Abstract
The non-genotoxic synthetic pyrethroid insecticide permethrin produced hepatocellular adenomas and bronchiolo-alveolar adenomas in female CD-1 mice, but not in male CD-1 mice or in female or male Wistar rats. Studies were performed to evaluate possible modes of action (MOAs) for permethrin-induced female CD-1 mouse liver and lung tumor formation. The MOA for liver tumor formation by permethrin involves activation of the peroxisome proliferator-activated receptor alpha (PPARα), increased hepatocellular proliferation, development of altered hepatic foci, and ultimately liver tumors. This MOA is similar to that established for other PPARα activators and is considered to be qualitatively not plausible for humans. The MOA for lung tumor formation by permethrin involves interaction with Club cells, followed by a mitogenic effect resulting in Club cell proliferation, with prolonged administration producing Club cell hyperplasia and subsequently formation of bronchiolo-alveolar adenomas. Although the possibility that permethrin exposure may potentially result in enhancement of Club cell proliferation in humans cannot be completely excluded, there is sufficient information on differences in basic lung anatomy, physiology, metabolism, and biologic behavior of tumors in the general literature to conclude that humans are quantitatively less sensitive to agents that increase Club cell proliferation and lead to tumor formation in mice. The evidence strongly indicates that Club cell mitogens are not likely to lead to increased susceptibility to lung tumor development in humans. Overall, based on MOA evaluation it is concluded that permethrin does not pose a tumorigenic hazard for humans, this conclusion being supported by negative data from permethrin epidemiological studies.
1. Introduction
The synthetic pyrethroid insecticide permethrin [(3-phenoxyphenyl)-methyl-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate], which is used for pest control, has been shown to produce liver and lung tumors in CD-1 female mice. The purpose of this review is to identify the modes of action (MOAs) by which permethrin produces these tumors and then to assess the relevance of these MOAs for human cancer hazards. In addition to the human hazard, possible cancer risk in humans is also discussed based on expected exposure levels and available epidemiological data for permethrin.
During the past 20 years, regulatory agencies and other organizations around the world have been evolving a framework to incorporate a scientific understanding of the tumorigenic processes in animal studies into regulatory decision-making for human risk assessment (Cohen and Arnold Citation2011). The International Life Sciences Institute (ILSI) [supported by US. Environmental Protection Agency (US.EPA) and Health Canada] and the International Programme on Chemical Safety (IPCS) of the World Health Organization (WHO) have developed a framework for the analysis of MOA for rodent toxicity and tumorigenicity findings along with an assessment of their human relevance (Sonich-Mullin et al. Citation2001; Meek et al. Citation2003; Cohen et al. Citation2004; Seed et al. Citation2005; Boobis et al. Citation2006, Citation2008; Holsapple et al. Citation2006; Meek, Boobis, et al. Citation2014; Meek, Palermo, et al. Citation2014). Based on the modified Bradford Hill considerations, the key (KE) and associative (AE) events (Andersen et al. Citation2014) for the proposed MOA for tumor formation in animals can be identified and then subsequently compared both qualitatively and quantitatively with effects in humans. Several case studies have been published illustrating the applicability of this framework for genotoxic and non-genotoxic cancer MOAs and for cancer and non-cancer endpoints. In fact, MOA analysis has been incorporated into the risk assessment guidelines/guidance of various regulatory agencies, including the US. EPA (US.EPA Citation2005a), the United Nations Conference on Environment and Development (McGregor et al. Citation2010), and the European Chemicals Agency (ECHA Citation2017).
An MOA for chemically-induced tumor formation comprises a biological sequence of events leading to tumorigenesis (Boobis et al. Citation2006). In developing an MOA, the component events may be defined as either KEs or as AEs. For the development of the MOAs for both permethrin-induced mouse liver and lung tumor formation, the following definitions of KEs and AEs (Andersen et al. Citation2014) have been used. A KE is an empirically observable causal step to the adverse outcome that is itself a necessary element of the MOA. While KEs are required events for the MOA, they often are not sufficient individually to induce the adverse outcome in the absence of other KEs. An AE is a biological process that itself is not a causal necessary KE for the MOA, but is a reliable indicator or marker for a KE. AEs can often be used as surrogate markers for a KE in an MOA evaluation or as indicators of exposure to a xenobiotic that has stimulated the molecular initiating event or a KE (Andersen et al. Citation2014).
In this review, the MOAs for tumor induction of mouse liver and lung by permethrin have first been analyzed through the procedures described in the IPCS framework (Boobis et al. Citation2006), employing data from experimental studies on permethrin and information on related chemicals from the literature. The human relevance of the mouse liver and lung tumorigenic response has then been assessed based upon the human relevance framework (Meek et al. Citation2003; Boobis et al. Citation2006).
2. Carcinogenicity data
Chemical substances are subjected to assessment of genotoxic and carcinogenic effects before being marketed to protect humans and the environment from health risks. For agrochemicals and biocides, two long-term rodent carcinogenicity studies are currently required from a regulatory perspective (OECD Citation2012). The carcinogenicity of permethrin has been studied in male and female Wistar rats and CD-1 mice in OECD guideline, Good Laboratory Practice (GLP) standard bioassays. These findings were assessed by the California Environmental Protection Agency in the US (Cal.EPA Citation1994) and the US.EPA (US.EPA Citation1988, Citation2000, Citation2002) as described below.
2.1. Wistar rat carcinogenicity study
Male and female Wistar rats were fed at dietary levels of 0 (control), 500, 1000, or 2500 ppm permethrin (cis:trans ratio 40:60) in the diet for 2 years. Furthermore, in another study, male and female Wistar rats were fed at dietary levels of 0 (control), 10, 50, or 250 ppm permethrin in the diet for 2 years. In both studies, there were no treatment-related increases in tumor incidences at any dose of the test material compared with control incidences (Ishmael and Lithfield Citation1988). The US.EPA and Cal.EPA concluded that there was no evidence of carcinogenicity in male and female Wistar rats (Cal.EPA Citation1994; US.EPA Citation2002).
2.2. Alderley Park mouse carcinogenicity study
Male and female Alderley Park (Swiss-derived) mice were fed permethrin (cis:trans ratio 40:60) at dietary levels of 0 (control), 250, 1000, or 2500 ppm for 98 weeks. A fairly high incidence of lung adenoma was seen in all groups with a slightly higher incidence than controls for the 2500 ppm group. Using Fisher’s exact test (5% level, one-sided), the difference between the control and 2500 ppm permethrin groups was not significant for either sex. With the Log-rank test, the increase was statistically significant for the 2500 ppm permethrin males (5% level) but not for females. For common tumors (spontaneous incidence is higher than 1%), such as mouse lung adenoma, a 1% level of statistical significance is considered necessary to avoid a false positive effect (Haseman Citation1983; US.FDA Citation2001; US.EPA Citation2005a; OECD Citation2012). Therefore, the higher incidence of lung adenoma in males was not considered to represent a carcinogenic effect (Ishmael and Lithfield Citation1988). Furthermore, there was no significant increase in liver tumors (Ishmael and Lithfield Citation1988). The US.EPA and Cal.EPA reported that this study was considered negative for an oncogenic effect (Cal.EPA Citation1994; US.EPA Citation2020a).
2.3. CD-1 mouse carcinogenicity study
Male and female CD-1 mice were fed permethrin (cis:trans ratio 40:60) at dietary levels of 0 (control), 20, 500, or 2000 ppm for males, and 0, 20, 2500, or 5000 ppm for females for 2 years (Ellison Citation1979). In 2002, the Cancer Assessment Review Committee (CARC) of the Health Effects Division of US.EPA reconsidered the 1995 Pathology Working Group (PWG) report on a reassessment of the lung tumor slides from the CD-1 mouse study (referred to as the 1995 lung PWG in this review) and determined it to be acceptable (US.EPA Citation2002). As shown in , the 1995 lung PWG report indicated that permethrin induced lung adenomas at 2500 and 5000 ppm in female mice, but did not increase the incidence of lung adenomas in male mice or the incidence of lung carcinomas in either sex. Permethrin also produced a dose-dependent increase in the incidence of mice exhibiting multifocal alveolar cell proliferation (considered as Club cell hyperplasia) in female CD-1 mice given permethrin at dietary levels of 2500 and 5000 ppm for two years [3/75 (4.0%), 5/76 (6.6%), 11/75 (14.7%), and 13/75 (17.3%) for control, 20, 2500, and 5000 ppm, respectively] (US.EPACitation1988); which were statistically significant at 2500 ppm (p < 0.05) and 5000 ppm (p < 0.01) by Fisher's exact probability test with a one-tailed test. In contrast, permethrin did not produce any dose-dependent increases in the incidence of alveolar cell proliferation in male mice given permethrin at dietary levels of up to 2000 ppm [1/75 (1.3%), 7/75 (9.3%), 5/74 (6.8%) and 1/75 (1.3%) for control, 20, 500, and 2000 ppm, respectively] (US.EPA Citation1988). While the effect at 20 ppm was just statistically significant (p = 0.0315), there was no dose-dependency.
Table 1. Incidences of tumors in liver and lung of male and female CD-1 mice treated with permethrin.
The CARC also reaffirmed a previous Carcinogenicity Peer Review Committee (CPRC) report that there were statistically significant increases in liver adenomas in male mice at all permethrin dose levels and in female mice given 2500 and 5000 ppm permethrin. However, treatment with permethrin did not result in any significant increase in hepatocellular carcinoma in either male or female mice. The combined incidence of hepatocellular adenoma and carcinoma was significantly increased in male mice given 500 ppm permethrin and in female mice given 2500 and 5000 ppm permethrin.
The background incidences of liver adenoma and carcinoma from the laboratory conducting the permethrin CD-1 mouse bioassay were reported to be 0–12 and 0–12%, respectively, for male mice, and 0–8 and 0–5%, respectively, for female mice (Cal.EPA Citation1994). Thus, while the observed control incidences for liver adenomas in male and female mice and for liver carcinoma for female mice were similar to the historical control data for the performing laboratory, the observed incidence of hepatocellular carcinoma in control male mice was much greater than the highest incidence of the historical control incidence range (12%). This suggests that the batch of male mice used for the permethrin CD-1 mouse bioassay under the environmental conditions employed had a high susceptibility to liver tumor formation. As shown in , the incidence of liver adenomas in male mice given 20, 500, and 2000 ppm permethrin was 27, 24, and 30%, respectively. There is thus a lack of a clear dose-response relationship in male mice for the formation of liver adenomas following treatment with permethrin and also for the combined incidences of liver adenomas and carcinomas. The lack of a clear dose-response for liver tumor formation in male mice suggests that the effects observed are not related to treatment with permethrin. Combined with the lack of a liver tumorigenic effect in another mouse bioassay at 250, 1000, and 2500 ppm in Swiss mice (Ishmael and Lithfield Citation1988), a treatment-related effect in male CD-1 mouse liver is considered unlikely.
Overall, this study produced clear dose-dependent evidence of permethrin-induced liver and lung tumor formation in female CD-1 mice, but only equivocal evidence of a non-dose-dependent increase in liver adenoma formation in male mice. However, as described below, a subsequent reevaluation of the liver slides (referred to as the 2019 liver PWG) from the CD-1 mouse study demonstrated that there was no treatment-related increase in the incidence of liver tumors in male mice after permethrin treatment (Quist et al. Citation2019).
2.4. Non-guideline mouse carcinogenicity study
In a non-guideline mouse carcinogenicity study, permethrin was administered to groups of 50 to 109 Crl:CD-1®(ICR)BR female mice in the diet at 0 or 5000 ppm for periods of 39, 52, 65, or 78 weeks (Barton et al. Citation2000; US.EPA Citation2000, Citation2002). Groups of mice from all treatment groups were examined immediately after each treatment interval and also following recovery (no permethrin treatment) at weeks 78 and 100. Groups of untreated mice (controls) were examined at each time point.
Significant increases were seen in the incidences of basophilic hepatocellular adenomas in female mice administered 5000 ppm in the diet for 39, 52, or 78 weeks followed by recovery to week 100 (7% to 10% compared to 1% in controls). The increased incidences were not treatment-duration related, and treatment with permethrin for 65 weeks resulted in no basophilic adenomas. Eosinophilic adenomas were increased after 78 weeks of treatment and after the recovery period (both 10% compared to 1–2% in controls). The incidences did not increase during the recovery period. No increases in hepatocellular carcinoma incidences were seen and the time to tumor onset for the adenomas was not different in treated animals compared to the controls.
Lung bronchiolo-alveolar adenoma incidences were increased after permethrin treatment and continued to be increased during the recovery periods compared to the controls (Barton et al. Citation2000; US.EPA Citation2000, Citation2002). Incidences of lung bronchiolo-alveolar adenoma after 39, 52, 65, and 78 weeks of treatment were 18, 33, 30, and 42%, respectively, with control incidences ranging from 8 to 10% (p < 0.01 for 52, 65, and 78 weeks treated groups). The incidences of lung bronchiolo-alveolar adenoma were 14, 43, 47, 49, and 49% for the control (100 weeks) and 39, 52, 65, and 78 weeks exposure (p < 0.01 for all treated groups) followed by recovery to week 100. The lung adenomas did not occur any earlier in the treated animals than in the control groups, and there was no increase in lung carcinomas in treated animals (i.e. the adenomas did not progress to carcinomas). The incidences of Club cell hyperplasia were significantly increased in the majority of the permethrin-treated mice (72–98% of animals tested) at all time points from 39 weeks onwards (i.e. before adenoma induction at 52 weeks), with only very low incidences being observed in control animals (1% at 78 weeks, 0.9% at 100 weeks). The incidences of Club cell hyperplasia as expected were significantly decreased during the recovery periods to weeks 78 and 100 (Barton et al. Citation2000; US.EPA Citation2000).
2.5. Reevaluation of the liver sections from the CD-1 mouse carcinogenicity study
The permethrin mouse carcinogenicity study is nearly 40 years old (Ellison Citation1979) and diagnostic pathology criteria for mouse liver lesions have changed. Therefore, a new PWG was convened to reevaluate all permethrin-induced mouse liver neoplasms employing current nomenclature and diagnostic criteria (referred to as the 2019 liver PWG in this review). The 2019 liver PWG review followed the procedures recommended in the EPA Pesticide Regulation (PR) Notice 94-5: Requests for Reconsiderations of Carcinogenicity Peer Review Decisions Based on Changes in Pathology Diagnoses (US.EPA Citation1994). As part of the PWG process, a peer review of all liver sections was conducted by Dr. Robert R. Maronpot before the PWG meeting. As required by US.EPA PR Notice 94-5, the 2019 liver PWG examined all slides containing sections of the liver for which there were differing diagnoses involving proliferative lesions (neoplasm or hyperplasia) between the Study and Reviewing Pathologists. Additionally, all sections of the liver were reexamined by the 2019 liver PWG that had an original diagnosis of hepatocellular adenoma, hepatocellular carcinoma, or hepatocholangiocarcinoma reported by either the Study or Reviewing Pathologist (Quist et al. Citation2019).
While a majority of liver proliferative lesions had features consistent with either hepatocellular adenoma or hepatocellular carcinoma resulting in a unanimous consensus among PWG members, some adenomas, especially in the males, had questionable areas of atypia, mitoses, necrosis, and/or trabecular formation resulting in considerable discussion among PWG members before a consensus was reached. However, the decision was frequently not unanimous for these difficult lesions. This is consistent with the previous statement that mouse liver adenomas may contain areas of atypia suggesting some may progress to malignancy (Harada et al. Citation1999). Thus, the 2019 liver PWG members thought that hepatocellular adenomas and carcinomas in this study represent a continuum and the combined incidence of adenomas and carcinomas is the best measure of any possible effect of permethrin on the mouse liver in this study, rather than evaluating adenomas and carcinomas separately (Quist et al. Citation2019).
The findings of the 2019 liver PWG analysis indicated that in female mice, compared to controls, treatment with 2500 and 5000 ppm permethrin produced a dose-dependent increase (p < 0.01) in hepatocellular adenomas (). This effect was not apparent in the female mice fed 20 ppm permethrin. In general, the hepatocellular adenomas in mice had typical features of adenoma and could be readily distinguished from hepatocellular carcinoma. There was no increase in the incidence of hepatocellular carcinomas in female mice treated with permethrin. However, since these hepatocellular neoplasms are considered a continuum, the combined incidence of hepatocellular adenomas and carcinomas was considered more appropriate for the evaluation. The incidence of females with either hepatocellular adenoma or carcinoma was similar to the results for adenoma only since they were mostly adenomas, and this was statistically significantly increased only in the 2500 and 5000 ppm permethrin groups (p < 0.001 by the poly-3 pair-wise comparisons) in a dose-related manner (p < 0.001 by the poly-3 trend test) (Quist et al. Citation2019).
Table 2. Incidences of hepatocellular tumors in CD-1 mice treated with permethrin based on the 2019 liver Pathology Working Group reevaluation.
In the male mice, a higher incidence of hepatocellular adenomas was observed in all treated groups compared to the control male mice; however, the increase was statistically significant only in the 500 and 2000 ppm permethrin groups at p < 0.05 by using the poly-3 statistical analysis for pair-wise comparisons (). Moreover, the poly-3 analysis demonstrated that there was no statistically significant trend for a dose-related increase in liver adenomas (p > 0.05). There was no increase in the incidence of hepatocellular carcinomas in male mice treated with permethrin, with the incidence of hepatocellular carcinomas in the high-dose (2000 ppm) permethrin group being somewhat lower than that observed in the 20 and 500 ppm treated groups. However, as mentioned above, since these hepatocellular neoplasms are considered a continuum, the combined incidence of hepatocellular adenomas and carcinomas was considered more appropriate for the evaluation (Quist et al. Citation2019). This was especially true in the males as there were several lesions classified as adenoma but had features suggestive of carcinoma, leading to disagreement between 2019 liver PWG pathologists (Quist et al. Citation2019). The incidence of males with either hepatocellular adenoma or carcinoma was statistically significantly increased only in the 500 and 2000 ppm permethrin groups based on p < 0.05 but not at the more appropriate value of p < 0.01 for common tumors, such as the liver tumors in this mouse strain (Haseman Citation1983; US.FDA Citation2001; US.EPA Citation2005a; OECD Citation2012). Furthermore, no dose-response was observed between 500 and 2000 ppm even at a 4-fold dose difference: 45% (34/75) at 500 ppm vs. 33% (25/75) at 2000 ppm. In addition to the lack of a significant trend (p > 0.05) for a dose-related increase in hepatocellular adenoma, the poly-3 statistical analysis demonstrated that there was no statistically significant trend (p > 0.05) for dose-related increases in either hepatocellular carcinoma or combined tumor types (Quist et al. Citation2019); a significance value of p < 0.005 would be taken as indicating a dose-related increase for common tumors (Lin and Ali Citation1994; US.FDA Citation2001).
We consider that the major issue for the liver tumors in CD-1 male mice is what constitutes a positive tumor response.
Concerning the incidences of “adenoma” and “adenoma or carcinoma” in males, statistical significance was observed at 500 and 2000 ppm but only at p < 0.05, not at p < 0.01 by the Poly-3 pair-wise comparisons. Moreover, there was no dose-dependent response at these two permethrin dose levels. Based on the 2019 liver PWG evaluation, there was no significant trend increase in liver tumors, with p > 0.05 (the Poly-3 trend test) (Quist et al. Citation2019).
Except for adenoma of the control group (where the incidence was equivalent to the highest incidence of the historical control data), the incidences for all groups (control, 20, 500, and 2000 ppm) based on the 2019 liver PWG data (hepatocellular adenoma in males; 9/75 (12%), 16/75 (25%), 19/75 (21%), 15/75 (20%), respectively; hepatocellular carcinoma; 13/75 (17%), 14/75 (19%), 16/75 (21%), 11/75 (15%), respectively] (Quist et al. Citation2019) are greater than historical control data for adenoma and carcinoma (0–12 and 0–12%, respectively) as previously reported by Cal.EPA (Citation1994).
