 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Methyl salicylate is the predominant constituent of oil of wintergreen and is used as a pesticide, a denaturant, an external analgesic, a fragrance ingredient, and a flavoring agent in products such as chewing gum, baked goods, syrups, candy, beverages, ice cream, and tobacco products; and it occurs naturally in some vegetables and berries. Methyl salicylate is of interest to the tobacco industry as oil of wintergreen is used as a flavorant in tobacco products. The purpose of this investigation was to conduct a critical review of the available literature for oral exposure to methyl salicylate, incorporating an analysis of the quality of the studies available and the current understanding of the mode of action. Following a review of all of the available literature, the most appropriate data sets for dose-response modeling were reported by Gulati et al. in which significant changes in reproductive/development endpoints were reported to occur after exposure to 500 mg/kg/d of methyl salicylate in male and female mice. Benchmark dose modeling was performed and the most sensitive endpoint, the number of litters per mating pair, was associated with a BMDL of 220 mg/kg/d. This BMDL was chosen as the point of departure and adjusted by a body weight scaling factor to derive a human equivalent dose. Based on the uncertainty factor analysis, the POD for methyl salicylate was adjusted by a UF of 3 for interspecies uncertainty to derive an allowable daily intake of 11 mg/kg/d.
Introduction
Methyl salicylate is the predominant constituent of oil of wintergreen (JECFA Citation2002) and is used as a pesticide, a denaturant, external analgesic, flavoring agent, and fragrance ingredient in a variety of products including chewing gum, baked goods, syrups, candy, beverages, and ice cream (CIR Citation2003; NFSA Citation2012). It also occurs naturally in some vegetables and berries. Methyl salicylate is of interest to the tobacco industry as oil of wintergreen is a flavorant in multiple tobacco products, including smokeless tobacco and cigarettes (Chen et al. Citation2010; Lisko et al. Citation2014). The goal of this investigation was to conduct a toxicity assessment for the oral consumption of methyl salicylate, considering both non-carcinogenic and carcinogenic endpoints.
Several regulatory agencies have established recommended toxicity levels for oral exposure to methyl salicylate. The United States Environmental Protection Agency’s (USEPA’s) Biopesticide and Pollution Prevention Division (USEPA Citation2005a), Norwegian Food Safety Authority (NFSA Citation2012), European Food Safety Authority (EFSA Citation2012), and the Joint FAO/WHO Expert Committee on Food Additives (JECFA Citation2002) all recommend an acceptable daily intake for methyl salicylate of 0.5 mg/kg/d.
While regulatory agencies have established recommended levels of methyl salicylate that appear to be safe, none of these assessments considered the quality of the available toxicity studies. If the available toxicity studies for methyl salicylate were not conducted according to certain study quality standards, then this could introduce a level of uncertainty into the toxicity assessment. In addition, the current recommended acceptable daily intakes (ADIs) do not appear to incorporate consideration of the mode of action of methyl salicylate following oral exposure. The purpose of this investigation was to conduct a toxicity assessment for oral exposure to methyl salicylate, incorporating an assessment of the quality of the studies available for methyl salicylate and the current understanding of the mode of action for methyl salicylate.
Methods
This investigation was composed of two main steps: hazard assessment and dose–response assessment. The hazard assessment was initiated with a review of the current regulatory agency information, both in the United States and internationally, to identify studies that have served as the basis for any current regulatory toxicity values for methyl salicylate. In addition, a comprehensive search of the peer-reviewed scientific literature was conducted to identify epidemiological and toxicological studies in which the potential for toxicity following oral exposure to methyl salicylate had been investigated. The hazard assessment included an evaluation of the study quality using a published methodology for assigning study quality criteria, to identify studies considered to be of the highest quality that could be relied upon for the identification of endpoints, both cancer and noncancer, reported following oral exposure to methyl salicylate. Second, a dose–response assessment was conducted on the most appropriate data set, which included identification of points of departure (PODs) including no observed adverse effect levels (NOAELs)/lowest observed adverse effect levels (LOAELs) and benchmark doses or concentrations estimated using Benchmark Dose (BMD) modeling, evaluation of the mode of action of methyl salicylate, determination of human relevance for each endpoint identified, an uncertainty analysis, and the derivation of a toxicity value for the oral consumption of methyl salicylate.
Hazard identification
A hazard assessment includes the identification and review of data pertinent to two questions (1) whether an agent may pose a hazard to humans and (2) under what circumstances an identified hazard may be expressed (USEPA Citation2005b). The hazard assessment step should include a well-defined literature search strategy, as well as methods for critically reviewing the literature identified. The National Research Council (NRC) (Citation2014) has recommended a systematic approach for hazard assessment that incorporates enhanced transparency and incorporates methods for assessing the quality of the studies identified. As part of the hazard assessment for methyl salicylate, an evaluation of the quality of the studies identified for consideration was also incorporated to ensure that the highest quality data currently available were incorporated into the dose–response assessment for methyl salicylate.
Identification of literature
Prior to conducting a search of the peer reviewed literature, a review of websites for authoritative bodies, both within the United States and internationally, was conducted to identify current recommended toxicity values for methyl salicylate and any published or nonpublished work that may have been relied upon in making decisions regarding potential effects following oral exposure to methyl salicylate.
Based on an initial review of the documents prepared by authoritative bodies, a literature search strategy was developed that included key words associated with common chemical names and synonyms for methyl salicylate identified using the database ChemSpider (Citation2015). These keywords and synonyms were used in combination with the CAS registry number for methyl salicylate and keywords relating to methyl salicylate exposure and relevant toxicity endpoints as well as its mode of action (). While this assessment focused on oral exposure studies for methyl salicylate, the literature search strategy was also developed to include studies that evaluated the potential for health effects following the inhalation route of exposure. These studies were also considered as they may provide additional information that would potentially address data gaps in the literature. Literature searching was conducted using the US National Library of Medicine’s Toxicology Data Network (TOXNET), Medline, and PubMed databases, and searching was limited to the literature published in English. The literature search strings included every possible combination between the categories listed in (Compound/Products, Exposure, and Endpoints).
Table 1. Literature search strategy keywords.
An initial screen of the titles and abstracts was performed using a defined set of inclusion and exclusion criteria that assist in the identification of those studies that could be considered for both hazard identification and dose–response modeling (). The focus was on studies that provided information on long-term exposure as these types of studies are largely relied upon for the development of acceptable levels of exposure to chemicals of interest (IPCS Citation1987; USEPA Citation1993, Citation1994, Citation2005b). Based on these criteria, the only in vivo studies included for further review were those in which the exposure was to methyl salicylate only, exposure was repeated dose, the route of exposure to methyl salicylate was reported to be oral or inhalation, and the study was conducted in humans or a relevant mammalian species (e.g. rats, mice, dogs, rabbits, guinea pigs, monkeys). The only in vitro studies that were included were those in which the exposure was to methyl salicylate only and the study was conducted in cell lines (bacterial, animal, or human) that have been relied upon by authoritative bodies to assess the potential for hazard.
Table 2. Inclusion and exclusion criteria.
Evaluation of study quality
Toxicity studies that were identified in the literature search and determined to be relevant based on inclusion and exclusion criteria were reviewed and assessed for study quality using criteria reported by Klimisch et al. (Citation1997). The Klimisch et al. (Citation1997) framework provides criteria for scoring studies on a scale of 1–4, with studies scored as a 1 being of the highest quality and studies scored as a 4 of the lowest quality. A detailed description of the criteria applied in the Klimisch et al. (Citation1997) study quality framework is provided in . The studies receiving the highest score of 1 would be those performed according to and complying with standard study guidelines or methods recommended by regulatory agencies such as the USEPA, the Organization for Economic Co-Operation and Development (OECD), or the European Union (EU) and preferably studies performed according to Good Laboratory Practices (GLP). GLP standards were developed to ensure transparency and consistency when conducting toxicity studies; however, the absence of GLP or the fact that a study was conducted prior to the implementation of GLP does not imply that the study was inadequately conducted. For this investigation, the absence of GLP was not used as a reason for excluding any data for consideration. Studies receiving a score of 2 were those that were not reported to have been conducted according to a published testing guideline and did not report GLP compliance. These studies would also be considered high quality because they would meet the requirements for a well-conducted toxicity study (e.g. appropriate number of animals per dose group; appropriate documentation of test chemical, dose, exposure methods). Studies of lower quality were given a score of 3 or 4. A score of 3 was given to studies for which the methods were not clearly defined or specified in the study, only one dose was administered and dose response could not be determined, or small numbers of animals per group were tested. A score of 4 was given to studies which do not provide sufficient experiment details (e.g. abstracts) or were secondary literature.
Table 3. Criteria from Klimisch et al. (Citation1997).
The higher quality in vivo and in vitro toxicological studies (i.e. those receiving quality scores of 1 or 2) were considered to provide the highest quality data. These should be given the most consideration when making conclusions regarding the potential for hazard. In addition, if the highest quality toxicological studies provide quantitative data for use in dose–response modeling, then these would be relied upon for the dose–response assessment. Data from toxicological studies with lower quality (scores of 3 or 4) were used as supporting information in the hazard assessment, but were not considered in the dose–response assessment. Studies that were identified that provided only mechanistic or mode of action data were used as supporting evidence only; therefore, no quality ratings were assigned to these studies.
Dose–response assessment
Utilizing the data identified in the hazard assessment, a dose–response assessment was performed to derive an acceptable level of methyl salicylate that an individual can be exposed to on a daily basis for a lifetime that would not be expected to result in any appreciable cancer or non-cancer health risk (USEPA Citation1993). Following a review of all the literature identified, a dose–response assessment was conducted utilizing the data from the most appropriate study to identify the most sensitive endpoint that could be relied upon to develop a point of departure (POD). An acceptable level or ADI was then estimated through the application of safety factors (for non-cancer effects) to the POD. For cancer effects, in the absence of an understanding of the mode of action, linear extrapolation from the POD to the dose or concentration associated with a one in a million risk (for cancer effects) is conducted. This approach assumes that the mode of action for the development of the cancer endpoint is a linear process.
Identification of the point of departure and dose–response modeling
The studies with the highest study quality scores were reviewed to determine if there were appropriate quantitative data sets that could be used to estimate a POD. The POD is defined as the estimated dose near the lower end of the observed range, without extrapolation to lower doses (USEPA Citation2005b). This POD, adjusted for potential uncertainty, could then be used to estimate an acceptable level of oral exposure, such as an oral Reference Dose (RfD) or an ADI. While a POD can be based on a LOAEL or a NOAEL, a POD can also be based on a benchmark dose (BMD) (USEPA Citation2002, Citation2005b, Citation2012). While a NOAEL, LOAEL, or BMD could all serve as the POD, the estimation of a BMD provides a more robust, informative POD, compared with using a NOAEL or LOAEL. BMDs, and their associated statistical confidence intervals (i.e. 95% lower limit on the BMD estimate, or BMDL), account for the shape of the dose-response curve over the continuum of observed exposures, rather than at a single dose level (USEPA Citation2002, Citation2012). They also inform the POD with respect to within-group variability of response and reward study designs that utilize a sufficiently large number of subjects and doses, and exposure ranges inclusive of the no response level and the maximally tolerated dose.
