Abstract
Extensive toxicology studies of synthetic vitreous fibers (SVFs) demonstrated that fiber dimension, durability/dissolution, and biopersistence are critical factors for risk of fibrogenesis and carcinogenesis. Lessons learned from the SVF experience provide useful context for predicting hazards and risk of nano-enabled advanced materials. This review provides (1) a historical toxicological overview of animal and in vitro toxicology studies of SVFs, (2) key findings that long durable fibers pose a risk of fibrogenic and tumorigenic responses and not short fibers or long soluble fibers, (3) in vitro and in vivo test methods for biodurability and biopersistence and associated predictive thresholds for fibrosis or tumors, and (4) recommendations for testing of advanced materials. Generally, SVFs (fiber lengths >20 µm) with in vitro fiber dissolution rates greater than 100 ng/cm2/hr (glass fibers in pH 7 and stone fibers in pH 4.5) and in vivo fiber clearance less than WT1/2 40 or 50 days were not associated with fibrosis or tumors. Long biodurable and biopersistent fibers exceeding these fiber dissolution and clearance thresholds may pose a risk of fibrosis and cancer. Fiber length-, durability-, and biopersistent-dependent factors that influence pathogenicity of mineral fibers are also expected to affect the biological effects of high aspect ratio nanomaterials (HARN). Only with studies aimed to correlate in vitro durability, in vivo biopersistence, and biological outcomes will it be determined whether similar or different in vitro fiber dissolution and in vivo half-life thresholds, which exempt carcinogenicity classification of SVFs, can also apply to HARNs.
Introduction
The 3Ds of fiber toxicology (dose, dimension, and durability) as the primary drivers for influencing hazard and disease risk have been well accepted for several decades. Recognition that fiber diameter, length, and durability can influence the extent to which fibers are deposited and retained in the lungs and present a risk of fibrogenic and carcinogenic disease emerged from the toxicological and epidemiologic experience with asbestos. The historical occupational and environmental experience with asbestos prompted caution in the research, regulatory, and industrial communities as new man-made fibers, including synthetic vitreous fibers (SVFs), were introduced into the market. It was through extensive animal and in vitro toxicology research of SVFs that the interplay between fiber dimension, durability, dissolution, and biopersistence (retention in the lungs) were identified as critical factors for SVFs not presenting a risk of fibrogenesis and carcinogenesis in occupational settings. Also, through this research, lung fiber clearance/biopersistence was correlated with fibrotic and tumorigenic responses in animals and fiber dissolution rates in vitro to identify critical thresholds for predicting hazards of novel SVFs in acellular in vitro systems and potentially limiting unnecessary animal testing. The use of acellular in vitro dissolution assays and animal testing, where needed, have been leveraged by industry as a safe-by-design tool to assure novel SVFs do not exceed dissolution/biopersistence thresholds and do not pose a health risk in occupational and end-use settings. Biopersistence is a determinant for hazard and risk that can generally be applied to all particles.
A reflection of the historical toxicological journey of SVFs is pertinent to modern day as well as industries outside SVF manufacturing. Advanced materials, such as engineered nanomaterials, are designed to have unique sizes, shapes and properties, which in some cases may include high-aspect ratio fibers (WHO respirable fibers – fiber length of >5 µm, diameter <3 µm, length:width ratio >3:1; generally long high-aspect ratio fibers have lengths >10–20 µm and aspect ratios >3–5). The Organization for Economic Co-operation and Development (OECD) recognized the need to design nanomaterials with safety in mind and formed a Working Party on Manufactured Nanomaterials (WPMN) in 2006 with the aim to ensure that the hazard, exposure, and human health risk assessment of nanomaterials is high quality and science-based through internationally harmonized standards (OECD Citation2018). Since the formation of the WPMN, the OECD has published nearly 100 reports outlining various methods to characterize the physicochemical properties, exposure and potential health risk of nanomaterials. The lessons learned from the historical perspective of the SVF experience provide useful context for evaluating and developing predictive tools to assess the hazards and risk of nano-enabled and advanced materials in a safety-by-design framework. As such, this review will (1) provide a historical toxicological overview of the animal and in vitro toxicology studies SVFs, (2) discuss key aspects of the historical research, which contributed to the understanding that long durable fibers pose a risk of fibrogenic and tumorigenic responses and not short fibers or long soluble fibers, (3) summarize available in vivo and in vitro test methods for biodurability and biopersistence and thresholds for solubility and lung clearance which are not associated with fibrosis or tumors, and (4) offer recommendations for hazard and toxicological testing of new advanced materials (e.g. high aspect ratio nanoparticles of sufficient length and durability).
Overview of fiber toxicology
Fiber dimension influences the location and extent to which fibers are inhaled, deposited in, and cleared from the lungs. While particles with an aerodynamic diameter of 10 µm and 4 µm will have 50% penetration to the thoracic and respiratory regions of the lung (Brown et al. Citation2013), the aerodynamic properties of fibers are driven by fiber diameter. The propensity of fibrous particles (with an aspect ratio [length-to-width ratio] of 3:1 or larger) to deposit in the respiratory tract is largely determined by fiber diameter, and, to a lesser extent, fiber length. Fibers with diameters less than 3 µm align with the airstream and aerodynamically behave like small isometric particles and deposit in the deep lung (Hesterberg and Hart Citation2000). While fibers up to 3.5 µm in diameter have been detected in the lungs of asbestos workers, fibers of this dimension may represent the very upper-bound limit of respirability (Gross et al. Citation1971). A number of studies have shown that nearly all fibers deposited in the pulmonary region of the lung are thinner than 0.7 µm (Harris and Timbrell Citation1975; Sussman et al. Citation1991a, Citation1991b; Strom and Yu Citation1994; Yu et al. Citation1994). It is noteworthy that most commercial fibrous glass products are comprised of fiber dimensions that do not penetrate the lungs to any great extent (Lippmann Citation1990). Fiber length has little impact on respirability up to a length of about 20 µm and the deposition of longer fibers is generally inversely related to fiber length. However, it is possible for fibers 100–300 µm in length (with sufficiently thin diameters) to reach the lower lung (Eastes et al. Citation1996).
Once fibers are deposited within the respiratory tract, the mechanism by which they are cleared from the lungs depends not only on the site of deposition, but also on the size and chemistry of the fiber itself (). In general, solid particles are cleared from the lungs through a variety of mechanisms: (1) sneezing, coughing, and removing mucus from the nasopharyngeal region; (2) direct or macrophage-mediated transport along the mucociliary escalator and subsequent elimination by the gastrointestinal tract; (3) direct or macrophage-mediated transport across the bronchiolar or alveolar epithelium and subsequent clearance by the systemic circulation or interstitial lymphatics; and (4) physicochemical processes, including dissolution, leaching, and physical breakdown of particles (Madl et al. Citation2018). illustrates the various mechanisms by which deposited fibers can be removed from the lungs.
Figure 1. Mechanisms by which respirable short or long fibers clear from or persist in the lungs and pose a risk of non-carcinogenic and carcinogenic health effects. Respirable long biodurable fibers accumulate and persist in the lungs because of reduced dissolution and breakage and evasion of direct or macrophage-mediated transport out of the lungs. Whereas respirable short fibers or respirable biosoluble fibers undergo dissolution and cellular/acellular transport mechanisms to clear from the lungs (in non-overload conditions) and not pose a risk pulmonary health effects.
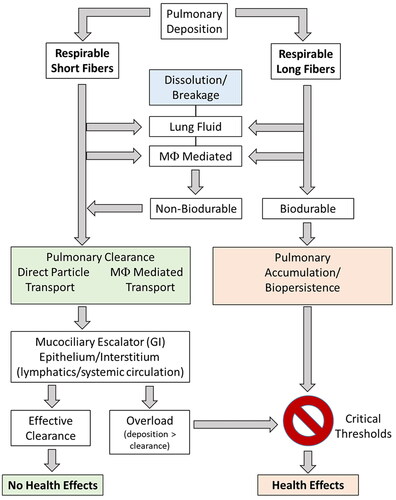
Macrophage-mediated clearance of deposited fibers is critical for clearance of “short” fibers, but also dissolution and breakage of “long” fibers. Human alveolar macrophages are approximately 14–21 µm in diameter and can readily engulf and clear fibers 15–20 µm in length, whereas rat pulmonary macrophages are 10.5–13 µm in diameter and are generally limited to clearance of fibers less than 7 µm in length (fibers >17 µm in length are generally too long to be completely engulfed) (Crapo et al. Citation1983; Lum et al. Citation1983; Sebring and Lehnert Citation1992; Stone et al. Citation1992; Krombach et al. Citation1997; Maxim et al. Citation2006). Fibers too long to be engulfed and cleared by macrophages must undergo dissolution and breakage in lung lining fluid (pH 7.4) and/or macrophage lysosomal fluid (pH 4.5) to be cleared from the lungs. Fibers that persist and are not completely engulfed by macrophages may cause “frustrated phagocytosis”, which triggers release of reactive oxidant species and acidic phagolysosomal contents into the surrounding tissue, stimulates inflammation, and initiates histopathological changes under chronic conditions (e.g. fibrosis, neoplasm). Thus, the biodurable and biopersistent nature of a fiber in the lungs will directly influence the extent to which a fiber will pose a hazard and risk of disease. While biodurability and biopersistence are terms that have been used interchangeably, they have distinct meanings. Biodurability refers to the fiber dissolution rate, which is typically measured in in vitro systems, and differs from biopersistence which encompass additional fiber removal mechanisms in vivo (Maxim et al. Citation2006). Biopersistence is defined as the ability of a fiber to remain in the lungs despite the various clearance mechanisms involved (e.g. alveolar macrophage clearance, dissolution, breakage, transport, mucociliary escalator) (Muhle et al. Citation1994; Muhle and Bellmann Citation1997; ). Biopersistence is measured in in vivo studies of laboratory animals or in human lung samples. Generally, the biopersistence and clearance half-life of fibers are measured by lung fiber burden analysis in which lung tissue samples are digested and the remaining fibers are counted. A detailed summary of methods for determining fiber dissolution rates in vitro and fiber biopersistence (and clearance) in vivo are provided in later sections.
When evaluating the effects of fibers in animal toxicology studies, there are a number of key anatomical and physiological differences between rodents and humans that need to be considered. First, as mentioned previously, the size of rat alveolar macrophages is smaller than that human alveolar macrophages. Thus, fibers which may elicit an inflammatory or fibrotic response in rats may not be expected to occur in humans because human alveolar macrophages are larger and can engulf longer fibers than alveolar macrophages in rodent species. Second, the rodent lifespan is 35-fold less than a human, and rats have a faster rate of aging and developing cancer than humans (Berry Citation1999). As such, the predicted tumor incidence in humans (e.g. asbestos and mesothelioma) is highly dependent on the clearance rate of durable fibers, whereas in rats, a similar dependence of fiber biopersistence-tumor incidence occurs at 17 times higher rate of elimination corresponding to less durable fibers (Berry Citation1999). Third, the anatomical features of the respiratory tract of a rat can limit, to some extent, the delivery and deposition of fibers in the lungs. Rats have highly convoluted nasal turbinates, are nose breathers, and have an asymmetrical airway branching pattern, which can enhance fiber interception deposition patterns in the nasal cavity and upper respiratory tract and limit fiber deposition in the lower respiratory tract. Generally, fibers with physical diameter of 1 µm or less are considered respirable in rats, whereas fibers with a diameter of 3 µm or less are respirable in humans (Hesterberg and Hart Citation2001). Fourth, macrophage-mediated clearance of insoluble particles is faster in rats compared humans, with reported half-times of 45–99 days in rats and 33–2500 days in humans (Muhle et al. Citation1990; Schlesinger Citation1995; Snipes Citation1995; ILSI Citation2005). Fifth, the method and extent of fiber exposures in animal toxicology studies may result in “overload” conditions, in which the lung’s normal mechanisms of particle clearance are overwhelmed by the sheer volume of material and result in responses that may be an artifact of dose rather than physicochemical properties of the inhaled material. All of these factors add complexity to understanding the physicochemical dynamics of fiber biodurability and biopersistence in evaluating effects in rodents and predicting responses in humans. A review of the historical animal toxicology studies of SVFs in the sections below provide a historical account as to how the state-of-the-science evolved from intrapleural and intraperitoneal studies to intratracheal instillation and inhalation studies that eventually led to the understanding that (1) long durable fibers (but not short fibers or long soluble fibers) pose a risk of fibrogenic and tumorigenic responses, and (2) correlations between fiber biopersistence and tumor incidence in vivo and fiber dissolution in vitro provide a basis for fiber biopersistence safety thresholds for newly developed SVFs.
The compilation of over three decades of fiber and SVF research has led to a regulatory framework (in the European Union) in which newly developed SVFs are exempted from classification as a carcinogen if a substance meets one of the following criteria (European Economic Community Citation1997):
A short-term biopersistence test by inhalation has shown that the fibers longer than 20 µm have a weighted halftime less than 10 days, or
A short-term biopersistence test by intratracheal instillation has shown that the fibers longer than 20 µm have a weighted halftime less than 40 days, or
An appropriate intraperitoneal test (single dose of 1 × 109 WHO fibers per animal) has shown no evidence of excess carcinogenicity, or
Absence of relevant pathogenicity or neoplastic changes in a suitable long-term inhalation test.
In the modern era of the 3Rs (reduce, refine, replace) of animal toxicology research, the application of acellular in vitro test methods to measure fiber biodurability or dissolution and predict in vivo biopersistence is paramount to designing SVFs for safe use. The historical experience and lessons learned with SVFs have important implications for the testing and safety evaluation of advanced materials, such as high-aspect ratio nanoparticles or nanofibers.
Classification and physicochemical characteristics of SVFs
SVFs are a class of materials that have major uses for commercial and residential insulation against heat and sound. SVFs are produced by melting various types of rock (stone), sand, slag, clay, or ceramic fibers and then blowing or extruding these raw materials into fibers of specific properties. Specific chemical and physical characteristics are driven by the end-use needs of each product, such as needs for high strength, high electrical resistivity or resistance to chemical agents. All SVFs are composed of a silicate backbone but vary considerably with respect to other components between classes of fibers and within an individual class (WHO Citation2000). In contrast to the crystalline structure of asbestos fibers, SVFs have no discernable crystalline structure and are comprised of an amorphous elemental/molecular arrangement making them susceptible to dissolution and breakage in the lung (NEHC Citation1997; Bernstein Citation2007). As a result of their amorphous nature, SVFs lack longitudinal cleavage planes and thus do not split lengthwise. Rather, in the fiber dissolution process, SVFs exhibit horizontal fractures, resulting in shorter fibers of the same diameter (Assuncao and Corn Citation1975; IARC Citation2002). SVFs remain vitreous when used at temperatures below 500 °C; as temperatures increase, depending upon composition, SVFs can flow, melt or devitrify. High-silica and low-alkali metal oxide compositions such as refractory ceramic fibers, alkaline earth silicate (AES) wools, and some rock wools will start to devitrify at temperatures above 900 °C (NEHC Citation1997).
SVFs are also manufactured with a variety of coatings, binders, sizing, and dedusting agents (Hamilton et al. Citation1994). The quantity of binder ranges from 0.5 to 1.5% by mass and varies depending on the intended use of the product (IARC Citation2002). The exception is high-density insulation wool products which may contain up to 25% binder by mass (IARC Citation2002). The composition and proportion of binder differs between products. Different types of oil are used as a lubricant in rock and slag wools, while glass fibers are usually a complex formulation containing lubricant, resin for binding, and one or more cation active agents for adhesion (WHO Citation2000). SVFs are categorized by filaments and wools, where filaments (e.g. continuous glass filaments) are drawn continuously during manufacturing and wools (e.g. glass wool, rock/stone wool, slag wool, refractory ceramic fibers, AES) are spun (WHO Citation2000).
Glass fibers
Glass fibers utilize finely powdered sand as the major silica source; aluminum oxides from either kaolin clay or synthetic sources are commonly added as well as calcium and magnesium oxides from dolomite and boric acid derived from calcium borate (NIOSH Citation1980; IARC Citation1988; TIMA Citation1990; NEHC Citation1997). Water soluble glass fibers typically contain a lower percentage of alkali metal oxides (K2O, Na2O3, Li2O) and a higher percentage of alkali earth metals (Ca, Mg, Fe), which confer lower chemical resistance (TIMA Citation1990). Stabilizers including oxides from aluminum, titanium and zinc contribute to the fiber’s durability; levels are dependent upon the intended use of the product (NRC Citation2000).
In addition, there are glass subtypes specific to end use needs. Electrical glass (E glass, 99% of which is continuous filament glass) has a low alkali content but a higher aluminum content (14.8% as Al2O3) making it insoluble in hydrochloric acid (NEHC Citation1997). Chemical glass (C glass) is designed to be chemically resistant to acids; high tensile strength glass (S glass) has 33% more tensile strength than E glass. Another version is alkali resistant (AR glass), which is capable of reinforcing 20–30 times its weight due to its zirconium content and is often found in cement reinforcement (NEHC Citation1997).
Rock wool
Rock or stone wools are produced by melting various igneous rocks including basalt, olivine, and diabase which are typically composed of 40–60% calcium and magnesium carbonate and are dissolvable in hydrochloric acid (NIOSH Citation1980; TIMA Citation1990; NEHC Citation1997). Rock wool is primarily composed of silicon dioxide (45–52%), calcium oxide (10–12%), magnesium oxide (8–15%), and aluminum oxide (8–13.5%) (NEHC Citation1997). Rock wool products also have a substantial non-fibrous component making up between 20–50% of the total mass of the product. Rock wool fibers have tensile strength ranging from 70 to 100 × 103 PSI and are optimal for high temperature insulation applications given their high melting point range (NEHC Citation1997; IARC Citation2002).
Slag wool
Slag wool recycles blast furnace waste (iron-ore slag is a common starting material) with the final composition dependent upon the metallic content of the slag starting material. Slag wools lack significant sodium and are typically slightly soluble in hydrochloric acid. Other materials are added to compensate for compositional deficiencies; for example, if acid oxides (silica) predominate, then limestone or a slag rich in calcium oxide is added (NEHC Citation1997). Similar to rock wools, slag wools have tensile strength ranging from 70 to 100 × 103 PSI (NEHC Citation1997). Non-fibrous material is slightly higher in slag wool than rock wool, with a range of 30–50% and also ideal for high temperature insulation applications as a result of their higher melting points (NEHC Citation1997).
Refractive ceramic fibers
Refractive ceramic fibers (RCFs) are the most specialized of the SVFs, with varied composition including kaolin clay based, aluminum silicate and metallic oxide blends (chromous or zirconia), and high purity aluminum silicates. Trace amounts of metal oxides, commonly boron, titanium, magnesium, and/or chromium are also present (TIMA Citation1990; NEHC Citation1997). RCFs are specifically designed for uses requiring high-temperature tolerance (1000–1460 °C), with higher percentages of alumina and zirconia oxides to allow these fibers to retain their physical properties at high temperatures (Maxim et al. Citation1999; EIPPCB Citation2013; Mast et al. Citation2000). AES wools were developed as an alternative to the aluminosilicate composition of traditional RCF, although they lack the high heat tolerance of traditional RCF (IARC Citation2002). One limitation, however, to increased levels of aluminum is a decreased dissolution rate (Maxim et al. Citation2006).
While RCF are manufactured as amorphous fibers, they will devitrify at higher temperatures. As temperatures reach 980–1100° C, the alumina-silica matrix is chemically changed to mullite, an aluminosilicate crystalline compound. Further increases in temperature result in excess silica crystallizing to cristobalite, with maximum conversion at 1200 °C and a viscous liquid at 1400 °C (IARC Citation1988; TIMA Citation1990; NEHC Citation1997).
Other fibers
Other fibers do not fit neatly into traditional fiber categories, such as X607, which is a high-silica (57.9%) fiber with glass-like properties that is manufactured similar to rock and slag wools (Hesterberg, Hart, et al. Citation1998). Originally developed to withstand higher temperatures than glass fiber (1300°F, as compared to 900°F for glass fibers), it is less expensive than RCF fibers and can be used to augment RCF insulation, which would still be in contact with higher heat surfaces (Hesterberg, Hart, et al. Citation1998). In contrast to RCF fibers, X607 (discussed in a later section) dissolves rapidly in vitro at pH 7.0, is cleared rapidly from the lungs, and does not demonstrate fibrogenic or tumorigenic activity (Hesterberg, Hart, et al. Citation1998).
