Abstract
An association between exposure to arsenic (As) and neurologic and behavioral effects has been reported in some studies, but no systematic review is available of the evidence linking As in drinking water and neurobehavioral effects after consideration of study quality and potential confounding, with focus on low-level circumstances of exposure. We conducted a systematic review and reported it in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, through a search of the databases PubMed, Web of Science, Scopus, and Embase. We included in the review the studies reporting results based on exposure from drinking water in humans. Endpoints were heterogeneous across studies, so we classified them into eight broad domains and developed an ad-hoc system to evaluate their methodological quality, based on three tiers. It was not possible to conduct meta-analysis because of the heterogeneity in exposure assessment and in the definition and assessment of outcomes. The search identified 18,518 articles. After elimination of duplicates and irrelevant articles, we retained 106 articles which reported results on As exposure and neurobehavioral effects, of which 22 reported risk estimates from exposure in drinking water (six among adults and 16 among children). None of the studies was conducted blindly. Among the studies in adults, two, which were conducted in highly exposed populations, were classified as high quality. These two studies were broadly consistent in reporting an association between exposure to As and decline in cognitive function; however, they provide no evidence of an association for exposure below 75 μg/L. The four lower-quality studies were based on populations with low exposure; these studies reported associations with inconsistent outcomes, few of which remained statistically significant after adjustment for multiple comparisons. Among the five high-quality studies of children, one reported an association between As in drinking water and intellectual function, whereas none of the other studies reported an association with different neurobehavioral indicators, after adjusting for potential confounders and multiple comparisons. Out of seven intermediate-quality studies, three reported an association with cognitive function or other outcomes; but sources of bias were not adequately controlled. The remaining studies were negative. The four low-quality studies did not contribute to the overall evidence because of methodological limitations. Our assessment of the available literature showed a lack of evidence for a causal association between exposure to As in drinking water and neurobehavioral effects. To clarify whether such an association exists, further studies prospectively evaluating changes in both the concentration of As in drinking water during the life course, and neurobehavioral outcomes, as well as appropriately controlling for potential confounders, are needed.
Introduction
Arsenic (As) is a metalloid naturally occurring in the environment. It is found in different valences (mostly +3 and +5) and in various organic and inorganic compounds, with the inorganic showing the higher toxicity for humans.
Exposure to high levels of inorganic As has been associated with cancer of lung, bladder, and skin (IARC Citation2012; Boffetta & Borron Citation2019). As has also been suggested to be associated with non-cancer adverse health outcomes, including cardiovascular (Moon et al. Citation2017), respiratory diseases (Sanchez et al. Citation2016, Citation2018), diabetes (Wang et al. Citation2014), and neurobehavioral effects (Mochizuki Citation2019; Tsai et al. Citation2003; Tolins et al. Citation2014; IOM Citation2015). A value of 10 μg of inorganic As/L in drinking water has been established by the World Health Organization for the cut off, based on inorganic As toxicity (WHO Citation2022).
As is widespread in the earth crust, from which it is distributed to the soil, water and air (IARC Citation2012). The main source of exposure to As for the general population is groundwater-derived drinking water, which contains mainly the inorganic form of As and primarily pentavalent (AsV, i.e. arsenate) (IARC Citation2012; Fatoki and Badmus Citation2022).
High levels of naturally occurring As are found in groundwater in a number of countries around the world (Khosravi-Darani et al. Citation2022). About 94–220 million people around the world are potentially exposed to high levels of As from groundwater, according to a recent study using a statistical learning approach aimed at modeling geogenic groundwater As concentrations, and based on estimates from nearly 80 previous reports. Most of these populations are located in South Asia, but additional areas, previously not identified, may be affected by groundwater As levels that are higher than the WHO cutoff level (Podgorski and Berg Citation2020). Studies on possible effects of As outcomes should therefore focus on exposure to As in drinking water.
3A number of systematic reviews were previously published on the possible effect of As on neurobehavioral effects (e.g. Brinkel et al. Citation2009; Dong and Su Citation2009; McClintock et al. Citation2012; Rodríguez-Barranco et al. Citation2013; Tsuji et al. Citation2015; Saghazadeh and Rezaei Citation2017; Wang et al. Citation2019; Khan et al. Citation2020; Hasanvand et al. Citation2020; Heng et al. Citation2022; Shiani et al. Citation2023; Li et al. Citation2023). However, most of these reviews relied on studies which did not measure direct exposure of As in drinking water, did not include an explicit assessment of the quality of the underlying studies and did not consider potential confounding. In addition, some of the reviews were restricted to specific world regions or countries (Brinkel et al. Citation2009; Dong and Su Citation2009; McClintock et al. Citation2012; Khan et al. Citation2020; Heng et al. Citation2022).
The aim of this study is to find out whether there is an association between exposure to As and neurobehavioral functions, with a focus on low-level exposure to As. For that purpose, we systematically summarize current literature on exposure to As from drinking water and neurobehavioral effects in both children and adults, taking into account study quality and potential residual confounding, and including a formal assessment of the strength of the evidence.
To our knowledge, our study is the first attempt to comprehensively assess the association between exposure to As in drinking water specifically, and broadly defined neurobehavioral effects, among both children and adults. Two previous meta-analyses that focused on exposure to As in drinking water (Rodríguez-Barranco et al. Citation2013; Hasanvand et al. Citation2020) considered only intelligence quotient (IQ) scores.
Materials and methods
Literature review methodology
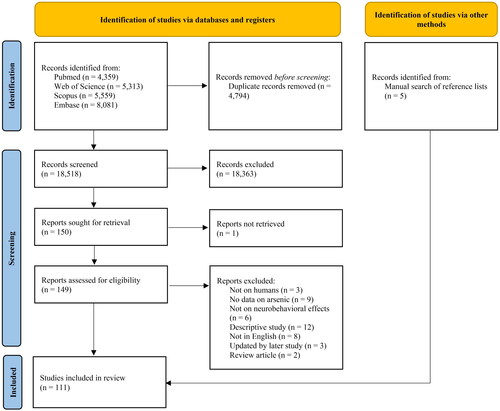
We conducted a systematic review and reported it herein based on the guidelines for Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) (Page et al. Citation2021) ( in Appendix). On 1 February 2022, a search was conducted in the databases PubMed, Web of Science, Scopus, and Embase, using the search string ((neurodevelopment* OR behavior OR behavior OR mental OR intelligence OR cognitive OR “attention deficit disorder with hyperactivity” OR ADHD) AND arsenic). Studies conducted in animals were excluded. This resulted in the identification of 18,518 articles. After excluding duplicates, we reviewed the title, abstract, and full text of the remaining articles and identified 106 English-written independent studies which reported results of human studies on the association between As exposure and one or more neurobehavioral effects. Furthermore, we carried out a manual search of reference lists of included articles and previous reviews, that allowed us to retrieve 5 additional studies, for a total number of 111 studies included in the review. The selection of the studies is described in a flowchart (). The list of studies retained for in depth assessment is included in .
Data extraction and synthesis of results
We used a standard form to abstract data on year of publication, parent study (if relevant), period of data collection or recruitment, country, study base, type of population (children [≤ 18 years], or adults), sex distribution, age (mean, median, range), sample size, source of As exposure, method for assessment of As exposure, As concentration (mean, median, categories), factors adjusted for in the analysis, outcomes, and detailed results. Emphasis was given to results among populations exposed to low-level (< 100 µg/L) As exposure in drinking water.
We classified the studies according to the source of As exposure for which results were reported, and reviewed in detail the studies reporting results based on exposure from drinking water. We reviewed and discussed the result of these studies independently, and drew our own conclusions regardless of the conclusions made by the original authors.
