Abstract
Even though exposure to chlorine gas has been quite frequent in the past few decades, no specific antidotes exist. This umbrella review aimed to investigate possible recommendations for treatment after a chlorine gas exposure. A published systematic review protocol that adapted the existing Navigation Guide methodology was used for including studies without comparator. Using PubMed, Web of Science, Google scholar for all potentially relevant systematic reviews, two authors independently included papers and extracted data. The risk of bias and quality of evidence was assessed by two independent review teams blinded to each other. A qualitative summary of the study findings was conducted for this overview. There were a total of 31 studies, from 4 systematic reviews, that met the inclusion criteria, comprising 3567 reported cases, with only two studies with comparators. Six studies reported pre-hospital management of patients after exposure to chlorine gas. With respect to the treatment, the most used were oxygen therapy, endotracheal intubation, β2-agonists, and corticosteroids. This review found a high quality of evidence for the effectiveness of pre-hospital management (i.e. exposure cessation) on survival at hospital discharge after exposure to chlorine gas. Oxygen administration was effective with moderate quality of evidence, as well as other types of treatment (e.g. β2, corticosteroids), but with a low level of evidence. This umbrella review highlighted the low level of evidence for existing treatments of chlorine gas poisoning. This project was supported by the French Pays de la Loire region and Angers Loire Métropole (TEC-TOP project). There is no award/grant number. The review protocol was registered on PROSPERO under the registration number CRD42021231524
1. Introduction
Chlorine is an irritant gas whose toxicity depends on the concentration, duration of exposure (Squadrito et al. Citation2010), preexisting respiratory conditions (D'Alessandro et al. Citation1996; White and Martin Citation2010; Kim et al. Citation2014) and whether the person exposed is a current or former smoker (D'Alessandro et al. Citation1996; White and Martin Citation2010; Kim et al. Citation2014). When inhaled, the respiratory tract is the primary initial target organ (Milanez Citation2020) with acute health consequences ranging from irritation of the upper respiratory airways (Winder Citation2001; Carpenter et al. Citation2016) to acute lung injury, which can lead to pulmonary obstruction (White and Martin Citation2010), reactive airway dysfunction syndrome (Winder Citation2001; Kim et al. Citation2014), acute respiratory distress syndrome (White and Martin Citation2010; Shin et al. Citation2017) and, rarely, death (Winder Citation2001; Kim et al. Citation2014). Further long-term damage may occur like reactive airway dysfunction syndrome (Donnelly and FitzGerald Citation1990; Schwartz et al. Citation1990; Schönhofer et al. Citation1996; Duncan et al. Citation2011). At low concentrations (<40ppm), victims develop mild to moderate mucous membrane irritation (throat and eyes) and reversible bronchospasm and increased airway resistance, clinically reflected by cough, dyspnea, and chest pain. At higher chlorine concentrations (>40ppm), distal areas of lungs are reached, leading to pulmonary edema, toxic pneumonitis and death, if the exposure is too long (>30min at 430 ppm) or too intense (>1000 ppm) (Winder Citation2001). Other organs may be affected such as eyes with an irritation of the conjunctivae and skin with burns (Mangat et al. Citation2012).
Exposure to chlorine gas commonly occurs in-home cleaning misadventures by mixing household products (Cevik et al. Citation2009; Mangat et al. Citation2012; Shin et al. Citation2017), with industrial accidents (Bellenger and Frizzi Citation2014; Kim et al. Citation2014; Carpenter et al. Citation2016), or at swimming-pool (swimming-pool tablets being often on chlorine) (Li et al. Citation2011; Vajner and Lung Citation2013; Matos et al. Citation2017). It has also been used as a chemical warfare agent. Hypochlorite being available in many detergents and chlorine-based bleach, those accidents can occur quite frequently. However, there is no specific antidote or pre-exposure countermeasures for chlorine toxicity in humans and no clear management has been established although its exposure has been quite frequent those last decades. In addition, different systematic reviews have been carried out and none have been able to define what management should be done. This umbrella review aimed to study possible recommendations for appropriate treatment that must be administered after exposure to chlorine gas.
2. Methods
To guide this umbrella review, we developed a systematic review protocol published and freely available (Nambiema et al. Citation2021).
2.1. Developed protocol
We applied the methodology of a systematic review protocol that has been specifically developed to consider the inclusion and integration of case reports/studies and case series (Nambiema et al. Citation2021). The systematic review protocol, used as our guiding methodological framework, was prepared in accordance with the usually structured methodology for systematic reviews (Preferred Reporting Items for Systematic Review and Meta-analysis [PRISMA], PRISMA-P [for Protocols] and Navigation guide) (Liberati et al. Citation2009; Woodruff and Sutton Citation2011; Lam et al. Citation2014; Woodruff and Sutton Citation2014; Moher et al. Citation2015; Shamseer et al. Citation2015; Page et al. Citation2021). The guide applies established systematic review methods from clinical medicine, including standard Cochrane methods for systematic reviews of interventions, to the field of environmental and occupational health. These methods ensure systematic and rigorous evidence on environmental and occupational risk factors synthesis that reduces bias and maximizes transparency (Woodruff and Sutton Citation2014). The need for additional methodological development and refinement of the relatively new Navigation Guide was recognized (Woodruff and Sutton Citation2014).
We registered the protocol in PROSPERO under the registration number CRD42021231524. This protocol adheres to the statement of PRISMA-P (Moher et al. Citation2015; Shamseer et al. Citation2015), with the abstract adhering to the reporting items for systematic reviews in journal and conference abstracts (PRISMA-A) (Beller et al. Citation2013; Page et al. Citation2021). Our review has been presented in concordance with the PRISMA guidelines (Liberati et al. Citation2009; Page et al. Citation2021).
The overall protocol differentiates itself by defining two independent teams of reviewers: a classical team and a case team. The classical team included studies from systematic reviews with control groups and an acceptable comparison group (case reports/studies and case series were excluded); in essence, a more traditional umbrella review where evidence from case reports/studies and case series were not considered. The case team included both classical studies and case reports/studies and case series; in essence, a comparison group to identify differences in umbrella review conclusions when including and integrating evidence from case reports/studies and case series. Both teams have identified studies that meet our inclusion criteria, conducted separate analyses and risk of bias evaluations, along with the overall quality of evidence assessments, and syntheses of strengths of evidence. Each team was blind to the other team's results during the process and the results. Once the umbrella review was completed, the results of each team were presented, evaluated, and compared.
2.2. Eligibility criteria
The eligibility criteria for the review were determined according to the criteria of PICOS (Population, Intervention, Comparison, Outcome and Setting) (Higgins et al. Citation2020):
2.2.1. Population
We included all types of systematic review studies about human chlorine gas exposure. Systematic reviews not translated in English, or not available were excluded. Within systematic reviews articles about human chlorine gas exposure were selected. Those not translated in English, or not available were excluded. Furthermore, articles with no treatment or no information about the management, off-topic articles, and physiopathology studies were not included. In addition, animal studies were not included for the methodological purpose of the review although the framework of toxicological systematic reviews is already proven adequate for retrieving this type of toxicant data.
2.2.2. Intervention
We considered systematic reviews that included studies where all treatments and management were applied.
2.2.3. Comparator
We included systematic reviews that included studies with a comparator. The accepted comparator included oxygen and exposure cessation. We compared two review methodologies: one included and the other excluded high-quality case reports/studies and case series.
