Abstract
The National Research Council’s vision of using adverse outcome pathways (AOPs) as a framework to assist with toxicity assessment for regulatory requirements of chemical assessment has continued to gain traction since its release in 2007. The need to expand the AOP knowledge base has gained urgency, with the U.S. Environmental Protection Agency’s directive to eliminate reliance on animal toxicity testing by 2035. To meet these needs, our goal was to elucidate the AOP for male-rat-specific kidney cancer. Male-rat-specific kidney tumors occur through the ability of structurally diverse substances to induce α2u-globulin nephropathy (α2u-N), a well-studied mode of action (MoA) not relevant in humans that results in kidney tumor formation in male rats. An accepted AOP may help facilitate the differentiation from other kidney tumors MoAs. Following identification and review of relevant in vitro and in vivo literature, both the MIE and subsequent KEs were identified. Based on the weight of evidence from the various resources, the confidence in this AOP is high. Uses of this AOP include hazard identification, development of in vitro assays to determine if the MoA is through α2u-N and not relevant to humans resulting in decreased use of animals, and regulatory applications.
Graphical Abstract
Introduction
Regulatory drivers, such as the United States Environmental Protection Agency’s (US EPA) directive to eliminate reliance on animal toxicity testing by 2035 (US EPA Citation2019), and technological advances are modernizing toxicology testing and risk assessment. New approach methods (NAMs) that reduce reliance on animal testing either for chemical hazard identification or studies to define the mode of action (MoA) and human relevance are inherent to this process. Confidence in NAMs can be increased by documenting biological response pathways, called adverse outcome pathways (AOPs) that temporally and physiologically link molecular and cellular biological events to apical outcomes in animal studies. Internationally, efforts are underway to document a wide range of biological key events (KEs) in a standardized manner to move toward both a consistent scientific understanding of relationships between KEs and alignment on levels of confidence needed to apply AOPs in decision-making processes (Becker et al. Citation2015; Perkins et al. Citation2015; Edwards et al. Citation2016).
To contribute to this effort, we propose an AOP based on well-documented pathological observations of empirical evidence obtained from a diverse set of non-genotoxic chemicals that cause kidney tumors in male but not female, rats or mice and also induces pathological observations unique to male rats, referred to as α2u-globulin (α2u) nephropathy (α2u-N) (Swenberg et al. Citation1989; Borghoff et al. Citation1990; US EPA Citation1991; IARC Citation1999; Swenberg and Lehman-McKeeman Citation1999). These pathological observations, α2u-N, are characterized by the accumulation of α2u, a low-molecular-weight protein that is synthesized in the liver of male rats, in the kidney.
Although humans do not synthesize α2u, they do synthesize other low molecular weight proteins in the same lipocalin superfamily as α2u based on limited sequence homology, a similar molecular weight, and a proposed function of transport (Brooks Citation1987; Pervaiz and Brew Citation1987; Löbel et al. Citation2001). However, Borghoff and Lagarde (Citation1993) demonstrated that humans do not possess a protein similar to a2u in their kidneys with respect to the relative abundance of this protein or ligand binding characteristics and therefore not be at risk of developing chemically induced protein-mediated nephropathy. Unlike male rats, female rats, NBR male rats, or mice do not synthesize α2u in the liver, which is the major source of this protein that is filtered at the glomerulus and contributes to the protein droplets observed in kidneys of untreated male rats. Rodents that do not synthesize a2u in their liver or synthesize a2u (i.e. mice) do not develop α2u-N or kidney tumors when exposed to the chemicals that cause these responses in the male rats (Short, Burnett, et al. Citation1989; Short, Steinhagen, et al. Citation1989; US EPA Citation1991; Dietrich and Swenberg Citation1992a, Citation1992b). Both the U.S. EPA (US EPA Citation1991) and the International Agency for Research on Cancer (IARC Citation1999) developed criteria for determining if a male rat kidney carcinogen operates through an α2u-N-specific MoA as described and outlined in these published documents.
Chemically-induced α2u-N that occurs in male rats is a well-delineated MoA that has been found to lead to kidney tumors in male rats with chronic exposure. This MoA is supported by multiple examples of non-genotoxic chemicals that have been shown to induce these kidney effects, defined by protein droplet formation in the P2 segment of the renal proximal tubules, epithelial cell shedding and cytolysis, regenerative cell proliferation, along with granular cast formation in the outer medulla, and linear mineralization in the papilla (Charbonneau et al. Citation1989; Borghoff et al. Citation1990, Citation2001, Citation2009). The objective of this evaluation is to characterize the evidence base for the α2u-N kidney tumor MoA in the framework of an AOP by identifying and providing support for, the molecular initiating event (MIE), key events (KEs), and key event relationships (KERs) based on the Organization for Economic Cooperation and Development (OECD) AOP User’s Handbook (OECD Citation2018). Building this AOP is a first step to expanding the AOP knowledge-based network to organize this well-recognized pathway in a framework that can subsequently be used to align on a consistent set of in vitro assays to determine the likelihood of a chemical to elicit the identified MIE and or subsequent KEs in the AOP.
Materials and methods
Targeted literature search and review
Consistent with the OECD AOP User’s Handbook (OECD Citation2018), the literature review incorporated elements of best practice for identification, evaluation, and prioritization of data sources. Additionally, data sources, including reviews (secondary literature) from authoritative bodies that describe in detail the MoA for male rat kidney tumors were used to determine the relevancy of the literature for consideration in the development of this AOP (US EPA Citation1991; IARC Citation1999).
As a starting point for the literature search, targeted literature searching was performed from PubMed on 1 October 2020 to identify key peer-reviewed publications using the terms “α2u,” “alpha2u,” α2u, or “α2u globulin.” The targeted literature search identified 116 articles that were screened by title and abstract to determine utility to confirm and establish the MIE, KEs, and KERs critical to the development of the AOP for kidney tumors that developed through the α2u-N MoA. The full-text literature identified to be potentially relevant, based on screening the title and abstract, was then critically reviewed and categorized using the available data to inform the MIE, KE, or KERs. While α2u-N has been studied for over 30 years, this male rat kidney tumor MoA has not been characterized in terms of an AOP framework in which the MIE and subsequent KEs associated with α2u-N and kidney tumors are identified.
Organization into the AOP framework
The AOP Framework and template, as described by the OECD’s AOP User’s Handbook (OECD Citation2018) were used to characterize, develop and review this AOP in a transparent and scientifically-based method. Each event (MIE, KE, and KER) is described in terms of both a general biological description and the specific measurement method(s). Based on the empirical evidence supporting each KE within the AOP, the domains of applicability are defined in the section entitled AOP assessment.
Assessment of relative confidence in the AOP
Recognizing that AOPs are living documents intended to be used in regulatory decision-making in a fit-for-purpose manner according to the relative confidence in the AOP (Edwards et al. Citation2016), this section summarizes the weight of the evidence (WOE) for this AOP through consideration of biological plausibility, empirical evidence supporting the KERs and essentiality of all KEs (Section AOP Assessment).