Animal toxicology studies of SVFs
Early studies by Pott (Pott and Friedrichs Citation1972; Pott et al. Citation1987) and Stanton et al. (Citation1972, Citation1981b), exposing rodents to fibrous dusts found a relationship between fiber dose, dimension, durability and resulting toxicity. From these experiments came the Stanton-Pott hypothesis, stating the severity of the biological effect of a given fiber is directly related to its dimensions, with longer and thinner fibers being more harmful (Hesterberg and Hart Citation2001). Early in vivo studies delivered fibers based on mass; although it was soon discovered that suspensions of fibers are rarely homogeneous with respect to size or mass. As study methodology evolved, the importance of size separating bulk material to obtain fibers suitably respirable for the target species was evident and became standard practice (Hesterberg et al. Citation1993). Fibers were also more clearly characterized in terms of fiber numbers, diameter and length distribution, and non-fibrous content. Later aerosol generation systems were also developed to minimize fiber breakage and non-fibrous dust and were monitored for temperature, relative humidity, and oxygen concentration throughout exposures (Hesterberg et al. Citation1993). The historical account of the toxicology studies of SVFs by method or route of exposure are provided in the sections below, whereas detailed summaries of individual studies are provided in Appendices Tables (Table A1 – Intraperitoneal Injection, Table A2 – Intrapleural Injection, Table A3 – Intratracheal Instillation, Table A4 – Whole-Body Inhalation, and Table A5 – Nose-Only Inhalation).
Table 1. Summary of in vitro dissolution, in vivo retention and biological responses of SVFs.
Table 2. Glass SVF biological effects corresponding to in vitro dissolution at pH 7.4, lung retention following inhalation or intratracheal instillation in .
Table 3. Stone SVF biological effects corresponding to in vitro dissolution at pH 4.5 or 7.4 and lung retention following inhalation in .
Intraperitoneal injection studies
Intraperitoneal (IP) and intrapleural injection ([IPL], discussed in the next section) involve injection of bolus doses into the intraperitoneal or intrapleural cavity, respectively. While this route of exposure and bolus dose method are not representative of inhalation exposures, early studies utilizing this method provided fiber hazard ranking information and was the foundation upon which fiber dimension (especially long thin fibers) was recognized as a driving factor for lung pathogenesis (Vorwald et al. Citation1951). While a brief review of early SVF studies are described herein, it is important to keep in mind that IP and IPL studies have a number of inherent limitations. IP or IPL injection or implantation studies generally deliver exceedingly high doses (typically defined by fiber mass rather than fiber number) in body compartments well beyond that would be experienced through inhalation and that bypass mechanisms of dissolution, breakage, and removal of deposited fibers from the lungs. As a result, IP and IPL dose administration bypass the normal clearance mechanisms of the lung (mucociliary clearance, pulmonary macrophages), and deliver doses directly to compartments (peritoneum, intrapleural space) that may overwhelm clearance and repair mechanisms and lead to tumors (which would not otherwise occur through inhalation). These early IP and IPL studies often resulted in false-positive results, producing a significant increase in mesotheliomas for most fibers (Roller et al. Citation1996; Miller et al. Citation1999), for which later studies conducted by inhalation or intratracheal instillation showed that certain non-biodurable and non-biopersistent fibers were non-carcingenic (IARC Citation2002; NTP Citation2011; OEHHA Citation2011). Further, these early studies lacked specifications for fiber selection and characterization, which was subsequently codified in guidelines for standardization of fiber selection and IP studies in the European Commission (EC) Directive 97/69/EC, adopted in 1997 (European Economic Community Citation1997). With careful fiber characterization, IP injection studies can be comparable in terms of hazard ranking to studies of carcinogenicity following chronic inhalation of fibers, provided that the delivered fibers are of similar biopersistence and length (Bernstein et al. Citation2001a, Citation2001b). Overall, evaluation of biopersistence of long fibers indicated that fibers longer than 20 µm are a good predictor of the tumor response in chronic IP studies (Bernstein et al. Citation2001b).
Glass fibers
While early IP injection studies of SVFs (MMVF10, MMVF11, JM104 and JM112 glass wool fibers) reported tumors in rodent species, these studies generally delivered high bolus doses that likely overloaded natural defenses and did not characterize fiber size distribution of injected fibers (Pott et al. Citation1976; Citation1988; Hesterberg et al. Citation1993; Pott et al. Citation1994; Bernstein et al. Citation1996; Roller et al. Citation1996; Miller et al. Citation1999; Searl et al. Citation1999). However, despite these shortcomings, there were indications that fiber size and dissolution may be important determinants of tumorigenicity. For example, acid or base treatment of glass fibers prior to IP injection showed a different propensity of tumor formation compared to untreated glass fibers (Pott et al. Citation1988). Pott et al. (Citation1988) showed that acid treatment of JM104 glass fibers (90% of fibers <8.4 µm long and <0.44 µm diameter) influenced the rodent tumor incidence following IP injection. While JM104 fibers treated with NaOH showed a similar tumor incidence (approximately 80%) as untreated fibers, JM104 fibers treated with HCl for either 2 or 24 h showed a lower tumor incidence of ∼60% and <10% respectively, suggesting that fiber durability (and perhaps biopersistence) was decreased for fibers in an acidic environment. Interestingly, SEM imaging did not show acid/base treatment altered fibers (Pott et al. Citation1988). JM475 glass fibers (median dimensions 3.2 μm × 0.18 μm) treated for 24 h with HCl and IP injected in rats resulted in a higher tumor incidence (50% versus 17–26%) compared to untreated JM475 glass fibers.
With other types of glass fibers, intraperitoneal injection studies comparing fibers of different sizes also showed that fiber dimension influences the incidence of tumors. Specialty glass fibers including either a thin, shorter B-09-0.6 (median dimensions 0.49 × 3.3 μm) or thicker, longer B-09-2.0 (median dimensions 1.2 × 10.5 μm) experimental glass wool had incidences of peritoneal mesotheliomas of 3% (100 mg B-09-0.6), 10% (300 mg B-09-0.6), 23% (150 mg B-09-2.0) and 53% (450 mg B-09-2.0); 0 to 1.4% (1/69 rats) mesotheliomas were detected in saline controls (Roller et al. Citation1996, Citation1997). Under the study conditions, exposure to longer and thicker fibers resulted in a greater number of tumors (Roller et al. Citation1996, Citation1997). In addition, IP studies showed that larger diameter glass fibers resulted in a lower tumor incidence compared to smaller diameter fibers. In these studies, JM475 glass fibers, which are typically used for filtration of air and liquids as opposed to insulation, were used. Although often grouped with E-glass, these fibers are both non-fibrogenic and non-carcinogenic and should not be equated to E-glass fibers (Bernstein Citation2007). Generally, these fibers are coded according to mean fiber diameter, with larger numbers indicating larger diameters (e.g. Johns Manville [JM] 110/475 fibers have a greater nominal diameter (1.9–3.0 μm) than JM100/475 fibers (0.28–0.38 μm). A single IP injection of 0.5 mg JM104/475 fibers resulted in a tumor rate of 17% (Muhle et al. Citation1987), whereas an IP injection of 8.3 mg 100/475 glass resulted in a mesothelioma incidence of 33% (Miller et al. Citation1999). Tumor incidences in these studies appear to be consistent with the slow dissolution rates of 9.1 ng/cm2/h for 100/475 fibers and 12 ng/cm2/h for 475 glass fibers (nominal diameter not provided) (Hesterberg, Chase, et al. Citation1998; Miller et al. Citation1999).
Other IP studies of glass fibers with higher dissolution rates show a lack of tumorigenicity. Intraperitoneal injection studies of biosoluble glass fibers (M, P, and V), and one soluble glass fiber (B) were administered by repeated intraperitoneal injection at doses of 0.5, 2.0, or 5 × 109 WHO fibers to female rats (Grimm et al. Citation2002). The tumor response in this study was not statistically significant or treatment related for any of the glass fibers. Considering that the dissolution rates for M, P, V, and B glass fibers are 103.7, 610, 450, and 580 ng/cm2/hr, respectively (Bernstein et al. Citation1996), the findings from Grimm et al. (Citation2002) are consistent with highly soluble fibers having low biopersistence and tumorigenic potential.
Rock and slag wools
Studies of rock and slag wools also appear to show similar findings as glass fibers in that fibers with higher dissolution rates show a lower propensity for tumor formation following IP injection. For example, mesothelioma incidences of MMVF21 (30 mg IP) showed a 97% mesothelioma incidence for two injections per week and 87% incidence for five injections per week, whereas R-stone E3 resulted in no tumor incidence for four weekly injections and 11% tumor incidence for nine weekly injections (Pott et al. Citation1993; Davis et al. Citation1996; Roller et al. Citation1996). While MMVF21 is known to be a more durable fiber (Kdis=21.8 ng/cm2/h in pH 7.4), results from equivalent dimension and higher dose suggest that R-stone E3 fibers (no Kdis data available) are less durable. These findings were further supported by a study by Miller et al. (Citation1999), in which MMVF21 stone wool (Kdis=21.8 ng/cm2/h in pH 7.4) showed a higher mesothelioma incidence (95 vs. 59%) compared to the more biosoluble fiber, MMVF22 (Kdis range of 52.8–400 ng/cm2/h in pH 7.4).
Other historical IP studies of stone wool fibers showed mixed results and did not always have corresponding in vitro fiber dissolution rates or fiber size characterization to put the in vivo biological effects into appropriate context of biodurability and biopersistence. For example, M stone wool fibers (Pott et al. Citation1993; Davis et al. Citation1996; Roller et al. Citation1996) and experimental rock wool B-20 fibers (Pott et al. Citation1993; Davis et al. Citation1996; Roller et al. Citation1996) do not have reported corresponding dissolution rates and showed a range of peritoneal mesothelioma incidence rates (6–63% for M-stone fibers depending on dose, 6–87% for B-20 fibers depending on dose) following IP injection. When administered by IP injection to female rats, finer B-20 fibers (B-20-0.6, median diameter, 0.3 µm) showed generally a greater incidence of mesotheliomas (30, 43, or 75% for 3.5, 8.5, or 25 mg single doses, respectively) compared to B-20-2.0 fibers with wider fiber dimensions (6 or 42%, 22 or 35%, for 6 or 18 single doses to female or male rats, respectively; or 60% for two 30 mg injections in males only) (Pott et al. Citation1993; Davis et al. Citation1996; Roller et al. Citation1996). The explanation for the greater tumorigenic response of B-20-0.6 fibers compared to B-20-2.0 fibers may likely be attributed to the larger doses of WHO fibers of the B-20-0.6 fiber treatment group.
Other stone wool fibers with demonstrated high solubility and low biodurability show a lack of tumorigenic potential. The highly biosoluble stone wool fiber (O) administered by repeated intraperitoneal injection (0.5, 2.0, or 5 × 109 WHO fibers per injection with either 2, 8, or 20 weekly injections) did not show statistically significant tumorigenic responses at any dose (Grimm et al. Citation2002). With an in vitro dissolution rate of Kdis = >500 ng/cm2/h, the Grimm et al. (Citation2002) study illustrates that highly soluble, non-biodurable fibers do not have a propensity for tumorigenesis (Hesterberg, Hart, et al. Citation1998; Maxim et al. Citation2006).
Refractory ceramic fibers
The tumorigenic potential of RCFs in IP toxicology studies is similarly dependent on fiber dissolution and biodurability. Early IP injection studies of Fiberfrax RCF wool and Manville RCF wool show varying tumor incidence rates in IP injection studies (Pott et al. Citation1987; Smith et al. Citation1987), however dissolution rates have not been reported on these fiber types to put the biological effects into context. In a 1999 study by Miller, RCF1 and RCF2 showed mesothelioma incidence of 88% and 72%, respectively, whereas RCF4 fibers showed no incidence of tumors. The dissolution rate of RCF1 and RCF2 of 4.4 and 3.1 ng/cm2/h at pH 7.4, respectively, signifies a relatively low dissolution rate and high biodurability, which is consistent with the high tumor incidence observed in IP animal studies. Interestingly, the dissolution rate for RCF4 is slower than either RCF1 or RCF2 at a pH of 7.4 at 0.5 ng/cm2/h, which is inconsistent with the observation of no tumors in these animals (Miller et al. Citation1999). However, RCF4 is a heat-treated fiber that had fewer long thin fibers compared to RCF1. The heat treatment of RCF4 may have altered the chemical structure and explain the discrepancy between the low dissolution rate in pH 7.4 in vitro and lack of tumors in animals.
In summary, IP studies of glass, stone, and RCF SVFs provide some but limited insight on the relationship between fiber size, durability, and risk for tumors. IP studies of SVFs were limited by extreme administered doses and lack of fiber size characterization. However, retrospectively considering these IP studies with more modern knowledge of fiber dissolution rate, there were indications that some SVFs (e.g. M, P, V, and B glass fibers) with high dissolution rates (Kdis of 100–400+ ng/cm2/h) had a low propensity for tumorigenicity.
Overall, the utility of the intraperitoneal assay for screening potential human hazards is limited. The route of exposure does not mimic any possible route in humans and the suggested, but arbitrary, dose of 109 WHO fibers is unrealistically high. In many cases, fiber burdens in earlier studies exceeded this level. In addition to the fiber clearance mechanisms being completely different between the peritoneal cavity and the lung, the IP methodology of toxicity testing has also been criticized as overly sensitive with a time scale of tumor production that is significantly shorter than that in humans. While IP toxicology studies may be generally insightful for hazard ranking of different fiber types, utilizing these assays for prediction or classification of the carcinogenicity is more tenuous. For intraperitoneal results to have significance, some relationship should exist between biopersistence, dose, and dose distribution of fibers in the lung. As discussed in more depth later, in the lung, the diameters of the long glass fibers (>20 µm) declined at a rate consistent with their exposure to a neutral pH, while the diameter of shorter fibers declined at a slower rate, consistent with exposure to a more acidic environment (phagolysosomes of alveolar macrophages). In the peritoneal cavity, regardless of length, glass fibers dissolved at the same rate (IARC Citation2002). At higher doses, excess material (greater than 1.5 mg) formed clumps of fibers that were either free in the peritoneal cavity or loosely bound to peritoneal organs. These nodules resulted in foreign body reactions with a granulomatous inflammatory response, which could be a nidus for subsequent neoplastic reactions. Thus, differences in both durability of a fiber in the peritoneal cavity and the presence of these nodules have implications for the use of intraperitoneal injections to assess potential carcinogenicity (Collier et al. Citation1994).
Intrapleural injection studies
Early IPL studies either injected or surgically implanted fibers directly into the pleural space to evaluate hazard of various fibers and model risk for pleural mesothelioma (Wagner Citation1963; Wagner and Berry Citation1969; Stanton and Wrench Citation1972). These studies had a number of limitations, such as the use of large, non-physiologically relevant quantities of fibers and bypassing normal defense mechanisms following inhalation. These early studies also typically lacked information on chemical composition of fibers and characterization of fibrous versus non-fibrous composition (IARC Citation2002). While IPL studies provide limited context for risk assessment, the information gained from these studies are useful from the perspective that biodurable fibers longer than 10–20 μm are most likely to induce a biological response (IARC Citation2002).
Glass fibers
While most studies directly placing fibers into the pleura used injection, Stanton implanted 40 mg of one of 17 glass fibers in 1.5 ml gelatin smeared onto coarse fibrous glass pledgets surgically into the left thoracic cavity. The incidence of pleural mesothelioma in animals that survived for more than 52 weeks varied from 0/28 to 20/29, and was dependent upon fiber size with the most carcinogenic fibers <1.5 μm in diameter and >8 μm in length. A key finding of this study was fibers less than or equal to 8 μm in length were inactivated by phagocytosis (Stanton et al. Citation1977). This research was basis of the Stanton hypothesis which indicates that the “carcinogenicity of inorganic particulates depends on dimension and durability rather than on physiochemical properties” (Stanton et al. Citation1981a). Increase in carcinogenicity was correlated with fibers longer than 8 μm, suggesting biodurability plays a key role for longer fibers. Interestingly, Wylie et al. (Citation1987) conducted a follow-up evaluation of the fiber size distribution characteristics of samples administered in the Stanton et al. (Citation1977) study because of the recognized variability of the dose-response relationship between long, thin fibers and carcinogenicity. After evaluating the fiber size distribution, sample reproducibility, and fiber-mass-number conversion of the Stanton samples, Wylie and colleagues concluded that additional factors besides size, shape, and abundance play a role in the development of tumors (Wylie et al. Citation1987).
Two studies investigated tumor incidence following a single intrapleural injection of various borosilicate fibers in mice or rats (Wagner et al. Citation1973; Davis et al. Citation1978). Wagner and colleagues administered to rats 20 mg by intrapleural injection of either borosilicate with 30% of fibers 1.5–2.5 μm in diameter (maximum diameter of 7 μm) and 60% >20 μm in length or borosilicate glass powder (with a diameter <8 μm). No tumor incidence was noted with glass fibers, one mesothelioma was identified in the glass powder exposed rats (Wagner et al. Citation1973). Davis and colleagues administered to mice 10 mg of two borosilicate glass fiber samples with average diameters of 0.05 and 3.5 μm, which had fiber lengths of several hundred microns or lengths of <20 μm. No pleural tumors were found in the glass fiber treated mice (Davis et al. Citation1978). For both of these studies, the large burden of fibers did not result in marked tumor incidence; variations in diameter also did not have an impact.
Single intrapleural injections of 20 mg of either a fine fraction (99% <0.5 μm diameter with a median diameter of 0.12 μm; 2% >20 μm in length with a median length of 1.7 μm) or course fraction (17% of fibers <1 μm in diameter with a median diameter of 1.8 μm; 10% >50 μm in length and a median length of 22 μm) JM100 glass fiber were delivered to rats (Wagner Citation1976). No pleural tumors were noted with the coarse fibers; 12.5% of rats injected with the fine fibers developed mesotheliomas (Wagner Citation1976). In a later study by the same group, 20 mg either English glass fiber dust with resin coating (70% fibers <5 μm in length; 85% <1 μm in diameter), English glass fiber without resin coating (57% <5 μm in length; 85% < 1 μm in diameter), or US JM 100 glass fiber (88% <5 μm in length; 98.5% ≤1 μm in diameter) were given via a single intrapleural injection. One mesothelioma occurred with English glass fiber dust; it was not clear if this occurred in the coated or uncoated group. Mesotheliomas were found in 4/48 (8.3%) animals in the glass fiber group (Wagner et al. Citation1984). Similar to the 1976 study, the finer JM100 glass fibers had a greater impact on tumor incidence. Another study delivered a single intrapleural injections of 20 mg JM104 glass fiber (mean length, 5.89 μm; mean diameter, 0.229 μm) to rats. Animals that received JM104 glass fiber had a mesothelioma incidence of 13.3% (Monchaux et al. Citation1981). With regard to glass fibers, a single exposure to finer fibers (JM100 or JM104) delivered by intrapleural injection induced a relatively low tumor incidence rates. Presence or absence of resin coating did not appear to impact fiber diameter although removal of coating appeared to result in an overall shorter fiber fraction.
Rock and slag wools
Rats received 20 mg either Swedish rock (stone) wool with resin coating (70% fibers <5 μm in length; 52% <0.6 μm in diameter), without resin coating (70% <5 μm in length; 58% <0.6 μm in diameter) or German slag wool with resin (67% <5 μm in length; 42% <0.6 μm in diameter) or without resin (80% <5 μm in length; 62% <0.6 μm in diameter), injected intrapleurally. Similar tumor incidences were seen in the rock wool groups with 3/48 with mesotheliomas in the resin group and 2/48 in the group without; no tumor induction was noted in the slag wool groups, either with or without resin (Wagner et al. Citation1984).
Refractory ceramic fibers
Rats received single intrapleural injections of 20 mg suspended solids of ceramic aluminum silicate RCF fiber, prepared by ball mill grinding (diameters between 0.5 and 1 μm, no length given). Of the 31 animals injected with RCF, three were found to have pleural mesotheliomas (9.7%). RCF fiber length was not described and it is unclear if ball milling resulted in lower levels of long fibers, which could have contributed to low tumor incidence. Electron microscopy images for RCF show large amounts of non-fibrous particulate, which may have confounded findings (Wagner et al. Citation1973).
Rats received a single intrapleural injection of 20 mg suspended solids of either fiber A made from kaolin (diameter ≤3 μm, 66%; length ≥10 μm, 80%) or fiber B made from alumina and silica (diameter ≤3 μm, 92%; length ≥10 μm, 46%), prepared by grinding, then sieving to remove large particles. No mesotheliomas were observed in animals treated with fiber A; fiber B rats had a single pleural (2.1%) and two peritoneal mesotheliomas in (4.1%), although the latter were deemed a result of a partial deposition of the dose in the peritoneum (Pigott and Ishmael Citation1992).
