Abstract
Anthropogenic chemicals are ubiquitous throughout the environment. Consequentially, humans are exposed to hundreds of anthropogenic chemicals daily. Current chemical risk assessments are primarily based on testing individual chemicals in rodents at doses that are orders of magnitude higher than that of human exposure. The potential risk from exposure to mixtures of chemicals is calculated using mathematical models of mixture toxicity based on these analyses. These calculations, however, do not account for synergistic or antagonistic interactions between co-exposed chemicals. While proven examples of chemical synergy in mixtures at low doses are rare, there is increasing evidence that, through non-conformance to current mixture toxicity models, suggests synergy. This review examined the published studies that have investigated exposure to mixtures of chemicals at low doses in mammalian in vivo systems. Only seven identified studies were sufficient in design to directly examine the appropriateness of current mixture toxicity models, of which three showed responses significantly greater than additivity model predictions. While the remaining identified studies were unable to provide evidence of synergistic toxicity, it became apparent that many results of such studies were not always explicable by current mixture toxicity models. Additionally, two data gaps were identified. Firstly, there is a lack of studies where individual chemical components of a complex mixture (>10 components) are tested in parallel to the chemical mixture. Secondly, there is a lack of dose-response data for mixtures of chemicals at low doses. Such data is essential to address the appropriateness and validity of future chemical mixture toxicity models.
1. Introduction
A wide variety of natural and anthropogenic chemicals are found throughout the environment in air, water, food, soil, and dust. Sources of such environmental chemicals (ECs) include agrochemical food residues, consumer products, industrial chemical effluent, occupational use, and pharmaceutical or recreational drugs, to name but a few. Biomonitoring of EC exposure is routinely performed by various agencies across the world, which commonly see a range of ECs in blood, plasma, and urine samples from across the general population. These include heterocyclic amines, organochlorides, polychlorinated biphenyls (PCBs), polychlorinated dibenzofurans, polycyclic aromatic hydrocarbons (PAHs), metals and metalloids, and volatile organic compounds (VOC) (U.S. HHS Citation2019). These components, at high doses, have a diverse range of toxicological profiles; many are known to be carcinogenic, hormonally active, hepatotoxic, nephrotoxic, and/or neurotoxic. Co-exposure to ECs from multiple sources, or accumulation of EC exposure over time, is of increasing concern to both regulators and the public, however, the exposome (the totality of EC exposure over a lifespan) is particularly difficult to risk assess (Rappaport Citation2011; Sarigiannis and Karakitsios Citation2018) and may be having an impact on increasingly prevalent chronic diseases and viral susceptibility (Tsatsakis et al. Citation2020).
Within traditional chemical risk-assessment, points of departure (PODs) such as the no observable adverse effect level (NOAEL), the highest administered dose which did not produce a statistically significant toxicological effect, are crucial values to determine for every chemical. PODs are defined relative to toxicological dose-response curves for individual chemicals and have been cornerstones of toxicology for decades (reviewed by Dorato and Engelhardt Citation2005). Indeed, the idea of a NOAEL was first conceived in the 16th century by the “father of toxicology” – Paracelsus (Temkin Citation1941). PODs are used to determine the tolerable daily intake (TDI) for a chemical, by dividing PODs by safety factors for inter- and intra-species variance (usually around 100–1000). While invaluable in the context of individual chemical toxicological assessments, the toxicological profile of a chemical mixture is not just the sum of the effects of the component chemicals but may be altered by additive and/or synergistic interactions between chemicals in the mixture, even where each component chemical is below accepted individual PODs (here described as “low dose” mixtures). This has led to calls for an urgent assessment of the effects of chemical mixtures on human and environmental health (Bergman et al. Citation2012; Ribeiro et al. Citation2017; Drakvik et al. Citation2020).
Assessing risk from exposure to chemical mixtures poses many unique problems, including the nearly infinite number of possible chemical combinations, interactions between chemicals within mixtures, and the attribution of responses to component chemicals (Bopp et al. Citation2018). There are two main approaches for evaluating risk from exposure to chemical mixtures: whole-mixture and component-based. Whole-mixture methodologies provide a comprehensive assessment of a specific mixture where unidentified chemical components and inter-component reactions are maintained. This “top-down” approach is analogous to the assessment methods used for individual chemicals (Hernández et al. Citation2017). Whole-mixture methodologies, however, are not always feasible, due to variation between chemical mixtures, changes due to chemical degradation or metabolism, and factors that affect environmental load, over time. In addition, whole-mixture methodologies do not identify which chemicals within the mixture are responsible for a response(s), nor any interactions between chemicals within the mixture. Component-based methodologies use a “bottom up” approach, where a limited number of chemicals with defined concentrations are mixed and tested, or toxicological information for defined chemical components within a mixture are analysed with additivity models, to assess the risk from mixed chemical exposure (Heys et al. Citation2016; Hernández et al. Citation2017). Additivity models, however, assume no compounding toxicodynamics or toxicokinetics, and the required toxicological information for such models can be inconsistent, or absent (Boobis et al. Citation2011) in particular regarding in vivo data pertaining to chemical interactions (synergy/antagonism) (Heys et al. Citation2016).
Current models to define and predict mixture toxicity fall into four categories: dose addition (DA), response addition (RA, also known as independent action), effect summation (ES), and integrated addition (IA) (Rider et al. Citation2018). Appropriate choice of model is critical for accurate predictions of mixture toxicity, however equally important is which chemicals within a mixture are grouped together for mixture risk assessment – a subject deeply debated (Kortenkamp Citation2020). Indeed, these two considerations are connected, and while similarities exist between toxicological models for chemical mixtures, the model which a chemical mixture is thought to follow most closely depends on the individual mixture components. Toxicological similarities between mixture components may occur at one or more levels (Rider et al. Citation2018). The highest form of similarity is chemicals that share a common active metabolite, as is the case of benzyl butyl phthalate and dibutyl phthalate (which assumes an inactive parent molecule). Next similar are compounds that share a molecular initiating event, and whose toxic events, therefore, occur via identical pathways, as with parathion and chlorpyrifos which are both acetylcholinesterase inhibitors. At the next level of similarity, which is less defined, chemicals share adverse outcome pathways (AOPs), but the convergence of different initiating events may occur at several key events in that pathway. For example, perchlorates and dioxin both reduce circulating thyroid hormone concentrations, but they act to reduce the production of and increase the elimination of, thyroid hormones, respectively (Boas et al. Citation2006). Less similar still are chemicals that induce toxicity within the same organ system but via different mechanisms. For example, caffeine and ephedrine can individually produce cardiotoxicity through separate mechanisms, but when co-administered act synergistically to produce enhanced toxicity (Dunnick et al. Citation2007). The toxic additivity of chemicals at this level of similarity within a mixture is a topic of debate, as they may or may not compound each other. Finally, toxicologically least similar are chemicals that share a common disease outcome. For example, diethylstilbestrol and cyclophosphamide are both recognised carcinogens but act via different mechanisms of action and can affect different organ systems. Here, the nature of the disease is of greater importance.
Reports indicate advances in terms of hazard identification, exposure and risk assessment, and subsequent risk management relative to co-exposure to chemicals in mixtures (reviewed by Bopp et al. (Citation2019)) as well as harmonisation of methodologies for mixture toxicity assessment (More and Hardy, Citation2019). However, much of the focus of this work has been on intentional mixtures, such as formulations within pesticides or cosmetic products, and does not cover doses below PODs (Rotter et al. Citation2018). Furthermore, even less attention has been paid to ‘real-life’ chemical exposure, which is characterised as being vastly more complex and varied mixtures, at doses far below PODs. This pattern of exposure is that to which the wider human and wildlife populations are exposed throughout their lifetimes. Regulatory agencies often review the literature on mixture toxicity. Although varying conclusions by different bodies, the general approach is to separate chemicals into groups with distinct modes of action before individually applying additive models to each group for risk assessment, noting that in the case of knowledge gaps dose additivity should be assumed and that interactions between chemical components are rare, and generally only occur at mid to high doses (U.S. EPA Citation2007; WHO Citation2009; SCHER/SCCS/SCENIHR Citation2013; NAS Citation2017; OECD Citation2018). Few publications, however, focus on EC mixtures around or below POD doses, or chemical interactions at these doses. A review of the literature concerning low-dose mixtures of pesticides from 1985-1998 concluded that exposure to such mixtures is not a source of concern to human health (Carpy et al. Citation2000). An EU commissioned a report on the state of the art of mixture toxicity risk assessment and concluded that generally, mixture toxicity risk assessments by dose addition models were reliable. However, they also noted several important examples of synergy which highlight the need for clarity as to which chemicals to group in cumulative risk assessments (Kortenkamp et al. Citation2009). A review of low EC dose synergy noted synergy as rare and that the effects of synergy did not exceed additivity models by more than a factor of 4 (Boobis et al. Citation2011). The most recent and most extensive review of low-dose mixtures was performed by the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC). Assessing mixture toxicity studies where component concentrations were at or below NOAELs and examining toxicity reported as more than expected based on current models of mixture exposure (i.e. more than additivity in the case of similar chemicals, and more than independent action in the case of dissimilar chemicals), ECETOC concluded that there is no evidence of a risk to human health from exposure to complex mixtures of components where each is below regulated levels (ECETOC Citation2012). However, to only consider effects provably greater than model predictions, rather than effects unsatisfactorily explained by model predictions, is tantamount to requiring proof of synergistic toxicity – a considerably larger endeavour. To place the burden of proof on the literature rather than the toxicity model might also be considered contrary to the traditionally conservative nature applied in toxicology. Still, despite this requirement, over 5% of studies reviewed by the ECETOC showed a deviation from additivity that suggested synergistic chemical effects (ECETOC Citation2012).
Here we critically review the literature which has investigated the effects of exposure to chemical mixtures in mammalian in vivo systems, with each component at or below respective POD doses. The review covers the period of 2000 to 2020, inclusive, as a resource collating the most recent literature for those interested in mixtures research and to identify and highlight areas in need of additional research.
2. Methodology
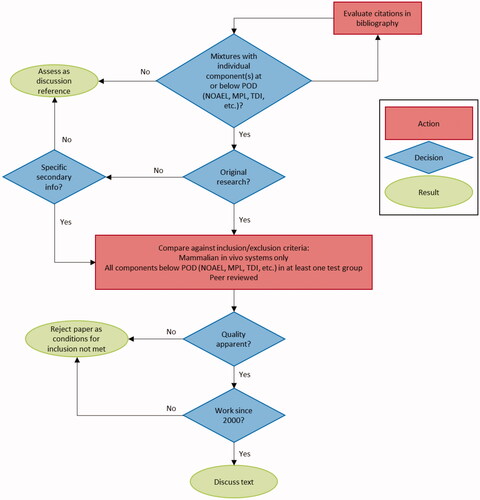
A literature search was conducted across multiple journal databases (PubMed, Google Scholar, and SCIRUS) for peer-reviewed studies published between 2000 and 2020 relating to low-dose mixtures of chemicals in mammalian in vivo systems. Low dose mixtures were defined as those in which all components were at or below their POD. Identified literature with at least one experimental group fulfilling these criteria was included for discussion. illustrates the logic underpinning the literature search and subsequent evaluation for inclusion. To identify any missed literature a wider search of non-scientific sources (Google) was performed, and publication histories of relevant authors or research groups were interrogated. For each identified study which met all inclusion criteria, detailed experimental information was extracted (mixture components, dosing levels and regime, route, species, duration, and timing). Reported significant outcomes relevant to exposure were captured and summarised. Results from the authors’ original analyses were used, and no statistical re-analysis was performed.
3. Results
3.1. General findings
Literature searches identified sixty-eight research articles which met the inclusion criteria. Fifty-four reported findings from studies that used component-based methodologies, and fourteen from studies that used whole-mixture methodologies. Detailed experimental information can be found in Supplementary Tables.
Thirty research articles that used component-based methodologies tested mixtures with at least one component at or close to its NOAEL. In nineteen of these research articles, this was the lowest dose tested. Twenty-one research articles that used component-based methodologies had at least one group exposed to a mixture with all components at or below TDI values. Research articles with component-based methodologies were divided into groups, firstly on the number of components (simple mixtures that contained ≤10 individual chemicals, complex mixtures that contained >10), and secondly simple mixtures were divided based on the commonality between the characteristics of individual mixture components. These secondary groups were antiandrogen mixtures, antiandrogen plus xenoestrogen mixtures, mixed endocrine disrupting mixtures, neurotoxic mixtures, and mixed mode of action mixtures.
