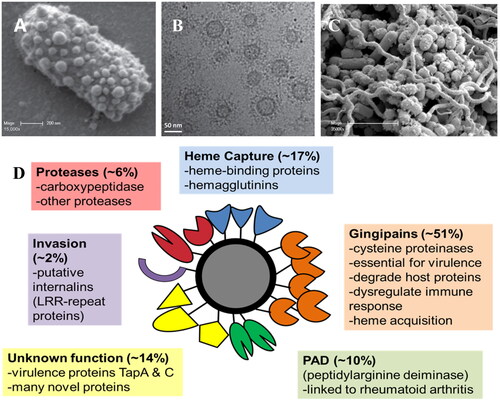
OMVs are spherical nanostructures released from the outer membrane of Gram-negative bacteria. Carrying a broad range of bacterial virulence factors, they have a pivotal role in bacterial growth, intercellular communication, biofilm formation, invasion and modulation of host defence () (Gui et al. Citation2016). P. gingivalis and other oral pathobionts including Tannerella forsythia, and Prevotella intermedia use the recently described Type IX Secretion System (T9SS) to load their outer membrane surface with a high density of proteinaceous virulence factors (Veith et al. Citation2022). These virulence factors include proteinases, adhesins, haemolysins, iron capture proteins and internalins, which are then differentially packaged depending on environmental conditions and released on OMVs in structurally stable, active forms (Veith et al. Citation2018). These OMVs also contain bacterial DNA and RNA that are transported intact into host cells when the OMVs are internalised, where they can influence host cell gene expression and regulation (Bitto et al. Citation2017; Han et al. Citation2019). In addition, OMVs are composed of LPS and other biomolecules containing pathogen-associated molecular patterns (PAMPs) that provoke an inflammatory response. P. gingivalis produces significant numbers of OMVs, especially when cultured as pathogenic polymicrobial biofilms (). The ratio of P. gingivalis OMVs to cells in bacterial culture was approximately 2000:1 by stationary growth phase (Cecil et al. Citation2016). These OMVs penetrate the subgingival pocket epithelium and at low concentrations distal from the bacterial biofilm stimulate the secretion of proinflammatory mediators, dysregulating the host response and causing tissue damage (O'Brien-Simpson et al. Citation2009). P. gingivalis OMVs induce significantly different responses in macrophages compared with whole cells, inducing substantial TNF-α, IL-12p70, IL-6, IL-10, IFN-γ, and nitric oxide, a response that is modulated to some extent by OMV sphingolipids (Fleetwood et al. Citation2017; Rocha et al. Citation2021). The release of OMVs by P. gingivalis increases its sphere of influence, orchestrating the production of available peptides, amino acids and haem in the host environment. Many of these essential nutrients are captured on the OMVs by binding proteins to enable transport in a bioactive form. They act as community goods, benefitting other oral pathobionts, including Treponema denticola and promoting periodontal disease-associated dysbiosis (Dashper et al. Citation2009; Tan et al. Citation2014; Gui et al. Citation2016).
Figure 3. (A) Scanning electron micrograph showing the high numbers of OMVs blebbing from the surface of a P. gingivalis cell. (B) Cryo-transmission electron micrograph showing P. gingivalis OMVs and their extensive surface layer of proteins (reproduced with permission from Gui et al. (Citation2016)). (C). Scanning electron micrograph of a pathogenic polymicrobial biofilm of P. gingivalis, T. denticola and T. forsythia showing high numbers of OMVs (reproduced with permission from Zhu et al. (Citation2013)). (D) The P. gingivalis OMV proteome is composed largely of Type IX Secretion System proteins that play roles in host tissue destruction, immune response dysregulation, internalisation and (micro)nutrient capture.
