Abstract
Chemical substances are subjected to assessment of genotoxic and carcinogenic effects before being marketed to protect man and the environment from health risks. For agrochemicals, the long-term rodent carcinogenicity study is currently required from a regulatory perspective. Although it is the current mainstay for the detection of nongenotoxic carcinogens, carcinogenicity studies are shown to have prominent weaknesses and are subject to ethical and scientific debate. A transition toward a mechanism-based weight-of-evidence approach is considered a requirement to enhance the prediction of carcinogenic potential for environmental (agro)chemicals. The resulting approach should make optimal use of innovative (computational) tools and be less animal demanding. To identify the various mode of actions (MOAs) underlying the nongenotoxic carcinogenic potential of agrochemicals, we conducted an extensive analysis of 411 unique agrochemicals that have been evaluated for carcinogenicity by the United States Environmental Protection Agency (US EPA) and the European Chemicals Agency (ECHA). About one-third of these substances could be categorized as nongenotoxic carcinogens with an average of approximately two tumor types per substance, observed in a variety of organs. For two-third of the tumor cases, an underlying MOA (network) could be identified. This analysis demonstrates that a limited set of MOA (networks) is underlying nongenotoxic carcinogenicity of agrochemicals, illustrating that the transition toward a MOA-driven approach appears manageable. Ultimately the approach should cover relevant MOAs and its associated key events; this will also facilitate the evaluation of the human relevance. This manuscript describes the results of the analysis while identifying knowledge gaps and necessities to achieve a mechanism-based weight-of-evidence approach.
Introduction
Chemical substances are subjected to hazard and risk assessments before being marketed to protect man and the environment from health risks. Amongst other toxicological endpoints, chemical risk assessments concern genotoxic and carcinogenic effects. From a regulatory perspective, cancer hazard assessment generally requires the performance of long-term carcinogenicity studies or combined chronic toxicity/carcinogenicity studies. However, these rodent studies are shown to have prominent weaknesses related to the limited translatability of rodent assays to man and a rather low reproducibility of 60–70% (Gottmann et al. Citation2001). Also from an ethical perspective, the default request for these studies for safety testing of chemicals is not desirable because of the high number of animals involved. Therefore, there is a strong need for alternative approaches for assessing the carcinogenic potential of substances that results in better prediction and a lower number of animals required (Doe et al. Citation2019).
Dependent on the product or industry sector and regulatory jurisdiction, different test strategies for cancer hazard identification are in place with corresponding information requirements laid down in various legislations (e.g. cosmetics (EC Citation2009; SCCS Citation2016), industrial chemicals [REACH; (EC Citation2008a), pharmaceuticals (ICH Citation1995; ICH Citation1997; ICH Citation2008), and biocides (EC 2012)]. For agrochemicals in the EU, data requirements for cancer hazard assessment are laid down in EC regulation 283/2013 (EC Citation2013). Herein it is described that all active substances have to be tested for genotoxic potential in a full set of in vitro and in vivo genotoxicity studies. In addition, it is a standard requirement to test each substance in a long-term carcinogenicity study in both rats and mice [TG 451; (OECD Citation2018a)]. In rats, this study is ideally combined with the oral long-term toxicity study [TG 453 (OECD Citation2018b)]. In case it can be scientifically justified that an additional TG 451 carcinogenicity study using mice as test species is not necessary, a scientifically validated alternative (transgenic) carcinogenicity model may be used instead.
In the case of nongenotoxic carcinogens, chronic disruption of physiological processes typically precedes tumor formation. Therefore, the assumption is that a point-of-departure such as a No-Observed-Adverse-Effect-Level (NOAEL) derived from 90-day sub-chronic toxicity studies in rodents would also be protective against cancer induced by nongenotoxic carcinogens. For Classification, Labeling and Packaging (CLP) purposes, carcinogenicity classification relies mainly on genetic toxicity endpoints based on the rationale that genetic damage will eventually lead to cancer (EC Citation2008b). The relevance of this binary approach is increasingly being questioned (Doe et al. Citation2019; Wolf et al. Citation2019), among others based on the observation that nongenotoxic carcinogens, while acting via other mechanisms than direct genetic damage, may remain undetected under the regulatory testing requirements currently in place (Hernandez et al. Citation2009).
For pharmaceuticals, the so-called ‘NegCarc’ (Negative for Endocrine, Genotoxicity and Chronic Study Associated Histopathologic Risk Factors for Carcinogenicity) approach has been proposed as an alternative approach to cancer hazard identification (Sistare et al. Citation2011). Using this approach for pharmaceuticals resulted in a reasonable negative predictive value (NPV) (ability to correctly predict a negative outcome) for carcinogenicity of 82% (Sistare et al. Citation2011). However, given the low number of carcinogenic pharmaceuticals, the NPV is of limited value since most substances will not be genotoxic or carcinogenic and will thus not induce histopathological changes in a sub-chronic study. Therefore, both a high NPV and positive predictive value (the ability of a study to correctly predict a positive outcome; PPV) are needed to be certain that there is a correlation between the effects in a 90-day study and a carcinogenicity study. This renders the sole consideration of the NPV as quality criterium potentially deceiving. From a regulatory perspective, the low sensitivity of 79% (PPV; Sistare et al. Citation2011) is a reason for concern and limits the immediate applicability of the approach.
Upon further evaluation of the available data, it was hypothesized, and subsequently proven, that inclusion of pharmacological properties relating to the mode of action (MOA) improved the performance of the approach (ICH 2013; van der Laan et al. Citation2016; van der Laan et al. Citation2016). In general, it was concluded that the NegCarc approach provides a promising approach but that incorporation of mechanistic information and well-defined criteria for a WoE evaluation will provide the required improvement of the performance of the approach (Bourcier Citation2015).
The studies of van der Laan et al. (Citation2016) and Woutersen et al. (Citation2016) clearly showed that the sole absence of effects in a sub-chronic toxicity study (90-day) is indicative but insufficient to conclude a compound is not carcinogenic. Therefore, a transition toward a mechanism-based approach is considered a requirement to enhance the prediction of carcinogenic potential for environmental chemicals including agrochemicals (Boobis et al. Citation2009; Julien et al. Citation2009; Meek et al. Citation2014). Development of such an approach should make optimal use of innovative (computational) tools and test methods that are preferably animal free, or at least (far) less animal demanding. Via implementation of the adverse outcome pathway (AOP) concept (Ankley et al. Citation2010; Edwards et al. Citation2016), the approach should cover a range of relevant pathways since broad knowledge of a MOA and associated key events (KEs) has proven valuable for evaluating the human relevance of information obtained from toxicity studies in animals (Meek et al. Citation2003; Boobis et al. Citation2006; Holsapple et al. Citation2006; Meek et al. Citation2014). Thus, the ultimate approach comprises a WoE evaluation that weighs the human relevance of pathways and allows for quantitative analysis with the highest level of sensitivity and specificity achievable.
To identify the various MOAs underlying the nongenotoxic carcinogenic potential of agrochemicals, we conducted an extensive analysis of data on 411 unique agrochemical active substances that have been evaluated for carcinogenicity by the United States Environmental Protection Agency (US EPA) and European Chemicals Agency (ECHA; CL inventory). This manuscript describes the results of this analysis while identifying knowledge gaps and exploring a way toward a novel mechanism-based approach.
Methodology
Data collection
For the identification of MOAs involved in the nongenotoxic carcinogenicity of agrochemicals, we compiled a list of agrochemicals based on the agrochemicals that have been evaluated for carcinogenicity by the US EPA (US EPA Annual Cancer Report Citation2016) and on the EU pesticides database (Carc classes 1 A, 1B and 2; (http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN). In total, this list comprises 411 unique agrochemicals. For all substances, toxicity assessment reports and documents were collected from the databases of the European Chemicals Agency (ECHA), the European Food Safety Authority (EFSA), the National Institute of Environmental Health Sciences (NIEHS), the United States Environmental Protection Agency (US EPA), the International Agency for Research on Cancer (IARC), the Joint FAO/WHO Meeting on Pesticide Residues (JMPR), and the Agency for Toxic Substances and Disease Registry (ATSDR).