Identified research articles that used a whole-mixture methodology were from studies that used one of two experimental models: the biosolid treated pasture (BTP) sheep model and a disinfection by-product model. As such, that literature was grouped by model type, and then for BTP articles also as a function of age; effects in the foetus, juveniles, and adult offspring.
3.2. Component-based methodologies
3.2.1. Antiandrogen mixtures
Six research articles tested mixtures of known antiandrogens and chemicals with antiandrogenic action. Phthalates were included as while not strictly antiandrogens, phthalates induce antiandrogenic effects through non-androgen receptor-mediated mechanisms. Effects on sexual development were reported in male rat offspring following in utero exposure via parental oral dosing with antiandrogen mixtures with individual chemicals at/below NOAEL. Exposure to a mixture of vinclozolin, procymidone, linuron, prochloraz, BBP, DBP, and DEHP at NOAEL resulted in increased areola numbers at post-natal day (PND) 13 (Rider et al. Citation2008). Nipple retention (NR) was observed following exposure to a mixture of vinclozolin, procymidone, linuron, prochloraz, BBP, DBP, and DEHP, DiBP, DiHP, and DPP at NOAEL (Rider et al. Citation2010). Exposure to a mixture of vinclozolin, finasteride, prochloraz, and DEHP at NOAEL resulted in reduced anogenital distances (AGD) at birth and increased nipple retention (NR) at PND13 (Christiansen et al. Citation2009). However, a study by Schneider et al. (Citation2017) reported no effects of chemical exposure on male or female offspring at PND1, 12, 20, or 83, following gestational and lactational exposure to a mixture of flutamide, prochloraz, and vinclozolin at TDI and NOAEL (parental oral and direct oral dosing after weaning). The apparent lack of an effect of exposure to the chemical mixture was despite the observation of multiple statistically significant physiological effects, both on the mothers, such as increased gestation length and lower oestradiol concentrations, and on male offspring, which exhibited decreased progesterone concentrations and decreased absolute cauda, epididymis, and relative bulbourethral gland weights. These effects were not attributed to chemical exposure due to the absence of significant effects in other organs which are known to be more sensitive to androgen disruption, and the limited magnitude of the observed effects. In the same study, however, rats were exposed to flutamide, prochloraz, and vinclozolin at LOAEL, both individually and as a mixture (Schneider et al. Citation2017). In these groups, they observed greater than additivity (i.e. potentially synergistic) in the effects of chemical mixture exposure with regards to increased NR and delayed sexual maturation in male offspring, and increased androstenedione in mothers (Schneider et al. Citation2017). This would indicate that the lack of effects seen at or below NOAEL may be due to the limited number of chemical components within the mixture, rather than a lack of synergy between mixture components.
As noted above, the authors reported no effects from co-exposure to flutamide, prochloraz, and vinclozolin at NOAEL or TDI, but greater than additive effects when the chemicals were administered at LOAEL (Schneider et al. Citation2017). More in-depth comparisons of chemical effects to additivity models were reported in the previous three studies (Rider et al. Citation2008, Citation2010; Christiansen et al. Citation2009), which investigated more complex mixtures of antiandrogens. Rider et al. (Citation2008) reported logistically regressed ED50 values for hypospadias and undescended testes from co-exposure to vinclozolin, procymidone, linuron, prochloraz, BBP, DBP, and DEHP, which differed with statistical significance to those from additivity models (DA, TEF, RA, IA), with each model predicating higher ED50 values than those observed, while models based on additivity (DA and/or TEF) were accurate for predicting AGD shortening, epididymal agenesis, ventral prostate weight reductions, epididymal and seminal vesicle weight, and gubernacular agenesis. On balance, Rider et al. (Citation2008) reported that additive toxicity models were generally accurate despite mechanistically distinct chemicals examined, which would have been risked assessed by RA or IA. Similarly, with regards to vinclozolin, finasteride, prochloraz, and DEHP co-exposure, the differences between effective doses for producing AGD shortening, cleft phallus, and malformations in general derived from observations and from additivity models (DA, RA) were statistically significant, with models predicting higher effective doses than those observed (Christiansen et al. Citation2009). However, Rider et al. (Citation2010) showed DA additivity model predictions to be accurate for all studied endpoints (AGD reduction, various malformations, NR, organ weights) except ventral prostate weight, where the effect magnitude was under-predicted. In Rider et al. (Citation2010) non-phthalate components were identically dosed as in Rider et al. (Citation2008), however, 6 phthalates were used instead of 3 while total phthalate dosage was maintained. That DA model predictions were accurate in Rider et al. (Citation2010) yet inaccurate in Rider et al. (Citation2008) suggests unknown interactions between phthalates and other mixture components, yet current guidelines would use an RA approach to these mixtures, which gave inaccurate predictions in both Rider et al. (Citation2008) and Rider et al. (Citation2010). Discrepancies between model predictions and observations despite dissimilarities between antiandrogenic mechanisms across mixture components support concerns regarding compounding and/or synergistic effects of antiandrogens on the foetus, and of improper considerations of additivity/synergy between mechanistically distinct antiandrogens by currently employed mixture toxicity models.
Two studies were identified that investigated mixtures containing only phthalate plasticisers. Exposure of juvenile male rats to a mixture of six phthalates (DMP, DEP, DBP, BBP, DEHP, and DnOP), at doses equivalent to 1x or 0.1x NOAEL, via dietary exposure for 15 weeks, resulted in reduced body weights and altered the weight of several vital organs (Gao et al. Citation2017). The exposed mice had lower testosterone and higher luteinizing hormone concentrations compared to the controls and the testes showed increased deciduous spermatids (at both dose levels), and reduced concentrations of several proteins involved in testicular development and spermatogenesis (at 1x NOAEL) (Gao et al. Citation2017). Effects on body weight and the reproductive system were also seen when male mice were exposed to a mixture of 3 phthalates with 2 alkyl-aromatics (DEHP, DBP, BBP, NP, and 4-tert-octylphenol) at doses equivalent to individual TDI values from conception to PND60 via drinking water. In this instance, the mixture exposed mice exhibited increased body weights and reduced relative testes weights compared to the controls (Buñay et al. Citation2018). Within the testes of exposed mice, the seminiferous tubules showed multiple signs of damage: tubule widths were reduced, germ cell exfoliation was increased, a greater proportion of tubules were without lumen, and steroidogenic gene expression was altered. Germ cells also showed signs of disruption: an increase in germ cell death, apoptotic signalling, and a greater proportion at an undeterminable stage of spermatogenesis due to the high level of degeneration/atrophy (Buñay et al. Citation2018). Of these two studies, only that of Buñay et al. (Citation2018) also tested for the effects of phthalates individually in parallel to the mixture (at TDI doses), where each effect observed in response to the mixture was also observed in response to an individual chemical, however, the number of animals affected was, in general, greater when exposed to the mixture of chemicals.
3.2.2. Antiandrogen plus xenoestrogen mixtures
Seven research articles tested mixtures of known antiandrogens and/or xenoestrogens. Six of these investigated offspring effects following gestational and lactational exposure to a mixture of genistein and vinclozolin via parental oral dosing, in rats. Both chemicals were administered at 1 mg/kg/day, which is below the NOAEL for vinclozolin and at the levels of genistein expected for animals on a soya-bean diet, which is accepted as below the TDI for genistein. Following exposure to this mixture, litter sizes were significantly smaller, and pups were significantly heavier than unexposed controls (Eustache et al. Citation2009). While litter sizes were also smaller, and with heavier pups, following exposure to genistein alone, the effect was greater when mothers were exposed to the mixture of genistein and vinclozolin. Mixture exposed male offspring exhibited reduced testosterone secretion upon ex vivo stimulation at PND3 (Lehraiki et al. Citation2011), AGD shortening and more frequent immature penile development at weaning, and decreased epididymis and seminal vesicle weights, sperm quality and quantity, and increased FSH and oestradiol at PND80 (Eustache et al. Citation2009). However, of these effects, all except reduced seminal vesicle weight and lower sperm quantity were also seen in animals dosed with either genistein or vinclozolin alone. Interestingly, microarray analysis of testicular gene expression in animals at PND80 also demonstrated a correlation between the effects of the low dose mixture and individual exposure to a 30x higher vinclozolin dose, which would suggest a synergistic action between the two chemicals when present in the mixture (Eustache et al. Citation2009). Mixture-exposed female offspring exhibited earlier vaginal opening, and multiple histopathological changes in mammary gland structure at PND35, including increased duct sizes, thicknesses, and cellular proliferation, however, some of these effects were also noted in animals dosed with either genistein or vinclozolin alone (Saad et al. Citation2011). Female rats exposed to genistein and vinclozolin from conception through weaning via parental oral dosing also exhibited fewer striated submandibular salivary ducts, an increased area of striated salivary ducts, and decreased proliferation with lower expression of growth factors EGF, NGF, and TGFα in submandibular salivary glands, however, most of these findings were also seen in animals exposed to just vinclozolin, although the effect size was greater when exposed to the mixture (Kouidhi et al. Citation2012).
Direct and multigenerational effects on bone formation in the paw and spine following gestational exposure to genistein and vinclozolin alone or in combination with BPA have been reported. Specifically, alterations in forepaw digit lengths of F1 (exposed in utero) and F2 (not directly exposed) males towards a more feminine digit length ratio have been reported following F1 gestational exposure to mixtures of various combinations of genistein at sub-NOAEL, vinclozolin at NOAEL or TDI, and BPA at TDI (Auger et al. Citation2013). Histopathological alterations in the vertebral growth plates (intertransverse apophysis width increases and v5 to v8 vertebral length shortening) have been reported in F1 females, but not males, at PND30-110 following similar exposure to genistein, vinclozolin and BPA, however these effects were also seen when F1 animals were exposed to individual chemicals and did not continue into the F2 generation (Auxietre et al. Citation2014). Interestingly, F1 males exposed to vinclozolin and/or genistein at sub-NOAEL, alone or as a mixture, were unaffected, while co-exposure of either vinclozolin at the lower TDI dose, or genistein, at sub-NOAEL, with BPA at TDI, resulted in histopathological alterations in the vertebral growth plates, but these findings were not present following exposure to genistein, vinclozolin and BPA, at these levels (Auxietre et al. Citation2014). In all the above studies, where individual chemicals were tested in parallel to mixtures of the chemicals, some effects were also noted from exposure to the individual chemicals, in some cases, effects were only noted from exposure to an individual chemical. However, mostly, effects were more numerous and/or greater in size when animals were exposed to the mixture.
While the above studies noted compounding effects from chemicals when administered as a mixture, compared to when administered individually, the remaining study concluded that co-exposure to certain chemicals could, through counteraction measures, result in fewer physiological effects. This conclusion was based on the observation that, compared to controls, mice exposed (via parental oral dosing during gestation and lactation) to sub-LOAEL doses of DEHP (daily) or BPA (daily), or TCDD (single treatment) had lower body weights (PND14 and PND21), increased relative brain weights at PND14, and lower absolute brain weights at PND42, with changes in midbrain dopaminergic nuclei at both PND14 and PND42. However, when animals were dosed with the chemicals as a mixture, body weight was unaffected, brain weight (relative and absolute) was increased at PND14, and there were no signs of changes in midbrain dopaminergic nuclei (Tanida et al. Citation2009).
3.2.3. Mixed endocrine disrupting chemical mixtures
Fourteen research articles tested mixtures of known EDCs. One study investigated the effects of drinking water disinfection by-products at higher levels than maximum contaminant levels (MCLs) but with two groups (500x and 1000x MCL) ≤NOAEL (0,5x and 1x, respectively). Pregnant rats and their offspring were exposed from F1 GD0 to F2 PND6 via drinking water. Water consumption was reduced in treated dams intermittently during gestation and consistently throughout lactation at 1000x MCL, and in F1 females at 500x and 1000x MCL. F1 pup weights were reduced in males at PND26 and in females at PNDs 21 and 26, and the onset of puberty was delayed in both F1 sexes at 1000x. No effects were seen in the F2 generation (Narotsky et al. Citation2015).