BMD modeling was performed on all data sets with adverse effects reported to be biologically significant and statistically significantly increased at any dose when compared to controls. All BMD modeling was performed using BMD Software (BMDS) version 2.6.01 from the USEPA (Citation2016). As part of the BMDS software (USEPA Citation2016), the BMDS models used were based on the type of results being considered (i.e. continuous, quantal, or nested). Quantal data are presented as a fraction or percent of a population with a condition or effect at a given dose or exposure level and BMDS (2012, 2016) uses probability density models including the Gamma, Logistic, Multistage, Probit, Weibull, and Multistage for quantal data. For continuous data, presented as means and standard deviations or standard errors, BMDS software (USEPA Citation2012, Citation2016) uses continuous models including the polynomial, power, linear, exponential and Hill models. Models used by USEPA (Citation2012, Citation2016) for use in modeling nested data (i.e. the response is either true or false, or outcomes are measured in the offspring of pregnant animals) include the NLogistic, NCTR, and Rai & Van Ryzin models. Based on recommendations from USEPA (Citation2012), model results were evaluated for goodness of fit and the BMD and BMDL of those models with the lowest Akaike information criterion (AIC) value that had adequate Chi-square p values (greater than .1) were considered as PODs. The Chi-square Goodness of Fit Test is “a statistical hypothesis test used to compare observed counts with predicted numbers of independent observations classified into two or more categories” (USEPA Citation2012). The AIC is defined as “a measure of information loss from a dose–response model that can be used to compare a specified set of models”. Among a set of models, the model with the lowest AIC is considered the best” (USEPA Citation2012). Finally, the lowest BMDL with the most appropriate model fit was chosen as the POD for the estimation of an acceptable daily intake of methyl salicylate (USEPA Citation2012).
Mode of action and human relevance assessment
The studies identified in the literature search providing mechanistic or mode of action information for methyl salicylate were considered separately in a mode of action assessment. While mode of action data are important in understanding the potential for hazard, a lack of evidence from human studies or the lack of human studies also elevates the importance of mode of action data for understanding the potential for human health effects when the toxicity observed is based on studies conducted in animals (i.e. are the effects reported in animal studies relevant to human health). Once the potential POD was identified for the development of the ADI, the mode of action data relevant to the endpoint associated with the POD were reviewed and considered in order to establish (1) whether the effects reported in animal models (in the absence of human data) are potentially relevant to humans and (2) what uncertainty factors should be applied to the POD.
Adjustments for uncertainty
Traditionally, authoritative bodies have applied factors to adjust the POD that are intended to account for several types of uncertainty. For this assessment, an uncertainty factor analysis was performed in accordance with the methods currently recommended by USEPA (Citation2002). This approach is applied by dividing the POD by factors ranging from 1 to 10 intended to adjust for various types of uncertainty. This includes consideration of five uncertainty factors: interspecies, intraspecies, chronic versus subchronic, LOAEL versus NOAEL, and incomplete database uncertainty factors. Each factor is given a value of 1, 3, or 10 depending on the quality of the information available for each uncertainty category and scientific judgment.
Interspecies (UFA) – uncertainty in extrapolating from laboratory animal data to humans.
Intraspecies (UFH) – variation in sensitivity among the members of the human population.
Chronic versus subchronic study duration (UFS) – uncertainty in extrapolating from data obtained in a study that is of less-than-lifetime exposure (subchronic) to a toxicity value that is defined as a lifetime exposure (chronic).
LOAEL versus NOAEL (UFL) – uncertainty in using a LOAEL rather than a NOAEL.
Incomplete database (UFD) – inability of any single study to adequately address all possible adverse outcomes in humans (e.g. incomplete database).
The uncertainty value chosen for each type of uncertainty was based on the quality of the available studies and scientific judgment (USEPA Citation2002). A factor of 10 is typically used to adjust for each type of uncertainty identified; however, if, based on the available data and scientific judgment, a lower uncertainty value can be justified, values of 3 or even 1 have been applied. In the case of interspecies (UFA) and intraspecies (UFH) uncertainty factors, each factor includes two components to account for pharmacokinetic differences (UF = 3) and pharmacodynamic differences (UF =3), for a total default UF value of 10 for each (Kroes et al. Citation1993; Beck & Clewell Citation2001; USEPA Citation2002; Dorne Citation2004). A reduction in the default UF of 10 should only be used if sufficient data are available to support the conclusion that the data set on which the POD is based is representative of the dose–response data for the most susceptible human subpopulation. In the case of the UFA, the USEPA (Citation2011a) has recently recommended using an allometric body weight scaling to the 3/4 power approach (BW3/4) to adjusted the POD to a human equivalent dose (HED). For this conversion the POD is multiplied by a dosimetric adjustment factor (DAF) calculated as follows:
(1)
(1)
where BWA is the body weight of the animal species tested and BWH is the human body weight. The ¼ exponent accounts for the application of BW3/4 scaling to exposure in units of mg/kg-d (BW3/4/BW1/1 = BW 1/4). Using this approach, a reduced UFA (default of 3) is applied to the HED. The reduction of the UFA from a default value of 10 to 3 with the use of BW3/4 scaling is based on the qualitative recognition that current scientific knowledge indicates that BW3/4 scaling addresses the potential for species differences in both kinetic and dynamic processes, which the UFA is intended to address (USEPA Citation2011a).
Derivation of an acceptable daily intake
Based on the POD, an acceptable daily intake was derived. For this investigation, the ADI was considered to be synonymous with the USEPA’s oral RfD and is defined as an estimate of the daily oral exposure to the human population that includes adjustments for uncertainty in the data, which is likely to be without an appreciable risk of adverse effects over the course of a lifetime (USEPA Citation2002). If the POD was based on a non-cancer endpoint or a cancer endpoint associated with a nonlinear mode of action, the ADI was derived from the POD divided by the appropriate uncertainty factors:
(2)
(2)
Results
Literature search
The literature search was conducted in two steps. First, a review of websites for authoritative bodies, both within the United States and internationally was conducted. Second, using the literature search strategy presented in , a search of published literature was conducted. The results of each step are detailed in the following sections.
Documents from authoritative bodies
Documents providing recommendations regarding ADIs for methyl salicylate were identified for four authoritative bodies: NFSA (Citation2012), EFSA (Citation2012), USEPA (Citation2005a), and JECFA (Citation1968, Citation2002). A review of these documents was conducted to identify studies that had been relied upon in making decisions regarding the potential toxicity of methyl salicylate. Although USEPA has not established a reference dose (RfD) for methyl salicylate, in 2005 the Biopesticide and Pollution Prevention Division of the Office of Pesticide Programs (OPP) (USEPA Citation2005a) developed a RfD for methyl salicylate as part of the re-registration eligibility document for methyl salicylate of 0.5 mg/kg/d based on the NOEL reported in a 2-year oral study in dogs and a 17-week feeding study in rats of 50 mg/kg/d (Webb & Hansen Citation1963). Based on the results of this study (discussed in more detail in section Chronic toxicity studies), USEPA (Citation2005a) identified a NOEL for both rats and dogs of 50 mg/kg/d. The documentation indicates that a UF of 10 was applied for interspecies extrapolation, resulting in an RfD of 0.5 mg/kg/d. It appears another UF of 10 must have been applied to result in the recommended RfD and it is likely that this factor was an adjustment for intraspecies uncertainty. However, USEPA (Citation2005a) does not document an adequate adjustment factor to support an RfD of 0.5 mg/kg/d.
JECFA (Citation2002) has also established an ADI for methyl salicylate of 0.5 mg/kg-body weight. According to JECFA (Citation2002), the ADI of 0.5 mg/kg-bodyweight is based on a no observed effect level (NOEL) of 50 mg/kg/d reported in a 2-year dietary study in dogs; however, the citation for the study that serves as the basis for the NOEL is not provided. JECFA concluded that the estimated intake for consumers of 0.1 mg/kg body weight is below the ADI of 0.5 mg/kg body weight and the safety of methyl salicylate as a flavoring agent would not raise concern at the currently estimated level of use. NFSA (Citation2012) relies upon the ADI established by JECFA (Citation2002) and, according to NFSA (Citation2012), the results of Webb and Hansen are relied upon in making recommendations regarding acceptable levels of methyl salicylate in food.
EFSA (Citation2012) also relies upon the results of the study conducted by Webb and Hansen (Citation1963), in which a NOAEL of 50 mg/kg/d was reported based on the results of a 2-year rat dietary study. The EFSA recommendations are for allowable concentrations of methyl salicylate in animal feed and not human exposure. The recommendations were species-specific and ranged from 8 mg methyl salicylate/kg body weight for chickens to 30 mg methyl salicylate/kg body weight for dogs.
Methyl salicylate also has United States Food and Drug Administration (USFDA)-specified uses as an indirect food additive (CFR Citation2015). No other documents developed by authoritative bodies providing recommendations on acceptable levels of methyl salicylate following oral exposure were identified.
Literature search results
The goal of the literature search was to identify all the peer-reviewed, published scientific literature presenting data in animals or humans related to repeated dose toxicity associated with oral exposure to methyl salicylate. In addition, search terms were included to identify data related to the mode of action of methyl salicylate, as these data would be relevant for drawing conclusions about the shape of the dose-response curve and the potential relevance to human health of endpoints identified in animals. While it was originally thought that searches of the peer reviewed literature could be limited to dates associated with the most recent authoritative reviews to the present, because the available regulatory documents contained only limited data that could be used for quantitative risk assessment, the literature search was not limited to any date range. The literature search conducted based on the key words presented in resulted in the identification of 18 toxicity studies for methyl salicylate and 116 mode of action studies. Also, as a result of the review of authoritative review articles, 15 additional toxicity studies and one additional mode of action study were identified. These studies were not identified in the original search because the citations were from an unpublished study or a book chapter. The titles and abstracts (where available) identified from the literature search and a thorough review of documents from authoritative bodies were screened for inclusion based on the criteria presented in . Following inclusion and exclusion screening of both the literature identified through the literature search and the unpublished studies identified in regulatory documents, a total of 26 toxicity studies and 25 mode of action studies were identified for critical review and consideration in the hazard and dose–response assessments which are presented in . Of these, three unpublished studies by Abbot and Harrisson (Citation1978), USFDA (Citation1966) and LaWall and Harrisson Research Laboratories Inc. (Citation1964) were not able to be obtained; however, summaries of the studies in CIR (Citation2003) were reviewed. Two of the 26 citations were available as abstracts only (Packman et al. Citation1961; Harrisson et al. Citation1963) and were obtained for review.
Table 4. Literature identified from authoritative reviews and literature searching.