We developed an ad-hoc system to evaluate the methodological quality of each study. Specifically, we evaluated how exposure was measured and reported, what confounders were considered, and whether bias was avoided. The rationale for using an ad-hoc system for the assessment of methodological quality was represented by the use of an evaluation system specifically thought and adapted to this field of scientific research (i.e. As and neurobehavioral effects), in an effort to provide a more detailed evaluation of sources of bias compared with that possible with available and commonly used scales for quality assessment, which usually have instead a general scope. Based on the quality, we sorted the articles by three tiers. Tier I includes studies with individual assessment of water As exposure, typically based on measurements of samples taken from the place of residence of subjects, use of standard instruments for outcome assessment, adjustment for main confounders, in particular indicators of socioeconomic status, and lack of other sources of bias. Tier II studies lack one or two of the following: (i) individual water As exposure, (ii) use of standard instruments for outcome assessment, (iii) adjustment for indicators of socioeconomic status and other important confounders, and (iv) lack of evidence of other sources of bias. Studies lacking three of four of these aspects were classified in Tier III. Tier III also includes studies with major methodological limitations, such as lack of adjustment for any confounder or high proportion of missing values, and studies for which key details of the design (e.g. As exposure level, instruments used for outcome or confounder assessment) are not available.
Finally, we applied the GRADE framework to assess the quality of such evidence from the studies both in adults and in children (Balshem et al. Citation2011).
The studies retained in the review included over seventy heterogenous tests for neurobehavioral outcomes. To be able to make comparisons and draw conclusions from the studies, we classified them into eight broad domains: (1) global cognitive functioning, (2) attention, (3) executive functioning, (4) language, (5) visuospatial functioning, (6) memory, (7) behavior, and (8) motor skills. Details on this classification are reported in .
It was not possible to conduct meta-analysis because of the heterogeneity in exposure assessment and in the definition and assessment of outcomes. In the review of each study, we included in square brackets our comments and re-analyses of original data, which were based on linear regression modeling of aggregate results reported in the original manuscripts.
Results
The distribution of studies identified in the literature search, according to source of exposure to As, is presented in . A total of 23 studies based on exposure to As from drinking water, published between 2003 and 2019, were included in the review. Out of the 23 studies, one (Desai et al. Citation2020) reported results on the correlation between water and urine As concentrations but no results on the outcome according to water concentration, and was excluded. All included studies had a cross-sectional design; selected characteristics are summarized in .
Table 1. Distribution of studies selected for review, by source of arsenic exposure.
Table 2. Main characteristics of the studies included in detailed review.
Several studies were conducted in the same populations (e.g. the FRONTIER Project: Gong et al. Citation2011; O’Bryant et al. Citation2011; Edwards, Hall, et al. Citation2014; Edwards, Johnson, et al. Citation2014. The Health Effects of Arsenic Longtitudinal Study (HEALS): Wasserman et al. Citation2004, Citation2007; Khan et al. Citation2011; Parvez et al. Citation2011; Khan et al. Citation2012). They were all included in the review because their results appeared to be independent.
Nine of the 22 studies (41%) were conducted in Bangladesh (Wasserman et al. Citation2004, Citation2007; Khan et al. Citation2011; Asadullah and Chaudhury Citation2011; Parvez et al. Citation2011; Khan et al. Citation2012; Nahar, Inaoka, Fujimura, Watanabe, et al. Citation2014; Rodrigues et al. Citation2016; Karim et al. Citation2019), five (23%) in the USA (Gong et al. Citation2011; O’Bryant et al. Citation2011; Edwards, Hall, et al. Citation2014; Wasserman et al. Citation2014; Edwards, Johnson, et al. Citation2014), three (14%) in India (Von Ehrenstein et al. Citation2007; Ghosh et al. Citation2017; Manju et al. Citation2017), three (14%) in China and Taiwan (Tsai et al. Citation2003; Wang et al. Citation2007; Liu et al. Citation2017), and two (9%) in other countries (one each in Mexico (Rocha-Amador et al. Citation2007) and Pakistan (Abbas et al. Citation2012)).
Six of the studies were conducted in adults (Gong et al. Citation2011; O’Bryant et al. Citation2011; Edwards, Hall, et al. Citation2014; Edwards, Johnson, et al. Citation2014; Liu et al. Citation2017; Karim et al. Citation2019), and 16 studies were conducted in children (Tsai et al. Citation2003; Wasserman et al. Citation2004; Rocha-Amador et al. Citation2007; Von Ehrenstein et al. Citation2007; Wasserman et al. Citation2007; Wang et al. Citation2007; Khan et al. Citation2011; Asadullah and Chaudhury Citation2011; Parvez et al. Citation2011; Abbas et al. Citation2012; Khan et al. Citation2012; Wasserman et al. Citation2014; Nahar, Inaoka, Fujimura, Watanabe, et al. Citation2014; Rodrigues et al. Citation2016; Ghosh et al. Citation2017; Manju et al. Citation2017).
Studies in adults – review
Tier I studies
Liu et al. (Citation2017) investigated the relationship between water As concentration and cognitive impairment among 483 subjects aged 40 or older from Shanxi and Jilin provinces of China using a Chinese version of the Mini-Mental State Examination (MMSE). The subjects were divided into four exposure groups, with mean levels, based on samples collected from individual homes, of 4 ± 2 μg/L, 25 ± 11 μg/L, 73 ± 15 μg/L, and 183 ± 88 μg/L. A total of 148 subjects were classified as cognitively impaired (no details provided). Compared to the category with lowest As concentration, the odds ratio (OR) of cognitive impairment, adjusted for sex, age, learning level, smoking status, alcohol consumption, body mass index (BMI), and marital status, were 1.11 (95% confidence interval [CI] 1.01–1.43), 1.32 (95% CI 1.21–2.75), and 4.01 (95% CI 2.77–11.03) in the categories with increasing As concentration. [The unadjusted OR for an increase of 10 μg/L As concentration, calculated based on the data reported in the article, was 1.07 (95% CI 1.03–1.11). No adjustment was made for factors associated with socioeconomic status other than education.]
Karim et al. (Citation2019) examined the association between As exposure in drinking water and adult cognitive impairment using the Bangla version of the MMSE and the serum levels of brain-derived neurotrophic factor (BDNF), a potential biomarker of cognitive impairment. The cross-sectional study included 693 adult (18–60 years old) subjects from non-endemic (N = 169) and endemic (N = 524) areas of As exposure in rural Bangladesh. Average MMSE score and serum BDNF level were lower in subjects from As-endemic area than in subjects from non-endemic area. In regression analyses, after adjustment for age, sex, BMI, education, smoking and income, the coefficient for a log(10) increase in As concentration was −0.021 (95% CI −0.026, −0.015) for MMSE score and −0.082 (95% CI −0.105, −0.059) for BDNF level. Corresponding results for a quartile increase were −0.113 (95% CI −0.161, −0.064) for MMSE score and −0.437 (95% CI −0.609, −0.265) for BDNF level. Similar results were obtained after excluding subjects from the non-endemic area.
