Abstract
Acute exposure to hydrogen sulfide initiates a series of hallmark biological effects that occur progressively at increasing exposure levels: odor perception, conjunctivitis, olfactory paralysis, “knockdown,” pulmonary edema, and apnea. Although effects of exposure to high concentrations of hydrogen sulfide are clear, effects associated with chronic, low-level exposure in humans is under debate, leading to uncertainty in the critical effect used in regulatory risk assessments addressing low dose exposures. This study integrates experimental animal, observational epidemiology, and occupational exposure evidence by applying a pathway-based approach. A hypothesized adverse outcome pathway (AOP) network was developed from 34 studies, composed of 4 AOPs sharing 1 molecular initiating events (MIE) and culminating in 4 adverse outcomes. A comparative assessment of effect levels and weight of evidence identified an AOP leading to a biologically-plausible, low-dose outcome relative to the other outcomes (nasal lesions, 30 ppm versus olfactory paralysis, >100 ppm; neurological effects, >80 ppm; pulmonary edema, >80 ppm). This AOP (i.e. AOP1) consists of the following key events: cytochrome oxidase inhibition (>10 ppm), neuronal cell loss (>30 ppm), and olfactory nasal lesions (defined as both neuronal cell loss and basal cell hyperplasia; >30 ppm) in rodents. The key event relationships in this pathway were supported by moderate empirical evidence and have high biological plausibility due to known mechanistic understanding and consistency in observations for diverse chemicals.
Introduction
Hydrogen sulfide (H2S) is a naturally occurring constituent in crude oil and natural gas and is emitted from sources such as volcanoes, sewers, and stagnant water. As such, occupational exposures can occur in oil and gas operations, but also in a myriad of other scenarios, including in sewage treatment plants, during agricultural tasks such as manure handling and during certain manufacturing processes (pulp and paper production, tanneries, viscose rayon production, etc.) (ACGIH Citation2010; ATSDR Citation2016). In acute, high exposure scenarios, this irritating, colorless gas induces a series of hallmark biological effects that occur progressively at increasing exposure levels, starting with effects like conjunctivitis (∼5–50 ppm) and olfactory paralysis (∼100 ppm) and progressing to “knockdown” (reversible acute central neurotoxicity; ∼500 ppm), pulmonary edema (∼200–750 ppm), and apnea (immediate paralysis of breathing; ∼1000 ppm) (Reiffenstein et al. Citation1992; Milby and Baselt Citation1999; Guidotti Citation2015). Human experience indicates that perception of “rotten eggs” at an approximate threshold of 0.1 ppm serves as an early warning signal (SCOEL Citation2007) to avoid/limit exposures.
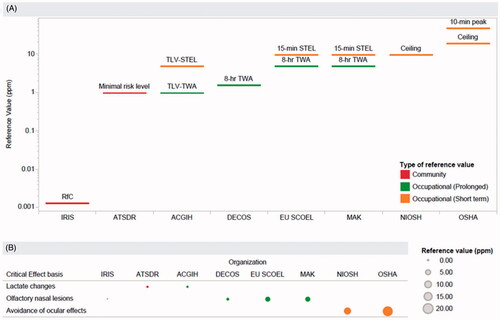
The derivation of a safe dose to protect against these effects can be based on human evidence; however, human studies often have limitations (e.g. limited exposure characterization, acute versus chronic considerations). In the case of H2S, human respiratory irritation is a key consideration to protect against short-term exposures (i.e. via the STEL), but human evidence assessing repeated, environmentally relevant exposures is lacking. Thus, olfactory nasal lesions observed in rats after subchronic inhalation exposure are often cited as hydrogen sulfide’s critical effect, due to the consistency with decreased olfactory function noted in humans (EPA US Citation2003; Health Council of the Netherlands Citation2006; SCOEL Citation2007; Hartwig Citation2013) (). Nonetheless, there are significant anatomical and functional differences across species, such as breathing patterns and nasal airflow characteristics, that create uncertainty in extrapolation of rodent nasal outcomes to humans (Harkema et al. Citation2006; Chamanza and Wright Citation2015), leading some organizations to select oxygen uptake and venous blood lactate concentration changes at 5 ppm as the critical effect (ACGIH Citation2010; ATSDR Citation2016) (). The divergent choices made across organizations exemplifies that a range of factors contribute to the uncertainty inherent to occupational exposure limit setting (Dourson et al. Citation1996; Deveau et al. Citation2015), which can include the physiological relevance of the animal model to of the human condition and functional adversity of the apical outcome.
Figure 1. Global reference values for hydrogen sulfide. A comparison of reference values (occupational (prolonged, short term) or community exposure) are shown in the top panel (A). The critical effect on which each value is based is shown in the bottom panel (B). IRIS: Integrated Risk Information System; ATSDR: Agency for Toxic Substances and Disease Registry; ACGIH: American Conference of Governmental Industrial Hygienists; DECOS: Dutch Expert Committee on Occupational Standards; EU SCOEL: European Union’s Scientific Committee on Occupational Exposure Limits; MAK: Maximale Arbeitsplatz Konzentration (MAK) (or Maximum Workplace Concentration) Commission; NIOSH: National Institute for Occupational Safety and Health; OSHA: Occupational Safety and Health Administration.
This review hypothesizes that consideration of the biological mechanisms leading to apical outcomes can increase the scientific justification supporting the selection of a point of departure, building on a previous case study in which a comparative weight of the evidence (WOE) assessment was conducted to identify an operant mode of action (MOA) for individual chemicals (Becker et al. Citation2017). Specifically, biological pathway analyses are applied as a means to identify a data-informed and biologically plausible point of departure for assessing human health risks associated with chronic exposures at environmentally relevant concentrations of H2S. The biological pathway analyses were conducted in a 2-step process, where mechanistic evidence was organized into an AOP network to facilitate identification of outcomes occurring at the lowest effect levels, followed by a comparative weight of evidence assessment in accordance with the World Health Organization/International Program on Chemical Safety’s Mode of Action/Species Concordance framework (Meek et al. Citation2014) to increase confidence (and reduce uncertainty) that the chosen effect is both human relevant and health-protective.
Materials and methods
Development of an AOP network
Using selected advisory agency reviews (EPA US Citation2003; SCOEL Citation2007; ACGIH Citation2010; ATSDR Citation2016), the biological effects data from the primary sources cited within these reviews were organized into key events leading to the hallmark adverse outcomes defined in the reviews as relevant for regulatory risk assessment. This is not a systematic review but relies on the extensive reviews conducted by credible organizations as a starting point.
Starting with the regulatory review, effects noted in the primary sources were categorized initially by level of biological organization. Next, effects were than sorted by tissue or organ system (e.g. nasal tissue, pulmonary tissue) and organized into linear pathways leading, following AOP development best practices (Villeneuve et al. Citation2014b; OECD Citation2018). Linear pathways were hypothesized based on professional judgment, linking potential key events identified in a tissue or organ system using a general understanding of biology (e.g. epithelial cell loss observed in pulmonary tissues might lead to accumulation of extravascular fluid in the lungs). The effects chosen as apical outcomes were those effects occurring at either tissue/organ or individual level of biological organization.
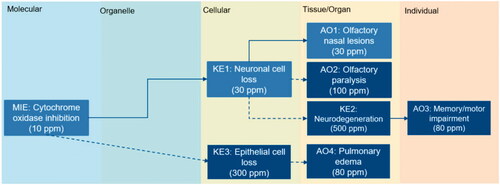
Throughout this article, the naming conventions for the MIEs, KEs, and AOs described in this AOP network will be consistent with the naming shown in . The AOPs are described in brief narrative form. For each AOP, the primary references and the basis for each effect level are documented in Supplementary Tables 1–4 to facilitate independent assessment of the interpretations and conclusions drawn in this analysis.
Assessment of each AOP in the network
The confidence in each AOP was assessed with regards to biological plausibility and empirical evidence following the recommendations in the OECD AOP Developer’s Handbook (OECD Citation2018). That is, biological plausibility was defined for each key event relationship (KER) by asking is there is a mechanistic (either structural or functional) relationship between the upstream and downstream key events consistent with established biological knowledge, where high biological plausibility is indicated by extensive previous documentation of a broadly accepted mechanistic basis, moderate is indicated by documentation of analogous biological relationships with incomplete scientific understanding, and weak is indicated by statistical association without function or structural understanding. The level of empirical support for a KER was assessed by asking if upstream key events occur at lower doses and earlier time points than downstream key events and/or if the frequency and incidence of an upstream KE is greater than downstream KE at the same dose, where high evidence is indicated by dependent change in both KEs following exposure to a wide range of stressors and extensive evidence with few inconsistencies, moderate indicated by demonstrated dependent change following exposure to a small number of stressors and some inconsistencies in the evidence, and low indicated by few or no studies documenting dependent change and/or lacking evidence. Based on the intended chemical-specific application, essentiality of the KEs was addressed as part of the chemical-specific assessment, rather than as part of the general AOP weight of the evidence assessment.