Study quality assessment
Study quality of the relevant studies identified from the literature searching was assessed using the approaches recommended by Klimisch et al. (Citation1997) (). Of the 26 toxicity studies noted in , once obtained and reviewed in more detail, six were found to be studies other than toxicity studies and were not included in the study quality assessment (Warknay & Takacas Citation1959; Sharpe et al. Citation1975; Overman & White Citation1983; Ungvary et al. Citation1983; Hutt et al. Citation1986; Infurna et al. Citation1990). A list of 20 studies that were assessed for study quality is presented in , along with the corresponding study quality score and the rationale for the score. Based on the application of study quality scores, no toxicity study identified for methyl salicylate received a study quality score of 1. Two studies (Gulati et al. Citation1984; Morrissey et al. Citation1989) received a score of 2 instead of 1 because the studies were not performed according to standard study guidelines recommended by regulatory agencies (e.g. USEPA or OECD). The study reported by Webb and Hansen (Citation1963) also received a study quality score of 2 because the study was conducted prior to the establishment of Good Laboratory Practices (GLP) and the study was not performed according to standard study guidelines recommended by regulatory agencies (e.g. USEPA or OECD). Gulati et al. (Citation1984), Morrissey et al. (Citation1989) and Webb and Hansen (Citation1963) were retained for further critical review for consideration in the dose-response assessment.
Table 5. Results of the study quality analysis.
One study, Collins et al. (Citation1971), received a score of 3 and was not considered further for quantitative analysis because the study was conducted prior to the establishment of GLP, the study was not performed according to standard study guidelines recommended by regulatory agencies, and the purity of the test substance was not reported. The remaining sixteen studies received a score of 4 and were not considered further for quantitative analysis (). These were mainly studies that were cited in authoritative reviews and were not publicly available. The information provided in the authoritative review was limited. It also included citations for which only abstracts for the studies were available, or the studies were review articles and did not provide any original data.
The following sections provide a summary of the studies that were considered for the dose–response assessment based on study quality scores of 1 or 2 and the inclusion of quantitative data that could be used for dose–response modeling. No studies received a study quality score of 1; however, two studies received study quality scores of 2. These include one chronic toxicity study conducted in rats (Webb & Hansen Citation1963) and one reproductive/developmental study conducted in two separate laboratories (Gulati et al. Citation1984; NTP Citation1984; Morrissey et al. Citation1989). When reviewing these studies, only those effects considered to be treatment related and biologically significant; and reported to be statistically significantly different from the control were considered as potential effects associated with methyl salicylate exposure and these were the only effects considered for evaluation in the dose–response assessment.
Chronic toxicity studies.
Webb and Hansen (Citation1963) conducted a chronic dietary study in which Osborne–Mendel rats were administered diets for 2 years containing 0, 0.1, 0.5, 1.0, or 2.0% methyl salicylate (). Each study group consisted of 25 male and 25 female rats, with the exception of the 2.0% group, which included 24 males and 26 females. Rats were fed ad libitum and weighed weekly. Hematologic examinations were performed at 3, 11, 17, and 22 months. Weights of the heart, liver, kidneys, spleen, and testes were recorded at study termination. Gross pathological and histopathological examinations of the thyroid, parathyroid, lung, heart, stomach, pancreas, spleen, liver, kidney, adrenal gland, lymph node, small intestine, bone marrow smear, leg bone, leg muscle, urinary bladder, testis and prostate (males), and ovary and uterus (females) were performed following necropsy. In animals administered the highest concentration, 50% died by week eight of the experiment and all were dead by week 49. A statistically significant decrease in body weight was reported for rats administered diets containing 1% or 2% methyl salicylate (through week 49 when all animals in the high concentration group were reported to have died). Absolute organ weights were not statistically significantly different from control animals; however, significant changes in organ weights relative to body weight were reported. Statistically significant increases in testis weight relative to body weight were reported in male rats administered diets containing 1.0% methyl salicylate. In addition, statistically significant increases in heart and kidney weight relative to body weight were reported in females administered diets containing 1.0% methyl salicylate, compared with controls. The authors reported that the incidence of an induced lesion in the leg bone of rats administered diets containing 2.0% methyl salicylate was the most prominent histopathological change. However, a histopathological examination of the leg bone was only conducted in a subset of animals (n = 10–11) and the results were reported as an increase of cancellous bone in the metaphysis of bones examined per dose group with no indication of how many bones per rat were examined and no information reported for the control group for this effect. Therefore, the data reported by the study authors appear to support the presence of a dose-related increase in the incidence of cancellous bone and the authors state that the increased incidence in the high dose group was very apparent compared with the controls; however, the data reported in the study were insufficient to determine if the incidence of these lesions was statistically significantly increased compared to control animals. The histopathological changes reported in the bone consisted of an increased amount of cancellous bone (i.e. trabecular bone or spongy bone) in the metaphysis. A subset of the bones of rats in the 0.5 and 1.0% groups were also examined and the same lesions in the bone were reported at a lesser degree of severity in two of 11 bones in the 1.0% dose group and one of 11 bones in the 0.5% dose group. In addition to histopathological changes noted in the bone, the authors reported similar types and numbers of tumors at all dose levels except the 2.0% dose group. These included mammary tumors and benign and malignant pituitary tumors; however, the authors did not report incidence data nor did they conclude that any of the tumors were treatment related. The authors also noted that pituitary tumors were reported in 22 females (occurrence by dose level was not specified) and only one male rat (dose level not specified).
Table 6. Summary or studies considered for evaluation in the QRA
Webb and Hansen (Citation1963) also performed a chronic study in beagle dogs (two/sex/dose) in which the animals were orally administered capsules containing 0, 50, 150, or 350 mg/kg/d of methyl salicylate 6 d a week for 2 years. The dogs were weighed weekly and hematological examinations were performed at 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years after the start of the experiment. One female dog in the 350 mg/kg/d group died of infectious canine hepatitis after 33 d of exposure and her replacement died of canine distemper after 19 weeks of exposure. At the time, the replacement was added to the high-dose group, a sibling was also added to the control group increasing the total number of dogs to 18, with three females and two males in the control and 350 mg/kg/d dose groups and two females and two males in the 50 and 150 mg/kg/d dose groups. No significant hematologic effects were noted at any of the examination time points. Necropsies were performed on all dogs dying or killed during the experiment or upon termination, and all major organs were weighed. In the surviving dogs in the high-dose group, microscopic examinations were conducted on the brain, pituitary, salivary gland, thyroid, parathyroid, thymus, lymph nodes, lung, heart, stomach, spleen, pancreas, kidney, adrenal, liver, gallbladder, small intestine, large intestine, urinary bladder, rib, skeletal muscle, testis and prostate or ovary, and uterus. Body weights of the dogs administered 150 or 350 mg/kg/d were reported to be decreased (statistical significance not specified) at the termination of the experiment; and enlarged livers were reported in dogs administered 150 or 350 mg/kg/d (statistical significance not specified). Although changes in body weight are reported, it is unclear if these changes were over the duration of the study or observed at terminal sacrifice. For all 18 dogs, including the two that died earlier, bone marrow smears, fat content of the liver and kidney, and adrenal glands were examined and no significant changes were reported. Necropsies were conducted on dogs that died prior to the end of the experiment and on dogs that were killed at termination. Results of histopathological examinations were only reported in the three dogs (two male and one surviving female) in the high-dose group. Microscopic examinations in these three dogs included examination of the brain, pituitary, salivary, spleen, pancreas, kidney, adrenal, liver, gallbladder, small intestine, large intestine, urinary bladder, rib, skeletal muscle, and testis and prostate or ovary and uterus. No significant histopathological effects were reported.
Reproductive/developmental toxicity studies.
Morrissey et al. (Citation1989) reported the methods and results from the Reproductive Assessment by Continuous Breeding (RACB) study, which includes 48 continuous breeding studies performed for various chemicals including methyl salicylate (). A review of Morrissey et al. (Citation1989) suggested that the information reported specifically for methyl salicylate was from studies conducted in two separate laboratories: Environmental Health Research Testing (Lab A) and Research Triangle Institute (Lab B). The methods and results from Lab A have been published in a detailed technical report (Gulati et al. Citation1984), while a summary of the methods and results for Lab B are provided in Morrissey et al. (Citation1989) and in an abstract reported by NTP (Citation1984) (). The study protocol for each lab consisted of four tasks: Task 1 – dose range finding study, Task 2 – continuous breeding phase, Task 3 – crossover mating phase, and Task 4 – second-generation phase.
In the study conducted at Lab A (Gulati et al. Citation1984), groups of male and female mice were administered methyl salicylate by gavage at doses of 0, 100, 250, or 500 mg/kg/d, while animals in Lab B (Morrissey et al. Citation1989; NTP Citation1984) were administered doses of 0, 25, 50, or 100 mg/kg/d by gavage. In Task 1, eight mice per sex per dose group were dosed for 14 days, and clinical signs, mortality, body weight gain, and food and water consumption were reported. In task 2 (continuous breeding phase), dose groups included 20 mice per sex per dose group with 40 mice per sex in the control group. During task 2, male and female mice were housed separately and dosed for 7 d before mating. The mice were then randomly paired for mating and housed together while receiving daily exposures for 98 d. Body weight, proportions of males, number or litters per pair, and number of live and dead pups were recorded within 12 h of birth in the first generation (F1) newborn pups, then the litters were discarded.
In task 3, the crossover mating phase, F0, mice continued to this phase only if any significant adverse effects on fertility were observed in the F1 pups. In order to determine if the effects seen were sex dependent, F0 mice in the high-dose group were mated with control mice in different combinations. Control males were mated with control females, control males were mated with high-dose females, and high-dose males were mated with control females. Litters were observed for litter size and sex, pup weight was measured and F0 mice were necropsied. Endpoints measured in F0 necropsied female mice included selected organ weights (brain, kidneys/adrenals, liver, ovary/oviducts, pituitary, reproductive tract, and uterus) and histology. In F0 males, selected organ weights (brain, kidneys/adrenals, and liver), histology, percentage motile sperm, epididymal sperm concentration and percentage of abnormal sperm were measured.
In task 4, the second-generation phase, mice were exposed to methyl salicylate for a 7 d premating period and then were randomly grouped into mating pairs and cohabitated for 98 d with continuous treatment. The F1 mice born within the next 21 d were exposed throughout lactation until weaning, and body weight, proportion of males, number of litters per pair, number of live, and dead pups were assessed. F1 mice were continually dosed after weaning and then mated, at about 74 d, from the same treatment group (20 mice/group/sex). Litter size, sex, and pup weight were recorded for second-generation (F2) mice.
The results from the dose-setting study (task 1) were not reported. For task 2, no statistically significant changes in body weights or fertility indices were reported following exposure to any dose of methyl salicylate (Gulati et al. Citation1984). A statistically significant decrease in the mean number of litters, the average number of pups per litter, the proportion of pups born alive, and mean live pup weights were reported to occur after exposure to 500 mg/kg/d of methyl salicylate (Gulati et al. Citation1984) (). A statistically significant decrease in live pup weights was reported to occur in females and in the adjusted live pup weight (adjusted for total number of live and dead pups per litter) in females and combined total pups (male and females) after exposure to 250 or 500 mg/kg/d of methyl salicylate (Gulati et al. Citation1984). For Lab B, NTP (Citation1984) and Morrissey et al. (Citation1989) reported no significant effects on body weight or reproductive indices in the F1 or F2 generation at any of the doses tested.
