Abstract
The potential toxic effects of short chrysotile and amphibole asbestos fibers with lengths <5 to ∼10 µm have been debated over the years. This stems from the large database of epidemiology, toxicology, and in-vitro studies, each of which often provides different information in understanding and differentiating the effects of short fibers. The epidemiology studies in which the cancer potency estimates were based upon relatively high exposure concentrations provide a conservative assessment that shorter fibers would have little if any effect, especially under controlled exposure or environmental conditions that may occur today. The QSAR models have shown that fiber aspect ratio and Mg content are excellent predictors of cancer potency and that short fibers/particles of amphibole would have no effect. The studies of motor vehicle mechanics and in particular workers who serviced chrysotile containing brakes with the majority of the fibers being short provides evidence that motor vehicle mechanics, including workers who were engaged in brake repair, are not at an increased risk of mesothelioma. Several inhalation toxicology studies clearly differentiated that short chrysotile and amphibole asbestos fibers did not produce a significant carcinogenic effect in the lung or pleural cavity. Because of dosing and lack of sensitivity to biosolubility, in vitro studies can be difficult to interpret; however, a number have differentiated short chrysotile and amphibole asbestos fibers from long fibers. Integral to understanding the importance of fiber length in determining possible health effects is an understanding of the biological and physiological function of the respiratory system. Short asbestos fibers, like innocuous dust, can be cleared through the tracheobronchial ciliated mucous transport, phagocytized by macrophages and cleared via the bronchial tree, and can also be removed through the lymphatic system. While the first two methods can remove them from the lung, with lymphatic transport through one-way valves, fibers are removed from the active area of the lung where the fiber-related disease has been shown to develop and can accumulate in lymphatic sumps and lymph nodes. While short asbestos fibers are present in most occupational or environmental exposures, the large body of studies strongly supports that they do not contribute to the health effects of asbestos exposure.
1. Introduction
The potential toxic effects of short elongated mineral fibers (EMF) of chrysotile and amphibole asbestos have been debated over the years. While there appears to be general agreement that long fibers are more toxic than short fibers, the potential effects of short fibers remain debated. This stems from the large database of epidemiology, toxicology, and in-vitro studies, each of which often provides different information in understanding and differentiating the effects of short fibers.
This review examines in detail the biological activity of short chrysotile and amphibole asbestos EMF as reported in publications and reports on short fiber asbestos that were identified through either a Google or PubMed search using the terms: “asbestos toxicity,” with either “epidemiology,” “animal,” “in vitro,” and “short or long.” Where possible, asbestos was further differentiated into chrysotile and amphibole asbestos. These studies were evaluated not only for assessing the effects of short fiber chrysotile and amphibole asbestos but also examining where possible the exposure and dosing regimes that were used, which often provide further insight on the applicability of the results reported.
The term asbestos is in itself ambiguous. Classically, it referred to what was termed regulated asbestos fibers, which included chrysotile, and the amphibole asbestos amosite, crocidolite, actinolite anthophyllite, and tremolite. Initially, there was little, or no differentiation between the two minerals named asbestos – chrysotile (serpentine) and amphibole asbestos.
Chrysotile was first described as being decomposed by acid by von Kobell (Citation1834). Chrysotile has the approximate composition Mg3Si205(OH)4 and is a sheet silicate composed of silicate and brucite layers (magnesium hydroxide octahedra). The different dimensions of these two components result in a structural mismatch in which the layers curl concentrically or spirally (Pauling Citation1930). The fiber walls are made up of ∼12–20 of these layers in which there is some mechanical interlocking. However, there is no chemical bonding as such between the layers. Each layer is about 7.3 A° thick, with the magnesium hydroxide part of each layer closest to the fiber surface and the silicon-oxygen tetrahedra “inside” the curl (Whittaker Citation1957, Citation1963; Tanji Citation1984; Titulaer et al. Citation1993, Table 2). Hargreaves and Taylor (Citation1946) reported that if fibrous chrysotile is treated with dilute acid, the magnesia can be completely removed. The hydrated silica, which remains, though fibrous in form, had completely lost the elasticity characteristic of the original chrysotile and had a structure that was “amorphous” or “glassy” in type.
In contrast, the amphibole asbestos class of fibers is formed as solid rods/fibers (Skinner et al. Citation1988; Whittaker Citation1960). The structure of amphibole is a double chain of tetrahedral silicate (Si4O11)6− with the silica on the outside of the fiber, which makes it very strong and durable. Crocidolite and amosite were the only amphiboles with significant industrial uses (Virta Citation2002). Tremolite, while not used commercially, has been found as a contaminant in other fibers or in other industrial minerals (e.g. chrysotile and talc). Due to the structural matrix of amphibole fibers, they have negligible solubility at any pH that might be encountered in an organism (Speil and Leineweber Citation1969).
A distinction is often made between asbestiform and non-asbestiform amphibole. In the asbestiform habit, fibers grow almost exclusively in one direction and exhibit narrow widths (on the order of 0.1 μm). Bundles of individual fibers can often occur. The most common occurrence of asbestos is in cross-fiber or slip fiber veins. In the former, the fiber axes are perpendicular to the walls of narrow openings in the host rock; in the latter, they are parallel. Asbestos rarely occurs as mass fiber bundles in which fibrillar growth is in many directions.
Non-fibrous amphibole asbestos is also sometimes referred to as cleavage fragments. When mechanical stress is applied to non-asbestiform minerals, as when rock extracted from quarries is ground up to produce aggregate, for example, they can split and release particles of different lengths called “cleavage fragments.” These particles can sometimes be counted as asbestos fibers, especially because of their dimensions.
With non-asbestiform amphibole, the mineral crystal growth tends not to grow with parallel alignment but to form multi-directional growth patterns instead. When pressure is applied in crushing the rock, the crystals fracture easily, fragmenting into prismatic particles called cleavage fragments. Some particles or cleavage fragments are acicular or needle-shaped as a result of the tendency of amphibole minerals to cleave along two dimensions but not along with the third (Langer et al. Citation1991).
In terms of the assessment of short fibers, there is no scientific basis for an absolute cutoff. In addition, as is discussed below, the available studies that address short fibers refer to a range of fiber lengths often up to 10 µm and often use a variety of size distributions having a broader distribution. Most long fiber samples include fibers longer than 15–20 µm, which, as discussed below, cannot be cleared by macrophages. Due to the log-normal distribution of fiber length in most aerosols, the long fiber samples will also include short fibers. There were no studies identified which examined uniquely the effects of intermediate length fractions of 5–15 µm.
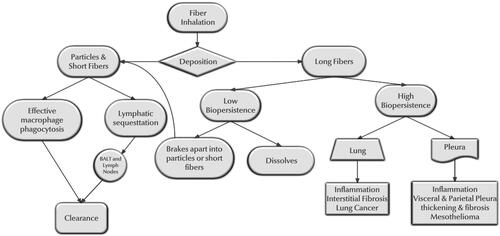
Short fibers may also be defined based on physiological deposition and clearance mechanisms in the respiratory tract. As discussed below, the deposition, cellular interaction, and clearance pathways are different depending upon fiber length, diameter, and biosolubility.
While epidemiology studies are important in determining the relationship of exposure to effect, the distribution of fiber length in most situations is broad, which impacts the ability of epidemiology studies to differentiate effect by length. In addition, populations were often exposed to mixtures of chrysotile and amphibole asbestos. Meta-analysis and quantitative structure-activity relationships (QSAR) models of epidemiology studies that have characterized the fiber dimensions provide evidence for differentiating effect by length and diameter. The toxicology of short fibers can be assessed more quantitatively through inhalation toxicology studies where the exposure conditions are well-characterized. In-vitro studies have also investigated differential effects, however, these systems are static and not sensitive to dissolution and the dose to the cell was often high and based on mass rather than fiber number making extrapolation more difficult.
The more inclusive term elongated mineral particles (EMP) has been used to describe a wide variety of mineral fibers without differentiation of composition or dimensions (IOM Citation2009; NOISH Citation2011). While the use of the term EMP has become more common, many of the studies reviewed predate this terminology and refer to fibers. To avoid confusion in referencing these studies, the term fiber is used differentiating whenever possible amphibole asbestos fibers from chrysotile. As such, the following terminology is used:
Short fibers (EMP) (<∼5–10 µm) of chrysotile: SFC
Short fibers (EMP) (<∼5–10 µm) amphibole asbestos: SFA
Longer fibers (EMP) >10–20 µm of chryostile: LFC
Longer fibers (EMP) >10–20 µm of amphibole asbestos: LFA
While due to the nature of mining, production, and use, there is often some overlap, this review address primarily asbestiform fibers.
2. Fiber distribution
Asbestos is a natural material that is mined. The mined ore is then milled, which is primarily carried out by fiberizers or crushers, which frees the fibers from the rock and separates them from each other. The separation process typically involves a jaw-type crusher. Following processing, the fibers are separated into numerous standard grades and cleansed further in this rock circuit. The fibers are then machine packaged either by compressing the material into a dense bundle or by blowing the material into bags (EPA Citation2020a).
Fibers are essentially cylindrical particles that have a length longer than the diameter. This length to diameter ratio is referred to as the aspect ratio. As asbestos fibers are produced through a milling or crushing procedure, the distribution of the length and diameter is usually best described by a bivariate (length and diameter) lognormal distribution (Cheng Citation1986). The mean values of such a bivariate lognormal distribution are described by geometric mean diameter (geometric standard deviation) and the geometric mean length (geometric standard deviation) (Ramachandran and Cooper Citation2011). shows a typical plot of fiber number concentration against fiber length showing the skewed nature of the lognormal distribution.
Figure 1. Typical plot of fiber number concentration against fiber length showing the skewed nature of the lognormal distribution.
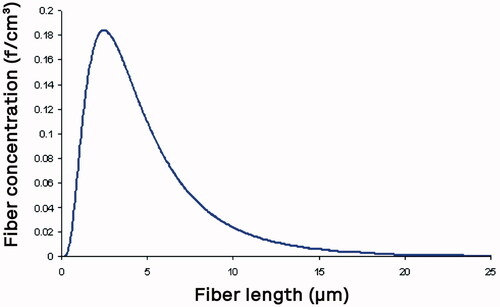
With the log-normal distribution of length, in most exposure situations, there would be considerably more shorter fibers present than long fibers. The question to be addressed is whether the larger number of short fibers is causing an effect or the fewer long fibers.
3. Human studies
Due to the sampling techniques available for measuring exposures during the first half of the 20th century when asbestos exposures were high, there was no systematic measurement of fiber length and usually not of fiber type. Fiber concentration measurements were initially performed using the midget impinger sampling and later by phase-contrast optical microscopy (PCOM) measurements (WHO Citation1997). Most studies that have assessed fiber potency from occupational exposure cohorts have relied upon exposure measurements made using the PCOM method. The PCOM method presents limitations as it does not measure the specific length or diameter of the fiber and does not identify the mineral composition of the fiber. In terms of resolution, as it is based upon optical microscopy, it does not assess fibers with a diameter ≤0.2 μm. As mentioned above, to facilitate counting, only fibers >5 µm in length with an aspect ratio of L/D > 3 and diameter <3 µm are counted. Finally, the method specifies that 100 fibers are counted without differentiating length. Because of the larger number of shorter fibers in the log-normal distribution of the aerosol, there will likely be a bias toward counting short fibers as opposed to long fibers.
Subsequent simulations of work environments or reanalysis of available filters, where available, by transmission electron microscopy (TEM) (e.g. Dement et al. Citation2009) have provided information for assessing the influence of fiber size. Conversion factors from PCOM to TEM have been proposed as well (Cherrie et al. Citation1989). As usually, 100 fibers were counted and sized by TEM, without differentiation of size range categories (Bernstein and Kunzendorf Citation2018), the sensitivity of finding fibers longer than 20 µm is often limited.
In a review of epidemiological morbidity or mortality studies, an expert panel set up by ATSDR (Citation2003) identified no studies specifically of fibers shorter than 5 µm. Studies of human/epidemiological data which assess the relationship of fiber length to cancer potency are based upon meta-analysis of studies with fiber size and mineral type information, on quantitative structure-activity relationships (QSAR) models, and on studies in which the vast majority of the airborne fibers were reported to be <10 µm in length.
3.1. Mixed exposures (chrysotile and amphibole)
Berman and Crump (Citation2008) performed a meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. In their analysis, the authors expanded the U.S. Environmental Protection Agency (Nicholson Citation1986) models for asbestos-related lung cancer and mesothelioma to allow the potency of fibers to depend upon their mineralogical types and sizes.
To address the major obstacle of the lack of data on the types and size destructions of fibers to which the study populations were exposed, concentrations were estimated using surrogate data, which included fiber size distributions obtained from air samples collected in various occupational environments and analyzed using TEM. The data was matched to the studies based on factors, such as location (in a limited number of cases), type of operation, and major fiber type. In addition to uncertainty as a result of the matching process, the size distribution measurements were limited to 10 µm, which, as the authors state, precluded assessing the importance of fibers longer than 40 µm, which have been described by Berman et al. (Citation1995) as especially potent. An additional constraint of the size distribution data was the absence of information on morphological types of fibers (e.g. fibrils or single-crystal fibers, bundles, clusters, or matrices). As the counting rules in the original studies (Dement and Harris Citation1979; Gibbs and Hwang Citation1980; Hwang and Gibbs Citation1981) were not clearly documented, it is neither known to what extent such complex fibers were included in the reported distributions nor whether such considerations were applied consistently across the studies. Thus, consideration of the effects of fiber morphology on potency cannot be addressed with the available data.
In addition, none of the studies from which the fiber size distributions were obtained contained information on the relative amounts of chrysotile and amphibole asbestos in each environment. It was necessary to assess this separately.
As there are few studies for which such surrogate data are available, the meta-analysis was limited in the number of studies included. The following studies were identified as potential sources of such data: Cherrie et al. (Citation1979), Dement and Harris (Citation1979), Winer and Cossette (Citation1979), Gibbs and Hwang (Citation1980), Hwang and Gibbs (Citation1981), Roberts and Zumwalde (Citation1982), Marconi et al. (Citation1984), Snyder et al. (Citation1987), Rood and Scott (Citation1989), and Dement et al. (Citation2008).