Defining questions for each component in the WOE were used to anchor the relative confidence for the overall conclusion for the AOP. Biological plausibility was assessed on the availability of a clear mechanistic relationship between upstream and downstream KEs; essentiality was assessed by confirming the prevention of a downstream KE after blocking an upstream KE, and empirical evidence was assessed on dose and temporal concordance across multiple KEs (OECD Citation2018). In alignment with the OECD guidance and due to the large number of chemicals associated with this MoA, and the fact that this MoA is recognized by regulatory agencies (US EPA Citation1991: IARC Citation1999), the quantitative data set was not reported in its entirety.
Results
AOP for kidney tumors in male rats
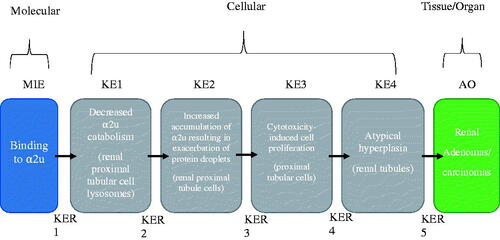
Based on data developed on structurally diverse chemicals, the MIE is identified as chemical bonding to the protein α2u, KE1 is the decreased rate of α2u catabolism leading to KE2, the exacerbation of α2u accumulation in the form of protein droplets within renal proximal tubular cells. Repeated exposure to chemicals that induce α2u-N leads to sustained cycles of cytotoxicity and compensatory proliferation in the proximal tubular cells (KE3). Continued, chronic exposure eventually leads to linear mineralization of the kidney papilla (associative event) and observations of atypical kidney tubular hyperplasia (KE4), in some cases progressing to a low incidence of kidney adenomas/carcinomas [adverse outcome (AO)] ( and ). The MIE, KEs, and AO are described in detail below, followed by a description of the domain of applicability of this AOP and an overall assessment of the confidence of the AOP.
Figure 1. Empirical evidence supporting the AOP. Effect levels shown in gray indicate the highest no effect level per study; effect levels in orange indicate the lowest observed effect level per study. Route of exposure shown by shape: circle, inhalation; square, oral. Short-term exposure is defined as <28 days; mid-term exposure is >28 and <300 days; long-term exposure is >300 days. 1,3-dichlorobenzene has only a single data point and so is not included in the graph. Full study details can be found in . ETBE: ethyl tertiary butyl ether; IP: isophorone; MIBK: methyl isobutyl ketone; MTBE: methyl-tert butyl ether; TBA: tertiary butyl alcohol.

Table 1 (A). Events: molecular initiating event (MIE), key events (KEs), and adverse outcome (AO).
Table 1 (B). Relationships between two key events (including MIE and adverse outcome).
The molecular initiating event (MIE): binding to α2u protein
The MIE is the reversible binding of a chemical to the α2u protein. α2u is a low-molecular-weight protein (∼18 700 Da) that is synthesized in the liver and secreted at a rapid rate into the blood of male, but not female, rats, with ∼50% of the protein filtered at the glomerulus being resorbed into the proximal tubule cells (Roy and Raber Citation1972; Roy et al. Citation1976). Chemical binding to α2u can decrease its rate of catabolism, leading to its accumulation in the form of protein droplets in proximal tubule cells. A diverse group of chemicals, or their metabolites, have been reported to bind to α2u, either in vivo or in vitro including the following: 1,4-dichlorobenzene (-2,5-dichlorophenol, metabolite), d-limonene (d-limonene 2-5-oxide, metabolite), methyl isobutyl ketone (MIBK), 2,2,4-trimethylpentane, (2,4,4-trimethyl-2-pentanol, metabolite), tertiary butyl alcohol (TBA), ethyl tertiary butyl ether (ETBE) (TBA, metabolite), and methyl tert-butyl ether (MTBE) (TBA, metabolite) (Borghoff and Lagarde Citation1993; Swenberg Citation1993; Prescott-Matthews et al. Citation1999; Borghoff et al. Citation2015, Citation2017). Following exposure to one of these chemicals, either the parent compound or metabolite were found to co-elute with α2u when purified from the male rat kidney (Lock et al. Citation1987; Charbonneau et al. Citation1989; Prescott-Matthews et al. Citation1999). Borghoff et al. (Citation1991) demonstrated chemical binding to α2u in vitro with several chemicals showing varying binding affinities that range from 10−7 to 10−4 M. Molecular modeling was then used to evaluate the critical components of these structures to bind to this protein, with the analysis demonstrating that binding is dependent on both hydrophobic interactions and hydrogen bonding. Examples of chemical stressors are provided in .
Table 2. Stressors (examples).
Key event 1: decreased rate of (α2u) catabolism (renal proximal tubular cell lysosomes)
When a chemical binds to α2u, it forms a complex with the α2u protein that can result in a reduction in its rate of catabolism (Lehman-McKeeman et al. Citation1990). α2u then accumulates in the phagolysomes of the renal proximal tubules (; Swenberg et al. Citation1989; Borghoff et al. Citation1990; US EPA Citation1991; IARC Citation1999; Swenberg and Lehman-McKeeman Citation1999). Although a decrease in the catabolism of this protein was directly measured in vitro in only one study (Lehman-McKeeman et al. Citation1990), this study demonstrated a response using isolated kidney cortical lysosomes with a number of the chemical stressors that cause α2u-N, or with their metabolite (e.g. d-limonene-1,2-oxide, isophorone, 2,5-dichlorophenol). Indirect measures of decreased catabolism were noted via studies that show an increase in α2u in the proximal tubule cells of exposed rats by immunohistochemical staining for α2u and or quantitation of α2u in kidney tissue (US EPA Citation1991, IARC Citation1999).
Key event 2: increased accumulation of α2u resulting in exacerbation of protein droplets (renal proximal tubular cells)
Under normal physiological conditions, α2u is degraded in the kidney by proteolytic enzymes within phagolysosomes. However, when the chemical initiator (chemical identified as binding to α2u) binds reversibly to α2u (MIE), it can decrease its rate of proteolytic hydrolysis, leading to its accumulation in the renal proximal tubular cells (summarized in , see reviews by Swenberg et al. Citation1989; Borghoff et al. Citation1990; US EPA Citation1991; IARC Citation1999; Swenberg and Lehman-McKeeman Citation1999). Increased accumulation of α2u has been evaluated by quantitating α2u in the kidney using an enzyme-linked immunosorbent assay (ELISA), semi-quantitating by grading the size and extent of α2u accumulation in the kidney in the form of protein droplets histologically, and/or immunohistochemically staining the droplets for α2u. The protein droplets reflect the accumulation of exfoliated renal tubule cells, specifically in the P2 segment of the renal proximal tubules, where the majority of protein is observed. Under specific exposure concentrations including both acute and repeated exposure, certain chemical stressors ( and ; citations listed in ) result in excessive protein droplet accumulation, described as crystal-like structures in the renal proximal tubules (i.e. 2,4,4-trimethyl pentane, d-limonene, 1,4-dichlorobenzene).
