Abstract
This article addresses issues of importance for occupational exposure limits (OELs) and chemical carcinogens with a focus on non-threshold carcinogens. It comprises scientific as well as regulatory issues. It is an overview, not a comprehensive review. A central topic is mechanistic research and insights, and its implications for cancer risk assessment. Alongside scientific advancements, the approaches of hazard identification and qualitative and quantitative risk assessment have developed over the years. The key steps in a quantitative risk assessment are outlined, with special attention given to the dose–response assessment and the derivation of an OEL using risk calculations or default assessment factors. The work procedures of several bodies performing cancer hazard identifications and quantitative risk assessments, as well as regulatory procedures to derive OELs for non-threshold carcinogens, are presented. Non-threshold carcinogens for which the European Union (EU) introduced binding OELs in 2017–2019 serve as illustrations together with some currently used strategies in the EU and elsewhere. Available knowledge supports the derivation of health-based OELs (Hb-OELs) for non-threshold carcinogens, and the use of a risk-based approach with low-dose linear extrapolation (linear non-threshold, LNT) as the default for non-threshold carcinogens. However, there is a need to develop methods that allow recent years’ advances in cancer research to be used for improving risk estimates. It is recommended that defined risk levels (terminology and numerical values) are harmonised, and that both collective and individual risks are considered and clearly communicated. Socioeconomic aspects should be dealt with transparently and separated from the scientific health risk assessment.
1. Introduction
In the European Union (EU), more than 30 million people were occupationally exposed to carcinogens in the early 1990s (15–19 member states) (EU-OSHA Citation2014). In 2012, about 120 000 newly diagnosed cancer cases and 80 000 cancer deaths were attributed to work-related exposure to carcinogenic substances (28 member states, EU-28) (RIVM Citation2016). Estimates indicate that 8% of all cancer cases in the EU-28 are caused by occupational exposure (based on 25 selected carcinogenic agents, mostly chemicals but also, e.g. solar and ionising radiation, and shift work) (RPA/FoBiG Citation2017). In light of this, the European Commission has proposed to further limit workers’ exposure to chemical carcinogens. This includes the update of binding occupational exposure limits (OELs) for carcinogens (Roadmap on Carcinogens Citation2021), and initiatives to revise the methodology for OEL setting (European Parliament Citation2022).
Efforts to regulate exposure to carcinogens at work were introduced in the second half of the twentieth century. Since then, knowledge about cancer development and chemical-induced carcinogenesis has advanced tremendously along with a parallel development in cancer risk assessment strategies, going from hazard identification to quantitative risk assessment. Concepts and strategies from the twentieth century are still influential; therefore, the narrative is partially presented with a historical perspective and with the ambition to give a foundation for currently used procedures for risk assessment and risk management in the work environment. Aspects addressed comprise scientific and regulatory issues including cancer mechanisms, genotoxic versus non-genotoxic carcinogens, the threshold concept, hazard identification versus quantitative risk assessment, and risk calculations versus default assessment factors. The work procedures of a number of bodies performing cancer hazard identifications or risk assessments are described, and risk estimates for binding OELs for non-threshold carcinogens introduced in the EU in 2017–2019 are provided. The scientific basis for the chemical agents in question is presented together with the European Commission’s rationales for the final limit values. Finally, recommendations are given regarding regulatory aspects in the OEL setting of carcinogens and future research directions.
This article, with minor modifications, has previously been published as a report by the Nordic Expert Group (NEG) for Criteria Documentation of Health Risks from Chemicals (Högberg and Järnberg Citation2022). The NEG document resulted in recommendations by NEG and these remain in Chapter 8.
The aim of the NEG document and this article is to define and describe critical issues in the risk assessment of carcinogens that are of importance for the derivation of OELs. These topics might also be of interest to scientists who conduct risk-related cancer research. To that end, the authors prepared this version for publication as a stand-alone article available through scientific databases.
2. From hazard identification to quantitative risk assessment of carcinogens
Knowledge about radiation-induced cancer initially influenced the regulation of chemical carcinogens, and for many years, the regulation in the work environment focussed on hazard identification of chemical carcinogens. In later years, focus has shifted toward an emphasis on risk and quantitative risk assessments. The chapter summarises this development.
2.1. The origin of low-dose, linear non-threshold extrapolation
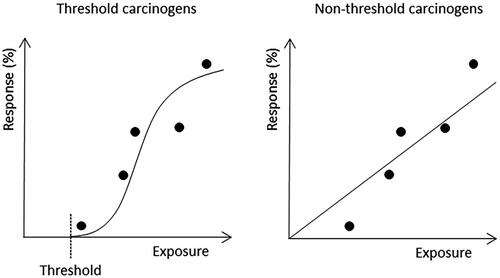
In analogy with radiation, and in the absence of specific knowledge, mutations were seen as the driving force also behind chemical-induced cancer. Muller’s classical experiments with radiation and Drosophila melanogaster published in 1927 came in focus (Calabrese Citation2017b). Muller (Citation1927) bred flies whose genomes contained particular genetic markers on the X-chromosome, and developed methods for quantification of lethal mutations induced by X-ray. In this way, he was able to show increasing effects with increasing doses. These experiments were interpreted to indicate that there was no threshold (dose/exposure level below which effects do not appear) (Henshaw Citation1946) but rather a linear dose–response relationship between radiation dose and mutational responses. This interpretation was supported by a study on tobacco plants, which compared the background frequency of phenotypic “variants” with the frequency induced by high-dose radiation. By employing simple probabilistic mathematics, the background variants could be explained by the very low natural background radiation (Olson and Lewis Citation1928). The tobacco plant data implied that even very low increases in exposures are associated with an increased risk for mutations and thus also an increased risk for cancer. Furthermore, it implied that no safe level of exposure could be defined for carcinogens. Given that a mutation theoretically can be induced by a single chemical-DNA adduct, these data were interpreted to indicate that carcinogens should be exempted from the idea that a threshold can be defined in the dose–response curve for toxic chemicals. Instead, the concept of linear non-threshold (LNT) dose–response was introduced for radiation as well as for many chemical carcinogens (Calabrese Citation2017b). For other carcinogens and most non-cancer responses, a threshold is anticipated (). For a review on radiation risk assessment, see Wojcik and Harms-Ringdahl (Citation2019).
Figure 1. Dose–response curves for threshold versus non-threshold carcinogens with five empirically documented dose levels (dots). The response is expressed as the percentage of a population (humans or laboratory animals) affected. The curves show that, guided by mechanistic knowledge, the same empirical data can be interpreted differently.
From a regulatory point of view, an initial strategy was to ban or substitute chemical carcinogens. Already in 1958, the United States (US) congress revised the law concerning food additives, which in effect led to the banning of any additive designated as carcinogenic (zero risk policy). This approach was later abandoned because of e.g. advances in analytical chemistry (Jasanoff Citation1982). For occupational exposures, an alternative strategy was to assign OELs as low as technically (reasonably) achievable (now often termed ALARA, Section 2.4). Differences in potency were neglected.
2.2. Initial strategies for assessment of carcinogenicity
Chemical-induced cancer is inherently hard to document in humans. It is a rare event even among heavily exposed humans, whereas “background” cancers are relatively common, in relevant, i.e. high age, groups (Armitage and Doll Citation1954; Tomasetti et al. Citation2017). Therefore, epidemiological studies require large exposure gradients and/or large groups of exposed and unexposed subjects to get sufficient statistical power to conclude that a given chemical is associated with cancer. Although recent technical advances have enabled the identification of characteristic mutational signatures for some carcinogens (Kucab et al. Citation2019), a remaining problem is that most carcinogen-induced tumours cannot be distinguished from those caused by random biological events. Furthermore, there is a long latency period between the start of exposure and clinical signs of cancer, for solid tumours 10–50 years and for blood cancers 0–20 years (Cherrie et al. Citation2017). During this period, an individual might experience multiple chemical exposures without signs of disease. This complicated, and still complicates, the interpretation of epidemiological studies of carcinogens. Thus, even if epidemiological data are important for reaching conclusions about carcinogenicity, causality is hard to prove. In addition, gathering conclusive data takes time, and the unavoidable delay, as compared to primary prevention, activates ethical issues.
These circumstances were hard to reconcile with the regulatory ambition to prevent even relatively rare cases of occupational cancer. To meet these challenges, 2-year animal studies, standardised bioassays employing mice and rats, were introduced in the 1970s (Cohen and Ellwein Citation1990). It was early recognised that close to life-long exposure to high doses was needed to get statistically significant results and to compensate for the practical necessity to use a limited number of animals (usually 50 per dose group). Facing the problem of detecting a cancer risk in humans of say 1 case per 100 000 (1 × 10−5), it can be argued that 50 animals give a limited statistical power. Although animal studies are important for proving causality and for corroborating epidemiology, the high doses used have been criticised for introducing effects that might not be valid for low doses and for humans (Section 2.3.2). There are also short-term in vivo test protocols, not often used today, but published data are still utilised in risk assessments. Many such tests emphasise initiation-promotion phases (Section 2.3.1), use pre-neoplastic endpoints and are less time-consuming than 2-year animal bioassays (Solano et al. Citation2016).
To compensate for limitations of the 2-year rodent cancer assay, simple high-throughput in vitro bacterial assays for mutagenicity were introduced. These tests were superior regarding speed, costs, and ethical issues, and much hope was given to them, as exemplified by the title “Carcinogens are mutagens: their detection and classification” of an influential article published by Ames (Citation1973).
2.3. Genotoxicity and the threshold concept
2.3.1. Tumour initiators and promoters
Positive responses in 2-year animal bioassays were partially interpreted in the light of results obtained from initiation-promotion animal research (Solano et al. Citation2016). Consistent with a long latency period for cancer development (Section 2.2), the initiation-promotion model emphasises two phases of cancer development. The initiation phase is associated with mutations caused by a mutagenic chemical (a tumour initiator) and leads to the appearance of “initiated cells”. Each of these cells with a presumed first mutation has the potential to constitute a single cell origin of a tumour. The promotion phase requires multiple doses of the same or a different chemical, over an extended period of time. The tumour promoter may target initiated cells to proliferate. During the promotion phase, the number of initiated cells thus multiply, and it was soon discovered that new mutations were acquired via indirect mechanisms such as stimulated cell replication. The discovery that chemicals can act as promoters gave rise to the concept of non-genotoxic carcinogens (Cohen and Ellwein Citation1990). Later, the terms genotoxic (direct and indirect genotoxic) and non-genotoxic carcinogens became established nomenclature to distinguish in risk assessments between non-threshold (DNA-reactive) and threshold (indirect genotoxic and non-genotoxic) carcinogens (Chapter 5).
2.3.2. Non-genotoxic carcinogens
It also became clear that many test substances that were positive in 2-year animal bioassays or active as tumour promoters were negative in in vitro tests for mutagenicity (Section 2.2) (Cohen and Ellwein Citation1990). Intense toxicological research during the 1970s–1980s confirmed that some chemicals given alone in high doses (not commonly experienced by humans) could cause cancer without being positive in mutagenicity tests. This insight indicated that not all carcinogens are suited for mathematical modelling based on an LNT dose–response. Even though supportive empirical data are missing for many substances, biologically based mechanistic considerations suggest that non-genotoxic carcinogens have a threshold in their dose–response curve.
It was for example shown that chemicals given in high doses may produce acute liver cell death, an effect that is compensated for by cell replication that increases the risk for indirect mutations and cancer (Cohen and Ellwein Citation1990). Another example from the 1980s is the delineation of mechanisms for dioxin-induced tumours. Thus, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was found to be an extremely potent liver carcinogen in animal bioassays, yet its mechanism of action included receptor binding and consistent activation of normal proliferative signalling pathways (Pääjärvi et al. Citation2005; Benigni and Bossa Citation2011).
Other discoveries were non-genotoxic mechanisms operating in animals, but seemingly not in humans. A well-studied example is that of α2-microglobulin. It is a protein found in male rats, and its production has been shown to be induced by chronic exposure to some chemicals. When excreted in urine, it kills kidney epithelial cells and to compensate for the lost epithelium, it is replaced by proliferating surviving cells. The continued proliferation leads to kidney cancer in male rats. Of importance for risk assessment is that α2-microglobulin is not produced in humans. Hence, if it can be shown that chemicals giving kidney tumours in male rats act via this mechanism, their carcinogenicity in humans can be questioned (Dietrich and Swenberg Citation1991).
2.3.3. Direct and indirect genotoxic carcinogens
Direct acting (non-threshold) genotoxic carcinogens (or their metabolites) interact directly with DNA, resulting in mutations (Benigni and Bossa Citation2011; Hartwig et al. Citation2020) (Section 2.1). However, many compounds indirectly induce DNA damage without direct interaction. One common mechanism for indirect genotoxicity is that mediated by reactive oxygen species (ROS) causing oxidative stress. A chemical may trigger ROS production in many ways: via redox-cycling toxicity, through inflammatory responses, or simply by activating signalling pathways (Wu et al. Citation2020; Zheng et al. Citation2020). ROS may react with DNA thereby causing oxidative damage or strand breaks and mutations. The chemical-induced oxidative mutagenic mode of action (MoA) is regarded as a threshold mechanism for cancer. Other MoAs conferring a threshold might depend on saturated, non-error-prone DNA repair (Bononi et al. Citation2017; Supek and Lehner Citation2017). Additional examples of effects leading to tumours, besides those sketched here and above (Section 2.3.2), are shown in the MoA taxonomy (Section 3.2.2). The MoA concept is used to bridge data gaps when all steps in chemical-induced cancer development are not fully characterised. Current categorisations of carcinogens for risk assessment purposes based on MoAs are described in Section 5.2.
2.3.4. Current strategies for assessment of mutagenicity, genotoxicity, and carcinogenicity
The threshold issue put emphasis on testing mutagenic activity of chemical carcinogens in mammalian cells or test animals, and not primarily in microorganisms. Employing mammalian experimental models to assess mutagenicity is time-consuming and new methods for monitoring genotoxicity (i.e. the many upstream events initiated by DNA damage that indicate an increased risk for mutations) were developed.
Chemical-induced effects in mammalian (including human) cells can be efficiently tested in assays based on e.g. micronuclei, DNA strand breaks (Comet assay), DNA adducts and DNA damage responses (DDR), i.e. cell signalling events such as phosphorylation of p53 or histone H2AX (γH2AX) (Hartwig et al. Citation2020; Wu et al. Citation2020). Also worth mentioning are efforts to use gene expression profiles as endpoints in high-throughput short-term test models that may predict carcinogenicity and mechanisms of action (Gusenleitner et al. Citation2014). For a more comprehensive review of genotoxicity testing, see Hartwig et al. (Citation2020).
Positive in vitro mutagenicity tests are nowadays regarded as supportive evidence and provide mechanistic insight for chemicals. In the EU, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) legislation requires in vitro gene mutation tests in bacteria as a standard information for chemicals on the market (ECHA Citation2017a).
Guidelines for the testing of chemicals are provided by the Organisation for Economic Co-operation and Development (OECD). They comprise around 150 of the most relevant and internationally agreed methods to assess the potential effects on human health, including genotoxicity and carcinogenicity (2-year animal bioassays) (OECD Citation2020). A consensus statement from an OECD Expert Group dealing with testing and assessment of non-genotoxic carcinogens is also available (Jacobs et al. Citation2020).
Although epidemiological studies have limited power to show cancer caused by specific agents against, e.g. a background of common tumour types, they are still crucial for reaching conclusions about carcinogenicity in humans. However, it is hardly possible to conclude that a given chemical is carcinogenic without understanding its chemical properties and biological effects.
2.4. Hazard identification and qualitative risk assessment
Despite obvious gaps in mechanistic knowledge and controversies raised by testing and research, the fundamental question whether a chemical is carcinogenic or not had to be addressed for adequate risk management. The International Agency for Research on Cancer (IARC) pioneered this task during the 1970s. The IARC approach was termed risk identification (nowadays called hazard identification). The basic structure of the stepwise procedure developed by IARC is still in use (IARC Citation2019) and considers all published literature on cancer, from epidemiology and animal studies to mechanistic evidence. The outcome is a classification based on strength of evidence for carcinogenicity for a given chemical agent or exposure (Section 5.1.1).
In the work environment, this strategy has been employed with the aim to ban or substitute carcinogens or to set OELs based on technical feasibility (instead of risk) or by demanding exposures to be “as low as reasonably achievable” (ALARA).
During the 1970s, when hazard identification was the dominating issue in the risk assessment of most carcinogens, the risk management strategy practiced, e.g. in Sweden resulted in drastically reduced OELs for carcinogens (Hansson Citation1998).
A related approach is a qualitative risk assessment. It is used when data are not available to support numerical estimates but permit risk ranking or separating into descriptive categories of risk in addition to the hazard identification (US EPA Citation2020).
2.5. Early development of quantitative risk assessment
The initial risk management strategy to ban or substitute chemical carcinogens (irrespective of their presumed MoA) (Section 2.1) was not feasible for all carcinogens. Thus, there was a need for methods to prioritise remaining environmental carcinogens according to potency. This demand was met during the 1980s by approaches to quantitatively estimate risks from exposure to, e.g. environmental carcinogens at ambient levels. The US Environmental Protection Agency (US EPA) was influential. An obvious problem was, and still is, that empirical risk data are rare at realistic levels of exposure (i.e. levels to which humans are typically exposed) (Melnick et al. Citation2002). In most cases, extrapolations from epidemiological studies of historically high exposure levels, or from animal bioassays, down to ambient exposure levels were practised with the assumption that the dose–response curve is without a threshold.
Sophisticated mathematical models were developed during the 1980s. Initial models focussed on the role of mutations and took advantage of contemporary knowledge about the number of mutations needed for cancer development in general. For example, there was statistical evidence indicating that death rates for several cancers increased proportionally with the 6th power of the age, and it was estimated that cancer was the delayed result of 6–7 mutations (Nordling Citation1953; Armitage and Doll Citation1954). These early extrapolation models for calculating risk levels can obviously be criticised for giving an impression of false exactness and precision, and in later years, more simplified models have been suggested by US EPA to acknowledge the uncertainty (US EPA Citation2005).
The non-threshold extrapolation models gave ways to prioritise risk management efforts for single chemical carcinogens in the general environment. Thus, the models made it possible to calculate extra cancer risks for ambient exposures for single carcinogens and to prioritise those with the highest calculated risks.
An additional step was to define acceptable cancer risks and to make comparisons with other risk factors in society, such as traffic accidents. In the US, the acceptable risk for carcinogen-induced cancer in the general population was set to one extra cancer case in a lifetime per million individuals, or 1 × 10−6. This level was apparently derived in the 1960s during the development of guidelines for safety testing of drugs. In 1973, the figure of 1 per 100 million (1 × 10−8) of developing cancer was put forward as safe and adopted by the US Food and Drug Administration, but was amended to 1 per million (1 × 10−6) in 1977. This risk level is far lower than what can be proved empirically for most carcinogens. It has been regarded as “essentially zero” and has become something of a gold standard (Kelly Citation1991; WHO Citation2001). Often extrapolations to such low risk levels mean that the exposure level at the point that marks the beginning of the low-dose extrapolation, the point of departure (PoD), is linearly scaled down by several orders of magnitude.
As further specified in Chapter 6, risk levels in the work environment are often much higher than levels that are accepted for the general population. A more elaborate description of current approaches to perform quantitative cancer risk assessments is presented in Chapter 4.
3. Mechanistic cancer research that may affect quantitative risk assessment
The scientific basis for the LNT approach and the use of the LNT policy as a whole have been questioned (Calabrese Citation2017b, Citation2017a). This criticism will not be further commented here as the LNT policy for several reasons are favoured by many bodies involved in OEL settings. However, highlighting some recent achievements in cancer research might be constructive. Novel mechanistic insight might be used to improve or modulate the LNT policy when practiced for risk assessment of single or groups of carcinogens. Some recent or ongoing mechanistic research that directly addresses, or indirectly may affect, quantitative risk assessment strategies are commented. Furthermore, the concepts hallmarks of cancer and MoA are presented. By employing text-mining tools, these concepts can be used for structuring previous and current scientific knowledge and for overviews.
