Abstract
The ILSI Health and Environmental Sciences Institute (HESI) has developed a framework to support a transition in the way in which information for chemical risk assessment is obtained and used (RISK21). The approach is based on detailed problem formulation, where exposure drives the data acquisition process in order to enable informed decision-making on human health safety as soon as sufficient evidence is available. Information is evaluated in a transparent and consistent way with the aim of optimizing available resources. In the context of risk assessment, cumulative risk assessment (CRA) poses additional problems and questions that can be addressed using the RISK21 approach. The focus in CRA to date has generally been on chemicals that have common mechanisms of action. Recently, concern has also been expressed about chemicals acting on multiple pathways that lead to a common health outcome, and non-chemical other conditions (non-chemical stressors) that can lead to or modify a common outcome. Acknowledging that CRAs, as described above, are more conceptually, methodologically and computationally complex than traditional single-stressor risk assessments, RISK21 further developed the framework for implementation of workable processes and procedures for conducting assessments of combined effects from exposure to multiple chemicals and non-chemical stressors. As part of the problem formulation process, this evidence-based framework allows the identification of the circumstances in which it is appropriate to conduct a CRA for a group of compounds. A tiered approach is then proposed, where additional chemical stressors and/or non-chemical modulating factors (ModFs) are considered sequentially. Criteria are provided to facilitate the decision on whether or not to include ModFs in the formal quantitative assessment, with the intention to help focus the use of available resources to have the greatest potential to protect public health.
Introduction
Current approaches to cumulative risk assessment (CRA)
The consideration of risks posed by exposures to chemicals is currently performed largely on an individual chemical basis. However, risk assessment is evolving to more explicitly address the complexity deriving from the reality of combined exposure to multiple chemicals and/or non-chemical stressors (Meek et al. Citation2011; Sexton Citation2012). This broadened scope forces risk assessors to go beyond basic hazard identification and characterization, and exposure assessment to consider complex combined exposures to various stressors for which there may be very little existing information. To date, a single unified and comprehensive approach has not been realized and may not be practical, given this complexity. However, the need for this type of approach to CRA is clear. Currently, the U.S. Food Quality Protection Act states that the U.S. Environmental Protection Agency (EPA) shall base risk upon “available information concerning the cumulative effects on infants and children of [pesticide] residues and other substances that have a common mechanism of toxicity”. Likewise, the U.S. Superfund Regulation, under the Preliminary Remediation Goals for Non-carcinogens and the European Union Regulation (EC) No. 1107/2009 call for cumulative non-cancer risks to be assessed. In addition, high-level recommendations such as those of the National Research Council (Citation2009) and EU DG SANCO (SCCS, SCHER, SCENIHR Citation2012) have emphasized inclusion of non-chemical stressors in CRA, while maintaining a focus on the decision context or problem formulation (NRC Citation2009) and emphasizing the need for risk assessors to identify priorities for risk management where co-exposures are expected (Meek et al. Citation2011).
The HESI RISK21 project
In 2010, the ILSI Health and Environmental Sciences Institute (HESI) convened representatives from academia, government and industry in response to calls from the U.S. National Academy of Sciences (NAS), Canadian Academies and the European Union, among others, to implement fundamental changes to the current unsustainable and inefficient risk assessment process. CRA was considered one of the top priorities in establishing innovative and transparent approaches to assessing the safety of chemical exposures.
The RISK21 team identified the following principles as guides (Embry et al. Citation2014; Pastoor et al. Citation2014):
Focus on problem formulation;
Utilize existing information;
Consider exposure early in the risk assessment process;
Use a tiered approach for development of data and decision-making.
Current CRA frameworks and approaches
The process of assessing risks from combined exposures has been described and developed in numerous frameworks and reports (e.g., USEPA Citation2007; EFSA Citation2008; NRC Citation2009; Meek et al. Citation2011, 2013; Price et al. Citation2012; SCCS, SCHER, SCENIHR Citation2012). Each provides, to some extent, a conceptual structure for identifying the fundamental elements and basic principles to evaluate cumulative risks from a combination of chemical and, at times, non-chemical stressors through the adoption of a tiered approach. As these frameworks have developed, it has become apparent that there are fundamentally two different perspectives to approach the conduct of a CRA. In one case, the objective is the prospective evaluation of potential health effects associated with a defined set of chemical stressors (stressor-based or prospective CRA), typically, but not exclusively, driven by and conducted for regulatory purposes, which focuses on a hypothetical population and exposure. However, in certain instances, a (sub)population of concern can be identified based on expected specific environmental conditions. In the other situation, the trigger for the assessment is the observation of a certain effect in a given population, where identification and consideration of all potential contributors to the observed effect are required (effect-based or retrospective CRA). A hybrid perspective has recently been described, where the focus is on identification and evaluation of vulnerable (at-risk) communities and populations disproportionately affected by cumulative consequences from exposure to multiple environmental stressors (Sexton Citation2015).
Objective
The objective of the cumulative risk component of the RISK21 multi-sector, international initiative was to develop a means—quantitative to the extent practical—of assessing the potential adverse health effects from combined exposure to multiple chemical and non-chemical stressors. An integral part of the approach is identifying when a CRA is appropriate and necessary, once both the likelihood of co-exposure and a common toxicity have been demonstrated or hypothesized. The companion paper (Solomon et al. Citation2016, this issue) describes the need to appropriately define the scope and purpose of the assessment in a problem formulation (PF) step prior to embarking on any CRA. With this as the foundation, the risk assessment approach proposed is to initially conduct a “chemical” CRA, involving an identified group of chemical stressors, i.e., two or more chemicals, followed by consideration of other chemical and non-chemical stressors, termed as modulating factors (ModFs, see below and Simon et al. Citation2014) which influence the risk posed by the chemical stressors. Therefore, evaluations focused only on non-chemical stressors or single chemicals with or without ModFs were considered out of scope. By creating manageable boundaries for this framework, previous work was extended and a unified approach developed, enabling integration of multiple perspectives (e.g., effects-based and stressor-based) within a decision-context. The aim has not been that of providing a definitive methodology to carry out CRA for which there is certainly a need (Gallagher et al. Citation2015). Rather, we provide an easy-to-follow, visual and transparent framework for addressing the difficult and complex issue of CRA.