Table 3. Summary of examples from the evidence base that establish support for the dose-responses of KERs.
Protein droplet accumulation was also reported in male rats following 14 and 90 days of oral exposure to MTBE (Robinson et al. Citation1990). Prescott-Mathews et al. (Citation1997) demonstrated an increase in protein droplet accumulation with droplet staining for α2u and an increase in the concentration of α2u in the kidney of male rats following 10 days of exposure to varying concentrations of MTBE. A time- and concentration-dependent increase in protein-droplet accumulation was measured in male rat kidneys, with evidence of protein droplet accumulation following inhalation to ETBE for 90 days (Medinsky et al. Citation1999). Alpha-2u immunoreactivity in these protein droplets was confirmed. Another study showed concentration-dependent accumulation of protein droplets in the kidneys of male rats, following 10-day inhalation to TBA (0, 230, 450, and 1750 ppm) (Borghoff et al. Citation2001). In a more recent study, rats were exposed via inhalation for 1 or 4 weeks to 0, 450, 900, or 1800 ppm MIBK for 6 h/day. An increase in protein droplet formation (also referred to as hyaline droplets in H&E stained kidney sections) was reported to occur following exposure to 450 ppm and higher, increasing in incidence and severity with increasing concentration (Borghoff et al. Citation2015). This KE is consistently reported to occur in multiple studies following in vivo exposure to different α2u chemical stressors across various exposure scenarios and duration (Phillips et al. Citation1987; Robinson et al. Citation1990; Medinsky et al. Citation1999; Prescott-Matthews Citation1999; Borghoff et al. Citation2001, Citation2009, Citation2015; Nemec et al. Citation2004). Several review articles and regulatory assessments also identify chemicals that cause this key event (see reviews by US EPA Citation1991; Swenberg Citation1993; IARC Citation1999; Swenberg and Lehman-McKeeman Citation1999).
Key event 3: cytotoxicity-induced cell proliferation (renal proximal tubular cells)
The accumulation of α2u (KE2) within the phagolysosome results in cytotoxicity of the kidney tubular cells (; see Borghoff et al. Citation1990; Hard et al. Citation1993; Swenberg and Lehman-McKeeman Citation1999; Borghoff et al. Citation2001). Male rat α2u-specific lesions result from recurring epithelial cell death, and ensuing cell shedding into the lumen within the renal proximal tubules, and result in the formation of granular casts at the change-over from the pars recta of the proximal tubules and the descending Thin-Loop of Henle due to cellular debris overload and subsequently the accumulation of cell mineralization products in the collecting duct and ductus of Bellini of the papilla (Hard et al. Citation2013; Borghoff et al. Citation2015). Compensatory cell proliferation takes place in an effort to overcome recurring cell death and is found to be specific to the site at which α2u accumulation occurs (Swenberg and Lehman-McKeeman Citation1999; Borghoff et al. Citation2001).
Many of the chemical stressors identified in and as operating through this MoA to cause kidney tumors in male rats demonstrated an increased and sustained cell proliferative response. Increased kidney cell proliferation has been shown to occur following TMP and DCB exposure (Eldridge et al. Citation1990; Lake et al. Citation1997) and shown to increase with dose and time in male rats only, following inhalation exposure for 90 days to ETBE (Medinsky et al. Citation1999), and up to 10 days following exposure to MTBE or TBA (Prescott-Mathews et al. Citation1997; Williams and Borghoff Citation2001). TBA was also reported to have an increased cell proliferation in the male rat kidneys, corresponding to increased concentrations of α2u (Williams and Borghoff Citation2001).
Key event 4: atypical hyperplasia (renal tubules)
Sustained regenerative cell proliferation described in KE3 leads to atypical hyperplasia in the presence of renal lesions characteristic of protein accumulation, such as granular cast formation and linear mineralization (; see NTP Citation1986, Citation2007; Borghoff et al. Citation2001, Doi et al. Citation2007; Saito et al. Citation2013). Multiple studies report atypical hyperplasia following exposure to different chemicals that also have reported previous KEs in this AOP, as summarized in and . Atypical renal tubular hyperplasia is described by Doi et al. (Citation2007) to consist of discrete, focal to multifocal proliferative lesions of renal tubules. It is noted that grading of hyperplasia is based on the size of the lesion, the number of affected tubular profiles present, and the complexity of the hyperplastic epithelium. Granulation cast formulation and linear mineralization are considered an associative event (AE) that acts as a reliable indicator of atypical hyperplasia but is not considered a biological process in and of itself that is necessary to progress through the overall pathway (Andersen et al. Citation2014; Simon et al. Citation2014). Linear mineralization is described as regularly arranged basophilic mineralized linear deposits that are distributed mainly in the lower half of the renal papillae. d-Limonene induced atypical renal tubule hyperplasia in male rats, but not females, as reviewed in Rice et al. (Citation1999). NTP (Citation2007) reports atypical renal tubule hyperplasia in male rats following inhalation exposure to MIBK for 2 years; Hagiwara et al. (Citation2011) report similar findings in male rats following oral exposure to ETBE for 4 weeks. Sustained regenerative cell proliferation leads to atypical hyperplasia, which is believed to be associated with the promotion of spontaneous mutations through clonal expansion.
Adverse outcome (AO): kidney adenomas/carcinomas
Sustained cell proliferation and clonal expansion of the renal tubular cells lead to a cellular environment leading to tumorigenic response (; see Borghoff et al. Citation1990, Citation2001, Citation2015; Borghoff and Lagarde Citation1993; Lehman-McKeeman et al. Citation1990; Swenberg Citation1993; Prescott-Matthews et al. Citation1999). The extent of this sustained cell proliferation is seen in the observation of atypical hyperplasia in the kidneys of male rats. Inhalation exposure to 3000 ppm MTBE caused an increased incidence of kidney tumors in male rats, but not female rats or mice (Bird et al. Citation1997; Prescott-Matthews et al. Citation1999). TBA was also reported to induce kidney tumors in male rats following exposure in drinking water (Cirvello et al. Citation1995; NTP Citation1995). A diverse group of chemicals has been identified to cause kidney tumors in male but not female rats (Swenberg et al. Citation1989; Borghoff et al. Citation1990; US EPA Citation1991; IARC Citation1999; Swenberg and Lehman-McKeeman Citation1999; Doi et al. Citation2007).
AOP assessment
Defining the chemical and biological domains of applicability
The MIE applies to several chemicals across diverse classes of chemicals, all of which bind reversibly as the parent chemical or a metabolite, to the α2u protein. Empirical evidence demonstrating activation of MIE and KEs was reviewed for 1,4-dichlorobenzene (2,5-dichlorophenol), isophorone, d-limonene (d-limonene 2-5-oxide), decalin, MIBK, ETBE (TBA), MTBE (TBA), TBA, and TMP (TMPOH) ().