Multiple research articles were identified that had investigated mixtures of endocrine disrupting pesticides at low doses. When juvenile rats were exposed, via a standard diet, to three azole fungicides (cyproconazole, epoxiconazole, and prochloraz) at individual doses equivalent to 1x NOAEL and 0.01x NOAEL (TDI) either no adverse effects were seen (Schmidt et al. Citation2016), or effects were noted on organ weights and circulating hormone levels, but these effects were not attributed to exposure to the chemical mixture as they were only seen at the 0.01x NOAEL dose and not at 1x NOAEL (Rieke et al. Citation2017). However, gestational, or gestational and lactational exposure to 5 fungicides of different classes (procymidone, mancozeb, epoxiconazole, tebuconazole, and prochloraz), via parental oral gavage, at individual doses equivalent to 0.083x – 1x NOAEL, did result in adverse reproductive effects. In fact, increased gestation duration, pup mortality and birthing complications were so severe at 0.75x and 1x NOAEL doses that this part of the experiment had to be discontinued (Jacobsen et al. Citation2010; Hass et al. Citation2012). Against the lower doses, the pups exhibited reduced birth weights, females had increased AGD, whereas males had decreased AGD, and increased incidence of genital dysgenesis and NR (Jacobsen et al. Citation2010; Hass et al. Citation2012). At weaning, relative liver weights were reduced in both sexes, and in males prostate and epididymis weights were reduced (Jacobsen et al. Citation2010). When allowed to mature, the relative weights of sexual organs were reduced (PND16 and 280), and reduced sperm counts, and increased prostate hyperplasia were observed (PND280) (Jacobsen et al. Citation2012). When the mixture dose-response data were compared to mixture toxicity models (DA and IA) using dose-response data from individual chemicals, a synergistic effect was reported for the mixture on gestation length and NR, and potentially also towards genital malformations however synergy could not be distinguished from a marked joint effect as no significant genital malformations were observed in response to chemicals individually (Hass et al. Citation2012). Mice that received, from conception to PND98, dietary exposure to a mixture of the pesticides atrazine, chlorpyrifos, and endosulfan, at doses equivalent to individual TDI values, expressed increased circulating glucose concentrations at PND21 and PND98, and by PND98 a significant reduction in body weight was observed (Demur et al. Citation2013). At PND21, the EC-exposed animals, of both sexes, were characterised by lower levels of many amino acids and nutrients compared to the controls, however, by PND98 this profile was reversed in the males (Demur et al. Citation2013). Effects on cognition from gestational exposure to atrazine in combination with 3 different EDCs (PFOA, BPA, and TCDD), all at doses equivalent to TDI values or below NOAEL values, have also been reported. The results indicated reduced environmental habituation in exposed mice of both sexes, and males were found to display decreased exploratory behaviour, short-term memory, and attention to task, relative to the controls (Sobolewski et al. Citation2014). In both above cases which looked at the effects of chemical mixtures including atrazine, effects from exposure to individual chemicals were also noted, however, the observations were of greater effect sizes when chemical exposure was to a mixture, which indicates at least additivity despite separate toxicological mechanisms.
Administration of a mixture of TCDD, PCB-153, DEHP, and BPA through dietary supplementation equivalent to TDI induced oestrogeno-mimetic and metabolic effects in mice, effects which were also shown to be influenced by diet. At 7-12 weeks old, various metabolic changes were observed in male and female offspring of parents exposed prior to mating, through gestation and lactation, and which received direct exposure after weaning, via supplementation of a standard (Labaronne et al. Citation2017) or high-fat-high-sucrose (HFHS) diet (Naville et al. Citation2013, Citation2015), with the EC mixture. These include changes in glucose tolerance, circulating and hepatic cholesterol, triglyceride, fatty acid levels, and adipose tissue mass. In the HFHS diet groups, changes in hepatic metabolic gene expression were reported in response to the mixed chemical exposure, which included increased aryl hydrocarbon receptor (Ahr) and peroxisome proliferator-activated receptor α (Pparα) gene expression, and alterations in the expression patterns of various xenobiotic transforming and metabolic enzymes (Naville et al. Citation2013, Citation2015). In animals which received the chemical mixture in the standard diet, enriched hepatic gene expression was found relative to drug and xenobiotic metabolism, steroid biosynthesis, and fatty acid metabolism (Labaronne et al. Citation2017). A follow-up study in juvenile male mice compared the effect of standard or HFHS with and without EC supplementation (Naville et al. Citation2019). Metabolic changes like those previously recorded against an HFHS diet were reported in response to EC exposure, however, several effects reversed in direction between standard and HFHS diets or were only present when administered in one type of diet (Naville et al. Citation2019). Studies in ovariectomised female mice receiving the same chemical exposure (TCDD, PCB-153, DEHP, and BPA at TDI equivalent doses in HFHS diet) demonstrated oestrogeno-mimetic effects from exposure to the chemical mixture from prior to conception to a juvenile age, with additional metabolic alterations including insulin and glucose tolerance (worsened with EC exposure in sham-operated but alleviated with EC exposure in ovariectomised mice) and increased hepatic triglycerides (Julien et al. Citation2018, Citation2019). EC mixture exposure after ovariectomy, with or without oestrogen replacement, also resulted in tissue and steroid replacement-specific effects on steroid hormone production and several oestrogen pathways (Julien et al. Citation2019). Consistent across these studies was the observation of an increase in hepatic oestrogen receptor expression, which was also observed in male mice exposed to the chemical mixture via standard and HFHS diets (Julien et al. Citation2018, Citation2019; Naville et al. Citation2019). None of the above studies which tested mixtures of TCDD, PCD153, DEHP, and BPA concurrently investigated the physiological effects of individual chemical exposure.
3.2.4. Neurotoxic mixtures
Five research articles tested mixtures of chemicals which alone can produce neurotoxic effects. Although the primary toxicity of organophosphorus insecticides is that of neurotoxicity, the identified research which investigated the effects of organophosphorus insecticide mixtures demonstrated hepatic and nephrotoxic effects. Adult male rats, exposed to a mixture of diazinon, chlorpyrifos, malathion, and profenofos for 28 days at doses equivalent to individual NOAEL values, via oral gavage, exhibited reduced body weight and increased relative liver weight (Mossa et al. Citation2011). Upon closer examination, liver damage was evident, as indicated by histopathological changes (dilatation and congestion of central veins sinusoids, dilated cystic bile ducts, oedemas, and hepatocyte degradation) as well as serum clinical chemistry indicative of liver damage (increased ALT, AST, ALP, and ChE) (Mossa et al. Citation2011). Whereas the exposure of rats to a mixture of dichlorvos, dimethoate, acephate, and phorate, at doses equivalent to respective individual NOAEL values, for 24 weeks, via drinking water, found no effect on hepatic antioxidative defence mechanisms or lipid peroxidation (Yang et al. Citation2012). However, increased circulating triglycerides, lipoproteins, and cholesterol concentrations, and a greater incidence of renal tubular epithelial cell swelling and granular degeneration have been reported following the same EC mixture exposure (Du et al. Citation2014). Organophosphorus insecticides were tested only individually in parallel with the chemical mixtures in the study by Yang et al. (Citation2012), where no effects were seen at NOAEL following exposure to individual chemicals or the mixture, and they did not compare observations to mixture toxicity models.
Despite pyrethroids being the most widely used commercial insecticide, only three research articles were identified that investigated low-dose pyrethroid mixtures. Mild signs of neurotoxicity were seen in adult rats orally dosed with a mixture of 11 pyrethroid pesticides (S-bioallethrin, permethrin, cypermethrin, deltamethrin, esfenvalerate, β-cyfluthrin, fenpropathrin, tefluthrin, λ-cyhalothrin, bifenthrin, resmethrin) at doses below individual NOAEL values. For 8 h after exposure to the pyrethroid mixture, at both 43% and 85% individual NOAEL values, animals displayed mild whole-body tremors and reduced motor activity (Wolansky et al. Citation2009). When rats were dosed with all pesticides simultaneously, the mixture produced effects which conformed well to DA model predictions based on the effects of each individual pesticide, but when the chemicals were dosed in three sets to align the effect maxima of individual chemicals (at 1, 2 or 4 h) the threshold was 3.7x slower than dose addition model predictions. As this reduction in threshold was not statistically significant (p = 0.07) the study concluded that the data supported the suitability of DA models for predicting the effects of this pesticide mixture (Wolansky et al. Citation2009) as opposed to there being a synergistic mixture effect. A similar conclusion was reached when it was shown that a mixture of tefluthrin, bifenthrin, cypermethrin, and deltamethrin dosed to juvenile rats at doses determined in a preliminary study to be below that which individually cause a lowering of body temperature, did not influence body temperature as a mixture (Mosquera Ortega et al. Citation2018). Similarly, no significant effects were found on ventilation efficiency, pulmonary perfusion, cardiac output, or metabolic rate in mice following inhalation of prallethrin and phenothrin, each at NOAEL, individually or as a mixture (Santiasih et al. Citation2020).
3.2.5. Mixed mode of action mixtures
Ten research articles studied mixtures of chemicals which alone can produce adverse effects through various modes of action. Nine of these investigated the physiological effects of exposure to mixtures of pesticides with mechanistically distinct modes of action. Two pesticide mixtures induced haematological changes. Dietary exposure to alphacypermethrin, bromopropylate, carbendazim and mancozeb (neurotoxic, hepatotoxic, EDC) at NOAEL equivalent doses, and chlorpyrifos ranging from 0.04x NOAEL to 1x NOAEL, lowered haematocrit, haemoglobin, and red blood counts, with liver and thyroid weights increased, and thymus weight reduced (Jacobsen et al. Citation2004). While exposure to a mixture of alachlor, captan, diazinon, endosulfan, maneb, and mancozeb (neurotoxic, hepatotoxic, EDC), at TDI doses via thrice weekly oral gavage, altered bone marrow colony compositions in terms of cell type proportions and haematopoietic protein levels, reduced liver weight and increased spleen weight, with distinct and distinguishable metabolic profiles for both exposure and sex (Merhi et al. Citation2010). Neither study investigated exposure to individual chemicals in parallel to the mixture.
Reproductive effects were reported in male and female rats following dietary exposure to a mixture of dicofol, dichlorvos, permethrin, endosulfan, and dieldrin (hepatotoxic, neurotoxic, EDC) at NOAEL equivalent doses. Specifically, male rats had reduced sperm motility and increased numbers of immotile sperm (Perobelli et al. Citation2010), while various effects were seen in female rats which appeared to be dependent on strain: decreased oestrous cycle and dioestrus period lengths in Lewis rats, decreased ovarian primordial and primary follicles, antral follicles, and corpora lutea in Sprague-Dawley rats, and interestingly, no statistically significant effects in Wistar rats, the most frequently used experimental strain (Pascotto et al. Citation2015). Individual chemicals were only tested in parallel to the mixture in males, where effects were also observed for some individual chemicals, but the effect size was greater when animals were exposed to the pesticide mixture (Perobelli et al. Citation2010). This could indicate synergistic effects between chemicals within the mixture, however, the study design did not provide dose response relationships appropriate to compare observations to mixture toxicity models. Reproductive effects have also been reported following gestational exposure via maternal dietary supplementation with cyromazine, MCPB, pirimicarb, quinoclamine, thiram, and ziram (reprotoxic, nephrotoxic, neurotoxic, teratogenic), at doses related to 0.05x − 0.375x of the benchmark doses for a 5% birthweight reduction, which was derived from regulatory draft assessment reports (DARs) (Hass et al. Citation2017; Svingen et al. Citation2018). Body weight was reduced in both dams and pups through gestation and from birth to PND16 (Hass et al. Citation2017) following EC mixture exposure, compared to untreated controls. EC exposed male offspring had reduced relative liver and retroperitoneal fat pad weights at PND16, but not at 5-6 months of age (Svingen et al. Citation2018), relative to untreated controls. Overall, at 5-6 months old, no statistically significant differences were observed in the male offspring, whereas the females showed significantly elevated plasma leptin concentrations relative to the controls (Svingen et al. Citation2018). Although pesticides were not tested individually in these studies, the empirically derived chemical mixture LOAEL for birthweight was used to determine the dose level to compare to DAR-derived LOAEL values. As each chemical was at between 0.2x − 0.09x respective LOAELs, a mixture of 6 chemicals producing the reported effects is in approximate agreement with DA model predictions, which supports the appropriateness of the model (Hass et al. Citation2017).
Hepatic effects were reported following 12 months of dietary exposure to a mixture of ziram, chlorpyrifos, thiacloprid, boscalid, thiofanate, and captan (neurotoxic, EDC, hepatotoxic) at TDI equivalent doses in wild type (WT) and Car knockout (KO) C57Bl/6J mice (Lukowicz et al. Citation2018). Exposure to the chemical mixture was associated with increased body weight gain in both strains of mice. WT mice exhibited increased hepatic steatosis and triglycerides, decreased fasting glucose and glucose tolerance, and altered glutathione redox states. KO mice did not exhibit the same hepatic responses, and predictably displayed differential hepatic expression of many detoxifying enzymes and circulating levels of metabolites. Interestingly, subsequent microarray analysis of WT livers identified over 500 genes was upregulated, and over 500 down-regulated, in both sexes in response to chemical exposure but less than 10% of the gene changes were common between the sexes. Pathway analysis also highlighted enrichment in differing pathways between sexes (Lukowicz et al. Citation2018). These results highlight that the effects of exposure to chemical mixtures can be sex-specific or sexually differentiated – an important element when considering studies in which only one sex was examined or where sex is either unconsidered or unreported.
