Abstract
This paper summarizes current challenges, the potential use of novel scientific methodologies, and ways forward in the risk assessment and risk management of mixtures. Generally, methodologies to address mixtures have been agreed; however, there are still several data and methodological gaps to be addressed. New approach methodologies can support the filling of knowledge gaps on the toxicity and mode(s) of action of individual chemicals. (Bio)Monitoring, modeling, and better data sharing will support the derivation of more realistic co-exposure scenarios. As knowledge and data gaps often hamper an in-depth assessment of specific chemical mixtures, the option of taking account of possible mixture effects in single substance risk assessments is briefly discussed. To allow risk managers to take informed decisions, transparent documentation of assumptions and related uncertainties is recommended indicating the potential impact on the assessment. Considering the large number of possible combinations of chemicals in mixtures, prioritization is needed, so that actions first address mixtures of highest concern and chemicals that drive the mixture risk. As chemicals with different applications and regulated separately might lead to similar toxicological effects, it is important to consider chemical mixtures across legislative sectors.
1. Introduction
Every day humans and the environment are exposed to low levels of hundreds of different chemicals present, for example, in food, consumer products, air, and water. To reduce the risk that these chemicals might pose to public and environmental health, their introduction onto the European market is tightly regulated, which implies a risk assessment that considers the impact on environmental and human health.
Contrary to realistic co-exposure scenarios, current risk assessments are mostly based on single chemicals, even though several EU regulations nowadays include requirements that specifically refer to chemical mixtures (Kienzler et al. Citation2016).
Indeed, concerns about the combined effects of chemicals have been growing over the past decade (McCarty and Borgert Citation2006; Heys et al. Citation2016). This was acknowledged by the European Commission (EC) in its Communication on “Combined effects of chemicals – chemical mixtures” (EC Citation2012), which stated that: “Current EU legislation does not provide for a comprehensive and integrated assessment of cumulative effects of different chemicals taking into account different routes of exposure. In the case where a mixture of concern is identified and where such a mixture contains chemical substances regulated under different pieces of EU legislation, no mechanism currently exists for promoting an integrated and coordinated assessment across the different pieces of legislation.”
Many overarching documents are available providing an overview of methodology and terminology, relevant EU legislative requirements, related guidance documents and international frameworks (OECD Citation2018). These are not repeated here, but readers may refer to Kienzler et al. (Citation2014, Citation2016); Meek et al. (Citation2011); Kortenkamp et al. (Citation2009) for further background information on the assessment of chemical mixtures.
Due to the large number of chemicals in use, combined with the limited amount of information on their toxicity and their near infinite number of possible combinations, the assessment of mixtures remains challenging (Kortenkamp et al. Citation2009; SCHER/SCENIHR/SCCS Citation2012).
This paper summarizes recent developments, challenges, and possible ways forward not only in mixture risk assessment (MRA) but also the risk management of mixtures. Risk assessment of combined exposures to multiple chemicals is addressed for both humans and the environment. In many cases, the same issues arise in considering mixtures, but in some cases, there might be specific aspects to consider and different approaches might be applicable for human or environmental mixture risk assessment. If such differences and peculiarities have to be considered, they are specifically addressed and highlighted throughout the review.
The paper focuses on combined exposure to multiple chemicals, although non-chemical stressors, such as nutrition, socioeconomic factors, habitat status, are briefly addressed.
2. Addressing challenges in mixture hazard assessment
2.1. Challenges in the hazard assessment of mixtures
The hazard of chemical mixtures can be assessed by testing the whole mixture – bearing in mind that it is not feasible to test all possible combinations of chemicals in mixtures – or based on the concentration and effect information of the individual components of the mixture. One of the main challenges in the toxicological assessment of mixtures is the need to address data gaps: whole mixture effect data are available for a limited number of mixtures only, and single chemical hazard, dose–response, and Mode of Action (MoA) information needed in component-based approaches is often lacking for many chemical classes (Backhaus and Karlsson Citation2014; Evans et al. Citation2015; Bopp et al. Citation2016, Citation2018a).
Another major challenge concerns the possible interactions between chemicals (i.e. synergistic or antagonistic effects) and their impact on the hazard of the chemical mixture. Chemicals may influence the combined activity, for example, by having an effect on each other’s uptake, metabolism, excretion, or toxicodynamics. This can modify the magnitude and sometimes also the nature of the toxic effect, which can be defined as the influence of one chemical on the biological action of another (either qualitatively or quantitatively) (Binderup et al. Citation2003). Interactions are thus defined as deviations from expected (additive) combined effects, leading to effects that are more than additive, that is, synergistic, or less than additive, that is, antagonistic, (see e.g. Kortenkamp et al. Citation2009). Two main questions need to be clarified to decide on the approach for addressing potential interactions in MRA: (1) how relevant are interactions, that is, how frequently do they occur at relevant exposure concentrations, and to what extent do the effects deviate from additivity-based predictions; and (2) which mechanisms are involved in toxicological interactions?
Currently, the relevance of interactions at realistic exposure levels in humans and the environment is unclear. Several authors have reviewed the evidence of interaction in real life mixtures, and concluded that interactions at lower concentration levels such as environmental concentrations are rare and, if observed, lead to relatively small deviations from additivity-based predictions (Boobis et al. Citation2011; Cedergreen Citation2014). Where deviations from additivity-based predictions were observed, these were mostly within a factor of 4. A new systematic literature review on the topic is currently ongoing and will provide further clarification (Martin et al. Citation2018).
2.2. Novel scientific approaches and ways forward in mixture hazard assessment
2.2.1. Use of new approach methodologies (NAMs)
NAMs can help address the above-mentioned data gaps. At the screening or scoping phase of the assessment, conservative values like a Threshold of Toxicological Concern (TTC) for humans and ecoTTC for environmental assessments can be applied (Price et al. Citation2009; Mons et al. Citation2013; Kienzler et al. Citation2019), or the known toxicity values of one component might be used as an approximation of all similar compounds in a group (Meek et al. Citation2011). This can be refined by more compound-specific information, based on in silico, in vitro or where necessary in vivo toxicity studies (Altenburger et al. Citation2003, Citation2012; Bopp et al. Citation2015; Kim and Kim Citation2015).
Smart strategies are needed to assess many compounds in a fast and cost-effective manner. The focus should be on non-animal models and computational tools, sometimes referred to as NAMs, to assess potential hazards, exposure and eventually risk. The ways in which NAMs can support the assessment of chemical mixtures are summarized in .
Table 1. Potential use of NAMs to support the hazard and risk assessment of chemical mixtures.
2.2.2. Integrating different types of toxicity data and grouping
An increasingly popular way of integrating and interpreting hazard data is to use the Adverse Outcome Pathway (AOP) framework. This provides a means of capturing existing knowledge concerning the linkage via key events (KEs) between a molecular initiating event (MIE) and an adverse outcome (AO), at a level of biological organization relevant to hazard and/or risk assessment (Ankley et al. Citation2010; OECD Citation2013). Although it is important to understand the quantitative relationships between upstream KEs and the AO to reliably use AOPs to estimate risk, qualitative AOPs are already used to support the hazard assessment of single chemicals in a regulatory context (ECHA and EFSA Citation2018).
AOPs can form networks that encompass multiple chemicals and endpoints (), thus supporting the hazard assessment of chemical mixtures. AOP networks provide a framework for mapping toxicity data for individual chemicals, which allow identification of: (1) which chemicals might lead to combined effects/AOs, (2) where information is missing, and (3) further targeted testing needs. AOP networks can thus help in integrating data from diverse types of testing (in vitro, in silico, and in vivo) and across different levels of biological organization, and thus support addressing gaps of toxicity data.
Figure 1. Mapping of different types of toxicity information for individual chemicals in a mixture onto AOP networks. This mapping can help identify (1) which chemicals might lead to combined effects/common adverse outcomes, (2) where information is missing, and (3) further targeted testing needs. MIE: molecular initiating event; KE: key event; AO: adverse outcome.