2.2.4. Outcomes
The primary outcome of this review included survival at hospital discharge and later available, without any sequelae (including asthma). The additional outcome was the proportion of sequelae.
2.2.5. Study design
All original human studies referenced in eligible systematic reviews and available in a language translated by online programs, i.e. Google Translate, (in English or French) were included. Thereafter, only the case team specified in its research strategy and included separately (human subjects research without controls) case reports/studies and case series.
2.2.6. Inclusion/exclusion criteria
For this overview, all studies of any design were included if they fulfilled all the eligibility criteria. To be integrated into the overall body of evidence, cases reports/studies and case series had to meet pre-defined criteria that were well-documented, scientifically rigorous, and followed ethical practices, following the CARE guidelines (for CAse Reports) (Gagnier et al. Citation2013; Riley et al. Citation2017) and the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports and for Case Series (Moola et al. Citation2020; Munn et al. Citation2020) that allows for the inclusion of case reports to facilitate completeness, transparency and data analysis in the systematic review process. Studies that were conducted using unethical practices were excluded.
2.3. Information sources and search strategy
For this overview, two electronic databases (PubMed, Web of Science) and the Internet search engine “Google Scholar” were searched for all systematic reviews published between January 1, 2007, and December 31, 2020, according to the eligibility criteria.
We developed detailed search strategies for each database to account for differences in controlled vocabulary and syntax rules. The final search equations used were as follows:
PubMed: ("chlorine gas" OR "chlorine-induced" OR" chlorine-exposed") AND ("therapy" OR "treatment" OR "post-exposure") AND review
Web of Science: ("chlorine gas" OR "chlorine-induced" OR" chlorine-exposed") AND ("therapy" OR "treatment" OR "post-exposure") AND review
Google Scholar: ("chlorine gas" OR "chlorine-induced" OR" chlorine-exposed") AND ("therapy" OR "treatment" OR "post-exposure") AND review
2.4. Selection process
Following a systematic search in all the above databases, each of the two independent teams of reviewers (the classic team and the case team) separately uploaded, in accordance with the eligibility criteria, the results of the literature search to the Covidence systematic review software (Covidence systematic review software, V.H.I Citationn.d.), a web-based tool that allows a double selection on the title, abstract, and then full text by independent review authors. Duplicates were identified and removed from all study uploaded records. Thereafter, two review authors form each team (GC and JBBR on one side and JB and NRD on the other) independently screened each title and abstract to exclude studies that did not meet the inclusion criteria (step 1). These authors then independently examined full‐text articles for eligibility (step 2). When necessary, information was requested from the publication authors to resolve eligibility issues. Where uncertainties or discrepancies arose, we resolved them through discussion, and with a third author (AD or AN) of the review where necessary.
Where a study record identified during the search was authored by a member of the reviewing research team, or where that member participated in the identified study, that study record was re-assigned to another reviewing team member.
2.5. Data collection process and items
Data were extracted directly from systematic reviews included in this umbrella review. Review authors piloted the data extraction form and made appropriate changes. All reviewing teams used standardized forms, and each review member independently extracted the data from included studies. To ensure consistency across reviewers, calibration exercises (reviewer training) were conducted prior to starting the reviews. Data extraction was performed electronically with the Covidence systematic review software (Covidence systematic review software, V.H.I Citationn.d.). We compared data for each study and resolved any discrepancies by discussion and by consulting a third review author when consensus could not be reached. We extracted the following data from each study as follows:
General information: study record (study authors, lead author contacts details, study year), study type (study design, study period, follow-up period)
Study characteristics: participants (total number, population description, inclusion/exclusion criteria, method of recruitment), exposure, and outcome
Source of population: geographic location, number of cases, age class, medical background (asthma, smoking, pulmonary affection), exposure circumstances, exposure duration
Outcome: discharge treatment, follow-up, a summary of the outcome
Comparator: group definition, exposure, treatment, treatment dose/ route/ administration time, outcome, follow-up
Management: preliminary measures, testing (besides vital signs and chest auscultation), endotracheal intubation, oxygen, β2-agonists, corticosteroids, others
Data to assess the risk of bias: source population representation, blinding, exposure assessment, outcome assessment, confounding, incomplete outcome data, selective outcome reporting, possible conflicts of interest for study authors, study funding sources, and other sources of bias
2.6. Dealing with missing data
When information was not available or further information was necessary, we contacted the authors of the studies, using the contact details provided in the principal study record, to attempt to obtain the missing data. If we did not receive a positive response from the principal study author, we sent follow-up emails twice, at two and four weeks. If we were unable to obtain the missing information after the follow-up emails, we excluded the study. In the event that a study met our inclusion criteria but did not provide useful outcome data for extraction or inclusion in the meta-analyses, we reported this in the “Characteristics of included studies” table and in the main text.
2.7. Risk of bias assessment
Review authors independently assessed the risk of bias within the included studies by using criteria described in the Navigation Guide tool for human studies to determine the methodological quality (Woodruff and Sutton Citation2011; Johnson et al. Citation2014). The Navigation Guide tool for human studies is based on the standard risk of bias assessment methods of Cochrane that reduces bias and maximize transparency (Higgins et al. Citation2021). This method covers nine risks of bias domains that have been used for human studies: (i) source population representation; (ii) blinding and intervention; (iii) exposure or intervention assessment; (iv) outcome assessment; (v) confounding; (vi) incomplete outcome data; (vii) selective outcome reporting; (viii) conflict of interest; and (ix) other sources of bias. For each section of the tool, the procedures undertaken for each study were described and the risk of bias was rated as “low risk”; “probably low risk”; “probably risk”; “high risk”; or “not applicable”. The risk of bias was assessed on the levels of the individual study and the entire body of evidence. Most of the text from these instructions and criteria for judging the risk of bias has been adopted verbatim or adapted from one of the latest Navigation Guide systematic reviews used by WHO/ILO (Woodruff and Sutton Citation2011; Johnson et al. Citation2014; Descatha et al. Citation2018). Two study authors (GC and JBBR on one side and JB and NRD on the other) independently judged (or assessed) the risk of bias by outcome according to each type of study. The toxicologist (AD) of the team was involved in the analysis and supervision of the task and resolved any disagreements that arose.
To our knowledge, there are no standard tools for assessing the risk of bias in case reports/studies and case series for systematic reviews for toxic exposure identification and risk management in toxicology. For these types of studies, the text from these instructions and criteria for judging the risk of bias has been adopted verbatim or adapted from one of the latest Navigation Guide systematic reviews (Lam et al. Citation2017), and further described in Supplementary material, Appendix 1 that distinguishes high-quality case reports/studies and case series for systematic reviews. To ensure consistency across reviewers, calibration exercises (reviewer training) were conducted prior to starting the risk of bias assessments for case reports/studies and case series.
2.8. Effect measures
Due to the important heterogeneity of the included studies with respect to population, treatment, and other factors, as well as study methods, and as specified in the protocol registered in PROSPERO, we decided that the data were not sufficiently homogeneous to conduct meta-analyses. Therefore, we were unable to quantify the effect measures.
2.9. Data synthesis
As specified previously, we decided that the data were not sufficiently homogeneous to conduct meta-analyses. Therefore, a systematic narrative synthesis, i.e. a qualitative summary of the study findings, was carried out for this overview with the information presented in the text and tables to summarize and explain the characteristics and findings of the included studies as well as exposure circumstances and its management.