Application of the AOP network in risk assessment
The AOP network was used to identify the health outcomes occurring at the lowest exposure levels to be considered as candidate health-protective critical effects. For each AOP containing a candidate outcome, a comparative weight of evidence assessment was done, summarizing the H2S-specific data to assess dose-temporal concordance of the pathway as a whole (versus individual KER assessments) to ultimately identify the most sensitive pathway best supported by empirical evidence for H2S.
Results
Development
Cytochrome oxidase inhibition leading to nasal tissue outcomes
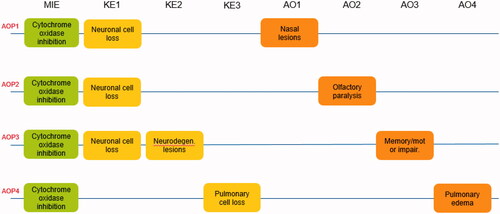
AOPs leading to nasal tissue outcomes were developed based on data indicating that the respiratory tract is sensitive to H2S exposure, as noted in a number of regulatory reviews (EPA US Citation2003; SCOEL Citation2007; ACGIH Citation2010; ATSDR Citation2016). Biomarkers of effects in nasal and lung tissue have been noted after inhalation exposure to H2S in a variety of studies, ranging from markers of cytotoxicity such as serum chemistry changes (e.g. lactate dehydrogenase, alkaline phosphatase), morphological changes (e.g. increased cellularity of nasal and bronchoalveolar lavage fluids), and histopathological changes (e.g. necrosis and exfoliation of respiratory and olfactory mucosal cells). Based on effects in the respiratory tract, two nasal-specific AOPs are proposed, initiated by inhibition of cytochrome oxidase and leading to distinct adverse outcomes: nasal lesions (AO1: defined by both neuronal cell loss and basal cell hyperplasia, where H2S-specific evidence is limited to animal studies), olfactory paralysis (AO2: loss of olfactory function, where H2S-specific evidence is limited to human experience) ().
Description of key events: nasal tissue outcomes
Oxidative phosphorylation is the key cellular pathway through which cells obtain energy, in the form of adenosine triphosphate (ATP). This pathway is mediated by a series of enzymes, including cytochrome oxidase, that are embedded in the inner mitochondrial membrane and generate an electrochemical gradient across this membrane (Kühlbrandt Citation2015; Cogliati et al. Citation2018). Due to the critical role in maintaining cellular energy, inhibition of any of the enzymes, including cytochrome oxidase (MIE), that are involved in oxidative phosphorylation can result in cellular stress responses such as a disruption to Ca + homeostasis and reactive oxygen species formation (Imaizumi et al. Citation2015; Bergman and Ben-Shachar Citation2016). The MIE can be measured in vitro as the rate of oxidation of reduced ferricytochrome c in tissue homogenates obtained from animals treated in vivo (Dorman et al. Citation2002).
In an environment of depleted energy, loss of viable cells (KE1) can occur as one of two distinct types. The first, apoptosis, is considered an active process, due to its dependence on the availability of ATP. Apoptosis includes the following sequential events: shrinkage, nuclear disassembly, and fragmentation of a cell into discrete bodies with intact plasma membranes. The second type, necrosis, is a more passive death pathway, characterized morphologically as a gain of cell volume, swelling of organelles, plasma membrane rupture, and loss of intracellular contents (Fink et al. Citation2005; Kroemer et al. Citation2009). In the nasal tissue, inhaled contaminants can damage distinct cell types, possibly due to increased deposition resulting from a combination of regional airflow and phys-chem properties of the inhaled substance. Alternatively, it is possible that certain cell populations (e.g. respiratory epithelium, olfactory epithelium, olfactory neurons) are uniquely susceptible to certain chemicals (Monticello et al. Citation1990; Harkema et al. Citation2006). KE1 can be measured as part of tissue histopathology visual inspection (i.e. reduction in thickness of the epithelial layer) or by immunohistochemical staining of tissue sections using cell-specific markers (Brenneman et al. Citation2000; Teeguarden Citation2017).
Of chemicals that target the olfactory epithelium, general categories of tissue damage have been described in animals, including degeneration, regeneration, post-degenerative atrophy, inflammation, respiratory metaplasia, and basal cell hyperplasia. The earliest change is generally degeneration of olfactory mucosa, usually by the loss of either sensory neurons (such as those in experimental studies on hydrogen sulfide; Brenneman et al. Citation2000) and 3-methylindole (Kim et al. Citation2010)). Olfactory neurons are unique in their ability to regenerate; however, under continued insult, this capacity can be exceeded, ultimately resulting in adaptive tissue remodeling or hyperplasia often with respiratory epithelia or basal cells (AO1) (Monticello et al. Citation1990; Hardisty et al. Citation1999; Teeguarden Citation2017). AO1 is defined as both neuronal cell loss and basal cell hyperplasia determined by visual histopathological assessment after in vivo rodent exposure (Hardisty et al. Citation1999).
A distinctly observed nasal tissue effect after exposure to inhaled chemical stressors is olfactory dysfunction (AO2), which can be characterized in humans as hyposmia (reduced sense of smell), anosmia (loss of odor perception), dysosmia (distorted odor perception), or paralysis (temporary loss of odor perception) (Stevenson Citation2010). Olfactory dysfunction can be reported clinically as change to the sense of smell or detected in rodent studies measured as change in behavior to avoid an odor or decreased in time to find food (Youngentob et al. Citation1997; Kim et al. Citation2010).
Cytochrome oxidase inhibition leading to neurological outcomes
The rationale for consideration of neurological effects was based on reports both in the clinical literature and in animal studies after exposure to H2S. Typical effects reported in humans include recurrent seizures, persistent headache, nausea, vomiting, fatigue, hearing impairment, movement disorders (e.g. spasticity, ataxia), altered psychological states, memory impairment, and vision impairment, among others (ACGIH Citation2010; Rumbeiha et al. Citation2016). In experimental animal studies, neurological effects after repeated exposure to H2S include changes in neuronal activity as measured by electroencephalographic activity (Skrajny et al. Citation1996), motor activity deficits (Struve et al. Citation2001), and functional deficits such as those measured by maze performance (Partlo et al. Citation2001; Struve et al. Citation2001). Based on these reported observations, we propose an AOP initiated via inhibition of cytochrome oxidase (MIE), which induces neuronal cell loss (KE1) in the brain, progressing to neurodegenerative lesions (KE2) and memory and/or motor deficits (AO3) ().
Description of key events: neurological outcomes
In addition to the biological description of the upstream key events in olfactory tissues discussed above, inhibition of cellular energy production (MIE) and neuronal cell loss (KE1) is relevant in neurological tissues as well, due to high energy requirements (Rugarli and Langer Citation2012; Bal-Price et al. Citation2018).
As a term, neurodegeneration combines two words: “neuro” in reference to nerve cells and “degeneration” in reference to progressive damage. As a diagnosis, neurodegeneration (KE2) is used to describe conditions that present with a loss of nerve structure and function and/or by neuronal cell loss. Multiple biological pathways can lead to neurodegeneration, including protein misfolding and aggregation, mitochondrial dysfunction, disrupted axonal transport, and neuroinflammation (Bal-Price et al. Citation2018; Wolfe Citation2018). Common clinical diseases (Alzheimer’s, Parkinson’s, Hungtindon’s) are defined by both neurodegeneration and progressive memory or motor loss (AO3) (Bal-Price et al. Citation2018).
Cytochrome oxidase inhibition leading to pulmonary outcomes
The final biological system considered as part of this network the pulmonary system. In human experience, pulmonary edema is relatively common after acute H2S exposure (Wasch et al. Citation1989; Tvedt et al. Citation1991; Schneider et al. Citation1998; SCOEL Citation2007). AOP4 is proposed to be initiated at the molecular level by inhibition of cytochrome oxidase, leading to cellular hypoxia and subsequent cell death in the pulmonary epithelium, ultimately leading to pulmonary edema ().
Description of key events: pulmonary outcomes
Normal lung physiology relies on circulation of both air and blood, which are relegated to their own conduits. The barrier between capillaries and the bronchoalveolar spaces consists of an epithelial cell layer. This barrier is semi-permeable, setting up a balance between the filtrate removed from pulmonary circulation and the fluid absorbed by the lymphatic system. Under normal conditions, this leaky epithelial membrane allows only a small volume of into the interstitial space. Under conditions of trauma, such as surgical ischemic/reperfusion injury, epithelial cell death (KE3) occurs, increasing capillary permeability and leading to excessive fluid translocation to the interstitial space (Baumann et al. Citation2007; Murray Citation2011; Assaad et al. Citation2018). KE3 can be detected in lung tissue histological and ultrastructural assessment (Lopez et al. Citation1988; Prior et al. Citation1990).
