Abstract
The induction of immunological responses that trigger bio-physiological symptoms in the respiratory tract following repeated exposure to a substance, is known as respiratory sensitization. The inducing compound is known as a respiratory sensitizer. While respiratory sensitization by high molecular weight (HMW) materials is recognized and extensively studied, much less information is available regarding low molecular weight (LMW) materials as respiratory sensitizers. Variability of symptoms presented in humans from such exposures, limited availability of (and access to) documented reports, and the absence of standardized and validated test models, hinders the identification of true respiratory sensitizers. This review aims to sort suspected LMW respiratory sensitizers based on available compelling, reasonable, inadequate, or questionable evidence in humans from occupational exposures and use this information to compose a reference list of reported chemical respiratory sensitizers for scientific research purposes. A list of 97 reported respiratory sensitizers was generated from six sources, and 52 LMW organic chemicals were identified, reviewed, and assigned to the four evidence categories. Less than 10 chemicals were confirmed with compelling evidence for induction of respiratory sensitization in humans from occupational exposures. Here, we propose the reference list for developing novel research on respiratory sensitization.
Introduction
Respiratory sensitization associated with occupational inhalation exposures is a serious health issue in occupational medicine with potentially life-threatening consequences. Occupational exposures and respiratory sensitization related to HMW substances, such as proteins, are well established (Wild and Lopez Citation2003; Raulf et al. Citation2018). In contrast, less is known about LMW chemicals. Although many have been reported as respiratory sensitizers, this class of chemicals lacks rigorous scientific scrutiny (Huby et al. Citation2000; Dufour et al. Citation2009; Krutz et al. Citation2020).
While there have been significant innovations in testing for skin sensitization with the development of multiple in vivo, in vitro, and in silico models that have been standardized, validated, and are now used to set guidelines and regulate exposures, research on respiratory sensitization is still in its infancy. There are no established animal models and due to bans or restrictions on animal testing of cosmetic products and their ingredients in some countries or regions around the world, the ability to validate innovative approaches to identify respiratory sensitizing chemicals and set sensitizing thresholds has been limited (Kimber et al. Citation1996; Arts Citation2020; Pemberton and Kimber Citation2021).
In the absence of adequate models to understand the immunology and mechanisms underlying respiratory sensitization, scientists and regulators are obliged to rely on the use of limited cellular assays or chemical analyses that are not designed to test for respiratory sensitization specifically. Using the expert judgment and the weight of evidence (WoE) approach, the European Chemical Agency (ECHA), German list of Maximum Concentrations and Biological Tolerance Values at the Workplace (DFG DF Citation2020) and UK Health and Safety Executive (UK-HSE Citation2001) have developed a list of compounds that are labeled as respiratory sensitizers. Also, other published lists have identified different LMW chemicals with the potential to be respiratory sensitizers (Jarvis et al. Citation2015; Dik et al. Citation2016; Krutz et al. Citation2021). However, these lists do not significantly overlap because there is a general lack of agreement on what criteria should be considered when categorizing a chemical as a respiratory sensitizer. Thus, the conventional approach is a hazard- and risk-based classification system that has led to the use of low-quality reports, extrapolation of structural chemistry, and WoE from skin sensitization tests to designate whether a chemical is a respiratory sensitizer. Such a conservative approach may potentially mislead research efforts to develop human-relevant models to identify respiratory sensitizers and accurately investigate respiratory sensitization. Furthermore, this lack of agreement has amplified confusion in the field, as any novel approaches to identify respiratory sensitizers are flawed due to the lack of an agreed-upon, positive control for a LMW respiratory sensitizer.
It is impossible to understand the mechanism of respiratory sensitization or identify new respiratory sensitizers without having a reference set of existing chemicals that are known respiratory sensitizers. It is challenging to identify LMW respiratory sensitizers as they have a broad spectrum of inhalation exposure-related respiratory effects in humans. With no accepted in vivo and in vitro approaches to evaluate and classify them based on their potential and potency, leveraging existing epidemiologic studies and reevaluating human data from workplace exposures is an opportunity for gaining more understanding (Isola et al. Citation2008; Kimber et al. Citation2010; Arts Citation2020). However, the challenge is to retrospectively evaluate published data, identify knowledge gaps, and apply the conclusions to categorize these chemicals based on the quality of their evidence (Pemberton and Kimber Citation2021). The medical history of human subjects adds another layer of complexity to data evaluation, especially whether an individual is predisposed or genetically susceptible to exhibit respiratory symptoms upon exposure to potential respiratory sensitizers (Venables et al. Citation1989; Bernstein Citation2003; Yucesoy et al. Citation2012). A comprehensive review carried out for the European Commission highlights that identifying the specific LMW chemical responsible for respiratory sensitization in an occupational setting is challenging due to complex exposure patterns and a clear causality to asthma is not always given (Bloemen et al. Citation2009). For example, highly reactive chemicals, such as diisocyanates, are also known to be respiratory irritants, which may complicate the assessment of biological responses as irritation or sensitization, especially in animal models (Pauluhn and Mohr Citation2005; ECHA-RAC Citation2020). An additional challenge is related to the development of animal-alternative models that account for the highly complex tissue architecture and bio-physiologic function of the human respiratory tract, which includes multiple cell types and systems, such as epithelial cells, inflammatory cells, sensory and autonomic nervous systems, secretory, and endocrinal systems (Van Lommel et al. Citation1999; Noguchi et al. Citation2020). Animal models have been used widely, but none of them are generally accepted nor validated due to the inability to distinguish between irritation and sensitization responses, and their human relevance is not well defined (Pauluhn Citation2008; Aun et al. Citation2017; Tanner and Single Citation2020).
Furthermore, another challenge is pinpointing respiratory sensitization symptoms in humans and identifying precise biomarkers in the test models directly associated with respiratory symptoms (Kimber et al. Citation2011). Respiratory sensitizers can induce a complex combination of symptoms, including respiratory irritation, inflammation, mucus secretion, cough and asthmatic-like bronchoconstriction and airway hyper reactivity. Depending on the chemical and its exposure, symptoms can additionally be classified as immediate or late-onset (Malo and Chan-Yeung Citation2009). An immunologic component of sensitization can be identified by measuring serum IgE and IgG antibodies. These symptoms can also be distinguished as local (rhinitis and airway inflammation) and systemic (asthma and immunologic reactions detected in the plasma samples).
The limited number of LMW chemicals identified as respiratory sensitizers, the broad variability in symptoms observed in the adult human population, and the absence of appropriate in vitro/in vivo models compound the difficulty of identifying respiratory sensitizers (Pemberton and Kimber Citation2021). Here, we have collected and evaluated existing epidemiological studies and case reports purporting to identify human respiratory sensitizers, focusing on LMW chemicals. We have identified those respiratory sensitizers that are supported by compelling evidence in humans and propose they should be used as positive controls in future studies of respiratory sensitization with high confidence. As such, this review is strictly limited to evaluating evidence observed in humans from occupational exposures and does not consider additional data or their chemical reactivities as WoE. This data analysis is not intended for use in hazard assessments or risk assessments of these chemicals.