In summary, while there are numerous limitations to the use of IPL technique for SVF exposure, including the non-physiological loads injected, some important data has come from the collection of historical studies. The end goal for these early studies was to build an understanding of the comparative effects of various kinds of fibers and understand which fiber characteristics, especially diameter and length, were responsible for fiber-induced disease. Stanton found increase probability of tumorigenesis was correlated with fibers longer than 8 µm and less than 0.25 µm in diameter and also noted fibers less than or equal to 8 μm in length were inactivated by phagocytosis (Stanton et al. Citation1977). The large 40 mg dose used in the Stanton study would result in a greater concentration than would be inhaled by an animal in a two-year bioassay and would be equivalent to 11 g fibrous material into the pleural cavity of a 70 kg human (Hesterberg et al. Citation2012). These studies also indicate fibers longer than 10–20 μm are those most likely to induce a biological response (Stanton et al. Citation1977; IARC Citation2002).
Intratracheal instillation studies
Intratracheal instillation (IT) studies have been used for many decades as a means to evaluate the pulmonary effects of precisely delivered substances to the respiratory system. As described elsewhere (Madl and Pinkerton Citation2009), IT studies have both advantages and disadvantages to characterizing the toxicology of a substance, in particular particles and fibers. One primary advantage is that the exact dose of the delivered substance can be characterized, however the main shortcomings to this type of administration is the bolus nature of the dose delivery and liquid media required for delivery that does not replicate the deposition or clearance patterns of inhaled particles or fibers. Further, the bolus dose delivered by IT instillation is often non-uniform and may result in fibers being deposited in small clumps in the small airways with few fibers reaching the alveolar region (Hamilton et al. Citation1994; Davis et al. Citation1996). This bolus effect often results in the formation of granulomatous lesions in the upper airway (Bernstein et al. Citation1980) and may have significant adverse effects on lung clearance mechanisms (Davis et al. Citation1996), complicating data interpretation (Maxim et al. Citation1999). Despite these limitations and challenges, IT studies may provide useful mechanistic and hazard ranking information. In accordance with the 1997 EU Directive, IT studies can be used to exempt SVFs from classification as a carcinogen providing that short-term biopersistence test by IT has shown that fibers longer than 20 µm have a weighted halftime less than 40 days (European Economic Community Citation1997). The following sections review the historical IT toxicology studies on SVF biodurability, biopersistence, and respiratory effects; a compilation of the in vitro dissolution, in vivo half-life, and biological effects from IT administration of SVFs are summarized in .
Glass fibers
Early IT studies provided limited or no information about fiber size distribution, dissolution kinetics, or lung clearance half-life, which in turn offered limited understanding for biodurability and biopersistence of different SVF types and dimensions. IT studies of JM100 glass fibers (1 instillation/wk for 5 wks, >94% ≥20 µm in length) showed no pulmonary tumors in rats or hamsters (Smith et al. Citation1987), whereas JM 104/475 glass fibers (one 0.5 mg instillation/wk for 20 wks) showed a 14.7% incidence of pulmonary tumors in rats (Pott et al. Citation1987). However, Pott et al. (Citation1987) did not report fiber size distribution (particularly the proportion of fibers >20 µm in length) and both studies did not provide information on dissolution rates or half-time data. While Pott et al. (Citation1988) also did not report dissolution or half-time data, acid treatment of JM104 glass fibers were observed to influence the lung fiber burdens following IT instillation of HCl or NaOH treated fibers. For example, fiber burdens of untreated, NaOH-treated, and HCl-treated JM104 glass fibers were 295 × 106/lung, 76 × 106/lung, and 31 × 106/lung, respectively, 9 months following IT instillation. Fiber burden data suggest greater biopersistence of untreated JM104 than of NaOH- or HCl treated JM104, however tumor incidences of the three test fibers did not follow a dose-response in terms of extent of retained fibers. While the Pott et al. (Citation1988) study illustrated that acid or base treatment can influence the extent that SVFs are retained in the lungs, the lack of fiber distribution, dissolution, and half-time data provided limited information about the physicochemical characteristics of SVFs that preclude or produce inflammatory, fibrotic or tumorigenic responses.
Additional studies during this same time period showed that fibers which had slower fiber dissolution rates in vitro translated to greater biopersistence and longer retention times in the lung. 100/475 and MMVF10 glass fibers have dissolution rates (Kdis) of 9.1 ng/cm2/hour and 122.4–300 ng/cm2/hour, respectively (Searl et al. Citation1999). Following a 4 day instillation in male rats using size-selected 100/475 or MMVF10 glass fibers, it was shown that the more durable 100/475 fibers were cleared from the lung more slowly than the more soluble MMVF10 fibers (no weighted half-time (WT1/2) values reported). As of note, clearance half-times are usually represented by a faster clearance phase (generally associated with tracheobronchial clearance) followed by a slower clearance phase (generally associated with pulmonary clearance). WT1/2 is an index of the complete clearance that includes both fast and slow clearance half-times (Bernstein et al. Citation1996). Interestingly, longer fibers cleared more rapidly than shorter fibers, supporting the hypothesis that long fibers undergo dissolution and disintegration into shorter fibers, which in turn are cleared by cellular, physical, and dissolution processes.
In a study of sheep, animals received a single IT instillation of MMVF11 and were evaluated 6, 40, 60, 180, 360, or 730 days post-treatment (Dufresne et al. Citation1999). Lung clearance decreased with time according to both a fast and a slow kinetic component. The diameter of MMVFs decreased over the course of the study, which was not observed with crocidolite fibers (which showed no change). No typical ferruginous bodies (particle or fiber with a coating of protein and iron regarded as support for metal-catalyzed oxidative stress in cell and tissue injury (Ghio et al. Citation2004)) were found in the group exposed to MMVF11 fibers, but were observed with the crocidolite fibers. Clearance of MMVF11 was thought to proceed through dissolution and macrophage translocation (Dufresne et al. Citation1999). The IT half-time of MMVF11 was calculated to be 84 days, and the dissolution rate at pH 7.4 was reported to range 71–100 ng/cm2/hour (Hesterberg et al. Citation1993; Bernstein et al. Citation1996).
Rock and slag wools
Similar to glass SVFs, historical IT studies of rock or slag SVFs demonstrated that long fibers with greater dissolution rates were more readily cleared from the lungs compared to durable SVFs with slower dissolution rates. Two studies evaluated biopersistence of HT stone wool (Kdis of 59 ng/cm2/hr at pH 7.4), MMVF30 or MMVF31 experimental stone wools (the latter two with chemical composition modified to increase biosolubility) in comparison to MMVF21 (Kdis range of 18–28.9 g/cm2/hr at pH 7.4) (Bellmann et al. Citation1994, Citation1995; Kamstrup et al. Citation1998; Searl et al. Citation1999; Maxim et al. Citation2002). Fiber burdens categorized by fiber count by length and diameter fractions were used to characterize whether dissolution, breakage or mechanical clearance were driving mechanisms for clearance from the lungs. While at 2 days post instillation MMVF21 lung burdens were higher than HT rock wool, fiber burdens were 41% for MMVF21 and 10% for HT stone wool of the initial dose at 12 months, and 31% of MMVF21, 39% of MMVF30 and 29% of MMVF31 of the initial dose at 18 months (Bellmann et al. Citation1994, Citation1995). Based on the fiber kinetics over the duration of these studies, the WT1/2 values for fibers >20 µm in length were 196, 133, 123 and 92 days for MMVF21, MMVF30, MMVF31, and HT wool, respectively (Bellmann et al. Citation1994, Citation1995). When considering available Kdis values, (range of 18–28.9 ng/cm2/hr for MMVF21 and 59 ng/cm2/hr for HT wool at pH 7.4), the relative dissolution rates did not rank (in relative terms) with the respective WT1/2 values, likely due to dissolution rates being measured in pH 7.4 instead of pH 4.5. Further, pathology data for IT instillation is limited; MMVF21 delivered to sheep resulted in alveolitis (beginning at 6 days and continued through 720 days), but no lesions throughout the entire 720 post instillation follow-up period (Dufresne et al. Citation1999). Although there were no lesions, the inflammatory response (alveolitis) may have impacted the in vivo clearance, thus potentially explaining the disconnect between the in vitro dissolution rates and in vivo clearance rates.
In another study of MMVF21 rock wool and MMVF22 slag wool fibers, faster in vitro dissolution rates followed a trend of faster in vivo fiber half-life (Searl et al. Citation1999). In a biopersistence study, male rats received 0.5 mg fibers per day for 4 days of size-selected MMVF21 rock wool or MMVF22 slag wool. More durable MMVF21 long fibers (>20 µm) cleared from the lung more slowly while longer fibers (>20 µm) of the less durable MMVF22 cleared more rapidly (consistent with dissolution rates of 28.9 and 52.8 ng/cm2/hr at pH 7.4 for MMVF21 and MMVF22, respectively). At 12 months, lung burdens of all fibers longer than >0.4 µm was 37% for MMVF21 and 21% for MMVF21 of the initial dose. Results from the Searl et al. (Citation1999) study supported that SVFs with a faster dissolution rate translated to faster clearance from the lungs, which occurred through a process of fiber dissolution, disintegration, and cellular removal.
Refractory ceramic fibers
Similar to the other SVF types previously reviewed, pulmonary clearance of RCF fibers also generally tracked with in vitro dissolution rates. In a biopersistence study, male Fischer 344 rats were exposed by IT instillation (0.5 mg fibers per day for 4 days) to size-selected RCF1, RCF2, and RCF4 fibers. The biopersistence of the different fibers was influenced by their dimensions and solubility; long fibers (>20 µm) tended to clear from the lung more slowly than shorter fibers (those >0.4 µm diameter and <20 µm length) for the more durable RCF1, RCF2 and RCF4 fibers, consistent with the hypothesis that short fibers are predominantly cleared by direct transport and cellular processes, whereas long fibers are cleared by a combination of dissolution, disintegration, and cellular mechanisms (Searl et al. Citation1999). While there is limited data on half-times for IT instillation of RCF fibers, one study reported a WT1/2 of 266.5 days for IT of RCF1 fibers which is consistent with slow dissolution of 3–7.6 ng/cm2/hour at pH 7.4 (Hesterberg, Hart, et al. Citation1998; Maxim et al. Citation1999). For RCF2 and RCF4, Kdis values of 3.1 and 0.5 ng/cm2/hr at pH 7.4, respectively, were identified.
Other fibers
In the 1990s, researchers became more sophisticated in their approaches to prepare and characterize fiber size selective IT doses, quantify clearance half-times in the lungs, and benchmark in vivo fiber clearance with in vitro fiber dissolution. The biopersistence of the hybrid fiber X607 was evaluated following a single intratracheal instillation of 2 mg sized fraction in 0.3 ml to Wistar rats. Fiber elimination correlated with fiber diameters, which supported dissolution, fiber breakage, and physical clearance as the mechanism of the fiber elimination. The half-time of fiber elimination from the lung following IT exposure was 47 days (all fiber lengths) (Muhle et al. Citation1994), however in another study of the same fibers delivered by inhalation, half-time of 9.8 days (fibers >20 µm in length) and a dissolution rate of 104 ng/cm2/hour at pH 7.4 and 4.3 ng/cm2/hour at pH 4.5 was observed (Bernstein et al. Citation1996).
In summary, IT toxicology studies in the 1990s began to illustrate a clear association between fiber dissolution and biopersistence of SVFs; higher fiber dissolution rates in vitro translated to a faster clearance from the lungs. IT studies of SVFs generally did not correlate in vitro dissolution kinetics to in vivo clearance kinetics or to biological effects. It was not until additional studies using inhalation delivery of SVFs (as described next) that researchers began to appreciate that fiber biodurability and dissolution not only influenced biopersistence, but also impacted the propensity of fibers to elicit lung inflammatory, fibrotic and tumorigenic responses.
Inhalation studies
Before inhalation studies of SVFs were initiated by the Research and Consulting Co (RCC, Itingen, Switzerland), many earlier studies were confounded by study design limitations. For example, fiber aerosols were delivered by whole body exposure, which could result in oral exposure through preening and potential confounding of digestive tract tumors. Aerosol concentrations were characterized by mass concentrations rather than fiber number concentration by fiber dimension. Significant non-fiber particulate concentrations were delivered with SVFs, which increased the potential for overload conditions and biological effects due to the extent of dose rather than the characteristics of the SVFs. Limitations also included lack of size selective fiber aerosol preparations that allow for inhalation and delivery of fibers to the deep rodent lung; fibers with diameters greater than 1 µm are unlikely to have appreciable deposition in the rodent lung. In the Smith et al. (Citation1987) study, RCF, slag wool, Certainteed glass, High Temp, and Manville insulation fibers of mean fiber diameters 1.8–6.1 µm were delivered by nose-only inhalation to rats and hamsters. While no significant tumor induction was noted with any of the SVFs, the relatively large fiber diameters suggest a low possibility of fibers reaching alveoli (Smith et al. Citation1987). Further, a high proportion of non-fiber particulate to fiber in the aerosol (4:1 for JM100, 33:1 for RCF, 38:1 for high temp glass wool) also suggests that any biological response would be highly confounded by exposure to non-particulate matter (Smith et al. Citation1987). Thus, for meaningful toxicological comparisons of different fiber types, comparable aerosol fiber dimensions and doses of respirable fibers (with low interference of non-fiber particulates) need to be delivered by inhalation. Based on experience up to the late 1980s, this was not a small technological and methodological undertaking.
By 1989, rodent inhalation studies began to address these limitations. The criteria, as described in Bernstein et al. (Citation1995), included: (1) highly rat respirable fibers (geometric mean diameter <1 µm), (2) large proportion of long fibers (arithmetic mean length >20 µm), (3) representative of fiber dimensions in occupational exposure, (4) aerosol generating system that does not destroy fibers, (5) nose-only flow past inhalation, and (6) characterization of fiber aerosol and lung burden by fiber dimension. During this time, key studies were initiated at the RCC in Switzerland to evaluate biological effects and biopersistence of fibers representative of each class of SVF, with a focus on three main protocols, including chronic inhalation to evaluate thoracic fibrogenesis and tumorigenesis during and following a 5 day exposure to characterize the maximum tolerated dose (MTD) and biopersistence (18 months for hamsters; 24 months for rats). While many studies included multiple species when evaluating the effects of SVFs, some animal models, such as hamsters, have less sensitivity for evaluating the pulmonary carcinogenicity of inhaled fibers (Maxim and McConnell Citation2001). For example, hamsters (but not rats) appear to be resistant to development of lung tumors from exposure to RCF and sensitive to the induction of mesothelioma from exposure to amphibole asbestos (McConnell Citation1994; McConnell et al. Citation1999). In the context of evaluating the potential for fibrosis, lung cancer, or mesothelioma, Warheit and Hartsky proposed that the rat is a preferred model, because the fibrogenic responses (at least in response to asbestos) in rats are qualitatively similar to humans, rats have low spontaneous tumor rates, and incidence of mesotheliomas in asbestos- and erionite-exposed rats mirrors that observed in humans (Warheit et al. Citation1994).
Early studies illustrated the importance of fiber length and also illustrated inhalation of more soluble fibers was less likely to result in a pathogenic response. To standardize the measurement of the clearance rate of fibers from the lung and better understand the relationship between biopersistence and toxicity, standardized biopersistence protocols (“Ispra Protocols”) were developed by the European Commission at the European Chemicals Bureau (ECB) (Bernstein and Riego Sintes Citation1999). These protocols specify male rats should be exposed to a well-defined rat respirable aerosol SVF for 6 h a day for a total of 5 days. The aerosol should have at least 100 fibers at lengths >20 μm/cm3 with diameters less than ∼0.8 μm. The clearance half-times are determined by fitting the data to either a single or double exponential curve based on criteria in the protocol. The clearance half-time of the fibers longer than 20 μm in length is reported either directly from the single exponential curve or as a weighted half-time combining the double exponential curves. Within the context of this protocol, the clearance halftime of SVFs longer than 20 μm ranged from a few days to less than 100 days. For SVFs, the European Commission has established a directive that states that if the inhalation biopersistence clearance half-time of a fiber is less than 10 days, it does not need to be classified as a carcinogen (Bernstein and Riego Sintes Citation1999). A compilation of the in vitro dissolution, in vivo half-life, and biological effects from inhalation exposure to SVFs are summarized in .
Glass fibers
A wide range of glass fibers have been tested in inhalation toxicology studies to understand the correlation between fiber lung clearance, biopersistence, fiber dissolution, and biological effects (including inflammation, fibrogenesis, and tumorigenesis). Among the historical studies, tested glass fibers have included MMVF10, MMVF11, MMVF10a (a thinner version of MMVF10), MMVF32, JM475 glass fiber (MMVF33), 104E, 100/475, JM902, JM901F, E-glass microfiber, and other glass fibers coded “A”, “B”, and “C” (Hesterberg et al. Citation1993; Bernstein et al. Citation1996; Hesterberg, Chase, et al. Citation1998; Hesterberg, Hart, et al. Citation1998; McConnell et al. Citation1999; Cullen et al. Citation2000; Hesterberg and Hart Citation2001; Hesterberg et al. Citation2002; Bellmann et al. Citation2003; Bernstein Citation2007). Many of these studies exposed the animals using flow-past nose only inhalation, whereby airborne fibers were generated by a rotating brush feed system which aerosolized fibers with minimal breakage (Bernstein et al. Citation1996; Hesterberg, Chase, et al. Citation1998; Hesterberg, Hart, et al. Citation1998; Hesterberg et al. Citation2002). The following collection of studies demonstrate that glass SVFs with low biopersistence, relatively fast clearance rates from the lungs, have correspondingly high dissolution rates in in vitro systems, and a negligible risk for lung fibrosis or tumors.
In 1993, Hesterberg et al. tested two standard glass fibers, MMVF10 and MMVF11, by aerosolizing and delivering size selected fibers to rats by nose-only inhalation for 6 h/day, 5 days/week with post-treatment follow-up up to 24 months. Macrophage infiltration was observed beginning at 3 months exposure, which was transient and resolved by 6 to 12 months after exposure cessation, and neither SVF significantly increased the incidence of mesotheliomas or lung tumors (Hesterberg et al. Citation1993). Lung clearance (WT1/2) of fibers longer than 20 µm has been reported ranging from 39 to 44 days for MMVF10 and 9 to 38 days for MMVF11; in vitro dissolution rates were considerably lower for MMVF11 either 100 and 25 ng/cm2/hr at pH 7 and 4.5 compared to MMVF10 at 300 and 329 ng/cm2/hr at pH 7 and 4.5, respectively (Bernstein et al. Citation1996; Hesterberg et al. Citation1996; Hesterberg, Hart, et al. Citation1998). While MMVF10 in vitro dissolution rates were higher and in vivo half-life was lower than MMVF11, both SVFs demonstrate a sufficient extent of lung clearance to not present a risk of tumors.
McConnell et al. (Citation1999) exposed male hamsters by nose-only inhalation 6 h a day, 5 days a week for 18 months to MMVF10a, (a thinner version of MMVF10) or JM475 glass fiber (MMVF33). MMVF10a resulted in lung inflammation with recovery at six weeks and no neoplasms, whereas MMVF33 showed inflammation and mild fibrosis by 26 weeks, progressing in severity by 52 weeks with a single incidence of mesothelioma. Fiber lung burdens (>20 µm length) for MMVF10a were 37% lower than MMVF33 at 6 h post-exposure. After 78 weeks of exposure and six weeks of recovery, MMVF10a fiber (>20 µm length) burden reduced by 95% of the initial dose, while the MMVF33 fiber burdens were only reduced by 40%. Cumulative fiber burdens were shown to be inversely related to in vitro dissolution rates for MMVF33 (Kdis 12 ng/cm2/hr at pH 7.4 and Kdis 12 ng/cm2/hr at pH 4.5) with higher cumulative lung burdens and more severe pulmonary effects; no dissolution data was available for the finer MMVF10a (McConnell et al. Citation1999).