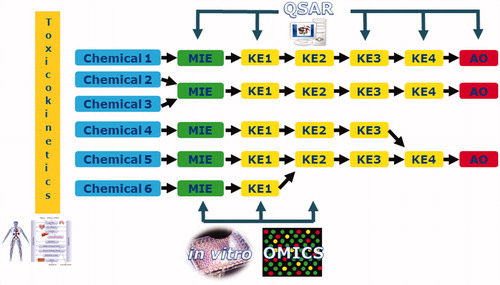
Furthermore, AOPs and their networks can be used for the grouping of chemicals according to their MoA, which is an important step in the assessment of mixtures (Bopp et al. Citation2015). Mixture components can affect pathways that converge at common intermediate steps in several ways, as illustrated by Ankley et al. (Citation2010). In a conservative approach, that is, at a screening level, the assumption that all mixture components will contribute to a combined effect can be applied (Meek et al. Citation2011). This grouping can be refined, for example, based on their MoA, chemical structure (for which a similar toxicity profile is to be expected), common target organ or AO (OECD Citation2018). At higher tiers, also toxicokinetic (TK) modeling could help in the grouping of components based on common target organs that the mixture components might reach.
Grouping rationales for assessing combined effects of chemicals can also be based on structural and physicochemical similarities, or the type of product/use, for example, in formulations, or exposure scenario, for example, co-emission from the same source or targeting similar population groups.
While human MRA aims to protect the individual, environmental MRA is performed to protect a wide range of environmental organisms, with the protection goal being the protection of populations rather than each individual (e.g. Nienstedt et al. Citation2012; Brown et al. Citation2017). This is achieved by investigating ecotoxicity to representative species, and risk assessment is then based on the most sensitive species. This is more challenging in the context of chemical mixtures, as different species show different sensitivities to the various chemicals in the mixture, and chemicals may have different MoAs in different species. When performing environmental MRA, it is, therefore, more relevant to look into effects on different trophic levels rather than looking into specific MoAs, although both might be closely linked, that is, a specific MOA will often target a specific trophic level.
2.2.3. Addressing the potential for interactions
In most regulatory frameworks where MRA is used, the concept of dose or concentration addition (CA) is applied as a default approach and assumed to adequately cover the risks (Kienzler et al. Citation2016). Interactions are considered on a case by case basis (Bopp et al. Citation2015).
A number of guidance documents (e.g. ECHA Citation2017a, Citation2017b; ATSDR Citation2018) include guidance on evidence that needs to be provided or on methods to address possible chemical interactions. The most commonly used prediction methods are based on the interaction-based Hazard Index (HIint) and the Combination Index. Despite the different approaches used, a final optimal approach has not been found yet (Sarigiannis and Hansen Citation2012; Rodea-Palomares et al. Citation2015). Prediction methods such as the HIint usually require a lot of information that is lacking for most compounds and their combinations; and even where available, the predictions are derived for binary combinations only.
The three non-food scientific committees of the European Commission (SCHER/SCENIHR/SCCS Citation2012) proposed that the potential for toxicologically significant synergistic effects should be considered in the cases where one compound can significantly change the uptake, metabolism or excretion rate of the other, or by targeting different aspects of cellular protections or repair mechanisms.
Most interactions observed so far are due to TK interactions, as shown for pesticides (Cedergreen Citation2014; Hernández et al. Citation2017). By investigating further the particular mechanisms underlying known effects, for example, via AOP networks, it might be possible to highlight mechanisms that could lead to synergisms. Furthermore, TK modeling for mixtures might help predict related interactions as described in Section 3.2.1 (Desalegn et al. Citation2019).
3. Addressing challenges in mixture exposure assessment
3.1. Challenges in the exposure assessment of mixtures
The challenge of assessing realistic co-exposure to multiple chemicals from single or multiple sources lies in taking into account multiple components, pathways, and routes of exposure. It should at least focus on the relevant target population(s), the timing of exposure, and the magnitude, sources and routes of exposure, along with the chemicals involved (Price et al. Citation2012a). The timing of exposure in terms of duration and frequency is an important factor in this evaluation, as some exposures might be separated in time, but could still lead to co-exposure, in particular for more persistent chemicals that bioaccumulate. Another major uncertainty is that the exposure to some chemicals might be missed, that is, the real (co)exposure situation is not completely known.
3.1.1. Simultaneous versus sequential exposure to multiple chemicals
As exposure to chemicals in the environment is often episodic and repeated, organisms can be exposed to toxicants simultaneously and/or sequentially. Thus, co-exposure may be sequential, and it has been shown that even the sequence of exposure can modify the toxicity observed (Ashauer et al. Citation2017). This implies that temporal aspects of exposure also need to be taken into account.
Generally, combined effects of chemicals can occur in a mixture even when they are present at concentrations where the individual chemicals show no effects (e.g. Silva et al. Citation2002). In addition, combined effects from simultaneous exposure to multiple chemicals at human-relevant levels were demonstrated (Hadrup et al. Citation2016). Moreover, possible combined effects from sequential exposure to multiple chemicals should not be overlooked considering also chemicals with dissimilar effects, for example, if chemicals act on pathways involved in carcinogenesis (Hanahan and Weinberg Citation2000, Citation2011). They might individually not be carcinogenic but may be capable of producing carcinogenic synergies over a lifetime, which might be missed using single substance risk assessment practices or standard co-exposure considerations (Goodson et al. Citation2015).
3.1.2. Critical time-windows
Another difficulty is that exposure at a given developmental life stage can have consequences for an organism later in life. Especially when exposure occurs prenatally during organogenesis, systems are particularly sensitive to disturbances. Prenatal exposure has been linked to many long-term adverse effects later in life, for example, following exposure to persistent organochlorine chemicals (Kristensen et al. Citation2016), phthalates and BPA (Ferguson et al. Citation2014). Observations from human epidemiological studies also emphasize the need to consider the critical windows of exposure. However, because these critical windows might be as narrow as specific trimesters during pregnancy (Swan et al. Citation2015; Martino-Andrade et al. Citation2016), they can be very difficult to identify.
3.1.3. Internal vs external co-exposure over time
While most MRAs focus on the external co-exposure composition, an additional layer of complexity is added by the fact that external and internal exposure can differ, both in terms of time patterns and concentrations. In addition to the external dose, the internal concentrations depend on the TK properties of the individual compounds, which determine whether a chemical or its metabolites reach a specific target organ at a relevant concentration and over a sufficient duration to produce an adverse effect.
As the TK properties of each of the constituents of the mixture might be different, an external sequential co-exposure can result in an internal simultaneous co-exposure (and vice versa), on the short to medium term, that is, a few days. On a longer timescale, exposure to bioaccumulative and persistent compounds, such as persistent organic pollutants (POPs), is particularly relevant, as they can remain in an organism even after cessation of (external) exposure, increasing the potential for (internal) co-exposure. Combined exposures to such compounds could be the result of exposures that occur over decades.
Gaps in exposure data are of similar concern as for toxicity data. Monitoring activities can capture occurrence data, however, they typically cover only a limited number of chemicals. The selection of chemicals is often driven by the identification of substances of specific concern in a specific location or based on specific legislation, for example, priority pollutants under the EU Water Framework Directive. Overlooking chemicals that are not analyzed or present at concentrations below the detection limit can lead to underestimations of mixture exposure and risk. Complementary use of monitoring data and modeling approaches is therefore needed.
3.2. Novel scientific approaches and ways forward in mixture exposure assessment
3.2.1. Complementary use of monitoring and modeling
Although the majority of chemicals lack (bio)monitoring data, the use of such data provide a realistic picture of co-exposure in a specific site or medium. Monitoring data are further used to identify temporal and spatial trends in exposure in longer-term monitoring programs. However, there are some limitations. The limits of detection (LOD) and quantification (LOQ) of the analytical methods used for monitoring will influence the results, as a chemical present below its LOD or LOQ will not be accurately quantified. One way to tackle this issue is to consider them present at their LOD in a conservative lower tier which can be refined by a fraction of the LOD at higher tiers. Examples can be found in Price et al. (Citation2012b) for human and environmental assessments from surface water and waste water effluents exposure, Gustavsson et al. (Citation2017) for environmental assessments of pesticides in rivers, and Reyes and Price (Citation2018) for assessment of phthalates based on human biomonitoring (HBM) data. Moreover, the selected chemicals that are monitored in targeted analyses will focus a MRA on those chemicals suspected to be relevant or that can be measured. Untargeted chemical analyses might help to identify other relevant chemicals. Modeling chemical exposure based on more generically available information might also help to prioritize which chemicals to further investigate.