Materials and methods
Building an inventory of respiratory sensitizers and data review
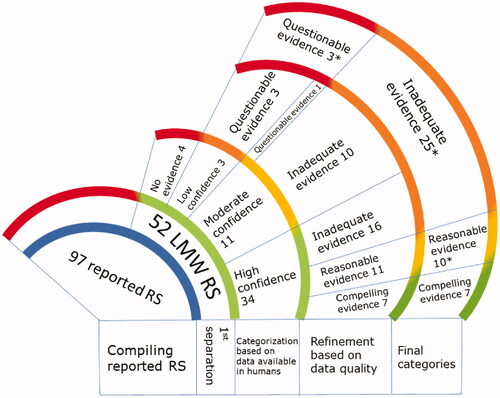
An inventory of 97 LMW chemicals reported to be respiratory sensitizers was generated from six sources (UK-HSE Citation2001; Jarvis et al. Citation2015; Dik et al. Citation2016; DFG DF Citation2020; ECHA Citation2021; Krutz et al. Citation2021). The following databases were searched for relevant publications and chemical information: PubMed, OECD Toolbox, REACH documents, ECHA entries, and additional nonpublic databases to which authors may have had access (e.g. Embase, Web of Science, and the Research Institute for Fragrance Materials (RIFM) database). Fifty-two of the 97 substances selected were identified as organic LMW chemicals with literature available for review. Materials, such as heavy metals, inorganic salts, proteins, polymers, and non-chemical entities (e.g. house dust mites) were not considered under this comprehensive review. Search terms were designed to inclusively capture general sensitization data in reports independent of whether data was captured in respiratory or dermal systems to ensure the best chance of obtaining all available respiratory sensitization reports, for example (“chemical name” or “chemical synonym” or “CAS RN”) and (sensit* or respiratory or allerg* or asthma or rhinitis). The literature search included years from 1930 to 2021. Pertinent references were identified by evaluating titles and abstracts using the inclusion and exclusion criteria. Reports were also extracted if the citations from the searched studies were determined to be relevant. The following inclusion and exclusion criteria were used to identify relevant references on 52 chemicals.
Inclusion and exclusion criteria search results
The report should specify or suspect the route of exposure of the chemicals as inhalation.
The chemical exposures must take place in an occupational setting. Exposures related to personal use of products containing the chemical of interest are excluded as they are considered as low confidence.
The data are presented in an epidemiological study, a clinical case report or any peer-reviewed scientific literature. Data obtained from intentional exposures in test subjects for pointed scientific evaluations are not considered.
The presented observations and evaluations are from adult human subjects and not from children, animals, or animal-alternative models.
Articles from languages other than English with no translations are not considered.
Scoring system
The gathered evidence was limited to epidemiological studies and clinical case reports from occupational exposures in humans. These reports were evaluated based on the following set criteria, summarized in .
Table 1. Summary of criteria for data evaluation and categorization of reported respiratory sensitizers.
Compelling Evidence – LMW chemicals having strong evidence in humans regarding occurrences, chemical identity, exposure route, and symptoms that are well-documented and evaluated and confirmed by a medical professional.
Requirements for evidence related to exposure
Confirmed report with “confirmation” in the form of documented exposure (with testing) or individual knowledge of direct exposure to a single chemical exposure (handled or processed) followed by testing of the individual or testing at the exposure location, AND
Reaction occurred within a reasonable period following exposure, or upon re-challenge with the suspected offending substance
AND
Only citations dealing with adult-aged workers and patients were considered.
Documented confirmation of a reaction in a medical setting including using patch tests, skin prick tests, serum IgE, pulmonary function testing, or other forms of medically supervised re-challenge and observations.
Data/observations collected by a qualified or authoritative source and not anecdotal but observational.
Reasonable evidence – LMW chemicals having strong evidence in humans regarding occurrences, exposure route and symptoms but with insufficient clarity on the specific chemical identity and subjects’ medical history showing prior incidences of atopy and/or respiratory issues.
Group of individuals in a shared location experiencing the same or similar respiratory symptoms with clear exposure primarily to the substance in question (may be in a mixture or other substances in occupational setting might be present, but the documented scenario contains reasonable evidence to believe the exposure was predominately to the substance in question), OR
The subjects’ medical history (atopic status or history of respiratory issues before the onset of symptoms) was considered to identify the possibility of predisposition to sensitization.
Inadequate evidence – LMW chemicals having limited single individual clinical case reports with documented observations not necessarily confirmed or evaluated by a medical professional.
Case reports involving a single (individual) case of respiratory sensitization were included and scored. However, these cases were flagged for detailed review to discern whether the report was based on the reaction of a single subject who may have unique susceptibilities not reflected in the broader population.
The substance being investigated was contained in a mixture. Thus, it was unclear whether exposure was predominantly to the substance in question, or whether additional substances, possibly also associated with respiratory sensitization, contribute to observed effects, OR
A substance belonging to a cluster of reported respiratory sensitizers. However, reports for that substance as a respiratory sensitizer were scored as Compelling evidence only if actual data on that substance were presented in the report.
Patient or colleague reports likely but not definite exposure, OR
Cases with ambiguous routes of exposure, OR
There is reason to believe an individual might have been exposed to a substance, but it cannot be definitively confirmed.
Reaction occurred outside a reasonable period following exposure.
No medical confirmation of sensitization exists or was conducted.
Symptoms/reactions were reported by a patient or colleague but not necessarily directly observed by a qualified health professional or another authoritative source.
Questionable evidence – LMW chemicals having anecdotal evidence that are summaries of self-reported exposure and symptoms and are not clinical case reports or epidemiological reports with expert confirmation or evaluation establishing an exposure-effect relationship.
The report consists solely of anecdotes or patient report without any observation of symptoms by an attending physician or other healthcare professional or authoritative source.
If the publication cites the primary source of a respiratory sensitization claim, then that source was checked and scored.
Results
A comprehensive literature review on 52 LMW chemicals was carried out to identify those respiratory sensitizers supported by compelling evidence of respiratory sensitization in humans. depicts the compelling evidence category, where the chemicals are recognized as the reference list among the reported respiratory sensitizers for use as positive controls in respiratory sensitization-related research.
Table 2. Compelling evidence.
Of the 52 chemicals reviewed, 51 have molecular weights below 500 g/mol. The chemical chlorhexidine (CAS #55-56-1) has a molecular weight above 500 g/mol and did not have any published reports that could be categorized into one of the four categories (see ). In addition, three chemicals did not have an associated CAS # and could not be specifically identified. Therefore, the following chemicals were removed from the final lists: diazonium tetrafluoroborate salt from category 2, penicillin from category 3, and diazonium chloride salts from category 4. As such, the final lists have seven chemicals in category 1 that are confirmed with compelling evidence of respiratory sensitization in humans (), 10 chemicals in category 2 with reasonable evidence of respiratory sensitization (), 25 in category 3 with inadequate evidence for respiratory sensitization (), 3 in category 4 with questionable evidence (), and the remaining four chemicals were uncategorized ().
Table 3. Reasonable evidence.
Table 4. Inadequate evidence.
Table 5. Questionable evidence.
Table 6. Uncategorized.
Apart from identifying a handful of chemicals as confirmed respiratory sensitizers, this review process also draws attention to chemicals from the same functional group scattered across the evidence-based categories. To demonstrate this observation, two chemicals from each category (except the questionable evidence category) representing an anhydride and a diisocyanate, are discussed having varying confidence levels as respiratory sensitizers.
Compelling evidence
The 7 chemicals listed in , show compelling evidence of inhalation exposure-based respiratory sensitization symptoms. The reports provide details on measured air concentration levels that the subjects were exposed to during their working hours. The symptoms experienced by subjects were evaluated and documented by medical professionals. The subjects were also evaluated using a combination of confirmatory tests, which included pulmonary function testing, patch testing, skin prick tests, serum antibody evaluations, or re-challenges. Overall, the evidence presented here provides a clear exposure-effect relationship for these chemicals that are expected to serve as reference chemicals for development of respiratory sensitization research.
Trimellitic anhydride (TMA)
There is strong evidence supporting TMA as a respiratory sensitizer (Zeiss et al. Citation1977, Citation1982; Bernstein et al. Citation1983; McGrath et al. Citation1984). The reports are detailed in terms of the patients’ clinical history, with well-documented symptoms for both physiological and immunological responses, identification of exposures and characterization of exposure concentrations, and confirmation of observations with respiratory re-challenge and/or skin tests. From all the reviewed reports, those providing strong evidence had a small number of subjects, about 5 out of 42 (Zeiss et al. Citation1977; McGrath et al. Citation1984) with a history of respiratory or other health issues before presenting with occupational exposure-related respiratory symptoms. In addition, reports from Patterson et al. (Citation1978) and Zeiss et al. (Citation1982) have well-documented observations in humans from an immunological perspective for TMA exposure-induced respiratory sensitization. Although these observations are variable, asthma-like symptoms may correlate with the presence of serum IgA and IgM antibodies, and rhinitis and immediate-type asthma may correlate with the presence of serum IgE antibodies. Thus, there is high confidence that this chemical is a confirmed respiratory sensitizer and is an ideal positive control for developing laboratory models to evaluate respiratory sensitization.