2.10. Subgroup analysis and investigation of heterogeneity
Owing to the absence of meta-analyses and the substantial heterogeneity of the included studies, we did not perform subgroup analyses. We planned to conduct analyses separately for intervention studies (RCT), observational studies with comparator, and observational studies without comparator but there were insufficient data. We also planned to investigate potential sources of heterogeneity, whether there was evidence for differences in effect estimates by country, study design or patient characteristics. However, we did not conduct such sensitivity analyses in the absence of meta-analyses to provide effect estimates.
2.11. Quality of evidence assessment
We assessed the quality of evidence using an adapted version of the Evidence Quality Assessment Tool in the Navigation Guide (Nambiema et al. Citation2021). This tool is based on the GRADE approach (Guyatt et al. Citation2008). The assessment of the entire body of evidence by the outcome was performed by two teams, again in a blinded fashion, one with the results of the case-series/control studies synthesis, the other without. Data synthesis was performed independently by the classical and case teams. At least two review authors in each team assessed the quality of evidence, with any disagreements resolved by a third review author. We downgraded the quality of evidence for the following five GRADE reasons (Balshem et al. Citation2011): (i) quality of study limitations (risk of bias); (ii) indirectness; (iii) inconsistency; (iv) imprecision; and (v) publication bias.
We graded the overall quality of evidence by the outcome, using the three Navigation Guide quality of evidence ratings: “high”, “moderate” and “low”. Within each of the relevant reasons for downgrading, we ranked any concern per reason as “none”, “serious” or “very serious”. Furthermore, and in accordance with the Navigation Guide, evidence ratings started at “high” for randomized control studies, “moderate” for observational studies and “low” for case reports/ series. Indeed, if case reports/studies and series might be considered, it is only on low evidence. However, not all studies without a comparator could reach a sufficient level of evidence. Concerning case reports/studies and case series, we start from the lowest point and therefore we cannot consider evidence higher than low. We downgraded the quality for no concern by null (0), for serious concern by one grade (−1), and for very serious concern by two grades (−2). We considered the following criteria for upgrading the quality of evidence, if appropriate: large magnitude of effect, dose-response gradient, and plausible confounding effect. Complete instructions for making the quality of evidence are presented in Supplementary material, Appendix 2.
3. Results
3.1. Description of included reviews
3.1.1. Study selection
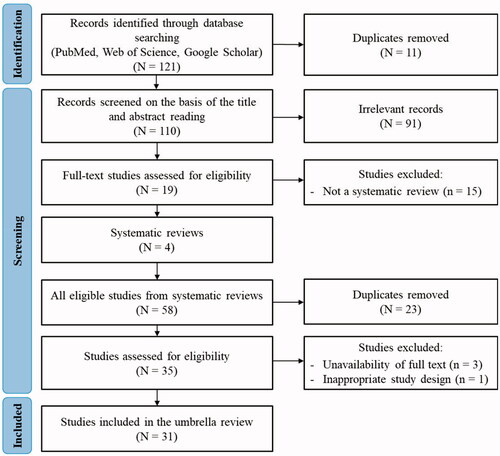
A total of 121 records were found using the search strategy. After duplicate removal, we identified 110 potentially relevant articles that were screened for eligibility. We excluded 91 of these records based on title and abstract. We, therefore, conducted a full-text review of 19 articles for eligibility, and finally 4 systematic reviews (SRs) (de Lange and Meulenbelt Citation2011; Govier and Coulson Citation2018; Huynh Tuong et al. Citation2019; Zellner and Eyer Citation2020) were included. From the 4 included SRs, we uploaded all articles (n = 58) for the second screening. After the authors removed duplicates, 35 articles were eligible. Of these 35 remaining articles, 4 were excluded for different reasons (Pino et al. Citation1993; Traub et al. Citation2002; Russell et al. Citation2006; Hoyle and Svendsen Citation2016) (). Finally, a total of 31 studies that met the inclusion criteria were included in this umbrella review. All included studies were present in the initial database search. Excluded articles are listed in including reasons for exclusion. The study selection process, which follows the PRISMA methodology (Page et al. Citation2021), is illustrated in .
Table 1. Characteristics of excluded studies.
3.1.2. Study characteristics
and show the characteristics of the included studies which cover a total of 3567 cases.
Table 2. Characteristics of included studies with a comparator.
Table 3. Characteristics of included studies without comparator.
3.1.2.1. Design
Of the thirty-one included studies, twenty-seven were case reports/studies (Chester et al. Citation1977; Gapany-Gapanavicius et al. Citation1982; Fleta et al. Citation1986; Vinsel Citation1990; Heidemann and Goetting Citation1991; Myers Citation1997; Parimon et al. Citation2004; Akdur et al. Citation2006; Vohra and Clark Citation2006; Howard et al. Citation2007; Babu et al. Citation2008; Cevik et al. Citation2009; Sever et al. Citation2009; Van Sickle et al. Citation2009; Ho et al. Citation2010; Li et al. Citation2011; Mangat et al. Citation2012; Vajner and Lung Citation2013; Bellenger and Frizzi Citation2014; Kim et al. Citation2014; Mackie et al. Citation2014; Carpenter et al. Citation2016; Warren et al. Citation2016; Matos et al. Citation2017; Shin et al. Citation2017), three were case series (Bosse Citation1994; Güloğlu et al. Citation2002; Mohan et al. Citation2010) and one study was randomized control study (Aslan et al. Citation2006). Two of the included studies were studies with comparators (Chester et al. Citation1977; Aslan et al. Citation2006).
3.1.2.2. Setting
The included studies were carried out in twelve geographic locations worldwide with the earliest study published in 1977 and the latest in 2017. The majority of studies were conducted in the USA (fourteen studies: (Chester et al. Citation1977; Vinsel Citation1990; Heidemann and Goetting Citation1991; Bosse Citation1994; Myers Citation1997; Parimon et al. Citation2004; Vohra and Clark Citation2006; Howard et al. Citation2007; Wenck et al. Citation2007; Babu et al. Citation2008; Van Sickle et al. Citation2009; Vajner and Lung Citation2013; Mackie et al. Citation2014; Warren et al. Citation2016)), followed by Turkey (five studies: (Güloğlu et al. Citation2002; Akdur et al. Citation2006; Aslan et al. Citation2006; Cevik et al. Citation2009; Sever et al. Citation2009)), China (two studies: (Ho et al. Citation2010; Li et al. Citation2011)), South Korea (two studies: (Kim et al. Citation2014; Shin et al. Citation2017)), Afghanistan (Bellenger and Frizzi Citation2014), Brazil(Matos et al. Citation2017), Canada (Mangat et al. Citation2012), India (Mohan et al. Citation2010), Israel (Gapany-Gapanavicius et al. Citation1982), Spain (Fleta et al. Citation1986), Sierra Leone (Carpenter et al. Citation2016), and Singapore (Ngo et al. Citation2007).