P. gingivalis OMVs: spheres of influence in the brain?
The relatively small size, large numbers, and surface enrichment in proteinases and adhesins compared to their parental cells render P. gingivalis OMVs more efficient in invading host tissues and diffusing via the bloodstream to remote organs (Ho et al. Citation2015). A recent study has demonstrated that P. gingivalis OMVs translocated into the brain after repeated injection into the abdominal cavity of mice for 12 weeks (Yoshida et al. Citation2022). OMVs from another oral pathobiont Actinobacillus actinomycetemcomitans have also been shown to traverse the blood brain barrier (BBB) into the mouse brain after peripheral administration where the encapsulated RNA elevated TNF-α expression (Han et al. Citation2019). P. gingivalis OMVs may disrupt and penetrate the BBB through several mechanisms. Enriched gingipains on OMV surfaces can cleave an array of protein constituents of the barrier, such as junctional proteins, integrins, and extracellular matrix (ECM) proteins (Zhao et al. Citation2015). P. gingivalis OMVs increase vascular permeability in vitro in a gingipain-dependent manner by cleaving a major endothelial adhesion protein, PECAM-1, which is found at the BBB adherens junction (Farrugia et al. Citation2020). Gingipains degrade β1-integrins (Qiu et al. Citation2018), the lack of which in endothelial cells leads to an immature BBB and prominent brain haemorrhage in mice (Yamamoto et al. Citation2015). These findings indicate a direct role for gingipains enabling P. gingivalis OMVs to cross the BBB paracellularly. Moreover, gingipains have a strong affinity for several ECM proteins and degrade them efficiently in vitro. Gingipains may disturb the functioning of ECM proteins in the basal membrane such as astrocytic laminins which play a key role in maintaining BBB integrity (Yao et al. Citation2014). P. gingivalis OMVs are also taken up into host cells via endocytosis and can degrade vicinal cellular proteins (Furuta et al. Citation2009). Therefore, circulating OMVs could be absorbed by brain endothelial cells, disrupting cellular and subcellular structures and functions, leading to cell death and leaks in the endothelial barrier. Another possibility is that peripheral macrophages, after phagocytosing OMVs, transport them or their components into the brain like a “Trojan horse.”
LPS and gingipains are influential in the complex pathomechanisms of P. gingivalis, and are increasingly appreciated as contributors to AD-related neuropathology after they reach the brain. Beside LPS and gingipains, aforementioned haem and iron acquisition systems, extracellular RNA, fimbriae, lipoproteins, and other components of OMVs have all been identified as virulence factors (Gui et al. Citation2016), and their potential roles in AD pathogenesis are only now starting to be explored or inferred.
LPS is a major structural component of Gram-negative bacterial OMVs. P. gingivalis LPS/lipoprotein stimulate pattern recognition receptors expressed on innate immune cells to activate immune responses. In the brain, P. gingivalis LPS/lipoprotein can contribute to neuroinflammation in a microglia-dependent manner. Although controversial, toll-like receptor (TLR) 2 and/or TLR4 on microglia are reported to be the targets for P. gingivalis LPS/lipoprotein preparations, which mediate the downstream NF-κB and STAT3 pathways and initiate a cascade of events involving increased expression and secretion of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, IL-17 and IL-23 (Nativel et al. Citation2017; Wu et al. Citation2017; Qiu et al. Citation2021). In particular, cathepsin B in microglial cells is induced by P. gingivalis LPS preparations through the NF-κB pathway resulting in the microglial production of IL-1β. Released IL-1β acts on neuronal IL-1 receptors which promotes the expression and processing of amyloid precursor protein (APP) and tau phosphorylation within neurons (Li et al. Citation2003; Villemagne et al. Citation2013). Cathepsin B responds to NF-κB signalling, further promoting the chronic activation of NF-κB pathway and microglia-mediated neuroinflammation (Wu et al. Citation2017). In addition, P. gingivalis LPS preparations induce glycogen synthase kinase 3β (GSK-3β) activation in microglia which, probably converging to NF-κB and STAT3 activation, increases the expression and secretion of TNF-α that act on neurons triggering AKT-GSK-3β-mediated tau hyperphosphorylation (Jiang et al. Citation2021). In contrast, P. gingivalis LPS treatment did not directly change the activity of GSK-3β in an APP-over-expressing neuroblastoma cell line, but it induced tau phosphorylation by upregulating the inactive form of protein phosphatase 2, a principal phosphatase for tau de-phosphorylation (Zeng et al. Citation2021). Further to this, a recent transcriptome study showed that P. gingivalis LPS treatment of human neuroblastoma cells affects an array of interconnected pathways involving cellular oxidative stress, inflammation and metabolism (Bahar and Singhrao Citation2021). Overall, these findings suggest a complex role for P. gingivalis LPS in AD pathophysiology, similar to its complex role in the pathology of periodontitis.