For more in-depth overviews, the reader is referred to articles on MoA-based risk assessment (Hartwig et al. Citation2020), molecular mechanisms of major preventable causes of cancer (Golemis et al. Citation2018), key mechanistic characteristics of substances classified by IARC as carcinogens (Smith et al. Citation2016), and historical aspects of cancer-causing agents and their effects in general (Blackadar Citation2016). In line with some epidemiologically based estimates of the proportion of spontaneous cancer cases, a mechanistic study indicated that about two thirds of all cancer can be explained by random/spontaneous mutations affecting “driver genes” in replicating stem cells (Tomasetti et al. Citation2017).
3.1. Current mechanistic research
The statistically based indications of about 6–7 mutations as rate-limiting mechanism for tumour development initially discussed during the 1950s (Nordling Citation1953; Armitage and Doll Citation1954) have been supported by several studies, e.g. by a seminal human study. This study showed an ordered sequence of mutational events, correlating with morphological alterations well known by pathologists, and which lead to hereditary colorectal cancer in humans (Kinzler and Vogelstein Citation1996).
In another study, mutations in morphologically healthy human tissue samples were investigated for very early indications of cancer development. The largest number of mutations were found in skin and lung, organs directly exposed to environmental stressors. These two organs also expressed high levels of a proliferation marker. In skin samples, the number of mutations was strongly correlated to markers of UV exposure (Yizhak et al. Citation2019). An interpretation of these data is that critical carcinogenic mechanisms, activated by environmental factors and modelled in initiation-promotion animal experiments (Section 2.3.1), also operate at an early stage of cancer development in humans and may affect the dose–response in a non-linear fashion.
Basic biological factors affecting, e.g. DNA adduct formation and DNA repair may vary in importance over a range of exposure levels (Jenkins et al. Citation2005), and may protect from mutations and effectively prevent, e.g. binding to DNA if a cell is exposed to low doses of a carcinogen. Besides DNA repair, the cell has mechanisms to eliminate itself (apoptosis) if overwhelmed with DNA damage. These mechanisms oppose cancer development at low doses of a carcinogen but may be overwhelmed at high doses or be disturbed by parallel exposure to other stressors, thus resulting in a sublinear dose–response.
Other mechanisms that may affect the shape of the dose–response curve for carcinogens include cellular senescence, replication stress, lineage infidelity, genomic instability, epigenetic changes, and autophagy.
Cellular senescence is characterised by the irreversible blocking of cell division and may prevent cancer development. However, the senescent cell phenotype produces chemokines and growth factors (Perez-Mancera et al. Citation2014), which provoke inflammation and tumour growth (Fontana et al. Citation2018; Golemis et al. Citation2018; Xu et al. Citation2018). Senescence may thus both prevent and stimulate cancer development and thereby affect the dose–response in an unpredictable way.
A publication from 2018 revealed the occurrence of cancer stem cells in hyperplastic nodules (pre-neoplastic lesions) in mammary glands (McGrail et al. Citation2018). This phenotype developed in response to replication stress and it seems reasonable to assume that cancer stem cells promote cancer growth. It has further been proposed that stem-like cells (i.e. de-differentiated, proliferative, and drug-tolerant cells) may develop via non-mutational mechanisms in response to toxicological stress induced by anti-cancer drugs (Huang Citation2021). If such non-mutational mechanisms are rate-limiting for tumours induced by environmental carcinogens, linearity cannot be expected.
A skin cancer study showed that cell lineage infidelity (deviations from e.g. an organ-specific cell fate) in normal stem cells occurs transiently in stressed wound healing. However, it may also stimulate cancer development as the infidelity programme may be hijacked by developing tumour cells via non-mutagenic mechanisms (Ge Y et al. Citation2017). The studied effect was provoked by wounding but may be relevant for chemical exposure.
Genomic instability (the increased tendency for mutations to occur during various types of cellular stress) leading to permanent genetic alterations may be common for many cancer types (Tubbs and Nussenzweig Citation2017), and efforts to characterise its importance in mathematical terms for the carcinogenesis of e.g. human lung cancer and colon cancer induced by radiation and other external factors have been presented (Kaiser et al. Citation2014; Li et al. Citation2019). Genomic instability may lead to hundreds of mutations. A role in experimental chemical carcinogenesis has been indicated (Liu et al. Citation2015). These examples challenge the concept that a certain order of 6–7 mutations explains the exponential increase of cancer with age, as suggested by Armitage and Doll (Citation1954) and Nordling (Citation1953) (Section 2.5).
There is a shortage of studies on the role of epigenetic changes (changes in gene function due to e.g. DNA methylation that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence) in cancer development. Chappell et al. analysed 28 genotoxic IARC Group 1 carcinogenic compounds or occupations (IARC Citation2012). There were published reports of epigenetic alterations for 12 of the 28 compounds. For three of these carcinogens (aflatoxins, benzene, and benzo[a]pyrene), 10 or more studies reported epigenetic effects while epigenetic studies were sparse for the other nine (Chappell et al. Citation2016).
In another study, the ratio between PoDs for cancer incidence and DNA methylation changes in animals exposed to both genotoxic and non-genotoxic carcinogens were investigated. The relationship was similar for seven of eight carcinogens (Kuppusamy et al. Citation2015) suggesting that epigenetic influence on the PoD is more common than previously anticipated. The relative importance of genetic versus epigenetic alterations in different organs may also be influential. In one study, epigenetic alterations were seen primarily as an effect of inflammation and the authors suggested a strategy to estimate the influence of DNA methylations (Yamashita et al. Citation2018).
Autophagy is a cellular catalytic process, which degrades cellular components. It may be activated in response to environmental challenges such as starvation, and protects cells from apoptosis and necrosis. It has been shown that inhibition of autophagy delays formation of premalignant foci in mesothelial cells challenged by asbestos (Xue et al. Citation2020). As asbestos-induced mesotheliomas exhibit long latency periods, the epigenetically controlled autophagy may strongly influence the asbestos-induced cancer incidence, and thus also the shape of the dose–response curve.
The molecular mechanisms outlined above may serve as additional indications that current models for quantitative risk estimates should not be expected to give exact risk figures. Furthermore, they underscore that, in the absence of low-dose epidemiological data, current scientific knowledge does not permit solid conclusions about cancer risks at the very low end of the dose–response curve. However, if any of these mechanisms were demonstrated for a given carcinogen, that information might be used to adjust quantitative risk estimates for that agent.
In summary, epigenetic effects may mimic the phenotypic effects of mutations but are expected to have a threshold. Research on stem cells and cancer stem cells may lead to new knowledge that improves the understanding of the dose–response relationship for carcinogens, as may research on autophagy and genomic instability in early cancer development. Only rarely have these new areas in cancer research been investigated from a risk assessment point of view.
3.2. Structuring mechanistic knowledge
One way to get an overview of the earlier and current mechanistic literature is to structure knowledge by taking advantage of the two concepts hallmarks of cancer (Section 3.2.1) and MoA (Section 3.2.2). In this way, recent research can be incorporated in ongoing risk assessment undertakings. The hallmarks concept is intended to facilitate cancer research and is based on phenotypic characteristics of cancer cells. MoAs refer to critical events activated by chemicals that may lead to carcinogenesis or other toxic effects. The MoA concept is thus of direct interest for risk assessors.
3.2.1. Hallmarks of cancer
Current views on general phenotypic alterations exhibited by malignant cells are codified in the well accepted cancer hallmark nomenclature (). Cancer hallmarks characterise the phenotype of fully developed cancer cells (Hanahan and Weinberg Citation2011). The hallmarks have been defined by experimental and human cancer research with the ambition to understand human cancer development, prevention, and treatment. Chemical carcinogens (or radiation) may have been used in the underlying experimental studies; however, the hallmarks are generally regarded relevant for all cancers including those that develop spontaneously or are driven by the “ageing factor”.
Figure 2. The hallmarks of cancer taxonomy. The circle represents the main 10 cancer hallmarks and the boxes indicate a subdivision of hallmarks into cellular processes. Adapted from Baker et al. (Citation2017) and Hanahan and Weinberg (Citation2011).
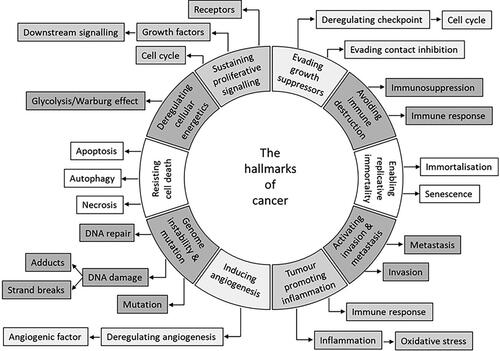
Of note for hallmarks is that mutations and epigenetic changes are “hallmark-enabling effects”. This indicates that both may have similar effects, e.g. on tumour suppressor genes and phenotypic alterations. Furthermore, the hallmarks may develop sequentially, but the timing and order of the appearance of each hallmark may differ with, e.g. tumour type.
Mutations in many different genes may give rise to the same hallmark (e.g. many mutated genes can cause sustained proliferative signalling or evade growth suppression). It is also generally assumed that the number of hallmarks varies with tumour type.
3.2.2. Mode of action
Earlier studies employing initiation-promotion protocols (Section 2.3.1) conveyed the message that chemicals can facilitate cancer development via many MoAs. Additional MoAs have been characterised since then. MoAs were gathered and put in taxonomies with the ambition to summarise well established and generally accepted lines of evidence for carcinogenic actions that can be used for analogous reasoning to support risk assessment of similar carcinogens. The use of MoAs in risk assessment was introduced as a way to circumvent gaps of detailed mechanistic knowledge (mechanism of action) for a single chemical. The concept “adverse outcome pathway” (AOP) is similar to MoA. AOP has been used preferentially in the field of ecotoxicology but has also been used, e.g. to develop an integrated approach to testing and assessment of non-genotoxic carcinogens (Jacobs et al. Citation2020). AOP is rarely, perhaps not at all, mentioned by the regulatory bodies cited in this document.
A MoA taxonomy for carcinogenic effects of chemical exposures summarises so far categorised critical toxicological mechanisms that may lead to or facilitate cancer development (Korhonen et al. Citation2012). In the taxonomy (), the division between genotoxic carcinogens and non-genotoxic/indirect acting genotoxic carcinogens is fundamental. This is of crucial importance for assessing whether a threshold or a non-threshold approach should be applied in dose–response modelling. As also seen in , there are many non-genotoxic/indirect genotoxic MoAs. As indicated above (Section 3.2.1), hallmark characteristics may be introduced by other mechanisms than mutations, and mutations are found in non-cancer tissue. Thus, chemical agents with non-genotoxic/indirect genotoxic MoAs may cause cancer even though they are not initiators, as was shown in studies using initiation-promotion protocols. However, these studies indicate that for most carcinogenic effects of chemicals acting via non-genotoxic/indirect genotoxic MoAs, relatively high and repetitive doses are needed and that a threshold dose must be exceeded for cancer to develop.
Figure 3. The mode of action (MoA) taxonomy for a given chemical carcinogen. Adapted from Kadekar et al. (Citation2012). The terms in the subnodes are intended to be used in literature searches.
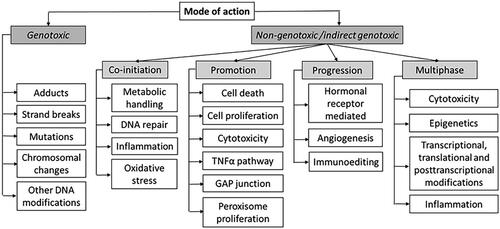
A MoA taxonomy should not be regarded as final as new MoAs can be characterised in the future. For example, a potentially novel MoA has been highlighted in later years. It has been indicated that at least some environmental factors (ethanol, UV) may alter DNA repair mechanisms to error-prone repair in active genes (Supek and Lehner Citation2017). Other mechanisms, triggered by environmental chemicals that affect DNA repair, have also been characterised (Feng et al. Citation2006; Bononi et al. Citation2017) further supporting the meaningfulness of such a MoA.
There are many overlapping aspects with regard to hallmarks and MoAs (compare e.g. Baker et al. (Citation2017) and Korhonen et al. (Citation2012)), and chemicals may thus enable many hallmark characteristics to develop via genotoxic or non-genotoxic/indirect genotoxic MoAs.
3.2.3. Text-mining tools
When a single suspected chemical has been studied comprehensively, an apprehension of carcinogenic properties, MoAs, detailed molecular mechanisms, exposure conditions, and so forth can be obtained by combining data from cancer epidemiology, biomarker studies, studies on polymorphisms, cancer test models and earlier and current basic research. The PubMed database (NIH Citation2020) currently comprises more than 30 million articles. Navigating this literature demands time and training in many scientific subdisciplines. Overviews can be facilitated by employing text analysis software (text-mining tools), which categorise articles found in e.g. PubMed into taxonomies such as those for hallmarks of cancer () or MoAs (), respectively (Korhonen et al. Citation2012; Baker et al. Citation2017). As PubMed is continuously updated, the text-mining tools capture historical as well as recent research trends, such as those exemplified in Section 3.1. The input can be single substances, natural mixtures, exposure scenarios, groups of chemicals and occupations. The output gives indications about, e.g. genotoxicity and other endpoints. More detailed information can also be obtained for risk assessment by comparing the toxicological profile of a studied chemical with a well-known reference compound or for grouping chemicals with similar properties (Ali et al. Citation2016). These automatic tools give an overview of the published literature within minutes and can greatly facilitate manual reading, but do not replace careful assessment of critical studies.
A text analysis of about 57 000 articles in PubMed of relevance for 22 polycyclic aromatic hydrocarbons (PAHs) showed that the results from the tool built on the hallmark taxonomy largely overlapped the results from the tool built on the MoA taxonomy (Ali et al. Citation2021). As the two tools not only utilise two different taxonomies but also separate computer algorithms, the results indicate robustness and that both tools can support risk assessments. An advantage with the hallmark tool might be that it captures more recent trends in cancer research, although the study (Ali et al. Citation2021) did not reflect that.
3.3. Mixed exposure
Interacting effects between carcinogens are difficult to show in epidemiological studies. However, experimental evidence indicates that carcinogens may interact in many ways, and e.g. data obtained by employing the initiation-promotion protocols often suggested synergistic carcinogenic responses by the combined exposure to an initiating chemical and to a promoter (Section 2.3.1).
In an effort to explore the hypothesis about interactions further, and focussing on low-dose exposures to mixtures of environmental chemicals, the actions of selected carcinogens on the hallmarks of cancer were reviewed. Of 85 chemicals, 15% had evidence for a threshold, 59% had low-dose effects (i.e. no support for a threshold), and 26% had no dose–response data. It was suggested “that the cumulative effects of individual (non-carcinogenic) chemicals acting on different pathways, and on a variety of related systems, organs, tissues and cells could plausibly conspire to produce carcinogenic synergies” (Goodson et al. Citation2015). Further studies are needed to confirm this hypothesis and to establish numerical risk estimates.
A recent literature study elaborated on the use of text-mining tools (Section 3.2.3) for PAH containing exposures. The MoA taxonomy on carcinogenic effects () was used. One finding was that diesel engine exhaust, cook oven emissions, and coal tar differed substantially in their toxicological profiles in published data and in MoAs assigned by the tools (Ali et al. Citation2021). This suggests that conclusions about interactions based on one type of mixed PAH exposure might not be valid for other exposures containing PAHs and that text-mining tools might help clarifying this.
4. Quantitative cancer risk assessments and derivation of OELs
The main features of a toxicological quantitative risk assessment of carcinogens are well established and are similar for many bodies, although differences exist relating to the series of steps involved.
The four key steps in a quantitative risk assessment (hazard identification, dose–response assessment, exposure assessment, and risk characterisation) are described in brief below. For a comprehensive review of the risk assessment process of carcinogens, the reader is referred to the following documentations (US EPA Citation2005; WHO/IPCS Citation2009; GR Citation2010, Citation2012b, Citation2021; COC Citation2012, Citation2018, Citation2020; ECHA Citation2012; EFSA Citation2012, Citation2017; AGS Citation2014b; ANSES Citation2014; SCOEL Citation2017a; IARC Citation2019). All steps are not necessarily performed by the same body, sometimes the first two steps are performed by a scientific body (Section 5.2), and the following two steps by a regulatory body (Section 6.3).
By combining epidemiological studies with animal and in vitro data, including mechanistic data, the risk assessor can approach the issues of “sufficient evidence for carcinogenicity” and non-threshold/threshold MoAs, and quantitate and characterise exposure and risk. Addressing these issues means grading the quality of key studies, balancing positive and negative findings, and extrapolating between species and exposure routes and from high to low doses. This requires training and expertise in a number of disciplines and is preferably performed by a group of experts rather than by a single expert.
4.1. Hazard identification
The hazard identification attempts to identify the potential for a substance to act as a human carcinogen. All relevant data are described and analysed. This includes a description and assessment of e.g. human and animal tumour data, study type, biological markers, confounders, bias, causality, and combined statistical evidence across studies. Other key data to be evaluated include physicochemical properties, toxicokinetics, structure–activity relationships, and mechanistic evidence. The predominant MoA(s) is described and its implications for a threshold/non-threshold approach.
4.2. Dose–response assessment
The dose–response assessment comprises different steps depending on whether animal or epidemiological data are selected for defining the PoD (Section 2.5). In case animal data are used (preferably well performed 2-year cancer bioassays), a standardisation of different experimental dosing regimens, toxicokinetic data and modelling, cross-species scaling and route extrapolation are performed. As regards epidemiological data, cohort studies and case-control studies are generally considered most appropriate to determine long-term cancer risks from a specified exposure. Combining statistical evidence across epidemiological studies (pooled studies and meta-analyses) may provide a more reliable outcome.
The observable dose–response range is first assessed to determine a representative measure of the carcinogenic activity that can serve as the starting point for low-dose extrapolation, the PoD. Subsequently, the extrapolation down to exposure levels of relevance for humans (in this case the work force) is performed. As will become apparent below, the approaches practiced vary. This may to a large extent be due to differences in the availability and choice of scientific data.
4.2.1. Dose–response models
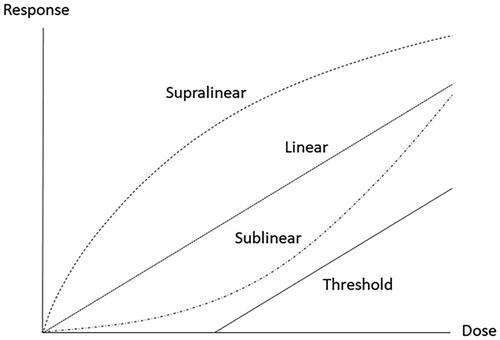
The shape and slope of the dose–response curve are essential to assess potency and predict the proportion of affected individuals at a certain exposure or dose level. Theoretical models describing different shapes of dose–response curves in the low-dose range are shown in , illustrating the concepts of linear, supralinear, sublinear/hockey-stick, and threshold dose–response. These theoretical models are applied to patterns observed in dose–response data from epidemiological or animal studies.
For non-genotoxic carcinogens, it is generally accepted that a threshold concentration exists which theoretically can be established. For most genotoxic carcinogens, the available data are likely inadequate for a threshold to be identified with sufficient confidence. The default assumption for such carcinogens is that there is no threshold for the carcinogenicity (Section 2.5). However, for some genotoxic carcinogens for which sufficient mechanistic information is available, it may be possible to conclude on a MoA-based threshold (ECHA Citation2019).
At high doses, sometimes even at the PoD, the direct genotoxic effect of a non-threshold carcinogen is likely amplified by threshold effects such as inflammation and cell death that cause additional, indirect DNA damage or adversely affect DNA repair (Balkwill et al. Citation2005; Alexandrov et al. Citation2016; Shi et al. Citation2017; Benesch et al. Citation2018; Golemis et al. Citation2018). Thus, the LNT procedure has a tendency to overestimate the risk at low doses in two ways: (1) by assuming no threshold, implying a risk at doses very close to zero and (2) by neglecting that MoAs that contribute to the response seen at the relatively high doses (from which the PoD is sometimes derived) might be ineffective at low doses. For further discussions of these issues, see Hartwig et al. (Citation2020).
The sublinear/hockey-stick model may reflect the response seen after exposure to high doses that overwhelm endogenous, physiological defence system (such as DNA repair). Yet another mechanism might be inflammation and ROS production, kicking in at high doses. The model has been applied to describe, e.g. the expected response to inhaled formaldehyde, a chemical also produced endogenously (Hartwig et al. Citation2020).
A model that describes the observed data are chosen, e.g. by curve fitting or based on mechanistic considerations. Thus, the choice of dose–response model is done also in the absence of empirical low-dose data in the literature. Subsequently, a point that can serve as the starting point for low-dose extrapolation has to be determined, the PoD (Section 4.2.2).
