Abstract
The recent classification of glyphosate as a probable human carcinogen by the International Agency for Research on Cancer (IARC) was arrived at without a detailed assessment of exposure. Glyphosate is widely used as an herbicide, which might result in exposures of the general public and applicators. Exposures were estimated from information in the open literature and unpublished reports provided by Monsanto Company. Based on the maximum measured concentration in air, an exposure dose of 1.04 × 10 − 6 mg/kg body mass (b.m.)/d was estimated. Assuming consumption of surface water without treatment, the 90th centile measured concentration would result in a consumed dose of 2.25 × 10 − 5 mg/kg b.m./d. Estimates by the Food and Agriculture Organization of the United Nations (FAO) of consumed doses in food provided a median exposure of 0.005 mg/kg b.m./d (range 0.002–0.013). Based on tolerance levels, the conservative estimate by the US Environmental Protection Agency (US EPA) for exposure of the general population via food and water was 0.088 mg/kg b.m./d (range 0.058–0.23). For applicators, 90th centiles for systemic exposures based on biomonitoring and dosimetry (normalized for penetration through the skin) were 0.0014 and 0.021 mg/kg b.m./d, respectively. All of these exposures are less than the reference dose and the acceptable daily intakes proposed by several regulatory agencies, thus supporting a conclusion that even for these highly exposed populations the exposures were within regulatory limits.
Introduction
The recent classification of glyphosate as a probable human carcinogen by the International Agency for Research on Cancer (IARC Citation2015) has generated considerable interest, particularly as the IARC classification was arrived at without a detailed assessment of risk to applicators and the general public. Glyphosate is widely used for control of weeds in agriculture, forestry, and in the management of public and private landscapes. These uses might result in exposures of the general public as well as applicators. Unfortunately, the IARC monograph merely focused on the potential hazards of glyphosate and not on the risks. Exposure is a critical component of risk assessment and, without measured values; it is difficult to provide guidance on the appropriate uses of glyphosate or, for that matter, any pesticide. It is also not possible to properly assess toxicity and hazard data for relevance to humans and the environment. As per their mandate, none of the IARC evaluations characterize exposures analytically or in the context of risk; the monograph on glyphosate (IARC Citation2015) summarizes several exposure studies from the open literature, but does not use these values to estimate risks. This is different from the approach used by most regulatory agencies such as the US EPA, the Food and Agricultural Agency (FAO) of the United Nations, and the European Food Safety Agency (EFSA) where exposures are compared to Reference Doses (RfDs) or Acceptable Daily Intake (ADIs).
There are several sources of exposure of humans to glyphosate in the environment. These are: air, water, application to crops and target weeds, and food. The following sections are an analysis of exposures of humans to glyphosate from these sources. Data for these exposures were obtained from papers published in the open literature and from unpublished reports provided by the Monsanto Company. These sources of information are listed in the references and summary data are provided in the Supplemental information (SI).
Methods
Unpublished reports of studies on exposure to glyphosate in applicators were provided by the Monsanto Company and covered uses in agriculture and forestry. Other data on exposures were obtained from the open literature as a result of searches in PubMed®, references in reviews, and Google Scholar®. These papers and reports were grouped into sources of exposures and the data analyzed as described below.
Air
Only one paper reported concentrations of glyphosate in air. In a study conducted in Iowa, Mississippi, and Indiana in 2007 and 2008, concentrations of glyphosate and its major environmental degradation, aminomethylphosphonic acid (AMPA) were measured in air and precipitation (Chang et al. Citation2011). Detections of AMPA were infrequent and the concentrations were small. These are not discussed further. The frequency of detection of glyphosate ranged from 60 to 100% in air and rainwater. Concentrations in air ranged from <0.01 to 9.1 ng/m3, while those in rain were from <0.1 to 2.5 μg/L. Unless rainwater was collected as drinking water, this would be an incomplete pathway for exposure of humans. Once in contact with soil, exposures would be via surface waters (see below). Concentrations in air were seasonal and the sources were likely associated with application to crops in the growing season. For estimation of human exposure, it was assumed that there was total absorption of glyphosate from the air into the body of a 70 kg human breathing 8 m3 air (half a day for an adult, US EPA Citation2009). These values were then used to calculate the systemic dose, based on a worst-case assumption of 100% uptake via the respiratory tract.