Berman and Crump (Citation2008) reported that “Although the best estimates of the potency of shorter fiber (5 < length <10 µm) is zero for the “all widths” and widths <0.4 µm metrics (or a small fraction of that of longer fibers for the widths >0.2 µm metric for mesothelioma), the hypothesis that these shorter fibers were non-potent could not be rejected for any of these metrics. Expansion of these metrics to include a category for fibers with lengths <5 µm did not find any consistent evidence for any potency of these shortest fibers for either lung cancer or mesothelioma.”
Korchevskiy et al. (Citation2019) developed a model that reconstructed the mesothelioma potency factors for various types of fibers based on chemical composition and dimensionality. The mesothelioma potency factors were modeled based upon the following data:
Chrysotile, Quebec – Hodgson and Darnton (Citation2000)
Amosite, South Africa – Darnton (Citation2010)
Crocidolite, South Africa – Hodgson and Darnton (Citation2000)
Crocidolite, Australia – Hodgson and Darnton (Citation2000)
Libby amphibole – Darnton (Citation2010)
Russian anthophyllite – Calculated based on Korchevskiy et al. (Citation2013)
Turkish erionite – Calculated based on Simonato et al. (Citation1989)
The following variables were included in the modeling analysis: Silicon oxide content, Ferrous, Ferric oxide content, Iron content expressed as the sum of Fe2O3 and FeO, Aluminum oxide content, Magnesium oxide content, Manganese oxide content, Potassium as potassium oxide content, Sodium as sodium oxide, Calcium as calcium oxide, and the Median aspect ratio minus 3.
The model which best predicted the mesothelioma potency factor included silicon oxide content, median aspect ratio minus 3, ferric oxide content, and magnesium oxide content (R = 0.998, R2 = 0.996, adjusted R2 = 0.988, p = 0.0077). There was only a slight difference in including the Ferric oxide content.
The authors reported that a combination of the aspect ratio of fibers and Mg fraction by itself predicted the mesothelioma potency factor with good accuracy, though with a slightly lower correlation coefficient of R = 0.988.
This analysis further supports the role of dimensionality in fiber toxic effects and, through the observed relationship with magnesium, the effect of biopersistence.
3.2. Amphibole exposure
Korchevskiy and Wylie (Citation2021, Citation2022) assessed the carcinogenic potency of elongated amphibole particles using quantitative structure-activity relationships (QSAR) models. The mesothelioma and lung cancer potency of amphibole particles were modeled based on their dimensional characteristics and mineral habit (asbestiform vs. non-asbestiform) using epidemiological data and detailed size information. This model was then applied to a large database that they established on dimensional and other relevant characteristics of elongate mineral particles based on dimensional and other relevant characteristics of elongate mineral particles. Included in this analysis was particle-by-particle information for 77 datasets, including dimensionality information for 114,098 particles, covering data for 45 mineral type categories. In the applied database, only particles longer than 2 µm were considered.
To assess the lung and mesothelioma carcinogenic potency from the dimensional characteristics of this dataset, the authors created a set of reference datasets that contained 19,509 particles (82% were simulated from published data, and 18% derived directly from raw laboratory data). The validation datasets included 738 particles, all of them from raw laboratory data. The carcinogenic potency was derived from lung (RL %) and mesothelioma (RM %), potency factors defined by Hodgson and Darnton (Citation2000) for crocidolite from Australia and South Africa, amosite from South Africa, and Na-Ca amphiboles from Libby, MT. Three additional types of particles (asbestiform fluoro-edenite, asbestiform anthophyllite, and non-asbestiform grunerite) were used as validation sets. The mesothelioma potencies for asbestiform anthophyllite and asbestiform fluoro-edenite were estimated as described in Korchevskiy et al. (Citation2020): lung cancer potencies were calculated by regressing the log-log relationship between mesothelioma and lung cancer potency from Hodgson and Darnton (Citation2000). Zero potencies for both mesothelioma and lung cancer was assumed for non-asbestiform grunerite from the Homestake mine based on Gamble and Gibbs (Citation2008) and Garabrant and Pastula (Citation2018).
The datasets used for the comparison of various habits of asbestiform minerals included 31,444, non-asbestiform 5058, and mixed 33,379 particles; 22.9% of the particles were from the simulation datasets, and 77.1% from raw data. These included data on dimensional characteristics from the following asbestiform, non-asbestiform, and mixed habit datasets (shown in parenthesis are the country/region from which they were sampled) (from Korchevskiy and Wylie Citation2021, ; repeated names indicates either difference in TEM/SEM or simulation measurements of either bullk ofr airborne samples):
3.2.1. Asbestiform
Cummingtonite-grunerite (Transvaal, SA; Transvaal, SA; Electric company Shipyard; Transvaal, SA; Transvaal, SA)
Riebeckite (Kuruman Hills, Cape SA; Cape Province, SA; Cape Province, SA; Cape Province, SA; Cape Province, SA; Wittenoom, Australia)
Anthophyllite (Finland; Russia; India)
Tremolite/Actinolite (Lone Pine, CA; Lone Pine, CA; Korea Metsovo, Greece; Udiapur, India; Fairfax, VA; Transvaal, SA)
Na-Ca Amphiboles (Libby, MN; Libby, MN; Libby, MN; Libby, MN)
Fluoro-edenite (Biancavilla, Italy; Biancavilla, Italy; Basilicata, Italy)
Hornblende (Goolwa, Australia)
3.2.2. Non-asbestiform
Cummingtonite-grunerite (Peter Mitchell mine, MN; Homestake, SD; Homestake, SD; Portugal)
Riebeckite (Long Valley, CA; St. Peter's Dome, CO)
Anthophyllite (Sweden)
Tremolite/Actinolite (Miners Bay, Canada; Gouverneur, NY; Balmat, NY; San Bernadina, CA; Enoree, SC; Shadwell, VA)
Fluoro-edenite (Sicily, Italy (Set 1); Sicily, Italy (Set 2))
3.2.3. Mixed
Tremolite/Actinolite (Barstow, CA; El Dorado, CA; Ontario, Canada; El Dorado, CA; Boulder City, NV; Boulder City, NV; Boulder City, NV; Rockhill Quarry, PA; Rockhill Quarry, PA; Calaveras Dam, CA)
Korchevskiy and Wylie (Citation2022) derived regression equations from the reference and validation datasets, which were applied to the data from the asbestiform, non-asbestiform, and mixed selections listed above to estimate the predicted potency factors for each category.
This analysis found that dimensional parameters and morphological habits are the main drivers for differentiating the cancer potency of amphibole elongated mineral particles. The mesothelioma potency was found to correlate best with specific surface area and lung cancer potency correlated best with particle aspect ratio. In addition, through a statistical evaluation of various criteria for carcinogenic potency in the scientific literature, Korchevskiy and Wylie (Citation2022) found that the Stanton criteria (fraction of particles longer than 8 μm and thinner than 0.25 μm) were a relevant predictor of the carcinogenic potency of EMPs, along with recently introduced EMPA and EMPB parametersFootnote1 (Wylie et al. Citation2020).
The authors also reported that the results suggest that asbestiform amphibole particles are typical of the particles found in the lung burden of mesothelioma patients and that non-asbestiform amphiboles, as a rule, are irrelevant for cancer risk assessment (non-asbestiform particles refer to “mineral fragments,” or “cleavage fragments”) and have negligible potency factors.
3.3. Chrysotile exposure
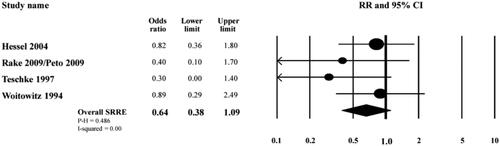
As shown in the toxicology study by Bernstein et al. (Citation2021) the chrysotile present in brake dust is largely short fiber <5 µm in length. Garabrant et al. (Citation2016), Goodman et al. (Citation2004) presented the results of a meta-analysis of the association between work as a motor vehicle mechanic and mesothelioma. This analysis was based on studies that reported relative risk estimates for mesothelioma among motor vehicle mechanics (in general), and those who were engaged in brake repair (specifically) and included ten case-control studies, one cohort study, and five proportionate mortality ratios (PMR)/standardized mortality odds ratio (SMOR) studies. The results of this meta-analysis provide evidence that motor vehicle mechanics, including workers who were engaged in brake repair, are not at an increased risk of mesothelioma.
The specific studies that examined mesothelioma risk among workers involved in brake dust repair are summarized in (reproduced from Garabrant et al. Citation2016). All four studies reported odds ratios (ORs) below 1.0, which is indicative of no statistical difference in mesothelioma risk between the workers involved with brake dust repair and the corresponding control group not involved in brake dust repair.
Figure 2. Meta-analysis of mesothelioma risk among workers involved with brake repair (reproduced from Garabrant et al. Citation2016).
4. Biopersistence
The most standardized study assessing the biopersistence of mineral fibers is the five-day inhalation or intratracheal installation study following a protocol similar to that established by the European Commission (Bernstein and Riego Citation1999). However, there are no biopersistence studies that have specifically examined samples of short fiber vs. long fiber asbestos.
As discussed below, the clearance of short and long fibers follows different pathways. Fibers that are sufficiently long and insoluble can frustrate the ability of the macrophage to dissolve, break apart or transport them, leading to inflammation and disease. Long fibers which remain in the lung are too large to be cleared by the lymphatic system. The biopersistence of these longer fibers has been associated with carcinogenic potential.
With short fibers, the lymphatic system is one of the major routes of clearance. However, in the standard biopersistence study, the entire lung is digested with no differentiation of where the fibers are in the lung. Short fibers can accumulate in the lymphatic system. With amphibole asbestos, there is often a difference in short-term clearance between long and short fibers. However, with the presence of long fibers, the developing inflammatory response has been shown to, in effect lock up the remaining short fibers as well (Bernstein et al. Citation2020a, Citation2020b). With chrysotile, the long fibers have been shown to be considerably less biopersistent and to quickly break apart into shorter fibers which augment the number of shorter fibers present (Bernstein et al. Citation2013; Bernstein and Kunzendorf Citation2018). Thus following lung digestion and quantification, the apparent biopersistence of the shorter chrysotile fibers is a function of the increase in numbers from the breakdown of the longer fibers and the accumulation in the lymphatic system. In these inhalation toxicology studies, such accumulation has not been associated with disease.
There are two primary factors that would likely influence the biopersistence of asbestos fibers. The first is the difference in biosolubility between the two mineral types chrysotile and amphibole asbestos. Amphibole asbestos is encased by a crystalline silica shell which has been shown to have little solubility at either pH 7.4 (lung surfactant) or ∼4.5 (macrophage phagolysosome), the two pH environments encountered in the lung. In contrast, chrysotile has been shown to dissociate into individual fibrils at pH 7.4 and to break down into amorphous silica particles at pH ∼4.5.
Chrysotile inhalation biopersistence studies performed with aerosol exposure concentrations at a few orders of magnitude higher than past human exposures have shown that even with significant long fiber (>20 µm) exposure, chrysotile breaks down into smaller particles and fibers which are cleared from the lung with half-times of ∼1 day to 15 days, following a 5 day exposure, depending on the source.
Bernstein et al. (Citation2020a) has determined the clearance half-times of short chrysotile following 90 days of exposure (6 h/d, 5 d/wk) at concentrations of 665 f/cm3 and 753 f/cm3. At the end of the 90 days exposure, the chrysotile fibers with length <5 µm were found to clear with half-times of 29 and 32 days and for fibers 5-20 µm in length with half-times of 28 and 42 days. This is faster than insoluble dust which are considered innocuous (Muhle et al. Citation1990; Stober et al. Citation1993; Oberdoester Citation1994; Yu et al. Citation1994; ECETOC Citation2013; Bevan et al. Citation2018).
There are no biopersistence studies of short fibers of amphibole asbestos. From their composition and the physiological mechanisms of macrophage and lymphatic clearance, they would likely clear at rates similar to insoluble dust. However, with amphibole asbestos aerosols which contain fibers longer than ∼20 µm, Bernstein et al. (Citation2021) has shown using confocal imaging that the intense inflammation caused by the long fibers, once inhaled, quickly locks up the shorter fibers that may deposit in the same regions. In those regions of the lung without such long fiber-initiated inflammation, short fibers have not been observed to accumulate.
5. Toxicology studies
Several inhalation toxicology studies have been designed to determine the differential response of short vs. long fibers. In general, the short fiber samples were produced by milling or grinding the original sample. In many early studies, the exposure concentration was set gravametrically at 10 mg/m3. Fiber concentrations were rarely reported using TEM at the time. A rough estimate can be determined based on a fiber’s cylindrical dimensions and density. For example, at 10 mg/m3, chrysotile (density 2.4 g/cm3) with a mean length of 3 µm and diameter of 0.1 µm would have ∼175,000 fibers/cm3 in the exposure atmosphere.
Vorwald et al. (Citation1951) reported on several inhalation and injection studies on guinea pigs, rabbits, cats, clogs, rats, and mice that were performed at Saranac Laboratory. The inhalation studies were whole body in a cubical dust room with aerosol generation using a rotating dust hopper. The injection studies used either dry asbestos or asbestos suspended in fluid with administration by intravenous, intraperitoneal, intratracheal, or another route.
The short fiber chrysotile samples were ball milled. In the inhalation studies with short fibers asbestos dust, a small quantity of unground short fiber asbestos was mixed with the ball mill product to generate a suitable dust cloud. The authors reported that “When that experiment failed to produce an accelerated tissue reaction, in comparison with the response initiated by Kings floats (a chrysotile which contained fibers from 1 mm to 1 micron and less in length as well as much particulate matter), it became apparent that the biological activity of asbestos is not increased by a reduction of fiber size. Thus the possibility arose that the tissue reaction observed was due solely to the relatively few long fibers of the unground asbestos and that the short fibers of asbestos had no more than a very insignificant role in the production of asbestosis, a concept not in accord with previous experiments concerning pneumonoconiosis.”
Another experiment used white rats and mice exposed to 100% ball-milled chrysotile with rats exposed for periods of up to 20 months and mice for periods of up to 12 months. The exposure concentration was 100–150 mppcf = 3530–5295 particles/cm3.Footnote2 Fiber size was determined at a magnification of 1300× and showed that 90 percent of the particles/fibers seen were smaller than ∼3 µm with only 1% >10 microns in length. The authors reported for this experiment that “In neither species, it even a suggestion of asbestosis develop and the reaction was limited to phagocytosis of inhaled particles by widely scattered dust cells which remained free in air spaces or were transported to the tracheobronchial lymph nodes. No asbestos bodies were found in the rats, but in the mice, there were a very few small, non-haustrated forms within phagocytes.”