Exclusion and inclusion criteria
In general, only studies that were considered to be of sufficient quality according to criteria laid down by the respective agencies were included. In case only summaries of assessment reports were to be found or solely assessment reports from one organization, data were deemed too little for analysis. An exemption to this rule was made when only reports of one organization (e.g. EFSA, US EPA) could be found but the report provided qualitatively acceptable and detailed information. In this case, the substance was included for analysis. Substances labeled too old generally presented with reports pre-1990 and often overlapped with cases with too little information. In case younger reports were available (>2000) but the assessment was solely based on carcinogenicity studies (pre-1990, a substance was still assigned to the category “too old/too little information”.
The OECD Test Guidelines for carcinogenicity studies (TG 451/453; (OECD Citation2018a) require that all substances under scrutiny (e.g. an agrochemical) are tested at dosages high enough for the identification of principal target organs and toxic effects while avoiding suffering, severe toxicity, morbidity and death (OECD Citation2014; OECD Citation2018b). However, too high doses can give rise to nonspecific general toxicity, compensatory cell proliferation where damage has occurred, and subsequent tumor formation. Consequently, when tumor formation is observed only at the highest dose, it can be due to the disruption of general physiology/homeostasis. In those cases where tumors were only observed at the highest dose tested, or the effect was denoted by the regulatory bodies as occurring ‘at excessive dose’, substances were labeled ‘high-dose’ yet still included for further analysis. These cases were further scrutinized on a case-by-case basis and remained included or were excluded based on expert judgment of among others the dosing range and circumstantial evidence of general toxicity.
Categorization of substances
Substances were categorized either as genotoxic carcinogens, nongenotoxic carcinogens or noncarcinogens based on the combined data in the available reports. In case multiple assessment reports were identified, combined data from all available reports were considered for categorization. Substances were labeled as potentially ‘genotoxic carcinogens’ and excluded for analysis when both in vitro (e.g. mammalian cell gene mutation, cytogenetic test, DNA repair assays) and in vivo (e.g. micronucleus and chromosomal aberrations) genotoxicity assays turned out positive. When a substance scored positive in in vitro genotoxicity assays and negative or equivocal in in vivo genotoxicity assays, it was included in further subcategorization. Substances for which no treatment-related increase in tumor formation was observed in long-term carcinogenicity studies were labeled ‘noncarcinogens’. Substances showing a treatment-related increase in the incidence of benign or malignant tumors in a long-term carcinogenicity study without in vivo evidence of genotoxicity were labeled as ‘nongenotoxic carcinogens’ (NGTXC). These substances are listed in Supplementary Table 1.
Subsequently, for every substance labeled ‘nongenotoxic carcinogen’, data were collected on the observed tumor type(s), sex and species of the test animal as well as available details on dose and (proposed) carcinogenic MOA. To harmonize tumor data, all included tumors were categorized according to standardized pathological nomenclature (INHAND guidelines) by a board-certified pathologist with ample experience in carcinogenicity studies.
Identification of MOAs for NGTXC
Where available, details on the carcinogenic MOA of substances were collected from the combined assessment reports. This was complemented with existing knowledge on MOAs as reported in the scientific literature or the AOP database (AOP wiki; www.aopwiki.org). Thus, a list of MOAs underlying the reported tumors was compiled. Tumors for which no underlying MOA could be deduced from the reports and/or literature were labeled ‘unknown’.
Results
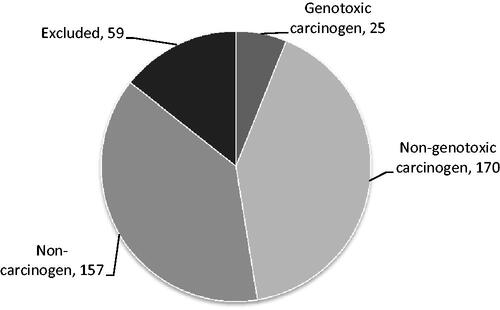
In total, data were collected on 411 unique substances. From this initial list, 59 substances were excluded based on the availability of too little or too old information. From the remaining 352 substances, 25 substances were excluded for analysis based on evidence of a genotoxic MOA underlying the observed carcinogenicity, and for 157 substances no treatment-related changes in tumor formation were observed; these were therefore designated as noncarcinogenic and also excluded for analysis. The remaining 170 substances were categorized as nongenotoxic carcinogen (; Supplementary Table S1) and were subjected to further analysis.
Figure 1. Distribution of the 411 substances over the different categories. Clockwise: Genotoxic carcinogen: positive in in vitro and in vivo genotoxicity/mutagenicity assays; Nongenotoxic carcinogen: substances with the reported treatment-related increase in incidence of benign or malignant tumors without in vivo evidence of genotoxicity; noncarcinogen: substance without reported treatment-related increase in incidence of benign or malignant tumors; Excluded: substance without sufficiently detailed or reliable tumor data available.
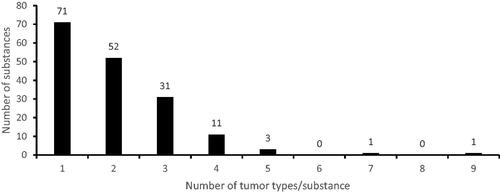
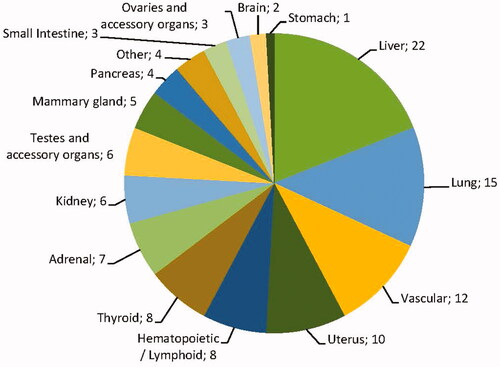
For the 170 substances considered as nongenotoxic carcinogen, 340 unique occasions of treatment-related tumor formation were registered. This clearly shows that on average more than one treatment-related tumor type occurred per substance. Indeed, less than 50% (71/170) of the substances was related to only one type of tumor whereas the largest fraction of the substances was related to the formation of two or three unique tumor types (83/170) (). Only in a small fraction of the cases four or more tumor types were observed (16/170) ().
Types of tumors/organs affected
Below, we describe the data on tumor formation per organ or organ system related to exposure to agrochemicals categorized as nongenotoxic carcinogens according to the criteria described in the Methodology section. This section is organized primarily based on the anatomical location of the tumor. The numbers listed relate to the occasions of treatment-related tumor formation. An overview of the distribution over different organs is given in .
Figure 3. Organ distribution of all 340 observed treatment-related tumors with a suspected nongenotoxic MOA. The category “Other” includes bone, skin, eye, and prostate tumors.
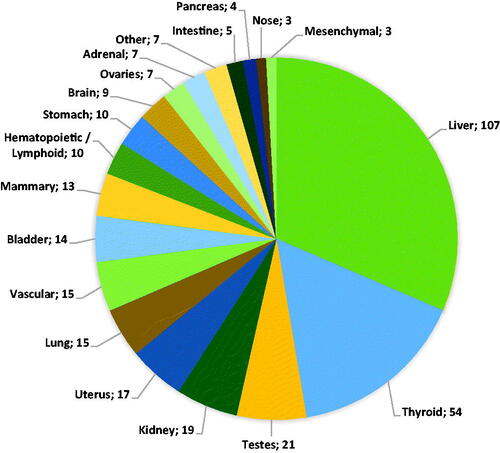
Liver
The largest fraction of tumors was observed in the liver comprising tumors categorized as hepatocellular adenoma/carcinoma (99/107) and cholangioma/cholangiocarcinoma (6/107). In addition, one case of interstitial cell adenoma was reported as well as one case of epithelial cell tumors (not further specified). All tumors in the category cholangioma/cholangiocarcinoma were reported in conjunction with tumors categorized as hepatocellular adenoma/carcinoma. In one case, an interstitial cell adenoma was reported but not further specified. Tumors were in most cases observed in either rats (21/107) or mice (65/107) although in 21 cases liver tumors were observed in both species. In 57 cases, tumors were observed in both sexes, in 27 cases only in male animals, in nine cases only in female animals, and in 14 cases the sex of the animals in which tumor formation was observed was not specified. None of the tumor categories was obviously linked to one sex.