Generally, liver tumors are well-known common tumors in CD-1 mice (Maronpot Citation2009), with a large variation in liver tumor incidence between different studies in this mouse strain (Rao et al. Citation1988; Giknis and Clifford Citation2005; Baldrick and Reeve Citation2007). In contrast to the historical control data reported by Cal.EPA, the incidences of hepatocellular adenoma and hepatocellular carcinoma in males reported by the 2019 liver PWG (Quist et al. Citation2019) were well within the historical control ranges presented in the above references (overall observed control ranges from these references are 1.7–39% for hepatocellular adenoma and 0–28% for hepatocellular carcinoma).
Importantly, the incidences of carcinoma and combined adenomas plus carcinomas in control male mice were 17 and 28%, respectively, which are higher than the highest incidence of historical control data for carcinoma (12%; Cal.EPA Citation1994) and combined tumors (24%; Cal.EPA Citation1994). This suggests that this particular batch of mice under the environmental conditions employed in this study were unusually sensitive to the development of liver tumors. Such a high spontaneous incidence may make for a larger variation, and such large variation may result in equivocal findings, such as statistical significance.
To reduce false-positives in carcinogenicity evaluations, p-values are one-sided and, for common tumors (background rate of at least 1%), a significance level of 0.01 for pair-wise comparisons and 0.005 for trend tests is considered appropriate (Haseman Citation1983; Lin and Ali Citation1994; US.FDA Citation2001; US.EPA Citation2005a; OECD Citation2012). Based on this judgment rule, no significant effects were observed for both pair-wise comparison and trend tests (Quist et al. Citation2019), demonstrating that the increased incidences of liver tumors in male mice in the permethrin carcinogenicity study are unlikely to be treatment-related effects (Kondo et al. Citation2019). The US.EPA agreed with this conclusion (US.EPA Citation2020a).
No tumorigenic response occurred in male Alderley Park (Swiss-derived) mice fed permethrin a dietary level of 0, 250, 1000, and 2500 ppm for 98 weeks (Ishmael and Lithfield Citation1988; Cal.EPA Citation1994).
Based on the permethrin carcinogenicity data described above, it is concluded that permethrin increased liver and lung tumors at 2500 and 5000 ppm in female mice but no treatment-related tumorigenic response occurred in male mice at the dose levels examined in the 2 year bioassay (Cal.EPA Citation1994; US.EPA Citation2002, Citation2020a; Quist et al. Citation2019). The increase in tumors in female mouse liver and lung were only in adenomas and not in carcinomas (Cal.EPA Citation1994; Barton et al. Citation2000; US.EPA Citation2000, Citation2002). Investigative MOA studies have thus been performed to evaluate the human relevance of the liver and lung tumors produced in female mice by permethrin (Yamada et al. Citation2017; Kondo et al. Citation2019). The MOAs for liver and lung tumor induction were developed and are considered separately as suggested by the IPCS framework (Boobis et al. Citation2006).
In many cases, it is clear that tumors arise secondary to some early occurring toxic event (Cohen and Arnold Citation2011). By examining the mechanisms involved in the early events, rather than having to rely on a 2-year bioassay, considerable progress can be made more quickly in delineating the actual mechanisms involved with possible carcinogenesis (Cohen and Arnold Citation2011). Several investigative studies were conducted to elucidate the early effects of permethrin on mouse liver and lung after short-term treatment with permethrin (Yamada et al. Citation2017; Kondo et al. Citation2019; Ogata et al. Citation2021). The batch of permethrin used in these studies had a cis:trans ratio of 40:60, which was the same cis:trans ratio as that used in the permethrin CD-1 mouse bioassay. In this paper, we comprehensively review the data obtained in the investigative MOA studies with permethrin and related literature studies to evaluate possible MOAs for liver and lung tumor production in female mice and evaluate human relevance.
3. Analysis for tumorigenic MOA in liver
3.1. Liver: postulated MOA for the induction of hepatocellular tumors in female mice
The MOA for induction of female mouse liver tumors by permethrin is postulated to involve activation (KE 1) of the peroxisome proliferator-activated receptor alpha (PPARα) which results in a pleiotropic response including hepatic peroxisome proliferation (AE 1), the stimulation of both peroxisomal and microsomal fatty acid oxidizing enzymes (AE 2) and increased hepatocellular proliferation (KE 2). Prolonged administration of permethrin to female mice results in altered liver foci (KE 3) and ultimately in liver tumor formation (KE 4).
3.2. Liver: postulated KEs and AEs in experimental animals
A substantial body of knowledge for the hepatic effects of PPARα activators is available to support the characterization of the MOA by which liver tumors arise in rats and mice and the relevance of this MOA to the assessment of human cancer hazard (Cattley et al. Citation1998; Cohen et al. Citation2003; Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018; Foreman et al. Citation2021a, Citation2021b). The KEs for PPARα activator-induced rodent liver tumor formation described by Corton et al. (Citation2014, Citation2018) were considered to comprise PPARα activation, alteration in cell growth pathways, perturbation of cell growth and survival, and selective clonal expansion of preneoplastic foci, which leads to the apical event of increases in hepatocellular adenomas and carcinomas. The postulated KEs for permethrin-induced mouse liver tumor formation are considered to be PPARα activation (KE 1), increased hepatocellular proliferation (KE 2), formation of preneoplastic foci (KE 3) and ultimately liver tumor formation (KE 4). AEs include hepatic peroxisome proliferation (AE 1) and induction of peroxisomal/microsomal fatty acid oxidizing enzymes (AE 2). Liver peroxisomes contain a fatty acid β-oxidation cycle of which the first rate-limiting enzyme, namely acyl-CoA oxidase, catalyzes the desaturation of acyl-CoAs to 2-trans-enoyl-CoAs (Lazarow and De Duve Citation1976) and is a target gene for PPARα (Rosen et al. Citation2008). In addition, liver microsomes contain cytochrome P450 (CYP)-dependent enzymes in the CYP4A subfamily which can catalyze the ω- and (ω-1)-hydroxylation of fatty acids. To test each of these postulated KEs and AEs, several parameters were evaluated in male and female mice in short-term and subchronic studies, and at interim and terminal sacrifices in a chronic study. In addition, studies were also performed in female wild-type (i.e. normal) mice and in mice lacking PPARα (PPARα knockout mice)(Kondo et al. Citation2019).
3.2.1. Activation of PPARα (KE 1)
Previous studies have demonstrated that PPARα activators do not produce hepatic peroxisome proliferation, increased cell proliferation, induction of peroxisomal/microsomal fatty acid oxidizing enzyme activities, and liver tumors in PPARα knockout mice (Lee et al. Citation1995; Peters et al. Citation1997, Citation1998; Hays et al. Citation2005; Foreman et al. Citation2021a, Citation2021b). The treatment of wild type female mice with 5000 ppm permethrin for 7 days resulted in peroxisome proliferation as assessed by ultrastructural examination, a significant increase in acyl-coenzyme A oxidase 1 (Acox1) mRNA levels, and a significant increase in induction of lauric acid hydroxylation activity and Cyp4a10 mRNA levels (Kondo et al. Citation2019). Unlike female wild-type mice, the treatment of PPARα knockout mice with 5000 ppm permethrin for 7 days did not result in these findings except for a small increase in lauric acid hydroxylation activity (Kondo et al. Citation2019). The small increase could be due to increased (ω-1)-hydroxylation, via constitutive androstane receptor (CAR) activation by permethrin, as the assay employed measured both ω- and (ω-1)-hydroxylation of lauric acid (Kondo et al. Citation2019). Finally, while permethrin treatment produced stimulation of replicative DNA synthesis (RDS) in wild-type mice, no increase was observed in PPARα knockout mice (Kondo et al. Citation2019). These findings strongly suggest that permethrin activates mouse PPARα in the liver. This conclusion is supported by the data related to AEs as discussed below.
3.2.2. Peroxisome proliferation (AE 1)
Hepatic peroxisome proliferation has been identified and characterized traditionally either by morphological or biochemical techniques (Cattley et al. Citation1998; Klaunig et al. Citation2003). As the term “peroxisome proliferator” implies, there is an increase in the number and volume fraction of peroxisomes in the cytoplasm of hepatocytes. Examination by electron microscopy was conducted on livers from female mice treated with 10 000 ppm permethrin for 7 and 14 days and revealed increased numbers and enlargement of peroxisomes (Kondo et al. Citation2019). These results demonstrated that permethrin can produce peroxisome proliferation in female mouse liver at the single high dose level examined. Evidence for increased peroxisome proliferation at the carcinogenic dose levels of 2500 and 5000 ppm was provided by effects on hepatic fatty acid oxidizing enzyme mRNA levels and enzyme activities as described below (AE 2).
3.2.3. Induction of peroxisomal/microsomal fatty acid oxidizing enzymes (AE 2)
Measurements of effects on either peroxisomal or microsomal fatty acid oxidizing enzymes are established biochemical markers of PPARα activation in rodent liver (Lake Citation2009). The induction of the peroxisomal fatty acid β-oxidation cycle can be measured as overall activity as cyanide-insensitive palmitoyl-CoA oxidation or by determining acyl-CoA oxidase activity, which is the first and rate-limiting enzyme of the cycle. Hepatic Acox1 mRNA levels in mice treated with permethrin were determined by real-time polymerase chain reaction (PCR). Permethrin statistically significantly increased Acox1 mRNA levels in female mice at 2500 ppm and higher (see Section 3.3. Liver: concordance of dose-response relationships below) (Kondo et al. Citation2019).
Global gene expression analysis was employed to examine the effect of permethrin on CYP enzyme expression in mouse liver. This analysis demonstrated a higher expression of mRNA levels for CYP4A enzymes in female mice treated with 5000 ppm of permethrin (Kondo et al. Citation2019). Furthermore, real-time PCR analysis showed that hepatic Cyp4a10 mRNA levels were markedly increased in female mice treated with permethrin at dietary levels of 2500 ppm and higher for 7 days (see footnote of ) (Kondo et al. Citation2019).
Table 3. Dose-response relationships for the hepatic effects of permethrin in female CD-1 mice.
Table 4. Temporality and dose-response for MOA key events related to CD-1 female mouse liver tumors by permethrin.
The induction of CYP4A-dependent microsomal fatty acid oxidation is normally determined with lauric acid as substrate, determined as lauric acid hydroxylation activity (Madan et al. Citation1999). Time-course, dose-response and recovery, sex differences, PPARα dependency, and species differences studies were performed (Kondo et al. Citation2019). Treatment with 5000 ppm of permethrin in female mice increased CYP4A enzyme activity after 3-days treatment and the induction was maintained throughout 28-days of treatment. In another study, significant increases in CYP4A enzyme activity in female mice were observed after 14-days treatment with 5000 and 10 000 ppm permethrin and 5000 ppm of the known PPARα agonist clofibrate, whereas treatment with 500 ppm of the CAR activator phenobarbital had no significant effect. In a dose-response study, significant increases in CYP4A enzyme activity were observed after 7-days treatment at permethrin dietary levels of 2500 ppm and higher (see Section 3.3. Liver: concordance of dose-response relationships below). The treatment of female mice with 10 000 ppm permethrin for 7-days resulted in a significant induction of CYP4A enzyme activity, however, following a recovery period of 5-weeks of no treatment, enzyme activity had returned to control levels. The induction of CYP4A enzyme activity in female PPARα knockout mice (2.5-fold control) was much less than in female wild-type mice (19.4-fold control) following treatment for 7-days with the highest carcinogenicity study dose level of 5000 ppm permethrin. No induction of CYP4A enzyme activity was observed in female rats following treatment for 7-days with the highest carcinogenicity study dose level of 2500 ppm permethrin (Kondo et al. Citation2019).
In addition to CYP enzyme induction, hepatocellular hypertrophy, particularly in the centrilobular region of the liver lobule, was observed at the carcinogenic dose levels of 2500 and 5000 ppm in female mice (Kondo et al. Citation2019). This is also a characteristic effect of PPARα activators in rodent liver (Maronpot et al. Citation2010; Corton et al. Citation2014).
3.2.4. Altered gene expression profile
Recently developed genomics technology has the potential to improve our understanding of an organism’s response to stressors, and there is general agreement that toxicogenomics will play an increasingly large role in human health risk assessment (Farmahin et al. Citation2017; Vachon et al. Citation2018). As part of a series of analyses for MOA for female mouse liver tumor formation by permethrin, the global gene expression profile in the liver after 14-days treatment was evaluated using DNA microarray technology. Clustering analysis evaluated in genes altered by permethrin treatment in the liver of female mice demonstrated that the profile of these genes is generally similar to that of the PPARα agonist clofibrate, rather than to that of the CAR activator phenobarbital and the hepatotoxicants thioacetamide and carbon tetrachloride (Kondo et al. Citation2019). Furthermore, the top 10 scored pathways identified on the altered genes sets by each chemical using MetaCore analysis system revealed a similarity between permethrin and clofibrate based on 6 out of 10 pathways overlapping. Interestingly, the overlapping pathways included processes of metabolism or biosynthesis of lipids that are known effects produced by PPARα activators. Furthermore, the MetaCore analysis demonstrated that the top Gene Ontology Processes by permethrin completely overlapped with those by clofibrate (Kondo et al. Citation2019). While these data are not designated as a specific AE, it strongly suggests that permethrin is a PPARα activator in female mouse liver as permethrin produced similar effects to the known PPARα activator clofibrate.
3.2.5. Increased hepatocellular proliferation (KE 2)
Increased cell proliferation is considered to be integral to the tumorigenic process. Many studies have shown that PPARα activators increase cell proliferation in rodent liver (Cohen et al. Citation2003; Klaunig et al. Citation2003; Lake Citation2009; Cohen and Arnold Citation2011; Corton et al. Citation2014, Citation2018). The observation of increased liver weight, particularly relative weight, is in support of the KE of increased cell proliferation. Previous studies with PPARα activators in the rat and mouse have demonstrated that the increase in relative liver weight after short-term treatment is due to both hepatocyte hypertrophy and hyperplasia (Klaunig et al. Citation2003; Lake Citation2009). A sensitive measure of cell proliferation is to determine the rate of S-phase activity of the cell cycle using 5-bromo-2′-deoxyuridine (BrdU) incorporation as a marker of DNA synthesis (Wood et al. Citation2015). Therefore, BrdU labeling indices were determined in the short-term MOA studies, focusing on time-course, dose-response, recovery and sex differences, PPARα dependency, and investigation of species differences (Kondo et al. Citation2019). In these investigations BrdU was administered in one experiment by intraperitoneal injection at 24 and 2 h before euthanization and in all other studies by osmotic minipumps using 7 day labeling periods. The results of these studies are summarized as follows:
In the experiment using intraperitoneal injection of BrdU, the treatment of female mice with 5000 ppm permethrin for 3-, 7-, 14-days produced a non-statistically significant increase in hepatocyte labeling index values, however, statistically significantly higher labeling index values were observed after the treatment of female mice with 5000 ppm permethrin for 28-days. This apparent increase appears attributable to low labeling index values in the 28-day control group
In the experiments using osmotic minipumps, significant increases in hepatocyte labeling index values were observed in female mice after treatment with 5000 and 10 000 ppm permethrin, 5000 ppm clofibrate, and 500 ppm phenobarbital for 7-days.
The increased labeling index values in female mice observed after treatment with permethrin, clofibrate, and phenobarbital were transient, as increases were only observed after 7-days and not after 14-days of treatment.
Significant increases in hepatocyte labeling index values in female mice were observed after 7-days treatment at permethrin dietary levels of 2500 ppm and higher (see Section 3.3. Liver: concordance of dose-response relationships below).
The treatment of female mice with 10 000 ppm permethrin for 7-days resulted in a significant increase in hepatocyte labeling index values, however, following a recovery period of 5-weeks of no treatment, labeling index values (with liver weight) had returned to control levels.
While the treatment of female wild-type mice with the highest carcinogenicity study dose level of 5000 ppm permethrin for 7-days resulted in a significant increase in hepatocyte labeling index values, no increase was observed in PPARα knockout mice.
No increase in hepatocyte labeling index values was observed in female rats following treatment for 7-days with the highest carcinogenicity study dose level of 2500 ppm permethrin.
As described above, increased BrdU labeling index values were observed only at the early times of treatment (i.e. 3 or 7 days) and returned to control levels or remained marginally higher when permethrin treatment was continued for a longer period, which is consistent with other PPARα (Corton et al. Citation2018) or CAR (Yamada et al. Citation2021) activators. The hepatocyte labeling index is only a measure of the percentage of hepatocyte nuclei undergoing RDS and takes no account of the increase in liver weight and hence the total number of hepatocytes per animal (Lake Citation2009; Cohen Citation2010; Yamada et al. Citation2021). Indeed, the treatment of rats with phenobarbital has been shown to result in a significant increase in the total number of hepatocytes per animal (Carthew et al. Citation1998a), with some increase in the total number of hepatocytes per liver also being observed in rats treated with the PPARα activator gemfibrozil (Carthew et al. Citation1998b). Based on studies with phenobarbital and other compounds, the sustained increase in liver weight in mice treated with permethrin will be associated with an overall increase in the number of hepatocytes per animal. Thus, although hepatocyte labeling index values return to control levels after continued permethrin treatment (Kondo et al. Citation2019), the total number of cell proliferations in treated animals will be enhanced due to the increase in the total number of hepatocytes per animal. The continued increase in total hepatocyte cell proliferation may lead to tumor formation as a result of critical errors being produced during cell replication and/or to the enhancement of spontaneously initiated pre-neoplastic hepatocytes (Schulte-Hermann et al. Citation1983; Wolf et al. Citation2019).
The effect of permethrin on RDS in mouse liver is similar to that of several other PPARα activators, with only very potent compounds (e.g. WY 14 643: [4-chloro-6-(2,3-xylidino)-2-pyrimidyl-thio] acetic acid) producing a sustained increase in labeling index values, albeit at lower rates than after acute exposures in rodent liver (Cohen et al. Citation2003; Klaunig et al. Citation2003; Lake Citation2009; Cohen and Arnold Citation2011; Corton et al. Citation2014, Citation2018). Furthermore, while treatment with permethrin produced an increase in RDS in wild-type mice, no increase was observed in PPARα knockout mice, demonstrating that the presence of PPARα is required for permethrin-induced stimulation of RDS in mouse liver (Kondo et al. Citation2019). Overall, there is direct and strong evidence in support of the KE of increased hepatocyte proliferation in the MOA for permethrin-induced mouse liver tumor formation.
3.2.6. Selective clonal expansion of preneoplastic foci (KE 3)
Altered liver foci have been described as a KE for PPARα activator-induced rodent liver tumor formation (Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018). However, while no data for increased altered liver foci were reported at the end of the 2-year permethrin CD-1 mouse bioassay, altered liver foci are considered to be the precursor lesions for subsequent tumor formation in rodent liver (Farber and Sarma Citation1987; Maronpot et al. Citation1987; Harada et al. Citation1989; Williams Citation1997; Thoolen et al. Citation2012; Corton et al. Citation2014, Citation2018), hence it is considered that such foci would develop before the appearance of liver tumors.
3.2.7. Liver tumor formation (KE 4)
Permethrin has been shown to produce liver tumors in CD-1 female mice (but not male mice) at dose levels examined in the carcinogenicity study (see Section 2. Carcinogenicity data).
3.3. Liver: concordance of dose-response relationships
Investigative studies were performed in female CD-1 mice at doses used in the 2-year bioassay (20, 2500, and 5000 ppm) and at lower (500 ppm) and higher (10 000 ppm) dose levels than those which produced liver tumors (2500 and 5000 ppm) in female mice (). The hepatic effects of permethrin were dose-dependent (Kondo et al. Citation2019).
3.3.1. Enzyme induction
The treatment of female mice with 2500, 5000, and 10 000 ppm permethrin resulted in significant increases in hepatic Acox1 mRNA levels, CYP4A enzyme activity, and Cyp4a10 mRNA levels (). The marked increases in enzyme activity at 2500, 5000, and 10 000 ppm were associated with centrilobular hepatocellular hypertrophy. Induction of hepatic microsomal CYP4A enzyme activity was also observed in the livers of female mice treated with 5000 ppm permethrin for 52 weeks (Barton et al. Citation2000; US.EPA Citation2002).