Toluene diisocyanate (TDI)
As evidenced from this review process, the epidemiological reports on TDI provide strong support for a preferred positive control in respiratory sensitization-related research projects. These reports are detailed epidemiological studies on occupational exposures to TDI and the resultant respiratory health effects in exposed workers. As per the observations in Elkins et al. (Citation1962), 15 work facilities using polyurethane were evaluated following reports of TDI exposure-related toxicity in workers. Although the authors mention TDI is most widely used in the manufacturing of polyurethanes, their evaluations at these 15 plants do not rule out the exposures to other mono or diisocyanates. However, a few other reports provide a clear correlation between TDI exposure and related health effects in workers from TDI manufacturing facilities (Porter et al. Citation1975; Weill et al. Citation1975; Butcher et al. Citation1977). A common observation is that the sensitized subjects appear to return to normal respiratory physiology eventually once they are removed from the area of exposure. Additionally, there is a possibility of a reduction in specific antibodies (IgE) in individuals that do not present with asthma-like symptoms from TDI exposures upon removal from the work areas or controlling the occupational TDI exposure concentrations to below a certain level (Porter et al. Citation1975; Karol et al. Citation1979; Butcher et al. Citation1982; Musk et al. Citation1982).
Reasonable evidence
The 10 chemicals listed in , had reasonable evidence of respiratory sensitization in humans from occupational exposures. The epidemiological or clinical case reports available provide sufficient evidence of respiratory sensitization effects in affected humans but there are still some areas that need further confirmation. For example, the exposure assessments do not identify a clear exposure–effect relationship for the responsible chemical. There are other potentially reactive chemicals being used concurrently in the facilities that may also be responsible for the symptoms observed in affected subjects. The medical history of the subjects also reveals a possible interference from preexisting health conditions, such as the atopic status and/or incidents of childhood asthma. This category of chemicals may present respiratory sensitization effects in exposed individuals due to cross reactivities from other similarly reactive chemicals being used simultaneously and may also affect individuals that are either predisposed or hypersusceptible to respiratory sensitization from such exposures.
Tetrachlorophthalic anhydride (TCPA)
The collected evidence points to an exposure-effect relationship from occupational exposures to TCPA; however, the confidence is low due to the absence of characterization of exposure concentrations in the workplace and/or added complexity because of the subjects’ medical history (Schlueter et al. Citation1978; Howe et al. Citation1983; Liss et al. Citation1993). Documented reports show the subjects having a history of respiratory illness or were active smokers at the time of the report. In addition, a series of publications provide snapshots in time along a 12-year journey of a follow-up study involving seven women (Howe et al. Citation1983; Venables et al. Citation1987; Venables and Newman Taylor Citation1990; Barker et al. Citation1998). Each of these reports provides some form of evidence for TCPA exposure-associated physiological and immunological symptoms; however, the primary publication by Howe et al. (Citation1983) did not characterize the workplace TCPA exposures. In some cases, it was observed that workplace exposures involved other chemicals in addition to TCPA, such as benzophenone tetracarboxylic dianhydride (BTDA), finely divided silica, glass fiber, fiberglass, epoxy resin, lactose, and blue pigment (Schlueter et al. Citation1978; Venables and Newman Taylor Citation1990). As such, this chemical is not an optimal choice to serve as a positive control for scientific research on respiratory sensitization.
Naphthalene diisocyanate (NDI)
Of all the reviewed reports, only one provides reliable evidential support for NDI as a respiratory sensitizer in humans. Alexandersson et al. (1986) reported observations in 23 subjects working at a rubber plant using NDI in the tire manufacturing process. The authors provide a detailed analysis of the workers’ symptoms along with characterized NDI exposures at their workstations. However, the subjects were not tested for evidence of sensitization by immune activation via confirmatory tests, such as serum antibodies or skin tests. Also, the report does not explicitly clarify if other isocyanates were being used at the plant which could have also contributed to the symptoms. Therefore, there is not enough confidence for NDI to be considered a positive control for scientific research on respiratory sensitization.
Inadequate evidence
The 25 chemicals listed in have very low confidence as reference chemicals. The available reports show insufficient evidence of respiratory sensitization in humans from occupational exposures to these chemicals. The details on confirmation of exposure–effect relationship for the responsible chemical and the specific inhalation route of exposure are lacking. There is no systematic evaluation or confirmation of symptoms by a medical professional, and the details regarding documentation of such tests are either missing or non-existent. Also, the scattered evidence available as individual case reports for these chemicals portrays an image of a low incidence of respiratory sensitization in humans. Thus, rendering these chemicals unsuitable for respiratory sensitization research.
Phthalic anhydride (PA)
In this review, much of the literature poorly supported the occupational cases of respiratory sensitization from PA exposures. Chester et al. (Citation1977) summarized findings in a single subject case report. However, when compared to the other published reports, more information is required to establish if the subject was hyper-susceptible to PA-based respiratory sensitization. There are detailed observations of occupational asthma supported with either a re-challenge test, cessation of symptoms upon withdrawal from the workstation, or confirmation from a skin test or specific serum antibody testing. It was noted that the subjects commonly experienced respiratory irritation and immediate-type respiratory sensitization; however, the medical histories or possible cross-reactivity from other anhydrides further complicate these reports (Kern Citation1939; Chester et al. Citation1977; Wernfors et al. Citation1986; Baur et al. Citation1995; Piirila, Keskinen, et al. Citation1997). Thus, there is very low confidence in PA for use as a positive control in developing scientific research for respiratory sensitization.
Isophorone diisocyanate (IPDI)
A few published reports are available on IPDI occupational exposure-related respiratory sensitization (ECHA Citation2010). All these case studies are summarized in REACH registered dossier reported on the ECHA website, and it is noted that the observations are from single individuals where the exposures were not characterized (Tyrer Citation1979; Clarke and Aldons Citation1981), and it was not established if the subjects had any prior respiratory problems which may have contributed to their symptoms (Germanaud et al. Citation2003). Full-text reports were not available for this review. Therefore, based on the REACH dossier summary, there is weak evidence of respiratory sensitization in humans from occupational exposure to IPDI and would not constitute an appropriate choice for a positive control in scientific research on respiratory sensitization.
Questionable evidence
The case reports evaluated for the three chemicals in this category did not have well-documented observations to make a clear connection between exposure and occurrence of symptoms. The reports are missing exposure characterization, specific antibody testing, respiratory re-challenge, or confirmation of the exposed chemical. The reports on formaldehyde and persulfate salts did not provide a systematic evaluation of the presented cases (Parra et al. Citation1992; Nilsson et al. Citation2016; Dahlgren and Talbott Citation2018). The review of an Occupational Health Care Report on ethyl acrylate by NIOSH highlights that although ethyl acrylate is considered a potentially toxic chemical, the subjects did not exhibit symptoms of respiratory sensitization upon exposure to ethyl acrylate (Cohen et al. Citation1974).
Uncategorized
The chemicals listed in do not have human evidence for respiratory sensitization in the form of an epidemiological or clinical case report.
Discussion
Generally, substances that cause respiratory sensitization in humans upon inhalation exposure are encountered primarily in occupational settings where a sizeable workforce presents with respiratory effects. The number of cases has dramatically diminished possibly through improvements in personnel safety, the identification of respiratory sensitizers in epidemiological studies, and measures taken to monitor occupational exposures (Paris et al. Citation2012; Ribeiro et al. Citation2014). However, as experimental models are yet to be developed, identifying true respiratory sensitizers is challenging, specifically for LMW chemicals. This is an even more significant challenge with the restrictions on animal testing of chemicals used in cosmetics products in some countries and regions worldwide.