Water
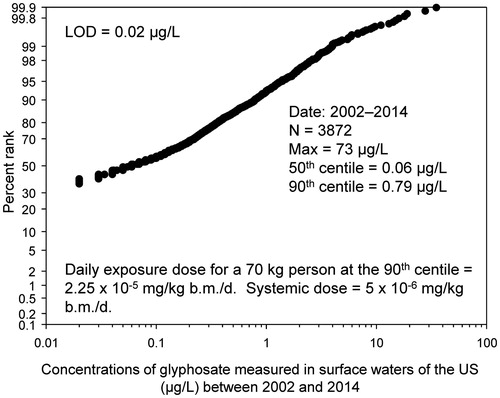
Glyphosate can enter surface waters through use on aquatic weeds, runoff from sprayed soils, and from drift of spray. Glyphosate is very soluble in water and, although it binds strongly to soils and sediments, small concentrations have been measured on surface waters in the United States. These measurements are part of the US Geological Survey (USGS) National Water-Quality Assessment (NAWQA) program (USGS Citation2015), which has been in place since the 1980s. Glyphosate was added to the large range of analytes measured in surface water in 2002. These data were downloaded from the NAWQA data warehouse (USGS Citation2015) and then sorted by concentration. All values measured across the US between 2002 and 2014 were pooled for the analysis. Where concentrations were less than the level of detection (0.02 μg glyphosate acid equivalents (a.e.)/L), these values were substituted with a dummy value of “zero”. The values were ranked from the smallest to the largest and a cumulative frequency distribution was derived. These values were processed using the Weibull formula to estimate ranks and plotted on a log-probability scale (Solomon and Takacs Citation2002). The 90th centile values were calculated from the raw data using the Excel function < =percentile>. Systemic dose was estimated from the assumption of consumption of 2 L of water per day by a 70 kg human with 20% absorption from the gastrointestinal (GI) tract (EFSA Citation2015). Although chlorine and ozone are highly effective for removing glyphosate and AMPA during purification of drinking water (Jönsson et al. Citation2013), it was assumed that treatment did not remove any glyphosate. The estimated concentrations are thus a worst-case.
Food and bystanders
Several studies have measured concentration of glyphosate in “bystanders” and people not involved in application of glyphosate. Bystanders are presumable exposed via food, water, and air (see above). It is also assumed that bystanders are exposed on a daily basis through the environment and/or food and drinking water, and that these exposures are constant and not episodic as in an applicator. Here, a single daily sample of urine is a reasonable surrogate for daily exposures, although uncertainty would be reduced with more frequent samples and analysis of total daily urinary output. Several of these studies were critically reviewed in 2015 (Niemann et al. Citation2015). This review was thorough, but the strengths of the methods of the original studies were variable. In addition, the authors did not correct for incomplete excretion of glyphosate (95%) as has been done for the applicator studies. In a study of farm and non-farm households in Iowa (Curwin et al. Citation2007), urine samples were analyzed from 95 adults and 117 children. A study in Europe (Mesnage et al. Citation2012) measured exposures in a farm family (two adults and three children). A report on the analysis of urine of 182 people from 18 countries (Hoppe Citation2013) provided data on concentrations in urine. In another study, urine concentrations of 40 male and female German students were measured (Markard Citation2014). The original study was in German and the value used here for the systemic dose is from the review of Niemann et al. (Citation2015). A study using enzyme linked immunosorbent assay (ELISA) analysis with an unstated level of quantitation (LOQ) was used to measure the concentrations of glyphosate in samples of urine from more than 300 individuals in the EU (most from Germany) (Krüger et al. Citation2014). A report of a study in the US on 35 individuals using an ELISA analysis (Honeycutt and Rowlands Citation2014) provided data from which a systemic dose of glyphosate was estimated.
Where the systemic dose was calculated, it was used. Where dietary exposures were provided, the urinary concentration was used to calculate the systemic dose on the assumption of 2 L of urine per day and a 60 kg person (Niemann et al. Citation2015).