Stanton and Wrench (Citation1972), Stanton (Citation1974), and Stanton et al. (Citation1977, Citation1981) were the first to systematically assess the effect of fiber length on mesothelioma incidence through the implantation of fibers on gelatin-coated glass substrates in the pleural cavity of rats. Over the course of the studies, more than 70 samples were evaluated, ranging from crocidolite to talc. Stanton (Citation1974) reported that the samples of asbestos and glass evaluated produced a tumor incidence that may be related to fibers below 2.5 µm in diameter and between 10 and 80 µm in length. The 1977 and 1981 publications included a wider variety of materials. The 1981 publication presented a summary of more than 10 years of particle characterization-implantation research. For many of the samples, the fiber measurements showed few fibers with diameters <0.25 µm and lengths longer than 8 µm (Table-Figure 1, Stanton et al. Citation1977 and Text-Figure 1, Stanton et al. Citation1981). When repeated samples from the same original fibers were analyzed the authors reported that considerable variation in counts occurred and stated that “Clearly, the method is subject to several errors; calibration of the electron microscope, deviation of particles from the assumed cylindrical shape, and sampling errors, especially where large particles are concerned, represent the major problems. Nevertheless, the estimates are probably valid to within one order of magnitude. Consequently, the counts are reported as the common log with the characteristic of the log representing the probable limit of accuracy (Text-Figure 1).” The log of the counts smooths out some of the variations to facilitate correlation. To include as many samples as possible, the correlation coefficients were only presented in the range <4, >4–8, and >8 µm. It would have been interesting to have presented correlations for subsets of the samples that had a larger number of longer fibers present.
Table 1. In vitro studies that differentiate the effects of short versus long fiber asbestos.
Table 2. Median diameter (and range) of the alveolar macrophage population and interstitial macrophage population for never smokers, smokers and individuals with COPD (from Dewhurst et al. Citation2017).
This concept was stated by Wylie et al. (Citation1987), who reexamined using STEM and optical images seven crocidolite samples used by Stanton et al. (Citation1981). reproduced from Wylie presents the tumor probability as a function of the log of the number of fibers per microgram for those samples that had sufficient fiber measurement data to determine what was called the index number – that is, the log of the number of particles longer than 8 µm with widths equal to or <0.25 µm. This figure illustrates the wide variation in the relationship with tumor probability. The correlation coefficient was r = 0.53 (r2 = 0.281). While significant, this represents a little more than 25% of the variation in the data. Stanton et al. (Citation1981), including all index numbers, reported an r = 0.80. As Wylie states, “Stanton was aware of the limitations in the characterization of the mineral particulates.”
Figure 3. Regression curve showing the relationship of Station criteria to tumor probability (reproduced from Wylie et al. Citation1987).
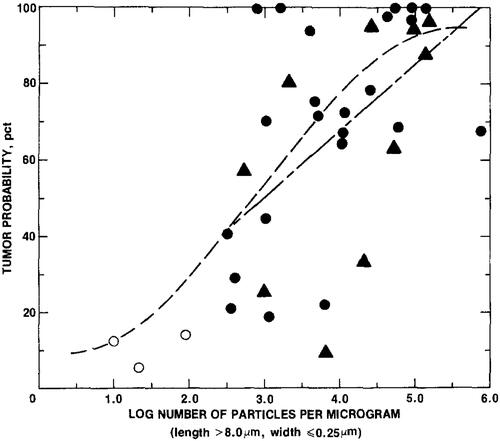
Stanton was instrumental in indicating the importance of fiber dimensions in tumor production. What has become known as the Stanton criteria of fibers L > 8 µm and W ≤ 0.25 µm should be viewed in context with the understanding of the limitations.
Davis et al. (Citation1986) investigated the pathogenicity of long vs. short fiber samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. The amosite sample was produced so that almost all fibers were <5 µm in length. This sample was compared to a raw amosite that contained a very high proportion of long fibers. The total dust concentration was 1.9 mg/m3 for the long fiber amosite and 11.6 mg/m3 for the short fiber amosite. The authors reported that “At the end of 12 months of dust inhalation, significantly more short fiber amosite was present in the lung tissue compared to the long, but while the long fiber dust caused the development of widespread pulmonary fibrosis, no fibrosis at all was found in animals treated with short fiber. One-third of animals treated with long fiber dust developed pulmonary tumors or mesotheliomas, but no pulmonary neoplasms were found in animals treated with short fiber dust.”
Davis et al. (Citation1986) also exposed animals by interpersonal injection. “Following intraperitoneal injection, the long fiber amosite produced mesotheliomas in 95% of animals with a mean induction period of ∼500 days. With short fiber dust, only single mesothelioma developed after 837 days.”
Davis and Jones (Citation1988) also performed a similar study on long and short chrysotile again prepared by ball milling. The exposure concentration was 13.9 mg/m3 short fiber chrysotile and 11.81 mg/m3 long fiber, with for each exposure group, 10 mg/m3 being respirable. However, ball milling chrysotile was more difficult than amosite, and while the short aerosol had more short fibers than the long aerosol, more than 5% of the fibers were longer than 5 µm with some longer than 20 µm. The fiber length distribution curves appeared parallel with no difference in slope (as did occur in the amosite study). The results did differentiate the long and short exposures; however, the short fiber group still showed a tumorigenic response.
Ilgren and Chatfield (Citation1997, Citation1998) reported on a chronic inhalation toxicology study that was performed by the US National Institute of Environmental Health Science (NIEHS) that included a short fiber chrysotile Coalinga. Fisher 344 rats were exposed 7 h/day, 5 days/week for 12 months with the mean exposure concentration of 7.78 ± 1.46 mg/m3. The fiber size of the aerosol as measured by TEM and reported as 63% <5 µm (dia 0.39 µm) and 77% <10 µm (dia 0.71 µm) as reported by Pinkerton et al. (Citation1983). Parenchymal fibrosis was scored with the average Wagner score of 1.9 (males) and 2.0 (females). Wagner grade 2 is defined as a few macrophages in the lumen of the terminal bronchioles and alveoli (McConnell and Davis Citation2002). The incidence of adenomas, carcinomas, or mesothelioma in the Coalinga exposed animals was not statistically different from the controls.
Platek et al. (Citation1985) and Stettler et al. (Citation2008) reported on a chronic inhalation toxicity study to evaluate the effects following exposure to short chrysotile asbestos fibers. Sprague-Dawley rats and Cynomolgus monkeys were exposed for 18 months, 7 h/day, 5 days/week to a specially prepared, chrysotile asbestos aerosol at a mean exposure concentration of 1 ± 0.28 mg/m3. The chrysotile was type 7TF1 obtained from the Johns Manville Sales Corporation (Denver, Colorado). Fibers were prepared by grinding for 24 h in a ceramic ball mill. The count median length of the fibers determined by SEM was 0.67 µm (geometric standard deviation = 1.87), the count median diameter was 0.09 µm, and the median aspect ratio (length:width) was 12. Only 46 (0.66%) of the 6940 fibers were >5 µm in length. The mean number of fibers >5 µm in length was 3.0 fibers/cm3. Through 24 months, the rats showed no fibrosis or pulmonary tumors compared to the controls. With the monkeys, the mean chamber exposure concentration was 1.0 mg/m3 with an average of 0.79 fibers/ml >5 μm in length. Fifteen monkeys (9 exposed and 6 controls) were maintained for 11.5 years following exposure, after which the lungs were examined grossly and microscopically with no lesions attributable to the inhalation exposure noted.
Wagner et al. (Citation1984, Citation1985) and Wagner (Citation1988) reported on inhalation and intrapleural inoculation studies of long and short erionite and crocidolite asbestos. The short fiber samples were milled to be <5 µm, with the majority <3 µm in length. The inhalation exposure concentration for the erionite was 10 mg/m3, mean gravimetric respirable dust concentration. None of the short fiber samples administered either by inhalation produced tumors following 12 months of exposure and a further 12 months of observation. By intrapleural inoculation, with long fiber crocidolite, there were over 90% tumors, while with short crocidolite, there was a single tumor. No tumors occurred in animals exposed to short erionite.
Ilgren and Chatfield (Citation1998) reported on the results of a lifetime investigation of F344 rats exposed by inhalation to Coalinga chrysotile that was performed at the National Institute of Environmental Health Sciences (NIEHS) and the National Toxicology Program (NTP) between 1978 and 1980. The Coalinga chrysotile exposure used in this study was unique in that almost all of the fibers were <5 µm in length. The exposure concentration was 7.78 ± 1.46 mg/m3. The study showed that animals exposed to these short fibers displayed no tumors above control levels.
Bernstein et al. (Citation2021) reported on the results from a multi-dose, 90-day inhalation toxicology study in the rat with lifetime post-exposure observation which showed a significant fundamental difference in pathological response and tumorgenicity between brake dust generated from brake pads manufactured with chrysotile or from chrysotile alone in comparison to the amphiboles, crocidolite and amosite asbestos. Three doses of brake dust containing chrysotile were used 0.20, 0.34, and 0.67 mg/m3. For the three doses, aerosol exposure concentration of the total number of fibers and those <5 µm (fibers/cm3) were: Low dose 36.44/34.02; mid-dose 48.83/43.98 and high dose 78.44/71.81 fibers/cm3. The chrysotile fibers present in the brake dust were largely smaller than 5 µm and showed no significant pathological, or tumorigenic response in the respiratory track compared to the air control group at exposure concentrations and deposited doses well above those at which humans have been exposed. Two doses of similar chrysotile to that used in the brakes were also exposed at similar gravimetric concentrations of 0.27 and 0.64 mg/m3. The total number of fibers/no fibers L < 5 µm were: Low dose 665/546 and High dose 753/520 fibers/cm3. At these exposure concentrations, the authors reported that there was no peribronchiolar inflammation, occasional very-slight interstitial fibrosis, and no exposure-related tumorigenic response.
6. In-vitro studies
In vitro cell culture studies of fibers are accessible, and these testing methods are valuable to elucidate possible mechanisms involved in pathogenesis. In vitro, cellular systems are most often static systems and not sensitive to differences in fiber solubility. There are usually performed with a single layer of a specific immortalized cell line. An immortalized cell line is a population of cells from a multicellular organism that would normally not proliferate indefinitely but, due to mutation, have evaded normal cellular senescence and instead can keep undergoing division. Immortalized cells are primary cells whose telomeres and/or tumor suppressor genes have been altered to facilitate continued cell division. The cells can therefore be grown for prolonged periods in vitro. This is in contrast to in-vivo inhalation studies where the fibers are exposed to the dynamic behavior of the respiratory system with systematic interaction and signaling between multiple cell types. In addition, the effect of lung surfactants and the fluid flows of the lung are not present.
Fiber number and dimensions are frequently not or are poorly characterized with the in vitro dosing often stated gravimetrically. Many in vitro studies were performed at concentrations to each cell that were significantly higher than that which would occur in either occupational or environmental exposures. High doses of fibers are often used to obtain a positive response, and it is difficult to extrapolate from these large short-term cellular exposures to the considerably lower-dose chronic exposures that occur in vivo.
Most important, however, is that these endpoints have not been validated as screening assays that are predictive of long-term pathological effects in vivo. While in-vitro tests may be useful tools to identify and evaluate possible mechanisms with fibers, these in-vitro test systems are of limited use in differentiating different fiber types (IARC Citation1999; Bernstein et al. Citation2005; Griesinger et al. Citation2016).
Several in vitro studies have been performed that compared short and long samples of chrysotile, amosite, and crocidolite asbestos fibers. As presented in (adapted and updated from Barlow et al. Citation2017), most of these studies exposed cell cultures on an equal mass basis, which resulted in a large difference in fiber dose for the short as compared to the long fibers.
All studies differentiated short from long fibers of either chrysotile or amphibole asbestos except that reported by Aalto and Heppleston (Citation1984). In this study, the amosite dosing was based on an equal mass of fibers. All fiber length fractions induced the production or release of fibrogenic factors; however, short fibers were more active than long fibers. Based on the fiber dimensions, the approximate amosite fiber dose was calculated from the dimensions (and density) presented in the publication. The short fiber amosite sample resulted in a dose of ∼1000 fibers/macrophage, while the long fiber samples had 100 fibers/macrophage.
One of the more recent studies on short and long amosite by Tomatis et al. (Citation2010) provided an evaluation of amosite fiber number per µg of the sample with a determination of the number of fibers administered per cell, which ranged between 0.1 and 10 for long fibers and between 1.5 and 150 for short fibers amosite, and was 2 f/cell for a synthetic glass fiber MMVF10. The dose-response range of this study was unique in that it was based upon the number of fibers administered per cell and was within plausible ranges of dosing. However, the in-vitro system was static with little sensitivity to dissolution effects. The authors stated that in terms of the number of fibers per cell, the short fiber amoiste at a dose of 0.35 μg/cm2 may be compared to long fiber amosite at 3.5 μg/cm2 and MMVF10 at 35 μg/cm2. The authors reported that long fiber amosite shows a higher free radical yield and stimulates more than short fibers the NO production by cells and reacts with ascorbic acid. This difference was associated with the presence of Fe2+ ions poorly coordinated to the surface. The short fibers showed only loosely bound Fe3+ ions as the pristine Fe2+ ions were oxidized during the grinding process converting long fibers into short fibers. Both length and these differences were considered determinants in the differential toxicity.