Table 7. Reproductive performance of fertile pairs during continuous breeding (task 2) methyl salicylate (Gulati et al. Citation1984).
Summary of the available data.
In summary, based on the highest quality studies identified, a chronic 2-year toxicity study conducted in rats and dogs (Webb & Hansen Citation1963) and a RACB study conducted in mice (Gulati et al. Citation1984; Morrissey et al. Citation1989) was available to assess the potential hazard from chronic oral exposure to methyl salicylate. While only minimal effects (changes in body weight) were noted in rats and dogs following two years of oral (capsule) exposure to methyl salicylate (Webb & Hansen Citation1963); statistically significant reproductive/developmental effects were reported by Morrissey et al. (Citation1989) and Gulati et al. (Citation1984) in a RACB study in mice, in which oral exposure occurred during gestational development, considered a sensitive window of exposure. These effects included significant decreases in the mean number of litters, the average number of pups per litter, the proportion of pups born alive and mean live pup weights reported to occur after oral exposure to methyl salicylate (Gulati et al. Citation1984; Morrissey et al. Citation1989).
While the studies or abstracts that received quality scores of 3 and 4 were not considered for the quantitative assessment, they were reviewed and considered qualitatively. The only study receiving a study quality score of 3 was a reproductive toxicity study conducted in rats fed methyl salicylate in the diet at concentrations of 0, 500, 1500, 3000, or 5000 ppm (Collins et al. Citation1971). The study received a quality score of three because it was performed prior to GLP, no testing guidelines were followed, and the purity of the test substance was not specified in the study report. However, the results reported by Collins et al. (Citation1971) in the second generation rats are comparable the results reported in Morrissey et al. (Citation1989) and Gulati et al. (Citation1984). Collins et al. (Citation1971) reported no treatment related effects in the parental group. A significant decrease in the fertility index was reported in the 5000 ppm groups in the second and third generations. Significant decreases in mean litter size, mean number of live born pups per mated female, and mean number of offspring that survived to day 4 per mated female were reduced at 3000 ppm and 5000 ppm in the second generation only. However, these same effects were not noted in the first and third generations at any concentration tested.
Fifteen studies received study quality scores of 4. Of the 15, 10 were considered review articles and did not report original study data, and were, therefore, not considered either qualitatively or quantitatively. One of the 15 receiving a score of 4 was only available in an abstract form (Packman et al. Citation1961). Packman et al. (Citation1961) exposed groups of rats to methyl salicylate in the diet at concentrations of 0, 700, or 2100 ppm for 2 years. The authors reported no treatment-related effects on growth, survival, or food consumption, no changes in blood or urine parameters and no increase in treatment related lesions following pathological evaluation.
Four of the 15 studies receiving a quality score of 4 (Harrisson et al. Citation1963; LaWall & Harrisson Research Laboratories, Inc. 1964; USFDA Citation1966; Abbot and Harrisson Citation1978) were unpublished studies that were not publically available; however, summaries were presented in the CIR (Citation2003) and NFSA (Citation2012). Abbot and Harrisson (Citation1978) and LaWall and Harrisson Research Laboratories, Inc. (Citation1964) exposed rats to various concentrations of methyl salicylate in the diet for durations ranging from 10 to 30 weeks. Abbot and Harrisson (Citation1978) reported increased bone density at 1.13% and 2% methyl salicylate and bone lesions at 2% methyl salicylate in the diet for 10 to 12 weeks, while LaWall and Harrisson Research Laboratories, Inc. (Citation1964) reported increased bone density in rats feds diets containing 11 250 and 20 000 ppm methyl salicylate for 30 weeks. A short summary of Harrisson et al. (Citation1963) can be found in the NFSA (Citation2012) and indicates increased bone density was reported in rats fed diets containing methyl salicylate for 10–12 weeks.
LaWall and Harrisson Research Laboratories, Inc. (Citation1964) and USFDA (Citation1966) also performed studies in dogs administered oral capsules of methyl salicylate at doses ranging from 50 to 800 mg/kg/d for 6–7.5 months. Survival was decreased in dogs dosed at 500 and 800 mg/kg/d and some increases in liver and kidney weight were noted at 150 mg/kg/d and higher; however, the pathologist reported no lesions or deleterious alterations at any dose tested. While the summaries of these unpublished studies presented in CIR (Citation2003) indicate that the results support those reported by Webb and Hansen (Citation1963) of increased incidence of cancellous bone in rats with an absence of the same results reported in dogs, the information reported in the CIR (Citation2003) was not adequate to perform a quantitative assessment of these results.
Dose–response assessment
Identification of study and endpoints for dose–response modeling
The ADI reported by JECFA (Citation1968, Citation2002) of 0.5 mg/kg/d is based on a NOEL of 50 mg/kg/d reported in a 2-year study in dogs. While JECFA (Citation2002) does not cite the study reference that is the basis of the ADI, it is assumed that the data that serve as the basis for the ADI are that reported in the only 2-year dog study identified in the literature and performed by Webb and Hansen (Citation1963). Results reported by Webb and Hansen (Citation1963) for both rats and dogs indicated that the only adverse effect was the increased incidence of cancellous bone reported in a subset of rats fed 2% methyl salicylate in the diet with no statistically significant adverse effects reported in dogs. An increased incidence of cancellous bone in the metaphysis was reported in a subset of rats (n = 10 or 11) and the incidence appeared to be dose related with an incidence rate of 1/11 bones, 2/11 bones, and 9/9 bones in the low-, mid-, and high-dose groups. However, no information concerning the incidence of cancellous bone in the control group was given in the study. While the authors state that the increased incidence in the high-dose group was very apparent compared with the controls, the data reported in the study were insufficient to determine if the incidence of these lesions was statistically significantly increased compared with control animals. In addition, it is unclear why all animals from a treatment group were not examined and whether or not each bone examined was from separate animals. In the absence of the incidence for the control group, it is not possible to conduct dose–response modeling on the incidence of cancellous bone. Therefore, this data set from Webb and Hansen (Citation1963) was not considered for the development of a POD. The biological impact of the increased amount of cancellous bone (also referred to as spongy bone or trabecular bone) in the metaphysis of rats is also unclear. No data were identified to clarify the biological significance of this bone lesion and determine the historical incidence of cancellous bone in rats.
The results from the RACB reproductive/developmental study for methyl salicylate (Gulati et al. Citation1984; Morrissey et al. Citation1989) were reviewed to determine if the data could be relied upon to quantify the potential toxicity of methyl salicylate (). The authors reported no statistically significant changes in body weights or reproductive indices in the F1 or F2 generation mice exposed to doses of 25, 50, or 100 mg/kg/d, compared with controls (NTP Citation1984). Gulati et al. (Citation1984) reported a statistically significant decrease in the number of litters per pair, the number of live pups per litter, and the proportion of pups born alive following administration of doses of 500 mg/kg/d (). A statistically significant decrease in live pup weight and adjusted live pup weight (adjusted for the total number of live and dead pups per litter by analysis of variance) was also reported following administration of doses of 250 or 500 mg/kg/d (). Following a review of the studies identified that had the appropriate criteria, data, and study quality scores (Webb & Hansen Citation1963; Gulati et al. Citation1984; Morrissey et al. Citation1989), it was determined that the reproductive/developmental endpoints reported by Gulati et al. (Citation1984) were the only results for which sufficient data were available to be used for dose-response modeling. Webb and Hansen (Citation1963) reported no significant effects in rats following chronic exposure; and the effects reported in dogs following oral exposure were not sufficiently reported for use in dose-response modeling.
Dose–response modeling and identification of point of departure (POD)
Based on the effects reported in Gulati et al. (Citation1984) and NTP (Citation1984), a POD using the NOAEL of 100 mg/kg/d could be assumed based on statistically significant decreases in the number of litters, number of pups per litter, proportion of pups born alive, or mean pup weight reported in animals administered 250 mg/kg/d. Following review of the data reported by Gulati et al. (Citation1984), it was determined that the endpoints for which the data necessary for BMD modeling were available included average number of litters per pair (), live pup weight adjusted for the average litter size (), and the proportion of pups born alive per fertile pair ( and ). Because many of the endpoints from developmental studies are reported with litter as the statistical unit, data from these types of studies are often best modeled using nested models (USEPA Citation2012). Nested models account for any intra-litter correlation, or the tendency of littermates to respond more similar to one another relative to the other litters in a dose group. If this correlation (which may vary with dose) is not estimated, variance estimates, and hence the confidence limits on benchmark responses and doses, will generally be miss-specified. In addition, these models often include provision for a litter-specific covariate, such as the litter size, which may be correlated with the outcome of interest (but not necessarily with the treatment) and may help clarify the response pattern (USEPA Citation2012).
Table 8. Litter data for individual mating pairs (Gulati et al. Citation1984).
The average litter size data () were modeled in BMDS (USEPA Citation2016) as continuous data with a benchmark response (BMR) of one standard deviation below the control mean to determine a BMD and a 95% lower bound confidence limit (BMDL). For continuous data, USEPA (Citation2012) recommends a BMR be defined based on the level of change in the endpoint at which the effect is considered to become biologically significant. However, in the absence of the level of response to consider adverse, a change in the mean equal to one control standard deviation from the control mean is recommended (USEPA Citation2012). Because the variance was not homogeneous across the dose groups, a non-homogeneous variance model was used. A nested model was not appropriate to use to model this particular endpoint because the nested model shows the risk of an effect nested within the litter; however, a change in litter size is the effect for this endpoint. Therefore, continuous models including the polynomial, power, linear, exponential, and Hill models were used (). Model results were then evaluated for goodness of fit and the BMD and BMDL of those models with the lowest Akaike information criterion (AIC) value that had an adequate Chi-square p value (greater than .1) were considered in identifying a POD for use in estimating an ADI. The linear and power models, which produced the same fit, were chosen as the best fit models for the litter per pair endpoint and produced a BMD of 428 mg/kg/d (0.428 g/kg/d) and a BMDL of 220 mg/kg/d (0.22 g/kg/d). The modeling results are summarized in and graphs of the BMDL output from both the linear and power models are presented in .
Figure 1. (a) Graph of benchmark modeling output for number of litters per mating pair reported in Gulati et al. (Citation1984). Linear model with BMR of 1 standard deviation for the BMD and 0.95 lower confidence limit for the BMDL. (b) Graph of benchmark modeling output for number of litters per mating pair reported in Gulati et al. (Citation1984). Power Model with BMR of 1 standard deviation for the BMD and 0.95 lower.
