 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This review is a hypothesis driven, mechanistic evaluation of the potential for octamethylcyclotetrasiloxane (D4) to produce any effects via endocrine modes of action. D4 is a volatile, lipophilic liquid used in the production of high molecular weight dimethylsiloxane polymers. These are used in a variety of industrial, medical, cleaning, and personal care products, and they may contain low levels of residual D4. Low concentrations of D4 are found in the environment and there is potential for low level human exposure. All of the measured environmental and workplace levels of D4 fall below no observed effect levels (NOEL). Most of the effects of high dose D4 involve the female reproductive system. In the mature intact female rat following chronic high dose exposure, D4 may cause inhibition of mating and ovulation, decreased live litter sizes, small increases in the estrogen to progesterone ratio primarily through decreases in progesterone, and increases in uterine hyperplasia. When endogenous estrogens are very low, high dose D4 causes increases in some uterine parameters. To assess whether these high dose effects can be attributed to an endocrine mode of action, endpoints are ranked for relevance and strength, consistent with published concepts. When sufficient information is available the level of activity of D4 for producing the observed effect is compared with that of potent endocrines. The conclusions reached are that all of the effects of D4 fall well short of any established criteria for D4 to be capable of producing any adverse effect via an endocrine mode of action.
Introduction
Because of its presence at low levels in consumer products and the biosphere, several studies in animals, cells in culture, and isolated receptors have examined the biological fate and effects of D4. The focus of this review is to catalogue those actions of D4 for which there is a hypothesis of a potential endocrine system-related mode of action and to determine the potential of these actions for producing adverse effects. The information in this review encompasses all of the available data, from the earliest report in Citation1988 to the present, on the potential endocrine system-related actions of D4. Most of the data for D4 and decamethylcyclopentasiloxane (D5) are from unpublished research reports from the Dow Corning Corporation (DCC). Some of this data has also been published. The author relied primarily on the original data for the preparation of this review. Most of the in vivo studies have employed the rat as the experimental subject. D4, only at high doses (e.g. ≥300 ppm for inhalational exposures), produces a variety of effects on female reproductive parameters. The possibility that any of these effects on the female reproductive system may be due to an endocrine mode of action is evaluated according to the weight of evidence, tier 1 guidelines of the United States Environmental Protection Agency endocrine disruptor screening program (U.S. EPA Citation2011) and the evidence for each potential endocrine system effect is prioritized consistent with the hypothesis-driven framework and endpoint rankings as described by Borgert et al. (Citation2011, Citation2014), de Peyster and Mihaich (Citation2014), Mihaich et al. (Citation2017), Neal et al. (Citation2017), and Mihaich and Borgert (Citation2018).
Properties of D4
D4 is a cyclic tetramer of dimethylsiloxane with an 8 membered, puckered ring of alternating silicone and oxygen atoms with a molecular weight of 296.62 g/mole. Each silicone has 2 methyl groups attached to it (). The octanol-water partition coefficient (logkow) for D4 is in the range of 6 demonstrating its high lipophilicity (U.S. EPA DSSTox database). Each oxygen of D4 has the capability of forming 1 equatorial hydrogen bond with water (NIH PubChem). This gives D4 a limited water solubility of 1.89 × 10−7 M at 23 °C (Varaprath et al. Citation1996). The solubility of D4 is lower in artificial sea water (1.11 × 10−7 M at 25 °C) (NIH PubChem). D4 is relatively volatile with a vapor pressure of 1.05 mm of Hg at 25 °C in air at 1 atm (U.S. EPA DSSTox database). This translates to a vapor saturation of 1382 ppm v/v, or 5.66 × 10−5 mol/l.
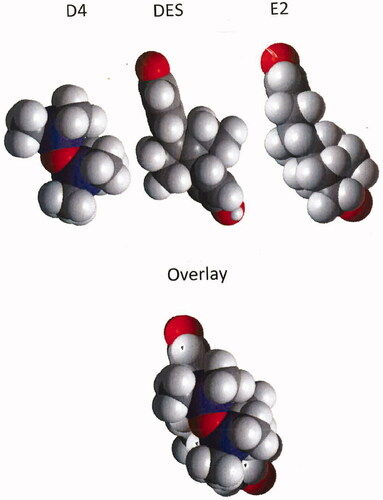
Figure 1. Space filling models of D4, DES, and E2 with the three molecules overlayed in the lower frame.
Exposure routes
The routes of exposure to D4 that are incidental or that have been used for experimental exposures in animals and humans include inhalation, oral, parenteral, dermal, and in the water and sediments for aquatic animals. Only the highest single or repeated doses of D4 (e.g. >70 ppm for 6 h/day for inhalation studies or >3.4 × 10−5 mol/kg/day for gavage or subcutaneous (sc) administration) have been shown to produce observable biological effects. In systems that are not, or cannot, be sealed air tight (e.g. in vivo exposures or in vitro cell-based receptor transcription assays) there is substantial evaporative loss of D4 (from surfaces or exhalation) over extended periods (e.g. 24 h) (DCC Citation2001). Dermal exposures (due, in part, to evaporative losses) have not been capable of producing body burdens of D4 sufficient to produce any observable biological effects (Jovanovic et al. Citation2008). Over 90% of D4 is lost to evaporation from the skin surface without being absorbed.
Oral exposures are by ingestion or gavage. In a study to determine if D4 could be administered to rats in their food it was found that when high levels of D4 were mixed with rat chow the animals would not eat it (DCC Citation1988). In some way the presence of large amounts of D4 in the food rendered it unpalatable to the animals. Gavage and parenteral exposures involve administering the material to the animal as a bolus dose. Due to its low water solubility and high lipophilicity much of the D4 administered by these routes remains relatively un-dispersed and, therefore, biologically less available (Sarangapani et al. Citation2003). Much of gavage administered D4 is excreted unchanged in the feces (DCC Citation1998c). Employing vegetable oil (corn oil or sesame oil) vehicles to dilute D4 for gavage administration produced higher uptake from the gastrointestinal tract (51.95% for corn oil) than neat (28.14%) or vehicles such as simethicone (12.11%) (DCC Citation1998c). The vegetable oil vehicles served to enhance the dispersibility of the material.
Experimental inhalation exposures have been nose only, mouth only (humans), or whole body. Vapor levels in air are highly sensitive to fluctuations in temperature, pressure, and equilibration time factors. Levels of D4 in air at 300 ppm and higher, used for experimental exposures, have been shown to be at least partly aerosol. Inhalation of lipophilic aerosols can produce a range of exposure artifacts (DCC Citation1995b; Pauluhn Citation2019). Due to grooming behavior and other factors whole body inhalation dosing in rats will result in some exposure by the oral route. Although probably of small consequence, this factor has not been addressed in any reports or publications. Inhalation dosing has generally been for 6 h as a single dose or 6 h/day, 5 days/week for the duration of the exposure period. Some inhalation exposures have been every day, and some have been for 16 h/day. During inhalation exposures food and sometimes water were not available to the experimental subject. In general, inhalation doses of D4 at less than 300 ppm have not been shown to produce any endocrine system-related effects.
Partitioning
Due to its high lipophilicity, getting and keeping D4 in solution in the aqueous phase of the analytical milieu is problematical. Lipophilic substances partition between water and less polar phases. This process is governed by partition or adsorption coefficients. These coefficients are simple ratios of the amount of substance in each phase, and as long as the two phases are not saturated the concentration in each phase will be a constant proportion of the total amount. Such phases could be proteins, lipoproteins, membrane lipid cores, cells, adipose tissue, aquatic sediments, etc. In most systems we don’t know the magnitudes of these coefficients. The overall effect will be a summation of the magnitude of each coefficient and the capacity of each phase. Even at low levels of D4 (near its aqueous solubility limit), where there are non-polar partitioning phases available, most of the D4 will be partitioned to these other phases and the aqueous concentration will be a small fraction of the total.
Distribution, metabolism and excretion of D4
The distribution and half lives of D4 found in various tissues following a single 6 h nose-only inhalation exposure of rats to [14C]D4 is shown in (Plotzke et al. Citation2000). These parameters, in general, have not been measured for other dosage forms. In rats 40–50% of inhaled doses are metabolized to polar metabolites in the liver (75–80% dimethylsilanediol and methylsilanetriol, approximately 15% tetramethyldisiloxane-1,3-diol, trimethyldisiloxane-1,3,3-triol, and dimethyldisiloxane-1,1,3,3-tetrol, plus minor amounts hexamethyltrisiloxane-1,5-diol and dimethyldisiloxane-1,1,1,3,3-pentol. These are excreted in the urine. About 35% of the dose is exhaled or evaporated from the skin as parent D4 and 10–15% of the dose is excreted unchanged in the feces (Plotzke et al. Citation2000).
Table 1. Retention and elimination of 6 h single dose nose-only inhalation exposure to [14C]D4.
Environmental levels of D4
Because of its presence at low levels in consumer products and its volatility, D4 can be found in the environment (Wang et al. Citation2013; McGoldrick et al. Citation2014; Krogseth et al. Citation2017; Lee et al. Citation2018; Nusz et al. Citation2018; Horii et al. Citation2021). In a study of 11 sewage treatment facilities from southeastern Canada Wang et al. (Citation2013) found low levels of D4 in sewage effluents (≤1.5 × 10−9 M), surface waters receiving sewage effluents (≤8 × 10−11 M), aquatic sediments (≤5 × 10−10 mol/kg dry wt.), and bio-solid amended soils (≤8 × 10−11 mol/kg dry wt.). These levels were among the highest environmental levels found from any study anywhere in the world (Lee et al. Citation2018). The levels of D4 in the surface waters receiving sewage effluents were well below the NOEL (1.5 × 10−8 M) for rainbow trout, sheepshead minnow, and Daphnia magna (Sousa et al. Citation1995).
D4 is degraded in soils, sediments, and sewage sludges by clay catalyzed hydrolysis and by aerobic and anaerobic microbial metabolism (Grumping et al. Citation1999; Xu Citation1999). In mammals and other vertebrates D4 is metabolized to polar metabolites in the liver (Plotzke et al. Citation2000). These are excreted in the urine for terrestrial animals or through the skin for aquatic animals (Varaprath et al. Citation1999; Andersen et al. Citation2001). D4 has been found at levels up to 1.22 ppm in landfill gases (Urban et al. Citation2009) and up to 8.3 ppm in wastewater treatment digester gases (Griffin Citation2004). In the open atmosphere D4 is subject to hydroxyl radical and UV catalyzed degradation reactions with an estimated half life of 10 days (Mueller et al. Citation1995; Surita and Tansel Citation2014). Levels of D4 in the open atmosphere have been measured at up to 3.7 × 10−6 ppm (Genualdi et al. Citation2011). In enclosed work place environments where products containing D4 are manufactured or in use atmospheric concentrations of D4 have been measured as high as 34 ppm (Gentry et al. Citation2017). The NOEL of D4 for whole body inhalational exposure of female Sprague-Dawley (SD) rats and their pups at 6 h/day for 28 days prior to mating through gestation day 21 and from lactation days 5–21 is >70 ppm (DCC Citation1995c).
D4 does not bio-accumulate beyond its partitioning tendency
Several ecological studies have found no bio-accumulation potential for D4 (e.g. Powell et al. Citation2017). After 3 weeks of daily dosing of rainbow trout with D4 in the feed at 5.06 × 10−5 mol/kg/day the body burden of D4 reached 4.32 × 10−7 mol/kg. This was estimated to be 70–75% of steady state accumulation (Woodburn et al. Citation2013). Calculated bio-magnification factors from this study were less than 1 demonstrating that D4 did not bio-accumulate with chronic dosing beyond its partitioning tendency.
All of the in vivo and in vitro studies of the biological actions of D4 have required levels of D4 in the animal or the assay system to be well in excess of its aqueous solubility limit to produce any measurable biological effects. In rats and other mammals, due to partitioning, following single or repeated exposures to high doses of D4 (e.g. ≥ 300 ppm for inhalation exposures), by various routes of administration, blood and plasma levels of D4 were hundreds of times the solubility limit of D4 (DCC Citation1998c). After 6 months of daily inhalational dosing of rats with D4 at 700 ppm for 6 h/day, 5 days per week, plasma levels were 4.4 × 10−5 M and abdominal adipose tissue levels were 4.2 × 10−3 mol/kg (DCC Citation1995d, Citation1995e, Citation2000a, Citation2019; Plotzke et al. Citation2000; Quinn, Dalu, et al. Citation2007; Jean and Plotzke Citation2017).