Thyroid
In the thyroid, the follicular cell adenoma and/or carcinoma was the most frequently reported tumor type (49/54). C-cell adenomas or carcinomas were also reported (4/54) and in one case thyroid tumors were reported but not further specified. C-cell adenomas or carcinomas were never observed in conjunction with other thyroid tumors. In 27 cases, thyroid tumors were observed in conjunction with observations of hepatocellular adenoma/carcinoma. In the majority of the cases (48/54) thyroid tumors were observed in rats only, in two cases tumors were observed in mice only and in four cases thyroid tumors were observed in both species. In 23 cases, tumors were solely observed in male animals, in five cases in female animals only, in 18 cases in both sexes and in eight cases the sex of the animals in which tumor formation was observed was unspecified.
Testes and accessory organs
Twenty-one cases of tumor formation in the testes and accessory organs were registered. The majority of these were Leydig cell adenomas and carcinomas (19/21). In addition, single cases of epididymal histiocytic sarcoma and preputial gland adenoma/carcinoma were observed. Almost all cases were observed in rats (20/21); only the epididymal histiocytic sarcoma was observed in mice. None of the cases included tumors in both rats and mice. Thirteen cases of tumor formation coincided with tumor formation in the liver.
Kidney
In the kidney, two types of tumors were observed, i.e. tubular cell adenoma/carcinoma (16/19) and transitional cell adenoma/carcinoma (2/19). In one case specification of the kidney tumors observed was absent resulting in a report of ‘renal tumors’. In 11 cases, tumors were observed in rats only, in five cases tumors were observed in mice and in three cases tumors were observed in both species. The majority of the cases of tumor formation were observed in male animals (13/19), in two cases tumors were observed in female only, in three cases tumors were observed in both sexes and in one case the sex was not specified.
Uterus
The most frequently reported cases of tumor formation in the uterus were categorized as endometrial polyps, adenoma, or carcinoma (13/17). In two cases, there were additional uterine tumor types observed. In one case, this was an unilateral luteoma, endometrial sarcoma, and squamous cell carcinoma, in another case Müllerian tumors. For one substance, four different tumor types were noted in both rat and mice, in the other cases tumors were solely observed in rats.
Lung
In the lung, only one type of tumor was observed which could be categorized as bronchiolar/alveolar adenoma/carcinoma (n = 15). These tumors were exclusively observed in mice. In two cases tumors were observed in males only, in seven cases in females only, and in six cases tumors were observed in both sexes.
Vascular
Tumors arising from the vascular system were observed in 16 (16/340) cases and comprised 15 cases of haemangiosarcoma and one case of haemangiopericytoma. The haemangiosarcomas were reported in various organs including the liver (5/15), spleen (5/15), small intestine (1/15), and lymph nodes (1/15). In two cases, haemangiosarcomas were reported but the organ was not specified. The case of haemangiopericytoma was reported to be found in the bladder. In 10 cases, tumors were reported in mice only (two cases female only, five cases male only, three cases both sexes), in two cases in rat only (male), and in three cases in both species (one case male only, two cases both sexes).
Bladder
Tumors noted in the bladder were in 12 out of 14 cases categorized as transitional cell papilloma or carcinoma. Additionally, two cases of mesenchymal adenoma/carcinoma were observed, in one case in addition to the transitional cell papilloma or carcinoma, while in the other case this was the sole type of tumor observed in the bladder. Observations of bladder tumors were either in mouse (6/14) or rat (8/14). The majority of the bladder tumors were observed in both sexes (9/14), in three cases in females only, in one case in male only and in one case sex was not specified.
Mammary gland
Thirteen cases of mammary tumors were reported for 10 individual substances. Most tumors could be categorized as adenoma or adenocarcinoma (8/12). In three cases, tumors could be categorized as fibroadenomas, in one case a carcinosarcoma was observed, and in one case mammary tumors were reported but not further specified. The majority of the tumors were observed in female rats (11/13), one case (adenoma/adenocarcinoma) in female mice, and one case (fibroadenoma) in male rats.
Hematopoietic/lymphoid
Tumors from hematopoietic/lymphoid origin comprise lymphoma (5/340) and leukemia-related tumors (5/340). In three cases, tumor formation was observed in rat only, in seven cases mouse only. In four cases, tumors were observed in male only (two cases rat, two cases mice), in six cases in female only (one case rat, five cases mice).
Stomach
Stomach tumors were observed in 10 cases of which eight were categorized as squamous cell papilloma or carcinoma of the fore stomach, one as gastric (adeno)carcinoma and one remained unidentified. Stomach tumors were observed in either rats or in mice (four uniquely in rats, five uniquely in mice), only in one case stomach tumors were observed in both species. In five cases, tumors were observed in both sexes, in three cases in females only, in one case in males only and in one case the sex was not specified.
Brain
In total, nine cases of brain tumor development were reported. The largest fraction (6/9) consisted of endocrine-related pituitary adenomas and carcinomas. In two cases, these tumors were observed in both sexes, in two cases in males only, in one case in females only, and in one case sex was not specified. One of the cases of pituitary adenomas or carcinomas was observed in mice (unspecified sex), all other cases were observed in rats. In four cases, pituitary tumors were observed in conjunction with liver and/or thyroid tumors. In addition to pituitary tumors, two cases of astrocytoma were observed (one case both sexes, one case males only; all cases rats) and one case of oligodendrocytoma in the hypothalamus (observed in male rat).
Ovaries and accessory organs
In seven cases, tumors were observed in the ovaries and accessory organs. In five cases, the tumors could be categorized as luteoma or granulosa/theca cell tumor and in two cases tubular adenomas were observed. In four cases, tumors were observed in rats, in three cases in mice. In none of the cases tumors were observed in both species.
Adrenals
Seven cases of tumor formation in the adrenal glands were reported for seven substances. In six cases, the tumors were categorized as phaeochromocytoma (benign/malignant). Additionally, one case of cortical adenoma or carcinoma was observed (female rats). In five cases, the adrenal tumors were observed in rats (four cases males only, one case females only), one case of phaeochromocytoma was observed in mice (both sexes).
Intestine
Five cases of tumor formation in the intestinal tract were reported, all in the small intestine. In four cases, the tumors could be categorized as duodenal adenoma or (adeno)carcinoma, in one case an undifferentiated smooth muscle sarcoma was observed. All intestinal tumors were observed in mice except for the sarcoma which was observed in rats. In four cases, tumors were observed in both sexes, in one case of duodenal adenoma or (adeno)carcinoma, tumor formation was observed in males only.
Pancreas
Four cases of tumor formation in the pancreas were reported for three substances. Two different types of tumors were observed, in three cases tumors were categorized as acinar cell adenoma/carcinoma (two cases males only, one case females only) and one case of islet cell adenoma/carcinoma (males only). Pancreas tumors were exclusively reported in rats. No association was found with a specific tumor type.
Nose
Tumors in the nasal cavity were reported for three substances. These tumors were categorized as respiratory cell adenomas and/or adenocarcinomas. In all three cases, the tumors were observed in rats of both sexes upon inhalation of the substance.
Mesenchymal
In three cases, tumors were categorized as mesenchymal: for one substance both leiomyosarcoma and osteosarcoma were reported (rat, both sexes), and for one substance fibrosarcoma was reported (both rat and mice, males only).
Eye
Three cases of tumors in the eye were reported which could be categorized as papilloma of the cornea, squamous cell carcinoma or in one case corneal tumors. All eye tumors were observed in male rats.
Prostate
In one case, tumor formation in the prostate was reported which could be categorized as prostate gland adenoma or carcinoma. This tumor was observed in rats.
Skin
In one case, tumor formation of the skin was reported which could be categorized as keratoacanthoma and was observed in rats of both sexes.