3.1.2.3. Population
The number of cases of chlorine poisoning reported in the included studies ranged from 1 to 1069 poisoned individuals for a total of 3567 cases. Of this total, there was no gender information for 2194 persons in five studies (Ngo et al. Citation2007; Sever et al. Citation2009; Van Sickle et al. Citation2009; Ho et al. Citation2010; Mackie et al. Citation2014), 40.4% were female, representing 555 cases of the 1373 persons with gender information. The age of participants in the included studies varied from newborn to 85 years old. In eleven of the included studies (Bosse Citation1994; Aslan et al. Citation2006; Ngo et al. Citation2007; Wenck et al. Citation2007; Cevik et al. Citation2009; Sever et al. Citation2009; Van Sickle et al. Citation2009; Ho et al. Citation2010; Mohan et al. Citation2010; Kim et al. Citation2014; Mackie et al. Citation2014), there was no available information concerning the number of children for a total of 3219 persons and more than half of cases were under the age of 18 representing a total of 193 cases of the 348 persons with age information.
3.1.2.4. Exposure and duration
In seven studies of the thirty-one included, the cases were due to inadvertent intoxication to mixing of cleaning products (Gapany-Gapanavicius et al. Citation1982; Bosse Citation1994; Akdur et al. Citation2006; Aslan et al. Citation2006; Howard et al. Citation2007; Cevik et al. Citation2009; Mangat et al. Citation2012; Shin et al. Citation2017). Thirteen included studies presented cases that were accidentally exposed at swimming pool settings (Heidemann and Goetting Citation1991; Bosse Citation1994; Myers Citation1997; Parimon et al. Citation2004; Vohra and Clark Citation2006; Ngo et al. Citation2007, Citation2007; Babu et al. Citation2008; Ho et al. Citation2010; Mohan et al. Citation2010; Li et al. Citation2011; Vajner and Lung Citation2013; Matos et al. Citation2017), three studies described cases of exposure to chlorine gas following an accidental leak from the chlorination system (Fleta et al. Citation1986; Vinsel Citation1990; Güloğlu et al. Citation2002). Cases of exposure to chlorine gas due to an occupational accident were presented in five studies (Chester et al. Citation1977; Bosse Citation1994; Bellenger and Frizzi Citation2014; Kim et al. Citation2014; Carpenter et al. Citation2016), those resulting from exposure following a train derailment were described in three included studies (Wenck et al. Citation2007; Van Sickle et al. Citation2009; Mackie et al. Citation2014). One study described cases accidentally exposed to chlorine gas due to chlorine tank explosion that was used for city water purification (Sever et al. Citation2009), and another study reported cases who inadvertently inhaled chlorine gas while mixing an industrial-grade chlorine solution with a sulfuric acid solution (Warren et al. Citation2016). Eight studies of the included studies reported estimates of the chlorine gas concentrations to which patients were exposed (Gapany-Gapanavicius et al. Citation1982; Fleta et al. Citation1986; Parimon et al. Citation2004; Akdur et al. Citation2006; Vajner and Lung Citation2013; Bellenger and Frizzi Citation2014; Kim et al. Citation2014; Carpenter et al. Citation2016).
Regarding the duration of the exposure, twelve of the thirty-one included studies reported this information (Gapany-Gapanavicius et al. Citation1982; Myers Citation1997; Parimon et al. Citation2004; Aslan et al. Citation2006; Ngo et al. Citation2007; Wenck et al. Citation2007; Cevik et al. Citation2009; Ho et al. Citation2010; Li et al. Citation2011; Mangat et al. Citation2012; Vajner and Lung Citation2013; Shin et al. Citation2017). This exposure time varied from a few seconds (Vinsel Citation1990; Myers Citation1997; Vajner and Lung Citation2013; Warren et al. Citation2016) to three days (Wenck et al. Citation2007).
3.1.2.5. Management of the chlorine gas exposure
Six of the included studies (Ngo et al. Citation2007; Wenck et al. Citation2007; Babu et al. Citation2008; Mangat et al. Citation2012; Vajner and Lung Citation2013; Carpenter et al. Citation2016) reported pre-hospital management of patients after exposure to chlorine gas. In respect to the treatment of people exposed to chlorine gas, the most commonly used treatments were: oxygen therapy used in all of the included studies except six of them (Vinsel Citation1990; Bosse Citation1994; Aslan et al. Citation2006; Wenck et al. Citation2007; Li et al. Citation2011; Warren et al. Citation2016), endotracheal intubation used in ten studies (Heidemann and Goetting Citation1991; Babu et al. Citation2008; Van Sickle et al. Citation2009; Li et al. Citation2011; Mangat et al. Citation2012; Bellenger and Frizzi Citation2014; Mackie et al. Citation2014; Warren et al. Citation2016; Matos et al. Citation2017; Shin et al. Citation2017), β2-agonists in twenty studies (Gapany-Gapanavicius et al. Citation1982; Bosse Citation1994; Myers Citation1997; Parimon et al. Citation2004; Akdur et al. Citation2006; Aslan et al. Citation2006; Vohra and Clark Citation2006; Howard et al. Citation2007; Ngo et al. Citation2007; Babu et al. Citation2008; Cevik et al. Citation2009; Sever et al. Citation2009; Van Sickle et al. Citation2009; Ho et al. Citation2010; Mohan et al. Citation2010; Vajner and Lung Citation2013; Bellenger and Frizzi Citation2014; Kim et al. Citation2014; Carpenter et al. Citation2016; Matos et al. Citation2017) and corticosteroids used in nineteen of the studies included in this review (Chester et al. Citation1977; Gapany-Gapanavicius et al. Citation1982; Fleta et al. Citation1986; Bosse Citation1994; Myers Citation1997; Parimon et al. Citation2004; Akdur et al. Citation2006; Aslan et al. Citation2006; Babu et al. Citation2008; Cevik et al. Citation2009; Sever et al. Citation2009; Van Sickle et al. Citation2009; Ho et al. Citation2010; Mohan et al. Citation2010; Vajner and Lung Citation2013; Kim et al. Citation2014; Mackie et al. Citation2014; Carpenter et al. Citation2016; Matos et al. Citation2017). In ten studies, at least one reported case was transferred to an intensive care unit (Myers Citation1997; Babu et al. Citation2008; Sever et al. Citation2009; Mohan et al. Citation2010; Li et al. Citation2011; Mangat et al. Citation2012; Vajner and Lung Citation2013; Kim et al. Citation2014; Mackie et al. Citation2014; Matos et al. Citation2017).
3.1.2.6. Outcome
Of the 31 included studies, twenty-seven investigated the “recovery or discharge” outcome (survival at hospital discharge), two studies (Wenck et al. Citation2007; Mangat et al. Citation2012) reporting cases due to chlorine gas exposure that led to death, two studies (Van Sickle et al. Citation2009; Mackie et al. Citation2014) reporting both “recovery or discharge” and “death” as an outcome and one study (Myers Citation1997) reporting an expected outcome (expected full recovery).
3.2. Risk of bias in studies
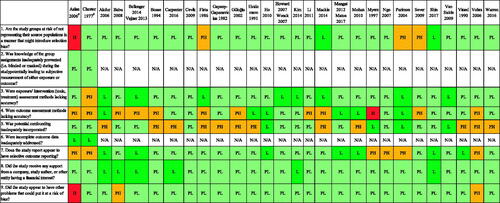
The risk of bias ratings for each domain of the 31 included studies are provided in , and the rationale for each rating for each domain by the included study is given in Appendix 3 in the supplementary material.