Neurobehavioral effects were reported in male and female rats exposed to diquat, imazamox, bentazone, imazethapyr, tepraloxydim, and acifluorfen (hepatotoxic, teratogenic, genotoxic) via drinking water (Tsatsakis et al. Citation2019b; Sergievich et al. Citation2020). Exposure to the mixture at 0.25x and 1x NOAEL doses resulted in decreased anxiety at 3, 6 and 12 months, with additional changes in research activity and problem-solving (Sergievich et al. Citation2020). Rats exposed to the same mixture but at TDI values for 9 months, either with 100% or 25% RDI of essential vitamins, saw reduced locomotor activity resulting from chemical mixture exposure or vitamin deficiency, but not both (i.e. in vitamin deficient controls and vitamin sufficient treated). Locomotor activity and spatial orientation activity were reported to be increased in exposed animals that received insufficient vitamins, which interestingly also did not present increased anxiety that was seen in control animals that received insufficient vitamins (Tsatsakis et al. Citation2019b). Neurobehavioral effects have also been observed in mice gestationally exposed to a mixture of the flame retardant DecaBDE and lead (EDC, neurotoxic) via subcutaneous osmotic pumps, at doses far below (<0.017x) TDI and reference doses, respectively (Chen et al. Citation2019). In this study, chemical exposure was associated with increased repetitive stereotyped behaviours and impaired spatial learning ability and these behavioural effects were accompanied by increased circulating levels of many cytokines and a reduction in the number of hippocampal neuronal cells. Chen et al. (Citation2019) also noted effects from exposure to DecaBDE or lead alone, especially lead which resulted in equally lowered hippocampal neuronal cell numbers as the mixture exposure group. While the effect size with regards to serum levels of pro-inflammation cytokines was greater when animals were exposed to the mixture, which suggests a synergistic action, the study design did not permit comparison of observations to mixture toxicity model predictions. These results may be of concern as the levels of lead were considerably lower than the accepted TDI.
3.2.6. Complex chemical mixtures
Eleven research articles tested complex chemical mixtures with various toxicological mechanisms. These ten research articles reported findings from five separate experiments, none of which tested mixture chemicals individually. Exposure to 16 organochloride pesticides and 2 heavy metals, for 70 consecutive days, via oral gavage, at the lowest investigated dose level (equivalent to individual TDI, reference dose (RfD), maximum residue limit (MRL), or NOAEL values) resulted in increased epididymal sperm counts and greater natural killer cell activity from ex vivo splenocytes from exposed male rats (Wade et al. Citation2002a). This was accompanied by multiple signs of thyroid toxicity: increased thyroid stimulating hormone (TSH), larger median thyroid follicle areas, and reduced hepatic thyroxine outer-ring deiodinase activity (Wade et al. Citation2002b). Hepatic effects have also been reported in rats following exposure to a mixture of 27 chemicals (8 Heavy metals, 4 pollutants, 3 pesticides, 2 food-derived carcinogens, 2 plasticisers, 2 preservatives, 2 surfactants, 1 disinfectant, 1 food additive, 1 photostabilizer, and 1 polycyclic musk) at values lower than TDI (Hadrup et al. Citation2016). In this study, chemical ratios were based on comparisons to measured concentrations in human samples, and certain chemical classes were grouped into one of that class (e.g. PCBs were represented by PCB153 at a level equal to the sum of all PCBs). As a result, the comparability to individual PODs was reduced. The results indicated that chemical mixture exposure was associated with increased liver weight, macrovesicular changes, and vacuolisation. Hepatic lipid metabolomics also indicated separate and distinguishable metabolic profiles for chemical mixture exposed and control animals, however, direct quantification indicated that only one, unidentified, lipid was significantly changed in response to chemical mixture exposure (Hadrup et al. Citation2016). While these studies support an effect of low-dose chemical mixtures on hepatic function, a study that used a Solt-Farber model of hepatocellular carcinoma demonstrated that a mixture of 12 pesticides of various chemical classes at doses equivalent to TDI had no effect on hepatic carcinogenesis (Perez-Carreon et al. Citation2009).
Two studies investigated the reproductive effects of gestational exposure to a complex mixture of chemicals with a diverse range of toxicological mechanisms. Gestational exposure of rats to 13 chemicals (3 plasticisers, 6 various pesticides, 2 pollutants, 1 preservative, and 1 analgesic) at doses equivalent to individual NOAEL values for AGD or NR, or where NOAEL values were not available LOAEL values divided by a conservative value, indicated specific effects on the reproductive system suggestive of at least additive, potentially synergistic, chemical action. Despite exposure to such low concentrations of mechanistically dissimilar chemicals, male offspring exhibited an increased number of nipples at birth and greater NR at PND13 (Christiansen et al. Citation2012). Administration of a mixture of 18 chemicals (5 pesticides, 3 pharmaceuticals, 9 phthalates, and 1 pollutant) to rats during gestation resulted in male offspring with multiple signs of developmental/reproductive toxicity. Reduced testes, epididymis, and levator ani plus bulbocavernosus muscles (LABC) weights (PND21) were seen in offspring exposed to the mixture from ≤0.125x individual NOAEL, reduced AGD (PND2) and glans penis weights (PND21) from ≤0.25x NOAEL, and increased NR (PND13), seminal vesicle weights, and total malformation rates (PND21) at ≤0.5x and ≤1x NOAEL, (Conley et al. Citation2018).
The remaining five research articles all reported findings from an 18-month rat study which exposed rats via drinking water to a mixture of 13 chemicals (4 preservatives, 4 insecticides, 1 herbicide, 1 fungicide, 1 plasticiser, 1 food additive, and 1 chelating agent) at doses equivalent to 0.25x, 1x and 5x TDI values. The reports detailed clinical observations and serum clinical chemistry at 6 and 12 months of exposure (Docea et al. Citation2018, Citation2019), behavioural tests (Tsatsakis et al. Citation2019c), oxidative stress findings in serum at 12 months of exposure and in organs at 18 months of exposure (Fountoucidou et al. Citation2019), and genotoxic, cytotoxic, and histopathological findings at 18 months of exposure (Tsatsakis et al. Citation2019a). Over the first 12 months, mixture-treated animals had increased weight gain, yet reduced food and water consumption (Docea et al. Citation2018, Citation2019). By 12 months, animals that received the lower two doses of the chemical mixture exhibited increased exploratory behaviour (Tsatsakis et al. Citation2019c). ALT and ALP were increased in animals of each exposure group at 6 and 12 months of exposure, which is indicative of liver damage (Docea et al. Citation2018, Citation2019). At 18 months of exposure deleterious histopathological findings were found in the liver and stomach of animals that received the chemical mixture at 1x and 5x TDI, and in testes, kidneys, lungs, and brains at all dose levels (Tsatsakis et al. Citation2019a). At 12 months of exposure, signs of oxidative stress or adaptive redox capacity were mixed within the chemical mixture exposed groups, but these animals had generally lower protein carbonyl levels than controls (Fountoucidou et al. Citation2019). By 18 months of exposure to the chemical mixture at 0.25x and 1x TDI most tissues showed lower protein carbonyls and thiobarbituric acid reactive substances and higher redox capacity, which suggests that compensatory mechanisms may have become operative in the exposed relative to control animals. However, at 5x TDI, protein and/or lipid oxidation biomarkers were generally increased relative to controls (Fountoucidou et al. Citation2019), indicating sufficient oxidative stress to overcome compensatory responses, and suggesting greater than additivity (potential synergy), as 13 chemicals at 5x TDI (approximately 0.05x NOAEL) would not be expected to elicit a response even by conservative DA modelling.
3.3. Whole-mixture methodologies
Fourteen research articles used whole-mixtures methodologies, and these were all associated with one of two experimental models: the biosolid treated pasture (BTP) sheep model and a concentrated drinking water model.
3.3.1. BTP sheep model
Eleven research articles used sheep reared on pastures fertilised using biosolids, a by-product of wastewater treatment. Due to the origins of biosolids they contain a diverse range of anthropogenic chemicals that encompass the human exposome (Rigby et al. Citation2020), which result in organ chemical loads of 0.5–200 μg/kg dry matter (Rhind et al. Citation2005, Citation2009, Citation2010; Bellingham et al. Citation2012; Filis et al. Citation2019). Due to a lack of toxicological studies in sheep, empirically determined PODs are not available. A series of studies have quantified the chemicals in biosolids, soil and herbage from BTP and blood and tissue samples from animals grazed on BTP. Chemical levels are small and not significantly different from control pastures; however, it must be noted that due to their ubiquitous nature many chemicals are also detectable in control pastures (Rhind et al. Citation2002, Citation2010, Citation2013; Evans et al. Citation2014). Chemical quantification and oral dosage estimations are limited to dioctyl phthalate, octyl phenol, and nonyl phenol, which were concluded to be below TDI (Rhind et al. Citation2002). As where residual chemical levels are monitored in crops and animals grazed on BTPs, they remain below human TDI values, the model was included in this review. Articles are presented further grouped as a function of age, i.e. effects on the foetus, juveniles, and adult offspring.
3.3.1.1. Effects in the foetus
Seven research articles which utilised the BTP sheep model investigated the effects on foetuses. Gestational exposure to BTPs was shown to cause lower body weights in exposed males (Paul et al. Citation2005) and female (Fowler et al. Citation2008) foetuses at gestation day (GD) 110. These studies also documented multiple reproductive effects of exposure, in both sexes. Exposed male foetuses showed lower circulating testosterone concentrations, and had testis with reduced weights, fewer Sertoli cells, Leydig cells, and gonocytes, and less androgen receptor expression (Paul et al. Citation2005). Exposed female foetuses showed decreased oocyte numbers and altered oocyte type ratios. Proteomic analysis identified differentially expressed ovarian proteins related to core cellular processes (Fowler et al. Citation2008). Alterations of the hypothalamic-pituitary axis have also been reported in both sexes at GD110, with reduced kisspeptin gene expression and protein levels in the hypothalamus and pituitary and altered pituitary cellular composition (Bellingham et al. Citation2009) in lambs exposed to the chemical mixture.
In two separate studies the timing of exposure to BTP and therefore gestational chemical exposure, prior to and/or during gestation, were investigated: prior to conception only (TC), gestation only (CT), and prior to conception and throughout gestation (TT), compared to controls (CC) where the mothers were never grazed on BTP. These studies reported that at GD110 foetal ovary weight was increased from TT exposure, yet effects on ovarian cell type counts and ratios were only seen in TC and CT (acute exposure) foetuses (Bellingham et al. Citation2013). All groups exposed to chemical mixtures, regardless of when exposure occurred, showed an increase in ovarian proteins involved in stress, oocyte maturation, and apoptosis, relative to the controls (CC). Ovarian proteomic pathway analysis identified enrichment in two functional networks: 1) cancer, gastrointestinal disease, and cellular movement, and 2) cancer, genetic disorder, and respiratory disease (Bellingham et al. Citation2013). The timing of exposure to the chemical mixture also alters the reported effects on the thyroid and the hypothalamic-pituitary axis (Hombach-Klonisch et al. Citation2013; Bellingham et al. Citation2016). The most severe thyroid effects of exposure to a mixture of chemicals were seen in CT and TC (acute exposure) groups, and males (Hombach-Klonisch et al. Citation2013). Thyroid weight was significantly increased, displayed reduced blood vessel area, reduced follicle numbers and areas, and increased cell proliferation in these acute BTP exposure groups. In the hypothalamic-pituitary axis, the most notable observations from BTP exposure were altered expression of gonadotropin-releasing hormone (GnRH) and its receptor (GnRHR), KISS1, the gene that encodes kisspeptin, and its receptor (KISS1R), oestrogen receptor, and androgen receptor. These expression changes were seen in most BTP exposed groups, for both sexes, although effects of exposure were not consistent between exposure group or sex (Bellingham et al. Citation2016).