In female Fischer-344 (F-344) rats there were no differences in adipose tissue radioactivity (9.57 × 10−4 mole equivalents/kg) after a single dose of [14C]D4 administered by whole body inhalation at 700 ppm for 6 h or after 14 days of daily dosing (6 h/day) of unlabeled D4 at 700 ppm followed by a single dose of [14C]D4 (DCC Citation2002b; Plotzke et al. Citation2000). One observation in this study is that D4 partitions into deep kinetic reservoirs, e.g. adipose tissue, with slow equilibration kinetics (Plotzke et al. Citation2000). The label from the single dose of labeled D4 following 14 days of unlabeled D4 may not have had time to fully equilibrate with the unlabeled D4 in these deep tissue reservoirs giving an underestimate of the body burden of D4. D4, analyzed in adipose tissue by gas chromatography/mass spectrometry following the single exposure provided a good match with the radioactivity measures. This analysis was not performed for the 15 day exposures. The D4 concentration in adipose tissue of female F-344 rats, measured by gas chromatography/mass spectrometry, after inhalation exposure to 700 ppm for 5 days, at 6 h/day, was 2.54 × 10−3 mol/kg (DCC Citation1999a). The higher levels of adipose tissue D4 in this study relative to the [14C]D4 study may be a reflection of incomplete equilibration of [14C]D4 with unlabeled D4 in this deep kinetic reservoir of the body. After 6 months of dosing of female F-344 rats with D4 by whole body inhalation at 700 ppm for 6 h/day, 5 days/week, abdominal adipose tissue concentrations were 4.2 × 10−3 mol/kg (Jean and Plotzke Citation2017). These studies indicate that, due to elimination of the parent compound through exhalation and in the feces, and through metabolism in the liver and excretion in the urine, D4 does not bio-accumulate beyond its partitioning tendency.
The endocrine system
The endocrine system is a chemical messenger system that transmits information and instructions from tissue to tissue in the body. These chemical signals, which generally travel through the blood, interact with receptors on, or in, their target cells to produce physiological alterations in these cells. Exogenous substances capable of directly interacting with and modifying the endocrine system can be classified as endocrine system modifiers. The ability of a substance to act as an endocrine system modifier, of itself, is not an adverse effect. An endocrine system modifier capable of producing an adverse effect or effects, due specifically to its endocrine mode of action may be classified as an endocrine disruptor. The World Health Organization International Programme on Chemical Safety (WHO/IPCS Citation2002) definition is: “A potential endocrine disruptor is an exogenous substance or mixture that possesses properties that might be expected to lead to endocrine disruption in an intact organism, or its progeny, or (sub)populations.” In 2017, the European Union (EU) set out scientific criteria for the determination of endocrine-disrupting properties pursuant to Regulation EU No 528/2012 of the European Parliament and Council (Citation2012). The European Commission then asked the European Food Safety Authority (EFSA) and the European Chemicals Agency (ECHA) to develop a guidance document for the implementation of the scientific criteria for the determination of endocrine‐disrupting properties (ECHA and EFSA Citation2018). According to the criteria defined in this guidance document a substance shall be considered as having endocrine disrupting properties if it meets all of the following criteria. “a) It shows an adverse effect in [an intact organism or its progeny]/[non-target organisms], which is a change in the morphology, physiology, growth, development, reproduction or life span of an organism, system or (sub)population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress, or an increase in susceptibility to other influences. b) It has an endocrine mode of action, i.e. it alters the function(s) of the endocrine system. c) The adverse effect is a consequence of the endocrine mode of action.” The U.S. EPA (Citation2011) has designated those substances that act directly (with or without metabolic activation) as receptor agonists or antagonists on estrogen, androgen, or thyroid hormone receptors, substances that activate or inhibit androgen or estrogen synthesis (steroidogenesis), and substances that directly interact with the hypothalamic-pituitary-gonadal axis (HPG) or the hypothalamic-pituitary-thyroid axis as substances in need of evaluation as potential endocrine disruptors.
In order for a chemical to act as a receptor agonist it must be capable of binding in the agonist binding site of its receptor and stimulating (activating) the receptor once bound. Receptor antagonists must be capable of binding to the receptor and interfering with the ability of the receptor to be stimulated by its agonist. Binding between receptors and their agonists or antagonists and the ability of the agonist to stimulate the receptor require that the two molecules “fit” together with very precise interactions. These include high degrees of specificity for size, shape, charge, and chemical properties. Co-activators, co-repressors, agents that interfere with transport or storage of receptor agonists or antagonists, and substances that act to enhance metabolic breakdown of receptor agonists or antagonists, are indirect secondary effects that do not meet the U.S. EPA criteria for evaluation as potential endocrine disruptors.
The rat estrous cycle
Most of the reproductive effects that have been observed for D4 occur only at high doses (e.g. ≥300 ppm for inhalation exposure) and have been associated with effects on the estrous cycle. Much of this research has employed the rat as the experimental subject. The normal estrous cycle in the laboratory rat, which is strongly influenced by the 12:12 h light-dark cycle, consists of 5 stages spanning 4 or 5 days. Proestrus lasts for 12 h (lights on to lights off, 6 AM–6 PM). Early estrus, late estrus, and metestrus last for about 12 h each, and diestrus lasts for 48 or 72 h (Long and Evans Citation1922). Low levels of progesterone and rising levels of estrogen in late diestrus promote the resumption of follicular development, which prepares some follicles for cyclic recruitment (Garris and Foreman Citation1984). The increased follicle stimulating hormone (FSH) levels during late proestrus and early estrus recruit several follicles in the ovaries. As these cycle-recruited follicles develop through late estrus, metestrus and diestrus they secrete moderate, gradually increasing levels of estrogen. During early proestrus these follicles grow rapidly and secrete rapidly increasing levels of estrogen that peak at about 9–11 AM on proestrus and then fall to low levels by the beginning of early estrus (Smith et al. Citation1975; Hebel and Stomberg Citation1986; Quinn, Dalu, et al. Citation2007). The rapid and sustained increase of estrogen to high levels during early proestrus releases the inhibitory effects exerted by moderate levels of estrogen on gonadotropin releasing hormone (GnRH) secretion from the hypothalamus. GnRH triggers surges of luteinizing hormone (LH), FSH, and prolactin (PRL) from the anterior pituitary. These gonadotropin surges peak late in the afternoon of proestrus initiating the events that lead to ovulation (Hashimoto et al. Citation1987; Quinn, Dalu, et al. Citation2007). The rise in LH causes estrogen levels to fall and progesterone levels to rise late in the afternoon of proestrus. Ovulation occurs at about 4 AM the morning of early estrus, 10–12 h after the LH peak. Rats ovulate spontaneously (mating not required) and following ovulation the remainder of the follicles develop into the corpora lutea. Female rats are sexually receptive only during late proestrus and early estrus. When no mating has occurred, the corpora lutea fail to develop into their fully functional secretory state. These corpora lutea achieve their maximum growth within 24 h and secrete progesterone that peaks during diestrus. Progesterone pauses the development of primordial follicles prior to their cyclic recruitment. Rising estrogen levels during late diestrus initiate the cessation of progesterone secretion and then the PRL surge of proestrus initiates the regression of these corpora lutea, which progressively involute before the third estrous cycle to form small scars (Yoshinaga Citation1973; Smith et al. Citation1975; McCracken et al. Citation1999). Vaginal stimulation, as during mating, relayed to the CNS by sensory neurons, induces twice daily surges of PRL from the anterior pituitary (Lu et al. Citation1979; Johnson Citation2007). These maintain the newly formed corpora lutea and their secretion of progesterone (Gunnet and Freeman Citation1983). Infertile mating leads to a state of pseudo-pregnancy lasting about 10 days (McCracken et al. Citation1999). Pseudo-pregnancy is characterized as a continued activity of fully functional corpora lutea, which secrete moderate levels of progesterone. Progesterone suppresses further estrous cycling.
The stress response
Several authors have commented on the potential for a number of the effects seen following the exposure to D4 as being secondary to activation of the stress response (e.g. Jean and Plotzke Citation2017; Dekant et al. Citation2017a, Citation2017b; Pauluhn Citation2021). Virtually any chemical substance will produce a stress response if the body burden approaches the limits that the organism or person can tolerate (e.g. De Boeck et al. Citation1997; Pruett et al. Citation2007). This was the case in all of the high dose studies of the effects of D4 (e.g. ≥300 ppm for inhalation exposures) and many of the biological actions that are produced only by high doses of D4 are most probably primarily due to stress-related effects on the female reproductive system. The stress response is a physiological, endocrine-based system that functions to maintain homeostasis in the face of forces that work to alter this state. Stress is commonly defined as a state of real or perceived (psychological) threat to homeostasis. The stress response is mediated by hormones of the hypothalamic-pituitary-adrenal axis (HPA). In this system neurons in the paraventricular nucleus of the hypothalamus respond to stressful stimuli by releasing corticotropin releasing factor (CRF) into the blood traveling to the anterior pituitary gland. CRF stimulates the anterior pituitary to produce proopiomelanocortin, which is fragmented to form adrenocorticotropic hormone, β-endorphin, α- and β-melanocyte stimulating hormone, and β-lipotropin. These are secreted into the general circulation. CRF is also produced and acts in peripheral tissues (Calogero et al. Citation1996). Adrenocorticotropic hormone induces glucocorticoid synthesis and release from the adrenal cortex. Glucocorticoid levels have been found to be elevated in response to D4 exposure (Wilson Citation1996; He et al. Citation2003; Jean and Plotzke Citation2017). D4, administered to 129 J/C57Bl/6J mice by gavage in corn oil at 3.37 × 10−3 mol/kg/day for 7 days elevated glucocorticoid levels by 6.3* fold (He, et al. Citation2003).
Pregnancy places the female under conditions of extreme physiological demand. The functional interactions between the HPA and the HPG serve to protect the female by preventing or terminating pregnancy when serious threats to homeostasis exist.
Body weight and food consumption effects of D4 are due to the stress response
Nose only inhalation exposure of rats involves constraining the animal in a tube with its nose held in position to receive the atmospheric exposure. Restraint stress is a well characterized phenomenon. Pauluhn and Mohr (Citation1999) showed that rats exposed to clean air by nose only exposure lost body weight and exhibited reduced food and water consumption compared to control rats that were held in their home cages with no food or water for the same periods of time. This indicates that the restraint stress alone is sufficient to cause weight loss and reduction of food consumption in the test animals. However, in both D4 and D5 exposure studies the higher doses (≥300 ppm for D4 or 160 ppm for D5) caused greater weight loss and greater decreases in food consumption as compared to the air only and lower dose exposed animals indicating that D4 and D5 caused greater levels of stress. In nearly every study in which body weights and food consumption were measured, rats exposed to high doses of D4 or D5 across multiple routes and durations of exposure exhibited reduced food consumption (e.g. ↓4.0%*, SD rats) and/or weight loss (e.g. ↓4.9%*, SD rats), or reduced weight gain, and sometimes hunched posture and gait abnormalities (DCC Citation1988, 1995a, 1995b, 1995c, Citation1996, 1997b, 1998a, 1998d, 1999b, 2001, 2004a, 2012; Quinn, Dalu, et al. Citation2007; Franzen et al. Citation2017). These effects generally resolved after the first 3 weeks of exposure even when daily dosing continued. An important exception to this finding was for the F1 generation offspring in a two generation exposure study (DCC Citation2001). Other than an increase in mean body weight on post-natal day 1 at only the 700 ppm dose these animals showed no changes in body weight from control groups (DCC Citation2001). Initial activation of the HPA is potentially a major cause of these effects. The non-persistence of body weight and food consumption deficits can be attributed to the ongoing actions of glucocorticoids and other hormones of the HPA to bring the body back into, and maintain, a state of homeostasis in the face of continued external forces that are working to disrupt the homeostatic state. D5 served as a negative control in some of the analyses of changes in uterine parameters. It is, therefore, rational to conclude that effects produced by both D4 and D5 are due to a nonspecific stress response.
Blood chemistry changes produced by D4 exposure are due to the stress response
Blood chemistry changes noted during chronic D4 exposures (up to 1000 ppm, by nose only inhalation, 6 h/day, 5 days per week, for 4 weeks) included increased glucose (1.22* fold), increased cholesterol (1.17* fold), increased albumin (1.05* fold), and increased total protein (1.04* fold) (DCC Citation1995a, Citation1995b). These changes were observed only in the highest doses and returned to normal after 1 month of recovery. Many of these blood chemistry changes are consistent with the stress response, which works to mobilize nutrients from storage and make them available to tissues to help those tissues respond to the stressful situation (Rabasa and Dickson Citation2016).