3.3.2. Cell proliferation
The treatment of female mice with the above bioassay dose levels of 2500, 5000, and 10 000 ppm permethrin resulted in significant increases in BrdU labeling index values (). Significant increases in absolute and relative liver weights were also observed at these permethrin dose levels. Increases in liver weight were also observed in female mice treated with 5000 ppm permethrin for 39, 52, 65, or 78 weeks (Barton et al. Citation2000; US.EPA Citation2002).
3.3.3. Tumor induction
While the treatment of female mice with 20 ppm permethrin did not affect liver tumor incidence, significant increases in liver adenomas and combined adenomas and carcinomas were observed in female mice given 2500 and 5000 ppm permethrin () (Quist et al. Citation2019). In the present study (Quist et al. Citation2019) and another study where female CD-1 mice were given 5000 ppm permethrin (Barton et al. Citation2000), there was no evidence of progression to hepatocellular carcinoma.
Overall, the various parameters described above that are related to the KEs and AEs in the proposed MOA for induction of liver tumors were also observed at or below the dose levels at which these tumors were observed in female mice in the 2-year permethrin carcinogenicity study ().
3.4. Liver: temporal association
Temporality and dose-responses for MOA KEs related to CD-1 female mouse liver tumors are shown in . If a KE (or KEs) is an essential element for carcinogenesis, it must precede the appearance of the tumors. Thus, it is critical in the evaluation of an MOA that effects on KEs and AEs occur before the appearance of tumors, and this is clearly the case with permethrin.
The induction of hepatic fatty acid oxidizing enzyme mRNA levels and/or activities were observed after as little as 1 week of treatment (Kondo et al. Citation2019). Increased liver weight and hepatocellular hypertrophy were shown to appear in short-term studies (3 days, and 1, 2, and 4 weeks) (Kondo et al. Citation2019), and these findings were also observed after longer treatment times (39, 52, 65, and 78 weeks) (Barton et al. Citation2000; US.EPA Citation2002). In addition, treatment with permethrin resulted in a transient stimulation of the rate of RDS, determined as the hepatocyte labeling index (Kondo et al. Citation2019). However, as described above, cell proliferation continues to be increased at longer times due to the increase in the total number of hepatocytes per animal. Like other PPARα activators (Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018), while increased liver weight with enzyme induction and enhanced cell proliferation were observed at an early stage of permethrin administration, the appearance of liver tumors occurred only after chronic administration of permethrin. As altered liver foci are the precursor lesions for subsequent tumor formation in rodent liver (Farber and Sarma Citation1987; Maronpot et al. Citation1987; Harada et al. Citation1989; Williams Citation1997; Thoolen et al. Citation2012; Corton et al. Citation2014, Citation2018), it is considered that such foci would develop before the appearance of liver tumors.
Overall, there is a logical temporal response for all the KEs and AEs for permethrin-induced mouse liver tumor formation, in which all effects on the KEs and AEs precede tumor formation.
3.5. Liver: strength, consistency, and specificity of association of tumor response with KEs
The treatment of female mice with permethrin results in a pleiotropic response. The hepatic effects of permethrin included increased liver weight, morphological evidence of hypertrophy, peroxisome proliferation, stimulation of fatty acid oxidizing enzyme activities and/or mRNA levels, and increased RDS. At dose levels examined in the carcinogenicity study with permethrin, these early KEs and AEs were observed in the livers of female mice after short-term treatment with permethrin, whereas liver tumors were only observed after chronic treatment ().
The relationship of the observed hepatic effects to permethrin treatment with higher dose levels was demonstrated in a recovery study, where the effects of permethrin were shown to be reversible on cessation of treatment (Kondo et al. Citation2019). Female mice were given 10 000 ppm permethrin for 7 days and 7 days followed by 5 weeks of no treatment. While permethrin increased liver weight, produced hepatocyte hypertrophy and peroxisome proliferation, stimulated RDS, and induced lauric acid hydroxylation activity in female mice after 7 days of treatment, no such effects were observed after 5 weeks of recovery (Kondo et al. Citation2019).
For male mice, the 2019 liver PWG (Quist et al. Citation2019) revealed that increases were only in the 500 and 2000 ppm permethrin groups based on p < 0.05 but not at the more appropriate value for statistical significance for common tumors of p < 0.01 for common tumors, such as the liver tumors in this strain (Haseman Citation1983; US.FDA Citation2001; US.EPA Citation2005a; OECD Citation2012). The results of the investigative studies demonstrate that the treatment of male mice with the greater than bioassay dose level of 2500 ppm permethrin produces similar effects on the KEs and AEs for the proposed MOA to those observed in female mice. However, the treatment of male mice with 20 and 500 ppm permethrin for 7 days resulted in no toxicologically significant alterations in these endpoints, with 2500 ppm being above the highest dose used in the chronic bioassay (2000 ppm) (Kondo et al. Citation2019).
Overall, there are strong parallels in the observed dose-response effects of permethrin on the KEs and AEs and the subsequent formation of liver tumors in female mice at permethrin dose levels of 2500 and 5000 ppm (Kondo et al. Citation2019). The effects on KEs and AEs were also observed in males given the above highest bioassay dose level of 2500 ppm permethrin, but no toxicologically significant effects were observed in male mice given 20 and 500 ppm permethrin (Kondo et al. Citation2019). These findings were consistent with the results of the PWG assessment of the male mouse liver tumors based on Haseman’s rule, that 20, 500 and 2000 ppm were all non-tumorigenic dose levels (Quist et al. Citation2019).
3.6. Liver: biological plausibility and coherence
The liver is by far the most common target tissue affected in rodent cancer bioassays (Huff et al. Citation1991; Gold et al. Citation2001). This may be due to the fact that the liver is the major site of metabolic activation of exogenous chemicals (Thoolen et al. Citation2010). Furthermore, the liver is the first organ exposed to the chemical following absorption from the gastrointestinal tract if administered orally, as in the case of the bioassays with permethrin. A recent analysis of 411 unique agrochemicals that have been evaluated for carcinogenicity by US.EPA and the ECHA identified 170 chemicals as non-genotoxic carcinogens, which produced 340 cases of treatment-related tumor formation (Heusinkveld et al. Citation2020). Of 340 cases, the largest fraction of tumors was observed in the liver (107 cases) comprising tumors categorized as hepatocellular adenoma/carcinoma (99 of 107 cases). Liver tumors were in most cases observed in either rats (21 of 107 cases) or mice (65 of 107 cases) although in 21 cases liver tumors were observed in both species, suggesting mice are more susceptible for liver tumor production by chemicals. This finding is most likely related to the marked sensitivity to liver tumor development in many strains of mice, even without treatment with exogenous chemicals (Maronpot Citation2009).
Several MOAs have been identified for the induction of liver tumors in rodents, including DNA reactivity, cytotoxicity, and consequent regenerative proliferation, activation of CAR, activation of PPARα, hormonal perturbation, and porphyria (Yamada et al. Citation2021). The proposed MOA for permethrin is biologically plausible and is coherent with the current understanding of the MOA for liver tumor formation by non-genotoxic PPARα activators. The MOA for PPARα activator-induced rat/mouse liver tumor formation is well defined in the literature and the KEs and AEs identified in the proposed MOA for permethrin-induced mouse liver tumor formation are similar to those described for other PPARα activators (Klaunig et al. Citation2003; Lake Citation2009; Cohen Citation2010; Corton et al. Citation2014, Citation2018). Moreover, the key role of PPARα in liver tumor formation has been demonstrated by studies in PPARα knockout mice (Klaunig et al. Citation2003; Corton et al. Citation2014, Citation2018; Foreman et al. Citation2021a, Citation2021b). While WY 14 643, bezafibrate and the high-affinity human PPARα agonist GW7647 ((2-(4-(2-(1-cyclohexanebutyl)-3-cyclohexylureido)ethyl)phenylthio)-2-methylpropionic acid) produced liver tumors in wild type mice, they did not significantly increase the incidence of liver tumors in PPARα knockout mice (Peters et al. Citation1997; Hays et al. Citation2005; Foreman et al. Citation2021a, Citation2021b). Permethrin also did not produce peroxisome proliferation, did not increase RDS, and did not induce Cyp4a10 and Acox1 mRNA levels in PPARα knockout mice (Kondo et al. Citation2019).
While permethrin produces liver tumors in the female mouse, it does not produce liver tumors in the male and female rats. The apparent species difference in susceptibility to permethrin-induced liver tumor formation appears to be attributable to differences in the hepatic effects of permethrin in the mouse and rat. In agreement with previous studies (Heder et al. Citation2001), permethrin was found to produce CYP2B induction in primary rat hepatocyte cultures (Kondo et al. Citation2020). Moreover, in a 7-day in vivo study the treatment of female rats with 2500 ppm permethrin resulted in increased liver weight, together with significant increases in Cyp2b1/2 mRNA levels and hepatic CYP2B enzyme activity (Kondo et al. Citation2019). These results are consistent with previous findings after long-term treatment of rats with permethrin where increased liver weight was associated with hypertrophy, increased smooth endoplasmic reticulum, and CYP enzyme induction (Ishmael and Lithfield Citation1988). However, the highest permethrin dose level of 2500 ppm did not result in liver tumor formation in this chronic rat study (Ishmael and Lithfield Citation1988). In the 7-day rat study treatment with 2500 ppm permethrin did not induce Cyp4a1 mRNA levels and CYP4A enzyme activity and did not stimulate RDS (Kondo et al. Citation2019). These findings demonstrate that permethrin is not a PPARα activator in the rat and while permethrin has some CAR activating activity, this does not result in liver tumor formation in rats.
3.7. Liver: other MOAs
As described above, there is strong evidence in support of a mitogenic effect via PPARα activation as the MOA for permethrin-induced female mouse liver tumor formation. Other known MOAs for rodent liver carcinogenesis, including genotoxicity, other receptors, cytotoxicity, etc., have been assessed from the data available from the current MOA studies and previous investigations performed with permethrin.
3.7.1. Genotoxicity as an MOA
Liver tumors can be produced in rodents by both genotoxic and non-genotoxic MOAs (Williams Citation1997; Cohen and Arnold Citation2011). Permethrin was tested in a standard battery of genotoxicity and mutagenicity tests in vitro and in vivo, and the findings have been summarized (US.EPA Citation2002). There was no indication of gene mutation either in the bacterial reverse mutation and mammalian lymphoma assays. Permethrin is also negative for clastogenicity in a mouse bone marrow micronucleus assay and does not cause unscheduled DNA synthesis in primary rat hepatocytes. There is no evidence of increased dominant lethal mutations in the germinal cells of male mice. Two published studies suggested that permethrin has clastogenic activity in cultured human lymphocytes and Chinese hamster ovary cells, but only in the absence of S9 activation and only at cytotoxic doses (Barrueco et al. Citation1992, Citation1994). Therefore, US.EPA concluded that permethrin has no genotoxic potential (US.EPA Citation2002). Recently, to investigate the genotoxic potential of permethrin in more detail, two in vivo studies were conducted on female mice to assess DNA damage in possible tumor target organs by the comet assay and micronucleus test (Matsuyama et al. Citation2018). For this, mice were administered permethrin at doses of 150, 300, or 600 mg/kg/day by gavage for 2 days, and their liver, lung, glandular stomach, peripheral blood, and bone marrow cells were examined for DNA damage by combined comet and micronucleus assays. There were no significant increases in percentage tail DNA in the organs examined and no increase in micronuclei in peripheral blood by flow cytometry. Taken together, these findings provide additional evidence that permethrin has no genotoxic, aneugenic, or clastogenic potential (Matsuyama et al. Citation2018). Thus, it is highly likely that the MOA for permethrin-induced liver tumors in mice is attributable to a non-genotoxic MOA and not via direct effects on DNA (Kondo et al. Citation2019).
3.7.2. CAR activation as an MOA
The MOA for liver tumor formation through CAR activation is well-established (Lake Citation2009, Citation2018; Elcombe et al. Citation2014; Yamada Citation2018; Yamada et al. Citation2021). KEs for the MOA for liver tumor formation by CAR activators include activation of CAR, altered gene expression specific to CAR activation, increased cell proliferation, and liver tumor formation; whereas AEs include liver hypertrophy and CYP2B induction (Elcombe et al. Citation2014). The treatment of female mice with 5000 ppm permethrin for periods of 3, 7, 14, and 28 days resulted in significant increases in hepatic CYP4A marker enzyme activity (lauric acid hydroxylation) expressed per unit of liver S9 protein, but had no significant effect on 7-pentoxyresorufin O-depentylase activity, which is a marker of CAR activation (Kondo et al. Citation2019). In another study, the treatment of female mice with 5000 and 10 000 ppm permethrin for 14 days resulted in significant increases in hepatic lauric acid hydroxylation activity and Cyp4a10 mRNA levels, whereas hepatic 7-pentoxyresorufin O-depentylase activity was unaffected and only small increases were observed in hepatic Cyp2b10 mRNA levels (Kondo et al. Citation2019). The treatment of female mice with 2500–10 000 ppm permethrin for 7 days resulted in marked statistically significant increases in hepatic Cyp4a10 mRNA levels, whereas only treatment with 10 000 ppm permethrin resulted in a small significant increase in hepatic Cyp2b10 mRNA levels (Kondo et al. Citation2019). Thus, the induction of hepatic lauric acid hydroxylation activity was much more marked than the induction of 7-pentoxyresorufin O-depentylase activity. Furthermore, global gene expression analysis demonstrated that the profile of genes altered by permethrin treatment in female mouse liver was generally similar to the profile of genes altered by the PPARα agonist clofibrate, rather than to the profile of genes altered by the CAR activator phenobarbital (Kondo et al. Citation2019). Overall, the available data demonstrate that the MOA for permethrin-induced female mouse liver tumor formation occurs through the activation of PPARα rather than through the activation of CAR.
3.7.3. Other MOAs
Gene expression profiling analysis of the livers of female mice treated with permethrin for 7 or 14 days demonstrated that permethrin did not induce significant alterations in either aryl hydrocarbon receptor (AhR) or pregnane X receptor (PXR) signaling (Kondo et al. Citation2019). To confirm the lack of effect of permethrin on AhR and PXR in female mouse liver, Cyp1a2 and Cyp3a11 mRNA levels were determined by quantitative real-time PCR in the livers of female mice treated with permethrin at 5000 and 10 000 ppm for 14 days (Kondo et al. Citation2019). This analysis demonstrated that permethrin did not affect the mRNA expression levels of Cyp1a2 or Cyp3a11 in female mouse liver, supporting previous evidence that permethrin has no effects on either AhR or PXR signaling. Utilizing histopathology and blood biochemistry there was no evidence of hepatocellular cytotoxicity (necrosis) following treatment with permethrin in several studies (Ishmael and Lithfield Citation1988; Kondo et al. Citation2019), or any evidence of sex hormone perturbations (Kunimatsu et al. Citation2002). In addition, there was no histologic evidence of iron accumulation suggesting that permethrin is not a porphyrogenic agent nor acting by the accumulation of iron. In addition, there is no evidence of an effect on immune or hematopoietic tissues in short or long-term studies (JMPR Citation1999), excluding immunosuppression as an MOA.
Overall, other known MOAs for rodent liver carcinogenesis are excluded and thus it is considered that the hepatic effects of permethrin in the female mouse and subsequent liver tumor formation are mediated by activation of the PPARα (Kondo et al. Citation2019).
3.8. Uncertainties, inconsistencies, and data gaps
While the effect on organelle proliferation (AE 1), determined by ultrastructural examination, was only examined at one high dose level (10 000 ppm), significant increases in markers of peroxisomal and microsomal fatty acid oxidizing enzymes (AE 2), namely Acox 1 and Cyp4a10 mRNA levels and CYP4A enzyme activity, were observed at the carcinogenic dose levels of 2500 and 5000 ppm, as well as at 10 000 ppm permethrin. While no morphologic data were obtained for the effect of permethrin on organelle proliferation at the carcinogenic dose levels of 2500 and 5000 ppm, this is not considered to represent a data gap as other studies have demonstrated good correlations between effects on organelle proliferation and fatty acid oxidizing enzyme activities (Lake Citation2009). As described above, permethrin increased hepatocyte proliferation at tumorigenic dose levels in female mice (KE 2). Previous studies with PPARα activators in rodent liver have demonstrated effects on both hepatocyte proliferation and apoptosis (Corton et al. Citation2014, Citation2018). However, the present studies just focused on effects on hepatocyte proliferation, as it is very difficult to determine effects on low levels of apoptosis in in vivo studies (Corton et al. Citation2018). It is considered that this does not constitute a significant data gap for the proposed MOA for permethrin-induced mouse liver tumor formation. Concerning the selective clonal expansion of preneoplastic foci (KE 3), increased incidence of foci has not been reported and the time course of the appearance of altered liver foci was not investigated for permethrin. As altered liver foci are the precursor lesions for subsequent tumor formation in rodent liver (Farber and Sarma Citation1987; Maronpot et al. Citation1987; Harada et al. Citation1989; Williams Citation1997; Thoolen et al. Citation2012; Corton et al. Citation2014, Citation2018), it is considered that such foci would develop before the appearance of liver tumors. Thus, this does not constitute a significant data gap.
3.9. Liver: assessment of postulated MOA
As described above, the KEs and AEs in the proposed MOA for permethrin-induced female mouse liver tumor formation have been identified. Because permethrin produces similar hepatic effects to other PPARα activators for which much literature data are available (Klaunig et al. Citation2003; Lake Citation2009; Cohen Citation2010; Corton et al. Citation2014, Citation2018; Foreman et al. Citation2021a, Citation2021b), it is considered that there is a high level of confidence in the proposed MOA for permethrin-induced mouse liver tumor formation.
Permethrin has been reported to produce liver tumors in female CD-1 mice at dietary levels of 2500 and 5000 ppm but not in male CD-1 mice at dietary levels of 20, 500, and 2000 ppm by the 2019 liver PWG (see Section 2. Carcinogenicity data). Examination of the data obtained in the investigative studies described in this review demonstrates that in female mice the effects on the KEs and AEs for permethrin-induced mouse liver tumor formation are clearly observed at permethrin dose levels of 2500 and 5000 ppm (). The treatment of male mice with 2500 ppm permethrin, which was higher than the highest dose level (2000 ppm) in the carcinogenicity study, resulted in similar effects on the KEs and AEs to those observed in female mice after treatment with 2500 and 5000 ppm permethrin (Kondo et al. Citation2019). These findings demonstrate that treatment with 2500 and 5000 ppm permethrin results in marked PPARα activation in female mice. However, in male mice toxicologically significant effects on markers of PPARα activation were only observed at the greater than the highest bioassay dose level of 2500 ppm (2000 ppm was determined to be the maximum tolerated dose (MTD) in the male mouse carcinogenicity study).
Table 5. Comparison of key and associative events for permethrin-induced liver tumor formation in mice, rats and humans.
As described in Section 2. Carcinogenicity data in the permethrin CD-1 bioassay, based on the findings by the 2019 liver PWG (Quist et al. Citation2019), liver adenoma formation in male mice did not exhibit a clear dose-response relationship (the incidence was higher at 500 than 2000 ppm), whereas a dose-dependent increase in liver adenomas, with 20 ppm being a no adverse effect level, was observed in female mice. An important issue with the male mice used in this bioassay was the 17% incidence of liver carcinoma observed in the control group, which is much greater than the 0–12% historical control range for the performing laboratory (Cal.EPA Citation1994). The high control incidence of carcinoma (17%) suggests that the male CD-1 mice used in the permethrin bioassay had a high susceptibility to liver tumor formation (the highest incidence of the historical background incidence was 12%), namely this particular batch of mice were unusually sensitive to the spontaneous development of liver tumors. The lack of a clear dose-response relationship for male mice for liver adenoma formation and also for the combined incidence of liver adenomas and carcinomas suggests that tumor formation was not related to treatment with permethrin. Indeed, the treatment of male mice with 20 and 500 ppm permethrin had no effect on the KEs measured and no toxicologically significant effects on the AEs measured, suggesting that the non-dose-dependent increase in liver tumors was observed in the bioassay were not related to permethrin treatment. Furthermore, based on the more appropriate evaluation using a significance value of p < 0.01 for common tumors (Haseman Citation1983; US.FDA Citation2001; US.EPA Citation2005a; OECD Citation2012), such as the liver tumors in this mouse strain, the higher incidence of liver tumors in male mice given permethrin 500 and 2000 ppm in this study are not considered to be statistically significant increases and therefore are not treatment-related (Quist et al. Citation2019).