P. gingivalis gingipains are cysteine proteinases of two types, lysine-specific (Kgp) and arginine-specific (RgpA/B) that are located on the surface of the outer membrane and on OMVs. Gingipains on OMVs outnumber those on bacterial cells by 3–5 fold (Ho et al. Citation2015) and exert a broad-spectrum proteolytic activity towards host tissues. This makes the gingipains a powerful weapon for OMVs to trigger AD pathology through many facets. Dominy and co-workers identified that gingipains cleaved tau in SH-SY5Y cells and some of the released peptides are related to NFT formation by nucleating paired helical filaments (Dominy et al. Citation2019). A later study provided similar findings that in vitro P. gingivalis infected neurons showed strong degradation of tau protein and an increased phospho-tau/tau ratio correlated with intraneuronally active gingipains (Haditsch et al. Citation2020). Further, it has been suggested that gingipains also hydrolyse Aβ peptides or proteins mediating Aβ internalisation and degradation, such as integrins (Verdier and Penke Citation2004), or can mediate the coaggregation of Aβ through its haemagglutinin-adhesin subdomain. Gingipains also have the potential to exploit the complement system to facilitate bystander injury of brain structures, such as synapse pruning and neuronal death observed in AD, which is attributed, at least in part, to microglial complement activation (Hong et al. Citation2016; Dejanovic et al. Citation2018). Orally inoculated P. gingivalis has been demonstrated to aid in activating complement responses of microglia with collateral neuronal damage in APOE-/- mouse brains (Poole et al. Citation2015). Gingipains may have a role herein, by proteolytically cleaving the complement regulatory proteins expressed on neurons which protect host cells from complement-mediated damage, as they do to oral epithelial cells by shedding CD46 (Mahtout et al. Citation2009). Also, gingipains are implicated in neuroinflammatory responses. Nakanishi and co-workers reported a partial contribution of gingipains to increased microglial expression of IL-6, TNF-α, and inducible nitric oxide synthase following the injection of P. gingivalis culture in murine somatosensory cortexes, and observed that gingipains mediated microglia migration into the infection sites by activating protease-activated receptor 2 (Liu et al. Citation2017) which was further confirmed in a human microglial cell line (Nonaka and Nakanishi Citation2020). It is hypothesised that intraneuronal gingipains are involved in the neuronal release of pro-inflammatory cytokines and Aβ deposition via inflammasome activation (Dominy et al. Citation2019). A study with young adult mice found that after repeated oral application of P. gingivalis, gingipains were detected in and around the nucleus of astrocytes, microglia and neurons, and extracellularly in murine hippocampi, accompanied by more degenerating neurons, extracellular Aβ plaques and neuroinflammation (Ilievski et al. Citation2018). Correspondingly, inhibition of gingipains reduces cerebral DNA load of P. gingivalis, Aβ1-42 production, neuroinflammation and the loss of hippocampal neurons, suggesting gingipains as an effective target for rescuing neurons and treating neurodegeneration in AD (Dominy et al. Citation2019). As gingipains target a multitude of cell surface receptors and proteins in a cell-type dependent manner (Guo et al. Citation2010), we propose gingipains are also involved in a novel mechanism of OMV facilitated AD deterioration by hydrolysis of iron-containing host proteins (see below).
P. gingivalis OMVs and the “rusty” brain
As the most abundant transition metal in the brain, iron has long been associated with the pathogenesis of AD. Iron may influence the production of Aβ, as intracellular iron levels modulate the translation of APP via an iron-regulatory element in the 5′-untranslated region of APP transcripts (Cho et al. Citation2010) and high iron levels promote the amyloidogenic processing of APP by activating γ-secretase (Li et al. Citation2013). Iron binds Aβ with a binding affinity eight times stronger than that of transferrin (Jiang et al. Citation2009), which results in not only the formation of toxic iron-Aβ aggregates but perturbation of iron homeostasis. Iron also binds tau and is related to the formation of NFTs. Several studies show that iron mediates tau hyperphosphorylation and dysfunction through the aberrant activation of proline-directed tau kinases (Lovell et al. Citation2004; Muñoz et al. Citation2006; Bautista et al. Citation2016). Fe3+ was shown to induce the aggregation of hyperphosphorylated tau in vitro, possibly through action on an iron-binding motif in tau (Yamamoto et al. Citation2002). On the other hand, tau and APP are involved in the stabilisation of ferroportin which mediates intracellular iron efflux. Therefore, dysfunction of tau and APP may result in cellular iron retention that is observed in AD.