This AOP biological domain of applicability is specific to sexually mature adult male rats (∼10–12 weeks, peak of α2u synthesis) which would be also the relevant life stage. The hepatic synthesis of α2u in male rats begins at the onset of sexual maturity and increases until ∼20 weeks of age (Vandoren et al. Citation1983; Roy et al. Citation1983). α2u is synthesized at a high level in the liver of male but not female rats (Roy et al. Citation1976; Burnett et al. Citation1989), although there are glands in female rats that synthesize α2u, this protein is only present at extremely low levels. α2u kidney concentrations are ∼120 times lower in female kidneys vs. male rat kidneys, blood, and urine (Vandoren et al. Citation1983; Williams and Borghoff Citation2001). Chemical binding to α2u and subsequent nephropathy has consistently been reported to occur only in male, but not female, rats (Alden Citation1986; Swenberg et al. Citation1989; Borghoff et al. Citation1990; Ridder et al. Citation1990; Dietrich and Swenberg Citation1991; Swenberg Citation1993; Doi et al. Citation2007; Aksu and Tanrikulu Citation2016).
α2u has not been detected in human kidneys; one study showed that a similar protein to α2u, could not be detected in human kidneys based on its abundance and chemical binding specificity (Borghoff and Lagarde Citation1993). In addition, mice excrete a mouse urinary protein similar to α2u, but its amino acid sequence homology is ∼90% that of rat α2u (Shaw et al. Citation1983) and ligand specificity and reabsorption in the kidney differs from rat α2u. Female rats, NBR male rats, or mice, which do not synthesize α2u in the liver, do not develop α2u-mediated nephropathy or kidney tumors when exposed to the chemicals that cause these responses in the male rats (Alden Citation1986; Short, Burnett, et al. Citation1989; Short, Steinhagen, et al. Citation1989; Swenberg et al. Citation1989; Borghoff et al. Citation1990; Ridder et al. Citation1990; Dietrich and Swenberg Citation1991, Citation1992a, Citation1992b; US EPA Citation1991; Swenberg Citation1993; Doi et al. Citation2007; Aksu and Tanrikulu Citation2016). Again supporting that this AOP’s taxonomic applicability is specific to the male rat (Rattus norvegicus).
Assessing the relative confidence in the AOP
The justification and support for the essentiality of the KEs based on biological plausibility (), essentiality (), and empirical evidence () are therefore provided below with detailed tables and summaries that are consistent with these OECD templates for assessment of confidence of an AOP. The confidence in KEs and KE relationships is summarized in .
Table 4. Summary assessment of the relative level of confidence in the overall AOP based on weight-of-evidence elements—support for the biological plausibility of KERs.
Table 5. Support for the essentiality of KEs.
Table 6. Empirical evidence for KERs.
WOE: biological plausibility
The biological plausibility of each KER within this pathway is considered to be strong (high, see ), based on the well-understood mechanistic basis of the KERs that are well-established, documented, and widely accepted in the scientific community. Briefly, the evidence to support the direct relationship between the MIE (chemical binding to α2u) and KE1 (reduction of α2u catabolism) (KER1) is supported by data reported for diverse chemical structures within the context of a well-defined MoA and based on empirical evidence in vitro and in vivo. Indirect data from multiple chemicals in vivo are available that demonstrate the reversible binding of the chemical to α2u (KER2) resulting in a chemical-protein complex. There is also consistent direct evidence in vivo for multiple chemicals for the formation of protein droplets (KER3), observed through the immunohistochemical staining of α2u within the droplet, and quantitation of α2u concentration in kidneys. It is also well-established and widely accepted there is a direct correlation between protein droplet accumulation, cytotoxicity, and regenerative cell proliferation (KER4); which then through sustained cell proliferation leads to the development of atypical hyperplasia.
WOE: essentiality
The essentiality of the early KEs in this pathway is considered strong (MIE, KE1, KE2), based on direct experimental evidence showing that elimination of one of the upstream KEs prevents progression through this pathway (). Later KEs (KE3, KE4, and AO) are considered moderately essential to this AOP as there was no direct experimental evidence available that illustrated the essentiality of these KEs. However, the lack of hepatic synthesis of α2u in female rats, and other species including humans, with the lack of the identified chemical stressors inducing KE2-KE4 and the direct empirical evidence for earlier KEs supports the essentiality of these KEs and the overall AOP.
WOE: empirical evidence
Individually, relationships between early KEs are supported by strong empirical evidence, based on available direct and indirect evidence across multiple chemicals demonstrating that binding to α2u is associated with decreased catabolism and protein droplet accumulation, eventually leading to cytotoxicity and compensatory cell proliferation in the kidney tissue (i.e. KERs between MIE, KE1, KE2, KE3) (). Relationships between the later KEs are considered to be supported by moderate evidence, mainly due to the difficulty quantitating atypical hyperplasia and the reported low incidence of kidney tumors in the available studies (i.e. relationship between KE3, KE4, and AO).
Collectively, this AOP was developed based on empirical evidence for 9 distinct chemicals with generally consistent dose-temporal patterns (). For example, acute (short) and sub-chronic (medium) exposure to ETBE at doses of ∼300–500 ppm induces KE2 but not KE3 and KE4 (Medinsky et al. Citation1999; Hagiwara et al. Citation2011). Induction of KE3 and KE4 is observed after relatively higher or relatively longer doses of ETBE (≥1000 ppm) (Medinsky et al. Citation1999; Hagiwara et al. Citation2011). Although this pattern of dose-responsiveness across KEs is also observed for MTBE, there is a noted inconsistency for TBA, where KE2 is not observed after acute exposure of 250 ppm, but KE3 occurs at this dose (Borghoff Citation2001). Given the integration of studies conducted under varying methods (e.g. test material, route of exposure, study duration, species), the dose-response supports moderate-to-strong confidence in this AOP.
WOE: limitations and/or inconsistencies
As noted above, not all KERs are supported by strong biological plausibility and empirical evidence. Further, a dose-response relationship has not been demonstrated directly in the MIE or KE1. However, Borghoff et al. (Citation1991) demonstrated different binding affinities of the chemical stressors to α2u in vitro, but a direct relationship between the amount of protein accumulation and binding affinity is difficult to measure in vivo, along with the complexity associated with either or both the parent and metabolite binding to the protein, and not necessarily both affecting catabolism (Lehman-McKeeman et al. Citation1990).
Discussion
The purpose of this assessment was to elucidate and assess the confidence in an AOP for male-rat-specific kidney toxicity of α2u-N induced kidney tumors through the evaluation of primary literature, reviews, and regulatory guidance.