The timing of maternal exposure to BTPs in early, middle, late, or the entirety of gestation, has also been demonstrated to cause differential effects in female foetuses (GD140) (Lea et al. Citation2016), including body weight reductions, altered relative weights for the uterus, thyroid, and liver, increases in AGD, and changes to circulating testosterone, and free T3 and T4 concentrations (Lea et al. Citation2016). Despite differences in the effects of the timing of mixed chemical exposure, females in all the exposed groups had a lower proportion of healthy type 1a follicles relative to the controls, with a concordant increase in atretic type 1 and 1a follicles (Lea et al. Citation2016). Transcriptomic and proteomic analyses of ovaries identified many differentially expressed genes and proteins, but with very little overlap between groups. Pathway analysis of transcriptomic data identified enrichment within cellular growth and differentiation, cell cycle regulation, cell death, cellular development, and cell movement functions. Pathway analysis of proteomic data identified enrichment within free radical scavenging, cell-to-cell signalling and interaction, small molecule biochemistry, drug metabolism, and protein synthesis functions (Lea et al. Citation2016).
3.3.1.2. Effects in juveniles
One paper that utilised the BTP sheep model investigated 5-month-old lambs. This study reported that following gestational and direct exposure to a chemical mixture through maternal and experimental subjects grazing on BTPs, lambs of both sexes had increased body weights at weaning and showed increased vocalisation and lower maximal activity levels while restrained compared to controls. Males that had been exposed to the chemical mixture also exhibited increased exploratory behaviours relative to the controls, suggestive of the ability of chemical mixtures in this model to affect cognitive ability (Erhard and Rhind Citation2004).
3.3.1.3. Effects in adults
Three research articles that utilised the BTP sheep model investigated the effects in adult sheep of gestational exposure to a chemical mixture. Homeostasis of bone tissue was disrupted by exposure, with bone mineral content, thickness, circumference, cross-sectional area, and cavity size all affected (Lind et al. Citation2009). Interestingly, the adult studies that have used the BTP model also allow for the observation of the effects of exposure to a chemical mixture following an extended period of non-exposure. Testicular morphology was altered in adult males (18 months old) following gestational exposure from conception, lactational exposure through weaning, and then direct exposure to BTPs until 7 months old. These rams exhibited a higher occurrence of Sertoli-cell-only seminiferous tubules, and lower numbers and volumes of germ cells (Bellingham et al. Citation2012). Interestingly these effects were not consistent in all animals, with only a subset of animals (5 of 12) showing a markedly altered phenotype, which may reflect the effects of mixed chemical exposure against the diverse genetic background in this outbred study population. Proteomic analysis of livers from these males and their female counterparts (maintained on treated pastures until 18 months) also identified differentially expressed proteins involved in detoxification and fatty-acid β-oxidation, as well as albumin and transferrin, in both sexes, with pathway analysis indicating dysregulation of cancer-related and lipid-related pathways (Filis et al. Citation2019).
3.3.2. Drinking water disinfection by-products
A series of whole-mixture studies were conducted by the U.S. Environmental Protection Agency which examined the effects of drinking water disinfection by-products (DBPs) in pregnant rats. Only a small percentage of the >600 identified DBPs have been toxicologically evaluated, but of those that have, many are cytotoxic and genotoxic at concentrations achievable in the disinfection process. For example, epidemiological studies indicate trihalomethanes (THM4; chloroform, bromodichloromethane, dibromochloromethane, and bromoform) at concentrations >50 μg/L are associated with an increased risk of bladder cancer (Costet et al. Citation2011). Published research on DBPs have focussed on some of the chemicals currently regulated in the US; specifically total THM4, HAA5 (5 haloacetic acids: chloroacetic acid, bromoacetic acid, dichloroacetic acid, dibromoacetic acid, trichloroacetic acid), and bromate, at 80, 60, and 10 μg/L, respectively. It is of note that in the EU only THM4 and bromate are regulated in this regard and that THM4 is allowed at levels up to 100 μg/L (Li and Mitch Citation2018; Andersson et al. Citation2019). Three research articles investigated the effects of DBP mixtures from various disinfection methods. The model used in these articles provides a mixture of DBPs at realistic ratios, at concentrations higher than maximum contaminant levels but lower than determined NOAELs for monitored chemicals. While not all the chemicals have determined PODs, these studies were included as regulatory assumed structure-activity relationships and chemical groupings for regulatory conditions can be applied.
Narotsky et al. (Citation2008) supplied water approximately 130x concentrated in DBPs, disinfected by either chlorination or ozonation, to pregnant rats, for 10 days during gestation (GD6–16) while controls received boiled, distilled, deionised water. While water consumption was increased and gestation lengths were reduced in dams that supplied concentrated water, no adverse developmental effects were reported in pups. In a similar experiment conducted by Narotsky et al. (Citation2012), two strains of rats (F344 and Sprague-Dawley) were exposed to water that had undergone chlorination and then concentration (chlor/conc), or concentration and then chlorination (conc/chlor), over gestation and lactation. Both concentration methods increase DBPs by around 120x. Dams of both strains given chlor/conc water from early gestation to weaning experienced diarrhoea and polyuria. Sprague-Dawley dams that received concentrated drinking water also had lower body weights at GD20 – PND6 and F344 dams exhibited increased gestation lengths. There were fewer live Sprague-Dawley offspring, with greater perinatal loss/mortality, by PND6, and both strains had reduced body weights at PND6. The authors concluded, however, that some of the effects noted may be related to the concentration of inorganic materials such as sodium and sulphate in the concentrated water. However, when dams were administered conc/chlor water, which was similar in DBP composition but with greatly reduced sodium and sulphate levels to the chlor/conc water, only increased water consumption by dams was noted. Finally, using the chlor/conc method, Narotsky et al. (Citation2013) performed a multi-generational study using water concentrated around 130x, with exposure starting at GD2 for the F1 generation and lasting until termination of the F2 generation. In this study, concentrated water consumption was associated with reduced caput sperm counts in adult F1 males, delayed puberty in juvenile F1 females, thyroid follicular hypertrophy in parental females as well as adult F1 females, and increased birthweights in F2 offspring. While these studies tested concentrated drinking water containing broadly similar concentrations and ratios of DBPs, there are also considerable differences between batches of concentrated drinking water used between studies, which may alter the toxic potential (Li and Mitch Citation2018).
4. Discussion
A key finding of this review was that in studies where low-dose chemical mixtures were tested in parallel with individual component chemicals, most studies reported that the response to mixture exposures was more numerous and/or greater in severity than the responses to individual chemicals. While it is not uncommon to see occasional mild signs of toxicity when individual chemicals are tested at, or close to, NOAEL levels, eight studies summarised in this review reported significant effects from individual chemicals at or below TDI values, theoretically two or three orders of magnitude away from an effective dose. The observation of physiological effects at these very low ‘safe’ doses calls into question the validity of the POD values used in the calculation of TDI values. Alternatively, these results could indicate the significant variation between experiments/laboratories, genomic drift between animal breeders that purport to supply the “same” strain of animals, strain differences in susceptibility, or differences in detection sensitivity. However, all the above still expose potential shortcomings in toxicity assessment. Observations where toxicological effects were more numerous and/or more severe following exposure to a mixture of chemicals rather than the chemical components individually suggest at least additivity between components of a chemical mixture. However, most studies did not employ experimental designs which allowed for direct comparisons to, and assessments of, mixture toxicity models, which can definitively distinguish between additivity and synergy. Of the seven which provided data appropriate for such comparisons, three reported responses significantly greater than all investigated additivity model predictions, strongly indicating synergy. Although the remaining studies were not appropriate for similar direct critiques of mixture toxicity models, indications of the appropriateness of the mixture toxicity models can be inferred, for example, where there are effects following TDI exposure, which current applications of mixture toxicity models cannot explain. While this work has shown some commonality between various studies, there were also disparities between others. The focus of this review on the most relevant research for translational considerations (mammalian, in vivo) also posed the largest limitation, as data and designs between papers were too disparate from each other, and the quantity of literature too small, for true comparative re-evaluations. In a recent systematic review and quantitative reappraisal by Martin et al. (Citation2021), which covered all living organisms, in vitro and in vivo, most mixtures were found to conform to dose additivity models. This agrees with many reviews which showed dose additivity-based models to be the most accurate across the whole dose-response curve, even when chemical components of a mixture are mechanistically distinct. A most notable example is the addictive nature of antiandrogenic chemicals with phthalates, provided by Howdeshell et al. Citation2017, where additive models accurately predict exposure outcomes, although many of the studies involved did not meet the inclusion criteria for this review. However, in twenty percent of the literature identified by Martin et al. (Citation2021) effects exceeded dose additivity models substantially, with synergistic interactions more than two-fold. These interactions were attributed to groupings already suspected of synergy (combinations of triazine, azole and pyrethroid pesticides), while also indicating new, potentially synergistic groupings (EDCs within metallic compounds).
Signs of toxicity may be expected from co-exposure to mixtures containing chemicals at or close to NOAEL, regardless of additivity type. At this dose level, an important consideration is an endpoint used to derive a NOAEL. Where NOAELs have been derived from endpoints dissimilar to those being investigated, study endpoints may have greater or lesser sensitivity to disruption. In these cases, compounds could be dosed either above or below NOAEL for study endpoints. In the latter case, greater additivity would be needed to cross the effect threshold, prohibitive to detecting effects. Similarly, experiments often used mixtures of too few components. As deviations from additivity are commonly small, simpler mixtures may not have the power to elicit an observable effect. Nearly half of the identified component-based literature used ≤5 mixture components. Additionally, some studies have used pilot data to generate dose-response curves for the specific endpoints being investigated, whereas others have used values derived in some form from regulatory studies or determinations. This could lead to contradictory findings; for example, vinclozolin NOAEL was determined at 4 and 5 mg/kg/d by Schneider et al. (Citation2017) and Christiansen et al. (Citation2009) respectively. It is impossible to know if this difference could go towards explaining the differences between the studies (greater than additivity at NOAEL in Christiansen et al. (Citation2009) and no effect at NOAEL in Schneider et al. (Citation2017)), although this was not considered a major issue as PODs were broadly similar for the same chemicals across most studies. Twenty-one of the thirty studies which examined mixtures at NOAEL values reported physiological effects that were attributed to chemical exposure. However, no toxicological or physiological effect should be expected from co-exposure to chemical mixtures where the components are present at or close to TDI. This review identified that in eighteen of the twenty-one studies that tested mixtures at or below TDI values toxicological or physiological effects were reported. The greatest number of chemicals tested at doses equivalent to TDI values was twenty-seven chemicals. In this example, even using dose addition for all components, this level of exposure would still be anticipated to be more than three-fold lower than a dose expected to be able to elicit an effect. This is an indication of interactions between chemical components within the chemical mixtures, unaccounted for by mixture toxicity models.
Of the literature identified that used a whole-mixture methodology, the BTP sheep model is the only which reflects actual human exposure as the drinking water by-products model is orders of magnitude away from realistic exposure. This lack of variation means that inter-species variance remains unaccounted for. While the BTP sheep model could be criticised for a lack of empirically determined PODs, limited quantification of individual chemicals, and no normalisation of dosages, it represents a real-world situation, with a chemical mixture used according to regulatory guidelines. Such use is deemed appropriate to ensure contaminant levels are below conservative calculations for acceptable human exposure, and thus also for other species for which there is a lack of empirically determined PODs. The BTP sheep model is also representative of human exposure in that many chemicals to which humans are exposed have little or no toxicological data in any species. This is a recognised problem that can not feasibly be solved by the traditional route of testing each chemical individually but will most likely rely on new and future methodologies, including read-across, quantitative structure-activity relationship (QSAR) analysis, machine learning, and artificial intelligence (NAS Citation2017; Aschner et al. Citation2022).
The most examined endpoints in identified research articles were reproductive and/or developmental (nineteen), endocrine disruption (fifteen), hepatic (twelve), and behavioural (eight). There is concern that current guidelines do not sufficiently account for the multitude and ubiquity that characterises human exposure, especially foetal EDC exposure, which could be contributing to current global health problems, including the decline in male reproductive health (Skakkebaek et al. Citation2001; Skakkebaek Citation2002; Bergman et al. Citation2012). It has been suggested that current inclusion criteria for chemicals in mixture risk assessments, based on shared mechanisms of action, may be too restrictive in terms of mixture risk assessments for male reproductive health (Kortenkamp Citation2020). Of the nineteen research articles that reported effects on reproduction and/or development after in utero exposure to chemical mixtures, ten used mixtures with individual chemicals at TDI or ≤0.25x NOAEL values. Common responses observed were gonadal dysgenesis, deleterious germ cell alterations, and altered AGD, and specifically in males increased areola number, NR, and genital malformations.