Where monitoring data are lacking, modeling opens additional opportunities. Especially in component-based approaches, modeled exposure concentrations can be helpful to delineate places and times where combined exposures may become relevant and should be managed to reduce risks. With a limited amount of information, conservative estimates of exposure can be predicted, for example, based on production volumes, sales information, number of users, and physicochemical properties. The assessment can then be refined based on available input data, from a combination of measured and modeled data using default values and conservative scenarios, to more sophisticated tailored scenarios incorporating more measured data, up to probabilistic exposure assessments (see tiered WHO/IPCS framework, Meek et al. Citation2011). Models can be used at different levels, allowing a balance to be found between accuracy of the predictions and spatial resolution considering available input information (e.g. Lindim et al. Citation2016; Brack et al. Citation2017; Oldenkamp et al. Citation2018). The use of monitoring and modeling is complementary as modeling can also improve the design of monitoring campaigns in terms of time and space. Monitoring data can be used for retrospective assessments or to evaluate the appropriateness of models used in prospective assessments.
TK information and modeling can further support the assessment of chemical mixtures with the main areas of application being: (1) determination of internal exposure concentrations, for example, enabling a relation between body concentrations and in vitro experiments (i.e. in vitro to in vivo extrapolations; IVIVE), of relevance for single chemicals as well as for chemical mixtures, (2) assessment of the simultaneous or sequential exposure to different mixture components, considering the probability that those reach the same target at the same time, (3) prediction of TK interactions among mixture components, and (4) use of physiologically based kinetic (PBK) modeling to interpret HBM data in toxicological risk assessment.
3.2.2. Better data sharing
The limited understanding of exposure to chemical mixtures was also recognized in the Commission Communication on chemical mixtures (EC Citation2012), which reports the need to promote “a more coherent approach to the generation, collection, storage and use of chemical monitoring data in relation to humans and the environment.” For this purpose, IPCHEM was created (http://ipchem.jrc.ec.europa.eu/), which represents the European Commission’s reference platform for chemical monitoring data collected across various media (environment, food and feed, humans, consumer products, and indoor air) by the European Commission bodies, EU Member States, international and national organizations, and research communities. The Platform supports a coordinated approach for collecting, storing, accessing, and comparing data related to the occurrence of chemicals, and their metabolites, in relation to humans and the environment. The use and further development of IPCHEM for assessing chemical mixtures and supporting MRA were discussed at an expert workshop in 2017 (Dalla Costa et al. Citation2018; Bopp et al. Citation2018a).
3.2.3. Aggregate exposure pathway (AEP) framework
While there is a lot of information on exposure potentially available in the scientific literature, a structured way to collect exposure information at different levels is missing. Analogous to the AOP concept, the AEP framework has been proposed to collect all relevant information of environmental fate and exposure of chemicals and stressors through the environment up to the individual or population (Teeguarden et al. Citation2016; Hines et al. Citation2018). It is specifically designed for organizing exposure data from multiple sources, enabling that information is readily available, and highlighting data gaps and uncertainties (Hines et al. Citation2018).
The AEP starts with the chemical(s) release into the environment from a source up to the Target Site Exposures (TSEs). The AEP consists of two entities: a Key Exposure State that describes the amount of a stressor at a given location and time, and a Key Transitional Relationship that describes either the transport of that stressor to a different location or the transformation of that stressor into a different molecule (Hines et al. Citation2018). Individual AEPs can be assembled into AEP networks to characterize the movement of multiple chemicals and transformations from one chemical to another (Hines et al. Citation2018). The TSE of the AEP corresponds to the same level of biological organization as the MIE of the AOP, thereby allowing, in theory, the prediction of the expected perturbation of the AOP based on in silico or in vitro dose–response information and to integrate exposure to dose-response data across multiple endpoints in multiple taxa.
The joint AEP–AOP framework could be used to put into context exposure models, biokinetic and dynamic modeling, the results from monitoring, biomonitoring, and in vitro testing. The framework could in the future integrate a range of computational models, such as multimedia fate models, PBK models, in vitro fate mathematical models and dynamic models. Examples of relevant available exposure models and TK models are provided in Table S1 in the Supplementary Information. To date, the new AEP approach has not gained broad acceptance by the regulatory community, but remains a promising scientific framework.
4. Addressing challenges in mixture risk assessment
4.1. Challenges in mixture risk assessment
4.1.1. Matching toxicity and exposure information in component-based approaches
In order to derive appropriate estimates of risks from chemical mixtures, the exposure and hazard assessment data need to be properly matched. For this purpose, it is important to consider the time frame and routes of exposure in the real exposure situation compared to the conditions used for the toxicity testing and hazard assessment. Typically, multiple exposure routes need to be combined, such as dermal, oral, and inhalation.
There are different methods to predict mixture risks from the mixture components. CA based concepts are frequently applied by default in the regulatory context. One major distinction is whether predictions are based on toxicological reference values that already incorporate assessment factors or whether predictions are based on points of departure (PODs). In the first case, the sum of risk quotients such as a Hazard Index can be calculated using acceptable or tolerable daily intake values for human health, or Predicted No Effect Concentrations (PNECs) for environmental assessment. In this case, the toxicological reference values are based on the lowest PODs for the critical effect for each chemical. This critical effect might differ among the mixture components, for example, could be based on neurotoxicity for one chemical and on liver toxicity for another chemical in the mixture, meaning that the reference values would not necessarily be based on a common effect. Especially in the context of environmental risk assessment, the use of PNECs might be critical, since the assessment factors applied vary significantly depending on the underlying data used to derive them and might make them incomparable. Using toxicological reference values or PNECs might be a good way for lower tier level assessments, which can then be refined by more endpoint-specific information considering different assessment factors. This was demonstrated, for example, for environmental MRA in Backhaus and Faust (Citation2012) and proposed in general in the WHO framework (Meek et al. Citation2011). In higher tier assessments, risk quotients can be based on toxicity values such as No Observed Adverse Effect Levels, Benchmark dose, or Effective Concentrations such as EC10. Using values based on the same type of effect, risk quotients and their sum, such as a Point of Departure Index, can be calculated for the mixture.
4.1.2. Considering other stressors to link to health and environmental effects
The ultimate goal of risk assessment is to predict or estimate the impact of a range of stressors on environmental or human health. For chemical stressors, this is based on information on toxicity and exposure of certain chemicals, often limited to historical contaminants. However, the compounds under investigation might not necessarily be the cause of biological effects and the stress of chemicals can be enhanced by non-chemical stressors (see e.g. Johnson and Sumpter Citation2016; Burton Citation2017). Where applicable, the influence of other factors such as dietary status, habitat conditions, etc. might be considered. It should be noted that there are often difficulties in linking effects from exposure to chemicals or chemical mixtures to human health or ecosystem effects when excluding non-chemical stressors.
For human health, the main limitations and difficulties in linking between (combined) chemical exposure and health effects are often difficulties in interpreting epidemiological studies due to, for example, sample size or the number of chemicals, confounding by socioeconomic factors or nutrition status etc. (Carpenter et al. Citation2002; Braun et al. Citation2016).
For ecosystem health, the main limitations and difficulties in linking between (combined) chemical exposure and ecosystem health are the focus on historical contaminants and often neglect of emerging pollutants and the relevance of non-chemical stress factors such as food availability, weather and climate conditions, changes in the morphology of the ecosystem such as hydromorphology, etc (Burkhardt-Holm et al. Citation2005).
4.1.3. Prioritizing mixtures and drivers
Because of the near infinite number of possible combinations of chemicals, and limited resources to assess them, there is a clear need to identify and prioritize mixtures of concern, in order to effectively act on them. Several criteria have been suggested to identify priority mixtures, focusing on chemicals that: (1) are expected to be present at concentrations close to their effect level, (2) are thought to act on the same pathway, (3) are assumed to have no threshold, or (4) are considered to be very persistent (SCHER/SCENIHR/SCCS Citation2012). More concrete methodology for prioritizing mixtures based on hazard, exposure and risks are needed, to risk manage mixtures of concern in a timely manner.
4.1.4. Uncertainties
The identification, characterization, and transparent documentation of uncertainties encountered in the different steps of hazard/risk assessment, including gaps in data and knowledge and assumptions made, is important in order to evaluate their impact on the assessment outcome, make informed decisions, and allow appropriate risk management measures (US EPA Citation2014; WHO/IPCS Citation2017; OECD Citation2018; EFSA Citation2018a, Citation2018b, Citation2018c, Citation2019). Compared with single chemical assessments, this is particularly relevant in the context of mixtures, as MRA often involves more assumptions, and uncertainties around single chemical assessments might add up. Therefore, uncertainties in MRA need to be evaluated both for the individual mixture compounds and the mixture as a whole (SCHER/SCENIHR/SCCS Citation2012).