MMVF11 glass wool biopersistence was compared to one of several experimental glass wools labeled A, C, or B-01/09 (Bernstein et al. Citation1996). Aerosolized fibers were delivered to Fischer 344 rats by nose-only inhalation for 6 h a day, 5 days a week and followed at post-exposure time points of 1 h, 1 or 5 day(s), or 4, 13, or 26 weeks. Generally, the WT1/2 were correlated with in vitro dissolution:
MMVF11 – WT1/2 13 days (fibers >20 µm long) – Kdis 71 ng/cm2/hr at pH 7.4 and 0.7 ng/cm2/hr at pH 4.5
C Glass – WT1/2 4.1 days (fibers >20 µm long) – Kdis 309 ng/cm2/hr at pH 7.4 and 6.2 ng/cm2/hr at pH 4.5
A Glass – WT1/2 3.5 days (fibers >20 µm long) – Kdis 129 ng/cm2/hr at pH 7.4 and 2.4 ng/cm2/hr at pH 4.5
B-01/09 – WT1/2 3.5 days (fibers >20 µm long) – Kdis 320 ng/cm2/hr at pH 7.4 and 11.8 ng/cm2/hr at pH 4.5
Cullen et al. (Citation2000) evaluated two special purpose glass microfibers, 104E and 100/475, in an inhalation study in which fibers were delivered to rats by nose-only inhalation for 12 months with recovery follow-up for an additional 12 months. At the end of the 12-month recovery period, fibers of all fiber lengths were 30% of the initial fiber burden for 104E fibers, resulting in lung tumors (7 carcinoma, 3 adenoma) and mesotheliomas. Persistence of 100/475 fibers was 28% of the initial burden but resulted in minimal fibrosis, lung adenomas and no mesotheliomas. While the chemical composition of 104E fibers were not significantly altered by up to 24 months of residence in lung tissue, the chemical composition of 100/475 was substantially altered (leaching of some components resulted in modified surface properties for 100/475 fibers) over the same time period. Overall, despite similar dissolution rates (9.1 ng/cm2/hr for 100/475 and 9 ng/cm2/hr for JM E-glass fibers [similar to 104E]), pathogenicity outcomes were markedly different. This is likely partly due to differences in numbers of long fibers (at 12 months of exposure, 83 × 106 104E fibers and 11 106 100/475 fibers >20 µm were measured) but also due to proportionately greater leaching of 100/475 fibers (Cullen et al. Citation2000).
Two types of glass wools developed for optimal biosolubility in the lung, JM902 (used for insulation and filtration) and JM901F (used for standard thermal and acoustical insulation) were compared to previous evaluations of JM901 glass wool and JM475 (MMVF33) (Hesterberg and Hart Citation2001). Rats received JM902, JM901F or JM475 by nose-only inhalation for 6 h a day over a total of 5 days, with evaluation at day 1, 14, and days 29–30 post treatment. After 5 days total exposure to JM902, WT1/2 was 6.8 days (fibers >20 μm length) and 90% clearance time (T90) was 33 days; for JM901F fibers, WT1/2 (fibers >20 μm in length) was 8.1 days and T90 was 38 days and, for JM475 fibers, WT1/2 (fibers >20 μm in length) was 49 days and T90 was 240 days. Inhalation of either JM902 and JM901F fibers induced pulmonary inflammation (primarily macrophage infiltration), which returned to normal at 3 weeks post-exposure. Lung clearance half-times for 902 and 901 F did not exceed the European Union (EU) criteria for noncarcinogenic fibers (WT1/2 F > 20 μm, <10 days), while the JM475 half-times of 49 days did exceed the EU criteria. Dissolution rates for JM902 and JM901F (Kdis- 500 and 150 ng/cm2/hr at pH 7.4, respectively) compared to JM475 at a substantially slower rate of 12 ng/cm2/hr at pH 7.4, further demonstrated that biosoluble (non-biodurable) fibers, such as 902 and 901 F, with a fast rate of lung clearance (WT1/2 <10 days for fibers >20 μm) should be less persistent than JM475 in the rat by inhalation (Hesterberg et al. Citation2002).
In a study of E-glass microfibers (Bellmann et al. Citation2003), lung retention was evaluated following 3 months of nose-only inhalation exposure in rats. A WT1/2 of 67 days (range of 56–78 days) was reported and after three months of exposure, increases in lung weight, polymorphonuclear leukocytes (PMN) in the bronchoalveolar lavage fluid (BALF), cell proliferation (BrdU-response) in terminal bronchiolar epithelium, and interstitial fibrosis were evaluated. The authors concluded that the cell proliferation assay may offer important predictive value for evaluating the potential for carcinogenicity (Bellmann et al. Citation2003).
In general, glass fibers have lower biopersistence than other forms of SVFs. The newer Fibers A, B, and C have the lowest reported WT1/2 values and are less biopersistent than either MMVF10 or MMVF11, while special purpose fibers MMVF33 and MMVF32 have the highest WT1/2 values (Bernstein Citation2007). Of the less biopersistent fibers, Fibers C and B have no alumina and have high levels of alkaline and alkaline earth metals, contributing to an increase in solubility. The more biopersistent fibers MMVF32 and MMVF33 have higher concentrations of alumina and silica (54.3% SiO2 and 13.9% Al2O3 and 58.6% SiO2 and 5.87% Al2O3, respectively), which contributes to slower dissolution rates (Bernstein Citation2007).
Based on these key inhalation studies, the risk of lung fibrosis or lung cancer following inhalation of glass fibers is negligible for biosoluble fibers; some tumor induction was found with continuous exposure at concentrations high enough to maintain a high lung burden, especially with high concentrations of fibers longer than 20 µm. Long fibers with higher concentrations of alumina and silica were associated with lower dissolution rates and slower clearance from the lungs. However, the risk of tumors was typically associated fibers with lung clearance rates (WT1/2) greater than 40–50 days and in vitro dissolution rates less than 100 ng/cm2/hr at pH 7.
Rock and slag wools
Historical animal inhalation studies of rock and slag wools show similar findings as glass fibers in that faster fiber lung clearance and dissolution rates (although at pH 4.5 instead of pH 7.4) are associated with a decreased risk of lung fibrosis and tumors. In these studies, a wide range of different stone and slag wools were tested, including MMVF21 (rock wool), MMVF22 (slag wool), MMVF34 (HT, high alumina, low-silica rock wool fiber), and experimental rock wool fibers (labeled F, G, H, and L). MMVF21 stone wool, with longer biopersistence (WT1/2 64.5 days), resulted in persistent inflammation and fibrosis, while other newer fibers like MMVF34 HT rock wool with a substantially lower WT1/2 of 6 days and inflammatory or fibrotic responses were not observed (Bernstein et al. Citation1996; Kamstrup et al. Citation1998). Both fibers have comparable in vitro dissolution rates at pH 7.5, but MMVF34 has a substantially higher Kdis at pH 4.5 of 620 ng/cm2/hr compared to that of MMVF21 at pH 4.5 of 59 ng/cm2/hr indicating MMVF34 fibers should be more easily cleared as long as doses do not exceed clearance mechanisms managed by alveolar macrophages (e.g. do not reach overload conditions) (Bernstein et al. Citation2005).
In a study of MMVF21 (rock wool) or MMVF22 (slag wool), male Fischer 344 rats were exposed by nose-only inhalation for 6 h/day, 5 days per week for up to 104 weeks at fiber concentrations of 3, 16 and 30 mg/m3 (McConnell Citation1994). Groups of six randomly selected animals were either evaluated for lung fiber burdens or removed from exposure (recovery groups) at 3, 6, 12, 18, and 24 months. For the 24-month exposure group, animals were held for lifetime observation until 20% survival (28 months). While there were some lung tumors documented with the MMVF21 and MMVF22 treatment groups, there was a difference in the tumor incidence rate at the different dose levels. For example, four lung adenomas (3.5%) and one lung carcinoma (0.8%) each were observed in the 3, 16 and 30 mg/m3 MMVF21 groups. In the MMVF22 group, one adenoma (0.9%) and one carcinoma (0.9%) were noted in the 3 mg/m3 dose; no tumors were observed in the 16 mg/m3 dose, and two pulmonary adenomas (2.6%) and one lung carcinoma (0.9%) was observed in the 30 mg/m3 treatment group. While size-selected stock fiber numbers were roughly equivalent (slightly more in MMVF21 samples), the retained lung burdens/mg of dry lung tissue after 24 months were greater for MMVF21 compared to MMVF22-treated animals. Across doses, lung burdens for MMVF22 were 72, 82, and 73% lower than MMVF21 animals for fibers >20 µm in length at the 30, 16, or 3 mg/m3 doses, respectively. In later studies, WT1/2 was determined to be 64.5 days for MMVF21 and 8.7 days for MMVF22, consistent with the lower lung burden seen at 24 months with MMVF22 (McConnell Citation1994; Bernstein et al. Citation1996). In general, the faster in vitro dissolution rate (Kdis 52.8 ng/cm2/hr at pH 7.4) and in vivo clearance (WT1/2 8.7 days) for MMVF22 translated to a lower tumor incidence rate compared to MMVF21, which showed a slower in vitro dissolution rate (Kdis- 23 and 59 ng/cm2/hr at pH 7.5 and 4.5, respectively), slower in vivo clearance rate (WT1/2 64.5 days) and modestly higher tumor incidence of 4.4–4.5% for 3, 9, and 30 mg/m3 (MMVF21) compared to 1.7% (3 mg/m3) and 2.6% (30 mg/m3) for MMVF22 (McConnell Citation1994; Bernstein et al. Citation1996; Kamstrup et al. Citation1998).
Two separate studies evaluated the pulmonary and fiber kinetic effects of either MMVF34 (HT, high alumina, low-silica rock wool fiber) or MMVF21 (rock wool) following nose-only inhalation exposure to male rats for 6 h/day, 5 days per week for 104 weeks with sacrifices at 13, 26, 52, and 104 weeks (Kamstrup et al. Citation2001). Minimal cellular changes were seen with MMVF21 at three months, minimal fibrosis at 18 months, and a 0.5% incidence of fibrosis in the second study at 24 months. Normal to minimal fibrosis was noted with MMVF34 in the first study, whereas in the second study, fibrosis incidence was 5.04% at 18 months but appeared to be resolving by 24 months. Tumor incidence was only evaluated in the later study and was found not to be statistically significant from controls (MMVF21 − 3.5% adenomas, 0.9% carcinoma; MMVF34 − 4.7% adenomas, 0 carcinomas) (Kamstrup et al. Citation2001). Lung burdens steadily increased for both fibers at each time point with MMVF34 fibers peaking at 18 months then declining by 24 months, while MMVF21 lung burdens continued to increase through 24 months. MMVF34 demonstrated WT1/2 values for WHO fibers and long fibers (L > 20 µm) of 25 and 6 days, respectively, while MMVF21 stone wool demonstrated a much slower clearance rate, with elimination halftimes of 65 days (WHO fibers) and 92 days (L > 20 µm) (Kamstrup et al. Citation1998). Lung fiber clearance of long fibers (L > 20 µm) was faster for MMVF34 compared to MMVF21, mild fibrosis occurred with MMVF21, whereas MMVF34 inhalation resulted in minimal to mild cellular changes (macrophage infiltration). The tumor incidence was similar for MMVF21 (3.5% adenoma, 0.9% carcinoma) and MMVF34 (4.7% adenomas, no carcinomas) with neither significantly different from controls (Kamstrup et al. Citation1998; Citation2001). In another study, MMVF34 showed significant leaching from fibers, further demonstrating characteristics of a fiber with low biopersistence (Hesterberg, Chase, et al. Citation1998). MMVF21, with a high silica and low alumina content, was significantly less soluble in vitro at pH 4.5 and therefore less susceptible to dissolution and breakage when exposed to an acidic environment. HT stone wool (MMVF34) was observed to have low biopersistence in the rat lung (weighted retention half-time of fibers >20 μm, 6 days), but has a slow dissolution rate in vitro at pH 7.4 (59 ng/cm2/h) and a substantially faster dissolution rate at pH 4.5 (620 ng/cm2/h) (Etherington et al. Citation1981; Kamstrup et al. Citation2001; IARC Citation2002).
Biopersistence of four experimental rock wools (labeled F, G, H, and L) was evaluated in rats (5 days of exposure, for 6 h per day) with post-exposure monitoring up to 1 h, 1 or 5 days, or 4, 13, or 26 weeks (Bernstein et al. Citation1996). The highest lung burdens were found at both 1-h and 26-weeks post exposure for F stone wool at 15.6 × 106 and 0.62 × 106 total fibers/lung, respectively; the lowest in L stone wool at 5.7 × 106 total fibers/lung at 1 h and 0.30 × 106 fibers/lung at 26 weeks. It is important to note, however, that both F and G stone wools had higher aerosol fiber numbers compared to L fibers. The WT 1/2 ranged from 5.4–45 days for fibers >20 μm in length, with L stone wool having the greatest lung persistence and G stone wool the shortest. Values for WT1/2 and Kdis at pH 7.4 and pH 4.5:
F rock wool – WT1/2 8.5 days (fibers >20 µm long) – Kdis 96 ng/cm2/hr at pH 7.4 and 5.9 ng/cm2/hr at pH 4.5
G rock wool – WT1/2 5.4 days (fibers >20 µm long) – Kdis 129 ng/cm2/hr at pH 7.4 and 4.1 ng/cm2/hr at pH 4.5
H rock wool – WT1/2 13 days (fibers >20 µm long) – Kdis 169 ng/cm2/hr at pH 7.4 and 5.5 ng/cm2/hr at pH 4.5
L rock wool – WT1/2 45 days (fibers >20 µm long) – Kdis 23 ng/cm2/hr at pH 7.4 and 342 ng/cm2/hr at pH 4.5
The clearance rate of WHO fibers was found to closely reflect the clearance rate of fibers in the 5–20 μm range and disappearance of fibers longer than 20 µm mediated by their dissolution rates at pH 7.4. Lack of correlation for clearance of shorter fibers at pH 4.5 is likely due to clearance mediated by macrophages and lymphatic transport to the lymph nodes and BALT as well as dissolution (Bernstein et al. Citation1996). Dissolution rates are also impacted by composition of these fibers, with L rock wool having a higher percentage of aluminum oxide (13.5%) as compared to G, F, and H rock wools (0.45, 3.15, or 3.9%, respectively), which is a factor known to decrease in vitro dissolution. In contrast, presence of higher concentrations of alkali and alkali Earth metals including magnesium and calcium oxides will increase dissolution. For example, F, G, and H stone wools have 2-2.5x calcium oxide content compared to L stone wool (Bernstein et al. Citation1996; Maxim et al. Citation2006).
In a study by Bellmann et al. (Citation2003), Wistar rats were exposed by nose-only to fiber aerosol concentrations of approximately 15, 50, and 150 fibers/cm3 (fiber length >20 μm) MMVF21 stone wool or 150 fibers/cm3 of a new high-temperature application fiber (calcium–magnesium–silicate fiber for CMS) and followed for 3 months post-exposure. After 3 months of recovery, long fiber fraction concentration was decreased to 63.9 and 3.0% compared to original lung burden for MMVF21 and CMS, respectively. Dose-dependent effects of MMVF21 exposure include increase in lung weight, in measured biochemical parameters and polymorphonuclear leukocytes (PMN) in the bronchoalveolar lavage fluid (BALF), in cell proliferation (BrdU-response) of terminal bronchiolar epithelium, and interstitial fibrosis. As CMS fibers are biosoluble (WT1/2 9.8 days, compared to 67 days for E-glass and 79 days for MMVF21), fibrogenic potential was anticipated to be low but was nonetheless detected. A possible explanation for fibrosis observed with the CMS fibers (despite its rapid in vivo clearance) is the presence of a nonfibrous particle fraction (mass of lung fibers 1 wk after exposure was 1.02 mg as compared to 1.879 mg for particles) of CMS (with the same chemical composition as the fibrous fraction) (Bellmann et al. Citation2003).
Overall, the historical inhalation toxicology studies of rock and slag wools show that some fibers can elicit fibrosis and tumors, however this generally associated with fibers with lung clearance half-times (WT1/2) greater than 40 or 50 days (MMVF21). With respect to in vitro dissolution data, most studies tested stone and slag wool fibers in pH 7.4 and far fewer studies conducted in vitro dissolution in pH 4.5. Given that in vivo fiber half-life of stone wool fibers is not well correlated with in vitro fiber dissolution at pH 7.4, further research is needed to characterize stone wool fibers and the correlations between in vivo fiber clearance and in vitro dissolution at pH 4.5. Despite this, fibers such as MMVF34 HT rock wool showed fast in vivo clearance (WT1/2 6 days), rapid in vitro dissolution (Kdis 620 ng/cm2/hr at pH 4.5), which translated to a lack of inflammatory and fibrotic responses. Thus, while more research is needed to bridge the gap between in vitro dissolution of stone and slag wool fibers at pH 4.5 and in vivo biopersistence, available studies suggest that stone and slag wool fibers with a fiber clearance half-life less than 10 days did not show fibrotic or tumorigenic responses.
Refractory ceramic fibers
A number of different RCF were evaluated in historical animal inhalation studies, including RCF1, RCF1a, kaolin-based RCF, zirconia based RCF, high-purity RCF, after-service RCF, and high purity aluminum zirconia silica (AZS) RCF (Hesterberg et al. Citation1993; Mast et al. Citation1994; Mast et al. Citation1995a, Citation1995b; Everitt et al. Citation1997; Hesterberg, Chase, et al. Citation1998; Hesterberg, Hart, et al. Citation1998; Brown et al. Citation2000; Bellmann et al. Citation2001; Muhle and Mangelsdorf Citation2003; Bernstein et al. Citation2005; Brown et al. Citation2005). While there are several inhalation animal studies which evaluated the pulmonary effects of RCF, very few studies assessed the relationship between in vivo fiber kinetics, in vitro fiber dissolution, and fibrogenic and tumorigenic potential of RCF.
Early studies of RCF1 showed pulmonary fibrosis, lung tumors (both adenomas and carcinomas), and mesotheliomas (Hesterberg et al. Citation1993; Everitt et al. Citation1997; Hesterberg, Chase, et al. Citation1998). RCF1 has negligible amounts of alkaline earth metals (slightly over 1%) that would normally confer increased solubility and relatively high levels of SiO2 and Al2O3 (47.7 and 48%, respectively) which increases biopersistence (Bernstein et al. Citation2005). While RCF1 and RCF1a have comparable compositions, RCF1 has the presence of a relatively large percent of non-fibrous particles (25%) as compared to other RCF1a formulations (2% non-fibrous particles) (Brown et al. Citation2000; Bellmann et al. Citation2001). The presence of the non-fibrous particles can not only have a significant effect on the clearance rates from the lungs, but also influences the risk of inflammation, fibrosis and tumors. For example, in a study by Bellmann et al. (Citation2001), female Wistar rats were exposed 6 h/day for 3 weeks to either RCF1a or RCF1 fiber aerosol of approximately 125 fibers (>20 µm long)/cm3. The results from this study indicated that particle fraction of RCF1 significantly enhanced adverse effects (Bellmann et al. Citation2001). In another Bellmann et al. study, a 3 week nose-only inhalation exposure to RCF1 and RCF1a (730 fibers/cm3 >20 µm length) resulted in more persistent inflammation with RCF1 compared to RCF1a, which showed transient increases in lymphocytes and decreases in macrophages that were resolved by 90 days post-exposure (Brown et al. Citation2000; Bellmann et al. Citation2001). The differential biological effects of RCF1 versus RCF1a were related to the increased biopersistence of RCF1 (and confounding of non-fibrous particulate). Alveolar clearance of tracer particles was slowed after RCF1 exposure (1,200 days clearance half time) compared to the exposure to RCF1a (80 days clearance half time) (Muhle and Mangelsdorf Citation2003). Thus, the significant composition of non-fibrous particles (25%) in the RCF1 formulation likely contributed to a lung overload condition, which slowed tracer particle clearance and further enhanced the fibrogenic and tumorigenic response. Additionally, in vivo half-life of RCF1a was 55 days (WT1/2, L > 20 µm; 90% clearance (T90) was 227 days for L > 20 µm) and in vitro dissolution (Kdis) at pH 7.4 was 3 ng/cm2/hr (which is still a relatively slow dissolution rate) (Hesterberg, Chase, et al. Citation1998).
Exposure to other RCF resulted in fibrosis (kaolin-based RCF, kaolin RCF, After Service RCF) and tumors (kaolin RCF, zirconia RCF, High-Purity RCF, After Service RCF), however there were no apparent correlations between lung burdens (retention) and tumor incidence (Mast et al. Citation1994). In another study by Mast et al. (Citation1995a, Citation1995b), kaolin-based RCF, zirconia-based RCF, high purity AZS size-selected RCF fibers, or an after-service heat-treated (2400 °F for 24 h) kaolin-based fibers resulted in inflammation, interstitial fibrosis (all fibers except after-service fiber), pulmonary neoplasms (bronchoalveolar adenomas and carcinomas combined), and pleural mesotheliomas (kaolin, AZS, high-purity, and after-service fibers). No biopersistence or dissolution data were available and no chemical composition data were listed. It is noteworthy that mathematical modeling data indicate impaired clearance occurred at concentrations at or exceeding 30 mg/m3 with an approximate half-life of 200 days; lower doses had estimated half-lives of 140 days (Mast et al. Citation1994).