3.2.1. Selection bias
This domain was assessed by determining whether the study groups represented their source populations in a way that did not introduce selection bias. The risk of selection bias was rated to be high for one study (Aslan et al. Citation2006) because only patients with reactive airways dysfunction syndrome after a chlorine exposure were included. We rated the risk of this selection bias as low for two studies (Mackie et al. Citation2014; Shin et al. Citation2017) and probably low for 24 studies because these studies described the cases reported in detail while providing indirect evidence on recruitment and enrollment procedures. We rated the risk of selection bias as probably high for three studies because these studies reported a low participant among the exposed persons (Fleta et al. Citation1986; Parimon et al. Citation2004; Sever et al. Citation2009).
3.2.2. Performance bias
For case reports/studies and case series, the risk of performance bias was not applicable in all studies because there was no control group except for two studies for which this bias was rated as probably low (Chester et al. Citation1977; Aslan et al. Citation2006). In addition, there was no blinding of participants and/or personnel in case reports/studies and case series.
3.2.3. Detection bias (blinding of exposure assessment)
For this domain, we rated the risk of detection bias as probably high for one study (Chester et al. Citation1977) because the exposure was not accurately described in the study (no more detail on the chlorine gas composition), as probably low for twenty studies, and as low for ten studies. We judged that in these other studies, the reporting with more details of exposure did not introduce a noteworthy risk of detection bias or introduced a low bias.
3.2.4. Detection bias (blinding of outcome assessment)
Of the 31 studies included in this review, one study (Myers Citation1997) did not provide information on the post-treatment fate of the patient exposed to chlorine. We consequently rated the detection bias of this study as high. For the other included studies, we judged 12 studies as probably high risk of bias, 11 studies as probably low risk of bias, and 7 studies as low risk of bias. For the 12 studies rated as having as probably high, there was a lack of precision about the assessment of outcome. The 11 studies rated as probably low risk provided information about the outcome without further follow-up information, knowing that very often patients may have long-term sequela that can sometimes lead to a relapse. For the seven studies, we rated as low risk of detection bias, because these studies assessed the outcome with a follow-up treatment or testing.
3.2.5. Confounding
With respect to the risk of confounding bias, case reports and series should be well documented on other settings or treatments that might explain the outcome, as there is no control. Of the included studies, 4 studies were rated as low risk of confounding, either because of appropriate assessment of important potential confounders or because of sufficient documentation on other contexts or treatments that might explain the outcome. We judged 15 studies as probably low risk of bias because studies did not account for the (other potentially) important and relevant confounders. Twelve studies did not assess all the (other potentially) important and relevant confounders such as the medical background. Based on these considerations, we judged these studies to have a probably high risk of confounding bias.
3.2.6. Attrition bias (incomplete outcome data)
We rated the risk of attrition bias as low for the two studies with comparator (Chester et al. Citation1977; Aslan et al. Citation2006) and judged that the risk of attrition bias was not applicable to the other included studies because there was no concern.
3.2.7. Selective outcome reporting (reporting bias)
We judged 6 studies to have a probably high risk of reporting bias, 16 studies as probably low risk of bias, and 9 studies as low risk of bias. None of the included studies had a pre-specified study protocol, but the 9 studies were judged to have a low risk of reporting bias because, all results described in the methods, abstract, and/or introduction section of these studies are of interest to the review were reported in a pre-specified manner. The 16 studies with probably low risk, selective bias is unlikely as these published studies have been reported in a pre-specified way. For the 6 studies with a probably high risk of selective bias, all outcomes described in the methods, abstract, and/or introduction section of these studies that are of interest to the review were not reported in a prespecified manner, and there is indirect evidence to suggest that they were not free of selective reporting.
3.2.8. Conflict of interest
Seven of the included studies did not receive financial support from a company or other entity with a financial interest in the study findings. All of them were funded by public research agencies or related organizations that were free from commercial interests in the study findings. We, therefore, rated these studies as having a low risk of bias. In 24 included studies, there was unspecified information regarding this risk of bias domain, but no conflict of interest was detected. We consequently judged these studies as having a probably low risk of bias from conflict of interest.
3.2.9. Other risk of bias
We did not find any evidence to assume the high or probably high risk of other types of risk of bias in 26 studies. We rated two studies (Vohra and Clark Citation2006; Babu et al. Citation2008) as having a probably high risk of this domain due to the absence of data on the duration of the treatment, and one study (Aslan et al. Citation2006) as having a high risk of other risks of bias because there is at least one important risk of bias. We judged that the risk of other risks of bias was not applicable to the two included studies because there was no concern.
3.3. Synthesis of results
Due to the important heterogeneity of the included studies, it was not possible to perform a meta-analysis. We, therefore, performed a narrative synthesis of the study's main findings for this overview.
3.3.1. Comparison: studies with pre-hospital care versus those without
Six of the included studies, with a total of 637 cases over 3567, reported pre-hospital management of patients after exposure to chlorine gas. However, the type of pre-hospital intervention and its duration differed between studies. In (Babu et al. Citation2008), the patient received supplemental oxygen and nebulized albuterol treatment on the route during her transfer to the hospital. In (Carpenter et al. Citation2016), the personal protective equipment of the patient was safely removed by a nurse before the initial clinical assessment. In (Mangat et al. Citation2012), the patient’s clothes were immediately removed after the chlorine inhalation, and he was placed in the shower for approximately 5 min until the ambulance arrived. In addition, ten liters per minute of high-flow oxygen was administered to the patient during transport to the hospital. In the study of Ngo (Ngo et al. Citation2007), patients, following exposure to chlorine gas and upon arrival, were instructed to remove all personal belongings and clothing and place them in a labeled plastic bag for decontamination. The study of Vajner (Vajner and Lung Citation2013) reported pre-hospital management with administration of 15–30 min of continuous nebulized albuterol by non-rebreather mask on the route to the hospital. In (Wenck et al. Citation2007), wet decontamination was used. Patients were hosed with cold water after removing their clothes. They were subsequently given disposable clothing or blankets.
Of the total number of 637 cases, 10 deaths were reported in two studies with the same number of deaths reported by other studies. Follow-up time, i.e. the duration between exposure and outcome assessment varied from 15 h to 32 days in studies reported pre-hospital care of patients after their exposure to chlorine gas compared to the duration ranging from 1 h to 1650 days (55 months) reported by studies that did not report pre-hospital care of patients after chlorine gas exposure.
3.3.2. Comparison: oxygen gas versus other treatments (β2, corticosteroids, …)
A total of 25 studies (24 case reports and 1 case series) with a total of 1512 reported cases provided treatment data on oxygen administration treatment and 30 studies (27 case reports, 2 case series and 1 randomized control study) with a total of 3567 reported cases provided treatment data only on other treatments. All included studies except six of them reported the use of oxygen gas as treatment of people exposed to chlorine gas for a total of 1506 cases. In most of these studies, patients’ oxygen saturation was improved following the administration. Among these cases, only 3 deaths (i.e. 0.2%) were recorded compared to 9 deaths (i.e. 1.2%) in the other studies. Specifically, studies that used oxygen in the treatment of patients include Van Sickle's study (Van Sickle et al. Citation2009), which reports 1 death out of 71 patients; the study of (Mangat et al. Citation2012), in which the treated patient died; and the study of (Mackie et al. Citation2014), in which there were 9 deaths (out of 1069), only 1 of which received oxygen, for a mortality rate of 0.09%. In some studies, no treatment was indicated for all or part of the reported cases, for a total of 5 studies with 1925 cases exposed to chlorine gas representing 54.0% of all cases reported in this review (Ngo et al. Citation2007; Wenck et al. Citation2007; Sever et al. Citation2009; Van Sickle et al. Citation2009; Kim et al. Citation2014).