4.2.2. Determination of the point of departure
There are currently two approaches available to determine the PoD, the traditional approach, using the no/lowest observed adverse effect level (NOAEL/LOAEL) and the benchmark dose (BMD) approach. The BMD is the dose/exposure level, estimated by curve-fitting, corresponding to a predetermined change in response called the benchmark response (BMR). The modelling will result in a confidence interval (normally 90%) for the estimated BMD, with the lower and upper confidence limits being designated BMDL and BMDU, respectively. Both the BMD and the BMDL are used as PoD ().
Figure 5. Schematic illustration of dose–response data and BMD modelling with the descriptors BMD, BMDL, and BMDU. Both the BMD and the BMDL are used as PoD. The solid and dotted curves show the best fit to the experimental data (Δ) and the confidence interval, respectively. BMD: benchmark dose BMDL; lower confidence limit of the BMD; BMDU: upper confidence limit of the BMD; PoD: point of departure.
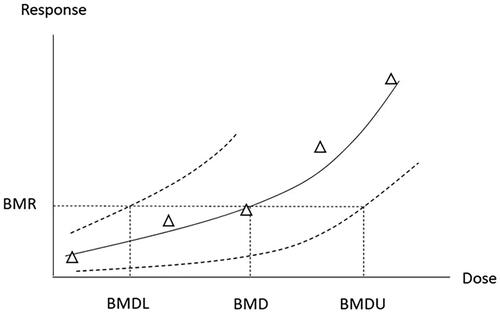
Some obvious advantages of the BMD approach over the classical NOAEL/LOAEL approach are that the BMD is not limited to the experimental doses, is less dependent on dose spacing, and takes into account the shape of the dose–response curve and statistical uncertainties from the quality of the data. Few data points (e.g. small number of animals) and high data variability decrease the statistical power and the likelihood of detecting a significant effect. This increases the likelihood that a given dose level is classified as a NOAEL. Thus, with the classical approach, a poor study with a low power tends to result in a higher NOAEL compared to a study with high power. In contrast, the BMD approach will give a lower BMDL for a low-power study. Disadvantages with the BMD approach are that it is more complex and time-consuming. Also, current OECD guidelines have been developed for the NOAEL approach and are therefore not optimal for BMD modelling, e.g. regarding the number of doses and animals (EFSA Citation2017; FoBiG Citation2019). Despite the obvious advantages, there is as yet no consensus regarding several aspects of the BMD procedure (Davis et al. Citation2011; Haber et al. Citation2018). Nonetheless, BMD modelling is nowadays regarded as state of the art for determining the PoD in risk assessment. Meanwhile, the NOAEL/LOAEL approach is still commonly used in the derivation of OELs. For in-depth information on the BMD approach, see the guidance documents from the European Food Safety Authority (EFSA) (EFSA Citation2017), US EPA (US EPA Citation2012), and the World Health Organization (WHO) (WHO/IPCS Citation2009).
For dichotomous (quantal) data such as cancer incidence data, the BMR of interest is relatively straightforward to define. Thus, when animal dichotomous cancer data are used, a 10% response, BMR10, is used by several bodies as the default PoD, with the corresponding dose descriptor being BMD10 or BMDL10 (ECHA Citation2012; GR Citation2012b; US EPA Citation2012; AGS Citation2014b; EFSA Citation2017; SCOEL Citation2017a). The use of BMR10 as default stems from estimations, see e.g. Sand et al. (Citation2011), showing that the median risk at the NOAEL is approximately 10%. EFSA states that the BMD approach can be used for dose–response assessment also for epidemiological data (to be elaborated in a separate guidance document). It is noted that the observed response in epidemiological studies is often below 10% and lower BMR values may therefore be used (EFSA Citation2017).
The BMR is defined as an increase from the background response predicted by the fitted model (not the observed background response). This also means that BMD modelling may be used even for data lacking a non-exposed control group provided the background response can be estimated (EFSA Citation2017).
BMD modelling is performed with specific software tools that typically include several statistical models for analysis of the toxicological data. The models are mathematical functions with parameters that are estimated by fitting the models to the data. Goodness of fit is evaluated and criteria for the choice of the best model have been developed. Instead of choosing a single “best” statistical model, model averaging is advocated by, e.g. EFSA as the preferred approach (EFSA Citation2017).
If data do not permit a BMD analysis, a single point estimate may be used as PoD. The minimum data requirements are then one incidence level significantly above the controls. One example of a single point estimate is the T25 (the dose or exposure level causing 25% increase in the incidence of a specified tumour type) method. The T25 approach was originally proposed as a practical method for the inclusion of potency considerations in carcinogen classification systems (Dybing et al. Citation1997), and is presently used within the EU for setting specific concentration limits for classification and labelling of mixtures with carcinogenic properties (Sanner et al. Citation2001; ECHA Citation2017b).
4.3. Exposure assessment
After the dose–response assessment follows assessment of exposure, which comprises a qualitative and quantitative assessment of the magnitude, frequency and duration of exposure and, if needed, the resulting internal dose. In addition to external exposure assessment (e.g. by air monitoring), exposure and internal dose may be assessed by biological monitoring.
4.4. Risk characterisation and derivation of OELs
The final step in a quantitative risk assessment is risk characterisation. In this step, the risk of effects among exposed in a particular setting is evaluated (e.g. the working population), based on the results of the previous steps. It should be noted that present quantitative risk assessment models do not account for exposure to multiple carcinogens (Sections 3.3 and 6.2).
A recommended OEL for non-threshold carcinogens may be derived either by low-dose risk calculation or by application of default assessment factors, both starting from the PoD.
4.4.1. Risk calculations
For non-threshold carcinogens, the LNT model is the default. The exposure levels corresponding to selected excess risk levels (e.g. 1 × 10−3 to 1 × 10−6) is estimated departing from the PoD. Guidelines for the calculations of cancer risk values have been published by several bodies, see e.g. the Dutch Expert Committee on Occupational Safety (DECOS) (GR Citation2012b). Examples of risk values obtained from occupational exposure to non-threshold carcinogens are presented in Chapter 7.
4.4.2. Default assessment factors
An alternative, and a way to circumvent the criticism of indiscriminately practicing the LNT method, is to use assessment factors. For example, EFSA uses default assessment factors to calculate margins of exposure (MoE, the ratio between the PoD and the human intake) for genotoxic carcinogens that cannot be eliminated or avoided. The applied factors are 100 for inter- and intra-species differences (may be split in sub-factors if chemical-specific data on toxicokinetics or toxicodynamics are available) and 100 for human variability in DNA repair and cell cycle control, and for taking into account that the BMDL is not a surrogate for a threshold. Assessment factors may be applied both to NOAELs/LOAELs or BMD(L)s, but EFSA explicitly states that it prefers the use of BMDL10 to the NOAEL/LOAEL approach. At BMD(L)10, this overall default assessment factor of 10 000 corresponds to an estimated lifetime risk level of one extra case per million (1 × 10−6). A compound with a calculated MoE of 10 000 or higher would then be of low health risk and therefore considered of low priority for risk management (EFSA Citation2005, Citation2012). This strategy can be seen as a way to acknowledge the many scientific data gaps, and to avoid the objection that numbers may give a false impression of exactness and robustness. Default assessment factors may also be used under the REACH Regulation when setting derived minimal effect levels (DMELs) for carcinogens in the work environment (large assessment factor approach) (ECHA Citation2012). For threshold carcinogens, like for all other substances with threshold effects, the use of assessment factors is the standard procedure.
5. Policies for cancer categorisation and risk assessment
5.1. Categorisation by strength of evidence for carcinogenicity
5.1.1. International Agency for Research on Cancer
IARC published its first monograph in 1972. Peer-reviewed epidemiological and experimental animal studies were scrutinised to evaluate the strength of evidence for carcinogenic activity. To this end, IARC developed criteria for the hazard identification. The criteria involve separate evaluations of human, animal, and (nowadays) mechanistic data and finally, a summarising evaluation that results in a classification of the agent in question. Four categories of carcinogens are used (IARC Citation2019) ().
Table 1. IARC categorisation of carcinogens.
It should be noted that IARC performs hazard identifications, but no quantitative risk assessments. From today’s perspective, it is perhaps hard to understand IARC’s policy, as MoAs, potency at low doses and other issues are critical in contemporary risk assessments (see e.g. The Lancet Oncology Citation2016; Hartwig et al. Citation2020). At earlier times, these issues were of less importance as the general view was that a chemical sufficiently proven to be a carcinogen should be banned or exposure be as low as reasonably achievable (ALARA, see Section 2.4). Nevertheless, IARC maintains its policy and its monographs are regarded as reliable and authoritative sources by scientists, governments, and non-governmental organisations around the world. The strategy developed by IARC has been influential and similar strategies are used by e.g. the EU (Section 5.1.2) and the American Conference of Governmental Industrial Hygienists (ACGIH) (Section 5.1.3). An IARC classification of 1, 2A, or 2B renders a cancer notation in the OEL list in e.g. Denmark (Arbejdstilsynet Citation2007).
However, recent criticism of cancer risk assessment policies has been raised because of seemingly contradictory classifications by different expert groups. For example, the IARC classification of glyphosate was put up against the risk assessment performed by EFSA (The Lancet Oncology Citation2016); IARC classified glyphosate as “probably carcinogenic to humans” (group 2A) (IARC Citation2017), whereas EFSA concluded that glyphosate is unlikely to be carcinogenic to humans (EFSA Citation2015). The two classifications may appear to be contradictory but can be explained by the fact that IARC evaluated the weight of evidence for carcinogenicity (hazard), whereas EFSA evaluated the cancer risk (hazard × exposure).
5.1.2. EU CLP Regulation
The EU Regulation on the classification, labelling and packaging of substances and mixtures (CLP) (European Parliament Citation2008) is based on the United Nations’ Globally Harmonised System of Classification and Labelling of Chemicals (GHS) (UN Citation2019). For substances of particular concern such as carcinogens and mutagens, CLP sets out a system for formal harmonisation of classifications at EU level. Hazard categories for carcinogens are shown in (European Parliament Citation2008).
Table 2. EU hazard categories for carcinogens.
5.1.3. American Conference of Governmental Industrial Hygienists
The ACGIH is a private not-for-profit non-governmental corporation. It uses five categories of carcinogenicity (ACGIH Citation2021) ().
Table 3. ACGIH categorisation of carcinogens.
5.1.4. US National Toxicology Program (NTP)
The US NTP evaluates substances and circumstances for cancer and non-cancer health effects, usually using rodent models. NTP has set the standard for animal bioassays for carcinogen testing (Section 2.2). Alternative test models are also used. Tested substances are classified in Reports on Carcinogens (RoCs). The NTP criteria for carcinogens (NTP Citation2019) are presented in .
Table 4. NTP criteria for listing an agent, substance, mixture, or exposure circumstance in the NTP Report on Carcinogens (NTP Citation2019).
5.1.5. MAK Commission
According to the German Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (known as the MAK Commission), advances in our understanding of the MoAs and the potency of carcinogens have enabled an improved differentiation of carcinogenic substances. Therefore, carcinogens are classified in five categories (summarised in ). In the methodology for the derivation of MAK-values (maximum workplace concentration), it is said that no scientifically justifiable MAK-value can be proposed in the absence of a NOAEL (DFG Citation2019). The benchmark approach is not mentioned. It should be noted that the MAK Commission approach is a hybrid in that categories 1–3 are largely based on strength of evidence, while categories 4–5 are based on MoA considerations.
Table 5. MAK Commission categorisation of carcinogens.
5.2. Categorisation of carcinogens by mode of action, and quantitative risk assessment
Several European expert committees (including the MAK Commission, see Section 5.1.5) use categorisation schemes based on mechanistic or MoA reasoning to separate threshold carcinogens from non-threshold carcinogens in quantitative risk assessments.
5.2.1. EU Scientific Committees
The scientific procedure in the EU is initiated by the European Commission, which decides on priority substances in need of new or revised OELs. These are discussed in the tripartite Working Party on Chemicals with Commission representatives. Subsequently, a scientific report is requested from the EU Scientific OEL Committee. The scientific part was initially performed by the Scientific Expert Group (SEG) and then for many years by its successor, the Scientific Committee on Occupational Exposure Limits (SCOEL) (Section 5.2.1.1). SCOEL or SEG was operative 1990–2018. From 2019, the activities are performed by the Committee for Risk Assessment (RAC) of the European Chemicals Agency (ECHA) (Section 5.2.1.3).
5.2.1.1. Scientific Committee on Occupational Exposure Limits
SCOEL developed a flowchart for distinguishing between strategies to be used in the quantitative risk assessment of individual carcinogens. Carcinogens were grouped in four categories (A–D) according to their MoA and considerations regarding threshold/non-threshold models (Bolt et al. Citation2004; Bolt and Huici-Montagud Citation2008; SCOEL Citation2013a). For chemical agents assigned to group A or B, BMD modelling was the preferred approach to determine the PoD ( and ). When epidemiological data were used to calculate lifetime risk, SCOEL preferred the use of incidence data over mortality data (SCOEL Citation2017a).
Figure 6. Grouping of chemical carcinogens according to SCOEL. Adapted from Bolt et al. (Citation2004), Bolt and Huici-Montagud (Citation2008), and SCOEL (Citation2013a, Citation2017a). MoA: mode of action; SCOEL: Scientific Committee on Occupational Exposure Limits.
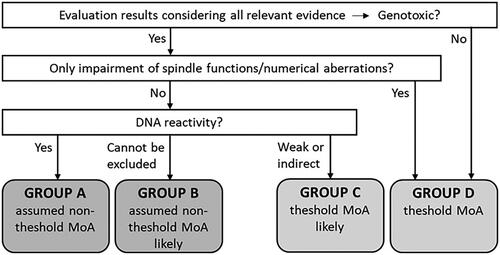
Table 6. SCOEL categorisation of carcinogens.
5.2.1.2. Joint Task Force
In 2015, SCOEL and ECHA were requested by the European Commission to create a Joint Task Force, composed of members from SCOEL and RAC, to address scientific aspects and methodologies related to occupational exposure to chemicals. One of the tasks was to perform a comparative critical assessment of the methodologies used by the two groups in relation to carcinogens. In particular, the concept of a “practical threshold” (as used by SCOEL) was discussed and it was agreed that a “mode of action based threshold” is a more appropriate description. The conclusions by the Joint Task Force (JTF Citation2017a, Citation2017b) constituted the basis for subsequent work prepared by ECHA (Section 5.2.1.3).
5.2.1.3. European Chemicals Agency/Committee for Risk Assessment
From 2019, ECHA has the task to support the Commission with scientific reports for OELs for chemical agents. Each report is subsequently evaluated by RAC who adopts an opinion, recommending OELs when possible.
Based on the Joint Task Force reports (Section 5.2.1.2), ECHA elaborated a guidance for preparing a scientific report for OELs (ECHA Citation2019). The starting point/default in the risk assessment of carcinogens is said to be a non-threshold MoA. A threshold approach can be followed only when subsequent analysis of the data allows refinement in the sense that the data overall actually points to a threshold. Without sufficient data to conclude this, the default stays a non-threshold MoA. High quality epidemiological data with sufficient statistical power should be used for excess cancer risk estimation of non-threshold carcinogens in preference to other data. ECHA prefers the use of incidence data in calculations of lifetime risk. When animal data are used, the T25 or BMD methodology is employed when linear exposure–response is assumed.
For carcinogens for which it might be possible to adapt a threshold approach, the SCOEL methodology and underlying principles for establishing MoA-based thresholds in general were considered appropriate and feasible for use under REACH with some adaptation (ECHA Citation2019).
ECHA stated that it may be useful for understanding the rationale for the OEL recommendation to refer to the SCOEL grouping system for carcinogens (SCOEL Citation2017a), but that the scheme is not a necessary step in the procedure (ECHA Citation2019). The resulting ECHA categorisation of carcinogens is summarised in .
Table 7. ECHA categorisation of carcinogens.
5.2.2. Dutch Expert Committee on Occupational Safety
The DECOS Subcommittee on the Classification of Carcinogenic Substances assesses whether substances to which employees can be exposed at their respective workplaces are carcinogenic. For agents known or presumed to be carcinogenic to man (EU categories 1A and 1B), the subcommittee will when possible indicate the MoA involved and classify the carcinogens in three groups (GR Citation2010, Citation2021) (). The DECOS then proceeds to derive health-based OELs (Hb-OELs) or calculate occupational cancer risk values. The committee prefers the use of epidemiological data over animal data, and the use of incidence data over mortality data in the calculations. Animal data are considered as starting point when no reliable epidemiological data are available. In such cases, the BMD approach is preferred and the PoD by default is BMD10 (GR Citation2012b, Citation2021).
Table 8. DECOS categorisation of carcinogens. Summarised from the Health Council of the Netherlands (GR Citation2010, Citation2012b, Citation2021).
5.2.3. French Agency for Food, Environmental and Occupational Health & Safety (ANSES)
ANSES has been entrusted to organise the national OEL committee. For non-threshold substances, the committee considers that applying an adjustment factor is not suitable for establishing an OEL. Mutagenic, carcinogenic, and genotoxic effects are considered non-threshold effects when there are no established MoAs with a threshold. Sometimes, a non-linear model that better satisfies the statistical criteria for data adjustment quality can be suggested.
For each substance considered to act through a non-threshold mechanism, the committee decides on the most coherent and reliable published model to adopt for quantitative risk assessment. Use of BMD modelling is strongly encouraged in the case of co-existing studies. If data permit, the OEL committee can decide to carry out its own risk assessment when no published risk assessment is considered satisfactory.
Based on the data, concentrations corresponding to three individual lifetime excess risk levels from work-life exposure are presented (1 × 10−4, 1 × 10−5, and 1 × 10−6) (ANSES Citation2014).
6. Regulatory approaches for non-threshold carcinogens and derivation of OELs
The OEL setting process in the EU and many countries involves three-party negotiations between employers, employees, and governments. Historically, numerical cancer risk estimates have not been addressed in these discussions. Included in the implicit acceptance of theoretically high risks is the notion that the work force consists mainly of healthy adults that undergo health examinations and surveillances. The employer has the responsibility to keep exposure and the number of exposed as low as possible, to apply suitable working procedures, measures, and protective equipment, and to inform and train the workers.
6.1. Feasibility approach
Traditionally, OELs for non-threshold carcinogens have been set at levels that are believed to be achievable at the current state of the art. These OELs are not entirely science or risk-based but have an element of socioeconomic and technical feasibility. The residual risk at exposure at the OELs is thus not communicated, and the level of protection may vary from substance to substance.
6.2. Risk-based approach
Quantitative cancer risk assessment strategies and defined accepted risk levels were introduced later for the work environment than for the general population. The methodology for deriving occupational cancer risk values was e.g. issued by the Health Council of the Netherlands in 1995 (GR Citation2012b).
A risk-based approach for OEL setting, based on predefined risk levels, has been introduced by individual EU member states, such as Germany and the Netherlands. For non-threshold carcinogens, the scientific basis for the derivation of an OEL is then an exposure–risk relationship. In the Netherlands, feasibility is taken into account in a separate step (SER Citation2020, Citation2021).
The EU procedure for deriving OELs for non-threshold carcinogens under the Carcinogens and Mutagens Directive (CMD) (European Parliament Citation2004) is at present only partly risk-based, in that the risk calculation performed by the scientific committee is not transformed to an OEL according to any predefined risk level. At present, there is no common nomenclature for predefined risk levels (Section 6.3), and no consensus regarding lifetime excess risk levels as basis for regulatory actions. To address these issues, the EU Parliament and the Council adopted an amendment of the CMD on 9 March 2022. It includes to further explore the possibilities of adopting a risk-based methodology with the aim of setting OELs at an exposure level corresponding to a risk of developing an adverse health effect, such as cancer. This covers the option of establishing the OELs in the range between an upper and a lower risk level. The Commission shall subsequently, and after appropriate consultation of relevant stakeholders, prepare Union guidelines on the methodology establishing risk-based limit values (European Parliament Citation2022).
In a guidance document, ECHA has stated that a lifetime excess cancer risk of 1 × 10−5 could be seen as an indicative tolerable risk level when setting DMELs for workers (ECHA Citation2012).