Actions of D4 to decrease thymus weights and increase adrenal weights are due to the stress response
In female F-344 rats exposure to D4 (1000 ppm, by nose only inhalation, 6 h/day, 5 days/week, for 4 weeks) decreased thymus weights by 29.4%* and increased adrenal weights by 112%* (DCC Citation1995a, Citation1995b, Citation1998a). Glucocorticoids cause a rapid involution of the thymus and activation of the HPA in response to stress is well documented to increase adrenal weights (Klein et al. Citation1992; Moleriu et al. Citation2014).
Statistics
In this manuscript and its tables * indicates a statistically significant (p ≤ 0.05) difference from control. # indicates a statistically significant (p ≤ 0.05) difference from the highest dose of the full agonist employed. Statistical tests employed and group sizes are noted for many of the individual studies. In several instances the author has converted group mean results to fold changes to streamline discussion of the findings.
Relative effect estimates
A weak agonist is a substance that has low efficacy for producing an effect on an endocrine receptor dependent process. Relative effect estimates for the weak agonist (RE) are calculated using EquationEquation (1)(1)
(1) .
(1)
(1)
[Af] and [Aw] are the highest concentrations employed of the full agonist and the weak agonist respectively. RRw is the ratio of response of the weak agonist relative to the response of the full agonist. The assumption here is that [Af] is ≥10 times its agonist affinity constant (KD). This calculation provides a conservative estimate of RE. Actual REs are probably lower.
Hypotheses and endpoint prioritization
The following six specific hypotheses are evaluated according to concepts described by the U.S. EPA for the results of tier 1 endocrine system screening assays (U.S. EPA Citation2011). The evidence for each hypothesis is prioritized consistent with the framework and endpoint rankings described by Borgert et al. (Citation2011) and Borgert et al. (Citation2014); de Peyster and Mihaich (Citation2014); Mihaich et al. (Citation2017); Neal et al. (Citation2017); Mihaich and Borgert (Citation2018).
Hypothesis 1: D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via an estrogenic mode of action
In vivo effects
Immature female rats, or mature ovaryectomized (OVX) rats or mice dosed with D4 by gavage, sc, or whole body inhalation showed increases in uterine weights, uterine fluid, uterine luminal and glandular epithelial cell heights, uterine peroxidase activity, and induction of the calbindin 9 K (CaBP-9K) gene (DCC Citation1998d, Citation2003, Citation2004a, 2004b, 2004c, Citation2012; Lee et al. Citation2015; McKim et al. Citation2001; He et al., Citation2003; Quinn, Dalu, et al. Citation2007; Quinn, Regan, et al. Citation2007).
Rank 1
Ethinyl estradiol (EE), coumestrol, and D4, administered by gavage in sesame oil for 4 days, produced dose-dependent increases in absolute wet and blotted uterine weights and in uterine epithelial cell heights in immature SD and F-344 rats (DCC Citation1998d). The antagonist, ICI 182 780 (ICI), of the alpha subtype of the nuclear estrogen receptor (ERα) at 4.94 × 10−6 mol/kg/day co-administered with EE completely blocked the effects of EE. D4 co-administered with EE caused a partial inhibition of the actions of EE. The doses of D4 that were tested were 3.37 × 10−3 mol/kg/day, 8.43 × 10−3 mol/kg/day, 1.68 × 10−2 mol/kg/day, and 3.37 × 10−2 mol/kg/day. The lowest dose caused an increase in liver weight in the F-344 rats, while 8.43 × 10−3 mol/kg/day and higher doses were necessary to cause effects on body weight gain and increases in uterine parameters. The results for the highest concentrations tested are shown in .
Table 2. Effects of EE, coumestrol, and D4 on some uterine parameters in immature rats.
These data support the conclusion that D4 is capable of producing ERα-related actions by altering some uterine parameters with much lower activity (REs 1.2 × 10−6–5.3 × 10−6) than EE and lower activity than the phytoestrogen coumestrol. These data also demonstrate that D4 has the ability to partially inhibit the actions of EE. ICI was not co-administered with D4 or coumestrol and there were no negative controls employed in this study. Statistical analyses used in this study were two-tailed analysis of variance (ANOVA) with Dunnet’s t and Levene’s tests followed by Welch’s t test with Bonferroni correction as needed. n = 12 per group.
Rank 1
D4 at 1.69 × 10−3 mol/kg/day or 3.37 × 10−3 mol/kg/day, EE at 1.01 × 10−8 mol/kg/day, or ICI at 4.94 × 10−9 mol/kg/day (administered 30 min prior to D4 or EE), dissolved in dimethylsulfoxide and diluted with corn oil, were administered sc to immature SD rats for 4 days. Uterine weights were increased 5 fold* by EE (Lee et al. Citation2015). This effect was completely blocked by ICI. D4 did not produce any increase in uterine weight at either dose. Uterine protein extracts analyzed by SDS polyacrylamide gel electrophoresis, western blotting, and chemiluminescence detection of antibody binding showed that EE increased uterine CaBP-9K protein expression 166* fold. CaBP-9K protein was increased 1.53*# fold and 2.27*# fold by D4 at 1.69 × 10−3 mol/kg/day and 3.37 × 10−3 mol/kg/day respectively. The REs for CaBP-9K induction by the two dose levels of D4 were 5.55 × 10−9 and 4.16 × 10−9, respectively. ICI decreased CaBP-9K about 90%* from the vehicle control value and completely blocked the increases in CaBP-9K expression produced by EE or D4. The data for CaBP-9K support the conclusion that D4 is capable of producing an ERα-related action, but with much lower activity than EE. There were no weak agonist controls employed in this study. Statistical analyses were one-way ANOVA and Tuckey’s test. n = 5 per group.
The Lee et al. study showed no D4-induced increases in uterine weights or cell heights. The indication is that the CaBP-9K induction assay is sufficiently more sensitive such that the small increases were found. Several uterus-specific ERα induction assays can be on the order of tens of fold more sensitive than assays that measure increases in uterine weight or uterine cell heights (Ashby Citation2001).
Rank 1
In OVX, 129 J/C57Bl/6J mice 17β-estradiol (E2) (4.6 × 10−8 mol/kg/day), administered by gavage in corn oil + 1% EtOH for 3 days, produced increases in absolute uterine weights of 4.93* fold (He et al. Citation2003). At doses of 8.43 × 10−3 mol/kg/day and higher D4 administered by gavage in corn oil for 3 days, produced dose-dependent increases in absolute uterine weights up to 61.0%*# of the increase found for E2. The RE of this effect for D4 is 2.12 × 10−6. The doses of D4 that were tested were 3.37 × 10−5, 3.37 × 10−4, 1.68 × 10−3, 3.37 × 10−3, 8.43 × 10−3, 1.68 × 10−2, and 3.37 × 10−2 mol/kg/day. The uterine weight increases produced by D4 or E2 were completely blocked when ICI was administered (by gavage in corn oil + 5% EtOH, at 3.30 × 10−5 mol/kg) 30 min prior to D4 or E2 each day. In addition, D4 and E2 produced no effect on uterine weight when administered to OVX, αERKO mice (knock-out mice genetically lacking ERα) of the same strain. Uterine peroxidase activity (measured as the rate of quaiacol oxidation) was increased 10.8* fold in the OVX mice by E2 or 5.0*# fold by D4. The RE of this effect for D4 is 1.17 × 10−6. Negative controls D5, hexamethylcyclotrisiloxane, decamethyltetrasiloxane, and octaphenylcyclotetrasiloxane) all failed to produce any effects in these analyses when administered by gavage at 1000 mg/kg/day. In this study statistical analyses were one-way ANOVA and Dunnett’s t test with n ≥ 6 per group.
These data demonstrate that D4 can produce ERα-related actions by causing alterations in some uterine parameters in vivo. The ability of D4 to produce these effects is much lower than that of E2 (RE 1.17 × 10−6). One problem with these data resides in the fact that the vehicles used for the E2 and ICI treatments were not the same as one another, and they were not the same as the vehicle for D4 and the control. An additional deficiency in this study was the lack of measurement of the effect of D4 and E2 combined.
Rank 1
EE and genistein produced dose-dependent increases in absolute wet and blotted uterine weights and increases in uterine luminal and glandular epithelial cell heights in OVX F-344 rats (DCC Citation2004a). D4 (tested only at 700 ppm by whole body inhalation exposure, for 16 h/day for 3 days) also produced increases in absolute wet and blotted uterine weights and increases in uterine luminal and glandular epithelial cell heights in this study. D4 co-administered with EE caused a partial inhibition of the action of EE. The results for the highest concentrations tested are shown in .
Table 3. Effects of EE, genistein, and D4 on some uterine parameters in OVX F-344 rats.
These data support the conclusion that D4 can produce ERα-related actions by causing effects on some uterine parameters in vivo. The ability of D4 to produce these effects is much lower than that of EE (REs 2.14 × 10−6–2.84 × 10−6). When co-administered with EE, D4 was able to partially inhibit the action of EE on uterine weight increases, but not on uterine cell height increases. A deficiency in this study was that the controls for the D4 dosed animals were different than the controls for the EE and genistein dosed animals. There were no negative controls and no use of ERα antagonists in this study. Statistical analyses employed were one-way ANOVA, Dunnett’s t, and Kruskal–Wallis with Shirley’s tests as needed. n ≥ 6 per group.
Rank 1
EE produced dose-dependent increases in absolute and relative, wet and blotted uterine weights and in uterine luminal and glandular epithelial cell heights in OVX SD and F-344 rats (DCC Citation2004b). D4 (tested only at 700 ppm by whole body inhalation exposure for 6 h/day for 3 days) also caused increases in absolute and relative, wet and blotted uterine weights and in uterine luminal and glandular epithelial cell heights in both rat strains. D4 co-administered with EE did not inhibit the abilities of EE to produce these effects. ICI (4.94 × 10−6 mol/kg) co-administered with EE completely blocked the uterine weight increases produced by EE alone. The results for the highest concentrations tested are shown in . These data support the conclusion that D4 can produce ERα-related actions by causing alterations in some uterine parameters in vivo. The ability of D4 to produce these effects is much lower than that of EE. Deficiencies in this study were the absence of weak agonist positive controls and no co-administration of the ERα antagonist ICI with D4. Statistical analyses employed were one-way ANOVA and least squares means with n = 8 per group.
Table 4. Effects of EE and D4 on some uterine parameters in OVX SD and F-344 rats.
Rank 1
D4, 4-hydroxytamoxifen, and EE produced increases in relative wet and blotted uterine weights and increases in uterine luminal and glandular epithelial cell heights in OVX F-344 rats (DCC Citation2012). Control animals received sesame oil vehicle by gavage. Control animals were exposed to filtered air for 6 or 16 h and sacrificed at the end of the exposure period. EE and 4-hydroxytamoxifen dosed animals were exposed to filtered air for 6 h and sacrificed at the end of the exposure period. The results are shown in . These data support the conclusion that D4 is capable of producing ERα-related actions by causing alterations in some uterine parameters in vivo. The ability of D4 to produce these effects is much lower than that of EE. D5, 160 ppm (6 or 16 h) was employed as a negative control. There was no ERα antagonist used in this study, and there was no measure of D4 and EE administered together. The controls for the 16 h/day D4 exposures and EE were not the same. Statistical analyses employed were one-way ANOVA, with Kruskal–Wallis, Levene’s, Shapiro–Wilk, Dunnett’s, Shirley’s, Fisher’s exact, Jonckheere–Terpstra, and Wilcoxon’s tests as needed with n = 6–8 per group.
Table 5. Effects of EE, 4-hydroxytamoxifen, and D4 on some uterine parameters in OVX F-344 rats.
Rank 1
In a chronic two generation D4 exposure study with SD rats there were no observed estrogen-mediated developmental effects in the F1 generation (DCC Citation2001). Dams were exposed to D4 by whole body inhalation at doses of 0, 70, 300, 500, and 700 ppm per day, 6 h per day, for 70 days prior to mating and through gestation day 21 and then the female F1 offspring were exposed after lactation day 4 through mating with a breeding competent male, gestation, and delivery of two successive litters. Anogenital distances in the male and female F1 pups were not affected, and there were no effects on vaginal patency in the F1 pups. Anogenital separation is a developmental estrogenic effect that is highly sensitive to pup weight (Heinrichs Citation1985; Gallavan et al. Citation1999). Mean pup weights were elevated only on post natal day one only in the 700 ppm dosage group. There were no other differences in mean pup weights and no differences in anogenital separation relative to the cube root of pup weight. Vaginal patency (time to opening of the vagina) in rat pups is also an estrogen-responsive developmental effect (Gellert Citation1978). Statistical tests employed in this study were one-way ANOVA with Dunnett’s test, and chi square test with Yates correction with n = 30 per group. Uterine parameters have not been examined in immature F1 generation females in any multi-generation D4 exposure study.