4.2. Novel scientific approaches and ways forward in mixture risk assessment
4.2.1. Better reporting of toxicity studies
To facilitate the prediction of combined effects and risks using component-based approaches, a simple lower tier approach can be to use reference values based on most sensitive adverse effects. However, in order to refine the assessment in higher tiers, toxicities of similar types need to be considered. Such data are often captured in toxicity studies, but databases often report only the driving/most sensitive effect and the additional information on other types of effect is difficult to find. A more detailed and comprehensive reporting of toxicity study data would greatly facilitate the refinement of risk assessment in component-based approaches.
4.2.2. Addressing non-chemical stressors
The link between chemical exposure and human or ecosystem health status is often difficult to establish because of other non-chemical stress factors. One way to take a more holistic approach is to use exposome studies that look at a wider range of exogenous and endogenous chemicals over longer periods. Examples include an application to urban populations (Andrianou and Makris Citation2018) and the linking between mechanistic information using the AOP concept and the (eco)exposome (Escher et al. Citation2017). The interplay with non-chemical stressors has been shown for combined stress resulting from physical stress (such as sunlight, heat, radiation, infectious disease, and noise) and chemical stress in Rider et al. (Citation2014).
The RISK21 framework (Moretto et al. Citation2017) shows one way of considering non-chemical modulating factors (ModF), which might influence the risk posed by the chemical stressors, in a risk assessment. First, a risk assessment for the chemical combined exposure is performed, then non-chemical ModF are addressed subsequently where needed. This is first done using reporting tables addressing for each ModF the impact on either exposure or toxicity, the strength, and direction of the impact and the strength of the evidence.
4.2.3. Prioritizing mixtures and identifying drivers of mixture risk
Prioritizing mixtures: Several priority setting exercises have been suggested for single chemicals for a variety of matrices or exposure pathways, for example, air pollutants (EEA Citation2017) or European river basin pollutants (Malaj et al. Citation2014). The methodology usually assesses the frequency of exceedances of safety thresholds in time and space as well as looking at the degree of exceedance. Other approaches focus on identifying prevalent combinations of chemicals by statistical analysis of co-exposure patterns (Kapraun et al. Citation2017). Considering use-dependent mixture exposures (modeling exposure concentrations, for example, based on agricultural, domestic and urban run-off scenarios) was suggested as a means of prioritizing environmental MRA in river catchments (Posthuma et al. 2018). Key patterns will arise from different land uses which can help identify typical chemical fingerprints and exposure time aspects. Such simplified signatures can be used when setting priorities. Nevertheless, even in land-use based approaches highly variable mixture compositions can occur in time and space; thus over-interpretation of lower tier methods needs to be avoided (Posthuma et al. 2018).
The majority of approaches that have been suggested are still retrospective, based on monitoring data (environmental chemical monitoring, wildlife or HBM), which automatically creates the limitation that it can only focus on chemicals chosen to be analyzed or able to be detected. As a consequence, only a small part of the overall chemicals will be addressed, and chemicals that were not included in the analysis will not be identified. Prospective approaches including exposure modeling can help identify priority mixtures at an earlier stage.
Identifying drivers of risk: As the risk is a combination of exposure and toxicity, both have to be considered to identify which compounds and what effect(s) are driving the risk. Whatever the goal of the risk assessment, it is important to keep in mind that these drivers might not be constant and can be, for example, site-specific, endpoint-specific, species-specific, or life-stage specific. Which drivers and effects to focus on might depend on previously defined prioritization aims.
The total toxicity can be estimated based on the compounds identified in a mixture, relying mostly on CA models. By comparing the contribution of the individual compounds to the overall effect, the main drivers of the risk can be identified. The currently often used Maximum Cumulative Ratio approach (MCR; Price and Han Citation2011; Price et al. Citation2012a) calculates the ratio of the overall sum of risk quotients to the risk quotient of the compound with the highest risk quotient. The lower the MCR, the lower the number of compounds that are significantly contributing to the overall risk. Using the MCR, the “nature of concern” of a mixture and related risk management actions can be decided on (Section 5.1). A review of case studies (Bopp et al. Citation2016) showed that the MCR was usually ranging between 1.2 and 5.8, depending on the number of analyzed compounds (Han and Price Citation2011; Backhaus and Karlsson Citation2014; De Brouwere et al. Citation2014; Price et al. Citation2014), that is, the number of chemicals that drive the mixture risk is usually low. In all the examples, the MCR decreased with increasing HI, indicating that the higher the predicted risk, the lower the number of substantially contributing chemicals. However, the overall assessment relies on the knowledge of the identity of the compounds and their contribution to the overall toxicity. In addition, these assessments usually ignore any potential for interaction.
Another option to determine the drivers of risk is top–down, that is, determine the overall toxicity of a mixture and then determine which compounds are present in the mixture that would explain the observed toxicity, for example, by Toxicity Identification and Evaluation (TIE) or Effect Directed Analysis (EDA). Both TIE and EDA can be used to determine whether the individual component can explain the (majority) of the toxicity observed for the more complex sample, or that additional compounds (not yet analyzed) are likely to contribute (Burgess et al. Citation2013). While for the whole organisms in TIE both independent action and CA approaches are used (as it frequently involves more complex models, e.g. Daphnia), in EDA most models for toxicity are based on CA as they usually focus on specific and relatively upstream events in the AOP by using, for example, in vitro assays (Brack et al. Citation2016). To ensure that deviations from the observed toxicity and the calculated toxicity are not due to (unknown) interaction factors or compounds lost during the refinement, reconstituted mixtures can be tested and compared to the calculated toxicity. While most TIE and EDA studies focus on a specific site and/or sample, a special case or combination can be made for “virtual EDA,” combining the overall effects and chemical analyses for several sites.
Thus, methods for identifying drivers of the mixture risk are available, but they require a lot of information on individual components or the whole mixture.
4.2.4. Tackling uncertainties in mixture risk assessment
For MRA, uncertainties can be related to the combined exposure, the chemical effects or specifically the grouping of chemicals for consideration in MRA. Examples of uncertainties and their possible impact on the risk assessment (over- or underestimation of the risk) are listed in .
Table 2. Uncertainties related to mixture risk assessment.
Overall, the main uncertainties concern the limited knowledge on what constitutes the combined exposure (chemicals, routes, concentrations, and timeframe), whether chemicals are overlooked in the assessment, lack of knowledge on the hazard/modes of action and potential interactions of the mixture components and thus specific mixture effects.
Frameworks proposed for MRA, such as the tiered approach by WHO IPCS (Meek et al. Citation2011) and the derived Cefic Mixtures Industry Ad-hoc Team (MIAT) decision tree (Price et al. Citation2012a) take account of uncertainties. Both OECD (Citation2018) and EFSA (Citation2018c) have developed overarching frameworks for mixture risk assessment including uncertainty considerations. So far, a comprehensive framework for characterizing uncertainties specifically related to the assessment of combined exposure to multiple chemicals does not exist.
The ECHA Read-Across Assessment Framework considerations on multi-constituent substances and substances of unknown or variable composition, complex reaction products or biological materials (UVCBs) (ECHA Citation2017c) describe uncertainties related to the application of read-across (Section 2.2.1) to intentional mixtures. The uncertainties concern the definition of the different mixture constituents and their respective concentrations, which would be required, still leaving uncertainty about potential interactions, preventing a definitive conclusion to be made. Furthermore, variability in the concentrations (changing the assumed dose–response of the chemicals) and the presence of unknown components add to the overall uncertainty.
5. Addressing challenges in the risk management of mixtures
5.1. Challenges in the risk management
5.1.1. The nature of the mixture concern
If a concern is identified from a combined exposure to multiple chemicals, the nature of the concern can be different. The overall risk might be driven by (1) chemicals that individually exceed safe levels, (2) a limited number of chemicals that contribute the biggest proportion to the overall risk, or (3) many components contributing each only a small fraction to the combined effect and overall risk. Depending on the scenario, different risk management options might be appropriate:
Individual chemicals exceed safe levels. Does not need to be addressed specifically in the context of mixtures, as those should be tackled appropriately by the usual single substance legislative measures.
Few compounds driving the risk. Risk management and mitigation actions in mixtures where clear drivers of the overall risk can be identified can focus on the few chemicals that are responsible for the majority of the risk in a similar way as for individual chemical management.