Another important issue is the largely uncharacterised risk associated with combined exposure to carcinogens (Section 3.3), which at present is not included in risk calculations. In January 2022; however, the EU launched a public consultation on the revision of REACH including to seek the views on the introduction of mixture assessment factors (MAFs) to regulate the risks of exposure to unintended combinations of chemicals (European Commission Citation2020b, Citation2022).
6.3. Some national strategies
6.3.1. Germany
The Committee on Hazardous Substances (Ausschuss für Gefahrstoffe, AGS) evaluates OEL proposals elaborated by other organisations, predominantly the MAK Commission (IFA Citation2020).
Previously, OELs were not specified for carcinogenic substances. Instead so-called technical reference concentrations (TRCs) were applied. The TRC of a carcinogenic substance was defined as the lowest possible concentration that could reasonably be achieved in accordance with the state of the art.
Some of the weaknesses with the TRC concept were that in practice, Hb-OELs and TRCs were perceived to be equally safe, and that the level of residual risk at the TRCs, which varied strongly from substance to substance, was not reflected (lack of transparency). To address these weaknesses, the AGS developed a risk-based concept for risk assessment of exposure to carcinogens, which defines three risk areas (high, medium, and low) with boundaries between the areas being referred to as tolerable and acceptable risks levels, respectively. The acceptable risk level means that 4 per 10 000 (4 × 10−4) persons exposed to a substance throughout their working life (8 h/day for 40 years) will develop cancer. The intention is to lower the acceptable risk level to 4 per 100 000 (4 × 10−5; change foreseen in 2022). This level stems from an accepted excess yearly risk of one per million (1 × 10−6), multiplied by 40 years of occupational exposure. The tolerable risk level is 4 per 1000 (4 × 10−3), which according to the AGS corresponds to the risk of developing lung cancer for a non-smoker unexposed to chemical carcinogens. For non-threshold carcinogens and carcinogens with an unknown MoA, linear extrapolation is the default method. The BMD approach is mentioned as an alternative to the T25 approach (AGS Citation2013b, Citation2014b). The AGS has performed risk calculations for several of the substances with new or revised EU binding OELs (Chapter 7).
The aim of the risk-based concept is to ensure that exposures are below the acceptable risk level. In the high-risk area (above the tolerable risk), there is a direct necessity of additional measures to reduce exposure at least to the medium risk area (between acceptable and tolerable risk) or use of the substance is prohibited. Measures include, e.g. that respiratory equipment must be provided and worn. In the medium risk area, the need for additional measures increases considerably as exposure approaches the tolerable risk level. However, regardless of the exposure level, the employer shall always ensure that minimum quantities of substances relevant to exposure are used (AGS Citation2014b). The risk-based concept has been introduced stepwise and is now referred to in the Hazardous Substances Ordinance (BAuA Citation2010).
6.3.2. Netherlands
Low-dose linear extrapolations are also used in the Netherlands for calculating risks from exposure to non-threshold carcinogens. So-called health-based calculated occupational cancer risk values are derived (Section 5.2.2). Such values are exposure levels corresponding to extra cancer risk levels from 40 years of occupational exposure that are predefined by the government (Minster of Social Affairs and Employment). Two risk levels have been defined, a prohibitive risk level of 4 × 10−3 and a target risk level of 4 × 10−5, respectively (GR Citation2012b), i.e. identical to the tolerable and acceptable risk levels promoted by the AGS (Section 6.3.1).
The legally binding OEL will preferably correspond to the target risk level. A temporarily higher OEL may be set due to problems of technical and economic feasibility, as considered by the tripartite OEL Subcommittee of the Social and Economic Council (SER). In practice, the established OELs vary between the exposure levels corresponding to the target risk level and the prohibitive risk level (GR Citation2012b; RIVM Citation2014).
If a substance is classified as a carcinogen (EU category 1), but lacks a legally binding OEL, the employer must determine the lowest possible OEL (implying that companies can set different OELs for the same substance). When exceeding this self-derived OEL, preventive measures should immediately be taken (SZM Citation1997).
6.3.3. France
The French OEL committee performs low-dose linear extrapolations for non-threshold carcinogens and performs risk calculations corresponding to risk levels of 1 × 10−4, 1 × 10−5, and 1 × 10−6 (Section 5.2.3). For the actual OEL setting, no defined risk levels have been identified. Based on the report from the OEL committee, the most protective OEL for which an analytical method is available (or can be adapted within 6 months) is selected. Socioeconomic feasibility and the need for a transitional period is assessed. OELs are established in agreement with the social partners. If industry is incapable of achieving the OEL, a higher value can be chosen (case-by-case decision). The legislation on the protection from the risks related to chemical agents including OEL setting is currently under revision (personal communication, N. Bessot, Ministère du Travail, de l’Emploi et de l’Insertion (Ministère du Travail Citation2008)).
6.3.4. United Kingdom
The Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment (COC) is an advisory committee whose remit is to advise on all aspects of the carcinogenicity of chemicals, at the request of government departments and agencies and the devolved administrations. If a putative carcinogen is found to be potentially genotoxic, the COC recommends a non‐threshold approach to risk assessment and that the approach of ALARP (as low as reasonably practicable) should always be adopted by risk managers, where possible, for exposure recommendations. In addition, the MoE approach could be used for risk characterisation, to aid risk communication and prioritise risk management when there are adequate carcinogenicity and exposure data. This could be supplemented in specific situations by the setting of a minimal risk level based on expert judgement.
Thus, the COC does not recommend the use of linear extrapolation for non-threshold carcinogens because the resultant cancer risk estimate has a degree of precision which does not reflect the uncertainties about the shape of the dose–response curve orders of magnitude below the doses administered in animal studies. In the MoE approach, a PoD (usually the BMDL10) is generated by modelling the dose–response data from an animal carcinogenicity study. The MoE (the ratio between the PoD and the estimated human exposure) is then calculated. A judgement can be made on the basis of the magnitude of the MoE. When other PoDs are used, for example, if based on human data, the MoE should be considered on a case-by-case basis (COC Citation2012, Citation2018) ().
Table 9. Banding of MoE values based on BMDL10 (PoD) from an animal cancer study to aid risk communication (COC Citation2018).
6.3.5. United States
The US National Institute for Occupational Safety and Health (NIOSH) has the mandate to recommend standards to the Occupational Safety and Health Administration (OSHA) who sets the legally enforceable regulatory limits, so-called permissible exposure levels (PELs).
For the past 20 plus years, NIOSH has subscribed to a carcinogen policy, which called for “no detectable exposure levels for proven carcinogenic substances”. Due to advances in science and in approaches for risk assessment and risk management, NIOSH published a revised policy in 2017, which states that for exposures that cannot be eliminated, NIOSH will calculate exposure levels corresponding to from 1 × 10−2 to 1 × 10−6 excess lifetime cancer deaths from a 45-year working life. The term recommended exposure limit (REL) is replaced by risk management limit for a carcinogen (RML-CA) (8-h time-weighted average, TWA) to acknowledge that there is no safe exposure level for most carcinogens. RML-CAs are set at the estimated 95% lower confidence limit of the exposure level corresponding to 1 × 10−4 excess lifetime risk, when analytically feasible. For exposures resulting in excess risks above this level, NIOSH will recommend additional actions to be taken. Potential thresholds can be adequately modelled by sublinear, but non-threshold, mathematical models (NIOSH Citation2017).
7. Non-threshold carcinogens with EU binding OELs 2017–2019
This chapter intends to illustrate some of the problems and considerations encountered when setting OELs for non-threshold carcinogens. For that purpose, the binding OELs issued by the EU in 2017–2019 were analysed (European Parliament Citation2017, Citation2019a, Citation2019b). For each carcinogen, the scientific basis for an OEL is summarised, followed by a brief description of the regulatory rationale for the binding OEL. Cancer risk assessments including numerical risk estimates are mainly taken from SCOEL and RAC, as these form the scientific basis for the EU OELs in question. For illustration and to give a more diverse picture of the scientific data and procedures, they are supplemented by assessments by other European Scientific Committees.
7.1. The EU procedure for setting OELs for carcinogens
The OEL setting process in the EU is initiated by a prioritisation of chemicals decided by the Commission based on engagement by member states and social partners. Thereafter, the scientific committee (in this case SCOEL or RAC) evaluates the prioritised agents. Before adoption of the scientific recommendation, it is subject to external public consultation. For non-threshold carcinogens, the EU procedure is in part risk-based, as the risk calculation performed by the scientific committee is not transformed to an OEL according to a predefined risk level. However, work has been initiated in the EU to introduce a fully risk-based methodology for the OEL setting of carcinogens (Section 6.2).
In the next step, three-party negotiations between employers, employees and governments (Chapter 6) take place. The tripartite body involved is the Advisory Committee on Safety and Health at Work (ACSH). The ACSH discusses the scientific recommendation and adopts an opinion for the OEL that is considered to be achievable by employers and still ensures adequate protection of workers’ health. For the carcinogenic substances of concern here, the Commission contracted specific studies (IOM Citation2011; RPA Citation2019a, Citation2019b) to evaluate the cost–benefit of introducing or revising an OEL, evaluations that were fed into the discussions of the ACSH. For the carcinogens with binding OELs issued in 2017–2019, the ACSH adopted opinions in 2012–2017 (ACSH Citation2012, Citation2013, Citation2016; European Commission Citation2016; ACSH Citation2017a, Citation2017b).
Before presenting its proposal, the Commission conducts an impact assessment, in which the rationale behind the proposed binding OEL is provided. In this step, up to three potential OEL values were compared with the baseline option for each chemical agent (no action taken, i.e. no OEL introduced or no change). Only cancer-related health impacts were considered (European Commission Citation2016, Citation2017, Citation2018, Citation2020a). The potential OELs evaluated were either based on existing, typical OELs among the EU member states or were suggested by the Commission (IOM Citation2011; RPA Citation2019a, Citation2019b). In all but two cases (chromium (VI) compounds and diesel engine exhaust), the impact assessment confirmed the level supported by the ACSH. For binding OELs, the legislative proposals from the Commission are finally adopted by the European Parliament and the Council. For chromium (VI) and diesel engine exhaust, the adopted binding OELs deviated from the Commission proposals.
7.2. Acrylamide
7.2.1. Scientific basis
IARC has classified acrylamide as a group 2A carcinogen in 1994 (IARC Citation2020). In 2012, SCOEL categorised acrylamide as a genotoxic carcinogen, for which the existence of a threshold cannot be sufficiently supported (group B). A reasonable quantitative cancer risk assessment for humans was not considered feasible because human cancer studies did not provide reliable figures as a basis for a risk quantitation, and the cancers observed in rats (testicular mesotheliomas, mammary tumours, glial cell tumours, thyroid tumours, and adrenal pheochromocytomas) are significantly influenced by species-specific factors. Dermal absorption was regarded important. A biological guidance value (BGV) was therefore recommended for haemoglobin adducts, 80 pmol/g globin (non-smokers), based on the 95% percentiles of European non-smoking populations. A skin notation was also recommended. SCOEL further stressed the importance of any regulation to protect against neurotoxicity, given the wealth of evidence for acrylamide-induced neurotoxicity in workers. A NOAEC for neurotoxicity of 0.1 mg/m3 was identified (SCOEL Citation2012b).
In contrast, the cancer risk assessment performed by the AGS used data on benign and malignant mammary tumours in female rats (drinking water study) for risk calculations. A BMD analysis of the rat data combined with LNT extrapolation, showed that excess lifetime cancer incidence risks of 4 × 10−5 and 4 × 10−3 correspond to 40 years of occupational exposure to 0.007 and 0.7 mg/m3, respectively (AGS Citation2012b).
In 2006, DECOS also departed from data from rats exposed to acrylamide via drinking water, and based its calculations on the incidence of mesothelioma of the tunica vaginalis (single point estimate model). The committee estimated that 40 years of occupational exposure to 1.6 and 160 µg/m3 confer excess lifetime cancer risks of 4 × 10−5 and 4 × 10−3, respectively (GR Citation2006). In a later advisory letter 2014, it is stated that the committee sees no reason to revise its previous report (GR Citation2014a).
7.2.2. Regulatory rationale for the OEL
In the EU impact assessment, OELs of 0.03 and 0.1 mg/m3 were evaluated (0.03 mg/m3 as typical current OEL and 0.1 mg/m3 being within the range of 0.07–0.1 mg/m3 agreed by the ACSH). Current exposure levels in the EU were estimated to be below 0.03 mg/m3. The proposed EU binding OEL was 0.1 mg/m3. Among EU countries, 13 had no or higher OELs. In line with the SCOEL recommendation, a skin notation was further proposed (ACSH Citation2012; European Commission Citation2016).
7.3. Arsenic and its inorganic compounds
7.3.1. Scientific basis
In 2012, IARC classified arsenic and inorganic arsenic compounds as group 1 carcinogens (IARC Citation2020). RAC concluded that the cancer MoA of arsenic and its inorganic compounds has not been established, but appears not to be related to direct DNA-reactive genotoxicity and that therefore possibly the arsenic carcinogenicity has a threshold. However, the available data did not allow the identification of threshold exposure levels for key events in the MoAs proposed in the scientific literature. Dose–response relationships were therefore derived by linear extrapolation. As the mechanistic evidence is suggestive of non-linearity, it was acknowledged that the excess risks in the low exposure range might be an overestimate (ECHA Citation2017d). RAC referred to calculations performed by DECOS (GR Citation2012a), based on epidemiological data from a copper smelter plant. DECOS had estimated the excess lifetime (40 years working life exposure) lung cancer mortality risk to be 1.4 × 10−4 per μg As/m3 (for the inhalable fraction), which recalculated means that a risk of 4 × 10−3 corresponds to an exposure level of 28 μg As/m3 (ECHA Citation2017d). A risk calculation performed by the AGS resulted in a risk of 4 × 10−3 from exposure to 8.3 μg As/m3 (AGS Citation2015c).
7.3.2. Regulatory rationale for the OEL
For the EU OEL setting, three OELs were evaluated, 10 μg/m3 (as put forward by the ACSH based on the RAC document), 25 μg/m3 and 50 μg/m3 (close to the average of member states’ OELs). In the impact assessment, the EU Commission concluded that the benefits outweighed the costs for all three evaluated OELs, and the most stringent value of 10 μg/m3 was therefore the preferred option (16 member states would have to introduce or update their OELs) (ACSH Citation2017b; European Commission Citation2018; RPA Citation2019b).
7.4. 1,3-Butadiene
7.4.1. Scientific basis
In 2012, IARC classified 1,3-butadiene as a group 1 carcinogen (IARC Citation2020). In its recommendation from 2007, SCOEL concluded that 1,3-butadiene should be treated as a possible human carcinogen, acting via a genotoxic mechanism (group A). The excess risk entailed in exposure during a working life to various concentrations of 1,3-butadiene was calculated using various models, based on epidemiological data on leukaemia. The results were illustrated as follows: in a population of 1000 adult males, occupational exposure to 1 ppm would cause from 0.0 to 10.78 extra leukaemia deaths in the ages 25–85 years (SCOEL Citation2007). Using the central estimate (10.78/2), an excess risk of 4 × 10−3 leukaemia deaths corresponds to an exposure level of 0.74 ppm.
The AGS performed linear extrapolation of epidemiological leukaemia mortality data with access to additional studies. The calculations showed excess lifetime risks of 4 × 10−5 and 4 × 10−3 following 35–40 years of occupational exposure to 0.02 and 2 ppm, respectively (AGS Citation2010a).
The DECOS concluded that 1,3-butadiene induces cancer by a stochastic genotoxic mechanism. Hence, the committee derived risk values, based on epidemiological data on leukaemia. Air concentrations of 0.1 mg/m3 (0.05 ppm) and 10 mg/m3 (5 ppm) were estimated to correspond to excess lifetime risks of cancer mortality of 4 × 10−5 and 4 × 10−3, respectively, from 40 years of occupational exposure (GR Citation2013).
7.4.2. Regulatory rationale for the OEL
In the EU impact assessment, three OELs were evaluated (0.5, 1, and 5 ppm, not clear how the options were chosen). Current exposure levels in the EU were estimated to be well below 2 ppm. A binding OEL of 1 ppm was proposed and was considered feasible while concerns were raised about potential impacts of an OEL below that level. National OELs would have to be introduced or revised in 23 member states (European Commission Citation2016).
7.5. Chromium (VI) compounds
7.5.1. Scientific basis
IARC classified in 2012 chromium (VI) compounds as group 1 carcinogens (IARC Citation2020). In 2004, SCOEL considered chromium (VI) compounds to be genotoxic carcinogens and referred to risk calculations where it was estimated that the excess lifetime risks of lung cancer were 0.1–0.6 and 0.5–3 per 1000 workers, respectively, after exposure to 1 and 5 µg/m3. SCOEL concluded that an exposure limit of 50 µg/m3 may well provide adequate protection for workers exposed to poorly soluble chromium (VI) compounds but consideration could be given to setting exposure limits at 25 or 10 µg/m3 for other chromium (VI) compounds (SCOEL Citation2004a).
In a re-evaluation in 2017, SCOEL categorised chromium (VI) compounds as non-threshold carcinogens (group A) and presented point estimates of four extra lung cancer cases/1000 at an exposure level of 1 µg/m3 and 20 extra lung cancer cases/1000 at 5 µg/m3 from 40 years of exposure, based on epidemiological data (SCOEL Citation2017d). Linear extrapolation of these figures by NEG suggests 4 × 10−5 extra lung cancer cases at an exposure level of 0.01 µg/m3.
Similar risk estimates had previously been derived by DECOS (GR Citation2016), the AGS (AGS Citation2014a), and RAC (ECHA Citation2013), although different assumptions and approaches were used.
7.5.2. Regulatory rationale for the OEL
In the case of chromium (VI), the Commission procedure was performed before the re-evaluation by SCOEL (SCOEL Citation2017d); in the EU impact assessment, OELs of 25 and 50 µg/m3 were suggested and evaluated by the Commission. The more stringent option was preferred as being more effective in reducing exposure and the number of deaths while being slightly more costly than the less stringent option. It is noted that the workers group in the ACSH had argued that even an OEL of 25 µg/m3 would correspond to a high cancer risk. In the amended CMD issued by the Parliament and the Council, the OEL for chromium (VI) compounds was set to 5 µg/m3 with a transitional period with 10 µg/m3 as the limit except for e.g. welding and plasma cutting where 25 µg/m3 should apply during the transitional period (ACSH Citation2012; European Commission Citation2016; European Parliament Citation2017).
7.6. 1,2-Dibromoethane (ethylene dibromide)
7.6.1. Scientific basis
In 1999, IARC classified 1,2-dibromoethane in group 2A (IARC Citation2020). In 2011, SCOEL categorised the substance as a genotoxic carcinogen without a threshold (group A). The quantitative data on carcinogenicity and the present state of toxicokinetic interspecies modelling did not permit a reasonable and reliable quantitative cancer risk assessment for humans. SCOEL noted that about 80 mg/m3 is carcinogenic in animals, assigned a skin notation and recommended that any exposure to this compound should be avoided (SCOEL Citation2011a).
In 2017, DECOS assumed a linear dose–response relationship and performed a risk calculation from a point estimate of incidence of nasal tumours in rats exposed by inhalation. Subsequently, the cancer risk from occupational exposure was calculated. The committee estimated that the air concentrations corresponding to excess lifetime cancer risks of 4 × 10−5 and 4 × 10−3 equal 0.002 and 0.2 mg/m3, respectively (GR Citation2017a).
7.6.2. Regulatory rationale for the OEL
For the setting of an EU OEL, the only value evaluated compared to the baseline option was 0.8 mg/m3 (within the range of national OELs in the EU, but 20 member states would have to introduce or update their OELs). There were no concerns about technical feasibility or overall costs, and 0.8 mg/m3 with a skin notation was proposed by the Commission as binding OEL (European Commission Citation2017).
7.7. 1,2-Dichloroethane (ethylene dichloride)
7.7.1. Scientific basis
In 1999, 1,2-dichloroethane was classified by IARC as a group 2B carcinogen (IARC Citation2020). SCOEL concluded that 1,2-dichloroethane is a genotoxic carcinogen with a non-threshold dose–response (group A). Employing the BMD approach on the combination of adenoma and fibroadenoma in mammary glands of inhalatory exposed female rats, SCOEL estimated that excess lifetime cancer risks of 1 × 10−5 and 1 × 10−3 equal exposure to 0.00386 and 0.386 ppm, respectively (SCOEL Citation2016). Recalculated this means that an excess lifetime cancer risk level of 4 × 10−5 corresponds to an exposure level of 0.015 ppm.