In vitro ERα transcription assays
In MCF-7 human breast cancer cells D4 increased the expression of mRNA for the pS2 gene (DCC Citation2000b). D4 increased luciferase expression in MCF-7 cells transfected to express luciferase in response to ERα activation (Quinn, Dalu, et al. Citation2007). D4 also increased CaBP-9K and progesterone receptor mRNA expression in rat pituitary GH3 cells (Lee et al. Citation2015).
Rank 2
In the MCF-7 human breast cancer cell line E2 (up to 3 × 10−10 M), bisphenol A (up to 2 × 10−5 M), and D4, at applied doses of 1 × 10−7 M and greater (up to 1 × 10−5 M) added to the culture as ethanol solutions with incubations for 24 or 48 h, produced dose-dependent increases in pS2 gene mRNA expression (DCC Citation2000b). D4 was progressively lost from these assays by evaporation plateauing at about 12 h at about 10% of the applied dose. There were no differences in the maximum level of pS2 reporter gene mRNA expression produced by the 3 compounds and there was no inhibition of E2 response when E2 and D4 were administered together. REs calculated from the linear portions of the slopes of the dose-response relationships, were 1.35 × 10−5 and 1.61 × 10−5 for D4 and bisphenol A respectively. These data support the conclusion that D4 is capable of causing an ERα-related action but with much lower activity than E2.
D4 showed the same maximal effect as E2 in the MCF-7 pS2 induction assay and it did not reduce the maximal effect of E2 in this assay. This is an indication that activation of only a small fraction of the receptors available in this system is required to produce the maximal effect. In an extremely sensitive system like this where there are spare receptors, full agonists and weak agonists are likely to be able to produce the same maximal effect and inhibition of the full agonist by the weak agonist will most likely not be observed (Matthews Citation1993).
Rank 2
In the MCF-7 human breast cancer cell line, transfected to express luciferase in response to ERα activation, EE (1 × 10−11 M − 3 × 10−8 M), bisphenol A (doses 1 × 10−7 M − 2 × 10−5 M), and D4 only at the highest dose tested 1 × 10−5 M) added to the culture as ethanol solutions with incubations for 24 or 48 h produced dose-dependent increases in luciferase expression (Quinn, Regan, et al. Citation2007). At the highest doses EE, bisphenol A, and D4 produced 37.6*, 9.6*#, and 6.4*# fold increases respectively in luciferase expression. REs were 1.71 × 10−7 and 6.15 × 10−7 for bisphenol A and D4 respectively. These data support the conclusion that D4 is capable of producing an ERα-related action, but with lower activity than bisphenol A and much lower activity than EE.
Rank 2
E2 at 1 × 10−9 M and D4 at 1 × 10−5 M in dimethylsulfoxide (0.1% in the media) produced 43.2* and 1.27*# fold increases respectively in CaBP-9K mRNA expression in rat pituitary GH3 cells in culture (Lee et al. Citation2015). The RE of D4 for this action was 3.0 × 10−7. ICI, at 1 × 10−7 M, reduced the control level of CaBP-9K to about one half* and when added 30 min before E2 or D4 completely blocked the increases produced by E2 or D4. In the same experiments E2 and D4 produced 4.5* and 1.4*# fold increases in progesterone receptor mRNA expression. The increases in progesterone receptor mRNA expression were completely blocked by ICI. The RE of D4 for this action was 4.5 × 10−6. These data support the conclusion that D4 is capable of producing some ERα-related actions, but with much lower activity than EE.
Displacement of E2 binding from isolated ERα
Rank 3
D4 at 4 × 10−5 M produced a displacement of 8%* of bound [3H]E2 from human recombinant ERα (He et al. Citation2003). The concentration of E2 required to displace the same amount of [3H]E2 was estimated to be about 5 × 10−11 M. The RE for D4 was ∼1.3 × 10−6 in this measurement.
Rank 3
D4, at a measured concentration of 4.5 × 10−7 M in air tight reaction vessels, produced a displacement of 18%* of [3H]E2 from purified human ERα (Quinn, Regan, et al. Citation2007). The concentration of unlabeled E2 required to produce the same level of displacement as that produced by D4 was estimated to be <1 × 10−11 M. The RE for D4 was ∼2.2 × 10−5 in this measurement.
The difference in the Quinn et al. report compared with the He, et al. report is probably due to evaporative losses of D4 during the incubations in the He et al. report. This procedure incubated the mixtures for 30 min at 37 °C without controlling for evaporative loss. The report by Quinn, Regan, et al. used air tight reaction vessels to prevent evaporative losses during the 4 h incubation period at 37 °C.
These displacements by D4 fall below the 50% cutoff specified by the U.S. EPA for consideration as potential endocrine disruptors (U.S. EPA Citation2011).
Discussion of hypothesis 1 results
As shown by Borgert et al. (Citation2018) weak agonists with REs below 1 × 10−4 are incapable of producing measurable effects in any but the most sensitive situations. D4 causes weak changes in some uterine parameters in naïve immature rats during the brief window in time when the uterine tissues are becoming sensitive to estrogen but endogenous estrogen levels are very low. The finding that estrogen-mediated developmental effects of D4 were not observed with chronic exposure in the two generation exposure study is an indication that the estrogen system undergoes adjustments that overcome any possible effects of a weak agonist that is not strong enough to upset the functional integrity of the system.
Each of the ranked in vivo and in vitro ERα-related actions produced by D4 is in the range of millions of times weaker than those produced by full agonists such as E2 or EE (REs generally in the 1–5 × 10−6 range). In addition, high doses of D4 (e.g. ≥ 300 ppm for inhalation exposures) produced small decreases in the maximum effects of E2 or EE in some of the measurements when the two compounds were administered in combination (REs in the 3–7 × 10−6 range, ).
Receptor interaction with the terminal oxygens of estrogens is critically important for the stimulation of the receptor. The wide range of structures that have been shown to produce stimulation of the ERα receptor (phytoestrogens, pesticides such as o,p-DDT and chlordecone, surfactants such as octyl and nonyl phenol, phthalates, polychlorinated biphenyls, and an extensive variety of others) is an indication that the lipophylic portion of the agonist binding site of this receptor is capable of accommodating a wide range of shapes. When the two terminal oxygens of these molecules are spaced appropriately to be able to interact with the receptor these molecules can produce some receptor stimulation. As shown for the Rank 3 data in hypothesis 1, D4 has a real, although poor, ability to displace E2 from isolated ERα. Examination of molecular models of D4 compared with those of E2 and diethylstilbestrol (DES) shows that D4 is approximately the same length as the lipophilic portions of these potent ERα agonists (). This means that D4 may be able to fulfill the size and shape requirements for being able to bind in the agonist binding site of ERα. However, the poor ability of D4 to displace bound E2 from ERα indicates that it does not “fit” very well into the agonist binding site. D4 lacks the terminal oxygen groups critical for ERα activation. Theoretically, two water molecules hydrogen bonded to oxygens of D4 at the 1 and 5 positions of the ring can provide OH groups (from the water molecules) located in approximately the same 3-dimensional positions as the 2 OH groups of E2 or DES (). It is, therefore, possible that D4-1,5-dihydrate can bind in the agonist binding site of ERα and produce limited stimulation of the receptor. Due to the relatively weak association of the hydration waters with D4 and the poor “fit” of the molecule in the agonist binding site, it is assumed that this molecular association will not be very effective at producing and stabilizing the stimulated conformation of the receptor resulting in very low potential for producing biological effects through this mechanism.
Other cyclic dimethylsiloxanes, e.g. D5, do not produce the ERα-related effects that have been shown for D4. These other dimethylsiloxanes lack the size and shape characteristics to allow them to enter and bind in the ERα agonist binding site (Paech et al. Citation1997). Similarly, D4 has been shown to be incapable of producing any activation-related effects with the beta isoform of the nuclear estrogen receptor (He et al. Citation2003) probably because it cannot fulfill the requirements for a proper “fit” in this receptor binding site.
Displacement of bound steroid hormones from carriers in the blood
Generally, only about 1–2% of steroid hormones in the blood are unbound. As shown by Jury et al. (Citation2000) some phytoestrogens and other lipophilic substances are capable of displacing bound E2 from human plasma steroid hormone binding globulin in vitro. As described under Partitioning high doses of D4 produce blood and plasma levels of D4 hundreds of times greater than its solubility limit demonstrating that most of the D4 in blood is adsorbed to or sequestered by blood components. It is probable that D4 is capable of displacing bound steroid hormones from their carriers in the blood similar to its ability to displace binding of E2 from isolated ERα receptors. Affinities of steroid hormones for binding to serum albumin (a major steroid hormone carrier in the rat) are on the order of 10,000 fold lower than for their receptors (Hammond Citation2016). In general, lower binding affinities correlate with reduced binding specificities (Eaton et al. Citation1995). D4 as a displacer could, therefore, be proportionately better able to displace steroid hormones from their blood carriers and other physiological reservoirs relative to its ability to displace bound E2 from ERα. If D4 displaces bound steroid hormones from their carriers in the blood and from other reservoirs it would make a larger proportion of these steroid hormones available for access to their receptors and for metabolic conversions. In addition, stress-related activation of the HPA has been shown to increase the levels of aromatase in a variety of tissues throughout the body (e.g. Zhao et al. Citation2004; Bell et al. Citation2014). This would be especially relevant in highly sensitive situations such as uterotropic assay systems, where estrogens are normally present at very low levels. Enhanced availability of endogenous androgens and enhanced conversion of these to estrogens could represent an important contribution to the observed abilities of D4 to cause changes in some uterine parameters.
One important difference between rats and humans is the absence of sex steroid hormone binding proteins in rat blood. The affinity of human sex steroid hormone binding protein for E2 is 1 × 10−8 M and for testosterone is 3 × 10−9 M (Martin et al. Citation1995). The affinity of E2 for sex steroid binding protein is about 10 fold lower than for nuclear estrogen receptors and the affinity of E2 for serum albumin is about 10,000 fold lower than for nuclear estrogen receptors (Hammond Citation2016). This means that D4 would be substantially less able to displace endogenous steroid hormones from blood carriers in humans compared with rats. If the displacement mechanism turns out to represent an important contribution to the ability of D4 to produce ERα-related effects it would be much less of a factor in humans.
Membrane G protein-coupled estrogen receptors (GPER)
GPERs activate protein kinases and other second messenger systems in the cell to accomplish rapid responses to estrogen (Zhou et al. Citation1996; Prossnitz and Barton Citation2009). The affinities of E2 for GPERs are relatively unaffected across widely divergent genera (3.3 × 10−9 M for humans versus 2.7 × 10−9 M for croaker fish) and these are about an order of magnitude lower than its affinity for ERα (1.3–6 × 10−10 M for humans and 4–6 × 10−10 M for croaker fish) (Thomas et al. Citation2010). This would require higher estrogen concentrations to activate relative to those required for activation of ERα, as during early proestrus. The increased rapidity of response, and the higher concentrations required, make estrogen binding to GPER in the hypothalamus a good candidate for the triggering mechanism for the pre-ovulatory surges of PRL, LH, and FSH. Due to the lower affinity of GPER for estrogens, D4 could be better able to displace estrogen binding to GPER relative to ERα rendering a greater proportion of the GPER agonist binding sites unavailable. However, there is no specific data for the ability of D4 to bind to and activate or inhibit GPER.
The foregoing ranked studies indicate that D4 is capable of producing some ERα-related effects, both in vivo and in vitro. D4 is generally millions of times weaker in these actions relative to endogenous estrogens. It is extremely unlikely that incidental exposures to D4 could ever be of sufficient magnitude to produce any detectable endocrine-related or other biological effects in humans or animals. Therefore, the hypothesis that D4 has sufficient magnitude of ability to upset the functional integrity of the endocrine system via an estrogenic mode of action is not supported. However, in a recent review from the Technical University of Denmark and the Danish Center for Endocrine Disruptors (CeHoS Citation2018) the authors concluded that “There is strong evidence for an estrogenic mode of action of D4, and strong evidence for adverse effects on female reproductive system that can be related to this estrogenic mode of action of D4 together with an endocrine mode of action through LH.” and recent regulatory actions in Europe have classified D4 as toxic for reproductive category 2 (Hass et al. Citation2018). Statements and conclusions that suggest adverse or toxic endocrine system-related effects from low dose exposures based solely on extrapolations of data from high dose exposures are reflective of the failure of the evaluators to examine the mechanisms involved in producing observed effects that occur only at high doses.