Under the auspices of the Food and Agricultural Organization of the United Nations, the Joint Meeting on Pesticide Residues (JMPR) conducts routine assessments of residues of pesticides in food (JMPR Citation2014). These are evaluated in relation to diets in various regions of the world and exposure via food compared to an ADI. In 2013, the JMPR reviewed dietary exposures to glyphosate, its major metabolites, and breakdown products (N-acetyl glyphosate, AMPA, and N-acetyl AMPA) and calculated the international estimated daily intakes (IEDI) of glyphosate for 13 regional food diets (JMPR Citation2014). These IEDIs were based on estimated mean residues from supervised trails under normal or good agricultural practice. These values were for a 60 kg person but were used without modification.
The US Environmental Protection Agency (US EPA) has calculated exposures to glyphosate using the Dietary Exposure Evaluation Model (DEEM, ver 7.81), which is based on tolerance levels for all commodities and modeled estimates of exposures from food and drinking water for the overall US population (US EPA Citation2012).
There is some uncertainty in all of these studies and approaches. All of the monitoring studies used relatively few participants (<300), which increases uncertainty and lack of raw data in most studies does not allow variance to be fully characterized. Modeling approaches (US EPA and JMPR) based on maximum residue limits and assumptions of good agricultural practices are also subject to uncertainty; however, the assumptions used are more likely to result in overestimation. However, proportion of foods consumed is based on the statistical analyses of diets and this does incorporate, but not quantify, uncertainty.
Applicators
A relatively large number of studies on exposures of applicators to glyphosate have been conducted (see SI for a full list). Older studies tended to use passive dosimetry, either as whole-body dosimeters or patches. Some of the studies with dosimeters used tracers (dyes or other surrogates) and others analyzed dosimeters for glyphosate itself. Some more recent studies used biological monitoring and some a mixture of biological monitoring and dosimeter-patches. For compounds, such as glyphosate, where the excretion kinetics is well understood, biological monitoring provides a measure of the actual amount of the chemical in the body. For this reason, data from these studies are most appropriate for risk assessment. However, data from dosimetry studies can be used to estimate systemic dose. This allows comparison of exposures from different studies to a benchmark for exposure i.e. the reference dose (RfD) or ADI.
For studies using dosimetry, the normalization to systemic dose was conducted using the procedure outlined in . This was done for the dosimetry studies listed in SI Table 1. The estimated systemic doses were ranked from smallest to largest and a cumulative frequency distribution was derived. These values were plotted on a log-probability scale as above. The 90th centile values were calculated from the raw data using the Excel® function < =percentile>.
Table 1. Procedure for normalization of dosimetry data to estimate systemic dose.
Where an applicator makes a single application, the systemic dose of glyphosate can be estimated from the total amount of glyphosate excreted in the urine over the four or five days following and including the day of application (Acquavella et al. Citation2004). Glyphosate is rapidly excreted and does not bioaccumulate. If applications are conducted every day, the amount excreted each day provides a time-weighted average for daily exposures. Because glyphosate is applied infrequently in normal agricultural practice, the assumption of a single initial exposure is appropriate for risk assessment.
The procedure of normalization for biomonitoring studies is complicated by the fact that many studies reported concentrations of glyphosate that are less than the LOQ, even on the day of application (d-0), when exposures would be expected to be greatest. Similarly, even if residues were detected on d-0, those on subsequent days might have values less than the LOQ. The common practice of using half the level of detection as a default value might be acceptable for the first observation day, but this fails to account for excretion that would reduce the amount in the body on each successive day. Use of half the LOQ on each day would grossly overestimate the systemic dose. Because of this, normalization of systemic doses was modeled using excretion kinetics and followed the steps outlined in .
Table 2. Procedure for normalization of biomonitoring data to estimate systemic dose of glyphosate.
If concentrations in urine are > LOQ for one or more days, the actual elimination rate for the individual can be used to correct for days where concentration is < LOQ. Unless already carried out in the study itself, these corrections were applied to the data in SI Table 2.
Because raw data were available for the studies on applicators, uncertainty could be considered. Total number of participants was large (249, See SI Table 2) and range of the values provided the upper and lower bounds of uncertainty. To be conservative, the 90th centiles of the data were used to characterize reasonable worst-case exposures.