Other fibers
A rapidly dissolving fiber with high silica and glass-like characteristics, X607, was evaluated for biopersistence (Hesterberg, Hart, et al. Citation1998). Following two years of nose-only inhalation exposure to rats, mean levels were 275 × 103 WHO fibers/dry lung (48 × 103 fibers longer than 20 µm/dry lung). X607 was neither fibrogenic nor tumorigenic and induced only minimal lung cellularity that reversed after exposure was terminated (78 weeks of exposure, 26 weeks recovery). Evidence of rapid leaching (loss of calcium), a WT1/2 of 9.6–9.8 days, and a rapid rate of in vitro dissolution (Kdis = 990 ng/cm2 per hour) as well as evidence of transverse fragmentation are indicative of the low biopersistence of X607 fibers (Hesterberg, Hart, et al. Citation1998).
In summary, the inhalation route of exposure is the most relevant for comparison to human exposure. Despite this, there has been controversy as to which route of exposure is most appropriate. Muhle and Pott (Citation2000) opined that the inhalation model in rats is not sufficiently sensitive to predict cancer risk (other than for asbestos) and proposed intraperitoneal injections would be more appropriate. In contrast, Maxim et al. (Citation2002) argued well-conducted inhalation studies of carcinogenicity are sufficiently sensitive and that rats may be more sensitive than humans to the carcinogenic potential of SVFs. Very-high-dose experiments in toxicology must always be suspected of causing effects through mechanisms irrelevant in more "normal" exposures (Hext Citation1994). In any case, available information suggest that in vitro fiber dissolution rates greater than 100 ng/cm2/hr of glass fibers in pH 7 and in vivo fiber clearance less than WT1/2 40 or 50 days are not associated with fibrosis or tumors. While there is some suggestion that these same thresholds apply to stone wool fibers, more research is needed to correlate in vitro dissolution rates of stone SVFs at pH 4.5 with in vivo clearance half-life and biological effects.
Correlations between biodurability, biopersistence and health effects
Dissolution, physical breakage, and clearance of SVFs from the lungs is a complex interaction between fiber length and chemical composition. As described above, through a series of inhalation toxicology studies of SVFs, generally referred to as the RCC studies, the relationship between exposure to long, durable fibers, and the development pulmonary disease became apparent.
The dissolution of SVFs is dictated by its chemistry in aqueous and lung fluids. The amorphous nature of SVF in a randomly oriented silicon oxygen tetrahedron aid in the dissolution of silicon (H4SiO4) and associated fiber elements into aqueous solution (Bernstein Citation2007). Once a fiber is deposited in the lungs, it will encounter the neutral pH of the lung lining fluid and the acidic environment of the macrophage phagolysosome. Lung lining fluid is critical to the function of the lung; it provides an important barrier to the exogenous environment, is comprised of phospholipids and proteins to reduce surface tension and maintain alveoli in an open formation for gas exchange, and modulates innate immune function (opsonization, enhancement of phagocytosis, and regulation of immune signaling) (Martin and Poynter Citation2016). The pH extracellular lung fluid is maintained at pH 7.4–7.5, whereas the pH of the macrophage phagolysosome is pH 4.5–5 (Oberdorster Citation2000; Effros et al. Citation2005). Glass fibers generally dissolve in the neutral pH of the lung lining fluid, whereas rock wool fibers readily dissolve in the acidic environment of the macrophage phagolysosome ().
Figure 2. Mechanisms of dissolution SVFs depend on the length of the fiber and chemical composition. Irrespective of the composition, short fibers are cleared by direct or macrophage-mediated transport out of the lungs. For long glass SVFs, dissolution and subsequent breakage into shorter fibers occurs in extracellular space and neutral pH 7.4 of the lung lining fluid. In contrast, stone SVFs generally undergo minimal dissolution at neutral pH, but are readily dissolved and broken into shorter fiber segments in the acidic environment (pH 4.5) of the macrophages phagolysosome.
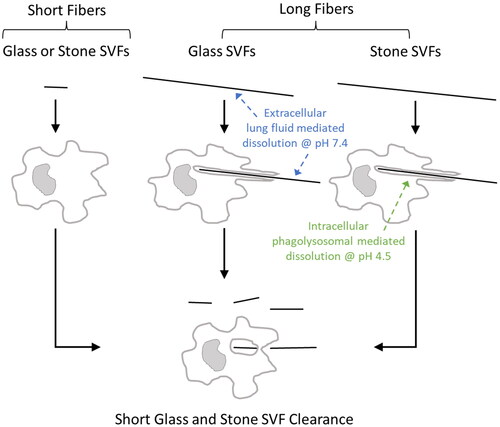
The differential processes of fiber dissolution of glass versus rock wool SVFs is important in considering the correlation between dissolution rates measured in vitro and biopersistence and health effects observed in vivo. With glass SVFs, long fibers (>20 µm in length) undergo dissolution in the lung lining fluid, which causes breakage into shorter fibers which are in turn cleared from the lungs through physical transport and/or macrophage phagocytosis ( and ). Because rock wool fibers undergo dissolution in acidic rather than neutral conditions, lung clearance of long rock wool fibers (>20 µm in length) is driven by dissolution and breakage at or near macrophage engulfment sites along the fiber, which in turn will result in removal from the lungs through similar physical transport/phagocytosis processes ( and ).
Dissolution rates (Kdis) are a function of fiber composition, and at pH 7.4 represent dissolution of long fibers not subject to alveolar macrophage removal, and at pH 4.5 generally signify dissolution of fibers in associated with macrophage phagocytosis (Maxim et al. Citation2006). For glass SVFs, dissolution occurs faster at neutral pH outside of the macrophage than inside the acidic environment of the macrophage phagolysosome, and thus long fibers are dissolved into shorter fibers outside of the macrophage, resulting in short parts of fibers inside the macrophage. For stone wool SVFs, dissolution occurs faster inside the acidic environment of the macrophage rather than the neutral pH of the lung lining fluid. As a result, stone wool fibers are dissolved inside the macrophage and fiber parts outside of the macrophage are mostly intact, which in turn are phagocytized by and dissolved in macrophages () (Bellmann et al. Citation2010). Although, long fibers that are partially phagocytosed by macrophages may also experience breakdown and weakening due to a release of an acidic milieu in the macrophage extracellular microenvironment.
SVF composition can vary depending on the desired commercial properties (tensile strength, elasticity, thermal conductivity, heat capacity, and end use temperature), which in turn can influence the propensity for dissolution. For example, CaO, MgO, Na2O, B2O3, and BaO can increase the dissolution rate of borosilicate glass fibers, whereas Al2O3 decreases the dissolution rate (Eastes et al. Citation2000). The ratio of alumina and silicon (Al3+/Al3+ + Si4+) can influence the dissolution behavior of SVFs. SVFs with an alumina content of less than 5% (ratio below about 0.07) corresponds to a decreasing dissolution rate at neutral pH, whereas for medium- and high-alumina fibers (ratio above 0.2), the dissolution rate increases with increasing Al3+/Al3+ + Si4+ ratio. In acidic pH 4.5, the dissolution rate is low until the Al3+/Al3+ + Si4+ ratio reaches about 0.25 (when Al3+ is about one-third of Si4+) (Guldberg et al. Citation2000). As a result of these physicochemical factors which influence biodurability and biopersistence, the EC adopted a criteria for composition for classification, labeling and exemption of SVFs as carcinogens referred to as “Note Q” (European Economic Community Citation1997). The basis for this regulation is that the chemical composition of vitreous fibers influences its biopersistence. SVFs are grouped according to the sum of weight of oxides of sodium, potassium, calcium, magnesium and barium. If this sum of oxides exceeds 18%, then carcinogen category 3 (suspected animal carcinogen) applies: if the sum is below 18%, then carcinogen category 2 (animal carcinogen) applies (European Economic Community Citation1997). Fibers classified as carcinogen category 3 can be exempt if it is demonstrated that biopersistence is low (T1/2 F > 20 µm = 10 days by inhalation, T1/2 F > 20 µm = 40 days by intratracheal instillation) (European Economic Community Citation1997).
Fiber length
Since the early 1970s, it has been acknowledged that long, thin fibers present a greater risk of disease than shorter fibers (Stanton Citation1973; Stanton et al. Citation1977; Wagner et al. Citation1980; Stanton et al. Citation1981b; Lippmann Citation1988; ATSDR Citation2002; ERG Citation2003). Stanton and colleagues conducted a series of pleural implantation experiments with asbestos and non-asbestos fibers and ultimately concluded that fibers less than or equal to 8 μm in length (with diameters of less than 1.5 μm) are inactivated by phagocytosis and have little or no mesotheliogenic potential (Stanton Citation1973; Stanton et al. Citation1977, Citation1981b). Following an analysis of fiber length distribution data from rat inhalation studies using amosite, brucite, chrysotile, crocidolite, erionite, and tremolite, Lippmann concluded that the concentration of fibers longer than 10 or 20 μm in length is a better predictor of lung tumor incidence than is the concentration of fibers longer than 5 μm (Lippmann Citation1994). Additionally, comparison of long (>6 µm length) versus short (3–5 µm length) erionite fibers (generally thought to be one of the most potent fiber types for mesothelioma risk) demonstrated that no cases of mesothelioma developed among 24 rats exposed by inhalation to shortened erionite and almost a 100 percent incidence of mesothelioma occurred among 27 rats exposed to longer-fiber erionite (Wagner Citation1990). From the Wagner research, it was suggested that short fibers will not cause mesothelioma regardless of the potency of longer fibers of the same fiber type. Further, the compilation of epidemiology, toxicology, and in vitro research of asbestos over the last 40 years has demonstrated that chrysotile and amphibole fibers with lengths <5–10 µm do not contribute to the health effects of asbestos (Bernstein Citation2022).
Studies by Donaldson et al. (Citation2010) have provided additional insights as to how biodurable, biopersistent long fibers induce effects on the pleura, whereas degradable and easily cleared fibers do not. As evidenced by “black spots” on the parietal pleural wall at autopsy of urban dwellers, a fraction of all deposited particles reach the pleura and through normal pathways of clearance exit the pleura through stomata in the parietal pleura. The stomatal openings (<10 µm in diameter) on the parietal pleura drain fluid from the pleural space into lung lymph nodes. It was proposed by Donaldson and others that if particles are too large or too elongated to navigate through the stomatal opening, accumulation can occur leading to inflammation and pleural pathology (Donaldson et al. Citation2010). While the impetus for this original hypothesis and research was to explore how high aspect ratio nanoparticles (HARN), such as carbon nanotubes, may fit into the classic fiber toxicology paradigm, it also has implications for understanding the biological effects of biodurable long fibers retained in the pleura space (Donaldson et al. Citation2010).
Using intrapleural or intraperitoneal injection in mice as a model to evaluate effects on mesothelial cells of the lining of the lung or peritoneum, respectively, has consistently shown across different particle types that long fibers (greater than 15–20 µm length) induce greater inflammatory and granulomatous reactions than short fibers (less than 5 µm in length) (Murphy et al. Citation2011; Osmond-McLeod et al. Citation2011). In particular, long amosite fibers injected directly in the pleural cavity showed inflammation and granuloma reactions leading to progressive fibrosis, whereas short amosite fibers did not (Murphy et al. Citation2011). Interestingly, polystyrene beads (generally considered an innocuous and inert particle) injected as 10 µm diameter sized particles into the pleura cavity, resulted in an inflammatory response, whereas quartz (a highly surface reactive particle), coal mine dust, or polystyrene beads of 3 µm in diameter or less showed no inflammatory response (Murphy et al. Citation2011). These studies have supported and confirmed the hypothesis that although a portion of all deposited particles clear through the pleura, the pathogenicity of high aspect ratio particles arises as a result of length-dependent retention of durable fibers at the stomata on the parietal pleura (Murphy et al. Citation2011).
Test methods for biodurability and biopersistence
Animal toxicology research of mineral fibers, such as asbestos, and SVFs underscore the correlation between fiber dissolution (biodurability), lung clearance (biopersistence), and health effects. Long durable fibers result in increased persistence in the lungs, which in turn leads to an increased risk of fibrosis and tumors. The historical research on SVFs has served as a basis for developing and utilizing in vivo test methods to characterize fiber biopersistence and in vitro test methods to assess biodurability with the overall intent to evaluate safety and restriction of SVFs that may present a risk for disease. As the historical research has illustrated, long durable fibers (>20 µm length) produce an increased incidence of fibrosis and tumors in animals exposed by intratracheal instillation or inhalation, whereas short fibers (<20 µm) of the same fiber type do not produce these same effects. However, soluble (non-durable) SVFs >20 µm length are cleared from the lungs and do not result in fibrosis or tumors following chronic exposures. illustrates that there is an inverse relationship between in vivo fiber half-life and dissolution of glass fibers at pH 7.4 in that slow dissolution rates of fibers >20 µm length translate to a longer residence time in the lungs, longer fiber half-lives, and increased propensity for disease (). It should be noted, however, that dissolution may also be influenced by fiber width (thicker fibers take longer to dissolve than thinner fibers). SVF fibers evaluated in the literature do not all have the same diameter, and thus, variations in the correlation between in vitro dissolution and in vivo half-life may be related the fiber dimensions as well as experimental setup of the dissolution assay (as well as other factors as discussed later). While fewer studies are available for stone wool fibers, available studies of in vitro dissolution at pH 4.5, in vivo biopersistence, and biological effects of stone wool fibers show similar trends – increasing dissolution at pH 4.5 was associated with shorter fiber half-lives, and decreased biological responses (, ). No in vitro dissolution-in vivo biopersistence patterns were observed at pH 7.4 with stone wool fibers. From these studies (), in vitro dissolution rate of 100 ng/cm2/hr or greater and an in vivo half-life less than 40 or 50 days following inhalation exposure correlated with a low probability of producing fibrosis and tumors in animal inhalation studies (Eastes and Hadley Citation1996). Considering the low likelihood that fibrosis and tumors will develop from inhaled fibers of >20 µm length with a half-life of 40 or 50 days or less, the EU Nota Q Directive 97/69/EEC criteria of a weighted half-life of less than 10 days from a short-term inhalation biopersistence test (fibers >20 µm length) to exempt carcinogenic classification of fibers is conservative and provides a margin of safety.
Figure 3. Correlation between in vitro dissolution at pH 7.4 (Kdis, ng/cm2/hr), in vivo clearance (WT1/2 fibers >20 µm in length) of glass SVFs, and corresponding biological effects (when available, ). The vertical dotted line represent the EU Note Q WT1/2 threshold (10 days for inhalation, 40 days for intratracheal instillation studies) requirement of exemption of classification for carcinogenicity. Glass SVFs (MMVF32, MMVF33) that demonstrated fibrotic and tumorigenic responses showed Kdis less than 100 ng/cm2/hr and WT1/2 (inhalation and/or intratracheal instillation) greater than 40–50 days. The confluence of the Kdis (>100 ng/cm2/hr, as illustrated by horizontal dotted line) and WT1/2 (>40–50 days) thresholds that are not associated with biological effects is represented by the blue shadowed area.
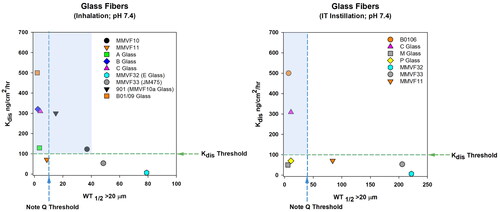
Figure 4. Correlation between in vitro dissolution at pH 4.5 (Kdis, ng/cm2/hr), in vivo clearance (WT1/2 fibers >20 µm in length) of stone SVFs, and corresponding biological effects (when available, ). The vertical dotted line represent the EU Note Q WT1/2 threshold (10 days for inhalation, 40 days for intratracheal instillation studies) requirement of exemption of classification for carcinogenicity. Stone SVF (MMVF21) that demonstrated the most significant fibrotic responses (tumorigenic responses were not significant) showed Kdis less than 100 ng/cm2/hr and WT1/2 (inhalation) greater than 40–50 days. The confluence of the Kdis (>100 ng/cm2/hr, as illustrated by horizontal dotted line) and WT1/2 (>40–50 days) thresholds that are not associated with biological effects is represented by the blue shadowed area. It is noteworthy that correlations were observed between in vitro Kdis at pH 4.5 (but not pH 7.4) and in vivo WT1/2 for stone SVFs, however limited data are available for in vitro dissolution at pH 4.5, in vivo clearance, and in vivo health effects () for a given stone SVF.
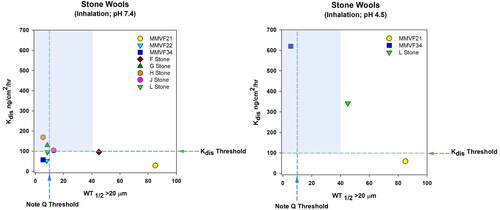
Fiber dissolution test methods
In vitro dissolution methods have been used for at least a couple decades to characterize the biodurabilty and dissolution behavior of SVFs, but also used as a tool to predict the biopersistence and biological effects in vivo. Static and dynamic flow systems have been used to measure the dissolution constant of SVFs. The general principal of these test methods is that dissolved amounts of silicon and other elements are measured in the effluent at regular time intervals in a system in which a static or continuous constant flow (dynamic) of buffer is moved across the sample of fibers. While a static system (a single finite volume of fluid is mixed with the SVF sample) is generally considered a more simplistic approach than a dynamic system (fluid actively flows across the SVF sample), there are complexities with both systems which can significantly influence consistency of the fiber dissolution rate and potential correlation with in vivo measures of biopersistence and biological effects. For example, fiber sample preparation (fiber mass, dimension, and surface area), buffer solution composition (ionic strength, complexing agents, buffer capacity), buffer volume, pH, ratio of flow rate to fiber surface area, temperature, system design features (fiber cassette characteristics, agitation, solution sampling and analytical methods), and analytical method can greatly influence the assay results. While a governmental standardized method has yet to be developed for evaluating SVF dissolution in vitro, the industry has a test method guideline “In-vitro acellular dissolution of man-made vitreous silicate fibres” (Sebastian et al. Citation2002). In the early 2000s, the European Insulation Manufacturers Association (EURIMA) undertook a round robin testing program to evaluate the inter- and intra-laboratory variability of two round robin testing regimens among nine and six different laboratories with two glass wool samples (traditional and soluble SVFs at pH 7.4) and two stone wool samples (traditional and soluble [high alumina/low silica] at pH 4.5) (Guldberg et al. Citation2003). A specified mass was placed in filter holds (“flow cells”), artificial lung fluid (Gamble’s liquid) continuously flowed through the flow cells, and silicon in the effluent was measured in intervals up to 14 days. The results from this round robin testing demonstrated that there was relatively good reproducibility within a given laboratory, but the inter-laboratory variability was relatively high, ranging from 24 to 61 percent. It was concluded that despite the difference between laboratories (which may be relatively unimportant in the broader solubility assessment), the method still may be adequately valid for ranking different fibers with respect to dissolution coefficients. It was also noted that it is important to test fibers in both neutral and acidic pH, because the neutral pH of the lung lining fluid was important for the dissolution of glass wool fibers (low alumina fibers), whereas the acidic environment was important for dissolution of stone wool fibers (high alumina/low silica fibers) (Guldberg et al. Citation2003).
Regulatory acceptability of an acellular in vitro dissolution assay will require that the test method has robust reliability and predictability for in vivo biopersistence and biological effects. In vitro dissolution (Kdis) values can vary significantly across SVFs, particularly between stone and glass fibers at a given pH. In a publication by Bellmann et al. (Citation2010) comparing calculated dissolution (Kdis) with in vivo biopersistence (WT1/2) for multiple SVF types showed that a Kdis threshold of 100 ng/cm2/hr or greater for glass fibers was consistently associated with an in vivo half-life less than 40 days for fibers >20 µm in length (). It is noteworthy that the Kdis values in Bellmann et al. (Citation2010) were modeled and not measured. It is also noteworthy that few SVF types would meet the Kdis 100 ng/cm2/hr and WT1/2 thresholds if WHO sized fibers were considered. This finding is consistent with the recommendation from Eastes and Hadley (Citation1996) that an in vitro dissolution rate of 100 ng/cm2/hr correlates with an acceptably low probability of producing fibrosis and tumors in animal inhalation studies. However, Bellmann et al. (Citation2010) showed that some stone wool fibers had a fast predicted Kdis (>100 ng/cm2/hr), but a relatively long half-life in in vivo biopersistence studies. While this disparity between calculated Kdis and in vivo clearance of some stone wool fibers needs to be further understood, it may be that actual measured in vitro dissolution of stone wool in buffered solution at pH 4.5 could better align with in vivo biopersistence. In fact, it was noted by Eastes et al. (Citation2000) that Kdis equations and coefficients for weight of oxides may not adequately fit for all rock and slag wool compositions, because of the lack of boron, lower silica concentrations, and higher and more variable alumina concentrations (0–50%) compared to glass wool fibers. Irrespective of the alignment of measured versus calculated Kdis or the variability that is observed with measured in vitro dissolution assays across different laboratories, continuing to correlate in vitro dissolution and in vivo biopersistence with the presence or lack of biological effects in inhalation and intratracheal instillation animal studies will increase confidence in acellular in vitro systems and reinforce critical in vitro Kdis thresholds to adequately predict health effects and product safety. Further, it may be that there is a reasonable range of variance of in vitro dissolution results across laboratories that can still be within acceptable thresholds which are correlated with in vivo biopersistence and responses.