Scientists have attempted to identify potential respiratory sensitizers by extrapolating chemical properties based on functional groups (Enoch et al. Citation2012) and reactivities, as well as by using analytical methods developed for identifying skin sensitizers as the WoE; for example, direct peptide reactivity assay (DPRA) (De Jong et al. Citation2009; Lalko et al. Citation2012; Krutz et al. Citation2021). Several studies suggest there is a mechanistic difference between skin sensitization and respiratory sensitization (Magnan et al. Citation2000; Lalko et al. Citation2011; Mizoguchi et al. Citation2017; Kimber et al. Citation2018). Previously published research by Lalko et al. (Citation2012) demonstrated that reported LMW respiratory sensitizers have a preferential Lysine reactivity, which is not observed for skin sensitizers. Therefore, the group proposed that the ratio of Cysteine to Lysine reactivity might indicate respiratory sensitizing potential for LMW respiratory allergens. However, when Krutz et al. (Citation2021) tested this hypothesis by compiling existing DPRA data on LMW respiratory sensitizers and skin sensitizers, their observations revealed that the anhydride chemicals in the list drove the skewed Lysine preferential reactivity. On our path to better understand mechanistic differences between skin sensitization and respiratory sensitization, we need to challenge results that might be biased due to the very limiting data set of LMW respiratory sensitizers.
In the study carried out for the European Commission by Bloemen et al. (Citation2009), the variability of cause and effect relationship and presented symptoms of respiratory sensitization in humans from occupational exposures in European regions was highlighted. The conclusions drawn from this report suggest an urgent need to clearly distinguish in a standardized way the symptoms of non-immunological and immunological asthma and tools to identify the LMW chemicals that induce respiratory sensitization and trigger immunological asthma. Additionally, in some instances of respiratory sensitization to LMW chemicals, the evidence needs to be captured systematically to help build a database of chemicals with high confidence of respiratory sensitization potential. Such a database could help further develop and validate alternative methods for identifying LMW respiratory sensitizers.
Despite the challenges of identifying respiratory sensitizers and understanding their underlying mechanisms, the test methods are required to reflect human relevance. As such, any assay developed for testing adverse effects in the respiratory system needs a model that represents the characteristics of, or some features of, a healthy adult human. Thus, many approaches rely upon human tissues or cells, such as human precision-cut lung slices and human bronchial epithelial cells. An extensive body of research is available on in vitro models used to answer specific research questions with regards to the respiratory tract, for example, the A549 cell line (Nova et al. Citation2020; Tran et al. Citation2020), which is an immortalized alveolar epithelial type 2 cell line isolated from human pulmonary adenocarcinoma. However, such models do not represent the complex tissue structure. They may underestimate biological and functional aspects of healthy tissues in humans (Swain et al. Citation2010) and thus limit their value for predicting in vivo effects (Suter Citation2006; McKim Citation2010).
Developing an appropriate test model needs careful evaluation by employing a reference and a training data set of respiratory sensitizers and establishing a robust foundation for good correlation with human sensitization and bio-physiologic response data. One way to develop such data sets in this direction is to evaluate chemicals reported upon in the existing epidemiological studies based on occupational exposure cases and utilize the chemicals supported with compelling evidence in humans as the reference list of positive controls ().
This review focused on the LMW chemicals whose categorization was based on published reports documenting occupational cases of respiratory sensitization in workers and did not include expert judgment or WoE. The evaluation process considered the occurrences in the human population in terms of occupational exposures, routes of exposures, chemical identity, and symptoms. There was high variability across compelling and reasonable evidence categories in terms of the concentrations and the duration of exposures encountered by subjects at their workplace. Also, it was observed that more than 50% (32) of the 52 reviewed chemicals that have previously been described as causing respiratory sensitization in humans from occupational inhalation exposure are either supported with poor quality reports or have no existing evidence in humans ().
The focus of this article was to identify chemicals as ideal positive controls for utilization in respiratory sensitization research, such as identifying underlying immunological mechanisms and developing and validating alternative methods. Therefore, the criteria for compelling evidence were restricted to observations made from occupational inhalation exposures in humans, with a sizeable number of individuals showing symptoms of respiratory sensitization. This approach is different from the evidence-based literature evaluation presented by Baur and Bakehe (Citation2014). Their publication presents an evaluation of all reported respiratory sensitizers, such as substances of biological and non-biological origin, and worksites, such as farms, breweries, and bakeries. They assigned high confidence to the evidence from a single worksite, scientific laboratories conducting animal experiments, which involved working with various animals. Additionally, as per their evaluation, only TDI was identified as a LMW respiratory sensitizer with a moderate level of evidence.
In this review, we evaluated reports that included details of confirmatory tests to validate the association between the exposed chemical and the subjects’ symptoms. In the absence of additional accompanying evidence of multiple subjects affected from the exposures, single individual case reports were considered to be low confidence. Additionally, the past medical history of the subjects (when available) was utilized to refine the categorization process for compelling evidence. However, the possibility of a subject’s genetic predisposition to respiratory sensitization or individual hypersusceptibility remains unexplored.
This evidence-based classification process determined that less than 10 reported respiratory sensitizers have compelling human data and are proposed here as positive controls for identifying innovative approaches to study respiratory sensitization. It is important to note that most of these reference chemicals are also skin sensitizers. However, unlike skin sensitizers, these reference respiratory sensitizers are extremely limited in the chemical space, with specific anhydrides and diisocyanate chemicals dominating this category. Thus, to fully understand the process and mechanisms of LMW respiratory sensitizers, it needs to be acknowledged that such research will be mainly focused on and biased by the minimal chemical space. This space could further extend if some instances of respiratory sensitization to LMW chemicals of different chemical spaces are captured with comprehensive and publicly available evidence. Also, as seen from the examples on anhydride and diisocyanate chemicals, extrapolating respiratory sensitizing properties based on their functional groups and chemical reactivities alone could potentially misinform the scientific research to understand the process of respiratory sensitization fully, thus hindering the development of novel models for accurately identifying respiratory sensitizers.
Along with knowledge gaps associated with the retrospective analyses, such as the lack of standard reporting guidelines for epidemiological reports of occupational exposure cases, there are two limitations of this comprehensive review: 1) articles in languages other than English were not reviewed, and 2) chemicals in the compelling evidence category were classified based on the availability of at least one strong evidence epidemiological report as specified by the criteria. It should also be noted that the evaluation process does not account for expert judgment since the purpose of this project is to identify respiratory sensitizers having high-quality evidence in humans. Despite the limitations, it is envisioned that the proposed reference list of respiratory sensitizers will inform and improve the efforts to develop novel research methods to identify true respiratory sensitizers by providing essential details and observations of known chemical exposures in humans.
Acknowledgments
We would like to express our gratitude to the reviewers for their thoughtful feedback and support in improving this manuscript. We would like to acknowledge the valuable contribution by Exponent, a consultancy, for conducting the literature search and assigning preliminary scores to the dataset. We also take this opportunity to acknowledge Dr. Sven-Eric Jordt at Duke University, Durham, NC for reviewing the preliminary categories, and Dr. Jane Rose at P&G, Cincinnati, OH who is a member of the RIFM Respiratory Core Team and Dr. W. Michael Foster an Environmental Consultant who is a member of the RIFM Respiratory Adjunct Group for their help in reviewing the draft manuscript. Additionally, we want to thank Dr. Glen Sipes at the University of Arizona and a Member of the Expert Panel for Fragrance Safety and Dr. Maria Dagli at the University of Sao Paolo and a Member of the Expert Panel for Fragrance Safety for reviewing the draft manuscript, and Gary Sullivan (RIFM Editorial Manager) for proofreading the manuscript.