Overall, it is considered that a plausible MOA has been established for permethrin-induced liver tumor formation in female mice given 2500 and 5000 ppm permethrin. No significant increases in liver tumor formation were observed in female mice given 20 ppm permethrin or in male mice given 20–2000 ppm permethrin.
3.10. Liver: human relevance of the proposed MOA
The IPCS Human Relevance Framework, which was developed from the ILSI Risk Sciences Institute (RSI) “Human Relevance Framework” (Meek et al. Citation2003) and modified by the IPCS Cancer Working Group (Boobis et al. Citation2006), presents a four-part approach to addressing a series of three questions and leading to a documented, logical conclusion regarding the human relevance of the MOA underlying animal tumors.
3.10.1. Question 1. Is the weight of evidence sufficient to establish an MOA in animals?
As described in detail above, there is clear evidence that permethrin activates PPARα in female mice at bioassay dose levels of 2500 and 5000 ppm, which results in a pleiotropic response including increased liver weight, centrilobular hepatocellular hypertrophy, peroxisome proliferation, increased peroxisomal/microsomal fatty acid oxidizing enzyme activities and/or mRNA levels, and increased RDS. The KEs and AEs for permethrin-induced female mouse liver tumor formation are similar to those described for other PPARα activators (Klaunig et al. Citation2003; Lake Citation2009; Cohen Citation2010; Corton et al. Citation2014, Citation2018). Hence the answer to question 1 is yes.
3.10.2. Question 2. Can human relevance of the MOA be reasonably excluded on the basis of fundamental, qualitative differences in KEs between experimental animals and humans?
Examination of the relevance of the MOA to humans requires not only information regarding the chemical of concern, but also an evaluation of the MOA for similarly acting compounds (Meek et al. Citation2003; Cohen et al. Citation2004). Thus, in this examination of permethrin with regard to human relevance of the animal data, reference can also be made to the evaluation of the MOA for other PPARα activators. The MOA of such compounds involves PPARα activation resulting in peroxisome proliferation, increased peroxisomal/microsomal fatty acid oxidizing enzymes, and increased hepatocellular proliferation; with prolonged administration resulting in altered liver foci and ultimately in liver tumor formation in rodents (Klaunig et al. Citation2003; Lake Citation2009; Cohen Citation2010; Corton et al. Citation2014, Citation2018). In analyzing the relevance of the animal MOA data to humans, a concordance table has been suggested as being of considerable value (Meek et al. Citation2003). This includes not only the data from permethrin in the mouse but also a similar listing for the rat and for the likely findings in humans ().
As described above, the sequence of KEs and AEs in the MOA for permethrin-induced mouse liver tumor formation include activation of PPARα (KE 1) evidenced by peroxisome proliferation (AE 1), the induction of peroxisomal/microsomal fatty acid oxidizing enzymes (AE 2); increased hepatocellular proliferation (KE 2); and ultimately liver tumor formation (KE 4). Effects on all these KEs and AEs have been demonstrated in female mice ().
Several studies have shown that PPARα is present in the human liver and that this receptor can be activated by drugs and other compounds (Klaunig et al. Citation2003; Corton et al. Citation2014, Citation2018). Hence, it is possible that at high doses permethrin could activate PPARα in the human liver. As no data are available for the hepatic effects of permethrin in humans, valuable information can be derived from in vitro studies employing cultured human hepatocytes. Investigations have also been performed with some other PPARα activators and species differences examined by performing studies with mouse, rat, and human hepatocytes (Kondo et al. Citation2020). Treatment with clofibrate, WY14643 and GW7647, included as positive controls, increased Cyp4a1, Cyp4a10, CYP4A11 mRNA levels in rat, mouse, and human hepatocytes, respectively, demonstrating that under the experimental conditions employed in these studies that the cultured hepatocyte preparations maintain enough PPARα signaling function to be able to respond to treatment with CYP4A enzyme inducers. Treatment with 5–1500 µM permethrin increased Cyp4a10 mRNA levels in female CD-1 mouse hepatocytes and a single experiment 1500 µM permethrin increased CYP4A11 mRNA levels in human hepatocytes. The treatment of human hepatocytes with permethrin also resulted in some induction of CYP2B6 mRNA levels. Permethrin at concentrations up to 1500 µM did not increase Cyp4a1 mRNA levels in female Wistar rat hepatocytes but did increase Cyp2b1/2 mRNA levels (Kondo et al. Citation2020).
The PPARα activator-mediated peroxisome proliferation response and associated regulation of peroxisomal genes/proteins and increased peroxisomal fatty acid β-oxidation activity are either absent or much reduced in the human liver. Evidence for this comes from studies with cultured human hepatocytes where little or no effect is observed on markers, such as ACOX1 mRNA, acyl-CoA oxidase protein, and acyl-CoA oxidase and/or palmitoyl-CoA oxidation activities (Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018). Moreover, studies in human subjects receiving hypolipidemic drugs (which are rodent PPARα activators), such as clofibrate, ciprofibrate, fenofibrate, and gemfibrozil have demonstrated that unlike rodents these compounds only produce small effects on hepatocellular peroxisome content in the human liver (Doull et al. Citation1999; Klaunig et al. Citation2003; Corton et al. Citation2014, Citation2018).
While the potential for compounds to increase cell proliferation in rodent liver can be examined by performing in vivo studies as described above, compounds can also be tested for mitogenic potential in vitro by conducting studies using primary hepatocyte cultures. For example, several compounds that can activate either PPARα or CAR in rodent liver can induce RDS in cultured mouse and rat hepatocytes (Corton et al. Citation2014, Citation2018; Yamada et al. Citation2021). As the stimulation of RDS is a critical KE in the proposed MOA for permethrin-induced mouse liver tumor formation (Kondo et al. Citation2019), the effect of permethrin on RDS has been studied in cultured human, mouse, and rat hepatocytes (Kondo et al. Citation2020). RDS was determined by incorporation of BrdU over the last 24 h of a 48 h treatment period. To serve as positive controls for induction of RDS, mouse, rat, and human hepatocytes were treated with epidermal growth factor (EGF), hepatocyte growth factor (HGF), phenobarbital, clofibrate, and WY-14643 (mouse only). Phenobarbital is known to induce RDS in mouse and rat but not in human hepatocytes, whereas EGF and HGF are known mitogenic agents which have previously been shown to increase RDS in cultured rodent and human hepatocytes (Yamada et al. Citation2021).
Using female CD-1 mouse primary hepatocyte cultures, statistically significant increases in RDS were observed after treatment with either 1 ng/ml EGF or 10 ng/ml HGF (Kondo et al. Citation2020). RDS was also increased in mouse hepatocytes by treatment with 500 µM phenobarbital, 10 µM WY-14643, and 25–500 µM permethrin. In contrast, while treatment of human hepatocytes with 10–100 ng/ml EGF and 50 and 100 ng/ml HGF resulted in concentration-dependent increases in RDS, the treatment of human hepatocytes with 5–1500 µM permethrin, 5–500 µM phenobarbital, or 5–500 µM clofibrate did not increase RDS. These results demonstrate that while permethrin induced Cyp4a10 mRNA levels in mouse hepatocytes and CYP4A11 mRNA levels in human hepatocytes, permethrin only induced RDS in mouse and not in human hepatocytes (Kondo et al. Citation2020). The results demonstrate a good agreement between the observed effects of permethrin in the in vivo and in vitro studies conducted in female mice and rats (Kondo et al. Citation2019, Citation2020). The results indicate that the in vitro cultured hepatocyte system is reliable for the prediction of the effects of permethrin in the human liver. A KE in the proposed MOA for permethrin-induced female mouse liver tumor formation is the stimulation of cell proliferation (Kondo et al. Citation2019).
summarizes the available female mouse, rat, and human data for the KEs and AEs in the proposed MOA for permethrin-induced female mouse liver tumor formation. As described above, high doses of permethrin could activate PPARα and possibly induce CYP4A enzymes. However, a key species difference is that while permethrin is clearly a mitogenic agent in female mouse liver, as demonstrated by both in vivo and in vitro studies, permethrin does not stimulate RDS in cultured human hepatocytes. Overall, while some of the KEs (e.g. PPARα activation) and AEs (e.g. CYP4A enzyme induction) in the proposed MOA for permethrin-induced female mouse liver tumor formation could occur in the human liver at high permethrin doses, the available data demonstrate that human hepatocytes are refractory to the mitogenic effects of permethrin (Kondo et al. Citation2020). It is therefore concluded that the MOA for permethrin-induced female mouse liver tumor formation is not plausible for humans.
Support for this conclusion comes from studies in the literature with other PPARα activators and from investigations with transgenic mice. There is generally consensus that species differences exist in the response to ligand activation of mouse vs. human PPARα (Corton et al. Citation2014, Citation2018). While levels of PPARα are lower in the human liver than in rodent liver, there are also differences in the range of genes affected by PPARα activators in that genes involved in cell proliferation are not activated in the human liver (Gonzalez and Shah Citation2008; McMullen et al. Citation2020). This was demonstrated in studies with wild-type (i.e. normal) and transgenic mice which contain human but not mouse PPARα (Cheung et al. Citation2004; Yang et al. Citation2008; Tang et al. Citation2018). Following treatment with PPARα activators, an induction of fatty acid metabolism genes and a decrease in serum triglyceride levels were observed in both wild-type and the mice expressing human PPARα. However, unlike wild-type mice, there was no induction of hepatic cell cycle genes and no stimulation of RDS in the PPARα-humanized mice (Cheung et al. Citation2004; Yang et al. Citation2008). Recently, as treatment periods in these studies were <1 year, the effect of long-term administration of a potent, high-affinity human PPARα agonist (GW7647) on hepatocarcinogenesis was investigated in wild-type, Pparα-null, or PPARA-humanized mice (Foreman et al. Citation2021a, Citation2021b). Two studies were performed with treatment being commenced on postnatal day 3 in one study and to adult mice in the other study. In wild-type mice, treatment with GW7647 increased relative liver weight, produced liver toxicity, increased the expression of known PPARα target genes, and resulted in liver tumor formation. In contrast, these effects were essentially absent or greatly diminished in Pparα-null mice and PPARA-humanized mice. While hepatocarcinogenesis was observed in both genotypes, the tumor incidences were not statistically significant from controls and appear to be related to the liver toxicity observed in these studies. Previous studies have demonstrated that Pparα-null mice have fatty livers and are more susceptible to liver toxicity on chemical exposure (Corton et al. Citation2018). The authors state that Ppara-null and PPARA-humanized mice are valuable tools for examining species differences in the mechanisms of PPARα-induced hepatocarcinogenesis, but background levels of liver cancer observed in aged Pparα-null and PPARA-humanized mice must be considered when interpreting results from studies that use these models (Foreman et al. Citation2021a, Citation2021b). In addition, as has been previously demonstrated in a genetically humanized CAR/PXR mouse model, caution is needed in the interpretation of data from genetically humanized mouse models, as the human genes operate in a mouse hepatocyte environment with the downstream genes being of the mouse, not human origin (Yamada Citation2018, Citation2021; Yamada et al. Citation2021).
Apart from the genetically PPARA-humanized mice, studies with non-human primates have demonstrated that rodent PPARα activators produce only small effects on markers of PPARα activation and do not appear to have any effect on hepatocyte proliferation (Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018). Furthermore, similar findings were also observed in the humanized chimeric mouse liver model (Tateno et al. Citation2015; de la Rosa Rodriguez et al. Citation2018). The usefulness of the humanized chimeric mouse liver model has been previously reviewed (Yamada Citation2018, Citation2021; Tateno and Kojima Citation2020; Yamada et al. Citation2021).
IARC evaluated the hypolipidemic drugs clofibrate (IARC Citation1996a) and gemfibrozil (IARC Citation1996b) and concluded that the mechanism responsible for the increased incidence of liver tumors in rodents with both compounds would not operate in humans. Human epidemiological data are also available for both clofibrate and gemfibrozil. The WHO trial on clofibrate comprised 208 000 man-years of observation. Furthermore, a meta-analysis of the results from six clinical trials on clofibrate also found no excess cancer mortality (IARC Citation1996a; Klaunig et al. Citation2003). Bonovas et al. carried out a meta-analysis of 17 randomized controlled trials and concluded that the four hypolipidemic drugs studied, namely clofibrate, bezafibrate, fenofibrate, and gemfibrozil, have a neutral effect on cancer outcomes, which include those in the liver (Bonovas et al. Citation2012). These findings support the conclusion that the MOA for hepatocarcinogenesis via PPARα-activation in rodents is not likely to occur in humans (Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018; Foreman et al. Citation2021a, Citation2021b).
The data obtained in the MOA studies with permethrin (Kondo et al. Citation2019, Citation2020) are in agreement with much literature data on other PPARα activators. Namely, studies performed in several laboratories have demonstrated that while PPARα activators, including some hypolipidemic drugs, can stimulate RDS in cultured rodent hepatocytes, human hepatocytes are refractory to the effects of such compounds (Klaunig et al. Citation2003; Lake Citation2009; Corton et al. Citation2014, Citation2018). Overall, for permethrin the key species difference between mice and humans is that human hepatocytes are refractory to the mitogenic effect of this compound. Thus, the MOA for permethrin-induced female mouse liver tumor formation is considered to be qualitatively not plausible for humans. This conclusion is similar to that of non-genotoxic CAR activators which produce liver tumors in rodents by a mitogenic MOA and also exhibit this species difference in stimulating RDS in rodent but not in human hepatocytes (Lake Citation2009, Citation2018; Elcombe et al. Citation2014; Yamada Citation2018, Citation2021; Yamada et al. Citation2021).
3.10.3. Question 3. Can human relevance of the MOA be reasonably excluded on the basis of quantitative differences in either kinetic or dynamic factors between experimental animals and humans?
As an examination of the available data demonstrates that the MOA for permethrin-induced female mouse liver tumor formation is qualitatively not plausible for humans, there is no need to consider quantitative differences in either kinetic or dynamic factors between mice and humans.
3.11. Liver conclusion: statement of confidence, analysis, and implications
Based on the data described above, there is strong evidence to support a plausible MOA for permethrin-induced liver tumor formation in female mice. This MOA involves activation of the PPARα (KE 1) evidenced by observable morphological changes of hepatocytes by both light microscopic and ultrastructural examinations and increased metabolic enzyme (CYP4A and Acyl-CoA) induction in hepatocytes (AEs 1 and 2), followed by a mitogenic effect resulting in hepatocyte proliferation (Key event 2), the development of altered hepatic foci (KE 3), and ultimately liver tumors (KE 4) (Kondo et al. Citation2019). In studies with cultured hepatocytes, permethrin was found to induce CYP4A enzyme mRNA levels in both mouse and at high concentrations in human hepatocytes. However, in marked contrast to the effect in mouse hepatocytes, permethrin did not stimulate RDS in human hepatocytes (Kondo et al. Citation2020). These data demonstrate an important species difference in that a critical KE in the MOA for mouse liver tumor formation does not operate in human hepatocytes. The data strongly support the conclusion that this MOA would be qualitatively not plausible for humans following exposure to permethrin. The proposed MOA is similar to that established for some other PPARα activators (Klaunig et al. Citation2003; Lake Citation2009; Cohen Citation2010; Corton et al. Citation2014, Citation2018), and epidemiological data are available for both permethrin (Rusiecki et al. Citation2009; Boffetta and Desai Citation2018) and other PPARα activators (Doull et al. Citation1999; Bonovas et al. Citation2012; Corton et al. Citation2014, Citation2018) that demonstrate that these compounds do not increase cancer mortality (see Section 5. Human exposure levels, human risk, and epidemiology of permethrin). Other possible MOAs for mouse liver tumor formation have been examined and excluded. It is therefore concluded that permethrin does not provide a liver tumorigenic hazard for humans.
4. Analysis for tumorigenic MOA in lung
4.1. Lung: postulated MOA for the induction of lung tumors in female mice
Club cells are known to be widely located from trachea through bronchiole to bronchiolar alveolar duct junction (Wong et al. Citation2009). In contrast to humans where Club cells are located in various sites (the trachea, bronchi, respiratory bronchiolar epithelium, and bronchiolo-alveolar epithelium), Club cells were reported to be located predominantly in the epithelium of the bronchiolo-alveoli in the mouse (Wong et al. Citation2009; Wang et al. Citation2012; Plopper and Hyde Citation2015). Functions of Club cells include detoxification of xenobiotics, inflammation control, clearance of environmental agents, and proliferation/differentiation to maintain the ciliated and non-ciliated pulmonary cell populations, effectively acting as a stem cell population. Club cells contain high levels of CYP enzymes that can contribute to the metabolism of a variety of substances (Reynolds and Malkinson Citation2010; Wang et al. Citation2012). Interestingly, the specific enrichment of CYP2F2 in mouse Club cells has also been demonstrated as a molecular initiating event accounting for mouse lung-specific metabolism of toxicants to mitogenic and/or cytotoxic metabolites that ultimately drive mouse lung-specific tumors (Cohen et al. Citation2020). Furthermore, transcriptomic MOA investigations for some substances reveal a likely convergence to later-in-life disruption of circadian control of Club cell biological processes as a plausible driver of mouse lung cancer outcomes (Cohen et al. Citation2020). Since Club cells are located very close to the alveolar region, they migrate to the alveolar region in case of severe lung injury and have been reported to be involved in alveolar bronchiolization (Park et al. Citation2021). These findings support the possibility that Club cells may be involved in mouse bronchiolo-alveolar adenoma formation by permethrin.
The proposed MOA for permethrin-induced female mouse lung tumor formation is postulated to involve interaction with Club cells (KE 1); increased Club cell proliferation (KE 2); increased Club cell hyperplasia (KE 3); and increased lung adenoma (KE 4) (Yamada et al. Citation2017; Ogata et al. Citation2021). AEs include interaction with Club cells (AE 1) and enzyme induction in Club cells (AE 2). Other known MOAs for rodent lung carcinogenesis (Cohen et al. Citation2020) have been compared with the data obtained from studies performed with permethrin. The proposed MOA for permethrin operates in female mice, but not in male mice or in male or female rats.
4.2. Lung: postulated KE in experimental animals
As described in the liver section, an MOA for chemically-induced tumor formation comprises a biological sequence of events leading to tumorigenesis (Boobis et al. Citation2006). Here the component events for the MOA for permethrin-induced lung tumor formation in female mice are defined as either KEs or as AEs as described below.
4.2.1. Interaction with Club cells (KE 1; AE 1)
Light microscopic examination of lung sections from female CD-1 mice given 5000 ppm permethrin for 3, 7, 14, and 28 days and 10 000 ppm permethrin for 7 and 14 days did not reveal any abnormalities. However, ultrastructural examination (EM) of lung sections by electron microscopy from female mice given 5000 ppm permethrin revealed morphological changes in Club cells (but not other types of cells), including dilatation of the smooth endoplasmic reticulum (SER) after 3 days and proliferation of the SER after 3, 7, 14, and 28 days (Yamada et al. Citation2017). Permethrin treatment also resulted in increased membrane blebbing, increased numbers of elongated mitochondria, and decreased secretory granules in Club cells (Yamada et al. Citation2017). In another study, female mice were given 5000 and 10 000 ppm permethrin for 7 and 14 days, and similar findings were observed. To determine dose-response, ultrastructural examination of Club cells were examined in two female animals from each dose group given 20, 500, 2500, 5000, and 10 000 ppm permethrin for 7 days. Increased mitochondria elongation or irregular shape and decreased secretory granules were observed at 2500 ppm and higher. Moreover, no change in mitochondria but slightly decreased secretory granules were observed in one female mouse has given 500 ppm permethrin, this dose level not being examined in the carcinogenicity study (Yamada et al. Citation2017).