Putting aside its role in plaques and NFTs, recently iron has been suggested as an effector of neurodegeneration acting downstream of AD hallmark proteinopathies (Ayton et al. Citation2021; Jakaria et al. Citation2021). This suggestion is supported by the finding that brain iron, within normal levels, was strikingly associated with the rate of cognition impairment in patients with established AD neuropathology (Ayton et al. Citation2021). In this regard, research is now focussing on ferroptosis, an iron-dependent cell death pathway, as a likely mechanism underlying neurodegeneration in AD.
Intriguingly, P. gingivalis is a haem/protoporphyrin IX auxotroph indispensably relying on host-derived haem and iron. To meet these basic needs for survival and growth, P. gingivalis has evolved efficient molecular machineries to capture haem and iron from the host, concomitant with a significant capacity to store haem on its surface and on OMVs. The characterisation of the P. gingivalis OMV proteome has demonstrated selective enrichment of proteins, as well as high levels of iron capture proteins, variations in nucleic acid content, and other compositional changes dependent on environmental conditions () (Veith et al. Citation2014, Citation2018). Most of the body iron is circulated in the blood as haemoglobin, which is preferentially captured by P. gingivalis OMVs through the concerted efforts of gingipains and haem-binding proteins such as HmuY (Smalley et al. Citation2011). In the circulatory system, P. gingivalis OMVs will become loaded with haem and iron as a result of their haemolytic capability, the proteolytic degradation of haemoglobin, and other iron-carrying plasma proteins including transferrin (Dashper et al. Citation2004; Gao et al. Citation2010). In this context, the transfer of iron by P. gingivalis OMVs from the bloodstream to the brain may provide one explanation for iron maldistribution observed in AD. Of particular note, the proliferation of P. gingivalis and the disruption of the epithelia during periodontitis progression () enables more OMVs to spread into circulation and cause a shift in iron distribution from blood to brain. In this way, OMVs may serve as a mechanistic link between chronic periodontitis and AD.
Recently, ferroptosis has been presented as a possible mechanism of neurodegeneration and cognitive impairment (Ayton et al. Citation2021; Jakaria et al. Citation2021). Ferroptosis is a newly discovered mechanism of programmed cell death characterised by iron-mediated phospholipid peroxidation (Dixon et al. Citation2012). It needs to be stressed that ferroptosis activation results from an increase in iron bioactivity, rather than iron amount (Ayton et al. Citation2021). Normally, excessive intracellular iron is stored by ferritin in a redox-inactive form. The liberation of iron from ferritin to the labile iron pool has a direct bearing on increased redox activity and cell overload of iron. P. gingivalis gingipains on OMVs hydrolyse ferritin and other iron-containing proteins releasing iron to enlarge the free iron pool and cumulative ferroptotic stress. This scenario is supported by the finding that gingipains cleaved human transferrin into free iron and iron-bound fragments which were able to catalyse the production of toxic hydroxyl radicals (Goulet et al. Citation2004). Albeit not necessary for the ferroptotic pathway, the increased iron burden that can be induced by OMVs sensitises the cell to ferroptotic death since more iron is liberated to catalyse the generation of poisonous lipid reactive oxygen species directly or in the form of iron-containing dioxygenase (Jakaria et al. Citation2021). Ferroptosis is essentially a result of an imbalance between two systems, iron-mediated production of lipid hydroperoxides and antioxidative host defense largely dependent on glutathione peroxidase 4 (GPX4) (Yang et al. Citation2016; Bersuker et al. Citation2019). As a main ferroptosis checkpoint, GPX4 directly detoxifies membranous lipid hydroperoxides, while oral injection of P. gingivalis LPS can lead to significantly decreased expression of GPX4 in the hippocampi of APP/PS1 mice (Zeng et al. Citation2021). These findings suggest that the surface components of OMVs may influence both systems to tip the redox balance of the cell towards lipid peroxidation and ferroptosis. Apart from iron, one should be aware that neurochemical anomalies of several other metals are observed in the AD brain, and the iron capture protein HmuY on OMVs shows binding affinity for some of the other dysregulated metals like copper and zinc (Wójtowicz et al. Citation2013).
Based on these findings we propose that OMVs are the nexus between the “infectious hypothesis” and “metal hypothesis” to produce a unifying hypothesis of AD linking oral pathobiont OMVs with the “rusty” brain.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Armstrong RA. 2019. Risk factors for Alzheimer’s disease. Folia Neuropathologica. 57(2):87–105.
- Ayton S, Portbury S, Kalinowski P, Agarwal P, Diouf I, Schneider JA, Morris MC, Bush AI. 2021. Regional brain iron associated with deterioration in Alzheimer’s disease: a large cohort study and theoretical significance. Alzheimers Dement. 17(7):1244–1256.