Declaration of interest
RIFM funded this project. NS is a RIFM scientist for the local respiratory toxicity endpoint. She helped with the conceptualization, development of methodology, data search, formal analysis, writing the original draft and developing the final manuscript. AMA is the research lead and VP at RIFM. She helped with conceptualization, reviewing and confirming methodology, reviewing the draft manuscript, and project supervision.
The RIFM Respiratory Core Team members are industry experts or consultants. They provide expert guidance on respiratory research projects. The RIFM Respiratory Adjunct Group members are experts from academia and consultants. They provide expert and technical guidance on respiratory research projects. The Expert Panel for Fragrance Safety is an independent panel of mixed expertise representing various endpoints for human health safety at RIFM. The Expert Panel for Fragrance Safety members review and approve all RIFM’s research projects and safety assessments. Two Expert Panel members (WD and ADF) liaise with the Respiratory Adjunct group. The RIFM Core Team and Adjunct group members (FB, WD, ADF, GFG, PG, CH, NLK, OL, CM, RP, KEP, KJR, PS, SS, and MW) helped with formal data analysis, writing – reviewing and editing the manuscript. GFG, RP, KEP, and KJR report financial support provided by RIFM. FB, PG, CH, NLK, OL, CM, PS, SS, and MW report no potential competing interests. Affiliations for all the authors are indicated on the title page. During the last 10 years, the authors were not involved in any legal, regulatory, or advocacy activities related to the article’s contents.
References
- Ait Bamai Y, Shibata E, Saito I, Araki A, Kanazawa A, Morimoto K, Nakayama K, Tanaka M, Takigawa T, Yoshimura T, et al. 2014. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci Total Environ. 485–486:153–163.
- Alexandersson R, Gustafsson P, Hedenstierna G, Rosen G. 1986. Exposure to naphthalene-diisocyanate in a rubber plant: symptoms and lung function. Arch Environ Health. 41(2):85–89.
- Alexandersson R, Hedenstierna G, Plato N, Kolmodin-Hedman B. 1987. Exposure, lung function, and symptoms in car painters exposed to hexamethylendiisocyanate and biuret modified hexamethylendiisocyanate. Arch Environ Health. 42(6):367–373.
- Anees W, Moore VC, Croft JS, Robertson AS, Burge PS. 2011. Occupational asthma caused by heated triglycidyl isocyanurate. Occup Med (Lond). 61(1):65–67.
- Arts J. 2020. How to assess respiratory sensitization of low molecular weight chemicals? Int J Hyg Environ Health. 225:113469.
- Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. 2017. Animal models of asthma: utility and limitations. J Asthma Allergy. 10:293–301.
- Barker RD, Harris JM, Welch JA, Venables KM, Newman Taylor AJ. 1998. Occupational asthma caused by tetrachlorophthalic anhydride: a 12-year follow-up. J Allergy Clin Immunol. 101(6 Pt 1):717–719.
- Baur X, Bakehe P. 2014. Allergens causing occupational asthma: an evidence-based evaluation of the literature. Int Arch Occup Environ Health. 87(4):339–363.
- Baur X, Czuppon AB, Rauluk I, Zimmermann FB, Schmitt B, Egen-Korthaus M, Tenkhoff N, Degens PO. 1995. A clinical and immunological study on 92 workers occupationally exposed to anhydrides. Int Arch Occup Environ Heath. 67(6):395–403.
- Baur X, Dewair M, Rommelt H. 1984. Acute airway obstruction followed by hypersensitivity pneumonitis in an isocyanate (MDI) worker. J Occup Med. 26(4):285–287.
- Belin L, Wass U, Audunsson G, Mathiasson L. 1983. Amines: possible causative agents in the development of bronchial hyperreactivity in workers manufacturing polyurethanes from isocyanates. Br J Ind Med. 40(3):251–257.
- Bernstein DI. 2003. Occupational asthma caused by exposure to low-molecular-weight chemicals. Immunol Allergy Clin North Am. 23(2):221–234, vi.
- Bernstein DI, Roach DE, McGrath KG, Larsen RS, Zeiss CR, Patterson R. 1983. The relationship of airborne trimellitic anhydride concentrations to trimellitic anhydride-induced symptoms and immune responses. J Allergy Clin Immunol. 72(6):709–713.
- Bloemen K, Verstraelen S, Schoeters G, Legiest B, Nemery B. 2009. The collection and evaluation of data on incidence and severity of skin and respiratory allergy related to exposure of chemicals from nonfood sources. Report. European Commission Health & Consumer Protection Directorate Contract SANCO/2008/C7/ 015. [accessed 3 September 2021. https://ec.europa.eu/health/scientific_committees/docs/vito_study_allergy_en.pdf.
- Bourke SJ, Convery RP, Stenton SC, Malcolm RM, Hendrick DJ. 1997. Occupational asthma in an isothiazolinone manufacturing plant. Thorax. 52(8):746–748.
- Bourne MS, Flindt ML, Walker JM. 1979. Asthma due to industrial use of chloramine. Br Med J. 2(6181):10–12.
- Burge PS, Richardson MN. 1994. Occupational asthma due to indirect exposure to lauryl dimethyl benzyl ammonium chloride used in a floor cleaner. Thorax. 49(8):842–843.
- Butcher BT, Jones RN, O’Neil CE, Glindmeyer HW, Diem JE, Dharmarajan V, Weill H, Salvaggio JE. 1977. Longitudinal study of workers employed in the manufacture of toluene-diisocyanate. Am Rev Respir Dis. 116(3):411–421.
- Butcher BT, O’Neil CE, Reed MA, Salvaggio JE, Weill H. 1982. Development and loss of toluene diisocyanate reactivity: immunologic, pharmacologic, and provocative challenge studies. J Allergy Clin Immunol. 70(4):231–235.
- Cakmak S, Dales RE, Hebbern C, Saravanabhavan G. 2014. The association between urinary phthalates and lung function. J Occup Environ Med. 56(4):376–381.
- Chester EH, Schwartz HJ, Payne CB, Jr., Greenstein S. 1977. Phthalic anhydride asthma. Clin Allergy. 7(1):15–20.
- Clarke CW, Aldons PM. 1981. Isophorone diisocyanate induced respiratory disease (IPDI). Aust NZ J Med. 11(3):290–292.
- Cohen SR, Maier AA, Flesch JP. 1974. Occupational health care report. 3. Ethyl acrylate. J Occup Med. 16(3):199–200.
- Dahlgren JG, Talbott PJ. 2018. Asthma from hair straightening treatment containing formaldehyde: two cases and a review of the literature. Toxicol Ind Health. 34(4):262–269.
- Dallongeville A, Costet N, Zmirou-Navier D, Le Bot B, Chevrier C, Deguen S, Annesi-Maesano I, Blanchard O. 2016. Volatile and semi-volatile organic compounds of respiratory health relevance in French dwellings. Indoor Air. 26(3):426–438.
- Day JH, Lees RE, Clark RH, Pattee PL. 1984. Respiratory response to formaldehyde and off-gas of urea formaldehyde foam insulation. Can Med Assoc J. 131(9):1061–1065.
- De Jong WH, Arts JH, De Klerk A, Schijf MA, Ezendam J, Kuper CF, Van Loveren H. 2009. Contact and respiratory sensitizers can be identified by cytokine profiles following inhalation exposure. Toxicology. 261(3):103–111.
- Deschamps D, Rosenberg N, Soler P, Maillard G, Fournier E, Salson D, Gervais P. 1992. Persistent asthma after accidental exposure to ethylene oxide. Br J Ind Med. 49(7):523–525.
- DFG DF. 2020. Sensitizing substances. List of MAK and BAT values. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co KGaA; p. 158–172.
- Di Stefano F, Siriruttanapruk S, McCoach JS, Burge PS. 1998. Occupational asthma due to glutaraldehyde. Monaldi Arch Chest Dis. 53(1):50–55.