Overall, EM evaluation of lung samples of female mice treated with permethrin for 7 days demonstrated that (1) permethrin induced morphological alterations only in Club cells, (2) the findings in Club cells were detected only by EM, not by light microscopy, without evidence of cytotoxicity, (3) the effects observed were associated with acceleration of xenobiotic metabolism and oxidative phosphorylation (Kitamura et al. Citation1987; Van Winkle et al. Citation1999), and (4) the EM findings were consistent with the global gene expression analysis of female mouse lung in permethrin 5000 and 10 000 ppm groups (i.e. altered gene expressions in metabolism-related processes) (Yamada et al. Citation2017). The ultrastructural changes in Club cells of female mice were observed at tumorigenic dose levels (2500 and 5000 ppm) and higher (10 000 ppm), but were not observed at the non-tumorigenic 20 ppm dose level (Yamada et al. Citation2017). In contrast to female mice, the treatment of male mice with a greater bioassay dose level of 5000 ppm permethrin for 7 days had little effect on Club cell ultrastructural morphology (Yamada et al. Citation2017).
Cypermethrin (a synthetic pyrethroid insecticide structurally related to permethrin) (US.EPA Citation2003) and isoniazid (an anti-tuberculosis drug) (Peacock and Peacock Citation1966; Strupp et al. Citation2016), which also produce lung tumors in mice, demonstrated similar EM findings and mitogenic effects on Club cells to those produced by permethrin (Yamada et al. Citation2017). Therefore, it is likely that these morphological effects on Club cells are chemically-induced precursor events indicative of an interaction with the cells following systemic chemical exposure (Cohen et al. Citation2020).
In the permethrin MOA studies, as EM was conducted only in a small number of animals (two mice per group), dose-response analysis may not be completely established. Thus, the morphological changes were defined as an AE for a biomarker of KE 1 (Interaction with Club cells), namely, AE 1. The morphologic changes were similar to those described for other mitogenic mouse lung tumorigens (Cohen et al. Citation2020).
4.2.2. Enzyme induction in Club cells (AE 2)
We examined the dose-response of altered gene expression to further support the EM findings (Ogata et al. Citation2021). As described above, in the lungs of female mice treated with permethrin for 7 days, the previous gene expression analysis at high dose levels (5000 and 10 000 ppm) demonstrated that permethrin treatment altered “metabolism-related process signaling” (Yamada et al. Citation2017). Using lung samples from the 7-day dose-response study (dose levels were 0, 20, 500, 2500, and 5000 ppm) and from the 7-day higher dose study (dose levels were 0, 5000, and 10 000 ppm; data previously reported in Yamada et al. Citation2017), additional analysis was conducted focusing on two points; (1) dose-response and (2) reproducibility. This new analysis demonstrated that permethrin at tumorigenic dose levels (2500 and 5000 ppm) affected some metabolism or detoxification processes in mouse lungs (Ogata et al. Citation2021), consistent with the previous findings (Yamada et al. Citation2017).
Three overlapping up-regulated genes (i.e. Aldehyde dehydrogenase 3A1 [Aldh3a1], oxidative stress-induced growth inhibitor 1 [Osgin1], and thioredoxin reductase 1 [Txnrd1]) were identified as genes affected by permethrin with dose-dependency and reproducibility, while there were no overlapping genes for down-regulation (Ogata et al. Citation2021). Subsequent PCR analysis demonstrated that expression of Aldh3a1, Osgin1, and Txnrd1 mRNA was significantly increased in female mice at the tumorigenic dose levels of 2500 and 5000 ppm, but not at the non-tumorigenic 20 ppm dose level. No increases in these three genes were observed in male mice at permethrin doses up to 2500 ppm (a greater than bioassay dose level) and only small increases in Osgn1 and Txnrd1 mRNA levels, but not Aldh3a1 mRNA level, were observed in female rats given 2500 ppm permethrin (the highest bioassay dose level) (Ogata et al. Citation2021). The difference in effects on Aldh3a1 mRNA induction between female mice and male mice and female rats suggests that the increase in Aldh3a1 mRNA may be specifically related to lung tumor formation in female mice.
Aldehyde dehydrogenase enzymes (ALDH) are phase I xenobiotic-metabolizing enzymes that are involved in the metabolism of both endogenous and exogenous aldehydes (Laskar and Younus Citation2019). ALDH3A1 is a cytosolic enzyme that is highly expressed in lungs and some other tissues (Muzio et al. Citation2012; Laskar and Younus Citation2019). Microarray analysis of lung tissues from nine inbred mouse strains revealed that Aldh3a1 was highly expressed in mouse strains that are susceptible to pulmonary adenoma formation (Stearns et al. Citation2012). High levels of ALDH3A1 expression have been reported in some mouse and human lung tumors (Patel et al. Citation2008; Muzio et al. Citation2012; Stearns et al. Citation2012; Pors and Moreb Citation2014). Several investigations have linked ALDH3A1 expression and enzyme activity with cell proliferation (Muzio et al. Citation2012; Pors and Moreb Citation2014; Laskar and Younus Citation2019). For example, the content and activity of ALDH3A1 were directly correlated with cell proliferation based on findings with specific inhibitors, antisense oligonucleotides, and knockdown of ALDH3A1 (Muzio et al. Citation2012). However, the precise mechanism(s) through which ALDH3A1 influences cell proliferation are not yet fully understood.
Club cell secretory protein (CCSP) is specifically localized in Club cells whereas the CYP enzyme CYP2F2 is known to be mainly if not exclusively localized in Club cells (Buckpitt et al. Citation1995; Singh and Katyal Citation1997; Shultz et al. Citation2001; Wang et al. Citation2012; Ahmad and Ahmad Citation2017; Cohen et al. Citation2020). Recently conducted immunohistochemical studies clearly demonstrated that the induction of increased ALDH3A1 is localized to Club cells (evidenced by co-staining with CCSP and CYP2F2) rather than other lung cells (Ogata et al. Citation2021). To more quantitatively evaluate the dose-dependency of the increased ALDH3A1 expression in Club cells, flow cytometry (FCM) analysis demonstrated that permethrin treatment increased: (1) the number of Club cells (identified by co-expression with CCSP and CYP2F2); (2) only Club cell proliferation (determined by co-expression with a marker of proliferation KI67); and (3) expression of ALDH3A1 levels per Club cell. Generally, these increases showed dose-dependency at 2500 and 5000 ppm although some of the increases were not statistically significant due to the small numbers of mice examined (Ogata et al. Citation2021). While immunohistochemical staining and FCM analysis were not conducted at 20 ppm, considering the PCR data showing no induction of Aldh3a1 mRNA, it is reasonable to conclude that the dose levels of permethrin that produce alterations in Club cells correlate with subsequent lung tumor induction in female mice.
The EM findings and the increased proliferation of the Club cells were first observed after 3-day and 7-day dietary treatment with permethrin, respectively; but were not observed after 1-day treatment (Yamada et al. Citation2017). The increased Aldh3a1 mRNA levels were observed after 1-day treatment, demonstrating that Aldh3a1 mRNA is increased before the morphological alterations (Ogata et al. Citation2021). These findings suggest that permethrin interacts with Club cells in the lung of female mice as a KE 1 for lung tumorigenesis.
4.2.3. Club cell proliferation (KE 2)
Increased cell proliferation represents a key step in the process of tumor formation (Cohen and Arnold Citation2011; Cohen et al. Citation2020). To investigate the effects of permethrin on Club cell proliferation, several experiments were performed. These investigations included time-course studies, dose-response, recovery and sex difference studies, strain difference studies, and species difference studies (Yamada et al. Citation2017). A sensitive measure of cell proliferation is to determine the rate of S-phase activity of the cell cycle using BrdU incorporation as a marker of RDS. BrdU labeling indices in lung sections were determined by counting the percentage of Club cell nuclei undergoing RDS. In these investigations, BrdU was administered in one experiment by intraperitoneal injection at 24 and 2 h before euthanization and in all other studies by osmotic minipumps using 7 day labeling periods (Yamada et al. Citation2017). The results of these studies are summarized as follows:
In the experiment using intraperitoneal injection of BrdU, the treatment of female mice with 5000 ppm permethrin resulted in significant increases in labeling index values after 14 and 28 days of treatment. Treatment with permethrin for 7 days produced a small non-statistically significant increase in BrdU labeling index values, whereas no increase was observed after 3 days of treatment.
The treatment of female mice with 5000 and 10 000 ppm permethrin for 7 and 14 days resulted in significant increases in RDS.
Following 7 days of treatment, increases in Club cell labeling index values were observed in female mice given permethrin dose levels of 2500 ppm in the diet and above (discussed in Section 4.3. Lung: concordance dose-response relationships), however, there were no dose-dependent increases in labeling index values in male mice.
The treatment of female mice with 2500 ppm isoniazid, as a positive control, for 7 days resulted in significant increases in BrdU labeling index values, thus confirming the potential responsiveness of the animals to a Club cell mitogen.
The treatment of female mice for 7 days with 10 000 ppm permethrin resulted in a significant increase in Club cell labeling index values, however, following a recovery period of 5 weeks of no treatment, labeling index values had returned to control levels.
Treatment with 10 000 ppm permethrin for 7 days increased Club cell labeling index values in several strains of female mice. Permethrin produced a greater stimulation of Club cell RDS in Avy strain mice, being more susceptible to chemically-induced lung tumor formation (Wolff et al. Citation1987; Yen et al. Citation1994).
No increase in Club cell labeling index values was observed in female rats following treatment for 7 days with the highest carcinogenicity study dietary level of 2500 ppm permethrin.
As shown above, treatment with both permethrin and isoniazid resulted in stimulation of RDS in Club cells of female CD-1 mice. Permethrin was also shown to increase Club cell labeling index values in other strains of female mice. In addition, there was no evidence by either light or electron microscopy of any marked cytotoxicity to mouse lung after treatment with permethrin (Yamada et al. Citation2017). The stimulation of Club cell RDS thus appears due to a mitogenic effect of permethrin rather than a regenerative proliferation following tissue injury. In addition to the BrdU labeling index analyses, the recent FCM analysis demonstrated that the percentage of KI67 labeled cell fraction (mostly Club cells as determined by CCSP) also tended to increase at 2500 and 5000 ppm (Ogata et al. Citation2021).
4.2.4. Club cell hyperplasia (KE 3)
In an evaluation of the data from the CD-1 mouse bioassay (Ellison Citation1979), US.EPA (Citation1988) reported that permethrin produced a dose-dependent increase in the incidence of mice exhibiting multifocal alveolar cell proliferation (considered as Club cell hyperplasia) in female CD-1 mice given permethrin at dietary levels of 2500 and 5000 ppm for two years. In contrast, permethrin did not produce any dose-dependent increases in the incidence of alveolar cell proliferation in male mice given permethrin at dietary levels of up to 2000 ppm (US.EPA Citation1988).
Female CD-1 mice were treated with 0 (control) and 5000 ppm permethrin for 39, 52, 65, and 78 weeks, with recovery studies (no permethrin treatment) to either 78 or 100 weeks being performed (Barton et al. Citation2000; US.EPA Citation2000, Citation2002). The treatment of female CD-1 mice (group sizes 50 or 75 animals) with 5000 ppm permethrin for 39, 52, 65, and 78 weeks resulted in significant increases in Club cell hyperplasia (p < 0.01 for all permethrin-treated groups) with percentage incidences of 72, 98, 89, and 86%, respectively, whereas incidences of Club cell hyperplasia were either absent or very low (range 0–1%) in control animals. Compared to mice treated with 5000 ppm permethrin for 39–78 weeks, the incidence of Club cell hyperplasia was significantly reduced (p < 0.01 all treated groups) in mice given 5000 ppm permethrin for 39, 52, and 65 weeks followed by recovery (no permethrin treatment) to 78 weeks and in mice given 5000 ppm permethrin for 39, 52, 65, and 78 weeks followed by recovery to 100 weeks. While the effect of the lower carcinogenic permethrin dose level of 2500 ppm was not examined in this study, proliferative lesions in the lung are known to evolve as a continuum rather than sharp demarcation between specific entities of bronchiolo-alveolar hyperplasia, adenoma, and carcinoma (Renne et al. Citation2009). The lesions of hyperplasia, adenoma, and adenocarcinoma of the mouse lung occur as a continuum, initially as bronchiolo-alveolar hyperplasia, which evolve into adenomas, and ultimately into adenocarcinomas. Adenomas and adenocarcinomas in mice do not arise de novo, but arise from hyperplasia. Most of the tumors that occur in mouse lungs involve one of these diagnoses, with other types of tumors occurring much less commonly (Cohen et al. Citation2020). More importantly, although often not being evident in a chronic study until later time points (e.g. after 12 months), hyperplasia is a required step in mouse lung tumorigenesis (Cohen et al. Citation2020). If the hyperplasia lesions progress to adenoma, the apparent incidence of hyperplasia will be less at the termination of the study, but the adenomas and adenocarcinomas will have arisen through the intermediate stage of hyperplasia. This is a well-established sequence in mouse lung tumorigenesis (Nikitin et al. Citation2004; Renne et al. Citation2009) and hence it is considered that the treatment of female mice with 2500 and 5000 ppm permethrin results in Club cell hyperplasia.
4.2.5. Increased lung adenoma (KE 4)
Permethrin was shown to increase the incidence of bronchiolo-alveolar adenomas in female CD-1 mice when given at dietary levels of 2500 and 5000 ppm (see Section 2. Carcinogenicity data). Treatment with permethrin had no effect on the incidence of lung bronchiolo-alveolar carcinoma in female mice. Permethrin did not increase the incidence of lung bronchiolo-alveolar adenoma or carcinoma in male mice when given at dietary levels of up to 2000 ppm (Yamada et al. Citation2017). Permethrin did not increase the incidence of lung bronchiolo-alveolar adenoma or carcinoma in female or male rats when given at dietary levels of up to 2500 ppm (Yamada et al. Citation2017).
4.3. Lung: concordance of dose-response relationships
shows temporality and dose-response for MOA KEs related to CD-1 female mouse lung tumors. Club cell proliferation was evaluated in female mice at bioassay dose levels (20, 2500, and 5000 ppm) and also at lower (500 ppm) and higher (10 000 ppm) dose levels than the bioassay dose levels. In addition, Club cell RDS was also evaluated in male mice at bioassay dose levels of 20 and 500 ppm, as well as at greater than bioassay dose levels of 2500 and 5000 ppm. The treatment of female mice with 20 and 500 ppm permethrin for 7 days had no significant effect on RDS, whereas increases in BrdU labeling index values were observed in some female mice given 2500, 5000, and 10 000 ppm permethrin. The increase at 5000 ppm was not statistically significant (p = 0.06) by analysis using all dose levels (including 500 and 10 000 ppm which were not tested in carcinogenicity study) (Yamada et al. Citation2017). However, when the data are limited to dose levels used in the carcinogenicity study (0, 20, 2500, and 5000 ppm), a statistically significant (p < 0.05) increase in BrdU labeling index was observed at 2500 and 5000 ppm after 7-day treatment (Ogata et al. Citation2021). Thus while there was some variation in response between animals, treatment with tumorigenic and higher dose levels of permethrin stimulated Club cell RDS in female mice. This Club cell proliferation was also confirmed by FCM analysis of lung cells employing KI67 as a cell proliferation marker (Ogata et al. Citation2021). In contrast, the treatment of male mice with permethrin did not result in any significant effect on Club cell RDS even at the greater than bioassay dose levels of 2500 and 5000 ppm (Yamada et al. Citation2017).
Table 6. Temporality and dose-response for MOA key and associative events for permethrin-induced female CD-1 mouse lung tumors.
In the permethrin mouse bioassay, the incidences of lung adenomas and multifocal alveolar cell proliferation (considered as Club cell hyperplasia) were increased in female mice given 2500 and 5000 ppm permethrin, but no increase was observed in male mice given up to 2000 ppm permethrin (US.EPA Citation1988, Citation2002). Thus, there is a good correlation between the dose-response relationships for the increases in Club cell RDS, Club cell hyperplasia, and the observed lung tumor response in female mice. RDS was not increased in female mice at the non-tumorigenic dose level of 20 ppm, with a dose level of between 500 and 2500 ppm representing a threshold for the mitogenic effect of permethrin in mouse lung Club cells (Yamada et al. Citation2017). Finally, in keeping with the observed sex difference for permethrin-induced mouse lung tumor formation, no significant increases in Club cell RDS were seen in male mice even at greater than bioassay dose levels of permethrin (Yamada et al. Citation2017).
4.4. Lung: temporal association
If a KE (or KEs) is an essential element for carcinogenesis, it must precede the appearance of the tumors (Sonich-Mullin et al. Citation2001; Boobis et al. Citation2006). Thus, it is critical in the evaluation of an MOA that effects on KEs occur before the appearance of tumors and this is clearly the case for permethrin-induced mouse lung tumors. Temporality for MOA KEs related to CD-1 female mouse lung tumors is shown in .
Multiple exposure time data at 1, 3, 7, 14, and 28 days and at 39, 52, 65, 78, and 104 weeks are available in which female CD-1 mice were fed diets containing 5000 ppm permethrin (Yamada et al. Citation2017). One-day treatment with permethrin did not induce any morphological alteration of Club cells by both light and electron microscopic examination (Yamada et al. Citation2017). However, morphological changes in Club cells were observed by EM, but not by light microscopy, after treatment with 5000 ppm permethrin for 3, 7, 14, and 28 days (Yamada et al. Citation2017). The treatment of female mice with 5000 ppm permethrin for 3 days did not produce any increase in Club cell RDS (as assessed by intraperitoneal administration of BrdU), whereas treatment for 7, 14, and 28 days resulted in increases in RDS (assessed by both intraperitoneal administrations of BrdU and by use of osmotic minipumps) (Yamada et al. Citation2017; Ogata et al. Citation2021). The chronic administration of 5000 ppm permethrin to female mice resulted in sustained Club cell hyperplasia which was observed after 39 (the earliest time point examined), 52, 65, and 78 weeks of treatment (Barton et al. Citation2000; US.EPA Citation2000, Citation2002). Increased incidences of Club cell hyperplasia were also observed in female CD-1 mice treated with 2500 and 5000 ppm permethrin for two years (US.EPA Citation1988).
Treatment with 2500 ppm permethrin resulted in a significant increase in Club cell RDS after 7- and 14-days treatment (2.1- and 1.6-fold control, respectively), and a small, but not statistically significant, increase (1.2-fold control) after 28-days treatment (Ogata et al. Citation2021). While the effect of 2500 ppm permethrin on Club cell RDS was less marked than that observed with 5000 ppm permethrin, an increase in cell proliferation was observed over an extended period. In contrast to female mice, permethrin treatment did not increase either RDS or tumor formation in male mice or in male and female rats.
Overall, there is a logical temporal response for all the KEs for permethrin-induced mouse lung tumor formation, in which all effects on the KEs precede tumor formation.
4.5. Lung: strength, consistency, and specificity of association of tumor response with KEs
The strength, consistency, and specificity of the association between the identified KEs and subsequent tumor formation are demonstrated by the data obtained in several studies (Barton et al. Citation2000; Yamada et al. Citation2017; Ogata et al. Citation2021). Apart from studies in CD-1 mice, permethrin was also shown to increase Club cell RDS in several other mouse strains. While permethrin increased RDS in female mouse Club cells, no such effect was observed in male mice, which correlates with the lack of tumor formation in male mice. The relationship of the observed effects on female mouse lung Club cells to permethrin treatment was demonstrated in a recovery study, where the effects of permethrin were shown to be reversible on cessation of treatment. Female CD-1 mice were given 10 000 ppm permethrin for 7 days followed by 5 weeks of no treatment. While permethrin produced ultrastructural changes in lung Club cells and stimulated RDS after 7 days of treatment, no such effects were observed after 5 weeks of recovery (Yamada et al. Citation2017).