Normalization of the RfD and ADI for systemic dose
Regulatory agencies set allowable limits for consumption of residues of glyphosate exposure based on toxicity studies. The US EPA RfD is 1.75 mg/kg body mass (b.m.)/day (US EPA Citation2012). The ADI for JMPR/WHO is 1 mg/kg b.m./d (JMPR Citation2014), while the ADI used by EFSA is 0.5 mg/kg b.m./d (EFSA Citation2015). In a recent review (summary published on 16 May 2016), JMPR (Citation2016) has reaffirmed their ADI of 1 mg/kg b.m./d. These values are suitable for comparison to the dietary intake, but for comparison to systemic doses as estimated from biological monitoring (urinary excretion), the ADIs and RfD were divided by five to account for only 20% absorption from the GI tract (EFSA Citation2015). These normalized values are 0.35, 0.2, and 0.1 mg/kg b.m./d, for US EPA, JMPR, and EFSA, respectively.
Results
Air
Based on the above assumptions of respiratory volume and total absorption, inhaling glyphosate in air at the maximum measured concentration would result in an exposure dose of 1.04 × 10 − 6 mg/kg b.m./d. This is about five orders of magnitude less than the systemic ADI proposed by EFSA (Citation2015).
Water
The cumulative frequency distribution of concentrations of glyphosate measured in surface waters of the US are shown in . The 90th centile was 0.79 μg/L. The maximum concentration measured was 73 μg/L. Consumption of 2 L of drinking water by a 70 kg person at the 90th centile concentration is estimated to result in a consumed dose of 2.25 × 10 − 5 mg/kg b.m./d, more than four orders of magnitude less than the EFSA ADI.
Food and bystanders
Estimates of the systemic dose resulting from exposures of bystanders and the general public to glyphosate are shown in . All of these systemic doses are more than 150-times less than the EFSA ADI, normalized for reduced uptake from the gut.
Table 3. Summary of exposures to glyphosate in bystanders and the general public.
Based on the estimates of daily intake from the FAO/JMPR, the minimum IEDI was 124 μg/person/d, the median was 301, and maximum was 762 (JMPR Citation2014). These values were normalized to a 60 kg person (0.002, 0.005, and 0.013 mg/kg b.m./d, respectively) for comparison to the ADI. Median exposures are 100-times less than the ADI suggested by EFSA.
The dietary exposure of the general population in the US was estimated by US EPA to be 0.088 mg/kg b.m./d and the range of values was from 0.058 to 0.23 mg/kg b.m./d across a range of age-groups from adults to toddlers. These values are all less than the ADI suggested by EFSA.
Applicators
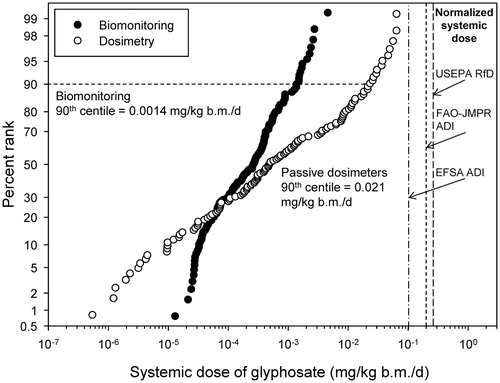
For the applicator studies, the corrections were applied as in or and the results are presented graphically in . Raw data are provided in SI Tables 1 and 2.
The range of values for systemic doses measured in the dosimeter studies (90th centile =0.021 mg/kg b.m./d) was greater than in the biomonitoring studies (90th centile =0.0014 mg/kg b.m./d). Given the corrections applied to the data, this is surprising; however, there are a number of assumptions used in the normalization of the systemic doses that might result in overestimation of exposure. These are likely in the amount of absorption though skin and the penetration of clothing. The assumption of 1% penetration through the skin is greater than the value of 0.7% suggested from observations in an in vitro model with human skin (Bo Nielsen et al. Citation2009). The 90th centile in the dosimetry studies was 0.021 mg/kg b.m./d; about five-times less than the systemic EFSA ADI.
The range of values for the systemic doses determined by biomonitoring was smaller than for the passive dosimeters and more accurately reflects the true exposures. The 90th centile was 0.0014 mg/kg b.m./d; about 70-times less than the systemic EFSA ADI.