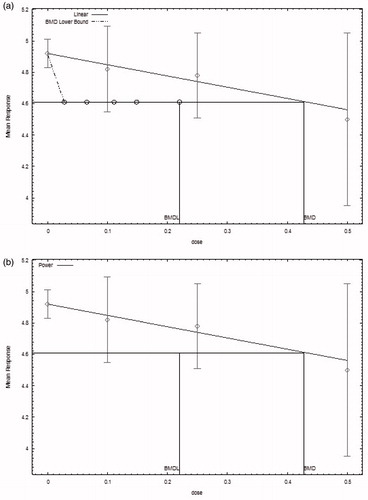
Table 9. BMD results from Gulati et al. (Citation1984).
The live pup weights (that were not adjusted for litter size) were not modeled (), because these weights do not account for the significant change noted in the average litter size in treated animals compared with untreated controls. The more appropriate data to model were the live pup weight adjusted for litter size (). Although it would be most appropriate to model this endpoint with a nested model, the available models in BMDS require specific information. First, the individual pup weights would be necessary and Gulati et al. (Citation1984) do not provide this information. In addition, the only nested models available in BMDS require dichotomized data, which would require a transformation of the continuous body weights for the pups to a dichotomized form. Because of this, the adjusted pup weight data () were modeled using the continuous models (not nested), provided in BMDS. The variance for the adjusted pup weight was homogeneous across dose groups so the homogenous variance option for continuous data was used for the BMDS models, with a BMR of one standard deviation below the control mean used to determine a BMD and a BMDL. The linear model was chosen as the model providing the best fit to the adjusted average pup weights, with an estimated BMD of 412 mg/kg/d (0.412 g/kg/d) and a BMDL of 297 mg/kg/d (0.297 g/kg/d). The modeling results are summarized in .
In addition to requiring dichotomous data, the nested models in BMDS only allow for an event in which the incidence is increasing. For the endpoint of proportion of pups born alive per fertile pair, the results are expressed as a decreasing proportion born alive with increasing concentration of methyl salicylate () (Gulati et al. Citation1984). This required some transformation of the individual data to convert the result to the number of dead pups in each litter (). The result would be an endpoint where adverse changes would be associated with an increase in incidence with increasing dose, rather than a decrease in incidence with increasing dose. The individual data were available for this endpoint () and were used in the BMD modeling. Because the BMD and BMDL for these nested models are computed in terms of a fixed litter size, both the mean litter size of the control group and the mean litter size over all dose groups were used. In addition, the models were fit both using the litter as a covariate and without the additional covariate. The NLogistic model using litter size as a covariate with the control group average litter size used to determine the BMD and BMDL provided the best fit model to the data, with a BMD of 2710 mg/kg/d (2.71 g/kg/d) and a BMDL of 770 mg/kg/d (0.77 g/kg/d). If the average litter size over all dose groups was used to determine the BMD and BMDL, the results were slightly different with a BMD of 1670 mg/kg/d (1.67 g/kg/d) and a BMDL of 600 mg/kg/d (0.60 g/kg/d). All modeling for this endpoint was conducted with a 95% confidence level with a BMR of 5% as recommended by Allen et al. (Citation1994), which concluded that benchmark doses developed from nested models with BMRs at the 5% level of risk were similar to no observed adverse effect levels determined by statistical tests of trend from a large dataset of reproductive/developmental studies. USEPA (Citation2012) also notes “most reproductive and developmental studies with nested study designs support a BMR of 5%”. The modeling results are presented in .
Table 10. Individual breeding pair BMD modeling results for Gulati et al. (Citation1984).
A BMDL based on the best model fit for each of the datasets modeled was determined. For decreases in the numbers of litters per mating pair, the best model fit was produced by both the linear and power models and produced a BMD of 428 mg/kg/d (0.428 g/kg/d) and a BMDL of 220 mg/kg/d (0.22 g/kg/d). Modeling results for the adjusted average pup weights indicated the exponential 3 model was the model providing the best fit with an estimated BMD of 415 mg/kg/d (0.415 g/kg/d) and a BMDL of 293 mg/kg/d (0.293 g/kg/d). Finally, for the number of dead pups per individual breeding pair, the NLogistic model provided the best fit and a BMD of 2710 mg/kg/d (2.71 g/kg/d) and a BMDL of 770 mg/kg/d (0.77 g/kg/d). Overall, the most sensitive endpoint determined from BMDS modeling was the number of litters per mating pair with a BMDL of 0.22 g/kg/d or 220 mg/kg/d. This BMDL will serve as the POD in the derivation of an allowable daily intake for methyl salicylate.
Mode of action and human relevance assessment
As the endpoints of interest for dose–response modeling were all reproductive/developmental endpoints, consideration of the available mode of action information associated with these types of effects following oral exposure to methyl salicylate was evaluated. The results from studies that focus on the mode of action of methyl salicylate were used to determine (1) whether the reproductive effects reported in animal models are relevant to humans and (2) what uncertainty factors should be applied to the POD estimated from Gulati et al. (Citation1984) in order to estimate an acceptable daily intake. The most conservative BMDL or POD of 220 mg/kg/d was derived based on a significant decrease in the number of litters per pair, in mice orally exposed to methyl salicylate during mating. No human data related to oral toxicity of methyl salicylate were identified in the literature. In addition, a review of the available literature indicated limited data are available related to the mode of action of methyl salicylate following oral exposure.
Once absorbed, the majority of methyl salicylate is converted to salicylic acid (Opdyke Citation1978). Salicylic acid is also the major metabolite of acetylsalicylic acid or aspirin for which mode of action data are readily available (Hutt et al. Citation1986). Therefore, similarities in the mode of action for methyl salicylate and acetylsalicylic acid can be assumed. Davison et al. (Citation1961) conducted a study to compare the toxicity and metabolism of methyl salicylate and salicylic acid. Davison et al. (Citation1961) concluded that the results indicated the toxicity and metabolism of both methyl salicylate and salicylic acid are comparable across multiple species (rats, dogs, and monkeys).
The mode of action for salicylic acid, the metabolite of both methyl salicylate and acetylsalicylic acid, has been well studied and is known to involve the inhibition of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) activity, which in turn leads to a decrease in the synthesis of prostaglandins and thromboxane (Patrono et al. Citation2001; Roberts & Morrow Citation2001; Vane & Botting Citation2003). The inhibition of COX-2 leads to the therapeutic effects associated with the use of salicylates as antipyretic, analgesic, and anti-inflammatory agents, and it can also lead to unwanted side effects due to decreased prostaglandin formation (Roberts & Morrow Citation2001). Prostaglandins play an important role in reproductive functions (Rudolph Citation1981). The inhibition of prostaglandins may interfere with uterine contractility, platelet function, and fetal vascular structure (Rudolph Citation1981). Offspring of women that ingested salicylates for long periods during pregnancy have been reported to have significantly reduced body weights (Roberts & Morrow Citation2001). In pregnant women administered aspirin during the third trimester, increased perinatal mortality, anemia, antepartum and postpartum hemorrhage, prolonged gestation, and complicated deliveries were reported (Roberts & Morrow Citation2001).
Similar reproductive effects to those reported following oral methyl salicylate exposure in animals have also been reported following oral exposure to acetylsalicylic acid. These effects include decreased litter size and fetal weights in rats orally exposed to salicylic acid during gestation (CIR Citation2003). Therefore, based on similar reproductive effects noted in animals and similar metabolism of methyl salicylate; along with the reported reproductive effects in humans following exposure to acetylsalicylic acid during pregnancy, it was assumed that the reproductive effects reported in rats following methyl salicylate exposure are relevant to humans. This is supported by the mode of action of salicylates, which involves the inhibition of prostaglandin synthesis which has been reported to interfere with uterine contractility, platelet function, and fetal vascular structure (Rudolph Citation1981).
Adjustments for uncertainty
Based on the study characteristics and the mode of action information available in animals following oral exposure to methyl salicylate, an uncertainty factor analysis was performed in accordance with the methods currently recommended by USEPA (Citation2002). As noted previously, a factor of 1–10 is commonly used to adjust for each type of uncertainty considered based on the available data and scientific judgment.
Interspecies uncertainty factor (UFA).
There are very limited data available to characterize the pharmacokinetic and/or pharmacodynamic differences between animals and humans following exposure to methyl salicylate. No data were identified to compare the pharmacokinetics of methyl salicylate in humans and mice, which is the species upon which the POD is based. Based on the data reported in Davison et al. (Citation1961), there appear to be some slight differences in the metabolism of methyl salicylate between humans and animals. Methyl salicylate administered orally to dogs was nearly completely hydrolyzed to salicylic acid within 1 h of administration (Davison et al. Citation1961). However, hydrolysis was slower in humans when compared with animals with 21% total salicylate measured in the plasma 90 min following oral administration of methyl salicylate compared with the negligible amounts noted in dogs at 60 min post-administration. Therefore, based on recommendations by USEPA (Citation2011a) an animal to human body weight scaling factor was calculated and applied to the POD to derive a HED. The DAF of 0.15 was estimated using the study specific mean maternal body weight at parturition reported by Gulati et al. (Citation1984) of 0.042 kg divided by the mean body weight for a pregnant female of 75 kg provided in the USEPA Exposure Factors Handbook (USEPA Citation2011b) and raised to the ¼ power as shown in EquationEquation (1)(1)
(1) . Because body weight scaling was applied, the default UFA of 3 as recommended by USEPA (Citation2011a) was used.
Intraspecies uncertainty factor (UFH).
When considering the pharmacokinetic variability component of the UFH, the only available data specifically for methyl salicylate is provided by Davison et al. (Citation1961). In this study, six volunteers were administered a single dose of methyl salicylate orally at an average dose of 7 mg/kg. Plasma levels of methyl salicylate, free salicylate, and total salicylate were measured at 15 and 90 min post-administration. The available results suggested limited variability in plasma concentrations among the volunteers. In addition, Davison et al. (Citation1961) also administered a single dose of acetylsalicylic acid to the same six volunteers and measured total salicylate in the plasma at 15 and 90 min post-exposure. Results indicated variability in total salicylate plasma concentrations in humans following exposure to methyl salicylate did not differ markedly from total salicylate plasma concentrations following administration of acetylsalicylic acid.
While limited pharmacokinetic data were available in humans exposed to methyl salicylate that could be used to characterize variability in human pharmacokinetics; based on the similarities in metabolism and mode of action between methyl salicylate and acetylsalicylic acid pharmacokinetic data available for acetylsalicylic acid were used as surrogate data to characterize the potential variability associated with the pharmacokinetics of methyl salicylate in humans. In a review of the pharmacokinetic data in humans for salicylates as a class of compounds, Needs and Brooks (Citation1985) concluded there were no significant differences in the pharmacokinetics of salicylates in the elderly or in children compared with young adults (Needs & Brooks Citation1985), indicating that there was limited human variability across age groups. In addition, human variability was limited when measured area under the curve (AUC) and peak serum concentrations in volunteers administered acetylsalicylic acid were compared across several studies (Gatti et al. Citation1989; Cerletti et al. Citation2003; Lucker et al. Citation2003).