Many compounds contributing to the risk. If the overall risk is a true mixture problem, that is, is caused by a larger number of chemicals that are individually present at levels far from raising a concern, the management options are most difficult. Many actors, across different sectors, might be involved (such as chemical producers) and scenario dependent decisions on appropriate measures will be needed.
Several studies show that usually risks from chemical mixtures are driven by a limited number of mixture components. Backhaus and Karlsson (Citation2014) investigated environmental risks from pharmaceuticals detected in waste water treatment plant effluents across Europe and identified that at maximum 10 compounds usually explained more than 95% of the overall risk. Posthuma et al. (Citation2016) found for Dutch rivers that 5–10 compounds are usually responsible for nearly the whole toxic pressure. The compounds driving the risk from the chemical mixtures might differ depending on the specific site/mixture composition, but the number of compounds responsible for the biggest fraction of the mixture effect seems to be always in the same range.
5.1.2. Differences in the way mixtures are addressed in different sectors
Under different pieces of chemical legislations across different sectors, there are different requirements and ways to address co-exposure and mixtures (Kienzler et al. Citation2014).
For example, for human health, the risk assessment of pharmaceuticals (Directive 2001/83/EC) requires an in-depth assessment of the potential interactions with other pharmaceuticals, dietary components or tobacco where co-exposure can be expected. For pesticide residues in food and feed (Regulation (EC) No 396/2005), co-exposure to various pesticide active substance residues via the diet specifically is taken into account in setting maximum residue levels. The methodology to group pesticides with common effects is currently being developed (EFSA Citation2014), but it does not (yet) take into account potential co-exposure via other (non-dietary) sources.
However, a comprehensive consideration of co-exposure is rather the exception than the rule in the current risk assessment processes, and is always restricted in time and in terms of types of chemicals to be considered, within a given regulatory framework. Usually only simultaneous co-exposure is taken into consideration and the exposure to (environmental) coincidental mixtures are not addressed. And while the environmental fate of pollutants such as pesticide or biocides active substances is modeled prospectively over several years, the final risk assessment is usually based on the individual active chemical without considering other chemicals concomitantly present in the media.
If mixtures are considered, in most cases it is required to assess a specific group of chemicals together using usually very similar, CA-based prediction methods. For component-based approaches, first of all it is important to decide on the scope of the MRA and the relevant chemicals to be included and the grouping rationale, if applicable. Moreover, related to risk management, it is important to take into account the limitations in chemicals co-exposure information. If an assessment is based on analytical measurements of exposure levels for the mixture components, there might be compounds that are below the LOD. A decision has to be made on how to deal with those chemicals, that is, to consider them as absent, as present at the LOD, or at a concentration in between, as outlined in Section 3.2.1.
In addition, the way for expressing risk needs to be agreed. This can be done using a simple Hazard Index like approach, where a value >1 would indicate a potential risk, in the same way as a Risk Quotient >1 would indicate a potential risk for single chemicals. In the case of a Hazard Index, a safety factor as foreseen in the respective legislative sector is already included. Another approach is to calculate a combined Margin of Exposure (cMoE) for such groups. Here, exposure levels are compared to the toxicity values and the margin between both is calculated. The choice of acceptable margins can be decided depending on the case or legislation specific context. An example is the application in the context of pesticide cumulative risk assessment, where a probabilistic assessment can be performed. A decision has to be taken on the Xth percentile of the population that should have a cMoE above a specific value Y.
The different uncertainties in the MRA, as described in Section 4.3, need to be identified, characterized, and transparently documented, in order to enable the risk manager to make informed decisions on appropriate risk management measures.
5.2. Novel scientific approaches and ways forward in mixture risk management
5.2.1. Mixture assessment factors
One way to address the uncertainties and data gaps regarding the mixture composition, nature of hazard, and co-exposure is to apply an additional safety factor [Mixture Assessment Factors (MAF)] in a single chemical risk assessment. This is supposed to cover generically for co-exposures without performing mixture specific assessments. Therefore, MAFs are often considered an easily applicable approach to protect from combined effects without the need to know all details by lowering the overall exposure levels.
The difficulty in using MAFs is the selection of an appropriate value. Due to the large differences in mixture composition and exposure patterns, it is difficult to derive generic MAFs that are neither overly conservative nor insufficiently protective. The use of a MAF has been investigated in particular in the context of environmental risk assessment. The Swedish Chemicals Agency (Kemi Citation2015) investigated possibilities to develop a protective MAF concept to cover the most important uncertainties of composition, hazard, and interaction. It was observed that single substance risk assessment and risk management and mitigation significantly lowered the risk of the mixture, although was insufficient to ensure protection against mixture effects. The MCR (Price and Han Citation2011) resulted as an adequate approximation for a MAF, which ranged from 2 to 17 in the investigated examples, highlighting the need to consider specific exposure scenarios. Looking at smaller areas, the number of chemicals that might (co)occur will be lower than when looking at larger scales where more chemicals will be in use (van Broekhuizen et al. Citation2016). Furthermore, the types of chemicals being most relevant contributors to mixture risks will differ across sites. The identification of land-use specific mixture patterns could support the derivation of area-specific MAFs if environmental risk assessment for a specific location needs to be performed.
5.2.2. Regulation of chemical mixtures across sectors?
The current chemicals legislative framework focuses on single substances in isolated sectors (Kienzler et al. Citation2014, Citation2016; Rotter et al. Citation2018). If mixtures are covered, they are mostly addressed within a specific regulatory sector (e.g. mixtures of pesticides, biocides, food additives, etc.). However, as humans and the environment are in reality exposed to chemicals via many different routes and sources, co-exposure to chemicals regulated under different sectorial legislations does occur, as can be confirmed by human and environmental (bio)monitoring data (e.g. available via IPCHEM). As illustrated in , there might be a concern regarding combined effects, even if the individual chemicals are present at safe levels of exposure. Such combined effects are not covered by single substance risk assessment and if mixture risk assessment is carried out only within specific sectors (Evans et al. Citation2016). Additional difficulties for regulation lie in the often unknown nature of the mixture or exposure pattern variations over time. New mixture case studies considering cross-sectorial mixtures for both humans based on HBM data and for the environment could help to further characterize the extent of the issue (Bopp et al. Citation2018a).
Figure 2. Mapping of chemicals and their mixtures to the risks they pose for various toxicological effects. For each chemical (1–9) the individual Risk Quotients are presented for different types of effect (effect 1-X, e.g. hepatotoxicity, neurotoxicity, etc.). Chemicals are grouped according to the legislative sector they are regulated under (e.g. REACH, pesticides, cosmetics, food contaminants, etc.). The Sum of Risk Quotients is illustrated for mixtures within each sector and in the last column for the cross-sectorial mixture.
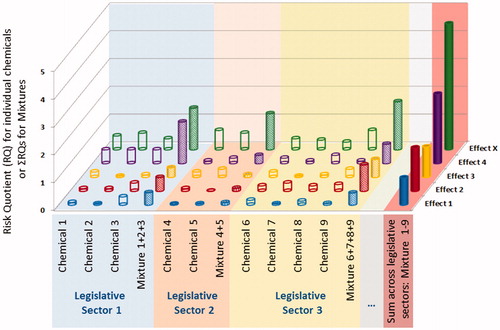
If a concern is identified from such cross-sectorial assessments, the important risk management question arises how to limit exposure: on which sector or even which chemical/source to impose restrictions, especially if single chemicals are complying with their individual safety thresholds. It would be difficult to take these risk management decisions, with several actors involved across different sectors and different legislations. A holistic approach and harmonization of MRA across sectors are needed (Kortenkamp and Faust Citation2018). Many of the approaches proposed within one sector could be applied in a similar way in other sectors.
6. Conclusions
As identified in the Commission Communication on chemical mixtures (EC Citation2012), there are several limitations or gaps that need to be addressed: (1) improved understanding of exposure to chemical mixtures, for both human populations and the environment, (2) improved understanding of toxicology of single substances and their mixtures, their modes of action, potential interactions, and (3) identification of mixtures that need to be tackled with priority (see also Bopp et al. Citation2018b).
The methodology of component-based approaches to predict combined effects and risks from information on the mixture composition (mixture components, their concentrations, their toxicological properties, and information on their modes of action) is well developed and is already applied in several pieces of EU chemicals legislation. In addition, several risk assessment frameworks have been developed [such as WHO/IPCS framework of 2009 (Meek et al. Citation2011), Price et al. decision tree of 2012] and serve as a valuable basis for building tailored schemes for specific purposes.