Using the same data and approach, the AGS estimated that the excess cancer risks were 4 × 10−5 at 0.08 mg/m3 (0.02 ppm) and 4 × 10−3 at 8 mg/m3 (2 ppm) (AGS Citation2015a). DECOS used the same study as SCOEL and the AGS, but based their risk calculations on the increase of adenocarcinomas in the mammary glands of female mice. By using BMD modelling, it was estimated that the air concentrations corresponding to excess lifetime cancer mortalities of 4 × 10−5 and 4 × 10−3 from 40 years of occupational exposure equal 0.126 and 12.6 mg/m3 (0.0315 and 3.15 ppm), respectively (GR Citation2019a).
7.7.2. Regulatory rationale for the OEL
In the EU impact assessment, three exposure levels were analysed, namely 1, 2, and 5 ppm; 1 and 5 ppm being typical national OELs and 2 ppm as proposed by the ACSH. The most stringent option (1 ppm) was considered (possibly) not technically feasible, and the mid-option (2 ppm) was proposed by the Commission as binding OEL with a skin notation (23 member states would have to introduce or update their OELs) (European Commission Citation2017).
7.8. Diesel engine exhaust
7.8.1. Scientific basis
IARC classified diesel engine exhaust as carcinogenic to humans (group 1) in 2014 (IARC Citation2020). In 2016, NEG and DECOS published a joint criteria document on diesel engine exhaust (Taxell and Santonen Citation2016) that was the lead document for assessments performed by other bodies. Shortly thereafter, SCOEL concluded in 2017 that although animal data support a threshold possibly at or below 0.02 mg/m3 of diesel exhaust particles (DEP), corresponding to 0.015 mg/m3 of elemental carbon (EC, exposure indicator), epidemiological data suggest significant cancer risks already at and below these exposure levels. The observation that chronic inflammation leading to secondary genotoxicity together with increased cell proliferation seemed to be predominant in rats would give a group C categorisation (practical threshold). However, category B (threshold cannot be supported) would also apply since a genotoxic activity could not be fully excluded and epidemiological studies showed a gradually increasing exposure–response relation starting at exposure levels close to background levels and were not indicative of a clear exposure threshold. SCOEL concluded that an OEL (for traditional diesel engine exhaust) that would be adequately protective for workers could not be established (SCOEL Citation2017b) and referred to a meta-analysis with estimated numbers of excess lung cancer deaths through 80 years of age for 45 years of occupational exposures, i.e. 17, 200, and 689 per 10 000 for 1, 10, and 25 μg EC/m3, respectively (Vermeulen et al. Citation2014).
Both DECOS and the Danish National Research Centre for the Working Environment (NRCWE) estimated cancer risk values based on the same meta-analysis (Vermeulen et al. Citation2014) with similar results. DECOS concluded that excess lung cancer risks of 4 × 10−5 and 4 × 10−3 from 40 years of occupational exposure equal 0.011 and 1.03 µg respirable EC/m3, respectively (GR Citation2019b). NRCWE (Citation2018) calculated the expected excess lung cancer risk to be 1 × 10−5 at 0.0045 μg/m3, and 1 × 10−3 at 0.45 μg/m3 of DEP (NRCWE Citation2018). NRCWE also calculated excess cancer risk values based on two 2-year inhalation studies in rats, which resulted in higher corresponding air concentrations than did the epidemiological data. NRCWE recommended using the human data to derive OELs (Saber et al. Citation2019). This example illustrates that animal data do not always result in higher cancer risk estimates than human data.
Lower risk estimates were derived in a pooled analysis from 2020 comprising 14 case-control studies. The lung cancer excess lifetime risks at age 80 associated with 45 years of occupational exposure to 1, 20, and 50 μg EC/m3 were 4, 99, and 300 per 10 000, respectively (Ge C et al. Citation2020).
7.8.2. Regulatory rationale for the OEL
In the contractors’ cost–benefit analysis, an OEL of 100 μg EC/m3 was evaluated, and was referred to as a typical OEL (IOM Citation2011). The opinion from the ACSH was not consensual. Any action by the Commission was withheld pending a legally clear definition of the agent. The Parliament and the Council then pre-empted the preparatory impact work of the Commission (European Commission Citation2020a) and set a binding OEL of 50 μg EC/m3 (European Parliament Citation2019a).
7.9. Epichlorohydrin
7.9.1. Scientific basis
In 1999, IARC classified epichlorohydrin as a group 2A carcinogen (IARC Citation2020). In consequence of a clear-cut direct genotoxicity, epichlorohydrin was categorised by SCOEL as a non-threshold carcinogen (group A). SCOEL strongly recommended that occupational exposure to epichlorohydrin should be avoided. It was further stated that an assessment of human cancer risks is associated with great uncertainties, and no quantitative risk assessment was therefore performed. A skin notation was recommended (SCOEL Citation2011b).
DECOS, however, calculated excess cancer risk values based on a rat inhalation study (incidence of squamous cell carcinoma in the nasal cavity and nasal and bronchial papilloma). By applying linear extrapolation, the committee estimated the excess lifetime cancer risks to be 4 × 10−5 and 4 × 10−3 from 40 years of occupational exposure to 0.19 mg/m3 and 19 mg/m3, respectively (GR Citation2000b).
Using the same study, the AGS estimated by linear extrapolation that the excess lifetime cancer risks were 4 × 10−5 and 4 × 10−3 at 0.23 and 23 mg/m3, respectively (AGS Citation2012a).
7.9.2. Regulatory rationale for the OEL
In the EU impact assessment, the only potential OEL evaluated was 1.9 mg/m3, which was considered typical of OELs in place in the member states (15 member states would still need to introduce or update their OELs). A binding OEL of 1.9 mg/m3 and a skin notation was proposed (European Commission Citation2017).
7.10. Ethylene oxide
7.10.1. Scientific basis
In 2012, IARC classified ethylene oxide as a group 1 carcinogen (IARC Citation2020). According to SCOEL the carcinogenicity of ethylene oxide is reasonably connected with its DNA alkylating capacity and resulting genotoxic properties. Although a non-linear dose–response (genotoxicity) relationship could reasonably be assumed based on arguments of MoA, a definite no-effect level based on dose–response data could not be defined. Thus, SCOEL provisionally categorised ethylene oxide into group B as a genotoxic carcinogen, for which a threshold is not sufficiently supported. SCOEL stated that the cancer risk assessment should preferably be based on epidemiological data on haematological malignancies (SCOEL Citation2012c), and referred to a large study by Valdez-Flores et al. In that study, occupational exposure at 0.286 ppm and 21.35 ppm ethylene oxide was estimated to result in an excess risk of lymphoid tumour mortality of 4 × 10−5 and 4 × 10−3, respectively (Valdez-Flores et al. Citation2011). A skin notation was recommended. SCOEL also stated that no genotoxic changes could so far be directly established in exposed humans at 1 ppm (SCOEL Citation2012c).
In contrast, the AGS based its risk assessment on animal data and took the BMD10 for lung tumours in mice as PoD. Derived human excess lifetime cancer risk values were 4 × 10−5 at 23.6 µg/m3 (11.8 ppb) and 4 × 10−3 at 2.36 mg/m3 (1.18 ppm) (AGS Citation2011).
In an advisory letter from 2014, DECOS recommended to use the cancer risk values derived by the AGS and noted that these values probably overestimate the cancer risk for humans (GR Citation2014b).
7.10.2. Regulatory rationale for the OEL
In the EU impact assessment, only 1 ppm was evaluated (typical national OEL). Current exposure levels in the EU were estimated to be below this value. The Commission proposed 1 ppm as the binding OEL (nine member states had no or a higher OEL). In line with the SCOEL Recommendation, a skin notation was introduced (SCOEL Citation2012c; European Commission Citation2016).
7.11. Hydrazine
7.11.1. Scientific basis
In 2018, IARC classified hydrazine as a group 2A carcinogen (IARC Citation2020). In 2010, SCOEL categorised hydrazine as a genotoxic carcinogen for which a threshold cannot be sufficiently supported (group B), but derivation of a reasonable quantitative risk assessment was not considered possible. A skin notation was recommended (SCOEL Citation2010a). In a re-evaluation in 2017, SCOEL maintained the group B categorisation and the skin notation. Based on the incidence of malignant thyroid tumours in rats (1-year inhalation exposure) and BMD modelling, SCOEL suggested an excess lifetime tumour risk after work-life exposure of 1 × 10−3 at 76 μg/m3 (SCOEL Citation2017e), i.e. a risk of 4 × 10−3 at 300 µg/m3. The AGS applied linear risk assessment to derive risk figures, based on the T25 for rat nasal tumour incidence data after inhalation exposure. The estimated excess lifetime cancer risks were 4 × 10−5 at 0.22 µg/m3 and 4 × 10−3 at 22 µg/m3 (AGS Citation2015d).
7.11.2. Regulatory rationale for the OEL
The impact assessment by the Commission referred to SCOEL (Citation2010a) who found the data insufficient to perform a risk calculation. Two options for an OEL were evaluated (0.013 and 0.13 mg/m3, typical national OELs). The proposed binding OEL of 0.013 mg/m3 was accompanied by a skin notation. There were few concerns regarding technical feasibility and compliance costs were small. At the time, 24 member states had no or a less stringent OEL and 75% of the workers were considered to be exposed above the proposed value (SCOEL Citation2010a; European Commission Citation2016).
7.12. 4,4′-Methylenedianiline (4,4′-diaminodiphenylmethane)
7.12.1. Scientific basis
Already in 1987, IARC classified 4,4′-methylenedianiline (MDA) as a group 2B carcinogen (IARC Citation2020). SCOEL categorised MDA as a non-threshold genotoxic carcinogen (group A) because of the experimentally proven carcinogenicity and genotoxicity. Accordingly, the derivation of an Hb-OEL was not possible, but a skin notation and a BGV was recommended based on proven skin permeability. No quantitative risk assessment was performed, but SCOEL referred to risk calculations performed by others, e.g. the AGS (SCOEL Citation2012a). The AGS applied linear risk assessment based on the T25 (incidence of rat liver tumours, oral dosing) to derive risk figures, as BMD modelling had failed. The excess cancer risks were 4 × 10−5 at 7.3 µg/m3 and 4 × 10−3 at 731 µg/m3 (AGS Citation2010b). Likewise, DECOS concluded that the animal data did not enable a reliable derivation of dose–response relationships and BMD modelling. By making a representative point estimate of the rat liver tumour incidence, it was estimated that the excess lifetime cancer risks were 4 × 10−5 and 4 × 10−3 at 16 and 1600 µg/m3, respectively (GR Citation2015).
7.12.2. Regulatory rationale for the OEL
In the EU impact assessment, two exposure levels were evaluated (0.08 and 0.8 mg/m3; both typical existing national OELs). Current exposure levels were estimated as at most 0.14 mg/m3 in manufacture and 0.07 mg/m3 in other industrial sectors. No significant compliance costs for companies were expected. A binding OEL of 0.08 mg/m3 was considered appropriate with a skin notation, meaning that 23 countries would have to introduce or update their OELs (European Commission Citation2017).
7.13. 4,4′-Methylenebis(2-chloroaniline)
7.13.1. Scientific basis
In 2012, IARC classified 4,4′-methylenebis(2-chloroaniline) (MOCA) as carcinogenic to humans (group 1) (IARC Citation2020). In 2010, SCOEL categorised MOCA as a group A carcinogen, i.e. a genotoxic carcinogen for which a threshold cannot be assigned. No quantitative risk assessment was performed. Skin uptake was considered the most significant route of exposure. A skin notation was therefore recommended and the relevance of biological monitoring was emphasised. Since MOCA was considered a non-threshold carcinogen, no health-based biological limit value (BLV) could be recommended. Instead, a BGV was put forward in 2013, corresponding to the detection limit of the analytical method (as background levels could not be detected). It was said that urinary levels of total MOCA below 5 μmol/mol creatinine could be reached in occupationally exposed populations, using good working practices at the workplace. Referring to calculations performed by DECOS (GR Citation2000a), this was said to correspond to an excess lifetime cancer risk of 3–4 × 10−6 (SCOEL Citation2013b). RAC made similar conclusions as SCOEL and recommended a skin notation and a BGV at the detection limit of the biomonitoring method. The committee, however, did calculate a unit risk for workers’ inhalation exposure as 9.65 × 10−6 per µg/m3 based on the T25 (lung cancer incidence data in rats following oral dosing) (ECHA Citation2017c). This corresponds to an excess lifetime risk of 4 × 10−3 from work-life exposure to 0.4 mg/m3.
7.13.2. Regulatory rationale for the OEL
In the EU impact assessment, binding OELs for MOCA of 5, 10, and 20 µg/m3 were evaluated (5 and 20 µg/m3 represented the lowest and median national OELs in the EU). Current exposure levels were typically below 5 µg/m3. All assessed OELs would bring similar health effects and costs as baseline (no OEL). The Commission proposed 10 µg/m3 (with a skin notation) as the option easiest to apply and enforce, as agreed in the ACSH (16 member states lacked OEL) (ACSH Citation2017a; European Commission Citation2018; RPA Citation2019a).
7.14. 2-Nitropropane
7.14.1. Scientific basis
In 1999, IARC classified 2-nitropropane as possibly carcinogenic to humans (group 2B) (IARC Citation2020). SCOEL categorised 2-nitropropane as a genotoxic carcinogen (group A) acting via a non-threshold MoA. A cancer risk assessment was performed based on a chronic rat inhalation study with a single dose. The T25 approach (incidence of hepatocellular nodules) with linear extrapolation resulted in a human excess lifetime cancer incidence risk of 1 × 10−3 at 0.644 mg/m3 (SCOEL Citation2017c).
SCOEL also referred to cancer risk assessments previously performed by DECOS (GR Citation1999a) and the AGS (Citation2015b) giving comparable risk numbers. Both organisations used the same critical study but slightly different approaches (SCOEL Citation2017c).
7.14.2. Regulatory rationale for the OEL
There was no SCOEL recommendation for 2-nitropropane when the Commission performed its impact assessment. The only OEL evaluated was 18 mg/m3, which was considered a typical value for existing OELs in the EU (14 member states had no limit or one that was less protective). It was considered likely that no worker in the EU was exposed above the proposed OEL of 18 mg/m3 (European Commission Citation2016).
7.15. Propylene oxide (1,2-epoxypropane)
7.15.1. Scientific basis
In 1994, IARC classified propylene oxide as a group 2B carcinogen (IARC Citation2020). SCOEL categorised, in 2010, propylene oxide in group C (genotoxic carcinogen for which a practical threshold is supported) with the primary aspect being the local carcinogenicity of the nasal tissue. Investigations into the MoA of rodent nasal carcinogenesis due to propylene oxide inhalation pointed to decisive contributions of several factors (including glutathione depletion) besides genotoxicity. SCOEL argued that only minimal local glutathione depletion in the nasal tissue of the rats occurs at 5 ppm. Based on the argument that the nasal epithelium of rodents is more susceptible to irritation and irritation-based carcinogenicity than that of humans and that 2 ppm was a NOAEL for sister chromatid exchange in humans, SCOEL suggested an OEL at 1 ppm (2.4 mg/m3) (SCOEL Citation2010b).
The AGS concluded that a threshold for the carcinogenic effect from propylene oxide could not be identified, but acknowledged that the dose–response relationship was sublinear. A risk level of 4 × 10−5 was estimated to equal a concentration of 4.8 mg/m3 (2 ppm), based on slight effects on the rat nasal mucosa and BMD modelling (AGS Citation2013a).
7.15.2. Regulatory rationale for the OEL
Propylene oxide was regarded by SCOEL to be a threshold carcinogen. In the EU impact assessment, OELs at 1 and 5 ppm were considered (1 ppm as recommended by SCOEL/ACSH and 5 ppm as a typical national OEL), and it was judged that most companies already complied with an OEL of 1 ppm. The proposed binding OEL was in agreement with this value (26 countries would have to introduce or revise their national OELs) (SCOEL Citation2010b; European Commission Citation2016).
7.16. o-Toluidine
7.16.1. Scientific basis
In 2012, o-toluidine was classified by IARC as a group 1 carcinogen (IARC Citation2020). SCOEL categorised o-toluidine as a non-threshold genotoxic carcinogen (group A). Based on chronic data on rats after dietary exposure (incidence of transitional-cell urinary bladder carcinoma) and BMD modelling, SCOEL estimated an excess tumour risk of 1 × 10−3 from 40 years of occupational exposure at 2.10 mg/m³ (0.48 ppm) (SCOEL Citation2017f).
The AGS (Citation2014b) has taken o-toluidine as an example where a risk calculation is not possible. The human data were considered insufficient, but still indicating a substantial cancer risk for humans. At the same time, results from qualified animal experiments indicated a low risk of cancer, which did not completely explain the quantitative information from the human data (AGS Citation2014b).
7.16.2. Regulatory rationale for the OEL
There was no SCOEL recommendation when the Commission performed its impact assessment for o-toluidine. The assessment considered potential OELs of 0.1 and 1 ppm, as typical values of existing national OELs. It was judged that 98% of the workers in EU were exposed to less than 0.1 ppm. The lowest value was put forward as binding OEL (25 member states had no or a less stringent limit value) (European Commission Citation2016).
7.17. Trichloroethylene
7.17.1. Scientific basis
In 2014, IARC classified trichloroethylene as a group 1 carcinogen (IARC Citation2020). SCOEL concluded in Citation2009 that trichloroethylene is a genotoxic carcinogen with a practical threshold (group C). The key target of human trichloroethylene toxicity and carcinogenesis was said to clearly be the kidney, and human renal cell cancer had been observed in several recent studies in highly and repetitively exposed workers, having used trichloroethylene mostly in metal degreasing activities. The MoA was considered to likely involve multiple pathways and with several lines of evidence suggesting a sublinear dose-tumour response. SCOEL further stated that tumours in kidneys had only been observed after occupational trichloroethylene exposure to very high, clearly nephrotoxic concentrations. Observations in experimental systems, as well as in occupationally exposed and diseased persons, led to the conclusion that human renal cell cancer risk is avoided if exposure to nephrotoxic concentrations does not occur, including concentrations leading to subclinical renal changes that can be monitored by urinary excretion of suitable marker proteins. An 8-h TWA of 10 ppm (NOAEC for human renal toxicity), a short-term exposure limit (STEL) of 30 ppm and a skin notation were recommended (SCOEL Citation2009).
In contrast, RAC concluded (in a trial exercise to improve the efficiency of the application for the authorisation process) that trichloroethylene should, in terms of the REACH Regulation, be considered as a non-threshold carcinogen. A sublinear approach was used to account for both the genotoxic mechanism and the cytotoxic co-carcinogenic mechanism that operates at higher levels. Thus, the dose–response curve was regarded to become steeper above the threshold level of 6 ppm for the cytotoxic effects. Referring to calculations based on occupational data performed by the AGS (Citation2008), the excess kidney cancer incidence risk from 40 years of occupational exposure at 6 ppm was 4 × 10−3 (ECHA Citation2014).
7.17.2. Regulatory rationale for the OEL
In the EU impact assessment, two exposure levels were evaluated (10 and 50 ppm as typical national OELs, the lower in agreement with the SCOEL Recommendation for an Hb-OEL). No concerns about feasibility, overall cost or competitiveness outside the EU had been raised by the ACSH. A binding OEL of 10 ppm with a STEL of 30 ppm and a skin notation was proposed as recommended by SCOEL (17 member states would have to introduce or update their OELs) (SCOEL Citation2009; European Commission Citation2017).
7.18. Vinyl bromide (bromoethylene)
7.18.1. Scientific basis
In 2008, vinyl bromide was classified by IARC as a group 2A carcinogen (IARC Citation2020). The same year SCOEL categorised vinyl bromide as a group A non-threshold carcinogen. The carcinogenic effects and MoA were considered similar to those of the established human carcinogen vinyl chloride. Reference was made to the assessed hepatic angiosarcoma risk of vinyl chloride of 3 × 10−4 for work-life exposure to 1 ppm, based on epidemiological data (SCOEL Citation2004b). It was advised to apply this risk assessment also to vinyl bromide, considering a threefold higher potency of vinyl bromide compared to vinyl chloride. The resulting angiosarcoma risk for work-life exposure to 1 ppm vinyl bromide amounted to 9 × 10−4 (SCOEL Citation2008).