Discerned MOAs
The mechanistic information we collected on the 170 substances categorized as nongenotoxic carcinogens (see Methodology section; Supplementary Table 1) was consolidated into a list of MOAs and MOA networks underlying the observed tumor formation (). Of all observed cases of treatment-related tumor formation (340), the majority (224) could be linked to a MOA or MOA network whereas for 116 cases of tumor formation the available information was insufficient to determine a MOA underlying tumor formation. lists the (networks of) MOAs, while each of the MOAs is discussed in more detail below.
Table 1. Summary table describing all discerned MOAs and MOA networks.
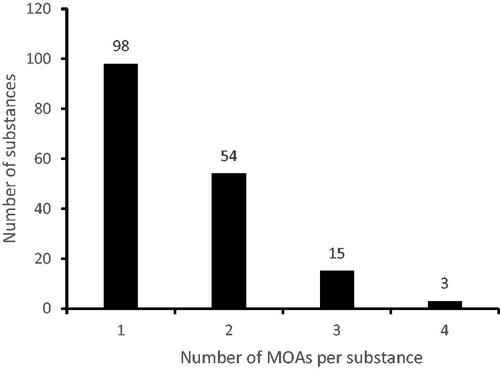
When the number of different MOAs per substance was counted it showed that 58% (98/170) of the substances activated a single MOA whereas 42% of the substances (72/170) activated two MOAs or more with a maximum of four MOAs (). The number of tumors related to a particular MOA is reported in alongside the number of substances related to these tumors. From these data, it can be appreciated that for substances related to the MOAs “sustained cytotoxicity” including “oxidative stress”, and “endocrine” more than one type of tumor was noted. The same holds for substances that evoked tumor formation with an unknown underlying MOA.
In some cases, clear evidence was presented for the MOA involved in tumor formation (e.g. induction of xenobiotic metabolism through nuclear receptor (CAR/PXR) activation; PPARα activation). Nevertheless, in many cases a presumptive MOA had to be deducted from combined information on the organ, type of tumor, and knowledge available in the literature (e.g. cytotoxicity as a MOA in (fore)stomach, intestine, bladder, and kidney tumors). In cases where no information was available and assigning a MOA would be speculation, no MOA was assigned.
For tumors in almost all organs presented in one or more MOAs could be identified except for tumors observed in the pancreas, and the majority of the vascular, hematopoietic/lymphoid, adrenal, lung, and uterus tumors.
Nuclear receptor activation
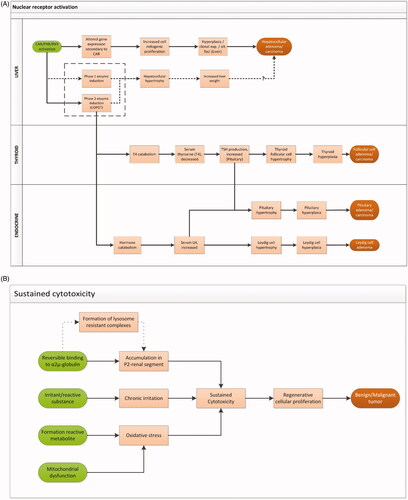
The largest fraction of tumors with an identifiable MOA (112/225, ) was related to induction of xenobiotic metabolism through nuclear receptor (CAR/PXR) activation (). Both CAR and PXR are involved in a wide range of functions and are known to “crosstalk”: they regulate the expression of analogous genes by stimulating similar response elements and show overlapping affinities for some ligands (Hernandez et al. Citation2009; Oladimeji et al. Citation2016). Related tumors include hepatocellular adenomas and carcinomas, follicular cell adenomas and carcinomas of the thyroid, Leydig cell adenomas of the testis, and pituitary adenomas and carcinomas. These tumors were attributable to a MOA network starting with induction of enzymes involved in xenobiotic metabolism in the liver upon activation of (primarily) the CAR and/or PXR nuclear receptor, leading to increased hepatocellular activity, escalating to mitogenic proliferation, hyperplasia, and the formation of hepatocellular adenomas and/or carcinomas (Elcombe et al. Citation2014; Peffer et al. Citation2018). In parallel, activation of Phase II enzymes may result in an increase in catabolism of thyroid hormones (via feedback routes leading to activation/stimulation of the HPT-axis and sustained hormone production in the thyroid) or steroid hormones (via feedback routes leading to activation/stimulation of the HPG axis and sustained steroid hormone production in the gonads). This may result in the induction of thyroid follicular cell adenomas and/or carcinomas and testis Leydig cell adenomas (Marty et al. Citation2015). In addition, enhanced hormone production in the pituitary will occur which may escalate to hyperplasia and lead eventually to the formation of pituitary adenomas and/or carcinomas (Papineni et al. Citation2015). Although there is a clear link between the tumor types described here, activation of the CAR/PXR/RXR receptor does not necessarily lead to the formation of tumors in all organs involved. In fact, several different combinations of tumors have been observed.
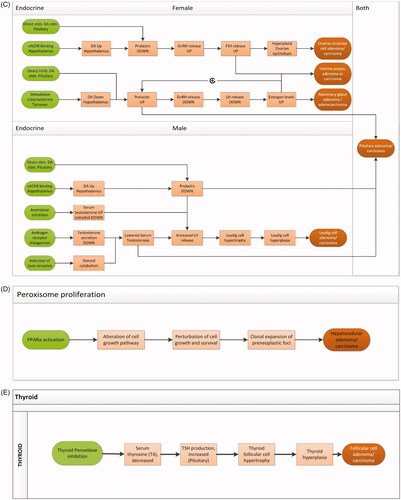
Figure 5. (A) MOA network describing the relationship between nuclear receptor activation in the liver and liver, thyroid, testis, and pituitary tumors (Elcombe et al. Citation2014; Marty et al. Citation2015; Papineni et al. Citation2015; Peffer et al. Citation2018). CAR: constitutive androstane receptor; PXR: pregnane X receptor; RXR: retinoid X receptor; exp.: expansion; alt.: alternated; UDPGT: uridine 5’-difoso-glucuronosyltransferase; T4: thyroxine; TSH: thyroid-stimulating hormone; LH: luteinizing hormone. (B) MOA describing tumor formation related to sustained cytotoxicity and oxidative stress (Meek et al. Citation2003; Cohen et al. Citation2010; Klaunig et al. Citation2010; Strupp et al. Citation2016). (C) MOA network describing the possible receptor-mediated pathways underlying endocrine-related tumors in male and female animals. (Cooper et al. Citation2007; Simpkins et al. Citation2011; Rasoulpour et al. Citation2015; van der Laan et al. Citation2016). (D) MOA network describing liver tumor formation related to PPAR activation (Klaunig et al. Citation2003; Corton et al. Citation2014). (E) MOA underlying follicular cell adenoma/carcinoma related to thyroid peroxidase inhibition (Motonaga et al. Citation2016).
Sustained cytotoxicity/oxidative stress
A well-known MOA for nongenotoxic carcinogenesis is sustained cytotoxicity and related regenerative proliferation (McGregor et al. Citation2006; Meek et al. Citation2014). In 61/340 of our tumor cases, sustained cytotoxicity and regenerative proliferation were the presumptive underlying MOA (). This MOA has been identified in several organs including liver, (fore) stomach, large and small intestine, kidney and bladder. These organs represent body compartments where exposure levels are elevated either as a result of metabolism (formation of reactive metabolites), or increased exposure related to excretion, or as a result of prolonged retention times of content.
Within the MOA sustained cytotoxicity, oxidative stress provides a central event (Klaunig et al. Citation2010; Klaunig Citation2018). In our dataset, it was only explicitly reported as underlying mechanism for 7 out of 340 cases of tumor formation, either related to formation of reactive metabolites or disturbance of mitochondrial function. This type of oxidative stress-related tumor formation was reported in liver (hepatocellular adenoma/carcinoma or hemangioma/hemangiosarcoma), spleen (hemangioma/hemangiosarcoma), and the lymphoid system (lymphoid tumor), related to three substances, mostly in mice, either in females alone (three cases) or in both sexes (three cases). In one case, oxidative stress-related tumors were observed in mice (both sexes) and rat (female).