Cypermethrin is a close structural analogue of permethrin and has been classified as a Category “C” carcinogen (possible human carcinogen) based on the formation of lung tumors in female mice (US.EPA Citation2003). Female CD-1 mice were given diets containing the highest bioassay dose level of 1600 ppm cypermethrin and also a higher dose level of 2100 ppm cypermethrin for 7 and 14 days (Yamada et al. Citation2017). As a positive control, female CD-1 mice were given diets containing 600 and 1000 ppm isoniazid for 7 and 14 days. Morphological examination of mouse lung sections by light microscopy did not reveal any changes following treatment with cypermethrin. However, EM revealed a proliferation of the SER of Club cells following treatment with cypermethrin for 7 and 14 days. In addition, treatment with cypermethrin and isoniazid for 14 days was shown to increase the numbers of elongated mitochondria in mouse lung Club cells. The treatment of mice with 1600 and 2100 ppm cypermethrin for 7 days resulted in increases in Club cell RDS to 149 and 179% of control, respectively, with treatment with 600 and 1000 ppm isoniazid producing large increases in Club cell RDS. Overall, the effects of cypermethrin on female CD-1 mouse lung Club cells were similar in this study to those of isoniazid and those produced in other studies by both permethrin and isoniazid. This study with a close structural analogue of permethrin also demonstrates the strength, consistency, and specificity of the association between enhanced Club cell RDS and mouse lung tumor formation.
4.6. Lung: biological plausibility and coherence
The lung is the second most common target tissue affected in mouse cancer bioassays (Huff et al. Citation1991; Gold et al. Citation2001). In a recent analysis of 411 unique agrochemicals described in the liver Section 3.6., it was reported that in the lung only one type of tumor was observed which could be categorized as bronchiolar/alveolar adenoma/carcinoma, and these tumors were exclusively observed in mice (Heusinkveld et al. Citation2020). As some mouse strains have high spontaneous background incidences of lung tumors, including the strain used in this permethrin bioassay (CD-1), and thus are sensitive to chemically-induced proliferative lesions (Manenti et al. Citation2003; Nikitin et al. Citation2004).
The proposed MOA for permethrin-induced mouse lung tumor formation is biologically plausible and is similar to that of other mouse lung inducers, such as isoniazid and flonicamid. Both isoniazid and flonicamid are mitogenic agents which stimulate Club cell RDS and subsequently produce mouse lung tumors (Cohen et al. Citation2020). Both isoniazid and flonicamid stimulated RDS without producing any evidence of necrosis. Isoniazid produced similar morphological changes in Club cells to those observed after treatment with permethrin and flonicamid, which have been shown to produce Club cell hyperplasia (US.EPA Citation1988, Citation2000, Citation2005b; Strupp et al. Citation2012, Citation2016). Thus, isoniazid and flonicamid are Club cell mitogens (US.EPA Citation2005b; Yamada et al. Citation2017). In addition to isoniazid and flonicamid, fluensulfone is also a mouse lung Club cell mitogen and has been shown to produce lung tumors in mice (Strupp et al. Citation2012, Citation2016). While permethrin produced lung tumors in female mice given 2500 and 5000 ppm, no lung tumors were observed in male and female rats given 2500 ppm permethrin (see Section 2. Carcinogenicity data). Permethrin is thus similar to several other mouse lung Club cell mitogens in which the mouse is susceptible to lung tumor formation by such chemicals, but lung tumors are not observed in the rat.
4.7. Lung: other MOAs
As described above, there is strong evidence in support of a mitogenic effect of permethrin on lung Club cells leading to hyperplasia as the MOA for permethrin-induced mouse lung tumor formation. Other known MOAs for rodent lung carcinogenesis have been assessed from the data available from the various studies performed with permethrin.
4.7.1. Genotoxicity as an MOA
As described for the liver MOA (Section 3.7. Liver: other MOAs), permethrin has no genotoxic potential (US.EPA Citation2002). Thus permethrin-induced mouse lung tumor formation is attributable to a non-genotoxic MOA. The findings from a recent in vivo comet assay and micronucleus test also strongly support that permethrin has no genotoxic, aneugenic, or clastogenic potential (Matsuyama et al. Citation2018).
4.7.2. Decreased cell deaths as an MOA
Increased cell proliferation can occur either by an increase in cell births or a decrease in cell deaths (Cohen and Arnold Citation2011). A decrease in cell deaths can be produced by either inhibiting apoptosis or cell differentiation, two cell death processes that will lead to an accumulation of cells. There is no evidence for such changes indicative of effects on apoptosis or differentiation in the mouse lung tumor model in general, nor with permethrin specifically, leaving an increase in cell births as the most plausible MOA for tumor formation.
4.7.3. Cytotoxicity as an MOA
Increased cell proliferation can be produced either by direct mitogenesis or by cytotoxicity, such as necrosis, which results in consequent regenerative proliferation. Some chemicals have been shown to produce lung tumors in mice by an MOA that involves cytotoxicity to Club cells (Cohen et al. Citation2020). Such chemicals result in regenerative hyperplasia and subsequently in lung tumor formation. The effects of non-genotoxic cytotoxic mouse lung Club cell carcinogens, such as coumarin, are known to reverse quickly due to tolerance (Born et al. Citation1998, Citation1999). The acute effect of permethrin on female mouse lung Club cells was investigated by giving permethrin both by gavage and in the diet for one day, with a single oral dose of coumarin being employed as a positive control. Acute treatment with permethrin did not induce cytotoxic effects in mouse lung Club cells, evaluated by either light microscopy or EM (Yamada et al. Citation2017). Furthermore, there was no evidence of necrosis or inflammatory changes in mouse lungs following permethrin treatment for up to 28 days (Yamada et al. Citation2017; Ogata et al. Citation2021). These findings constitute clear evidence to exclude a cytotoxic MOA for permethrin-induced mouse lung tumor formation, thus leaving increased mitogenesis as the only plausible MOA for producing increased cell proliferation.
4.8. Lung: uncertainties, inconsistencies, and data gaps
The tumors occur in the periphery of the lungs where terminal bronchioles and alveoli are in intimate contact. Depending on the tissue section examined, a sufficient number of terminal bronchioles may or may not be included. The tumors displace normal bronchiolar and alveolar tissues and, based on histopathology, it is not possible to determine whether the tumors arise from alveolar tissue or terminal bronchiolar tissue. Although there is no direct evidence that the mouse lung tumors caused by permethrin are derived from Club cells, the results of the ultrastructural and cell proliferation studies clearly demonstrate that permethrin treatment affects only Club cells, but not other lung cell types (Yamada et al. Citation2017). While only two animals per treatment group were examined in the ultrastructural studies, the effects were dose-dependent and this is not considered to represent a data gap. A recent study extends the previously proposed MOA by clearly demonstrating that Club cells are the primary initial target of permethrin administration, as demonstrated by effects on ALDH3A1 levels by immunohistochemistry and FCM and by increases in the cell proliferation marker KI67 by FCM (Ogata et al. Citation2021). Overall, it is considered that the primary target for tumorigenesis of the lung by permethrin is the Club cell.
Although disruption of key circadian clock genes has been identified as a driver of mouse lung tumorigenesis (Cohen et al. Citation2020), there is no evidence of permethrin effects on circadian clock genes (Ogata et al. Citation2021). The studies with other mouse lung-specific tumorigens, such as styrene and dichloromethane have shown impacts on the clock genes only later in life (Cohen et al. Citation2020). Since gene expression analyses were conducted only in a short-term study (i.e. 7-day treatment) with permethrin, the lack of effects by permethrin on the clock genes may reflect the short-term nature of the study (Ogata et al. Citation2021).
4.9. Assessment of postulated MOA
As described above, the KEs in the MOA for permethrin-induced mouse lung tumor formation have been identified and shown to exhibit a strong dose and temporal consistency with tumor formation. Considering (1) the timing of the gene expression changes (before the Club cell morphological changes and increased RDS), (2) a dose-response consistent with tumor appearance, and (3) relatively large induction, the increase in ALDH3A1 levels suggests that it might be either a KE (an empirically observable causal precursor step to the adverse outcome that is itself a necessary element of the MOA) or an AE (a biological process that is not a causal necessary KE for the MOA but is a reliable indicator or marker for a KE).
While some studies have demonstrated a link between ALDH3A1 induction and cell proliferation (Muzio et al. Citation2012; Pors and Moreb Citation2014; Laskar and Younus Citation2019), a causal relationship between ALDH3A1 induction and Club cell proliferation has not been fully established. Overall, it is reasonable to consider that ALDH3A1 induction is an indicator of acceleration of xenobiotic metabolism in female mouse lungs following permethrin treatment and thus represents an AE for the MOA for permethrin-induced mouse lung tumor formation (Ogata et al. Citation2021). As such, the increases in ALDH3A1 levels is considered to be an AE for subsequent lung tumor formation, being similar to the induction of CYP2B and CYP4A subfamily enzymes which are AEs for rodent liver tumor formation, respectively, by mitogenic CAR and PPARα activators (Corton et al. Citation2018; Lake Citation2018; Yamada Citation2018; Yamada et al. Citation2021). While the precise molecular mechanism for permethrin-induced Club cell proliferation is unknown, a detailed mechanistic understanding is not necessary for a conclusion regarding the proposed MOA (Cohen et al. Citation2003, Citation2004).
The proposed MOA for permethrin-induced mouse lung tumor formation is similar to the mitogenic MOAs previously described for the formation of mouse lung tumors by isoniazid, flonicamid, and fluensulfone (US.EPA Citation2005b; Strupp et al. Citation2012, Citation2016; Cohen et al. Citation2020). Furthermore, cypermethrin, a close structural analogue to permethrin, also increased Club cell proliferation and lung tumors in female mice (Yamada et al. Citation2017). It is considered that there is a high level of confidence in the proposed MOA for permethrin-induced mouse lung tumor formation.
4.10. Lung: human relevance of the proposed MOA
As for the liver, the human applicability of the proposed MOA for permethrin-induced mouse lung tumors was evaluated using the ILSI RSI “Human Relevance Framework” (Meek et al. Citation2003) as modified by the IPCS Cancer Working Group (Boobis et al. Citation2006).
4.10.1. Question 1. Is the weight of evidence sufficient to establish an MOA in animals?
As described above, there is clear evidence that following systemic exposure permethrin interacts with Club cells and results in the observable morphological changes in Club cells by EM followed by a mitogenic effect resulting in Club cell proliferation. Prolonged administration of permethrin results in Club cell hyperplasia and subsequently in the formation of bronchiolo-alveolar adenomas. The KEs and AEs for permethrin-induced female mouse liver tumor formation are similar to those described for other Club cell mitogens (US.EPA Citation2005b; Strupp et al. Citation2012, Citation2016; Cohen et al. Citation2020). Hence, the answer to question 1 is yes.
4.10.2. Question 2. Can human relevance of the MOA be reasonably excluded on the basis of fundamental, qualitative differences in KEs between experimental animals and humans?
The current understanding of increased Club cell proliferation as a risk factor for tumor formation (Cohen et al. Citation2020) was considered to assess the human relevance of the KEs in the proposed MOA for permethrin-induced mouse lung tumor formation (Yamada et al. Citation2017; Ogata et al. Citation2021). For mouse-specific lung tumor production, CYP2F2 metabolism is a key initial metabolic gateway that is “open” only in mouse lung (Cruzan et al. Citation2018) and thus, for rats and humans, that gateway is qualitatively (or possibly quantitatively) “closed” to initiation of subsequent events progressing to lung cancer (cell proliferation, hyperplasia) (Cohen et al. Citation2020). Based on a preliminary in vitro permethrin metabolism study using mouse lung microsomes there is no evidence that permethrin-induced mouse lung tumors are CYP2F2-mediated. However, the effect of permethrin in Cyp2f2 knockout mice needs to be evaluated. While there are substantial quantitative dynamic differences between mice and humans, the fundamental mechanisms involved in the stimulation of cell proliferation in mouse lung Club cells cannot be completely excluded in humans. Therefore, a further analysis focusing on quantitative differences between mouse and human should be conducted.
4.10.3. Question 3. Can human relevance of the MOA be reasonably excluded on the basis of quantitative differences in either kinetic or dynamic factors between experimental animals and humans?
To put mouse lung tumors into perspective with regard to human relevance, it is critical to take into account similarities and differences between mouse and human lungs and airways, the histogenesis of the tumors, and the biologic behavior of the tumors (Cohen et al. Citation2020). Although there are several similarities, there are also numerous differences that greatly impact the interpretation of the results of rodent studies, including differences in anatomy, physiology, metabolism, kinetics, and pathogenesis, in addition to significant differences in the biological behavior of the tumors that develop. Details are shown in a recent extensive review (Cohen et al. Citation2020).
As shown in , important quantitative differences in KEs or AEs clearly exist between mice, rat, and humans. Based on these species differences, the postulated MOA for mouse lung tumor formation is considered to be not plausible for humans, with humans being expected to be substantially less sensitive than mice to the pulmonary effects of permethrin. Indeed, because permethrin did not produce any effects on Club cells or produce lung tumors in the rat, based on the proposed MOA in mice it is highly unlikely that permethrin would produce lung tumors in humans ().
Table 7. Summary of known species differences in characteristics related to lung tumor formation in mice, rats and humans.
Table 8. Comparison of key and associative events for permethrin-induced lung tumor formation in mice, rats and humans.
There are numerous chemicals that increase the incidence of lung tumors in long-term bioassays in mice, but not in rats (Cohen et al. Citation2020). The MOAs for mouse lung-specific non-genotoxic carcinogens involve increased early-stage bronchiolo-alveolar cell proliferation, either due to mitogenicity or cytotoxicity (cell death) with consequent regenerative proliferation (Cohen et al. Citation2020). The proposed MOA for permethrin-induced mouse lung tumor formation is similar to the mitogenic MOAs previously described for isoniazid (Cohen et al. Citation2020), flonicamid (US.EPA Citation2005b), and fluensulfone (Strupp et al. Citation2012, Citation2016). Like permethrin (Yamada et al. Citation2017), none of these other mouse lung mitogenic tumorigens produced any proliferative effect in the rat lung (US.EPA Citation2005b; Strupp et al. Citation2012, Citation2016; Cohen et al. Citation2020). Several human epidemiology studies have demonstrated that, in subjects given isoniazid to treat tuberculosis, there is no marked effect on the incidence of lung or other tumors due to isoniazid treatment (IARC Citation1987; Cohen et al. Citation2020), the tumorigenic dose of isoniazid in mice (600 ppm, 75 mg/kg/day; Yamada et al. Citation2017) being somewhat higher than the pharmacological dose in humans (7–25 mg/kg/day; WHO Citation2014).
Indeed, as with isoniazid, epidemiological studies with permethrin have not shown an increased incidence of lung or liver tumors in humans, nor any other tumors (see description of permethrin epidemiology studies below, Section 5. Human exposure levels, human risk, and epidemiology of permethrin).
4.11. Lung conclusion: statement of confidence, analysis, and implications
Based on the data described above, there is strong evidence to support a plausible MOA for permethrin-induced lung tumor formation in female mice. This MOA involves interaction with Club cells (KE 1) evidenced by observable morphological changes only in Club cells by EM, and increased metabolic enzyme induction in Club cells (ALDH3A1) (AE 1), followed by a mitogenic effect resulting in increased Club cell proliferation (KE 2), with prolonged administration producing Club cell hyperplasia (KE 3), and subsequently the formation of bronchiolo-alveolar adenomas (KE 4). The proposed MOA for permethrin-induced mouse lung tumor formation is similar to that previously described for isoniazid, flonicamid, and fluensulfone. While permethrin, isoniazid, flonicamid, and fluensulfone produce lung tumors in mice by a mitogenic MOA, these compounds do not produce lung tumors in rats. Although the possibility that permethrin exposure may potentially result in enhancement of Club cell proliferation in humans cannot be completely excluded, there is sufficient information on differences in basic lung anatomy, physiology, metabolism, and biology in the general literature to conclude that humans are quantitatively less sensitive to agents that increase Club cell proliferation and lead to tumor formation in mice (Cohen et al. Citation2020). The evidence strongly indicates that chemicals having such an effect in mice do not have this effect in rats and are not likely to lead to an increase in susceptibility to lung tumor development in humans. Other possible MOAs for mouse lung tumor formation have been examined and excluded. It is therefore concluded that permethrin does not provide a lung tumorigenic hazard for humans ().
5. Human exposure levels, human risk, and epidemiology of permethrin
In addition to analysis of qualitative/quantitative differences in response in mice vs. humans based on MOA considerations, a quantitative difference in external exposure levels (which is not generally the basis for the analysis under the WHO/IPCS MOA/human relevance framework) is discussed here.
Permethrin was first synthesized in 1973, first marketed in 1977, and first registered in the United States in 1979 (Rusiecki et al. Citation2009). It has long been widely used in agriculture, in public health, and in many US. homes and gardens. Chronic human exposure of the US population to permethrin has been reported to be 0.000184 mg/kg/day (US.EPA Citation2009) and 0.000785 mg/kg/day (US.EPA Citation2020b), which is some 1 940 000 and 455 000 times lower than the dose of 357 mg/kg/day (2500 ppm) of permethrin that produced female mouse liver and lung tumors (Yamada et al. Citation2017; Kondo et al. Citation2019). The Acceptable Daily Intake for permethrin in humans is 0.05 mg/kg/day (WHO Citation2019) which is over 7000 times lower than the observed dose levels at which the mitogenic and tumorigenic effects (around 360 mg/kg/day) occur in the liver and lung of female mice fed 2500 ppm permethrin (Yamada et al. Citation2017; Kondo et al. Citation2019; Ogata et al. Citation2021).
Based on the permethrin toxicology data including the MOA data (Yamada et al. Citation2017; Kondo et al. Citation2019, Citation2020), the latest evaluation by the US.EPA concluded that permethrin has been reclassified as “Suggestive Evidence of Carcinogenic Potential” by the oral route, and a separate quantification of human cancer risk using linear-approach is no longer required; the acute reference dose (RfD) will adequately account for all repeated exposure/chronic toxicity, including carcinogenicity, which could result from exposure to permethrin (US.EPA Citation2020a, Citation2020b). For all registered uses of permethrin in US, the US.EPA concluded that there are no concerns for dietary or residential risks, acute aggregate (combined food and drinking water exposures) risk, and the short-term aggregate (combined residential, food, and drinking water) risk (US.EPA Citation2020b). Furthermore, all screening-level occupational handler inhalation risks estimated are not of concern using engineering controls (for aerial applicators) or baseline personal protective equipment and no respirator (US.EPA Citation2020b). Therefore, permethrin would not be expected to induce tumors in humans at usual levels of exposure. Even though liver and lung tumors were increased in the permethrin female mouse 2-year study, the conclusion of this analysis is that permethrin does not pose a tumorigenic risk to humans based on the currently accepted MOA considerations by the US.EPA (US.EPA Citation2020a, Citation2020b). This conclusion is supported by epidemiology data from the US Agricultural Health Study and from several other epidemiological studies (Rusiecki et al. Citation2009; Boffetta and Desai Citation2018) that showed no increase in liver or lung tumors or any other type of tumor.
6. Conclusions
Based on the data described in this review, plausible MOAs have been established for both permethrin-induced liver and lung tumor formation in female CD-1 mice. In male mice, the 2019 liver PWG concluded that the liver tumors were not treatment-related (Quist et al. Citation2019), with the CARC agreeing with this conclusion (US.EPA Citation2020a). Moreover, permethrin did not increase the incidence of lung tumors in male mice and did not increase the incidences of liver, lung, or other tumors in male or female rats (Cal.EPA Citation1994; US.EPA Citation2002, Citation2020a, Citation2020b). The proposed MOAs for permethrin-induced female mouse liver and lung tumor formation were then evaluated using the IPCS framework (). Both the proposed female CD-1 mouse liver tumor MOA and the proposed lung tumor MOA were found to satisfy the conditions of dose and temporal concordance, biological plausibility, coherence, strength, consistency, and specificity as described in the IPCS framework. Alternative MOAs for both permethrin-induced liver and lung tumor formation were excluded. For liver tumors, it is concluded that the proposed MOA could not qualitatively operate (Question 2) in humans (). The proposed MOA for permethrin-induced liver tumor formation is similar to that established for several other PPARα activators. For lung tumors, although the postulated MOA could potentially operate qualitatively in humans, there are marked quantitative species differences in lung anatomy, physiology, metabolism, histogenesis, tumor behavior, and susceptibility to tumor formation. Thus, it is concluded that the proposed MOA for permethrin-induced lung tumor formation could not quantitatively operate (Question 3) in humans (). Based on the MOA evaluations described in this paper, neither the liver nor the lung MOAs for mouse permethrin-induced tumor formation is considered to be relevant for humans. Thus, permethrin poses no carcinogenic hazard for humans and therefore humans have no carcinogenic risk via permethrin exposure (). The conclusion that permethrin poses no carcinogenic risk for humans is also supported by available exposure data and by epidemiology investigations; human exposure being orders of magnitude lower than levels that produce liver and lung tumors in female CD-1 mice.