This assessment developed a high confidence AOP for kidney tumors in male rats based on an MIE of reversible binding of chemical stressors to the α2u-globulin protein. This chemical binding leads to a reduction in the catabolism rate of α2u (KE1); resulting in the accumulation of the α2u in the form of protein droplets in the lysosomes of RPT cells (KE2). Exacerbation of α2u accumulation in the RPT cells results in cytotoxicity and compensatory cell proliferation (KE3). Chronic cytotoxicity and regenerative cell proliferation leads to atypical hyperplasia (KE4) that is indicated by mineralization of the RPTs (AE) leading to, with continued chronic exposure, the formation of kidney adenomas and carcinomas in male rats (AO).
Collectively, there is moderate-to-strong support in the WOE for the overall assessment of this AOP based on the essentiality of the KEs, biological plausibility, and empirical evidence of the KERs. Considering the empirical evidence, some inconsistencies are noted. Inhalation exposure of MIBK for 1 week, but not 4 weeks activated KE3 at lower but not higher doses (450 ppm but not 900 ppm) (Borghoff Citation2015) and inhalation exposure to TBA (250, 450, or 1750 ppm) for 10 days activated the downstream (KE3) but not the upstream KEs (KE2) at 250 ppm (Borghoff et al. Citation2001). However, inconsistencies, such as these are best described as limited when compared to the totality of data across other chemical stressors and are thought to be influenced by the sensitivity of the assay and the overall mild induction of α2u-N and sensitivity of the assays that measure these early events. The overall WOE still ranges in strength from moderate to strong. Consequently, the AOP as a whole is considered to have high confidence.
Based on the high overall confidence, the AOP for α2u-N MoA for kidney tumors could be used to evaluate the likelihood that chemicals act through this pathway or to determine the human relevance of kidney findings in animal studies. With an available AOP of high confidence, this tool can be used to develop new approaches to screen for this particular outcome after chemical exposure using biological events that occur earlier than the adverse outcome itself Characterization of the MIE as an indicator for the AO supports the creation of rapid screening of new and emerging chemicals through in vitro assays that can be used to identify more targeted testing strategies for hazard characterization. A similar approach has been applied to developmental neurotoxicity, where a network of AOPs has been developed with the intent to progress the development of in vitro and in silico testing approaches for this outcome (Bal-Price et al. Citation2015). Given the complexity of the developing brain, a series of AOPs were developed, with a focus on the identification of common KEs to reduce the overall resource investment in testing while increasing the likelihood that developmental perturbations are identified. The development of in vitro test batteries based on this common KE is envisioned to be used to prioritize higher-tier testing in animals in a data-informed, resource-conscious manner.
In the case of kidney outcomes, the AOP described here is envisioned to eventually be one in a series of kidney-focused AOPs, that could be used to confirm male rat-specific observations without progressing to chronic exposure studies. Although this pathway has long been recognized, it remains relevant and timely for regulatory risk assessment activities, particularly for hazard identification, prioritization of chemicals of potential concern for human health, product stewardship, and regulatory submissions.
A second commonly observed and interfering kidney lesion is chronic progressive nephropathy (CPN), a progressive rat kidney pathology that often co-occurs with α2u-N. CPN is considered a risk factor for the development of atypical tubule hyperplasia and renal tubule tumors. The hallmark features of CPN are histopathological events with progressive severity. It has been suggested that the likely cause underlying the association of CPN with renal tubule neoplasia is CPN-associated renal tubule cell proliferation (Hard et al. Citation2013). Distinct from α2u-N, scientific consensus has not been reached on whether the biological events leading to CPN are indeed specific to rodents, and thus the co-occurrence of α2u-N and CPN can be considered to be potentially human-relevant, as in the recent case of ETBE and TBA (US EPA Citation2021). An area of future work is to develop a comparative set of KEs that can be used to inform the outstanding questions of whether the biological events leading to CPN qualitatively occur in cells derived from human tissues.
The availability of a network of AOPs leading to kidney outcomes, specifically for both α2u- and CPN-mediated effects, might have helped clarify this question of human relevance earlier in the IRIS review process by providing a transparent and scientifically validated alternative to characterize the relative likelihood that the kidney effects in question were mediated via one of these pathways.
To that end, our analysis represents the first fully elucidated and empirically supported AOP for α2u-N-mediated kidney tumors in male rats established according to OECD guidance (OECD Citation2018). Earlier work with a similar concept is stated to be under development in a public database (AOP-Wiki), entitled “Alpha2u-microglobulin cytotoxicity leading to kidney tubular adenomas and carcinomas (in male rat)” and listing a similar order of key events and biological applicability domain. However, no detailed characterization is available (Wood Citation2020). The KEs identified by Wood (Citation2020) and in this manuscript are well-established in the literature and have a broad acceptance in the scientific community and by both EPA and IARC (US EPA Citation1991; Swenberg and Lehman-McKeeman Citation1999). The novelty of the work herein is the integration of mechanistic evidence from a variety of sources and methodologies into the AOP with an explicit statement of confidence in the WOE. This work takes into consideration biological plausibility, essentiality, and empirical evidence for the KEs and also characterizes the dose-response for several chemicals.
In the future, this AOP could be applied to risk assessment in a few different ways. Firstly, it could be used to clarify the mechanistic basis of kidney effects observed in animal studies to assess the potential for human relevance. Building on the ETBE example above, in vitro assays could be developed and used to investigate ETBE’s ability to activate early KEs in the AOP: in vitro binding affinity assays to evaluate the interaction between the chemical and α2u (as described in Borghoff et al. Citation1991), measurements to quantify the reduction of lysosomal degradation of chemically-bound α2u (as described in Lehman-McKeeman et al. Citation1990), or possibly ex vivo kidney slices or 3 D microphysiological systems of the proximal tubule to assess subcellular localization of α2u in phagolysosomes (Saitta et al. Citation2019; Yeung and Himmelfarb Citation2019). Using, such assays may help to reduce the number of animals used to confirm if a chemical is likely to cause kidney tumors in male rats through the α2u-N MoA vs. conducting chronic in vivo animal studies. Kidney tissues from different rodent species, strains, and sexes could be assessed, including human-derived tissues or cell lines, to inform the species-specificity of observed changes in these in vitro assays. Secondly, the availability of a defined AOP with specified in vitro assays for alternate kidney mechanisms would allow research programs to make comparative assessments of the likelihood that each mechanism contributes to apical kidney outcomes. Drawing from existing animal studies on a diverse set of chemistries to develop this kidney-specific AOP is a step toward a paradigm shift in regulatory toxicology, where data-driven testing strategies may be developed based on screening MIEs drawn from a library of AOPs rather than by conducting a standard set of resource-intensive toxicology studies. In this way, the establishment of a library of high-confidence AOPs can facilitate achieving regulatory targets set out by organizations, such as the US EPA (US EPA Citation2019).