Conclusions
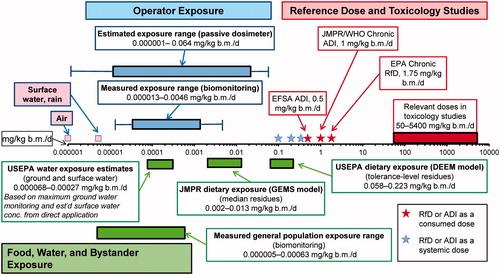
Even when using a number of reasonable worst-case assumptions, systemic doses of glyphosate in human applicators, bystanders, and the general public are small. Exposures to glyphosate in the general public are less than EFSA’s ADI. The same conclusion applies to applicators. As an overall summary, exposures and ADIs are compared graphically in . It should be noted that the ADIs and RFDs used in this assessment are derived from the most sensitive response in long-term feeding studies in the most sensitive laboratory test species and that an uncertainty factor is applied to these values. Furthermore, the biomonitoring exposures measured in applicators aggregate all sources of exposures (air, food, water, and dermal contact) and are still less than the most conservative ADI. Based on the current RfDs and ADIs, there is no hazard and no intolerable risk from exposure to glyphosate via its normal use in agriculture and management of weeds in landscapes.
Supplemental material
Supplemental material for this article is available online here.
Solomon_Supplemental_Material.docx
Download MS Word (102.9 KB)Acknowledgments
The author gratefully acknowledges the extensive comments offered by five reviewers selected by the Editor and presented anonymously to the author. These comments were useful in revising the paper. I thank Monsanto Inc. for providing access to reports from exposure studies for glyphosate in applicators. I wish to thank the authors of the other papers in this series for their constructive suggestions and comments.
Declaration of interest
The employment affiliation of the author is shown on the cover page. However, it should be recognized that the author participated in the review process and preparation of this paper as an independent professional and not as a representative of his employer. Keith R. Solomon previously served as an independent consultant for the Monsanto Company on the European Glyphosate Task Force. KS has not been involved in any litigation procedures involving Monsanto Company and glyphosate. KS’s recruitment and evaluation of the data was organized and conducted by Intertek Scientific & Regulatory Consultancy (Intertek). KS acted as a consultant for Intertek. Intertek (previously Cantox) is a consultancy firm that provides scientific and regulatory advice, as well as safety and efficacy evaluations for the chemical, food and pharmaceutical industries.
While Intertek Scientific & Regulatory Consultancy has not previously worked on glyphosate related matters for the Monsanto Company, previous employees of Cantox had worked in this capacity. Funding for this evaluation was provided by the Monsanto Company which is a primary producer of glyphosate and products containing this active ingredient. Neither any Monsanto company employees nor any attorney reviewed any of the Expert Panel’s manuscripts prior to submission to the journal.
This article is part of a supplement, sponsored and supported by Intertek Scientific & Regulatory Consultancy. Funding for the sponsorship of this supplement was provided to Intertek by the Monsanto Company, which is a primary producer of glyphosate and products containing this active ingredient.
References
- Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M. 2004. Glyphosate biomonitoring for farmers and their families: results from the farm family exposure study. Environ Health Perspect. 112:321–326.
- Bo Nielsen J, Ahm Sorensen J, Nielsen F. 2009. The usual suspects-influence of physicochemical properties on lag time, skin deposition, percutaneous penetration of nine model compounds. J Toxicol Environ Health A. 72:315–323.
- Chang FC, Simcik MF, Capel PD. 2011. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ Toxicol Chem. 30:548–555.
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MC. 2007. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann Occup Hyg. 51:53–65.
- EFSA. 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate (EFSA-Q-2014-00546, EFSA-Q-2015-00279, approved on 30 October 2015 by European Food Safety Authority). EFSA J. 13:4302; p. 107. doi:10.2903/j.efsa.2015.4302. Available from: http://www.efsa.europa.eu/en/efsajournal/pub/4302.
- Honeycutt Z, Rowlands H. 2014. Glyphosate testing report: findings in American mothers’ breast milk, urine and water. Moms Across America & Sustainable Pulse; p. 19. Available from: https://d3n8a8pro7vhmx.cloudfront.net/yesmaam/pages/774/attachments/original/1396803706/Glyphosate__Final__in_the_breast_milk_of_American_women_Draft6_.pdf?1396803706.