The relevance of systems biology approaches in a toxicological context without additional confirmation of biological effect has previously been questioned (Schneider et al. Citation2015). The use of omics data was seen in six component-based research articles and four whole-mixture research articles. However, of the research articles which used omics technologies, four of the six component-based research articles, and three of the four whole-mixture research articles, also had strong supporting morphological data, therefore this was not considered a factor for exclusion.
5. Conclusions
The basis of compounding toxicity from chemical mixtures at low doses, especially at or below TDI values, remains a subject of debate. While there have been great advancements in mixture toxicity assessment, with some acceptance within regulatory bodies, there remains a lack of harmonisation as well as a lack of dose coverage to those far below individually determined NOAEL values. The present work represents a collation and analysis of research articles reporting experiments testing chemical mixtures with individual components at doses believed unable to elicit effects alone. This review, however, did not address other (controversial) aspects of low-dose chemical mixture exposure, such as hormetic, non-monotonic, or biphasic responses.
While the extensive ECETOC literature review concluded no substantial evidence of mixture toxicity not already accounted for (ECETOC Citation2012), this review includes many studies which tested mixtures at TDI levels and were published after the ECETOC review. In addition, it should be noted that the ECETOC review focussed on the identification of studies that provided evidence of effects greater than additivity model predictions, rather than evidence of effects unaccounted for by additivity model predictions. With this view, most of the literature identified here also falls short, like experimental designs which would have made this possible were typically not employed. However, of those who could, around half found additivity model predictions were inaccurate, and responses significantly greater than additivity model predictions were reported. Additionally, at doses around TDI, this fact is somewhat immaterial, especially when applied to real-world situations, where co-exposure occurs to thousands of chemicals. It is in this respect that current methodological approaches for cumulative risk assessment fall short, and as such a novel paradigm has been suggested focussing on complex mixtures of chemicals at individual doses around TDI (Tsatsakis et al. Citation2016, Citation2017) and assessing risk using a real-life risk simulation (RLRS) approach (Hernández et al. Citation2020). However, it is logistically impossible to truly simulate real-life exposure by component-based methodologies. For this the BTP sheep model is most realistic, however, it was not accepted by ECETOC due to the lack of empirically determined chemical concentrations and dose calculations, despite being common practice on fields growing crops for human consumption where chemical loads within crops remain below human TDIs. Additionally, due to the expansive chemical nature of biosolids and the limitations of current analytical techniques for such mass chemical quantification, such empirical determination is impractical. Finally, whole-mixture studies cannot give answers to the question of synergy, which can only be addressed through carefully designed component-based studies, but rather give snapshots of an extremely complex and dynamic exposure. Thus, there is little extra to be gained from precise quantification of individual chemicals and exact calculations of dosages resulting from BTP exposure without a greater understanding of biological and chemical interactions between mixture components.
With these considerations in mind, it can be concluded that although no unequivocal evidence to refute conclusions from previous reviews, there is strong evidence to support additional research in this area. However, there were two data gaps identified which would facilitate a greater understanding of low-dose chemical mixture toxicity, especially for complex chemical mixtures. Firstly, within the literature, there was a lack of data on complex mixtures (with >10 components) at TDI values with data on individual components generated in parallel, likely because of greatly increased study requirements to generate such data. This data gap has been previously identified as impeding improvements to mixture risk assessments (Evans et al. Citation2015). Secondly, there is a lack of mixture dose-response data at very low-doses, with very few research articles reporting more than two concentrations less than or equal to NOAEL. This data gap deprives analyses of response curves and PODs for mixtures with which to compare to individual components, and thus leaves no basis to assert evidence for or against current additivity models. Without these gaps being addressed, results cannot be interpreted regarding the nature of any combination effects, nor on the type of interactions or non-interactions occurring. Thus, the appropriateness of current mixture toxicity assessments cannot be critically examined further.
Author contributions
CSE – Conceptualisation, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. NPE and MB – Conceptualisation, Writing – review & editing.
| Abbreviations | ||
| AGD | = | Anogenital distances |
| Ahr | = | Arylhydrocarbon receptor |
| ALP | = | Alkaline phosphatase |
| ALT | = | Alanine transaminase |
| AOP | = | Adverse outcome pathway |
| AST | = | Aspartate transaminase |
| BBP | = | Benzyl butyl phthalate |
| BMD | = | Benchmark dose |
| BPA | = | Bisphenol A |
| BTP | = | Biosolid treated pasture |
| Car | = | Constitutive androstane receptor |
| ChE | = | Cholinesterase |
| DA | = | Dose addition |
| DAR | = | Draft assessment report |
| DBP | = | Dibutyl phthalate |
| DecaBDE | = | Decabromodiphenyl ether |
| DEHP | = | Diethylhexyl phthalate |
| DEP | = | Diethyl phthalate |
| DMP | = | Dimethyl phthalate |
| DnOP | = | Di-n-octyl phthalate |
| EC | = | Environmental chemical |
| ECETOC | = | European centre for ecotoxicology and toxicology of chemicals |
| EDC | = | Endocrine disrupting chemical |
| EGF | = | Epidermal growth factor |
| ES | = | Effect summation |
| FSH | = | Follicle-stimulating hormone |
| GD | = | Gestation day |
| GnRH | = | Gonadotropin releasing hormone |
| GnRHR | = | Gonadotropin releasing hormone receptor |
| HFHS | = | High-fat-high-sucrose |
| HPT-axis | = | Hypothalamic–pituitary–thyroid axis |
| IA | = | Integrated addition |
| KISS1 | = | Kisspeptin 1 |
| KISS1R | = | Kisspeptin 1 receptor |
| KO | = | Knockout |
| LOAEL | = | Lowest observable adverse effect level |
| MCPB | = | 4-(4-chloro-2-methylphenoxy)butanoic acid |
| MRL | = | Maximum residue limit |
| NGF | = | Nerve growth factor |
| NOAEL | = | No observable adverse effect level |
| NP | = | Nonylphenol |
| NR | = | Nipple retention |
| PAH | = | Polycyclic aromatic hydrocarbon |
| PCB | = | Polychlorinated biphenyl |
| PFOA | = | Perfluorooctanoic acid |
| PND | = | Post-natal day |
| PO | = | Per os (by mouth) |
| POD | = | Point of departure |
| Pparα | = | Peroxisome proliferator-activated receptor alpha |
| QSAR | = | Quantitative structure-activity relationship |
| RA | = | Response addition |
| RDI | = | Recommended daily intake |
| RfD | = | Reference dose |
| SC | = | Subcutaneous |
| TCDD | = | Tetrachlorodibenzo-p-dioxin |
| TDI | = | Tolerable daily intake |
| TEF | = | Toxic equivalency factor |
| TGFα | = | Transforming growth factor alpha |
| TSH | = | Thyroid-stimulating hormone |
| VOC | = | Volatile organic compound |
| WT | = | Wild type. |
Supplemental Material
Download MS Excel (33.1 KB)Acknowledgements
The authors have no further acknowledgements to declare. The manuscript was prepared solely by the authors, whose contributions are declared herein. We would like to thank the anonymous reviewers for their insight and valuable input which has improved the manuscript to its current form.
Declaration of interest
The authors have no conflicts of interest to declare. The author’s affiliations are as shown on the cover page. The motivation for the preparation of this manuscript was a part of the completion of doctoral training for CSE, which was paid for by the University of Glasgow, and utilised funding from NIEHS detailed herein. The authors have not participated in, nor anticipate participation in, any legal, regulatory, or advocacy proceedings related to the contents of the paper. The authors had sole responsibility for the preparation of this manuscript. Opinions and conclusions expressed within are those of the authors and are not necessarily those of any sponsoring entity.
Additional information
Funding
References
- Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC, Eustache F. 2013. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proc Biol Sci. 280(1768):20131532.
- Andersson A, Ashiq MJ, Shoeb M, Karlsson S, Bastviken D, Kylin H. 2019. Evaluating gas chromatography with a halogen-specific detector for the determination of disinfection by-products in drinking water. Environ Sci Pollut Res Int. 26(8):7305–7314.
- Aschner M, Mesnage R, Docea AO, Paoliello MMB, Tsatsakis A, Giannakakis G, Papadakis GZ, Vinceti SR, Santamaria A, Skalny AV, et al. 2022. Leveraging artificial intelligence to advance the understanding of chemical neurotoxicity. Neurotoxicology. 89:9–11.
- Auxietre T-A, Dumontier M-F, Balguy I, Frapart Y, Canivenc-Lavier M-C, Berges R, Boudalia S, Auger J, Corvol M-T, Savouret J-F. 2014. Sub-NOAEL amounts of vinclozolin and xenoestrogens target rat chondrogenesis in vivo. Biochimie. 99:169–177.
- Bellingham M, Amezaga MR, Mandon-Pepin B, Speers CJ, Kyle CE, Evans NP, Sharpe RM, Cotinot C, Rhind SM, Fowler PA. 2013. Exposure to chemical cocktails before or after conception – the effect of timing on ovarian development. Mol Cell Endocrinol. 376(1–2):156–172.
- Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, Sharpe RM, Evans NP. 2009. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the Kisspeptin/GPR54 system in ovine hypothalamus and pituitary gland. Environ Health Perspect. 117(10):1556–1562.
- Bellingham M, Fowler PA, MacDonald ES, Mandon‐Pepin B, Cotinot C, Rhind S, Sharpe RM, Evans NP. 2016. Timing of maternal exposure and foetal sex determine the effects of low-level chemical mixture exposure on the foetal neuroendocrine system in sheep. J Neuroendocrinol. 28(12):jne.12444.
- Bellingham M, McKinnell C, Fowler PA, Amezaga MR, Zhang Z, Rhind SM, Cotinot C, Mandon-Pepin B, Evans NP, Sharpe RM. 2012. Foetal and post-natal exposure of sheep to sewage sludge chemicals disrupts sperm production in adulthood in a subset of animals. Int J Androl. 35(3):317–329.
- Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. 2012. State of the science of endocrine disrupting chemicals. Geneva: Switzerland.
- Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. 2006. Environmental chemicals and thyroid function. Eur J Endocrinol. 154(5):599–611.
- Boobis A, Budinsky R, Collie S, Crofton K, Embry M, Felter S, Hertzberg R, Kopp D, Mihlan G, Mumtaz M, et al. 2011. Critical analysis of literature on low-dose synergy for use in screening chemical mixtures for risk assessment. Crit Rev Toxicol. 41(5):369–383.
- Bopp SK, Barouki R, Brack W, Dalla Costa S, Dorne J-L, Drakvik PE, Faust M, Karjalainen TK, Kephalopoulos S, van Klaveren J, et al. 2018. Current EU research activities on combined exposure to multiple chemicals. Environ Int. 120:544–562.
- Bopp SK, Kienzler A, Richarz A-N, van der Linden SC, Paini A, Parissis N, Worth AP. 2019. Regulatory assessment and risk management of chemical mixtures: challenges and ways forward. Crit Rev Toxicol. 49(2):174–189.
- Buñay J, Larriba E, Patiño-Garcia D, Cruz-Fernandes L, Castañeda-Zegarra S, Rodriguez-Fernandez M, Del Mazo J, Moreno RD. 2018. Differential effects of exposure to single versus a mixture of endocrine-disrupting chemicals on steroidogenesis pathway in mouse testes. Toxicol Sci. 161(1):76–86.
- Carpy SA, Kobel W, Doe J. 2000. Health risk of low-dose pesticides mixtures: a review of the 1985-1998 literature on combination toxicology and health risk assessment. J Toxicol Environ Heal Part B. 3:1–25.
- Chen Y, Liu S, Xu H, Zheng H, Bai C, Pan W, Zhou H, Liao M, Huang C, Dong Q. 2019. Maternal exposure to low dose BDE209 and Pb mixture induced neurobehavioral anomalies in C57BL/6 male offspring. Toxicology. 418:70–80.
- Christiansen S, Kortenkamp A, Axelstad M, Boberg J, Scholze M, Jacobsen PR, Faust M, Lichtensteiger W, Schlumpf M, Burdorf A, et al. 2012. Mixtures of endocrine disrupting contaminants modelled on human high end exposures: an exploratory study in rats. Int J Androl. 35(3):303–316.
- Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, Hass U. 2009. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ Health Perspect. 117(12):1839–1846.
- Conley JM, Lambright CS, Evans N, Cardon M, Furr J, Wilson VS, Gray LE. 2018. Mixed “antiandrogenic” chemicals at low individual doses produce reproductive tract malformations in the male rat. Toxicol Sci. 164(1):166–178.