Pulmonary edema (AO4) is defined as the accumulation of extravascular water in the lungs. Two main types of pulmonary edema are characterized based on the preceding biological events: cardiogenic, which develops from increased capillary pressure due to heart failure, and noncardiogenic, which is associated with increased capillary permeability (Murray Citation2011; Assaad et al. Citation2018). Noncardiogenic pulmonary edema has been reported in association with central neurologic insult, in which increased capillary permeability arises due to localized ischemic insult in brain trigger zones (Baumann et al. Citation2007; Davison et al. Citation2012). AO4 can be measured as the ratio of lung weight immediately after removal from the thoracic cavity and after drying, as an estimate of amount of fluid in the lung (Prior et al. Citation1990).
Assessment
Biological plausibility
The early key events in AOP1-3 are well established across a broad chemical range (). Disruption of oxidative phosphorylation via cytochrome oxidase inhibition (MIE) initiates mitochondrial dysfunctional and cell death (KE1), as observed for chemicals such as rotenone (Moon et al. Citation2005), methylmercury (Yee and Choi Citation1996), and paraquat (Tawara et al. Citation1996). For neurons in particular, cell survival (KE1) is critically dependent on the integrity and functionality of mitochondria (MIE) as the main source of cellular energy. The specialized functions of neurons require a significant amount of energy, to maintain membrane potential and neurotransmitter loading and release from synaptic vesicles (Kann and Kovács Citation2007; Rugarli and Langer Citation2012).
Table 1. Assessment of biological plausibility for individual key event relationships.
Moving to downstream key events in AOP1, nasal cell-specific susceptibility to individual cell stressors is a well-documented phenomena, and those chemicals targeting neurons (KE1) (e.g. methyl bromide, zinc sulfate, chlorine, rotenone) induce lesions (AO1) described by necrosis of the olfactory epithelium and regenerative basal cell hyperplasia (Monticello et al. Citation1990; Hardisty et al. Citation1999; Harkema et al. Citation2006; Sasajima et al. Citation2015; Teeguarden Citation2017).
Specific to nasal outcomes, AOP1, which starts with inhibition of cytochrome oxidase and leads to olfactory nasal lesions, is considered to have high biological plausibility, based on known mechanistic understanding and the consistency in observations for diverse chemicals for the 2 KERs ().
Considering the unique KER in AOP2, chemicals are observed that both target olfactory neurons (KE1) and induce a loss of olfactory function (AO2) in humans. For example, wartime experience has shown that sulfur mustard can induce anosmia in humans; mechanistically, rodent inhalation studies have shown that sulfur mustard analogues induce oxidative damage to the olfactory epithelium resulting in neuron loss (O'Neill et al. Citation2011). Occupational exposures to nickel have reported associations with hyposmia and anosmia, which has been hypothesized to be induced by nickel-induced apoptosis of olfactory sensory neurons observed after intranasal instillation of nickel sulfate in mice (Jia et al. Citation2010). Studies assessing the cellular basis leading to loss of smell have suggested that disrupted regeneration of neurons in the olfactory epithelium are associated both with clinical symptoms (Huart et al. Citation2013; Boesveldt et al. Citation2017) and with mechanistic animal studies, assessing a variety of chemical agents such as sulfur mustard, nickel, and 3-methylindole (Jia et al. Citation2010; Kim et al. Citation2010; O'Neill et al. Citation2011).
Specific to nasal outcomes, AOP2, which starts with inhibition of cytochrome oxidase and leads to olfactory paralysis, the reviewed evidence supports a conclusion of moderate biological plausibility for AOP2, based on the limited experience of chemicals inducing both neuronal cell loss and degrees of olfactory dysfunction (). Although some instances have been identified, the relationship is neither extensively documented nor has broad scientific acceptance.
Considering KER leading to neurological outcomes (AOP3), the indirectly adjacent KER between inhibition of oxidative phosphorylation (MIE) and the neurological symptoms of memory/motor loss (AO3) have been implicated in a number of clinical outcomes, including Parkinson’s disease (Brown and Borutaite Citation2004), Alzheimer’s disease (McManus et al. Citation2011), and neurodegeneration (Wallace Citation1999). These outcomes are defined clinically by loss of neurons in specific regions of the brain (e.g. dopaminergic neurons in the substantia nigra) (KE1) or by neurodegeneration (KE2) (Fahn Citation2003; Wolfe Citation2018). Of note, the evidence supporting the relationship between mitochondrial dysfunction mediated by inhibition of oxidative phosphorylation to neurological effects (i.e. parkinsonian-like motor deficits, AO3) has already been characterized in an OECD-endorsed AOP with moderate to strong biological plausibility for each assessed KER (Bal-Price et al. Citation2018).
Specific to neurological outcomes, the reviewed evidence supports a conclusion of high biological plausibility for AOP3, based both on the biological understanding gained through clinical disease defined by loss of neurons leading to memory/motor loss and mechanistic understanding across a diverse chemical space for all KERs (1 indirect and 3 direct, ).
Considering the KEs leading to pulmonary outcomes (AOP4), clinical evidence derived from human literature characterizes pulmonary edema (AO4) as arising from localized ischemic insult (MIE), which results in increased capillary permeability, permitting extravascular fluid to leak into the lungs (Baumann et al. Citation2007). This general etiology of pulmonary edema is not derived from the H2S literature, but rather from non-chemical specific clinical medicine. Further, noncardiogenic pulmonary edema (AO4) is often associated clinically with central neurologic insult, presumably due to the subsequent disruption to cellular respiration (MIE).
Considering the adjacent KERs, it has been demonstrated that disruption of cellular respiration via inhibition of enzymes responsible for oxidative phosphorylation (MIE) leads to cell death (KE3) (Imaizumi et al. Citation2015). However, there is a lack of established biological knowledge that this relationship occurs in pulmonary tissues specifically. Similarly, limited studies were found in which a relationship was observed between pulmonary cytotoxicity (KE3) and pulmonary edema (AO4), despite a reasonable mechanistic explanation that leakage of fluid into the lungs can occur after death of either alveolar cells or capillary endothelial cells.
Specific to pulmonary outcomes, the reviewed evidence supports a conclusion of moderate biological plausibility, due to the limited chemical space in which most of these relationships are observed (). Although the relationship between MIE and AO4 is considered high due to the demonstrated clinical evidence and mechanistic understanding, the 2 direct KERs in AOP3 are considered to have moderate biological plausibility since these relationships have not been evaluated or observed for many chemicals.
Empirical evidence
In all of the individual pathways, the upstream KERs (inhibition of cytochrome oxidase leading to neuronal or epithelial cell loss) are considered to be supported by strong experimental evidence (), with multiple chemical stressors inducing a reduction in cell viability after inhibition of cytochrome oxidase in a dose- and temporally consistent manner. As an example, H2S inhibited cytochrome oxidase approximately 10–20% in rats treated with 10–30 ppm for 4 h versus control rats after acute exposure (Khan et al. Citation1990; Dorman et al. Citation2002). Histopathological detection of cell death was only observed at higher concentrations (i.e. necrosis observed in 1/5, 3/5 and 4/5 rats at 80, 200, and 400 ppm, but not at 30 ppm (Brenneman et al. Citation2002)) or longer exposures (subchronic exposure: necrosis observed at 30 and 70 ppm with an severity score of 1.4 and 2.4 (max: 3), respectively; Brenneman et al. Citation2000).
Table 2. Assessment of empirical evidence for individual key event relationships.
The empirical evidence underpinning the downstream KERs in each of the AOPs ranges is less extensive, characterized as weak to moderate based on the targeted literature searches ().
Limitations and/or conflicting evidence
Inconsistencies were identified through consideration of animal studies aimed at investigating the hypothesis that olfactory neuron loss (KE1) can affect odor detection (AO2). Although the studies above did note chemical-induced olfactory functional deficits in animals for sulfur mustard, nickel and 3-methylindole, destruction of approximately 95% of rat olfactory epithelium by methyl bromide did not negatively impact performance in an odor differential response task when group performance was assessed (Youngentob et al. Citation1997). Some individual rats did perform significantly better with an intact olfactory epithelium. It is possible that rodents have a higher ability to maintain near-normal odor detection during continual damage than humans, due to differences in due to differences in the relative percentage of nasal epithelium committed to olfaction (humans, 3% versus rodents, 50%) (Youngentob et al. Citation1997; Harkema et al. Citation2006; O'Neill et al. Citation2011).
Application
AOPs are tools to organize biological effects data, to link early key events with adverse outcomes that are relevant for regulatory risk assessment. Application of the AOP in risk assessment decision-making requires a fit between the level of confidence supporting these linkages and the type of decision being made. In this case, the objective is to identify a critical effect that is protective against all likely effects and is relevant to humans. For H2S, mapping the effects cited in existing regulatory risk assessments identified pathways leading to tissue-specific outcomes, each with differing levels of confidence (concluded based on empirical evidence and biological plausibility). To identify a critical effect with high confidence that can be used to derive occupational exposure levels, individual AOPs were assessed to identify the effect that occurs comparatively at the lowest dose with the highest confidence.