- Dijkman JH, Vooren PH, Kramps JA. 1981. Occupational asthma due to inhalation of chloramine-T. I. Clinical observations and inhalation-provocation studies. Int Arch Allergy Appl Immunol. 64(4):422–427.
- Dik S, Rorije E, Schwillens P, van Loveren H, Ezendam J. 2016. Can the direct peptide reactivity assay be used for the identification of respiratory sensitization potential of chemicals? Toxicol Sci. 153(2):361–371.
- Dufour MH, Lemière C, Prince P, Boulet LP. 2009. Comparative airway response to high- versus low-molecular weight agents in occupational asthma. Eur Respir J. 33(4):734–739.
- Durham SR, Graneek BJ, Hawkins R, Taylor AJ. 1987. The temporal relationship between increases in airway responsiveness to histamine and late asthmatic responses induced by occupational agents. J Allergy Clin Immunol. 79(2):398–406.
- ECHA. 2010. Registration dossier - isophorone diisocyanate. [accessed 10 April 2021]. https://echa.europa.eu/cs/registration-dossier/-/registered-dossier/14516/7/5/1.
- ECHA. 2021. List of respiratory sensitizers. [accessed 10 April]. https://ec.europa.eu/health/scientific_committees/docs/annex6_respiratory.pdf.
- ECHA-RAC. 2020. Opinion on scientific evaluation of occupational exposure limits for Diisocyanates. ECHA/RAC/A77-O-0000006826-64-01/F. [accessed 21 December 2021]. https://echa.europa.eu/documents/10162/4ea3b5ee-141b-63c9-8ffd-1c268dda95e9
- Elkins HB, Mc CG, Brugsch HG, Fahy JP. 1962. Massachusetts experience with toluene di-isocyanate. Am Ind Hyg Assoc J. 23:265–272.
- Enoch SJ, Seed MJ, Roberts DW, Cronin MT, Stocks SJ, Agius RM. 2012. Development of mechanism-based structural alerts for respiratory sensitization hazard identification. Chem Res Toxicol. 25(11):2490–2498.
- Fawcett IW, Taylor AJ, Pepys J. 1977. Asthma due to inhaled chemical agents–epoxy resin systems containing phthalic acid anhydride, trimellitic acid anhydride and triethylene tetramine. Clin Exp Allergy. 7(1):1–14.
- Gannon PF, Bright P, Campbell M, O’Hickey SP, Burge PS. 1995. Occupational asthma due to glutaraldehyde and formaldehyde in endoscopy and x ray departments. Thorax. 50(2):156–159.
- Germanaud J, Proffit V, Janvoie B, Lemarie E, Lasfargues G. 2003. Pneumopathy due to isocyanate hypersensitivity: recognition as an occupational disease. Rev Mal Respir. 20(3 Pt 1):443–449.
- Grammer LC, Eggum P, Silverstein M, Shaughnessy MA, Liotta JL, Patterson R. 1988. Prospective immunologic and clinical study of a population exposed to hexamethylene diisocyanate. J Allergy Clin Immunol. 82(4):627–633.
- Hagberg S, Ljungkvist G, Andreasson H, Karlsson S, Barregard L. 2005. Exposure to volatile methacrylates in dental personnel. J Occup Environ Hyg. 2(6):302–306.
- Hagmar L, Bellander T, Bergoo B, Simonsson BG. 1982. Piperazine-induced occupational asthma. J Occup Med. 24(3):193–197.
- Hagmar L, Welinder H. 1986. Prevalence of specific IgE antibodies against piperazine in employees of a chemical plant. Int Arch Allergy Appl Immunol. 81(1):12–16.
- Herman JJ. 1983. Intractable sneezing due to IgE-mediated triethanolamine sensitivity. J Allergy Clin Immunol. 71(3):339–344.
- Hoey JR, Turcotte F, Couet S, L’Abbe KA. 1984. Health risks in homes insulated with urea formaldehyde foam. Can Med Assoc J. 130(2):115–117.
- Howe W, Venables KM, Topping MD, Dally MB, Hawkins R, Law JS, Taylor AJ. 1983. Tetrachlorophthalic anhydride asthma: evidence for specific IgE antibody. J Allergy Clin Immunol. 71(1 Pt 1):5–11.
- Huby RDJ, Dearman RJ, Kimber I. 2000. Why are some proteins allergens? Toxicol Sci. 55(2):235–246.
- Hur GY, Koh DH, Choi GS, Park HJ, Choi SJ, Ye YM, Kim KS, Park HS. 2008. Clinical and immunologic findings of methylene diphenyl diisocyanate-induced occupational asthma in a car upholstery factory. Clin Exp Allergy. 38(4):586–593.
- Isola D, Kimber I, Sarlo K, Lalko J, Sipes IG. 2008. Chemical respiratory allergy and occupational asthma: what are the key areas of uncertainty? J Appl Toxicol. 28(3):249–253.
- Jachuck SJ, Bound CL, Steel J, Blain PG. 1989. Occupational hazard in hospital staff exposed to 2 percent glutaraldehyde in an endoscopy unit. Occup Med. 39(2):69–71.
- Jarvis J, Seed MJ, Stocks SJ, Agius RM. 2015. A refined QSAR model for prediction of chemical asthma hazard. Occup Med (Lond). 65(8):659–666.
- Jones M, Graham C, Taylor AN, Sarlo K, Hoyle V, Karol MH. 1998. Immunologic cross-reactivity between respiratory chemical sensitizers: reactive dyes and cyanuric chloride. J Allergy Clin Immunol. 102(5):835–840.
- Kanerva L, Keskinen H, Autio P, Estlander T, Tuppurainen M, Jolanki R. 1995. Occupational respiratory and skin sensitization caused by polyfunctional aziridine hardener. Clin Exp Allergy. 25(5):432–439.
- Karol MH, Sandberg T, Riley EJ, Alarie Y. 1979. Longitudinal study of tolyl-reactive IgE antibodies in workers hypersensitive to TDI. J Occup Med. 21(5):354–358.
- Kern RA. 1939. Asthma and allergic rhinitis due to sensitization to phthalic anhydride: report of a case. J Allergy. 10(2):164–165.
- Kimber I, Basketter DA, Dearman RJ. 2010. Chemical allergens-what are the issues? Toxicology. 268(3):139–142.
- Kimber I, Basketter DA, Gerberick GF, Ryan CA, Dearman RJ. 2011. Chemical allergy: translating biology into hazard characterization. Toxicol Sci. 120( 1):S238–S268.
- Kimber I, Bernstein IL, Karol MH, Robinson MK, Sarlo K, Selgrade MK. 1996. Workshop overview. Identification of respiratory allergens. Fundam Appl Toxicol. 33(1):1–10.
- Kimber I, Poole A, Basketter DA. 2018. Skin and respiratory chemical allergy: confluence and divergence in a hybrid adverse outcome pathway. Toxicol Res (Camb). 7(4):586–605.
- Kramps JA, van Toorenenbergen AW, Vooren PH, Dijkman JH. 1981. Occupational asthma due to inhalation of chloramine-T. II. Demonstration of specific IgE antibodies. Int Arch Allergy Appl Immunol. 64(4):428–438.
- Krutz NL, Kimber I, Maurer-Stroh S, Gerberick GF. 2020. Determination of the relative allergenic potency of proteins: hurdles and opportunities. Crit Rev Toxicol. 50(6):521–530.
- Krutz NL, Kimber I, Ryan CA, Kern PS, Gerberick GF. 2021. Critical evaluation of low-molecular weight respiratory sensitizers and their protein reactivity potential toward lysine residues. Toxicol Sci. 182(2):346–354.
- Lalko JF, Kimber I, Dearman RJ, Gerberick GF, Sarlo K, Api AM. 2011. Chemical reactivity measurements: potential for characterization of respiratory chemical allergens. Toxicol In Vitro. 25(2):433–445.