However, component-based approaches can potentially lead to underestimations of risk when the composition of a mixture is not fully known, which is usually the case, except for clearly defined, intentionally manufactured products (Tang et al. Citation2014; Neale et al. Citation2015). Moreover, it is important to keep in mind that the compounds under investigation might not necessarily be the (sole) cause of biological effects and the stress of chemicals can be enhanced by non-chemical stressors (e.g. Johnson and Sumpter Citation2016; Burton Citation2017).
Data gaps are an issue for both hazard and exposure assessment. Some of the hazard assessment challenges can be addressed by using NAMs, which enable a mechanistic understanding of the (combined) effects and facilitate the grouping in component-based approaches. Mechanistic understanding and TK modeling can help to assess the potential for interactions, in particular, synergistic effects. Whole mixture approaches can partly overcome issues with unknown mixture composition and unknown potential for interactions, as they measure directly the combined effect of the complete mixture. Therefore, whole mixture approaches, and related effect-based methods, are increasingly being used in situations of unknown and varying composition, such as surface and waste water (Brack et al. Citation2017; Napierska et al. Citation2018). There is an ongoing discussion whether the setting of thresholds, such as environmental quality standards could or should be effect-based (Escher et al. Citation2015, Citation2018). However, with the increasing number of effect-based methods, it is important to identify how these tests can be used to complement chemical monitoring without increasing the overall monitoring burden.
Exposure data gaps can be addressed by the complementary use of prospective modeling and retrospective monitoring approaches. In general, more open data sharing will further improve exposure and hazard assessment. TK related modeling can support extrapolations between species and exposure routes, while also simulating the timing of environmental exposure(s) and matching the timing to the response of interest.
In addition to scientific challenges in the hazard, exposure, and risk assessment of mixtures, one major challenge is to develop appropriate risk management measures. If a particular mixture raises a concern, restrictions may need to be placed on chemicals that individually do not pose a risk. Addressing these challenges might require a complete rethink of the way in which the risk assessment and management of chemical mixtures is currently performed.
Declaration of interest
All coauthors are employed by the European Commission Joint Research Centre. One coauthor (Sander van der Linden) has recently changed to work for ECHA (European Chemicals Agency) on 1 August 2018, but was working for JRC for the entire drafting period. The manuscript resulted from an institutional project financed under the institutional JRC work program. Therefore, we have no financial or non-financial competing interests to declare. No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental material for this article is available online here.
Table_S1.docx
Download MS Word (34.9 KB)Acknowledgements
The authors gratefully acknowledge three anonymous reviewers for their helpful comments and suggestions on the manuscript.
References
- Altenburger R, Backhaus T, Boedeker W, Faust M, Scholze M. 2013. Simplifying complexity: mixture toxicity assessment in the last 20 years. Environ Toxicol Chem. 32:1685–1687.
- Altenburger R, Nendza M, Schüürmann G. 2003. Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ Toxicol Chem. 22:1900–1915.
- Altenburger R, Scholz S, Schmitt-Jansen M, Busch W, Escher BI. 2012. Mixture toxicity revisited from a toxicogenomic perspective. Environ Sci Technol. 46:2508–2522.
- Andrianou XD, Makris KC. 2018. The framework of urban exposome: application of the exposome concept in urban health studies. Sci Tot Environ. 636:963–967.
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 29:730–741.
- Ashauer R, O’Connor I, Escher BI. 2017. Toxic mixtures in time - the sequence makes the poison. Environ Sci Technol. 51:3084–3092.
- ATSDR (Agency for Toxic Substances and Disease Registry). 2018 Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/interactionprofiles/ipga.html.
- Backhaus T, Blanck H, Faust M. 2010. Hazard and risk assessment of chemical mixtures under REACH - state of the art, gaps and options for improvement. Swedish Chemicals Agency Report PM 3/10 1–91. Order No. 510968.
- Backhaus T, Faust M. 2012. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ Sci Technol. 46:2564–2573.
- Backhaus T, Karlsson M. 2014. Screening level mixture risk assessment ofpharmaceuticals in STP effluents. Water Res. 49:157–165.
- Binderup ML, Dalgaard M, Dragsted LO, Hossaini A, Ladefoged O, Lam HR, Larsen JC, Madsen C, Meyer O, Selzer Rasmussen E, et al. 2003. Combined Actions and Interactions of Chemicals in Mixtures-The Toxicological Effects of Exposure to Mixtures of Industrial and Environmental Chemicals, Fødevare Rapport 2003:12, 1st Edition, 1st Circulation, August 2003, Danish Veterinary and Food Administration, Søborg, Denmark.
- Boobis A, Budinsky R, Collie S, Crofton K, Embry M, Felter S, Hertzberg R, Kopp D, Mihlan G, Mumtaz M, et al. 2011. Critical analysis of literature on low-dose synergy for use in screening chemical mixtures for risk assessment. Crit Rev. Toxicol. 41(5):369–383.
- Bopp S, Berggren E, Kienzler A, van der Linden S, Worth A. 2015. Scientific methodologies for the assessment of combined effects of chemicals – a survey and literature review. JRC Technical Report. EUR 27471 EN, 64 pp. Publications Office of the European Union, Luxembourg. https://doi.org/10.2788/093511.
- Bopp SK, Kienzler A, van der Linden S, Lamon L, Paini A, Parissis N, Richarz A-N, Triebe J, Worth A. 2016. Review of case studies on the human and environmental risk assessment of chemical mixtures future needs. JRC Technical Report. EUR 27968 EN; 89 pp. Publications Office of the European Union, Luxembourg.
- Bopp SK, Barouki R, Brack W, Dalla Costa S, Dorne JLCM, Drakvik PE, Faust M, Karjalainen TK, Kephalopoulos S, van Klaveren J, et al. 2018a. Current EU research activities on combined exposure to multiple chemicals. Env Int. 120:544–562.
- Bopp S, Richarz A, Worth A, Berggren E, Whelan M. 2018b. Something from nothing? Ensuring the safety of chemical mixtures. Policy Brief. JRC Science for Policy Brief. 2pp. doi: 10.2760/618648 ISBN 978-92-79-86747-7.
- Brack W, Ait-Aissa S, Burgess RM, Busch W, Creusot N, Di Paolo C, Escher BI, Mark Hewitt L, Hilscherova K, Hollender J, et al. 2016. Effect-directed analysis supporting monitoring of aquatic environments — an in-depth overview. Sci Tot Environ. 544:1073–1118.
- Brack W, Dulio V, Ågerstrand M, Allan I, Altenburger R, Brinkmann M, Bunke D, Burgess RM, Cousins I, Escher BI, et al. 2017. Towards the review of the European Union Water Framework management of chemical contamination in European surface water resources. Sci Tot Environ.576:720–737.
- Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 124:A6–A9.
- Brown AR, Whale G, Jackson M, Marshall S, Hamer M, Solga A, Kabouw P, Galay-Burgos M, Woods R, Nadzialek S, et al. 2017. Toward the definition of specific protection goals for the environmental risk assessment of chemicals: a perspective on environmental regulation in Europe. Integr Environ Assess Manag. 13:17–37.
- Burgess RM, Ho KT, Brack W, Lamoree M. 2013. Effects-directed analysis (EDA) and toxicity identification evaluation (TIE): complementary but different approaches for diagnosing causes of environmental toxicity. Environ Toxicol Chem. 32:1935–1945.
- Burkhardt-Holm P, Giger W, Guttinger H, Ochsenbein U, Peter A, Scheurer A, Segner H, Staub E, Suter MJF. 2005. Where have all the fish gone? Environ Sci Technol. 39:441A–447A.
- Burton GA. 2017. The focus on chemicals alone in human-dominated ecosystems is inappropriate. Integr Environ Assess Manag. 13:568–572.
- Carpenter DO, Arcaro K, Spink DC. 2002. Understanding the human health effects of chemical mixtures. Environ Health Perspect.110:25–42.
- Cedergreen N. 2014. Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE. 9(5): e96580. doi:10.1371/journal.pone.0096580.
- Dalla Costa S, Kephalopoulos S, Bopp S, Kienzler A, Richarz A, Van der Linden S, Korytar P, Backhaus T, Lebret E, Van Klaveren J, et al. 2018. JRC workshop on IPCHEM supporting the assessment of chemical mixtures. Final report. JRC workshop report, Joint Research Centre, Ispra, Italy. 52 pp. JRC112027.