Chemical-specific WOE assessment
Because the problem formulation for this analysis is specific to H2S as a chemical stressor, a comparative WOE assessment was done specifically using H2S data. Regarding nasal outcomes, AOP1 integrates empirical evidence from twelve studies. Assessing the key event relationships in AOP1, consistency in temporal- and dose-responsiveness is observed between key events (). That is, later key events are observed at doses/time points exceeding those at which the early key events occur (detailed list of individual studies/effect levels can be found in Supplementary Table 1). Specifically, the most upstream key event, MIE, was observed at the lowest dose, 10 ppm (Khan et al. Citation1998), compared to downstream KEs: KE1, 30 ppm (Brenneman et al. Citation2000; Dorman et al. Citation2004); AO1, 30 ppm (Brenneman et al. Citation2000); and AO2, 80 ppm (Dorman et al. Citation2004). However, direct evidence to assess the KEs in AOP1 collectively in a single study was not identified. Based on the consistency in this dose-temporal pattern across the AOP, together with the high biological plausibility, AOP1 (olfactory nasal lesions) is considered to have higher confidence for use as a potential point of departure for H2S exposure limits.
Figure 3. Dose–response and temporal concordance analysis of (A) AOP1 (nasal tissue outcomes: olfactory nasal lesions), (B) AOP2 (nasal tissue outcomes: olfactory paralysis), (C) AOP3 (neurological outcomes), and (D) AOP4 (pulmonary outcomes). +: 0–33% inhibition or mild severity; /++: 34–66% inhibition or moderate severity; /+++: 67–100% inhibition or massive severity; *observed and reported qualitatively.
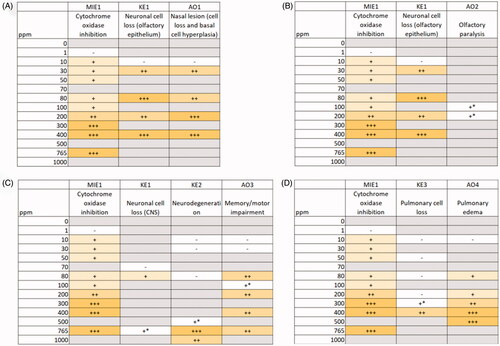
The second pathway related to nasal outcomes, AOP2, integrates empirical evidence from 13 studies, where 11 studies that provide supporting data for MIE and KE1 and were conducted in experimental animals (detailed list of effect levels per study in Supplementary Table 2). The remaining 2 studies report effects observed in humans, with estimated doses based on case reports (Beauchamp et al. Citation1984; van Aalst et al. Citation2000). Limited conclusions can be drawn on the dose-concordance between neuronal cell loss (KE2), measured in experimental animal studies, and olfactory paralysis (AO2), due to the uncertainty in quantitative effects levels at which olfactory paralysis occurs in humans. The minimal doses at which neuronal cell loss is observed appear to be lower than the minimal doses at which olfactory paralysis is observed (), however, this assessment is complicated by the timing at which these effects are observed after exposure. Temporally, olfactory paralysis has been reported to occur within minutes (Beauchamp et al. Citation1984); however, experimental animal data were not identified that evaluated neuronal cell loss (the upstream key event) at this time frame. Experimental animal studies only made histopathological assessments ≥24 h after acute exposure (Lund and Wieland Citation1966; Brenneman et al. Citation2002; Anantharam et al. Citation2017), limiting the ability to directly assess the essentiality of these KEs collectively. Considering the lack of data by which the dose-dependence of the key events in this pathway can be assessed after H2S exposure, AOP2 (olfactory paralysis) has lower confidence for use as a potential point of departure for H2S exposure limits.
Regarding neurological outcomes, AOP3 integrates H2S-specific empirical evidence from 14 studies supporting the key events in this AOP. Assessing the key events in AOP2 together, the empirical evidence is concluded to be moderate. While each individual key event shows dose-responsive pattern itself, there is a gap in available evidence in the mid-dose range and at least one noted inconsistency, where memory/motor deficits appear to occur at lower doses than neurodegeneration (; more detail provided in Supplementary Table 3). Specifically, no evidence of neurodegeneration was observed at 80 ppm after 90 days of treatment in mice and 2 strains of rats (Sprague Dawley and Fischer 344) (CIIT Citation1983a, Citation1983b, Citation1983c), despite the observation of decreased learning performance (treated animals made more tries to complete radial arm maze task versus control, p = 0.01) at this dose (Partlo et al. Citation2001) after 5 days of exposure. As noted, there are no studies assessing neuronal cell loss in CNS tissues and neurodegeneration between 80 ppm and 500 ppm, limiting concordance and essentiality conclusions. Given the noted inconsistency in dose-temporal patterns, AOP3 (neurological outcomes) has lower confidence for use as a potential point of departure for H2S exposure limits.
Regarding pulmonary outcomes, AOP4 integrates H2S-specific empirical evidence from 14 studies supporting the key events in this AOP. After acute exposures in animals, H2S produced cytotoxicity in the pulmonary epithelium at high doses as indicated by changes in cellularity of bronchoalveolar gavage fluid and histological changes such as necrosis and exfoliation of mucosal cells lining the airways (Lopez et al. Citation1987, Citation1988; Prior et al. Citation1990; Khan et al. Citation1991; Wu et al. Citation2011). Pulmonary edema observations ranged from slight congestion in the lungs after 1 h exposure to 78 ppm H2S (Kohno et al. Citation1991) to moderate/massive pulmonary edema after 4 h exposure to >200 ppm H2S (Prior et al. Citation1990; Green et al. Citation1991). Although the severity of pulmonary edema is observed with increasing dose-responsiveness, the key events relationships do not display the expected dose- and temporal-responsiveness; that is, the downstream KE (pulmonary edema) is observed at doses below that which the upstream KE (pulmonary cell loss) occurs: 80–100 ppm (; Supplementary Table 4). AOP3 is supporting by low empirical evidence, suggesting that pulmonary edema induced by H2S is not initiated via pulmonary cell loss. Given this inconsistency and lack of direct evidence to assess essentiality of the KEs, AOP4 (pulmonary outcomes) has lower confidence for use as a potential point of departure for H2S exposure limits.
Comparative assessment of effect levels
Based on the H2S-specific data, the minimum effect level at which each adverse outcome occurred was compared to identify the effects occurring at the lowest doses for consideration as health-protective critical effects. As noted earlier, the series of key events leading to H2S’s hallmark effects are thought to start with the inhibition of cytochrome oxidase (MIE), a key component in cellular respiration. The molecular events are proposed to lead to a series of tissue-specific downstream events (e.g. nasal lesions (AO1), olfactory paralysis (AO2), memory/motor impairment (AO3), pulmonary edema (AO4)). At high doses (>400 ppm), mortality occurs. Of the networked tissue- and individual-level effects occurring at doses below those at which mortality is the key concern, the key events in AOP1 are induced by H2S at the lowest doses: cytochrome oxidase inhibition (MIE, 10 ppm), neuronal cell loss (KE1, 30 ppm), and olfactory nasal lesions (AO1, 30 ppm). Comparatively, the other potential adverse outcomes occur at higher effect levels: olfactory paralysis (>100 ppm), pulmonary edema (≥80 ppm), neurodegenerative lesions (≥500 ppm) (). Comparison of effect levels suggests that the key events in AOP1 are likely candidates for consideration as the critical effect to be used in the derivation of H2S exposure limits.
Comparative assessment of confidence
Three different measures of confidence were assessed: biological plausibility of the KERs, empirical evidence of the KERs, and a comparative WOE assessment for the AOPs as a whole. Considering all three measures, the pathway leading to nasal outcomes, AOP1, is comparatively supported by strong WOE, where individual KERs are considered to have high biological plausibility and moderate empirical evidence based on multiple chemical stressor data and the pathway demonstrates an expected pattern of dose-/temporal-concordance after exposure to H2S (). Each of the remaining AOPs has gaps or inconsistencies in at least one measure of confidence; for example, AOP2 is considered to have lower confidence based on the chemical-specific assessment of dose-/temporal-concordance, due to the lack of data evaluating the anticipated timing at which neuronal cell death (KE2) can be detected relative to the observations of olfactory paralysis (AO3). AOP3 and AOP4 are considered to have lower confidence, also due to the H2S-specific pathway WOE assessment, due to inconsistent dose-response across KE in the pathway (i.e. downstream AO are observed at lower doses than upstream KEs).
Table 3. Comparative assessment of confidence measures for the AOPs.