Figure 1. Assessment of the relevance of the permethrin-induced liver and lung tumors in female CD-1 mice to humans based on the IPCS framework (Meek et al. Citation2003; Boobis et al. Citation2006; Meek, Boobis, et al. Citation2014; Meek, Palermo, et al. Citation2014). Based on evaluation using the IPCS framework both the mouse liver and lung MOAs for permethrin-induced tumor formation can be considered to be not relevant for humans, and thus permethrin has no carcinogenic hazard for humans. In addition, levels of human environmental exposure to permethrin are orders of magnitude lower than those required to produce liver and lung tumors in mice. That permethrin does not produce tumors in humans is supported by the results of both permethrin epidemiological studies (Rusiecki et al. Citation2009; Boffetta and Desai Citation2018) and from studies with other nongenotoxic agents which produce either liver (Doull et al. Citation1999; Bonovas et al. Citation2012; Corton et al. Citation2014, Citation2018) or lung (IARC Citation1987; Cohen et al. Citation2020) tumors in mice by similar MOAs to those established for permethrin.
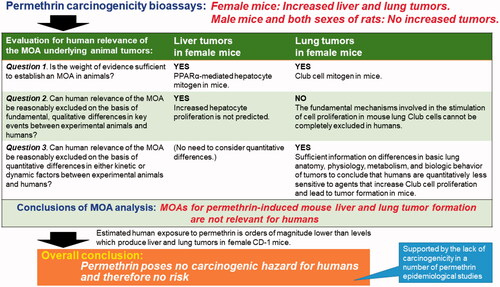
Previously, permethrin was classified as “likely to be carcinogenic to humans” based on female mouse lung and liver tumors, with a low-dose extrapolation approach for the quantification of human cancer risk (US.EPA Citation2002). Recently, based on the data for MOA analysis (Yamada et al. Citation2017; Kondo et al. Citation2019, Citation2020), US.EPA re-classified permethrin as "suggestive evidence of carcinogenic potential” based on lung adenomas in female mice and concluded that quantification of human cancer risk is not required (US.EPA Citation2020a, Citation2020b). The CARC of the US.EPA considered that the submitted data for lung did not adequately support the proposed MOA at 2500 ppm (US.EPA Citation2020a). More recently, however, new additional data were developed (Ogata et al. Citation2021). The data clearly demonstrated that Club cells are the primary target of permethrin in the lung, and Club cell proliferation was observed at 2500 ppm and higher up to 28 days of permethrin dosing (Ogata et al. Citation2021). As discussed above, permethrin does not pose a tumorigenic hazard to humans. This conclusion is supported by available negative epidemiological data (Rusiecki et al. Citation2009; Boffetta and Desai Citation2018). Therefore, permethrin should not be classified as "suggestive evidence of carcinogenic potential,” and may be classified as “not likely to be carcinogenic to humans.”
| Abbreviations | ||
| Acox1 | = | acyl-Coenzyme A oxidase 1 |
| AE | = | associative event |
| AhR | = | aryl hydrocarbon receptor |
| ALDH | = | aldehyde dehydrogenase enzymes |
| ALDH3A1 | = | aldehyde dehydrogenase 3A1 |
| BrdU | = | 5-bromo-2′-deoxyuridine |
| Cal.EPA | = | California Environmental Protection Agency (in US) |
| CAR | = | constitutive androstane receptor |
| CARC | = | Cancer Assessment Review Committee |
| CCSP | = | club cell secretory protein |
| CYP | = | cytochrome P450 |
| ECHA | = | European Chemicals Agency |
| EGF | = | epidermal growth factor |
| EM | = | ultrastructural examination |
| FCM | = | flow cytometry |
| GW7647 | = | (2-(4-(2-(1-cyclohexanebutyl)-3-cyclohexylureido)ethyl)phenylthio)-2-methylpropionic acid |
| HGF | = | hepatocyte growth factor |
| IPCS | = | International Programme on Chemical Safety |
| ILSI | = | International Life Sciences Institute |
| KE | = | key event |
| KI67 | = | marker of proliferation KI67 |
| MOA | = | mode of action |
| MTD | = | maximum tolerated dose |
| Osgin1 | = | oxidative stress induced growth inhibitor 1 |
| PCR | = | polymerase chain reaction |
| PPARα | = | peroxisome proliferator-activated receptor alpha |
| PXR | = | pregnane X receptor |
| PWG | = | Pathology Working Group |
| RDS | = | replicative DNA synthesis |
| RSI | = | Risk Sciences Institute |
| SER | = | smooth endoplasmic reticulum |
| Txnrd1 | = | thioredoxin reductase 1 |
| US.EPA | = | US Environmental Protection Agency |
| US.FDA | = | US Food and Drug Administration |
| WHO | = | World Health Organization |
| WY-14643 | = | 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid |
Acknowledgements
The authors gratefully acknowledge members of the Environmental Health Science Laboratory, Sumitomo Chemical Company, Ltd. for their encouragement to collect data for this manuscript; and also to Dr. Keiko Ogata for the internal review of the manuscript; and to the external reviewers selected by the Editor and anonymous to the authors whose comments were valuable in revising and refining the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of interest
The affiliations of the authors are shown on the cover page. Dr. Tomoya Yamada is employed by Sumitomo Chemical Company, Ltd. Prof. Samuel M. Cohen and Prof. Brian G. Lake consult for Sumitomo Chemical Company, Ltd. regarding research on chemical-induced carcinogenicity as well as other matters. The manuscript was written as part of the authors’ normal employment, but the authors have sole responsibility for the writing and content of this paper. The views presented in this manuscript are those of the authors based on many years of research in the respective areas of the investigation reported in this paper. This work was supported by the Sumitomo Chemical Company, Ltd. (https://www.sumitomo-chem.co.jp/english/company/). This company is interested in the human relevance of the MOA for chemical-induced rodent tumor formation because some company products (e.g. pesticides) have carcinogenicity in rodents. Permethrin is also one of the company products. Some of the research on permethrin produced by Sumitomo Chemical Company, Ltd. has been utilized by the company in regulatory submissions to provide MOA information for interpreting the relevance to humans of the liver and lung tumors in mice. An additional review of the paper for internal approval purposes was conducted by Dr. Kyoko Odawara, a research director of Environmental Health Science Laboratory, Sumitomo Chemical Company, Ltd. No external funding was obtained for manuscript preparation. None of the authors has appeared in any legal or regulatory proceedings related to the contents of this paper. No potential conflict of interest was reported by the authors.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Ahmad S, Ahmad A. 2017. Chapter 6 – epithelial regeneration and lung stem cells. In: Sidhaye VK, Koval M, editors. Lung epithelial biology in the pathogenesis of pulmonary disease. Boston (MA): Academic Press; p. 91–102.
- Andersen ME, Preston RJ, Maier A, Willis AM, Patterson J. 2014. Dose-response approaches for nuclear receptor-mediated modes of action for liver carcinogenicity: results of a workshop. Crit Rev Toxicol. 44(1):50–63.
- Baldrick P, Reeve L. 2007. Carcinogenicity evaluation: comparison of tumor data from dual control groups in the CD-1 mouse. Toxicol Pathol. 35(4):562–569.
- Barrueco C, Herrera A, Caballo C, de la Pena E. 1992. Cytogenetic effects of permethrin in cultured human lymphocytes. Mutagenesis. 7(6):433–437.
- Barrueco C, Herrera A, Caballo C, de la Pena E. 1994. Induction of structural chromosome aberrations in human lymphocyte cultures and CHO cells by permethrin. Teratog Carcinog Mutagen. 14(1):31–38.
- Barton S, Robinson S, Martin T. 2000. Permethrin technical 100 week carcinogenicity/reversibility study in mice with administration by the diet: Lab Project Number: 452695: A954264. Unpublished study prepared by Inveresk Research.
- Boffetta P, Desai V. 2018. Exposure to permethrin and cancer risk: a systematic review. Crit Rev Toxicol. 48(6):433–442.
- Bonovas S, Nikolopoulos GK, Bagos PG. 2012. Use of fibrates and cancer risk: a systematic review and meta-analysis of 17 long-term randomized placebo-controlled trials. PLOS One. 7(9):e45259.
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. 2006. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 36(10):781–792.
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 38(2):87–96.
- Born SL, Fix AS, Caudill D, Lehman-McKeeman LD. 1998. Selective Clara cell Injury in mouse lung following acute administration of coumarin. Toxicol Appl Pharmacol. 151(1):45–56.
- Born SL, Fix AS, Caudill D, Lehman-McKeeman LD. 1999. Development of tolerance to Clara cell necrosis with repeat administration of coumarin. Toxicol Sci. 51(2):300–309.
- Buckpitt A, Chang AM, Weir A, Van Winkle L, Duan X, Philpot R, Plopper C. 1995. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol. 47(1):74–81.
- Cal.EPA. 1994. Permethrin (permethrin tick repellent), risk characterization document, medical toxicology and worker health and safety branches. Sacramento (CA): Department of Pesticide Regulation, California Environmental Protection Agency; [accessed 2021 Nov 25]. https://www.cdpr.ca.gov/docs/risk/rcd/permet_s3.pdf
- Carlson GP. 2008. Critical appraisal of the expression of cytochrome P450 enzymes in human lung and evaluation of the possibility that such expression provides evidence of potential styrene tumorigenicity in humans. Toxicology. 254(1–2):1–10.
- Carthew P, Edwards RE, Nolan BM. 1998a. The quantitative distinction of hyperplasia from hypertrophy in hepatomegaly induced in the rat liver by phenobarbital. Toxicol Sci. 44(1):46–51.
- Carthew P, Edwards RE, Nolan BM. 1998b. New approaches to the quantitation of hypertrophy and hyperplasia in hepatomegaly. Toxicol Lett. 102–103:411–415.
- Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, et al. 1998. Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regul Toxicol Pharmacol. 27(1 Pt 2):47–60.
- Cheung C, Akiyama TE, Ward JM, Nicol CJ, Feigenbaum L, Vinson C, Gonzalez FJ. 2004. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor alpha. Cancer Res. 64(11):3849–3854.
- Cohen SM, Meek ME, Klaunig JE, Patton DE, Fenner-Crisp PA. 2003. The human relevance of information on carcinogenic modes of action: overview. Crit Rev Toxicol. 33(6):581–589.
- Cohen SM, Klaunig J, Meek ME, Hill RN, Pastoor T, Lehman-McKeeman L, Bucher J, Longfellow DG, Seed J, Dellarco V, et al. 2004. Evaluating the human relevance of chemically induced animal tumors. Toxicol Sci. 78(2):181–186.
- Cohen SM. 2010. Evaluation of possible carcinogenic risk to humans based on liver tumors in rodent assays: the two-year bioassay is no longer necessary. Toxicol Pathol. 38(3):487–501.
- Cohen SM, Arnold LL. 2011. Chemical carcinogenesis. Toxicol Sci. 120(Supplement 1):S76–S92.
- Cohen SM, Zhongyu Y, Bus JS. 2020. Relevance of mouse lung tumors to human risk assessment. J Toxicol Environ Health B Crit Rev. 23(5):214–241.
- Corton JC, Cunningham ML, Hummer BT, Lau C, Meek B, Peters JM, Popp JA, Rhomberg L, Seed J, Klaunig JE. 2014. Mode of action framework analysis for receptor-mediated toxicity: the peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit Rev Toxicol. 44(1):1–49.
- Corton JC, Peters JM, Klaunig JE. 2018. The PPARα-dependent rodent liver tumor response is not relevant to humans: addressing misconceptions. Arch Toxicol. 92(1):83–119.
- Cruzan G, Bus J, Banton M, Gingell R, Carlson G. 2009. Mouse specific lung tumors from CYP2F2-mediated cytotoxic metabolism: an endpoint/toxic response where data from multiple chemicals converge to support a mode of action. Regul Toxicol Pharmacol. 55(2):205–218.
- Cruzan G, Bus JS, Andersen ME, Carlson GP, Banton MI, Sarang SS, Waites R. 2018. Based on an analysis of mode of action, styrene-induced mouse lung tumors are not a human cancer concern. Regul Toxicol Pharmacol. 95:17–28.
- de la Rosa Rodriguez MA, Sugahara G, Hooiveld G, Ishida Y, Tateno C, Kersten S. 2018. The whole transcriptome effects of the PPARα agonist fenofibrate on livers of hepatocyte humanized mice. BMC Genomics. 19(1):443.
- Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. 1999. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA risk assessment guidelines. Regul Toxicol Pharmacol. 29(3):327–357.
- ECHA. 2017. Guidance on the application of the CLP criteria, Guidance to regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures, version 5.0; [accessed 2021 Nov 25]. https://echa.europa.eu/documents/10162/23036412/clp_en.pdf/58b5dc6d-ac2a-4910-9702-e9e1f5051cc5
- Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, et al. 2014. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: a case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol. 44(1):64–82.
- Ellison T. 1979. Analysis of physical observations, twenty-four month oral carcinogenicity study of FMC 33297 in mice. East Millstone, NJ: Bio/dynamics, Inc. Bio/dynamics Study Number: 76-1695, FMC Study Number: ACT 115.35, October 9, 1979. Unpublished.
- Farber E, Sarma DS. 1987. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 56(1):4–22.
- Farmahin R, Williams A, Kuo B, Chepelev NL, Thomas RS, Barton-Maclaren TS, Curran IH, Nong A, Wade MG, Yauk CL. 2017. Recommended approaches in the application of toxicogenomics to derive points of departure for chemical risk assessment. Arch Toxicol. 91(5):2045–2065.
- Foreman JE, Koga T, Kosyk O, Kang BH, Zhu X, Cohen SM, Billy LJ, Sharma AK, Amin S, Gonzalez FJ, et al. 2021a. Diminished hepatocarcinogenesis by a potent, high-affinity human PPARα agonist in PPARA-humanized mice. Toxicol Sci. 183(1):70–80.
- Foreman JE, Koga T, Kosyk O, Kang BH, Zhu X, Cohen SM, Billy LJ, Sharma AK, Amin S, Gonzalez FJ, et al. 2021b. Species differences between mouse and human PPARα in modulating the hepatocarcinogenic effects of perinatal exposure to a high-affinity human PPARα agonist in mice. Toxicol Sci. 183(1):81–92.
- Gervasi PG, Longo V, Naldi F, Panattoni G, Ursino F. 1991. Xenobiotic-metabolizing enzymes in human respiratory nasal mucosa. Biochem Pharmacol. 41(2):177–184.
- Giknis MLA, Clifford CB. 2005. Spontaneous neoplastic lesions in the Crl:CD-1(ICR) mouse in control groups from 18 month to 2 year studies. Charles River Laboratories; [accessed 2021 Nov 25]. https://www.criver.com/sites/default/files/resources/SpontaneousNeoplasticLesionsintheCrlCD-1ICRMouseinControlGroupsfrom18Monthto2YearStudies%E2%80%94March2005.pdf
- Gold LS, Manley NB, Slone TH, Ward JM. 2001. Compendium of chemical carcinogens by target organ: results of chronic bioassays in rats, mice, hamsters, dogs, and monkeys. Toxicol Pathol. 29(6):639–652.
- Gonzalez FJ, Shah YM. 2008. PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 246(1):2–8.
- Green T, Mainwaring GW, Foster JR. 1997. Trichloroethylene-induced mouse lung tumors: studies of the mode of action and comparisons between species. Fundam Appl Toxicol. 37(2):125–130.
- Green T, Lee R, Toghill A, Meadowcroft S, Lund V, Foster J. 2001. The toxicity of styrene to the nasal epithelium of mice and rats: studies on the mode of action and relevance to humans. Chem Biol Interact. 137(2):185–202.
- Green T, Toghill A, Foster JR. 2001. The role of cytochromes P-450 in styrene induced pulmonary toxicity and carcinogenicity. Toxicology. 169(2):107–117.
- Hahn FF, Gigliotti A, Hutt JA. 2007. Comparative oncology of lung tumors. Toxicol Pathol. 35(1):130–135.
- Harada T, Maronpot RR, Morris RW, Boorman GA. 1989. Observations on altered hepatocellular foci in National Toxicology Program two-year carcinogenicity studies in rats. Toxicol Pathol. 17(4 Pt 1):690–708.
- Harada T, Enomoto A, Boorman GA, Maronpot RR. 1999. Liver and gallbladder. In: Maronpot RR, Boorman GA, Gaul BW, editors. Pathology of the mouse: reference and atlas. 1st ed. St. Louis (MO): Cache River Press; p. 119–183.
- Haseman JK. 1983. A reexamination of false-positive rates for carcinogenesis studies. Fundam Appl Toxicol. 3(4):334–339.
- Hays T, Rusyn I, Burns AM, Kennett MJ, Ward JM, Gonzalez FJ, Peters JM. 2005. Role of peroxisome proliferator-activated receptor-alpha (PPARalpha) in bezafibrate-induced hepatocarcinogenesis and cholestasis. Carcinogenesis. 26(1):219–227.
- Heder AF, Hirsch-Ernst KI, Bauer D, Kahl GF, Desel H. 2001. Induction of cytochrome P450 2B1 by pyrethroids in primary rat hepatocyte cultures. Biochem Pharmacol. 62(1):71–71.
- Heusinkveld H, Braakhuis H, Gommans R, Botham P, Corvaro M, van der Laan JW, Lewis D, Madia F, Manou I, Schorsch F, et al. 2020. Towards a mechanism-based approach for the prediction of nongenotoxic carcinogenic potential of agrochemicals. Crit Rev Toxicol. 50(9):725–739.
- Holsapple MP, Pitot HC, Cohen SM, Cohen SH, Boobis AR, Klaunig JE, Pastoor T, Dellarco VL, Dragan YP. 2006. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 89(1):51–56.
- Huff J, Cirvello J, Haseman J, Bucher J. 1991. Chemicals associated with site-specific neoplasia in 1394 long-term carcinogenesis experiments in laboratory rodents. Environ Health Perspect. 93:247–270.
- IARC. 1987. IARC Monographs on the evaluation of the carcinogenic risks to humansIs. Vol. 1 to 42. Supplement 7, Isonicotinic acid hydrazide (isoniazid). Lyon: IARC Press; p. 227–229; [accessed 2021 Nov 25]. https://monographs.iarc.who.int/wp-content/uploads/2018/06/Suppl7.pdf
- IARC. 1996a. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 66. Clofibrate. Lyon: IARC Press; p. 391–426; [accessed 2021 Nov 25]. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Pharmaceutical-Drugs-1996
- IARC. 1996b. IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 66. Gemfibrozil. Lyon: IARC Press; p. 427–444; [accessed 2021 Nov 25]. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Pharmaceutical-Drugs-1996
- Ishmael J, Lithfield MH. 1988. Chronic toxicity and carcinogenic evaluation of permethrin in rats and mice. Fundam Appl Toxicol. 11(2):308–322.
- JMPR. 1999. Permethrin, toxicological evaluations, pesticide residues in food. Joint meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group; [accessed 2021 Nov 25]. https://inchem.org/documents/jmpr/jmpmono/v99pr07.htm
- Kitamura H, Inayama Y, Ito T, Yabana M, Piegorsch WW, Kanisawa M. 1987. Morphologic alteration of mouse Clara cells induced by glycerol: ultrastructural and morphometric studies. Exp Lung Res. 12(4):281–302.
- Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, et al. 2003. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 33(6):655–780.