- De Brouwere K, Cornelis C, Arvanitis A, Brown T, Crump D, Harrison P, Jantunen M, Price P, Torfs R. 2014. Application of the maximum cumulative ratio (MCR) as a screening tool for the evaluation of mixtures in residential indoor air. Sci Tot Environ. 479–480:267–276.
- Dewalque L, Charlier C, Pirard C. 2014. Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol Lett. 231:161–168.
- Desalegn A, Bopp S, Asturiol D, Lamon L, Worth A, Paini A 2019. Role of Physiologically Based Kinetic modelling in addressing environmental chemical mixtures - A review. Comp Tox. In press. doi:10.1016/j.comtox.2018.09.001.
- EC (European Commission). 2012. Communication from the commission to the council - the combination effects of chemicals. Chemical mixtures. Brussels, 31.5.2012, COM(2012) 252 final.
- ECHA. (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC); Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, et al. 2018. Guidance for the identification of endocrine disruptors in the context of regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA J. 16:5311. ECHA-18-G-01-EN
- ECHA (European Chemicals Agency). 2017a. Guidance on the biocidal products regulation, Volume III Human health - assessment & evaluation, Helsinki, Finland: European Chemicals Agency. (Parts B + C), Version 3.0, November 2017. Reference: ECHA-17-G-25-EN.
- ECHA (European Chemicals Agency). 2017b. Guidance on the biocidal products regulation - Volume IV Environment - assessment & evaluation, European Chemicals Agency, Helsinki, Finland. (Parts B + C) (Vol. II).
- ECHA (European Chemicals Agency). 2017c. Read-Across Assessment Framework (RAAF) — considerations on multi-constituent substances and UVCBs. Helsinki, Finland: European Chemicals Agency.
- EEA (European Environment Agency). 2017. Air quality in Europe — 2017. European Environment Agency, Copenhagen, Denmark. Report No 13/2017.
- EFSA Panel on Plant Protection Products and their residues (PPR). 2013. Scientific opinion on the relevance of dissimilar mode of action and its appropriate application for cumulative risk assessment of pesticides. EFSA Journal. 2013;11(12):3472, 40 pp.
- EFSA Panel on Plant Protection Products and their Residues (PPR). 2013. Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile (2014 update).. EFSA Journal 2013;11(7):3293, 131:3293.
- EFSA (European Food Safety Authority) Scientific Committee. 2018a. Guidance on uncertainty analysis in scientific assessments. EFSA J. 16:5123.
- EFSA (European Food Safety Authority) Scientific Committee 2018b. The principles and methods behind EFSA’s Guidance on Uncertainty Analysis in Scientific Assessment. EFSA J. 16:5122.
- EFSA (European Food Safety Authority) Scientific Committee. 2018c. Draft guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. Published for public consultation 26 June–15 September 2018. http://www.efsa.europa.eu/sites/default/files/consultation/consultation/180626-1-ax1.pdf
- EFSA (European Food Safety Authority); Hart A, Maxim L, Siegrist M, Von Goetz N, da Cruz C, Merten C, Mosbach-Schulz O, Lahaniatis M, Smith A, Hardy A. 2019. Guidance on communication of uncertainty in scientific assessments. EFSA J. 17:5520.
- Escher BI, Neale PA, Leusch FDL. 2015. Effect-based trigger values for in vitro bioassays: reading across from existing water quality guideline values. Water Res. 81:137–148.
- Escher BI, Hackermüller J, Polte T, Scholz S, Aigner A, Altenburger R, Böhme A, Bopp SK, Brack W, Busch W, et al. 2017. From the exposome to mechanistic understanding of chemical-induced adverse effects. Environ Int. 99:97–106.
- Escher BI, Aït-Aïssa S, Behnisch PA, Brack W, Brion F, Brouwer A, Buchinger S, Crawford SE, Du Pasquier D, Hamers T, et al. 2018. Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci Tot Environ. 628–629:748–765.
- Evans RM, Scholze M, Kortenkamp A. 2015. Examining the feasibility of mixture risk assessment: a case study using a tiered approach with data of 67 pesticides from the Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Food Chem Toxicol. 84:260–269.
- Evans RM, Martin OV, Faust M, Kortenkamp A. 2016. Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci Total Environ. 543:757–764.
- Ferguson KK, Peterson KE, Lee JM, Mercado-García A, Blank-Goldenberg C, Téllez-Rojo MM, Meeker JD. 2014. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol. 47:70–76.
- Goodson WH, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, Lasfar A, Carnero A, Azqueta A, Amedei A, et al. 2015. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis. 36:S254–S296.
- Gustavsson M, Kreuger J, Bundschuh M, Backhaus T. 2017. Pesticide mixtures in the Swedish streams: environmental risks, contributions of individual compounds and consequences of single-substance oriented risk mitigation. Sci Tot Environ. 598:973–983.
- Hadrup N, Svingen T, Mandrup K, Skov K, Pedersen M, Frederiksen H, Frandsen HL, Vinggaard AM. 2016. Juvenile male rats exposed to a low-dose mixture of twenty-seven environmental chemicals display adverse health effects. PLoS One. 11:e0162027.
- Han X, Price PS. 2011. Determining the maximum cumulative ratios for mixtures observed in ground water wells used as drinking water supplies in the United States. Int J Environ Res Public Health. 8:4729–4745.
- Han X, Price PS. 2013. Applying the maximum cumulative ratio methodology to biomonitoring data on dioxin-like compounds in the general public and two occupationally exposed populations. J Expo Sci Environ Epidemiol. 23:343–349.
- Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell. 100:57–70.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144:646–674.
- Hernández AF, Gil F, Lacasaña M. 2017. Toxicological interactions of pesticide mixtures: an update. Arch Toxicol. 91:3211–3223.
- Heys KA, Shore RF, Pereira MG, Jones KC, Martin FL. 2016. Risk assessment of environmental mixture effects. RSC Adv. 6:47844–47857.
- Hines DE, Edwards SW, Conolly RB, Jarabek AM. 2018. A case study application of the aggregate exposure pathway (AEP) and adverse outcome pathway (AOP) frameworks to facilitate the integration of human health and ecological end points for cumulative risk assessment (CRA). Environ Sci Technol. 52:839–849.
- IGHRC. 2009. Chemical Mixtures: A Framework for Assessing Risk to Human Health (CR14). Cranfield University, UK: Institute of Environment and Health. http://www.iehconsulting.co.uk/IEH_Consulting/IEHCPubs/IGHRC/cr14.pdf
- Johnson AC, Sumpter JP. 2016. Are we going about chemical risk assessment for the aquatic environment the wrong way? Environ Toxicol Chem. 35:1609–1616.
- Kapraun DF, Wambaugh JF, Ring CL, Tornero-Velez R, Woodrow Setzer R. 2017. A method for identifying prevalent chemical combinations in the U.S. population. Environ Health Perspect 125:1–16.
- Kemi. 2015. An additional assessment factor (MAF) – a suitable approach for improving the regulatory risk assessment of chemical mixtures? Stockholm. Report 5/15. Swedish Chemicals Agency, Stockholm, Sweden.
- Kienzler A, Berggren E, Bessems J, Bopp S, van der Linden S, Worth A. 2014. Assessment of mixtures - review of regulatory requirements and guidance. JRC Science for Policy Report, EUR 26675EN. Luxembourg: Publications Office of the European Union.
- Kienzler A, Bopp SK, van der Linden S, Berggren E, Worth A. 2016. Regulatory assessment of chemical mixtures: Requirements, current approaches and future perspectives. Regul Toxicol Pharmacol 80:321–334.
- Kienzler A, Bopp SK, Halder M, Embry M, Worth A. 2019. Application of new statistical distribution approaches for environmental mixture risk assessment: a case study. Submitted to Sci Tot Env.
- Kim J, Kim S. 2015. State of the art in the application of QSAR techniques for predicting mixture toxicity in environmental risk assessment. SAR QSAR Environ Res. 26:41–59.
- Kortenkamp A, Backhaus T, Faust M. 2009. State of the Art Report on Mixture Toxicity. Final Report. 22 December 2009. Study Contract Number 070307/2007/485103/ETU/D.1
- Kortenkamp A, Faust M. 2010. Combined exposures to anti-androgenic chemicals: steps towards cumulative risk assessment. Int J Androl. 33:463–472.
- Kortenkamp A, Faust M. 2018. Regulate to reduce chemical mixture risk. Science. 361:224–225.