- Bahar B, Kanagasingam S, Tambuwala MM, Aljabali AAA, Dillon SA, Doaei S, Welbury R, Chukkapalli SS, Singhrao SK. 2021. Porphyromonas gingivalis (W83) infection induces Alzheimer’s disease-like pathophysiology in obese and diabetic mice. J Alzheimers Dis. 82(3):1259–1275.
- Bahar B, Singhrao SK. 2021. An evaluation of the molecular mode of action of trans-resveratrol in the Porphyromonas gingivalis lipopolysaccharide challenged neuronal cell model. Mol Biol Rep. 48(1):147–156.
- Bautista E, Vergara P, Segovia J. 2016. Iron-induced oxidative stress activates AKT and ERK1/2 and decreases Dyrk1B and PRMT1 in neuroblastoma SH-SY5Y cells. J Trace Elem Med Biol. 34:62–69.
- Bennett JP, Jr, Keeney PM, Brohawn DG. 2019. RNA sequencing reveals small and variable contributions of infectious agents to transcriptomes of postmortem nervous tissues from amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson’s disease subjects, and increased expression of genes from disease-activated microglia. Front Neurosci. 13:235.
- Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. 2019. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 575(7784):688–692.
- Beydoun MA, Beydoun HA, Hossain S, El-Hajj ZW, Weiss J, Zonderman AB. 2020. Clinical and bacterial markers of periodontitis and their association with incident all-cause and Alzheimer’s disease dementia in a large national survey. J Alzheimers Dis. 75(1):157–172.
- Bitto NJ, Chapman R, Pidot S, Costin A, Lo C, Choi J, D’Cruze T, Reynolds EC, Dashper SG, Turnbull L, et al. 2017. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci Rep. 7(1):7072.
- Cecil JD, O'Brien-Simpson NM, Lenzo JC, Holden JA, Chen Y-Y, Singleton W, Gause KT, Yan Y, Caruso F, Reynolds EC, et al. 2016. Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PLoS One. 11(4):e0151967.
- Cho H-H, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, Rogers JT. 2010. Selective Translational control of the alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem. 285(41):31217–31232.
- Dashper SG, Ang C-S, Veith PD, Mitchell HL, Lo AWH, Seers CA, Walsh KA, Slakeski N, Chen D, Lissel JP, et al. 2009. Response of Porphyromonas gingivalis to heme limitation in continuous culture. J Bacteriol. 191(3):1044–1055.
- Dashper SG, Cross KJ, Slakeski N, Lissel P, Aulakh P, Moore C, Reynolds EC. 2004. Hemoglobin hydrolysis and heme acquisition by Porphyromonas gingivalis. Oral Microbiol Immunol. 19(1):50–56.
- Dejanovic B, Huntley MA, De Mazière A, Meilandt WJ, Wu T, Srinivasan K, Jiang Z, Gandham V, Friedman BA, Ngu H, et al. 2018. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron. 100(6):1322–1336. e7.
- Díaz-Zúñiga J, More J, Melgar-Rodríguez S, Jiménez-Unión M, Villalobos-Orchard F, Muñoz-Manríquez C, Monasterio G, Valdés JL, Vernal R, Paula-Lima A, et al. 2020. Alzheimer’s disease-like pathology triggered by Porphyromonas gingivalis in wild type rats is serotype dependent. Front Immunol. 11:588036.
- Ding Y, Ren J, Yu H, Yu W, Zhou Y. 2018. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun Ageing. 15(1):1–8.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 149(5):1060–1072.
- Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, et al. 2019. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 5(1):eaau3333.
- Farrugia C, Stafford G, Murdoch C. 2020. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J Dent Res. 99(13):1494–1501.
- Fleetwood AJ, Lee MKS, Singleton W, Achuthan A, Lee M-C, O'Brien-Simpson NM, Cook AD, Murphy AJ, Dashper SG, Reynolds EC, et al. 2017. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front Cell Infect Microbiol. 7:351.
- Furuta N, Takeuchi H, Amano A. 2009. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infect Immun. 77(11):4761–4770.
- Gao J-L, Nguyen K-A, Hunter N. 2010. Characterization of a hemophore-like protein from Porphyromonas gingivalis. J Biol Chem. 285(51):40028–40038.