When considering interspecies and intraspecies variability, the default factor of 10 for each is comprised of two components, pharmacokinetic differences and pharmacodynamics differences, each having a default value of 3 (Kroes et al. Citation1993; Beck & Clewell Citation2001; USEPA Citation2002; Dorne Citation2004). However, in cases where there are sufficient pharmacokinetic and/or pharmacodynamic data available, a reduction in the default UFA or UFH to 3 or 1 could be justified. Therefore, because there are sufficient data available to suggest limited human pharmacokinetic variability for methyl salicylate and the pharmacokinetic component of the UFH was decreased from 3 to 1.
No data were identified that could be used to characterize human variability in the pharmacodynamics of methyl salicylate. However, the endpoint relied upon is from a sensitive subpopulation. The UFA incorporates a factor to account for the potential that humans are more sensitive than animals. The available pharmacokinetic data also suggest limited variability across subpopulations. Therefore, the pharmacodynamic component of the UFH was decreased from 3 to 1. The total UFH would be 1, which is composed of a factor of 1 for pharmacokinetics and 1 for pharmacodynamics.
Chronic versus subchronic uncertainty (UFS).
The POD is based on a reproductive endpoint reported in a well-conducted continuous breeding reproductive study (Gulati et al. Citation1984). While the exposure duration in reproductive studies is less than chronic, the duration or exposure and the timing of exposure in these types of studies are designed to occur during critical windows of reproductive and development physiology. Therefore, no uncertainty factors were applied to the POD to account for less than chronic exposure and the UFS was 1.
LOAEL versus NOAEL uncertainty (UFL).
Because a BMDL is being recommended as the POD, which is considered a NOAEL equivalent, no uncertainty factor adjustment will be made. The UFL was 1.
Incomplete database uncertainty (UFD).
The UFD is intended to account for any underestimates of an acceptable daily intake due to incomplete characterization of the chemical’s toxicity. In the case of methyl salicylate, the database characterizing toxicity associated with oral exposure includes chronic/carcinogenicity studies in multiple species, as well as, reproductive/developmental studies conducted in mice. Therefore, the data are considered complete and no adjustment for database insufficiency will be made to the POD. The UFD will be 1.
Based on the uncertainty factor analysis, the POD for methyl salicylate will be adjusted by a total UF of 3 (3 for UFA, 1 for UFH, and 1 for UFS, UFL, and UFD).
Estimation of an acceptable daily intake
An ADI is an estimate that includes adjustments for uncertainty in the data, of the daily oral exposure to the human population that is likely to be without an appreciable risk of adverse effects over the course of a lifetime. An ADI is derived from a POD, which is the dose–response point equivalent to the beginning of a low-dose extrapolation and includes body weight scaling adjustment (DAF) and an adjustment for uncertainty using UFs. An oral ADI of 11 mg/kg/d was derived using the BMDL estimated from data reported in Gulati et al. (Citation1984) of 220 mg/kg/d based on significant decreases in the number of litters per pair in rats orally exposed to methyl salicylate during mating (). The BMDL was converted to a HED by multiplying by the DAF (BW3/4 scaling factor) and adjusted by the uncertainty factors determined in the uncertainty factor analysis of 3 for UFA, 1 for UFH, and 1 for UFS, UFL, and UFD. EquationEquations (3)(3)
(3) and Equation(4)
(4)
(4) were used to calculate the ADI:
(3)
(3)
(4)
(4)
Discussion/conclusions
Several authoritative agencies have recommended an ADI for ingestion of methyl salicylate, which is used as a denaturant, external analgesic, flavoring agent, and fragrance ingredient in a variety of products including chewing gum, baked goods, syrups, candy, beverages and ice cream (CIR Citation2003; NFSA Citation2012). JECFA (Citation1968, Citation2002) recommended an ADI of 0.5 mg/kg-body weight for methyl salicylate based on a NOEL of 50 mg/kg/d reported in a 2-year study in dogs; however, the specific study was not cited (JECFA Citation1968, Citation2002). JECFA (Citation2002) also reports an estimated daily per capita intake for methyl salicylate of 0.7 mg/kg-body weight. In addition, NFSA (Citation2012) relies upon the JECFA (Citation2002) ADI of 0.5 mg/kg/d in making recommendations regarding acceptable levels of methyl salicylate in food. In making recommendations regarding concentrations of methyl salicylate in animal feed, EFSA (Citation2012) relies upon a study conducted by Webb and Hansen (Citation1963), which appears to be the same study relied upon by JECFA (Citation2002) for recommending an ADI. Methyl salicylate also has USFDA-specified uses as an indirect food additive (CIR Citation2003).
Based on a review of the available published literature and summaries of unpublished literature identified through review documents for methyl salicylate, while chronic toxicity studies were available, no studies were identified that reported statistically significant increases in tumors following oral exposure to methyl salicylate. The studies relied upon by JECFA (Citation2002), NFSA (Citation2012), and EFSA (Citation2012) were identified for review of non-cancer endpoints. However, studies were also identified that evaluate the potential reproductive/developmental toxicity of methyl salicylate following oral exposure (Gulati et al. Citation1984; NTP Citation1984; Morrissey et al. Citation1989; CIR Citation2003) which were available at the time the ADIs from EFSA, JECFA and NFSA were recommended but do not appear to have been considered by authoritative agencies when developing ADI’s for methyl salicylate.
When considering the currently recommended ADI’s for methyl salicylate, several points should be considered that could increase uncertainty in these ADI’s. Based on current recommendations from the NRC (Citation2014) in reviewing the processes applied by the EPA in estimating acceptable intake of chemicals that approaches be developed to identify studies in a systematic, defined manner and that study quality be considered. The literature search strategy used to identify the studies and the criteria used to assess study quality should be well documented. This ensures that all the available toxicological literature have been considered and the highest quality data currently available were incorporated into the dose-response assessment. A literature search strategy, the results of the literature search, and an assessment of study quality based on criteria recommended by Klimisch et al. (Citation1997) were all incorporated into this investigation. The study quality analysis was conducted and a transparent set of study quality criteria were used to determine the highest quality studies receiving scores of 1 or 2, which were considered for evaluation for the dose response assessment. In order to receive a quality score of 1 or 2, the study must have been conducted according to a published testing guideline and preferably according to GLP. Studies receiving a score of 2 were those that were not reported to be conducted according to published guidelines; however, met the reporting requirements outlined by Klimisch et al. (Citation1997) (). For this investigation, no studies received a score of 1. Studies receiving a score of 2 included the chronic toxicity study relied upon by EFSA (Citation2012) (and presumably JECFA (Citation2002) and NFSA (Citation2012)) conducted in rats and dogs (Webb and Hansen Citation1963); and one reproductive/developmental study conducted in two laboratories (Gulati et al. Citation1984; NTP Citation1984; Morrissey et al. Citation1989).
A second consideration that should be addressed when comparing the current recommended ADIs to the ADI proposed in this assessment is the ADI recommended by JECFA (Citation2002), NFSA (Citation2012) and EFSA (Citation2012) is based on a NOEL or no observed effect level, for changes in body weights, an endpoint that is not typically considered adverse in the absence of accompanying histopathological changes when it is the only significant effect reported in a study (USEPA Citation1988). In addition, while a POD can be based on a NOEL, a POD based on the estimation of a BMD provides a more robust, informative POD because BMDs account for the shape of the dose-response curve over the continuum of observed exposures, rather than at a single dose level.
Webb and Hansen (Citation1963) reported no significant effects in rats following chronic exposure; and the studies conducted in dogs following oral exposure do not provide adequate detail to allow for the use in a dose response assessment. However, based on the available data provided by Gulati et al. (Citation1984), BMD modeling could be conducted for selected reproductive/developmental endpoints. These reproductive effects of methyl salicylate do not appear to have been considered for the ADIs recommended by JECFA (Citation2002), NFSA (Citation2012) and EFSA (Citation2012), even though reproductive/developmental effects are typically considered more sensitive endpoints because they occur in sensitive subpopulations and the studies by Morrissey et al. (Citation1989) and Gulati et al. (Citation1984) were published and available at the time the JECFA, EFSA, and NFSA documents were published. This could possibly be because the ADI developed by JECFA of 0.5 mg/kg/d was higher than the expected daily consumption rate of 0.1 mg/kg/d. This could also be because a NOEL approach was used for the estimation of the POD for the ADIs and the NOAEL for reproductive effects would have been higher at 100 mg/kg/d reported by Gulati et al. (Citation1984) compared with the NOEL for body weight changes in dogs of 50 mg/kg/d. The reproductive data may also not have been considered because many of the endpoints from developmental studies are reported as nested data with litter as the statistical unit. These data can be more difficult to model and are best modeled using nested models (USEPA Citation2012), which account for intra-litter correlation.
Benchmark modeling, using either continuous or nested models where appropriate, was conducted on datasets associated with decreased average pup weight, adjusted average pup weights, and the number of dead pups per individual breeding pair (Gulati et al. Citation1984). BMDLs for each dataset were chosen based on the best model fit and ranged from a BMDL of 220 mg/kg/d for decreases in average pup weight to a BMDL of 770 mg/kg/d for the number of dead pups per individual breeding pair. Overall, a BMDL of 220 mg/kg/d associated with the most sensitive endpoint (i.e. the number of litters per mating pair) was chosen as the BMDL to serve as the POD in the derivation of an ADI for methyl salicylate.
Due to a lack of relevant epidemiological studies in humans following oral exposure to methyl salicylate, a review of the mode of action was conducted to determine if the available data suggested that the most sensitive endpoint identified in animals (i.e. reproductive toxicity) was relevant to humans. Limited mode of action data were available for methyl salicylate; however, both methyl salicylate and acetylsalicylic acid are metabolized to the same active metabolite (i.e. salicylic acid) and there are data to characterize the mode of action for acetylsalicylic acid (Opdyke Citation1978; Hutt et al. Citation1986). The mode of action for salicylic acid involves the inhibition of COX-1 and COX-2 activity, leading to an inhibition in the synthesis of prostaglandins (Patrono et al. Citation2001; Roberts & Morrow Citation2001; Vane & Botting Citation2003). Prostaglandins play an important role in reproductive functions, and their inhibition has been linked to changes in uterine contractility, platelet function, and fetal vascular structure (Rudolph Citation1981). Therefore, based on the available mode of action data for acetylsalicylic acid and the fact that similar reproductive toxicity has been reported in both humans and animals following exposure to acetylsalicylic acid (Roberts & Morrow Citation2001; CIR Citation2003), the reproductive endpoints reported in mice following oral exposure to methyl salicylate were considered relevant to humans.