Tier II studies
O’Bryant et al. (Citation2011) examined the association between current and cumulative As exposure from drinking water and detailed neuropsychological functioning in 434 adults and elders from two rural counties in Western Texas (FRONTIER Study). As concentration was estimated based on place of residence and statewide As data. Average As concentration in the two counties was 3.0 μg/L and 7.4 μg/L, the maximum estimated concentration was 15.6 μg/L. Long-term As exposure was calculated based on concentration at the current address multiplied by the years of residence. Neuropsychological tests included the MMSE, the Executive Interview (EXIT25), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), separately for immediate memory, visuospatial, language, attention, and delayed memory functions, the Trails Making Test (TMTA and TMTB), the Controlled Oral Word Association Test (COWAT), including both phonemic (FAS) and categorical (Animal Naming) verbal fluency, and the Clock Drawing Task (CLOX1 and 2). In regression analyses adjusted for age, gender, education, language of administration, selenium, and the presence of APOE4 (a gene which increases Alzheimer’s risk), long-term As exposure (scale unspecified, possibly 1 μg/L) was associated with lower scores in the following indicators: MMSE (coefficient = −0.003, standard error [SE] 0.001, p = 0.004), CLOX2 (coefficient = −0.001, SE 0.001, p = 0.038), FAS B (coefficient = −0.012, SE 0.004, p = 0.002), BANS language (coefficient = −0.005, SE 0.002, p = 0.017), TMTA (coefficient = 0.034, SE 0.014, p = 0.016), EXIT25 (coefficient = 0.006, SE 0.002, p < 0.001), and RBANS immediate memory (coefficient = −0.010, SE 0.003, p = 0.003). Current As concentration was associated with CLOX1 (coefficient = −0.225, SE 0.0080, p = 0.005). [Limitations of this study include confusing reporting of key aspects of the design, and the lack of adjustment for potential confounders, in particular those related to socioeconomic status, with the exception of education. Multiple comparisons were not taken into account (if a Bonferroni correction is applied, only the association between cumulative As exposure and EXIT25 score would remain statistically significant. The correlation between different measures of cognitive function (and therefore their potential reciprocal confounding) is not reported or discussed. The discrepancy in results between current and long-term exposure was not explained. No details are reported on the timing of measurement of As in groundwater and that of administration of the tests.]
Edwards, Hall, et al. (Citation2014) conducted an analysis of 527 subjects included in the FRONTIER Study. Exposure to As in drinking water was negatively associated with language (p < 0.001) and executive functioning (EXIT25; p < 0.001). These associations remained statistically significant after accounting for multiple comparisons using Bonferroni correction. No association was detected with immediate memory (p = 0.60), visuospatial skills (p = 0.05), attention skills (p = 0.09), and delayed memory index (p = 0.06), RBANS total index (p = 0.39) and MMSE (p = 0.07). These associations were stronger among those with the AA genotype of the AS3MT gene, while among those with GG genotype, As levels were positively associated with visuospatial functioning. [The overlap between this study and those by O’Bryant et al. (Citation2011) and Gong et al. (Citation2011) is unclear; stratification by AS3MT genotype resulted in a large number of comparisons which were not accounted for. The correlation between different measures of cognitive function (and therefore their potential reciprocal confounding) was not reported or discussed. The authors did not adjust for several potential confounders, in particular those related to socioeconomic status, with the exception of education. Exposure relied on geographic information systems (GIS) data and was not assessed at individual level. Overall, this study does not provide evidence independent from that of the study by O’Bryant et al. (Citation2011).]
Edwards, Johnson, et al. (Citation2014) conducted a cross-sectional study to investigate the association between As groundwater levels and neuropsychological function in 733 subjects with Alzheimer’s disease, 127 subjects with Mild Cognitive Impairment, and 530 subjects with normal cognition from the Texas Alzheimer’s Research and Care Consortium (TARCC) study. GIS analyses were used to estimate regional-specific groundwater As concentrations. Results were adjusted for age, gender, education, obesity, hyperlipidemia, hypertension, diabetes, and selenium level. In the full study population, As concentrations were positively associated with language abilities (p = 0.008) and memory (verbal immediate p = 0.008, verbal delayed p < 0.001, visual immediate p = 0.02, and visual delayed p < 0.001). In subjects with normal cognition, As concentration was positively associated with MMSE (p = 0.03). [The study included a large number of comparisons: after taking into account multiple comparisons using the Bonferroni method, the only association that remained significant was between As exposure and Wechsler Memory Scale–Third Edition (WMS-III) Visual Reproduction (VR) II in patients with Alzheimer’s disease. The correlation between different measures of cognitive function (and therefore their potential reciprocal confounding) is not reported or discussed. No adjustment was made for factors associated with socioeconomic status other than education.]
Tier III studies
Gong et al. (Citation2011) conducted analysis of 299 participants from the FRONTIER Study. These authors compared Folstein MMSE score between subjects with As concentration up to 10 μg/L and those with concentration 10–11 μg/L and higher than 11 μg/L. There was a difference between unadjusted means of low and intermediate concentration groups and between the low and high concentration groups (p = 0.03). [Results were not adjusted for any potential confounder, including age or sex. No details were reported on key aspects of the design of the study. The overlap with the other analyses of the FRONTIER study (O’Bryant et al. Citation2011; Edwards, Hall, et al. Citation2014) is unclear.]
Studies in adults – GRADE evaluation
The results from studies in adults are too sparse for an evaluation under the GRADE framework.
Studies in children – review
Tier 1 studies
Wasserman et al. (Citation2004) conducted a cross-sectional study of intellectual function in 201 children at the age of 10 years from Araihazar, a rural area of Bangladesh as part of the HEALS study (Ahsan et al. Citation2006). Median As exposure was 118 µg/L. Tube wells at each child’s home were sampled and analyzed for water concentrations of As and manganese. Children’s intellectual function on tests drawn from the Wechsler Intelligence Scale for Children version III (WISC-III), was assessed by summing weighted items across domains to create Verbal, Performance, and Full-Scale raw scores. After adjustment for maternal education, maternal intelligence, house type, television access, height, and head circumference, the resulting associations with water As concentration were:
Full-scale intellectual function: coefficient for one log unit of As concentration = –1.64, p < 0.01.
performance score: coefficient = −1.45, p < 0.001.
verbal score: coefficient = –0.19, p > 0.05.
In a log-linear regression model, the authors estimated decrease of −3.8 score in the full scale at 10 μg/L As, and −6.4 score at 50 μg/L, compared to unexposed. [The rationale for the choice of a log-linear regression model is unclear.]
Von Ehrenstein et al. (Citation2007) conducted a cross-sectional study of 351 children aged 5 to 15 years from West Bengal, India, in 2001–2003. Lifetime exposure to As in drinking water was assessed using analyses of urinary samples as well as samples from 409 wells, and was categorized in tertiles. Intellectual function was assessed with 6 subtests from the WISC for Children as well as with the Total Sentence Recall test, the Colored Progressive Matrices test, and a pegboard test. Adjustment was done for age, sex, BMI and mother’s age, maternal and paternal education, father’s occupation, number of rooms in the house, type of house-building material. After adjustment, no association was found for any of the 10 tests used in the study, for peak As concentration during life or for average As concentration during pregnancy, with exposure categorized in tertiles. In a continuous analysis, the coefficients of the linear regressions for an increase in 100 μg/L (both sources of exposure) ranged from −0.03 to 0.02 in both analyses, none being significantly different from 0.
Wasserman et al. (Citation2007) replicated the study by Wasserman et al. (Citation2004) on 301 6-year old children, using the Wechsler Preschool and Primary Scale of Intelligence III. As exposure categories were defined as quartiles: 0.1–20.9; 21–77.9; 78–184.9; 185–864 µg/L. The coefficient for one log unit of As concentration was −0.48 (95% CI −0.95, −0.01) for Performance score, −0.18 (95% CI −0.72, 0.37) for Verbal score, −0.54 (95% CI −1.09, 0.01) for Processing Speed score, and −1.06 (95% CI −2.18, 0.06) for Full scale. [None of the regression coefficient is statistically significant after adjustment for multiple comparisons using the Bonferroni method.]
Parvez et al. (Citation2011) investigated the association between water As concentration and motor function in 303 children in Bangladesh, 8–11 years of age, of whom 151 were from a low-exposure area (mean As concentration, 2.7 μg/L) and 152 from a high exposure area (mean 83.5 μg/L). Participants were children of members of the HEALS cohort study. Motor function was assessed using the Bruininks-Oseretsky test version 2 (Bruininks and Bruininks Citation2005), in four subscales: fine manual control (FMC), manual coordination (MC), body coordination (BC), and strength and agility (SA)—which can be summarized with a total motor composite score (TMC). Adjustment was done for sex, school attendance, head circumference, mother’s intelligence score, blood lead, selenium, and manganese. After the adjustment, the regression coefficients for one unit of log-transformed As level were −0.54 (95% CI −1.03, −0.05) for FMC, −0.15 (95% CI −0.52, 0.30) for MC, −0.43 (95% CI −0.77, −0.06) for BC, −0.11 (95% CI −0.28, 0.18) for SA, and −1.18 (95% CI −2.13, −0.10) for TMC. [None of the regression coefficient was statistically significant after adjustment for multiple comparisons based on Bonferroni method.]