- Genco RJ, Borgnakke WS. 2013. Risk factors for periodontal disease. Periodontol 2000. 62(1):59–94.
- Goulet V, Britigan B, Nakayama K, Grenier D. 2004. Cleavage of human transferrin by Porphyromonas gingivalis gingipains promotes growth and formation of hydroxyl radicals. Infect Immun. 72(8):4351–4356.
- Gu Y, Wu Z, Zeng F, Jiang M, Teeling JL, Ni J, Takahashi I. 2020. Systemic exposure to lipopolysaccharide from Porphyromonas gingivalis induces bone loss-correlated Alzheimer’s disease-like pathologies in middle-aged mice. J Alzheimers Dis. 78(1):61–74.
- Gui MJ, Dashper SG, Slakeski N, Chen Y-Y, Reynolds EC. 2016. Spheres of influence: Porphyromonas gingivalis outer membrane vesicles. Mol Oral Microbiol. 31(5):365–378.
- Guo Y, Nguyen K-A, Potempa J. 2010. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 54(1):15–44.
- Haditsch U, Roth T, Rodriguez L, Hancock S, Cecere T, Nguyen M, Arastu-Kapur S, Broce S, Raha D, Lynch CC, et al. 2020. Alzheimer’s disease-like neurodegeneration in Porphyromonas gingivalis infected neurons with persistent expression of active gingipains. J Alzheimers Dis. 75(4):1361–1376.
- Hajishengallis G, Lamont RJ. 2012. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 27(6):409–419.
- Han E-C, Choi S-Y, Lee Y, Park J-W, Hong S-H, Lee H-J. 2019. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood–brain barrier in mice. FASEB J. 33(12):13412–13422.
- Han YW, Wang X. 2013. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 92(6):485–491.
- Ho M-H, Chen C-H, Goodwin JS, Wang B-Y, Xie H. 2015. Functional advantages of Porphyromonas gingivalis vesicles. PLoS One. 10(4):e0123448.
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. 2016. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 352(6286):712–716.
- Hu Y, Li H, Zhang J, Zhang X, Xia X, Qiu C, Liao Y, Chen H, Song Z, Zhou W, et al. 2020. Periodontitis induced by P. gingivalis-LPS is associated with neuroinflammation and learning and memory impairment in Sprague-Dawley rats. Front Neurosci. 14:658.
- Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O'Brien-Simpson NM, Reynolds EC, Watanabe K, et al. 2018. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 13(10):e0204941.
- Ishida N, Ishihara Y, Ishida K, Tada H, Funaki-Kato Y, Hagiwara M, Ferdous T, Abdullah M, Mitani A, Michikawa M, et al. 2017. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech Dis. 3(1):15–17.
- Jakaria M, Belaidi AA, Bush AI, Ayton S. 2021. Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J Neurochem. 159(5):804–825.
- Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. 2013. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun. 81(7):2288–2295.
- Jiang D, Li X, Williams R, Patel S, Men L, Wang Y, Zhou F. 2009. Ternary complexes of iron, amyloid-β, and nitrilotriacetic acid: binding affinities, redox properties, and relevance to iron-induced oxidative stress in Alzheimer’s disease. Biochemistry. 48(33):7939–7947.
- Jiang M, Zhang X, Yan X, Mizutani S, Kashiwazaki H, Ni J, Wu Z. 2021. GSK3β is involved in promoting Alzheimer’s disease pathologies following chronic systemic exposure to Porphyromonas gingivalis lipopolysaccharide in amyloid precursor proteinNL-F/NL-F knock-in mice. Brain Behav Immun. 98:1–12.
- Laugisch O, Johnen A, Maldonado A, Ehmke B, Bürgin W, Olsen I, Potempa J, Sculean A, Duning T, Eick S, et al. 2018. Periodontal pathogens and associated intrathecal antibodies in early stages of Alzheimer’s disease. J Alzheimers Dis. 66(1):105–114.
- Lennon MJ, Makkar SR, Crawford JD, Sachdev PS. 2019. Midlife hypertension and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 71(1):307–316.
- Li X, Liu Y, Zheng Q, Yao G, Cheng P, Bu G, Xu H, Zhang Y-W 2013. Ferritin light chain interacts with PEN-2 and affects γ-secretase activity. Neurosci Lett. 548:90–94.
- Li Y, Liu L, Barger SW, Griffin WST. 2003. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 23(5):1605–1611.