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Halldorsson TI, Rantakokko P, Kiviranta H, Toft G. 2016. Prenatal exposure to persistent organochlorine pollutants and female reproductive function in young adulthood. Environ Int. 92–93:366–372.
- Lindim C, van Gils J, Cousing IT. 2016. A large scale model for simulating the fate & transport of organic contaminants in river basins. Chemosphere. 144:803–810.
- Malaj E, von der Ohe PC, Grote M, Kühne R, Mondy CP, Usseglio-Polatera P, Brack W, Schäfer RB. 2014. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc Natl Acad Sci U S A. 111:9549–9554.
- Martin OV, Bopp S, Ermler S, Kienzler A, McPhie J, Paini A, Parrisis N, Richarz AN, Scholze M, van der Linden S, et al. 2018. Protocol for a systematic review of ten years of research on interactions in chemical mixtures of environmental pollutants. doi:10.5281/zenodo.1319759.
- Martino-Andrade AJ, Liu F, Sathyanarayana S, Barrett ES, Redmon JB, Nguyen RHN, Levine H, Swan SH. 2016. Timing of prenatal phthalate exposure in relation to genital endpoints in male newborns. Andrology. 4:585–593.
- Marx C, Mühlbauer V, Krebs P, Kuehn V. 2015. Environmental risk assessment of antibiotics including synergistic and antagonistic combination effects. Sci Tot Environ. 524–525:269–279.
- McCarty LS, Borgert CJ. 2006. Review of the toxicity of chemical mixtures: theory, policy, and regulatory practice. Regul Toxicol Pharmacol. 45:119–143.
- Meek ME, Boobis AR, Crofton KM, Heinemeyer G, Raaij MV, Vickers C. 2011. Risk assessment of combined exposure to multiple chemicals: A WHO/IPCS framework. Regul Toxicol Pharmacol. 60:S1–S14.
- Mons MN, Heringa MB, van Genderen J, Puijker LM, Brand W, van Leeuwen CJ, Stoks P, van der Hoek JP, van der Kooij D. 2013. Use of the Threshold of Toxicological Concern (TTC) approach for deriving target values for drinking water contaminants. Water Res. 47:1666–1678.
- Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, Embry MR. 2017. A framework for cumulative risk assessment in the 21st century. Crit Rev Toxicol. 47:85–97.
- Napierska D, Sanseverino I, Loos R, Cortés LG, Niegowska M, Lettieri T. 2018. Modes of action of the current priority substances list under the water framework directive and other substances of interest review of the relevant modes of action. EUR29008 EN, Publications Office of the European Union, Luxembourg, ISBN 978-92-79-77301-3, doi:10.2760/226911.
- Neale PE, Ait-Aissa S, Brack W, Creusot N, Denison MS, Deutschmann B, Hilscherova K, Hollert H, Krauss M, Novak J, et al. 2015. Linking in vitro effects and detected organic micropollutants in surface water using mixture-toxicity modeling. Environ Sci Technol. 49:14614–14624.
- Nienstedt KM, Brock TC, van Wensem J, Montforts M, Hart A, Aagaard A, Alix A, Boesten J, Bopp SK, Brown C, et al. 2012. Development of a framework based on an ecosystem services approach for deriving specific protection goals for environmental risk assessment of pesticides. Sci Total Environ. 415:31–38.
- OECD. 2013. Guidance document on developing and assessing adverse outcome pathways. Series on testing and assessment no. 184. Organisation for Economic Co-operation and Development, Paris, France. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2013)6&doclanguage=en.
- OECD. 2018. Considerations for assessing the risks of combined exposure to multiple chemicals. Series on testing and assessment no. 296. Environment, Health and Safety Division, Environment Directorate. Paris, France: Organisation for Economic Co-operation and Development.
- Oldenkamp R, Hoeks S, Čengić M, Barbarossa V, Burns EE, Boxall ABA, Ragas AMJ. 2018. A high-resolution spatial model to predict exposuire to pharmaceuticals in european surface waters: ePiE. Environ Sci Technol. 52:12494–12503.
- Posthuma L, De Zwart D, Keijzers R, Postma J. 2016. Watersysteemanalyse met de Ecologische Sleutelfactor Toxiciteit Deel 2. Kalibratie: toxische druk en ecologische effecten op macrofauna. STOWA rapport number: 2016-15 B. Stichting toegepast Onderzoek Waterbeheer, Amersfoort, The Netherlands. ISBN 978.90.5773.727.5
- Posthuma L, Brown CD, de Zwart D, Diamond J, Dyer SD, Holmes CM, Marshall S, Burton Jr. GA. 2018. Prospective mixture risk sssessment and management prioritizations for river catchments with diverse land uses. Env Tox Chem. 37:715–728.
- Price PS, Hollnagel HM, Zabik JM. 2009. Characterizing the noncancer toxicity of mixtures using concepts from the TTC and quantitative models of uncertainty in mixture toxicity. Risk Anal. 29:1534–1548.
- Price PS, Han X. 2011. Maximum cumulative ratio (MCR) as a tool for assessing the value of performing a cumulative risk assessment. Int J Environ Res Public Health. 8:2212–2225.
- Price P, Dhein E, Hamer M, Han X, Heneweer M, Junghans M, Kunz P, Magyar C, Penning H, Rodriguez C. 2012a. A decision tree for assessing effects from exposures to multiple substances. Environ Sci Eur. 24:26.
- Price P, Han X, Junghans M, Kunz P, Watts C, Leverett D. 2012b. An application of a decision tree for assessing effects from exposures to multiple substances to the assessment of human and ecological effects from combined exposures to chemicals observed in surface waters and waste water effluents. Environ Sci Eur. 24:36.
- Price P, Zaleski R, Hollnagel H, Ketelslegers H, Han X. 2014. Assessing the safety of co-exposure to food packaging migrants in food and water using the maximum cumulative ratio and an established decision tree. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 31:414–421.
- Reyes JM, Price PS. 2018. An analysis of cumulative risks based on biomonitoring data for six phthalates using the Maximum Cumulative Ratio. Environ Int. 112:77–84.
- Rider CV, Boekelheide K, Catlin N, Gordon CG, Morata T, Selgrade MJK, Sexton K, Simmons JE. 2014. Cumulative risk: toxicity and interactions of physical and chemical stressors. Toxicol Sci.137:3–11.
- Rodea-Palomares I, Gonzalez-Pleiter M, Martin-Betancor K, Rosal R, Fernandez-Pinas F. 2015. Additivity and interactions in ecotoxicity of pollutant mixtures: some patterns, conclusions, and open questions. Toxics. 3:342–369.
- Rotter S, Beronius A, Boobis AR, Hanberg A, van Klaveren J, Luijten M, Machera K, Nikolopoulou D, van der Voet H, Zilliacus J, et al. 2018. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: the potential EuroMix contribution. Crit Rev Toxicol. 48:796–781. doi:10.1080/10408444.2018.1541964
- Sarigiannis DA, Hansen U. 2012. Considering the cumulative risk of mixtures of chemicals - a challenge for policy makers. Environ Health. 11(Suppl 1):S18.
- SCHER, SCENIHR, SCCS. 2012. Toxicity and assessment of chemical mixtures. European Union. doi:10.2772/21444
- Silva E, Rajapakse N, Kortenkamp A. 2002. Something from “nothing”-eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 36:1751–1756.
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, Redmon JB. 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 30:963–972.
- Tang JYM, Busetti F, Charrois JWA, Escher BI. 2014. Which chemicals drive biological effects in waste water and recycled water? Water Res. 60:289–299.
- Teeguarden JG, Tan YM, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanguay RL, et al. 2016. Completing the link between exposure science and toxicology for improved environmental health decision making: the aggregate exposure pathway framework. Environ Sci Technol. 50:4579–4586.
- US EPA (Environmental Protection Agency). 2014 Apr. Framework for human health risk assessment to inform decision making. U.S. Environmental Protection Agency, Risk Assessment Forum. EPA/100/R-14/001. http://www.epa.gov/sites/production/files/2014-12/documents/hhra-framework-final-2014.pdf
- van Broekhuizen FA, Posthuma L, Traas TP. 2016. Addressing combined effects of chemicals in environmental safety assessment under REACH - a thought starter. RIVM Letter report 2016-0162, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
- WHO International Programme on Chemical Safety (IPCS). 2017. Guidance document on evaluating and expressing uncertainty in hazard characterization. 2nd ed. Geneva, Switzerland: World Health Organization. Harmonization Project Document No. 11.