Terminology and scope
Given the various definitions that have been proposed for CRA and related terms over the last decade, a brief discussion on terminology is provided to ensure clarity. Definitions of CRA are presented in , which have been proposed by a number of prominent organizations; these definitions illustrate the overlap in concept but the specificity in terminology.
Table 1. Definitions of CRA.
Each definition includes some variation of “combined risks from aggregate exposures” from “agents or stressors”. For the purposes of this RISK21 framework, this terminology was viewed as too broad and unsuitable within the context of the HESI RISK21 project, i.e., assessing the adverse health effects from combined exposure to multiple chemical and non-chemical stressors. A narrower definition is proposed to clarify the boundaries within which the framework applies.
A chemical CRA (CCRA) is defined as an appraisal—quantitative to the extent practical—of the adverse health effects from combined exposure to multiple chemical stressors and all relevant chemical or non-chemical ModFs (see below).
In addition, the NRC (Citation2009) report and others (Sexton Citation2012, Citation2015; Gallagher et al. Citation2015) propose incorporation of the concepts and factors of vulnerability, susceptibility and sensitivity1 into CRA. Indeed, the NRC does not consider that a CRA is completed until such factors are considered and quantitatively incorporated into the assessment. RISK21 recognizes that these factors need to be addressed and suggests that they can be broken down into their simple elementary constituents. Therefore, the term “modulating factor” (ModF), adapted from the RISK21 companion paper on quantitative key events/dose–response framework (Q-KEDRF) (Simon et al. Citation2014), is introduced where individual non-chemical contributory factors are listed and, when combined, may collectively comprise the determinants of susceptibility and/or vulnerability. While it is recognized that the latter terms are widely used and concisely convey the message, their use is not recommended in CRA. In fact, “quantification” of the overall impact of non-chemical stressors on modifying the response is much simpler when individual factors are addressed separately. Alternatively, the resulting combined effects can be expressed as a single “value” for susceptibility and for vulnerability. However, it should be clearly explained that this “value” is the result of combining different factors for which evidence is provided.
ModFs represent biological, environmental and individual factors, including control mechanisms or host factors and other chemicals, that can modulate the response to an identified (group of) chemical stressor(s) thus altering the probability or magnitude of the adverse outcome. ModFs fall into four main categories including: host factors, lifestyle, environment and other chemicals. The latter are chemicals that do not share the mode of action (MOA) or toxicological endpoint (i.e.: belong to the same cumulative assessment group) but modify the effect by e.g., interfering with the toxicokinetics of one or more of the chemicals in the initially considered chemicals in cumulative assessment group. Categorization of potential ModFs is discussed in the companion paper (Solomon et al. Citation2016, this issue). It should be noted that this list is not meant to be exhaustive, not least because some ModFs can apply to very specific situations (see e.g., DeFur et al. Citation2007; NAS/NRC Citation2009; Sacks et al. Citation2011; Gallagher et al. Citation2015).
Within this context both chemicals and the traditionally termed “non-chemical stressors” can modulate a response to an individual chemical as well as to a common chemical assessment group (CCAG) that is defined as follows:
A CCAG is a group of chemicals sharing both evidence for likelihood of co-exposure within a relevant timeframe AND evidence for dose-additive response.
These definitions clarify the context of the framework described herein where multiple chemical stressors serve as the starting point, and chemical and non-chemical ModFs are subsequently considered as necessary. This approach is most appropriate for any organization or body dealing with chemicals. However, it can be adapted also for those interested in personal and life-style factors, provided that there is a way to quantify the determinants that enter in the risk assessment, as described below for the ModF. Gallagher et al. (Citation2015), that summarize EPA efforts in this respect, also indicate that both increased likelihood of experiencing an adverse outcome or developing a more severe one, and increased exposures for geographical and social reasons to stressors should be considered.
RISK21 cumulative framework approach
This framework builds upon existing knowledge and approaches, and involves a systematic approach to make the problem tractable. Furthermore, the approach is based on five key elements/principles which are also illustrated in .
Figure 1. General conceptual framework of the proposed RISK21 approach to CRA.
Step 1. Preliminary assessment of the need, or lack thereof, to conduct the risk assessment.
Step 2. Problem formulation (PF) (see Solomon et al. Citation2016, this issue).
Step 3. Detailed evaluation of the data on co-exposure and toxicity on the basis of the PF.
Step 4a. Use of the RISK21 matrix, where individual values are plotted. It is intended that after Mitigation/Refinement (oval in the middle), this step is reiterated.
Step 4b. Plot individual values or IC-normalized values. Refine/Mitigate and reiterate, if needed.
Step 5. Apply appropriate CRA methodology and plot. Refine/Mitigate and reiterate, if needed.
Step 6. Apply ModFs, either to individual compounds or to the IC-normalized values and conclude, either directly or after mitigation, on CRA. Note that, if judged that the margin of exposure is so high that consideration of ModFs will not significantly change the safety judgment, the calculation of the impact of such ModFs may not be performed and the plot needs not to be re-drawn, and an appropriate explanation given. All Refinements include, if needed, reiteration of “gatekeeping” and PF.