One case of follicular cell adenoma/carcinoma, observed in both male mice and male rats, was reported to relate to oxidative stress as a result of metabolism of the iodine-containing substance. This specific case involves oxidation of the excess iodine by endogenous peroxidases including TPO leading to cytotoxicity (Kanno et al. Citation1992; Krohn et al. Citation2007; Zimmermann and Galetti Citation2015). Upon prolonged exposure to the substance sustained cytotoxicity may lead to tumor formation. Although recognized, this MOA is rather poorly understood.
A specific type of cytotoxicity-related tumors is provided by renal tumors related to the induction of α2u-globulin nephropathy via accumulation of lysosome-resistant chemical protein complexes in the P2 segment of the kidney. Depending on the level of sustained cytotoxicity, regenerative proliferation may ultimately lead to tumor (tubular cell adenoma/carcinoma) formation (Swenberg Citation1993; ). This MOA is considered specific for male rats since the α2u-globulin protein is exclusively formed in male rats and has no human analogue (US EPA Citation1991; Borghoff and Lagarde Citation1993; IARC Citation1999; Doi et al. Citation2007). In our dataset, three cases of kidney tumors were related to the occurrence of α2u-globulin nephropathy.
Endocrine-related MOAs
Twenty-nine cases of tumors in endocrine organs were reported (20 substances) for which the presumed MOA was endocrine-function dependent but could not be related to induction of enzymes involved in xenobiotic metabolism. These tumors were observed in various organs such as the mammary gland, uterus, and ovaries as well as in the testis. Tumor types observed in relation to this category were Leydig cell adenomas or carcinomas in the testes, luteoma/granulosa cell/theca cell tumors in the ovaries and accessory organs, uterine endometrial tumors (polyps/adenoma/carcinoma), and mammary adenomas or adenocarcinomas (). MOAs underlying the tumors in this category were various with the largest fraction related to androgen or estrogen receptor antagonism (11/29). Additionally, both stimulatory MOAs [prolactin release, gonadotropin-releasing hormone (GnRH) agonism] and inhibitory MOAs (suppression of LH release and aromatase inhibition) were described (summarized in ). These are MOAs with a variable level of complexity and understanding. It should therefore be noted that this network of putative MOAs contains the information provided by the various reports, where possible substantiated with data from the literature. It comprises therefore a simplified and incomplete network that contains a number of uncertainties such as the MOA underlying the occurrence of uterine polyps. To clarify the role of the various putative KEs and their interconnection in , data would be required on individual hormone levels to be able to evaluate the level of disturbance of hormonal balance, including hormones that are currently not mentioned such as progesterone.
Depending on the molecular initiating event (MIE), also pituitary adenomas or carcinomas can be expected based on overstimulation of pituitary hormone production through increased prolactin signaling. However, only for one compound the occurrence of mammary adenomas or adenocarcinomas coincided with observation of pituitary adenoma or carcinoma.
PPAR-alpha activation
In addition to the MOA on nuclear receptor-related enzyme induction, another receptor-related MOA is apparent which may lead to liver tumors (hepatocellular adenoma/carcinoma): activation of peroxisome proliferator-activated receptor alpha (PPARα) (11/340). Activation of PPARα leads to an alteration of cell growth pathways, and perturbation of cell growth and survival, which leads to clonal expansion of preneoplastic foci in the liver and may ultimately lead to the development of hepatocellular adenoma/carcinoma (Corton et al. Citation2014; ). Modulating factors that are considered to play a role in this pathway are among others oxidative stress and activation of the transcription factor NF-kB (Corton et al. Citation2014).
Thyroid peroxidase inhibition as endocrine mediated model
Another MOA could be discriminated in the database underlying thyroid follicular cell tumors that are not related to induction of liver enzymes. This MOA (observed and described for four substances) is related to specific substance-mediated inhibition of the enzyme thyroid peroxidase resulting in reduced production of T4, leading to decreased serum T4 levels which in turn activates the HPT axis leading to increased TSH production (Motonaga et al. Citation2016; ). In all four cases, these thyroid-specific tumors were observed both in rats and in mice.
Tumors without identifiable MOA
A substantial number of tumors were observed without an identifiable MOA (116/340; related to 74 unique substances). These are tumors for which no MOA is presented in the reports studied and the reported data provided insufficient clues to draw a conclusion on a possible underlying MOA. These so-called “known–unknowns” originate from several different organ systems () including vascular tumors (hemangiomas/hemangiosarcomas) in various organs, several tumors of the digestive system (liver, intestines, pancreas), lung tumors, lymphomas, and leukemia-related tumors, brain tumors, phaeochromocytoma in the adrenals, tumors of endocrine tissues (mammary gland, ovaries, testes, and uterus), and C-cell tumors of the thyroid gland.
Discussion
The present manuscript comprises an analysis of an unbiased selection of agrochemicals for which carcinogenicity study reports are publicly available. This selection includes among others herbicides, insecticides, acaricides, and additives to pesticide formulas listed in the US- and EU- databases. About one-third of these substances could be categorized as nongenotoxic carcinogens with an average of approximately two tumor types per substance. In the analysis, only tumors that show a treatment-related tumor response have been included. Despite the wide variety of tumors in various organs distilled from the carcinogenicity studies, a prevalence of MOAs involved in carcinogenesis could be seen. Scrutiny of the unknowns will probably result in additional MOAs or more elaborate networks. Yet, the limited number of MOAs clearly illustrates that the movement toward a MOA-driven approach appears manageable. Understanding the MOAs underlying the nongenotoxic carcinogenic potential of agrochemicals is the first step toward the development of a mechanism-based approach to predict carcinogenic potential of agrochemicals without the need for a long-term rodent carcinogenicity study.
Observed MOAs
The largest fraction of the MOAs identified appears related to receptor- or enzyme-mediated mechanisms such as activation of a CAR/PXR-mediated cascade () (Elcombe et al. Citation2014; Papineni et al. Citation2015; Felter et al. Citation2018). Other frequently observed MOAs include cytotoxicity leading to regenerative proliferation (including α2u-globulin nephropathy and oxidative stress; (Swenberg Citation1993; Doi et al. Citation2007; Zimmermann and Galetti Citation2015; Proctor et al. Citation2018) and perturbation of hormonal regulation (). Less frequently observed MOAs include peroxisome proliferation (through activation of PPARα; (Corton et al. Citation2014; Felter et al. Citation2018) and more specific MOAs dealing with hormonal perturbation like inhibition of thyroid peroxidase () (Hurley Citation1998). For one-third of the tumors reported, mechanistic information was not presented and could not be deduced from the study reports. The first and simplest explanation for a tumor to be categorized as ‘unknown’ is that reports on carcinogenicity studies can be relatively data-poor regarding underlying mechanisms of tumor formation. Therefore, it is likely that part of these unknowns (e.g. the hepatocellular adenomas or carcinomas) is in fact related to one of the already identified MOAs or MOA networks. For other types of tumors, MOAs may be known e.g. for pharmaceuticals but no data has been presented to substantiate a MOA. Likely examples of this are adrenal phaeochromocytoma and pancreatic acinar cell adenomas or carcinomas in rats for which disruption of calcium homeostasis and peroxisome proliferation provide known MOAs, respectively (Klaunig et al. Citation2003; van der Laan et al. Citation2016). However, it is important to notice in this respect that certain MOAs such as immune suppression or -modulation appear missing while immune-related tumors (lymphomas) have been observed. This may very well illustrate a disconnect between parameters assessed and potential MOAs. A similar disconnect may also explain the absence of MOAs related to deregulation of epigenetic mechanisms. This might not be surprising, since parameters for deregulation of epigenetic mechanisms are not part of the information requirements for regulatory carcinogenicity assessments. Since it is well known that deregulation of epigenetic mechanisms may play a role in tumor formation (Herceg et al. Citation2013; Thomson et al. Citation2014; Jacobs et al. Citation2020), this is another issue to follow-up when further developing the MOA-driven approach.