A previous risk calculation by DECOS based on rat data (incidence of hepatic angiosarcomas) resulted in an excess lifetime risk of 4 × 10−3 after 40 years of occupational exposure to 1.2 mg/m3 (GR Citation1999b). Recalculated, this gives a risk of 1.5 × 10−2 at 1 ppm.
7.18.2. Regulatory rationale for the OEL
In the EU impact assessment, two potential OELs (1 and 5 ppm, typical national OELs) were evaluated. Exposure levels were judged to be low, with the highest exposure probably being below 1 ppm. The number of exposed workers were less than a few hundred and possibly close to zero. Referring to the risk calculations performed by SCOEL for vinyl chloride, a binding OEL of 1 ppm was proposed (22 national OELs would have to be introduced or revised) (SCOEL Citation2008; European Commission Citation2016). Thus, the threefold higher potency of vinyl bromide compared to vinyl chloride was not taken into account.
7.19. Vinyl chloride (chloroethylene)
7.19.1. Scientific basis
IARC classified vinyl chloride as a group 1 carcinogen in 2012 (IARC Citation2020). SCOEL used linear extrapolation of epidemiological data to assess the risk of hepatic angiosarcoma from working lifetime. Derived excess risk values were 3 × 10−4, 6 × 10−4, and 9 × 10−4 at 1, 2, and 3 ppm, respectively. Independent data, derived from animal experiments and using physiologically based pharmacokinetic (PBPK) modelling, pointed to a similar order of magnitude, and thus confirmed this approach (SCOEL Citation2004b).
DECOS concluded that vinyl chloride induces cancer in animals and humans via a mutagenic MoA (stochastic genotoxic substance). The committee used epidemiological data (incidence of liver angiosarcomas) and estimated by linear extrapolation that lifetime excess cancer risks of 4 × 10−5 and 4 × 10−3 correspond to 40 years of occupational exposure to 0.65 and 65.5 mg/m3 (0.25 and 25 ppm), respectively (GR Citation2017b).
Also the AGS used epidemiological data on liver angiosarcomas and estimated that the lifetime excess cancer risks from 40 years of exposure are 4 × 10−5 and 4 × 10−3 at 1 and 100 mg/m3 (0.4 and 40 ppm), respectively (AGS Citation2020).
7.19.2. Regulatory rationale for the OEL
In the EU impact assessment, two options for an OEL (1 and 2 ppm) were evaluated versus the OEL of 3 ppm currently in place. A binding OEL of 1 ppm was put forward (25 member states had less stringent values). It was stated that this option was the closest to the limit recommended by SCOEL (SCOEL Citation2004b; European Commission Citation2016). It should be noted that SCOEL considered vinyl chloride to be a non-threshold carcinogen, and therefore only presented the calculated excess risk at 1 ppm.
7.20. Wood dust
7.20.1. Scientific basis
In 2012, IARC classified wood dust as a group 1 carcinogen (IARC Citation2020). SCOEL stated (Citation2003) that the mechanism underlying the carcinogenic action of wood dust was unknown, but that the causal role of exposure to wood dust for the development of sinonasal cancer had been unambiguously established by numerous epidemiological studies carried out in populations of varying geographical origin, exposed for different periods and in several fields of activity. However, a quantitative risk assessment was not considered realistic because of lack of good-quality quantitative exposure–response data. It was noted that very few studies had been conducted on workers exposed to average concentrations of wood dust below 0.5 mg/m3, mainly because such low levels were rarely observed in the wood industry. At exposure levels between 0.5 and 1 mg/m3 (total dust), several studies indicated an increased incidence of sinonasal cancer. SCOEL therefore concluded that 0.5 mg/m3 (total dust) (1 mg/m3 as inhalable dust) is probably below the levels to which the cases of sinonasal cancers had been exposed. It was also said that hardwood dust seemed particularly dangerous regarding sinonasal cancer, but that is was impossible to identify the role of each type of wood in cancer development (SCOEL Citation2003).
7.20.2. Regulatory rationale for the OEL
In the EU impact assessment, the present OEL of 5 mg/m3 was evaluated along with two lower values, 3 and 1 mg/m3 (as inhalable dust). A binding OEL of 1 mg/m3 was considered to impose a disproportionate burden on, in particular small and medium-sized, enterprises. The preferred option was 3 mg/m3 which would also lead to a substantial reduction in health costs, but have a low or non-existent negative impact on firms (18 national OELs would have to be revised) (European Commission Citation2016). Finally, the limit was set at 3 mg/m3, to be lowered to 2 mg/m3 in 2023 (European Parliament Citation2017).
7.21. Summary and discussion
The carcinogens described in this chapter and for which the EU introduced binding OELs 2017–2019 are summarised in . Lifetime excess cancer risk estimates at the binding OEL in the EU are expressed as numbers of extra cancer cases per 1000 exposed during a full working life of 40 years. They were calculated by NEG, using LNT extrapolation, based on numerical risk estimates presented by the various expert committees referred to in this chapter.
Table 10. EU binding OELs for non-threshold carcinogens introduced in 2017–2019 (European Parliament Citation2017, Citation2019a, Citation2019b) and the corresponding risk levels (excess cancer cases/1000 workers) estimated by European expert committees. Estimated number of exposed workers are from the EU impact assessments (European Commission Citation2016, Citation2017, Citation2018, Citation2020a).
So far, the EU procedure is in part a risk-based approach, as the EU scientific committee (previously SCOEL and at present RAC) deliver risk calculations. However, the risk calculations are not transformed to OELs according to any predefined risk level, and the calculated risk at the binding OELs therefore varies (for a recent EU initiative regarding the use of a risk-based approach, see Section 6.2).
Thus, binding OELs in the EU have an element of socioeconomic and technical feasibility considerations. These limits are minimum requirements, and member states may set lower OELs. The CMD stipulates that the exposure (to carcinogens) should always be reduced to a minimum. Many of the carcinogens presented in this chapter have a skin notation. Regarding this, the Directives amending the CMD have fully followed the SCOEL and RAC recommendations.
As shown in , an excess cancer risk of one per 1000 (1 × 10−3) is exceeded for most of these chemicals at the binding OEL. For diesel engine exhaust, chromium (VI) compounds and 2-nitropropane the calculated risks even exceed one per 100 (1 × 10−2). The number of exposed workers differ enormously from close to zero (vinyl bromide) up to 3–4 million (MDA, diesel engine exhaust, hardwood dust).
8. Recommendations for the setting of OELs for carcinogens
In this chapter, NEG gives recommendations regarding regulatory aspects in the OEL setting of carcinogens in the light of the critical issues/steps described in the preceding chapters.
8.1. Threshold and non-threshold carcinogens
Historically, regulators have handled all carcinogens in the same way. With the discovery that carcinogens may act via fundamentally different mechanisms (Section 2.3), it has become important to distinguish between threshold and non-threshold carcinogens.
From a biological and mechanistic point of view, there is most likely a threshold at very low doses, even for directly DNA-reactive carcinogens. However, for most such substances there are not sufficient data to confirm that there is a threshold and even less data to determine the threshold dose and the shape of the dose–response curve. Considering the precautionary principle, LNT extrapolation (i.e. simply drawing a straight line from the PoD down to dose zero) remains the best option in these cases. One of the advantages of the LNT approach is that it most likely does not underestimate the risk at low, workplace-relevant, exposure levels. Also, the use of LNT conveys the message that an administrative policy is followed, and that the quantitative assessment is not based on exact biological knowledge. The exposure levels corresponding to predefined risk levels (or the cancer risk estimates at relevant exposure levels) should be clearly described, along with a description of the underlying exposure–response data.
For threshold carcinogens, NEG supports the approach taken by SCOEL (Citation2017a) and ECHA (Citation2019). Thus, for a carcinogen with a threshold MoA, an Hb-OEL can be derived based on the relevant PoD and applying appropriate assessment factors. However, limited data for a single carcinogen in combination with the general lack of knowledge about cancer mechanisms sometimes make it hard to exclude a non-threshold MoA. As for non-threshold carcinogens, a detailed description of the exposure–response data (including cancer risk estimates if possible) should be included. This is particularly important when the final OEL is set above the recommended Hb-OEL (for feasibility reasons). In such cases, dose–response data are needed for the socioeconomic assessment.
Recommendation
For a non-threshold carcinogen, LNT extrapolation should be used to calculate the cancer risk at different exposure levels, unless data clearly point to another shape of the dose–response curve.
For a threshold carcinogen, it is appropriate to derive an Hb-OEL by applying assessment factors.
8.2. Uncertainty of the risk estimates
A problem with non-threshold carcinogens is that estimated risks at different exposure levels (and the OEL) are generally uncertain due to insufficient knowledge about basic mechanisms. Meanwhile, as also pointed out, e.g. by the Institute of Occupational Medicine (IOM) and the COC (IOM Citation2011; COC Citation2012, Citation2018), expression of a numerical value for the risk estimate may convey a false sense of precision. This creates obstacles for risk assessors and regulators and makes communication with stakeholders difficult. Moreover, uncertainties in the risk calculations may lead to improper prioritisation and/or improper balancing of benefits (reduced cancer risk) versus costs of control measures (IOM Citation2011).
An additional problem with some of the “high-risk” carcinogens (i.e. high estimated cancer risk at the OEL) is that epidemiological data are meagre or lacking (sometimes because very few are exposed), therefore, any quantitative risk estimate has to be based on animal data. The extrapolations (animal to man, high dose to low dose) adds uncertainty that needs to be addressed and clearly declared.
In spite of these concerns, NEG recommends use of the LNT approach for non-threshold carcinogens, as there are few alternatives with today’s knowledge (Section 8.1). Still, uncertainties, including the presence or absence of a threshold, the shape of the dose–response curve and lack of solid epidemiological data should be declared. In case of essential gaps of knowledge, such as lack of epidemiological data, this should be explained, and extra caution should be practiced.
Recommendation
The scientific uncertainties in the cancer risk assessment, including the presence or absence of a threshold, the (assumed) shape of the dose–response curve and lack of solid epidemiological data should be clearly described.
8.3. Harmonised terminology and defined risk levels
So far, there is no consensus among risk assessors and regulators on how to define cancer risk levels for OEL setting, regarding terminology as well as numerical values. NEG recommends striving toward harmonisation stepwise, by:
Constructing and reaching consensus on a framework for risk levels, such as the “Traffic light model” (). In this model, there are two risk levels and, thus, three risk areas, labelled with green, yellow and red.
Reaching consensus on the terminology in the framework. If two risk levels are used, NEG recommends the terms “low risk level” and “high risk level”. The terms “acceptable” and “tolerable” risk (used e.g. by Germany, AGS Citation2013b) are less suitable as the two have a similar semantic meaning and are easily confused. Indeed, several dictionaries lists the two words as synonyms. The three risk areas could be named “low”, “medium,” and “high”, in accordance with the German terminology (AGS Citation2013b).
Reaching consensus on the numerical values of the risk levels. NEG does not take a stand on the numerical values; the numbers presented in should be seen as examples.
Figure 7. The “Traffic light model”, an example of a framework for visualising and communicating cancer risk levels at work. Adapted from AGS (Citation2013b). NEG does not take a stand on the numerical values presented. aCommittee on Hazardous Substances (AGS). The intention is to lower the present acceptable risk level from 4 × 10−4 to 4 × 10−5 (AGS Citation2013b); change foreseen in 2022. bHealth Council of the Netherlands (GR Citation2012b).
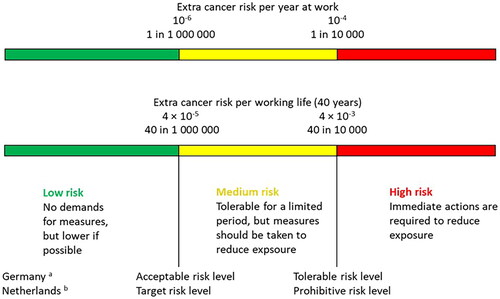
Recommendation
Construct a framework and harmonise the wording for risk levels. If two risk levels are used, the wordings “low” and “high” are recommended.
Define and harmonise the numerical values of the risk levels.
8.4. Collective risk versus individual risk
Binding OELs have an element of socioeconomic and technical feasibility (see e.g. Chapter 7). In this context, it is important to consider both the collective risk (estimated total number of workers that will get cancer from exposure at work at the OEL) and the individual risk. Disregarding the individual risk violates the individual’s right to a healthy workplace. Thus, individual risks should be transparently communicated. This is particularly important for some non-threshold carcinogens with high theoretical excess cancer risk (assuming 40–45 years of exposure at work, 8 h/day, 5 days/week) at the current EU binding OEL ().
Recommendation
The collective cancer risk (estimated total number of workers that will get cancer from 40 to 45 years of work exposure at the OEL) should be considered and clearly communicated.
The individual cancer risk should also be considered and clearly communicated. It may be visualised with the “Traffic light model” (Section 8.3).
8.5. Socioeconomic aspects of the OEL setting
Socioeconomic aspects are obviously important when setting OELs. Vested interests may impact the OEL, in particular in the final stages and when the scientific basis is weak, as it is for many non-threshold carcinogens.
NEG is of the opinion that the socioeconomic impact assessment should be done clearly separated from the health risk assessment, ideally by a second, independent committee. Furthermore, the socioeconomic assessment should be clearly communicated. Relevant non-cancer effects should be assessed and reported.
Recommendation
The socioeconomic impact assessment should be done separately from the health risk assessment, ideally by a second, independent committee.
The socioeconomic impact assessment and how it influenced the OEL setting should be clearly communicated.
8.6. Additional measures
Although not in focus of this review, there are obviously a number of measures in addition to OELs that can and should be taken to reduce the risk of occupational cancer. The EU Chemical Agents Directive (CAD) recommends a hierarchy of control measures to prevent or reduce exposure to dangerous substances, with the complete elimination of the substance at the top, i.e. the STOP principles (Substitution, Technical measures, Organisational measures, Personal protective equipment) (European Parliament Citation1998; EU-OSHA Citation2018; ECHA Citation2021; European Parliament Citation2022). In addition, the EU initiative Roadmap on Carcinogens comprises four pillars and 12 challenges to prevent workers from getting exposed to carcinogens (Roadmap on Carcinogens Citation2020) ().
Figure 8. Four pillars and 12 challenges presented by the EU initiative Roadmap on Carcinogens. Adapted from Roadmap on Carcinogens (Citation2020).
NEG recommends that more emphasis is made on such measures, including for example substitution, technical solutions, education, and the ALARA principle. These measures should be communicated in close connection to the OEL value.
Recommendation
For carcinogens, in particular non-threshold carcinogens, additional measures should be communicated and emphasised in close connection to the OEL. Such measures include, e.g. substitution, technical solutions, education, and the ALARA principle.
8.7. Support research on cancer mechanisms and low-dose risk
Most cancer risk estimates for chemical exposure are uncertain due to insufficient knowledge about the basic cancer mechanisms. Data gaps are bridged by simplifications, assumptions, and analogy reasoning. More detailed mathematical modelling seems unrealistic as long as carcinogenic mechanisms in general, and mechanisms activated by carcinogenic chemicals in particular, remain incompletely understood. Novel research trends (Section 3.1) indicate that a detailed scenario for cancer development and its rate limiting steps is far from clear. Today’s knowledge is also hard to capture in unifying mathematical models, as was assumed in the 1950s, e.g. by Nordling (Citation1953) and Armitage and Doll (Citation1954).
Further research on cancer mechanisms and low-dose risk is therefore urgently needed, and scientists and institutions in occupational medicine and toxicology should be encouraged and supported to orient their research along these lines. In this work, both basic and mechanistic research, supported by long-term efforts, are of vital importance.
Recommendation
Scientists and institutions in occupational medicine and toxicology should be encouraged and supported to orient their research toward cancer mechanisms and low-dose risk.
9. Research needs
As discussed in this document, methods currently used for calculating risk levels are imprecise. Improvements taking advantage of recent years’ achievements in cancer research are needed. Below are some examples of research that might lead to better models for estimating risks or for modulation of risk estimates for single or groups of carcinogens:
Studies on early indicators of mutagenic activity in combination with stimulated proliferation in e.g. the bronchial epithelium. It is crucial to focus on mutations, and not just genotoxicity markers, by employing new techniques such as sensitive mutational analysis, see e.g. Yizhak et al. (Citation2019). Complementing exposome studies are important. High-risk carcinogens with limited or no epidemiological data are of highest concern.
Studies on mutational signatures as exemplified in Kucab et al. (Citation2019). These types of studies should be continued by analysis of additional carcinogens.
Research involving genomic instability and replication stress that focuses on the development of techniques that make it possible to study interactions between DNA damage and other stressors causing, e.g. replications at low doses. Of particular interest is the relationship between DNA damage and actual mutations.
How other recent discoveries in cancer research (exemplified in Section 3.1) might affect the dose–response. Other mechanisms that might affect the shape of the dose–response curve for carcinogens include cellular senescence, lineage infidelity, epigenetic changes, and autophagy. For example, the study on the role of autophagy in asbestos-induced mesothelioma (Xue et al. Citation2020) should be followed up by similar studies employing other carcinogens. Also dose–response issues are of direct interest, e.g. whether autophagy operates at high doses of asbestos only, or also at common human exposure levels.
More comprehensive studies of possible influences of chemical carcinogens on stem cells. For example, can combinations of carcinogens, e.g. “initiators” and “promoters” activate mutagenic stem cell responses, or can carcinogens affect hormonal responses to chemicals in stem cells?
Studies on whether fewer years of exposure, as in the work environment compared to the general environment, linearly compensate the risk for higher exposure levels. For example, does a low exposure for 80 years confer the same risk as a doubled exposure for 40 years?
| Abbreviations | ||
| ACGIH | = | American Conference of Governmental Industrial Hygienists |
| ACSH | = | Advisory Committee on Safety and Health at Work |
| AGS | = | Ausschuss für Gefahrstoffe (Committee on Hazardous Substances) |
| ALARA | = | as low as reasonably achievable |
| ALARP | = | as low as reasonably practicable |
| ANSES | = | Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail (French Agency for Food, Environmental and Occupational Health & Safety) |
| AOP | = | adverse outcome pathway |
| BAT | = | Biologischer Arbeitsstoff‐Toleranzwert (biological tolerance value) |
| BGV | = | biological guidance value |
| BLV | = | biological limit value |
| BMD | = | benchmark dose |
| BMD10 | = | BMD corresponding to a 10% extra risk |
| BMDL | = | lower confidence limit of the BMD |
| BMDL10 | = | lower confidence limit of the BMD10 |
| BMDU | = | upper confidence limit of the BMD |
| BMR | = | benchmark response |
| BMR10 | = | BMR of 10% |
| CAD | = | Chemical Agents Directive |
| CLP | = | Classification, Labelling and Packaging (of substances and mixtures) |
| CMD | = | Carcinogens and Mutagens Directive |
| COC | = | Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment |
| DDR | = | DNA damage responses |
| DECOS | = | Dutch Expert Committee on Occupational Safety |
| DEP | = | diesel exhaust particles |
| DMEL | = | derived minimal effect level |
| EC | = | elemental carbon |
| ECHA | = | European Chemicals Agency |
| EFSA | = | European Food Safety Authority |
| EPA | = | Environmental Protection Agency |
| EU | = | European Union |
| GHS | = | Globally Harmonised System of Classification and Labelling of Chemicals |
| Hb-OEL | = | health-based occupational exposure limit |
| IARC | = | International Agency for Research on Cancer |
| IOM | = | Institute of Occupational Medicine |
| LNT | = | linear non-threshold |
| LOAEC | = | lowest observed adverse effect concentration (at inhalation) |
| LOAEL | = | lowest observed adverse effect level |
| MAF | = | mixture assessment factor |
| MAK | = | Maximale Arbeitsplatzkonzentration (maximum workplace concentration) |
| MDA | = | 4,4′-methylenedianiline |
| MoA | = | mode of action |
| MOCA | = | 4,4′-methylenebis(2-chloroaniline) |
| MoE | = | margin of exposure (sometimes also called margin of safety) |
| NIOSH | = | National Institute for Occupational Safety and Health |
| NOAEC | = | no observed adverse effect concentration (at inhalation) |
| NOAEL | = | no observed adverse effect level |
| NRCWE | = | National Research Centre for the Working Environment |
| NTP | = | National Toxicology Program |
| OECD | = | Organisation for Economic Co-operation and Development |
| OEL | = | occupational exposure limit |
| OSHA | = | Occupational Safety and Health Administration |
| PAH | = | polycyclic aromatic hydrocarbon |
| PBPK | = | physiologically based pharmacokinetic |
| PEL | = | permissible exposure limit |
| PoD | = | point of departure |
| RAC | = | Committee for Risk Assessment |
| REACH | = | Registration, Evaluation, Authorisation and Restriction of Chemicals |
| REL | = | recommended exposure limit |
| RML-CA | = | risk management limit for a carcinogen |
| RoC | = | Report on Carcinogens |
| ROS | = | reactive oxygen species |
| SCOEL | = | Scientific Committee on Occupational Exposure Limits |
| SEG | = | Scientific Expert Group |
| SER | = | Sociaal-Economische Raad (Social and Economic Council) |
| STEL | = | short-term exposure limit |
| TCDD | = | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TRC | = | technical reference concentration |
| TWA | = | time-weighted average |
| US | = | United States |
| WHO | = | World Health Organization |
Acknowledgements
This publication is a slightly modified version of a document written by the authors in dialog with the Nordic Expert Group (NEG) and published in Arbete och Hälsa (Nr 2022;56(2)). The authors express their deep gratitude for the cooperation with NEG, which included the following experts: Gunnar Johanson (Sweden, chair), Merete Drevvatne Bugge (Norway), Helge Johnsen (Norway), Gry Koller (Norway), Anne Thoustrup Saber (Denmark), Piia Taxell (Finland), Mattias Öberg (Sweden), Anna-Karin Alexandrie (Sweden, secretary), and Jill Järnberg (Sweden, secretary).