One argument that has been put forth to justify the hazard classification is that even low doses of potential endocrine system modifiers (at NOEL levels) can be hazardous since we are being exposed to mixtures of chemicals that may have similar activities, the additive effects of which can place us in the hazard zone. This argument has no basis in reality. Taking data for D4 from , we find that the apparent α value (efficacy term) and apparent dissociation constant for D4 (KDD4) for the effect of producing increases in absolute uterine weights (E) are approximately 0.44 and 4.3 × 10−3 mol/kg respectively. Applying these values to EquationEquation (2)(2)
(2) for the effect of a weak agonist added to a strong agonist (equation 113 from Matthews Citation1993) when the strong agonist is present at its KD concentration and D4 is present at its highest observed NOEL (3.4 × 10−5 mol/kg) gives a 0.03% change in effect from the strong agonist alone.
(2)
(2)
If we add 9 additional weak agonists with similar α and KD values at their observed highest NOELs we might, in the most extreme circumstance, achieve a cumulative effect of up to approximately 1% relative to the strong agonist alone. This level of change is irrelevant. Only when exogenous agonist ERs reach at least 1 × 10−4 relative to endogenous agonists will these have sufficient potential alone or in combinations to be able to upset the functional integrity of the endocrine system (Borgert et al. Citation2018).
Hypothesis 2: D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via an androgenic mode of action
Only very minor effects have been shown to be produced by D4 on the reproductive system in males. No effects have been found for D4 exposure in pre-pubertal males.
Rank 1
There was no effect in the Hershberger assay with castrated male F-344 rats exposed to D4 at 700 ppm by whole body inhalation for 16 h/day for 10 days (Quinn, Regan, et al. Citation2007).
Rank 1
Male F-344 rats had a 34.5%* decreased survival rate when exposed to D4 by whole body inhalation, at 700 ppm, for 6 h/day, 5 days/week from 50 to 108 weeks of age (DCC Citation2001; Burns-Naas et al. Citation2002; Jean and Plotzke Citation2017). This was attributed to increased mononuclear cell leukemia, a common finding in aged F344 rats. There were also D4-induced increases in relative testis weights (1.28* fold) and in testis interstitial cell hyperplasia (15%*) in these studies. Due to the relatively high aging-related incidence of testis interstitial cell adenoma in this rat strain, and the absence of any other established androgenic effects it is unlikely that D4 is producing its effects on the testis through any action on androgen receptors.
Rank 3
In some D4 dosing studies the presence of ejaculatory plugs in the caging was noted, but the numbers of plugs did not exceed the average daily production for unexposed, unrestrained rats (DCC Citation1995c, Citation1997b, Citation2001). Spontaneous ejaculation occurs in several mammalian species. In some species, including rats and mice, this is evidenced by the presence of white, waxy appearing ejaculatory plugs found on the trays under cage floor screens. Ejaculatory plugs are essential for successful fertilization in rats. They have a sperm reservoir on the rostral surface and during copulation they are deposited in the vagina in close apposition to the cervix. They serve to seal the vagina and insure completion of trans-cervical migration of sufficient numbers of sperm to complete fertilization (Blandau and Jordan Citation1941). On average, unrestrained, single housed male rats produce 2–3 ejaculatory plugs per day (Reynolds et al. Citation2010). Normally, the animals will consume these plugs if they can access them and, therefore, they are not observed. The most probable explanation for the presence of these ejaculatory plugs is that they contained sufficient amounts of D4 to render them unpalatable to the animals (Reynolds et al. Citation2010).
Based on these findings the hypothesis that D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via an androgenic mode of action is not supported.
Hypothesis 3: D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via a thyroid hormone mode of action
Rank 1
In a two generation study, after exposure to D4 at 700 ppm for 70 days at 6 h/day by whole body inhalation, relative thyroid weights were increased in F0 generation male SD rats by 140%* (DCC Citation1997a, Citation2001). Lower D4 doses produced no significant effect. These thyroid weight changes resolved 1-month post exposure. D4 caused thyroid follicular hypertrophy in F1 females in these studies. D4 at high doses (e.g. 700 ppm) produces blood and plasma levels of D4 hundreds of times greater than its solubility limit. This demonstrates that most of the D4 in blood or plasma is adsorbed to or sequestered by blood components. It is possible that D4 may interfere with binding of thyroid hormone to thyroid hormone carriers in the blood. Decreased blood carrying capacity for thyroid hormone could result in reductions in thyroid hormone availability. D4 induction of liver enzymes (DCC 1995a, 1995b, Citation1997a, Citation1998b, Citation1998c, Citation1998d, Citation1998e, Citation1999a, Citation2001, Citation2000c, Citation2019; Zhang et al. Citation2000) can reduce thyroid hormone availability by accelerating the metabolic breakdown of thyroid hormone (Hood et al. Citation1999). Various causes of thyroid hormone deficiency, e.g. iodine deficiency, can cause thyroid gland hypertrophy (Denef et al. Citation1981).
Discussion of hypothesis 3 results
The thyroid related effects of D4 occur only at high doses and only after prolonged exposures. Mechanisms other than an endocrine mode of action can possibly explain these effects. Therefore the hypothesis that D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via a thyroid hormone mode of action is not adequately supported.
Hypothesis 4: D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via a steroidogenic mode of action
Rank 1
In estrus cycle staged SD rats whole body inhalation of D4 at 700 ppm for 6 h/day for the 3 days leading into proestrus increased E2 levels by 2.68* fold (DCC Citation2002a). E2 levels at the end of diestrus were increased by 1.14* fold. No differences were found in E2 levels at 10 AM of proestrus.
Rank 1
At doses of 3.37 × 10−3 mol/kg/day and greater D4, administered to 129 J/C57Bl/6J mice by gavage in corn oil for 7 days produced dose-dependent decreases (up to 45%*) in serum E2 (He et al. Citation2003). This study did not control for the stages of the estrous cycle. Therefore, the changes in E2 levels cannot be interpreted.
Rank 1
Whole body inhalation exposure of F-344 rats to D4 at 700 ppm for 6 h/day, 5 days/week from 50 to 108 weeks of age showed alterations reflective of an estrogen dominant character (Jean and Plotzke Citation2017). These included endometrial hyperplasia (↑31.4%*), non-significant increases in benign endometrial adenomas, and increases in absolute and relative uterine weights (1.46* fold and 1.53* fold respectively). Vaginal lavage sampling showed extended proestrus-estrus during weeks 50–79 and increased frequency of proestrus-estrus during weeks 80–108. Vaginal histology showed increased vaginal epithelial thickness (Jean et al. Citation2017). Dopamine inhibits PRL secretion from the pituitary (MacLeod and Lehmeyer Citation1974). Chronic stress increases hypothalamic synthesis and release of dopamine. Inhibition of PRL secretion decreases ovarian progesterone synthesis and release thereby increasing the estrogen to progesterone ratio, ending pseudo-pregnancy, and persistently stimulating the uterine endometria (Alison et al. Citation1990). These changes do not, necessarily, require changes in estrogen levels.
Discussion of hypothesis 4 results
Blood levels of ovarian estrogens fluctuate widely according to the stage of the estrous cycle. Gonadotrophic hormones, feedback regulation, and metabolic breakdown all contribute to control of ovarian estrogen levels across the estrous cycle. Available data do not permit an evaluation of the ability of D4 to influence blood ovarian estrogen levels. Therefore, the hypothesis that D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via a steroidogenic mode of action is not supported.
Hypothesis 5: D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via direct effects on the HPG
Rank 1
In a two generation exposure study with SD rats, dams were exposed to D4 by whole body inhalation at doses of 0, 70, 300, 500, and 700 ppm per day, 6 h per day, for 70 days prior to mating and through gestation day 21 and then the F1 female offspring were exposed after lactation day 4 through mating with a breeding competent male, gestation, and delivery of two successive litters (DCC Citation2001). The F1 generation females exposed at 700 ppm showed decreases in mating. Lower dose groups were not different from the controls. The number of control females with no evidence of mating was 3.3% compared with 26.7%* for the 700 ppm D4 treated animals. All of the control females that mated became pregnant compared with only 77.3%* of the mated 700 ppm D4 treated animals.
Reproductive effects that were observed in this study were live litter size and fertility index reductions for the F0 and F1 dams at the 500 and 700 ppm dosages. Inhalational dosages of 300 ppm and lower were not different from controls. Statistical tests employed in this study were one-way ANOVA with Dunnett’s test, and chi square test with Yates correction. n = 30 per group.
Rank 1
D4, administered by whole body inhalation at 700 or 900 ppm to estrous staged SD rats, for 6 h/day, for the 3 days leading to proestrus, decreased the number of mated animals that ovulated by 47%* and 61%* respectively (DCC Citation2002a, Citation2019; Quinn, Dalu, et al. Citation2007). In mated animals that failed to ovulate, D4 at 700 ppm decreased LH surge levels at 6 PM on proestrus by 44.4%* while D4 at 900 ppm decreased LH surge levels at 4, 6, and 8 PM on proestrus by 33.4%*, 54.9%*, and 43.0%* respectively. Statistical analyses employed were one-way ANOVA with Dunnett’s, Tukey’s, and Kruskal–Wallis tests as needed. n = 20 or 30 per group.
Rank 1
D4, administered to F-344 female rats by whole body inhalation for 6 h/day at 700 ppm from days −3 to +3 of gestation decreased corpora lutea by 13.1%* (DCC Citation1999b). Statistical analyses employed were two-tailed ANOVA with Dunnett’s, Kruskal–Wallis, and Mann–Whitney U tests as needed. n ≥ 25 per group.
Rank 1
Female SD rats exposed to 700 ppm D4 by whole body inhalation for 6 h on just the day before mating had increased rates (4.3%*) of anovulatory matings (DCC Citation1999b; Meeks et al. Citation2007).
Rank 1
Whole body inhalation exposure to D4 at 700 or 900 ppm for 6 h on the third day after the beginning of sc implant administration of E2 in OVX SD rats decreased peak LH levels to below the minimum observed for the control animals in 40%* and 70%* of animals in the 700 and 900 ppm exposure groups respectively (DCC Citation1998f). Statistical analyses employed in this study were two-tailed ANOVA with Dunnett’s t test. n = 4–50 per group. The use of E2 implants in OVX rats provides evidence against any inhibitory action of D4 on estrogen synthesis as a cause of reduced LH surge peak levels.
Rank 1
In SD rats that became pregnant, whole body inhalation exposure to D4 at 700 ppm for 6 h/day for 28 days prior to mating and continuing until gestation day 20 did not significantly decrease the level of the LH surge and did not affect the number of corpora lutea that were produced (DCC Citation1995c, Citation1996, Citation2019; Meeks et al. Citation2007; Franzen et al. Citation2017). However, these animals displayed decreased numbers (↓27.3%*) of live fetuses due to decreased numbers of implantation sites (↓21.5%*), increased numbers of pre- and post-implantation losses, and increased numbers of implantation sites not accounted for (implantations not producing a fetus) (↑2.43* fold).
Discussion of hypothesis 5 results
D4 in extended whole body inhalation studies, with F-344 rats, at 700 ppm resulted in nasal cavity lesions typical of a mild irritant and the lungs showed alveolar histiocytosis and focal interstitial inflammation (Jean and Plotzke Citation2017; DCC Citation2001). The chronic stress caused by large body burdens of D4 and by inflammatory irritation in the nasal cavity and lungs may be the cause of observed implantation decreases and losses. Nasal cavity and lung irritants and their associated stress responses have been shown to cause uterine implantation deficits. Mice exposed to polluted air (particulate matter and NO2) displayed decreased live litter sizes and increased implantation failures versus clean air during early development (Mohallem et al. Citation2005). Exposure of women to ambient particulate matter in air (aerodynamic diameter ≤ 10 μm) during the pre-conceptional period and early pregnancy showed a 2.6* fold increase in risk of miscarriage (Perin et al. Citation2010). Uterine endometria secrete a variety of cytokines and other paracrine factors that have important functions in the processes of implantation (Singh et al. Citation2011). Glucocorticoids interfere with the synthesis, secretion, and actions of a number of cytokines including those involved in the processes of implantation and maintenance of placental function (Schimmer and Parker Citation1996). Disregulation of cytokines and their functions leads to absolute or partial failure of implantation and abnormal placental function (Guzeloglu-Kayisli et al. Citation2009).
The effects of D4 administration on peak LH surge levels are most likely due to stress-induced inhibition of GnRH release from the hypothalamus. CRF, glucocorticoids and β-endorphin act in the brain to inhibit GnRH and LH release (Rivier and Vale Citation1984; Gambacciani et al. Citation1986; Rivier and Rivest Citation1991). Effects of D4 to inhibit GPER activation may also contribute to these effects. Individual variations in LH surge levels in the female rats in response to D4 can result in the spectrum of effects noted for mating and ovulation. Those animals with no or minimal reductions of LH surge levels mated, ovulated normally, and became pregnant. Intermediate reductions of LH surge levels did not affect mating but reduced ovulation. At the high end of this range the number of corpora lutea was reduced and at the low end there was no ovulation. Larger reductions of the LH surge disrupted mating behavior.