A remainder of the unknowns will comprise tumors in, e.g. the brain (astrocytoma, oligodendrocytoma), the vascular system (haemangiosarcoma), and the immune system (lymphomas). These tumors present a relevant data gap since these tumors are potentially relevant to humans while the underlying substance-related MOAs are unknown.
The importance of a MOA-driven approach
An important topic of discussion, in particular in the area of carcinogenicity, is the human relevance of tumors observed in rodents (Papineni et al. Citation2015; Peffer et al. Citation2018). To address this issue, the WHO International Programme on Chemical Safety developed a conceptual framework for assessment of species concordance (Boobis et al. Citation2006; Meek et al. Citation2014). According to this framework, assessment of human relevance should address the plausibility of a hypothesized MOA based on a thorough weight-of-evidence evaluation including mode-of-action/species concordance analysis. Obviously, this requires a fundamental understanding of toxicokinetic and toxicodynamic differences between species. In this respect, it is likely that only a small number of human-irrelevant MOAs exists, due to qualitative, i.e. physiological, differences between animals and humans. Therefore, insight in quantitative differences is key as illustrated by the cases described below.
Some of the observed tumor types are expected to be likely related to a species-specific MOA. For example, 15 substances were found to induce lung tumors (bronchiolar/alveolar adenoma/carcinoma) upon oral exposure. In all cases, lung tumors were solely observed in mice. Although none of the reports did provide details on the underlying MOA or data from which an underlying MOA could be deduced, literature on 2 of the 15 substances learned that CYP2F2-related metabolism of substances in the lung presents a MOA via which oral exposure to a substance can result in the formation of lung tumors (Strupp et al. Citation2012; Strupp et al. Citation2016; Yamada et al. Citation2017). This MOA is based on the formation of reactive metabolites due to CYP2F2 activity in the Club cells of the lung, leading to cytotoxicity and regenerative proliferation. Although claimed mouse-specific, no robust molecule-specific human data are known to substantiate this. In fact, the human lung is known to express CYP2F isoforms capable of metabolizing chemical compounds (Buckpitt and Bahnson Citation1986; Cruzan et al. Citation2012). This indicates that the species-specificity for these lung tumors is strictly speaking based on quantitative differences in toxico-kinetics and -dynamics instead of qualitative species differences.
Another example of quantitative differences between rodents (rats in this case) and humans is the thyroid hormone metabolism and production. In rats, induction of Phase II liver enzymes can lead to thyroid and pituitary tumors via increased thyroid hormone metabolism and subsequent increased TSH production via (over)stimulation of the HPT axis. The pathway of thyroid hormone production and feedback to the pituitary is preserved across species but there are quantitative differences between rats and humans, which render humans less susceptible to thyroid and pituitary tumors via thyroid hormone metabolism. In humans, T4 binds to globulin in the plasma (plasma T4 half-life of 5–9 days) whereas in rats the plasma protein globulin is lacking and therefore T4 has a lower half-life (approximately 12 h). Due to the longer half-life, humans are considered less vulnerable to degradation and clearance of T4 compared to rats (Hurley Citation1998; Papineni et al. Citation2015) and hence less susceptible to this type of thyroid and/or pituitary tumor induction. The quantitative nature of this difference is further illustrated by clinical observation of pituitary enlargement in humans following severe pharmacologically induced increases in TSH concentration (Pappy et al. Citation2016). Another example of a species-specific tumor type is provided by kidney tumors in male rats as a result of sustained tubular cell proliferation-related to α2u-globulin nephropathy. Alpha2u-globulin nephropathy provides a generally accepted MOA responsible for renal tumors in (male) rats (US EPA Citation1991; Borghoff and Lagarde Citation1993; Swenberg Citation1993; IARC Citation1999). However, within the scope of this article, it is important to also take notice of the observation that the association between the observation of α2u-globulin nephropathy in 90-day toxicity studies and the occurrence of renal tumors in the chronic carcinogenicity study appears weak (Doi et al. Citation2007). This indicates that the use of α2u droplet detection as predictor for renal tumors is limited and should probably be coupled to the use of, e.g. BrdU to detect cell proliferation.
Forestomach tumors provide a clear example of a tumor type where physiological or anatomical differences between humans and rodents are important (Proctor et al. Citation2007). In rodents, the forestomach has a particular function in food retention, this results in local prolonged high levels of exposure to food-borne chemicals which may result in local (chronic) irritation or cytotoxicity. Since humans are lacking a forestomach, this specific type of tumor is considered not relevant for humans. The underlying MOA (cytotoxicity leading to regenerative proliferation) is nonetheless certainly human relevant. Therefore, data on absorption, distribution, metabolism, and excretion in humans should determine which organs are potentially at risk in humans as a direct point of contact tissue site.
These examples clearly illustrate that human relevance is not a simple question with a binary answer, in particular when considering both quantitative differences and (apparent) qualitative differences. Evaluation of human relevance also includes consideration of quantitative differences in kinetic or dynamic factors between experimental animals and humans (Boobis et al. Citation2006; Boobis et al. Citation2009; Meek et al. Citation2014). This implies that differences in route of administration and possible consequences with regard to target tissue(s) need to be considered, as well as careful evaluation of the dose- and time-dependencies in responses across the KEs involved. Another important factor to be taken into account is the relative magnitude of the biological perturbation of each KE relative to the other KEs within a MOA. These considerations underline the importance of identification of known MOAs related to tumor formation, regardless of claimed species specificity. In many cases, the data revealed that more than one MOA is activated by a single substance, underpinning the importance of obtaining insight into the MOAs involved in carcinogenesis and assembling networks of these MOAs. Such a collection of (networks of) MOAs will enable determining which MOAs are activated and which are not.
Summary and outlook
Given the limitations of the rodent carcinogenicity studies and the desire to develop chemicals that do not pose a health risk to humans, there is a strong demand for a MOA-driven approach to assess/predict carcinogenic potential of agrochemicals. Such an approach starts with a comprehensive overview of MOAs underlying nongenotoxic carcinogenesis as well as the analysis of crucial knowledge gaps. This article is a first effort to develop such an overview. Whether or not a MOA-driven approach provides a viable alternative to the chronic carcinogenicity assay stands or falls with the completeness of the testing strategy. It is expected that a new approach will not revolve around the tumor as a morphological endpoint but rather encompasses the detection of nongenotoxic carcinogenic properties of a chemical substance through a combination of computational, in vitro, and in vivo evidence (e.g. hormonal and proliferation studies) from sub-chronic data. Databases such as the Toxcast/Tox21 database can provide important data on substance-induced effects in this respect, but will need careful interpretation for various reasons. High-throughput screening approaches such as ToxCast mainly inform on MIEs, while also information on KEs may be needed to allow for a more comprehensive understanding of the MOAs involved. Furthermore, meaningful interpretation of in vitro data and incorporation of in vitro techniques in a MOA-driven approach strongly depends on the possibilities for relevant in vitro to in vivo extrapolation and a detailed understanding of important caveats of in vitro models such as lack of biological barriers, organ-organ interactions, and relevant metabolism. Based on the biological role of the key aspects tested in human tumor formation, as well as their threshold of activation, human relevance can be defined. This will then be grounded on the simple assumption that early mechanistic endpoints will be human relevant unless clearly dismissed from a qualitative point of view.
Moreover, an alternative approach should combine a high negative and a high positive predictive value. For pharmaceuticals, an approach focusing on NPV appears adequate. However, in contrast to pharmaceuticals, agrochemicals provide a class of chemicals that is relatively data poor when it comes to data on pharmacological or nonpesticidal MOAs. Therefore, any MOA-driven approach in this respect should include evidence for both the activation as well as the absence of activation of relevant MOAs.