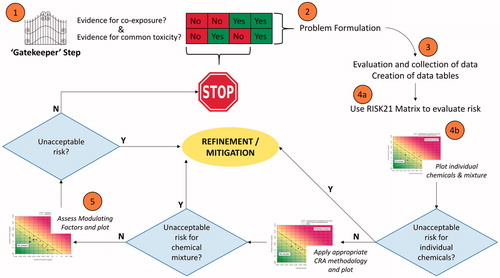
A gatekeeper step for determining whether a CRA is necessary;
Problem formulation;
Evaluation and collection of toxicity and exposure data in a tier-wise approach using evidence/concordance tables;
Use of the RISK21 Matrix to assess risk
Evaluate individual chemicals;
Evaluate cumulative risk;
Evaluation of ModFs in a stepwise manner through an iterative process.
The development of evidence tables facilitates defining the CCAG. Once the CCAG is determined, cumulative exposure and toxicity are estimated using appropriate methods and plotted on the RISK21 matrix. If the assessment indicates that the combined risk to the chemicals is acceptable, then consideration can be paid to the impact of ModFs. If the risk is determined not to be acceptable, then a return to the PF phase at this stage can help refine the assessment, identify direct information needs or develop a risk mitigation strategy.
Gatekeeper step
A starting assumption of this framework approach is that interactions are not likely to occur to a significant extent at doses/exposure levels of individual chemicals at or below their no-effect levels, and therefore only dose-additivity would be expected for compounds with similar MOA (Boobis et al. Citation2011; ECETOC Citation2012). Also, if any single chemical is likely to be present at doses or concentrations above its health-based guidance values, such as its reference value, or any other appropriate value (e.g., TTC for data-poor compounds), it would be premature to start a CRA until the risk from that individual chemical had been addressed.
Scientific evidence should be assembled to facilitate a transparent determination concerning the likelihood of co-exposure AND the likelihood of common toxicity for all chemicals being considered. Both pieces of information are necessary to justify the need to assess cumulative risk and are emphasized as part of an initial “gatekeeper” step. This step establishes a minimum set of conditions that trigger the need for a CRA and requires that sufficient evidence is available to justify moving into the full PF phase, details of which can be found in Solomon et al. (Citation2016, this issue).
While the top-down (effects-based) and bottom-up (stressor-based) approaches differ, the “gatekeeper” step can be adapted to either situations. In the latter case, based on observed effects in a given population, exposures might be investigated and the approach is mainly driven by the observed effect(s). In the former case, compounds are known (e.g., predefined by a specific regulation or monitoring campaign) and the approach is mainly driven by the measured or hypothesized exposures.
It is stressed again that, as a first step, each individual chemical should be evaluated to assess the risk associated with its estimated/measured exposure. If this is considered unacceptable, it is not advisable to proceed to any consideration for CRA before taking risk management measures for that chemical.
A CRA is deemed not necessary or prudent if the totality of information is such that it does not indicate co-exposure AND common toxicity (). Therefore, the “gatekeeper” step reinforces the need to assess and utilize all existing information, i.e., information in-hand, in determining whether a CRA is necessary or at least should be considered.
Given a sufficient rationale can be established via available evidence on likelihood of co-exposure AND common toxicity, a full PF should ensue (Solomon et al. Citation2016, this issue). As shown in , the process of CRA does not start if both exposure and common toxicity do not (likely) occur, i.e., a “yes” from both sides is required to trigger CRA.
It should be noted that this approach could also be applied to any regulated group of chemicals. Even when CRA is mandated by an existing regulation, the approach will indicate where and when the assessment can be ended because no concern is evident, which does not necessarily mean that the CRA needs to be conducted up to the highest tier. This may seem to contradict the RISK21 approach (‘exposure first’); however, in this approach, there is already an exposure consideration (i.e., dietary exposure and all other exposures for which there is reliable information), although not refined. In any case, once the initial toxicity information is available, the next step would be to seek information on exposure in order to decide if and how to proceed with the assessment.
Problem formulation
A CRA is ultimately only conducted after following a detailed PF stage which considers the goals of the risk management, the purpose of the assessment, the scope and depth of the analysis, the analytical approach and the resources available for the assessment (Solomon et al. Citation2016, this issue). PF is critical as it clearly identifies the question(s) being asked once a preliminary determination has been made that a CRA is necessary. In fact, the PF phase allows further refinement of, perhaps, the most fundamental question facing risk assessors and managers during the PF phase: “In which circumstances and under what conditions is a CRA necessary or even appropriate?”. In proceeding, the objectives and scope defined by the PF stage help to focus the risk assessment and potentially constrain the approach. These include a refinement of the combination of exposures to the stressors identified at the gatekeeper step and the use of higher tier information to exclude some of the initially identified stressors, e.g., from mechanistic information or more accurate assessment of exposure.
Evaluation and collection of toxicity and exposure data in a tier-wise approach using evidence/concordance tables
After the PF, a concordance table () can be populated with details and refined in a tiered manner. A tiered approach for CRA has been described by various organizations, such as WHO/IPCS (Meek et al. Citation2011). A tiered approach is also utilized in the RISK21 framework, such that results obtained from the lower tiers for both toxicity and exposure can inform the necessary resources required for the higher tiers and whether this should be for exposure, toxicity or both. However, the process can be started at any tier depending on the available information and the needs of risk management. It is also not necessary to move to a higher tier if a decision can be made using lower tier information (Embry et al. Citation2014).
Table 2. CRA evidence/concordance table (with hypothetical examples included for illustrative purposes).
Evidence demonstrating co-exposure is based on modeling or detection in environmental or biological samples and includes considerations of context and temporality. Toxicological data may suggest or confirm that dose-additivity applies, based on QSAR (or other) models, common target organ, common apical effect, common MOA or adverse outcome pathway (AOP) or interaction data.