Rodrigues et al. (Citation2016) investigated 524 children who were enrolled in a prospective birth cohort established to study the effects of prenatal and early childhood As exposure in the Sirajdikhan and Pabna districts of Bangladesh. Water was collected from the family’s primary drinking source during the first trimester of pregnancy and at ages 1, 12, and 20–40 months. As levels were highly correlated within each child, and for this analysis levels measured in the samples collected at 20-40 months were used. At age 20–40 months, neurodevelopmental outcomes (cognitive and fine motor functions) were assessed using an adapted version of the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III). Median water As concentrations were lower in Sirajdikhan than Pabna (1.5 vs 25.7 μg/L, p < 0.0001). After adjustment for maternal age, maternal education, child’s gender, exposure to secondhand smoke, home score, maternal Raven score, and child hematocrit levels, increased water As level was not associated with decreased cognitive scores either in Pabna (coefficient for one log unit increase in As level = −0.06, SE = 0.03, p = 0.05) or in Sirajdikhan (coefficient −0.002, SE = 0.02, p = 0.93). No association was detected with fine motor score either in Sirajdikhan (coefficient = −0.05, SE = 0.03, p = 0.09) or in Pabna (coefficient = 0.02, SE = 0.03, p = 0.48).
Tier II studies
Tsai et al. (Citation2003) studied 49 junior school students from Taiwan (average age 13.5 years) exposed to As from well water and 60 controls matched on age, sex, education, body weight, height, BMI, and socioeconomic status. Exposed students were divided into two groups, with the 29 students in the high exposure group exposed to 185 ± 225 ppb As in well water and the 20 students in the low exposure exposed to 131 ± 344 ppb As in well water. Four neurobehavioral tests (continuous performance test, symbol digit, pattern memory, and switching attention) were applied: continuous performance was associated with low exposure (p = 0.001); switching attention was associated with high and low exposure (p < 0.0001); symbol digit showed no associations and pattern memory was associated with high exposure (p = 0.003). [Matching variables were not adjusted for in the analysis, generating potential confounding.]
Rocha-Amador et al. (Citation2007) studied the association between exposure to As in drinking water and intelligence using the Revised Mexican Version of Wechsler Intelligence Scale (WISC-RM) in 132 children aged 6–10 years from three rural communities in Mexico. Water samples and urine samples were collected in each child’s home. The average As concentrations in the drinking water from the three communities were 5.8 ± 1.3 μg/L, 169 ± 0.9 μg/L, and 194 ± 1.3 μg/L (Rocha-Amador et al. Citation2007). After adjustment for blood lead, mother education, socioeconomic status, height-for-age, and transferrin saturation, the correlation coefficients between As concentration and Global Cognitive Function were −4.30 (p < 0.01) for Performance IQ, −6.40 (p < 0.01) for Verbal IQ and −6.15 (p < 0.01) for Full IQ. [Socioeconomic status and mother education were inversely correlated with As concentration. Age of children was not adjusted for in the analysis.]
Asadullah and Chaudhury (Citation2011) examined 7710 grade 8 students from 316 school across Bangladesh (Asadullah and Chaudhury Citation2011). Exposure to As was based on participants reporting whether their household well was contaminated (with threshold at 50 μg/L, 12% of students reported use of contaminated well). Scores of two mathematics tests were used as outcome, one mathematics competence for primary school and one for secondary school. After adjustment for age, sex, father’s and mother’s education, availability of appliances in the household, home construction material, and travel time to school from home, the regression coefficient for the primary school score was −0.085 (p = 0.01), that for the secondary school score was −0.044 (p = 0.08). [The approach for exposure assessment is prone to misclassification.]
Khan et al. (Citation2011) conducted an analysis of behavior of a subset of 201 children out of 304 children who participated in the HEALS study conducted in Araihazar, a rural area of Bangladesh studied by Wasserman et al. (Citation2004; Citation2007). Average As concentration was 43.7 ± 67.0 μg/L, with median of 14.0 and range of 0.0–371.1 μg/L. Participation rate was 66%. Children were rated by their school teachers on externalizing and internalizing items of classroom behavior using the standardized Child Behavior Checklist-Teacher’s Report Form (CBCL-TRF). Water As concentration was not associated with CBCL-TRF score: the coefficients of regression for one log unit of water As concentration, adjusted for water Mn concentration, sex, maternal education, arm circumference, and BMI, and controlled for within-teacher correlations in rating the children, were −0.29 (95% CI −0.65, 0.07, p = 0.12) for TRF internalizing score, −0.45 (95% CI −1.62, 0.73, p = 0.46) for TRF externalizing score, and −0.78 (95% CI −2.18, 0.62, p = 0.28) for total score. [The assessment of outcome was not blind and may result in bias.]
Khan et al. (Citation2012) conducted a cross-sectional study in Araihazar, a rural area of Bangladesh as part of the HEALS (Ahsan et al. Citation2006), 840 children aged 8–11 agreed to participate in this study to investigate the association between water As concentration and academic achievement in mathematics and languages. These children were included in a longitudinal study of school-based educational intervention, in which As concentrations in drinking water was measured (average 119 μg/L, SD 147.5). Annual scores in languages (Bangla and English) and mathematics were obtained from the schools’ records. Water As concentration was not associated with any of the scores of three academic achievement tests, after adjusting for water manganese concentration, school-grade, maternal education, paternal education, head circumference, and within-teacher correlations in rating the children. The coefficients in the regression model based on dichotomized As concentration (details not provided) were: Bangla language: −1.71 (95% CI −4.77, 1.34); English language: −0.73 (9%% CI −4.32, 2.86), and Mathematics: 0.56 (95% CI −2.98, 4.10).
Wasserman et al. (Citation2014) studied the association between As in drinking water and intelligence, using the WISC-IV scale, in 272 children in grades 3–5 from three Maine, US school districts (average exposure 9.9 μg/L). Adjustment was done for maternal IQ and education, home environment, school district, and number of siblings, and for multiple comparisons based on the Bonferroni method. After the adjustment, exposure to water As concentration between 5 and 10 μg/L was associated with Full Scale IQ, but not with Working memory, Perceptual reasoning, Verbal comprehension, or Processing speed, compared exposure to concentration below 5 μg/L. In the regression analyses comparing 10–20 and >20 μg/L vs. <5 μg/L As in water, the associations became weaker and non-significant.
Nahar, Inaoka, Fujimura, Watanabe, et al. (Citation2014) investigated the relationship between As exposure and IQ (measured using Raven’s Standard Progressive Matrices) and social competence (SC, measured using the Texas Social Behavior Inventory, Form A) in 213 adolescents aged 14–15 years recruited in Sonargaonthana, Bangladesh. Subjects were classified according to As concentration in drinking water (0.8–10, 10–50, 50–100, and >100 μg/L). IQ percentile levels were 52.2, 43.4, 44.0, and 40.7 in the four As categories. This corresponded to −0.88 percentile score per 10 μg/L increase in As concentration (95% CI −0.84, −0.93, p < 0.001), calculated based on the raw numbers reported in the article. Corresponding values for SC total score were 38.6, 37.6, 36.1, and 35.9; corresponding to −0.23 score value per 10 μg/L increase (95% CI −0.22, −0.24). The authors state that differences between the four As groups persisted for IQ after adjustment for socioeconomic factors, including parental education, occupation, and income, while the differences for SC were no longer present. [Limited details are provided on multivariate adjustment, which hampers the interpretation of the results.]