Decreases in LH surge levels and stress-induced disregulation of cytokines necessary for regulating implantation and maintenance of placental function are sufficient to account for the changes induced by D4. Therefore, the hypothesis that D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via direct effects on the HPG is not supported.
Hypothesis 6: D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via a dopaminergic mode of action
Rank 1
In OVX F-344 rats, 24 h after reserpine-induced dopamine depletion, D4 (700 ppm, 6 h nose only inhalation exposure) completely* inhibited PRL release from the anterior pituitary (DCC Citation2005a). D5 (160 ppm, 6 h nose only inhalation exposure) inhibited PRL release from the anterior pituitary by 57%*. No inhalation exposure with air only control was run. Pretreatment with sulpiride (7.32 × 10−5 mol/kg), a dopamine D2 receptor antagonist, completely reversed these effects. Glucocorticoids stimulate the synthesis and release of dopamine in the brain and this effect is likely sufficient to overcome the dopamine depletion and explain the abilities of both D4 and D5 to inhibit PRL release (Lewis et al. Citation1987; Czyrak et al. Citation2003). Statistical analyses performed in this study were one-way ANOVA with Shapiro–Wilk, Levene’s and Kruskal–Wallis tests as needed. n = 6–10 per group.
Rank 2
D4 and D5 (administered at 1 × 10−5 M as ethanol solutions) completely blocked maitotoxin-induced PRL release from pituitary tumor-derived MMQ cells in culture (DCC Citation2005b). Dopamine (1 × 10−5 M) also decreased PRL release from these cells to levels below the controls. Dopamine (1 × 10−5 M) interfered with the cell viability measures employed. Maitotoxin administration decreased cell viability by 22% at the lowest dose (1.5 ng/mL) but not at the highest dose (3.0 ng/mL). There is no stress response involved in this system. There were, however, numerous flaws in this study, which substantially diminish its validity. Due to structural considerations it is unlikely that both D4 and D5 are functioning as dopamine D2 receptor agonists.
Rank 3
In a technical report from the Silicones Environmental, Health and Safety Council there was no effect of D4 or D5 on [125I]iodosulpiride binding to D2 receptors in isolated striatal membranes from F344 rat brain (Baker Citation2010).
Based on these findings the hypothesis that D4 has sufficient magnitude of ability to upset the functional activity of the endocrine system via a dopaminergic mode of action is not supported.
Discussion
D4, only at high doses (e.g. ≥300 ppm), exhibits weak ability to interact with and stimulate the ERα receptor. It also has weak ability to displace endogenous estrogens from this receptor. Therefore, in situations where sensitivity to estrogens is high and endogenous estrogen concentrations are very low some ERα-mediated effects, e.g. increases in some uterine parameters, can be observed. Increases in some uterine parameters are not, of themselves, adverse events. They merely provide information on the potential ability of a chemical to influence these sensitive estrogen-responsive processes in a naïve animal.
Other possible, unconfirmed, effects of D4 on the endocrine system include interference with binding of thyroid hormones to their carriers in the blood and displacement of binding of estrogens and other steroids to their carriers and other storage sites combined with stress-related enhancement of conversion of endogenous androgens to estrogens. The latter of these possibilities could represent an important contribution to the ability of high dose D4 to influence some ERα mediated processes. Humans have blood sex steroid hormone binding proteins, which are absent in rats, and these have substantially higher affinities for steroid hormones than serum albumin the primary steroid hormone carrier in rat blood. The affinity difference would make the human much less susceptible to this possible mechanism for a D4 effect on estrogen dependent processes relative to the rat. Even if confirmed, these possible effects are indirect and not endocrine-mediated (Marty et al. Citation2018). No other endocrine system-related effects have been found for D4.
The stress response serves to protect the female by preventing or terminating pregnancy when stressful conditions exist that would compromise the female’s ability to successfully bring a pregnancy to term. Virtually any chemical substance will produce a stress response if the body burden approaches the limits that the organism or person can tolerate. This was the case in all of the high dose studies of the effects of D4 and many of the biological actions that are produced only by high doses of D4 are most probably primarily due to stress-related effects on the female reproductive system. According to all of the regulatory definitions stress-related effects cannot be classified as having an endocrine mode of action.
Assuming proper manufacturing practices and adequate ventilation in workplace environments, only low levels of D4 will be found in consumer products, the workplace, or the environment. D4 can be degraded by a variety of mechanisms and, therefore, it has no ability to bio-accumulate or persist over the long term in the environment. Exposure levels in the workplace or the environment are unlikely to ever approach the NOEL for producing any of the observed endocrine system-related actions.
Conclusions
This thorough, hypothesis driven, mechanistic review of the data and endpoints reveals that D4, only at high doses, and only in short term exposure studies in naïve animals (e.g. ≥300 ppm for inhalational exposures), can cause alterations in some uterine parameters with REs generally in the range of 1–5 × 10−6. Estrogen dependent development effects were not observed in animals that had been chronically exposed to D4. The large D4 concentrations required to produce any measurable effects on uterine parameters are not found in the workplace or in any other environment. Therefore, it is highly unlikely that exposure to D4 at levels that humans or creatures in the biosphere may be exposed to will be of sufficient magnitude to produce any endocrine system-related adverse effects.
Acknowledgements
The author gratefully acknowledges the American Chemistry Council, and its affiliate the Global Silicones Council, for the provision of resources including the full set of research reports from the Dow Corning Corporation on the biological effects of D4 and D5 as well as other information sources. The author also wishes to express his profound gratitude and thanks to Christopher Borgert, President & Principal Scientist, Applied Pharmacology and Toxicology, Inc.; Tracy Guerrero, Director, American Chemistry Council – Silicones, Environmental, Health, and Safety Center; Ellen Mihaich, President, Environmental and Regulatory Resources, LLC; and Kathleen Plotzke, Chief Health and Environmental Scientist, Consumer Solutions Business, Dow Corning Corporation for their critical reviews of the manuscript and for their many helpful suggestions.
Declaration of interest
The Global Silicones Council is an association that brings together all the major global manufacturers via the three Regional Silicone Industry Associations located in North America, Europe, and Japan. The Silicones Environmental, Health and Safety Center, comprised of North American silicone chemical producers and importers, is a not-for-profit trade sector group within the American Chemistry Council, whose mission is to promote the safe use of silicones through product stewardship and environmental, health, and safety research. John C. Matthews, Ph.D., taught biochemistry, toxicology, and pharmacology for 36 years. His primary research area was determining the effects and mechanisms of aging and stress-enhanced sensitivity to neurotoxicants as they relate to the development of neurodegenerative diseases such as Alzheimer’s disease. Based on his book and at least two of his coauthored publications (Matthews Citation1993; Borgert et al. Citation2013, Citation2018) the American Chemistry Council on behalf of the Global Silicones Council identified him as having the appropriate scientific background, credentials, and expertise to prepare this critical, comprehensive, unbiased review of the endocrine system-related actions of D4. Beyond the funding received for the work in preparing this review the author reports no conflict of interest with any topic or issue discussed in this review. The author has not been involved in any legal or regulatory proceedings related to the contents of this paper. The findings and conclusions are strictly those of the author and not those of the American Chemistry Council or the Global Silicones Council.
Additional information
Funding
References
- Alison R, Morgan K, Montgomery C. 1990. Ovary. In: Boorman G, Eustis S, Elwell M, Montgomery C, Mackenzie W, editors. Pathology of the Fischer rat. San Diego (CA): Academic Press, p. 429–442.
- Andersen ME, Sarangapani R, Reitz RH, Gallavan RH, Dobrev ID, Plotzke KP. 2001. Physiological modeling reveals novel pharmacokinetic behavior for inhaled octamethylcyclotetrasiloxane in rats. Toxicol Sci. 60(2):214–231.
- Ashby J. 2001. Increasing the sensitivity of the rodent uterotropic assay to estrogens with particular reference to bisphenol A. Environ Health Perspect. 109(11):1091–1094.
- Baker S. 2010. Potential for octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane to interact with and activate the dopamine D2 receptor in rat striatal membranes. Silicones Environmental, Health and Safety Council report.
- Bell JR, Bernasochi GE, Varma U, Boon WC, Ellem SJ, Risbridger GP, Delbridge LMD. 2014. Aromatase transgenic upregulation modulates basal cardiac performance and the response to ischemic stress in male mice. Am J Physiol Heart Circ Physiol. 306(9):H1265–H1274.
- Blandau RJ, Jordan ES. 1941. A technique for the artificial insemination of the white lab rat. J Lab Clin Med. 26:1361–1363.
- Borgert CJ, Baker SP, Matthews JC. 2013. Potency matters: thresholds govern endocrine activity. Regul Toxicol Pharmacol. 67(1):83–88.
- Borgert CJ, Matthews JC, Baker SP. 2018. Human-relevant potency threshold (HRPT) for ERα agonism. Arch Toxicol. 92(5):1685–1702.
- Borgert CJ, Mihaich EM, Ortego LS, Bentley KS, Holmes CM, Levine SL, Becker RA. 2011. Hypothesis-driven weight of evidence framework for evaluating data within the US EPA's Endocrine Disruptor Screening Program. Regul Toxicol Pharmacol. 61(2):185–191.
- Borgert CJ, Stuchal LD, Mihaich EM, Becker RA, Bentley KS, Brausch JM, Coady K, Geter DR, Gordon E, Guiney PD, et al. 2014. Relevance weighting of tier 1 endocrine screening endpoints by rank order. Birth Defects Res B Dev Reprod Toxicol. 101(1):90–113.
- Burns-Naas LA, Meeks RG, Kolesar GB, Mast RW, Elwell MR, Hardisty JF, Thevenaz P. 2002. Inhalation toxicology of octamethylcyclotetrasiloxane (D4) following a 3-month nose-only exposure in Fischer 344 rats. Int J Toxicol. 21(1):39–53.
- Calogero AE, Burrello N, Negri-Cesi P, Papale L, Palumbo MA, Cianci A, Sanfilippo S, D'Agata R. 1996. Effects of corticotropin-releasing hormone on ovarian estrogen production in vitro. Endocrinology. 137(10):4161–4166.
- Czyrak A, Maćkowiak M, Chocyk A, Fijał K, Wedzony K. 2003. Role of glucocorticoids in the regulation of dopaminergic neurotransmission. Pol J Pharmacol. 55(5):667–674.
- De Boeck G, Vlaeminck A, Blust R. 1997. Effects of sublethal copper exposure on copper accumulation, food consumption, growth, energy stores, and nucleic acid content in common carp. Arch Environ Contam Toxicol. 33(4):415–422.
- Dekant W, Bridges J, Scialli AR. 2017b. A quantitative weight of evidence assessment of confidence in modes-of-action and their human relevance. Regul Toxicol Pharmacol. 90:51–71.
- Dekant W, Scialli AR, Plotzke K, Klaunig JE. 2017a. Biological relevance of effects following chronic administration of octamethyltetrasiloxane (D4) in Fischer 344 rats. Toxicol Lett. 279:S42–S53.
- Denef J-F, Haumont S, Cornette C, Beckers C. 1981. Correlated functional and morphometric study of thyroid hyperplasia induced by iodine deficiency. Endocrinology. 108(6):2352–2858.
- de Peyster A, Mihaich E. 2014. Hypothesis-driven weight of evidence analysis to determine potential endocrine activity of MTBE. Regul Toxicol Pharmacol. 69(3):348–370.
- Dow Corning Corporation [DCC]. 1988. Feasability studies to determine the palatability of octamethylcyclotetrasiloxane in rats. Report: 1988-0005-1757.
- Dow Corning Corporation [DCC]. 1995a. 3-Month repeated dose inhalation toxicity study with octamethylcyclotetrasiloxane in rats with 1-month recovery period. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1995-10000-40152.
- Dow Corning Corporation [DCC]. 1995b. 1-Month repeated dose inhalation toxicity study with octamethylcyclotetrasiloxane in rats. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1995-10000-40168.
- Dow Corning Corporation [DCC]. 1995c. An inhalation range-finding reproductive toxicity study octamethylcyclotetrasiloxane (D4) in rats. Ashland (OH): WIL Research Labs, Inc., Report No. 1995-10000-40919.
- Dow Corning Corporation [DCC]. 1995d. A pilot study for the determination of 14C-octamethylcyclotetrasiloxane (D4) pharmacokinetics in Fischer 344 rats following a single nose- only vapor inhalation exposure to 700 ppm 14C-D4. Midland (ML): Dow Corning Health and Environmental Sciences. Report No. 1995-10000-40998.