Donaldson et al. (Citation1993), Dogra and Donaldson (Citation1995), Donaldson and Golyasnya (Citation1995), Hill et al. (Citation1995), Riganti et al. (Citation2003), Tomatis et al. (Citation2010), and Boyles et al. (Citation2015) all used the same short and long fiber amosite samples that were prepared for the amosite short fiber chronic inhalation study (Davis et al. Citation1986). These studies included rat alveolar macrophage (cytotoxicity), Chinese hamster ovary cells (chromosomal aberration), rat alveolar macrophages (superoxide anions), human type 2 alveolar epithelial cells (epithelial cell injury assay), human lung epithelial cells (measurement of PPP activity; lipid peroxidation assay; cytotoxicity; enzyme activity assay), human lung epithelial cells (NO synthesis and NO synthase (NOS) activity), macrophages (cytotoxicity), respectively and all differentiated short from long fibers. Tilkes and Beck (Citation1980) and Kaw et al. (Citation1982) used the same short and long samples of amosite, chrysotile, and crocidolite in rat phagocytic ascetic tumor cells (cytotoxicity) and mouse peritoneal macrophages (cytotoxicity), also differentiating effects from short from long fibers for each. Woodworth et al. (Citation1983) used a hamster tracheal organ culture-related squamous metaplasia to assess fiber geometry. Tilkes and Beck (Citation1983) used guinea pig lung macrophages with short and long (chrysotile and crocidolite and reported a length- and dose-dependent cytotoxicity with long, very thin fibers of glass, chrysotile, crocidolite, and synthetic fluoro-amphiboles were all toxic in the test system. Hesterberg and Barrett (Citation1984), using Syrian hamster embryo cells, reported that milling chrysotile greatly reduced its cytotoxicity and eliminated its transforming potency. Marsh and Mossman (Citation1988), using hamster tracheal epithelial cells using chrysotile and crocidolite, reported that the potency of ODC induction was directly related to fiber length. Goodglick and Kane (Citation1990), using peritoneal macrophages using crocidolite at relatively high exposures, reported that the release of reactive oxygen species was stimulated by both long and short crocidolite; however, long fibers were found to be more toxic than short fibers.
7. Inhalation and pulmonary response to short asbestos fibers
Humans can breathe through their mouth as well as their nose. Rodents that are often used for inhalation toxicology studies, however, are mandatory nasal breathers. In humans, inhaled fibers can be inhaled through the larynx and trachea and enter the tracheal, bronchial airways which are designed to conduct air to and from the alveoli for respiration. The primary (main) bronchi divide into secondary lobar bronchi. The airways continue to fan out into 17–21 generations of bronchioles, with the terminal bronchioles being the last generation of conducting airways that give rise to respiratory bronchioles, which ultimately lead to the alveoli (Hyde et al. Citation2009). Fibers that land on the bronchial tree can be cleared through ciliated mucous transport and eventually swallowed or expectorated. Fiber movement in this region is largely by diffusion. Each respiratory bronchiole divides into two through eleven alveolar ducts; each duct gives rise to five through six alveolar sacs.
Depending on aerodynamic diameter, <∼20% of the fibers inhaled by nose-breathing and <∼30% by mouth breathing will deposit in the alveoli (Dai and Yu Citation1998). The alveoli are lined by a dynamic layer of surfactant which is secreted by the type II alveolar epithelial cells, which have as well microvilli on their surface (Mason and Lewis Citation2011; Crouch and Wright Citation2001). Fibers that deposit in the alveoli will come into contact with pulmonary surfactant and will likely be coated by surfactant.
Our understanding of the roles of fiber dose, dimension (length and diameter), and durability (biopersistence) on fiber toxicology has evolved largely through the study of asbestos and synthetic vitreous fibers (SVF). Numerous groups and publications have detailed the importance of the 3 parameters, dose, dimension, and durability associated with potential fiber-related pathology. These include Hammad (Citation1984), Hart et al. (Citation1994); USEPA (Citation1995), Bernstein et al. (Citation1996); ATSDR (Citation2003, Citation2004, Citation2018), Donaldson et al. (Citation2006, Citation2011), and SCOEL (Citation2012).
The differential paradigm of short and long fiber inhalation, deposition, and response is summarized in . The aerodynamic diameter (which is ∼3 times the fiber’s width) determines whether and where in the respiratory tract the fiber can deposit.
The fiber length determines in conjunction with biodurability the potential clearance within the respiratory tract. Two transitional ranges are important. The first is whether the fiber is short and thin enough to be cleared by the lymphatic system, and the second is whether it is short enough to be phagocytized by the alveolar macrophages.
For longer fibers, the biopersistence determines whether the longer fibers which have the potential of frustrating phagocytosis by the macrophage will dissolve or disintegrate into shorter fibers and particles or persist. This differentiates chrysotile from amphibole asbestos. Amphibole asbestos is relatively insoluble at either acid or neutral pH at body temperature (Speil and Leineweber Citation1969).
Choi and Smith (Citation1972) reported on the kinetics of chrysotile dissolution in water (neutral pH) at ambient and body temperatures. They found that the magnesium cations may be continuously liberated from chrysotile fibers leaving behind a silica skeleton that has the elements of the original structure and that the chrysotile dissolves congruently in water. The experimental evidence suggested that the rate-controlling step is the removal of the brucite layer from the fiber surface of chrysotile and that the smaller the particle size, the more magnesium is liberated from the chrysotile in water suspension. Chrysotile has been shown to be acid soluble (Kobell Citation1834; Whittaker Citation2009; Gualtieri et al. Citation2019). The macrophage phagolysosome produces an acid environment which is considered instrumental in the biosolubility of chrysotile. However, these studies are ex-vivo, and mineral dissolution rates alone do not seem not to fully model the dissolution/breakage that has been observed in-vivo (see biopersistence section). It is likely that a combination of the dissolution of the brucite layer combined with the effect of the macrophage acid environment weakens the thin rolled structure of the chrysotile fibril resulting in breakage into shorter fibers and particles. Finally, biopersistence will also have an impact on the number of shorter fibers remaining.
With fibers long enough to frustrate the ability of the macrophage to clear them and which are not bio-soluble, will persist in the lung and initiate an inflammatory cascade. Chronic inflammation has been shown to be a precursor in the pathogenesis of lung cancer and is associated with the development of cancer (Hanahan and Weinberg Citation2011; Conway et al. Citation2016). In contrast to acute inflammation, which is important for clearing infection, healing wounds, maintaining tissue homeostasis, and clearing short fibers, inflammation associated with tumor development is often low in grade and chronic (Yang and Lin Citation2017). With fiber-related inflammation and carcinogenesis, long durable fibers, such as the crocidolite and amosite fibers, once inhaled, are present as physical impediments, which the macrophage fails to successfully phagocytize and clear. With such fibers, the frustrated phagocytosis releases the proteolytic enzymes, which can break down the connective tissue of the lung (Fels and Cohn Citation1986). Grecian et al. (Citation2018) and Michel et al. (Citation2012) have reviewed the relationship of the cross-talk between macrophages and natural killer cells, such as neutrophils, and the role of neutrophils in cancer. In the subchronic inhalation study discussed above (Bernstein et al. Citation2021), has shown through collagen quantification and histopathological examination the important role of inflammation in association with tumorigenesis of the long fibers of amosite and crocidolite asbestos. The inflammation also extended to the visceral and parietal pleura with significant increases in thickening and collagen formation.
The lack of inflammation and pathogenesis in the control and chrysotile containing brake dust groups of this study and the lack of persistence in response in the chrysotile groups, which largely resolved by 24 months, provides a further explanation as to why the exposures in these groups did not produce a carcinogenic response in this study. The chrysotile in the brake dust groups was largely short fibers.
7.1. Macrophage clearance
The alveolar macrophage in humans is larger than that found in rats and hamsters. Krombach et al. (Citation1997) reported the following diameters for alveolar macrophages:
Hamster 13.6 ± 0.4 μm (n = 8)
Rat 13.1 ± 0.2 μm (n = 12)
Monkeys 15.3 ± 0.5 μm (n = 7
Human volunteers 21.2 ± 0.3 μm (n = 10)
The authors stated that the alveolar macrophages (AM) from humans were significantly larger (p < 0.05) than those from all other species studied, corresponding to a 4-fold larger cell volume of human AM (4990 ± 174 μm3) compared to hamster (1328 ± 123 μm3) and rat (1166 ± 42 μm3) AM.
More recently, Dewhurst et al. (Citation2017) examined the characteristics of lung macrophage subpopulations in COPD patients and controls. They differentiated as well macrophages located in the alveolar spaces [alveolar macrophages (AM)] and macrophages located in the alveolar walls or peripheral tissue [interstitial macrophages (IM)]. The median diameter of the alveolar macrophage population was significantly larger than the interstitial macrophage population for never smokers. As shown in , alveolar macrophage size is increased in COPD patients compared to controls.
7.2. Lymphatic clearance
The peri-bronchoalveolar lymphatics wind around the airways to the level of the respiratory bronchioles and also surround the pulmonary arteries and veins. The lymphatics are involved in removing fluid and particles from the alveolar lumina. Open intercellular junctions composed of the over-lapping and free edges of neighboring endothelial cells are considered to act as “one-way inlet flap valves” together with the help of the anchoring filaments, thus allowing fluid or particulate matter to enter and not to leave the lymphatic capillary lumen (Lauweryns and Baert Citation1977).
Most important is the existence throughout the lung and pleural lymphatic system of one-way valves which direct the flow. The visceral pleura is abundantly endowed with lymphatic vessels. These lymphatics form a plexus of intercommunicating vessels that run over the surface of the lung toward the hilum and also penetrate the lung to join the bronchial lymph vessels by passing through the interlobular septa. The larger lymphatic vessels in the visceral pleura are equipped with one-way valves directing flow toward the hilum of the lung. All lymph draining from the visceral pleura, therefore, reaches the lung root, either through the lymphatic vessels of the lobular and lobar lung septae, all by flowing along the visceral pleural surface to the lung hilum (Hallifax and Rahman Citation2016; Bernstein and Pavlisko, Citation2017).
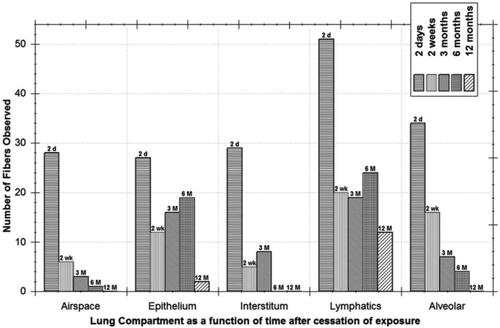
Bernstein et al. (Citation2004) quantified, using 3D confocal microscopy imaging, the uptake and clearance of chrysotile fibers following a 5 day (6 h/day) exposure to Brazilian chrysotile at an exposure concentration of 2098 WHOFootnote3 fibers/cm3. Quantified were the numbers of fibers observed as a function of time in the airspace, bronchial and alveolar epithelium, interstitium, lymphatics, and alveolar macrophages (). The lymphatics had the highest clearance rate surpassing that of alveolar macrophage clearance. The authors also demonstrated using confocal imaging that chrysotile fibers of ∼5 µm in length were also observed in the distal lymphatics, both free and in phagocytic lymphocytes (Figures 9(b,e), Bernstein et al. Citation2004). Fibers <5 µm were not reported; however, these results suggest that the shorter fibers would also be cleared similarly.
Figure 5. Quantification of the numbers of chrysotile fibers observed as a function of time after a 5-day exposure in the airspace, bronchial and alveolar epithelium, interstitium, lymphatics, and alveolar macrophages (reproduced from Figure 11, Bernstein et al. Citation2004).
7.3. Pleural clearance
7.3.1. Parietal pleura
On the parietal pleura, lymphatic channels communicate with the pleural space via stomas measuring 2–12 μm in humans that are found predominantly along the mediastinal parietal pleura and intercostal surfaces, particularly in the lower chest. These stomas are the primary pathway for the elimination of fluid and material from the pleural space. The flow through the parietal pleura lymphatic channels is aided by one-way valves that prevent fluid from flowing back into the pleura (Wang Citation1985; Miura et al. Citation2000; Bertin and Deslauriers Citation2011; Negrini and Moriondo Citation2013, Light and Lee Citation2016). The pleural liquid is conveyed to the regional lymph nodes, including the intercostal, parasternal, diaphragmatic, and posterior mediastinal lymph nodes (Broaddus et al. Citation1988; Yalcin et al. Citation2013). From there, lymph is carried to the thoracic duct and right lymphatic duct (Krenke and Mierzejewski Citation2020).
7.3.2. Visceral pleura
The visceral pleura does not have stomata and thus does not act in the clearance of pleural fluid.
The visceral pleura has an extensive lymphatic plexus that drains from the lung surface toward the hilum. The plexus also penetrates internally to join lymph channels along the interlobular septa. All drainage from the visceral pleura eventually reaches the lung root (Finley and Rusch Citation2011).
Alveolar structure and the lung lymphatic system: Around the alveoli, the lymphatic ducts wind around the blood vessels surrounding the alveoli. The surfactant in the alveoli enters into the lymphatic ducts through a system of one-way valves, which are designed to assure that the flow of fluid is into the lymphatic ducts then up through lymphatic channels exiting into the bloodstream.
In addition, there is an extensive lymphatic network below the visceral pleura. Again one-way valves assure that the lymphatic fluid flow is away from the visceral pleura and leads eventually to the hilar lymph node region.
Riquet (Citation2007) described the lymphatics of the lung as a visceral pleural network and a parenchymatous peri-bronchovascular plexus, located mainly in the connective tissue surrounding the airways and their bronchial artery vascular supply. Lymphatics originate from capillaries located between the alveolar walls and the interlobular, pleural, peribronchial, or perivascular connective sheets. They form lymphatic collecting vessels called “collectors.” The lymphatic collectors contain smooth muscles and one-way valves every 2–10 mm along their length. The segments within two valves, the lymphangions, act as a small pump, with smooth muscle contraction in one lymphangion forcing fluid into the next. The valves prevent retrograde flow. The visceral pleura lymphatic collectors course over the surface of the lung toward the lung hilum, where they generally anastomose with the lymphatic collectors of the peribronchovascular plexus (the latter, strictly speaking, being the bronchial lymphatics). They also can travel further along the bronchi and the lower part of the trachea without anastomosing and enter directly into the mediastinum (Lauweryns Citation1970; Lauweryns and Baert Citation1977; Riquet et al. Citation1989; Drake et al. Citation1991).
Bronchus-associated lymphoid tissue (BALT) describes small clusters of cells found near large airways, usually bronchi. BALT is especially prominent in rodents following inhalation exposure which accumulate and likely transport particles cleared by the lymphatic system. These cellular accumulations are not present in the healthy lungs of rodents or humans but can be induced following infections or other antigenic events. In humans, a similar pattern of cells has been described and is called IALC for isolated aggregations of lymphoid cells (Macklin Citation1955; Semmler-Behnke et al. Citation2007; Randall Citation2010; Van Winkle et al. Citation2018, Margaris and Black Citation2012).
8. Discussion
8.1. Reviews and reports
The Agency for Toxic Substances and Disease Registry (ATSDR Citation2004) reported on the findings of an expert panel that was convened to evaluate the influence of fiber length on the health effects of asbestos and synthetic vitreous fibers. The panel of experts concluded that: “Given findings from epidemiologic studies, laboratory animal studies, and in vitro genotoxicity studies, combined with the lung’s ability to clear short fibers, the panelists agreed that there is a strong weight of evidence that asbestos and SVFs shorter than 5 μm are unlikely to cause cancer in humans.”