- Costet N, Villanueva CM, Jaakkola JJK, Kogevinas M, Cantor KP, King WD, Lynch CF, Nieuwenhuijsen MJ, Cordier S. 2011. Water disinfection by-products and bladder cancer: is there a European specificity? A pooled and meta-analysis of European Case-Control Studies. Occup Environ Med. 68(5):379–385.
- Demur C, Métais B, Canlet C, Tremblay-Franco M, Gautier R, Blas-Y-Estrada F, Sommer C, Gamet-Payrastre L. 2013. Dietary exposure to a low dose of pesticides alone or as a mixture: the biological metabolic fingerprint and impact on hematopoiesis. Toxicology. 308:74–87.
- Docea AO, Gofita E, Goumenou M, Calina D, Rogoveanu O, Varut M, Olaru C, Kerasioti E, Fountoucidou P, Taitzoglou I, et al. 2018. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem Toxicol. 115:470–481.
- Docea AO, Goumenou M, Calina D, Arsene AL, Dragoi CM, Gofita E, Pisoschi CG, Zlatian O, Stivaktakis PD, Nikolouzakis TK, et al. 2019. Adverse and hormetic effects in rats exposed for 12 months to low dose mixture of 13 chemicals: RLRS part III. Toxicol Lett. 310:70–91.
- Dorato MA, Engelhardt JA. 2005. The no-observed-adverse-effect-level in drug safety evaluations: use, issues, and definition(s). Regul Toxicol Pharmacol. 42(3):265–274.
- Drakvik E, Altenburger R, Aoki Y, Backhaus T, Bahadori T, Barouki R, Brack W, Cronin MTD, Demeneix B, Hougaard Bennekou S, et al. 2020. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ Int. 134:105267.
- Du L, Li S, Qi L, Hou Y, Zeng Y, Xu W, Wang H, Zhao X, Sun C. 2014. Metabonomic analysis of the joint toxic action of long-term low-level exposure to a mixture of four organophosphate pesticides in rat plasma. Mol BioSyst. 10(5):1153–1161.
- Dunnick JK, Kissling G, Gerken DK, Vallant MA, Nyska A. 2007. Cardiotoxicity of Ma Huang/caffeine or ephedrine/caffeine in a rodent model system. Toxicol Pathol. 35(5):657–664.
- ECETOC. 2012. Effects of chemical co-exposures at doses relevant for human safety assessments. European Centre for Ecotoxicology and Toxicology of Chemicals. Technical report No. 115. p. 1–208. https://www.ecetoc.org/wp-content/uploads/2021/10/ECETOC-TR-115-and-Appendix-B.zip
- Erhard HW, Rhind SM. 2004. Prenatal and postnatal exposure to environmental pollutants in sewage sludge alters emotional reactivity and exploratory behaviour in sheep. Sci Total Environ. 332(1–3):101–108.
- Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, Berges R, Cravedi JP, Vaiman D, Auger J. 2009. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 117(8):1272–1279.
- Evans NP, Bellingham M, Sharpe RM, Cotinot C, Rhind SM, Kyle C, Erhard H, Hombach-Klonisch S, Lind PM, Fowler PA. 2014. Reproduction symposium: does grazing on biosolids-treated pasture pose a pathophysiological risk associated with increased exposure to endocrine disrupting compounds? J Anim Sci. 92(8):3185–3198.
- Evans RM, Scholze M, Kortenkamp A. 2015. Examining the feasibility of mixture risk assessment: a case study using a tiered approach with data of 67 pesticides from the Joint FAO/WHO Meeting on Pesticide Residues (JMPR). Food Chem Toxicol. 84:260–269.
- Filis P, Walker N, Robertson L, Eaton-Turner E, Ramona L, Bellingham M, Amezaga MR, Zhang Z, Mandon-Pepin B, Evans NP, et al. 2019. Long-term exposure to chemicals in sewage sludge fertilizer alters liver lipid content in females and cancer marker expression in males. Environ Int. 124:98–108.
- Fountoucidou P, Veskoukis AS, Kerasioti E, Docea AO, Taitzoglou IA, Liesivuori J, Tsatsakis A, Kouretas D. 2019. A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: the time and dose issue. Toxicol Lett. 317:24–44.
- Fowler PA, Dorà NJ, McFerran H, Amezaga MR, Miller DW, Lea RG, Cash P, McNeilly AS, Evans NP, Cotinot C, et al. 2008. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol Hum Reprod. 14(5):269–280.
- Gao H-T, Xu R, Cao W-X, Qian L-L, Wang M, Lu L, Xu Q, Yu S-Q. 2017. Effects of six priority controlled phthalate esters with long-term low-dose integrated exposure on male reproductive toxicity in rats. Food Chem Toxicol. 101:94–104.
- Hadrup N, Svingen T, Mandrup K, Skov K, Pedersen M, Frederiksen H, Frandsen HL, Vinggaard AM. 2016. Juvenile male rats exposed to a low-dose mixture of twenty-seven environmental chemicals display adverse health effects. PLOS One. 11(9):e0162027–22.
- Hass U, Boberg J, Christiansen S, Jacobsen PR, Vinggaard AM, Taxvig C, Poulsen ME, Herrmann SS, Jensen BH, Petersen A, et al. 2012. Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod Toxicol. 34(2):261–274.
- Hass U, Christiansen S, Axelstad M, Scholze M, Boberg J. 2017. Combined exposure to low doses of pesticides causes decreased birth weights in rats. Reprod Toxicol. 72:97–105.
- Hernández AF, Docea AO, Goumenou M, Sarigiannis D, Aschner M, Tsatsakis A. 2020. Application of novel technologies and mechanistic data for risk assessment under the real-life risk simulation (RLRS) approach. Food Chem Toxicol. 137:111123.
- Hernández AF, Gil F, Lacasaña M. 2017. Toxicological interactions of pesticide mixtures: an update. Arch Toxicol. 91(10):3211–3223.
- Heys KA, Shore RF, Pereira MG, Jones KC, Martin FL. 2016. Risk assessment of environmental mixture effects. RSC Adv. 6(53):47844–47857.
- Hombach-Klonisch S, Danescu A, Begum F, Amezaga MR, Rhind SM, Sharpe RM, Evans NP, Bellingham M, Cotinot C, Mandon-Pepin B, et al. 2013. Peri-conceptional changes in maternal exposure to sewage sludge chemicals disturbs fetal thyroid gland development in sheep. Mol Cell Endocrinol. 367(1–2):98–108.
- Howdeshell KL, Hotchkiss AK, Gray LE. 2017. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int J Hyg Environ Health. 220(2 Pt A):179–188.
- Jacobsen PR, Axelstad M, Boberg J, Isling LK, Christiansen S, Mandrup KR, Berthelsen LO, Vinggaard AM, Hass U. 2012. Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod Toxicol. 34(2):237–250.
- Jacobsen PR, Christiansen S, Boberg J, Nellemann C, Hass U. 2010. Combined exposure to endocrine disrupting pesticides impairs parturition, causes pup mortality and affects sexual differentiation in rats. Int J Androl. 33(2):434–442.
- Jacobsen H, Østergaard G, Lam HR, Poulsen ME, Frandsen H, Ladefoged O, Meyer O. 2004. Repeated dose 28-day oral toxicity study in Wistar rats with a mixture of five pesticides often found as residues in food: alphacypermethrin, bromopropylate, carbendazim, chlorpyrifos and mancozeb. Food Chem Toxicol. 42(8):1269–1277.
- Julien B, Pinteur C, Vega N, Labaronne E, Vidal H, Naville D, Le Magueresse-Battistoni B. 2018. Evidence for estrogeno-mimetic effects of a mixture of low-dose pollutants in a model of ovariectomized mice. Environ Toxicol Pharmacol. 57:34–40.
- Julien B, Pinteur C, Vega N, Vidal H, Naville D, Le Magueresse-Battistoni B. 2019. Estrogen withdrawal and replacement differentially target liver and adipose tissues in female mice fed a high-fat high-sucrose diet: impact of a chronic exposure to a low-dose pollutant mixture⋆. J Nutr Biochem. 72:108211.
- Kortenkamp A. 2020. Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol Cell Endocrinol. 499:110581.
- Kortenkamp A, Backhaus T, Faust M. 2009. State of the art report on mixture toxicity – final report. ECHA 070307/2007/485103/ETU/D.1
- Kouidhi W, Desmetz C, Nahdi A, Bergès R, Cravedi J-P, Auger J, El May M, Canivenc-Lavier MC. 2012. Utero and lactational exposure to low-dose genistein-vinclozolin mixture affects the development and growth factor mRNA expression of the submandibular salivary gland in immature female rats. Toxicol Pathol. 40(4):593–604.
- Labaronne E, Pinteur C, Vega N, Pesenti S, Julien B, Meugnier-Fouilloux E, Vidal H, Naville D, Le Magueresse-Battistoni B. 2017. Low-dose pollutant mixture triggers metabolic disturbances in female mice leading to common and specific features as compared to a high-fat diet. J Nutr Biochem. 45:83–93.
- Lea RG, Amezaga MR, Loup B, Mandon-Pépin B, Stefansdottir A, Filis P, Kyle C, Zhang Z, Allen C, Purdie L, et al. 2016. The fetal ovary exhibits temporal sensitivity to a ‘real-life’ mixture of environmental chemicals. Sci Rep. 6(22279):1–13.
- Lehraiki A, Messiaen S, Berges R, Canivenc-Lavier M-C, Auger J, Habert R, Levacher C. 2011. Antagonistic effects of gestational dietary exposure to low-dose vinclozolin and genistein on rat fetal germ cell development. Reprod Toxicol. 31(4):424–430.
- Li X-F, Mitch WA. 2018. Drinking Water Disinfection Byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol. 52(4):1681–1689.
- Lind PM, Gustafsson M, Hermsen SA, Larsson S, Kyle CE, Örberg J, Rhind SM. 2009. Exposure to pastures fertilised with sewage sludge disrupts bone tissue homeostasis in sheep. Sci Total Environ. 407(7):2200–2208.
- Lukowicz C, Ellero-Simatos S, Régnier M, Polizzi A, Lasserre F, Montagner A, Lippi Y, Jamin EL, Martin J-F, Naylies C, et al. 2018. Metabolic effects of a chronic dietary exposure to a low-dose pesticide cocktail in mice: sexual dimorphism and role of the constitutive androstane receptor. Environ Health Perspect. 126(6):067007.
- Martin O, Scholze M, Ermler S, McPhie J, Bopp SK, Kienzler A, Parissis N, Kortenkamp A. 2021. Ten years of research on synergisms and antagonisms in chemical mixtures: a systematic review and quantitative reappraisal of mixture studies. Environ Int. 146:106206.
- Merhi M, Demur C, Racaud-Sultan C, Bertrand J, Canlet C, Estrada FBY, Gamet-Payrastre L. 2010. Gender-linked haematopoietic and metabolic disturbances induced by a pesticide mixture administered at low dose to mice. Toxicology. 267(1–3):80–90.
- More SJ, Hardy A, Benford D, Bennekou SH, Bragard C, Halldorsson TI, Hernández-Jerez AF, Koutsoumanis K, Naegeli H, Schlatter JR, et al. 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 17:e05634.
- Mosquera Ortega ME, Romero DM, Pato AM, Sosa-Holt CS, Ridolfi A, Villaamil Lepori E, Wolansky MJ. 2018. Relationship between exposure, body burden and target tissue concentration after oral administration of a low-dose mixture of pyrethroid insecticides in young adult rats. Toxicology. 409:53–62.
- Mossa A-TH, Refaie AA, Ramadan A. 2011. Effect of exposure to mixture of four organophosphate insecticides at no observed adverse effect level dose on rat liver: the protective role of vitamin C. Res J Environ Toxicol. 5(6):323–335.
- Narotsky MG, Best DS, Rogers EH, McDonald A, Sey YM, Simmons JE. 2008. Integrated disinfection by-products mixtures research: assessment of developmental toxicity in Sprague-Dawley rats exposed to concentrates of water disinfected by chlorination and ozonation/postchlorination. J Toxicol Environ Health A. 71(17):1216–1221.
- Narotsky MG, Klinefelter GR, Goldman JM, Best DS, McDonald A, Strader LF, Suarez JD, Murr AS, Thillainadarajah I, et al. 2013. Comprehensive assessment of a chlorinated drinking water concentrate in a rat multigenerational reproductive toxicity study. Environ Sci Technol. 47:10653–10659.
- Narotsky MG, Klinefelter GR, Goldman JM, DeAngelo AB, Best DS, McDonald A, Strader LF, Murr AS, Suarez JD, George MH, et al. 2015. Reproductive toxicity of a mixture of regulated drinking-water disinfection by-products in a multigenerational rat bioassay. Environ Health Perspect. 123(6):564–570.