Tier III studies
Wang et al. (Citation2007) studied 720 children aged 8–12 years from rural villages in Shanxi province, China. The children were exposed to As in drinking water at concentrations of 142 ± 106 μg/L (medium-As group, N = 253) and 190 ± 183 μg/L (high-As group, N = 91), compared with a control group that was exposed to low concentrations of As (2 ± 3 μg/L, N = 196). A standardized IQ test was modified for children in rural China, based on the classic Raven Progressive Matrices test. The mean IQ scores were 104.8 ± 14.7 in the control group, 100.6 ± 15.6 in the medium-As group, and 95.1 ± 16.6 in the high-As group. [No potential confounders were accounted for in this study, all analyses were based on grouped data.]
Abbas et al. (Citation2012) compared the intellectual score, measured using Raven Progressive Matrices, in children and adults from 3 areas with As in drinking water and one control area from Pakistan (number of subjects not reported). The concentrations of As in the drinking water was above permissible level (not specified) in 35%, 47%, and 56% of the water used by subjects in the exposed areas. The total score was similar in the four groups (average total score, 26 in the control area, and 22–23 in the exposed areas). [The data provided do not allow to test for the statistical significance of the differences, not to fit a regression model to estimate the dose-response relationship between As in drinking water and intellectual function score. Multiple aspects of the design of the study are not provided, complicating the interpretation of the results.]
Ghosh et al. (Citation2017) conducted a cross-sectional study in an As contaminated area in West Bengal, India using the Raven Progressive Matrices to estimate IQ and a Memory Power test administered by teachers. Total of 142 children were selected for the study, including 114 children aged 9–11, living in the area with high exposure for at least 5 years, and 28 children with low exposure. Water samples were collected and analyzed. The average As concentration was 50.6 μg/L in the high exposure group and 6 μg/L in the low exposure group. Adjustment was done for sex, urinary As, water manganese, water iron, BMI, and head circumference. After the adjustment, the regression coefficients for an increase of 10 μg/L As concentration in drinking water were −3.08 (SD 1.34) for Raven Progressive Matrices score and −0.062 (SE 0.027, p = 0.02) for Memory Power score. [The results were not adjusted for socioeconomic indicators; it is not clear whether children with low exposure were recruited from the same area as the high exposure group.]
Manju et al. (Citation2017) studied 20 school children of age 10–14 years from each of two villages in Karnataka, India, with high (90 μg/L) and low (“negligible”) level of As in drinking water. IQ assessment was done using the Raven’s Standard Progressive Matrices. The mean IQ tests score in the control group and study group was 30.55 and 17.95, respectively (p < 0.001). [No adjustment was made for any potential confounder. It is unclear whether the As level reported referred to the study subjects or were ecologic results.]
Studies in children – GRADE evaluation
Studies in children show a lack of evidence of an effect of As exposure from drinking water on neurobehavioral outcomes. The initial quality of the evidence was scored low because it was based only on cross-sectional studies. Among the factors reducing the level of evidence, risk of bias was considered moderate, inconsistency was not detected, indirectness, and imprecision were considered absent, and publication bias could not be assessed. Among the factors increasing the level, there were no large effects nor positive results on dose-response, while any residual confounding would have likely generated a spurious effect if such effect is absent. The final quality of evidence for the lack of an association between water As exposure and neurobehavioral effects (mainly cognitive function) is therefore considered of moderate quality.
Discussion
Overall, our assessment of available literature showed a lack of evidence for a causal association between exposure to As in drinking water and neurobehavioral effects.
Our systematic review identified 22 studies, which reported results on neurobehavioral effects in subjects exposed to As in drinking water. All studies were cross-sectional, and measured the prevalence of the outcomes of interest. In most studies, the assessment of exposure to As in drinking water and that of prevalence of neurobehavioral effects were conducted at the same point in time. Since it is possible that As concentrations have decreased over time, especially in highly exposed populations, the lack of a time lag between measurement of exposure and outcome likely results, in cross-sectional studies of adults, in a bias away from the null, since effects possibly associated with exposure to high levels of As in drinking water, are attributed to the currently measured exposure levels, which are lower than the ones relevant for the etiology of the conditions.
In only a few studies, exposure assessment preceded outcome assessment considerably since study subjects were members of prospective cohorts whose exposure was measured at baseline. In none of the available studies was there an explicit statement that outcome was assessed blindly with respect to the As exposure status of subjects. This possible source of bias is particularly relevant in studies comparing subjects living in two or more areas with different exposure levels, since the allocation of study subjects was likely to be known to the investigators measuring the outcomes. A further limitation of the available studies is the availability of a single measure of exposure, typically a single tap water sample. In this case, however, the resulting bias is likely to be toward the null, since the resulting misclassification is likely to operate non-differentially with respect to outcome.
The studies varied greatly in terms of protection from bias. To address this heterogeneity, we classified the studies in tiers, to account for possible effects of selection, information, and reporting bias. We performed an adjustment for multiple comparisons of the reported results, albeit crudely based on Bonferroni method, because of the large number of tests performed in many studies, in the absence of a strategy for prioritization.
Six studies were conducted in adults, of which two (Liu et al. Citation2017; Karim et al. Citation2019), were classified in Tier I. Both studies were conducted in highly exposed populations, and were broadly consistent in reporting an association between exposure to As and decline in cognitive function, measured with MMSE. Although a formal quantitative comparison cannot be made, they provided no evidence of an association for exposure below approximately 75 μg/L. However, since these were cross sectional studies, it is possible that if the exposures were, indeed, causing the decline in cognition function, the levels causing the effect were those occurring in the past and likely higher than the reported values.
The other four studies of adults (Gong et al. Citation2011; O’Bryant et al. Citation2011; Edwards, Hall, et al. Citation2014; Edwards, Johnson, et al. Citation2014) studied two US populations with low exposures. These cross-sectional studies used ecological assessment of As exposure, and were characterized by a large number of tested associations. These studies reported associations between exposure to As and various outcomes, some of which with p < 0.05; however, only few of the associations remained valid after our adjustment for multiple comparisons. Results of Gong et al. (Citation2011), were not adjusted for any potential confounder. Overall, the studies in low-exposed populations did not provide evidence of an association with the different measures of cognitive ability.
The 16 studies in children varied greatly in terms of protection from bias; we tried to address this heterogeneity by classifying the studies in tiers. Of the studies of children classified in Tier I, one (Wasserman et al. Citation2004) reported results showing an association between measured level of As in drinking water (medial level, 118 µg/L) and intellectual function. None of the remaining Tier I studies (Von Ehrenstein et al. Citation2007; Wasserman et al. Citation2007; Parvez et al. Citation2011; Rodrigues et al. Citation2016) resulted in an association between drinking water As concentration and neurobehavioral indicators, after adjustment for potential confounders and multiple comparisons. Of note, the studies by Wasserman et al. (Citation2004, Citation2007) were conducted in the same population using the same instruments, although the former included older children than the latter (average age 10.0 vs. 6.1).
Two of the children studies classified in Tier II (Rocha-Amador et al. Citation2007; Nahar, Inaoka, Fujimura, Watanabe, et al. Citation2014) reported an association between drinking water As concentration and IQ. In addition, Tsai et al. (Citation2003) reported results showing inconsistent associations between As concentration and other neurobehavioral tests, mainly assessing attention. In none of these studies, however, sources of bias were adequately controlled. In three additional studies classified in Tier II (Khan et al. Citation2011; Khan et al. Citation2012; Wasserman et al. Citation2014), the results were mainly negative. Another study classified in Tier II (Asadullah and Chaudhury Citation2011) considered two outcomes, but only one of which was found to be associated with water As concentration.