- Lalko JF, Kimber I, Gerberick GF, Foertsch LM, Api AM, Dearman RJ. 2012. The direct peptide reactivity assay: selectivity of chemical respiratory allergens. Toxicol Sci. 129(2):421–431.
- Lam S, Chan-Yeung M. 1980. Ethylenediamine-induced asthma. Am Rev Respir Dis. 121(1):151–155.
- Lee HS, Wang YT, Cheong TH, Tan KT, Chee BE, Narendran K. 1991. Occupational asthma due to maleic anhydride. Br J Ind Med. 48(4):283–285.
- Leffler CT, Milton DK. 1999. Occupational asthma and contact dermatitis in a spray painter after introduction of an aziridine cross-linker. Environ. Health Perspect. 107(7):599–601.
- Lindstrom M, Alanko K, Keskinen H, Kanerva L. 2002. Dentist's occupational asthma, rhinoconjunctivitis, and allergic contact dermatitis from methacrylates. Allergy. 57(6):543–545.
- Liss GM, Bernstein D, Genesove L, Roos JO, Lim J. 1993. Assessment of risk factors for IgE-mediated sensitization to tetrachlorophthalic anhydride. J Allergy Clin Immunol. 92(2):237–247.
- Lozewicz S, Davison AG, Hopkirk A, Burge PS, Boldy DA, Riordan JF, McGivern DV, Platts BW, Davies D, Newman Taylor AJ. 1985. Occupational asthma due to methyl methacrylate and cyanoacrylates. Thorax. 40(11):836–839.
- Maestre-Batlle D, Huff RD, Schwartz C, Alexis NE, Tebbutt SJ, Turvey S, Bolling AK, Carlsten C. 2020. Dibutyl phthalate augments allergen-induced lung function decline and alters human airway immunology. A randomized crossover study. Am J Respir Crit Care Med. 202(5):672–680.
- Magnan AO, Mely LG, Camilla CA, Badier MM, Montero-Julian FA, Guillot CM, Casano BB, Prato SJ, Fert V, Bongrand P, et al. 2000. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Increased IFN-gamma-producing CD8(+) T cells in asthma. Am J Respir Crit Care Med. 161(6):1790–1796.
- Malo JL, Chan-Yeung M. 2009. Agents causing occupational asthma. J Allergy Clin Immunol. 123(3):545–550.
- Malo JL, Pineau L, Cartier A. 1985. Occupational asthma due to azobisformamide. Clin Allergy. 15(3):261–264.
- Marquardt W, Seiss M, Hickel R, Reichl FX. 2009. Volatile methacrylates in dental practices. J Adhes Dent. 11(2):101–107.
- McCullagh SF. 1968. Allergenicity of piperazine: a study in environmental aetiology. Br J Ind Med. 25(4):319–325.
- McGrath KG, Roach D, Zeiss CR, Patterson R. 1984. Four-year evaluation of workers exposed to trimellitic anhydride. A brief report. J Occup Med. 26(9):671–675.
- McKim JM. Jr. 2010. Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance. Comb Chem High Throughput Screen. 13(2):188–206.
- Meuleman L, Goossens A, Linders C, Rochette F, Nemery B. 1999. Sensitization to triglycidylisocyanurate (TGIC) with cutaneous and respiratory manifestations. Allergy. 54(7):752–756.
- Mizoguchi I, Ohashi M, Chiba Y, Hasegawa H, Xu M, Owaki T, Yoshimoto T. 2017. Prediction of chemical respiratory and contact sensitizers by OX40L expression in dendritic cells using a novel 3D coculture system. Front Immunol. 8:929.
- Moller DR, Gallagher JS, Bernstein DI, Wilcox TG, Burroughs HE, Bernstein IL. 1985. Detection of IgE-mediated respiratory sensitization in workers exposed to hexahydrophthalic anhydride. J Allergy Clin Immunol. 75(6):663–672.
- Morfeld P, Yong M. 2019. Effect of occupational exposure to cyanuric chloride on respiratory morbidity: cross-sectional analyses of respiratory symptoms and longitudinal analyses of lung function parameters. J Occup Environ Med. 61(1):e1–e11.
- Musk AW, Peters JM, DiBerardinis L, Murphy RL. 1982. Absence of respiratory effects in subjects exposed to low concentrations of TDI and MDI. J Occup Med. 24(10):746–750.
- Nagy L, Orosz M. 1984. Occupational asthma due to hexachlorophene. Thorax. 39(8):630–631.
- Nakazawa T, Matsui S. 1990. Ethylenediamine-induced late asthmatic responses. J Asthma. 27(4):207–212.
- Nielsen J, Welinder H, Horstmann V, Skerfving S. 1992. Allergy to methyltetrahydrophthalic anhydride in epoxy resin workers. Br J Ind Med. 49(11):769–775.
- Nielsen J, Welinder H, Ottosson H, Bensryd I, Venge P, Skerfving S. 1994. Nasal challenge shows pathogenetic relevance of specific IgE serum antibodies for nasal symptoms caused by hexahydrophthalic anhydride. Clin Exp Allergy. 24(5):440–449.
- Nilsson PT, Marini S, Wierzbicka A, Karedal M, Blomgren E, Nielsen J, Buonanno G, Gudmundsson A. 2016. Characterization of hairdresser exposure to airborne particles during hair bleaching. Ann Occup Hyg. 60(1):90–100.
- Noguchi M, Furukawa KT, Morimoto M. 2020. Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease. Dis Model Mech. 13(12):dmm046920.
- Normand JC, Grange F, Hernandez C, Ganay A, Davezies P, Bergeret A, Prost G. 1989. Occupational asthma after exposure to azodicarbonamide: report of four cases. Br J Ind Med. 46(1):60–62.
- Nova Z, Skovierova H, Strnadel J, Halasova E, Calkovska A. 2020. Short-term versus long-term culture of A549 cells for evaluating the effects of lipopolysaccharide on oxidative stress, surfactant proteins and cathelicidin LL-37. Int J Mol Sci. 21(3):1148.
- Oie L, Hersoug LG, Madsen JO. 1997. Residential exposure to plasticizers and its possible role in the pathogenesis of asthma. Environ Health Perspect. 105(9):972–978.
- Paris C, Ngatchou-Wandji J, Luc A, McNamee R, Bensefa-Colas L, Larabi L, Telle-Lamberton M, Herin F, Bergeret A, Bonneterre V, et al. 2012. Work-related asthma in France: recent trends for the period. Occup Environ Med. 69(6):391–397.
- Parra FM, Igea JM, Quirce S, Ferrando MC, Martin JA, Losada E. 1992. Occupational asthma in a hairdresser caused by persulphate salts. Allergy. 47(6):656–660.
- Patterson R, Zeiss CR, Pruzansky JJ. 1982. Immunology and immunopathology of trimellitic anhydride pulmonary reactions. J Allergy Clin Immunol. 70(1):19–23.
- Patterson R, Zeiss CR, Roberts M, Pruzansky JJ, Wolkonsky P, Chacon R. 1978. Human antihapten antibodies in trimellitic anhydride inhalation reactions. Immunoglobulin classes of anti-trimellitic anhydride antibodies and hapten inhibition studies. J Clin Invest. 62(5):971–978.
- Pauluhn J. 2008. Brown Norway rat asthma model of diphenylmethane-4,4'-diisocyanate (MDI): analysis of the elicitation dose-response relationship. Toxicol Sci. 104(2):320–331.
- Pauluhn J, Mohr U. 2005. Experimental approaches to evaluate respiratory allergy in animal models. Exp Toxicol Pathol. 56(4–5):203–234.
- Pemberton MA, Kimber I. 2021. Classification of chemicals as respiratory allergens based on human data: requirements and practical considerations. Regul Toxicol Pharmacol. 123:104925.
- Pepys J, Pickering CA, Loudon HW. 1972. Asthma due to inhaled chemical agents-piperazine dihydrochloride. Clin Allergy. 2(2):189–196.