In conclusion, this work established a high-confidence AOP for male rat kidney tumors through the accumulation of the male rat-specific protein α2u. This AOP will be useful both for chemical screening using NAMs developed for measuring the MIE with critical KEs (e.g. decreased α2u catabolism) for use in either prioritization of chemical testing or hazard characterization in regulatory settings.
| Abbreviations | ||
| α2u | = | α2u-globulin (α2u) |
| α2u-N | = | α2u nephropathy |
| AE | = | associative event |
| AO | = | adverse outcome |
| AOP | = | adverse outcome pathway |
| CPN | = | chronic progressive nephropathy |
| Da | = | daltons |
| DCB | = | dichlorobenzene |
| ELISA | = | enzyme-linked immunosorbent assay |
| ETBE | = | ethyl tertiary butyl ether |
| IARC | = | International Agency for Research on Cancer |
| KE | = | key event |
| KER | = | key event relationships |
| MIBK | = | methyl isobutyl ketone |
| MIE | = | molecular initiating event |
| MoA | = | mode of action |
| MTBE | = | methyl tert-butyl ether |
| NAM | = | new approach methods |
| NBR | = | NCI-Black-Reiter |
| OECD | = | Organization for Economic Coordination and Development |
| RPT | = | Renal proximal tubule |
| TBA | = | tertiary butyl alcohol |
| TMP | = | 2,2,4-Trimethylpentane |
| TMPOH | = | 2,4,4-Trimethylpentane-2,4,4-trimethyl-2-pentanol |
| U.S. EPA | = | United States Environmental Protection Agency |
| WOE | = | weight of the evidence |
Acknowledgments
The authors wish to gratefully acknowledge API’s Toxicology Methods & Research Group for their encouragement to write this manuscript and for subsequent review of this manuscript, specifically Pearl Moy (Chevron and member) who provided helpful editorial comments; API’s Health and Product Stewardship Sub-Committee for financial support and review of the manuscript; and to the external reviewers selected by the Editor and anonymous to the authors whose comments were extremely valuable in revising and refining the manuscript.
Declaration of interest
KG is an employee of ExxonMobil Biosciences, Inc., a separately incorporated but wholly owned affiliate of Exxon Mobil Corporation. SS is an employee of Shell International and is free to express his own scientific opinion and ideas. Both ExxonMobil and Shell manufacture gasoline and both are members of the American Petroleum Institute (API), a trade association that represents all segments of oil and gas development, including manufacturers of gasoline. ETBE and TBA (chemicals reviewed in this manuscript) are fuel oxygenates that have historically been added to gasoline and have recently been reviewed by the US EPA’s IRIS Program. JR was an employee of API during the development of this manuscript but is currently employed elsewhere. At API, JR managed both the API Toxicology Methods and Research Group, on which KG participates, and the API Health and Product Stewardship Sub-Committee, on which SS acts as the chair. This Group and Sub-Committee commissioned and funded this work, as well as reviewed and approved the manuscript. AF and SB are employees of ToxStrategies, Inc., a scientific consulting firm. ToxStrategies, Inc. was retained as paid consultants to produce the API-funded report. An early version of this work was presented by AF and SB as an e-poster at the Society of Toxicology 59th Annual Meeting (#1997), which was also approved by the API Group before the presentation. The work of all Authors on this manuscript was done as part of their normal employment. Before submission, this manuscript underwent internal, legal review. This internal review did not impact the text, which was written by the Authors. Some Authors have participated in regulatory proceedings on chemicals cited in this manuscript: KG and JR provided public comments to the US EPA on ETBE and TBA IRIS assessments on behalf of API and SB provided public comments on behalf of Lyondell Chemical Company; SS/SB participated in trade association panels sponsoring research to inform IARC assessments, and SB participated in IARC reviews for several of these chemicals. In addition to regulatory proceedings, readers should be aware that chemicals mentioned in this publication that support the proposed AOP have been, currently are, or possibly will be, involved in civil litigation. This civil ligation may involve API member companies.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Aksu S, Tanrikulu F. 2016. Differentiation of protein species of alpha-2u-globulin according to database entries: a half-theoretical approach. J Proteomics. 134:186–192.
- Alden CL. 1986. A review of unique male rat hydrocarbon nephropathy. Toxicol Pathol. 14(1):109–111.
- Andersen ME, Preston RJ, Maier A, Willis AM, Patterson J. 2014. Dose-response approaches for nuclear receptor-mediated modes of action for liver carcinogenicity: results of a workshop. Crit Rev Toxicol. 44(1):50–63.
- Bal-Price A, Crofton KM, Leist M, Allen S, Arand M, Buetler T, Delrue N, FitzGerald RE, Hartung T, Heinonen T, et al. 2015. International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes. Arch Toxicol. 89(2):269–287.
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, et al. 2015. Increasing scientific confidence in adverse outcome pathways: application of tailored bradford-hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 72(3):514–537.
- Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, Andrews LS. 1997. Oncogenicity studies of inhaled methyl tertiary butyl ether in CD-1 mice and F-344 rats. J Appl Toxicol. 17(S1):S45–S55.
- Borghoff SJ, Lagarde WH. 1993. Assessment of binding of 2,4,4-trimethyl-2-pentanol to low-molecular-weight proteins isolated from kidneys of male rats and humans. Toxicol Appl Pharmacol. 119(2):228–235.
- Borghoff SJ, Miller AB, Bowen JP, Swenberg JA. 1991. Characteristics of chemical binding to alpha 2u-globulin in vitro-evaluating structure-activity relationships. Toxicol Appl Pharmacol. 107(2):228–238.
- Borghoff SJ, Short BG, Swenberg JA. 1990. Biochemical mechanisms and pathobiology of alpha 2u-globulin nephropathy. Annu Rev Pharmacol Toxicol. 30:349–367.
- Borghoff SJ, Prescott JS, Janszen DB, Wong BA, Everitt JI. 2001. Alpha 2u-globulin nephropathy, renal cell proliferation, and dosimetry of inhaled tert-butyl alcohol in male and female F-344 rats. Toxicol Sci. 61(1):176–186.
- Borghoff SJ, Hard GC, Berdasco NM, Gingell R, Green SM, Gulledge W. 2009. Methyl isobutyl ketone (MIBK) induction of alpha2u-globulin nephropathy in male, but not female rats. Toxicology. 258(2–3):131–138.
- Borghoff SJ, Poet T, Green S, Davis J, Hughes B, Mensing T, Sarang SS, Lynch AM, Hard GC. 2015. Methyl isobutyl ketone exposure-related increases in specific measures of α2u-globulin (α2u) nephropathy in male rats along with in vitro evidence of reversible protein binding. Toxicology. 333:1–13.
- Borghoff SJ, Ring C, Banton MI, Leavens TL. 2017. Physiologically based pharmacokinetic model for ethyl tertiary-butyl ether and tertiary-butyl alcohol in rats: contribution of binding to α2u-globulin in male rats and high-exposure nonlinear kinetics to toxicity and cancer outcomes. J Appl Toxicol. 37(5):621–640.
- Brooks DE. 1987. The major androgen-regulated secretory proteins of the rat epididymis bear sequence homology with members of the alpha 2u-globulin superfamily. Biochem Int. 14(2):235–240.