Four studies of children (Wang et al. Citation2007; Abbas et al. Citation2012; Ghosh et al. Citation2017; Manju et al. Citation2017) were classified in Tier III. The results of three of them (Wang et al. Citation2007; Ghosh et al. Citation2017; Manju et al. Citation2017) reported associations between As concentration and various neurobehavioral indicators, while the results of Abbas et al. (Citation2012) were not easily interpretable. Because of important methodological limitations, including lack of control for possible confounding factors, these studies do not contribute to the overall assessment of the evidence.
Our conclusions do not match those of some previous reviews of the association between As and neurobehavioral effects. Brinkel et al. (Citation2009), McClintock et al. (Citation2012), Rodríguez-Barranco et al. (Citation2013), Khan et al. (Citation2020), and Heng et al. (Citation2022) concluded that As exposure might be associated with neurological effects with particular reference to developmental disabilities and cognitive deficits among children, while evidence on behavioral effects was deemed less clear (Rodríguez-Barranco et al. Citation2013). In our opinion, these previous reviews did not pay sufficient attention to protection from bias, including weaknesses of cross-sectional design, effect of adjustment for confounders, and statistical significance arising from multiple comparisons in the underlying studies. None of the high-quality studies reported associations with neurobehavioral outcomes in populations exposed to concentrations lower than 100 µg/L.
Heng et al. (Citation2022) indicated that the period of exposure could be relevant, with post-natal exposure to As being associated with worse children’s neurodevelopment, while findings were mixed for pre-natal exposure to As. This is in contrast with a previous publication of Tsuji et al. (Citation2015), who concluded that the overall evidence did not show a causal dose-response relationship at low exposure. Tsuji et al. (Citation2015) specifically reviewed the evidence on the association between pre- and post-natal exposure to low-level of As (defined as As concentration < 100 µg/L in drinking water or equivalent biomarker level), and cognitive, behavioral, or motor/sensory function in children. These authors noted that a number of studies failed to adjust for some potentially relevant confounders, such as nutritional deficiencies, maternal IQ, and exposure to other neurotoxicants.
In previous meta-analyses, inverse associations of As were reported with IQ scores (Dong and Su Citation2009; Rodríguez-Barranco et al. Citation2013; Hasanvand et al. Citation2020) and positive or conflicting associations with autism spectrum disorder (Saghazadeh and Rezaei Citation2017; Wang et al. Citation2019; Shiani et al. Citation2023) and Alzheimer dementia (Li et al. Citation2023). The limitations discussed above, including weaknesses related to the cross-sectional design and other biases, residual confounding, and multiple comparisons were not adequately considered in these meta-analyses. In addition, previous systematic reviews and meta-analyses mostly relied on studies assessing As exposure in biological samples, such as blood, serum, hair, and urine, rather than drinking water. Measures of As in biological samples do not reflect only As ingested through drinking water, they also depend on other source of intake and their performance will depend on the context (e.g. acute and chronic exposure) (Rivera-Núñez et al. Citation2010; Choi et al. Citation2022). In addition, the organism’s capacity to metabolize As might differ between individuals, perhaps modifying As concentration in biological samples and thus individual susceptibility even for exposure to the same levels of As in drinking water (Kobayashi and Agusa Citation2019).
To our knowledge, our study is the first attempt to comprehensively assess the association between exposure to As in drinking water specifically, and broadly defined neurobehavioral effects, among both children and adults. Two previous meta-analyses that focused on exposure to As in drinking water (Rodríguez-Barranco et al. Citation2013; Hasanvand et al. Citation2020) considered only IQ scores.
Although the degree and detail of adjustment for potential confounders varied between studies, it is important to notice that in most cases such adjustments weakened the positive associations between As exposure and various outcomes, suggesting that some degree of residual confounding may be present. This is particularly important in low-income populations, in which poor conditions, such as low socioeconomic status, parental education, malnutrition, and BMI are associated with low cognitive function (Von Stumm and Plomin Citation2015; Eilertsen et al. Citation2016) and may also be associated with As level in drinking water (Rocha-Amador et al. Citation2007; Nahar, Inaoka, Fujimura Citation2014; Tsuji et al. Citation2015). An additional factor which should be considered is physical exercise, which has been associated with cognitive function (Tomporowski and Pesce Citation2019; Moore et al. Citation2022). In addition, a decrease in cognitive function over time (“negative Flynn effect”) has been observed in several populations (Dutton et al. Citation2016; Graves et al. Citation2021).
The comprehensive search strategy and rigorous assessment of available evidence carried out in this study in order to evaluate whether there is a causal relationship between As in drinking water and neurobehavioral effects, allowed us to identify published studies from a large number of records initially retrieved. Unfortunately, the heterogeneity in outcome and exposure definitions, as well as statistical methods employed by the primary studies included in this review, prevented us from performing a quantitative assessment of the association between As in drinking water and neurobehavioral effects through a meta-analysis. This heterogeneity also prevented us from assessing the occurrence of publication bias, which may have led to overestimate of the associations in systematic reviews and meta-analyses (Higgins et al. Citation2019). The restriction of our review to reports written in English might have led to the exclusion of relevant studies reported in other languages. However, there is evidence suggesting that this issue may be limited and, more importantly, it may not lead to overestimation of investigated associations (Morrison et al. Citation2012; Dechartres et al. Citation2018)
In summary, the available studies show lack of evidence for an association between exposure to drinking water As and neurobehavioral effects (mainly cognitive function) in children, both in high- and low-exposure populations. Evidence in adults is more limited than in children, and shows no effects at low levels of exposure. Caution should be used to generalize results of studies conducted in highly exposed populations, because of the possible role of confounders and effect modifiers, which are specific of such populations. Given the limitations of the studies included in our review, however, further studies prospectively measuring concentration of As in drinking water during the life course, and neurobehavioral outcomes, and appropriately controlling for potential confounders, with particular reference to socioeconomic status, nutritional deficiencies, and co-exposures with other contaminants, are needed to clarify this potential association.
| Abbreviation list | ||
| As | = | arsenic |
| BC | = | body coordination |
| BDNF | = | brain-derived neurotrophic factor |
| BMI | = | body mass index |
| BSID-III | = | Bayley Scales of Infant and Toddler Development–Third Edition (BSID-III) |
| CBCL-TRF | = | Child Behavior Checklist-Teacher’s Report Form |
| CI | = | confidence interval |
| CLOX | = | Clock Drawing Task |
| COWAT | = | Controlled Oral Word Association Test |
| EXIT25 | = | Executive Interview |
| FMC | = | fine manual control |
| GIS | = | geographic information systems |
| IARC | = | International Agency for Research on Cancer |
| IQ | = | intelligence quotient |
| MC | = | manual coordination |
| MMSE | = | Mini-Mental State Examination |
| OR | = | odds ratio |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RBANS | = | Repeatable Battery for the Assessment of Neuropsychological Status |
| SA | = | strength and agility |
| SC | = | social competence |
| SD | = | standard deviation |
| SE | = | standard error |
| TMC | = | total motor composite score |
| TMT | = | Trails Making Test |
| VR | = | Visual Reproduction |
| WISC-III | = | Wechsler Intelligence Scale for Children, version III |
| WISC-IV | = | Wechsler Intelligence Scale for Children, version IV |
| WMS-III | = | Wechsler Memory Scale–Third Edition |
Acknowledgments
The authors thank Dr. Michal Eldan (Arsenic Science Task Force), who reviewed an early draft of the manuscript and provided non-binding comments. The authors gratefully acknowledge the comments of the Editor and the external reviewers selected by the Editor who were anonymous to the authors.