When considering the non-cancer effects of short fibers, the panel concluded that laboratory animal studies, epidemiologic studies, and in vitro studies generally suggest that asbestos and SVF pathogenicity increases with fiber length, but there are several notable exceptions. In laboratory animals at sufficiently high doses, which may not be relevant to human environmental exposures, inflammation and fibrosis were observed. Four epidemiological studies (Churg et al. Citation1989; Churg Citation1990; Nayebzadeh et al. Citation2001) suggested that short fibers in the lung at death were associated with fibrosis. These associations in these publications were fairly weak and largely attributed to short amphibole fibers. As an example, Nayebzadeh et al. (Citation2001) found a correlation of tremolite fibers <5 µm with R = 0.44, thus explaining <20% of the association with fibrosis. Churg et al. (Citation1989), examining 21 cases, found a positive correlation of tremolite grade with fibrosis and a negative association with length. However, Churg et al. (Citation1989) counted by TEM, 100 sequentially encountered chrysotile and/or tremolite asbestos fibers which preferentially count short fibers, which were larger in number than long fibers. In addition, the correlation coefficient was R = 0.46, accounting for 21% of the association with fibrosis.
The US Environmental Protection Agency (EPA) has recently conducted an assessment to evaluate the dangers associated with high-density products made with chrysotile and, in particular, brake shoes and sealing gaskets which could be found on the US market in which the EPA identified cancer risks from inhalation exposure to chrysotile asbestos (EPA Citation2020a, Citation2020b, Citation2020c, Citation2020d). While the Science Advisory Committee on Chemicals (SACC), peer-reviewed the document, many questions remained unanswered. As reviewed by Paustenbach et al. (Citation2021), the EPA did not consider more than 15 epidemiology studies as discussed above, which indicated that exposures to encapsulated chrysotile asbestos in brakes and gaskets, which were generally in commerce from ∼1950 to 1985, did not increase the incidence of any asbestos-related disease. Instead, the EPA based their evaluation of the chrysotile mesothelioma hazard based on populations in two chrysotile textile facilities where mixed exposure to chrysotile and commercial amphibole asbestos (amosite and crocidolite) occurred.
As Paustenbach et al. (Citation2021) has shown, ∼96% of the person-time accrued for the North Carolina textile cohorts was from workers at plants three and four (Garabrant Citation2020), which also had documented use of amosite and crocidolite asbestos (UNARCO Citation1954; UNARCO Citation2012).
It also remains unclear why exceedingly high exposures 1940s–late 1970s to longer textile fibers (Dement et al. Citation2009; Loomis et al. Citation2009; Elliott et al. Citation2012; Loomis et al. Citation2012, Citation2019) were used to assess lifetime doses of chrysotile asbestos that were around 2–5 f/cc-years as occurred for auto mechanics in the 1950s–1985 and as presented above was exposed predominantly to short fibers (Paustenbach et al. Citation2003, Citation2004; Finley et al. Citation2007). The resulting suggested inhalation unit risk for chrysotile was not greatly different than that for amosite even though evaluation of mesothelioma potency has shown that amphibole asbestos is considerably more potent than chrysotile (Berman et al. Citation1995; Hodgson and Darnton Citation2000; Berman and Crump Citation2008; Garabrant and Pastula Citation2018; Wylie et al. Citation2020). The importance of length and diameter has been stated by many researchers (Pott Citation1978; Berman et al. Citation1995; Berman and Crump Citation2008; Loomis et al. Citation2010; Lippmann Citation2014; Wylie et al. Citation2020).
Korchevskiy et al. (Citation2019) reported on the development of an empirical model that would reconstruct mesothelioma potency factors for various types of fibers based on their chemical composition and dimensionality. The authors modeled the mesothelioma potency factors estimated by Hodgson and Darnton method based upon the chemical composition and dimensionality metrics (aspect ratios) for Quebec chrysotile, South Africa amosite, South Africa and Australian crocidolite, Russian anthophyllite, Libby amphiboles, and Turkey erionite. The mesothelioma potency factor was highly correlated with a function of the aspect ratio, silicon dioxide, iron, and magnesium (R2 = 0.994). However, a combination of the aspect ratio of fibers and Mg fraction also predicted the model mesothelioma potency factor with good accuracy (R = 0.988). Through this analysis, the authors proposed the possibility that the biopersistence of asbestos fibers in the human respiratory system is approximately inversely proportional to magnesium content.
Barlow et al. (Citation2017) has reviewed asbestos fiber length in relation to disease and found that studies reported over the last several decades have consistently supported the conclusions that there is very little if any risk associated with exposure to fibers <5 µm and that exposure to fibers longer than 10 µm and perhaps 20 µm are required to significantly increase the risk of developing asbestos-related disease in humans.
Boulanger et al. (Citation2014) also reviewed fiber size toxicity through the quantification of short and long asbestos fibers to assess asbestos exposure. Included was a review of air sampling measurements collected in the city environment and buildings with asbestos-containing materials (ACM) and reported that the majority of fibers were <5 µm in length with concentrations sometimes ≥ 0.01 f/cm3. The authors concluded that the pathogenicity of short asbestos fibers could not be completely ruled out, especially in high exposure situations. There was no consistent differentiation between chrysotile and amphibole asbestos but rather the generalized use of the term asbestos.
Gualtieri et al. (Citation2019) presented a structure model to explain the toxic action of chrysotile in terms of structural, chemical, and physical properties using in vitro cytotoxicity tests and alkaline comet assay tests. The cytotoxicity effects of acid-leached chrysotile fibers were tested on THP-1 (monocytes) and Met-5A (benign immortalized mesothelial cells) cell lines. Cells were dosed at concentrations of 25–100 µg/ml without any details provided of the number of fibers administered to the cells in these static systems. The authors reported that the in vitro tests indicate a significant cyto/genotoxicity of the acid-leached chrysotile in both investigated cells lines. There was no discussion of the limitations of using static in-vitro methods or of the fiber dose to the cells.
Gualtieri (Citation2021) presented a novel approach to correlate how and to what extent the physical/crystal‐chemical and morphological parameters (including length, chemistry, biodurability, and surface properties) of mineral fibers are associated with major adverse effects with an emphasis on asbestos. The authors state that the model assumes that chrysotile is less potent for the induction of MM and low exposures to chrysotile do not present a detectable risk to health compared to amphibole asbestos (Roggli Citation1995; Hodgson and Darnton Citation2000; Roggli and Vollmer Citation2008; Mossman et al. Citation2011; Bernstein et al. Citation2013; Garabrant and Pastula Citation2018). The model fiber characteristics include that chrysotile is not biodurable and easily leached in vivo in the lungs, whereas amphibole asbestos fibers are biodurable (Jaurand et al. Citation1977; Morgan Citation1994; Bernstein et al. Citation2013) and induce chronic inflammation responsible for adverse effects. The authors viewed this as an initial model and plan to verify the model predictions with in vitro toxicity tests and eventually in vivo testing. However, the use of in-vitro testing due to the difficulties and biases described in this review may not be the best validation. A recent exchange between Gualtieri and Di Giuseppe Citation2022 and Wylie and Korchevskiy (Citation2022) concerning the letter to the editor by Wylie and Korchevskiy (Citation2021) on the carcinogenicity of fibrous glaucophane has highlighted this. Wylie and Korchevskiy (Citation2021, Citation2022) emphasized that epidemiology should be the basis for the validation of structural models and pointed out that the model developed by Gualtieri (Citation2021) does not correctly predict the potency of fibrous glaucophane.
8.2. In vitro
In vitro studies can provide valuable information depending upon how they are performed. Many in vitro studies, however, have been reported with little if any information on the number of fibers actually dosed per cell in the cell cultures. Most often, dosing is stated as µg/cm2 applied to the cell culture dish. As is illustrated below, this can result in very high dosing to each cell far above that which would be expected to occur in human occupational or environmental exposures.
The number of fibers applied can be estimated based on the cylindrical dimensions and density of the fiber. If fiber has an average diameter of 0.1 µm and length of 3 µm, with a density of 2.4 g/cm3 (chrysotile), there would be more than 10 million fibers per µg.
In the publication by Huang (Citation1979), the authors stated that they used a Flacon cell dish (100 mm) with 105 cells per dish and applied 10 µg/cm2 to the dish. As shown in , ∼500,000 fibers were applied to each cell.
Table 3. In-vitro dosing and extrapolated fiber exposure in Huang (Citation1979).
In a more recent study by Levresse et al. (Citation2000), the authors stated that “Normal RPMC from Sprague–Dawley rats were cultured according to a method described elsewhere (Jaurand et al. Citation1981).”
Jaurand et al. (Citation1981) state “5 × 105 cells were then put into a Falcon tissue culture flask (25 cm2).”
The authors in this study reported the length and diameter were 1.7 ± 2.2 and 0.05 ± 0.04 μm, respectively, for chrysotile and 2.1 ± 3.6 and 0.19 ± 0.12 μm for crocidolite.
The cells were exposed to 0.5, 2, 5, or 10 μg/cm2 chrysotile, stating that “Briefly, cells were plated in 75 cm2 tissue culture flasks (Costar, Dutscher, Brumath, France).”
For the low dose of 0.5 µg/cm2, as shown in , ∼70,000 fibers were applied per cell.
Table 4. In-vitro dosing and extrapolated fiber exposure in Levresse et al. (Citation2000).
Huang et al. (Citation2011) summarized a large series of over 80 in vitro studies. The greatest detail provided in dosing was by µg/cm2 applied. The above analysis of two of these studies chosen at random shows that the number of fibers applied per cell is certainly excessive and far exceeds the few fibers per cell that are occasionally seen following exposure to ∼250 WHO fibers/cm3 of chrysotile (Bernstein et al. Citation2021).
At these concentrations, dispersion and agglomeration can also be important. Cohen et al. (Citation2014) reported on evidence of the effect of agglomeration on the dose delivered to the cell.
8.3. Clearance of short fibers from the lung
In contrast to longer fibers (>∼20 µm length) which are too long to be fully engulfed by the macrophage or enter the lymphatic system and can only be removed either by dissolution or breakage, short thin chrysotile or amphibole asbestos fibers, especially those shorter than 5 µm in length can be removed by these routes. If a short fiber is phagocytized by a macrophage, it can be transported to the ciliated bronchi and cleared by the ciliated mucous bronchial clearance. An equally important route of clearance is by entry through the lymphatic valves into the lymphatic system. As presented above, the study by Bernstein et al. (Citation2004), which quantified using confocal microscopy the differential clearance between these routes, has shown that lymphatic clearance is the most important for short, thin fibers.
Distributed throughout the lymphatic system are 1-way valves (every 2–10 mm), which assure that the lymph flow is in the direction of the hilum. Distal lymph nodes located within the lobes along the segmental bronchi lymphatics probably develop during the years following birth, as does bronchial and bronchiolar lymphoid tissue (Riquet Citation2007).
Bronchus-associated lymphoid tissue (BALT) in rodents and isolated aggregations of lymphoid cells (IALC) in humans describe small clusters of cells found near large airways, usually bronchi. BALT and IALC increase following inhalation exposure which accumulates and likely transport particles cleared by the lymphatic system. Through the one-way valves, the lymphatics reach the larger peri-tracheobronchial lymph nodes. The lymphoid tissue and lymph nodes can accumulate the short fibers. Dodson et al. (Citation2000) reported that even in non-occupationally exposed individuals, short non-commercial amphibole asbestos fibers could be found.
Short fibers in both inhalation toxicology studies and human tissue burden studies may be cleared from the active area of the lung but may be stored in the lymphatic tissue. Lung digestion studies which will include the lymphatics, cannot differentiate wherein the lung the fibers were located, and thus the attribution of shorter fibers remaining in the lung to possible disease should not be based on such studies.
The role of lymphatics is also important when determining fiber concentration in the pleural cavity and associating this with the possible disease. The extensive lymphatic network just below the visceral pleura can be an important source for short fibers. Upon necropsy, if the visceral pleura of the lung is dissected, which is often the case to reach the pleural cavity, the extensive network of one-way valves and the pumping action of the lymphangions will no longer be present, allowing the draining of the fluid into the pleural cavity effectively contaminating the area with short fibers.
Barlow et al. (Citation2017) has summarized human studies which document the abundance of short fiber compared to longer fibers. “Several groups have documented fiber concentration and size retained in the lung tissue of those occupationally exposed to asbestos (Churg and Wiggs Citation1984, Citation1986, Citation1987; Langer and Nolan Citation1994; Tossavainen et al. Citation1994; Suzuki and Yuen Citation2002). These groups reported that shorter fibers were more abundant in the human lung of those occupationally exposed to asbestos than longer fibers, regardless of fiber type. One group suggested, based on these data, that either the carcinogenic size range is much broader than thought or a small number of fibers in certain size ranges can induce tumors in humans (Churg and Wiggs Citation1984).”
However, these authors did not assess where in the lung the short fibers were located. Lung digestion studies cannot differentiate fiber location in the lung. In addition, as presented above, biopsy and especially autopsy samples will have a very high likelihood of disturbing the homoeostatic pressure balances in the lung. As presented above, the lymphatic system is important in the removal of short fibers from the active area of the lung, where the fiber-related disease has been shown to develop. The extensive network of lymphatic sumps and nodes in the human lung in which fibers have been shown to accumulate the shorter fibers that enter the lymphatics. Confocal microscopy as discussed above does provide the means to differentiate the routes of fiber clearance in the lung without producing artifacts through the cross-contamination of fluid and tissues through surgical procedures.
8.4. Assigning causality based upon fiber number
As discussed above, because of the log-normal distribution of fiber length, there are usually many more short fibers than longer fibers present. This is augmented through the use of fiber counting/sizing protocols in which 100 fibers are counted without differentiating size categories (that is, individual counting rules for fibers <5 µm, 5–20 µm, >20 µm).
As an example, Suzuki et al. (Citation2005) reported pathological evidence that short, thin asbestos fibers contribute to the development of human malignant mesothelioma. The authors reported that the samples were taken at autopsy or biopsy. Suzuki et al. state (in of the publication) that for the digested tissues 3.7% of the chrysotile found and ∼50% of the amphiboles (amosite, crocidolite, tremolite, and anthophyllite) were longer than 5 µm in length. By ashing 0.3% of the chrysotile and between 43 and 75% of the amphiboles were longer than 5 µm. However, the authors provided no basis other than fiber numbers to associate cause and effect.