Available evidence for both exposure and “common” toxicity in a CCAG will have different strengths and levels of uncertainty, depending on available data. It should be noted that both environmental and occupational exposure can and should be included in the assessment (Williams et al. Citation2012; Lentz et al. Citation2015). Therefore, the definition of a CCAG may be subject to refinement, based on increasing exposure and/or toxicological information. However, the nature and extent of refinement needed will depend on the outcome of assessments at lower tiers (Gallagher et al. Citation2015). In certain instances, refinement can be performed by collection of more information, more sophisticated data analysis, or modeling; in some cases, it will require additional studies. This RISK21 approach, as described for single chemicals in Embry et al. (Citation2014), is applicable also in the context of CRA.
There is currently lack of agreement on the criteria to determine when it is toxicologically appropriate to include a chemical in a group for CRA. However, the proposed framework is always applicable, regardless of the criteria used. In fact, it accommodates groups based on the lowest tier, where the only criterion for considering substances in the same CCAG is co-exposure, to subsequent refinements based on in silico, in vitro or in vivo methods, where evidence for a common target organ or apical effect serves as the basis for grouping, or, further, to grouping based on identification of common mode/mechanism of action or AOP. Similarly, information on hazard characterization can be as minimal as the TTC or as detailed as a dose–response relationship for key events of a MOA.
Building evidence tables () helps to identify those chemicals which form the CCAG. These tables also present the strength of evidence or the uncertainty associated with inclusion of a chemical in the CCAG. In addition, tables also should be presented for those stressors which are either included as ModFs (see below) or can be excluded from the assessment altogether, presenting the considerations and uncertainties that led to the decision.
Use of the RISK21 matrix to assess risk
CRA methodologies
There are several methodologies that have been proposed to carry out CRA as summarized in . Most methodologies deal simultaneously with toxicity and exposure. For instance, the HI, CRI, RfPI and the MOET provide a value (adimensional) that is derived for each component of the CCAG based on toxicological data and estimated/measured exposure. Usually these are deterministic point estimates, but they can also be expressed probabilistically. The TEF/RPF approach, instead, provides potency values for each component of the group that are used to “normalize” the dose in relation to the toxicity of the “index compound” (IC). Consequently, the exposure to each compound is expressed as equivalents of the IC and then these can be simply added up and the total IC-equivalent exposure compared to the toxicity of the IC, as in a single-chemical assessment.
Table 3. Summary of the characteristics of the commonly used methods to conduct CRA (for more details see e.g., EFSA Citation2008).
Plot information on the RISK21 matrix
Given the way the RISK21 matrix is designed, the application of safety/assessment/uncertainty factors to the Reference Point (RfP)/Point of Departure (PoD, e.g., NOAEL or BMD) is not recommended, although it can be done after adjusting the matrix. Rather the RfP or PoD (single deterministic value, range, or distribution) and exposure (single deterministic value, range or distribution) should be plotted on the matrix as in the “standard” RISK21 approach. Please note that the TTC values already incorporate a SF of 100; therefore, if plotting both TTCs and specific RfPs on the same matrix, the TTC values should be multiplied by 100 to enable direct comparison. There should be as many dots, clouds or boxes on the matrix as the number of compounds (see ). In , two lines define three areas: the area of potential concern for any individual compound (to the right of the solid line), the area of no concern or irrelevance for CRA (to the left of the dashed line) and the area in which compounds should be considered for CRA (between the solid and dashed lines). In this case, the cutoff for concern for individual compounds has been set at 1/100th of the RfP, in line with conventional risk analysis policy (this is analogous to comparing reference values). The cutoff for the need for inclusion in CRA has been set at 1/100th of the level of concern for the chemical alone, i.e., the chemical would add less than 1% to the combined effect. However, these cutoff points, i.e., the positions of the lines, are for illustrative purposes only, and in practice would be decided in dialog with risk managers and the decision makers, in a manner consistent with legislative mandates and/or regulatory policy as documented in the PF. This is consistent with the scheme proposed by Gallagher et al. (Citation2015), although they propose a specific cutoff for dismissing a chemical from CRA.
Figure 2. Application of the RISK21 matrix to a CCAG. In (A), individual compounds are plotted and two lines define three areas: the area of potential concern for any individual compound (to the right of the solid line), the area of no concern or irrelevance for grouping into a CCAG (to the left of the dashed line) and the area of no concern for the individual compound that should, however, be considered for CRA (between the solid and dashed lines). The cutoff points, i.e., the position of the lines have been set in this figure at a ratio toxicity/exposure of 100 for the solid line, 1:10,000 for the dashed line, but these can vary according to the specific situation and risk management decisions or policy. (B) The refinement obtained by excluding compounds falling into the left-hand area and by gathering additional, more accurate and refined data, either on exposure or toxicity, or both, for compounds that fall in the ``between lines'' area, and if appropriate in the ``potential concern'' area. The black star and diagonal dotted line represent the sum of the IC-equivalents relative to the IC (arrow) after normalization of the other components of the CCAG.
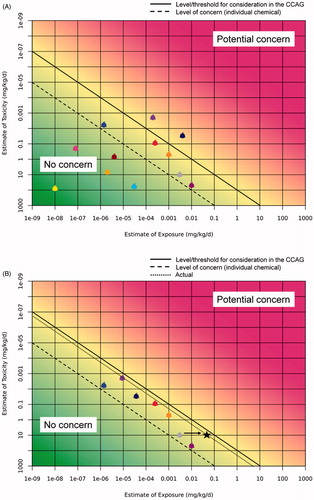
The next step depends on decisions made during PF. It may be that CRA would not be appropriate until the risk of those chemicals in the area “potential concern” has been addressed. This could be achieved by the refinement of the information, either exposure or toxicity, or both, for these compounds, as described in the conventional RISK21 approach (Embry et al. Citation2014). If concern still remains for some or all of these compounds, depending on the PF, it may still be considered necessary to proceed with CRA for those compounds that fall in the “between lines” area (including any that were individually of concern prior to refinement). represents the situation after such refinement, and the exclusion of compounds falling into the left-hand area (deep green). In respect of the latter, this is essentially a different way of presenting the maximum cumulative ratio (MCR) approach as proposed by Price and Han (Citation2011), and Price et al. (Citation2012); this is a tool that helps in the identification of the compound(s) that drive(s) the risk and those compounds that can be excluded from further consideration in CRA. The matrix also provides a graphical representation of a sensitivity analysis performed to identify the drivers of risk, which is frequently done for pesticide residue risk assessment (see e.g., EFSA Citation2009; USEPA Citation2002) and see below the worked example and ensuing considerations. For transparency and communication purposes the risk assessors might consider to present both in the final report.