Two studies (a case report and a randomized controlled study) compared 2 treatments. The case report (Chester et al. Citation1977) compared oxygen therapy (received in outpatient care: patient 1) versus therapy with adrenocorticosteroids and oxygen (administered in the hospital: patient 2) between two sisters who in the same home, received similar massive toxic exposure to chlorine gas. Both patients were examined the day after exposure and at intervals of approximately 1 month, 1 year, and 2 years. Patient 2 became asymptomatic at the end of two years, with values returning to normal, and was discharged from further examination, whereas patient 1was followed for 55 months. As for the randomized controlled trial (Aslan et al. Citation2006), the authors investigated the effect of nebulized sodium bicarbonate on the treatment and quality of life of victims exposed to chlorine gas. Two groups of patients have been compared: patients in the treatment group received nebulized treatment (4 cm3 of 4.20% sodium bicarbonate solution) whereas those in the control group received a nebulized placebo. The authors concluded that the treatment group had significantly higher forced expiratory volume in 1 s values at 120 and 240 min. In addition, significantly more improvement in quality of life questionnaire scores occurred in the treatment group compared to the control group.
3.4. Quality of evidence
In terms of GRADE assessments, we graded the certainty of the evidence for all intervention categories, as starting at the lowest point of evidence because of case reports/series, using the three Navigation Guide quality of evidence ratings (see our published protocol (Nambiema et al. Citation2021)).
We upgraded the quality of evidence for the exposure cessation as high (by 4 levels) reflecting the large magnitude of effect (+2 levels) and the dose-response relationship (+2 levels) between the duration before the exposure cessation and the outcome. We did not have any serious concerns regarding the risk of bias in the body of evidence on this intervention category for the outcome. We also had no serious concerns regarding indirectness, inconsistency, and imprecision of evidence. “High” certainty of the evidence was finally attributed to the effect of exposure cessation on the outcome ().
Table 4. Summary of findings.
Concerning the intervention category, oxygen gas administration, we had serious concerns about the risk of bias and imprecision. We, therefore, downgraded by 2 levels (−2). We did not have any serious concerns regarding evidence of inconsistency and indirectness in the body of evidence on this intervention category for the outcome. We upgraded the evidence by 3 levels (+3) regarding a large magnitude of effect (+3) and a dose-response relationship (+1). In summary, we started at “low” for case reports/series, downgraded by 2 levels (−2) for evidence of inconsistency and indirectness, upgraded by 3 levels (+3) for large magnitude of effect and dose-response relationship, to a final rating of “moderate” ().
As above, we had serious concerns about the risk of bias and imprecision for the intervention category, other treatments (e.g. β2, corticosteroids). We, therefore, downgraded by 2 levels (−2). We upgraded 2 levels (+2) for evidence of the large magnitude of effect and therefore concluded with a final rating of “low” ().
4. Discussion
The aim of this review was to investigate possible recommendations for appropriate treatment that must be administered after exposure to chlorine gas. To the best of our knowledge, this review is the first to specifically address the incorporation of case reports/studies and case series in an umbrella review.
Chlorine gas exposure is a relevant issue since it can happen in various aspects of our lives (workplace, home, swimming pool, and even at war when used as a chemical weapon, …) (Zellner and Eyer Citation2020). Case reports showed that each hospital had its own protocol of management. Some used oxygen therapy and bronchodilators (Bellenger and Frizzi Citation2014) and/or corticosteroids (Gapany-Gapanavicius et al. Citation1982; Fleta et al. Citation1986; Cevik et al. Citation2009; Carpenter et al. Citation2016) and/or nebulized sodium (Bosse Citation1994; Howard et al. Citation2007).
As shown in the table of summary of findings (), our umbrella review found the high quality of evidence of the effectiveness of pre-hospital management (i.e. exposure cessation) on survival at hospital discharge after exposure to chlorine gas. This review also found that oxygen administration to patients exposed to chlorine gas was effective with moderate quality of evidence, as well as other types of treatment (e.g. β2, corticosteroids), but with a low level of evidence. Finally, the results based on the only randomized controlled trial presented in this review did not allow us to conclude on the effectiveness of the treatments used.
Depending on the importance of the exposure, clinical effects can be mild or severe with acute respiratory distress syndrome (ARDS) and even death. Due to its intermediate water solubility, chlorine can cause upper airway injury as well as alveolar injury. In water-based environments, such as the moist lining of airways, chorine will form hydrochloric and hypochlorous acids. This last one further decomposes into hydrochloric acid and oxygen-free radicals which may damage the alveolar epithelial cells and adjacent capillary endothelial cells. This can clinically manifest as bronchospasm, acute lung injury (ALI) with subjects complaining of dyspnea (Greenfield et al. Citation2002). However, even though bronchodilators are commonly used to treat bronchospasm, only low interest in their utilization was found. The same goes for corticosteroids (oral, intravenous, or inhaled). They were given because of their anti-inflammatory properties in order to block possible hematogenous inflammatory mediators that may appear after exposure to chlorine, during cell destruction (de Lange and Meulenbelt Citation2011).
As for the use of nebulized sodium, it was not possible to conclude on its benefit. It is frequently associated with other treatments like humidified oxygen and bronchodilators (Huynh Tuong et al. Citation2019) making it impossible to assess a potential beneficial effect on the symptoms (Bosse Citation1994; Aslan et al. Citation2006; Howard et al. Citation2007; Cevik et al. Citation2009). In Vinsel’s study (Vinsel Citation1990), three male patients exposed to chlorine gas were only treated with a nebulized solution of 3.75% sodium bicarbonate. Prompt relief of their symptoms had been described without any other treatment administration. However, due to the insufficient number of patients and the lack of data on their medical history, it does not change our conclusion.
The results of this current review show that the most effective management seems to be the preliminary measures for which we have concluded a high interest in their execution. These consist mainly in decontamination, getting the person out of the contaminated area and contacting the poison center. If those actions were done shortly after the exposure, we can assume the number of hospitalization due to chlorine gas would decrease.
However, our study had some limitations. Although we tried to look into systematic reviews for a large period of time (from 2007 to 2020), almost all reported studies were case reports except one randomized controlled study (Aslan et al. Citation2006). This can be explained by the fact that it would be unethical to expose voluntarily persons to this irritant gas to evaluate its management. Another explanation could be that due to the low rate of mortality and the fact that people usually feel better within hours after the exposure, a randomized controlled study is not seen as a priority. However, death (Van Sickle et al. Citation2009; Mackie et al. Citation2014) and some long-term effects may occur like reactive airways dysfunction syndrome (Evans Citation2005) which can affect significatively the quality of life of the exposed people. This review has also limitations related primarily to the constraints of case reports/studies and case series. These are descriptive studies. In addition, a case series is subject to selection bias because the clinician or researcher selects the cases themselves and may represent outliers in clinical practice. Finally, we must note that case reports/studies and case series do not provide independent proof, and therefore, the findings of this separate evidence stream (case reports/studies and case series) will only be considered if evidence from RCTs and observational studies is not available. Case reports/studies and case series will not be used to upgrade or downgrade the strength of other evidence streams if evidence from RCTs and observational studies is available. In any case, it is very important to remember that these kinds of studies (case reports/studies and case series) are there to quickly alert agencies of the need to take immediate action to prevent further harm. The strength of our umbrella review is also limited since we could not do a quantitative analysis. This can also be explained by the lack of randomized controlled studies. As we analyzed a series of cases, data were not homogeneous with some data missing or incomplete (e.g. lack of medical background, the exact protocol used to treat patients).