As mentioned above, Bernstein et al. (Citation2021) reported on the results of a 90-day inhalation exposure which included chrysotile and amosite and crocidolite asbestos. At 12 months following the 90-day exposure, the chrysotile exposed rats had 5% of fibers in the lung >5 µm, similar to what Suzuki et al. reported 63% of amosite and 77% of crocidolite were longer than 5 µm. With chrysotile, there was only a very-slight interstitial inflammatory response, peribronchiolar inflammation, occasional very-slight interstitial fibrosis, and no exposure-related tumorigenic response. Crocidolite and amosite induced dose-related lung tumor response, which correlated to the number of fibers L > 20 µm. Confocal microscopy quantified extensive inflammatory response and collagen development in the lung, visceral and parietal pleura, as well as pleural adhesions. The assigning of cause and effect based solely on fiber numbers can be very misleading.
In addition, concerning the Suzuki studies, the ATSDR report (Citation2003) stated that they must always be regarded with suspicion, particularly when the study lacks controls and the finding is in what is in fact, tissues of new growth, such as plaques and tumors (Case Citation1994).
9. Conclusions
Epidemiology, toxicology, and in-vitro studies provide strong support that short chrysotile and amphibole asbestos fibers with lengths <5–10 µm do not contribute to the health effects of asbestos. The epidemiology studies in which the cancer potency estimates were based upon relatively high exposure concentrations provide a conservative assessment that shorter fibers would have little if any effect, especially under controlled exposure or environmental conditions that may occur today.
The QSAR models have shown that fiber aspect ratio and Mg content are excellent predictors of cancer potency and that short fibers/particles of amphibole would have no effect. The studies of motor vehicle mechanics and in particular, workers who serviced chrysotile containing brakes with the majority of the fibers being short provides evidence that motor vehicle mechanics, including workers who were engaged in brake repair, are not at an increased risk of mesothelioma. Several inhalation toxicology studies clearly differentiated that short chrysotile and amphibole asbestos fibers did not produce a significant carcinogenic effect in the lung or pleural cavity. Nearly all these studies were performed at exposure concentrations far exceeding those that occurred with uncontrolled human exposures in the past. Because of dosing and lack of sensitivity to biosolubility, in vitro studies can be difficult to interpret; however, a number have differentiated short chrysotile and amphibole asbestos fibers from long fibers.
Integral to understanding the importance of fiber length in determining possible health effects is an understanding of the biological and physiological function of the respiratory system. Short asbestos fibers, like innocuous dust, can be cleared through the tracheal-bronchial ciliated mucous transport, phagocytized by macrophages and cleared via the bronchial tree, and can also be removed through the lymphatic system. While the first two methods can remove them from the lung, with lymphatic transport through one-way valves, fibers are removed from the active area of the lung where the fiber-related disease has been shown to develop and can accumulate in lymphatic sumps and lymph nodes.
While short asbestos fibers are present in most occupational or environmental exposures, the large body of studies strongly supports that they do not contribute to the health effects of asbestos exposure.
Acknowledgments
I am pleased to acknowledge the anonymous reviewers the Editor selected. Besides these anonymous reviewers and the author, no individual has reviewed any draft material included in the paper and any drafts of the paper.
Declaration of interest
This study was initiated and funded by the International Chrysotile Association (ICA) to promote a further update on the science of short fibers. David Bernstein is an independent consultant specializing in inhalation toxicology. He has appeared twice as an expert witness in litigation concerned with alleged health effects of exposure to chrysotile and has been deposed seven times. ICA does not intend to use this publication for litigation purposes. Dr. Bernstein has published more than 20 peer-reviewed papers on asbestos over the past 20 years. The work product, including the conclusions drawn, is exclusively those of the author, Dr. Bernstein, and has not been influenced by anyone other than the author. Aside from the author, no one, including the International Chrysotile Association or its members, has reviewed, commented on, or revised this paper before its submission.
Notes
1 The EMPA index is defined as the fraction of elongated mineral particles (EMPs) with aspect ratio (AR) ≥ 3, length > 5 μm and width ≤ 0.15 μm to total EMPs with AR ≥ 3 and length > 5 μm. The EMPB index is the fraction of EMPs with AR ≥ 3, length >5 μm and width ≤ 0.25 μm to all EMPs with AR ≥ 3, length >5 μm (Wylie et al., 2020).
2 mppcf × 35.3 = million particles per cubic meter = particles per c.c. (Occupational Safety and Health Admin., Labor § 1910.1001).
3 Fibers with length ≥5 μm, diameter ≤3 μm (WHO, Citation1985).
References
- Aalto M, Heppleston AG. 1984. Fibrogenesis by mineral fibers: an in-vitro study of the roles of the macrophage and fiber length. Br J Exp Pathol. 65(1):91–99.
- ATSDR. 2003. Report on the expert panel on health effects of asbestos and synthetic vitreous fibers: the influence of fiber length prepared for: Agency for Toxic Substances and Disease Registry Division of Health Assessment and Consultation, Atlanta, GA. Lexington (MA): Eastern Research Group, Inc.
- ATSDR. 2004. Toxicological profile for synthetic vitreous fibers additional resources. Public Health Service Agency for Toxic Substances. http://www.atsdr.cdc.gov/toxprofiles/tp161.html.
- ATSDR. 2018. Asbestos toxicity, agency for toxic substances and disease registry case studies in environmental medicine (CSEM). January 29, 2014. [accessed 2022 March 1]. http://www.atsdr.cdc.gov/csem/asbestos_2014/docs/asbestos.pdf.
- Barlow CA, Grespin M, Best EA. 2017. Asbestos fiber length and its relation to disease risk. Inhal Toxicol. 29(12–14):541–554.
- Berman DW, Crump KS, Chatfield EJ, Davis JM, Jones AD. 1995. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 15(2):181–195.
- Berman DW, Crump KS. 2008. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol. 38(sup1):49–73.
- Bernstein D, Castranova V, Donaldson K, Fubini B, Hadley J, Hesterberg T, Kane A, Lai D, McConnell EE, Muhle H, et al. 2005. Testing of fibrous particles: short-term assays and strategies. Inhal Toxicol. 17(10):497–537.
- Bernstein D, Dunnigan J, Hesterberg T, Brown R, Velasco JA, Barrera R, Hoskins J, Gibbs A. 2013. Health risk of chrysotile revisited. Crit Rev Toxicol. 43(2):154–183.
- Bernstein D, Riego J. 1999. Methods for the determination of the hazardous properties for human health of man-made mineral fibres (MMMF). EUR 18748 EN. JRC18422; [accessed 2022 Feb 16]. https://publications.jrc.ec.europa.eu/repository/handle/JRC18422.
- Bernstein DM, Kunzendorf P. 2018. Standardized methods for preparation and bi-variate length & diameter counting/sizing of aerosol and tissue digestion fiber samples. Toxicol Appl Pharmacol. 361:174–184.
- Bernstein DM, Rogers R, Smith P. 2004. The biopersistence of Brazilian chrysotile asbestos following inhalation. Inhal Toxicol. 16(11–12):745–761.
- Bernstein DM, Toth B, Rogers RA, Kling DE, Kunzendorf P, Phillips JI, Ernst H. 2020a. Evaluation of the exposure, dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to TiO2, chrysotile, crocidolite or amosite asbestos in a 90-day quantitative inhalation toxicology study – interim results part 1: experimental design, aerosol exposure, lung burdens and BAL. Toxicol Appl Pharmacol. 387:114856.
- Bernstein DM, Toth B, Rogers RA, Kling DE, Kunzendorf P, Phillips JI, Ernst H. 2020b. Evaluation of the dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to TiO2, chrysotile, crocidolite or amosite asbestos in a 90-day quantitative inhalation toxicology study – interim results part 2: histopathological examination, Confocal microscopy and collagen quantification of the lung and pleural cavity. Toxicol Appl Pharmacol. 387:114847.
- Bernstein DM, Toth B, Rogers RA, Kunzendorf P, Phillips JI, Schaudien D. 2021. Final results from a 90-day quantitative inhalation toxicology study evaluating the dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to TiO2, chrysotile, crocidolite or amosite asbestos: Histopathological examination, confocal microscopy and collagen quantification of the lung and pleural cavity. Toxicol Appl Pharmacol. 424:115598.
- Bernstein DM, Mast R, Anderson R, Hesterberg TW, Musselman R, Kamstrup O, Hadley J. 1994. An experimental approach to the evaluation of the biopersistence of respirable synthetic fibers and minerals. Environ Health Perspect. 102(Suppl 5):15–18.
- Bernstein DM, Morscheidt C, Grimm HG, Thévenaz P, Teichert U. 1996. Evaluation of soluble fibers using the inhalation biopersistence model, a nine-fiber comparison. Inhal Toxicol. 8(4):345–385.
- Bernstein DM, Pavlisko EN. 2017. Differential pathological response and pleural transport of mineral fibers. EMU Notes Mineral. 18:417–434.
- Bertin F, Deslauriers J. 2011. Anatomy of the pleura: reflection lines and recesses. Thorac Surg Clin. 21(2):165–171, vii.
- Bevan RJ, Kreiling R, Levy LS, Warheit DB. 2018. Toxicity testing of poorly soluble particles, lung overload and lung cancer. Regul Toxicol Pharmacol. 100:80–91.
- Boulanger G, Andujar P, Pairon JC, Billon-Galland MA, Dion C, Dumortier P, Brochard P, Sobaszek A, Bartsch P, Paris C, et al. 2014. Quantification of short and long asbestos fibers to assess asbestos exposure: a review of fiber size toxicity. Environ Health. 13:59.
- Boyles MS, Young L, Brown DM, MacCalman L, Cowie H, Moisala A, Smail F, Smith PJ, Proudfoot L, Windle AH, et al. 2015. Multi-walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol In Vitro. 29(7):1513–1528.
- Broaddus VC, Wiener-Kronish JP, Berthiaume Y, Staub NC. 1988. Removal of pleural liquid and protein by lymphatics in awake sheep. J Appl Physiol. 64(1):384–390.
- Case BW. 1994. Biological indicators of chrysotile exposure. Ann Occup Hyg. 38(4):503–518. 410–411.
- Cheng Y-S. 1986. Bivariate lognormal distribution for characterizing asbestos fiber aerosols. Aerosol Sci Technol. 5(3):359–368.
- Cherrie J, Addison J, Dodgson J. 1989. Comparative studies of airborne asbestos in occupational and non-occupational environments using optical and electron microscope techniques. IARC Sci Publ. (Vol. 90):304–309.
- Cherrie JW, Dodgson J, Groat S, Carson M. 1979. Comparison of optical and electron microscopy for evaluating airborne asbestos. Edinburgh: Institute of Occupational Medicine (U.S. Department of Commerce).
- Choi I, Smith RW. 1972. Kinetic study of dissolution of asbestos fibers in water. J Colloid Interface Sci. 40(2):253–262.
- Churg A, Wiggs B. 1984. Fiber size and number in amphibole asbestos-induced mesothelioma. Am J Pathol. 115(3):437–442.
- Churg A, Wiggs B. 1986. Fiber size and number in workers exposed to processed chrysotile asbestos, chrysotile miners, and the general population. Am J Ind Med. 9(2):143–152.
- Churg A, Wiggs B. 1987. Types, numbers, sizes, and distribution of mineral particles in the lungs of urban male cigarette smokers. Environ Res. 42(1):121–129. PMID: 3803330.
- Churg A, Wright JL, DePaoli L, Wiggs B. 1989. Mineralogic correlates of fibrosis in chrysotile miners and millers. Am Rev Respir Dis. 139(4):891–896.
- Churg A. 1990. The distribution of amosite asbestos in the periphery of the normal human lung. Br J Ind Med. 47(10):677–681.
- Cohen JM, Teeguarden JG, Demokritou P. 2014. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part Fibre Toxicol. 11:20.
- Conway EM, Pikor LA, Kung SHY, Hamilton MJ, Lam S, Lam WL, Bennewith KL. 2016. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 193 (2):116–130.
- Crouch E, Wright JR. 2001. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 63:521–554.
- Dai YT, Yu CP. 1998. Alveolar deposition of fibers in rodents and humans. J Aerosol Med. 11(4):247–258.
- Darnton A. 2010. HSE assessment of recently updated information relating to cohorts with chrysotile exposure; [accessed 2019 Feb 15]. http://www.hse.gov.uk/aboutus/meetings/iacs/acts/watch/271010/watchoctober-2010-additional-chrysotile-exposure.pdf.
- Davis JM, Addison J, Bolton RE, Donaldson K, Jones AD, Smith T. 1986. The pathogenicity of long versus short fiber samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 67(3):415–430.
- Davis JM, Jones AD. 1988. Comparisons of the pathogenicity of long and short fibers of chrysotile asbestos in rats. Br J Exp Pathol. 69(5):717–737.
- Dement JM, Kuempel E, Zumwalde R, Smith R, Stayner L, Loomis D. 2008. Development of a fiber size-specific job-exposure matrix for airborne asbestos fibers. Occup Environ Med. 65(9):605–612.
- Dement JM, Myers D, Loomis D, Richardson D, Wolf S. 2009. Estimates of historical exposures by phase contrast and transmission electron microscopy in North Carolina USA asbestos textile plants. Occup Environ Med. 66(9):574–583.
- Dement JM, Harris RL. 1979. Estimates of pulmonary and gastrointestinal deposition for occupational fiber exposure. NTIS PB80-149644. U.S. HEW Contract 78-2438.
- Dewhurst JA, Lea S, Hardaker E, Dungwa JV, Ravi AK, Singh D. 2017. Characterisation of lung macrophage subpopulations in COPD patients and controls. Sci Rep. 7(1):7143.
- Dodson RF, Huang J, Bruce JR. 2000. Asbestos content in the lymph nodes of nonoccupationally exposed individuals. Am J Ind Med. 37(2):169–174.
- Dogra S, Donaldson K. 1995. Effect of long and short fibre amosite asbestos on in vitro TNF production by rat alveolar macrophages: the modifying effect of lipopolysaccharide. Ind Health. 33(3):131–141.
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. 2006. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 92(1):5–22.
- Donaldson K, Golyasnya N. 1995. Cytogenetic and pathogenic effects of long and short amosite asbestos. J Pathol. 177(3):303–307.