Declaration of interest
Dr. Paolo Boffetta has received financial support from the Arsenic Science Task Force, managed by B&C Consortia Management, for the preparation of this manuscript. The Arsenic Science Task Force is an organization of companies and trade associations that fund research and analyses to inform ongoing scientific and regulatory assessments of As. The article was prepared following an invitation from the Task Force to the authors, who have been active in independent research on health effects of As. The responsibility for the preparation and content of this manuscript rests only with the authors, and the conclusions and interpretations expressed are entirely those of the authors and not of any institution or commercial entity. The authors have not participated in and do not anticipate participation in any legal, regulatory, or advocacy proceedings related to the contents of the article.
Data availability
The work was performed on publicly available data retrieved from the studies included in the review and which are available upon request from the authors.
References
- Abbas S, Ahmad Qure EM, Ahmad F, Vehra S, Khan AU. 2012. An assessment of relationship between arsenic in drinking water, health status and intellectual functioning of children in district Kasur. Pakistan J of Nutrition. 11(2):150–153. doi: 10.3923/pjn.2012.150.153.
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H, Levy D, Van Geen A, Howe G, Graziano J. 2006. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 16(2):191–205. doi: 10.1038/sj.jea.7500449.
- Asadullah MN, Chaudhury N. 2011. Poisoning the mind: arsenic contamination of drinking water wells and children’s educational achievement in rural Bangladesh. Econ Educ Rev. 30(5):873–888. doi: 10.1016/j.econedurev.2011.05.001.
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. 2011. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015.
- Brinkel J, Khan MH, Kraemer A. 2009. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health. 6(5):1609–1619. doi: 10.3390/ijerph6051609.
- Bruininks R, Bruininks B. 2005. Bruininks-Oseretsky test of motor proficiency. 2nd ed. Minneapolis (MN): NCS Pearson.
- Boffetta P, Borron C. 2019. Low-level exposure to arsenic in drinking water and risk of lung and bladder cancer: a systematic review and dose–response meta-analysis. Dose-Response. 17(3):1559325819863634. doi: 10.1177/1559325819863634.
- Choi JW, Song YC, Cheong NY, Lee K, Kim S, Lee KM, Ji K, Shin MY, Kim S. 2022. Concentrations of blood and urinary arsenic species and their characteristics in general Korean population. Environ Res. 214(Pt 2):113846. doi: 10.1016/j.envres.2022.113846.
- Committee on the Review of Clinical Guidance for the Care of Health Conditions Identified by the Camp Lejeune Legislation; Board on the Health of Select Populations; Institute of Medicine. 2015. Characterization of neurobehavioral effects. Review of VA clinical guidance for the health conditions identified by the camp lejeune legislation. Washington (DC): National Academies Press (US); p. 35–50.
- Dechartres A, Atal I, Riveros C, Meerpohl J, Philippe R. 2018. Association between publication characteristics and treatment effect estimates a meta-epidemiologic study. Ann Intern Med. 169(6):385–393. doi: 10.7326/M18-1517.
- Desai G, Barg G, Vahter M, Queirolo EI, Peregalli F, Mañay N, Millen AE, Yu J, Kordas K. 2020. Executive functions in school children from Montevideo, Uruguay and their associations with concurrent low-level arsenic exposure. Environ Int. 142:105883. doi: 10.1016/j.envint.2020.105883.
- Dong J, Su SY. 2009. The association between arsenic and children’s intelligence: a meta-analysis. Biol Trace Elem Res. 129(1–3):88–93. doi: 10.1007/s12011-008-8298-1.
- Dutton E, Van der Linden D, Lynn R. 2016. The negative Flynn effect: a systematic literature review. Intelligence. 59:163–169. doi: 10.1016/j.intell.2016.10.002.
- Edwards M, Hall J, Gong G, O’Bryant SE. 2014. Arsenic exposure, AS3MT polymorphism, and neuropsychological functioning among rural dwelling adults and elders: a cross-sectional study. Environ Health. 13(1):15. doi: 10.1186/1476-069X-13-15.
- Edwards M, Johnson L, Mauer C, Barber R, Hall J, O’Bryant S. 2014. Regional specific groundwater arsenic levels and neuropsychological functioning: a cross-sectional study. Int J Environ Health Res. 24(6):546–557. doi: 10.1080/09603123.2014.883591.
- Eilertsen T, Thorsen AL, Holm SEH, Bøe T, Sørensen L, Lundervold AJ. 2016. Parental socioeconomic status and child intellectual functioning in a Norwegian sample. Scand J Psychol. 57(5):399–405. doi: 10.1111/sjop.12324.
- Fatoki JO, Badmus JA. 2022. Arsenic as an environmental and human health antagonist: a review of its toxicity and disease initiation. J Hazard Mater Adv. 5:100052. doi: 10.1016/j.hazadv.2022.100052.
- Ghosh SB, Chakraborty D, Mondal NK. 2017. Effect of arsenic and manganese exposure on intellectual function of children in arsenic stress area of Purbasthali, Burdwan, West Bengal. Expo Health. 9(1):1–11. doi: 10.1007/s12403-016-0216-8.
- Gong G, Hargrave KA, Hobson V, Spallholz J, Boylan M, Lefforge D, O’Bryant SE. 2011. Low-level groundwater arsenic exposure impacts cognition: a project FRONTIER study. J Environ Health. 74(2):16–22.
- Graves LV, Drozdick L, Courville T, Farrer TJ, Gilbert PE, Delis DC. 2021. Cohort differences on the CVLT-II and CVLT3: evidence of a negative Flynn effect on the attention/working memory and learning trials. Clin Neuropsychol. 35(3):615–632. doi: 10.1080/13854046.2019.1699605.
- Hasanvand M, Mohammadi R, Khoshnamvand N, Jafari A, Palangi HS, Mokhayeri Y. 2020. Dose-response meta-analysis of arsenic exposure in drinking water and intelligence quotient. J Environ Health Sci Eng. 18(2):1691–1697. doi: 10.1007/s40201-020-00570-0.
- Heng YY, Asad I, Coleman B, Menard L, Benki-Nugent S, Were FH, Karr CJ, McHenry MS. 2022. Heavy metals and neurodevelopment of children in low and middle-income countries: a systematic review. PLoS One. 17(3):e0265536. doi: 10.1371/journal.pone.0265536.
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. 2019. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: John Wiley & Sons.
- International Agency for Research on Cancer. 2012. Arsenic, metals, fibres, and dusts. IARC monographs on the evaluation of carcinogenic risks to humans volume 100C. Lyon: IARC.
- Karim Y, Siddique AE, Hossen F, Rahman M, Mondal V, Banna HU, Hasibuzzaman MM, Hosen Z, Islam MS, Sarker MK, et al. 2019. Dose-dependent relationships between chronic arsenic exposure and cognitive impairment and serum brain-derived neurotrophic factor. Environ Int. 131:105029. doi: 10.1016/j.envint.2019.105029.
- Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, van Geen A, et al. 2011. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect. 119(10):1501–1506. doi: 10.1289/ehp.1003397.
- Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, van Geen A, Graziano JH, et al. 2012. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology. 33(1):91–97. doi: 10.1016/j.neuro.2011.12.002.
- Khan KM, Chakraborty R, Bundschuh J, Bhattacharya P, Parvez F. 2020. Health effects of arsenic exposure in Latin America: an overview of the past eight years of research. Sci Total Environ. 710:136071. doi: 10.1016/j.scitotenv.2019.136071.
- Kobayashi Y, Agusa T. 2019. Arsenic metabolism and toxicity in humans and animals: racial and species differences. Arsen contam Asia. Singapore: Springer; p. 13–28.
- Khosravi-Darani K, Rehman Y, Katsoyiannis IA, Kokkinos E, Zouboulis AI. 2022. Arsenic exposure via contaminated water and food sources. Water. 14(12):1884. doi: 10.3390/w14121884.