Despite these limitations, case reports/studies and case series are the first line of evidence because they are where new issues and ideas emerge (hypothesis-generating) and can contribute to a change in clinical practice (Graham et al. Citation2011; Buonfrate et al. Citation2013; Nissen and Wynn Citation2014). We, therefore, believe that data from case reports/studies and case series, when synthesized and presented with completeness and transparency, may provide important details that are relevant to systematic review recommendations. Another strength of this current review is that it was based on a protocol that was adapted from the Navigation Guide with the intent of integrating the case reports/studies and case series in systematic review recommendations while following traditional systematic review methodology to the greatest extent possible.
This review showed the value of using case reports/series in systematic reviews, some of which may provide relevant knowledge that should be considered in systematic review recommendations when data from RCTs and observational studies are not available. Practically, this review is the first to effectively incorporate case reports/studies and case series in systematic reviews that synthesize evidence for clinicians, researchers, and drug developers. However, it is important to note that promoting the need to synthesize these types of studies (case reports/studies and case series) in a formal systematic review, should not deter or delay immediate action from being taken when a few small studies report a plausible causal association between exposure and disease, such as, in the event of an epidemic or a side effect of a newly marketed medicine (Nissen and Wynn Citation2014). It also is very important to remember that these kinds of studies (case reports/studies and case series) are there to quickly alert agencies of the need to take immediate action to prevent further harm.
This umbrella review revealed the effectiveness of pre-hospital care following chlorine gas exposure with a high level of evidence, oxygen administration with a moderate level of evidence, and other treatments (e.g. β2, corticosteroids) with a low level of evidence. Pre-hospital care for chlorine inhalation should include decontamination of the patient's body, including the face, and removal of contaminated clothing. We believe that this analysis offers some insights into better management practices for emergency departments worldwide and could highlight more effective systems.
Author Contributors
The systematic review was designed by AD and GS. GC, JBBR, JB and NRD were significantly involved in the selection process, data extraction, and quality assessment of the articles included in this review. AN and GC wrote the manuscript. AD and GS significantly contributed to the improvement of the first draft of the manuscript. All authors reviewed and commented on the final manuscript. All authors read and approved the final manuscript to be published.
Acknowledgments
We thank Dr. Gaël Le Roux and Dr. Corinne Dano, both affiliated with CHU Angers, University of Angers, and Poisoning Control Center, Clinical Data Center of Angers in France, for their review on the manuscript.
Declaration of interest
The authors declare that they have no competing interests associated with this manuscript.
Supplemental material
Supplemental data for this article is available online here.
Additional information
Funding
References
- Akdur O, Durukan P, Ikizceli I, Ozkan S, Avsarogullari L. 2006. A rare complication of chlorine gas inhalation: pneumomediastinum. Emerg Med J. 23(11):e59.
- Aslan S, Kandiş H, Akgun M, Çakır Z, Inandı T, Görgüner M. 2006. The effect of nebulized NaHCO treatment on “RADS” due to chlorine gas inhalation. Inhalation Toxicol. 18(11):895–900.
- Babu RV, Cardenas V, Sharma G. 2008. Acute respiratory distress syndrome from chlorine inhalation during a swimming pool accident: a case report and review of the literature. J Intensive Care Med. 23(4):275–280.
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. 2011. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 64(4):401–406.
- Bellenger SR, Frizzi JD. 2014. Sevoflurane as a therapy for acute chlorine gas exposure in an austere healthcare environment: a case report. AANA J. 82(3):223–226.
- Beller EM, Glasziou PP, Altman DG, Hopewell S, Bastian H, Chalmers I, Gøtzsche PC, Lasserson T, Tovey D. 2013. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 10(4):e1001419.
- Bosse GM. 1994. Nebulized sodium bicarbonate in the treatment of chlorine gas inhalation. J Toxicol Clin Toxicol. 32(3):233–241.
- Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, Bisoffi Z. 2013. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 13(1):78.
- Carpenter A, Cox AT, Marion D, Phillips A, Ewington I. 2016. A case of a chlorine inhalation injury in an Ebola treatment unit. J R Army Med Corps. 162(3):229–231.
- Cevik Y, Onay M, Akmaz I, Sezigen S. 2009. Mass casualties from acute inhalation of chlorine gas. South Med J. 102(12):1209–1213.
- Chester EH, KaimaL PJ, Payne CB, Kohn PM. 1977. Pulmonary injury following exposure to chlorine gas. Possible beneficial effects of steroid treatment. Chest. 72(2):247–250.
- Covidence systematic review software, V.H.I, n.d. Covidence systematic review software, V.H.I. Melbourne, Australia. www.covidence.org.
- D'Alessandro A, Kuschner W, Wong H, Boushey HA, Blanc PD. 1996. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest. 109(2):331–337.
- Descatha A, Sembajwe G, Baer M, Boccuni F, Di Tecco C, Duret C, Evanoff BA, Gagliardi D, Ivanov ID, Leppink N, et al. 2018. WHO/ILO work-related burden of disease and injury: protocol for systematic reviews of exposure to long working hours and of the effect of exposure to long working hours on stroke. Environ Int. 119:366–378.
- Donnelly SC, FitzGerald MX. 1990. Reactive Airways Dysfunction Syndrome (RADS) due to chlorine gas exposure. Ir J Med Sci. 159(9–12):275–277.
- Duncan MA, Drociuk D, Belflower-Thomas A, Van Sickle D, Gibson JJ, Youngblood C, Daley WR. 2011. Follow-up assessment of health consequences after a chlorine release from a train Derailment-Graniteville, SC, 2005. J Med Toxicol. 7(1):85–91.
- Evans RB. 2005. Chlorine: state of the art. Lung. 183(3):151–167.
- Fleta J, Calvo C, Zuñiga J, Castellano M, Bueno M. 1986. Intoxication of 76 children by chlorine gas. Hum Toxicol. 5(2):99–100.
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. 2013. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 7(1):223.
- Gapany-Gapanavicius M, Yellin A, Almog S, Tirosh M. 1982. Pneumomediastinum. A complication of chlorine exposure from mixing household cleaning agents. JAMA. 248(3):349–350.
- Govier P, Coulson JM. 2018. Civilian exposure to chlorine gas: a systematic review. Toxicol Lett. 293:249–252.
- Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. 2011. Clinical practice guidelines we can trust. Washington, D.C.: National Academies Press.
- Greenfield RA, Brown BR, Hutchins JB, Iandolo JJ, Jackson R, Slater LN, Bronze MS. 2002. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci. 323(6):326–340.
- Güloğlu C, Kara İH, Erten PG. 2002. Acute accidental exposure to chlorine gas in the Southeast of Turkey: a study of 106 cases. Environ Res. 88(2):89–93.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. 2008. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 336(7650):924–926.
- Heidemann SM, Goetting MG. 1991. Treatment of acute hypoxemic respiratory failure caused by chlorine exposure. Pediatr Emerg Care. 7(2):87–88.
- Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., and Welch, V., eds., 2020. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020).
- Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., and Welch, V., eds., 2021. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021).