Declaration of interest
Johan Högberg has not participated in and do not anticipate participation in any legal, regulatory, or advocacy proceedings related to the contents of the paper. Jill Järnberg has, as representative of SWEA, participated in discussions between Swedish authorities on a definition of risk levels for non-threshold carcinogens, including the traffic light model (no decision yet taken). However, the views expressed in this article are those of the authors’ and of the Nordic Expert Group, and not necessarily those of SWEA. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals (NEG) is financed by the Swedish Work Environment Authority and the Norwegian Ministry of Labour and Social Inclusion. Johan Högberg’s work was financially supported by FORTE (Swedish Research Council for Health, Working Life and Welfare) Sweden (project nr 2018-00610) and FORMAS (Swedish Research Council for Sustainable Development) Sweden (project nr 2022-00995).
References
- [ACGIH] American Conference of Governmental Industrial Hygienists. 2021. TLVs and BEIs. Based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati (OH): ACGIH.
- [ACSH] Advisory Committee on Safety and Health at Work. 2012. Opinion on the approach and content of an envisaged proposal by the Commission on the Amendment of Directive 2004/37/EC on carcinogens and mutagens at the workplace. Doc. 2011/12. Luxembourg, Luxembourg: European Commission, Employment and Social Affairs and Inclusion.
- [ACSH] Advisory Committee on Safety and Health at Work. 2013. Supplementary opinion on the approach and content of an envisaged proposal by the Commission on the Amendment of Directive 2004/37/EC on carcinogens and mutagens at the workplace. Doc. 727/13. Luxembourg, Luxembourg: European Commission, Employment and Social Affairs and Inclusion.
- [ACSH] Advisory Committee on Safety and Health at Work. 2016. Supplementary opinion No. 2 on the approach and content of an envisaged proposal by the Commission on the amendment of Directive 2004/37/EC on carcinogens and mutagens at the workplace. Doc. 2016/13. Luxembourg, Luxembourg: European Commission, Employment and Social Affairs and Inclusion.
- [ACSH] Advisory Committee on Safety and Health at Work. 2017a. Fourteenth annual activity report of the Advisory Committee on Safety and Health at Work. Doc. 800/19-EN. Luxembourg, Luxembourg: European Commission, Employment and Social Affairs and Inclusion.
- [ACSH] Advisory Committee on Safety and Health at Work. 2017b. Opinion on an EU occupational exposure limit value for arsenic acid and its salts as well as inorganic arsenic compounds in the scope of Directive 2004/37/EC (CMD). Doc. 1334/17. Luxembourg, Luxembourg: European Commission, Employment and Social Affairs and Inclusion.
- [AGS] Ausschuss für Gefahrstoffe. 2008. Begründung zu Expositions-Risiko-Beziehung für Trichlorethylen in BekGS 910 [Exposure–risk relationship for trichloroethylene in BekGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2010a. Exposure–risk relationship for 1,3-butadiene in BekGS 910. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2010b. Exposure–risk relationship for 4,4′-methylenedianiline in BekGS 910. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2011. Exposure–risk relationship for ethylene oxide in BekGS 910. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2012a. Begründung zu Epichlorhydrin in BekGS 910. [Exposure–risk relationship for epichlorohydrin in BekGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2012b. Begründung zu Expositions-Risiko-Beziehung für Acrylamid in BekGS 910 [Exposure–risk relationship for acrylamide in BekGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2013a. Begründung zu Propylenoxid in TRGS 900. [Exposure–risk relationship for propylene oxide in TRGS 900]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2013b. The risk-based concept for carcinogenic substances developed by the Committee for Hazardous Substances. From limit-value orientation to an action-oriented approach. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2014a. Begründung zu Chrom VI in TRGS 910. [Exposure–risk relationship for chromium VI in TRGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2014b. Technical rules for hazardous substances (TRGS) 910. Risk-related concept of measures for activities involving carcinogenic hazardous substances. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2015a. Begründung zu 1,2-dichlorethan in TRGS 910. [Exposure–risk relationship for 1,2-dichloroethane in TRGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2015b. Begründung zu 2-nitropropan in TRGS 910. [Exposure–risk relationship for 2-nitropropane in TRGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2015c. Begründung zu ERB Arsenverbindungen in TRGS 910 [Exposure–risk relationship for arsenic compounds in TRGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2015d. Begründung zu ERB hydrazin in TRGS 910. [Exposure–risk relationship for hydrazine in TRGS 910]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- [AGS] Ausschuss für Gefahrstoffe. 2020. Begründung zu Chlorethylen (Vinylchlorid) in TRGS 900 [Exposure–risk relationship for chloroethylene (vinyl chloride) in TRGS 900]. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health), AGS (Committee on Hazardous Substances).
- Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, Totoki Y, Fujimoto A, Nakagawa H, Shibata T, et al. 2016. Mutational signatures associated with tobacco smoking in human cancer. Science. 354(6312):618–622.
- Ali I, Dreij K, Baker S, Högberg J, Korhonen A, Stenius U. 2021. Application of text mining in risk assessment of chemical mixtures: a case study of polycyclic aromatic hydrocarbons (PAHs). Environ Health Perspect. 129(6):67008.
- Ali I, Guo Y, Silins I, Högberg J, Stenius U, Korhonen A. 2016. Grouping chemicals for health risk assessment: a text mining-based case study of polychlorinated biphenyls (PCBs). Toxicol Lett. 241:32–37.
- Ames BN. 1973. Carcinogens are mutagens: their detection and classification. Environ Health Perspect. 6:115–118.
- [ANSES] Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. 2014. Expert appraisal on recommending occupational exposure limits for chemical agents. Reference document for the derivation and the measurement of exposure limit values for chemical agents in the workplace (OELs). Collective expert appraisal report. Request 2009-SA-0339. Maisons-Alfort Cedex, France: ANSES (French Agency for Food, Environmental and Occupational Health & Safety).
- Arbejdstilsynet. 2007. Grænseværdier for stoffer og materialer. Grænseværdier for stoffer og materialer [Limit values for substances and materials]. At-vejledning. Stoffer og materialer–C.0.1. Copenhagen, Denmark: Arbejdstilsynet (Work Environment in Denmark).
- Armitage P, Doll R. 1954. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 8(1):1–12.
- Baker S, Ali I, Silins I, Pyysalo S, Guo Y, Högberg J, Stenius U, Korhonen A. 2017. Cancer Hallmarks Analytics Tool (CHAT): a text mining approach to organize and evaluate scientific literature on cancer. Bioinformatics. 33(24):3973–3981.
- Balkwill F, Charles KA, Mantovani A. 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 7(3):211–217.
- [BAuA] Bundesanstalt Für Arbeitsschutz und Arbeitsmedizin. 2010. Hazardous Substances Ordinance (Gefahrstoffverordnung–GefStoffV). 2010, 26 Nov. (Federal Law Gazette I, p. 1643–1644). Updated 2021. Article 10. Special protective measures for activities involving hazardous substances classified in categories 1A or 1B for carcinogenicity, germ cell mutagenicity or reproductive toxicity. Dortmund, Germany: BAuA (Federal Institute for Occupational Safety and Health).
- Benesch MGK, MacIntyre ITK, McMullen TPW, Brindley DN. 2018. Coming of age for autotaxin and lysophosphatidate signaling: clinical applications for preventing, detecting and targeting tumor-promoting inflammation. Cancers. 10(3):73.
- Benigni R, Bossa C. 2011. Mechanisms of chemical carcinogenicity and mutagenicity: a review with implications for predictive toxicology. Chem Rev. 111(4):2507–2536.
- Blackadar CB. 2016. Historical review of the causes of cancer. World J Clin Oncol. 7(1):54–86.
- Bolt HM, Foth H, Hengstler JG, Degen GH. 2004. Carcinogenicity categorization of chemicals—new aspects to be considered in a European perspective. Toxicol Lett. 151(1):29–41.
- Bolt HM, Huici-Montagud A. 2008. Strategy of the Scientific Committee on Occupational Exposure Limits (SCOEL) in the derivation of occupational exposure limits for carcinogens and mutagens. Arch Toxicol. 82(1):61–64.
- Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, Pellegrini L, Signorato V, Olivetto F, Pastorino S, et al. 2017. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature. 546(7659):549–553.
- Calabrese EJ. 2017a. The mistaken birth and adoption of LNT: an abridged version. Dose Response. 15(4):1559325817735478.
- Calabrese EJ. 2017b. Obituary notice: LNT dead at 89 years, a life in the spotlight. Environ Res. 155:276–278.
- Chappell G, Pogribny IP, Guyton KZ, Rusyn I. 2016. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: a systematic literature review. Mutat Res Rev Mutat Res. 768:27–45.
- Cherrie JW, Hutchings S, Gorman Ng M, Mistry R, Corden C, Lamb J, Sánchez Jiménez A, Shafrir A, Sobey M, van Tongeren M, et al. 2017. Prioritising action on occupational carcinogens in Europe: a socioeconomic and health impact assessment. Br J Cancer. 117(2):274–281.
- [COC] Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment. 2012. A strategy for the risk assessment of chemical carcinogens. COC/G1–version 4. London (UK): Public Health England.
- [COC] Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment. 2018. COC guidance statement G06–version 1.1. Cancer risk characterisation methods. Chilton (UK): Public Health England, Centre for Radiation, Chemical and Environmental Hazards.
- [COC] Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment. 2020. COC guidance statement G05–version 2-0. Defining a point of departure and potency estimates in carcinogenic dose response. London (UK): Public Health England.
- Cohen SM, Ellwein LB. 1990. Cell proliferation in carcinogenesis. Science. 249(4972):1007–1011.
- Davis JA, Gift JS, Zhao QJ. 2011. Introduction to benchmark dose methods and U.S. EPA's Benchmark Dose Software (BMDS) version 2.1.1. Toxicol Appl Pharmacol. 254(2):181–191.
- [DFG] Deutsche Forschungsgemeinschaft. 2019. List of MAK and BAT values 2019. Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area. Report 55. DFG (German Research Foundation). Weinheim, Germany: Wiley-VCH.
- Dietrich DR, Swenberg JA. 1991. The presence of alpha 2u-globulin is necessary for d-limonene promotion of male rat kidney tumors. Cancer Res. 51(13):3512–3521.
- Dybing E, Sanner T, Roelfzema H, Kroese D, Tennant RW. 1997. T25: a simplified carcinogenic potency index: description of the system and study of correlations between carcinogenic potency and species/site specificity and mutagenicity. Pharmacol Toxicol. 80(6):272–279.
- [ECHA] European Chemicals Agency. 2012. Guidance on information requirements and chemical safety assessment. Chapter R.8: characterisation of dose [concentration]–response for human health. Version: 2.1. Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2013. Application for authorisation: establishing a reference dose response relationship for carcinogenicity of hexavalent chromium. RAC/27/2013/06 Rev.1. Committee for Risk Assessment (RAC). Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2014. Application for authorisation: establishing a reference dose response relationship for carcinogenicity of trichloroethylene. RAC/28/2014/07 rev 2 Final. Committee for Risk Assessment (RAC). Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2017a. Guidance on information requirements and chemical safety assessment. Chapter R.7a: endpoint specific guidance. Version 6.0. Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2017b. Guidance on the application of the CLP criteria. Guidance to Regulation (EC) No. 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0. Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2017c. Opinion on 4,4′-methylene-bis-[2-chloroaniline] (MOCA). ECHA/RAC/A77-O-0000001412-86-147/F. Committee for Risk Assessment (RAC). Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2017d. Opinion on arsenic acid and its inorganic salts. ECHA/RAC/A77-O-0000001412-86-148/F. Committee for Risk Assessment (RAC). Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2019. Guidance on information requirements and chemical safety assessment. Appendix to chapter R.8: guidance for preparing a scientific report for health-based exposure limits at the workplace. Version 1.0. Helsinki, Finland: ECHA.
- [ECHA] European Chemicals Agency. 2021. Substances of concern: why and how to substitute? Helsinki, Finland: ECHA.
- [EFSA] European Food Safety Authority. 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 282:1–31.
- [EFSA] European Food Safety Authority. 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J. 10(3):2579.
- [EFSA] European Food Safety Authority. 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 13(11):4302.
- [EFSA] European Food Safety Authority. 2017. EFSA Scientific Committee, Hardy EFSA, Benford A, Halldorsson D, Jeger T, Knutsen MJ, More KH, Mortensen S, Naegeli A, Noteborn H, et al. Update: use of the benchmark dose approach in risk assessment. EFSA J. 15(1):4658.
- [EU-OSHA] European Agency for Safety and Health at Work. 2014. Exposure to carcinogens and work-related cancer: a review of assessment methods. European Risk Observatory Report. Luxembourg, Luxembourg: Publication Office of the European Union.
- [EU-OSHA] European Agency for Safety and Health at Work. 2018. Healthy workplaces. Manage dangerous substances. Bilbao, Spain: EU-OSHA.
- European Commission. 2016. Commission staff working document. Impact assessment. accompanying the document proposal for a directive of the European Parliament and of the Council amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. SWD(2016) 152 final. Brussels, Belgium: European Commission.
- European Commission. 2017. Commission staff working document. Impact assessment. accompanying the document proposal for a directive of the European Parliament and of the Council amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. SWD(2017) 7 final. Brussels, Belgium: European Commission.
- European Commission. 2018. Commission staff working document. Impact assessment. accompanying the document proposal for a directive of the European Parliament and of the Council amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. SWD(2018) 88 final. Brussels, Belgium: European Commission.
- European Commission. 2020a. Commission staff working document. Impact assessment. Accompanying the document proposal for a directive of the European Parliament and of the Council amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. SWD(2020) 183 final. Brussels, Belgium: European Commission.
- European Commission. 2020b. Commission staff working document. Progress report on the assessment and management of combined exposures to multiple chemicals (chemical mixtures) and associated risks. SWD(2020) 250 final. Brussels, Belgium: European Commission.
- European Commission. 2022. Chemicals: commission seeks views on revision of REACH, the EU’s chemicals legislation. Brussels, Belgium: European Commission, Directorate-General for Environment.
- [European Parliament] European Parliament and Council of the European Union. 1998. Council Directive 98/24/EC of 7 April 1998 on the protection of the health and safety of workers from the risks related to chemical agents at work (fourteenth individual Directive within the meaning of Article 16(1) of Directive 89/391/EEC). Consolidated Version: 26/07/2019. Off J Eur Union L 131:11–23.
- [European Parliament] European Parliament and Council of the European Union. 2004. Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens or mutagens at work (Sixth individual Directive within the meaning of Article 16(1) of Council Directive 89/391/EEC) (codified version). Off J Eur Union L 158:50–76.
- [European Parliament] European Parliament and Council of the European Union. 2008. Regulation (EC) No. 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No. 1907/2006. Off J Eur Union L 353(1):1–1355.
- [European Parliament] European Parliament and Council of the European Union. 2017. Directive (EU) 2017/2398 of the European Parliament and of the Council of 12 December 2017 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Off J Eur Union L 345:87–95.
- [European Parliament] European Parliament and Council of the European Union. 2019a. Directive (EU) 2019/130 of the European Parliament and of the Council of 16 January 2019 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Off J Eur Union L 30:112–120.
- [European Parliament] European Parliament and Council of the European Union. 2019b. Directive (EU) 2019/983 of the European Parliament and of the Council of 5 June 2019 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Off J Eur Union L 164:23–29.
- [European Parliament] European Parliament and Council of the European Union. 2022. Directive (EU) 2022/431 of the European Parliament and of the Council of 9 March 2022 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Off J Eur Union L 88:1–14.
- Feng Z, Hu W, Hu Y, Tang MS. 2006. Acrolein is a major cigarette-related lung cancer agent: preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 103(42):15404–15409.
- [FoBiG] Forschungs- und Beratungsinstitut Gefahrstoffe. 2019. Benchmark dose modelling. Research project F2437: derivation of occupational exposure limits for airborne chemicals–comparison of methods and protection levels. Prepared on behalf of Federal Institute for Occupational Safety and Health (BAuA) by Kaiser E and Schneider K. Freiburg, Germany: FoBiG.
- Fontana L, Nehme J, Demaria M. 2018. Caloric restriction and cellular senescence. Mech Ageing Dev. 176:19–23.
- Ge C, Peters S, Olsson A, Portengen L, Schuz J, Almansa J, Ahrens W, Bencko V, Benhamou S, Boffetta P, et al. 2020. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. A pooled exposure–response analysis of 14 case-control studies. Am J Respir Crit Care Med. 202(3):402–411.
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, Lu CP, Polak L, Yuan S, Elemento O, et al. 2017. Stem cell lineage infidelity drives wound repair and cancer. Cell. 169(4):636–650.e14.
- Golemis EA, Scheet P, Beck TN, Scolnick EM, Hunter DJ, Hawk E, Hopkins N. 2018. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 32(13–14):868–902.
- Goodson WH, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, Lasfar A, Carnero A, Azqueta A, Amedei A, et al. 2015. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis. 36(Suppl. 1):S254–S296.
- [GR] Gezondheidsraad. 1999a. 2-Nitropropane. Health based calculated occupational cancer risk values. Publication no. 1999/13OSH. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 1999b. Vinylbromide. Health based calculated occupational cancer risk values. Publication no. 1999/15OSH. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2000a. 4,4′-Methylene bis (2-chloroaniline). Health-based calculated occupational cancer risk values. Publication no. 2000/09OSH. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2000b. Epichlorohydrin (1-chloro-2,3-epoxypropane). Health-based calculated occupational cancer risk values. Publication no. 2000/10OSH. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2006. Acrylamide. Health-based calculated occupational cancer risk values. Publication no. 2006/05OSH. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2010. Guideline to the classification of carcinogenic compounds. Publication no. A10/07E. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2012a. Arsenic and inorganic arsenic compounds. Health-based recommendation on occupational exposure limits. Publication no. 2012/32. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2012b. Guideline for the calculation of risk values for carcinogenic compounds. Publication no. 2012/16E. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2013. 1,3-Butadiene. Health-based calculated occupational cancer risk values. Publication no. 2013/08. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2014a. Advisory letter acrylamide. Publication no. 2014/20E. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2014b. Advisory letter ethylene oxide. Publication no. 2014/26E. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2015. 4,4-Methylenedianiline. Health-based calculated occupational cancer risk values. Publication no. 2015/28. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2016. Hexavalent chromium compounds. Health-based recommendation on occupational exposure limits. Publication no. 2016/13E. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2017a. 1,2-Dibromoethane. Health-based recommendation on occupational exposure limits. Publication no. 2017/22. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2017b. Vinyl chloride monomer. Health-based calculated occupational cancer risk values. Publication no. 2017/01. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2019a. 1,2-Dichloroethane. Publication no. 2019/16. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2019b. Diesel engine exhaust. Publication no. 2019/02. The Hague: GR (Health Council of the Netherlands).
- [GR] Gezondheidsraad. 2021. Guideline for recommending classifications and health-based occupational exposure limits. The Hague: GR (Health Council of the Netherlands).