If only nonstandard reference points are available, such as the LOAEL, consideration should be given as to how these should be used, if at all; if this are used, the procedure used to derive an adjusted value for use in the matrix should be clearly stated (e.g., application of a safety factor of 3 or 10) and the compounds involved clearly identified in the text and in the matrix.
In view of the above, the HI/aHI/CRI approach is not entirely appropriate for the matrix because SFs are applied to the RfPs before comparison with exposure. However, if this is known, the decision lines can be adjusted appropriately, as long as consistent metrics are used for all compounds in the matrix. Either individual RVs or RfPs can be represented in the matrix, but the HI/aHI/CRI and RfPI/MOET cannot be represented directly on the matrix, although this can be represented as a diagonal line. In contrast, the RPF approach allows the plotting of individual exposure values, before normalization, as points, ranges or distributions, as well as the representation of the IC-equivalent as shown in (arrow to star).
Within the RPF approach itself, the level of refinement can vary, including the distribution of RPFs (if based on BMD), the RfP of the IC, exposure of each individual compound, or the combined distribution of IC-normalized exposures.
The TEF approach is used for compounds sharing a MOA, which are generally present as mixtures, although of variable composition; the most well-known example being the dioxins and dioxin-like compounds. With the TEF approach, there will only be one point (deterministic), a box (range) or a “cloud” (probabilistic) in the matrix since exposure is expressed as the sum of IC-equivalents, obtained by multiplying the exposure of each congener.
Consideration of ModFs
Once the assessment has been performed for all components of the CCAG, if combined exposure is considered not to be of concern, ModFs should be considered as per goals defined during PF. As reported in Simon et al. (Citation2014), ModFs should be characterized with respect to their effects on biological processes and key events within a MOA, or on exposure. While this will not always be applicable because of lack of specific information, it is proposed that for each ModF identified as relevant for the CCAG, a table be prepared that summarizes its impact in terms of strength of evidence and direction of impact, i.e., higher/lower exposure, more/less severe toxic response, because of e.g., agonism/antagonism, different levels of endogenous active substances and, possibly, its (semi)quantitation. In the example presented in (not related to ) three ModFs for organophosphate insecticides (OP) are reported: (1) PON1 polymorphism (see Simon et al. Citation2014) which is known to modify metabolism of an OP in different ways depending on the compound; (2) A vegetarian diet that increases the intake of residues of OPs; (3) Exposure to carbamates that also affects acetylcholinesterase activity, with a rapid recovery, hence only simultaneous exposure is relevant.
Figure 3. Application of the RISK21 matrix to the effects of ModFs. In (A), the ModFs are applied to the IC-equivalent because there was no evidence that ModFs were compound-dependent. The arrows indicate that the shift can be in any direction. (B) can be used when the ModFs are different for each individual compound. In this example, the RPF should be reassessed and the IC-equivalents recalculated in order to obtain a single combined estimate. Note that the compound indicated with * can be excluded for further evaluation because its falls into the “no concern” or irrelevance for grouping into a CCAG area.
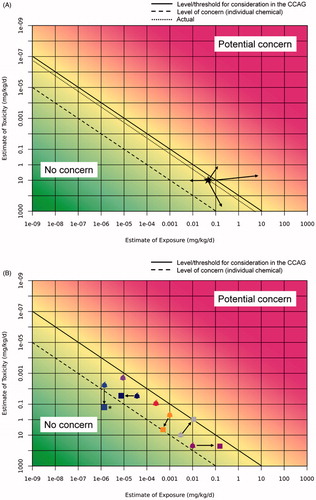
Table 4. Example of ModFs description in a reporting table for organophosphates (OPs). For ModF#1 a separate entry for each compound is needed because “direction & magnitude” differ, while for the others no differences are envisaged.
Unless there is evidence to the contrary (see ModF1 in ), ModFs can be applied directly to the CCAG as a whole, via the summed IC-equivalents () or ModFs can be considered for each individual compound within the CCAG (). ModFs may increase or decrease exposure, toxicity or both. It is conceivable that certain ModFs affect the outcome in a different direction and/or to a different extent for individual compounds as graphically shown in . If this is the case, ModFs should be applied to each compound individually. Quantitation of the effects of ModFs may indicate the possibility of excluding a compound from the CCAG as it no longer adds significantly to the total risk. On the other hand, it might be judged that the margin of exposure is so high that consideration of ModFs will not significantly change the safety judgment and therefore an explanation can be provided for not performing the actual calculation of the impact of such ModFs.
A case study: residues of the triazole fungicides
In an EFSA opinion (EFSA Citation2009), a CRA case study of triazoles present as food residues was carried out. Acute (cranio-facial malformations) and chronic (hepatotoxicity) effects were addressed and respective two separate CCAGs consequently formed, based upon each of the two endpoints. It should be noted that EFSA explicitly stated that this case study was not a formal risk assessment, that the choice of triazoles was based on the chemical structure, and that hepatotoxicity was broadly defined as any effect on the liver. Regarding the latter point, EFSA recognized that grouping refinement was possible but that was not the purpose of the exercise as carried out. On the exposure side, data from supervised field trials and residue monitoring studies were used. The former included the Highest Residue (HR) for acute exposure assessment, and the supervised trials median residue (STMR) for chronic exposure (). The approach used for assessing combined effect was that of the RPF and deterministic and probabilistic exposure assessments were carried out. We present here the application of the proposed framework to this case study, where the PF defined the hypothetical assumption that a registrant has requested approval for marketing of the triazole bitertanol. Therefore, CRA including all other hepatotoxic triazoles, as necessary, needs to be carried out.