- Ho M, Yang C-C, Cheung W, Liu C, Tsai K. 2010. Chlorine gas exposure manifesting acute lung injury. J Intern Med Taiwan. 21:210–215.
- Howard C, Ducre B, Burda AM, Kubic A. 2007. Management of chlorine gas exposure. J Emerg Nurs. 33(4):402–404.
- Hoyle GW, Svendsen ER. 2016. Persistent effects of chlorine inhalation on respiratory health: persistent chlorine-induced lung disease. Ann NY Acad Sci. 1378(1):33–40.
- Huynh Tuong A, Despréaux T, Loeb T, Salomon J, Mégarbane B, Descatha A. 2019. Emergency management of chlorine gas exposure – a systematic review. Clin Toxicol. 57(2):77–98.
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. 2014. The Navigation Guide – evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 122(10):1028–1039.
- Kim J-A, Yoon S-Y, Cho S-Y, Yu J-H, Kim H-S, Lim G-I, Kim J-S. 2014. Acute health effects of accidental chlorine gas exposure. Ann Occup Environ Med. 26(1):29.
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. 2014. The Navigation Guide – evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect. 122(10):1040–1051.
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ. 2017. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ Health Perspect. 125(8):086001.
- de Lange DW, Meulenbelt J. 2011. Do corticosteroids have a role in preventing or reducing acute toxic lung injury caused by inhalation of chemical agents? Clin Toxicol. 49(2):61–71.
- Li B, Jia L, Shao D, Liu H, Nie S, Tang W, Xu B, Hu Z, Sun H. 2011. Pneumomediastinum from acute inhalation of chlorine gas in 2 young patients. Am J Emerg Med. 29(3):357.e1–e4.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 62(10):e1–e34.
- Mackie E, Svendsen E, Grant S, Michels JE, Richardson WH. 2014. Management of chlorine gas-related injuries from the Graniteville, South Carolina, Train Derailment. Disaster Med Public Health Prep. 8(5):411–416.
- Mangat HS, Stewart TL, Dibden L, Tredget EE. 2012. Complications of chlorine inhalation in a pediatric chemical burn patient: a case report. J Burn Care Res. 33(4):e216–e221.
- Matos AM, Oliveira RR, de Lippi MM, Takatani RR, Oliveira Filho W. d. 2017. Use of noninvasive ventilation in severe acute respiratory distress syndrome due to accidental chlorine inhalation: a case report. Revista Brasileira de Terapia Intensiva. 29(1):105–110.
- Milanez S. 2020. Chapter 22 - Chlorine. In: Gupta RC, editor. Handbook of toxicology of chemical warfare agents. 3rd ed. United States: Elsevier.
- Mohan A, Kumar SN, Rao MH, Bollineni S, Manohar IC. 2010. Acute accidental exposure to chlorine gas: clinical presentation, pulmonary functions and outcomes. Indian J Chest Dis Allied Sci. 52(3):149–152.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 4:1–1.
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K. 2020. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). JBI manual for evidence synthesis. JBI. Available from: https://synthesismanual.jbi.global, https://doi.org/https://doi.org/10.46658/JBIMES-20-08
- Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. 2020. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 18(10):2127–2133.
- Myers SJ. 1997. Chlorine inhalation in a pediatric patient. J Emerg Nurs. 23(6):583–585.
- Nambiema A, Sembajwe G, Lam J, Woodruff T, Mandrioli D, Chartres N, Fadel M, Le Guillou A, Valter R, Deguigne M, et al. 2021. A protocol for the use of case reports/studies and case series in systematic reviews for clinical toxicology. Front Med. 8:708380.
- Ngo A, Ponampalam R, Leong M, Han LS. 2007. Chlorine and its impact on an emergency department. Prehosp Disaster Med. 22(2):136–139.
- Nissen T, Wynn R. 2014. The clinical case report: a review of its merits and limitations. BMC Res Notes. 7:264.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71.
- Parimon T, Kanne JP, Pierson DJ. 2004. Acute inhalation injury with evidence of diffuse bronchiolitis following chlorine gas exposure at a swimming pool. Respiratory Care. 49(3):4.
- Pino F, Puerta H, D'Apollo R, Ferrer M, Arias I, Irastorza IM, Ramirez MS. 1993. Effectiveness of morphine in non-cardiogenic pulmonary edema due to chlorine gas inhalation. Vet Hum Toxicol. 35(1):36.
- Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, et al. 2017. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 89:218–235.
- Russell D, Blain PG, Rice P. 2006. Clinical management of casualties exposed to lung damaging agents: a critical review. Emerg Med J. 23(6):421–424.
- Schönhofer B, Voshaar T, Köhler D. 1996. Long-term lung sequelae following accidental chlorine gas exposure. Respiration. 63(3):155–159.
- Schwartz DA, Smith DD, Lakshminarayan S. 1990. The pulmonary sequelae associated with accidental inhalation of chlorine gas. Chest. 97(4):820–825.
- Sever M, Mordeniz C, Sever F, Dokur M. 2009. Accidental chlorine gas intoxication: evaluation of 39 patients. J Clin Med Res. 1(5):274–279.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle PG, Stewart L. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 349(jan02 1):g7647–g7647.
- Shin H-J, Chang J-S, Ahn S, Kim T-O, Park C-K, Lim J-H, Oh I-J, Kim Y-I, Lim S-C, Kim Y-C, et al. 2017. Acute respiratory distress syndrome and chemical burns after exposure to chlorine-containing bleach: a case report. J Thorac Dis. 9(1):E17–E20.
- Squadrito GL, Postlethwait EM, Matalon S. 2010. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol. 299(3):L289–L300.
- Traub SJ, Hoffman RS, Nelson LS. 2002. Case report and literature review of chlorine gas toxicity. Vet Hum Toxicol. 44(4):235–239.
- Vajner JE, Lung D. 2013. Case files of the University of California San Francisco Medical Toxicology Fellowship: acute chlorine gas inhalation and the utility of nebulized sodium bicarbonate. J Med Toxicol. 9(3):259–265.
- Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, et al. 2009. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med. 27(1):1–7.
- Vinsel PJ. 1990. Treatment of acute chlorine gas inhalation with nebulized sodium bicarbonate. J Emerg Med. 8(3):327–329.
- Vohra R, Clark RF. 2006. Chlorine-related inhalation injury from a swimming pool disinfectant in a 9-year-old girl. Pediatric Emergency Care. 22(4):254–257.
- Warren B, Royall N, Smith H, Bhullar IS. 2016. Novel treatment of acute respiratory distress syndrome after chlorine gas inhalation injury. Am Surg. 82(8):219–220.
- Wenck MA, Van Sickle D, Drociuk D, Belflower A, Youngblood C, Whisnant MD, Taylor R, Rudnick V, Gibson JJ. 2007. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. Public Health Rep. 122(6):784–792.
- White CW, Martin JG. 2010. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 7(4):257–263.
- Winder C. 2001. The toxicology of chlorine. Environ Res. 85(2):105–114.
- Woodruff TJ, Sutton P. 2014. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect. 122(10):1007–1014.
- Woodruff TJ, Sutton P. 2011. An evidence-based medicine methodology to bridge the gap between clinical and environmental health sciences. Health Aff. 30(5):931–937.
- Zellner T, Eyer F. 2020. Choking agents and chlorine gas – history, pathophysiology, clinical effects and treatment. Toxicol Lett. 320:73–79.