An uncertainty factor analysis for methyl salicylate resulted in a total recommended uncertainty factor of 3. This was based on uncertainty factors of 1 representing uncertainty associated with study length (UFS), use of LAOEL/NOAEL (UFL) and database insufficiencies (UFD); and 3 for uncertainty associated with intraspecies (UFA) and 1 for uncertainty associated with interspecies (UFH). The factor of 3 for UFA is the default UFA recommended by USEPA (Citation2011a) used in combination with a body weight scaling adjustment to the POD. There are sufficient data to suggest limited human variability in the pharmacokinetics associated with methyl salicylate (Davison et al. Citation1961) and salicylates as a class of compounds (Needs & Brooks Citation1985; Gatti et al. Citation1989; Cerletti et al. Citation2003; Lucker et al. Citation2003). There are limited pharmacodynamic data for methyl salicylate; however, the available pharmacokinetic data suggest limited variability across subpopulations and the endpoint relied upon for the POD is from a sensitive subpopulation. Therefore, the pharmacodynamic component of the UFH was decreased from 3 to 1.
In conclusion, while the only ADIs recommended by authoritative bodies were based on NOELs for body weight changes which were the lowest doses tested from chronic studies conducted in dogs and rats, through a critical review of the available peer-reviewed literature, a reproductive/developmental study was identified that could be used to derive a BMDL. BMD analysis of the data reported by Gulati et al. (Citation1984) indicated the lowest BMDL associated with the best model fit was 220 mg/kg/d. Therefore, the BMDL of 220 mg/kg/d was considered as a POD and adjusted by a DAF of 0.15 based on study specific animal body weight reported by Gulati et al. (Citation1984) and a total uncertainty factor of 3 to derive an ADI for methyl salicylate of 11 mg/kg/d.
In comparison with the ADI recommended by JECFA (Citation2002), which is assumed to be based on the body weight changes and bone effects in rats at 50 mg/kg/d reported by Webb and Hansen (Citation1963), the POD of 220 mg/kg/d based on the data reported by Gulati et al. (Citation1984) is greater than four times higher. A NOEL or no observed effect level is a level at which no effects are noted; however, those effects are not necessarily toxicologically significant or considered to be precursors to adverse effects (Lewis et al. Citation2002). A NOAEL or no observed adverse effect level is a level at which there are no statistically significant or biologically significant adverse effects (Lewis et al. Citation2002; USEPA Citation2002). Because JECFA (2002) defines the POD as a NOEL with no other information as to the endpoint or even the study on which the POD is based, it must be assumed that the POD is based on the results of Webb and Hansen (Citation1963) and, based on the definition of a NOEL, the effects that serve as the basis of the POD were not considered adverse. The POD proposed in this investigation of 220 mg/kg/d is based on the most sensitive, biologically significant adverse effect reported in both of the studies reviewed and considered for use in estimating the ADI. The four-fold difference in the POD used by JECFA and the POD recommended in this assessment is likely due to two reasons: (1) the POD used by JECFA (2002) is likely based on effects that are not considered biologically significant or adverse that are occurring at lower doses than the adverse reproductive effects reported in Gulati et al. Citation1984), and (2) the POD recommended in this assessment is based on the results of benchmark modeling that provides a more robust, informative POD compared with the NOAEL that accounts for the shape of the dose–response curve over the continuum of observed exposures, rather than at a single dose level (USEPA Citation2002, Citation2012). The differences in the PODs resulted in the recommended ADI in this investigation of 11 mg/kg/d being 22 times higher than the current ADI recommended by JECFA (Citation2002) of 0.5 mg/kg-body weight.
Declaration of interest
The conclusions of this assessment are those of the authors. The employment affiliation of the authors is as shown on the cover page. All authors are employed by Ramboll Environ, a global environmental consultancy. This project was supported by funding provided by Altria Client Services (ALCS), LLC, which provides professional services and support to the Altria Group and its operating companies. Altria Group consists of a family of companies that are leading manufacturers and producers of tobacco products and wines. Methyl salicylate is used in some of these tobacco products as an additive.
ALCS had no role in study design, data collection and analysis, decision to publish, or preparation of any portion of the manuscript. ALCS were given the opportunity to review a draft manuscript while it was under review and consideration by the journal; however, no comments or suggested revisions were provided by the funders. The analyses, opinions, and conclusions presented in this manuscript are exclusively those of the authors and do not necessarily represent the view of ALCS. The authors’ scientific conclusions and professional judgments were not subject to the funders’ control.
There are no conflicts of interest for any of the authors to disclose related to the submission of this manuscript. None of the authors have been involved or appeared in regulatory or legal proceedings related to the contents of this paper. It is anticipated that regulatory authorities will consider the contents of this review in making regulatory decision on the use of methyl salicylate in multiple products.
Acknowledgements
The authors gratefully acknowledge the extensive and very insightful comments offered by three independent reviewers selected by the Editor and presented anonymously to the authors. The final manuscript reflects revisions suggested by these reviewers to not only clarify questions in the assessment, but also incorporate additional references to regulatory guidance for the approaches and critical decision points made in conducting the assessment. The authors greatly appreciate this input as it resulted in a stronger, more transparent assessment.
References
- Abbot DD, Harrisson JWE. 1978. Methyl salicylate: studies of osseous changes in the rat, reproduction in the rat and mouse, and liver and kidney effects in the dog. Unpublished communication to the Flavor and Extract Manufacturers Association (As cited in CIR 2003, NFSA 2012).
- Allen BC, Kavlock RJ, Kimmel CA, Faustman EM. 1994. Dose-response assessment for developmental toxicity. III. Statistical Models. Fund Appl Toxicol. 23:496–509.
- Battino M, Ferreiro MS, Armeni T, Politi A, Bompadre S, Massoli A, Bullon P. 2005. In vitro antioxidant activities of antioxidant-enriched toothpastes. Free Radic Res. 39:343–350.
- Beck BD, Clewell HJ III. 2001. Uncertainty/safety factors in health risk assessment: opportunities for improvement. Hum Ecol Risk Assess. 7:203–207.
- Cerletti C, Dell'Elba G, Manarini S, Pecce R, Di Castelnuovo A, Scorpiglione N, Feliziani V, de Gaetano G. 2003. Pharmacokinetic and pharmacodynamic differences between two low dosages of aspirin may affect therapeutic outcomes. Clin Pharmacokinet. 42:1059–1070.
- ChemSpider. 2015. Search and share chemistry [Internet]. Royal Society of Chemistry. [cited 2016 Apr 14]. Available from: http://www.chemspider.com/Chemical-Structure.13848808.html?rid =56c5d453-3418-4c23-9d07-6dbb9d658fd2.
- Chen C, Isabelle LM, Pickworth WB, Pankow JF. 2010. Levels of mint and wintergreen flavorants: smokeless tobacco products vs. confectionary products. Food Chem Toxicol. 49:755–763.
- Code of Federal Regulations. 2015. From the U.S. Government Printing Office via GPA access. Title 21, vol. 3, part 175. [cited 2016 Aug 31]; Available from: http://www.ecfr.gov/cgi-bin/text-idx?SID=a52bd4f6a793316bc823387b87c80829&mc=true&node=pt21.3.175&rgn=div5#se21.3.175_1105.
- [CIR] Cosmetic Ingredient Review. 2003. Safety assessment of salicylic acid, butyloctyl salicylate, calcium salicylate, C12-15 alkyl salicylate, capryloyl salicylic acid, hexyldodecyl salicylate, isocetyl salicylate, isodecyl salicylate, magnesium salicylate, MEA- salicylate, ethylhexyl salicylate, potassium salicylate, methyl salicylate, myristyl salicylate, sodium salicylate, TEA- salicylate, and tridecyl salicylate. Int J Toxicol. 22 Suppl 3:1–108.
- [CIR] Cosmetic Ingredient Review. 2008. Final report of the safety assessment of alcohol denaturants, including SD alcohol 3-A, SD alcohol 30, SD alcohol 39, SD alcohol 39-B, SD alcohol 39-C, SD alcohol 40, SD alcohol 40-B, and SD alcohol 40-C, and the denaturants, quassin, brucine sulfate/brucine, and denatonium benzoate. Int J Toxicol. 27 Suppl 1:1–43.
- Collins TF, Hansen WH, Keeler HV. 1971. Effect of methyl salicylate on rat reproduction. Toxicol Appl Pharmacol. 18:755–765.
- Crossland AM. 1948. Methyl salicylate poisoning. Can Med Assoc J. 58:75–77.
- Daston GP, Rehnberg BF, Carver B, Rogers E, Kavlock R. 1988. Functional teratogens of the rat kidney. I. Colchicine, dinoseb, and methyl salicylate. Fundam Appl Toxicol. 11:381–400.
- Davis LE, Westfall BA. 1972. Species differences in biotransformation and excretion of salicylate. Am J Vet Res. 33:1253–1262.
- Davison C, Zimmerman EF, Smith P. 1961. On the metabolism and toxicity of methyl salicylate. J Pharmacol Exp Ther. 132:207–211.
- Dorne JL. 2004. Impact of inter-individual differences in drug metabolism and pharmacokinetics on safety evaluation. Fundam Clin Pharmacol. 18:609–620.
- [EFSA] European Food Safety Authority. 2012. Scientific opinion on the safety and efficacy of benzyl alcohols, aldehydes, acids, esters and acetals (chemical group 23) when used as flavorings for all animal species. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA J. 10:2785. doi: 10.2903/j.efsa.2012.2785.
- Gatti G, Barzaghi N, Attardo Parrinello G, Vitiello B, Perucca E. 1989. Pharmacokinetics of salicylic acid following administration of aspirin tablets and three different forms of soluble aspirin in normal subjects. Int J Clin Pharm Res. 9:385–389.
- Graham GG, Roberts MS, Day RO, Rainsford KD. 2004. Pharmacokinetics and metabolism of the salicylates. In: Rainsford KD, editor. Aspirin and related drugs. New York: Taylor and Francis, p. 97–155 (Chapter 4).
- Gulati DK, Choudhury H, Chambers R, Sabharwal PS, Lamb JIV. 1984. Methyl salicylate: fertility assessment in cd-1 mice when administered by gavage. National Toxicology Program, National Institutes of Environmental Health Sciences. NTP-85-022. Available from: https://www.ntis.gov/products/ntrl/.
- Harrisson JWE, Abbott DD, and Packman EW. 1963. Salicylates and other hydroxybenzoates: effect upon osseous tissue of young rats. Federation Proceedings, vol. 22, p. 554.
- Hutt AJ, Caldwell J, Smith R. 1986. The metabolism of aspirin in man: a population study. Xenobiotica. 16:239–249.
- Infurna R, Beyer B, Twitty L, Koehler G, Daughtrey W. 1990. Evaluation of the dermal absorption and teratogenic potential of methyl salicylate in a petroleum-based grease. East Millstone (NJ): Exxon Biomedical Sciences, Inc. Teratology. 41:566.
- [IPCS] International Program on Chemical Safety. (1987). Environmental Health Criteria 70. Principles for the safety assessment of food additives and contaminants in food. World Health Organization, Geneva. Available from: http://www.inchem.org/documents/ehc/ehc/ehc70.htm#SectionNumber:5.5.
- Ishidate M, Sofuni T, Yoshikawa D, Hayashi M, Nohmi T, Sawada M, Matsuoka A. 1984. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol. 22:623–636.