Rationale for selection of a point of departure
For the purposes of deriving an OEL, all studies assessing potential health effects due to H2S exposure were organized into a proposed adverse outcome pathway (AOP) network, to incorporate the mechanistic data into the decision-making process and to increase confidence in the selection of the critical effect that will serve as the point of departure. Initially, the lowest effect levels of all possible tissue- and individual-level key events were compared to identify candidate critical effects that would be protective of the range of possible adverse outcomes, finding that the KE in AOP1 leading to olfactory nasal lesions may be appropriate as protective points of departure. Next, weight of evidence considerations were used to distinguish which outcome would provide greater confidence for use in risk assessment decision-making. Based on this qualitative assessment, the AOP leading to nasal lesions was assessed to have high biological plausibility, moderate empirical evidence supporting the KERs, and high dose-temporal concordance across the entire pathway. Comparatively, the weight of evidence is strongest for nasal lesions, according to each measure (biological plausibility, KER empirical evidence, and pathway dose-temporal concordance), lending confidence to the selection of these KE as potential points of departure in the derivation of H2S exposure limits.
Discussion
One step in the development of occupational exposure limits is the selection of the critical effect, or point of departure, associated with exposure to a chemical. This choice can vary across organizations and may reflect both problem formulation (e.g. is the exposure limit relevant for intermittent use or continuous use over an 8-h work day) and risk policy (e.g. is higher weight placed on animal studies with controlled experimental conditions or human experience) (Deveau et al. Citation2015). In practice, this can lead different organizations to select distinct outcomes, as is the case with H2S, partially contributing to a range of global exposure limits for H2S (0.001–10 ppm) (as depicted and referenced in ). To move toward harmonization, new evidence integration methods provide a means to incorporate mechanistic information into the selection of a point of departure and increase confidence in this selection via added biological context. To this end, an adverse outcome pathway approach was applied to integrate mechanistic information, demonstrating consistency in both response across experimental conditions and quantitative effect levels across related biological events.
Using this approach, an AOP network proposed based on H2S-specific effects, organized around one MIE, cytochrome oxidase inhibition, as the often-cited biological event triggering multiple outcomes in distinct tissues (olfactory, nervous, pulmonary) (Beauchamp et al. Citation1984; Reiffenstein et al. Citation1992; ACGIH Citation2010; Guidotti Citation2015). The KERs in the AOP network were assessed in terms of biological plausibility and empirical evidence based on multiple chemical stressors that activate the same MIE (inhibition of cytochrome oxidase), including potassium cyanide, sodium azide, and beta amyloid peptides. Finally, the likelihood that H2S acts via each AOP was assessed via consideration of the dose-/temporal-concordance of the key events collectively. Based on the 3 measures of confidence, the AOP leading to olfactory nasal lesions was assessed to have the strongest WOE in comparison to the other 3 AOPS, where the KERs were considered to be have high biological plausibility and supported by moderate empirical evidence and the KEs collectively exhibited a consistent dose-/temporal-pattern (). Consequently, the KEs in this AOP (cytochrome oxidase inhibition, neuronal cell loss, and olfactory nasal lesions) are viable candidates as potential points of departure for the derivation of an occupational exposure limit for H2S.
Although the AOP network is based on a single MIE, it’s unlikely that this is the sole interaction responsible for the diverse biological signaling mediated by H2S, considering that H2S interacts with a network of enzymes and is recognized as an endogenous neuromodulator (Reiffenstein et al. Citation1992; Zhang and Bian Citation2014). Hypothesized pathways initiated by alternate molecular events were considered but not included here due to limited supporting mechanistic data. For example, the release of various neurotransmitters, including glutamate, gamma-aminobutyric acid (GABA) and noradrenaline (Savolainen et al. Citation1980; Kombian et al. Citation1988; Hannah et al. Citation1989; Warenycia et al. Citation1989; Nicholson et al. Citation1998) were considered as potential MIEs that might lead to CNS toxicity, an outcome observed after acute, high-exposure conditions (Milby and Baselt Citation1999; Guidotti Citation2015). However, the studies identified in our search did not evaluate a common set of neurotransmitters in specific tissue regions, preventing identification of specific key event relationships. It is possible that a targeted systematic review could be conducted to tease out key events with more specificity.
The AOPs developed in this analysis do not clearly reflect the human effects (i.e. oxygen uptake and venous blood lactate concentration) that are cited as the critical effect basis for H2S exposure limits recommended by some organizations that have set exposure limits (ACGIH Citation2010; ATSDR Citation2016). These effects in human volunteers were observed in a series of 5 publications assessing physiological responses to H2S over short time periods during exercise, conducted following similar but distinct protocols (Bhambhani and Singh Citation1991; Bhambhani et al. Citation1994, Citation1996a, Citation1996b, Citation1997). Individually, the studies identified statistically significant responses, but examination of the five studies collectively reveals no pattern of reproducible adverse effects in the range of 0.5–10 ppm. That is, changes in oxygen uptake are observed but not consistently; Bhambhani and Singh (Citation1991) found increased oxygen uptake at 5 ppm under conditions of exhaustive exercise, but no significant changes in two different studies at the same dose under moderate exercise (Bhambhani et al. Citation1994, Citation1996b). At 10 ppm, decreased oxygen uptake was identified with moderate exercise (Bhambhani et al. Citation1997). Its plausible that increased blood lactate reflects a shift in the ratio of cellular energy production via oxidative phosphorylation relative to glycolysis, consistent with the MIE. In this respect, increased blood lactate is more consistent with a biomarker of MIE rather than a key event in and of itself. Beyond this observation, the current analysis did not identify a mechanistic linkage between the physiological responses observed in the human volunteer studies and the adverse outcomes noted in animal studies or to the biological hallmarks indicative of human exposure to H2S.
Biological response pathway assessments have proven to be a useful tool in chemical risk assessment in wide-ranging applications, including clarification that carcinogenic effects in animals are qualitatively or quantitatively not possible in humans (Holsapple et al. Citation2006; Proctor et al. Citation2007), informing chemical grouping for the purposes of cumulative risk assessment (Pistollato et al. Citation2020), and providing the mechanistic context for integrated assessment and testing approaches (Patlewicz et al. Citation2014). Although sometimes referred to interchangeably, a key distinguishing factor between adverse outcome pathway (AOP)-based analysis and mode of action (MOA)-based analyses is that AOPs are intended to be “chemically agnostic” whereas MOAs are chemical-specific (Villeneuve et al. Citation2014a). In other words, AOPs are descriptions of general biological responses that should hold true regardless of chemical stressor, while MOAs focus on the likelihood of a specific chemical to initiate and progress through a defined set of key events, taking into account toxicokinetic/toxicodynamic considerations that impact the target-tissue dose required to “tip” a biological system from one key event to the next. The current analysis applies these frameworks in a step-wise process to utilize the strength of each framework, first relying on the AOP framework to organize all evidence (human, animal, mechanistic) into a series of hypothesized key event relationships describing biological pathways and then using the MOA framework to comparatively assess the weight of the evidence supporting each pathway to assess the fit between confidence in the pathway and application in decision-making. The AOP development phase was done iteratively, where the network was hypothesized initially using only H2S data but additional, targeted literature searches were conducted to inform biological plausibility and empirical evidence assessments of KERs.
One potential limitation of AOP development is lack of clarity in the evidence identification process, especially when development and assessment are done in multiple rounds of literature searching as described above. Even though AOPs are considered chemically agnostic in nature, the KERs within an AOP are hypothesized and assessed using data specific to one chemical or a group of chemicals. Clear documentation of the literature search strategy as part of the AOP development process has been highlighted as an opportunity to increase the potential utility of AOPs in regulatory decision-making (Kleinstreuer et al. Citation2016; Leist et al. Citation2017), and collaborative initiatives are ongoing to align on best practices in the application of systematic literature review tools during AOP development (de Vries et al. Citation2021, accepted in press). The current study relied on existing systematic reviews on a well-studied chemical, however, in practice, these reviews are older and do not reflect more recently published studies. In future studies, systematic literature review tools such as SWIFT-Review, would offer improved transparency for the follow-up searches on individual KERs.
One final point that warrants discussion is that the conclusions drawn in this study may be considered contradictory to a MOA-based assessment of similar tumors (nasal neuroblastomas and nasal respiratory epithelial adenomas) induced by naphthalene inhalation exposure in rodents. For H2S, the MOA-based conclusion drawn here is that the key events leading to olfactory nasal lesions in rodents are relevant to humans, while the cytotoxicity-induced nasal tumors occurring after naphthalene exposure are concluded not to be relevant to human risk assessment due to quantitative MOA differences between species (Rhomberg et al. Citation2010; Piccirillo et al. Citation2012; Bailey et al. Citation2016). Two key reasons support that these conclusions are not truly contradictory. First, naphthalene’s cytotoxic and tumorigenic responses are dependent on metabolic activation, primarily oxidation of naphthalene via CYP2F (Bogen et al. Citation2008). On the other hand, the fast-acting effects associated with H2S, such as near-immediate olfactory paralysis and knockdown, do not appear to be associated with metabolism. Second, there is limited human epidemiological evidence consistent with nasal or lung tumor risk for naphthalene (Piccirillo et al. Citation2012), whereas there is ample human evidence indicating H2S induces nasal tissue effects in humans (i.e. olfactory paralysis). Therefore while these chemicals do have common KEs within their MOAs, they fundamentally function by different MOAs due to toxicokinetic differences.