- Donaldson K, Miller BG, Sara E, Slight J, Brown RC. 1993. Asbestos fiber length-dependent detachment injury to alveolar epithelial cells in vitro: role of a fibronectin-binding receptor. Int J Exp Pathol. 74(3):243–250.
- Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. 2011. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine. 6(1):143–156.
- Drake RE, Weiss D, Gabel JC. 1991. Active lymphatic pumping and sheep lung lymph flow. J Appl Physiol. 71(1):99–103.
- ECETOC. 2013. Technical Report No. 122, poorly soluble particles/lung overload. Brussels: ECETOC AISBL European Centre for Ecotoxicology and Toxicology of Chemicals; [accessed 2022 Feb 2].
- Elliott L, Loomis D, Dement J, Hein MJ, Richardson D, Stayner L. 2012. Lung cancer mortality in North Carolina and South Carolina chrysotile asbestos textile workers. Occup Environ Med. 69(6):385–390.
- EPA. 2020a. Asbestos: industry profile final report prepared for U.S. Environmental Protection Agency Office of Air Quality Planning and Standards Innovative Strategies and Economics Group MD-15 Research Triangle Park, NC. Research Triangle Park (NC): Research Triangle Institute Center for Economics Research. https://www.epa.gov/sites/default/files/2020-07/documents/asbestos_ip.pdf.
- EPA. 2020b. Draft risk evaluation for asbestos. Office of Chemical Safety and Pollution Prevention. Washington (DC): Environmental Protection Agency. https://www.regulations.gov/document/EPA-HQ-OPPT-2019-0501-0002.
- EPA. 2020c. Risk evaluation for asbestos, part I: chrysotile asbestos. Office of Chemical safety and Pollution Prevention. Washington (DC): Environmental Protection Agency. https://www.epa.gov/sites/default/files/2020-12/documents/1_risk_evaluation_for_asbestos_part_1_chrysotileasbestos.pdf.
- EPA. 2020d. Summary of external peer review and public comments for asbestos and disposition for asbestos part 1: chrysotile asbestos – response to support risk evaluation for asbestos part I: chrysotile asbestos. Washington (DC): Environmental Protection Agency. https://www.epa.gov/sites/default/files/2020-12/documents/2_summary_ofexternal_peer_review_and_public_comments_and_disposition.pdfEPA.
- Fels AO, Cohn ZA. 1986. The alveolar macrophage. J Appl Physiol. 60 (2):353–369.
- Finley BL, Richter RO, Mowat FS, Mlynarek S, Paustenbach DJ, Warmerdam JM, Sheehan PJ. 2007. Cumulative asbestos exposure for US automobile mechanics involved in brake repair (circa 1950s–2000). J Expo Sci Environ Epidemiol. 17(7):644–655.
- Finley DJ, Rusch VW. 2011. Anatomy of the pleura. Thorac Surg Clin. 21(2):157–163, vii.
- Gamble JF, Gibbs GW. 2008. An evaluation of the risks of lung cancer and mesothelioma from exposure to amphibole cleavage fragments. Regul Toxicol Pharmacol. 52(1 Suppl):S154–S156.
- Garabrant D. 2020. Comments on US EPA Office of chemical safety and pollution prevention and toxics draft risk evaluation for asbestos, dated March 2020 (EPA document # EPA-740-R1-8012). Washington (DC): Comments submitted by The University of Michigan, Ann Arbor, Michigan.
- Garabrant DH, Alexander DD, Miller PE, Fryzek JP, Boffetta P, Teta MJ, Hessel PA, Craven VA, Kelsh MA, Goodman M. 2016. Mesothelioma among motor vehicle mechanics: an updated review and meta-analysis. ANNHYG. 60(8):1036–1026.
- Garabrant DH, Pastula ST. 2018. A comparison of asbestos fiber potency and elongate mineral particle (EMP) potency for mesothelioma in humans. Toxicol Appl Pharmacol. 361:127–136.
- Gibbs GW, Hwang CY. 1980. Dimensions of airborne asbestos fibers. In: Wagner JC, editor. Biological effects of mineral fibers. Lyon: IARC Scientific Publication; p. 69–78.
- Goodglick LA, Kane AB. 1990. Cytotoxicity of long and short crocidolite asbestos fibers in vitro and in vivo. Cancer Res. 50(16):5153–5163.
- Goodman M, Teta MJ, Hessel PA, et al. 2004. Mesothelioma and lung cancer among motor vehicle mechanics: a metaanalysis. Ann Occup Hyg. 48:309–326.
- Grecian R, Whyte MKB, Walmsley SR. 2018. The role of neutrophils in cancer. Br Med Bull. 128(1):5–14.
- Griesinger C, Desprez B, Coecke S, Casey W, Zuang V. 2016. Validation of alternative in vitro methods to animal testing: concepts, challenges, processes and tools. Adv Exp Med Biol. 856:65–132.
- Gualtieri AF. 2021. Bridging the gap between toxicity and carcinogenicity of mineral fibres by connecting the fibre crystal-chemical and physical parameters to the key characteristics of cancer. Curr Res Toxicol. 2:42–52.
- Gualtieri AF, Di Giuseppe D. 2022. Letter to the Editor: Comments on the paper of Wylie and Korchevskiy – carcinogenicity of fibrous glaucophane: how should we fill the data gaps? Curr Res Toxicol. 3:100063.
- Gualtieri AF, Lusvardi G, Pedone A, Di Giuseppe D, Zoboli A, Mucci A, Zambon A, Filaferro M, Vitale G, Benassi M, et al. 2019. Structure model and toxicity of the product of biodissolution of chrysotile asbestos in the lungs. Chem Res Toxicol. 32(10):2063–2077.
- Gualtieri AF, Zoboli A, Filaferro M, Benassi M, Scarfì S, Mirata S, Avallone R, Vitale G, Bailey M, Harper M, et al. 2021. In vitro toxicity of fibrous glaucophane. Toxicology. 454:152743.
- Hallifax RJ, Rahman NM. 2016. Pleural embryology and gross structure, circulation, lymphatics, and nerves. In: Light RW, Lee YCG, editors. Pleural diseases edition. 3rd ed. Boca Raton (FL): CRC Press; Taylor & Francis Group; p. 13–26.
- Hammad YY. 1984. Deposition and elimination of MMMF. In: Biological effects of manmade mineral fibers. Copenhagen: World Health Organization; p. 126–142.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144(5):646–674. Mar 4.
- Hargreaves A, Taylor WH. 1946. An X-ray examination of decomposition products of chrysotile asbestos and serpentine. Mineral Mag J Mineral Soc. 27(193):204–216.
- Hart GA, Kathman LM, Hesterberg TW. 1994. In vitro cytotoxicity of asbestos and man-made vitreous fibers: roles of fiber length, diameter and composition. Carcinogenesis. 15(5):971–977.
- Hesterberg TW, Barrett JC. 1984. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 44(5):2170–2180.
- Hill IM, Beswick PH, Donaldson K. 1995. Differential release of superoxide anions by macrophages treated with long and short fibre amosite asbestos is a consequence of differential affinity for opsonin. Occup Environ Med. 52(2):92–96.
- Hodgson JT, Darnton A. 2000. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg. 44(8):565–601. https://www.ecetoc.org/wp-content/uploads/2014/06/ECETOC_2013_Annual_Report.pdf.
- Huang SL. 1979. Amosite, chrysotile and crocidolite asbestos are mutagenic in Chinese hamster lung cells. Mutat Res. 68(3):265–274.
- Huang SX, Jaurand MC, Kamp DW, Whysner J, Hei TK. 2011. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J Toxicol Environ Health B Crit Rev. 14(1–4):179–245.
- Hwang CY, Gibbs GW. 1981. The dimensions of airborne asbestos fibres—I. Crocidolite from Kuruman area, Cape Province, South Africa. Ann Occup Hyg. 24(1):23–41.
- Hyde DM, Hamid Q, Irvin CG. 2009. Anatomy, pathology, and physiology of the tracheobronchial tree: emphasis on the distal airways. J Allergy Clin Immunol. 124(6 Suppl):S72–S77.
- IARC. 1999. Mechanisms of fiber carcinogenesis. IARC Scientific Publication No. 140 (Kane AB, Boffetta P, Saracci R, Wilbourn JD, editors). Lyon, France: World Heath Organization. ISBN-13 (Print Book) 978-92-832-2140-1, ISBN: 92832-2140-0.
- Ilgren E, Chatfield E. 1997. Coalinga fibre – a short, amphibole-free chrysotile part 1 evidence for a lack of fibrogenic activity. Indoor Built Environ. 6:264–276.
- Ilgren E, Chatfield E. 1998. Coalinga fiber – a short, amphibole-free chrysotile: part 2: evidence for lack of tumorigenic activity. Indoor Built Environ. 7(1):18–31.
- IOM. 2009. Institute of Medicine (US) and National Research Council (US) Committee for the review of the NIOSH research roadmap on asbestos fibers and other elongate mineral particles. In: Nelson AR, Liverman CT, Eide EA, Abt E, editors. A review of the NIOSH roadmap for research on asbestos fibers and other elongate mineral particles. Washington (DC): National Academies Press (US).
- Jaurand MC, Bernaudin JF, Renier A, Kaplan H, Bignon J. 1981. Rat pleural mesothelial cells in culture. In Vitro. 17(2):98–106.
- Jaurand MC, Bignon J, Sebastien P, Goni J. 1977. Leaching of chrysotile asbestos in human lungs. Correlation with in vitro studies using rabbit alveolar macrophages. Environ Res. 14(2):245–254.
- Kaw JL, Tilkes F, Beck EG. 1982. Reaction of cells cultured in vitro to different asbestos dusts of equal surface area but different fibre length. Br J Exp Pathol. 63(1):109–115.
- Kobell F. 1834. Ueber den schillernden Asbest von Reichenstein in Schlesien. J Prakt Chemie. 2:297–298.
- Korchevskiy A, Rasmuson JO, Rasmuson EJ, Strode RD. 2020. Inhalation unit risk (IUR) of asbestos based on available science. Inhal Toxicol. 32(9–10):372–374.
- Korchevskiy A, Rasmuson JO, Rasmuson EJ. 2019. Empirical model of mesothelioma potency factors for different mineral fibers based on their chemical composition and dimensionality. Inhal Toxicol. 31(5):180–191.
- Korchevskiy AA, Rasmuson EJ, Rasmuson JO, Strode RD. 2013. Asbestos mining in Russia: approaches to public health risk assessment. Preprint 13-117 in: Mining: It’s All About People. Pre-prints of the Society for Mining, Metallurgy & Exploration Annual Meeting; February 24–27; Denver, Colorado.
- Korchevskiy AA, Wylie AG. 2022. Dimensional characteristics of the major types of amphibole mineral particles and the implications for carcinogenic risk assessment. Inhal Toxicol. 34(1–2):24–38.
- Korchevskiy AA, Wylie AG. 2021. Dimensional determinants for the carcinogenic potency of elongate amphibole particles. Inhal Toxicol. 33(6–8):244–259.
- Krenke R, Mierzejewski M. 2020. Anatomy and physiology of the pleural space. In: Janes SM, editor. Encyclopedia of respiratory medicine. 2nd ed. Amsterdam, Netherlands: Elsevier.; p. 318–340.
- Krombach F, Münzing S, Allmeling AM, Gerlach JT, Behr J, Dörger M. 1997. Cell size of alveolar macrophages: an interspecies comparison. Environ Health Perspect. 105(Suppl 5):1261–1263.
- Langer AM, Nolan RP, Addison J. 1991. Distinguishing between amphibole asbestos fibers and elongate cleavage fragments of their non-asbestos analogues. In: Brown RC, Hoskins JA, Johnson NF, editors. Mechanisms in fibre carcinogenesis. NATO ASI series (series A: life sciences). Vol. 223. Boston (MA): Springer..
- Langer AM, Nolan RP. 1994. Chrysotile biopersistence in the lungs of persons in the general population and exposed workers. Environ Health Perspect. 102(Suppl 5):235–239.
- Lauweryns JM, Baert JH. 1977. Alveolar clearance and the role of the pulmonary lymphatics. Am Rev Respir Dis. 115(4):625–683.
- Lauweryns JM. 1970. The juxta-alveolar lymphatics in the human adult lung. Histologic studies in 15 cases of drowning. Am Rev Respir Dis. 102(6):877–885.
- Levresse V, Renier A, Levy F, Broaddus VC, Jaurand M. 2000. DNA breakage in asbestos-treated normal and transformed (TSV40) rat pleural mesothelial cells. Mutagenesis. 15(3):239–244.
- Light RW, Lee YCG. 2016. Textbook of pleural diseases. 3rd ed. New York: Taylor & Francis.
- Lippmann M. 2014. Toxicological and epidemiological studies on effects of airborne fibers: coherence and public [corrected] health implications. Crit Rev Toxicol. 44(8):643–695.
- Loomis D, Dement J, Richardson D, Wolf S. 2010. Asbestos fibre dimensions and lung cancer mortality among workers exposed to chrysotile. Occup Environ Med. 67(9):580–584.
- Loomis D, Dement JM, Elliott L, Richardson D, Kuempel ED, Stayner L. 2012. Increased lung cancer mortality among chrysotile asbestos textile workers is more strongly associated with exposure to long thin fibres. Occup Environ Med. 69(8):564–568.
- Loomis D, Dement JM, Wolf SH, Richardson DB. 2009. Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup Environ Med. 66(8):535–542.
- Loomis D, Richardson DB, Elliott L. 2019. Quantitative relationships of exposure to chrysotile asbestos and mesothelioma mortality. Am J Ind Med. 62(6):471–477.
- Macklin CC. 1955. Pulmonary sumps, dust accumulations, alveolar fluid and lymph vessels. Acta Anat. 23(1):1–33.
- Marconi A, Menichini E, Paoletti L. 1984. A comparison of light microscopy and transmission electron microscopy results in the evaluation of the occupational exposure to airborne chrysotile fibres. Ann Occup Hyg. 28(3):321–331.
- Margaris KN, Black RA. 2012. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface. 9(69):601–612.
- Marsh JP, Mossman BT. 1988. Mechanisms of induction of ornithine decarboxylase activity in tracheal epithelial cells by asbestiform minerals. Cancer Res. 48(3):709–714.