- Dow Corning Corporation [DCC]. 1995e. Pharmacokinetics of 14C-octamethylcyclotetrasiloxane (D4) in the rat following single nose-only vapor inhalation exposure to 14C-D4 at three dose levels. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1995-10000-40999.
- Dow Corning Corporation [DCC]. 1996. An inhalation range-finding reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in rats. Ashland (OH): WIL Research Labs, Inc. Report No. 1996-10000-41337.
- Dow Corning Corporation [DCC]. 1997a. An inhalation range-finding reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in male rats. Ashland (OH): WIL Research Labs, Inc. Report No. 1997-10000-43725.
- Dow Corning Corporation [DCC]. 1997b. An inhalation range-finding reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in male rats. Ashland (OH): WIL Research Labs, Inc. Report No. 1997-10000-43726.
- Dow Corning Corporation [DCC]. 1998a. An inhalation range-finding reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in female rats using multiple exposure regimens. Ashland (OH): WIL Research Labs, Inc. Report No. 1998-10000-44490.
- Dow Corning Corporation [DCC]. 1998b. Effects of repeated whole body exposure to octamethylcyclotetrasiloxane (D4) vapors on hepatic microsomal CYP2B1/2 induction in female Fischer 344 rats. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1998-10000-44687.
- Dow Corning Corporation [DCC]. 1998c. An oral gavage study to compare the absorption potential of 14C-octamethylcyclotetrasiloxane (D4) in Fischer 344 rats when delivered in various carriers. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1998-10000-44815.
- Dow Corning Corporation [DCC]. 1998d. Estrogenic and antiestrogenic activity of octamethylcyclotetrasiloxane (D4) in Sprague Dawley and Fischer 344 immature female rats using a uterotropic assay. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1998-10000-45425.
- Dow Corning Corporation [DCC]. 1998e. Evaluation of octamethylcyclotetrasiloxane (D4) as a potential inhibitor of human cytochrome P450 enzymes. Unpublished data submitted by Silicones Environmental, Health and Safety Council of North America. p. 74.
- Dow Corning Corporation [DCC]. 1998f. An inhalation study of the effects of octamethylcyclotetrasiloxane (D4) exposure on the preovulatory LH surge in ovaryectomized female rats. Ashland (OH): WIL Research Labs, Inc. Report No. 1998-10000-50592.
- Dow Corning Corporation [DCC]. 1999a. Effects of repeated whole body inhalation exposure to octamethylcyclotetrasiloxane (D4) vapors on hepatic microsomal CYP2B1/2 induction in female Fischer 344 rats: a dose-response study. Midland (MI): Dow Corning Corporation Health and Environmental Sciences. Report No. 1999-10000-44687.
- Dow Corning Corporation [DCC]. 1999b. An inhalation reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in female rats using multiple and single day exposure regimens. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 1999- 10000-47049.
- Dow Corning Corporation [DCC]. 2000a. Disposition of octamethylcyclotetrasiloxane (D4) in Female Fischer 344 and Sprague Dawley IGS rats following a single nose-only vapor inhalation exposure to 700 ppm 14C-D4. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 2000-10000-48876.
- Dow Corning Corporation [DCC]. 2000b. Evaluation of potential estrogenic properties of octamethylcyclotetrasiloxane (D4) using the MCF-7 cell line: amendment to final report No. 2000-10000-48477. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 2000-10000-50345.
- Dow Corning Corporation [DCC]. 2000c. Evaluation of decamethylcyclopentasiloxane (D5) as a potential inhibitor of human and rat cytochrome P450 enzymes. Unpublished data submitted by Silicones Environmental, Health and Safety Council of North America. p. 118.
- Dow Corning Corporation [DCC]. 2001. A two-generation inhalation reproductive toxicity and developmental neurotoxicity study of octamethylcyclotetrasiloxane (D4) in rats. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 2001-10000-50855.
- Dow Corning Corporation [DCC]. 2002a. Effects of octamethylcyclotetrasiloxane (D4) on LH surge and levels of various sex hormones in female Sprague-Dawley rats. Midland (MI): Dow Corning Health and Environmental Sciences. Report No. 2000-10000-51695.
- Dow Corning Corporation [DCC]. 2002b. Disposition of octamethylcyclotetrasiloxane (D4) in female Fischer 344 and Sprague-Dawley IGS rats following fourteen repeat nose-only vapor inhalation exposures to 700 ppm D4 followed by a single nose-only vapor inhalation exposure to 700 ppm 14C-D4 on day fifteen. Auburn (MI): Dow Corning Health and Environmental Sciences. Report No. 2002-10000-51831.
- Dow Corning Corporation [DCC]. 2003. Evaluation of octamethylcyclotetrasiloxane (D4) with the rat uterotropic assay using ovariectomized adult Fischer 344 rats. Report No. 2003-I0000-53146.
- Dow Corning Corporation [DCC]. 2004a. Evaluation of octamethylcyclotetrasiloxane (D4) with the rat uterotropic assay using ovariectomized adult Fischer 344 rats. Auburn (MI): Dow Corning Health and Environmental Sciences. Report No. 2004-STEC-2426.
- Dow Corning Corporation [DCC]. 2004b. The rat uterotropic assay evaluation of octamethylcyclotetrasiloxane (D4) in two strains of rats exposed for 6 hours by whole-body inhalation. Auburn (MI): Dow Corning Health and Environmental Sciences. Report No. 2004- I0000-53624.
- Dow Corning Corporation [DCC]. 2004c. Evaluation of decamethylcyclopentasiloxane (D5) with the rat uterotropic assay using ovariectomized adult Fischer 344 rats. Report No. 2004-I0000- 54570.
- Dow Corning Corporation [DCC]. 2005a. Effect of cyclic siloxanes on dopamine receptor regulation of serum prolactin levels in Fischer 344 rats. Auburn (MI): Dow Corning Health and Environmental Sciences. Report No. 2005-STEC-2802.
- Dow Corning Corporation [DCC]. 2005b. Effect of cyclic siloxanes on dopamine receptor regulation of prolactin release from rat pituitary tumor-derived transformed cell lines. Auburn (MI): Dow Corning Health and Environmental Science. Report No. 2005-STEC-2824.
- Dow Corning Corporation [DCC]. 2012. Potential for uterine proliferation in the Fischer 344 rat with octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane: effect of vapor inhalation exposure duration. Auburn (MI): Dow Corning Health and Environmental Sciences. Report No. 2012-STEC-4135.
- Dow Chemical Company [DCC]. 2019. Octamethylcyclotetrasiloxane (D4): toxicokinetic study in estrous cycle staged nonpregnant Crl:CD(SD) rats following repeated inhalation exposure. Study No. 171006. Sponsor: Silicones Environmental, Health and Safety Center.
- Eaton BE, Gold L, Zichi BA. 1995. Let’s get specific: the relationship between specificity and affinity. Chem Biol. 2(10):633–638.
- European Chemicals Agency and European Food Safety Authority [ECHA and EFSA]. 2018. Guidance for the identification of endocrine disruptors in biocides and pesticides. [accessed 2021 Apr 26]. [https://doi.org/https://doi.org/10.2903/j.efsa.2018.5311].
- European Parliament and Council 2012. Regulation (EU) No. 528/2012 of the European Parliament and the council of 22 May 2012 – concerning the making available on the market and use of biocides products. Official J E U. 167/1.
- Franzen A, Greene T, Van Landingham C, Gentry R. 2017. Toxicology of octamethylcyclotetrasiloxane (D4). Toxicol Lett. 279:2–22.
- Gallavan RH, Holson JF, Stump DJ, Knapp JF, Reynolds VL. 1999. Interpreting the toxicological significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 13(5):383–390.
- Gambacciani M, Yen SSC, Rasmussen DD. 1986. GnRH release from the mediobasal hypothalamus: in vitro inhibition by corticotropin-releasing factor. Neuroendocrinology. 43(4):533–536.
- Garris DR, Foreman D. 1984. Follicular growth and atresia during the last half of the luteal phase of the guinea pig estrous cycle: relation to serum progesterone and estradiol levels and utero-ovarian blood flow. Endocrinology. 115(1):73–77.
- Gellert RJ. 1978. Kepone, mirex, dieldrin, and aldrin: estrogenic activity and the induction of persistent vaginal estrous and anovulation in rats following neonatal treatment. Environ Res. 16(1–3):131–138.
- Gentry R, Franzen A, Van Landingham C, Greene T, Plotzke K. 2017. A global human health risk assessment for octamethylcyclotetrasiloxane (D4). Toxicol Lett. 279:23–41.
- Genualdi S, Harner T, Cheng Y, Macleod M, Hansen KM, van Egmond R, Shoeib M, Lee SC. 2011. Global distribution of linear and cyclic volatile methyl siloxanes in air. Environ Sci Technol. 45(8):3349–3354.
- Griffin P. 2004. Severn Trent Water wastewater treatment plant at Minworth. ST-seminar.
- Grumping K, Michalke K, Hirner AV, Hensel R. 1999. Microbial degradation of octamethylcyclotetrasiloxane. Appl Environ Microbiol. 65(5):2276–2278.
- Gunnet JW, Freeman ME. 1983. The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev. 4(1):44–61.
- Guzeloglu-Kayisli O, Kayisli UM, Taylor HS. 2009. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Seminars in Reprod Med. 27:62–79.
- Hammond GL. 2016. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 230(1):R13–R25.
- Hashimoto I, Isomoto N, Eto M, Kawaminami M, Sunazuka C, Ueki N. 1987. Preovulatory secretion of progesterone, luteinizing hormone, and prolactin in 4-day and 5-day cycling rats. Biol Reprod. 36(3):599–605.
- Hass U, Christiansen S, Andersen M, Rosenburg S, Mandrup K, Egebjerg S, Nikolov N. 2018. List of endocrine disruptive chemicals: final report, December 12th, 2017. Danish Center on Endocrine Disruptors. [accessed 2021 Sep 3]. Available at: http://cend.dk/files/DKED-list-final2018.pdf.
- He B, Rhodes-Brower S, Miller MR, Munson AE, Germolec DR, Walker VR, Korach KS, Meade BJ. 2003. Octamethylcyclotetrasiloxane exhibits estrogenic activity in mice via ERalpha. Toxicol Appl Pharmacol. 192(3):254–261.
- Hebel R, Stomberg MW. 1986. Anatomy and embryology of the laboratory rat. Wörthsee (Germany): Biomed Verlag. p. 91.
- Heinrichs WL. 1985. Current laboratory approaches for assessing female reproductive toxicity. In: Dixon RL, editor. Reproductive toxicology. New York: Raven Press, p. 95–108.
- Hood A, Hashmi R, Klaassen CD. 1999. Efects of microsomal enzyme inducers on thyroid-follicular cell proliferation, hyperplasia, and hypertrophy. Toxicol Appl Pharmacol. 160(2):163–170.
- Horii Y, Ohtsuka N, Minomo K, Takemine S, Motegi M, Hara M. 2021. Distribution characteristics of methylsiloxanes in atmospheric environment of Saitama, Japan: diurnal and seasonal variations `and emission source apportionment. Sci Tot Environ. 754:142399.
- Jean PA, Plotzke KP. 2017. Chronic toxicity and oncogenicity of octamethylcyclotetrasiloxane (D4) in the Fischer 344 rat. Toxicol Lett. 279:75–95.
- Jean PA, Sloter ED, Plotzke KP. 2017. Effects of chronic exposure to octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane in the aging female Fischer 344 rat. Toxicol Lett. 279:54–74.
- Johnson MH. 2007. Essential reproduction. 6th ed. New York (NY): Wiley-Blackwell, p. 316.
- Jovanovic ML, McMahon JM, McNett D, Tobin JM, Plotzke KP. 2008. In vitro and in vivo percutaneous absorption of 14C-octamethylcyclotetrasiloxane (14C-D4) and 14C-decamethylcyclopentasiloxane (14C-D5). Regul Toxicol Pharmacol. 50(2):239–248.
- Jury HH, Zacharewski TR, Hammond GL. 2000. Interactions between human plasma sex hormone-binding globulin and xenobiotic ligands. J Steroid Biochem Mol Biol. 75(2–3):167–176.
- Klein F, Lemaire V, Sandi C, Vitiello S, Van der Logt J, Laurent PE, Neveu P, Le Moal M, Mormede P. 1992. Prolonged increase of corticosterone secretion by chronic social stress does not necessarily impair immune functions. Life Sci. 50(10):723–731.
- Krogseth IS, Undeman E, Evenset A, Christensen GN, Whelan MJ, Breivik K, Warner NA. 2017. Elucidating the behavior of cyclic volatile methylsiloxanes in a subarctic freshwater food web: a modeled and measured approach. Environ Sci Technol. 51(21):12489–12497.