Figure 5. Correlation of fiber dissolution rate (Kdis, calculated) and fiber clearance from the lungs of animals (WT1/2, weighted half-life) for multiple SVF types show that a Kdis threshold of 100 ng/cm2/hr or greater for glass fibers was consistently associated with an in vivo half-life less than 40 days for fibers >20 µm in length. This is consistent with the Note Q threshold requirements for exemption of fibers for carcinogenicity classification in the EU. Some stone wool fibers were predicted to have a relatively fast Kdis (>100 ng/cm2/hr), but showed a relatively long half-life. While this disparity between calculated Kdis and in vivo clearance of some stone wool fibers needs to be further understood, it may be that actual measured in vitro dissolution of stone wool in buffered solution at pH 4.5 could better align with in vivo biopersistence. It is noteworthy that few SVF types would meet the Kdis 100 ng/cm2/hr and WT1/2 thresholds if WHO sized fibers were considered.
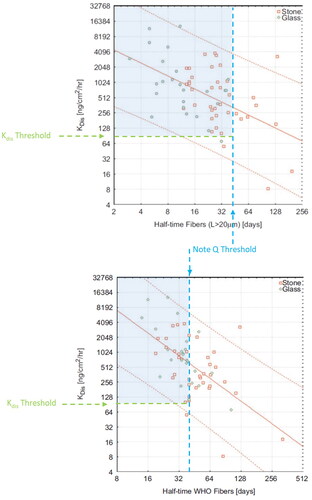
Biopersistence test methods
In vivo inhalation and intratracheal instillation studies to evaluate biopersistence and biological effects of SVFs are still the gold standard upon which to exempt classification and labeling of SVFs for carcinogenicity. However, with the mission to reduce the use of animal testing, international regulatory agencies, including in Europe and the U.S., have committed to supporting the development of alternative testing strategies in the reduction, refinement, and replacement of use of animals in research. In fact, in 2013, EU executed a complete ban on animal testing for finished cosmetics products and cosmetic ingredients (European Commission Citation2009). As a result of this directive, the European center for the validation of alternative methods (ECVAM) serves an important role in the development, validation, and international recognition of alternative methods to reduce, refine, and replace the use of animals in testing. While there do not appear to be any initiatives to replace the animal testing requirement of SVFs for exemption under the EU Nota Q Directive, standardization of in vitro methods to predict the effects in animals and humans would be beneficial to reduce resources and safeguard product safety and innovation in the event that regulators seek to reduce animal testing of SVFs.
In 1999 as a part of the Directive 97/69/EC, the European Commission issued animal testing protocols for four tests, the results of which exonerate synthetic mineral fibers for classification as carcinogens (Bernstein and Riego Sintes Citation1999). These four animal testing protocols include (1) Biopersistence of Fibers – Short term exposure by inhalation [ECB/TM/26 rev.7], (2) Biopersistence of Fibers – Intratracheal instillation [ECB/TM/27 rev. 7], (3) Carcinogenicity of synthetic mineral fibers after intraperitoneal injection in rats [ECB/TM/18(97) rev. 1], and (4) Chronic inhalation toxicity of synthetic mineral fibers in rats [ECB/TM/17(97) rev. 2]. For the inhalation study protocol, rats (Fischer 344 or Wistar) are exposed by nose-only inhalation 6 h/day for 5 days to fiber test atmospheres optimized for rat respirability (aspect ratio 3:1, 100 fibers/cm3 >20 µm length, gravimetric mean concentration of fibers 0.8 µm should not exceed 40 mg/m3, all particulates should not exceed 60 mg/m3). Subgroups of animals (at least 7 per group/time point) are evaluated for fiber and particulate burden (which involve counting of fibers/particles of a digested tissue sample) at various time intervals (1, 2, 3, 14 days, 4 weeks, 3, 6, and 12 months) for determination of the time for removing 50% of fibers (T1/2) which are longer than 20 µm. T1/2 of other fiber length fractions <5 µm and 5–20 µm (WHO fibers) should also be determined. MMVF21 and MMVF10a are recommended as a validation material (Bernstein and Riego Sintes Citation1999).
For the IT study protocol, rats (Fischer 344 or Wistar) are intratracheally administered fibers (0.5 mg and 2 mg) for 4 consecutive days and examined for lung fiber burden at post-exposure time points comparable to the inhalation study protocol (Bernstein and Riego Sintes Citation1999). Instilled fibers should be suspended in saline and have a mean aspect ratio of 3:1, an upper limit of 3 µm (95% less than 3 µm), and at least a 20% of WHO (L > 5 µm, D < 3 µm) fibers should have lengths >20 µm and geometric mean diameter as close to 0.8 µm as possible. Similar to the inhalation study protocol, IT studies should report the T1/2 of fibers longer than 20 µm, as well as of lengths <5 µm and 5-20 µm (WHO fibers). The IP injection study protocol involves evaluation of the tumorigenic response in the peritoneal cavity in rats following one or more IP injections of a well characterized SVF. Animals (Wistar rats, 50 per treatment group) are followed throughout their lifetime until a total of 20% survival occurs in the exposure group. Criteria for the fiber sample preparation are the same as that outlined for the IT study protocol with delivery of a single dose of 1 x 109 WHO fibers per animal. Histopathology is assessed by reporting the number, type, and percentage of each type of lesion.
Finally, the chronic inhalation toxicity study involves inhalation of SVF for 2 years for evaluation of fiber lung burden by fiber size fraction and prevalence of fibrosis, adenocarcinoma, and mesothelioma. Wistar rats (100 per group) are exposed by inhalation to at least 100 fibers/cm3 (L > 20 µm with GMD close to 0.8 µm) for 6 h/day, 5 day/week for 104 consecutive weeks to a well characterized fiber test atmosphere optimized for rat respirability and followed over their lifetime. Fibers such as E-glass, MMVF21, or MMVF10a are recommended as a validation material. Similar to the inhalation biopersistence protocol, fiber burden of fibers <5, 5–20 and >20 µm in length should be reported, along with the histopathology (Bernstein and Riego Sintes Citation1999).
The more recent inhalation studies of SVFs (as described in the previous sections) generally followed these EC protocols and support that a half-life of 10 days or faster is a conservative threshold for which the tumorigenic risk is negligible. Interestingly, when WT1/2 of SVFs are compared for fibers administered by inhalation versus IT administration, there is a comparable relative ranking between the exposure methods, but the actual WT1/2 values vary significantly. and clearly show that WT1/2 of MMVF32, MMVF33, MMVF11, and C Glass fibers by inhalation exposure have a similar ranking as exposures by IT instillation. However, the actual WT1/2 values are dramatically different. For example, MMVF11 WT1/2 >20 µm is 8.7–11 days by inhalation and 84 days by IT, whereas MMVF32 WT1/2 >20 µm is 79 days by inhalation and 222 days by IT. The method of exposure for biopersistence studies may have implications for carcinogenicity exemption of SVFs. For example, MMVF11 by inhalation may be exempt according to Nota Q (based on criteria WT1/2 >20 µm 10 days), but not by intratracheal instillation (based on criteria WT1/2 >20 µm 40 days). While the EC protocols provide an opportunity to evaluate and exempt novel SVFs should they meet established thresholds, these types of studies require significant time and resources. Thus, standardizing and gaining regulatory acceptance of in vitro dissolution assays to predict in vivo effects would not only conserve animals for testing, but also provide more efficient research and development and safety assessment for novel SVFs for the marketplace. The lessons learned from the SVF research can also provide insights to other industries, such as engineered high aspect ratio nanomaterials, as they seek to design advanced but safe products for the marketplace. The following section will review the existing state of the science for biodurability and biopersistence methods of advanced nanomaterials and potential considerations and lessons learned that can be gained from the SVF industry experience on these topics. Additionally, considerations from the nanomaterials industry will be reviewed for the potential standardization of in vitro biodurability methods of SVFs for predicting biopersistence and health effects.
Applicability of SVF biodurability and biopersistence methods for advanced materials
The OECD WPMN has recommended testing methods to address biodurability and biopersistence of nanomaterials including: (1) dissolution and dispersion stability for environmental testing (OECD Citation2020b), (2) biodurability to characterize dissolution and biodegradation and predict toxicity and health effects (OECD Citation2018), and (3) biopersistence/biodurability to induce lysosomal membrane permeabilization (LMP) as a predictor of long-term toxic effects (OECD Citation2020a). LMP is the loss of membrane integrity which allows the release of luminal contents into the cytosol and can activate multiple stress response pathways including regulated cell death.
While biodurability and biopersistence methods developed for SVFs have some applicability for advanced nanomaterials, nanoparticles or nanofibers have exceptional physicochemical characteristics that require careful consideration. Nanomaterials span a seemingly endless array of sizes, shapes, surface chemistries, aggregation and agglomeration states, which can change from in situ conditions in their production to aqueous or airborne conditions in testing assays (e.g. in vitro or in vivo) or environmental exposure scenarios (e.g. occupational and consumer product settings). Thus, standardizing testing regimens to correlate dissolution behaviors of nanomaterials in simulated lung fluid to lung deposition-clearance-retention patterns (and ultimately correlate to biological effects) is no small challenge.
In the Guidance Document for the Testing of Dissolution and Dispersion Stability of Nanomaterials and the Use of the Data for Further Environmental Testing and Assessment Strategies, two methods are described for the determination of solubility of substances, a static batch test and a dynamic test (OECD Citation2020b). These test methods are intended to evaluate the dissolution rate and dispersion of nanomaterials in environmental aqueous media, which has limited applicability to the complex physicochemical milieu of the lung lining fluid. The primary limitation with static assays is with the restricted volume, of which the supernatant may become supersaturated inhibiting further dissolution (Campopiano et al. Citation2014). In the dynamic system, test media composition (pH, ionic or organic compounds), flow rates, test duration, sample concentration (mass, surface area), and test conditions (agitation) are study design features which can influence dissolution rates.
For both nanomaterials and SVFs, solubility (or biodurability) and dissolution rate can inform on further hazard testing strategies, but characterization (chemical composition, size distribution, morphology, surface modifications) of the test material is critical for appropriate interpretation of test results. Many of the dissolution studies of nanoparticles or nanofibers unfortunately do not report dissolution rates or constants so correlating physicochemical characteristics with dissolution kinetics is not possible (Utembe et al. Citation2015). Dissolution can be greatly influenced by media characteristics (pH, ionic strength, presence of dissolve organic matter, concentration of polyvalent ions, temperature, equilibrium time, analytical method), and intrinsic properties of the material (particle size, morphology, composition, crystallinity, surface modifications (Utembe et al. Citation2015). Additionally, ions and molecules (such as sulphides, chlorides, proteins, enzymes, polysaccharides) in the test media can influence dissolution (Utembe et al. Citation2015). As described in an extensive review of the chemistry and composition of simulated biological fluids, Innes and colleagues noted that particle and fiber dissolution is not always driven by pH and is not only driven by fluid composition (Innes et al. Citation2021). Thus, selecting an appropriate simulated biological fluid for in vitro dissolution studies is complex and needs validation, alignment and correlation with the in vivo experience (clearance, biopersistence). Additional challenges with nanomaterials are generating a representative sample for in vitro dissolution assays and inhalation studies particularly given that these materials have a high propensity for agglomeration. While the same laws of surface-area-normalized-dissolution-rate as is observed with SVFs and micro-sized particles may also apply to nanomaterials, resolution on the drivers for nanomaterial dissolution cannot be resolved until systematic material characterization is coupled with measured dissolution kinetics. Ultimately, correlations of in vitro nanomaterial dissolution kinetics with in vivo biopersistence kinetics will provide support of biodurability and biopersistence outcomes as predictive tools for health effects (similar to what have been established with SVFs). However, testing procedures and material characterization (as noted above) will likely need to be extensively investigated and harmonized before interpolations can be made for nanomaterial dissolution and health effects.
The OECD guidance document, Assessment of Biodurability of Nanomaterials and Their Surface Ligands, provides a compilation of biodurability information of pristine and functionalized nanomaterials in biological and environmental media in vitro and in vivo with a brief summary of methods for stability and halftimes (OECD Citation2018). Nanomaterial surface modifications (e.g. surfactants, ligands, coatings) were proposed as the primary determinants of nanomaterial biodurability. Nanomaterial surface modifications, such as use of surfactants, capping agents or attached ligands (small molecules or polymers that carry different functional groups) have been shown to influence dissolution or biodegradation (OECD Citation2018). Similar to larger particles and fibers, however, dissolution of nanomaterials is also expected to be affected by physicochemical properties, including size, shape, surface area, surface ligands, morphology, crystallinity, aggregation and agglomeration. Although there is not extensive research on correlations between nanomaterial dissolution rate, biopersistence, and health effects, there are examples (CeO2 nanoparticles) in which low dissolution rate nanomaterials persist in the lungs and cause lung inflammation and granulomas (Keller et al. Citation2014).
As of present, in vivo biopersistence studies of high dissolution nanomaterials (e.g. ZnO, CuO) are generally lacking to determine whether nanomaterials follow a similar pattern as SVFs: high dissolution translates to low biopersistence and low risk of health effects, whereas low dissolution is associated with high biopersistence and increased risk of health effects. Nanomaterial dissolution and release of free or complexed metal ions (which induce cytotoxicity, oxidative stress, lysosome destabilization, inflammation) may suggest a more complex interplay between nanomaterial biodurability, biopersistence, and adverse health effects than what is observed with SVFs. Further research is needed on whether nanomaterial in vitro dissolution and toxicity assays may correlate and be predictive of in vivo persistence and long-term biological responses.
The OECD guidance document (as titled) further explores the Ability of Biopersistent/Biodurable Manufactured Nanomaterials (MNs) to Induce Lysosomal Membrane Permeabilization (LMP) as a Prediction of their Long-Term Toxic Effect as an alternative testing strategy for safety assessment (OECD Citation2020a). Lysosomal and autophagy dysfunction has been hypothesized as a mechanism of nanomaterial induced toxicity and pathology (Gulumian and Andraos Citation2018). Nanomaterials enter the cell through endocytic mechanisms into vesicular structures (endosomes, phagosomes) that merge into lysosomes. The nanomaterials are in turn degraded by different hydrolytic enzymes in an acidic environment. Biodurable nanomaterials, however, are resistant to biodegradation and may induce lysosomal and autophagy dysfunction and subsequent long-term toxicity. A common form of phagolysosomal dysfunction is LMP which has been associated with assembly and activation of inflammasome NLRP3 and chronic inflammation leading to pathological effects such as fibrosis and cancer (Sayan and Mossman Citation2016). This phenomenon is not specific to nanosized particles as crystalline micro-sized particles have been shown to induce LMP with subsequent NLRP3 inflammasome activation and has been suggested as the underlying mechanism for silica-induced fibrosis (Cassel et al. Citation2008; Dostert et al. Citation2008; Hornung et al. Citation2008; Franchi et al. Citation2009; Biswas et al. Citation2014). Because of the lack of understanding and potential complexities at play between nanomaterial durability, biopersistence, and health effects, research will need to first focus on the development of acellular in vitro dissolution assays that can predict nanoparticle biopersistence kinetics in vivo before cellular in vitro assays are able to predict long-term effects in humans.
As described by Donaldson et al. (Citation2013), the biologically effective dose (BED) (internal dose that drives the critical pathophysiological outcomes) is critical for safety evaluation of any substance. For fibers, BED is driven by fiber dimension and durability, which influences the deposition, retention, persistence and fiber-cellular interaction that may or may not lead to toxicological effects. Whereas for nanomaterials, other physicochemical factors may come into play when considering the appropriate BED. For example, toxicity potential of metal ions released during nanoparticle dissolution in the acidic environment of the phagolysosome has been shown to influence inflammation. Soluble zinc ions (but not zinc nanoparticles or magnesium ions) elicited inflammation in rats following intratracheal instillation (Flink et al. Citation1992; Cho et al. Citation2011, Citation2012). However, it should be noted that the effects of metal nanoparticles versus metal ions have not been evaluated or validated in inhalation studies. These findings suggest that the relative toxicity of compositional ions of high solubility nanoparticles is important for evaluating the toxicity and BED for nanomaterials and their dissolved ions. Allocating the relative toxicity of released ions versus nanoparticles may be challenging due to in vitro assay differences (media, concentration, duration, ion-particle interactions) (Peijnenburg et al. Citation2020). Further, the electric and oxidative potential of a particle can impact cellular membrane integrity, oxidative balance, and proinflammatory responses (Donaldson et al. Citation2013).
Understanding the impact of fiber dimension of high aspect ratio nanoparticles or HARN is important for defining biopersistence and the BED. The rigidity of fibers to maintain a high aspect ratio or their ability to coil up into a bundle or to agglomerate (which will modify the length) may influence macrophage phagocytosis (frustrated phagocytosis and lysosome instability) and in turn in vivo biopersistence and pathogenicity (Donaldson et al. Citation2011; Kane et al. Citation2018). In fact, a study by Donaldson et al. (Citation2011) demonstrated that long rigid carbon nanotubes (>15 µm length) produced inflammation and fibrosis in mice exposed by intraperitoneal injection, whereas short or tangled carbon nanotubes or carbon nanotubes incubated in Gambles solution for 10 weeks (which reduced the fiber mass and fiber lengths [<15 µm] and formed tightly agglomerated bundles) produced minimal inflammogenic responses (Osmond-McLeod et al. Citation2011).
While corollaries between asbestos and manufactured nanofibers, nanotubes, nanorods, and nanowires have been made, the fiber length-, durability-, and biopersistent-dependent factors that influence pathogenicity of mineral fibers are also expected to affect the biological effects of HARN. Additionally, unique nanomaterial shapes, such as graphene nanoplatelets, which can have a relatively low aerodynamic diameter (3 µm) but large actual size (30 µm), may present a risk of respiratory effects due to respirability and deposition of relatively large particles in the lungs that cannot be easily engulfed and cleared by macrophages (Donaldson et al. Citation2013). While the fiber toxicology paradigm (dose, dimension, and durability) which have been garnered from research of asbestos and SVFs are anticipated to apply to HARN, other factors such as toxicity of metal ions, surface reactivity (electric or oxidative potential), and protein corona formation in biological fluids (which appears to mitigate cellular toxicity) may also need to be considered particularly for HARN which have moderate or greater biopersistence (Arts et al. Citation2015). Only with toxicological studies aimed to correlate in vitro durability (and other measures of HARN reactivity), in vivo biopersistence, and biological outcomes will it be determined whether similar or different in vitro fiber dissolution and in vivo half-life thresholds, which exempt carcinogenicity classification of SVFs, can also be applied to HARNs.
Conclusions
In vitro dissolution assays in both neutral and acidic pH have been used for several decades to characterize the biodurability of SVFs and predict the biopersistence and biological effects in animals and humans. While in vitro dissolution data of SVFs were not always reported for correlation with in vivo clearance and biological effects, available information suggest that generally in vitro fiber dissolution rates greater than 100 ng/cm2/hr of glass fibers in pH 7 and stone fibers in pH 4.5 and in vivo fiber clearance less than WT1/2 40 or 50 days do not present a risk of fibrotic and tumorigenic responses. The epidemiologic studies of SVF manufacturing worker cohorts have supported that SVFs do not present a risk of nonmalignant or malignant respiratory disease (Stone et al. Citation2004; Egnot et al. Citation2020), thus further reinforcing the utility of fiber physicochemical, dissolution, and biopersistence characterization methods that have been applied for understanding the safety and risks of SVFs.