- Liu J, Wang Y, Guo J, Sun J, Sun Q. 2020. Salvianolic acid B improves cognitive impairment by inhibiting neuroinflammation and decreasing Aβ level in Porphyromonas gingivalis-infected mice. Aging. 12(11):10117–10128. ),
- Liu Y, Wu Z, Nakanishi Y, Ni J, Hayashi Y, Takayama F, Zhou Y, Kadowaki T, Nakanishi H. 2017. Infection of microglia with Porphyromonas gingivalis promotes cell migration and an inflammatory response through the gingipain-mediated activation of protease-activated receptor-2 in mice. Sci Rep. 7(1):11759.
- Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR. 2004. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimers Dis. 6(6):659–671.
- Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, Hashim A, Hoch S, Curtis MA, Nussbaum G, et al. 2014. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 15(6):768–778.
- Mahtout H, Chandad F, Rojo JM, Grenier D. 2009. Porphyromonas gingivalis mediates the shedding and proteolysis of complement regulatory protein CD46 expressed by oral epithelial cells. Oral Microbiol Immunol. 24(5):396–400.
- Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, Osorio M, Wahl SM. 2012. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 39(4):294–303.
- Muñoz P, Zavala G, Castillo K, Aguirre P, Hidalgo C, Núñez MT. 2006. Effect of iron on the activation of the MAPK/ERK pathway in PC12 neuroblastoma cells. Biol Res. 39(1):189–190.
- Nativel B, Couret D, Giraud P, Meilhac O, d’Hellencourt CL, Viranaïcken W, Da Silva CR. 2017. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci Rep. 7(1)15789:.
- Ng HM, Kin LX, Dashper SG, Slakeski N, Butler CA, Reynolds EC. 2016. Bacterial interactions in pathogenic subgingival plaque. Microb Pathog. 94:60–69.
- Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB. 2009. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 80(11):1206–1211.
- Noble JM, Scarmeas N, Celenti RS, Elkind MSV, Wright CB, Schupf N, Papapanou PN. 2014. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One. 9(12):e114959.
- Nonaka S, Nakanishi H. 2020. Secreted gingipains from Porphyromonas gingivalis induce microglia migration through endosomal signaling by protease-activated receptor 2. Neurochem Int. 140:104840.
- O'Brien-Simpson NM, Pathirana RD, Walker GD, Reynolds EC. 2009. Porphyromonas gingivalis RgpA-Kgp proteinase-adhesin complexes penetrate gingival tissue and induce proinflammatory cytokines or apoptosis in a concentration-dependent manner. Infect Immun. 77(3):1246–1261.
- Panzarella V, Mauceri R, Baschi R, Maniscalco L, Campisi G, Monastero R. 2020. Oral health status in subjects with amnestic mild cognitive impairment and Alzheimer’s disease: data from the Zabút aging project. J Alzheimers Dis. 87(1):173–183.
- Patel S, Howard D, Chowdhury N, Derieux C, Wellslager B, Yilmaz Ö, French L. 2021. Characterization of human genes modulated by Porphyromonas gingivalis highlights the ribosome, hypothalamus, and cholinergic neurons. Front Immunol. 12:2165.
- Poole S, Singhrao SK, Chukkapalli S, Rivera M, Velsko I, Kesavalu L, Crean S. 2015. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE-/-mice brains. J Alzheimers Dis. 43(1):67–80.
- Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. 2013. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 36(4):665–677.
- Qiu C, Yuan Z, He Z, Chen H, Liao Y, Li S, Zhou W, Song Z. 2021. Lipopolysaccharide preparation derived from Porphyromonas gingivalis induces a weaker immuno-inflammatory response in BV-2 microglial cells than Escherichia coli by differentially activating TLR2/4-mediated NF-κB/STAT3 signaling pathways. Front Cell Infect Microbiol. 11:606986.
- Qiu Q, Zhang F, Wu J, Xu N, Liang M. 2018. Gingipains disrupt F‐actin and cause osteoblast apoptosis via integrin β1. J Periodontal Res. 53(5):762–776.
- Rocha FG, Ottenberg G, Eure ZG, Davey ME, Gibson FC. 2021. Sphingolipid-containing outer membrane vesicles serve as a delivery vehicle to limit macrophage immune response to Porphyromonas gingivalis. Infect Immun. 89(4):e00614-20.
- Singhrao SK, Harding A, Simmons T, Robinson S, Kesavalu L, Crean S. 2014. Oral inflammation, tooth loss, risk factors, and association with progression of Alzheimer’s disease. J Alzheimers Dis. 42(3):723–737.