- Burnett VL, Short BG, Swenberg JA. 1989. Localization of alpha 2u-globulin within protein droplets of male rat kidney: immunohistochemistry using perfusion-fixed, GMA-embedded tissue sections. J Histochem Cytochem. 37(6):813–818.
- Charbonneau M, Strasser J, Lock EA, Turner MJ, Swenberg JA. 1989. Involvement of reversible binding to alpha 2u-globulin in 1,4-dichlorobenzene-induced nephrotoxicity. Toxicol Appl Pharmacol. 99(1):122–132.
- Cirvello JD, Radovsky A, Heath JE, Farnell DR, Lindamood C. 1995. Toxicity and carcinogenicity of t-butyl alcohol in rats and mice following chronic exposure in drinking water. Toxicol Ind Health. 11(2):151–165.
- Dietrich DR, Swenberg JA. 1991. Alpha2u-globulin is necessary for d-limonene promotion of male rat kidney tumors. Cancer Res. 51(13):3512–3521.
- Dietrich DR, Swenberg JA. 1992a. The presence of alpha2 glоbulin is necessary for d-limonene promotion of male rat kidney tumors. Cancer Res. 51:3512–3521.
- Dietrich DR, Swenberg JA. 1992b. NCI-Black-Reiter (NBR) male rats fail to develop kidney disease following exposure to agents that induce a-2-globulin nephropathy. Fundam Appl Toxicol. 16(4):749–762.
- Doi AM, Hill G, Seely J, Hailey JR, Kissling G, Bucher JR. 2007. Alpha 2u-globulin nephropathy and renal tumors in national toxicology program studies. Toxicol Pathol. 35(4):533–540.
- Edwards SW, Tan Y-M, Villeneuve DL, Meek ME, McQueen CA. 2016. Adverse outcome pathways-organizing toxicological information to improve decision making. J Pharmacol Exp Ther. 356(1):170–181.
- Eldridge SR, Tilbury LF, Goldsworthy TL, Butterworth BE. 1990. Measurement of chemically induced cell proliferation in rodent liver and kidney: a comparison of 5-bromo-2′-deoxyuridine and [3H]thymidine administered by injection or osmotic pump. Carcinogenesis. 11(12):2245–2251.
- Hagiwara A, Doi Y, Imai N, Nakashima H, Ono T, Kawabe M, Furukawa F, Tamano S, Nagano K, Fukushima S. 2011. Medium-term multi-organ carcinogenesis bioassay of ethyl tertiary-butyl ether in rats. Toxicology. 289(2–3):160–166.
- Hard GC, Rodgers IS, Baetcke KP, Richards WI, McGaughy RE, Valcovic LR. 1993. Hazard evaluation of chemicals that cause accumulation of alpha2u-globulin hyaline droplet nephropathy and tubule neoplasia in the kidneys of male rats. Enviro Health Persp. 99:313–349.
- Hard GC, Banton MI, Bretzlaff RS, Dekant W, Fowles JR, Mallett AK, McGregor DB, Roberts KM, Sielken RL, Valdez-Flores C, et al. 2013. Consideration of rat chronic progressive nephropathy in regulatory evaluations for carcinogenicity. Toxicol Sci. 132(2):268–275.
- Hard GC, Cohen SM, Ma J, Yu F, Arnold LL, Banton MI. 2019. Histopathology re-examination of the NTP toxicity/carcinogenicity studies of tert-butyl alcohol to identify renal tumor and toxicity modes of action. Regul Toxicol Pharmacol. 102:65–73.
- [IARC] International Agency for Research on Cancer. 1999. Consensus report. In: Capen CC, Dybing E, Rice JM, Willbourn JD, editors. Species difference in thyroid, kidney, and urinary bladder carcinogenesis. Lyon: IARC Scientific Publication (No. 147).
- Kanerva RL, Ridder GM, Lefever FR, Alden CL. 1987. Comparison of short-term renal effects due to oral administration of decalin or d-limonene in young adult male Fischer-344 rats. Food Chem Toxicol. 25(5):335–345.
- Lake BG, Cunninghame ME, Price RJ. 1997. Comparison of the hepatic and renal effects of 1,4-dichlorobenzene in the rat and mouse. Fundam Appl Toxicol. 39(1):67–75.
- Lehman-McKeeman LD, Rivera-Torres MI, Caudill D. 1990. Lysosomal degradation of alpha 2u-globulin and alpha 2u-globulin-xenobiotic conjugates. Toxicol Appl Pharmacol. 103(3):539–548.
- Löbel D, Strotmann J, Jacob M, Breer H. 2001. Identification of a third rat odorant-binding protein (OBP3). Chem Senses. 26(6):673–680.
- Lock EA, Charbonneau M, Strasser J, Swenberg JA, Bus JB. 1987. 2,4,-Trimethyl-induced nephropathy II. The reversible binding of a TMP metabolite to a kidney protein fraction containing α2u-globulin. Toxicol Appl Pharmacol. 91(2):182–192.
- Medinsky MA, Wolf DC, Cattley RC, Wong B, Janszen DB, Farris GM, Wright GA, Bond JA. 1999. Effects of a thirteen-week inhalation exposure to ethyl tertiary butyl ether on fischer-344 rats and CD-1 mice. Toxicol Sci. 51(1):108–118.
- Nemec MD, Pitt JA, Topping DC, Gingell R, Pavkov KL, Rauckman EJ, Harris SB. 2004. Inhalation two-generation reproductive toxicity study of methyl isobutyl ketone in rats. Int J Toxicol. 23(2):127–143.
- [NTP] National Toxicology Program. 1986. NTP technical report on the toxicology and carcinogenesis studies of isophorone CAS NO. 78-599-1) in F344 rats and B6aC3F1 mice (gavage studies).
- [NTP] National Toxicology Program. 1990. NTP technical report on the toxicology and carcinogenesis studies of d-limonene (CAS NO. 5989-27-5) in F344/N rats and B6C3F1 mice (gavage studies).
- [NTP] National Toxicology Program. 1995. NTP technical report on the toxicology and carcinogenesis studies of t-butyl alcohol (CAS NO. 75-65-0) in F344/N rats and B6C3F1 mice (drinking water studies).
- [NTP] National Toxicology Program. 2005. NTP toxicology and carcinogenesis studies of decalin (CAS No. 91-17-8) in F344/N rats and B6C3F1 mice and a toxicology study of decaline in male NBR rats (inhalation studies). NTP Technical Report 513.
- [NTP] National Toxicology Program. 2007. NTP toxicology and carcinogenicity studies of methyl isobutyl ketone (CAS no. 108-10-1) in F344/N rats and B6C3F1 mice inhalation studies. NTP Technical Report Series 538. p. 1–236.
- [OECD] Organisation for Economic Co-operation and Development. 2018. User’s handbook supplement to the guidance document for developing and assessing AOPs. ENV/JM/MONO(2016)12.