Conclusions
A biological pathway-based approach is a means to incorporate different lines of evidence into a single framework to increase confidence in the identification of a biologically plausible effect that can be used to derive a health-protective exposure limit. In this analysis, animal and human data from 34 studies identified from existing systematic literature reviews on H2S were used to propose an Adverse Outcome Pathway (AOP) network, composed of 4 AOPs sharing 1 molecular initiating event (MIE) and 4 potential outcomes. Potential health-protective critical effects (cytochrome oxidase inhibition, neuronal cell loss, olfactory nasal tumors) were identified through consideration of effect levels to distinguish lower- from higher-dose outcomes, followed by a more focused assessment of dose-temporal concordance specific to the H2S-data.
Acknowledgments
The authors would like to gratefully acknowledge Bruce Copley and Felix Boachie (former epidemiologist and current industrial hygienist, respectively, at ExxonMobil Biomedical Sciences, Inc.) for initial discussions on organizing hydrogen sulfide effects data into the AOP framework; Jessica Ryman-Rasmussen (policy advisor at American Petroleum Institute) for discussions on the topic of hydrogen sulfide exposure limits; James Freeman, Robert Barter (retired and current toxicologists, ExxonMobil Biomedical Sciences, Inc.) and members of ExxonMobil’s Occupational Exposure Limit committee for their encouragement to write this manuscript; Christine Palermo (toxicologist at ExxonMobil Biomedical Sciences, Inc.) for her review and comment on the draft manuscript which helped in particular to clarify the weight of evidence considerations; to Bette Meek (Associate Director, Chemical Risk Assessment at the University of Ottawa) and Brigitte Landesmann (scientist at the European Commission, Joint Research Centre) for review of the preprint version of this manuscript and comments related to the application of the AOP framework in decision-making; and to the external reviewers selected by the Editor and anonymous to the Authors whose comments were valuable in revising and refining the manuscript.
Declaration of interest
The authors are current (KOG) or retired (RJL) employees of ExxonMobil Biomedical Sciences, Inc., a separately incorporated but wholly owned affiliate of Exxon Mobil Corporation. This company processes crude oil and natural gas that contains hydrogen sulfide as a naturally occurring component. The manuscript was written as part of the authors’ normal employment and was the sole responsibility of the authors. No external funding was obtained for manuscript preparation. None of the authors has appeared in any legal or regulatory proceedings related to the contents of this paper.
Supplemental material
Supplemental data for this article can be accessed here.
References
- ACGIH 2010. Hydrogen sulphide. Documentations of the threshold limit values and biological exposure indices, Seventh Edition – 2010. Cincinnati, Ohio: American Conference of Governmental Industrial Hygienists (ACGIH).
- Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. 2002. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 161(5):1783–1796.
- Anantharam P, Whitley EM, Mahama B, Kim DS, Imerman PM, Shao D, Langley MR, Kanthasamy A, Rumbeiha WK. 2017. Characterizing a mouse model for evaluation of countermeasures against hydrogen sulfide-induced neurotoxicity and neurological sequelae. Ann NY Acad Sci. 1400(1):46–64.
- Assaad S, Kratzert WB, Shelley B, Friedman MB, Perrino A. 2018. Assessment of pulmonary edema: principles and practice. J Cardiothorac Vasc Anesth. 32(2):901–914.
- ATSDR. 2016. Toxicological profile for hydrogen sulfide/carbonyl sulfide. Atlanta (GA): US Department of Health and Human Services, Public Health Service.
- Bailey LA, Nascarella MA, Kerper LE, Rhomberg LR. 2016. Hypothesis-based weight-of-evidence evaluation and risk assessment for naphthalene carcinogenesis. Crit Rev Toxicol. 46(1):1–42.
- Bal-Price A, Leist M, Schildknecht S, Tschudi-Monnet F, Paini A, Terron A. 2018. Adverse outcome pathway on inhibition of the mitochondrial complex I of nigro-striatal neurons leading to parkinsonian motor deficits. OECD Series on Adverse Outcome Pathways, No. 7. Paris: OECD Publishing. DOI:https://doi.org/10.1787/b46c3c00-en.
- Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet P-F. 1998. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol. 19(4):573–581.
- Baumann A, Audibert G, McDonnell J, Mertes PM. 2007. Neurogenic pulmonary edema. Acta Anaesthesiol Scand. 51(4):447–455.
- Beauchamp R, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA, Leber P. 1984. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 13(1):25–97.
- Becker RA, Dellarco V, Seed J, Kronenberg JM, Meek B, Foreman J, Palermo C, Kirman C, Linkov I, Schoeny R, et al. 2017. Quantitative weight of evidence to assess confidence in potential modes of action. Regul Toxicol Pharmacol. 86:205–220.
- Bergman O, Ben-Shachar D. 2016. Mitochondrial Oxidative Phosphorylation System (OXPHOS) deficits in schizophrenia: possible interactions with cellular processes. Can J Psychiatry. 61(8):457–469.
- Bhambhani Y, Burnham R, Snydmiller G, MacLean I. 1997. Effects of 10-ppm hydrogen sulfide inhalation in exercising men and women. Cardiovascular, metabolic, and biochemical responses. J Occup Environ Med. 39(2):122–129.
- Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Lovlin R. 1996a. Effects of 10-ppm hydrogen sulfide inhalation on pulmonary function in healthy men and women. J Occup Environ Med. 38(10):1012–1017.
- Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Martin T. 1994. Comparative physiological responses of exercising men and women to 5 ppm hydrogen sulfide exposure. Am Ind Hyg Assoc J. 55(11):1030–1035.
- Bhambhani Y, Burnham R, Snydmiller G, MacLean I, Martin T. 1996b. Effects of 5 ppm hydrogen sulfide inhalation on biochemical properties of skeletal muscle in exercising men and women. Am Ind Hyg Assoc J. 57(5):464–468.
- Bhambhani Y, Singh M. 1991. Physiological effects of hydrogen sulfide inhalation during exercise in healthy men. J Appl Physiol (1985). 71(5):1872–1877.
- Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, Martens J, Ngai J, Duffy VB. 2017. Anosmia – a clinical review. Chem Senses. 42(7):513–523.
- Bogdanffy MS, VAN DER Meulen HCD, Beems RB, Feron VJ, Cascieri TC, Tyler TR, Vinegar MB, Rickard RW. 1994. Chronic toxicity and oncogenicity inhalation study with vinyl acetate in the rat and mouse1. Toxicol Sci. 23(2):215–229.
- Bogen KT, Benson JM, Yost GS, Morris JB, Dahl AR, Clewell HJ, Krishnan K, Omiecinski CJ. 2008. Naphthalene metabolism in relation to target tissue anatomy, physiology, cytotoxicity and tumorigenic mechanism of action. Regul Toxicol Pharm. 51(2):27–36.
- Brenneman KA, James RA, Gross EA, Dorman DC. 2000. Olfactory neuron loss in adult male CD rats following subchronic inhalation exposure to hydrogen sulfide. Toxicol Pathol. 28(2):326–333.
- Brenneman KA, Meleason DF, Sar M, Marshall MW, James RA, Gross EA, Martin JT, Dorman DC. 2002. Olfactory mucosal necrosis in male CD rats following acute inhalation exposure to hydrogen sulfide: reversibility and the possible role of regional metabolism. Toxicol Pathol. 30(2):200–208.
- Brown GC, Borutaite V-B. 2004. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 1658(1–2):44–49.
- Burté F, Carelli V, Chinnery PF, Yu-Wai-Man P. 2015. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 11(1):11–24.
- Chamanza R, Wright J. 2015. A review of the comparative anatomy, histology, physiology and pathology of the nasal cavity of rats, mice, dogs and non-human primates. Relevance to inhalation toxicology and human health risk assessment. J Comp Pathol. 153(4):287–314.
- Chi H, Chang H-Y, Sang T-K. 2018. Neuronal cell death mechanisms in major neurodegenerative diseases. IJMS. 19(10):3082.
- Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. 2015. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases [Review]. Environmental factors in neurodegeneration. Front Cell Neurosci. 9:124.
- CIIT. 1983a. 90-Day vapor inhalation toxicity study of hydrogen sulfide in B6C3F1 mice. Research Triangle Park, NC. Chemical Industry Institute of Toxicology CIIT docket #42063.
- CIIT. 1983b. 90-Day vapor inhalation toxicity study of hydrogen sulfide in Fischer 344 rats. Research Triangle Park, NC. Chemical Industry Institute of Toxicology CIIT docket #22063.