- Narotsky MG, Pressman JG, Miltner RJ, Speth TF, Teuschler LK, Rice GE, Richardson SD, Best DS, McDonald A, Hunter ES, et al. 2012. Developmental toxicity evaluations of whole mixtures of disinfection by-products using concentrated drinking water in rats: gestational and lactational effects of sulfate and sodium. Birth Defects Res B Dev Reprod Toxicol. 95(3):202–212.
- NAS 2017. Using 21st century science to improve risk-related evaluations, using 21st century science to improve risk-related evaluations. Washington, D.C: National Academies Press.
- Naville D, Gaillard G, Julien B, Vega N, Pinteur C, Chanon S, Vidal H, Le Magueresse-Battistoni B. 2019. Chronic exposure to a pollutant mixture at low doses led to tissue-specific metabolic alterations in male mice fed standard and high-fat high-sucrose diet. Chemosphere. 220:1187–1199.
- Naville D, Labaronne E, Vega N, Pinteur C, Canet-Soulas E, Vidal H, Le Magueresse-Battistoni B. 2015. Metabolic outcome of female mice exposed to a mixture of low-dose pollutants in a diet-induced obesity model. PLOS ONE. 10(4):e0124015.
- Naville D, Pinteur C, Vega N, Menade Y, Vigier M, Le Bourdais A, Labaronne E, Debard C, Luquain-Costaz C, Bégeot M, et al. 2013. Low‐dose food contaminants trigger sex‐specific, hepatic metabolic changes in the progeny of obese mice. Faseb J. 27(9):3860–3870.
- OECD 2018. Considerations for assessing the risks of combined exposure to multiple chemicals, series on testing and assessment No. 296, Environment, Health and Safety Division, Environment Directorate.
- Pascotto VM, Guerra MT, Franci JAA, de Camargo JLV, Kempinas WG, Franchi CAS. 2015. Effects of a mixture of pesticides on the adult female reproductive system of Sprague-Dawley, Wistar, and Lewis Rats. J Toxicol Environ Heal Part A. 78(9):602–616.
- Paul C, Rhind SM, Kyle CE, Scott H, McKinnell C, Sharpe RM. 2005. Cellular and hormonal disruption of fetal testis development in sheep reared on pasture treated with sewage sludge. Environ Health Perspect. 113(11):1580–1587.
- Perez-Carreon JI, Dargent C, Merhi M, Fattel-Fazenda S, Arce-Popoca E, Villa-Treviño S, Rouimi P. 2009. Tumor promoting and co-carcinogenic effects in medium-term rat hepatocarcinogenesis are not modified by co-administration of 12 pesticides in mixture at acceptable daily intake. Food Chem Toxicol. 47(3):540–546.
- Perobelli JE, Martinez MF, da Silva Franchi CA, Fernandez CDB, de Camargo JLV, Kempinas WDG. 2010. Decreased sperm motility in rats orally exposed to single or mixed pesticides. J Toxicol Environ Health A. 73(13–14):991–1002.
- Rappaport SM. 2011. Implications of the exposome for exposure science. J Expo Sci Environ Epidemiol. 21(1):5–9.
- Rhind SM, Kyle CE, MacKie C, McDonald L. 2009. Accumulation of endocrine disrupting compounds in sheep fetal and maternal liver tissue following exposure to pastures treated with sewage sludge. J Environ Monit. 11(8):1469–1476.
- Rhind SM, Kyle CE, Mackie C, McDonald L, Zhang Z, Duff EI, Bellingham M, Amezaga MR, Mandon-Pepin B, Loup B, et al. 2010. Maternal and fetal tissue accumulation of selected endocrine disrupting compounds (EDCs) following exposure to sewage sludge-treated pastures before or after conception. J Environ Monit. 12(8):1582–1593.
- Rhind SM, Kyle CE, Ruffie H, Calmettes E, Osprey M, Zhang ZL, Hamilton D, McKenzie C. 2013. Short- and long-term temporal changes in soil concentrations of selected endocrine disrupting compounds (EDCs) following single or multiple applications of sewage sludge to pastures. Environ Pollut. 181:262–270.
- Rhind SM, Kyle CE, Telfer G, Duff EI, Smith A. 2005. Alkyl phenols and diethylhexyl phthalate in tissues of sheep grazing pastures fertilized with sewage sludge or inorganic fertilizer. Environ Health Perspect. 113(4):447–453.
- Rhind SM, Smith A, Kyle CE, Telfer G, Martin G, Duff E, Mayes RW. 2002. Phthalate and alkyl phenol concentrations in soil following applications of inorganic fertiliser or sewage sludge to pasture and potential rates of ingestion by grazing ruminants. J Environ Monitor. 4(1):142–148.
- Ribeiro E, Ladeira C, Viegas S. 2017. EDCs mixtures: a stealthy hazard for human health? Toxics. 5(1):5–17.
- Rider CV, Dinse GE, Umbach DM, Simmons JE, Hertzberg RC. 2018. Predicting mixture toxicity with models of additivity. In: Chemical mixtures and combined chemical and nonchemical stressors. Cham: Springer International Publishing; p. 235–270.
- Rider CV, Furr J, Wilson VS, Gray LE. 2008. A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl. 31(2):249–262.
- Rider CV, Furr JR, Wilson VS, Gray LE. Jr 2010. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl. 33(2):443–462.
- Rieke S, Heise T, Schmidt F, Haider W, Bednarz H, Niehaus K, Mentz A, Kalinowski J, Hirsch-Ernst KI, Steinberg P, et al. 2017. Mixture effects of azole fungicides on the adrenal gland in a broad dose range. Toxicology. 385:28–37.
- Rigby H, Dowding A, Fernandes A, Humphries D, Jones NR, Lake L, Petch RG, Reynolds CK, Rose M, Smith SR. 2020. Concentrations of organic contaminants in industrial and municipal bioresources recycled in agriculture in the UK. Sci Total Environ. 765:142787.
- Rotter S, Beronius A, Boobis AR, Hanberg A, van Klaveren J, Luijten M, Machera K, Nikolopoulou D, van der Voet H, Zilliacus J, et al. 2018. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: the potential EuroMix contribution. Crit Rev Toxicol. 48(9):796–814.
- Saad HES, Meduri G, Phrakonkham P, Bergès R, Vacher S, Djallali M, Auger J, Canivenc-Lavier MC, Perrot-Applanat M. 2011. Abnormal peripubertal development of the rat mammary gland following exposure in utero and during lactation to a mixture of genistein and the food contaminant vinclozolin. Reprod Toxicol. 32(1):15–25.
- Santiasih I, Titah HS, Hermana J. 2020. The effects of particulate matters inhalation exposures of prallethrin and d-phenothrin mixture in mice (Mus musculus) against exhaled carbon dioxide concentration. Toxicol Res. 36(1):59–67.
- Sarigiannis DA, Karakitsios SP. 2018. Modeling complex exposures. In: Chemical mixtures and combined chemical and nonchemical stressors. Cham: Springer International Publishing, p. 81–125.
- SCHER/SCCS/SCENIHR. 2013. Toxicity and assessment of chemical mixtures. Eur. Comm.
- Schmidt F, Marx-Stoelting P, Haider W, et al. 2016. Combination effects of azole fungicides in male rats in a broad dose range. Toxicology. 355–356:54–63.
- Schneider S, Fussell KC, Melching-Kollmuss S, Buesen R, Gröters S, Strauss V, Jiang X, van Ravenzwaay B. 2017. Investigations on the dose–response relationship of combined exposure to low doses of three anti-androgens in Wistar rats. Arch Toxicol. 91(12):3961–3989.
- Schneider S, Fussell KC, Melching-Kollmuss S, Gröters S, Strauss V, Siddeek B, Benahmed M, Frericks M, van Ravenzwaay B. 2015. Low dose/dose response relationship of hormonally active substances and their mixture – testing endocrine disruptors in classical and molecular endpoints at human-relevant exposure levels. Toxicol Lett. 238(2):S52.
- Sergievich AA, Khoroshikh PP, Artemenko AF, Zakharenko AM, Chaika VV, Kodintsev VV, Stroeva OA, Lenda EG, Tsatsakis A, Burykina TI, et al. 2020. Behavioral impacts of a mixture of six pesticides on rats. Sci Total Environ. 727:138491.
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. 2001. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 16(5):972–978.
- Skakkebaek NE. 2002. Endocrine disrupters and testicular dysgenesis syndrome. Horm Res Paediatr. 57(2):43–43.
- Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, Cory-Slechta DA. 2014. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology. 45:121–130.
- Svingen T, Ramhøj L, Mandrup K, Christiansen S, Axelstad M, Vinggaard AM, Hass U. 2018. Effects on metabolic parameters in young rats born with low birth weight after exposure to a mixture of pesticides. Sci Rep. 8(1):305.
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, Tabuchi Y, Nanmori T, Qi W-M, Inamoto T, et al. 2009. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett. 189(1):40–47.
- Temkin CL. 1941. Seven defences, the reply to certain calumniations of his enemies. In: Sigerist HE (ed) Four treatises of Theophrastus von Hohenheim called Paracelsus. The Institute of the History of Medicine, The Johns Hopkins Press.
- Tsatsakis AM, Docea AO, Calina D, Buga AM, Zlatian O, Gutnikov S, Kostoff RN, Aschner M. 2019c. Hormetic Neurobehavioral effects of low dose toxic chemical mixtures in real-life risk simulation (RLRS) in rats. Food Chem Toxicol. 125:141–149.
- Tsatsakis A, Docea AO, Constantin C, Calina D, Zlatian O, Nikolouzakis TK, Stivaktakis PD, Kalogeraki A, Liesivuori J, Tzanakakis G, et al. 2019a. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicol Lett. 316:154–170.
- Tsatsakis AM, Docea AO, Tsitsimpikou C. 2016. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem Toxicol. 96:174–176.
- Tsatsakis AM, Kouretas D, Tzatzarakis MN, Stivaktakis P, Tsarouhas K, Golokhvast KS, Rakitskii VN, Tutelyan VA, Hernandez AF, Rezaee R, et al. 2017. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum Exp Toxicol. 36(6):554–564.
- Tsatsakis A, Petrakis D, Nikolouzakis TK, Docea AO, Calina D, Vinceti M, Goumenou M, Kostoff RN, Mamoulakis C, Aschner M, et al. 2020. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem Toxicol. 141:111418.
- Tsatsakis A, Tyshko NV, Docea AO, Shestakova SI, Sidorova YS, Petrov NA, Zlatian O, Mach M, Hartung T, Tutelyan VA. 2019b. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes – a real-life risk simulation approach. Toxicol Lett. 315:96–106.
- U.S. EPA 1986. Guidelines for the health risk assessment of chemical mixtures. Fed Regist. 51:34014–34025.
- U.S. EPA 2007. Concepts, methods, and data sources for cumulative health risk assessment of multiple chemicals, exposures and effects: a resource document (Final Report). U.S. Environmental Protection Agency, National Center Environmental Assessment, Cincinnati, OH EPA/600/R-06/013F
- U.S. HHS 2019. Fourth national report on human exposure to environmental chemicals. Updated tables, January 2019, Volume one. Fourth National Report on Human Exposure to Environmental Chemicals 1–529.
- Wade MG, Foster WG, Younglai EV, McMahon A, Leingartner K, Yagminas A, Blakey D, Fournier M, Desaulniers D, Hughes CL. 2002a. Effects of subchronic exposure to a complex mixture of persistent contaminants in male rats: systemic, immune, and reproductive effects. Toxicol Sci. 67(1):131–143.
- Wade MG, Parent S, Finnson KW, Foster W, Younglai E, McMahon A, Cyr DG, Hughes C. 2002b. Thyroid toxicity due to subchronic exposure to a complex mixture of 16 organochlorines, lead, and cadmium. Toxicol Sci. 67(2):207–218.
- WHO 2009. Assessment of combined exposures to multiple chemicals: report of a WHO/IPCS International Workshop. IPCS Harmon. Proj. Doc. no. 7.
- Wolansky MJ, Gennings C, DeVito MJ, Crofton KM. 2009. Evidence for dose-additive effects of pyrethroids on motor activity in rats. Environ Health Perspect. 117(10):1563–1570.
- Yang J, Cao J, Sun X, Feng Z, Hao D, Zhao X, Sun C. 2012. Effects of long-term exposure to low levels of organophosphorous pesticides and their mixture on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochem Funct. 30(2):122–128.