- Li K, Li A, Mei Y, Zhao J, Zhou Q, Li Y, Yang M, Xu Q. 2023. Trace elements and Alzheimer dementia in population-based studies: a bibliometric and meta-analysis. Environ Pollut. 318:120782. doi: 10.1016/j.envpol.2022.120782.
- Liu J, Gao Y, Liu H, Sun J, Liu Y, Wu J, Li D, Sun D. 2017. Assessment of relationship on excess arsenic intake from drinking water and cognitive impairment in adults and elders in arsenicosis areas. Int J Hyg Environ Health. 220(2 Pt B):424–430. doi: 10.1016/j.ijheh.2016.12.004.
- Manju R, Hegde AM, Parlees P, Keshan A. 2017. Environmental arsenic contamination and its effect on intelligence quotient of school children in a historic gold mining area Hutti, North Karnataka, India: a pilot study. J Neurosci Rural Pract. 8(3):364–367.
- McClintock TR, Chen Y, Bundschuh J, Oliver JT, Navoni J, Olmos V, Lepori EV, Ahsan H, Parvez F. 2012. Arsenic exposure in Latin America: biomarkers, risk assessments and related health effects. Sci Total Environ. 429:76–91. doi: 10.1016/j.scitotenv.2011.08.051.
- Mochizuki H. 2019. Arsenic neurotoxicity in humans. Int J Mol Sci. 20(14):3418. doi: 10.3390/ijms20143418.
- Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, Rahman M, Sohel N, D'Ippoliti D, Wade TJ, et al. 2017. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol. 46(6):1924–1939. doi: 10.1093/ije/dyx202.
- Moore D, Jung M, Hillman CH, Kang M, Loprinzi PD. 2022. Interrelationships between exercise, functional connectivity, and cognition among healthy adults: a systematic review. Psychophysiology. 59(6):e14014. doi: 10.1111/psyp.14014.
- Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. 2012. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 28(2):138–144. doi: 10.1017/S0266462312000086.
- Nahar MN, Inaoka T, Fujimura M, Watanabe C, Shimizu H, Tasmin S, Sultana N. 2014. Arsenic contamination in groundwater and its effects on adolescent intelligence and social competence in Bangladesh with special reference to daily drinking/cooking water intake. Environ Health Prev Med. 19(2):151–158. doi: 10.1007/s12199-013-0369-z.
- Nahar MN, Inaoka T, Fujimura M. 2014. A consecutive study on arsenic exposure and intelligence quotient (IQ) of children in Bangladesh. Environ Health Prev Med. 19(3):194–199. doi: 10.1007/s12199-013-0374-2.
- O'Bryant SE, Edwards M, Menon CV, Gong G, Barber R. 2011. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: a project FRONTIER study. Int J Environ Res Public Health. 8(3):861–874. doi: 10.3390/ijerph8030861.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. doi: 10.1136/bmj.n71.
- Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, et al. 2011. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 119(11):1665–1670. doi: 10.1289/ehp.1103548.
- Podgorski J, Berg M. 2020. Global threat of arsenic in groundwater. Science. 368(6493):845–850. doi: 10.1126/science.aba1510.
- Rivera-Núñez Z, Meliker JR, Linder AM, Nriagu JO. 2010. Reliability of spot urine samples in assessing arsenic exposure. Int J Hyg Environ Health. 213(4):259–264. doi: 10.1016/j.ijheh.2010.03.003.
- Rocha-Amador D, Navarro ME, Carrizales L, Morales R, Calderón J. 2007. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad Saúde Pública. 23(4):S579–S587. doi: 10.1590/S0102-311X2007001600018.
- Rodrigues EG, Bellinger DC, Valeri L, Hasan MOSI, Quamruzzaman Q, Golam M, Kile ML, Christiani DC, Wright RO, Mazumdar M. 2016. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health. 15(1):44. doi: 10.1186/s12940-016-0127-y.
- Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, Rojas-García A. 2013. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 454–455:562–577. doi: 10.1016/j.scitotenv.2013.03.047.
- Saghazadeh A, Rezaei N. 2017. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog Neuropsychopharmacol Biol Psychiatry. 79(Pt B):340–368. doi: 10.1016/j.pnpbp.2017.07.011.
- Sanchez TR, Perzanowski M, Graziano JH. 2016. Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: a systematic review. Environ Res. 147:537–555. doi: 10.1016/j.envres.2016.02.009.
- Sanchez TR, Powers M, Perzanowski M, George CM, Graziano JH, Navas-Acien A. 2018. A meta-analysis of arsenic exposure and lung function: is there evidence of restrictive or obstructive lung disease? Curr Environ Health Rep. 5(2):244–254. doi: 10.1007/s40572-018-0192-1.
- Shiani A, Sharafi K, Omer AK, Kiani A, Karamimatin B, Massahi T, Ebrahimzadeh G. 2023. A systematic literature review on the association between exposures to toxic elements and an autism spectrum disorder. Sci Total Environ. 857(Pt 2):159246. doi: 10.1016/j.scitotenv.2022.159246.
- Tolins M, Ruchirawat M, Landrigan P. 2014. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann. Glob. Health. 80(4):303–314. doi: 10.1016/j.aogh.2014.09.005.
- Tomporowski PD, Pesce C. 2019. Exercise, sports, and performance arts benefit cognition via a common process. Psychol Bull. 145(9):929–951. doi: 10.1037/bul0000200.
- Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. 2003. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology. 24(4–5):747–753. doi: 10.1016/S0161-813X(03)00029-9.
- Tsuji JS, Garry MR, Perez V, Chang ET. 2015. Low-level arsenic exposure and developmental neurotoxicity in children: a systematic review and risk assessment. Toxicology. 337:91–107. doi: 10.1016/j.tox.2015.09.002.
- von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A, Hira-Smith M, Ghosh N, Lahiri S, Haque R, et al. 2007. Children’s intellectual function in relation to arsenic exposure. Epidemiology. 18(1):44–51. doi: 10.1097/01.ede.0000248900.65613.a9.
- Von Stumm S, Plomin R. 2015. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence. 48:30–36. doi: 10.1016/j.intell.2014.10.002.
- Wang M, Hossain F, Sulaiman R, Ren X. 2019. Exposure to inorganic arsenic and lead and autism spectrum disorder in children: a systematic review and meta-analysis. Chem Res Toxicol. 32(10):1904–1919. doi: 10.1021/acs.chemrestox.9b00134.
- Wang SX, Wang ZH, Cheng XT, Li J, Sang ZP, Zhang XD, Han LL, Qiao XY, Wu ZM, Wang ZQ. 2007. Arsenic and fluoride exposure in drinking water: children’s IQ and growth in Shanyin Country, Shanxi Province, China. Environ Health Perspect. 115(4):643–647. doi: 10.1289/ehp.9270.
- Wang W, Xie Z, Lin Y, Zhang D. 2014. Association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis. J Epidemiol Community Health. 68(2):176–184. doi: 10.1136/jech-2013-203114.
- Wasserman GA, Liu X, LoIacono NJ, Kline J, Factor-Litvak P, Van Geen A, Mey JL, Levy D, Abramson R, Schwartz A, et al. 2014. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ Health. 13(1):23. doi: 10.1186/1476-069X-13-23.
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Hussain I, et al. 2004. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 112(13):1329–1333. doi: 10.1289/ehp.6964.
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Levy D, et al. 2007. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Perspect. 115(2):285–289. doi: 10.1289/ehp.9501.
- World Health Organization. 2022. Guidelines for drinking-water quality: fourth edition incorporating the first and second addenda. Geneva: WHO.
Appendix
Table A1. Preferred reporting Items for systematic reviews and meta-analyses (PRISMA) checklist.
Table A2. List of studies selected for review.
Table A3. Outcome categories included in the studies retained in the systematic review.
Categories
1. Global cognitive functioning
2. Attention
3. Executive functioning
4. Language
5. Visuospatial functioning
6. Memory
7. Behavior
8. Motor skills