- Mason RJ, Lewis JF. 2011. Pulmonary surfactant. In: Mason RJ, Courtney Broaddus V, Martin TR, King TE Jr., Schraufnagel D, Murray JF, Nadel JA, editors. Chapter 11 in Murray and Nadel's textbook of respiratory medicine. 5th ed. Philadelphia, PA: Saunders, An Imprint of Elsevier. https://www.elsevier.ca/ca/product.jsp?isbn=978145570873.
- McConnell EE, Davis JM. 2002. Quantification of fibrosis in the lungs of rats using a morphometric method. Inhal Toxicol. 14(3):263–272.
- Michel T, Hentges F, Zimmer J. 2012. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 3:403.
- Miura T, Shimada T, Tanaka K, Chujo M, Uchida Y. 2000. Lymphatic drainage of carbon particles injected into the pleural cavity of the monkey, as studied by video-assisted thoracoscopy and electron microscopy. J Thorac Cardiovasc Surg. 120(3):437–447.
- Morgan A. 1994. The removal of fibres of chrysotile asbestos from lung. Ann Occup Hyg. 38(4):643–646.
- Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. 2011. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev. 14(1–4):76–121.
- Muhle H, Crentzenberg O, Bellmann B, Heinrich U, Mermelstein R. 1990. Dust overloading of the lungs: investigation of various materials, species differences, and irreversibility effects. J Aerosol Med. 3:S111–S128.
- Nayebzadeh A, Dufresne A, Case B, Vali H, Williams-Jones AE, Martin R, Normand C, Clark J. 2001. Lung mineral fibers of former miners and millers from Thetford-Mines and asbestos regions: a comparative study of fiber concentration and dimension. Arch Environ Health. 56(1):65–76.
- Negrini D, Moriondo A. 2013. Pleural function and lymphatics. Acta Physiol. 207(2):244–259.
- Nicholson WJ. 1986. Airborne asbestos health assessment update. Report 600/8-84-003F. Washington (DC): U.S. Environmental Protection Agency.
- NOISH. 2011. Asbestos fibers and other elongate mineral particles: state of the science and roadmap for research, revised edition. Current Intelligence Bulletin 62. Centers for Disease Control and Prevention National Institute for Occupational Safety and Health. Publication No. 2011-159; [accessed 2022 Feb 2]. https://www.cdc.gov/niosh/docs/2011-159/pdfs/2011-159.pdf.
- Oberdoester G. 1994. Macrophage associated responses to chrysotile. Ann Occup Hyg. 38:601–615.
- Pauling L. 1930. The structure of the chlorites. Proc Natl Acad Sci USA. 16(9):578–582.
- Paustenbach D, Brew D, Ligas S, Heywood J. 2021. A critical review of the 2020 EPA risk assessment for chrysotile and its many shortcomings. Crit Rev Toxicol. 51(6):509–539.
- Paustenbach DJ, Finley BL, Lu ET, Brorby GP, Sheehan PJ. 2004. Environmental and occupational health hazards associated with the presence of asbestos in brake linings and pads (1900 to present): a “state-of-the-art” review. J Toxicol Environ Health B Crit Rev. 7(1):25–80.
- Paustenbach DJ, Richter RO, Finley BL, Sheehan PJ. 2003. An evaluation of the historical exposures of mechanics to asbestos in brake dust. Appl Occup Environ Hyg. 18(10):786–804.
- Pinkerton KE, Brody AR, McLaurin DA, Adkins B, Jr, O'Connor RW, Pratt PC, Crapo JD. 1983. Characterization of three types of chrysotile asbestos after aerosolization. Environ Res. 31(1):32–53.
- Platek SF, Groth DH, Ulrich CE, Stettler LE, Finnell MS, Stoll M. 1985. Chronic inhalation of short asbestos fibers. Fundam Appl Toxicol. 5(2):327–340.
- Pott F. 1978. Some aspects on the dosimetry of the carcinogenic potency of asbesatos and other fibrous susts. Staub Reinhalt. 38. (12):486–490.
- Ramachandran G, Cooper DW. 2011. Size distribution data analysis and presentation. In: Kulkarni P, Baron PA, Willeke K, editors. Chapter 22 in aerosol measurement: principles, techniques, and applications. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc.
- Randall TD. 2010. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv Immunol. 107:187–241.
- Riganti C, Aldieri E, Bergandi L, Tomatis M, Fenoglio I, Costamagna C, Fubini B, Bosia A, Ghigo D. 2003. Long and short fiber amosite asbestos alters at a different extent the redox metabolism in human lung epithelial cells. Toxicol Appl Pharmacol. 193(1):106–115.
- Riquet M, Hidden G, Debesse B. 1989. Direct lymphatic drainage of lung segments to the mediastinal nodes. J Thorac Cardiovasc Surg. 97(4):623–632.
- Riquet M. 2007. Bronchial arteries and lymphatics of the lung. Thorac Surg Clin. 17(4):619–638, viii.
- Roberts DR, Zumwalde RD. 1982. Industrial hygiene summary report of asbestos exposure assessment for brake mechanics. NIOSH Reports No. IWS-32-4A, Industrial Hygiene Section, NTIS# PB 87-105433. http://www.ntis.gov/#.
- Roggli VL. 1995. Malignant mesothelioma and duration of asbestos exposure: correlation with tissue mineral fibre content. Ann Occup Hyg. 39(3):363–374.
- Roggli VL, Vollmer RT. 2008. Twenty-five years of fiber analysis: what have we learned? Hum Pathol. 39(3):307–315.
- Rood AP, Scott RM. 1989. Size distributions of chrysotile asbestos in a friction products factory as determined by transmission electron microscopy. Ann Occup Hyg. 33(4):583–590.
- SCOEL. 2012. Recommendation from the Scientific Committee on Occupational Exposure Limits for man-made-mineral fibers (MMMF) with no indication for carcinogenicity and not specified elsewhere. SCOEL/SUM/88.
- Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdörster G, Kreyling WG. 2007. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ Health Perspect. 115(5):728–733.
- Simonato L, Baris R, Saracci R, Skidmore J, Winkelmann R. 1989. Relation of environmental exposure to erionite fibers to risk of respiratory cancer. In: Bignon J, Peto J, Saracci R, editors. Non-occupational exposure to mineral fibers. Vol. 90. Lyon: World Health Organization, International Agency for Research on Cancer (IARC), Scientific Publications; p. 398–405.
- Skinner HCW, Ross M, Frondel C. 1988. Asbestos and other fibrous materials – mineralogy, crystal chemistry, and health effects. New York (NY): Oxford University Press; p. 204.
- Snyder J, Virta R, Segreti J. 1987. Evaluation of the phase contrast microscopy method for the detection of fibrous and other elongated mineral particulates by comparison with a STEM technique. Am Ind Hyg Assoc J. 48(5):471–477.
- Speil S, Leineweber JP. 1969. Asbestos minerals in modern technology. Environ Res. 2(3):166–208.
- Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, Smith A. 1981. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 67(5):965–975.
- Stanton MF, Laynard M, Tegeris A, Miller E, May M, Kent E. 1977. Carcinogenicity of fibrous glass: pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst. 58(3):587–603.
- Stanton MF, Wrench C. 1972. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 48(3):797–821.
- Stanton MF. 1974. Editorial: fiber carcinogenesis: is asbestos the only hazard? J Natl Cancer Inst. 52(3):633–634.
- Stettler LE, Sharpnack DD, Krieg EF. 2008. Chronic inhalation of short asbestos: lung fiber burdens and histopathology for monkeys maintained for 11.5 years after exposure. Inhal Toxicol. 20(1):63–73.
- Stober W, McClellan RO, Morrow P. 1993. Approaches to modeling disposition of inhaled particles and fibers in the lung. In: Gardner DE, Crapo JD, McClellan RO. Toxicology of the lung. 2nd ed. New York (NY): Raven Press; p. 527–602.
- Suzuki Y, Yuen SR, Ashley R. 2005. Short, thin asbestos fibers contribute to the development of human malignant mesothelioma: pathological evidence. Int J Hyg Environ Health. 208(3):201–210.
- Suzuki Y, Yuen SR. 2002. Asbestos fibers contributing to the induction of human malignant mesothelioma. Ann N Y Acad Sci. 982:160–176.
- Tanji T, Yada K, Akatsuka Y. 1984. Alternation of clino- and orthochrysotile in a single fiber as revealed by high-resolution electron microscopy. Clays Clay Miner. 32(5):429–432.
- Tilkes F, Beck EG. 1980. Comparison of length-dependent cytotoxicity of inhalable asbestos and man-made mineral fibres. IARC Sci Publ. (Vol. 30):475–483.
- Tilkes F, Beck EG. 1983. Sep Macrophage functions after exposure to mineral fibers. Environ Health Perspect. 51:67–72.
- Titulaer MK, van Miltenburg JC, Jansen JBH, Geus JW. 1993. Characterization of tubular chrysotile by thermoporometry, nitrogen sorption, drifts, and TEM. Clays Clay Miner. 41(4):496–513.
- Tomatis M, Turci F, Ceschino R, Riganti C, Gazzano E, Martra G, Ghigo D, Fubini B. 2010. High aspect ratio materials: role of surface chemistry vs. length in the historical “long and short amosite asbestos fibers”. Inhal Toxicol. 22(12):984–998.
- Tossavainen A, Karjalainen A, Karhunen PJ. 1994. Retention of asbestos fibers in the human body. Environ Health Perspect. 102(Suppl 5):253–255.
- UNARCO. 1954. Specifications on UNARCO Insubestos Felt. CRMC Marshville Files 000444.
- UNARCO. 2012. Insutape Inventory. CRMC-Marshville-002758.
- USEPA. 1995. EPA-748-R-001 workshop report on chronic inhalation toxicity and carcinogenicity testing of respirable fibrous particles, May 8–10, 1995 Omni Europa Hotel. Chapel Hill (NC): U.S. Environmental Protection Agency in collaboration with the National Institute of Environmental Health Sciences National Institute for Occupational Safety and Health Occupational Safety and Health Administration Assembled by Research Evaluation Associates, Inc.
- Van Winkle LS, Kelty JS, Smiley-Jewell S, Pinkerton KE, McQueen C. 2018. 15.04 tracheobronchial airways. In: Comprehensive toxicology. 3rd ed. Amsterdam, Netherlands: Elsevier Science; p. 30–46.
- Virta RL. 2002. Asbestos: geology, mineralogy, mining, and uses. U.S. Department of the Interior U.S. Geological Survey. Prepared in cooperation with Kirk-Othmer Encyclopedia of Chemical Technology. Online Edition. New York, NY: Wylie-Interscience, a division of John Wiley & Sons, Inc.
- Vorwald AJ, Durkan TM, Pratt PC. 1951. Experimental studies of asbestosis. AMA Arch Ind Hyg Occup Med. 3(1):1–43.
- Wagner JC, Griffiths DM, Hill RJ. 1984. The effect of fibre size on the in vivo activity of UICC crocidolite. Br J Cancer. 49(4):453–458.
- Wagner JC, Skidmore JW, Hill RJ, Griffiths DM. 1985. Erionite exposure and mesotheliomas in rats. Br J Cancer. 51(5):727–730.
- Wagner JC. 1988. Biological effects of short fibers. Proceedings of the VIIth International Pneumoconioses Conference, August 23–26, 1988, Pittsburgh, Pennsylvania, USA. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 90-108, 1990 Nov (Part II); p. 835–839.
- Wang NS. 1985. Anatomy and physiology of the pleural space. Clin Chest Med. 6(1):3–16.
- Whittaker EJW. 1957. The structure of chrysotile. V. Diffuse reflexions and fibre texture. Acta Cryst. 10(3):149–156.
- Whittaker EJW. 1960. The crystal chemistry of the amphiboles. Acta Cryst. 13(4):291–298.
- Whittaker EJW. 1963. Research report: chrysotile fibers –filled or hollow tubes? Mathematical interpretation may resolve conflicting evidence. Chem Eng News. 41:34–35.
- Whittaker EJW. 2009. Structure and properties of asbestos. In: Eichhorn S, editor. Handbook of textile fibre structure. Volume 2. Natural, regenerated, inorganic, and specialist fibres. Sawston (UK): Woodhead Publishing; p. 425–449. (Reprinted from Hearle JWS, Peters, RH (eds). 1963. Asbestos. In: Fibre structure. Copyright Butterworth & Co. (Elsevier) and the Textile Institute; p. 594–620).
- WHO. 1985. Reference methods for measuring airborne man-made mineral fibers (MMMF): WHO/EURO MMMF reference scheme. Copenhagen: WHO.
- WHO. 1997. Determination of airborne fibre number concentrations: a recommended method by phase-contrast optical microscopy (membrane filter method). Geneva: World Health Organization.
- Winer AA, Cossette M. 1979. The effect of aspect ration on fiber counts: a preliminary study. Ann NY Acad Sci. 330(1):661–672.
- Woodworth CD, Mossman BT, Craighead JE. 1983. Interaction of asbestos with metaplastic squamous epithelium developing in organ cultures of hamster trachea. Environ Health Perspect. 51:27–33.
- Wylie A, Korchevskiy A. 2022. Letter to the Editor: epidemiology holds a key to the validation of toxicological models for elongate mineral particles. Curr Res Toxicol. 3:100062.
- Wylie AG, Korchevskiy A, Segrave A, Duane A. 2020. Modeling mesothelioma risk factors from amphibole fiber dimensionality: mineralogical and epidemiological perspective. J Appl Toxicol. 40(4):515–524.
- Wylie AG, Korchevskiy AA. 2021. Carcinogenicity of fibrous glaucophane: how should we fill the data gaps? Curr Res Toxicol. 2:202–203.
- Wylie AG, Virta RL, Segreti JM. 1987. Characterization of mineral population by index particle: implication for the Stanton hypothesis. Environ Res. 43(2):427–439.
- Yalcin NG, Choong CK, Eizenberg N. 2013. Anatomy and pathophysiology of the pleura and pleural space. Thorac Surg Clin. 23(1):1–10, v.
- Yang L, Lin PC. 2017. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 47:185–195.
- Yeager H Jr., Russo DA, Yañez M, Gerardi D, Nolan RP, Kagan E, Langer AM. 1983. Cytotoxicity of a short-fiber chrysotile asbestos for human alveolar macrophages: preliminary observations. Environ Res. 30(1):224–232.
- Yu CP, Zhang L, Oberdorster G, Mast CW, Glass LR, Utell MJ. 1994. Clearance of refractory ceramic fibers (RCF) from the rat lung: development of a model. Environ Res. 65(2):243–253.