In order to make this approach work, it is important to identify MOAs for the tumor types with a currently unknown MOA. Subsequently, data on KEs in the respective MOAs has to be collected to identify which KE are suitable for inclusion in the new MOA-driven approach. The completeness and applicability of the collected data can then be assessed in case studies. In a next step, assays should be identified for detecting the selected KE. These assays may comprise in vitro, in silico and/or short term (up to 90 days) in vivo endpoints, combined with knowledge on toxicokinetics where applicable or best suited. Based on the network of endpoints that will arise from these steps, a weight-of-evidence approach could be defined to predict carcinogenic potential of agrochemicals without the need for a long-term rodent carcinogenicity assay. Once this approach is designed, a virtual waiver program for agrochemicals should be applied to test the approach for its robustness and to work toward regulatory acceptance. This should ultimately result in improved safety testing with the least use of animals, a higher human relevance, and thus provide a cost-effective improvement of hazard and risk assessment.
Supplemental Material
Download MS Excel (18.1 KB)Acknowledgments
The authors gratefully acknowledge Dr. Andrea Terron, European Food Safety Authority, Parma, Italy, and Dr. Stephanie Melching-Kolmuss, BASF, Limburgerhof, Germany, for being sparring partners throughout the project. Also, the authors would like to thank Dr. Shalenie den Braver-Sewradj, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands, for critical proofreading of the manuscript. The authors note with appreciation the value of four sets of comments and critiques provided by reviewers selected by the Editor and anonymous to the authors.
Declaration of interest
The authors declare that they have no conflict of interest. The employment affiliations of the authors are shown on the cover page. Data gathering and analysis was a joint task conducted by HH, HB, RG and ML. HH, HB and ML wrote the manuscript. PB, MC, JWL, DL, FM, IM, FS, GW, RW, RC and JM all provided written contributions pertaining to their expertise and participated in the preparation and editing of the manuscript. HH acted as corresponding author. This manuscript describes the results obtained in the project “Predicting carcinogenicity of agrochemicals”, that was funded by the European Partnership for Alternative Approaches to animal testing (EPAA, Brussels, Belgium). The project was coordinated by ML with RC and JM acting as co-lead. PB, MC, JWL, DL, FM, FS, GW, RW, RC and JM were part of the project team’s discussion group as representants of the various stakeholders in the field. IM acted as representant of the funder. HH, HB, RG, and ML are employed by the National Institute for Public Health and the Environment (RIVM), a governmental knowledge institute of the Dutch Ministry of Health, Welfare and Sport. Funding was received solely by the RIVM and solely used to fund HH, HB, RG, and ML. None of the other authors received compensation. The funder had no influence on the manuscript’s content. None of the authors have been involved in legal or regulatory matters related to the contents of the article.
Supplemental material
Supplemental material for this article is available online here.
References
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 29(3):730–741.
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. 2006. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 36(10):781–792.
- Boobis AR, Daston GP, Preston RJ, Olin SS. 2009. Application of key events analysis to chemical carcinogens and noncarcinogens. Crit Rev Food Sci Nutr. 49(8):690–707.
- Boobis AR, Cohen SM, Doerrer NG, Galloway SM, Haley PJ, Hard GC, Hess FG, Macdonald JS, Thibault S, Wolf DC, et al. 2009. A data-based assessment of alternative strategies for identification of potential human cancer hazards. Toxicol Pathol. 37(6):714–732.
- Borghoff SJ, Lagarde WH. 1993. Assessment of binding of 2,4,4-trimethyl-2-pentanol to low-molecular-weight proteins isolated from kidneys of male rats and humans. Toxicol Appl Pharmacol. 119(2):228–235.
- Bourcier T, McGovern T, Stavitskaya L, Kruhlak N, Jacobson-Kram D. 2015. Improving Prediction of Carcingenicity to reduce, refine and replace the use of experimental animals. J Am Assoc Lab Anim Sci. 54(2):163–169.
- Buckpitt AR, Bahnson LS. 1986. Naphthalene metabolism by human lung microsomal enzymes. Toxicology. 41(3):333–341.
- Cohen SM, Gordon EB, Singh P, Arce GT, Nyska A. 2010. Carcinogenic mode of action of folpet in mice and evaluation of its relevance to humans. Crit Rev Toxicol. 40:531–545.
- Cooper RL, Laws SC, Das PC, Narotsky MG, Goldman JM, Lee Tyrey E, Stoker TE. 2007. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol. 80(2):98–112.
- Corton JC, Cunningham ML, Hummer BT, Lau C, Meek B, Peters JM, Popp JA, Rhomberg L, Seed J, Klaunig JE. 2014. Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit Rev Toxicol. 44(1):1–49.
- Cruzan G, Bus J, Hotchkiss J, Harkema J, Banton M, Sarang S. 2012. CYP2F2-generated metabolites, not styrene oxide, are a key event mediating the mode of action of styrene-induced mouse lung tumors. Regul Toxicol Pharmacol. 62(1):214–220.
- Doe JE, Boobis AR, Dellarco V, Fenner-Crisp PA, Moretto A, Pastoor TP, Schoeny RS, Seed JG, Wolf DC. 2019. Chemical carcinogenicity revisited 2: current knowledge of carcinogenesis shows that categorization as a carcinogen or non-carcinogen is not scientifically credible. Regul Toxicol Pharmacol. 103:124–129.
- Doi AM, Hill G, Seely J, Hailey JR, Kissling G, Bucher JR. 2007. alpha 2u-globulin nephropathy and renal tumors in national toxicology program studies. Toxicol Pathol. 35(4):533–540.
- EC. 2008a. Council Regulation (EC) No 440/2008 of 30 May 2008 laying down test methods pursuant to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Official Journal, L 142.
- EC. 2008b. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006.
- EC. 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Official Journal, L 342.
- EC. 2013. Commission Regulation (EU) No 284/2013 of 1 March 2013 setting out the data requirements for plant protection products, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. Official Journal, L 93.
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA. 2016. Adverse outcome pathways-organizing toxicological information to improve decision making. J Pharmacol Exp Ther. 356(1):170–181.
- Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, Goodman JI, et al. 2014. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: a case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol. 44(1):64–82.
- Felter SP, Foreman JE, Boobis A, Corton JC, Doi AM, Flowers L, Goodman J, Haber LT, Jacobs A, Klaunig JE, et al. 2018. Human relevance of rodent liver tumors: key insights from a toxicology forum workshop on nongenotoxic modes of action. Regul Toxicol Pharmacol. 92:1–7.
- Gottmann E, Kramer S, Pfahringer B, Helma C. 2001. Data quality in predictive toxicology: reproducibility of rodent carcinogenicity experiments. Environ Health Perspect. 109(5):509–514.
- Herceg Z, Lambert MP, van Veldhoven K, Demetriou C, Vineis P, Smith MT, Straif K, Wild CP. 2013. Towards incorporating epigenetic mechanisms into carcinogen identification and evaluation. Carcinogenesis. 34(9):1955–1967.
- Hernandez JP, Mota LC, Baldwin WS. 2009. Activation of CAR and PXR by dietary, environmental and occupational chemicals alters drug metabolism, intermediary metabolism, and cell proliferation. Curr Pharmacogen Person Med. 7(2):81–105.
- Hernandez LG, van Steeg H, Luijten M, van Benthem J. 2009. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat Res. 682(2-3):94–109.
- Holsapple MP, Pitot HC, Cohen SM, Cohen SH, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, Dragan YP. 2006. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 89(1):51–56.
- Hurley PM. 1998. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ Health Perspect. 106(8):437–445.
- IARC 1999. Species differences in thyroid, kidney and Urinary bladder carcinogenesis. In Capen CD, Rice JM, Wilbourn JD (eds.) IARC Scientific Publications. Lyon: IARC.
- ICH 1995. Guideline S1A: need for carcinogenicity studies of pharmaceuticals.
- ICH 1997. Guideline S1B: testing for carcinogenicity of pharmaceuticals.
- ICH 2008. Guideline S1C (R2): dose selection for carcinogenicity studies of pharmaceuticals.
- International Conference on Harmonization (IC0H). 2013. Regulatory notice document: proposed change to rodent carcinogenicity testing of pharmaceuticals.
- Jacobs MN, Colacci A, Corvi R, Vaccari M, Aguila MC, Corvaro M, Delrue N, Desaulniers D, Ertych N, Jacobs A, et al. 2020. Chemical carcinogen safety testing: OECD expert group international consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch Toxicol. 94(8):2899–2923.