- Lee D, Ahn C, An B-S, Jeung E-B. 2015. Induction of the estrogenic marker calbindin-D9k by octamethylcyclotetrasiloxane. Int J Environ Res Pub Health. 12(11):1461–1462.
- Lee S-Y, Lee S, Choi M, Kannan K, Moon H-B. 2018. An optimized method for the analysis of cyclic and linear siloxanes and their distribution in surface and core sediments from industrialized bays in Korea. Environ Pollut. 236:111–118.
- Lewis AG, Harrington CA, Chikaraishi DM. 1987. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci USA. 84:3350–3354.
- Long JA, Evans HM. 1922. The estrous cycle in the rat and its associated phenomena. Mem Univ Calif. 6:1–148.
- Lu KH, Hopper BR, Vargo TM, Yen SSC. 1979. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 21(1):193–203.
- MacLeod RM, Lehmeyer JE. 1974. Studies on the mechanism of the dopamine-mediated inhibition of prolactin secretion. Endocrinology. 94(4):1077–1085.
- Martin ME, Haourigui M, Pelissero C, Benassayag C, Nunez EA. 1995. Interactions between phytoestrogens and human sex streroid binding protein. Life Sci. 58(5):429–436.
- Marty MS, Borgert C, Coady K, Green R, Levine SL, Mihaich E, Ortego L, Wheeler JR, Yi KD, Zorrilla LM. 2018. Distinguishing between endocrine disruption and non-specific effects on endocrine systems. Regul Toxicol Pharmacol. 99:142–158.
- Matthews JC. 1993. Fundamentals of receptor, enzyme, and transport kinetics. Boca Raton (FL): CRC Press.
- McCracken JA, Custer EE, Lamsa JC. 1999. Luteolysis: a neuroendocrine-mediated event. Physiol Rev. 79(2):263–323.
- McGoldrick DJ, Letcher RJ, Barresi E, Keir MJ, Small J, Clark MG, Sverko E, Backus SM. 2014. Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ Pollut. 193:254–261.
- McKim JM, Jr, Wilga P, Breslin W, Plotzke K, Gallavan R, Meeks R. 2001. Potential estrogenic and antiestrogenic activity of the cyclic siloxane octamethylcyclotetrasiloxane (D4) and the linear siloxane hexamethyldisiloxane (HMDS) in immature rats using the uterotrophic assay. Toxicol Sci. 63(1):37–46.
- Meeks RG, Stump DG, Siddiqui WH, Holson JF, Plotzke KP, Reynolds VL. 2007. An inhalation reproductive toxicity study of octamethylcyclotetrasiloxane (D4) in female rats using multiple and single day exposure regimens. Reprod Toxicol. 23(2):192–201.
- Mihaich E, Capdevielle M, Urbach-Ross D, Slezak B. 2017. Hypothesis-driven weight-of-evidence analysis of endocrine disruption potential: a case study with triclosan. Crit Rev Toxicol. 47(4):263–285.
- Mihaich EM, Borgert CJ. 2018. Hypothesis-driven weight-of-evidence analysis for the endocrine disruption potential of benzene. Regul Toxicol Pharmacol. 100:7–15.
- Mohallem SV, Ja de Araujo Lobo D, Pesquero CR, Assuncao JV, de Andre PA, Saldiva PHN, Dolhnikoff M. 2005. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ Res. 98(2):196–202.
- Moleriu RD, Zaharie D, Moatar-Moleriu LC, Gruia AT, Mic AA, Mic FA. 2014. Insights into the mechanisms of thymus involution and regeneration by modeling the glucocorticoid-induced perturbation of thymocyte populations dynamics. J Theor Biol. 348:80–99.
- Mueller JA, Di Toro DM, Maiello JA. 1995. Fate of octamethylcyclotetrasiloxane (OMCTS) in the atmosphere and in sewage treatment plants as an estimation of aquatic exposure. Environ Toxicol Chem. 14(10):1657–1666.
- Neal BH, Bus J, Marty MS, Coady K, Williams A, Staveley J, Lamb JC. 2017. Weight-of-the-evidence evaluation of 2,4-D potential for interactions with the estrogen, androgen and thyroid pathways and steroidogenesis. Critical Rev Toxicol. 47(5):352–408.
- National Library of Medicine; National Center for Biotechnology Information; PubChem [NIH]. [accessed 2020 Apr 30]. https://pubchem.ncbi.nlm.nih.gov/compound/Octamethylcyclotetrasiloxane#section=Chemical-and-Physical-Properties.
- Nusz JB, Fairbrother A, Daley J, Burton GA. 2018. Use of multiple lines of evidence to provide a realistic toxic substances control act ecological risk evaluation based on monitoring data: D4 case study. Sci Total Environ. 636:1382–1395.
- Paech K, Webb P, Kuiper GJM, Nilsson S, Gustafsson J-A, Kushner PJ, Scanlan TS. 1997. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 277(5331):1508–1510.
- Pauluhn J. 2019. Expert review of D4 inhalation toxicology and consumer risks. Silicones Environmental, Health and Safety Center. Report 1-45.
- Pauluhn J. 2021. Inhalation toxicity of cyclic semi-volatile methylsiloxanes: disentangling the conundrum of phase-specific adaptations from adverse outcomes. Regul Toxicol Pharmacol. 122:104923.
- Pauluhn J, Mohr U. 1999. Repeated 4-week inhalation exposure of rats: effect of low-, intermediate-, and high-humidity chamber atmospheres. Exp Toxicol Pathol. 51(2):178–187.
- Perin PM, Maluf M, Czeresnia CE, Januário DANF, Saldiva PHN. 2010. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril. 93(1):301–303.
- Plotzke KP, Crofoot SD, Ferdinandi ES, Beattie JG, Reitz RH, McNett DA, Meeks RG. 2000. Disposition of radioactivity in Fischer 344 rats after single and multiple inhalation exposure to [14C]octamethylcyclotetrasiloxane ([14C]D4). Drug Metab Dispos. 28(2):192–204.
- Powell DE, Suganuma N, Kobayashi K, Nakamura T, Ninomiya K, Matsumura K, Omura N, Ushioka S. 2017. Trophic dilution of cyclic volatile methylsiloxanes (cVMS) in the pelagic marine food web of Tokyo Bay, Japan. Sci Total Environ. 578:366–382.
- Prossnitz ER, Barton M. 2009. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 89(3–4):89–97.
- Pruett S, Hebert P, Lapointe J-M, Reagan W, Lawton M, Kawabata TT. 2007. Characterization of the action of drug-induced stress responses on the immune system: evaluation of biomarkers for drug-induced stress in rats . J Immunotoxicol. 4(1):25–38.
- Quinn AL, Dalu A, Meeker LS, Jean PA, Meeks RG, Crissman JW, Gallavan RH, Jr., Plotzke KP. 2007. Effects of octamethylcyclotetrasiloxane (D4) on the luteinizing hormone (LH) surge and levels of various reproductive hormones in female Sprague-Dawley rats. Reprod Toxicol. 23(4):532–540.
- Quinn AL, Regan JM, Tobin JM, Marinik BJ, McMahon JM, McNett DA, Sushynski CM, Crofoot SD, Jean PA, Plotzke KP. 2007. In vitro and in vivo evaluation of the estrogenic, androgenic, and progestagenic potential of two cyclic siloxanes. Toxicol Sci. 96(1):145–153.
- Rabasa C, Dickson SL. 2016. Impact of stress on metabolism and energy balance. Curr Opin Behav Sci. 9:71–77.
- Reynolds VL, Stump DG, Holson JF. 2010. Assessing the toxicological significance of ejaculatory plugs as a clinical observation in rat toxicology studies: CNS-mediated increases in plug production vs. gustatory-mediated decreases in plug consumption. Reprod Toxicol. 29(3):271–278.
- Rivier C, Rivest S. 1991. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms . Biol Reprod. 45(4):523–532.
- Rivier C, Vale W. 1984. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology. 114(3):914–921.
- Sarangapani R, Teeguarden J, Andersen ME, Reitz RH, Plotzke KP. 2003. Route-specific differences in distribution characteristics of octamethylcyclotetrasiloxane in rats: analysis using PBPK models. Toxicol Sci. 71(1):41–52.
- Schimmer BP, Parker KL. 1996. Adrenocorticopic hormone: adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Hardman JE, Limbird LE, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill, p. 1459–1485.
- Singh M, Chaudhry P, Asselin E. 2011. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol. 210(1):5–14.
- Smith MS, Freeman ME, Neill JD. 1975. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 96(1):219–226.
- Sousa JV, McNamara PC, Putt AE, Machado MW, Surprenant DC, Hamelink JL, Kent DJ, Silberhorn EM, Hobson JF. 1995. Effects of octamethylcyclotetrasiloxane (OMCTS) on freshwater and marine organisms. Environ Toxicol Chem. 14(10):1639–1647.
- Surita SC, Tansel B. 2014. Emergence and fate of cyclic polydimethylsiloxanes (D4,D5) in municipal waste streams: release mechanisms, partitioning and persistence in air, water, soil and sediments. Sci Tot. Environ. 468-469:46–52.
- Technical University of Denmark and the Danish Centre for Endocrine Disruptors [CeHoS]. 2018. Octamethylcyclotetrasiloxane (D4), CAS no. 556-67-2. [accessed 2021 Apr 8]. [https://edlists.org/sites/edlists.org/files/media/document/D4.pdf].
- Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg H. 2010. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1, GPER, in mammals and fish. Steroids. 75(8-9):595–602.
- United States Environmental Protection Agency [U.S. EPA]. 2011. Weight-of-evidence: Evaluating results of EDSP tier 1 screening to identify the need for tier 2 testing. EPA-HQ-OPPT-2010-0877-0021. [accessed 2021 Aug 5]. Available at: http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2010-0877-0021.
- United States Environmental Protection Agency [U.S. EPA]. DSSTox database. [accessed 2020 Apr 30]. https://comptox.epa.gov/dashboard/dsstoxdb/results?search=DTXSID7027205#propertiesOctamethylcyclotetrasiloxane.
- Urban W, Lohmann H, Gomez JIS. 2009. Catalytically upgraded landfill gas as a cost-effective alternative for fuel cells. J Power Sources. 193(1):359–366.
- Varaprath S, Frye CL, Hamelink J. 1996. Aqueous solubility of permethylsiloxanes (silicones). Environ Toxicol Chem. 15(8):1263–1265.
- Varaprath S, Salyers KL, Plotzke KP, Nanavati S. 1999. Identification of metabolites of octamethylcyclotetrasiloxane (D4) in rat urine. Drug Metab Distrib. 27(11):1267–1273.
- Wang D-G, Steer H, Tait T, Williams Z, Pacepavicius G, Young T, Ng T, Smyth SA, Kinsman L, Alaee M. 2013. Concentrations of volatile methylsiloxanes in biosolid amended soil, influent, effluent, receiving water, and sediment of wastewater treatment plants in Canada. Chemosphere. 93(5):766–773.
- World Health Organization International Programme on Chemical Safety [WHO/IPCS]. 2002. Global assessment of the state-of-the-science of endocrine disruptors. World Health Organization, Geneva. [accessed 2020 Aug 21]. http://www.who.int/ipcs/publications/newissues/endocrinedisruptors/en/.
- Wilson SD. 1996. Modulation of immune and neuroendocrine function by siloxanes [Dissertation]. Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, Virginia.
- Woodburn K, Drottar K, Domoradzki J, Durham J, McNett D, Jezowski R. 2013. Determination of the dietary biomagnification of octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane with the rainbow trout (Oncorhynchus mykiss). Chemosphere. 93(5):779–788.
- Xu S. 1999. Fate of methylsiloxanes in soil 1. The degradation pathway. Environ Sci Technol. 33(4):603–608.
- Yoshinaga K. 1973. Gonadotropin induced hormone secretion and structural changes in the ovary during the nonpregnant reproductive cycle. In: Field J, editor: Handbook of physiology. Volume II (Endocrinol), Section 7, Part 1. Am Physiol Soc. Baltimore: Williams and Wilkins, p. 363–388.
- Zhang J, Falany JL, Xie X, Falany CN. 2000. Induction of rat hepatic drug metabolizing enzymes by dimethylcyclosiloxanes. Chem Biol Interact. 124(2):133–147.
- Zhao H, Tian Z, Cheng L, Chen B. 2004. Electroacupuncture enhances extragonadal aromatization in ovariectomized rats. Reprod Biol Endocrinol. 2(1):18–27.
- Zhou Y, Watters JJ, Dorsa DM. 1996. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 137(5):2163–2166.