- Piirila P, Estlander T, Hytonen M, Keskinen H, Tupasela O, Tuppurainen M. 1997. Rhinitis caused by ninhydrin develops into occupational asthma. Eur Respir J. 10(8):1918–1921.
- Piirila P, Estlander T, Keskinen H, Jolanki R, Laakkonen A, Pfaffli P, Tupasela O, Tuppurainen M, Nordman H. 1997. Occupational asthma caused by triglycidyl isocyanurate (TGIC). Clin Exp Allergy. 27(5):510–514.
- Piirila P, Keskinen H, Anttila S, Hyvonen M, Pfaffli P, Tuomi T, Tupasela O, Tuppurainen M, Nordman H. 1997. Allergic alveolitis following exposure to epoxy polyester powder paint containing low amounts (<1%) of acid anhydrides. Eur Respir J. 10(4):948–951.
- Porter CV, Higgins RL, Scheel LD. 1975. A retrospective study of clinical, physiologic and immunologic changes in workers exposed to toluene diisocyanate. Am Ind Hyg Assoc J. 36(3):159–168.
- Raulf M, Quirce S, Vandenplas O. 2018. Addressing molecular diagnosis of occupational allergies. Curr Allergy Asthma Rep. 18(1):6.
- Ribeiro M, Tarlo SM, Czyrka A, Vernich L, Luce CE, Liss GM. 2014. Diisocyanate and non-diisocyanate sensitizer-induced occupational asthma frequency during 2003 to 2007 in Ontario. Canada J Occup Environ Med. 56(9):1001–1007.
- Rosenman KD, Bernstein DI, O’Leary K, Gallagher JS, D’Souza L, Bernstein IL. 1987. Occupational asthma caused by himic anhydride. Scand J Work Environ Health. 13(2):150–154.
- Schlueter DP, Banaszak EF, Fink JN, Barboriak J. 1978. Occupational asthma due to tetrachlorophthalic anhydride. J Occup Med. 20(3):183–188.
- Slovak AJ. 1981. Occupational asthma caused by a plastics blowing agent, azodicarbonamide. Thorax. 36(12):906–909.
- Stenton SC, Dennis JH, Walters EH, Hendrick DJ. 1990. Asthmagenic properties of a newly developed detergent ingredient: sodium iso-nonanoyl oxybenzene sulphonate. Br J Ind Med. 47(6):405–410.
- Suter W. 2006. Predictive value of in vitro safety studies. Curr Opin Chem Biol. 10(4):362–366.
- Swain RJ, Kemp SJ, Goldstraw P, Tetley TD, Stevens MM. 2010. Assessment of cell line models of primary human cells by Raman spectral phenotyping. Biophys J. 98(8):1703–1711.
- Tanner L, Single AB. 2020. Animal models reflecting chronic obstructive pulmonary disease and related respiratory disorders: translating pre-clinical data into clinical relevance. J Innate Immun. 12(3):203–225.
- Tanser AR, Bourke MP, Blandford AG. 1973. Isocyanate asthma: respiratory symptoms caused by diphenyl-methane di-isocyanate. Thorax. 28(5):596–600.
- Tran E, Shi T, Li X, Chowdhury AY, Jiang D, Liu Y, Wang H, Yan C, Wallace WD, Lu R, et al. 2020. Development of novel in vitro human alveolar epithelial cell models to study distal lung biology and disease. bioRxiv.
- Tran S, Francis H, Hoyle J, Niven R. 2009. Occupational asthma and the paper recycling industry. Occup Med (Lond). 59(4):277–279.
- Tyrer FH. 1979. Hazards of spraying with two-pack paints containing isocyanates. J Soc Occup Med. 29(1):22–24.
- UK-HSE. 2001. Asthmagen? Critical assessments of the evidence for agents implicated in occupational asthma. [accessed 10 April 2021]. https://www.hse.gov.uk/asthma/substances.htm.
- Uriarte SA, Fernandez-Nieto M, Sastre J. 2013. Occupational asthma due to polyvinyl chloride and methyl methacrylate in a plumber. J Investig Allergol Clin Immunol. 23(6):437–438.
- Van Lommel A, Bolle T, Fannes W, Lauweryns JM. 1999. The pulmonary neuroendocrine system: the past decade. Arch Histol Cytol. 62(1):1–16.
- Vandenplas O, Cartier A, Lesage J, Cloutier Y, Perreault G, Grammer LC, Shaughnessy MA, Malo JL. 1993. Prepolymers of hexamethylene diisocyanate as a cause of occupational asthma. J Allergy Clin Immunol. 91(4):850–861.
- Vandenplas O, Rifflart C, Evrard G, Thimpont J, Seed M, Agius R. 2017. Occupational asthma caused by an epoxy amine hardener. Occup Med (Lond). 67(9):722–724.
- Venables KM, Dally MB, Nunn AJ, Stevens JF, Stephens R, Farrer N, Hunter JV, Stewart M, Hughes EG, Newman Taylor AJ. 1989. Smoking and occupational allergy in workers in a platinum refinery. Br Med J. 299(6705):939–942.
- Venables KM, Newman Taylor AJ. 1990. Exposure-response relationships in asthma caused by tetrachlorophthalic anhydride. J Allergy Clin Immunol. 85(1 Pt 1):55–58.
- Venables KM, Topping MD, Nunn AJ, Howe W, Newman Taylor AJ. 1987. Immunologic and functional consequences of chemical (tetrachlorophthalic anhydride)-induced asthma after four years of avoidance of exposure. J Allergy Clin Immunol. 80(2):212–218.
- Verraes S, Michel O. 1995. Occupational asthma induced by ethylene oxide. Lancet. 346(8987):1434–1435.
- Wang CW, Chen SC, Wu DW, Chen HC, Lin HH, Su H, Shiea JT, Lin WY, Hung CH, Kuo CH. 2021. Effect of dermal phthalate levels on lung function tests in residential area near a petrochemical complex. Environ Sci Pollut Res. 28(21):27333–27344.
- Weill H, Salvaggio J, Neilson A, Butcher B, Ziskind M. 1975. Respiratory effects in toluene diisocyanate manufacture: a multidisciplinary approach. Environ Health Perspect. 11:101–108.
- Welinder H, Hagmar L, Gustavsson C. 1986. IgE antibodies against piperazine and N-methyl-piperazine in two asthmatic subjects. Int Arch Allergy Appl Immunol. 79(3):259–262.
- Wernfors M, Nielsen J, Schutz A, Skerfving S. 1986. Phthalic anhydride-induced occupational asthma. Int Arch Allergy Appl Immunol. 79(1):77–82.
- Wild LG, Lopez M. 2003. Occupational asthma caused by high-molecular-weight substances. Immunol Allergy Clin North Am. 23(2):235–250, vii.
- Yucesoy B, Johnson VJ, Lummus ZL, Kissling GE, Fluharty K, Gautrin D, Malo JL, Cartier A, Boulet LP, Sastre J, et al. 2012. Genetic variants in antioxidant genes are associated with diisocyanate-induced asthma. Toxicol Sci. 129(1):166–173.
- Zeiss CR, Kanellakes TM, Bellone JD, Levitz D, Pruzansky JJ, Patterson R. 1980. Immunoglobulin E-mediated asthma and hypersensitivity pneumonitis with precipitating anti-hapten antibodies due to diphenylmethane diisocyanate (MDI) exposure. J Allergy Clin Immunol. 65(5):347–352.
- Zeiss CR, Patterson R, Pruzansky JJ, Miller MM, Rosenberg M, Levitz D. 1977. Trimellitic anhydride-induced airway syndromes: clinical and immunologic studies. J Allergy Clin Immunol. 60(2):96–103.
- Zeiss CR, Wolkonsky P, Pruzansky JJ, Patterson R. 1982. Clinical and immunologic evaluation of trimellitic anhydride workers in multiple industrial settings. J Allergy Clin Immunol. 70(1):15–18.