Table 5. Hepatotoxic triazoles. The toxicity data are the respective NOAELs for hepatotoxicity. The exposure data are from monitoring data, except for bitertanol for which the highest supervised trial median residue (STMR) is plotted.
plots all the hepatotoxic triazoles using monitoring data (see EFSA Citation2009 for detailed rationale for this) except for bitertanol where the NOAEL and the highest STMR (in tomato) were used. According to the criteria described above, a refinement before carrying out CRA can be done by excluding the compounds falling to the left of the diagonal line (note the different position of the line in ). As shown in only two compounds remain for CRA consideration (bitertanol and difenoconazole). When these are expressed as IC-equivalents (black dot) there appears to be no concern. Including all of the compounds that were omitted increases the overall margin of exposure by only 33%.
Figure 4. Plots of hepatotoxic triazoles (based on data from ). (A) is a plot of all of the hepatotoxic triazoles. The line represents a margin of 1:10,000. (B) A refinement of the hepatotoxic triazole group for CRA, by exclusion of compounds falling in the “no-concern for CRA” area (i.e., to the left of the 1:100 line). The black dot represents the IC-equivalent (cyproconazole) exposure vs the IC NOAEL.
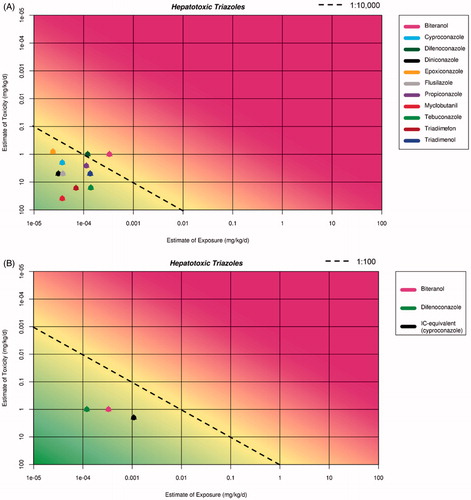
The next step would be the consideration of ModFs. In this case, in order to result in any concern, ModF(s) would need to increase the response and/or exposure by more than one order of magnitude, because of the distance of the summed IC-equivalents from the red line of concern. This situation is very unlikely to occur given the impact of most ModFs at the levels of exposure occurring as residues in food: in fact, residue levels are unlikely to be significantly modified by processing the food and difference in sensitivity to toxicity to these compounds well within one order of magnitude. Therefore, the assessment can be stopped here and there appears to be no need to refine the exposure assessment for bitertanol by carrying out monitoring of residues (except for enforcement purposes).
This short example shows that the framework can be applied in a regulatory setting and that the use of the matrix clearly visualizes the process and helps in taking the decision to the next step. It also shows that preliminary, less sophisticated evaluation could have given the same information in terms of the “decision” to be taken.
Conclusions
The proposed framework to address CRA is based on the concepts described in the previous RISK21 papers where the roadmap and matrix have been explained in detail (Embry et al. Citation2014; Pastoor et al. Citation2014). It also takes advantage of other technical papers from RISK21, where approaches to and refinement of exposure (Dellarco et al. Citation2016) and toxicological (Simon et al. Citation2014) assessments have been described. Given the complexity of CRA, it is of utmost importance to have an efficient gate-keeping step and a clear PF (Solomon et al. Citation2016, this issue) to avoid unnecessary resource-intensive evaluations. The proposed framework, with the tables and visualization matrix, enables a transparent, graphical representation of the process, of its iterations and of the outcome that will help both the conduct of the assessment and its communication to both specialists and to a wider, more general, audience. The importance of stakeholder involvement and understanding, particularly for CRA, has been recently stressed (Gallagher et al. Citation2015), based on EPA experiences, and the visualization tool proposed by RISK21 may be helpful.
The effects-based general approach to CRA (also called top-down approach) and the approach based on stressors (also called bottom-up approach) can be addressed in a unified manner by the RISK21 CRA. In fact, gate-keeping and PF use existing exposure and toxicological information to inform on the need for a full CRA in either a specific situation (e.g., observed effects), or in a less defined setting (e.g., known possible effects of a given group of compounds). Indeed, the framework is generally coherent with most regulatory requirements, and helps to reduce this complex issue to manageable dimensions. In certain regulatory domains, CRA is required to be carried out almost irrespective of a prior evaluation of exposure (EC Citation2009). However, the proposed approach will help in reducing to the minimum the amount of information and resources needed to reach a safety or risk decision for exposure to multiple chemicals, by integrating exposure information early in the process.
Consistent with the overall RISK21 objectives, no definitive methods for the definition of CCAGs have been proposed. Instead, the recommended approach is sufficiently flexible so as to accommodate future developments in this field. It should be stressed that the application of the framework enables the identification of the precision that is needed to take a decision. In a number of cases, such precision will be provided by very limited toxicological information and, hence, the use of broadly defined CCAGs will be sufficient for the purpose, with no need to devote resources for a more refined process of inclusion and exclusion of compounds from a CCAG.
The modifying effects of chemicals not directly belonging to a CCAG and of non-chemical agents or stressors are also taken into account in a quantitative way, to the extent possible. These additional factors should be accompanied by explanatory tables and a graphical representation. Therefore, even where only qualitative or semiquantitative assessments are possible, or undertaken, the instruments developed clearly communicate that these factors have been considered and integrated in the CRA.