- CIIT. 1983c. 90-Day vapor inhalation toxicity study of hydrogen sulfide in Sprague-Dawley rats. Research Triangle Park, NC. Chemical Industry Institute of Toxicology CIIT docket #32063.
- Cogliati S, Lorenzi I, Rigoni G, Caicci F, Soriano ME. 2018. Regulation of mitochondrial electron transport chain assembly. J Mol Biol. 430(24):4849–4873.
- Davison DL, Terek M, Chawla LS. 2012. Neurogenic pulmonary edema. Crit Care. 16(2):212.
- de Vries RBM, Angrish M, Browne P, Brozek J, Rooney A, Wikoff DS, Whaley P, Edwards SW, Morgan RL, Druwe IL, et al. 2021. Applying evidence-based methods to the development and use of adverse outcome pathways. ALTEX – Alternatives to Animal Experimentation (In press). DOI:https://doi.org/10.14573/altex.2101211
- Deveau M, Chen CP, Johanson G, Krewski D, Maier A, Niven KJ, Ripple S, Schulte PA, Silk J, Urbanus JH, et al. 2015. The global landscape of occupational exposure limits—implementation of harmonization principles to guide limit selection. J Occup Environ Hyg. 12(Sup1):S127–S144.
- Dorman DC, Moulin FJM, McManus BE, Mahle KC, James RA, Struve MF. 2002. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci: Off J Soc Toxicol. 65(1):18–25.
- Dorman DC, Struve MF, Gross EA, Brenneman KA. 2004. Respiratory tract toxicity of inhaled hydrogen sulfide in Fischer-344 rats, Sprague-Dawley rats, and B6C3F1 mice following subchronic (90-day) exposure. Toxicol Appl Pharmacol. 198(1):29–39.
- Dorman DC, Struve MF, Wong BA, Gross EA, Parkinson C, Willson GA, Tan Y-M, Campbell JL, Teeguarden JG, Clewell HJ, et al. 2008. Derivation of an inhalation reference concentration based upon olfactory neuronal loss in male rats following subchronic acetaldehyde inhalation. Inhal Toxicol. 20(3):245–256.
- Dourson ML, Felter SP, Robinson D. 1996. Evolution of science-based uncertainty factors in noncancer risk assessment. Regul Toxicol Pharmacol. 24(2 Pt 1):108–120.
- Durham SK, Castleman WL. 1985. Pulmonary lesions induced by 3-methylindole in mice. Am J Pathol. 121(1):128–137.
- EPA US. 2003. Toxicological review of hydrogen sulphide (CAS No. 7783-06-4). US Environmental Protection Agency. EPA/635/R-03/005.
- Fahn S. 2003. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 991(1):1–14.
- Fink SL, Cookson BT. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immunity. 73(4):1907–1916.
- Gandhirajan RK, Rödder K, Bodnar Y, Pasqual-Melo G, Emmert S, Griguer CE, Weltmann K-D, Bekeschus S. 2018. Cytochrome C oxidase inhibition and cold plasma-derived oxidants synergize in melanoma cell death induction. Sci Rep. 8(1):12734.
- Glass DC. 1990. A review of the health effects of hydrogen sulphide exposure. Ann Occup Hyg. 34(3):323–327.
- Godoy JA, Rios JA, Zolezzi JM, Braidy N, Inestrosa NC. 2014. Signaling pathway cross talk in Alzheimer's disease. Cell Commun Signal. 12(1):23.
- Gorman AM. 2008. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 12(6A):2263–2280.
- Green F, Schurch S, De Sanctis G, Wallace J, Cheng S, Prior M. 1991. Effects of hydrogen sulfide exposure on surface properties of lung surfactant. J Appl Physiol (1985). 70(5):1943–1949.
- Guidotti TL. 2015. Hydrogen sulfide intoxication. Handbook of clinical neurology. New York, NY: Elsevier; p. 111–133.
- Hannah RS, Hayden LJ, Roth SH. 1989. Hydrogen sulfide exposure alters the amino acid content in developing rat CNS. Neurosci Lett. 99(3):323–327.
- Hardisty JF, Garman RH, Harkema JR, Lomax LG, Morgan KT. 1999. Histopathology of nasal olfactory mucosa from selected inhalation toxicity studies conducted with volatile chemicals. Toxicol Pathol. 27(6):618–627.
- Harkema JR, Carey SA, Wagner JG. 2006. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 34(3):252–269.
- Hartwig A. 2013. Hydrogen sulfide [MAK Value Documentation, 2013]. The MAK-collection for occupational health and safety: Part I: MAK value documentations. New York, NY: John Wiley & Sons.
- Health Council of the Netherlands. 2006. Hydrogen sulphide; Health-based recommended occupational exposure limit in the Netherlands; publication no. 2006/07OSH.
- Holsapple MP, Pitot HC, Cohen SM, Cohen SH, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, Dragan YP. 2006. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 89(1):51–56.
- Huart C, Rombaux P, Hummel T. 2013. Plasticity of the human olfactory system: the olfactory bulb. Molecules. 18(9):11586–11600.
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. 2012. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice . Sci Transl Med. 4(159):159ra147.
- Imaizumi N, Lee KK, Zhang C, Boelsterli UA. 2015. Mechanisms of cell death pathway activation following drug-induced inhibition of mitochondrial complex I. Redox Biol. 4:279–288.
- Jia C, Roman C, Hegg CC. 2010. Nickel sulfate induces location-dependent atrophy of mouse olfactory epithelium: protective and proliferative role of purinergic receptor activation. Toxicol Sci. 115(2):547–556.
- Kann O, Kovács R. 2007. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 292(2):C641–C657.
- Khan A, Coppock R, Schuler M, Prior M. 1998. Biochemical effects of subchronic repeated exposures to low and moderate concentrations of hydrogen sulfide in Fischer 344 rats. Inhalation Toxicol. 10(11):1037–1044.
- Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE. 1990. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol. 103(3):482–490.
- Khan AA, Yong S, Prior MG, Lillie LE. 1991. Cytotoxic effects of hydrogen sulfide on pulmonary alveolar macrophages in rats. J Toxicol Environ Health. 33(1):57–64.
- Kim J-W, Hong S-L, Lee CH, Jeon E-H, Choi A-R. 2010. Relationship between olfactory function and olfactory neuronal population in C57BL6 mice injected intraperitoneally with 3-methylindole. Otolaryngol Head Neck Surg. 143(6):837–842.
- Kleinstreuer NC, Sullivan K, Allen D, Edwards S, Mendrick DL, Embry M, Matheson J, Rowlands JC, Munn S, Maull E, et al. 2016. Adverse outcome pathways: from research to regulation scientific workshop report. Regul Toxicol Pharmacol. 76:39–50.
- Kohno M, Tanaka E, Nakamura T, Shimojo N, Misawa S. 1991. Influence of the short-term inhalation of hydrogen sulfide in rats. Eisei Kagaku. 37(2):103–106.
- Kombian S, Warenycia M, Mele F, Reiffenstein R. 1988. Effects of acute intoxication with hydrogen sulfide on central amino acid transmitter systems. Neurotoxicology. 9(4):587–595.
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke E, Blagosklonny M, El-Deiry W, Golstein P, Green DJCd, et al. Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16(1):3–11.
- Kühlbrandt W. 2015. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 13(1):89.
- Leist M, Ghallab A, Graepel R, Marchan R, Hassan R, Bennekou SH, Limonciel A, Vinken M, Schildknecht S, Waldmann T, et al. 2017. Adverse outcome pathways: opportunities, limitations and open questions. Arch Toxicol. 91(11):3477–3505.
- Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl A, Wong-Riley M. 2006. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 139(2):639–649.
- Lopez A, Prior M, Lillie LE, Gulayets C, Atwal OS. 1988. Histologic and ultrastructural alterations in lungs of rats exposed to sub-lethal concentrations of hydrogen sulfide. Vet Pathol. 25(5):376–384.
- Lopez A, Prior M, Yong S, Albassam M, Lillie LE. 1987. Biochemical and cytologic alterations in the respiratory tract of rats exposed for 4 hours to hydrogen sulfide. Fundam Appl Toxicol: Off J Soc Toxicol. 9(4):753–762.
- Lund O, Wieland H. 1966. Pathologic-anatomic findings in experimental hydrogen sulfide poisoning (H2S). A study on rhesus monkeys. Internationales Archiv Fur Arbeitsmedizin. 22(1):46–54.
- Mattson MP. 2004. Pathways towards and away from Alzheimer's disease. Nature. 430(7000):631–639.
- McManus MJ, Murphy MP, Franklin JL. 2011. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 31(44):15703–15715.
- Meek M, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34(1):1–18.
- Milby TH, Baselt RC. 1999. Hydrogen sulfide poisoning: clarification of some controversial issues. Am J Ind Med. 35(2):192–195.
- Monticello TM, Morgan KT, Uraih L. 1990. Nonneoplastic nasal lesions in rats and mice. Environ Health Perspect. 85:249–274.