- [JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1968. Specifications for the identity and purity of food additives and their toxicological evaluation: some flavoring substances and non-nutritive sweetening agents. Eleventh report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization Technical Report Series No. 383, FAO Nutrition Meetings Report Series No. 44, Geneva.
- [JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2002. Evaluation of certain food additives and contaminants. Fifty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 909. Geneva: World Health Organization.
- Klimisch HJ, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol. 25:1–5.
- Kroes R, Munro I, Poulsen E. 1993. Workshop on the scientific evaluation of the safety factor for the acceptable daily intake (ADI): editorial summary. Food Addit Contam. 10:269–273.
- Kuboyama N, Fujii A. 1992. Mutagenicity of analgesics, their derivatives, and anti-inflammatory drugs with S-9 mix of several animal species. J Nihon Univ School Dentistry. 34:183–195.
- Lapczynski A, Jones L, McGinty D, Bhatia S, Letizia C, Api A. 2007. Fragrance material review on methyl salicylate. Food Chem Toxicol. 45(Suppl 1):S428–S452.
- LaWall and Harrisson Research Laboratories, Inc. 1964. Study of Methyl Salicylate on rats. L&H 58188. (Submitted by FDA in response to an FOI request – 1998; 120 pages) (As cited in CIR 2003).
- Levy G, Weintraub L, Matsuzawa T, Oles S. 1966. Absorption, metabolism and excretion of salicylic phenolic glucuronide in rats. J Pharm Sci. 55:1319–1320.
- Lewis RW, Billington R, Debryune E, Gamer A, Land B, Carpanini F. 2002. Recognition of adverse and nonadverse effects in toxicity studies. Toxicol Pathol. 30:66–74.
- Lisko JG, Stanfill SB, Watson CH. 2014. Quantitation of ten flavor compounds in unburned tobacco products. Anal Methods. 6:4698–4704.
- Lucker PW, Birkel M, Hey B, Loose I, Schaefer A. 2003. Pharmacokinetic interaction study of a fixed combination of 500 mg acetylsalicylic acid/30 mg pseudoephedrine versus each of the single active ingredients in healthy male volunteers. Arzneimittel-Forsch. 53:260–265.
- Montgomery PR, Berger LG, Mitenko P, Sitar D. 1986. Salicylate metabolism: effects of age and sex in adults. Clin Pharmacol Therap. 39:571–576.
- Morrissey RE, Lamb J, Morris R, Chapin R, Gulati D, Heindel J. 1989. Results and evaluations of 48 continuous breeding reproduction studies conducted in mice. Developmental and Reproductive Toxicology Group, NIEHS, NTP Program, Research Triangle Park, North Carolina, Analytical Sciences Inc., Durham, North Carolina, Environmental Health Research and Testing, Inc., Lexington, Kentucky. Fund Appl Toxicol. 13:747–777.
- Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 8(Suppl 7):1–119.
- Murugesh N, Kumar VR, Vembar S, Damodaran C. 1981. Studies on erythrocyte membrane. V. Haemolytic effect of methylsalicylate and its possible mechanism. Toxicol Lett. 9:225–229.
- Needs CJ, Brooks PM. 1985. Clinical pharmacokinetics of the salicylates. Clin Pharmacokinet. 10:164–177.
- [NFSA] Norwegian Food Safety Authority. 2012. Risk profile methyl salicylate CAS no. 119-36-8.
- [NRC] National Research Council of the National Academies. 2014. Review of EPA’s Integrated Risk Information (IRIS) Process. Committee to Review the IRIS Process Board on Environmental Studies and Toxicology Division on Earth and Life Studies.
- [NTP] National Toxicology Program. 1984. Abstract for RACB82104 – Methyl salicylate CAS no. 119-36-8. NTIS #RACB82104. Available from: http://ntp.niehs.nih.gov/testing/types/repro/abstracts/racb/index-8.html.
- Opdyke DL. 1978. Acute oral toxicity: rat; acute dermal toxicity: rabbit; and primary dermal irritation: rabbit for methyl salicylate, fragrance raw materials monographs. Food Cosmet Toxicol. 16:821–825.
- Opdyke DL. 1979a. Monographs on fragrance raw materials. Food Cosmet Toxicol. 17:357–390.
- Opdyke DL. 1979b. Monographs on fragrance raw materials. Food Cosmet Toxicol. 17:(Special Issue V):695–923.
- Overman DO, White JA. 1983. Comparative teratogenic effects of methyl salicylate applied orally or topically to hamsters. Teratology. 28:421–426.
- Packman EW, Abbott DD, Wagner BM, Harrisson JWE. 1961. Chronic oral toxicity of oil sweet birch (methyl salicylate). Pharmacologist. 3:62 (As cited in CIR 2003).
- Patrono C, Coller B, Dalen J, Fitzgerald G, Fuster V, Gent M, Hirsh J, Roth G. 2001. Platelet-active drugs: the relationships among dose, effectiveness, and side effects. Chest. 119(1 Suppl):39S–63S.
- Rainsford K. 2004a. History and development of the salicylates. In: Rainsford K, editor. In aspirin and related drugs. New York: Taylor and Francis; p. 1–24.
- Rainsford K. 2004b. Pharmacology and biochemistry of salicylates and related drugs. In: Rainsford K, editor. In aspirin and related drugs. New York: Taylor and Francis; p. 215–366.
- Rainsford K. 2004c. Side effects and toxicology of the salicylates. In: Rainsford K, editor. In aspirin and related drugs. New York: Taylor and Francis; p. 367–554.
- Roberts LJIII, Morrow JD. 2001. Analgesic-antipyretic and anti-inflammatory agents and drugs employed in the treatment of gout. In: Hardman JG, Limbird LE, editors. Goodman & Gilman's the pharmacological basis of therapeutics. New York, (NY): McGraw-Hill; p. 687–729.
- Rotilio D, Joseph D, Hatmi M, Vargaftig B. 1984. Structural requirements for preventing the aspirin- and the arachidonate-induced inactivation of platelet cyclo-oxygenase: additional evidence for distinct enzymatic sites. Eur J Pharmacol. 97:197–208.
- Rudolph AM. 1981. Effects of aspirin and acetaminophen in pregnancy and in the newborn. Arch Intern Med. 141:358–363.
- Sharpe GL, Larsson KS, Thalme B. 1975. Studies on closure of the ductus arteriosus. XII. In utero effect of indomethacin and sodium salicylate in rats and rabbits. Prostaglandins. 9:585–596.
- Ungvary G, Tatrai E, Lorincz M, Barcza G. 1983. Combined embryotoxic action of toluene, a widely used industrial chemical, and acetylsalicylic acid (aspirin). Teratology. 27:261–269.
- [USEPA] United States Environmental Protection Agency. 1985. Reference values for risk assessment. United States Environmental Protection Agency, Washington, (DC): Environmental Criteria and Assessment Office.
- [USEPA] United States Environmental Protection Agency. 1988. Research and Development: General Quantitative Risk Assessment Guidelines for Noncancer Health Effects. Prepared for Risk Assessment Forum. Sixth Draft. ECAD-CIN-538.
- [USEPA] United States Environmental Protection Agency. 1993. Reference dose (RfD): Description and use in health risk assessments. Background document 1A, March 15, 1993. Integrated Risk Information System (IRIS), United States Environmental Protection Agency. Available from: http://www.epa.gov/iris/rfd.htm.
- [USEPA] United States Environmental Protection Agency. 1994. Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. United States Environmental Protection Agency, Office of Research and Development. EPA/600/8 90/066F.
- [USEPA] United States Environmental Protection Agency. 2002. A review of the reference dose and reference concentration processes. Final report. Risk Assessment Forum, United States Environmental Protection Agency, Washington, DC. EPA/630/P-02/002F.
- [USEPA] United States Environmental Protection Agency. 2005a. Reregistration eligibility decision for methyl salicylate. United States Environmental Protection Agency. OPP Chemical Code: 076601. Issued September 27, 2005.
- [USEPA] United States Environmental Protection Agency. 2005b. Guidelines for carcinogen risk assessment. United States Environmental Protection Agency. EPA/630/P-03/001F.
- [USEPA] Untied States Environmental Protection Agency. 2011a. Recommended Use of BW3/4 as the Default Method in Derivation of the Oral Reference Dose. EPA/100/R11/001.
- [USEPA] Untied States Environmental Protection Agency. 2011b. Exposure Factors Handbook: 2011 Edition. EPA/600/R-090/052F. Available at: Available from: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid =236252.
- [USEPA] United States Environmental Protection Agency. 2012. Benchmark Dose Technical Guidance. Risk Assessment Forum. United States Environmental Protection Agency. EPA/100/R-12/001.
- [USEPA] Untied States Environmental Protection Agency. 2016 Benchmark Dose Software (BMDS). Available at: https://www.epa.gov/bmds.
- [USFDA] United States Food and Drug Administration. 1966. Chronic oral toxicity study of Methyl Salicylate using dogs and reproductive and developmental effects oral study of Methyl Salicylate using rats. Studies dated May. Submitted by FDA in response to an FOI request – 1998; 33 pages (As cited in CIR 2003).
- Vane JR, Botting RM. 2003. The mechanism of action of aspirin. Thromb Res. 110:255–258.
- Verstraelen S, Nelissen I, Hooyberghs J, Witters H, Schoeters G, Van Cauwenberge P, Van Den Heuvel R. 2009a. Gene profiles of a human alveolar epithelial cell line after in vitro exposure to respiratory (non-)sensitizing chemicals: identification of discriminating genetic markers and pathway analysis. Toxicol Lett. 185:16–22.
- Verstraelen S, Nelissen I, Hooyberghs J, Witters H, Schoeters G, Van Cauwenberge P, Van Den Heuvel R. 2009b. Gene profiles of a human bronchial epithelial cell line after in vitro exposure to respiratory (non-)sensitizing chemicals: identification of discriminating genetic markers and pathway analysis. Toxicology. 255:151–159.
- Vigano G, Garagiola U, Gaspari F. 1991. Pharmacokinetic study of a new oral buffered acetylsalicylic acid (ASA) formulation in comparison with plain ASA in healthy volunteers. Int J Clin Pharm Res. 11:129–135.
- Warknay J, Takacas E. 1959. Experimental production of congenital malformations in rats by salicylate poisoning. Am J Pathol. 35:315–331.
- Webb WK, Hansen WH. 1963. Chronic and subacute toxicology and pathology of methyl salicylate in dogs, rats, and rabbits. Toxicol Appl Pharm. 5:576–587.
- Wolowich WR, Hadley CM, Kelley M, Walson P, Casavant M. 2003. Plasma salicylate from methyl salicylate cream compared to oil of wintergreen. J Toxicol-Clin Toxic. 41:355–358.
- Zhang Z, Jia C, Hu Y, Sun L, Jiao J, Zhao L, Zhu D, LI J, Tian Y, Bai H, et al. 2012. The estrogenic potential of salicylate esters and their possible risks in foods and cosmetics. Toxicol Lett. 209:146–153.