While the fiber length-, durability-, and biopersistent-dependent factors that influence pathogenicity of mineral fibers are also expected to affect the biological effects of HARN, other factors such as toxicity of metal ions, surface reactivity, protein corona formation, particle shape (e.g. straight versus curly), and surface modifications may also need to be considered for HARN. As in vitro biodurability and biodissolution assays are being developed for HARN, the following are recommended for consideration:
Material characterization – Complete physicochemical characterization, including concentration, mass, dimension, surface area, morphology, crystallinity, surface ligand modification, aggregation, agglomeration, fiber rigidity, electric and oxidative potential, protein corona formation;
Assay design – System selection which aligns with the intended assay purpose (e.g. static system as a screening tool, dynamic system as representative biological systems), as well as characterization of design features (fiber cassette characteristics, flow rate, equilibrium time, agitation method/frequency, temperature, test duration, solution sampling and analytical methods) that may influence dissolution rates;
Media composition – Characterization of buffer solution compositions representing both the neutral pH of the lung lining fluid and acidic pH of the macrophage phagolysosome, in addition to the understanding the impact of ionic strength, complexing agents, organic matter, buffer capacity, buffer volume, and flow rate on material dissolution rates;
Particle versus ion interactions – Toxicity characterization of particles in pristine and degraded states, as well as released metal ions free or complexed with organic binders.
Understanding the primary factors which influence HARN dissolution and biodurability in in vitro systems will provide a foundation upon which to bridge in vivo biopersistence and biological responses, which in turn can be used to identify critical thresholds to adequately predict health effects and product safety. Only with toxicological studies aimed to correlate in vitro durability (and other measures of HARN reactivity), in vivo biopersistence, and biological outcomes will it be determined whether similar or different in vitro fiber dissolution and in vivo half-life thresholds, which exempt carcinogenicity classification of SVFs, can also be applied to HARNs.
| Abbreviations | ||
| 3Ds | = | dose, dimension, and durability |
| 3Rs | = | reduce, refine, replace |
| AES | = | alkaline earth silicate |
| AR | = | alkali resistant |
| AZS | = | aluminum zirconia silica |
| BALF | = | bronchoalveolar lavage fluid |
| BALT | = | bronchus-associated lymphoid tissue |
| BED | = | biologically effective dose |
| BrdU | = | bromodeoxyuridine |
| CMS | = | calcium–magnesium–silicate |
| EC | = | European Commission |
| ECB | = | European Chemicals Bureau |
| EURIMA | = | European Insulation Manufacturers Association |
| ECVAM | = | European Center for the Validation of Alternative Methods |
| GI | = | gastrointestinal |
| GMD | = | geometric mean diameter |
| HARN | = | high aspect ratio nanomaterials |
| HT | = | high temperature |
| IP | = | intraperitoneal |
| IPL | = | intrapleural |
| IT | = | intratracheal |
| Kdis | = | dissolution rate |
| L | = | length |
| LMP | = | lysosomal membrane permeabilization |
| MΦ | = | macrophage |
| MMVF | = | man-made vitreous fibers |
| MTD | = | maximum tolerated dose |
| NLRP3 | = | nucleotide-binding doman (NOD)-like receptor protein 3 |
| SVF | = | synthetic vitreous fiber |
| OECD | = | Organization for Economic Co-operation and Development |
| PMN | = | polymorphonuclear leukocytes |
| PSI | = | pounds per square inch |
| RCC | = | Research and Consulting Co |
| RCF | = | refractive ceramic fibers |
| WHO | = | World Health Organization |
| WPMN | = | Working Party on Manufactured Nanomaterials |
| WT1/2 | = | weighted half-life |
Acknowledgements
We appreciate and acknowledge the four anonymous reviewers the Editor selected. Besides these anonymous reviewers, the Editor, and the authors, no individual has reviewed any drafts of the paper.
Declaration of interest
AKM and HCO are employed by ChemRisk, a consulting firm providing scientific advice to the government, corporations, law firms, and various scientific/professional organizations. Funding for this manuscript was provided, in part, by the North American Insulation Manufacturers Association (NAIMA). AKM has also been engaged by NAIMA to provide general scientific consulting and expert advice on scientific matters. This article was prepared and written exclusively by the authors without review or comment by NAIMA employees, counsel, or associated members. The preparation of the paper, including conduct of the literature review, review of the individual papers, integration and synthesis of the findings, the conclusions drawn, and recommendations made are the exclusive professional work product of the authors and may not necessarily be those of their employer or the financial sponsor.
References
- Adachi S, Kawamura K, Kimura K, Takemoto K. 1991. Tumorigenicity of fine man-made fibers after intratracheal administrations to hamsters. Env Res. 54(1):52–73.
- Adachi S, Kawamura K, Kimura K, Takemoto K. 1992. Tumor incidence was not related to the thickness of visceral pleural in female Syrian hamsters intratracheally administered amphibole asbestos or manmade fibers. Environ Res. 58(1):55–65.
- Arts JHE, Hadi M, Irfan M-A, Keene AM, Kreiling R, Lyon D, Maier M, Michel K, Petry T, Sauer UG, et al. 2015. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul Toxicol Pharmacol. 71(2 Suppl): s1–s27.
- Assuncao J, Corn M. 1975. The effects of milling on diameters and lengths of fibrous glass and chrysotile asbestos fibers. Am Ind Hyg Assoc J. 36(11):811–819.
- ATSDR. 2002. Expert panel on the health effects of asbestos and synthetic vitreous fibers (SVF): the influence of fiber length. Premeeting Comments; New York, NY, October 29–30, 2002. Atlanta, GA: U.S. Dept. of Health and Human Services-Public Health Service-Agency for Toxic Substances and Disease Registry (ATSDR).
- Bellmann B, Muhle H, Creutzenberg O, Ernst H, Brown RC, Sebastien P. 2001. Effects of nonfibrous particles on ceramic fiber (RCF1) toxicity in rats. Inhal Toxicol. 13(10):877–901.
- Bellmann B, Muhle H, Creutzenberg O, Ernst H, Muller M, Bernstein DM, Riego Sintes JM. 2003. Calibration study on subchronic inhalation toxicity of man-made vitreous fibers in rats. Inhal Toxicol. 15(12):1147–1177.
- Bellmann B, Muhle H, Kamstrup O, Draeger UF. 1994. Investigation on the durability of man-made vitreous fibers in rat lungs. Environ Health Perspect. 102 Suppl 5(Suppl 5):185–189.
- Bellmann B, Muhle H, Kamstrup O, Draeger UF. 1995. Investigation on the biodurability of chemically different stone wool fibres. Exp Toxicol Pathol. 47(2–3):195–201.
- Bellmann B, Schaeffer HA, Muhle H. 2010. Impact of variations in the chemical composition of vitreous mineral fibers on biopersistence in rat lungs and consequences for regulation. Inhal Toxicol. 22(10):817–827.
- Bernstein DM. 2007. Synthetic vitreous fibers: a review toxicology, epidemiology and regulations. Crit Rev Toxicol. 37(10):839–886.
- Bernstein DM. 2022. The health effects of short fiber chrysotile and amphibole asbestos. Crit Rev Toxicol. 52(2):89–112.
- Bernstein DM, Chevalier J, Smith P. 2005. Comparison of Calidria chrysotile asbestos to pure tremolite: final results of the inhalation biopersistence and histopathology examination following short-term exposure. Inhal Toxicol. 17(9):427–449.
- Bernstein DM, Drew RT, Kuschner M. 1980. Experimental approaches for exposure to sized glass fibers. Environ Health Perspect. 34:47–57.
- Bernstein DM, Morscheidt C, Grimm HG, Thévenaz P, Teichert U. 1996. Evaluation of soluble fibers using the inhalation biopersistence model, a nine-fiber comparison. Inhalation Toxicol. 8(4):345–385.
- Bernstein DM, Riego Sintes JM. 1999. Methods for the determination of the hazardous properties for the human health of manmade mineral fibres (MMMF). Document No.: EUR18748EN. Ispra, Italy: European Commission Joint Research Centre-Institute for Health and Consumer Protection-Toxicology and Chemical Substances. https://publications.jrc.ec.europa.eu/repository/handle/JRC18422
- Bernstein DM, Riego Sintes JM, Ersboell BK, Kunert J. 2001a. Biopersistence of synthetic mineral fibers as a predictor of chronic inhalation toxicity in rats. Inhal Toxicol. 13(10):823–849.
- Bernstein DM, Riego Sintes JM, Ersboell BK, Kunert J. 2001b. Biopersistence of synthetic mineral fibers as a predictor of chronic intraperitoneal injection tumor response in rats. Inhal Toxicol. 13(10):851–875.
- Bernstein DM, Thevenaz P, Fleissner H, Anderson R, Hesterberg TW, Mast R. 1995. Evaluation of the oncogenic potential of man-made vitreous fibres: the inhalation model. Ann Occup Hyg. 39(5):661–672.
- Bermudez E, Mangum J, Moss O, Wong B, Everitt J. 2003. Pleural dosimetry and pathobiological responses in rats and hamsters exposed subchronically to MMVF 10a fiberglass. Toxicol Sci. 74(1):165–173.
- Berry G. 1999. Models for mesothelioma incidence following exposure to fibers in terms of timing and duration of exposure and the biopersistence of the fibers. Inhal Toxicol. 11(2):111–130.
- Biswas R, Hamilton RF, Jr., Holian A. 2014. Role of lysosomes in silica-induced inflammasome activation and inflammation in absence of MARCO. J Immunol Res. 2014:304180.
- Brown JS, Gordon T, Price O, Asgharian B. 2013. Thoracic and respiratory particle definitions for human health risk assessment. Part Fibre Toxicol. 10(12):12–12.
- Brown RC, Bellmann B, Muhle H, Davis JM, Maxim LD. 2005. Survey of the biological effects of refractory ceramic fibres: overload and its possible consequences. Ann Occup Hyg. 49(4):295–307.
- Brown RC, Sebastien P, Bellmann B, Muhle H. 2000. Particle contamination in experimental fiber preparations. Inhal Toxicol. 12(Suppl 3):99–107.
- Campopiano A, Cannizzaro A, Angelosanto F, Astolfi ML, Ramires D, Olori A, Canepari S, Iavicoli S. 2014. Dissolution of glass wool, rock wool and alkaline earth silicate wool: morphological and chemical changes in fibers. Regul Toxicol Pharmacol. 70(1):393–406.
- Carthew P, Edwards RE, Dorman BM, Brown RC, Young J, Laskowski JJ, Wagner JC. 1995. Intrapleural administration of vitreous high duty ceramic fibres and heated devitrified ceramic fibres does not give rise to pleural mesothelioma in rats. Hum Exp Toxicol. 14(8):657–661.
- Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 105(26):9035–9040.
- Cho WS, Duffin R, Howie SE, Scotton CJ, Wallace WA, Macnee W, Bradley M, Megson IL, Donaldson K. 2011. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part Fibre Toxicol. 8(1):27.
- Cho WS, Duffin R, Poland CA, Duschl A, Oostingh GJ, Macnee W, Bradley M, Megson IL, Donaldson K. 2012. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology. 6(1):22–35.
- Collier CG, Morris KJ, Launder KA, Humphreys JA, Morgan A, Eastes W, Townsend S. 1994. The behavior of glass fibers in the rat following intraperitoneal injection. Regul Toxicol Pharmacol. 20(3 Pt 2): s89–103.
- Crapo JD, Young SL, Fram EK, Pinkerton KE, Barry BE, Crapo RO. 1983. Morphometric characteristics of cells in the alveolar region of mammalian lungs. Am Rev Respir Dis. 128(2 Pt 2): s42–46.
- Cullen RT, Searl A, Buchanan D, Davis JM, Miller BG, Jones AD. 2000. Pathogenicity of a special-purpose glass microfiber (E glass) relative to another glass microfiber and amosite asbestos. Inhal Toxicol. 12(10):959–977.
- Davis JMG. 1976. Pathological aspects of the injection of glass fiber into the pleural and peritoneal cavities of rats and mice. In: Occupational exposure to fibrous glass: Proceedings of a Symposium, June 26-27, 1974. College Park, MD: U.S. Dept. of Health, Education, and Welfare–Center for Disease Control–National Institute for Occupational Safety and Health (NIOSH)–Division of Criteria Documentation and Standards Development. p. 141–150.
- Davis JM, Beckett ST, Bolton RE, Collings P, Middleton AP. 1978. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 37(5):673–688.
- Davis JM, Dungworth DL, Boorman GA. 1996. Concordance in diagnosis of mesotheliomas. Toxicol Pathol. 24(5):662–663.
- Donaldson K, Murphy F, Schinwald A, Duffin R, Poland CA. 2011. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine. 6(1):143–156.
- Donaldson K, Murphy FA, Duffin R, Poland CA. 2010. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 7(1):5.
- Donaldson K, Schinwald A, Murphy F, Cho WS, Duffin R, Tran L, Poland C. 2013. The biologically effective dose in inhalation nanotoxicology. Acc Chem Res. 46(3):723–732.
- Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. 2008. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 320(5876):674–677.
- Dufresne A, Perrault G, Yamato H, Masse S, Begin R. 1999. Clearance of man made mineral fibres from the lungs of sheep. Occup Environ Med. 56(10):684–690.
- Eastes W, Hadley J. 1996. A mathematical model of fiber carcinogenicity and fibrosis in inhalation and intraperitoneal experiments in rats. Inhal Toxicol. 8(4):323–343.
- Eastes W, Hadley J, Bender J. 1996. Assessing the biological activity of fibres: insights into the role of fibre durability. J Occup Health Safety Aust NZ. 12(3):381–385.
- Eastes W, Morris KJ, Morgan A, Launder KA, Collier CG, Davis JA, Mattsom SM, Hadley JG. 1995. Dissolution of glass fibers in the rat lung following intratracheal instillation. Inhal Toxicol. 7(2):197–213.
- Eastes W, Potter R, Hadley J. 2000. Estimating rock and slag wool fiber dissolution rate from composition. Inhal Toxicol. 12(12):1127–1139.
- Effros RM, Wesson JA, Mason RJ, Broaddus VC, Murray JF, Nadel JA. 2005. Acid-base balance, Chapter 7. In: Mason RJ, Broaddus VC, Murray JF, Nadel J, editors. Murray and Nadel’s textbook of respiratory medicine. Fourth edition. Philadelphia, PA: Elsevier Inc. p. 171–182.
- Egnot NS, Benson SM, Vater MF, Hazan R, Patel O, Marsh GM. 2020. Systematic review and meta-analysis of epidemiologic literature evaluating the association between exposure to man-made vitreous fibers and respiratory tract cancers. Regul Toxicol Pharmacol. 112(104585):104512–104585.
- EIPPCB, Scalet, BM, Garcia Munoz M, Sissa A, Delgado Sancho L, Roudier S, et al. 2013. Best available techniques (BAT) reference document for the manufacture of glass : industrial emissions Directive 2010/75/EU: integrated pollution prevention and control, Publications Office. https://data.europa.eu/doi/10.2791/70161
- ERG. 2003. Report on the expert panel on health effects of asbestos and synthetic vitreous fibers: the influence of fiber length. March 17, 2003. Atlanta, GA: Agency for Toxic Substances and Disease Registry, Division of Health Assessment and Consultation.
- Etherington DJ, Pugh D, Silver IA. 1981. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 40(10–11):1625–1636.
- European Commission. 2009. Regulation (EC) No. 1223/2009 of the European Parliament of the Council of 30 November 2009 on cosmetic products (Recast). Official Journal of the European Union, L342/59. Retrieved from: https://health.ec.europa.eu/system/files/2016-11/cosmetic_1223_2009_regulation_en_0.pdf#:∼:text=This%20Regulation%20aims%20at%20simplifying,of%20protection%20of%20human%20health.
- European Economic Community. 1997. Commission Directive 97/69/EC of 5 December 1997 adapting to technical progress for the 23rd time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Official Journal of the European Communities. L343/19. Retrieved from: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31997L0069.
- Everitt JI, Gelzleichter TR, Bermudez E, Mangum JB, Wong BA, Janszen DB, Moss OR. 1997. Comparison of pleural responses of rats and hamsters to subchronic inhalation of refractory ceramic fibers. Environ Health Perspect. 105 Suppl 5(Suppl 5):1209–1213.
- Flink EB, Dedhia HV, Dinsmore J, Doshi HM, Banks D, Hshieh P. 1992. High-dose magnesium sulfate attenuates pulmonary oxygen toxicity. Crit Care Med. 20(12):1692–1698.
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 10(3):241–247.
- Ghio AJ, Churg A, Roggli VL. 2004. Ferruginous bodies: implications in the mechanism of fiber and particle toxicity. Toxicol Pathol. 32(6):643–649.
- Grimm HG, Bernstein DM, Attia M, Richard J, De Reydellet A. 2002. Experience from a long-term carcinogenicity study with intraperitoneal injection of biosoluble synthetic mineral fibers. Inhal Toxicol. 14(8):855–882.
- Gross P, Kaschak M, Tolker EB, Babyak MA, de Treville RT. 1970. The pulmonary reaction to high concentrations of fibrous glass dust. A preliminary report. Arch Environ Health. 20(6):696–704.
- Gross P, Tuma J, DeTreville RT. 1971. Lungs of workers exposed to fiber glass. A study of their pathologic changes and their dust content. Arch Environ Health. 23(1):67–76.
- Guldberg M, de Meringo A, Kamstrup O, Furtak H, Rossiter C. 2000. The development of glass and stone wool compositions with increased biosolubility. Regul Toxicol Pharmacol. 32(2):184–189.
- Guldberg ML, Madsen A, Sebastien K, Fellman J, Potter R, Bauer J, Searl A, Maquin B, Jubb G. 2003. In-vitro dissolution of vitreous silicate fibres according to EURIMA test guideline – results of two Round Robins. Glass Sci Technol. 76(4):199–205.
- Gulumian M, Andraos C. 2018. In search of a converging cellular mechanism in nanotoxicology and nanomedicine in the treatment of cancer. Toxicol Pathol. 46(1):4–13.
- Hamilton RD, Miiller WC, Christensen DR, Anderson R, Hesterberg TW. 1994. Characterization of exposure and dose of manmade vitreous fiber in experimental studies. Environ Health Perspect. 102(Suppl 5):109–112.
- Harris RL, Jr., Timbrell V. 1975. The influence of fibre shape in lung deposition-mathematical estimates. Inhaled Part. 4(Pt 1):75–89.
- Hesterberg TW, Axten C, McConnell EE, Oberdörster G, Everitt J, Miiller WC, Chevalier J, Chase GR, Thevenaz P. 1997. Chronic inhalation study of fiber glass and amosite asbestos in hamsters: twelve-month preliminary results. Environ Health Perspect. 105(Suppl 5):1223–1229.
- Hesterberg TW, Anderson R, Bernstein DM, Bunn WB, Chase GA, Jankousky AL, Marsh GM, McClellan RO. 2012. Product stewardship and science: safe manufacture and use of fiber glass. Regul Toxicol Pharmacol. 62(2):257–277.
- Hesterberg TW, Chase G, Axten C, Miller WC, Musselman RP, Kamstrup O, Hadley J, Morscheidt C, Bernstein DM, Thevenaz P. 1998. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol Appl Pharmacol. 151(2):262–275.
- Hesterberg TW, Hart GA. 2000. Lung biopersistence and in vitro dissolution rate predict the pathogenic potential of synthetic vitreous fibers. Inhal Toxicol. 12(Suppl 3):91–97.
- Hesterberg TW, Hart GA. 2001. Synthetic vitreous fibers: a review of toxicology research and its impact on hazard classification. Crit Rev Toxicol. 31(1):1–53.
- Hesterberg TW, Hart GA, Chevalier J, Miiller WC, Hamilton RD, Bauer J, Thevenaz P. 1998. The importance of fiber biopersistence and lung dose in determining the chronic inhalation effects of X607, RCF1, and chrysotile asbestos in rats. Toxicol Appl Pharmacol. 153(1):68–82.
- Hesterberg TW, Hart GA, Miiller WC, Chase G, Rogers RA, Mangum JB, Everitt JI. 2002. Use of short-term assays to evaluate the potential toxicity of two new biosoluble glasswool fibers. Inhal Toxicol. 14(3):217–246.
- Hesterberg TW, Miiller WC, McConnell EE, Chevalier J, Hadley JG, Bernstein DM, Thevenaz P, Anderson R. 1993. Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundam Appl Toxicol. 20(4):464–476.
- Hesterberg TW, Miiller WC, Musselman RP, Kamstrup O, Hamilton RD, Thevenaz P. 1996. Biopersistence of man-made vitreous fibers and crocidolite asbestos in the rat lung following inhalation. Fundam Appl Toxicol. 29(2):269–279.