- Perkins EJ, Antczak P, Burgoon L, Falciani F, Garcia-Reyero N, Gutsell S, Hodges G, Kienzler A, Knapen D, McBride M, et al. 2015. Adverse outcome pathways for regulatory applications: examination of four case studies with different degrees of completeness and scientific confidence. Toxicol Sci. 148(1):14–25.
- Pervaiz S, Brew K. 1987. Homology and structure-function correlations between alpha 1-acid glycoprotein and serum retinol-binding protein and its relatives. FASEB J. 1(3):209–214.
- Phillips RD, Moran EJ, Dodd DE, Fowler EH, Kary CD, O'Donoghue J. 1987. A 14-week vapor inhalation toxicity study of methyl isobutyl ketone. Fundam Appl Toxicol. 9(3):380–388.
- Prescott-Mathews JS, Wolf DC, Wong BA, Borghoff SJ. 1997. Methyl tert-butyl ether causes alpha2u-globulin nephropathy and enhanced renal cell proliferation in male Fischer-344 rats. Toxicol Appl Pharmacol. 143(2):301–314.
- Prescott-Matthews JS, Poet TS, Borghoff SJ. 1999. Evaluation of the in vivo interaction of methyl tert-butyl ether with alpha2u-globulin in male F-344 rats. Toxicol Appl Pharmacol. 102:524–536.
- Rice JM, Baan RA, Blettner M, Genevois-Charmeau C, Grosse Y, McGregor DB, Partensky C, Wilbourn JD. 1999. Rodent tumors of urinary bladder, renal cortex, and thyroid gland in IARC monographs evaluations of carcinogenic risk to humans. Toxicol Sci. 49(2):166–171.
- Ridder GM, Von Bargen EC, Alden CL, Parker R. 1990. Increased hyaline droplet formation in male rats exposed to decalin is dependent on the presence of alpha2u-globulin. Fundam Appl Toxicol. 15(4):732–743.
- Robinson M, Bruner RH, Olson GR. 1990. Fourteen- and ninety-day oral toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley rats. J Am Coll Toxicol. 9(5):525–540.
- Roy AK, Raber DL. 1972. Immunofluorescent localization of 2u-globulin in the hepatic and renal tissues of rat. J Histochem Cytochem. 20(2):89–96.
- Roy AK, Schiop MJ, Dowbenko DJ. 1976. Regulation of the hepatic synthesis of alpha2u-globulin and its corresponding messenger RNA in maturing rats. FEBS Lett. 70(1):137–140.
- Roy AK, Nath TS, Motwani NM, Chatterjee B. 1983. Age-dependent regulation of the polymorphic forms of alpha2u-globulin. J Biol Chem. 258:10121–10127.
- Saito A, Sasaki T, Kasai T, Katagiri T, Nishizawa T, Noguchi T, Aiso S, Nagano K, Fukushima S. 2013. Hepatotumorigenicity of ethyl tertiary-butyl ether with 2-year inhalation exposure in F344 rats. Arch Toxicol. 87(5):905–914.
- Saitta B, Jalili MF, Zohoorkari H, Rao R, Hallows KR, Baty CJ, Pastor-Soler NM. 2019. Ex vivo kidney slice preparations as a model system to study signaling cascades in kidney epithelial cells. Methods Cell Biol. 153:185–203.
- Shaw PH, Held WA, Hastie ND. 1983. The gene family for major urinary proteins: expression in several secretory tissues of the mouse. Cell. 32(3):755–761.
- Short BG, Burnett VL, Swenberg JA. 1989. Elevated proliferation of proximal tubule cells and localization of accumulated alpha 2u-globulin in F344 rats during chronic exposure to unleaded gasoline or 2,2,4-trimethylpentane. Toxicol Appl Pharmacol. 101(3):414–431.
- Short BG, Steinhagen WI, Swenberg JA. 1989. Promoting effects of unleaded gasoline and 2,2,4-trimethylpentane on the development of atypical cell foci and renal tubular cell tumors in rats exposed to N-ethyl-N-hydroxyethylnitrosamine. Cancer Res. 49(22):6369–6378.
- Simon TW, Simons SS Jr., Preston RJ, Boobis AR, Cohen SM, Doerrer NG, Fenner-Crisp PA, McMullin TS, McQueen CA, Rowlands JC. 2014. The use of mode of action information in risk assessment: quantitative key events/dose-response framework for modeling the dose-response for key events. Crit Rev Toxicol. 44 Suppl 3:17–43.
- Swenberg JA. 1993. Alpha 2u-globulin nephropathy: Review of the cellular and molecular mechanisms involved and their implications for human risk assessment. Environ Health Persp. 101(Suppl 6):39–44.
- Swenberg JA, Lehman-McKeeman LK. 1999. Alpha2u-urinary globulin associated nephropathy as a mechanism of renal tubule cell carcinogensis in male rats. In: Capen CC, Dybing E, Rice JM, Wilbourn JD, editors. Species differences in thyroid, kidney and urinary bladder carcinogenesis. IARC Scientific Publications No. 147. Lyon: International Agency for Research on Cancer.
- Swenberg JA, Short B, Borghoff S, Strasser J, Charbonneau M. 1989. The comparative pathobiology of alpha 2u-globulin nephropathy. Toxicol Appl Pharmacol. 97(1):35–46.
- U.S. EPA. 1991. Alpha2u-globulin: association with chemically induced renal toxicity and neoplasia in the male rat (Risk Assessment Forum). Washington, DC: U.S. Environmental Protection Agency. EPA/625/391/019F; NTIS PB92143668.
- U.S. EPA. 2019. Memorandum from Andrew R. Wheeler, Administrator to Associate Deputy Administrator. Directive to prioritize efforts to reduce animal testing (2019). Available from: https://www.epa.gov/sites/production/files/2019-09/documents/image2019-09-09-231249.pdf.
- U.S. EPA. 2021. Toxicological review of ethyl tertiary butyl ether. EPA/635/R-20/400Fa [accessed 2021 Sep 7]. Available from: https://iris.epa.gov/static/pdfs/1034tr.pdf.
- Vandoren G, Mertens B, Heyns W, Van Baelen H, Rombauts W, Verhoeven G. 1983. Different forms of alpha 2u-globulin in male and female rat urine. Eur J Biochem. 134(1):175–181.
- Williams TM, Borghoff SJ. 2001. Characterization of tert-butyl alcohol binding to alpha2u-globulin in F-344 rats. Toxicol Sci. 62(2):228–235.
- Wood C. 2020. Alpha2u-microglobulin cytotoxicity leading to renal tubular adenomas and carcinomas (in male rat) [accessed 2020 Jul 3]. Available from: https://aopwiki.org/aops/105.
- Yeung CK, Himmelfarb J. 2019. Kidneys on chips: emerging technology for preclinical drug development. Clin J Am Soc Nephrol. 14(1):144–146.