- Hoppe H-W. 2013. Determination of glyphosate residue in human urine samples from 18 European countries. Bremen (Germany): medical laboratory Bremen. (Report Glyphosate MLHB-2013-06-06); p. 18. Available from: https://www.bund.net/fileadmin/bundnet/pdfs/gentechnik/130612_gentechnik_bund_glyphosat_urin_analyse.pdf.
- IARC. 2015. Glyphosate. In: Some organophosphate insecticides and herbicides: diazinon, glyphosate, malathion, parathion, tetrachlorvinphos. IARC working group, March 3-10, 2015. Lyon (France): World Health Organization (WHO), International Agency for Research on Cancer (IARC). (IARC monographs on the evaluation of carcinogen risks to humans, vol 112); p. 1–92. Available from: http://monographs.iarc.fr/ENG/Monographs/vol112/index.php.
- JMPR. 2014. 5.21. Glyphosate (158) and metabolites. In: Pesticide residues in food 2013. Joint FAO/WHO meeting on pesticide residues and the WHO core assessment group on pesticide residues, Geneva, 17 to 26 September 2013. Rome: Food and Agriculture Organization of the United Nations/Geneva, World Health Organization (WHO). (FAO Plant Production and Protection Paper No. 219); p. 225–228, 484–486. Available from: http://www.fao.org/publications/card/en/c/299ca869-ae51-5093-8407-9cb30782b9f5/.
- JMPR. 2016. Summary report for diazinon, glyphosate, malathion. Geneva, Switzerland: Food and agriculture organization of the United Nations/Geneva, World Health Organization (WHO). Joint FAO/WHO meeting on pesticide residues (JMPR); p. 6. Available from: http://www.who.int/foodsafety/jmprsummary2016.pdf?ua =1.
- Jönsson J, Camm R, Hall T. 2013. Removal and degradation of glyphosate in water treatment: a review. Aqua. 62:395–408.
- Krüger M, Schledorn P, Shrödl W, Wolfgang Hoppe H, Lutz W, Shehata AA. 2014. Detection of glyphosate residues in animals and humans. J Environ Anal Toxicol. 4:210. doi: 10.4172/2161-0525.1000210.
- Markard C. 2014. Ergebnisse der Vorstudie HBM von Glyphosat. Dessau-Roßlau (Germany): Federal Environmental Agency (UBA), Umweltprobenbank des Bundes. [Unpublished Report provided to] Berlin (Germany): German Federal Institute for Risk Assessment (BfR).
- Mesnage R, Moesch C, Grand R, Lauthier G, Vendômois J, Gress S, Séralini G. 2012. Glyphosate exposure in a farmer’s family. J Environ Protect. 3:1001–1003.
- Niemann L, Sieke C, Pfeil R, Solecki R. 2015. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J Verbr Lebensm. 10:3–12.
- Solomon KR, Takacs P. 2002. Probabilistic risk assessment using species sensitivity distributions. In: Posthuma L, Suter GW, Traas T, editors. Species sensitivity distributions in ecotoxicology. Boca Raton (FL): CRC Press; p. 285–313.
- US EPA. 2009. Exposure factors handbook: review draft. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Research and Development, National Center for Environmental Assessment. (No. EPA/600/R-09/052A); p. 1265. Available from: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid =209866.
- US EPA. 2012. Glyphosate. section 3 registration concerning the application of glyphosate to carrots, sweet potato, teff, oilseeds (crop group (CG) 20) and to update the CG definitions for bulb vegetable (CG 3-07), fruiting vegetable (CG 8-10), citrus fruit (CG 10-10), porne fruit (CG 11-10), berry (CG 13-07), human health risk assessment. Washington (DC): U.S. Environmental Protection Agency (US EPA), Office of Chemical Safety and Pollution Prevention. (No. Decision No.: 459870); p. 28.
- USGS. 2015. NAWQA Database. Reston (VA): United States Geological Survey (USGS); [cited 2015 Sep 2]. Available from: http://cida.usgs.gov/nawqa_public/apex/f?p=136:1:0.
- Wester RC, Melendres J, Sarason R, McMaster J, Maibach HI. 1991. Glyphosate skin binding, absorption, residual tissue distribution, and skin decontamination. Fundam Appl Toxicol. 16:725–732.