In addition, the matrix and roadmap, as explained in the previous papers (Embry et al. Citation2014; Pastoor et al. Citation2014) can also be applied to better consider risk-risk tradeoffs, taking into account possible “beneficial” ModFs. The incorporation of specific conditions that will “reduce” the magnitude of the estimated risk may prevent unnecessary and possibly costly risk management decisions which could serve to divert attention and effort away from more significant problems impacting public health and/or the environment. The impact of possible risk management options, such as restrictions on use in certain scenarios or the suspension of approval of a specific compound can also be readily visualized in terms of the absolute and relative change in the summed IC-equivalents.
Declaration of interest
This publication was authored collectively by participants of the ILSI HESI Risk Assessment in the 21st Century (RISK21) Technical Committee’s Cumulative Risk Sub-Team, whose work is supported by HESI, a nonprofit institution whose mission is to collaboratively identify and help to resolve global health and environment challenges through the engagement of scientists from academia, government, industry, NGOs and other strategic partners. HESI receives funding and in-kind support from member companies and other non-industry organizations to support projects. The employment affiliation of the authors is shown on the cover page. These individuals had the sole responsibility for the writing and content of the paper, with additional input provided by other participants of the RISK21 Technical Committee’s Cumulative Risk Sub-Team (see acknowledgments). The individual authors worked as professionals in preparing the article and not as agents of their employers. Travel expenses were provided for academic and government committee participants to attend committee meetings, and they did not receive any other compensation. This work has been presented at numerous international meetings, workshops and symposia to scientists and regulators from academia, industry and government.
| Abbreviations | ||
| aHI | = | acute hazard index |
| ADME | = | absorption, distribution, metabolism, excretion |
| AOP | = | adverse outcome pathway |
| CCAG | = | common chemical assessment group |
| CCRA | = | chemical cumulative risk assessment |
| CRA | = | cumulative risk assessment |
| CRI | = | Cumulative Risk Index |
| EFSA | = | European Food Safety Authority |
| HI | = | hazard index |
| HESI | = | Health and Environmental Sciences Institute |
| IC | = | index compound |
| ILSI | = | International Life Sciences Institute |
| MCR | = | maximum cumulative ratio |
| MOA | = | mode of action |
| ModF | = | modulating factor |
| MOET | = | combined margin of exposure |
| IPCS | = | International Program on Chemical Safety |
| NAS | = | United States National Academy of Sciences |
| NRC | = | National Research Council |
| ModFs | = | modulating factors |
| PF | = | problem formulation |
| POD | = | point of departure |
| RfP | = | reference point |
| RfPI | = | Reference Point Index |
| RISK21 | = | Risk Assessment in the 21st Century |
| RPF | = | relative potency factor |
| TEF | = | toxicity equivalence factor |
| USEPA | = | United States Environmental Protection Agency |
| WHO | = | World Health Organization |
Acknowledgements
The authors gratefully acknowledge the government, academic and industry scientists of the HESI RISK21 Technical Committee for their contributions to this work. A full list can be accessed at www.risk21.org. In addition, the authors gratefully acknowledge the extensive comments offered by five reviewers selected by the Editor and anonymous to the authors. The comments were helpful in revising the paper.
Note
Notes
1 There are many definitions of sensitivity, susceptibility and vulnerability in the literature (among others, Kasperson et al. Citation1995; American Lung Association Citation2001; Kleeberger & Ohtuska 2005; Pope & Dockery, Citation2006; DeFur et al., Citation2007; Porta Citation2008; NRC 2009; Sacks et al. Citation2011). The following definitions proposed by NRC (2009) have been used in this paper:Sensitivity: The degree to which the outputs of a quantitative assessment are affected by changes in selected input parameters or assumptions.Susceptibility: The capacity to be affected. Variation in risk reflects susceptibility. A person can be at greater or lesser risk relative to the individual in the population who is at median risk because of such characteristics as age, sex, genetic attributes, socioeconomic status, prior exposure to harmful agents and stress.Vulnerability: The intrinsic predisposition of an exposed element (person, community, population or ecologic entity) to suffer harm from external stresses and perturbations; it is based on variations in susceptibility to disease, psychological and social factors, and adaptive measures to anticipate and reduce future harm, and to recover from an insult.
References
- American Lung Association. 2001. Urban air pollution and health inequities: a workshop report. Environ Health Perspect. 109:S357–S374.
- Boobis A, Budinsky R, Collie S, Crofton K, Embry M, Felter S, Hertzberg R, Kopp D, Mihlan G, Mumtaz M, et al. 2011. Critical analysis of literature on low-dose synergy for use in screening chemical mixtures for risk assessment. Crit Rev Toxicol. 41:369–383.
- deFur PL, Evans GW, Cohen Hubal EA, Kyle AD, Morello-Frosch RA, Williams DR. 2007. Vulnerability as a function of individual and group resources in CRA. Environ Health Perspect. 115:817–824.
- Dellarco M, Zaleski R, Gaborek BJ, Qian H, Bellin CA, Egeghy P, Heard N, Jolliet O, Lander DR, Sunger N, et al. 2016. Using exposure bands for rapid decision-making in the RISK21 tiered exposure assessment; in preparation.
- EC (European Commission). 2009. Regulation No. 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC; [Internet]. [cited 2016 Jul 20]. Available from: http://www.eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32009R1107.
- ECETOC. 2012. Effects of chemical co-exposures at doses relevant for human safety assessment. Technical Report no. 115.
- EFSA (European Food Safety Authority). 2009. Scientific opinion on risk assessment for a selected group of pesticides from the triazole group to test possible methodologies to assess cumulative effects from exposure through food from these pesticides on human health. EFSA J. 7:1167.