- Julien E, Boobis AR, Olin SS, Ilsi Research Foundation Threshold Working G, Ilsi Research Foundation Threshold Working Group. 2009. The key events dose-response framework: a cross-disciplinary mode-of-action based approach to examining dose-response and thresholds. Crit Rev Food Sci Nutr. 49(8):682–689.
- Kanno J, Onodera H, Furuta K, Maekawa A, Kasuga T, Hayashi Y. 1992. Tumor-promoting effects of both iodine deficiency and iodine excess in the rat thyroid. Toxicol Pathol. 20(2):226–235.
- Klaunig JE. 2018. Oxidative stress and cancer. Curr Pharm Des. 24(40):4771–4778.
- Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, et al. 2003. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 33(6):655–780.
- Klaunig JE, Kamendulis LM, Hocevar BA. 2010. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 38(1):96–109.
- Krohn K, Maier J, Paschke R. 2007. Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 3(10):713–720.
- Marty MS, Papineni S, Coady KK, Rasoulpour RJ, Pottenger LH, Eisenbrandt DL. 2015. Pronamide: weight of evidence for potential estrogen, androgen or thyroid effects. Regul Toxicol Pharmacol. 72(2):405–422.
- McGregor D, Bolt H, Cogliano V, Richter-Reichhelm HB. 2006. Formaldehyde and glutaraldehyde and nasal cytotoxicity: case study within the context of the 2006 IPCS Human Framework for the Analysis of a cancer mode of action for humans. Crit Rev Toxicol. 36(10):821–835.
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34(1):1–18.
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE. 2003. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 33(6):591–653.
- Motonaga K, Ota M, Odawara K, Saito S, Welsch F. 2016. A comparison of potency differences among thyroid peroxidase (TPO) inhibitors to induce developmental toxicity and other thyroid gland-linked toxicities in humans and rats. Regul Toxicol Pharmacol. 80:283–290.
- NTP (National Toxicology Program). 2016. Report on Carcinogens. 14th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.
- OECD. 2014. Guidance Document 116 on the conduct and design of chronic toxicity and carcinogenicity studies, supporting test guidelines 451, 452 and 453. 2nd ed. OECD series on testing and assessment, No. 116. Paris: OECD Publishing. https://doi.org/10.1787/9789264221475-en
- OECD. 2018a. Test No. 451: Carcinogenicity studies, OECD guidelines for the testing of chemicals, Section 4. Paris: OECD Publishing. https://doi.org/10.1787/9789264071186-en.
- OECD. 2018b. Test No. 453: Combined chronic toxicity/carcinogenicity studies, OECD guidelines for the testing of chemicals, Section 4. Paris: OECD Publishing. https://doi.org/10.1787/9789264071223-en.
- Oladimeji P, Cui H, Zhang C, Chen T. 2016. Regulation of PXR and CAR by protein-protein interaction and signaling crosstalk. Expert Opin Drug Metab Toxicol. 12(9):997–1010.
- Papineni S, Marty MS, Rasoulpour RJ, LeBaron MJ, Pottenger LH, Eisenbrandt DL. 2015. Mode of action and human relevance of pronamide-induced rat thyroid tumors. Regul Toxicol Pharmacol. 71(3):541–551.
- Pappy AL, 2nd, Oyesiku N, Ioachimescu A. 2016. Severe TSH elevation and pituitary enlargement after changing thyroid replacement to compounded T4/T3 therapy. J Investig Med High Impact Case Rep. 4(3):2324709616661834.
- Peffer RC, LeBaron MJ, Battalora M, Bomann WH, Werner C, Aggarwal M, Rowe RR, Tinwell H. 2018. Minimum datasets to establish a CAR-mediated mode of action for rodent liver tumors. Regul Toxicol Pharmacol. 96:106–120.
- Proctor DM, Gatto NM, Hong SJ, Allamneni KP. 2007. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci. 98(2):313–326.
- Proctor DM, Suh M, Chappell G, Borghoff SJ, Thompson CM, Wiench K, Finch L, Ellis-Hutchings R. 2018. An adverse outcome pathway (AOP) for forestomach tumors induced by non-genotoxic initiating events. Regul Toxicol Pharmacol. 96:30–40.
- Rasoulpour RJ, Andrus AK, Marty MS, Zhang F, Thomas J, LeBaron MJ, Papineni S, Pottenger LH, Eisenbrandt DL. 2015. Pronamide: human relevance of liver-mediated rat leydig cell tumors. Regul Toxicol Pharmacol. 72(2):394–404.
- SCCS 2016. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation; 9th revision (SCCS/1564/15), 25 April 2016.
- Simpkins JW, Swenberg JA, Weiss N, Brusick D, Eldridge JC, Stevens JT, Handa RJ, Hovey RC, Plant TM, Pastoor TP, et al. 2011. Atrazine and breast cancer: a framework assessment of the toxicological and epidemiological evidence. Toxicol Sci. 123(2):441–459.
- Sistare FD, Morton D, Alden C, Christensen J, Keller D, Jonghe SD, Storer RD, Reddy MV, Kraynak A, Trela B, et al. 2011. An analysis of pharmaceutical experience with decades of rat carcinogenicity testing: support for a proposal to modify current regulatory guidelines. Toxicol Pathol. 39(4):716–744.
- Strupp C, Banas DA, Cohen SM, Gordon EB, Jaeger M, Weber K. 2012. Relationship of metabolism and cell proliferation to the mode of action of fluensulfone-induced mouse lung tumors: analysis of their human relevance using the IPCS framework. Toxicol Sci. 128(1):284–294.
- Strupp C, Bomann W, Cohen SM, Weber K. 2016. Relationship of metabolism and cell proliferation to the mode of action of fluensulfone-induced mouse lung tumors. II: Additional mechanistic studies. Toxicol Sci. 154(2):296–308.
- Swenberg JA. 1993. Alpha 2u-globulin nephropathy: review of the cellular and molecular mechanisms involved and their implications for human risk assessment. Environ Health Perspect. 101 (Suppl 6):39–44.
- Thomson JP, Moggs JG, Wolf CR, Meehan RR. 2014. Epigenetic profiles as defined signatures of xenobiotic exposure. Mutat Res Genet Toxicol Environ Mutagen. 764-765:3–9.
- US EPA. 1991. Alpha 2u-globulin: association with chemically induced renal toxicity and neoplasia in the male rat. In Baetcke KPH (ed.) Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.
- van der Laan JW, Buitenhuis WH, Wagenaar L, Soffers AE, van Someren EP, Krul CA, Woutersen RA. 2016. Prediction of the carcinogenic potential of human pharmaceuticals using repeated dose toxicity data and their pharmacological properties. Front Med (Lausanne). 3:45.
- van der Laan JW, Kasper P, Silva Lima B, Jones DR, Pasanen M. 2016. Critical analysis of carcinogenicity study outcomes. Relationship with pharmacological properties. Crit Rev Toxicol. 46(7):587–614.
- Wolf DC, Cohen SM, Boobis AR, Dellarco VL, Fenner-Crisp PA, Moretto A, Pastoor TP, Schoeny RS, Seed JG, Doe JE. 2019. Chemical carcinogenicity revisited 1: a unified theory of carcinogenicity based on contemporary knowledge. Regul Toxicol Pharmacol. 103:86–92.
- Woutersen RA, Soffers AE, Kroese ED, Krul CA, van der Laan JW, van Benthem J, Luijten M. 2016. Prediction of carcinogenic potential of chemicals using repeated-dose (13-week) toxicity data. Regul Toxicol Pharmacol. 81:242–249.
- Yamada T, Kondo M, Miyata K, Ogata K, Kushida M, Sumida K, Kawamura S, Osimitz TG, Lake BG, Cohen SM. 2017. An evaluation of the human relevance of the lung tumors observed in female mice treated with permethrin based on mode of action. Toxicol Sci. 157(2):465–486.
- Zimmermann MB, Galetti V. 2015. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res. 8:8.