- Hext PM. 1994. Current perspectives on particulate induced pulmonary tumours. Hum Exp Toxicol. 13(10):700–715.
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 9(8):847–856.
- Hurbankova M, Cerna S, Gergelova P, Wimmerova S. 2005. Influence of refractory ceramic fibres – asbestos substitute - on the selected parameters of bronchoalveolar lavage 6 months after intratracheal instillation to W-rats. Biomed Pap Med. 149(2):367–371.
- IARC. 1988. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 43: man-made mineral fibres and radon. Chapter 2. Production, use, occurrance and analysis. Lyon, France: World Health Organization (WHO)-International Agency for Research on Cancer (IARC).
- IARC. 2002. IARC monographs on the evaluation of the carcinogenic risk of humans: man-made vitreous fibers. Vol. 81. Lyon: International Agency for Research on Cancer.
- ILSI. 2005. Testing of fibrous particles: short-term assays and strategies. Report of an International Life Sciences Institute (ILSI) Risk Science Institute Working Group. Inhal Toxicol. 17:497–537.
- Innes E, Yiu HHP, McLean P, Brown W, Boyles M. 2021. Simulated biological fluids - a systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit Rev Toxicol. 51(3):217–248.
- Kamstrup O, Davis JM, Ellehauge A, Guldberg M. 1998. The biopersistence and pathogenicity of man-made vitreous fibres after short- and long-term inhalation. Ann Occup Hyg. 42(3):191–199.
- Kamstrup O, Ellehauge A, Chevalier J, Davis JM, McConnell EE, Thevenaz P. 2001. Chronic inhalation studies of two types of stone wool fibers in rats. Inhal Toxicol. 13(7):603–621.
- Kamstrup O, Ellehauge A, Collier CG, Davis JM. 2002. Carcinogenicity studies after intraperitoneal injection of two types of stone wool fibres in rats. Ann Occup Hyg. 46(2):135–142.
- Kane AB, Hurt RH, Gao H. 2018. The asbestos-carbon nanotube analogy: an update. Toxicol Appl Pharmacol. 361:68–80.
- Keller J, Wohlleben W, Ma-Hock L, Strauss V, Gröters S, Küttler K, Wiench K, Herden C, Oberdörster G, van Ravenzwaay B, et al. 2014. Time course of lung retention and toxicity of inhaled particles: short-term exposure to nano-Ceria. Arch Toxicol. 88(11):2033–2059.
- Kovacikova Z, Hurbankova M, Tatrai E, Cerna S. 2005. Effect of intratracheal fibres exposure on the rat lung. Biomed Pap Med. 149(2):363–365.
- Krombach F, Munzing S, Allmeling AM, Gerlach JT, Behr J, Dorger M. 1997. Cell size of alveolar macrophages: an interspecies comparison. Environ Health Perspect. 105:1261–1263.
- Le Bouffant L, Daniel H, Henin JP, Martin JC, Normand C, Tichoux G, Trolard F. 1987. Experimental study on long-term effects of inhaled MMMF on the lungs of rats. Ann Occup Hyg. 31(4B):765–790.
- Lee KP, Barras CE, Griffith FD, Waritz RS, Lapin CA. 1981. Comparative pulmonary responses to inhaled inorganic fibers with asbestos and fiberglass. Environ Res. 24(1):167–191.
- Lippmann M. 1988. Asbestos exposure indices. Environ Res. 46(1):86–106.
- Lippmann M. 1990. Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect. 88:311–317.
- Lippmann M. 1994. Deposition and retention of inhaled fibres: effects on incidence of lung cancer and mesothelioma. Occup Environ Med. 51(12):793–798.
- Lum H, Tyler WS, Hyde DM, Plopper CG. 1983. Morphometry of in situ and lavaged pulmonary alveolar macrophages from control and ozone-exposed rats. Exp Lung Res. 5(1):61–77.
- Madl AK, Pinkerton KE. 2009. Health effects of inhaled engineered and incidental nanoparticles. Crit Rev Toxicol. 39(38):639–658.
- Madl AK, Sun RM, Silva T, Kadir , Pinkerton KE. 2018. Particle toxicities. Chapter 15. In: Charlene A. McQueen, editor. Comprehensive Toxicology. 3rd Edition. Oxford: Elsevier. p. 263–301.
- Martin RA, Poynter ME. 2016. The immunobiology of asthma. Encyclop Immunobiol. 5:295–305.
- Mast RW, Hesterberg TW, Glass LR, McConnell EE, Anderson R, Bernstein DM. 1994. Chronic inhalation and biopersistence of refractory ceramic fiber in rats and hamsters. Environ Health Perspect. 102(Suppl 5):207–209.
- Mast RW, McConnell EE, Anderson R, Chevalier J, Kotin P, Bernstein DM, Thevenaz P, Glass LR, Miiller WC, Hesterberg TW. 1995a. Studies on the chronic toxicity (inhalation) of four types of refractory ceramic fiber in male Fischer 344 rats. Inhal Toxicol. 7(4):425–467.
- Mast RW, McConnell EE, Hesterberg TW, Chevalier J, Kotin P, Thevenaz P, Bernstein DM, Glass LR, Miiller W, Anderson R. 1995b. Multiple-dose chronic inhalation toxicity study of size-separated kaolin refractory ceramic fiber in male Fischer 344 rats. Inhal Toxicol. 7(4):469–502.
- Mast RW, Yu CP, Oberdorster G, McConnell EE, Utell MJ. 2000. A retrospective review of the carcinogenicity of refractory ceramic fiber in two chronic fischer 344 rat inhalation studies: an assessment of the MTD and implications for risk assessment. Inhal Toxicol. 12(12):1141–1172.
- Maxim LD, Boymel P, Chase GR, Bernstein DM. 2002. Indices of fiber biopersistence and carcinogen classification for synthetic vitreous fibers (SVFs). Regul Toxicol Pharmacol. 35(3):357–378.
- Maxim LD, Hadley JG, Potter RM, Niebo R. 2006. The role of fiber durability/biopersistence of silica-based synthetic vitreous fibers and their influence on toxicology. Regul Toxicol Pharmacol. 46(1):42–62.
- Maxim LD, Mast RW, Utell MJ, Yu CP, Boymel PM, Zoitos BK, Cason JE. 1999. Hazard assessment and risk analysis of two new synthetic vitreous fibers. Regul Toxicol Pharmacol. 30(1):54–74.
- Maxim LD, McConnell EE. 2001. Interspecies comparisons of the toxicity of asbestos and synthetic vitreous fibers: a weight-of-the-evidence approach. Regul Toxicol Pharmacol. 33(3):319–342.
- McConnell EE. 1994. Synthetic vitreous fibers-inhalation studies. Regul Toxicol Pharmacol. 20(3 Pt 2):S22–S34.
- McConnell EE, Axten C, Hesterberg TW, Chevalier J, Miiller WC, Everitt J, Oberdorster G, Chase GR, Thevenaz P, Kotin P. 1999. Studies on the inhalation toxicology of two fiberglasses and amosite asbestos in the Syrian golden hamster. Part II. Results of chronic exposure. Inhal Toxicol. 11(9):785–835.
- Miller BG, Searl A, Davis JM, Donaldson K, Cullen RT, Bolton RE, Buchanan D, Soutar CA. 1999. Influence of fibre length, dissolution and biopersistence on the production of mesothelioma in the rat peritoneal cavity. Ann Occup Hyg. 43(3):155–166.
- Monchaux G, Bignon J, Jaurand MC, Lafuma J, Sebastien P, Masse R, Hirsch A, Goni J. 1981. Mesotheliomas in rats following inoculation with acid-leached chrysotile asbestos and other mineral fibres. Carcinogenesis. 2(3):229–236.
- Muhle H, Bellmann B. 1997. Significance of the biodurability of man-made vitreous fibers to risk assessment. Environ Health Perspect. 105(Suppl 5):1045–1047.
- Muhle H, Bellmann B, Pott F. 1994. Comparative investigations of the biodurability of mineral fibers in the rat lung. Environ Health Perspect. 102(Suppl 5):163–168.
- Muhle H, Creutzenberg O, Bellmann B, Heinrich U, Mermelstein R. 1990. Dust overloading of lungs: investigations of various materials, species differences, and irreversibility of effects. J Aerosol Med. 3:S-111–S–128.
- Muhle H, Mangelsdorf I. 2003. Inhalation toxicity of mineral particles: critical appraisal of endpoints and study design. Toxicol Lett. 140–141:223–228.
- Muhle H, Pott F. 2000. Asbestos as reference material for fibre-induced cancer. Int Arch Occup Environ Health. 73:S53–S59.
- Muhle H, Pott F, Bellmann B, Takenaka S, Ziem U. 1987. Inhalation and injection experiments in rats to test the carcinogenicity of MMMF. Ann Occup Hyg. 31(4B):755–764.
- Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, Prina-Mello A, Volkov Y, Li S, et al. 2011. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 178(6):2587–2600.
- NEHC. 1997. Man-made vitreous fibers (Technical manual NEHC-TM6290.91-1 Rev). A. Norfolk, VA: Navy Environmental Health Center (NEHC). p. 110.
- NIOSH. 1980. Workplace exposure to asbestos: review and recommendations. DHHS (NIOSH) Publication No. 81-103. NIOSH-OSHA Asbestos Work Group April 1980. Washington, DC: U.S. Department of Health and Human Resources, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health (NIOSH). p. 1–47.
- NRC. 2000. Review of the U.S. Navy’s exposure standard for manufactured vitreous fibers. Washington, DC: National Research Council Subcommittee on Manufactured Vitreous, Fibers (NRC). National Academies Press (US).
- NTP. 2011. Certain glass wool fibers (inhalable). National Toxicology Program (NTP). Rep Carcinog. 12:207–215.
- Oberdorster G. 2000. Determinants of the pathogenicity of man-made vitreous fibers (MMVF). Int Arch Occup Environ Health. 73:S60–S68.
- OECD. 2018. Assessment of biodurability of nanomaterials and their surface ligands. Series on the safety of manufactured nanomaterials. Paris, France: Environment Directorate, Organisation for Economic Cooperation and Development (OECD) (No. 86, ENV/JM/MONO(2018)11).
- OECD. 2020a. Ability of biopersistent/biodurable manufactured nanomaterials (MNs to induce lysosomal membrane permeabilization (LMP) as a prediction of their long-term toxic effects, Series on the Safety of Manufactured Nanomaterials. Paris, France: Environment Directorate, Organisation for Economic Cooperation and Development (OECD) (No. 92, ENV/JM/MONO(2020)32).
- OECD. 2020b. Guidance document for the testing of dissolution and dispersion stability of nanomaterials and the use of the data for further environmental testing and assessment strategies. Series on Testing and Assessment. Paris, France: Environment Directorate, Organisation for Economic Cooperation and Development (OECD) (No. 318, ENV/JM/MONO(2020)9).
- OEHHA. 2011. Modification of the listing of glasswool fibers (airborne particles of respirable size) to glass wool fibers (inhalable and biopersistent). Sacramento, CA: California Office of Environmental Health Hazard Assessment (OEHHA).
- Osmond-McLeod MJ, Poland CA, Murphy F, Waddington L, Morris H, Hawkins SC, Clark S, Aitken R, McCall MJ, Donaldson K. 2011. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part Fibre Toxicol. 8(1):15.
- Peijnenburg W, Ruggiero E, Boyles M, Murphy F, Stone V, Elam DA, Werle K, Wohlleben W. 2020. A method to assess the relevance of nanomaterial dissolution during reactivity testing. Materials. 13(10):2235.
- Pigott GH, Ishmael J. 1992. The effects of intrapleural injections of alumina and aluminosilicate (ceramic) fibres. Int J Exp Pathol. 73(2):137–146.
- Pott F. 1998. Carcinogenicity of fibers in experimental animals data and evaluation. Assessment of Inhalation Hazards. ILSI Monographs, Springer Nature. p. 243–253.
- Pott F, Friedrichs KH. 1972. Tumors in the rat following intraperitoneal injections of fibrous dust. Naturwissenschaften. 59(7):318.
- Pott F, Friedrichs KH, Huth F. 1976. Results of animal experiments concerning the carcinogenic effect of fibrous dusts and their interpretation with regard to the carcinogenesis in humans (author’s transl). Zentralbl Bakteriol Orig B. 162(5–6):467–505.
- Pott F, Matscheck A, Ziem U, Muhle H, Huth F. 1988. Animal experiments with chemically treated fibres. Ann Occup Hyg. 32:353–359.
- Pott F, Roller M, Althoff GH, Kamino K, Bellmann B, Ulm K. 1993. Estimation of the carcinogenicity of inhaled fibres. VDI Berichte, Kolloquium Faserformige Staube, Conference Paper No. 1075, pp. 17–77 (in German). Dusseldorf: VD-Verlag.
- Pott F, Roller M, Kamino K, Bellmann B. 1994. Significance of durability of mineral fibers for their toxicity and carcinogenic potency in the abdominal cavity of rats in comparison with the low sensitivity of inhalation studies. Environ Health Perspect. 102(Suppl 5):145–150.
- Pott F, Ziem U, Reiffer FJ, Huth F, Ernst H, Mohr U. 1987. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp Pathol. 32(3):129–152.
- Roller M, Pott F, Kamino K, Althoff GH, Bellmann B. 1996. Results of current intraperitoneal carcinogenicity studies with mineral and vitreous fibres. Exp Toxicol Pathol. 48(1):3–12.
- Roller M, Pott F, Kamino K, Althoff GH, Bellmann B. 1997. Dose-response relationship of fibrous dusts in intraperitoneal studies. Environ Health Perspect. 105(Suppl 5):1253–1256.
- Sayan M, Mossman BT. 2016. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 13(1):51.
- Schlesinger R. 1995. Deposition and clearance of inhaled particles. In McClellan RO, Henderson RF, editors. Concepts in inhalation toxicology. Washington, DC: Taylor and Francis. p. 191–224.
- Searl A, Buchanan D, Cullen RT, Jones AD, Miller BG, Soutar CA. 1999. Biopersistence and durability of nine mineral fibre types in rat lungs over 12 months. Ann Occup Hyg. 43(3):143–153.
- Sebastian K, Fellman J, Potter R, Bauer J, Searl A, de Meringo A, de Reydellet A, Jubb G, Moore M, Preininger R. 2002. EURIMA test guideline: in-vitro acellular dissolution of man-made vitreous silicate fibres. Glass Sci Technol. 75(5):263–270.
- Sebring RJ, Lehnert BE. 1992. Morphometric comparisons of rat alveolar macrophages, pulmonary interstitial macrophages, and blood monocytes. Exp Lung Res. 18(4):479–496.
- Smith DM, Ortiz LW, Archuleta RF, Johnson NF. 1987. Long-term health effects in hamsters and rats exposed chronically to man-made vitreous fibres. Ann Occup Hyg. 31(4B):731–754.
- Snipes M. 1995. Pulmonary retention of particles and fibers: biokinetics and effects of exposure concentration. In McClellan OO, Henderson RF, editors. Concepts in inhalation toxicology. Washington, DC: Taylor and Francis. p. 191–224.
- Stanton MF. 1973. Some etiological considerations of fibre carcinogenesis. In Bogovski P, Timbrell V, Gilson JC, Wagner JC, editors. Biological effects of asbestos: Proceedings of a Working Conference. IARC Scientific Publications No. 8. Lyon: International Agency for Research on Cancer. p. 289–294.
- Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, Smith A. 1981a. Relation of particle dimension of carcingenicity in amphibole asbestosis and other fibrosis minerals. J Natl Cancer Inst. 67:965–975.
- Stanton MF, Layard M, Tegeris A, Miller E, May M, Morgan E, Smith A. 1981b. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 67(5):965–975.
- Stanton MF, Laynard M, Tegeris A, Miller E, May M, Kent E. 1977. Carcinogenicity of fibrous glass: pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst. 58(3):587–603.
- Stanton MF, Wrench C. 1972. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 48(3):797–821.
- Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. 1992. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 6(2):235–243.
- Stone RA, Youk AO, Marsh GM, Buchanich JM, Smith T. 2004. Historical cohort study of U.S. man-made production workers IX: summary of 1992 mortality follow up and analysis of respiratory system cancer among female workers. J Occup Environ Med. 46(1):55–67.
- Strom KA, Yu CP. 1994. Mathematical modeling of silicon-carbide whisker deposition in the lung-comparison between rats and humans. Aerosol Sci Technol. 24(3):193–209.
- Sussman R, Cohen B, Lippmann M. 1991a. Asbestos fiber deposition in a human tracheobronchial cast. I. Experimental. Inhal Toxicol. 3(2):145–160.
- Sussman RG, Cohen BS, Lippmann M. 1991b. Asbestos fiber deposition in a human trancheobronchial cast. II. Empirical Model. Inhal Toxicol. 3(2):161–179.
- TIMA. 1990. Health and safety aspects of man-made vitreous fibers. Information, data, comments and recommendations regarding occupational exposure to man-made vitreous fibers. Submitted by Thermal Insulation Manufacturers Association (TIMA) in Response to NIOSH request for comments and secondary data relevant to occupational exposure to synthetic and natural mineral fibers. Fed Regist. 55(5073):347.
- Topinka JB, Loli P, Dusinska M, Hurbankova M, Kovacikova Z, Volkovova K, Kazimirova A, Barancokova M, Tatrai E, Wolff T, et al. 2006. Mutagenesis by man-made mineral fibres in the lung of rats. Mutat Res. 595(1-2):174–183.
- Utembe W, Potgieter K, Stefaniak AB, Gulumian M. 2015. Dissoluation and biodurability: important parameters needed for risk assessment of nanomaterials. Part Fibre Toxicol. 12(11):11–12.
- Vorwald AJ, Durkan TM, Pratt PC. 1951. Experimental Studies of Asbestosis. Arch Indust Hyg Occup Med. 3(1):1–43.
- Wagner JC. 1963. Asbestosis in experimental animals. Br J Ind Med. 20(1):1–12.
- Wagner JC. 1976. Tumours in experimental animals following exposure to asbestos dust. Ann Anat Pathol. 21(2):211–214.
- Wagner JC. 1990. Biological effects of short fibers. In Proceedings of the VIIth International Pneumoconiosis Conference Part II, November. DHHS (NIOSH) Publication No. 90-108 Part II. Animal Models- Pneumoconiosis II. November 1990. Washington, DC: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. p. 835–839.
- Wagner JC, Berry G. 1969. Mesotheliomas in rats following inoculation with asbestos. Br J Cancer. 23(3):567–581.
- Wagner JC, Berry G, Hill RJ, Munday DE, Skidmore JW. 1980. Animal experiments with man-made mineral fibres. IARC Sci Publ. (30):361–362.
- Wagner JC, Berry G, Timbrell V. 1973. Mesotheliomata in rats after inoculation with asbestos and other materials. Br J Cancer. 28(2):173–185.
- Wagner JC, Griffiths DM, Hill RJ. 1984. The effect of fibre size on the in vivo activity of UICC crocidolite. Br J Cancer. 49(4):453–458.
- Warheit D, Hartsky MA, et al. 1994. Influences of gender, species and strain differences in pulmonary toxicological assessments of inhaled particles and/or fibers. In Mohr U, Dungworth DL, Mauderly JL editors. Toxic and carcinogenic effects of solid particles in the respiratory tract. Washington, DC: ILSI Press.
- WHO. 2000. Air quality guidelines - second edition. Chapter 8.2: Man-made vitreous fibres. Air quality guidelines for Europe 2nd Edition WHO Regional Publications European Series No 91. Copenhagen, Denmark: World Health Organization (WHO) Regional Office for Europe; p. 18. Retrieved February 15, 2022 from: https://www.euro.who.int/__data/assets/pdf_file/0004/123088/AQG2ndEd_8_2MMVF.pdf.
- Wylie AG, Virta RL, Segreti JM. 1987. Characterization of mineral population by index particle: IMPLICATION for the Stanton hypothesis. Environ Res. 43(2):427–439.
- Yu CP, Zhang L, Oberdorster G, Mast RW, Glass LR, Utell MJ. 1994. Deposition of refractory ceramic fibers (RCF) from the rat lung: development of a model. Environ Res. 65(2):243–253.
Appendix A
Table A1. Summary of intraperitoneal injection toxicology studies of SVFs.
Table A2. Summary of intrapleural injection toxicology studies of SVFs.
Table A3. Summary of intratracheal instillation toxicology studies of SVFs.
Table A4. Summary of whole-body inhalation toxicology studies of SVFs.
Table A5. Summary of nose-only inhalation toxicology studies of SVFs.