- Smalley JW, Byrne DP, Birss AJ, Wojtowicz H, Sroka A, Potempa J, Olczak T. 2011. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PLoS One. 6(2):e17182.
- Stein PS, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, Dawson D 3rd. 2012. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dementia. 8(3):196–203.
- Tan KH, Seers CA, Dashper SG, Mitchell HL, Pyke JS, Meuric V, Slakeski N, Cleal SM, Chambers JL, McConville MJ, et al. 2014. Porphyromonas gingivalis and Treponema denticola exhibit metabolic symbioses. PLoS Pathog. 10(3):e1003955.
- Tang Z, Liang D, Cheng M, Su X, Liu R, Zhang Y, Wu H. 2021. Effects of Porphyromonas gingivalis and its underlying mechanisms on Alzheimer-like tau hyperphosphorylation in Sprague-Dawley rats. J Mol Neurosci. 71(1):89–100.
- Van Dyke TE, Bartold PM, Reynolds EC. 2020. The nexus between periodontal inflammation and dysbiosis. Front Immunol. 11:511.
- Veith PD, Chen Y-Y, Gorasia DG, Chen D, Glew MD, O'Brien-Simpson NM, Cecil JD, Holden JA, Reynolds EC. 2014. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 13(5):2420–2432.
- Veith PD, Glew MD, Gorasia DG, Cascales E, Reynolds EC. 2022. The type IX secretion system and its role in bacterial function and pathogenesis. J Dent Res. 101(4):374–383.
- Veith PD, Luong C, Tan KH, Dashper SG, Reynolds EC. 2018. Outer membrane vesicle proteome of Porphyromonas gingivalis is differentially modulated relative to the outer membrane in response to heme availability. J Proteome Res. 17(7):2377–2389.
- Verdier Y, Penke B. 2004. Binding sites of amyloid β-peptide in cell plasma membrane and implications for Alzheimer’s disease. Curr Protein Pept Sci. 5(1):19–31.
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, et al. 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12(4):357–367.
- Wójtowicz H, Bielecki M, Wojaczyński J, Olczak M, Smalley JW, Olczak T. 2013. The Porphyromonas gingivalis HmuY haemophore binds gallium (III), zinc (II), cobalt (III), manganese (III), nickel (II), and copper (II) protoporphyrin IX but in a manner different to iron (III) protoporphyrin IX. Metallomics. 5(4):343–351.
- Wu X, Chen J, Xu M, Zhu D, Wang X, Chen Y, Wu J, Cui C, Zhang W, Yu L, et al. 2017. 16S rDNA analysis of periodontal plaque in chronic obstructive pulmonary disease and periodontitis patients. J Oral Microbiol. 9(1):1324725.
- Wu Z, Ni J, Liu Y, Teeling JL, Takayama F, Collcutt A, Ibbett P, Nakanishi H. 2017. Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav Immun. 65:350–361.
- Xue L, Zou X, Yang X-Q, Peng F, Yu D-K, Du J-R. 2020. Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp Neurol. 326:113176.
- Yamamoto A, Shin R-W, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. 2002. Iron (III) induces aggregation of hyperphosphorylated τ and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem. 82(5):1137–1147.
- Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D, Adams RH, et al. 2015. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 6(1):1–14.
- Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. 2016. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 113(34):E4966–E4975.
- Yao Y, Chen Z-L, Norris EH, Strickland S. 2014. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 5(1):1–12.
- Yoshida K, Yoshida K, Seyama M, Hiroshima Y, Mekata M, Fujiwara N, Kudo Y, Ozaki K. 2022. Porphyromonas gingivalis outer membrane vesicles in cerebral ventricles activate microglia in mice. Oral Dis. doi: 10.1111/odi.14413.
- Zeng Q, Fang Q, Zhou X, Yang H, Dou Y, Zhang W, Gong P, Rong X. 2021. Cofilin 2 acts as a protein link between chronic periodontits and Alzheimer’s disease. Front Mol Neurosci. 14:203.
- Zhang J, Yu C, Zhang X, Chen H, Dong J, Lu W, Song Z, Zhou W. 2018. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 15(1):1–14.
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. 2015. Establishment and dysfunction of the blood-brain barrier. Cell. 163(5):1064–1078.
- Zhu Y, Dashper SG, Chen Y-Y, Crawford S, Slakeski N, Reynolds EC. 2013. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One. 8(8):e71727.