- Kondo M, Miyata K, Nagahori H, Sumida K, Osimitz TG, Cohen SM, Lake BG, Yamada T. 2019. Involvement of peroxisome proliferator-activated receptor-alpha in liver tumor production by permethrin in the female mouse. Toxicol Sci. 168(2):572–696.
- Kondo M, Kikumoto H, Osimitz TG, Cohen SM, Lake BG, Yamada T. 2020. An evaluation of the human relevance of the liver tumors observed in female mice treated with permethrin based on mode of action. Toxicol Sci. 175(1):50–63.
- Kunimatsu T, Yamada T, Ose K, Sunami O, Kamita Y, Okuno Y, Seki T, Nakatsuka I. 2002. Lack of (anti-) androgenic or estrogenic effects of three pyrethroids (esfenvalerate, fenvalerate, and permethrin) in the Hershberger and uterotrophic assays. Regul Toxicol Pharmacol. 35(2 Pt 1):227–237.
- Lake BG. 2009. Species differences in the hepatic effects of inducers of CYP2B and CYP4A subfamily forms: relationship to rodent liver tumour formation. Xenobiotica. 39(8):582–596.
- Lake BG. 2018. Human relevance of rodent liver tumour formation by constitutive androstane receptor (CAR) activators. Toxicol Res (Camb). 7(4):697–717.
- Laskar AA, Younus H. 2019. Aldehyde toxicity and metabolism: the role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab Rev. 51(1):42–64.
- Lazarow PB, De Duve C. 1976. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA. 73(6):2043–2046.
- Lee S, Pineau T, Drago J, Lee E, Owens J, Kroetz D, Fernandez-Salguero P, Westphal H, Gonzalez F. 1995. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 15(6):3012–3022.
- Lewis DFV, Ito Y, Lake BG. 2009. Molecular modelling of CYP2F substrates: comparison of naphthalene metabolism by human, rat and mouse CYP2F subfamily enzymes. Drug Metabol Drug Interact. 24(2–4):229–257.
- Lewis JL, Nikula KJ, Novak R, Dahl AR. 1994. Comparative localization of carboxylesterase in F344 rat, beagle dog, and human nasal tissue. Anat Rec. 239(1):55–64.
- Lin KK, Ali MW. 1994. Statistical review and evaluation of animal tumorigenecity studies. In: Buncher CR, Tsay JY, editors. Statistics in the pharmacutical industry. 2nd ed. New York (NY): Marcel Dekker, Inc.; p. 19–57.
- Lumsden AB, McLean A, Lamb D. 1984. Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax. 39(11):844–849.
- Madan A, DeHaan R, Mudra D, Carroll K, LeCluyse E, Parkinson A. 1999. Effect of cryopreservation on cytochrome P-450 enzyme induction in cultured rat hepatocytes. Drug Metab Dispos. 27(3):327–335.
- Manenti G, Galbiati F, Noci S, Dragani TA. 2003. Outbred CD-1 mice carry the susceptibility allele at the pulmonary adenoma susceptibility 1 (Pas1) locus. Carcinogenesis. 24(6):1143–1148.
- Maronpot RR, Haseman JK, Boorman GA, Eustis SE, Rao GN, Huff JE. 1987. Liver lesions in B6C3F1 mice: the National Toxicology Program, experience and position. Arch Toxicol Suppl. 10:10–26.
- Maronpot RR. 2009. Biological basis of differential susceptibility to hepatocarcinogenesis among mouse strains. J Toxicol Pathol. 22(1):11–33.
- Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, Singh B, Ward JM. 2010. Hepatic enzyme induction: histopathology. Toxicol Pathol. 38(5):776–795.
- Matsuyama R, Kitamoto S, Tomigahara Y. 2018. Lack of genotoxic potential of permethrin in mice evaluated by the comet assay and micronucleus test. Toxicol Environ Chem. 100(1):92–102.
- Mattes PM, Mattes WB. 1992. alpha-Naphthyl butyrate carboxylesterase activity in human and rat nasal tissue. Toxicol Appl Pharmacol. 114(1):71–76.
- McDowell EM, Barrett LA, Glavin F, Harris CC, Trump BF. 1978. The respiratory epithelium. I. Human bronchus. J Natl Cancer Inst. 61(2):539–549.
- McGregor D, Boobis A, Binaglia M, Botham P, Hoffstadt L, Hubbard S, Petry T, Riley A, Schwartz D, Hennes C. 2010. Guidance for the classification of carcinogens under the globally harmonised system of classification and labelling of chemicals (GHS). Crit Rev Toxicol. 40(3):245–285.
- McMullen PD, Bhattacharya S, Woods CG, Pendse SN, McBride MT, Soldatow VY, Deisenroth C, LeCluyse EL, Clewell RA, Andersen ME. 2020. Identifying qualitative differences in PPARα signaling networks in human and rat hepatocytes and their significance for next generation chemical risk assessment methods. Toxicol In Vitro. 64:104463.
- Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE. 2003. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 33(6):591–653.
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 34(1):1–18.
- Meek ME, Palermo CM, Bachman AN, North CM, Jeffrey Lewis R. 2014. Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 34(6):595–606.
- Muzio G, Maggiora M, Paiuzzi E, Oraldi M, Canuto RA. 2012. Aldehyde dehydrogenases and cell proliferation. Free Radic Biol Med. 52(4):735–746.
- Nikitin AY, Alcaraz A, Anver MR, Bronson RT, Cardiff RD, Dixon D, Fraire AE, Gabrielson EW, Gunning WT, Haines DC, et al. 2004. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 64(7):2307–2316.
- OECD. 2012. Guidance document 116 on the Conduct and design of chronic toxicity and carcinogenicity studies, supporting test guidelines 451, 452 and 453. 2nd ed. OECD series on testing and assessment, No. 116, NV/JM/MONO(2011)47. Paris: OECD Publishing; [accessed 2021 Nov 25]. https://www.oecd-ilibrary.org/environment/guidance-document-116-on-the-conduct-and-design-of-chronic-toxicity-and-carcinogenicity-studies-supporting-test-guidelines-451-452-and-453_9789264221475-en
- Oesch F, Fabian E, Landsiedel R. 2019. Xenobiotica-metabolizing enzymes in the lung of experimental animals, man and in human lung models. Arch Toxicol. 93(12):3419–3489.
- Ogata K, Liu Y, Ohara A, Kawamoto K, Kondo M, Kobayashi K, Fukuda T, Asano H, Kitamoto S, Lake BG, et al. 2021. Club cells are the primary target for permethrin-induced mouse lung tumor formation. Toxicol Sci. 184(1):15–32.
- Park SY, Hong JY, Lee SY, Lee SH, Kim MJ, Kim SY, Kim KW, Shim HS, Park MS, Lee CG, et al. 2021. Club cell-specific role of programmed cell death 5 in pulmonary fibrosis. Nat Commun. 12(1):2923.
- Patel M, Lu L, Zander DS, Sreerama L, Coco D, Moreb JS. 2008. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer. 59(3):340–349.
- Peacock A, Peacock PR. 1966. The results of prolonged administration of isoniazid to mice, rats and hamsters. Br J Cancer. 20(2):307–325.
- Peters JM, Cattley RC, Gonzalez FJ. 1997. Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 18(11):2029–2033.
- Peters JM, Aoyama T, Cattley RC, Nobumitsu U, Hashimoto T, Gonzalez FJ. 1998. Role of peroxisome proliferator-activated receptor alpha in altered cell cycle regulation in mouse liver. Carcinogenesis. 19(11):1989–1994.
- Plopper CG, Hill LH, Mariassy AT. 1980a. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp Lung Res. 1(2):171–180.
- Plopper CG, Mariassy AT, Hill LH. 1980b. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung: I. A comparison of rabbit, guinea pig, rat, hamster, and mouse. Exp Lung Res. 1(2):139–154.
- Plopper CG, Hyde DM. 2015. Chapter 7 – epithelial cells of the bronchiole. In: Parent RA, editors. Comparative biology of the normal lung. 2nd ed. San Diego (CA): Academic Press; p. 83–92.
- Pors K, Moreb JS. 2014. Aldehyde dehydrogenases in cancer: an opportunity for biomarker and drug development? Drug Discov Today. 19(12):1953–1963.
- Poteracki J, Walsh KM. 1998. Spontaneous neoplasms in control Wistar rats: a comparison of reviews. Toxicol Sci. 45(1):1–8.
- Quist E, Boorman G, Cullen J, Maronpot R, Remick A, Swenberg JA, Freshwater L, Hardisty J. 2019. Hepatocellular neoplasms in CD-1 mice chronically exposed to permethrin: results from a 2-year oral carcinogenicity study. Toxicol Pathol. 47(1):11–17.
- Rao GN, Birnbaum LS, Collins JJ, Tennant RW, Skow LC. 1988. Mouse strains for chemical carcinogenicity studies: overview of a workshop. Fundam Appl Toxicol. 10(3):385–394.
- Renne R, Brix A, Harkema J, Herbert R, Kittel B, Lewis D, March T, Nagano K, Pino M, Rittinghausen S, et al. 2009. Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol. 37(7 Suppl):5S–73S.
- Reynolds SD, Malkinson AM. 2010. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol. 42(1):1–4.
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. 2008. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci. 103(1):46–56.
- Rusiecki JA, Patel R, Koutros S, Beane-Freeman L, Landgren O, Bonner MR, Coble J, Lubin J, Blair A, Hoppin JA, et al. 2009. Cancer incidence among pesticide applicators exposed to permethrin in the agricultural health study. Environ Health Perspect. 117(4):581–586.
- Saghir SA, Rick DL, McClymont EL, Zhang F, Bartels MJ, Bus JS. 2009. Mechanism of ethylbenzene-induced mouse-specific lung tumor: metabolism of ethylbenzene by rat, mouse, and human liver and lung microsomes. Toxicol Sci. 107(2):352–366.
- Schulte-Hermann R, Timmermann-Trosiener I, Schuppler J. 1983. Promotion of spontaneous preneoplastic cells in rat liver as a possible explanation of tumor production by nonmutagenic compounds. Cancer Res. 43(2):839–844.
- Seed J, Carney EW, Corley RA, Crofton KM, DeSesso JM, Foster PM, Kavlock R, Kimmel G, Klaunig J, Meek ME, et al. 2005. Overview: using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol. 35(8–9):664–672.
- Shultz MA, Morin D, Chang AM, Buckpitt A. 2001. Metabolic capabilities of CYP2F2 with various pulmonary toxicants and its relative abundance in mouse lung subcompartments. J Pharmacol Exp Ther. 296(2):510–519.
- Singh G, Katyal SL. 1997. Clara cells and Clara cell 10 kD protein (CC10). Am J Respir Cell Mol Biol. 17(2):141–143.
- Smith MN, Greenberg SD, Spjut HJ. 1979. The Clara cell: a comparative ultrastructural study in mammals. Am J Anat. 155(1):15–30.
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, et al. 2001. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol. 34(2):146–152.
- Stearns TM, Cario CL, Savage HS, Sundberg JP, Paigen B, Berndt A. 2012. Early gene expression differences in inbred mouse strains with susceptibility to pulmonary adenomas. Exp Mol Pathol. 93(3):455–461.
- Strupp C, Banas DA, Cohen SM, Gordon EB, Jaeger M, Weber K. 2012. Relationship of metabolism and cell proliferation to the mode of action of fluensulfone-induced mouse lung tumors: analysis of their human relevance using the IPCS framework. Toxicol Sci. 128(1):284–294.
- Strupp C, Bomann W, Cohen SM, Weber K. 2016. Relationship of metabolism and cell proliferation to the mode of action of fluensulfone-induced mouse lung tumors. II: additional mode of action studies. Toxicol Sci. 154(2):296–308.
- Tang Y, M Vanlandingham M, Wu Y, Beland FA, Olson GR, Fang J-L. 2018. Role of peroxisome proliferator-activated receptor alpha (PPARα) and PPARα-mediated species differences in triclosan-induced liver toxicity. Arch Toxicol. 92(11):3391–3402.
- Tateno C, Yamamoto T, Utoh R, Yamasaki C, Ishida Y, Myoken Y, Oofusa K, Okada M, Tsutsui N, Yoshizato K. 2015. Chimeric mice with hepatocyte-humanized liver as an appropriate model to study human peroxisome proliferator-activated receptor-α. Toxicol Pathol. 43(2):233–248.
- Tateno C, Kojima Y. 2020. Characterization and applications of chimeric mice with humanized livers for preclinical drug development. Lab Anim Res. 36(2):2.
- Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, et al. 2010. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38(7 Suppl):5S–81S.
- Thoolen B, Ten Kate FJ, van Diest PJ, Malarkey DE, Elmore SA, Maronpot RR. 2012. Comparative histomorphological review of rat and human hepatocellular proliferative lesions. J Toxicol Pathol. 25(3):189–199.
- Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. 2004. World Health Organization classification of tumours. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press; p. 82–87.
- US.EPA. 1988. Memorandum. 52 Page(s). Esther Rinde. Toxicology Branch I. Peer Review of Permethrin. Registration Data info removed from pages 9–15, US. EPA. Attachment(s) Following: October 14, 1988. Memorandum. John Doherty. Toxicology Branch I. Peer Review of Permethrin. Addendum: Historical control data for lung & liver tumors for the CD-1 mouse strain compiled by the Bio/Dynamics Laboratory for studies terminating in 1979 to 1982; [accessed 2022 Jan 19]. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/109701/109701-153.pdf
- US.EPA. 1994. PRN 94-5: requests for re-considerations of carcinogenicity peer review decisions based on changes in pathology diagnoses; [accessed 2022 Jan 19]. https://www.epa.gov/pesticide-registration/prn-94-5-requests-re-considerations-carcinogenicity-peer-review-decisions#policy
- US.EPA. 2000. Data evaluation record: Permethrin/109701. Study type: carcinogenicity – mouse (OPPTS 870.4200b/OECD 451). MRID 45597105 (Main Study), 45597104; [accessed 2022 Jan 19]. https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-109701_undated_a.pdf
- US.EPA. 2002. Memorandum. Permethrin: Report of the Cancer Assessment Review Committee (Third Evaluation). Health Effects Division, Office of Pesticides Program, US. EPA, TXR No. 0051220; [accessed 2022 Jan 19]. https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-109701_23-Oct-02_a.pdf
- US.EPA. 2003. Memorandum. Cypermethrin and Zeta-cypermethrin – 5th Report of the Hazard Identification Assessment Review Committee. TXR NO. 0051578.
- US.EPA. 2005a. Guidelines for carcinogen risk assessment. Risk Assessment Forum, Washington, DC. EPA/639/P-03/001F; [accessed 2021 Nov 25]. https://www3.epa.gov/airtoxics/cancer_guidelines_final_3-25-05.pdf
- US.EPA. 2005b. Memorandum. Flonicamid: Report of the Cancer Assessment Review Committee, PC Cord: 128016. Health Effects Division, Office of Pesticides Program, US. EPA, TXR No.0052013; [accessed 2021 Nov 25]. https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/128016/128016-2005-02-24a.pdf
- US.EPA. 2009. Permethrin Facts. EPA document No. 738-F-09-001; [accessed 2021 Nov 25]. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-109701_1-Aug-09.pdf
- US.EPA. 2020a. Memorandum. Permethrin. Report of the Cancer Assessment Review Committee (Fourth Evaluation). PC Cord: 109701. Health Effects Division, Office of Pesticides Program, US. EPA. TXR No. 0057953; [accessed 2022 Feb 15]. https://www.regulations.gov/document/EPA-HQ-OPP-2011-0039-0124
- US.EPA. 2020b. Memorandum. Permethrin: Human Health Risk Assessment for New Use on “Fruit, Small, Vine Climbing, Except Fuzzy Kiwifruit, Subgroup 13-07F”; Multiple Crop Group Conversions/Expansions; and the Establishment of a Tolerance without a U.S. Registration for Tea, AND the Revised Draft Risk Assessment (DRA) for Registration Review; [accessed 2021 Nov 25]. https://www.regulations.gov/document/EPA-HQ-OPP-2011-0039-0130
- US.FDA. 2001. Center for Drug Evaluation and Research, Guidance for Industry: Statistical Aspects of the Design, Analysis, and Interpretation of Chronic Rodent Carcinogenicity Studies of Pharmaceuticals; [accessed 2021 Nov 25]. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079272.pdf
- Vachon J, Page-Lariviere F, Sirard MA, Rodriguez MJ, Levallois P, Campagna C. 2018. Availability, quality, and relevance of toxicogenomics data for human health risk assessment: a scoping review of the literature on trihalomethanes. Toxicol Sci. 163(2):364–373.
- Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG. 1999. Early events in naphthalene-induced acute Clara cell toxicity: comparison of membrane permeability and ultrastructure. Am J Respir Cell Mol Biol. 21(1):44–53.
- Vassallo JD, Hicks SM, Born SL, Daston GP. 2004. Roles for epoxidation and detoxification of coumarin in determining species differences in Clara cell toxicity. Toxicol Sci. 82(1):26–33.
- Wang XY, Keefe KM, Jensen-Taubman SM, Yang D, Yan K, Linnoila RI. 2012. Novel method for isolation of murine Clara cell secretory protein-expressing cells with traces of stemness. PLOS One. 7(8):e43008.
- WHO. 2014. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2nd ed. Geneva: World Health Organization (WHO); [accessed 2022 Jan 20]. https://www.who.int/publications/i/item/9789241548748
- WHO. 2019. World Health Organization (WHO) specifications and evaluations for public health pesticides, Permethrin (40:60 cis:trans isomer ratio); [accessed 2021 Nov 25]. https://extranet.who.int/pqweb/sites/default/files/vcp-documents/WHOVC-SP_Permethrin%20%2840%2060%20cis%20trans%20isomer%20ratio%29_2019.pdf
- Williams GM. 1997. Chemicals with carcinogenic activity in the rodent liver; mechanistic evaluation of human risk. Cancer Lett. 117(2):175–188.
- Wolf DC, Cohen SM, Boobis AR, Dellarco VL, Fenner-Crisp PA, Moretto A, Pastoor TP, Schoeny RS, Seed JG, Doe JE. 2019. Chemical carcinogenicity revisited 1: a unified theory of carcinogenicity based on contemporary knowledge. Regul Toxicol Pharmacol. 103:86–92.
- Wolff GL, Roberts DW, Morrissey RL, Greenman DL, Allen RR, Campbell WL, Bergman H, Nesnow S, Frith CH. 1987. Tumorigenic responses to lindane in mice: potentiation by a dominant mutation. Carcinogenesis. 8(12):1889–1897.
- Wong AP, Keating A, Waddell TK. 2009. Airway regeneration: the role of the Clara cell secretory protein and the cells that express it. Cytotherapy. 11(6):676–687.
- Wood CE, Hukkanen RR, Sura R, Jacobson-Kram D, Nolte T, Odin M, Cohen SM. 2015. Scientific and Regulatory Policy Committee (SRPC) review: interpretation and use of cell proliferation data in cancer risk assessment. Toxicol Pathol. 43(6):760–775.
- Yamada T, Kondo M, Miyata K, Ogata K, Kushida M, Sumida K, Kawamura S, Osimitz TG, Lake BG, Cohen SM. 2017. An evaluation of the human relevance of the lung tumors observed in female mice treated with permethrin based on mode of action. Toxicol Sci. 157(2):465–486.
- Yamada T. 2018. Case examples of an evaluation of the human relevance of the pyrethroids/pyrethrins-induced liver tumours in rodents based on the mode of action. Toxicol Res (Camb). 7(4):681–696.
- Yamada T. 2021. Review/featured article Application of humanized mice to toxicology studies: evaluation of the human relevance of the mode of action for rodent liver tumor formation by activators of the constitutive androstane receptor (CAR). J Toxicol Pathol. 34(4):283–297.
- Yamada T, Cohen SM, Lake BG. 2021. Critical evaluation of the human relevance of the mode of action for rodent liver tumor formation by activators of the constitutive androstane receptor (CAR). Crit Rev Toxicol. 51(5):373–394.
- Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. 2008. The PPAR alpha-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicol Sci. 101(1):132–139.
- Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. 1994. Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. FASEB J. 8(8):479–488.