- Gusenleitner D, Auerbach SS, Melia T, Gómez HF, Sherr DH, Monti S. 2014. Genomic models of short-term exposure accurately predict long-term chemical carcinogenicity and identify putative mechanisms of action. PLOS One. 9(7):e102579.
- Haber LT, Dourson ML, Allen BC, Hertzberg RC, Parker A, Vincent MJ, Maier A, Boobis AR. 2018. Benchmark dose (BMD) modeling: current practice, issues, and challenges. Crit Rev Toxicol. 48(5):387–415.
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144(5):646–674.
- Hansson SO. 1998. Setting the limit: occupational health standards and the limits of science. New York: Oxford University Press.
- Hartwig A, Arand M, Epe B, Guth S, Jahnke G, Lampen A, Martus HJ, Monien B, Rietjens I, Schmitz-Spanke S, et al. 2020. Mode of action-based risk assessment of genotoxic carcinogens. Arch Toxicol. 94(6):1787–1877.
- Henshaw PS. 1946. Genetic injury. Radiology. 46:62–65.
- Högberg J, Järnberg J. 2022. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals. 154. Approaches for the setting of occupational exposure limits (OELs) for carcinogens. Vol. 56. Gothenburg, Sweden: University of Gothenburg. Arbete och Hälsa; p. 2.
- Huang S. 2021. Reconciling non-genetic plasticity with somatic evolution in cancer. Trends Cancer. 7(4):309–322.
- [IARC] International Agency for Research on Cancer. 2012. Chemical agents and related occupations. IARC monographs on the evaluation of carcinogenic risks to humans Vol. 100F. Lyon, France: IARC.
- [IARC] International Agency for Research on Cancer. 2017. Glyphosate. In: some organophosphate insecticides and herbicides. IARC monographs on the evaluation of carcinogenic risks to humans Vol. 112. Lyon, France: IARC; p. 321–399.
- [IARC] International Agency for Research on Cancer. 2019. IARC monographs on the identification of carcinogenic hazards to humans. Preamble. Lyon, France: IARC.
- [IARC] International Agency for Research on Cancer. 2020. List of classifications. Agents classified by the IARC monographs. Vol. 1–127. Lyon, France: IARC.
- [IFA] Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung. 2020. GESTIS–International limit values for chemical agents. Limit Values Germany–AGW. Sankt Augustin, Germany: IFA (Institute for Occupational Safety and Health of the German Social Accident Insurance).
- [IOM] Institute of Occupational Medicine. 2011. Health, socio-economic and environmental aspects of possible amendments to the EU Directive on the protection of workers from the risks related to exposure to carcinogens and mutagens at work. IOM research project: P937/99, May 2011. Edinburgh (UK): IOM.
- Jacobs MN, Colacci A, Corvi R, Vaccari M, Aguila MC, Corvaro M, Delrue N, Desaulniers D, Ertych N, Jacobs A, et al. 2020. Chemical carcinogen safety testing: OECD Expert Group International Consensus on the development of an integrated approach for the testing and assessment of chemical non-genotoxic carcinogens. Arch Toxicol. 94(8):2899–2923.
- Jasanoff S. 1982. Science and the limits of administrative rule-making: lessons from the OSHA Cancer Policy. Osgoode Hall Law J. 20:537–559.
- Jenkins GJ, Doak SH, Johnson GE, Quick E, Waters EM, Parry JM. 2005. Do dose response thresholds exist for genotoxic alkylating agents? Mutagenesis. 20(6):389–398.
- [JTF] Joint Task Force. 2017a. ECHA Committee for Risk Assessment (RAC) and Scientific Committee on Occupational Exposure Limits (SCOEL) on scientific aspects and methodologies related to the exposure of chemicals at the workplace. Final version. Luxembourg, Luxembourg and Helsinki, Finland.
- [JTF] Joint Task Force. 2017b. ECHA Committee for Risk Assessment (RAC) and Scientific Committee on Occupational Exposure Limits (SCOEL) on scientific aspects and methodologies related to the exposure of chemicals at the workplace. Task 2. Final report. Luxembourg, Luxembourg and Helsinki, Finland.
- Kadekar S, Silins I, Korhonen A, Dreij K, Al-Anati L, Högberg J, Stenius U. 2012. Exocrine pancreatic carcinogenesis and autotaxin expression. PLOS One. 7(8):e43209.
- Kaiser JC, Meckbach R, Jacob P. 2014. Genomic instability and radiation risk in molecular pathways to colon cancer. PLOS One. 9(10):e111024.
- Kelly KE. 1991. The myth of 10−6 as a definition of acceptable risk. Updated from a paper originally presented at the 84th Annual Meeting Air & Waste Management Association, Vancouver, Canada; June 16–21.
- Kinzler KW, Vogelstein B. 1996. Lessons from hereditary colorectal cancer. Cell. 87(2):159–170.
- Korhonen A, Seaghdha DO, Silins I, Sun L, Högberg J, Stenius U. 2012. Text mining for literature review and knowledge discovery in cancer risk assessment and research. PLOS One. 7(4):e33427.
- Kucab JE, Zou X, Morganella S, Joel M, Nanda AS, Nagy E, Gomez C, Degasperi A, Harris R, Jackson SP, et al. 2019. A compendium of mutational signatures of environmental agents. Cell. 177(4):821–836.e16.
- Kuppusamy SP, Kaiser JP, Wesselkamper SC. 2015. Epigenetic regulation in environmental chemical carcinogenesis and its applicability in human health risk assessment. Int J Toxicol. 34(5):384–392.
- Li L, Zhang X, Tian T, Pang L. 2019. Mathematical modelling the pathway of genomic instability in lung cancer. Sci Rep. 9(1):14136.
- Liu X, He Y, Li F, Huang Q, Kato TA, Hall RP, Li CY. 2015. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell. 58(2):284–296.
- McGrail DJ, Lin CC, Dai H, Mo W, Li Y, Stephan C, Davies P, Lu Z, Mills GB, Lee JS, et al. 2018. Defective replication stress response is inherently linked to the cancer stem cell phenotype. Cell Rep. 23(7):2095–2106.
- Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Gallo M, Reuhl K, Ho SM, Brown T, et al. 2002. Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review. Environ Health Perspect. 110(4):427–431.
- [Ministère du Travail] Ministère du Travail, des Relations sociales et de la Solidarité. 2008. Les valeurs limites d'exposition professionnelle: un outil concret pour la prévention de risques chimiques. In: Conditions de travail [Occupational exposure limit values: a concrete tool for the prevention of chemical risks. In: Working conditions]. Bilan 2007, Chapter 7, p. 171–190. Paris, France: Ministère du Travail, des Relations sociales et de la Solidarité [Ministry of Labour, Social Relations and Solidarity].
- Muller HJ. 1927. Artificial transmutation of the gene. Science. 66(1699):84–87.
- [NIH] National Institutes of Health. 2020. PubMed database. Bethesda (MD): NIH, National Library of Medicine (NLM), National Center for Biotechnology Information (NCBI).
- [NIOSH] National Institute for Occupational Safety and Health. 2017. Current intelligence bulletin 68: NIOSH chemical carcinogen policy. By Whittaker C, Rice F, McKernan L, Dankovic D, Lentz TJ, MacMahon K, Kuempel E, Zumwalde R, Schulte P, on behalf of the NIOSH Carcinogen and RELs Policy Update Committee. DHHS (NIOSH) Publication No. 2017-100. Cincinnati (OH): US Department of Health and Human Services, Centers for Disease, Control and Prevention, NIOSH.
- Nordling CO. 1953. A new theory on cancer-inducing mechanism. Br J Cancer. 7(1):68–72.
- [NRCWE] National Research Centre for the Working Environment. 2018. Diesel exhaust particles: scientific basis for setting a health-based occupational exposure limit. By Thoustrup Saber A, Hadrup N, Søs Poulsen S, Raun Jacobsen N, Vogel U. Copenhagen, Denmark: NRCWE.
- [NTP] National Toxicology Program. 2019. Report on carcinogens process & listing criteria. Research Triangle Park (NC): US Department of Health and Human Services, Public Health Service.
- [OECD] Organisation for Economic Cooperation and Development. 2020. OECD guidelines for the testing of chemicals, Section 4. Health effects. OECD iLibrary; https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788.
- Olson A, Lewis G. 1928. Natural reactivity and the origin of species. Nature. 121(3052):673–674.
- Pääjärvi G, Viluksela M, Pohjanvirta R, Stenius U, Högberg J. 2005. TCDD activates Mdm2 and attenuates the p53 response to DNA damaging agents. Carcinogenesis. 26(1):201–208.
- Perez-Mancera PA, Young AR, Narita M. 2014. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 14(8):547–558.
- [RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2014. Overview of methodologies for the derivation of occupational exposure limits for non-threshold carcinogens in the EU. RIVM letter report 2014-0153. By Pronk MEJ. Bilthoven, The Netherlands: RIVM (National Institute for Public Health and the Environment).
- [RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2016. Work-related cancer in the European Union. Size, impact and options for further prevention. RIVM Letter report 2016-0010. By Jongeneel WP, Eysink PED, Theodori D, Hamberg-van Reenen HH, Verhoeven JK. Bilthoven, The Netherlands: RIVM (National Institute for Public Health and the Environment).
- Roadmap on Carcinogens. 2020. New strategy 2020–2024 taking up challenges; [accessed 2021 Jun]. https://roadmaponcarcinogens.eu/roadmap-challenges-2020-2024/.
- Roadmap on Carcinogens. 2021. Beating cancer: better protection of workers against cancer-cancer chemicals; [accessed 2021 Jun]. https://roadmaponcarcinogens.eu/beating-cancer-better-protection-of-workers-against-cancer-causing-chemicals/.
- [RPA] Risk and Policy Analysts. 2019a. Final report for 4,4′-methylene-bis(2-chloroaniline) (MOCA). Third study on collecting most recent information for a certain number of substances with the view to analyse the health, socio-economic and environmental impacts in connection with possible amendments of Directive 2004/37/EC (Ref: VC/2017/0011). Ref. Ares(2018)1092875–27/02/2018. Luxembourg, Luxembourg: Publications Office of the European Union.
- [RPA] Risk and Policy Analysts. 2019b. Final report for inorganic arsenic compounds incl. arsenic acid and its salts. Third study on collecting most recent information for a certain number of substances with the view to analyse the health, socio-economic and environmental impacts in connection with possible amendments of Directive 2004/37/EC (Ref: VC/2017/0011). Ref. Ares(2018)1092729–27/02/2018. Luxembourg, Luxembourg: Publications Office of the European Union.
- [RPA/FoBiG] Risk and Policy Analysts/Forschungs- und Beratungsinstitut Gefahrstoffe. 2017. The cost of occupational cancer in the EU-28. Final report prepared for European Trade Union Institute (ETUI). Nov 2017. RPA/FoBiG; [accessed 2021 Dec]. https://www.etui.org/publications/reports/the-cost-of-occupational-cancer-in-the-eu-28.
- Saber AT, Poulsen SS, Hadrup N, Jacobsen NR, Vogel U. 2019. Commentary: the chronic inhalation study in rats for assessing lung cancer risk may be better than its reputation. Part Fibre Toxicol. 16(1):44.
- Samet JM, Chiu WA, Cogliano V, Jinot J, Kriebel D, Lunn RM, Beland FA, Bero L, Browne P, Fritschi L, et al. 2020. The IARC monographs: updated procedures for modern and transparent evidence synthesis in cancer hazard identification. J Natl Cancer Inst. 112(1):30–37.
- Sand S, Portier CJ, Krewski D. 2011. A signal-to-noise crossover dose as the point of departure for health risk assessment. Environ Health Perspect. 119(12):1766–1774.
- Sanner T, Dybing E, Willems MI, Kroese ED. 2001. A simple method for quantitative risk assessment of non-threshold carcinogens based on the dose descriptor T25. Pharmacol Toxicol. 88(6):331–341.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2003. Recommendation from the Scientific Committee on Occupational Exposure Limits: risk assessment for wood dust. SCOEL/SUM/102 final. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2004a. Recommendation from the Scientific Committee on Occupational Exposure Limits: risk assessment for hexavalent chromium. SCOEL/SUM/86. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2004b. Recommendation from the Scientific Committee on Occupational Exposure Limits: risk assessment for vinyl chloride. SCOEL/SUM/109 final. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2007. Recommendation from the Scientific Committee on Occupational Exposure Limits for 1,3-butadiene. SCOEL/SUM/75. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2008. Recommendation from the Scientific Committee on Occupational Exposure Limits for vinyl bromide. SCOEL/SUM/155. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2009. Recommendation from the Scientific Committee on Occupational Exposure Limits for trichloroethylene. SCOEL/SUM/142. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2010a. Recommendation from the Scientific Committee on Occupational Exposure Limits for hydrazine. SCOEL/SUM/164. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2010b. Recommendation from the Scientific Committee on Occupational Exposure Limits for propylene oxide. SCOEL/SUM/161. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2011a. Recommendation from the Scientific Committee on Occupational Exposure Limits for 1,2-dibromoethane (ethylene dibromide). SCOEL/SUM/166. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2011b. Recommendation from the Scientific Committee on Occupational Exposure Limits for epichlorohydrin. SCOEL/SUM/169. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2012a. Recommendation from the Scientific Committee on Occupational Exposure Limits for 4,4′-diaminodiphenylmethane [MDA]. SCOEL/SUM/107. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2012b. Recommendation from the Scientific Committee on Occupational Exposure Limits for acrylamide. SCOEL/SUM/139. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2012c. Recommendation from the Scientific Committee on Occupational Exposure Limits for ethylene oxide. SCOEL/SUM/160. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2013a. Methodology for the derivation of occupational exposure limits. Key documentation (version 7), June 2013. Scientific Committee on Occupational Exposure Limits. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2013b. Recommendation from the Scientific Committee on Occupational Exposure Limits for 4,4′-methylene-bis-(2-chloroaniline) [MOCA]. SCOEL/SUM/174. June 2010/Annex March 2013. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2016. Recommendation from the Scientific Committee on Occupational Exposure Limits for 1,2-dichloroethane (ethylene dichloride). SCOEL/REC/302. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2017a. Methodology for derivation of occupational exposure limits of chemical agents. The general decision-making framework of the Scientific Committee on Occupational Exposure Limits (SCOEL). Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2017b. Opinion from the Scientific Committee on Occupational Exposure Limits for diesel engine exhaust. SCOEL/OPIN/403. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2017c. Recommendation from the Scientific Committee on Occupational Exposure Limits for 2-nitropropane. SCOEL/REC/300. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2017d. Recommendation from the Scientific Committee on Occupational Exposure Limits for chromium VI compounds. SCOEL/REC/386. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2017e. Recommendation from the Scientific Committee on Occupational Exposure Limits for hydrazine. SCOEL/REC/164. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SCOEL] Scientific Committee on Occupational Exposure Limits. 2017f. Recommendation from the Scientific Committee on Occupational Exposure Limits for o-toluidine, 2-methylaniline. SCOEL/REC/301. Luxembourg, Luxembourg: European Commission, Employment, Social Affairs and Inclusion.
- [SER] Sociaal-Economische Raad. 2020. Working together on the future of the limit values for carcinogens in Europe. European conference on occupational exposure limit (OELs) values for carcinogens, 10–11 February, 2020, The Hague, Netherlands. The Hague, Netherlands: SER (Social and Economic Council).
- [SER] Sociaal-Economische Raad. 2021. Towards a harmonized risk-based approach for OELs in the EU for carcinogens without a threshold. The Hague, Netherlands: SER (Social and Economic Council).
- Shi Q, Godschalk RWL, van Schooten FJ. 2017. Inflammation and the chemical carcinogen benzo[a]pyrene: partners in crime. Mutat Res Rev Mutat Res. 774:12–24.
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert PF, et al. 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. 124(6):713–721.
- Solano ML, Rocha NS, Barbisan LF, Franchi CA, Spinardi-Barbisan AL, de Oliveira ML, Salvadori DM, Ribeiro LR, de Camargo JL. 2016. Alternative multiorgan initiation-promotion assay for chemical carcinogenesis in the Wistar rat. Toxicol Pathol. 44(8):1146–1159.
- Supek F, Lehner B. 2017. Clustered mutation signatures reveal that error-prone DNA repair targets mutations to active genes. Cell. 170(3):534–547.e23.
- [SZM] Ministerie van Sociale Zaken en Werkgelegenheid. 1997. Besluit van 15 januari 1997, houdende regels in het belang van de veiligheid, de gezondheid en het welzijn in verband met de arbeid (Arbeidsomstandighedenbesluit) [Decree of 15 January 1997, containing rules in the interest of safety, health and well-being in connection with work (Working Conditions Decree)]. Dutch: Staatsblad.
- Taxell P, Santonen T. 2016. The Nordic Expert Group for criteria documentation of health risks from chemicals and the Dutch Expert Committee on Occupational Safety. 149. Diesel engine exhaust. Vol. 49. Gothenburg, Sweden: University of Gothenburg. Arbete och Hälsa; p. 6.
- The Lancet Oncology. 2016. When is a carcinogen not a carcinogen? Lancet Oncol. 17(6):681.
- Tomasetti C, Li L, Vogelstein B. 2017. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 355(6331):1330–1334.
- Tubbs A, Nussenzweig A. 2017. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 168(4):644–656.
- [UN] United Nations. 2019. Globally harmonized system of classification and labelling of chemicals (GHS). 8th ed. New York: UN.
- [US EPA] US Environmental Protection Agency. 2005. Guidelines for carcinogen risk assessment. Risk Assessment Forum. EPA/630/P-03/001F. Washington (DC): US EPA.
- [US EPA] US Environmental Protection Agency. 2012. Benchmark dose technical guidance. Risk Assessment Forum. EPA/100/R-12/001 June 2012. Washington (DC): US EPA.
- [US EPA] US Environmental Protection Agency. 2020. Terms & acronyms: qualitative risk assessment. Washington (DC): US EPA.
- Valdez-Flores C, Sielken RL Jr, Teta MJ. 2011. Quantitative cancer risk assessment for ethylene oxide inhalation in occupational settings. Arch Toxicol. 85(10):1189–1193.
- Vermeulen R, Silverman DT, Garshick E, Vlaanderen J, Portengen L, Steenland K. 2014. Exposure–response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environ Health Perspect. 122(2):172–177.
- [WHO] World Health Organization. 2001. Chapter 10: acceptable risks by Hunter PR, Fewtrell L. In: Fewtrell L, Bartram J, editors. Water quality. Guidelines, standards and health: assessment of risk and risk management for water-related infectious disease. London (UK): IWA Publishing; p. 207–228.
- [WHO/IPCS] World Health Organization/International Programme on Chemical Safety. 2009 (updated 2020). Principles and methods for the risk assessment of chemicals in food. Environmental health criteria 240. A joint publication of the Food and Agriculture Organization of the United Nations and the World Health Organization. Geneva, Switzerland: WHO/IPCS.
- Wojcik A, Harms-Ringdahl M. 2019. Radiation protection biology then and now. Int J Radiat Biol. 95(7):841–850.
- Wu R, Högberg J, Adner M, Ramos-Ramírez P, Stenius U, Zheng H. 2020. Crystalline silica particles cause rapid NLRP3-dependent mitochondrial depolarization and DNA damage in airway epithelial cells. Part Fibre Toxicol. 17(1):39.
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, et al. 2018. Senolytics improve physical function and increase lifespan in old age. Nat Med. 24(8):1246–1256.
- Xue J, Patergnani S, Giorgi C, Suarez J, Goto K, Bononi A, Tanji M, Novelli F, Pastorino S, Xu R, et al. 2020. Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy. Proc Natl Acad Sci U S A. 117(41):25543–25552.
- Yamashita S, Kishino T, Takahashi T, Shimazu T, Charvat H, Kakugawa Y, Nakajima T, Lee YC, Iida N, Maeda M, et al. 2018. Genetic and epigenetic alterations in normal tissues have differential impacts on cancer risk among tissues. Proc Natl Acad Sci U S A. 115(6):1328–1333.
- Yizhak K, Aguet F, Kim J, Hess JM, Kübler K, Grimsby J, Frazer R, Zhang H, Haradhvala NJ, Rosebrock D, et al. 2019. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science. 364(6444):eaaw0726.
- Zheng F, Gonçalves FM, Abiko Y, Li H, Kumagai Y, Aschner M. 2020. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 34:101475.