- Moon Y, Lee KH, Park J-H, Geum D, Kim K. 2005. Mitochondrial membrane depolarization and the selective death of dopaminergic neurons by rotenone: protective effect of coenzyme Q10. J Neurochem. 93(5):1199–1208.
- Murray J. 2011. Pulmonary edema: pathophysiology and diagnosis. Int J Tuberc Lung Dis. 15(2):155–160.
- Nicholson RA, Roth SH, Zhang A, Zheng J, Brookes J, Skrajny B, Bennington R. 1998. Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. J Toxicol Environ Health, Part A. 54(6):491–507.
- OECD. 2018. Users' handbook supplement to the guidance document for developing and assessing adverse outcome pathways. OECD Series on Adverse Outcome Pathways, No. 1. Paris: OECD Publishing. DOI:https://doi.org/10.1787/5jlv1m9d1g32-en.
- Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. 2008. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci. 28(24):6239–6249.
- Ohta S, Ohsawa I. 2006. Dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer's disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. J Alzheimers Dis. 9(2):155–166. ].
- O'Neill HC, Orlicky DJ, Hendry-Hofer TB, Loader JE, Day BJ, White CW. 2011. Role of reactive oxygen and nitrogen species in olfactory epithelial injury by the sulfur mustard analogue 2-chloroethyl ethyl sulfide. Am J Respir Cell Mol Biol. 45(2):323–331.
- Partlo LA, Sainsbury RS, Roth SH. 2001. Effects of repeated hydrogen sulphide (H2S) exposure on learning and memory in the adult rat. Neurotoxicology. 22(2):177–189.
- Patlewicz G, Kuseva C, Kesova A, Popova I, Zhechev T, Pavlov T, Roberts DW, Mekenyan O. 2014. Towards AOP application-implementation of an integrated approach to testing and assessment (IATA) into a pipeline tool for skin sensitization. Regul Toxicol Pharmacol. 69(3):529–545.
- Piccirillo VJ, Bird MG, Lewis RJ, Bover WJ. 2012. Preliminary evaluation of the human relevance of respiratory tumors observed in rodents exposed to naphthalene. Regul Toxicol Pharmacol. 62(3):433–440.
- Pistollato F, de Gyves EM, Carpi D, Bopp SK, Nunes C, Worth A, Bal-Price A. 2020. Assessment of developmental neurotoxicity induced by chemical mixtures using an adverse outcome pathway concept. Environ Health. 19(1):23.
- Prior M, Green F, Lopez A, Balu A, De Sanctis GT, Fick G. 1990. Capsaicin pretreatment modifies hydrogen sulphide-induced pulmonary injury in rats. Toxicol Pathol. 18(2):279–288.
- Proctor DM, Gatto NM, Hong SJ, Allamneni KP. 2007. Mode-of-action framework for evaluating the relevance of rodent forestomach tumors in cancer risk assessment. Toxicol Sci. 98(2):313–326.
- Reiffenstein RJ, Hulbert WC, Roth SH. 1992. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 32:109–134.
- Rhomberg LR, Bailey LA, Goodman JE. 2010. Hypothesis-based weight of evidence: a tool for evaluating and communicating uncertainties and inconsistencies in the large body of evidence in proposing a carcinogenic mode of action-naphthalene as an example. Crit Rev Toxicol. 40(8):671–696.
- Ricci JE, Waterhouse N, Green DR. 2003. Mitochondrial functions during cell death, a complex (I-V) dilemma. Cell Death Differ. 10(5):488–492.
- Romashko J, Horowitz S, Franek WR, Palaia T, Miller EJ, Lin A, Birrer MJ, Scott W, Mantell LL. 2003. MAPK pathways mediate hyperoxia-induced oncotic cell death in lung epithelial cells. Free Radic Biol Med. 35(8):978–993.
- Rugarli EI, Langer T. 2012. Mitochondrial quality control: a matter of life and death for neurons. Embo J. 31(6):1336–1349.
- Rumbeiha W, Whitley E, Anantharam P, Kim DS, Kanthasamy A. 2016. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: challenges for translational neuroprotective research. Ann N Y Acad Sci. 1378(1):5–16.
- Sasajima H, Miyazono S, Noguchi T, Kashiwayanagi M. 2015. Intranasal administration of rotenone in mice attenuated olfactory functions through the lesion of dopaminergic neurons in the olfactory bulb. NeuroToxicology. 51:106–115.
- Savolainen H, Tenhunen R, Elovaara E, Tossavainen A. 1980. Cumulative biochemical effects of repeated subclinical hydrogen sulfide intoxication in mouse brain. Int Arch Occup Environ Health. 46(1):87–92. ].
- Schneider JS, Tobe EH, Mozley PD Jr, Barniskis L, Lidsky TI. 1998. Persistent cognitive and motor deficits following acute hydrogen sulphide poisoning. Occup Med (Lond). 48(4):255–260.
- SCOEL. 2007. Recommendation from the Scientific Committee on Occupational Exposure Limits (SCOEL) for Hydrogen Sulphide. European Commission. SCOEL/SUM/124.
- Selvatici R, Previati M, Marino S, Marani L, Falzarano S, Lanzoni I, Siniscalchi A. 2009. Sodium azide induced neuronal damage in vitro: evidence for non-apoptotic cell death. Neurochem Res. 34(5):909–916.
- Skrajny B, Reiffenstein RJ, Sainsbury RS, Roth SH. 1996. Effects of repeated exposures of hydrogen sulphide on rat hippocampal EEG. Toxicol Lett. 84(1):43–53.
- Stevenson RJ. 2010. An initial evaluation of the functions of human olfaction. Chem Senses. 35(1):3–20.
- Struve MF, Brisbois JN, James RA, Marshall MW, Dorman DC. 2001. Neurotoxicological effects associated with short-term exposure of Sprague-Dawley rats to hydrogen sulfide. Neurotoxicology. 22(3):375–385.
- Tawara T, Fukushima T, Hojo N, Isobe A, Shiwaku K, Setogawa T, Yamane Y. 1996. Effects of paraquat on mitochondrial electron transport system and catecholamine contents in rat brain. Arch Toxicol. 70(9):585–589. ].
- Teeguarden JG. 2017. AOP136: intracellular acidification induced olfactory epithelial injury leading to site of contact nasal tumors (status as of 5 July 2019: "Open for citation & Comment"). Last modified 20 March 2017. https://aopwiki.org/aops/136.
- Tvedt B, Edland A, Skyberg K, Forberg O. 1991. Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: affection of motor function, memory, vision and hearing. Acta Neurol Scand. 84(4):348–351.
- van Aalst JA, Isakov R, Polk JD, Van Antwerp AD, Yang M, Fratianne RB. 2000. Hydrogen sulfide inhalation injury. J Burn Care Rehabil. 21(3):248–253.
- Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW. 2006. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J Biol Chem. 281(40):29468–29478.
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. 2014a. Adverse Outcome Pathway (AOP) Development I: strategies and principles. Toxicol Sci. 142(2):312–320.
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. 2014b. Adverse outcome pathway development II: best practices. Toxicol Sci. 142(2):321–330.
- Wallace DCJS. 1999. Mitochondrial diseases in man and mouse. Science. 283(5407):1482–1488.
- Warenycia MW, Smith KA, Blashko CS, Kombian SB, Reiffenstein RJ. 1989. Monoamine oxidase inhibition as a sequel of hydrogen sulfide intoxication: increases in brain catecholamine and 5-hydroxytryptamine levels. Arch Toxicol. 63(2):131–136.
- Wasch HH, Estrin WJ, Yip P, Bowler R, Cone JE. 1989. Prolongation of the P-300 latency associated with hydrogen sulfide exposure. Arch Neurol. 46(8):902–904.
- Wei H, Leeds PR, Qian Y, Wei W, Chen R-w, Chuang D-M. 2000. β-Amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur J Pharmacol. 392(3):117–123.
- Wolfe MS. 2018. Chapter 1 – Solving the puzzle of neurodegeneration. In: Wolfe MS, editor. The molecular and cellular basis of neurodegenerative diseases. London: Academic Press; p. 1–22.
- Wu N, Du X, Wang D, Hao F. 2011. Myocardial and lung injuries induced by hydrogen sulfide and the effectiveness of oxygen therapy in rats. Clin Toxicol. 49(3):161–166.
- Yee S, Choi BH. 1996. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 17(1):17–26.
- Youngentob SL, Schwob JE, Sheehe PR, Youngentob LM. 1997. Odorant threshold following methyl bromide-induced lesions of the olfactory epithelium. Physiol Behav. 62(6):1241–1252.
- Zhang X, Bian J-S. 2014. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci. 5(10):876–883.
- Ziabreva I, Campbell G, Rist J, Zambonin J, Rorbach J, Wydro MM, Lassmann H, Franklin RJM, Mahad D. 2010. Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. Glia. 58(15):1827–1837.