- EFSA (European Food Safety Authority). 2013. Scientific opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile. EFSA J. 11:3293; [Internet]. [cited 2016 Jul 20]. Available from: http://www.efsa.europa.eu/en/efsajournal/doc/3293.pdf.
- EFSA (European Food Safety Authority). 2008. Opinion of the scientific panel on plant protection products and their residues to evaluate the suitability of existing methodologies and, if appropriate, the identification of new approaches to assess cumulative and synergistic risks from pesticides to human health with a view to set MRLs for those pesticides in the frame of Regulation (EC) 396/2005. EFSA J. 704:1–84; [Internet]. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/705.htm.
- Embry MR, Bachman AN, Bell DR, Boobis AR, Cohen SM, Dellarco M, Dewhurst IC, Doerrer NG, Hines RN, Moretto A, et al. 2014. Risk assessment in the 21st century: roadmap and matrix. Crit Rev Toxicol. 44:6–16.
- Gallagher SS, Rice GE, Scarano LJ, Teuschler LK, Bollweg G, Martin L. 2015. Cumulative risk assessment lessons learned: a review of case studies and issue papers. Chemosphere. 120:697–705.
- Kasperson JX, Kasperson RE, Turner BL. 1995. Regions at risk: comparisons of threatened environments. Tokyo: United Nations University Press.
- Lentz TJ, Dotson GS, Williams PR, Maier A, Gadagbui B, Pandalai SP, Lamba A, Hearl F, Mumtaz M. 2015. Aggregate exposure and cumulative risk assessment – integrating occupational and non-occupational risk factors. J Occup Environ Hyg. 12 Suppl 1. S112–S126.
- Meek ME, Boobis AR, Crofton KM, Heinemeyer G, Raaij MV, Vickers C. 2011. Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework. Regul Toxicol Pharmacol. 60:S1–S14.
- NRC (National Research Council). 2009. Science and decisions: advancing risk assessment. Washington (DC): The National Academies Press.
- Pastoor TP, Bachman AN, Bell DR, Cohen SM, Dellarco M, Dewhurst IC, Doe JE, Doerrer NG, Embry MR, Hines RN, et al. 2014. A 21st century roadmap for human health risk assessment. Crit Rev Toxicol. 44:1–5.
- Pope CA III, Dockery DW. 2006. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 56:709–742.
- Porta M, editor. 2008. A dictionary of epidemiology. 5th ed. New York: Oxford University Press.
- Price PS, Dhein E, Hamer M, Han X, Heneweer M, Junghans M, Kunz P, Magyar C, Penning H, Rodriguez C. 2012. A decision tree for assessing effects from exposures to multiple substances. Environ Sci Eur. 24:26–37.
- Price PS, Han X. 2011. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int J Environ Res Public Health. 8:2212–2225.
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, Ross M. 2011. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 119:446–454.
- Salinas JJ, Shah M, Abdelbary B, Gay JL, Sexton K. 2012. Application of a novel method for assessing cumulative risk burden by county. Int J Environ Res Public Health. 9:1820–1835.
- SCCS, SCHER, SCENIHR. 2012. Joint opinion on the use of the threshold of toxicological concern (TTC) approach for human safety assessment of chemical substances with focus on cosmetics and consumer products, 8 June 2012; [Internet]. [cited 2014 May 6]. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_092.pdf.
- Sexton K. 2012. Cumulative risk assessment: an overview of methodological approaches for evaluating combined health effects from exposure to multiple environmental stressors. Int J Environ Res Public Health. 9:370–390.
- Sexton K. 2015. Cumulative health risk assessment: finding new ideas and escaping from the old ones. Hum Ecol Risk Assess. 21:934–951.
- Simon TW, Simons SS, Preston RJ, Boobis AR, Cohen SM, Doerrer NG, Fenner-Crisp PA, McMullin TS, McQueen CA, Rowlands JC. 2014. The use of mode of action information in risk assessment: quantitative key events/dose-response framework for modeling the dose-response for key events. Crit Rev Toxicol. 44:17–43.
- Solomon KR, Wilks M, Bachman A, Boobis AR, Moretto A, Pastoor TP, Phillips R, Embry MR. 2016. Problem formulation for risk assessment of combined exposures to chemicals and other stressors. Crit Rev Toxicol., this issue
- Su JG, Morello-Frosch R, Jesdale BM, Kyle AD, Shamasunder B, Jerrett M. 2009. An index for assessing demographic inequalities in cumulative environmental hazards with application to Los Angeles, California. Environ Sci Technol. 43:7626–7634.
- U.S. Environmental Protection Agency (EPA). 2012. The Community-Focused Exposure and Risk Screening Tool (EJSEAT); [Internet]. [cited 2012 Apr 17]. Available from: http://www.epa.gov/heasd/c-ferst/.
- USEPA. 2002. Guidance on cumulative risk assessment of pesticide chemicals that have a common mechanism of toxicity; [Internet]. [cited 2016 Jul 20]. Available from: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/guidance-cumulative-risk-assessment-pesticide.
- USEPA. 2007. Concepts, methods and data sources for cumulative health risk assessment of multiple chemicals, exposures and effects: a resource document. Cincinnati (OH): U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment. Report EPA/600/R-06/014F.
- Williams PRD, Dotson GS, Maier A. 2012. Cumulative risk assessment (CRA): transforming the way we assess health risks. Environ Sci Technol. 46:10868–10874.
- World Health Organization (WHO). 2010b. Urban HEART: user manual. Kobe, Japan: WHO Publications; [Internet]. [cited 2011 Aug 5]. Available from: http://www.who.or.jp/urbanheart/index.html.
- Zartarian VG, Schultz BD, Barzyk TM, Smuts M, Hammond DM, Medina-Vera M, Geller AM. 2011. The Environmental Protection Agency’s community-focused exposure and risk screening tool (C-FERST) and its potential use for environmental justice efforts. Am J Public Health. 101:S286–S294.
